Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis
Michael M H Yang
Rebecca L Hartley
Alexander A Leung
Paul E Ronksley
Nathalie Jetté
Steven Casha
Jay Riva-Cambrin
Correspondence to Dr Michael M H Yang;minhan.yang@ucalgary.ca
Received 2018 Jul 2; Revised 2019 Feb 8; Accepted 2019 Feb 22; Collection date 2019.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See:http://creativecommons.org/licenses/by-nc/4.0/.
Abstract
Objectives
Inadequate postoperative pain control is common and is associated with poor clinical outcomes. This study aimed to identify preoperative predictors of poor postoperative pain control in adults undergoing inpatient surgery.
Design
Systematic review and meta-analysis
Data sources
MEDLINE, Embase, CINAHL and PsycINFO were searched through October 2017.
Eligibility criteria
Studies in any language were included if they evaluated postoperative pain using a validated instrument in adults (≥18 years) and reported a measure of association between poor postoperative pain control (defined by study authors) and at least one preoperative predictor during the hospital stay.
Data extraction and synthesis
Two reviewers screened articles, extracted data and assessed study quality. Measures of association for each preoperative predictor were pooled using random effects models.
Results
Thirty-three studies representing 53 362 patients were included in this review. Significant preoperative predictors of poor postoperative pain control included younger age (OR 1.18 [95% CI 1.05 to 1.32], number of studies, n=14), female sex (OR 1.29 [95% CI 1.17 to 1.43], n=20), smoking (OR 1.33 [95% CI 1.09 to 1.61], n=9), history of depressive symptoms (OR 1.71 [95% CI 1.32 to 2.22], n=8), history of anxiety symptoms (OR 1.22 [95% CI 1.09 to 1.36], n=10), sleep difficulties (OR 2.32 [95% CI 1.46 to 3.69], n=2), higher body mass index (OR 1.02 [95% CI 1.01 to 1.03], n=2), presence of preoperative pain (OR 1.21 [95% CI 1.10 to 1.32], n=13) and use of preoperative analgesia (OR 1.54 [95% CI 1.18 to 2.03], n=6). Pain catastrophising, American Society of Anesthesiologists status, chronic pain, marital status, socioeconomic status, education, surgical history, preoperative pressure pain tolerance and orthopaedic surgery (vs abdominal surgery) were not associated with increased odds of poor pain control. Study quality was generally high, although appropriate blinding of predictor during outcome ascertainment was often limited.
Conclusions
Nine predictors of poor postoperative pain control were identified. These should be recognised as potentially important factors when developing discipline-specific clinical care pathways to improve pain outcomes and to guide future surgical pain research.
PROSPERO registration number
CRD42017080682.
Keywords: postoperative pain, preoperative predictors, surgery, pain, pain scales, meta-analysis
Strengths and limitations of this study.
This systematic review provides a comprehensive meta-analysis on a large number of preoperative patient prognostic factors for poor acute postoperative pain control.
The inclusion of multiple surgical specialties and articles representing diverse geographical locations increases the generalisability of the findings.
There were a variety of definitions for poor postoperative pain control, timing of pain assessment and thresholds used to categorise continuous preoperative variables making the clinical and statistical interpretation of the meta-analysis more challenging.
For certain preoperative variables, the number of studies included were few and may be underpowered to detect significant differences.
Introduction
Since 1999, when the Joint Commission on Accreditation of Healthcare Organizations set the standard for the appropriate assessment and management of pain, pain has been recognised as the fifth vital sign.1 With the ageing and growing population, the number of surgeries has increased to an excess of 280 million procedures performed globally every year.2–8 Numerous studies suggest poor acute postoperative pain control is common and often inadequately treated.9–12 Importantly, ineffective postoperative pain control is associated with poor outcomes including increased length-of-stay, sleep disturbance, prolonged time to first mobilisation and increased opioid use.11 13 14 Further, poor postoperative pain control is associated with delirium in the elderly, development of chronic pain syndromes, cardiopulmonary and thromboembolic complications.10 11 15–17 Postoperative pain may be improved by understanding the preoperative predictors of poor pain control by allowing the use of anticipatory and individualised treatments.18 19
A previous systematic review reported a limited number of predictors of poor postoperative pain control including age, anxiety, preoperative pain and surgery type.20 However, quantitative analysis was not possible due to variability in the reporting of measures of associations and study design heterogeneity of the included studies. Since its publication was nearly a decade ago, many additional studies have been published with improved methodological rigour;21–24 thus, providing a new opportunity to provide an updated summary of the literature and to generate pooled estimates of risk. The goal of this study was to systematically identify significant preoperative predictors of poorly controlled acute postoperative pain and to quantify the associated risks. We focused on acute postoperative pain experienced during the surgical hospitalisation. This meta-analysis is important to help to identify predictors that could inform future surgical pain research and aid in the development of discipline-specific clinical care pathways (eg, enhanced recovery after surgery programmes) to improve pain outcomes.
Methods
This review was reported according to the Meta-analyses Of Observational Studies in Epidemiology standards for systematic reviews and meta-analyses of observational studies. This review was also conducted based on ana priori protocol registered with PROSPERO International Prospective Register of Systematic Review (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017080682).25–27
Patient and public involvement
Patients and the public were not involved in the development of this systematic review.
Search strategy
A search strategy was developed using thePeer Review of Electronic Search Strategy28 in consultation with two research librarians. We focused on the keywords ‘pain’, ‘pain measurement’, ‘surgery’ and ‘predictors’. We searched MEDLINE (1950 to 13 October 2017), Embase (1980 to 13 October 2017), CINAHL (1937 to 13 October 2017) and PsycINFO (1967 to 13 October 2017) (onlinesupplementary appendix S1, online supplemental information). To maximise sensitivity for studies of prognosis, search filters were not used, and no restrictions were placed on date or language of publication.29 30 Our search was repeated using Google and Google Scholar for the grey literature. Bibliographies of included studies were searched by hand for other relevant articles. A local pain specialist was also consulted to identify any potential ongoing studies or unpublished data.
bmjopen-2018-025091supp001.pdf (72.9KB, pdf)
Study inclusion
We included observational studies (cohort and cross-sectional) reporting on adults (≥18 years old) undergoing surgery and admitted for at least 24 hours following their procedure (eg, excluded ambulatory surgery/procedures, dental procedures, carpal tunnel release, and so on), and studies that assessed for the association between preoperative patient-level predictors and poor postoperative pain control (as defined by individual study authors). Only inpatient procedures were included to minimise the heterogeneity of the surgical population as well as providing more reliable pain outcomes. Perioperative predictors were not assessed because our primary aim was to inform clinicians evaluating patients in the preoperative clinical setting where perioperative risk factors may not be known or modifiable. No interventional studies were included.
Studies were required to report an assessment of pain during the inpatient period using a validated pain scale. Previous studies have demonstrated that the visual analogue scale (VAS), numeric rating scale (NRS) and verbal rating scale (VRS) for pain are highly correlated with each other, and thus, they were considered comparable in the present study.31 To facilitate pooling of data, we only included studies that reported a measure of association, such as an OR or relative risk (RR), as well as studies with raw data where an OR could be manually calculated. Conference abstracts, reviews, protocols and secondary publications (of studies already included in our review) were excluded. Two reviewers (MMHY and RLH) independently reviewed titles, abstracts and full-text articles of the retrieved studies in duplicate. Discrepancies were resolved by consensus. Inter-rater agreement was evaluated using Cohen’s κ statistic for the full-text review stage.
Data extraction
Study information, such as author, year and country of publication, sample size, pain scale used, the definition of poorly controlled postoperative pain, number of predictors adjusted for in a multivariable regression model (where applicable) and the average age of the sample population, were extracted. Both unadjusted and most adjusted effect estimates were recorded whenever multiple estimates were presented. For studies that reported their results in distinct strata (eg, young vs old age, or moderate vs severe pain), each stratum was treated as an independent study for the pooled analysis (no patients were analysed in duplicate).23 32–34 Non-English studies were data-extracted with the help of a translator.
Study quality assessment
We used a component-based approach to assess the quality of included studies.35 The following variables were considered to be the most important quality indicators for studies of prognosis (definition of quality indicators are in onlinesupplementary table S1, online supplemental information)35: description of population, non-biased selection, adequate follow-up (eg, postoperative pain measurements were recorded for at least 80% of study participants), predictor measurement, outcome measurement and ascertainment, adjustment for confounding variables (operationalised as adjusting for at least three potential confounders), precision of reported results (eg, reporting of CIs), as well as the use of an appropriate reference standard (eg, definition of poor postoperative pain control provided).29 35 36 Data extraction and assessment of study quality were performed in duplicate; discrepancies were resolved by consensus. If a study presented unclear data, the corresponding author was emailed with a follow-up email after 2 weeks if a response was not received.
bmjopen-2018-025091supp002.pdf (82.7KB, pdf)
Statistical analysis
We used ORs as the common measure of association. RRs were converted to OR using the formula, OR=RR/(1/[1/(1−Po)]+Po), where Po is the incidence of the outcome of interest in the non-exposed group.37 When raw data were presented, ORs were manually calculated. For the primary analysis, the most adjusted ORs were used to determine the pooled estimates. The analysis was then repeated using the least adjusted effect estimates. Pooled estimates, expressed as ORs (with 95% CIs), were determined for each preoperative predictor associated with poor postoperative pain control levels using the DerSimonian and Laird random effects model and visualised using forest plots. A random effects model was chosen due to the variability in surgical specialties, definitions of poor postoperative pain and the reported timing of postoperative pain assessment in the included studies. Meta-analysis was performed using the ‘metan’ command within STATA V.15. Level of significance was set at alpha=0.05.
Between-study heterogeneity was examined and quantified using the Cochran’s Q test and I2 statistic.38 Stratified analysis and meta-regression were performed to explore for potential sources of heterogeneity based on ana priori list of factors related to study quality and clinical prognosis. Stratification was conducted on the following variables: degree of statistical adjustment (eg, operationalised as adjustment for <3 vs ≥3 variables), definition of poor postoperative pain control (moderate vs severe pain; moderate pain: 3–6, severe pain: >6 on an 11-point scale; studies not using a numeric scale [eg, morphine requirements as the definition for poor pain control] were considered moderate pain), surgical discipline, blinding of predictors when assessing pain scores and location of pain assessment (eg, postanaesthetic care unit vs ward). Preoperative factors only reported in a single study could not be pooled and therefore, were not included in the final analyses. We did not assess for publication bias because conventional tools used to examine for publication bias, such as funnel plots, are intended to detect small study effects. Small study effects are challenging to interpret for meta-analyses of observational studies, such as ours, where multiple sources of heterogeneity may be present, such as those arising from true clinical differences (eg, different surgical disciplines/procedures) or bias inherent to individual studies (eg, residual confounding and lack of blinding).30
Results
Literature search and study characteristics
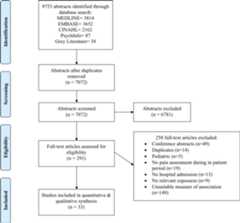
We identified 9753 articles through the electronic database and grey literature search (figure 1). Consultation with a pain expert and searching of the grey literature yielded 38 articles. After initial screening, 291 articles were included for full-text review. Full-text review resulted in the inclusion of 33 articles for data extraction with excellent inter-rater reliability (κ=0.83 [95% CI 0.71 to 0.91]). No unpublished studies were identified and included in the final analysis.
Figure 1.
Systematic review and meta-analysis flow diagram. All database and grey literature search was performed on 13 October 2017.
The 33 included studies represented 53 362 patients with publication dates ranging between 2002 and 2017 (study characteristics of included studies are intable 1).19 21–24 32–34 39–63 Twenty-six studies were prospective cohort studies (79%) and seven were retrospective cohort studies (21%). Most studies were conducted in Europe (17/33 studies, 51.5%), followed by Asia (8/33 studies, 24.2%). Studies involving a mixture of specialties (11/33 studies, 33.3%) and general surgery (10/33 studies, 30.3%) had the largest representation. A variety of thresholds were used to define poor pain control on a standard 11-point scale (0–10) across studies; the most common definition of significant postoperative pain was ≥4 out of 10 (13/33 studies, 39.4%) followed by > or ≥5 out of 10 (7/33 studies, 21.1%). NRS, VAS and VRS scales for pain were used in 57.6%, 42.4% and 3.0% of studies, respectively. The most common time-interval when postoperative pain was measured was between 24–48 hours (19/33 studies, 57.6%). The mean number of predictors (including preoperative and perioperative variables) explored per study was 10.0 (SD 5.73, range 1–19) (table 1). There was a lack of dedicated prognostic studies evaluating predictors of postoperative pain control in most surgical subspecialties including neurosurgery, spine surgery, otolaryngology and plastic surgery.
Table 1.
Study characteristics of included studies
| Author, year | Country of origin | Sample size | Incidence of poor postoperative pain control (%) | Mean age in years (SD) | Study design | Setting of pain assessment | Pain scale* | Definition of poor pain control | Time of assessment† | Specialty | Pathology | No. of predictors examined |
| Alveset al, 2 01339 | Brazil | 139 | Not stated | 51.7 (11.8) | PCS | Ward | VAS | >30 | 24 | GS | Breast cancer | 3 |
| Auburnet al, 200840 | France | 342 | 41.5 | 48 (18) | PCS | PACU | VAS and NRS | Morphine>0.15 mg/kg in PACU | <24 hours | Mixed | Mixed | 3 |
| Baudicet al, 201641 | France | 100 | 14.0 | 55.2 (12.1) | PCS | Ward | BPI | ≥3 | 48 | GS | Breast cancer | 9 |
| Beliiet al, 201442 | Moldova | 176 | Not stated | Not stated | PCS | Ward | NRS | ≥5 | 24 | GS | Abdominal pathologies | 3 |
| Borgeset al, 201643 | Brazil | 1062 | 78.4 | 25.1 (5.7) | PCS | Ward | NRS | ≥5 | Immediate postoperative period | Obstetric | Non-emergent caesarean section | 14 |
| Camuoet al, 200244 | Brazil | 346 | 43.4 | 44.3 (9.6) | PCS | PACU | VAS | >30 | 24 | GS | Abdominal pathologies | 15 |
| Duanet al, 201745 | China | 1002 | 15.5 | 49.5 (11.6) | PCS | Ward | NRS | ≥4 | 24 | Mixed | Mixed | 3 |
| Genovet al, 201546 | Russia | 321 | Not stated | Not stated | RCS | PACU | VAS | >4 | 12 | Mixed | Mixed | 1 |
| Gerbershagenet al, 201447 | Germany | 22 963 | 24.5 | 55.2‡ | PCS | Ward | NRS | ≥7 | 24 | Mixed | Mixed | 3 |
| Gorkemet al, 201621 | Turkey | 80 | Not stated | 29.7 (5.8) | PCS | Ward | VAS | >40 | 18 | Obstetric | Non-emergent caesarean section | 16 |
| Jae Chulet al, 2015§32 | Korea | 10 575 | Not stated | Young: 31.8 (5.8) Old: 74.8 (4.4) | RCS | Ward | NRS | >4 | 48 | Mixed | Mixed | 5 |
| Jasimet al, 201748 | Malaysia | 400 | Not stated | 30.4 (4.8) | RCS | PACU and Ward | VAS | Not stated | 12 | Obstetric | Non-emergent caesarean section | 7 |
| Katzet al, 200522 | USA | 109 | 54.1 | 58.2 (12) | PCS | Ward | NRS | ≥5 | 48 | GS | Breast cancer | 17 |
| Kimet al, 201649 | UK | 156 | 42.3 | 64.4 (10.9) | PCS | Ward | NRS | ≥5 | 48 | GS | Gastric tumours (endoscopic resection) | 11 |
| Lesinet al, 201650 | Croatia | 226 | 19.9 | 67 (13) | PCS | Ward | NRS | ≥5 | 6 | Ophtho | Ophthalmologic pathologies | 19 |
| Liuet al, 2012§23 | USA | 897 | At rest: 22.4 Movement: 39.0 | 67 (11) | RCS** | Ward | NRS at rest & with activity | >4 | 24 | Orthopaedic | Primary total hip or knee replacement | 17 |
| Lunnet al, 201351 | Denmark | 92 | 39.1 | Median 66 (IQR 13) | PCS | Ward | VAS (with activity) | ≥60 | 6–24 | Orthopaedic | Total knee arthroplasty | 4 |
| Mamieet al, 200452 | Switzerland | 304 | 25.1 | 45‡ | PCS | Ward | VAS | >5 | 24 | Mixed | Abdominal and orthopaedic pathologies | 10 |
| Meiet al, 201053 | Germany | 1736 | 28.5 | Not stated | PCS | PACU | NRS | >4 | After extubation | Mixed | Mixed | 10 |
| Murrayet al, 201654 | South Africa | 1231 | 61.9 | 44¶ | PCS | Ward | VAS | >40 | 24 | Mixed | Mixed | 8 |
| Nishimuraet al, 201724 | Japan | 64 | 48.4 | 60 (11) | PCS | Ward | VAS | >40 | 6–60 | GS | Partial mastectomy for cancer | 8 |
| Orbach-Zingeret al, 201755 | Israel | 245 | Good sleeper: 12.8 Poor sleeper: 27.5 | Good sleeper: 34.9 (4.9) Poor sleeper: 34.1 (4.9) | PCS | Ward | VRS | >7 | 24 | Obstetric | Non-emergent caesarean section | 3 |
| Perssonet al, 2017§33 | Sweden | 152 | Not stated | Median 49 (IQR 29) | PCS | PACU | VAS | >40 | 1.5 | GS | Laparoscopic cholecystectomy | 2 |
| Petrovicet al, 201456 | Serbia | 90 | 48.9 | High pain group: 64.2 (3.8) Low pain group: 69 (3.9) | PCS | Ward | NRS | ≥5 | 12 | Orthopaedic | Total hip arthroplasty | 15 |
| Radinovicet al, 201457 | Serbia | 234 | Not stated | 71.2 (8.3) | PCS | PACU | NRS | ≥7 | 1 | Orthopaedic | Hip fractures | 14 |
| Rakelet al, 2012§34 | USA | 215 | Moderate pain: 46.0 Severe pain: 27.0 | 61.7 (9.8) | PCS | Ward | NRS (0–21) | 8–14 (moderate) 15–20 (severe) | 48 | Orthopaedic | Total knee arthroplasty | 17 |
| Rehberget al, 201719 | Switzerland | 198 | 44.9 | 57.5 (12.5) | PCS | Ward | NRS | >3 | 24 | GS | Breast cancer | 15 |
| Robledaet al, 201458 | Spain | 127 | 61.0 | 71.0 (18) | RCS | PACU | NRS | ≥4 | Immediate in PACU | Orthopaedic | Femur fractures and prosthetics | 15 |
| Sananslipet al, 201659 | Thailand | 340 | 28.5 | 54.8 (17.8) | PCS | Ward | NRS | ≥4 | 24–48 | Mixed | Mixed | 12 |
| Sommeret al, 201060 | Netherlands | 1300 | 30.2 | 56 (15.5) | PCS | Ward | VAS | >40 | 24 | Mixed | Mixed | 15 |
| Storesundet al, 201661 | Norway | 336 | 67.3 | 52¶ | RCS** | PACU | VAS or vNRS | ≥4 | At the time of transfer out of PACU | Orthopaedic | Ankle fractures | 15 |
| Tigheet al, 201462 | USA | 7731 | 60.9 | Female: 56.4¶ Male: 56.6¶ | RCS | Ward | NRS | ≥7 | 24 | Mixed | Mixed | 1 |
| Zhaoet al, 201463 | China | 73 | 58.9 | Median 43 (IQR 57) | PCS | PACU and Ward | VAS | >30 | 24 | GS | Haemorrhoids | 12 |
*Pain measured at rest, unless otherwise stated.
†Time of assessment measured in hours.
‡Authors’ estimate (study only included age ranges).
§Studies that divided their data set into two groups when evaluating predictors: Jae Chulet al: young versus old age group; Liuet al: NRS at rest versus with activity; Perssonet al: female versus male and Rakelet al: moderate versus severe pain outcome.
¶Variance not stated.
**Labelled as a cross-sectional study design by study authors, but methodology more represent a retrospective cohort study design.
BPI, brief pain index (0–10); GS, general surgery; Mixed, more than one specialty or pathology; NRS, numeric rating scale for pain (0–10); PCS, prospective cohort study; RCS, retrospective cohort study; VAS, visual analogue scale for pain (0–100 mm); vNRS, verbal numeric rating scale for pain (0–10); PACU, Post-anesthesia care unit.
Assessment of study quality
The overall methodological quality of the included studies was generally high except for the use of a blinded outcome assessment (figure 2). In 25 studies (76%), there was either no blinding or no reporting on whether there was blinding of predictors during outcome ascertainment. The lack of blinding of predictors during outcome ascertainment in the majority of studies could lead to increased risk of misclassification bias. Twelve studies (36%) did not adjust for at least three potential confounders, five studies (15%) did not provide definitions of preoperative predictors and four studies (12%) did not define how their sample was selected.
Figure 2.
Assessment of study quality. (1) Adequate description of population, (2) non-biased selection, (3) adequate predictor measurement, (4) adequate outcome measurement, (5) blinded outcome assessment (to predictor), (6) adequate statistical adjustment, (7) precision of results, (8) reference standard and (9) low loss to follow-up. Green: low risk of bias, yellow: unclear risk of bias and red: high risk of bias.
Preoperative predictors of poor postoperative pain control
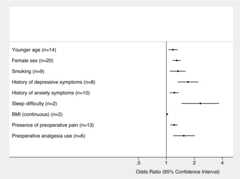
Of the 23 variables examined, nine statistically significant preoperative predictors of poor postoperative pain control were found: younger age (OR 1.18 [95% CI 1.05 to 1.32]), female sex (OR 1.29 [95% CI 1.17 to 1.43]), smoking (OR 1.33 [95% CI 1.09 to 1.61]), history of depressive symptoms (OR 1.71 [95% CI 1.32 to 2.22]), history of anxiety symptoms (OR 1.22 [95% CI 1.09 to 1.36]), sleep difficulties (OR 2.32 [95% CI 1.46 to 3.69]), higher body mass index (BMI) as a continuous variable (OR 1.02 [95% CI 1.01 to 1.03]), presence of preoperative pain (OR 1.21 [95% CI 1.10 to 1.32]) and use of preoperative analgesia (OR 1.54 [95% CI 1.18 to 2.03]). Pooled ORs and definition for each preoperative variable are given intable 2. Summary forest plots of significant preoperative predictors of poor postoperative pain control are shown infigure 3. Significant heterogeneity was detected in five of these predictors (female sex, younger age, the presence of preoperative pain, history of anxiety symptoms and smoking) with I2 values ranging from 50.4% to 82.4% (table 2). Detailed forest plots for each significant preoperative predictor are shown in onlinesupplementary figures S1–S3.
Table 2.
Pooled ORs and definitions of preoperative predictors of poor postoperative pain control
| Preoperative predictor | No. of studies included in the pooled estimate | No. of patients | OR (95% CI) | P value | I2 statistic | Definition |
| Younger age | 14 | 5577 | 1.18 (1.05 to 1.32) | <0.001 | 79.7%* | Authors’ cut-off (range ≤31 to <70 years) |
| Female sex | 20 | 48 753 | 1.29 (1.17 to 1.43) | <0.001 | 71%* | Female sex |
| Smoking | 9 | 15 764 | 1.33 (1.09 to 1.61) | 0.005 | 55.8%* | Self-reported (any amount) |
| History of depressive symptoms | 8 | 3042 | 1.71 (1.32 to 2.21) | 0.018 | 12.6% | Self-reported, any use of antidepressants or at least moderate score on depression scale (Hamilton Depression Rating Scale≥19, Montgomery-Asberg Depression Rating Scale>13 and Geriatric Depression Scale>6) |
| History of anxiety symptoms | 10 | 2598 | 1.22 (1.09 to 1.36) | 0.001 | 82.4%* | Self-reported or moderate to severe score on anxiety scale (State Anxiety Inventory≥30 to >46, Hamilton Anxiety Scale≥25 and numeric rating scale for anxiety≥5) |
| Sleep difficulty | 2 | 549 | 2.32 (1.46 to 3.69) | <0.001 | 0% | Self-reported chronic sleep difficulties or score >5 on the Pittsburgh Sleep Quality Index |
| BMI (continuous) | 2 | 1095 | 1.02 (1.01 to 1.03) | <0.001 | 0% | BMI as a continuous variable |
| Presence of preoperative pain | 13 | 4733 | 1.21 (1.10 to 1.32) | <0.001 | 50.4%* | Self-reported, any preoperative pain |
| Preoperative analgesia use | 6 | 2448 | 1.54 (1.18 to 2.03) | 0.002 | 44.0% | Self-reported use of preoperative analgesia or opioids |
| Age (continuous) | 9 | 26 846 | 0.97 (0.93 to 1.01) | 0.16 | 93.5%* | Age as a continuous variable |
| Higher education | 8 | 2272 | 0.97 (0.69 to 1.38) | 0.89 | 43.4% | Authors’ cut-off from self-reported levels of education (range: >9 years of education to college or postgraduate degree) |
| History of surgery | 8 | 3954 | 1.15 (0.97 to 1.37) | 0.10 | 33.9% | Any self-reported previous surgical history |
| Alcohol use | 5 | 3851 | 0.89 (0.72 to 1.11) | 0.29 | 26.2% | Self-reported alcohol use (range from any to dependence) |
| Low ASA physical status | 5 | 3629 | 0.94 (0.59 to 1.51) | 0.80 | 79.0%* | ASA I compared with II or III |
| High BMI (dichotomous) | 5 | 1926 | 1.23 (0.98 to 1.55) | 0.069 | 66.5%* | Authors’ cut-off (range from >30 to >40 kg/m2) |
| Chronic pain | 4 | 1583 | 0.96 (0.65 to 1.42) | 0.84 | 59.5% | Self-reported chronic pain |
| Diabetes | 4 | 1287 | 1.02 (0.73 to 1.42) | 0.90 | 0% | Self-reported history of diabetes |
| Pain Catastrophizing Scale (continuous) | 4 | 407 | 1.02 (0.98 to 1.05) | 0.37 | 64.8%* | Pain Catastrophizing Scale scores as a continuous variable |
| Marital status | 3 | 1571 | 1.42 (0.62 to 3.23) | 0.41 | 60.1% | Self-reported as single or not married |
| Orthopaedic procedure | 3 | 10 879 | 1.06 (0.72 to 1.57) | 0.77 | 76.3%* | Orthopaedic procedure compared with abdominal surgery |
| Preoperative pressure pain tolerance | 3 | 536 | 0.85 (0.69 to 1.06) | 0.14 | 81.0%* | Preoperative pressure pain tolerance as measured by Wagner Force Ten Digital Force Gauge FPX 50 or hand-held pressure algometer (Somedic AB, Farsta, Sweden) |
| Low socioeconomic status | 2 | 1288 | 0.85 (0.49 to 1.47) | 0.56 | 0% | Brazilian Economic Classification Criteria Classes D or E or monthly family net income less than US$750 |
| Pain Catastrophizing Scale (dichotomous) | 2 | 1476 | 1.47 (0.67 to 3.22) | 0.34 | 73.0% | Authors’ cut-off (range from ≥ or >15) |
*Significant Cochran’s Q test (p<0.05).
ASA, American Society of Anesthesiologists; BMI, body mass index.
Figure 3.
Summary forest plot for significant preoperative predictors of poor postoperative pain control. ORs are shown with 95% CIs. The number of studies included in the meta-analysis for each predictor is indicated. BMI, body mass index.
bmjopen-2018-025091supp003.pdf (1.5MB, pdf)
Non-significant preoperative predictors of poor postoperative pain control
Fourteen predictors were not significant in the final analysis: Pain Catastrophizing Scale (exaggerated negative perception to painful stimuli) as a dichotomous variable, marital status, high BMI as a dichotomous variable, any previous surgical history, orthopaedic surgery compared with abdominal surgery, diabetes, pain catastrophising as a continuous variable, higher education, age as a continuous variable, chronic pain, American Society of Anesthesiologists physical status, alcohol use, preoperative pressure pain tolerance and low socioeconomic status (table 2). Detailed forest plots for each non-significant preoperative predictor are shown in onlinesupplementary figures S4–S8.
Preoperative variables reported in only one study (and hence were excluded from the meta-analyses) included: patient weight, surgeon’s anticipated pain level, self-assessment of good health, generalised self-efficacy scale, sedentary lifestyle, employment status, short portable mental status questionnaire, preoperative delirium (confusion assessment method), constipation, rectal volume, body image scale, history of cancer, hypertension, heart disease, preoperative anaemia, anticonvulsant medication, home sedatives, electrical pain threshold, heat pain threshold, von Frey pain intensity, blood type, preoperative 24 hours urinary cortisol level, thoracic surgery, spine surgery, head and neck surgery, and total knee replacement.
Stratified meta-analysis and meta-regression
Stratified meta-analyses (according to the level of statistical adjustment, the definition of poor pain, surgical discipline, blinding of predictors and location of pain assessment) showed no differences in the pooled estimates and therefore, did not explain the significant level of heterogeneity observed between studies. These results were corroborated by meta-regression. Repeating the analysis using least adjusted versus most adjusted models also found similar pooled results for each preoperative predictor.
Discussion
In this systematic review and meta-analysis of 33 studies, we identified nine preoperative predictors that were negatively associated with pain control after surgery: young age, female sex, smoking, history of depressive symptoms, history of anxiety symptoms, sleep difficulties, higher BMI, presence of preoperative pain and use of preoperative analgesia. The most well-studied predictors were female sex (number of studies, n=20), young age (n=14) and the presence of preoperative pain (n=13). The strongest negative prognostic factors were a history of sleeping difficulties (number of studies, n=2) and depression (n=8), which were independently associated with approximately twofold higher odds of poor postoperative pain control. Our findings are consistent with and extend the results of the previous systematic review by Ipet al.20 In addition to the predictors previously described, we identified six additional preoperative predictors of poor postoperative pain control.20
Previous reports have been inconsistent in their conclusions regarding the association of female sex with worse pain prognosis after surgery.20 60 Some have observed higher pain scores in females,47 50 53 54 whereas others failed to find such a difference between sexes.34 57 59 In this meta-analysis, we found females had an approximately 30% increased odds of poor postoperative pain control compared with males. Sex differences may potentially relate to complex psychosocial and biological factors, such as an increased willingness of women to communicate pain,64 and subjective differences in pain perception and experience.20 Indeed, females are reported to require 11% greater doses of morphine on average compared with males in order to achieve adequate postoperative analgesia.65 Furthermore, younger age (as a dichotomous variable) was found to be a significant predictor for poor postoperative pain control. When examined as a continuous variable, the point estimate also suggested older age was protective (eg, for every decade of age, there was an associated 30% decrease in the odds for poor postoperative pain control), though this association was not statistically significant. Notably, studies examining age as a continuous variable may not have been able to detect a statistically significant difference because the majority of these studies were restricted to older patients and few examined younger subjects. Further, it is possible that the association between age and postoperative pain is non-linear. While sex and age are non-modifiable risk factors, this knowledge can still be used to anticipate pain trajectories and individualise analgesia requirements in the perioperative period.
Novel risk factors identified in this study included smoking, history of depressive symptoms, preoperative analgesic use and higher BMI. Smoking has been previously reported to be a negative prognostic factor for pain control and a predictor of increased use of opioid analgesia.66 67 Our finding implicating this modifiable risk factor in the setting of surgical pain supports the undertaking of future interventional studies evaluating the impact of preoperative smoking cessation programmes on postoperative pain control. The presence of depression (whether self-reported or measured with a validated scale) was also associated with worse pain outcomes. Importantly, a wide spectrum of depression was represented by the included studies, and even included subjects with relatively mild depressive symptoms.44 Thus, even mild or moderate levels of depressive symptoms may be associated with an increased odds of poor postoperative pain control. The use of preoperative analgesia, especially opioid therapy, has been linked to poor postoperative pain control in numerous studies.23 68 This may be due to greater preoperative severity of pain, opioid-induced hyperalgesia and central or peripheral sensitisation to pre-existing nociception.23 69 Further research on the impact of modifying these risk factors in the preoperative and perioperative period is needed to determine its effect on improving postoperative pain outcomes.
Strengths and limitations
The strengths of our study are the comprehensive search of the literature, inclusion of 33 articles (resulting in data on more than 53 000 patients), and the ability to generate pooled estimates for a large number of prognostic factors. The inclusion and stratification by multiple surgical specialties and the diversity of geographic locations increase the generalisability of the findings. However, the findings from the present report should be interpreted in the context of the study design. First, the primary studies included in our systematic review and meta-analysis were observational in nature. As is inherent to all observational designs, residual confounding cannot be excluded. This was particularly the case for unadjusted estimates. Nonetheless, we found that the most adjusted models yielded broadly similar results to the least adjusted estimates. Further, we performed meta-analyses on studies that had appreciable heterogeneity as it pertains to definition of poor postoperative pain control (which was variably defined by individual study authors), surgical procedure/specialty, timing and instrument used for pain assessment and threshold used to categorise continuous preoperative predictors between studies (eg, young vs old). Outcome heterogeneity may have been a potential source of bias if, for example, a particular predictor was associated with an increased risk of postoperative pain with one instrument (or cut-off) and a decreased risk of pain using a different instrument (or cut-off). In such cases, a pooled analysis might fail to detect either finding. Although we do not believe this issue biased our findings, future studies should attempt to standardise definitions (common data elements) to facilitate comparisons between studies. For significant predictors that were evaluated by a limited number of studies (eg, sleep difficulty), future studies should be performed to ensure reproducibility. Finally, there was significant statistical heterogeneity between studies, which could not be explained by stratified analysis or meta-regression based on a variety of clinical and study design factors (and the results should be interpreted with caution for surgical discipline as there were limited number of studies in each group). This heterogeneity was likely a product of important clinical differences as the included studies differed widely in surgery type and case-mix. Additional research may further define the influence of specific types of surgery on pain control.
Conclusion
In conclusion, we identified and described nine predictors of poor postoperative pain control in patients undergoing surgery requiring hospital admission. Early identification of predictors in patients at risk of poor postoperative pain control may allow for more individualised interventions, better pain management and decrease reliance on pain medications (particularly opioids). Increased awareness of these predictors can also aid in the development of personalised discipline-specific clinical care pathways (eg, multimodal analgesic strategies and enhanced recovery after surgery programmes) to reduce the length of stay and perioperative medical complications by improving postoperative pain outcomes. In addition, there is a lack of dedicated research in certain specialties, such as spine surgery, plastic surgery and otolaryngology, which should warrant further investigation. Although acute postoperative pain is common, no standard criteria exist to classify outcomes. Future work is needed to develop consensus criteria for acute postoperative pain outcomes, ideally as an international, multicentre collaborative using the Delphi method. Future prospective (observational or interventional) studies on acute postoperative pain control should consider addressing the predictors found in this review.
Supplementary Material
Acknowledgments
We thank Dr Diane Lorenzetti and Lorraine Toews for their assistance in developing the electronic database search strategy, Dr Kelly Shinkaruk for her assistance in our literature search and Dr Alina Makoyeva for translating the non-English articles.
Footnotes
Contributors: MMHY: conception and design of work; acquisition, analysis and interpretation of data; drafting initial draft of manuscript; and critical review and final approval of manuscript. RLH: design of work; acquisition, analysis and interpretation of data; and critical review and final approval of manuscript. AAL: design of work; analysis and interpretation of data; and critical review and final approval of manuscript. PER: design of work; analysis and interpretation of data; and critical review and final approval of manuscript. NJ: design of work; interpretation of data; and critical review and final approval of manuscript. SC: design of work; interpretation of data; and critical review and final approval of manuscript. JR-C: design of work; interpretation of data; and critical review and final approval of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. MMHY has received scholarship funding from the Canadian Institute of Health Research. MMHY and RLH has received salary funding from the Clinical Investigator Program, University of Calgary.
Competing interests: None declared.
Ethics approval: This study did not require ethical approval as the data used have been published previously, and hence are already in the public domain.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extracted data and statistical code will be made available by contacting the corresponding author.
Patient consent for publication: Not required.
References
- 1.Berry PH, Dahl JL. The new JCAHO pain standards: implications for pain management nurses. Pain Manag Nurs2000;1:3–12. 10.1053/jpmn.2000.5833 [DOI] [PubMed] [Google Scholar]
- 2.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet2008;372:139–44. 10.1016/S0140-6736(08)60878-8 [DOI] [PubMed] [Google Scholar]
- 3.Ciol MA, Deyo RA, Howell E, et al. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc1996;44:285–90. 10.1111/j.1532-5415.1996.tb00915.x [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, Maggard MA, et al. The aging population and its impact on the surgery workforce. Ann Surg2003;238:170–7. 10.1097/01.SLA.0000081085.98792.3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Total knee and hip replacement surgery projections show meteoric rise by 2030. Orthopaedic procedures set to continue gaining widespread acceptance as means to restore quality-of-life. Annual Meeting of the American Academy of Orthopaedic Surgery2006. [Google Scholar]
- 6.McCarty CA, Keeffe JE, Taylor HR. The need for cataract surgery: projections based on lens opacity, visual acuity, and personal concern. Br J Ophthalmol1999;83:62–5. 10.1136/bjo.83.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa JA, Wang TS, Yeo HL, et al. The maturation of a specialty: Workforce projections for endocrine surgery. Surgery2007;142:876–83. 10.1016/j.surg.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Wu JM, Kawasaki A, Hundley AF, et al. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am J Obstet Gynecol2011;205:e1-30. e5:23010.1016/j.ajog.2011.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalkman CJ, Visser K, Moen J, et al. Preoperative prediction of severe postoperative pain. Pain2003;105:415–23. 10.1016/S0304-3959(03)00252-5 [DOI] [PubMed] [Google Scholar]
- 10.Radinovic K, Milan Z, Markovic-Denic L, et al. Predictors of severe pain in the immediate postoperative period in elderly patients following hip fracture surgery. Injury2014;45(8):e9210.1016/j.injury.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 11.Caumo W, Broenstrub JC, Fialho L, et al. Risk factors for postoperative anxiety in children. Acta Anaesthesiol Scand2000;44:782–9. 10.1034/j.1399-6576.2000.440703.x [DOI] [PubMed] [Google Scholar]
- 12.Sommer M, de Rijke JM, van Kleef M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol2008;25:267–74. 10.1017/S0265021507003031 [DOI] [PubMed] [Google Scholar]
- 13.Peters CL, Shirley B, Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty2006;21:132–8. 10.1016/j.arth.2006.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Sriwatanakul K, Weis OF, Alloza JL, et al. Analysis of narcotic analgesic usage in the treatment of postoperative pain. JAMA1983;250:926–9. 10.1001/jama.1983.03340070032022 [DOI] [PubMed] [Google Scholar]
- 15.Katz J, Jackson M, Kavanagh BP, et al. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain1996;12:50–5. 10.1097/00002508-199603000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg1998;86:781–5. 10.1213/00000539-199804000-00019 [DOI] [PubMed] [Google Scholar]
- 17.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand1999;43:563–7. 10.1034/j.1399-6576.1999.430513.x [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Schnabel A, Yahiaoui-Doktor M, et al. Age and preoperative pain are major confounders for sex differences in postoperative pain outcome: A prospective database analysis. PLoS One2017;12:e017865910.1371/journal.pone.0178659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehberg B, Mathivon S, Combescure C, et al. Prediction of Acute Postoperative Pain Following Breast Cancer Surgery Using the Pain Sensitivity Questionnaire: A Cohort Study. Clin J Pain2017;33:57–66. 10.1097/AJP.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 20.Ip HY, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology2009;111:657–77. 10.1097/ALN.0b013e3181aae87a [DOI] [PubMed] [Google Scholar]
- 21.Gorkem U, Togrul C, Sahiner Y, et al. Preoperative anxiety may increase postcesarean delivery pain and analgesic consumption. Minerva Anestesiol2016;82:974–80. [PubMed] [Google Scholar]
- 22.Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain2005;119(1-3):16–25. 10.1016/j.pain.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 23.Liu SS, Buvanendran A, Rathmell JP, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop2012;36:2261–7. 10.1007/s00264-012-1623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura D, Kosugi S, Onishi Y, et al. Psychological and endocrine factors and pain after mastectomy. Eur J Pain2017;21:1144–53. 10.1002/ejp.1014 [DOI] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 26.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ2015;350:g764710.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol2009;62:e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 28.McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol2016;75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 29.Altman DG.Systematic reviews of evaluations of prognostic variables. BMJ2001;323:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey-Smith G, Altman D. Systematic reviews in health care: meta-analysis in context. John Wiley & Sons, 2008. [Google Scholar]
- 31.Katz J, Melzack R. Measurement of pain. Surg Clin North Am1999;79:231–52. 10.1016/S0039-6109(05)70381-9 [DOI] [PubMed] [Google Scholar]
- 32.Jae Chul K, Jinae L, So Yeon K, et al. Postoperative Pain and Intravenous Patient-Controlled Analgesia-Related Adverse Effects in Young and Elderly Patients: A Retrospective Analysis of 10,575 Patients. Medicine2015;94:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson AK, Dyrehag LE, Åkeson J. Prediction of Postoperative Pain From Electrical Pain Thresholds After Laparoscopic Cholecystectomy. Clin J Pain2017;33:126–31. 10.1097/AJP.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 34.Rakel BA, Blodgett NP, Bridget Zimmerman M, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain2012;153:2192–203. 10.1016/j.pain.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med2006;144:427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 36.Laupacis A, Wells G, Richardson WS, et al. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. JAMA1994;272:234–7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Yu KF, Kai FY. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves ML, Vieira JE, Mathias LA, et al. Preoperative coping mechanisms have no predictive value for postoperative pain in breast cancer. Braz J Psychiatry2013;35:364–8. 10.1590/1516-4446-2012-0934 [DOI] [PubMed] [Google Scholar]
- 40.Aubrun F, Valade N, Coriat P, et al. Predictive factors of severe postoperative pain in the postanesthesia care unit. Anesth Analg2008;106:1535–41. 10.1213/ane.0b013e318168b2ce [DOI] [PubMed] [Google Scholar]
- 41.Baudic S, Jayr C, Albi-Feldzer A, et al. Effect of Alexithymia and Emotional Repression on Postsurgical Pain in Women With Breast Cancer: A Prospective Longitudinal 12-Month Study. J Pain2016;17:90–100. 10.1016/j.jpain.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 42.Belii N, Moghildea V, Sandru S, et al. Anxiety, but not pain catastrophizing, represents a risk factor for severe acute postoperative pain: A prospective, observational, cohort study. Jurnalul Roman de Anestezie Terapie Intensiva/Romanian Journal of Anaesthesia and Intensive Care2014;21:19–26. [Google Scholar]
- 43.Borges NC, Pereira LV, de Moura LA, et al. Predictors for Moderate to Severe Acute Postoperative Pain after Cesarean Section. Pain Res Manag 20162016:578381710.1155/2016/5783817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caumo W, Schmidt AP, Schneider CN, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand2002;46:1265–71. 10.1034/j.1399-6576.2002.461015.x [DOI] [PubMed] [Google Scholar]
- 45.Duan G, Guo S, Zhang Y, et al. Effects of Epidemiological Factors and Pressure Pain Measurements in Predicting Postoperative Pain: A Prospective Survey of 1,002 Chinese Patients. Pain Physician2017;20:E903–E14. [PubMed] [Google Scholar]
- 46.Genov PG, Smirnova OV, Glushchenko NS, et al. [Predicting of postoperative pain level and morphine consumption by preoperative pressure pain assessment in patients before elective surgery]. Anesteziol Reanimatol2015;60:11–16. [PubMed] [Google Scholar]
- 47.Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology2014;120:1237–45. 10.1097/ALN.0000000000000108 [DOI] [PubMed] [Google Scholar]
- 48.Jasim HH, Sulaiman S, Khan AH, Ah K, et al. Factors Affecting Post Caesarean Pain Intensity among Women in the Northern Peninsular of Malaysia. J Clin Diagn Res2017;11:IC07–IC11. 10.7860/JCDR/2017/25364.10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SY, Jung SW, Choe JW, et al. Predictive Factors for Pain After Endoscopic Resection of Gastric Tumors. Dig Dis Sci2016;61:3560–4. 10.1007/s10620-016-4325-9 [DOI] [PubMed] [Google Scholar]
- 50.Lesin M, Dzaja Lozo M, Duplancic-Sundov Z, et al. Risk factors associated with postoperative pain after ophthalmic surgery: a prospective study. Ther Clin Risk Manag2016;12:93–102. 10.2147/TCRM.S97024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunn TH, Gaarn-Larsen L, Kehlet H. Prediction of postoperative pain by preoperative pain response to heat stimulation in total knee arthroplasty. Pain2013;154:1878–85. 10.1016/j.pain.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 52.Mamie C, Bernstein M, Morabia A, et al. Are there reliable predictors of postoperative pain?Acta Anaesthesiol Scand2004;48:234–42. 10.1111/j.0001-5172.2004.00298.x [DOI] [PubMed] [Google Scholar]
- 53.Mei W, Seeling M, Franck M, et al. Independent risk factors for postoperative pain in need of intervention early after awakening from general anaesthesia. Eur J Pain2010;14:149.e1–149.e7. 10.1016/j.ejpain.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 54.Murray AA, Retief FW. Acute postoperative pain in 1 231 patients at a developing country referral hospital: incidence and risk factors. Southern African Journal of Anaesthesia and Analgesia2016;22:19–24. 10.1080/22201181.2015.1115608 [DOI] [Google Scholar]
- 55.Orbach-Zinger S, Fireman S, Ben-Haroush A, et al. Preoperative sleep quality predicts postoperative pain after planned caesarean delivery. Eur J Pain2017;21:787–94. 10.1002/ejp.980 [DOI] [PubMed] [Google Scholar]
- 56.Petrovic NM, Milovanovic DR, Ignjatovic Ristic D, et al. Factors associated with severe postoperative pain in patients with total hip arthroplasty. Acta Orthop Traumatol Turc2014;48:615–22. 10.3944/AOTT.2014.14.0177 [DOI] [PubMed] [Google Scholar]
- 57.Radinovic K, Milan Z, Markovic-Denic L, et al. Predictors of severe pain in the immediate postoperative period in elderly patients following hip fracture surgery. Injury2014;45:1246–50. 10.1016/j.injury.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 58.Robleda G, Sillero-Sillero A, Puig T, et al. Influence of preoperative emotional state on postoperative pain following orthopedic and trauma surgery. Rev Lat Am Enfermagem2014;22:785–91. 10.1590/0104-1169.0118.2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanansilp V, Dejarkom S, Deetayart S. Postoperative Pain Management and the Risk Factors in Major Operation: A Baseline Study of Acute Pain Service, Siriraj Hospital. J Med Assoc Thai2016;99:549–56. [PubMed] [Google Scholar]
- 60.Sommer M, de Rijke JM, van Kleef M, et al. Predictors of acute postoperative pain after elective surgery. Clin J Pain2010;26:87–94. 10.1097/AJP.0b013e3181b43d68 [DOI] [PubMed] [Google Scholar]
- 61.Storesund A, Krukhaug Y, Olsen MV, et al. Females report higher postoperative pain scores than males after ankle surgery. Scand J Pain2016;12:85–93. 10.1016/j.sjpain.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 62.Tighe PJ, Riley JL, Fillingim RB. Sex differences in the incidence of severe pain events following surgery: a review of 333,000 pain scores. Pain Med2014;15:1390–404. 10.1111/pme.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y, Ding JH, Yin SH, et al. Predictors of early postoperative pain after stapled haemorrhoidopexy. Colorectal Dis2014;16:O206–O211. 10.1111/codi.12531 [DOI] [PubMed] [Google Scholar]
- 64.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med2001;16:266–75. 10.1046/j.1525-1497.2001.016004266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aubrun F, Salvi N, Coriat P, et al. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology2005;103:156–60. 10.1097/00000542-200507000-00023 [DOI] [PubMed] [Google Scholar]
- 66.Chiang HL, Chia YY, Lin HS, et al. The Implications of Tobacco Smoking on Acute Postoperative Pain: A Prospective Observational Study. Pain Res Manag2016;2016:1–7. 10.1155/2016/9432493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Creekmore FM, Lugo RA, Weiland KJ. Postoperative opiate analgesia requirements of smokers and nonsmokers. Ann Pharmacother2004;38:949–53. 10.1345/aph.1D580 [DOI] [PubMed] [Google Scholar]
- 68.Kampe S, Wendland M, Welter S, et al. Independent Predictors for Higher Postoperative Pain Intensity During Recovery After Open Thoracic Surgery: A Retrospective Analysis in 621 Patients. Pain Med2018;19:pnx238–pnx38. 10.1093/pm/pnx238 [DOI] [PubMed] [Google Scholar]
- 69.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology2006;104:570–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-025091supp001.pdf (72.9KB, pdf)
bmjopen-2018-025091supp002.pdf (82.7KB, pdf)
bmjopen-2018-025091supp003.pdf (1.5MB, pdf)