Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants1
Georgia Tooulakou
Andreas Giannopoulos
Dimosthenis Nikolopoulos
Panagiota Bresta
Elissavet Dotsika
Malvina G Orkoula
Christos G Kontoyannis
Costas Fasseas
Georgios Liakopoulos
Maria I Klapa
George Karabourniotis
Address correspondence tokarab@aua.gr.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: George Karabourniotis (karab@aua.gr).
This work was supported by Empeirikos Foundation, by FORTH/ICE-HT (funding the metabolomic experiments), and by the Greek Scholarship Foundation (as a scholarship to G.T.).
G.K. conceived and coordinated the study; G.K., G.L., D.N., and M.I.K. contributed to the design of the experiments; G.T. and A.G. performed the plant experiments as part of their PhD dissertations supervised by G.K., D.N., and G.L.; P.B. contributed to the plant experiments and the statistical analysis of the CaOx size distribution; M.G.O. and C.G.K. recorded the Raman spectra of CaOx crystals; E.D. carried out the stable carbon isotope composition measurements and C.F. the microscopy measurements; M.I.K. coordinated the metabolomic analyses; G.T. performed the metabolomic data acquisition; M.I.K. and G.T. carried out the multivariate statistical analysis of the metabolomic data; G.T., M.I.K., G.L., and G.K. drafted and G.K. finalized the manuscript.
Received 2016 Jan 22; Accepted 2016 Jun 2; Issue date 2016 Aug.
A new photosynthetic path named “alarm photosynthesis” uses mesophyll calcium oxalate crystals as the CO2 source when stomata are closed, providing adaptive advantages under drought conditions.
Abstract
Calcium oxalate crystals are widespread among animals and plants. In land plants, crystals often reach high amounts, up to 80% of dry biomass. They are formed within specific cells, and their accumulation constitutes a normal activity rather than a pathological symptom, as occurs in animals. Despite their ubiquity, our knowledge on the formation and the possible role(s) of these crystals remains limited. We show that the mesophyll crystals of pigweed (Amaranthus hybridus) exhibit diurnal volume changes with a gradual decrease during daytime and a total recovery during the night. Moreover, stable carbon isotope composition indicated that crystals are of nonatmospheric origin. Stomatal closure (under drought conditions or exogenous application of abscisic acid) was accompanied by crystal decomposition and by increased activity of oxalate oxidase that converts oxalate into CO2. Similar results were also observed under drought stress inDianthus chinensis,Pelargonium peltatum, andPortulacaria afra. Moreover, inA. hybridus, despite closed stomata, the leaf metabolic profiles combined with chlorophyll fluorescence measurements indicated active photosynthetic metabolism. In combination, calcium oxalate crystals in leaves can act as a biochemical reservoir that collects nonatmospheric carbon, mainly during the night. During the day, crystal degradation provides subsidiary carbon for photosynthetic assimilation, especially under drought conditions. This new photosynthetic path, with the suggested name “alarm photosynthesis,” seems to provide a number of adaptive advantages, such as water economy, limitation of carbon losses to the atmosphere, and a lower risk of photoinhibition, roles that justify its vast presence in plants.
In animals and humans, high levels of dietary oxalate act either as an antinutrient or as the main pathogenesis parameter of hyperoxaluria (Nakata, 2012;Aggarwal et al., 2013) or urolithiasis since 70% of all urinary calculi are composed mainly of calcium oxalate (Vijaya et al., 2013). On the other hand, in photosynthetic organisms, the accumulation of calcium oxalate crystals at high levels is a normal activity tightly integrated with metabolism (Franceschi and Nakata, 2005). The huge variation in distribution among organs, tissues, and cells among plant species suggests multiple independent origins of crystal formation and its functions (Horner and Wagner, 1995). The production of calcium oxalate crystals has a long evolutionary history and probably evolved independently in major clades of symbiotic fungi and several times in the Plantae, as part of the overall process of biomineralization (Horner and Wagner, 1995;Franceschi and Nakata, 2005). Although this independent evolution of the biomineralization process has led to the development of alternative strategies encompassing the formation of other biominerals aside from CaOx crystals, oxalic acid metabolism itself seems to remain significant in defense reactions, irrespective of CaOx presence. For example, the grasses of the Poaceae family, where CaOx crystals are normally absent (Prychid and Rudall, 1999), have adopted the silicon biomineralization strategy (providing protection against biotic and abiotic stress factors), while the enzymes of oxalate metabolism participate in the resistance against pathogen attack. It is also known that germins, which have oxalate oxidase activity, seem to be specific for the Poaceae family and they have an important role in apoplastic H2O2 production induced by abiotic stresses, wounding, and pathogen infection (Lane, 1994;Kärkönen and Kuchitsu, 2015).
Even though the CaOx crystals have intrigued plant scientists for a long time, their functional roles are still poorly understood. Currently, it is considered (Nakata, 2012;Webb, 1999;Franceschi and Loewus, 1995;He et al., 2014) that CaOx crystals are formed for (1) high-capacity regulation or sequestration of calcium, (2) ion balance, (3) detoxification from heavy metals and/or oxalate, (4) light collection and reflection, and (5) plant protection against herbivores. Most of these hypotheses focus on calcium ions, whereas oxalate is considered as a counterion required for calcium binding. On the other hand, plants may not have adopted this relatively convoluted mechanism of calcium sequestration, if not to use the organic part of the crystals for other purposes. It may be reasonable to assume that the oxalate represents a rich source of carbon that could be utilized in the form of CO2 in photosynthesis. This idea was formulated previously (Karpilov et al., 1978;Loewus, 1999) but has never been proven experimentally, probably because the fate of the CO2 released from the crystals is difficult to trace. If this hypothesis is true, the crystals should exhibit dynamic behavior that, in turn, should be influenced by the adequacy of the atmospheric carbon supply to the photosynthetic cells. Indeed, complete or partial degradation of the CaOx crystals has been observed (Webb, 1999;Franceschi and Horner, 1980;Calmes and Piquemal, 1977;Ilarslan et al., 2001;Tilton and Horner, 1980), thus supporting their dynamic nature. However, in most cases, these fluctuations in the crystal volume were linked to calcium demands and not to carbon cycling.
Amaranthus hybridus (rough pigweed, slim amaranth) is a summer annual C4 weed species. Its aggressiveness has been related to highly efficient use of water under drought conditions (Weaver and McWilliams, 1980).A. hybridus was selected as a model system for this study because of the occurrence of distinct large crystal cells localized within the mesophyll between the veins (Fig. 1A), reaching a high content (14–21% of dry weight).
Figure 1.
Anatomy and treatment effects on CaOx crystals and histochemical detection of oxalate oxidase activity. A, Scanning electron micrograph showing a transverse section of pigweed leaf grown in field. Leaves of this C4 plant exhibit typical Kranz anatomy. A large crystal cell (magenta arrows) is located within mesophyll between bundle sheaths (yellow arrows). B and C, Chlorine-bleached leaves depicting crystal (magenta arrows) distribution and size; control leaf (B), ΑΒΑ-treated leaf (C). D to F, The 30-μm-thick leaf cryosections histochemically tested for presence of oxalate oxidase activity (unstained leaf [D]; stained control leaf [E]; stainedABA-treated leaf [F]). Bars = 100 μm.
RESULTS AND DISCUSSION
FT-Raman analysis (Kontoyannis et al., 1997) validated that isolated spherical crystal aggregates (drusses;Supplemental Fig. S1) are comprised almost exclusively of calcium oxalate monohydrate (Supplemental Fig. S2). Diurnal fluctuations in the volume of CaOx crystals are reported here (Fig. 2A), although seasonal fluctuation of crystal dimensions (Calmes and Piquemal, 1977) and diurnal fluctuation of oxalate leaf contents (Seal and Sen, 1970;Jones and Ford, 1972) have been previously reported. All the above confirm that CaOx crystals are a dynamic system. According toFigure 2A, after midday, when stomata gradually closed, the mean crystal volume decreased, whereas a complete recovery was attained during the night (Fig. 2A). These observations suggest that the carbon in the CaOx crystals is derived from an alternative source other than the photosynthetic CO2 fixation, with dark respiration being a prominent possibility. This is further supported by the fact that the13C composition of the isolated crystals (−7.06 ± 0.12,n = 3) was higher than leaf biomass (−16.98 ± 0.22,n = 3), suggesting a lower13C discrimination in the CaOx crystals compared to the leaf bulk organic carbon. This result agrees with previous findings in other species (Raven et al., 1982;Rivera and Smith, 1979). Metabolic profiling indicated the presence of oxalate in the xylem sap (Supplemental Fig. S3), suggesting the root as another possible source of the CaOx crystal carbon. As previously reported, roots not only contain CaOx crystals but they can also metabolize oxalate (Franceschi, 1989;Horner et al., 2000;Kausch and Horner, 1984,1985).
Figure 2.
Diurnal fluctuations in crystal volume, stomatal conductance, and PSII operating efficiency of control andABA-treated leaves. A, Diurnal fluctuations in stomatal conductance and crystal volume in pigweed leaves. Plants were grown in a growth chamber under controlled conditions (error bars representse of mean;n = 5). B to D, Effect ofABA treatment on leaf parameters (stomatal conductance [gs] [B]; calcium oxalate crystal volume [C]; PSII operating efficiency [D]). In B to D, cut leaves remained for a time period under constant light either in artificial sap solution (control leaves, gray cycles) or in artificial sap solution containing 200 μmABA (ABA-treated leaves, magenta cycles). Error bars representse of mean;n = 5. In B to D, asterisks denote significant differences between treatments (Student’s two-tailedt test; ***P < 0.001; **P < 0.01; *P < 0.05).
Crystal decomposition during the day (Fig. 2A) might be related to carbon demands for photosynthesis, the latter being, in turn, influenced by the stomatal function. In order to examine these dependencies, abscisic acid (ABA), a hormonal regulator of plant responses to water stress (Cutler et al., 2010), was applied exogenously on cut leaves. Cut leaves were used to exclude potential interference from root-borne oxalate. We opted for a 6-h-long time-series experiment, measuring the metabolic profile of leaves harvested every hour from both the control and theABA-treated leaves. Both control andABA-treated leaves did not demonstrate any macroscopic deterioration during the experiment. In the latter, stomata remained closed throughout the 6-h period (Fig. 2B), whereas a gradual reduction of the crystal volume was recorded (Fig. 2C; also compareFig. 1, B and C). Despite stomatal closure, theABA-treated leaves did not exhibit any substantial metabolic decline compared to the control leaves (Fig. 3B). The metabolic stability of ABA-treated leaves was also indicated by the relatively small number of metabolites significantly different between the ABA-treated and the control leaves, i.e. the “significant” metabolites of this comparison (Fig. 4). In addition, the variation in the ABA-treated leaf metabolic profile during the experiment was clearly different, but smaller, than that observed in the control leaves (Fig. 3A). The observed significant decrease in the concentration of dissacharides, e.g. Suc, in the ABA-treated compared to the control leaves (Fig. 4) is consistent with a reduction in the photosynthetic activity expected due to the CO2 starvation. In addition, the significant increase in the concentration of Val agrees with previous observations in ABA-treated or water-stressed plants (Dugas et al., 2011). Finally, Glu plays a signaling role in the activated ionotropic receptors that act as amino acid-gated Ca2+ channels (Forde, 2014), and these receptors interact with ABA (Kang et al., 2004). Thus, the observed increase in the concentration of this amino acid may also be related with the regulation of the massive Ca2+ flow, which follows crystal decomposition.
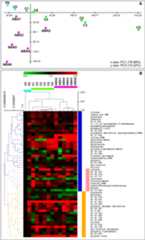
Figure 3.
Metabolic profiling data of control andABA-treated leaves. A, Principal component analysis (A) and hierarchical clustering analysis (HCL; B), Pearson correlation distance metric, of metabolic profiles of control andABA-treated leaves. Color scale of the HCL heat map corresponds to a value range from 0 to 3 for relative concentration of a metabolite at a particular time point and treatment group with respect to its concentration at zero time point (C0). C1-C6 and ABA1-6 depict, respectively, metabolic profile of control andABA-treated leaves at 1 to 6 h of treatment; P0 depicts leaf metabolic profile of original plant.
Figure 4.
Profiling data in significant metabolites of control andABA-treated leaves. A, Hierarchical clustering of metabolic profiles (Pearson distance metric) based on eleven metabolites with significantly increased (POS, positive) or decreased (NEG, negative) concentration inABA-treated compared to control leaves, according to significance analysis for microarrays (SAM). B, The time profile of 11 significant metabolites in control andABA-treated leaves. Numbering (e.g. POS1 and NEG3) indicates hierarchy in significance among metabolites of each group, with 1 depicting most statistically significant (seeSupplemental Methods). Color codes, value definitions, and symbols are same as inFigure 3.
The decomposition of crystals should be accompanied by the release of considerable amounts of oxalate in ABA-treated leaves. However, oxalate was not detected, suggesting its rapid breakdown into CO2 and H2O2 by oxalate oxidase (Lane, 1994). Histochemical detection indicated that this enzyme is mainly localized in the spongy mesophyll cells and, indeed, its activity increased due to the ABA treatment (Fig. 1, D–F). Moreover, the histochemical localization of catalase, the enzyme that cleaves H2O2 to H2O and O2, resembled that of oxalate oxidase, but it exhibited strong staining in both the control and the ABA-treated leaves (Supplemental Fig. S4). In combination, these observations further support the hypothesis that crystal decomposition is correlated with oxalate oxidase and catalase activities. The produced CO2 could be photosynthetically assimilated while the toxic H2O2 could be scavenged by catalase. Then, the photosynthetic metabolism in the ABA-treated leaves would remain active, despite their closed stomata and the accompanied CO2 starvation (Fig. 2B). However, the closed stomata did not permit direct photosynthetic activity measurements in the ABA-treated leaves. Chlorophyll fluorescence measurements showed that the operational efficiency of the PSII photochemistry (ΔF/Fm′) in the ABA-treated leaves remained substantial despite the CO2 starvation conditions (Fig. 2D). However, the lower ΔF/ Fm′ values compared to the control leaves along with the metabolic profiling measurements suggested that the CO2 supply from the breakdown of the oxalate that could be derived from the crystal decomposition is lower than the CO2 supply from the open stomata. Indeed, considering the mean photosynthetic activity of the plants used in our experiments (i.e. 13 μmol m−2 s−1) and the mean leaf CaOx content (i.e. 18% of dry weight), we estimated that under stomatal closure, the amount of CO2 that can be released from complete crystal decomposition could support the above photosynthetic rate for 70 min or maintain a low photosynthetic rate of 3 μmol CO2 m−2 s−1 for 4 to 5 h. These estimates may be conservative because a considerable amount of crystals (located in the stem and in the bundle sheaths) and any root-borne oxalate were not included in the calculations. In addition to oxalate released during crystal decomposition, high amounts of Ca2+ ions are also released. A possible mechanism for their quenching could be based on Ca-binding proteins given that the occurrence of such proteins in crystal cells has already been reported (Franceschi et al., 1993;Webb, 1999). Calcium ion management during crystal recycling could be a subject of further studies.
The correlation between the CO2 starvation conditions and the CaOx crystal decomposition was reinforced by a whole plant experiment, in which four plant species possessing CaOx crystals and representing different functional groups (C3, C4, and Crassulacean acid metabolism [CAM] plants; see “Materials and Methods”) were exposed to drought under controlled conditions (A. hybridus,Dianthus chinensis,Pelargonium peltatum, andPortulacaria afra;Table I). Water deficit caused a reduction in leaf water potential and a decrease in stomatal conductance. Importantly, in accordance with all previous observations, this decrease was accompanied by a significant reduction in the crystal volume in all plants (Table I). Oxalate oxidase activity was increased in all cases, except forD. chinensis. In this plant, the activity of the enzyme was very high irrespective of the treatment (Table I). The observed differences in plant responses may be explained by the different tolerance to water stress, as it was indicated by the water potential values.
Table I. Means comparison of leaf water potential, stomatal conductance, oxalate oxidase activity, and crystal volume in leaves of well-watered and water-stressed plants.
Plants were grown in a growth chamber under controlled conditions. Taking into account that during the night a total crystal volume recovery occurs (Fig. 2A), measurements were conducted at the end of the light period. Errors representse;n = 5. Asterisks denote statistically significant differences between treatments (Student’s two-tailedt test; ***P < 0.001; **P < 0.01; *P < 0.05). n.d., Not detected; WW, well watered; WS, water stressed; FW, fresh weight.
| Treatment | Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. hybridus (C4) | D. chinensis (C3) | P. peltatum (C3) | P. afra (Succulent) | |||||||||
| WW | WS | WW | WS | WW | WS | WW | WS | |||||
| Parameter | ||||||||||||
| Water potential (Ψw) MPa | −0.976 | −1.416 | ** | −0.66 | −1.1 | ** | −0.28 | −0.64 | ** | −0.17 | −0.38 | *** |
| ±0.077 | ±0.097 | ±0.014 | ±0.152 | ±0.062 | ±0.002 | ±0.010 | ±0.028 | |||||
| Stomatal conductance (gs) mol H2O m−2 s−1 | 0.330 | 0.008 | *** | 0.236 | 0.008 | ** | 0.148 | 0.007 | *** | 0.044 | 0.009 | ** |
| ±0.019 | ±0.001 | ±0.038 | ±0.001 | ±0.014 | ±0.001 | ±0.008 | ±0.001 | |||||
| Oxalate oxidase activity nmol H2O2 min−1 g−1 FW | 39.2 | 120.9 | * | 8,622.1 | 6,038.7 | * | n.d. | 10.8 | n.d. | 241.4 | ||
| ±1.8 | ±26.9 | ±709.4 | ±718.9 | ±3.9 | ±25.0 | |||||||
| Crystal volume μm3 | 11,580 | 560 | *** | 12,383 | 7,729 | ** | 8,942 | 6,004 | * | 3,756 | 1,863 | *** |
| ±499 | ±19 | ±904 | ±885 | ±1120 | ±164 | ±248 | ±229 | |||||
According to our results, the formation of the CaOx crystals within specialized cells can act as a biochemical mechanism that stores carbon in the form of calcium oxalate, mainly during nighttime, when stomata are closed and photosynthetic reactions are inoperative. During the day, the degradation of the crystals provides subsidiary carbon for photosynthetic assimilation, especially under conditions of carbon starvation. The signal is probably given by the hormoneABA. Hence, we suggest this new photosynthetic path to be called “alarm photosynthesis.” This pathway for photosynthetic carbon assimilation could be added to the already known C4 and CAM photosynthetic metabolic pathways and can be operational in numerous plant species, irrespective of their functional (i.e. C3, C4, or CAM) group. Alarm photosynthesis as a syndrome could have evolved multiple times independently in various plant lineages, as in the case of CAM and C4 syndromes, which are both considered as convergent adaptations to stressful environments (Edwards and Ogburn, 2012). The data of this study support the idea that this is also the case for the alarm photosynthesis, indicating water stress and the consequent carbon starvation within mesophyll tissues as the ultimate selective factor.
The function of alarm photosynthesis may provide a number of competitive advantages: (1) It contributes to carbon and water economy, since carbon acquisition from the atmosphere is associated with water losses. For this reason, we predict that at the interspecific level the frequency of occurrence and the intensity of alarm photosynthesis will tend to be higher in xerophytes (e.g. cacti that are known to contain large quantities of CaOx crystals; seeGarvie, 2006). Recent data support that this trend may also exist at the intraspecific level. According toBrown et al. (2013), the number of the CaOx crystals in the phyllodes of theAcacia species increases with aridity. (2) Leaf photosynthesis can maintain a basal level of activity during partial or full stomatal closure. Low CO2 levels in combination with high light intensities could increase the photooxidative risk and lead to instability of the PSII (Murata et al., 2007;van Rensen, 2002). Thus, alarm photosynthesis could act as a quenching valve for the energy excess accumulated in the electron transport chain, when the light reactions are not in pace with photosynthetic CO2 assimilation from the atmosphere. (3) Storage of carbon in the form of CaOx crystals confers some metabolic advantages, considering that these deposits are metabolically and osmotically inactive. Only if needed, CO2 and calcium ions can be released.
CONCLUSION
Drought, in combination with coincident high temperature and light intensity, represents the most important environmental stress factor that limits plant survival and crop productivity (Chaves et al., 2003;Flexas et al., 2006). Research into drought resistance mechanisms has become of great significance, as climate change scenarios predict intensified drought conditions in many parts of the world and concomitant yield reduction (Long and Ort, 2010). Plants in arid regions or seasonally dry environments face a constant trade-off between acquiring atmospheric carbon dioxide for photosynthesis while losing water vapor or saving water with a subsequent reduction of photosynthesis and thus growth. Alarm photosynthesis allows the utilization of large CaOx crystals as dynamic internal carbon pools, irrespective of the availability of the atmospheric CO2 and thus preventing water losses. This function probably acts harmoniously with the other already known stress adapted CAM and C4 syndromes and may play a crucial role in the surviving of plants under drought conditions, further justifying the extensive occurrence of CaOx crystals in the Plant Kingdom.
Future investigations on the contribution of alarm photosynthesis in drought tolerance and the mechanisms controlling crystal size will lead to a better understanding of the responses of wild and cultivated plants in a warmer and water limiting planet. Additionally, a systematic quantification of calcium oxalate crystals and an evaluation of the intensity of alarm photosynthesis at the interspecific level in different climatic regions of the planet are needed. Taking into account the estimations that a large tree may produce annually up to several hundred kilograms of calcium oxalate in its aboveground tissues (Horner and Wagner, 1995;Cailleau et al., 2011; see alsoGarvie 2006), calcium oxalate crystals as part of the phytomineralization process could represent a considerable carbon sink with a long residence time at the ecosystem as well as at the global level. The discovery of alarm photosynthesis gives rise to the investigation of its potential role in global carbon cycle and the effect of climate change on this process.
MATERIALS AND METHODS
Plant Material
Pigweed plants (Amaranthus hybridus, Amaranthaceae [C4 plant]) were grown from seeds in a growth chamber. China pink plants (Dianthus chinensis, Caryophyllaceae [C3 plant]), ivy-leaf geranium plants (Pelargonium peltatum, Geraniaceae [C3 plant]), and elephant bush plants (Portulacaria afra, Portulacaceae [succulent, probably facultative CAM plant]) were obtained from a commercial nursery. All plants were transplanted (A. hybridus plants 10 d after showing) in pots filled with a commercial soil mixture (fertilized peat mixture, pH 5.5–6.5, organic matter 80%, clay 20 kg/m3, perlite 5–10%) and grown under a 12/12-h photoperiod, radiation 400 μmol quanta m−2 s−1 (Powerstar HQI-BT-400W/D; OSRAM), day/night temperature 30/19°C, and air relative humidity 75%.
Microscopy
For scanning electron microscopy, small pieces of leaves were fixed in 3% glutaraldehyde in 0.1m phosphate buffer, pH 7.2, for 24 to 36 h at 4°C, dehydrated with a 30 to 100% acetone gradient, critical point dried, mounted on stubs with self-adhesive double-sided carbon discs (Agar Scientific), and sputter coated with gold. Observations and digital micrographs were taken with a JEOL JSM-6360 scanning electron microscope at 20 kV.
For light microscopy, fresh leaves were cut with a cryotome (Leica CM 1850) and observed directly or used for histochemical staining. Sections were observed and photographed with an Olympus BX40 light microscope equipped with an Olympus DP70 digital camera. Images of histochemical staining were enhanced with image processing software (Adobe Photoshop CS5) by correcting for brightness and contrast. These corrections were equally applied to all images (control andABA-treated leaves).
Measurements of Crystal Density and Size
Crystal density and size were measured in leaves bleached in sodium hypochlorite solution (2%) for 24 h. Images of isolated crystals or crystals in chlorine-bleached leaves were obtained using a Axiolab microscope (Carl Zeiss) equipped with a digital camera (DSC-S75; Sony), and dimensions were measured by digital image analysis (Image Pro Plus v. 5.1.0.20; MediaCybernetics).
Crystal Isolation
CaOx crystals were isolated by leaf tissue rupture in a homogenizer with isopropanol. After crystal precipitation, pellet was cleared by sequential washings with pure isopropanol and isolation after precipitation until microscopic observation revealed the absence of cell debris (Supplemental Fig. S1).
Histochemical Detection and Enzyme Assays
Cryosections were stained for oxalate oxidase activity using 0.6 mg mL−1 4-chloro-1-naphthol solution in succinate buffer (25 mm succinate and 3.5 mm Fe-Na EDTA, pH adjusted to 4.0 with NaOH) containing 1 unit mL−1 peroxidase (horseradish peroxidase; MP Biomedicals) and 2.5 mm oxalate. Blank staining was done using the same solution omitting oxalate (Dumas et al., 1995). Staining for catalase activity was done using 3,3-diaminobenzidine (Frederick and Newcomb, 1969). Briefly, sections were incubated with 1 mg mL−1 3,3-diaminobenzidine solution in Tris-HCl buffer (200 mm Tris adjusted to pH 9.0 with 100 mm HCl) and 0.06% H2O2 for 60 min at 30°C. Sections were washed with Tris-HCl buffer and observed directly. Blank staining was done by preincubating sections in 50 mm 3-amino-1,2,4-triazole solution in Tris-HCl buffer for 30 min and incubating in the staining solution containing 50 mm 3-amino-1,2,4-triazole. Oxalate oxidase activity in leaf extracts was measured using 3-methyl-2-benzothiazolinone hydrazone andΝ,Ν-dimethylaniline (Laker et al., 1980;Goyal et al., 1999). Extracts were prepared by homogenizing 1 g of leaf tissue in 6 mL phosphate buffer (100 mm phosphate and 500 mm Suc, pH 7.0) at 4°C and centrifuging at 15,000g for 15 min. Activity was tested in both the supernatant and the pellet (suspended in 6 mL of extraction buffer) by adding 225 μL of extract, 20 μL of 300 mm oxalate solution, and 2.5 units of horseradish peroxidase in aqueous solution (5 μL), in 2.7 mL reaction buffer (25 mm succinate and 2.5 mm FeNa EDTA, pH 4.0 adjusted with NaOH). Reaction mixture was incubated at 35°C for 30 min under dim light and stirring. Absorbance was measured during incubation in a double-beam spectrophotometer (UV-160A; Shimadzu) at 600 nm and at 5-min intervals using a nonextract photometric blank. Due to the membrane localization of oxalate oxidase inA. hybridus (Goyal et al., 1999), activity was only detected in pellet suspensions and not in supernatants.
FT-Raman Spectra
Raman spectroscopy of crystals was done as described elsewhere (Kontoyannis et al., 1997). Spectra of isolated crystals were recorded using a FRA-106/S FT-Raman (Bruker) with the following characteristics: The laser excitation line used was the 1064 nm of a Nd:YAG laser. A secondary filter was used to remove the Rayleigh line. The scattered light was collected at an angle of 180° (backscattering). The system was equipped with a LN2-cooled Ge detector (D 418). The power of the incident laser was 370 mW, and the focused laser spot had a diameter of 100 μm. The recorded spectra were the sum of 300 scans, and the typical spectral resolution was 4 cm−1. To verify Raman instrument calibration, the cyclohexane bands at 1029 and 1158 cm−1 were used.
Stable Carbon Isotope Composition of Isolated Crystals and Whole Leaves
Samples from control leaves or isolated crystals were analyzed with a ThermoScientific Delta V Plus mass spectrometer. The samples were introduced into a Thermo-Flash elemental analyzer where CO2 and N2 gas were produced by combustion at 1020°C. The gases, moved along in a continuous flow of helium, were separated by a GC column and introduced into a continuous flow gas source mass spectrometer for carbon and nitrogen isotopic ratio determination. The isotopic ratios are expressed for carbon as δ13C versus PDB (a marine carbonate). Analytical precision was 0.1‰ for δ13C.
Photosynthetic and Transpiration Parameters
Stomatal conductance (gs) was measured with a portable porometer (AP4; Delta-T Devices) calibrated on the same day. Operational efficiency of PSII photochemistry (Genty et al., 1989; ΔF/ Fm′) was measured with a portable chlorophyll fluorometer (PAM 2100; Walz) using red LED measuring light (650 nm, PFD < 0.15 μmol quanta m−2 s−1). Saturation pulse (white halogen; PFD approximately 15,000 μmol quanta m−2 s−1) lasted for 0.8 s. Net photosynthetic activity was measured using a portable photosynthesis system (ADC Pro Plus; ADC BioScientific).
ABA Treatment
Leaves from 20 plants (three leaves per plant) were cut under water and placed in petri dishes filled with artificial xylem sap (30 leaves) or with artificial xylem sap containing 200 μmABA (Sigma-Aldrich; 30 leaves). All leaves were placed under light (400 μmol quanta m−2 s−1; Powerstar HQI-BT-400W/D; OSRAM) at 30°C for 6 h. Measurements of gs and chlorophyll fluorescence parameters were undertaken every hour using five leaves from each treatment. Immediately after, leaves were removed for microscopic examination and measurement of crystal size.
Water Stress Treatment and Water Potential Measurements
Ten plants were grown as described previously. After 30 d of growth, water stress was imposed on five plants by retarding irrigation for 7 d, whereas the rest of the plants received normal irrigation. Leaf water potential was measured using a Scholander pressure chamber.
Metabolomic Data Acquisition and Analysis
The gas chromatography-mass spectrometry metabolic profile acquisition of the control andABA-treated leaves was carried out after appropriate adaptation of previously published protocol (Dutta et al., 2009;Kanani et al., 2010). The metabolic profile of each physiological state/time point was estimated from the mean profile of two separate leaves harvested at the particular state, and the profile of each leaf was measured at least three times. Specific details are provided inSupplemental Methods and Supplemental Data Set S1. After appropriate data correction, normalization, and filtering (Kanani et al., 2010;Kanani and Klapa, 2007), profiles of 55 metabolite derivatives were considered in the multivariate statistical analysis using the hierarchical clustering analysis, principal component analysis, and significance analysis for microarrays algorithms implemented in the “omic” data analysis software TM4 MeV (v4.8.1) software (Saeed et al., 2003,2006). Further details on the metabolomic data normalization, filtering, and statistical analysis can be found inSupplemental Methods.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Isolated crystals from control leaves of pigweed plants using polarizing microscopy.
Supplemental Figure S2. Typical Raman spectrum of isolated crystals of pigweed leaves.
Supplemental Figure S3. MS-reconstructed chromatogram of xylem sap, indicating presence of oxalate peak.
Supplemental Figure S4. Histochemical detection of catalase activity.
Supplemental Figure S5. Paired SAM curve normalized with C0 metabolic profiles of AΒΑ treated with respect to control leaves considered in metabolomic analysis.
Supplemental Data File S1. Raw data set of metabolomics analysis (as Excel file).
Supplementary Material
Acknowledgments
We thank Associate Professor Garifalia Economou and Dr. Petros Vahamidis (Laboratory of Agronomy, Department of Crop Science, Agricultural University of Athens) for measurements of water potential.
Glossary
- ABA
abscisic acid
References
- Aggarwal KP, Narula S, Kakkar M, Tandon C (2013) Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res Int2013: 292953. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brown SL, Warwick NWM, Prychid CJ (2013) Does aridity influence the morphology, distribution and accumulation of calcium oxalate crystals inAcacia (Leguminosae: Mimosoideae)?Plant Physiol Biochem73: 219–228 [DOI] [PubMed] [Google Scholar]
- Cailleau G, Braissant O, Verrecchia EP (2011) Turning sunlight into stone: the oxalate-carbonate pathway in a tropical tree ecosystem. Biogeosci Disc8: 1077–1108 [Google Scholar]
- Calmes J, Piquemal M (1977) Seasonal variations of calcium oxalate crystals in tissues ofVigne vierge.Can J Bot55: 2075–2078 [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol30: 239–264 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol61: 651–679 [DOI] [PubMed] [Google Scholar]
- Dugas DV, Monaco MK, Olsen A, Klein RR, Kumari S, Ware D, Klein PE (2011) Functional annotation of the transcriptome ofSorghum bicolor in response to osmotic stress and abscisic acid. BMC Genomics12: 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas B, Freyssinet G, Pallett KE (1995) Tissue-specific expression of germin-like oxalate oxidase during development and fungal infection of barley seedlings. Plant Physiol107: 1091–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta B, Kanani H, Quackenbush J, Klapa MI (2009) Time-series integrated “omic” analyses to elucidate short-term stress-induced responses in plant liquid cultures. Biotechnol Bioeng102: 264–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. Int J Plant Sci173: 724–733 [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribs-Carbó M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant127: 343–352 [Google Scholar]
- Forde BG. (2014) Glutamate signalling in roots. J Exp Bot65: 779–787 [DOI] [PubMed] [Google Scholar]
- Franceschi VR. (1989) Calcium oxalate formation is a rapid and reversible process inLemna minor L. Protoplasma148: 130–137 [Google Scholar]
- Franceschi VR, Horner HT Jr (1980) A microscopic comparison of calcium oxalate crystal idioblasts in plant parts and callus cultures ofPsychotria punctata (Rubiaceae). Z Pflanzenphysiol97: 449–455 [Google Scholar]
- Franceschi VR, Li X, Zhang D, Okita TW (1993) Calsequestrinlike calcium-binding protein is expressed in calcium-accumulating cells ofPistia stratiotes. Proc Natl Acad Sci USA90: 6986–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Loewus F (1995) Oxalate biosynthesis and function in plants and fungi.InKhan SR, ed, Oxalate in Biological Systems, CRC Press, Boca Raton, FL, pp 53–72 [Google Scholar]
- Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol56: 41–71 [DOI] [PubMed] [Google Scholar]
- Frederick SE, Newcomb EH (1969) Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol43: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie LAJ. (2006) Decay of cacti and carbon cycling. Naturwissenschaften93: 114–118 [DOI] [PubMed] [Google Scholar]
- Genty B, Brintais J-M, Baker NR (1989) The relationship between the quantum yield of photosyntetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta990: 87–92 [Google Scholar]
- Goyal L, Thakur M, Pundir CS (1999) Purification and properties of a membrane bound oxalate oxidase fromAmaranthus leaves. Plant Sci142: 21–28 [Google Scholar]
- He H, Veneklaas EJ, Kuo J, Lambers H (2014) Physiological and ecological significance of biomineralization in plants. Trends Plant Sci19: 166–174 [DOI] [PubMed] [Google Scholar]
- Horner HT, Wagner BL (1995) Calcium oxalate formation in higher plants.InKhan SR, ed, Oxalate in Biological Systems, CRC Press, Boca Raton, FL, pp 53–72. [Google Scholar]
- Horner HT Jr, Kausch AP, Wagner BL (2000) Ascorbic acid: a precursor of oxalate in crystal idioblasts ofYucca torreyi in liquid root culture. Int J Plant Sci161: 861–868 [Google Scholar]
- Ilarslan H, Palmer RG, Horner HT Jr (2001) Calcium oxalate crystals in developing seeds of soybean. Ann Bot (Lond)88: 243–257 [PubMed] [Google Scholar]
- Jones RJ, Ford CW (1972) Some factors affecting the oxalate content of the tropical grassSetaria sphacelata.Aust J Exp Agric Anim Husb12: 400–406 [Google Scholar]
- Kanani H, Dutta B, Klapa MI (2010) Individual vs. combinatorial effect of elevated CO2 conditions and salinity stress onArabidopsis thaliana liquid cultures: comparing the early molecular response using time-series transcriptomic and metabolomic analyses. BMC Syst Biol4: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanani HH, Klapa MI (2007) Data correction strategy for metabolomics analysis using gas chromatography-mass spectrometry. Metab Eng9: 39–51 [DOI] [PubMed] [Google Scholar]
- Kang J, Mehta S, Turano FJ (2004) The putativeglutamate receptor 1.1 (AtGLR1.1) inArabidopsis thaliana regulates abscisic acid biosynthesis and signaling to control development and water loss. Plant Cell Physiol45: 1380–1389 [DOI] [PubMed] [Google Scholar]
- Kärkönen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry112: 22–32 [DOI] [PubMed] [Google Scholar]
- Karpilov YS, Kuzmin AN, Bil KY (1978) Distribution of glycolytic enzymes in assimilating tissues of C4 leaves and their link with characteristics of photosynthetic and photorespiratory reactions. Sov Plant Physiol25: 891–897 [Google Scholar]
- Kausch AP, Horner HT Jr (1984) Differentiation of raphide crystal idioblasts in isolated root cultures ofYucca torreyi (Agavaceae). Can J Bot62: 1474–1484 [Google Scholar]
- Kausch AP, Horner HT Jr (1985) Absence of CeCl3-detectable peroxisomal glycolate-oxidase activity in developing raphide crystal idioblasts in leaves ofPsychotria punctata Vatke and roots ofYucca torreyi L. Planta164: 35–43 [DOI] [PubMed] [Google Scholar]
- Kontoyannis CG, Bouropoulos N, Koutsoukos PG (1997) Use of raman spectroscopy for the quantitative analysis of calcium oxalate hydrates: application to urinary stones. Appl Spectrosc51: 64–67 [Google Scholar]
- Laker MF, Hofmann AF, Meeuse BJ (1980) Spectrophotometric determination of urinary oxalate with oxalate oxidase prepared from moss. Clin Chem26: 827–830 [PubMed] [Google Scholar]
- Lane BG. (1994) Oxalate, germin, and the extracellular matrix of higher plants. FASEB J8: 294–301 [DOI] [PubMed] [Google Scholar]
- Loewus F. (1999) Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry52: 193–210 [Google Scholar]
- Long SP, Ort DR (2010) More than taking the heat: crops and global change. Curr Opin Plant Biol13: 241–248 [DOI] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta1767: 414–421 [DOI] [PubMed] [Google Scholar]
- Nakata PA. (2012) Plant calcium oxalate crystal formation, function, and its impact on human health. Front Biol7: 254–266 [Google Scholar]
- Prychid CJ, Rudall PJ (1999) Calcium oxalate crystals in monocotyledons: A review of their structure and systematics. Ann Bot (Lond)84: 725–739 [Google Scholar]
- Raven JA, Griffiths H, Glidewell SM, Preston T (1982) The mechanism of oxalate biosynthesis in higher plants: Investigations with the stable isotopes18O and13C. Proc R Soc Lond B Biol Sci216: 87–101 [Google Scholar]
- Rivera ER, Smith BN (1979) Crystal morphology and carbon/carbon composition of solid oxalate in cacti. Plant Physiol64: 966–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques34: 374–378 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J (2006) TM4 microarray software suite. Methods Enzymol411: 134–193 [DOI] [PubMed] [Google Scholar]
- Seal SN, Sen SP (1970) The photosynthetic production of oxalic acid inOxalis corniculata.Plant Cell Physiol11: 119–128 [Google Scholar]
- Tilton VR, Horner HT Jr (1980) Calcium oxalate raphide crystals and crystalliferous idioblasts in the carpels ofOrnithogalum caudatum.Ann Bot (Lond)46: 533–539 [Google Scholar]
- van Rensen JJS. (2002) Role of bicarbonate at the acceptor side of photosystem II. Photosynth Res73: 185–192 [DOI] [PubMed] [Google Scholar]
- Vijaya T, Sathish Kumar M, Ramarao NV, Naredra Babu A, Ramarao N (2013) Urolithiasis and its causes-short review. J Phytopharmacol2: 1–6 [Google Scholar]
- Weaver SE, McWilliams EL (1980) The biology of Canadian weeds. 44.Amaranthus retroflexus L.,A. powellii S. Wats. andA. hybridus L. Can J Plant Sci60: 1215–1234 [Google Scholar]
- Webb MA. (1999) Cell-mediated crystallization of calcium oxalate in plants. Plant Cell11: 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.