Chromatin regulation in drug addiction and depression
- PMID:19877494
- PMCID: PMC2834246
- DOI: 10.31887/DCNS.2009.11.3/wrenthal
Chromatin regulation in drug addiction and depression
Abstract
Alterations in gene expression are implicated in the pathogenesis of several neuropsychiatric disorders, including drug addiction and depression. Increasing evidence indicates that changes in gene expression in neurons, in the context of animal models of addiction and depression, are mediated in part by epigenetic mechanisms that alter chromatin structure on specific gene promoters. This review discusses recent findings from behavioral, molecular, and bioinformatic approaches that are being used to understand the complex epigenetic regulation of gene expression in brain by drugs of abuse and by stress. These advances promise to open up new avenues for improved treatments of these disorders.
Las alteraciones en la expresión génica están implicadas en la patogénesis de varios trastornos neuropsiquiátricos, incluyendo la adicción a drogas y la depresión. Existe una evidencia creciente que indica que los cambios en la expresión génica en las neuronas, en el contexto de modelos animales de adicción y depresión, están mediados en parte por mecanismos epigenéticos que alteran la estructura de la cromatina de genes promotores especificos. Esta revisión discute hallazgos recientes que provienen de aproximaciones conductuales, moleculares y bioinformáticas los cuales están siendo utilizados para comprender la compleja regulación epigenética de la expresión génica en el cerebro por las drogas de abuso y por el estrés. Estos avances prometen establecer nuevos caminos para mejores tratamientos de estos trastornos.
Des modifications de l'expression génique sont impliquées dans la pathogenèse de plusieurs maladies neuropsychiatriques, y compris la toxicomanie et la dépression. Des modèles animaux de ces pathologies montrent de plus en plus que des variations de l'expression des gènes dans les neurones sont transmises en partie par des mécanismes épigénétiques qui changent la structure de la chromatine sur les promoteurs spécifiques des gènes. Cet article analyse les résultats récents des approches comportementales, moléculaires et bioinformatiques utilisées pour comprendre la régulation épigénétique complexe de l'expression génique dans le cerveau par l'abus de substances et par le stress. Ces avancées ouvrent de nouvelles voies pour mieux traiter ces maladies.
Figures
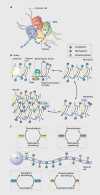

References
- Bird A. Perceptions of epigenetics. . Nature. 2007;447:396–398. - PubMed
- Surani MA., Hayashi K., Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747–762. - PubMed
- Luger K., Richmond TJ. The histone tails of the nucleosome. . Curr Opin Genet Dev. 1998;8:140–146. - PubMed
- Felsenfeld G., Groudine M. Controlling the double helix. . Nature. 2003;421:448–453. - PubMed
- Li B., Carey M., Workman JL. The role of chromatin during transcription. . Cell. 2007;128:707–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical