rhenium
Our editors will review what you’ve submitted and determine whether to revise the article.
rhenium (Re),chemical element, a very raremetal of Group 7 (VIIb) of theperiodic table and one of the densest elements. Predicted by the Russian chemistDmitry Ivanovich Mendeleyev (1869) as chemically related tomanganese, rhenium was discovered (1925) by the German chemists Ida and Walter Noddack and Otto Carl Berg. The metal and itsalloys have found limited application as turbine blades infighter-jet engines, fountain pen points, high-temperaturethermocouples (withplatinum),catalysts, electrical contact points, and instrument-bearing points and in electrical components, such as in flashbulb filaments as analloy withtungsten.
Rhenium does not occur free in nature or as acompound in any distinctmineral; instead it is widely distributed in small amounts in other minerals, usually in concentrations averaging about 0.001 parts per million.Chile is the world leader in rhenium recovery, followed by theUnited States, Poland, Uzbekistan, and Kazakhstan.
Rhenium occurs up to about 20 parts per million inmolybdenite and to a lesser extent in sulfidiccopper ores. The recovery of rhenium is aided by the concentration of its volatile heptoxide (Re2O7) in the flue dust and gases given off during the smelting of molybdenite ore or from its concentration with the platinum metals in the anode sludge during electrolytic copper refining. The black metal powder isextracted from the gases and dust by leaching or scrubbing them with water to dissolve the oxide, Re2O7, which in turn can be converted to ammonium perrhenate, NH4ReO4, and then reduced to the metal withhydrogen. The powder may be compressed and sintered into bars in hydrogen at elevated temperatures. Cold-working andannealing permit the fabrication of wire or foil.
- Key People:
- Ida Noddack

Rhenium metal is silvery white and extremely hard; it resists wear and corrosion very well and has one of the highest melting points of the elements. (Themelting point of rhenium, 3,180°C [5,756 °F], is exceeded only by those of tungsten andcarbon.) The metal powder slowly oxidizes in air above 150 °C (300 °F) and rapidly at higher temperatures to form the yellow heptoxide, Re2O7. The metal is not soluble inhydrochloric acid and dissolves only slowly in other acids. There is evidence for the existence of rhenium in each of the oxidation states from −1 to +7; the most common states are +3, +4, +5, and especially +7. Rhenium’s most characteristic and importantcompounds are formed in the oxidation states +4 and +7, although compounds are known in all formal oxidation states from −1 to +7. Perrhenicacid (HReO4) and its anhydride, the heptoxide, and the perrhenates are common stable compounds in which rhenium is in the +7 state. Natural rhenium is a mixture of the stableisotope rhenium-185 (37.4 percent) and the radioactive rhenium-187 (62.6 percent, 4.1 × 1010-year half-life).
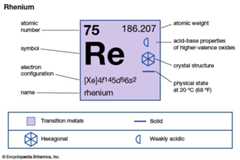
| atomic number | 75 |
|---|---|
| atomic weight | 186.2 |
| melting point | 3,180 °C (5,756 °F) |
| boiling point | 5,627 °C (10,161 °F) |
| specific gravity | 20.5 at 20 °C (68 °F) |
| oxidation states | +1, +2, +3, +4, +5, +6, +7 |
| electron configuration | [Xe]4f145d56s2 |