
Effect of hyperbaric oxygen treatment on ischaemia-reperfusion injury in rats detorsioned after experimental ovarian torsion
Eralp Bulutlar
Ali Yilmaz
Gizem Berfin Uluutku Bulutlar
Dr Eralp Bulutlar, Başakşehir Çam and Sakura City Hospital, Başakşehir Olimpiyat Bulvarı Yolu, 34480 Başakşehir, İstanbul, Turkey,eralpbulutlar@hotmail.com
Corresponding author.
Received 2021 Mar 15; Accepted 2023 Dec 26; Collection date 2024 Mar.
Abstract
Introduction
This study aimed to investigate whether hyperbaric oxygen treatment (HBOT) could ameliorate ischaemia-reperfusion injury in a rat model of ovarian torsion-detorsion.
Methods
Twenty-seven rats were divided among four groups: surgical sham rats (S) (n = 6) underwent identical anaesthesia and surgical incisions to other groups (n = 7 per group) but with no ovary intervention; torsion rats (T) underwent laparotomy, ovarian torsion, relaparotomy and sacrifice after three hours; torsion and detorsion rats (T/DT) underwent laparotomy, ovarian torsion (three hours), relaparotomy and detorsion, and sacrifice after one week; torsion, detorsion, hyperbaric oxygen rats (T/DT/HBOT) underwent laparotomy, ovarian torsion, relaparotomy and detorsion, and sacrifice after one week during which HBOT was provided 21 times (100% oxygen at 600 kPa for 50 min). In all groups blood collection for markers of oxidative stress or related responses, and ovary collection for histology were performed after sacrifice.
Results
When the T/DT, and T/DT/HBOT groups were compared, 8-hydroxy-2′-deoxyguanosine (a marker of oxidative damage to DNA) and malondialdehyde (a product of lipid peroxidation) levels were lower in the T/DT/HBOT group. Anti-Mullerian hormone levels were higher in the T/DT/HBOT group compared to the T/DT group. In addition, oedema, vascular occlusion, neutrophilic infiltration and follicular cell damage were less in the T/DT/HBOT group than in the T/DT group.
Conclusions
When biochemical and histopathological findings were evaluated together, HBOT appeared reduce ovarian ischaemia / reperfusion injury in this rat model of ovarian torsion-detorsion.
Keywords: Animal model, Antioxidants, Experimental, Hyperbaric research, Inflammation
Introduction
Ovarian torsion may be defined as the impairment of ovarian perfusion and occurrence of ischaemic changes, as a result of rotation of the ovary around the infundibulo-pelvic and utero-ovarian ligament.[1,2] Since the majority of the cases occur in the reproductive period, the protection of ovarian function is extremely important in terms of fertility and women’s health in general. Historically, the standard treatment for ovarian torsion has been salpingo-oophorectomy on the affected side, due to concerns about the risk of thromboembolism. However, in observational studies, it has been noted that ovarian function continued in cases where detorsion had been undertaken.[3]
Minimising ischaemia-reperfusion injury in detorsion cases in which ovarian tissue is preserved has become a new area of interest amongst clinicians and scientists.[3-5] We have not been able to identify reports of hyperbaric oxygen treatment (HBOT) being used in this context. The promising results of HBOT in testicular torsion,[6-9] which has similar mechanisms as cases of ovarian torsion, inspired this study. Should HBOT prove beneficialin vivo, this might justify a clinical study.
Methods
The study was approved by the Ethics Committee of Animal Experiments in the Health Sciences University of Hamidiye (approval number 46418926-605.02).
ANIMALS
Twenty-seven female Sprague-Dawley rats of about four months age, weighing 200–250 g, were used. Experimental design elements suggested by ARRIVE guidelines 2.0 were followed.[10]
ANAESTHESIA
For surgical interventions anaesthesia was provided intraperitoneally with 80 mg·kg-1 ketamine hydrochloride and 20 mg·kg-1 xylazine hydrochloride. Where necessary, ketamine (25 mg·kg-1) was repeated (based on checking reflex responses) to keep the anaesthesia depth of the rats constant.
PROCEDURE
The rats were divided into four groups: In the surgical sham group (S), six rats’ laparotomy incisions were closed after the uterus and adnexa were seen. After three hours relaparotomy was performed and bilateral ovaries were removed. In the torsion group (T) (n = 7), rats underwent laparotomy, exposing the ovaries which were tied with 5/0 polydioxanone suture approximately 1 cm below the adnexal structure containing the tubal and ovarian vessels, in order to create an ovarian ischaemia model. Three hours after skin closure, both ovaries were removed by relaparotomy. Both S and T groups were sacrificed after blood and tissue samples were taken at relaparotomy. In the torsion/detorsion group (T/DT) (n = 7) after performing the ischaemia intervention as above, the ovaries were reperfused by suture removal during relaparotomy at the third hour. The rats were housed in their cages for one week without any other treatment. In the torsion/detorsion/hyperbaric oxygen (T/DT/HBOT) group (n = 7) rats underwent an identical procedure to the T/DT group but subsequently underwent HBOT sessions for one week (as below), in a pressure chamber designed for animals.
The HBOT protocol was designed in accordance with previous literature relevant to our study.[11] That study investigated the effect of HBOT (1,000 kPa) in testicular torsion in rats. In the present study we restricted the treatment pressure to 600 kPa (absolute). In our protocol, the pressure was increased to 600 kPa over 10 min and maintained for 50 min using oxygen. Compression began slowly to minimise discomfort. Thereafter, decompression was conducted linearly to ambient pressure at a rate of 200 kPa·min-1. The chamber underwent continuous ventilation to avoid accumulation of carbon dioxide. In the first two days following surgery, four sessions of 50 minutes were applied. On the 3rd, 4th, and 5th days, three sessions of 50 minutes each were applied. On the 6th and 7th days, 2 sessions of 50 minutes were applied.[12] That is, 21 sessions of HBO were given over seven days, and the daily treatment sessions program can be summarised as 4/4/3/3/3/2/2.7,12 At the end of the 7th day, both ovaries were removed by relaparotomy and blood samples taken after which the rats were sacrificed.
Blood samples underwent enzyme-linked immunosorbent assay (Bioassay Laboratory brand trade kit, China) conducted by staff unaware of group allocations. Five assays were conducted: 8-hydroxy-2′-deoxyguanosine (8-OHDG), one of the oxidative damage products when reactive oxygen species damage DNA;[13] malondialdehyde (MDA), a product of lipid peroxidation (higher levels indicate a greater degree of oxidative damage);[14] superoxide dismutase (SOD), the only enzyme in the organism that utilises the superoxide free radical as a substrate (an increase indicates antioxidant capacity as well as indirectly indicating the mitochondrial extent of oxidative damage); glutathione peroxidase (GSH-Px), found in the cell cytoplasm (an increase protects cells against oxidative damage caused by H2O2);[15] and anti-Mullerian hormone (AMH), an ovarian hormone (a decrease indicates a decrease in ovarian reserve).[16]
The ovarian tissues were fixed in 10% formaldehyde for 24 hours after which 4 µm sections were prepared from paraffin blocks and stained with hematoxylin eosin (H&E). The sections were examined with a light microscope for ischaemia-reperfusion injury and the results were evaluated semi-quantitatively as 0 – no damage, 1 – mildly damaged, 2 – moderately damaged, 3 – severely damaged, in respect of oedema, follicular cell damage, vascular congestion, haemorrhage, neutrophil infiltration and cohesion failure. The examining pathologist was blinded to group allocation.
STATISTICAL ANALYSIS
The ‘E value’ method was used to determine the number of animals to be used in our study. According to this analysis, the E value should be between 10 and 20.[17] The E value (effectively the degrees of freedom for analysis of variance) was calculated from total number of animals – total number of groups. Assuming use of six rats per group in four groups, one of which is the control group, the total number of animals needed was 24 and the E value was 24-4 = 20.[17]
Statistical analyses were undertaken with the Statistical Package for Social Sciences, version 22.0 (SPSS Inc, Chiago, III, USA). Individual group biochemical parameters were assessed with the 1-sample Kolmogorov-Smirnov Z test and found normally distributed. The data were therefore expressed as means and standard deviations (SD). Analysis of variance was performed on the biochemical data to examine differences among groups. If a significant group effect was found, a Tukey honestly significant difference (HSD) test was used to identify the location of differences between groups. Statistical significance was defined asP < 0.05. Tissue damage scores were compared by nonparametric analysis, and statistical significance was assessed by Kruskal-Wallis followed by a Bonferroni-corrected Mann-Whitney U test.
Results
8-OHDG VALUES
The one-way ANOVA test showed a statistically significant difference between the mean 8-OHDG levels of the groups (P < 0.05). The values in the T/DT/HBOT group were significantly decreased compared to the T/DT group (2.42 [SD 0.54] vs 2.79 [0.43] ng.ml-1),P < 0.05. Group T had the lowest data compared to the other three groups 1.28 (0.17) ng.ml-1 (Figure 1).
Figure 1.

Comparison of 8-OHDG values between groups; data are mean and standard deviation; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group
MDA VALUES
The one-way ANOVA test showed a statistically significant difference between the mean MDA levels of the groups (P < 0.05). The values in the T/DT/HBOT group were significantly decreased compared to the T/DT group (1.20 [0.19] vs 2.03 [0.59] nmol.ml-1),P < 0.05. In addition, the significantly lower value in Group T compared to Group T/DT (0.76 [0.24] vs 2.03 [0.59] nmol.ml-1),P < 0.05 showed that reperfusion injury was more prominent than ischaemic injury (Figure 2).
Figure 2.

Comparison of MDA values between groups; data are mean and standard deviation; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group
GSH-PX VALUES
The one-way ANOVA test showed a statistically significant difference between the mean GSH-Px levels of the groups (P < 0.05). The values in the T/DT group were higher compared to the T/DT/HBOT group (142.74 [28.22] vs 98.37 [42.99] U.ml-1) but the difference was statistically insignificant (P = 0.085). While the differences between the other three groups were statistically insignificant, the significant decrease in the torsion-only group (T) was considered an interesting result. (Figure 3).
Figure 3.
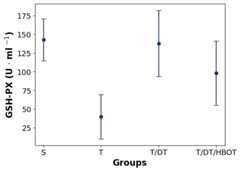
Comparison of GSH-Px values between groups; data are mean and standard deviation; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group
SOD VALUES
The one-way ANOVA test showed a statistically significant difference between the mean SOD levels of the groups (P < 0.05). Similar to the GSH-Px result, the only result significantly different from the other groups was the low value in the torsion-only group (T) (0.94 [0.11] ng.ml-1). The differences between the other three groups were statistically insignificant (P = 0.833) (Figure 4).
Figure 4.

Comparison of SOD values between groups; data are mean and standard deviation; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group
AMH VALUES
The one-way ANOVA test showed a statistically significant difference between the means of the AMH levels of the groups (P < 0.05). The highest AMH value was found in Group S, and the lowest AMH value was found in Group T (Figure 5). One potentially exciting finding was that the AMH value in Group T/DT/HBOT was higher than Group T/DT (2.95 [0.56] ng·ml-1 vs 2.10 [0.97] ng.ml-1) although this difference was not statistically significant.
Figure 5.

Comparison of AMH values between groups; data are mean and standard deviation; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group
PATHOLOGICAL ANALYSIS OF OVARIAN TISSUE
The histopathological damage grades are summarised inTable 1.
Table 1. Comparison of discrete scores for histopathological variables between groups; data are numbers of ovaries in each category; score key: 0 – no damage, 1 – mildly damaged, 2 – moderately damaged, 3 – severely damaged; L – left; R – right; S – surgical control group; T – surgery plus ovarian torsion group; T/DT – surgery plus torsion plus detorsion (reperfusion) group; T/DT/HBOT – surgery plus torsion plus detorsion (reperfusion) plus hyperbaric oxygen treatment group.
| Histologic parameter | Score | Group S | Group T | Group T/DT | Group T/DT/HBOT | ||||
| R | L | R | L | R | L | R | L | ||
| Oedema | 0 | 6 | 6 | 2 | 3 | 0 | 0 | 1 | 0 |
| 1 | 0 | 0 | 5 | 4 | 4 | 3 | 6 | 7 | |
| 2 | 0 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vascular congestion | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1 | 3 | 3 | 2 | 1 | 1 | 1 | 7 | 6 | |
| 2 | 1 | 1 | 4 | 5 | 6 | 3 | 0 | 0 | |
| 3 | 0 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | |
| Neutrophilic infiltration | 0 | 6 | 6 | 1 | 0 | 0 | 0 | 6 | 5 |
| 1 | 0 | 0 | 5 | 4 | 0 | 4 | 1 | 2 | |
| 2 | 0 | 0 | 1 | 3 | 7 | 3 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Follicular cell damage | 0 | 6 | 6 | 7 | 7 | 4 | 5 | 7 | 5 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 2 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cohesion failure | 0 | 6 | 6 | 2 | 0 | 0 | 0 | 1 | 1 |
| 1 | 0 | 0 | 5 | 5 | 7 | 7 | 6 | 6 | |
| 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Haemorrhage | 0 | 6 | 6 | 7 | 6 | 4 | 4 | 3 | 3 |
| 1 | 0 | 0 | 0 | 0 | 3 | 3 | 4 | 4 | |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Oedema
No oedema was found in Group S. Severe oedema was observed in Group T/DT, while moderate oedema was observed in Group T/DT/HBOT and Group T. The increased oedema in Group T/DT was significantly greater than in Group T/DT/HBOT and Group T (P < 0.05).
Vascular congestion
Relatively mild vascular congestion was seen in Group S, with the most severe congestion seen in Group T/DT. Severe vascular congestion was also observed in Group T. Vascular congestion in Group T/DT/HBOT was significantly less than both Group T/DT and Group T (P < 0.05).
Neutrophil infiltration
No neutrophilic infiltration was observed in Group S. While severe infiltration was observed in Group T/DT and Group T, mild infiltration was observed in Group T/DT/HBOT and these differences with other groups were statistically significant (P < 0.05) (Figure 6).
Figure 6.
Post-reperfusion neutrophilic infiltration; A – mild infiltration in Group T/DT/HBOT (surgery plus torsion plus detorsion [reperfusion] plus hyperbaric oxygen treatment); B – severe infiltration in Group T/DT (surgery plus torsion plus detorsion [reperfusion])
Follicular cell damage
Follicular damage was not seen in Group S and Group T. Moderate damage was observed in Group T/DT, while mild damage was observed in Group T/DT/HBOT.
Haemorrhage
No haemorrhage was observed in Group S. Moderate haemorrhage was observed in the right ovary of only one rat in Group T. This has been interpreted as a surgical complication. Although the haemorrhage seen in Group T/DT was higher than seen in Group T/DT/HBOT, this difference was statistically insignificant (P = 0.71) (Figure 7).
Figure 7.
Post-reperfusion haemorrhage; A – moderate haemorrhage in Group T/DT/HBOT (surgery plus torsion plus detorsion [reperfusion] plus hyperbaric oxygen treatment); B – severe haemorrhage in Group T/DT (surgery plus torsion plus detorsion [reperfusion])
Cohesion failure
Cohesion failure was not seen in Group S. Mild loss of cohesion was observed in Group T, Group T/DT and Group T/DT/HBOT, and there were no significant differences between groups.
Discussion
The significant decrease in 8-OHDG values in Group T/DT/HBOT compared to Group T/DT (P < 0.05) indicated that HBOT reduced DNA damage resulting from reperfusion-induced oxidative stress. The highest MDA value was found in Group T/DT, while the values in Group S and Group T/DT/HBOT were similar and statistically significantly lower compared to Group T/DT (P < 0.05). This result is further evidence that HBOT may have suppressed reperfusion-induced oxidative stress. There is no obvious basis for the lower MDA values in Group T compared with Group S, and specifically targeted studies would be required to understand this clearly. Nevertheless, the fact that MDA in Group T/DT is significantly higher than Group S suggests that the secondary oxidative damage that follows ovarian ischemia-reperfusion is greater than after a simple laparotomy and anaesthetic.
Group T had lower GSH-Px and SOD values compared to the other three groups. The likely reason for this result is the reperfusion damage suffered by Groups T/DT and T/DT/HBOT, whereas Group T’s rats were sacrificed prior to reperfusion damage. The reperfusion damage might have caused a secondary anti-oxidant capacity increase in the rat system. It is acknowledged that this explanation is somewhat inconsistent with the high GSH-Px and SOD values measured in Group S. Further studies are necessary to determine the cause of these results.
The significantly lower AMH level of Group T compared to Group S (P < 0.05) can be explained by isolation of the ovaries such that the AMH levels in the plasma fell during the period of ischaemia. The fact that Group T/DT was lower than Group S (P < 0.05) but higher than Group T (P < 0.05) is perhaps explained by backwashing of AMH into plasma from a previously isolated (but dysfunctional) ovary after ischaemia and reperfusion. There was a trend toward increased AMH levels in the T/DT/HBOT group compared to the T/DT group, perhaps suggesting some degree of protection of ovarian reserve by HBOT, but this difference was statistically insignificant.
The most severe histologic oedema was seen in Group T/DT, with significantly less oedema in Group T/DT/HBOT suggesting an anti-inflammatory effect of HBOT in this context. Perhaps not surprisingly, HBOT exposure was also associated with reduced vascular congestion and reduced neutrophilic infiltration. The latter finding was consistent with previously reported oxygen-dose-dependent reduction in expression of adhesion molecules on cultured neutrophils activated in an in vitro ischaemia-reperfusion simulation.[18] When follicular cell damage and haemorrhage were examined, No follicular cell damage or haemorrhage was observed in Groups S and T (bleeding in one Group T ovary was thought to be surgical artefact). There was a decrease in the follicular cell damage in Group T/DT/HBOT compared to Group T/DT supporting a protective effect of HBOT during ovarian ischaemia-reperfusion.
These results, collectively indicate a potential role in for HBOT in protecting the ovaries from reperfusion injury after detorsioning. However, there are some obvious limitations in extrapolating beyond our small study in rats, not least being the possibility that results in an animal model may not translate into humans. We also acknowledge that the HBOT regimen was highly atypical of human treatment paradigms with oxygen being administered at 600 kPa and the treatment frequency being much higher than in typical clinical practice. We selected the study parameters to be consistent with previous successful work in a rat model of testicular torsion-detorsion, but further dose-finding studies, perhaps with a narrower outcome focus (concentrating on those outcome measures that appeared to benefit here), would be required to explore whether clinically relevant treatment regimens also seem effective.
Conclusions
Our study has demonstrated that HBOT appears to reduce ovarian damage both biochemically and histopathologically in this ovarian ischaemia-reperfusion model. As a ‘first of type’ study with small sample sizes, it is clear that more comprehensive studies will be needed to further clarify effects and to optimise HBOT schedules and timing. We see potential for clinical testing following more comprehensivein vivo studies.
Footnotes
Conflicts of interest and funding: nil
References
- McWilliams GDE, Hill MJ, Dietrich CS 3rd. Gynecologic emergencies. Surg Clin North Am. 2008;88:265–83. doi: 10.1016/j.suc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Tielli A, Scala A, Alison M, Vo Chieu VD, Farkas N, Titomanlio L, et al. Ovarian torsion: diagnosis, surgery, and fertility preservation in the pediatric population. Eur J Pediatr. 2022;181:1405–11. doi: 10.1007/s00431-021-04352-0. [DOI] [PubMed] [Google Scholar]
- Bristow RE, Nugent AC, Zahurak ML, Khouzhami V, Fox HE. Impact of surgeon specialty on ovarian-conserving surgery in young females with an adnexal mass. J Adolesc Health. 2006;39:411–6. doi: 10.1016/j.jadohealth.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Anders JF, Powell EC. Urgency of evaluation and outcome of acute ovarian torsion in pediatric patients. Arch Pediatr Adolesc Med. 2005;159:532–5. doi: 10.1001/archpedi.159.6.532. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–7. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Alp BF, Cebi G, Özdemir A, Irkilata HC, Uzun G. Hyperbaric oxygen treatment for unilateral testicular torsion in a child. Diving Hyperb Med. 2014;44:161–2. [PubMed] [Google Scholar]
- Karlı G, Erginel B, Yanar F, Üstyol EA, Ozluk Y, Karadeniz MS, et al. Comparison of hyperbaric oxygen and ozone treatment for ischemia/re-perfusion injury in an experimental testicular torsion model. Ulus Travma Acil Cerrahi Derg. 2023;29:259–65. doi: 10.14744/tjtes.2023.98861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolski JM, Mazolewski PJ, Stephenson LL, Texter J, Grigoriev VE, Zamboni WA, et al. Effect of hyperbaric oxygen therapy on testicular ischemia-reperfusion injury. J Urol. 1998;160:601–4. [PubMed] [Google Scholar]
- Zhang Y, Lv Y, Liu YJ, Yang C, Hu HJ, Meng XE, et al. Hyperbaric oxygen therapy in rats attenuates ischemia-reperfusion testicular injury through blockade of oxidative stress, suppression of inflammation, and reduction of nitric oxide formation. Urology. 2013;82:489.e9–489.e15. doi: 10.1016/j.urology.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617–24. doi: 10.1111/bph.15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alici B, Kalkan M, Tunç B, Çeti̇nkaya M, Aktaş Ş, Uzun H, et al. Testiküler torsiyonda hiperbarik oksijen tedavisinin etkinliği. Türk Üroloji Dergisi/Turkish Journal of Urology. 2004;30(3):273–8. [Google Scholar]
- Konak M, Cincik H, Erkul E, Kucukodaci Z, Gungor A, Ozdemir S, et al. The protective effects of different treatments on rat salivary glands after radiotherapy. Eur Arch Otorhinolaryngol. 2016;273:4501–6. doi: 10.1007/s00405-016-4159-2. [DOI] [PubMed] [Google Scholar]
- Graille O, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci. 2020;21(11):3743. doi: 10.3390/ijms21113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Di Clemente N, Racine C, Pierre A, Taieb J. Anti-müllerian hormone in female reproduction. Endocr Rev. 2021;42:753–82. doi: 10.1210/endrev/bnab012. [DOI] [PubMed] [Google Scholar]
- Charan J,Kantharia ND.How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–6. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras JA, Stahl GL, Svoboda KK, Reenstra WR. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol. 2000;278(2):C292–302. doi: 10.1152/ajpcell.2000.278.2.C292. [DOI] [PubMed] [Google Scholar]

