
Sonic Hedgehog Signaling Controls Thalamic Progenitor Identity and Nuclei Specification in Mice
Tou Yia Vue
Krista Bluske
Amin Alishahi
Lin Lin Yang
Naoko Koyano-Nakagawa
Bennett Novitch
Yasushi Nakagawa
Correspondence should be addressed to Yasushi Nakagawa at the above address.nakagawa@umn.edu
Corresponding author.
Received 2009 Feb 6; Revised 2009 Mar 6; Accepted 2009 Mar 9.
Abstract
The mammalian thalamus is located in the diencephalon and is composed of dozens of morphologically and functionally distinct nuclei. The majority of these nuclei project axons to the neocortex in unique patterns and play critical roles in sensory, motor, and cognitive functions. It has been assumed that the adult thalamus is derived from neural progenitor cells located within the alar plate of the caudal diencephalon. Nevertheless, how a distinct array of postmitotic thalamic nuclei emerge from this single developmental unit has remained largely unknown. Our recent studies found that these thalamic nuclei are in fact derived from molecularly heterogeneous populations of progenitor cells distributed within at least two distinct progenitor domains in the caudal diencephalon. In this study, we investigated how such molecular heterogeneity is established and maintained during early development of the thalamus and how early signaling mechanisms influence the formation of postmitotic thalamic nuclei. By using mouse genetics andin utero electroporation, we provide evidence that Sonic hedgehog (Shh), which is normally expressed in ventral and rostral borders of the embryonic thalamus, plays a crucial role in patterning progenitor domains throughout the thalamus. We also show that increasing or decreasing Shh activity causes dramatic reorganization of postmitotic thalamic nuclei through altering the positional identity of progenitor cells.
Introduction
The thalamus is located in the vertebrate diencephalon and plays critical roles in controlling animal behavior. Each of the thalamic nuclei exhibits a unique pattern of gene expression and connectivity. In mammals, a vast majority of thalamic nuclei send axons to unique sets of distinct neocortical areas. For example, principal sensory nuclei (visual, somatosensory, and auditory) establish topographic and area-specific thalamocortical projections to primary sensory areas in the neocortex (Jones, 2007). Despite the functional importance of the thalamus, however, molecular mechanisms that control the specification of thalamic nuclei are not well understood. A particularly intriguing question is how a single developmental unit in the alar plate of caudal diencephalon [prosomere 2 in the study byPuelles and Rubenstein (2003)] can generate such a diverse array of postmitotic nuclei at later stages of development.
Numerous studies have shown that ventral-to-dorsal gradient of Shh activity specifies different neuronal subtypes throughout the embryonic CNS (Fuccillo et al., 2006). In the diencephalon, Shh is not only expressed in the basal plate, which is ventral to the thalamus, but is also expressed in the zona limitans intrathalamica (ZLI), a dorsally extending group of cells located immediately rostral to the early embryonic thalamus (Puelles and Rubenstein, 1993;Shimamura et al., 1995).In ovo electroporation and grafting studies in chick showed that ectopic expression of Shh in the caudal diencephalon and mesencephalon induces the expression ofGbx2 and reducesPax6 (Kiecker and Lumsden, 2004;Vieira et al., 2005). Conversely, inhibition of Shh signaling by a dominant-negative form of Shh receptor Ptc1 reducedNkx2.2,Ptc1, andGbx2 expression (Kiecker and Lumsden, 2004). Together, these studies establish the importance of Shh signaling in the global regionalization of the diencephalon, particularly its role in specifying the identity of the thalamus as a whole. To date, however, whether Shh signaling also controls the identity of progenitor cells within the thalamus along the rostrocaudal axis and subsequently contributes to the specification of different thalamic nuclei has remained largely unknown.
We recently reported molecular heterogeneity of thalamic progenitor cells in the mouse and analyzed the postmitotic fate of each progenitor cell population using genetic lineage tracing methods (Vue et al., 2007). We proposed that the thalamic ventricular zone is marked by the expression of the basic-helix-loop-helix (bHLH) transcription factor Olig3, and is divided into two distinct progenitor domains, pTH-R and pTH-C (Fig. 1A,B). The pTH-R domain is rostroventrally located within the thalamic primordium, expresses transcription factors Nkx2.2 and Mash1, and contributes largely to nuclei that do not project to the cortex. These nuclei were previously not considered to be part of the postmitotic thalamus. The other domain, pTH-C, expresses Ngn1 and Ngn2 and gives rise to all the classical thalamic nuclei projecting to the cortex. Within pTH-C, Olig2, a bHLH protein, is expressed in a high-rostroventral to low-caudodorsal gradient, while the homeodomain protein Dbx1 is expressed in the opposite gradient. Because Nkx2.2, Olig2, and Dbx1 are known to be differentially regulated by Shh in the ventral spinal cord (Briscoe and Novitch, 2008), our previous study predicted that differential Shh signaling may impart not only the specification of pTH-R and pTH-C, but also the molecular heterogeneity of progenitor cells within the pTH-C domain. To test this hypothesis, we used conditional gene activation or deletion as well asin utero electroporation in mice to increase or decrease Shh activity in thalamic progenitor cells in temporally and spatially restricted manners. We found that altering the level of Shh activity shifts the positional gene expression patterns in both pTH-R and pTH-C. In addition, this shift results in reorganization of postmitotic thalamic nuclei.
Figure 1.

Thalamic progenitor cells are exposed to graded activity of Shh signaling.A, A schematic of the location of the thalamus (TH) in the caudal diencephalon of the mouse embryo. Embryo is facing left. The thalamus is bordered by the pretectum caudally, the habenula dorsally, the basal plate ventrally, and by the ZLI rostrally. The basal plate and ZLI are sources of Shh (blue). Red dashed line shows the curvature of the anterior–posterior (AP) axis of the diencephalon. The two vertical lines (1 and 2) represent the planes of frontal sections shown inC–J.B, Expression patterns of bHLH (Olig2, Olig3, Ngn1/2, Mash1) and homeodomain (Nkx2.2, Dbx1) transcription factors in the thalamic ventricular zone. Olig3 is expressed in the entire thalamic ventricular zone and the ZLI. Expression of Mash1, Nkx2.2, Ngn1, and Ngn2 divides the thalamus into two distinct progenitor domains, a small rostral domain (pTH-R) and a much larger caudal domain (pTH-C). Olig2 and Dbx1 are expressed in opposing gradients within pTH-C.C–J, Graded patterns ofGli1 andPtc1 expression in thalamic progenitor cells. Frontal sections through the thalamus of E10.5 (C,D,G,H) and E12.5 (E,F,I,J) wild-type brains. Top panels (C–F) are sections at a more dorsal and rostral level (plane 1 inA) and the bottom panels (G–J) are for a more ventral and caudal level (plane 2 inA), showing similar results.C,G, Double immunofluorescence for Olig3 and Shh at E10.5 to show the location of the thalamus. Olig3 expression caudal to the ZLI marks the entire thalamic ventricular zone.D,H,In situ hybridization forGli1 on sections adjacent toC andG.Gli1 expression is higher closer to the ZLI and decreases in a graded manner throughout the thalamic ventricular zone. Arrowheads indicate dorsal and caudal boundary of the thalamus delineated by Olig3 expression.E,I,In situ hybridization forGli1 at E12.5, showing a similar gradient to E10.5.F,J,In situ hybridization forPtc1 on adjacent sections toE andI, respectively, again showing similar gradients. Asterisk and dotted lines inE andF mark the thalamic ventricular zone, indicating that expression ofGli1 andPtc1 is restricted to progenitor cells. 3V, Third ventricle; LV, lateral ventricle; PT, pretectum; TH, thalamus; PTh, prethalamus. Scale bar, 500 μm.
Materials and Methods
Mice.
Care and experimentation on mice were done in accordance with the Institutional Animal Care and Use Committee of the University of Minnesota. Noon of the day on which the vaginal plug was found was counted as embryonic day 0.5 (E0.5). Stages of early embryos were confirmed by morphology (Kaufman, 1992). To generateNesCre/+; R26SmoM2/+ compound heterozygous mice, we crossed heterozygousNestin-Cre (NesCre/+) transgenic mice (Tronche et al., 1999) with homozygousROSA26-stop-SmoM2-EYFP (R26SmoM2/SmoM2) mice (Jeong et al., 2004) and identified Cre-positive embryos by PCR. Cre-negative littermates were used as controls.NesCre/+; R26SmoM2/+ mice died by the end of the first postnatal day (P0). For Shh conditional knock-out, we crossedNesCre/+; Shhc/+ mice withShhc/c mice.Shhc is a conditional allele forShh (Lewis et al., 2001). PCR was done to identifyNesCre allele as well as floxed and wild-type alleles of Shh.Smo conditional knock-out mice were generated by crossingNesCre/+; Smoc/+ mice withSmoc/c mice (Long et al., 2001). Postmitotic fates of Olig2-expressing progenitor cells were analyzed by crossingOlig2Cre/+ mice (Dessaud et al., 2007) withR26-stop-EGFP reporter mice.Olig2 mutant embryos were obtained by crossing betweenOlig2IRES-EGFP/+ mice (Mukouyama et al., 2006). For analysis of normal gene expression (Fig. 1) andin utero electroporation, timed pregnant CD1 mice (Charles River) were used. For all the other mice, colonies were maintained in C57B/6J background.
Production ofOlig3Cre/+ mice.
We producedOlig3Cre/+ mice by homologous recombination in embryonic stem (ES) cells. In short, R1 ES cells were transfected with a targeting vector so that the entire coding region of theOlig3 gene is replaced by a cassette encoding Cre recombinase followed by frt-neo-frt (FNF) sequence (seeFig. 3K). G418-resistant clones were selected and analyzed by Southern blot analysis for homologous recombinants. Two positive clones were microinjected into blastocysts obtained from the mating of C57BL/6NCrl female mice with (C57BL/6J × DBA/2J) F1 male mice (performed at Transgenic Animal Model Core of University of Michigan), and male chimeras were further bred with C57BL/6J females to produce heterozygousOlig3Cre/+ mice.Olig3Cre/+ mice were bred withR26SmoM2/SmoM2 mice to obtainOlig3Cre/+; R26SmoM2/+ mice. To produce conditionalSmo knock-out mice, we crossedOlig3Cre/+; Smoc/+ andSmoc/c mice. To monitor the spatial and temporal patterns of Cre-mediated recombination,NesCre/+ orOlig3Cre/+ mice were bred withR26-stop-EYFP reporter mice (Srinivas et al., 2001), and embryos were analyzed for the expression of EYFP and various progenitor cell markers.
Figure 3.

Ectopic SmoM2 expression causes caudodorsal expansion of pTH-R marker genes Nkx2.2 and Mash1 in thalamic progenitors.A–H, Frontal sections ofNesCre/+; R26SmoM2/+ (E–H) andCre− control (A–D) embryos at E12.5. Midline is to the left. Immunofluorescence for Shh shows the location of the ZLI, which is similar betweenNesCre/+; R26SmoM2/+ (A) and control brains (E).B,F, High-magnification views of the boxed areas inA andE, respectively. These are adjacent toC/D andG/H, respectively. Double immunofluorescence for Nkx2.2 and Olig2 (C,G) and double immunofluorescence for Mash1 and Ngn2 (D,H) show that in the control brain, Nkx2.2 and Mash1 are expressed in pTH-R and form a distinct boundary with pTH-C, which expresses Olig2 and Ngn2 (C,D). InNesCre/+; R26SmoM2/+ brains, both Nkx2.2 and Mash1 are expanded caudodorsally, increasing the size of pTH-R. Only inNesCre/+; R26SmoM2/+ embryos, there is an additional domain between pTH-R and pTH-C that contain both pTH-R markers Nkx2.2/Mash1 and pTH-C markers Olig2/Ngn2. These two sets of markers are not colocalized (arrows inG,H). Dotted lines outline the ZLI in each panel. Arrowheads indicate the domain with both pTH-R and pTH-C markers, which we refer to as pTH-R/C.I,J, Analysis of E12.5 embryos electroporated withpCAG-SmoM2-EYFP plasmid at E11.5 in the thalamus. Double immunofluorescence of Nkx2.2 and YFP on frontal sections. Right side was transfected. Left side is control. Nkx2.2 is ectopically induced in the thalamic progenitor cells only on the transfected side (double arrows inI). Transfection occurred only in the thalamus, not in the prethalamus, as shown by YFP expression (J).K, Strategy for making theOlig3Cre mice. The exon encoding for Olig3 was replaced by the Cre-frt-neo-frt (Cre-FNF) cassette.L–O, Analysis of recombination inOlig3Cre mice.Olig3Cre/+ mice were bred withROSA26-stop-EYFP mice and embryos were analyzed at E11.5. Double immunofluorescence of YFP (L) and Olig3 (M) shows that YFP is expressed in the thalamus, where endogenous Olig3 is expressed. Low-magnification images of the same embryo indicate the specificity of Cre-mediated recombination in the forebrain (N,O). Neither other regions of the diencephalon or the neocortex have undergone recombination (arrows).P–S, Frontal sections ofOlig3Cre/+; R26SmoM2/+ embryos at E12.5, showing that thalamus-specific, ectopic expression of SmoM2 causes the same marker changes in progenitor cells as seen inNesCre/+; R26SmoM2/+ embryos (A–H). pTH-R is expanded (bracket), and the mixed zone (pTH-R/C) emerges between pTH-R and pTH-C (arrowheads). Scale bar, 200 μm forA–M andP–S, 500 μm forN andO.
In utero electroporation.
In utero electroporation was performed as described previously (Saito and Nakatsuji, 2001). Timed pregnant (E10.5 or E11.5) CD1 females were anesthetized by intraperitoneal injection of pentobarbital sodium (2.0 mg for 30 g dams). Embryos were visualized by illumination of the uterus with fiber optics, and a pulled micropipette (1B120F-6, World Precision Instruments) loaded with DNA was inserted into the third ventricle of each embryo. Approximately 0.5 μl of the DNA solution was injected with Picospritzer III (Parker Instruments). Four pulses of square-wave current were applied (35 V, 30 ms on and 100 ms off), using CUY21EDIT electroporator (NEPAGENE) and paddle- or needle-type electrodes. We used 4 μg/μlpCAG-Olig2 (from Masato Nakafuku, Cincinnati Children's Hospital Medical Center, Cincinnati, OH) plus 0.5 μg/μl pCAG-nuclear EGFP and 2 μg/μlpCAG-SmoM2-EYFP (from Andrew McMahon, Harvard University, Cambridge, MA). After the injection, the dam was sutured and allowed to recover until analysis.
In situ hybridization and immunohistochemistry.
In situ hybridization and immunohistochemistry were done based onVue et al. (2007). In this study, goat anti-Sox1 antibody from R&D Systems was used at 1:100. cDNAs forBHLHB4,Gli1,Ptc1,Dlx2,Sox14, andPdlim3 were obtained from Open Biosystems.Irx2 andSpry1/Spry2 cDNAs were obtained from Peter Gruss (Max Planck Society, Munich, Germany) and Gail Martin (University of California, San Francisco, San Francisco, CA), respectively. Sox1-positive cells in the intergeniculate leaflet (IGL) nucleus were counted and compared betweenOlig3Cre/+;Smoc/c and control embryos. Serial sections from each brain were collected onto 8 slides, and Sox1-positive cells within NPY-expressing IGL nucleus were counted with ImageJ software. Average cell counts per slide were compared between the two genotypes (4 thalami in each group), and Student'st test was performed to test the significance of difference. An SEM was used for presentation of data.
Analysis of cell proliferation in the thalamic ventricular zone of SmoM2-expressing or Shh knock-out brains.
Brains of E11.5Olig3Cre/+; R26SmoM2/+ and control litter mates, or E12.5NesCre/+; Shhc/c and control littermates were cryosectioned at 20 μm thickness and then immunostained with anti-phospho histone H3 (anti-PH3) antibody (1:100, Millipore #06-570) to label cells in M-phase. PH3-positive cells situated caudal to the ZLI and within the Olig3-expressing domain in the diencephalon were manually counted, and the numbers were compared betweenOlig3Cre/+; R26SmoM2/+ orNesCre/+; Shhc/c with their respective controls. Student'st test was performed to test the significance of difference, and an SEM was used for presentation of data.
Anterograde labeling of retinogeniculate axons and their targets in the thalamus.
Approximately 2 μl of cholera toxin B subunit [CTB (5 μg/μl), List Biological Laboratories or Invitrogen] was injected into the right retina of neonatalOlig3Cre/+; R26SmoM2/+ mice and their control littermates. After an overnight survival period, pups were perfused with 4% paraformaldehyde and processed for cryosectioning. Goat anti-CTB antibody (1:100, List Biological Laboratories) was used to detect the retinal ganglion cell axons as well as their targets in the contralateral diencephalon, the dorsal lateral geniculate (dLG) and ventral lateral geniculate (vLG) nuclei as well as IGL. We compared the areas of terminations by measuring the dLG betweenCre+ pups andCre− controls. The border between dLG and IGL was identified by DAPI staining and immunohistochemistry with anti-Sox2. Scanned images were analyzed by ImageJ software for area measurement. We measured the area of every section containing dLG (21–29 sections per brain, 20 μm thickness) and calculated the estimated volume of dLG by summing the values of area × thickness of each section. Student'st test was performed to test the significance of difference, and an SEM was used for presentation of data.
Results
Shh signaling is graded within the thalamic progenitor zone
Within the diencephalon, Shh is expressed in the ZLI and the basal plate, which directly border the thalamus rostrally and ventrally, respectively (Fig. 1A). In this study we asked whether differential Shh signaling is responsible for setting up the expression patterns of the various transcription factors that we described previously (Fig. 1B) and later contributes to the specification of different thalamic nuclei. To determine whether the embryonic thalamus is indeed exposed to graded Shh activity, we analyzed the expression ofGli1 andPtc1 in mouse embryos from E10.5 to E12.5.Gli1 andPtc1 are direct target genes of the Shh transduction pathway, and their expression levels reflect the levels of Shh activity in the cell (Agren et al., 2004;Bai et al., 2004). We predicted that if thalamic progenitor cells are differentially patterned by Shh signaling, then bothGli1 andPtc1 should be expressed in graded manners across pTH-R and pTH-C. Indeed, at both E10.5 and E12.5,Gli1 andPtc1 were expressed in a high-rostroventral to low-caudodorsal gradient in wild-type brains (Fig. 1D–F,H–J; thalamus is defined by Shh and Olig3 inC andG); both genes were expressed at the highest levels in pTH-R, and their expression gradually tapered off caudally and dorsally away from the ZLI and the basal plate. With this finding, we hypothesized that the highest level of Shh signaling defines pTH-R, and progressively lower levels define different positional identity of progenitor cells within pTH-C depending on the distance from the ZLI and the basal plate. It was also evident that expression ofGli1 andPtc1 is found only in progenitor cells of the thalamic ventricular zone (Fig. 1E,F,I,J) and not in postmitotic cells in the mantle zone, demonstrating that Shh signaling, whose direct output is gene activation mediated by Gli transcription factors, is restricted to neural progenitor cells.
Ectopic enhancement of Shh signaling strongly inducesGli1 andPtc1 in the entire thalamic progenitor domains
Based on the above findings, we examined whether increased Shh activity in thalamic progenitor cells can cause a rostroventral shift in their positional identity. More specifically, we predicted that if caudodorsal progenitors are exposed to a higher level of Shh activity than normal, these progenitor cells would express rostroventral molecular markers rather than caudodorsal ones. To test this possibility, we crossedNestin-Cre (NesCre/+) transgenic mice (Tronche et al., 1999) withROSA26-stop-SmoM2-EYFP (R26SmoM2/SmoM2) mice (Jeong et al., 2004) to express the fusion protein of SmoM2-EYFP broadly in neural progenitor cells, including those in the thalamus.SmoM2 carries a missense mutation in theSmoothened (Smo) gene, which encodes a transmembrane Shh effector. SmoM2-expressing cells undergo cell-autonomous activation of Shh signaling independent of ligand binding (Xie et al., 1998). Thus, we expected that mis-expression of SmoM2 would cause elevated Shh signaling in all thalamic progenitor cells. By comparingNesCre/+; R26SmoM2/+ and Cre-negative,R26SmoM2/+ controls and wild-type embryos, we found that Cre-negative control and wild-type embryos show the same gene expression and morphology at all stages. In contrast, brains ofNesCre/+; R26SmoM2/+ mice were larger in size than Cre-negative littermates, especially in the dorsal telencephalon.
Analysis of SmoM2-EYFP expression as detected by anti-EGFP antibody showed that inNesCre/+; R26SmoM2/+ embryos, Cre-mediated recombination starts to occur in the diencephalon by E9.5 (Fig. 2A–C,F–H). At this stage, Shh expression in the ZLI has just started (Fig. 2A,F), andGli1 andPtc1 were not clearly detectable in control embryos (Fig. 2D,E). However, inNesCre/+; R26SmoM2/+ embryos,Gli1 andPtc1 were already expressed ectopically in thalamic progenitor cells near the ZLI (Fig. 2I,J). Subsequently, recombination progresses through the thalamus into the more caudodorsal region, and by E10.0, SmoM2-EYFP andGli1 expression covered the entire thalamus labeled by Olig3 (Fig. 2K,L,M,N). By E10.5, most embryos we analyzed showed broad expression of SmoM2-EYFP andGli1 in the entire diencephalon except the most dorsal portion (Fig. 2P,Q). Analysis of recombination usingR26-stop-EYFP reporter mice showed a similar rostroventral to caudodorsal progression of EYFP expression (supplemental Fig. S1A–D, available atwww.jneurosci.org as supplemental material). These results demonstrate that inNesCre/+; R26SmoM2/+ embryos, Shh signaling is elevated in a rostroventral to caudodorsal direction, and by E10.0, there is a uniform enhancement in signaling within the entire thalamic ventricular zone.
Figure 2.
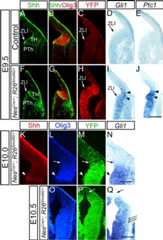
Ectopic expression of SmoM2 in neural progenitor cells elevates the level of Shh signaling throughout the entire thalamic ventricular zone.A–Q, Frontal sections ofNesCre/+; R26SmoM2/+ (F–Q) andCre− control (A–E) embryos at E9.5 (A–J), E10.0 (K–N), and E10.5 (O–Q). Midline is to the left.A–J,Controls (A–E) are compared withNesCre/+; R26SmoM2/+ littermates (F–J) at E9.5.A,B,F,G, Double immunofluorescence for Shh and Olig3 to show the locations of the ZLI and thalamus, which are similar between control andNesCre/+; R26SmoM2/+ embryos. EYFP immunofluorescence shows that Cre-mediated misexpression ofSmoM2-EYFP in the thalamus starts to occur at E9.5, but only in the vicinity of the ZLI (C,H, arrowheads).C andH are adjacent toB andG, respectively.In situ hybridization analysis forGli1 andPtc1 at E9.5 (D,E,I,J) shows thatGli1 andPtc1 are not yet expressed inCre− controls (D,E), but in SmoM2-expressing brain, bothGli1 andPtc1 are prematurely induced in the thalamus (I,J, arrowheads) as well as in the prethalamus.K–N, InNesCre/+; R26SmoM2/+ embryos at E10.0, recombination as indicated by SmoM2-YFP expression spans the entire thalamus marked by Olig3 expression.Gli1 expression follows this. Arrowhead indicates the ZLI, and arrow shows the caudodorsal border of the thalamus.O–Q, InNesCre/+; R26SmoM2/+ embryos at E10.5, recombination has further progressed, and SmoM2-EYFP expression is found in most of the diencephalon. The arrow indicates the most caudal–dorsal part of the diencephalon that has not yet undergone recombination. Triple arrows show that the mantle zone of the thalamus does not expressGli1. Scale bar, 200 μm forA–N, 500 μm forO–Q.
Increased Shh activity induces rostroventral markers in thalamic progenitor cells
We then asked whether the increased Shh signaling inNesCre/+; R26SmoM2/+ embryos alters the expression profile of transcription factors in the thalamus. InNesCre/+; R26SmoM2/+ embryos, expression of Shh in the ZLI was not affected (Fig. 3A,B,E,F). Nonetheless, expression of Nkx2.2 and Mash1, two markers for the rostral progenitor domain, pTH-R, was expanded (Fig. 3C,D,G,H). This expansion results in more than two-fold increase in the size of pTH-R and disrupts the normally distinct gene expression boundary between pTH-R and pTH-C. The once distinct boundary is now replaced by a “mixed zone (pTH-R/C)” in which some cells expressing only pTH-R markers (Nkx2.2 and Mash1) are intermingled with cells expressing only pTH-C markers (Ngn2 and Olig2) (Fig. 3G,H). Such a zone of mixed pTH-R and pTH-C identity was not seen in control embryos (Fig. 3C,D). Although expression of SmoM2-EYFP appeared ubiquitous and homogeneous throughout the thalamic ventricular zone ofNesCre/+; R26SmoM2/+ embryos at E11.5 (supplemental Fig. S1E–H, available atwww.jneurosci.org as supplemental material), it is possible that only a small number of cells in this zone expressed ectopic SmoM2 early enough to take on the pTH-R fate, whereas the remaining cells were irreversibly determined to become pTH-C progenitor cells.
SmoM2 acts cell autonomously to enhance Shh signaling (Xie et al., 1998). Thus, the observed changes in marker expression are likely to be caused by intrinsic changes in thalamic patterning. Nonetheless, since most CNS progenitor cells are forced to express SmoM2 inNesCre/+; R26SmoM2/+ embryos, it is possible that some secondary, indirect effects from outside the thalamus may have contributed to the expansion of pTH-R and/or the formation of the “mixed zone.” To investigate this possibility, we took two additional approaches to further restrict the SmoM2 expression spatially and temporally within the thalamus.
First, we performedin utero electroporation of SmoM2 plasmid (pCAG-SmoM2-EYFP) at E11.5 and analyzed the embryos a day later. We found that similar toNesCre/+; R26SmoM2/+ embryos, both Nkx2.2 (Fig. 3I,J) and Mash1 (data not shown) are expanded and are ectopically expressed more caudally on the electroporated side compared with the control side. As a second approach, we generatedOlig3Cre mice (Fig. 3K), so that we can express Cre recombinase in pTH-R, pTH-C, and the ZLI, but not in surrounding regions of the forebrain such as the prethalamus, the habenula, the pretectum, the diencephalic basal plate or the telencephalon. Cre-mediated recombination as assessed withR26-stop-EYFP reporter mice (Fig. 3L–O) andROSA26-stop-SmoM2-EYFP mice (supplemental Fig. S1I,J, available atwww.jneurosci.org as supplemental material) faithfully followed the expression of the endogenousOlig3 gene. Thus,Olig3Cre mice are useful in thalamus-specific gene manipulation. We found that brains ofOlig3Cre/+; R26SmoM2/+ are morphologically indistinguishable from control littermates, and some of these pups survive postnatally. Neuronal differentiation as determined by the expression of TuJ1, a neuronal marker, was also not affected in these embryos (supplemental Fig. S2A,B, available atwww.jneurosci.org as supplemental material). Additionally, analysis of cell proliferation inOlig3Cre/+; R26SmoM2/+ embryos at E11.5 revealed that BrdU-incorporation and phospho-histone H3 (PH3), an M-phase marker, also appeared normal (supplemental Fig. S2A,B, available atwww.jneurosci.org as supplemental material). Quantification of PH3-positive cells in Olig3-expressing thalamic progenitor cells showed that there was no significant difference in number betweenOlig3Cre/+; R26SmoM2/+ and control embryos (4440 ± 206.0,n = 2 forOlig3Cre/+; R26SmoM2/+, 4906 ± 449.5,n = 2 forCre− controls;p = 0.45).
Analysis of progenitor markers inOlig3Cre/+; R26SmoM2/+ embryos showed similar changes in gene expression compared withNesCre/+; R26SmoM2/+ embryos; pTH-R was expanded, and a “mixed zone (pTH-R/C)” was formed between pTH-C and the expanded pTH-R (Fig. 3P–S). The results of these two additional approaches,in vivo electroporation and the use ofOlig3Cre mice, further reinforce our conclusion that it is the intrinsic increase in Shh signaling that causes the caudodorsal shift of pTH-R markers in thalamic progenitor cells.
We next examined how the pTH-C domain was affected by the increase in Shh signaling. First, inNesCre/+; R26SmoM2/+ embryos, Olig3 was expanded caudodorsally into the pretectal/habenular region at E12.5 (Fig. 4A,G). At this stage,Gbx2 andRORα, markers of the postmitotic thalamus derived from the pTH-C domain (Vue et al., 2007), were also expanded accordingly (supplemental Fig. S3A–C,F–H, available atwww.jneurosci.org as supplemental material). In contrast, Pax7, which is normally expressed in the pretectum and habenula, but not in thalamic progenitor cells, was not detected inNesCre/+; R26SmoM2/+ embryos (Fig. 4E,K). Moreover, expression of Mash1 in the caudal pretectum was replaced by Ngn2, which normally spans from pTH-C only into the rostral pretectum (Fig. 4F,L).Irx2 andBHLHB4, early postmitotic markers of the pretectum, were also reduced at E12.5 (supplemental Fig. S3D,E,I,J, available atwww.jneurosci.org as supplemental material). These results show that a high Shh signal in the caudal diencephalon induces the thalamic progenitor domain, pTH-C, at the expense of the pretectum and habenula. Olig2, which is normally expressed in a rostral–ventral to caudal–dorsal gradient within pTH-C, was homogeneously expressed in this expanded pTH-C domain ofNesCre/+; R26SmoM2/+ embryos (Fig. 4B,H). Another marker gene,Pdlim3, which is normally expressed in pTH-R as well as the rostroventral part of pTH-C (Fig. 4C) (Gray et al., 2004), also expanded (Fig. 4C,I). These results demonstrate that enhanced Shh signaling in the entire diencephalon transformed it into the rostral–ventral thalamus, which is composed of the pTH-R domain and the rostral–ventral part of the pTH-C domain (summarized inFig. 9A,B1,B2,C1,C2).
Figure 4.

Ectopic SmoM2 expression in neural progenitor cells causes the caudal diencephalon to acquire rostral thalamic identity.A–L, Frontal sections ofNesCre/+; R26SmoM2/+ (G–L) andCre− control (A–F) embryos at E12.5. Midline is to the left. InNesCre/+; R26SmoM2/+ thalamus, Olig3 expression maintains its rostral boundary between the ZLI and the prethalamus, but is expanded caudodorsally (A,G; arrows inG indicate the area of expansion). Similarly, Olig2 andPdlim3, both of which are expressed in the rostroventral part of pTH-C inCre− embryos (B,C), are significantly expanded inNesCre/+; R26SmoM2/+ embryos (H,I; arrows show the extent of expansion).Dbx1, which is normally expressed in the caudal pTH-C and the rostral pretectum (D, arrow), is not significantly altered inNesCre/+; R26SmoM2/+ embryos (J, arrows). Pax7 is expressed in the pretectum and the habenula ofCre− controls (E, arrow), but is reduced inNesCre/+; R26SmoM2/+ embryos (K, no expression between arrowheads). Ngn2 is expressed in pTH-C and the rostral pretectum, whereas Mash1 is expressed in the caudal pretectum (F, arrows to show Mash1 expression). InNesCre/+; R26SmoM2/+ brains, Ngn2 is induced in the caudal pretectum and Mash1 is repressed (L, no expression between the arrows).M–R,Olig3Cre/+; R26SmoM2/+ (P–R) andCre− control (M–O) embryos at E12.5. Midline is to the left. InOlig3Cre/+; R26SmoM2/+ embryos, Olig3 expression is not expanded, unlike inNesCre/+; R26SmoM2/+ embryos (M,P, arrow showing the caudodorsal boundary of pTH-C), indicating that SmoM2 expression is indeed limited to the thalamus. Within the thalamus, however,Ptc1 expression is increased and now occupies the entire pTH-R and pTH-C (N,Q). Olig2 is also induced more caudodorsally within pTH-C (O,R). Arrow inM–R indicates the caudal–dorsal limit of gene expression, showing that unlike in theCre− control,Ptc1 and Olig2 are now expressed in the entire pTH-C inOlig3Cre/+; R26SmoM2/+ embryos.S,T, E12.5 embryo in whichpCAG-SmoM2-EYFP was electroporated into two focal diencephalic regions at E11.5. The right side was electroporated. Olig2 is ectopically expressed in the caudal group of electroporated cells in an isolated manner (arrow). Scale bar, 200 μm.
Figure 9.

Summary of the current study.A, Side view of the embryonic thalamus. The curved anterior–posterior (A–P) axis of the brain is shown in a red dotted line. The black vertical line indicates the orientation of frontal sections used throughout this study. In a given section, the top part is more dorsal and caudal, while the bottom part is more ventral and rostral.B1,C1,D1, Schematic frontal sections showing the progenitor domains at early embryonic stages in wild type (WT),NesCre/+; R26SmoM2/+ (SmoM2), andNesCre/+; Shhc/c (Shh cko) mice. Color gradients within pTH-C indicate the graded differential patterns of gene expression within this domain, which are altered when Shh signal is increased (C1) or decreased (D1).B2,C2,D2, Differential gene expression patterns in each genotype.B3,C3,D3, Schematic frontal sections indicating the postmitotic thalamus at late embryonic stages. The graded color of pTH-C-derived thalamus corresponds to the overall lineage relationship with the progenitor domains shown inB1,C1, andD1. Alterations in SmoM2-expressing mice and Shh cko mice indicate that the changes in the mantle zone are consistent with those observed in progenitor cells.
Surprisingly, the expression of Dbx1, which is normally expressed in the caudodorsal part of pTH-C as well as in the pretectum and habenula, was not significantly reduced inNesCre/+; R26SmoM2/+ embryos (Fig. 4D,J), suggesting that Dbx1 is positively regulated by some unknown mechanisms operating in the caudodorsal diencephalon. Expression ofDlx2, a prethalamic marker, was not ectopically induced in the thalamic progenitor cells inNesCre/+; R26SmoM2/+ embryos (data not shown), consistent with a previous study showing that the thalamus and the prethalamus are distinctly prepatterned and express different sets of genes in response to Shh signaling (Kiecker and Lumsden, 2004).
To ensure that the above changes were also not the product of indirect effects, we again analyzed embryos electroporated with thepCAG-SmoM2-EYFP plasmid, as well asOlig3Cre/+; R26SmoM2/+ embryos. In electroporated embryos where an isolated population of progenitor cells in the pretectum were transfected, Olig2 was ectopically induced (Fig. 4S,T). InOlig3Cre/+; R26SmoM2/+ embryos, although the pretectum and the habenula are spared and the extent of Olig3 expression was similar to controls (Fig. 4M,P),Ptc1 and Olig2 were homogeneously elevated within the entire pTH-C domain (Fig. 4N,O,Q,R), demonstrating that thalamus-specific elevation of Shh signaling intrinsically induced the rostral pTH-C markers.
Expression of rostral postmitotic thalamic markers is expanded in SmoM2-expressing embryos
Does increased Shh signaling in thalamic progenitor cells lead to reorganization of postmitotic thalamus? To address this question, we examined the expression of postmitotic markers that are differentially expressed within nuclei of the late embryonic thalamus (Fig. 5).
Figure 5.

Ectopic SmoM2 expression causes a rostroventral shift of postmitotic thalamus.A–L, Frontal sections ofNesCre/+; R26SmoM2/+ (G–L) andCre− control embryos (A–F) at E16.5. Midline is to the left. In control embryos, Nkx2.2 andSox14 expression delineates the lateral vLG and IGL (A–C, arrowhead), which are derived from pTH-R.B, High-magnification image of the boxed region inA. InNesCre/+; R26SmoM2/+ embryos, Nkx2.2 andSox14 expression is expanded caudally and dorsally (G–I, arrowhead).H, High-magnification image of the boxed areas inG, showing that some cells ectopically expressing Nkx2.2 (H, arrowheads) are intermingled with Sox2-expressing cells (J). InCre− controls, Sox2 is highly expressed in rostroventral part of the postmitotic thalamus, including VP and dLG, but not in more caudodorsal part (D, asterisk). InNesCre/+; R26SmoM2/+ embryos, Sox2 is robustly expanded caudodorsally (J, arrowheads). Similarly,RORα expression, which corresponds to rostrolateral nuclei such as VP and dLG (E), is also expanded caudodorsally inNesCre/+; R26SmoM2/+ embryos (K, arrowheads). Note that the medial part of the thalamus that is negative forRORα in control is also negative in SmoM2 embryos (E,K, asterisk). The caudodorsal expression border ofGbx2 is elongated near the midline in SmoM2 embryos (F,L, arrow), but overall,Gbx2 expression is reduced, especially from the medial dorsal part (F,L, asterisk).M,N, E17.5 embryo in whichpCAG-SmoM2-EYFP plasmid was electroporated at E11.5 into the thalamus. The right side was electroporated, and the left side is the control. On the electroporated side, Sox2 is ectopically induced in a caudodorsal region (M, arrow), while this induction did not occur on the control side. Conversely,Gbx2 is downregulated where Sox2 is induced (N, arrow).O–T,Olig3Cre/+; R26SmoM2/+ andCre− control embryos at E18.5. Midline is to the left. InOlig3Cre/+; R26SmoM2/+ embryos, Sox2 expression is expanded and now occupies the entire thalamic mantle zone (R). The Sox2-negative, caudodorsal region (asterisk inO) is undetectable inOlig3Cre/+; R26SmoM2/+ embryos. The caudodorsal boundary of the thalamus is outlined by a dotted line. Similarly, expression ofRORα is expanded (P,S). Unlike Sox2,RORα is not induced in the medial part of the thalamic mantle zone (P,S, asterisk).Gbx2 expression is dramatically reduced inOlig3Cre/+; R26SmoM2/+ embryos (Q,T).U–X, Postnatal day 1 thalamus ofOlig3Cre/+; R26SmoM2/+ (W,X) andCre− control (U,V) pups injected with CTB in the contralateral retina at P0. dLG nucleus is delineated as dashed lines by CTB immunohistochemistry (V,X) and DAPI staining (U,W). dLG is expanded by ∼3.6 times (see Results). Scale bar, 200 μm forB,H; 500 μm for other panels.
First, we analyzed nuclei that are derived from the pTH-R domain. We previously found that progenitor cells in pTH-R generate part of the vLG as well as the IGL, two nuclei that do not contain neurons projecting to the neocortex (Vue et al., 2007). Consistent with this fate analysis and the expanded pTH-R markers inNesCre/+; R26SmoM2/+ as well asOlig3Cre/+; R26SmoM2/+ embryos, vLG/IGL was expanded caudodorsally along the lateral surface of the diencephalon (Fig. 5A,G forNesCre/+; R26SmoM2/+, and data not shown forOlig3Cre/+; R26SmoM2/+). These expanded nuclei are marked by expression of Nkx2.2,Sox14 (Fig. 5A–C,G–I), and Sox1 (data not shown). The expanded Nkx2.2 andSox14 expression is consistent with previous studies using chick and mouse embryos (Hashimoto-Torii et al., 2003;Kiecker and Lumsden, 2004;Vieira et al., 2005) and establishes that IGL and the lateral part of vLG are specified by high Shh activity in pTH-R. Nkx2.2-positive cells were also found in a more caudolateral region that is away from the large cluster of expanded vLG/IGL cells (Fig. 5H, arrowheads). These cells did not express Sox2 but intermingled with Sox2-expressing cells (Fig. 5, compareG,H,J). This intermingling of two molecularly different cell types reflects our earlier observation that two completely separate progenitor cell populations, one with pTH-R identity and the other with pTH-C identity, are mixed in the SmoM2-expressing, thalamic ventricular zone (Fig. 3G,H).
Next, we tested whether markers of thalamic nuclei that are derived from the pTH-C domain are also shifted in SmoM2-expressing embryos. In control embryos, Sox2 is expressed in the rostroventral part of the pTH-C-derived thalamic mantle zone (Fig. 5D,M,O) (Vue et al., 2007). In E16.5NesCre/+; R26SmoM2/+ brains, Sox2 expression was expanded caudally and dorsally, occupying essentially the entire diencephalic mantle zone (Fig. 5D,J). Similarly, another rostral nucleus marker,RORα, which is expressed more specifically in ventral posterior nucleus (VP) and dLG (Nakagawa and O'Leary D, 2003), was expanded caudally and dorsally inNesCre/+; R26SmoM2/+ brains compared with controls (Fig. 5E,K). To determine whether the expansion of these rostral nucleus markers is accompanied by the repression of caudal nucleus markers, we analyzed the expression ofGbx2. AlthoughGbx2 is initially expressed in the entire postmitotic thalamus (supplemental Fig. S3A,B,F,G, available atwww.jneurosci.org as supplemental material), its expression later becomes restricted to medial and caudodorsal nuclei and is nearly complementary to that of Sox2 andRORα (Fig. 5F,N,Q). InNesCre/+; R26SmoM2/+ brains,Gbx2 expression was dramatically reduced, particularly in regions where both Sox2 andRORα are ectopically induced (Fig. 5L).
To better demonstrate the local effects of enhanced Shh signal on postmitotic thalamic development, we again analyzed SmoM2-electroporated embryos andOlig3Cre/+; R26SmoM2/+ embryos. When caudodorsal thalamus was electroporated at E11.5 and analyzed at E17.5, Sox2 was highly induced in the cohort of cells on the transfected side (Fig. 5M).Gbx2 was specifically repressed in these Sox2-expressing cells, but its expression on the control side was unaffected (Fig. 5M,N). InOlig3Cre/+; R26SmoM2/+ brains at E18.5, the overall morphology of the thalamic mantle zone was normal. However, Sox2 was ubiquitously expressed in the entire thalamus, not just in rostroventral nuclei as seen in the control (Fig. 5O,R).RORα was also significantly expanded caudodorsally in the lateral part of the thalamus (Fig. 5P,S). Furthermore,Gbx2 expression is almost completely absent inOlig3Cre/+; R26SmoM2/+ brains (Fig. 5Q,T). These data demonstrate that inOlig3Cre/+; R26SmoM2/+ embryos, the entire postmitotic thalamus is dominated by rostroventral identity.
Although the expansion ofRORα implicates that rostroventrally located nuclei, VP, and dLG are increased in size inOlig3Cre/+; R26SmoM2/+ embryos, we used criteria other than molecular markers that would definitively prove that individual nuclei are altered. For this purpose, we used anatomical criteria to define thalamic nuclei. Specifically, we injected anterograde axon tracer CTB to the retina of the neonatalOlig3Cre/+; R26SmoM2/+ mice to label retinogeniculate axons in the dLG nucleus. Because Olig3 is not expressed in the retina or along the retinogeniculate pathway to the thalamus,Olig3Cre/+; R26SmoM2/+ mice provide a particularly useful model to evaluate the specific effects of enhanced Shh signal in thalamic progenitor cells on the size of postnatal thalamic nuclei. We found that the volume of the dLG nucleus as estimated by the CTB-labeled region in the thalamus, was increased by ∼3.6 times inCre+ pups (0.086 ± 0.008 mm3 inCre+ pups,n = 3; 0.024 ± 0.003 mm3 inCre− pups,n = 2;p = 0.0091) (Fig. 5U–X).
In summary, we demonstrated that increasing Shh signaling in CNS progenitor cells results in expansion of rostral–ventral thalamic progenitor pools, pTH-R, and rostroventral pTH-C, at the expense of more caudal–dorsal thalamus as well as the pretectum and habenula (summarized inFig. 9B1,B2,C1,C2). When the manipulation of the Shh signal was restricted to thalamic progenitor cells, pTH-R and rostroventral pTH-C domain still expanded and caudal–dorsal pTH-C was shrunken. These results strongly indicate that the high Shh signal biases thalamic progenitor cells toward more rostral–ventral fates in a cell-intrinsic manner. This conclusion is consistent with our observation that the number of PH3+ cells in the entire Olig3-expressing domain was similar betweenOlig3Cre/+; R26SmoM2/+ and control embryos (supplemental Fig. S2, available atwww.jneurosci.org as supplemental material). Compatible with changes in progenitor identity, postmitotic thalamic nuclei are expanded or shrunken based on their putative locations of origin (summarized inFig. 9B3,C3).
BecauseGli1 andPtc1 are not ectopically expressed in the mantle zone ofSmoM2-expressing brains, the altered organization of the postmitotic thalamus is unlikely to be due to the direct activation of Shh signaling in postmitotic cells. Instead, we conclude that shifted identity of thalamic progenitor cells resulted in corresponding alteration of the identity of postmitotic thalamic nuclei.
Overexpression of the bHLH transcription factor Olig2 partially mimics the effect of SmoM2 expression in the thalamus
If Shh signal directly controls the identity of thalamic progenitor cells and indirectly influences the specification of postmitotic thalamic nuclei, it is likely that transcription factors that are regulated by Shh signal in progenitor cells translate their positional information onto their postmitotic progeny. Olig2 is normally expressed in the rostroventral portion of pTH-C, and is induced in progenitor cells that ectopically express SmoM2. These SmoM2-expressing embryos also showed induction of Sox2 andRORα caudally and dorsally throughout the thalamic mantle zone. Thus, it is possible that Olig2 functions to specify rostroventrally located thalamic nuclei that express Sox2 andRORα. To test this possibility, we first performed genetic lineage tracing of Olig2-expressing progenitor cells (supplemental Fig. S4A–C, available atwww.jneurosci.org as supplemental material). We crossedOlig2Cre/+ mice (Dessaud et al., 2007) withROSA26-stop-EGFP reporter mice, and analyzed E14.5 compound heterozygous embryos. At E11.5, GFP was expressed in the rostroventral part of pTH-C, as well as pTH-R (supplemental Fig. S4A, available atwww.jneurosci.org as supplemental material). Expression of GFP in pTH-R suggests that there is transient expression ofOlig2 in this progenitor domain before E10.5, which is similar to the observation in ventral spinal cord (Dessaud et al., 2007). At E14.5, EGFP was expressed in the rostroventral portion of the postmitotic thalamus, which largely overlapped with Sox2 expression (supplemental Fig. S4B,C, available atwww.jneurosci.org as supplemental material). VP and the ventral part of medial geniculate (MGv) nuclei were two nuclei that had particularly strong EGFP expression. In contrast, EGFP was not found in more caudodorsal nuclei. Thus, Olig2-expressing thalamic progenitor cells give rise to rostroventral part of the postmitotic thalamus, consistent with the possibility that Olig2 plays a role in the specification of rostroventral nuclei such as VP and MGv.
We then tested whether Olig2 is necessary and/or sufficient for the specification of these nuclei. When we ectopically expressed Olig2 byin utero electroporation at E10.5 in the caudodorsal diencephalon, Sox2 was induced in the mantle zone only on the transfected side at E13.5 and E14.5 (supplemental Fig. S4E,F, available atwww.jneurosci.org as supplemental material), which partially mimicked the effect of expressing SmoM2. Cell proliferation and neuronal differentiation did not seem to be affected in Olig2-transfected cells, and overexpressing EGFP alone did not induce Sox2 expression (data not shown). These results suggest that Olig2 mediates the Shh signal and confers at least part of the rostroventral identity to the postmitotic thalamus. In contrast, Olig2 null mice did not show altered expression of Sox2 or progenitor markers such as Mash1, Ngn2, and Dbx1 (data not shown). Thus, Olig2 is not necessary but is sufficient to induce at least some molecular features of the rostroventral thalamic nuclei.
Attenuation of Shh signal in thalamic progenitor cells reduces the expression of rostroventral markers and increases caudodorsal markers
Previous studies on chick embryos showed that ectopic expression of Ptc1Δloop2, a dominant-negative form of the Shh receptor, Ptc1, reduced the expression ofNkx2.2 andGbx2, and increased the expression ofPax6, which is normally expressed highly in the caudal diencephalon (Kiecker and Lumsden, 2004). It was also reported thatGli2/3 double knock-out mice showed reduced expression ofGbx2, whereasGli1 mutant mice showed reducedSox14 expression (Hashimoto-Torii et al., 2003). These studies demonstrated the requirement of Shh signaling in establishing the identity of the thalamus as a whole. However, it is still unknown whether Shh is required for the patterned expression of progenitor markers in pTH-R and pTH-C, or for the normal organization of postmitotic thalamic nuclei. Shh null mice show pronounced reduction in size of the diencephalon by E9.0, which reflects the early role of Shh in cell proliferation and survival (Ishibashi and McMahon, 2002). Therefore, we analyzed conditionalShh knock-out mice by using theNesCre allele to avoid such early effects. Conditional Shh mutant mice were smaller than control littermates, but they were postnatally viable and have relatively normal gross morphology of the brain (data not shown). The smaller brain size is reflected in part by a significant decrease in the number of PH3-positive progenitor cells in the thalamic ventricular zone of conditionalShh knock-out (NesCre/+; Shhc/c) embryos (3437 ± 146.0,n = 2 forNesCre/+; Shhc/c, 6605 ± 472.0,n = 2 forCre− controls;p = 0.024) (supplemental Fig. S2C,D, available atwww.jneurosci.org as supplemental material).
InNesCre/+; Shhc/c embryos, Shh expression in the forebrain was lost by E10.5 (data not shown;Machold et al., 2003). Consistent with the loss of Shh in the ZLI and the basal plate,Ptc1 (data not shown) andGli1 mRNA was undetectable at E10.5 and E12.5 inNesCre/+; Shhc/c embryos (Fig. 6B,G and not shown). Furthermore, markers for the pTH-R domain were not induced. At E12.5, we observed a complete loss of Nkx2.2 (Fig. 6C,H) and Mash1 (Fig. 6D,I) within the thalamic ventricular zone, although their expression in the prethalamic progenitor domain remained at very low levels (Fig. 6H,I). Thus,NesCre/+ mice are useful in deleting theShh gene in the brain when Shh signal is still critically required for the induction and/or maintenance of the expression of pTH-R markers. In addition to Nkx2.2 and Mash1, expression of Olig2, a rostroventral pTH-C marker, was also lost in pTH-C ofNesCre/+; Shhc/c embryos, although it was weakly detected in the prethalamus (Fig. 6C,H, arrowhead for the prethalamus). In contrast, markers that are expressed throughout the pTH-C domain, such as Olig3 (Fig. 6A,F) and Ngn2 (Fig. 6D,I) were still expressed inNesCre/+; Shhc/c embryos at E12.5 and earlier stages, although the size and intensity of the expression of Olig3 seemed to be reduced. Dbx1, which is normally restricted to the caudodorsal pTH-C, was expanded rostroventrally inNesCre/+; Shhc/c thalamus (Fig. 6E,J). A pretectal marker, Pax7, was still expressed (data not shown). Together, these findings demonstrate that Shh activity is required for progenitor cell identity in pTH-R and rostroventral pTH-C and for suppressing the caudodorsal pTH-C identity (summarized inFig. 9B1,B2,D1,D2).
Figure 6.
Shh is required for the normal organization of thalamic progenitor domains. Frontal sections ofNesCre/+; Shhc/c forebrain (F–J) andNesCre/+; Shhc/+ controls at E12.5 (A–E). Midline is to the left. Shh is expressed in the ZLI of control embryos (A, arrow) but not in theNesCre/+; Shhc/c mice (F, arrow). Olig3 expression is maintained inNesCre/+; Shhc/c embryos, but the domain is smaller and density of Olig3-positive cells in this domain also seems reduced (F).Gli1, a target gene of Shh signaling, is lost in the thalamic progenitor cells ofNesCre/+; Shhc/c embryos (G), while it is expressed in a graded manner in the control (B). Expression of Nkx2.2 and Olig2 is found in controls (C, arrow for Nkx2.2, double arrows for Olig2), but is completely lost inNesCre/+; Shhc/c embryos (H). A reduced number of Olig2-expressing cells are still found in the prethalamus (C,H, arrowhead). Mash1 expression in the pTH-R domain is detected in the control but is lost inNesCre/+; Shhc/c embryos (D,I, arrowhead). Mash1 expression in the posterior pretectum is maintained (D,I, arrow). Expression of Ngn2 is intact and now occupies the entire thalamus, including the most rostroventral region (I, arrowhead). Dbx1 expression is limited to the rostral pretectum and caudodorsal part of pTH-C in control embryos (E). The expression domain of Dbx1 is expanded rostroventrally inNesCre/+; Shhc/c embryos (E,J, arrow). Scale bar, 200 μm.
Next, to more directly test the intrinsic requirement of Shh signaling in the thalamus, we conditionally knocked out the gene encoding Smo, a transmembrane effector of Shh signaling. Two Cre drivers,NesCre/+ andOlig3Cre/+, were used. BothNesCre/+; Smoc/c andOlig3Cre/+; Smoc/c mice showed normal gross morphology of the brain and survived postnatally. We found thatNesCre/+; Smoc/c embryos show lack ofPtc1 expression in the thalamus at E12.5 (Fig. 7B,G) despite the normal expression of Shh in the ZLI (Fig. 7A,F). In these embryos, we also found that Olig3 expression was reduced in the thalamic ventricular zone (Fig. 7A,F), and Mash1 and Olig2 were undetectable in pTH-R and pTH-C, respectively (Fig. 7C,D,H,I). Furthermore, Dbx1 expression was expanded rostroventrally (Fig. 7E,J). However, unlike inNesCre/+; Shhc/c embryos, Nkx2.2 was still detectable though not as robustly as in the control (Fig. 7C,H).
Figure 7.

Smo is required for the normal organization of thalamic progenitor domains. Frontal sections ofNesCre/+; Smoc/c andNesCre/+; Smoc/+ control embryos (A–J) andOlig3Cre/+; Smoc/c andOlig3Cre/+; Smoc/+ control embryos (K–T) at E12.5. Midline is to the left.A–J,NesCre/+; Smoc/c embryos show normal expression of Olig3 and Shh (A,F), butPtc1 (B,G, arrow) and Olig2 (C,H, arrow) are lost in thalamic progenitor cells. Nkx2.2 is still detectable in a reduced number (C,H double arrows). Mash1 expression in pTH-R is undetectable inNesCre/+; Smoc/c embryo (D,I, arrow), whereas it is still detectable in the prethalamus (D,I, double arrows). Dbx1 expression is expanded inNesCre/+; Smoc/c embryos (E,J). Arrows inE andJ indicate the boundaries of the expression domain of Dbx1.K–T,Olig3Cre/+; Smoc/c embryos show normal expression of Olig3 and Shh (K,P).Ptc1 expression is reduced in the thalamus (L,Q, arrow) but not in the prethalamus (L,Q, double arrows). Mash1 expression in the thalamus is reduced inOlig3Cre/+; Smoc/c embryos (M,R, double arrow). In high-magnification views ofM andR, Ngn2, a pTH-C marker, is now expressed more rostroventrally. Ngn2-expressing cells are intermingled with Mash1-expressing cells, forming a mixed zone of pTH-R/C at the expense of pTH-R (N,S, bracket). Expression of Dbx1 is expanded inOlig3Cre/+; Smoc/c embryos, reaching near the rostroventral border of the thalamus (O,T, arrowhead). Olig2 expression is still detected in the pTH-C domain ofOlig3Cre/+; Smoc/c embryos (O,T). Scale bar, 100 μm forN,S; 200 μm for other panels.
Olig3Cre/+; Smoc/c embryos at E12.5 showed normal expression of Shh in the ZLI (Fig. 7K,P) and reduction ofPtc1 expression in the thalamus but not in the prethalamus (Fig. 7L,Q), compatible with the restricted deletion ofSmo and reduced Shh signal specific to the thalamus. The reduction ofPtc1 expression in the thalamus was less significant than inNesCre/+; Smoc/c embryos, wherePtc1 was undetectable in the thalamus and the prethalamus (Fig. 7G). Nevertheless,Olig3Cre/+; Smoc/c embryos showed reduction of Mash1-expressing cells in the thalamus (Fig. 7M,N,R,S). In addition, the decreased number of Mash1-expressing cells were intermingled with Ngn2-expressing cells, giving rise to the mixed zone (pTH-R/C) instead of pure pTH-R as we found in the control (Fig. 7N,S). Although Nkx2.2 and Olig2 were not consistently reduced in expression, Dbx1 expression was grossly expanded rostroventrally (Fig. 7O,T), as in theNesCre/+; Shhc/c andNesCre/+; Smoc/c embryos. Together, these results indicate that the Shh signal is intrinsically required for the induction and/or maintenance of molecular characteristics of the pTH-R domain and for the suppression of caudodorsal part of the pTH-C domain.
The dramatic changes in the progenitor cell identity with conditional deletion of theShh orSmo gene prompted us to examine which populations of postmitotic thalamus are affected in the absence ofShh orSmo. In E17.5NesCre/+; Shhc/c embryos, as expected from the loss of pTH-R markers at E12.5 (Fig. 6C,D,H,I), Nkx2.2 (Fig. 8A,C,F,H) andSox14 (data not shown) in vLG/IGL were both dramatically reduced, confirming the requirement of Shh signaling for the vLG/IGL lineage. Similar changes were found inNesCre/+; Smoc/c embryos (Fig. 8K–N). We next analyzed the pTH-C derivative. Expression of Sox2 (Fig. 8B,G) andRORα (Fig. 8D,I) was profoundly reduced inNesCre/+; Shhc/c embryos. In contrast,Gbx2 expanded and occupied almost the entire thalamic mantle zone (Fig. 8E,J). Again, similar changes were observed inNesCre/+; Smoc/c embryos (Fig. 8K,M and data not shown). InOlig3Cre/+; Smoc/c mice, IGL, as marked by Sox1 and neuropeptide Y (NPY) expression, was dramatically reduced in size and cell number. Sox1-positive cells in this nucleus was reduced by ∼70% (Olig3Cre/+; Smoc/+: 337.5 ± 13.80 cells/slide,Olig3Cre/+; Smoc/c: 103 ± 24.93 cells/slide,n = 4;p = 0.0002). This reduction likely reflects the reduction of Mash1-positive cells in the pTH-R at E12.5 (Fig. 7M,N,R,S).
Figure 8.

Shh signaling is required for the normal organization of postmitotic thalamus.A–P, Frontal sections ofNesCre/+; Shhc/c andNesCre/+; Shhc/+ control embryos at E17.5 (A–J),NesCre/+; Smoc/c andNesCre/+; Smoc/+ control embryos at E17.5 (K–N), andOlig3Cre/+; Smoc/c andOlig3Cre/+; Smoc/+ control embryos at E18.5 (O,P). Midline is to the left.A–J, Immunohistochemistry for Nkx2.2 (A,F), Sox2 (B,G), andin situ hybridization ofRORα (D,I) andGbx2 (E,J).C,H, High-magnification images ofA/B andF/G, respectively, showing the double immunofluorescence of Nkx2.2 and Sox2. Nkx2.2 is expressed in vLG/IGL in the control but is undetectable inNesCre/+; Shhc/c brains (A,C,F,H; see arrowhead inC,H). Sox2 is expressed in the rostroventral part of the postmitotic thalamus, including VP and dLG in control embryos (B). InNesCre/+; Shhc/c thalamus, Sox2 expression is severely reduced to a small, lateral region (G). Expression ofRORα shows an even more dramatic reduction inNesCre/+; Shhc/c mice (D,I).Gbx2 is expressed in many thalamic nuclei, but is excluded from the rostroventral nuclei such as VP and dLG in control embryos (E). InNesCre/+; Shhc/c brain,Gbx2 is expanded, reaching the most rostroventral part of the thalamus in these mice (J, arrowheads).K–N, Immunohistochemistry for Nkx2.2 and Sox2 shows that Sox2 expression is dramatically reduced inNesCre/+; Smoc/c embryos at E17.5. High-magnification images of the boxed areas inK andM clearly show the loss of Nkx2.2 in the vLG/IGL region ofNesCre/+; Smoc/c embryos (L,N).O,P, Immunohistochemistry for the two IGL markers, Sox1 and NPY, shows that both markers are severely reduced inOlig3Cre/+; Smoc/c embryos at E18.5. Po, Posterior nucleus; LP, lateral posterior nucleus. Scale bar, 500 μm forC,H; 500 μm for other panels.
In summary, decreasing Shh signaling resulted in reduction of rostral–ventral progenitor pools, pTH-R, and the rostroventral part of the pTH-C domain, and expansion of caudodorsal progenitor pools (summarized inFig. 9B1,B2,D1,D2). This change in thalamic progenitor cell identity corresponds to alterations in postmitotic populations (Fig. 9B3,D3). Although conditional deletion ofShh orSmo with two different Cre lines caused different degrees of reduction in Shh signal, we observed consistent changes in the progenitor cell populations as well as their postmitotic derivatives. These observations confirm the cell-intrinsic requirement of Shh signal in the specification of rostral–ventral progenitor cells in the thalamus.
Relationship between Shh and Fgf signaling pathways in thalamic patterning
A recent study byKataoka and Shimogori (2008) reported that Fgf8 is expressed in the dorsal midline of the caudal diencephalon and that this expression domain continues to a region immediately rostral to the dorsal tip of the ZLI, suggesting that Fgf signaling may have a role in thalamic patterning. Focalin vivo electroporation of Fgf8 near the ZLI increased the size of pTH-R and its derivative, “Rim domain,” although overexpression of Fgf8 did not change the expression pattern ofShh orPtc1 (Kataoka and Shimogori, 2008). These results suggest that Fgfs may play a patterning role independent of Shh signaling in the thalamus by regulating gene expression in pTH-R progenitor cells. Alternatively, Fgf8 may be downstream of the Shh pathway and mediates part of the roles that Shh plays. In fact, Shh induces the expression of Fgf4 in early limb development (Laufer et al., 1994), and completeShh null embryos show reduced expression ofFgf15 in mouse diencephalon at E8.5 (Ishibashi and McMahon, 2002). To assess the role of Shh in Fgf8 expression and Fgf signal in the thalamus, we analyzed the expression ofFgf8 and its downstream targets,Spry1 andSpry2 (Liu et al., 2003) inNesCre/+; R26SmoM2/+ andNesCre/+; Shhc/c embryos (supplemental Fig. S4, available atwww.jneurosci.org as supplemental material). We found that in both control andNesCre/+; R26SmoM2/+ embryos,Fgf8 is expressed in the dorsal midline as well as in the region immediately rostral to the dorsal part of the ZLI (supplemental Fig. S4B,F, available atwww.jneurosci.org as supplemental material). Expression ofSpry1 andSpry2 was also similar in the control and SmoM2-expressing embryos (supplemental Fig. S4C,D,G,H, available atwww.jneurosci.org as supplemental material). In addition,NesCre/+; Shhc/c embryos at E12.5 showed no significant reduction of expression ofFgf8,Spry1, orSpry2 (supplemental Fig. S4J–L, available atwww.jneurosci.org as supplemental material) near the ZLI. These results indicate that the changes to the pTH-R domain observed inNesCre/+; R26SmoM2/+ andNesCre/+; Shhc/c embryos are not due to alterations inFgf8 expression or Fgf signal.
Discussion
In this study, we used two lines of mice expressing Cre recombinase to conditionally increase or decrease Shh signaling in the thalamus, and performedin vivo electroporation to elevate the signal. When Shh signaling was globally increased using theNesCre allele, the entire caudal alar diencephalon, but not the surrounding brain regions including the midbrain, took on the molecular properties of the thalamus, which is consistent with previous studies using chick embryos (Vieira et al., 2005). Our current study further demonstrates that the differential Shh signal plays a crucial role not only in the specification of the most rostroventral progenitor domain of the thalamus (pTH-R) and its postmitotic derivatives, but also in the patterning within the caudodorsal thalamic progenitor domain (pTH-C) and the formation of the cortex-projecting thalamic nuclei (summarized inFig. 9).
A high Shh activity positively regulates the identity of the pTH-R domain and its postmitotic derivatives in the thalamus
Previous studies revealed that a high Shh activity positively regulates the expression ofNkx2.2 andSox14 in chick and mouse thalamus (Hashimoto-Torii et al., 2003;Kiecker and Lumsden, 2004;Vieira et al., 2005). We find that in the mouse, bothNkx2.2 andSox14 are expressed in the lineage of the rostral progenitor domain, pTH-R. In our present study, ectopic expression of SmoM2 resulted in increased Shh activity in thalamic progenitor cells as shown by the expression of the target genesGli1 andPtc1, and expansion of pTH-R markers. Postmitotic populations derived from pTH-R progenitor cells also increased. Conversely, conditionally deletingShh using theNesCre allele completely abolished the expression of Nkx2.2 and Mash1 as well as the postmitotic markers of the pTH-R derivative. Deleting theSmo gene with eitherNesCre orOlig3Cre allele also reduced expression of pTH-R markers in progenitor cells, and resulted in corresponding decrease in its postmitotic derivative, further confirming the cell-autonomous requirement of Shh signaling in this cell lineage. Interestingly, even when SmoM2 is ectopically expressed in the entire thalamic progenitor domains either withNesCre orOlig3Cre allele, only part of the thalamus became pTH-R; the caudodorsal part still expressed pTH-C markers (Fig. 3G,H,R,S). Because the Cre-mediated recombination seems to progress in a rostroventral to caudodorsal direction in these Cre mice, it is possible that the rostroventral part starts to express SmoM2 earlier than caudodorsal part. If SmoM2 can induce ectopic expression of pTH-R markers only before a certain stage of development, the differential onset of SmoM2 expression across the thalamic ventricular zone might explain the partial transformation of pTH-C into pTH-R. In addition, caudodorsally located thalamic progenitor cells may be less sensitive to enhanced Shh signaling than rostroventral cells. This may explain whyin vivo electroporation of SmoM2 at E11.5 caused the ectopic expression of pTH-R markers only in the most rostroventral part of pTH-C (Fig. 3I,J).
However, it is also possible that establishment of pTH-R identity requires not only high Shh signaling, but also activity of other signaling molecules that are expressed in or near the ZLI or the basal plate. An intriguing possibility is Fgf8, which was recently shown to play an independent role in specifying pTH-R and its derivatives (Kataoka and Shimogori, 2008). In this current study, we found that increasing Shh activity does not enhance the expression ofFgf8 or its downstream targets,Spry1 andSpry2, and that sustained expression of Shh is not required for the expression ofFgf8,Spry1, orSpry2 in the diencephalon. Thus, if activation of both Shh and Fgf8 pathways is required for the expression of pTH-R markers, the lack of increased Fgf8 signaling in SmoM2-expressing thalamus may account for the limited expansion of pTH-R domain. However, since Fgf8 is expressed only near the dorsal tip of the ZLI, not in the entire ZLI, it is unclear whether endogenous Fgf8 signaling is required for all the progenitor cells in the pTH-R domain. Further studies are needed to identify the collaboration of signaling pathways responsible for the pTH-R identity.
How does Shh activity control the patterning of the pTH-C domain and the cortex-projecting thalamic nuclei?
Compared with the contribution of Shh signaling to the pTH-R lineage, understanding the role of Shh activity in the caudodorsal progenitor domain, pTH-C, is more challenging. The pTH-C domain contains highly heterogeneous progenitor populations characterized by graded expression of transcription factors such as Olig2 or Dbx1. In addition, the many dozens of distinct, cortex-projecting thalamic nuclei that are generated from this domain are not always identifiable by marker gene expression alone, and the use of cytoarchitectural features to discriminate individual nuclei is also limited. Because of these difficulties, how these nuclei are patterned and specified during embryogenesis is not well understood. We approached this question by first identifying and characterizing the molecular differences within pTH-C, which we found contributes to all the cortex-projecting thalamic nuclei. This analysis indicated that heterogeneity within this progenitor domain may contribute to the specification of multiple thalamic nuclei (Vue et al., 2007). Our current study now shows for the first time that the level of Shh signaling controls the identity of pTH-C progenitor cells, and that this in turn regulates the sizes and identity of postmitotic thalamic nuclei.
We showed that high Shh signaling positively regulates the expression of Olig2 andPdlim3 in the rostral part of pTH-C. Conversely, conditional deletion ofShh orSmo with theNesCre/+ allele diminishes the expression of Olig2 in pTH-C, while it expands the expression of Dbx1, a marker for caudodorsal pTH-C. In addition, increasing or decreasing Shh signaling altered regional patterns of gene expression in the postmitotic thalamus in a way that was consistent with the changes in progenitor cells. Among the postmitotic markers we analyzed,RORα and Sox2 are expressed more strongly in the nuclei that are derived from the rostroventral part of the pTH-C domain. In SmoM2-expressing embryos, Sox2 andRORα are expanded to occupy the entire rostrocaudal extent of the postmitotic thalamus, consistent with the expanded expression of Olig2 andPdlim3. Shh andSmo knock-out brains show the opposite overall changes. These results clearly demonstrate that differential Shh activity controls not only the identity of pTH-C progenitor cells but also the spatial organization of the postmitotic thalamic nuclei. When SmoM2 was conditionally expressed using theOlig3Cre allele, the dLG nucleus, which is derived from the rostroventral part of pTH-C, was indeed expanded based on anterograde axon tracing from the retina. This confirms an intrinsic role of Shh signaling in the formation of a specific thalamic nucleus that projects to the cortex.
A recent study bySzabó et al. (2009) usedFoxb1-Cre mice to broadly deleteShh in the developing CNS and found grossly abnormal thalamus and aberrant expression of postmitotic markers at E18.5. They also found that markers broadly expressed in thalamic progenitor cells such as Olig3 and Ngn2 were still expressed in the mutant mice. Based on these observations, they argued that Shh is mainly required in postmitotic cells in the thalamus to promote migration and aggregation into nuclei as well as axonal extension to the cortex rather than neuronal fate acquisition. In contrast, our current study strongly indicates that Shh acts primarily on thalamic progenitor cells. First, markers that are normally expressed in graded manners within the pTH-C domain such as Olig2,Pdlim3, and Dbx1 were significantly affected when Shh activity was altered. The observed alteration of the postmitotic thalamus reflected the changes in the expression of these progenitor cell markers. Second, direct target genes of Shh signaling such asGli1 andPtc1 were expressed only in thalamic progenitors, even though SmoM2 was mis-expressed in both progenitor and postmitotic cells after Cre-mediated recombination.Gli1 andPtc1 expression was diminished in progenitor cells when the Shh signal was perturbed. Based on these observations, we conclude that Shh acts primarily on thalamic progenitor cells, and the graded Shh signal is essential to confer thalamic progenitors with position-dependent molecular heterogeneity, which forms a basis for generating a diverse set of postmitotic thalamic nuclei. Although the study bySzabó et al. (2009) and our current study used different Cre lines to delete theShh gene, both observed the complete absence of the pTH-R marker Nkx2.2. Given the early and broad deletion ofShh in their conditional mutant mice, it is also likely that expression of graded pTH-C markers were altered as well. Thus, we think the different conclusions of the two studies have resulted from different interpretations of potentially similar data. Although we cannot completely rule out the possibility that Shh plays some role in postmitotic differentiation of thalamic neurons such as migration or axonal projection, demonstration of such roles would require different sets of experiments where Shh signal is altered in postmitotic cell-specific, conditional gene targeting in mice, or byin vitro assays that exclude the involvement of progenitor cells.
If Shh signaling regulates the expression of transcription factors in thalamic progenitor cells, then do these transcription factors play a functional role in specifying the formation of the various nuclei along the rostral–caudal and dorsal–ventral axes? We found by overexpression experiments that Olig2 can induce rostroventral thalamic nucleus marker Sox2 in the dorsal–caudal thalamus. However, Olig2 mutant mice show normal expression of Sox2, indicating that other factors are also involved. Further studies are needed to identify such genes and to test the individual roles of each of the other transcription factors.
In conclusion, we believe that this study provides a unique perspective in neural development in several respects. First, we showed how Shh patterns a single developmental unit in the embryonic diencephalon (pTH-C) to generate multiple neuronal groups that are grossly arranged in similar rostrocaudal and dorsoventral locations to those of their precursor cells. This feature makes the developing thalamus a good model system to study how early patterning events contribute to the specification of multiple neuronal fates that share some basic properties such as the projection to the neocortex and use of glutamate as neurotransmitter. Second, connectivity and functions of thalamocortical projections are relatively well understood, especially for those from the principal sensory thalamic nuclei. In this study, by usingOlig3Cre mice, we were able to generate postnatally viable mice in which Shh signaling was specifically enhanced or reduced in the thalamus without primarily affecting the development of the cortex or brain regions along the thalamocortical pathway. These mice will allow us to examine at the systems level the neuroanatomical and functional consequences of altering the early patterning of the thalamus.
Footnotes
This work was supported by Minnesota Medical Foundation, Grant-in-Aid Program of University of Minnesota Graduate School, National Institute of Neurological Disorders and Stroke Grant R01 NS049357, and Whitehall Foundation. We thank Aya Taniguchi, Anu Jayabalu, Christina Kazemzadeh, Lauren Bolopue, and Melody Lee for help in gene targeting, maintaining mouse colonies, and genotyping embryos; Yasuhiko Kawakami and members of the Nakagawa and Koyano laboratories for discussion; James Briscoe, Martyn Goulding, Peter Gruss, Gail Martin, Andrew McMahon, and Masato Nakafuku for plasmids; Andras Nagy for R1 ES cells; Jun-ichi Miyazaki for CAG promoter; Tomomi Shimogori and Tetsuichiro Saito for advice onin utero electroporation; Kyuson Yun forNestinCre/+ mice; and Developmental Studies Hybridoma Bank for antibodies. R26SmoM2/SmoM2,Shhc/c, andSmoc/c mice were developed by Andrew McMahon's laboratory and were obtained from The Jackson Laboratory. University of Minnesota Mouse Genetics Facility provided neomycin-resistant fibroblasts for ES cell feeders, and Thom Sanders, Elizabeth Hughes, and Tina Jones and University of Michigan Transgenic Animal Model Core contributed to the production ofOlig3Cre chimeras. We thank Paul Letourneau and Steve McLoon for critical reading of early versions of the manuscript and Masato Nakafuku for encouragement and helpful discussion.
References
- Agren M, Kogerman P, Kleman MI, Wessling M, Toftgård R. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. 2004;330:101–114. doi: 10.1016/j.gene.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Motoyama J, Hui CC, Kuroiwa A, Nakafuku M, Shimamura K. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120:1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, McMahon AP. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development. 2002;129:4807–4819. doi: 10.1242/dev.129.20.4807. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Cambridge UP: New York; 2007. The thalamus. [Google Scholar]
- Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Development. 2008;135:2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. San Diego: Academic; 1992. Atlas of mouse development. [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Liu A, Li JY, Bromleigh C, Lao Z, Niswander LA, Joyner AL. FGF17b and FGF18 have different midbrain regulatory properties from FGF8b or activated FGF receptors. Development. 2003;130:6175–6185. doi: 10.1242/dev.00845. [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Deneen B, Lukaszewicz A, Novitch BG, Wichterle H, Jessell TM, Anderson DJ. Olig2+ neuroepithelial motoneuron progenitors are not multipotent stem cells in vivo. Proc Natl Acad Sci U S A. 2006;103:1551–1556. doi: 10.1073/pnas.0510658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, O'Leary DD. Dynamic patterned expression of orphan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci. 2003;25:234–244. doi: 10.1159/000072271. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó NE, Zhao T, Zhou X, Alvarez-Bolado G. The role of Sonic hedgehog of neural origin in thalamic differentiation in the mouse. J Neurosci. 2009;29:2453–2466. doi: 10.1523/JNEUROSCI.4524-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284:351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Vue TY, Aaker J, Taniguchi A, Kazemzadeh C, Skidmore JM, Martin DM, Martin JF, Treier M, Nakagawa Y. Characterization of progenitor domains in the developing mouse thalamus. J Comp Neurol. 2007;505:73–91. doi: 10.1002/cne.21467. [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
