
Can plant litter affect net primary production of a typical steppe in Inner Mongolia?
Jing Wang
Mengli Zhao
Walter D Willms
Guodong Han
Zhongwu Wang
Yongfei Bai
Received 2010 Feb 24; Accepted 2010 Dec 27; Issue date 2011 Apr.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Abstract
Question: Litter (dead leaves or stems) affects production by conserving soil moisture. However, that role is not clear for grasslands where most precipitation falls during the growing season when the demand for water is high. Our question was: Does litter affect forage production in such an environment?
Location: Typical steppe, Inner Mongolia.
Methods: We examined the role of plant litter in two experiments where litter was either removed or added in a protected or heavily grazed site, respectively, in autumn and in spring in a split plot design. The treatments (control, moderate and heavy litter application) were applied once in five replications but repeated at new locations in each of 3 years. This was done to examine only the direct effect of litter on annual net primary production and selected plant characteristics and not potential secondary effects. We also measured soil moisture and soil temperature.
Results: Removing litter caused a reduction in the amount of grass (Leymus chinensis) that was produced, but litter addition caused an inconsistent effect among years, with moderate applications producing the most positive effects. Litter removal resulted in shorter and less dense plants ofL. chinensis andCarex duriuscula, while heavy litter addition in autumn reduced plant height of bothCleistogenes squarrosa andC. duriuscula.
Conclusions: Litter was effective for enhancing soil moisture status and reducing soil heat units in the typical steppe of Inner Mongolia. Therefore, litter mass may serve as an index of grassland health in such environments.
Keywords: Growing degree‐days,Leymus chinensis, Litter addition, Litter removal, Soil moisture,Stipa grandis
Introduction
Of the 3.93 million km2 of grasslands in China, 7 89 000 km2 are found in Inner Mongolia and most of these (90%) are considered to be in various stages of degradation (Jiang et al. 2006), which is generally believed to be caused by overgrazing. Grazing affects the plant community directly through disturbances such as defoliation and trampling and indirectly by selective grazing and litter removal, which can result in a change in species composition, and warmer and drier soils. Heavy grazing pressure has, therefore, led to grassland degradation.
Litter is the dead, above‐ground, plant material that is either attached to the crown or unattached and fragmented. It is an important component of the semi‐arid grassland ecosystem, where it ameliorates the soil temperature and interacts with the hydrological cycle, thereby affecting available soil water for plant growth. Litter can improve soil water by trapping snow (Willms & Chanasyk 2006), enhancing water infiltration and reducing evaporation by acting as an insulating layer on the soil surface (Willms et al. 1993;Dormaar & Carefoot 1996). However, litter also intercepts precipitation and prevents a portion of it from reaching the soil surface (Naeth et al. 1991;Pierson et al. 2002). The semi‐arid grasslands are water‐limited and any effect on soil water will impact other ecosystem processes, including plant growth and competitive interactions.
The shading effect of litter may also affect the production dynamics of the plant community by limiting seedling recruitment (Sydes & Grime 1981) and inhibiting tillering (Weaver & Rowland 1952;Knapp & Seastedt 1986). Litter may also produce warmer soil temperatures in autumn, thereby extending the growing season; conversely, it may impede soil warming in spring (Knapp & Seastedt 1986;Facelli & Pickett 1991). The net effect will depend on the presence of other constraints on the plants' ability to grow.
Litter is an important attribute of grasslands and its presence has been used as a key indicator of grazing effects (Adams et al. 2004). Litter mass was positively associated with above‐ground production (ANPP) in semi‐arid grasslands (Willms et al. 1993;Deutsch et al. 2010) through its effect on enhancing available soil water to the plant. However, its effectiveness depends on the climatic conditions within a year (Willms et al. 1993) and the characteristics of the plant community. The effectiveness of litter mass among communities is inversely correlated with soil water; therefore, it is most effective where water deficits are greatest and least effective where available soil water is high. However, this relationship is likely not linear because too much litter will impede production in the more mesic tallgrass prairie (Knapp & Seastedt 1986). Thus, the effectiveness of litter on ANPP reaches a threshold after which it declines with increasing litter mass.
Production on the typical steppe in Inner Mongolia is water‐limited and could be enhanced with water conservation strategies (Bai et al.2004,2008). However, litter may not be as effective on the typical steppe as it is on the Northern Great Plains because of differences in precipitation distribution. In Inner Mongolia, about 70% of annual precipitation occurs in June, July and August, with only about 4% occurring from December to March. This compares with summer and winter precipitation, for the same months, of 38% and 20%, respectively, of total annual precipitation at two widely separated sites in the mixed prairie in Canada (W.D. Willms, pers. observ.). Therefore, snow capture and retention is relatively less important on the typical steppe than in the mixed prairie, while the coincidence of the majority of precipitation with the growing season ensures that the plant is able to use it with maximum efficiency. These dynamics may contribute to forage production, which is about twice as high on the typical steppe as on the mixed prairie, despite receiving about 18% less precipitation, while ANPP in the mixed prairie may increase by up to 60% along a gradient of increasing litter mass (Willms et al. 1993). Similar observations of litter mass and ANPP are not available for the typical steppe.
Overgrazing on the typical steppe has resulted in the loss of biodiversity, decreased levels of productivity and deteriorated ecological conditions (Bao 2002;Bai et al 2007). The effects of overgrazing are direct, caused by the disruption of plant energy relations, and indirect through modifying the plant environment, which is affected largely by litter reduction. The two factors can produce similar effects in species shifts and productivity thereby making the causes difficult to separate.
We conducted two studies to examine the hypothesis that litter can enhance ANPP in the short term in an environment where most precipitation occurs during the growing season. We address this hypothesis by testing the effects of litter removal or addition on ANPP in an ungrazed and in a previously heavily grazed community, respectively. We also examine the effect of litter on soil moisture and soil temperature in order to explain a possible vegetation response.
Methods
Site description
The study was conducted at the Inner Mongolia Grassland Ecosystem Research Station of the Chinese Academy of Sciences (43°33′12″N‐43°33′35″N, 116°42′26″E‐116°42′31″E) located in the typical steppe of the Inner Mongolia Plateau. The average altitude is 1400 m above sea level. This area belongs to the continental middle temperate semi‐arid zone (Chen 1988). The winters are cold and dry while the summers are warm and wet. The long‐term (1970–2007) mean annual temperature at the study area is 0.4 °C, with mean monthly temperatures ranging from −21.4 °C in January to 19.0 °C in July. Mean annual precipitation is 337 mm. About 80% of annual precipitation occurs between May and August, which coincides with the highest temperatures. The soils are classified as Chestnut sandy loam, while the dominant plant species wereLeymus chinensis (Trin.) Tzvel andStipa grandis P. A. Smirn. Heavy grazing pressure reduced the concentration of soil organic carbon and total soil nitrogen by about 33% compared to an ungrazed control in a nearby (100 km) typical steppe grassland (Q.B. Wang, unpubl. data).
Experimental methods
We examined the role of plant litter (dead leaves or stems) on a typical steppe in Inner Mongolia in a 3‐year experiment, in two separate studies where litter was either removed or added, in a protected or heavily grazed site, respectively. Only the first year effects were observed to ensure that subsequent responses were not the result of shifts in species composition, which might occur when the grass environment is altered.
The litter removal study was conducted in an exclosure that had been protected from grazing since 1983, while the litter addition study was conducted in a heavily grazed site that was adjacent to the exclosure. The latter study was fenced to exclude livestock in the year before the treatments were applied.
The treatments were applied in the spring of 2005 to 2007 and in the autumn of 2002 to 2006. The spring treatments could not be applied in 2003 because of travel restrictions (due to the SARS virus) and in 2004 because personnel could not be recruited to visit the field station. Therefore, in order to balance the experimental units, we focussed only on the vegetation data sampled from 2005 to 2007, representing treatment applications made in autumn 2004 to spring 2007.
In both studies, the effects of litter treatment (control, moderate and heavy) and season of treatment (spring or autumn) were assessed in a split‐plot design, with the main plots (2 × 6 m) represented by the litter treatments, which were arranged in a randomized complete block design (five blocks), and the subplots (2 × 3 m) represented by the season of treatment. Trials utilizing this design were repeated in each of 3 years at contiguous new locations for both studies. For any year of measurement, the autumn in the previous year was paired with the spring treatments to ensure that the effects applied only to the first subsequent growing season. The trials among years were separated by a 1‐m buffer.
The litter treatments were applied when the plants were dormant. For these treatments, litter from the removal experiment was transferred to the addition experiment so that litter from the heavy removal was applied to the heavy addition. This was done to facilitate the treatments but also to apply an amount that represents a normal mass for that community.
Heavy litter removal consisted of raking the fallen (fragmented) and standing (attached to the crown) litter after cutting the latter to near ground level. In the moderate treatment, only the fallen litter was removed by raking. The raking procedure removed most fallen litter that was longer than 2 cm and left most of the mulch. The removed litter was harvested by plot and evenly applied by hand to the assigned plot in the litter addition experiment.
Vegetation and soil sampling
From 2003 to 2007, ANPP was estimated at peak standing crop by clipping plants of each species at ground level in two 20 × 50 cm quadrats centered in each plot. Fallen litter was harvested from each quadrat by raking. The clipped samples were separated by species into current year production (ANPP) and standing litter. The litter was added to the fallen litter in a composite sample of all species. ANPP of each species and litter was bagged, dried at 65 °C for 48 h and weighed. Species composition was determined for each quadrat based on species weight as a proportion of total mass.
We sampled plant height and density ofLeymus chinensis (Trin.) Tzvelev,Cleistogenes squarrosa (Trin.) Keng,Koeleria cristata (L.) Pers.,Stipa grandis P.Smirn, andCarex duriuscula C.A.Meyer. Plant height was estimated by species in each quadrat before they were harvested. This was done by visually estimating average canopy height and measuring to the top using a meter stick. Plant densities were determined by counting the number of plants of each species as the plants were being harvested, and when they could be distinguished more readily.
Surface soil temperatures were sampled in the litter removal trial from each treatment in four replicates using a HOBO® four external channel data logger with temperature sensors (model: TMC20‐HD; Onset Computer Corp., Cape Cod, MA, US). A single sensor was installed in a representative location of each plot at a depth of 2 cm and held in place with a wooden pin. The sensors were installed from May to September in 2004 and 2005. The data logger was programmed to record temperatures every hour. Growing degree‐days (GDD) were calculated for each year using 0 °C base temperature (Frank & Hofmann 1989).
Soil water was sampled at monthly intervals from May to July in 2005, May to September in 2006 and July to September in 2007 from each plot using a hand‐held coring tool (2‐cm diameter). A single core was extracted from each plot near the end of each month in four increments: 0–10 cm, 10–20 cm, 20–30 cm and 30–40 cm. The soil samples were placed in plastic bags and immediately weighed after being taken from the field. They were then air‐dried indoors at room temperature (∼20 °C) for about 2 weeks and then reweighed. During the drying process, the samples were kept in the bag, which was rolled down to expose the samples. Soil water was calculated as a percentage of dry weight.
Statistical analysis
The effect of litter removal on ANPP was tested by selected functional groups and their totals. The functional groups were rhizomatous grass, bunch grass, forb and annual species. Tests were also conducted on the proportion of total grass to total biomass.
The first‐year effects of litter removal or addition on ANPP, proportion of grass, and plant morphological characteristics were tested with analysis of variance for a split‐plot design using a mixed model (Mixed Procedure, SAS, Version 9.1.3, SAS Institute Inc., Cary, NC, US). Litter removal and addition were analysed separately because of their unique site and treatment characteristics. The effect of litter treatment (main plot), season (subplot) and year were treated as fixed effects, with block nested in year. The interaction of block (random effect) with the main effects produced the error terms (Table 1). The data were tested for normality (Shapiro‐Wilk test) and the residuals were plotted and examined for outliers. If necessary, outliers were either removed or the data were adjusted using log transformation (+1); the transformation was usually applied when numerous values were zero. Paired means were compared using a protected LSD0.05. For all analyses of variance, the size effects (η2) were calculated as: SStreat/(SStreat+SSerror).
Table 1.
Components of the statistical model used for analyses of vegetation variables and their degrees of freedom.
| Source of variation | Degrees of freedom |
|---|---|
| Year (Y) | 2 |
| Error a; Replicate (R) × Y | 4 |
| Litter treatment (T) | 2 |
| Y × T | 4 |
| Error b; R × Y × T | 24 |
| Season (S) | 1 |
| Y × S | 2 |
| Error c; R × Y × S | 12 |
| T × S | 2 |
| T × S × Y | 4 |
| Error d; Residual | 32 |
| Total variation | 89 |
Species differences (by weight) amonga priori groups were examined using indicator species analysis with PC‐ORD (Version 5, MjM Software, OR, US). This analysis calculates the proportional abundance of a species in a group relative to its abundance in all groups, and the proportional frequency of the species in each group, which are then combined by multiplication to yield an indicator value. This value is then evaluated with a Monte Carlo method (McCune et al. 2002). For our analysis, we used 4999 randomizations in the Monte Carlo test.
Several comparisons were made using indicator species analysis: (1) between the litter treatment sites (addition or removal) of the control plots only in order to describe site characteristics; and (2) among treatments at each site by year or years combined to determine whether litter removal or addition affected species composition. Replicates were averaged for all analyses.
Summary data of species richness and diversity (Shannon's diversity index;McCune et al. 2002) were calculated for each plot using PC‐ORD. The effects of litter treatment (in both the litter removal and addition study), year of treatment, and season of treatment on these indices were evaluated using a mixed model with analysis of variance similar to that described above. The means were compared using the protected LSD0.05.
The effect of litter removal on soil water was analysed separately for each depth and each year. The data were analysed similar to that described above for a mixed model but month was a repeated measure. In this case, four co‐variance structure matrices (autoregressive, heterogeneous autoregressive, compound symmetry and heterogeneous compound symmetry) were tested with Akaike's information criterion to select the best structure. Treatment means were separated using Fisher's protected LSD0.05.
Results
Experimental conditions
Of the study years, 2005 had the least precipitation either during the growing season or annually, while 2007 had the highest average temperature in both periods (Table 2). Graminoids comprised 69% and 86% of the species by weight in the litter removal and addition sites, respectively. The composition (percentage of total biomass at each site) of species that differed (P<0.05) between the two sites (removal, addition) wasAgropyron cristatum (Linn.) Gaertn (0%, 22%),S. grandis (3%, 14%),K. cristata (7%, 3%),L. chinensis (24%, 3%),Artemisia frigida Willd. (7%, 2%),Kochia prostrata (L.) Schrad. (1%, 0%) andPotentilla bifurca L. (6%, 0%). The composition of other species that were similar (P>0.05) between the two sites wereC. duriuscula (16%, 24%),C. squarrosa (18%, 21%),Allium ramosum L. (2%, 2%),Chenopodium glaucum L. (3%, 3%) andPotentilla acaulis L. (2%, 4%). The only rhizomatous grasses found at the site wereL. chinensis andPoa pratensis L. but the latter was present at less than 1% in the removal site and was not detected in the addition site.
Table 2.
Precipitation and temperatures measured at the Inner Mongolia Grassland Ecosystem Research Station, Chinese Academy of Sciences, Inner Mongolia (43°32′30″N, 116°33′29″E).
| Year | Growing season | Annual | ||||||
|---|---|---|---|---|---|---|---|---|
| Apr | May | Jun | Jul | Aug | Sep | Apr–Sep | ||
| Precipitation (mm) | ||||||||
| 2004 | 6 | 23 | 29 | 50 | 133 | 53 | 294 | 325 |
| 2005 | 2 | 11 | 32 | 58 | 27 | 13 | 143 | 166 |
| 2006 | 5 | 35 | 71 | 55 | 34 | 68 | 268 | 304 |
| 2007 | 2 | 46 | 27 | 52 | 48 | 19 | 194 | 222 |
| 12‐year mean | 9 | 36 | 46 | 83 | 72 | 33 | 280 | 315 |
| Mean temperature (°C) | ||||||||
| 2004 | 6.0 | 11.1 | 18.4 | 18.9 | 16.3 | 11.8 | 13.8 | 1.9 |
| 2005 | 4.4 | 10.5 | 17.8 | 20.1 | 19.2 | 12.6 | 14.1 | 0.7 |
| 2006 | 2.2 | 12.0 | 16.4 | 18.6 | 19.6 | 11.6 | 13.4 | 1.4 |
| 2007 | 3.1 | 11.4 | 20.4 | 20.7 | 18.8 | 13.6 | 14.7 | 2.1 |
| 12‐year mean | 4.3 | 11.7 | 17.4 | 19.9 | 17.9 | 12.1 | 13.9 | 1.4 |
The litter mass in the moderate and heavy removal treatments was about 70% and 44%, respectively, of the control (Fig. 1,P<0.01), and was consistent among years and between spring and autumn treatments. Litter addition contributed about 168% and 241% more litter in the moderate and heavy addition treatments, respectively, than in the control (Fig. 1;P=0.02). While the trends of litter mass within season were the same, the magnitude was different (P<0.05).
Figure 1.
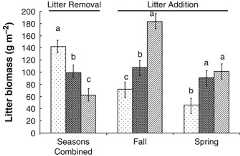
Litter mass following treatment after the first growing season in relation to litter removal or addition ( – control;
– control; – moderate;
– moderate; – heavy) in aLeymus chinensis plant community of Inner Mongolia. Means (±1 SE) having the same letter within a subset of litter removal or addition are not different (P>0.05).
– heavy) in aLeymus chinensis plant community of Inner Mongolia. Means (±1 SE) having the same letter within a subset of litter removal or addition are not different (P>0.05).
Experimental results
Of the functional groups, only grass biomass was affected (P<0.05) by litter removal, while litter addition had no effect (P>0.05) on total biomass or the biomass of grass or forbs (Table 3). Litter removal at either moderate or heavy rates reduced the ANPP of rhizomatous grass by 43% (P<0.05) and 54% (P<0.05), respectively, which was reflected in a reduction of about 30% (P<0.05) in total grass ANPP (Table 3). Annual plants did not exhibit a response to litter removal, although there was a tendency (P=0.08) to increase with moderate removal. The biomass of annual species was about 81% higher (P<0.05) in the spring removal treatments than the autumn removal treatments. The proportion of grass in relation to total biomass decreased (P<0.05) with increased litter removal (Fig. 2).
Table 3.
Means and analysis of variance on the effects, their probability (P) and size (η2) of litter removal or addition in aLeymus chinensis plant community of Inner Mongolia on yields of herbage biomass of major functional groups in relation to season of treatment over 3 years.1Means having a different lower case letter within a subset of a column are different (P>0.05).2Means in paired subsets of columns ([spring+autumn]/2) within a year having a different uppercase letter are different (P>0.05).
| 2005 | 2006 | 2007 | All years | All | Effect | P | η2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | |||||
| Removal | ||||||||||||
| Total biomass | Biomass (g m−2) | |||||||||||
| Control (0) | 105 | 89 | 124 | 164 | 146 | 178 | 125 | 144 | 135 | Year (Y) | <0.01 | 0.57 |
| Moderate | 106 | 89 | 131 | 160 | 160 | 175 | 132 | 142 | 137 | Season (S) | 0.83 | 0.01 |
| Heavy | 87 | 64 | 152 | 145 | 170 | 139 | 136 | 116 | 126 | Treatment (T) | 0.64 | 0.01 |
| se | 21 | 12 | 9 | Y × S | 0.37 | 0.05 | ||||||
| S × T | 0.20 | 0.02 | ||||||||||
| Y × T | 0.90 | 0.04 | ||||||||||
| Total grass | Biomass (g m−2) | |||||||||||
| Control (0) | 79 | 55 | 65 | 66 | 73 | 71 | 72 | 64 | 68 a1 | Year (Y) | 0.71 | 0.11 |
| Moderate | 48 | 45 | 32 | 62 | 66 | 41 | 49 | 49 | 49 b | Season (S) | 0.52 | 0.02 |
| Heavy | 40 | 37 | 46 | 59 | 62 | 37 | 49 | 44 | 47 b | Treatment (T) | <0.01 | 0.25 |
| se | 12 | 7 | 6 | Y × S | 0.14 | 0.16 | ||||||
| S × T | 0.81 | 0.01 | ||||||||||
| Y × T | 0.88 | 0.09 | ||||||||||
| Forbs | Biomass (g m−2) | |||||||||||
| Control (0) | 26 | 35 | 59 | 96 | 74 | 107 | 63 | 79 | 71 | Year (Y) | <0.01 | 0.48 |
| Moderate | 27 | 44 | 99 | 92 | 77 | 118 | 68 | 84 | 76 | Season (S) | 0.37 | 0.02 |
| Heavy | 47 | 24 | 101 | 76 | 108 | 101 | 74 | 67 | 71 | Treatment (T) | 0.88 | 0.05 |
| se | 19 | 11 | 9 | Y × S | 0.56 | 0.01 | ||||||
| S × T | 0.31 | 0.03 | ||||||||||
| Y × T | 0.86 | 0.06 | ||||||||||
| Addition | ||||||||||||
| Total biomass | Biomass (g m−2) | |||||||||||
| Control (0) | 82 | 56 | 134 | 116 | 74 | 60 B | 97 | 77 | 87 | Year (Y) | <0.01 | 0.72 |
| Moderate | 49 | 44 | 109 | 118 | 99 | 94 A | 85 | 85 | 85 | Season (S) | 0.14 | 0.10 |
| Heavy | 68 | 60 | 97 | 108 | 93 | 70 AB | 86 | 79 | 82 | Treatment (T) | 0.84 | 0.02 |
| se | 12 | 8 | 5 | Y × S | 0.51 | 0.06 | ||||||
| S × T | 0.39 | 0.08 | ||||||||||
| Y × T | 0.04 | 0.36 | ||||||||||
| Total grass | Biomass (g m−2) | |||||||||||
| Control (0) | 48 | 36 | 54 | 36 | 60 | 38 B | 54 | 37 | 45 | Year (Y) | <0.01 | 0.34 |
| Moderate | 40 | 37 | 55 | 32 | 78 | 86 A | 58 | 52 | 55 | Season (S) | 0.10 | 0.13 |
| Heavy | 33 | 50 | 59 | 43 | 55 | 56 B | 49 | 50 | 49 | Treatment (T) | 0.25 | 0.06 |
| se | 10 | 5 | 4 | Y × S | 0.19 | 0.04 | ||||||
| S × T | 0.29 | 0.03 | ||||||||||
| Y × T | 0.03 | 0.14 | ||||||||||
| Forbs | Biomass (g m−2) | |||||||||||
| Control (0) | 10 | 20 | 75 | 62 A | 14 | 11 | 33 | 31 | 32 | Year (Y) | <0.01 | 0.82 |
| Moderate | 8 | 7 | 53 | 41 B | 21 | 17 | 28 | 22 | 25 | Season (S) | 0.43 | 0.03 |
| Heavy | 22 | 10 | 38 | 59 B | 24 | 14 | 28 | 28 | 28 | Treatment (T) | 0.25 | 0.11 |
| se | 7 | 4 | 3 | Y × S | 0.81 | 0.01 | ||||||
| S × T | 0.80 | 0.03 | ||||||||||
| Y × T | 0.04 | 0.30 | ||||||||||
Figure 2.
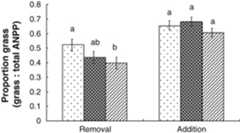
Litter removal or addition ( – control;
– control; – moderate;
– moderate; – heavy) effects on the ratio of above‐ground net primary production (ANPP) of grass relative to total ANPP in aLeymus chinensis community of a typical steppe in Inner Mongolia. Means (±1 SE) having the same letter within the subset of litter removal or addition are not different (P>0.05).
– heavy) effects on the ratio of above‐ground net primary production (ANPP) of grass relative to total ANPP in aLeymus chinensis community of a typical steppe in Inner Mongolia. Means (±1 SE) having the same letter within the subset of litter removal or addition are not different (P>0.05).
The effect of litter addition was expressed for total biomass and total grass with moderate addition only in 2007 (Table 3). Moderate litter addition yielded about 44% more total biomass and 67% more total grass (P<0.05). The biomass of forbs was reduced (P<0.05) by about 30% with either moderate or heavy litter addition only in 2006 (Table 3). The proportion of grass to total biomass was affected (P<0.01) by litter removal but not by litter addition (P>0.05;Fig. 2).
Year contributed the greatest η2 on the ANPP of all functional groups, except for total grass with litter removal (Table 3). In the latter case, litter removal treatments explained the greatest variation while all other effects were not significant (P>0.05).
Species composition differences were not detected (P>0.05) among treatments within either the litter removal or addition study using indicator species analysis when years were pooled (data not shown). However, when analysed by year,L. chinensis was different (P<0.05) among litter removal treatments in 2005 when its' relative abundance in the control, moderate and heavy litter removal treatments was 54%, 31% and 16%, respectively, its relative frequency was 100%, 80% and 70%, respectively, and its' indicator value was 54%, 25% and 11%, respectively. The observed maximum indicator value (53.5%) was higher(P=0.04) than the indicator value from randomized groups (39.0%) as determined by the Monte Carlo test of significance.L. chinensis also differed among litter addition treatments in 2007 when its' relative abundance in the control, moderate and heavy litter addition treatments was 9%, 77%, and 14%, respectively, relative frequency was 50%, 90%, and 20%, respectively, and indicator value was 5%, 69% and 3%, respectively. The observed maximum indicator value (68.9%) was higher (P=0.01) than the indicator value from randomized groups (37.7%), as determined by the Monte Carlo test of significance.
Litter removal had no effect on species richness, evenness or diversity. However, litter addition in spring reduced (P<0.05) both species richness (6 and 5 species m−2, in the control and heavy litter application treatments, respectively; SE=0.58) and diversity (Shannon's diversity index=1.38 and 1.17 in the control and heavy litter application treatments, respectively; SE=0.064) but increased (P<0.05) species diversity if applied in autumn (Shannon's diversity index=1.06 and 1.23 in the control and heavy litter application treatments, respectively; SE=0.064).
Of the grass species evaluated for plant density or height response to litter removal, onlyL. chinensis andC. duriuscula were affected (P<0.05).L. chinensis plant heights were similar (treatment means within a subset having the same letter were not different;P>0.05) among litter removal treatments in 2005 (22a, 26a and 24a cm in the heavy, moderate and control, respectively) and were equal to or greater (P≤0.05) in the control than the litter removal treatments in 2006 and 2007 (31b, 27a and 30ab cm in 2006 and 23a, 21a and 27b cm in 2007, respectively). Plant heights ofC. duriuscula were similar (P>0.05) among treatments in 2005 and 2006 but greater (P<0.05) in the control (10 cm) than the moderate (7 cm) or heavy (7 cm) removal treatments in 2007. Plant density ofL. chinensis was reduced (P<0.05) with autumn litter removal (3.7a, 2.1a and 6.4b plants … 0.1 m−2 in the heavy, moderate and control, respectively) but increased (3.7b, 3.0ab and 1.8a plants … 0.1 m−2, respectively) with spring litter removal.
Litter addition reduced plant height inC. squarrosa andC. duriuscula but this was influenced (P<0.05) by season. Spring addition had no observed effect (P>0.05) but heavy autumn addition resulted in shorter plants (P<0.05) of bothC. squarrosa (17 and 22 cm for heavy and control, respectively) andC. duriuscula (9 and 11 cm for heavy and control, respectively).
The effect of heavy litter addition was also influenced (P>0.05) by season forS. grandis andK. cristata plants. Plant densities ofS. grandis were reduced (P<0.05) with heavy litter applications in autumn (2.5 and 6.9 plants … 0.1 m−2 in the heavy and control, respectively) but spring application had no effect (P>0.05), while the density ofK. cristata plants increased with heavy litter application in autumn (4.5 and 1.9 plants … 0.1 m−2, respectively) but decreased with spring application (2.7 and 5.8 plants … 0.1 m−2, respectively).
Most variation in soil moisture was expressed by the month of sampling (4,5), while litter removal affected soil water content in the surface soil (0–10 cm;Table 4) but not in the next three layers (10–20, 20–30 and 30–40 cm; data not shown). Water content was not affected by litter in 2005, but in 2006 and 2007 litter removal reduced soil water on average over the sampling period and specifically in June and July 2006 (Table 4). The treatment response to litter removal was consistent (P>0.05) among years. Litter addition resulted in higher (P<0.05) soil moisture only in 2007 in May and June (Table 5).
Table 4.
Soil moisture (0 to 10 cm soil depth) response in relation to litter removal treatments as influenced by season (spring or autumn) and sampling month, and their size effects (η2), over 3 years in aLeymus chinensis plant community of Inner Mongolia.1Data not collected.2Means having the same letter within a subset of a column are not different (P>0.05).
| Year | Treatment | Month | Average | Effect | Probability | η2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| May | Jun | Jul | Aug | Sep | ||||||
| Soil moisture (%) | ||||||||||
| 2005 | Heavy | –1 | – | 2.3 | 2.3 | 1.4 | 2.0 | Month (M) | <0.01 | 0.51 |
| Moderate | – | – | 2.3 | 2.9 | 1.3 | 2.2 | Treatment (T) | 0.65 | 0.02 | |
| Control (0) | – | – | 2.4 | 2.9 | 1.2 | 2.2 | M × T | 0.34 | 0.08 | |
| se | – | – | 0.22 | 0.31 | 0.09 | 0.14 | Season (S) | 0.02 | 0.13 | |
| M × S | <0.01 | 0.19 | ||||||||
| T × S | 0.82 | 0.01 | ||||||||
| 2006 | Heavy | 1.1 a2 | 1.1 b | 3.8 ab | 1.6 a | 11.8 a | 3.9 ab | Month (M) | <0.01 | 0.96 |
| Moderate | 0.9 a | 1.3 ab | 3.6 b | 1.6 a | 9.8 b | 3.5 b | Treatment (T) | 0.01 | 0.11 | |
| Control (0) | 0.9 a | 1.6 a | 4.9 a | 1.6 a | 11.4 a | 4.1 a | M × T | 0.02 | 0.24 | |
| se | 0.14 | 0.15 | 0.43 | 0.10 | 0.48 | 0.14 | Season (S) | 0.01 | 0.15 | |
| M × S | <0.01 | 0.15 | ||||||||
| T × S | 0.68 | <0.01 | ||||||||
| 2007 | Heavy | 5.6 | 2.6 | 1.3 | – | – | 3.2 b | Month (M) | <0.01 | 0.90 |
| Moderate | 6.6 | 2.5 | 1.7 | – | – | 3.6 ab | Treatment (T) | 0.04 | 0.16 | |
| Control (0) | 7.0 | 3.0 | 1.7 | – | – | 3.9 a | M × T | 0.07 | 0.11 | |
| se | 0.5 | 0.15 | 0.13 | – | – | 0.20 | Season (S) | 0.18 | 0.08 | |
| M × S | 0.11 | 0.01 | ||||||||
| T × S | 0.43 | 0.05 | ||||||||
Table 5.
Soil moisture (0 to 10 cm soil depth) response in relation to litter addition treatments as influenced by season (spring or autumn) and sampling month, and their size effects (η2), over 3 years in aLeymus chinensis plant community of Inner Mongolia.1Data not collected.2Means having the same letter within a subset of a column are not different (P>0.05).
| Year | Treatment | Month | Average | Effect | Probability | η2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| May | Jun | Jul | Aug | Sep | ||||||
| Soil moisture (%) | ||||||||||
| 2005 | Heavy | –1 | – | 1.6 | 2.7 | 1.4 | 1.9 | Month (M) | <0.01 | 0.32 |
| Moderate | – | – | 1.8 | 2.5 | 2.2 | 2.1 | Treatment (T) | 0.56 | 0.02 | |
| Control (0) | – | – | 1.6 | 3.0 | 1.3 | 2.0 | M × T | 0.39 | 0.09 | |
| se | – | – | 0.14 | 0.25 | 0.09 | 0.17 | Season (S) | 0.13 | 0.06 | |
| M × S | 0.20 | 0.09 | ||||||||
| T × S | 0.42 | 0.03 | ||||||||
| 2006 | Heavy | 1.1 | 1.6 | 3.5 | – | 10.0 | 4.0 | Month (M) | <0.01 | 0.93 |
| Moderate | 1.6 | 2.3 | 4.0 | – | 9.7 | 4.4 | Treatment (T) | 0.18 | 0.03 | |
| Control (0) | 2.1 | 1.8 | 3.8 | – | 9.9 | 4.4 | M × T | 0.51 | 0.05 | |
| se | 0.26 | 0.38 | 0.38 | – | 0.39 | 0.17 | Season (S) | <0.01 | 0.23 | |
| M × S | <0.01 | 0.24 | ||||||||
| T × S | 0.12 | 0.04 | ||||||||
| 2007 | Heavy | 5.4 ab2 | 2.5 ab | 1.2 a | – | – | 3.0 a | Month (M) | <0.01 | 0.93 |
| Moderate | 5.8 a | 2.6 a | 0.9 a | – | – | 3.1 a | Treatment (T) | 0.02 | 0.23 | |
| Control (0) | 4.5 b | 2.2 b | 1.0 a | – | – | 2.6 b | M × T | 0.03 | 0.18 | |
| se | 0.35 | 0.12 | 0.11 | – | – | 0.14 | Season (S) | 0.02 | 0.13 | |
| M × S | 0.25 | 0.01 | ||||||||
| T × S | 0.45 | 0.02 | ||||||||
Litter removal increased (P<0.05) the heat units in the soil (0–2 cm) by about 5% and 11% in 2004 in the moderate and heavy removal treatments, respectively, and by 6% (P<0.05) in the heavy removal treatment in 2005 (Fig. 3). The magnitude of the treatment response was not consistent among years but the trends were consistent. The effects of treatment, year and their interaction were all significant (P<0.05; SE=65).
Figure 3.

The effects of litter removal treatment ( – control;
– control; – moderate;
– moderate; – heavy) on the growing degree‐days (GDD, base 0°C, 0 to 2 cm soil depth) over 2 years from May to September in aLeymus chinensis plant community of Inner Mongolia. Means (±1 SE) having the same letter, or no letter, within a subset of either litter removal or addition are not different (P>0.05).
– heavy) on the growing degree‐days (GDD, base 0°C, 0 to 2 cm soil depth) over 2 years from May to September in aLeymus chinensis plant community of Inner Mongolia. Means (±1 SE) having the same letter, or no letter, within a subset of either litter removal or addition are not different (P>0.05).
Discussion
Litter is an important component on the typical steppe of Inner Mongolia and its removal through overgrazing represents an indirect, but critical, effect that leads to warmer and drier soils and a reduction in the composition of rhizomatous grasses. Thus, it defines one process of degradation, which can result in eventual desertification. However the effects of litter were not observed in every year because of different inter‐annual growing conditions, which explained the greatest variation on the ANPP of most functional groups.
The effect of litter on ANPP is related to its ability to conserve soil moisture by reducing evaporation and ensuring its availability to the plant. This assumes that water is available for conservation and that conserving it will benefit plant production. In our study, a relatively weak response to litter removal or addition indicates that these assumptions were relatively unimportant for total ANPP, possibly because most precipitation occurred during the warmest months of the growing season when water demand by plants was high. Therefore, available soil moisture would have a shortened residence time in the soil and be less vulnerable to evaporation. Furthermore, the lack of winter precipitation also indicates that the potential snow retention properties of litter is irrelevant, which is reflected in the absence of an effect produced by litter removal or addition in autumn or spring.
While the time of soil sampling had an overwhelming influence on soil moisture, nevertheless, soil moisture tended to be reduced in response to litter removal, which was particularly evident in 2006, a year with near average precipitation, and was less evident in 2005 and 2007 when precipitation was considerably below average. The apparent link of litter to moisture effectiveness was also noted byWillms et al. (1993) who reported a significant ANPP response to litter when precipitation was near average but not during a drought or when precipitation was high. Therefore, litter may not be effective when soil moisture is severely deficient or in abundant supply. The latter condition may have occurred in September 2006, a month with relatively high rainfall, lower temperatures and near the end of the growing season, resulting in similar soil moisture among treatments.
The reduction in soil moisture with litter removal is linked to higher soil temperatures and a reduction ofL. chinensis, a moisture‐sensitive species (Wang & Gao 2003). Litter moderates the soil temperature by creating a barrier to heat flow during the day and energy loss at night. Although these values only represent four sampling points per treatment, they support the contention that evaporative losses can be increased with litter removal and, therefore, may affect plant production. GDD has also been associated with plant development (Frank & Hofmann 1989) and can shift the competitive relationships among species.
The rhizomatous grass, consisting almost entirely ofL. chinensis, was the only functional group that responded to litter removal, suggesting a response to soil moisture.L. chinensis favours more mesic conditions (Wang & Gao 2003), declined to less than 50% ANPP and exhibited a reduction in plant height and density when litter was removed in autumn. However, we could not detect a reciprocal recovery ofL. chinensis with litter addition, possibly because of its very small representation in the overgrazed community. Historical heavy grazing pressure is expected to produce a more xeric environment (Wang & Ripley 1997), presumably in response to litter removal (Deutsch et al. 2010).
Our observations forL. chinensis appear to be consistent with the conclusion ofDerner & Briske (2001) that rhizomatous plants in semi‐arid grasslands are more efficient at accumulating nutrients per unit rhizome than bunch grasses. The inconsistent response ofL. chinensis to litter removal among years may be related to its sensitivity to available soil moisture. Precipitation in 2005 was almost 50% of the long‐term average, which may have produced unusual stress on the species and a more distinct response to litter removal. Our inability to detect differences in soil moisture may reflect the greater proportional losses of water through transpiration. This was not the case in 2006, when precipitation was near the long‐term average and excess soil moisture was not utilized until August.
The bunchgrasses, represented in this study primarily byC. squarrosa,S. grandis andK. cristata, are more tolerant of a xeric environment thanL. chinensis (Chen et al. 2005) and might be expected to replace it as litter was removed andL. chinensis declined. However, we could not detect a strong shift in the density of bunchgrasses, which should be expected with plants of this growth form.
Plant height tends to be a more sensitive indicator of environmental change than plant density, but plant height may not readily distinguish between the effects of increased light at the crown or reduced soil moisture, as both can produce shorter plants. Under these conditions, litter addition would be expected to produce taller plants, which was observed forC. squarrosa andC. duriuscula plants with litter application in spring but not in autumn. A possible interpretation may be related to a physical suppression by litter with autumn application, as observed bySmoliak (1965) on the mixed prairie. However, this effect was not observed with litter addition in spring, suggesting the possibility of another mechanism.
The mass of residual litter after grazing provides a good indication of shifts in composition that might be expected despite the direct impact of defoliation. We expect that the greatest response would occur during the first year after treatments, but an additive response is likely with repeated applications of either litter removal or addition. Litter management provides a means for optimizing forage for livestock production and a strategy for adapting to an expected warmer and drier climate (Wang et al. 2003;IPCC 2007). Litter accumulation on a mixed prairie site following 70 years of protection from grazing resulted in an accumulation of almost 3000 kg of litter ha‐1, which was associated with 29% more ANPP than on contiguous grazed sites where litter mass was about 950 kg ha‐1 (Willms et al. 2002). The residual mass of litter after grazing provides an index of grazing pressure and has a direct and indirect effect not only on ANPP but also on the quality of habitat for wildlife and watershed characteristics. Therefore, litter biomass provides crucial support for the production of ecosystem goods and services in this grassland and should be incorporated into an index of health for grazed semi‐arid grasslands, as has been done byAdams et al. (2004).
Conclusion
About 90% of the grassland in Inner Mongolia is degraded to varying stages. Presently, local governments have implemented a policy of ‘forbidden grazing’ in spring, which is directed toward recharging carbohydrate reserves in the plants and maintaining a healthy plant community. However, species shifts and loss of forage production can also occur when insufficient litter is left on the grassland to modify the soil environment and conserve soil moisture for plant growth. This requires that grasslands are managed throughout the year to avoid overgrazing, whether during the growing season or during dormancy.
Acknowledgements
Numerous summer students from the College of Ecology and Environmental Science, Inner Mongolia Agricultural University, assisted with data collection over the study period. Greatly appreciated was the logistical support obtained from staff of the Inner Mongolian Grassland Ecosystem Research Station where the study was conducted. The authors acknowledge funding for the project from the Chinese National Key Fundamental Research and Development Fund (No.2007CB106800), the Chinese National Nature Science Foundation (No.30860196, 31070414), the Chinese National Eleventh Five‐Year Plan Technology Support Project (2008BAD95B03) and the National Key Laboratory of Grassland Science and Range Resources.
Co‐ordinating Editor: Helge Bruelheide
Zhao, M. (corresponding author,menglizhao@yahoo.com);Wang, J. (jing.wang@agr.gc.ca);Han, G. (nmghangd@yahoo.com) &Wang, Z. (zhongwuwang1979@yahoo.com): Department of Grassland Science, College of Ecology and Environment Science, Inner Mongolia Agricultural University, 306 Zhaowuda Road, Hohhot, Inner Mongolia 010018, PR China Willms, W.D. (Walter.Willms@agr.gc.ca): Agriculture and Agri‐Food Canada, Lethbridge Research Centre, 5403‐1st Avenue South, Lethbridge, Alberta, Canada T1J 4B1 Bai, Y. (yfbai@ibcas.ac.cn): State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, PR China
References
- Adams, B.W., Ehlert, G., Stone, C., Alexander, M., Lawrence, D., Willoughby, M., Moisey, D., Hincz, C. & Burkinshaw, A.2004. Range health assessment for grassland, forest and tame pasture. Public Lands and Forests Division, Alberta Sustainable Resource Development, Edmonton, AL, CA. [Google Scholar]
- Bai, Y.F., Han, X.G., Wu, J.G., Chen, Z.Z. & Li, L.H.2004. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature431: 181–184. [DOI] [PubMed] [Google Scholar]
- Bai, Y.F., Wu, J.G., Pan, Q.M., Huang, J.H., Wang, Q.B., Li, F.S., Buyantuyev, A. & Han, X.G.2007. Positive linear relationship between productivity and diversity: evidence from the Eurasian Steppe. Journal of Applied Ecology44: 1023–1034. [Google Scholar]
- Bai, Y.F., Wu, J.G., Xing, Q., Pan, Q.M., Huang, J.H., Yang, D.L. & Han, X.G.2008. Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology89: 2140–2153. [DOI] [PubMed] [Google Scholar]
- Bao, Y.2002. Problems the animal husbandry of the Inner Mongolian Grassland is facing and the way to deal with them. Journal of Inner Mongolia Normal University (Philosophy & Social Science)31: 44–47. [Google Scholar]
- Chen, S.P., Bai, Y.F., Zhang, L.X. & Han, X.G.2005. Comparing physiological responses of two dominant grass species to nitrogen addition in Xilin River Basin of China. Environmental Experimental Botany53: 65–75. [Google Scholar]
- Chen, Z.Z.1988. Topography and climate of Xilin River basin. Research on Grassland Ecosystems3: 13–22. [Google Scholar]
- Derner, J.D. & Briske, D.D.2001. Below‐ground carbon and nitrogen accumulation in perennial grasses: a comparison of caespitose and rhizomatous growth forms. Plant and Soil237: 117–127. [Google Scholar]
- Deutsch, E.S., Bork, E.W. & Willms, W.D.2010. Soil moisture and plant growth responses to litter and defoliation impacts in Parkland grasslands. Agriculture Ecosystems and Environment135: 1–9. [Google Scholar]
- Dormaar, J.F. & Carefoot, J.M.1996. Implications of crop residue management and conservation tillage on soil organic matter. Canadian Journal of Plant Science76: 627–634. [Google Scholar]
- Facelli, J.M. & Pickett, S.T.A.1991. Plant litter: its dynamics and effects on plant community structure. Botanical Review57: 1–32. [Google Scholar]
- Frank, A.B. & Hofmann, L.1989. Relationship among grazing management, growing degree days, and morphological development for native grasses on the Northern Great Plains. Journal of Range Management42: 199–202. [Google Scholar]
- IPCC2007.Climate change 2007, Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK.
- Jiang, G.M., Han, X.G. & Wu, J.G.2006. Restoration and management of the Inner Mongolia grassland require a sustainable strategy. Ambio35: 269–270. [DOI] [PubMed] [Google Scholar]
- Knapp, A.K. & Seastedt, T.R.1986. Detritus accumulation limits productivity of tallgrass prairie. BioScience36: 622–668. [Google Scholar]
- McCune, B., Grace, J.B. & Urban, D.L.2002. Analysis of ecological communities. MJM Press, Gleneden Beach, OR, US. [Google Scholar]
- Naeth, M.A., Bailey, A.W., Chanasyk, D.S. & Pluth, D.J.1991. Water holding capacity of litter and soil organic matter in Mixed Prairie and fescue grassland ecosystems of Alberta. Journal of Range Management44: 13–17. [Google Scholar]
- Pierson, F.B., Spaeth, K.E., Weltz, M.A. & Carson, D.H.2002. Hydraulic response of diverse western rangelands. Journal of Range Management55: 558–570. [Google Scholar]
- Smoliak, S.1965. Effects of manure, straw and inorganic fertilizers on Northern Great Plains ranges. Journal of Range Management18: 11–15. [Google Scholar]
- Sydes, C. & Grime, J.P.1981. Effects of tree leaf litter on herbaceous vegetation in deciduous woodland: II. An experimental investigation. Journal of Ecology69: 249–262. [Google Scholar]
- Wang, R. & Gao, Q.2003. Climate‐driven changes in shoot density and shoot biomass inLeymus chinensis (Poaceae) on the North‐East China Transect (NECT). Global Ecology and Biogeography12: 249–259. [Google Scholar]
- Wang, R. & Ripley, E.A.1997. Effects of grazing on aLeymus chinensis grassland on the Songnen Plain of north‐eastern China. Journal of Arid Environments36: 307–318. [Google Scholar]
- Wang, R., Gao, Q. & Chen, Q.2003. Effects of climatic change on biomass and biomass allocation inLeymus chinensis (Poaceae) along the North‐East China Transect (NECT). Journal of Arid Environments54: 653–665. [Google Scholar]
- Weaver, J.E. & Rowland, N.W.1952. Effect of excessive natural mulch on the development, yield, and structure of a native grassland. Botanical Gazette114: 1–19. [Google Scholar]
- Willms, W.D. & Chanasyk, D.S.2006. Grazing effects on snow accumulation on rough Fescue grasslands. Rangeland Ecology and Management59: 400–405. [Google Scholar]
- Willms, W.D., McGinn, S.M. & Dormaar, J.F.1993. Influence of litter on herbage production in the Mixed Prairie. Journal of Range Management46: 320–324. [Google Scholar]
- Willms, W.D., Dormaar, J.F., Adams, B.W. & Douwes, H.E.2002. Response of the Mixed Prairie to protection from grazing. Journal of Range Management55: 210–216. [Google Scholar]