Epithelial sodium channel (ENaC) family: Phylogeny,structure-function, tissue distribution, and associated inheriteddiseases
Israel Hanukoglu
Aaron Hanukoglu
Corresponding author: Israel Hanukoglu, Laboratory of CellBiology, Faculty of Natural Sciences, Ariel University, Ariel 40700 Israel. Tel:+972 3 9066293,mbiochem@gmail.com
Issue date 2016 Apr 1.
Abstract
The epithelial sodium channel (ENaC) is composed of three homologoussubunits and allows the flow of Na+ ions across high resistanceepithelia, maintaining body salt and water homeostasis. ENaC dependentreabsorption of Na+ in the kidney tubules regulates extracellularfluid (ECF) volume and blood pressure by modulating osmolarity. Inmulti-ciliated cells, ENaC is located in cilia and plays an essential role inthe regulation of epithelial surface liquid volume necessary for cilialtransport of mucus and gametes in the respiratory and reproductive tractsrespectively.
The subunits that form ENaC (named as alpha, beta, gamma and delta,encoded by genes SCNN1A, SCNN1B, SCNN1G, and SCNN1D) are members of theENaC/Degenerin superfamily. The earliest appearance of ENaC orthologs is in thegenomes of the most ancient vertebrate taxon, Cyclostomata (jawless vertebrates)including lampreys, followed by earliest representatives of Gnathostomata (jawedvertebrates) including cartilaginous sharks. Among Euteleostomi (bonyvertebrates), Actinopterygii (ray finned-fishes) branch has lost ENaC genes.Yet, most animals in the Sarcopterygii (lobe-finned fish) branch includingTetrapoda, amphibians and amniotes (lizards, crocodiles, birds, and mammals),have four ENaC paralogs. We compared the sequences of ENaC orthologs from 20species and established criteria for the identification of ENaC orthologs andparalogs, and their distinction from other members of the ENaC/Degenerinsuperfamily, especially ASIC family. Differences between ENaCs and ASICs aresummarized in view of their physiological functions and tissue distributions.Structural motifs that are conserved throughout vertebrate ENaCs arehighlighted. We also present a comparative overview of the genotype-phenotyperelationships in inherited diseases associated with ENaC mutations, includingmultisystem pseudohypoaldosteronism (PHA1B), Liddle syndrome, cysticfibrosis-like disease and essential hypertension.
Keywords: Ion channels, Epithelia, Evolution, Transmembrane proteins, Kidney, Renin-angiotensin-aldosterone system
1. Introduction
As it is well known, 60–70 % of the human body weight iswater. About 2/3 of this water is within the cells (intracellular fluid, ICF) andthe remaining 1/3 fills the extracellular spaces and the vascular bed in thecirculatory system (extracellular fluid, ECF) (Ruthand Wassner, 2006). The cell membrane, as a semi-permeable barrier, ispermeable to water molecules. Yet, the net movement of water between ECF and ICFdepends on the relative osmolarity of these compartments and the permeability of themembranes (Fischbarg, 2010). In mostvertebrates, the osmolarity of both the ECF and ICF is determined mainly by theconcentration of electrolytes (dissolved salt ions carrying a net charge, mainlyNa+, K+, Ca+2, Mg+2,Cl−, HCO3−,PO43−, SO42−). Inthe ECF, Na+ is the electrolyte with the highest concentration and thusit is the major determinant of the osmolarity of the ECF (Takei, 2000). Osmolarity-dependent volume changes may lead toshrinking or swelling of cells. To prevent damage from such changes and to protectthe nervous system, mammals maintain a common osmotic set-point near 300 mosmol/L(Bourque, 2008). Thus, in vertebrates,the regulation of water and electrolyte homeostasis is highly interdependent (Ruth and Wassner, 2006).
The processes of absorption, secretion and excretion of water and solutestake place in epithelial cell layers that cover the internal and external surfacesof the body. In terms of permeability properties, epithelia are classified into twogroups as leaky- and tight-epithelia (Fischbarg,2010;Reddy and Stutts, 2013).Leaky epithelia are located generally in an isoosmotic environment as in the smallintestine and proximal kidney tubules and are highly permeable to water. In contrastto leaky epithelia, the cells in tight epithelia are connected by complex tightjunctions that reduce the permeability of the epithelia (Capaldo et al., 2014;Reddyand Stutts, 2013).
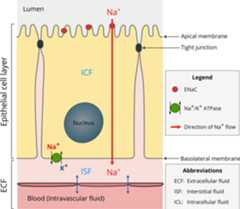
The epithelial sodium channel (ENaC), that is the focus of this review, islocated mostly in tight or high-resistance epithelia. As a constitutively activechannel, ENaC allows the flow of Na+ ions from the lumen into theepithelial cell, across the apical cell membrane (Garty and Palmer, 1997;Kashlan andKleyman, 2011;Kellenberger and Schild,2015) (Fig. 1). The absorbedNa+ ions are then pumped out of the cell into the interstitial fluidby the action of Na+/K+ ATPase located on the basolateralmembrane (Fig. 1). As ENaC modulates the amountof Na+ in the ECF, it has a central role in the regulation of ECF volumeand blood pressure (Büsst, 2013;Rossier et al., 2015). The activity ofENaC is regulated by the renin-angiotensin-aldosterone system (Asher et al., 1996;Bhalla andHallows, 2008;Büsst,2013;Rossier et al., 2015) and acomplex variety of extracellular factors including Na+,Cl−, protons, shear stress and proteases (Bhalla and Hallows, 2008;Kashlan and Kleyman, 2012,2011;Kellenberger and Schild,2015).
Fig. 1.
Schematic illustration of the location and function of ENaC inepithelia.
The subunits that form ENaC constitute a family within the ENaC/Degenerinsuperfamily. In addition to ENaC, this superfamily includes acid-sensing ionchannels (ASICs) (Deval and Lingueglia, 2015;Kellenberger and Schild, 2015,2002;Lin etal., 2015;Omerbašić etal., 2014;Waldmann and Lazdunski,1998), pickpocket genes in the Diptera order including Drosophila andmosquitoes (Zelle et al., 2013), degenerinsubunits involved in sensory transduction in nematodes such as Caenorhabditiselegans (Eastwood and Goodman, 2012;Liddle et al., 1963), and peptide-gated HydraNa+ channels (HyNaC) in hydrozoans (Gründer and Assmann, 2015).
The first sequences of ENaC subunits were based on cDNAs cloned from mRNAsisolated from rat and human tissues (Canessa et al.,1994b;Lingueglia et al., 1993;McDonald et al., 1995,1994;Voilleyet al., 1995,1994;Waldmann et al., 1995). Later development ofrapid genome sequencing techniques has led to the determination of the sequences ofENaC/Degenerin superfamily members in a growing number of species. This reviewconcentrates on the sequences and phylogenetic relationships of ENaC paralogs andorthologs across species and with other homologous proteins that have been mostlyrevealed by genome sequences of many species.
In biology, the word "homology" is also used to describefunctional equivalence and not just sequence and structural similarity. Thus, afterinter-species sequence comparisons, we shall also present the physiologicalimplications of the currently available information about ENaC phylogeneticdistribution and function.
2. Nomenclature of ENaC homologs
2.1. Definitions: Homolog, paralog, ortholog
In studies of protein evolution, the word "homologous" isused to describe proteins that share significant sequence similarity that isassumed to derive from a common ancestral origin. This concept of homologycovers both proteins that are homologous across species as well as proteins thatare present in multiple copies in the genome of a single species. To distinguishbetween these two types of homologous proteins, two separate terms were coinedby Walter Fitch (Fitch, 1970):orthologous and paralogous. Within the genome of a single species, there aremany genes that represent duplicate copies encoding isoforms of proteins withsimilar functions. The most common example is the globin family that includesα-globin, β-globin, and myoglobin. Homologous proteins thatexist "in parallel" within one species are called"paralogs", a hybrid word combining "parallel"with "homolog". The word "ortholog" is used forhomologous proteins that originate from a single ancestral gene in the lastcommon ancestor of the compared species. Continuing the globin example, theortholog of human α-globin is any of the α-globins in relatedprimates. Further examples of these terms are provided by (Koonin, 2005).
2.2. ENaC paralogs
In the human genome there are nine genes that encode for ENaC paralogs.These paralogs are grouped into two families based on their homology: 1.Non-voltage gated sodium channel family that is composed of four genes encodingENaC homologs and 2. acid-sensing (proton-gated) ion channels (ASIC) family thatis composed of five homologous genes. The four ENaC genes have been assignedabbreviations as SCNN1A, SCNN1B, SCNN1G, and SCNN1D by the Human GenomeOrganization (HUGO) Gene Nomenclature Committee (http://www.genenames.org/)following the Greek letters assigned to the four ENaC subunits α,β, γ, and δ (Table1 andTable 2). The second"N" in "SCNN1" was added to distinguish betweenthe NON-voltage gated ENaC and the SCN1 symbol assigned to the "sodiumchannel, voltage-gated, type I" that is expressed in neurons and muscle.The UniProt protein database (UniProt,2014) uses an abbreviated code for ENaC subunits (SCNNA, SCNNB, SCNNGand SCNND) to which the abbreviated species name is appended (Table 2). For the mouse genome, theconvention for gene nomenclature starts with an uppercase letter, followed byall lowercase letters as shown inTable1. For mouse, the gene for SCNN1D is not listed as it was not found inthe mouse genome (Giraldez et al.,2012). Another common name for ENaC subunits is"amiloride-sensitive sodium channel" as ENaC is inhibited byamiloride (Garty and Palmer, 1997;Kashlan and Kleyman, 2011).
Table 1.
Characteristics of the genes and transcripts encoding for ENaCsubunits.*
| Species | Gene | Chro. | CCDS code | Ensembl Transcript ID | Pre-spliced (nt) | Exons | Coding exons |
|---|---|---|---|---|---|---|---|
| Human | SCNN1A | 12p | 8543.1 | ENST00000228916 | 28,703 | 13 | 12 |
| SCNN1B | 16p | 10609.1 | ENST00000343070 | 79,030 | 13 | 12 | |
| SCNN1G | 16p | 10608.1 | ENST00000300061 | 34,169 | 13 | 12 | |
| SCNN1D | 1p | - | ENST00000400928 | 10,806 | 16 | 13 | |
| Mouse | Scnn1a | 6 | 39641.2 | ENSMUST00000081440 | 23,603 | 12 | 12 |
| Scnn1b | 7 | 21804.1 | ENSMUST00000033161 | 53,691 | 13 | 12 | |
| Scnn1g | 7 | 21803.1 | ENSMUST00000000221 | 33,971 | 13 | 12 | |
| Rat | Scnn1a | 4 | - | ENSRNOT00000067271 | 23,137 | 12 | 12 |
| Scnn1b | 1 | - | ENSRNOT00000067138 | 54,743 | 13 | 12 | |
| Scnn1g | 1 | - | ENSRNOT00000024057 | 33,957 | 13 | 12 |
Based on the NCBI CCDS, and Ensembl databases.
Table 2.
Length and mass of human and mouse ENaC subunits.
| Species | Subunit | Gene | CCDS code | Uniprot name | Length* (aa) | Mass* (Da) |
|---|---|---|---|---|---|---|
| Human | Alpha | SCNN1A | 8543.1 | SCNNA_HUMAN | 669 | 75,704 |
| Beta | SCNN1B | 10609.1 | SCNNB_HUMAN | 640 | 72,659 | |
| Gamma | SCNN1G | 10608.1 | SCNNG_HUMAN | 649 | 74,270 | |
| Delta | SCNN1D | - | SCNND_HUMAN | 638 | 70,215 | |
| Mouse | Alpha | Scnn1a | 39641.2 | SCNNA_MOUSE | 699 | 78,893 |
| Beta | Scnn1b | 21804.1 | SCNNB_MOUSE | 638 | 72,197 | |
| Gamma | Scnn1g | 21803.1 | SCNNG_MOUSE | 655 | 74,635 | |
| Rat | Alpha | Scnn1a | - | SCNNA_RAT | 698 | 78,888 |
| Beta | Scnn1b | - | SCNNB_RAT | 638 | 71,995 | |
| Gamma | Scnn1g | - | SCNNG_RAT | 650 | 74,066 | |
Based on Uniprot database.
As detailed below, the HUGO nomenclature appears to be sufficient fornaming ENaC orthologs in other vertebrate species whose genomes have beensequenced.
2.3. ASICs and other homologs
The five genes that code for the five Acid-Sensing Ion Channel (ASIC)subunits in the human genome have been numbered as ASIC1, ASIC2, ASIC3, ASIC4and ASIC5 by the HUGO Gene Nomenclature. The same abbreviation is used by theUniProt database (e.g. ASIC1_HUMAN). These channels were previously called asACCN and BNaC (García-Añoveroset al., 1997). One example of the proliferation of names is ASIC5.The product of this gene was initially named "brain, liver, intestineNa+ channel" (BLINaC) in mouse and rat. The homologous protein in humanswas found to be expressed in the intestine. Therefore, it was named"intestine Na+ channel (INaC)" in humans (Schaefer et al., 2000). A more recent study renamed thesame protein as "bile acid-sensitive ion channel" (BASIC) (Lefèvre et al., 2014). Althoughreferred to as ASIC5, it is not an acid-activated ion channel. The multiplicityof names for one protein emphasizes the need to adhere to names standardized byinternational nomenclature.
Many of the ENaC homologs were named based on the proteincharacteristics such as, sites of expression (e.g. "INaC","BLINaC"), physiologic consequences of activating mutations(e.g. "degenerin"), ligand interactions (e.g."FMRFamide-activated", "amiloride-sensitive","acid-sensing"), organism (e.g. HyNaC for channels in Hydra) andoriginal gene name (e.g. pickpocket in Drosophila). As noted with ASIC5, the useof different terms to name homologous proteins results in unrelated names forproteins that are highly homologous or orthologous. Moreover, homologousproteins may be expressed in different cell types and fulfill multiple functionsin different species, as observed with ENaC/Degenerin superfamily members. Thus,assignment of one name for a protein may not be relevant for an orthologousprotein in a different species.
As an alternative to naming proteins based on functionalcharacteristics, HUGO has taken the approach of a serial numbering system basedon homologous groupings (e.g. ASIC1… ASIC5, SCNN1A…SCNN1D). Inour view, this is a better approach for the nomenclature of ENaC/Degenerinsuperfamily, as it provides identical names to orthologs across species. In thecurrent genomic era, protein sequences are predicted based on genomic sequenceanalysis that includes comparisons between predicted and known proteinsequences. This approach of naming proteins based on sequence homology avoidsthe problems of names associated with protein characteristics.
In numerous invertebrate Metazoan species there is a multitude of highlydivergent proteins that show sequences homologous to ENaC/Degenerin superfamilymembers, but clearly represent different families based on low sequencesimilarity. As there is no standardized nomenclature for these proteins, in thisreview we used the names as in the original database records.
3. Chromosomal location and intron-exon organization of ENaC genes
In the human genome, SCNN1A encoding the α subunit is located on theshort arm of chromosome 12 (12p) (Voilley et al.,1994). The genes SCNN1B and SCNN1G encoding the β and theγ subunits are located side by side on the short arm of chromosome 16 (16p)(Shimkets et al., 1994;Voilley et al., 1995). The SCNN1D geneencoding the δ subunit is located in chromosome 1p (Table 1).
In the mouse genome, the gene Scnn1a is located on chromosome 6, and Scnn1band Scnn1g are juxtaposed at a region of chromosome 7 that shares synteny with thehuman chromosome 16 (Brooker et al., 1995;Pathak et al., 1996) (Table 1). Mouse genome appears to have lost thegene for the delta subunit (Giraldez et al.,2012). Yet, as detailed in Section 6, most vertebrate genomes have a genethat encodes for the delta subunit.
Sequencing of the α, β, and γ genes of the humangenome revealed that all three genes include 13 exons but only 12 of these containtranslated sequence (Fig. 2) (Table 1) (Ludwig et al., 1998;Saxena et al.,2002,1998;Thomas et al., 1996). In the human somatic chromosomes, theaverage number of exons per coding gene ranges from 8.5 to 13.5 (Hubé and Francastel, 2015).
Fig. 2.
Intron-exon organization of the human ENaC genes, SCNN1A, SCNN1B,SCNN1G and SCNN1D and their primary transcripts based on the NCBI Homo sapiensAnnotation Release 107 (2015-03-13). The name of each gene and its chromosomallocation are noted at the left-edge of the diagrams. Under each exon-intron map,there are two coordinates: the upper one specifies the chromosomal coordinates,and the lower one specifies the position of the nucleotide (in kb) starting atthe 5'-end of the RNA transcript (marked as 0). The codes above thediagrams represent the ID numbers of the RNA transcript (starting with NM_) andthe encoded protein (starting with NP_) in the NCBI Gene database. For SCNN1A,two transcripts are shown as examples of alternative splicing products. Notes:1) SCNN1A coordinates are given in a scale that descends from left-to-rightbecause the gene is located in the reverse strand of the chromosome. 2) Thex-axis for SCNN1B intron #1 includes a break between 5 kb and 45 kbmarks. Display of the full sequence (i.e., without a break) would lead to thevisible merger of exons 9 and 10 and hence disappearance of the intron 9 becauseof the short size of intron 9. Additional information about the genes and theirproducts is provided inTable 1 andTable 2.
In all three genes, SCNN1A, SCNN1B and SCNN1G, the introns are located atidentical positions in the coding sequence (Saxenaet al., 1998). The SCNN1D gene structure, revealed by the human genomesequencing project, includes at least 16 exons 13 of which are protein coding (Table 1). Despite the conservation of theintron positions within the coding sequence, the sizes of the introns have divergedgreatly resulting in significant differences between gene lengths (Fig. 2). The sizes of the primary transcriptsprior to splicing range from 10,806 bp (for SCNN1D) to 79,030 (for SCNN1B) (Table 1) (Fig.2). Among the four genes, the longest intron is intron #1 ofSCNN1B (Fig. 2, note that there is a break inthe x-axis of nucleotide position). In both SCNN1A and SCNN1B genes, the longestintrons are intron #1 or #2 closest to the 5'-end of thetranscription initiation site (Fig. 2). Thisrepresents a general trend that in genomes the longest introns appear at the5'-end of the gene (Zhu et al.,2009).
Analyses of the RNA transcripts of the genes encoding ENaC subunits haveprovided evidence for alternative RNA splicing products and multiple translationinitiation sites (see Ensembl records listed inTable 1) (Berman et al., 2015;Bremner et al., 2002;Thomas et al., 2002). Alternative splicing iscommon in vertebrates and is thought to contribute to a higher level of phenotypiccomplexity in mammals (Kim et al., 2007). Incases where there was more than one isoform sequence for a gene, we used the UniProtCanonical Sequence or an NCBI Consensus CDS (CCDS) as the representative sequencefor the gene in homology analyses for paralogs and orthologs.
4. Assembly of ENaC with paralogs
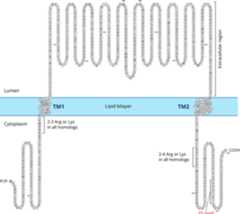
Previous studies have established that ENaC paralogs serve as subunits thatform the channel (Canessa et al., 1994b;Kashlan and Kleyman, 2011). The mostsalient common feature of ENaC paralogs is the presence of two segments thatfunction as two transmembrane (TM) segments embedded in the membrane, referred to asTM1 and TM2 (Fig. 3). In membrane-bound form,the amino (N) and the carboxy (C) termini of ENaC are intracellular, and a largeextracellular segment, comprising about 70% of the amino acids of eachsubunit, connects the TM segments.
Fig. 3.
Schematic illustration of the transmembrane localization of an ENaCsubunit. The sequence shown is of human α subunit (seeTable 1). All homologous ENaC subunits havetwo transmembrane segments. The TM segments for this figure was predicted by thePhobius program (seeTable 3) and drawnusing Protter (Omasits et al., 2014).The extracellular domain includes about 70% of the sequence of aminoacids of an ENaC subunit.
Although the structure of ENaC is not known, the strong hydrophobicity ofthe TM segments and homology with the resolved ASIC1 structure (Jasti et al., 2007) allows prediction of theTM segments (Table 3) (Fig. 3). In humans, the four ENaC subunits show significantsequence similarity in large segments of the extracellular region (Fig. 4). The most divergent parts of the ENaCparalogs are the N- and C-termini (Fig. 4).
Table 3.
Intracellular, extracellular and transmembrane (TM) segments of humanENaC subunits. The position1 of TM1 was predicted using Phobius software (Käll et al., 2004). The positionof TM2 is based on homology with the ASIC1 structure (Jasti et al., 2007).
| Cytoplasmic N-ter | TM1 | Extracellular | TM2 | Cytoplasmic C-ter | |
|---|---|---|---|---|---|
| Alpha | 1–84 | 85–106 | 107–543 | 544–575 | 576–669 |
| Beta | 1–49 | 50–70 | 71–514 | 515–546 | 547–640 |
| Gamma | 1–53 | 54–79 | 80–523 | 524–555 | 556–649 |
| Delta | 1–87 | 88–107 | 108–520 | 521–552 | 553–638 |
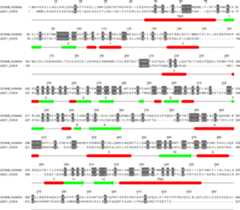
Fig. 4.
Aligned sequences of human α, β, γ andδ-ENaC subunits and conserved positions of introns in all four subunits.Residues that are identical in all four subunits are shaded. The numbers (2 to12) below the sequences mark the position and number of the intron located in orat the end of the codon of the specific residue above the number. In the5' portion of the gene encoding δ-ENaC subunit there areadditional introns that are not shown here. The sequences were aligned using theClustalW2 program, and the alignment of some residues in the amino and carboxytermini were manually edited to eliminate some gaps without affecting percentidentity score. TM1 and TM2 mark the predicted transmembrane segments of theproteins.
The resolved structures of chicken ASIC1 revealed a homotrimer composed ofthree identical subunits (Baconguis et al.,2014;Jasti et al., 2007) (Fig. 5). In contrast to ASIC1 structure,independent lines of evidence indicate that ENaC is assembled as a heterotrimercomposed of α (or δ), β and γ subunits:
Specific mutations in any one of the three genes coding for theα, β, and γ-ENaC were shown to result in anautosomal recessive disorder termed multi-system pseudohypoaldosteronismtype I (PHA) (Chang et al., 1996;Hanukoglu, 1991). Theunderlying mechanism of multi-system PHA is the unresponsiveness toaldosterone in target organs expressing ENaC including kidney, sweat andsalivary glands, reproductive and respiratory tracts (Enuka et al., 2012;Hanukoglu, 1991). In affectedpatients the disease is characterized by severe hyponatremia,hyperkalemia, dehydration and acidosis that starts in infancy andcontinues later in life with varying severity (Belot et al., 2008;Chang et al., 1996;Edelheitet al., 2010,2005;Hanukoglu and Hanukoglu,2010;Hanukoglu,1991;Strautnieks et al.,1996). So far, no case of PHA has been identified that iscaused by a mutation in the SCNN1D gene encoding δ-ENaC.
Gene knockout studies inactivating the genes coding for theα, β, and γ subunits in mice showed that allthree subunits are essential for survival. All gene knockout micewithout either α, β, or γ subunits (genotype:−/−) die within < 50 hours after birth, withrespiratory insufficiency or kidney dysfunction leading to hyperkalemia,metabolic acidosis and severe dehydration (Barker et al., 1998;Bonny and Hummler, 2000;Hummler et al., 1996).
Robust expression of ENaC activity in Xenopus oocytes requiresall three subunits (α, β, and γ) (Canessa et al., 1994b;Edelheit et al., 2014,2011;Giraldez et al., 2007). Expression of one or twoENaC subunits in Xenopus oocytes yields either minimal or no detectablechannel activity (Canessa et al.,1994b;Edelheit et al.,2011;Giraldez et al.,2007).
Assessment of the stoichiometry of ENaC subunits usingfluorescently labeled subunits, and imaging of ENaC-antibody complexesby atomic force microscopy indicated that the subunits are assembled asheterotrimers with a ratio of 1:1:1 (Staruschenko et al., 2005;Stewart et al., 2011).
Post-translation processing of the channel, including N-glycanmaturation and furin-dependent cleavage, requires expression of allthree subunits (Hughey et al.,2004).
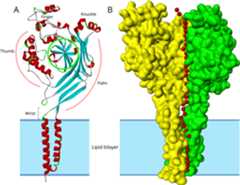
Fig 5.
A. Ribbon structure model of subunit A of chicken ASIC1 (PDB ID: 2QTS).Segments in helical conformation are red colored and segments in sheetconformation are blue colored.
B. The surface structure of subunits A and B of ASIC1. The fourhydrophobic helices of the A and B subunits are embedded in the lipid bilayermarked by gray shading. The third subunit (C) surface is not shown to allowvisibility of the central pore predicted by the Porewalker software. Red coloredsmall spheres represent water molecules placed at the center of the predictedpore and extracellular vestibule in each 3 Angstrom slice of 2QTS calculated byPorewalker.
Studies examining the structure of ENaC by molecular modeling andsite-directed mutagenesis of conserved residues support the concept that ENaCstructure is homologous to ASIC1 channel. In contrast to ASIC1 that functions as ahomotrimer, ENaC is an obligate heterotrimer (Edelheit et al., 2014;Kashlan andKleyman, 2011;Stockand et al.,2008). A study based on mutagenesis of Cl− inhibitorysites suggests that the clockwise orientation of the subunits isαγβ, when viewed from the top of the channel (Collier and Snyder, 2011).
In summary, the three paralogs encoding the α (or δ),β and γ subunits are essential for the assembly of functionalchannels. As summarized below, these three paralogs are highly conserved in allvertebrates. The evolutionary conservation of these genes provides further evidencethat the subunits encoded by these genes are essential for the assembly of theheterotrimeric channel. The tissue distribution of the δ subunit isdifferent from that of other subunits and its activity has been studied less.Excellent reviews by Giraldez et al. and Ji et al. summarize the characteristics ofδ-ENaC (Giraldez et al., 2012;Ji et al., 2012).
4.1. Trimeric structure and channel pore
In the trimeric structure of ASIC1, one of the issues that have beenintensively studied is the location of the channel pore through which ions flowacross the membrane. ASIC1 has six transmembrane segments - three of each of TM1and TM2. The structure of ASIC1 revealed that the TM1 and TM2 helices areorganized in two separate concentric triads. The central pore is formed by thetriad of TM2s. TM1s form a triad around the TM2 triad (Baconguis et al., 2014;Gonzales et al., 2009;Li et al.,2011). Most studies on ENaC suggest a similar organization of the TMsegments in ENaC as well (Tolino et al.,2011). Section 11 on conserved motifs presents the properties ofthese segments in detail.
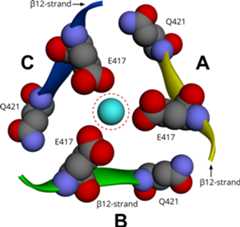
One of the major unresolved questions in ENaC function is the path(s) ofions into the channel pore in the membrane as described above. On top of thechannel pore, the extracellular regions of the three subunits form a tripartitefunnel with rotational symmetry (Fig. 5)(Baconguis et al., 2014;Jasti et al., 2007). However, the threesubunits are not completely tightly juxtaposed along their entire lengths andthere are fenestrations between the subunits above the pore around the regioncalled "extracellular vestibule" (Baconguis et al., 2014). The hollow space along the centralaxis of rotational symmetry of this channel has been called a"vestibule". This vestibule leads from the top opening in thelumen to the channel pore embedded in the membrane. Under differentcrystallization conditions, segments of this vestibule may be constricted orexpanded (Baconguis et al., 2014). Thesedifferent states suggest that dynamic vestibule constriction and expansion mayregulate ion flow into the channel pore.
The extracellular and central segments of the vestibule are surroundedby beta-strands of the palm domain two of which are connected to the TM helices(β1 to TM1 and β12 to TM2) (Fig.5). Thus, changes in the angles of TM helices may effect constrictionof the vestibule. Conversely, movement of the β1 and β12 strandsmay effect opening or closing of the channel gate by modulating the position ofthe TM helices. For ASIC1, there is evidence that the movement of the coiledlinker region immediately prior to β1 and β12 strands may effectchannel opening and closing (Li et al.,2010;Springauf et al.,2011). The dynamics of these parts are also affected by the interactionsbetween the thumb and finger domains (Gwiazdaet al., 2015;Yang et al.,2009). There is a variety of intracellular and extracellular factorsthat can affect the dynamics of these segments, e.g. cytoplasmic Ca2+(Gu, 2008), binding to actin andother cytoskeletal proteins (Ilatovskaya etal., 2012;Sasaki et al.,2014), phosphoinositides that serve as second messengers inintracellular signaling cascades (Hille et al.,2015;Pochynyuk et al.,2008), extracellular ions, including Na+ andCl−, pH and cleavage by extracellular proteases (Kashlan and Kleyman, 2012;Kellenberger and Schild, 2015).
5. Homology between ENaC and ASIC paralogs
To assess the similarity of the ENaC and ASIC sequences, Fasta format of theselected sequences were downloaded from the Uniprot database. Multiple sequencealignments were carried out by the CLUSTALW software (version 2.1) with defaultparameters (http://www.genome.jp/tools/clustalw/) (Chenna et al., 2003). Percent identity figures were calculatedusing GeneDoc (Nicholas and Deerfield,1997). Sequence alignments for the figures were generated using the Jalviewprogram (Waterhouse et al., 2009).
Among the four human ENaC subunits, greatest similarity exists between theα and δ subunits (34% identity) and the β andγ subunits (34% identity) (Table4). The percent identity between other pairs (e.g. α vs.β or γ) is between 23–27% (Table 4). Since the N- and C-termini of ENaC subunits showdivergence, we also determined the sequence identity in the extracellular regions ofENaC subunits. These values indicate a 2–6% higher sequence identityin the extracellular regions (Table 5), ascompared to the full-length sequences of ENaC subunits (Table 4).
Table 4.
Percent identity between human ENaC and ASIC subunits along theirentire sequences.
| SCNNA | SCNNB | SCNNG | SCNND | ASIC1 | ASIC2 | ASIC3 | ASIC4 | |
|---|---|---|---|---|---|---|---|---|
| SCNNB_HUMAN | 26 | |||||||
| SCNNG_HUMAN | 27 | 34 | ||||||
| SCNND_HUMAN | 34 | 23 | 23 | |||||
| ASIC1_HUMAN | 13 | 16 | 15 | 16 | ||||
| ASIC2_HUMAN | 13 | 15 | 15 | 13 | 64 | |||
| ASIC3_HUMAN | 14 | 14 | 15 | 14 | 46 | 45 | ||
| ASIC4_HUMAN | 12 | 12 | 11 | 14 | 35 | 31 | 32 | |
| ASIC5_HUMAN | 12 | 14 | 13 | 13 | 22 | 22 | 21 | 17 |
The sequences were aligned using ClustalW2 program (version2.1).
Table 5.
Percent identity between human ENaC and ASIC subunits in the conservedcentral segment including TM1 + extracellular domain + TM2 (seeFig. 3 andFig.4).
| SCNNA | SCNNB | SCNNG | SCNND | ASIC1 | ASIC2 | ASIC3 | ASIC4 | |
|---|---|---|---|---|---|---|---|---|
| SCNNB_HUMAN | 31 | |||||||
| SCNNG_HUMAN | 33 | 36 | ||||||
| SCNND_HUMAN | 37 | 28 | 28 | |||||
| ASIC1_HUMAN | 16 | 19 | 18 | 18 | ||||
| ASIC2_HUMAN | 16 | 18 | 17 | 17 | 74 | |||
| ASIC3_HUMAN | 17 | 17 | 16 | 17 | 52 | 53 | ||
| ASIC4_HUMAN | 15 | 17 | 15 | 17 | 49 | 47 | 46 | |
| ASIC5_HUMAN | 16 | 17 | 16 | 16 | 26 | 27 | 26 | 25 |
In contrast to ENaC subunits, the sequence identities between human ENaC andhuman ASIC subunits are much lower: in the range of 11 to 16% (Table 4). Thus, clearly ENaC and ASIC paralogsbelong to distinct families as marked by the demarcation lines inTable 4. Percent sequence identity between ASICsubunits themselves ranges from 17 to 64% (Table 4). Similar to ENaC, the extracellular segments of ASIC subunitsshare higher identity than the whole sequences (compareFig. 7 vs.Fig. 6 forASIC), reflecting divergence of N- and C- terminal sequences (see Section 11).
Fig. 7.
Secondary structures in the sequence of chicken ASIC1. The positions ofthe structures were taken from the PDB file of 2QTS. The numbering of thestructures is based on (Jasti et al.,2007). Note that some short stretches of helix and β-strandare not numbered. For comparison of sequence conservation, human β-ENaCis globally aligned with the ASIC1 sequence and identical residues were graycolor shadowed. Note that most but not all secondary structures are associatedwith conserved sequences.
Fig. 6.
Topology diagram of chicken ASIC1 structure. The cylinders representhelical segments, and the arrows represent β-strands. The transmembrane(TM), and secondary structural domains (palm, β-ball, finger, thumb andknuckle) were colored distinctly and named as in (Jasti et al., 2007). Certain features of the diagram wereadopted from previous diagrams (Eastwood andGoodman, 2012;Kashlan and Kleyman,2011).
Comparisons of the sequences of all four ENaC paralogs from six species (inaddition to human) indicate that the degree of sequence identity between the fourparalogs within each species is quiet similar to that observed in the human genome(compareTable 4 andTable 6).
Table 6.
Percent sequence identity between four paralogous ENaC subunits(α, β, γ and δ) in six species (for comparisonof human paralogs seeFig. 4 andTable 4).
| α | β | γ | ||
|---|---|---|---|---|
| Rhesus | β | 24 | ||
| γ | 27 | 31 | ||
| δ | 25 | 16 | 18 | |
| Bovine | β | 28 | ||
| γ | 28 | 34 | ||
| δ | 38 | 23 | 25 | |
| Tasmanian D. | β | 26 | ||
| γ | 27 | 32 | ||
| δ | 37 | 21 | 22 | |
| Xenopus | β | 29 | ||
| γ | 31 | 30 | ||
| δ | 39 | 27 | 28 | |
| Alligator | β | 27 | ||
| γ | 29 | 34 | ||
| δ* | 39 | 25 | 27 | |
| Coelacanth | β | 26 | ||
| γ | 29 | 29 | ||
| δ | 43 | 24 | 27 |
Named by us as the δ-subunit. Named as "alphalike" in the original report.
In the CATH protein structural domain database (Sillitoe et al., 2015), ASIC and ENaC channels are listed astwo separate families within the Superfamily number 2.60.470.10 titled"Acid-sensing ion channels like domains". CATH classification systemis mostly based on specific local structural domains. The domain selected for theclassification is mainly the "palm" domain based on the ASIC1structure (2QTS). The palm domain is composed of a complex of β-sheets.Therefore within the CATH database, the channel is included under Class 2 for"Mainly beta" type domains. Since the ASIC1 structure is anintricate complex of α-helices and β-sheets this classification doesnot take into account the full structural view of the channels.
5.1. Sites of divergence among ENaC and ASIC paralogs
The divergence of N- and C- termini of ENaC/Degenerin superfamilymembers (noted above) represents a general trend in protein families. Previousstudies on other proteins have shown that changes in protein domain architectureare most common in the N- and C-termini of proteins (Björklund et al., 2005;Forslund and Sonnhammer, 2012). In contrast to α-and δ-ENaC, the N- and C-termini of human β- and γ-ENaCare highly conserved. The structures of these terminal segments are currentlynot known, but there are studies indicating that these cytoplasmic domainsinteract, either directly or indirectly, with other cytoplasmic and cytoskeletalproteins such as syntaxin (Berdiev et al.,2004;Condliffe et al., 2003),actin (Copeland et al., 2001), ubiquitinligase Nedd4 and protein kinases (Asher et al.,2001;Bobby et al., 2013;Shi et al., 2002).
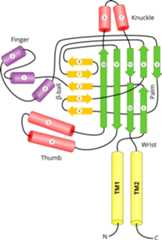
Since the structure of the extracellular region of ASIC1 has beenresolved and in this region there is a significant homology between ASIC1 andENaC subunits, we shall present the sites of divergence in this region in termsof the secondary structural segments of ASIC1. The original study on the crystalstructure of ASIC1 noted that ASIC1 structure resembles a hand holding a ball(Jasti et al., 2007). Hence, domainswithin the extracellular regions are referred to as palm, thumb, knuckle, fingerand β-ball (Jasti et al., 2007).The palm and β-ball domains are formed by non-contiguousβ-strands and loops, and are in close proximity to the membrane (Fig. 6). More peripheral domains (thumb,knuckle and finger) are formed by contiguous α-helices and loops (Fig. 6).
To facilitate location of divergent regions in ENaC relative to thestructural domains of ASIC1 inFig. 7 weprovide an alignment of the β-subunit with ASIC1 sequence includingmarking of the positions of the secondary structural elements according to thePDB ID 2QTS (Fig. 7).
In the extracellular region of ENaC subunits, there are several highlydivergent segments where insertions/deletions are found (Fig. 4 andFig. 7). Onedivergent area is in the finger domain in between helix #1 andβ-strand #3 (Fig. 4,Fig. 6 andFig.7). This segment is divergent in four ENaC paralogs and ischaracterized by poorly aligned sequences including large insertions anddeletions (Fig. 4 andFig. 7). This "finger" domain shows thehighest variability among ENaC/Degenerin superfamily members indicating thatthis region may have an important role in conferring functional specificity(Eastwood and Goodman, 2012;Kashlan and Kleyman, 2011). For example,the α and γ-subunit finger domains have inhibitory tracts thatare released following proteolytic processing (Bruns et al., 2007;Carattino etal., 2008a,2006;Kashlan et al., 2011;Passero et al., 2010).
Another divergent segment in ENaC starts at about residue 376 of thehuman β-ENaC and includes an insertion of three residues (Fig. 4). In alignment with ASIC1 this regionis located in the region between β-9 and α-4 (Fig. 7). This is the region that connects thepalm domain of ASIC1 to the thumb domain (Jastiet al., 2007). This region has been proposed to transmitconformational changes in the periphery of the extracellular region to thechannel pore and gate (Jasti et al.,2007;Li et al., 2011;Shi et al., 2011). Other divergent areasinclude the knuckle domain and the loop connecting the β-6 andβ-7 strands. Residues in the β-6 - β-7 loop of theα subunit have been proposed to function as an extracellularNa+ binding site that is involved in Na+self-inhibition (Kashlan et al.,2015).
In conclusion, it appears that areas of divergence that are seen in ENaCand ASIC1 comparisons are located in the connecting segments within the fingerand thumb domains. In additions to these, there are a few other sequencedifferences but the sequence homology predominates especially in theβ-strand segments in the palm and β ball domains (Fig. 6, andFig. 7).
It is interesting that the most divergent areas within members of theENaC/Degenerin family are in the periphery of the extracellular region. There isgrowing evidence that these divergent areas have sites of direct interactionwith extracellular regulatory factors that modulate channel activity, such asproteases (Bruns et al., 2007;Vallet et al., 1997), inhibitory peptidereleased by proteases (Carattino et al.,2006;Kashlan et al., 2010),extracellular chloride (Cl−) ions (Collier and Snyder, 2011), extracellular Na+(Chraibi and Horisberger, 2002;Edelheit et al., 2014;Winarski et al., 2010), protons (Collier et al., 2012;Krauson et al., 2013), and laminar shear stress induced byfluid flow (Shi et al., 2012). As thedifferent ENaC/Degenerin family members are regulated by distinct factors,evolutionary divergence within the peripheral domains may have been a key factorin allowing this family to evolve with different functional properties.
6. Phylogenetic distribution of ENaC orthologs
Determination of genomic sequences of many eukaryotic species has providedENaC gene sequences from a broad spectrum of vertebrates. Comparison of ENaC geneand protein sequences across species is useful from several perspectives. Knowledgeabout ENaC orthologs across species can contribute to our understanding of thesignificance and function of ENaC subunits. Conservation of a gene across speciessuggests an important physiological function for the organism (see for example(Studer et al., 2011)). Secondly,comparisons of the sequences of the ENaC subunits enhance our understanding of thestructural and functional importance of conserved sequence segments. Thirdly, theabsence of an ENaC gene in a species is important information as the species may usealternative subunits or channels to fulfill the homeostatic functions of ENaC.
The Ensembl genome database (release 79) of vertebrate and eukaryoticspecies currently includes 540 genes homologous to ENaC family members, 188 of whichencode one of the four ENaC subunits. The remainder represents ASICs or other familymembers from different species. A BLAST search of the UniProt protein database showsthat ENaC subunits are found in vertebrates. BLAST search of UniProt bacteria, fungiand plant protein sequence databases did not reveal orthologs of human ENaCsubunits. Here we provide a summary of the appearance of ENaC genes in Metazoanspecies.
6.1. Cyclostomata and Chondrichthyes (cartilaginous fishes)
In the phylogeny of vertebrates, the most ancient taxon is Cyclostomata,i.e. jawless vertebrates. Lampreys and hagfishes are common extant species thatrepresent this taxon. These fishes have only cartilaginous elements as aprimitive skeleton that supports their body parts (Shimeld and Donoghue, 2012). The genome of sea lampreyincludes three genes that code for the orthologs of α, β andγ-ENaC, but apparently does not include a gene for the delta subunit(Table 7) (Smith et al., 2013). The sequence of lamprey αsubunit is not complete (S4RTA3_PETMA).
Table 7.
Presence (+) or absence (−) of genes encoding SCNN1A, SCNN1B,SCNN1G, and SCNN1D in non-mammalian vertebrates.
| Taxon | Example species | A | B | G | D | Reference |
|---|---|---|---|---|---|---|
| Cyclostomata (jawless vertebrates) | ||||||
| Petromyzontidae(lampreys) | Petromyzon marinus (Sea lamprey) | + | + | + | − | (Smith et al.,2013) |
| Gnathostomata (jawed vertebrates) | ||||||
| Chondrichthyes(cartilaginous fishes) | Callorhinchus milii (Elephant shark) | + | + | + | − | (Venkatesh et al.,2007) |
| Euteleostomi (bony vertebrates) | ||||||
| Actinopterygii(ray-finned fishes) | Danio rerio (Zebrafish) | − | − | − | − | (Venkatesh et al.,2007) |
| Coelacanthiformes(lobe-finned fishes) | Latimeria chalumnae (Coelacanth) | + | + | + | + | (Amemiya et al.,2013) |
| Dipnoi(lungfishes) | Neoceratodus forsteri (Lungfish) | + | + | + | ? | (Uchiyama et al.,2012) |
| Euteleostomi: Tetrapoda: Amphibia: Batrachia:Anura (frogs and toads) | ||||||
| Pipidae (tonguelessfrogs) | Xenopus tropicalis (Frog) | + | + | + | + | (Hellsten et al.,2010) |
| Euteleostomi: Tetrapoda: Amniota: Sauropsida:Sauria: Archelosauria: Archosauria: Crocodylia | ||||||
| Alligatorinae(alligators) | Alligator mississippiensis (American alligator) | + | + | + | + | (Green et al.,2014) |
| Euteleostomi: Tetrapoda: Amniota: Sauropsida:Sauria: Archelosauria: Archosauria: Dinosauria: Aves (birds) | ||||||
| Galliformes(fowls) | Gallus gallus (Chicken) | + | + | + | + | (Chicken-Genome,2004) |
| Galliformes | Meleagris gallopavo (Turkey) | + | + | + | + | (Dalloul et al.,2010) |
| Gruiformes | Eurypyga helias (Sunbittern) | + | + | + | + | (Zhang et al.,2014) |
| Passeriformes (perchingbirds) | Taeniopygia guttata (Zebra finch) | + | + | + | + | (Warren et al.,2010) |
| Passeriformes | Ficedula albicollis (Flycatcher) | + | + | + | + | (Ellegren et al.,2012) |
| Piciformes | Picoides pubescens (Downy woodpecker) | + | + | + | + | (Zhang et al.,2014) |
| Spheniscidae(penguins) | Aptenodytes forsteri (Emperor penguin) | + | + | + | + | (Zhang et al.,2014) |
| Euteleostomi: Tetrapoda: Amniota: Sauropsida:Sauria: Archelosauria: Testudines (turtles) | ||||||
| Trionychidae(soft-shelled turtles) | Pelodiscus sinensis (Soft-shelled turtle) | + | + | + | + | (Z. Wang et al.,2013) |
| Euteleostomi: Tetrapoda: Amniota: Sauropsida:Sauria: Lepidosauria | ||||||
| Squamata (lizards andsnakes) | Anolis carolinensis (Green anole lizard) | + | + | + | + | (Alföldi et al.,2011) |
Next steps in the evolution of vertebrates include the development ofjaw and skeleton leading to the formation of Gnathostomata (jawed vertebrates)(Donoghue et al., 2006;Kawasaki and Weiss, 2006;Kuratani, 2012). The earliestrepresentatives of this branch include cartilaginous fish species, includingrays and sharks. The genome of the cartilaginous elephant shark has beendetermined and it includes three orthologous ENaC genes (Venkatesh et al., 2007) (Table 7).
6.2. Euteleostomi (bony vertebrates)
In evolution, the development of jaw is followed by the development ofbony fishes. The clade of Euteleostomi (bony vertebrates) includes two branches:
Actinopterygii (ray-finned fishes): The"ray-finned" description is based on spinyprojections in the fins of these fishes.
Comparison of shark, human and teleost ray-finned fishgenomes has revealed that 154 genes (including ENaC paralogs) thathave orthologs in the shark genome are not present in ray-finnedfish genomes (Venkatesh et al.,2007). Thus, the whole clade of Actinopterygii (rayfinned-fishes), which includes Zebrafish, do not have ENaC genes.However, they have ASIC genes. During the course of evolution, ENaCgenes may have been lost at the onset of the branch of ray-finnedfishes for lack of a functional need or were replaced functionallyby alternative genes and proteins (Uchiyama et al., 2014;Venkatesh et al., 2007).
The EnsemblCompara GeneTree shows one"SCNN1A" gene for Lepisosteus oculatus (spotted gar)that is a freshwater ray-finned fish. Our comparison of this proteinwith human ASIC and ENaC paralogs showed that it is more homologousto ASIC than ENaC paralogs. UniProt database includes 9 proteinfragments from the spotted-gar genome that show homology to"amiloride-sensitive sodium channel family". Ourcomparison of 4 partial sequences (with lengths >400residues) from the UniProt database with human ASIC and ENaCparalogs showed that all four sequences share 49–61%sequence identity with human ASIC1, while they share13–15% with human ENaC paralogs. Therefore, thenaming of the single ray-finned fish spotted gar protein(ENSLOCP00000013400) as "SCNN1A" appears to be inerror. Thus with the elimination of this case, so far ray-finnedfish genomes do not appear to have ENaC orthologs as notedabove.
Sarcopterygii (lobe-finned fish): The"lobe-finned" description was given because of theirfleshy paired fins which are considered an early form of limbdevelopment in tetrapod vertebrates with four limbs. Therefore, thisclade also includes all Tetrapoda species.
Sarcopterygii includes two ancient taxa with extant fishes:Coelacanthiformes (lobe finned fishes, coelacanth) and Dipnoi(lungfishes) (Table 7). Thethree ENaC genes are present in the genomes of these fish (Amemiya et al., 2013;Uchiyama et al., 2014,2012). Tetrapoda is considereda branch that emerged in parallel to Dipnoi.
6.3. Amphibia
In the evolutionary ladder, the development of bony vertebrates wasfollowed by the emergence of tetrapods with four limbs. Amphibians (frogs, toadsand salamanders) represent the first class of tetrapods. Xenopus tropicalis(frog) genome includes genes encoding the four ENaC paralogs (Hellsten et al., 2010) (Table 7).
6.4. Sauropsida
The second group of tetrapods is Amniota (amniotes) characterized byhaving an egg or embryo covered with an amniotic membrane. Amniotes include twoclades: Sauropsida that includes birds and reptiles, and Mammalia (mammals).
The genome sequences of three crocodilians have been recently reported(Green et al., 2014). Currently,NCBI Genome database Genome Assembly and Annotation report (including a list ofpredicted proteins) is available only for Alligator mississippiensis (Americanalligator). Search of this database for amiloride-sensitive sodium channelyielded four sequences (XP_006258424.1,XP_006268483.1,XP_006268484.1, andXP_006277862.1). In this report, the first and the fourth sequences were namedas "amiloride-sensitive sodium channel subunit alpha-like",while the second and the third sequences were named as "…subunit beta" and "… subunit gamma". Percentidentities of these four sequences are shown onTable 6. These results show that the second sequence(XP_006277862.1) that was labeled as "alpha-like", matches otherdelta-ENaC sequences in terms of its percent identity with the other alligatorENaC subunits (Table 6) and human ENaCsubunits (results not shown). Thus, we conclude that this alligator has fourENaC paralogs including one gene coding for the delta subunit.
The Ensembl (release 79) Gene Tree view includes two reptiles:soft-shell turtle and green anole lizard (Table7). Both of these genome sequences also include four genes coding forthe four ENaC paralogs (Table 7).
Bird genomes that are listed in Ensembl (release 79) Gene Tree view,include four genes coding for ENaC subunits. The recently determined genome ofsunbittern (Eurypyga helias) (Zhang et al.,2014) is not yet included in the Ensembl database. Similar to thecase of alligator genome noted above, NCBI Genome database Genome Assembly andAnnotation report includes four amiloride-sensitive sodium channel entries oneof which was listed as "…alpha-like". Our sequenceidentity analysis unequivocally classifies this "alpha-like" asthe δ subunit. Therefore, this genome also includes four ENaC heterologs(Table 7). Since birds andcrocodilians are considered evolutionary descendants of dinosaurs (Green et al., 2014), it is likely thatdinosaurs also had four genes coding for ENaC subunits.
6.5. Mammalia
The class of Mammalia includes three taxa: egg-laying mammals(Monotremata), marsupials (Metatheria) and placental mammals (Eutheria). Innearly all mammals in these three clades, there are four ENaC genes (Table 8). Ensembl genome database (release79) includes 38 mammalian species, including 34 placental mammals, 3 marsupials(opossum, Tasmanian devil, wallaby) and egg-laying platypus. All of thesespecies have four paralogs of ENaC with the exception of the mouse genome thatappears to have lost the gene for the delta subunit (Ensembl Gene Tree for ENaChomologs). The rat genome, that is a very close phylogenetic relative of themouse, includes four ENaC paralogs, but the δ subunit sequence ispresently available only as a fragment (NCBI Accession:NC_005104.4).
Table 8.
Presence (+) or absence (−) of genes encoding SCNN1A, SCNN1B,SCNN1G, and SCNN1D in mammals.
| Taxon | Example species | A | B | G | D | Reference | |
|---|---|---|---|---|---|---|---|
| Monotremata (egg-laying mammals) | Ornithorhynchus anatinus (Platypus) | + | + | + | + | (Warren et al.,2008) | |
| Metatheria (marsupials) | |||||||
| Diprotodontia | Macropus eugenii (tammar wallaby) | + | + | + | + | (Renfree et al.,2011) | |
| Didelphimorphia | Monodelphis domestica (opossum) | + | + | + | + | (Mikkelsen et al.,2007) | |
| Dasyuridae | Sarcophilus harrisii (Tasmanian devil) | + | + | + | + | (Miller et al.,2011) | |
| Eutheria (placental mammals) | |||||||
| Afrotheria | |||||||
| Elephantidae (elephants) | Loxodonta africana (African elephant) | + | + | + | + | Elephant genome project | |
| Tenrecidae (tenrecs) | Echinops telfairi (hedgehog) | + | + | + | + | ||
| Boreoeutheria: Laurasiatheria: Carnivora(carnivores) | |||||||
| Canidae (dog, coyote, wolf, fox) | Canis lupus familiaris (dog) | + | + | + | + | (Lindblad-Toh et al.,2005) | |
| Felidae (cat family) | Felis catus (domestic cat) | + | + | + | + | (Pontius et al.,2007) | |
| Boroeutheria: Laurasiatheria: Cetartiodactyla(whales, hippos, ruminants, pigs, camels etc.) | |||||||
| Cetacea (whales) | Orcinus orca (killer whale) | + | + | + | + | Marine mammal genomics Ensembl | |
| Boreoeutheria: Laurasiatheria: Cetartiodactyla:Ruminantia | |||||||
| Bovinae | Bos taurus (cow) | + | + | + | + | (Elsik et al.,2009) | |
| Caprinae | Ovis aries (sheep) | + | + | + | + | Sheep Genomics Consortium | |
| Boreoeutheria: Laurasiatheria: Perissodactyla(odd-toed ungulates) | |||||||
| Equidae (horses) | Equus caballus (horse) | + | + | + | + | (Wade et al.,2009) | |
| Boreoeutheria: Euarchontoglires: Rodentia | |||||||
| Muridae | Mus musculus (mouse) | + | + | + | − | (Takada et al.,2013) | |
| Muridae | Rattus norvegicus (rat) | + | + | + | + | (Saar et al.,2008) | |
| Boreoeutheria: Euarchontoglires: Primates | |||||||
| Hominidae | Pan troglodytes (chimpanzee) | + | + | + | + | Chimpanzee Sequencing and Analysis Consortium | |
| Hominidae | Gorilla gorilla | + | + | + | + | (Scally et al.,2012) | |
| Hominidae | Pongo abelii (Sumatran orangutan) | + | + | + | + | (Locke et al.,2011) | |
| Cercopithecidae (Old World monkeys) | Macaca mulatta | + | + | + | + | (Zimin et al.,2014) | |
| Platyrrhini (New World monkeys) | Callithrix jacchus (marmoset) | + | + | + | + | (Worley et al.,2014) | |
In the Ensembl (release 79) Gene Tree view, there are only one to threeENaC paralogs for some mammalian species. Our examination of the genome in eachof these cases showed that in most cases the genome sequence does include themissing paralog(s); in other cases the genome sequence is incomplete.
6.6. Summary for Tetrapoda
For the genomes of tetrapods where sequence information is available,including amphibians and amniotes (lizards, crocodiles, birds, and mammals)there are four paralogs of ENaC with the exception of mouse that has lost thegene for the delta subunit (Table 7 andTable 8) (Giraldez et al., 2012).
7. Homologs in invertebrates
As noted in the introduction, invertebrate species have many genes encodingpolypeptides homologous to ASIC/ENaC such as mec and deg genes in C. elegans, andpickpocket genes in Drosophila (Table 9). Inglobal (end-to-end) sequence alignment, homologous C. elegans (CAEEL) proteins shareup to 16% sequence identity with ENaC subunits from 18 vertebrate species(Table 10). In contrast, among ENaCsubunits, percent identities are 40–95% depending on the taxonomicdistance (Table 11 andTable 12).
Table 9.
Sodium channel families within the DEG/ENaC superfamily.
| Channel/gene name* | Phylum | Genus / species | Reference | |
|---|---|---|---|---|
| Invertebrates | Annelida (annelid worms) | Helobdella (leech) | (Simakov etal., 2013) | |
| Pickpocket (ppk) | Arthropoda | Drosophila Anopheles Triboliumcastaneum | (Zelle etal., 2013) (Holt etal., 2002) (Kim etal., 2009) | |
| Hydra Na+ channel (HyNaC) | Cnidaria | Hydra | (Gründer and Assmann, 2015) | |
| Sp-Scnnla Sp-Scnnlg | Echinodermata | Strongylocentrotus (sea urchin) | Ensembl database | |
| FMRFamide-activated amiloride-sensitive sodiumchannel (FaNaC) | Mollusca | Aplysia (sea hare) Crassostrea(oyster) Helix aspersa (snail) Planorbella trivolvis | (Furukawa etal., 2006) (Zhang etal., 2012) (Lingueglia et al., 2006) | |
| Degenerin (deg) (mec) (unc) | Nematoda | C. elegans Toxocaracanis Trichuris suis | (Eastwood andGoodman et al., 2012) (Zhu et al., 2015) (Jex et al., 2014) | |
| Putative FMRFamide-gated Na+ channel | Platyhelminthes (flatworms) | Schistosoma mansoni Echinococcus | (Protasio etal., 2012) (Zheng etal., 2013) | |
| C3Y149_BRAFL C3ZNH4_BRAFL | Chordata | Branchiostoma floridae (Florida lancelet) | (Putnam etal., 2008) | |
| Vertebrates | acid-sensing ion channel (ASIC) | Chordata | Wide distribution | (Deval andLingueglia et al., 2015) |
| Epithelial Na Channel (ENaC) | Chordata | Wide distribution | This review |
Names for the retrieval of sequence records from the Uniprotdatabase.
Table 10.
Percent identity between globally aligned amino acid sequences ofselected metazoan ENaC homologs and α ENaC subunit sequences from 18vertebrate species (seeTable 7 andTable 8 for the full names of thespecies).
| Human | Chimp. | Rhesus | Eleph. | Bovine | Dog | Mouse | Rabbit | Orca | Tasman. | Platypus | Chick | Flycat. | Alligator | Turtle | Xenopus | Lungfish | Coelacanth | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAEEL-deg1 | 12 | 12 | 12 | 13 | 13 | 13 | 12 | 13 | 13 | 13 | 12 | 13 | 12 | 13 | 13 | 12 | 13 | 12 |
| CAEEL-del1 | 16 | 15 | 15 | 15 | 15 | 15 | 16 | 16 | 15 | 16 | 15 | 14 | 14 | 15 | 15 | 15 | 15 | 13 |
| CAEEL-mec4 | 14 | 14 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 14 | 12 | 14 | 14 | 14 | 14 | 13 | 13 | 12 |
| CAEEL-mec10 | 14 | 14 | 13 | 14 | 13 | 14 | 14 | 14 | 14 | 14 | 13 | 13 | 14 | 14 | 13 | 12 | 13 | 12 |
| CAEEL-unc8 | 15 | 15 | 15 | 15 | 14 | 15 | 15 | 14 | 15 | 15 | 14 | 14 | 14 | 14 | 14 | 14 | 13 | 13 |
| CAEEL- unc105 | 14 | 14 | 14 | 13 | 12 | 13 | 13 | 13 | 13 | 12 | 11 | 14 | 13 | 13 | 13 | 12 | 12 | 12 |
| CAEEL-asic1 | 11 | 11 | 10 | 11 | 11 | 10 | 10 | 11 | 11 | 11 | 12 | 11 | 11 | 11 | 11 | 12 | 11 | 11 |
| STRPU-Scnn1a | 18 | 17 | 17 | 17 | 18 | 17 | 17 | 18 | 18 | 16 | 15 | 16 | 17 | 17 | 17 | 17 | 15 | 15 |
| STRPU- Scnn1bL | 15 | 14 | 14 | 14 | 15 | 14 | 15 | 15 | 15 | 16 | 15 | 14 | 14 | 14 | 13 | 15 | 15 | 15 |
| STRPU-Scnn1g | 16 | 15 | 14 | 16 | 16 | 15 | 15 | 15 | 16 | 15 | 16 | 14 | 14 | 14 | 15 | 16 | 15 | 14 |
Sequences were selected from the Uniprot database. Speciesabbreviation is followed by the gene symbol. Species: Caenorhabditis elegans(CAEEL); S. purpuratus (STRPU, sea urchin).
Table 11.
Percent identity between globally aligned amino acid sequences ofα-ENaC orthologs from 18 species of Vertebrata (seeTable 7 andTable 8 for the full names of the species).
The first species (Human) listed on the header row is not listed inthe first column to avoid including self-comparisons (e.g. Human vs. Human)that obviously equal 100%.
Table 12.
Percent identity between globally aligned amino acid sequences ofβ- and γ-ENaC orthologs from 18 species.
| SCNN1B | Human | Chimp. | Rhesus | Eleph. | Bovine | Dog | Mouse | Rabbit | Orca | Tasman. | Platypus | Chick | Flycat. | Alligator | Turtle | Xenopus | Lungfish |
| Chimpanzee | 87 | ||||||||||||||||
| Rhesus | 97 | 85 | |||||||||||||||
| Elephant | 82 | 72 | 82 | ||||||||||||||
| Bovine | 85 | 75 | 84 | 81 | |||||||||||||
| Dog | 87 | 77 | 87 | 84 | 86 | ||||||||||||
| Mouse | 83 | 73 | 83 | 80 | 80 | 82 | |||||||||||
| Rabbit | 85 | 75 | 85 | 80 | 83 | 85 | 81 | ||||||||||
| Orca | 83 | 73 | 83 | 81 | 89 | 86 | 80 | 81 | |||||||||
| Tasmanian | 75 | 65 | 75 | 74 | 75 | 75 | 74 | 75 | 73 | ||||||||
| Platypus | 75 | 66 | 74 | 74 | 74 | 75 | 75 | 73 | 75 | 79 | |||||||
| Chick | 61 | 54 | 61 | 59 | 61 | 62 | 60 | 59 | 61 | 63 | 65 | ||||||
| Flycatcher | 64 | 56 | 64 | 62 | 64 | 64 | 62 | 63 | 63 | 66 | 67 | 82 | |||||
| Alligator | 63 | 56 | 63 | 62 | 63 | 64 | 61 | 62 | 63 | 66 | 67 | 78 | 78 | ||||
| Turtle | 61 | 53 | 61 | 58 | 60 | 62 | 59 | 61 | 60 | 61 | 63 | 71 | 70 | 74 | |||
| Xenopus | 57 | 51 | 58 | 57 | 57 | 58 | 56 | 57 | 56 | 59 | 60 | 59 | 58 | 59 | 56 | ||
| Lungfish | 52 | 46 | 52 | 52 | 51 | 53 | 54 | 52 | 52 | 51 | 53 | 52 | 54 | 53 | 49 | 54 | |
| Coelacanth | 48 | 43 | 48 | 47 | 49 | 48 | 49 | 48 | 49 | 49 | 51 | 49 | 49 | 50 | 45 | 47 | 49 |
| SCNN1G | Human | Chimp. | Rhesus | Eleph. | Bovine | Dog | Mouse | Rabbit | Orca | Tasman. | Platypus | Chick | Flycat. | Alligator | Turtle | Xenopus | Lungfish |
| Chimpanzee | 89 | ||||||||||||||||
| Rhesus | 92 | 83 | |||||||||||||||
| Elephant | 83 | 73 | 78 | ||||||||||||||
| Bovine | 85 | 76 | 80 | 81 | |||||||||||||
| Dog | 88 | 79 | 83 | 84 | 85 | ||||||||||||
| Mouse | 85 | 75 | 80 | 79 | 82 | 85 | |||||||||||
| Rabbit | 86 | 77 | 81 | 81 | 82 | 87 | 86 | ||||||||||
| Orca | 78 | 70 | 74 | 77 | 84 | 81 | 77 | 78 | |||||||||
| Tasmanian | 72 | 64 | 68 | 74 | 72 | 73 | 71 | 73 | 68 | ||||||||
| Platypus | 75 | 67 | 71 | 76 | 75 | 75 | 75 | 74 | 70 | 76 | |||||||
| Chick | 63 | 56 | 59 | 63 | 62 | 63 | 62 | 62 | 60 | 61 | 66 | ||||||
| Flycatcher | 59 | 52 | 56 | 60 | 59 | 60 | 59 | 59 | 57 | 58 | 62 | 86 | |||||
| Alligator | 64 | 56 | 60 | 64 | 63 | 63 | 63 | 64 | 59 | 63 | 66 | 81 | 76 | ||||
| Turtle | 62 | 55 | 60 | 62 | 62 | 62 | 62 | 62 | 59 | 62 | 65 | 79 | 74 | 79 | |||
| Xenopus | 55 | 48 | 52 | 55 | 55 | 55 | 55 | 55 | 52 | 54 | 55 | 57 | 54 | 58 | 57 | ||
| Lungfish | 50 | 44 | 48 | 51 | 51 | 50 | 51 | 49 | 48 | 50 | 51 | 51 | 50 | 52 | 52 | 52 | |
| Coelacanth | 55 | 49 | 52 | 53 | 55 | 53 | 54 | 54 | 51 | 54 | 55 | 55 | 53 | 55 | 54 | 54 | 57 |
Table 10 includes only comparisonswith the α ENaC subunit. Comparisons with the β and γsequences from the same 18 species show a highly similar range of identity (data notshown), i.e., there is no significant difference in the similarity of any CAEELhomolog to any of the three ENaC subunits. Similarly, C. elegans homologs share onlya low (<15%) sequence identity with the human ASIC isoforms. Thus,these homologs represent a family(s) separate from the ENaC as well as ASICfamilies. InTable 9 we note only a fewreferences for the Deg family of proteins in nematodes. The UniProt protein databaseincludes many polypeptides that belong to this family in various worms. As these areoutside the scope of this review we will not further relate to these sequences.
In addition to the nematode and arthropod species, BLAST search of UniProtprotein database shows significant sequence identity in the range of13–22% between predicted protein sequences from the genome ofStrongylocentrotus purpuratus (purple sea urchin) (unpublished yet; available atEnsembl database) (Table 9) and vertebrateENaC subunit sequences. Sea urchins belong to the phylum Echinodermata(echinoderms). In the records of this genome, some of these homologs have beenassigned names as such as "amiloride-sensitive sodium channel subunitalpha", "…beta" and"…gamma". Multi-sequence comparisons of these proteins withvertebrate ENaC sequences show up to 18% partial sequence identity andreveal large areas of sequence insertions. These echinoderm sequences likelyconstitute an additional family within the ENaC/Degenerin superfamily. Thefunctional characteristics of these proteins have not been determined, and webelieve that it is premature yet to call these proteins with names that imply adirect orthologous relationship with vertebrate ENaC subunits. Moreover, sea urchinhomologs show greater sequence identity with vertebrate ASIC paralogs than withENaC. Gene Tree display in Ensembl Metazoa Genome database links between these seaurchin proteins and Deg type proteins from invertebrate species listed inTable 9.
Among invertebrates the taxon that is closest to vertebrates isCephalochordata that includes lancelets. Cephalochordata and Vertebrata are two ofthe subphyla of Chordata (Table 9). Thegenome sequence of Florida lancelet (amphioxus) has been determined (Putnam et al., 2008). BLAST search of thepredicted lancelet proteins using ENaC sequences yields many homologous fragments.Most of these lancelet sequences share greater homology with human ASIC(7–24%) than with ENaC paralogs. As many of these sequences are inthe status of homology predicted proteins, it is too early to make definitivestatements regarding phylogenetic relationships. Nonetheless, the lancelet sequencesdo not appear to be direct orthologs of human ENaC paralogs.
In summary, among invertebrate species, there are many members of theENaC/Degenerin superfamily that clearly differ from ENaC. Thus, these homologs donot appear to be direct orthologs or ancestors of ENaC. As we discuss below, theancestors of ENaC apparently emerged prior to the branching of the first vertebratesbut there is not an apparent direct ancestor of ENaC among the invertebratesequences available at present. The total number of eukaryotic species is estimatedas ~8.7 million (Mora et al., 2011)and only a few percent are vertebrate species. Hence, determination of moreinvertebrate genomes may lead to the findings of new families within theENaC/Degenerin superfamily.
The multiplicity and divergence of invertebrate sequences that show homologyto "amiloride-sensitive sodium channels" require extended efforts toclassify these proteins into families based on their homology and phylogeneticdistance among other metazoan sequences.
8. Homology between ENaC orthologs
To determine the degree of conservation and sites of divergence of ENaCorthologs, we examined in more detail 20 species for which full sequence of thethree ENaC subunits are available.Table 11andTable 12 show results for 18 speciesrather than 20 we analyzed (omitted gorilla and rat) because of page and font sizelimitations. The species selected included representatives of primates (rhesus,chimpanzee, and human), elephant, ruminants (cow), carnivores (dog), rodents(mouse), leporids (rabbit), whales (killer whale Orca), marsupials (Tasmaniandevil), egg-laying mammals (platypus), birds (chicken and flycatcher), reptiles(alligator and turtle), amphibians (Xenopus), lobe finned fishes (coelacanth), andlungfish.
Table 11 presents the percentsequence identity for α subunit orthologs from 18 species with headers thatmark taxonomic classification. Each cell of the table gives percent identity betweentwo sequences from the species listed in the respective header and the first column.To determine the percent identity in the conserved extracellular domain of αsubunit orthologs, we also compared the sequences of the extracellular domain (Fig. 3). On the average across species, sequenceidentity is ~9% higher in this central segment, than the sequenceidentity along the entire length of the orthologous proteins (data not shown).Table 12 shows the percent identity betweenthe entire sequences of β- (upper table) and γ subunit orthologs(lower table) in 18 species.
Global alignment of α sequences from 20 species showed that N- andC-termini of orthologous α subunits are divergent across species (seeSection 11). Similar to the α subunit, δ subunit orthologs also showhigh divergence at their N- and C-termini. In contrast, the N- and C-termini of theβ and γ subunits are well conserved (see Section 11).
By the comparisons presented here we also wanted to examine if the rate ofevolutionary change of ENaC orthologs among different species is similar for thethree subunits. Previous studies have indicated that interacting proteins showsimilar patterns and dynamics of evolution (Lemoset al., 2005). Since the three subunits (α, β andγ) assemble to form a tight complex of a functional channel, we hypothesizedthat the rate of divergence as measured by the sequence identity would be similaracross species for all three subunits.
A cursory comparison of the figures inTables 11 and12 shows that foreach pair of species the percent identity for all three subunits are similar. Forexample, percent identity between human and turtle α, β andγ sequences is 56, 61 and 62% respectively. The correlation betweenthe sequence identities among α and β subunits and β andγ subunits was r=0.96 and r=0.97 respectively (Fig. 8). Thus, as measured by the percent identity, the divergence ofthe three subunits has proceeded at similar levels during the species evolution.
Fig. 8.
Correlation between the sequence identities among α, βand γ subunits of ENaC for 20 species relative to human ENaC. A)Correlation of the extent of identity of α and β subunits withtheir human counterparts. B) Correlation of the extent of identity of βand γ subunits with their human counterparts. The x, y coordinates ofeach point are percent identities between human sequence and the sequence ofanother species for the subunit indicated in the x and y axes. The sequenceswere from human, chimpanzee, gorilla, rhesus, elephant, bovine, dog, mouse, rat,rabbit, orca, Tasmanian devil, platypus, chicken, flycatcher, alligator, turtle,Xenopus, lungfish, and coelacanth.
For all three ENaC subunits (α, β, and γ), sequenceidentity between orthologs is consistent with the phylogenetic distance betweenspecies.
The following list represents some highlights of this phylogeny relatedhomology:
ENaC subunits of extant species within the same taxonomic familyshare generally >87–96% sequence identity.Example: Human and chimpanzee (family Hominidae) (Table 11 and12).
All placental mammal sequences, including marine mammal Orcinusorca (killer whale), share >70% identity (Tables 10 and11).
Birds and reptiles share a common ancestor (Green et al., 2014). Consistentwith this phylogenetic relationship, chicken, and flycatcher ENaCsequences share the highest identity (70–81%) withalligator and turtle (Table 11and12). In contrast, sequenceidentity between ENaC sequences from birds versus mammalian species islower, ranging between 50 to 66% (Tables 11 and12).
ENaC orthologs in the amphibian Xenopus, share47–59% identity with the sequences from amniotic animals(Table 11 and12).
The orthologs in coelacanth that are descendants of the earliestforms of vertebrates share about 39–55% identity withthe ENaC sequences from other Vertebrata species (Table 11 and12).
Lungfish (Table 8),considered a species closest to tetrapods, share 49–57%identity with coelacanth sequence and 39–54% identitywith other vertebrates (Table 11and12).
8.1. Insertions and deletions in orthologs
In phylogenetic comparisons above, we noted that in some ENaC/Degenerinhomologs, in addition to sequence divergence, there are majorinsertions/deletions (extending for tens to hundreds of residues) relative toENaC. Thus, we concluded that such proteins belong to different families withinthe ENaC/Degenerin superfamily. The major differences in the functions of thesefamilies of proteins are associated with specific structural features built uponthe major common scaffold of these channels. Whereas ENaC is constitutivelyactive and functions in transport of Na+ across epithelia andconsequently regulates extracellular fluid volume, ASIC and degenerin typechannels fulfill mainly sensory functions (Ben-Shahar, 2011) (see Section 14). The large insertions in thefinger domain (Fig. 6) of DEG family ofproteins are apparently part of the complex of mechano-sensitivity of thesechannels (Eastwood and Goodman,2012).
In the alignment of α subunit sequences from tetrapod species,it can be seen that the N- and C-termini show divergence (see Section 11).However, the extracellular regions do not have major insertions and deletions.Several sequences have deletion/insertion of 2–6 residues relative tothe human ortholog. Nearly all of these are located at sites of sequencedivergence when compared with ASIC1 (see Section 5.1).
Alignments of β-ENaC sequences also show no majorinsertions/deletions for 20 species. The anole lizard β-ENaC has a16-residue insert starting at residue 406. The status of this protein iscurrently "uncharacterized protein" implying it may haveerrors.
Alignment of γ subunit sequences from 20 species shows highhomology in the extracellular region, with the exception of the chimpanzeesequence that has a ~65 residue deletion. Such a deletion is not foundin other mammalian species, and could reflect an error.
Overall, ENaC family orthologs are highly conserved throughout thespectrum of vertebrate species. The degree of their sequence identity is relatedto their phylogenetic/taxonomic distance. ENaC orthologs do not have majorinsertions/deletions and can be readily distinguished from members of otherfamilies within the ENaC/Degenerin superfamily by their high percent of sequenceidentity.
9. Identifying ENaC family members within the ENaC/Degenerin superfamily
Members of the ENaC/Degenerin superfamily are readily identified by theircommon structural features: a large extracellular region connecting twotransmembrane domains, and relatively short intracellular N- and C-termini (Fig. 3). Beyond these common structural features,the proteins share sequence homology of varying degrees, depending on theirsubfamily and the phylogenetic distance between species. Among vertebrates, thereare two subfamilies: ASIC and ENaC. Analyses presented above show that ENaC paralogsin vertebrate species can be readily distinguished from ASIC paralogs.
In phylogenetic comparisons, we noted that some homologs are marked as ENaCorthologs in genome analysis. However, our analyses indicate that these are ASICrather than ENaC orthologs. As more genome sequences are determined,misclassification of orthologs may occur. To avoid this problem, we formulatedthresholds of sequence identity that can clearly distinguish ENaC orthologs fromother members of ENaC/Degenerin superfamily.
9.1. Threshold for orthologs
The sequences of ENaC orthologs across species show a high degree ofconservation with the lowest sequence identity of 39% between tetrapodspecies and lobe-finned fish coelacanth in global alignment (Tables 10 and11). The termini of α subunit orthologs are moredivergent, while the sequences of the extracellular region have about10% higher sequence identity. Thus, in a case where the classificationof a sequence is unclear, extracellular regions should be compared. Secondly,insertion/deletion of a large segment (>10 residues) should raiseconcerns regarding subfamily classification (see Section 8.1).
Protein structure database SCOP employed a minimal criteria of30% sequence identity for assignment of proteins into the same proteinfamily (Murzin et al., 1995). CATHdatabase uses >35% sequence similarity as the criteria forclassification as members of a family (Sillitoeet al., 2015). The observation that among ENaC orthologs sequenceidentity is >38%, matches the requirements of these twodatabases for the classification of these proteins as members of the same familyof ENaC. As sequence identity with ASIC homologs (seeTable 4) and other Degenerin type proteins are generallyless than 20%, these proteins represent members of families differentfrom ENaC.
9.2. Threshold for paralogs
Multisequence comparisons presented here show a consistent picture.Global alignments within species show that ENaC paralogs generally share>20% sequence identity with one another (Table 4 andTable 6).In contrast, all four ENaC subunits share less than 20% sequenceidentity with ASIC. This also extends to other homologs, such as Degenerins.Thus within species, 20% sequence identity appears as the cut-off pointfor the ENaC family as opposed to membership in the ASIC family amongvertebrates.
10. Pedigree of ENaC family members
By definition, paralogous proteins emerge as a result of a duplication of agene in a genome and then diverge as a result of accumulation of mutations induplicate copies at evolutionary time scale. There are several strong lines ofevidence that the four ENaC subunits share a common ancestor:
All four ENaC subunits share the highest homology amongthemselves as compared to other families.
The genes for all four ENaC subunits have introns in the samelocations (Fig. 4) (Saxena et al., 1998) while manyintrons of other homologs are at different positions.
Within the ENaC family, two pairs appear to have distinct ancestors: 1) theα and δ subunits, and 2) the β and γ subunits.Apparently, an ancestral ENaC sequence underwent a gene duplication that resulted inthe formation of two ancestral genes that again underwent independent duplicationevents. The result is four paralogous genes coding for the four ENaC subunits. Theevidence for two duplication events includes the following:
Within each pair of subunits (α and δ; βand γ), there is higher sequence identity than with the otherpair of subunits (Table 4 andTable 6).
The genes encoding the β and γ subunits are inadjacent locations on the same chromosome (Brooker et al., 1995), providing evidence that theyresulted from a local gene duplication event.
The information provided above on the human genome and other speciesrepresents a picture that is true for vertebrates in general. The Ensembl genomedatabase (release 79) of vertebrate and eukaryotic species currently includes 540homologs of ENaC. A phylogenetic "Gene Tree" constructed for these540 ENaC homologs using EnsemblCompara GeneTrees paralogy prediction method (Vilella et al., 2009) presents a picture thatis consistent with the information provided above.
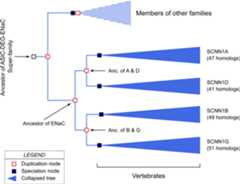
InFig. 9 we present a hypothetical"pedigree" for the ENaC paralogous genes based on the Ensembl GeneTree. A phylogenetic tree is analogous to a pedigree. But, phylogeny differs frompedigree in that while in a pedigree ancestor is known, in phylogeny the ancestor isdeduced based on homology relationships. The Ensembl Gene Tree predicts a commonancestral gene for all the ENaC homologs that was duplicated. These duplicate geneswere once again duplicated to generate the ancestral genes from which the four ENaCgenes derive (Fig. 9).
Fig. 9.
A hypothetical phylogenetic tree for paralogs of ENaC."Anc." is used as an abbreviation for"Ancestor". A "duplication node" represents agene duplication event that yields two genes within one genome. A"speciation node" represents the formation of a new species thatcarries the gene of interest. By the convention of Ensembl Gene Tree, collapsedtrees for paralogs are shown in blue color. The figure is based on a Gene Treeconstructed for 540 ENaC homologs in the Ensembl genome database (release 79) ofvertebrate and eukaryotic species using EnsemblCompara GeneTrees paralogyprediction method. The figure includes several modifications from the Gene Tree:The nodes for C. elegans degenerins and one homolog from a fish were omittedfrom the figure, and the positions of the nodes were modified to show branchesin parallel. The number of homologs in each collapsed branch is written on theright side of the collapsed tree marking.
As noted in Section 6, the genes coding for the α, β andγ subunits are present in all vertebrates, except ray-finned fishes,starting with the most ancient jawless vertebrate species such as lamprey (Table 7). SCNN1D gene coding for the δsubunit appears only in Euteleostomi (bony vertebrates) (Table 7).
The widespread phylogenetic spread of the four ENaC subunits providesevidence that the gene duplications that resulted in the formation of these subunitsrepresent an ancient event that preceded the evolution of vertebrates. There isstrong evidence for two rounds of whole-genome duplication (2R-WGD) prior to thediversification of the vertebrates (Cañestroet al., 2013;Putnam et al.,2008). 2R-WGD could result in the generation of four copies of duplicatedgenes. Yet, it is assumed that duplicate copies of many genes were lost afterinitial duplication. Currently, we do not know whether all paralogs of ENaC aredescendants of this 2R-WGD event. Duplicated genes may also originate as a result oflocal gene duplication events, independent of whole genome duplication (Cañestro et al., 2013). The SCNN1B andSCNN1G genes coding for the β and γ subunits most likely representproducts of a local duplication event as they are immediate chromosomal neighbors. Arecent review provides a general overview of the evolution of ENaC and otherfunctionally related proteins such as Na+-K+-ATPase andrenin-angiotensin-aldosterone system proteins and enzymes (Rossier et al., 2015).
11. Conserved sequence motifs and their functions
Alignments of ENaC orthologs from different species reveal many segments aswell as single isolated residues that are conserved in all species. The conservationof these residues and sequence segments suggests that these residues fulfillimportant functional roles. In this section, we shall summarize conserved sequencemotifs and their functions as well as other important functional sites.
11.1. Cytoplasmic amino terminus
As can be seen in the alignments of the human ENaC sequences, theN-termini of α and δ subunits show heterogeneity in both theirsequence and length (Fig. 4). A similarpattern of heterogeneity is observed in the alignment of the N-terminalsequences from 20 species (Fig. 10). Incontrast to the α and δ subunits, the β and γsubunits from 20 species are highly conserved and most are of similar length(Fig. 10).
Fig. 10.
Comparison of α, β, and γ sequences in theN-terminal, pre-TM1, and TM1 segments from twenty species. For each subunit,residues that are identical in at least 19 out of 20 species (95%identity) are shaded. The location of the predicted TM1 is shown above thesequences. The α subunits have N-termini of highly variable lengths (thenumbers at the beginning of each sequence marks the number of additionalresidues that did not fit into the page), with little or no sequenceconservation in this variable region. In contrast, the β and γsubunit N-termini are mostly of similar length and show a high degree ofconservation within a ~40 residues-long segment prior to the TM1. Therow of red letters, in between the β and γ sequence groups, markthe residues that are identical in both β and γ subunits. Infour sequences (β: chicken; γ: gorilla, chicken and coelacanth)2–5 residues prior to the first methionine were deleted to be consistentwith other Uniprot sequences that start with Met as the first translated codon.There may be also sequencing errors in the unusually short platypus αsequence, and flycatcher γ sequence.
Chalfant et al. examined activities of rat ENaC subunits withN-terminal deletions. They found that deletion of residues 2–67 in theN-terminus of the α subunit reduced endocytosis of ENaC and increasedthe half-life of the channel in the membrane, suggesting that the N-terminuscontains an endocytotic motif (Chalfant et al.,1999). Deletion of longer segments of α, β, andγ N-terminus (94, 50, and 94 residues respectively), drastically reduceENaC activity (Bachhuber et al.,2005).
Yue et al. noted that the N-terminal segments of β andγ subunits contain a stretch of basic residues that is characteristic ofPhosphatidylinositol 4,5-bisphosphate (PIP2) binding sites in other proteins(Yue et al., 2002). Mutation of 4 ofthese basic residues to nonpolar residues in the β but not in theγ subunit drastically reduced ENaC currents in the Xenopus oocyteexpression system (Kunzelmann et al.,2005).
In a stretch of about 30 to 40 residues prior to the start of TM1, allfour human ENaC paralogs have some strictly conserved residues (Fig. 4 andFig. 10). These residues are conserved in the four human ENaCparalogs (Fig. 4) as well as in all 20species examined (Fig. 10). Theconservation of these sequences across species suggests that this region has animportant functional role. A Gly37Ser missense mutation in this region of theβ subunit causes multi-system PHA and this mutation reduces the openprobability (Po) of ENaC (Gründer et al., 1997). Mutations of the correspondingresidue in the α and γ subunits also reduced channel activity,suggesting that this site has an important role in regulating channel gating(Gründer et al., 1997).Gründer et al. also examined the roles of the residues flanking the keyGly residue by systematic alanine mutagenesis of 28 residues (H77 to H104 in ratα subunit) (Gründer et al.,1999). The expression of ENaC with these mutant subunits in Xenopusoocytes showed that most mutants decreased channel activity, likely due to areduction in channel Po (Gründer et al., 1999). The stretch of ten residues from T92to C101 showed the highest sensitivity to alanine mutagenesis, with G95, H94 andR98 mutants showing the strongest reduction (Gründer et al., 1999). These residues are conserved in all20 species with the exception of the platypus, which has an exceptionally shortN-terminus (Uniprot ID: F7F7U2_ORNAN) (Fig.10). Since the status of this sequence is marked as an"uncharacterized protein" it may have an error.
11.2. TM1
The resolved structure of ASIC1, and sequence similarities betweenASICs and ENaCs provide important clues about the stretch of ENaC residues thatform TM1. We have also used algorithms to predict the location of the TM1 (Fig. 4 andTable 3). Relative to the alignment with ASIC1 sequence, predictedTM1 segment starts three residues (KKK in human β subunit) after thestart of the ASIC1 TM1 (cf.Fig. 4,Fig. 7, andFig. 10). According to this prediction, TM1 is preceded by2–3 Arg/Lys residues that are conserved in all ENaC orthologs (Fig. 3, andFig. 10). Studies on the distributions of charged residues inα-helical TM segments indicated that positively charged residues Arg andLys are present at much higher proportions on the cytoplasmic side of the TMsegment of proteins. This trend was named the "positive-inside"rule (von Heijne, 1992). A recent studyhas shown that in 191 transmembrane α-helical segments, the residues Argand Lys are present at highest proportion just before the start of the lipidbilayer (Pogozheva et al., 2014). Atthis location, the positively charged residues interact with the polar headgroups of membrane lipids and contribute to the strength of membrane anchoringof ENaC. The conserved appearance of Arg and Lys just before the predicted TM1provide support for the predicted location of the TM1 (Table 3,Fig. 3, andFig. 10).
In the predicted TM1 location, a tryptophan (W) appears as one of thefirst three residues conserved in all 20 ENaC orthologs (Fig. 10). In ASIC1, a Trp appears as the third residue fromthe beginning of the first helix (Fig. 7).Analysis of 191 α-helical TM proteins showed that aromatic residues Trpand Tyr are predominantly located at the membrane-water interface (Pogozheva et al., 2014). These aromaticamino acids are known to partition into the interface region of membranes.Hence, it has been suggested that they contribute strongly to the anchoring andprecise positioning of TM segments in the lipid bilayer (Hong et al., 2007). The appearance of Trp at the beginningof the TM1 in all ENaC orthologs in 20 species provides further support for thepredicted location of the TM1 (Fig. 3,Fig. 4 andFig. 10). Analyses of TM1 of the α subunit of ENaCby tryptophan-scanning mutagenesis suggested two functionally different regions.N-terminal tryptophan residues altered both channel activity and cationselectivity, with a periodicity consistent with a helical structure. WhileC-terminal tryptophan residues also affected activity and selectivity, there wasno apparent periodicity (Kashlan et al.,2006).
11.3. Extracellular region
The extracellular region, as revealed in the resolved structures ofASIC1, has a complex structure that resembles an outstretched hand holding aball (Figs. 3 and4). Hence, domains within the extracellular regions arereferred to as palm, thumb, knuckle, finger and β ball (Jasti et al., 2007) (Fig. 7). The palm and β ball domains are formed bynon-contiguous β strands and loops, and are in close proximity to themembrane (Figs. 3 and4). More peripheral domains (thumb, knuckle and finger) areformed by contiguous α helices and loops, and are poorly conserved amongENaC/Degenerin family members, when compared with other parts of theextracellular region. Based on sequence homology and predicted secondarystructure, it is likely that the structural features of the extracellular regionof ASIC1 is shared among all members of the ENaC/Degenerin superfamily. This isone of the key defining features of this ion channel family. Below we presentsome of these conserved segments the functions of which have been examined.
Protease cleavage sites
Proteases activate ENaC by cleaving the α and γsubunits at multiple sites within their extracellular finger domains,releasing imbedded inhibitory tracts (Kleyman et al., 2009;Rossierand Stutts, 2009;Vuagniaux etal., 2002). Serine proteases represent one of the largest genefamilies with 175 predicted genes in the human genome (Szabo and Bugge, 2011). Despite variations in thecleavage sequence specificity of these enzymes, there is a commondenominator of one or more Arg or Lys residue immediately preceding thecleavage site (Antalis et al.,2010).
Furin, a member of the proprotein convertase family of serineproteases, cleaves the α subunit twice at sites (RSTR (proximal) andRSAR (distal)) flanking an inhibitory tract (LPHPLQRL) (mouse sequences)(Carattino et al., 2008b,2006;Hughey et al., 2004;Sheng etal., 2006). Furin cleaves the γ subunit once (RKRR), andcleavage by a second protease at a distal site releases another inhibitorytract (RFLNLIPLLVF) (Bruns et al.,2007;Passero et al.,2010). A polybasic RKRK sequence is one of the distal sitestargeted by some of the non-furin proteases that cleave the γsubunit and activate ENaC (Bruns et al.,2007;Passero et al.,2011;Patel et al.,2012). The sequences of the inhibitory tracts in both α andγ subunits are conserved in mammals (marked with blue shading inFig. 11). While there is adivergence of the sequence of the homologous segment in Sauropsida,Amphibia, lungfish and coelacanth, the fact that amphibian ENaC is activatedby proteases (Alli et al., 2012)suggests that this intrinsic inhibitory tract has evolved over time. Atpresent, we are not aware of evidence that other members of theENaC/Degenerin family are activated by proteases.
Fig. 11.
Serine protease cleavage sites in the extracellular domain ofα, β and γ ENaC subunits from 20 species. Key basicamino acids (Arg (R) and Lys (K)) in the putative cleavage site are marked withyellow shading. The sequences of the respective subunits from 20 species werealigned by CLUSTALW. The conserved sequences of the inhibitory tracts located inbetween the two SP sites are marked light blue background. The residues of thesubstrate protein that are recognized by proteases are numbered based on theirposition relative to the cleaved peptide bond. P1 marks the putativeresidue after which the peptide bond is cleaved by the SP (Antalis et al., 2010).
The α and γ subunit sequences from 20 speciesindicate that the key protease cleavage sites are strongly conserved in allspecies with the exception of lungfish and coelacanth (Fig. 11). InFig.11, β and γ subunit sequences have been alignedtogether as these two proteins are products of paralogous duplicated genes.It is noteworthy that in the β sequences the protease cleavagemotifs are missing (Fig. 11). Inmammals and marsupials, there is a gap instead of the protease cleavagemotifs (Fig. 11). Considering thatboth genes are apparently the descendants of the same gene, either theprotease cleavage motifs were deleted from the SCNN1B gene or were lateradded to the SCNN1G gene.
Disulfide bonds
Within the extracellular regions of ENaC family members, there are16 highly conserved cysteine residues that likely form eight disulfide bonds(Firsov et al., 1999;Jasti et al., 2007;Sheng et al., 2007). Based on thedisulfide bonds in the resolved ASIC1 structure, these include fivedisulfide bonds in the thumb domain (Sherwood et al., 2012). The 16 extracellular cysteine residuesare conserved in all 20 species examined with the exception of the γsubunit of chimpanzee and the β subunit of coelacanth. As notedabove, there are two large gaps in the chimpanzee γ subunitsequence. These gaps probably reflect an error in sequence. The structuralimportance of the conserved cysteines has been demonstrated by site-directedmutagenesis experiments (Firsov et al.,1999;Sheng et al.,2007). There are additional Cys residues in the finger domain offamily members in C. elegans, which may form additional disulfide bonds.
Sites of N-linked glycosylation
During the process of translation of proteins that are membranebound or secreted, oligasacccharides may be attached to the N4 of theasparagine residue at the start of a consensus sequence composed of threeamino acids: Asn-Xaa-Ser/Thr. The extracellular region of rat α,β, and γ subunits were shown to have such sites that areglycosylated (Canessa et al., 1994a;Snyder et al., 1994). Alignmentof the sequences of subunits from 20 species show that most of the sitesidentified in the rat sequences are conserved in mammals, but not in birdsand lower species. Since the studies on glycosylated residues were carriedout using rat subunits, we note here the conserved sites according to therat subunit residue numbering. Thus, the homologs of the following ratresidues are conserved as the first residue in N-glycosylation consensussequence in most mammals: α subunit: N259, N320, N339, N424, N538;β subunit: N135, N141, N146, N197, N205, N258, N362, N376, N482;γ subunit: N210, N249, N272, N292, N498.
Knuckle domain
In the ASIC1 model, the knuckle domain is composed of two helices(α6 and α7) that are located at the top of each subunit(Figs. 3–5). The sequence of this region isconserved within each subunit, but shows divergence between ENaCs and ASICs(Fig. 7). In all three subunitsthere is a positively charged residue (Arg or Lys) at a position thatcorresponds to the end of the α7 helix in ASIC1 (Fig. 7). Mutation of this residue (K498in the human γ subunit) to alanine was shown significantly to reducesurface expression of ENaC (Edelheit et al.,2014). Recently Chen et al. showed that deletion of the entireknuckle domain (including the conserved Arg/Lys) in mouse β orγ subunit drastically reduced ENaC surface expression andconsequently ENaC function (Chen et al.,2015). These consistent findings suggest that the conservedcharged residues in this domain may be involved in binding to other proteinsthat are involved in the transport of ENaC subunits.
In contrast to effects in β or γ subunits, deletionof the knuckle domain in the α subunit resulted in channelactivation as a result of a loss of Na+ self-inhibition (Chen et al., 2015).
Palm domain
A stretch of 15 residues prior to TM2 form the twoβ-strands (β11 and β-12) that are a centralcomponent of the palm domain (Fig. 6andFig. 7). This region is highlyconserved in all three subunits and includes three charged residues that areconserved in all three subunits in all 20 species examined (Fig. 12). These residues are homologousto A413, E417 and Q421 in cASIC1 (Fig.12). In cASIC1, the R-group of E417 protrudes into the centralvestibule (Fig. 13) and has beenimplicated in proton binding and functional conformational changes in ASIC(Ishikita, 2011). In all threeENaC subunits, the homologous residues are positively charged Lys or Arg(K534, R505 and R514 in human α, β and γ subunitsrespectively) in all 20 species (Fig.12). An R514A mutant in human γ subunit significantlyleads to a decrease in sodium feedback inhibition consistent with anincrease in channel open probability (Edelheit et al., 2014). The other two residues (A413 and Q421)are located at the interface between subunits (Fig. 13) (Jasti etal., 2007). Because of the strict conservation of homologousresidues in ENaC subunits, these residues are probably located atsymmetrical sites in ENaC subunits as they are in ASIC1 (Fig. 13). Site-directed mutagenesisstudies using human ENaC subunits showed that mutation of these residues toalanine also leads to a decrease in sodium feedback inhibition that controlschannel open probability (Po) (Edelheit et al., 2014,2011). A human γ subunit variant in the palm domain(L511Q) is associated with an increase in channel open probability (Chen et al., 2013).
Fig. 12.
Comparison of α, β, and γ sequences in thepre-TM2 and TM2 segment from twenty species. For each subunit, residues that areidentical in at least 19 out 20 species (95% identity) are shaded. Thelocation of the TM2 based on homology to ASIC1 is shown above the sequences. Inthe preTM2 region, only three charged residues are conserved in all threesubunits. The positions of these charged residues are marked at the top of thealignments by the corresponding cASIC1 homologs, Ala413, Glu417 and Gln421.Column headers: Deg: degenerin or "Deg" residue. Ami: amiloridebinding residues. Sel.: selectivity filter.
Fig. 13.
Location of the cASIC1 E417 and Q421 in ASIC1 structure (PDB ID: 2QTS).The three ribbon structures shown represent the β12-strand region (fromL414 to K423) of all three subunits of chicken ASIC1, termed in order A, B, andC (PDB 2QTS). For each subunit, only two residues, E417, and Q421, are shown inCPK style. In 20 species examined, the residue homologous to E417 is an arginineor lysine (K534 in α, R505 in β and R514 in γ subunit ofhuman ENaC). The space in the center of the figure is part of the vestibulealong the three-fold axis of symmetry that is thought to be part of the ionpathway.
11.4. TM2
Prior to the report on ASIC1 structure, the location of the TM2 in ENaCsubunits was predicted by various software based on hydrophobicity of thisregion (Canessa et al., 1994a;Saxena et al., 1998). After thepublication of the ASIC1 structure in 2007, the helical TM2 segment of ASIC1 hasbeen generally adopted as the location of TM2 in ENaC subunits as well, based onthe strong sequence homology in this region and subsequent empirical studies(Kashlan and Kleyman, 2011). Whilethere is overlap between earlier predictions and ASIC1 structure based segment,a segment identified as "pre-M2" (i.e. before the TM2) inearlier work (Kellenberger et al., 1999;Schild et al., 1997), resides withinTM2. The terms used in earlier studies should be examined to avoid confusionabout regions studied.
Fig. 12 presents alignments ofα, β and γ subunits in the TM2 segments in 20 specieswhere the TM2 was marked based on homology with ASIC1. These alignments showthat TM2 is highly conserved in all subunits.
A number of key functional sites are within the TM2 region of ENaCsubunits, including the channel gate, amiloride binding site and selectivityfilter.Fig. 10 illustrates how thesesites are conserved through evolution. Functional studies as well as resolvedASIC1 structures suggest that the channel gate is within the outer part of thesecond membrane spanning domain, within the region encompassing an LLSN motifthat is conserved among ENaC subunits across species (Fig. 12). The Ser in this motif is a site where theintroduction of large residues has been found to dramatically increase channelopen probability (Kellenberger et al.,2002;Sheng et al., 2001a;Snyder et al., 1999). This site hasbeen referred to as the degenerin or "Deg" site, as theintroduction of large residues in mechanosensitive ENaC/Degenerin family memberin C. elegans results in neurodegeneration, in association with an increase inchannel open probability (Goodman et al.,2002;Sherwood et al.,2012).
One of the characteristics of αβγ ENaC is itsinhibition by relatively low concentrations of amiloride (Kleyman and Cragoe, 1988). Other family members, includingδβγ and ASICs, are inhibited by amiloride or itsderivatives at higher concentrations of these drugs (Table 13) (Diochot et al.,2007;Ji et al., 2012). Anamiloride binding site has been described at a site in the secondmembrane-spanning domain, consisting of a Ser in the α subunit, and aGly in the beta and gamma subunits (Fig.12). The introduction of specific mutations at these sites led to aprofound loss of the efficacy of amiloride (Kashlan et al., 2005;Schild etal., 1997).
Table 13.
Functional characteristics of ASIC and ENaC type channels.
| ENaC (αβγ) | ASIC | |
|---|---|---|
| Channel structure | Hetero-trimer | Homo- / hetero-trimer |
| Channel gating | Constitutively active | H+ activated |
| pH EC50 | Species dependent | 4.8–6.7* |
| Na+/K+ permeability ratio* | >100 | 5–14 |
| Permeable to larger cations | No | Yes |
| Protease activation | Yes | No |
| Amiloride IC50 | 0.1 µM | 10–100 µM |
| Amiloride Ki | 0.35 µM | |
| Extracellular Na+ inhibition | Yes | No |
| Shear stress activation | Yes | No |
| Main functions | Na+ reabsorption across highresistance epithelia. Maintenance of body salt and waterhomeostasis. Kidney: Regulation of ECF volume,blood pressure and electrolyte homeostasis. Respiratoryairway: Regulation of airway surface liquid (ASL) volume,composition and mucociliary clearance. Reproductivetract: Regulation of epithelial fluid volume necessary forcilial transport of gametes, fertilization andimplantation. Skin and exocrine glands:Na+ reabsorption. Taste buds: Salttaste perception. | Nociception Mechanosensation Synapticplasticity Fear-related behavior Seizure termination |
Source: (Gründer andPusch, 2015)
Another defining characteristic of ENaC is its cation selectivity. Withregard to its ability to discriminate Na+ and K+, ENaC isthe most Na selective mammalian ion channel (Table 13). A three-residue selectivity filter, consisting of aG/S-X-S motif, is present in the TM2 of ENaC subunits (Fig. 12). The introduction of specific mutations in thefirst or third residue of this motif resulted in channels that allow for modestK+ permeation (Kellenberger etal., 1999;Sheng et al.,2000;Snyder et al., 1999).
At the distal end of TM2 there are three charged residues (within thestretch of EMAELVFD in human α subunit) that are conserved in all 20species examined (Fig. 12). Mutation ofthese acidic residues reduced channel conductance (Langloh et al., 2000;Sheridan et al., 2005) and also affect ion selectivity (Sheng et al., 2001b).
11.5. Carboxy terminus
In general, the cytoplasmic C-terminus contains sites of interactionwith other proteins, signal transduction molecules, and ions that regulate ENaCfunction.
Motifs involved in signal transduction
In accordance with the "positive inside" rule notedabove (in Subsection 11.2), the region after TM2 is enriched in positivelycharged Arg and Lys in all three subunits in all 20 species examined (Fig. 12). The proximity of theseresidues to the cytoplasmic side of the membrane allows interactions ofthese residues with polar heads of membrane phospholipids andphosphoinositides concentrated at the cytosolic surface of membranes (Di Paolo and De Camilli, 2006). Thisregion in the β and γ subunits has a role in the binding ofphosphotidylinositol triphosphate which has an allosteric effect on ENaCopen probability (Pochynyuk et al.,2007,2005). It isnoteworthy that positively charged residues at the same analogous region inP2X receptor channels fulfill similar roles (Bernier et al., 2012).
One of the common mechanisms of membrane protein regulation isphosphorylation/dephosphorylation of critical residues by intracellularsignal transduction systems. It is likely that the residues in theC-terminus of ENaC subunits may be substrates for such reactions. Forexample, Volk et al. showed that the activity of ENaC expressed in oocytescould be enhanced by activation of protein kinase C (PKC) by phorbol esterand that this effect was dependent on the presence of an intact C-terminusof α subunit (Volk et al.,2000). The C-terminus also is phosphorylated by the kinases SGK,casein kinase 2, and ERK (Diakov andKorbmacher, 2004;Shi et al.,2002;Yang et al.,2006).
An important regulator of ENaC activity is chloride ions.Truncation of the C-terminus of the β but not of α andγ subunits reduced cytoplasmic Cl− inhibition ofENaC, suggesting that this segment is essential for down-regulation of ENaCby CFTR (Bachhuber et al., 2005;Ji et al., 2000).
Interaction with cytoskeletal elements
Cytoskeletal elements such as microtubules, actin filaments, andassociated proteins form an essential part of the complex network ofproteins involved in intracellular transport of proteins includingendocytosis (Anitei and Hoflack,2012). As ENaC subunits are transported to the cell membrane via thetrans-Golgi network (Butterworth,2010), cytoplasmic termini of ENaC may interact with cytoskeletalelements during transport and in the cell membrane itself. Indeed, theC-terminus of the α subunit was shown to bind to spectrin that islocated in the intracellular side of the membrane. The sequence responsiblefor binding to the SH3 domain of α-spectrin (PPLALTAPPPA in ratα subunit) (Rotin et al.,1994) starts prior to the PY motif (see below) and partiallyoverlaps with it (Fig. 12). This motifis conserved only in mammals (Fig.12). In addition to spectrin, there is also evidence for directinteraction of F-actin with the carboxy terminus of the α subunit(Mazzochi et al., 2006;Sasaki et al., 2014).
PY motif
The most conserved motif in the C-terminus of ENaC subunits is thePY motif (Fig. 14). The consensussequence for the PY motif is PPPXYXXL that is located 65–70 residuesafter the end of the TM2 in α, β and γ subunits.Delta ENaC orthologs do not have a conserved PY motif. In the 20 species weexamined the PY motif is conserved strictly in nearly all species (Fig. 14). Turtle β subunitsequence has a short C-terminus that may be a genome sequencing error. Incontrast to the β and γ subunits, in Sauropsida, Amphibiaand fishes the PY motif has been lost in the α subunit (Fig. 14). The consistent lack of PYmotif in these species makes it very unlikely that the lack of the motif isa sequencing error.
Fig. 14.
Conservation of the PY motif in the C-termini of α, βand γ ENaC subunits from 20 species.
The PY motif is recognized by the WW domains in Nedd4-2 that is anE3 ubiquitin-protein ligase (Rotin andStaub, 2011). WW domains are ~40 residue long segmentsthat are characterized by a conserved sequence that includes two tryptophans(W). Such modules are found in many proteins and bind to proline-richsequences such as PY motif (Macias et al.,2002). With its WW domains, Nedd4-2 catalyzes ligation ofubiquitin to the ENaC subunit leading to the internalization of ENaC andeventual degradation in a proteasome or lysosome (Rotin and Staub, 2011).
Missense mutations in the β or γ subunit PY motifor truncations that lead to a loss of this motif in these subunits, causeLiddle syndrome (see section 15.2). Mutation or deletion of the PY motifreduces the rate of ENaC ubiquitylation and consequent internalization,leading to accumulation of ENaC in the membrane (Lu et al., 2007). This in turn leads to enhancedabsorption of filtered Na+ and consequently increases bloodvolume and blood-pressure (Rotin,2008).
12. Tissue distribution of ENaC
Alpha, beta and gamma subunits of ENaC
Most of the studies on tissue localization of ENaC subunits have beencarried out by immunohistochemical studies using antibodies generated againstsmall segments of expressed proteins or synthetic peptides that represent shortsegments of ENaC subunits (Brouard et al.,1999;Coric et al., 2004,2003;Duc et al., 1994;Hager et al.,2001;Masilamani et al.,1999;Tousson et al., 1989;S. Wang et al., 2013). These studiesprovided evidence for the localization of ENaC in kidney, lung, salivary glands,skin, placenta and the colon. Expression of ENaC subunits has been also examinedby in situ hybridization of tissue sections using cDNA probes (Greig et al., 2003). But, this approachdoes not provide an image of the intracellular localization of the subunitsthemselves.
To enhance the immunofluorescence signal, we generated polyclonalantibodies against the complete extracellular region of ENaC subunits (Enuka et al., 2012). These antibodiesallowed us to visualize ENaC expression in the bronchial epithelia of human lungand the female reproductive tract extending from the uterus to the fallopiantube at a high resolution by immunofluorescence and 3D confocal microscopy(Enuka et al., 2012).
The expression and sites of localization of ENaC in the kidney nephrontubules have been recently extensively reviewed (Rossier, 2014) and the complexities of this subject arebeyond the scope of the present review.
Tissue specificity of expression of ENaC subunits has been alsoinvestigated by large-scale microarray and high-throughput RNA sequencingexperiments. The results of these studies can be accessed via the EMBL-EBIExpression Atlas database of gene expression (Petryszak et al., 2014). Experiments included in the ExpressionAtlas (https://www.ebi.ac.uk/gxa/home) report many tissues wherein ENaCsubunits are expressed at varying levels. Consistent with theimmunohistochemical studies, these studies report highest levels of expressionfor the α, β and γ subunits in the kidney, lung, andcolon. Other tissues reported include the fallopian tube, esophagus, placenta,prostate, skin, stomach, thyroid, tongue and vagina (Expression Atlas).
Some recent studies have reported immunolocalization of ENaC subunitsin astrocytes in the brain (Miller and Loewy,2013), human eye (Krueger et al.,2012), nasal mucosa (Jiang et al.,2015), mouse ear (Morris et al.,2012), rat muscle (Simon et al.,2010), vascular endothelium (Kusche-Vihrog et al., 2014), vascular smooth muscle cells (Drummond et al., 2008), lymphocytes (Ottaviani et al., 2002) and platelets(Cerecedo et al., 2014).
There is also evidence for the expression of ENaC in mammary epithelia(Wang and Schultz, 2014). Thefunctional significance of ENaC in organs, such as kidney, lung and therespiratory tract, sweat and salivary glands and the reproductive tract whereENaC is expressed at relatively high levels, has been established, based on thefacts that mutations in ENaC genes result in major dysfunction in these tissuesin multi-system PHA (Chang et al., 1996;Enuka et al., 2012;Hanukoglu, 1991;Hanukoglu et al., 2008). However, even in most severecases of multi-system PHA there does not appear to be an abnormal function inthe eye, ear, muscle or neural tissue function. Thus, the physiologicalsignificance of the low-level expression of ENaC in other tissues remains to beestablished.
Expression of ENaC has also been detected in the lingual epithelium andin taste receptor cells (TRCs) (Chandrashekar etal., 2010;Kretz et al.,1999). Most mammals can sense five basic tastes, sweet, sour, bitter,umami and salt by TRCs specific for each taste. In fungiform taste buds of micethere are amiloride-sensitive TRCs that are responsive specifically to NaCl(Shigemura et al., 2008). Ingenetically engineered mice lacking the α subunit specifically in TRCs,the neural response to low NaCl (<120 mM) in a subpopulation of TRCs wasabolished while response to high NaCl remained intact (Chandrashekar et al., 2010). These studies suggest thatENaC in TRCs plays an essential role in the salt-taste receptor system (Chandrashekar et al., 2010;Oka et al., 2013).
In the mammalian order Cetacea that includes whales and dolphins, tastereceptor buds have atrophied and appear in degenerate form (Tinker, 1988). Sequencing of the variouscetacean genes for the receptors of the five tastes revealed that the receptorgenes for four tastes are non-functional pseudogenes because of accumulation ofmutations (Zhu et al., 2014). However,in contrast to these, the three ENaC subunits, α, β, andγ, that also serve as salt taste receptor have remained intact andfunctional. As the authors note, the conservation of ENaC genes in Cetacea isbecause of the significance of ENaC in osmoregulation and other physiologicalfunctions and it is still not known whether Cetacea are capable of sensing saltytaste (Zhu et al., 2014).
Delta subunit of ENaC
The first report on the cloning of the human δ subunit alsoshowed that in northern blots the highest levels of its expression are observedin brain, pancreas, testis and ovary with only low levels in the kidney and lung(Waldmann et al., 1995). Consistentwith these initial results, the Expression Atlas database results show that theexpression of the delta subunit gene (SCNN1D) is relatively much lower in thekidney and lung but highest in neural tissues, (including cerebral cortex,cerebellum, hippocampus, hypothalamus and pituitary gland) and testis(Expression Atlas, 53 GTEx). Expression of the δ subunit has been alsodetected in the human nasal epithelium (Bangel-Ruland et al., 2010) and eye (Krueger et al., 2012). Overall, the tissue distributionpattern of the δ subunit is distinctly different from that of α,β and γ subunits.
ASICs
ASIC subunits are expressed mainly in the central and peripheralnervous systems and the gastrointestinal tract. ASIC tissue distribution hasbeen recently extensively reviewed (Holzer,2015;Lin et al., 2015;Zha, 2013).
13. Subcellular and cilial localization
As an ion channel on the apical side of the epithelium, ENaC would beexpected to be localized on the apical membrane of cells. Indeed, in human polarizedepithelial cells in the female reproductive tract and the respiratory airways,immunohistochemical and immunofluorescence studies show that ENaC is localized atthe apical surface of the cells (Enuka et al.,2012). Studies on subcellular localization of α, β andγ subunits in mice and rat kidney nephron and in cultured Madin-Darby CanineKidney (MDCK) epithelial cell line have yielded varying results probably in partbecause of hormonal and Na+ treatment conditions (Ackermann et al., 2010;Bao etal., 2014;Chanoux et al., 2013;Hager et al., 2001).
ENaC on cilia
A cilium is a finger like protrusion on the cell surface that has amicrotubular skeleton called axoneme. The ciliary membrane that covers theaxoneme is continuous with the cell membrane (Satir and Christensen, 2007). Most cells in mammals contain a singlecilium (primary cilium) that is built upon the microtubular structure of acentriole. In three major organs, the lung, the reproductive tract, and thecentral nervous system, the epithelial surface of many cells have multiple cilia(200–300 per cell). These cilia are motile and beat in concert at apredetermined direction generating waves that move the fluid, particles andcells in the lumen of the epithelium (Brooks andWallingford, 2014).
Using antibodies we generated, we showed that in multi-ciliated cellsin the human bronchus and the female reproductive tract (extending from theuterus to the fimbria of fallopian tube), ENaC is specifically located in cilia(Enuka et al., 2012). This was alsoconfirmed by co-localization of ENaC immunofluorescence with that ofcilia-specific β-tubulin IV (Enuka etal., 2012).
The discovery that ENaCs are highly expressed in multi-ciliated cellsin the lung and the reproductive tract and that they are specifically locatedover the entire length of cilia has increased our understanding of the functionof ENaC in epithelia with motile cilia (Enuka etal., 2012). The depth of the fluid that bathes the cilia in the lumenhas to be precisely regulated for normal cilial function (Choi et al., 2015;Tilleyet al., 2015). Since Na+ is the major solute in the ECF,regulation of ENaC activity directly affects osmolarity of the periciliary fluidand consequently the flow and volume of the fluid in the lumen. Cilial locationallows ENaC to serve as a sensor and regulator of osmolarity of the periciliaryfluid along the entire length of the cilia. Thus, ENaC mediated changes inosmolarity would then modulate the fluid volume on the epithelial surface (Enuka et al., 2012). Since all airwayepithelia are Na+ absorptive, ENaC plays a key role in pulmonaryepithelia, but it should be noted that in the airway epithelium there areadditional ion channels and transporters that contribute to the regulation ofthe composition and volume of the periciliary liquid (Hollenhorst et al., 2011).
The functional importance of ENaC in the multi-ciliated cells of therespiratory airway is illustrated by the contrasting phenotypes of two diseases:1) In multi-system pseudohypoaldosteronism (see section 15.1), theloss-of-function of ENaC leads to an increase in the volume of airway surfaceliquid (ASL) (Kerem et al., 1999). 2) Incystic fibrosis (see section 15.3), dysfunction of the chloride transporter CFTR(Cystic Fibrosis Transmembrane Conductance Regulator) leads to reducedinhibition of ENaC by chloride ions. Enhanced activity of ENaC contributes tothe drastic reduction of the airway surface liquid (ASL) volume observed incystic fibrosis. Normally, the undulating movement of the cilia rapidly movesthe mucus gel on top of the cilial layer, and together with the mucus, it pushesinhaled particles and microbes in the respiratory airways for clearance via themouth (Rogers, 2007;Tilley et al., 2015). When ASL volume isreduced, clearance of foreign particles, dust, microbes and viruses is impairedcontributing to the chronic infections characteristic of cystic fibrosis.
In epithelia with motile-cilia, the interaction between ENaC and CFTRin the respiratory airways has been extensively studied because of theinvolvement of these channels in cystic fibrosis (Althaus, 2013). There are conflicting views regarding the mechanismof CFTR and ENaC interactions (Nagel et al.,2005). Some studies suggested that CFTR interacts directly with ENaC(Berdiev et al., 2009). But, whileENaC is located on the cilial surface, CFTR is located on the apical membraneoutside of cilial borders (Enuka et al.,2012). Thus, the mechanism of CFTR action in multi-ciliated cellscannot be via direct interaction with ENaC. There is evidence that chloride ionsinhibit ENaC (Bachhuber et al., 2005).Thus, CFTR may regulate ENaC activity via its modulation ofCl− levels.
14. Functional differences between ENaC and ASIC
As noted above, ENaCs and ASICs are the only families that are expressed invertebrates. While ENaCs and ASICs share similar structures, there are majordifferences in their functional characteristics as observed in heterologousexpression systems as well as differences in the physiologic roles in vertebrates(Gründer and Pusch, 2015;Kellenberger and Schild, 2015). The majordifferences are outlined inTable 13.
ENaCs are constitutively active and facilitate the bulk transport ofNa+ across high resistance epithelia in many organs (see Section 12).Transport of sodium is accompanied by a flow of fluid as a result of osmolaritychanges. The physiological consequences of these effects depend on the tissue whereENaC is expressed.
ENaC in the distal nephron has an important role in regulatingextracellular fluid volume and renal K+ secretion (Rossier et al., 2002). In the respiratory airway and alveoli,ENaC has a major role in regulating the volume of the airway and alveolar fluids(Chambers et al., 2007;Eaton et al., 2009). In the female reproductivetract, ENaC modulates uterine fluid absorption during the reproductive cycles (Ruan et al., 2014;Salleh et al., 2005). Fertilization of the oocyte in theoviduct and fallopian tube requires transport of the oocyte to the ampulla region ofthe tube. This process is dependent on the ciliary beating along the oviduct inaddition to smooth muscle contractions (Coy et al.,2012). Similarly, the transport of embryo to the uterus is also dependenton cilial motion (Lyons et al., 2006). AsENaC is richly expressed on cilia in multi-ciliated cells in the oviduct (seeSection 13) and in the uterine glands in the endometrium, reproductive processes ofovum and embryo transport and implantation are dependent on ENaC function (Enuka et al., 2012). In the tongue, ENaC hasbeen identified as the salt taste "receptor" (Chandrashekar et al., 2010;Oka et al., 2013) (see Section 12).
In contrast to ENaC, ASICs are H+-gated ion channels that areare closed in the resting state, and rapidly desensitize following activation. Theyare expressed in mammalian central and peripheral nervous systems and have roles innociception, mechanosensation, fear-related behavior and seizure termination (Chen et al., 1998;Deval and Lingueglia, 2015;Price et al., 2001,2000;Waldmann and Lazdunski, 1998;Ziemann et al., 2009,2008). ASICs also play an important role in synaptic functionregulating neural plasticity in pathological conditions (Zha, 2013). ASICs are also expressed in sensory neurons in thegastrointestinal tract, and play a role in acid-sensing within the gastrointestinaltract and may be involved in human sour taste sensing (Holzer, 2015).
15. Diseases associated with ENaC mutations
In this section, we shall briefly describe hereditary diseases that havebeen associated with mutations in the genes coding for ENaC subunits. The majorcharacteristics of these diseases are summarized inTable 14.
Table 14.
Characteristics of hereditary disorders that result from mutations orpolymorphisms in ENaC subunits.
| Multi system PHA | Liddle syndrome | Cystic fibrosis-like disease | |
|---|---|---|---|
| Inheritance | Autosomal recessive | Autosomal dominant | Polygenetic mechanism. ENaC associationin some cases. |
| Mutations in | α, β and γsubunits | PY motif at the C-terminus of β andγ subunits | Heterozygous variants in α, βor γ subunit genes. ENaC / CFTR genotype (seeTable 15). |
| ENaC activity | Loss of function | Gain of function | Increased or decreased activity |
| Water and electrolyte metabolism | Hypovolemia (dehydration), hyponatremia,hyperkalemia | Volume expansion, hypokalemia (usually) | Rarely dehydration |
| Acid-base balance | Metabolic acidosis during recurrent saltwasting episodes | Metabolic alkalosis | Rarely metabolic alkalosis |
| Blood pressure | Hypotension during recurrent salt wastingepisodes | Hypertension | Mostly normal |
| Renin-aldosterone system | Hyperreninemia, hyperaldosteronism | Hyporeninemia, Normal-high aldosterone | Mostly normal aldosterone and PRA |
| End organs involved | Kidneys, sweat and salivary glands,respiratory tract, reproductive system, colon | Kidney | Respiratory tract, sweat glands. Lessfrequent: pancreatic and gastrointestinal tract. |
| Kidneys | Impaired Na+ reabsorption resultingin severe salt loss | Increased Na+ and fluidreabsorption | Normal renal function |
| Respiratory tract | Recurrent lower pulmonary tract infections,chronic rhinitis. No chronic lung disease | Normal | Chronic, moderate to severebronchitis/bronchiectasis/sinusitis. P. aeruginosainfections |
| Sweat and salivary glands | Increased chloride (>>60mmol/L) Aggravates renal salt wasting specifically in hotenvironments. | Normal | Borderline to highly increased sweat chloride(40–>60 mmol/L), rarely normal. Normal salivarychloride. |
| Reproductive tract | Impaired ciliary function in endometrium andfallopian tube, impaired fertility | Normal | Normal |
| Age of onset/presentation | Infancy | Childhood/young adulthood Rare in infancy | Infancy, childhood, young adulthood |
| Outcome | High mortality in infancy. Decreasingfrequency and severity of salt wasting episodes with age. | Premature death in undiagnosed youngadults | Variable. Depends on severity of pulmonaryinvolvement. No long term data in most studies |
| Therapy | High NaCl supplementation lifelong | Diuretics that block ENaC (amiloride ortriamterene) and low salt diet | Variable |
15.1. Multi-system pseudohypoaldosteronism type 1 (PHA1B)
Type I pseudohypoaldosteronism (PHA) is a syndrome of unresponsiveness(also called resistance) to mineralocorticoid hormone aldosterone. This diseasewas first described by Cheek and Perry as an aldosterone unresponsivenesssyndrome resulting in salt wasting in a child (Cheek and Perry, 1958). Subsequently over 50 studies reported manycases of PHA with characteristics of aldosterone resistance with varying degreesof salt wasting. In 1991, Hanukoglu established that PHA includes twoindependent syndromes (called renal and multi-system forms of PHA) that differin their pathogenesis, mode of inheritance, the involvement of aldosteronetarget organs and the severity of salt wasting (Hanukoglu, 1991). Later studies confirmed this distinction and thesetwo forms have been assigned two separate entries in the OMIM database(http://omim.org/): 1) Renal form (PHA1A): OMIM #177735,inherited as an autosomal-dominant disease; and 2) multi-system form (PHA1B):OMIM #264350, inherited as an autosomal-recessive disease.
As commonly observed for other steroid hormone resistance diseases,initially the cause of PHA was suspected to be a mutation(s) in themineralocorticoid receptor (MR) gene (Armaniniet al., 1985). However, analysis of the linkage between PHA andpolymorphisms adjacent to the mineralocorticoid receptor gene on chromosome 4 in10 consanguineous families excluded mutations in the MR gene in multi-system PHApatients (Chung et al., 1995). By furtherhomozygosity mapping, multi-system PHA locus was mapped to two regions codingfor SCNN1A and SCNN1B and SCNN1G in chromosomes 12p and 16p respectively (Strautnieks et al., 1996). Indeed,sequencing of the genes coding for the α, β and γsubunits revealed that the multi-system form results from mutations in thesethree genes (Chang et al., 1996). Twoyears later Geller et al. reported that the milder renal form (PHA1A) is causedby mutations in the mineralocorticoid receptor gene (Geller et al., 1998).
Clinical presentation
Patients affected by multi-system PHA lose salt from allaldosterone-responsive target organs expressing ENaC including kidney, sweatand salivary glands and respiratory tract (Hanukoglu, 1991). This may necessitate frequent hospitalizationsespecially during infancy and childhood for severe hyponatremia,hyperkalemia, acidosis and dehydration (Edelheit et al., 2005;Hanukoglu, 1991). These patients also exhibit recurrentpulmonary symptoms such as congestion, wheezing, recurrent lower respiratorytract infections and chronic rhinorrhea due to impaired lung fluidabsorption causing excessive airway surface liquid (Hanukoglu et al., 1994;Kerem et al., 1999;Schaedel et al., 1999). They may also fail to conceive due toimpaired ENaC expression in cilia lining the fallopian tube and theendometrial mucosa (Enuka et al.,2012). This form carries a high mortality risk especially ininfancy, although mortality has been observed even in older patients (Hanukoglu, 1991;Porter et al., 2003;Saxena et al., 2002). Disease manifestations are life long, yetthe severity and frequency of salt wasting episodes improve with age (Adachi et al., 2010;Hanukoglu and Hanukoglu, 2010;Hanukoglu et al., 2008).
PHA1B patients require high amounts of sodium chloride (up to 45g/day) life long, to prevent recurrent salt wasting episodes from multipleorgans (Hanukoglu and Hanukoglu,2010;Hanukoglu et al.,2008;Hogg et al., 1991).The severity of PHA manifestations improves with age, depending on thenature of the mutations, environmental factors such as ambient temperaturesand degree of compliance with the therapy (Adachi et al., 2010;Hanukogluet al., 2008).
Genotype-phenotype relationships
Multi-system PHA patients characterized to date carry homozygous orcompound heterozygous mutations in the genes coding for the α,β and γ subunits (Belot etal., 2008;Bonny et al.,2002;Chang et al., 1996;Edelheit et al., 2005;Kellenberger et al., 1999;Saxena et al., 2002;Strautnieks et al., 1996;J. Wang et al., 2013;Welzel et al., 2013). Nonsense,frameshift, and abnormal splicing mutations are associated with a severephenotype (Edelheit et al., 2005).Generally, missense mutations result in a milder phenotype (Hanukoglu et al., 2008). Functionalexpression of subunits with frameshift mutations showed these mutationsreduce but do not necessarily eliminate ENaC activity (Edelheit et al., 2010).
Transient severe salt loss (severe hyponatremia and hyperkalemia)was reported in a premature baby with a homozygous missense mutation in theSCNN1A gene but not in his brother born at term who carried the samemutation (Dirlewanger et al., 2011).In the neonatal period, the human kidney is characterized by an impairedability to regulate water and sodium homeostasis and premature babies areeven more susceptible to blood volume and electrolyte changes when ENaCactivity is partial (Martinerie et al.,2009).
In cases where the mutant subunit has been expressed together withthe other two wild-type subunits, a correlation has been observed betweenthe in vitro sodium conductance activity of the mutated channel and theclinical severity of the disease (Hanukogluet al., 2008).
Subjects carrying the mutations in one allele (e.g. parents) areasymptomatic. Rarely, increased sweat sodium and chloride concentrationshave been observed in carriers of a mutation in the α subunitwithout additional hormonal or clinical phenotypes (Riepe et al., 2009).
15.2. Liddle syndrome
Liddle syndrome (OMIM #177200) is an autosomal dominantdisorder characterized by early-onset hypertension associated with hypokalemia,metabolic alkalosis, and low levels of plasma renin activity (PRA) andaldosterone (Bogdanović et al.,2012;Hansson et al., 1995;Liddle et al., 1963) (seeTable 14). The degree of phenotypicexpression may vary even within the same family (Bogdanović et al., 2012;Findling et al., 1997).
Liddle syndrome is caused by missense mutations in the PY motif ofβ or γ subunit of ENaC (Fig.14), and nonsense or frameshift mutations that result in truncationof the C-terminus leading to the loss of the PY motif in these subunits (Furuhashi et al., 2005;Hansson et al., 1995;Ma et al., 2008;Schild et al., 1996;Shimkets et al., 1994). Mutation or elimination of the PY motifdisrupts ubiquitin ligase Nedd4-2 binding to the PY motif (Rotin and Staub, 2011). This leads to accumulation ofactive channels at the cell surface and increased Na+ reabsorption inthe kidney, resulting in elevated blood volume and blood pressure. Although inthe α subunit there is also a PY-motif (Fig. 14), so far no case of Liddle syndrome has been reported with amutation in the α subunit.
In addition to mutations in the PY motif region, two mutations in theC-terminus (β subunit, R563Q) and the TM2 segment (γ subunit,N530S) have been reported to be associated with Liddle syndrome phenotype. Inthe β subunit, an R563Q mutation is associated with low plasma reninactivity (PRA), low aldosterone and hypertension in a minority of theindividuals carrying the variant (Rayner etal., 2003). In the γ subunit, Hiltunen et al. found an N530Smutation in a patient who developed Liddle syndrome like symptoms at the age of25 years (Hiltunen et al., 2002). Yet,in the same study the N530S mutation was also found in a healthy person withnormal blood pressure (Hiltunen et al.,2002). Thus, the authors raise the possibility that the γN530S mutation may not be the sole cause of hypertension and that there may beadditional factors (such as other genes or environmental factors) responsiblefor the phenotype observed (Hiltunen et al.,2002). Functional expression of ENaC carrying the N530S mutationshowed that the mutation increases ENaC open probability two fold. The increasedENaC activity was observed without a change in the surface expression of ENaCrelative to the wild type (Hiltunen et al.,2002). It is probable that more mutations will be found that increaseENaC activity and that are associated with Liddle syndrome like symptoms,similar to the two cases noted above.
Generally Liddle syndrome is a rare disease. However, an extensivestudy that included a sample of 330 Chinese young hypertensive patients revealedthat 1.5% of the patients had mutations associated with a loss of the PYmotif (Wang et al., 2015). In aretrospective study in a cohort of 149 hypertensive US veterans, 6% werefound to have biochemical abnormalities compatible with Liddle syndrome (Tapolyai et al., 2010). Thus, Liddlesyndrome may be a common cause of monogenic hypertension in some populations(Padmanabhan et al., 2015).
Undiagnosed and untreated individuals with Liddle syndrome are at highrisk of premature cardiovascular morbidity and mortality. Diuretics that blockENaC activity (amiloride or triamterene) and low salt diet, are usually adequateto control hypertension.
15.3. Cystic fibrosis-like disease
Cystic fibrosis (CF) is an autosomal recessive disease caused bymutations in the cystic fibrosis transmembrane conductance regulator (CFTR)gene. CFTR dysfunction affects epithelial chloride transport in multiple organsincluding the digestive system, sweat glands, pancreas, and the reproductivetract, but, progressive lung disease continues to be the major cause ofmorbidity and mortality.
Respiratory tract infections result from dehydration of airway surfacesthat reduces mucociliary clearance and creates an environment conducive tobacterial infections leading to progressive respiratory insufficiency andeventually respiratory failure.
In the lumen of the respiratory tract, ENaC function is essential fornormal mucociliary clearance (Althaus,2013;Hobbs et al., 2013). Asnoted in section 13, ENaC is expressed in the respiratory tract, located on thesurface of cilia and regulates the volume of luminal fluid (Enuka et al., 2012). PHA1B patients withENaC mutations suffer frequently from lower respiratory tract infections (Hanukoglu et al., 1994;Kerem et al., 1999;Schaedel et al., 1999). Increased airway-specificexpression of the β subunit of ENaC results in CF-like symptoms in mice(Mall et al., 2004). This is thoughtto result in over-expression of channels composed of only α andβ subunits, which have a high intrinsic open probability (Mall et al., 2010). Therefore, it has beensuggested that mutations in ENaC genes may be involved in some forms of cysticfibrosis. To examine the hypothesis that ENaC mutations may be associated withthe degree of severity of CF, a French group screened genomic DNA of 56 CFpatients for the presence of variants in SCNN1B and SCNN1G genes (Viel et al., 2008). By using denaturinghigh-performance liquid chromatography (DHPLC), they found 4 missense mutationsin three patients out of 56 (T313M and G589S in β, and L481Q and V546Iin γ subunit). However, nasal potential difference measurements did notindicate a functional effect of these variants on Na+ transport, atleast in the nasal epithelium of these patients. Thus, the authors concludedthat variants in SCNN1B and SCNN1G genes are not associated with CF severity inthe cohort examined (Viel et al.,2008).
Cases that present with classical features of cystic fibrosis (such aschronic lung infections with elevated sweat chloride concentration), but withoutCFTR mutation or a single allele CFTR mutation, have been referred to as cysticfibrosis-like (CF-like) disease (Table14). These cases lack a genetic diagnosis, and a commonly suspectedcause is mutations in ENaC genes (Collawn etal., 2012).
Screening 185 patients with non-classic CF, Sheridan at al. identified20 patients who had elevated sweat chloride concentrations, and pulmonarydisease but without CFTR mutations (Sheridan etal., 2005). Sequencing of the ENaC genes (SCNN1A, B or G) revealedthat two of the patients carry compound heterozygous mutations in the SCNN1Bgene: a missense mutation (P267L) with a splice site mutation in one patient andtwo missense mutations (G294S and E539K) in the other. Neither patient hadabnormal renin or aldosterone levels. In functional expression studies, P267Land E539K mutants showed decreased activity and G294S mutant showed increasedactivity (Sheridan et al., 2005). Theauthors concluded that the compound heterozygous mutations identified in theβ ENaC genes that have a mild effect on ENaC activity are associatedwith CF-like disease without causing severe renal salt loss (Sheridan et al., 2005).
In a multi-center European study, 30 ENaC variants were found in 76patients with CF-like disease (Azad et al.,2009). Only two (hypoactive F61L and hyperactive V114I in SCNN1A) ofthe 30 variants were found in patients but not in the control populations. ENaCsubunit variants had a significantly higher frequency in the patients ascompared to controls. The variant W493R in the α subunit showed the mostsignificant difference, and in functional expression in Xenopus oocytes showedover four-fold higher ENaC activity. Thus, the authors concluded that thesevariants may be involved in CF-like disease by a polygenetic mechanism (Azad et al., 2009). In a study including 99Italian patients with CFTR-related diseases, 12 ENaC variants were found, butthe allele frequency of these variants was not significantly different fromcontrols (Amato et al., 2012).
Fajac et al. screened a group of 55 patients with diffuse idiopathicbronchiectasis (permanent dilation of the airways as a result of chronicbronchial infection) by sequencing SCNN1B and SCNN1G exons and identified fiveheterozygous missense variants (S82C, P369T, N288S in β, and G183S,E197K in γ subunit) in eight patients (Fajac et al., 2008). The S82C mutation was found in three unrelatedpatients who were also heterozygous for a CFTR mutation. The authors thusconcluded that trans-heterozygous mutations in ENaC and CFTR may be responsiblefor the CF-like symptoms.
In a study including 60 Rwandan children with CF-like symptoms, fivepatients were found to have a heterozygous CFTR mutation. Two of these patientshad a missense ENaC variant (V573I in α, V348M and G442V in βsubunit); of these only V348M was not found in the control group (Mutesa et al., 2009). Functionalexpression of the V348M mutant showed that the mutation enhances ENaC activity(Rauh et al., 2013). Since the fullENaC subunit gene sequences were not determined in 55 of the patients, therelationship between ENaC variants and CF-like disease cannot be determined forthe whole group.
A recent study on CF-like phenotypes examined the sequences of fivegenes (CFTR, SCNN1A, SCNN1B, SCNN1G and SERPINA1) in six patients by whole exomesequencing (Ramos et al., 2014). Theauthors detected three missense variants in SCNN1A (R204W, A357T, C641F) and onemissense (R563Q) in SCNN1B, and four additional nucleotide variants. Two ofthese mutants (C641F and R563Q) appeared also in two CF controls but not inhealthy controls. The authors suggest that the variants that appear at a higherfrequency in patients with CF-like phenotype than in controls may be responsiblefor this phenotype. By their family analysis they also stress the importance ofgenetic /environmental factors in the development of CF-like disease (Ramos et al., 2014).
Brenan et al. examined 33 nonwhite, non-Hispanic patients with aCF-like disease whose CFTR gene analysis was non-diagnostic (19 with nomutations in CFTR gene and 14 with a heterozygous change in the CFTR gene)(Brennan et al., 2015). Sequencing ofthe exons and introns of SCNN1A, SCNN1B, and SCNN1G in all patients revealed 21variants. Since the variants found in conjunction with a CFTR mutation werecommon polymorphisms, the authors concluded that there is no conclusiveassociation of ENaC genetic variants with CF in their cohort.
InTable 15 we listed all themissense mutations identified in the studies reviewed above. Among the threeENaC genes, most of the variants have been observed in SCNN1A and SCNN1B.Proportionately, the highest number of mutants is in SCNN1A. Two of the majorstudies examined only two genes: SCNN1B and SCNN1G, thus the number of variantsin SCNN1A inTable 15 isunderrepresented. In our analysis of PHA1B mutants, we observed a similar trendthat most of the PHA1B causing mutants appear in SCNN1A and only a few in SCNN1G(Edelheit et al., 2005).
Table 15.
Missense mutations found in patients with a CF-like phenotype.
| Variant | Change in function | Ethnicity | References | |
|---|---|---|---|---|
| SCNN1A | ||||
| V14G | Spanish | (Ramos et al.,2014) | ||
| F61L | ↓ ENaC activity | Caucasian | (Azad et al.,2009) | |
| V114I | ↑ ENaC activity | Caucasian | (Azad et al.,2009) | |
| R181W | ↑ ENaC activity | Caucasian | (Azad et al., 2009;Sheridan et al., 2005) | |
| R204W | Spanish | (Ramos et al.,2014) | ||
| A304P | Spanish | (Ramos et al.,2014) | ||
| A334T | ↓ ENaC activity | Caucasian, nonwhite | (Amato et al., 2012;Azad et al., 2009;Brennan et al., 2015) | |
| A357T | Spanish | (Ramos et al.,2014) | ||
| W493R | ↑ ENaC activity | Caucasian | (Azad et al.,2009) | |
| V573I | African, nonwhite | (Brennan et al.,2015;Mutesa et al.,2009) | ||
| C618F | ↑ ENaC activity | Nonwhite | (Brennan et al.,2015) | |
| C641F | Spanish | (Ramos et al.,2014) | ||
| T663A | ↓ ENaC activity | Caucasian, nonwhite | (Amato et al., 2012;Azad et al., 2009;Brennan et al., 2015) | |
| SCNN1B | ||||
| S82C | None | Caucasian | (Azad et al., 2009;Fajac et al., 2008;Sheridan et al., 2005) | |
| P267L | ↓ ENaC activity | (Sheridan et al.,2005) | ||
| N288S | Caucasian | (Fajac et al.,2008) | ||
| G294S | ↑ ENaC activity | (Sheridan et al.,2005) | ||
| T313M | No change in NPD* | French | (Viel et al.,2008) | |
| V348M | ↑ ENaC activity | African | (Mutesa et al.,2009) | |
| P369T | High basal NPD | Caucasian | (Fajac et al.,2008) | |
| R388C | Nonwhite | (Brennan et al.,2015) | ||
| G442V | African, nonwhite | (Brennan et al.,2015;Mutesa et al.,2009) | ||
| E539K | ↓ ENaC activity | (Sheridan et al.,2005) | ||
| R563Q | Spanish | (Ramos et al.,2014) | ||
| G589S | None, normal NPD | Caucasian, French | (Azad et al., 2009;Viel et al., 2008) | |
| T594M | Nonwhite | (Brennan et al.,2015) | ||
| SCNN1G | ||||
| G183S | African, nonwhite | (Brennan et al.,2015;Fajac et al.,2008) | ||
| E197K | None | Caucasian | (Azad et al., 2009;Fajac et al., 2008) | |
| L481Q | Normal NPD | French | (Viel et al.,2008) | |
| V546I | Normal NPD | French | (Viel et al.,2008) |
NPD: Nasal potential difference.
As detailed in the studies cited, many of the variants are also presentin control groups. Yet, some of the mutants that were shown to affect adverselyENaC activity have been reported in independent studies (Table 15). The total number of cases is still too small toreach definitive conclusions about the role of these variants/mutants in causingCF-like disease. In any case, the number of variants that may be associated withCF-like disease represents a small percentage of the total patients in eachcohort and differs between ethnic groups.
15.4. Hypertension
In modern industrial societies, hypertension has emerged as one of mostwidespread health problems (Toka et al.,2013). A minority of the cases of hypertension can be ascribed tomonogenic conditions such as Liddle's syndrome or Gordon'ssyndrome (Padmanabhan et al., 2015). Theremainder of the cases is generally grouped as "essentialhypertension" with multifactorial etiology, including multiple genetic,humoral, environmental and dietary factors (Suand Menon, 2001). The major systems that are responsible for theregulation of blood pressure include the renin-angiotensin-aldosterone systemand Na+ transporters in the kidney (Padmanabhan et al., 2015;Rossier,2014;Soundararajan et al.,2010;Su and Menon, 2001).Moreover, the Liddle syndrome described above firmly established the importanceof ENaC in blood pressure regulation. Therefore, several large-scale studieshave examined the association of ENaC variants with essential hypertension. Liuet al. examined 2880 Chinese subjects (GenSalt study) and found an associationof blood pressure with SCNN1B and SCNN1G SNPs and variants (Liu et al., 2015). Rayner et al. screened139 South African black hypertensives for the R563Q variant of β subunitand found that the variant was significantly associated with hypertension (Rayner et al., 2003). In a study in aFinland, the authors sequenced only exon 13 (that codes for TM2 and theC-terminal segment) of β and γ subunits (seeFig. 7) in 27 hypertensive patients. Thethree identified variants were then screened in 347 hypertensives. The frequencyof all variants in the hypertensives was significantly higher (~3 fold)than that in two control groups. Functional expression of one variant (βG589S) showed slightly enhanced activity in Xenopus oocytes (Hannila-Handelberg et al., 2005).
Ambrosius et al. reported a small but significant association between acommon αT663A variant and normal blood pressure (Ambrosius et al., 1999). The αA663 variant reducesENaC activity and functional expression in Xenopus oocytes (Samaha et al., 2004;Tong et al., 2006), but the change in activity wasopposite of that predicted by the results of Ambrosius et al. Furthermore, noassociation was found between αT663A variant and blood pressure in asample of 247 Japanese hypertensives (Sugiyamaet al., 2001). Nonetheless, homozygous A663 allele appears to have adifferential effect on lung function (Foxx-Lupoet al., 2011).
Another polymorphism with conflicting reports is β-T594M. In asample of black hypertensives from London, 8.3% (17 out of 206) wereheterozygous for the β T594M variant, but in the control group only2.1% had the same variant (Baker et al.,1998). However, in a much larger sample, including 1666 Jamaicanblacks, no association was found between the β T594M allele andhypertension (Hollier et al., 2006).This variant did not alter ENaC activity in a heterologous expression system(Persu et al., 1998)
Persu et al. identified seven variants in the β subunits of,mostly white, 525 probands of hypertensive families, but could not identify anassociation between a variant and essential hypertension (Persu et al., 1998).
In summary, currently there does not appear to be a clear associationbetween ENaC variants and essential hypertension. Yet, this area of research isjust at its beginnings and requires examination of larger sets of SNPs andvariants in selected populations. Some of the past studies have examined onlyspecific segments (such as exon 13) of subunits. This approach skews theresults. The SNPs that are accumulating in whole genome sequencing will presentlarger and more comprehensive databases for future examination. It is likelythat such studies will reveal new variants associated with hypertension similarto that found in the Chinese GenSalt study. Since the majority of the variantsso far screened do not seem to be associated with hypertension, the proportionof ENaC variants associated with hypertension would be expected to be small.
Highlights.
A comprehensive review of the structure and function of four ENaCsubunits from an evolutionary perspective.
Comparison of the sequences of ENaC homologs and identification ofstructural motifs conserved throughout vertebrates.
Establishing criteria for distinguishing ENaC family members fromother families within the ENaC/Degenerin superfamily including ASIC, deg,mec, unc, ppk type gene products.
Review of tissue-specific expression and functions of ENaC paralogsand inherited diseases associated with mutations in ENaC genes.
Acknowledgments
This review and the corresponding Gene Wiki articles are written as part ofthe Gene Wiki Review series - a series resulting from a collaboration between thejournal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported byNational Institutes of Health (GM089820). Additional support for Gene Wiki Reviewsis provided by Elsevier, the publisher of GENE.
We are grateful to Prof. Thomas Kleyman (University of Pittsburgh) forvaluable discussions. This research was funded in part by a grant from the UnitedStates-Israel Binational Science Foundation (BSF).
Abbreviations
- ASIC
acid-sensing ion channel
- ASL
airway surface liquid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial sodium channel
- ECF
extracellular fluid
- ICF
intracellular fluid
- PHA
pseudohypoaldosteronism
- PRA
plasma renin activity
- TM
transmembrane
- TRCs
taste receptor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an uneditedmanuscript that has been accepted for publication. As a service to our customerswe are providing this early version of the manuscript. The manuscript willundergo copyediting, typesetting, and review of the resulting proof before it ispublished in its final citable form. Please note that during the productionprocess errors may be discovered which could affect the content, and all legaldisclaimers that apply to the journal pertain.
References
- Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid andglucocorticoid receptors in rat kidney: differential effect ofcorticosteroids along the distal tubule. Am J Physiol Renal Physiol. 2010;299:F1473–F1485. doi: 10.1152/ajprenal.00437.2010. [DOI] [PubMed] [Google Scholar]
- Adachi M, Asakura Y, Muroya K, Tajima T, Fujieda K, Kuribayashi E, Uchida S. Increased Na reabsorption via the Na-Cl cotransporter inautosomal recessive pseudohypoaldosteronism. Clin Exp Nephrol. 2010;14:228–232. doi: 10.1007/s10157-010-0277-0. [DOI] [PubMed] [Google Scholar]
- Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, Russell P, Lowe CB, Glor RE, Jaffe JD, Ray DA, Boissinot S, Shedlock AM, Botka C, Castoe TA, Colbourne JK, Fujita MK, Moreno RG, ten Hallers BF, Haussler D, Heger A, Heiman D, Janes DE, Johnson J, de Jong PJ, Koriabine MY, Lara M, Novick PA, Organ CL, Peach SE, Poe S, Pollock DD, de Queiroz K, Sanger T, Searle S, Smith JD, Smith Z, Swofford R, Turner-Maier J, Wade J, Young S, Zadissa A, Edwards SV, Glenn TC, Schneider CJ, Losos JB, Lander ES, Breen M, Ponting CP, Lindblad-Toh K. The genome of the green anole lizard and a comparative analysiswith birds and mammals. Nature. 2011;477:587–591. doi: 10.1038/nature10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alli AA, Song JZ, Al-Khalili O, Bao H-F, Ma H-P, Alli AA, Eaton DC. Cathepsin B is secreted apically from Xenopus 2F3 cells andcleaves the epithelial sodium channel (ENaC) to increase itsactivity. J Biol Chem. 2012;287:30073–30083. doi: 10.1074/jbc.M111.338574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus M. ENaC inhibitors and airway re-hydration in cystic fibrosis: stateof the art. Curr Mol Pharmacol. 2013;6:3–12. doi: 10.2174/18744672112059990025. [DOI] [PubMed] [Google Scholar]
- Amato F, Bellia C, Cardillo G, Castaldo G, Ciaccio M, Elce A, Lembo F, Tomaiuolo R. Extensive molecular analysis of patients bearing CFTR-relateddisorders. J. Mol. diagnostics. 2012;14:81–89. doi: 10.1016/j.jmoldx.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation toaldosterone and potassium excretion and risk forhypertension. Hypertension. 1999;34:631–637. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- Amemiya CT, Alföldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SMJ, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff J-N, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The African coelacanth genome provides insights into tetrapodevolution. Nature. 2013;496:311–316. doi: 10.1038/nature12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory andendocytic pathways. Nat. Cell Biol. 2012;14:11–19. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities inthe pericellular microenvironment. Biochem J. 2010;428:325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanini D, Kuhnle U, Strasser T, Dorr H, Butenandt I, Weber PC, Stockigt JR, Pearce P, Funder JW. Aldosterone-receptor deficiency inpseudohypoaldosteronism. N Engl J Med. 1985;313:1178–1181. doi: 10.1056/NEJM198511073131902. [DOI] [PubMed] [Google Scholar]
- Asher C, Chigaev A, Garty H. Characterization of interactions between Nedd4 and beta andgammaENaC using surface plasmon resonance. Biochem Biophys Res Commun. 2001;286:1228–1231. doi: 10.1006/bbrc.2001.5508. [DOI] [PubMed] [Google Scholar]
- Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channelsubunits. Am. J. Physiol. 1996;271:C605–C611. doi: 10.1152/ajpcell.1996.271.2.C605. [DOI] [PubMed] [Google Scholar]
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, des Georges M, Stanke F, De Boeck K, Dupont L, Balascáková M, Hjelte L, Lebecque P, Radojkovic D, Castellani C, Schwartz M, Stuhrmann M, Schwarz M, Skalicka V, de Monestrol I, Girodon E, Férec C, Claustres M, Tümmler B, Cassiman J-J, Korbmacher C, Cuppens H. Mutations in the amiloride-sensitive epithelial sodium channel inpatients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K. Cl-interference with the epithelial Na+ channelENaC. J Biol Chem. 2005;280:31587–31594. doi: 10.1074/jbc.M504347200. [DOI] [PubMed] [Google Scholar]
- Baconguis I, Bohlen CJ, Goehring A, Julius D, Gouaux E. X-ray structure of acid-sensing ion channel 1-snake toxin complexreveals open state of a Na(+)-selective channel. Cell. 2014;156:717–729. doi: 10.1016/j.cell.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND, MacGregor GA. Association of hypertension with T594M mutation in beta subunitof epithelial sodium channels in black people resident inLondon. Lancet. 1998;351:1388–1392. doi: 10.1016/s0140-6736(97)07306-6. [DOI] [PubMed] [Google Scholar]
- Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber W-M. Characterization of the epithelial sodium channel delta-subunitin human nasal epithelium. Am J Respir Cell Mol Biol. 2010;42:498–505. doi: 10.1165/rcmb.2009-0053OC. [DOI] [PubMed] [Google Scholar]
- Bao H-F, Thai TL, Yue Q, Ma H-P, Eaton AF, Cai H, Klein JD, Sands JM, Eaton DC. ENaC activity is increased in isolated, split-open corticalcollecting ducts from protein kinase Cα knockoutmice. Am J Physiol Renal Physiol. 2014;306:F309–F320. doi: 10.1152/ajprenal.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B. Role of gammaENaC subunit in lung liquid clearance andelectrolyte balance in newborn mice. Insights into perinatal adaptation andpseudohypoaldosteronism. J Clin Invest. 1998;102:1634–1640. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belot A, Ranchin B, Fichtner C, Pujo L, Rossier BC, Liutkus A, Morlat C, Nicolino M, Zennaro MC, Cochat P. Pseudohypoaldosteronisms, report on a 10-patientseries. Nephrol Dial Transpl. 2008;23:1636–1641. doi: 10.1093/ndt/gfm862. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y. Sensory functions for degenerin/epithelial sodium channels(DEG/ENaC) Adv Genet. 2011;76:1–26. doi: 10.1016/B978-0-12-386481-9.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdiev BK, Jovov B, Tucker WC, Naren AP, Fuller CM, Chapman ER, Benos DJ. ENaC subunit-subunit interactions and inhibition by syntaxin1A. Am J Physiol Renal Physiol. 2004;286:F1100–F1106. doi: 10.1152/ajprenal.00344.2003. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol. Biosyst. 2009;5:123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JM, Brand C, Awayda MS. A long isoform of the epithelial sodium channel alpha subunitforms a highly active channel. Channels (Austin) 2015;9:30–43. doi: 10.4161/19336950.2014.985478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L-P, Blais D, Boué-Grabot É, Séguéla P. A dual polybasic motif determines phosphoinositide binding andregulation in the P2X channel family. PLoS One. 2012;7:e40595. doi: 10.1371/journal.pone.0040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinicalimplications. J Am Soc Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- Björklund AK, Ekman D, Light S, Frey-Skött J, Elofsson A. Domain rearrangements in protein evolution. J Mol Biol. 2005;353:911–923. doi: 10.1016/j.jmb.2005.08.067. [DOI] [PubMed] [Google Scholar]
- Bobby R, Medini K, Neudecker P, Lee TV, Brimble MA, McDonald FJ, Lott JS, Dingley AJ. Structure and dynamics of human Nedd4-1 WW3 in complex with theαENaC PY motif. Biochim Biophys Acta. 2013;1834:1632–1641. doi: 10.1016/j.bbapap.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Bogdanović R, Kuburović V, Stajić N, Mughal SS, Hilger A, Ninić S, Prijić S, Ludwig M. Liddle syndrome in a Serbian family and literature review ofunderlying mutations. Eur J Pediatr. 2012;171:471–478. doi: 10.1007/s00431-011-1581-8. [DOI] [PubMed] [Google Scholar]
- Bonny O, Hummler E. Dysfunction of epithelial sodium transport: from human tomouse. Kidney Int. 2000;57:1313–1318. doi: 10.1046/j.1523-1755.2000.00968.x. [DOI] [PubMed] [Google Scholar]
- Bonny O, Knoers N, Monnens L, Rossier BC. A novel mutation of the epithelial Na+ channel causes type 1pseudohypoaldosteronism. Pediatr Nephrol. 2002;17:804–808. doi: 10.1007/s00467-002-0945-8. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemicosmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Bremner HR, Freywald T, O’Brodovich HM, Otulakowski G. Promoter analysis of the gene encoding the beta-subunit of therat amiloride-sensitive epithelial sodium channel. Am J Physiol Lung Cell Mol Physiol. 2002;282:L124–L134. doi: 10.1152/ajplung.2002.282.1.L124. [DOI] [PubMed] [Google Scholar]
- Brennan M-L, Pique LM, Schrijver I. Assessment of epithelial sodium channel variants in nonwhitecystic fibrosis patients with non-diagnostic CFTR genotypes. J Cyst Fibros. 2015 doi: 10.1016/j.jcf.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Brooker DR, Kozak CA, Kleyman TR. Epithelial Sodium Channel GenesScnn1bandScnn1gAre Closely Linkedon Distal Mouse Chromosome 7. Genomics. 1995;29:784–786. doi: 10.1006/geno.1995.9934. [DOI] [PubMed] [Google Scholar]
- Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol. 2014;24:R973–R982. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard M, Casado M, Djelidi S, Barrandon Y, Farman N. Epithelial sodium channel in human epidermal keratinocytes:expression of its subunits and relation to sodium transport anddifferentiation. J Cell Sci. 1999;112(Pt 1):3343–3352. doi: 10.1242/jcs.112.19.3343. [DOI] [PubMed] [Google Scholar]
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- andprostasin-dependent release of an inhibitory peptide from thegamma-subunit. J Biol Chem, The Journal of biological chemistry. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- Büsst CJ. Blood pressure regulation via the epithelial sodium channel: fromgene to kidney and beyond. Clin Exp Pharmacol Physiol. 2013;40:495–503. doi: 10.1111/1440-1681.12124. [DOI] [PubMed] [Google Scholar]
- Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membranetrafficking. Biochim Biophys Acta. 2010;1802:1166–1177. doi: 10.1016/j.bbadis.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intactcells. Am J Physiol. 1994a;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of threehomologous subunits. Nature. 1994b;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Albalat R, Irimia M, Garcia-Fernàndez J. Impact of gene gains, losses and duplication modes on the originand diversification of vertebrates. Semin. Cell Dev. Biol. 2013;24:83–94. doi: 10.1016/j.semcdb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Farkas AE, Nusrat A. Epithelial adhesive junctions. F1000Prime Rep. 2014;6:1. doi: 10.12703/P6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gammasubunit has a dominant role in channel activation. J Biol Chem. 2008a;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the alpha-subunit of theepithelial sodium channel. Am J Physiol Renal Physiol. 2008b;294:F47–F52. doi: 10.1152/ajprenal.00399.2007. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived fromproteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–18907. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- Cerecedo D, Martínez-Vieyra I, Alonso-Rangel L, Benítez-Cardoza C, Ortega A. Epithelial sodium channel modulates platelet collagenactivation. Eur J Cell Biol. 2014;93:127–136. doi: 10.1016/j.ejcb.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Chalfant ML, Denton JS, Langloh AL, Karlson KH, Loffing J, Benos DJ, Stanton BA. The NH(2) terminus of the epithelial sodium channel contains anendocytic motif. J Biol Chem. 1999;274:32889–32896. doi: 10.1074/jbc.274.46.32889. [DOI] [PubMed] [Google Scholar]
- Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of largeairways. Respir Physiol Neurobiol. 2007;159:256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste inmice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Grunder S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause saltwasting with hyperkalaemic acidosis, pseudohypoaldosteronism type1. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- Chanoux Ra, Shubin CB, Robay A, Suaud L, Rubenstein RC. Hsc70 negatively regulates epithelial sodium channel traffickingat multiple sites in epithelial cells. Am J Physiol Cell Physiol. 2013;305:C776–C787. doi: 10.1152/ajpcell.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek DB, Perry JW. A salt wasting syndrome in infancy. Arch Dis Child. 1958;33:252–256. doi: 10.1136/adc.33.169.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, protongated ionchannel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kleyman TR, Sheng S. Gain-of-function variant of the human epithelial sodiumchannel. Am J Physiol Renal Physiol. 2013;304:F207–F213. doi: 10.1152/ajprenal.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ray EC, Yates ME, Buck TM, Brodsky JL, Kinlough CL, Winarski KL, Hughey RP, Kleyman TR, Sheng S. Functional roles of clusters of hydrophobic and polar residues inthe epithelial Na+ channel knuckle domain. J Biol Chem. 2015 doi: 10.1074/jbc.M115.665398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series ofprograms. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicken-Genome. Sequence and comparative analysis of the chicken genome provideunique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Choi H-C, Kim CSK, Tarran R. Automated acquisition and analysis of airway surface liquidheight by confocal microscopy. Am J Physiol Lung Cell Mol Physiol. 2015;309:L109–L118. doi: 10.1152/ajplung.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperaturedependence and effect of extracellular proteases. J Gen Physiol. 2002;120:133–145. doi: 10.1085/jgp.20028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Hanukoglu A, Rees M, Thompson R, Dillon M, Hanukoglu I, Bistritzer T, Kuhnle U, Seckl J, Gardiner RM. Exclusion of the locus for autosomal recessivepseudohypoaldosteronism type 1 from the mineralocorticoid receptor generegion on human chromosome 4q by linkage analysis. J Clin Endocrinol Metab. 1995;80:3341–3345. doi: 10.1210/jcem.80.11.7593448. [DOI] [PubMed] [Google Scholar]
- Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lungdisease? Am J Physiol Lung Cell Mol Physiol. 2012;302:L1141–L1146. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DM, Peterson ZJ, Blokhin IO, Benson CJ, Snyder PM. Identification of extracellular domain residues required forepithelial Na+ channel activation by acidic pH. J Biol Chem. 2012;287:40907–40914. doi: 10.1074/jbc.M112.417519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl-inhibitory residues suggests a trimeric alpha gamma beta channelarchitecture. J Biol Chem. 2011;286:6027–6032. doi: 10.1074/jbc.M110.198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe SB, Carattino MD, Frizzell RA, Zhang H. Syntaxin 1A regulates ENaC via domain-specificinteractions. J Biol Chem. 2003;278:12796–12804. doi: 10.1074/jbc.M210772200. [DOI] [PubMed] [Google Scholar]
- Copeland SJ, Berdiev BK, Ji HL, Lockhart J, Parker S, Fuller CM, Benos DJ. Regions in the carboxy terminus of alpha-bENaC involved in gatingand functional effects of actin. Am J Physiol Cell Physiol. 2001;281:C231–C240. doi: 10.1152/ajpcell.2001.281.1.C231. [DOI] [PubMed] [Google Scholar]
- Coric T, Hernandez N, de la R, osa DA, Shao D, Wang T, Canessa CM. Expression of ENaC and serum- and glucocorticoid-induced kinase 1in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2004;286:G663–G670. doi: 10.1152/ajpgi.00364.2003. [DOI] [PubMed] [Google Scholar]
- Coric T, Zhang P, Todorovic N, Canessa CM. The extracellular domain determines the kinetics ofdesensitization in acid-sensitive ion channel 1. J Biol Chem. 2003;278:45240–45247. doi: 10.1074/jbc.M304441200. [DOI] [PubMed] [Google Scholar]
- Coy P, García-Vázquez FA, Visconti PE, Avilés M. Roles of the oviduct in mammalian fertilization. Reproduction. 2012;144:649–660. doi: 10.1530/REP-12-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul RA, Long JA, Zimin AV, Aslam L, Beal K, Blomberg LA, Bouffard P, Burt DW, Crasta O, Crooijmans RPMA, Cooper K, Coulombe RA, De S, Delany ME, Dodgson JB, Dong JJ, Evans C, Frederickson KM, Flicek P, Florea L, Folkerts O, Groenen MAM, Harkins TT, Herrero J, Hoffmann S, Megens H-J, Jiang A, de Jong P, Kaiser P, Kim H, Kim K-W, Kim S, Langenberger D, Lee M-K, Lee T, Mane S, Marcais G, Marz M, McElroy AP, Modise T, Nefedov M, Notredame C, Paton IR, Payne WS, Pertea G, Prickett D, Puiu D, Qioa D, Raineri E, Ruffier M, Salzberg SL, Schatz MC, Scheuring C, Schmidt CJ, Schroeder S, Searle SMJ, Smith EJ, Smith J, Sonstegard TS, Stadler PF, Tafer H, Tu ZJ, Van Tassell CP, Vilella AJ, Williams KP, Yorke JA, Zhang L, Zhang H-B, Zhang X, Zhang Y, Reed KM. Multi-platform next-generation sequencing of the domestic turkey(Meleagris gallopavo): genome assembly and analysis. PLoS Biol. 2010;8:e1000475. doi: 10.1371/journal.pbio.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Lingueglia E. Acid-Sensing Ion Channels and nociception in the peripheral andcentral nervous systems. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membranedynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Diakov A, Korbmacher C. A novel pathway of epithelial sodium channel activation involvesa serum- and glucocorticoid-inducible kinase consensus motif in the Cterminus of the channel’s alpha-subunit. J Biol Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Dirlewanger M, Huser D, Zennaro MC, Girardin E, Schild L, Schwitzgebel VM. A homozygous missense mutation in SCNN1A is responsible for atransient neonatal form of pseudohypoaldosteronism type 1. Am J Physiol Endocrinol Metab. 2011;301:E467–E73. doi: 10.1152/ajpendo.00066.2011. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ, Downs JP. Early evolution of vertebrate skeletal tissues and cellularinteractions, and the canalization of skeletal development. J. Exp. Zool. B. Mol. Dev. Evol. 2006;306:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducersin vascular smooth muscle. Physiology (Bethesda) 2008;23:23–31. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha,beta, and gamma subunits in aldosterone-responsive epithelia from the rat:localization by in situ hybridization andimmunocytochemistry. J Cell Biol. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood AL, Goodman MB. Insight into DEG/ENaC channel gating from genetics andstructure. Physiology (Bethesda) 2012;27:282–290. doi: 10.1152/physiol.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolarfunction in health and disease. Annu Rev Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- Edelheit O, Ben-Shahar R, Dascal N, Hanukoglu A, Hanukoglu I. Conserved charged residues at the surface and interface ofepithelial sodium channel subunits--roles in cell surface expression and thesodium self-inhibition response. FEBS J. 2014;281:2097–2111. doi: 10.1111/febs.12765. [DOI] [PubMed] [Google Scholar]
- Edelheit O, Hanukoglu I, Dascal N, Hanukoglu A. Identification of the roles of conserved charged residues in theextracellular domain of an epithelial sodium channel (ENaC) subunit byalanine mutagenesis. Am J Physiol Renal Physiol. 2011;300:F887–F897. doi: 10.1152/ajprenal.00648.2010. [DOI] [PubMed] [Google Scholar]
- Edelheit O, Hanukoglu I, Gizewska M, Kandemir N, Tenenbaum-Rakover Y, Yurdakök M, Zajaczek S, Hanukoglu A. Novel mutations in epithelial sodium channel (ENaC) subunit genesand phenotypic expression of multisystempseudohypoaldosteronism. Clin Endocrinol (Oxf) 2005;62:547–553. doi: 10.1111/j.1365-2265.2005.02255.x. [DOI] [PubMed] [Google Scholar]
- Edelheit O, Hanukoglu I, Shriki Y, Tfilin M, Dascal N, Gillis D, Hanukoglu A. Truncated beta epithelial sodium channel (ENaC) subunitsresponsible for multi-system pseudohypoaldosteronism support partialactivity of ENaC. J Steroid Biochem Mol Biol. 2010;119:84–88. doi: 10.1016/j.jsbmb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Ellegren H, Smeds L, Burri R, Olason PI, Backström N, Kawakami T, Künstner A, Mäkinen H, Nadachowska-Brzyska K, Qvarnström A, Uebbing S, Wolf JBW. The genomic landscape of species divergence in Ficedulaflycatchers. Nature. 2012;491:756–760. doi: 10.1038/nature11584. [DOI] [PubMed] [Google Scholar]
- Elsik CG, Tellam RL, Worley KC, Gibbs RA, Muzny DM, Weinstock GM, Adelson DL, Eichler EE, Elnitski L, Guigo R, Hamernik DL, Kappes SM, Lewin HA, Lynn DJ, Nicholas FW, Reymond A, Rijnkels M, Skow LC, Zdobnov EM, Schook L, Womack J, Alioto T, Antonarakis SE, Astashyn A, Chapple CE, Chen H-C, Chrast J, Camara F, Ermolaeva O, Henrichsen CN, Hlavina W, Kapustin Y, Kiryutin B, Kitts P, Kokocinski F, Landrum M, Maglott D, Pruitt K, Sapojnikov V, Searle SM, Solovyev V, Souvorov A, Ucla C, Wyss C, Anzola JM, Gerlach D, Elhaik E, Graur D, Reese JT, Edgar RC, McEwan JC, Payne GM, Raison JM, Junier T, Kriventseva EV, Eyras E, Plass M, Donthu R, Larkin DM, Reecy J, Yang MQ, Chen L, Cheng Z, Chitko-McKown CG, Liu GE, Matukumalli LK, Song J, Zhu B, Bradley DG, Brinkman FSL, Lau LPL, Whiteside MD, Walker A, Wheeler TT, Casey T, German JB, Lemay DG, Maqbool NJ, Molenaar AJ, Seo S, Stothard P, Baldwin CL, Baxter R, Brinkmeyer-Langford CL, Brown WC, Childers CP, Connelley T, Ellis SA, Fritz K, Glass EJ, Herzig CTA, Iivanainen A, Lahmers KK, Bennett AK, Dickens CM, Gilbert JGR, Hagen DE, Salih H, Aerts J, Caetano AR, Dalrymple B, Garcia JF, Gill CA, Hiendleder SG, Memili E, Spurlock D, Williams JL, Alexander L, Brownstein MJ, Guan L, Holt RA, Jones SJM, Marra MA, Moore R, Moore SS, Roberts A, Taniguchi M, Waterman RC, Chacko J, Chandrabose MM, Cree A, Dao MD, Dinh HH, Gabisi RA, Hines S, Hume J, Jhangiani SN, Joshi V, Kovar CL, Lewis LR, Liu Y-s, Lopez J, Morgan MB, Nguyen NB, Okwuonu GO, Ruiz SJ, Santibanez J, Wright RA, Buhay C, Ding Y, Dugan-Rocha S, Herdandez J, Holder M, Sabo A, Egan A, Goodell J, Wilczek-Boney K, Fowler GR, Hitchens ME, Lozado RJ, Moen C, Steffen D, Warren JT, Zhang J, Chiu R, Schein JE, Durbin KJ, Havlak P, Jiang H, Liu Y, Qin X, Ren Y, Shen Y, Song H, Bell SN, Davis C, Johnson AJ, Lee S, Nazareth LV, Patel BM, Pu L-L, Vattathil S, Williams RL, Curry S, Hamilton C, Sodergren E, Wheeler DA, Barris W, Bennett GL, Eggen A, Green RD, Harhay GP, Hobbs M, Jann O, Keele JW, Kent MP, Lien S, McKay SD, McWilliam S, Ratnakumar A, Schnabel RD, Smith T, Snelling WM, Sonstegard TS, Stone RT, Sugimoto Y, Takasuga A, Taylor JF, Van Tassell CP, MacNeil MD, Abatepaulo ARR, Abbey CA, Ahola V, Almeida IG, Amadio AF, Anatriello E, Bahadue SM, Biase FH, Boldt CR, Carroll JA, Carvalho WA, Cervelatti EP, Chacko E, Chapin JE, Cheng Y, Choi J, Colley AJ, de Campos TA, De Donato M, Santos IKFdM, de Oliveira CJF, Deobald H, Devinoy E, Donohue KE, Dovc P, Eberlein A, Fitzsimmons CJ, Franzin AM, Garcia GR, Genini S, Gladney CJ, Grant JR, Greaser ML, Green JA, Hadsell DL, Hakimov HA, Halgren R, Harrow JL, Hart EA, Hastings N, Hernandez M, Hu Z-L, Ingham A, Iso-Touru T, Jamis C, Jensen K, Kapetis D, Kerr T, Khalil SS, Khatib H, Kolbehdari D, Kumar CG, Kumar D, Leach R, Lee JC-M, Li C, Logan KM, Malinverni R, Marques E, Martin WF, Martins NF, Maruyama SR, Mazza R, McLean KL, Medrano JF, Moreno BT, More DD, Muntean CT, Nandakumar HP, Nogueira MFG, Olsaker I, Pant SD, Panzitta F, Pastor RCP, Poli MA, Poslusny N, Rachagani S, Ranganathan S, Razpet A, Riggs PK, Rincon G, Rodriguez-Osorio N, Rodriguez-Zas SL, Romero NE, Rosenwald A, Sando L, Schmutz SM, Shen L, Sherman L, Southey BR, Lutzow YS, Sweedler JV, Tammen I, Telugu BPVL, Urbanski JM, Utsunomiya YT, Verschoor CP, Waardenberg AJ, Wang Z, Ward R, Weikard R, Welsh TH, White SN, Wilming LG, Wunderlich KR, Yang J, Zhao F-Q. The Genome Sequence of Taurine Cattle: A Window to RuminantBiology and Evolution. Science. 2009;324:522–528. doi: 10.1126/science.1169588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed onmotile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 2012;137:339–353. doi: 10.1007/s00418-011-0904-1. [DOI] [PubMed] [Google Scholar]
- Fajac I, Viel M, Sublemontier S, Hubert D, Bienvenu T. Could a defective epithelial sodium channel lead tobronchiectasis. Respir. Res. 2008;9:46. doi: 10.1186/1465-9921-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening andsuppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab. 1997;82:1071–1074. doi: 10.1210/jcem.82.4.3862. [DOI] [PubMed] [Google Scholar]
- Firsov D, Robert-Nicoud M, Gruender S, Schild L, Rossier BC. Mutational analysis of cysteine-rich domains of the epitheliumsodium channel (ENaC). Identification of cysteines essential for channelexpression at the cell surface. J Biol Chem. 1999;274:2743–2749. doi: 10.1074/jbc.274.5.2743. [DOI] [PubMed] [Google Scholar]
- Fischbarg J. Fluid transport across leaky epithelia: central role of the tightjunction and supporting role of aquaporins. Physiol Rev. 2010;90:1271–1290. doi: 10.1152/physrev.00025.2009. [DOI] [PubMed] [Google Scholar]
- Fitch WM. Distinguishing homologous from analogous proteins. Syst. Zool. 1970;19:99–113. [PubMed] [Google Scholar]
- Forslund K, Sonnhammer ELL. Evolution of protein domain architectures. Methods Mol Biol. 2012;856:187–216. doi: 10.1007/978-1-61779-585-5_8. [DOI] [PubMed] [Google Scholar]
- Foxx-Lupo WT, Wheatley CM, Baker SE, Cassuto NA, Delamere NA, Snyder EM. Genetic variation of the alpha subunit of the epithelial Na+channel influences exhaled Na+ in healthy humans. Respir Physiol Neurobiol. 2011;179:205–211. doi: 10.1016/j.resp.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Kitamura K, Adachi M, Miyoshi T, Wakida N, Ura N, Shikano Y, Shinshi Y, Sakamoto K, Hayashi M, Satoh N, Nishitani T, Tomita K, Shimamoto K. Liddle’s syndrome caused by a novel mutation in theproline-rich PY motif of the epithelial sodium channelbeta-subunit. J Clin Endocrinol Metab. 2005;90:340–344. doi: 10.1210/jc.2004-1027. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Miyawaki Y, Abe G. Molecular cloning and functional characterization of the AplysiaFMRFamide-gated Na+ channel. Pflugers Arch. 2006;451:646–656. doi: 10.1007/s00424-005-1498-z. [DOI] [PubMed] [Google Scholar]
- García-Añoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodiumchannels related to degenerins and epithelial sodiumchannels. Proc Natl Acad Sci U S A. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, andregulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Geller DS, Rodriguez-Soriano J, Vallo Boado A, Schifter S, Bayer M, Chang SS, Lifton RP. Mutations in the mineralocorticoid receptor gene cause autosomaldominant pseudohypoaldosteronism type I. Nat Genet. 1998;19:279–281. doi: 10.1038/966. [DOI] [PubMed] [Google Scholar]
- Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, González-Hernández T, Alvarez de la Rosa D. Cloning and functional expression of a new epithelial sodiumchannel delta subunit isoform differentially expressed in neurons of thehuman and monkey telencephalon. J. Neurochem. 2007;102:1304–1315. doi: 10.1111/j.1471-4159.2007.04622.x. [DOI] [PubMed] [Google Scholar]
- Giraldez T, Rojas P, Jou J, Flores C, Alvarez de la Rosa D. The epithelial sodium channel δ-subunit: new notes for anold song. Am J Physiol Renal Physiol. 2012;303:F328–F338. doi: 10.1152/ajprenal.00116.2012. [DOI] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels andP2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed formechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Green RE, Braun EL, Armstrong J, Earl D, Nguyen N, Hickey G, Vandewege MW, St. John JA, Capella-Gutierrez S, Castoe TA, Kern C, Fujita MK, Opazo JC, Jurka J, Kojima KK, Caballero J, Hubley RM, Smit AF, Platt RN, Lavoie CA, Ramakodi MP, Finger JW, Suh A, Isberg SR, Miles L, Chong AY, Jaratlerdsiri W, Gongora J, Moran C, Iriarte A, McCormack J, Burgess SC, Edwards SV, Lyons E, Williams C, Breen M, Howard JT, Gresham CR, Peterson DG, Schmitz J, Pollock DD, Haussler D, Triplett EW, Zhang G, Irie N, Jarvis ED, Brochu CA, Schmidt CJ, McCarthy FM, Faircloth BC, Hoffmann FG, Glenn TC, Gabaldon T, Paten B, Ray DA. Three crocodilian genomes reveal ancestral patterns of evolutionamong archosaurs. Science. 2014;346:1254449–1254449. doi: 10.1126/science.1254449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig ER, Mathialahan T, Boot-Handford RP, Sandle GI. Molecular and functional studies of electrogenic Na(+) transportin the distal colon and rectum of young and elderly subjects. Gut. 2003;52:1607–1615. doi: 10.1136/gut.52.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S, Assmann M. Peptide-gated ion channels and the simple nervous system ofHydra. J Exp Biol. 2015;218:551–561. doi: 10.1242/jeb.111666. [DOI] [PubMed] [Google Scholar]
- Gründer S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies aconserved glycine that is involved in the gating of the epithelial sodiumchannel. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S, Jaeger NF, Gautschi I, Schild L, Rossier BC. Identification of a highly conserved sequence at the N-terminusof the epithelial Na+ channel alpha subunit involved ingating. Pflugers Arch. 1999;438:709–715. doi: 10.1007/s004249900119. [DOI] [PubMed] [Google Scholar]
- Gründer S, Pusch M. Biophysical properties of acid-sensing ion channels(ASICs) Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Gu Y. Effects of [Ca2+]i and pH on epithelial Na+ channel activity ofcultured mouse cortical collecting ducts. J Exp Biol. 2008;211:3167–3173. doi: 10.1242/jeb.019646. [DOI] [PubMed] [Google Scholar]
- Gwiazda K, Bonifacio G, Vullo S, Kellenberger S. Extracellular Subunit Interactions Control Transitions BetweenFunctional States of Acid-sensing Ion Channel 1a. J Biol Chem. 2015 doi: 10.1074/jbc.M115.641688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frøkiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization ofalpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol. 2001;280:F1093–F1106. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- Hannila-Handelberg T, Kontula K, Tikkanen I, Tikkanen T, Fyhrquist F, Helin K, Fodstad H, Piippo K, Miettinen HE, Virtamo J, Krusius T, Sarna S, Gautschi I, Schild L, Hiltunen TP. Common variants of the beta and gamma subunits of the epithelialsodium channel and their relation to plasma renin and aldosterone levels inessential hypertension. BMC Med Genet, BMC medical genetics. 2005;6:4. doi: 10.1186/1471-2350-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channelgamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11:76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A. Type I pseudohypoaldosteronism includes two clinically andgenetically distinct entities with either renal or multiple target organdefects. J Clin Endocrinol Metab. 1991;73:936–944. doi: 10.1210/jcem-73-5-936. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A, Bistritzer T, Rakover Y, Mandelberg A. Pseudohypoaldosteronism with increased sweat and salivaelectrolyte values and frequent lower respiratory tract infections mimickingcystic fibrosis. J Pediatr. 1994;125:752–755. doi: 10.1016/s0022-3476(94)70071-0. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A, Edelheit O, Shriki Y, Gizewska M, Dascal N, Hanukoglu I. Renin-aldosterone response, urinary Na/K ratio and growth inpseudohypoaldosteronism patients with mutations in epithelial sodium channel(ENaC) subunit genes. J Steroid Biochem Mol Biol. 2008;111:268–274. doi: 10.1016/j.jsbmb.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A, Hanukoglu I. Clinical improvement in patients with autosomal recessivepseudohypoaldosteronism and the necessity for saltsupplementation. Clin Exp Nephrol. 2010;14:518–519. doi: 10.1007/s10157-010-0326-8. [DOI] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS. The genome of the Western clawed frog Xenopustropicalis. Science. 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Dickson EJ, Kruse M, Vivas O, Suh B-C. Phosphoinositides regulate ion channels. Biochim Biophys Acta. 2015;1851:844–856. doi: 10.1016/j.bbalip.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen TP, Hannila-Handelberg T, Petäjäniemi N, Kantola I, Tikkanen I, Virtamo J, Gautschi I, Schild L, Kontula K. Liddle’s syndrome associated with a point mutation in theextracellular domain of the epithelial sodium channel gammasubunit. J Hypertens. 2002;20:2383–2390. doi: 10.1097/00004872-200212000-00017. [DOI] [PubMed] [Google Scholar]
- Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cysticfibrosis lung disease? J Physiol. 2013;591:4377–4387. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RJ, Marks JF, Marver D, Frolich JC. Long term observations in a patient withpseudohypoaldosteronism. Pediatr Nephrol. 1991;5:205–210. doi: 10.1007/BF01095953. [DOI] [PubMed] [Google Scholar]
- Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J. Biomed. Biotechnol. 2011;2011:174306. doi: 10.1155/2011/174306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollier JM, Martin DF, Bell DM, Li J-L, Chirachanchai MG, Menon DV, Leonard D, Wu X, Cooper RS, McKenzie C, Victor RG, Auchus RJ. Epithelial sodium channel allele T594M is not associated withblood pressure or blood pressure response to amiloride. Hypertension. 2006;47:428–433. doi: 10.1161/01.HYP.0000200704.45994.ff. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JMC, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin J-J, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anophelesgambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid-sensing ion channels in gastrointestinalfunction. Neuropharmacology. 2015;94:72–79. doi: 10.1016/j.neuropharm.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Park S, Jiménez RHF, Rinehart D, Tamm LK. Role of aromatic side chains in the folding and thermodynamicstability of integral membrane proteins. J. Am. Chem. Soc. 2007;129:8320–8327. doi: 10.1021/ja068849o. [DOI] [PubMed] [Google Scholar]
- Hubé F, Francastel C. Mammalian introns: when the junk generates moleculardiversity. Int. J. Mol. Sci. 2015;16:4429–4452. doi: 10.3390/ijms16034429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependentproteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance inalpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Ilatovskaya DV, Pavlov TS, Neguliyaev YA, Starushchenko A. Mechanisms of epithelial sodium channel (ENaC) regulation bycortactin: involvement of dynamin. Cell Tissue Biol. 2012;6:52–59. [PubMed] [Google Scholar]
- Ishikita H. Proton-binding sites of acid-sensing ion channel1. PLoS One. 2011;6:e16920. doi: 10.1371/journal.pone.0016920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution andlow pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jex AR, Nejsum P, Schwarz EM, Hu L, Young ND, Hall RS, Korhonen PK, Liao S, Thamsborg S, Xia J, Xu P, Wang S, Scheerlinck J-PY, Hofmann A, Sternberg PW, Wang J, Gasser RB. Genome and transcriptome of the porcine whipworm Trichurissuis. Nat Genet. 2014;46:701–706. doi: 10.1038/ng.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H-L, Zhao R-Z, Chen Z-X, Shetty S, Idell S, Matalon S. δ ENaC: a novel divergent amiloride-inhibitable sodiumchannel. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1013–L1026. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the beta- and gamma-ENaC subunits areinvolved in the functional interactions between cystic fibrosistransmembrane conductance regulator and epithelial sodiumchannel. J Biol Chem. 2000;275:27947–27956. doi: 10.1074/jbc.M002848200. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xu J, Chen Y, Shi J, Zhang C, Li J. Expression and distribution of epithelial sodium channel in nasalpolyp and nasal mucosa. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-014-3477-5. [DOI] [PubMed] [Google Scholar]
- Käll L, Krogh A, Sonnhammer ELL. A combined transmembrane topology and signal peptide predictionmethod. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain ofthe epithelial Na+ channel alpha subunit reveals a mechanism of channelactivation by proteases. J Biol Chem, The Journal of biological chemistry. 2011;286:649–660. doi: 10.1074/jbc.M110.167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Blobner BM, Zuzek Z, Tolino M, Kleyman TR. Na+ inhibits the epithelial Na+ channel by binding to a site inan extracellular acidic cleft. J Biol Chem. 2015;290:568–576. doi: 10.1074/jbc.M114.606152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Boyd CR, Argyropoulos C, Okumura S, Hughey RP, Grabe M, Kleyman TR. Allosteric inhibition of the epithelial Na+ channel throughpeptide binding at peripheral finger and thumb domains. J Biol Chem. 2010;285:35216–35223. doi: 10.1074/jbc.M110.167064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Kleyman TR. Epithelial Na+ channel regulation by cytoplasmic andextracellular factors. Exp Cell Res. 2012;318:1011–1019. doi: 10.1016/j.yexcr.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structureof a family member. Am J Physiol Renal Physiol. 2011;301:F684–F696. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Maarouf AB, Kussius C, Denshaw RM, Blumenthal KM, Kleyman TR. Distinct structural elements in the first membrane-spanningsegment of the epithelial sodium channel. J Biol Chem. 2006;281:30455–30462. doi: 10.1074/jbc.M604615200. [DOI] [PubMed] [Google Scholar]
- Kashlan OB, Sheng S, Kleyman TR. On the interaction between amiloride and its putativealpha-subunit epithelial Na+ channel binding site. J Biol Chem. 2005;280:26206–26215. doi: 10.1074/jbc.M503500200. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Weiss KM. Evolutionary genetics of vertebrate tissue mineralization: theorigin and evolution of the secretory calcium-binding phosphoproteinfamily. J. Exp. Zool. B. Mol. Dev. Evol. 2006;306:295–316. doi: 10.1002/jez.b.21088. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channelENaC. J Physiol. 2002;543:413–424. doi: 10.1113/jphysiol.2002.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Schild L. A single point mutation in the pore region of the epithelial Na+channel changes ion selectivity by modifying molecularsieving. Proc Natl Acad Sci U S A. 1999;96:4170–4175. doi: 10.1073/pnas.96.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. International union of basic and clinical pharmacology. XCI.structure, function, and pharmacology of acid-sensing ion channels and theepithelial Na+ channel. Pharmacol. Rev. 2015;67:1–35. doi: 10.1124/pr.114.009225. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: avariety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. Pulmonary epithelial sodium-channel dysfunction and excess airwayliquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- Kim E, Magen A, Ast G. Different levels of alternative splicing amongeukaryotes. Nucleic Acids Res. 2007;35:125–131. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Murphy T, Xia J, Caragea D, Park Y, Beeman RW, Lorenzen MD, Butcher S, Manak JR, Brown SJ. BeetleBase in 2010: revisions to provide comprehensive genomicinformation for Tribolium castaneum. Nucleic Acids Res. 2009;38:D437–D442. doi: 10.1093/nar/gkp807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodiumchannels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of iontransport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Koonin EV. Orthologs, paralogs, and evolutionary genomics. Annu Rev Genet. 2005;39:309–338. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- Krauson AJ, Rued AC, Carattino MD. Independent Contribution of Extracellular Proton Binding Sites toASIC1a Activation. J Biol Chem. 2013;288:34375–34383. doi: 10.1074/jbc.M113.504324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the threepore-forming subunits of the amiloride-sensitive epithelial sodium channelin taste buds of the rat. J Histochem Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- Krueger B, Schlötzer-Schrehardt U, Haerteis S, Zenkel M, Chankiewitz VE, Amann KU, Kruse FE, Korbmacher C. Four subunits (αβγδ) of theepithelial sodium channel (ENaC) are expressed in the human eye in variouslocations. Invest. Ophthalmol. Vis. Sci. 2012;53:596–604. doi: 10.1167/iovs.11-8581. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport viahydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Evolution of the vertebrate jaw from developmentalperspectives. Evol. Dev. 2012;14:76–92. doi: 10.1111/j.1525-142X.2011.00523.x. [DOI] [PubMed] [Google Scholar]
- Kusche-Vihrog K, Tarjus A, Fels J, Jaisser F. The epithelial Na+ channel: a new player in thevasculature. Curr Opin Nephrol Hypertens. 2014;23:143–148. doi: 10.1097/01.mnh.0000441054.88962.2c. [DOI] [PubMed] [Google Scholar]
- Langloh AL, Berdiev B, Ji HL, Keyser K, Stanton BA, Benos DJ. Charged residues in the M2 region of alpha-hENaC play a role inchannel conductance. Am J Physiol Cell Physiol. 2000;278:C277–C291. doi: 10.1152/ajpcell.2000.278.2.C277. [DOI] [PubMed] [Google Scholar]
- Lefèvre CMT, Diakov A, Haerteis S, Korbmacher C, Gründer S, Wiemuth D. Pharmacological and electrophysiological characterization of thehuman bile acid-sensitive ion channel (hBASIC) Pflugers Arch. 2014;466:253–263. doi: 10.1007/s00424-013-1310-4. [DOI] [PubMed] [Google Scholar]
- Lemos B, Bettencourt BR, Meiklejohn CD, Hartl DL. Evolution of proteins and gene expression levels are coupled inDrosophila and are independently associated with mRNA abundance, proteinlength, and number of protein-protein interactions. Mol Biol Evol. 2005;22:1345–1354. doi: 10.1093/molbev/msi122. [DOI] [PubMed] [Google Scholar]
- Li T, Yang Y, Canessa CM. Outlines of the pore in open and closed conformations describethe gating mechanism of ASIC1. Nat. Commun. 2011;2:399. doi: 10.1038/ncomms1409. [DOI] [PubMed] [Google Scholar]
- Li T, Yang Y, Canessa CM. Asn415 in the beta11-beta12 linker decreases proton-dependentdesensitization of ASIC1. J Biol Chem. 2010;285:31285–31291. doi: 10.1074/jbc.M110.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle GW, Bledsoe T, Coppage WSJ. A familial renal disorder simulating primary aldosteronism butwith negligible aldosterone secretion. Trans. Assoc. Am. Phys. 1963;76:199–213. [Google Scholar]
- Lin S-H, Sun W-H, Chen C-C. Genetic exploration of the role of acid-sensing ionchannels. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin C-W, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli K-P, Parker HG, Pollinger JP, Searle SMJ, Sutter NB, Thomas R, Webber C, Baldwin J, Abebe A, Abouelleil A, Aftuck L, Ait-Zahra M, Aldredge T, Allen N, An P, Anderson S, Antoine C, Arachchi H, Aslam A, Ayotte L, Bachantsang P, Barry A, Bayul T, Benamara M, Berlin A, Bessette D, Blitshteyn B, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Brown A, Cahill P, Calixte N, Camarata J, Cheshatsang Y, Chu J, Citroen M, Collymore A, Cooke P, Dawoe T, Daza R, Decktor K, DeGray S, Dhargay N, Dooley K, Dooley K, Dorje P, Dorjee K, Dorris L, Duffey N, Dupes A, Egbiremolen O, Elong R, Falk J, Farina A, Faro S, Ferguson D, Ferreira P, Fisher S, FitzGerald M, Foley K, Foley C, Franke A, Friedrich D, Gage D, Garber M, Gearin G, Giannoukos G, Goode T, Goyette A, Graham J, Grandbois E, Gyaltsen K, Hafez N, Hagopian D, Hagos B, Hall J, Healy C, Hegarty R, Honan T, Horn A, Houde N, Hughes L, Hunnicutt L, Husby M, Jester B, Jones C, Kamat A, Kanga B, Kells C, Khazanovich D, Kieu AC, Kisner P, Kumar M, Lance K, Landers T, Lara M, Lee W, Leger J-P, Lennon N, Leuper L, LeVine S, Liu J, Liu X, Lokyitsang Y, Lokyitsang T, Lui A, Macdonald J, Major J, Marabella R, Maru K, Matthews C, McDonough S, Mehta T, Meldrim J, Melnikov A, Meneus L, Mihalev A, Mihova T, Miller K, Mittelman R, Mlenga V, Mulrain L, Munson G, Navidi A, Naylor J, Nguyen T, Nguyen N, Nguyen C, Nguyen T, Nicol R, Norbu N, Norbu C, Novod N, Nyima T, Olandt P, O’Neill B, O’Neill K, Osman S, Oyono L, Patti C, Perrin D, Phunkhang P, Pierre F, Priest M, Rachupka A, Raghuraman S, Rameau R, Ray V, Raymond C, Rege F, Rise C, Rogers J, Rogov P, Sahalie J, Settipalli S, Sharpe T, Shea T, Sheehan M, Sherpa N, Shi J, Shih D, Sloan J, Smith C, Sparrow T, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Stone S, Sykes S, Tchuinga P, Tenzing P, Tesfaye S, Thoulutsang D, Thoulutsang Y, Topham K, Topping I, Tsamla T, Vassiliev H, Venkataraman V, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Yang S, Yang X, Young G, Yu Q, Zainoun J, Zembek L, Zimmer A, Lander ES. Genome sequence, comparative analysis and haplotype structure ofthe domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Deval E, Lazdunski M. FMRFamide-gated sodium channel and ASIC channels: a new class ofionotropic receptors for FMRFamide and related peptides. Peptides. 2006;27:1138–1152. doi: 10.1016/j.peptides.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+channel. A new channel type with homologies to Caenorhabditis elegansdegenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Liu F, Yang X, Mo X, Huang J, Chen J, Kelly TN, Hixson JE, Rao DC, Gu CC, Shimmin LC, Rice TK, Li J, Schwander K, He J, Liu D-P, Gu D. Associations of epithelial sodium channel genes with bloodpressure: the GenSalt study. J Hum Hypertens. 2015;29:224–228. doi: 10.1038/jhh.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke DP, Hillier LW, Warren WC, Worley KC, Nazareth LV, Muzny DM, Yang S-P, Wang Z, Chinwalla AT, Minx P, Mitreva M, Cook L, Delehaunty KD, Fronick C, Schmidt H, Fulton LA, Fulton RS, Nelson JO, Magrini V, Pohl C, Graves TA, Markovic C, Cree A, Dinh HH, Hume J, Kovar CL, Fowler GR, Lunter G, Meader S, Heger A, Ponting CP, Marques-Bonet T, Alkan C, Chen L, Cheng Z, Kidd JM, Eichler EE, White S, Searle S, Vilella AJ, Chen Y, Flicek P, Ma J, Raney B, Suh B, Burhans R, Herrero J, Haussler D, Faria R, Fernando O, Darré F, Farré D, Gazave E, Oliva M, Navarro A, Roberto R, Capozzi O, Archidiacono N, Della Valle G, Purgato S, Rocchi M, Konkel MK, Walker JA, Ullmer B, Batzer MA, Smit AFA, Hubley R, Casola C, Schrider DR, Hahn MW, Quesada V, Puente XS, Ordoñez GR, López-Otín C, Vinar T, Brejova B, Ratan A, Harris RS, Miller W, Kosiol C, Lawson HA, Taliwal V, Martins AL, Siepel A, Roychoudhury A, Ma X, Degenhardt J, Bustamante CD, Gutenkunst RN, Mailund T, Dutheil JY, Hobolth A, Schierup MH, Ryder OA, Yoshinaga Y, de Jong PJ, Weinstock GM, Rogers J, Mardis ER, Gibbs RA, Wilson RK. Comparative and demographic analysis of orang-utangenomes. Nature. 2011;469:529–533. doi: 10.1038/nature09687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulateschannel internalization, sorting and mobilization from subapicalpool. Traffic. 2007;8:1246–1264. doi: 10.1111/j.1600-0854.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Bolkenius U, Wickert L, Marynen P, Bidlingmaier F. Structural organisation of the gene encoding the alpha-subunit ofthe human amiloride-sensitive epithelial sodium channel. Hum Genet. 1998;102:576–581. doi: 10.1007/s004390050743. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tubecilia. Hum Reprod Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- Ma B-G, Chen L, Ji H-F, Chen Z-H, Yang F-R, Wang L, Qu G, Jiang Y-Y, Ji C, Zhang H-Y. Characters of very ancient proteins. Biochem Biophys Res Commun. 2008;366:607–611. doi: 10.1016/j.bbrc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Macias MJ, Wiesner S, Sudol M. WW and SH3 domains, two different scaffolds to recognizeproline-rich ligands. FEBS Lett. 2002;513:30–37. doi: 10.1016/s0014-5793(01)03290-2. [DOI] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cysticfibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O’Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines differentairway phenotypes in liddle compared with betaENaC-overexpressingmice. J Biol Chem. 2010;285:26945–26955. doi: 10.1074/jbc.M110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinerie L, Pussard E, Foix-L’Hélias L, Petit F, Cosson C, Boileau P, Lombès M. Physiological partial aldosterone resistance in humannewborns. Pediatr Res. 2009;66:323–328. doi: 10.1203/PDR.0b013e3181b1bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gammasubunit proteins in rat kidney. J Clin Invest, The Journal of clinical investigation. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the alpha-subunit of theamiloride-sensitive epithelial sodium channel binds toF-actin. J Biol Chem. 2006;281:6528–6538. doi: 10.1074/jbc.M509386200. [DOI] [PubMed] [Google Scholar]
- McDonald FJ, Price MP, Snyder PM, Welsh MJ. Cloning and expression of the beta- and gamma-subunits of thehuman epithelial sodium channel. Am J Physiol. 1995;268:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- McDonald FJ, Snyder PM, McCray PB, Welsh MJ. Cloning, expression, and tissue distribution of a humanamiloride-sensitive Na+ channel. Am J Physiol. 1994;266:L728–L734. doi: 10.1152/ajplung.1994.266.6.L728. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, Jurka J, Kamal M, Mauceli E, Searle SMJ, Sharpe T, Baker ML, Batzer MA, Benos PV, Belov K, Clamp M, Cook A, Cuff J, Das R, Davidow L, Deakin JE, Fazzari MJ, Glass JL, Grabherr M, Greally JM, Gu W, Hore TA, Huttley GA, Kleber M, Jirtle RL, Koina E, Lee JT, Mahony S, Marra MA, Miller RD, Nicholls RD, Oda M, Papenfuss AT, Parra ZE, Pollock DD, Ray DA, Schein JE, Speed TP, Thompson K, VandeBerg JL, Wade CM, Walker JA, Waters PD, Webber C, Weidman JR, Xie X, Zody MC, Graves JAM, Ponting CP, Breen M, Samollow PB, Lander ES, Lindblad-Toh K. Genome of the marsupial Monodelphis domestica reveals innovationin non-coding sequences. Nature. 2007;447:167–177. doi: 10.1038/nature05805. [DOI] [PubMed] [Google Scholar]
- Miller RL, Loewy AD. ENaC γ-expressing astrocytes in the circumventricularorgans, white matter, and ventral medullary surface: sites for Na+regulation by glial cells. J. Chem. Neuroanat. 2013;53:72–80. doi: 10.1016/j.jchemneu.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, Hayes VM, Ratan A, Petersen DC, Wittekindt NE, Miller J, Walenz B, Knight J, Qi J, Zhao F, Wang Q, Bedoya-Reina OC, Katiyar N, Tomsho LP, Kasson LM, Hardie R-A, Woodbridge P, Tindall EA, Bertelsen MF, Dixon D, Pyecroft S, Helgen KM, Lesk AM, Pringle TH, Patterson N, Zhang Y, Kreiss A, Woods GM, Jones ME, Schuster SC. Genetic diversity and population structure of the endangeredmarsupial Sarcophilus harrisii (Tasmanian devil) Proc Natl Acad Sci U S A. 2011;108:12348–12353. doi: 10.1073/pnas.1102838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in theocean? PLoS Biol. 2011;9:e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LM, DeGagne JM, Kempton JB, Hausman F, Trune DR. Mouse middle ear ion homeostasis channels and intercellularjunctions. PLoS One. 2012;7:e39004. doi: 10.1371/journal.pone.0039004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for theinvestigation of sequences and structures. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- Mutesa L, Azad AK, Verhaeghe C, Segers K, Vanbellinghen JF, Ngendahayo L, Rusingiza EK, Mutwa PR, Rulisa S, Koulischer L, Cassiman JJ, Cuppens H, Bours V. Genetic analysis of Rwandan patients with cystic fibrosis-likesymptoms: identification of novel cystic fibrosis transmembrane conductanceregulator and epithelial sodium channel gene variants. Chest. 2009;135:1233–1242. doi: 10.1378/chest.08-2246. [DOI] [PubMed] [Google Scholar]
- Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R. CFTR fails to inhibit the epithelial sodium channel ENaCexpressed in Xenopus laevis oocytes. J Physiol. 2005;564:671–682. doi: 10.1113/jphysiol.2004.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KB, Deerfield DWII. GeneDoc: Analysis and visualization of geneticvariation. EMBnew.News. 1997;4:14. [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJP, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization andintegration with experimental proteomic data. Bioinformatics. 2014;30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- Omerbašić D, Schuhmacher L-N, Bernal Sierra Y-A, Smith ESJ, Lewin GR. ASICs and mammalian mechanoreceptor function. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Ottaviani E, Franchini A, Mandrioli M, Saxena A, Hanukoglu A, Hanukoglu I. Amiloride-sensitive epithelial sodium channel subunits areexpressed in human and mussel immunocytes. Dev. Comp. Immunol. 2002;26:395–402. doi: 10.1016/s0145-305x(01)00097-0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and Molecular Aspects of Hypertension. Circ Res. 2015;116:937–959. doi: 10.1161/CIRCRESAHA.116.303647. [DOI] [PubMed] [Google Scholar]
- Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of theepithelial sodium channel. Am J Physiol Renal Physiol, American journal of physiology. Renalphysiology. 2010;299:F854–F861. doi: 10.1152/ajprenal.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passero CJ, Mueller GM, Myerburg MM, Carattino MD, Hughey RP, Kleyman TR. TMPRSS4-dependent activation of the epithelial sodium channelrequires cleavage of the gamma subunit distal to the furin cleavagesite. Am J Physiol Renal Physiol, American journal of physiology. Renalphysiology. 2011 doi: 10.1152/ajprenal.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Nachannel. Am J Physiol Renal Physiol. 2012;303:F540–F550. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak BG, Shaughnessy JD, Meneton P, Greeb J, Shull GE, Jenkins NA, Copeland NG. Mouse chromosomal location of three epithelial sodium channelsubunit genes and an apical sodium chloride cotransportergene. Genomics. 1996;33:124–127. doi: 10.1006/geno.1996.0168. [DOI] [PubMed] [Google Scholar]
- Persu A, Barbry P, Bassilana F, Houot AM, Mengual R, Lazdunski M, Corvol P, Jeunemaitre X. Genetic analysis of the beta subunit of the epithelial Na+channel in essential hypertension. Hypertension. 1998;32:129–137. doi: 10.1161/01.hyp.32.1.129. [DOI] [PubMed] [Google Scholar]
- Petryszak R, Burdett T, Fiorelli B, Fonseca NA, Gonzalez-Porta M, Hastings E, Huber W, Jupp S, Keays M, Kryvych N, McMurry J, Marioni JC, Malone J, Megy K, Rustici G, Tang AY, Taubert J, Williams E, Mannion O, Parkinson HE, Brazma A. Expression Atlas update--a database of gene and transcriptexpression from microarray- and sequencing-based functional genomicsexperiments. Nucleic Acids Res. 2014;42:D926–D932. doi: 10.1093/nar/gkt1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel byphosphatidylinositides. Curr Opin Nephrol Hypertens. 2008;17:533–540. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol3,4,5-trisphosphate binding site in the epithelial Na+channel. J Biol Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation ofthe epithelial Na+ channel. J Gen Physiol. 2007;130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogozheva ID, Mosberg HI, Lomize AL. Life at the border: adaptation of proteins to anisotropicmembrane environment. Protein Sci. 2014;23:1165–1196. doi: 10.1002/pro.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius JU, Mullikin JC, Smith DR, Lindblad-Toh K, Gnerre S, Clamp M, Chang J, Stephens R, Neelam B, Volfovsky N, Schäffer AA, Agarwala R, Narfström K, Murphy WJ, Giger U, Roca AL, Antunes A, Menotti-Raymond M, Yuhki N, Pecon-Slattery J, Johnson WE, Bourque G, Tesler G, O’Brien SJ. Initial sequence and comparative analysis of the catgenome. Genome Res. 2007;17:1675–1689. doi: 10.1101/gr.6380007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Kershaw M, Kirk J, Trevelyan N, Shaw NJ. The use of sodium resonium inpseudohypoaldosteronism. Arch Dis Child. 2003;88:1138–1139. doi: 10.1136/adc.88.12.1138-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touchsensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection ofcutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, De Silva N, Velarde GS, Anderson TJC, Clark RC, Davidson C, Dillon GP, Holroyd NE, LoVerde PT, Lloyd C, McQuillan J, Oliveira G, Otto TD, Parker-Manuel SJ, Quail MA, Wilson RA, Zerlotini A, Dunne DW, Berriman M. A systematically improved high quality genome and transcriptomeof the human blood fluke Schistosoma mansoni. PLoS Negl. Trop. Dis. 2012;6:e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DEK, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu J-K, Benito-Gutiérrez EL, Dubchak I, Garcia-Fernàndez J, Gibson-Brown JJ, Grigoriev IV, Horton AC, de Jong PJ, Jurka J, Kapitonov VV, Kohara Y, Kuroki Y, Lindquist E, Lucas S, Osoegawa K, Pennacchio LA, Salamov AA, Satou Y, Sauka-Spengler T, Schmutz J, Shin-I T, Toyoda A, Bronner-Fraser M, Fujiyama A, Holland LZ, Holland PWH, Satoh N, Rokhsar DS. The amphioxus genome and the evolution of the chordatekaryotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Ramos MD, Trujillano D, Olivar R, Sotillo F, Ossowski S, Manzanares J, Costa J, Gartner S, Oliva C, Quintana E, Gonzalez MI, Vazquez C, Estivill X, Casals T. Extensive sequence analysis of CFTR, SCNN1A, SCNN1B, SCNN1G andSERPINA1 suggests an oligogenic basis for cystic fibrosis-likephenotypes. Clin. Genet. 2014;86:91–95. doi: 10.1111/cge.12234. [DOI] [PubMed] [Google Scholar]
- Rauh R, Soell D, Haerteis S, Diakov A, Nesterov V, Krueger B, Sticht H, Korbmacher C. A mutation in the β-subunit of ENaC identified in apatient with cystic fibrosis-like symptoms has a gain-of-functioneffect. Am J Physiol Lung Cell Mol Physiol. 2013;304:L43–L55. doi: 10.1152/ajplung.00093.2012. [DOI] [PubMed] [Google Scholar]
- Rayner BL, Owen EP, King JA, Soule SG, Vreede H, Opie LH, Marais D, Davidson JS. A new mutation, R563Q, of the beta subunit of the epithelialsodium channel associated with low-renin, low-aldosteronehypertension. J Hypertens. 2003;21:921–926. doi: 10.1097/00004872-200305000-00016. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Stutts MJ. Status of fluid and electrolyte absorption in cysticfibrosis. Cold Spring Harb. Perspect. Med. 2013;3:a009555. doi: 10.1101/cshperspect.a009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree MB, Papenfuss AT, Deakin JE, Lindsay J, Heider T, Belov K, Rens W, Waters PD, Pharo EA, Shaw G, Wong ESW, Lefèvre CM, Nicholas KR, Kuroki Y, Wakefield MJ, Zenger KR, Wang C, Ferguson-Smith M, Nicholas FW, Hickford D, Yu H, Short KR, Siddle HV, Frankenberg SR, Chew KY, Menzies BR, Stringer JM, Suzuki S, Hore TA, Delbridge ML, Patel HR, Mohammadi A, Schneider NY, Hu Y, O’Hara W, Al Nadaf S, Wu C, Feng Z-P, Cocks BG, Wang J, Flicek P, Searle SMJ, Fairley S, Beal K, Herrero J, Carone DM, Suzuki Y, Sugano S, Toyoda A, Sakaki Y, Kondo S, Nishida Y, Tatsumoto S, Mandiou I, Hsu A, McColl KA, Lansdell B, Weinstock G, Kuczek E, McGrath A, Wilson P, Men A, Hazar-Rethinam M, Hall A, Davis J, Wood D, Williams S, Sundaravadanam Y, Muzny DM, Jhangiani SN, Lewis LR, Morgan MB, Okwuonu GO, Ruiz SJ, Santibanez J, Nazareth L, Cree A, Fowler G, Kovar CL, Dinh HH, Joshi V, Jing C, Lara F, Thornton R, Chen L, Deng J, Liu Y, Shen JY, Song X-Z, Edson J, Troon C, Thomas D, Stephens A, Yapa L, Levchenko T, Gibbs RA, Cooper DW, Speed TP, Fujiyama A, Graves JAM, O’Neill RJ, Pask AJ, Forrest SM, Worley KC. Genome sequence of an Australian kangaroo, Macropus eugenii,provides insight into the evolution of mammalian reproduction anddevelopment. Genome Biol. 2011;12:R81. doi: 10.1186/gb-2011-12-8-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepe FG, van Bemmelen MX, Cachat F, Plendl H, Gautschi I, Krone N, Holterhus PM, Theintz G, Schild L. Revealing a subclinical salt-losing phenotype in heterozygouscarriers of the novel S562P mutation in the alpha subunit of the epithelialsodium channel. Clin Endocrinol (Oxf) 2009;70:252–258. doi: 10.1111/j.1365-2265.2008.03314.x. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Physiology of airway mucus secretion and pathophysiology ofhypersecretion. Respir. Care. 2007;52:1134–1146. discussion 1146–9. [PubMed] [Google Scholar]
- Rossier BC. Epithelial sodium channel (ENaC) and the control of bloodpressure. Curr Opin Pharmacol. 2014;15:33–46. doi: 10.1016/j.coph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: thestory of our internal environment revisited. Physiol Rev. 2015;95:297–340. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance:interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serineproteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- Rotin D. Role of the UPS in Liddle syndrome. BMC Biochem. 2008;9(Suppl 1) doi: 10.1186/1471-2091-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Bar-Sagi D, O’Brodovich H, Merilainen J, Lehto VP, Canessa CM, Rossier BC, Downey GP. An SH3 binding region in the epithelial Na+ channel (alpha rENaC)mediates its localization at the apical membrane. EMBO J. 1994;13:4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Staub O. Role of the ubiquitin system in regulating iontransport. Pflugers Arch. 2011;461:1–21. doi: 10.1007/s00424-010-0893-2. [DOI] [PubMed] [Google Scholar]
- Ruan YC, Chen H, Chan HC. Ion channels in the endometrium: regulation of endometrialreceptivity and embryo implantation. Hum Reprod Update. 2014;20:517–529. doi: 10.1093/humupd/dmu006. [DOI] [PubMed] [Google Scholar]
- Ruth JL, Wassner SJ. Body composition: salt and water. Pediatr Rev. 2006;27:181–187. doi: 10.1542/pir.27-5-181. [DOI] [PubMed] [Google Scholar]
- Saar K, Beck A, Bihoreau M-T, Birney E, Brocklebank D, Chen Y, Cuppen E, Demonchy S, Dopazo J, Flicek P, Foglio M, Fujiyama A, Gut IG, Gauguier D, Guigo R, Guryev V, Heinig M, Hummel O, Jahn N, Klages S, Kren V, Kube M, Kuhl H, Kuramoto T, Kuroki Y, Lechner D, Lee Y-A, Lopez-Bigas N, Lathrop GM, Mashimo T, Medina I, Mott R, Patone G, Perrier-Cornet J-A, Platzer M, Pravenec M, Reinhardt R, Sakaki Y, Schilhabel M, Schulz H, Serikawa T, Shikhagaie M, Tatsumoto S, Taudien S, Toyoda A, Voigt B, Zelenika D, Zimdahl H, Hubner N. SNP and haplotype mapping for genetic analysis in therat. Nat Genet. 2008;40:560–566. doi: 10.1038/ng.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleh N, Baines DL, Naftalin RJ, Milligan SR. The hormonal control of uterine luminal fluid secretion andabsorption. J Membr Biol, The Journal of membrane biology. 2005;206:17–28. doi: 10.1007/s00232-005-0770-7. [DOI] [PubMed] [Google Scholar]
- Samaha FFFF, Rubenstein RCRC, Yan W, Ramkumar M, Levy DIDI, Ahn YJYJ, Sheng S, Kleyman TRTR. Functional polymorphism in the carboxyl terminus of thealpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Yui N, Noda Y. Actin directly interacts with different membrane channel proteinsand influences channel activities: AQP2 as a model. Biochim Biophys Acta. 2014;1838:514–520. doi: 10.1016/j.bbamem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammaliancilia. Annu Rev Physiol, Annual review of physiology. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Saxena A, Hanukoglu I, Saxena D, Thompson RJ, Gardiner RM, Hanukoglu A. Novel mutations responsible for autosomal recessive multisystempseudohypoaldosteronism and sequence variants in epithelial sodium channelalpha-, beta-, and gamma-subunit genes. J Clin Endocrinol Metab. 2002;87:3344–3350. doi: 10.1210/jcem.87.7.8674. [DOI] [PubMed] [Google Scholar]
- Saxena A, Hanukoglu I, Strautnieks SS, Thompson RJ, Gardiner RM, Hanukoglu A. Gene structure of the human amiloride-sensitive epithelial sodiumchannel beta subunit. Biochem Biophys Res Commun. 1998;252:208–213. doi: 10.1006/bbrc.1998.9625. [DOI] [PubMed] [Google Scholar]
- Scally A, Dutheil JY, Hillier LW, Jordan GE, Goodhead I, Herrero J, Hobolth A, Lappalainen T, Mailund T, Marques-Bonet T, McCarthy S, Montgomery SH, Schwalie PC, Tang YA, Ward MC, Xue Y, Yngvadottir B, Alkan C, Andersen LN, Ayub Q, Ball EV, Beal K, Bradley BJ, Chen Y, Clee CM, Fitzgerald S, Graves TA, Gu Y, Heath P, Heger A, Karakoc E, Kolb-Kokocinski A, Laird GK, Lunter G, Meader S, Mort M, Mullikin JC, Munch K, O’Connor TD, Phillips AD, Prado-Martinez J, Rogers AS, Sajjadian S, Schmidt D, Shaw K, Simpson JT, Stenson PD, Turner DJ, Vigilant L, Vilella AJ, Whitener W, Zhu B, Cooper DN, de Jong P, Dermitzakis ET, Eichler EE, Flicek P, Goldman N, Mundy NI, Ning Z, Odom DT, Ponting CP, Quail MA, Ryder OA, Searle SM, Warren WC, Wilson RK, Schierup MH, Rogers J, Tyler-Smith C, Durbin R. Insights into hominid evolution from the gorilla genomesequence. Nature. 2012;483:169–175. doi: 10.1038/nature10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedel C, Marthinsen L, Kristoffersson AC, Kornfalt R, Nilsson KO, Orlenius B, Holmberg L. Lung symptoms in pseudohypoaldosteronism type 1 are associatedwith deficiency of the alpha-subunit of the epithelial sodiumchannel. J Pediatr. 1999;135:739–745. doi: 10.1016/s0022-3476(99)70094-6. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Sakai H, Mattei M, Lazdunski M, Lingueglia E. Molecular cloning, functional expression and chromosomallocalization of an amiloride-sensitive Na(+) channel from human smallintestine. FEBS Lett. 2000;471:205–210. doi: 10.1016/s0014-5793(00)01403-4. [DOI] [PubMed] [Google Scholar]
- Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial Na channelsubunits as a target sequence for mutations causing channel activation foundin Liddle syndrome. EMBO J. 1996;15:2381–2387. [PMC free article] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, andgamma subunits of the epithelial sodium channel (ENaC) involved in amilorideblock and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relievingNa+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–F1496. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelialsodium channel. J Biol Chem. 2000;275:8572–8581. doi: 10.1074/jbc.275.12.8572. [DOI] [PubMed] [Google Scholar]
- Sheng S, Li J, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial sodium channel pore region. structure and role ingating. J Biol Chem. 2001a;276:1326–1334. doi: 10.1074/jbc.M008117200. [DOI] [PubMed] [Google Scholar]
- Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of theepithelial Na+ channel in Na+ self-inhibition. J Biol Chem. 2007;282:20180–20190. doi: 10.1074/jbc.M611761200. [DOI] [PubMed] [Google Scholar]
- Sheng S, McNulty KA, Harvey JM, Kleyman TR. Second transmembrane domains of ENaC subunits contribute to ionpermeation and selectivity. J Biol Chem. 2001b;276:44091–44098. doi: 10.1074/jbc.M108522200. [DOI] [PubMed] [Google Scholar]
- Sheridan MB, Fong P, Groman JD, Conrad C, Flume P, Diaz R, Harris C, Knowles M, Cutting GR. Mutations in the beta-subunit of the epithelial Na+ channel inpatients with a cystic fibrosis-like syndrome. Hum Mol Genet. 2005;14:3493–3498. doi: 10.1093/hmg/ddi374. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ionchannels. Am J Physiol Cell Physiol. 2012;303:C699–C710. doi: 10.1152/ajpcell.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitatedby an ERK-mediated phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- Shi S, Carattino MD, Kleyman TR. Role of the wrist domain in the response of the epithelial sodiumchannel to external stimuli. J Biol Chem. 2012;287:44027–44035. doi: 10.1074/jbc.M112.421743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Ghosh DD, Okumura S, Carattino MD, Kashlan OB, Sheng S, Kleyman TR. Base of the thumb domain modulates epithelial sodium channelgating. J Biol Chem, The Journal of biological chemistry. 2011;286:14753–14761. doi: 10.1074/jbc.M110.191734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura N, Ohkuri T, Sadamitsu C, Yasumatsu K, Yoshida R, Beauchamp GK, Bachmanov AA, Ninomiya Y. Amiloride-sensitive NaCl taste responses are associated withgenetic variation of ENaC alpha-subunit in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R66–R75. doi: 10.1152/ajpregu.00420.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM, Donoghue PCJ. Evolutionary crossroads in developmental biology: cyclostomes(lamprey and hagfish) Development. 2012;139:2091–2099. doi: 10.1242/dev.074716. [DOI] [PubMed] [Google Scholar]
- Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Ulick S, Milora RV, Findling JW. Liddle’s syndrome: heritable human hypertension caused bymutations in the beta subunit of the epithelial sodiumchannel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Sillitoe I, Lewis TE, Cuff A, Das S, Ashford P, Dawson NL, Furnham N, Laskowski RA, Lee D, Lees JG, Lehtinen S, Studer RA, Thornton J, Orengo CA. CATH: comprehensive structural and functional annotations forgenome sequences. Nucleic Acids Res. 2015;43:D376–D381. doi: 10.1093/nar/gku947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakov O, Marletaz F, Cho S-J, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo D-H, Larsson T, Lv J, Arendt D, Savage R, Osoegawa K, de Jong P, Grimwood J, Chapman JA, Shapiro H, Aerts A, Otillar RP, Terry AY, Boore JL, Grigoriev IV, Lindberg DR, Seaver EC, Weisblat DA, Putnam NH, Rokhsar DS. Insights into bilaterian evolution from three spiraliangenomes. Nature. 2013;493:526–531. doi: 10.1038/nature11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor tomechanotransduction in mammalian muscle spindles. J Physiol. 2010;588:171–185. doi: 10.1113/jphysiol.2009.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, Morgan JR, Buxbaum JD, Sachidanandam R, Sims C, Garruss AS, Cook M, Krumlauf R, Wiedemann LM, Sower SA, Decatur WA, Hall JA, Amemiya CT, Saha NR, Buckley KM, Rast JP, Das S, Hirano M, McCurley N, Guo P, Rohner N, Tabin CJ, Piccinelli P, Elgar G, Ruffier M, Aken BL, Searle SMJ, Muffato M, Pignatelli M, Herrero J, Jones M, Brown CT, Chung-Davidson Y-W, Nanlohy KG, Libants SV, Yeh C-Y, McCauley DW, Langeland JA, Pancer Z, Fritzsch B, de Jong PJ, Zhu B, Fulton LL, Theising B, Flicek P, Bronner ME, Warren WC, Clifton SW, Wilson RK, Li W. Sequencing of the sea lamprey (Petromyzon marinus) genomeprovides insights into vertebrate evolution. Nat Genet. 2013;45:415–421. 421e1–421e2. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodiumchannel. J Biol Chem. 1994;269:24379–24383. [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na(+) channels. J Biol Chem. 1999;274:28484–28490. doi: 10.1074/jbc.274.40.28484. [DOI] [PubMed] [Google Scholar]
- Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators inhypertension. J Biol Chem, The Journal of biological chemistry. 2010;285:30363–30369. doi: 10.1074/jbc.R110.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springauf A, Bresenitz P, Gründer S. The interaction between two extracellular linker regions controlssustained opening of acid-sensing ion channel 1. J Biol Chem. 2011;286:24374–24384. doi: 10.1074/jbc.M111.230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J. 2005;88:3966–3975. doi: 10.1529/biophysj.104.056804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic force microscopy reveals the architecture of theepithelial sodium channel (ENaC) J Biol Chem. 2011;286:31944–31952. doi: 10.1074/jbc.M111.275289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by theacid-sensing ion channel 1 structure. IUBMB Life. 2008;60:620–628. doi: 10.1002/iub.89. [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Thompson RJ, Hanukoglu A, Dillon MJ, Hanukoglu I, Kuhnle U, Seckl J, Gardiner RM, Chung E. Localisation of pseudohypoaldosteronism genes to chromosome16p12.2–13.11 and 12p13.1-pter by homozygositymapping. Hum Mol Genet. 1996;5:293–299. doi: 10.1093/hmg/5.2.293. [DOI] [PubMed] [Google Scholar]
- Studer RA, Person E, Robinson-Rechavi M, Rossier BC. Evolution of the epithelial sodium channel and the sodium pump aslimiting factors of aldosterone action on sodium transport. Physiol Genomics. 2011;43:844–854. doi: 10.1152/physiolgenomics.00002.2011. [DOI] [PubMed] [Google Scholar]
- Su YR, Menon AG. Epithelial sodium channels and hypertension. Drug Metab Dispos. 2001;29:553–556. [PubMed] [Google Scholar]
- Sugiyama T, Kato N, Ishinaga Y, Yamori Y, Yazaki Y. Evaluation of selected polymorphisms of the Mendelianhypertensive disease genes in the Japanese population. Hypertens. Res. 2001;24:515–521. doi: 10.1291/hypres.24.515. [DOI] [PubMed] [Google Scholar]
- Szabo R, Bugge TH. Membrane-anchored serine proteases in vertebrate cell anddevelopmental biology. Annu Rev Cell Dev Biol. 2011;27:213–235. doi: 10.1146/annurev-cellbio-092910-154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T, Ebata T, Noguchi H, Keane TM, Adams DJ, Narita T, Shin-I T, Fujisawa H, Toyoda A, Abe K, Obata Y, Sakaki Y, Moriwaki K, Fujiyama A, Kohara Y, Shiroishi T. The ancestor of extant Japanese fancy mice contributed to themosaic genomes of classical inbred strains. Genome Res. 2013;23:1329–1238. doi: 10.1101/gr.156497.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y. Comparative physiology of body fluid regulation in vertebrateswith special reference to thirst regulation. Jpn J Physiol, The Japanese journal of physiology. 2000;50:171–186. doi: 10.2170/jjphysiol.50.171. [DOI] [PubMed] [Google Scholar]
- Tapolyai M, Uysal A, Dossabhoy NR, Zsom L, Szarvas T, Lengvárszky Z, Fülöp T. High prevalence of liddle syndrome phenotype among hypertensiveUS Veterans in Northwest Louisiana. J Clin Hypertens (Greenwich) 2010;12:856–860. doi: 10.1111/j.1751-7176.2010.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Doggett NA, Fisher R, Stokes JB. Genomic organization and the 5’ flanking region of thegamma subunit of the human amiloride-sensitive epithelial sodiumchannel. J Biol Chem. 1996;271:26062–26066. doi: 10.1074/jbc.271.42.26062. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Loftus RW, Liu KZ, Itani OA. Genomic organization of the 5’ end of human beta-ENaC andpreliminary characterization of its promoter. Am J Physiol Renal Physiol. 2002;282:F898–F909. doi: 10.1152/ajprenal.00268.2001. [DOI] [PubMed] [Google Scholar]
- Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker SW. Whales of the world. E. J. Brill. 1988 [Google Scholar]
- Toka HR, Koshy JM, Hariri A. The molecular basis of blood pressure variation. Pediatr Nephrol. 2013;28:387–99. doi: 10.1007/s00467-012-2206-9. [DOI] [PubMed] [Google Scholar]
- Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ionchannel 1a. J Biol Chem. 2011;286:16297–16307. doi: 10.1074/jbc.M110.202366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the humanepithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–F827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- Tousson A, Alley CD, Sorscher EJ, Brinkley BR, Benos DJ. Immunochemical localization of amiloride-sensitive sodiumchannels in sodium-transporting epithelia. J Cell Sci, Journal of cell science. 1989;93(Pt 2):349–362. doi: 10.1242/jcs.93.2.349. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Konno N, Shibuya S, Nogami S. Cloning and expression of the epithelial sodium channel and itsrole in osmoregulation of aquatic and estivating African lungfishProtopterus annectens. Comp Biochem Physiol A Mol Integr Physiol. 2014;183:1–8. doi: 10.1016/j.cbpa.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Maejima S, Yoshie S, Kubo Y, Konno N, Joss JMP. The epithelial sodium channel in the Australian lungfish,Neoceratodus forsteri (Osteichthyes: Dipnoi) Proc. Biol. Sci. 2012;279:4795–4802. doi: 10.1098/rspb.2012.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniprot. UniProt: a hub for protein information. Nucleic Acids Res. 2014;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitivesodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Kirkness EF, Loh Y-H, Halpern AL, Lee AP, Johnson J, Dandona N, Viswanathan LD, Tay A, Venter JC, Strausberg RL, Brenner S. Survey sequencing and comparative analysis of the elephant shark(Callorhinchus milii) genome. PLoS Biol. 2007;5:e101. doi: 10.1371/journal.pbio.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel M, Leroy C, Hubert D, Fajac I, Bienvenu T. ENaCbeta and gamma genes as modifier genes in cysticfibrosis. J Cyst Fibros. 2008;7:23–29. doi: 10.1016/j.jcf.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Vilella AJ, Severin J, Ureta-Vidal A, Heng L, Durbin R, Birney E. EnsemblCompara GeneTrees: Complete, duplication-awarephylogenetic trees in vertebrates. Genome Res. 2009;19:327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, Bassilana F, Mignon C, Merscher S, Mattéi MG, Carle GF, Lazdunski M, Barbry P. Cloning, chromosomal localization, and physical linkage of thebeta and gamma subunits (SCNN1B and SCNN1G) of the human epithelialamiloride-sensitive sodium channel. Genomics. 1995;28:560–565. doi: 10.1006/geno.1995.1188. [DOI] [PubMed] [Google Scholar]
- Voilley N, Lingueglia E, Champigny G, Mattéi MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties,pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci U S A. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk KA, Husted RF, Snyder PM, Stokes JB. Kinase regulation of hENaC mediated through a region in theCOOH-terminal portion of the alpha-subunit. Am J Physiol Cell Physiol. 2000;278:C1047–C1054. doi: 10.1152/ajpcell.2000.278.5.C1047. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction. Hydrophobicity analysisand the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-boundchannel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- andglucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CM, Giulotto E, Sigurdsson S, Zoli M, Gnerre S, Imsland F, Lear TL, Adelson DL, Bailey E, Bellone RR, Blöcker H, Distl O, Edgar RC, Garber M, Leeb T, Mauceli E, MacLeod JN, Penedo MCT, Raison JM, Sharpe T, Vogel J, Andersson L, Antczak DF, Biagi T, Binns MM, Chowdhary BP, Coleman SJ, Della Valle G, Fryc S, Guérin G, Hasegawa T, Hill EW, Jurka J, Kiialainen A, Lindgren G, Liu J, Magnani E, Mickelson JR, Murray J, Nergadze SG, Onofrio R, Pedroni S, Piras MF, Raudsepp T, Rocchi M, Røed KH, Ryder OA, Searle S, Skow L, Swinburne JE, Syvänen AC, Tozaki T, Valberg SJ, Vaudin M, White JR, Zody MC, Lander ES, Lindblad-Toh K. Genome sequence, comparative analysis, and population genetics ofthe domestic horse. Science. 2009;326:865–867. doi: 10.1126/science.1178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novelamiloride-sensitive Na+ channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEGfamily of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu T, Yin L, Li J, Yu L, Shen Y, Yu Y, Shen Y, Fu Q. Novel mutations in the SCNN1A gene causingPseudohypoaldosteronism type 1. PLoS One. 2013;8:e65676. doi: 10.1371/journal.pone.0065676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-P, Yang K-Q, Jiang X-J, Wu H-Y, Zhang H-M, Zou Y-B, Song L, Bian J, Hui R-T, Liu Y-X, Zhou X-L. Prevalence of Liddle Syndrome Among Young Hypertension Patientsof Undetermined Cause in a Chinese Population. J Clin Hypertens (Greenwich) 2015 doi: 10.1111/jch.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Schultz BD. Cholera toxin enhances Na(+) absorption across MCF10A humanmammary epithelia. Am J Physiol Cell Physiol. 2014;306:C471–C484. doi: 10.1152/ajpcell.00181.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, He G, Yang Y, Liu Y, Diao R, Sheng K, Liu X, Xu W. Reduced expression of Enac in Placenta tissues of patients withsevere preeclampsia is related to compromised trophoblastic cell migrationand invasion during pregnancy. PLoS One. 2013;8:e72153. doi: 10.1371/journal.pone.0072153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pascual-Anaya J, Zadissa A, Li W, Niimura Y, Huang Z, Li C, White S, Xiong Z, Fang D, Wang B, Ming Y, Chen Y, Zheng Y, Kuraku S, Pignatelli M, Herrero J, Beal K, Nozawa M, Li Q, Wang J, Zhang H, Yu L, Shigenobu S, Wang J, Liu J, Flicek P, Searle S, Wang J, Kuratani S, Yin Y, Aken B, Zhang G, Irie N. The draft genomes of soft-shell turtle and green sea turtle yieldinsights into the development and evolution of the turtle-specific bodyplan. Nat Genet. 2013;45:701–706. doi: 10.1038/ng.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong L, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TAF, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin Y-C, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backström N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Völker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AFA, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu W, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang S-P, Li X, Graves T, Fulton L, Nelson J, Chinwalla A, Hou S, Mardis ER, Wilson RK. The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grützner F, Belov K, Miller W, Clarke L, Chinwalla AT, Yang S-P, Heger A, Locke DP, Miethke P, Waters PD, Veyrunes F, Fulton L, Fulton B, Graves T, Wallis J, Puente XS, López-Otín C, Ordóñez GR, Eichler EE, Chen L, Cheng Z, Deakin JE, Alsop A, Thompson K, Kirby P, Papenfuss AT, Wakefield MJ, Olender T, Lancet D, Huttley GA, Smit AFA, Pask A, Temple-Smith P, Batzer MA, Walker JA, Konkel MK, Harris RS, Whittington CM, Wong ESW, Gemmell NJ, Buschiazzo E, Vargas Jentzsch IM, Merkel A, Schmitz J, Zemann A, Churakov G, Kriegs JO, Brosius J, Murchison EP, Sachidanandam R, Smith C, Hannon GJ, Tsend-Ayush E, McMillan D, Attenborough R, Rens W, Ferguson-Smith M, Lefèvre CM, Sharp JA, Nicholas KR, Ray DA, Kube M, Reinhardt R, Pringle TH, Taylor J, Jones RC, Nixon B, Dacheux J-L, Niwa H, Sekita Y, Huang X, Stark A, Kheradpour P, Kellis M, Flicek P, Chen Y, Webber C, Hardison R, Nelson J, Hallsworth-Pepin K, Delehaunty K, Markovic C, Minx P, Feng Y, Kremitzki C, Mitreva M, Glasscock J, Wylie T, Wohldmann P, Thiru P, Nhan MN, Pohl CS, Smith SM, Hou S, Nefedov M, de Jong PJ, Renfree MB, Mardis ER, Wilson RK. Genome analysis of the platypus reveals unique signatures ofevolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor andanalysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel M, Akin L, Büscher A, Güran TP, Hauffa B, Högler W, Leonards J, Karges B, Kentrup H, Kirel B, Senses EEY, Tekin N, Holterhus P-M, Riepe FG. Five novel mutations in the SCNN1A gene causing autosomalrecessive pseudohypoaldosteronism type 1. Eur J Endocrinol. 2013;168:707–715. doi: 10.1530/EJE-12-1000. [DOI] [PubMed] [Google Scholar]
- Winarski KL, Sheng N, Chen J, Kleyman TR, Sheng S. Extracellular allosteric regulatory subdomain within the gammasubunit of the epithelial Na+ channel. J Biol Chem, The Journal of biological chemistry. 2010;285:26088–26096. doi: 10.1074/jbc.M110.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley KC, Warren WC, Rogers J, Locke D, Muzny DM, Mardis ER, Weinstock GM, Tardif SD, Aagaard KM, Archidiacono N, Rayan NA, Batzer MA, Beal K, Brejova B, Capozzi O, Capuano SB, Casola C, Chandrabose MM, Cree A, Dao MD, de Jong PJ, del Rosario RC-H, Delehaunty KD, Dinh HH, Eichler EE, Fitzgerald S, Flicek P, Fontenot CC, Fowler RG, Fronick C, Fulton LA, Fulton RS, Gabisi RA, Gerlach D, Graves TA, Gunaratne PH, Hahn MW, Haig D, Han Y, Harris RA, Herrero J, Hillier LW, Hubley R, Hughes JF, Hume J, Jhangiani SN, Jorde LB, Joshi V, Karakor E, Konkel MK, Kosiol C, Kovar CL, Kriventseva EV, Lee SL, Lewis LR, Liu Y, Lopez J, Lopez-Otin C, Lorente-Galdos B, Mansfield KG, Marques-Bonet T, Minx P, Misceo D, Moncrieff JS, Morgan MB, Nazareth LV, Newsham I, Nguyen NB, Okwuonu GO, Prabhakar S, Perales L, Pu L-L, Puente XS, Quesada V, Ranck MC, Raney BJ, Raveendran M, Deiros DR, Rocchi M, Rodriguez D, Ross C, Ruffier M, Ruiz SJ, Sajjadian S, Santibanez J, Schrider DR, Searle S, Skaletsky H, Soibam B, Smit AFA, Tennakoon JB, Tomaska L, Ullmer B, Vejnar CE, Ventura M, Vilella AJ, Vinar T, Vogel J-H, Walker JA, Wang Q, Warner CM, Wildman DE, Witherspoon DJ, Wright RA, Wu Y, Xiao W, Xing J, Zdobnov EM, Zhu B, Gibbs RA, Wilson RK. The common marmoset genome provides insight into primate biologyand evolution. Nat Genet. 2014;46:850–857. doi: 10.1038/ng.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yu Y, Li W-G, Yu F, Cao H, Xu T-L, Jiang H. Inherent dynamics of the acid-sensing ion channel 1 correlateswith the gating mechanism. PLoS Biol. 2009;7:e1000151. doi: 10.1371/journal.pbio.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L-M, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMPinvolves putative ERK phosphorylation sites in the C termini of thechannel’s beta- and gamma-subunit. J Biol Chem. 2006;281:9859–9868. doi: 10.1074/jbc.M512046200. [DOI] [PubMed] [Google Scholar]
- Yue GG, Malik B, Eaton DCDC, Yue GG, Eaton DCDC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulatesepithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- Zelle KM, Lu B, Pyfrom SC, Ben-Shahar Y. The genetic architecture of degenerin/epithelial sodium channelsin Drosophila. G3 (Bethesda) 2013;3:441–450. doi: 10.1534/g3.112.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. Acid-sensing ion channels: trafficking and synapticfunction. Mol. Brain. 2013;6:1. doi: 10.1186/1756-6606-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PWH, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Lošo T, Du Y, Sun X, Zhang S, Liu B, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, Du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinberg CEW, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J. The oyster genome reveals stress adaptation and complexity ofshell formation. Nature. 2012;490:49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li B, Li C, Gilbert MTP, Jarvis ED, Wang J. Comparative genomic data of the Avian PhylogenomicsProject. Gigascience. 2014;3:26. doi: 10.1186/2047-217X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhang W, Zhang L, Zhang Z, Li J, Lu G, Zhu Y, Wang Y, Huang Y, Liu J, Kang H, Chen J, Wang L, Chen A, Yu S, Gao Z, Jin L, Gu W, Wang Z, Zhao L, Shi B, Wen H, Lin R, Jones MK, Brejova B, Vinar T, Zhao G, McManus DP, Chen Z, Zhou Y, Wang S. The genome of the hydatid tapeworm Echinococcusgranulosus. Nat Genet. 2013;45:1168–1175. doi: 10.1038/ng.2757. [DOI] [PubMed] [Google Scholar]
- Zhu K, Zhou X, Xu S, Sun D, Zhou K, Yang G. The loss of taste genes in cetaceans. BMC Evol. Biol. 2014;14:218. doi: 10.1186/s12862-014-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Zhang W, Yang S, Chen J-Q, Tian D. Patterns of exon-intron architecture variation of genes ineukaryotic genomes. BMC Genomics. 2009;10:47. doi: 10.1186/1471-2164-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X-Q, Korhonen PK, Cai H, Young ND, Nejsum P, von Samson-Himmelstjerna G, Boag PR, Tan P, Li Q, Min J, Yang Y, Wang X, Fang X, Hall RS, Hofmann A, Sternberg PW, Jex AR, Gasser RB. Genetic blueprint of the zoonotic pathogen Toxocaracanis. Nat. Commun. 2015;6:6145. doi: 10.1038/ncomms7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide andacidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin AV, Cornish AS, Maudhoo MD, Gibbs RM, Zhang X, Pandey S, Meehan DT, Wipfler K, Bosinger SE, Johnson ZP, Tharp GK, Marçais G, Roberts M, Ferguson B, Fox HS, Treangen T, Salzberg SL, Yorke JA, Norgren RB. A new rhesus macaque assembly and annotation for next-generationsequencing analyses. Biol Direct. 2014;9:20. doi: 10.1186/1745-6150-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]