Interplay between CO Disproportionation and Oxidation:On the Origin of the CO Reaction Onset on Atomic Layer Deposition-GrownPt/ZrO2 Model Catalysts
Verena Pramhaas
Matteo Roiaz
Noemi Bosio
Manuel Corva
Christoph Rameshan
Erik Vesselli
Henrik Grönbeck
Günther Rupprechter
Email:guenther.rupprechter@tuwien.ac.at.
Received 2020 Sep 10; Revised 2020 Nov 4; Issue date 2021 Jan 1.
This is an open access article published under a Creative Commons Attribution (CC-BY)License, which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited.
Abstract
Pt/ZrO2 model catalysts were prepared by atomic layerdeposition (ALD) and examined at mbar pressure byoperando sum frequency generation (SFG) spectroscopy and near-ambient pressureX-ray photoelectron spectroscopy (NAP-XPS) combined with differentiallypumped mass spectrometry (MS). ALD enables creating model systemsranging from Pt nanoparticles to bulk-like thin films. Polarization-dependentSFG of CO adsorption reveals both the adsorption configuration andthe Pt particle morphology. By combining experimental data withab initio density functional theory (DFT) calculations,we show that the CO reaction onset is determined by a delicate balancebetween CO disproportionation (Boudouard reaction) and oxidation.CO disproportionation occurs on low-coordinated Pt sites, but onlyat high CO coverages and when the remaining C atom is stabilized bya favorable coordination. Thus, under the current conditions, initialCO oxidation is found to be strongly influenced by the removal ofcarbon deposits formed through disproportionation mechanisms ratherthan being determined by the CO and oxygen inherent activity. Accordingly,at variance with the general expectation, rough Pt nanoparticles areseemingly less active than smoother Pt films. The applied approachenables bridging both the “materials and pressure gaps”.
Keywords: Pt nanoparticles, catalysis, insitu spectroscopy, operando, SFG, NAP-XPS, DFT
The adsorption and catalyticoxidation of CO on Pt are among the most frequently examined surfaceprocesses due to their environmental and industrial relevance. Ptexhibits superior catalytic properties for various applications, suchas (preferential) CO oxidation for emission control or cleaning ofhydrogen streams for fuel cells.1−5 Despite efforts to replace expensive Pt by cheaper materials, itsactivity can typically not be matched. Thus, the focus is rather onreducing the Pt amount, e.g., by using Pt atoms, clusters, and smallnanoparticles6−13 (or alloys and core–shell structures14,15) on suitable support materials. It is, however, still challengingto obtain detailed knowledge about increasingly smaller nanoparticles,especially about their inherent activity and metal/support interaction.16−21
In recent years, significant advances have been made in modelcatalysis,enabling surface characterization at (near) atmospheric pressure,overcoming the “pressure gap”,22−31 but bridging the “materials gap” is evenly important.Previous single-crystal studies have provided fundamental insight,but they cannot fully mimic nanoparticles25,32,33 (with support effects being apparently inaccessible),which is why more realistic model systems are required, such as oxide-supportednanoparticles/islands11,16,25,34 or inverse systems.35−37
In thiscontribution, we present Pt/ZrO2 model catalystsprepared by atomic layer deposition (ALD) that were examined at mbarpressures byoperando sum frequency generation (SFG)spectroscopy and near-ambient pressure X-ray photoelectron spectroscopy(NAP-XPS), with simultaneous mass spectrometry (MS) product analysis,and complemented by density functional theory (DFT) calculations.
ALD has been widely used in industrial manufacturing,38,39 especially for dielectrics and microelectronics, and is receivingincreasing attention for (upscalable) catalyst preparation.40 The current model catalysts consist of a zirconiafilm, ALD-grown (400 cycles) on a Si (100) wafer, and Pt depositsprepared by different numbers of ALD cycles (10–250; see transmissionelectron microscopy (TEM) images inFigure1
Figure 1.

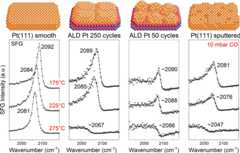
(a) Cross-sectional TEM micrographs of 250 cycle and 50 cycle Ptfilms. The 250 cycle film consists of large uniform and planar islands(∼10 nm in thickness), whereas the 50 cycle film is made upof individual Pt particles (size of about 8 nm). (b) SFG spectra intwo different polarization combinations (ppp and ssp) of adsorbedCO (10 mbar at 150 °C); the surface morphology of islands/particles(see models) can be assessed by comparing the ppp to ssp intensity(ppp (ssp) has a higher intensity if the C=O bond is parallel(tilted) to the macroscopic surface normal).
The standard cleaning used for single crystals in ultrahigh vacuum(UHV), i.e., sputtering/annealing, could not be applied as it wouldhave destroyed the ALD samples. Inspired by the (re-)activation oftechnological catalysts, all samples were thus cleaned from carbonaceousresidues by heating in 10 mbar O2 to 400 °C and in20 mbar CO/O2 (1:1) to 300 °C.
The Pt morphologywas then addressed by polarization-dependentSFG of CO adsorption (10 mbar CO at 150 °C;Figure1b; see theSupporting Information for SFG theory and fit values). Thespectra show the on-top CO resonance region, as no other binding geometrieswere observed. Two polarization combinations were employed: ppp (hasits maximum intensity for C=O bonds parallel to the macroscopicsurface normal; black inFigure1b) and ssp (has its maximum intensity for C=Obonds inclined with respect to the macroscopic surface normal, i.e.,around 30–40° depending on molecular polarizability; redinFigure1b).44,45 Due to the angular dependence, the resulting intensity ratioIppp/Issp for COis expected to decrease with increasing bond inclination of the molecules.46,47 Because the Pt film of the 250 cycle sample consisted of planarislands with a uniform height of about 10 nm, adsorbed CO was mostlyperpendicular to the ZrO2/Si(100) surface so that the COpeak intensity was high in ppp and very low in ssp (ratio of 17.4).In light of our previous study of CO/Pt(111),47 assuming an identical optical interface model, this would correspondto an average CO tilt angle of ∼5° (relative to the macroscopicsurface normal), although this value is just meant to show a trend.In contrast, the 50 ALD cycle Pt film consisted of small particles(about 8 nm) with multiple facets, many of which are no longer parallelto the substrate. On these inclined facets, CO still adsorbs perpendicularly,but the CO bonds are inclined with respect to the macroscopic surfacenormal. Accordingly, the SFG intensity is lower in ppp, resultingin a much lowerIppp/Issp ratio of 2.3. Following the sameassumptions as above, the average CO tilt angle would be ∼40°(note that this again is just to show the trend). This rough estimateagrees with the facet inclination and ratio from TEM images.
Apart from the intensity, the peak position and peak symmetry/asymmetryare noteworthy. The peak position depends on the coordination of thePt adsorption site and the CO surface coverage (inducing chemicaland dipole–dipole interactions).27,48,49 As the coverage should be (nearly) the same underidentical pressure and temperature conditions, the 3 cm–1 difference points to slightly rougher surfaces for the nanoparticlesample. The (a)symmetry of an SFG signal depends on the amplitudesAr orAnr and phasedifference ϕ between resonant (adsorbed CO) and nonresonantsignal contributions50−52 (see theSupporting Information). The nonresonant backgroundAnr mayoriginate from Pt surface defects (changing the electron localizationat the surface) and electronic contributions of the ZrO2/Si(100) substrate. Comparing the spectra of the 250 and 50 cyclesamples, the intensities of the resonant and nonresonant contributionsare much more similar in the case of the particulate film (smallerparticle size, more defects, and more metal/oxide interface), whilethe resonance phase relative to the nonresonant background, as obtainedby means of the quantitative deconvolution of the data, is found tobe similar for both CO-Pt systems. This leads to a more asymmetricline shape for the 50 cycle Pt, directly reflecting its surface morphology.
In order to further characterize the ALD-prepared model catalysts,they were compared to Pt single crystals at different temperatures.Figure2 shows the SFG pppspectra of smooth (UHV annealed to 800 °C) Pt(111), 250 and 50ALD cycle Pt/ZrO2, and sputtered Pt(111) in 10 mbar CO.Respective fitting values are given in theSupporting Information (our system has a spectral accuracy of 2 cm–1). At 175 °C, the characteristic on-top CO onPt(111) was observed at 2092 cm–1, matching thesaturation coverage.49,53 The 250 cycle sample exhibiteda continuous Pt surface (Figure1 andFigures S2 and S3),but was still rougher than the annealed Pt(111), as indicated by theredshifted wavenumber (2089 cm–1) and increasedpeak asymmetry. Adsorbed CO on the 50 cycle Pt/ZrO2 sample,consisting of 8 nm (connected) particles, exhibited a similar wavenumber,indicating identical coordination, but lower intensity and higherasymmetry due to the inclined facets. The higher number of low-coordinated(step/kink) sites54,55 on the ALD samples was confirmedby comparison with the CO spectra of sputtered Pt(111) (2081 cm–1) and Pt(110) (2075 cm–1) (Figure S9), showing even lower wavenumbers.53,56 Apparently, the ALD Pt catalysts exhibit roughness intermediatebetween the annealed and sputtered Pt(111).
Figure 2.
SFG spectra (ppp polarization)displaying on-top CO on differentPt surfaces, acquired in 10 mbar CO at the indicated temperatures.The surface roughness increases from left to right, as indicated bythe decreasing intensity and redshift of resonance positions. Theindicated values were obtained from data fits (solid lines). For roughsurfaces, spectra at 275 °C showed a diminishing on-top CO, whichwas irreversible upon cooldown (Figure S10). This cannot be explained by a decrease in CO coverage, as forPt(111), the spectrum remained almost unchanged.
Now, turning to the CO adsorption at higher temperatures (225/275°C), the decreased CO coverage induced a redshift.24,48,49,53 For Pt(111), the on-top CO signal redshifted, but the intensitywas similar to that at 175 °C. Analogously, the CO signals ofthe ALD samples and sputtered Pt(111) exhibited small redshifts at225 °C (and a minor intensity loss). However, at 275 °C,the rougher surfaces showed a pronounced intensity loss, peak shift,and phase alteration. Previously, a similar observation on polycrystallinePt foil57 was explained by CO desorption,but CO is more strongly bonded to steps/defects than terraces.58,59 Furthermore, the spectral changes were irreversible upon cooldownin CO (seeFigure S10) and ϕ changedsignificantly, ruling out simple adsorption/desorption and rathersuggesting a permanent modification/blocking of the adsorption sites.It has been reported that stepped Pt surfaces or small Pt particles/clustersmay cause CO dissociation, forming a carbon overlayer,60 in line with the current observation. Indeed,the CO spectrum of the 250 cycle Pt at 10 mbar/225 °C agreesquite well with the reported values of Pt(557) at 30 mbar/250 °C,which showed CO dissociation at 275 °C. The dissociation hypothesisis supported by the fact that adding O2 to CO and heatingreversed the spectral change by reoxidizing carbon to CO2 (Figure S11). Nevertheless, CO dissociationon Pt has been controversially discussed for a long time.
Thismotivated the DFT calculations of CO dissociation on smoothand rough Pt surfaces (Figure3). CO dissociation on Pt(111) and Pt(211) is strongly endothermicand barriers >3 eV have been reported in the low coverage limit,61 making this process improbable. For a more faciledissociation, the adsorbed state of CO should be destabilized, whereasthe final state needs to be stabilized. A destabilization of the adsorbedstate is achieved by increasing the coverage (gas pressure), whereasthe final state is stabilized by CO2 formation. In particular,direct CO2 formation according to the Boudouard reaction(CO* + CO* → CO2 + C*) hinders the backward C–Oassociation reaction. Similar contributions (local coverage and finalstate) to favoring the Boudouard reaction have been reported for PtSn61 and Cu.62,63 For efficient CO–COcoupling and reaction, the C weight of the 2π* orbital on onereacting molecule should overlap with the O weight on the other molecule.This may be accomplished on stepped and kinked surfaces, so we consideredtwo model structures: a Pt adatom coordinated with two CO moleculeson Pt(100) and Pt(410). The barriers were evaluated at coverages obtainedfrom a thermodynamic analysis (Figures S14 and S15). The barriers for the reactions are 1.8 and 2.1 eV fordissociation at the adatom and Pt(410), respectively. For the reactionat the adatom (Figure3b–d), one of the CO molecules on the Pt adatom is reactingwith a CO on the (100) facet. The transition state is a bent O–C–Ostructure (Figure3c), whereas the final state (Figure3d) is gas-phase CO2 and the remaining carbonatom is in a highly coordinated position. A fourfold coordinated Con Pt(100) is (per carbon atom) as stable as graphite, thus stabilizingthe final state.64 The reaction path onPt(410) is different as both reacting CO molecules are below the step(Figure3e–g).The 2π*–2π* match is, in this case, enabled byan initial bending of the CO close to the step, and the final stateagain has a fourfold coordinated C atom. Accordingly, on the rougherPt surfaces, CO dissociation is facilitated by a barrier that is lowerthan 1 eV as compared to the smooth surfaces.
Figure 3.
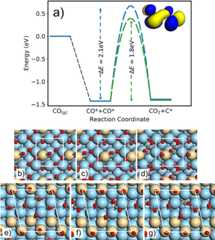
(a) Potential energydiagram for CO disproportionation over anadatom on Pt(100) (green) and Pt(410) (blue). The dissociation isevaluated at the CO coverage given by a thermodynamic analysis, andthe zero level is the saturation coverage minus one CO molecule andone CO molecule in the gas phase. The inset shows the HOMO orbitalfor (CO–CO)2–. Panels (b)–(d) and(e)–(g) show the initial, transition, and final states fordissociation at an adatom on Pt(100) and Pt(410), respectively. Atomiccolor code: C (dark gray), O (red), Pt adatom or top layer (beige),and Pt (light blue).
Turning to CO oxidationon the Pt/ZrO2 model catalysts,Figure4a,b shows the SFGppp spectra acquired in a reaction mixture of 10 mbar CO and 20 mbarO2 from 150 to 500 °C. At 150 °C, the Pt particleswere on-top CO-covered (poisoned) and thus inactive, with 2091 cm–1 indicating high-coordination sites for the 250 cyclesample. The 50 cycle sample showed a 10 cm–1 redshift,indicating lower coordination/higher roughness, with lower intensityand more asymmetry, due to the nanoparticle morphology. Upon temperatureincrease, the peak of adsorbed CO decreased and redshifted (due todecreasing CO coverage) and finally disappeared when the Pt surfaceswere fully oxygen-covered and thus active: at 400 °C for thePt thin film, but at 450 °C for the Pt nanoparticles. The temperature-dependentshifts were 2091–2080 cm–1 for the smootherPt film and 2081–2074 cm–1 for the rougherPt nanoparticles, the latter wavenumbers indicating stronger bondingon the rougher surfaces. The ignition temperatures were corroboratedby the simultaneously acquired CO2-MS traces inFigure4c,d and are in accordancewith values reported in the literature31,65 (note thatMS measurements without simultaneous SFG, “laser-off”,ruled out any laser-induced effects;Figure S16). As long as adsorbed CO is present, the oxidation reaction is largelyinhibited, as the Pt surface can only be in a single stable state,either CO-poisoned (inactive) or O-covered (active).66 Accordingly, both SFG and MS indicated that about 50 °Chigher temperature was required for ignition on the rougher (50 cycle)Pt nanoparticles. This is unexpected, because rougher Pt surfacesare generally considered to be more active in CO oxidation,9,66 as their low-coordinated sites (steps, kinks, and edges) bind bothoxygen and CO stronger than terraces. The O-covered active state canbe more easily established on rougher surfaces (indicated by the lowerignition temperature and higher CO tolerance) despite the higher COoxidation barriers67 (according to theBrønsted–Evans–Polanyi relation68). However, in the current case, the inherent activity isnot the only important factor, as shown in the following.
Figure 4.

(a, b) SFGspectra (ppp polarization) acquired in 10 mbar CO +20 mbar O2 (batch mode) for the 250 and 50 cycle ALD Ptsamples. The spectra redshift at higher temperatures due to reducedCO coverage until the surfaces fully switch to oxygen coverage. (c,d) Derivatives of the mass spectrometry data of CO and CO2. For the rougher 50 cycle film, both the disappearance of CO inSFG and the onset of CO2 production are shifted to highertemperature. (e, f) Temperature-dependent evolution of adsorbed COand carbon deduced from C 1s NAP-XPS (1 mbar CO, 2 mbar O2; flow mode).
To further examine the reactiononset, NAP-XPS was applied as thesecondoperando technique (which again rules outlaser-induced effects). Due to technical limitations, the pressurewas limited to 1 mbar CO and 2 mbar O2. The C 1s spectraacquired during CO oxidation (Figure S17) detected graphitic carbon (284.7 eV),69 adsorbed CO (286.2 eV),59,70 a weak shoulder (around288 eV; likely carbonate on zirconia), and gas-phase CO (∼291eV).Figure4e,f displaysthe fitted peak areas vs (increasing) temperature for adsorbed COand carbon. In analogy to SFG, for smoother (250 cycles) Pt films,CO fully disappears at a temperature 50 °C lower than for rougher(50 cycles) Pt nanoparticles (the absolute temperatures are lowerdue to the 10-fold lower pressure).
However, NAP-XPS showedthat much more carbon was present duringthe reaction on the 50 cycle (rough) Pt nanoparticles, which evenincreased during the first two temperature steps, clearly indicatingCO disproportionation (Figure4f). Atomic carbon apparently poisons the (low-coordinated)active sites for oxygen activation until it is removed by oxygen athigher temperatures. This effect explains the higher reaction onsettemperature of the Pt nanoparticles despite their presumably moreactive surface. In contrast, the smoother 250 cycle Pt film was muchless affected by C poisoning, yielding a lower reaction onset temperature.After ignition, both Pt surfaces were O-covered and showed the expectedhysteresis upon lowering the temperature (Figure S19). CO readsorbed at a comparably lower temperature paralleledby less coking (due to cooling in an oxygen-containing atmosphere).Throughout all experiments, theoperando Pt 4f spectrarevealed that Pt remained metallic (Figure S17).
In summary, combining the ALD model catalyst preparation,operando SFG/NAP-XPS/MS spectroscopy, and DFT calculationsenabled us to build another bridge across the “materials andpressure gap”. Few ALD Pt deposition cycles produced Pt nanoparticleswith multiple inclined facets, whereas more deposition cycles (≥125)led to more uniform Pt films. The polarization-dependent SFG revealedthe molecular orientation of CO (relative to the macroscopic surfacenormal) and thus both the morphology and roughness of different ALD-grownPt model catalysts. Upon CO adsorption at mbar pressure around 275°C, Pt(111) did not show CO disproportionation, whereas rougherPt particles/films and sputtered Pt(111) did. According to the DFTcalculations, direct CO dissociation is unfeasible even at steppedPt surfaces. Dissociation instead occurs at high coverages via a disproportionationreaction at low-coordinated sites that structurally promote CO–COcoupling and stabilize the remaining C atom. The effect of surfaceroughness on the CO oxidation was monitored at mbar pressure and elevatedtemperature by correlating theoperando SFG and NAP-XPSspectra with the MS reactivity data. Different from the general expectation,the reaction onset temperature was higher for the smaller/rougherPt nanoparticles than for the smooth Pt surfaces. The rougher surfaceswere poisoned by carbon coking, detected by NAP-XPS, and explainedby DFT calculations via CO disproportionation on favorable sites.Only after the removal of the carbon deposits did the rough Pt surfacesbecome active, but at 50 °C higher temperature than for smoothPt. Upon cooldown, the smooth Pt films exhibited a wider hysteresiswindow and were hardly affected by CO disproportionation. Future studiesof ALD Pt particles and films on different support materials shouldreveal whether reducible supports facilitate activation at lower temperatureand reduce/suppress the initial carbon poisoning.
Acknowledgments
G.R. acknowledges funding by the Austrian ScienceFund (FWF; projects DK+ Solids4Fun W1243, SFB FOXSI F4502-N16 andSingle Atom Catalysis I4434-N) and TU Wien (IP 2008 “SFG Spectroscopy”).H.G. acknowledges financial support from the Swedish Research Council(2016-05234). The calculations were performed at C3SE (Göteborg)through an SNIC grant. We are grateful to Ole Bethge, Emmerich Bertagnolli(ZNMS) and Stefan Löffler (USTEM) of TU Wien for the help withALD deposition and TEM imaging, respectively. We thank MAX IV forproviding beamtime (20180016) and Andrey Shavorskiy (MAX IV), ThomasHaunold, and Raffael Rameshan (both TU Wien) for the assistance.
Glossary
Abbreviations
- ALD
atomic layer deposition
- DFT
density functional theory
- HOMO
highest occupiedmolecular orbital
- MS
mass spectrometry
- NAP-XPS
near-ambient pressure X-ray photoelectron spectroscopy
- SFG
sum frequency generation
- TEM
transmission electronmicroscopy
- UHV
ultrahighvacuum
Supporting Information Available
The Supporting Information isavailable free of charge athttps://pubs.acs.org/doi/10.1021/acscatal.0c03974.
Model catalysts;operando methods;measurements of CO adsorption and dissociation; DFT calculations;measurements of CO oxidation (PDF)
Author Present Address
⊥ Lloyd’sRegister EMEA, Galleria Arrigo Protti 1, 34121 Trieste,Italy (M.R.).
Author Present Address
# Chair ofAnalytical Chemistry II, Ruhr-University Bochum, 44801Bochum, Germany (M.C.).
Author Contributions
SFG measurementswere performed by V.P., M.R., M.C., and E.V. and NAP-XPS was conductedby V.P. and C.R. DFT calculations were carried out by N.B and H.G.G.R. acquired funding and coordinated the experimental work. Finalinterpretation and manuscript preparation were led by V.P. and G.R.,with contributions from all authors.
The authors declarenocompeting financial interest.
Supplementary Material
References
- Somorjai G. A.; Li Y., Introduction to surface chemistryand catalysis; John Wiley & Sons: 2010. [Google Scholar]
- Ertl G.; Freund H.-J.Catalysis and SurfaceScience. Phys. Today1999, 52, 32–38. 10.1063/1.882569. [DOI] [Google Scholar]
- Carrette L.; Friedrich K. A.; Stimming U.Fuel Cells: Principles, Types, Fuels,and Applications. ChemPhysChem2000, 1, 162–193. . [DOI] [PubMed] [Google Scholar]
- Liu K.; Wang A.; Zhang T.Recent Advancesin Preferential Oxidationof CO Reaction over Platinum Group Metal Catalysts. ACS Catal.2012, 2, 1165–1178. 10.1021/cs200418w. [DOI] [Google Scholar]
- vanSpronsen M. A.; Frenken J. W. M.; Groot I. M. N.Surface scienceunder reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev.2017, 46, 4347–4374. 10.1039/C7CS00045F. [DOI] [PubMed] [Google Scholar]
- Liu L.; Corma A.Metal Catalysts forHeterogeneous Catalysis: From Single Atoms toNanoclusters and Nanoparticles. Chem. Rev.2018, 118, 4981–5079. 10.1021/acs.chemrev.7b00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.; Li J.; Zhang T.Heterogeneoussingle-atom catalysis. Nat. Rev. Chem.2018, 2, 65–81. 10.1038/s41570-018-0010-1. [DOI] [Google Scholar]
- Qiao B.; Wang A.; Yang X.; Allard L. F.; Jiang Z.; Cui Y.; Liu J.; Li J.; Zhang T.Single-atom catalysisof CO oxidation using Pt1/FeOx. Nat. Chem.2011, 3, 634–641. 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- Gotterbarm K.; Späth F.; Bauer U.; Bronnbauer C.; Steinrück H.-P.; Papp C.Reactivity of Graphene-SupportedPt Nanocluster Arrays. ACS Catal.2015, 5, 2397–2403. 10.1021/acscatal.5b00245. [DOI] [Google Scholar]
- Podda N.; Corva M.; Mohamed F.; Feng Z.; Dri C.; Dvorák F.; Matolin V.; Comelli G.; Peressi M.; Vesselli E.Experimental and Theoretical Investigationof the RestructuringProcess Induced by CO at Near Ambient Pressure: Pt Nanoclusters onGraphene/Ir(111). ACS Nano2017, 11, 1041–1053. 10.1021/acsnano.6b07876. [DOI] [PubMed] [Google Scholar]
- Bruix A.; Lykhach Y.; Matolínová I.; Neitzel A.; Skála T.; Tsud N.; Vorokhta M.; Stetsovych V.; Ševčíková K.; Mysliveček J.; Fiala R.; Václavå M.; Prince K. C.; Bruyère S.; Potin V.; Illas F.; Matolín V.; Libuda J.; Neyman K. M.Maximum Noble-Metal Efficiency inCatalytic Materials: Atomically Dispersed Surface Platinum. Angew. Chem., Int. Ed.2014, 53, 10525–10530. 10.1002/anie.201402342. [DOI] [PubMed] [Google Scholar]
- Jones J.; Xiong H.; DeLaRiva A. T.; Peterson E. J.; Pham H.; Challa S. R.; Qi G.; Oh S.; Wiebenga M. H.; Pereira Hernández X. I.; Wang Y.; Datye A. K.Thermallystable single-atom platinum-on-ceria catalysts via atom trapping. Science2016, 353, 150. 10.1126/science.aaf8800. [DOI] [PubMed] [Google Scholar]
- Vesselli E.; Peressi M., Chapter 8 - NanoscaleControl of Metal Clusters on Templating Supports.In Studies in Surface Science and Catalysis., Fornasiero P.; Cargnello M., Eds. Elsevier: 2017; Vol. 177, pp. 285–315.
- Liu J.; Lucci F. R.; Yang M.; Lee S.; Marcinkowski M. D.; Therrien A. J.; Williams C. T.; Sykes E. C. H.; Flytzani-Stephanopoulos M.Tackling COPoisoning with Single-Atom Alloy Catalysts. J. Am. Chem. Soc.2016, 138, 6396–6399. 10.1021/jacs.6b03339. [DOI] [PubMed] [Google Scholar]
- Alayoglu S.; Nilekar A. U.; Mavrikakis M.; Eichhorn B.Ru-Pt core-shell nanoparticlesfor preferential oxidation of carbon monoxide in hydrogen. Nat. Mater.2008, 7, 333–338. 10.1038/nmat2156. [DOI] [PubMed] [Google Scholar]
- Campbell C. T.Ultrathinmetal films and particles on oxide surfaces: structural, electronicand chemisorptive properties. Surf. Sci. Rep.1997, 27, 1–111. 10.1016/S0167-5729(96)00011-8. [DOI] [Google Scholar]
- Vayssilov G. N.; Lykhach Y.; Migani A.; Staudt T.; Petrova G. P.; Tsud N.; Skála T.; Bruix A.; Illas F.; Prince K. C.; Matolín V.; Neyman K. M.; Libuda J.Support nanostructureboosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater.2011, 10, 310–315. 10.1038/nmat2976. [DOI] [PubMed] [Google Scholar]
- Suchorski Y.; Kozlov S. M.; Bespalov I.; Datler M.; Vogel D.; Budinska Z.; Neyman K. M.; Rupprechter G.The role ofmetal/oxide interfaces for long-range metal particle activation duringCO oxidation. Nat. Mater.2018, 17, 519–522. 10.1038/s41563-018-0080-y. [DOI] [PubMed] [Google Scholar]
- Pacchioni G.; Freund H.-J.Controlling the charge state of supportednanoparticlesin catalysis: lessons from model systems. Chem.Soc. Rev.2018, 47, 8474–8502. 10.1039/C8CS00152A. [DOI] [PubMed] [Google Scholar]
- Freund H.-J.; Pacchioni G.Oxide ultra-thinfilms on metals: new materials forthe design of supported metal catalysts. Chem.Soc. Rev.2008, 37, 2224–2242. 10.1039/b718768h. [DOI] [PubMed] [Google Scholar]
- An K.; Alayoglu S.; Musselwhite N.; Plamthottam S.; Melaet G.; Lindeman A. E.; Somorjai G. A.EnhancedCO OxidationRates at the Interface of Mesoporous Oxides and Pt Nanoparticles. J. Am. Chem. Soc.2013, 135, 16689–16696. 10.1021/ja4088743. [DOI] [PubMed] [Google Scholar]
- Somorjai G.A.Surfacescience at high pressures. Z. Phys. Chem.1996, 197, 1–19. 10.1524/zpch.1996.197.Part_1_2.001. [DOI] [Google Scholar]
- Baldelli S.; Eppler A. S.; Anderson E.; Shen Y. R.; Somorjai G. A.Surfaceenhanced sum frequency generation of carbon monoxide adsorbed on platinumnanoparticle arrays. J. Chem. Phys.2000, 113, 5432–5438. 10.1063/1.1290024. [DOI] [Google Scholar]
- Rupprechter G.; Dellwig T.; Unterhalt H.; Freund H.-J.CO adsorption onNi(100) and Pt(111) studied by infrared–visible sum frequencygeneration spectroscopy: design and application of an SFG-compatibleUHV–high-pressure reaction cell | SpringerLink. Top. Catal.2001, 15, 19–26. 10.1023/A:1009063611629. [DOI] [Google Scholar]
- Freund H.-J.; Bäumer M.; Libuda J.; Risse T.; Rupprechter G.; Shaikhutdinov S.Preparation and characterization of model catalysts:from ultrahigh vacuum to in situ conditions at the atomic dimension. J. Catal.2003, 216, 223–235. 10.1016/S0021-9517(02)00073-8. [DOI] [Google Scholar]
- Rupprechter G.Sum FrequencyLaser Spectroscopy during Chemical Reactions on Surfaces. MRS Bull.2007, 32, 1031–1037. 10.1557/mrs2007.212. [DOI] [Google Scholar]
- Rupprechter G., SumFrequency Generation and Polarization–Modulation Infrared ReflectionAbsorption Spectroscopy of Functioning Model Catalysts from UltrahighVacuum to Ambient Pressure. In Adv. Catal., Gates B. C.; Knözinger H., Eds. Academic Press: 2007; Vol. 51, pp. 133–263. [Google Scholar]
- Somorjai G. A.; York R. L.; Butcher D.; Park J. Y.The evolution ofmodel catalytic systems; studies of structure, bonding and dynamicsfrom single crystal metal surfaces to nanoparticles, and from lowpressure (<10–3 Torr) to high pressure (>10–3 Torr) to liquid interfaces. Phys. Chem. Chem. Phys.2007, 9, 3500–3513. 10.1039/B618805B. [DOI] [PubMed] [Google Scholar]
- Salmeron M.; Schlögl R.Ambient pressurephotoelectron spectroscopy: A newtool for surface science and nanotechnology. Surf. Sci. Rep.2008, 63, 169–199. 10.1016/j.surfrep.2008.01.001. [DOI] [Google Scholar]
- Rupprechter G.; Weilach C.Spectroscopic studiesof surface-gas interactions andcatalyst restructuring at ambient pressure: mind the gap!. J. Phys.: Condens. Matter2008, 20, 184019. 10.1088/0953-8984/20/18/184019. [DOI] [Google Scholar]
- Gao F.; Wang Y.; Cai Y.; Goodman D. W.CO Oxidation onPt-Group Metals from Ultrahigh Vacuum to Near Atmospheric Pressures.2. Palladium and Platinum. J. Phys. Chem. C2009, 113, 174–181. 10.1021/jp8077985. [DOI] [Google Scholar]
- Jiang T.; Mowbray D. J.; Dobrin S.; Falsig H.; Hvolbæk B.; Bligaard T.; Nørskov J. K.Trends in CO Oxidation Rates forMetal Nanoparticles and Close-Packed, Stepped, and Kinked Surfaces. J. Phys. Chem. C2009, 113, 10548–10553. 10.1021/jp811185g. [DOI] [Google Scholar]
- Sauer J.; Freund H.-J.Models in Catalysis. Catal. Lett.2015, 145, 109–125. 10.1007/s10562-014-1387-1. [DOI] [Google Scholar]
- Anic K.; Wolfbeisser A.; Li H.; Rameshan C.; Föttinger K.; Bernardi J.; Rupprechter G.Surface Spectroscopyon UHV-Grownand Technological Ni–ZrO2 Reforming Catalysts: FromUHV to Operando Conditions. Top. Catal.2016, 59, 1614–1627. 10.1007/s11244-016-0678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek K.; Fuchs M.; Klötzer B.; Reichl W.; Rupprechter G.Studies ofmetal–support interactions with “real” and “inverted”model systems: reactions of CO and small hydrocarbons with hydrogenon noble metals in contact with oxides. Top.Catal.2000, 13, 55–66. 10.1023/A:1009072519733. [DOI] [Google Scholar]
- Palomino R. M.; Gutiérrez R. A.; Liu Z.; Tenney S.; Grinter D. C.; Crumlin E.; Waluyo I.; Ramírez P. J.; Rodriguez J. A.; Senanayake S. D.InverseCatalysts for CO Oxidation:Enhanced Oxide–Metal Interactions in MgO/Au(111), CeO2/Au(111), and TiO2/Au(111). ACSSustainable Chem. Eng.2017, 5, 10783–10791. 10.1021/acssuschemeng.7b02744. [DOI] [Google Scholar]
- Rameshan C.; Li H.; Anic K.; Roiaz M.; Pramhaas V.; Rameshan R.; Blume R.; Hävecker M.; Knudsen J.; Knop-Gericke A.; Rupprechter G.In situ NAP-XPS spectroscopy during methane dry reformingon ZrO2/Pt(1 1 1) inverse model catalyst. J. Phys.: Condens. Matter2018, 30, 264007. 10.1088/1361-648X/aac6ff. [DOI] [PubMed] [Google Scholar]
- Ritala M.; Niinistö J.IndustrialApplications of Atomic Layer Deposition. ECSTrans.2009, 25, 641–652. 10.1149/1.3207651. [DOI] [Google Scholar]
- Kääriäinen T.; Cameron D.; Kääriäinen M. L.; Sherman A., ALDApplications and Industry. In Atomic LayerDeposition: Principles, Characteristics, and Nanotechnology Applications,Second Edition, Scrivener Publishing LLC: 2013; pp. 215–242. [Google Scholar]
- Lu J.; Elam J. W.; Stair P. C.Atomiclayer deposition—Sequentialself-limiting surface reactions for advanced catalyst “bottom-up”synthesis. Surf. Sci. Rep.2016, 71, 410–472. 10.1016/j.surfrep.2016.03.003. [DOI] [Google Scholar]
- Lee H.-B.-R.; Mullings M. N.; Jiang X.; Clemens B. M.; Bent S. F.Nucleation-ControlledGrowth of Nanoparticles by Atomic Layer Deposition. Chem. Mater.2012, 24, 4051–4059. 10.1021/cm3014978. [DOI] [Google Scholar]
- ALD NanoSolutions.https://www.aldnanosolutions.com/.
- Ding K.; Gulec A.; Johnson A. M.; Schweitzer N. M.; Stucky G. D.; Marks L. D.; Stair P. C.Identificationofactive sites in CO oxidation and water-gas shift over supported Ptcatalysts. Science2015, 350, 189. 10.1126/science.aac6368. [DOI] [PubMed] [Google Scholar]
- Wang H.-F.; Gan W.; Lu R.; Rao Y.; Wu B.-H.Quantitative spectraland orientational analysis in surface sum frequency generation vibrationalspectroscopy (SFG-VS). Int. Rev. Phys. Chem.2005, 24, 191–256. 10.1080/01442350500225894. [DOI] [Google Scholar]
- Lu R.; Gan W.; Wu B.-H.; Chen H.; Wang H.-F.Vibrational PolarizationSpectroscopy of CH Stretching Modes of the Methylene Group at theVapor/Liquid Interfaces with Sum Frequency Generation. J. Phys. Chem. B2004, 108, 7297–7306. 10.1021/jp036674o. [DOI] [Google Scholar]
- Galletto P.; Unterhalt H.; Rupprechter G.The molecular orientation of CO onPd(111): a polarization-dependent SFG study. Chem. Phys. Lett.2003, 367, 785–790. 10.1016/S0009-2614(02)01811-0. [DOI] [Google Scholar]
- Li X.; Roiaz M.; Pramhaas V.; Rameshan C.; Rupprechter G.Polarization-DependentSFG Spectroscopy of Near Ambient Pressure CO Adsorption on Pt(111)and Pd(111) Revisited. Top. Catal.2018, 61, 751–762. 10.1007/s11244-018-0949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Yates J. T. Jr.Terrace width effect on adsorbatevibrations- A comparison of Pt(335) and Pt(112) for chemisorptionof CO. Surf. Sci.1995, 327, 193–201. 10.1016/0039-6028(94)00849-3. [DOI] [Google Scholar]
- Tüshaus M.; Schweizer E.; Hollins P.; Bradshaw A. M.Yet anothervibrat1onalstudy of the adsorption system Pt{111}-CO. J.Electron Spectrosc. Relat. Phenom.1987, 44, 305–316. 10.1016/0368-2048(87)87031-7. [DOI] [Google Scholar]
- Shen Y. R.Surfaceproperties probed by second-harmonic and sum-frequency generation. Nature1989, 337, 519–525. 10.1038/337519a0. [DOI] [Google Scholar]
- Morkel M.; Unterhalt H.; Klüner T.; Rupprechter G.; Freund H.-J.Interpreting intensitiesin vibrational sum frequencygeneration (SFG) spectroscopy: CO adsorption on Pd surfaces. Surf. Sci.2005, 586, 146–156. 10.1016/j.susc.2005.05.009. [DOI] [Google Scholar]
- Höbel F.; Bandara A.; Rupprechter G.; Freund H.-J.Deactivation ofPd particles supported on Nb2O5/Cu3Au(100): SFG and TPD studies from UHV to 100mbar. Surf. Sci.2006, 600, 963–970. 10.1016/j.susc.2005.12.039. [DOI] [Google Scholar]
- Rupprechter G.; Dellwig T.; Unterhalt H.; Freund H.-J.High-Pressure CarbonMonoxide Adsorption on Pt(111) Revisited: A Sum Frequency GenerationStudy. J. Phys. Chem. B2001, 105, 3797–3802. 10.1021/jp003585s. [DOI] [Google Scholar]
- Hayden B. E.; Kretzschmar K.; Bradshaw A. M.; Greenler R. G.An infrared studyof the adsorption of CO on a stepped platinum surface. Surf. Sci.1985, 149, 394–406. 10.1016/0039-6028(85)90071-8. [DOI] [Google Scholar]
- Backus E. H. G.; Bonn M.A quantitative comparisonbetween reflection absorptioninfrared and sum-frequency generation spectroscopy. Chem. Phys. Lett.2005, 412, 152–157. 10.1016/j.cplett.2005.06.118. [DOI] [Google Scholar]
- Klünker C.; Balden M.; Lehwald S.; Daum W.CO stretching vibrationson Pt(111) and Pt(110) studied by sum frequency generation. Surf. Sci.1996, 360, 104–111. 10.1016/0039-6028(96)00638-3. [DOI] [Google Scholar]
- Härle H.; Mendel K.; Metka U.; Volpp H. R.; Willms L.; Wolfrum J.Temperature dependence(90–440 K) of the vibrationalspectra of CO adsorbed on platinum(111) studied by sum-frequency generation. Chem. Phys. Lett.1997, 279, 275–281. 10.1016/S0009-2614(97)01040-3. [DOI] [Google Scholar]
- Jørgensen M.; Grönbeck H.Scaling Relationsand Kinetic Monte Carlo SimulationsTo Bridge the Materials Gap in Heterogeneous Catalysis. ACS Catal.2017, 7, 5054–5061. 10.1021/acscatal.7b01194. [DOI] [Google Scholar]
- Tränkenschuh B.; Fritsche N.; Fuhrmann T.; Papp C.; Zhu J. F.; Denecke R.; Steinrück H.-P.A site-selectivein situ study ofCO adsorption and desorption on Pt(355). J.Chem. Phys.2006, 124, 074712 10.1063/1.2168441. [DOI] [PubMed] [Google Scholar]
- McCrea K.; Parker J. S.; Chen P.; Somorjai G.Surface structure sensitivityof high-pressure CO dissociation on Pt(557), Pt(100) and Pt(111) usingsum frequency generation surface vibrational spectroscopy. Surf. Sci.2001, 494, 238–250. 10.1016/S0039-6028(01)01469-8. [DOI] [Google Scholar]
- Vandichel M.; Grönbeck H.A dimer pathfor CO dissociation on PtSn. Catal. Sci. Technol.2019, 9, 695–701. 10.1039/C8CY01989D. [DOI] [Google Scholar]
- Olmos-Asar J. A.; Monachino E.; Dri C.; Peronio A.; Africh C.; Lacovig P.; Comelli G.; Baldereschi A.; Peressi M.; Vesselli E.CO on Supported Cu Nanoclusters:Coverage and Finite Size Contributions to the Formation of Carbidevia the Boudouard Process. ACS Catal.2015, 5, 2719–2726. 10.1021/cs501361h. [DOI] [Google Scholar]
- Ng M. L.; Abild-Pedersen F.; Kaya S.; Mbuga F.; Ogasawara H.; Nilsson A.Low Barrier Carbon Induced CO Dissociation on SteppedCu. Phys. Rev. Lett.2015, 114, 246101. 10.1103/PhysRevLett.114.246101. [DOI] [PubMed] [Google Scholar]
- Trinchero A.; Hellman A.; Grönbeck H.Methane oxidation over Pd and Ptstudied by DFT and kinetic modeling. Surf. Sci.2013, 616, 206–213. 10.1016/j.susc.2013.06.014. [DOI] [Google Scholar]
- Deutschmann O.; Schmidt R.; Behrendt F.; Warnat J.Numerical modelingof catalytic ignition. ymp. (Int.) Combust.1996, 26, 1747–1754. 10.1016/S0082-0784(96)80400-0. [DOI] [Google Scholar]
- Vogel D.; Spiel C.; Suchorski Y.; Trinchero A.; Schlögl R.; Grönbeck H.; Rupprechter G.Local CatalyticIgnition during CO Oxidation on Low-Index Pt and Pd Surfaces: A CombinedPEEM, MS, and DFT Study. Angew. Chem., Int.Ed.2012, 51, 10041–10044. 10.1002/anie.201204031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alonso F. J.; McCarthy D. N.; Nierhoff A.; Hernandez-Fernandez P.; Strebel C.; Stephens I. E. L.; Nielsen J. H.; Chorkendorff I.The Effectof Size on the Oxygen Electroreduction Activity of Mass-Selected PlatinumNanoparticles. Angew. Chem., Int. Ed.2012, 51, 4641–4643. 10.1002/anie.201200586. [DOI] [PubMed] [Google Scholar]
- Jørgensen M.; Grönbeck H.The Site-Assembly Determines CatalyticActivity ofNanoparticles. Angew. Chem., Int. Ed.2018, 57, 5086–5089. 10.1002/anie.201802113. [DOI] [PubMed] [Google Scholar]
- Pazhetnov E. M.; Koshcheev S. V.; Boronin A. I.Formation Mechanismand Structureof Monatomic Carbon Films in Ethylene Decomposition on the Pt(111)Surface According to XPS Data. Kinet. Catal.2003, 44, 414–419. 10.1023/A:1024455204643. [DOI] [Google Scholar]
- Toyoshima R.; Yoshida M.; Monya Y.; Suzuki K.; Amemiya K.; Mase K.; Mun B. S.; Kondoh H.A high-pressure-induceddense CO overlayer on a Pt (111) surface: a chemical analysis usingin situ near ambient pressure XPS. Phys. Chem.Chem. Phys.2014, 16, 23564–23567. 10.1039/C4CP04318A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.