WO2025147390A1 - Entangled hydrogel network - Google Patents
Entangled hydrogel networkDownload PDFInfo
- Publication number
- WO2025147390A1 WO2025147390A1PCT/US2024/060731US2024060731WWO2025147390A1WO 2025147390 A1WO2025147390 A1WO 2025147390A1US 2024060731 WUS2024060731 WUS 2024060731WWO 2025147390 A1WO2025147390 A1WO 2025147390A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- null
- polymer
- ionically charged
- hydrocarbyl group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/28—Materials for coating prostheses
- A61L27/34—Macromolecular materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/52—Hydrogels or hydrocolloids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/20—Materials or treatment for tissue regeneration for reconstruction of the heart, e.g. heart valves
Definitions
- ENTANGLED HYDROGEL NETWORKS CROSS-REFERENCE TO RELATED APPLICATION[0001] This application claims the benefit of U.S. Application No.63/617,620, filed December 4, 2024, the entire disclosure which is incorporated by reference for all purposes.
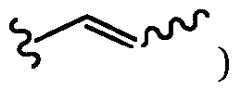
- FIELD[0002] The present application concerns aspects of chemical compositions that can be used in implantable medical devices. More specifically, the present application relates to entangled hydrogel networks for implantable medical devices to reduce or prevent tissue overgrowth and/or biofouling in implantable medical devices.
- BACKGROUND[0003] The heart can suffer from various valvular diseases or malformations that result in significant malfunctioning of the heart and ultimately require the replacement of the native heart valve with an artificial valve.
- Human heart valveswhich include the aortic, pulmonary, mitral, and tricuspid valves, function essentially as one-way valves operating in synchronization with the pumping heart.
- the valvesallow blood to flow downstream but block blood from flowing upstream.
- Diseased heart valvesexhibit impairments such as narrowing of the valve or regurgitation, which inhibits the valves’ ability to control blood flow.
- Such impairmentsreduce the heart’s blood-pumping efficiency and can be a debilitating and life-threatening condition.
- valve insufficiencycan lead to conditions such as heart hypertrophy and dilation of the ventricle.
- extensive effortshave been made to develop methods and apparatuses to repair or replace impaired heart valves.
- Prosthesesexist to correct problems associated with impaired heart valves.
- mechanical and tissue-based heart valve prosthesescan be used to replace impaired native heart valves.
- substantial efforthas been dedicated to developing replacement heart valves, particularly tissue-based replacement heart valves that can be delivered with less trauma to the patient than through open-heart surgery.
- Replacement valvesare being designed to be delivered through minimally invasive procedures and even percutaneous procedures.
- Such replacement valvesoften include a tissue-based valve body that is connected to an expandable frame that is then delivered to the native valve’s annulus.
- a replacement valvee.g., any foreign body
- different surfaces of the valvecan adsorb various proteins and ultimately elicit a foreign body response leading to cellular accumulation and tissue growth around the valve.
- the overgrowth of various tissuescan lead to structural valve deterioration and reduced implant effectiveness.
- transcatheter valvesexhibit faster leaflet encapsulation and tissue overgrowth.
- surface chemistrycontrols cell adhesion, modulates cell- cell interaction and cellular functions.
- Non-hemolytic polymer coatingscan be used to encourage endothelialization and promote anticoagulation which will lead to less tissue overgrowth over time.
- alkylis a subset of “alkyl” and refers to an alkyl group substituted by an aryl group, wherein the point of attachment is on the alkyl moiety.
- heteroarylrefers to a radical of a 5–14 membered monocyclic or polycyclic (e.g., bicyclic, tricyclic) 4n+2 aromatic ring system (e.g., having 6, 10, or 14 ⁇ electrons shared in a cyclic array) having ring carbon atoms and 1–4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur (“5–14 membered heteroaryl”).
- R 1ais O-CH2CH2N(CH3)2. In other implementations R 1a is O-(2-ethylhexyl).
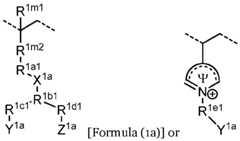
- the first ionically charged polymerincludes repeating units of formula (1a), optionally in combination with repeating units of formula (1e) and/or formula (1f). In some implementations the first ionically charged polymer includes repeating units of formula (1b), optionally in combination with repeating units of formula (1e) and/or formula (1f). In certain implementations the first ionically charged polymer includes repeating units of formula (1a) and formula (1b), optionally in combination with repeating units of formula (1e) and/or formula (1f).
- the first ionically charged polymerincludes repeating units of formula (1a) and formula (1c), optionally in combination with repeating units of formula (1e) and/or formula (1f).
- the first ionically charged polymerincludes repeating units having the formula: wherein R 1a1 is (CH 2 CH 2 O) n , R 1d1 is null, and Z 1a is N + (CH 3 ) 3 , in combination with 2- units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof.
- the first ionically charged polymercontains units having the above formula in combination with 2-ethylhexyl methacrylate units and N,N- dimethylaminoethyl methacrylate units.
- the first ionically charged polymerincludes 2- methacryloyloxyethyl phosphorylcholine (MPC) units, sulfobetaine methacrylate (SBMA) units, carboxybetaine methacrylate (CBMA) units, or any combination thereof, optionally in combination with 2-ethylhexyl methacrylate units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof.
- MPC2- methacryloyloxyethyl phosphorylcholine
- SBMAsulfobetaine methacrylate
- CBMAcarboxybetaine methacrylate
- the repeating unit of formula (2d)may include repeating units having the formula: , or a [0128]
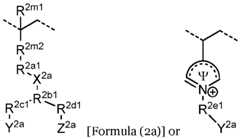
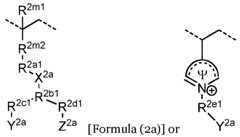
- X 2bis N + (CH3)2, and Y 2b is not H.
- X 2bis -O-PO 2 – -O-, and Y 2b is H.
- the repeating unit of formula (2c)includes units having the formula: , R 2f2 is O or NH; Y 2b is carboxylate; sulfonate, or phosphonate; and Z 2b is H or -N + (R n1 ) 3 .
- the repeating unit of formula (2c)includes units having the formula: , or a implementations n is 2.
- the second ionically charged polymercan include neutral repeating units of vinyl alcohol, formula (2e), formula (2f), or a combination thereof: [ Formula (1f)], wherein R 2m5 is H or CH3; R 2m6 is H or CH3; and R 2a is OR 2b or N(R 2b ) 2 , wherein R 2b is in each case independently selected from H, C 1– 12 hydrocarbyl, wherein R 2b may be substituted one or more times by OH, OC1–6 alkyl, NH 2 , NH(C 1–6 alkyl), or N(C 1–6 alkyl) 2 .
- R 2ais O-CH2CH2N(CH3)2. In other implementations R 2a is O-(2-ethylhexyl).
- the second ionically charged polymerincludes repeating units of formula (2a), optionally in combination with repeating units of formula (2e) and/or formula (2f). In some implementations the second ionically charged polymer includes repeating units of formula (2b), optionally in combination with repeating units of formula (2e) and/or formula (2f). In certain implementations the second ionically charged polymer includes repeating units of formula (2a) and formula (2b), optionally in combination with repeating units of formula (2e) and/or formula (2f).
- the second ionically charged polymerincludes repeating units of formula (2a) and formula (2c), optionally in combination with repeating units of formula (2e) and/or formula (2f).
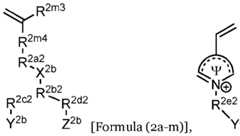
- the second ionically charged polymerincludes repeating units having the formula: wherein R 2a2 is (CH2CH2O)n, R 2c2 is null, and Y 2b is sulfonate, carboxylate or phosphonate, with 2-ethylhexyl methacrylate units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof.
- the repeating unit of formula (Xd)may include repeating units having the formula: , or a [0150]
- X Ybis N + (CH 3 ) 2 , and Y Yb is not H.
- X Ybis -O-PO2 – -O-, and Y Yb is H.
- the repeating unit of formula (Xc)includes units having the formula: , R Yf2 is O or NH; Y Yb is carboxylate; sulfonate, or phosphonate; and Z Yb is H or -N + (R n1 ) 3 .
- the repeating unit of formula (Xc)includes units having the formula: , or a implementations n is 2.
- the brush polymercan include neutral repeating units of vinyl alcohol, formula (Xe), formula (Xf), or a combination thereof: [Formula (Xf)], wherein R Ym5 is H or CH 3 ; R Ym6 is H or CH3; and R Ya is OR Yb or N(R Yb )2, wherein R Yb is in each case independently selected from H, C1– 12 hydrocarbyl, wherein R Yb may be substituted one or more times by OH, OC1–6 alkyl, NH 2 , NH(C 1–6 alkyl), or N(C 1–6 alkyl) 2 .
- R Yais O-CH 2 CH 2 N(CH 3 ) 2 . In other implementations R Ya is O-(2-ethylhexyl).
- the brush polymeris a poly(2-acrylamido-2-methyl- 1-propanesulfonic acid) (“PAAMPS”).
- PAAMPSpoly(2-acrylamido-2-methyl- 1-propanesulfonic acid)
- the first ionically charged polymeris a copolymer of (2-acrylamido-2-methyl-1-propanesulfonic acid) and vinyl alcohol (“PAAMPS-PVA”).
- the second ionically charged polymerincludes repeating units of formula (Xa) and formula (Xb), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In some implementations the second ionically charged polymer includes repeating units of formula (Xa) and formula (2c), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In certain implementations, the brush polymer includes repeating units of formula (Xe) and/or formula (Xf), and does not include repeating units of (Xa), (Xb), (Xc), or (Xd).
- the first ionically charged polymeris one of poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)], poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), a combination thereof, or a copolymer thereof.
- the first ionically charged polymeris a copolymer including the aforementioned ionic component in combination with polyvinyl alcohol, polyvinylpyrrolidone, or a combination thereof.
- the second ionically charged polymeris one of poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)], poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), a combination thereof, or a copolymer thereof.
- the brush charged polymeris a copolymer including the aforementioned ionic component in combination with polyvinyl alcohol, polyvinylpyrrolidone, or a combination thereof.
- the first ionically charged polymeris poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)],
- the second ionically charged polymeris poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), and the brush polymer is poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate).
- the entangled hydrogel networkcan be prepared by forming the second ionically charged polymer in the presence of the first ionically charged polymer, wherein the first ionically charged polymer can be crosslinked.
- the first ionically charged polymeris combined with second ionically charged polymer-precursor(s) in an aqeuous solvent.
- the compound of formula 2a-m, formula 2b-m, or combination thereofis polymerized in the presence of a crosslinker.
- exemplary crosslinkinersinclude bis(meth)acrylates, bis(meth)acrylamides, diimides, and divinyl compounds.
- the compound of formula 2a-m, formula 2b-m, or combination thereofis polymerized in the absence of a crosslinker, and then crosslinked in a separate step following formation of the second ionically charged polymer.
- the compound of formula 2a-m, formula 2b-m, or combination thereofis polymerized in the presence of an initiator.
- the initiatorcan be a photoinitiator or a thermal initiator.
- photoinitiatorsinclude: acetophenone, 2- phenylacetophenone, 2-hydroxy-2-methylpro piophenone, 4-hydroxybenzophenone, 4,4- dihydroxybenzophenone, 4-(dimethylamino) benzophenone 4-ethoxyacetophenone, 4- phenoxyacetophenone, hexafluorophosphate, anthraquinone, anthraquinone-2-sulfonic acid, sodium salt monohydrate; tricarbonylchromium, benzoin based initiators, benzoin methyl ether, benzoin isobutyl ether, 2-hydroxy-4-(2-hydroxy ethoxy )-2- methylpropiophenone, benzil ketal based initiators dialkoxyacetophenone, hydroxyphenone, phenyl ketone, aminoalkylphenone, acylphosphine oxide, benzophenone, thioxanthone, azobisisobutyronitrile, lithium phenyl
- the initiator in the prepolymer solutionranges between 0.01 wt % to 10 wt %.
- the compound of formula 2a-m, formula 2b-m, or combination thereofis polymerized by exposure to actinic radiation, for example UV irradiation.
- the polymerizationcan be carried out in solution with a 100 to 300:1:1 mol ratio between the monomer(s), initiator and the catalyst, respectively. In a typical synthesis, a flask can be charged with catalyst, then monomer and solvent. In some implementations, the solution is degassed and second monomer is added. In some implementations the molar ratio of the different monomers is from 1:5 to 1:3.
- the reaction mixturemaintained at a temperature between 50–100 °C for 1–24 hours.
- the final polymeris poured into different solvent, filtered and finally purified through column chromatography/filtration (e.g., through Florisil® magnesium silicate) with another solvent.
- the first ionically charged polymer (whether crosslinked or not) and compound of formula 2a-m, formula 2b-m, or combination thereofcan be provided in a reaction media (e.g., a solution or suspension with one or more solvents), or the first ionically charged polymer and compound of formula 2a-m, formula 2b-m, or combination thereof (and optional crosslinking agent) is disposed as a film on the surface of valve component, and then polymerized.
- a reaction mediae.g., a solution or suspension with one or more solvents
- the resulting entangled hydrogelmay then be disposed on the surface of a valve component.
- the second ionically charged polymermay be crosslinked prior to disposition on the valve surface.
- the second ionically charged polymermay be crosslinked subsequent to disposition on the valve surface.
- the second ionically charged polymeris not crosslinked.
- the compound of formula 1a-m, formula 1b-m, or combination thereofis polymerized in the presence of a crosslinker, as defined above.
- the compound of formula 1a-m, formula 1b-m, or combination thereofis polymerized in the presence of an initiator, as defined above.
- compound of formula 1a-m, formula 1b-m, or combination thereofcan be provided in a reaction media (e.g., a solution or suspension with one or more solvents), or the compound of formula 1a-m, formula 1b-m, or combination thereof (and optional crosslinking agent) is disposed as a film on the surface of valve components, and then polymerized.
- the resulting first ionically charged polymermay then be disposed on the surface of a valve component.
- the first ionically charged polymermay be crosslinked prior to disposition on the valve surface.
- the first ionically charged polymermay be crosslinked subsequent to disposition on the valve surface.
- the first ionically charged polymeris not crosslinked.
- the entangled hydrogel networkis applied to a surface of a valve component by grafting the entangled hydrogel to the surface or coating the entangled hydrogel on the surface.
- conjugationsmay be accomplished using nucleophile/electrophile chemistry or cycloaddition chemistry (including click-cycloaddition chemistry).
- the conjugationis accomplished using a reaction between a first functional group selected from an alcohol, thiol, amine, or carboxylate and a second functional group selected from an isocyanate, epoxide, activated carboxylate (e.g., acid chloride, NHS ester), and a Michael acceptor (e.g., an alkene or alkyne or acetylene directly bonded to an electron withdrawing group like a ketone, ester, cyano, nitro, sulfone, or sulfonyl).
- a first functional groupselected from an alcohol, thiol, amine, or carboxylate
- a second functional groupselected from an isocyanate, epoxide, activated carboxylate (e.g., acid chloride, NHS ester)
- a Michael acceptore.g
- the conjugationis accomplished using a click chemistry: Cu(I)-catalyzed azide-alkyne click chemistry reaction, strain-promoted azide-alkyne click chemistry reaction (SPAAC), cycloaddition between tetrazine and alkene (trans-cyclooctene).
- the alkyneis a cyclooctyne like a dibenzocyclooctyne or bicyclo[6.1.0]nonyne.
- the prerequisite reactive groupmay be included in the entangled hydrogel by including the relevant monomer in the polymerization reaction.
- a brush polymercan be directly formed on the entangled hydrogel network.
- the entangled hydrogelis first functionalized with an anchor compound using the chemistries disclosed above.
- the anchor compoundinclude a polymerizable group, for example a radically labile group such as a (meth)acrylate or meth(acrylamide).
- the entangled hydrogelis functionalized with the radically labile group it can be combined with one or more appropriate monomers (e.g., (meth)acrylates, (meth)acylamides, and/or vinyl compounds) and subjected to conditions suitable to polymerize the monomers.
- the brush polymeris not covalently conjugated to the entangled hydrogel, but rather mechanically conjugated.
- a portion of the brush polymeris physically entangled with the entangled network.
- the entangled hydrogelmay be swelled in a solution including the brush polymer. A portion of the brush polymer will penetrate the hydrogel.
- various surfactants, plasticizers, and the likemay be included in the solvent to facilitate penetration (a penetration aid) of the brush polymer.
- the penetration aidcan include polyethylene glycol, polypropylene oxide, pectin, carrageenan, polylysine, gelatins (including gelatin type A), agarose, PEO-PPO-PEO copolymers (Pluronics® polymers), poly(phosphazene), hyaluronans, chitosans, agar, heparin, sulfate, cellulose, alginates (including alginate sulfate), collagen, dextrans (including dextran sulfate), poly(hydroxyethyl methacrylate), (poly(methyl methacrylate, poly(N-isopropylacrylamide), poly(lactic acid), poly(lactic-co-glycolic acid), poly(N- vinylpyrrolidone), PL(G)A-PEO-PL(G)
- the exemplary implantable medical devicecan comprise a frame 1000, as shown in FIG.1.
- the frame 1000is shown in an expanded configuration.
- the frame 1000can include a frame portion 1002.
- frame portion 1002will include an entangled hydrogel network.
- Frame portion 1002can include an inner frame side 1002a and an outer frame side 1002b.
- inner frame side 1002acan include an entangled hydrogel network, while outer frame side 1002b does not.
- outer frame side 1002bcan include an entangled hydrogel network, while inner frame side 1002a does not.
- both inner frame side 1002a and outer frame side 1002bcan include an entangled hydrogel network.
- inner frame side 1002a and outer frame side 1002bcan include the same entangled hydrogel network, while in other implementations inner frame side 1002a will include a different entangled hydrogel network than outer frame side 1002b.
- inner skirt 2016includes an entangled hydrogel network.
- Inner skirt 2016can have an inner side 2016a and an outer side 2016b.
- inner skirt, inner side 2016aincludes an entangled hydrogel network.
- inner skirt, outer side 2016bincludes an entangled hydrogel network.
- both inner skirt, inner side 2016a and inner skirt, outer side 2016bcan include an entangled hydrogel network.
- inner skirt, inner side 2016a and inner skirt, outer side 2016bcan include the same entangled hydrogel network.
- inner skirt, inner side 2016a and inner skirt, outer side 2016bcan include different entangled hydrogel networks.
- the implantable medical devices disclosed hereincan comprise a mitral replacement valve or a tricuspid replacement valve, among other forms of valves (e.g., aortic replacement valves, pulmonary replacement valves, or other valves).
- the implantable medical devices disclosed hereincan include prosthetic heart valves or other forms of implants, such as stents or filters or diagnostic devices, among others.
- the implantable medical devicescan be expandable implants configured to move from a compressed or undeployed state to an expanded or deployed state.
- the implantable medical devicescan be compressible implants configured to be compressed inward to have a reduced outer profile and to move the implant to the compressed or undeployed state.
- Various forms of delivery apparatusescan be utilized with the examples disclosed herein.
- Example 13The device of any example herein, particularly Examples 1–12, wherein the outer surface of the inner skirt comprises the entangled hydrogel network, and the inner surface of the inner skirt does not comprise the entangled hydrogel network.
- Example 14The device of any example herein, particularly Examples 1–13, wherein the inner surface and outer surface of the inner skirt comprise the entangled hydrogel network.
- Example 15The device of any example herein, particularly Examples 1–14, wherein the inner surface of the frame comprises the entangled hydrogel network.
- Example 16The device of any example herein, particularly Examples 1–15, wherein the inner surface of the frame comprises the entangled hydrogel network, and the outer surface of the frame does not comprise the entangled hydrogel network.
- Example 17The device of any example herein, particularly Examples 1–16, wherein the outer surface of the frame comprises the entangled hydrogel network.
- Example 18The device of any example herein, particularly Examples 1–17, wherein the outer surface of the frame comprises the entangled hydrogel network, and the inner surface of the frame does not comprise the entangled hydrogel network.
- Example 19The device of any example herein, particularly Examples 1–18, wherein the inner surface and outer surface of the frame comprise the entangled hydrogel network.
- Example 23The device of any example herein, particularly Examples 1–22, wherein the outer surface of the outer skirt comprises the entangled hydrogel network, and the inner surface of the outer skirt does not comprise the entangled hydrogel network.
- Example 24The device of any example herein, particularly Examples 1–23, wherein the inner surface and outer surface of the outer skirt comprise the entangled hydrogel network.
- Example 25The device of any example herein, particularly Examples 1–24, wherein the outer surface of the frame comprises a covered portion covered by the outer skirt, and an exposed portion that is not covered by the outer skirt.
- Example 26The device of any example herein, particularly Examples 1–25, wherein the exposed portion of the outer surface of the frame comprises the entangled hydrogel network.
- Example 27The device of any example herein, particularly Examples 1–26, wherein the inner surface of the frame comprises the entangled hydrogel network, and the outer surface of the frame does not comprise the entangled hydrogel network.
- Example 28The device of any example herein, particularly Examples 1–27, wherein each leaflet comprises the entangled hydrogel network.
- Example 29The device of any example herein, particularly Examples 1–28, wherein the leaflet structure comprises two or three leaflets.
- Example 30The device of any example herein, particularly Examples 1–29, wherein the leaflet structure comprises pericardial tissue (e.g., bovine pericardial tissue), biocompatible synthetic polymers (e.g., polyurethane, polyethylene terephthalate), or a combination thereof.
- pericardial tissuee.g., bovine pericardial tissue
- biocompatible synthetic polymerse.g., polyurethane, polyethylene terephthalate
- Example 31The device of any example herein, particularly Examples 1–30, wherein the frame comprise a metal (pure titanium, cobalt–chromium-nickel or molybdenum alloys such as Elgiloy® alloy, stainless steel (316L), nickel ⁇ titanium alloy (nitinol), platinum, and tantalum alloys or magnesium alloys, a metal-based frame with ceramic-coated, or a combination thereof.
- metalpure titanium, cobalt–chromium-nickel or molybdenum alloys such as
- Example 32The device of any example herein, particularly Examples 1–31, wherein the frame comprises nickel-titanium alloy, cobalt-chromium alloy, nickel-cobalt- chromium alloy, nickel-cobalt-chromium-molybdenum alloy, stainless steel, [0230]
- Example 33The device of any example herein, particularly Examples 1–32, wherein the skirt comprises of the following polymer yarns that can be constructed into textile (wovens or knit warps) such as polyhydroxyalkanoates (PHAs) and polyethylene furanoate (PEF) polyethylene terephthalate (PET) polyamides of high molecular weight such as Nylon 6, 6,6, 12, 610, 1010.
- PHAspolyhydroxyalkanoates
- PEFpolyethylene furanoate
- PETpolyethylene terephthalate
- Example 34The device of any example herein, particularly Examples 1–33, wherein the inner skirt is sutured to the frame with sutures, wherein said sutures comprise an ionically charged polymer coating.
- Example 35The device of any example herein, particularly Examples 1–34, comprising a first entangled hydrogel network and a second entangled hydrogel network, wherein the inner surface of the frame comprises the first entangled hydrogel network, and the inner skirt comprises the second entangled hydrogel network.
- Example 36The device of any example herein, particularly Examples 1–35, wherein the first entangled hydrogel network is mechanically bonded to the frame, and the second entangled hydrogel network is covalently bonded to the inner skirt.
- Example 37The device of any example herein, particularly Examples 1–36, wherein the entangled hydrogel network is covalently incorporated into the polymeric structure or attached to the surface.
- Example 38The device of any example herein, particularly Examples 1–37, wherein the entangled hydrogel network is mechanically bonded to the surface.
- Example 39The device of any example herein, particularly Examples 1–38, wherein the entangled hydrogel network has a thickness from 10 nm – 100 ⁇ m, from 10 nm – 1,000 nm, from 10 nm – 500 nm, from 50 nm – 500 nm, from 50 nm – 250 nm, from 250 nm – 500 nm, from 500 nm – 2,500 nm, from 500 nm – 1,500 nm, from 1 ⁇ m – 100 ⁇ m, from 1 ⁇ m – 5 ⁇ m, from 1 ⁇ m – 10 ⁇ m, from 5 ⁇ m – 15 ⁇ m, from 10 ⁇ m – 25 ⁇ m, from 10 ⁇ m – 50 ⁇ m, from 25 ⁇ m – 50 ⁇ m, from 50 ⁇ m – 75 ⁇ m, or from 75 ⁇ m – 100 ⁇ m.
- Example 40The device of any example herein, particularly Examples 1–39, wherein the entangled hydrogel network comprises a first ionically charged polymer and a second ionically charged polymer.
- Example 41The device of any example herein, particularly Examples 1–40, wherein the entangled hydrogel network comprises a first ionically charged polymer, a second ionically charged polymer, and a third ionically charged polymer.
- Example 42The device of any example herein, particularly Examples 1–41, wherein the first ionically charged polymer is a crosslinked polymer or is not a crosslinked polymer.
- Example 43The device of any example herein, particularly Examples 1–42, wherein the first ionically charged polymer comprises a backbone and one or more side chains.
- Example 44The device of any example herein, particularly Examples 1–43, wherein the first ionically charged polymer is a homopolymer or copolymer.
- Example 45The device of any example herein, particularly Examples 1–44, wherein the first ionically charged polymer is a cationic polymer, anionic polymer, or zwitterionic polymer.
- Example 46The device of any example herein, particularly Examples 1–45, wherein the first ionically charged polymer has an ionically charged polymer backbone.
- Example 47The device of any example herein, particularly Examples 1–46, wherein the backbone of the first ionically charged polymer comprises quaternary ammonium groups.
- Example 48The device of any example herein, particularly Examples 1–47, wherein the backbone of the first ionically charged polymer comprises phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof.
- Example 49The device of any example herein, particularly Examples 1–48, wherein the first ionically charged polymer comprises a polyalkylene backbone, a polyether backbone, a polyurethane backbone, or combination thereof.
- Example 50The device of any example herein, particularly Examples 1–49, wherein the first ionically charged polymer comprises a neutral polymer backbone and ionically charged side chains.
- Example 51The device of any example herein, particularly Examples 1–50, wherein the first ionically charged polymer comprises a neutral polymer backbone and a combination of ionically charged side chains and neutral side chains.
- Example 52The device of any example herein, particularly Examples 1–51, wherein the first ionically charged polymer is a random copolymer.
- Example 53The device of any example herein, particularly Examples 1–52, wherein the first ionically charged polymer comprises a zwitterionic side chains and neutral side chains.
- Example 54The device of any example herein, particularly Examples 1–53, wherein the first ionically charged polymer comprises cationic side chains and anionic side chains.
- Example 55The device of any example herein, particularly Examples 1–54, wherein the first ionically charged polymer comprises a poly(meth)acrylate, a poly(meth)acrylamide, a polyolefin, or a combination thereof.
- Example 56The device of any example herein, particularly Examples 1–55, wherein the first ionically charged polymer comprises side chains comprising phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof.
- Example 58The device of any example herein, particularly Examples 1–57, wherein the first repeating unit in the first ionically charged polymer includes units having the formula: , or a [0256]
- Example 59The device of any example herein, particularly Examples 1–58, X 1a is N + (CH3)2, and Y 1a is not H.
- Example 60The device of any example herein, particularly Examples 1–58, X 1a is -O-PO2 – -O-, and Y 1a is H.
- Example 61The device of any example herein, particularly Examples 1–60, wherein the first repeating unit in the first ionically charged polymer includes units having the formula:
- Example 62The device of any example herein, particularly Examples 1–61, wherein the first repeating unit in the first ionically charged polymer includes units having the formula: , wherein R 1f1 is O
- Example 64The device of any example herein, particularly Examples 1–63, wherein the second repeating unit in the first ionically charged polymer includes units having the formula: , or a combination
- Example 65The device of any example herein, particularly Examples 1–64, X 1b is N + (CH3)2, and Y 1b is not H.
- Example 66The device of any example herein, particularly Examples 1–64, X 1b is -O-PO 2 – -O-, and Y 1b is H.
- Example 69The device of any example herein, particularly Examples 1–68, wherein the first ionically charged polymer further comprises neutral repeating units of formula (1e), formula (1f), or a combination thereof: [Formula (1f)], wherein, R 1m5 is H or CH3; R 1m6 is H or CH 3 ; and R 1a is OR 1b or N(R 1b )2, wherein R 1b is in each case independently selected from H, C1–12 hydrocarbyl, wherein R 1b may be substituted one or more times by OH, OC 1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2.
- Example 84The device of any example herein, particularly Examples 1–83, wherein the second ionically charged polymer comprises a neutral polymer backbone and ionically charged side chains.
- Example 85The device of any example herein, particularly Examples 1–84, wherein the second ionically charged polymer comprises a neutral polymer backbone and a combination of ionically charged side chains and neutral side chains.
- Example 86The device of any example herein, particularly Examples 1–85, wherein the second ionically charged polymer is a random copolymer.
- Example 147The method of any example herein, particularly Examples 145–146, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof further comprises a crosslinker, or does not include a crosslinker.
- Example 148The method of any example herein, particularly Examples 145–147, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof further comprises a crosslinker.
- Example 149The method of any example herein, particularly Examples 145–148, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof comprises an initiator.
- Example 160The method of any example herein, particularly Examples 145–159, further comprising forming a brush polymer conjugated to the entangled hydrogel network.
- Example 161The method of any example herein, particularly Examples 145–160, wherein forming a brush polymer comprises: conjugating a polymer to the entangled hydrogel network; polymerizing a brush polymer precursor in the presence of the entangled hydrogel network; or a combination thereof.
- Example 162The method of any example herein, particularly Examples 145–161, wherein the entangled polymer network comprises initiator groups that are reactive with the brush polymer precursor.
- Example 163The method of any example herein, particularly Examples 145–162, wherein the initiator group comprises a radically transferrable atom, an isocyanate, a carboxylic acid, a primary amine, a thiol, a Michael acceptor, or a combination thereof.
- Example 164The method of any example herein, particularly Examples 145–163, further comprising reacting the entangled polymer network with an anchor compound comprising an initiator group or masked initiator group.
- Example 165A device prepared according to the method of any example herein, particularly Examples 145–164.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Medicinal Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Dispersion Chemistry (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
ENTANGLED HYDROGEL NETWORKS CROSS-REFERENCE TO RELATED APPLICATION [0001] This application claims the benefit of U.S. Application No.63/617,620, filed December 4, 2024, the entire disclosure which is incorporated by reference for all purposes. FIELD [0002] The present application concerns aspects of chemical compositions that can be used in implantable medical devices. More specifically, the present application relates to entangled hydrogel networks for implantable medical devices to reduce or prevent tissue overgrowth and/or biofouling in implantable medical devices. BACKGROUND [0003] The heart can suffer from various valvular diseases or malformations that result in significant malfunctioning of the heart and ultimately require the replacement of the native heart valve with an artificial valve. Human heart valves, which include the aortic, pulmonary, mitral, and tricuspid valves, function essentially as one-way valves operating in synchronization with the pumping heart. The valves allow blood to flow downstream but block blood from flowing upstream. Diseased heart valves exhibit impairments such as narrowing of the valve or regurgitation, which inhibits the valves’ ability to control blood flow. Such impairments reduce the heart’s blood-pumping efficiency and can be a debilitating and life-threatening condition. For example, valve insufficiency can lead to conditions such as heart hypertrophy and dilation of the ventricle. Thus, extensive efforts have been made to develop methods and apparatuses to repair or replace impaired heart valves. [0004] Prostheses exist to correct problems associated with impaired heart valves. For example, mechanical and tissue-based heart valve prostheses can be used to replace impaired native heart valves. More recently, substantial effort has been dedicated to developing replacement heart valves, particularly tissue-based replacement heart valves that can be delivered with less trauma to the patient than through open-heart surgery. Replacement valves are being designed to be delivered through minimally invasive procedures and even percutaneous procedures. Such replacement valves often include a tissue-based valve body that is connected to an expandable frame that is then delivered to the native valve’s annulus. [0005] Upon implantation of a replacement valve (e.g., any foreign body), different surfaces of the valve can adsorb various proteins and ultimately elicit a foreign body response leading to cellular accumulation and tissue growth around the valve. The overgrowth of various tissues can lead to structural valve deterioration and reduced implant effectiveness. In comparison with many surgical valves, transcatheter valves exhibit faster leaflet encapsulation and tissue overgrowth. [0006] It is widely accepted that surface chemistry controls cell adhesion, modulates cell- cell interaction and cellular functions. Non-hemolytic polymer coatings can be used to encourage endothelialization and promote anticoagulation which will lead to less tissue overgrowth over time. The chemical attachment and durability of those polymer coatings on the commonly used surfaces like polyester (PET), polyurethanes, PTFE and metals have been described in the scientific literature. [0007] There remains a need for improved implantable devices that are resistant to protein adsorption, tissue overgrowth, and encapsulation. There remains a need for improved implantable devices that do not trigger a foreign body response post-implantation. There remains a need for improved transcatheter valves that resist leaflet encapsulation and tissue overgrowth. There remains a need for improved implantable devices that accelerate reendothelialization post implantation. [0008] These needs and others are at least partially satisfied by the present disclosure. SUMMARY [0009] Some of the aspects of the present disclosure relate to cardiovascular repair devices including an entangled hydrogel network on at least one surface. In some instances the devices can be a prosthetic valve, a patch, a clasp, a sleeve, a conduit, or a band. [0010] The entangled hydrogel networks include two or more entangled polymers forming a hydrogel network. In some aspects, the entangled hydrogel networks include at least one ionically charged polymers. In some aspects, the entangled hydrogel networks include at least two ionically charged polymers. In further aspects, the entangled hydrogel networks include a brush polymer extending from the hydrogel core. [0011] Some aspects relate to methods of reducing foreign body response to a medical device following implantation. Some aspects relate to methods for accelerating reendothelialization post implantation. [0012] Additional aspects of the disclosure will be set forth, in part, in the detailed description, figures, and claims which follow, and in part will be derived from the detailed description or can be learned by practice of the disclosure. It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive of the disclosure as disclosed. BRIEF DESCRIPTION OF THE DRAWINGS [0013] FIGURE 1 depicts an exemplary frame according to the disclosure. [0014] FIGURE 2 depicts an exemplary valve according to the disclosure. [0015] FIGURE 3A depicts an exemplary valve according to the disclosure. [0016] FIGURE 3B depicts an exemplary valve according to the disclosure. DETAILED DESCRIPTION [0017] The present disclosure can be understood more readily by reference to the following detailed description, examples, drawings, and claims, and their previous and following description. However, before the present articles, systems, and/or methods are disclosed and described, it is to be understood that this disclosure is not limited to the specific or exemplary aspects of articles, systems, and/or methods disclosed unless otherwise specified, as such can, of course, vary. It is also to be understood that the terminology used herein is for the purpose of describing particular aspects only and is not intended to be limiting. [0018] The following description of the disclosure is provided as an enabling teaching of the disclosure in its best, currently known aspect. To this end, those skilled in the relevant art will recognize and appreciate that many changes can be made to the various aspects of the disclosure described herein while still obtaining the beneficial results of the present disclosure. It will also be apparent that some of the desired benefits of the present disclosure can be obtained by selecting some of the features of the present disclosure without utilizing other features. Accordingly, those of ordinary skill in the pertinent art will recognize that many modifications and adaptations to the present disclosure are possible and can even be desirable in certain circumstances and are a part of the present disclosure. Thus, the following description is again provided as illustrative of the principles of the present disclosure and not in limitation thereof. DEFINITIONS [0019] As used in this application and in the claims, the singular forms “a,” “an,” and “the” include the plural forms unless the context clearly dictates otherwise. Thus, for example, reference to a “polymer” includes aspects having two or more such polymer unless the context clearly indicates otherwise. [0020] It is also to be understood that the terminology used herein is for the purpose of describing particular aspects only and is not intended to be limiting. As used in the specification and in the claims, the term “comprising” can include the aspects “consisting of” and “consisting essentially of.” Additionally, the term “includes” means “comprises.” [0021] For the terms “for example,” “exemplary,” and “such as,” and grammatical equivalents thereof, the phrase “and without limitation” is understood to follow unless explicitly stated otherwise. [0022] Ranges can be expressed herein as from “about” one particular value and/or to “about” another particular value. When such a range is expressed, another aspect includes from the one particular value and/or to the other particular value. Similarly, when values are expressed as approximations, by use of the antecedent “about,” it will be understood that the particular value forms another aspect. It should be further understood that the endpoints of each of the ranges are significant both in relation to the other endpoint and independently of the other endpoint. Unless stated otherwise, the term “about” means within 5% (e.g., within 2% or 1%) of the particular value modified by the term “about.” [0023] Throughout this disclosure, various aspects of the disclosure can be presented in a range format. It should be understood that the description in range format is merely for convenience and brevity and should not be construed as an inflexible limitation on the scope of the disclosure. Accordingly, the description of a range should be considered to have specifically disclosed all the possible subranges as well as individual numerical values within that range. For example, a description of a range such as from 1 to 6 should be considered to have specifically disclosed subranges such as from 1 to 3, from 1 to 4, from 1 to 5, from 2 to 4, from 2 to 6, from 3 to 6, etc., as well as individual numbers within that range, for example, 1, 2, 2.7, 3, 4, 5, 5.3, 6 and any whole and partial increments therebetween. This applies regardless of the breadth of the range. [0024] As used herein, the terms “optional” or “optionally” mean that the subsequently described event or circumstance can or cannot occur and that the description includes instances where said event or circumstance occurs and instances where it does not. [0025] Further, the terms “coupled” and “associated” generally mean electrically, electromagnetically, and/or physically (e.g., mechanically or chemically) coupled or linked and do not exclude the presence of intermediate elements between the coupled or associated items. [0026] It will be understood that when an element is referred to as being “connected” or “coupled” to another element, it can be directly connected or coupled to the other element, or intervening elements can be present. In contrast, when an element is referred to as being “directly connected” or “directly coupled” to another element, there are no intervening elements present. Other words used to describe the relationship between elements or layers should be interpreted in a like fashion (e.g., “between” versus “directly between,” “adjacent” versus “directly adjacent,” “on” versus “directly on”). [0027] It will be understood that although the terms “first,” “second,” etc., can be used herein to describe various elements, components, regions, layers and/or sections. These elements, components, regions, layers, and/or sections should not be limited by these terms. These terms are only used to distinguish one element, component, region, layer, or section from another element, component, region, layer, or a section. Thus, a first element, component, region, layer, or section discussed below could be termed a second element, component, region, layer, or section without departing from the teachings of example aspects. A second element may exist in an embodiment that does not include a first element. [0028] Spatially relative terms, such as, “beneath,” “below,” “lower,” “above,” “upper,” “upward,” “downward,” “top,” “bottom,” and the like, can be used herein for ease of description to describe one element or feature’s relationship to another element(s) or feature(s) as illustrated in the figures. It will be understood that the spatially relative terms are intended to encompass different orientations of the device in use or operation in addition to the orientation depicted in the figures. For example, if the device in the figures is turned over, elements described as “below” or “beneath” other elements or features would then be oriented “above” the other elements or features. Thus, the term “below” can encompass both an orientation of above and below. The device can be otherwise oriented (rotated 90 degrees or at other orientations), and the spatially relative descriptors used herein are interpreted accordingly. [0029] Terms such as “proximal,” “distal,” “radially outward,” “radially inward,” “outer,” “inner,” and “side” describe the orientation and/or location of portions of the components or elements within a consistent but arbitrary frame of reference which is made clear by reference to the text and the associated drawings describing the components or elements under discussion. Such terminology can include the words specifically mentioned above, derivatives thereof, and words of similar import. Similarly, the terms “first,” “second,” and other such numerical terms referring to structures neither imply a sequence nor order unless clearly indicated by the context. [0030] As used herein, the term “and/or” includes any and all combinations of one or more of the associated listed items. It is also understood that the term “and/or” includes where one or another of the associated listed items is present and the aspects where both of the associated listed items are present or any combinations of the associated listed items are present. [0031] As used herein, the term or phrase “effective,” “effective amount,” or “conditions effective to” refers to such amount or condition that is capable of performing the function or property for which an effective amount or condition is expressed. As will be pointed out below, the exact amount or particular condition required will vary from one aspect to another, depending on recognized variables such as the materials employed and the processing conditions observed. Thus, it is not always possible to specify an exact “effective amount” or “condition effective to.” However, it should be understood that an appropriate effective amount will be readily determined by one of ordinary skill in the art using only routine experimentation. [0032] As used herein, the term “substantially” means that the subsequently described event or circumstance completely occurs or that the subsequently described event or circumstance generally, typically, or approximately occurs. [0033] Still further, the term “substantially” can, in some aspects, refer to at least about 90 %, at least about 91 %, at least about 92 %, at least about 93 %, at least about 94 %, at least about 95 %, at least about 96 %, at least about 97 %, at least about 98 %, at least about 99 %, or about 100 % of the stated property, component, composition, or other condition for which substantially is used to characterize or otherwise quantify an amount. [0034] As used herein, the term “substantially,” in, for example, the context “substantially identical” or “substantially similar,” refers to a method or a system, or a component that is at least about 90%, at least about 91%, at least about 92%, at least about 93%, at least about 94%, at least about 95%, at least about 96%, at least about 97%, at least about 98%, at least about 99%, or about 100% by similar to the method, system, or the component it is compared to. [0035] As used herein “(meth)acrylic acid” covers both methacrylic acid and acrylate acid. The same convention is used for (meth)acrylates and (meth)acrylamides. [0036] As used herein “ionically charged” indicates the designated compound or functional groups is characterized by one or more cationically or anionically charged atoms. As used herein, a zwitterionic group (having both cationically and anionically charged atoms) is considered ionically charged even though the net charge might be zero. [0037] The term “alkyl” refers to a radical of a straight-chain or branched hydrocarbon group having a specified range of carbon atoms (e.g., a “C1–16 alkyl” can have from 1 to 16 carbon atoms). An alkyl group can be saturated or unsaturated, e.g., an alkenyl or alkynyl group. Unless specified to the contrary, an “alkyl” group includes both saturated alkyl groups and unsaturated alkyl groups. [0038] When a range of values is listed, it is intended to encompass each value and sub- range within the range. For example, “C1–6 alkyl” is intended to encompass C1, C2, C3, C4, C5, C6, C1–6, C1–5, C1–4, C1–3, C1–2, C2–6, C2–5, C2–4, C2–3, C3–6, C3–5, C3–4, C4–6, C4–5, and C5–6 alkyl. [0039] The term “alkoxy” refers to an alkyl group, as defined herein, appended to the parent molecular moiety through an oxygen atom. [0040] The term “heteroalkyl” refers to an alkyl group, which further includes at least one heteroatom (e.g., 1, 2, 3, or 4 heteroatoms) selected from oxygen, nitrogen, or sulfur within (e.g., inserted between adjacent carbon atoms of) and/or placed at one or more terminal position(s) of the parent chain. By way of example, a C1–6 heteroalkyl) group includes, but is not limited to, the following structures: . group includes, but it not limited to, the following structures: . group one or more or . Unless otherwise specified, each instance of an alkenyl group is independently unsubstituted (an “unsubstituted alkenyl”) or substituted (a “substituted alkenyl”) with one or more substituents. In an alkenyl group, a C=C double bond for which the stereochemistry is not specified (e.g., -CH=CHCH3 or ) may be an (E)- or (Z)-double bond. [0043] The term “alkynyl” of a straight-chain or branched hydrocarbon group having one or more carbon-carbon triple bonds (e.g., 1, 2, 3, or 4 triple bonds). Unless otherwise specified, each instance of an alkynyl group is independently unsubstituted (an “unsubstituted alkynyl”) or substituted (a “substituted alkynyl”) with one or more substituents. A terminal alkynyl group indicates the group includes the moiety: -C≡CH. [0044] The term “carbocyclyl,” “cycloalkyl,” or “carbocyclic” refers to a radical of a non- aromatic cyclic hydrocarbon group. A carbocyclyl group can either be monocyclic (“monocyclic carbocyclyl”) or polycyclic (e.g., a fused, bridged or spiro ring system such as a bicyclic system (“bicyclic carbocyclyl”) or tricyclic system (“tricyclic carbocyclyl”)), and can be saturated or can contain one or more carbon-carbon double or triple bonds. [0045] The term “heterocyclyl” refers to a ring system that includes at least one heteroatom in the cycle. A heterocyclyl group can either be monocyclic (“monocyclic heterocyclyl”) or polycyclic (e.g., a fused, bridged or spiro ring system such as a bicyclic system (“bicyclic heterocyclyl”) or tricyclic system (“tricyclic heterocyclyl”)), and can be saturated or can contain one or more carbon-carbon double or triple bonds. Heterocyclyl polycyclic ring systems can include one or more heteroatoms in one or both rings. “Heterocyclyl” also includes ring systems wherein the heterocyclyl ring, as defined above, is fused with one or more carbocyclyl groups wherein the point of attachment is either on the carbocyclyl or heterocyclyl ring, or ring systems wherein the heterocyclyl ring, as defined above, is fused with one or more aryl or heteroaryl groups, wherein the point of attachment is on the heterocyclyl ring, and in such instances, the number of ring members continue to designate the number of ring members in the heterocyclyl ring system. Unless otherwise specified, each instance of heterocyclyl is independently unsubstituted (an “unsubstituted heterocyclyl”) or substituted (a “substituted heterocyclyl”) with one or more substituents. [0046] The term “aryl” refers to a radical of a monocyclic or polycyclic (e.g., bicyclic or tricyclic) 4n+2 aromatic ring system (e.g., having 6, 10, or 14 π electrons shared in a cyclic array) having 6–14 ring carbon atoms and zero heteroatoms provided in the aromatic ring system (“C6–14 aryl”). “Aryl” also includes ring systems wherein the aryl ring, as defined above, is fused with one or more carbocyclyl or heterocyclyl groups wherein the radical or point of attachment is on the aryl ring, and in such instances, the number of carbon atoms continue to designate the number of carbon atoms in the aryl ring system. Unless otherwise specified, each instance of an aryl group is independently unsubstituted (an “unsubstituted aryl”) or substituted (a “substituted aryl”) with one or more substituents. [0047] “Aralkyl” is a subset of “alkyl” and refers to an alkyl group substituted by an aryl group, wherein the point of attachment is on the alkyl moiety. [0048] The term “heteroaryl” refers to a radical of a 5–14 membered monocyclic or polycyclic (e.g., bicyclic, tricyclic) 4n+2 aromatic ring system (e.g., having 6, 10, or 14 π electrons shared in a cyclic array) having ring carbon atoms and 1–4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur (“5–14 membered heteroaryl”). In heteroaryl groups that contain one or more nitrogen atoms, the point of attachment can be a carbon or nitrogen atom, as valency permits. Heteroaryl polycyclic ring systems can include one or more heteroatoms in one or both rings. “Heteroaryl” includes ring systems wherein the heteroaryl ring, as defined above, is fused with one or more carbocyclyl or heterocyclyl groups wherein the point of attachment is on the heteroaryl ring, and in such instances, the number of ring members continue to designate the number of ring members in the heteroaryl ring system. “Heteroaryl” also includes ring systems wherein the heteroaryl ring, as defined above, is fused with one or more aryl groups wherein the point of attachment is either on the aryl or heteroaryl ring, and in such instances, the number of ring members designates the number of ring members in the fused polycyclic (aryl/heteroaryl) ring system. Polycyclic heteroaryl groups wherein one ring does not contain a heteroatom (e.g., indolyl, quinolinyl, carbazolyl, and the like) the point of attachment can be on either ring, e.g., either the ring bearing a heteroatom (e.g., 2-indolyl) or the ring that does not contain a heteroatom (e.g., 5-indolyl). [0049] Affixing the suffix “-ene” to a group indicates the group is a divalent moiety, e.g., alkylene is the divalent moiety of alkyl, alkenylene is the divalent moiety of alkenyl, alkynylene is the divalent moiety of alkynyl, heteroalkylene is the divalent moiety of heteroalkyl, heteroalkenylene is the divalent moiety of heteroalkenyl, heteroalkynylene is the divalent moiety of heteroalkynyl, carbocyclylene is the divalent moiety of carbocyclyl, heterocyclylene is the divalent moiety of heterocyclyl, arylene is the divalent moiety of aryl, and heteroarylene is the divalent moiety of heteroaryl. [0050] The term “hydrocarbyl” is a generic term encompassing alkyl, heteroaryl, carbocyclyl, aryl, heterocyclyl, heteroaryl, and combinations thereof. Hydrocarbyl also embraces polyvalent, e.g., divalent, trivalent, etc. linking groups as well. [0051] A group is optionally substituted unless expressly provided otherwise. The term “optionally substituted” refers to being substituted or unsubstituted. In general, the term “substituted” means that at least one hydrogen present on a group is replaced with a permissible substituent, e.g., a substituent which upon substitution results in a stable compound, e.g., a compound which does not spontaneously undergo transformation such as by rearrangement, cyclization, elimination, or other reaction. Unless otherwise indicated, a “substituted” group has a substituent at one or more substitutable positions of the group, and when more than one position in any given structure is substituted, the substituent is either the same or different at each position. The term “substituted” is contemplated to include substitution with all permissible substituents of organic compounds and includes any of the substituents described herein that results in the formation of a stable compound. The present invention contemplates any and all such combinations in order to arrive at a stable compound. For purposes of this invention, heteroatoms such as nitrogen may have hydrogen substituents and/or any suitable substituent as described herein which satisfy the valencies of the heteroatoms and results in the formation of a stable moiety. The invention is not intended to be limited in any manner by the exemplary substituents described herein. [0052] The term “halo” or “halogen” refers to fluorine (fluoro, -F), chlorine (chloro, -Cl), bromine (bromo, -Br), or iodine (iodo, -I). [0053] The term “amino” refers to the group -NH2. The term “substituted amino,” by extension, refers to a monosubstituted amino, a disubstituted amino, or a trisubstituted amino. The term “monosubstituted amino” refers to an amino group wherein the nitrogen atom that is directly attached to the parent molecule is substituted with one hydrogen and one group other than hydrogen. The term “disubstituted amino” refers to an amino group wherein the nitrogen atom that is directly attached to the parent molecule is substituted with two groups other than hydrogen. The term “trisubstituted amino” refers to an amino group wherein the nitrogen atom (which is typically cationic in such configurations) that is directly attached to the parent molecule is substituted with three groups. [0054] The term “oxo” refers to the group =O, and the term “thiooxo” refers to the group =S. [0055] The term “cyano” refers to the group -CN. [0056] The term “azide” and “azido” refer to the group -N3.[0057] As used herein, a chemical bond depicted: represents either a single, double, or triple bond, valency permitting. By way of example, [0058] Unless shown only as solid lines and not as or isomer, e.g., each enantiomer, diastereomer, and meso compound, and a mixture of isomers, such as a racemic or scalemic mixture. Unless stated to the contrary, a formula depicting one or more stereochemical features does not exclude the presence of other isomers. [0059] Compounds disclosed herein may exist as one or more tautomers. Tautomers are interconvertible structural isomers that differ in the position of one or more protons or other labile atom. By way of example: O OH . [0060] The prevalence will depend both on the specific chemical compound as well as its local chemical environment. Unless specified to the contrary, the depiction of one tautomeric form is inclusive of all possible tautomeric forms. [0061] Unless stated to the contrary, a substituent drawn without explicitly specifying the point of attachment indicates that the substituent may be attached at any possible atom. For example, in a benzofuran depicted as: , [0062] the substituent may be present at any one of the six possible carbon atoms. [0063] As used herein, the term “null,” when referring to a possible identity of a chemical moiety, indicates that the group is absent, and the two adjacent groups are directly bonded to one another. By way of example, for a genus of compounds having the formula CH3-X-CH3, if X is null, then the resulting compound has the formula CH3-CH3. [0064] As used herein, the designation of a polyvalent moiety without specifying the specific order of attachment is intended to cover all possible arrangements. By way of example, a compound represented by the formula: A-X-B, wherein X is NHC(=O) embraces both: . [0065] Physiologically and salts are salts that retain the desired biological activity of impart undesirable toxicological effects. Examples of such salts are acid addition salts formed with inorganic acids, for example, hydrochloric, hydrobromic, sulfuric, phosphoric, and nitric acids and the like; salts formed with organic acids such as acetic, oxalic, tartaric, succinic, maleic, fumaric, gluconic, citric, malic, methanesulfonic, p-toluenesulfonic, napthalenesulfonic, and polygalacturonic acids, and the like; salts formed from elemental anions such as chloride, bromide, and iodide; salts formed from metal hydroxides, for example, sodium hydroxide, potassium hydroxide, calcium hydroxide, lithium hydroxide, and magnesium hydroxide; salts formed from metal carbonates, for example, sodium carbonate, potassium carbonate, calcium carbonate, and magnesium carbonate; salts formed from metal bicarbonates, for example, sodium bicarbonate and potassium bicarbonate; salts formed from metal sulfates, for example, sodium sulfate and potassium sulfate; and salts formed from metal nitrates, for example, sodium nitrate and potassium nitrate. Pharmaceutically acceptable and non- pharmaceutically acceptable salts may be prepared using procedures well known in the art, for example, by reacting a sufficiently basic compound such as an amine with a suitable acid comprising a physiologically acceptable anion. Alkali metal (for example, sodium, potassium, or lithium) or alkaline earth metal (for example, calcium) salts of carboxylic acids can also be made. [0066] Although the operations of exemplary aspects of the disclosed method can be described in a particular, sequential order for convenient presentation, it should be understood that disclosed aspects can encompass an order of operations other than the particular, sequential order disclosed. For example, operations described sequentially can, in some cases, be rearranged or performed concurrently. Further, descriptions and disclosures provided in association with one particular aspect are not limited to that aspect and can be applied to any aspect disclosed. [0067] Moreover, for the sake of simplicity, the attached figures cannot show the various ways (readily discernable, based on this disclosure, by one of ordinary skill in the art) in which the disclosed system, method, and apparatus can be used in combination with other systems, methods, and apparatuses. Additionally, the description sometimes uses terms such as “produce” and “provide” to describe the disclosed method. These terms are high-level abstractions of the actual operations that can be performed. The actual operations that correspond to these terms can vary depending on the particular implementation and are, based on this disclosure, readily discernible by one of ordinary skill in the art. [0068] Disclosed herein are cardiovascular repair devices having at least one entangled hydrogel network. The networks promote reendothelialization, reduce or prevent encapsulation (including leaflet encapsulation), and/or reduce or prevent tissue overgrowth. Exemplary devices which may include entangled hydrogel networks include prosthetic valves, patches, clasps, sleeves, conduits, bands, and the like. The devices may be derived from synthetic materials (e.g., ePFTE) and/or biological materials (bovine pericardial tissue). Exemplary devices include cardiovascular patches and conduits (e.g., pulmonic, aortic, and mitral annulus conduit, or pulmonic, aortic, mitral anulus conduits). [0069] In certain implementations the device is pericardial patch. In certain implementations, both faces of the pericardial patch include an entangled hydrogel network. In certain implementations, only one face of the pericardial patch includes an entangled hydrogel network. In certain implementations the pericardial patch is a bovine pericardial patch. In certain implementations the pericardial patch is a ePTFE or polyester pericardial patch. [0070] In certain implementations the device is an annuloplasty device. In certain implementations, the annuloplasty device includes a sleeve and one or more anchors. In certain implementations the sleeve includes an entangled hydrogel network. In certain implementations the one or more anchors includes an entangled hydrogel network. In certain implementations both the sleeve and the one or more anchors include an entangled hydrogel network. [0071] In certain implementations, the device is a prosthetic valve including the following components: [0072] an annular frame having an inner surface and an outer surface, comprising an inflow end and an outflow end and being radially collapsible and expandable between a radially collapsed configuration and a radially expanded configuration; [0073] a valvular structure comprising a plurality of leaflets positioned within the frame; [0074] an inner skirt positioned along at least a portion of the inner surface of the frame, wherein the inner skirt has an inner surface and an outer surface; [0075] wherein at least one of the frame, inner skirt, or one or more leaflets include an entangled hydrogel network. [0076] In some implementations the valve can also include an outer skirt positioned along at least a portion of the outer surface of the frame, wherein the outer skirt has an inner surface and an outer surface. [0077] In some implementations, the entangled hydrogel network will be disposed on one or more surfaces of the aforementioned components. In some implementations, an entangled hydrogel network may be disposed on one or more surfaces of a further component not listed. [0078] In certain implementations the entangled hydrogel network may be covalently bonded to said surfaces. In some implementations the entangled hydrogel network may be mechanically bonded to said surfaces. Mechanical bonding includes non-covalent bonding such as hydrogen-bond interactions, electrostatic interactions, Van der Waals interactions, and combinations thereof. In such implementations the entangled hydrogel network can be applied to the surface using various coating methods, for example dip coating, spray coating, ultrasonic coating, plasma deposition or a combination thereof. In some implementations the entangled hydrogel network may be physically entrapped in said surface. In such implementations, the component in question can be extruded or otherwise formed from a polymer melt including the entangled hydrogel network, which may then be brought to the surface via annealing. In some implementations, multiple components and/or surfaces of the valve may include entangled hydrogel network, and in each case the network may be disposed on the surface in any of the aforementioned modalities. [0079] In certain implementations an entangled hydrogel network is disposed on the inner skirt. In further implementations, an entangled hydrogel network is disposed on the inner skirt, but not on the outer skirt. In further implementations, an entangled hydrogel network is disposed on the outer skirt, but not on the inner skirt. In other implementations, an entangled hydrogel network is disposed on both the inner skirt and outer skirt. In such implementations, the same entangled hydrogel network may be disposed on both the inner skirt and outer skirt, while in other implementations, a first entangled hydrogel network may be disposed on the inner skirt, while a different, second entangled hydrogel network may be disposed on the outer skirt. [0080] In certain implementations, when an entangled hydrogel network is disposed on the inner skirt, it may be disposed on the inner surface of the inner skirt (but not the outer surface of the inner skirt), on the outer surface of the inner skirt (but not the inner surface of the inner skirt), or both the inner surface and outer surface of the inner skirt. In such implementations, the same entangled hydrogel network may be disposed on both the inner surface and outer surface of the inner skirt while in other implementations, a first entangled hydrogel network may be disposed on the inner surface of the inner skirt, while a different, second entangled hydrogel network may be disposed on the outer surface of the inner skirt. [0081] In certain implementations, when an entangled hydrogel network is disposed on the outer skirt, it may be disposed on the inner surface of the outer skirt (but not the outer surface of the outer skirt), on the outer surface of the outer skirt (but not the inner surface of the outer skirt), or both the inner surface and outer surface of the outer skirt. In such implementations, the same entangled hydrogel network may be disposed on both the inner surface and outer surface of the outer skirt while in other implementations, a first entangled hydrogel network may be disposed on the inner surface of the outer skirt, while a different, second entangled hydrogel network may be disposed on the outer surface of the outer skirt. [0082] The skirt (inner or outer) can be composed of a variety of materials. In some implementations the skirt can be made from polymer yarns constructed into textiles, including wovens or knit wraps. Exemplary materials include polyhydroxyalkanoates (PHAs), polyethylene furanoate (PEF), polyethylene terephthalate (PET), and/or polyamides of high molecular weight such as Nylon 6, 6,6, 12, 6,10, 6,12, or 10,10. [0083] In certain implementations, when an entangled hydrogel network is disposed on the frame, it may be disposed on the inner surface of the frame (but not the outer surface of the frame), on the outer surface of the frame (but not the inner surface of the frame), or both the inner surface and outer surface of the frame. In such implementations, the same entangled hydrogel network may be disposed on both the inner surface and outer surface of the frame while in other implementations, a first entangled hydrogel network may be disposed on the inner surface of the frame, while a different, second entangled hydrogel network may be disposed on the outer surface of the frame. [0084] The frame can be composed of a variety of materials. In some implementations the frame includes a metal (which includes alloys as well as ceramic coated metals and alloys). Exemplary metals include pure titanium, cobalt-chromium alloy, cobalt-chromium- nickel alloy, nickel-cobalt-chromium-molybdenum alloy, molybdenum alloys, stainless steel, nickel‑titanium alloy, (nitinol), platinum, tantalum alloys, magnesium alloys, or a combination thereof. [0085] In some implementations, the frame can be partially covered by the outer skirt. In such implementations, an entangled hydrogel network can be disposed on the portion of the frame not covered by the outer skirt (e.g., the exposed portion). In certain implementations, an entangled hydrogel network can be disposed on the uncovered portion of the frame, and an entangled hydrogel network (which may the same or different) can be disposed on the outer skirt (either the inner surface, the outer surface, or both). [0086] In some implementations, the prosthetic valve will include two or three leaflet. In some implementations, an entangled hydrogel network will be disposed on each leaflet present in the valve. The leaflet may be composed of a variety of different materials. In some implementations the leaflet includes pericardial tissue (e.g., bovine pericardial tissue), a biocompatible synthetic polymer (e.g., a polyurethane, polyolefin such as polyethylene terephthalate, or combination thereof). In some implementations the leaflet can include a combination of pericardial tissue and synthetic polymers. ENTANGLED HYDROGEL NETWORK [0087] The entangled hydrogel networks disclosed herein include at least two, three, or four separate, entangled ionically charged or nonionic polymers. In some implementations, an entangled hydrogel network includes two ionically charged polymers (a first ionically charged polymer and a second ionically charged polymer). In other implementations an entangled hydrogel network includes three ionically charged polymers (a first ionically charged polymer, a second ionically charged polymer, and a third ionically charged polymer). In some implementations the various ionically charged polymers present in the entangled hydrogel network will be compositionally different (e.g., having different monomeric constituents). In some implementations the various ionically charged polymers present in the entangled hydrogel network will have different molecular weights and/or charge density, but composed of the same monomeric constituents. In some implementations, one or more of the ionically charged polymers can be a crosslinked polymer. In certain implementations, when the entangled hydrogel network includes two different ionically charged polymers, one of the ionically charged polymers can be crosslinked and the other is not (e.g., the first ionically charged polymer is crosslinked and the second ionically charged polymer is not crosslinked). In some implementations, both the first and second ionically charged polymers are crosslinked and have different crosslinking density and/or crosslinking chemistries from one another. In certain implementations, one of the ionically charged polymers will have a greater degree of crosslinking than the other ionically charged polymer. [0088] In some implementations, the first ionically charged polymer can have a degree of crosslinking from 0.1–1%, from 0.1–0.5%, or from 0.5–1%, and the second ionically charged polymer can have a degree of crosslinking from 1–5%, from 1–2.5%, or from 2.5–5%. In certain implementations, the second ionically charged polymer can have a degree of crosslinking from 0.1–1%, from 0.1–0.5%, or from 0.5–1%, and the first ionically charged polymer can have a degree of crosslinking from 1–5%, from 1–2.5%, or from 2.5–5%. Unless specified to the contrary, degree of crosslinking refers to the wt.% of the crosslinking agent to the uncrosslinked polymer. [0089] In some implementations, the entangled hydrogel network includes polymer brushes. The polymer brush includes one or more brush polymers extending away from the entangled network. In certain implementations a single type of brush polymer extends from the entangled network. In some implementations, the brush polymer is an ionically charged polymer. In some implementations two or more different brush polymers extend from the entangled hydrogel network. When two or more brush polymers extend from the entangled hydrogel network, each brush polymer may be ionically charged, or one or more polymer is ionically charged and one or more polymers is not. In certain implementations the brush polymer is a non-crosslinked polymer that is conjugated to the entangled hydrogel network at a single site. [0090] In some implementations, the entangled hydrogel network can include one or more additional polymers not covalently joined to any of the ionically charged polymers or brush polymer. In some instance the additional polymer includes PEG/polypropylene oxide (PPO), pectin, carrageenan, polylysine, gelatins (including gelatin type A), agarose, PEO- PPO-PEO copolymers (Pluronics® polymers), poly(phosphazene), hyaluronans, chitosans, agar, heparin, sulfate, cellulose, alginates (including alginate sulfate), collagen, dextrans (including dextran sulfate), poly(hydroxyethyl methacrylate) (PHEMA), poly(methyl methacrylate) (PMMA), poly(N isopropylacrylamide) (PNIPAAm), poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) poly(methacrylates), poly(N-vinylpyrrolidone), PL(G)A-PEO- PL(G)A copolymers, poly(ethylene imine), polyethylene glycol (PEG)-thiol, PEG-acrylate, acrylamide, PEG, polypropylene oxide (PPO), polyacrylic acid, (PLGA), polycaprolactone (PCL), poly(vinylsulfonic acid) (PVSA), poly(L-aspartic acid), poly(L-glutamic acid), or a combination thereof. [0091] The entangled hydrogel networks may be present on the device in a variety of thicknesses. In some implementations the entangled hydrogel network has a thickness from 0.1 to 150 microns, from 0.1 to 10 microns, from 1 to 25 microns, from 5 to 50 microns, from 25 to 50 microns, from 50 to 100 microns, or from 100 to 150 microns. [0092] The entangled hydrogel networks can be characterized by water holding capacity. Water holding capacity refers to the maximum amount of water that can be absorbed into the hydrogel, which may be determined by swelling the hydrogel in water until it reaches a constant mass. In certain implementations, the entangled hydrogel network can have a water holding capacity between 10–1,000%, relative to the dry weight of the entangled hydrogel network. In some implementations, the water holding capacity can be from 10–50%, from 25–75%, from 50–100%, from 50–200%, from 100–250%, from 100–500%, from 100– 1,000%, from 250–750%, or from 500–1,000%. A lower water holding capacity indicates the entangled hydrogel network has more stable physical dimensions than a corresponding entangled hydrogel network having a higher water holding capacity. In certain implementations, the leaflets include the entangled hydrogel network, and the entangled hydrogel network has a lower water holding capacity, for example from 10–50%, from 25– 75%, from 50–100%, from 50–200%, or from 100–250%. In certain implementations, the skirt and/or frame includes the entangled hydrogel network, and the entangled hydrogel network has a higher water holding capacity, for example from 100–500%, from 100– 1,000%, from 250–750%, or from 500–1,000%. In certain implementations the device is a prosthetic valve as described herein, including an entangled hydrogel network with a low water holding capacity on the leaflets, and an entangled hydrogel network with a high water holding capacity on the frame and/or skirt. [0093] In certain implementations, the entangled hydrogel networks may be characterized by elasticity and stress relaxation. In certain implementations, the entangled hydrogel networks will completely recover from fixed strains of at least 5%, at least 10%, at least 15%, at least 20%, or at least 25%. [0094] The entangled hydrogel network may be characterized by its modulus. In certain implementations, the entangled hydrogel network is present on the leaflets and has a lower modulus, for example from 0.01–35 MPa, from 0–5 MPa, from 2–8 MPa, from 5–10 MPa, from 5–15 MPa, from 10–25 MPa, or from 20–35 Mpa. In certain implementations, the entangled hydrogel network is present on the frame and/or skirt and has a higher modulus, for example from 100–1,000 Mpa, from 100–250 MPa, from 100–500 MPa, from 250–500 MPa, from 250–750 MPa, or from 500–1,000 MPa. In certain implementations the device is a prosthetic valve as described herein, including an entangled hydrogel network with a low modulus on the leaflets, and an entangled hydrogel network with a high modulus on the frame and/or skirt. First ionically charged polymer [0095] In certain implementations the first ionically charged polymer is composed of a backbone and side chains. In certain implementations the first ionically charged polymer is a (meth)acrylate polymer. In certain implementations the first ionically charged polymer is derived from a single monomer precursor (e.g., a homopolymer) while in other implementations the first ionically charged polymer is derived from two of more monomer precursors (e.g., a copolymer). In some implementations the first ionically charged polymer is a random copolymer. In some implementations the first ionically charged polymer is a block copolymer. [0096] In certain implementations the first ionically charged polymer includes an ionically charged polymer backbone. Suitable ionic groups that may occur in the backbone include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, and combinations thereof. In certain implementations, the first ionically charged polymer is a polyurethane in which either, or both, the diol/diamine precursor and the di-isocyanate precursor include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0097] In other implementations, the backbone of the first ionically charged polymer is not ionically charged. Exemplary non-ionically charged backbones include polyalkylene backbones (including poly(meth)acrylates, poly(meth)acrylamides, polyvinyls, and other polyolefins, polyether backbones and polyurethane backbones. In such instances, the first ionically charged polymer will include one or more ionically charged side chains. In some implementations, the first ionically charged polymer can be a polyalkylene (e.g., poly(meth)acrylate, poly(meth)acrylamide, polyvinyl) having a mixture of ionically charged and neutral side chains (e.g., a copolymer). In some implementations, the first ionically charged polymer is a random copolymer. [0098] In certain implementations the first ionically charged polymer includes repeating units of formula (1a), formula (1b), or a combination thereof: [Formula (1b)], R1m1 is H or CH3; R1m2 is null, C(=O)O, or C(=O)NH; R1a1 is null, (CH2CH2O)x, or a C2–15 hydrocarbyl group; R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C2–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is independently H, carboxylate, sulfonate, or phosphonate; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z1a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. [0099] The skilled person understands that the aforementioned repeating units can be derived from compounds of formula (1a-m) and (1b-m): [Formula (1b-m)], R1m1 is H or CH3; R1m2 is null, C(=O)O, or C(=O)NH; R1a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C1–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is independently H, carboxylate, sulfonate, or phosphonate; wherein said groups may be masked with protecting groups; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z1a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. [0100] In certain implementations, the repeating unit of formula (1b) may include repeating units having the formula: , or a N+(CH3)2, and Y1a is not H. In other implementations X1a is -O-PO2–-O-, and Y1a is H. [0101] In certain implementations, the repeating unit of formula (1a) includes units having the formula: , R1f1 is independently O or NH; Y1a is independently carboxylate; sulfonate, or phosphonate; and Z1a is independently H or -N+(Rn1)3. [0102] In certain implementations, the repeating unit of formula (1a) includes units having the formula: wherein R1a1 is (CH2CH2O)n, R1d1 is null, and Z1a is N+(CH3)3. [0103] In certain implementations, the repeating unit of formula (1a) includes units having the formula: , wherein R1m1 is H, R1f1 is NH, R1a1 is C(CH3)2, R1b1 is CH, R1c1 is null, and Y1a is SO3H. [0104] In certain implementations the repeating unit of formula (1a) includes units having the formula:
, or a combinati implementations n is 2. Second repeating unit [0105] In certain implementations the first ionically charged polymer includes repeating units of formula (1c), formula (1d), or a combination thereof: [Formula (1d)], R1m3 is H or CH3; R1m4 is null, C(=O)O, or C(=O)NH; R1a2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e2 is a C1–15 hydrocarbyl group; wherein any two or more of R1a2, R1b2, R1c2, and R1d2 can together form a ring; Y1b is independently H, carboxylate, sulfonate, or phosphonate; X1b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z1b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R1a2, R1b2, R1c2, and R1d2 is not null; and when X1b is null then at least one of Z1b and Y1b is not H. [0106] In certain implementations, the repeating unit of formula (1d) may include repeating units having the formula: , or a N+(CH3)2, and Y1b is not H. In other - , [0107] In certain implementations, the repeating unit of formula (3a) includes units having the formula: , R1f2 is independently O or NH; Y1b is independently carboxylate; sulfonate, or phosphonate; and Z1b is independently H or -N+(Rn1)3. [0108] In certain implementations the repeating unit of formula (1c) includes units having the formula: , or a implementations n is 2. Neutral repeating unit [0109] In certain implementations, the first ionically charged polymer can include neutral repeating units of vinyl alcohol, or of formula (1e), formula (1f), or a combination thereof: [Formula (1f)], wherein R1m5 is H or CH3; R1m6 is H or CH3; and R1a 2, wherein R1b is in each case independently selected from H, C1–12 hydrocarbyl, wherein R1b may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. When R1a is OR1b then R1b is not H. [0110] In certain implementations R1a is O-CH2CH2N(CH3)2. In other implementations R1a is O-(2-ethylhexyl). [0111] In certain implementations the first ionically charged polymer includes repeating units of formula (1a), optionally in combination with repeating units of formula (1e) and/or formula (1f). In some implementations the first ionically charged polymer includes repeating units of formula (1b), optionally in combination with repeating units of formula (1e) and/or formula (1f). In certain implementations the first ionically charged polymer includes repeating units of formula (1a) and formula (1b), optionally in combination with repeating units of formula (1e) and/or formula (1f). In some implementations the first ionically charged polymer includes repeating units of formula (1a) and formula (1c), optionally in combination with repeating units of formula (1e) and/or formula (1f). [0112] In certain implementations, the first ionically charged polymer includes repeating units having the formula: wherein R1a1 is (CH2CH2O)n, R1d1 is null, and Z1a is N+(CH3)3, in combination with 2- units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof. In certain implementation the first ionically charged polymer contains units having the above formula in combination with 2-ethylhexyl methacrylate units and N,N- dimethylaminoethyl methacrylate units. [0113] In certain implementations, the first ionically charged polymer includes 2- methacryloyloxyethyl phosphorylcholine (MPC) units, sulfobetaine methacrylate (SBMA) units, carboxybetaine methacrylate (CBMA) units, or any combination thereof, optionally in combination with 2-ethylhexyl methacrylate units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof. [0114] In certain implementations, the first ionically charged polymer is a poly(2- acrylamido-2-methyl-1-propanesulfonic acid) (“PAAMPS”). In certain implementations, the first ionically charged polymer is a copolymer of (2-acrylamido-2-methyl-1-propanesulfonic acid) and vinyl alcohol (“PAAMPS-PVA”). [0115] In implementations in which the first ionically charged polymer is not crosslinked, the first ionically charged polymer can have a molecular weight from 2,500– 250,000 Da, from 2,500–100,000 Da, from 2,500–50,000 Da, from 2,500–10,000 Da, from 5,000–50,000 Da, from 25,000–100,000 Da, from 25,000–75,000 Da, from 50,000– 100,000 Da, or from 50,000–100,000 Da. [0116] In certain embodiments, the first ionically charged polymer is a crosslinked polymer. The degree of crosslinking may be characterized by the mass of the crosslinker relative to the total mass of the polymer (or monomer constituents when the crosslinker is added at the polymerization stage). In certain implementations, the first ionically charged polymer may have a degree of crosslinking from 0.1–5%, from 0.1–1%, from 0.1–0.5%, from 1–2.5%, from 1–5%, or from 2.5–5 %. Second ionically charged polymer [0117] In certain implementations the second ionically charged polymer is composed of a backbone and side chains. In certain implementations the second ionically charged polymer is a (meth)acrylate polymer. In certain implementations the second ionically charged polymer is derived from a single monomer precursor (e.g., a homopolymer) while in other implementations the second ionically charged polymer is derived from two of more monomer precursors (e.g., a copolymer). In some implementations the second ionically charged polymer is a random copolymer. In some implementations the second ionically charged polymer is a block copolymer. [0118] In certain implementations the second ionically charged polymer includes an ionically charged polymer backbone. Suitable ionic groups that may occur in the backbone include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, and combinations thereof. In certain implementations, the second ionically charged polymer is a polyurethane in which either, or both, the diol/diamine precursor and the di-isocyanate precursor include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0119] In other implementations, the backbone of the second ionically charged polymer is not ionically charged. Exemplary non-ionically charged backbones include polyalkylene backbones (including poly(meth)acrylates, poly(meth)acrylamides, polyvinyls, and other polyolefins), polyether backbone and polyurethane backbones. In such instances, the second ionically charged polymer will include one or more ionically charged side chains. In some implementations, the second ionically charged polymer can be a polyalkylene (e.g., poly(meth)acrylate, poly(meth)acrylamide, polyvinyl) having a mixture of ionically charged and neutral side chains (e.g., a copolymer). In some implementations, the second ionically charged polymer is a random copolymer. [0120] In certain implementations the second ionically charged polymer includes repeating units of formula (2a), formula (2b), or a combination thereof: [Formula (2b)], R2m1 is H or CH3; R2m2 is null, C(=O)O, or C(=O)NH; R2a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e1 is a C1–15 hydrocarbyl group; wherein any two or more of R2a1, R2b1, R2c1, and R2d1 can together form a ring; Y2a is H, carboxylate; sulfonate, or phosphonate; X2a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z2a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R2a1, R2b1, R2c1, and R2d1 is not null; and when X2a is null then at least one of Z2a and Y2a is not H. [0121] In certain implementations, the repeating unit of formula (2b) may include repeating units having the formula: , or a combination [0122] In certain implementations, X2a is N+(CH3)2, and Y2a is not H. In other implementations X2a is -O-PO2–-O-, and Y2a is H. [0123] In certain implementations, the repeating unit of formula (2a) includes units having the formula: , R2f1 is O or NH; Y2a is carboxylate; sulfonate, or phosphonate; and Z2a is H or -N+(Rn1)3. [0124] In certain implementations, the repeating unit of formula (2a) includes units having the formula: , wherein R2m1 is H, R2f1 is NH, 2, R2b1 is CH, R2c1 is null, and Y2a is SO3H. [0125] In certain implementations the repeating unit of formula (2a) includes units having the formula: , or a implementations n is 2. Second repeating unit [0126] In certain implementations the second ionically charged polymer includes repeating units of formula (2c), formula (2d), or a combination thereof: [Formula (2d)], R2m3 is H or CH3; R2m4 is null, C(=O)O, or C(=O)NH; R2a2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e2 is a C1–15 hydrocarbyl group; wherein any two or more of R2a2, R2b2, R2c2, and R2d2 can together form a ring; Y2b is H, carboxylate; sulfonate, or phosphonate; X2b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z2b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R2a2, R2b2, R2c2, and R2d2 is not null; and when X2b is null then at least one of Z2b and Y2b is not H. [0127] In certain implementations, the repeating unit of formula (2d) may include repeating units having the formula: , or a [0128] In certain implementations, X2b is N+(CH3)2, and Y2b is not H. In other implementations X2b is -O-PO2–-O-, and Y2b is H. [0129] In certain implementations, the repeating unit of formula (2c) includes units having the formula: , R2f2 is O or NH; Y2b is carboxylate; sulfonate, or phosphonate; and Z2b is H or -N+(Rn1)3. [0130] In certain implementations the repeating unit of formula (2c) includes units having the formula: , or a implementations n is 2. [0131] In certain implementations, the second ionically charged polymer can include neutral repeating units of vinyl alcohol, formula (2e), formula (2f), or a combination thereof: [Formula (1f)], wherein R2m5 is H or CH3; R2m6 is H or CH3; and R2a is OR2b or N(R2b)2, wherein R2b is in each case independently selected from H, C1– 12 hydrocarbyl, wherein R2b may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. [0132] In certain implementations R2a is O-CH2CH2N(CH3)2. In other implementations R2a is O-(2-ethylhexyl). [0133] In certain implementations the second ionically charged polymer includes repeating units of formula (2a), optionally in combination with repeating units of formula (2e) and/or formula (2f). In some implementations the second ionically charged polymer includes repeating units of formula (2b), optionally in combination with repeating units of formula (2e) and/or formula (2f). In certain implementations the second ionically charged polymer includes repeating units of formula (2a) and formula (2b), optionally in combination with repeating units of formula (2e) and/or formula (2f). In some implementations the second ionically charged polymer includes repeating units of formula (2a) and formula (2c), optionally in combination with repeating units of formula (2e) and/or formula (2f). [0134] In certain implementations, the second ionically charged polymer includes repeating units having the formula: wherein R2a2 is (CH2CH2O)n, R2c2 is null, and Y2b is sulfonate, carboxylate or phosphonate, with 2-ethylhexyl methacrylate units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof. In certain implementation the second ionically charged polymer contains units having the above formula in combination with 2-ethylhexyl methacrylate units and N,N-dimethylaminoethyl methacrylate units. [0135] In certain implementations, the second ionically charged polymer includes 2- methacryloyloxyethyl phosphorylcholine (MPC) units, sulfobetaine methacrylate (SBMA) units, carboxybetaine methacrylate (CBMA) units, or any combination thereof, optionally in combination with 2-ethylhexyl methacrylate units, N,N-dimethylaminoethyl methacrylate units, poly(ethylene)glycol methacrylate units, 2-hydroxyethyl methacrylate units, vinylpyrrolidone units, or a combination thereof. [0136] In certain implementations, the second ionically charged polymer is a poly(2- acrylamido-2-methyl-1-propanesulfonic acid) (“PAAMPS”). In certain implementations, the second ionically charged polymer is a copolymer of (2-acrylamido-2-methyl-1- propanesulfonic acid) and vinyl alcohol (“PAAMPS-PVA”). [0137] In implementations in which the second ionically charged polymer is not crosslinked, the second ionically charged polymer can have a molecular weight from 2,500– 250,000 Da, from 2,500–100,000 Da, from 2,500–50,000 Da, from 2,500–10,000 Da, from 5,000–50,000 Da, from 25,000–100,000 Da, from 25,000–75,000 Da, from 50,000– 100,000 Da, or from 50,000–100,000 Da. [0138] In certain embodiments, the second ionically charged polymer is a crosslinked polymer. The degree of crosslinking may be characterized by the mass of the crosslinker relative to the total mass of the polymer (or monomer constituents when the crosslinker is added at the polymerization stage). In certain implementations, the second ionically charged polymer may have a degree of crosslinking from 0.1–5%, from 0.1–1%, from 0.1–0.5%, from 1–2.5%, from 1–5%, or from 2.5–5%. Brush polymer [0139] In certain implementations the brush polymer is composed of a backbone and side chains. In certain implementations the brush polymer is a (meth)acrylate polymer. In certain implementations the brush polymer is derived from a single monomer precursor (e.g., a homopolymer) while in other implementations the brush polymer is derived from two of more monomer precursors (e.g., a copolymer). In some implementations the brush polymer is a random copolymer. In some implementations the brush polymer is a block copolymer. [0140] In certain implementations the brush polymer includes an ionically charged polymer backbone. Suitable ionic groups that may occur in the backbone include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, and combinations thereof. In certain implementations, the brush polymer is a polyurethane in which either, or both, the diol/diamine precursor and the di-isocyanate precursor include phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0141] In other implementations, the backbone of the brush polymer is not ionically charged. Exemplary non-ionically charged backbones include polyalkylene backbones (including poly(meth)acrylates, poly(meth)acrylamides, polyvinyls, and other polyolefins), polyether backbone and polyurethane backbones. In such instances, the brush polymer will include one or more ionically charged side chains. In some implementations, the brush polymer can be a polyalkylene (e.g., poly(meth)acrylate, poly(meth)acrylamide, polyvinyl) having a mixture of ionically charged and neutral side chains (e.g., a copolymer). In some implementations, the brush polymer is a random copolymer. [0142] In certain implementations the brush polymer includes repeating units of formula (Xa), formula (Xb), or a combination thereof: [Formula (Xb)], wherei RXm1 is H or CH3; RXm2 is null, C(=O)O, or C(=O)NH; RXa1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXb1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXc1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXd1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXe1 is a C1–15 hydrocarbyl group; wherein any two or more of RXa1, RXb1, RXc1, and RXd1 can together form a ring; YXa is H, carboxylate; sulfonate, or phosphonate; is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; ZXa is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of RXa1, RXb1, RXc1, and RXd1 is not null; and when XXa is null then at least one of ZXa and YXa is not H. [0143] In certain implementations, the repeating unit of formula (Xb) may include repeating units having the formula: , or a combinatio [0144] In certain implementations, XXa is N+(CH3)2, and YXa is not H. In other implementations XXa is -O-PO2–-O-, and YXa is H. [0145] In certain implementations, the repeating unit of formula (Xa) includes units having the formula: , RXf1 is O or NH; YXa is carboxylate; sulfonate, or phosphonate; and ZXa is H or -N+(Rn1)3. [0146] In certain implementations, the repeating unit of formula (Xa) includes units having the formula: , wherein R2xm1 is H, Rxf1 is NH, 2, Rxb1 is CH, Rxc1 is null, and Yxa is SO3H. [0147] In certain implementations the repeating unit of formula (Xa) includes units having the formula: , or a implementations n is 2. [0148] In certain implementations the brush polymer includes repeating units of formula (Xc), formula (Xd), or a combination thereof: [Formula (Xd)], wherein RYm3 is H or CH3; RYm4 is null, C(=O)O, or C(=O)NH; RYa2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYb2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYc2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYd2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYe2 is a C1–15 hydrocarbyl group; wherein any two or more of RYa2, RYb2, RYc2, and RYd2 can together form a ring; YYb is H, carboxylate; sulfonate, or phosphonate; XYb is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; ZYb is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of RYa2, RYb2, RYc2, and RYd2 is not null; and when XYb is null then at least one of ZYb and YYb is not H. [0149] In certain implementations, the repeating unit of formula (Xd) may include repeating units having the formula: , or a [0150] In certain implementations, XYb is N+(CH3)2, and YYb is not H. In other implementations XYb is -O-PO2–-O-, and YYb is H. [0151] In certain implementations, the repeating unit of formula (Xc) includes units having the formula: , RYf2 is O or NH; YYb is carboxylate; sulfonate, or phosphonate; and ZYb is H or -N+(Rn1)3. [0152] In certain implementations the repeating unit of formula (Xc) includes units having the formula: , or a implementations n is 2. [0153] In certain implementations, the brush polymer can include neutral repeating units of vinyl alcohol, formula (Xe), formula (Xf), or a combination thereof: [Formula (Xf)], wherein RYm5 is H or CH3; RYm6 is H or CH3; and RYa is ORYb or N(RYb)2, wherein RYb is in each case independently selected from H, C1– 12 hydrocarbyl, wherein RYb may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. [0154] In certain implementations RYa is O-CH2CH2N(CH3)2. In other implementations RYa is O-(2-ethylhexyl). [0155] In certain implementations, the brush polymer is a poly(2-acrylamido-2-methyl- 1-propanesulfonic acid) (“PAAMPS”). In certain implementations, the first ionically charged polymer is a copolymer of (2-acrylamido-2-methyl-1-propanesulfonic acid) and vinyl alcohol (“PAAMPS-PVA”). [0156] In certain implementations the brush polymer can have a molecular weight from 2,500–250,000 Da, from 2,500–100,000 Da, from 2,500–50,000 Da, from 2,500–10,000 Da, from 5,000–50,000 Da, from 25,000–100,000 Da, from 25,000–75,000 Da, from 50,000–100,000 Da, or from 50,000–100,000 Da. [0157] In certain implementations the brush polymer includes repeating units of formula (Xa), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In some implementations the brush polymer includes repeating units of formula (Xb), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In certain implementations the second ionically charged polymer includes repeating units of formula (Xa) and formula (Xb), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In some implementations the second ionically charged polymer includes repeating units of formula (Xa) and formula (2c), optionally in combination with repeating units of formula (Xe) and/or formula (Xf). In certain implementations, the brush polymer includes repeating units of formula (Xe) and/or formula (Xf), and does not include repeating units of (Xa), (Xb), (Xc), or (Xd). [0158] In certain implementations, the first ionically charged polymer is one of poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)], poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), a combination thereof, or a copolymer thereof. In certain implementations, the first ionically charged polymer is a copolymer including the aforementioned ionic component in combination with polyvinyl alcohol, polyvinylpyrrolidone, or a combination thereof. [0159] In certain implementations, the second ionically charged polymer is one of poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)], poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), a combination thereof, or a copolymer thereof. In certain implementations, the second ionically charged polymer is a copolymer including the aforementioned ionic component in combination with polyvinyl alcohol, polyvinylpyrrolidone, or a combination thereof. [0160] In certain implementations, the brush polymer is one of poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2-(methacryloyloxy)ethyl dimethyl- (3-sulfopropyl)ammonium hydroxide)], poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), a combination thereof, or a copolymer thereof. In certain implementations, the brush charged polymer is a copolymer including the aforementioned ionic component in combination with polyvinyl alcohol, polyvinylpyrrolidone, or a combination thereof. [0161] In certain implementations, the first ionically charged polymer is poly(lysine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), poly[2- (methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide)], the second ionically charged polymer is poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate), and the brush polymer is poly(sulfobetaine vinyl imidazole), poly(sulfobetaine acrylamide), poly(carboxybetaine methacrylate). Method of manufacture [0162] In certain implementations the entangled hydrogel network can be prepared by forming the second ionically charged polymer in the presence of the first ionically charged polymer, wherein the first ionically charged polymer can be crosslinked. In certain implementations the first ionically charged polymer is combined with second ionically charged polymer-precursor(s) in an aqeuous solvent. In some implementations the second ionically charged polymer-precursor(s) includes a compound of formula (2a-m), formula (2b-m), or a combination thereof: [Formula (2b-m)], R2m3 is H or CH3; R2m4 is null, C(=O)O, or C(=O)NH; R2a2 is null, (CH2CH2O)x, or a C2–15 hydrocarbyl group; R2b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e2 is a C2–15 hydrocarbyl group; wherein any two or more of R2a2, R2b2, R2c2, and R2d2 can together form a ring; Y2b is H, carboxylate; sulfonate, or phosphonate; X2b is null, -O-PO2-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O–, wherein n is 2–4; Z2b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R2a2, R2b2, R2c2, and R2d2 is not null; and when X2b is null then at least one of Z2b and Y2b is not H. [0163] In certain implementations, the compound of formula 2a-m, formula 2b-m, or combination thereof is polymerized in the presence of a crosslinker. Exemplary crosslinkiners include bis(meth)acrylates, bis(meth)acrylamides, diimides, and divinyl compounds. In some implementations, the crosslinker is methylene bisacrylamide, ethylene glycol dimethacrylate, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), tetraethylene glycol dimethacrylate, trimethylolpropane trimethacrylate, ethoxylated trimethylol triacrylate, ethoxylated pentaerythritol tetracrylate, glycol (PEG)-thiol/PEG- acrylate, acrylamide/N,N'-bis(acryloyl)cystamine (BACy), (meth)acrylate-oligolactide-PEO- oligolactide (meth)acrylate, N,N'-bis(acryloyl)cystamine, bisacrylamide, diacrylate, diallylamine, triallylamine, divinyl sulfone, diethylene glycol diallyl ether, ethylene glycol diacrylate, polyethylene glycol diacrylate, 1,4-phenylenediacryloyl chloride or a combination thereof. In other implementations, the compound of formula 2a-m, formula 2b-m, or combination thereof is polymerized in the absence of a crosslinker, and then crosslinked in a separate step following formation of the second ionically charged polymer. [0164] In certain implementations the compound of formula 2a-m, formula 2b-m, or combination thereof is polymerized in the presence of an initiator. The initiator can be a photoinitiator or a thermal initiator. Thermal radical initiators include: 1,1-azobis (cyclohexanecarbonitrile), ammonium persulfate, benzoyl peroxide, ditamylperoxide, terbutyl peroxybenzoate, dicumylperoxide, azobisisobutyronitrile (AIBN), sodium metabisulfite, 4,4-azobis(4-cyanovaleric acid), 2,2-azobis(2,4-dimethylpentanenitrile), 2,2- azobis(cyclohexanecarbonitdle). Examples of photoinitiators include: acetophenone, 2- phenylacetophenone, 2-hydroxy-2-methylpro piophenone, 4-hydroxybenzophenone, 4,4- dihydroxybenzophenone, 4-(dimethylamino) benzophenone 4-ethoxyacetophenone, 4- phenoxyacetophenone, hexafluorophosphate, anthraquinone, anthraquinone-2-sulfonic acid, sodium salt monohydrate; tricarbonylchromium, benzoin based initiators, benzoin methyl ether, benzoin isobutyl ether, 2-hydroxy-4-(2-hydroxy ethoxy )-2- methylpropiophenone, benzil ketal based initiators dialkoxyacetophenone, hydroxyphenone, phenyl ketone, aminoalkylphenone, acylphosphine oxide, benzophenone, thioxanthone, azobisisobutyronitrile, lithium phenyl-2,4,6-trimethylbenzoylphosphinate, 1-hydroxy- cyclohexylphenylketone or blends, benzophenone tetracarboxylicdianhydride, methybenzoylformate, phenanthrenequinone, ferrocene, triarylsulfonium hexafluorophosphate salts mixed with 30–60% propylene carbonate. The initiator in the prepolymer solution ranges between 0.01 wt % to 10 wt %. [In certain implementations, the compound of formula 2a-m, formula 2b-m, or combination thereof is polymerized by exposure to actinic radiation, for example UV irradiation. [0165] The polymerization can be carried out in solution with a 100 to 300:1:1 mol ratio between the monomer(s), initiator and the catalyst, respectively. In a typical synthesis, a flask can be charged with catalyst, then monomer and solvent. In some implementations, the solution is degassed and second monomer is added. In some implementations the molar ratio of the different monomers is from 1:5 to 1:3. The reaction mixture maintained at a temperature between 50–100 °C for 1–24 hours. The final polymer is poured into different solvent, filtered and finally purified through column chromatography/filtration (e.g., through Florisil® magnesium silicate) with another solvent. [0166] The first ionically charged polymer (whether crosslinked or not) and compound of formula 2a-m, formula 2b-m, or combination thereof can be provided in a reaction media (e.g., a solution or suspension with one or more solvents), or the first ionically charged polymer and compound of formula 2a-m, formula 2b-m, or combination thereof (and optional crosslinking agent) is disposed as a film on the surface of valve component, and then polymerized. [0167] When the compound of formula 2a-m, formula 2b-m, or combination thereof are crosslinked in a reaction media, the resulting entangled hydrogel may then be disposed on the surface of a valve component. In some implementations, the second ionically charged polymer may be crosslinked prior to disposition on the valve surface. In some implementations, the second ionically charged polymer may be crosslinked subsequent to disposition on the valve surface. In further implementations, the second ionically charged polymer is not crosslinked. [0168] In certain implementations the first entangled polymer can be prepared by polymerizing a mixture including compound of formula (1a-m), formula (1b-m), or a combination thereof: [Formula (1b-m)], wher R1m1 is H or CH3; R1m2 is null, C(=O)O, or C(=O)NH; R1a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C1–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is H, carboxylate; sulfonate, or phosphonate; wherein said groups may be masked with protecting groups; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z1a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. [0169] In certain implementations, the compound of formula 1a-m, formula 1b-m, or combination thereof is polymerized in the presence of a crosslinker, as defined above. [0170] In certain implementations the compound of formula 1a-m, formula 1b-m, or combination thereof is polymerized in the presence of an initiator, as defined above. [0171] In some implementations, compound of formula 1a-m, formula 1b-m, or combination thereof can be provided in a reaction media (e.g., a solution or suspension with one or more solvents), or the compound of formula 1a-m, formula 1b-m, or combination thereof (and optional crosslinking agent) is disposed as a film on the surface of valve components, and then polymerized. [0172] When the compound of formula 1a-m, formula 1b-m, or combination thereof are crosslinked in a reaction media, the resulting first ionically charged polymer may then be disposed on the surface of a valve component. In some implementations, the first ionically charged polymer may be crosslinked prior to disposition on the valve surface. In some implementations, the first ionically charged polymer may be crosslinked subsequent to disposition on the valve surface. In further implementations, the first ionically charged polymer is not crosslinked. [0173] In some implementations, the entangled hydrogel network is applied to a surface of a valve component by grafting the entangled hydrogel to the surface or coating the entangled hydrogel on the surface. In some implementations, the coating includes dip coating, spraying coating, ultrasonic coating, annealing the entangled hydrogel network, or a combination thereof. In some implementations the entangled hydrogel is covalently grafted to the surface. The surface may be functionalized to increase the reactivity towards the entangled hydrogel, for example by e-beam treatment and the like [0174] Once applied to the surface, the entangled hydrogel may be contacted with a solution including the brush polymer at a concentration from 0.1–5% by weight for a time sufficient to conjugate the brush polymer. Unreacted brush polymer may be removed by washing with solvent. [0175] In some implementations, a pre-formed brush polymer can be conjugated to the entangled hydrogel. Such conjugations may be accomplished using nucleophile/electrophile chemistry or cycloaddition chemistry (including click-cycloaddition chemistry). In some implementations the conjugation is accomplished using a reaction between a first functional group selected from an alcohol, thiol, amine, or carboxylate and a second functional group selected from an isocyanate, epoxide, activated carboxylate (e.g., acid chloride, NHS ester), and a Michael acceptor (e.g., an alkene or alkyne or acetylene directly bonded to an electron withdrawing group like a ketone, ester, cyano, nitro, sulfone, or sulfonyl). In some implementations the conjugation is accomplished using a click chemistry: Cu(I)-catalyzed azide-alkyne click chemistry reaction, strain-promoted azide-alkyne click chemistry reaction (SPAAC), cycloaddition between tetrazine and alkene (trans-cyclooctene). In some implementations the alkyne is a cyclooctyne like a dibenzocyclooctyne or bicyclo[6.1.0]nonyne. The prerequisite reactive group may be included in the entangled hydrogel by including the relevant monomer in the polymerization reaction. While the reactive group may be dispersed through the entangled hydrogel, due to sterics and other factors the brush polymer will only react with those functional groups at the surface of the entangled hydrogel. The brush polymer may be provided with the necessary functional group using conventional chemistries. [0176] In some implementations, a brush polymer can be directly formed on the entangled hydrogel network. In certain implementations the entangled hydrogel is first functionalized with an anchor compound using the chemistries disclosed above. The anchor compound include a polymerizable group, for example a radically labile group such as a (meth)acrylate or meth(acrylamide). Once the entangled hydrogel is functionalized with the radically labile group it can be combined with one or more appropriate monomers (e.g., (meth)acrylates, (meth)acylamides, and/or vinyl compounds) and subjected to conditions suitable to polymerize the monomers. [0177] In certain implementations, the brush polymer is not covalently conjugated to the entangled hydrogel, but rather mechanically conjugated. In such embodiments, a portion of the brush polymer is physically entangled with the entangled network. In certain implementations, the entangled hydrogel may be swelled in a solution including the brush polymer. A portion of the brush polymer will penetrate the hydrogel. In certain embodiments, various surfactants, plasticizers, and the like may be included in the solvent to facilitate penetration (a penetration aid) of the brush polymer. In certain implementations, the penetration aid can include polyethylene glycol, polypropylene oxide, pectin, carrageenan, polylysine, gelatins (including gelatin type A), agarose, PEO-PPO-PEO copolymers (Pluronics® polymers), poly(phosphazene), hyaluronans, chitosans, agar, heparin, sulfate, cellulose, alginates (including alginate sulfate), collagen, dextrans (including dextran sulfate), poly(hydroxyethyl methacrylate), (poly(methyl methacrylate, poly(N-isopropylacrylamide), poly(lactic acid), poly(lactic-co-glycolic acid), poly(N- vinylpyrrolidone), PL(G)A-PEO-PL(G)A copolymers, poly(ethylene imine), polyethylene glycol (PEG)-thiol, poly(L-aspartic acid), poly(L-glutamic acid), 2-methylmaleic acid, itaconic acid, 2-methylitaconic, or a combination thereof. After a suitable period, the entangled hydrogel may be washed to remove penetration aid and non-conjugated brush polymer. IMPLANTABLE MEDICAL DEVICE [0178] It is understood that any of entangled hydrogel networks described above can be used in an implantable medical device. [0179] In certain aspects, the exemplary implantable medical device can comprise a frame 1000, as shown in FIG.1. In such an aspect, the frame 1000 is shown in an expanded configuration. The frame 1000 can include a frame portion 1002. In certain implementations frame portion 1002 will include an entangled hydrogel network. Frame portion 1002 can include an inner frame side 1002a and an outer frame side 1002b. In certain implementations inner frame side 1002a can include an entangled hydrogel network, while outer frame side 1002b does not. In certain implementations outer frame side 1002b can include an entangled hydrogel network, while inner frame side 1002a does not. In other implementations both inner frame side 1002a and outer frame side 1002b can include an entangled hydrogel network. In certain implementations inner frame side 1002a and outer frame side 1002b can include the same entangled hydrogel network, while in other implementations inner frame side 1002a will include a different entangled hydrogel network than outer frame side 1002b. Frame portion 1002 can have an upper region 1004, an intermediate region 1006, and a lower region 1008 (and corresponding upper region, inner side, upper region, outer side; intermediate region, inner side, intermediate region, outer side, lower region, inner side, and lower region outside side (not shown)). In certain implementations one or more of frame portions 1004, 1006, and 1008 will include an entangled hydrogel network. In certain implementations each of frame portions 1004, 1006, and 1008 will include an entangled hydrogel network, and in some implementations the same entangled network is present on each portion. In certain aspects, the upper region 1004 can include an entangled hydrogel network, which can be on the inner side, outer side, or both. When both sides of upper region 1004 include an entangled hydrogel network, both sides can include the same network, or each can have a different network. In certain aspects, the intermediate region 1006 can include an entangled hydrogel network, which can be on the inner side, outer side, or both. When both sides of intermediate region 1006 include an entangled hydrogel network, both sides can include the same network, or each can have a different network. In certain aspects, the lower region 1008 can include an entangled hydrogel network, which can be on the inner side, outer side, or both. When both sides of lower region 1008 include an entangled hydrogel network, both sides can include the same network, or each can have a different network. [0180] A longitudinal axis (not shown) of the frame 1000 can be defined as the central axis that extends through the center of the frame 1000 between the upper and lower ends of the frame 1000. In some aspects, the frame 1000 can be oriented such that the upper region 1004 is a proximal portion, and the lower region 1008 is a distal portion. The frame 1000 can include a plurality of anchoring members 1010. In some aspects, the frame 1000 can be oriented such that the plurality of anchoring members 1010 are a distal anchoring members. In certain implementations, the anchors can include an entangled hydrogel network. In still further aspects, the one or more anchors of the medical device are positioned within the disclosed herein article. [0181] In still further aspects, the implantable device is a prosthetic valve configured to be deployed to a native valve of the heart. Exemplary prosthetic valves are shown in FIGS. 2, 3A, and 3B and described below in more detail. [0182] Referring to FIG.2, in this aspect, the prosthetic valve 2000, comprises a frame 2002, as described in FIG.1. The valve further comprises a plurality of prosthetic leaves 2020, an inner skirt 2016 and an outer skirt 2018. The outer skirt or the sealing element can be used for a paravalvular leak seal. Inner skirt 2016 can have an inner side 2016a and an outer side 2016b. The implantable medical device has an inflow end 2040 and an outflow end 2030. In certain implementations, the prosthetic leaves 2020 include an entangled hydrogel network. In certain implementations inner skirt 2016 includes an entangled hydrogel network. Inner skirt 2016 can have an inner side 2016a and an outer side 2016b. In certain implementations inner skirt, inner side 2016a includes an entangled hydrogel network. In certain implementations inner skirt, outer side 2016b includes an entangled hydrogel network. In certain implementations, when one side of the inner skirt includes an entangled hydrogel network, the other side will not. In certain implementations, both inner skirt, inner side 2016a and inner skirt, outer side 2016b can include an entangled hydrogel network. In some implementations inner skirt, inner side 2016a and inner skirt, outer side 2016b can include the same entangled hydrogel network. In other implementations inner skirt, inner side 2016a and inner skirt, outer side 2016b can include different entangled hydrogel networks. In certain implementations an outer skirt 2018 includes an entangled hydrogel network. Outer skirt 2018 can include an inner side (not shown) and outer side 2018b. In certain implementations the outer skirt, inner 8 includes an entangled hydrogel network. In certain implementations, when one side of the inner skirt includes an entangled hydrogel network, the other side will not. In certain implementations, both the outer skirt, inner side and outer skirt, outer side 2018b can include an entangled hydrogel network. In some implementations the outer skirt, inner side and outer skirt, outer side 2018b can include the same entangled hydrogel network. In other implementations the inner skirt, inner side and outer skirt, outer side 2018b can include different entangled hydrogel networks. [0183] In still further aspects, the implantable medical device is configured to be deployed to a native valve of a heart, wherein the prosthetic valve further comprises the plurality of prosthetic valve leaflets as shown herein, wherein the one or more anchors are coupled to the plurality of prosthetic valve leaflets, and each is configured to anchor to a portion of the heart. [0184] Some exemplary prosthetic devices are shown in FIG.3A and 3B. In one aspect is an implantable prosthetic valve 3100, comprising: an annular frame 3040, wherein the frame has an inflow end 3020 and an outflow end 3030, and a central longitudinal axis 2050 extending from the inflow end to the outflow end. An exemplary prosthetic heart valve 3100 is also described in U.S. Patent No.10,463,484, titled “Prosthetic Heart Valve Having Leaflet Inflow Below Frame,” which is incorporated herein by reference. The illustrated prosthetic valve 3100 is adapted to be implanted in the native aortic annulus, although in other aspects, it can be adapted to be implanted in the other native annuluses of the heart (the mitral valve, pulmonary valve, and tricuspid valve). [0185] In still further aspects, the implantable prosthetic valve also comprises an inner skirt 3005 having an inner surface (not numbered) and an outer surface 3005b and is positioned along the inner surface of the frame. As described earlier, either surface of the inner skirt may include an entangled hydrogel network, either the same or different. In still further aspects, the valve comprises a leaflet structure 3060 comprising one or more leaflets, having an inner surface and an outer surface and positioned at the inner surface of the annular frame. In some implementations the inner surface of the leaflets includes an entangled hydrogel network. In some implementation the outer surface of the leaflets includes an entangled hydrogel network. In some implementations, both the outer surface and inner surface of the leaflets include an entangled hydrogel network. In such implementations, the inner surface and outer surface may include the same entangled hydrogel network, or may include different hydrogel networks. In still further aspects, the valve can optionally comprise an outer skirt. The outer skirt 3009 as shown, for example, in FIG.3 has an inner surface (not numbered) and an outer surface 3009b and is positioned at the outer surface of the annular frame 3040. As described earlier, either surface of the outer skirt may include an entangled hydrogel network, either the same or different. The outer skirt can also be attached to the frame with one or more sutures 3007. The valve can also comprise additional sutures, for example, 3006 and 3014 that run along various portions of the device. In still further aspects, the sutures can be used to attach at least a portion of the inner skirt and/or at least a portion of the leaflet structure and/or at least a portion of the outer skirt, if present, to at least a frame of an implantable prosthetic valve. In yet still further aspects, the sutures can be used to attach various components of the valve together. In some implementations, the sutures include an entangled hydrogel network. [0186] In certain aspects, the prosthetic valve 3100 can comprise a leaflet structure 3060. In certain aspects, the leaflet structure can comprise one or more leaflets, each of which can be arranged to collapse in a tricuspid arrangement. The certain aspects, an edge of the leaflet structure 3060 can have an undulating, curved scalloped shape. Moreover, by virtue of the scalloped shape, folds and ripples at the belly of each leaflet, which can cause early calcification in those areas, can be eliminated or at least minimized. Leaflets can have various other shapes and/or configurations in other aspects. It is understood, however, that the leaflets of the leaflet structure need not have a V-shaped or scalloped inflow edge, and instead, each leaflet can have a square or rectangular shape defining a straight inflow edge. As described earlier, either surface of the leaflets may include an entangled hydrogel network, either the same or different. [0187] As shown in FIG.3A and 3B, the leaflets 3060 can be secured with the one or more sutures 3014 to the frame 3040. The frame 3040 can be made of any of various suitable plastically-expandable materials (e.g., stainless steel, cobalt chromium, etc.) or self- expanding materials (e.g., nitinol) as is known in the art. When constructed of a plastically- expandable material, the frame 3040 can be crimped to a radially compressed state on a delivery catheter and then expanded inside a patient by an inflatable balloon or equivalent expansion mechanism. When constructed of a self-expandable material, the frame 3040 can be crimped to a radially compressed state and restrained in the compressed state by insertion into a sheath or equivalent mechanism of a delivery catheter. Once inside the body, the valve can be advanced from the delivery sheath, which allows the valve to expand to its functional size. Suitable plastically-expandable materials that can be used to form the frame 3040 include, without limitation, stainless steel, a nickel-based alloy (e.g., a cobalt- chromium or a nickel-cobalt-chromium alloy), polymers, or combinations thereof. In particular aspects, frame 3040 can be made of a nickel-cobalt-chromium-molybdenum alloy, such as MP35N™ alloy (tradename of SPS Technologies), which is equivalent to UNS R30035 (covered by ASTM F562-02). MP35N™/UNS R30035 comprises 35% nickel, 35% cobalt, 20% chromium, and 10% molybdenum by weight. In aspects when MP35N alloy is used as the frame material, less material is needed to achieve the same or better performance in radial and crush force resistance, fatigue resistance, and corrosion resistance. Moreover, since less material is required, the crimped profile of the frame can be reduced, thereby providing a lower profile valve assembly for percutaneous delivery to the treatment location in the body. [0188] The leaflets can be sutured together to form the leaflet structure 3060, which can then be secured to the frame 3040. It is understood that when the leaflets are sutured together, each of the leaflets can comprise any of the disclosed herein sutures if desired. However, also disclosed herein are the aspects, when different from the disclosed one or more sutures are used to secure the leaflet structure within the valve. In still further exemplary and unlimiting aspects, the leaflets of the leaflet structure 3060 can be secured to one another at their adjacent sides to form commissures 3011 of the leaflet structure. It is understood that sutures disclosed herein can also be used to form such commissures. Yet, in other aspects, sutures different from those disclosed herein sutures can also be used. [0189] In still further exemplary aspects, the one or more leaflets of the leaflet structure 3060 can be attached to the inner skirt 3005, with sutures 3014. The suture 3014 can track the curvature of the bottom edge of the leaflet structure 3060 and are collectively referred to as the scallop line. [0190] The inner skirt 3005 can have a plurality of functions, which can include assisting in securing the leaflet structure 3060 and/or the outer skirt 3009 to the frame 3040 and to assist in forming a good seal between the valve 3100 and the native annulus by blocking the flow of blood below the lower edges of the leaflets. It is understood that any known in the art configuration/construction of the skirt can be used. For example, and without limitation, the configuration/construction of the skirt can comprise a textile that is braided, knitted, woven and/or nonwoven. The inner skirt 3005 can comprise a tough, tear-resistant material such as polyethylene terephthalate (PET), although various other synthetic or natural materials can be used. The thickness of the skirt is desirably less than 6 mils or 0.15 mm, and desirably less than 4 mils or 0.10 mm, and even more desirably about 2 mils or 0.05 mm, and even still more desirably about 1.1 mils or 0.03 mm. In particular aspects, the skirt 3005 can have a variable thickness, for example, the skirt can be thicker at its edges than at its center. In one implementation, the skirt 3005 can comprise a PET skirt having a thickness of about 0.07 mm at its edges and about 0.06 mm at its center. The thinner skirt can provide for better crimping performances while still providing good perivalvular sealing. In still further aspects, the inner skirt 3005 can have any of the disclosed coatings herein. In certain aspects, only an inner surface of the inner skirt 3005 can have the disclosed herein coating. While in other aspects, both surfaces of the inner skirt can have the same coating. [0191] In still further aspects, the implantable medical device is configured to be deployed to a native valve of a heart, wherein the prosthetic valve further comprises the plurality of prosthetic valve leaflets as shown herein, wherein the one or more anchors are coupled to the plurality of prosthetic valve leaflets, and each is configured to anchor to a portion of the heart. [0192] Features of an implant that can be utilized are disclosed in U.S. Patent Application No.16/028,172, the entire content of which is incorporated by reference herein. Additional details and example designs for an implant and prosthesis that can be utilized in examples herein are described in U.S. Patent Nos.8,403,983, 8,414,644, 8,652,203 and U.S. Patent Publication Nos.2011/0313515, 2012/0215303, 2014/0277390, 2014/0277422, 2014/0277427, 2018/0021129, and 2018/0055629, the entirety of these patents and publications are hereby incorporated by reference and made a part of this specification. Further details and examples of a replacement heart valve or prosthesis and its method of implantation are described in U.S. Publication Nos.2015/0328000 and 2016/0317301, the entirety of each of which is hereby incorporated by reference and made a part of this specification. [0193] The implantable medical devices disclosed herein can comprise a mitral replacement valve or a tricuspid replacement valve, among other forms of valves (e.g., aortic replacement valves, pulmonary replacement valves, or other valves). The implantable medical devices disclosed herein can include prosthetic heart valves or other forms of implants, such as stents or filters or diagnostic devices, among others. The implantable medical devices can be expandable implants configured to move from a compressed or undeployed state to an expanded or deployed state. The implantable medical devices can be compressible implants configured to be compressed inward to have a reduced outer profile and to move the implant to the compressed or undeployed state. [0194] Various forms of delivery apparatuses can be utilized with the examples disclosed herein. The delivery apparatuses as disclosed herein can be utilized for aortic, mitral, tricuspid, and pulmonary replacement and repair as well. The delivery apparatuses can comprise delivery apparatuses for the delivery of other forms of implants, such as stents or filters or diagnostic devices, among others. [0195] The implantable medical devices and the systems disclosed herein can be used in transcatheter aortic valve implantation (TAVI) or replacement of other native heart valves (e.g., mitral, tricuspid, or pulmonary). The delivery apparatuses and the systems disclosed herein can be utilized for transarterial access, including transfemoral access, to a patient’s heart. The delivery apparatuses and systems can be utilized in transcatheter percutaneous procedures, including transarterial procedures, which can be transfemoral or transjugular. Transapical procedures, among others, can also be utilized. Other procedures can be utilized as desired. Features of aspects can be modified, substituted, excluded, or combined across examples as desired. [0196] Still further disclosed is a method comprising: deploying a prosthetic valve to a native valve of a patient’s heart, the prosthetic valve comprising: a plurality of prosthetic valve leaflets, one or more anchors coupled to the plurality of prosthetic valve leaflets and each configured to anchor to a portion of the patient’s heart, wherein each of the one or more anchors is inserted into the disclosed herein woven article. In such aspects, the one or more anchors comprise ventricular anchors. In still further aspects, the method further comprises hooking each of the one or more anchors around a native valve leaflet. In still further aspects, the native valve is a native mitral valve or a native tricuspid valve. EXEMPLARY ASPECTS [0197] In view of the described processes and compositions, hereinbelow are described certain more particularly described aspects of the disclosures. These particularly recited aspects should not, however, be interpreted to have any limiting effect on any different claims containing different or more general teachings described herein, or that the “particular” aspects are somehow limited in some way other than the inherent meanings of the language and formulas literally used therein. [0198] Example 1: A cardiovascular repair device comprising an entangled hydrogel network on at least one surface, wherein the device is optionally a prosthetic valve, a patch, a clasp, a sleeve, a conduit, or a band. [0199] Example 2: The device of any example herein, particular example 1, wherein the device is an implantable prosthetic valve comprising: an annular frame having an inner surface and an outer surface, comprising an inflow end and an outflow end and being radially collapsible and expandable between a radially collapsed configuration and a radially expanded configuration; a valvular structure comprising a plurality of leaflets positioned within the frame; an outer skirt positioned along at least a portion of the outer surface of the frame, wherein the outer skirt has an inner surface and an outer surface; an inner skirt positioned along at least a portion of the inner surface of the frame, wherein the inner skirt has an inner surface and an outer surface; wherein at least one of the frame, outer skirt, inner skirt, or one or more leaflets comprises an entangled hydrogel network. [0200] Example 3: The device of any example herein, particularly Examples 1–2, wherein entangled hydrogel network is covalently bonded to the surface, mechanically bonded to the surface, or compounded with the surface of the device. [0201] Example 4: The device of any example herein, particularly Examples 1–3, wherein entangled hydrogel network is covalently bonded to the surface, mechanically bonded to the surface, or compounded with the surface. [0202] Example 5: The device of any example herein, particularly Examples 1–4, wherein the inner skirt comprises the entangled hydrogel network. [0203] Example 6: The device of any example herein, particularly Examples 1–5, wherein the inner skirt comprises the entangled hydrogel network, and the outer skirt does not comprise the entangled hydrogel network. [0204] Example 7: The device of any example herein, particularly Examples 1–6, wherein the outer skirt comprises the entangled hydrogel network. [0205] Example 8: The device of any example herein, particularly Examples 1–7, wherein the outer skirt comprises the entangled hydrogel network, and the inner skirt does not comprise the entangled hydrogel network. [0206] Example 9: The device of any example herein, particularly Examples 1–8, wherein the outer skirt and the inner skirt comprise the entangled hydrogel network. [0207] Example 10: The device of any example herein, particularly Examples 1–9, wherein the inner surface of the inner skirt comprises the entangled hydrogel network. [0208] Example 11: The device of any example herein, particularly Examples 1–10, wherein the inner surface of the inner skirt comprises the entangled hydrogel network, and the outer surface of the inner skirt does not comprise the entangled hydrogel network. [0209] Example 12: The device of any example herein, particularly Examples 1–11, wherein the outer surface of the inner skirt comprises the entangled hydrogel network. [0210] Example 13: The device of any example herein, particularly Examples 1–12, wherein the outer surface of the inner skirt comprises the entangled hydrogel network, and the inner surface of the inner skirt does not comprise the entangled hydrogel network. [0211] Example 14: The device of any example herein, particularly Examples 1–13, wherein the inner surface and outer surface of the inner skirt comprise the entangled hydrogel network. [0212] Example 15: The device of any example herein, particularly Examples 1–14, wherein the inner surface of the frame comprises the entangled hydrogel network. [0213] Example 16: The device of any example herein, particularly Examples 1–15, wherein the inner surface of the frame comprises the entangled hydrogel network, and the outer surface of the frame does not comprise the entangled hydrogel network. [0214] Example 17: The device of any example herein, particularly Examples 1–16, wherein the outer surface of the frame comprises the entangled hydrogel network. [0215] Example 18: The device of any example herein, particularly Examples 1–17, wherein the outer surface of the frame comprises the entangled hydrogel network, and the inner surface of the frame does not comprise the entangled hydrogel network. [0216] Example 19: The device of any example herein, particularly Examples 1–18, wherein the inner surface and outer surface of the frame comprise the entangled hydrogel network. [0217] Example 20: The device according to of any example herein, particularly Examples 1–19, wherein the inner surface of the outer skirt comprises the entangled hydrogel network. [0218] Example 21: The device according of any example herein, particularly Examples 1–20, wherein the inner surface of the outer skirt comprises the entangled hydrogel network, and the outer surface of the outer skirt does not comprise the entangled hydrogel network. [0219] Example 22: The device of any example herein, particularly Examples 1–21, wherein the outer surface of the outer skirt comprises the entangled hydrogel network. [0220] Example 23: The device of any example herein, particularly Examples 1–22, wherein the outer surface of the outer skirt comprises the entangled hydrogel network, and the inner surface of the outer skirt does not comprise the entangled hydrogel network. [0221] Example 24: The device of any example herein, particularly Examples 1–23, wherein the inner surface and outer surface of the outer skirt comprise the entangled hydrogel network. [0222] Example 25: The device of any example herein, particularly Examples 1–24, wherein the outer surface of the frame comprises a covered portion covered by the outer skirt, and an exposed portion that is not covered by the outer skirt. [0223] Example 26: The device of any example herein, particularly Examples 1–25, wherein the exposed portion of the outer surface of the frame comprises the entangled hydrogel network. [0224] Example 27: The device of any example herein, particularly Examples 1–26, wherein the inner surface of the frame comprises the entangled hydrogel network, and the outer surface of the frame does not comprise the entangled hydrogel network. [0225] Example 28: The device of any example herein, particularly Examples 1–27, wherein each leaflet comprises the entangled hydrogel network. [0226] Example 29: The device of any example herein, particularly Examples 1–28, wherein the leaflet structure comprises two or three leaflets. [0227] Example 30: The device of any example herein, particularly Examples 1–29, wherein the leaflet structure comprises pericardial tissue (e.g., bovine pericardial tissue), biocompatible synthetic polymers (e.g., polyurethane, polyethylene terephthalate), or a combination thereof. [0228] Example 31: The device of any example herein, particularly Examples 1–30, wherein the frame comprise a metal (pure titanium, cobalt–chromium-nickel or molybdenum alloys such as Elgiloy® alloy, stainless steel (316L), nickel‑titanium alloy (nitinol), platinum, and tantalum alloys or magnesium alloys, a metal-based frame with ceramic-coated, or a combination thereof. [0229] Example 32: The device of any example herein, particularly Examples 1–31, wherein the frame comprises nickel-titanium alloy, cobalt-chromium alloy, nickel-cobalt- chromium alloy, nickel-cobalt-chromium-molybdenum alloy, stainless steel, [0230] Example 33: The device of any example herein, particularly Examples 1–32, wherein the skirt comprises of the following polymer yarns that can be constructed into textile (wovens or knit warps) such as polyhydroxyalkanoates (PHAs) and polyethylene furanoate (PEF) polyethylene terephthalate (PET) polyamides of high molecular weight such as Nylon 6, 6,6, 12, 610, 1010. [0231] Example 34: The device of any example herein, particularly Examples 1–33, wherein the inner skirt is sutured to the frame with sutures, wherein said sutures comprise an ionically charged polymer coating. [0232] Example 35: The device of any example herein, particularly Examples 1–34, comprising a first entangled hydrogel network and a second entangled hydrogel network, wherein the inner surface of the frame comprises the first entangled hydrogel network, and the inner skirt comprises the second entangled hydrogel network. [0233] Example 36: The device of any example herein, particularly Examples 1–35, wherein the first entangled hydrogel network is mechanically bonded to the frame, and the second entangled hydrogel network is covalently bonded to the inner skirt. [0234] Example 37: The device of any example herein, particularly Examples 1–36, wherein the entangled hydrogel network is covalently incorporated into the polymeric structure or attached to the surface. [0235] Example 38: The device of any example herein, particularly Examples 1–37, wherein the entangled hydrogel network is mechanically bonded to the surface. [0236] Example 39: The device of any example herein, particularly Examples 1–38, wherein the entangled hydrogel network has a thickness from 10 nm – 100 µm, from 10 nm – 1,000 nm, from 10 nm – 500 nm, from 50 nm – 500 nm, from 50 nm – 250 nm, from 250 nm – 500 nm, from 500 nm – 2,500 nm, from 500 nm – 1,500 nm, from 1 µm – 100 µm, from 1 µm – 5 µm, from 1 µm – 10 µm, from 5 µm – 15 µm, from 10 µm – 25 µm, from 10 µm – 50 µm, from 25 µm – 50 µm, from 50 µm – 75 µm, or from 75 µm – 100 µm. [0237] Example 40: The device of any example herein, particularly Examples 1–39, wherein the entangled hydrogel network comprises a first ionically charged polymer and a second ionically charged polymer. [0238] Example 41: The device of any example herein, particularly Examples 1–40, wherein the entangled hydrogel network comprises a first ionically charged polymer, a second ionically charged polymer, and a third ionically charged polymer. [0239] Example 42: The device of any example herein, particularly Examples 1–41, wherein the first ionically charged polymer is a crosslinked polymer or is not a crosslinked polymer. [0240] Example 43: The device of any example herein, particularly Examples 1–42, wherein the first ionically charged polymer comprises a backbone and one or more side chains. [0241] Example 44: The device of any example herein, particularly Examples 1–43, wherein the first ionically charged polymer is a homopolymer or copolymer. [0242] Example 45: The device of any example herein, particularly Examples 1–44, wherein the first ionically charged polymer is a cationic polymer, anionic polymer, or zwitterionic polymer. [0243] Example 46: The device of any example herein, particularly Examples 1–45, wherein the first ionically charged polymer has an ionically charged polymer backbone. [0244] Example 47: The device of any example herein, particularly Examples 1–46, wherein the backbone of the first ionically charged polymer comprises quaternary ammonium groups. [0245] Example 48: The device of any example herein, particularly Examples 1–47, wherein the backbone of the first ionically charged polymer comprises phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0246] Example 49: The device of any example herein, particularly Examples 1–48, wherein the first ionically charged polymer comprises a polyalkylene backbone, a polyether backbone, a polyurethane backbone, or combination thereof. [0247] Example 50: The device of any example herein, particularly Examples 1–49, wherein the first ionically charged polymer comprises a neutral polymer backbone and ionically charged side chains. [0248] Example 51: The device of any example herein, particularly Examples 1–50, wherein the first ionically charged polymer comprises a neutral polymer backbone and a combination of ionically charged side chains and neutral side chains. [0249] Example 52: The device of any example herein, particularly Examples 1–51, wherein the first ionically charged polymer is a random copolymer. [0250] Example 53: The device of any example herein, particularly Examples 1–52, wherein the first ionically charged polymer comprises a zwitterionic side chains and neutral side chains. [0251] Example 54: The device of any example herein, particularly Examples 1–53, wherein the first ionically charged polymer comprises cationic side chains and anionic side chains. [0252] Example 55: The device of any example herein, particularly Examples 1–54, wherein the first ionically charged polymer comprises a poly(meth)acrylate, a poly(meth)acrylamide, a polyolefin, or a combination thereof. [0253] Example 56: The device of any example herein, particularly Examples 1–55, wherein the first ionically charged polymer comprises side chains comprising phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0254] Example 57: The device of any example herein, particularly Examples 1–56, wherein the first ionically charged polymer comprises a first repeating unit of formula (1a), formula (1b), or a combination thereof: [Formula (1b)], wherein R1m1 is H or CH3; R1m2 is null, C(=O)O, or C(=O)NH; R1a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C1–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is H, carboxylate; sulfonate, or phosphonate; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z1a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. [0255] Example 58: The device of any example herein, particularly Examples 1–57, wherein the first repeating unit in the first ionically charged polymer includes units having the formula: , or a [0256] Example 59: The device of any example herein, particularly Examples 1–58, X1a is N+(CH3)2, and Y1a is not H. [0257] Example 60: The device of any example herein, particularly Examples 1–58, X1a is -O-PO2–-O-, and Y1a is H. [0258] Example 61: The device of any example herein, particularly Examples 1–60, wherein the first repeating unit in the first ionically charged polymer includes units having the formula:
, R1f1is O or NH; Y1a is carboxylate; sulfonate, or phosphonate; and Z1a is H or -N+(Rn1)3. [0259] Example 62: The device of any example herein, particularly Examples 1–61, wherein the first repeating unit in the first ionically charged polymer includes units having the formula: , wherein R1f1 is O [0260] Example 63: The device of any example herein, particularly Examples 1–62, wherein the first ionically charged polymer further comprises a second repeating unit of formula (1c), formula (1d), or a combination thereof: [Formula (1d)], wherein R1m3 is H or CH3; R1m4 is null, C(=O)O, or C(=O)NH; R1a2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e2 is a C1–15 hydrocarbyl group; wherein any two or more of R1a2, R1b2, R1c2, and R1d2 can together form a ring; Y1b is H, carboxylate; sulfonate, or phosphonate; X1b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z1b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R1a2, R1b2, R1c2, and R1d2 is not null; and when X1b is null then at least one of Z1b and Y1b is not H. [0261] Example 64: The device of any example herein, particularly Examples 1–63, wherein the second repeating unit in the first ionically charged polymer includes units having the formula: , or a combination [0262] Example 65: The device of any example herein, particularly Examples 1–64, X1b is N+(CH3)2, and Y1b is not H. [0263] Example 66: The device of any example herein, particularly Examples 1–64, X1b is -O-PO2–-O-, and Y1b is H. [0264] Example 67: The device of any example herein, particularly Examples 1–66, wherein the second repeating unit in the first ionically charged polymer includes units having the formula: , R1f2 is O or NH; Y1b is carboxylate; sulfonate, or phosphonate; and Z1b is H or -N+(Rn1)3. [0265] Example 68: The device of any example herein, particularly Examples 1–67, wherein the second repeating unit in the first ionically charged polymer includes repeating units having the formula:
, or a combinati wherein R1f2 is O or NH. [0266] Example 69: The device of any example herein, particularly Examples 1–68, wherein the first ionically charged polymer further comprises neutral repeating units of formula (1e), formula (1f), or a combination thereof: [Formula (1f)], wherein, R1m5 is H or CH3; R1m6 is H or CH3; and R1a is OR1b or N(R1b)2, wherein R1b is in each case independently selected from H, C1–12 hydrocarbyl, wherein R1b may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. [0267] Example 70: The device of any example herein, particularly Examples 1–69, wherein R1a is O-(2-ethylhexyl). [0268] Example 71: The device of any example herein, particularly Examples 1–69, wherein R1a is O-CH2CH2N(CH3)2. [0269] Example 72: The device of any example herein, particularly Examples 1–71, wherein the first ionically charged polymer consists of the first repeating unit and the neutral repeating unit. [0270] Example 73: The device of any example herein, particularly Examples 1–71, wherein the first ionically charged polymer consists of the first repeating unit and second repeating unit. [0271] Example 74: The device of any example herein, particularly Examples 1–71, wherein the first ionically charged polymer consists of the first repeating unit, second repeating unit, and neutral repeating unit. [0272] Example 75: The device of any example herein, particularly Examples 1–74, wherein the second ionically charged polymer is a crosslinked polymer or is not a crosslinked polymer. [0273] Example 76: The device of any example herein, particularly Examples 1–75, wherein the second ionically charged polymer comprises a backbone and one or more side chains. [0274] Example 78: The device of any example herein, particularly Examples 1–77, wherein the second ionically charged polymer is a homopolymer or copolymer. [0275] Example 79: The device of any example herein, particularly Examples 1–78, wherein the second ionically charged polymer is a cationic polymer, anionic polymer, or zwitterionic polymer. [0276] Example 80: The device of any example herein, particularly Examples 1–79, wherein the second ionically charged polymer has an ionically charged polymer backbone. [0277] Example 81: The device of any example herein, particularly Examples 1–80, wherein the backbone of the second ionically charged polymer comprises quaternary ammonium groups. [0278] Example 82: The device of any example herein, particularly Examples 1–81, wherein the backbone of the second ionically charged polymer comprises phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0279] Example 83: The device of any example herein, particularly Examples 1–82, wherein the second ionically charged polymer comprises a polyalkylene backbone, a polyether backbone, a polyurethane backbone, or combination thereof. [0280] Example 84: The device of any example herein, particularly Examples 1–83, wherein the second ionically charged polymer comprises a neutral polymer backbone and ionically charged side chains. [0281] Example 85: The device of any example herein, particularly Examples 1–84, wherein the second ionically charged polymer comprises a neutral polymer backbone and a combination of ionically charged side chains and neutral side chains. [0282] Example 86: The device of any example herein, particularly Examples 1–85, wherein the second ionically charged polymer is a random copolymer. [0283] Example 87: The device of any example herein, particularly Examples 1–86, wherein the second ionically charged polymer comprises a zwitterionic side chains and neutral side chains. [0284] Example 88: The device of any example herein, particularly Examples 1–87, wherein the second ionically charged polymer comprises cationic side chains and anionic side chains. [0285] Example 89: The device of any example herein, particularly Examples 1–88, wherein the second ionically charged polymer comprises a poly(meth)acrylate, a poly(meth)acrylamide, a polyolefin, or a combination thereof. [0286] Example 90: The device of any example herein, particularly Examples 1–89, wherein the second ionically charged polymer comprises side chains comprising phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0287] Example 91: The device of any example herein, particularly Examples 1–90, wherein the second ionically charged polymer includes first repeating units of formula (2a), formula (2b), or combination thereof: [Formula (2b)], R2m1 is H or CH3; R2m2 is null, C(=O)O, or C(=O)NH; R2a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e1 is a C1–15 hydrocarbyl group; wherein any two or more of R2a1, R2b1, R2c1, and R2d1 can together form a ring; Y2a is H, carboxylate; sulfonate, or phosphonate; X2a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z2a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R2a1, R2b1, R2c1, and R2d1 is not null; and when X2a is null then at least one of Z2a and Y2a is not H. [0288] Example 92: The device of any example herein, particularly Examples 1–91, wherein the repeating unit of formula (2b) includes units having the formula: , or a [0289] Example 93: The device of any example herein, particularly Examples 1–92, X2a is N+(CH3)2, and Y2a is not H. [0290] Example 94: The device of any example herein, particularly Examples 1–92, X2a is -O-PO2–-O-, and Y2a is H. [0291] Example 95: The device of any example herein, particularly Examples 1–94, wherein the repeating unit of formula (2a) includes units having the formula:
, R2f1 is O or NH; Y2a is carboxylate; sulfonate, or phosphonate; and Z2a is H or -N+(Rn1)3. [0292] Example 96: The device of any example herein, particularly Examples 1–95, wherein the first repeating unit in the second ionically charged polymer includes units having the formula: , [0293] Examples 1–96, wherein the second ionically charged polymer further comprises a second repeating unit of formula (2c), formula (2d), or a combination thereof: [Formula (2d)], wherein R2m3 is H or CH3; R2m4 is null, C(=O)O, or C(=O)NH; R2a2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e2 is a C1–15 hydrocarbyl group; wherein any two or more of R2a2, R2b2, R2c2, and R2d2 can together form a ring; Y2b is H, carboxylate; sulfonate, or phosphonate; X2b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z2b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R2a2, R2b2, R2c2, and R2d2 is not null; and when X2b is null then at least one of Z2b and Y2b is not H. [0294] Example 98: The device of any example herein, particularly Examples 1–97, wherein the repeating unit of formula (2d) includes units having the formula: , or a combination [0295] Example 99: The device of any example herein, particularly Examples 1–98, wherein X2b is N+(CH3)2, and Y2b is not H. [0296] Example 100: The device of any example herein, particularly Examples 1–98, wherein X2b is -O-PO2–-O-, and Y2b is H. [0297] Example 101: The device of any example herein, particularly Examples 1–100, wherein repeating unit of formula (2c) includes units having the formula: R2m2 , R2f2 is O or NH; Y2b is carboxylate; sulfonate, or phosphonate; and Z2b is H or -N+(Rn1)3. [0298] Example 102: The device of any example herein, particularly Examples 1–101, wherein the repeating unit of formula (2c) includes units having the formula:
, or a combinati wherein R2f2 is O or NH. [0299] Example 103: The device of any example herein, particularly Examples 1–102, wherein the second ionically charged polymer further comprises neutral repeating units of formula (2e), formula (2f), or a combination thereof: R2m5 [Formula (1f)], wherein, R2m5 is H or CH3; R2m6 is H or CH3; and R2a is OR2b or N(R2b)2, wherein R2b is in each case independently selected from H, C1– 12 hydrocarbyl, wherein R2b may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. [0300] Example 104: The device of any example herein, particularly Examples 1–103, wherein R2a is O-(2-ethylhexyl). [0301] Example 105: The device of any example herein, particularly Examples 1–103, wherein R2a is O-CH2CH2N(CH3)2. [0302] Example 106: The device of any example herein, particularly Examples 1–105, wherein the second ionically charged polymer consists of the first repeating unit and third repeating unit. [0303] Example 107: The device of any example herein, particularly Examples 1–105, wherein the second ionically charged polymer consists of the first repeating unit and second repeating unit. [0304] Example 108: The device of any example herein, particularly Examples 1–105, wherein the second ionically charged polymer consists of the first repeating unit, second repeating unit, and third repeating unit. [0305] Example 109: The device of any example herein, particularly Examples 1–108, wherein the weight ratio of the first ionically charged polymer to the second ionically charged polymer is from 10:1 to 1:10, from 5:1 to 1:5, from 2.5:1 to 1:2.5, from 1.5:1 to 1:1.5, from 10:1 to 5:1, from 10:1 to 1:1, from 5:1 to 1:1, from 2.5:1 to 1:1, from 1:1 to 1:2.5, from 1:1 to 1:5, from 1:1 to 1:10, or from 1:5 to 1:10. [0306] Example 110: The device of any example herein, particularly Examples 1–109, wherein the first ionically charged polymer and the second ionically charged polymer are covalently bonded to one another, or are not covalently bonded to one another. [0307] Example 111: The device of any example herein, particularly Examples 1–110, wherein the entangled hydrogel network further comprises polymer brushes. [0308] Example 112: The device of any example herein, particularly Examples 1–111, wherein the polymer brushes comprise a brush polymer, wherein the brush polymer is not a crosslinked polymer. [0309] Example 113: The device of any example herein, particularly Examples 1–112, wherein the brush polymer comprises a backbone and one or more side chains. [0310] Example 114: The device of any example herein, particularly Examples 1–113, wherein the brush polymer is a homopolymer or copolymer. [0311] Example 115: The device of any example herein, particularly Examples 1–114, wherein the brush polymer is a cationic polymer, anionic polymer, or zwitterionic polymer. [0312] Example 116: The device of any example herein, particularly Examples 1–115, wherein the brush polymer has an ionically charged polymer backbone. [0313] Example 117: The device of any example herein, particularly Examples 1–116, wherein the backbone of the brush polymer comprises quaternary ammonium groups. [0314] Example 118: The device of any example herein, particularly Examples 1–117, wherein the backbone of the brush polymer comprises phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0315] Example 119: The device of any example herein, particularly Examples 1–118, wherein brush polymer comprises a polyalkylene backbone, a polyether backbone, a polyurethane backbone, or combination thereof. [0316] Example 120: The device of any example herein, particularly Examples 1–119, wherein the brush polymer comprises a neutral polymer backbone and ionically charged side chains. [0317] Example 121: The device of any example herein, particularly Examples 1–120, wherein the brush polymer comprises a neutral polymer backbone and a combination of ionically charged side chains and neutral side chains. [0318] Example 122: The device of any example herein, particularly Examples 1–121, wherein the brush polymer is a random copolymer. [0319] Example 123: The device of any example herein, particularly Examples 1–122, wherein the brush polymer comprises a zwitterionic side chains and neutral side chains. [0320] Example 124: The device of any example herein, particularly Examples 1–123, wherein the brush polymer comprises cationic side chains and anionic side chains. [0321] Example 125: The device of any example herein, particularly Examples 1–124, wherein the brush polymer comprises a poly(meth)acrylate, a poly(meth)acrylamide, a polyolefin, or a combination thereof. [0322] Example 126: The device of any example herein, particularly Examples 1–125, wherein the brush polymer comprises side chains comprising phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. [0323] Example 127: The device of any example herein, particularly Examples 1–126, wherein the brush polymer comprises repeating units of formula (Xa), formula (Xb), or a combination thereof: [Formula (Xb)], RXm1 is H or CH3; RXm2 is null, C(=O)O, or C(=O)NH; RXa1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXb1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXc1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXd1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXe1 is a C1–15 hydrocarbyl group; wherein any two or more of RXa1, RXb1, RXc1, and RXd1 can together form a ring; YXa is H, carboxylate; sulfonate, or phosphonate; XXa is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, 2; ZXa is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of RXa1, RXb1, RXc1, and RXd1 is not null; and when XXa is null then at least one of ZXa and YXa is not H. [0324] Example 128: The device of any herein, particularly Examples 1–127, wherein the repeating unit of formula (Xb) includes repeating units having the formula: , or a [0325] Example 129: The device of any example herein, particularly Examples 1–128, wherein XXa is N+(CH3)2, and YXa is not H. [0326] Example 130: The device of any example herein, particularly Examples 1–128, wherein XXa is -O-PO2–-O-, and YXa is H. [0327] Example 131: The device of any example herein, particularly Examples 1–130, wherein the repeating unit of formula (Xa) includes repeating units having formula:
, RXf1 is O or NH; YXa is carboxylate; sulfonate, or phosphonate; and ZXa is H or -N+(Rn1)3. [0328] Example 132: The device of any example herein, particularly Examples 1–131, wherein the repeating unit of formula (Xa) includes repeating units having the formula: , [0329] Examples 1–132, wherein the brush polymer further comprises repeating units of formula (Xc), formula (Xd), or a combination thereof: [Formula (Xd)], wherei RYm3 is H or CH3; RYm4 is null, C(=O)O, or C(=O)NH; RYa2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYb2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYc2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYd2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RYe2 is a C1–15 hydrocarbyl group; wherein any two or more of RYa2, RYb2, RYc2, and RYd2 can together form a ring; YYb is H, carboxylate; sulfonate, or phosphonate; XYb is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; ZYb is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of RYa2, RYb2, RYc2, and RYd2 is not null; and when XYb is null then at least one of ZYb and YYb is not H. [0330] Example 134: The device of any example herein, particularly Examples 1–133, wherein the repeating unit of formula (Xd) includes repeating units having the formula: , or a combinatio [0331] Example 135: The device of any example herein, particularly Examples 1–134, wherein XYb is N+(CH3)2, and YYb is not H. [0332] Example 136: The device of any example herein, particularly Examples 1–134, wherein XYb is -O-PO2–-O-, and YYb is H. [0333] Example 137: The device of any example herein, particularly Examples 1–136, the repeating unit of formula (Xc) includes units having the formula: , RYf2 is O or NH; YYb is carboxylate; sulfonate, or phosphonate; and ZYb is H or -N+(Rn1)3. device of any example herein, particularly Examples 1–137, wherein the repeating unit of formula (Xc) includes units having the formula:
, or a combinati wherein R2f2 is O or NH. [0335] Example 139: The device of any example herein, particularly Examples 1–138, wherein the brush polymer further comprises neutral repeating units of formula (Xe), formula (Xf), or a combination thereof: [Formula (Xf)], wherein, RYm5 is H or CH3; RYm6 is H or CH3; and RYa is ORYb or N(RYb)2, wherein RYb is in each case independently selected from H, C1– 12 hydrocarbyl, wherein RYb may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl). [0336] Example 140: The device of any example herein, particularly Examples 1–139, wherein RYb is O-(2-ethylhexyl). [0337] Example 141: The device of any example herein, particularly Examples 1–140, wherein Ryb is O-CH2CH2N(CH3)2. [0338] Example 142: The device of any example herein, particularly Examples 1–141, wherein the brush polymer consists of the first repeating unit and the neutral repeating unit. [0339] Example 143: The device of any example herein, particularly Examples 1–141, wherein the brush polymer consists of the first repeating unit and second repeating unit. [0340] Example 144: The device of any example herein, particularly Examples 1–141, wherein the brush polymer consists of the first repeating unit, second repeating unit, and the neutral repeating unit. [0341] Example 145: A method of making the device of any example herein, particularly Examples 1–144, comprising polymerizing a mixture comprising: (a) the first ionically charged polymer; (b) a monomer precursor of the second ionically charged polymer; to provide an entangled hydrogel network; wherein the first ionically charged polymer is disposed on the surface of a component of the device, or wherein the entangled hydrogel network is applied to the surface of a component of the device. [0342] Example 146: The method of any example herein, particularly Example 145, wherein the monomer precursor of the second ionically charged polymer is a compound of formula (2a-m), formula (2b-m), or a combination thereof: [Formula (2b-m)], R2m3 is H or CH3; R2m4 is null, C(=O)O, or C(=O)NH; R2a2 is null, (CH2CH2O)x, or a C2–15 hydrocarbyl group; R2b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e2 is a C1–15 hydrocarbyl group; wherein any two or more of R2a2, R2b2, R2c2, and R2d2 can together form a ring; Y2b is H, carboxylate; sulfonate, or phosphonate; X2b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z2b is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R2a2, R2b2, R2c2, and R2d2 is not null; and when X2b is null then at least one of Z2b and Y2b is not H. [0343] Example 147: The method of any example herein, particularly Examples 145–146, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof further comprises a crosslinker, or does not include a crosslinker. [0344] Example 148: The method of any example herein, particularly Examples 145–147, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof further comprises a crosslinker. [0345] Example 149: The method of any example herein, particularly Examples 145–148, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof comprises an initiator. [0346] Example 150: The method of any example herein, particularly Examples 145–149, wherein the first ionically charged polymer is prepared by polymerizing a mixture comprising compound of formula (1a-m), formula (1b-m), or a combination thereof: [Formula (1b-m)], R1m1 is H or CH3; R1m2 is null, C(=O)O, or C(=O)NH; R1a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C1–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is H, carboxylate; sulfonate, or phosphonate; wherein said groups may be masked with protecting groups; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4, preferably 2; Z1a is H or -N+(Rn1)3; x is 1–100, for example 1–25, 1–5, 1–10, 5–15, 10–25, 25–50, 50–75, or 75–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Y is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. [0347] Example 151: The method of any example herein, particularly Examples 145–150, wherein the mixture comprising compound of formula (1a-m), formula (1b-m), or a combination thereof is disposed in the surface of a component of the device, or the ionically charged hydrogel is applied to the surface of a component of the device. [0348] Example 152: The method of any example herein, particularly Examples 145–151, wherein the mixture comprising a compound of formula (1a-m), formula (1b-m), or a combination thereof further comprises a crosslinker, or does not include a crosslinker. [0349] Example 153: The method of any example herein, particularly Examples 145–152, wherein the mixture comprising a compound of formula (1a-m), formula (1b-m), or a combination thereof further comprises a crosslinker, preferably methylene bisacrylamide, ethylene glycol dimethacrylate, tetraethylene glycol dimethacrylate, trimethylolpropane trimethacrylate, or a combination thereof. [0350] Example 154: The method of any example herein, particularly Examples 145–153, wherein the mixture comprising a compound of formula (1a-m), formula (1b-m), or a combination thereof comprises an initiator. [0351] Example 155: The method of any example herein, particularly Examples 145–154 wherein applying the entangled hydrogel network to at least one component of the device comprises: coating at least one surface of the device with the entangled hydrogel network; grafting the entangled hydrogel network to at least one surface of the device; or a combination thereof. [0352] Example 156: The method of any example herein, particularly Examples 145–155, wherein the coating comprises dip coating, spraying coating, ultrasonic coating, or annealing the entangled hydrogel network to the surface of the device. [0353] Example 157: The method of any example herein, particularly Examples 145–156, wherein the grafting comprises chemical conjugation of the entangled hydrogel network to the surface of the device. [0354] Example 158: The method of any example herein, particularly Examples 145–157, wherein the chemical conjugation comprises providing a solution comprising the entangled hydrogel network and a crosslinker on the surface of the device. [0355] Example 159: The method of any example herein, particularly Examples 145–158, wherein the crosslinker comprises glutaraldehyde, genipin, a bis-carboxylic acid, a bis- anhydride, an N-hydroxysuccinimide ester, an imidoester, a carbodiimide, a bis-acrylate, or a combination thereof. [0356] Example 160: The method of any example herein, particularly Examples 145–159, further comprising forming a brush polymer conjugated to the entangled hydrogel network. [0357] Example 161: The method of any example herein, particularly Examples 145–160, wherein forming a brush polymer comprises: conjugating a polymer to the entangled hydrogel network; polymerizing a brush polymer precursor in the presence of the entangled hydrogel network; or a combination thereof. [0358] Example 162: The method of any example herein, particularly Examples 145–161, wherein the entangled polymer network comprises initiator groups that are reactive with the brush polymer precursor. [0359] Example 163: The method of any example herein, particularly Examples 145–162, wherein the initiator group comprises a radically transferrable atom, an isocyanate, a carboxylic acid, a primary amine, a thiol, a Michael acceptor, or a combination thereof. [0360] Example 164: The method of any example herein, particularly Examples 145–163, further comprising reacting the entangled polymer network with an anchor compound comprising an initiator group or masked initiator group. [0361] Example 165: A device prepared according to the method of any example herein, particularly Examples 145–164. [0362] Although several aspects of the disclosure have been disclosed in the foregoing specification, it is understood by those skilled in the art that many modifications and other aspects of the disclosure will come to mind to which the disclosure pertains, having the benefit of the teaching presented in the foregoing description and associated drawings. Thus, it is understood that the disclosure is not limited to the specific aspects disclosed hereinabove and that many modifications and other aspects are intended to be included within the scope of the appended claims. Moreover, although specific terms are employed herein, as well as in the claims which follow, they are used only in a generic and descriptive sense and not for the purposes of limiting the described disclosure nor the claims which follow. Therefore, we claim as our disclosure all that comes within the scope and spirit of these claims.
Claims
WE CLAIM: 1. A cardiovascular repair device comprising an entangled hydrogel network on at least one surface, wherein the device is optionally a valve. 2. The device of claim 2, wherein the entangled hydrogel network comprises a first ionically charged polymer and a second ionically charged polymer, and optionally a third ionically charged polymer. 3. The device of claim 2, wherein the first ionically charged polymer has an ionically charged polymer backbone. 4. The device of claim 2, wherein the first ionically charged polymer comprises a first repeating unit of formula (1a), formula (1b), or a combination thereof: ], R1b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R1e1 is a C1–15 hydrocarbyl group; wherein any two or more of R1a1, R1b1, R1c1, and R1d1 can together form a ring; Y1a is H, carboxylate; sulfonate, or phosphonate; X1a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z1a is H or -N+(Rn1)3; x is 1–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R1a1, R1b1, R1c1, and R1d1 is not null; and when X1a is null then at least one of Z1a and Y1a is not H. 5. The device of claim 2, wherein the first ionically charged polymer further comprises neutral repeating units of formula (1e), formula (1f), or a combination thereof: [Formula (1e)] [Formula (1f)], wherein, R1m5 is H or CH3; R1m6 is H or CH3; and R1a is OR1b or N(R1b)2, wherein R1b is in each case independently selected from H, C1–12 hydrocarbyl, wherein R1b may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. 6. The device of claim 2, wherein the second ionically charged polymer is a cationic polymer, anionic polymer, or zwitterionic polymer. 7. The device of claim 2, wherein the second ionically charged polymer has an ionically charged polymer backbone. 8. The device of claim 2, wherein the second ionically charged polymer includes first repeating units of formula (2a), formula (2b), or combination thereof: [Formula (2b)], R2m1 is H or CH3; R2m2 is null, C(=O)O, or C(=O)NH; R2a1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2b1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e1 is a C1–15 hydrocarbyl group; wherein any two or more of R2a1, R2b1, R2c1, and R2d1 can together form a ring; Y2a is H, carboxylate; sulfonate, or phosphonate; X2a is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z2a is H or -N+(Rn1)3; x is 1–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R2a1, R2b1, R2c1, and R2d1 is not null; and when X2a is null then at least one of Z2a and Y2a is not H. 9. The device of claim 2, wherein the second ionically charged polymer further comprises neutral repeating units of formula (2e), formula (2f), or a combination thereof: R2m5 O R2a[Formula (1f)], wherein, R2m5 is H or CH3; R2m6 is H or CH3; and R2a is OR2b or N(R2b)2, wherein R2b is in each case independently selected from H, C1– 12 hydrocarbyl, wherein R2b may be substituted one or more times by OH, OC1– 6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl)2. 10. The device of claim 2, wherein the weight ratio of the first ionically charged polymer to the second ionically charged polymer is from 10:1 to 1:10, from 5:1 to 1:5, from 2.5:1 to 1:2.5, from 1.5:1 to 1:1.5, from 10:1 to 5:1, from 10:1 to 1:1, from 5:1 to 1:1, from 2.5:1 to 1:1, from 1:1 to 1:2.5, from 1:1 to 1:5, from 1:1 to 1:10, or from 1:5 to 1:10. 11. The device of claim 1, wherein the entangled hydrogel network further comprises polymer brushes. 12. The device of claim 11, wherein the backbone of the brush polymer comprises quaternary ammonium groups, phosphoryl choline groups, carboxybetaine groups, sulfobetaine groups, or a combination thereof. 13. The device of claim 11, wherein the brush polymer comprises repeating units of formula (Xa), formula (Xb), or a combination thereof: [Formula [Formula (Xb)], wherein RXm1 is H or CH3; RXm2 is null, C(=O)O, or C(=O)NH; RXa1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXb1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXc1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXd1 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; RXe1 is a C1–15 hydrocarbyl group; wherein any two or more of RXa1, RXb1, RXc1, and RXd1 can together form a ring; YXa is H, carboxylate; sulfonate, or phosphonate; XXa is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; ZXa is H or -N+(Rn1)3; x is 1–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of RXa1, RXb1, RXc1, and RXd1 is not null; and when XXa is null then at least one of ZXa and YXa is not H. 14. The device of claim 11, wherein the brush polymer further comprises neutral repeating units of formula (Xe), formula (Xf), or a combination thereof: ], RYm5 is H or CH3; RYm6 is H or CH3; and RYa is ORYb or N(RYb)2, wherein RYb is in each case independently selected from H, C1– 12 hydrocarbyl, wherein RYb may be substituted one or more times by OH, OC1–6 alkyl, NH2, NH(C1–6 alkyl), or N(C1–6 alkyl). 15. A method of making the device of claim 2, comprising polymerizing a mixture comprising: (a) the first ionically charged polymer; (b) a monomer precursor of the second ionically charged polymer; to provide an entangled hydrogel network; wherein the first ionically charged polymer is disposed on the surface of a component of the device, or wherein the entangled hydrogel network is applied to the surface of a component of the device. 16. The method of claim 15, wherein the monomer precursor of the second ionically charged polymer is a compound of formula (2a-m), formula (2b-m), or a combination thereof: [Formula (2b-m)], R2m3 is H or CH3; R2m4 is null, C(=O)O, or C(=O)NH; R2a2 is null, (CH2CH2O)x, or a C2–15 hydrocarbyl group; R2b2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2c2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2d2 is null, (CH2CH2O)x, or a C1–15 hydrocarbyl group; R2e2 is a C1–15 hydrocarbyl group; wherein any two or more of R2a2, R2b2, R2c2, and R2d2 can together form a ring; Y2b is H, carboxylate; sulfonate, or phosphonate; X2b is null, -O-PO2–-O-, -N+(Rn1)2-, or -N+(Rn1)2-(CH2)n-O-PO2–-O-, wherein n is 2–4; Z2b is H or -N+(Rn1)3; x is 1–100, Rn1 is in each case selected from C1–6 alkyl, wherein two or more of Rn1 can together form a ring; Ψ is any heteroaryl ring; wherein at least one of R2a2, R2b2, R2c2, and R2d2 is not null; and when X2b is null then at least one of Z2b and Y2b is not H. 17. The method of claim 16, wherein the mixture comprising a compound of formula (2a-m), formula (2b-m), or a combination thereof further comprises a crosslinker. 18. The claim 15 wherein applying the entangled hydrogel network to at least one component of the device comprises: (a) coating at least one surface of the device with the entangled hydrogel network; (b) grafting the entangled hydrogel network to at least one surface of the device; or (c) a combination thereof. 19. The method of claim 15, further comprising forming a brush polymer conjugated to the entangled hydrogel network. 20. The method of claim 19, wherein forming a brush polymer comprises: (a) conjugating a polymer to the entangled hydrogel network; (b) polymerizing a brush polymer precursor in the presence of the entangled hydrogel network; (c) or a combination thereof. 21. The method of claim 20, wherein the entangled polymer network comprises initiator groups that are reactive with the brush polymer precursor. 22. A device prepared according to the method of any one of claims 15–21.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202463617620P | 2024-01-04 | 2024-01-04 | |
| US63/617,620 | 2024-01-04 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2025147390A1true WO2025147390A1 (en) | 2025-07-10 |
Family
ID=94386275
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/060731PendingWO2025147390A1 (en) | 2024-01-04 | 2024-12-18 | Entangled hydrogel network |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2025147390A1 (en) |
Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010065958A1 (en)* | 2008-12-05 | 2010-06-10 | Semprus Biosciences Corp. | Layered non-fouling, antimicrobial, antithrombogenic coatings |
| WO2011156589A2 (en)* | 2010-06-09 | 2011-12-15 | Semprus Biosciences Corp. | Non-fouling, anti-microbial, anti-thrombogenic graft-from compositions |
| US20110313515A1 (en) | 2010-06-21 | 2011-12-22 | Arshad Quadri | Replacement heart valve |
| US20120215303A1 (en) | 2009-09-29 | 2012-08-23 | Cardiaq Valve Technologies, Inc. | Replacement heart valve and method |
| US8403983B2 (en) | 2008-09-29 | 2013-03-26 | Cardiaq Valve Technologies, Inc. | Heart valve |
| US8414644B2 (en) | 2009-04-15 | 2013-04-09 | Cardiaq Valve Technologies, Inc. | Vascular implant and delivery system |
| US8652203B2 (en) | 2010-09-23 | 2014-02-18 | Cardiaq Valve Technologies, Inc. | Replacement heart valves, delivery devices and methods |
| US20140277390A1 (en) | 2013-03-14 | 2014-09-18 | CardiAQ Value Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue and methods of delivery |
| US20140277427A1 (en) | 2013-03-14 | 2014-09-18 | Cardiaq Valve Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue and methods of delivery |
| US20140277422A1 (en) | 2013-03-14 | 2014-09-18 | Cardiaq Valve Technologies, Inc. | Prosthesis with outer skirt |
| US20150328000A1 (en) | 2014-05-19 | 2015-11-19 | Cardiaq Valve Technologies, Inc. | Replacement mitral valve with annular flap |
| US20180021129A1 (en) | 2016-07-21 | 2018-01-25 | Edwards Lifesciences Corporation | Replacement heart valve prosthesis |
| US20180055629A1 (en) | 2016-08-26 | 2018-03-01 | Edwards Lifesciences Corporation | Multi-portion replacement heart valve prosthesis |
| US10463484B2 (en) | 2016-11-17 | 2019-11-05 | Edwards Lifesciences Corporation | Prosthetic heart valve having leaflet inflow below frame |
| WO2020106338A2 (en)* | 2018-08-14 | 2020-05-28 | University Of Washington | Zwitterionic double network hydrogels |
| WO2022212812A1 (en)* | 2021-04-02 | 2022-10-06 | Cornell University | Zwitterionic triple-network hydrogel compositions and methods of use |
- 2024
- 2024-12-18WOPCT/US2024/060731patent/WO2025147390A1/enactivePending
Patent Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8403983B2 (en) | 2008-09-29 | 2013-03-26 | Cardiaq Valve Technologies, Inc. | Heart valve |
| WO2010065958A1 (en)* | 2008-12-05 | 2010-06-10 | Semprus Biosciences Corp. | Layered non-fouling, antimicrobial, antithrombogenic coatings |
| US8414644B2 (en) | 2009-04-15 | 2013-04-09 | Cardiaq Valve Technologies, Inc. | Vascular implant and delivery system |
| US20120215303A1 (en) | 2009-09-29 | 2012-08-23 | Cardiaq Valve Technologies, Inc. | Replacement heart valve and method |
| WO2011156589A2 (en)* | 2010-06-09 | 2011-12-15 | Semprus Biosciences Corp. | Non-fouling, anti-microbial, anti-thrombogenic graft-from compositions |
| US20110313515A1 (en) | 2010-06-21 | 2011-12-22 | Arshad Quadri | Replacement heart valve |
| US8652203B2 (en) | 2010-09-23 | 2014-02-18 | Cardiaq Valve Technologies, Inc. | Replacement heart valves, delivery devices and methods |
| US20140277427A1 (en) | 2013-03-14 | 2014-09-18 | Cardiaq Valve Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue and methods of delivery |
| US20140277390A1 (en) | 2013-03-14 | 2014-09-18 | CardiAQ Value Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue and methods of delivery |
| US20140277422A1 (en) | 2013-03-14 | 2014-09-18 | Cardiaq Valve Technologies, Inc. | Prosthesis with outer skirt |
| US20150328000A1 (en) | 2014-05-19 | 2015-11-19 | Cardiaq Valve Technologies, Inc. | Replacement mitral valve with annular flap |
| US20180021129A1 (en) | 2016-07-21 | 2018-01-25 | Edwards Lifesciences Corporation | Replacement heart valve prosthesis |
| US20180055629A1 (en) | 2016-08-26 | 2018-03-01 | Edwards Lifesciences Corporation | Multi-portion replacement heart valve prosthesis |
| US10463484B2 (en) | 2016-11-17 | 2019-11-05 | Edwards Lifesciences Corporation | Prosthetic heart valve having leaflet inflow below frame |
| WO2020106338A2 (en)* | 2018-08-14 | 2020-05-28 | University Of Washington | Zwitterionic double network hydrogels |
| WO2022212812A1 (en)* | 2021-04-02 | 2022-10-06 | Cornell University | Zwitterionic triple-network hydrogel compositions and methods of use |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11980543B2 (en) | Mitral valve prosthesis for transcatheter valve implantation | |
| EP2830537B1 (en) | Dual valve prosthesis for transcatheter valve implantation | |
| EP3506855B1 (en) | Heart valve prosthesis and separate support flange for attachment thereto | |
| US11723764B2 (en) | Valve prostheses having an integral centering mechanism and methods of use thereof | |
| EP2830535B1 (en) | Dual valve prosthesis for transcatheter valve implantation | |
| EP2967853B1 (en) | Heart valve prosthesis | |
| AU2015355237B2 (en) | Segmented transcatheter valve prosthesis having an unsupported valve segment | |
| US8926690B2 (en) | Heart valve prosthesis | |
| EP3431041B1 (en) | Heart valve prosthesis | |
| US9066800B2 (en) | Dual valve prosthesis for transcatheter valve implantation | |
| US20230248516A1 (en) | Balloon expandable frame for transcatheter implantation of a cardiac valve prosthesis | |
| US20140088680A1 (en) | Prosthesis for Transcatheter Valve Implantation | |
| EP3852683A1 (en) | Transcatheter pulmonic regenerative valve | |
| WO2021078694A1 (en) | Expandable stent having outflow commissure posts for transcatheter implantation of a cardiac valve prosthesis | |
| WO2021025979A1 (en) | Rotary application of fibrous material to medical devices | |
| WO2025147390A1 (en) | Entangled hydrogel network |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24847201 Country of ref document:EP Kind code of ref document:A1 |