Sequencing the unsequenceable: expanded CGG-repeat alleles of the fragile X gene
- PMID:23064752
- PMCID: PMC3530672
- DOI: 10.1101/gr.141705.112
Sequencing the unsequenceable: expanded CGG-repeat alleles of the fragile X gene
Abstract
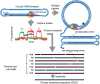
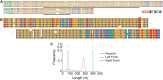
The human fragile X mental retardation 1 (FMR1) gene contains a (CGG)(n) trinucleotide repeat in its 5' untranslated region (5'UTR). Expansions of this repeat result in a number of clinical disorders with distinct molecular pathologies, including fragile X syndrome (FXS; full mutation range, greater than 200 CGG repeats) and fragile X-associated tremor/ataxia syndrome (FXTAS; premutation range, 55-200 repeats). Study of these diseases has been limited by an inability to sequence expanded CGG repeats, particularly in the full mutation range, with existing DNA sequencing technologies. Single-molecule, real-time (SMRT) sequencing provides an approach to sequencing that is fundamentally different from other "next-generation" sequencing platforms, and is well suited for long, repetitive DNA sequences. We report the first sequence data for expanded CGG-repeat FMR1 alleles in the full mutation range that reveal the confounding effects of CGG-repeat tracts on both cloning and PCR. A unique feature of SMRT sequencing is its ability to yield real-time information on the rates of nucleoside addition by the tethered DNA polymerase; for the CGG-repeat alleles, we find a strand-specific effect of CGG-repeat DNA on the interpulse distance. This kinetic signature reveals a novel aspect of the repeat element; namely, that the particular G bias within the CGG/CCG-repeat element influences polymerase activity in a manner that extends beyond simple nearest-neighbor effects. These observations provide a baseline for future kinetic studies of repeat elements, as well as for studies of epigenetic and other chemical modifications thereof.
Figures




Similar articles
- FMR1 CGG repeat lengths mediate different regulation of reporter gene expression in comparative transient and locus specific integration assays.Sølvsten C, Nielsen AL.Sølvsten C, et al.Gene. 2011 Oct 15;486(1-2):15-22. doi: 10.1016/j.gene.2011.06.034. Epub 2011 Jul 13.Gene. 2011.PMID:21767618
- Transcription-associated R-loop formation across the human FMR1 CGG-repeat region.Loomis EW, Sanz LA, Chédin F, Hagerman PJ.Loomis EW, et al.PLoS Genet. 2014 Apr 17;10(4):e1004294. doi: 10.1371/journal.pgen.1004294. eCollection 2014 Apr.PLoS Genet. 2014.PMID:24743386Free PMC article.
- CGG-repeat dynamics andFMR1 gene silencing in fragile X syndrome stem cells and stem cell-derived neurons.Zhou Y, Kumari D, Sciascia N, Usdin K.Zhou Y, et al.Mol Autism. 2016 Oct 6;7:42. doi: 10.1186/s13229-016-0105-9. eCollection 2016.Mol Autism. 2016.PMID:27713816Free PMC article.
- [FMR1 PREMUTATION CARRIERS - ARE THEY REALLY ASYMPTOMATIC?].Elizur S, Berkenstadt M, Ries-Levavi L, Gruber N, Pinhas-Hamiel O, Hassin-Baer S, Raas-Rothschild A, Raanani H, Cukierman-Yaffe T, Orvieto R, Cohen Y, Gabis L.Elizur S, et al.Harefuah. 2018 Apr;157(4):241-244.Harefuah. 2018.PMID:29688643Review.Hebrew.
- Fragile X syndrome: the FMR1 CGG repeat distribution among world populations.Peprah E.Peprah E.Ann Hum Genet. 2012 Mar;76(2):178-91. doi: 10.1111/j.1469-1809.2011.00694.x. Epub 2011 Dec 21.Ann Hum Genet. 2012.PMID:22188182Free PMC article.Review.
Cited by
- Exploring possible DNA structures in real-time polymerase kinetics using Pacific Biosciences sequencer data.Sawaya S, Boocock J, Black MA, Gemmell NJ.Sawaya S, et al.BMC Bioinformatics. 2015 Jan 28;16:21. doi: 10.1186/s12859-014-0449-0.BMC Bioinformatics. 2015.PMID:25626999Free PMC article.
- The second-tier status of fragile X syndrome testing for unexplained intellectual disability/global developmental delay in the era of next-generation sequencing.Zhang W, Li D, Pang N, Jiang L, Li B, Ye F, He F, Chen S, Liu F, Peng J, Yin J, Yin F.Zhang W, et al.Front Pediatr. 2022 Jul 22;10:911805. doi: 10.3389/fped.2022.911805. eCollection 2022.Front Pediatr. 2022.PMID:35935362Free PMC article.
- Pulse-Field capillary electrophoresis of repeat-primed PCR amplicons for analysis of large repeats in Spinocerebellar Ataxia Type 10.Hashem V, Tiwari A, Bewick B, Teive HAG, Moscovich M, Schüele B, Bushara K, Bower M, Rasmussen A, Tsai YC, Clark T, McFarland K, Ashizawa T.Hashem V, et al.PLoS One. 2020 Mar 11;15(3):e0228789. doi: 10.1371/journal.pone.0228789. eCollection 2020.PLoS One. 2020.PMID:32160188Free PMC article.
- [Fragile X syndrome and FMR1-dependent diseases - clinical presentation, epidemiology and molecular background].Landowska A, Rzońca S, Bal J, Gos M.Landowska A, et al.Dev Period Med. 2018;22(1):14-21. doi: 10.34763/devperiodmed.20182201.1421.Dev Period Med. 2018.PMID:29641417Free PMC article.Review.Polish.
- De novo repeat interruptions are associated with reduced somatic instability and mild or absent clinical features in myotonic dystrophy type 1.Cumming SA, Hamilton MJ, Robb Y, Gregory H, McWilliam C, Cooper A, Adam B, McGhie J, Hamilton G, Herzyk P, Tschannen MR, Worthey E, Petty R, Ballantyne B; Scottish Myotonic Dystrophy Consortium; Warner J, Farrugia ME, Longman C, Monckton DG.Cumming SA, et al.Eur J Hum Genet. 2018 Nov;26(11):1635-1647. doi: 10.1038/s41431-018-0156-9. Epub 2018 Jul 2.Eur J Hum Genet. 2018.PMID:29967337Free PMC article.
References
- Braida C, Stefanatos RK, Adam B, Mahajan N, Smeets HJ, Niel F, Goizet C, Arveiler B, Koenig M, Lagier-Tourenne C, et al. 2010. Variant CCG and GGC repeats within the CTG expansion dramatically modify mutational dynamics and likely contribute toward unusual symptoms in some myotonic dystrophy type 1 patients. Hum Mol Genet 19: 1399–1412 - PubMed
- Chen LS, Tassone F, Sahota P, Hagerman PJ 2003. The (CGG)n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet 12: 3067–3074 - PubMed
- Chen L, Hadd A, Sah S, Filipovic-Sadic S, Krosting J, Sekinger E, Pan R, Hagerman PJ, Stenzel TT, Tassone F, et al. 2010. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn 12: 589–600 - PMC - PubMed
- Chen L, Hadd AG, Sah S, Houghton JF, Filipovic-Sadic S, Zhang W, Hagerman PJ, Tassone F, Latham GJ 2011. High-resolution methylation polymerase chain reaction for fragile X analysis: Evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet Med 13: 528–538 - PMC - PubMed
Publication types
MeSH terms
Substances
Related information
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources