Parasitic inhibition of cell death facilitates symbiosis
Bart A Pannebakker
Benjamin Loppin
Coen P H Elemans
Lionel Humblot
Fabrice Vavre
To whom correspondence should be addressed. E-mail:bart.pannebakker@ed.ac.uk
Edited by Nancy A. Moran, University of Arizona, Tucson, AZ, and approved November 9, 2006
Author contributions: B.A.P., B.L., and F.V. designed research; B.A.P., B.L., and F.V. performed research; B.A.P., B.L., C.P.H.E., L.H., and F.V. analyzed data; and B.A.P., B.L., C.P.H.E., and F.V. wrote the paper.
Received 2006 Sep 7; Issue date 2007 Jan 2.
Abstract
Symbiotic microorganisms have had a large impact on eukaryotic evolution, with effects ranging from parasitic to mutualistic. Mitochondria and chloroplasts are prime examples of symbiotic microorganisms that have become obligate for their hosts, allowing for a dramatic extension of suitable habitats for life. Out of the extraordinary diversity of bacterial endosymbionts in insects, most are facultative for their hosts, such as the ubiquitousWolbachia, which manipulates host reproduction. Some endosymbionts, however, have become obligatory for host reproduction and/or survival. In the parasitoid waspAsobara tabida the presence ofWolbachia is necessary for host oogenesis, but the mechanism involved is yet unknown. We show thatWolbachia influences programmed cell death processes (a host regulatory feature typically targeted by pathogens) inA. tabida, making its presence essential for the wasps' oocytes to mature. This suggests that parasite strategies, such as bacterial regulation of host apoptosis, can drive the evolution of host dependence, allowing for a swift transition from parasitism to mutualism.
Keywords: apoptosis,Asobara tabida,Wolbachia
Symbiosis is an important factor in evolution as a source of both evolutionary novelty and ecological diversity (1,2). Symbiotic microorganisms in particular have had a large impact on eukaryotic evolution, with effects ranging from parasitic to mutualistic. Mitochondria and plant chloroplasts are prime examples of free-living bacteria that have become obligate symbiotic partners for their hosts, allowing for a dramatic extension of suitable habitats for life (3). The last two decades of symbiont research have highlighted an extraordinary diversity of bacterial endosymbionts in arthropods, such as the ubiquitousWolbachia, which manipulates host reproduction (4).Wolbachia bacteria are cytoplasmically inherited, intracellular parasites that persevere in a host population by manipulating their host's reproductive system in extreme ways (5), such as parthenogenesis (6), the induction of cytoplasmic incompatibility between individuals (7), or male killing (8). Despite these extreme manipulations, most hosts remain independent ofWolbachia for successful reproduction, rendering their parasitism facultative.
For the parasitoid waspAsobara tabida (Hymenoptera: Braconidae), however,Wolbachia is an obligate symbiotic partner. Female wasps that are cured of theirWolbachia fail to produce any mature oocyte, thus renderingWolbachia infection essential for reproduction (9,10). However, the mechanism that underlies this dramatic transition from facultative parasitism to obligate mutualism is yet unknown. Here we show that removal ofWolbachia affects host programmed cell death, which renders it an obligate symbiotic partner inA. tabida.
Results and Discussion
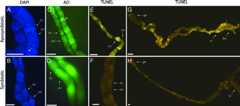
We observed pyknotic nuclei (Fig. 1A andB), one of the hallmarks of apoptosis, in the ovarioles ofA. tabida females whereWolbachia was antibiotically removed (so-called aposymbiotic females). Furthermore, both acridine orange staining (Fig. 1C andD) and the apoptosis-specific TUNEL assay (Fig. 1E–H) show that absence ofWolbachia bacteria in aposymbiotic females results in copious apoptosis of the nurse cells of mid-stage egg chambers, while at this stage apoptosis does not occur in the ovarioles of symbiotic control females (Fig. 2) (Mann–WhitneyU test:W = 464;n = 45;P ≪ 0.001). Apoptosis is an essential component of insect oogenesis and occurs at several stages during oogenesis in response to both developmental and environmental stimuli (11). InDrosophila there are checkpoints during early and mid-oogenesis at which various environmental stress factors can trigger cell death in egg chambers (11). Our results show that, inA. tabida, the presence ofWolbachia is essential to progress egg chambers past the mid-oogenesis checkpoint by preventing apoptosis of nurse cells and, as such, allows for oocyte maturation. Whether this modification of programmed cell death is controlled byWolbachia or is host-derived requires further investigation. Nevertheless,Wolbachia clearly interacts with apoptotic pathways during oogenesis.
Fig. 1.
Copious apoptosis occurs inA. tabida ovaries after removal of the bacterial endosymbiontWolbachia. (A andB) DAPI staining of mid-stage egg chambers reveals pyknotic nuclei (arrowhead) inWolbachia-uninfected or aposymbiotic females (A) but not inWolbachia-infected, symbiotic females (B). (C andD) Acridine orange staining reveals apoptosis in mid-stage nurse cells (arrowhead) of aposymbiotic females (C) but fails to show apoptosis in egg chambers of symbiotic females (D). (E–H). Apoptosis-specific TUNEL staining shows brightly stained apoptotic nuclei in nurse cells from mid-stage oocytes in aposymbiotic females (E) but not in symbiotic females (F). An overview of TUNEL-stained ovaries from an aposymbiotic female shows copious apoptotic nuclei in mid-stage egg chambers (H), whereas ovaries from a symbiotic female show only sporadic apoptotic nuclei in the germarium and in the nurse cells of late-stage oocytes (G). ge, germarium; vi, vitellarium; bo, basal region of ovarioles; nc, nurse cells; oo, oocyte. (Scale bars: 50 μm.)
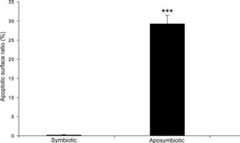
Fig. 2.
Mid-stage oocytes from aposymbioticA. tabida females show copious apoptosis. Mean apoptotic surface ratios (%) of mid-stage oocytes from symbiotic (n = 16) and aposymbiotic (n = 29)A. tabida females are shown. Aposymbiotic females show a significantly higher apoptotic surface ratio than symbiotic females (Mann–WhitneyU test:W = 464;n = 45; ***,P ≪ 0.001). Error bars indicate SEM.
Immune responses against intracellular bacteria frequently involve apoptosis of infected cells (12), and, in turn, intracellular bacteria can inhibit host cell apoptosis to circumvent the immune system (13). Several other well known intracellular pathogens are able to control apoptotic pathways in their host. WhereasSalmonella sp. and the causative agent of Legionnaire's disease,Legionella pneumophila, induce apoptosis in host cells, the sexually transmitted human disease agentChlamydia and the causative agent of Rocky Mountain spotted fever,Rickettsia ricketsii (a close relative ofWolbachia), are both able to either block or induce apoptosis of host cells depending on the stage of the infection (14). The necessary alterations to the cell physiology ofA. tabida most likely occurred after initial invasion of theWolbachia, because (i) they would have been destructive forA. tabida withoutWolbachia's presence and (ii) earlier comparisons within the genusAsobara show thatWolbachia-dependent oogenesis is a derived character (15).
Bacterial inhibition of apoptosis can result in a severe reduction in functionality of the infected host tissues. This is particularly relevant in the ovaries, where the highest densities ofWolbachia bacteria are found (16) and where apoptosis is an essential component of oogenesis (11). Selection is expected to favor a swift recovery of reduced ovarian functionality by coevolution between the two partners. Theoretically, at least three scenarios can lead to restoration of ovarian functionality: (i)Wolbachia ceases to manipulate apoptosis and evades the host immune system by hiding within membrane-bound compartments inside insect cells (13). (ii)Wolbachia permanently takes over control of the host apoptotic pathway. Permanent control becomes possible when in infected hosts the nuclear genes controlling apoptosis are no longer under selection, thus allowing for the accumulation of mutations in these genes. An example of this is found inDrosophila melanogaster, whereWolbachia restores several host mutations, among which is the mutantSxlf4, which closely resembles the phenotype observed inA. tabida (17,18). (iii) Host gene expression modifies to take into account the bacterial manipulation of the apoptotic pathway. The first scenario does not directly result in the observed complete dependence of the host onWolbachia. Both latter scenarios, however, although indistinguishable inA. tabida without further genetic information, undoubtedly lead to complete host dependence. As such, regulation of host programmed cell death is a likely mechanism in this system to explain the evolutionary shift from facultative parasitism toward obligate mutualism.
Future work could address the generality of apoptosis manipulation in host–Wolbachia associations and the molecular mechanisms underlying these manipulations. Recent findings show that parasites and mutualists often share common strategies and molecular tools, e.g., secretion systems, that have long been exclusively described as virulence factors (13,19). Because the host apoptotic pathway is targeted both by parasitic and mutualistic bacteria (12,20), apoptosis can be a general mechanism of molecular and cellular interaction in host–bacteria associations; initially to evade the host immune system and later to manipulate host development. InA. tabida, however, manipulation of the apoptotic pathway appears to be at the origin of the host dependence and the evolution of obligate mutualism. It has been proposed that endosymbionts have been slaved by their hosts (21); however, our results show that endosymbionts can also hijack their host by becoming obligatory for reproduction without providing any further advantage.
Materials and Methods
Insect Strains and Antibiotic Curing.
TheA. tabida lines used in the experiment originated from Pierrefeu, Var, France.A. tabida is naturally infected by three strains ofWolbachia (22). However, the line we used is singly infected by thewAtab3 strain, the only one required for oogenesis completion (10). The wasps were reared under noncompetition conditions in vials containing standardDrosophila medium (23) at 20°C, a controlled photoperiod of 12 h:12 h light:dark and 70% relative humidity. Aposymbiotic females were obtained by antibiotic treatment. Antibiotics (rifampicin; Aventis, Paris, France) were applied to the wasps through the developingDrosophila larvae: 150 μl of 2% rifampicin solution was added to 1.5 g of standardDrosophila medium (final rifampicin concentration: 2 mg/g). The endoparasitic wasp larvae developed into aposymbiotic females within the treatedDrosophila larvae. This technique is described in more detail in ref.9.
Acridine Orange and DAPI Staining.
To establish the occurrence of apoptosis, ovaries from symbiotic (n = 50) and aposymbiotic (n = 50) females were dissected in PBS-T (0.1% Triton X-100, pH 7.4) and stained in 0.25 μg/ml acridine orange (Sigma-Aldrich Chemicals, Munich, Germany). They were subsequently rinsed in PBS-T, mounted on glass slides, and embedded in VECTASHIELD HardSet containing 1.5 μg/ml DAPI (Vector Laboratories, Burlingame, CA). Observations were made by using an Axio Imager.Z1 fluorescence microscope (Zeiss, Oberkochen, Germany).
TUNEL Assay.
TUNEL was performed to specifically identify apoptotic cells in the ovaries (24). The ovaries from symbiotic (n = 50) and aposymbiotic (n = 50) females were dissected in PBS-T (0.1% Triton X-100, pH 7.4) and fixed in PBS containing 4% formaldehyde plus 0.1% Triton X-100 (Sigma-Aldrich Chemicals) for 30 min. After fixation the samples were rinsed three times in PBS-T, incubated in PBS-T containing proteinase K (20 μg/ml) for 10 min, and washed three times in PBS-T for 5 min each. Thein situ labeling of fragmented genomic DNA was done by using theIn Situ Cell Death Detection Kit TMR Red (Roche, Mannheim, Germany) for 3 h at 37°C. Hereafter, the ovaries were washed six times in PBS-T in the dark. Finally, the ovaries were mounted in VECTASHIELD HardSet with DAPI (0.2 μg/ml; Vector Laboratories) and observed under an Axio Imager.Z1 fluorescence microscope.
Image Analysis.
To quantify the amount of apoptosis in mid-stage oocytes of symbiotic and aposymbiotic females we measured the ratio of TUNEL-labeled surface over the total surface of the oocyte (apoptotic surface ratio) in TUNEL assay images (e.g.,Fig. 1E andF). A total of 29 oocytes from aposymbiotic females and 16 oocytes from symbiotic females were measured. All image-processing software was custom-developed in MATLAB version 7.1 (MathWorks, Natick, MA).
The apoptotic surface ratios were tested for differences between the symbiotic and aposymbiotic females by using a Mann–WhitneyU test in R statistical software (version 2.3.0) (25).
Acknowledgments
We thank P. Mavingui for stimulating discussions and M. E. Huigens, R. Stouthamer, P. M. Brakefield, S. A. West, and three anonymous referees for their critical comments. We thank the Centre National de la Recherche Scientifique for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Maynard Smith J. Nature. 1989;341:284–285. doi: 10.1038/341284a0. [DOI] [PubMed] [Google Scholar]
- 2.Wernegreen JJ. PLoS Biol. 2004;2:e68. doi: 10.1371/journal.pbio.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margulis L. Symbiosis in Cell Evolution. New York: Freeman; 1981. [Google Scholar]
- 4.Stouthamer R, Breeuwer JAJ, Hurst GDD. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill SL, Hoffmann AA, Werren JH. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 6.Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH. Nature. 1993;361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 7.Boyle L, O'Neill SL, Robertson HM, Karr TL. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 8.Hurst GDD, Jiggins FM, von der Schulenburg JHG, Bertrand D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN. Proc R Soc London Ser B. 1999;266:735–740. [Google Scholar]
- 9.Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. Proc Natl Acad Sci USA. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dedeine F, Vavre F, Shoemaker DD, Bouletreau M. Evolution (Lawrence, Kans) 2004;58:2167–2174. doi: 10.1111/j.0014-3820.2004.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 11.McCall K. Dev Biol. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Zychlinsky A, Sansonetti P. J Clin Invest. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batut J, Andersson SGE, O'Callaghan D. Nat Rev Microbiol. 2004;2:933–945. doi: 10.1038/nrmicro1044. [DOI] [PubMed] [Google Scholar]
- 14.Gao LY, Abu Kwaik Y. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- 15.Dedeine F, Bouletreau M, Vavre F. Heredity. 2005;95:394–400. doi: 10.1038/sj.hdy.6800739. [DOI] [PubMed] [Google Scholar]
- 16.Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F. Genetics. 2004;168:181–189. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starr DJ, Cline TW. Nature. 2002;418:76–79. doi: 10.1038/nature00843. [DOI] [PubMed] [Google Scholar]
- 18.Clark ME, Heath BD, Anderson CL, Karr TL. Genetics. 2006;173:727–734. doi: 10.1534/genetics.105.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale C, Moran NA. Cell. 2006;126:453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 21.Douglas AE, Smith DC. Trends Ecol Evol. 1989;4:350–352. doi: 10.1016/0169-5347(89)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M. Mol Biol Evol. 1999;16:1711–1723. doi: 10.1093/oxfordjournals.molbev.a026084. [DOI] [PubMed] [Google Scholar]
- 23.David JR. Drosophila Inf Serv. 1962;36:128. [Google Scholar]
- 24.Nezis IP, Stravopodis DJ, Papassideri I, Margaritis LH. Cell Motil Cytoskeleton. 2001;48:224–233. doi: 10.1002/1097-0169(200103)48:3<224::AID-CM1011>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Ihaka R, Gentleman R. J Comp Graph Stat. 1996;5:299–314. [Google Scholar]