Alpha Halogenation of Carboxylic Acids
Carboxylic acids withαhydrogenatoms can be brominated in the presence of catalytic amounts of phosphorus (or a phosphorus tribromide) formingα-bromo carboxylic acids. This is known as theHell–Volhard–Zelinski (or HVZ) reaction:

Now, let’s understand how this happens. First, why do we need the phosphorous?
When discussing thealpha halogenation of ketones and aldehydes, we saw that these reactions go viaformation of enols or enolates and therefore,carboxylic acids, despite havingαhydrogens, cannot be halogenated by this method. The problem is that carboxylic acids are deprotonated before the removal of α protons and therefore, theydon’t readilyform enols.
Instead, what happens is the carboxylic acid first reacts with PBr3 to form an acid bromide, which now establishes an equilibrium with an enol. After this, it follows the same mechanism as the α halogenation of ketones and aldehydes. Finally, the carboxylic acid is obtained by the hydrolysis of the acid bromide:

Now, as far as how the reaction works when phosphorous is used with bromine, the answer is thein-situ reaction of these twoforming phosphorous tribromide which then acts like we have seen above.
The same result can be achieved if PCl3 is used instead of PBr3.
Alpha Chlorination and Iodination of Carboxylic Acids
The Hell–Volhard–Zelinski reaction was developed by the three of them in 1881 while an efficient method forchlorination and iodinationof carboxylic acids was onlyestablished nearly a century later by David N. Harpp at McGill University.
It is a similar strategy and relies on the use of N-chlorosuccinimide (NCS) and thionyl chloride for in-situ conversion of the carboxylic acid into acid chlorides:
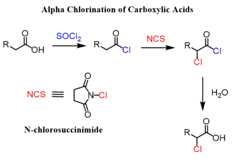
Thealpha iodination is achieved by using molecular iodine in a similar reaction:

The monohalogenation is used by using stoichiometric amounts of reactants and if more than one molar equivalent of the halogen is used,α,α-dihalo acids orα,α,α-trihalo acids are obtained.
Reactions ofα-Halo Acids andα-Halo Acid Halides
Just likeacid chlorides, he a-bromo acid bromides are very reactive towards many nucleophile and further transformations such as to esters and amides are possible:

α-Halo Acids can be converted into amino acids by reacting them with ammonia:

Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem

