Naming Esters
In the IUPACnomenclature of carboxylic acids, we learned that their salts are named by replacing the suffix “ic acid” or “oic acid” with “ate”. For example, sodium acetate, potassium butyrate, etc.
The good news is that esters follow the same pattern, and instead of the metal ion, we use the alkyl group connected to the RCO (acyl) fragment.
For example:

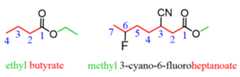
The substituents are numbered based on the position of the COOR group and placed in alphabetical order:
Naming Esters on a Ring
To name an ester on a ring, we need to refer to the corresponding carboxylic acid. For example, the suffix of cyclopentanecarboxylic acid is changed to carboxy”late” and the alkyl group is added at the beginning:

If substituents are also present, the numbering starts from the carbon connected to the COOH group and goes in the direction that minimizes the numbering of the substituents:

Below are some practice examples for naming carboxylic acids and their different derivatives.
Practice
Using the priority of functional groups, name each of the following compounds containing a carboxylic acid derivative:

This video is a fragment of thesummary quiz on IUPAC nomenclature.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to theanswers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps,and the powerful set ofOrganic Chemistry 1 and 2 Summary Study Guides.
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives

