Aminoacyl tRNA synthetases and their connections to disease
- PMID:18682559
- PMCID: PMC2516211
- DOI: 10.1073/pnas.0802862105
Aminoacyl tRNA synthetases and their connections to disease
Abstract
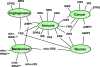
Aminoacylation of transfer RNAs establishes the rules of the genetic code. The reactions are catalyzed by an ancient group of 20 enzymes (one for each amino acid) known as aminoacyl tRNA synthetases (AARSs). Surprisingly, the etiology of specific diseases-including cancer, neuronal pathologies, autoimmune disorders, and disrupted metabolic conditions-is connected to specific aminoacyl tRNA synthetases. These connections include heritable mutations in the genes for tRNA synthetases that are causally linked to disease, with both dominant and recessive disease-causing mutations being annotated. Because some disease-causing mutations do not affect aminoacylation activity or apparent enzyme stability, the mutations are believed to affect functions that are distinct from aminoacylation. Examples include enzymes that are secreted as procytokines that, after activation, operate in pathways connected to the immune system or angiogenesis. In addition, within cells, synthetases form multiprotein complexes with each other or with other regulatory factors and in that way control diverse signaling pathways. Although much has been uncovered in recent years, many novel functions, disease connections, and interpathway connections of tRNA synthetases have yet to be worked out.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
- Verspohl EJ, Hohmeier N, Lempka M. Diadenosine tetraphosphate (Ap4A) induces a diabetogenic situation: Its impact on blood glucose, plasma insulin, gluconeogenesis, glucose uptake and GLUT-4 transporters. Pharmazie. 2003;58:910–915. - PubMed
- Nishimura A, et al. Diadenosine 5′, 5‴-P1, P4-tetraphosphate (Ap4A) controls the timing of cell division in Escherichia coli. Genes Cells. 1997;2:401–413. - PubMed
- Romby P, Springer M. Bacterial translational control at atomic resolution. Trends Genet. 2003;19:155–161. - PubMed
- Sampath P, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: Gene-specific silencing of translation. Cell. 2004;119:195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
