QM/MM Studies of the Matrix Metalloproteinase 2 (MMP2) Inhibition Mechanism of (S)-SB-3CT and its Oxirane Analogue
- PMID:21076643
- PMCID: PMC2976054
- DOI: 10.1021/ct100382k
QM/MM Studies of the Matrix Metalloproteinase 2 (MMP2) Inhibition Mechanism of (S)-SB-3CT and its Oxirane Analogue
Abstract
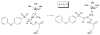
SB-3CT, (4-phenoxyphenylsulfonyl)methylthiirane, is a potent, mechanism-based inhibitor of the gelatinase sub-class of the matrix metalloproteinase (MMP) family of zinc proteases. The gelatinase MMPs are unusual in that there are several examples where both enantiomers of a racemic inhibitor have comparable inhibitory abilities. SB-3CT is one such example. Here, the inhibition mechanism of the MMP2 gelatinase by the (S)-SB-3CT enantiomer and its oxirane analogue is examined computationally, and compared to the mechanism of (R)-SB-3CT. Inhibition of MMP2 by (R)-SB-3CT was shown previously to involve enzyme-catalyzed C-H deprotonation adjacent to the sulfone, with concomitant opening by β-elimination of the sulfur of the three-membered thiirane ring. Similarly to the R enantiomer, (S)-SB-3CT was docked into the active site of MMP2, followed by molecular dynamics simulation to prepare the complex for combined quantum mechanics and molecular mechanics (QM/MM) calculations. QM/MM calculations with B3LYP/6-311+G(d,p) for the QM part (46 atoms) and the AMBER force field for the MM part were used to compare the reaction of (S)-SB-3CT and its oxirane analogue in the active site of MMP2 (9208 atoms). These calculations show that the barrier for the proton abstraction coupled ring opening reaction of (S)-SB-3CT in the MMP2 active site is 4.4 kcal/mol lower than its oxirane analogue, and the ring opening reaction energy of (S)-SB-3CT is only 1.6 kcal/mol less exothermic than its oxirane analogue. Calculations also show that the protonation of the ring-opened products by water is thermodynamically much more favorable for the alkoxide obtained from the oxirane, than for the thiolate obtained from the thiirane. In contrast to (R)-SB-3CT and the R-oxirane analogue, the double bonds of the ring-opened products of (S)-SB-3CT and its S-oxirane analogue have the cis-configuration. Vibrational frequency and intrinsic reaction path calculations on a reduced size QM/MM model (2747 atoms) provide additional insight into the mechanism. These calculations yield 5.9 and 6.7 for the deuterium kinetic isotope effect for C-H bond cleavage in the transition state for the R and S enantiomers of SB-3CT, in good agreement with the experimental results.
Figures




References
- Lei H, Furth EE, Kalluri R, Wakenell P, Kallen CB, Jeffrey JJ, Leboy PS, Strauss JF., 3rd Biol Reprod. 1999;60:183–189. - PubMed
- Liu CH, Wu PS. Biotechnol Lett. 2006;28:1725–1730. - PubMed
- Woessner JF., Jr FASEB J. 1991;5:2145–2154. - PubMed
- Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Mol Cell Endocrinol. 2002;191:45–56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous