Maternal Caloric Restriction Implemented during the Preconceptional and Pregnancy Period Alters Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Overweight and Increased Adiposity at Adulthood in Male Rat Offspring
- PMID:27847471
- PMCID: PMC5088205
- DOI: 10.3389/fnbeh.2016.00208
Maternal Caloric Restriction Implemented during the Preconceptional and Pregnancy Period Alters Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Overweight and Increased Adiposity at Adulthood in Male Rat Offspring
Abstract
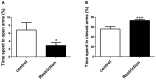
Exposure to inadequate nutritional conditions in critical windows of development has been associated to disturbances on metabolism and behavior in the offspring later in life. The role of the endocannabinoid system, a known regulator of energy expenditure and adaptive behaviors, in the modulation of these processes is unknown. In the present study, we investigated the impact of exposing rat dams to diet restriction (20% less calories than standard diet) during pre-gestational and gestational periods on: (a) neonatal outcomes; (b) endocannabinoid content in hypothalamus, hippocampus and olfactory bulb at birth; (c) metabolism-related parameters; and (d) behavior in adult male offspring. We found that calorie-restricted dams tended to have a reduced litter size, although the offspring showed normal weight at birth. Pups from calorie-restricted dams also exhibited a strong decrease in the levels of anandamide (AEA), 2-arachidonoylglycerol (2-AG), arachidonic acid (AA) and palmitoylethanolamide (PEA) in the hypothalamus at birth. Additionally, pups from diet-restricted dams displayed reduced levels of AEA in the hippocampus without significant differences in the olfactory bulb. Moreover, offspring exhibited increased weight gain, body weight and adiposity in adulthood as well as increased anxiety-related responses. We propose that endocannabinoid signaling is altered by a maternal caloric restriction implemented during the preconceptional and pregnancy periods, which might lead to modifications of the hypothalamic and hippocampal circuits, potentially contributing to the long-term effects found in the adult offspring.
Keywords: behavior; development; endocannabinoids; hippocampus; hypothalamus; maternal undernutrition; metabolism; rat.
Figures







References
- Antonelli T., Tomasini M. C., Tattoli M., Cassano T., Tanganelli S., Finetti S., et al. . (2005). Prenatal exposure to the CB1 receptor agonist WIN 55,212–2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 15, 2013–2020. 10.1093/cercor/bhi076 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
