Crystal structure of RimI fromSalmonella typhimurium LT2, the GNAT responsible for Nα-acetylation of ribosomal protein S18
Matthew W Vetting
David C Bareich
Michael Yu
John S Blanchard
Received 2008 Apr 17; Revised 2008 Jun 20; Accepted 2008 Jun 20.
Abstract
The three ribosomal proteins L7, S5, and S18 are included in the rare subset of prokaryotic proteins that are known to be Nα-acetylated. The GCN5-related N-acetyltransferase (GNAT) protein RimI, responsible for the Nα-acetylation of the ribosomal protein S18, was cloned fromSalmonella typhimurium LT2 (RimIST), overexpressed, and purified to homogeneity. Steady-state kinetic parameters for RimIST were determined for AcCoA and a peptide substrate consisting of the first six amino acids of the target protein S18. The crystal structure of RimIST was determined in complex with CoA, AcCoA, and a CoA-S-acetyl-ARYFRR bisubstrate inhibitor. The structures are consistent with a direct nucleophilic addition–elimination mechanism with Glu103 and Tyr115 acting as the catalytic base and acid, respectively. The RimIST-bisubstrate complex suggests that several residues change conformation upon interacting with the N terminus of S18, including Glu103, the proposed active site base, facilitating proton exchange and catalysis.
Keywords: protein Nα-acetylation, bisubstrate inhibition, GNAT structure, ribosomal protein
The bacterial ribosome is a complex biological machine composed of three ribosomal RNA (rRNA)1 molecules and 55 proteins. However, it is poorly appreciated that the complexity of the ribosome is increased by post-translational modifications to both the rRNA and ribosomal proteins. While these modifications, which include methylation and acetylation, have been known for decades, a complete understanding of how, when, and why these modifications are made and their contribution to the structure and function of the ribosome remain unknown. A better understanding of how ribosomal modifications are made, at what stage in ribosome biosynthesis they are made, and their influence on the structure or function of the ribosome would be useful for a complete understanding of ribosome assembly and function.
Acetylation of the alpha amino group of protein N-terminal amino acids is very common in eukaryotes. In some cells, estimates have shown more than half of all proteins are Nα-acetylated (Driessen et al. 1985;Bradshaw et al. 1998). However, the number of Nα-acetylated proteins in prokaryotes is limited to seven known examples (Table 1). It is not known why ribosomal proteins are overrepresented in this list, nor is the function of any of the acetylations known. Furthermore, except for RimI, RimJ, and RimL, which Nα-acetylate the ribosomal proteins S18, S5, and L12, respectively, the acetyltransferases responsible for the other Nα-acetylations are not known.
Table 1.
Prokaryotic proteins known to be Nα-acetylated
RimI, RimJ, and RimL were all initially identified using a nitrosoguanidine mutagenesis strategy yielding bacteria with a temperature-sensitive growth phenotype and alterations in the ribosomal proteins as assessed by two-dimensional SDS-PAGE gels (Isono and Isono 1978). The Isono group later determined that the temperature-sensitive growth phenotype was independent of the acetylation of S5, S18, and L12, and when cultured in rich media,Escherichia coli can survive without each of the Nα-acetyltransferases; however, these post-translational modifications may have other functions that are not essential for viability. Furthermore, using their strains with mutations in the acetyltransferases, the Isono group determined that each acetyltransferase is specific for only one ribosomal protein (Cumberlidge and Isono 1979;Isono and Isono 1980,1981).
The structural and biochemical data on ribosomal proteins sheds little light on the possible function of their Nα-acetylation. Several mutations in the 16S ribosomal RNA were found to impair acetylation of S5, and since these ribosomal RNA mutations are proximal to S5 on the ribosome, it suggests that S5 may be acetylated while assembled in the 70S ribosome or 30S subunit (Poot et al. 1997). Furthermore, underacetylation of S5 was also observed when ribosomal protein S4, a protein S5 associates with on the ribosome, was altered by drastic gene mutations (Cumberlidge and Isono 1979). Ribosomal protein L12 and its Nα-acetylated version, L7, are commonly referred to as L7/L12. Interestingly, the relative proportion of L7 to L12 is known to vary with growth phase, with L12 predominant in early log phase, whereas L7 predominates at the stationary phase (Ramagopal and Subramanian 1974). In general, L7/L12 appears to be involved in all ribosome reactions where GTP is hydrolyzed (Brot et al. 1974), and is the binding site for EF-G and the antibiotic thiostrepton (Joseph 2003). The ribosomal protein S18, the substrate for RimI, is found in the central domain of the 30S subunit of the ribosome, and interacts so tightly with the ribosomal protein S6 (8.7 nM) that it is effectively a functional heterodimer (Recht and Williamson 2001). The N-terminal 15 amino acids of S18 are not seen in the crystal structure of the 30S subunit of the bacterial ribosome, and are likely to be solvent exposed (Brodersen et al. 2002).
The prokaryotic RIM Nα-acetyltransferases and eukaryotic Nα-acetyltransferases, by sequence comparisons, all appear to belong to the GCN5-related N-acetyltransferase (GNAT) family, which acetylate a diverse set of substrates with primary amines, ranging from small molecules to large proteins (Vetting et al. 2005a). One hallmark of GNAT proteins is the very low sequence homology between family members with different target substrates. While RimJ and RimL appear to exhibit a distant sequence homology, RimI appears to be unique especially in light of its smaller size. For example, in the case ofSalmonella typhimurium LT2, the sequence identity between RimI (148 residues) and RimJ (194 residues) and RimL (178 residues) is 19% and 20%, respectively, while the sequence identity between RimJ and RimL is 23%. This is despite the fact that RimI and RimJ are termed ribosomal alanine Nα-acetyltransferases, while RimL is termed a serine Nα-acetyltransferase. Based on the low sequence identity it is most likely that the RIM proteins do not have a common ancestor, and evolved independent of each other. In addition, the designation of the RIM proteins as serine or alanine Nα-acetyltransferases may be an artifact of the organism from which they were first purified, and RIM proteins from other organisms may have different Nα-terminal amino acid targets. The structure of RimL fromSalmonella typhimurium LT2 was recently determined in complex with CoA and AcCoA; however, only a model of its interaction with its substrate L12 was proposed (Vetting et al. 2005b). Determination of enzyme functional groups, which are crucial to substrate specificity and catalysis require three-dimensional structures of all relevant substrates. It is often the case that structures of these complexes are not possible due to protein dynamics, limitations in “crystallization space,” and the requirement that the substrates are not turned over. Bisubstrate inhibitors can be utilized to overcome these difficulties as they are often good mimics of the active substrates but do not exhibit turnover and have additive or synergistic binding characteristics when compared to the individual substrates. Bisubstrate inhibitors have been successfully used to obtain structural information with other GNAT family members (Poux et al. 2002;Wolf et al. 2002;Hegde et al. 2007;Magalhaes et al. 2008). In the case of the research presented here, the bisubstrate analog was enzymatically synthesized using RimIST, chloroacetyl-CoA, and a peptide substrate. We demonstrate that the structure of an enzyme–bisubstrate complex produced in this manner is useful for providing intermolecular details between conserved residues of the enzyme and its target substrate. This is the first description of the interactions of an Nα-acetyltransferase with its peptide substrate.
Results and Discussion
RimIST kinetics
The peptide Ala1-Arg2-Tyr3-Phe4-Arg5-Arg6 (S181#x2013;6) representing the first six N-terminal residues of S18 (minus the initiator methionine) was used as a substrate, as efforts to express and purify full-length S18 fromSalmonella typhimurium LT2 were unsuccessful. The initial velocity pattern of the reciprocal S181–6 concentration at different fixed concentrations of AcCoA resulted in an intersecting initial velocity pattern, with kinetic parameters ofkcat = 19 ± 1 min−1,KAcCoA = 0.35 ± 0.08 μM, andKS18(1–6) = 2.2 ± 0.2 mM (Supplemental Fig. 1). Similar experiments performed with RimL Nα-acetyltransferase fromSalmonella typhimurium (RimLST) with its full-length ribosomal protein substrate L12, yielded akcat = 22 ± 1 min−1 and aKL12 of 1.0 ± 0.2 μM (Vetting et al. 2005b), suggesting that the S181–6 peptide is a reasonable substrate, but perhaps lacks some of the required functionalities to mimic the kinetics of the full-length substrate.
Recently, we published a detailed theory of the analysis of bisubstrate inhibition, and specifically its application to N-acetyltransferases, and RimIST was utilized as an example of such an application (Yu et al. 2006). ChloroacetylCoA and the S181–6 peptide were added to preparations of purified RimIST to produce the bisubstrate catalytically, CoA-S-acetyl-S181–6 in which the CoA thiol is covalently linked through the methyl group of the Nα-acetylated S181–6 peptide. The bisubstrate was linearly competitive against acetylCoA with aKi value of 4.1 μM and linearly noncompetitive against S181–6 (Yu et al. 2006). Based on the analysis of the bisubstrate inhibition pattern, RimIST was determined to follow an ordered kinetic mechanism, with AcCoA binding first followed by S181–6 (Yu et al. 2006).
Structure determination
The structure was determined by single isomorphous replacement with anomalous scattering. Crystals belonging to crystal form I were plentiful and easily obtained; however, they tended to be nonisomorphous with a highly variable C-axis making crystal form I not conducive to phase determination. Crystal form II, while more difficult to reproduce, yielded data sets which proved to be isomorphous and were successfully used in phasing. In all three structures the entire RimIST primary sequence (148 residues) was visualized, with the addition of three C-terminal residues (K149, L150, H151) from the C-terminal affinity tag, while the remaining nine histidines that comprise the remainder of the C-terminal tag were not observed in electron density maps. SeeTable 2 for model composition and refinement statistics.
Table 2.
Data collection and refinement statistics
Monomer fold
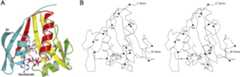
RimIST has a mixed αβ structure with a central seven-stranded β-sheet bounded by four α-helices (Fig. 1). The central β-sheet comprises mostly antiparallel strands except for strands 4 and 5, which are parallel. The order of the strands within the β-sheet is linear with respect to the sequence except at the C terminus, where β7 is positioned between β5 and β6. The β-sheet takes on a V-appearance, with strands β1–β4 making up one arm and β5–β7 constituting the other. The structure confirms that RimIST is a member of the GCN5-related N-acetyltransferase (GNAT) family of proteins. The splay between β4 and β5, which creates the V-like appearance of the central β-sheet, is distinctive of GNAT proteins, and is presumably a critical structural feature in promoting acetyl-transfer (see below).
Figure 1.
Structure of RimIST. (A) Ribbon illustration of the RimIST-bisubstrate complex. Bisubstrate is shown as sticks. From the N terminus secondary structural elements are colored green (β1, α1, α2), yellow (β2–β4), red (α3, β5), and blue (α4, β6). The yellow and red structural elements are strictly conserved among GNAT family members. (B) Stereo diagram of the RimIST–bisubstrate complex. Every twentieth residue is labeled, and every tenth residue is marked by a filled sphere. Bisubstrate is shown as sticks.
Structural homology searches using SSM (Krissinel and Henrick 2004) produced a long list of other GNAT proteins, with the highest ranked being those with a similar β-strand composition at the C terminus. The two highest ranked proteins were PDB:ID 1VHS (Badger et al. 2005), a putative phosphinothricin N-acetyltransferase (RMSD = 1.75 over 142 aligned residues and 18% sequence identity), and PDB:ID 2PSW (Schuetz et al. 2007), a human MAK3 homolog (RMSD = 1.83 over 141 aligned residues and 19% sequence identity). The GNAT structure with the highest sequence similarity to RimIST is PDB:ID 2AE6 (Kim et al. 2005), a putative acetyltransferase fromEnterococcus faecalis (RMSD = 1.94 over 129 aligned residues and 28% sequence identity). GNAT proteins tend to have highly variable protein sequences, and significant sequence similarity only occurs in homologs with similar substrate targets. None of the structure or sequence alignments produced by SSM indicates that these proteins would have the same substrate as RimIST; therefore, the structure presented here is the first determination of a RimI protein.
Oligomeric state
GNATS with higher order oligomeric structures are typically dimers constructed by adjoining the central β-sheet of each monomer into one continuous β-sheet (Burk et al. 2003;Vetting et al. 2005a). Often the active site contains residues from both monomers, and therefore it is crucial to determine the oligomeric state of the structure to describe important residues accurately. Examination of protein–protein interfaces in the two crystal forms revealed a common trimer interface. In both crystal forms there is one complete trimer in the ASU, while in crystal form II the additional monomer per ASU forms an identical trimer when acted upon by a threefold crystal symmetry operation. In contrast, analysis by gel filtration of the C terminally tagged RimIST indicates that RimIST is monomeric in solution (data not shown). The observed trimer interface buries an average of 914 Å2 solvent-accessible surface area per monomer, and involves residues from the α2/β2 loop, the β3/β4 loop, and the α3/β5 loop. These regions of the structure, however, are not conserved among RimI sequences (Supplemental Fig. 2). Therefore, the observed trimer is most probably a strong crystal contact at the similar pH of crystal forms I and II, and therefore, not physiologically relevant. Since the active site of RimIST is ∼20 Å away from this crystal contact it most likely has no effect on the binding of the bisubstrate analog.
AcCoA/CoA complex
The adenine and ribose moiety of CoA rests against α4 making minimal direct contacts (Supplemental Fig. 3). In the various complexes described here the specific conformation and orientation of the adenine and ribose varies slightly, consistent with its minimal interaction with the protein. In some of the complexes the guanidinium groups of Arg82 and Arg77 directly interact with the 3′-phosphate of the ribose, while in others the interaction is by long-range electrostatics. These positively charged residues are not strictly conserved in all RimI proteins, and it is not uncommon among GNAT enzymes that the adenine and ribose moieties are not utilized in the CoA binding affinity. The only conserved sequence among all GNATS has the consensus sequence {Q/R}-x-x-G-x-{G/A} (Neuwald and Landsman 1997), which constructs the “P-loop” between β4 and α3. The P-loop is utilized to coordinate the pyrophosphate moiety of CoA by orienting several consecutive backbone atoms in the same direction. In RimIST, the sequence is Gln76-Arg77-Arg78-Gly79-Leu80-Gly81 and there are four direct and three water-mediated hydrogen bonds between the main-chain atoms of these residues and the diphosphate moiety of CoA. The CoA molecule assumes an L-shape with a 90° bend in the pantothenate moiety due to its interactions with His23 and β4. His23 is the only histidine in the active-site pocket, and the plane of its side chain rests against the bend in the pantothenate. Despite its close proximity to the catalytic center, His23 most likely does not participate in chemistry, since other RimI homologs have a phenylalanine or tyrosine at this position. There is often a hydrophobic residue at this position in GNATS (Dyda et al. 2000), supporting the role of His23 as promoting the correct conformation of CoA and providing van der Waals contacts to improve binding affinity. The pantothenate is further guided into the active site by its interaction with β4. The splay between β4 and β5 generates open hydrogen bonding, which is utilized to form a pseudo antiparallel β-strand interaction with the peptide character of the pantothenate moiety of CoA. This is carried all the way to the thioester of AcCoA where the carbonyl of the acetyl group is hydrogen bonded to the amide backbone of Ile69. The acetyl group is nearly coplanar with the β-sheet, with the methyl group slightly tilted toward a hydrophobic cavity bound by hydrophobic side chains originating from β4, β5, and α3. The acetyl group is ∼4.5 Å from the first disrupted hydrogen bond produced by the splay between β4 and β5. AcCoA therefore occupies the majority of the area created by the splay between β4 and β5, and amine-containing substrates are forced to interact perpendicular to the plane of the β-sheet.
Bisubsubstrate structure
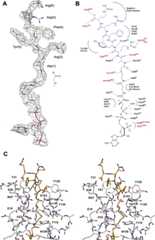
Attempts to crystallize a ternary complex of RimIST with CoA, and various 6–14 amino acid-long peptides corresponding to the S18 N-terminal sequence were unsuccessful. Crystals similar to forms I and II were always obtained, data sets were collected, and no residual density suggesting the position of bound peptide were ever observed. In contrast to the difficulties encountered with obtaining a ternary complex, the crystallization and structure determination of RimIST with a bisubstrate analog was straightforward. The enzyme was mixed with threefold excess bisubstrate, and co-crystallized. Crystals were obtained which were isomorphous with the AcCoA structure (crystal form I). The structure was initially refined with CoA in the model. There was unambiguous electron density for the peptide portion of the bisubstrate analog adjacent to the sulfhydryl of CoA. The electron density was of sufficient quality to fit the entire main chain of the peptide accurately and all of the side chains except the terminal arginines, which were included in the model in common rotamer positions (Fig. 2A). The average B-factor for the bisubstrate is 44 Å2, while neighboring protein side chains have B-factors in the 20–30s. The higher average B-factor for the bisubstrate is skewed by the two ends of the bisubstrate, with higher B-factors for the adenosine and the terminal arginines of peptide (∼50–65 Å), versus lower B-factors for the pantothenate and first two residues of peptide (∼25–35).
Figure 2.
Interactions of the bisubstrate inhibitor with with RimIST. (A) Final 2Fo–Fc electron density for the bisubstrate contoured at 1σ. (B) Detailed schematic of interactions of the bisubstrate with RimIST. The peptide portion is shown in blue and CoA in black. Side-chain interactions originating from RimIST, which coordinate the bisubstrate, are shown in red. (C) Stereo diagram illustrating the interactions of the peptide portion of the bisubstrate with RimIST. Atoms are colored by atom type, with the protein carbons colored gray and the bisubstrate carbons colored brown.
The peptide portion of the bisubstrate binds orthogonal to the β-sheet and therefore orthogonal to the pantothenate portion of CoA within a crevice, which has β4 and β5 as its floor and α2 and the β6/β7 loop as its walls (Fig. 2B,C). There are six residues from RimIST, which provide side-chain hydrogen bonds to the peptide. The side chain of Ser28 and Thr31 forms hydrogen bonds with the C-terminal carboxylate of the peptide. Ser28 and Thr31 are not conserved residues among RimI sequences, and the C-terminal carboxylate would not be part of the natural substrate so this interaction is nonphysiological. The side-chain hydroxyls of Tyr129 and Tyr130 hydrogen bond to the backbone amide of Tyr3–S181–6 and the backbone carbonyl of Ala1–S181–6, respectively. The side-chain nitrogen of Trp27 is hydrogen bonded to the backbone carbonyl of Tyr3–S181–6. Finally, Glu103 forms a salt link with the guanidinium group of Arg2–S181–6. Tyr129, Tyr130, Trp27, and Glu103 are strictly conserved among all RimI homologs. There are two hydrogen bonds from RimIST main-chain atoms to the main-chain atoms of the peptide. The backbone carbonyl of Phe67 is hydrogen bonded to the backbone amide of Arg2–S181–6 and the backbone carbonyl of Glu103 is hydrogen bonded to what would be the N-terminal amine of Ala1–S181–6. These main-chain atoms of RimIST are available for hydrogen bonding due to the splaying of β4 and β5. The nonpolar portions of Phe4–S181–6, Tyr3–S181–6, Arg2–S181–6, and Ala1–S181–6 form van der Waals contacts with the active site. Phe4–S181–6 rests against a small depression on the α2 crevice wall created by the side chains of Trp27, Thr31, Phe67, and Asn35. Tyr3–S181–6 rests against the side chains of Tyr129, Tyr130, and Pro131 of the β6/β7 wall with the side-chain hydroxyl of Tyr3–S181–6 pointing out into the solvent. The side chain of Arg2–S181–6 stacks against the side chain of Phe67. The Cβ carbon of Ala1–S181–6 is 4.1 Å from His23CE1 and 4.5 Å from Tyr130OH.
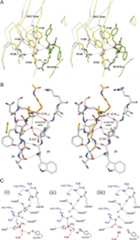
Individual subunits within each “complex” maintain similar conformations for secondary structures and side chains with RMSDs of <0.5 Å over all Cα. RMSDs between the various complexes vary from 0.6 to 1.6 Å over all Cα atoms. The majority of the movements are concentrated in the secondary structures and side chains directly abutting the active site. The largest movements are in residues 24–36 (α1/α2 loop, α2), 105–119 (α4), and 124–141 (β6–β7 loop). The most critical movements occur in the β6–β7 loop. In the RimIST–CoA complex the side chain of Glu103 is hydrogen bonded to the side chain of Arg126 and Tyr130, and the side chain of Tyr129 is pointing out into the solvent. In the RimIST bisubstrate complex this loop flexes toward the peptide and the side chain of Glu103 is released from Arg126 and Tyr130, changes conformation, and forms a salt link with the guanidinium group of Arg2–S181–6 (Fig. 3A). The side-chain hydroxyls of Tyr129 and Tyr130 move ∼4.0 and 2.1 Å, respectively, to make new hydrogen bonds with the peptide backbone of the bisubstrate. In addition to coordinating the arginine of the peptide, the newly positioned side chain of Glu103 is now in position to accept a proton from the primary amine of the substrate through a conserved water molecule (Fig. 3B; see below).
Figure 3.
Reaction mechanism. (A) Movements in the β6–β7 loop upon binding bisubstrate. Superposition of the RimIST–CoA complex (white carbons) with the RimIST (yellow carbons)–bisubstrate (green carbons) complex. (B) Proton wire. Illustration of the conserved water molecule positioned by the splay between β4 and β5. Hydrogen bonds between β4 and β5 are colored green, hydrogen bonds to the conserved water are colored black, and other hydrogen bonds from the exposed backbone atoms of RimIST to the bisubstrate are colored purple. (C) Proposed reaction mechanism. (i) Ternary complex, nucleophilic attack on the carbonyl carbon of AcCoA; (ii) collapse of the tetrahedral intermediate; (iii) product complex. The enzyme is proposed to return to the resting state through water-mediated deprotonation of Glu103 and protonation of Tyr115 either concomitant with or after release of acetylated peptide.
The bisubstrate inhibitor can be viewed as an Nα-acetylated peptide covalently joined to CoA through the methylene of the acetyl group. Due to the manner in which they are adjoined one would expect the enzyme must flex, or portions of the bisubstrate, must be slightly displaced to interact with each other when compared to a peptide–AcCoA ternary complex. This could potentially weaken the interaction between the protein and a true “tetrahedral” intermediate mimic, and one would expect that the bisubstrate inhibitor would not exhibit the full synergistic binding affinities of the two substrates. The structure of RimIST with the bisubstrate compound demonstrates how the covalent nature of the bisubstrate is accommodated (Supplemental Fig. 4). The terminal portion of the pantothenate moiety is displaced away from β4 toward β5 and α4 with the sulfur group of the bisubstrate ∼1.2 Å from its position in the AcCoA complex. This appears to result in a displacement of α4 through interactions of the sulfur of the bisubstrate with the side-chain hydroxyl of Tyr105 and the side chain of Asn108 with a carbonyl of the pantothenate. Despite these perturbations of the active site, the bisubstrate analog appears be a relatively good mimic of a ternary complex. The carbonyl of the keto group of the bisubstrate makes the same hydrogen bond as the acetyl group of AcCoA with the backbone amide of Ile69. A superposition of the AcCoA complex and the bisubstrate complex shows the Nα-amine in an ideal position for in-line attack on the si-face of the acetyl group of AcCoA with anNα-amine to carbonyl carbon distance of ∼1.8 Å. All of the polar atoms of the first two residues, Ala1–S181–6 and Arg2–S181–6 are hydrogen bonded to RimIST, suggesting the peptide is not dramatically displaced due to the direct coordination of the peptide with CoA.
Reaction mechanism
Members of the GNAT family of acetyltransferases typically catalyze the direct transfer of the acetyl group. There are no cysteines located within the active site suggesting RimIST catalyzes a direct transfer. In addition, a comparison of the RimIST–AcCoA and RimIST–bisubstrate structure suggests that the Nα-amine of peptide would be correctly positioned for a direct interaction with the acetyl group of AcCoA to form a tetrahedral intermediate. The polarization of the acetyl group during the formation of the tetrahedral intermediate would be stabilized by its interaction with the amide backbone of Ile69 (Fig. 3C). In previous GNAT structures, a semiconserved tyrosine residue or bulk solvent is typically implicated in the protonation of the sulfhydryl of CoA, while a Glu or an Asp either directly or through intervening water molecules is implicated in the deprotonation of the amine (Vetting et al. 2005a). In the case of RimIST Tyr115 is within hydrogen bonding distance of the sulfhydryl of AcCoA, and therefore is the most likely active site acid, while Glu103, through an intervening water molecule, could act as the active site base. The intervening water molecule is held by the first carbonyl and amide groups exposed due to the splaying of β4 from β5. As such, it is held in the correct geometry to pass a proton from the amine of substrate to an active site base, in this case the side chain of Glu103. While the final destination of the proton, that is, the proposed active site base, differs depending on the GNAT, a conserved water molecule has been observed in the structure of many GNAT proteins where it could act as the initial proton acceptor (Vetting et al. 2005a). In the case of RimIST, the structural rearrangement, following the binding of peptide (rotation of Glu103), completes the “proton wire” required for accepting a proton from the Nα-amine.
It is interesting to note that the backbone carbonyls and amides exposed due to the splaying of β4 from β5 in GNAT proteins have been proposed to have numerous functions: coordination of the pantothenate of AcCoA, positioning the acetyl group for nucleophilic attack, providing hydrogen bonding for the primary amine of the target substrate, stabilizing the tetrahedral intermediate, and coordination of a water molecule in an ideal geometry to shuttle protons away from the reaction site. Yet, while of such apparent great importance to the functionality of the enzyme, the production of this gross structural topological feature is not directed from a set of conserved residues or primary sequence homology, but is hidden in the randomness of convergent evolution.
Materials and Methods
Cloning and expression of RimIst
RimI (NP_463414) was cloned by polymerase chain reaction (PCR) fromSalmonella typhimurium LT2 genomic DNA into the expression vector pET23a(+) (Novagen), which had been previously modified to provide a C-terminal decahistidine tag (added amino acids: KLHHHHHHHHHH). The construct was confirmed by DNA sequencing. Rosetta2 (DE3) pLysS (Novagen) cells harboring the pET23a(+)–RimIST–C-10-His plasmid were grown in 4 × 1 L of LB media, supplemented with 100 μg/mL ampicillin + 30 μg/mL chloramphenicol, at 37°C until an OD600 of 0.6 was reached. The cultures were induced to express the RimIST by the addition of 1 mM isopropyl 1-thio-β-D-galactopyranoside and allowed to grow overnight at 16°C. Cells were harvested by centrifugation and resuspended in a minimal volume of 20 mM Tris hydroxymethyl aminomethane (Tris) pH 8.0 + 1 M NaCl, brought up to 25 mL with the same buffer and frozen at −20°C.
RimIST purification
The frozen cells were thawed and sonicated in an ice bath in the presence of the protease inhibitor mixture Complete (EDTA free) (Roche Applied Science), 0.2 mg/mL lysozyme (Sigma), and 0.1 mg/mL DNase (Roche Applied Science). The lysate was clarified by centrifugation for 10 min at 47,000g. The supernatant was loaded onto a 5-mL Ni HiTrap column, previously equilibrated, and washed with buffer A (20 mM Tris pH 8.0 + 15 mM imidazole + 1 M NaCl). Bound protein was eluted with a gradient of buffer B (20 mM Tris pH 8.0 + 300 mM imidazole + 1 M NaCl). Fractions found to be pure by Coomassie stained SDS-PAGE were pooled, concentrated by ultrafiltration, and dialyzed overnight against 1000-fold greater volume of 20 mM Tris pH 8.5 + 100 mM ammonium sulfate + 0.1 mM EDTA + 0.01 mM DTT. Glycerol was added to final concentration of 10% and RimIST (20 mg/mL) was frozen and stored at −80°C. Analytical gel filtration was performed with a HiPrep 26/60 Sephacryl S-200 high-resolution gel filtration column eluted at 1 mL/min with 20 mM Tris 8.5 + 100 mM ammonium sulfate and calibrated with BioRad gel filtration standards.
Enzyme kinetics
Reaction rates were determined by continuously monitoring the increase in absorbance at 412 nM due to the formation of 5-thio-2-nitrobenzoate resulting from the reaction between sulfhydryl group of the product of the acetyl transfer reaction, CoA-SH, and 5,5′ dithiobis(2-nitrobenzoic acid) (DTNB) in a UVIKON XL spectrophotometer equipped with thermospacers and connected to a constant temperature, circulating water bath. Assays were performed in 50 mM K2HPO4, pH 7.25, 100 μM DTNB, and at a temperature of 25°C. Reactions were initiated by the addition of 80 nM enzyme. For every enzymatic reaction, a corresponding nonenzymatic control reaction was carried out. The nonenzymatic rate was then subtracted from the enzymatic rate. In all cases, the background was close to zero.
Crystallization
Homogenous preparations of the RimIST with the C-terminal 10-His tag were used in all crystallization trials. The screens were prepared using vapor diffusion under oil in 96-well polystyrene microplates (Corning: 3795). Each condition consisted of a combination of 2 μL of protein and 2 μL of screen reagent combined under 150 μL of Fisher brand silicon oil. Crystallization experiments were stored at 18°C with the oil exposed to room humidity. Two distinct crystal forms were observed in the initial screens.
Crystal form I (AcCoA/bisubstrate)
Rod-shaped crystals with a triganol cross section grew from drops containing 2 μL of RimIST (8.8 mg/mL, 20 mM Tris pH 8.5, 100 mM (NH4)2SO4, 0.1 mM EDTA, 0.01 mM DTT, 5 mM AcCoA) with 2 μL of reservoir solution (15%–30% Peg400 (w/v), 200 mM K/phosphate, pH 4–5.5). Crystals had approximate dimensions of 0.1 × 0.1 × 0.3 mm and grew to maximum dimensions in 2–3 d. Crystal form I was briefly soaked (∼10 sec) in 25% Peg400, 100 mM (NH4)2SO4, 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) pH 5.25 before vitrification in liquid nitrogen. Crystal form I belongs to space groupR32 with three protein molecules per ASU with a solvent content of 62%.
Crystal form II
Large misshapen cylindrical crystals of RimIST grew from drops containing 2 μL of RimIST (8.8 mg/mL, 20 mM Tris pH 8.5, 100 mM (NH4)2SO4, 0.1 mM EDTA, 0.01 mM DTT, 5 mM CoA) and 2 μL of precipitant solution (2.2–2.8 M (NH4)2SO4, 100 mM MES pH 4.5–5.5) in a vapor diffusion under oil experiment. Crystals had approximate dimensions of 0.2 × 0.2 × 0.3 mm and grew to maximum dimensions in 1 wk. Crystal form II was briefly (∼10 sec) soaked in 2.2 M (NH4)2SO4, 100 mM MES pH 5.25, 20% (v/v) propylene glycol before vitrification in liquid nitrogen. Crystal form II belongs to space groupP321 with four protein molecules per ASU yielding a solvent content of 68%.
Bisubstrate complex
To form the bisubstrate inhibitor complex, 3 mol of bisubstrate were added to each mole of purified RimIST and incubated for 1 h on ice. The bisubstrate complex (6 mg/mL RimIST) crystallized in 15% Peg400 (w/v), 100 mM NaCitrate pH 5.5, 100 mM (NH4)2SO4 and belong to crystal form I. Crystals were briefly (∼10 sec) soaked in 30% PEG400 (w/v), 100 mM NaCitrate pH 5.5, 100 mM (NH4)2SO4 prior to vitrification in liquid nitrogen.
All X-ray data were collected at 113 K on an R-Axis IV++ image plate detector using CuKα radiation from a Rigaku RU-H3R X-ray generator. Data sets were integrated using MOSFLM (Leslie 2006) and scaled using SCALA (Evans 2006). Data statistics are shown inTable 2.
Structure determination
Crystal form II was phased by single isomorphous replacement with anomalous scattering (SIRAS). A single crystal was soaked in 10 mM trimethyl lead acetate, 2.2 M (NH4)2SO4, 100 mM MES pH 5.2, 20% propylene glycol for a period of 3 min before vitrification in liquid nitrogen. Since Pb+3 has a relatively large anomalous signal (f″ = 8.5) at CuKα a full 360° data set was collected in order to maximize the signal to noise and collect complete and redundant anomalous data. The program SOLVE/RESOLVE (Terwilliger 2002) was used to locate four Pb+3 binding sites, calculate initial SIRAS phases, and perform density modification. The SIRAS phases (mean figure of merit = 0.45, 30–2.8 Å) and resolution of crystal form II (2.4 Å) were of sufficient quality that the autobuilding program ARP/WARP (Perrakis 1997) was able to build 92% of the structure. Crystal form I was phased using a partially refined model from crystal form II and the molecular replacement package AMORE (Navaza 1994). The RimIST–bisubstrate complex was isomorphous with the form I–AcCoA complex. Structures were completed by a similar protocol with iterative rounds of REFMAC (Murshudov et al. 1997) refinement and manual rebuilding within the molecular graphics program COOT (Emsley and Cowtan 2004). Structures were refined with strict NCS restraints until convergence of the crystallographicR-factor, at which point they were completely removed. Waters were added by identification of peaks, which were >3.5σ in FO–FC difference maps with suitable geometry for hydrogen bonding. Translation/Libration/Screw (TLS) refinement (Winn et al. 2001) was used in the last steps of refinement, using each subunit as a different TLS group. All of the diffraction data were used throughout the refinement process, except the 5% randomly selected data for calculating Rfree. In the final two–three rounds of refinement protein atoms were refined using stereochemical restraints for CoA-S-acetyl–S181–6, and were generated using the server PRODGR2 (Schuttelkopf and van Aalten 2004). The quality of the model was validated using MOLPROBITY (Davis et al. 2004) and PROCHECK (Laskowski et al. 1993). Statistics for data collection and refinement are listed inTable 2.Figures 1–3 were prepared using PyMOL (DeLano Scientific).
Protein Data Bank deposition
The atomic coordinates and structure factors (code 2CNS, 2CNT, 2CNM) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Acknowledgments
Peptide synthesis was performed by the Proteomics Resource Center of the Rockefeller University. This work was supported by NIH Grant AI33696 to J.S.B.
Footnotes
Supplemental material: seewww.proteinscience.org
Reprint requests to: John S. Blanchard, Department of Biochemistry, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA; e-mail:blanchar@aecom.yu.edu; fax: (718) 430-8565.
Abreviations: GNAT, GCN5 related N-acetyltransferases; rRNA, ribosomal RNA; RimLST, RimL N-acetyltransferase fromSalmonella typhimurium; RimIST, RimI fromSalmonella typhimurium LT2; bisubstrate inhibitor, CoA-S-acetyl-S181–6; RMSD, root-mean-squared deviation.
Article and publication are athttp://www.proteinscience.org/cgi/doi/10.1110/ps.035899.108.
References
- Badger, J., Sauder, J.M., Adams, J.M., Antonysamy, S., Bain, K., Bergseid, M.G., Buchanan, S.G., Buchanan, M.D., Batiyenko, Y., Christopher, J.A., et al. Structural analysis of a set of proteins resulting from a bacterial genomics project. Proteins. 2005;60:787–796. doi: 10.1002/prot.20541. [DOI] [PubMed] [Google Scholar]
- Bradshaw, R.A., Brickey, W.W., Walker, K.W. N-Terminal processing: The methionine aminopeptidase and Nα-acetyl transferase families. Trends Biochem. Sci. 1998;23:263–267. doi: 10.1016/s0968-0004(98)01227-4. [DOI] [PubMed] [Google Scholar]
- Brodersen, D.E., Clemons W.M., Jr, Carter, A.P., Wimberly, B.T., Ramakrishnan, V. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: Structure of the proteins and their interactions with 16 S RNA. J. Mol. Biol. 2002;316:725–768. doi: 10.1006/jmbi.2001.5359. [DOI] [PubMed] [Google Scholar]
- Brot, N., Tate, W.P., Caskey, C.T., Weissbach, H. The requirement for ribosomal proteins L7 and L12 in peptide-chain termination. Proc. Natl. Acad. Sci. 1974;71:89–92. doi: 10.1073/pnas.71.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk, D.L., Ghuman, N., Wybenga-Groot, L.E., Berghuis, A.M. X-ray structure of the AAC(6′)-Ii antibiotic resistance enzyme at 1.8 Å resolution; examination of oligomeric arrangements in GNAT superfamily members. Protein Sci. 2003;12:426–437. doi: 10.1110/ps.0233503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberlidge, A.G., Isono, K. Ribosomal protein modification in Escherichia coli. I. A mutant lacking the N-terminal acetylation of protein S5 exhibits thermosensitivity. J. Mol. Biol. 1979;131:169–189. doi: 10.1016/0022-2836(79)90072-x. [DOI] [PubMed] [Google Scholar]
- Davis, I.W., Murray, L.W., Richardson, J.S., Richardson, D.C. MOLPROBITY: Structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 2004;32:W615–W619. doi: 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen, H.P., de Jong, W.W., Tesser, G.I., Bloemendal, H. The mechanism of N-terminal acetylation of proteins. CRC Crit. Rev. Biochem. 1985;18:281–325. doi: 10.3109/10409238509086784. [DOI] [PubMed] [Google Scholar]
- Dyda, F., Klein, D.C., Hickman, A.B. GCN5-related N-acetyltransferases: A structural overview. Annu. Rev. Biophys. Biomol. Struct. 2000;29:81–103. doi: 10.1146/annurev.biophys.29.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley, P., Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- Hegde, S.S., Chandler, J., Vetting, M.W., Yu, M., Blanchard, J.S. Mechanistic and structural analysis of human spermidine/spermine N1-acetyltransferase. Biochemistry. 2007;46:7187–7195. doi: 10.1021/bi700256z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono, S., Isono, K. Mutations affecting the structural genes and the genes coding for modifying enzymes for ribosomal proteins in Escherichia coli. Mol. Gen. Genet. 1978;165:15–20. doi: 10.1007/BF00270371. [DOI] [PubMed] [Google Scholar]
- Isono, K., Isono, S. Ribosomal protein modification in Escherichia coli. II. Studies of a mutant lacking the N-terminal acetylation of protein S18. Mol. Gen. Genet. 1980;177:645–651. doi: 10.1007/BF00272675. [DOI] [PubMed] [Google Scholar]
- Isono, S., Isono, K. Ribosomal protein modification in Escherichia coli. III. Studies of mutants lacking an acetylase activity specific for protein L12. Mol. Gen. Genet. 1981;183:473–477. doi: 10.1007/BF00268767. [DOI] [PubMed] [Google Scholar]
- Joseph, S. After the ribosome structure: How does translocation work? RNA. 2003;9:160–164. doi: 10.1261/rna.2163103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y., Hatzos, C., Moy, S., Collart, F., Joachimiak, A. Midwest Center for Structural Genomics. Rutgers University; New Brunswick, NJ: 2005. Crystal structure of acetyltransferase of GNAT family from Enterococcus faecalis V583. Protein Data Bank, Research Collaboratory for Structural Bioinformatics. [Google Scholar]
- Klussmann, S., Franke, P., Bergmann, U., Kostka, S., Wittmann-Liebold, B. N-terminal modification and amino-acid sequence of the ribosomal protein HmaS7 from Haloarcula marismortui and homology studies to other ribosomal proteins. Biol. Chem. Hoppe Seyler. 1993;374:305–312. doi: 10.1515/bchm3.1993.374.1-6.305. [DOI] [PubMed] [Google Scholar]
- Krissinel, E., Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., MacArthur, M.W., Moss, D.S., Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- Laursen, R.A., L'Italien, J.J., Nagarkatti, S., Miller, D.L. The amino acid sequence of elongation factor Tu of Escherichia coli. The complete sequence. J. Biol. Chem. 1981;256:8102–8109. [PubMed] [Google Scholar]
- Leslie, A.G. The integration of macromolecular diffraction data. Acta Crystallogr. D Biol. Crystallogr. 2006;62:48–57. doi: 10.1107/S0907444905039107. [DOI] [PubMed] [Google Scholar]
- Magalhaes, M.L., Vetting, M.W., Gao, F., Freiburger, L., Auclair, K., Blanchard, J.S. Kinetic and structural analysis of bisubstrate inhibition of the Salmonella enterica aminoglycoside 6′-N-acetyltransferase. Biochemistry. 2008;47:579–584. doi: 10.1021/bi701957c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras, B., Consalvi, V., Chiaraluce, R., Politi, L., De Rosa, M., Bossa, F., Scandurra, R., Barra, D. The protein sequence of glutamate dehydrogenase from Sulfolobus solfataricus, a thermoacidophilic archaebacterium. Is the presence of N-E-methyllysine related to thermostability? Eur. J. Biochem. 1992;203:81–87. doi: 10.1111/j.1432-1033.1992.tb19831.x. [DOI] [PubMed] [Google Scholar]
- Murshudov, G.N., Vagin, A.A., Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Navaza, J. An automated package for molecular replacement. Acta Crystallogr. 1994;A50:157–163. [Google Scholar]
- Neuwald, A.F., Landsman, D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- Okkels, L.M., Muller, E.C., Schmid, M., Rosenkrands, I., Kaufmann, S.H., Andersen, P., Jungblut, P.R. CFP10 discriminates between nonacetylated and acetylated ESAT-6 of Mycobacterium tuberculosis by differential interaction. Proteomics. 2004;4:2954–2960. doi: 10.1002/pmic.200400906. [DOI] [PubMed] [Google Scholar]
- Perrakis, A. wARP: Improvement and extension of crystallographic phases by weighted averaging of multiple-refined dummy atomic models. Acta Crystallogr. 1997;D53:448–455. doi: 10.1107/S0907444997005696. [DOI] [PubMed] [Google Scholar]
- Poot, R.A., Jeeninga, R.E., Pleij, C.W., van Duin, J. Acetylation of ribosomal protein S5 affected by defects in the central pseudoknot in 16S ribosomal RNA? FEBS Lett. 1997;401:175–179. doi: 10.1016/s0014-5793(96)01467-6. [DOI] [PubMed] [Google Scholar]
- Poux, A.N., Cebrat, M., Kim, C.M., Cole, P.A., Marmorstein, R. Structure of the GCN5 histone acetyltransferase bound to a bisubstrate inhibitor. Proc. Natl. Acad. Sci. 2002;99:14065–14070. doi: 10.1073/pnas.222373899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal, S., Subramanian, A.R. Alteration in the acetylation level of ribosomal protein L12 during growth cycle of Escherichia coli. Proc. Natl. Acad. Sci. 1974;71:2136–2140. doi: 10.1073/pnas.71.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht, M.I., Williamson, J.R. Central domain assembly: Thermodynamics and kinetics of S6 and S18 binding to an S15-RNA complex. J. Mol. Biol. 2001;313:35–48. doi: 10.1006/jmbi.2001.5018. [DOI] [PubMed] [Google Scholar]
- Schuetz, A., Walker, J.R., Antoshenko, T., Wu, H., Bernstein, G., Loppnau, P., Weigelt, J., Sundstrom, M., Arrowsmith, C.H., Edwards, A.M., et al. Rutgers University; New Brunswick, NJ: 2007. Human MAK3 homolog in complex with CoA. PDB:ID 2PSW. Protein Data Bank, Research Collaboratory for Structural Bioinformatics. [Google Scholar]
- Schuttelkopf, A.W., van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Terhorst, C., Wittmann-Liebold, B., Moller, W. 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2, isolated from 50-S ribosomes of Escherichia coli. Eur. J. Biochem. 1972;25:13–19. doi: 10.1111/j.1432-1033.1972.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Terwilliger, T.C. Automated structure solution, density modification and model building. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1937–1940. doi: 10.1107/s0907444902016438. [DOI] [PubMed] [Google Scholar]
- Vetting, M.W., de Carvalho, L.P., Roderick, S.L., Blanchard, J.S. A novel dimeric structure of the RimL Nα-acetyltransferase from Salmonella typhimurium. J. Biol. Chem. 2005b;280:22108–22114. doi: 10.1074/jbc.M502401200. [DOI] [PubMed] [Google Scholar]
- Vetting, M.W., de Carvalho, L.P.S., Yu, M., Hegde, S.S., Magnet, S., Roderick, S.L., Blanchard, J.S. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 2005a;433:212–226. doi: 10.1016/j.abb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Winn, M.D., Isupov, M.N., Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold, B., Greuer, B. The primary structure of protein S5 from the small subunit of the Escherichia coli ribosome. FEBS Lett. 1978;95:91–98. doi: 10.1016/0014-5793(78)80059-3. [DOI] [PubMed] [Google Scholar]
- Wolf, E., De Angelis, J., Khalil, E.M., Cole, P.A., Burley, S.K. X-ray crystallographic studies of serotonin N-acetyltransferase catalysis and inhibition. J. Mol. Biol. 2002;317:215–224. doi: 10.1006/jmbi.2001.5371. [DOI] [PubMed] [Google Scholar]
- Yaguchi, M. Primary structure of protein S18 from the small Escherichia coli ribosomal subunit. FEBS Lett. 1975;59:217–220. doi: 10.1016/0014-5793(75)80378-4. [DOI] [PubMed] [Google Scholar]
- Yu, M., Magalhaes, M.L., Cook, P.F., Blanchard, J.S. Bisubstrate inhibition: Theory and application to N-acetyltransferases. Biochemistry. 2006;45:14788–14794. doi: 10.1021/bi061621t. [DOI] [PubMed] [Google Scholar]