The E2 Reaction Mechanism
E2 is the most common elimination mechanism. The “2” indicates that both the alkyl halide and the base participate in the rate equation. This is somewhat similar to theSN2 mechanism:

Notice that the beta hydrogen and the bromine leaving group are at 180°, meaning they are in anantiperiplanar orientation. This arrangement is a key requirement for E2 elimination, which we will explore further in later discussions.
Let’s put them next to each other and compare these mechanisms:

What are the common features?
First, notice that all the bonds are broken and formed in a single step, indicating aconcerted process. This means that the rate of the reaction linearly depends on the concentration of both reactants since one molecule of the alkyl halide and the base/nucleophile appear in the transition state.
Overall, the reaction is biomolecular – second order:

Thedotted lines in the transition state of the E2 reaction indicate that theC-H and C-Br bonds are partially broken and, at the same time, theO-H and C=C π bonds are partially formed.
The negative charge is shared between the base and the leaving group, each bearing a partial negative charge.
How are the E2 and SN2 mechanisms different?
The key difference between theSN2 and E2 reactions is that the nucleophile in theSN2 mechanism attacks the carbon connected to the leaving group (ɑ-carbon) while in E2, the base attacks one of the β-hydrogens.
The result is a replacement of the leaving group with a nucleophile, in the SN2, and anewly-formed π bond in the E2 reaction.
These outcomes are true for any substitution and elimination reaction regardless of whether it follows the SN1/SN2 or E1/E2 mechanism.
Reactivity of the Substrate in E2 Reactions
Despite the common features, the reactivity of alkyl halides is opposite in E2 andSN2 reactions. In E2 reactions, itincreases with the number of alkyl groups on the substrate –the more substituted, the more reactive:

To understand this pattern, remember that the base needs to only access a small proton, while the nucleophile needs to access a carbon atom, which is often hindered by neighboring hydrogens and neighboring carbon atoms. This is why larger molecules lose their nucleophilicity while retaining the base strength:

Another factor favoring the E2 mechanism forsterically hindered alkyl halides is the stability of the resulting alkenes –the more substituted alkenes are more stable:

How is the stability of alkenes related to the reactivity of alkyl halides in the E2 mechanism?
To understand this, we need to take a closer look at the transition state:

Notice that thetransition state of the E2 mechanism resembles an alkene (the double bond is already partially formed), thus, increasing the number of alkyl groups makes the forming alkene more stable, which lowers the activation energy. Therefore, the reaction goes faster, and the resulting alkene, being more substituted, is more stable.
The Base in E2 Reactions
We have seen above that the base appears in the rate equation of E2 reactions:

This means therate of the E2 reaction increases with the concentration and the strength of the base.
The list of common strong bases used in E2 reactions is shown below:
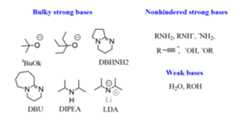
There are bulky (sterically hindered) and small (sterically unhindered) bases. All of them are suitable for E2 reactions, but they are used selectively mainly to control the regiochemistry of the E2 reaction (Zaitsev’s and Hoffman products).
Another thing to keep in mind when choosing a base is the factthat small bases can also serve as nucleophiles and perform anSN2 reaction.
In short, to push the reaction to E2 over SN2, heat needs to be applied if asterically unhindered base is used.
- Check also this article ondeciding between the SN2 and E2 mechanisms for more details and examples.
- The competition between nucleophilic substitution and elimination reactions (SN1, SN2, E1a, and E2) is addressed in thefollowing post.
Weak bases lead to elimination by theE1 mechanism. The good news is that there are mainly two types of weak bases – water and alcohols.

Choosing betweenE1 and E2 mechanisms is covered inthis post.
The Effect of the Leaving Group in E2 Reactions
Just like in any substitution and elimination reaction, the bond to the leaving group is partially broken in the transition state. Therefore, in E2 reactions as well,the better the leaving group, the faster the E2 reaction.The most common leaving groups in nucleophilic substitution and elimination reactions are shown below:

The Role of Solvent in E2 Reactions
As we discussed above, the stronger the base, the faster the E2 elimination occurs. Therefore,polar aprotic solvents increase the rate of E2 reactions.
This is because polar aprotic solvents do not interact with the base (no hydrogen bonding), thus leaving the base naked and more reactive:

Polar protic solvents, on the other hand, do formhydrogen bonding with the base, making itless reactive, so they are not preferred for doing E2 reactions.
The effect ofpolar protic and aprotic solvents on the nucleophilicity and basicity is covered inthis article.
Practice
The following reaction exhibits first order rate in each reactant. What happens to the rate of the when:

a) The concentration of 2-bromobutane is doubled and [EtONa] remains the same?
b) The concentration of 2-bromobutane is tripled and [EtONa] is halved?
Sodium ethoxide is a strong base and its reaction with a secondary alkyl halide will mainly yield an elimination product through E2 mechanism which can also be seen from the reaction equation. The rate of the E2 reaction depends both on the substrate and the base and therefore the following changes will be observed:
a) The rate doubles
b) The rateincreases 1.5 times (3 x 1/2 = 3/2)
Identify the β hydrogens and draw the curved arrow mechanism for each of the following E2 reactions.
The substrate in each reaction has two types of ß-hydrogens; the one(s) that yield, in an elimination reaction, theZaitsev product and the one(s) that yield the Hofmann product. These hydrogens are marked in blue and red colors respectively.
For this exercise, you need to identify the correct ß-hydrogen that participated in the reaction based on the position of the double bond in the product.
More material and practice about the Zaitsev and Hofman (regiochemistry of E2 reaction) products can be found here.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
You can find the new comprehensive set of problems on the E2 eliminationhere.
Check Also
- General Features of Elimination
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- SN2 and E2 Rates of Cyclohexanes
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism with Practice Problems
- Regioselectivity of E1 Reactions
- Stereoselectivity of E1 Reactions
- How to tell if it is E2 or E1 Mechanism
- SN1 vs E1 Reactions
- Dehydration of Alcohols by E1 and E2 Elimination
- Mesylates and Tosylates as Good Leaving Groups
- Mitsunobu Reaction
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Polar Protic and Polar Aprotic Solvents
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
- The Hammond Postulate
- The E1cB Elimination Mechanism
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides


