aluminum
Our editors will review what you’ve submitted and determine whether to revise the article.
- The Essential Chemical Industry - online - Aluminum
- Royal Society of Chemistry - Periodic Table - Aluminium
- Los Alamos National Laboratory - Aluminum
- Energy Education - Aluminum
- National Center for Biotechnology Information - PubChem - Aluminum
- Lenntech - Aluminum - Al
- The Minerals, Metals and Materials Society - Production and Processing of Aluminum
- Also spelled:
- aluminium
aluminum (Al),chemical element, a lightweight silvery whitemetal of main Group 13 (IIIa, orboron group) of theperiodic table. Aluminum is the most abundant metallic element inEarth’s crust and the most widely used nonferrous metal. Because of its chemical activity, aluminum never occurs in the metallic form in nature, but itscompounds are present to a greater or lesser extent in almost allrocks, vegetation, andanimals. Aluminum is concentrated in the outer 16 km (10 miles) of Earth’s crust, of which itconstitutes about 8 percent by weight; it is exceeded in amount only byoxygen andsilicon. The name aluminum is derived from the Latin wordalumen, used to describe potash alum, or aluminum potassium sulfate, KAl(SO4)2∙12H2O.
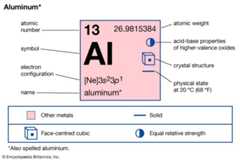
| atomic number | 13 |
|---|---|
| atomic weight | 26.9815384 |
| melting point | 660 °C (1,220 °F) |
| boiling point | 2,467 °C (4,473 °F) |
| specific gravity | 2.70 (at 20 °C [68 °F]) |
| valence | 3 |
| electron configuration | 1s22s22p63s23p1 |
Occurrence and history
Aluminum occurs inigneous rocks chiefly as aluminosilicates infeldspars,feldspathoids, andmicas; in the soil derived from them as clay; and upon further weathering asbauxite and iron-richlaterite. Bauxite, a mixture of hydrated aluminum oxides, is the principal aluminum ore. Crystalline aluminum oxide (emery,corundum), which occurs in a few igneous rocks, is mined as a natural abrasive or in its finer varieties asrubies andsapphires. Aluminum is present in othergemstones, such astopaz,garnet, andchrysoberyl. Of the many other aluminum minerals,alunite andcryolite have some commercial importance.
Before 5000bce people in Mesopotamia were making fine pottery from a clay that consisted largely of an aluminumcompound, and almost 4,000 years ago Egyptians and Babylonians used aluminumcompounds in various chemicals and medicines.Pliny refers to alumen, now known as alum, a compound of aluminum widely employed in the ancient andmedieval world to fix dyes in textiles. In the latter half of the 18th century, chemists such asAntoine Lavoisier recognized alumina as the potential source of a metal.

Crude aluminum was isolated (1825) by Danish physicistHans Christian Ørsted by reducing aluminum chloride withpotassium amalgam. British chemistSir Humphry Davy had prepared (1809) aniron-aluminumalloy byelectrolyzing fusedalumina (aluminum oxide) and had already named the element aluminum; the word later was modified to aluminium in England and some other European countries. German chemistFriedrich Wöhler, using potassium metal as the reducing agent, produced aluminum powder (1827) and small globules of the metal (1845), from which he was able to determine some of its properties.
The new metal was introduced to the public (1855) at the ParisExposition at about the time that it became available (in small amounts at great expense) by thesodium reduction of molten aluminum chloride through the Deville process. Whenelectric power became relatively plentiful and cheap, almost simultaneouslyCharles Martin Hall in theUnited States andPaul-Louis-Toussaint Héroult in France discovered (1886) the modern method of commercially producing aluminum: electrolysis of purified alumina (Al2O3) dissolved in molten cryolite (Na3AlF6). During the 1960s aluminum moved into first place, ahead ofcopper, in world production of nonferrous metals. For more specific information about the mining, refining, and production of aluminum,seealuminum processing.
Uses and properties
Aluminum is added in small amounts to certain metals to improve their properties for specific uses, as in aluminum bronzes and mostmagnesium-base alloys; or, for aluminum-basealloys, moderate amounts of other metals andsilicon are added to aluminum. The metal and its alloys are used extensively for aircraft construction, building materials, consumer durables (refrigerators, air conditioners, cooking utensils), electrical conductors, and chemical andfood-processing equipment.
Pure aluminum (99.996 percent) is quite soft and weak; commercial aluminum (99 to 99.6 percent pure) with small amounts of silicon and iron is hard and strong. Ductile and highlymalleable, aluminum can be drawn into wire or rolled into thin foil. The metal is only about one-third as dense as iron or copper. Though chemically active, aluminum is nevertheless highly corrosion-resistant, because in air a hard, tough oxide film forms on its surface.
Aluminum is an excellent conductor ofheat andelectricity. Itsthermal conductivity is about one-half that of copper; its electrical conductivity, about two-thirds. It crystallizes in the face-centred cubic structure. All natural aluminum is the stableisotope aluminum-27. Metallic aluminum and its oxide and hydroxide are nontoxic.
Aluminum is slowly attacked by most diluteacids and rapidly dissolves in concentratedhydrochloric acid. Concentratednitric acid, however, can be shipped in aluminum tank cars because it renders the metal passive. Even very pure aluminum is vigorously attacked byalkalies such as sodium and potassium hydroxide to yieldhydrogen and the aluminateion. Because of its greataffinity for oxygen, finely divided aluminum, if ignited, will burn incarbon monoxide orcarbon dioxide with the formation of aluminum oxide and carbide, but, at temperatures up to red heat, aluminum is inert tosulfur.
Aluminum can be detected in concentrations as low as one part per million by means of emissionspectroscopy. Aluminum can be quantitatively analyzed as the oxide (formula Al2O3) or as a derivative of the organicnitrogen compound 8-hydroxyquinoline. The derivative has the molecular formula Al(C9H6ON)3.
Compounds
Ordinarily, aluminum is trivalent. At elevated temperatures, however, a few gaseous monovalent and bivalentcompounds have been prepared (AlCl, Al2O, AlO). In aluminum theconfiguration of the three outerelectrons is such that in a few compounds (e.g., crystalline aluminum fluoride [AlF3] and aluminum chloride [AlCl3]) the bareion, Al3+, formed by loss of these electrons, is known to occur. The energy required to form the Al3+ ion, however, is very high, and, in the majority of cases, it is energetically more favourable for the aluminum atom to form covalent compounds by way ofsp2 hybridization, asboron does. The Al3+ ion can be stabilized by hydration, and the octahedral ion [Al(H2O)6]3+ occurs both in aqueous solution and in several salts.
A number of aluminum compounds have important industrial applications.Alumina, which occurs in nature ascorundum, is also prepared commercially in large quantities for use in the production of aluminum metal and the manufacture of insulators, spark plugs, and various other products. Upon heating, alumina develops a porous structure, which enables it to adsorb water vapour. This form of aluminum oxide, commercially known as activated alumina, is used for drying gases and certain liquids. It also serves as a carrier forcatalysts of various chemical reactions.
Anodic aluminum oxide (AAO), typically produced via the electrochemical oxidation of aluminum, is a nanostructured aluminum-based material with a very unique structure. AAO contains cylindrical pores that provide for a variety of uses. It is a thermally and mechanically stable compound while also being optically transparent and an electrical insulator. The pore size and thickness of AAO can easily be tailored to fit certain applications, including acting as a template for synthesizing materials into nanotubes and nanorods.
Another major compound isaluminum sulfate, a colourless salt obtained by the action ofsulfuric acid on hydrated aluminum oxide. The commercial form is a hydratedcrystalline solid with thechemical formula Al2(SO4)3. It is used extensively in paper manufacture as a binder for dyes and as a surface filler. Aluminum sulfatecombines with the sulfates of univalent metals to form hydrated double sulfates calledalums. The alums, double salts of formula MAl(SO4)2 ·12H2O (whereM is a singly charged cation such as K+), also contain the Al3+ ion; M can be thecation ofsodium,potassium,rubidium,cesium, ammonium, orthallium, and the aluminum may be replaced by a variety of other M3+ ions—e.g.,gallium,indium,titanium,vanadium,chromium,manganese,iron, orcobalt. The most important of such salts is aluminum potassium sulfate, also known as potassium alum or potash alum. These alums have many applications, especially in the production of medicines, textiles, and paints.
The reaction of gaseouschlorine with molten aluminum metal producesaluminum chloride; the latter is the most commonly usedcatalyst inFriedel-Crafts reactions—i.e.,synthetic organic reactions involved in the preparations of a wide variety of compounds, including aromatic ketones and anthroquinone and its derivatives. Hydrated aluminum chloride, commonly known asaluminum chlorohydrate, AlCl3∙H2O, is used as a topical antiperspirant or body deodorant, which acts by constricting the pores. It is one of several aluminum salts employed by the cosmetics industry.
Aluminum hydroxide, Al(OH)3, is used to waterproof fabrics and to produce a number of other aluminum compounds, including salts called aluminates that contain the AlO−2 group. With hydrogen, aluminum formsaluminum hydride, AlH3, a polymeric solid from which arederived the tetrohydroaluminates (important reducing agents).Lithium aluminum hydride (LiAlH4), formed by the reaction of aluminum chloride with lithium hydride, is widely used in organic chemistry—e.g., to reduce aldehydes and ketones to primary and secondary alcohols, respectively.