Boiling Point and Melting Point in Organic Chemistry
In this post, we will talk about themeltingandboiling points of organic compounds and their correlation with intermolecular forces such as dipole-dipole,London dispersion (also known asVan der Waals) interactions, andhydrogen bonding. We discussed these infractions inthe previous post, so today, the focus will be more from the perspective of physical properties.
Let’s put the relative strength of intermolecular interactions right before we get started. In general, for compounds of approximately the same molecular mass, you can follow thistrend for the strength of intermolecular interactions:

We will discuss the strength and effect of each interaction typical of covalent compounds below. Theionic bonding is the strongest intermolecular interaction characteristic of inorganic compounds, which, as a result, have very high melting points. These, however, are not so relevant in organic chemistry, and therefore, we won’t focus on them as much in this article.
Boiling Point and Dipole-Dipole Interactions
We mentioned in the previous post thatstronger intermolecular interactions increase the boiling andmelting points, but how exactly they affect the physical properties might be your next question.
Thedefinition of boiling point states that it is the temperature when thevapor pressure of the compound equals the atmospheric pressure. In other words, we can say it is the temperature at which there is so much of the compound evaporated that it creates a pressure equal to the external pressure. In a simpler perspective, let’s say that it is the temperature when theliquid turns into a gas, even though this process can occur at a large scale of temperature and pressure combination.
Now, to turn into the gas phase, the molecules should overcome the intermolecular interactions and escape the liquid surface, andthe stronger these interactions,the harder it is for the molecules to overcome them.

Let’s first discuss the effect ofdipole-dipole interactions on the boiling point of organic covalent compounds. Dipole-dipole interactions are not so strong (weaker than ionic and covalent bonding).
However, in reality, we never deal with two or three molecules, but rather we work in the mole scale. And this means, despite being weak,dipole-dipole interactions contributesignificantly to thephysical properties of compounds when thousands or millions of molecules chain together through this electrostatic interaction. Let’s show this with an example of a common organic solvent, acetone:

And now let’s compare theboiling points of Isobutylene and acetone. They have a similar structure and only differ in that one of the carbon atoms is replaced by an oxygen. This difference, however, introduces a polar covalent bond and therefore,dipole-dipole interactions, whichelevate the boiling and melting point of the compound:
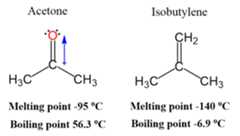
Isobutylene, on the other hand, is anonpolar molecule lacking dipole-dipole interactions since it only consists of C-C and C-H bonds.
Boiling Point and Hydrogen Bonding
You can check theprevious post for more details about the hydrogen bonding. In short, it is another type ofintermolecularelectrostatic interaction that occurs between a hydrogen atom bonded to an electronegativeatom, such as O, N, or F, that is attracted to a lone pairof electrons on an atom in another molecule.

As expected, hydrogen bonding affects the physical properties, which we can see, for example, by comparing the boiling point of ethanol shown above and dimethyl ether. These two areconstitutional isomers, meaning they have the same chemical formula and therefore, molecular mass.
However, despite having the same molecular mass, these compounds have completely different physical and chemical properties:

The reason for this difference is the lack of hydrogen bonding in the ether since it lacks hydrogen atom(s) connected to an electronegative atom.
Similar behavior can be seen when comparing the boiling points of three isomeric amines: Trimethylamine, Ethylmethylamine, and Propylamine. These are examples oftertiary, secondary, and primary amine,s which are defined based on the number of alkyl groups connected to the nitrogen.

Notice that theboiling point increases as we go from the tertiary to the primary amine as a result ofincreasing the number of hydrogen bonds per nitrogen. Propylamine hastwo hydrogens connected to the nitrogen, and each can make a hydrogen bond with a neighboring nitrogen atom from another molecule. This makes a stronger intermolecular interaction and therefore, more energy is needed to break it pushes the molecules to the gas phase. Thetertiary amine, on the other hand, hasno hydrogens and boils at a lot lower temperature.
London orVan Der Waals forces
Another factor that influences the boiling point is the surface of the molecule. Thelarger this surface, the stronger the intermolecular interactions, and thus, thehigher the boiling point. This can be seen by comparing the boiling points of pentane, 2-methylbutane, and 2,2-dimethylpropane:

Again, all three areconstitutional isomers and have identical molecular weights. In addition, they alsolack dipole-dipole interactions since there is no polar covalent bond present.
However, theboiling point decreases quite significantly as we move towards themore branched isomers. And this is a demonstration of adirect relationship between thesurface area and the boiling point. Pentane isunbranched and provides alarge surface for intermolecular interactions. The 2-methylbutane has one substituent, so it is a little more branched than pentane. This reduces the surface for intermolecular interactions and lowers the boiling point by about 8oC. Thehighly branched 2,2-dimethylpropane, on the other hand, lacks this surface interaction and has thelowest boiling point.
The analog of this can be the regular stacking of regular vs crumpled paper sheets.

To summarize,branching tends todecrease the boiling pointof alkanes with the same molecular weight.
All these examples demonstrate the importance of the molecular surface in intermolecular interactions, which directly affect the boiling point of a compound. However, you might have noticed one important thing missing here.
What interaction are we talking about?
We just said that themolecules are nonpolar and therefore lack dipole-dipole interaction, sowhat type of interactions increase the boiling point with a larger surface?
These are theVan Der Waals forces, also known asLondon dispersion forces, which are theweakest intramolecular interactions and occur as an electrostatic interaction oftemporary dipole moments formed in the molecule right at the time when they get in a close enough distance:
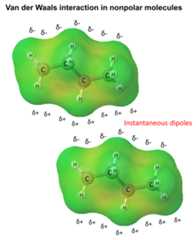
You can read more aboutVan Der Waals forces inthis article.
Melting Points
Just like we simplified the boiling point to explain the effect of intermolecular interactions on it, let’s formulate that themelting pointof a compound is the temperature at which it is convertedfrom the solid to the liquid phase.
In general,the melting pointof compounds with similar molecular weightincreases with stronger intermolecular interactions.
For example,pentane has avery low melting point compared tobutanal since it only relies onLondon dispersion forces, while butanal contains a polar C=O bond and therefore exhibits dipole-dipole interactions:

Potassium tert-butoxide, being an ionic compound, has the highest melting point.1-butanol is the second because of the OH group and thus, hydrogen bonding. Remember, the order of increasing intramolecular interactions in covalent compounds:
Symmetry and Melting Point
Aside from the intermolecular interactions, however, the melting point also depends on how the molecules are packed or arranged in the solid form. Themore symmetrical they are, the better they pack and form a perfect crystal lattice, which results in ahigher melting point. So, as the molecules fit tightly, more energy is required to break the lattice and melt them apart.
Let’s go back to the examples we discussed for the boiling point. Remember, theboiling pointincreases with less branching because of the increased surface area. So, pentane had a higher point than 2,2-dimethylpropane; 36.1oC vs 9.5oC.
Interestingly, thepattern is not observed for the melting points. 2,2-dimethylpropane has ahigher melting point since it ismore symmetrical than pentane, and when in the solid phase (before melting) its molecules arebetter packed.

We can also see the effect of symmetry by comparing the melting point temperatures of the butanol isomers. All of these are constitutional isomers capable of hydrogen bonding. However, compared to the other isomeric alcohols, thetert-Butyl alcohol has a muchhigher melting point because of itssymmetrical structure and therefore, compact packing in the solid phase:

And another great example to illustrate the importance of packing and molecular symmetry is the comparison of the melting and boiling points ofcis– and transalkenes.In general,trans alkenes have a higher melting point.
For example, despite being nonpolar, thetrans isomer of 1,2-dichloroethane has a higher melting point (−50oC) than thecis isomer (−80oC) because ofhigher symmetry, which allows for compact packing in the solid phase.
In contrast, the cis isomer is a polar molecule with a higher boiling point (60oC vs 48 oC ) because of the net molecular dipole moment andintermolecular dipole-dipole interactions.

Summarizing it, remember that given the samefunctional groups, theboiling and melting points would naturally be expected toincrease with the molecular mass (size) of the molecule. Also,stronger intermolecular interactions presumehigher boiling and melting points. However,for the melting point, you also need to consider the factor ofsymmetry. Moresymmetry means tighter packing in the solid phases and therefore, ahigher melting point.
In thenext article, we will also discuss the effect of intermolecular interactions on thesolubility of organic compounds.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- Bonding Patterns in Organic Chemistry
- How to Determine the Number of Lone Pairs
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
Practice
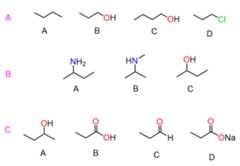
- Determine the compound with a higher boiling point in each set:

For each set, rank the compounds from the highest to the lowest boiling point.
Why would benzene have a lower boiling point but a much higher melting point than ethylbenzene?
6 thoughts on “ Boiling Point and Melting Point in Organic Chemistry”
It’s really helpful
ReplyThank you ,This is helpful to studies .
ReplyHi. May I ask is the boiling point of sec-butyl higher or lower than the isobutyl and why? Thank you
Reply


