Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles
- PMID:25775542
- PMCID: PMC4418852
- DOI: 10.1073/pnas.1423598112
Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles
Abstract
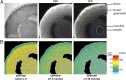
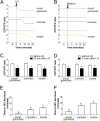
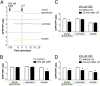
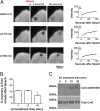
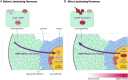
Meiosis in mammalian oocytes is paused until luteinizing hormone (LH) activates receptors in the mural granulosa cells of the ovarian follicle. Prior work has established the central role of cyclic GMP (cGMP) from the granulosa cells in maintaining meiotic arrest, but it is not clear how binding of LH to receptors that are located up to 10 cell layers away from the oocyte lowers oocyte cGMP and restarts meiosis. Here, by visualizing intercellular trafficking of cGMP in real-time in live follicles from mice expressing a FRET sensor, we show that diffusion of cGMP through gap junctions is responsible not only for maintaining meiotic arrest, but also for rapid transmission of the signal that reinitiates meiosis from the follicle surface to the oocyte. Before LH exposure, the cGMP concentration throughout the follicle is at a uniformly high level of ∼2-4 μM. Then, within 1 min of LH application, cGMP begins to decrease in the peripheral granulosa cells. As a consequence, cGMP from the oocyte diffuses into the sink provided by the large granulosa cell volume, such that by 20 min the cGMP concentration in the follicle is uniformly low, ∼100 nM. The decrease in cGMP in the oocyte relieves the inhibition of the meiotic cell cycle. This direct demonstration that a physiological signal initiated by a stimulus in one region of an intact tissue can travel across many layers of cells via cyclic nucleotide diffusion through gap junctions could provide a general mechanism for diverse cellular processes.
Keywords: cyclic GMP; gap junctions; luteinizing hormone; oocyte meiosis; ovarian follicle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
- Holt JE, Lane SIR, Jones KT. The control of meiotic maturation in mammalian oocytes. Curr Top Dev Biol. 2013;102:207–226. - PubMed
- Hunzicker-Dunn M, Mayo K. Gonadotropin signaling in the ovary. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th Ed. Academic; San Diego: 2015. pp. 895–945.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
