WO2025106930A1 - Circular polyribonucleotides encoding glucagon-like peptide 2 (glp-2) and uses thereof - Google Patents
Circular polyribonucleotides encoding glucagon-like peptide 2 (glp-2) and uses thereofDownload PDFInfo
- Publication number
- WO2025106930A1 WO2025106930A1PCT/US2024/056294US2024056294WWO2025106930A1WO 2025106930 A1WO2025106930 A1WO 2025106930A1US 2024056294 WUS2024056294 WUS 2024056294WWO 2025106930 A1WO2025106930 A1WO 2025106930A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- seq
- circular polyribonucleotide
- sequence
- polypeptide
- domain
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/605—Glucagons
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Definitions
- Glucagon-like peptide (GLP)-2is a peptide hormone derived from tissue-specific posttranslational processing of the pro-glucagon peptide.
- GLP-2 and GLP-2 analogshave been used to treat intestinal diseases, such as short bowel syndrome.
- the present disclosureis based, at least in part, on the development of circular polyribonucleotides and modified RNAs encoding a GLP-2 polypeptide or analog thereof. These circular polyribonucleotides and modified RNAs provide for prolonged and stable expression of the GLP-2 polypeptide or analog thereof in a subject (e.g., subjects having a GLP- 2-related disease, e.g., intestinal diseases such as short bowel syndrome).
- circular polyribonucleotidesincluding a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence.
- GLP-2 polypeptide or analog thereofincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- the GLP-2 polypeptide or analog thereofincludes an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- the sequence encoding the polypeptideincludes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- the sequence encoding the polypeptideincludes a nucleic acid sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- the GLP-2 analogincludes teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681.
- the half-life extension moietyincludes a peptide moiety.
- the peptide moietyincludes an Fc domain, an albumin polypeptide, a nanobody, or a post-translational modification site.
- the Fc domainincludes an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain.
- the Fc domainincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identical to SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain).
- the Fc domainincludes an amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain).
- the IgG4 Fc domainincludes an amino acid sequence of SEQ ID NO: 21.
- the sequence encoding the polypeptideincludes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 domain), or SEQ ID NO: 22 (IgG4 Fc domain).
- the sequence encoding the polypeptideincludes a nucleic acid sequence of SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain).
- the Fc domainincludes a mutation.
- the Fc domainincludes the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain), except the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain) includes an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L284A/L285A; M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL), and V308P.
- the albumin polypeptideincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23.
- the peptide moietyincludes a nanobody that binds to albumin.
- the circular polyribonucleotideincludes one or more modifications.
- the one or more modificationsinclude one or more modifications to a portion of the sequence encoding the GLP-2 polypeptide or analog thereof.
- the one or more modificationsinclude a modification to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof.
- the circular polyribonucleotideincludes a translation initiation sequence operably linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide includes an internal ribosome entry site (IRES) operatively linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide includes a translation termination sequence. In some embodiments, the circular polyribonucleotide lacks a translation termination sequence. In some embodiments, the circular polyribonucleotide includes a stagger element at a 3’ end of the sequence encoding the polypeptide. In some embodiments, the stagger element is configured to stall a ribosome during rolling circle translation.
- the circular polyribonucleotideincludes a replication element. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1
- the circular polyribonucleotideincludes a 5’ untranslated region operably linked to the sequence encoding the polypeptide.
- the circular polyribonucleotidedoes not include a poly(A) sequence operably linked to the sequence encoding the polypeptide.
- the circular polyribonucleotidedoes not include a 5’ untranslated region operably linked to the sequence encoding the polypeptide.
- the secretion signal sequenceis a liver-specific secretion signal sequence, where the liver-specific secretion signal includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to any one of SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87
- the secretion signal sequenceincludes an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia luciferase secretion signal sequence, or a erythropoietin (EPO) secretion signal sequence.
- the IL-2 secretion signal sequenceincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 29.
- the proglucagon secretion signal sequenceincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 31.
- the Gaussia luciferase secretion signal sequenceincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 33.
- the EPO secretion signal sequenceincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 35. LRN Ref. No.: LRN23-107WO Fish Ref.
- the sequence encoding the polypeptideincludes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to one of SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, or SEQ ID NO: 39.
- the circular polyribonucleotideincludes at least one spacer sequence.
- the polypeptideincludes a protease cleavage site and the at least one spacer sequence encodes the protease cleavage site.
- the protease cleavage siteis positioned between (a) the secretion signal and (b) the GLP-2 polypeptide or analog thereof and the half-life extension moiety.
- the polypeptideincludes at least one linker sequence.
- the at least one linker sequenceencodes an amino acid sequence selected from (GS)x, (GGS)x, (GGGGS)x (SEQ ID NO: 141), (GGSG)x (SEQ ID NO: 142), and (SGGG)x (SEQ ID NO: 143), where x is an integer from 1 to 50.
- the at least one linker sequenceis disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety.
- the circular polyribonucleotideencodes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. In some embodiments, the circular polyribonucleotide includes an amino acid sequence of SEQ ID NO: 1. In some embodiments, the circular polyribonucleotide includes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. In some embodiments, the circular polyribonucleotide includes a nucleic acid sequence of SEQ ID NO: 2.
- compositionsincluding any of the circular polyribonucleotides described herein and a pharmaceutically acceptable excipient.
- the circular polyribonucleotideis formulated as a lipid nanoparticle.
- the circular polyribonucleotideis formulated for unencapsulated delivery or natural particle delivery.
- the pharmaceutical compositionis formulated for intravenous, subcutaneous, intrahepatic, or intramuscular administration.
- the pharmaceutical compositionis formulated for local administration.
- the pharmaceutical compositionis formulated for systemic administration. LRN Ref. No.: LRN23-107WO Fish Ref.
- the pharmaceutical compositionincludes one or more of the following features: (i) a concentration of the circular polyribonucleotide of between 0.1 ng/mL and 500 mg/mL; (ii) a concentration of deoxyribonucleotide of no more than 100 ng/mL; (iii) a concentration of protein of less than 100 ng of protein per milligram (mg) of the circular polyribonucleotide; (iv) an A260/A280 absorbance ratio of from about 1.6 to 2.3 as measured by a spectrophotometer; (v) substantially free of a process-related impurity selected from a cell protein, a cell deoxyribonucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material; and (vi) a reduced level of one or more markers of an immune or inflammatory response after purification compared to prior to purification.
- GLP-2-related diseaseis selected from the group consisting of: short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy- induced mucositis, and intestinal ischemia–reperfusion injury.
- SBSshort bowel syndrome
- SBS-IFshort bowel syndrome with intestinal failure
- TPNtotal parenteral nutrition
- TPNtotal parenteral nutrition
- increasing intestinal growthincludes increasing crypt cell growth.
- methods of increasing intestinal absorption of fluids and nutrients in a subject in need thereofincluding administering to the subject an effective amount of any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein.
- the subjecthas short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury.
- any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described hereinis administered one or more times to the subject.
- any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described hereinis administered locally. In some embodiments, any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein is administered systemically. In some embodiments, the method includes administering one or more additional therapeutic agents to the subject. In some embodiments, the subject is a human subject. Also provided herein are kits including: (a) (i) a composition including any of the circular polyribonucleotides described herein or (ii) any of the pharmaceutical compositions described herein; and (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof.
- circular polyribonucleotidesincluding a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety. Also provided herein are circular polyribonucleotides including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety, where the half-life extension moiety is an Fc domain including a M252Y/S254T/T256E (YTE) mutation.
- GLP-2glucagon-like peptide-2
- YTEM252Y/S254T/T256E
- modified linear RNAsincluding a sequence encoding a polypeptide including a glucagon-like peptide-2 (GLP-2) or analog thereof. Also provided herein are modified linear RNAs including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence. In some embodiments, the modified linear RNA further includes a half-life extension moiety (e.g., any of the half-life extension moieties described herein). All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication, patent, LRN Ref.
- eachwhen used in reference to a collection of items, is intended to identify an individual item in the collection but does not necessarily refer to every item in the collection, unless expressly stated otherwise, or unless the context of the usage clearly indicates otherwise.
- all technical and scientific terms used hereinhave the same meaning as commonly understood by one of ordinary skill in the art to which this invention pertains. Although methods and materials similar or equivalent to those described herein can be used to practice the invention, suitable methods and materials are described below. All publications, patent applications, patents, and other references mentioned herein are incorporated by reference in their entirety. In case of conflict, the present specification, including definitions, will control.
- the materials, methods, and examplesare illustrative only and not intended to be limiting.
- FIG. 1includes a graph showing activity of teduglutide in cells treated with circular RNA encoding teduglutide-Fc or recombinant teduglutide.
- FIGs. 1includes a graph showing activity of teduglutide in cells treated with circular RNA encoding teduglutide-Fc or recombinant teduglutide.
- FIG. 2A-2Binclude graphs showing expression (FIG. 2A) and activity (FIG. 2B) of teduglutide-Fc in mammalian cells transfected with circular RNA constructs encoding teduglutide-Fc.
- FIG. 3includes a graph showing levels of teduglutide-Fc in mice injected with circular RNA encoding teduglutide-Fc and reported levels of glepaglutide, teduglutide, apraglutide, and HM15912 in rats injected with recombinant glepaglutide, teduglutide, apraglutide, or HM15912.
- FIGs. 4A-4Finclude data from characterization of various LNP formulations including expression levels of teduglutide-Fc (FIG. 4A), complete blood cell count (CBC) (FIGs. 4B-4C), ALT levels (FIG. 4D), IFN ⁇ levels (FIG. 4E), and cytokine levels (FIG. 4F).
- FIGs. 5A-5Ginclude dose response data in mice injected with LNP formulations of circular RNA encoding teduglutide-Fc. The data include expression of GLP2-Fc (FIGs. 5A-B), cytokine levels (FIG. 5C-F), and anti-target antibody(ATA) response (FIG. 5G) FIGs.
- FIG. 6A-Finclude data from characterization of GLP-2 Fc expressed from circular RNA in non-human primates including Teduglutide-Fc expression (FIG. 6A), antibody production (FIG. 6B), complete blood count, including lymphocyte count (FIG. 6C), cytokine responses (FIG. 6D), CRP levels (FIG. 6E), and activate partial thromboplastin (FIG. 6F).
- FIG. 6ATeduglutide-Fc expression
- FIG. 6Bantibody production
- FIG. 6Ccomplete blood count, including lymphocyte count (FIG. 6C), cytokine responses (FIG. 6D), CRP levels (FIG. 6E), and activate partial thromboplastin (FIG. 6F).
- DETAILED DESCRIPTIONProvided herein are circular polyribonucleotides and modified RNAs encoding a GLP-2 polypeptide or analog thereof and related pharmaceutical compositions and kits.
- Circular polyribonucleotides and modified RNAs described hereincomprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and optionally, (iii) a secretion signal sequence.
- the circular polyribonucleotides and modified RNAscomprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof and (ii) a half-life extension moiety.
- the circular polynucleotides and modified RNAscomprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence.
- the circular polyribonucleotides and modified RNAs described hereinare useful in the treatment or prevention of a GLP-2-related disease (e.g., short bowel syndrome).
- Circular Polyribonucleotides/Modified RNAscomprise a sequence encoding a polypeptide comprising: (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and optionally, (iii) a secretion signal sequence, each of which is described herein.
- a circular polyribonucleotide or a modified RNAcomprises a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof and (ii) a half-life extension moiety.
- a circular polyribonucleotide or a modified RNAcomprise a sequence encoding a polypeptide comprising a sequence encoding a (i) a GLP- 2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence.
- a circular polyribonucleotide or modified RNAcan include two or more copies of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- Non-limiting additional sequences that can be included in a circular polyribonucleotide and a modified RNAare described herein.
- modified RNAor a “linear modified RNA” refers to an RNA molecule that includes one or more (e.g., 2, 3, 4, 5, 10 or more) modified ribonucleotides described herein or modifications any of the exemplary modifications known in the art.
- modified ribonucleotidesinclude 5-methylcytidine, N1- methylpseudouridine, 5-methoxyuridine, and pseudouridine.
- a non-limiting example of a sequence encoding a polypeptide that can be present in any of the circular polyribonucleotides or modified RNAs described hereinare provided in Table 1 (SEQ ID NO: 2).
- a non-limiting example of a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequenceis provided in Table 1 (SEQ ID NO: 1). Table 1.
- SEQ ID NO: 1SEQ ID NO: 1
- Table 1Non-limiting example of a polypeptide and a sequence encoding a polypeptide SEQ Description Sequence ID NO: G T K P A LRN Ref. No.: LRN23-107WO Fish Ref.
- the polypeptidecomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. In some embodiments, the polypeptide comprises an amino acid sequence of SEQ ID NO: 1. In some embodiments, the sequence (encoding the polypeptide) comprises a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. In some embodiments, the sequence (encoding the polypeptide) comprises a sequence of SEQ ID NO: 2.
- GLP-2GLP-2 and Analogs Thereof Pro-glucagon is cleaved to glucagon by prohormone convertase 2 (PC2) in pancreatic ⁇ - cells (and other organs), and it is cleaved to glucagon-like peptide-2 (GLP-2) by prohormone convertase 1 (PC1).
- GLP-2is a 33 amino acid peptide-encoded carboxyterminal to the sequence of GLP-1 in the proglucagon gene.
- GLP-2also significantly enhances the surface area of the mucosal epithelium via stimulation of crypt cell proliferation and inhibition of apoptosis in the enterocyte and crypt compartments.
- GLP-1 and GLP-2are secreted from gut endocrine cells and promote nutrient absorption through distinct mechanisms of action. GLP-2 is also known to regulate gastric motility, gastric acid secretion, intestinal hexose transport, and increases the barrier function of the gut epithelium. GLP-2 has been shown to reduce mortality and decrease mucosal injury, cytokine expression, and bacterial septicemia in LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the setting of small and large bowel inflammation. GLP-2 also enhances nutrient absorption and gut adaptation in rodents or humans with short bowel syndrome.
- GLP-2 analogrefers to a polypeptide having one or more alterations (e.g., substitutions, deletions, and/or insertions) in an amino acid sequence of wild-type GLP-2 and having at least partial GLP-2 activity as compared to a wild-type GLP-2 polypeptide.
- Non- limiting examples of GLP-2 analogsinclude teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681. Further non-limiting examples of GLP-2 analogs are described in WO 2020/020904, which is incorporated herein by reference in its entirety. Non-limiting examples of amino acid sequences of pro-glucagon, GLP-2, and GLP-2 analogs are shown in Table 2 below. Non-limiting examples of nucleic acid sequence encoding pro-glucagon, GLP-2, and GLP-2 analogs are also shown in Table 2 below. Table 2.
- the GLP-2 polypeptide or analog thereofcomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- the GLP-2 polypeptide or analog thereofcomprises an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- the sequence (encoding the GLP-2 polypeptide or analog thereof)comprises a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- the sequence (encoding the GLP-2 polypeptide or analog thereof)comprises a sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- the GLP-2 polypeptide or analog thereofcomprises an amino acid sequence that is a functional fragment of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. LRN Ref. No.: LRN23-107WO Fish Ref.
- the GLP-2 polypeptide or analog thereofcomprises a functional fragment that has a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
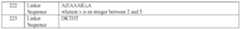
- the GLP-2 polypeptide or analog thereofcomprises an amino acid sequence of SEQ ID NO: 13.
- the amino acid at position X 1is norleucine.
- the amino acid at position X 2is D-phenylalanine.
- GLP-2 polypeptide or analog thereofcomprises an amino acid sequence of SEQ ID NO: 167.
- the amino acid at positions X1, X2, X3, and X 4 of SEQ ID NO: 167can be any amino acid.
- each of X1, X2, X3, and X4can independently be any of amino acids alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, or valine, with the proviso that the combination of X 1 of histidine, X 2 of alanine, X 3 of aspartic acid, and X4 of glycine is excluded.
- a “functional fragment”is a fragment that retains at least 50% (e.g., at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%) of the activity of the wildtype polypeptide from which it is derived.
- a functional fragmentcan have an activity greater than the wildtype polypeptide from which it is derived.
- the GLP-2 polypeptide or analog thereofcomprises an amino acid sequence having at least one mutation.
- substitutionrefers to a substitution of a residue within a sequence (e.g., a nucleic acid or an amino acid sequence) with another residue, or a deletion or insertion of one or more residues within a sequence (e.g., a nucleic acid or an amino acid sequence). Substitutions are typically described herein by identifying the original residue followed by the position of the residue within the sequence and by the identity of the newly substituted residue. Various methods for making the amino acid substitutions provided herein are well known in the art, and are provided by, e.g., Green and Sambrook, Molecular Cloning: A Laboratory Manual (4 th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2012).
- the GLP-2 polypeptide or analog thereofcan comprise any number and/or any type of substitutions.
- the sequence encoding the GLP-2 polypeptide or analog thereofcomprises one or more mutations as compared to the wild type sequence.
- the one or more mutationscan be silent, i.e., they do not modify the amino acid sequence of any encoded protein (or otherwise result in a substituted amino acid sequence).
- the one or more mutations in a nucleic acid sequencecan result in modifications to the encoded amino acid sequence of GLP-2 or analog thereof, resulting in GLP-2 or analog thereof having one or more amino acid substitutions (e.g., having 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 or more amino acid substitutions) relative to the wild-type protein sequence.
- the polypeptidecomprises (i) a GLP-2 polypeptide or analog thereof (e.g., any of the GLP-2 polypeptides or analogs thereof described herein or known in the art) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein or known in the art).
- the polypeptidecomprises (i) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein or known in the art), (ii) a GLP-2 polypeptide or analog thereof (e.g., any of the exemplary GLP- 2 polypeptides or analogs thereof described herein or known in the art), and (iii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein or known in the art).
- a secretion signal sequencee.g., any of the exemplary secretion signal sequences described herein or known in the art
- GLP-2 polypeptide or analog thereofe.g., any of the exemplary GLP- 2 polypeptides or analogs thereof described herein or known in the art
- a half-life extension moietye.g., any of the exemplary half-life extension moieties described herein or known in the art.
- the polypeptidemay further comprise an epitope tag for detection or purification (e.g., a HiBiT-tag) and/or a fluorescent tag for visualization (e.g., a GFP-tag).
- modified linear RNAsincluding a sequence encoding a polypeptide including a glucagon-like peptide-2 (GLP-2) or analog thereof.
- modified linear RNAsincluding a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence.
- the modified linear RNAincludes a half-life extension moiety (e.g., any of the half-life extension moieties described herein).
- a half-life extension moietye.g., any of the half-life extension moieties described herein.
- the polypeptideencoded by a sequence in a circular polyribonucleotide or a modified RNA described herein
- the term “half-life extension moiety”refers to any moiety that extends the circulating serum LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 half-life of a polypeptide comprising a GLP-2 polypeptide or analog thereof in vivo.
- Circulating serum half-liferefers to the amount of time it takes for the concentration or amount of a polypeptide comprising a GLP-2 polypeptide or analog thereof in the serum to be reduced by half (i.e., 50%).
- the half-life extension moietyis a peptide moiety.
- Non-limiting examples of a half-life extension moietyinclude an antibody or fragment thereof (e.g., an Fc domain, e.g., an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain), a serum protein or fragment thereof (e.g., albumin or fragment thereof (e.g., human serum albumin or a fragment thereof), fibronectin or fragment thereof (e.g., human fibronectin or a fragment thereof), or transferrin or fragment thereof (e.g., human transferrin or a fragment thereof), and antibodies or an antigen-binding fragments thereof or peptides that bind to a serum protein (e.g., an antibody or antigen-binding fragment thereof that binds to human albumin, human fibronectin, or human transferrin).
- an antibody or fragment thereofe.g., an Fc domain, e.g., an IgG1 Fc domain, an IgG2
- Antigen-binding fragments of antibodiesinclude, e.g., single-chain antibodies (scFv), single-domain antibodies (also called nanobodies), Fab fragments, Fab ⁇ fragments, and F(ab ⁇ )2 Fab2 fragments, diabodies, triabodies, and tetrabodies.
- scFvsingle-chain antibodies
- nanobodiessingle-domain antibodies
- Fab fragmentsFab ⁇ fragments
- F(ab ⁇ )2 Fab2 fragmentsdiabodies, triabodies, and tetrabodies.
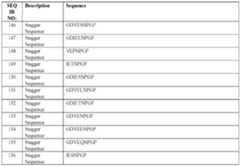
- Non-limiting examples of amino acid sequences (and corresponding nucleic acid sequences) of half-life extension moietiesare shown in Table 3 below. Table 3.
- Non-limiting examples of sequences of half-life extension moietiesSEQ Description Sequence ID E L S C C G G C G A C C G T E L LRN Ref. No.: LRN23-107WO Fish Ref.
- the half-life extension moietycomprises an antibody or fragment thereof.
- Non-limiting examples of antibody fragmentsinclude a Fab, a Fab ⁇ , a F(ab ⁇ )2, a Fc, a Fv, an scFv, a diabody, a triabody, a tetrabody, and a nanobody.
- the half-life extension moietycomprises an Fc domain (e.g., an IgG1 Fc domain (e.g., a human IgG1 Fc domain), an IgG2 Fc domain (e.g., a human IgG2 Fc domain), an IgG3 Fc domain (e.g., a human IgG3 Fc domain, or an IgG4 Fc domain (e.g., a human IgG4 Fc domain).
- an Fc domaine.g., an IgG1 Fc domain (e.g., a human IgG1 Fc domain)
- an IgG2 Fc domaine.g., a human IgG2 Fc domain
- an IgG3 Fc domaine.g., a human IgG3 Fc domain
- an IgG4 Fc domaine.g., a human IgG4 Fc domain
- the half-life extension moietycomprises an Fc domain comprising an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 15 (human IgG1 Fc domain), SEQ ID NO: 17 (human IgG2 Fc domain), SEQ ID NO: 19 (human IgG3 Fc domain), or SEQ ID NO: 21 (human IgG4 Fc domain).
- the half-life extension moietycomprises an Fc domain comprising an amino acid sequence of SEQ ID NO: 15 (human IgG1 Fc domain), SEQ ID NO: 17 (human IgG2 Fc domain), SEQ ID NO: 19 (human IgG3 Fc domain), or SEQ ID NO: 21 (human IgG4 Fc domain).
- the sequence encoding the Fc domaincomprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID LRN Ref. No.: LRN23-107WO Fish Ref.
- the sequence encoding the Fc domaincomprises a nucleic acid sequence of SEQ ID NO: 16 (human IgG1 Fc domain), SEQ ID NO: 18 (human IgG2 Fc domain), SEQ ID NO: 20 (human IgG3 Fc domain), or SEQ ID NO: 22 (human IgG4 Fc domain).
- the sequence encoding the Fc domaincomprises a nucleic acid sequence of SEQ ID NO: 16 (human IgG1 Fc domain), SEQ ID NO: 18 (human IgG2 Fc domain), SEQ ID NO: 20 (human IgG3 Fc domain), or SEQ ID NO: 22 (human IgG4 Fc domain).
- the Fc domaincomprises an amino acid sequence having at least one substitution.
- the Fc domaincan comprise any number and/or any type of substitution.
- the Fc domaincan comprise any substitution known in the art or described herein.
- Non-limiting examples of substitutions that can be included in an Fc domaininclude M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P.
- the Fc domaincan comprise the amino acid sequence of SEQ ID NO: 15 (human IgG1 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P.
- the Fc domaincan comprise the amino acid sequence of SEQ ID NO: 17 (human IgG2 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P.
- the Fc domaincan comprise the amino acid sequence of SEQ ID NO: 19 (human IgG3 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P.
- the Fc domaincan comprise the amino acid sequence of SEQ ID NO: 21 (human IgG4 Fc domain), except that it comprises an amino acid substitution(s) comprising an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P.
- the half-life extension moietycomprises an albumin polypeptide or a functional fragment thereof (e.g., a human albumin polypeptide or fragment thereof), a LRN Ref.
- the albumin polypeptide or a functional fragment thereofcomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23. In some embodiments, the albumin polypeptide comprises an amino acid sequence of SEQ ID NO: 23.
- the albumin polypeptidecomprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype albumin polypeptide (e.g., wildtype human albumin polypeptide, e.g., SEQ ID NO: 23).
- the albumin polypeptidecan comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution.
- the albumin polypeptide or a functional fragment thereofis encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 24. In some embodiments, the albumin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 24. In some embodiments, the fibronectin polypeptide or a functional fragment thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 25. In some embodiments, the fibronectin polypeptide comprises an amino acid sequence of SEQ ID NO: 25.
- the fibronectin polypeptidecomprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype fibronectin polypeptide (e.g., wildtype human fibronectin polypeptide, e.g., SEQ ID NO: 25).
- the fibronectin polypeptidecan comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution.
- the fibronectin polypeptide or a functional fragment thereofis encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 26. In some embodiments, the fibronectin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 26. In some embodiments, the transferrin polypeptide or a functional fragment thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 99% identical to SEQ ID NO: 27.
- the transferrin polypeptidecomprises an amino acid sequence of SEQ ID NO: 27. In some embodiments, the transferrin polypeptide comprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype transferrin polypeptide (e.g., wildtype human transferrin polypeptide, e.g., SEQ ID NO: 27).
- the transferrin polypeptidecan comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution.
- the transferrin polypeptide or a functional fragment thereofis encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 28. In some embodiments, the transferrin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 28.
- the half-life extension moietycomprises an antibody or antigen- binding fragment thereof (e.g., any of the exemplary types of antigen-binding antibody fragments described herein or known in the art) that binds to a serum protein (e.g., albumin (e.g., human albumin), fibronectin (e.g., human fibronectin), or transferrin (e.g., human transferrin)).
- a serum proteine.g., albumin (e.g., human albumin), fibronectin (e.g., human fibronectin), or transferrin (e.g., human transferrin)).
- the half-life extension moietycomprises antigen-binding antibody fragments, e.g., single-chain antibodies (scFv), single-domain antibodies (also called nanobodies), Fab fragments, Fab ⁇ fragments, and F(ab ⁇ )2 Fab2 fragments, diabodies, triabodies, and tetrabodies, that bind to albumin (e.g., human albumin), fibronectin (e.g., human fibronectin), or transferrin (e.g., human transferrin).
- the half-life extension moietycomprises a nanobody that binds specifically to human albumin, human fibronectin, or human transferrin.
- Non-limiting examples of antibodies, antigen-binding fragments, and peptides that bind to a serum protein that can be used as half-life extension moietiesare provided in WO 2013/128027; US 2015/0037334; US 9,376,495; US 8,349,569; US 10,407,508; and Michot, N., et al, Albumin binding Nanofitins, a new scaffold to extend half-life of biologics – a case study with exenatide peptide, Peptides, 152 (2022), the contents of each of which is incorporated herein by reference in its entirety.
- the half-life extension moietycan comprise one or more post- translational modification site(s).
- a post-translational modification siteincludes amino acid(s) in the polypeptide where a cellular enzyme can modify the amino acid to add a post-translational modification (e.g., phosphorylation, glycosylation, hydroxylation, methylation, acetylation, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 deamidation, and lipidation (e.g., N-myristoylation, palmitoylation, glycosylphosphatidylinositol (GPI)-anchor addition) that results in an increase in the half-life of the polypeptide.
- a post-translational modificatione.g., phosphorylation, glycosylation, hydroxylation, methylation, acetylation, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 deamidation
- lipidatione.g., N-myristo
- the polypeptide(encoded by the circular polyribonucleotides and modified RNAs described herein) can further comprise a secretion signal sequence.
- secretion signal sequencerefers to a polypeptide sequence (e.g., between about 10 and about 30 amino acids in length) that is present at the N-terminus of a protein and that targets at least a portion of the protein to the cellular secretory pathway.
- a sequence encoding a secretion signal sequence(e.g., any of the exemplary secretion signal sequences described herein or known in the art) is operably linked to a sequence encoding a GLP-2 polypeptide or analog thereof (and a sequence encoding the half- life extension moiety).
- the sequence encoding the secretion signal sequencecan be operably linked at a 5’ end of a sequence encoding a GLP-2 polypeptide or analog thereof.
- the secretion signal sequenceis a liver-specific secretion signal sequence.
- Non-limiting examples of a secretion signal sequenceinclude an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia lucifersase secretion signal sequence, and an erythropoietin (EPO) secretion signal sequence.
- Non-limiting examples of amino acid sequences (and corresponding nucleic acid sequences) of an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia lucifersase secretion signal sequence, and an EPO secretion signal sequenceare shown in Table 4 below. Table 4.
- Non-limiting examples of signal secretion sequencesSEQ Description Sequence ID NO: C LRN Ref. No.: LRN23-107WO Fish Ref.
- an IL-2 secretion signal sequencecomprises an amino acid sequence of SEQ ID NO: 29.
- a proglucagon secretion signal sequencecomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 31.
- a proglucagon secretion signal sequencecomprises an amino acid sequence of SEQ ID NO: 31.
- a Gaussia luciferase secretion signal sequencecomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 33.
- a Gaussia luciferase secretion signal sequencecomprises an amino acid sequence of SEQ ID NO: 33.
- an EPO secretion signal sequencecomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 35.
- an EPO secretion signal sequencecomprises an amino acid sequence of SEQ ID NO: 35.
- a signal secretion sequencecomprises an amino acid sequence having at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 29, SEQ ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO:
- a signal secretion sequencecomprises an amino acid sequence of SEQ ID NO: 29, SEQ ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91,
- the sequence encoding a secretion signal sequencecomprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, or SEQ ID NO: 36.
- the sequence encoding a secretion signal sequencecomprises a nucleic acid sequence of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, or SEQ ID NO: 36.
- the sequence encoding a secretion signal sequencecomprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, SEQ ID NO: 44, SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ ID NO: 68, SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, SEQ ID NO: 78, SEQ ID NO: 80, SEQ ID NO: 82, SEQ ID NO: 84, SEQ ID NO:
- the sequence encoding a secretion signal sequencecomprises a nucleic acid sequence of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, SEQ ID NO: 44, SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ ID NO: 68, SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, SEQ ID NO: 78, SEQ ID NO: 80, SEQ ID NO: 82, SEQ ID NO: 84, SEQ ID NO: 86, SEQ ID NO: 88, SEQ ID NO: 90, SEQ ID NO: 30
- a “spacer” or “spacer sequence” or “spacer region”refers to any contiguous nucleotide sequence (e.g., of one or more nucleotides) that provides distance or flexibility between two adjacent polynucleotide regions.
- the circular polyribonucleotide or linear modified RNA as disclosed hereinincludes at least one spacer sequence.
- the circular polyribonucleotide or linear modified RNAincludes 1, 2, 3, 4, 5, 6, 7, or more spacer sequences (e.g., identical or different spacer sequences).
- a first spacer sequence and a second spacer sequenceare about the same length. In some embodiments, a first spacer sequence and a second spacer sequence (e.g., a third spacer sequence, a fourth spacer sequence, a fifth spacer sequence, etc.) are different lengths.
- the spacer sequencecan be a nucleic acid sequence having low GC content, e.g., less than 65%, 60%, 55%, 50%, 55%, 50%, 45%, 40%, 39%, 38%, 37%, 36%, 35%, 34%, 33%, 32%, 31 %, 30%, 29%, 28%, 27%, 26%, 25%, 24%, 23%, 22%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11 %, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2% or 1 %, across the full length of the spacer, or across at least 50%, 60%, 70%, 80%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% contiguous nucleic acid residues of the spacer.
- the spacer sequenceis substantially free of a secondary structure, such as less than 40 kcal/mol, less than -39, -38, -37, -36, -35, -34, -33, -32, -31, -30, -29, -28, -27, -26, -25, -24, -23, -22, -20, - 19, -18, -17, -16, -15, -14, -13, -12, -11, -10, -9, -8, -7, -6, -5, -4, -3, -2 or -1 kcal/mol.
- a secondary structuresuch as less than 40 kcal/mol, less than -39, -38, -37, -36, -35, -34, -33, -32, -31, -30, -29, -28, -27, -26, -25, -24, -23, -22, -20, - 19, -18
- the spacer sequencecomprises: (i) a polyA region comprising 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine residues; (ii) a polyAC region comprising between 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine or cytosine residues; (iii) a polyAU region comprising between 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%,
- the spacer sequencecan be a non-coding sequence or a coding sequence.
- a start codonmay be provided in the coding sequence of an adjacent sequence.
- a start codonmay be provided in the spacer sequence.
- the spaceris operably linked to another sequence described herein.
- the circular polyribonucleotide or modified linear RNAincludes an IRES operably linked to an expression sequence encoding a polypeptide.
- the circular polyribonucleotidefurther includes a spacer region between the IRES and the 3’ exon fragment or the 5’ exon fragment.
- the spacer regionmay be, e.g., at least 5 (e.g., at least 10, at least 15, at least 20) ribonucleotides in length.
- the spacer regionmay be, e.g., from 5 to 500 (e.g., 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 200, 250, 300, 350, 400, 450, or 500) ribonucleotides.
- the spacer regionincludes a polyA sequence.
- the spacer regionincludes a polyA-C sequence.
- the spacer regionincludes a polyA-G sequence. In some embodiments, the spacer region includes a polyA- T sequence. In some embodiments, the spacer region includes a polyU sequence. In some embodiments, the spacer region includes a random sequence. In some embodiments, the spacer region includes a random sequence.
- the circular polyribonucleotideis from 50 to 20,000, e.g., 100 to 20,000, e.g., 200 to 20,000, e.g., 300 to 20,000 (e.g., 50, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,100, 1,200, 1,300, 1,400, 1,500, 1,600, 1,700, 1,800, 1,900, 2,000, 2,500, 3,000, 3,500, 4,000, 5,000, 6,000, 7,000, 8,000, 9,000, 10,000, 11 ,000, 12,000, 13,000, 14,000, 15,000, 16,000, 17,000, 18,000, 19,000, or 20,000) ribonucleotides in length.
- the circular polyribonucleotideis, e.g., at least 25, at least 50, at least 100, at least 125, at least 1150, at least 200, at least 300, at least 400, at least 500, at least 1,000, at least 2,000, at least 3,000, at least 4,000, or at least 5,000 ribonucleotides in length.
- a spacercan be specifically engineered depending on the IRES.
- an RNA folding computer softwaresuch as RNAFold, can be utilized to guide designs of the various elements of the vector, including the spacers.
- the polyribonucleotideincludes a 5’ spacer sequence (e.g., between the 5’ annealing region and the polyribonucleotide cargo).
- the 5’ spacer sequenceis at least 10 nucleotides in length.
- the 5’ spacer LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 sequenceis at least 15 nucleotides in length.
- the 5’ spacer sequenceis at least 30 nucleotides in length.
- the 5’ spacer sequenceis at least 7, 8, 9, 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 25 or 30 nucleotides in length.
- the 5’ spacer sequenceis no more than 100, 90, 80, 70, 60, 50, 45, 40, 35 or 30 nucleotides in length. In some embodiments the 5’ spacer sequence is between 20 and 50 nucleotides in length. In certain embodiments, the 5’ spacer sequence is 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 , 32, 33, 34, 35, 3637, 38, 39, 40, 41 , 42, 43, 44, 45, 46, 47, 48, 49 or 50 nucleotides in length. In one embodiment, the 5’ spacer sequence is a polyA sequence. In another embodiment, the 5’ spacer sequence is a polyA-C sequence.
- the 5’ spacer sequenceincludes a polyA-G sequence. In some embodiments, the 5’ spacer sequence includes a polyA-T sequence. In some embodiments, the 5’ spacer sequence includes a random sequence. In some embodiments, the polyribonucleotide includes a 3’ spacer sequence (e.g., between the 3’ annealing region and the polyribonucleotide cargo). In some embodiments, the 3’ spacer sequence is at least 10 nucleotides in length. In another embodiment, the 3’ spacer sequence is at least 15 nucleotides in length. In a further embodiment, the 3’ spacer sequence is at least 30 nucleotides in length.
- the 3’ spacer sequenceis at least 7, 8, 9, 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 25 or 30 nucleotides in length. In some embodiments, the 3’ spacer sequence is no more than 100, 90, 80, 70, 60, 50, 45, 40, 35 or 30 nucleotides in length. In some embodiments the 3’ spacer sequence is from 20 to 50 nucleotides in length. In certain embodiments, the 3’ spacer sequence is 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 , 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 , 42, 43, 44, 45, 46, 47, 48, 49 or 50 nucleotides in length.
- the 3’ spacer sequenceis a polyA sequence.
- the 5’ spacer sequenceis a polyA-C sequence.
- the 5’ spacer sequenceincludes a polyA-G sequence.
- the 5’ spacer sequenceincludes a polyA-T sequence.
- the 5’ spacer sequenceincludes a random sequence.
- the polyribonucleotideincludes a 5’ spacer sequence, but not a 3’ spacer sequence.
- the polyribonucleotideincludes a 3’ spacer sequence, but not a 5’ spacer sequence.
- the polyribonucleotideincludes neither a 5’ spacer sequence, nor a 3’ spacer sequence.
- polyribonucleotide LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1does not include an IRES sequence.
- polyribonucleotidedoes not include an IRES sequence, a 5’ spacer sequence or a 3’ spacer sequence.
- the spacer sequenceincludes at least 3 ribonucleotides, at least 4 ribonucleotides, at least 5 ribonucleotides, at least about 8 ribonucleotides, at least about 10 ribonucleotides, at least about 12 ribonucleotides, at least about 15 ribonucleotides, at least about 20 ribonucleotides, at least about 25 ribonucleotides, at least about 30 ribonucleotides, at least about 40 ribonucleotides, at least about 50 ribonucleotides, at least about 60 ribonucleotides, at least about 70 ribonucleotides, at least about 80 ribonucleotides, at least about 90 ribonucleotides, at least about 100 ribonucleotides, at least about 120 ribonucleotides, at least about 150 ribonucleotides, at least 3 rib
- the circular polyribonucleotide or modified RNAfurther comprises a sequence encoding a linker sequence.
- the circular polyribonucleotide or modified RNAcan comprise a sequence encoding a linker sequence disposed between the sequence encoding the GLP-2 polypeptide or analog thereof and the sequence encoding the half- life extension moiety.
- the polypeptidecomprises a linker sequence disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety in the polypeptide.
- linker sequencesare shown in Table 6 below. Additional examples of linker sequences are well known in the art (See e.g., Chen, X., Fusion Protein Linkers: Property, Design and Functionality, Adv Drug Deliv Rev., 65(10): 1357-1369 (2013), the content of which is incorporated herein by reference in its entirety). Table 6.
- Non-limiting examples of linker sequencesSEQ ID Description Sequence NO: LRN Ref. No.: LRN23-107WO Fish Ref.
- any of the circular polyribonucleotides or elements thereof described hereincan include one or more modifications (e.g., any of the exemplary modifications described herein or known in the art).
- Any of the modified RNAs described hereincan include one or more modifications (e.g., any of the exemplary modifications described herein or known in the art).
- the one or more modificationscan be included at any position in the circular polyribonucleotide or modified RNA.
- the sequence encoding a GLP-2 polypeptide or analog thereofcan comprise one or more modifications (e.g., the sequence encoding a GLP-2 polypeptide or analog thereof comprises one or more modified bases).
- the circular polyribonucleotide or modified RNAcan comprise a post-transcriptional modification (e.g., capping, cleavage, polyadenylation, and splicing).
- a circular polyribonucleotide or elements thereof, or a modified RNA or elements thereofcan include one or more nucleoside modifications (e.g., any of those described in Rozenski et al., Nucl. Acids Res. 27: 196-197, 1999).
- the circular polyribonucleotide or modified RNAcomprises one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) modification(s) to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof.
- the circular polyribonucleotide or modified RNAcan include one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) pyrimidine nucleobases having one or more atoms substituted with an amino, a thiol, an alkyl (e.g., methyl or ethyl), or halo (e.g., chloro or fluoro) group.
- pyrimidine nucleobaseshaving one or more atoms substituted with an amino, a thiol, an alkyl (e.g., methyl or ethyl), or halo (e.g., chloro or fluoro) group.
- the circular polyribonucleotide or modified RNAcan include one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) ribonucleotides that have been modified into deoxyribonucleic acids (DNAs), threose nucleic acids (TNAs), glycol nucleic acids (GNAs), peptide nucleic acids (PNAs), and/or locked nucleic acids (LNAs).
- the circular polyribonucleotide or modified RNAincludes at least one (e.g., two, three, four, or five) N(6)methyladenosine (m6A) modification to increase translation efficiency.
- m6AN(6)methyladenosine
- the N(6)methyladenosine (m6A) modificationcan LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 reduce immunogenicity (e.g., reduce the level of one or more marker of an immune or inflammatory response) of the circular polyribonucleotide or modified RNA.
- a modificationmay include a chemical or cellular induced modification.
- intracellular RNA modificationsare described in Lewis et al., Nature Reviews Mol. Cell Biol. 18:202-210, 2017, herein incorporated by reference.
- chemical modifications to the ribonucleotides of a circular polyribonucleotide or modified RNAmay enhance immune evasion.
- the circular polyribonucleotide or modified RNAmay be synthesized and/or modified by methods well established in the art, such as those described in “Current protocols in nucleic acid chemistry,” Beaucage, S.L. et al. (Eds.), John Wiley & Sons, Inc., New York, NY, USA, herein incorporated by reference.
- Modificationsinclude, for example, end modifications, e.g., 5’ end modifications (phosphorylation (mono-, di- and tri-), conjugation, inverted linkages), 3’ end modifications (conjugation, DNA nucleotides, inverted linkages), base modifications (e.g., replacement with stabilizing bases, destabilizing bases, or bases that base pair with an expanded repertoire of partners), removal of bases (abasic nucleotides), or conjugated bases.
- the modified ribonucleotide basesmay also include 5- methylcytidine, N1-methylpseudouridine, 5- methoxyuridine, and pseudouridine.
- base modificationsmay modulate expression, immune response, stability, subcellular localization, to name a few functional effects, of the circular polyribonucleotide or modified RNA.
- the modificationincludes a bi-orthogonal nucleotide, e.g., an unnatural base. See, e.g., Kimoto et al., Chem. Comm. (Camb) 53:12309, 2017, which is herein incorporated by reference.
- one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) ribonucleotides of the circular polyribonucleotide or modified RNAcan include a sugar modification (e.g., at the 2’ position or 4’ position) and optionally, a backbone modification, including modification or replacement of the phosphodiester linkages.
- a sugar modificatione.g., at the 2’ position or 4’ position
- a backbone modificationincluding modification or replacement of the phosphodiester linkages.
- Specific examples of circular polyribonucleotides or modified RNAsinclude, but are not limited to, circular polyribonucleotides or modified RNAs including modified backbones or no natural internucleoside linkages, such as internucleoside modifications, including modification or replacement of the phosphodiester linkages.
- Circular polyribonucleotides and modified RNAs having modified backbonesinclude, among others, those that do not have a phosphorus atom in LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the backbone.
- the circular polyribonucleotide or modified RNAwill include ribonucleotides with a phosphorus atom in its internucleoside backbone.
- Modified backbones of circular polyribonucleotides and modified RNAscan include, for example, phosphorothioates, chiral phosphorothioates, phosphorodithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates, such as 3’-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates, such as 3’-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3'- 5’ linkages, 2’-5’ linked analogs of these, and those having inverted polarity, wherein the adjacent pairs of nucleoside units are linked 3’-5’ to 5’-3’ or 2’-5’ to 5’-2’.
- the circular polyribonucleotides or modified RNAsmay be negatively- or positively-charged.
- the modified nucleotide(s), which may be incorporated into the circular polyribonucleotides or modified RNAs,can be modified on the internucleoside linkage (e.g., phosphate backbone).
- phosphate backbonethe phrases “phosphate” and “phosphodiester” are used interchangeably.
- Backbone phosphate groupscan be modified by replacing one or more of the oxygen atoms with a different substituent.
- modified nucleosides and nucleotidescan include the wholesale replacement of an unmodified phosphate moiety with another internucleoside linkage as described herein.
- modified phosphate groupsinclude, but are not limited to, phosphorothioate, phosphoroselenates, boranophosphates, boranophosphate esters, hydrogen phosphonates, phosphoramidates, phosphorodiamidates, alkyl or aryl phosphonates, and phosphotriesters.
- Phosphorodithioateshave both non-linking oxygens replaced by sulfur.
- the phosphate linkercan also be modified by the replacement of a linking oxygen with nitrogen (bridged phosphoramidates), sulfur (bridged phosphorothioates), and carbon (bridged methylene -phosphonates).
- A-thio substituted phosphate moietiescan be included to confer stability to circular polyribonucleotides and modified RNAs through the unnatural phosphorothioate backbone linkages.
- Phosphorothioate RNAshave increased nuclease resistance and subsequently a longer half-life in a cellular environment.
- a modified nucleosidecan include an alpha-thio-nucleoside (e.g., 5’-O-(l-thiophosphate)-adenosine, 5’-O-(l-thiophosphate)-cytidine (a-thio-cytidine), 5’-O- (l-thiophosphate)-guanosine, 5’-O-(l-thiophosphate)-uridine, or 5’-O- (1-thiophosphate)- pseudouridine).
- alpha-thio-nucleosidee.g., 5’-O-(l-thiophosphate)-adenosine, 5’-O-(l-thiophosphate)-cytidine (a-thio-cytidine), 5’-O- (l-thiophosphate)-guanosine, 5’-O-(l-thiophosphate)-uridine, or 5’-O- (1-thiophosphate)- pseudouridine).
- a circular polyribonucleotide or modified RNAmay or may not be uniformly modified along the entire length of the molecule.
- nucleotidee.g., naturally-occurring nucleotides, purine or pyrimidine, or any one or more or all of A, G, U, C, I, pU
- the circular polyribonucleotide or modified RNAincludes a pseudouridine.
- the circular polyribonucleotide or modified RNAincludes an inosine, which may aid in the immune system characterizing the circular polyribonucleotide or modified RNA as an endogenous RNA versus a viral RNA.
- the incorporation of inosinemay also mediate improved RNA stability/reduced degradation. See for example, Yu, Z. et al., Cell Res. 25, 1283–1284, 2015, which is incorporated by reference in its entirety.
- all or substantially all nucleotides in a circular polyribonucleotide or modified RNAare modified.
- the modificationmay include an m6A, which may augment expression; an inosine, which may attenuate an immune response; pseudouridine, which may increase RNA stability, or translational readthrough (stagger element); an m5C, which may increase stability; and a 2,2,7- trimethylguanosine, which aids subcellular translocation (e.g., nuclear localization).
- m6Awhich may augment expression
- pseudouridinewhich may increase RNA stability, or translational readthrough (stagger element)
- an m5Cwhich may increase stability
- a 2,2,7- trimethylguanosinewhich aids subcellular translocation (e.g., nuclear localization).
- Different sugar modifications, nucleotide modifications, and/or internucleoside linkagesmay exist at various positions in a circular polyribonucleotide or modified RNA.
- nucleotide analogs or other modification(s)may be located at any position(s) of the circular polyribonucleotide or modified RNA, such that the function of the circular polyribonucleotide or modified RNA is not LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 substantially decreased.
- a modificationcan be included in a coding sequence or a non-coding sequence.
- the circular polyribonucleotide or modified RNAmay include about 1% to about 100% modified nucleotides (either in relation to overall nucleotide content, or in relation to one or more types of nucleotide, e.g., any one or more of A, G, U, or C) or any intervening percentage (e.g., about 1% to about 10%, about 1% to about 15%, about 1% to about 20%, from about 1% to about 25%, about 1% to about 30%, about 1% to about 40%, about 1% to about 50%, about 1% to about 60%, about 1% to about 70%, about 1% to about 80%, about 1% to about 90%, about 1% to about 95%, about 10% to about 20%, about 10% to about 25%, about 10% to about 30%, about 10% to about 40%, about 10% to about 50%, about 10% to about 60%, about 10% to about 70%, about 10% to about 80%, about 10% to about 90%, about 10% to about 95%, about 10% to about 100%, about 20% to about 25%, about 20% to about 25%
- the circular polyribonucleotides or modified RNAs described hereinincludes one or more internal ribosome entry site(s) (IRES(s)).
- an IRESis operably linked to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- an IRESis located at or proximal to the LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 5’ end of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- a suitable IRESincludes an RNA sequence capable of engaging a eukaryotic ribosome.
- an IRESis at least about 50 nucleotides, at least about 100 nucleotides, at least about 200 nucleotides, at least about 250 nucleotides, at least about 350 nucleotides, or at least about 500 nucleotides.
- an IRESis about 50 nucleotides to about 500 nucleotides, about 50 nucleotides to about 450 nucleotides, about 50 nucleotides to about 400 nucleotides, about 50 nucleotides to about 350 nucleotides, about 50 nucleotides to about 300 nucleotides, about 50 nucleotides to about 250 nucleotides, about 50 nucleotides to about 200 nucleotides, about 50 nucleotides to about 150 nucleotides, about 50 nucleotides to about 100 nucleotides, about 100 nucleotides to about 500 nucleotides, about 100 nucleotides to about 450 nucleotides, about 100 nucleotides to about 400 nucleotides, about 100 nucleotides to about 350 nucleotides, about 100 nucleotides to about 300 nucleotides, about 100 nucleotides to about 250 nucleotides, about 100 nucle
- the IRESis derived from the DNA of an organism including, but not limited to, a virus, a mammal, and Drosophila.
- a virusa mammal
- DrosophilaSuch viral DNA may be derived from, but LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 not limited to, picornavirus complementary DNA (cDNA), encephalomyocarditis virus (EMCV) cDNA, and poliovirus cDNA.
- Drosophila DNA from which an IRES is derivedincludes, but is not limited to, an Antennapedia gene from Drosophila melanogaster.
- nucleic acidin the context of a nucleic acid, i.e., for a nucleic acid “derived from” (another) nucleic acid, means that the nucleic acid, which is derived from (another) nucleic acid, shares e.g. at least 60%, 70%, 80%, 81 %, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% sequence identity with the nucleic acid from which it is derived.
- sequence identityis typically calculated for the same types of nucleic acids, i.e., for DNA sequences or for RNA sequences.
- a DNAis “derived from” an RNA or if an RNA is “derived from” a DNA
- the RNA sequenceis converted into the corresponding DNA sequence (in particular by replacing the uracils (U) by thymidines (T) throughout the sequence) or, vice versa
- the DNA sequenceis converted into the corresponding RNA sequence (in particular by replacing the T by U throughout the sequence).
- sequence identity of the DNA sequences or the sequence identity of the RNA sequencesis determined.
- nucleic acid “derived from” a nucleic acidalso refers to nucleic acid, which is modified in comparison to the nucleic acid from which it is derived, e.g., in order to increase RNA stability even further and/or to prolong and/or increase protein production.
- the term “derived from”means that the amino acid sequence, which is derived from (another) amino acid sequence, shares e.g.
- the IRESis an IRES of Taura syndrome virus, Triatoma virus, Theiler's encephalomyelitis virus, simian Virus 40, Solenopsis invicta virus 1, Rhopalosiphum padi virus, Reticuloendotheliosis virus, human poliovirus 1, Plautia stall intestine virus, Kashmir bee virus, Human rhinovirus 2, Homalodisca coagulata virus- 1, Human Immunodeficiency Virus type 1, Homalodisca coagulata virus- 1, Himetobi P virus, Hepatitis C virus, Hepatitis A virus, Hepatitis GB virus, foot and mouth disease virus, Human enterovirus 71, Equine rhinitis virus, Ectropis obliqua picorna-like virus, Encephalomyocarditis virus (EMCV), Drosophila C Virus, Crucifer tobamo virus, Cricket paralysis virus, Bovine viral diarrhea virus 1, Black Queen Cell Virus,
- EMCVEncephal
- the IRESis an IRES of Coxsackievirus B3 (CVB3).
- the IRESis an IRES of Encephalomyocarditis virus.
- the IRES sequencehas more than 90% sequence identify with one of the foregoing IRES sequences.
- the IRES sequencemay have the sequence of wild-type CVB3 IRES sequence having the nucleic acid sequence of: TTAAAACAGCCTGTGGGTTGATCCCACCCACAGGCCCATTGGGCGCTAGCACTCTGG TATCACGGTACCTTTGTGCGCCTGTTTTATACCCCCTCCCCCAACTGTAACTTAGAAG TAACACACACCGATCAACAGTCAGCGTGGCACACCAGCCACGTTTTGATCAAGCAC TTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACGCGGTTGAAGGAGAAAGCG TTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAACACCGTGGAAGTTGCAGAGT GTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGATGAGTCACCGCATTCCCCAC GGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCCATGGGGAAACCCATGGGAC GCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGCTAGTTGGTAGTCCTCCGGCCGGCCGGGG
- the IRES sequencemay have a modified sequence in comparison to the wildtype IRES sequence.
- the last nucleotide of the wild-type IRESwhen the last nucleotide of the wild-type IRES is not a cytosine nucleic acid residue, the last nucleotide of the wild-type IRES sequence may be modified such that it is a cytosine residue.
- the IRES sequencemay be a CVB3 IRES sequence wherein the terminal adenosine residue is modified to cytosine residue.
- the modified CVB3 IRESmay have the nucleic acid sequence of: TTAAAACAGCCTGTGGGTTGATCCCACCCACAGGCCCATTGGGCGCTAGCACTCTGG TATCACGGTACCTTTGTGCGCCTGTTTTATACCCCCTCCCCCAACTGTAACTTAGAAG TAACACACACCGATCAACAGTCAGCGTGGCACACCAGCCACGTTTTGATCAAGCAC TTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACGCGGTTGAAGGAGAAAGCG TTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAACACCGTGGAAGTTGCAGAGT GTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGATGAGTCACCGCATTCCCCAC GGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCCATGGGGAAACCCATGGGGAAACCCATGGGAC GCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGCTAGTTGGTAGTCCTCCGGCC CC CCTGAATGCGGC
- the modified CVB3 IRESmay have the nucleic acid sequence of: CCAACTGTAACTTAGAAGTAACACACACCGATCAACAGTCAGCGTGGCACACCAGC CACGTTTTGATCAAGCACTTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACG CGGTTGAAGGAGAAAGCGTTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAAC ACCGTGGAAGTTGCAGAGTGTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGAT GAGTCACCGCATTCCCCACGGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCC ATGGGGAAACCCATGGGACGCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGC TAGTTGGTAGTCCTCCGGCCCCTGAATGCGGCTAATCCTAACTGCGGAGCACACACC CTCAAGCCAGAGGGCAGTGTGTCGTAACGGGCAACTCTGCAGCGGAACCGACTACT TTGGGTGTCCGTGTTTCATTTTATTATTGGGTAG
- the IRES sequenceis an encephalomyocarditis virus (EMCV) IRES.
- the ECMV IRESmay have the nucleic acid sequence of: ACGTTACTGGCCGAAGCCGCTTGGAATAAGGCCGGTGTGCGTTTGTCTATATGTTAT TTTCCACCATATTGCCGTCTTTTGGCAATGTGAGGGCCCGGAAACCTGGCCCTGTCTT CTTGACGAGCATTCCTAGGGGTCTTTCCCCTCTCGCCAAAGGAATGCAAGGTCTGTT GAATGTCGTGAAGGAAGCAGTTCCTCTGGAAGCTTCTTGAAGACAAACAACGTCTGT AGCGACCCTTTGCAGGCAGCGGAACCCCCCACCTGGCGACAGGTGCCTCTGCGGCC AAAAGCCACGTGTATAAGATACACCTGCAAAGGCGGCACAACCCCAGTGCCACGTT GTGAGTTGGATAGTTGTGGAAAGAGTCAAATGGCTCTCCTCAAGCGTATTCAACAAG GGGCTGAAGGATGCCCAGAAGGTACCCCATTGTATGGGATCTGATCTGGGGCCTCG GTGCACATGCTTTACATGTGTTTAGTC
- the IRES sequenceis an Enterovirus 71 (EV71) IRES.
- the IRES sequenceis a wild-type EV71 sequence having the nucleic acid sequence of: TTAAAACAGCTGTGGGTTGTCACCCACCCACAGGGTCCACTGGGCGCTAGTACACTG GTATCTCGGTACCTTTGTACGCCTGTTTTATACCCCCTCCCTGATTTGCAACTTAGAA GCAACGCAAACCAGATCAATAGTAGGTGTGACATACCAGTCGCATCTTGATCAAGC ACTTCTGTATCCCCGGACCGAGTATCAATAGACTGTGCACACGGTTGAAGGAAA ACGTCCGTTACCCGGCTAACTACTTCGAGAAGCCTAGTAACGCCATTGAAGTTGCAG AGTGTTTCGCTCAGCACTCCCCCCGTGTAGATCAGGTCGATGAGTCACCGCATTCCC CACGGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCTATGGGGTAACCCATAG GACGCTCTAATACGGACATGGCGACCGTGGCTGCGTTGCC
- the terminal guanosine residue of the EV71 IRES sequenceis modified to a cytosine residue.
- the polyribonucleotideincludes at least one IRES flanking at least one (e.g., 2, 3, 4, 5 or more) expression sequence. In some embodiments, the IRES flanks both sides of at least one (e.g., 2, 3, 4, 5 or more) expression sequence. In some embodiments, the polyribonucleotide includes one or more IRES sequences on one or both sides of each expression sequence, leading to separation of the resulting peptide(s) and or polypeptide(s).
- a polyribonucleotide described hereinmay include a first IRES operably linked to a first expression sequence and a second IRES operably linked to a second expression sequence.
- an exemplary construct described aboveincludes a construct with two IRES sequences each of which is followed by an ORF sequence.
- a spacer sequenceis disposed between the first IRES-ORF sequence and the second IRES-ORF sequence.
- both the first ORF and the second ORF sequenceencode a GLP-2 polypeptide or analog thereof (e.g., GLP-2 Fc).
- the first ORFencodes a GLP-2 polypeptide or analog thereof (e.g., GLP-2 Fc) and the second ORF encodes a different polypeptide.
- the circular polyribonucleotides or modified RNAs described hereincan include a modified IRES, such as those described in WO 2020/198403, which is incorporated herein by reference in its entirety or an IRES, such as those described in Fan et al. Nature Communications 13(1):3751, 2022 doi: 10.1038/s41467-022-31327-y; Chen et al. Mol. Cell 81:1-19, 2021; Jopling et al.
- the circular polyribonucleotides or modified RNAs described hereincan include at least one translation initiation sequence.
- the circular polyribonucleotide or modified RNAincludes a translation initiation sequence operably linked to LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- the translation initiation sequenceincludes, e.g., a start codon.
- the translation initiation sequenceincludes a Kozak or Shine-Dalgarno sequence.
- the circular polyribonucleotide or modified RNAincludes the translation initiation sequence, e.g., Kozak sequence, adjacent to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- the translation initiation sequencee.g., Kozak sequence
- the circular polyribonucleotide or modified RNAincludes at least one translation initiation sequence adjacent to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- the translation initiation sequenceprovides conformational flexibility to the circular polyribonucleotide or modified RNA. In some embodiments, the translation initiation sequence is within a substantially single stranded region of the circular polyribonucleotide or modified RNA.
- the circular polyribonucleotidemay include more than 1 start codon such as, but not limited to, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 25, at least 30, at least 35, at least 40, at least 50, at least 60, or more than 60 start codons.
- translationmay initiate on the first start codon or may initiate downstream of the first start codon.
- the circular polyribonucleotide or modified RNAmay initiate at a codon that is not the first start codon, e.g., AUG.
- Translation of the circular polyribonucleotide or modified RNAmay initiate at an alternative translation initiation sequence, such as, but not limited to, ACG, AGG, AAG, CTG/CUG, GTG/GUG, ATA/AUA, ATT/AUU, or TTG/UUG.
- translationbegins at an alternative translation initiation sequence under selective conditions, e.g., stress-induced conditions.
- the translation of the circular polyribonucleotide or modified RNAmay begin at alternative translation initiation sequence, such as ACG.
- alternative translation initiation sequencesuch as ACG.
- the circular polyribonucleotide or modified RNA translationmay begin at alternative translation initiation sequence, CTG/CUG.
- the circular polyribonucleotide or modified RNA translationmay begin at alternative translation initiation sequence, GTG/GUG.
- the circular polyribonucleotide or modified RNAmay begin translation at a repeat-associated non-AUG (RAN) sequence, such as an alternative translation initiation sequence that includes short stretches of repetitive RNA, e.g., CGG, GGGGCC, CAG, or CTG.
- Nucleotides flanking a codon that initiates translationmay affect the translation efficiency and the length and/or the structure of the circular polyribonucleotide or modified RNA. Masking any of the nucleotides flanking a codon that initiates translation may be used to alter the position of translation initiation, translation efficiency, and length, and/or structure of the circular polyribonucleotide or modified RNA.
- a masking agentmay be used near the start codon or alternative start codon in order to mask or hide the codon to reduce the probability of translation initiation at the masked start codon or alternative start codon.
- masking agentsinclude antisense locked nucleic acids (LNA) oligonucleotides and exon-junction complexes (EJCs).
- LNAantisense locked nucleic acids
- EJCsexon-junction complexes
- a masking agentmay be used to mask a start codon of the circular polyribonucleotide or modified RNA in order to increase the likelihood that translation will initiate at an alternative start codon.
- translationis initiated under selective conditions, such as, but not limited to, viral-induced selection in the presence of GRSF-1 and the circular polyribonucleotide or modified RNA includes GRSF-1 binding sites.
- translationis initiated by eukaryotic initiation factor 4A (eIF4A) treatment with Rocaglates. Translation may be repressed by blocking 43S scanning, leading to premature, upstream translation initiation and reduced protein expression from transcripts bearing the RocA–eIF4A target sequence.
- eIF4Aeukaryotic initiation factor 4A
- translationmay be repressed by blocking 43S scanning, leading to premature, upstream translation initiation and reduced protein expression from transcripts bearing the RocA–eIF4A target sequence.
- the circular polyribonucleotides or modified RNAscan further include at least one translation termination sequence.
- the translation termination sequenceis operably linked to the 3’ end of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein).
- the circular polyribonucleotide or modified RNAincludes a termination element at the end of a sequence encoding the polypeptide (e.g., any of the polypeptides described herein).
- a circular polyribonucleotide or modified RNAcan include two or more termination elements in succession.
- translation termination sequencesinclude an in-frame nucleotide triplet that signals termination of translation, e.g., UAA, UGA, or UAG.
- one or more translation termination sequences in the circular polyribonucleotide or modified RNAare frame-shifted termination elements, such as but not limited to, off-frame or -1 and + 1 shifted reading frames (e.g., hidden stop) that may terminate translation.
- Frame-shifted termination elementsinclude nucleotide triples, e.g., TAA, TAG, or TGA, that appear in the second and third reading frames of the sequence encoding the polypeptide.
- Frame-shifted termination elementsmay be important in preventing misreads of the sequence encoding the polypeptide, which is often detrimental to a cell.
- the termination elementis a stop codon.
- Further examples of translation termination sequencesare described in paragraphs [0169] - [0170] of WO2019/118919, which is hereby incorporated by reference in its entirety.
- Stagger ElementsA circular polyribonucleotide of the disclosure can include a cleavage domain (e.g., a stagger element or a cleavage sequence).
- the circular polyribonucleotideincludes a stagger sequence or a stagger element.
- stagger elementor a “stagger sequence” is a moiety, such as a nucleotide sequence, that induces ribosomal pausing during translation.
- a stagger sequencecan be included to induce ribosomal pausing during translation.
- the stagger sequencemay include a 2A-like or CHYSEL (cis-acting hydrolase element) sequence.
- the stagger elementencodes a sequence with a C- terminal consensus sequence that is X 1 X 2 X 3 EX 5 NPGP (SEQ ID NO: 144), where X 1 is absent or G or H, X2 is absent or D or G, X3 is D, V, I, S, or M, and X5 is any amino acid.
- the stagger elementcomprises a non-conserved sequence of amino-acids with a strong alpha-helical propensity followed by the consensus sequence -D(V/I)EXNPGP (SEQ ID LRN Ref. No.: LRN23-107WO Fish Ref.
- stagger elementsinclude any of the stagger sequences shown in Table 7.
- a stagger element described hereinterminates translation and/or cleaves an expression product between G and P of the consensus sequence described herein.
- the circular polyribonucleotide or modified RNAincludes at least one stagger sequence to terminate translation and/or cleave the encoded polypeptide.
- the circular polyribonucleotide or modified RNAincludes a stagger element adjacent to at least one sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein).
- the circular polyribonucleotide or modified RNAincludes a stagger element after each sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the circular polyribonucleotide or modified RNA includes a stagger element present on one or both sides of each sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein), leading to translation of individual peptide(s) and or polypeptide(s) from each expression sequence.
- Table 7Non-limiting examples of stagger elements SEQ Description Sequence ID LRN Ref. No.: LRN23-107WO Fish Ref.
- the stagger elementmay include a chemical moiety, such as glycerol, a non-nucleic acid linking moiety, a chemical modification, a modified nucleic acid, or any combination thereof.
- Replication ElementsReplication of a circular polyribonucleotide can occur by generating a complement circular polyribonucleotide.
- the circular polyribonucleotidecan include a motif to initiate transcription, where transcription is driven by either endogenous cellular machinery (DNA-dependent RNA polymerase) or an RNA-depended RNA polymerase encoded by the circular polyribonucleotide.
- the product of the rolling-circle transcriptional eventcan be cut by a ribozyme to generate either complementary or propagated circular polyribonucleotide at unit length.
- the ribozymecan be encoded by the circular polyribonucleotide, its complement, or by an RNA sequence in trans.
- the encoded ribozymecan include a sequence or motif that regulates (inhibits or promotes) activity of the ribozyme to control circular polyribonucleotide propagation.
- unit-length sequencescan be ligated into a circular form by a cellular RNA ligase.
- the circular polyribonucleotideincludes a replication element that aids in self-amplification.
- replication elementsinclude HDV replication domains and replication competent circular RNA sense and/or antisense ribozymes, such as antigenomic 5’- CGGGUCGGCAUGGCAUCUCCACCUCCUCGC GGUCCGACCUGGGCAUCCGAAGGAGGACGCACGUCCACUCGGAUGGCUAAGGGAG LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 AGCCA-3’ (SEQ ID NO: 165) or genomic 5’- UGGCCGGCAUGGUCCCAGCCUCCUCGCUGGCGCCGGCUGGGCAACAUU CCGAGGGGACCGUCCCCUCGGUAAUGGCGAAUGGGACCCA-3’ (SEQ ID NO: 166).
- antigenomic 5- CGGGUCGGCAUGGCAUCUCCACCUCCUCGC GGUCCGACCUGGGCAUCCGAAGGAGGACGCACGUCCACUCGGAUGGCUAAGGGAG LRN Ref. No.: LRN23-107WO Fish Ref. No
- the circular polyribonucleotideincludes at least one cleavage sequence as described herein to aid in replication.
- a cleavage sequence within the circular polyribonucleotidecan cleave long transcripts replicated from the circular polyribonucleotide to a specific length that can subsequently circularize to form a complement to the circular polyribonucleotide.
- the circular polyribonucleotideincludes at least one ribozyme sequence to cleave long transcripts replicated from the circular polyribonucleotide to a specific length, where another encoded ribozyme cuts the transcripts at the ribozyme sequence. Circularization forms a complement to the circular polyribonucleotide.
- the circular polyribonucleotideis substantially resistant to degradation, e.g., by exonucleases.
- a “substantially resistant” circular polyribonucleotidecan refer to one that has at least 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% resistance as compared to a reference polyribonucleotide.
- the circular polyribonucleotidereplicates within a cell.
- the circular polyribonucleotidereplicates within in a cell at a rate of about 10%- 20%, 20%-30%, 30%-40%, 40%-50%, 50%-60%, 60%-70%, 70%-75%, 75%-80%, 80%-85%, 85%-90%, 90%-95%, 95%-99%, or any percentage therebetween.
- the circular polyribonucleotidereplicates within a cell and is passed to daughter cells.
- a cellpasses at least one circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%.
- a cell undergoing meiosispasses the circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%. In some embodiments, a cell undergoing mitosis passes the circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%. In some embodiments, the circular polyribonucleotide replicates within the host cell. In some embodiments, the circular polyribonucleotide is capable of replicating in a mammalian cell, e.g., a human cell. LRN Ref.
- the circular polyribonucleotidereplicates in the host cell, the circular polyribonucleotide does not integrate into the genome of the host, e.g., with the host’s chromosomes. In some embodiments, the circular polyribonucleotide has a negligible recombination frequency, e.g., with the host’s chromosomes.
- the circular polyribonucleotidehas a recombination frequency, e.g., less than about 1.0 cM/Mb, 0.9 cM/Mb, 0.8 cM/Mb, 0.7 cM/Mb, 0.6 cM/Mb, 0.5 cM/Mb, 0.4 cM/Mb, 0.3 cM/Mb, 0.2 cM/Mb, 0.1 cM/Mb, or less, e.g., with the host’s chromosomes.
- a recombination frequencye.g., less than about 1.0 cM/Mb, 0.9 cM/Mb, 0.8 cM/Mb, 0.7 cM/Mb, 0.6 cM/Mb, 0.5 cM/Mb, 0.4 cM/Mb, 0.3 cM/Mb, 0.2 cM/Mb, 0.1 cM/Mb, or less, e.
- a DNA molecule used to produce a circular RNA moleculecan include a DNA sequence of a naturally occurring nucleic acid sequence, a modified version thereof, or a DNA sequence encoding a synthetic polypeptide not normally found in nature (e.g., chimeric molecules or fusion proteins).
- DNA and RNA moleculescan be modified using a variety of techniques including, but not limited to, classic mutagenesis techniques and recombinant techniques, such as site- directed mutagenesis, chemical treatment of a nucleic acid molecule to induce mutations, restriction enzyme cleavage of a nucleic acid fragment, ligation of nucleic acid fragments, polymerase chain reaction (PCR) amplification or mutagenesis of selected regions of a nucleic acid sequence, synthesis of oligonucleotide mixtures and ligation of mixture groups to "build" a mixture of nucleic acid molecules and combinations thereof.
- the circular polyribonucleotidesmay be prepared according to any available technique, including, but not limited to chemical synthesis and enzymatic synthesis.
- a linear primary construct or linear polyribonucleotide for circularizationmay be cyclized or concatenated to create a circular RNA described herein.
- the linear polyribonucleotide for circularizationmay be cyclized in vitro prior to formulation and/or delivery.
- the circular polyribonucleotidemay be in a mixture with linear polyribonucleotides.
- the linear polyribonucleotideshave the same nucleic acid sequence as the circular polyribonucleotides.
- the mechanism of cyclization or concatenationmay occur through methods such as, e.g., chemical, enzymatic, splint ligation, or ribozyme-catalyzed methods.
- the newly formed 5’-3’ LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 linkagemay be an intramolecular linkage or an intermolecular linkage.
- a splint ligasesuch as a SplintR® ligase, can be used for splint ligation.
- a single stranded polynucleotidesuch as a single-stranded DNA or RNA
- splintcan be designed to hybridize with both termini of a linear polyribonucleotide, so that the two termini can be juxtaposed upon hybridization with the single-stranded splint.
- Splint ligasecan thus catalyze the ligation of the juxtaposed two termini of the linear polyribonucleotide, generating a circular polyribonucleotide.
- a DNA or RNA ligasemay be used in the synthesis of the circular polynucleotides.
- the ligasemay be a circ ligase or circular ligase.
- either the 5' or 3' end of the linear polyribonucleotidecan encode a ligase ribozyme sequence such that during in vitro transcription, the resultant linear polyribonucleotide for circularization includes an active ribozyme sequence capable of ligating the 5' end of the linear polyribonucleotide for circularization to the 3' end of the linear polyribonucleotide for circularization.
- the ligase ribozymemay be derived from the Group I Intron, Hepatitis Delta Virus, Hairpin ribozyme or may be selected by SELEX (systematic evolution of ligands by exponential enrichment).
- a linear polyribonucleotidemay be cyclized or concatenated by using at least one non-nucleic acid moiety.
- the at least one non-nucleic acid moietymay react with regions or features near the 5' terminus or near the 3' terminus of the linear polyribonucleotide for circularization in order to cyclize or concatenate the linear polyribonucleotide for circularization.
- the at least one non-nucleic acid moietymay be located in or linked to or near the 5' terminus or the 3' terminus of the linear polyribonucleotide for circularization.
- the non-nucleic acid moietiesmay be homologous or heterologous.
- the non-nucleic acid moietymay be a linkage such as a hydrophobic linkage, ionic linkage, a biodegradable linkage, or a cleavable linkage.
- the non-nucleic acid moietyis a ligation moiety.
- the non-nucleic acid moietymay be an oligonucleotide or a peptide moiety, such as an aptamer or a non-nucleic acid linker as described herein.
- a linear polyribonucleotide for circularizationmay include a 5' triphosphate of the nucleic acid converted into a 5' monophosphate, e.g., by contacting the 5' triphosphate with RNA 5' pyrophosphohydrolase (RppH) or an ATP diphosphohydrolase LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 (apyrase).
- the 5’ end of at least a portion of the linear polyribonucleotidesincludes a monophosphate moiety.
- a population of polyribonucleotides including circular and linear polyribonucleotidesis contacted with RppH prior to digesting at least a portion of the linear polyribonucleotides with a 5’ exonuclease and/or a 3’ exonuclease.
- converting the 5' triphosphate of the linear polyribonucleotide for circularization into a 5' monophosphatemay occur by a two-step reaction including: (a) contacting the 5' nucleotide of the linear polyribonucleotide for circularization with a phosphatase (e.g., Antarctic Phosphatase, Shrimp Alkaline Phosphatase, or Calf Intestinal Phosphatase) to remove all three phosphates; and (b) contacting the 5' nucleotide after step (a) with a kinase (e.g., Polynucleotide Kinase) that adds a single phosphate.
- a phosphatasee.g., Antarctic Phosphatase, Shrimp Alkaline Phosphatase, or Calf Intestinal Phosphatase
- a kinasee.g., Polynucleotide
- linear polyribonucleotides for circularizationmay be cyclized or concatenated by self-splicing.
- the linear polyribonucleotidemay include a sequence that mediates self- ligation.
- the linear polyribonucleotidesmay include loop E sequence (e.g., in PSTVd) to self-ligate.
- the linear polyribonucleotidemay include a HDV sequence, e.g., HDV replication domain conserved sequence, GGCUCAUCUCGACAAGAGGCGGCAGUCCUCAGUACUCUUACUCUUUUCUGUAAAG AGGAGACUGCUGGACUCGCCGCCCAAGUUCGAGCAUGAGCC (SEQ ID NO: 170) or GGCUAGAGGCGGCAGUCCUCAGUACUCUUACUCUUUUCUGUAAAGAGGAGACUG CUGGACUCGCCGCCCGAGCC (SEQ ID NO: 171), to self-ligate.
- a HDV sequencee.g., HDV replication domain conserved sequence, GGCUCAUCUCGACAAGAGGCGGCAGUCCUCAGUACUCUUACUUUUCUGUAAAG AGGAGACUGCUGGACUCGCCGCCCAAGUUCGAGCAUGAGCC (SEQ ID NO: 170) or GGCUAGAGGCGGCAGUCCUCAGUACUCUUACUUUUCUGUAAAGAGGAGAC
- the linear polyribonucleotidesmay include a self-circularizing intron, e.g., a 5' and 3’ slice junction, or a self-circularizing catalytic intron such as a Group I, Group II, or Group III Introns.
- group I intron self- splicing sequencesmay include self-splicing permuted intron-exon sequences derived from T4 bacteriophage gene td, and the intervening sequence (IVS) rRNA of Tetrahymena, cyanobacterium Anabaena pre-tRNA-Leu gene, or a Tetrahymena pre-rRNA.
- the polyribonucleotideincludes catalytic intron fragments, such as a 3′ half of Group I catalytic intron fragment and a 5′ half of Group I catalytic intron fragment.
- the first and second annealing regionsmay be positioned within the catalytic intron fragments.
- Group I catalytic intronsare self-splicing ribozymes that catalyze their own excision from mRNA, tRNA, and rRNA precursors via two-metal ion phorphoryl transfer mechanism. LRN Ref. No.: LRN23-107WO Fish Ref.
- the RNAitself self-catalyzes the intron removal without the requirement of an exogenous enzyme, such as a ligase.
- the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragmentare from a cyanobacterium Anabaena pre-tRNA-Leu gene, or a Tetrahymena pre-rRNA.
- the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragmentare from a Cyanobacterium Anabaena pre-tRNA-Leu gene, and the 3’ exon fragment includes the first annealing region and the 5’ exon fragment includes the second annealing region.
- the first annealing regionmay include, e.g., from 5 to 50, e.g., from 10 to 15 (e.g., 10, 11, 12, 13, 14, or 15) ribonucleotides and the second annealing region may include, e.g., from 5 to 50, e.g., from 10 to 15 (e.g., 10, 11, 12, 13, 14, or 15) ribonucleotides.
- the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragmentare from a Tetrahymena pre-rRNA, and the 3′ half of Group I catalytic intron fragment includes the first annealing region and the 5’ exon fragment includes the second annealing region. In some embodiments, the 3′ exon includes the first annealing region and the 5’ half of Group I catalytic intron fragment includes the second annealing region.
- the first annealing regionmay include, e.g., from 6 to 50, e.g., from 10 to 16 (e.g., 10, 11, 12, 13, 14, 15, or 16) ribonucleotides
- the second annealing regionmay include, e.g., from 6 to 50, e.g., from 10 to 16 (e.g., 10, 11, 12, 13, 14, 15, or 16) ribonucleotides.
- the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragmentare from a cyanobacterium Anabaena pre-tRNA-Leu gene, a Tetrahymena pre-rRNA, or a T4 phage td gene.
- the 3′ half of Group I catalytic intron fragment and the 5’ Group I catalytic intron fragmentare from a T4 phage td gene.
- the 3′ exon fragmentmay include the first annealing region and the 5’ half of Group I catalytic intron fragment may include the second annealing region.
- the first annealing regionmay include, e.g., from 2 to 16 (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16) ribonucleotides
- the second annealing regionmay include, e.g., from 2 to 16 (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16) ribonucleotides.
- the 3′ half of Group I catalytic intron fragmentis the 5’ terminus of the linear polynucleotide.
- the 5′ half of Group I catalytic intron fragmentis the 3’ terminus of the linear polyribonucleotide.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AACAACAGATAACTTACAGCTAGTCGGAAGGTGCAGAGACTCGACGGGAGCTACCC TAACGTCAAGACGAGGGTAAAGAGAGTCCAATTCTCAAAGCCAATAGGCAGTAG CGAAAGCTGCGGGAGAATG-3’ (SEQ ID NO: 172).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCTCTCAAACTCAGGGA AACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCCGAAGTAGTAATTAG TAAGTT-3’ (SEQ ID NO: 173)).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 172 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 173.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- CTTCTGTTGATATGGATGCAGTTCACAGACTAAATGTCGGTCGGGGAAGATGTATTC TTCTCATAAGATATAGTCGGACCTCTCCTTAATGGGAGCTAGCGGATGAAGTGATGC AACACTGGAGCCGCTGGGAACTAATTTGTATGCGAAAGTATATTGATTAGTTTTGGA GTACTCG-3’ (SEQ ID NO: 174).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AAATAGCAATATTTACCTTTGGAGGGAAAAGTTATCAGGCATGCACCTGGTAGCTAG TCTTTAAACCAATAGATTGCATCGGTTTAAAAGGCAAGACCGTCAAATTGCGGGAA AGGGGTCAACAGCCGTTCAGTACCAAGTCTCAGGGGAAACTTTGAGATGGCCTTGC AAAGGGTATGGTAATAAGCTGACGGACATGGTCCTAACCACGCAGCCAAGTCCTAA GTCAACAGAT-3’ (SEQ ID NO: 175).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 174 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 175.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- GGTTCTACATAAATGCCTAACGACTATCCCTTTGGGGAGTAGGGTCAAGTGACTCGA AACGATAGACAACTTGCTTTAACAAGTTGGAGATATAGTCTGCTCTGCATGGTGACA TGCAGCTGGATATAATTCCGGGGTAAGATTAACGACCTTATCTGAACATAATG-3’ (SEQ ID NO: 176).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TAATTGAGGCCTGAGTATAAGGTGACTTATACTTGTAATCTATCTAAACGGGGAACC TCTCTAGTAGACAATCCCGTGCTAAATTGTAGGACT-3’ (SEQ ID NO: 177).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 176 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 177.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TAAACAACTAACAGCTTTAGAAGGTGCAGAGACTAGACGGGAGCTACCCTAACGGA TTCAGCCGAGGGTAAAGGGATAGTCCAATTCTCAACATCGCGATTGTTGATGGCAGC GAAAGTTGCAGAGAGAATGAAAATCCGCTGACTGTAAAGGTCGTGAGGGTTCGAGT CCCTCCGCCCCCA-3’ (SEQ ID NO: 178).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ACGGTAGACGCAGCGGACTTAGAAAACTGGGCCTCGATCGCGAAAGGGATCGAGTG GCAGCTCTCAAACTCAGGGAAACCTAAAACTTTAAACATTMAAGTCATGGCAATCC TGAGCCAAGCTAAAGC-3’ (SEQ ID NO: 179).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 178 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 179.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TTAAACTCAAAATTTAAAATCCCAAATTCAAAATTCCGGGAAGGTGCAGAGACTCG ACGGGAGCTACCCTAACGTAAAGCCGAGGGTAAAGGGAGAGTCCAATTCTCAAAGC LRN Ref. No.: LRN23-107WO Fish Ref.
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ATGGTAGACGCTACGGACTTAGAAAACTGAGCCTTGATAGAGAAATCTTTTAAGTG GAAGCTCTCAAATTCAGGGAAACCTAAATCTGAATACAGATATGGCAATCCTGAGC CAAGCCCAGAAAATTTAGACTTGAGATTTGATTTTGGAG-3’ (SEQ ID NO: 181).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 180 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 181.
- the 3’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- GGCTTTCAATTTGAAATCAGAAATTCAAAATTCAGGGAAGGTGCAGAGACTCGACG GGAGCTACCCTAACGTAAAGGCGAGGGTAAAGGGAGAGTCCAATTCTTAAAGCCTG AAGTTGTGCAAGCAACAAGGCAACAGTGAAAGCTGTGGAAGAATGAAAATCCGTTG ACCTTAAACGGTCGTGGGGGTTCAAGTCCCCCCACCCCC-3’ (SEQ ID NO: 182).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ATGGTAGACGCTACGGACTTAGAAAACTGAGCCTTGATAGAGAAATCTTTCAAGTG GAAGCTCTCAAATTCAGGGAAACCTAAATCTGAATACAGATATGGCAATCCTGAGC CAAGCCCGGAAATTTTAGAATCAAGATTTTATTTT-3’ (SEQ ID NO: 183).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 182 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 183. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGAAATGGAGAAGGTGTAGAGACTGGAAGGCAGGCACCCTAACGTTAAAGGCGAG GGTGAAGGGACAGTCCAGACCACAAACCAGTAAATCTGGGCAGCGAAAGCTGTAGA TGGTAAGCATAACCCGAAGGTCAGTGGTTCAAATCCACTTCCCGCCACCAAATTAAA AAAACAATAA-3’ (SEQ ID NO: 184).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGAAATGGAGAAGGTGTAGAGACTGGAAGGCAGGCACCCTAACGTTAAAGGCGAG GGTGAAGGGACAGTCCAGACCACAAACCAGTAAATCTGGGCAGCGAAAGCTGTAGA TGGTAAGCATAACCCGAAGGTCAGTGGTTCAAATCCACTTCCCGCCACCAAATTAAA AAAACAATAA-3’ (SEQ ID NO: 185).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 184 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 185. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ACAACAGATAACTTACTAACTTACAGCTAGTCGGAAGGTGCAGAGACTCGACGGGA GCTACCCTAACGTCAAGACGAGGGTAAAGAGAGAGTCCAATTCTCAAAGCCAATAG GCAGTAGCGAAAGCTGCGGGAGAATGAAAATCCGTAGCGTCTAAACGGTCGTGTGG GTTCAAGTCCCTCCACCCCCA-3’ (SEQ ID NO: 186).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGACGCTACGGACTTAAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCT CTCAAACTCAGGGAAACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCC GAAGTAGTAATTAGTAAGTTAGTAAGTT-3’ (SEQ ID NO: 187).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 186 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 187. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AACAACAGATAACTTACTAGTTACTAGTCGGAAGGTGCAGAGACTCGACGGGAGCT ACCCTAACGTCAAGACGAGGGTAAAGAGAGAGTCCAATTCTCAAAGCCAATAGGCA GTAGCGAAAGCTGCGGGAGAATGAAAATCCGTAGCGTCTAAACGGTCGTGTGGGTT CAAGTCCCTCCACCCCCA-3’ (SEQ ID NO: 188).
- the 5’ half of Group I catalytic intron fragmenthas at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 AGACGCTACGGACTTAAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCT CTCAAACTCAGGGAAACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCC GAAGTAGTAATTAGTAAGTT-3’ (SEQ ID NO: 189).
- the 3’ half of Group I catalytic intron fragmenthas the sequence of SEQ ID NO: 188 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 189.
- the Group I catalytic intron fragmentis from the T4 phage nrdB gene or nrdD gene.
- the 3′ half of Group I catalytic intron fragment ofincludes a sequence having at least 80% sequence identity to 5’- TTGCAAAACAAGGTTCAACGACTAGTCTTCGGACGTAGGGTCAAGCGACTCGAAAT GGGGAGAATCCCTCCGGGATTGTGATATAGTCTGGACTGCATGGTAACATGCAGCA GTTCATAAGAGAACGGGTTGAGAATTAGCGAGCTCAATCGAACATACG-3’ (SEQ ID NO: 190).
- the 3′ half of Group I catalytic intron fragment of (A)includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TTGCAAAACAAGGTTCAACGACTAGTCTTCGGACGTAGGGTCAAGCGACTCGAAAT GGGGAGAATCCCTCCGGGATTGTGATATAGTCTGGACTGCATGGTAACATGCAGCA GTTCATAAGAGAACGGGTTGAGAATTAGCGAGCTCAATCGAACATACG-3’ (SEQ ID NO: 191).
- the 5′ half of Group I catalytic intron fragment from the T4 phage nrdB geneincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TTGCAAAACAAGGTTCAACGACTAGTCTTCGGACGTAGGGTCAAGCGACTCGAAAT GGGGAGAATCCCTCCGGGATTGTGATATAGTCTGGACTGCATGGTAACATGCAGCA GTTCATAAGAGAACGGG
- the 3′ half of Group I catalytic intron fragmentis from the T4 phage nrdB gene and the 5′ half of Group I catalytic intron fragment is from the T4 phage nrdB gene.
- the 5′ half of Group I catalytic intronincludes a sequence having at least 80% sequence identity to 5’- AAAATGCGCCTTTAAACGGTAACGTTTATCGAAAACTCCTTTAATTGCTGGAAAGTC CTTTATGGAAAACTAGCAGCCAAGGTTTTGCTT-3’ (SEQ ID NO: 192).
- the 5′ half of Group I catalytic intronincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- AAAATGCGCCTTTAAACGGTAACGTTTATCGAAAACTCCTTTAATTGCTGGAAAGTC CTTTATGGAAAACTAGCAGCCAAGGTTTTGCTT-3’ (SEQ ID NO: 193).
- the 3′ half of Group I catalytic intron fragmentis from the T4 phage nrdD gene.
- the 3′ half of Group I catalytic intron fragmentincludes a sequence having at least 80% sequence identity to 5’- CAGTAGCTGTAAATGCCCAACGACTATCCCTGATGAATGTAAGGGAGTAGGGTCAA GCGACCCGAAACGGCAGACAACTCTAAGAGTTGAAGATATAGTCTGAACTGCATGG TGACATGCAGCTGTTTATCCTCGTATAAATATGAATACGAGGTGAAACGATGAAATG AATTACATTGTTTCATATAAACGGGTAGAGAAGTAGCGAACTCTACTGAACACATTG -3’ (SEQ ID NO: 194).
- the 3′ half of Group I catalytic intron fragmentincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- CAGTAGCTGTAAATGCCCAACGACTATCCCTGATGAATGTAAGGGAGTAGGGTCAA GCGACCCGAAACGGCAGACAACTCTAAGAGTTGAAGATATAGTCTGAACTGCATGG TGACATGCAGCTGTTTATCCTCGTATAAATATGAATACGAGGTGAAACGATGAAATG AATTACATTGTTTCATATAAACGGGTAGAGAAGTAGCGAACTCTACTGAACACATTG -3’ (SEQ ID NO: 195).
- the 5′ half of Group I catalytic intron fragmentis from the T4 phage nrdD gene.
- the 3′ half of Group I catalytic intron fragmentis from the T4 phage nrdD gene and the 5′ half of Group I catalytic intron fragment is from the T4 phage nrdD gene.
- the 5′ half of Group I catalytic intronincludes a sequence having at least 80% sequence identity to 5’- TAACGTAAGTCAAGCTCATGTAAAATCTGCCTAAAACGGGAAACTCTCACTGAGAC AATCCGTTGCTAAATCAG-3’ (SEQ ID NO: 196).
- the 5′ half of Group I catalytic intronincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TAACGTAAGTCAAGCTCATGTAAAATCTGCCTAAAACGGGAAACTCTCACTGAGAC AATCCGTTGCTAAATCAG-3’ (SEQ ID NO: 197).
- the 3’ exon fragmentincludes a sequence having at least 80% sequence identity to 5’- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 GTACCTTTAACTTCCATAAGAACATGGAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 198).
- the 3’ exon fragmentincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- GTACCTTTAACTTCCATAAGAACATGGAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 199). In some embodiments, the 3’ exon fragment includes a sequence having at least 80% sequence identity to 5’- GTACCTTTAACTTCCAAAAGATACATAAAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 200.
- the 3’ exon fragmentincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- GTACCTTTAACTTCCAAAAGATACATAAAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 201).
- the 5’ exon fragmentincludes a sequence having at least 80% sequence identity to 5’-TTTTTATGTATCTTTTGCGT-3’ (SEQ ID NO: 202).
- the 5’ exon fragmentincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’-TTTTTATGTATCTTTTGCGT-3’ (SEQ ID NO: 203).
- the 3’ exon fragmentIncludes a sequence having at least 80% sequence identity to 5’- ATGAAGTGAACACGTTATTCAGTTCAAACGGACAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 204). In some embodiments, the 3’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- ATGAAGTGAACACGTTATTCAGTTCAAACGGACAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 205).
- the 3’ exon fragmentincludes a sequence having at least 80% sequence identity to 5’- ATGAAGTGAACACGTTACATAAGCTTGGAATGCAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 206).
- the 3’ exon fragmentincludes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- ATGAAGTGAACACGTTACATAAGCTTGGAATGCAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 207).
- the 5’ exon fragmentincludes a sequence having at least 80% sequence identity to 5’- TGCATTCCAAGCTTATGAGT -3’ (SEQ ID NO: 208). In some embodiments, the 5’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TGCATTCCAAGCTTATGAGT -3’ (SEQ ID NO: 209).
- a linear polyribonucleotide for circularizationmay be cyclized or concatenated by a non-nucleic acid moiety that causes an attraction between atoms, molecular surfaces at, near, or linked to the 5’ and 3’ ends of the linear polyribonucleotide for circularization.
- one or more linear polyribonucleotidesis cyclized or concatenated by intermolecular forces or intramolecular forces.
- intermolecular forcesinclude dipole-dipole forces, dipole-induced dipole forces, induced dipole- induced dipole forces, Van der Waals forces, and London dispersion forces.
- intramolecular forcesinclude covalent bonds, metallic bonds, ionic bonds, resonant bonds, agnostic bonds, dipolar bonds, conjugation, hyperconjugation and antibonding.
- a linear polyribonucleotide for circularizationmay include a ribozyme RNA sequence near the 5’ terminus and near the 3’ terminus.
- the ribozyme RNA sequencemay covalently link to a peptide when the sequence is exposed to the remainder of the ribozyme.
- the peptides covalently linked to the ribozyme RNA sequence near the 5’ terminus and the 3 ‘terminusmay associate with each other causing a linear polyribonucleotide to cyclize or concatenate.
- the peptides covalently linked to the ribozyme RNA near the 5’ terminus and the 3’ terminusmay cause the linear primary construct or linear mRNA to cyclize or concatenate after being subjected to ligation using various methods known in the art such as, but not limited to, protein ligation.
- Non-limiting examples of ribozymes for use in the linear primary constructs or linear polyribonucleotides of the present invention or a non-exhaustive listing of methods to incorporate or covalently link peptidesare described in U.S. Patent Publication No. U.S. 2003/0082768, the contents of which is here in incorporated by reference in its entirety.
- chemical methods of circularizationmay be used to generate the circular polyribonucleotide.
- Such methodsmay include but are not limited to click chemistry (e.g., alkyne and azide-based methods, or clickable bases), olefin metathesis, phosphoramidate ligation, hemiaminal-imine crosslinking, base modification, and any combination thereof.
- click chemistrye.g., alkyne and azide-based methods, or clickable bases
- olefin metathesise.g., alkyne and azide-based methods, or clickable bases
- phosphoramidate ligatione.g., phosphoramidate ligation
- hemiaminal-imine crosslinkinghemiaminal-imine crosslinking
- base modificatione.g., base modification, and any combination thereof.
- the 5’-end and the 3’-end of a linear polyribonucleotide for circularizationincludes chemically reactive groups that, when close together, may form a new covalent linkage between the 5’-end and the 3’-end of
- the 5’-endmay contain an NHS-ester reactive group and the 3’-end may contain a 3’-amino-terminated nucleotide such that in an organic solvent the 3’-amino-terminated nucleotide on the 3’-end of a linear RNA molecule will undergo a nucleophilic attack on the 5’-NHS-ester moiety forming a new 5’-/3’-amide bond.
- the circular polyribonucleotidemay be produced using a deoxyribonucleotide template transcribed in a cell-free system (e.g., by in vitro transcription) to a produce a linear RNA.
- the linear polyribonucleotideproduces a splicing-compatible polyribonucleotide, which may be self-spliced to produce a circular polyribonucleotide.
- the disclosureprovides a method of producing a circular polyribonucleotide (e.g., in a cell-free system) by providing a linear polyribonucleotide; and self- splicing linear polyribonucleotide under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide; thereby producing a circular polyribonucleotide.
- the disclosureprovides a method of producing a circular polyribonucleotide by providing a deoxyribonucleotide encoding the linear polyribonucleotide; transcribing the deoxyribonucleotide in a cell-free system to produce the linear polyribonucleotide; optionally purifying the splicing-compatible linear polyribonucleotide; and self-splicing the linear polyribonucleotide under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide, thereby producing a circular polyribonucleotide.
- the disclosureprovides a method of producing a circular polyribonucleotide by providing a deoxyribonucleotide encoding a linear polyribonucleotide; transcribing the deoxyribonucleotide in a cell-free system to produce the linear polyribonucleotide, wherein the transcribing occurs in a solution under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide, thereby producing a circular polyribonucleotide.
- the linear polyribonucleotidecomprises a 5’ split- intron and a 3’ split-intron (e.g., a self-splicing construct for producing a circular LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 polyribonucleotide).
- the linear polyribonucleotidecomprises a 5’ annealing region and a 3’ annealing region.
- Suitable conditions for in vitro transcriptions and or self-splicingmay include any conditions (e.g., a solution or a buffer, such as an aqueous buffer or solution) that mimic physiological conditions in one or more respects.
- suitable conditionsinclude between 0.1-100mM Mg2+ ions or a salt thereof (e.g., 1-100mM, 1-50mM, 1-20mM, 5- 50mM, 5-20 mM, or 5-15mM). In some embodiments, suitable conditions include between 1- 1000mM K+ ions or a salt thereof such as KCl (e.g., 1-1000mM, 1-500mM, 1-200mM, 50- 500mM, 100-500mM, or 100-300mM).
- KCle.g., 1-1000mM, 1-500mM, 1-200mM, 50- 500mM, 100-500mM, or 100-300mM.
- suitable conditionsinclude between 1-1000mM Cl- ions or a salt thereof such as KCl (e.g., 1-1000mM, 1-500mM, 1- 200mM, 50- 500mM, 100-500mM, or 100-300mM).
- suitable conditionsinclude between 0.1-100mM Mn2+ ions or a salt thereof such as MnCl2 (e.g., 0.1-100mM, 0.1- 50mM, 0.1-20mM, 0.1-10mM, 0.1-5mM, 0.1-2mM, 0.5- 50mM, 0.5-20 mM, 0.5-15mM, 0.5- 5mM, 0.5-2mM, or 0.1-10mM).
- suitable conditionsinclude dithiothreitol (DTT) (e.g., 1-1000 ⁇ M, 1-500 ⁇ M, 1-200 ⁇ M, 50- 500 ⁇ M, 100-500 ⁇ M, 100-300 ⁇ M, 0.1- 100mM, 0.1-50mM, 0.1-20mM, 0.1-10mM, 0.1-5mM, 0.1-2mM, 0.5- 50mM, 0.5-20 mM, 0.5- 15mM, 0.5-5mM, 0.5-2mM, or 0.1-10mM).
- DTTdithiothreitol
- suitable conditionsinclude between 0.1mM and 100mM ribonucleoside triphosphate (NTP) (e.g., 0.1-100 mM, 0.1-50mM, 0.1-10mM, 1- 100mM, 1-50mM, or 1-10mM).
- NTPribonucleoside triphosphate
- suitable conditionsinclude a pH of 4 to 10 (e.g., pH of 5 to 9, pH of 6 to 9, or pH of 6.5 to 8.5).
- suitable conditionsinclude a temperature of 4°C to 50°C (e.g., 10°C to 40°C, 15 °C to 40°C, 20°C to 40°C, or 30°C to 40°C),
- the linear polyribonucleotideis produced from a deoxyribonucleic acid, e.g., a deoxyribonucleic acid described herein, such as a DNA vector, a linearized DNA vector, or a cDNA.
- the linear polyribonucleotideis transcribed from the deoxyribonucleic acid by transcription in a cell-free system (e.g., in vitro transcription).
- the circular polyribonucleotidemay be produced in a cell, e.g., a prokaryotic cell or a eukaryotic cell.
- an exogenous polyribonucleotideis provided to a cell (e.g., a linear polyribonucleotide described herein or a DNA molecule encoding for the transcription of a linear polyribonucleotide described here).
- the linear polyribonucleotidesmay be transcribed in the cell from an exogenous DNA molecule provided to the cell.
- polyribonucleotidemay be transcribed in the cell from an exogenous recombinant DNA molecule transiently provided to the cell.
- the exogenous DNA moleculedoes not integrate into the cell’s genome.
- the linear polyribonucleotideis transcribed in the cell from a recombinant DNA molecule that is incorporated into the cell’s genome.
- the cellis a prokaryotic cell.
- the prokaryotic cell including the polyribonucleotides described hereinis a bacterial cell or an archaeal cell.
- the prokaryotic cell including the polyribonucleotides described hereinmay be E coli, halophilic archaea (e.g., Haloferax volcaniii), Sphingomonas, cyanobacteria (e.g., Synechococcus elongatus, Spirulina (Arthrospira) spp., and Synechocystis spp.), Streptomyces, actinomycetes (e.g., Nonomuraea, Kitasatospora, or Thermobifida), Bacillus spp.
- E colihalophilic archaea
- Sphingomonase.g., cyanobacteria (e.g., Synechococcus elongatus, Spirulina (Arthrospira) spp., and Synechocystis spp.)
- Streptomycese.g., Nonom
- the prokaryotic cellsmay be grown in a culture medium.
- the prokaryotic cellsmay be contained in a bioreactor.
- the cellis a eukaryotic cell.
- the eukaryotic cellis a unicellular eukaryotic cell.
- the unicellular eukaryoticis a unicellular fungal cell such as a yeast cell (e.g., Saccharomyces cerevisiae and other Saccharomyces spp., Brettanomyces spp., Schizosaccharomyces spp., Torulaspora spp, and Pichia spp.).
- the unicellular eukaryotic cellis a unicellular animal cell.
- a unicellular animal cellmay be a cell isolated from a multicellular animal and grown in culture, or the daughter cells thereof.
- the unicellular animal cellis dedifferentiated.
- the unicellular eukaryotic cellis a unicellular plant cell.
- a unicellular plant cellmay be a cell isolated from a multicellular plant and grown in culture, or the daughter cells thereof. In some embodiments, the unicellular plant cell is dedifferentiated. In some embodiments, the unicellular plant cell is from a plant callus. In embodiments, the unicellular cell is a plant cell protoplast. In some embodiments, the unicellular eukaryotic cell is a unicellular eukaryotic algal cell, such as a unicellular green alga, a diatom, an euglenid, or a dinoflagellate.
- Non-limiting examples of unicellular eukaryotic algae of interestinclude Dunaliella salina, Chlorella vulgaris, Chlorella zofingiensis, Haematococcus pluvialis, Neochloris oleoabundans and other Neochloris spp., Protosiphon botryoides, Botryococcus LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 braunii, Cryptococcus spp., Chlamydomonas reinhardtii and other Chlamydomonas spp.
- the unicellular eukaryotic cellis a protist cell.
- the unicellular eukaryotic cellis a protozoan cell.
- the eukaryotic cellis a cell of a multicellular eukaryote.
- the multicellular eukaryotemay be selected from the group consisting of a vertebrate animal, an invertebrate animal, a multicellular fungus, a multicellular alga, and a multicellular plant.
- the eukaryotic organismis a human.
- the eukaryotic organismis a non-human vertebrate animal.
- the eukaryotic organismis an invertebrate animal.
- the eukaryotic organismis a multicellular fungus. In some embodiments, the eukaryotic organism is a multicellular plant. In embodiments, the eukaryotic cell is a cell of a human or a cell of a non-human mammal such as a non-human primate (e.g., monkeys, apes), ungulate (e.g., bovids including cattle, buffalo, bison, sheep, goat, and musk ox; pig; camelids including camel, llama, and alpaca; deer, antelope; and equids including horse and donkey), carnivore (e.g., dog, cat), rodent (e.g., rat, mouse, guinea pig, hamster, squirrel), or lagomorph (e.g., rabbit, hare).
- a non-human primatee.g., monkeys, apes
- ungulatee.g., bovids
- the eukaryotic cellis a cell of a bird, such as a member of the avian taxa Galliformes (e.g., chickens, turkeys, pheasants, quail), Anseriformes (e.g., ducks, geese), Paleaognathae (e.g., ostriches, emus), Columbiformes (e.g., pigeons, doves), or Psittaciformes (e.g., parrots).
- avian taxa Galliformese.g., chickens, turkeys, pheasants, quail
- Anseriformese.g., ducks, geese
- Paleaognathaee.g., ostriches, emus
- Columbiformese.g., pigeons, doves
- the eukaryotic cellis a cell of an arthropod (e.g., insects, arachnids, crustaceans), a nematode, an annelid, a helminth, or a mollusc.
- the eukaryotic cellis a cell of a multicellular plant, such as an angiosperm plant (which can be a dicot or a monocot) or a gymnosperm plant (e.g., a conifer, a cycad, a gnetophyte, a Ginkgo), a fern, horsetail, clubmoss, or a bryophyte.
- the eukaryotic cellis a cell of a eukaryotic multicellular alga.
- the eukaryotic cellsmay be grown in a culture medium.
- the eukaryotic cellsmay be contained in a bioreactor.
- any method of producing a circular polyribonucleotide described hereinmay be performed in a bioreactor.
- a bioreactorrefers to any vessel in which a chemical or biological process is carried out which involves organisms or biochemically active substances derived from such organisms. Bioreactors may be compatible with the cell-free methods for production of circular RNA described herein.
- a vessel for a bioreactormay include a culture flask, a dish, or a bag that may be single use (disposable), autoclavable, or sterilizable.
- a LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 bioreactormay be made of glass, or it may be polymer-based, or it may be made of other materials.
- bioreactorsinclude, without limitation, stirred tank (e.g., well mixed) bioreactors and tubular (e.g., plug flow) bioreactors, airlift bioreactors, membrane stirred tanks, spin filter stirred tanks, vibromixers, fluidized bed reactors, and membrane bioreactors.
- the mode of operating the bioreactormay be a batch or continuous processes.
- a bioreactoris continuous when the reagent and product streams are continuously being fed and withdrawn from the system.
- a batch bioreactormay have a continuous recirculating flow, but no continuous feeding of reagents or product harvest.
- Some methods of the present disclosureare directed to large-scale production of circular polyribonucleotides.
- the methodmay be performed in a volume of 1 liter (L) to 50 L, or more (e.g., 5 L, 10 L, 15 L, 20 L, 25 L, 30 L, 35 L, 40 L, 45 L, 50 L, or more).
- the methodmay be performed in a volume of 5 L to 10 L, 5 L to 15 L, 5 L to 20 L, 5 L to 25 L, 5 L to 30 L, 5 L to 35 L, 5 L to 40 L, 5 L to 45 L, 10 L to 15 L, 10 L to 20 L, 10 L to 25 L, 20 L to 30 L, 10 L to 35 L, 10 L to 40 L, 10 L to 45 L, 10 L to 50 L, 15 L to 20 L, 15 L to 25 L, 15 L to 30 L, 15 L to 35 L, 15 L to 40 L, 15 L to 45 L, or 15 to 50 L.
- a bioreactormay produce at least 1g of circular RNA.
- a bioreactormay produce 1-200g of circular RNA (e.g., 1-10g, 1-20g, 1-50g, 10-50g, 10-100g, 50-100g, or 50-200g of circular RNA).
- the amount producedis measured per liter (e.g., 1-200g per liter), per batch or reaction (e.g., 1-200g per batch or reaction), or per unit time (e.g., 1-200g per hour or per day).
- more than one bioreactormay be utilized in series to increase the production capacity (e.g., one, two, three, four, five, six, seven, eight, or nine bioreactors may be used in series).
- circularization efficiencyis at least about 10%, at least about 15%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%, at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, or 100%. In some embodiments, circularization efficiency is at least about 40%. In some embodiments, circularization efficiency is between about 10% and about 100%; for example, circularization efficiency is about 15%, about 20%, about 25%, about 30%, about 35%, about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, LRN Ref.
- circularization efficiencyis between about 20% and about 80%. In some embodiments, circularization efficiency is between about 30% and about 60%. In some embodiments, circularization efficiency is about 40%.
- the linear polyribonucleotideis substantively enriched or pure (e.g., purified) prior to self-splicing the linear polyribonucleotide. In other embodiments, the linear polyribonucleotide is not purified prior to self-splicing the linear polyribonucleotide.
- the resulting circular polyribonucleotideis purified.
- Purificationmay include separating or enriching the desired reaction product from one or more undesired components, such as any unreacted stating material, byproducts, enzymes, or other reaction components.
- purification of linear polyribonucleotide following LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 transcription in a cell-free systemmay include separation or enrichment from the DNA template prior to self-splicing the linear polyribonucleotide.
- Purification of the circular RNA product following splicingmay be used to separate or enrich the circular polyribonucleotide from its corresponding linear polyribonucleotide.
- Methods of purification of polyribonucleotidesare known to those of skill in the art and include enzymatic purification or by chromatography. In some embodiments, the methods of purification result in a circular polyribonucleotide that has less than 50% (e.g., less than 40%, 30%, 20%, 10%, 5%, 4%, 3%, 2%, or 1%) linear polyribonucleotides.
- the reference criterion for the amount of linear polyribonucleotide molecules present in a preparationis the presence of no more than 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 600 ng/ml, 1 ⁇ g/ ml, 10 ⁇ g/ml, 50 ⁇ g/ml, 100 ⁇ g/ml, 200 g/ml, 300 ⁇ g/ml, 400 ⁇ g/ml, 500 ⁇ g/ml, 600 ⁇ g/ml, 600 ⁇ g/ml, 500
- the reference criterion for the amount of circular polyribonucleotide molecules present in a preparationis at least 30% (w/w), 40% (w/w), 50% (w/w), 60% (w/w), 70% (w/w), 80% (w/w), 85% (w/w), 90% (w/w), 91% (w/w), 92% (w/w), 93% (w/w), 94% (w/w), 95% (w/w), 96% (w/w), 97% (w/w), 98% (w/w), 99% (w/w), 99.1% (w/w), 99.2% (w/w), 99.3% (w/w), 99.4% (w/w), 99.5% (w/w), 99.6% (w/w), 99.7% (w/w), 99.8% (w/w), 99.9% (w/w), or 100% (w/w) molecules of the total ribonucleotide molecules in the preparation.
- the reference criterion for the amount of linear polyribonucleotide molecules present in a preparationis no more than 0.5% (w/w), 1% (w/w), 2% (w/w), 5% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), 40% (w/w), 50% (w/w) linear polyribonucleotide molecules of the total ribonucleotide molecules in the preparation.
- the reference criterion for the amount of nicked polyribonucleotide molecules present in a preparationis no LRN Ref.
- the reference criterion for the amount of combined nicked and linear polyribonucleotide molecules present in a preparationis no more than 0.5% (w/w), 1% (w/w), 2% (w/w), 5% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), 40% (w/w), 50% (w/w) combined nicked and linear polyribonucleotide molecules of the total ribonucleotide molecules in the preparation.
- a preparatione.g., pharmaceutical preparation
- a preparationis an intermediate preparation of a final circular polyribonucleotide drug product.
- a preparationis a drug substance or active pharmaceutical ingredient (API).
- a preparationis a drug product for administration to a subject.
- a preparatione.g., pharmaceutical preparation
- a preparation (e.g., pharmaceutical preparation) of circular polyribonucleotidesis (before, during or after the reduction of linear polyribonucleotide) further processed to substantially remove DNA, protein contamination (e.g., cell protein such as a host cell protein or protein process impurities), endotoxin, mononucleotide molecules, and/or a process-related impurity.
- compositions and pharmaceutical compositionscomprising (i) any of the circular polyribonucleotides or modified RNAs described herein and (ii) a pharmaceutically acceptable excipient.
- Pharmaceutical compositionscan comprise one or more additional therapeutic agents, e.g., therapeutically and/or prophylactically active agents.
- additional therapeutic agentse.g., therapeutically and/or prophylactically active agents.
- Methods of making pharmaceutical compositions including circular polyribonucleotides or modified RNAsare described in WO 2020/181013, which is incorporated herein by reference in its entirety.
- Pharmaceutical compositions provided hereinare suitable for administration to humans or any other animal, e.g., to non-human animals, e.g., non-human mammals.
- compositions suitable for administration to humansin order to render the compositions suitable for administration to various animals is well understood.
- subjects that can be administered any of the pharmaceutical compositions described hereininclude humans, non-human primate, mammals (e.g., commercially-relevant mammals, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 such as cattle, pigs, horses, sheep, cats, dogs, mice, and rats), and birds (e.g., including commercially relevant birds such as poultry, chickens, ducks, geese, and/or turkeys).
- Pharmaceutical compositions provided hereincan be formulated for any suitable mode of administration.
- Non-limiting examplesinclude formulation of the pharmaceutical composition for intravenous, subcutaneous, intrahepatic, or intramuscular administration.
- a pharmaceutical compositionis formulated for local administration.
- a pharmaceutical compositionis formulated for systemic administration.
- Pharmaceutical compositions provided hereincan include an unencapsulated circular polyribonucleotide of modified RNA, a partially encapsulated circular polyribonucleotide or modified RNA, or a completely encapsulated circular polyribonucleotide or modified RNA. Any of the circular polyribonucleotides or modified RNAs described herein can be included in a pharmaceutical composition with a carrier or without a carrier.
- compositions described hereincan be formulated with a carrier, e.g., pharmaceutical carrier and/or a polymeric carrier, e.g., a liposome.
- a carriere.g., pharmaceutical carrier and/or a polymeric carrier, e.g., a liposome.
- the circular polyribonucleotide or modified RNAcan be formulated as a lipid nanoparticle.
- the circular polyribonucleotide or modified RNAis formulated for unencapsulated delivery or natural nanoparticle delivery.
- the natural nanoparticleis composed of a natural source selected from a plant, bacteria, animal, insect, archaea, or fungi.
- the circular polyribonucleotideis formulated within (a) a plurality of lipid nanoparticles (LNP) comprising synthetic structural lipids and an ionizable lipid or within (b) a natural nanoparticle comprising natural lipids and an ionizable lipid.
- LNPlipid nanoparticles
- One or more naturally occurring and/or synthetic lipid compoundsmay be used in the preparation of a lipid-based carrier (or lipid nanoformulation).
- a lipid-based carrier (or lipid nanoformulation)may be a lipid particle that has a complexity characterized by comprising a wide variety of lipids, including both synthetic lipids or lipids extracted from one or more natural sources (such as plants or bacteria).
- Natural lipidscan be derived from a variety of arthropod, fungi, plant, archaea, or bacteria, or one or more parts thereof (e.g., segments, organs, eggs, spores, mycelium, tissue, membrane or cell wall).
- the lipid-based carrieris a modified natural nanoparticle.
- modified natural particlerefers to a natural lipid LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 nanoparticles composition including both naturally derived lipids and one or more synthetically derived lipids.
- modified natural nanoparticlesmay include ionizable lipids, non-cationic lipids, sterols, and PEGylated lipids. More embodiments of natural lipids, complex lipid particles, and methods of producing and delivering natural nanoparticles may be found in PCT Application No. PCT/US2023/021094, filed on May 5, 2023, PCT Application No. PCT/US24/25134, filed on April 18, 2024, and PCT Application No. PCT/US24/25270, filed on April 18, 2024, the contents of which are incorporated herein by reference in their entirety.
- the modified natural nanoparticleis plant-based, e.g. as described in International Patent Publication Nos.
- the modified natural nanoparticleis bacteria-derived, e.g. as described in International Patent Publication Nos. WO2023/096858.
- more than one ionizable lipidcan be used for the ionizable lipid component. More embodiments of ionizable lipids may be found in PCT Application No. PCT/US22/50725, filed on November 22, 2022, PCT Application No.
- Suitable lipids for use in the RNA composition and methods for making and using thereofinclude the lipids as described in J. McClellan, M. C. King, Cell 2010, 141, 210-217 and in Whitehead et al., Nature Communications (2014) 5:4277, which is incorporated herein by reference in its entirety.
- the ionizable lipidmay be 1,1’-((2-(4-(2-((2-(bis(2- hydroxydodecyl)amino)ethyl) (2-hydroxydodecyl)amino)ethyl)piperazin-1- yl)ethyl)azanediyl)bis(dodecan-2-ol) (C12-200), MD1 (cKK-E12), OF2, EPC, ZA3-Ep10, TT3, LP01, 5A2-SC8, Lipid 5, SM-102 (Lipid H), or ALC-315.
- the ionizable lipid includedis LP01.
- the natural nanoparticlemay include one or more exogenous lipids, e.g., lipids that are exogenous to the natural source (e.g., originating from a source that is not the source or source part from which the particle is produced).
- the lipid compositionmay include 0%, less than 1%, or at least 1%, 2%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or more than 95% exogenous lipid.
- the exogenous lipide.g., ionizable lipid
- the exogenous lipidis added to the preparation prior to step (b), e.g., mixed with extracted natural lipids prior to step (b).
- the term “exogenous lipid”refers to a lipid that is exogenous to the natural source (e.g., plant, bacteria), i.e., a lipid originates from a source that is not the natural source from which the lipids are extracted (e.g., a lipid that is added to the lipid particle formulation using method described herein).
- the term “exogenous lipid”does not exclude a natural-derived lipid (such as a plant-derived sterol).
- an exogenous lipidcan be a natural-derived lipid (such as a plant-derived sterol that is exogenous to the plant source from which the lipids are extracted, e.g., an exogenous lipid can be a plant derived sterol that is added to the lipid particle formulation).
- an exogenous lipidcan be a natural- derived lipid that is exogenous to the particular natural source from which the lipids are extracted (e.g., a bacteria-derived lipid that is exogenous to the plant source from which the lipids are extracted, or vice versa).
- An exogenous lipidmay be a cell-penetrating agent, may be capable of increasing delivery of one or more polynucleotides by the lipid formulation (e.g. lipid nanoparticle or natural nanoparticle) to a cell, and/or may be capable of increasing loading (e.g., loading efficiency or loading capacity) of a polynucleotide.
- the exogenous lipidmay be a stabilizing lipid.
- the exogenous lipidmay be a structural lipid (e.g., a synthetic structural lipid).
- Exemplary exogenous lipidsinclude ionizable lipids, synthetic structural lipids, sterols, and PEGylated lipids.
- Exogenous lipidsmay also include cationic lipids.
- cationic lipidrefers to an amphiphilic LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 molecule (e.g., a lipid or a lipidoid) that is positively charged, containing a cationic group (e.g., a cationic head group).
- the term “ionizable lipid”refers to an amphiphilic molecule (e.g., a lipid or a lipidoid, e.g., a synthetic lipid or lipidoid) containing a group (e.g., a head group) that can be ionized, e.g., dissociated to produce one or more electrically charged species, under a given condition (e.g., pH).
- the natural nanoparticlemay contain 3-1000 lipids extracted from one or more natural (e.g., plant, bacteria) sources.
- the natural nanoparticlecontains at least 10 natural lipids belonging to one or more of the classes selected from the group consisting of fatty acyls (FA), fatty acyl conjugates, phospholipids, glycerolipids, glycolipids, glycerophospholipids, sphingolipids, waxes, and sterol.
- the lipid nanoparticle or natural nanoparticle compositioncomprises one or more ionizable lipids, one or more structural lipids (synthetic or natural), a sterol, and one or more PEG-modified lipids.
- the natural nanoparticlemay further comprise a neutral lipid as a helper lipid.
- the natural lipidsmay be used in combination with a neutral lipid as a structural lipid component.
- the particlehas a size of less than about 200 nm.
- the RNA and particle compositionhas an N:P ratio of at least 3, for instance, an N:P ratio of 3 to 100, 3 to 50, 3 to 30, 3 to 20, 3 to 15, 3 to 12, 6 to 30, 6 to 20, 6 to 15, or 6 to 12.
- pharmaceutical compositions described hereincan be formulated without a carrier, e.g., in a naked delivery formulation.
- a naked delivery formulationrefers to a formulation that is free from a carrier.
- a naked delivery formulationcan be free of any or all of: transfection reagents, cationic carriers, carbohydrate carriers, nanoparticle carriers, or protein carriers.
- a naked delivery formulationcan be free from phtoglycogen octenyl succinate, phytoglycogen beta-dextrin, anhydride-modified phytoglycogen beta-dextrin, lipofectamine, polyethylenimine, poly(trimethylenimine), poly(tetramethylenimine), polypropylenimine, aminoglycoside-polyamine, dideoxy-diamino-b-cyclodextrin, spermine, spermidine, poly(2-dimethylamino)ethyl methacrylate, poly(lysine), poly(histidine), poly(arginine), cationized gelatin, dendrimers, chitosan, l,2-Dioleoyl-3-Trimethylammonium- LRN Re
- the naked delivery formulationcan comprise a non-carrier excipient.
- a non-carrier excipientmay comprise an inactive ingredient that does not exhibit an active cell-penetrating effect.
- a non-carrier excipientmay comprise a buffer, for example PBS.
- a non-carrier excipientmay be a solvent, a non-aqueous solvent, a diluent, a suspension aid, a surface active agent, an isotonic agent, a thickening agent, an emulsifying agent, a preservative, a polymer, a peptide, a protein, a cell, a hyaluronidase, a dispersing agent, a granulating agent, a disintegrating agent, a binding agent, a buffering agent, a lubricating agent, or an oil.
- the naked delivery formulationcan comprise a diluent (e.g., a parenterally acceptable diluent).
- a diluentcan be a liquid diluent or a solid diluent.
- a diluentcan be an RNA solubilizing agent, a buffer, or an isotonic agent.
- Non- limiting examples of an RNA solubilizing agentinclude water, ethanol, methanol, acetone, formamide, and 2-propanol.
- Examples of a bufferinclude 2-(N-morpholino)ethanesulfonic acid (MES), Bis-Tris, 2-[(2-amino-2-oxoethyl)-(carboxymethyl)amino]acetic acid (ADA), N-(2- Acetamido)-2-aminoethanesulfonic acid (ACES), piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 2-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]ethanesulfonic acid (TES), 3- (N-morpholino)propanesulfonic acid (MOPS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Tris, Tricine, Gly-Gly, Bicine, or phosphate.
- MES2-(N-morpholino)ethanesulfonic acid
- Bis-Tris2-[(2-amino-2
- Non-limiting examples of an isotonic agentinclude glycerin, mannitol, polyethylene glycol, propylene glycol, trehalose, or sucrose.
- Purity of the pharmaceutical composition comprising the circular polyribonucleotide or modified RNAcan be measured using any method known in the art, e.g., chromatography, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 electrophoresis, mass spectrometry, fluorescence, light scattering, refractive index, microscopy, circular dichroism (CD) spectroscopy, spectrophotometry, or surface plasmon resonance (SPR).
- the pharmaceutical composition comprising the circular polyribonucleotide or modified RNAis at least 30% (w/w), 40% (w/w), 50% (w/w), 60% (w/w), 70% (w/w), 80% (w/w), 85% (w/w), 90% (w/w), 91% (w/w), 92% (w/w), 93% (w/w), 94% (w/w), 95% (w/w), 96% (w/w), 97% (w/w), 98% (w/w), 99% (w/w), or 100% (w/w) pure on a mass basis.
- a concentration of the circular polyribonucleotide or modified RNA in the pharmaceutical compositionis at least 0.1 ng/mL, 0.5 ng/mL, 1 ng/mL, 5 ng/mL, 10 ng/mL, 50 ng/mL, 0.1 ⁇ g/mL, 0.5 ⁇ g/mL, 1 ⁇ g/mL, 2 ⁇ g/mL, 5 ⁇ g/mL, 10 ⁇ g/mL, 20 ⁇ g/mL, 30 ⁇ g/mL, 40 ⁇ g/mL, 50 ⁇ g/mL, 60 ⁇ g/mL, 70 ⁇ g/mL, 80 ⁇ g/mL, 100 ⁇ g/mL, 200 ⁇ g/mL, 300 ⁇ g/mL, 500 ⁇ g/mL, 1 mg/mL, 2 mg/mL, 3 mg/mL, 5 mg/mL, 10 mg/mL, 100 mg/mL, or 500 mg/mL.
- the pharmaceutical compositionis substantially free of mononucleotide or has a mononucleotide content of no more than 1 pg/ml, 10 pg/ml, 0.1 ng/ml, 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 1000 ⁇ g/mL, 5000 ⁇ g/mL, 10,000 ⁇ g/mL, or 100,000 ⁇ g/mL.
- the pharmaceutical compositionhas a mononucleotide content of no more than 0.1% (w/w), 0.2% (w/w), 0.3% (w/w), 0.4% (w/w), 0.5% (w/w), 0.6% (w/w), 0.7% (w/w), 0.8% (w/w), 0.9% (w/w), 1% (w/w), 2% (w/w), 3% (w/w), 4% (w/w), 5% (w/w), 6% (w/w), 7% (w/w), 8% (w/w), 9% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), or any percentage therebetween of total nucleotides on a mass basis, wherein total nucleotide content is the total mass of deoxyribonucleotide molecules and ribonucleotide molecules.
- the pharmaceutical compositionhas a linear RNA content, e.g., linear RNA counterpart or RNA fragments, of no more than 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 600 ng/ml, 1 ⁇ g/ml, 10 ⁇ g/ml, 50 ⁇ g/ml, 100 ⁇ g/ml, 200 g/ml, 300 ⁇ g/ml, 400 ⁇ g/ml, 500 ⁇ g/ml, 600 ⁇ g/ml, 700 ⁇ g/ml,
- the pharmaceutical compositionhas a nicked RNA content of no more than 10% (w/w), 9.9% (w/w), 9.8% (w/w), 9.7% (w/w), 9.6% (w/w), 9.5% (w/w), 9.4% (w/w), 9.3% (w/w), 9.2% (w/w), 9.1% (w/w), 9% (w/w), 8% (w/w), 7% (w/w), 6% (w/w), 5% (w/w), 4% (w/w), 3% (w/w), 2% (w/w), 1% (w/w), 0.5% (w/w), or 0.1% (w/w), or percentage therebetween.
- the pharmaceutical compositionis substantially free of DNA content, e.g., template DNA or cell DNA (e.g., host cell DNA), has a DNA content, as low as zero, or has a DNA content of no more than 1 pg/ml, 10 pg/ml, 0.1 ng/ml, 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 1000 ⁇ g/mL, 5000 ⁇ g/mL, 10,000 ⁇ g/mL, or 100,000 ⁇ g/mL.
- DNA contente.g., template
- the pharmaceutical compositionis substantially free of an impurity (e.g., a cell protein, a cell nucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material, protein contamination, or endotoxin contamination).
- the pharmaceutical compositioncomprises a concentration of protein that is less than 0.1 ng, 1 ng, 5 ng, 10 ng, 15 ng, 20 ng, 25 ng, 30 ng, 35 ng, 40 ng, 50 ng, 60 ng, 70 ng, 80 ng, 90 ng, 100 ng, 200 ng, 300 ng, 400 ng, or 500 ng of protein per milligram (mg) of the circular polyribonucleotide.
- an impuritye.g., a cell protein, a cell nucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material, protein contamination, or endotoxin contamination.
- the pharmaceutical compositioncomprises a concentration of protein that is
- the pharmaceutical compositionhas an A260/A280 absorbance ratio from about 1.6 to about 2.3, e.g., as measured by spectrophotometer. In some embodiments, the A260/A280 absorbance ratio is about 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5, or any number therebetween. In some embodiments, the pharmaceutical composition comprises an amount of endotoxin that is less than 20 EU/kg (weight), 10 EU/kg, 5 EU/kg, 1 EU/kg, or is below a predetermined threshold, e.g., the pharmaceutical composition comprises a level of endotoxin below a limit of detection by a specified method.
- the pharmaceutical compositionis a sterile drug product or substantially free of microorganisms (e.g., supports growth of fewer than 100 viable microorganisms as tested under aseptic conditions).
- the pharmaceutical compositioncomprises a bioburden of less than 100 CFU/100 mL, 50 CFU/100 mL, 40 CFU/100 mL, 30 CFU/100 mL, 200 CFU/100 mL, 10 CFU/100 mL, or 10 CFU/100 mL before sterilization.
- the pharmaceutical composition comprising the circular polyribonucleotide that has undergone a purification stepproduces a reduced level of one more markers of an immune or inflammatory response after administration to a subject compared to a pharmaceutical composition comprising an unpurified circular polyribonucleotide.
- the one or more markers of an immune or inflammatory responseis a cytokine or immune response related gene.
- the one or more markers of an immune or inflammatory responseis expression of a gene, such as RIG-I, MDA5, PKR, IFN-beta, OAS, and OASL. III.
- Circular Polyribonucleotides and Modified RNAsAlso provided herein are methods for treating or reducing the risk of developing a GLP- 2-related disease in a subject that include administering to the subject a therapeutically effective amount of any of the circular polyribonucleotides or modified RNAs described herein, or any of the pharmaceutical compositions described herein.
- GLP-2 related diseaserefers to any disease in which cells engage in aberrant GLP-2 signaling, e.g., aberrant insulin signaling.
- Non-limiting examples of GLP-2-related diseasesinclude short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury.
- SBSshort bowel syndrome
- SBS-IFshort bowel syndrome with intestinal failure
- TPNtotal parenteral nutrition
- an effective amount of any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereinis administered to a subject (e.g., a mammalian subject, e.g., a human) having or at risk for developing a GLP-2 related disease (e.g., short bowel syndrome) via a suitable route (e.g., local administration and/or systemic administration).
- a subjecte.g., a mammalian subject, e.g., a human
- a suitable routee.g., local administration and/or systemic administration.
- the subjecthas been previously identified or diagnosed as having a GLP-2- related disease.
- the subjecthas been previously identified as having an elevated risk of developing a GLP-2-related disease. LRN Ref.
- subjectrefers to a subject who needs treatment as described herein.
- the subjectis a human or a non-human mammal (e.g., cat, dog, horse, cow, goat, or sheep).
- a human subject who needs treatmentcan be a human patient having, suspected of having, or at risk for having a GLP-2-related disease, e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury
- An “effective amount” or a “therapeutically effective amount” as used hereinrefers to the amount of each active agent required to confer therapeutic effect on the subject, either alone or in combination with one or more other active agents.
- Effective amountsvary, as recognized by those skilled in the art, depending on the particular condition being treated, the severity of the condition, the individual patient parameters including age, physical condition, size, weight, the duration of the treatment, the nature of concurrent therapy (if any), the specific route of administration and like factors within the knowledge and expertise of the health practitioner. These factors are well known in the medical field. It is generally preferred that a maximum dose of the individual components or combinations thereof be used, that is, the highest safe dose according to sound medical judgment. It will be understood by those of ordinary skill in the art, however, that a patient may insist upon a lower dose or tolerable dose for medical reasons, psychological reasons, or virtually any other reason. Empirical considerations such as the half-life of an agent will generally contribute to the determination of the dosage.
- Frequency of administrationcan be determined and adjusted over the course of therapy, and is generally, but not necessarily, based on treatment and/or suppression and/or amelioration and/or delay of a GLP-2-related disease (e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof).
- a GLP-2-related diseasee.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof).
- dosages of any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereincan be determined empirically in individuals who have been given one or more administration(s) of the circular polyribonucleotide LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 or modified RNA.
- individualsare given incremental dosages of any of the circular polyribonucleotides, modified RNAs, and pharmaceutical compositions described herein and an indicator and/or a symptom of a GLP-2-related disease can be followed to assess efficacy.
- Any suitable dosing regimencan be used in methods described herein.
- the dosage regimendepends on the pattern of pharmacokinetic decay that the practitioner wishes to achieve.
- dosing frequencyis once every day, once every other day, once every week, or longer. In some embodiments, dosing frequency is multiple times per day.
- the treatmentcan be sustained until a desired suppression of symptoms occurs or until sufficient therapeutic levels are achieved to alleviate a GLP-2-related disease, or a symptom thereof.
- dosing regimens(including inhibitor used) can vary over time.
- a circular or linear polyribonucleotide described hereinmay be administered in a single dose or in multiple doses to a cell, tissue or subject.
- a circular or linear polydeoxyribonucleotide described hereinmay be administered in a single dose or in multiple doses to a cell, tissue or subject.
- a method of administering multiple doses of a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- a pharmaceutical compositioncomprises providing two or more compositions, over a period of time, to a cell, tissue or subject (e.g. a mammal).
- multiple doses of a composition of a nucleic acid molecule described hereinmay be administered to a subject over a defined time course.
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- a pharmaceutical compositione.g., in a pharmaceutical composition
- the methods according to this aspect of the inventioncomprise sequentially administering to a subject multiple doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition).
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- compositions of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- a pharmaceutical compositione.g., in a pharmaceutical composition
- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1e.g., hours, days, weeks or months.
- the present inventionprovides methods which comprise sequentially administering to the subject a single initial dose of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition), followed by one or more secondary doses of the composition, and optionally followed by one or more tertiary doses of the composition.
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- initial doserefers to the temporal sequence of administration of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition).
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- the “initial dose”is the dose which is administered at the beginning of the treatment regimen (also referred to as the “baseline dose”); the “secondary doses” are the doses which are administered after the initial dose; and the “tertiary doses” are the doses which are administered after the secondary doses.
- the initial, secondary, and tertiary dosesmay all contain the same amount of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition), and in certain embodiments, may differ from one another in terms of frequency of administration.
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- the amount of a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- a pharmaceutical compositione.g., in a pharmaceutical composition
- one or more (e.g., 2, 3, 4, or 5) dosesare administered at the beginning of the treatment regimen as “loading doses” followed by subsequent doses that are administered on a less frequent basis (e.g., “maintenance doses”).
- each secondary and/or tertiary doseis administered after the immediately preceding dose.
- the immediately preceding dosemeans, in a sequence of multiple administrations, the dose of the composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 composition) which is administered to a subject prior to the administration of the very next dose in the sequence with no intervening doses.
- a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- each secondary and/or tertiary doseis administered every day, every 2 days, 3 days, 4 days, 5 days, 6 days, or 7 days after the immediately preceding dose. In certain embodiments, each secondary and/or tertiary dose is administered every 0.5 weeks, 1 week, 2 weeks, 3 weeks, or 4 weeks after the immediately preceding dose.
- the methods according to this aspect of the inventionmay comprise administering to a subject any number of secondary and/or tertiary doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition).
- a composition of a nucleic acid molecule described hereine.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide
- only a single secondary doseis administered to the subject.
- two or more (e.g., 2, 3, 4, 5, 6, 7, 8, or more) secondary dosesare administered to the subject.
- tertiary doseonly a single tertiary dose is administered to the subject.
- two or more (e.g., 2, 3, 4, 5, 6, 7, 8, or more) tertiary dosesare administered to the subject.
- the frequency at which the secondary and/or tertiary doses are administered to a subjectcan vary over the course of the treatment regimen. The frequency of administration may also be adjusted during the course of treatment.
- multiple dosesare provided to produce a level of the composition or express a level of the GLP-2 or GLP-2 analog in a cell, tissue or subject.
- multiple dosesare provided to produce or maintain a level of the composition, or to produce or maintain a level of the GLP-2 or GLP-2 analog, in a cell, tissue or subject for a period of time, for instance, for at least 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150 days, or at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 18, 21, or 24 months, or at least 1, 2, 3, 4, or 5 years.
- the methodcomprises providing (e.g., administering) at least a first composition and a second composition to the cells, tissue, or subject (e.g., a mammal, e.g., a human).
- the methodfurther comprises providing (e.g., administering) a third composition, fourth composition, fifth composition, sixth composition, seventh composition, eighth composition, ninth composition, tenth composition, or more.
- additional compositionsare provided for the duration of the life of the cell.
- additional compositionsare provided (e.g., administered) while the cell, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 tissue or subject obtains a benefit from the composition, e.g., stimulated insulin secretion, altered metabolism, improved glucose homeostasis, reduced body weight, improved liver function, and/or amelioration of fibrosis.
- a first composition in a multiple dosing regimencomprises a first amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein.
- a second composition in a multiple dosing regimencomprises a second amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein.
- a third composition, a fourth composition, a fifth composition, a sixth composition, a seventh composition, an eighth composition, a ninth composition, a tenth composition, or more in a multiple dosing regimencomprises a third, fourth, fifth, sixth, seventh, eighth, ninth, tenth or more amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein.
- the second amount of the nucleic acid moleculee.g., circular polyribonucleotide
- the second amount of the nucleic acid moleculeis the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the third amount of the nucleic acid moleculeis the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid moleculeis the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the second amount of the nucleic acid moleculeis less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the third amount of the nucleic acid moleculeis less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid moleculeis less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the second amount of the nucleic acid moleculeis greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the third amount of the nucleic acid moleculeis greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid moleculeis greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide).
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the second compositionvaries by no more than 1%, 5%, 10%, 15%, 20%, or 25% of an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the second composition is no more than 1%, 5%, 10%, 15%, 20%, or 25% less than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second compositionis from 0.1-fold to 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second composition is 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent compositionis 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second compositionis from 0.1-fold to 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second compositionis 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent compositionis 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent compositionis from 0.1-fold to 1000-fold higher or lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- an amount of the nucleic acid moleculee.g., circular LRN Ref. No.: LRN23-107WO Fish Ref.
- No.: 56929-0005WO1 polyribonucleotide) of a subsequent compositione.g., after a first composition of an amount of nucleic acid molecule (e.g., circular polyribonucleotide)
- a subsequent compositione.g., after a first composition of an amount of nucleic acid molecule (e.g., circular polyribonucleotide)
- an amount of nucleic acid moleculee.g., circular polyribonucleotide
- a first compositioncomprises 1-fold nucleic acid molecule (e.g., circular polyribonucleotide), a second composition comprises 5-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to the first composition, and a third composition comprises 0.2-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to the first composition.
- the second compositioncomprises at least 5-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to an amount of nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition.
- the first compositioncomprises a higher amount of the nucleic acid molecule (e.g., circular polyribonucleotide) than the second composition. In some embodiments, the first composition comprises a higher amount of the nucleic acid molecules (e.g., circular polyribonucleotides) than the third, fourth, fifth, sixth, seventh, eighth, ninth, or tenth composition. In some embodiments, the plurality (e.g., two or more) of compositions of a nucleic acid molecule (e.g., circular polyribonucleotide) encoding a GLP-2 or GLP-2 analog, which are administered in a multiple dosing regimen as described herein, are the same compositions.
- the nucleic acid moleculee.g., circular polyribonucleotide
- the plurality (e.g., two or more) of compositions of a nucleic acid molecule (e.g., circular polyribonucleotide) encoding a GLP-2 or GLP-2 analog, which are administered in a multiple dosing regimen as described herein,are different compositions.
- the same compositionscomprise the nucleic acid molecules (e.g., circular polyribonucleotides) encoding the same GLP-2 or GLP-2 analog.
- the different compositionscomprise the nucleic acid molecules (e.g., circular polyribonucleotides) encoding different a GLP-2 or GLP-2 analog, or a combination thereof.
- a composition of the nucleic acid molecule (e.g., a circular polyribonucleotide) disclosed hereincan induce a response in a subject.
- the responsemay be stimulated insulin secretion, altered metabolism, improved glucose homeostasis, reduced body weight, improved liver function, and/or amelioration of fibrosis.
- the method of administering the nucleic acid molecule (e.g., circular polyribonucleotide) provided hereinincludes administering LRN Ref. No.: LRN23-107WO Fish Ref.
- the method provided hereinincludes administering to a subject in need thereof the nucleic acid molecule for at least 3 times, with an interval of about 7 days.
- a level of the GLP-2 or GLP-2 analoge.g., a plasma GLP-2 or GLP-2 level
- a level with variation of less than 50%, 40%, 30%, 20%, or 10%is maintained at a level with variation of less than 50%, 40%, 30%, 20%, or 10% for a period of longer than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, or 20 weeks after the last dose.
- a level of the GLP-2 or GLP-2 analoge.g., a plasma GLP-2 or GLP-2 level
- a first levelis maintained at a first level for a period of longer than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19, or 20 weeks after the second, third, fourth, fifth, sixth, seventh, eight, or the last dose, wherein the first level is higher than a level of the GLP-2 or GLP-2 analog measured shortly after the first dose (e.g., measured about 12, 24, 36, or 48 hours after the first dose).
- a level of the GLP-2 or GLP-2 analoge.g., a plasma GLP-2 or GLP-2 level
- a level of the GLP-2 or GLP-2 analogis maintained at a first level for a period of longer than 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20 weeks after the second, third, fourth, fifth, sixth, seventh, eight, or the last dose, wherein the first level is higher than a level of the GLP-2 or GLP-2 analog measured shortly after the first dose (e.g., measured about 12, 24, 36, or 48 hours after the first dose).
- the term “treating”refers to the application or administration of a composition including one or more active agents to a subject who has a GLP-2-related disease (e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy- induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof), a symptom of a GLP-2-related disease, and/or an increased risk of developing a GLP-2-related disease, with LRN Ref. No.: LRN23-107WO Fish Ref.
- a GLP-2-related diseasee.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia
- No.: 56929-0005WO1the purpose to cure, heal, alleviate, relieve, alter, remedy, ameliorate, improve, or affect the GLP-2-related disease, the symptom of the GLP-2 related disease, and/or the risk of developing a GLP-2-related disease.
- Alleviating a GLP-2-related diseaseincludes delaying the development or progression of the disease, and/or reducing disease severity. Alleviating the disease does not necessarily require curative results.
- “delaying” the development of a GLP-2-related diseasee.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof
- SBSshort bowel syndrome
- SBS-IFshort bowel syndrome with intestinal failure
- TPNtotal parenteral nutrition
- This delaycan be of varying lengths of time, depending on the history of the GLP-2-related disease and/or individuals being treated.
- a method that “delays” or alleviates the development of a GLP-2-related disease and/or delays the onset of the GLP-2-related diseaseis a method that reduces probability of developing one or more symptoms of the GLP-2-related disease in a given time frame and/or reduces extent of the symptoms in a given time frame, when compared to not using the method. Such comparisons are typically based on clinical studies, using a number of subjects sufficient to give a statistically significant result. “Development” or “progression” of a disease means initial manifestations and/or ensuing progression of the GLP-2-related disease. Development of the GLP-2-related disease can be detectable and assessed using standard clinical techniques known in the art. However, development also refers to progression that may be undetectable.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereinis administered to a subject in an amount sufficient to increase levels of GLP-2-mediated signaling by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more).
- LRN RefLRN Ref.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereinis administered to a subject in an amount sufficient to increase intestinal growth in a subject by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more).
- increasing intestinal growthincludes increasing intestinal tissue and/or cells.
- the cellsare crypt cells.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereinis administered to a subject in an amount sufficient to increase intestinal absorption of fluids and nutrients in a subject by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more).
- methods for treating a GLP-2-related diseaseinvolve restoring, at least in part, expression and/or activity of GLP-2 using any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described hereincan be administered using any suitable method for achieving delivery of the circular polyribonucleotide or modified RNA to the subject in need thereof.
- the route of administrationcan depend on various factors such as the type of GLP-2-related disease to be treated and the site of the disease.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical composition described hereincan be administered topically, locally, nasally, parenterally, buccally, or by inhalation.
- Parenteral administrationincludes, but is not limited to, subcutaneous, intracutaneous, intravenous, intramuscular, or intrasynovial injection, or infusion techniques.
- any of the circular polyribonucleotides, modified RNAs, or pharmaceutical composition described hereinis administered locally (e.g., intramuscular injection). In some embodiments, any of the circular polyribonucleotides, the modified RNAs, or the pharmaceutical composition described herein is administered systemically (e.g., intravenous infusion). In some embodiments, any of the circular polyribonucleotides, the modified RNAs, or the pharmaceutical compositions described herein is administered one or more times to the subject. Alternatively, or in addition, any of the circular polyribonucleotides, the modified RNAs, or the LRN Ref. No.: LRN23-107WO Fish Ref.
- kits described hereincan be administered as part of a combination therapy with an additional therapeutic agent.
- Any therapeutic agent suitable for treating a GLP-2-related diseasecan be used as an additional therapeutic agent in methods and/or pharmaceutical compositions described herein.
- additional therapeutic agentsinclude anti-inflammatory agents (e.g., steroids, such as corticosteroids)), immunosuppressants (e.g., methotrexate, cyclosporine), or insulin.
- anti-inflammatory agentse.g., steroids, such as corticosteroids
- immunosuppressantse.g., methotrexate, cyclosporine
- insuline.g., insulin
- no other agentsare administered.
- kitsthat can be used, e.g., in any of the methods described herein.
- kitsinclude (a) (i) a composition comprising any of the circular polyribonucleotides described herein or any of the modified RNAs described herein or (ii) any of the pharmaceutical compositions described herein; and optionally, (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof.
- Nucleic AcidsThe present disclosure further provides nucleic acids (e.g., DNA or RNA) encoding any of the polypeptides described herein.
- the nucleic acidencodes a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the GLP-2 polypeptides or analogs described herein), (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein), and (iii) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein).
- GLP-2glucagon-like peptide-2
- a half-life extension moietye.g., any of the exemplary half-life extension moieties described herein
- a secretion signal sequencee.g., any of the exemplary secretion signal sequences described herein.
- the nucleic acidencodes a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP- 2 polypeptides or analogs described herein) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein).
- the nucleic acidencodes a polypeptide comprising a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of exemplary the GLP-2 polypeptides or analogs described herein).
- the present disclosurealso provides polypeptides generated from any of the circular polyribonucleotides, any of the modified RNAs, and any of the nucleic acids described herein.
- the polypeptidecomprises (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein), (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein), and (iii) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein).
- GLP-2glucagon-like peptide-2
- a half-life extension moietye.g., any of the exemplary half-life extension moieties described herein
- a secretion signal sequencee.g., any of the exemplary secretion signal sequences described herein.
- the polypeptidecomprises (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein).
- the polypeptidecomprises a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein).
- the polypeptidescan include an IgG constant domain (e.g., IgG1, IgG2, IgG3, IgG4), a serum protein or fragment thereof (e.g., albumin or fragment thereof (e.g., human serum albumin or a fragment thereof), fibronectin or fragment thereof (e.g., human fibronectin or a fragment thereof), or transferrin or fragment thereof (e.g., human transferrin or a fragment thereof)), and an antibody or an antigen-binding fragment thereof that binds to a serum protein (e.g., an antibody or antigen-binding fragment thereof that binds to human albumin, human fibronectin, or human transferrin).
- IgG constant domaine.g., IgG1, IgG2, IgG3, IgG4
- a serum protein or fragment thereofe.g., albumin or fragment thereof (e.g., human serum albumin or a fragment thereof), fibronectin or fragment thereof (e.g.,
- the IgG constant domainincludes a half-life extension substitution (e.g., any of the half-life extension substitutions described herein).
- a half-life extension substitutione.g., any of the half-life extension substitutions described herein.
- GGGGSGGGGS3 (SEQ ID NO: 141) linker (also referred to as teduglutide-Fc). Recombinant teduglutide was used as a control.
- the circular RNA constructwas transfected into HeLa cells using LipofectamineTM MessengerMAXTM (Invitrogen LMRNA001) according to the manufacturer’s instructions. Activity was measured using a G protein-coupled receptor (GPCR) assay in which cyclic adenosine monophosphate (cAMP) production were measured with a fluorescently labelled cAMP probe.
- GPCRG protein-coupled receptor
- teduglutide-Fc expressed from circular RNAshowed approximately 3-fold reduced activity level as compared to recombinant teduglutide in a GPCR assay.
- a functionally active GLP-2 analogcan be expressed from circular RNA.
- Example 2Expression of Functionally Active GLP-2 Analog from Circular RNA in Mammalian Cells The expression of the GLP-2 analog teduglutide from various circular RNA constructs (Table 8) was tested in mammalian cells (HEK293T cells). Circular RNA constructs were transfected into HeLa cells using LipofectamineTM MessengerMAXTM (Invitrogen LMRNA001) according to the manufacturer’s instructions.
- Table 9Circular RNA constructs Construct Polypeptide IRES C-Terminal CE 5’ Spacer 3’ Spacer K274(Shire Modified ne poly(adenosine-uridine) spacer sequence having a length of 120 nucleotides; CE: circularization element; Anabaena2.3: A specific intron based circularization element; Anabaena2: A specific intron based circularization element.
- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1Taken together, these results demonstrate that functionally active teduglutide can be expressed from various circular RNA constructs.
- Example 3Pharmacokinetic (PK) Profiles of GLP-2 Fc Expressed from Circular RNA Compared to Recombinant GLP-2 Analogs
- the PK profile of teduglutide-Fc expressed from circular RNA encoding teduglutide-Fcwas evaluated in mice. Circular RNA encoding teduglutide-Fc (1.5 mg/kg) was injected into mice via subcutaneous injection in the interscapular area. Blood was collected from the tail-vein of each mouse. Serum was collected from blood and the concentration and pharmacokinetic (PK) parameters for teduglutide-Fc were determined.
- PKPharmacokinetic
- PK profile of teduglutide-Fc expressed from circular RNA in micewas then compared to the reported PK profiles of various recombinant GLP-2 analogs.
- Reported PK profileswere obtained from Hargrove et al., “Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome,” J Pharmacol Exp Ther;373(2):193-203 (2020) and Choi et al., “HM15912, a Novel Long-acting Glucagon-like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome,” J Pharmacol Exp Ther; JPET-AR- 2022-001381 (2022).
- Example 4Characterization of Lipid Nanoparticle (LNP) Formulations Various LNP formulations were tested for tolerability and expression of teduglutide-Fc. Circular RNA encoding teduglutide-Fc (4133_3) was formulated in different LNPs. LNP formulations (0.75 mg/kg) were injected into mice. Negative control mice were injected with PBS. Blood was collected from the tail-vein at 4 hours, 6 hours, and 24 hours after injection. Expression of teduglutide-Fc at 6 and 24 hours (FIG. 4A), complete blood cell count (CBC) at 24 hours (FIGs. 4B-4C), ALT levels at 24 hours (FIG. 4D), IFN ⁇ levels at 4 hours (FIG.
- Example 5Dose Response in Mice Circular RNA (7297) and mRNA containing the modified nucleoside N1- methylpseudoiridine (m1 ⁇ ) (7377) encoding teduglutide-Fc was formulated into LNPs (LRN28). A dose response of the LNP formulations of teduglutide-Fc was evaluated in either WT or NSG (NOD-scid IL2Rg null ) mice.
- Circular RNA in LNPs encoding teduglutide-Fc (1, 0.3, 0.1, or 0.03 mg/kg) in WT and NSG mice and m1 ⁇ mRNA in LNP encoding teduglutide-Fc (0.3 mg/kg) in NSG mice onlywere injected via subcutaneous injection in the interscapular area. Blood was collected from the tail-vein of each mouse at baseline and 6h, D1, D2, D10, D17, and D28 after subcutaneous administration.
- Expression of GLP2-Fc(FIGs. 5A-B), cytokine levels (FIGs. 5C- F), and anti-target antibody(ATA) response (FIG. 5G) were measured at various timepoints.
- Example 6Characterization of GLP-2 Fc Expressed from Circular RNA in Non-Human Primates Circular RNA (7297) and mRNA containing the modified nucleoside N1- methylpseudoiridine (m1 ⁇ ) (7377) encoding teduglutide-Fc was formulated into LNPs (LRN28).
- the circular RNA formulationshad 2 purification levels: pre-Reverse Phase (pre-RP) or reverse phase (RP).
- pre-RPpre-Reverse Phase
- RPreverse phase
- the LNP formulations of teduglutide-Fcwere intravenously dosed in non-human primates (NHPs) at 0.5 mg/kg with or without steroids, as shown in Table 11.
- the steroidif given, was dexamethasone intramuscularly administered at 1mpk 24h and 1h pre-dose and 24h LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 post-dose.
- Bloodwas collected at baseline (D -14), post-infusion, 6hr, 24h, 48h, 72h, D5, D7, D14, D21, and D28.
- Teduglutide-Fc expressionFIG. 6A
- antibody productionFIG. 6B
- complete blood countincluding lymphocyte count (FIG. 6C), cytokine responses (FIG. 6D), CRP levels (FIG. 6E), and activate partial thromboplastin (FIG. 6F) were measured.
- Embodiment 1is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence.
- GLP-2glucagon-like peptide-2
- Embodiment 2is the circular polyribonucleotide of embodiment 1, wherein the GLP-2 polypeptide or analog thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- Embodiment 3is the circular polyribonucleotide of embodiment 1 or 2, wherein the GLP- 2 polypeptide or analog thereof comprises an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
- Embodiment 4is the circular polyribonucleotide of any one of embodiments 1-3, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- Embodiment 5is the circular polyribonucleotide of any one of embodiments 1-4, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14.
- Embodiment 6is the circular polyribonucleotide of any one of embodiments 1-5, wherein the GLP-2 analog comprises teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681.
- Embodiment 7is the circular polyribonucleotide of any one of embodiments 1-6, wherein the half-life extension moiety comprises a peptide moiety.
- Embodiment 8is the circular polyribonucleotide of embodiment 7, wherein the peptide moiety comprises an Fc domain, an albumin polypeptide, a nanobody, or a post-translational modification site.
- Embodiment 9is the circular polyribonucleotide of embodiment 8, wherein the Fc domain comprises an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain.
- Embodiment 10is the circular polyribonucleotide of embodiment 8 or 9, wherein the Fc domain comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identical to SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain).
- Embodiment 11is the circular polyribonucleotide of any one of embodiments 8-10, wherein the Fc domain comprises an amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain).
- Embodiment 12the circular polyribonucleotide of embodiment 11, wherein the IgG4 Fc domain comprises an amino acid sequence of SEQ ID NO: 21.
- Embodiment 13is the circular polyribonucleotide of any one of embodiments 8-12, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain).
- Embodiment 14is the circular polyribonucleotide of embodiment 13, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain).
- Embodiment 15is the circular polyribonucleotide of any one of embodiments 8-14, wherein the Fc domain comprises a mutation.
- Embodiment 16is the circular polyribonucleotide of any one of embodiments 8-10, 13, and 15, wherein the Fc domain comprises the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain), except the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain) comprises an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L284A/L285A; M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL), and V308P.
- Embodiment 17is the circular polyribonucleotide of embodiment 8, wherein the albumin polypeptide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23.
- Embodiment 18is the circular polyribonucleotide of embodiment 8, wherein the peptide moiety comprises a nanobody that binds to albumin.
- Embodiment 19is the circular polyribonucleotide of any one of embodiments 1-18, wherein the circular polyribonucleotide comprises one or more modifications.
- Embodiment 20is the circular polyribonucleotide of embodiment 19, wherein the one or more modifications comprise one or more modifications to a portion of the sequence encoding the GLP-2 polypeptide or analog thereof.
- Embodiment 21is the circular polyribonucleotide of embodiment 19 or embodiment 20, wherein the one or more modifications comprise a modification to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof.
- Embodiment 22is the circular polyribonucleotide of any one of embodiments 1-21, wherein the circular polyribonucleotide further comprises a translation initiation sequence operably linked to the sequence encoding the polypeptide.
- Embodiment 23is the circular polyribonucleotide of any one of embodiments 1-22, wherein the circular polyribonucleotide further comprises an internal ribosome entry site (IRES) operatively linked to the sequence encoding the polypeptide.
- IRSinternal ribosome entry site
- Embodiment 24is the circular polyribonucleotide of any one of embodiments 1-23, wherein the circular polyribonucleotide further comprises a translation termination sequence.
- Embodiment 2is the circular polyribonucleotide of any one of embodiments 1-24, wherein the circular polyribonucleotide lacks a translation termination sequence.
- Embodiment 26is the circular polyribonucleotide of any one of embodiments 1-25, wherein the circular polyribonucleotide further comprises a stagger element at a 3’ end of the sequence encoding the polypeptide.
- Embodiment 27is the circular polyribonucleotide of embodiment 26, wherein the stagger element is configured to stall a ribosome during rolling circle translation.
- Embodiment 28is the circular polyribonucleotide of any one of embodiments 1-27, wherein the circular polyribonucleotide further comprises a replication element.
- Embodiment 29is the circular polyribonucleotide of any one of embodiments 1-28, wherein the circular polyribonucleotide further comprises a 5’ untranslated region operably linked to the sequence encoding the polypeptide.
- Embodiment 30is the circular polyribonucleotide of any one of embodiments 1-29, wherein the circular polyribonucleotide does not comprise a poly(A) sequence operably linked to the sequence encoding the polypeptide.
- Embodiment 31is the circular polyribonucleotide of any one of embodiments 1-30, wherein the circular polyribonucleotide does not comprise a 5’ untranslated region operably linked to the sequence encoding the polypeptide.
- Embodiment 32is the circular polyribonucleotide of any one of embodiments 1-31, wherein the secretion signal sequence is a liver-specific secretion signal sequence, wherein the LRN Ref. No.: LRN23-107WO Fish Ref.
- No.: 56929-0005WO1 liver-specific secretion signalcomprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to any one of SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO
- Embodiment 33is the circular polyribonucleotide of any one of embodiments 1-31, wherein the secretion signal sequence comprises an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia luciferase secretion signal sequence, or a erythropoietin (EPO) secretion signal sequence.
- Embodiment 34is the circular polyribonucleotide of embodiment 33, wherein the IL-2 secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 29.
- Embodiment 35is the circular polyribonucleotide of embodiment 33, wherein the proglucagon secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 31.
- Embodiment 36is the circular polyribonucleotide of embodiment 33, wherein the Gaussia luciferase secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 33.
- Embodiment 37is the circular polyribonucleotide of embodiment 33, wherein the EPO secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 35.
- Embodiment 38is the circular polyribonucleotide of embodiment 33, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 90%, 95%, 99%, or 100% identical to one of SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, or SEQ ID NO: 39.
- Embodiment 39is the circular polyribonucleotide of any one of embodiments 1-38, wherein the circular polyribonucleotide further comprises at least one spacer sequence.
- Embodiment 40is the circular polyribonucleotide of embodiment 39, wherein the polypeptide comprises a protease cleavage site and the at least one spacer sequence encodes the protease cleavage site.
- Embodiment 41is the circular polyribonucleotide of embodiment 40, wherein the protease cleavage site is positioned between (a) the secretion signal and (b) the GLP-2 polypeptide or analog thereof and the half-life extension moiety.
- Embodiment 42is the circular polyribonucleotide of any one of embodiments 1-41, wherein the polypeptide further comprises at least one linker sequence.
- Embodiment 43is the circular polyribonucleotide of embodiment 42, wherein the at least one linker sequence encodes an amino acid sequence selected from (GS) x , (GGS) x , (GGGGS) x (SEQ ID NO: 141), (GGSG)x (SEQ ID NO: 142), and (SGGG)x (SEQ ID NO: 143), wherein x is an integer from 1 to 50.
- Embodiment 44is the circular polyribonucleotide of embodiment 42 or 43, wherein the at least one linker sequence is disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety.
- Embodiment 45is the circular polyribonucleotide of any one of embodiments 1-44, wherein the circular polyribonucleotide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1.
- Embodiment 46is the circular polyribonucleotide of any one of embodiments 1-45, wherein the circular polyribonucleotide comprises an amino acid sequence of SEQ ID NO: 1.
- Embodiment 47is the circular polyribonucleotide of any one of embodiments 1-46, wherein the circular polyribonucleotide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2.
- Embodiment 48is the circular polyribonucleotide of any one of embodiments 1-47, wherein the circular polyribonucleotide comprises a nucleic acid sequence of SEQ ID NO: 2.
- Embodiment 49is a pharmaceutical composition comprising the circular polyribonucleotide of any one of embodiments 1-48, and a pharmaceutically acceptable excipient.
- Embodiment 50is the pharmaceutical composition of embodiment 49, wherein the circular polyribonucleotide is formulated as a lipid nanoparticle.
- Embodiment 51is the pharmaceutical composition of embodiment 49, wherein the circular polyribonucleotide is formulated for unencapsulated delivery or natural particle delivery.
- Embodiment 52is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for intravenous, subcutaneous, intrahepatic, or intramuscular administration.
- Embodiment 53is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for local administration.
- Embodiment 54is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for systemic administration.
- Embodiment 55is the pharmaceutical composition of any one of embodiments 49-54, wherein the pharmaceutical composition comprises one or more of the following features: (i) a concentration of the circular polyribonucleotide of between 0.1 ng/mL and 500 mg/mL; (ii) a concentration of deoxyribonucleotide of no more than 100 ng/mL; (iii) a concentration of protein of less than 100 ng of protein per milligram (mg) of the circular polyribonucleotide; (iv) an A260/A280 absorbance ratio of from about 1.6 to 2.3 as measured by a spectrophotometer; (v) substantially free of a process-related impurity selected from a cell protein, a
- Embodiment 56is a method of treating a glucagon-like peptide-2 (GLP-2)-related disease in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55.
- Embodiment 57is the method of embodiment 56, wherein the GLP-2-related disease is selected from the group consisting of: short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total LRN Ref. No.: LRN23-107WO Fish Ref.
- SBSshort bowel syndrome
- SBS-IFshort bowel syndrome with intestinal failure
- pediatric SBSpediatric SBS
- adult SBSmajor small bowel resection
- Embodiment 58is a method of increasing intestinal growth in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55.
- Embodiment 59is the method of embodiment 59, wherein increasing intestinal growth comprises increasing crypt cell growth.
- Embodiment 60is a method of increasing intestinal absorption of fluids and nutrients in a subject in need thereof comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55.
- Embodiment 61is the method of any one of embodiments 58-60, wherein the subject has short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury.
- SBSshort bowel syndrome
- SBS-IFshort bowel syndrome with intestinal failure
- TPNtotal parenteral nutrition
- Embodiment 62is the method of any one of embodiments 56-61, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered one or more times to the subject.
- Embodiment 63is the method of any one of embodiments 56-62, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered locally.
- Embodiment 64is the method of any one of embodiments 56-62, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered systemically.
- Embodiment 65is the method of any one of embodiments 56-64, further comprising administering one or more additional therapeutic agents to the subject.
- Embodiment 66is the method of any one of embodiments 56-65, wherein the subject is a human subject.
- Embodiment 67is a kit comprising: (a) (i) a composition comprising the circular polyribonucleotide of any one of embodiments 1-48 or (ii) the pharmaceutical composition of any one of embodiments 49-55; and (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof.
- Embodiment 68is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety.
- Embodiment 69is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety, wherein the half-life extension moiety is an Fc domain comprising a M252Y/S254T/T256E (YTE) mutation.
- Embodiment 70is a modified linear RNA comprising a sequence encoding a polypeptide comprising a glucagon-like peptide-2 (GLP-2) or analog thereof.
- Embodiment 71is a modified linear RNA comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence.
- Embodiment 72is the modified linear RNA of embodiment 70 or 71, further comprising a half-life extension moiety.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biophysics (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Toxicology (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Endocrinology (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 CIRCULAR POLYRIBONUCLEOTIDES ENCODING GLUCAGON-LIKE PEPTIDE 2 (GLP-2) AND USES THEREOF CROSS-REFERENCE TO RELATED APPLICATIONS This application claims the benefit of priority to U.S. Provisional Patent Application No. 63/600,527, filed on November 17, 2023, and U.S. Provisional Patent Application No. 63/614,264, filed on December 22, 2023, the content of each of which is hereby incorporated by reference in its entirety. SEQUENCE LISTING This application contains a Sequence Listing that has been submitted electronically as an XML file named 56929-0005WO1_SL_ST26.xml. The XML file, created on November 12, 2024, is 228,547 bytes in size. The material in the XML file is hereby incorporated by reference in its entirety. TECHNICAL FIELD The present disclosure generally relates to compositions including circular polyribonucleotides and modified RNAs, and their use in treatment and/or prevention of GLP-2- related diseases, such as short bowel syndrome. BACKGROUND Glucagon-like peptide (GLP)-2 is a peptide hormone derived from tissue-specific posttranslational processing of the pro-glucagon peptide. GLP-2 and GLP-2 analogs have been used to treat intestinal diseases, such as short bowel syndrome. SUMMARY The present disclosure is based, at least in part, on the development of circular polyribonucleotides and modified RNAs encoding a GLP-2 polypeptide or analog thereof. These circular polyribonucleotides and modified RNAs provide for prolonged and stable expression of the GLP-2 polypeptide or analog thereof in a subject (e.g., subjects having a GLP- 2-related disease, e.g., intestinal diseases such as short bowel syndrome). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Thus, provided herein are circular polyribonucleotides including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence. In some embodiments, the GLP-2 polypeptide or analog thereof includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. In some embodiments, the GLP-2 polypeptide or analog thereof includes an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. In some embodiments, the sequence encoding the polypeptide includes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. In some embodiments, the sequence encoding the polypeptide includes a nucleic acid sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. In some embodiments, the GLP-2 analog includes teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681. In some embodiments, the half-life extension moiety includes a peptide moiety. In some embodiments, the peptide moiety includes an Fc domain, an albumin polypeptide, a nanobody, or a post-translational modification site. In some embodiments, the Fc domain includes an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain. In some embodiments, the Fc domain includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identical to SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). In some embodiments, the Fc domain includes an amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). In some embodiments, the IgG4 Fc domain includes an amino acid sequence of SEQ ID NO: 21. In some embodiments, the sequence encoding the polypeptide includes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 domain), or SEQ ID NO: 22 (IgG4 Fc domain). In some embodiments, the sequence encoding the polypeptide includes a nucleic acid sequence of SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain). In some embodiments, the Fc domain includes a mutation. In some embodiments, the Fc domain includes the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain), except the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain) includes an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L284A/L285A; M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL), and V308P. In some embodiments, the albumin polypeptide includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23. In some embodiments, the peptide moiety includes a nanobody that binds to albumin. In some embodiments, the circular polyribonucleotide includes one or more modifications. In some embodiments, the one or more modifications include one or more modifications to a portion of the sequence encoding the GLP-2 polypeptide or analog thereof. In some embodiments, the one or more modifications include a modification to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof. In some embodiments, the circular polyribonucleotide includes a translation initiation sequence operably linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide includes an internal ribosome entry site (IRES) operatively linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide includes a translation termination sequence. In some embodiments, the circular polyribonucleotide lacks a translation termination sequence. In some embodiments, the circular polyribonucleotide includes a stagger element at a 3’ end of the sequence encoding the polypeptide. In some embodiments, the stagger element is configured to stall a ribosome during rolling circle translation. In some embodiments, the circular polyribonucleotide includes a replication element. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the circular polyribonucleotide includes a 5’ untranslated region operably linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide does not include a poly(A) sequence operably linked to the sequence encoding the polypeptide. In some embodiments, the circular polyribonucleotide does not include a 5’ untranslated region operably linked to the sequence encoding the polypeptide. In some embodiments, the secretion signal sequence is a liver-specific secretion signal sequence, where the liver-specific secretion signal includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to any one of SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91, SEQ ID NO: 93, SEQ ID NO: 95, SEQ ID NO: 97, SEQ ID NO: 99, SEQ ID NO: 101, SEQ ID NO: 103, SEQ ID NO: 105, SEQ ID NO: 107, SEQ ID NO: 109, SEQ ID NO: 111, SEQ ID NO: 113, SEQ ID NO: 115, SEQ ID NO: 117, SEQ ID NO: 119, SEQ ID NO: 121, SEQ ID NO: 123, SEQ ID NO: 125, SEQ ID NO: 127, SEQ ID NO: 129, SEQ ID NO: 131, SEQ ID NO: 133, SEQ ID NO: 135, SEQ ID NO: 137, or SEQ ID NO: 139. In some embodiments, the secretion signal sequence includes an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia luciferase secretion signal sequence, or a erythropoietin (EPO) secretion signal sequence. In some embodiments, the IL-2 secretion signal sequence includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 29. In some embodiments, the proglucagon secretion signal sequence includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 31. In some embodiments, the Gaussia luciferase secretion signal sequence includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 33. In some embodiments, the EPO secretion signal sequence includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 35. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the sequence encoding the polypeptide includes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to one of SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, or SEQ ID NO: 39. In some embodiments, the circular polyribonucleotide includes at least one spacer sequence. In some embodiments, the polypeptide includes a protease cleavage site and the at least one spacer sequence encodes the protease cleavage site. In some embodiments, the protease cleavage site is positioned between (a) the secretion signal and (b) the GLP-2 polypeptide or analog thereof and the half-life extension moiety. In some embodiments, the polypeptide includes at least one linker sequence. In some embodiments, the at least one linker sequence encodes an amino acid sequence selected from (GS)x, (GGS)x, (GGGGS)x (SEQ ID NO: 141), (GGSG)x (SEQ ID NO: 142), and (SGGG)x (SEQ ID NO: 143), where x is an integer from 1 to 50. In some embodiments, the at least one linker sequence is disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety. In some embodiments, the circular polyribonucleotide encodes an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. In some embodiments, the circular polyribonucleotide includes an amino acid sequence of SEQ ID NO: 1. In some embodiments, the circular polyribonucleotide includes a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. In some embodiments, the circular polyribonucleotide includes a nucleic acid sequence of SEQ ID NO: 2. Also provided herein are pharmaceutical compositions including any of the circular polyribonucleotides described herein and a pharmaceutically acceptable excipient. In some embodiments, the circular polyribonucleotide is formulated as a lipid nanoparticle. In some embodiments, the circular polyribonucleotide is formulated for unencapsulated delivery or natural particle delivery. In some embodiments, the pharmaceutical composition is formulated for intravenous, subcutaneous, intrahepatic, or intramuscular administration. In some embodiments, the pharmaceutical composition is formulated for local administration. In some embodiments, the pharmaceutical composition is formulated for systemic administration. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the pharmaceutical composition includes one or more of the following features: (i) a concentration of the circular polyribonucleotide of between 0.1 ng/mL and 500 mg/mL; (ii) a concentration of deoxyribonucleotide of no more than 100 ng/mL; (iii) a concentration of protein of less than 100 ng of protein per milligram (mg) of the circular polyribonucleotide; (iv) an A260/A280 absorbance ratio of from about 1.6 to 2.3 as measured by a spectrophotometer; (v) substantially free of a process-related impurity selected from a cell protein, a cell deoxyribonucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material; and (vi) a reduced level of one or more markers of an immune or inflammatory response after purification compared to prior to purification. Also provided herein are methods of treating a glucagon-like peptide-2 (GLP-2)-related disease in a subject in need thereof, the method including administering to the subject an effective amount of any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein. In some embodiments, the GLP-2-related disease is selected from the group consisting of: short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy- induced mucositis, and intestinal ischemia–reperfusion injury. Also provided herein are methods of increasing intestinal growth in a subject in need thereof, the method including administering to the subject an effective amount of any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein. In some embodiments, increasing intestinal growth includes increasing crypt cell growth. Also provided herein are methods of increasing intestinal absorption of fluids and nutrients in a subject in need thereof including administering to the subject an effective amount of any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein. In some embodiments, the subject has short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. In some embodiments, any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein is administered one or more times to the subject. In some embodiments, any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein is administered locally. In some embodiments, any of the circular polyribonucleotides described herein or any of the pharmaceutical compositions described herein is administered systemically. In some embodiments, the method includes administering one or more additional therapeutic agents to the subject. In some embodiments, the subject is a human subject. Also provided herein are kits including: (a) (i) a composition including any of the circular polyribonucleotides described herein or (ii) any of the pharmaceutical compositions described herein; and (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof. Also provided herein are circular polyribonucleotides including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety. Also provided herein are circular polyribonucleotides including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety, where the half-life extension moiety is an Fc domain including a M252Y/S254T/T256E (YTE) mutation. Also provided herein are modified linear RNAs including a sequence encoding a polypeptide including a glucagon-like peptide-2 (GLP-2) or analog thereof. Also provided herein are modified linear RNAs including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence. In some embodiments, the modified linear RNA further includes a half-life extension moiety (e.g., any of the half-life extension moieties described herein). All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication, patent, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 patent application, or item of information was specifically and individually indicated to be incorporated by reference. To the extent publications, patents, patent applications, and items of information incorporated by reference contradict the disclosure contained in the specification, the specification is intended to supersede and/or take precedence over any such contradictory material. Where values are described in terms of ranges, it should be understood that the description includes the disclosure of all possible sub-ranges within such ranges, as well as specific numerical values that fall within such ranges irrespective of whether a specific numerical value or specific sub-range is expressly stated. The term “each,” when used in reference to a collection of items, is intended to identify an individual item in the collection but does not necessarily refer to every item in the collection, unless expressly stated otherwise, or unless the context of the usage clearly indicates otherwise. Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention pertains. Although methods and materials similar or equivalent to those described herein can be used to practice the invention, suitable methods and materials are described below. All publications, patent applications, patents, and other references mentioned herein are incorporated by reference in their entirety. In case of conflict, the present specification, including definitions, will control. In addition, the materials, methods, and examples are illustrative only and not intended to be limiting. The details of one or more embodiments of the invention are set forth in the accompanying drawings and the description below. Other features, objects, and advantages of the invention will be apparent from the description and drawings, and from the claims. BRIEF DESCRIPTION OF THE DRAWINGS The following drawings illustrate certain embodiments of the features and advantages of this disclosure. These embodiments are not intended to limit the scope of the appended claims in any manner. Like reference symbols in the drawings indicate like elements. FIG. 1 includes a graph showing activity of teduglutide in cells treated with circular RNA encoding teduglutide-Fc or recombinant teduglutide. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 FIGs. 2A-2B include graphs showing expression (FIG. 2A) and activity (FIG. 2B) of teduglutide-Fc in mammalian cells transfected with circular RNA constructs encoding teduglutide-Fc. FIG. 3 includes a graph showing levels of teduglutide-Fc in mice injected with circular RNA encoding teduglutide-Fc and reported levels of glepaglutide, teduglutide, apraglutide, and HM15912 in rats injected with recombinant glepaglutide, teduglutide, apraglutide, or HM15912. FIGs. 4A-4F include data from characterization of various LNP formulations including expression levels of teduglutide-Fc (FIG. 4A), complete blood cell count (CBC) (FIGs. 4B-4C), ALT levels (FIG. 4D), IFNα levels (FIG. 4E), and cytokine levels (FIG. 4F). FIGs. 5A-5G include dose response data in mice injected with LNP formulations of circular RNA encoding teduglutide-Fc. The data include expression of GLP2-Fc (FIGs. 5A-B), cytokine levels (FIG. 5C-F), and anti-target antibody(ATA) response (FIG. 5G) FIGs. 6A-F include data from characterization of GLP-2 Fc expressed from circular RNA in non-human primates including Teduglutide-Fc expression (FIG. 6A), antibody production (FIG. 6B), complete blood count, including lymphocyte count (FIG. 6C), cytokine responses (FIG. 6D), CRP levels (FIG. 6E), and activate partial thromboplastin (FIG. 6F). DETAILED DESCRIPTION Provided herein are circular polyribonucleotides and modified RNAs encoding a GLP-2 polypeptide or analog thereof and related pharmaceutical compositions and kits. Circular polyribonucleotides and modified RNAs described herein comprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and optionally, (iii) a secretion signal sequence. In some embodiments, the circular polyribonucleotides and modified RNAs comprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof and (ii) a half-life extension moiety. In some embodiments, the circular polynucleotides and modified RNAs comprise a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence. The circular polyribonucleotides and modified RNAs described herein are useful in the treatment or prevention of a GLP-2-related disease (e.g., short bowel syndrome). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 I. Circular Polyribonucleotides/Modified RNAs Circular polyribonucleotides and modified RNAs described herein comprise a sequence encoding a polypeptide comprising: (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and optionally, (iii) a secretion signal sequence, each of which is described herein. In some embodiments, a circular polyribonucleotide or a modified RNA comprises a sequence encoding a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof and (ii) a half-life extension moiety. In some embodiments, a circular polyribonucleotide or a modified RNA comprise a sequence encoding a polypeptide comprising a sequence encoding a (i) a GLP- 2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence. In some embodiments, a circular polyribonucleotide or modified RNA can include two or more copies of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). Non-limiting additional sequences that can be included in a circular polyribonucleotide and a modified RNA are described herein. As used herein a “modified RNA” or a “linear modified RNA” refers to an RNA molecule that includes one or more (e.g., 2, 3, 4, 5, 10 or more) modified ribonucleotides described herein or modifications any of the exemplary modifications known in the art. Non- limiting examples of modified ribonucleotides include 5-methylcytidine, N1- methylpseudouridine, 5-methoxyuridine, and pseudouridine. A non-limiting example of a sequence encoding a polypeptide that can be present in any of the circular polyribonucleotides or modified RNAs described herein are provided in Table 1 (SEQ ID NO: 2). A non-limiting example of a polypeptide comprising (i) a GLP-2 polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence is provided in Table 1 (SEQ ID NO: 1). Table 1. Non-limiting example of a polypeptide and a sequence encoding a polypeptide SEQ Description Sequence ID NO: G T K P A LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 C C C C C C G C G C G C C G C In some embodiments, the polypeptide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. In some embodiments, the polypeptide comprises an amino acid sequence of SEQ ID NO: 1. In some embodiments, the sequence (encoding the polypeptide) comprises a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. In some embodiments, the sequence (encoding the polypeptide) comprises a sequence of SEQ ID NO: 2. (i) GLP-2 and Analogs Thereof Pro-glucagon is cleaved to glucagon by prohormone convertase 2 (PC2) in pancreatic α- cells (and other organs), and it is cleaved to glucagon-like peptide-2 (GLP-2) by prohormone convertase 1 (PC1). GLP-2 is a 33 amino acid peptide-encoded carboxyterminal to the sequence of GLP-1 in the proglucagon gene. Importantly, GLP-2 also significantly enhances the surface area of the mucosal epithelium via stimulation of crypt cell proliferation and inhibition of apoptosis in the enterocyte and crypt compartments. Both GLP-1 and GLP-2 are secreted from gut endocrine cells and promote nutrient absorption through distinct mechanisms of action. GLP-2 is also known to regulate gastric motility, gastric acid secretion, intestinal hexose transport, and increases the barrier function of the gut epithelium. GLP-2 has been shown to reduce mortality and decrease mucosal injury, cytokine expression, and bacterial septicemia in LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the setting of small and large bowel inflammation. GLP-2 also enhances nutrient absorption and gut adaptation in rodents or humans with short bowel syndrome. Activation of GLP-2 receptor signaling in heterologous cells promotes resistance to apoptotic injury in vitro via a G protein-coupled receptor expressed in gut endocrine cells of the stomach, small bowel, and colon. The cytoprotective, reparative, and energy-retentive properties of GLP-2 suggests that GLP-2 may potentially be useful for the treatment of human disorders characterized by injury and/or dysfunction of the intestinal mucosal epithelium. A “GLP-2 analog” refers to a polypeptide having one or more alterations (e.g., substitutions, deletions, and/or insertions) in an amino acid sequence of wild-type GLP-2 and having at least partial GLP-2 activity as compared to a wild-type GLP-2 polypeptide. Non- limiting examples of GLP-2 analogs include teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681. Further non-limiting examples of GLP-2 analogs are described in WO 2020/020904, which is incorporated herein by reference in its entirety. Non-limiting examples of amino acid sequences of pro-glucagon, GLP-2, and GLP-2 analogs are shown in Table 2 below. Non-limiting examples of nucleic acid sequence encoding pro-glucagon, GLP-2, and GLP-2 analogs are also shown in Table 2 below. Table 2. Non-limiting examples of sequences of pro-glucagon, GLP-2, and GLP-2 analogs SEQ Description Sequence ID Q Y R T A G T A T A C G g LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 g g a g g In some embodiments, the GLP-2 polypeptide or analog thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. In some embodiments, the GLP-2 polypeptide or analog thereof comprises an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. In some embodiments, the sequence (encoding the GLP-2 polypeptide or analog thereof) comprises a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. In some embodiments, the sequence (encoding the GLP-2 polypeptide or analog thereof) comprises a sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. In some embodiments, the GLP-2 polypeptide or analog thereof comprises an amino acid sequence that is a functional fragment of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the GLP-2 polypeptide or analog thereof comprises a functional fragment that has a sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. In some embodiments, the GLP-2 polypeptide or analog thereof comprises an amino acid sequence of SEQ ID NO: 13. In some embodiments, the amino acid at position X1 is norleucine. In some embodiments, the amino acid at position X2 is D-phenylalanine. In some embodiments, GLP-2 polypeptide or analog thereof comprises an amino acid sequence of SEQ ID NO: 167. In some embodiments, the amino acid at positions X1, X2, X3, and X4 of SEQ ID NO: 167 (e.g., positions 1, 2, 3, and 4 of SEQ ID NO: 5) can be any amino acid. For example, each of X1, X2, X3, and X4 can independently be any of amino acids alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, or valine, with the proviso that the combination of X1 of histidine, X2 of alanine, X3 of aspartic acid, and X4 of glycine is excluded. As used herein a “functional fragment” is a fragment that retains at least 50% (e.g., at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%) of the activity of the wildtype polypeptide from which it is derived. In some embodiments, a functional fragment can have an activity greater than the wildtype polypeptide from which it is derived. In some embodiments, the GLP-2 polypeptide or analog thereof comprises an amino acid sequence having at least one mutation. The term “substitution” refers to a substitution of a residue within a sequence (e.g., a nucleic acid or an amino acid sequence) with another residue, or a deletion or insertion of one or more residues within a sequence (e.g., a nucleic acid or an amino acid sequence). Substitutions are typically described herein by identifying the original residue followed by the position of the residue within the sequence and by the identity of the newly substituted residue. Various methods for making the amino acid substitutions provided herein are well known in the art, and are provided by, e.g., Green and Sambrook, Molecular Cloning: A Laboratory Manual (4th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2012). The GLP-2 polypeptide or analog thereof can comprise any number and/or any type of substitutions. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the sequence encoding the GLP-2 polypeptide or analog thereof comprises one or more mutations as compared to the wild type sequence. The one or more mutations can be silent, i.e., they do not modify the amino acid sequence of any encoded protein (or otherwise result in a substituted amino acid sequence). Alternatively, the one or more mutations in a nucleic acid sequence can result in modifications to the encoded amino acid sequence of GLP-2 or analog thereof, resulting in GLP-2 or analog thereof having one or more amino acid substitutions (e.g., having 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 or more amino acid substitutions) relative to the wild-type protein sequence. In some embodiments, the polypeptide comprises (i) a GLP-2 polypeptide or analog thereof (e.g., any of the GLP-2 polypeptides or analogs thereof described herein or known in the art) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein or known in the art). In some embodiments, the polypeptide comprises (i) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein or known in the art), (ii) a GLP-2 polypeptide or analog thereof (e.g., any of the exemplary GLP- 2 polypeptides or analogs thereof described herein or known in the art), and (iii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein or known in the art). Non-limiting examples of half-life extension moieties are described herein. In some embodiments, the polypeptide may further comprise an epitope tag for detection or purification (e.g., a HiBiT-tag) and/or a fluorescent tag for visualization (e.g., a GFP-tag). Also provided herein are modified linear RNAs including a sequence encoding a polypeptide including a glucagon-like peptide-2 (GLP-2) or analog thereof. Also provided herein are modified linear RNAs including a sequence encoding a polypeptide including (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence. In some embodiments, the modified linear RNA includes a half-life extension moiety (e.g., any of the half-life extension moieties described herein). (ii) Half-Life Extension Moieties In some embodiments, the polypeptide (encoded by a sequence in a circular polyribonucleotide or a modified RNA described herein) comprises a half-life extension moiety. The term “half-life extension moiety” refers to any moiety that extends the circulating serum LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 half-life of a polypeptide comprising a GLP-2 polypeptide or analog thereof in vivo. Circulating serum half-life refers to the amount of time it takes for the concentration or amount of a polypeptide comprising a GLP-2 polypeptide or analog thereof in the serum to be reduced by half (i.e., 50%). In some embodiments, the half-life extension moiety is a peptide moiety. Non-limiting examples of a half-life extension moiety include an antibody or fragment thereof (e.g., an Fc domain, e.g., an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain), a serum protein or fragment thereof (e.g., albumin or fragment thereof (e.g., human serum albumin or a fragment thereof), fibronectin or fragment thereof (e.g., human fibronectin or a fragment thereof), or transferrin or fragment thereof (e.g., human transferrin or a fragment thereof), and antibodies or an antigen-binding fragments thereof or peptides that bind to a serum protein (e.g., an antibody or antigen-binding fragment thereof that binds to human albumin, human fibronectin, or human transferrin). Antigen-binding fragments of antibodies include, e.g., single-chain antibodies (scFv), single-domain antibodies (also called nanobodies), Fab fragments, Fabʹ fragments, and F(abʹ)2 Fab2 fragments, diabodies, triabodies, and tetrabodies. Non-limiting examples of amino acid sequences (and corresponding nucleic acid sequences) of half-life extension moieties are shown in Table 3 below. Table 3. Non-limiting examples of sequences of half-life extension moieties SEQ Description Sequence ID E L S C C G G C G A C C G T E L LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 S C C G G C G A C C G T E L S C C G G C G A C C G T E L S C C G G C G A C C G T Q C P Q A N D K S LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 A T T A T A T T G T A T T T A A T A A T C C G G A T G C A C G T G A T T A K R G R P C N T N Y I A G A T G LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 T A C T T G A C A C C G C C C T T T A G T C T T C C C T A Y N T L I K S L A F C T C T G C T C C T T A T A T LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 T C A C G C G G T T A A C A T T T A T G A C In some embodiments, the half-life extension moiety comprises an antibody or fragment thereof. Non-limiting examples of antibody fragments include a Fab, a Fabʹ, a F(abʹ)2, a Fc, a Fv, an scFv, a diabody, a triabody, a tetrabody, and a nanobody. In some embodiments, the half-life extension moiety comprises an Fc domain (e.g., an IgG1 Fc domain (e.g., a human IgG1 Fc domain), an IgG2 Fc domain (e.g., a human IgG2 Fc domain), an IgG3 Fc domain (e.g., a human IgG3 Fc domain, or an IgG4 Fc domain (e.g., a human IgG4 Fc domain). In some embodiments, the half-life extension moiety comprises an Fc domain comprising an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 15 (human IgG1 Fc domain), SEQ ID NO: 17 (human IgG2 Fc domain), SEQ ID NO: 19 (human IgG3 Fc domain), or SEQ ID NO: 21 (human IgG4 Fc domain). In some embodiments, the half-life extension moiety comprises an Fc domain comprising an amino acid sequence of SEQ ID NO: 15 (human IgG1 Fc domain), SEQ ID NO: 17 (human IgG2 Fc domain), SEQ ID NO: 19 (human IgG3 Fc domain), or SEQ ID NO: 21 (human IgG4 Fc domain). In some embodiments, the sequence encoding the Fc domain comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 NO: 16 (human IgG1 Fc domain), SEQ ID NO: 18 (human IgG2 Fc domain), SEQ ID NO: 20 (human IgG3 Fc domain), or SEQ ID NO: 22 (human IgG4 Fc domain). In some embodiments, the sequence encoding the Fc domain comprises a nucleic acid sequence of SEQ ID NO: 16 (human IgG1 Fc domain), SEQ ID NO: 18 (human IgG2 Fc domain), SEQ ID NO: 20 (human IgG3 Fc domain), or SEQ ID NO: 22 (human IgG4 Fc domain). In some embodiments, the Fc domain comprises an amino acid sequence having at least one substitution. The Fc domain can comprise any number and/or any type of substitution. The Fc domain can comprise any substitution known in the art or described herein. Non-limiting examples of substitutions that can be included in an Fc domain include M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. In some embodiments, the Fc domain can comprise the amino acid sequence of SEQ ID NO: 15 (human IgG1 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. In some embodiments, the Fc domain can comprise the amino acid sequence of SEQ ID NO: 17 (human IgG2 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. In some embodiments, the Fc domain can comprise the amino acid sequence of SEQ ID NO: 19 (human IgG3 Fc domain), except that it comprises an amino acid substitution(s) selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. In some embodiments, the Fc domain can comprise the amino acid sequence of SEQ ID NO: 21 (human IgG4 Fc domain), except that it comprises an amino acid substitution(s) comprising an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L234A/L235A (LALA); M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. In some embodiments, the half-life extension moiety comprises an albumin polypeptide or a functional fragment thereof (e.g., a human albumin polypeptide or fragment thereof), a LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 fibronectin polypeptide or fragment thereof (e.g., a human fibronectin polypeptide or fragment thereof), or a transferrin polypeptide or fragment thereof (e.g., a human transferrin polypeptide or fragment thereof). In some embodiments, the albumin polypeptide or a functional fragment thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23. In some embodiments, the albumin polypeptide comprises an amino acid sequence of SEQ ID NO: 23. In some embodiments, the albumin polypeptide comprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype albumin polypeptide (e.g., wildtype human albumin polypeptide, e.g., SEQ ID NO: 23). The albumin polypeptide can comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution. In some embodiments, the albumin polypeptide or a functional fragment thereof is encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 24. In some embodiments, the albumin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 24. In some embodiments, the fibronectin polypeptide or a functional fragment thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 25. In some embodiments, the fibronectin polypeptide comprises an amino acid sequence of SEQ ID NO: 25. In some embodiments, the fibronectin polypeptide comprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype fibronectin polypeptide (e.g., wildtype human fibronectin polypeptide, e.g., SEQ ID NO: 25). The fibronectin polypeptide can comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution. In some embodiments, the fibronectin polypeptide or a functional fragment thereof is encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 26. In some embodiments, the fibronectin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 26. In some embodiments, the transferrin polypeptide or a functional fragment thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 99% identical to SEQ ID NO: 27. In some embodiments, the transferrin polypeptide comprises an amino acid sequence of SEQ ID NO: 27. In some embodiments, the transferrin polypeptide comprises an amino acid sequence having at least one substitution (e.g., one, two, three, four, five, six, seven, eight, nine, or ten amino acid substitutions and/or deletions) as compared to the corresponding wildtype transferrin polypeptide (e.g., wildtype human transferrin polypeptide, e.g., SEQ ID NO: 27). The transferrin polypeptide can comprise one, two, three, four, five, six, seven, eight, nine, or ten of any type of substitution. In some embodiments, the transferrin polypeptide or a functional fragment thereof is encoded by a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 28. In some embodiments, the transferrin polypeptide is encoded by a nucleic acid sequence of SEQ ID NO: 28. In some embodiments, the half-life extension moiety comprises an antibody or antigen- binding fragment thereof (e.g., any of the exemplary types of antigen-binding antibody fragments described herein or known in the art) that binds to a serum protein (e.g., albumin (e.g., human albumin), fibronectin (e.g., human fibronectin), or transferrin (e.g., human transferrin)). For example, the half-life extension moiety comprises antigen-binding antibody fragments, e.g., single-chain antibodies (scFv), single-domain antibodies (also called nanobodies), Fab fragments, Fabʹ fragments, and F(abʹ)2 Fab2 fragments, diabodies, triabodies, and tetrabodies, that bind to albumin (e.g., human albumin), fibronectin (e.g., human fibronectin), or transferrin (e.g., human transferrin). In some embodiments, the half-life extension moiety comprises a nanobody that binds specifically to human albumin, human fibronectin, or human transferrin. Non-limiting examples of antibodies, antigen-binding fragments, and peptides that bind to a serum protein that can be used as half-life extension moieties are provided in WO 2013/128027; US 2015/0037334; US 9,376,495; US 8,349,569; US 10,407,508; and Michot, N., et al, Albumin binding Nanofitins, a new scaffold to extend half-life of biologics – a case study with exenatide peptide, Peptides, 152 (2022), the contents of each of which is incorporated herein by reference in its entirety. In some embodiments, the half-life extension moiety can comprise one or more post- translational modification site(s). A post-translational modification site includes amino acid(s) in the polypeptide where a cellular enzyme can modify the amino acid to add a post-translational modification (e.g., phosphorylation, glycosylation, hydroxylation, methylation, acetylation, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 deamidation, and lipidation (e.g., N-myristoylation, palmitoylation, glycosylphosphatidylinositol (GPI)-anchor addition) that results in an increase in the half-life of the polypeptide. (iii) Secretion Signal Sequences In some embodiments, the polypeptide (encoded by the circular polyribonucleotides and modified RNAs described herein) can further comprise a secretion signal sequence. The term “secretion signal sequence” refers to a polypeptide sequence (e.g., between about 10 and about 30 amino acids in length) that is present at the N-terminus of a protein and that targets at least a portion of the protein to the cellular secretory pathway. In some embodiments, a sequence encoding a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein or known in the art) is operably linked to a sequence encoding a GLP-2 polypeptide or analog thereof (and a sequence encoding the half- life extension moiety). For example, the sequence encoding the secretion signal sequence can be operably linked at a 5’ end of a sequence encoding a GLP-2 polypeptide or analog thereof. In some embodiments, the secretion signal sequence is a liver-specific secretion signal sequence. Non-limiting examples of a secretion signal sequence include an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia lucifersase secretion signal sequence, and an erythropoietin (EPO) secretion signal sequence. Non-limiting examples of amino acid sequences (and corresponding nucleic acid sequences) of an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia lucifersase secretion signal sequence, and an EPO secretion signal sequence are shown in Table 4 below. Table 4. Non-limiting examples of signal secretion sequences SEQ Description Sequence ID NO: C LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 G C C T C LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 G T G C G C C C C LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 T T G C T C T G G LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 C T C T C C C C C LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 A C C C G G , g q p sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 29. In some embodiments, an IL-2 secretion signal sequence comprises an amino acid sequence of SEQ ID NO: 29. In some embodiments, a proglucagon secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 31. In some embodiments, a proglucagon secretion signal sequence comprises an amino acid sequence of SEQ ID NO: 31. In some embodiments, a Gaussia luciferase secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 33. In LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 some embodiments, a Gaussia luciferase secretion signal sequence comprises an amino acid sequence of SEQ ID NO: 33. In some embodiments, an EPO secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to SEQ ID NO: 35. In some embodiments, an EPO secretion signal sequence comprises an amino acid sequence of SEQ ID NO: 35. In some embodiments, a signal secretion sequence comprises an amino acid sequence having at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 29, SEQ ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91, SEQ ID NO: 93, SEQ ID NO: 95, SEQ ID NO: 97, SEQ ID NO: 99, SEQ ID NO: 101, SEQ ID NO: 103, SEQ ID NO: 105, SEQ ID NO: 107, SEQ ID NO: 109, SEQ ID NO: 111, SEQ ID NO: 113, SEQ ID NO: 115, SEQ ID NO: 117, SEQ ID NO: 119, SEQ ID NO: 121, SEQ ID NO: 123, SEQ ID NO: 125, SEQ ID NO: 127, SEQ ID NO: 129, SEQ ID NO: 131, SEQ ID NO: 133, SEQ ID NO: 135, SEQ ID NO: 137, or SEQ ID NO: 139. In some embodiments, a signal secretion sequence comprises an amino acid sequence of SEQ ID NO: 29, SEQ ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91, SEQ ID NO: 93, SEQ ID NO: 95, SEQ ID NO: 97, SEQ ID NO: 99, SEQ ID NO: 101, SEQ ID NO: 103, SEQ ID NO: 105, SEQ ID NO: 107, SEQ ID NO: 109, SEQ ID NO: 111, SEQ ID NO: 113, SEQ ID NO: 115, SEQ ID NO: 117, SEQ ID NO: 119, SEQ ID NO: 121, SEQ ID NO: 123, SEQ ID NO: 125, SEQ ID NO: 127, SEQ ID NO: 129, SEQ ID NO: 131, SEQ ID NO: 133, SEQ ID NO: 135, SEQ ID NO: 137, or SEQ ID NO: 139. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the sequence encoding a secretion signal sequence comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, or SEQ ID NO: 36. In some embodiments, the sequence encoding a secretion signal sequence comprises a nucleic acid sequence of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, or SEQ ID NO: 36. In some embodiments, the sequence encoding a secretion signal sequence comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, or 99% identical to one of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, SEQ ID NO: 44, SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ ID NO: 68, SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, SEQ ID NO: 78, SEQ ID NO: 80, SEQ ID NO: 82, SEQ ID NO: 84, SEQ ID NO: 86, SEQ ID NO: 88, SEQ ID NO: 90, SEQ ID NO: 92, SEQ ID NO: 94, SEQ ID NO: 96, SEQ ID NO: 98, SEQ ID NO: 100, SEQ ID NO: 102, SEQ ID NO: 104, SEQ ID NO: 106, SEQ ID NO: 108, SEQ ID NO: 110, SEQ ID NO: 112, SEQ ID NO: 114, SEQ ID NO: 116, SEQ ID NO: 118, SEQ ID NO: 120, SEQ ID NO: 122, SEQ ID NO: 124, SEQ ID NO: 126, SEQ ID NO: 128, SEQ ID NO: 130, SEQ ID NO: 132, SEQ ID NO: 134, SEQ ID NO: 136, SEQ ID NO: 138, or SEQ ID NO: 140. In some embodiments, the sequence encoding a secretion signal sequence comprises a nucleic acid sequence of SEQ ID NO: 30, SEQ ID NO: 32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, SEQ ID NO: 44, SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ ID NO: 68, SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, SEQ ID NO: 78, SEQ ID NO: 80, SEQ ID NO: 82, SEQ ID NO: 84, SEQ ID NO: 86, SEQ ID NO: 88, SEQ ID NO: 90, SEQ ID NO: 92, SEQ ID NO: 94, SEQ ID NO: 96, SEQ ID NO: 98, SEQ ID NO: 100, SEQ ID NO: 102, SEQ ID NO: 104, SEQ ID NO: 106, SEQ ID NO: 108, SEQ ID NO: 110, SEQ ID NO: 112, SEQ ID NO: 114, SEQ ID NO: 116, SEQ ID NO: 118, SEQ ID NO: 120, SEQ ID NO: 122, SEQ ID NO: 124, SEQ ID NO: 126, SEQ ID NO: 128, SEQ ID NO: 130, SEQ ID NO: 132, SEQ ID NO: 134, SEQ ID NO: 136, SEQ ID NO: 138, or SEQ ID NO: 140. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 (iv) Spacer Sequences As used herein, a “spacer” or “spacer sequence” or “spacer region” refers to any contiguous nucleotide sequence (e.g., of one or more nucleotides) that provides distance or flexibility between two adjacent polynucleotide regions. In some embodiments, the circular polyribonucleotide or linear modified RNA as disclosed herein includes at least one spacer sequence. In some embodiments, the circular polyribonucleotide or linear modified RNA includes 1, 2, 3, 4, 5, 6, 7, or more spacer sequences (e.g., identical or different spacer sequences). In some embodiments, a first spacer sequence and a second spacer sequence (e.g., a third spacer sequence, a fourth spacer sequence, a fifth spacer sequence, etc.) are about the same length. In some embodiments, a first spacer sequence and a second spacer sequence (e.g., a third spacer sequence, a fourth spacer sequence, a fifth spacer sequence, etc.) are different lengths. The spacer sequence can be a nucleic acid sequence having low GC content, e.g., less than 65%, 60%, 55%, 50%, 55%, 50%, 45%, 40%, 39%, 38%, 37%, 36%, 35%, 34%, 33%, 32%, 31 %, 30%, 29%, 28%, 27%, 26%, 25%, 24%, 23%, 22%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11 %, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2% or 1 %, across the full length of the spacer, or across at least 50%, 60%, 70%, 80%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% contiguous nucleic acid residues of the spacer. In some embodiments, the spacer sequence is substantially free of a secondary structure, such as less than 40 kcal/mol, less than -39, -38, -37, -36, -35, -34, -33, -32, -31, -30, -29, -28, -27, -26, -25, -24, -23, -22, -20, - 19, -18, -17, -16, -15, -14, -13, -12, -11, -10, -9, -8, -7, -6, -5, -4, -3, -2 or -1 kcal/mol. In some embodiments, the spacer sequence comprises: (i) a polyA region comprising 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine residues; (ii) a polyAC region comprising between 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine or cytosine residues; (iii) a polyAU region comprising between 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine or uridine residues; or (iii) a polyAG region comprising between 80% to 100% (e.g., 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, and 100%) adenosine or guanosine residues. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 The spacer sequence can be a non-coding sequence or a coding sequence. When the spacer is a non-coding sequence, a start codon may be provided in the coding sequence of an adjacent sequence. When the spacer is a coding sequence, a start codon may be provided in the spacer sequence. In some embodiments, the spacer is operably linked to another sequence described herein. In some embodiments, the circular polyribonucleotide or modified linear RNA includes an IRES operably linked to an expression sequence encoding a polypeptide. In some embodiments, the circular polyribonucleotide further includes a spacer region between the IRES and the 3’ exon fragment or the 5’ exon fragment. The spacer region may be, e.g., at least 5 (e.g., at least 10, at least 15, at least 20) ribonucleotides in length. The spacer region may be, e.g., from 5 to 500 (e.g., 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 200, 250, 300, 350, 400, 450, or 500) ribonucleotides. In some embodiments, the spacer region includes a polyA sequence. In some embodiments, the spacer region includes a polyA-C sequence. In some embodiments, the spacer region includes a polyA-G sequence. In some embodiments, the spacer region includes a polyA- T sequence. In some embodiments, the spacer region includes a polyU sequence. In some embodiments, the spacer region includes a random sequence. In some embodiments, the spacer region includes a random sequence. In some embodiments, the circular polyribonucleotide is from 50 to 20,000, e.g., 100 to 20,000, e.g., 200 to 20,000, e.g., 300 to 20,000 (e.g., 50, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,100, 1,200, 1,300, 1,400, 1,500, 1,600, 1,700, 1,800, 1,900, 2,000, 2,500, 3,000, 3,500, 4,000, 5,000, 6,000, 7,000, 8,000, 9,000, 10,000, 11 ,000, 12,000, 13,000, 14,000, 15,000, 16,000, 17,000, 18,000, 19,000, or 20,000) ribonucleotides in length. In embodiments, the circular polyribonucleotide is, e.g., at least 25, at least 50, at least 100, at least 125, at least 1150, at least 200, at least 300, at least 400, at least 500, at least 1,000, at least 2,000, at least 3,000, at least 4,000, or at least 5,000 ribonucleotides in length. A spacer can be specifically engineered depending on the IRES. In some embodiments, an RNA folding computer software, such as RNAFold, can be utilized to guide designs of the various elements of the vector, including the spacers. In some embodiments, the polyribonucleotide includes a 5’ spacer sequence (e.g., between the 5’ annealing region and the polyribonucleotide cargo). In some embodiments, the 5’ spacer sequence is at least 10 nucleotides in length. In another embodiment, the 5’ spacer LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 sequence is at least 15 nucleotides in length. In a further embodiment, the 5’ spacer sequence is at least 30 nucleotides in length. In some embodiments, the 5’ spacer sequence is at least 7, 8, 9, 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 25 or 30 nucleotides in length. In some embodiments, the 5’ spacer sequence is no more than 100, 90, 80, 70, 60, 50, 45, 40, 35 or 30 nucleotides in length. In some embodiments the 5’ spacer sequence is between 20 and 50 nucleotides in length. In certain embodiments, the 5’ spacer sequence is 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 , 32, 33, 34, 35, 3637, 38, 39, 40, 41 , 42, 43, 44, 45, 46, 47, 48, 49 or 50 nucleotides in length. In one embodiment, the 5’ spacer sequence is a polyA sequence. In another embodiment, the 5’ spacer sequence is a polyA-C sequence. In some embodiments, the 5’ spacer sequence includes a polyA-G sequence. In some embodiments, the 5’ spacer sequence includes a polyA-T sequence. In some embodiments, the 5’ spacer sequence includes a random sequence. In some embodiments, the polyribonucleotide includes a 3’ spacer sequence (e.g., between the 3’ annealing region and the polyribonucleotide cargo). In some embodiments, the 3’ spacer sequence is at least 10 nucleotides in length. In another embodiment, the 3’ spacer sequence is at least 15 nucleotides in length. In a further embodiment, the 3’ spacer sequence is at least 30 nucleotides in length. In some embodiments, the 3’ spacer sequence is at least 7, 8, 9, 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 25 or 30 nucleotides in length. In some embodiments, the 3’ spacer sequence is no more than 100, 90, 80, 70, 60, 50, 45, 40, 35 or 30 nucleotides in length. In some embodiments the 3’ spacer sequence is from 20 to 50 nucleotides in length. In certain embodiments, the 3’ spacer sequence is 10, 11 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 , 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 , 42, 43, 44, 45, 46, 47, 48, 49 or 50 nucleotides in length. In one embodiment, the 3’ spacer sequence is a polyA sequence. In another embodiment, the 5’ spacer sequence is a polyA-C sequence. In some embodiments, the 5’ spacer sequence includes a polyA-G sequence. In some embodiments, the 5’ spacer sequence includes a polyA-T sequence. In some embodiments, the 5’ spacer sequence includes a random sequence. In one embodiment, the polyribonucleotide includes a 5’ spacer sequence, but not a 3’ spacer sequence. In another embodiment, the polyribonucleotide includes a 3’ spacer sequence, but not a 5’ spacer sequence. In another embodiment, the polyribonucleotide includes neither a 5’ spacer sequence, nor a 3’ spacer sequence. In another embodiment, the polyribonucleotide LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 does not include an IRES sequence. In a further embodiment, the polyribonucleotide does not include an IRES sequence, a 5’ spacer sequence or a 3’ spacer sequence. In some embodiments, the spacer sequence includes at least 3 ribonucleotides, at least 4 ribonucleotides, at least 5 ribonucleotides, at least about 8 ribonucleotides, at least about 10 ribonucleotides, at least about 12 ribonucleotides, at least about 15 ribonucleotides, at least about 20 ribonucleotides, at least about 25 ribonucleotides, at least about 30 ribonucleotides, at least about 40 ribonucleotides, at least about 50 ribonucleotides, at least about 60 ribonucleotides, at least about 70 ribonucleotides, at least about 80 ribonucleotides, at least about 90 ribonucleotides, at least about 100 ribonucleotides, at least about 120 ribonucleotides, at least about 150 ribonucleotides, at least about 200 ribonucleotides, at least about 250 ribonucleotides, at least about 300 ribonucleotides, at least about 400 ribonucleotides, at least about 500 ribonucleotides, at least about 600 ribonucleotides, at least about 700 ribonucleotides, at least about 800 ribonucleotides, at least about 900 ribonucleotides, or at least about 100 ribonucleotides. Exemplary spacer sequences are shown in Table 5 below. Table 5. Non-limiting examples of spacer sequences SEQ ID Description Sequence NO: U A A A (v) Linkers LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the circular polyribonucleotide or modified RNA further comprises a sequence encoding a linker sequence. For example, the circular polyribonucleotide or modified RNA can comprise a sequence encoding a linker sequence disposed between the sequence encoding the GLP-2 polypeptide or analog thereof and the sequence encoding the half- life extension moiety. In such instances, the polypeptide comprises a linker sequence disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety in the polypeptide. Non-limiting examples of linker sequences are shown in Table 6 below. Additional examples of linker sequences are well known in the art (See e.g., Chen, X., Fusion Protein Linkers: Property, Design and Functionality, Adv Drug Deliv Rev., 65(10): 1357-1369 (2013), the content of which is incorporated herein by reference in its entirety). Table 6. Non-limiting examples of linker sequences SEQ ID Description Sequence NO: LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 (vi) Modifications Any of the circular polyribonucleotides or elements thereof described herein can include one or more modifications (e.g., any of the exemplary modifications described herein or known in the art). Any of the modified RNAs described herein can include one or more modifications (e.g., any of the exemplary modifications described herein or known in the art). The one or more modifications can be included at any position in the circular polyribonucleotide or modified RNA. For example, the sequence encoding a GLP-2 polypeptide or analog thereof can comprise one or more modifications (e.g., the sequence encoding a GLP-2 polypeptide or analog thereof comprises one or more modified bases). In some embodiments, the circular polyribonucleotide or modified RNA can comprise a post-transcriptional modification (e.g., capping, cleavage, polyadenylation, and splicing). A circular polyribonucleotide or elements thereof, or a modified RNA or elements thereof can include one or more nucleoside modifications (e.g., any of those described in Rozenski et al., Nucl. Acids Res. 27: 196-197, 1999). In some embodiments, the circular polyribonucleotide or modified RNA comprises one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) modification(s) to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof. In some embodiments, the circular polyribonucleotide or modified RNA can include one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) pyrimidine nucleobases having one or more atoms substituted with an amino, a thiol, an alkyl (e.g., methyl or ethyl), or halo (e.g., chloro or fluoro) group. In some embodiments, the circular polyribonucleotide or modified RNA can include one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) ribonucleotides that have been modified into deoxyribonucleic acids (DNAs), threose nucleic acids (TNAs), glycol nucleic acids (GNAs), peptide nucleic acids (PNAs), and/or locked nucleic acids (LNAs). In some embodiments, the circular polyribonucleotide or modified RNA includes at least one (e.g., two, three, four, or five) N(6)methyladenosine (m6A) modification to increase translation efficiency. In some embodiments, the N(6)methyladenosine (m6A) modification can LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 reduce immunogenicity (e.g., reduce the level of one or more marker of an immune or inflammatory response) of the circular polyribonucleotide or modified RNA. In some embodiments, a modification may include a chemical or cellular induced modification. For example, some non-limiting examples of intracellular RNA modifications are described in Lewis et al., Nature Reviews Mol. Cell Biol. 18:202-210, 2017, herein incorporated by reference. In some embodiments, chemical modifications to the ribonucleotides of a circular polyribonucleotide or modified RNA may enhance immune evasion. The circular polyribonucleotide or modified RNA may be synthesized and/or modified by methods well established in the art, such as those described in “Current protocols in nucleic acid chemistry,” Beaucage, S.L. et al. (Eds.), John Wiley & Sons, Inc., New York, NY, USA, herein incorporated by reference. Modifications include, for example, end modifications, e.g., 5’ end modifications (phosphorylation (mono-, di- and tri-), conjugation, inverted linkages), 3’ end modifications (conjugation, DNA nucleotides, inverted linkages), base modifications (e.g., replacement with stabilizing bases, destabilizing bases, or bases that base pair with an expanded repertoire of partners), removal of bases (abasic nucleotides), or conjugated bases. The modified ribonucleotide bases may also include 5- methylcytidine, N1-methylpseudouridine, 5- methoxyuridine, and pseudouridine. In some embodiments, base modifications may modulate expression, immune response, stability, subcellular localization, to name a few functional effects, of the circular polyribonucleotide or modified RNA. In some embodiments, the modification includes a bi-orthogonal nucleotide, e.g., an unnatural base. See, e.g., Kimoto et al., Chem. Comm. (Camb) 53:12309, 2017, which is herein incorporated by reference. In some embodiments, one or more (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) ribonucleotides of the circular polyribonucleotide or modified RNA can include a sugar modification (e.g., at the 2’ position or 4’ position) and optionally, a backbone modification, including modification or replacement of the phosphodiester linkages. Specific examples of circular polyribonucleotides or modified RNAs include, but are not limited to, circular polyribonucleotides or modified RNAs including modified backbones or no natural internucleoside linkages, such as internucleoside modifications, including modification or replacement of the phosphodiester linkages. Circular polyribonucleotides and modified RNAs having modified backbones include, among others, those that do not have a phosphorus atom in LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the backbone. In particular embodiments, the circular polyribonucleotide or modified RNA will include ribonucleotides with a phosphorus atom in its internucleoside backbone. Modified backbones of circular polyribonucleotides and modified RNAs can include, for example, phosphorothioates, chiral phosphorothioates, phosphorodithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates, such as 3’-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates, such as 3’-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3'- 5’ linkages, 2’-5’ linked analogs of these, and those having inverted polarity, wherein the adjacent pairs of nucleoside units are linked 3’-5’ to 5’-3’ or 2’-5’ to 5’-2’. Various salts, mixed salts, and free acid forms of the circular polyribonucleotides and modified RNAs are also included. In some embodiments, the circular polyribonucleotides or modified RNAs may be negatively- or positively-charged. The modified nucleotide(s), which may be incorporated into the circular polyribonucleotides or modified RNAs, can be modified on the internucleoside linkage (e.g., phosphate backbone). Herein, in the context of the polynucleotide backbone, the phrases “phosphate” and “phosphodiester” are used interchangeably. Backbone phosphate groups can be modified by replacing one or more of the oxygen atoms with a different substituent. Further, the modified nucleosides and nucleotides can include the wholesale replacement of an unmodified phosphate moiety with another internucleoside linkage as described herein. Examples of modified phosphate groups include, but are not limited to, phosphorothioate, phosphoroselenates, boranophosphates, boranophosphate esters, hydrogen phosphonates, phosphoramidates, phosphorodiamidates, alkyl or aryl phosphonates, and phosphotriesters. Phosphorodithioates have both non-linking oxygens replaced by sulfur. The phosphate linker can also be modified by the replacement of a linking oxygen with nitrogen (bridged phosphoramidates), sulfur (bridged phosphorothioates), and carbon (bridged methylene -phosphonates). A-thio substituted phosphate moieties can be included to confer stability to circular polyribonucleotides and modified RNAs through the unnatural phosphorothioate backbone linkages. Phosphorothioate RNAs have increased nuclease resistance and subsequently a longer half-life in a cellular environment. Phosphorothioate linkages in a circular polyribonucleotide or LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 modified RNA is expected to reduce the innate immune response through weaker binding/activation of cellular innate immune molecules. In specific embodiments, a modified nucleoside can include an alpha-thio-nucleoside (e.g., 5’-O-(l-thiophosphate)-adenosine, 5’-O-(l-thiophosphate)-cytidine (a-thio-cytidine), 5’-O- (l-thiophosphate)-guanosine, 5’-O-(l-thiophosphate)-uridine, or 5’-O- (1-thiophosphate)- pseudouridine). Other internucleoside linkages that may be employed according to the present disclosure, including internucleoside linkages which do not contain a phosphorous atom, are described herein. A circular polyribonucleotide or modified RNA may or may not be uniformly modified along the entire length of the molecule. For example, one or more or all types of nucleotide (e.g., naturally-occurring nucleotides, purine or pyrimidine, or any one or more or all of A, G, U, C, I, pU) may or may not be uniformly modified in the circular polyribonucleotide or modified RNA, or in a given predetermined subregion thereof. In some embodiments, the circular polyribonucleotide or modified RNA includes a pseudouridine. In some embodiments, the circular polyribonucleotide or modified RNA includes an inosine, which may aid in the immune system characterizing the circular polyribonucleotide or modified RNA as an endogenous RNA versus a viral RNA. The incorporation of inosine may also mediate improved RNA stability/reduced degradation. See for example, Yu, Z. et al., Cell Res. 25, 1283–1284, 2015, which is incorporated by reference in its entirety. In some embodiments, all or substantially all nucleotides in a circular polyribonucleotide or modified RNA (or in a given subregion thereof) are modified. In some embodiments, the modification may include an m6A, which may augment expression; an inosine, which may attenuate an immune response; pseudouridine, which may increase RNA stability, or translational readthrough (stagger element); an m5C, which may increase stability; and a 2,2,7- trimethylguanosine, which aids subcellular translocation (e.g., nuclear localization). Different sugar modifications, nucleotide modifications, and/or internucleoside linkages (e.g., backbone structures) may exist at various positions in a circular polyribonucleotide or modified RNA. One of ordinary skill in the art will appreciate that the nucleotide analogs or other modification(s) may be located at any position(s) of the circular polyribonucleotide or modified RNA, such that the function of the circular polyribonucleotide or modified RNA is not LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 substantially decreased. A modification can be included in a coding sequence or a non-coding sequence. In some embodiments, the circular polyribonucleotide or modified RNA may include about 1% to about 100% modified nucleotides (either in relation to overall nucleotide content, or in relation to one or more types of nucleotide, e.g., any one or more of A, G, U, or C) or any intervening percentage (e.g., about 1% to about 10%, about 1% to about 15%, about 1% to about 20%, from about 1% to about 25%, about 1% to about 30%, about 1% to about 40%, about 1% to about 50%, about 1% to about 60%, about 1% to about 70%, about 1% to about 80%, about 1% to about 90%, about 1% to about 95%, about 10% to about 20%, about 10% to about 25%, about 10% to about 30%, about 10% to about 40%, about 10% to about 50%, about 10% to about 60%, about 10% to about 70%, about 10% to about 80%, about 10% to about 90%, about 10% to about 95%, about 10% to about 100%, about 20% to about 25%, about 20% to about 30%, about 20% to about 40%, about 20% to about 50%, about 20% to about 60%, about 20% to about 70%, about 20% to about 80%, about 20% to about 90%, about 20% to about 95%, about 20% to about 100%, about 30% to about 40%, about 30% to about 50%, about 30% to about 60%, about 30% to about 70%, about 30% to about 80%, about 30% to about 90%, about 30% to about 100%, about 40% to about 50%, about 40% to about 60%, about 40% to about 70%, about 40% to about 80%, about 40% to about 90%, about 40% to about 100%, about 50% to about 60%, about 50% to about 70%, about 50% to about 80%, about 50% to about 90%, about 50% to about 95%, about 50% to about 100%, about 60% to about 70%, about 60% to about 80%, about 60% to about 90%, about 60% to about 100%, about 70% to about 80%, about 70% to about 90%, about 70% to about 95%, about 70% to about 100%, about 80% to about 90%, about 80% to about 95%, about 80% to about 100%, about 90% to about 95%, about 90% to about 100%, and about 95% to about 100%). (vii) Internal Ribosome Entry Site (IRES) In some embodiments, the circular polyribonucleotides or modified RNAs described herein includes one or more internal ribosome entry site(s) (IRES(s)). In some embodiments, an IRES is operably linked to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, an IRES is located at or proximal to the LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 5’ end of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). A suitable IRES includes an RNA sequence capable of engaging a eukaryotic ribosome. In some embodiments, an IRES is at least about 50 nucleotides, at least about 100 nucleotides, at least about 200 nucleotides, at least about 250 nucleotides, at least about 350 nucleotides, or at least about 500 nucleotides. In some embodiments, an IRES is about 50 nucleotides to about 500 nucleotides, about 50 nucleotides to about 450 nucleotides, about 50 nucleotides to about 400 nucleotides, about 50 nucleotides to about 350 nucleotides, about 50 nucleotides to about 300 nucleotides, about 50 nucleotides to about 250 nucleotides, about 50 nucleotides to about 200 nucleotides, about 50 nucleotides to about 150 nucleotides, about 50 nucleotides to about 100 nucleotides, about 100 nucleotides to about 500 nucleotides, about 100 nucleotides to about 450 nucleotides, about 100 nucleotides to about 400 nucleotides, about 100 nucleotides to about 350 nucleotides, about 100 nucleotides to about 300 nucleotides, about 100 nucleotides to about 250 nucleotides, about 100 nucleotides to about 200 nucleotides, about 100 nucleotides to about 150 nucleotides, about 150 nucleotides to about 500 nucleotides, about 150 nucleotides to about 450 nucleotides, about 150 nucleotides to about 400 nucleotides, about 150 nucleotides to about 350 nucleotides, about 150 nucleotides to about 300 nucleotides, about 150 nucleotides to about 250 nucleotides, about 150 nucleotides to about 200 nucleotides, about 200 nucleotides to about 500 nucleotides, about 200 nucleotides to about 450 nucleotides, about 200 nucleotides to about 400 nucleotides, about 200 nucleotides to about 350 nucleotides, about 200 nucleotides to about 300 nucleotides, about 200 nucleotides to about 250 nucleotides, about 250 nucleotides to about 500 nucleotides, about 250 nucleotides to about 450 nucleotides, about 250 nucleotides to about 400 nucleotides, about 250 nucleotides to about 350 nucleotides, about 250 nucleotides to about 300 nucleotides, about 300 nucleotides to about 500 nucleotides, about 300 nucleotides to about 450 nucleotides, about 300 nucleotides to about 400 nucleotides, about 300 nucleotides to about 350 nucleotides, about 350 nucleotides to about 500 nucleotides, about 350 nucleotides to about 450 nucleotides, about 350 nucleotides to about 400 nucleotides, about 400 nucleotides to about 500 nucleotides, about 400 nucleotides to about 450 nucleotides, or about 450 nucleotides to about 500 nucleotides. In some embodiments, the IRES is derived from the DNA of an organism including, but not limited to, a virus, a mammal, and Drosophila. Such viral DNA may be derived from, but LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 not limited to, picornavirus complementary DNA (cDNA), encephalomyocarditis virus (EMCV) cDNA, and poliovirus cDNA. In some embodiments, Drosophila DNA from which an IRES is derived includes, but is not limited to, an Antennapedia gene from Drosophila melanogaster. Herein, the term “derived from” in the context of a nucleic acid, i.e., for a nucleic acid “derived from” (another) nucleic acid, means that the nucleic acid, which is derived from (another) nucleic acid, shares e.g. at least 60%, 70%, 80%, 81 %, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% sequence identity with the nucleic acid from which it is derived. The skilled person is aware that sequence identity is typically calculated for the same types of nucleic acids, i.e., for DNA sequences or for RNA sequences. Thus, it is understood, if a DNA is “derived from” an RNA or if an RNA is “derived from” a DNA, in a first step the RNA sequence is converted into the corresponding DNA sequence (in particular by replacing the uracils (U) by thymidines (T) throughout the sequence) or, vice versa, the DNA sequence is converted into the corresponding RNA sequence (in particular by replacing the T by U throughout the sequence). Thereafter, the sequence identity of the DNA sequences or the sequence identity of the RNA sequences is determined. Preferably, a nucleic acid “derived from” a nucleic acid also refers to nucleic acid, which is modified in comparison to the nucleic acid from which it is derived, e.g., in order to increase RNA stability even further and/or to prolong and/or increase protein production. In the context of amino acid sequences, the term “derived from” means that the amino acid sequence, which is derived from (another) amino acid sequence, shares e.g. at least 60%, 70%, 75%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% sequence identity with the amino acid sequence from which it is derived. In some embodiments, if present, the IRES is an IRES of Taura syndrome virus, Triatoma virus, Theiler's encephalomyelitis virus, simian Virus 40, Solenopsis invicta virus 1, Rhopalosiphum padi virus, Reticuloendotheliosis virus, human poliovirus 1, Plautia stall intestine virus, Kashmir bee virus, Human rhinovirus 2, Homalodisca coagulata virus- 1, Human Immunodeficiency Virus type 1, Homalodisca coagulata virus- 1, Himetobi P virus, Hepatitis C virus, Hepatitis A virus, Hepatitis GB virus, foot and mouth disease virus, Human enterovirus 71, Equine rhinitis virus, Ectropis obliqua picorna-like virus, Encephalomyocarditis virus (EMCV), Drosophila C Virus, Crucifer tobamo virus, Cricket paralysis virus, Bovine viral diarrhea virus 1, Black Queen Cell Virus, Aphid lethal paralysis virus, Avian encephalomyelitis LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 virus, Acute bee paralysis virus, Hibiscus chlorotic ringspot virus, Classical swine fever virus, Human FGF2, Human SFTPA1, Human AML1/RUNX1, Drosophila antennapedia, Human AQP4, Human AT1R, Human BAG-l, Human BCL2, Human BiP, Human c-IAPl , Human c- myc, Human eIF4G, Mouse NDST4L, Human LEF1, Mouse HIF1 alpha, Human n-myc, Mouse Gtx, Human p27kipl, Human PDGF2/c-sis, Human p53, Human Pim-l, Mouse Rbm3, Drosophila reaper, Canine Scamper, Drosophila Ubx, Human UNR, Mouse UtrA, Human VEGF-A, Human XIAP, Salivirus, Cosavirus, Parechovirus, Drosophila hairless, S. cerevisiae TFIID, S. cerevisiae YAP1, Human c-src, Human FGF-l, Simian picomavirus, Turnip crinkle virus, Aichivirus, Crohivirus, Echovirus 11, an aptamer to eIF4G, Coxsackievirus B3 (CVB3), or Coxsackievirus A (CVB1/2). In yet another embodiment, the IRES is an IRES of Coxsackievirus B3 (CVB3). In a further embodiment, the IRES is an IRES of Encephalomyocarditis virus. In some embodiments, the IRES sequence has more than 90% sequence identify with one of the foregoing IRES sequences. In some embodiments, The IRES sequence may have the sequence of wild-type CVB3 IRES sequence having the nucleic acid sequence of: TTAAAACAGCCTGTGGGTTGATCCCACCCACAGGCCCATTGGGCGCTAGCACTCTGG TATCACGGTACCTTTGTGCGCCTGTTTTATACCCCCTCCCCCAACTGTAACTTAGAAG TAACACACACCGATCAACAGTCAGCGTGGCACACCAGCCACGTTTTGATCAAGCAC TTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACGCGGTTGAAGGAGAAAGCG TTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAACACCGTGGAAGTTGCAGAGT GTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGATGAGTCACCGCATTCCCCAC GGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCCATGGGGAAACCCATGGGAC GCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGCTAGTTGGTAGTCCTCCGGCC CCTGAATGCGGCTAATCCTAACTGCGGAGCACACACCCTCAAGCCAGAGGGCAGTG TGTCGTAACGGGCAACTCTGCAGCGGAACCGACTACTTTGGGTGTCCGTGTTTCATT TTATTCCTATACTGGCTGCTTATGGTGACAATTGAGAGATCGTTACCATATAGCTATT GGATTGGCCATCCGGTGACTAATAGAGCTATTATATATCCCTTTGTTGGGTTTATACC ACTTAGCTTGAAAGAGGTTAAAACATTACAATTCATTGTTAAGTTGAATACAGCAAA (SEQ ID NO: 227). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 The IRES sequence may have a modified sequence in comparison to the wildtype IRES sequence. In some embodiments, when the last nucleotide of the wild-type IRES is not a cytosine nucleic acid residue, the last nucleotide of the wild-type IRES sequence may be modified such that it is a cytosine residue. For example, the IRES sequence may be a CVB3 IRES sequence wherein the terminal adenosine residue is modified to cytosine residue. In some embodiments, the modified CVB3 IRES may have the nucleic acid sequence of: TTAAAACAGCCTGTGGGTTGATCCCACCCACAGGCCCATTGGGCGCTAGCACTCTGG TATCACGGTACCTTTGTGCGCCTGTTTTATACCCCCTCCCCCAACTGTAACTTAGAAG TAACACACACCGATCAACAGTCAGCGTGGCACACCAGCCACGTTTTGATCAAGCAC TTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACGCGGTTGAAGGAGAAAGCG TTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAACACCGTGGAAGTTGCAGAGT GTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGATGAGTCACCGCATTCCCCAC GGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCCATGGGGAAACCCATGGGAC GCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGCTAGTTGGTAGTCCTCCGGCC CCTGAATGCGGCTAATCCTAACTGCGGAGCACACACCCTCAAGCCAGAGGGCAGTG TGTCGTAACGGGCAACTCTGCAGCGGAACCGACTACTTTGGGTGTCCGTGTTTCATT TTATTCCTATACTGGCTGCTTATGGTGACAATTGAGAGATCGTTACCATATAGCTATT GGATTGGCCATCCGGTGACTAATAGAGCTATTATATATCCCTTTGTTGGGTTTATACC ACTTAGCTTGAAAGAGGTTAAAACATTACAATTCATTGTTAAGTTGAATACAGCAAC (SEQ ID NO: 168). In some embodiments, the modified CVB3 IRES may have the nucleic acid sequence of: CCAACTGTAACTTAGAAGTAACACACACCGATCAACAGTCAGCGTGGCACACCAGC CACGTTTTGATCAAGCACTTCTGTTACCCCGGACTGAGTATCAATAGACTGCTCACG CGGTTGAAGGAGAAAGCGTTCGTTATCCGGCCAACTACTTCGAAAAACCTAGTAAC ACCGTGGAAGTTGCAGAGTGTTTCGCTCAGCACTACCCCAGTGTAGATCAGGTCGAT GAGTCACCGCATTCCCCACGGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCC ATGGGGAAACCCATGGGACGCTCTAATACAGACATGGTGCGAAGAGTCTATTGAGC TAGTTGGTAGTCCTCCGGCCCCTGAATGCGGCTAATCCTAACTGCGGAGCACACACC CTCAAGCCAGAGGGCAGTGTGTCGTAACGGGCAACTCTGCAGCGGAACCGACTACT TTGGGTGTCCGTGTTTCATTTTATTCCTATACTGGCTGCTTATGGTGACAATTGAGAG ATCGTTACCATATAGCTATTGGATTGGCCATCCGGTGACTAATAGAGCTATTATATA LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 TCCCTTTGTTGGGTTTATACCACTTAGCTTGAAAGAGGTTAAAACATTACAATTCATT GTTAAGTTGAATACAGCAAC (SEQ ID NO: 228) In some embodiments, the IRES sequence is an encephalomyocarditis virus (EMCV) IRES. In some embodiments, the ECMV IRES may have the nucleic acid sequence of: ACGTTACTGGCCGAAGCCGCTTGGAATAAGGCCGGTGTGCGTTTGTCTATATGTTAT TTTCCACCATATTGCCGTCTTTTGGCAATGTGAGGGCCCGGAAACCTGGCCCTGTCTT CTTGACGAGCATTCCTAGGGGTCTTTCCCCTCTCGCCAAAGGAATGCAAGGTCTGTT GAATGTCGTGAAGGAAGCAGTTCCTCTGGAAGCTTCTTGAAGACAAACAACGTCTGT AGCGACCCTTTGCAGGCAGCGGAACCCCCCACCTGGCGACAGGTGCCTCTGCGGCC AAAAGCCACGTGTATAAGATACACCTGCAAAGGCGGCACAACCCCAGTGCCACGTT GTGAGTTGGATAGTTGTGGAAAGAGTCAAATGGCTCTCCTCAAGCGTATTCAACAAG GGGCTGAAGGATGCCCAGAAGGTACCCCATTGTATGGGATCTGATCTGGGGCCTCG GTGCACATGCTTTACATGTGTTTAGTCGAGGTTAAAAAACGTCTAGGCCCCCCGAAC CACGGGGACGTGGTTTTCCTTTGAAAAACACGATGATAATA (SEQ ID NO: 229). In some embodiments, the IRES sequence is an Enterovirus 71 (EV71) IRES. In some embodiments, the IRES sequence is a wild-type EV71 sequence having the nucleic acid sequence of: TTAAAACAGCTGTGGGTTGTCACCCACCCACAGGGTCCACTGGGCGCTAGTACACTG GTATCTCGGTACCTTTGTACGCCTGTTTTATACCCCCTCCCTGATTTGCAACTTAGAA GCAACGCAAACCAGATCAATAGTAGGTGTGACATACCAGTCGCATCTTGATCAAGC ACTTCTGTATCCCCGGACCGAGTATCAATAGACTGTGCACACGGTTGAAGGAGAAA ACGTCCGTTACCCGGCTAACTACTTCGAGAAGCCTAGTAACGCCATTGAAGTTGCAG AGTGTTTCGCTCAGCACTCCCCCCGTGTAGATCAGGTCGATGAGTCACCGCATTCCC CACGGGCGACCGTGGCGGTGGCTGCGTTGGCGGCCTGCCTATGGGGTAACCCATAG GACGCTCTAATACGGACATGGCGTGAAGAGTCTATTGAGCTAGTTAGTAGTCCTCCG GCCCCTGAATGCGGCTAATCCTAACTGCGGAGCACATACCCTTAATCCAAAGGGCA GTGTGTCGTAACGGGCAACTCTGCAGCGGAACCGACTACTTTGGGTGTCCGTGTTTC TTTTTATTCTTGTATTGGCTGCTTATGGTGACAATTAAAGAATTGTTACCATATAGCT ATTGGATTGGCCATCCAGTGTCAAACAGAGCTATTGTATATCTCTTTGTTGGATTCAC ACCTCTCACTCTTGAAACGTTACACACCCTCAATTACATTATACTGCTGAACACGAA GCG (SEQ ID NO: 169). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the terminal guanosine residue of the EV71 IRES sequence is modified to a cytosine residue. In some embodiments, the polyribonucleotide includes at least one IRES flanking at least one (e.g., 2, 3, 4, 5 or more) expression sequence. In some embodiments, the IRES flanks both sides of at least one (e.g., 2, 3, 4, 5 or more) expression sequence. In some embodiments, the polyribonucleotide includes one or more IRES sequences on one or both sides of each expression sequence, leading to separation of the resulting peptide(s) and or polypeptide(s). For example, a polyribonucleotide described herein may include a first IRES operably linked to a first expression sequence and a second IRES operably linked to a second expression sequence. For example, an exemplary construct described above includes a construct with two IRES sequences each of which is followed by an ORF sequence. In some embodiments, a spacer sequence is disposed between the first IRES-ORF sequence and the second IRES-ORF sequence. In some embodiments, both the first ORF and the second ORF sequence encode a GLP-2 polypeptide or analog thereof (e.g., GLP-2 Fc). In some embodiments, the first ORF encodes a GLP-2 polypeptide or analog thereof (e.g., GLP-2 Fc) and the second ORF encodes a different polypeptide. In some embodiments, the circular polyribonucleotides or modified RNAs described herein can include a modified IRES, such as those described in WO 2020/198403, which is incorporated herein by reference in its entirety or an IRES, such as those described in Fan et al. Nature Communications 13(1):3751, 2022 doi: 10.1038/s41467-022-31327-y; Chen et al. Mol. Cell 81:1-19, 2021; Jopling et al. Oncogene 20:2664-2670, 2001; Baranick et al. PNAS 105(12):4733-4738, 2008; Lang et al. Molecular Biology of the Cell 13(5):1792-1801, 2002; Dorokhov et al. PNAS 99(8):5301-5306, 2002; Wang et al. Nucleic Acids Research 33(7):2248- 2258, 2005; Petz et a. Nucleic Acids Research 35(8):2473-2482, 2007; and WO 2021/263124 A2, each of, which is hereby incorporated by reference in its entirety. (viii) Translation initiation sequence In some embodiments, the circular polyribonucleotides or modified RNAs described herein can include at least one translation initiation sequence. In some embodiments, the circular polyribonucleotide or modified RNA includes a translation initiation sequence operably linked to LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the translation initiation sequence includes, e.g., a start codon. In some embodiments, the translation initiation sequence includes a Kozak or Shine-Dalgarno sequence. In some embodiments, the circular polyribonucleotide or modified RNA includes the translation initiation sequence, e.g., Kozak sequence, adjacent to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the translation initiation sequence, e.g., Kozak sequence, is present on one or both sides of each sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein), leading to separation of the expression products. In some embodiments, the circular polyribonucleotide or modified RNA includes at least one translation initiation sequence adjacent to the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the translation initiation sequence provides conformational flexibility to the circular polyribonucleotide or modified RNA. In some embodiments, the translation initiation sequence is within a substantially single stranded region of the circular polyribonucleotide or modified RNA. The circular polyribonucleotide may include more than 1 start codon such as, but not limited to, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 25, at least 30, at least 35, at least 40, at least 50, at least 60, or more than 60 start codons. In such embodiments, translation may initiate on the first start codon or may initiate downstream of the first start codon. In some embodiments, the circular polyribonucleotide or modified RNA may initiate at a codon that is not the first start codon, e.g., AUG. Translation of the circular polyribonucleotide or modified RNA may initiate at an alternative translation initiation sequence, such as, but not limited to, ACG, AGG, AAG, CTG/CUG, GTG/GUG, ATA/AUA, ATT/AUU, or TTG/UUG. In some embodiments, translation begins at an alternative translation initiation sequence under selective conditions, e.g., stress-induced conditions. As a non-limiting example, the translation of the circular polyribonucleotide or modified RNA may begin at alternative translation initiation sequence, such as ACG. As another non-limiting example, the circular polyribonucleotide or modified RNA translation may begin at alternative translation initiation sequence, CTG/CUG. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 As yet another non-limiting example, the circular polyribonucleotide or modified RNA translation may begin at alternative translation initiation sequence, GTG/GUG. As yet another non-limiting example, the circular polyribonucleotide or modified RNA may begin translation at a repeat-associated non-AUG (RAN) sequence, such as an alternative translation initiation sequence that includes short stretches of repetitive RNA, e.g., CGG, GGGGCC, CAG, or CTG. Nucleotides flanking a codon that initiates translation may affect the translation efficiency and the length and/or the structure of the circular polyribonucleotide or modified RNA. Masking any of the nucleotides flanking a codon that initiates translation may be used to alter the position of translation initiation, translation efficiency, and length, and/or structure of the circular polyribonucleotide or modified RNA. In some embodiments, a masking agent may be used near the start codon or alternative start codon in order to mask or hide the codon to reduce the probability of translation initiation at the masked start codon or alternative start codon. Non-limiting examples of masking agents include antisense locked nucleic acids (LNA) oligonucleotides and exon-junction complexes (EJCs). In some embodiments, a masking agent may be used to mask a start codon of the circular polyribonucleotide or modified RNA in order to increase the likelihood that translation will initiate at an alternative start codon. In some embodiments, translation is initiated under selective conditions, such as, but not limited to, viral-induced selection in the presence of GRSF-1 and the circular polyribonucleotide or modified RNA includes GRSF-1 binding sites. In some embodiments, translation is initiated by eukaryotic initiation factor 4A (eIF4A) treatment with Rocaglates. Translation may be repressed by blocking 43S scanning, leading to premature, upstream translation initiation and reduced protein expression from transcripts bearing the RocA–eIF4A target sequence. (ix) Translation Termination Sequences In some embodiments, the circular polyribonucleotides or modified RNAs can further include at least one translation termination sequence. In some embodiments, the translation termination sequence is operably linked to the 3’ end of the sequence encoding the polypeptide (e.g., any of the exemplary polypeptides described herein). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the circular polyribonucleotide or modified RNA includes a termination element at the end of a sequence encoding the polypeptide (e.g., any of the polypeptides described herein). In some embodiments, a circular polyribonucleotide or modified RNA can include two or more termination elements in succession. Generally, translation termination sequences include an in-frame nucleotide triplet that signals termination of translation, e.g., UAA, UGA, or UAG. In some embodiments, one or more translation termination sequences in the circular polyribonucleotide or modified RNA are frame-shifted termination elements, such as but not limited to, off-frame or -1 and + 1 shifted reading frames (e.g., hidden stop) that may terminate translation. Frame-shifted termination elements include nucleotide triples, e.g., TAA, TAG, or TGA, that appear in the second and third reading frames of the sequence encoding the polypeptide. Frame-shifted termination elements may be important in preventing misreads of the sequence encoding the polypeptide, which is often detrimental to a cell. In some embodiments, the termination element is a stop codon. Further examples of translation termination sequences are described in paragraphs [0169] - [0170] of WO2019/118919, which is hereby incorporated by reference in its entirety. (x) Stagger Elements A circular polyribonucleotide of the disclosure can include a cleavage domain (e.g., a stagger element or a cleavage sequence). In some embodiments, the circular polyribonucleotide includes a stagger sequence or a stagger element. As used herein, the term “stagger element” or a “stagger sequence” is a moiety, such as a nucleotide sequence, that induces ribosomal pausing during translation. To avoid production of a continuous expression product, e.g., peptide or polypeptide, while maintaining rolling circle translation, a stagger sequence can be included to induce ribosomal pausing during translation. The stagger sequence may include a 2A-like or CHYSEL (cis-acting hydrolase element) sequence. In some embodiments, the stagger element encodes a sequence with a C- terminal consensus sequence that is X1X2X3EX5NPGP (SEQ ID NO: 144), where X1 is absent or G or H, X2 is absent or D or G, X3 is D, V, I, S, or M, and X5 is any amino acid. In some embodiments, the stagger element comprises a non-conserved sequence of amino-acids with a strong alpha-helical propensity followed by the consensus sequence -D(V/I)EXNPGP (SEQ ID LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 NO: 145), where X is any amino acid. Some non-limiting examples of stagger elements include any of the stagger sequences shown in Table 7. In some embodiments, a stagger element described herein terminates translation and/or cleaves an expression product between G and P of the consensus sequence described herein. As one non-limiting example, the circular polyribonucleotide or modified RNA includes at least one stagger sequence to terminate translation and/or cleave the encoded polypeptide. In some embodiments, the circular polyribonucleotide or modified RNA includes a stagger element adjacent to at least one sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the circular polyribonucleotide or modified RNA includes a stagger element after each sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein). In some embodiments, the circular polyribonucleotide or modified RNA includes a stagger element present on one or both sides of each sequence encoding a polypeptide (e.g., any of the exemplary polypeptides described herein), leading to translation of individual peptide(s) and or polypeptide(s) from each expression sequence. Table 7. Non-limiting examples of stagger elements SEQ Description Sequence ID LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the stagger element may include a chemical moiety, such as glycerol, a non-nucleic acid linking moiety, a chemical modification, a modified nucleic acid, or any combination thereof. (xi) Replication Elements Replication of a circular polyribonucleotide can occur by generating a complement circular polyribonucleotide. In some embodiments, the circular polyribonucleotide can include a motif to initiate transcription, where transcription is driven by either endogenous cellular machinery (DNA-dependent RNA polymerase) or an RNA-depended RNA polymerase encoded by the circular polyribonucleotide. The product of the rolling-circle transcriptional event can be cut by a ribozyme to generate either complementary or propagated circular polyribonucleotide at unit length. The ribozyme can be encoded by the circular polyribonucleotide, its complement, or by an RNA sequence in trans. In some embodiments, the encoded ribozyme can include a sequence or motif that regulates (inhibits or promotes) activity of the ribozyme to control circular polyribonucleotide propagation. In some embodiments, unit-length sequences can be ligated into a circular form by a cellular RNA ligase. In some embodiments, the circular polyribonucleotide includes a replication element that aids in self-amplification. Examples of such replication elements include HDV replication domains and replication competent circular RNA sense and/or antisense ribozymes, such as antigenomic 5’- CGGGUCGGCAUGGCAUCUCCACCUCCUCGC GGUCCGACCUGGGCAUCCGAAGGAGGACGCACGUCCACUCGGAUGGCUAAGGGAG LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 AGCCA-3’ (SEQ ID NO: 165) or genomic 5’- UGGCCGGCAUGGUCCCAGCCUCCUCGCUGGCGCCGGCUGGGCAACAUU CCGAGGGGACCGUCCCCUCGGUAAUGGCGAAUGGGACCCA-3’ (SEQ ID NO: 166). In some embodiments, the circular polyribonucleotide includes at least one cleavage sequence as described herein to aid in replication. A cleavage sequence within the circular polyribonucleotide can cleave long transcripts replicated from the circular polyribonucleotide to a specific length that can subsequently circularize to form a complement to the circular polyribonucleotide. In another embodiment, the circular polyribonucleotide includes at least one ribozyme sequence to cleave long transcripts replicated from the circular polyribonucleotide to a specific length, where another encoded ribozyme cuts the transcripts at the ribozyme sequence. Circularization forms a complement to the circular polyribonucleotide. In some embodiments, the circular polyribonucleotide is substantially resistant to degradation, e.g., by exonucleases. A “substantially resistant” circular polyribonucleotide can refer to one that has at least 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% resistance as compared to a reference polyribonucleotide. In some embodiments, the circular polyribonucleotide replicates within a cell. In some embodiments, the circular polyribonucleotide replicates within in a cell at a rate of about 10%- 20%, 20%-30%, 30%-40%, 40%-50%, 50%-60%, 60%-70%, 70%-75%, 75%-80%, 80%-85%, 85%-90%, 90%-95%, 95%-99%, or any percentage therebetween. In some embodiments, the circular polyribonucleotide replicates within a cell and is passed to daughter cells. In some embodiments, a cell passes at least one circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%. In some embodiments, a cell undergoing meiosis passes the circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%. In some embodiments, a cell undergoing mitosis passes the circular polyribonucleotide to daughter cells with an efficiency of at least 25%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, or 99%. In some embodiments, the circular polyribonucleotide replicates within the host cell. In some embodiments, the circular polyribonucleotide is capable of replicating in a mammalian cell, e.g., a human cell. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 While in some embodiments the circular polyribonucleotide replicates in the host cell, the circular polyribonucleotide does not integrate into the genome of the host, e.g., with the host’s chromosomes. In some embodiments, the circular polyribonucleotide has a negligible recombination frequency, e.g., with the host’s chromosomes. In some embodiments, the circular polyribonucleotide has a recombination frequency, e.g., less than about 1.0 cM/Mb, 0.9 cM/Mb, 0.8 cM/Mb, 0.7 cM/Mb, 0.6 cM/Mb, 0.5 cM/Mb, 0.4 cM/Mb, 0.3 cM/Mb, 0.2 cM/Mb, 0.1 cM/Mb, or less, e.g., with the host’s chromosomes. (xii) Production Methods The disclosure provides methods for producing circular polyribonucleotides described herein, including, e.g., recombinant technology or chemical synthesis. For example, a DNA molecule used to produce a circular RNA molecule can include a DNA sequence of a naturally occurring nucleic acid sequence, a modified version thereof, or a DNA sequence encoding a synthetic polypeptide not normally found in nature (e.g., chimeric molecules or fusion proteins). DNA and RNA molecules can be modified using a variety of techniques including, but not limited to, classic mutagenesis techniques and recombinant techniques, such as site- directed mutagenesis, chemical treatment of a nucleic acid molecule to induce mutations, restriction enzyme cleavage of a nucleic acid fragment, ligation of nucleic acid fragments, polymerase chain reaction (PCR) amplification or mutagenesis of selected regions of a nucleic acid sequence, synthesis of oligonucleotide mixtures and ligation of mixture groups to "build" a mixture of nucleic acid molecules and combinations thereof. The circular polyribonucleotides may be prepared according to any available technique, including, but not limited to chemical synthesis and enzymatic synthesis. In some embodiments, a linear primary construct or linear polyribonucleotide for circularization may be cyclized or concatenated to create a circular RNA described herein. In some embodiments, the linear polyribonucleotide for circularization may be cyclized in vitro prior to formulation and/or delivery. In some embodiments, the circular polyribonucleotide may be in a mixture with linear polyribonucleotides. In some embodiments, the linear polyribonucleotides have the same nucleic acid sequence as the circular polyribonucleotides. The mechanism of cyclization or concatenation may occur through methods such as, e.g., chemical, enzymatic, splint ligation, or ribozyme-catalyzed methods. The newly formed 5’-3’ LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 linkage may be an intramolecular linkage or an intermolecular linkage. For example, a splint ligase, such as a SplintR® ligase, can be used for splint ligation. According to this method, a single stranded polynucleotide (splint), such as a single-stranded DNA or RNA, can be designed to hybridize with both termini of a linear polyribonucleotide, so that the two termini can be juxtaposed upon hybridization with the single-stranded splint. Splint ligase can thus catalyze the ligation of the juxtaposed two termini of the linear polyribonucleotide, generating a circular polyribonucleotide. In some embodiments, a DNA or RNA ligase may be used in the synthesis of the circular polynucleotides. As a non-limiting example, the ligase may be a circ ligase or circular ligase. In some embodiments, either the 5' or 3' end of the linear polyribonucleotide can encode a ligase ribozyme sequence such that during in vitro transcription, the resultant linear polyribonucleotide for circularization includes an active ribozyme sequence capable of ligating the 5' end of the linear polyribonucleotide for circularization to the 3' end of the linear polyribonucleotide for circularization. The ligase ribozyme may be derived from the Group I Intron, Hepatitis Delta Virus, Hairpin ribozyme or may be selected by SELEX (systematic evolution of ligands by exponential enrichment). In another example, a linear polyribonucleotide may be cyclized or concatenated by using at least one non-nucleic acid moiety. In one aspect, the at least one non-nucleic acid moiety may react with regions or features near the 5' terminus or near the 3' terminus of the linear polyribonucleotide for circularization in order to cyclize or concatenate the linear polyribonucleotide for circularization. In another aspect, the at least one non-nucleic acid moiety may be located in or linked to or near the 5' terminus or the 3' terminus of the linear polyribonucleotide for circularization. The non-nucleic acid moieties may be homologous or heterologous. As a non-limiting example, the non-nucleic acid moiety may be a linkage such as a hydrophobic linkage, ionic linkage, a biodegradable linkage, or a cleavable linkage. As another non-limiting example, the non-nucleic acid moiety is a ligation moiety. As yet another non-limiting example, the non-nucleic acid moiety may be an oligonucleotide or a peptide moiety, such as an aptamer or a non-nucleic acid linker as described herein. In some embodiments, a linear polyribonucleotide for circularization may include a 5' triphosphate of the nucleic acid converted into a 5' monophosphate, e.g., by contacting the 5' triphosphate with RNA 5' pyrophosphohydrolase (RppH) or an ATP diphosphohydrolase LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 (apyrase). In some embodiments, the 5’ end of at least a portion of the linear polyribonucleotides includes a monophosphate moiety. In some embodiments, a population of polyribonucleotides including circular and linear polyribonucleotides is contacted with RppH prior to digesting at least a portion of the linear polyribonucleotides with a 5’ exonuclease and/or a 3’ exonuclease. Alternately, converting the 5' triphosphate of the linear polyribonucleotide for circularization into a 5' monophosphate may occur by a two-step reaction including: (a) contacting the 5' nucleotide of the linear polyribonucleotide for circularization with a phosphatase (e.g., Antarctic Phosphatase, Shrimp Alkaline Phosphatase, or Calf Intestinal Phosphatase) to remove all three phosphates; and (b) contacting the 5' nucleotide after step (a) with a kinase (e.g., Polynucleotide Kinase) that adds a single phosphate. In another aspect, linear polyribonucleotides for circularization may be cyclized or concatenated by self-splicing. In some embodiments, the linear polyribonucleotide may include a sequence that mediates self- ligation. In some embodiments, the linear polyribonucleotides may include loop E sequence (e.g., in PSTVd) to self-ligate. In some embodiments, the linear polyribonucleotide may include a HDV sequence, e.g., HDV replication domain conserved sequence, GGCUCAUCUCGACAAGAGGCGGCAGUCCUCAGUACUCUUACUCUUUUCUGUAAAG AGGAGACUGCUGGACUCGCCGCCCAAGUUCGAGCAUGAGCC (SEQ ID NO: 170) or GGCUAGAGGCGGCAGUCCUCAGUACUCUUACUCUUUUCUGUAAAGAGGAGACUG CUGGACUCGCCGCCCGAGCC (SEQ ID NO: 171), to self-ligate. In another embodiment, the linear polyribonucleotides may include a self-circularizing intron, e.g., a 5' and 3’ slice junction, or a self-circularizing catalytic intron such as a Group I, Group II, or Group III Introns. Nonlimiting examples of group I intron self- splicing sequences may include self-splicing permuted intron-exon sequences derived from T4 bacteriophage gene td, and the intervening sequence (IVS) rRNA of Tetrahymena, cyanobacterium Anabaena pre-tRNA-Leu gene, or a Tetrahymena pre-rRNA. In some embodiments, the polyribonucleotide includes catalytic intron fragments, such as a 3′ half of Group I catalytic intron fragment and a 5′ half of Group I catalytic intron fragment. The first and second annealing regions may be positioned within the catalytic intron fragments. Group I catalytic introns are self-splicing ribozymes that catalyze their own excision from mRNA, tRNA, and rRNA precursors via two-metal ion phorphoryl transfer mechanism. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Importantly, the RNA itself self-catalyzes the intron removal without the requirement of an exogenous enzyme, such as a ligase. In some embodiments, the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragment are from a cyanobacterium Anabaena pre-tRNA-Leu gene, or a Tetrahymena pre-rRNA. In some embodiments, the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragment are from a Cyanobacterium Anabaena pre-tRNA-Leu gene, and the 3’ exon fragment includes the first annealing region and the 5’ exon fragment includes the second annealing region. The first annealing region may include, e.g., from 5 to 50, e.g., from 10 to 15 (e.g., 10, 11, 12, 13, 14, or 15) ribonucleotides and the second annealing region may include, e.g., from 5 to 50, e.g., from 10 to 15 (e.g., 10, 11, 12, 13, 14, or 15) ribonucleotides. In some embodiments, the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragment are from a Tetrahymena pre-rRNA, and the 3′ half of Group I catalytic intron fragment includes the first annealing region and the 5’ exon fragment includes the second annealing region. In some embodiments, the 3′ exon includes the first annealing region and the 5’ half of Group I catalytic intron fragment includes the second annealing region. The first annealing region may include, e.g., from 6 to 50, e.g., from 10 to 16 (e.g., 10, 11, 12, 13, 14, 15, or 16) ribonucleotides, and the second annealing region may include, e.g., from 6 to 50, e.g., from 10 to 16 (e.g., 10, 11, 12, 13, 14, 15, or 16) ribonucleotides. In some embodiments, the 3′ half of Group I catalytic intron fragment and the 5’ half of Group I catalytic intron fragment are from a cyanobacterium Anabaena pre-tRNA-Leu gene, a Tetrahymena pre-rRNA, or a T4 phage td gene. In some embodiments, the 3′ half of Group I catalytic intron fragment and the 5’ Group I catalytic intron fragment are from a T4 phage td gene. The 3′ exon fragment may include the first annealing region and the 5’ half of Group I catalytic intron fragment may include the second annealing region. The first annealing region may include, e.g., from 2 to 16 (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16) ribonucleotides, and the second annealing region may include, e.g., from 2 to 16 (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16) ribonucleotides. In some embodiments, the 3′ half of Group I catalytic intron fragment is the 5’ terminus of the linear polynucleotide. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the 5′ half of Group I catalytic intron fragment is the 3’ terminus of the linear polyribonucleotide. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AACAACAGATAACTTACAGCTAGTCGGAAGGTGCAGAGACTCGACGGGAGCTACCC TAACGTCAAGACGAGGGTAAAGAGAGAGTCCAATTCTCAAAGCCAATAGGCAGTAG CGAAAGCTGCGGGAGAATG-3’ (SEQ ID NO: 172). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCTCTCAAACTCAGGGA AACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCCGAAGTAGTAATTAG TAAGTT-3’ (SEQ ID NO: 173)). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 172 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 173. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- CTTCTGTTGATATGGATGCAGTTCACAGACTAAATGTCGGTCGGGGAAGATGTATTC TTCTCATAAGATATAGTCGGACCTCTCCTTAATGGGAGCTAGCGGATGAAGTGATGC AACACTGGAGCCGCTGGGAACTAATTTGTATGCGAAAGTATATTGATTAGTTTTGGA GTACTCG-3’ (SEQ ID NO: 174). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AAATAGCAATATTTACCTTTGGAGGGAAAAGTTATCAGGCATGCACCTGGTAGCTAG TCTTTAAACCAATAGATTGCATCGGTTTAAAAGGCAAGACCGTCAAATTGCGGGAA AGGGGTCAACAGCCGTTCAGTACCAAGTCTCAGGGGAAACTTTGAGATGGCCTTGC AAAGGGTATGGTAATAAGCTGACGGACATGGTCCTAACCACGCAGCCAAGTCCTAA GTCAACAGAT-3’ (SEQ ID NO: 175). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 174 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 175. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- GGTTCTACATAAATGCCTAACGACTATCCCTTTGGGGAGTAGGGTCAAGTGACTCGA AACGATAGACAACTTGCTTTAACAAGTTGGAGATATAGTCTGCTCTGCATGGTGACA TGCAGCTGGATATAATTCCGGGGTAAGATTAACGACCTTATCTGAACATAATG-3’ (SEQ ID NO: 176). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TAATTGAGGCCTGAGTATAAGGTGACTTATACTTGTAATCTATCTAAACGGGGAACC TCTCTAGTAGACAATCCCGTGCTAAATTGTAGGACT-3’ (SEQ ID NO: 177). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 176 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 177. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TAAACAACTAACAGCTTTAGAAGGTGCAGAGACTAGACGGGAGCTACCCTAACGGA TTCAGCCGAGGGTAAAGGGATAGTCCAATTCTCAACATCGCGATTGTTGATGGCAGC GAAAGTTGCAGAGAGAATGAAAATCCGCTGACTGTAAAGGTCGTGAGGGTTCGAGT CCCTCCGCCCCCA-3’ (SEQ ID NO: 178). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ACGGTAGACGCAGCGGACTTAGAAAACTGGGCCTCGATCGCGAAAGGGATCGAGTG GCAGCTCTCAAACTCAGGGAAACCTAAAACTTTAAACATTMAAGTCATGGCAATCC TGAGCCAAGCTAAAGC-3’ (SEQ ID NO: 179). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 178 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 179. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- TTAAACTCAAAATTTAAAATCCCAAATTCAAAATTCCGGGAAGGTGCAGAGACTCG ACGGGAGCTACCCTAACGTAAAGCCGAGGGTAAAGGGAGAGTCCAATTCTCAAAGC LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 CTGAAGTTGCTGAAGCAACAAGGCAGTAGTGAAAGCTGCGAGAGAATGAAAATCCG TTGACTGTAAAAAGTCGTGGGGGTTCAAGTCCCCCCACCCCC-3’ (SEQ ID NO: 180). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ATGGTAGACGCTACGGACTTAGAAAACTGAGCCTTGATAGAGAAATCTTTTAAGTG GAAGCTCTCAAATTCAGGGAAACCTAAATCTGAATACAGATATGGCAATCCTGAGC CAAGCCCAGAAAATTTAGACTTGAGATTTGATTTTGGAG-3’ (SEQ ID NO: 181). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 180 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 181. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- GGCTTTCAATTTGAAATCAGAAATTCAAAATTCAGGGAAGGTGCAGAGACTCGACG GGAGCTACCCTAACGTAAAGGCGAGGGTAAAGGGAGAGTCCAATTCTTAAAGCCTG AAGTTGTGCAAGCAACAAGGCAACAGTGAAAGCTGTGGAAGAATGAAAATCCGTTG ACCTTAAACGGTCGTGGGGGTTCAAGTCCCCCCACCCCC-3’ (SEQ ID NO: 182). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ATGGTAGACGCTACGGACTTAGAAAACTGAGCCTTGATAGAGAAATCTTTCAAGTG GAAGCTCTCAAATTCAGGGAAACCTAAATCTGAATACAGATATGGCAATCCTGAGC CAAGCCCGGAAATTTTAGAATCAAGATTTTATTTT-3’ (SEQ ID NO: 183). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 182 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 183. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGAAATGGAGAAGGTGTAGAGACTGGAAGGCAGGCACCCTAACGTTAAAGGCGAG GGTGAAGGGACAGTCCAGACCACAAACCAGTAAATCTGGGCAGCGAAAGCTGTAGA TGGTAAGCATAACCCGAAGGTCAGTGGTTCAAATCCACTTCCCGCCACCAAATTAAA AAAACAATAA-3’ (SEQ ID NO: 184). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGAAATGGAGAAGGTGTAGAGACTGGAAGGCAGGCACCCTAACGTTAAAGGCGAG GGTGAAGGGACAGTCCAGACCACAAACCAGTAAATCTGGGCAGCGAAAGCTGTAGA TGGTAAGCATAACCCGAAGGTCAGTGGTTCAAATCCACTTCCCGCCACCAAATTAAA AAAACAATAA-3’ (SEQ ID NO: 185). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 184 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 185. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- ACAACAGATAACTTACTAACTTACAGCTAGTCGGAAGGTGCAGAGACTCGACGGGA GCTACCCTAACGTCAAGACGAGGGTAAAGAGAGAGTCCAATTCTCAAAGCCAATAG GCAGTAGCGAAAGCTGCGGGAGAATGAAAATCCGTAGCGTCTAAACGGTCGTGTGG GTTCAAGTCCCTCCACCCCCA-3’ (SEQ ID NO: 186). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AGACGCTACGGACTTAAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCT CTCAAACTCAGGGAAACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCC GAAGTAGTAATTAGTAAGTTAGTAAGTT-3’ (SEQ ID NO: 187). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 186 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 187. In some embodiments, the 3’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- AACAACAGATAACTTACTAGTTACTAGTCGGAAGGTGCAGAGACTCGACGGGAGCT ACCCTAACGTCAAGACGAGGGTAAAGAGAGAGTCCAATTCTCAAAGCCAATAGGCA GTAGCGAAAGCTGCGGGAGAATGAAAATCCGTAGCGTCTAAACGGTCGTGTGGGTT CAAGTCCCTCCACCCCCA-3’ (SEQ ID NO: 188). In some embodiments, the 5’ half of Group I catalytic intron fragment has at least 80% (e.g., at least 85%, 90%, 95%, 97%, 99%, or 100%) sequence identity to the sequence of 5’- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 AGACGCTACGGACTTAAATAATTGAGCCTTAGAGAAGAAATTCTTTAAGTGGATGCT CTCAAACTCAGGGAAACCTAAATCTAGCTATAGACAAGGCAATCCTGAGCCAAGCC GAAGTAGTAATTAGTAAGTT-3’ (SEQ ID NO: 189). In some embodiments, the 3’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 188 and the 5’ half of Group I catalytic intron fragment has the sequence of SEQ ID NO: 189. In some embodiments, the Group I catalytic intron fragment is from the T4 phage nrdB gene or nrdD gene. In some embodiments, the 3′ half of Group I catalytic intron fragment of includes a sequence having at least 80% sequence identity to 5’- TTGCAAAACAAGGTTCAACGACTAGTCTTCGGACGTAGGGTCAAGCGACTCGAAAT GGGGAGAATCCCTCCGGGATTGTGATATAGTCTGGACTGCATGGTAACATGCAGCA GTTCATAAGAGAACGGGTTGAGAATTAGCGAGCTCAATCGAACATACG-3’ (SEQ ID NO: 190). In some embodiments, the 3′ half of Group I catalytic intron fragment of (A) includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TTGCAAAACAAGGTTCAACGACTAGTCTTCGGACGTAGGGTCAAGCGACTCGAAAT GGGGAGAATCCCTCCGGGATTGTGATATAGTCTGGACTGCATGGTAACATGCAGCA GTTCATAAGAGAACGGGTTGAGAATTAGCGAGCTCAATCGAACATACG-3’ (SEQ ID NO: 191). In some embodiments, the 5′ half of Group I catalytic intron fragment from the T4 phage nrdB gene. In some embodiments, the 3′ half of Group I catalytic intron fragment is from the T4 phage nrdB gene and the 5′ half of Group I catalytic intron fragment is from the T4 phage nrdB gene. In some embodiments, the 5′ half of Group I catalytic intron includes a sequence having at least 80% sequence identity to 5’- AAAATGCGCCTTTAAACGGTAACGTTTATCGAAAACTCCTTTAATTGCTGGAAAGTC CTTTATGGAAAACTAGCAGCCAAGGTTTTGCTT-3’ (SEQ ID NO: 192). In some embodiments, the 5′ half of Group I catalytic intron includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- AAAATGCGCCTTTAAACGGTAACGTTTATCGAAAACTCCTTTAATTGCTGGAAAGTC CTTTATGGAAAACTAGCAGCCAAGGTTTTGCTT-3’ (SEQ ID NO: 193). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the 3′ half of Group I catalytic intron fragment is from the T4 phage nrdD gene. In some embodiments, the 3′ half of Group I catalytic intron fragment includes a sequence having at least 80% sequence identity to 5’- CAGTAGCTGTAAATGCCCAACGACTATCCCTGATGAATGTAAGGGAGTAGGGTCAA GCGACCCGAAACGGCAGACAACTCTAAGAGTTGAAGATATAGTCTGAACTGCATGG TGACATGCAGCTGTTTATCCTCGTATAAATATGAATACGAGGTGAAACGATGAAATG AATTACATTGTTTCATATAAACGGGTAGAGAAGTAGCGAACTCTACTGAACACATTG -3’ (SEQ ID NO: 194). In some embodiments, the 3′ half of Group I catalytic intron fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- CAGTAGCTGTAAATGCCCAACGACTATCCCTGATGAATGTAAGGGAGTAGGGTCAA GCGACCCGAAACGGCAGACAACTCTAAGAGTTGAAGATATAGTCTGAACTGCATGG TGACATGCAGCTGTTTATCCTCGTATAAATATGAATACGAGGTGAAACGATGAAATG AATTACATTGTTTCATATAAACGGGTAGAGAAGTAGCGAACTCTACTGAACACATTG -3’ (SEQ ID NO: 195). In some embodiments, the 5′ half of Group I catalytic intron fragment is from the T4 phage nrdD gene. In some embodiments, the 3′ half of Group I catalytic intron fragment is from the T4 phage nrdD gene and the 5′ half of Group I catalytic intron fragment is from the T4 phage nrdD gene. In some embodiments, the 5′ half of Group I catalytic intron includes a sequence having at least 80% sequence identity to 5’- TAACGTAAGTCAAGCTCATGTAAAATCTGCCTAAAACGGGAAACTCTCACTGAGAC AATCCGTTGCTAAATCAG-3’ (SEQ ID NO: 196). In some embodiments, the 5′ half of Group I catalytic intron includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TAACGTAAGTCAAGCTCATGTAAAATCTGCCTAAAACGGGAAACTCTCACTGAGAC AATCCGTTGCTAAATCAG-3’ (SEQ ID NO: 197). In some embodiments, the 3’ exon fragment includes a sequence having at least 80% sequence identity to 5’- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 GTACCTTTAACTTCCATAAGAACATGGAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 198). In some embodiments, the 3’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- GTACCTTTAACTTCCATAAGAACATGGAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 199). In some embodiments, the 3’ exon fragment includes a sequence having at least 80% sequence identity to 5’- GTACCTTTAACTTCCAAAAGATACATAAAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 200. In some embodiments, the 3’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- GTACCTTTAACTTCCAAAAGATACATAAAAATCATGGAAGGTAATGCCAAG-3’ (SEQ ID NO: 201). In some embodiments, the 5’ exon fragment includes a sequence having at least 80% sequence identity to 5’-TTTTTATGTATCTTTTGCGT-3’ (SEQ ID NO: 202). In some embodiments, the 5’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’-TTTTTATGTATCTTTTGCGT-3’ (SEQ ID NO: 203). In some embodiments, the 3’ exon fragment Includes a sequence having at least 80% sequence identity to 5’- ATGAAGTGAACACGTTATTCAGTTCAAACGGACAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 204). In some embodiments, the 3’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- ATGAAGTGAACACGTTATTCAGTTCAAACGGACAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 205). In some embodiments, the 3’ exon fragment includes a sequence having at least 80% sequence identity to 5’- ATGAAGTGAACACGTTACATAAGCTTGGAATGCAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 206). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the 3’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- ATGAAGTGAACACGTTACATAAGCTTGGAATGCAGACTCCTTTTGTAACA -3’ (SEQ ID NO: 207). In some embodiments, the 5’ exon fragment includes a sequence having at least 80% sequence identity to 5’- TGCATTCCAAGCTTATGAGT -3’ (SEQ ID NO: 208). In some embodiments, the 5’ exon fragment includes a sequence having at least 85%, 90%, 95%, 97%, 99%, or 100% sequence identity to 5’- TGCATTCCAAGCTTATGAGT -3’ (SEQ ID NO: 209). In another aspect, a linear polyribonucleotide for circularization may be cyclized or concatenated by a non-nucleic acid moiety that causes an attraction between atoms, molecular surfaces at, near, or linked to the 5’ and 3’ ends of the linear polyribonucleotide for circularization. In one embodiment, one or more linear polyribonucleotides is cyclized or concatenated by intermolecular forces or intramolecular forces. Non-limiting examples of intermolecular forces include dipole-dipole forces, dipole-induced dipole forces, induced dipole- induced dipole forces, Van der Waals forces, and London dispersion forces. Non-limiting examples of intramolecular forces include covalent bonds, metallic bonds, ionic bonds, resonant bonds, agnostic bonds, dipolar bonds, conjugation, hyperconjugation and antibonding. In some embodiments, a linear polyribonucleotide for circularization may include a ribozyme RNA sequence near the 5’ terminus and near the 3’ terminus. The ribozyme RNA sequence may covalently link to a peptide when the sequence is exposed to the remainder of the ribozyme. In one aspect, the peptides covalently linked to the ribozyme RNA sequence near the 5’ terminus and the 3 ‘terminus may associate with each other causing a linear polyribonucleotide to cyclize or concatenate. In another example, the peptides covalently linked to the ribozyme RNA near the 5’ terminus and the 3’ terminus may cause the linear primary construct or linear mRNA to cyclize or concatenate after being subjected to ligation using various methods known in the art such as, but not limited to, protein ligation. Non-limiting examples of ribozymes for use in the linear primary constructs or linear polyribonucleotides of the present invention or a non-exhaustive listing of methods to incorporate or covalently link peptides are described in U.S. Patent Publication No. U.S. 2003/0082768, the contents of which is here in incorporated by reference in its entirety. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In another aspect, chemical methods of circularization may be used to generate the circular polyribonucleotide. Such methods may include but are not limited to click chemistry (e.g., alkyne and azide-based methods, or clickable bases), olefin metathesis, phosphoramidate ligation, hemiaminal-imine crosslinking, base modification, and any combination thereof. In some chemical methods, the 5’-end and the 3’-end of a linear polyribonucleotide for circularization includes chemically reactive groups that, when close together, may form a new covalent linkage between the 5’-end and the 3’-end of the molecule. The 5’-end may contain an NHS-ester reactive group and the 3’-end may contain a 3’-amino-terminated nucleotide such that in an organic solvent the 3’-amino-terminated nucleotide on the 3’-end of a linear RNA molecule will undergo a nucleophilic attack on the 5’-NHS-ester moiety forming a new 5’-/3’-amide bond. In another aspect, the circular polyribonucleotide may be produced using a deoxyribonucleotide template transcribed in a cell-free system (e.g., by in vitro transcription) to a produce a linear RNA. The linear polyribonucleotide produces a splicing-compatible polyribonucleotide, which may be self-spliced to produce a circular polyribonucleotide. In some embodiments, the disclosure provides a method of producing a circular polyribonucleotide (e.g., in a cell-free system) by providing a linear polyribonucleotide; and self- splicing linear polyribonucleotide under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide; thereby producing a circular polyribonucleotide. In some embodiments, the disclosure provides a method of producing a circular polyribonucleotide by providing a deoxyribonucleotide encoding the linear polyribonucleotide; transcribing the deoxyribonucleotide in a cell-free system to produce the linear polyribonucleotide; optionally purifying the splicing-compatible linear polyribonucleotide; and self-splicing the linear polyribonucleotide under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide, thereby producing a circular polyribonucleotide. In some embodiments, the disclosure provides a method of producing a circular polyribonucleotide by providing a deoxyribonucleotide encoding a linear polyribonucleotide; transcribing the deoxyribonucleotide in a cell-free system to produce the linear polyribonucleotide, wherein the transcribing occurs in a solution under conditions suitable for splicing of the 3’ and 5’ splice sites of the linear polyribonucleotide, thereby producing a circular polyribonucleotide. In some embodiments, the linear polyribonucleotide comprises a 5’ split- intron and a 3’ split-intron (e.g., a self-splicing construct for producing a circular LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 polyribonucleotide). In some embodiments, the linear polyribonucleotide comprises a 5’ annealing region and a 3’ annealing region. Suitable conditions for in vitro transcriptions and or self-splicing may include any conditions (e.g., a solution or a buffer, such as an aqueous buffer or solution) that mimic physiological conditions in one or more respects. In some embodiments, suitable conditions include between 0.1-100mM Mg2+ ions or a salt thereof (e.g., 1-100mM, 1-50mM, 1-20mM, 5- 50mM, 5-20 mM, or 5-15mM). In some embodiments, suitable conditions include between 1- 1000mM K+ ions or a salt thereof such as KCl (e.g., 1-1000mM, 1-500mM, 1-200mM, 50- 500mM, 100-500mM, or 100-300mM). In some embodiments, suitable conditions include between 1-1000mM Cl- ions or a salt thereof such as KCl (e.g., 1-1000mM, 1-500mM, 1- 200mM, 50- 500mM, 100-500mM, or 100-300mM). In some embodiments, suitable conditions include between 0.1-100mM Mn2+ ions or a salt thereof such as MnCl2 (e.g., 0.1-100mM, 0.1- 50mM, 0.1-20mM, 0.1-10mM, 0.1-5mM, 0.1-2mM, 0.5- 50mM, 0.5-20 mM, 0.5-15mM, 0.5- 5mM, 0.5-2mM, or 0.1-10mM). In some embodiments, suitable conditions include dithiothreitol (DTT) (e.g., 1-1000 μM, 1-500 μM, 1-200μM, 50- 500μM, 100-500μM, 100-300μM, 0.1- 100mM, 0.1-50mM, 0.1-20mM, 0.1-10mM, 0.1-5mM, 0.1-2mM, 0.5- 50mM, 0.5-20 mM, 0.5- 15mM, 0.5-5mM, 0.5-2mM, or 0.1-10mM). In some embodiments, suitable conditions include between 0.1mM and 100mM ribonucleoside triphosphate (NTP) (e.g., 0.1-100 mM, 0.1-50mM, 0.1-10mM, 1- 100mM, 1-50mM, or 1-10mM). In some embodiments, suitable conditions include a pH of 4 to 10 (e.g., pH of 5 to 9, pH of 6 to 9, or pH of 6.5 to 8.5). In some embodiments, suitable conditions include a temperature of 4°C to 50°C (e.g., 10°C to 40°C, 15 °C to 40°C, 20°C to 40°C, or 30°C to 40°C), In some embodiments the linear polyribonucleotide is produced from a deoxyribonucleic acid, e.g., a deoxyribonucleic acid described herein, such as a DNA vector, a linearized DNA vector, or a cDNA. In some embodiments, the linear polyribonucleotide is transcribed from the deoxyribonucleic acid by transcription in a cell-free system (e.g., in vitro transcription). In another aspect, the circular polyribonucleotide may be produced in a cell, e.g., a prokaryotic cell or a eukaryotic cell. In some embodiments, an exogenous polyribonucleotide is provided to a cell (e.g., a linear polyribonucleotide described herein or a DNA molecule encoding for the transcription of a linear polyribonucleotide described here). The linear polyribonucleotides may be transcribed in the cell from an exogenous DNA molecule provided to the cell. The linear LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 polyribonucleotide may be transcribed in the cell from an exogenous recombinant DNA molecule transiently provided to the cell. In some embodiments, the exogenous DNA molecule does not integrate into the cell’s genome. In some embodiments, the linear polyribonucleotide is transcribed in the cell from a recombinant DNA molecule that is incorporated into the cell’s genome. In some embodiments, the cell is a prokaryotic cell. In some embodiments, the prokaryotic cell including the polyribonucleotides described herein is a bacterial cell or an archaeal cell. For example, the prokaryotic cell including the polyribonucleotides described herein may be E coli, halophilic archaea (e.g., Haloferax volcaniii), Sphingomonas, cyanobacteria (e.g., Synechococcus elongatus, Spirulina (Arthrospira) spp., and Synechocystis spp.), Streptomyces, actinomycetes (e.g., Nonomuraea, Kitasatospora, or Thermobifida), Bacillus spp. (e.g., Bacillus subtilis, Bacillus anthracis, Bacillus cereus), betaproteobacteria (e.g., Burkholderia), alphaproteobacterial (e.g., Agrobacterium), Pseudomonas (e.g., Pseudomonas putida), and enterobacteria. The prokaryotic cells may be grown in a culture medium. The prokaryotic cells may be contained in a bioreactor. In some embodiments, the cell is a eukaryotic cell. In some embodiments, the eukaryotic cell is a unicellular eukaryotic cell. In some embodiments, the unicellular eukaryotic is a unicellular fungal cell such as a yeast cell (e.g., Saccharomyces cerevisiae and other Saccharomyces spp., Brettanomyces spp., Schizosaccharomyces spp., Torulaspora spp, and Pichia spp.). In some embodiments, the unicellular eukaryotic cell is a unicellular animal cell. A unicellular animal cell may be a cell isolated from a multicellular animal and grown in culture, or the daughter cells thereof. In some embodiments, the unicellular animal cell is dedifferentiated. In some embodiments, the unicellular eukaryotic cell is a unicellular plant cell. A unicellular plant cell may be a cell isolated from a multicellular plant and grown in culture, or the daughter cells thereof. In some embodiments, the unicellular plant cell is dedifferentiated. In some embodiments, the unicellular plant cell is from a plant callus. In embodiments, the unicellular cell is a plant cell protoplast. In some embodiments, the unicellular eukaryotic cell is a unicellular eukaryotic algal cell, such as a unicellular green alga, a diatom, an euglenid, or a dinoflagellate. Non-limiting examples of unicellular eukaryotic algae of interest include Dunaliella salina, Chlorella vulgaris, Chlorella zofingiensis, Haematococcus pluvialis, Neochloris oleoabundans and other Neochloris spp., Protosiphon botryoides, Botryococcus LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 braunii, Cryptococcus spp., Chlamydomonas reinhardtii and other Chlamydomonas spp. In some embodiments, the unicellular eukaryotic cell is a protist cell. In some embodiments, the unicellular eukaryotic cell is a protozoan cell. In some embodiments, the eukaryotic cell is a cell of a multicellular eukaryote. For example, the multicellular eukaryote may be selected from the group consisting of a vertebrate animal, an invertebrate animal, a multicellular fungus, a multicellular alga, and a multicellular plant. In some embodiments, the eukaryotic organism is a human. In some embodiments, the eukaryotic organism is a non-human vertebrate animal. In some embodiments, the eukaryotic organism is an invertebrate animal. In some embodiments, the eukaryotic organism is a multicellular fungus. In some embodiments, the eukaryotic organism is a multicellular plant. In embodiments, the eukaryotic cell is a cell of a human or a cell of a non-human mammal such as a non-human primate (e.g., monkeys, apes), ungulate (e.g., bovids including cattle, buffalo, bison, sheep, goat, and musk ox; pig; camelids including camel, llama, and alpaca; deer, antelope; and equids including horse and donkey), carnivore (e.g., dog, cat), rodent (e.g., rat, mouse, guinea pig, hamster, squirrel), or lagomorph (e.g., rabbit, hare). In embodiments, the eukaryotic cell is a cell of a bird, such as a member of the avian taxa Galliformes (e.g., chickens, turkeys, pheasants, quail), Anseriformes (e.g., ducks, geese), Paleaognathae (e.g., ostriches, emus), Columbiformes (e.g., pigeons, doves), or Psittaciformes (e.g., parrots). In embodiments, the eukaryotic cell is a cell of an arthropod (e.g., insects, arachnids, crustaceans), a nematode, an annelid, a helminth, or a mollusc. In embodiments, the eukaryotic cell is a cell of a multicellular plant, such as an angiosperm plant (which can be a dicot or a monocot) or a gymnosperm plant (e.g., a conifer, a cycad, a gnetophyte, a Ginkgo), a fern, horsetail, clubmoss, or a bryophyte. In embodiments, the eukaryotic cell is a cell of a eukaryotic multicellular alga. The eukaryotic cells may be grown in a culture medium. The eukaryotic cells may be contained in a bioreactor. In some embodiments, any method of producing a circular polyribonucleotide described herein may be performed in a bioreactor. A bioreactor refers to any vessel in which a chemical or biological process is carried out which involves organisms or biochemically active substances derived from such organisms. Bioreactors may be compatible with the cell-free methods for production of circular RNA described herein. A vessel for a bioreactor may include a culture flask, a dish, or a bag that may be single use (disposable), autoclavable, or sterilizable. A LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 bioreactor may be made of glass, or it may be polymer-based, or it may be made of other materials. Examples of bioreactors include, without limitation, stirred tank (e.g., well mixed) bioreactors and tubular (e.g., plug flow) bioreactors, airlift bioreactors, membrane stirred tanks, spin filter stirred tanks, vibromixers, fluidized bed reactors, and membrane bioreactors. The mode of operating the bioreactor may be a batch or continuous processes. A bioreactor is continuous when the reagent and product streams are continuously being fed and withdrawn from the system. A batch bioreactor may have a continuous recirculating flow, but no continuous feeding of reagents or product harvest. Some methods of the present disclosure are directed to large-scale production of circular polyribonucleotides. For large-scale production methods, the method may be performed in a volume of 1 liter (L) to 50 L, or more (e.g., 5 L, 10 L, 15 L, 20 L, 25 L, 30 L, 35 L, 40 L, 45 L, 50 L, or more). In some embodiments, the method may be performed in a volume of 5 L to 10 L, 5 L to 15 L, 5 L to 20 L, 5 L to 25 L, 5 L to 30 L, 5 L to 35 L, 5 L to 40 L, 5 L to 45 L, 10 L to 15 L, 10 L to 20 L, 10 L to 25 L, 20 L to 30 L, 10 L to 35 L, 10 L to 40 L, 10 L to 45 L, 10 L to 50 L, 15 L to 20 L, 15 L to 25 L, 15 L to 30 L, 15 L to 35 L, 15 L to 40 L, 15 L to 45 L, or 15 to 50 L. In some embodiments, a bioreactor may produce at least 1g of circular RNA. In some embodiments, a bioreactor may produce 1-200g of circular RNA (e.g., 1-10g, 1-20g, 1-50g, 10-50g, 10-100g, 50-100g, or 50-200g of circular RNA). In some embodiments, the amount produced is measured per liter (e.g., 1-200g per liter), per batch or reaction (e.g., 1-200g per batch or reaction), or per unit time (e.g., 1-200g per hour or per day). In some embodiments, more than one bioreactor may be utilized in series to increase the production capacity (e.g., one, two, three, four, five, six, seven, eight, or nine bioreactors may be used in series). In some embodiments, circularization efficiency is at least about 10%, at least about 15%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%, at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, or 100%. In some embodiments, circularization efficiency is at least about 40%. In some embodiments, circularization efficiency is between about 10% and about 100%; for example, circularization efficiency is about 15%, about 20%, about 25%, about 30%, about 35%, about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 and about 99%. In some embodiments, circularization efficiency is between about 20% and about 80%. In some embodiments, circularization efficiency is between about 30% and about 60%. In some embodiments, circularization efficiency is about 40%. Additional methods of making the circular polyribonucleotides described herein are described in, for example, Khudyakov & Fields, Artificial DNA: Methods and Applications, CRC Press (2002); in Zhao, Synthetic Biology: Tools and Applications, (First Edition), Academic Press (2013); Muller and Appel, from RNA Biol, 2017, 14(8):1018-1027; and Egli & Herdewijn, Chemistry and Biology of Artificial Nucleic Acids, (First Edition), Wiley-VCH (2012). Other methods of making circular polyribonucleotides are described, for example, in International Publication No. WO2023/044006, International Publication No. WO2022/247943, U.S. Patent No. 11000547, International Publication No. 2018/191722, International Publication No. WO2019/236673, International Publication No. WO2020/023595, International Publication No. WO2022/204460, International Publication No. WO2022/204464, International Publication No. WO2022/204466, and International Publication No. 2022/261490, the contents of each of which are herein incorporated by reference in their entirety). Additional methods of synthesizing circular polyribonucleotides are also described elsewhere (see, e.g., U.S. Patent No. 6210931, U.S. Patent No. 5773244, U.S. Patent No. 5766903, U.S. Patent No. 5712128, U.S. Patent No. 5426180, U.S. Patent Publication No. US20100137407, International Publication No. WO1992/001813, International Publication No. WO2010/084371, and Petkovic et al., Nucleic Acids Res. 43:2454-65 (2015); the contents of each of which are herein incorporated by reference in their entirety). (xiii) Purification Methods One or more purification steps may be included in the methods described herein. For example, in some embodiments, the linear polyribonucleotide is substantively enriched or pure (e.g., purified) prior to self-splicing the linear polyribonucleotide. In other embodiments, the linear polyribonucleotide is not purified prior to self-splicing the linear polyribonucleotide. In some embodiments, the resulting circular polyribonucleotide is purified. Purification may include separating or enriching the desired reaction product from one or more undesired components, such as any unreacted stating material, byproducts, enzymes, or other reaction components. For example, purification of linear polyribonucleotide following LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 transcription in a cell-free system (e.g., in vitro transcription) may include separation or enrichment from the DNA template prior to self-splicing the linear polyribonucleotide. Purification of the circular RNA product following splicing may be used to separate or enrich the circular polyribonucleotide from its corresponding linear polyribonucleotide. Methods of purification of polyribonucleotides are known to those of skill in the art and include enzymatic purification or by chromatography. In some embodiments, the methods of purification result in a circular polyribonucleotide that has less than 50% (e.g., less than 40%, 30%, 20%, 10%, 5%, 4%, 3%, 2%, or 1%) linear polyribonucleotides. In some embodiments, the reference criterion for the amount of linear polyribonucleotide molecules present in a preparation (e.g., pharmaceutical preparation) is the presence of no more than 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 600 ng/ml, 1 µg/ ml, 10 µg/ml, 50 µg/ml, 100 µg/ml, 200 g/ml, 300 µg/ml, 400 µg/ml, 500 µg/ml, 600 µg/ml, 700 µg/ml, 800 µg/ml, 900 µg/ml, 1 mg/ml, 1.5 mg/ml, or 2 mg/ml of linear polyribonucleotide molecules. In some embodiments, the reference criterion for the amount of circular polyribonucleotide molecules present in a preparation (e.g., pharmaceutical preparation) is at least 30% (w/w), 40% (w/w), 50% (w/w), 60% (w/w), 70% (w/w), 80% (w/w), 85% (w/w), 90% (w/w), 91% (w/w), 92% (w/w), 93% (w/w), 94% (w/w), 95% (w/w), 96% (w/w), 97% (w/w), 98% (w/w), 99% (w/w), 99.1% (w/w), 99.2% (w/w), 99.3% (w/w), 99.4% (w/w), 99.5% (w/w), 99.6% (w/w), 99.7% (w/w), 99.8% (w/w), 99.9% (w/w), or 100% (w/w) molecules of the total ribonucleotide molecules in the preparation. In some embodiments, the reference criterion for the amount of linear polyribonucleotide molecules present in a preparation (e.g., pharmaceutical preparation) is no more than 0.5% (w/w), 1% (w/w), 2% (w/w), 5% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), 40% (w/w), 50% (w/w) linear polyribonucleotide molecules of the total ribonucleotide molecules in the preparation. In some embodiments, the reference criterion for the amount of nicked polyribonucleotide molecules present in a preparation (e.g., pharmaceutical preparation) is no LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 more than 0.5% (w/w), 1% (w/w), 2% (w/w), 5% (w/w), 10% (w/w), or 15% (w/w) nicked polyribonucleotide molecules of the total ribonucleotide molecules in the preparation. In some embodiments, the reference criterion for the amount of combined nicked and linear polyribonucleotide molecules present in a preparation (e.g., pharmaceutical preparation) is no more than 0.5% (w/w), 1% (w/w), 2% (w/w), 5% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), 40% (w/w), 50% (w/w) combined nicked and linear polyribonucleotide molecules of the total ribonucleotide molecules in the preparation. In some embodiments, a preparation (e.g., pharmaceutical preparation) is an intermediate preparation of a final circular polyribonucleotide drug product. In some embodiments, a preparation (e.g., pharmaceutical preparation) is a drug substance or active pharmaceutical ingredient (API). In some embodiments, a preparation (e.g., pharmaceutical preparation) is a drug product for administration to a subject. In some embodiments, a preparation (e.g., pharmaceutical preparation) of circular polyribonucleotides is (before, during or after the reduction of linear polyribonucleotide) further processed to substantially remove DNA, protein contamination (e.g., cell protein such as a host cell protein or protein process impurities), endotoxin, mononucleotide molecules, and/or a process-related impurity. II. Pharmaceutical Compositions Provided herein are compositions and pharmaceutical compositions comprising (i) any of the circular polyribonucleotides or modified RNAs described herein and (ii) a pharmaceutically acceptable excipient. Pharmaceutical compositions can comprise one or more additional therapeutic agents, e.g., therapeutically and/or prophylactically active agents. Methods of making pharmaceutical compositions including circular polyribonucleotides or modified RNAs are described in WO 2020/181013, which is incorporated herein by reference in its entirety. Pharmaceutical compositions provided herein are suitable for administration to humans or any other animal, e.g., to non-human animals, e.g., non-human mammals. Modification of pharmaceutical compositions suitable for administration to humans in order to render the compositions suitable for administration to various animals is well understood. Non-limiting examples of subjects that can be administered any of the pharmaceutical compositions described herein include humans, non-human primate, mammals (e.g., commercially-relevant mammals, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 such as cattle, pigs, horses, sheep, cats, dogs, mice, and rats), and birds (e.g., including commercially relevant birds such as poultry, chickens, ducks, geese, and/or turkeys). Pharmaceutical compositions provided herein can be formulated for any suitable mode of administration. Non-limiting examples include formulation of the pharmaceutical composition for intravenous, subcutaneous, intrahepatic, or intramuscular administration. In some embodiments, a pharmaceutical composition is formulated for local administration. In some embodiments, a pharmaceutical composition is formulated for systemic administration. Pharmaceutical compositions provided herein can include an unencapsulated circular polyribonucleotide of modified RNA, a partially encapsulated circular polyribonucleotide or modified RNA, or a completely encapsulated circular polyribonucleotide or modified RNA. Any of the circular polyribonucleotides or modified RNAs described herein can be included in a pharmaceutical composition with a carrier or without a carrier. In some embodiments, pharmaceutical compositions described herein can be formulated with a carrier, e.g., pharmaceutical carrier and/or a polymeric carrier, e.g., a liposome. In such instances, the circular polyribonucleotide or modified RNA can be formulated as a lipid nanoparticle. In some embodiments, the circular polyribonucleotide or modified RNA is formulated for unencapsulated delivery or natural nanoparticle delivery. In some embodiments, the natural nanoparticle is composed of a natural source selected from a plant, bacteria, animal, insect, archaea, or fungi. In some embodiments the circular polyribonucleotide is formulated within (a) a plurality of lipid nanoparticles (LNP) comprising synthetic structural lipids and an ionizable lipid or within (b) a natural nanoparticle comprising natural lipids and an ionizable lipid. One or more naturally occurring and/or synthetic lipid compounds may be used in the preparation of a lipid-based carrier (or lipid nanoformulation). As described herein, a lipid-based carrier (or lipid nanoformulation) may be a lipid particle that has a complexity characterized by comprising a wide variety of lipids, including both synthetic lipids or lipids extracted from one or more natural sources (such as plants or bacteria). Natural lipids can be derived from a variety of arthropod, fungi, plant, archaea, or bacteria, or one or more parts thereof (e.g., segments, organs, eggs, spores, mycelium, tissue, membrane or cell wall). In some embodiments, the lipid-based carrier is a modified natural nanoparticle. As used herein, the term "modified natural particle" refers to a natural lipid LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 nanoparticles composition including both naturally derived lipids and one or more synthetically derived lipids. In some embodiments, modified natural nanoparticles may include ionizable lipids, non-cationic lipids, sterols, and PEGylated lipids. More embodiments of natural lipids, complex lipid particles, and methods of producing and delivering natural nanoparticles may be found in PCT Application No. PCT/US2023/021094, filed on May 5, 2023, PCT Application No. PCT/US24/25134, filed on April 18, 2024, and PCT Application No. PCT/US24/25270, filed on April 18, 2024, the contents of which are incorporated herein by reference in their entirety. In some embodiments, the modified natural nanoparticle is plant-based, e.g. as described in International Patent Publication Nos. WO2011/097480, WO2013/070324, WO2017/004526, WO2020/041784, WO2021/041301, WO2023/069498, WO2023/122080, or WO2024/102434. In some embodiments, the modified natural nanoparticle is bacteria-derived, e.g. as described in International Patent Publication Nos. WO2023/096858. In the lipid nanoparticle or natural nanoparticle composition, more than one ionizable lipid can be used for the ionizable lipid component. More embodiments of ionizable lipids may be found in PCT Application No. PCT/US22/50725, filed on November 22, 2022, PCT Application No. PCT/US23/16300, filed on March 24, 2023, PCT Application No. PCT/US22/50111, filed on November 16, 2022, and PCT Application No. PCT/US23/31669, filed on August 31, 2023, the contents of which are incorporated herein by reference in their entirety. Other suitable lipids for use in the RNA composition and methods for making and using thereof include the lipids as described in International Patent Publication WO2016/118725, WO2016/118724, WO2013/063468, WO2016/205691, WO2015/184256, WO2016/004202, WO2015/199952, WO2017/004143, WO2017/075531, WO2017/117528, WO2017/049245, WO2017/173054, WO2015/095340, WO2016118724, WO2016118725, WO2016187531, WO2017176974, WO2018078053, WO2019027999, WO2019036030, WO2019089828, WO2019099501, WO2020072605, WO2020081938, WO2020118041, WO2020146805, or WO2020219876 each of which is incorporated herein by reference in its entirety. Other suitable lipids for use in the RNA composition and methods for making and using thereof include the lipids as described in J. McClellan, M. C. King, Cell 2010, 141, 210-217 and in Whitehead et al., Nature Communications (2014) 5:4277, which is incorporated herein by reference in its entirety. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the ionizable lipid may be 1,1’-((2-(4-(2-((2-(bis(2- hydroxydodecyl)amino)ethyl) (2-hydroxydodecyl)amino)ethyl)piperazin-1- yl)ethyl)azanediyl)bis(dodecan-2-ol) (C12-200), MD1 (cKK-E12), OF2, EPC, ZA3-Ep10, TT3, LP01, 5A2-SC8, Lipid 5, SM-102 (Lipid H), or ALC-315. In one embodiment, the ionizable lipid included is LP01. The natural nanoparticle may include one or more exogenous lipids, e.g., lipids that are exogenous to the natural source (e.g., originating from a source that is not the source or source part from which the particle is produced). The lipid composition may include 0%, less than 1%, or at least 1%, 2%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or more than 95% exogenous lipid. In some examples, the exogenous lipid (e.g., ionizable lipid) is added to amount to 25% or 40% (w/w) of total lipids in the preparation. In some examples, the exogenous lipid is added to the preparation prior to step (b), e.g., mixed with extracted natural lipids prior to step (b). As used herein, the term “exogenous lipid” refers to a lipid that is exogenous to the natural source (e.g., plant, bacteria), i.e., a lipid originates from a source that is not the natural source from which the lipids are extracted (e.g., a lipid that is added to the lipid particle formulation using method described herein). The term “exogenous lipid” does not exclude a natural-derived lipid (such as a plant-derived sterol). That is to say, an exogenous lipid can be a natural-derived lipid (such as a plant-derived sterol that is exogenous to the plant source from which the lipids are extracted, e.g., an exogenous lipid can be a plant derived sterol that is added to the lipid particle formulation). As another example, an exogenous lipid can be a natural- derived lipid that is exogenous to the particular natural source from which the lipids are extracted (e.g., a bacteria-derived lipid that is exogenous to the plant source from which the lipids are extracted, or vice versa). An exogenous lipid may be a cell-penetrating agent, may be capable of increasing delivery of one or more polynucleotides by the lipid formulation (e.g. lipid nanoparticle or natural nanoparticle) to a cell, and/or may be capable of increasing loading (e.g., loading efficiency or loading capacity) of a polynucleotide. In some embodiments, the exogenous lipid may be a stabilizing lipid. In some embodiments, the exogenous lipid may be a structural lipid (e.g., a synthetic structural lipid). Exemplary exogenous lipids include ionizable lipids, synthetic structural lipids, sterols, and PEGylated lipids. Exogenous lipids may also include cationic lipids. As used herein, the term “cationic lipid” refers to an amphiphilic LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 molecule (e.g., a lipid or a lipidoid) that is positively charged, containing a cationic group (e.g., a cationic head group). As used herein, the term “ionizable lipid” refers to an amphiphilic molecule (e.g., a lipid or a lipidoid, e.g., a synthetic lipid or lipidoid) containing a group (e.g., a head group) that can be ionized, e.g., dissociated to produce one or more electrically charged species, under a given condition (e.g., pH). In some embodiments, the natural nanoparticle may contain 3-1000 lipids extracted from one or more natural (e.g., plant, bacteria) sources. In some embodiments, the natural nanoparticle contains at least 10 natural lipids belonging to one or more of the classes selected from the group consisting of fatty acyls (FA), fatty acyl conjugates, phospholipids, glycerolipids, glycolipids, glycerophospholipids, sphingolipids, waxes, and sterol. In some embodiments, the lipid nanoparticle or natural nanoparticle composition comprises one or more ionizable lipids, one or more structural lipids (synthetic or natural), a sterol, and one or more PEG-modified lipids. In some embodiments, the natural nanoparticle may further comprise a neutral lipid as a helper lipid. In some embodiments, the natural lipids may be used in combination with a neutral lipid as a structural lipid component. In some embodiments, the particle has a size of less than about 200 nm. In some embodiments, the RNA and particle composition has an N:P ratio of at least 3, for instance, an N:P ratio of 3 to 100, 3 to 50, 3 to 30, 3 to 20, 3 to 15, 3 to 12, 6 to 30, 6 to 20, 6 to 15, or 6 to 12. In some embodiments, pharmaceutical compositions described herein can be formulated without a carrier, e.g., in a naked delivery formulation. A naked delivery formulation refers to a formulation that is free from a carrier. In some embodiments, a naked delivery formulation can be free of any or all of: transfection reagents, cationic carriers, carbohydrate carriers, nanoparticle carriers, or protein carriers. For example, a naked delivery formulation can be free from phtoglycogen octenyl succinate, phytoglycogen beta-dextrin, anhydride-modified phytoglycogen beta-dextrin, lipofectamine, polyethylenimine, poly(trimethylenimine), poly(tetramethylenimine), polypropylenimine, aminoglycoside-polyamine, dideoxy-diamino-b-cyclodextrin, spermine, spermidine, poly(2-dimethylamino)ethyl methacrylate, poly(lysine), poly(histidine), poly(arginine), cationized gelatin, dendrimers, chitosan, l,2-Dioleoyl-3-Trimethylammonium- LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Propane (DOTAP), N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), l-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium chloride (DOTIM), 2,3-dioleyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-l-propanaminium trifluoroacetate (DOSPA), 3B—[N—(N\N′-Dimethylaminoethane)-carbamoyl]Cholesterol Hydrochloride (DC-Cholesterol HC1), diheptadecylamidoglycyl spermidine (DOGS), N,N- distearyl-N,N-dimethylammonium bromide (DDAB), N-(1,2-dimyristyloxyprop-3-yl)-N,N- dimethyl-N-hydroxyethyl ammonium bromide (DMRIE), N,N-dioleyl-N,N-dimethylammonium chloride (DODAC), human serum albumin (HSA), low-density lipoprotein (LDL), high-density lipoprotein (HDL), or globulin. In some embodiments, the naked delivery formulation can comprise a non-carrier excipient. In some embodiments, a non-carrier excipient may comprise an inactive ingredient that does not exhibit an active cell-penetrating effect. In some embodiments, a non-carrier excipient may comprise a buffer, for example PBS. In some embodiments, a non-carrier excipient may be a solvent, a non-aqueous solvent, a diluent, a suspension aid, a surface active agent, an isotonic agent, a thickening agent, an emulsifying agent, a preservative, a polymer, a peptide, a protein, a cell, a hyaluronidase, a dispersing agent, a granulating agent, a disintegrating agent, a binding agent, a buffering agent, a lubricating agent, or an oil. In some embodiments, the naked delivery formulation can comprise a diluent (e.g., a parenterally acceptable diluent). A diluent can be a liquid diluent or a solid diluent. In some embodiments, a diluent can be an RNA solubilizing agent, a buffer, or an isotonic agent. Non- limiting examples of an RNA solubilizing agent include water, ethanol, methanol, acetone, formamide, and 2-propanol. Examples of a buffer include 2-(N-morpholino)ethanesulfonic acid (MES), Bis-Tris, 2-[(2-amino-2-oxoethyl)-(carboxymethyl)amino]acetic acid (ADA), N-(2- Acetamido)-2-aminoethanesulfonic acid (ACES), piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 2-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]ethanesulfonic acid (TES), 3- (N-morpholino)propanesulfonic acid (MOPS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Tris, Tricine, Gly-Gly, Bicine, or phosphate. Non-limiting examples of an isotonic agent include glycerin, mannitol, polyethylene glycol, propylene glycol, trehalose, or sucrose. Purity of the pharmaceutical composition comprising the circular polyribonucleotide or modified RNA can be measured using any method known in the art, e.g., chromatography, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 electrophoresis, mass spectrometry, fluorescence, light scattering, refractive index, microscopy, circular dichroism (CD) spectroscopy, spectrophotometry, or surface plasmon resonance (SPR). In some embodiments, the pharmaceutical composition comprising the circular polyribonucleotide or modified RNA is at least 30% (w/w), 40% (w/w), 50% (w/w), 60% (w/w), 70% (w/w), 80% (w/w), 85% (w/w), 90% (w/w), 91% (w/w), 92% (w/w), 93% (w/w), 94% (w/w), 95% (w/w), 96% (w/w), 97% (w/w), 98% (w/w), 99% (w/w), or 100% (w/w) pure on a mass basis. In some embodiments, a concentration of the circular polyribonucleotide or modified RNA in the pharmaceutical composition is at least 0.1 ng/mL, 0.5 ng/mL, 1 ng/mL, 5 ng/mL, 10 ng/mL, 50 ng/mL, 0.1 μg/mL, 0.5 μg/mL, 1 µg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL, 30 μg/mL, 40 μg/mL, 50 μg/mL, 60 μg/mL, 70 μg/mL, 80 μg/mL, 100 μg/mL, 200 μg/mL, 300 μg/mL, 500 μg/mL, 1 mg/mL, 2 mg/mL, 3 mg/mL, 5 mg/mL, 10 mg/mL, 100 mg/mL, or 500 mg/mL. In some embodiments, the pharmaceutical composition is substantially free of mononucleotide or has a mononucleotide content of no more than 1 pg/ml, 10 pg/ml, 0.1 ng/ml, 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 1000 μg/mL, 5000 μg/mL, 10,000 μg/mL, or 100,000 μg/mL. In some embodiments, the pharmaceutical composition has a mononucleotide content of no more than 0.1% (w/w), 0.2% (w/w), 0.3% (w/w), 0.4% (w/w), 0.5% (w/w), 0.6% (w/w), 0.7% (w/w), 0.8% (w/w), 0.9% (w/w), 1% (w/w), 2% (w/w), 3% (w/w), 4% (w/w), 5% (w/w), 6% (w/w), 7% (w/w), 8% (w/w), 9% (w/w), 10% (w/w), 15% (w/w), 20% (w/w), 25% (w/w), 30% (w/w), or any percentage therebetween of total nucleotides on a mass basis, wherein total nucleotide content is the total mass of deoxyribonucleotide molecules and ribonucleotide molecules. In some embodiments, the pharmaceutical composition has a linear RNA content, e.g., linear RNA counterpart or RNA fragments, of no more than 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 600 ng/ml, 1 μg/ml, 10 μg/ml, 50 μg/ml, 100 μg/ml, 200 g/ml, 300 μg/ml, 400 μg/ml, 500 μg/ml, 600 μg/ml, 700 μg/ml, 800 μg/ml, 900 μg/ml, 1 mg/ml, 1.5 mg/ml, 2 mg/ml, 5 mg/mL, 10 mg/mL, 50 mg/mL, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 100 mg/mL, 200 mg/mL, 300 mg/mL, 400 mg/mL, 500 mg/mL, 600 mg/mL, 650 mg/mL, 700 mg/mL, or 750 mg/mL. In some embodiments, the pharmaceutical composition has a nicked RNA content of no more than 10% (w/w), 9.9% (w/w), 9.8% (w/w), 9.7% (w/w), 9.6% (w/w), 9.5% (w/w), 9.4% (w/w), 9.3% (w/w), 9.2% (w/w), 9.1% (w/w), 9% (w/w), 8% (w/w), 7% (w/w), 6% (w/w), 5% (w/w), 4% (w/w), 3% (w/w), 2% (w/w), 1% (w/w), 0.5% (w/w), or 0.1% (w/w), or percentage therebetween. In some embodiments, the pharmaceutical composition is substantially free of DNA content, e.g., template DNA or cell DNA (e.g., host cell DNA), has a DNA content, as low as zero, or has a DNA content of no more than 1 pg/ml, 10 pg/ml, 0.1 ng/ml, 1 ng/ml, 5 ng/ml, 10 ng/ml, 15 ng/ml, 20 ng/ml, 25 ng/ml, 30 ng/ml, 35 ng/ml, 40 ng/ml, 50 ng/ml, 60 ng/ml, 70 ng/ml, 80 ng/ml, 90 ng/ml, 100 ng/ml, 200 ng/ml, 300 ng/ml, 400 ng/ml, 500 ng/ml, 1000 μg/mL, 5000 μg/mL, 10,000 μg/mL, or 100,000 μg/mL. In some embodiments, the pharmaceutical composition is substantially free of an impurity (e.g., a cell protein, a cell nucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material, protein contamination, or endotoxin contamination). In some embodiments, the pharmaceutical composition comprises a concentration of protein that is less than 0.1 ng, 1 ng, 5 ng, 10 ng, 15 ng, 20 ng, 25 ng, 30 ng, 35 ng, 40 ng, 50 ng, 60 ng, 70 ng, 80 ng, 90 ng, 100 ng, 200 ng, 300 ng, 400 ng, or 500 ng of protein per milligram (mg) of the circular polyribonucleotide. In some embodiments, the pharmaceutical composition has an A260/A280 absorbance ratio from about 1.6 to about 2.3, e.g., as measured by spectrophotometer. In some embodiments, the A260/A280 absorbance ratio is about 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5, or any number therebetween. In some embodiments, the pharmaceutical composition comprises an amount of endotoxin that is less than 20 EU/kg (weight), 10 EU/kg, 5 EU/kg, 1 EU/kg, or is below a predetermined threshold, e.g., the pharmaceutical composition comprises a level of endotoxin below a limit of detection by a specified method. In some embodiments, the pharmaceutical composition is a sterile drug product or substantially free of microorganisms (e.g., supports growth of fewer than 100 viable microorganisms as tested under aseptic conditions). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, the pharmaceutical composition comprises a bioburden of less than 100 CFU/100 mL, 50 CFU/100 mL, 40 CFU/100 mL, 30 CFU/100 mL, 200 CFU/100 mL, 10 CFU/100 mL, or 10 CFU/100 mL before sterilization. In some embodiments, the pharmaceutical composition comprising the circular polyribonucleotide that has undergone a purification step produces a reduced level of one more markers of an immune or inflammatory response after administration to a subject compared to a pharmaceutical composition comprising an unpurified circular polyribonucleotide. In some embodiments, the one or more markers of an immune or inflammatory response is a cytokine or immune response related gene. In some embodiments, the one or more markers of an immune or inflammatory response is expression of a gene, such as RIG-I, MDA5, PKR, IFN-beta, OAS, and OASL. III. Uses of Circular Polyribonucleotides and Modified RNAs Also provided herein are methods for treating or reducing the risk of developing a GLP- 2-related disease in a subject that include administering to the subject a therapeutically effective amount of any of the circular polyribonucleotides or modified RNAs described herein, or any of the pharmaceutical compositions described herein. The term “GLP-2 related disease” refers to any disease in which cells engage in aberrant GLP-2 signaling, e.g., aberrant insulin signaling. Non-limiting examples of GLP-2-related diseases include short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. To practice a method disclosed herein, an effective amount of any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein is administered to a subject (e.g., a mammalian subject, e.g., a human) having or at risk for developing a GLP-2 related disease (e.g., short bowel syndrome) via a suitable route (e.g., local administration and/or systemic administration). In some embodiments of any of the methods described herein, the subject has been previously identified or diagnosed as having a GLP-2- related disease. In some embodiments of any of the methods described herein, the subject has been previously identified as having an elevated risk of developing a GLP-2-related disease. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 The term “subject” refers to a subject who needs treatment as described herein. In some embodiments, the subject is a human or a non-human mammal (e.g., cat, dog, horse, cow, goat, or sheep). A human subject who needs treatment can be a human patient having, suspected of having, or at risk for having a GLP-2-related disease, e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury An “effective amount” or a “therapeutically effective amount” as used herein refers to the amount of each active agent required to confer therapeutic effect on the subject, either alone or in combination with one or more other active agents. Effective amounts vary, as recognized by those skilled in the art, depending on the particular condition being treated, the severity of the condition, the individual patient parameters including age, physical condition, size, weight, the duration of the treatment, the nature of concurrent therapy (if any), the specific route of administration and like factors within the knowledge and expertise of the health practitioner. These factors are well known in the medical field. It is generally preferred that a maximum dose of the individual components or combinations thereof be used, that is, the highest safe dose according to sound medical judgment. It will be understood by those of ordinary skill in the art, however, that a patient may insist upon a lower dose or tolerable dose for medical reasons, psychological reasons, or virtually any other reason. Empirical considerations such as the half-life of an agent will generally contribute to the determination of the dosage. Frequency of administration can be determined and adjusted over the course of therapy, and is generally, but not necessarily, based on treatment and/or suppression and/or amelioration and/or delay of a GLP-2-related disease (e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof). In some embodiments, dosages of any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein can be determined empirically in individuals who have been given one or more administration(s) of the circular polyribonucleotide LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 or modified RNA. For example, individuals are given incremental dosages of any of the circular polyribonucleotides, modified RNAs, and pharmaceutical compositions described herein and an indicator and/or a symptom of a GLP-2-related disease can be followed to assess efficacy. Any suitable dosing regimen can be used in methods described herein. In some embodiments, the dosage regimen depends on the pattern of pharmacokinetic decay that the practitioner wishes to achieve. In some embodiments, dosing frequency is once every day, once every other day, once every week, or longer. In some embodiments, dosing frequency is multiple times per day. For repeated administrations over several days or longer, depending on the condition, the treatment can be sustained until a desired suppression of symptoms occurs or until sufficient therapeutic levels are achieved to alleviate a GLP-2-related disease, or a symptom thereof. In some embodiments, dosing regimens (including inhibitor used) can vary over time. A circular or linear polyribonucleotide described herein may be administered in a single dose or in multiple doses to a cell, tissue or subject. A circular or linear polydeoxyribonucleotide described herein may be administered in a single dose or in multiple doses to a cell, tissue or subject. A method of administering multiple doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition) comprises providing two or more compositions, over a period of time, to a cell, tissue or subject (e.g. a mammal). According to certain embodiments, multiple doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition) may be administered to a subject over a defined time course. The methods according to this aspect of the invention comprise sequentially administering to a subject multiple doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition). As used herein, “sequentially administering” means that each dose of composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition) is administered to the subject at a different point in time, e.g., LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 on different days separated by a predetermined interval (e.g., hours, days, weeks or months). In some embodiments, the present invention provides methods which comprise sequentially administering to the subject a single initial dose of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition), followed by one or more secondary doses of the composition, and optionally followed by one or more tertiary doses of the composition. The terms “initial dose,” “secondary doses,” and “tertiary doses,” refer to the temporal sequence of administration of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition). Thus, the “initial dose” is the dose which is administered at the beginning of the treatment regimen (also referred to as the “baseline dose”); the “secondary doses” are the doses which are administered after the initial dose; and the “tertiary doses” are the doses which are administered after the secondary doses. The initial, secondary, and tertiary doses may all contain the same amount of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition), and in certain embodiments, may differ from one another in terms of frequency of administration. In certain embodiments, the amount of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition) contained in the initial, secondary and/or tertiary doses varies from one another (e.g., adjusted up or down as appropriate) during the course of treatment. In certain embodiments, one or more (e.g., 2, 3, 4, or 5) doses are administered at the beginning of the treatment regimen as “loading doses” followed by subsequent doses that are administered on a less frequent basis (e.g., “maintenance doses”). In certain embodiments, each secondary and/or tertiary dose is administered after the immediately preceding dose. The phrase “the immediately preceding dose,” as used herein, means, in a sequence of multiple administrations, the dose of the composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 composition) which is administered to a subject prior to the administration of the very next dose in the sequence with no intervening doses. In certain embodiments, each secondary and/or tertiary dose is administered every day, every 2 days, 3 days, 4 days, 5 days, 6 days, or 7 days after the immediately preceding dose. In certain embodiments, each secondary and/or tertiary dose is administered every 0.5 weeks, 1 week, 2 weeks, 3 weeks, or 4 weeks after the immediately preceding dose. The methods according to this aspect of the invention may comprise administering to a subject any number of secondary and/or tertiary doses of a composition of a nucleic acid molecule described herein (e.g., a circular polyribonucleotide, a linear polyribonucleotide, a circular polydeoxyribonucleotide, a linear polydeoxyribonucleotide) (e.g., in a pharmaceutical composition). For example, in certain embodiments, only a single secondary dose is administered to the subject. In other embodiments, two or more (e.g., 2, 3, 4, 5, 6, 7, 8, or more) secondary doses are administered to the subject. Likewise, in certain embodiments, only a single tertiary dose is administered to the subject. In other embodiments, two or more (e.g., 2, 3, 4, 5, 6, 7, 8, or more) tertiary doses are administered to the subject. In certain embodiments, the frequency at which the secondary and/or tertiary doses are administered to a subject can vary over the course of the treatment regimen. The frequency of administration may also be adjusted during the course of treatment. In some embodiments, multiple doses are provided to produce a level of the composition or express a level of the GLP-2 or GLP-2 analog in a cell, tissue or subject. In some embodiments, multiple doses are provided to produce or maintain a level of the composition, or to produce or maintain a level of the GLP-2 or GLP-2 analog, in a cell, tissue or subject for a period of time, for instance, for at least 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150 days, or at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 18, 21, or 24 months, or at least 1, 2, 3, 4, or 5 years. In some embodiments, the method comprises providing (e.g., administering) at least a first composition and a second composition to the cells, tissue, or subject (e.g., a mammal, e.g., a human). In some embodiments, the method further comprises providing (e.g., administering) a third composition, fourth composition, fifth composition, sixth composition, seventh composition, eighth composition, ninth composition, tenth composition, or more. In some embodiments, additional compositions are provided for the duration of the life of the cell. In some embodiments, additional compositions are provided (e.g., administered) while the cell, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 tissue or subject obtains a benefit from the composition, e.g., stimulated insulin secretion, altered metabolism, improved glucose homeostasis, reduced body weight, improved liver function, and/or amelioration of fibrosis. In some embodiments, a first composition in a multiple dosing regimen comprises a first amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein. In some embodiments, a second composition in a multiple dosing regimen comprises a second amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein. In some embodiments, a third composition, a fourth composition, a fifth composition, a sixth composition, a seventh composition, an eighth composition, a ninth composition, a tenth composition, or more in a multiple dosing regimen comprises a third, fourth, fifth, sixth, seventh, eighth, ninth, tenth or more amount of the nucleic acid molecule (e.g., circular polyribonucleotide) disclosed herein. In some embodiments, the second amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the third amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is the same as the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the second amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the third amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is less than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the second amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the third amount of the nucleic acid molecule (e.g., circular polyribonucleotide) is greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, the fourth, fifth, sixth, seventh, eighth, ninth, tenth, or more amount of the nucleic acid molecule (e.g., circular LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 polyribonucleotide) is greater than the first amount of the nucleic acid molecule (e.g., circular polyribonucleotide). In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the second composition varies by no more than 1%, 5%, 10%, 15%, 20%, or 25% of an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the second composition is no more than 1%, 5%, 10%, 15%, 20%, or 25% less than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of the first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second composition is from 0.1-fold to 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second composition is 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent composition (e.g., a composition administered after a first composition) is 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold higher than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second composition is from 0.1-fold to 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a second composition is 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent composition (e.g., a composition administered after a first composition) is 0.1-fold, 1-fold, 5-fold, 10-fold, 100-fold, or 1000-fold lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a subsequent composition (e.g., after a first composition of an amount of nucleic acid molecule (e.g., circular polyribonucleotide)) is from 0.1-fold to 1000-fold higher or lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, an amount of the nucleic acid molecule (e.g., circular LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 polyribonucleotide) of a subsequent composition (e.g., after a first composition of an amount of nucleic acid molecule (e.g., circular polyribonucleotide)) is 0.1-fold, 1-fold, 5-fold, 10-fold, 100- fold, or 1000-fold higher or lower than an amount of the nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. For example, a first composition comprises 1-fold nucleic acid molecule (e.g., circular polyribonucleotide), a second composition comprises 5-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to the first composition, and a third composition comprises 0.2-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to the first composition. In some embodiments, the second composition comprises at least 5-fold nucleic acid molecule (e.g., circular polyribonucleotide) compared to an amount of nucleic acid molecule (e.g., circular polyribonucleotide) of a first composition. In some embodiments, the first composition comprises a higher amount of the nucleic acid molecule (e.g., circular polyribonucleotide) than the second composition. In some embodiments, the first composition comprises a higher amount of the nucleic acid molecules (e.g., circular polyribonucleotides) than the third, fourth, fifth, sixth, seventh, eighth, ninth, or tenth composition. In some embodiments, the plurality (e.g., two or more) of compositions of a nucleic acid molecule (e.g., circular polyribonucleotide) encoding a GLP-2 or GLP-2 analog, which are administered in a multiple dosing regimen as described herein, are the same compositions. In some embodiments, the plurality (e.g., two or more) of compositions of a nucleic acid molecule (e.g., circular polyribonucleotide) encoding a GLP-2 or GLP-2 analog, which are administered in a multiple dosing regimen as described herein, are different compositions. In some embodiments, the same compositions comprise the nucleic acid molecules (e.g., circular polyribonucleotides) encoding the same GLP-2 or GLP-2 analog. In some embodiments, the different compositions comprise the nucleic acid molecules (e.g., circular polyribonucleotides) encoding different a GLP-2 or GLP-2 analog, or a combination thereof. A composition of the nucleic acid molecule (e.g., a circular polyribonucleotide) disclosed herein can induce a response in a subject. In some embodiments, the response may be stimulated insulin secretion, altered metabolism, improved glucose homeostasis, reduced body weight, improved liver function, and/or amelioration of fibrosis. In some embodiments, in a multiple dosing regimen, the method of administering the nucleic acid molecule (e.g., circular polyribonucleotide) provided herein includes administering LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 to a subject in need thereof the nucleic acid molecule for multiple times (multiple doses), e.g., at least 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 30, 40, 50, 60, 100, 150, 200, or 500 times, with an interval of from 1 day to 56 days, such as about 49 days, 42 days, 35 days, 28 days, 21 days, 14 days, or 7 days. In some embodiments, in a multiple dosing regimen, the method provided herein includes administering to a subject in need thereof the nucleic acid molecule for at least 3 times, with an interval of about 7 days. In some embodiments, in a subject that receives administration of multiple doses of the nucleic acid molecule (e.g., at least 3, 4, 5, 6, 7, 8, or 9 doses) provided herein, a level of the GLP-2 or GLP-2 analog (e.g., a plasma GLP-2 or GLP-2 level) is maintained at a level with variation of less than 50%, 40%, 30%, 20%, or 10% for a period of longer than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, or 20 weeks after the last dose. In some embodiments, in a subject that receives administration of multiple doses of the nucleic acid molecule (e.g., at least 3, 4, 5, 6, 7, 8, or 9 doses) provided herein, a level of the GLP-2 or GLP-2 analog (e.g., a plasma GLP-2 or GLP-2 level) is maintained at a first level for a period of longer than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19, or 20 weeks after the second, third, fourth, fifth, sixth, seventh, eight, or the last dose, wherein the first level is higher than a level of the GLP-2 or GLP-2 analog measured shortly after the first dose (e.g., measured about 12, 24, 36, or 48 hours after the first dose). In some embodiments, in a subject that receives administration of multiple doses of the nucleic acid molecule (e.g., at least 3 doses) provided herein with an interval of about 7 days, a level of the GLP-2 or GLP-2 analog (e.g., a plasma GLP-2 or GLP-2 level) is maintained at a first level for a period of longer than 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20 weeks after the second, third, fourth, fifth, sixth, seventh, eight, or the last dose, wherein the first level is higher than a level of the GLP-2 or GLP-2 analog measured shortly after the first dose (e.g., measured about 12, 24, 36, or 48 hours after the first dose). As used herein, the term “treating” refers to the application or administration of a composition including one or more active agents to a subject who has a GLP-2-related disease (e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy- induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof), a symptom of a GLP-2-related disease, and/or an increased risk of developing a GLP-2-related disease, with LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 the purpose to cure, heal, alleviate, relieve, alter, remedy, ameliorate, improve, or affect the GLP-2-related disease, the symptom of the GLP-2 related disease, and/or the risk of developing a GLP-2-related disease. Alleviating a GLP-2-related disease includes delaying the development or progression of the disease, and/or reducing disease severity. Alleviating the disease does not necessarily require curative results. As used herein, “delaying” the development of a GLP-2-related disease (e.g., short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, intestinal ischemia–reperfusion injury, or a combination thereof) means to defer, hinder, slow, retard, stabilize, and/or postpone progression of the GLP-2 related disease. This delay can be of varying lengths of time, depending on the history of the GLP-2-related disease and/or individuals being treated. A method that “delays” or alleviates the development of a GLP-2-related disease and/or delays the onset of the GLP-2-related disease is a method that reduces probability of developing one or more symptoms of the GLP-2-related disease in a given time frame and/or reduces extent of the symptoms in a given time frame, when compared to not using the method. Such comparisons are typically based on clinical studies, using a number of subjects sufficient to give a statistically significant result. “Development” or “progression” of a disease means initial manifestations and/or ensuing progression of the GLP-2-related disease. Development of the GLP-2-related disease can be detectable and assessed using standard clinical techniques known in the art. However, development also refers to progression that may be undetectable. For purposes of this disclosure, development or progression refers to the biological course of the symptoms. “Development” includes occurrence, recurrence, and onset. As used herein, “onset” or “occurrence” of a GLP-2- related disease includes initial onset and/or recurrence. In some embodiments, any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein is administered to a subject in an amount sufficient to increase levels of GLP-2-mediated signaling by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more). LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 In some embodiments, any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein is administered to a subject in an amount sufficient to increase intestinal growth in a subject by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more). In some embodiments, increasing intestinal growth includes increasing intestinal tissue and/or cells. In some embodiments, the cells are crypt cells. In some embodiments, any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein is administered to a subject in an amount sufficient to increase intestinal absorption of fluids and nutrients in a subject by at least 5% (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or more). In some embodiments, methods for treating a GLP-2-related disease involve restoring, at least in part, expression and/or activity of GLP-2 using any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein. Any of the circular polyribonucleotides, modified RNAs, or pharmaceutical compositions described herein can be administered using any suitable method for achieving delivery of the circular polyribonucleotide or modified RNA to the subject in need thereof. The route of administration can depend on various factors such as the type of GLP-2-related disease to be treated and the site of the disease. In some embodiments, any of the circular polyribonucleotides, modified RNAs, or pharmaceutical composition described herein can be administered topically, locally, nasally, parenterally, buccally, or by inhalation. Parenteral administration includes, but is not limited to, subcutaneous, intracutaneous, intravenous, intramuscular, or intrasynovial injection, or infusion techniques. In some embodiments, any of the circular polyribonucleotides, modified RNAs, or pharmaceutical composition described herein is administered locally (e.g., intramuscular injection). In some embodiments, any of the circular polyribonucleotides, the modified RNAs, or the pharmaceutical composition described herein is administered systemically (e.g., intravenous infusion). In some embodiments, any of the circular polyribonucleotides, the modified RNAs, or the pharmaceutical compositions described herein is administered one or more times to the subject. Alternatively, or in addition, any of the circular polyribonucleotides, the modified RNAs, or the LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 pharmaceutical compositions described herein can be administered as part of a combination therapy with an additional therapeutic agent. Any therapeutic agent suitable for treating a GLP-2-related disease can be used as an additional therapeutic agent in methods and/or pharmaceutical compositions described herein. Non-limiting examples of additional therapeutic agents include anti-inflammatory agents (e.g., steroids, such as corticosteroids)), immunosuppressants (e.g., methotrexate, cyclosporine), or insulin. Alternatively, in some embodiments, no other agents are administered. IV. Kits The present disclosure also provides kits that can be used, e.g., in any of the methods described herein. Such kits include (a) (i) a composition comprising any of the circular polyribonucleotides described herein or any of the modified RNAs described herein or (ii) any of the pharmaceutical compositions described herein; and optionally, (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof. V. Nucleic Acids The present disclosure further provides nucleic acids (e.g., DNA or RNA) encoding any of the polypeptides described herein. In some embodiments, the nucleic acid encodes a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the GLP-2 polypeptides or analogs described herein), (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein), and (iii) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein). In some embodiments, the nucleic acid encodes a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP- 2 polypeptides or analogs described herein) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein). In some embodiments, the nucleic acid encodes a polypeptide comprising a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of exemplary the GLP-2 polypeptides or analogs described herein). VI. Polypeptides LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 The present disclosure also provides polypeptides generated from any of the circular polyribonucleotides, any of the modified RNAs, and any of the nucleic acids described herein. In some embodiments, the polypeptide comprises (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein), (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein), and (iii) a secretion signal sequence (e.g., any of the exemplary secretion signal sequences described herein). In some embodiments, the polypeptide comprises (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein) and (ii) a half-life extension moiety (e.g., any of the exemplary half-life extension moieties described herein). In some embodiments, the polypeptide comprises a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof (e.g., any of the exemplary GLP-2 polypeptides or analogs described herein). In some embodiments, the polypeptides can include an IgG constant domain (e.g., IgG1, IgG2, IgG3, IgG4), a serum protein or fragment thereof (e.g., albumin or fragment thereof (e.g., human serum albumin or a fragment thereof), fibronectin or fragment thereof (e.g., human fibronectin or a fragment thereof), or transferrin or fragment thereof (e.g., human transferrin or a fragment thereof)), and an antibody or an antigen-binding fragment thereof that binds to a serum protein (e.g., an antibody or antigen-binding fragment thereof that binds to human albumin, human fibronectin, or human transferrin). In some embodiments, the IgG constant domain includes a half-life extension substitution (e.g., any of the half-life extension substitutions described herein). EXAMPLES In order that the disclosure described may be more fully understood, the following examples are set forth. The examples described in this application are offered to illustrate the methods provided herein and are not to be construed in any way as limiting their scope. Example 1: GLP-2 Analog Expressed from Circular RNA is Less Active Than Recombinant GLP-2 Analog The activity of the GLP-2 analog teduglutide expressed from a circular RNA construct was tested in mammalian cells. The circular RNA construct included teduglutide linked to IgG4 LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 YTE via a (GGGGS)3 (SEQ ID NO: 141) linker (also referred to as teduglutide-Fc). Recombinant teduglutide was used as a control. The circular RNA construct was transfected into HeLa cells using Lipofectamine™ MessengerMAX™ (Invitrogen LMRNA001) according to the manufacturer’s instructions. Activity was measured using a G protein-coupled receptor (GPCR) assay in which cyclic adenosine monophosphate (cAMP) production were measured with a fluorescently labelled cAMP probe. As shown in FIG.1, teduglutide-Fc expressed from circular RNA showed approximately 3-fold reduced activity level as compared to recombinant teduglutide in a GPCR assay. These results demonstrate that a functionally active GLP-2 analog can be expressed from circular RNA. Example 2: Expression of Functionally Active GLP-2 Analog from Circular RNA in Mammalian Cells The expression of the GLP-2 analog teduglutide from various circular RNA constructs (Table 8) was tested in mammalian cells (HEK293T cells). Circular RNA constructs were transfected into HeLa cells using Lipofectamine™ MessengerMAX™ (Invitrogen LMRNA001) according to the manufacturer’s instructions. Expression was measured using RT-qPCR at 3 days post-transfection using the Luna® Universal One-Step RT-qPCR kit following manufacturer’s instructions. Activity was measured using a GPCR assay in which cAMP production was measured with a fluorescently labelled cAMP probe. Table 8: Circular RNA constructs Construct Name Description 2860_0 IL2_K274(Shire GLP2 Fc)_G4S_HiBit 3539 0 IL2 K274(Shir GLP2 F ) G4S HiBit LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 linker; G4S: linker sequence mutation As shown in FIG.2A, expression levels of teduglutide from circular RNA constructs was similar to the expression level of teduglutide from mRNA. As shown in FIG. 2B, teduglutide expressed from the circular RNA constructs described in Table 9 showed robust activity in a GPCR assay. Table 9: Circular RNA constructs Construct Polypeptide IRES C-Terminal CE 5’ Spacer 3’ Spacer K274(Shire Modified ne poly(adenosine-uridine) spacer sequence having a length of 120 nucleotides; CE: circularization element; Anabaena2.3: A specific intron based circularization element; Anabaena2: A specific intron based circularizationelement. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Taken together, these results demonstrate that functionally active teduglutide can be expressed from various circular RNA constructs. Example 3: Pharmacokinetic (PK) Profiles of GLP-2 Fc Expressed from Circular RNA Compared to Recombinant GLP-2 Analogs The PK profile of teduglutide-Fc expressed from circular RNA encoding teduglutide-Fc was evaluated in mice. Circular RNA encoding teduglutide-Fc (1.5 mg/kg) was injected into mice via subcutaneous injection in the interscapular area. Blood was collected from the tail-vein of each mouse. Serum was collected from blood and the concentration and pharmacokinetic (PK) parameters for teduglutide-Fc were determined. The PK profile of teduglutide-Fc expressed from circular RNA in mice was then compared to the reported PK profiles of various recombinant GLP-2 analogs. Reported PK profiles were obtained from Hargrove et al., “Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome,” J Pharmacol Exp Ther;373(2):193-203 (2020) and Choi et al., “HM15912, a Novel Long-acting Glucagon-like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome,” J Pharmacol Exp Ther; JPET-AR- 2022-001381 (2022). As shown in FIG. 3 and Table 10, higher levels of teduglutide-Fc were detected in mice injected with circular RNA encoding teduglutide-Fc compared to reported levels of teduglutide and HM15912 in rats. The Cmax (µg/mL) of teduglutide-Fc was approximately 10-fold higher in mice injected with circular RNA encoding teduglutide-Fc compared to rats injected with recombinant teduglutide. Table 10: PK parameters for mice injected with circular RNA encoding GLP-2 Fc or recombinant GLP-2 analog GLP-2 Fc Analog Dose Species* Cmax Tmax Terminal t1/2 (mg/kg) (g/mL) (hr) (hr) 3539 1.5 CD1 mouse 30.5 24 60 LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Thus, these results demonstrate that expression of teduglutide-Fc from circular RNA can achieve a preferred PK profile compared to recombinant teduglutide. Example 4: Characterization of Lipid Nanoparticle (LNP) Formulations Various LNP formulations were tested for tolerability and expression of teduglutide-Fc. Circular RNA encoding teduglutide-Fc (4133_3) was formulated in different LNPs. LNP formulations (0.75 mg/kg) were injected into mice. Negative control mice were injected with PBS. Blood was collected from the tail-vein at 4 hours, 6 hours, and 24 hours after injection. Expression of teduglutide-Fc at 6 and 24 hours (FIG. 4A), complete blood cell count (CBC) at 24 hours (FIGs. 4B-4C), ALT levels at 24 hours (FIG. 4D), IFNα levels at 4 hours (FIG. 4E), and cytokine levels at 24 hours (FIG. 4F) were determined. Example 5: Dose Response in Mice Circular RNA (7297) and mRNA containing the modified nucleoside N1- methylpseudoiridine (m1ψ) (7377) encoding teduglutide-Fc was formulated into LNPs (LRN28). A dose response of the LNP formulations of teduglutide-Fc was evaluated in either WT or NSG (NOD-scid IL2Rgnull) mice. Circular RNA in LNPs encoding teduglutide-Fc (1, 0.3, 0.1, or 0.03 mg/kg) in WT and NSG mice and m1ψ mRNA in LNP encoding teduglutide-Fc (0.3 mg/kg) in NSG mice only were injected via subcutaneous injection in the interscapular area. Blood was collected from the tail-vein of each mouse at baseline and 6h, D1, D2, D10, D17, and D28 after subcutaneous administration. Expression of GLP2-Fc (FIGs. 5A-B), cytokine levels (FIGs. 5C- F), and anti-target antibody(ATA) response (FIG. 5G) were measured at various timepoints. Example 6: Characterization of GLP-2 Fc Expressed from Circular RNA in Non-Human Primates Circular RNA (7297) and mRNA containing the modified nucleoside N1- methylpseudoiridine (m1ψ) (7377) encoding teduglutide-Fc was formulated into LNPs (LRN28). The circular RNA formulations had 2 purification levels: pre-Reverse Phase (pre-RP) or reverse phase (RP). The LNP formulations of teduglutide-Fc were intravenously dosed in non-human primates (NHPs) at 0.5 mg/kg with or without steroids, as shown in Table 11. The steroid, if given, was dexamethasone intramuscularly administered at 1mpk 24h and 1h pre-dose and 24h LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 post-dose. Blood was collected at baseline (D -14), post-infusion, 6hr, 24h, 48h, 72h, D5, D7, D14, D21, and D28. Teduglutide-Fc expression (FIG. 6A), antibody production (FIG. 6B), complete blood count, including lymphocyte count (FIG. 6C), cytokine responses (FIG. 6D), CRP levels (FIG. 6E), and activate partial thromboplastin (FIG. 6F) were measured. Blood counts and safety levels measured throughout were comparable. Table 11: Experimental Design D OTHER EMBODIMENTS It is to be understood that while the invention has been described in conjunction with the detailed description thereof, the foregoing description is intended to illustrate and not limit the scope of the invention, which is defined by the scope of the appended claims. Other aspects, advantages, and modifications are within the scope of the following claims. Embodiment 1 is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence. Embodiment 2 is the circular polyribonucleotide of embodiment 1, wherein the GLP-2 polypeptide or analog thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Embodiment 3 is the circular polyribonucleotide of embodiment 1 or 2, wherein the GLP- 2 polypeptide or analog thereof comprises an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. Embodiment 4 is the circular polyribonucleotide of any one of embodiments 1-3, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. Embodiment 5 is the circular polyribonucleotide of any one of embodiments 1-4, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. Embodiment 6 is the circular polyribonucleotide of any one of embodiments 1-5, wherein the GLP-2 analog comprises teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681. Embodiment 7 is the circular polyribonucleotide of any one of embodiments 1-6, wherein the half-life extension moiety comprises a peptide moiety. Embodiment 8 is the circular polyribonucleotide of embodiment 7, wherein the peptide moiety comprises an Fc domain, an albumin polypeptide, a nanobody, or a post-translational modification site. Embodiment 9 is the circular polyribonucleotide of embodiment 8, wherein the Fc domain comprises an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain. Embodiment 10 is the circular polyribonucleotide of embodiment 8 or 9, wherein the Fc domain comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identical to SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). Embodiment 11 is the circular polyribonucleotide of any one of embodiments 8-10, wherein the Fc domain comprises an amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). Embodiment 12 the circular polyribonucleotide of embodiment 11, wherein the IgG4 Fc domain comprises an amino acid sequence of SEQ ID NO: 21. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Embodiment 13 is the circular polyribonucleotide of any one of embodiments 8-12, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain). Embodiment 14 is the circular polyribonucleotide of embodiment 13, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain). Embodiment 15 is the circular polyribonucleotide of any one of embodiments 8-14, wherein the Fc domain comprises a mutation. Embodiment 16 is the circular polyribonucleotide of any one of embodiments 8-10, 13, and 15, wherein the Fc domain comprises the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain), except the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain) comprises an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L284A/L285A; M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL), and V308P. Embodiment 17 is the circular polyribonucleotide of embodiment 8, wherein the albumin polypeptide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23. Embodiment 18 is the circular polyribonucleotide of embodiment 8, wherein the peptide moiety comprises a nanobody that binds to albumin. Embodiment 19 is the circular polyribonucleotide of any one of embodiments 1-18, wherein the circular polyribonucleotide comprises one or more modifications. Embodiment 20 is the circular polyribonucleotide of embodiment 19, wherein the one or more modifications comprise one or more modifications to a portion of the sequence encoding the GLP-2 polypeptide or analog thereof. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Embodiment 21 is the circular polyribonucleotide of embodiment 19 or embodiment 20, wherein the one or more modifications comprise a modification to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof. Embodiment 22 is the circular polyribonucleotide of any one of embodiments 1-21, wherein the circular polyribonucleotide further comprises a translation initiation sequence operably linked to the sequence encoding the polypeptide. Embodiment 23 is the circular polyribonucleotide of any one of embodiments 1-22, wherein the circular polyribonucleotide further comprises an internal ribosome entry site (IRES) operatively linked to the sequence encoding the polypeptide. Embodiment 24 is the circular polyribonucleotide of any one of embodiments 1-23, wherein the circular polyribonucleotide further comprises a translation termination sequence. Embodiment 2 is the circular polyribonucleotide of any one of embodiments 1-24, wherein the circular polyribonucleotide lacks a translation termination sequence. Embodiment 26 is the circular polyribonucleotide of any one of embodiments 1-25, wherein the circular polyribonucleotide further comprises a stagger element at a 3’ end of the sequence encoding the polypeptide. Embodiment 27 is the circular polyribonucleotide of embodiment 26, wherein the stagger element is configured to stall a ribosome during rolling circle translation. Embodiment 28 is the circular polyribonucleotide of any one of embodiments 1-27, wherein the circular polyribonucleotide further comprises a replication element. Embodiment 29 is the circular polyribonucleotide of any one of embodiments 1-28, wherein the circular polyribonucleotide further comprises a 5’ untranslated region operably linked to the sequence encoding the polypeptide. Embodiment 30 is the circular polyribonucleotide of any one of embodiments 1-29, wherein the circular polyribonucleotide does not comprise a poly(A) sequence operably linked to the sequence encoding the polypeptide. Embodiment 31 is the circular polyribonucleotide of any one of embodiments 1-30, wherein the circular polyribonucleotide does not comprise a 5’ untranslated region operably linked to the sequence encoding the polypeptide. Embodiment 32 is the circular polyribonucleotide of any one of embodiments 1-31, wherein the secretion signal sequence is a liver-specific secretion signal sequence, wherein the LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 liver-specific secretion signal comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to any one of SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91, SEQ ID NO: 93, SEQ ID NO: 95, SEQ ID NO: 97, SEQ ID NO: 99, SEQ ID NO: 101, SEQ ID NO: 103, SEQ ID NO: 105, SEQ ID NO: 107, SEQ ID NO: 109, SEQ ID NO: 111, SEQ ID NO: 113, SEQ ID NO: 115, SEQ ID NO: 117, SEQ ID NO: 119, SEQ ID NO: 121, SEQ ID NO: 123, SEQ ID NO: 125, SEQ ID NO: 127, SEQ ID NO: 129, SEQ ID NO: 131, SEQ ID NO: 133, SEQ ID NO: 135, SEQ ID NO: 137, or SEQ ID NO: 139. Embodiment 33 is the circular polyribonucleotide of any one of embodiments 1-31, wherein the secretion signal sequence comprises an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia luciferase secretion signal sequence, or a erythropoietin (EPO) secretion signal sequence. Embodiment 34 is the circular polyribonucleotide of embodiment 33, wherein the IL-2 secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 29. Embodiment 35 is the circular polyribonucleotide of embodiment 33, wherein the proglucagon secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 31. Embodiment 36 is the circular polyribonucleotide of embodiment 33, wherein the Gaussia luciferase secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 33. Embodiment 37 is the circular polyribonucleotide of embodiment 33, wherein the EPO secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 35. Embodiment 38 is the circular polyribonucleotide of embodiment 33, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 90%, 95%, 99%, or 100% identical to one of SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, or SEQ ID NO: 39. Embodiment 39 is the circular polyribonucleotide of any one of embodiments 1-38, wherein the circular polyribonucleotide further comprises at least one spacer sequence. Embodiment 40 is the circular polyribonucleotide of embodiment 39, wherein the polypeptide comprises a protease cleavage site and the at least one spacer sequence encodes the protease cleavage site. Embodiment 41 is the circular polyribonucleotide of embodiment 40, wherein the protease cleavage site is positioned between (a) the secretion signal and (b) the GLP-2 polypeptide or analog thereof and the half-life extension moiety. Embodiment 42 is the circular polyribonucleotide of any one of embodiments 1-41, wherein the polypeptide further comprises at least one linker sequence. Embodiment 43 is the circular polyribonucleotide of embodiment 42, wherein the at least one linker sequence encodes an amino acid sequence selected from (GS)x, (GGS)x, (GGGGS)x (SEQ ID NO: 141), (GGSG)x (SEQ ID NO: 142), and (SGGG)x (SEQ ID NO: 143), wherein x is an integer from 1 to 50. Embodiment 44 is the circular polyribonucleotide of embodiment 42 or 43, wherein the at least one linker sequence is disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety. Embodiment 45 is the circular polyribonucleotide of any one of embodiments 1-44, wherein the circular polyribonucleotide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. Embodiment 46 is the circular polyribonucleotide of any one of embodiments 1-45, wherein the circular polyribonucleotide comprises an amino acid sequence of SEQ ID NO: 1. Embodiment 47 is the circular polyribonucleotide of any one of embodiments 1-46, wherein the circular polyribonucleotide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. Embodiment 48 is the circular polyribonucleotide of any one of embodiments 1-47, wherein the circular polyribonucleotide comprises a nucleic acid sequence of SEQ ID NO: 2. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Embodiment 49 is a pharmaceutical composition comprising the circular polyribonucleotide of any one of embodiments 1-48, and a pharmaceutically acceptable excipient. Embodiment 50 is the pharmaceutical composition of embodiment 49, wherein the circular polyribonucleotide is formulated as a lipid nanoparticle. Embodiment 51 is the pharmaceutical composition of embodiment 49, wherein the circular polyribonucleotide is formulated for unencapsulated delivery or natural particle delivery. Embodiment 52 is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for intravenous, subcutaneous, intrahepatic, or intramuscular administration. Embodiment 53 is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for local administration. Embodiment 54 is the pharmaceutical composition of any one of embodiments 49-51, wherein the pharmaceutical composition is formulated for systemic administration. Embodiment 55 is the pharmaceutical composition of any one of embodiments 49-54, wherein the pharmaceutical composition comprises one or more of the following features: (i) a concentration of the circular polyribonucleotide of between 0.1 ng/mL and 500 mg/mL; (ii) a concentration of deoxyribonucleotide of no more than 100 ng/mL; (iii) a concentration of protein of less than 100 ng of protein per milligram (mg) of the circular polyribonucleotide; (iv) an A260/A280 absorbance ratio of from about 1.6 to 2.3 as measured by a spectrophotometer; (v) substantially free of a process-related impurity selected from a cell protein, a cell deoxyribonucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material; and (vi) a reduced level of one or more markers of an immune or inflammatory response after purification compared to prior to purification. Embodiment 56 is a method of treating a glucagon-like peptide-2 (GLP-2)-related disease in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55. Embodiment 57 is the method of embodiment 56, wherein the GLP-2-related disease is selected from the group consisting of: short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. Embodiment 58 is a method of increasing intestinal growth in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55. Embodiment 59 is the method of embodiment 59, wherein increasing intestinal growth comprises increasing crypt cell growth. Embodiment 60 is a method of increasing intestinal absorption of fluids and nutrients in a subject in need thereof comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55. Embodiment 61 is the method of any one of embodiments 58-60, wherein the subject has short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. Embodiment 62 is the method of any one of embodiments 56-61, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered one or more times to the subject. Embodiment 63 is the method of any one of embodiments 56-62, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered locally. Embodiment 64 is the method of any one of embodiments 56-62, wherein the circular polyribonucleotide of embodiments 1-48 or the pharmaceutical composition of any one of embodiments 49-55 is administered systemically. Embodiment 65 is the method of any one of embodiments 56-64, further comprising administering one or more additional therapeutic agents to the subject. Embodiment 66 is the method of any one of embodiments 56-65, wherein the subject is a human subject. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 Embodiment 67 is a kit comprising: (a) (i) a composition comprising the circular polyribonucleotide of any one of embodiments 1-48 or (ii) the pharmaceutical composition of any one of embodiments 49-55; and (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof. Embodiment 68 is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety. Embodiment 69 is a circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half-life extension moiety, wherein the half-life extension moiety is an Fc domain comprising a M252Y/S254T/T256E (YTE) mutation. Embodiment 70 is a modified linear RNA comprising a sequence encoding a polypeptide comprising a glucagon-like peptide-2 (GLP-2) or analog thereof. Embodiment 71 is a modified linear RNA comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence. Embodiment 72 is the modified linear RNA of embodiment 70 or 71, further comprising a half-life extension moiety.
Claims
LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 WHAT IS CLAIMED IS: 1. A circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, (ii) a half-life extension moiety, and (iii) a secretion signal sequence. 2. The circular polyribonucleotide of claim 1, wherein the GLP-2 polypeptide or analog thereof comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. 3. The circular polyribonucleotide of claim 1 or 2, wherein the GLP-2 polypeptide or analog thereof comprises an amino acid sequence of any one of SEQ ID NO: 3, SEQ ID NO: 5, SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. 4. The circular polyribonucleotide of any one of claims 1-3, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. 5. The circular polyribonucleotide of any one of claims 1-4, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of any one of SEQ ID NO: 4, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, or SEQ ID NO: 14. 6. The circular polyribonucleotide of any one of claims 1-5, wherein the GLP-2 analog comprises teduglutide, glepaglutide, dapiglutide, apraglutide, HM15912, and SHP-681. 7. The circular polyribonucleotide of any one of claims 1-6, wherein the half-life extension moiety comprises a peptide moiety. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 8. The circular polyribonucleotide of claim 7, wherein the peptide moiety comprises an Fc domain, an albumin polypeptide, a nanobody, or a post-translational modification site. 9. The circular polyribonucleotide of claim 8, wherein the Fc domain comprises an IgG1 Fc domain, an IgG2 Fc domain, an IgG3 Fc domain, or an IgG4 Fc domain. 10. The circular polyribonucleotide of claim 8 or 9, wherein the Fc domain comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identical to SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). 11. The circular polyribonucleotide of any one of claims 8-10, wherein the Fc domain comprises an amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain). 12. The circular polyribonucleotide of claim 11, wherein the IgG4 Fc domain comprises an amino acid sequence of SEQ ID NO: 21. 13. The circular polyribonucleotide of any one of claims 8-12, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain). 14. The circular polyribonucleotide of claim 13, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence of SEQ ID NO: 16 (IgG1 Fc domain), SEQ ID NO: 18 (IgG2 Fc domain), SEQ ID NO: 20 (IgG3 Fc domain), or SEQ ID NO: 22 (IgG4 Fc domain). 15. The circular polyribonucleotide of any one of claims 8-14, wherein the Fc domain comprises a mutation. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 16. The circular polyribonucleotide of any one of claims 8-10, 13, and 15, wherein the Fc domain comprises the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain), except the amino acid sequence of SEQ ID NO: 15 (IgG1 Fc domain), SEQ ID NO: 17 (IgG2 Fc domain), SEQ ID NO: 19 (IgG3 Fc domain), or SEQ ID NO: 21 (IgG4 Fc domain) comprises an amino acid substitution selected from one or more of: M252Y/S254T/T256E (YTE); L284A/L285A; M428L/N434S (LS); T307A/E380A/N434A (AAA); T250Q/M428L (QL); and V308P. 17. The circular polyribonucleotide of claim 8, wherein the albumin polypeptide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 23. 18. The circular polyribonucleotide of claim 8, wherein the peptide moiety comprises a nanobody that binds to albumin. 19. The circular polyribonucleotide of any one of claims 1-18, wherein the circular polyribonucleotide comprises one or more modifications. 20. The circular polyribonucleotide of claim 19, wherein the one or more modifications comprise one or more modifications to a portion of the sequence encoding the GLP-2 polypeptide or analog thereof. 21. The circular polyribonucleotide of claim 19 or claim 20, wherein the one or more modifications comprise a modification to a sugar, a nucleobase, an internucleoside linkage, or a combination thereof. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 22. The circular polyribonucleotide of any one of claims 1-21, wherein the circular polyribonucleotide further comprises a translation initiation sequence operably linked to the sequence encoding the polypeptide. 23. The circular polyribonucleotide of any one of claims 1-22, wherein the circular polyribonucleotide further comprises an internal ribosome entry site (IRES) operatively linked to the sequence encoding the polypeptide. 24. The circular polyribonucleotide of any one of claims 1-23, wherein the circular polyribonucleotide further comprises a translation termination sequence. 25. The circular polyribonucleotide of any one of claims 1-24, wherein the circular polyribonucleotide lacks a translation termination sequence. 26. The circular polyribonucleotide of any one of claims 1-25, wherein the circular polyribonucleotide further comprises a stagger element at a 3’ end of the sequence encoding the polypeptide. 27. The circular polyribonucleotide of claim 26, wherein the stagger element is configured to stall a ribosome during rolling circle translation. 28. The circular polyribonucleotide of any one of claims 1-27, wherein the circular polyribonucleotide further comprises a replication element. 29. The circular polyribonucleotide of any one of claims 1-28, wherein the circular polyribonucleotide further comprises a 5’ untranslated region operably linked to the sequence encoding the polypeptide. 30. The circular polyribonucleotide of any one of claims 1-29, wherein the circular polyribonucleotide does not comprise a poly(A) sequence operably linked to the sequence encoding the polypeptide. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 31. The circular polyribonucleotide of any one of claims 1-30, wherein the circular polyribonucleotide does not comprise a 5’ untranslated region operably linked to the sequence encoding the polypeptide. 32. The circular polyribonucleotide of any one of claims 1-31, wherein the secretion signal sequence is a liver-specific secretion signal sequence, wherein the liver-specific secretion signal comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to any one of SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO: 43, SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55, SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, SEQ ID NO: 77, SEQ ID NO: 79, SEQ ID NO: 81, SEQ ID NO: 83, SEQ ID NO: 85, SEQ ID NO: 87, SEQ ID NO: 89, SEQ ID NO: 91, SEQ ID NO: 93, SEQ ID NO: 95, SEQ ID NO: 97, SEQ ID NO: 99, SEQ ID NO: 101, SEQ ID NO: 103, SEQ ID NO: 105, SEQ ID NO: 107, SEQ ID NO: 109, SEQ ID NO: 111, SEQ ID NO: 113, SEQ ID NO: 115, SEQ ID NO: 117, SEQ ID NO: 119, SEQ ID NO: 121, SEQ ID NO: 123, SEQ ID NO: 125, SEQ ID NO: 127, SEQ ID NO: 129, SEQ ID NO: 131, SEQ ID NO: 133, SEQ ID NO: 135, SEQ ID NO: 137, or SEQ ID NO: 139. 33. The circular polyribonucleotide of any one of claims 1-31, wherein the secretion signal sequence comprises an IL-2 secretion signal sequence, a proglucagon secretion signal sequence, a Gaussia luciferase secretion signal sequence, or a erythropoietin (EPO) secretion signal sequence. 34. The circular polyribonucleotide of claim 33, wherein the IL-2 secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 29. 35. The circular polyribonucleotide of claim 33, wherein the proglucagon secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 31. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 36. The circular polyribonucleotide of claim 33, wherein the Gaussia luciferase secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 33. 37. The circular polyribonucleotide of claim 33, wherein the EPO secretion signal sequence comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to SEQ ID NO: 35. 38. The circular polyribonucleotide of claim 33, wherein the sequence encoding the polypeptide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 99%, or 100% identical to one of SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, or SEQ ID NO: 39. 39. The circular polyribonucleotide of any one of claims 1-38, wherein the circular polyribonucleotide further comprises at least one spacer sequence. 40. The circular polyribonucleotide of claim 39, wherein the polypeptide comprises a protease cleavage site and the at least one spacer sequence encodes the protease cleavage site. 41. The circular polyribonucleotide of claim 40, wherein the protease cleavage site is positioned between (a) the secretion signal and (b) the GLP-2 polypeptide or analog thereof and the half-life extension moiety. 42. The circular polyribonucleotide of any one of claims 1-41, wherein the polypeptide further comprises at least one linker sequence. 43. The circular polyribonucleotide of claim 42, wherein the at least one linker sequence encodes an amino acid sequence selected from (GS)x, (GGS)x, (GGGGS)x (SEQ ID NO: 141), (GGSG)x (SEQ ID NO: 142), and (SGGG)x (SEQ ID NO: 143), wherein x is an integer from 1 to 50. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 44. The circular polyribonucleotide of claim 42 or 43, wherein the at least one linker sequence is disposed between the GLP-2 polypeptide or analog thereof and the half-life extension moiety. 45. The circular polyribonucleotide of any one of claims 1-44, wherein the circular polyribonucleotide comprises an amino acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 1. 46. The circular polyribonucleotide of any one of claims 1-45, wherein the circular polyribonucleotide comprises an amino acid sequence of SEQ ID NO: 1. 47. The circular polyribonucleotide of any one of claims 1-46, wherein the circular polyribonucleotide comprises a nucleic acid sequence that is at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO: 2. 48. The circular polyribonucleotide of any one of claims 1-47, wherein the circular polyribonucleotide comprises a nucleic acid sequence of SEQ ID NO: 2. 49. A pharmaceutical composition comprising the circular polyribonucleotide of any one of claims 1-48, and a pharmaceutically acceptable excipient. 50. The pharmaceutical composition of claim 49, wherein the circular polyribonucleotide is formulated as a lipid nanoparticle. 51. The pharmaceutical composition of claim 49, wherein the circular polyribonucleotide is formulated for unencapsulated delivery or natural particle delivery. 52. The pharmaceutical composition of any one of claims 49-51, wherein the pharmaceutical composition is formulated for intravenous, subcutaneous, intrahepatic, or intramuscular administration. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 53. The pharmaceutical composition of any one of claims 49-51, wherein the pharmaceutical composition is formulated for local administration. 54. The pharmaceutical composition of any one of claims 49-51, wherein the pharmaceutical composition is formulated for systemic administration. 55. The pharmaceutical composition of any one of claims 49-54, wherein the pharmaceutical composition comprises one or more of the following features: (i) a concentration of the circular polyribonucleotide of between 0.1 ng/mL and 500 mg/mL; (ii) a concentration of deoxyribonucleotide of no more than 100 ng/mL; (iii) a concentration of protein of less than 100 ng of protein per milligram (mg) of the circular polyribonucleotide; (iv) an A260/A280 absorbance ratio of from about 1.6 to 2.3 as measured by a spectrophotometer; (v) substantially free of a process-related impurity selected from a cell protein, a cell deoxyribonucleic acid, an enzyme, a reagent component, a gel component, or a chromatographic material; and (vi) a reduced level of one or more markers of an immune or inflammatory response after purification compared to prior to purification. 56. A method of treating a glucagon-like peptide-2 (GLP-2)-related disease in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of claims 1-48 or the pharmaceutical composition of any one of claims 49-55. 57. The method of claim 56, wherein the GLP-2-related disease is selected from the group consisting of: short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 58. A method of increasing intestinal growth in a subject in need thereof, the method comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of claims 1-48 or the pharmaceutical composition of any one of claims 49-55. 59. The method of claim 58, wherein increasing intestinal growth comprises increasing crypt cell growth. 60. A method of increasing intestinal absorption of fluids and nutrients in a subject in need thereof comprising administering to the subject an effective amount of a circular polyribonucleotide of any one of claims 1-48 or the pharmaceutical composition of any one of claims 49-55. 61. The method of any one of claims 58-60, wherein the subject has short bowel syndrome (SBS), short bowel syndrome with intestinal failure (SBS-IF), pediatric SBS, adult SBS, major small bowel resection, total parenteral nutrition (TPN)-induced intestinal hypoplasia, small and large intestinal inflammation, Crohn’s disease, chemotherapy-induced mucositis, and intestinal ischemia–reperfusion injury. 62. The method of any one of claims 56-61, wherein the circular polyribonucleotide of claims 1-48 or the pharmaceutical composition of any one of claims 49-55 is administered one or more times to the subject. 63. The method of any one of claims 56-62, wherein the circular polyribonucleotide of claims 1-48 or the pharmaceutical composition of any one of claims 49-55 is administered locally. 64. The method of any one of claims 56-62, wherein the circular polyribonucleotide of claims 1-48 or the pharmaceutical composition of any one of claims 49-55 is administered systemically. LRN Ref. No.: LRN23-107WO Fish Ref. No.: 56929-0005WO1 65. The method of any one of claims 56-64, further comprising administering one or more additional therapeutic agents to the subject. 66. The method of any one of claims 56-65, wherein the subject is a human subject. 67. A kit comprising: (a) (i) a composition comprising the circular polyribonucleotide of any one of claims 1- 48 or (ii) the pharmaceutical composition of any one of claims 49-55; and (b) instructions for administering the composition or the pharmaceutical composition to a subject in need thereof. 68. A circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half- life extension moiety. 69. A circular polyribonucleotide comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a half- life extension moiety, wherein the half-life extension moiety is an Fc domain comprising a M252Y/S254T/T256E (YTE) mutation. 70. A modified linear RNA comprising a sequence encoding a polypeptide comprising a glucagon-like peptide-2 (GLP-2) or analog thereof. 71. A modified linear RNA comprising a sequence encoding a polypeptide comprising (i) a glucagon-like peptide-2 (GLP-2) polypeptide or analog thereof, and (ii) a secretion signal sequence. 72. The modified linear RNA of claim 70 or 71, further comprising a half-life extension moiety.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363600527P | 2023-11-17 | 2023-11-17 | |
| US63/600,527 | 2023-11-17 | ||
| US202363614264P | 2023-12-22 | 2023-12-22 | |
| US63/614,264 | 2023-12-22 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2025106930A1true WO2025106930A1 (en) | 2025-05-22 |
Family
ID=93742939
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/056294PendingWO2025106930A1 (en) | 2023-11-17 | 2024-11-15 | Circular polyribonucleotides encoding glucagon-like peptide 2 (glp-2) and uses thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2025106930A1 (en) |
Citations (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992001813A1 (en) | 1990-07-25 | 1992-02-06 | Syngene, Inc. | Circular extension for generating multiple nucleic acid complements |
| US5426180A (en) | 1991-03-27 | 1995-06-20 | Research Corporation Technologies, Inc. | Methods of making single-stranded circular oligonucleotides |
| US5712128A (en) | 1992-01-13 | 1998-01-27 | Duke University | Enzymatic RNA molecules |
| US5766903A (en) | 1995-08-23 | 1998-06-16 | University Technology Corporation | Circular RNA and uses thereof |
| US5773244A (en) | 1993-05-19 | 1998-06-30 | Regents Of The University Of California | Methods of making circular RNA |
| US6210931B1 (en) | 1998-11-30 | 2001-04-03 | The United States Of America As Represented By The Secretary Of Agriculture | Ribozyme-mediated synthesis of circular RNA |
| US20030082768A1 (en) | 1998-04-17 | 2003-05-01 | Whitehead Institute For Biomedical Research | Use of a ribozyme to join nucleic acids and peptides |
| US20100137407A1 (en) | 2007-05-09 | 2010-06-03 | Riken | Single-chain circular rna and method of producing the same |
| WO2010084371A1 (en) | 2009-01-26 | 2010-07-29 | Mitoprod | Novel circular interfering rna molecules |
| WO2011097480A1 (en) | 2010-02-05 | 2011-08-11 | University Of Louisville Research Foundation, Inc. | Exosomal compositions and methods for the treatment of disease |
| US8349569B2 (en) | 2009-03-31 | 2013-01-08 | Takara Bio Inc. | Anti-fibronectin fragment monoclonal antibody |
| WO2013063468A1 (en) | 2011-10-27 | 2013-05-02 | Massachusetts Institute Of Technology | Amino acid derivates functionalized on the n- terminal capable of forming drug incapsulating microspheres |
| WO2013070324A1 (en) | 2011-11-07 | 2013-05-16 | University Of Louisville Research Foundation, Inc. | Edible plant-derived microvesicle compositions for diagnosis and treatment of disease |
| WO2013128027A1 (en) | 2012-03-01 | 2013-09-06 | Amgen Research (Munich) Gmbh | Long life polypeptide binding molecules |
| WO2015095340A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Lipids and lipid compositions for the delivery of active agents |
| WO2015184256A2 (en) | 2014-05-30 | 2015-12-03 | Shire Human Genetic Therapies, Inc. | Biodegradable lipids for delivery of nucleic acids |
| WO2015199952A1 (en) | 2014-06-25 | 2015-12-30 | Acuitas Therapeutics Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2016004202A1 (en) | 2014-07-02 | 2016-01-07 | Massachusetts Institute Of Technology | Polyamine-fatty acid derived lipidoids and uses thereof |
| US9376495B2 (en) | 2011-05-21 | 2016-06-28 | Macrogenics, Inc. | Deimmunized serum-binding domains and their use in extending serum half-life |
| WO2016118725A1 (en) | 2015-01-23 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016118724A1 (en) | 2015-01-21 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016187531A1 (en) | 2015-05-21 | 2016-11-24 | Ohio State Innovation Foundation | Benzene-1,3,5-tricarboxamide derivatives and uses thereof |
| WO2016205691A1 (en) | 2015-06-19 | 2016-12-22 | Massachusetts Institute Of Technology | Alkenyl substituted 2,5-piperazinediones and their use in compositions for delivering an agent to a subject or cell |
| WO2017004143A1 (en) | 2015-06-29 | 2017-01-05 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017004526A1 (en) | 2015-07-02 | 2017-01-05 | University Of Louisville Research Foundation, Inc. | EDIBLE PLANT-DERIVED MICROVESICLE COMPOSITIONS FOR DELIVERY OF miRNA AND METHODS FOR TREATMENT OF CANCER |
| WO2017049245A2 (en) | 2015-09-17 | 2017-03-23 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of therapeutic agents |
| WO2017075531A1 (en) | 2015-10-28 | 2017-05-04 | Acuitas Therapeutics, Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017117528A1 (en) | 2015-12-30 | 2017-07-06 | Acuitas Therapeutics, Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017173054A1 (en) | 2016-03-30 | 2017-10-05 | Intellia Therapeutics, Inc. | Lipid nanoparticle formulations for crispr/cas components |
| WO2017176974A1 (en) | 2016-04-06 | 2017-10-12 | Ohio State Innovation Foundation | Biodegradable amino-ester nanomaterials for nucleic acid delivery |
| WO2018078053A1 (en) | 2016-10-26 | 2018-05-03 | Curevac Ag | Lipid nanoparticle mrna vaccines |
| US20180191722A1 (en) | 2017-01-05 | 2018-07-05 | Mastercard International Incorporated | Systems and Methods for Use in Managing Access to User Profiles, and Content Blocks Included Therein |
| WO2019027999A1 (en) | 2017-07-31 | 2019-02-07 | Ohio State Innovation Foundation | Biomimetic nanomaterials and uses thereof |
| WO2019036030A1 (en) | 2017-08-17 | 2019-02-21 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| WO2019089828A1 (en) | 2017-10-31 | 2019-05-09 | Acuitas Therapeutics, Inc. | Lamellar lipid nanoparticles |
| WO2019099501A1 (en) | 2017-11-14 | 2019-05-23 | Ohio State Innovation Foundation | Benzene-1,3,5-tricarboxamide derived ester lipids and uses thereof |
| WO2019118919A1 (en) | 2017-12-15 | 2019-06-20 | Flagship Pioneering, Inc. | Compositions comprising circular polyribonucleotides and uses thereof |
| US10407508B2 (en) | 2013-07-08 | 2019-09-10 | Nanjing Legend Biotech., Ltd. | Compositions and methods for increasing protein half-life in a serum |
| WO2019236673A1 (en) | 2018-06-06 | 2019-12-12 | Massachusetts Institute Of Technology | Circular rna for translation in eukaryotic cells |
| WO2020023595A1 (en) | 2018-07-24 | 2020-01-30 | Mayo Foundation For Medical Education And Research | Circularized engineered rna and methods |
| WO2020020904A1 (en) | 2018-07-23 | 2020-01-30 | Zealand Pharma A/S | Therapeutic uses of glp-2 agonists |
| WO2020041784A1 (en) | 2018-08-24 | 2020-02-27 | Flagship Pioneering Innovations Vi, Llc. | Methods for manufacturing plant messenger packs |
| WO2020072605A1 (en) | 2018-10-02 | 2020-04-09 | Intellia Therapeutics, Inc. | Ionizable amine lipids |
| WO2020081938A1 (en) | 2018-10-18 | 2020-04-23 | Acuitas Therapeutics, Inc. | Lipids for lipid nanoparticle delivery of active agents |
| WO2020118041A1 (en) | 2018-12-05 | 2020-06-11 | Intellia Therapeutics, Inc. | Modified amine lipids |
| US20200199192A1 (en)* | 2017-08-22 | 2020-06-25 | Shire-Nps Pharmaceuticals, Inc. | Glp-2 fusion polypeptides and uses for treating and preventing gastrointestinal conditions |
| WO2020146805A1 (en) | 2019-01-11 | 2020-07-16 | Acuitas Therapeutics, Inc. | Lipids for lipid nanoparticle delivery of active agents |
| WO2020181013A1 (en) | 2019-03-04 | 2020-09-10 | Flagship Pioneering Innovations Vi, Llc | Circular polyribonucleotides and pharmaceutical compositions thereof |
| WO2020198403A2 (en) | 2019-03-25 | 2020-10-01 | Flagship Pioneering Innovations Vi, Llc | Compositions comprising modified circular polyribonucleotides and uses thereof |
| WO2020219876A1 (en) | 2019-04-25 | 2020-10-29 | Intellia Therapeutics, Inc. | Ionizable amine lipids and lipid nanoparticles |
| WO2021041301A1 (en) | 2019-08-24 | 2021-03-04 | Flagship Pioneering Innovations Vi, Llc | Modification of plant messenger packs with charged lipids |
| US11000547B2 (en) | 2015-06-05 | 2021-05-11 | Dana-Farber Cancer Institute, Inc. | Compositions related to rna in circularized form |
| WO2021263124A2 (en) | 2020-06-25 | 2021-12-30 | The Board Of Trustees Of The Leland Stanford Junior University | Genetic elements driving circular rna translation and methods of use |
| JP2022001381A (en) | 2020-06-01 | 2022-01-06 | ザ・ボーイング・カンパニーThe Boeing Company | Systems and methods for compensating for springback in structures formed by incremental seat forming |
| WO2022204460A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Compositions and methods for producing circular polyribonucleotides |
| WO2022204464A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Production of circular polyribonucleotides in a eukaryotic system |
| WO2022204466A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Production of circular polyribonucleotides in a prokaryotic system |
| WO2022247943A1 (en) | 2021-05-28 | 2022-12-01 | Shanghai Circode Biomed Co., Ltd. | Constructs and methods for preparing circular rnas and use thereof |
| WO2022261490A2 (en) | 2021-06-10 | 2022-12-15 | Orna Therapeutics, Inc. | Circular rna compositions and methods |
| WO2023044006A1 (en) | 2021-09-17 | 2023-03-23 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for producing circular polyribonucleotides |
| WO2023069498A1 (en) | 2021-10-22 | 2023-04-27 | Senda Biosciences, Inc. | Mrna vaccine composition |
| WO2023096858A1 (en) | 2021-11-23 | 2023-06-01 | Senda Biosciences, Inc. | A bacteria-derived lipid composition and use thereof |
| WO2023122080A1 (en) | 2021-12-20 | 2023-06-29 | Senda Biosciences, Inc. | Compositions comprising mrna and lipid reconstructed plant messenger packs |
| WO2023122789A1 (en)* | 2021-12-23 | 2023-06-29 | Flagship Pioneering Innovations Vi, Llc | Circular polyribonucleotides encoding antifusogenic polypeptides |
| WO2023168405A2 (en)* | 2022-03-03 | 2023-09-07 | The Trustees Of The University Of Pennsylvania | Viral vectors encoding glp-2 receptor agonist fusions and uses thereof in treating short bowel syndrome |
| WO2024102434A1 (en) | 2022-11-10 | 2024-05-16 | Senda Biosciences, Inc. | Rna compositions comprising lipid nanoparticles or lipid reconstructed natural messenger packs |
| WO2024192420A1 (en) | 2023-03-15 | 2024-09-19 | Flagship Pioneering Innovations Vi, Llc | Compositions comprising polyribonucleotides and uses thereof |
- 2024
- 2024-11-15WOPCT/US2024/056294patent/WO2025106930A1/enactivePending
Patent Citations (68)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992001813A1 (en) | 1990-07-25 | 1992-02-06 | Syngene, Inc. | Circular extension for generating multiple nucleic acid complements |
| US5426180A (en) | 1991-03-27 | 1995-06-20 | Research Corporation Technologies, Inc. | Methods of making single-stranded circular oligonucleotides |
| US5712128A (en) | 1992-01-13 | 1998-01-27 | Duke University | Enzymatic RNA molecules |
| US5773244A (en) | 1993-05-19 | 1998-06-30 | Regents Of The University Of California | Methods of making circular RNA |
| US5766903A (en) | 1995-08-23 | 1998-06-16 | University Technology Corporation | Circular RNA and uses thereof |
| US20030082768A1 (en) | 1998-04-17 | 2003-05-01 | Whitehead Institute For Biomedical Research | Use of a ribozyme to join nucleic acids and peptides |
| US6210931B1 (en) | 1998-11-30 | 2001-04-03 | The United States Of America As Represented By The Secretary Of Agriculture | Ribozyme-mediated synthesis of circular RNA |
| US20100137407A1 (en) | 2007-05-09 | 2010-06-03 | Riken | Single-chain circular rna and method of producing the same |
| WO2010084371A1 (en) | 2009-01-26 | 2010-07-29 | Mitoprod | Novel circular interfering rna molecules |
| US8349569B2 (en) | 2009-03-31 | 2013-01-08 | Takara Bio Inc. | Anti-fibronectin fragment monoclonal antibody |
| WO2011097480A1 (en) | 2010-02-05 | 2011-08-11 | University Of Louisville Research Foundation, Inc. | Exosomal compositions and methods for the treatment of disease |
| US9376495B2 (en) | 2011-05-21 | 2016-06-28 | Macrogenics, Inc. | Deimmunized serum-binding domains and their use in extending serum half-life |
| WO2013063468A1 (en) | 2011-10-27 | 2013-05-02 | Massachusetts Institute Of Technology | Amino acid derivates functionalized on the n- terminal capable of forming drug incapsulating microspheres |
| WO2013070324A1 (en) | 2011-11-07 | 2013-05-16 | University Of Louisville Research Foundation, Inc. | Edible plant-derived microvesicle compositions for diagnosis and treatment of disease |
| WO2013128027A1 (en) | 2012-03-01 | 2013-09-06 | Amgen Research (Munich) Gmbh | Long life polypeptide binding molecules |
| US20150037334A1 (en) | 2012-03-01 | 2015-02-05 | Amgen Research (Munich) Gmbh | Long life polypeptide binding molecules |
| US10407508B2 (en) | 2013-07-08 | 2019-09-10 | Nanjing Legend Biotech., Ltd. | Compositions and methods for increasing protein half-life in a serum |
| WO2015095340A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Lipids and lipid compositions for the delivery of active agents |
| WO2015184256A2 (en) | 2014-05-30 | 2015-12-03 | Shire Human Genetic Therapies, Inc. | Biodegradable lipids for delivery of nucleic acids |
| WO2015199952A1 (en) | 2014-06-25 | 2015-12-30 | Acuitas Therapeutics Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2016004202A1 (en) | 2014-07-02 | 2016-01-07 | Massachusetts Institute Of Technology | Polyamine-fatty acid derived lipidoids and uses thereof |
| WO2016118724A1 (en) | 2015-01-21 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016118725A1 (en) | 2015-01-23 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016187531A1 (en) | 2015-05-21 | 2016-11-24 | Ohio State Innovation Foundation | Benzene-1,3,5-tricarboxamide derivatives and uses thereof |
| US11000547B2 (en) | 2015-06-05 | 2021-05-11 | Dana-Farber Cancer Institute, Inc. | Compositions related to rna in circularized form |
| WO2016205691A1 (en) | 2015-06-19 | 2016-12-22 | Massachusetts Institute Of Technology | Alkenyl substituted 2,5-piperazinediones and their use in compositions for delivering an agent to a subject or cell |
| WO2017004143A1 (en) | 2015-06-29 | 2017-01-05 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017004526A1 (en) | 2015-07-02 | 2017-01-05 | University Of Louisville Research Foundation, Inc. | EDIBLE PLANT-DERIVED MICROVESICLE COMPOSITIONS FOR DELIVERY OF miRNA AND METHODS FOR TREATMENT OF CANCER |
| WO2017049245A2 (en) | 2015-09-17 | 2017-03-23 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of therapeutic agents |
| WO2017075531A1 (en) | 2015-10-28 | 2017-05-04 | Acuitas Therapeutics, Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017117528A1 (en) | 2015-12-30 | 2017-07-06 | Acuitas Therapeutics, Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017173054A1 (en) | 2016-03-30 | 2017-10-05 | Intellia Therapeutics, Inc. | Lipid nanoparticle formulations for crispr/cas components |
| WO2017176974A1 (en) | 2016-04-06 | 2017-10-12 | Ohio State Innovation Foundation | Biodegradable amino-ester nanomaterials for nucleic acid delivery |
| WO2018078053A1 (en) | 2016-10-26 | 2018-05-03 | Curevac Ag | Lipid nanoparticle mrna vaccines |
| US20180191722A1 (en) | 2017-01-05 | 2018-07-05 | Mastercard International Incorporated | Systems and Methods for Use in Managing Access to User Profiles, and Content Blocks Included Therein |
| WO2019027999A1 (en) | 2017-07-31 | 2019-02-07 | Ohio State Innovation Foundation | Biomimetic nanomaterials and uses thereof |
| WO2019036030A1 (en) | 2017-08-17 | 2019-02-21 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| US20200199192A1 (en)* | 2017-08-22 | 2020-06-25 | Shire-Nps Pharmaceuticals, Inc. | Glp-2 fusion polypeptides and uses for treating and preventing gastrointestinal conditions |
| WO2019089828A1 (en) | 2017-10-31 | 2019-05-09 | Acuitas Therapeutics, Inc. | Lamellar lipid nanoparticles |
| WO2019099501A1 (en) | 2017-11-14 | 2019-05-23 | Ohio State Innovation Foundation | Benzene-1,3,5-tricarboxamide derived ester lipids and uses thereof |
| WO2019118919A1 (en) | 2017-12-15 | 2019-06-20 | Flagship Pioneering, Inc. | Compositions comprising circular polyribonucleotides and uses thereof |
| WO2019236673A1 (en) | 2018-06-06 | 2019-12-12 | Massachusetts Institute Of Technology | Circular rna for translation in eukaryotic cells |
| WO2020020904A1 (en) | 2018-07-23 | 2020-01-30 | Zealand Pharma A/S | Therapeutic uses of glp-2 agonists |
| WO2020023595A1 (en) | 2018-07-24 | 2020-01-30 | Mayo Foundation For Medical Education And Research | Circularized engineered rna and methods |
| WO2020041784A1 (en) | 2018-08-24 | 2020-02-27 | Flagship Pioneering Innovations Vi, Llc. | Methods for manufacturing plant messenger packs |
| WO2020072605A1 (en) | 2018-10-02 | 2020-04-09 | Intellia Therapeutics, Inc. | Ionizable amine lipids |
| WO2020081938A1 (en) | 2018-10-18 | 2020-04-23 | Acuitas Therapeutics, Inc. | Lipids for lipid nanoparticle delivery of active agents |
| WO2020118041A1 (en) | 2018-12-05 | 2020-06-11 | Intellia Therapeutics, Inc. | Modified amine lipids |
| WO2020146805A1 (en) | 2019-01-11 | 2020-07-16 | Acuitas Therapeutics, Inc. | Lipids for lipid nanoparticle delivery of active agents |
| WO2020181013A1 (en) | 2019-03-04 | 2020-09-10 | Flagship Pioneering Innovations Vi, Llc | Circular polyribonucleotides and pharmaceutical compositions thereof |
| WO2020198403A2 (en) | 2019-03-25 | 2020-10-01 | Flagship Pioneering Innovations Vi, Llc | Compositions comprising modified circular polyribonucleotides and uses thereof |
| WO2020219876A1 (en) | 2019-04-25 | 2020-10-29 | Intellia Therapeutics, Inc. | Ionizable amine lipids and lipid nanoparticles |
| WO2021041301A1 (en) | 2019-08-24 | 2021-03-04 | Flagship Pioneering Innovations Vi, Llc | Modification of plant messenger packs with charged lipids |
| JP2022001381A (en) | 2020-06-01 | 2022-01-06 | ザ・ボーイング・カンパニーThe Boeing Company | Systems and methods for compensating for springback in structures formed by incremental seat forming |
| WO2021263124A2 (en) | 2020-06-25 | 2021-12-30 | The Board Of Trustees Of The Leland Stanford Junior University | Genetic elements driving circular rna translation and methods of use |
| WO2022204460A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Compositions and methods for producing circular polyribonucleotides |
| WO2022204464A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Production of circular polyribonucleotides in a eukaryotic system |
| WO2022204466A1 (en) | 2021-03-26 | 2022-09-29 | Flagship Pioneering Innovations Vii, Llc | Production of circular polyribonucleotides in a prokaryotic system |
| WO2022247943A1 (en) | 2021-05-28 | 2022-12-01 | Shanghai Circode Biomed Co., Ltd. | Constructs and methods for preparing circular rnas and use thereof |
| WO2022261490A2 (en) | 2021-06-10 | 2022-12-15 | Orna Therapeutics, Inc. | Circular rna compositions and methods |
| WO2023044006A1 (en) | 2021-09-17 | 2023-03-23 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for producing circular polyribonucleotides |
| WO2023069498A1 (en) | 2021-10-22 | 2023-04-27 | Senda Biosciences, Inc. | Mrna vaccine composition |
| WO2023096858A1 (en) | 2021-11-23 | 2023-06-01 | Senda Biosciences, Inc. | A bacteria-derived lipid composition and use thereof |
| WO2023122080A1 (en) | 2021-12-20 | 2023-06-29 | Senda Biosciences, Inc. | Compositions comprising mrna and lipid reconstructed plant messenger packs |
| WO2023122789A1 (en)* | 2021-12-23 | 2023-06-29 | Flagship Pioneering Innovations Vi, Llc | Circular polyribonucleotides encoding antifusogenic polypeptides |
| WO2023168405A2 (en)* | 2022-03-03 | 2023-09-07 | The Trustees Of The University Of Pennsylvania | Viral vectors encoding glp-2 receptor agonist fusions and uses thereof in treating short bowel syndrome |
| WO2024102434A1 (en) | 2022-11-10 | 2024-05-16 | Senda Biosciences, Inc. | Rna compositions comprising lipid nanoparticles or lipid reconstructed natural messenger packs |
| WO2024192420A1 (en) | 2023-03-15 | 2024-09-19 | Flagship Pioneering Innovations Vi, Llc | Compositions comprising polyribonucleotides and uses thereof |
Non-Patent Citations (26)
| Title |
|---|
| BARANICK ET AL., PNAS, vol. 105, no. 12, 2008, pages 4733 - 4738 |
| CHEN ET AL., MOL. CELL, vol. 81, 2021, pages 1 - 19 |
| CHEN, X.: "Fusion Protein Linkers: Property, Design and Functionality", ADV DRUG DELIV REV., vol. 65, no. 10, 2013, pages 1357 - 1369, XP028737352, DOI: 10.1016/j.addr.2012.09.039 |
| CHOI ET AL.: "HM15912, a Novel Long-acting Glucagon-like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome", J PHARMACOL EXP THER |
| CHOI JAEHYUK ET AL: "HM15912, a Novel Long-Acting Glucagon-Like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome", THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS, vol. 384, no. 2, 1 February 2023 (2023-02-01), pages 277 - 286, XP093244029, ISSN: 0022-3565, DOI: 10.1124/jpet.122.001381* |
| DOROKHOV ET AL., PNAS, vol. 99, no. 8, 2002, pages 5301 - 5306 |
| EGLIHERDEWIJN: "Chemistry and Biology of Artificial Nucleic Acids", 2012, COLD SPRING HARBOR LABORATORY PRESS |
| FAN ET AL., NATURE COMMUNICATIONS, vol. 13, no. 1, 2022, pages 3751 |
| HARGROVE DIANE M. ET AL: "Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome", THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS, vol. 373, no. 2, 13 April 2020 (2020-04-13), pages 193 - 203, XP055813396, ISSN: 0022-3565, DOI: 10.1124/jpet.119.262238* |
| HARGROVE ET AL.: "Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome", J PHARMACOL EXP THER, vol. 373, no. 2, 2020, pages 193 - 203, XP055813396, DOI: 10.1124/jpet.119.262238 |
| J. MCCLELLANM. C. KING, CELL, vol. 141, 2010, pages 210 - 217 |
| JOPLING ET AL., ONCOGENE, vol. 20, 2001, pages 2664 - 2670 |
| KHUDYAKOVFIELDS: "Artificial DNA: Methods and Applications", 2002, CRC PRESS |
| KIMOTO ET AL., CHEM. COMM. (CAMB, vol. 53, 2017, pages 12309 |
| LANG ET AL., MOLECULAR BIOLOGY OF THE CELL, vol. 13, no. 5, 2002, pages 1792 - 1801 |
| LEWIS ET AL., NATURE REVIEWS MOL. CELL BIOL., vol. 18, 2017, pages 202 - 210 |
| MICHOT, N. ET AL.: "Albumin binding Nanofitins, a new scaffold to extend half-life of biologics - a case study with exenatide peptide", PEPTIDES, vol. 152, 2022, XP087045947, DOI: 10.1016/j.peptides.2022.170760 |
| MULLERAPPEL, RNA BIOL, vol. 14, no. 8, 2017, pages 1018 - 1027 |
| PETKOVIC ET AL., NUCLEIC ACIDS RES., vol. 43, 2015, pages 2454 - 65 |
| PETZ, NUCLEIC ACIDS RESEARCH, vol. 35, no. 8, 2007, pages 2473 - 2482 |
| R. ALEXANDER WESSELHOEFT ET AL: "Engineering circular RNA for potent and stable translation in eukaryotic cells", NATURE COMMUNICATIONS, vol. 9, no. 1, 6 July 2018 (2018-07-06), XP055622096, DOI: 10.1038/s41467-018-05096-6* |
| ROZENSKI ET AL., NUCL. ACIDS RES., vol. 27, 1999, pages 196 - 197 |
| WANG ET AL., NUCLEIC ACIDS RESEARCH, vol. 33, no. 7, 2005, pages 2248 - 2258 |
| WHITEHEAD ET AL., NATURE COMMUNICATIONS, vol. 5, 2014, pages 4277 |
| YU, Z. ET AL., CELL RES., vol. 25, 2015, pages 1283 - 1284 |
| ZHAO: "Synthetic Biology: Tools and Applications", 2013, ACADEMIC PRESS |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7618577B2 (en) | Compositions Comprising Modified Circular Polyribonucleotides and Uses Thereof | |
| AU2018385757B2 (en) | Compositions comprising circular polyribonucleotides and uses thereof | |
| JP7284833B2 (en) | Modification of mammalian cells with artificial microRNAs to alter their properties and compositions of their products | |
| EP3935162A1 (en) | Circular polyribonucleotides and pharmaceutical compositions thereof | |
| KR20240099185A (en) | Compositions and methods for producing circular polyribonucleotides | |
| EP4096682A1 (en) | Compositions for translation and methods of use thereof | |
| KR20240118881A (en) | Circular polyribonucleotide encoding an antifusogenic polypeptide | |
| WO2021155171A1 (en) | Delivery of compositions comprising circular polyribonucleotides | |
| KR20250035055A (en) | Compositions and methods for targeting PCSK9 | |
| IL296718A (en) | RNAi agents for inhibiting expression of pnpla3, their pharmaceutical compositions and methods of use | |
| US20240415980A1 (en) | Crispr/cas-related methods and compositions for knocking out c5 | |
| WO2025106930A1 (en) | Circular polyribonucleotides encoding glucagon-like peptide 2 (glp-2) and uses thereof | |
| WO2024192420A1 (en) | Compositions comprising polyribonucleotides and uses thereof | |
| WO2025106915A1 (en) | Circular polyribonucleotides encoding glucagon-like peptide 1 (glp-1) and uses thereof | |
| KR20240117569A (en) | Coronavirus immunogen composition and uses thereof | |
| WO2025179198A1 (en) | Circular polyribonucleotides and unmodified linear rnas with reduced immunogenicity | |
| EA049851B1 (en) | RNAi AGENTS FOR INHIBITING PNPLA3 EXPRESSION, THEIR PHARMACEUTICAL COMPOSITIONS AND METHODS OF APPLICATION | |
| TW202339775A (en) | Engineered adar-recruiting rnas and methods of use thereof | |
| WO2024216162A1 (en) | Synthetic circuits and uses thereof | |
| CN120435298A (en) | Compositions and methods for producing cyclic polyribonucleotides | |
| EA048586B1 (en) | METHOD FOR PRODUCING A PHARMACEUTICAL COMPOSITION CONTAINING RING POLYRIBONUCLEOTIDES |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24817027 Country of ref document:EP Kind code of ref document:A1 |