Substance P modulates NMDA responses and causes long-term protein synthesis-dependent modulation of the lamprey locomotor network
- PMID:9614253
- PMCID: PMC6792700
- DOI: 10.1523/JNEUROSCI.18-12-04800.1998
Substance P modulates NMDA responses and causes long-term protein synthesis-dependent modulation of the lamprey locomotor network
Abstract
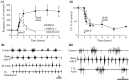
Tachykinin immunoreactivity is found in a ventromedial spinal plexus in the lamprey. Neurons in this plexus project bilaterally and are thus in a position to modulate locomotor networks on both sides of the spinal cord. We have examined the effects of the tachykinin substance P on NMDA-evoked locomotor activity. Brief (10 min) application of tachykinin neuropeptides results in a prolonged concentration-dependent (>24 hr) modulation of locomotor activity, shown by the increased burst frequency and more regular burst activity. These effects are blocked by the tachykinin antagonist spantide II. There are at least two phases to the burst frequency modulation. An initial phase (approximately 2 hr) is associated with the protein kinase C-dependent potentiation of cellular responses to NMDA. The long-lasting phase (>2 hr) appears to be protein synthesis-dependent, with protein synthesis inhibitors causing the increased burst frequency to recover after washing for 2-3 hr. The modulation of the burst regularity is caused by a separate effect of tachykinins, because unlike the burst frequency modulation it does not require the modulation of NMDA receptors for its induction and is blocked by H8, an inhibitor of cAMP- and cGMP-dependent protein kinases. The effects of substance P were mimicked by the dopamine D2 receptor antagonist eticlopride. The effects of eticlopride were blocked by the tachykinin antagonist spantide II, suggesting that eticlopride may endogenously release tachykinins. Because locomotor activity in vitro corresponds to that during swimming in intact animals, we suggest that endogenously released tachykinins will result in prolonged modulation of locomotor behavior.
Figures










References
- Alford S, Grillner S. CNQX and DNQX block non-NMDA synaptic transmission but not NMDA-evoked locomotion in lamprey spinal cord. Brain Res. 1990;506:297–302. - PubMed
- Barthe J-Y, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Exp Brain Res. 1997;115:485–492. - PubMed
- Ben Ari Y, Aniksztejn L, Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci. 1992;15:333–339. - PubMed
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources