Identification and Expression of theBacillus subtilis Fructose-1,6-Bisphosphatase Gene (fbp)
Yasutaro Fujita
Ken-Ichi Yoshida
Yasuhiko Miwa
Nobuo Yanai
Eishi Nagakawa
Yasuhiro Kasahara
Corresponding author. Mailing address: Department of Biotechnology, Faculty of Engineering, Fukuyama University, 985 Sanzo, Higashimura-cho, Fukuyama-shi, Hiroshima 729-0292, Japan. Phone: (81)849 36 2111. Fax: (81)849 36 2023. E-mail:yfujita@bt.fubt.fukuyama-u.ac.jp.
Series information
Note
Received 1998 Mar 12; Accepted 1998 Jun 9.
Abstract
TheBacillus subtilis fbp gene encoding fructose-1,6-bisphosphatase (FBPase) was originally identified asyydE. Thefbp gene was expressed at a fairly constant level in cells undergoing glycolysis or gluconeogenesis.fbp transcription was initiated 94 bp upstream of the translation initiation codon, resulting in a 2.4-kb monocistronic transcript. Interestingly,B. subtilis FBPase exhibited no significant similarity to other FBPases in protein sequence databases.
Fructose-1,6-bisphosphatase (EC3.1.3.11) (FBPase) is a well-known enzyme involved in gluconeogenesis. TheBacillus subtilis FBPase appears to be a constitutive enzyme and has been purified and characterized (4). The enzyme is very labile in the absence of Mn2+ and is inhibited by AMP, but this inhibition can be overcome by phosphoenolpyruvate. Consequently, a change in the relative intracellular concentrations of AMP and phosphoenolpyruvate in glycolysis and gluconeogenesis may control FBPase activity (4). TheBacillus licheniformis FBPase also exhibits properties similar to those of theB. subtilis enzyme (13). In contrast to enterobacteria such asEscherichia coli (2,22), aB. subtilis lacking FBPase is able to grow on gluconeogenic carbon sources such as malate and glycerol (5,6).
Thefbp (formerlyfdp) gene required for FBPase synthesis is located betweengntK andpurA (6,7), but the gene itself has not been identified. Completion of the sequencing of theB. subtilis genome (10,11,19–21) allowed us to identify theyydE gene asfbp.
B. subtilis strains.
TheB. subtilis strains used are listed in Table1. To construct strains YF311 and YF312, we used plasmid pMutin1, which was provided by V. Vagner (Institut National de la Recherche Agronomique, Jouy-en-Josas, France). Integration of plasmid pMutin1 into the target gene through a single crossover allows the gene’s promoter to be monitored with alacZ reporter gene and places the downstream genes under the control of thespac promoter (1).fbp sequences were amplified by PCR using DNA from strain 1A1 as a template with primer pairs designed to generate flankingHindIII andBglII sites. The resulting PCR products were cleaved withHindIII andBglII and then ligated with plasmid pMutin1, which had been doubly digested withHindIII andBamHI. The ligated DNAs were doubly digested withBamHI andBglII to avoid self-ligation and then used for the transformation ofE. coli C600 (17) to ampicillin resistance (100 μg/ml) on 2× YT plates (17). The correct cloning of PCR products into plasmid pMutin1 was confirmed by DNA sequencing. The resulting plasmids were used for the transformation (6) ofB. subtilis 1A1 to erythromycin resistance (0.3 μg/ml) on tryptose blood agar base (Difco) plates containing 0.18% glucose. Correct integration of a single copy of each of the plasmids into thefbp locus of the chromosome through a single crossover was confirmed by Southern blot analysis (17).
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| 1A1 (= 168trpC2)a | trpC2 | 19 |
| 60015 | trpC2 metC7 | 5 |
| YF062 | bfd-1 fbp-74 glp hisA1 leuA8 metB5 trpC2 | 6 |
| YF311 | trpC2 fbp::pMutin1 | This study |
| YF312 | trpC2 Pfbp::pMutin1 (Pspac-fbp) | This study |
This strain was obtained from theBacillus Genetic Stock Center (Columbus, Ohio).
Identification of thefbp gene.
TheB. subtilis genome sequence (10,11,19–21) allowed us to determine the end points of the Δigf deletion (6). This deletion was 63 kb in size, affecting 58 genes fromyycR toiolI, with an 18-kb chromosomal segment at 76° replacing the 63-kb region (3). Thefbp gene is located within Δigf, with the gene order beingfbp,gntK, andiol (6,7). Thus, thefbp gene must be one of the genes betweenyycR andgntP.
As noted above,B. subtilis can bypass FBPase (5,6), so only strains (such as YF062) carrying bothfbp-74 andbfd-1, with defective FBPase and unable to bypass it (6), are unable to grow on gluconeogenic carbon sources such as Casamino Acids (Difco). To identify thefbp gene, we examined the ability of PCR products covering various parts of theyycR-to-gntP region to transform strain YF062 (fbp-74 bfd-1) to Fbp+ (able to grow on Casamino Acids) (Fig.1);bfd-1 is located outside Δigf (3,6). Fbp+ transformants were selected on N medium plates (6) containing 0.5% Casamino Acids, 0.1% Na3 citrate, 0.05 mM MnCl2, and 50 μg of tryptophan per ml. All PCR products able to transformfbp-74 tofbp+ contained theyydE gene, strongly suggesting thatyydE might befbp.
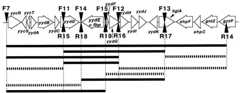
FIG. 1.
Identification of theB. subtilis fbp gene by examination of the transformation offbp-74 tofbp+ of PCR products covering various parts of theyycR-to-gntP region. The 19 genes (yycR togntP) are denoted by large open arrows indicating the direction of transcription. The PCR products were synthesized with various pairs of the F and R series primers. The 5′ ends of the 20-bp hybridizing sequences of primers F7, F11, F12, F13, F14, and F15 (the complementary sequences are found in the GSDB, DDBJ, EMBL, and NCBI databases under accession no.D78193 [10]) are positions 18592, 13433, 8124, 3321, 11851, and 9341, respectively, whereas the 5′ ends of the 20-bp hybridizing sequences of primers R15, R16, R17, R18, and R19 (accession no.D78193) are positions 13411, 8102, 3376, 11832, and 9322, respectively. The 5′ end of the 20-bp hybridizing sequence of primer R14 (the complementary sequence can be found under the accession no. ofAB005554 orD45242 [20]) is position 2693. Transformation of strain YF062 to Fbp+ with various PCR products was carried out as described previously (6). The products (F7-R14, F11-R17, F7-R16, F11-R16, F14-R17, and F7-R19) exhibited Fbp+ transforming activities of 553, 311, 442, 252, 74, and 166 Fbp+ colonies per ng of DNA, respectively, whereas the other products had no transforming activity (less than 5 colonies per ng of DNA); each value is the average of two independent experiments, with two different dilutions of each culture on a total of four plates. The products transformingfbp-74 tofbp+ are shown by the solid black bars, whereas those possessing no transforming activity are shown by the broken bars.
Disruption of thefbp gene.
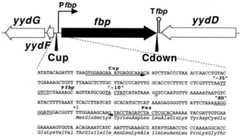
TheyydE gene contains a potential Shine-Dalgarno (SD) sequence preceding an AUG translation initiation codon at nucleotide (nt) −90 (10), as well as a second potential SD sequence preceding a UUG translation initiation codon at nt +1 (Fig.2). Thus, two PCR products carrying nt −58 to +263 and nt +78 to +418 with and without the second SD, respectively, were used for the integration of plasmid pMutin1 through a single crossover into theyydE locus of strain 1A1, resulting in strains YF312 (Pspac-fbp) and YF311 (fbp::pMutin1).
FIG. 2.
Promoter region of theB. subtilis fbp gene. A map of thefbp gene and its neighboring genes is shown at the top of the figure. Pfbp and Tfbp denote the putativefbp promoter and terminator, respectively. The locations of the Cup and Cdown primers used to obtain a PCR product for the cloning offbp inE. coli are also indicated. The nucleotide sequence of the upstream region and the 5′ part of thefbp gene are shown under the map. The −10 and −35 regions of Pfbp and an SD sequence forfbp are indicated. The putative transcription initiation nucleotide, G, is doubly underlined. The sequence and complementary sequence for hybridization of the Cup and Pex primers are indicated by arrows.
B. subtilis FBPase is considered to be a constitutive enzyme (4,5), but in contrast to FBPase activity in cells of the wild-type strain 1A1 (56 nmol/min per mg of protein), strain YF311 lacked FBPase (less than 1 nmol/min per mg of protein). However, FBPase synthesis was induced in cells of strain YF312 upon the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to the medium (data not shown). The fact that pMutin1 integration intoyydE affected FBPase activity clearly indicated that theyydE gene isfbp. Moreover, pMutin1 integration through the fragment from nt −58 to +263 caused the replacement of thefbp promoter with thespac promoter, whereas pMutin1 integration through a single crossover with the fragment from nt +78 to +418 disruptedfbp. The results suggested that the assignment of the second SD sequence and a UUG initiation codon forfbp is correct. Thus, thefbp gene likely comprises 1,923 nt (641 amino acids), which is 90 nt shorter thanyydE is thought to be (10).
To identify thefbp-74 mutation, we determined 2,488 bp of DNA sequence (nt −445 to +2043; containing the putativefbp promoter and coding region) of strains 60015 and YF062 (fbp-74) by successive cycle sequencing of PCR products amplified from these strains, using chromosomal DNAs as templates; the Δigf region was restored by transformation with DNA from strain 60015 in the process of isolation of strain YF062 (6). The 2,488-bp sequence of strain 60015 perfectly matched the sequence reported for theB. subtilis genome (10,11). However, that of strain YF062 carried one mismatch, a substitution of G at nt +1406 by A, causing the substitution of Asp for Gly in residue 469 of Fbp. It seems likely that this amino acid substitution renders strain YF062 to have defective FBPase.
Expression of thefbp gene inE. coli.
To determine whether thefbp gene encodes FBPase or a positive regulator of its synthesis, we attempted to clone and express this gene inE. coli and to characterize the protein synthesized. Thefbp coding region (nt −237 to +2186) carrying thefbp promoter as described below was amplified by PCR using a primer pair (Cup and Cdown) (Fig.2) and DNA from strain 1A1 as a template to produce a PCR product with flankingBamHI andPstI sites at the respective ends. The PCR product was digested withBamHI andPstI and then ligated with plasmid pUC118 or pUC119 (17), which had been doubly digested with these endonucleases. The ligated DNAs were used for the transformation ofE. coli XL1-blue (17) to produce white colonies on 2× YT plates containing 50 μg of ampicillin per ml, 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside per ml, and 0.1 mM IPTG. The resultant plasmids pUC118 and pUC119 carrying thefbp gene were designated plasmids pFBP118 and pFBP119, respectively.
The levels ofB. subtilis FBPase inE. coli XL1-blue carrying the various plasmids were determined as described previously (4,5). Cells carrying plasmids pFBP118 and pFBP119 synthesized similar large amounts of FBPase (5.02 and 5.46 mmol/min per mg of protein), respectively, whereas those bearing plasmid pUC118 or pUC119 synthesized no detectable FBPase (less than 1 nmol/min per mg of protein). Since the orientations of thefbp gene were the same as and opposite that of the plasmid’slac promoter in plasmids pFBP118 and pFBP119, respectively, the results suggest thatfbp is expressed from its own promoter efficiently inE. coli.
B. subtilis FBPase requires phosphoenolpyruvate and Mn2+ for its activation and stability, respectively (4). When lysates fromE. coli strains overexpressing Fbp were prepared and assayed in the absence of phosphoenolpyruvate and Mn2+, the lysates showed 14 and 2.3 times less enzyme activity, respectively (data not shown). Analysis of these lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequent Western blotting analysis (8) showed that the lysate of the cells bearing plasmid pFBP118 or pFBP119 contained an abundant protein with a molecular mass of 74 kDa (data not shown), which is very close to that of the Fbp protein (74,390 Da). This protein cross-reacted with anti-FBPase (data not shown), which was produced in a rabbit against purified FBPase (4,5). These results clearly indicate that thefbp gene is the structural gene of FBPase.
Expression of thefbp gene.
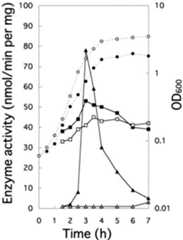
FBPase is required for gluconeogenesis. However, this enzyme was synthesized even when cells were grown on glucose as the sole carbon source, although its synthesis was somewhat lower in cells growing on glucose than in cells growing on malate (5). WhenB. subtilis is cultivated in NSMP medium (5), cells initially carrying out glycolysis enter gluconeogenic growth in the middle of the logarithmic phase (5) when catabolic operons such asiol (18) are released from catabolite repression (16). Consequently, we monitored FBPase synthesis in cells of strain 1A1 during both logarithmic and stationary growth phases (Fig.3). The activity of FBPase increased in the early logarithmic growth phase (up to an optical density at 600 nm [OD600] of 0.5) and then gradually decreased slightly in the late logarithmic and stationary phases when cells were cultivated without glucose. As observed previously (16), inositol dehydrogenase was induced in the middle of the logarithmic phase after consumption of the glycolytic carbohydrates in NSMP medium and then its expression decreased in the late logarithmic and stationary phases, probably due to consumption of themyo-inositol in the medium. When glucose was added to NSMP medium, levels of FBPase synthesized were more constant, and inositol dehydrogenase was not induced due to catabolite repression. These results indicate that expression of thefbp gene is relatively constant regardless of the necessity of FBPase. However,fbp expression is significantly dependent on the growth phase of the cell, but at present, we cannot explain this regulation.
FIG. 3.
Synthesis of FBPase during glycolytic and gluconeogenic growth. Cells of strain 1A1 were grown in NSMP medium with (open symbols) and without (closed symbols) 10 mM glucose, and the OD600 (•, ○) was monitored during growth at 37°C in a shaking water bath. At the indicated times, the cells (OD600 units = 9.0) were harvested and lysed in the presence of phosphoenolpyruvate and Mn2+ by lysozyme treatment and brief sonication (5). FBPase (■, □) and inositol dehydrogenase (▴, ▵) were assayed as described previously (4,5,16).
To investigatefbp transcription by means of Northern blot and primer extension analyses, RNA was prepared as follows.B. subtilis cells (strain 1A1) were grown in NSMP medium with or without 10 mM glucose, and an 80-ml sample was harvested at an OD600 of 1. The RNA was then purified by a modified version of a procedure described elsewhere (15), using glass beads for cell disruption (9). A 0.2-ml portion of TE buffer (10 mM Tris-Cl [pH 7.4] and 1 mM Na-EDTA) containing 10 mM sodium iodoacetate was added to suspend the cell pellet. The cell suspension was mixed with 0.5 ml of 0.5-mm-diameter glass beads, 0.4 ml of phenol, and 0.8 ml of detergent solution (0.6% cetyltrimethylammonium bromide, 50 mM sodium acetate, 1 mM dithiothreitol), and then the cells were lysed by vigorous shaking with a Mini-Beadbeater (Biospec Products) for 1.5 min at 65°C. Following reextraction of the aqueous phase with phenol-chloroform-isoamyl alcohol (125/24/1, by volume), crude RNA was recovered by precipitation with an equal volume of isopropanol in the presence of 0.3 M sodium acetate at 4°C for 5 min. During incubation of crude RNA in 1 M sodium acetate at −20°C for 1 h, RNA was reprecipitated, and the RNA pellet was washed thoroughly with ethanol. The final RNA was dissolved in 50 μl of water containing 10 units of ribonuclease inhibitor (Bethesda Research Laboratories).
Thefbp gene is supposed to be monocistronic, because the direction offbp transcription is opposite that of the neighboring genes (Fig.1 and2). To confirm this, we determined the sizes of thefbp transcripts by Northern blotting (Fig.4A), using a32P-labeled PCR product (nt −58 to +263) as a DNA probe. We detected two transcripts which were 2.4 and 2.2 kb in size, with the 2.4-kb one being dominant (Fig.4A). There was no significant difference between the amounts of the two transcripts in the cells grown with and without glucose (Fig.4A).
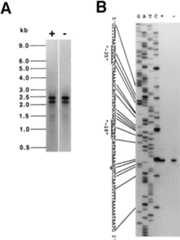
FIG. 4.
Analysis of thefbp transcript. (A) Northern blot analysis. Northern blotting was performed essentially as described previously (17,18), using the DNA probe (nt −58 to +263) and total RNA samples prepared from the cells grown in the presence (+) and absence (−) of glucose as described in the text. The positions of the RNA size markers are indicated to the left of the panel. (B) Mapping of the 5′ end of the transcript. Primer extension was carried out essentially as described previously (17,18), using total RNAs prepared from the cells grown in the presence (+) and absence (−) of glucose and the Pex primer (Fig.2). The dideoxy cycle sequencing ladders (lanes G, A, T, and C) were created with the same primer using the PCR product (nt −543 to +326), which had been amplified from strain 1A1 DNA, as the template. The part of the nucleotide sequence of the noncoding strand corresponding to these ladders is shown with the −35 and −10 regions of the putativefbp promoter (underlined); the putative transcription start nucleotide, G, is doubly underlined.
To determine the 5′ ends of the transcripts, we performed primer extension (Fig.4B) using a primer corresponding to nt +12 to +31 (Pex [Fig.2]). When32P-labeled cDNAs extended from the primer were subjected to urea-polyacrylamide gel electrophoresis, we detected only one clear band corresponding to G at nt −94 (Fig.4B). Again, there was no significant difference in the density of the bands obtained using RNAs in the cells grown with and without glucose. The transcription start site at nt −94 was preceded by the −35 and −10 sequences with significant similarity to those recognized by ςA (Fig.2 and4B).
Thefbp gene is followed by a sequence which may well be a ρ-independent transcription terminator, andfbp is located between nt +2220 and +2263 (AAAAAAGTCCATTCTA-12 bases-TAGGGTGGACCTTTTT).fbp transcription beginning at nt −94 and ending at this putative terminator will produce an approximately 2,360-nt product, the size of which is close to the size of the dominant transcript (2.4 kb) detected in Northern blotting. Presumably, the 2.2-kb transcript detected in Northern blotting is a degradation product of the 2.4-kb transcript.
We conclude from these results that thefbp gene is monocistronic, transcription of which is most likely to be initiated from G at nt −94, and thatfbp transcription is not affected by the availability of glucose in the medium.
Homology search ofB. subtilis FBPase.
We searched for proteins similar toB. subtilis FBPase in a nonredundant protein sequence database (Institute for Chemical Research, Kyoto University), using the FASTA program (14). Surprisingly, this search failed to reveal any protein exhibiting significant similarity to theB. subtilis FBPase (scores of less than 125). This indicates that theB. subtilis FBPase is unique among many FBPases in the protein sequence databases, which include those of enteric bacteria, yeasts, plants, and animals.
Although theB. subtilis FBPase showed no significant similarity to the other FBPase, these enzymes catalyze the same reaction and might exhibit small regions of homology. Therefore, local similarity betweenB. subtilis andE. coli FBPases was searched for by use of the LFASTA program (14). The resultant best score of 55 (16.1% identity in 137-amino-acid overlap) is low, so that it is very difficult to judge whether this similarity indicates any functional relationship between the two enzymes.
B. subtilis can bypass FBPase (5,6), suggesting that the enzyme involved in this bypassing might be an ortholog of FBPases in the databases. However, this possibility was eliminated by the failure of an ortholog search for FBPases of other organisms among the 4,100 proteins encoded by the completeB. subtilis 168 genome (11,12). For example, the FASTA search of aB. subtilis ortholog forE. coli FBPase gave scores of less than 113. It is quite interesting that among theB. subtilis proteins which are known or supposed to be involved in glycolysis and gluconeogenesis, only FBPase has no ortholog in the protein sequence databases at present (12). TheB. subtilis FBPase gene might have arisen by convergent evolution independently of other members of the FBPase family. However, orthologs of theB. subtilis FBPase might be found at least in theBacillus genus, because the properties of theB. licheniformis enzyme are very similar to those of theB. subtilis one (4,13).
Acknowledgments
We thank A. Yamamoto and H. Nakahara for their help in the experiments. We are also grateful to N. Ogasawara and H. Mori for providing DNA sequence prior to publication and for performing the local homology search, respectively.
This work was supported in part by a grant, JSPS-RFTF96L00105, from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Ehrlich, S. D., and V. Vagner. Personal communication.
- 2.Fraenkel D G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967;93:1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita, Y. Unpublished data.
- 4.Fujita Y, Freese E. Purification and properties of fructose-1,6-bisphosphatase of Bacillus subtilis. J Biol Chem. 1979;254:5340–5349. [PubMed] [Google Scholar]
- 5.Fujita Y, Freese E. Isolation and properties of a Bacillus subtilis mutant unable to produce fructose-bisphosphatase. J Bacteriol. 1981;145:760–767. doi: 10.1128/jb.145.2.760-767.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita Y, Fujita T. Genetic analysis of a pleiotropic deletion mutation (Δigf) in Bacillus subtilis. J Bacteriol. 1983;154:864–869. doi: 10.1128/jb.154.2.864-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita Y, Fujita T. Effect of mutations causing gluconate kinase or gluconate permease deficiency on expression of the Bacillus subtilis gnt operon. J Bacteriol. 1989;171:1751–1754. doi: 10.1128/jb.171.3.1751-1754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita Y, Shindo K, Miwa Y, Yoshida K. Bacillus subtilis inositol dehydrogenase-encoding gene (idh): sequence and expression in Escherichia coli. Gene. 1991;108:121–125. doi: 10.1016/0378-1119(91)90496-x. [DOI] [PubMed] [Google Scholar]
- 9.Igo M M, Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986;191:615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- 10.Kasahara Y, Nakai S, Ogasawara N. Sequence analysis of the 36-kb region between gntZ and trnY genes of Bacillus subtilis genome. DNA Res. 1997;4:155–159. doi: 10.1093/dnares/4.2.155. [DOI] [PubMed] [Google Scholar]
- 11.Kunst F, Ogasawara N, et al. The complete genome sequence of the Gram-positive bacterium, Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 12.Ogiwara, A. Personal communication.
- 13.Opheim D J, Bernlohr R W. Purification and regulation of fructose-1,6-bisphosphatase from Bacillus licheniformis. J Biol Chem. 1975;250:3024–3033. [PubMed] [Google Scholar]
- 14.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pujic P. Etude systématique du chromosome de Bacillus subtilis. Thèse de Doctorat. Paris, France: Université Paris 7; 1997. [Google Scholar]
- 16.Ramaley R, Fujita Y, Freese E. Purification and properties of Bacillus subtilis inositol dehydrogenase. J Biol Chem. 1979;254:7684–7690. [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Yoshida K, Aoyama D, Ishio I, Shibayama T, Fujita Y. Organization and transcription of the myo-inositol operon, iol, of Bacillus subtilis. J Bacteriol. 1997;179:4591–4598. doi: 10.1128/jb.179.14.4591-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida K, Sano H, Miwa Y, Ogasawara N, Fujita Y. Cloning and nucleotide sequencing of a 15 kb region of the Bacillus subtilis genome containing the iol operon. Microbiology. 1994;140:2289–2298. doi: 10.1099/13500872-140-9-2289. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Seki S, Fujimura M, Miwa Y, Fujita Y. Cloning and sequencing of a 36-kb region of the Bacillus subtilis genome between the gnt and iol operons. DNA Res. 1995;2:61–69. doi: 10.1093/dnares/2.2.61. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Shindo K, Sano H, Seki S, Fujimura M, Yanai N, Miwa Y, Fujita Y. Sequencing of a 65 kb region of the Bacillus subtilis genome containing the lic and cel loci, and creation of a 177 kb contig covering the gnt-sacXY region. Microbiology. 1996;142:3113–3123. doi: 10.1099/13500872-142-11-3113. [DOI] [PubMed] [Google Scholar]
- 22.Yu M T, Kaney A R, Atwood K C. Genetic mapping of fructose-1,6-diphosphatase mutants in Escherichia coli. J Bacteriol. 1965;90:1150–1152. doi: 10.1128/jb.90.4.1150-1152.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]