Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline
- PMID:10510461
- PMCID: PMC1571643
- DOI: 10.1038/sj.bjp.0702800
Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline
Abstract
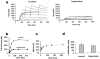
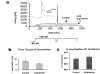
1 Tricyclic antidepressants (TCAs) are associated with cardiovascular side effects including prolongation of the QT interval of the ECG. In this report we studied the effects of two TCAs (imipramine and amitriptyline) on ionic current mediated by cloned HERG potassium channels. 2 Voltage clamp measurements of HERG currents were made from CHO cells transiently transfected with HERG cDNA. HERG-encoded potassium channels were inhibited in a reversible manner by both imipramine and amitriptyline. HERG tail currents (IHERG) following test pulses to +20 mV were inhibited by imipramine with an IC50 of 3.4+/-0.4 microM (mean+/-s.e.mean) and a Hill coefficient of 1.17+/-0.03 (n = 5). 3 microM amitriptyline inhibited IHERG by 34+/-6% (n = 3). The inhibition showed only weak voltage dependence. 3 Using an 'envelope of tails' comprised of pulses to +20 mV of varying durations, the tau of activation was found to be 155+/-30 ms for control and 132+/-26 ms for 3 microM imipramine (n = 5). Once maximal channel activation was achieved after 320 ms (as demonstrated by maximal tail currents), further prolongation of depolarization did not increase imipramine-mediated HERG channel inhibition. 4 Taking current measurements every second during a 10 s depolarizing pulse from -80 mV to 0 mV, block was observed during the first pulse in the presence of imipramine and the level of IHERG block was similar throughout the pulse (n=5). 5 A three pulse protocol (two depolarizing pulses to +20 mV separated by 20 ms at -80 mV) revealed that imipramine did not significantly alter the kinetics of IHERG inactivation. The tau of inactivation was 8+/-2 ms and 5.6+/-0.4 ms (n = 5) in the absence and presence of 3 microM imipramine, respectively, and currents inactivated to a similar extent. 6 Our data are consistent with TCAs causing components of block of the HERG channel in both the closed and open states. Any component of open channel block occurs rapidly upon depolarization. Inhibition of IHERG by the prototype TCAs imipramine and amitriptyline may suggest a mechanism for QT prolongation associated with risks of arrhythmia and sudden death that accompany high concentrations of TCAs following overdose.
Figures



References
- ABBOTT G.W., SESTI F., SPLAWSKI I., BUCK M., LEHMANN M.H., TIMOTHY K.W., KEATING M.T., GOLDSTEIN S.A.N. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
- BALSER J.R., BENNETT P.B., HONDEGHEM L.M., RODEN D.M. Suppression of time-dependent outward current in guinea-pig ventricular myocytes. Actions of quinidine and amiodarone. Circ. Res. 1991;69:519–529. - PubMed
- BIGGER J.T., GIARDINA E.G., PEREL J.M., KANTOR S.J., GLASSMAN A.H. Cardiac antiarrhythmic effect of imipramine hydrochloride. N. Engl. J. Med. 1977;296:206–208. - PubMed
- CASIS O., SANCHEZ-CHAPULA J.A. Disopyramide, imipramine, and amitriptyline bind to a common site on the transient outward K+ channel. J. Cardiovasc. Pharm. 1998;32:521–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
