Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms
- PMID:33060197
- PMCID: PMC7808408
- DOI: 10.1126/science.abe9403
Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms
Abstract
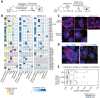
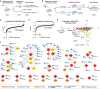
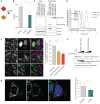
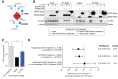
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a grave threat to public health and the global economy. SARS-CoV-2 is closely related to the more lethal but less transmissible coronaviruses SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV). Here, we have carried out comparative viral-human protein-protein interaction and viral protein localization analyses for all three viruses. Subsequent functional genetic screening identified host factors that functionally impinge on coronavirus proliferation, including Tom70, a mitochondrial chaperone protein that interacts with both SARS-CoV-1 and SARS-CoV-2 ORF9b, an interaction we structurally characterized using cryo-electron microscopy. Combining genetically validated host factors with both COVID-19 patient genetic data and medical billing records identified molecular mechanisms and potential drug treatments that merit further molecular and clinical study.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures






Comment in
- Conserved host-pathogen interactions identify novel treatment options in betacoronavirus infections.Lukassen S, Eils R.Lukassen S, et al.Signal Transduct Target Ther. 2021 Feb 10;6(1):57. doi: 10.1038/s41392-021-00480-z.Signal Transduct Target Ther. 2021.PMID:33563888Free PMC article.No abstract available.
- Olanzapine, risperidone and quetiapine: Do these atypical antipsychotics have a protective effect for SARS-CoV-2?Prokopez CR, Farinola R, Vallejos M, Lopredo LS, Sfriso LE, Chiapella LC, Arce C, Corral RM, Cuesta MJ, Alomo M.Prokopez CR, et al.Schizophr Res. 2022 Mar;241:140-141. doi: 10.1016/j.schres.2022.01.035. Epub 2022 Jan 24.Schizophr Res. 2022.PMID:35123336Free PMC article.No abstract available.
Similar articles
- Crystal structure of SARS-CoV-2 Orf9b in complex with human TOM70 suggests unusual virus-host interactions.Gao X, Zhu K, Qin B, Olieric V, Wang M, Cui S.Gao X, et al.Nat Commun. 2021 May 14;12(1):2843. doi: 10.1038/s41467-021-23118-8.Nat Commun. 2021.PMID:33990585Free PMC article.
- Phosphorylation of SARS-CoV-2 Orf9b Regulates Its Targeting to Two Binding Sites in TOM70 and Recruitment of Hsp90.Brandherm L, Kobaš AM, Klöhn M, Brüggemann Y, Pfaender S, Rassow J, Kreimendahl S.Brandherm L, et al.Int J Mol Sci. 2021 Aug 26;22(17):9233. doi: 10.3390/ijms22179233.Int J Mol Sci. 2021.PMID:34502139Free PMC article.
- In-vitro acetylation of SARS-CoV and SARS-CoV-2 nucleocapsid proteins by human PCAF and GCN5.Hatakeyama D, Masuda T, Miki R, Ohtsuki S, Kuzuhara T.Hatakeyama D, et al.Biochem Biophys Res Commun. 2021 Jun 11;557:273-279. doi: 10.1016/j.bbrc.2021.03.173. Epub 2021 Apr 8.Biochem Biophys Res Commun. 2021.PMID:33894414Free PMC article.
- Nucleoside Analogs and Nucleoside Precursors as Drugs in the Fight against SARS-CoV-2 and Other Coronaviruses.Borbone N, Piccialli G, Roviello GN, Oliviero G.Borbone N, et al.Molecules. 2021 Feb 13;26(4):986. doi: 10.3390/molecules26040986.Molecules. 2021.PMID:33668428Free PMC article.Review.
- The lethal internal face of the coronaviruses: Kidney tropism of the SARS, MERS, and COVID19 viruses.Motavalli R, Abdelbasset WK, Rahman HS, Achmad MH, Sergeevna NK, Zekiy AO, Adili A, Khiavi FM, Marofi F, Yousefi M, Ghoreishizadeh S, Shomali N, Etemadi J, Jarahian M.Motavalli R, et al.IUBMB Life. 2021 Aug;73(8):1005-1015. doi: 10.1002/iub.2516. Epub 2021 Jun 30.IUBMB Life. 2021.PMID:34118117Free PMC article.Review.
Cited by
- Recent progress in mass spectrometry-based strategies for elucidating protein-protein interactions.Low TY, Syafruddin SE, Mohtar MA, Vellaichamy A, A Rahman NS, Pung YF, Tan CSH.Low TY, et al.Cell Mol Life Sci. 2021 Jul;78(13):5325-5339. doi: 10.1007/s00018-021-03856-0. Epub 2021 May 27.Cell Mol Life Sci. 2021.PMID:34046695Free PMC article.Review.
- Cyclin D3 restricts SARS-CoV-2 envelope incorporation into virions and interferes with viral spread.Gupta RK, Mlcochova P.Gupta RK, et al.EMBO J. 2022 Nov 17;41(22):e111653. doi: 10.15252/embj.2022111653. Epub 2022 Oct 10.EMBO J. 2022.PMID:36161661Free PMC article.
- A network view of human immune system and virus-human interaction.Tang K, Tang J, Zeng J, Shen W, Zou M, Zhang C, Sun Q, Ye X, Li C, Sun C, Liu S, Jiang G, Du X.Tang K, et al.Front Immunol. 2022 Oct 26;13:997851. doi: 10.3389/fimmu.2022.997851. eCollection 2022.Front Immunol. 2022.PMID:36389817Free PMC article.
- A glimpse into viral warfare: decoding the intriguing role of highly pathogenic coronavirus proteins in apoptosis regulation.Cheng L, Rui Y, Wang Y, Chen S, Su J, Yu XF.Cheng L, et al.J Biomed Sci. 2024 Jul 13;31(1):70. doi: 10.1186/s12929-024-01062-1.J Biomed Sci. 2024.PMID:39003473Free PMC article.Review.
- SARS-CoV-2-infected hiPSC-derived cardiomyocytes reveal dynamic changes in the COVID-19 hearts.Li X, Hu H, Liu W, Zhang Q, Wang Y, Chen X, Zhu Y, Hu Z, Wang M, Ma J, Leng L.Li X, et al.Stem Cell Res Ther. 2023 Dec 12;14(1):361. doi: 10.1186/s13287-023-03603-1.Stem Cell Res Ther. 2023.PMID:38087340Free PMC article.
References
- Beigel J. H., Tomashek K. M., Dodd L. E., Mehta A. K., Zingman B. S., Kalil A. C., Hohmann E., Chu H. Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R. W., Dierberg K., Tapson V., Hsieh L., Patterson T. F., Paredes R., Sweeney D. A., Short W. R., Touloumi G., Lye D. C., Ohmagari N., Oh M.-d., Ruiz-Palacios G. M., Benfield T., Fätkenheuer G., Kortepeter M. G., Atmar R. L., Creech C. B., Lundgren J., Babiker A. G., Pett S., Neaton J. D., Burgess T. H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H. C., ACTT-1 Study Group Members , Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 383, 1813–1826 (2020). 10.1056/NEJMoa2007764 - DOI - PMC - PubMed
- Gordon D. E., Jang G. M., Bouhaddou M., Xu J., Obernier K., White K. M., O’Meara M. J., Rezelj V. V., Guo J. Z., Swaney D. L., Tummino T. A., Hüttenhain R., Kaake R. M., Richards A. L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B. J., Braberg H., Fabius J. M., Eckhardt M., Soucheray M., Bennett M. J., Cakir M., McGregor M. J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z. Z. C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I. T., Melnyk J. E., Chorba J. S., Lou K., Dai S. A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C. J. P., Perica T., Pilla K. B., Ganesan S. J., Saltzberg D. J., Rakesh R., Liu X., Rosenthal S. B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.-P., Liu Y., Wankowicz S. A., Bohn M., Safari M., Ugur F. S., Koh C., Savar N. S., Tran Q. D., Shengjuler D., Fletcher S. J., O’Neal M. C., Cai Y., Chang J. C. J., Broadhurst D. J., Klippsten S., Sharp P. P., Wenzell N. A., Kuzuoglu-Ozturk D., Wang H.-Y., Trenker R., Young J. M., Cavero D. A., Hiatt J., Roth T. L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R. M., Frankel A. D., Rosenberg O. S., Verba K. A., Agard D. A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H. S., Fujimori D. G., Ideker T., Craik C. S., Floor S. N., Fraser J. S., Gross J. D., Sali A., Roth B. L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K. M., Shoichet B. K., Krogan N. J., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020). 10.1038/s41586-020-2286-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Related information
Grants and funding
- R01 GM024485/GM/NIGMS NIH HHS/United States
- T32 AI060537/AI/NIAID NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- R01 HG008742/HG/NHGRI NIH HHS/United States
- R01 AI122747/AI/NIAID NIH HHS/United States
- F32 CA239333/CA/NCI NIH HHS/United States
- MR/V03541X/1/MRC_/Medical Research Council/United Kingdom
- R01 AI128214/AI/NIAID NIH HHS/United States
- R35 GM122481/GM/NIGMS NIH HHS/United States
- MC_UU_12016/2/MRC_/Medical Research Council/United Kingdom
- U19 AI135972/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- BB/J014443/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- F30 AI143401/AI/NIAID NIH HHS/United States
- R01 AI120694/AI/NIAID NIH HHS/United States
- T32 GM008284/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U19 AI135990/AI/NIAID NIH HHS/United States
- F32 GM137463/GM/NIGMS NIH HHS/United States
- 201366/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- R01 NS089713/NS/NINDS NIH HHS/United States
- R35 GM118099/GM/NIGMS NIH HHS/United States
- MC_PC_19026/MRC_/Medical Research Council/United Kingdom
- R01 HG009979/HG/NHGRI NIH HHS/United States
- P01 AI120943/AI/NIAID NIH HHS/United States
- MC_UU_00018/1/MRC_/Medical Research Council/United Kingdom
- P50 AI150476/AI/NIAID NIH HHS/United States
- MC_UU_12014/2/MRC_/Medical Research Council/United Kingdom
- K99 GM138753/GM/NIGMS NIH HHS/United States
- F32 CA239336/CA/NCI NIH HHS/United States
- R01 AI143292/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
