Crucial role of copper in detection of metal-coordinating odorants
- PMID:22328155
- PMCID: PMC3295281
- DOI: 10.1073/pnas.1111297109
Crucial role of copper in detection of metal-coordinating odorants
Abstract
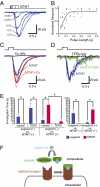
Odorant receptors (ORs) in olfactory sensory neurons (OSNs) mediate detection of volatile odorants. Divalent sulfur compounds, such as thiols and thioethers, are extremely potent odorants. We identify a mouse OR, MOR244-3, robustly responding to (methylthio)methanethiol (MeSCH(2)SH; MTMT) in heterologous cells. Found specifically in male mouse urine, strong-smelling MTMT [human threshold 100 parts per billion (ppb)] is a semiochemical that attracts female mice. Nonadjacent thiol and thioether groups in MTMT suggest involvement of a chelated metal complex in MOR244-3 activation. Metal ion involvement in thiol-OR interactions was previously proposed, but whether these ions change thiol-mediated OR activation remained unknown. We show that copper ion among all metal ions tested is required for robust activation of MOR244-3 toward ppb levels of MTMT, structurally related sulfur compounds, and other metal-coordinating odorants (e.g., strong-smelling trans-cyclooctene) among >125 compounds tested. Copper chelator (tetraethylenepentamine, TEPA) addition abolishes the response of MOR244-3 to MTMT. Histidine 105, located in the third transmembrane domain near the extracellular side, is proposed to serve as a copper-coordinating residue mediating interaction with the MTMT-copper complex. Electrophysiological recordings of the OSNs in the septal organ, abundantly expressing MOR244-3, revealed neurons responding to MTMT. Addition of copper ion and chelator TEPA respectively enhanced and reduced the response of some MTMT-responding neurons, demonstrating the physiological relevance of copper ion in olfaction. In a behavioral context, an olfactory discrimination assay showed that mice injected with TEPA failed to discriminate MTMT. This report establishes the role of metal ions in mammalian odor detection by ORs.
Conflict of interest statement
Conflict of interest statement: The authors declare a potential conflict of interest. The authors plan to submit a patent application relevant to the work.
Figures



References
- Devos M, Patte F, Rouault J, Laffort P, Van Gemert LJ, editors. Standardized Human Olfactory Thresholds. New York: IRL Press; 1990.
- Laska M, Bautista RM, Höfelmann D, Sterlemann V, Salazar LT. Olfactory sensitivity for putrefaction-associated thiols and indols in three species of non-human primate. J Exp Biol. 2007;210:4169–4178. - PubMed
- Block E. Reactions of Organosulfur Compounds. New York: Academic Press; 1978.
- Crabtree RH. Copper(I)—possible olfactory binding-site. J Inorg Nucl Chem. 1978;40:1453.
- Day JC. New nitrogen bases with severe steric hindrance due to flanking tert-butyl groups. cis-2,6-Di-tert-butylpiperidine. Possible steric blocking of olfaction. J Org Chem. 1978;43:3646–3649.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
