The effect of VMAT2 inhibitor GZ-793A on the reinstatement of methamphetamine seeking in rats
Kristin M Alvers
Joshua S Beckmann
Guangrong Zheng
Peter A Crooks
Linda P Dwoskin
Michael T Bardo
Corresponding Author: Michael T. Bardo, Department of Psychology, 741 S. Limestone, BBSRB, Room 447, University of Kentucky, USA.mbardo@uky.edu
Issue date 2012 Nov.
Abstract
Rationale
The vesicular monoamine transporter 2 (VMAT2) has been identified as a potential target for the treatment of methamphetamine (METH) abuse. GZ-793A is a potent and selective VMAT2 inhibitor that has been shown to block the primary and conditioned rewarding effects of METH, while demonstrating no abuse liability when given alone.
Objectives
The aim of the current study was to determine if GZ-793A attenuates METH- or cue-induced reinstatement of METH-seeking after a period of extinction. The effect of acute GZ-793A on locomotor activity also was assessed.
Methods
After a period of extinction, rats were administered GZ-793A (15 mg/kg, s.c.) 15 min prior to a priming injection of METH or re-exposure to cues associated with METH infusions. GZ-793A also was administered 20 min prior to an injection of METH (0.5 mg/kg, s.c.) or saline to determine its effect on locomotor behavior.
Results
Pretreatment with GZ-793A (15 mg/kg) decreased cue-induced reinstatement, without demonstrating any response suppressive effects when administered in the absence of reinstating stimuli. GZ-793A also decreased methamphetamine-induced reinstatement; however, response suppressant effects of GZ-793A were obtained when the compound was presented alone. In this latter experiment, GZ-793A may have reduced responding for the conditioned reinforcing effects of the contingently available cues rather than having non-specific effects on baseline responding. GZ-793A had no effect on locomotor activity when administered alone or with METH.
Conclusions
GZ-793A and related VMAT2 inhibitors may be promising leads for reducing the risk of relapse to METH use following exposure to drug-associated cues.
Keywords: Methamphetamine, Reinstatement, Relapse, GZ-793A, VMAT2, locomotor activity
Methamphetamine (METH) use remains a substantial public health risk in the US, despite reports that rates of use are declining. According to the National Institute of Drug Abuse, (NIDA) in 2009, approximately 1.2 million people aged 12 and older reported past year abuse of METH. In addition, 68,000 annual emergency department visits have been attributed to METH [Community Epidemiology Work Group 2009]. Although illicit psychostimulant use accounts for a considerable portion of admissions to drug abuse treatment programs, a significant number of treatment-seeking abusers resume use within 6 months of community- or hospital-based treatment (Ramo and Brown 2008). Because of the chronic nature of addiction, prevention of relapse is regarded as one of the most significant challenges to the successful treatment of substance use disorders (O’Brien 1997).
The vesicular monoamine transporter 2 (VMAT2) may offer a novel therapeutic target for METH abuse (Dwoskin and Crooks 2002;Zheng et al. 2006). METH alters the synthesis, storage and release of dopamine (DA), a monoamine identified as mediating, at least in part, the rewarding effect of abused drugs (Wise and Rompre 1989;Ross and Peselow 2009). Through inhibition of VMAT2, coupled with inhibition of monoamine oxidase (MAO) and activation of the precursor enzyme tyrosine hydroxylase, methamphetamine increases cytosolic DA available for release by reversal of the DA transporter (DAT;Sulzer et al. 2005;Guillot and Miller 2009). However, selective inhibition of VMAT2, without concurrent inhibition of MAO, allows for enhanced intracellular DA metabolism and less DA available for release by METH via reversal of DAT (Miller et al. 2001;Dwoskin and Crooks 2002;Nickell et al. 2009).
Lobeline, an alkaloidal constituent of the plantLobelia inflata, and its des-oxy defunctionalized analog lobelane, have been identified previously as VMAT2 inhibitors (Dwoskin and Crooks 2002;Miller et al. 2004). Both lobeline and lobelane significantly reduce METH-evoked DA overflow from rat striatal slices along with decreasing METH self-administration and METH-induced hyperactivity in rats (Harrod et al. 2001;Miller et al. 2001;Neugebauer et al. 2007;Nickell et al. 2009). However, problems associated with the non-selectivity of lobeline for VMAT2 (Dwoskin and Crooks 2002) and the rapid development of behavioral tolerance to lobelane across repeated treatment (Neugebauer et al. 2007) has prompted the search for additional VMAT2 inhibitor candidates to treat METH abuse.
GZ-793A [(R)-cis-N-(2,3–dihydroxypropyl)-2,6-di-(4-methoxyphenethyl) piperidine hydrochloride], a structural N-dihydroxyl analog of lobelane (Fig. 1), demonstrates greater selectivity and potency at VMAT2 than lobeline and lobelane (Horton et al. 2011). GZ-793A dose-dependently blocks METH-induced DA release from rat striatal slices (Horton et al. 2011). In behavioral studies, GZ-793A decreases METH self-administration and conditioned place preference (CPP) at doses that have no effect on food-maintained behavior. GZ-793A also does not engender self-administration behavior or produce CPP, suggesting that it lacks abuse potential (Beckmann et al. 2011). However, while effective in specifically reducing METH self-administration, it is not clear if GZ-793A would be effective in preventing relapse among individuals undergoing METH abstinence.
Fig. 1.

Chemical structures for lobeline, lobelane and GZ-793A
The current study examined the ability of GZ-793A to attenuate reinstatement of METH-seeking behavior following a period of abstinence, elicited by either (1) a priming injection of METH, or (2) the presentation of cues paired with METH infusions during self-administration. Because DA has been implicated in drug- and cue-induced reinstatement (Shaham et al. 2003;Bossert et al. 2005), and because GZ-793A reduces METH-evoked striatal DA release (Horton et al. 2011), it was hypothesized that GZ-793A would attenuate reinstatement of METH-seeking in both reinstatement models. Also, to assess the specificity of GZ-793A in altering METH-seeking, we determined the ability of GZ-793A to alter locomotor behavior and to reduce METH-induced hyperactivity.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats, 250–275 g on arrival (Harlan Industries, Indianapolis, IN), were housed individually in a temperature-controlled colony maintained on a 14:10 hrlight: dark cycle. All experiments were performed during the light cycle. Different rats were used for each experiment. Rats in the reinstatement experiments were food-restricted to 85% of their free-feeding body weight during initial lever training for food reinforcement. Rats were otherwise provided ad libitum food and water in the home cage. Experimental procedures were in accord with University of Kentucky Institutional Animal Care and Use Committee (IACUC) and the 1996 National Institute of HealthGuide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23).
Apparatus
Operant conditioning experiments were conducted in operant conditioning chambers (28 cm x 24 cm x 25 cm; ENV- 008CT; MED Associates, St. Albans, VT) contained within sound-attenuating chambers (ENV- 018M; MED Associates). The front and back walls of the chamber were constructed of clear Plexiglas, the side walls were aluminum, and the floor was constructed of metal rods. A white house light (28 V) on the left side wall illuminated the chamber, while a ventilation fan on the right side of the sound-attenuating chamber masked extra-chamber sounds. A pellet dispenser (ENV-203M-45; MED Associates) was centered on the right wall and delivered individual food pellets (45 mg, Bio-Serv, F0021, Frenchtown, NJ) into a recessed tray situated 2 cm above the floor. Retractable levers were located 6 cm above the floor on each side of the dispenser, and a white stimulus light (28 V, 3 cm diameter) was located 3 cm above each lever. For intravenous infusions, a pump (PHM-100; MED Associates) was located on the outside wall of the sound-attenuating chamber. Plastic tubing connected the pump to a swivel located inside the chamber, which was attached to an additional length of tubing surrounded by a spring covered leash. MED-PC software (MED Associates) was used for experimental programming and data collection.
Measurement of locomotor activity occurred in activity chambers (42 × 42 × 30 cm) with clear acrylic walls and floor. Chambers contained a horizontal 16 × 16 grid of photo beam sensors located 2.5 cm apart and 7 cm above the floor. Activity was recorded automatically using Versamax System software (AccuScan Instruments Inc., Columbus, OH).
Drugs
d-Methamphetamine HCl was obtained from Sigma (St. Louis, MO). GZ-793A [(R)-cis-N-(2,3–dihydroxypropyl)-2,6-di-(4-methoxyphenethyl)piperidine hydrochloride] was synthesized by the methods described inHorton et al. (2011); the chemical structure of GZ-793A is provided inFigure 1. Methamphetamine and GZ-793A were dissolved in sterile saline (SAL; 0.9% NaCl). All doses are expressed as the salt weight.
Surgical Procedures
Rats were anesthetized with 100 mg/kg ketamine (i.p.) and 5 mg/kg diazepam (i.p.) to implant a chronic indwelling jugular catheter. Catheters were constructed of silastic tubing, with one end inserted and secured into the jugular vein, and the other attached to a metal cannula. The cannula exited sub cutaneously and was fastened to the skull with dental acrylic and jewelers screws. Animals were given one week to recover, and catheters were flushed daily with a mixture of gentamicin (0.2 ml), heparin (0.6 ml) and SAL to maintain patency.
METH Self-Administration Training
The general methods for self-administration training were based on those described by previous studies (Harrod et al, 2001; Beckmann et al, 2011). Rats were trained initially to respond for food pellets. They received a single session of magazine training, in which ~20 pellets were automatically dispensed into the magazine over a period of ~10 minutes, in the presence of both stimulus lights. Following 2 sessions of auto shaping (Carroll and Lac, 1993), rats were then trained to respond on one lever (active lever) on a fixed ratio 1 (FR1) schedule of reinforcement. Responses on the active lever delivered a single food pellet in the absence of any stimulus lights. Responses on the inactive lever were recorded, but had no programmed consequence. On the following days, the response requirement was increased to FR 3 and FR 5. Rats then were surgically implanted with a chronic indwelling jugular catheters as previously described.
METH self-administration training began using a FR 1, 20-s signaled time out schedule during daily 60-min sessions, across 3 days. Responses on the active lever resulted in an infusion of METH (0.05 mg/kg/infusion), paired with illumination of the stimulus lights above both levers. The stimulus lights remained on during the time out, and responses on either lever had no programmed consequence. The ratio requirement was increased to a FR3 (3 sessions) and to a terminal FR 5 on subsequent sessions until stability was reached; escalating the FR schedule allowed for greater discrimination between the active and inactive lever to be observed. Stability was defined as at least 10 infusions, a 2:1 ratio of responding on the active lever to the inactive lever, and ≤ 20% variability in number of responses across 3 consecutive sessions. On average, rats underwent 13.96 (± 0.51) sessions of METH self-administration and were receiving 14.40 (± 0.99) infusions of METH (0.05 mg/kg/infusion) upon stability.
METH-Induced Reinstatement
Following acquisition of stable METH self-administration, rats underwent daily extinction sessions that were identical to self-administration training, except completion of the ratio requirement (FR 5) delivered an infusion of SAL, instead of METH, paired with the stimulus lights. Extinction lasted a minimum of 10 sessions, or until stable responding was achieved. Stability was determined by ≤10 infusions and ≤ 20% variability in number of responses across 3 consecutive days. On average, rats received 11.67 (± 0.66) extinction sessions.
Two groups of rats were used to assess the effects of GZ-793A on the reinstatement of METH-seeking behavior. The first groups of rats (n=9) received a pretreatment dose of 15 mg/kg (s.c.) GZ-793A. The effects of this dose on responding prompted the use of a second group of rats (n=9) who were administered a lower pretreatment dose of GZ-793A (10 mg/kg, s.c.). Both doses have been shown previously to reduce the number of self-administered METH infusions; however, 15 mg/kg (s.c.) reduced the number of infusions by approximately half, while 10 mg/kg (s.c.) only reduced the number of infusions by approximately 25%.
Reinstatement was determined using a within-subject 2 × 2 design in which pretreatment drug (SAL vs. GZ-793A) and reinstatement drug (SAL vs. METH) were the repeated measures. Rats were pretreated with either GZ-793A or SAL 15 min prior to a priming injection of either METH (0.5 mg/kg, s.c.) or saline administered immediately before the start of the reinstatement session. Each rat received all 4 treatment conditions (SAL-SAL, SAL-METH, GZ-793A-SAL and GZ-793A-METH) using a Latin-square design to control for order effects. Tests of reinstatement were conducted once every 4 days, with 3 days of extinction (no injections) between each reinstatement session.
Cue-Induced Reinstatement of METH-Seeking
In another group of rats, following acquisition of stable self-administration, rats (n=7) underwent daily extinction sessions which were identical to self-administration training, except completion of the ratio requirement (FR5) delivered an infusion of SAL without illumination of the stimulus lights. Extinction lasted at least 10 sessions, at which point the group achieved the aforementioned stability criteria.
Reinstatement of METH-seeking behavior was determined using a within-subject 2 × 2 design in which pretreatment drug (SAL vs. GZ-793A) and reinstatement stimulus (CUE vs. NO CUE) were the repeated measures. Rats were pretreated with either GZ-793A (15 mg/kg, s.c.) or SAL 15 min prior to the reinstatement session. During the session, completion of the ratio requirement resulted in illumination of the previously METH-paired stimulus lights (CUE condition) or no illumination of the stimulus lights (NO CUE condition). Each subject received all 4 treatment conditions (SAL-NO CUE, SAL-CUE, GZ-793A-NO CUE, GZ-793A-CUE) using a Latin-square design to control for order effects. Tests of reinstatement were conducted once every 4 days, with 3 days of extinction (no injections or stimulus light illumination) between each reinstatement session.
Effects of GZ-793A on Locomotor Activity
In another group of rats, the effects of GZ-793A (15 mg/kg, s.c.) on locomotor activity was assessed using a 2 × 2 between-subject design, in which the pretreatment drug (SAL or GZ-793A) and the test drug (SAL or METH) served as the factors. There were 6 rats per treatment condition (SAL-SAL, GZ-793A-SAL, SAL-METH, GZ-793A-METH). Rats were first placed into the locomotor activity chambers to habituate for 60 min. The following day, rats were administered a pretreatment injection of either SAL or GZ-793A, followed 20 min later by an injection of either SAL or METH (0.5 mg/kg, s.c.). Immediately following the second injection, rats were placed in the locomotor activity chamber for 60 min.
Data Analysis
Separate two-way repeated measures analysis of variance (ANOVA) was used to analyze the reinstatement of METH-seeking. Significant interactions were subjected to post hoc analyses using pairwise comparisons with a Tukey test. Differences in active lever responding between the first and last extinction session were determined with paired t-tests. Locomotor activity was measured by total distance travelled (cm) and analyzed using a two-way ANOVA.
RESULTS
METH-Induced Reinstatement
Replacing METH infusions with saline infusions reduced responding on the active lever significantly during extinction (Fig. 2a). A paired t-test revealed a significant decrease in active lever presses from the first to last extinction session [t(17) = 2.10,p< 0.005].
Fig. 2.
a Mean (± SEM) responses on the active lever on the last day of maintenance of methamphetamine self-administration, as well as the first and the last day of extinction. ** p< 0.01 compared to the first day.b Responding on the previously active lever during methamphetamine-induced reinstatement testing with 10 mg/kg of GZ-793A.c Responding on the previously active lever during methamphetamine-induced reinstatement testing with 15 mg/kg of GZ-793A.
In both experiments (10 or 15 mg/kg GZ-793A pretreatment), ANOVA revealed a significant main effect of reinstatement drug [F(1, 8) = 7.08,p < 0.05,Fig. 2b;F(1, 8) = 11.57,p < 0.01,Fig. 2c], indicating that a non-contingent priming injection of METH (0.5 mg/kg) reinstated responding on the active lever. There was also a significant main effect of pretreatment following either 10 mg/kg of GZ-793A [F(1, 8) =10.41,p < 0.05] or 15 mg/kg of GZ-793A [F(1, 8) = 9.50,p < 0.05], indicating that both doses decreased responding on the active lever regardless of the reinstatement drug. There was no significant pretreatment drug × reinstatement drug interaction in either experiment.
Table 1 shows the number of responses on the inactive lever during the reinstatement tests. There were no significant main effects or interaction for pretreatment drug or reinstatement stimulus on inactive lever presses.
Table 1.
Mean number of responses (± SEM) on the inactive lever during reinstatement testing and the extinction session preceding each reinstatement test.
| GZ-793A (mg/kg) | Cue- Induced Reinstatement | |||
|---|---|---|---|---|
| Sal- No Cue | Sal- Cue | GZ-793A- No Cue | GZ-793A- Cue | |
| Extinction 15 | 8.57 ± 2.25 | 9.29 ± 3.18 | 7.43 ± 1.67 | 7.86 ± 1.82 |
| 7.14 ± 1.81 | 15.29 ± 4.68 | 4.14 ± 1.22 | 5.29 ± 1.80 | |
| Methamphetamine- Induced Reinstatement | ||||
| Sal- Sal | Sal- Meth | GZ-793A- Sal | GZ-793A- Meth | |
| Extinction 10 | 2.78 ± 0.55 | 6.44 ± 1.80 | 6.56 ± 1.71 | 6.89 ± 1.67 |
| 4.22 ± 1.08 | 4.33 ± 2.05 | 4.00 ± 1.27 | 2.33 ± 0.50 | |
| Extinction 15 | 7.44 ± 1.58 | 7.0 ± 1.39 | 6.56 ± 1.38 | 6.56 ± 1.84 |
| 4.44 ± 1.03 | 8.44 ± 3.95 | 3.00 ± 1.33 | 7.78 ± 5.00 | |
Cue-Induced Reinstatement of METH-Seeking
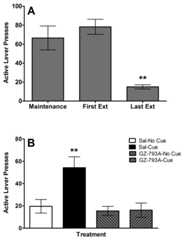
Omission of METH infusions and the associated stimulus lights reduced responding on the active lever during extinction (Fig. 3a). There was a significant decrease in active lever presses from the first to last extinction session [t(7) = 2.45,p< 0.005].
Fig. 3.
a Mean (± SEM) responses on the active lever on the last day of maintenance of methamphetamine self-administration, as well as the first and the last day of extinction training. ** p< 0.01 compared to the first day of extinction.b Responding on the previously active lever during cue-induced reinstatement testing with 15 mg/kg of GZ-793A. ** p< 0.01 compared to all other groups.
Fig. 3b illustrates that when rats were provided contingent re-exposure to METH-associated cues, responding on the active lever was reinstated and pretreatment with GZ-793A was able to block completely this response. ANOVA revealed a significant main effect of pretreatment drug [F(1, 6) = 13.06,p ≤ 0.01], reinstatement stimulus [F(1, 6) = 9.43,p ≤ 0.05], and a pretreatment drug × reinstatement stimulus interaction [F(1, 6) = 8.07,p < 0.05]. Post-hoc analysis revealed an increase in responding during the SAL-CUE condition, compared to all other conditions (p < 0.05). No other pairwise comparisons were statistically significant.Table 1 shows the number of responses on the inactive lever during the reinstatement tests. There were no significant main effects or interaction for pretreatment drug or reinstatement stimulus on inactive lever presses.
Effects of GZ-793A on Locomotor Activity
A two-way ANOVA revealed that there were no differences between groups during the habituation session (Fig. 4a). On the test day, there was a significant main effect of test drug [F(1, 5) = 34.58,p < 0.01], but no effect of pretreatment or interaction (Fig. 4b), indicating that pretreatment with GZ-793A (15 mg/kg), either alone or in combination with METH, did not alter locomotor activity.
Fig. 4.
a Total distance traveled during the initial habituation session.bTotal distance traveled on the test day, following pretreatment with SAL or GZ-793A, followed by treatment with SAL or METH.
DISCUSSION
The potent and selective VMAT2 inhibitor, GZ-793A, has been shown previously to block the primary and conditioned reinforcing effects of METH, while demonstrating low abuse liability (Beckmann et al. 2011). The present study characterized further the effects of GZ-793A on drug-seeking behavior by assessing its ability to attenuate drug- and cue-induced reinstatement of METH seeking.
In accordance with others (Harrod et al. 2003;Shelton and Beardsley 2008;Rogers et al. 2008), METH-seeking was elicited following a priming injection of METH or by contingent presentation of METH-associated cues after a period of extinction. Pretreatment with GZ-793A (15 mg/kg) was able to block completely cue-induced reinstatement. GZ-793A (10 or 15 mg/kg) pretreatment also modestly decreased METH-induced reinstatement; however, this interpretation must be tempered because this effect was accompanied by a nonspecific decrease in responding when GZ-793A (10 or 15 mg/kg) was given alone. The response suppressant effect of GZ-793A in the METH-induced reinstatement experiment is unique, as the compound has failed to demonstrate the effect otherwise. For example, pretreatment alone did not affect responding in the absence of METH-associated cues or responding on the inactive lever. Moreover, it did not show nonspecific suppressant effects on locomotor behavior. This is also in agreement with previous findings from our laboratory that pretreatment with GZ-793A (15 mg/kg) has no effect on responding for cocaine or food pellets (Beckmann et al. 2011).
One potential explanation for theGZ-793A-induced response suppressant effect relates to the presence of the cue lights. In the METH-induced reinstatement experiments, the cue lights were presented contingently during both extinction and reinstatement test sessions. As indicated, GZ-793A decreases responding for METH-associated cues; therefore, when GZ-793A was presented alone (GZ-793A-SAL condition) it may have reduced any remaining conditioned reinforcing effects of the cues following the end of extinction, rather than having non-specific effects on responding. Indeed, the level of extinction in the METH-induced reinstatement experiment (~50%) was less than that of extinction in the cue-induced reinstatement experiment (~25%), suggesting that the presence of the cues in the former experiment maintained some reinforcing effects by the end of extinction. The presence of the cues during reinstatement presents some interpretational difficulty because one cannot parse the contributions of the cue and the METH priming injection to the reinstated response. However, presenting drug-associated cues concurrent with the drug itself (i.e. a priming injection) is a clinically relevant design.
While the neural mechanisms for drug- and cue-induced reinstatement are distinct, both are mediated in part by the mesocorticolimbic DA system (Shaham et al. 2003). For example, non-contingent priming injections of amphetamine or cocaine that reinstate extinguished responding are accompanied by an increase DA efflux in NAc (Di Ciano et al. 2001;Ranaldi et al. 1999;Neisewander et al. 1996). Exposure to amphetamine- or cocaine-paired cues also increases DA levels (Gratton and Wise, 1994;Kiyatkin and Stein, 1996;Ito et al. 2000); however, evidence indicates that cue-induced DA release occurs exclusively with non-contingent presentation of drug-paired cues and not with contingent presentation (Willuhn et al. 2010;Di Ciano et al. 2001;Neisewander et al. 1996;Ito et al. 2000).Willuhn et al. (2010) suggest that drug-seeking is maintained by a “preresponse” DA signal (an indicator of motivation to obtain drug) that is maintained after extinction of a conditioned stimulus. Thus, one possible explanation for the results of the current study is that GZ-793A may reduce the preresponse DA signal and its ability to elicit reinstatement (Willuhn et al. 2010).Horton et al. (2011) demonstrated that GZ-793Adose-dependently blocks METH-induced DA release from rat striatal slices and does not increase extracellular DA when administered alone. Based on that neurochemical work, our results suggest further that DA release may occur with response-dependent exposure to drug-paired cues after a period of extinction. While these results contrast with a report byHarrod et al. (2003) showing that the VMAT2 inhibitor lobeline does not attenuate METH-induced reinstatement, GZ-793A has increased selectivity for VMAT2 relative to lobeline (Horton et al. 2011), which may account for its significant effect of reinstatement in the current report.
The current results suggest that GZ-793A has low abuse liability, as it did not reinstate responding when administered in the absence of a METH-priming injection or METH-associated cues. This supports previous findings that GZ-793A is not self-administered and does not support CPP (Beckmann et al. 2011). Also, acute administration of GZ-793A had no effect on locomotor activity when administered alone or in conjunction with METH. This finding was surprising given that VMAT2 inhibitors lobelineand tetrabenazine are able to inhibit amphetamine- and methamphetamine-induced hyperactivity (Miller et al. 2001;Meyer et al. 2011). Also, GZ-793A reduces striatal extracellular DA release (Horton et al. 2011) which is assumed to underlie the psychomotor activating effects of psychostimulants (Wise and Bozarth 1987). However, DA release and locomotor activity do not always correlate directly and locomotor activity has been questioned as an adequate model of drug craving in humans (Di Ciano et al. 1998). Nonetheless, evidence from our laboratory indicates that GZ-793A is able to attenuate other measures of drug-reward (i.e. self-administration, CPP, reinstatement).
There is currently no Food and Drug Administration (FDA) approved medications specific to the treatment of METH dependence; treatment instead involves the use of medications approved for other purposes (Yahyavi-Firouz-Abadi and See 2009;Elkashef et al. 2008). Some dopaminergic agents have shown moderate success in reducing METH use (bupropion, modafinil, risperidone,d-amphetamine), but overall, evidence for efficacious treatment is weak (Karil et al. 2010). As such, there continues to be a need for novel pharmacotherapies, specifically those that curb cue- and METH-induced reinstatement (Elkashef et al. 2008). GZ-793A demonstrates the ability to attenuate these forms of reinstatement, adding to the already positive findings from our laboratory and representing a promising lead for the development of VMAT2 inhibitors as a potential novel pharmacotherapeutic approach to reduce METH dependence and relapse.
Acknowledgments
This research was supported by the National Institute of Drug Abuse grants DA13519 and DA016176. We thank Emily Denehy for technical assistance. The University of Kentucky holds a patent on GZ-793A, and a potential royalty stream to LPD, GZ and PAC may occur consistent with University of Kentucky policy.
Footnotes
Disclosure:
The other authors have no disclosures.
References
- Beckmann JS, Denehy ED, Zheng G, Crooks PA, Dwoskin LP, Bardo MT. GZ-793A, a selective an potent vesicular monoamine transporter-2 blocker, specifically blocks methamphetamine reward in rats. Psychopharmacology (Berl) (in press) [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Community Epidemiology Work Group. NIH Publication No 10–7421. Bethesda, MD: 2009. Proceedings of the Community Epidemiology Work Group: Volume I. Highlights and Executive Summary. [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. The relationship between dopamine oxidation currents in the nucleus accumbens and conditioned increases in motor activity in rats following repeated administration of d-amphetamine or cocaine. Eur J Neurosci. 1998;10:1113–1120. doi: 10.1046/j.1460-9568.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–158. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacology of methamphetamine addiction: an update. SubstAbus. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–179. [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 2003;165:397–404. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- Horton DB, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Novel N-1,2-dihydroxypropyl analogs of lobelane inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release. J Pharmacol Exp Ther. 2011;339:286–297. doi: 10.1124/jpet.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J ClinPharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Conditioned changes in nucleus accumbens dopamine signal established by intravenous cocaine in rats. NeurosciLett. 1996;211:73–76. doi: 10.1016/0304-3940(96)12731-2. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Horton DB, Neugebauer NM, Wooters TE, Nickell JR, Dwoskin LP, Bardo MT. Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: locomotor activity, drug discrimination and self-administration. Neuropharmacology. 2011;61:849–856. doi: 10.1016/j.neuropharm.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296:1023–1034. [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm SD, Dwoskin LP. Lobeline analogs with enhanced affinity and selectivity for plasmalemma and vesicula monoamine transporters. J Pharmacol Exp Ther. 2004;310:1035–45. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castañeda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571:33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2009;332:612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Brown SA. Classes of substance abuse relapse situations: a comparison of adolescents and adults. Psychol Addict Behav. 2008;22:372–379. doi: 10.1037/0893-164X.22.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009;32:269–76. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Effect of drug-paired exteroceptive stimulus presentations on methamphetamine reinstatement in rats. Pharmacol Biochem Behav. 2008;90:434–440. doi: 10.1016/j.pbb.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. ProgNeurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top BehavNeurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987:469–492. [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, See RE. Anti-relapse medications: Preclinical models for drug addiction treatment. Pharmacol Ther. 2009;124:235–247. doi: 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. AAPS J. 2006;8:E682–692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]