Neuropathology of the Mcoln1(-/-) knockout mouse model of mucolipidosis type IV
- PMID:19151629
- PMCID: PMC4232971
- DOI: 10.1097/NEN.0b013e3181942cf0
Neuropathology of the Mcoln1(-/-) knockout mouse model of mucolipidosis type IV
Abstract
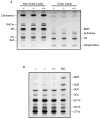
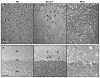
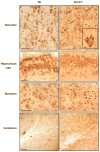
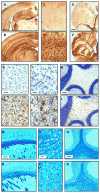
The recently developed Mcoln1(-/-) knockout mouse provides a novel model for analyzing mucolipin 1 function and mucolipidosis type IV disease. Here we characterize the neuropathology of Mcoln1(-/-) mouse at the end stage. Evidence of ganglioside accumulation, including increases in GM2, GM3, and GD3 and redistribution of GM1, was found throughout the central nervous system (CNS) independent of significant cholesterol accumulation. Unexpectedly, colocalization studies using immunofluorescence confocal microscopy revealed that GM1 and GM2 were present in separate vesicles within individual neurons. While GM2 was significantly colocalized with LAMP2, consistent with late-endosomal/lysosomal processing, some GM2-immunoreactivity occurred in LAMP2-negative sites, suggesting involvement of other vesicular systems. P62/Sequestosome 1 (P62/SQSTM1) inclusions were also identified in the CNS of the Mcoln1(-/-) mouse, suggesting deficiencies in protein degradation. Glial cell activation was increased in brain, and there was evidence of reduced myelination in cerebral and cerebellar white matter tracts. Autofluorescent material accumulated throughout the brains of the knockout mice. Finally, axonal spheroids were prevalent in white matter tracts and Purkinje cell axons. This neuropathological characterization of the Mcoln1(-/-) mouse provides an important step in understanding how mucolipin 1 loss of function affects the CNS and contributes to mucolipidosis type IV disease.
Figures




References
- Bargal R, Avidan N, Ben-Asher E, et al. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–23. - PubMed
- Sun M, Goldin E, Stahl S, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–78. - PubMed
- Zeevi DA, Frumkin A, Bach G. TRPML and lysosomal function. Biochim Biophys Acta. 2007;1772:851–58. - PubMed
- Berman ER, Livni N, Shapira E, et al. Congenital Cornea clouding with abnormal systemic storage bodies: A new variant of Mucolipidosis. J Pediatr. 1974;84:519–26s. - PubMed
- Chitayat D, Meunier CM, Hodgkinson KA, et al. Mucolipidosis type IV: Clinical manifestations and natural history. Am J Med Genet. 1991;41:313–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
