Comparative PharmacologicalEffects of Lisuride andLysergic Acid Diethylamide Revisited
Grant C Glatfelter
Eline Pottie
John S Partilla
Christophe P Stove
Michael H Baumann
Email:grant.glatfelter@nih.gov.
Received 2023 Aug 17; Accepted 2024 Jan 12; Revised 2023 Dec 12; Collection date 2024 Mar 8.
Abstract

Lisuride is a non-psychedelic serotonin (5-HT) 2A receptor(5-HT2A) agonist and analogue of the psychedelic lysergicacid diethylamide(LSD). Lisuride also acts as an agonist at the serotonin 1A receptor(5-HT1A), a property known to counter psychedelic effects.Here, we tested whether lisuride lacks psychedelic activity due toa dual mechanism: (1) partial agonism at 5-HT2A and (2)potent agonism at 5-HT1A. Thein vitro effects of lisuride, LSD, and related analogues on 5-HT2A signaling were characterized by using miniGαq andβ-arrestin 2 recruitment assays. The 5-HT1A- and5-HT2A-mediated effects of lisuride and LSD were also comparedin male C57BL/6J mice. Thein vitro results confirmedthat LSD is an agonist at 5-HT2A, with high efficacy andpotency for recruiting miniGαq and β-arrestin2. By contrast, lisuride displayed partial efficacy for both functionalend points (6–52% of 5-HT or LSDEmax) and antagonized the effects of LSD. The mouse experiments demonstratedthat LSD induces head twitch responses (HTRs)(ED50 = 0.039mg/kg), while lisuride suppresses HTRs (ED50 = 0.006 mg/kg).Lisuride also produced potent hypothermia and hypolocomotion (ED50 = 0.008–0.023 mg/kg) that was blocked by the 5-HT1A antagonist WAY100635 (3 mg/kg). Blockade of 5-HT1A prior to lisuride restored basal HTRs, but it failed to increaseHTRs above baseline levels. HTRs induced by LSD were blocked by lisuride(0.03 mg/kg) or the 5-HT1A agonist 8-OH-DPAT (1 mg/kg).Overall, our findings show that lisuride is an ultrapotent 5-HT1A agonist in C57BL/6J mice, limiting its use as a 5-HT2A ligand in mouse studies examining acute drug effects. Resultsalso indicate that the 5-HT2A partial agonist-antagonistactivity of lisuride explains its lack of psychedelic effects.
Keywords: 5-HT2A, lisuride, LSD, psychedelic, 5-HT1A
Lisuride is a close structuralanalogue of the psychedelic ergoline lysergic acid diethylamide (LSD,structures shown inFigure1
Figure 1.
Chemical structures of lisuride and LSD (A) and heatmapof functionalparameters for each of the tested substances in both assay formats.(B) EC50 values forin vitro functionalpotency in βarr2 and miniGαq assays as wellasEmax values relative to theEmax of LSD (C) and serotonin (D, 5-HT), whichwere arbitrarily defined as 100%. Darker colors indicate higher potencies(lower EC50 values) and efficacies. Data are from at leastthree biologically independent experiments, each performed in duplicate.
In addition to its 5-HT2A agonist activity,5,20,21 lisuride has many other biologicaltargets.22 These include dopamine receptors(D1- and D2-like),22,23 non-5-HT2A serotonin receptor subtypes (5-HT1A, 5-HT2C, and others),5,20 as well asother sites (adrenergic and histamine receptors).22 From a clinical perspective, the dopamine receptor agonistproperties of lisuride are thought to mediate its efficacy in treatingearly symptoms of Parkinson’s disease.24 Previous experiments in rats showed that lisuride is a more potentagonist at 5-HT1A than LSD, and this property distinguishesthe two drugs.16,20,25 Finally, studies in mice demonstrate that the 5-HT2A-mediatedHTR is suppressed by the 5-HT1A agonist activity of certainpsychedelic compounds,26−32 including the ergoline lysergic acid morpholide (LSM-775).31
At present, it is unclear whether thelack of psychedelic effectsof lisuride is due to weak signaling efficacy at 5-HT2A, effects exerted at non-5-HT2A targets, or perhaps bothmechanisms. Here, we hypothesized that lisuride is devoid of psychedelicactions due to a two-pronged mechanism: (1) partial agonism at 5-HT2A, coupled with (2) potent agonism at 5-HT1A. First,we examined thein vitro 5-HT2A functionalactivities of lisuride, LSD, and three structurally related ergolinesin assays measuring G-protein and β-arrestin 2 (βarr2)recruitment. Next, we compared thein vivo effectsof lisuride and LSD in mice to evaluate 5-HT2A-mediatedHTR and 5-HT1A-mediated hypothermia and locomotor suppression.Given the other known targets of lisuride, we also investigated thepossible role of D1-like, D2-like, and 5-HT2C receptor activity in the acute effects of lisuride. Site-selectivereceptor antagonists were employed to discern the role of specificreceptor subtypes in mediatingin vivo effects.
Results and Discussion
Functional Assessments of Lisuride and LSD at 5-HT2AIn Vitro
The 5-HT2A activatingpotential of lisuride was compared to LSD and several ergoline analogues.To this end, two differentin vitro assays were employed,which monitor the recruitment of either βarr2 or miniGαq by 5-HT2A, utilizing NanoBiT functional complementationtechniques.33,34 In this study, we opted to testonly βarr2, rather than both βarr1 and βarr2, becauseof the reported relevance of the latter in LSD-stimulated behaviorsin mice.35 Further, βarr2 has recentlybeen reported to be required for LSD-induced reopening of the “socialreward learning critical period” in mouse behavioral studies.36 The employed NanoBiT technology is specificallydesigned to monitor protein–protein interactions37 and consists of two split subunits of a nanoluciferaseenzyme. In the assays used here, one subunit of the enzyme is fusedto the C-terminus of the 5-HT2A, and the other subunitto the N-terminus of either βarr2 or the engineered miniGαq protein (as described by Nehmé et al.).38 Upon activation of the receptor, the intracellularprotein is brought closer to the receptor protein, thereby allowingfunctional complementation of the enzyme subunits and a concomitantluminescent signal in the presence of the enzyme substrate.37 Thein vitro functional potencies(expressed as EC50 values) and efficacies (expressed asEmax values relative to the maximal responseof LSD and 5-HT) obtained in both recruitment assays are providedinFigure1B–D,with associated curves (%LSD) shown inFigures2A,B andS1. LSDwas included as a reference compound forEmax in addition to 5-HT because the present study aimed to directlycompare lisuride to LSD.
Figure 2.
(A,B) Concentration–response curves foreffects of lisuride(A) and LSD (B) in 5-HT2Ain vitro recruitmentassays. (C, D) Effects of lisuride on the functional activity of LSDfor 5-HT2A-mediated β-arrestin 2 (C) and miniGαq (D) recruitment assays. Concentration–response curvesfor cells that were preincubated for 5 min with either lisuride ora corresponding solvent control, after which a concentration rangeof LSD (green curve), 100 nM LSD (purple curve), or the correspondingsolvent control (blue curve) was added. Data in all panels are themean ± SEM of three biologically independent experiments, eachperformed in duplicate, and normalized relative to theEmax of LSD. LIS = lisuride.
In addition to lisuride, the structurally relatedergolines 2-bromolysergicacid diethylamide (2-Br-LSD), 6-allyl-6-nor-lysergic acid diethylamide(AL-LAD), and lysergic acid methylpropylamide (LAMPA) were includedin the current experiments (structures shown inFigure S1). DOI and serotonin were also included as referencecompounds to enable comparison of the current results with those obtainedpreviously. Results for reference agonists LSD, serotonin, and DOIalign with previously published data obtained with the same assaysand with data obtained using a cell line stably expressing the assaycomponents of the βarr2 assay (Figures1 andS1).33,34,39−43 Specifically, low nanomolar EC50 valueswere observed for LSD in both 5-HT2A receptor recruitmentassays. Serotonin exhibited a similar EC50 value and ahigherEmax value in the βarr2assay relative to LSD. Serotonin also displayed weaker potency buthigherEmax values in the miniGαq recruitment assay relative to LSD. The last reference compound,DOI, displayed higher potency and efficacy in the βarr2 assay,with a similar potency and an increased efficacy in the miniGαq assay vs LSD.
The data revealed notable functionaldifferences among the variousergoline analogues (Figures1,2, andS1). Compared to LSD, lisuride displayed partial agonist effects inthe βarr2 recruitment assay (52 and 45% of LSD and serotoninEmax respectively) with a slightly reduced potency.In the miniGαq assay, lisuride was even weaker, exhibitingan efficacy of only 15% relative to LSD, indicative of a relativepreference toward βarr2 recruitment. When compared to DOI orserotonin as reference agonists, the relative efficacy of lisuridein the miniGαq assay would be even lower (yieldinganEmax of 8% for lisuride when theEmax of DOI is set at 100%, or anEmax of merely 6% when theEmax of serotonin is set at 100%). These findings indicate a strong preferenceof lisuride toward βarr2 recruitment, relative to either DOIor serotonin.
2-Br-LSD displayed a slightly higher potency thanLSD in both recruitmentassays, but efficacies were strongly reduced relative to LSD, witha lowerEmax in the βarr2 (47.7%of LSDEmax) and miniGαq (23.5% of LSDEmax) recruitment assays.It is interesting that we found the 5-HT2A efficacy profileof 2-Br-LSD to be quite similar to that of lisuride, and 2-Br-LSDis devoid of psychedelic effects in humans and animals.44 These observations still hold true when thedata are normalized to the serotonin reference instead of LSD (Figure1C). Relative to LSDas a reference, potencies for AL-LAD were similar to those of LSDin both assays, but the efficacies were higher, yielding values of137 and 167% for βarr2 and miniGαq recruitment,respectively. The data with AL-LAD are noteworthy, since in the assayformat used, it displayed a slight preference toward recruitment ofminiGαq over βarr2. However, when normalizedrelative to serotonin, which is the most efficacious agonist in theminiGαq setup, both LSD and AL-LAD display efficaciesbelow 100%. In that scenario, all of the investigated compounds aremore efficacious in the βarr2 recruitment assay than in theminiGαq assay. Irrespective of whether LSD or serotoninis used as a reference compound, AL-LAD remains the most efficaciousagonist in the βarr2 assay. The third analogue, LAMPA, displayedEC50 values similar to those of LSD and slightly decreasedEmax values in both assays (87–89% ofLSDEmax).
Based on the weak efficacyof lisuride in both recruitment assays,we assessed the ability of lisuride to antagonize the effects of LSDat 5-HT2Ain vitro. To this end, cellswere preincubated with a concentration range of lisuride, to which100 nM LSD was added, in both assays. The results of that experimentare shown in the purple curves ofFigure2C,D. The green and blue curves result fromcontrol conditions, involving a concentration range of LSD after “preincubation”with the solvent control of lisuride (in green), and a concentrationrange of lisuride to which the solvent control of LSD was added (inblue).
Following preincubation with a low concentration of lisuride,100nM LSD was still able to induce the recruitment of βarr2 tothe same extent as when no lisuride was present. When the concentrationof lisuride was increased, the activity of LSD was reduced to thelevel that coincided with the upper plateau of the concentration–responsecurve of lisuride in this assay. The results indicate that lisurideis capable of antagonizing the effect of LSD down to the level atwhich it can maximally induce βarr2 recruitment itself. Thus,in the βarr2 assay, lisuride acted as a partial agonist/antagonistto block LSD-mediated activity at 5-HT2A. For the miniGαq recruitment assay, the results were slightly different, consistentwith a weaker partial agonism displayed by lisuride in that setup.Again, in the presence of a low concentration of lisuride, 100 nMLSD was able to induce the recruitment of miniGαq to the receptor to the same extent as when no lisuride was present.Increasing lisuride concentrations strongly reduced this recruitmentof miniGαq, indicating that lisuride can fully antagonizethe LSD-induced functional activity at 5-HT2A in this assay.
We must acknowledge some differences between the presently usedand other 5-HT2A receptor functional assays. Allin vitro assays used to study 5-HT2A activationinherently entail both advantages and limitations (see Pottie andStove).45 For example, assays like thepresently used miniGq recruitment assay measure an eventupstream of distal downstream signaling cascades, which can be advantageousto directly study 5-HT2A activation. However, measurementof signaling events proximal to receptor activation may not fullyrecapitulate downstream signal amplification of assays measuring eventsmore distal to the receptor. Conversely, distal downstream signalingcascades may have undergone substantial signal amplification, makingit difficult to distinguish between full and partial agonists. Moreover,different experimental setups may have an impact on the obtained data,with the temperature at which the assay is performed and the readouttime as specific examples in the context of 5-HT2A signalinginduced by ergolines.46,47 Considerations inherent to thecurrently used recruitment assay are the fact that both the receptorand cytosolic protein are fused to small enzyme fragments, which mayinfluence receptor function, and the use of the artificially engineeredminiGq protein. It is therefore important to note differencesbetween this assay and other assay systems measuring 5-HT2A receptor activation.
Affinities of Lisuride and LSD at 5-HT1A and 5-HT2A Receptors in Mouse Brain
We next assessed the bindingaffinities (Ki values) of lisuride andLSD at the 5-HT1A and 5-HT2A receptors. To thisend, competition binding assays were carried out in mouse brain tissueusing the 5-HT1A agonist [3H]8-OH-DPAT or 5-HT2A antagonist [3H]M100907. Lisuride and LSD displayedhigh affinities at both receptors in the low nM range (2–6nM,Table1 andFigure S2), consistent with previous radioligandbinding studies.48−50 Additionally, control compounds 8-OH-DPAT and DOIdisplayed the expected low nM inhibition constants for [3H]8-OH-DPAT (1 nM) and [3H]M100907 (6 nM) binding, respectively(Table1 andFigure S2).
Table 1. Inhibition Constants (Ki) in Mouse Brain Binding Assays as well as Potency(ED50) and Maximum Observed Effects (Emax) forIn Vivo Tests in Mice ComparingLisuride and LSDa.
| affinity-mouse brain | potency & efficacy–mouse | ||||
|---|---|---|---|---|---|
| [3H]8-OH-DPAT binding m5-HT1A | [3H]M100907 binding m5-HT2A | HTR | temperature Δ | hypolocomotion | |
| ligand | Ki (nM) | Ki (nM) | ED50 (μg/kg s.c.) | ED50 (μg/kg s.c.) | ED50 (μg/kg s.c.) |
| lisuride | 2.2 (1.5–3.1) | 6.0 (4.9–7.1) | 6b (0.8–16) | 23 (17–32) | 8 (5–15) |
| Emax = −5.4 °C | Emax = 364 cm | ||||
| LSD | 2.1 (1.2–3.6) | 1.6 (1.3–1.9) | 39 (25–58) | 1620 (1218–2144) | 840 (475–1505) |
| Emax = 52 HTR | Emax = −3.1 °C | Emax = 758 cm | |||
m5-HT1A and m5-HT2A = mouse receptors and s.c. = subcutaneous drug administration.Parameters reported are expressed with 95% confidence intervals (CI)noted in parentheses.Ki values with 95%CI for DOI and 8-OH-DPAT at 5-HT2A and 5-HT1A receptors were 6.3 nM (4.5–8.6) and 0.8 nM (0.5–1.1),respectively.
Inhibitionof basal spontaneousHTR.
Dose–Response of the Effects of Lisuride and LSD in Mice
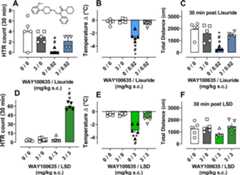
Mouse studies were conducted to test the dose-related (0.001–3mg/kg s.c.) acute effects of lisuride and LSD on HTR, body temperature,and locomotor activity over a 30 min testing session, using our previouslyreported methods.27,28,51 The resulting ED50 andEmax values can be found inTable1, while mean effects for each dose and information about statisticalcomparisons can be found inTable S1. Lisuridedisplayed potent dose-related decreases in the HTR (ED50 = 6 μg/kg), along with hypothermia (ED50 = 23 μg/kg)and hypolocomotion (ED50 = 8 μg/kg) vs vehicle controls(Figure3A–CandTable1). In contrast,LSD induced robust dose-related increases in the HTR (ED50 = 39 μg/kg), while showing hypothermic and locomotor suppressiveeffects similar to lisuride at doses that were ∼70–100times higher (ED50 = 800–1600 μg/kg) (Figure3D–F andTable1). Remarkably, theeffects of lisuride were similar to those of the prototypical 5-HT1A agonist, 8-OH-DPAT, with lisuride being ∼6–30times more potent across measures (Figure S3A–C and Table S2). Time-course plots of distance traveled duringthe session further illustrate the similarity in locomotor suppressioninduced by lisuride (0.01–3 mg/kg) and 8-OH-DPAT (0.1–30mg/kg) vs LSD (0.3–3 mg/kg;Figure S4). Overall, these results from mice complement and extend previousfindings from rats showing that lisuride produces 8-OH-DPAT-like effects,and does so much more potently than LSD.16,25
Figure 3.
Acutedose-related effects of lisuride and LSD in mice. Dose–responsecurves for lisuride (A–C) and LSD (D–F) were obtainedfor effects on HTR, body temperature, and distance traveled over the30 min session. All values are the mean ± standard error of themean (SEM) ofn = 5–7.* =p < 0.05 vs vehicle control (0 mg/kg). More details regardingthe pharmacological parameters determined and statistical comparisonsmade can be found in theMaterials and Methods section andTables1 andS1.
In this study, lisuride failed to elicit any increasein the HTRand potently (ED50 = 6 μg/kg) reduced spontaneousHTR events relative to vehicle controls. This observation is consistentwith findings from other groups that used much higher doses of lisuridein mice (≥400 μg/kg).2,3,7 Similar to lisuride, 8-OH-DPAT also reduced basalHTR events vs. vehicle controls (ED50 = 33 μg/kg).LSD showed the opposite effect, stimulating HTR with a typical invertedU-shaped dose–response curve, where the high doses (0.3–3mg/kg) reduced total HTRs and compressed the time-course of effects(Figure S5A). The descending limb of theLSD HTR curve corresponded with the hypolocomotive and hypothermiceffects seen at high doses and underscores the importance of usingwide dose ranges when studying drug-induced HTRs. Notably, the observedpotency for LSD-induced HTR shown here (39 μg/kg), is similarto the potency (ED50 = 53 μg/kg) and total numberof events observed by other laboratories using the same strain ofmice.2,3,35,87
Our dose–response studies show thatlisuride produces verypotent 5-HT1A agonist-like effects52,53 in the popularly used C57BL/6J mouse strain. Additionally, lisurideshowed high potency for the reduction of spontaneous HTR. The presentdata show that the ED50 of lisuride to reduce spontaneousHTR events is ∼42–67 times lower than the lowest dosesof lisuride (≥250–400 μg/kg) commonly employedin prior mouse studies.2,3,7,8 As a result, the present data indicate thatconclusions drawn about the pharmacology of lisuride in mouse studiesusing doses higher than ∼5 μg/kg may be confounded byits potent 5-HT1A agonist-like effects, limiting hypothesistesting regarding its 5-HT2A-mediated effects. Findingsfrom other recent studies of lisuride in mice support this, showingthat lisuride produces potent acute 5-HT syndrome-like effects onmotor activity and rearing behavior (0.01–4 mg/kg).9,54 These effects can affect the expression of the 5-HT2A-mediated HTR and other acutely expressed behaviors in rodents.
In contrast to the data shown here in mice, some studies have reportedthat lisuride induces hyperlocomotion in rats. For example, one studyfound that lisuride increases motor activity at doses of 200–500μg/kg in Wistar rats,55 while othersreported lisuride-induced hyperlocomotion in Sprague–Dawleyrats, along with hypothermic effects.56,57 Drug discriminationexperiments in Sprague–Dawley rats show that lisuride disruptsresponse rates at doses above 50–100 μg/kg, and similardoses evoke elements of the 5-HT behavioral syndrome, such as 5-HT1A-mediated ambulation, flat body posture, and forepaw treading.16,58 Thus, the apparent differences in behavioral manifestations of lisuridein rats vs mice could be related to the occurrence of elements ofthe 5-HT syndrome in rats, namely, ambulation and forepaw treading,which would be scored as locomotor activity. Others have postulatedthat lisuride has biphasic dose–response effects on locomotoractivity in rats, with increased activity at high doses.59 In the least shrew, lisuride produced hyperlocomotionin addition to a robust HTR,60 and in othermouse studies, lisuride potently reduced motor activity and rearing(0.01–4 mg/kg).9,54 Taken together, the summed behavioralfindings demonstrate key species differences in the effects of lisurideon motor activity, whereby the drug reliably suppresses locomotionin mice but enhances locomotion in rats, especially at high doses.
Role of 5-HT1A Receptors in the Effects of Lisurideand LSD
Given that we found lisuride to produce potent 8-OH-DPAT-likeeffects in mice, we next sought to verify whether these effects weremediated by 5-HT1A receptors. Additionally, since antagonismof 5-HT1A activity has been reported to enhance the 5-HT2A-mediated HTR induced by certain psychedelics,28,31 we also sought to test whether lisuride might induce HTR under conditionsof 5-HT1A blockade. For these experiments, mice were pretreatedwith saline vehicle or the 5-HT1A antagonist WAY100635(3 mg/kg s.c.), 30 min prior to administration of lisuride (0.02 mg/kgs.c.) or the comparator drugs LSD (3 mg/kg s.c. and 8-OH-DPAT (1 mg/kgs.c.) We chose these specific doses of lisuride, LSD, and 8-OH-DPATbecause they produced similar efficacious reductions in locomotionand body temperature in the dose–response studies. Importantly,WAY100635 pretreatment did not produce any effect on locomotor activityor change in body temperature vs vehicle controls over the first 30min prior to lisuride, LSD, or 8-OH-DPAT administration (Figure S6 and Tables S3 and S4).
Our resultsshow that lisuride alone produced a significant reduction in basalHTR compared with the vehicle and antagonist control conditions. Thisreduction in HTR was prevented when mice were pretreated with WAY100635(Figure4A andTables S3 and S4), but the number of HTRs wasnot elevated above control levels. The hypothermia and hypolocomotioninduced by lisuride were also attenuated by WAY100635 pretreatment(Figure4B,C andTables S3 and S4). Interestingly, WAY100635 pretreatmentprior to a high dose of LSD, which normally suppresses HTR (i.e.,3 mg/kg, corresponding to the descending limb of the HTR dose–responsecurve), produced a robust increase in HTR that was significant comparedto all other groups, including vehicle + LSD (Figures4D andS5B, Tables S3 and S4). The vehicle + LSD group also produced significanthypothermia vs all other groups, an effect that was blocked in theWAY100635 + LSD group (Figure4E andTables S3 and S4). It isnoteworthy that the vehicle + LSD group did not exhibit significantlydecreased locomotion relative to the other groups. The lack of effectof LSD on locomotion in this experiment differs from the findingsin the dose–response studies and may be due to the longer habituationperiod used in the antagonist reversal studies (Figure4F andTables S3 and S4).28,61 Lastly, data for the effects of WAY100635pretreatment in mice receiving 8-OH-DPAT mirrored the results forlisuride. Specifically, WAY100635 pretreatment reversed the 8-OH-DPAT-inducedeffects on basal HTR, hypothermia, and hypolocomotion (Figure S3D–F and Tables S3 and S4).
Figure 4.
Effects ofWAY100635 pretreatment on acute effects of lisuride(A–C) and LSD (D–F). All data are mean with SEM (n = 5) and individual points plotted for total HTR count,body temperature change, and distance traveled. Bold points and symbolsrepresent statistically significant differences (p < 0.05)via Tukey’s post-test as follows:*vs 0/0, #vs 3/0, ∧vs 0/lisuride or LSD, +vs 3/lisuride orLSD. More details regarding statistical comparisons can be found intheMaterials and Methods section andTables S3 and S4.
Collectively, the results from these mouse studiessupport thehypothesis that the hypothermic and hypolocomotor effects of the compoundstested here involve 5-HT1A receptor activation. Furthermore,lisuride and 8-OH-DPAT suppress basal spontaneous HTR by a mechanisminvolving 5-HT1A. Interestingly, we found that the blockadeof 5-HT1A completely reversed the suppression of HTR byhigh-dose LSD (3 mg/kg) and unmasked a fully efficacious HTR response.Consistent with our findings, a prior study showed that the LSD analogueLSM-775 does not increase HTR in mice unless mice are pretreated withthe 5-HT1A antagonist WAY100635.31 In previous human studies, pretreating study participants with pindololto antagonize 5-HT1A receptors enhanced the psychedelicsubjective effects of intravenousN,N-dimethyltryptamine administration.62 Suchresults support a growing body of evidence that shows an inhibitoryinfluence of 5-HT1A activation on psychedelic-like drugeffects in rodents and humans.26−31,62,63
It is important to note that the inhibitory effect of lisurideon basal HTR was abrogated when blocking its 5-HT1A agonistactivity, but no increase in the HTR above baseline control levelswas observed. González-Maeso et al. previously used 5-HT1A knockout mice to test the role of this receptor in modulatingHTR produced by LSD or lisuride.2 In 5-HT1A knockout mice, lisuride (0.4 mg/kg) did not induce the HTR,in agreement with the findings presented here. However, the effectsof LSD (0.24 mg/kg) on HTR were not enhanced in 5-HT1A knockoutmice, in apparent contrast to our results. One possible explanationfor the discrepancy is that a global 5-HT1A knockout maytrigger neuroadaptive mechanisms during development which compensatefor the lack of this receptor, to functionally replace it. Anotherpossibility for the discrepancy may be the different doses of LSDused between studies. At the lower dose of LSD used in the González-Maesoet al. study, 5-HT1A receptor activity may have less ofa regulatory influence on 5-HT2A-mediated behavioral effectssuch as HTR. Regardless, the inability of lisuride to induce HTR inWAY100635-pretreated or 5-HT1A knockout mice suggests 5-HT1A-independent mechanisms in mediating the non-psychedelicproperties of the drug.
Effects of M100907, Lisuride, and 8-OH-DPAT on the LSD-InducedHTR
We next carried out experiments to verify that LSD (administereds.c. at 0.1 mg/kg) produces the HTR in a 5-HT2A-dependentmanner. To test this, the 5-HT2A receptor antagonist64 M100907 (0.01 mg/kg) was administered 30 minprior to LSD. The vehicle + LSD group produced the expected increasein HTR, which was significantly higher than all other groups, withthe M100907 + LSD group exhibiting a response indistinguishable fromvehicle controls (Figures5A andS5C and Tables S5 and S6).In accordance with previous studies from our laboratory, pretreatmentwith 0.01 mg/kg M100907 did not induce any effect on body temperatureor locomotor activity before or after LSD administration (Figure S7A – C, Figure S8A – B, Table S5, Table S6).27,28 The finding that LSD-inducedHTR is 5-HT2A-dependent is consistent with many other studiesusing pharmacological and genetic manipulations in mice, as well asclinical studies where the subjective effects of psilocybin or LSDare attenuated by pretreatment with ketanserin.2,35,65−69
Figure 5.
Effects of M100907 (A, structure shown), lisuride (B),and 8-OH-DPAT(C, structure shown) pretreatment on LSD-induced HTR. Data shown aremean with SEM (n = 6–8) and individual pointsplotted for total HTR count. Bold points and symbols represent statisticallysignificant differences (p < 0.05)via Tukey’s post-test as follows:*vs 0/0, #vs pretreatment/0, ∧vs 0/LSD, +vs pretreatment/LSD. More details regardingstatistical comparisons can be found in theMaterialsand Methods section andTables S5 and S6.
It has previously been shown that pretreatmentor coadministrationof 5-HT1A agonists reduces the effects of psychedelicsin preclinical and clinical studies.29,30,63 Additionally, a higher dose of lisuride (0.4 mg/kg)has been shown to block LSD-induced HTR in mice.2 Therefore, we next sought to test whether a lower doseof lisuride that induces 5-HT1A-mediated effects can attenuatethe 5-HT2A-mediated HTR by LSD. Mice were pretreated withlisuride (0.03 mg/kg s.c.) or 8-OH-DPAT (1 mg/kg s.c.) 15 min priorto administration of LSD (0.1 mg/kg s.c.) to monitor HTR, temperaturechanges, and locomotor activity for 30 min. Pretreatment with eitherlisuride or 8-OH-DPAT attenuated the LSD-induced HTR (Figures5B–C andS5D–E and Tables S5 and S6). As expected,lisuride or 8-OH-DPAT pretreatment also produced hypolocomotor andhypothermic effects that were observable at the time point 15 minafter administration of the drugs. These effects persisted throughthe end of the 30 min testing period after LSD administration (Figures S7 and S8 and Tables S5 and S6). Despiteits 5-HT1A activity at higher doses, 0.1 mg/kg LSD didnot influence the effects of lisuride or 8-OH-DPAT on body temperatureor locomotor activity in these experiments.
Role of D1, D2, and 5-HT2C Receptorsin the Effects of Lisuride
In addition to its affinity for5-HT2A and 5-HT1A, lisuride has other receptortargets that potentially influence its biological effects, includingits discriminative stimulus properties (e.g., dopamine receptors andother serotonin receptors).16,18,70−73 Given that we found that (i) lisuride does not produce an increasein the HTR (Figure3), (ii) lisuride fails to induce HTRs under conditions of 5-HT1A blockade (Figure4), and (iii) lisuride antagonizes LSD-induced HTR (Figure5),2 we sought to test whether other pharmacologically relevanttargets of lisuride might be responsible for its lack of psychedelic-likeeffects in mice. These experiments explored the effects of pretreatmentswith antagonists at D1-like (SCH23390, 0.01 mg/kg s.c.),D2-like (eticlopride, 0.03 mg/kg s.c.), or 5-HT2C (SB242084, 3 mg/kg s.c.) receptors as well as some antagonist combinationsgiven 15 min prior to lisuride (0.03 mg/kg s.c.).
The resultsshown inFigure6 indicatethat none of the pretreatments with antagonists acting at D1, D2, D1 + D2, 5-HT2C, 5-HT2C + 5-HT1A, or D1 + D2 + 5-HT1A unveiled an increase in HTR activityof lisuride (Tables S7–S10). Infact, several of the antagonist pretreatments or their combinationssignificantly reduced basal HTR relative to saline vehicle controls,analogous to the vehicle+lisuride and antagonists+lisuride conditions.These results suggest that there is no masking of latent HTR by thesevarious targetsin vivo. Notably, many neurotransmittersystems can influence the HTR produced by psychedelics in mice,74,75 so blockade of some of the targets assessed may influence criticalcircuitry responsible for mice to display the HTR.
Figure 6.
Effects of blockade ofvarious non-5-HT2A receptor targetsof lisuride on the HTR. Effects of SCH23390 (A), eticlopride (B),SCH23390 + eticlopride (C), SB242084 (D), SB242084 + WAY100635 (E),and WAY100635 + eticlopride + SCH23390 (F) pretreatment on lisuride-inducedreductions in basal HTR. Data shown are mean with SEM (n = 4–6) and individual points plotted for total HTR count.Symbols and bold points represent statistically significant differences(p < 0.05)via Tukey’spost-test as follows:*vs 0/0, #vs pretreatment/0, ∧vs 0/lisuride,+vs pretreatment/lisuride. More details regardingstatistical comparisons can be found in theMaterialsand Methods section andTables S7–S10.
Pretreatment with the D1-like receptorantagonist SCH23390alone resulted in decreased locomotor activity 45 min post administration(as assessed in the 30 min post lisuride time point) but had no effecton body temperature or the effects of lisuride (Figures S9A–C and S10A–B and Tables S7–S8). Similarly, a D2-like receptor blockade with eticlopridereduced locomotor activity after 45 min. Pretreatment with eticlopridedid not affect lisuride-induced hypolocomotion but did partially attenuatelisuride-induced hypothermia (Figures S9D–F and S10C–D and Tables S7–S8). These results showthat the activity of lisuride at D2-like receptors canmodulate the pathways responsible for its effects on body temperature.When both D1-like and D2-like receptors wereblocked, the observed effects resembled the effects of eticlopridealone, with the combination partially attenuating the hypothermiainduced by lisuride (Figures S9G–I and S10E–F and Tables S7–S8).
5-HT2C receptor blockade with SB242084 did not significantlyinfluence the locomotor effects of lisuride despite a trend for motorstimulation produced by the antagonist alone (Figure S11A–C and Tables S9–S10). Interestingly,5-HT2C blockade seemed to enhance lisuride-induced hypothermia(Figure S12A–B and Tables S9–S10). Combining a 5-HT2C antagonist with the 5-HT1A antagonist, WAY100635, produced significant hyperlocomotion priorto lisuride administration, without altering effects on body temperature(Figures S11D–F and S12C,D and Tables S9–S10). Lastly, blocking 5-HT1A + D1 + D2 receptors together significantly reduced locomotor activity postlisuride administration (Figure S11G–I and Tables S9–S10), and partially attenuated lisuride-inducedhypothermia (Figure S12E–F and Tables S9–S10). The overall results from these studies reveal that the blockadeof many non-5-HT2A receptor targets of lisuride could notunmask suppressed psychedelic-like effects of the drug.
Despitethe known dopaminergic effects of lisuride, blockade ofthe D1-like and D2-like receptor activitiesof lisuride in the present study did not reveal any 5-HT2A-mediated psychedelic-like effects on the HTR. González-Maesoet al. similarly investigated the effects of D1-like andD2-like agonists on effects of LSD, showing that thesetargets likely do not play a role in differential effects of lisuridevs LSD.2 Blocking combinations of D1-like, D2-like, or 5-HT2C receptorswith or without additional 5-HT1A blockade further failedto reveal any HTR increase by lisuride. These data refute the hypothesisthat non-5-HT2A receptor targets inhibit lisuride fromproducing psychedelic-like effects.
Integration ofIn Vitro andIn Vivo Findings
Taken together, the results from this study confirmand extend previous findings related to the pharmacological effectsof lisuride and LSD. We found that lisuride is a weak partial agonistat 5-HT2A, with particularly low efficacy at recruitingGαq. Consistent with its low efficacy at 5-HT2A, lisuride could antagonize signaling at this receptor inducedby LSD (Figure2).One other study found that lisuride blocks the effects of LSD on theelectrical properties of somatosensory neurons and induction of immediateearly gene expression in mouse brain.2 Moreover,the same previous study and our present data (Figure5B) both indicate that lisuride is capableof blocking the 5-HT2A-mediated effects of LSD on HTR inmice. Furthermore, a recent study has shown that βarr1 and βarr2agonist-like signaling at 5-HT2A does not play a significantrole in the behavioral effects of lisuride in mice.54 Together, these findings provide compelling and convergentevidence supporting the hypothesis that the partial agonist/antagonistprofile of lisuride at 5-HT2A can be linked to its antagonismof the LSD-induced HTR and its inability to induce HTR. Supportingthis notion, Lewis et al. recently reported that 2-Br-LSD, a non-hallucinogenicLSD analogue with a potential application for the treatment of mooddisorders, acted as a potent but partial 5-HT2A agonist.44 In the study presented here, we found that lisurideand 2-Br-LSD displayed nearly equivalent partial agonist effects inβarr2 and miniGαq recruitment assays. Similarto lisuride, 2-Br-LSD does not induce the HTRin vivo.44
In addition to lisuride, ourstudy evaluated thein vitro functional activityof several related ergolines. AL-LAD has been detected in recreationaldrug markets in both powdered and blotter forms.76 Its synthesis and psychedelic properties in humans aredocumented in TiHKAL.77 In drug discriminationstudies, AL-LAD exhibited a higher potency than LSD in rats trainedto discriminate LSD from saline, and AL-LAD induces HTR in mice witha slightly lower potency than LSD.76,78 Scarce pharmacologicalinformation is available about the related compound, LAMPA. In a studyassessing the effects of 100 μg p.o. of LAMPA in six psychedelic-experiencedsubjects, two subjects reported effects reminiscent of a thresholddose of LSD, whereas four others did not report subjective effects.79 Data obtained for LAMPA in HTR experiments indicatea potency for psychedelic-like effects approximately 3-fold lowerthan that of LSD (ED50 = 116vs 39 μg/kg).3,80 The presentin vitro functional activities forthese compounds generally agree with the existing literature, showingthat LSD, AL-LAD, and LAMPA exhibit differences in their activationof 5-HT2A, as assessed by βarr2 and miniGαq recruitment assays.
Several hypotheses have been proposedto explain mechanistic differencesbetween psychedelic and non-psychedelic 5-HT2A ligands.In a study comparing effects of the non-psychedelic 5-HT2A agonist, 2,5-dimethoxy-4-methyl-α-ethylphenethylamine (Ariadne),to those of the structurally related psychedelic 2,5-dimethoxy-4-methyl-amphetamine(DOM), Ariadne displayed a decrease in signaling potency and efficacyin several 5-HT2A-coupled functional pathwaysinvitro.81 No apparent change inpreference toward either pathway was reported. In the same study,the authors proposed a “5-HT2A signaling efficacyhypothesis” to explain the lack of psychedelic effects forAriadne, with a caveat that the hypothesis may be restricted to thecomparison of structurally related analogues. Other evidence supportsthe idea that signaling bias at 5-HT2A may differentiate5-HT2A agonists with and without psychedelic-like effects.A recent drug screening and optimization effort identified Gαq-biased 5-HT2A agonists with anxiolytic-like andantidepressant effects in mouse models, but which lack psychedelic-likeeffects.82 On the other hand, a differentstudy found βarr2-biased 5-HT2A partial agoniststhat produce antidepressant activity without psychedelic-like effects.8 The latter study posited that drugs producingpsychedelic-like effects require high transduction efficiency in bothβarr2 and Gαq signaling pathways,8 which is consistent with the present observationsfor lisuride and a previous study of 2-Br-LSD44 vs other psychoactive ergolines, including LSD. Adding to the complexity,a recently published study highlights the importance of Gαq signaling in mediating the psychedelic-like effects of some5-HT2A agonists in mice, showing that a "Gαq-efficacy threshold" of >70%, as measured in aninvitro BRET G protein dissociation assay, is required fora compound to produce the HTR.87
Two previous studies reported partial 5-HT2A agonistefficacy for lisuride and LSD, when compared to the endogenous neurotransmitterserotonin, in a phosphatidyl inositol hydrolysis assay (13–16and 22–32% relative to serotonin, respectively).16,21 In the psychLight assay, which is based on a conformational changeof 5-HT2A induced by psychedelic substances, lisuride didnot display detectable activity, whereas LSD acted as a partial agonist.83 Of note, other studies report higher relativeefficacies of lisuride (and LSD) relative to serotonin as a referenceagonist. For example, Cussac et al. reported efficacies for lisurideof 40.7 and 48.6% relative to serotonin in a GTPγS and a Ca2+ mobilization assay, respectively.84 Similarly, Egan et al. reported that LSD and lisuride display similarEmax values of 32 and 25% relative to serotoninin a PI hydrolysis assay.5 Meanwhile, recentdata from Wallach et al. found that LSD is an efficacious agonistat 5-HT2A for Gαq signaling and dose-dependentlyinduces the HTR in mice, while lisuride does not achieve >70% theEmax of 5-HT and does not induce the HTR.87 Regardless of apparent differences across assays,together the collective findings suggest that there may be an “efficacythreshold” in one or both 5-HT2A signaling pathwaysthat is required to produce psychedelic subjective effects in humansand psychedelic-like effects in rodents.
Conclusions
In summary, the results presented heredemonstrate that lisurideinduces potent 5-HT1A-mediated effects in mice that confoundits ability to be used acutely as a “non-psychedelic”or “non-hallucinogenic” 5-HT2A agonist. Importantly,antagonism of 5-HT1A did not reveal latent psychedelic-likeactivity for lisuride, contrary to what has been observed for otherpsychedelic 5-HT2A agonists. At the receptor level, lisuridedisplayed weak partial agonism in bothin vitro assaysthat monitor the recruitment of intracellular proteins to 5-HT2A, with particularly low efficacy in the miniGαq recruitment assay, which set it apart from LSD and relatedpsychedelic analogues. Thus, the summed findings suggest that weakpartial agonist activity at 5-HT2A likely underlies thenon-psychedelic nature of lisuride.
Materials and Methods
Materials and Reagents
In Vitro Studies
Lisuride maleate(#4052) was purchased from Bio-Techne and dissolved in DMSO. The analyticalstandards of AL-LAD (#30442), LAMPA (#31500), and 2-Br-LSD (#36791)were procured from Sanbio (distributor for Cayman Chemical), whilelysergic acid diethylamide and DOI hydrochloride were purchased fromChiron AS. DMEM (Dulbecco’s modified Eagle’s medium,GlutaMAX), OptiMEM, penicillin/streptomycin, and HBSS (Hank’sBalanced Salt Solution) were purchased from Fisher Scientific. Poly-d-lysine hydrobromide and serotonin were purchased from Sigma-Aldrich.FuGENE HD transfection reagent and NanoGlo Live Cell Reagent werepurchased from Promega.
In Vivo Studies
Lisuride maleate (#4052),8-OH-DPAT hydrobromide (#0529), (+)-SCH 23390 hydrochloride (#0925),and SB 242084 (#2901) were all procured from Tocris Biosciences. (+)-Lysergicacid diethylamide (+)-tartrate (2:1) was generously provided by theNational Institute on Drug Abuse Drug Supply Program. (+)-M100907freebase was generously provided by the laboratory of Kenner Rice,Ph.D. for antagonist studies. WAY100635 maleate (#14599) and DOI hydrochloride(#13885) were purchased from Cayman Chemical Company. (−)-Eticlopridehydrochloride (#E-101) was purchased from Research Biochemicals International.All drugs were administered subcutaneously as the weight of the saltdissolved or diluted in a sterile 0.9% saline vehicle at an injectionvolume of 0.01 mL/g body weight. For the administration of drug combinations,the appropriate concentrations of drug solutions were diluted togetherand coadministered as a combined solution in a single injection.
5-HT2A Functional Assays
The protocols forthe functional complementation assays monitoring the recruitmentof β-arrestin 2 (βarr2) or the engineered miniGαq protein (as described by Nehmé et al.)38 to 5-HT2A have been described previously.33,34,40,41 Human Embryonic Kidney (HEK) 293T cells were routinely culturedin a humidified atmosphere at 37 °C and 5% CO2. Theculturing medium was DMEM containing 10% heat-inactivated fetal bovineserum (FBS), 100 IU/mL of penicillin, 100 μg/mL of streptomycin,and 0.25 μg/mL amphotericin B. For transient transfection, thecells were seeded in a 6-well plate at a density of 500,000 cellsper well. The following day, the cells are transfected with 1.65 μgof 5-HT2A-LgBiT and 1.65 μg of either SmBiT-βarr2or SmBiT-miniGαq, utilizing FuGENE HD transfectionreagent in a 3:1 FuGENE/DNA ratio. The transfection mixture is preparedin OptiMEM, according to the manufacturer’s protocol.
24 h post transfection, the cells are reseeded into a poly-d-lysine-coated white 96-well plate. The readout takes place on thenext day (in total 48 h post transfection). The cells are rinsed twicewith HBSS, and 100 μL of HBSS is pipetted into each well, towhich 25 μL of NanoGlo Live Cell Substrate is added (diluted1/20 in LCS dilution buffer, according to the manufacturer’sprotocol). The plate is transferred to a Tristar2 LB 942multimode microplate reader (Berthold Technologies GmbH & Co.,Germany), where luminescence is monitored until equilibration of thesignal. Subsequently, 10 μL of the 13.5× concentrated agonistsolutions are added, to obtain in-well concentrations ranging from10–5 to 10–11 M, alongside theappropriate solvent controls. Luminescence is continuously monitoredfor 2 h. Each experiment additionally included LSD, serotonin, andDOI as reference agonists for normalization and comparability withprevious results. Data are gathered in three independent experiments,each including at least two replicates for each solvent control orligand concentration point.
Mouse Studies
Male C57BL/6J mice (#000664) were purchasedfrom the Jackson Laboratory and group housed under a 12:12 light–darkcycle (lights on at 7AM) for 1–2 weeks for facility acclimationwith ad libitum access to food and water. After acclimation and underbrief isoflurane immobilization, the mice were subcutaneously implantedwith a temperature transponder (14 mm × 2 mm, model IPTT-300,Bio Medic Data Systems, Inc.) and allowed 1 week to recover priorto experiments as previously described.27,85 All mouseexperiments were approved by the Animal Care and Use Committee infacilities at the National Institute on Drug Abuse Intramural ResearchProgram in Baltimore, MD.
Mouse studies were conducted as describedpreviously with little modification.27,28 Briefly, cohortsof 12 C57BL/6J male mice (2–5 months, one cohort per drug)were tested for acute dose-related effects of lisuride, 8-OH-DPAT,or LSD once per week for 4–5 weeks. Similarly, separate cohortsof mice were used to test the effects of antagonist pretreatmentson the effects of lisuride, 8-OH-DPAT, and LSD. For acute effectsin dose–response studies, various doses of each test drug (0.001–30mg s.c.) were administered and the HTR was recorded and scored from30 min video recordings (GoPro Hero 7, 120 frames/s, 960P resolution).51 At the same time, locomotor activity was recordedvia modified photobeam array chambers (TruScan, CoulbourneInstruments) and pre (just prior to drug administration after a 5min chamber acclimation) to post-test session body temperature (30min post drug administration) was measured using a hand-held device(Bio Medic Data Systems, Inc.) to read implanted transponders. Forantagonist studies, antagonist drugs were administered either 15 or30 min prior to test drugs, and the same end points measured in dose–responsestudies were monitored.
Data Analyses
In Vitro Studies
Data are analyzedas described before in more detail.86 Inbrief, the data are plotted in Microsoft Excel as time-luminescenceprofiles, corrected for interwell variability, and the area underthe curve (AUC) is calculated. Upon subtracting the AUC of the correspondingsolvent control (“blank”), the data are normalized inGraphPad Prism software (San Diego, CA), with the maximal response(Emax – as defined by a three parametricnonlinear regression) of reference agonist LSD or 5-HT defined as100% in each individual experiment. The normalized data from the individualexperiments are pooled, and the means of the data points are usedto fit sigmoidal concentration–response curves through threeparametric nonlinear regression, allowing one to calculate EC50 andEmax values.
In Vivo Studies
Nonlinear regressionwith either three- or four-parameter fits were utilized to constructconcentration- and dose–response curves as well as to determinepotencies and maximum values (ED50,Emax). In some cases (LSD HTR, lisuride locomotor activity),a “bell-shaped” nonlinear regression function was mostappropriate to visually depict biphasic data. In these instances,the rising phase of the curves was used to determine ED50 potency values separately. Dose–response data for mean HTRcount, temperature change (°C), and locomotor activity (distancetraveled cm) were compared to saline vehicle control micevia one-way ANOVA with Dunnett’s post-test. Effectsin antagonist studies using the same end points were also comparedvia one-way ANOVA, but with Tukey’s post-test to assess allpotential treatment group comparisons. Further information regardingstatistical comparisons in mouse studies (group means,n values,p values,F statistics,degrees of freedom, etc.) can be found in the figure legends or intheSupporting Information.
Acknowledgments
Specialthanks goes to K. Rice and A. Sulima for generouslyproviding the M100907 used to complete a portion of the present study.The authors also thank the National Institute on Drug Abuse Drug SupplyProgram for their continued support of our research program.
Glossary
Abbreviations
- 5-HT
serotoninor 5-hydroxy-tryptamine
- 5-HT1A
serotonin 1A receptor
- 5-HT2A
serotonin 2A receptor
- 5-HT2C
serotonin2C receptor
- D1
dopamine D1 receptor
- D2
dopamine D2 receptor
- LSD
lysergic acid diethylamide
- LSM-775
lysergic acid morpholide
- DOI
2,5-dimethoxy-4-iodoamphetamine
- AL-LAD
6-allyl-6-nor-lysergicacid diethylamide
- LAMPA
lysergic acid methylpropylamide
- 2-Br-LSD
2-bromolysergic acid diethylamide
- HTR
head twitch response
- ariadne
2,5-dimethoxy-4-methyl-α-ethylphenethylamine
- DOM
2,5-dimethoxy-4-methyl-amphetamine
Supporting Information Available
The Supporting Informationis available free of charge athttps://pubs.acs.org/doi/10.1021/acsptsci.3c00192.
In vitro functional assay concentration–responsecurves extended; concentration–response curves for mouse brainbinding assays; mouse dose–response statistical table; mousedata for 8-OH–DPAT; mouse potency values for 8-OH-DPAT; locomotoractivity time-course plots; HTR time-course plots; effects of WAY100635on locomotor activity and body temperature; descriptive statisticsfor WAY100635 antagonist experiments; descriptive statistics for M100907,lisuride, and 8-OH-DPAT antagonist experiments; locomotor activityand temperature change plots for M100907, lisuride, and 8-OH-DPATantagonist experiments; descriptive statistics for D1/D2/5-HT2C antagonist experiments; and locomotor activityand temperature change plots for D1/D2/5-HT2C antagonist experiments (PDF)
Author Contributions
§ G.C.G. and E.P. contributed equally to thiswork. Study design:G.C.G., E.P., C.P.S., M.H.B. 5-HT2A functional assays:E.P. Mouse brain binding assays: J.S.P. Mouse experiments: G.C.G.The manuscript was originally drafted by G.C.G. with help from E.P.and critically reviewed by C.P.S. and M.H.B. The final version wasapproved by all authors.
This work wassupported by National Institute of Drug Abuse Intramural ResearchProgram funds to M.H.B. (DA000522–16).
The authors declare nocompeting financial interest.
Supplementary Material
References
- Herrmann W. M.; Horowski R.; Dannehl K.; Kramer U.; Lurati K.Clinical Effectivenessof Lisuride Hydrogen Maleate: A Double-Blind Trial Versus Methysergide. Headache: J. Head Face Pain1977, 17, 54–60. 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- González-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A.Hallucinogens Recruit Specific Cortical5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron2007, 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A.Characterization of the head-twitchresponse inducedby hallucinogens in mice: detection of the behavior based on the dynamicsof head movement. Psychopharmacology2013, 227, 727–739. 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowski R.Psychiatricside-effects of high-dose lisuride therapy in parkinsonism. Lancet1986, 328, 510 10.1016/s0140-6736(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Egan C. T.; Herrick-Davis K.; Miller K.; Glennon R. A.; Teitler M.Agonist activityof LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology1998, 136, 409–414. 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Pieri L.; Keller H. H.; Burkard W.; Da Prada M.Effects of lisurideand LSD on cerebral monoamine systems and hallucinosis. Nature1978, 272, 278–280. 10.1038/272278a0. [DOI] [PubMed] [Google Scholar]
- dela Fuente Revenga M.; Jaster A. M.; McGinn J.; Silva G.; Saha S.; González-Maeso J.Tolerance and Cross-Toleranceamong Psychedelic and Nonpsychedelic 5-HT(2A) Receptor Agonists inMice. ACS Chem. Neurosci.2022, 13, 2436–2448. 10.1021/acschemneuro.2c00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D.; Yu J.; Wang H.; Luo Z.; Liu X.; He L.; Qi J.; Fan L.; Tang L.; Chen Z.; Li J.; Cheng J.; Wang S.Structure-based discovery of nonhallucinogenicpsychedelic analogs. Science2022, 375, 403–411. 10.1126/science.abl8615. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Liu J.; Yao Y.; Yan H.; Su R.Rearing behaviour inthe mouse behavioural pattern monitor distinguishes the effects ofpsychedelics from those of lisuride and TBG. Front. Pharmacol.2023, 14, 1021729 10.3389/fphar.2023.1021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zhu H.; Gao H.; Tian X.; Tan B.; Su R.G(s) signalingpathway distinguishes hallucinogenic and nonhallucinogenic 5-HT(2A)Ragonists induced head twitch response in mice. Biochem. Biophys. Res. Commun.2022, 598, 20–25. 10.1016/j.bbrc.2022.01.113. [DOI] [PubMed] [Google Scholar]
- Qu Y.; Chang L.; Ma L.; Wan X.; Hashimoto K.Rapid antidepressant-likeeffect of non-hallucinogenic psychedelic analog lisuride, but nothallucinogenic psychedelic DOI, in lipopolysaccharide-treated mice. Pharmacol., Biochem. Behav.2023, 222, 173500 10.1016/j.pbb.2022.173500. [DOI] [PubMed] [Google Scholar]
- González-Maeso J.; Yuen T.; Ebersole B. J.; Wurmbach E.; Lira A.; Zhou M.; Weisstaub N.; Hen R.; Gingrich J. A.; Sealfon S. C.Transcriptomefingerprints distinguish hallucinogenicand nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effectsin mouse somatosensory cortex. J. Neurosci.2003, 23, 8836–8843. 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J.; Sealfon S. C.Agonist-traffickingand hallucinogens. Curr. Med. Chem.2009, 16, 1017–1027. 10.2174/092986709787581851. [DOI] [PubMed] [Google Scholar]
- Banerjee A. A.; Vaidya V. A.Differential signalingsignatures evoked by DOI versuslisuride stimulation of the 5-HT(2A) receptor. Biochem. Biophys. Res. Commun.2020, 531, 609–614. 10.1016/j.bbrc.2020.08.022. [DOI] [PubMed] [Google Scholar]
- Karaki S.; Becamel C.; Murat S.; la Cour C. M.; Millan M. J.; Prézeau L.; Bockaert J.; Marin P.; Vandermoere F.Quantitativephosphoproteomics unravels biased phosphorylation of serotonin 2Areceptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol. Cell. Proteomics2014, 13, 1273–1285. 10.1074/mcp.M113.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D.; Kurrasch-Orbaugh D. M.; Selken J. R.; Cumbay M. G.; Lisnicchia J. G.; Nichols D. E.Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1Areceptor-mediated behavioral effects overlap its other propertiesin rats. Psychopharmacology2002, 164, 93–107. 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A.LSD but not lisuridedisrupts prepulse inhibition inrats by activating the 5-HT(2A) receptor. Psychopharmacology2010, 208, 179–189. 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. A.; Callahan P. M.; Craigmyle N. A.; Appel J. B.Discriminative stimulusproperties of clonidine: substitution by ergot derivatives. Eur. J. Pharmacol.1985, 119, 225–229. 10.1016/0014-2999(85)90299-7. [DOI] [PubMed] [Google Scholar]
- White F. J.Comparativeeffects of LSD and lisuride: clues to specific hallucinogenic drugactions. Pharmacol., Biochem. Behav.1986, 24, 365–379. 10.1016/0091-3057(86)90367-9. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A.; Cussac D.; Quentric Y.; Touzard M.; Verrièle L.; Carpentier N.; Millan M. J.Differential actions of antiparkinsonagents at multiple classes of monoaminergic receptor. III. Agonistand antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptorsubtypes. J. Pharmacol. Exp. Ther.2002, 303, 815–822. 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- Rabin R. A.; Regina M.; Doat M.; Winter J. C.5-HT2A receptor-stimulatedphosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol., Biochem. Behav.2002, 72, 29–37. 10.1016/S0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Millan M. J.; Maiofiss L.; Cussac D.; Audinot V.; Boutin J. A.; Newman-Tancredi A.Differential actions of antiparkinsonagents at multipleclasses of monoaminergic receptor. I. A multivariate analysis of thebinding profiles of 14 drugs at 21 native and cloned human receptorsubtypes. J. Pharmacol. Exp. Ther.2002, 303, 791–804. 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A.; Cussac D.; Audinot V.; Nicolas J. P.; De Ceuninck F.; Boutin J. A.; Millan M. J.Differentialactions of antiparkinsonagents at multiple classes of monoaminergic receptor. II. Agonistand antagonist properties at subtypes of dopamine D(2)-like receptorand alpha(1)/alpha(2)-adrenoceptor. J. Pharmacol.Exp. Ther.2002, 303, 805–814. 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- Goetz C. G.; Koller W. C.; Poewe O.; et al. DA agonists -- ergotderivatives: Lisuride. Mov. Disord.2002, 17, S74–S78. 10.1002/mds.5565. [DOI] [PubMed] [Google Scholar]
- Millan M.J.; Bervoets K.; Colpaert F. C.5-hydroxytryptamine (5-HT)1A receptorsand the tail-flick response. I. 8-hydroxy-2-(di-n-propylamino) tetralinHBr-induced spontaneous tail-flicks in the rat as an in vivo modelof 5-HT1A receptor-mediated activity. J. Pharmacol.Exp. Ther.1991, 256, 973–982. [PubMed] [Google Scholar]
- Klein L. M.; Cozzi N. V.; Daley P. F.; Brandt S. D.; Halberstadt A. L.Receptorbinding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamineanalogs. Neuropharmacology2018, 142, 231–239. 10.1016/j.neuropharm.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Pottie E.; Partilla J. S.; Sherwood A. M.; Kaylo K.; Pham D. N. K.; Naeem M.; Sammeta V. R.; DeBoer S.; Golen J. A.; Hulley E. B.; Stove C. P.; Chadeayne A. R.; Manke D. R.; Baumann M. H.Structure-ActivityRelationshipsfor Psilocybin, Baeocystin, Aeruginascin, and Related Analogues toProduce Pharmacological Effects in Mice. ACSPharmacol. Transl. Sci.2022, 5, 1181–1196. 10.1021/acsptsci.2c00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Naeem M.; Pham D. N. K.; Golen J. A.; Chadeayne A. R.; Manke D. R.; Baumann M. H.Receptor BindingProfiles for TryptaminePsychedelics and Effects of 4-Propionoxy-N,N-dimethyltryptamine inMice. ACS Pharmacol. Transl. Sci.2023, 6, 567–577. 10.1021/acsptsci.2c00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Pandey U.; Glennon R. A.Do functional relationshipsexist between 5-HT1A and 5-HT2 receptors?. Pharmacol.,Biochem. Behav.1990, 36, 901–906. 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Schreiber R.; Brocco M.; Audinot V.; Gobert A.; Veiga S.; Millan M. J.(1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-inducedhead-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT)2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonistsand 5-HT1A agonists. J. Pharmacol. Exp. Ther.1995, 273, 101–112. [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Twamley B.; Westphal F.; Elliott S. P.; Wallach J.; Stratford A.; Klein L. M.; McCorvy J. D.; Nichols D. E.; Halberstadt A. L.Returnof the lysergamides. PartIV: Analytical and pharmacological characterization of lysergic acidmorpholide (LSM-775). Drug Test. Anal.2018, 10, 310–322. 10.1002/dta.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Botvinnik A.; Shahar O.; Wolf G.; Yakobi C.; Saban M.; Salama A.; Lotan A.; Lerer B.; Lifschytz T.Effect ofpsilocybin on marble burying in ICR mice:role of 5-HT1A receptors and implications for the treatment of obsessive-compulsivedisorder. Transl. Psychiatry2023, 13, 164 10.1038/s41398-023-02456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Cannaert A.; Van Uytfanghe K.; Stove C. P.Setup of a Serotonin2A Receptor (5-HT2AR) Bioassay: Demonstration of Its ApplicabilityTo Functionally Characterize Hallucinogenic New Psychoactive Substancesand an Explanation Why 5-HT2AR Bioassays Are Not Suited for UniversalActivity-Based Screening of Biofluids for New Psychoactive Substances. Anal. Chem.2019, 91, 15444–15452. 10.1021/acs.analchem.9b03104. [DOI] [PubMed] [Google Scholar]
- Pottie E.; Dedecker P.; Stove C. P.Identification ofpsychedelic newpsychoactive substances (NPS) showing biased agonism at the 5-HT(2A)Rthrough simultaneous use of β-arrestin 2 and miniGα(q)bioassays. Biochem. Pharmacol.2020, 182, 114251 10.1016/j.bcp.2020.114251. [DOI] [PubMed] [Google Scholar]
- Rodriguiz R. M.; Nadkarni V.; Means C. R.; Pogorelov V. M.; Chiu Y.-T.; Roth B. L.; Wetsel W. C.LSD-stimulatedbehaviorsin mice require β-arrestin 2 but not β-arrestin 1. Sci. Rep.2021, 11, 17690 10.1038/s41598-021-96736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardou R.; Sawyer E.; Song Y. J.; Wilkinson M.; Padovan-Hernandez Y.; de Deus J. L.; Wright N.; Lama C.; Faltin S.; Goff L. A.; Stein-O’Brien G. L.; Dölen G.Psychedelics reopen the social reward learning criticalperiod. Nature2023, 618, 790–798. 10.1038/s41586-023-06204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A. S.; Schwinn M. K.; Hall M. P.; Zimmerman K.; Otto P.; Lubben T. H.; Butler B. L.; Binkowski B. F.; Machleidt T.; Kirkland T. A.; Wood M. G.; Eggers C. T.; Encell L. P.; Wood K. V.NanoLuc ComplementationReporterOptimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol.2016, 11, 400–408. 10.1021/acschembio.5b00753. [DOI] [PubMed] [Google Scholar]
- Nehmé R.; Carpenter B.; Singhal A.; Strege A.; Edwards P. C.; White C. F.; Du H.; Grisshammer R.; Tate C. G.Mini-G proteins: Novel tools for studying GPCRs intheir active conformation. PLoS One2017, 12, e0175642 10.1371/journal.pone.0175642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Cannaert A.; Stove C. P.In vitrostructure-activity relationshipdetermination of 30 psychedelic new psychoactive substances by meansof β-arrestin 2 recruitment to the serotonin 2A receptor. Arch. Toxicol.2020, 94, 3449–3460. 10.1007/s00204-020-02836-w. [DOI] [PubMed] [Google Scholar]
- Pottie E.; Kupriyanova O. V.; Brandt A. L.; Laprairie R. B.; Shevyrin V. A.; Stove C. P.Serotonin 2A Receptor (5-HT(2A)R)Activation by 25H-NBOMe Positional Isomers: In Vitro Functional Evaluationand Molecular Docking. ACS Pharmacol. Transl.Sci.2021, 4, 479–487. 10.1021/acsptsci.0c00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulie C. B. M.; Pottie E.; Simon I. A.; Harpsøe K.; D’Andrea L.; Komarov I. V.; Gloriam D. E.; Jensen A. A.; Stove C. P.; Kristensen J. L.Discovery of β-Arrestin-Biased25CN-NBOH-Derived 5-HT2A Receptor Agonists. J. Med. Chem.2022, 65, 12031–12043. 10.1021/acs.jmedchem.2c00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Kupriyanova O. V.; Shevyrin V. A.; Stove C. P.Synthesisand FunctionalCharacterization of 2-(2,5-Dimethoxyphenyl)-N-(2-fluorobenzyl)ethanamine(25H-NBF) Positional Isomers. ACS Chem. Neurosci.2021, 12, 1667–1673. 10.1021/acschemneuro.1c00124. [DOI] [PubMed] [Google Scholar]
- Pottie E.; Poulie C. B. M.; Simon I. A.; Harpsøe K.; D’Andrea L.; Komarov I. V.; Gloriam D. E.; Jensen A. A.; Kristensen J. L.; Stove C. P.Structure–ActivityAssessmentand In-Depth Analysis of Biased Agonism in a Set of Phenylalkylamine5-HT2A Receptor Agonists. ACS Chem. Neurosci.2023, 14, 2727–2742. 10.1021/acschemneuro.3c00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V.; Bonniwell E. M.; Lanham J. K.; Ghaffari A.; Sheshbaradaran H.; Cao A. B.; Calkins M. M.; Bautista-Carro M. A.; Arsenault E.; Telfer A.; Taghavi-Abkuh F. F.; Malcolm N. J.; El Sayegh F.; Abizaid A.; Schmid Y.; Morton K.; Halberstadt A. L.; Aguilar-Valles A.; McCorvy J. D.A non-hallucinogenic LSD analog with therapeutic potentialfor mood disorders. Cell Rep.2023, 42, 112203 10.1016/j.celrep.2023.112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Stove C. P.In vitro assaysfor the functional characterizationof (psychedelic) substances at the serotonin receptor 5-HT(2A) R. J. Neurochem.2022, 162, 39–59. 10.1111/jnc.15570. [DOI] [PubMed] [Google Scholar]
- Wacker D.; Wang S.; McCorvy J. D.; Betz R. M.; Venkatakrishnan A. J.; Levit A.; Lansu K.; Schools Z. L.; Che T.; Nichols D. E.; Shoichet B. K.; Dror R. O.; Roth B. L.CrystalStructure of an LSD-Bound Human Serotonin Receptor. Cell2017, 168, 377–389.e12. 10.1016/j.cell.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G. P.; Jain M. K.; Slocum S. T.; Roth B. L.5-HT2A SNPs Alterthe Pharmacological Signaling of Potentially Therapeutic Psychedelics. ACS Chem. Neurosci.2022, 13, 2386–2398. 10.1021/acschemneuro.1c00815. [DOI] [PubMed] [Google Scholar]
- Janowsky A.; Eshleman A. J.; Johnson R. A.; Wolfrum K. M.; Hinrichs D. J.; Yang J.; Zabriskie T. M.; Smilkstein M. J.; Riscoe M. K.Mefloquine and psychotomimetics shareneurotransmitterreceptor and transporter interactions in vitro. Psychopharmacology2014, 231, 2771–2783. 10.1007/s00213-014-3446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E.; Frescas S.; Marona-Lewicka D.; Kurrasch-Orbaugh D. M.Lysergamidesof isomeric 2,4-dimethylazetidines map the binding orientation ofthe diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide(LSD). J. Med. Chem.2002, 45, 4344–4349. 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Rickli A.; Moning O. D.; Hoener M. C.; Liechti M. E.Receptor interactionprofiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol.2016, 26, 1327–1337. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Glatfelter G. C.; Chojnacki M. R.; McGriff S. A.; Wang T.; Baumann M. H.AutomatedComputer Software Assessment of 5-Hydroxytryptamine 2A Receptor-MediatedHead Twitch Responses from Video Recordings of Mice. ACS Pharmacol. Transl. Sci.2022, 5, 321–330. 10.1021/acsptsci.1c00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach J.; Cao A. B.; Calkins M. M.; Heim A. J.; Lanham J. K.; Bonniwell E. M.; Hennessey J. J.; Bock H. A.; Anderson E. I.; Sherwood A. M.; Morris H.; de Klein R.; Klein A. K.; Cuccurazzu B.; Gamrat J.; Fannana T.; Zauhar R.; Halberstadt A. L.; McCorvy J. D.Identification of 5-HT2A receptorsignaling pathways associated with psychedelic potential. Nat. Commun.2023, 14, 8221. 10.1038/s41467-023-44016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl R.; Bert B.; Fink H.; Fox M. A.Animal models ofthe serotonin syndrome: A systematic review. Behav. Brain Res.2013, 256, 328–345. 10.1016/j.bbr.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Blanchard R. J.; Griebel G.; Guardiola-Lemaître B.; Brush M. M.; Lee J.; Blanchard D. C.An EthopharmacologicalAnalysis of Selective Activationof 5-HT1A Receptors: The Mouse 5-HT1A Syndrome. Pharmacol., Biochem. Behav.1997, 57, 897–908. 10.1016/S0091-3057(96)00472-8. [DOI] [PubMed] [Google Scholar]
- Pogorelov V. M.; Rodriguiz R. M.; Roth B. L.; Wetsel W. C.The G proteinbiasedserotonin 5-HT2A receptor agonist lisuride exerts anti-depressantdrug-like activities in mice. Front. Mol. Biosci.2023, 10, 1233743 10.3389/fmolb.2023.1233743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink H.; Morgenstern R.Locomotor effects of lisuride: aconsequence of dopaminergicand serotonergic actions. Psychopharmacology1985, 85, 464–468. 10.1007/BF00429666. [DOI] [PubMed] [Google Scholar]
- Carruba M. O.; Ricciardi S.; Chiesara E.; Spano P. F.; Mantegazza P.Toleranceto some behavioural effects of lisuride, a dopamine receptor agonist,and reverse tolerance to others, after repeated administration. Neuropharmacology1985, 24, 199–206. 10.1016/0028-3908(85)90074-7. [DOI] [PubMed] [Google Scholar]
- Jiang K.; Liu X.; Su R.Contrasting effectsof DOI and lisuride on impulsivedecision-making in delay discounting task. Psychopharmacology2022, 239, 3551–3565. 10.1007/s00213-022-06229-y. [DOI] [PubMed] [Google Scholar]
- Fiorella D.; Rabin R. A.; Winter J. C.Role of5-HT2A and 5-HT2C receptorsin the stimulus effects of hallucinogenic drugs II: reassessment ofLSD false positives. Psychopharmacology1995, 121, 357–363. 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Adams L. M.; Geyer M. A.Patterns of explorationin rats distinguish lisuridefrom lysergic acid diethylamide. Pharmacol.,Biochem. Behav.1985, 23, 461–468. 10.1016/0091-3057(85)90022-X. [DOI] [PubMed] [Google Scholar]
- Darmani N. A.; Mock O. B.; Towns L. C.; Gerdes C. F.The head-twitchresponse in the least shrew (Cryptotis parva) is a 5-HT2- and nota 5-HT1C-mediated phenomenon. Pharmacol., Biochem.Behav.1994, 48, 383–396. 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A.Effect of Hallucinogens on Unconditioned Behavior. Curr. Top. Behav. Neurosci.2018, 36, 159–199. 10.1007/7854_2016_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R. J.Human psychopharmacologyof N,N-dimethyltryptamine. Behav. Brain Res.1995, 73, 121–124. 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- Pokorny T.; Preller K. H.; Kraehenmann R.; Vollenweider F. X.Modulatoryeffect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol.2016, 26, 756–766. 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Casey A. B.; Cui M.; Booth R. G.; Canal C. E.″Selective″ serotonin5-HT(2A) receptor antagonists. Biochem. Pharmacol.2022, 200, 115028 10.1016/j.bcp.2022.115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Westphal F.; Stratford A.; Elliott S. P.; Hoang K.; Wallach J.; Halberstadt A. L.Returnof the lysergamides. Part I: Analytical and behavioural characterizationof 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test. Anal.2016, 8, 891–902. 10.1002/dta.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster A. M.; Elder H.; Marsh S. A.; de la Fuente Revenga M.; Negus S. S.; González-Maeso J.Effects of the 5-HT(2A)receptor antagonist volinanserin on head-twitch response and intracranialself-stimulation depression induced by different structural classesof psychedelics in rodents. Psychopharmacology2022, 239, 1665–1677. 10.1007/s00213-022-06092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A. M.; Klaiber A.; Holze F.; Istampoulouoglou I.; Duthaler U.; Varghese N.; Eckert A.; Liechti M. E.KetanserinReverses the Acute Response to LSD in a Randomized, Double-Blind,Placebo-Controlled, Crossover Study in Healthy Participants. Int. J. Neuropsychopharmacol2023, 26, 97–106. 10.1093/ijnp/pyac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller K. H.; Herdener M.; Pokorny T.; Planzer A.; Kraehenmann R.; Stämpfli P.; Liechti M. E.; Seifritz E.; Vollenweider F. X.The Fabricof Meaning and Subjective Effects in LSD-Induced States Depend onSerotonin 2A Receptor Activation. Curr. Biol.2017, 27, 451–457. 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Bäbler A.; Vogel H.; Hell D.Psilocybin inducesschizophrenia-likepsychosis in humans via a serotonin-2 agonist action. NeuroReport1998, 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Cunningham K. A.; Callahan P. M.; Appel J. B.Discriminativestimulus propertiesof lisuride revisited: involvement of dopamine D2 receptors. J. Pharmacol. Exp. Ther.1987, 241, 147–151. [PubMed] [Google Scholar]
- Holohean A. M.; White F. J.; Appel J. B.Dopaminergic and serotonergic mediationof the discriminable effects of ergot alkaloids. Eur. J. Pharmacol.1982, 81, 595–602. 10.1016/0014-2999(82)90349-1. [DOI] [PubMed] [Google Scholar]
- Kimura K.; Akai T.; Nakamura K.; Yamaguchi M.; Nakagawa H.; Oshino N.Dual activation by lisuride of centralserotonin 5-HT(1A) and dopamine D(2) receptor sites: drug discriminationand receptor binding studies. Behav. Pharmacol.1991, 2, 105–112. [PubMed] [Google Scholar]
- White F. J.; Appel J. B.The role of dopamineand serotonin in the discriminativestimulus effects of lisuride. J. Pharmacol.Exp. Ther.1982, 221, 421–427. [PubMed] [Google Scholar]
- Canal C. E.; Morgan D.Head-twitch responsein rodents induced by the hallucinogen2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluationof mechanisms, and its utility as a model. DrugTest. Anal.2012, 4, 556–576. 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E.SerotonergicPsychedelics: Experimental Approaches for Assessing Mechanisms ofAction. In New Psychoactive Substances; Springer, 2018; Vol. 252, pp 227–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Westphal F.; Elliott S. P.; Wallach J.; Colestock T.; Burrow T. E.; Chapman S. J.; Stratford A.; Nichols D. E.; Halberstadt A. L.Returnof the lysergamides. PartII: Analytical and behavioural characterization of N(6) -allyl-6-norlysergicacid diethylamide (AL-LAD) and (2’S,4’S)-lysergic acid2,4-dimethylazetidide (LSZ). Drug Test. Anal.2017, 9, 38–50. 10.1002/dta.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin A. T.; Shulgin A.. TiHKAL: The Continuation; Transform Press, 1997. [Google Scholar]
- Hoffman A.J.; Nichols D. E.Synthesisand LSD-like discriminative stimulus propertiesin a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives. J. Med. Chem.1985, 28, 1252–1255. 10.1021/jm00147a022. [DOI] [PubMed] [Google Scholar]
- Abramson H. A.The Use of LSD inPsychotherapy and Alcoholism; Bobbs-Merrill, 1967. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Klein L. M.; Chatha M.; Valenzuela L. B.; Stratford A.; Wallach J.; Nichols D. E.; Brandt S. D.Pharmacologicalcharacterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide(ECPLA). Psychopharmacology2019, 236, 799–808. 10.1007/s00213-018-5055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. J.; Bock H. A.; Serrano I. C.; Bechand B.; Vidyadhara D. J.; Bonniwell E. M.; Lankri D.; Duggan P.; Nazarova A. L.; Cao A. B.; Calkins M. M.; Khirsariya P.; Hwu C.; Katritch V.; Chandra S. S.; McCorvy J. D.; Sames D.PharmacologicalMechanism of the Non-hallucinogenic 5-HT2A Agonist Ariadne and Analogs. ACS Chem. Neurosci.2023, 14, 119–135. 10.1021/acschemneuro.2c00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. L.; Confair D. N.; Kim K.; Barros-Álvarez X.; Rodriguiz R. M.; Yang Y.; Kweon O. S.; Che T.; McCorvy J. D.; Kamber D. N.; Phelan J. P.; Martins L. C.; Pogorelov V. M.; DiBerto J. F.; Slocum S. T.; Huang X.-P.; Kumar J. M.; Robertson M. J.; Panova O.; Seven A. B.; Wetsel A. Q.; Wetsel W. C.; Irwin J. J.; Skiniotis G.; Shoichet B. K.; Roth B. L.; Ellman J. A.Bespoke librarydocking for 5-HT2A receptor agonists with antidepressant activity. Nature2022, 610, 582–591. 10.1038/s41586-022-05258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.; Ly C.; Dunlap L. E.; Vargas M. V.; Sun J.; Hwang I. W.; Azinfar A.; Oh W. C.; Wetsel W. C.; Olson D. E.; Tian L.Psychedelic-inspired drug discoveryusing an engineered biosensor. Cell2021, 184, 2779–2792.e18. 10.1016/j.cell.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D.; Boutet-Robinet E.; Ailhaud M.-C.; Newman-Tancredi A.; Martel J.-C.; Danty N.; Rauly-Lestienne I.Agonist-directedtrafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSVreceptors mediated Gq/11 activation and calcium mobilisation in CHOcells. Eur. J. Pharmacol.2008, 594, 32–38. 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Glatfelter G. C.; Partilla J. S.; Baumann M. H.Structure-activityrelationshipsfor 5F-MDMB-PICA and its 5F-pentylindole analogs to induce cannabinoid-likeeffects in mice. Neuropsychopharmacology2022, 47, 924–932. 10.1038/s41386-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Tosh D. K.; Gao Z.-G.; Jacobson K. A.; Stove C. P.Assessmentof biased agonism at the A3 adenosine receptor using β-arrestinand miniGαi recruitment assays. Biochem.Pharmacol.2020, 177, 113934 10.1016/j.bcp.2020.113934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.