Substitution and Elimination Reactions
Let’s understand the most common words you have been hearing the past couple of days that will last towards the end of the semester:Substitution and Elimination Reactions
So, what are we substituting and eliminating?
These terms refer to the halogen and a hydrogen atom inalkyl halides.
Here is what you can have in mind for a general representation of substitution and elimination reactions:
1) Substitution: The halogen is replaced by the nucleophile
2) Elimination: The halogen is removed from the alkyl halide (electrophile) together with an adjacent hydrogen
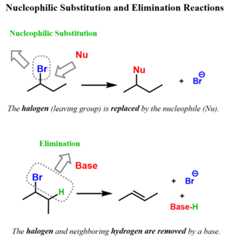
Notice that we named thealkyl halide “an electrophile”, and we can see that it reacts with a nucleophile and a base. So,why do we call them electrophiles, and why do they react with electron-rich species such as nucleophiles and bases?
Recall that halogens are more electronegative than carbon; therefore, thecarbon-halogen bond is a polar covalent bond. This means thecarbon connected to the halogen is electron-deficient, and that’s why we put apartial positive charge on it:

So, for a general picture, visualizealkyl halides as species where we have a positive center. To be more scientific, we call this anelectrophilic center – a center/atom that likes/wants electrons. Because of this,alkyl halides are known as electrophiles.
These electrons are provided by the nucleophiles or the base, as these are electron-rich species with a lone pair(s) of electrons, that can also be negatively charged (recallformal charges):

This, by far, is not the complete list of nucleophiles and bases you will see in your organic chemistry class, so feel free to checkthis article for a larger list of these species.
So, in a simple scheme (which, by the way, is what it boils down to no matter how deep we go into the topic),the cause of substitution and elimination reactions (and most chemical reactions) is the electronic attractive forces that are caused bythe polar nature of certain bonds in the molecule.
Electrophiles are the “positives “+”, and they react with the “negatives”, which are the nucleophiles and bases.
For example, even if you have never heard the terms substitution and elimination reactions, you may have a good shot guessing what atoms will be attracted if we mix, let’s2-bromobutane with athiolate ion (R–S⁻), such as ethanethiolate:

Yes, you are absolutely right, the negatively charged sulfur is going to attack the carbon with a partial positive charge.
Now, by doing so, it provides electrons to the carbon, which means it ismaking a new covalent bond. So, what do you think is going to happen to the carbon? Is it going to have five bonds (don’t forget about omitted hydrogens in bond line structures)?

We know thatcarbon cannot have five bonds, so to make this bond, itmust break one of the four bonds that the carbon already has, and it turns out,the easiest is the one with thehalogen:
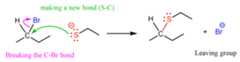
The reason for this selective kick out of halogen is that, once again, they are more electronegative than carbon, thus can better handle the electrons of the C-halogen bond that they take with them:

In a more organic chemistry language, we say that they are abetter leaving group than a negatively charged carbon or hydrogen would have been because theystabilize the negative charge better. Recall, the concept of acid-base chemistry – the H-Br bond dissociates incomparably better than any C-H bond because the resulting halide ion is quite stable and can handle the negative charge.
And just like this, we have shown what is called anSN2 substitution reaction, where the nucleophile attacks and kicks out the leaving group. This substitution reaction of 2-bromobutane with ethyl thiolate was an example of anSN2 substitution reaction:

To read more aboutgood and weak nucleophiles, as well aswhat makes a good leaving group, refer to the article dedicated to theSN2 mechanism here.
Let’s now see what types of substitution and elimination reactions there are and how they work.
There are two main types of substitution and elimination reactions:
1) When thenucleophile and base are very reactive (read aggressive), they attack thealkyl halide right away. These are theSN2 substitution and E2 elimination reactions.
2) When thenucleophile and base are weak, they don’t die to attack the alkyl halide. These are theSN1 and E1 reactions.
The SN2 Substitution Reaction
We have seen by the example of the reaction between2-bromobutane andethanethiolate that inSN2 reactions, the strong/good nucleophiles attack the carbon with a partial positive charge to make a new bond, and the C–X bond of the halogen breaks at the same time, making the halide ion a leaving group. Here are some more examples ofSN2 nucleophilic substitution reactions:

Notice that when the halogen is connected to a chiral center, theconfiguration of that center changes fromR to S and vice versa. This is what we call aninversion of configuration, which is a keycharacteristic feature of theSN2 mechanism that sets it apart from the SN1 mechanism, which we will discuss in a little bit.

Another characteristic of theSN2 reaction is that the nucleophilic attack and the loss of the leaving group happen simultaneously. Thesetypes of mechanisms are known asconcerted mechanisms.
There are more details andnuances to each of thesubstitution andelimination reactions that we overviewed today. We cannot cover all of this in one, so check the linked articles for more details, mechanisms, examples, and practice problems for each mechanism.
The E2 Elimination Mechanism
Although E2 is an elimination reaction, it is closer to theSN2 substitution mechanism thanSN1 is. Both SN2 and E2 are concerted, bimolecular mechanisms – meaning the all the bond breaking and making happen at the same time, and both the nucleophile/base and alkyl halide (substrate in general) participate in the rate-determining step.
Here is acomparison of an SN2 and E2 reaction that 2-bromobutane undergoes when reacted with a thiolate and alkoxide ion:

The key difference is that in the E2 reaction, theelectron-rich species, which is a base as it removes the halogen with a hydrogen, attacks the neighboringhydrogen instead of the electrophilic carbon.
The driving force, or at least asignificant contributor to it, is still the electrophilic nature of the carbon that now takes the electrons of the C–H bond, instead of those of thenucleophile in theSN2 mechanism.
The SN1 Mechanism
At the beginning of this discussion, we mentioned that substitution and elimination reactions can bedivided into two groups depending on whether a strong or weaknucleophile/base is used.
The ones we have seen so far are good nucleophiles and strong bases, and as mentioned earlier, they are very reactive and attack theelectrophile right away as it is.
On the other hand, if we have a weak nucleophile or a base, which is most often water and alcohols, the reaction between them and theelectrophile does not occur right away. They are not so reactive and don’t attack the electrophile unless the latter becomes more reactive.
So, how does thealkyl halide become more reactive?
This happens byloss of the leaving group, which forms acarbocation intermediate. Notice that in carbocations, the carbon lacks an octet and has an actual positive charge compared to the partial positive charge in the alkyl halides.

So, as the nucleophile is weak and lazy to attack the electrophile, the latter ionizes by breaking the carbon–halogen bond to form a carbocation intermediate. Once the carbocation is formed, the nucleophile then attacks it, forming a new C–Nu bond. For example, this is how tertiary alkyl halides 1-iodo-1-methylcyclohexane andtert-butyl bromide react with NaCl and water, respectively:

So, what do we see? First, unlike theSN2, theSN1 mechanism is a stepwise process: first, loss of a leaving group, and only after that a nucleophilic attack.
The E1 Elimination
TheE1 mechanism is closer to the SN1 mechanism because, like we saw in the comparison between theSN2 and E2 reactions, instead of attacking the electrophilic carbon, attacks a β-hydrogen, forming an alkene as the final product:

Very often, a mixture of products is formed, and there is no universal, clear-cut cut between the E1 and SN1 reactions. For example, the ratio of the alkene and alcohol formed in the reaction shown above will depend, for example, on the temperature.
Check this article for a more detailedcomparison of SN1 and E1 reactions.
Summarizing the Overview of Substitution and Elimination Reactions
Substitution and elimination reactions are two of the most important types of reactions in organic chemistry, especially in the Organic Chemistry 1 course. These reactions occur onalkyl halides, which, in a general description, are referred to as thesubstrate.
- In asubstitution reaction, the halogen is replaced by another group, which is the nucleophile that attacks the electrophilic carbon.
- In anelimination reaction, the halogen and a neighboring hydrogen are removed, resulting in the formation of adouble bond.
Substitution and elimination reactions can followdifferent mechanisms, depending on the strength of the nucleophile or base involved, as well as the structure of the alkyl halide.
- SN2 and E2 reactions occur withstrong nucleophiles and bases and proceed in a single, concerted step.
- SN1 and E1 reactions involveweaker nucleophiles or bases and proceed through acarbocation intermediate in a stepwise manner.
Each of these mechanisms has its own set of rules, trends, and exceptions. While this post provides a broad overview, there are manynuances, details, and variations that we can’t cover all at once. For a deeper understanding, including examples, step-by-step mechanisms, and practice problems, be sure to explore thelinked articles on each reaction type.
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- SN1 vs E1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
The Elimination Reactions can be found on the Organic Chemistry 1 and 2Topics page.
Practice
Identify the nucleophile and leaving group in the following substitution reactions:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Identify the nucleophile and leaving group and draw the products in the following substitution reactions:

This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Draw the mechanism for the following reaction assuming a concerted process is taking place:

This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Draw the mechanism for the following reaction assuming a concerted process is taking place:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Draw the mechanism for the following reaction assuming a concerted process is taking place:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Draw the mechanism for the following reaction assuming a stepwise process is taking place:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Draw the mechanism for the following reaction assuming a stepwise process is taking place:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Label the ɑ and β carbons in each alkyl halide:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Locate the β hydrogens and draw all possible elimination (regioisomers with different location of the double bond) products formed when each alkyl halide is treated with sodium ethoxide (CH3CH2O–Na+):
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Using curved arrows, draw the mechanism for the following elimination reactions, assuming a concerted process is taking place:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.


