Next-Generation MDMA Analogue SDMA: Pharmacological and Metabolic Insights
- PMID:41329099
- PMCID: PMC12715756
- DOI: 10.1021/acschemneuro.5c00782
Next-Generation MDMA Analogue SDMA: Pharmacological and Metabolic Insights
Abstract
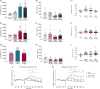
3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy, shows promise in treating depression and post-traumatic stress disorder (PTSD), resulting in breakthrough status. However, concerns regarding MDMA's abuse potential and cytotoxicity have sparked interest in developing safer analogues with similar therapeutic benefits. This study investigated the pharmacological properties of MDMA analogues in which the 1,3-benzodioxole group is replaced by a 1,3-benzoxathiole, termed SDA and SDMA, compared to MDA and MDMA throughin silico,in vitro, andin vivo assays.In vitro experiments using human embryonic kidney (HEK293) cells examined the interactions with monoamine transporters. SDA and SDMA showed similar profiles to MDMA at the serotonin transporter (SERT), while both inhibited dopamine (DAT) and norepinephrine (NET) transporters more potently, in line within silico molecular docking fitness scores of binding. SDA and SDMA also showed increased potency in evoking efflux through SERT and DAT acting as partial releasers. SDA and SDMA exhibited a similar interaction profile with 5-HT2 receptors compared with their respective analogues. Metabolism studies revealed faster clearance rates for SDA and SDMA, in contrast to MDA and MDMA, which exhibited only weak degradation. In contrast to MDMA's rewarding effects, SDMA did not induce significant effects in mice, while SDA only produced a significant preference for the drug-paired compartment at the lowest dose tested. Moreover, while SDMA shares similar locomotor and hyperthermic profiles as MDMA in mice, SDA induced increased hyperlocomotion and more sustained hyperthermia. In conclusion, these findings suggest that SDMA, with enhanced metabolic profiles and reduced abuse potential, is a promising candidate for further studies.
Keywords: MDMA; analogues; hepatic metabolism; monoamine receptors; monoamine transporters; pharmacological profile.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
