WO2025101914A1 - Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors having improved therapeutic index against solid tumors - Google Patents
Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors having improved therapeutic index against solid tumorsDownload PDFInfo
- Publication number
- WO2025101914A1 WO2025101914A1PCT/US2024/055148US2024055148WWO2025101914A1WO 2025101914 A1WO2025101914 A1WO 2025101914A1US 2024055148 WUS2024055148 WUS 2024055148WWO 2025101914 A1WO2025101914 A1WO 2025101914A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- compound
- cancer
- mixture
- pharmaceutically acceptable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/53—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with three nitrogens as the only ring hetero atoms, e.g. chlorazanil, melamine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- the disclosurerelates to a new class of small molecules having a substituted aminobenzyl quinazoline structure or a substituted aminobenzyl quinoline structure, which function as dual inhibitors of EGFR proteins and PI3K proteins.
- the disclosurefurther relates to the use of the compounds described herein as therapeutics for the treatment of diseases and conditions mediated by EGFR proteins and/or PI3K proteins, such as cancer and other diseases.
- BACKGROUND[005] Every year more than 830,000 patients are diagnosed with head and neck cancer worldwide and at least 430,000 patients die from this disease (see e.g., Cramer, J.D., et al.,Nat Rev Clin Oncol, 2019.16(11): p.669-683).
- HNSCCssquamous cell carcinomas
- EGFR overexpressionis an early and frequent molecular change in HNSCC, a change that has been shown to be associated with reduced survival (see e.g., Grandis, J.R. and D.J. Tweardy, Cancer Research, 1993.53(15): p.3579-3584).
- Cetuximabremains the only U.S. FDA-approved EGFR-targeted therapy available for HNSCC.
- a fundamental problem in EGFR-targeted therapy in HNSCCis patient selection, since a consistent mechanism for resistance has not been identified.
- PI3K mutationswhich are particularly common in HPV+ head and neck cancers, confer increased resistance to EGFR inhibition (see e.g., Simpson, D.R., L.K. Mell, and E.E.W. Cohen, Oral Oncology, 2015.51(4): p.291-298; Young, N.R., et al., Molecular Oncology, 2013.7(3): p.359-368).
- PIK3CAhas therefore emerged as a candidate biomarker of EGFR resistance.
- the PI3K/AKT/mTOR pathwaywhich supports tumor cell survival and progression, is aberrantly activated in a large percentage of human tumors (see e.g., Yap, T.A., et al., Current Opinion in Pharmacology, 2015.23: p.98-107; Liu, P., et al., Nature Reviews Drug Discovery, 2009.8: p.627-644; Janku, F., T.A. Yap, and F. Meric-Bernstam, Nature Reviews Clinical Oncology, 2018.15: p.273-291).
- Squamous cancersshow a particularly high incidence of genomic alterations in this pathway, encompassing PIK3CA mutations and other alterations, for instance in PIK3R1, PTEN, and AKT, that result in activation of this pathway.
- PI3K inhibitorsthat have received regulatory approval for the treatment of solid tumors are copanlisib (panPI3K) and alpelisib (PI3K ⁇ -selective). Lack of progress can largely be attributed to unacceptable toxicities, which are in part driven by the need for high exposures to elicit monotherapy activity.
- Anti-EGFR treatmenthas been shown to reverse acquired and intrinsic resistance to PI3K ⁇ inhibition in HNSCC (see e.g., Elkabets, M., et al., Cancer Cell, 2015.27(4): p.533-546).
- AXLwas shown to interact with EGFR to activate PLC ⁇ and PKC, leading to activation of mTOR in a PI3K-independent manner.
- EGFR and PI3Kemerge as potential co-targeting candidates to test this concept, since these oncogenic kinases drive adaptive resistance across a broad spectrum of human cancers, as exemplified by squamous head and neck cancers (HNSCC).
- EGFR and PI3Kare each known to mediate resistance to inhibition of the other.
- the clinical activity of cetuximabwhich is the only approved kinase-targeted therapy for this disease, is modest.
- Molecular aberrations in the PI3K/mTOR pathway leading to its dysregulationare found in up to 80% of HNSCC and confer increased resistance to EGFR inhibition.
- Treatment failures in the PI3K fieldhave been attributed to unacceptable toxicities, in part driven by the need for high exposures to elicit monotherapy activity.
- the PI3K ⁇ inhibitor alpelisib(Piqray TM ) is the only approved clinical agent in this target class, based on its activity against PIK3CA mutated advanced breast cancer.
- Hyperglycemia, hyperinsulinemia, insulin resistance and body weight lossare a dose limiting toxicity observed in subjects treated with PI3K inhibitors.
- Some of the problems associated with the use of PI3K inhibitorsinclude: hyperinsulinemia antagonizes PI3K inhibition by stimulating tumor IR and IGF1 R hybrid receptor signaling; hyperglycemia overrides the metabolic action of PI3K inhibitors in tumors by increasing glucose utilization and aerobic glycolysis, thus promoting tumor glucose uptake which drives cell growth and replication irrespective of PI3K inhibition, and the T1 D-like symptoms induced by PI3K inhibitors are dose limiting and severely limit tumor target coverage.
- PI3K inhibitorsare not normalized by drugs used to manage diabetes (e.g., treatment of CD-17 scid mice with GSK690693 potently induces hyperglycemia.
- This negative side effectwas not prevented or reduced by several drug used to treat clinical diabetes including rosiglitazone maleate, vildagliptin, metformin, and Exendin-4; see for example, Crouthamel et al., Clin Cancer Res, 15:217-225, 2009).
- Hyperglycemia induced by AKT inhibitors(a direct PI3K target) can be partially resolved by fasting in rodents, but hyperinsulinemia is not reduced.
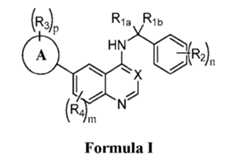
- the compounds of Formula Iare substituted benzylamino compounds and may have advantages over the analogous unsubstituted benzylamino compounds or analogous phenylamino compounds.
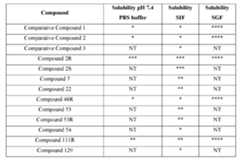
- the compounds of Formula Imay possess properties selected from one or more of increased solubility in pH 7.4 aqueous buffer solution, increased solubility in simulated intestinal fluid (SIF), and increased solubility in simulated gastric fluid (SGF), as compared to the analogous unsubstituted benzylamino compounds or analogous phenylamino compounds.
- Increased solubility in the mediums described abovecan be indicative of increased bioavailability in a biological system, such as a human subject. Further, an increase in bioavailability may allow for equivalent biological activity of smaller doses, compared to the required dose of a less soluble active pharmaceutical ingredient (API).
- APIactive pharmaceutical ingredient
- the disclosureincludes a compound of Formula I Formula I or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring A is selected from phenyl or a 5 or 6 membered heteroaryl; X is N or C-R 5 ; R 1a is selected from the group consisting of H or C 1-6 alkyl; R 1b is selected from the group consisting of C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’) 2 , C(O)R’, C(O)OR’, C(O)N(R’) 2 , halo, CN, and NO 2 , wherein each C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and
- the disclosureincludes a pharmaceutical composition comprising a compound or salt according to Formula (I) described herein and a pharmaceutically acceptable excipient.
- the disclosureincludes a method of modulating the activity of an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound, salt or a composition described herein.
- the disclosureincludes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound, salt or a composition described herein.
- the EGFR and/or PI3K mediated diseaseis a cancer.
- FIG. 1Ais a chemical structure of COMPOUND 2R ((R)-N-(2-chloro-5-(4-((1- phenylethyl)amino)quinazolin-6-yl)pyridin-3-yl)methane-sulfonamide).
- Figure 1Bis a crystal structure of EGFR co-complexed with COMPOUND 2R (PDB Code 8SC7) solved at 2.0 Angstroms.
- Figure 1Cshows the secondary structure of the crystal structure displayed in Fig.1B with a view of COMPOUND 2R bound to EGFR from the ATP binding site. Graphics were generated by Molegro Virtual Docker 5.557.
- Figure 1Dis a crystal structure of PI3K ⁇ co-complexed with COMPOUND 2R (PDB Code 8SC8) solved at 2.7 Angstroms.
- Figure 1Eshows the secondary structure of the crystal structure displayed in Fig.1D with a view of COMPOUND 2R bound to PI3K ⁇ from the ATP binding site. Graphics were generated by Molegro Virtual Docker 5.5.
- Figure 1Fis a table of biochemical potency of COMPOUND 2R and approved PI3K inhibitors against purified HER and PI3K family members. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. Enzyme inhibition curves are shown in Figures 17, 18, and 19. [0025]
- Figure 1Gdepicts kinase selectivity of COMPOUND 2R. Selectivity was determined against a panel of 482 protein and lipid kinases by carrying out single point testing at a final concentration of 10 ⁇ M COMPOUND 2R. Kinases inhibited by >80% at 10 ⁇ M were re-tested in dose response assays to determine IC 50 values.
- FIG. 1Hdepicts a western immunoblot of EGFR and PI3K/mTOR pathway targets in EGF-stimulated CAL-33 cells treated for 2 hours with COMPOUND 2R over an eight-point dose response. Data are representative of two independent experiments. Quantification of phosphorylated kinase expression was carried out by densitometry analysis to determine EC50 values.
- Figure 2Adepicts a western immunoblot showing protein expression from a related to the pharmacodynamic modulation of EGFR and PI3K/mTOR pathway expression in subcutaneous CAL-33 tumors excised from mice treated with a single oral dose of 100 mg/kg COMPOUND 2R. Tumors were harvested at the indicated time point (3 mice/time point) followed by immunoblot analysis.
- Figure 2Bshows quantification of phosphorylated kinase expression of the individual bands shown in Fig.2A. by densitometry analysis.
- Figure 2Cdepicts antitumor efficacy of COMPOUND 2R against subcutaneous CAL-33 xenografts.
- Figure 2Eshows the effects of CAL-33 treatment model on survival quantitated by sacrificing individual mice when tumor burden reached an equivalent size of 1000 mm3.
- a one- way ANOVA comparison between all groupswas carried out to determine statistical significance.
- ****p-value ⁇ 0.0001
- Figure 2Fshows the effects of CAL-27 treatment model on survival quantitated by sacrificing individual mice when tumor burden reached an equivalent size of 500 mm3.
- a one- way ANOVA comparison between all groupswas carried out to determine statistical significance.
- FIG. 2Gdepicts a western immunoblot showing pharmacodynamic modulation of protein expression in the pEGFR and PI3K/mTOR pathway after a single oral dose of 100 mg/kg COMPOUND 2R in mice with subcutaneous NCI 848979-319-R patient-derived xenografts.
- FIG. 2Hshows protein expression as percent of control for the phosphorylated kinase expression from the individual immunoblot bands in Fig.2G as quantified by densitometry analysis.
- Figure 2Ishows percent expression as percent of control for cleaved PARP from the individual immunoblot band in Fig.2G as quantified by densitometry analysis.
- Figure 2Jdepicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CA E545K mutation (245127-232-R).
- Figure 2Kdepicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CA E545A mutation (354836-022-R).
- FIG. 1depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CA G364R mutation (455876-151-R).
- the HNSCC PDX graftwas obtained from the NCI PDX biorepository.
- Figure 2Mdepicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CA E726K mutation (848979-319-R).
- Figure 2Ndepicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CA H1047L mutation (944545-341-R).
- Figure 2Odepicts Waterfall plot of the best individual response of HNSCC PDX- implanted mice treated with COMPOUND 2R in Figs.2J-2N. The percent increase in tumor burden observed in the vehicle treated mice during these studies ranged between 773% and 912% for all five models.
- Figure 2Qshows days of treatment study for six different mice. The letters “SD” indicates stable disease, meaning that it does not qualify for tumor shrinkage or tumor growth. The letters “CR” indicates complete response and requires disappearance of the tumor and lack of palpable tumor.
- Figure 2Tshows the mean body weight change in mice between both drug-treated groups and the vehicle control group over 14 days of treatment.
- Figure 3Bshows the best antitumor response seen in individual animals from each group in Fig.3A is shown in the waterfall plot. The percent increase in tumor burden observed in the vehicle treated mice was 887 %.
- Figure 3Eshows the best antitumor response seen in individual animals from each group in Fig.3D is shown in the waterfall plot. The percent increase in tumor burden observed in the vehicle treated mice was 844%.
- Figure 4Cshows the best antitumor response seen in individual animals from each group is shown in the waterfall plots at the bottom.
- Figure 4Dshows the best antitumor response seen in individual animals from each group is shown in the waterfall plots at the bottom.
- the percent increase in tumor burden observed in the vehicle treated mice in the MIA PaCa-2 pancreatic model during this studywas 970% (Fig. 4B).
- Figure 5Eshows the best antitumor response seen in individual animals from each group from Fig.5D is shown in the waterfall plots. The percent increase in tumor burden observed in the vehicle treated mice was 679%.
- Figure 5Fshows extension of survival of KPC-implanted mice treated daily with COMPOUND 2R or alpelisib as described in Fig.5D.
- Figure 6Adepicts COMPOUND 2R competitive binding and transactivation of PPAR ⁇ .
- Figure 6Cdepicts the effects of COMPOUND 2R on PPAR ⁇ target gene expression. The indicated PPAR ⁇ target gene expression levels were analyzed in quadruplicate by quantitative RT-PCR analysis of total RNA extracts from 3T3-L1 cells treated with differentiation medium containing rosiglitazone or COMPOUND 2R for 8 or 24 hours.
- Figure 6Dshows a western immunoblot of PPAR ⁇ expression was carried out in total cell lysates from 3T3-L1 adipocytes at 7 days post induction of differentiationThe two bands representing PPAR ⁇ 1 (53 kDa) and PPAR ⁇ 2 (57 kDa) were upregulated in the 3T3-L1 cells differentiated in the presence of 10 ⁇ M COMPOUND 2R and the reference compound rosiglitazone at 1 ⁇ M.
- Figure 6Edepicts the crystal structure of PPAR ⁇ co-complexed with COMPOUND 2R (PDB Code 8SC9) solved at 1.9 Angstroms.
- Figure 6Fshows the secondary structure of the crystal structured displayed in Fig.6E and a view of COMPOUND 2R bound to PPAR ⁇ from the ligand binding pocket is shown in the right panel. Graphics were generated by Molegro Virtual Docker 5.5.
- Figure 7Adepicts the structural features of NVP-AEE788 bound to EGFR used in the design of COMPOUND 2R.
- Figure 7Bdepicts the structural features omipalisib bound to PI3K ⁇ used in the design of COMPOUND 2R.
- COMPOUND 2RThe computational design of COMPOUND 2R was based on analysis of x- ray crystal structures of NVP-AEE788 (Fig.7A) and omipalisib kinase inhibitors bound to their respective targets.
- the flipped binding mode of the uinazoline core between EGFR (Fig.7A) and PI3K ⁇was leveraged to design a potent and selective inhibitor of both enzyme families.
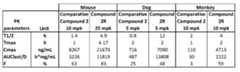
- Figure 8Adepicts the pharmacokinetic analysis of COMPOUND 2R in mice.
- COMPOUND 2Rexhibits high oral bioavailability and a dose-dependent increase in plasma concentrations in mice.
- FIG. 8Bshows data of pharmacokinetic parameters after single treatment of 5 mg/kg COMPOUND 2R (IV).
- Figure 8Cshows data of pharmacokinetic parameters after single treatment of 25 mg/kg COMPOUND 2R (PO).
- Figure 8Dshows data of pharmacokinetic parameters after single treatment of 100 mg/kg COMPOUND 2R (PO).
- Figure 9depicts tumor response rates and survival data for CAL-33 and CAL-27 xenografts.
- FIG. 10Adepicts a western immunoblot showing baseline protein expression in five HNSCC PDX tumor models.
- Tumors from five HNSCC models from the NCI Patient-Derived Models Repository (PDMR)were lysed and baseline protein expression was analyzed for proteins related to EGFR and PI3K/mTOR signaling through immunoblotting. Lysates were normalized for total protein concentration and equivalents amounts of protein were added to each lane for expression analysis of a given kinase.
- PDMRPatient-Derived Models Repository
- Figure 10Bshows data summarizing the genomic alterations in the tumor models as summarized in the NCI PDMR database.
- Figure 11depicts a western immunoblot showing pharmacodynamic modulation of EGFR and PI3K/mTOR pathway expression in subcutaneous NCI 944545-341-R patient-derived xenografts. Mice were treated daily with COMPOUND 2R orally at 100 mg/kg for five days. At two hours after the fifth dose, tumors were excised and immunoblot analysis was carried out (3 mice/group).
- Figure 12Adepicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models.
- FIG. 12Bdepicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models.
- FIG. 12Cdepicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models.
- FIG. 12Ddepicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models.
- FIG. 13Adepicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R.
- mice bearing subcutaneous HNSCC PDX tumorswere dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group).
- Mean group body weight changes ( ⁇ SEM)were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment.
- Figure 13Bdepicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R.
- mice bearing subcutaneous HNSCC PDX tumorswere dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group).
- Mean group body weight changes ( ⁇ SEM)were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment.
- Figure 13Cdepicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R.
- mice bearing subcutaneous HNSCC PDX tumorswere dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group).
- Mean group body weight changes ( ⁇ SEM)were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment.
- Figure 13Ddepicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R.
- mice bearing subcutaneous HNSCC PDX tumorswere dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group).
- Mean group body weight changes ( ⁇ SEM)were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment.
- Figure 15Adepicts a western immunoblot showing pharmacodynamic modulation of protein expression in the EGFR and PI3K/mTOR pathway in subcutaneous UM CRC 14-929 patient-derived xenografts. Mice were treated orally with COMPOUND 2R at 100 mg/kg, trametinib at 1 mg/kg or the combination. At two hours after a single dose, tumors were excised and immunoblot analysis was carried out (3 mice/group). [0093] Figure 15B depicts the quantification of phosphorylated kinase expression was carried out by densitometry analysis of the individual bands in Fig.15A.
- Figure 16Adepicts an overlay of COMPOUND 2R x-ray binding modes with commercial PPAR ⁇ agonist rosiglitazone and pioglitazone. This figure shows an overlay of x- ray crystal binding modes of COMPOUND 2R, rosiglitazone (PDB Code 4EMA) and pioglitazone (PDB Code 2XKW).
- Figure 16Bdepicts the compound structure of COMPOUND 2R (MTX-531) for comparison with the structural overlay in Fig.16A.
- Figure 16Cdepicts the compound struct of rosiglitazone for comparison with the structural overlay in Fig.16A.
- Figure 16Ddepicts the compound structure or pioglitazone for comparison with the structural overlay in Fig.16A.
- Figure 17Adepicts line graph of the inhibitory activity of COMPOUND 2R against EGFR (ErbB1). The biochemical potency of COMPOUND 2R was evaluated against purified HER family members. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 17Bdepicts line graph of the inhibitory activity of COMPOUND 2R against HER2 (ErbB2). The biochemical potency of COMPOUND 2R was evaluated against purified HER family members. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 17Cdepicts line graph of the inhibitory activity of COMPOUND 2R against HER4 (ErbB4).
- the biochemical potency of COMPOUND 2Rwas evaluated against purified HER family members. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme
- Figure 18Adepicts the line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3C ⁇ /PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 18Bdepicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3C ⁇ /PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 18Cdepicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3C ⁇ /PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 18Ddepicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3C ⁇ /PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 19Adepicts the inhibitory activity of COMPOUND 2R against FRAP (mTOR). The biochemical potency of COMPOUND 2R was evaluated against purified FRAP1. Nanomolar IC 50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme.
- Figure 19Bdepicts the inhibitory activity of COMPOUND 2R against DNA-PK.
- FIG. 20Adepicts the broad kinome screening of COMPOUND 2R.
- the inhibitory potency of COMPOUND 2Rwas determined against a panel of 482 protein and lipid kinases by carrying out single point testing at a final concentration of 10 ⁇ M COMPOUND 2R.
- Figure 20Bdepicts COMPOUND 2R IC 50 values for nine kinases identified in a broad kinome screening as inhibited by >80% at 10 ⁇ M (shown in bold font in Fig.20A).
- Figure 21Adepicts a line graph representing the potency of COMPOUND 2R on the downstream effector pEGFR ⁇ 1068 within the EGFR and PI3K pathways in CAL-33 cells.
- Densitometry using ImageJ softwarewas performed for immunoblots of pEGFR ⁇ 1068 in CAL- 33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 ⁇ M.
- Pixel intensity of the band of interestwas normalized to the loading control.
- the ratio of the normalized pixel intensity of treated to control valueswas multiplied by 100.
- the IC 50 value of 3.131e-007was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad.
- Figure 21Bdepicts a line graph representing the potency of COMPOUND 2R on the downstream effector pAKTS473 within the EGFR and PI3K pathways in CAL-33 cells.
- Densitometry using ImageJ softwarewas performed for immunoblots of pAKT S473 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 ⁇ M.
- Pixel intensity of the band of interestwas normalized to the loading control.
- the ratio of the normalized pixel intensity of treated to control valueswas multiplied by 100.
- the IC 50 value of 4.279e-007was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad.
- Figure 21Cdepicts a line graph representing the potency of COMPOUND 2R on the downstream effector pAKT T308 within the EGFR and PI3K pathways in CAL-33 cells.
- Densitometry using ImageJ softwarewas performed for immunoblots of pAKTT308 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 ⁇ M.
- Pixel intensity of the band of interestwas normalized to the loading control.
- the ratio of the normalized pixel intensity of treated to control valueswas multiplied by 100.
- the IC 50 value of 2.979e-007was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad.
- Figure 21Ddepicts a line graph representing the potency of COMPOUND 2R on the downstream effector pS6 S235/236 in the EGFR and PI3K pathways in CAL-33 cells.
- Densitometry using ImageJ softwarewas performed for immunoblots of pS6S235/236 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 ⁇ M.
- Pixel intensity of the band of interestwas normalized to the loading control.
- the ratio of the normalized pixel intensity of treated to control valueswas multiplied by 100.
- the IC 50 value of 5.970e-006was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad.
- Figure 21Edepicts a line graph representing the potency of COMPOUND 2R on the downstream effector p4E-BP1S65 of the EGFR and PI3K pathways in CAL-33 cells.
- Densitometry using ImageJ softwarewas performed for immunoblots of p4E-BP1 S65 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 ⁇ M.
- Pixel intensity of the band of interestwas normalized to the loading control.
- the ratio of the normalized pixel intensity of treated to control valueswas multiplied by 100.
- the IC 50 value of 3.390e-007was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad.
- Figure 22Adepicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 245127 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas.
- Figure 22Bdepicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 354836 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis.
- Figure 22Cdepicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 455876 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas.
- Figure 22Ddepicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 848979 model.
- Compound efficacywas determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas.
- Figure 22Edepicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 944545 model.
- Compound efficacywas determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas.
- Figure 23depicts a line graph showing the selection of a combination dosage of erlotinib and alpelisib in mice. Mice were treated with the combination of erlotinib and alpelisib daily via oral gavage for 5 weeks (8 mice/group). The dosage of erlotinib was held constant at 50 mg/kg and the dosage of alpelisib was varied (12.5, 25 and 50 mg/kg). Body weights were recorded 2-3 times per week.
- FIG. 24Adepicts body weight change of CRC NCI CN0375-F725 PDX subcutaneous tumor-bearing mice in response to daily oral treatment with COMPOUND 2R in combination with the MEK inhibitor trametinib.
- FIG. 25Adepicts COMPOUND 2R activity in PPAR ⁇ time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays.
- COMPOUND 2Rwas titrated across a 10-point concentration range to test for competitive binding to PPAR ⁇ against GW7647 in a TR-FRET assay. COMPOUND 2R failed to displace the reference ligands demonstrating specificity for PPAR ⁇ .
- Figure 25Bdepicts COMPOUND 2R activity in PPAR ⁇ time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays. COMPOUND 2R was titrated across a 10-point concentration range to test for competitive binding PPAR ⁇ against GW0742 in a TR-FRET assay. COMPOUND 2R failed to displace the reference ligands demonstrating specificity for PPAR ⁇ .
- TR-FRETtime-resolved fluorescence resonance energy transfer
- Figure 26Adepicts electron density maps of the COMPOUND 2R binding site of PPAR ⁇ in the initial Fo-Fc difference electron density map of the model (contoured at 3 ⁇ ) resulting from refinement of the initial model prior to modelling of the compound with BUSTER. Shown is the region of compound binding site in chain B.
- Figure 26Bdepicts the final 2Fo-Fc electron density map (contoured at 1.0 ⁇ ) of the COMPOUND 2R binding site of PPAR ⁇ resulting from refinement of the final model with BUSTER. Shown is the region of the compound 2R binding site.
- Figure 27depicts a line graph showing blood glucose levels in athymic mice following a single dose of alpelisib or MTX-531(Compound 2R).
- Figure 28depicts a line graph showing oral glucose tolerance testing in fasted mice following a single oral dose of alpelisib or MTX-531(Compound 2R).
- Figure 29depicts a line graph showing oral glucose tolerance testing in fasted mice after five days of PI3K inhibitor dosing.
- Figure 30depicts a bar graph representing adiponectin levels in blood from C57/BL6 mice treated with MTX-531 (Compound 2R) versus the PPAR gamma agonist rosiglitazone.
- Figure 31depicts a bar graph representing adiponectin levels in blood from athymic nude mice treated with MTX-531 (Compound 2R) versus comparator PI3K and PPAR gamma targeted agents.
- MTX-531Compound 2R
- comparator PI3K and PPAR gamma targeted agentsDETAILED DESCRIPTION OF THE DISCLOSURE
- the present disclosureprovides a method for preventing, treating, reducing, inhibiting or controlling a neoplasia, tumor or cancer and/or the establishment of metastases in a subject involving administering a compound of Formula I or a pharmaceutically acceptable salt thereof.
- the chemical elementsare identified in accordance with the Periodic Table of the Elements, CAS version, Handbook of Chemistry and Physics, 98th Ed.
- an "effective amount”is defined as the amount required to confer a therapeutic effect on the treated patient, and is typically determined based on age, surface area, weight, and condition of the patient. The interrelationship of dosages for animals and humans (based on milligrams per meter squared of body surface) is described by Freireich et al., Cancer Chemother.
- Body surface areamay be approximately determined from height and weight of the patient. See, e.g., Scientific Tables, Geigy Pharmaceuticals, Ardsley, New York, 537 (1970).
- patientrefers to a mammal, including a human.
- pharmaceutically acceptable saltrefers to any salt (e.g., obtained by reaction with an acid or a base) of a compound of the present disclosure that is physiologically tolerated in the target patient (e.g., a mammal). Salts of the compounds of the present disclosure may be derived from inorganic or organic acids and bases.
- acidsinclude, but are not limited to, hydrochloric, hydrobromic, sulfuric, nitric, perchloric, fumaric, maleic, phosphoric, glycolic, lactic, salicylic, succinic, toluene-p-sulfonic, tartaric, acetic, citric, methanesulfonic, ethanesulfonic, formic, benzoic, malonic, sulfonic, naphthalene-2-sulfonic, benzenesulfonic acid, and the like.
- Other acidssuch as oxalic, while not in themselves pharmaceutically acceptable, may be employed in the preparation of salts useful as intermediates in obtaining the compounds of the disclosure and their pharmaceutically acceptable acid addition salts.
- pharmaceutically acceptable carrierincludes any and all solvents, dispersion media, coatings, surfactants, antioxidants, preservatives ⁇ e.g., antibacterial agents, antifungal agents), isotonic agents, absorption delaying agents, salts, preservatives, drugs, drug stabilizers, gels, binders, excipients, disintegration agents, lubricants, sweetening agents, flavoring agents, dyes, such like materials and combinations thereof, as would be known to one of ordinary skill in the art (see, for example, Remington's Pharmaceutical Sciences, 18th Ed. Mack Printing Company, 1990, pp.1289-1329).
- pharmaceutically acceptable carrieror “pharmaceutical acceptable excipient” includes any material which, when combined with an active ingredient, allows the ingredient to retain biological activity and is non-reactive with the subject's immune system, and can include any and all solvents, diluents, carriers, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents, and the like that are physiologically compatible, non-toxic, and does not interfere with the mechanism of action of the compound of Formula I or a pharmaceutically acceptable salt thereof.
- the pharmaceutical acceptable excipientis suitable for intravenous, intramuscular, subcutaneous, parenteral, spinal or epidermal administration (e.g., by injection or infusion).
- a compound of Formula I or a pharmaceutically acceptable salt thereofmay be coated in a material to protect the compound from the action of acids and other natural conditions that may inactivate the compound.
- Pharmaceutically acceptable excipientsinclude sterile aqueous solutions or dispersions and sterile powders for the extemporaneous preparation of sterile injectable solutions or dispersion.
- the use of such media and agents for pharmaceutically active substancesis known in the art. Except insofar as any conventional media or agent is incompatible with the active compound, use thereof in the pharmaceutical compositions of the disclosure is contemplated. Supplementary active compounds can also be incorporated into the compositions.
- stable or chemically feasiblerefers to compounds that are not substantially altered when subjected to conditions to allow for their production, detection, and preferably their recovery, purification, and use for one or more of the purposes disclosed herein.
- a stable compound or chemically feasible compoundis one that is not substantially altered when kept at a temperature of 40 °C or less, in the absence of moisture or other chemically reactive conditions, for at least a week.
- the methods of treatment of the disclosurecomprise administering a safe and effective amount of a compound described herein or a pharmaceutically-acceptable salt thereof to a patient in need thereof.
- the term "subject”is intended to include human and non-human animals, and is used synonymously with the term "patient".
- Preferred subjectsinclude human patients in need of enhancement of an immune response that may be beneficial in the patient’s treatment of cancer and/or cancer metastasis.
- the methodsare particularly suitable for treating human patients having a disorder that can be treated by augmenting the T-cell mediated immune response.
- the methodsare particularly suitable for treatment of cancer cells in vivo.
- administeringrefers to the physical introduction of a therapeutic agent to a subject (e.g., a composition or formulation comprising the therapeutic agent), using any of the various methods and delivery systems known to those skilled in the art.
- Exemplary routes of administrationinclude intravenous, intramuscular, subcutaneous, intraperitoneal, spinal or other parenteral routes of administration, for example by injection or infusion.
- parenteral administrationmeans modes of administration other than enteral and topical administration, usually by injection, and includes, without limitation, intravenous, intramuscular, intraarterial, intrathecal, intralymphatic, intralesional, intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal, epidural and intrasternal injection and infusion, as well as in vivo electroporation.
- the therapeutic agentis administered via a non- parenteral route, in some aspects, orally.
- Other non-parenteral routesinclude a topical, epidermal or mucosal route of administration, for example, intranasally, vaginally, rectally, sublingually or topically.
- Administeringcan also be performed, for example, once, a plurality of times, and/or over one or more extended periods.
- Treatmentor “therapy” of a subject refers to any type of intervention or process performed on, or the administration of an active agent to, the subject with the objective of reversing, alleviating, ameliorating, inhibiting, slowing down progression, development, severity or recurrence of a symptom, complication or condition, or biochemical indicia associated with a disease.
- Response Evaluation Criteria In Solid TumorsRECIST is a measure for treatment efficacy and are established rules that define when tumors respond, stabilize, or progress during treatment.
- RECIST 1.1is the current guideline to solid tumor measurement and definitions for objective assessment of change in tumor size for use in adult and pediatric cancer clinical trials.
- effective treatmentrefers to treatment producing a beneficial effect, e.g., amelioration of at least one symptom of a disease or disorder.
- a beneficial effectcan take the form of an improvement over baseline, i.e., an improvement over a measurement or observation made prior to initiation of therapy according to the method.
- a beneficial effectcan also take the form of arresting, slowing, retarding, or stabilizing of a deleterious progression of a marker of solid tumor.
- Effective treatmentcan refer to alleviation of at least one symptom of a solid tumor.
- Such effective treatmentcan, e.g., reduce patient pain, reduce the size and/or number of lesions, reduce the size of the tumor mass, can reduce or prevent metastasis of a tumor, and/or can slow tumor growth.
- reduceor other forms of the word, such as “reducing” or “reduction,” is meant lowering of an event or characteristic (e.g., tumor growth). It is understood that this is typically in relation to some standard or expected value, in other words it is relative, but that it is not always necessary for the standard or relative value to be referred to.
- “reduces tumor growth”means reducing the rate of growth of a tumor relative to a standard or a control.
- an effective amountrefers to an amount of an agent that provides the desired biological, therapeutic, and/or prophylactic result. That result can be reduction, amelioration, palliation, lessening, delaying, and/or alleviation of one or more of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system.
- an effective amountcomprises an amount sufficient to cause a tumor to shrink and/or to decrease the growth rate of the tumor (such as to suppress tumor growth) or to delay other unwanted cell proliferation.
- an effective amountis an amount sufficient to prevent or delay tumor recurrence.
- An effective amountcan be administered in one or more administrations.
- the effective amount of the drug or compositioncan: (i) reduce the number of cancer cells; (ii) reduce tumor size; (iii) inhibit, retard, slow to some extent and can stop cancer cell infiltration into peripheral organs; (iv) inhibit (i.e., slow to some extent and can stop tumor metastasis; (v) inhibit tumor growth; (vi) prevent or delay occurrence and/or recurrence of tumor; and/or (vii) relieve to some extent one or more of the symptoms associated with the cancer.
- an “effective amount”is the amount of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, alone or the amount of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and the amount an additional therapeutic agent (e.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein), in combination, clinically proven to affect a significant decrease in cancer or slowing of progression of cancer, such as an advanced solid tumor.
- an additional therapeutic agente.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein
- the terms “fixed dose,” “flat dose,” and “flat-fixed dose”are used interchangeably and refer to a dose that is administered to a patient without regard for the weight or body surface area (BSA) of the patient.
- the fixed or flat doseis therefore not provided as a mg/kg dose, but rather as an absolute amount of the agent (e.g., an amount in ⁇ g or mg).
- fixed dose combinationwith regard to a composition of the invention means that two or more different inhibitors as described herein (e.g., a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof (e.g a first inhibitor), and the amount an additional therapeutic agent (e.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein; i.e. a second inhibitor) in a single composition are present in the composition in particular (fixed) ratios with each other.
- an additional therapeutic agente.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein; i.e. a second inhibitor
- the fixed doseis based on the weight (e.g., mg) of the first and second inhibitors. In certain aspects, the fixed dose is based on the concentration (e.g., mg/mL) of the first and second inhibitors. In some aspects, the ratio is at least about 1:1, about 1:2, about 1:3, about 1:4, about 1:5, about 1:6, about 1:7, about 1:8, about 1:9, about 1:10, about 1:15, about 1:20, about 1:30, about 1:40, about 1:50, about 1:60, about 1:70, about 1:80, about 1:90, about 1:100, about 1:120, about 1:140, about 1:160, about 1:180, about 1:200, about 200:1, about 180:1, about 160:1, about 140:1, about 120:1, about 100:1, about 90:1, about 80:1, about 70:1, about 60:1, about 50:1, about 40:1, about 30:1, about 20:1, about 15:1, about 10:1, about 9:1, about 8:1, about 7:1, about 6
- Dosing intervalmeans the amount of time that elapses between multiple doses of a formulation disclosed herein being administered to a subject. Dosing interval can thus be indicated as ranges.
- Dosing frequencyrefers to the frequency of administering doses of a formulation disclosed herein in a given time. Dosing frequency can be indicated as the number of doses per a given time, e.g., once a week or once in two weeks, etc.
- the terms “about once a week,” “once about every week,” “once about every two weeks,” or any other similar dosing interval terms as used hereinmeans approximate number, and “about once a week” or “once about every week” can include every seven days ⁇ two days, i.e., every five days to every nine days.
- the dosing frequency of “once a week”thus can be every five days, every six days, every seven days, every eight days, or every nine days.
- “Once about every three weeks”can include every 21 days ⁇ 3 days, i.e., every 25 days to every 31 days.
- a dosing interval of once about every six weeks or once about every twelve weeksmeans that the first dose can be administered any day in the first week, and then the next dose can be administered any day in the sixth or twelfth week, respectively.
- a dosing interval of once about every six weeks or once about every twelve weeksmeans that the first dose is administered on a particular day of the first week (e.g., Monday) and then the next dose is administered on the same day of the sixth or twelfth weeks (i.e., Monday), respectively.
- “Such as”has the same meaning as “such as but not limited to.”
- “include”has the same meaning as “include but not limited to,” while “including” has the same meaning as “including but not limited to.”
- Terms of degreesuch as “about”, “substantially”, and “approximately” as used herein mean a reasonable amount of deviation of the modified term such that the end result is not significantly changed.
- tumorrefers to a cell or population of cells whose growth, proliferation or survival is greater than growth, proliferation or survival of a normal counterpart cell, e.g. a cell proliferative or differentiative disorder. Typically, the growth is uncontrolled.
- malignancyrefers to invasion of nearby tissue.
- MTX-531described in the specification herein, for example in the Examples, and Figures; refers to a compound of Formula (I) referred to herein as “Compound 2R” or “COMPOUND 2R”.
- MTX-531(Compound 2R) is found in Table 1 and and and Fig.1 is known as N-(2-chloro-5-(4-((1R-phenylethyl)amino)quinazolin-6- yl)pyridin-3-yl)methanesulfonamide.
- Effective amount or “therapeutically effective amount” as disclosed in the present inventionrefers to the amount or dose of the compound or a pharmaceutically acceptable salt thereof according to the present invention which, upon single or multiple dose administration to the subject, provides the desired effect in the subject under diagnosis or treatment.
- itmay include an amount of a compound of the present disclosure that is sufficient to treat, prevent or inhibit a disease or condition such as cancer, in a subject that has in addition to cancer, a disease or condition associated with type II diabetes and/or hyperglycemia, (as used herein, the term “hyperglycemia” refers to higher than normal fasting blood glucose concentration, optionally at least 125 mg/dL) and/or insulin resistance.
- a disease or conditionsuch as cancer
- hyperglycemiarefers to higher than normal fasting blood glucose concentration, optionally at least 125 mg/dL
- insulin resistanceinsulin resistance.
- the amount of a given compound of the present disclosure that will correspond to such an amountwill vary depending upon various factors, such as the given compound, the composition, the route of administration, the type of disease or disorder, the identity of the subject or host being treated, and the like, but can nevertheless be routinely determined by one skilled in the art.
- a “therapeutically effective amount”is an amount sufficient to have a desired effect on a subject, such as have a therapeutic benefit with respect to the treatment of cancer.
- “Minimum effective dose” as disclosed in the present inventionrefers to the minimum administration dose at which the drug effect is produced. Specifically, as the drug effect experiment of multiple administrations on db/db mice in the present invention is concerned, it refers to the minimum dose at which the blood glucose AUC of the group taken the test compound is significantly different (p ⁇ 0.05) from that of the Vehicle group.
- “Efficacy dose” as disclosed in the present inventionrefers to the administration dose at which the drug effect is produced.
- “Vehicle group” as disclosed in the present inventionrefers to the vehicle control group.
- “Treatment” as disclosed in the present inventionincludes attenuating, inhibiting, reversing, slowing, delaying or halting the progression or severity of an existing condition, disease, disorder or symptom, for example, cancer.
- “Prevention” as disclosed in the present inventionincludes reducing the risk of acquiring a particular disease, disease condition or disorder.
- the term “primate”includes humans (male or female) and non-human primates.
- the term “non-human” primateincludes monkeys, such as a Cynomolgus Monkey.
- the term “subject”includes humans and animals, which includes pets.
- the terms “pet” and “companion animal”are interchangeable and include non-human primates, rodent animals (especially rodent mammals), and non-human and non-rodent animals (especially non- human and non-rodent mammals).
- the term “rodent animal” or “rodent mammal”includes mice and rats.
- the term “non-human and non-rodent animal” or “non-human and non-rodent mammal”means an animal or a mammal that is neither human nor rodent.
- non-human and non-rodent animalor “non-human and non-rodent mammal” includes, but is not limited to, dogs, cats, rabbits, pigs, alpacas, horses, sheep and bovines.
- EGFRis an acronym for epidermal growth factor receptor, a protein that helps cells grow and is involved in cell signaling pathways that control cell division and survival. EGFR is also involved in various processes related to cancer, such as cell proliferation, apoptosis, angiogenesis, and metastasis.
- the term EGFRcan include human and animal variants of EGFR.
- the term EGFRrelates to human EGFR, which is illustratively exemplified as UniProt P00533, and has an amino acid sequence as provided in SEQ ID NO: 1.
- PI3Kis an acronymm for the term “Phosphatidylinositol-3 kinase”.
- PI3Kis a family of enzymes that send signals in cells and help control cell growth.
- the term PI3Kincludes all of the class I PI3K human enzymes, including, human PI3K-alpha, PI3K-beta, PI3K-delta, and PI3K-gamma.
- compounds of the present disclosureinhibit a PI3K enzyme, for example, human PI3K-gamma which is illustratively exemplified as UniProt P48736, and has an amino acid sequence as provided in SEQ ID NO: 2.
- compounds of the present disclosureinhibit a PI3K enzyme, for example, human PI3K-alpha which is illustratively exemplified as UniProt P42336, and has an amino acid sequence as provided in SEQ ID NO: 4.
- compounds of the present disclosureinhibit a PI3K enzyme, for example, human PI3K-beta, which is illustratively exemplified as UniProt P42338, and has an amino acid sequence as provided in SEQ ID NO: 5.
- compounds of the present disclosureinhibit a PI3K enzyme, for example, human PI3K-delta which is illustratively exemplified as UniProt O00329, and has an amino acid sequence as provided in SEQ ID NO: 6.
- a PI3K enzymefor example, human PI3K-delta which is illustratively exemplified as UniProt O00329, and has an amino acid sequence as provided in SEQ ID NO: 6.
- PPAR-gammais an acronym for the term “Peroxisome proliferator- activated receptor gamma (PPAR- ⁇ or PPARG.
- PPAR-gammais a transcription factor and nuclear receptor that regulates gene expression and controls how the body uses glucose and lipids.
- PI3Kis human PI3K-gamma which is illustratively exemplified as UniProt P37231, and has an amino acid sequence as provided in SEQ ID NO: 3.
- an "alkyl” grouprefers to a saturated aliphatic hydrocarbon group containing 1-12 (e.g., 1-8, 1-6, or 1-4) carbon atoms. An alkyl group can be straight or branched.
- alkyl groupsinclude, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, n-heptyl, or 2-ethylhexyl.
- an "alkenyl” grouprefers to an aliphatic carbon group that contains 2-8 (e.g., 2-12, 2-6, or 2-4) carbon atoms and at least one double bond. Like an alkyl group, an alkenyl group can be straight or branched.
- alkenyl groupexamples include, but are not limited to allyl, isoprenyl, 2-butenyl, and 2-hexenyl.
- an "alkynyl” grouprefers to an aliphatic carbon group that contains 2-8 (e.g., 2-12, 2-6, or 2-4) carbon atoms and has at least one triple bond.
- An alkynyl groupcan be straight or branched. Examples of an alkynyl group include, but are not limited to, propargyl and butynyl.
- an “alkylene” grouprefers to a bivalent alkyl group that connects to two attachment points simultaneously, wherein the alkylene unit can be bivalent on the same carbon or two different carbons of the alkyl moiety.
- alkylene groupsare, without limitation, methylene, ethylene, propylene, and butylene, as well as branched structures, such as –CH 2 (CH 2 )- (1,1-ethylene) and – CH 2 CH 2 (CH 2 )- (1,2-propylene).
- an “aryl” grouprefers to a mono-, bi-, or tri-cyclic ring system wherein all rings in the system are aromatic and contain no heteroatoms in the ring.
- aryl groupsinclude, but are not limited to phenyl, naphthyl, anthracenyl, and tetracenyl.
- a "carbocycle” or “carbocyclyl” grouprefers to a mono-, bi-, or tricyclic (fused or bridged) hydrocarbon ring system that contains no heteroatoms in the ring structures, wherein at least one of the rings in the system is non-aromatic, and can be completely saturated or partially unsaturated.
- the terms “carbocycle” or “carbocyclyl”encompass a "cycloalkyl” group and a "cycloalkenyl” group, each of which is set forth below.
- a "cycloalkyl” grouprefers to a saturated carbocyclic mono-, bi-, or tricyclic (fused or bridged) ring system of 3-20 (e.g., 5-10) carbon atoms.
- cycloalkyl groupsinclude cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, adamantyl, norbornyl, cubyl, octahydro-indenyl, decahydro-naphthyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl, bicyclo[3.3.1]nonyl, bicyclo[3.3.2.]decyl, bicyclo[2.2.2]octyl, adamantyl, or ((aminocarbonyl)cycloalkyl)cycloalkyl.
- a "cycloalkenyl” grouprefers to a non-aromatic carbocyclic mono-, bi, or tricyclic (fused or bridged) ring system of 3-20 (e.g., 4-8) carbon atoms, wherein at least one ring in the system has one or more double bonds.
- cycloalkenyl groupsinclude cyclopentenyl, 1,4-cyclohexa-di-enyl, cycloheptenyl, cyclooctenyl, hexahydro-indenyl, octahydro-naphthyl, cyclohexenyl, cyclopentenyl, bicyclo[2.2.2]octenyl, or bicyclo[3.3.1]nonenyl.
- heterocycleand “heterocyclyl” are used interchangeably and refer to a mono-, bi-, or tricyclic (fused or bridged) non-aromatic hydrocarbon ring system that contains at least one heteroatom in the ring structure and can be completely saturated or partially unsaturated.
- heterocycleand “heterocyclyl” encompass a “heterocycloalkyl” group and a “heterocycloalkenyl” group, each of which is set forth below.
- heterocycloalkylrefers to a 3-20 membered mono-, di-, or tricylic (fused or bridged) (e.g., 5- to 10-membered) saturated ring structure, in which one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof).
- heterocycloalkyl groupexamples include piperidyl, piperazyl, tetrahydropyranyl, tetrahydrofuryl, 1,4- dioxolanyl, 1,4-dithianyl, 1,3-dioxolanyl, oxazolidyl, isoxazolidyl, morpholinyl, thiomorpholyl, octahydrobenzofuryl, octahydrochromenyl, octahydrothiochromenyl, octahydroindolyl, octahydropyrindinyl, decahydroquinolinyl, octahydrobenzo[b]thiopheneyl, 2-oxa- bicyclo[2.2.2]octyl, 1-aza-bicyclo[2.2.2]octyl, 3-aza-bicyclo[3.2.1]octyl, and 2,6-dioxa-
- a "heterocycloalkenyl” grouprefers to a 3-20 membered mono-, di-, or tricylic (fused or bridged) (e.g., 5- to 10-membered) non-aromatic ring structure, in which one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof), and wherein at least one of the ring structures has one or more double bonds.
- a “heteroaryl” grouprefers to a monocyclic, bicyclic, or tricyclic ring system having 4 to 15 ring atoms wherein one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof) and in which the monocyclic ring system is aromatic or at least one of the rings in the bicyclic or tricyclic ring systems is aromatic.
- a heteroaryl groupincludes a benzofused ring system having 2 to 3 rings.
- a benzofused groupincludes benzo fused with one or two 4 to 8 membered heterocycloaliphatic moieties (e.g., indolizyl, indolyl, isoindolyl, 3H-indolyl, indolinyl, benzo[b]furyl, benzo[b]thiophenyl, quinolinyl, or isoquinolinyl).
- heterocycloaliphatic moietiese.g., indolizyl, indolyl, isoindolyl, 3H-indolyl, indolinyl, benzo[b]furyl, benzo[b]thiophenyl, quinolinyl, or isoquinolinyl.
- heteroarylexamples include azetidinyl, pyridyl, 1H-indazolyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, tetrazolyl, benzofuryl, isoquinolinyl, benzothiazolyl, xanthene, thioxanthene, phenothiazine, dihydroindole, benzo[1,3]dioxole, benzo[b]furyl, benzo[b]thiophenyl, indazolyl, benzimidazolyl, benzthiazolyl, puryl, cinnolyl, quinolyl, quinazolyl,cinnolyl, phthalazyl, quinazolyl, quinoxalyl, isoquinolyl, 4H-quinolizyl, benzo-1,2,5-thiadiazolyl, or 1,
- monocyclic heteroarylsinclude furyl, thiophenyl, 2H- pyrrolyl, pyrrolyl, oxazolyl, thazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,3,4- thiadiazolyl, 2H-pyranyl, 4-H-pranyl, pyridyl, pyridazyl, pyrimidyl, pyrazolyl, pyrazyl, or 1,3,5- triazyl.
- bicyclic heteroarylsinclude indolizyl, indolyl, isoindolyl, 3H- indolyl, indolinyl, benzo[b]furyl, benzo[b]thiophenyl, quinolinyl, isoquinolinyl, indolizinyl, isoindolyl, indolyl, benzo[b]furyl, bexo[b]thiophenyl, indazolyl, benzimidazyl, benzthiazolyl, purinyl, 4H-quinolizyl, quinolyl, isoquinolyl, cinnolyl, phthalazyl, quinazolyl, quinoxalyl, 1,8- naphthyridyl, or pteridyl.
- cyclic moietyand “cyclic group” refer to mono-, bi-, and tri- cyclic ring systems including cycloaliphatic, heterocycloaliphatic, aryl, or heteroaryl, each of which has been previously defined.
- a "bridged bicyclic ring system”refers to a bicyclic heterocyclicaliphatic ring system or bicyclic cycloaliphatic ring system in which the rings are bridged.
- bridged bicyclic ring systemsinclude, but are not limited to, adamantanyl, norbornanyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl, bicyclo[3.3.1]nonyl, bicyclo[3.2.3]nonyl, 2-oxabicyclo[2.2.2]octyl, 1-azabicyclo[2.2.2]octyl, 3-azabicyclo[3.2.1]octyl, and 2,6-dioxa- tricyclo[3.3.1.0.3.7]nonyl. [00181] As used herein, an "alkoxy" group refers to an alkyl-O- group where "alkyl" has been defined previously.
- a "carbonyl”refers to -C(O)-.
- a “carboxy”refers to -C(O)OH.
- an “ester”refers to –C(O)O-W, in which W is, for example, alkyl, carbocyclyl, or heterocyclyl.
- compounds of the disclosuremay optionally be substituted with one or more substituents, such as are illustrated generally herein, or as exemplified by particular classes, subclasses, and species of the disclosure.
- an optionally substituted groupcan have a substituent at each substitutable position of the group, and when more than one position in any given structure can be substituted with more than one substituent selected from a specified group, the substituent can be either the same or different at every position.
- a ring substituentsuch as a heterocycloalkyl, can be bound to another ring, such as a cycloalkyl, to form a spiro- bicyclic ring system, e.g., both rings share one common atom.
- substituents envisioned by this disclosureare those combinations that result in the formation of stable or chemically feasible compounds.
- treatin reference to a condition means: (1) to ameliorate, diminish or counteract the condition or one or more of the biological manifestations of the condition, (2) to interfere with (a) one or more points in the biological cascade that leads to or is responsible for the condition or (b) one or more of the biological manifestations of the condition, (3) to alleviate one or more of the symptoms or effects associated with the condition, or (4) to slow the progression of the condition or one or more of the biological manifestations of the condition.
- structures depicted hereinare also meant to include compounds that differ only in the presence of one or more isotopically enriched atoms.
- isotopesthat can be incorporated into compounds of the disclosure and pharmaceutically acceptable salts thereof include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, sulphur, fluorine, iodine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 15 N, 17 0, 18 0, 31 P, 32 P, 35 S, 18 F, 36 Cl, 123 I and 125 I.
- isotopesof hydrogen, carbon, nitrogen, oxygen, phosphorous, sulphur, fluorine, iodine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 15 N, 17 0, 18 0, 31 P, 32 P, 35 S, 18 F, 36 Cl, 123 I and 125 I.
- Compounds of the present disclosure and pharmaceutically acceptable salts of said compounds that contain the aforementioned isotopes and/or other isotopes of other atomsare within the scope of the present disclosure.
- Isotopically-labelled compounds of the present disclosurefor example those into which radioactive isotopes, such as 3 H and 14 C, are incorporated, are useful in drug and/or substrate tissue distribution assays.
- Tritiated hydrogen ( 3 H) and carbon-14 ( 14 C) isotopesare particularly preferred for their ease of preparation and detectability.
- 11 C and 18 F isotopesare particularly useful in PET (positron emission tomography), and 125 l isotopes are particularly useful in SPECT (single photon emission computerized tomography), all useful in brain imaging.
- Isotopically labeled compounds of the disclosurecan generally be prepared by carrying out the procedures disclosed in the schemes and/or in the examples below, and substituting a readily available isotopically labeled reagent for a non-isotopically labeled reagent.
- structures depicted hereinare also meant to include all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or conformational)) forms of the structure.
- “Isomer”refers to compounds that have the same composition and molecular weight but differ in physical and/or chemical properties; for example (Z) and (E) double bond isomers, and (Z) and (E) conformational isomers.
- the structural differencemay be in constitution (geometric isomers) or in the ability to rotate the plane of polarized light (stereoisomers); for example, the R and S configurations for each asymmetric center.
- the compounds of the disclosuremay contain one or more asymmetric centers, also referred to as chiral centers, and may, therefore, exist as individual enantiomers, diastereomers, or other stereoisomeric forms, or as mixtures thereof. All such isomeric forms are included within the present disclosure, including mixtures thereof.
- Chiral centersmay also be present in a substituent such as an alkyl group.
- a substituentsuch as an alkyl group.
- the structureis intended to encompass any stereoisomer and all mixtures thereof.
- compounds of the disclosure containing one or more chiral centersmay be used as racemic mixtures, enantiomerically enriched mixtures, or as enantiomerically pure individual stereoisomers.
- Individual stereoisomers of a compound of the disclosure which contain one or more asymmetric centersmay be resolved by methods known to those skilled in the art.
- such resolutionmay be carried out (1) by formation of diastereoisomeric salts, complexes or other derivatives; (2) by selective reaction with a stereoisomer-specific reagent, for example by enzymatic oxidation or reduction; or (3) by gas-liquid or liquid chromatography in a chiral environment, for example, on a chiral support such as silica with a bound chiral ligand or in the presence of a chiral solvent.
- a stereoisomer-specific reagentfor example by enzymatic oxidation or reduction
- gas-liquid or liquid chromatographyin a chiral environment, for example, on a chiral support such as silica with a bound chiral ligand or in the presence of a chiral solvent.
- the disclosureincludes a compound of Formula I or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring A is selected from phenyl or a 5 or 6 membered heteroaryl; X is N or C-R 5 ; R 1a is selected from the group consisting of H or C 1-6 alkyl; R 1b is selected from the group consisting of C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’) 2 , C(O)R’, C(O)OR’, C(O)N(R’) 2 , halo, CN, and NO 2 , wherein each C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or hetero
- R 1ais H.
- R 1bis C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC 1-6 alkyl, OH, NH 2 , NHC 1-6 alkyl, N(C 1-6 alkyl) 2 , C(O)C 1-6 alkyl, C(O)OC 1-6 alkyl, C(O)NH 2 , C(O)NHC 1-6 alkyl, C(O)N(C 1-6 alkyl) 2 , halo, CN, or NO 2 , wherein each C 1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with OC 1-6 alkyl, oxo, OH, halo, CN, NH 2 , NHC 1-6 alkyl, N(C 1-6 alkyl) 2 , C(C 1-6 alkyl) 2 , C(C
- R 1bis C 1-6 alkyl, OC 1-6 alkyl, OH, NH 2 , NHC 1-6 alkyl, N(C 1-6 alkyl) 2 , C(O)C 1-6 alkyl, C(O)NH 2 , C(O)NHC 1-6 alkyl, C(O)N(C 1-6 alkyl) 2 , halo, or CN, wherein each C 1-6 alkyl is optionally and independently substituted with OC 1-6 alkyl, OH, halo, or CN.
- R 1bis C 1-6 alkyl, OC 1-6 alkyl, OH, halo, or CN, wherein each C 1-6 alkyl is optionally and independently substituted with OH, CN, or halo.
- R 1bis C 1-6 alkyl, C 1-6 alkyl-OH, or CN.
- R 1bis methyl, CN, or CH 2 OH.
- R 1a and R 1btogether with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C 3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with C 1-6 alkyl, OC 1-6 alkyl, oxo, OH, halo, CN, C(O)C 1-6 alkyl, C(O)OC 1-6 alkyl, C(O)NH 2 , C(O)NHC 1-6 alkyl, or C(O)N(C 1-6 alkyl) 2 , wherein each C 1-6 alkyl is optionally and independently substituted with one or substituents selected from halo, alkoxy, CN, or NH 2 .
- R 1a and R 1btogether with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C 3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with oxo, OH, halo, or CN.
- R 1a and R 1btogether with the methylene moiety to which they are attached, form an unsubstituted spirocyclic ring selected from a C 3-7 cycloalkyl or 3-7 membered heterocycloalkyl.
- R 1a and R 1btogether with the methylene moiety to which they are attached, form a [00211]
- each R 2 substituentis independently selected from halo, OH, C 1-6 alkyl, haloalkyl, OC 1-6 alkyl, CN, NH 2 , C(O)C 1-6 alkyl, and C(O)NH 2 .
- each R 2 substituentis independently selected from halo and OH.
- nis 0 or 1.
- nis 0.
- one R 2 substituent and R 1btogether with the phenyl group to which R 2 is attached and the carbon atom to which R 1b is attached, form a bicyclic group having the general structure wherein Ring B is a C 3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C 1-6 alkyl, haloalkyl, OC 1-6 alkyl, CN, NH 2 , C(O)C 1-6 alkyl, C(O)OC 1-6 alkyl, or C(O)NH 2 .
- Ring Bis a C 4-6 cycloalkyl or 4-6 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, OC 1-6 alkyl, CN, or NH 2 , C(O)C 1-6 alkyl.
- Ring Bis a C 4-5 cycloalkyl or 5 membered heterocycloalkyl, each of which is optionally substituted with OH.
- each R 3is independently selected from C 1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO 2 , OH, OC 1-6 alkyl, N(C 1- 6 alkyl) 2 , NH(C 1-6 alkyl), NH 2 , C(O)H, C(O)C 1-6 alkyl, COOH, C(O)OC 1-6 alkyl, C(O)NH 2 , C(O)NH(C 1-6 alkyl), C(O)NH(C 3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C 1-6 alkyl)(OH), C(O)N(C 1-6 alkyl) 2 , OC(O)OC 1-6 alkyl, OC(O)NH 2 , OC(O)NH 2 , OC(O)NH 2 , OC(O)NH 2 , OC
- each R 3is independently selected from C 1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO 2 , OH, OC 1-6 alkyl, N(C1- 6 alkyl) 2 , NH(C 1-6 alkyl), NH 2 , C(O)H, C(O)C 1-6 alkyl, COOH, C(O)OC 1-6 alkyl, C(O)NH 2 , C(O)NH(C 1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C 1-6 alkyl)(OH), C(O)N(C 1-6 alkyl) 2 , OC(O)OC 1-6 alkyl, OC(O)NH 2 , OC(O)NH(C 1-6 alkyl), OC(O)N(C 1-6 alkyl),
- each R 3is independently selected from C 1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC 1-6 alkyl, NH 2 , C(O)H, C(O)NH 2 , C(O)NH(C 1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C 1-6 alkyl)(OH), C(O)N(C 1-6 alkyl) 2 , NHC(O)NH 2 , NHC(O)NH(C 1-6 alkyl), NHC(O)N(C 1-6 alkyl) 2 , N(C 1-6 alkyl)C(O)NH 2 , N(C 1-6 alkyl)C(O)C(O)N(C 1-6 alkyl), N(C 1-6 alkyl)C(O)N(
- each R 3is independently selected from C 1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC 1-6 alkyl, NH 2 , C(O)H, C(O)NH 2 , C(O)NH(C 1-6 alkyl), C(O)NH(C 3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C 1-6 alkyl)(OH), C(O)N(C 1-6 alkyl) 2 , NHC(O)NH(C 1-6 alkyl), SO 2 (C 1-6 alkyl), NHSO 2 (C 1-6 alkyl), wherein each C 1-6 alkyl, heteroaryl, and heterocycloalkyl are each optionally and independently substituted with one or more R” substituents.
- each R 3is independently selected from C 1-6 alkyl, triazolyl, oxadiazolyl, halo, CN, OH, OC 1-6 alkyl, NH 2 , C(O)H, C(O)NH 2 , C(O)NH(C 1-6 alkyl), C(O)NH(C 3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C 1-6 alkyl)(OH), C(O)N(C 1-6 alkyl) 2 , NHC(O)NH(C 1-6 alkyl), SO 2 (C 1-6 alkyl), NHSO 2 (C 1-6 alkyl), wherein each C 1-6 alkyl, triazolyl and oxadiazolyl, are each optionally and independently substituted with one or more substituents independently selected from oxo, OH, halo, C 1-6 alkyl, NH 2 , NHC(O)NH(C 1-6 alkyl
- each R 3 substituentis independently selected from halo, CN, NH 2 , OH, C(O)H, C(O)N(CH 3 ) 2 , C(O)NH(CH 3 ), C(O)NH(Et), C(O)NH(isopropyl), C(O)NH(tert-butyl), C(O)NH(cyclopropyl), C(O)NCH 3 (CN), C(O)NH(OH), C(O)NCH 3 (OH), C(O)NH 2 , NHC(O)NHCH 3 , NHS(O) 2 CH 3 , S(O) 2 CH 3 , methyl, methoxy, NHS(O) 2 CH 2 CH 2 N(CH 3 ) 2 , CF 3 , CH 2 OH, C(CH 3 ) 2 OH, , , , [00225]
- the compound of Formula Iis a compound of Formula Ia or a pharmaceutically acceptable salt or
- the ringis a phenyl, pyridyl, pyrimidyl, pyrazyl, or triazyl, which is optionally substituted by R 3 .
- the ring[00228] In one embodiment, p is 0, 1, or 2.
- pis 1 or 2.
- the ringis selected from
- two R 3 substituents, together with Ring A, to which they are attachedform a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more R” substituents.
- two R 3 substituents, together with Ring A, to which they are attachedform a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C 1-6 alkyl, halo, OH, OC 1-6 alkyl, oxo, CN, NH 2 , NH(C 1-6 alkyl), and N(C 1-6 alkyl) 2 .
- two R 3 substituentstogether with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C 1-6 alkyl, oxo, and NH 2 .
- the compound of Formula Iis a compound of Formula Ib or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring C is a phenyl, a 5-6 membered heteroaryl, or a 5-6 membered heterocyclic ring; and X’ is CH, C-R”, or N.
- Ring C of Formula Ibis a phenyl ring.
- Ring C of Formula Ibis a 5 membered heteroaryl or a 5 membered heterocyclic ring.
- Ring C of Formula Ibis pyrrole, pyrazole, imidazole, triazole, oxazole, hydrofuran, dihydrofuran, hydropyrrole, dihydropyrrole, hydroimidazole, dihydroimidazole, hydrooxazole, dihydrooxazole, or [00238]
- the compound of Formula Iis a compound of Formula Ic-1, Formula Ic-2, or Formula Ic-3: or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein each X’ is independently CH or N.
- the ring of Formula Ic-1is [00240] In another embodiment, the ring of Formula Ic-2 is or [00241] In another embodiment, the ring of Formula Ic-3 is [00242] In a further embodiment, Ring A is a bicyclic group selected from [00243] In one embodiment, m is 0. [00244] In another embodiment, X is N. [00245] In another embodiment, X is C-H or C-CN. [00246] In a further embodiment, X is C-CN.
- the disclosureincludes a pharmaceutical composition comprising a compound of Formula I, Ia, Ib, Ic-1, Ic-2, or Ic-3, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof described herein and a pharmaceutically acceptable excipient.
- the disclosureincludes a method of modulating the activity of an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound, salt or a composition described herein.
- the disclosureincludes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound, salt or a composition described herein.
- the EGFR and/or PI3K mediated diseaseis a cancer.
- the canceris selected from neoplasm, giant and spindle cell carcinoma; small cell carcinoma; papillary carcinoma; squamous cell carcinoma; lymphoepithelial carcinoma; basal cell carcinoma; pilomatrix carcinoma; transitional cell carcinoma; papillary transitional cell carcinoma; adenocarcinoma, gastrinoma, cholangiocarcinoma, hepatocellular carcinoma; combined hepatocellular carcinoma and cholangiocarcinoma, trabecular adenocarcinoma, adenoid cystic carcinoma; adenocarcinoma in adenomatous polyp; adenocarcinoma, familial polyposis coli, solid carcinoma; carcinoid tumor, bronchiolo-alveolar adenocarcinoma, papillary adenocarcinoma, chromophobe carcinoma; acidophil carcinoma; oxyphilic adenocarcinoma, basophil carcinoma; clear cell aden
- the canceris selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, (cancers of the ovaries and uterus), thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, breast cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma.
- the canceris selected from breast cancer, carcinomas of the head and neck, head and neck cancer, and skin cancer.
- the head and neck canceris squamous head and neck cancer.
- the breast canceris triple negative breast cancer.
- the canceris a metastatic or a malignant cancer.
- the compound of Formula Iis a compound selected from the compounds listed in Table 1, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof.
- a compound or salt described hereinhas a solubility that is at least 0.5 times more soluble that a compound of Formula X or X’: wherein R 2 , R 3 , R 4 , X, m, n, p, and Ring A are defined as for Formula I, as measured in simulated intestinal fluid pH of 6.8.
- the compound or saltis at least 2 times more soluble that a compound of Formula X or X’.
- the compound or saltis at least 5 times more soluble that a compound of Formula X or X’.
- the compound or saltis at least 10 times more soluble that a compound of Formula X or X’.
- the compound of Formula Xis Comparative Compound 2
- the compound of Formula X’is Comparative Compound 1 or Comparative Compound 2:
- Table 1Compounds of the disclosure listed by name and structure Docket No.262340-557381 Docket No.262340-557381 Docket No.262340-557381 Docket No.262340-557381
- the disclosureprovides a compound of Formula Ia-1 or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein, each X’ is N, C-R 3 , or CH; R 1a is selected from the group consisting of H or C 1-6 alkyl; R 1b is selected from the group consisting of
- R 1ais H.
- R 1bis C 1-6 alkyl, C 1-6 alkyl-OH, or CN.
- R 1bis methyl, CN, or CH 2 OH.
- each R 2 substituentis independently selected from halo and OH.
- nis 0.
- each R 3 substituentis independently selected from halo, CN, NH 2 , OH, C(O)H, C(O)N(CH 3 ) 2 , C(O)NH(CH 3 ), C(O)NH(Et), C(O)NH(isopropyl), C(O)NH(tert-butyl), C(O)NH(cyclopropyl), C(O)NCH 3 (CN), C(O)NH(OH), C(O)NCH 3 (OH), C(O)NH 2 , NHC(O)NHCH 3 , NHS(O) 2 CH 3 , S(O) 2 CH 3 , methyl, methoxy, NHS(O) 2 CH 2 CH 2 N(CH 3 ) 2 , CF 3 , CH 2 OH, C(CH 3 ) 2 OH, [00270]
- the ringis a phenyl, pyridyl, pyrimidyl, pyrazyl
- the ring[00271] In a further embodiment, the ring [00272] In one embodiment, p is 1 or 2. [00273] In one embodiment, the compound of Formula Ia-1 is a compound of Formula Ia- 2 [00274] In another embodiment, the ring of Formula Ia-2 is selected from
- the compound of Formula Ia-1is a compound selected from Compounds 1, 2, 2R, 2S, 3-6, 11, 14-16, 21, 22, 29-33, 36, 37, 45, 107-111, 111R, 111S, 112, 113, 118, 132-135, 138, 141, 142, and 148.
- the disclosureincludes a pharmaceutical composition comprising a compound or salt described herein, and a pharmaceutically acceptable excipient.
- the disclosureincludes a method of modulating an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound or salt described herein.
- the disclosureincludes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound or salt described herein.
- the EGFR and/or PI3K mediated diseaseis a cancer.
- the canceris selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma.
- the canceris a metastatic or a malignant cancer.
- the canceris selected from breast cancer, carcinomas of the head and neck, head and neck cancer, and skin cancer.
- the head and neck canceris squamous head and neck cancer.
- the breast canceris triple negative breast cancer.
- the disclosureprovides a compound of Formula I, wherein the compound of Formula I has an increased solubility compared to a compound of Formula X or a compound of Formula X’ Wherein R 1a , R 1b , R 2 , R 3 , R 4 , X, m, n, p, and Ring A are all defined herein.
- R 1ais hydrogen and R 1b is C 1-6 alkyl.
- R 1ais hydrogen and R 1b is methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, t- butyl, pentyl, or hexyl.
- R 1ais hydrogen and R 1b is methyl.
- the compound of Formula Iis selected from a compound listed in Table 1.
- the compound of Formula Iis selected from Compound 2, 2R, 2S, 8, 10, 22, 48, 48R, 48S, 111, 111R, and 111S.
- the compound of Formula Iis selected from Compound 2R, 48R, and 111R.
- the compound of Formula X’is Comparative Compound 1 or Comparative Compound 3, which have the structures below. Comparative Compound 1 Comparative Compound 3
- the compound of Formula Xis Comparative Compound 2, which has the structure Comparative Compound 2
- the compound of Formula Iis at least 0.5 times more soluble than a compound of Formula X or a compound of Formula X’.
- the compound of Formula Iis at least twice as soluble as a compound of Formula X or a compound of Formula X’.
- the compound of Formula Iis at least 5.0 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 10 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 50 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 100 times more soluble than a compound of Formula X or a compound of Formula X’.
- the compound of Formula Ihas increased solubility over a compound of Formula X or a compound of Formula X’ in phosphate buffered saline (PBS) at about pH 7.4.
- PBSphosphate buffered saline
- the compound of Formula Ihas increased solubility over a compound of Formula X or a compound of Formula X’ in simulated intestinal fluid (SIF).
- the compound of Formula Ihas increased solubility over a compound of Formula X or a compound of Formula X’ in simulated gastric fluid (SGF).
- a pharmaceutically acceptable derivativeincludes, but is not limited to, pharmaceutically acceptable prodrugs, salts, esters, salts of such esters, or any other adduct or derivative that upon administration to a patient in need is capable of providing, directly or indirectly, a compound as otherwise described herein, or a metabolite or residue thereof.
- the term "pharmaceutically acceptable salt”refers to those salts that are, within the scope of sound medical judgement, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like.
- Pharmaceutically acceptable saltsare well known in the art. For example, S. M. Berge et al., describe pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences, 1977, 66, 1-19, incorporated herein by reference.
- Pharmaceutically acceptable salts of the compounds of this disclosureinclude those derived from suitable inorganic and organic acids and bases.
- Examples of pharmaceutically acceptable, nontoxic acid addition saltsinclude salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- inorganic acidssuch as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid
- organic acidssuch as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- saltsinclude adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate,
- Salts derived from appropriate basesinclude alkali metal, alkaline earth metal, ammonium and N + (C 1-4 alkyl) 4 salts. This disclosure also envisions the quaternization of any basic nitrogen-containing groups of the compounds disclosed herein. Water or oil-soluble or dispersable products may be obtained by such quaternization.
- Representative alkali or alkaline earth metal saltsinclude sodium, lithium, potassium, calcium, magnesium, and the like.
- a compound of Formula I, or a pharmaceutically acceptable salt thereofcan be formulated as pharmaceutical compositions comprising a therapeutically or prophylactically effective amount of the compound or salt, and one or more pharmaceutically compatible (acceptable) ingredients.
- compositions of a compound of Formula I, or a pharmaceutically acceptable salt thereof, and pharmaceutical excipientsare provided in which an effective amount of the compound or salt, is in admixture with the excipients, suitable for administration to a mammal.
- a compound of Formula I, or a pharmaceutically acceptable salt thereofis formulated for administration to a human.
- the present disclosureprovides a pharmaceutical composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, formulated for administration to a human subject in need thereof.
- the formulated composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereofwill generally comprise one or more pharmaceutically compatible (acceptable) ingredients.
- Exemplary pharmaceutical or non-pharmaceutical compositionstypically include one or more carriers (e.g., sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like). Water is a more typical carrier when the pharmaceutical composition is administered intravenously. Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions.
- carrierse.g., sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like.
- Wateris a more typical carrier when the pharmaceutical composition is administered intravenously.
- Saline solutions and aqueous dextrose and glycerol solutionscan also be employed as liquid carriers, particularly for injectable solutions.
- Suitable excipientsinclude, for example, amino acids, starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol, and the like.
- the compositionif desired, can also contain minor amounts of wetting or emulsifying agents, or pH buffering agents. These compositions can take the form of solutions, suspensions, emulsion, tablets, pills, capsules, powders, sustained-release formulations and the like. Examples of suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences" by E. W. Martin.
- compositionswill typically contain a therapeutically effective amount of a compound of Formula I, or a pharmaceutically acceptable salt thereof, in purified form, together with a suitable amount of carrier so as to provide the form for proper administration to the subject.
- the formulationscorrespond to the mode of administration.
- the pharmaceutically acceptable carrier or vehiclecan be particulate, so that the compositions are, for example, in tablet or powder form.
- the carrier(s)can be liquid, with the compositions being, for example, an oral syrup, flavored water, or injectable liquid.
- the compositionis preferably in solid or liquid form, where semi-solid, semi-liquid, suspension and gel forms are included within the forms considered herein as either solid or liquid.
- the compositioncan be formulated into a powder, granule, compressed tablet, pill, capsule, chewing gum, wafer or the like form.
- a solid compositiontypically contains one or more inert diluents.
- binderssuch as carboxymethylcellulose, ethyl cellulose, microcrystalline cellulose, or gelatin
- excipientssuch as starch, lactose or dextrins, disintegrating agents such as alginic acid, sodium alginate, Primogel, corn starch and the like
- lubricantssuch as magnesium stearate or Sterotex
- glidantssuch as colloidal silicon dioxide
- sweetening agentssuch as sucrose or saccharin, a flavoring agent such as peppermint, methyl salicylate or orange flavoring, and a coloring agent.
- compositionWhen the composition is in the form of a capsule, e.g., a gelatin capsule, it can contain, in addition to materials of the above type, a liquid carrier such as polyethylene glycol, cyclodextrin or fatty oil.
- a liquid carriersuch as polyethylene glycol, cyclodextrin or fatty oil.
- the compositioncan be in the form of a liquid, e.g., an elixir, syrup, solution, emulsion or suspension.
- the liquidcan be useful for oral administration or for delivery by injection.
- a compositioncan comprise one or more of a sweetening agent, preservatives, dye/colorant, and flavor enhancer.
- the compositionis formulated into a powder and the end user mixes the power in aqueous solution for oral administration.
- compositions for administration by injectioninclude one or more of a surfactant, preservative, wetting agent, dispersing agent, suspending agent, buffer, stabilizer and isotonic agent.
- a surfactantpreservative, wetting agent, dispersing agent, suspending agent, buffer, stabilizer and isotonic agent.
- capsulesmay be prepared from gelatin (e.g., Type A, Type B), carrageenan (e.g., kappa, iota, lambda) and/or modified cellulose (e.g., hydroxypropyl methyl cellulose, methyl cellulose, hydroxypropyl methyl cellulose acetate succinate, hydroxypropyl methyl cellulose phthalate, cellulose acetate phthalate), and optionally one or more excipients such as oils (e.g., fish oil, olive oil, corn oil, soybean oil, coconut oil, tri-, di- and monoglycerides), plasticizers (e.g., glycerol, glycerin, sorbitol, polyethylene glycol, citric acid, citric acid esters such as triethylcitrate, polyalcohols), co-solvents (e.g., triacetin, propylene carbonate, ethyl lactate, propylene glycol, ole
- Capsulesmay be hard or soft.
- hard capsulesinclude ConiSnap®, DRcaps®, OceanCaps®, Pearlcaps®, Plantcaps®, DUOCAP®, Vcaps®, and Vcaps®. Plus capsules available from Capsugel®.
- Hard capsulesmay be prepared, for example, by forming two telescoping capsule halves, filling one of the halves with a fill comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, and sealing the capsule halves together.
- the fillmay be in any suitable form, such as dry powder, granulation, suspension or liquid.
- soft capsulesinclude soft gelatin (also called softgel or soft elastic) capsules, such as SGcaps®.
- Soft capsulesmay be prepared, for example, by rotary die, plate, reciprocating die or Accogel® machine method.
- the capsulemay be a liquid-filled hard capsule or a soft-gelatin capsule.
- Tabletscan be made by compression or molding, optionally with one or more accessory ingredients.
- Compressed tabletscan be prepared by compressing in a suitable machine a compound of formula (I) or pharmaceutically acceptable salt thereof in a free-flowing form such as a powder or granules, optionally mixed with a binder, lubricant, inert diluent, preservative, surface-active or dispersing agent.
- Molded tabletscan be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
- the tabletscan be optionally coated or scored and can be formulated so as to provide sustained, extended, delayed or controlled release. Methods of formulating such sustained, extended, delayed or controlled release compositions are known in the art and disclosed in issued U.S. patents, including but not limited to U.S. Pat. Nos.4,369,174, 4,842,866, and the references cited therein.
- Coatingsfor example enteric coatings, can be used for delivery of compounds to the intestine (see, e.g., U.S. Pat.
- the pharmaceutical compositionis formulated for parenteral administration.
- Examples of a pharmaceutical composition suitable for parenteral administrationinclude aqueous sterile injection solutions and non-aqueous sterile injection solutions, each containing, for example, anti-oxidants, buffers, bacteriostatic agents and/or solutes that render the formulation isotonic with the blood of the intended recipient; and aqueous sterile suspensions and non-aqueous sterile suspensions, each containing, for example, suspending agents and/or thickening agents.
- the formulationscan be presented in unit-dose or multi-dose containers, for example, sealed ampules or vials, and can be stored in a freeze dried (lyophilized) condition requiting only the addition of a sterile liquid carrier, such as water, immediately prior to use.
- the pharmaceutical compositionis formulated for intravenous administration.
- the pharmaceutical compositionfurther includes a pharmaceutically acceptable excipient.
- a pharmaceutically acceptable excipientmay be any substance, not itself a therapeutic agent, used as a carrier, diluent, adjuvant, binder, and/or vehicle for delivery of a therapeutic agent to a patient, or added to a pharmaceutical composition to improve its handling or storage properties or to permit or facilitate formation of a compound or pharmaceutical composition into a unit dosage form for administration.
- Pharmaceutically acceptable excipientsare known in the pharmaceutical arts and are disclosed, for example, in Remington: The Science and Practice of Pharmacy, 21.sup.st Ed. (Lippincott Williams & Wilkins, Baltimore, Md., 2005).
- pharmaceutically acceptable excipientscan provide a variety of functions and can be described as wetting agents, buffering agents, suspending agents, lubricating agents, emulsifiers, disintegrants, absorbents, preservatives, surfactants, colorants, flavorants, and sweeteners.
- Examples of pharmaceutically acceptable excipientsinclude without limitation: (1) sugars, such as lactose, glucose and sucrose; (2) starches, such as corn starch and potato starch; (3) cellulose and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose, cellulose acetate, hydroxypropyl methylcellulose, and hydroxypropylcellulose; (4) powdered tragacanth; (5) malt; (6) gelatin; (7) talc; (8) excipients, such as cocoa butter and suppository waxes; (9) oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10) glycols, such as propylene glycol; (11) polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; (12) esters, such as ethyl oleate and ethyl laurate; (13) agar; (14)
- compositionscan be non-toxic in the amounts used. It will be evident to those of ordinary skill in the art that the optimal dosage of the active ingredient(s) in the pharmaceutical composition will depend on a variety of factors. Relevant factors include, without limitation, the type of animal (e.g., human), the particular form of a compound of Formula I, or a pharmaceutically acceptable salt thereof, the manner of administration, the composition employed, and the severity of the disease or condition being treated. [00311] In addition to administering the compound as a raw chemical, the compounds of the disclosure may be administered as part of a pharmaceutical preparation containing suitable pharmaceutically acceptable carriers comprising excipients and auxiliaries which facilitate processing of the compounds into preparations which can be used pharmaceutically.
- suitable pharmaceutically acceptable carrierscomprising excipients and auxiliaries which facilitate processing of the compounds into preparations which can be used pharmaceutically.
- compositions of the disclosuremay be administered to any patient which may experience the beneficial effects of the compounds of the disclosure.
- the compounds and pharmaceutical compositions thereofmay be administered by any means that achieve their intended purpose.
- administrationmay be by parenteral, subcutaneous, intravenous, intramuscular, intraperitoneal, transdermal, buccal, intrathecal, intracranial, intranasal or topical routes.
- administrationmay be by the oral route.
- the dosage administeredwill be dependent upon the age, health, and weight of the recipient, kind of concurrent treatment, if any, frequency of treatment, and the nature of the effect desired.
- compositions of the present disclosureare manufactured in a manner which is itself known, for example, by means of conventional mixing, granulating, dragee-making, dissolving, or lyophilizing processes.
- pharmaceutical preparations for oral usecan be obtained by combining the active compounds with solid excipients, optionally grinding the resulting mixture and processing the mixture of granules, after adding suitable auxiliaries, if desired or necessary, to obtain tablets or dragee cores.
- Suitable excipientsare, in particular, fillers such as saccharides, for example lactose or sucrose, mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example tricalcium phosphate or calcium hydrogen phosphate, as well as binders such as starch paste, using, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, tragacanth, methyl cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, and/or polyvinyl pyrrolidone.
- fillerssuch as saccharides, for example lactose or sucrose, mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example tricalcium phosphate or calcium hydrogen phosphate, as well as binders such as starch paste, using, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, tragacanth, methyl cellulose,
- disintegrating agentsmay be added such as the above- mentioned starches and also carboxymethyl-starch, cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof, such as sodium alginate.
- Auxiliariesare, above all, flow-regulating agents and lubricants, for example, silica, talc, stearic acid or salts thereof, such as magnesium stearate or calcium stearate, and/or polyethylene glycol.
- Dragee coresare provided with suitable coatings which, if desired, are resistant to gastric juices.
- concentrated saccharide solutionsmay be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, polyethylene glycol and/or titanium dioxide, lacquer solutions and suitable organic solvents or solvent mixtures.
- suitable cellulose preparationssuch as acetylcellulose phthalate or hydroxypropylmethyl- cellulose phthalate, are used.
- Dye stuffs or pigmentsmay be added to the tablets or dragee coatings, for example, for identification or in order to characterize combinations of active compound doses.
- Other pharmaceutical preparationswhich can be used orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer such as glycerol or sorbitol.
- the push-fit capsulescan contain the active compounds in the form of granules which may be mixed with fillers such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers.
- the active compoundsare in one embodiment dissolved or suspended in suitable liquids, such as fatty oils, or liquid paraffin.
- stabilizersmay be added.
- Possible pharmaceutical preparations which can be used rectallyinclude, for example, suppositories, which consist of a combination of one or more of the active compounds with a suppository base.
- Suitable suppository basesare, for example, natural or synthetic triglycerides, or paraffin hydrocarbons.
- gelatin rectal capsuleswhich consist of a combination of the active compounds with a base.
- Possible base materialsinclude, for example, liquid triglycerides, polyethylene glycols, or paraffin hydrocarbons.
- Suitable formulations for parenteral administrationinclude aqueous solutions of the active compounds in water-soluble form, for example, water-soluble salts and alkaline solutions.
- suspensions of the active compounds as appropriate oily injection suspensionsmay be administered.
- Suitable lipophilic solvents or vehiclesinclude fatty oils, for example, sesame oil, or synthetic fatty acid esters, for example, ethyl oleate or triglycerides or polyethylene glycol-400.
- Aqueous injection suspensionsmay contain substances which increase the viscosity of the suspension include, for example, sodium carboxymethyl cellulose, sorbitol, and/or dextran.
- the suspensionmay also contain stabilizers.
- the topical compositions of this disclosureare formulated in one embodiment as oils, creams, lotions, ointments and the like by choice of appropriate carriers.
- Suitable carriersinclude vegetable or mineral oils, white petrolatum (white soft paraffin), branched chain fats or oils, animal fats and high molecular weight alcohol (greater than C12).
- the carriersmay be those in which the active ingredient is soluble.
- Emulsifiers, stabilizers, humectants and antioxidantsmay also be included as well as agents imparting color or fragrance, if desired.
- transdermal penetration enhancerscan be employed in these topical formulations. Examples of such enhancers can be found in U.S. Pat. Nos.3,989,816 and 4,444,762; each herein incorporated by reference in its entirety.
- Ointmentsmay be formulated by mixing a solution of the active ingredient in a vegetable oil such as almond oil with warm soft paraffin and allowing the mixture to cool.
- a vegetable oilsuch as almond oil
- a typical example of such an ointmentis one which includes about 30% almond oil and about 70% white soft paraffin by weight.
- Lotionsmay be conveniently prepared by dissolving the active ingredient, in a suitable high molecular weight alcohol such as propylene glycol or polyethylene glycol.
- a suitable high molecular weight alcoholsuch as propylene glycol or polyethylene glycol.
- the compound of Formula Ior a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, can be employed under a variety of conditions and therapeutic uses to treat a variety of diseases or conditions related to the aberrant expression of PI3K and/or EGFR activity, including cancer.
- the subjectWhen administered to such a subject, the administration of the compound of Formula I, Compound 2R, or a pharmaceutically acceptable salt thereof, or a combination disclosed herein, the subject does not significantly elevate any one of: blood glucose, blood insulin or hyperglycemia in the subject, after one or more administrations of the compound or combination.
- the compound of Formula Ior a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, or Compound 2R or a salt thereof, can be employed under a variety of conditions and therapeutic uses to treat a variety of diseases or conditions related to the aberrant expression of PI3K and/or EGFR activity, for example, cancer, or the treatment of diseases associated with a tumor, cancer or a neoplastic growth.
- Exemplary cancers that can be treated with the compounds of Formula I, for example, Compound 2Rare illustrated herein.
- the subject with cancer, or likely to develop cancermay be a subject that does not metabolize glucose normally, for example may have a metabolic disease that is characterized by hyperglycemia caused by insulin resistance.
- the subject to be treatedis one that may respond to inhibition of PI3K and/or EGFR and which also has diabetes type I or diabetes type II.
- the subject to be treatedmay also include subjects that have cancer that is sensitive to a PI3K and/or EGFR inhibitor and also has dyslipidaemia, obesity and/or hepatic steatosis associated with insulin resistance and diabetes mellitus.
- the subject to be treatedmay also include subjects that have cancer that is sensitive to a PI3K and/or EGFR inhibitor and also has hyperglycemia, insulin intolerance and/or elevated glucose levels above normal healthy levels when tested after fasting, for example the subject may have diabetes, and/or obesity, and/or a condition associated with improper glucose metabolism, for example, glucose tolerance disorder, diabetes mellitus (especially Type II diabetes mellitus, obesity diabetes mellitus), abnormal lipid metabolism (synonymous with hyperlipidemia above), hypertension, heart failure, hyperuricemia, fatty liver (including non- alcoholic hepatitis), coronary heart disease (myocardial infarction, angina pectoris), cerebral infarction (cerebral thrombosis, transient ischemic attack), bone/joint disease (knee osteoarthritis, hip osteoarthritis, deformed spondylitis, low back pain), sleep apnea syndrome/Pickwick syndrome, menstrual disorders (abnormal menstrual
- the subject with canceris a subject with diabetes type 1, diabetes type 2, diabetic retinopathy, metabolic syndrome, insulin resistance, pancreatic dysfunction, and wherein administration of the compound or pharmaceutically acceptable salt thereof of Formula I, does not significantly elevate blood glucose or cause hyperglycemia in the subject, after one or more administrations of the compound or pharmaceutically acceptable salt thereof, wherein hyperglycemia is defined as a plasma or whole blood glucose measurement greater than 125 mg/dL while fasting and greater than 200 mg/dL, 2 hours postprandial, during a 75-g oral glucose tolerance test (OGTT) using a blood glucose meter employing strips comprising glucose oxidase, hexokinase, or combinations thereof.
- OGTToral glucose tolerance test
- the exact amount requiredwill vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular agent, its mode of administration, and the like.
- the compounds of the disclosureare preferably formulated in dosage unit form for ease of administration and uniformity of dosage.
- dosage unit formrefers to a physically discrete unit of agent appropriate for the patient to be treated. It will be understood, however, that the total daily usage of the compounds and compositions of the present disclosure will be decided by the attending physician within the scope of sound medical judgment.
- the specific effective dose level for any particular patient or organismwill depend upon a variety of factors including the disorder being treated and the severity of the disorder; the activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed, and like factors well known in the medical arts.
- the term “patient”, as used herein,means an animal, preferably a mammal, and most preferably a human.
- patientand “subject” are used interchangeably.
- compositions of this disclosurecan be administered to humans and other animals orally, rectally, parenterally, intracisternally, intravaginally, intraperitoneally, topically (as by powders, ointments, drops or patch), bucally, as an oral or nasal spray, or the like, depending on the severity of the disease, for example, a cancer, being treated.
- a disclosed method of inhibiting replication of cancer cells in a subjectcan comprise repeating the administration of the compounds of the present disclosure or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure .
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered systemically to the subject.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure,can be administered to the subject at least two times.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered to the subject two or more times.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered at routine or regular intervals.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered to the subject one time per day, or two times per day, or three or more times per day.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered to the subject daily, or one time per week, or two times per week, or three or more times per week, etc.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosurecan be administered to the subject weekly, or every other week, or every third week, or every fourth week, etc.
- a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosureor a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, can be administered to the subject monthly, or every other month, or every third month, or every fourth month, etc.
- the repeated administration of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosureoccurs over a pre-determined or definite duration of time.
- the repeated administration of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosureoccurs over an indefinite period of time.
- the compounds or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosuremay be administered orally or parenterally to the subject in need thereof, at therapeutically effective amounts or prophylactically effective amounts, of about wherein the therapeutically effective amount of the compound according to Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is selected from the group consisting of 0.01 mg/kg to about 1,000 mg/kg, 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80
- the method of treating a cancer, or an advanced cancer, or a metastatic cancercomprises oral or parenteral administration of a compound according to Formula I, for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient, wherein the daily dose ranges from about 1 mg per day to about 1,000 mg per day, and all values and integers therebetween, dosed according to a daily dosing regimen or intermittently.
- a compound according to Formula Ifor example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient, wherein the daily dose ranges from about 1 mg per day to about 1,000 mg per day, and all values and integers therebetween, dosed according to a daily dosing regimen
- the daily doseis administered as fractionated oral or parenteral dosages twice or three, or four times per day.
- daily doses or unit dosesmay include therapeutically effective doses.
- the subject to be treatedhas a cancer that can be treated with the compounds of the present disclosure, and also have impaired glucose metabolism, such as hyperglycemia, and/or insulin resistance, and/or is overweight, and/or is obese, and/or or has any form of metabolic syndrome associated with defective glucose utilization and glucose metabolism, and/or requires medication to provide glycemic control [00332] While treatment regimen and therapeutically effective doses may vary according to the subject’s age, weight, health condition, severity of disease and past treatments administered to treat the cancer, or an advanced cancer, or a metastatic cancer, in some embodiments, the daily dose is administered 1, 2, 3, or 4 times per 28-day treatment cycle, on an intermittent schedule for 3 weeks on and one week off.
- the daily doseis administered on day 1, 8, and 15 of a 28-day treatment cycle.
- the daily dose or therapeutic dosesmay be administered as multiple cycles of treatment which may be administered for a time period sufficient to effect at least a partial tumor response.
- the patientis administered a daily dose for a time period of 28 day cycles, until the patient no longer shows therapeutic benefit or clinical signs of toxicity preclude continuation of therapy.
- Some illustrative time periodsmay include one or more of the following: wherein the time period is at least 6 months; wherein the time period is at least 12 months; wherein the time period is sufficient to effect a complete tumor response is effected in the patient.
- the patientis administered a treatment cycle comprising administering a daily dose of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient for 21 days followed by no administration for seven days, wherein the patient is administered one to twelve treatment cycles per year.
- the compounds of Formula (I), for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosuremay be administered orally or parenterally to the subject in need thereof, at therapeutically effective amounts or prophylactically effective amounts, of about 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 500 mg/kg,
- the pharmaceutical compositions of the present disclosurecan be tested in clinical studies. Suitable clinical studies can be, for example, open label, dose escalation studies in patients with cancer. Such studies prove in particular the synergism of the active ingredients of the combination of the invention. The beneficial effects on cancer can be determined directly through the results of these studies which are known as such to a person skilled in the art. Such studies can be, in particular, suitable to compare the effects of a monotherapy using the active ingredients and a combination of the invention. In various embodiments, the dose of a compound or pharmaceutically acceptable salt thereof of Formula I, is escalated until the Maximum Tolerated Dosage is reached.
- a compound of Formula (I), for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosureis administered in a fixed dose and the dose of a secondary active agent is escalated.
- the dose of a secondary active agentis a fixed dose
- Each patientcan receive doses of the compounds either daily or intermittently.
- the efficacy of the treatmentcan be determined in such studies, e.g., after 12, 18 or 24 weeks of treatment, by evaluation of symptom scores every 4 to 6 weeks. It is envisioned that the administration of a combination therapy of the present disclosure will result not only in a beneficial effect, e.g.
- a synergistic therapeutic effecte.g. with regard to alleviating, delaying progression of or inhibiting the symptoms, but also in further surprising beneficial effects, e.g. fewer side-effects, an improved quality of life or a decreased morbidity, compared with a monotherapy applying only one of the pharmaceutically active ingredients used in the combination of the invention.
- a further benefitcan be that lower and/or less frequent doses of one or both of the active ingredients of the combination of the invention can be used, which can diminish the incidence or severity of side- effects. This is in accordance with the desires and requirements of the patients to be treated.
- Dosage amounts and dosing regimens for the pharmaceutical compositions of the present disclosurecan be adjusted to provide the optimum desired response (e.g., a therapeutic response). For example, a single bolus can be administered, or several divided doses (multiple or repeat or maintenance) can be administered over time and the dose can be proportionally reduced or increased as indicated by the exigencies of the therapeutic situation. It is especially advantageous to formulate parenteral compositions in dosage unit form for ease of administration and uniformity of dosage.
- Dosage unit form as used hereinrefers to physically discrete units suited as unitary dosages for the mammalian patients to be treated; each unit containing a predetermined quantity of active compound calculated to produce the desired therapeutic effect in association with the required pharmaceutical carrier.
- the specification for the dosage unit forms of the present disclosurewill be dictated primarily by the unique characteristics of the of the compounds of Formula I, as it pertains to their dual inhibitory action on pan-PI3K and/mTOR and EGFR targets, and the particular therapeutic effect to be achieved.
- the dose and dosing regimenis adjusted in accordance with methods well-known in the therapeutic arts as it pertains to oncotherapy. That is, the maximum tolerable dose can be readily established, and the effective amount providing a detectable therapeutic benefit to a patient may also be determined, as can the temporal requirements for administering each agent to provide a detectable therapeutic benefit to the patient.
- dosage valuesmay vary with the type and severity of the condition to be alleviated, and may include single or multiple doses. It is to be further understood that for any particular subject, specific dosage regimens should be adjusted over time according to the patient need and the professional judgment of the person administering or supervising the administration of the compositions, and that dosage ranges set forth herein are exemplary only and are not intended to limit the scope or practice of the claimed composition.
- the dosage regimen with the compositions of this inventionmay be based on a variety of factors, including the type of disease, the age, weight, sex, medical condition of the patient, the severity of the condition, the route of administration, and the particular compound or mixtures of compounds disclosed herein, and in some embodiments, combinations of the compounds disclosed herein in combination with a secondary anti -cancer agents employed.
- the dosage regimencan vary widely, but can be determined routinely using standard methods. For example, doses may be adjusted based on pharmacokinetic or pharmacodynamic parameters, which may include clinical effects such as toxic effects and/or laboratory values.
- the present inventionencompasses intra-patient dose-escalation as determined by the skilled artisan.
- Liquid dosage forms for oral administrationinclude, but are not limited to, pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs.
- the liquid dosage formsmay contain inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
- inert diluentscommonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzy
- the oral compositionscan also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- adjuvantssuch as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- injectable preparationsfor example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparationmay also be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- the acceptable vehicles and solventsthat may be employed are water, Ringer’s solution, U.S.P. and isotonic sodium chloride solution.
- sterile, fixed oilsare conventionally employed as a solvent or suspending medium.
- any bland fixed oilcan be employed including synthetic mono- or diglycerides.
- fatty acidssuch as oleic acid are used in the preparation of injectables.
- the injectable formulationscan be sterilized, for example, by filtration through a bacterial-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
- compositions for rectal or vaginal administrationare preferably suppositories which can be prepared by mixing the compounds of this disclosure with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- Solid dosage forms for oral administrationinclude capsules, tablets, pills, powders, and granules.
- the active compoundis mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar--agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cety
- the dosage formmay also comprise buffering agents.
- Solid compositions of a similar typemay also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the solid dosage forms of tablets, dragees, capsules, pills, and granulescan be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- embedding compositionsexamples include polymeric substances and waxes. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polethylene glycols and the like. [00347]
- the active compoundscan also be in microencapsulated form with one or more excipients as noted above.
- the solid dosage forms of tablets, dragees, capsules, pills, and granulescan be prepared with coatings and shells such as enteric coatings, release controlling coatings and other coatings well known in the pharmaceutical formulating art.
- the active compoundmay be admixed with at least one inert diluent such as sucrose, lactose or starch.
- inert diluentsuch as sucrose, lactose or starch.
- Such dosage formsmay also comprise, as is normal practice, additional substances other than inert diluents, e.g., tableting lubricants and other tableting aids such a magnesium stearate and microcrystalline cellulose.
- the dosage formsmay also comprise buffering agents. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions that can be used include polymeric substances and waxes.
- Dosage forms for topical or transdermal administration of a compound of this disclosureinclude ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants or patches.
- the active componentis admixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives or buffers as may be required.
- Ophthalmic formulation, eardrops, and eye dropsare also contemplated as being within the scope of this disclosure.
- the present disclosurecontemplates the use of transdermal patches, which have the added advantage of providing controlled delivery of a compound to the body.
- Such dosage formsare prepared by dissolving or dispensing the compound in the proper medium.
- Absorption enhancerscan also be used to increase the flux of the compound across the skin.
- the ratecan be controlled by either providing a rate controlling membrane or by dispersing the compound in a polymer matrix or gel.
- the present disclosuremay be used to treat a neoplastic disease, such as solid or non-solid cancers.
- treatmentencompasses the reduction, control and/or inhibition of a neoplastic disease.
- diseasesinclude a sarcoma, carcinoma, adenocarcinoma, melanoma, myeloma, blastoma, glioma, lymphoma or leukemia.
- Exemplary cancersinclude, for example, carcinoma, sarcoma, adenocarcinoma, melanoma, neural (blastoma, glioma), mesothelioma and reticuloendothelial, lymphatic or hematopoietic neoplastic disorders (e.g., myeloma, lymphoma or leukemia).
- a neoplasm, tumor or cancerincludes a lung adenocarcinoma, lung carcinoma, diffuse or interstitial gastric carcinoma, colorectal, adenocarcinoma, prostate adenocarcinoma, esophagus carcinoma, breast carcinoma, for example, triple negative breast cancer, pancreatic cancer, e.g. pancreatic adenocarcinoma, carcinomas of the cervix, ovarian adenocarcinoma, adenocarcinoma of the adrenal gland, adenocarcinoma of the endometrium or uterine adenocarcinoma, and carcinomas of the head and neck.
- pancreatic cancere.g. pancreatic adenocarcinoma, carcinomas of the cervix, ovarian adenocarcinoma, adenocarcinoma of the adrenal gland, adenocarcinoma of the endometrium or uterine adenocarcinoma
- Neoplasia, tumors and cancersinclude benign, malignant, metastatic and non- metastatic types, and include any stage (I, II, III, IV or V) or grade (G1, G2, G3, etc.) of neoplasia, tumor, or cancer, or a neoplasia, tumor, cancer or metastasis that is progressing, worsening, stabilized or in remission.
- Cancers that may be treated according to the disclosureinclude but are not limited to cells or neoplasms of the bladder, blood, bone, bone marrow, brain, breast, colon, esophagus, gastrointestinal system, gum, head, kidney, liver, lung, nasopharynx, neck, ovary, prostate, skin, stomach, testis, tongue, or uterus.
- the cancermay specifically be of the following histological type, though it is not limited to the following: neoplasm, malignant; carcinoma; carcinoma, undifferentiated; giant and spindle cell carcinoma; small cell carcinoma; papillary carcinoma; squamous cell carcinoma; lymphoepithelial carcinoma; basal cell carcinoma; pilomatrix carcinoma; transitional cell carcinoma; papillary transitional cell carcinoma; adenocarcinoma, gastrinoma, malignant; cholangiocarcinoma, hepatocellular carcinoma; combined hepatocellular carcinoma and cholangiocarcinoma, trabecular adenocarcinoma, adenoid cystic carcinoma; adenocarcinoma in adenomatous polyp; adenocarcinoma, familial polyposis coli, solid carcinoma; carcinoid tumor, malignant; bronchiolo-alveolar adenocarcinoma, papillary adenocarcinoma, chromophobe carcinoma
- the neoplastic diseasemay be tumors associated with a cancer selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma.
- the tumormay be metastatic or a malignant tumor.
- the neoplastic disease to be treatedis pancreatic cancer, colorectal cancer and/or carcinomas of the head and neck.
- the cancercomprises colorectal cancer, pancreatic cancer, breast cancer.
- the compounds of the present invention, or their pharmaceutically acceptable salts, solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofare effective against cancers which harbor at least one mutation selected from: a BRAF mutation, a KRAS mutation, and a NRAS mutation, for example, wherein the cancer harbors a KRASG12 mutation, and/or a KRASA136 mutation.
- the cancerharbors a BRAFV600 mutation.
- Combination Therapies[00353] Some embodiments of the present disclosure provide methods for administering an effective amount of a compound of the disclosure and at least one additional therapeutic agent (including, but not limited to, chemotherapeutic antineoplastics, apoptosis-modulating agents, antimicrobials, antivirals, antifungals, and anti-inflammatory agents) and/or therapeutic technique (e.g., surgical intervention, and/or radiotherapies).
- the additional therapeutic agent(s)is an anticancer agent.
- the present disclosureprovides for the treatment of certain cancers to a subject in need, by administering a therapeutically effective combination comprising a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, pan-RAS, NRAS, MAPK, ALK, BRAF or MEK inhibitor.
- These various combination therapiesmay provide a "synergistic effect", i.e., the effect achieved when the active ingredients used together is greater than the sum of the effects that results from using the compounds separately.
- combination therapy methodsare particularly effective against a cancer that is resistant or refractory to treatment using the P13K and EGFR inhibitor (i.e. a compound or a pharmaceutically acceptable salt thereof of Formula I) alone, another anti-cancer agent alone, or the P13K and EGFR inhibitor in combination with another anti-cancer agent.
- P13K and EGFR inhibitori.e. a compound or a pharmaceutically acceptable salt thereof of Formula I
- another anti-cancer agentalone
- P13K and EGFR inhibitorin combination with another anti-cancer agent.
- a number of suitable secondary anticancer agentsare contemplated for use in the methods of the present disclosure.
- anticancer agentssuch as: agents that induce apoptosis; polynucleotides (e.g., anti-sense, ribozymes, siRNA); polypeptides (e.g., enzymes and antibodies); biological mimetics; alkaloids; alkylating agents; antitumor antibiotics; antimetabolites; hormones; platinum compounds; monoclonal or polyclonal antibodies (e.g., antibodies conjugated with anticancer drugs, toxins, defensins), toxins; radionuclides; biological response modifiers (e.g., interferons (e.g., IFN- ⁇ ) and interleukins (e.g., IL-2)); adoptive immunotherapy agents; hematopoietic growth factors; agents that induce tumor cell differentiation (e.g., all-trans-retinoic acid); gene therapy reagents (e.g., antisense therapy reagents and nucleo
- the secondary anticancer agent to be used in therapeutically effective combinationsmay include one or more KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, and MEK inhibitors.
- secondary, anticancer agentscomprise agents that induce or stimulate apoptosis.
- Agents that induce apoptosisinclude, but are not limited to, radiation (e.g., X-rays, gamma rays, UV); tumor necrosis factor (TNF)-related factors (e.g., TNF family receptor proteins, TNF family ligands, TRAIL, antibodies to TRAIL-R1 or TRAIL-R2); kinase inhibitors (e.g., epidermal growth factor receptor (EGFR) kinase inhibitor, vascular growth factor receptor (VGFR) kinase inhibitor, fibroblast growth factor receptor (FGFR) kinase inhibitor, platelet-derived growth factor receptor (PDGFR) kinase inhibitor, and Bcr-Abl kinase inhibitors (such as GLEEVEC)); antisense molecules; antibodies (e.g., HERCEPTIN, RITUXAN, ZEVALIN, and AVASTIN); anti-estrogens (e.g., raloxifene and tam
- compositions and methods of the present disclosureprovide a compound of the disclosure and at least one anti-hyperproliferative or antineoplastic agent selected from alkylating agents, antimetabolites, and natural products (e.g., herbs and other plant and/or animal derived compounds).
- at least one anti-hyperproliferative or antineoplastic agentselected from alkylating agents, antimetabolites, and natural products (e.g., herbs and other plant and/or animal derived compounds).
- Alkylating agents suitable for use in the present compositions and methodsinclude, but are not limited to: 1) nitrogen mustards (e.g., mechlorethamine, cyclophosphamide, ifosfamide, melphalan (L-sarcolysin); and chlorambucil); 2) ethylenimines and methylmelamines (e.g., hexamethylmelamine and thiotepa); 3) alkyl sulfonates (e.g., busulfan); 4) nitrosoureas (e.g., carmustine (BCNU); lomustine (CCNU); semustine (methyl-CCNU); and streptozocin (streptozotocin)); and 5) triazenes (e.g., dacarbazine (DTIC; dimethyltriazenoimid- azolecarboxamide).
- nitrogen mustardse.g., mechlorethamine, cyclophosphamide
- antimetabolites suitable for use in the present compositions and methodsinclude, but are not limited to: 1) folic acid analogs (e.g., methotrexate (amethopterin)); 2) pyrimidine analogs (e.g., fluorouracil (5-fluorouracil; 5-FU), floxuridine (fluorode-oxyuridine; FudR), and cytarabine (cytosine arabinoside)); and 3) purine analogs (e.g., mercaptopurine (6-mercaptopurine; 6-MP), thioguanine (6-thioguanine; TG), and pentostatin (2’-deoxycoformycin)).
- folic acid analogse.g., methotrexate (amethopterin)
- pyrimidine analogse.g., fluorouracil (5-fluorouracil; 5-FU), floxuridine (fluorode-oxyuridine; FudR), and cytarabine
- chemotherapeutic agents suitable for use in the compositions and methods of the present disclosureinclude, but are not limited to: 1) vinca alkaloids (e.g., vinblastine (VLB), vincristine); 2) epipodophyllotoxins (e.g., etoposide and teniposide); 3) antibiotics (e.g., dactinomycin (actinomycin D), daunorubicin (daunomycin; rubidomycin), doxorubicin, bleomycin, plicamycin (mithramycin), and mitomycin (mitomycin C)); 4) enzymes (e.g., L-asparaginase); 5) biological response modifiers (e.g., interferon-alfa); 6) platinum coordinating complexes (e.g., cisplatin (cis-DDP) and carboplatin); 7) anthracenediones (e.g., mitoxantrone
- vinca alkaloidse
- any oncolytic agent that is routinely used in a cancer therapy contextfinds use in the compositions and methods of the present disclosure.
- the U.S. Food and Drug Administrationmaintains a formulary of oncolytic agents approved for use in the United States. International counterpart agencies to the U.S.F.D.A. maintain similar formularies.
- Table 2provides a list of exemplary antineoplastic agents approved for use in the U.S. Those skilled in the art will appreciate that the “product labels” required on all U.S. approved chemotherapeutics describe approved indications, dosing information, toxicity data, and the like, for the exemplary agents. [00362] Table 2.
- Anticancer agentsfurther include compounds which have been identified to have anticancer activity.
- the additional active agent that finds utility when combined with a compound of Formula I, or a pharmaceutically acceptable salt thereof or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofis an inhibitor of KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEK.
- Examplesinclude, but are not limited to, 3-AP, 12-O- tetradecanoylphorbol-13-acetate, 17AAG, 852A, ABI-007, ABR-217620, ABT-751, ADI-PEG 20, AE-941, AG-013736, AGRO100, alanosine, AMG 706, antibody G250, antineoplastons, AP23573, apaziquone, APC8015, atiprimod, ATN-161, atrasenten, azacitidine, BB-10901, BCX-1777, bevacizumab, BG00001, bicalutamide, BMS 247550, bortezomib, bryostatin-1, buserelin, calcitriol, CCI-779, CDB-2914, cefixime, cetuximab, CG0070, cilengitide, clofarabine, combretastatin A4 phosphate, CP-675,206, CP-724
- each of the active agents in the combinations described hereincan include amounts of each agent that are found to be clinically relevant amounts that provide therapeutic benefit in the aggregate when dosed in combination.
- the additional active agentmay be dosed or provided in compositions which are dosed with the compounds of the present disclosure in amounts that do cause adverse effects and that may be titrated when studied in a clinical trial of the combination.
- the combinationmay be provided in compositions that may be administered separately or formulated in a single composition and may be dosed in amounts that are either therapeutically effective amounts individually, or when one or both of the active agents of the combination are dosed at sub-optimal or sub-therapeutic levels, if the combination as a whole is therapeutically effective.
- the amount of the additional active agent when used in a therapeutically effective combination with the compounds of Formula I, (for example, any compound as provided in Table 1 herein) or their pharmaceutically acceptable salts or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofmay range from about 0.1 mg/kg to about 500 mg/kg body weight of the patient, or preferably, ranging from about 1 mg/kg to about 200 mg/kg body weight of the patient, and all ranges therebetween.
- the additional active agent that is an inhibitor of KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEKcan include one or more of: sotorasib, sorafenib, panitumumab, encorafenib, trametinib, cobimetinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, divarasib, adagrasib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, glecirasib, ERAS-007, ulixertinib, ASTX029, ravoxertinib, PLX4720 (Plexxikon), ERAS-007, LY3537982, ASTX029,
- the present disclosureprovides methods for administering a compound of the disclosure with radiation therapy.
- the disclosureis not limited by the types, amounts, or delivery and administration systems used to deliver the therapeutic dose of radiation to an animal.
- the animalmay receive photon radiotherapy, particle beam radiation therapy, other types of radiotherapies, and combinations thereof.
- the radiationis delivered to the animal using a linear accelerator.
- the radiationis delivered using a gamma knife.
- the source of radiationcan be external or internal to the animal. External radiation therapy is most common and involves directing a beam of high-energy radiation to a tumor site through the skin using, for instance, a linear accelerator.
- Internal radiation therapyinvolves implanting a radiation-emitting source, such as beads, wires, pellets, capsules, particles, and the like, inside the body at or near the tumor site including the use of delivery systems that specifically target cancer cells (e.g., using particles attached to cancer cell binding ligands). Such implants can be removed following treatment, or left in the body inactive.
- a radiation-emitting sourcesuch as beads, wires, pellets, capsules, particles, and the like
- Such implantscan be removed following treatment, or left in the body inactive.
- Types of internal radiation therapyinclude, but are not limited to, brachytherapy, interstitial irradiation, intracavity irradiation, radioimmunotherapy, and the like.
- the animalmay optionally receive radiosensitizers (e.g., metronidazole, misonidazole, intra-arterial Budr, intravenous iododeoxyuridine (IudR), nitroimidazole, 5- substituted-4-nitroimidazoles, 2H-isoindolediones, [[(2-bromoethyl)-amino]methyl]-nitro-1H- imidazole-1-ethanol, nitroaniline derivatives, DNA-affinic hypoxia selective cytotoxins, halogenated DNA ligand, 1,2,4 benzotriazine oxides, 2-nitroimidazole derivatives, fluorine- containing nitroazole derivatives, benzamide, nicotinamide, acridine-intercalator, 5- thiotretrazole derivative, 3-nitro-1,2,4-triazole, 4,5-dinitroimidazole derivative, hydroxylated texaphrins,
- Radiosensitizersenhance the killing of tumor cells. Radioprotectors protect healthy tissue from the harmful effects of radiation.
- Any type of radiationcan be administered to an animal, so long as the dose of radiation is tolerated by the animal without unacceptable negative side-effects.
- Suitable types of radiotherapyinclude, for example, ionizing (electromagnetic) radiotherapy (e.g., X-rays or gamma rays) or particle beam radiation therapy (e.g., high linear energy radiation).
- Ionizing radiationis defined as radiation comprising particles or photons that have sufficient energy to produce ionization, i.e., gain or loss of electrons (as described in, for example, U.S.5,770,581 incorporated herein by reference in its entirety).
- the effects of radiationcan be at least partially controlled by the clinician.
- the dose of radiationis fractionated for maximal target cell exposure and reduced toxicity.
- the total dose of radiation administered to an animalis about .01 Gray (Gy) to about 100 Gy.
- about 10 Gy to about 65 Gye.g., about 15 Gy, 20 Gy, 25 Gy, 30 Gy, 35 Gy, 40 Gy, 45 Gy, 50 Gy, 55 Gy, or 60 Gy
- a complete dose of radiationcan be administered over the course of one day, the total dose is ideally fractionated and administered over several days.
- radiotherapyis administered over the course of at least about 3 days, e.g., at least 5, 7, 10, 14, 17, 21, 25, 28, 32, 35, 38, 42, 46, 52, or 56 days (about 1-8 weeks).
- a daily dose of radiationwill comprise approximately 1-5 Gy (e.g., about 1 Gy, 1.5 Gy, 1.8 Gy, 2 Gy, 2.5 Gy, 2.8 Gy, 3 Gy, 3.2 Gy, 3.5 Gy, 3.8 Gy, 4 Gy, 4.2 Gy, or 4.5 Gy), or 1-2 Gy (e.g., 1.5-2 Gy).
- the daily dose of radiationshould be sufficient to induce destruction of the targeted cells. If stretched over a period, in one embodiment, radiation is not administered every day, thereby allowing the animal to rest and the effects of the therapy to be realized.
- radiationdesirably is administered on 5 consecutive days, and not administered on 2 days, for each week of treatment, thereby allowing 2 days of rest per week.
- radiationcan be administered 1 day/week, 2 days/week, 3 days/week, 4 days/week, 5 days/week, 6 days/week, or all 7 days/week, depending on the animal’s responsiveness and any potential side effects.
- Radiation therapycan be initiated at any time in the therapeutic period. In one embodiment, radiation is initiated in week 1 or week 2, and is administered for the remaining duration of the therapeutic period. For example, radiation is administered in weeks 1- 6 or in weeks 2-6 of a therapeutic period comprising 6 weeks for treating, for instance, a solid tumor.
- Antimicrobial therapeutic agentsmay also be used as therapeutic agents in the present disclosure. Any agent that can kill, inhibit, or otherwise attenuate the function of microbial organisms may be used, as well as any agent contemplated to have such activities. Antimicrobial agents include, but are not limited to, natural and synthetic antibiotics, antibodies, inhibitory proteins (e.g., defensins), antisense nucleic acids, membrane disruptive agents and the like, used alone or in combination.
- the present disclosureprovides a method for treating a cancer in a subject in need thereof, comprising, administering to the subject, a therapeutically effective combination comprising a compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, NRAS, MAPK, ALK, BRAF, or MEK inhibitor.
- the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofis found in table 1, as provided above.
- the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofis selected from Compound 1, 2R or 2S, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof.
- the compound or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereofis Compound 2R, which can be used in a method of treating cancer, by combining said compound with at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor to form a therapeutically effective combination for the treatment of cancer.
- the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitorcomprises: sotorasib, sorafenib, panitumumab, encorafenib, trametinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, PLX4720 (company ?), PD0325901 (Company ?), PD184352(Company ?), E6201 (Esai Co Ltd), GDC-0623 (RG 7421 Genentech Inc), CH5126766 (RO5126766 - Chugai Pharmaceutical Co.), HL-085(Shanghai Kechow Pharma, Inc.), SHR7390 (
- the amount of the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor for use in the combinationcan be any amount that has therapeutic benefit to the subject in need of the combination treatment, which can include a daily dose, a therapeutically effective daily dose selected from about 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 20 mg/kg, 0.05 mg/kg to 10 mg/kg, 0.1 mg/kg to 75
- a compound of the disclosure and one or more therapeutic agents or anticancer agentsare administered to an animal under one or more of the following conditions: at different periodicities, at different durations, at different concentrations, by different administration routes, etc.
- the compoundis administered prior to the therapeutic or anticancer agent, e.g., 0.5, 1, 2, 3, 4, 5, 10, 12, or 18 hours, 1, 2, 3, 4, 5, or 6 days, or 1, 2, 3, or 4 weeks prior to the administration of the therapeutic or anticancer agent.
- the compoundis administered after the therapeutic or anticancer agent, e.g., 0.5, 1, 2, 3, 4, 5, 10, 12, or 18 hours, 1, 2, 3, 4, 5, or 6 days, or 1, 2, 3, or 4 weeks after the administration of the anticancer agent.
- the compound and the therapeutic or anticancer agentare administered concurrently but on different schedules, e.g., the compound is administered daily while the therapeutic or anticancer agent is administered once a week, once every two weeks, once every three weeks, or once every four weeks.
- the compoundis administered once a week while the therapeutic or anticancer agent is administered daily, once a week, once every two weeks, once every three weeks, or once every four weeks.
- single or multiple administrations of the pharmaceutical compositionsare administered depending on the dosage and frequency as required and tolerated by the patient.
- the dosagecan be administered once but may be applied periodically until either a therapeutic result is achieved or until side effects warrant discontinuation of therapy.
- the combination therapycomprises administering to the subject with a cancer, a therapeutically effective combination comprising a compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I, and at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor simultaneously, either in the same pharmaceutical composition or in separate pharmaceutical compositions.
- the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula Iand at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor are administered sequentially, i.e., the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor is administered either prior to or after the administration of the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I.
- the administrations of the combination, i.e. a compound of Formula I of the present invention, or a pharmaceutically acceptable salt thereof and at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitorare concurrent, i.e., the administration period of the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor and the compound of Formula I, of the present invention, or a pharmaceutically acceptable salt thereof overlap with each other.
- the administrations of the at least one KRAS, NRAS, MAPK, ALK, BRAF, or MEK inhibitor and the compound of Formula Iare non-concurrent.
- the administration of the at least one KRAS, NRAS, pan- RAS, MAPK, ALK, or MEK inhibitoris terminated before the compound of Formula I is administered.
- the administration of the compound of Formula Iis terminated before the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor is administered.
- Toxicity and therapeutic index of the pharmaceutical compositions of the inventioncan be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., for determining the LD 50 (the dose lethal to 50% of the population) and the ED 50 (the dose therapeutically effective in 50% of the population).
- the dose ratio between toxic and therapeutic effective doseis the therapeutic index and it can be expressed as the ratio LD 50 /ED 50 .
- Compositions that exhibit large therapeutic indicesare generally preferred.
- the methods described hereinmay be used in combination with other conventional anti-cancer therapeutic approaches directed to treatment or prevention of proliferative disorders, such approaches including, but not limited to immunotherapy, chemotherapy, small molecule kinase inhibitor targeted therapy, surgery, radiation therapy, and stem cell transplantation.
- such methodscan be used in prophylactic cancer prevention, prevention of cancer recurrence and metastases after surgery, and as an adjuvant of other conventional cancer therapy.
- chemotherapeutic compoundshave been shown to have anti- neoplastic activities. These compounds have been used as pharmaceutical agents in chemotherapy to shrink solid tumors, prevent metastases and further growth, or decrease the number of malignant T-cells in leukemic or bone marrow malignancies.
- chemotherapyhas been effective in treating various types of malignancies, many anti-neoplastic compounds induce undesirable side effects, for example, known ⁇ PI3K inhibitors, for example, Alpelisib have been known to induce adverse side effects such as hyperglycemia.
- hyperinsulinemiathat occurs in patients with Alpelisib-induced hyperglycemia may provide breast cancer cells with a survival mechanism and reduce the efficacy of Alpelisib as demonstrated in preclinical studies. Patients with an absolute or relative deficiency in insulin, and those with insulin resistance or pancreatic dysfunction, may experience exacerbated or prolonged hyperglycemia. Furthermore, if not successfully managed, hyperglycemia can necessitate ⁇ - selective PI3K inhibitor dose reductions, treatment interruptions, or treatment discontinuation. Optimal hyperglycemia management is therefore required to maintain Alpelisib dose intensity and maximize treatment benefit.
- the compounds of Formula Ifor example, compounds, or their pharmaceutical acceptable salts, such as those exemplified in Table 1, herein, do not substantially increase hyperglycemia, blood glucose or blood insulin when administered in therapeutically effective amounts to subjects with cancer, as shown in the examples and experiments of the present disclosure. It has been shown that when two or more different treatments are combined, the treatments may work synergistically and allow reduction of dosage of each of the treatments, thereby reducing the detrimental side effects exerted by each compound at higher dosages.
- malignancies that are refractory to a treatmentmay respond to a combination therapy of compounds or their pharmaceutically acceptable salts, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I, (for example, the compounds or their salts of Table 1) in combination with at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor.
- the term hyperglycemiagenerally refers to a blood glucose greater than 125 mg/dL while fasting and greater than 180 mg/dL 2 hours postprandial.
- hyperglycemiais defined as a plasma or whole blood glucose measurement greater than 125 mg/dL while fasting and greater than 200 mg/dL, 2 hours postprandial, during a 75-g oral glucose tolerance test (OGTT) using a blood glucose meter employing strips comprising glucose oxidase, hexokinase, or combinations thereof.
- OGTToral glucose tolerance test
- a patienthas impaired glucose tolerance, or pre-diabetes, with a fasting plasma glucose of 100 mg/dL to 125 mg/dL.
- a patientis termed diabetic with a fasting blood glucose of greater than 125 mg/dL.
- administeringdoes not significantly increase the blood glucose, or insulin or cause hyperglycemia in a patient, when administered the compounds of Formula I, or a pharmaceutically acceptable salts, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof as part of a cancer treatment regimen.
- not significantly increasing blood glucose levels, or blood insulin levels after one or more administrations of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitormeans that the blood or plasma glucose levels or the blood or plasma insulin levels in the patient do not rise more than about 30%, or about 29%, or about 28%, or about 27%, or about 26%, or about 25%, or about 24%, or about 23%, or about 22%, or about 21%, or about 20%, or about 19%, or about 18%, or about 17%, or about 16%, or about 15%, or about 14%, or about 13%, or about 12%, or about 11%, or about 10%, or about 5%, or about 1% of the value of blood or plasma glucose or blood or plasma insulin as a result of the administration alone, when measured around one to two hours after administration, when compared to a measurement of blood or plasma glucose or insulin taken just prior (e.g.1 minute to about 30 minutes
- the blood or plasma glucose levels or the blood or plasma insulin levelsdoes not significantly rise as a result of the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor alone, i.e.
- the blood or plasma glucose levels or the blood or plasma insulin levelsdoes not rise more than about 0.1% to about 20% of the levels of the patient's blood or plasma glucose levels or the blood or plasma insulin levels measured just immediately (1-60 mins) prior to the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEK inhibitor.
- the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitordoes not significantly rise as a result of the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor alone, i.e.
- the blood or plasma glucose levels or the blood or plasma insulin levelsdoes not rise more than about 0.1% to about 20% of the levels of the patient's blood or plasma glucose levels or the blood or plasma insulin levels measured just immediately (1-60 mins) prior to the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor.
- Methods for the measurement of blood glucose and insulinare well known in the art.
- a patient's blood or plasma glucose level or amountcan be measured using any glucose sensor known in the art.
- glucose sensoras used herein is a broad term, and is to be given its ordinary and customary meaning to a person of ordinary skill in the art (and is not to be limited to a special or customized meaning), and refers without limitation to any mechanism (e.g., enzymatic or non-enzymatic) by which glucose can be quantified.
- some embodimentsutilize a membrane that contains glucose oxidase that catalyzes the conversion of oxygen and glucose to hydrogen peroxide and gluconate, as illustrated by the following chemical reaction: Glucose + O 2 Gluconate + H 2 O 2 .
- hydrogen peroxidereacts with the surface of the working electrode producing two protons (2H + ), two electrons (2e) and one molecule of oxygen (O 2 ), which produces the electronic current being detected by a sensor relative and proportional to the amount of glucose present at the time of sensing.
- measuring a patient's (for example, a human patient) glucose (whether using the patient's whole blood or plasma) levelcan be typically accomplished using two generally established methods.
- One methoduses an electronic blood glucose meter. A sample of blood is obtained by piercing the skin of a patient with a lancet. The sample of blood is then placed on a chemically-active test-strip, which interfaces with the blood glucose meter. Within several seconds of inserting the test-strip into the blood glucose meter, the blood glucose level of the user is read and shown on the digital display of the blood glucose meter.
- Another methodcan involve the measurement of whole blood or plasma glucose concentration using a colorimetric method based on enzymatic oxidation of glucose in the presence of glucose oxidase.
- the produced hydrogen peroxidereacts with phenol and 4-aminophenazone in a reaction catalyzed by peroxidase to form a red-violet quinoneimine dye as indicator.
- the intensity of the final coloris directly proportional to the glucose concentration in the sample, and can be measured at 505 nm.
- the obtained absorbance measurementscan then be determined and compared using a blood/plasma glucose standard curve.
- the blood glucose meter methodprovides an accurate snapshot of a user's blood glucose level at a single moment in time.
- a CGM systemgenerally includes a sensor, a transmitter, and a receiver (such as a handheld receiver). Some CGM systems use a glucose oxidase sensor. Some CGM devices use glucose binding protein (GBP) sensors. GBP CGM sensors may be preferred because they may be able to provide faster response times and better stability at lower cost than glucose oxidase based sensors.
- GBPglucose binding protein
- blood glucose levels in a patientcan be measured using a handheld device, for example, a CONTOUR®Next (Bayer HealthCare LLC, Mishawaka, IN USA) blood glucose monitor, using blood glucose strips (CONTOUR®NEXT Test Strips - Bayer HealthCare LLC, Mishawaka, IN USA).
- Other blood glucose measurementscan employ the glucose-oxidase method for example, (InfinityTM Glucose (Ox) TR15221 Thermo Scientific, USA) as described in, for example, Christiansen M et. al. A new, wireless-enabled blood glucose monitoring system that links to a smart mobile device: accuracy and user performance evaluation. J. Diabetes Sci Technol.2017; 11:567-573, 3. Bernstein, et al. A New Test Strip Technology Platform for Self-Monitoring of Blood Glucose. J Diabetes Sci Technol 2013;7(5):1388-1399 the disclosures of which are incorporated by reference herein in their entireties.
- the compound of formula G5can be synthesized by reacting a compound of formula G3 with a compound of formula G4 under nucleophilic aromatic substitution conditions. It will be also understood that the order of reactions as specified in Scheme 1 can also be reversed, so that a compound of formula G2 reacts with a compound of formula G3 to form an intermediate product, followed by reacting the intermediate product with a compound of formula G4 to provide a compound of Formula I. It will also be understood that the compounds represented by formula G4 can possess a stereocenter, wherein R 1a and R 1b are different. Further, a compound of G4 can be a single enantiomer or a racemic mixture of enantiomers, which will provide an enantiomeric or racemic compound of Formula I, respectively.
- Compounds of Formula Ican also be synthesized according to Scheme 2. Following the scheme, a compound of Formula G6 first undergoes nucleophilic substitution with an amine of Formula G7. The resulting compound of Formula G8, where Xa is a group appropriate for functional group interconversion to the tetramethyl-1,3,2-dioxaborolane, is converted to a compound of Formula G9. Exposure of the compound of Formula of G9 to a compound of Formula G10 under coupling conditions, for example palladium catalyzed coupling conditions provides a compound of Formula I.
- Step 2Synthesis of 6-(5-amino-3-pyridyl)-N-(1-phenylethyl)quinazolin-4- amine (3a)
- 5-amino-3-pyridyl) boronic acid, 2a(402.32 mg, 1.83 mmol, 2 eq) in DMF (4 mL) and H 2 O (1.5 mL) was added 6-bromo-N-(1-phenylethyl) quinazolin-4-amine (300 mg, 914.06 ⁇ mol, 1 eq), K 3 PO 4 (582.07 mg, 2.74 mmol, 3 eq) and Pd(PPh 3 ) 4 (105.63 mg, 91.41 ⁇ mol, 0.1 eq), the reaction was stirred at 100 o C for 4 h under N2.
- Step 3Synthesis of N-methylsulfonyl-N-[5-[4-(1- phenylethylamino)quinazolin-6-yl]-3-pyridyl]methanesulfonamide (4a)
- Step 4Synthesis of N-[5-[4-(1-phenylethylamino)quinazolin-6-yl]-3- pyridyl]methanesulfonamide (Compound 1) [00406] To a stirred solution of N-methylsulfonyl-N-[5-[4-(1-phenylethylamino) quinazolin-6-yl]-3-pyridyl] methanesulfonamide, 4a, (40 mg, 80.39 ⁇ mol, 1 eq) in MeOH (2 mL) was added K 2 CO 3 (22.22 mg, 160.78 ⁇ mol, 2 eq), the reaction was stirred at 60 °C for 2 h.
- Step 2Synthesis of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (3b) [00411] To a stirred solution of 2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)pyridin-3-amine, 2c, (27 g, 106.08 mmol, 1 eq) in DCM (200 mL) was added TEA (42.94 g, 424.33 mmol, 59.06 mL, 4 eq), MsCl (31.170 g, 272.11 mmol, 21.06 mL, 2.57 eq) at 0 o C.
- Step 2Synthesis of N-[2-chloro-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]methanesulfonamide (Compound 2)
- Compound 2bTo a stirred solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine, 2b, (50 mg, 152.34 ⁇ mol, 1 eq) in DMF (2 mL) and H 2 O (0.5 mL) was added N-[2-chloro-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (62.57 mg, 152.34 ⁇ mol, 1 eq), K 3 PO 4 (97.01 mg, 457.03 ⁇ mol, 3 eq) and Pd(PPh 3 ).
- Step 1Synthesis of (5-amino-6-methyl-3-pyridyl) boronic acid (2) [00424] To a stirred solution of 5-bromo-2-methyl-pyridin-3-amine, 1d, (1 g, 5.35 mmol, 1 eq) in dioxane (5 mL) was added BPD (1.63 g, 6.42 mmol, 1.2 eq), AcOK (1.57 g, 16.04 mmol, 3 eq) and Pd(dppf)Cl 2 .CH 2 Cl 2 (436.62 mg, 534.65 ⁇ mol, 0.1 eq), the reaction was purged with Ar 3 times, and stirred at 100 °C for 16 h under Ar.
- Step 2Synthesis of 6-(5-amino-6-methyl-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (3d)
- 6-bromo-N-(1-phenylethyl)quinazolin-4-amine, 2d, (420.59 mg, 1.28 mmol, 1 eq) in DMF (4 mL) and H 2 O (1.5 mL)was added (5-amino-6-methyl- 3-pyridyl)boronic acid (194.51 mg, 1.28 mmol, 1 eq), K 3 PO 4 (815.12 mg, 3.84 mmol, 3 eq) and Pd(PPh 3 ) 4 (147.91 mg, 128.00 ⁇ mol, 0.1 eq), the reaction was stirred at 100 °C for 4 h under N2.
- Step 3Synthesis of N-[2-methyl-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]-N-methylsulfonyl-methanesulfonamide (4d)
- TEATEA
- Step 4Synthesis of N-[2-methyl-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]methanesulfonamide (Compound 4)
- Step 2Synthesis of N-(1-phenylethyl)-6-(3H-triazolo [4,5-b]pyridin-6- yl)quinazolin-4-amine (Compound 24) [00445] To a stirred solution of N-(1-phenylethyl)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinazolin-4-amine, 2e, (150 mg, 399.71 ⁇ mol, 1 eq) in DMF (2.5 mL) and H 2 O (0.5 mL) was added K 3 PO 4 (254.54 mg, 1.20 mmol, 3 eq), palladium; triphenylphosphane (46.19 mg, 39.97 ⁇ mol, 0.1 eq) and 6-bromo-3H-trazolo[4,5-b]pyridine (63.64 mg, 319.77 ⁇ mol,
- Step 1Synthesis of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (2f)
- a solution of 4-bromoaniline (184.88 g, 1.07 mol, 1 eq) in toluene (1.5 L)was added ethyl (E)-2-cyano-3-ethoxy-prop-2-enoate (200 g, 1.18 mol, 1.1 eq), the mixture was stirred at 110 °C for 6 h.
- Step 3Synthesis of N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3- pyridyl]methanesulfonamide (4f) [00458] To a stirred solution of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (4 g, 9.74 mmol, 1 eq) in dioxane (30 mL) and H 2 O (6 mL) was added 6-bromo-4-hydroxy-quinoline-3-carbonitrile, 3f, (3.15 g, 12.66 mmol, 1.3 eq), Cs 2 CO 3 (9.52 g, 29.22 mmol, 3 eq) ,Pd(dppf)Cl 2 (712.64 mg, 973.94 ⁇ mol,
- Step 4Synthesis of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (5f) [00460] To a solution of POCl 3 (18 mL) was added N-[2-chloro-5-(3-cyano-4-hydroxy-6- quinolyl)-3-pyridyl]methanesulfonamide (2.5 g, 6.67 mmol, 1 eq), the mixture was purged with N 2 3 times, and stirred at 130 °C for 16 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrate in vacuum.
- Step 5Synthesis of N-[2-chloro-5-[3-cyano-4-(1-phenylethylamino)-6- quinolyl]-3-pyridyl]methanesulfonamide (Compound 48) [00462] To a stirred solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (80 mg, 203.43 ⁇ mol, 1 eq) in MeCN (3 mL) was added 1- phenylethanamine (24.65 mg, 203.43 ⁇ mol, 25.95 ⁇ L, 1 eq), and TEA (32.94 mg, 325.50 ⁇ mol, 45.30 ⁇ L, 1.6 eq), and the mixture was stirred at 90 °C for 2 h.
- Example 16Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 53) [00467] Step 1: Synthesis of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (2g) [00468] To a stirred solution of 6-bromo-4-chloro-quinoline-3-carbonitrile (1 g, 3.74 mmol, 1 eq) in i-PrOH (15 mL) was added TEA (605.22 mg, 5.98 mmol, 832.49 ⁇ L, 1.6 eq) 1- phenylethanamine (543.59 mg, 4.49 mmol, 572.20 ⁇ L, 1.2 eq), the mixture was stirred at 80 °C for 12 h.
- Step 2Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 53)
- 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile500 mg, 1.42 mmol, 1 eq
- DMF5 mL
- H 2 O1 mL
- 2-aminopyrimidin-5- yl)boronic acid(197.20 mg, 1.42 mmol, 1 eq)
- Pd(PPh 3 ) 4(1.64 g, 1.42 mmol, 1 eq)
- K 3 PO 4903.96 mg, 4.26 mmol, 3 eq
- Compound 53Rwas synthesized using the analogous procedure for Compound 53 in Example 16 with chiral starting material.
- Step 2Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 54) [00477] To a stirred solution of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (500 mg, 1.42 mmol, 1 eq) in DMF (5 mL) and H 2 O (1 mL) was added 5-(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine (348.02 mg, 1.42 mmol, 1 eq), Pd(PPh 3 ) 4 (164.04 mg, 142.00 ⁇ mol, 0.1 eq) and K 3 PO 4 (903.96 mg, 4.26 mmol, 3 eq), the mixture was bubbled with N 2 for 1 minute, and stirred at 100 o C for 3 h.
- Example 18Synthesis of 4-[[(1R)-1-phenylethyl] amino]-6-(1H- pyrazolo [3, 4-b] pyridin-5-yl) quinoline-3-carbonitrile (Compound 54R) [00479]
- Step 1Synthesis of 6-bromo-4-chloro-quinoline-3-carbonitrile (2i)
- DMF293.47 mg, 4.02 mmol, 308.92 ⁇ L, 0.1 eq
- Step 3Synthesis of 4-[[(1R)-1-phenylethyl] amino]-6-(1H- pyrazolo [3, 4-b] pyridin-5-yl) quinoline-3-carbonitrile (Compound 54R) [00484] To a solution of 6-bromo-4-[[(1R)-1-phenylethyl] amino] quinoline-3-carbonitrile (1.57 g, 4.46 mmol, 1 eq) in DMF (15 mL) and H 2 O (3 mL) was added Cs 2 CO 3 (4.36 g, 13.37 mmol, 3 eq), Pd(dppf)Cl 2 (326.15 mg, 445.73 ⁇ mol, 0.1 eq) and 5-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl) -1H-pyrazolo [3,4-b]pyridine (1.09 g
- Step 2Synthesis of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinoline-3-carbonitrile (3j) [00501] To a solution of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile, 2j, (7 g, 19.87 mmol, 1 eq) in dioxane (80 mL) was added AcOK (5.85 g, 59.61 mmol, 3 eq), Pd(dppf)Cl 2 .CH 2 Cl 2 (1.62 g, 1.99 mmol, 0.1 eq), BPD (6.06 g, 23.84 mmol, 1.2 eq), the mixture was purged with Ar then the reaction was stirred at 110 °C for 8 h.
- Step 3Synthesis of 6-(5,6-dimethoxy-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 68) [00503] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile, 3j, (230 mg, 576.02 ⁇ mol, 1 eq) in DMF (0.5 mL) and H 2 O (0.1 mL) was added Cs 2 CO 3 (563.04 mg, 1.73 mmol, 3 eq), Pd(dppf)Cl 2 (42.15 mg, 57.60 ⁇ mol, 0.1 eq) and 5-bromo-2,3-dimethoxy-pyridine (163.28 mg, 748.83 ⁇ mol, 1.3 eq), the mixture was
- Step 1Synthesis of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (2k)
- a solution of 4-bromoaniline (184.88 g, 1.07 mol, 1 eq) in toluene (1.5 L)was added ethyl (E)-2-cyano-3-ethoxy-prop-2-enoate (200 g, 1.18 mol, 1.1 eq), the mixture was stirred at 110 o C for 6 h.
- Step 2Synthesis of 6-bromo-4-hydroxy-quinoline-3-carbonitrile (3k)
- a solution of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate, 2k, (40 g, 135.53 mmol, 1 eq) in Ph 2 O (400 mL)was stirred at 270 o C for 8 h.
- the reaction mixturewas poured into MTBE (200 mL). The reaction mixture was filtered, and filter cake was concentrated in vacuum.
- Step 3Synthesis of N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3- pyridyl]methanesulfonamide (4k)
- Step 3Synthesis of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (1.7 g, 4.14 mmol, 1 eq) in dioxane (30 mL), H 2 O (5 mL) was added 6-bromo-4-hydroxy-quinoline-3-carbonitrile, 3k, (1.55 g, 6.21 mmol, 1.5 eq), Pd(dppf)
- Step 4Synthesis of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (5k)
- Step 5Synthesis of N-[2-chloro-5-[3-cyano-4-[[(1R)-1-(4-fluorophenyl) ethyl]amino]-6-quinolyl]-3-pyridyl]methanesulfonamide (Compound 97R)
- N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide, 5k, (100 mg, 254.29 ⁇ mol, 1 eq) in i-PrOH (3 mL)was added (1R)-1-(4-fluorophenyl)ethanamine (35.39 mg, 254.29 ⁇ mol, 1 eq), TEA (41.17 mg, 406.87 ⁇ mol, 56.63 ⁇ L, 1.6 eq), the mixture was stirred at 80 °C for 16 h.
- Compound 97Swas synthesized using the same procedure as Compound 97R in Example 29, but substituting (1S)-1-(4-fluorophenyl)ethanamine for (1R)-1-(4- fluorophenyl) ethanamine.
- Example 30Synthesis of N-[2-chloro-5-[3-cyano-4-(indan-1-ylamino)-6- quinolyl]-3-pyridyl]methanesulfonamide (Compound 101) [00524] To a solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (70 mg, 178.01 ⁇ mol, 1 eq) in i-PrOH (1 mL) was added TEA (54.04 mg, 534.02 ⁇ mol, 74.33 ⁇ L, 3 eq) and indan-1-amine (23.71 mg, 178.01 ⁇ mol, 22.80 ⁇ L, 1 eq).
- Step 1Synthesis of N-[(1S)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4- amine (2l)
- a solution of 4-chloro-6-iodo-quinazoline (2 g, 6.88 mmol, 1 eq) in i-PrOH (20 mL)was added (1S)-1-(4-fluorophenyl)ethanamine (1.05 g, 7.57 mmol, 1.1 eq), TEA (1.11 g, 11.02 mmol, 1.53 mL, 1.6 eq), the mixture was stirred at 80 °C for 16 h.
- Step 2Synthesis of N-[2-chloro-5-[4-[[(1S)-1-(4-fluorophenyl)ethyl]amino] quinazolin-6-yl]-3-pyridyl]methanesulfonamide (Compound 111S) [00537] To a stirred solution of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (2.09 g, 5.09 mmol, 1 eq) in DMF (15 mL) /H 2 O (3 mL) was added N-[(1S)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4-amine, 2l, (2 g, 5.09 mmol, 1 eq
- Compound 111Rwas synthesized using the same procedure as Compound 111S in Example 34, but substituting (1R)-1-(4-fluorophenyl)ethanamine for (1S)-1-(4- fluorophenyl)ethanamine.
- Step 2Synthesis of 6-(2-aminopyrimidin-5-yl)-N-[(1R)-1-(4-fluorophenyl) ethyl]quinazolin-4-amine (Compound 121R)
- Step 2To a stirred solution of N-[(1R)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4- amine, 2m, (300 mg, 762.98 ⁇ mol, 1 eq) in DMF (5 mL), H 2 O (1 mL) was added 5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-amine (168.67 mg, 762.98 ⁇ mol, 1eq), K 3 PO 4 (485.86 mg, 2.29 mmol, 3 eq), Pd(PPh 3 ) 4 (88.17 mg, 76.30 ⁇ mol, 0.1
- Example 36Synthesis of 4-(1-phenylethylamino)-6-(3-quinolyl)quinoline-3- carbonitrile (Compound 122) [00546] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 ⁇ mol, 1 eq) in DMF (2.5 mL) and H 2 O (0.5 mL) was added Cs 2 CO 3 (244.80 mg, 751.33 ⁇ mol, 3 eq), Pd(dppf)Cl 2 (18.33 mg, 25.04 ⁇ mol, 0.1 eq) and 3-bromoquinoline (52.11 mg, 250.44 ⁇ mol, 33.62 ⁇ L, 1 eq), the mixture was bubbled with N2, and stirred at 100°C for 3h.
- Step 2Synthesis of N-[(1R)-1-phenylethyl]-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (3n) [00571] To a stirred solution of 6-bromo-N-[(1R)-1-phenylethyl]quinazolin-4-amine, 2n, (5.6 g, 17.06 mmol, 1 eq) in dioxane (60 mL) was added BPD (5.20 g, 20.47 mmol, 1.2 eq), Pd(dppf)Cl 2 .CH 2 Cl 2 (1.39 g, 1.71 mmol, 0.1 eq) and AcOK (5.02 g, 51.19 mmol, 3 eq), the reaction was stirred at 110 °C for 12 h under N 2 .
- Step 3Synthesis of methyl 5-[4-[[(1R)-1-phenylethyl]amino]quinazolin-6- yl]pyridine-3-carboxylate (Compound 135)
- N-[(1R)-1-phenylethyl]-6- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine60 mg, 159.88 ⁇ mol, 1 eq
- K 3 PO 4(101.81 mg, 479.65 ⁇ mol, 3 eq)
- Pd(dppf)Cl 211.70 mg, 15.99 ⁇ mol, 0.1 eq
- Step 3Synthesis of N-[(1R)-1-phenylethyl]-6-[3-(4H-1,2,4-triazol-3- yl)phenyl]quinazolin-4-amine (Compound 137)
- 3-(3-bromophenyl)-1H-1,2,4-triazole35.82 mg, 159.88 ⁇ mol, 1 eq
- DMF1 mL
- H 2 O0.2 mL
- N-[(1R)-1-phenylethyl]-6-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine3p, (60mg, 159.88 ⁇ mol, 1 eq), Cs 2 CO 3 (156.28 mg, 479.65 ⁇ mol, 3 eq) and Pd(dppf)Cl 2 (11.70 mg, 15
- Step 2Synthesis of 2-[(6-imidazo[1,2-a]pyrazin-3-ylquinazolin-4-yl)amino]- 2-phenyl-ethanol (Compound 140) [00592] To a solution of 2-[(6-iodoquinazolin-4-yl)amino]-2-phenyl-ethanol, 2r, (200 mg, 511.24 ⁇ mol, 1 eq) in dioxane (2 mL) was added imidazo[1,2-a]pyrazine (121.80 mg, 1.02 mmol, 2 eq), PPh 3 (13.41 mg, 51.12 ⁇ mol, 0.1 eq), Cs 2 CO 3 (333.14 mg, 1.02 mmol, 2 eq) and Pd(OAc) 2 (5.74 mg, 25.56 ⁇ mol, 0.05 eq).
- Comparative compound 2is a dual enzyme inhibitor against EGFR and PI3K.
- the description and synthesis of Comparative Compound 2 and Comparative Compound 3can be found in International Application No. PCT/US2015/065827 filed on December 15, 2015 and is referenced as Mol 211 and Mol 167 respectively, the disclosure of which is incorporated herein by reference in its entirety.
- Example 53Affinity of selected compounds of the disclosure for PI3Ka, EGFR, and DNA-PK enzymes.
- the Z ⁇ -LYTE® biochemical assayemploys a fluorescence-based, coupled- enzyme format and is based on the differential sensitivity of phosphorylated and non- phosphorylated peptides to proteolytic cleavage (Fig.6A and 6B).
- the peptide substrateis labeled with two fluorophores—one at each end—that make up a FRET pair.
- the kinasetransfers the gamma-phosphate of ATP to a single tyrosine, serine or threonine residue in a synthetic FRET-peptide.
- a site-specific proteaserecognizes and cleaves non-phosphorylated FRET-peptides.
- FRET-peptidessuppresses cleavage by the Development Reagent. Cleavage disrupts FRET between the donor (i.e., coumarin) and acceptor (i.e., fluorescein) fluorophores on the FRET-peptide, whereas uncleaved, phosphorylated FRET-peptides maintain FRET.
- a ratiometric methodwhich calculates the ratio (the Emission Ratio) of donor emission to acceptor emission after excitation of the donor fluorophore at 400 nm, is used to quantitate reaction progress. [00600] A significant benefit of this ratiometric method for quantitating reaction progress is the elimination of well-to-well variations in FRET-peptide concentration and signal intensities.
- the assayyields very high Z ⁇ -factor values (>0.7) at a low percent phosphorylation.
- Both cleaved and uncleaved FRET-peptidescontribute to the fluorescence signals and therefore to the Emission Ratio.
- the extent of phosphorylation of the FRET-peptidecan be calculated from the Emission Ratio.
- the Emission Ratiowill remain low if the FRET-peptide is phosphorylated (i.e., no kinase inhibition) and will be high if the FRET-peptide is non- phosphorylated (i.e., kinase inhibition).
- the ADAPTA universal kinase assayis a homogenous, fluorescent based immunoassay for the detection of ADP. In contrast to ATP depletion assays, the ADAPTA assay is extremely sensitive to ADP formation such that a majority of the signal change occurs in the first 10-20% conversion of ATP to ADP. This makes the ADAPTA universal kinase assay ideally suited for use with low activity kinases.
- the principle of the ADAPTA universal kinase assayis outlined below. The assay itself can be divided into two phases: a kinase reaction phase, and an ADP detection phase.
- kinase reaction phaseall components required for the kinase reaction are added to the well, and the reaction is allowed to incubate for 60 minutes.
- a detection solutionconsisting of a europium labeled anti-ADP antibody, an Alexa Fluor® 647 labeled ADP tracer, and EDTA (to stop the kinase reaction) is added to the assay well.
- ADP formed by the kinase reactionin the absence of an inhibitor will displace the Alexa Fluor® 647 labeled ADP tracer from the antibody, resulting in a decrease in the TR-FRET signal.
- Test CompoundsThe Test Compounds are screened in 1% DMSO (final) in the well. For 10 point titrations, 3-fold serial dilutions are conducted from the starting concentration.
- Peptide/Kinase MixturesAll Peptide/Kinase Mixtures are diluted to a 2X working concentration in the appropriate Kinase Buffer.
- ATP SolutionAll ATP Solutions are diluted to a 4X working concentration in Kinase Buffer (50 mM HEPES pH 7.5, 0.01% BRIJ-35, 10 mM MgCl 2 , 1 mM EGTA). ATP Km apparent is previously determined using a Z ⁇ -LYTE® assay.
- Development Reagent SolutionThe Development Reagent is diluted in Development Buffer.
- 10X Novel PKC Lipid Mix2 mg/mL Phosphatidyl Serine, 0.2 mg/mL DAG in 20 mM HEPES, pH 7.4, 0.3% CHAPS. For 5 mL 10X Novel PKC Lipid Mix: 1.
- Assay ProtocolBar-coded Corning, low volume NBS, black 384-well plate (Corning Cat. #4514) 1.2.5 ⁇ L – 4X Test Compound or 100 nL 100X plus 2.4 ⁇ L kinase buffer. 2.5 ⁇ L – 2X Peptide/Kinase Mixture.3.2.5 ⁇ L – 4X ATP Solution.4.30-second plate shake.5.
- each data pointuses 100 nL – 100X test compound in 100% DMSO. Commonly, 100 nL of a 10 ⁇ M solution of test compound is used for each experiment, which is equivalent to 1 picomole of test compound.
- a 10 ⁇ M single- point assayuses 100 picomoles of test compound, and a 10-point titration uses about 200 picomoles of test compound – 100 picomoles for the initial test and another 100 picomoles for the serial dilution.
- ADP formationis determined by calculating the emission ratio from the assay well. The emission ratio is calculated by dividing the intensity of the tracer (acceptor) emission by the intensity of the Eu (donor) emission at 615 nm as shown in the equation below.
- the ADAPTA technologymeasures ADP formation (i.e. conversion of ATP to ADP) it can be used to measure any type of ATP hydrolysis, including intrinsic ATPase activity of kinases.
- the substrateis water, not a lipid or peptide.
- the SelectScreen® servicescreens CHUK in this way, so a substrate is not included in the kinase reaction.
- a reference for using intrinsic ATPase activity to screen for kinase inhibitorsis provided below.
- Substrate/Kinase MixturesAll Substrate/Kinase Mixtures are diluted to a 2X working concentration in the appropriate Kinase Buffer (see section Kinase Specific Assay Conditions for a complete description).
- ATP SolutionAll ATP Solutions are diluted to a 4X working concentration in water. ATP Km apparent is previously determined using a radiometric assay except when no substrate is available in which case an Adapta® assay is conducted.
- Detection MixThe Detection Mix is prepared in TR-FRET Dilution Buffer. The Detection mix consists of EDTA (30 mM), Eu-anti-ADP antibody (6 nM) and ADP tracer.
- the detection mixcontains the EC60 concentration of tracer for 5-150 ⁇ M ATP.
- Assay ProtocolBar-coded Corning, low volume, white 384-well plate (Corning Cat. #4512)1.2.5 ⁇ L – 4X Test Compound in 30 mM HEPES or 100 nL 100X in 100% DMSO plus 2.4 ⁇ L 30 mM HEPES.2.2.5 ⁇ L – 4X ATP Solution.3.5 ⁇ L – 2X Substrate/Kinase Mixture.4.30-second plate shake.5.1-minute centrifuge at 1000 x g.6.60-minute Kinase Reaction incubation at room temperature.7.5 ⁇ L – Detection Mix.8.30-second plate shake.9.
- each data pointuses 100 nL – 100X test compound in 100% DMSO.
- 100 nL of a 10 ⁇ M solution of test compoundis used for each experiment, which is equivalent to 1 picomole of test compound.
- a 10 ⁇ M single- point assayuses 100 picomoles of test compound
- a 10-point titrationuses about 200 picomoles of test compound – 100 picomoles for the initial test and another 100 picomoles for the serial dilution.
- Example 54Effect of Compound 2 on tumor volumes in xenograft mice.
- Xenograft StudiesFemale NCR nude mice (CrTac:NCr-Foxn1nu from Taconic), 6-7 weeks old, were implanted subcutaneously with 5x10 6 cells in a 1:1 serum-free media/Matrigel® mixture into the region of the right axilla. Mice were randomized into treatment groups and treatments initiated when tumors reached 100 to 200 mg. Compound 2R was administered daily by oral gavage as a fine suspension in 1:2 propylene glycol in 1% Tween80/Na3PO4 based upon individual animal body weight (0.2 mL/20 g).
- liver Microsomes PreparationThe appropriate concentrations of microsome working solutions were prepared in 100 mM potassium phosphate buffer. Cold (4°C) acetonitrile (ACN) containing 200 ng/mL tolbutamide and 200 ng/mL labetalol as internal standards (IS) was used as the stop solution [00632] Assay Procedure: Pre-warm empty 'Incubation' plates T60 and NCF60 for 10 minutes. Dilute liver microsomes to 0.56 mg/mL in 100 mM phosphate buffer.
- ACNacetonitrile
- the biological stability of the compounds of the disclosurecan be measured by determining its half-life in the presence microsomes.
- Table 5is the half-life of selected compounds of the disclosure in the presence of human liver microsomes (HLM) or mouse liver microsomes (MLM) as described above.
- half-lifeis presented as “++++” (value is greater than 60 minutes), “+++” (value is greater than 30 minutes and less than or equal to 60 minutes), “++” (value is greater than 15 minutes and less than or equal to 30 minutes) and “+” (value is 15 minutes or less).
- Table 5Half-life of selected compounds of the disclosure in HLM or MLM.
- Simulated Intestinal FluidPrepared by dissolving 6.8 g of KH 2 PO 4 into about 500 mL ultrapure water and adjust the solution to a pH 6.8 with 0.1 M NaOH.10 g trypsin is then dissolved into ultrapure water. The two solutions are mixed well and diluted with ultrapure water to a final volume of 1000 mL.
- Procedure for solubility determination15 ⁇ L of stock solution (10 mM) of each sample was placed in order into their proper 96-well rack.485 ⁇ L of (SIF, SGF, PBS 7.4, FESSIF, or FESSGF) was added into each vial of the cap-less Solubility Sample plate.
- the assaywas performed in duplicate. Add one stir stick to each vial and seal using a molded PTFE/Silicone plug. Then the solubility sample plate was transferred to the Eppendorf Thermomixer Comfort plate shaker and shaken at 25°C at 1100 rpm for 2 hours. After completion of the 2 hours, plugs were removed and the stir sticks were removed using a big magnet, the samples from the Solubility Sample plate were transferred into the filter plate. Using the Vacuum Manifold, all the samples were filtered. Aliquot of 5 ⁇ L and 5 ⁇ L DMSO were taken from the filtrate followed by addition of 490 ⁇ L of a mixture of H 2 O and acetonitrile containing internal standard (1:1).
- Solubility values of the test compound and control compoundwere calculated as follows: [00646] Any value of the compounds that was not within the specified limits was rejected and the experiment was repeated. [00647]
- the solubility data for selected compounds of the disclosureis provided in Table 6.
- the solubility data in Table 6is presented as “****” (value is greater than 200 ⁇ M), “***” (value is greater than 100 ⁇ M and less than or equal to 200 ⁇ M), “**” (value is greater than 20 ⁇ M and less than or equal to 100 ⁇ M) and “*” (value is 20 ⁇ M or less).
- NTis “not tested.”
- Table 6Solubility data for selected compounds of the disclosure.
- the results in Table 6show that the solubility of the compounds of the present disclosure are significantly improved compared to Comparative Compounds 1-3. Seven out of ten compounds tested show an improvement in solubility in simulated intestinal fluid, with Compounds 2R and 2S being at least 5 times as soluble. Compound 2R also shows the same improvement in solubility in PBS buffer.
- APIactive pharmaceutical ingredient
- Example 57Assessment of the pharmacokinetics of Compound 2R
- the IV and PO solution formulations for Comparative Compound 2contained 20% (w/v) propylene glycol, 75% (w/v) 50 mM trisodium phosphate (pH 12), and 5% (w/v) Kolliphor EL.
- the formulation for Compound 2Rcontained 1:2 propylene glycol: 1%Tween 80/Na 3 PO 4 (50 mM).
- Oral PK analysisrevealed a similar trend in mice as reflected by 3-fold enhanced exposure with Compound 2R relative to Comparative Compound 2.
- improvement in exposure in response to oral treatment with Compound 2Rwas especially strong in both dogs (>28-fold) and monkeys (>74-fold) as shown by AUClast/D values of 13,808 vs 487 h*mg/ml (dogs) and 2222 vs 30 h*mg/ml (monkeys).
- Compound 2Rdramatically improves therapeutic outcomes in combination with RAS pathway intervention in BRAF and KRAS mutated colorectal and pancreatic cancers.
- Compound 2R regimenswere well tolerated and efficacious over a wide range of doses in preclinical models.
- Compound 2R regimenssurprisingly and unexpectedly, did not produce a hyperglycemic response as commonly seen with PI3K inhibitors.
- EGFR crystallization[00658] The EGFR construct used for crystallization (G696-G1022) was produced as described previously (PDB Code 2GS2)58. Crystals of apo-EGFR were obtained using hanging- drop vapor diffusion set-ups and EGFR at a concentration of 5.6 mg/mL (25 mM Tris/HCl, 50 mM NaCl, 2 mM DTT, pH 7.5).
- the protein solution (2 pL)was mixed with 0.6 pL of reservoir solution (0.10 M HEPES/NaOH pH 6.70, 1.05 M Na-Succinate pH 7.00) and equilibrated at 12°C over 0.4 mL of reservoir solution.
- Well diffracting crystalswere selected for data collection after 13 days. Crystals were soaked for 3.5 hours with 1 mM Compound 2R in 30% solubilizing mix 4 from the Molecular Dimensions CryoSol kit.
- PI3K ⁇ crystallization[00660] The PIK3CG construct used for crystallization (S144-A1102) was produced as described previously (PDB Code 1E8Y)59.
- PI3KyApo crystals of PI3Ky were obtained using hanging- drop vapor diffusion set-ups.
- PI3Kywas used at a concentration of 7.1 mg/mL (20 mM Tris/HCl, 0.5 mM ammonium sulfate, 5 mM DTT, 0.02 % (v/v) CHAPS, 1 % ethylene glycol, pH 7.2).1 pL of the protein solution was mixed with 1 pL of reservoir solution (100 mM Tris/HCl pH8.2, 0.2 M lithium sulfate, 15 % (w/v) PEG 4000) and equilibrated at 20°C over 300 pL of reservoir solution. Well diffracting crystals were selected for soaking after 20 days.
- Crystalswere soaked with 5 mM Compound 2R for 16 hours in 30% ligand solubilization mix SM1 from molecular dimensions (MD1-90) diluted in reservoir solution.
- PPAR ⁇ crystallization[00662] The PPAR ⁇ construct used for crystallization (L232-Y505 of UniProt entry P37231) was produced as described previously (PDB entry 3SZ1)60. Crystals of apo-PPARy were obtained using sitting-drop vapour diffusion set-ups.
- Biochemical kinase assays[00672] Biochemical kinase assays [00673] All biochemical kinase profiling was carried out at ThermoFisher Scientific. Most kinases were screened using Z-Lyte or LanthaScreenTM assays. Z-Lyte assays, employ a fluorescence-based, coupled-enzyme format, which is based on the differential sensitivity of phosphorylated and non-phosphorylated peptides to proteolytic cleavage. This radiometric method yields very high Z’-factor values (>0.7) at a low percent phosphorylation.
- the LanthaScreen formatis another FRET approach that detects binding of a fluorescent tracer to a kinase with a terbium-labeled GST antibody.
- Screening of lipid kinaseswas carried out using the Adapta universal kinase assay format, which is a fluorescent based immunoassay for the detection of ADP.
- a known inhibitorusually staurosporine for protein kinases and PI-103 for the PIK3CA/PIK3R isoforms, was titrated on the same plate as Compound 2R, to ensure that it was inhibited within the expected IC 50 range previously determined. All assays were carried out at the ATP Km apparent, which was previously determined for that kinase.
- Dose response curveswere curve fit to model number 205 (sigmoidal dose response model) using XLfit graphing software from IDBS.
- Nuclear receptor assays[00675] LanthaScreen time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays were carried out at Thermo-Fisher Scientific for screening Compound 2R against the family of PPAR nuclear hormone receptors. Compounds were screened in 1% DMSO (final concentration) and 3-fold serial dilutions were conducted for 10- point titrations.
- TR-FRETFluorescence resonance energy transfer
- a known inhibitorwas titrated on each plate: GW1929 (PPAR ⁇ ), GW7647 (PPAR ⁇ ), and GW0742 (PPAR( ⁇ ), to ensure that reference compounds were displaced within an expected IC 50 range previously determined.
- Dose response curveswere curve fit to model number 205 (sigmoidal dose-response model) using XLfit graphing software from IDBS.
- Intracellular effects on the PPAR ⁇ nuclear hormone receptorwere determined at Thermo-Fisher Scientific using GeneBLAzerTM Beta-lactamase reporter technology.
- PPAR-gamma-UAS-bla HEK 293T cellswere thawed and resuspended in Assay Media (DMEM phenol red free, 2% CD-treated FBS, 0.1 mM NEAA, 1 mM sodium pyruvate, 100 U/mL/100pg/mL Pen/Strep) to a concentration of 9.4 x 105 cells/mL.4 pL (picoliters) of a 10x serial dilution of rosiglitazone (control agonist starting concentration, 316 nM) or Compound 2R was added to appropriate wells of a 384-well poly-D-lysine assay plate.32 pL of cell suspension (30,000) cells was added to each well followed by 4 pL of assay media to bring the final assay volume to 40 pL.
- Assay MediaDMEM phenol red free, 2% CD-treated FBS, 0.1 mM NEAA, 1 mM sodium pyruv
- Sotorasib(C-1499) was purchased from Chemgood.
- Bovine insulin (I0516) and methylisobutylxanthine (IBMX; I5879)were purchased from MilliporeSigma.
- Dexamethasone(API-04) was purchased from G Biosciences.
- CAL-27 and CAL-33 cell lineswere obtained from Leibniz Institute DSMZ- German Collection of Microorganisms and Cell Cultures.
- MIA PaCa-2 and 3T3-L1 cell lineswere obtained from the American Type Culture Collection.
- KPC tumorsoriginated from 65671 cells (FVB/N strain), which were kindly provided by Marina Pasca di Magliano, University of Michigan61.
- the following modelswere received from the NCI Patient-Derived Models Repository (PDMR) as cryopreserved cells: 135848-042-T (lot# CK3998), 455876-151-R (lot# CK9041), 848979-319-R (lot# CK9558), and CN0375-F725-PDC (lot# CK5838).
- the following modelswere received from the NCI PDMR as cryopreserved tumor fragments: 245127-232-R (lot# JC2149), 354836-022-R (lot# JK0388) and 944545-341-R (lot# MA1086).
- the PDX model CRC 14-929was established at the University of Michigan (Leopold lab)62.. [00679] All patients provided informed written consent and samples were procured with approval of the University of Michigan Institutional Review Board (HUM00065489). Demographic information on all the PDX models used in this study are provided in Table 10. [00680] Table 10. Demographic Data for Patient-Derived Xenograft Models. [00681] Cell culture [00682] RPMI 1640 medium (11875-093), DMEM (11965-092), penicillin streptomycin (15150-122), GlutaMax (35050-061), sodium pyruvate (11360-070) and PBS (10010-023) were all obtained from Gibco/Thermo Fisher Scientific.
- FBSwas obtained from Cytivia (SH3039603).
- Bovine Calf Serum (CBS)was purchased from ATCC (30-2022). Cells were grown in the appropriate medium and maintained at 37°C in a humidified incubator with 5-10% CO 2 . SOPs issued by the NCI PDMR were used to thaw, expand, and maintain the PDC lines obtained from there. All cell lines were determined to be Mycoplasma free using Lonza’s MycoAlert Mycoplasma Detection kit. Cell lines were authenticated by STR analysis (American Type Culture Collection, human and mouse STR profiling service). [00683] Adipocyte Differentiation [00684] 3T3-L1 cells were differentiated according to the methods published previously.
- Cellswere seeded at a density of 6.0 x 10 5 cells per T-75 flask (Corning) in DMEM supplemented with 10% CBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate and grown to confluency. The culture medium was refreshed and the cells were grown for 48 hours.
- the cellswere cultured for 48 hours in differentiation induction medium containing DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate, 1.0 ⁇ M dexamethasone, 0.5 mM IBMX, 1.0 ⁇ g/mL insulin containing DMSO, Rosiglitazone 1 mM or Compound 2R 10 mM. Thereafter, the medium was changed to DMEM, 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate, 1.0 mg/mL insulin every 48 hrs until 7 days post-induction.
- NP-40 lysis buffer50 mM Tris pH 7.5, 1% NP-40, 150 mM NaCl, 10% glycerol, 1 mM EDTA
- protease and phosphatase inhibitors(Roche). Tumors were minced and manually homogenized in NP-40 lysis buffer. Lysates rocked at 4°C for 30-60 minutes and cleared by centrifugation.
- Equal amounts of protein (10–20 ⁇ g) in normalized lysateswere resolved by SDS–PAGE, transferred to 0.2 ⁇ m or 0.45 ⁇ m polyvinyldifluoride (PVDF) membrane (Millipore Sigma), probed with specific primary and secondary antibodies, and detected by chemiluminescence with ECL detection reagents (Cytiva).
- PVDFpolyvinyldifluoride
- anti-p- EGFR(tyr1068) #3777, anti-EGFR #2646 (1:10,000), anti-p-AKT (thr308) #13038, anti-p-AKT (ser473) #4060, anti-AKT #9272 (1:5000), anti-pS6 (ser235/236) # 4857, anti-S6 #2217 (1:10,000), anti-p-PRAS40 (thr246) #2997, anti-PRAS40 #2691 (1:10,000), anti-p-4E-BP1 (ser65) #9451), anti-4E-BP1 #9644, anti-p-p70 S6K (thr389) #97596, anti-p70 S6K #9202, anti- PPAR ⁇ #2443, anti-cleaved PARP #9541, all from Cell Signaling Technologies; anti-ii-actin HRP conjugated #197277 (1:10,000) and anti-vinculin #129002 (1:
- peroxidase-conjugated AffiniPure goat anti-rabbit IgG(Jackson ImmunoResearch Laboratories, 111-035-003) was used at a 1:10,000 dilution.
- imageswere converted to grayscale and saved as .tif files.
- ImageJ softwarehttps://imagej.nih.gov/ij/ was used to create a rectangular ROI around the widest band for each protein on a given blot. The same ROI was applied to each band for a given protein and histograms were generated indicating the intensity of each band. This signal intensity was determined for both the phosphorylated protein and the housekeeping protein for each target protein of interest. Data was exported to Microsoft Excel.
- the level of phosphorylation for a given protein for each samplewas determined by dividing the signal intensity of the phosphorylated protein band by the signal intensity of the housekeeping protein band. The level of phosphorylation for each target protein was averaged within the control treatment group. Percent inhibition of phosphorylated protein expression for compound treated samples were compared to the average of the control sample by taking the signal intensity of the treated sample, dividing it by the average signal intensity of the control sample and multiplying by 100. Standard error of the mean was calculated for each replicate within a given treatment condition. GraphPad Prism was used to plot the results.
- First strand cDNAwas synthesized using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s instructions. Briefly, 20 ⁇ L 5X VILO Reaction Mix, 10 ⁇ L 10X SuperScript Enzyme Mix and 65 ⁇ L of DEPC-treated dH2O were added to 5 ⁇ g of RNA in a 100 ⁇ L reaction volume. PCR reactions were amplified in the VeritiTM 96-well Thermal Cycler (Applied Biosystems) under the following conditions: 10 min at 25°C, 90 min at 42°C, 5 min at 85°C. PCR reactions without reverse transcriptase were included to detect genomic DNA contamination.
- Quantitative PCR reactionswere performed in quadruplicate using mouse-specific Taqman Gene Expression Single Tube Assays (Applied Biosystems) for lipoprotein lipase (LPL) (Mm00434764_m1), PPAR ⁇ (Mm00440940_m1) and adiponectin (Mm04933656_m1).
- LPLlipoprotein lipase
- PPAR ⁇Mm00440940_m1
- adiponectinMm04933656_m1
- the housekeeping gene, RLPL0(Mm00725448_s1), was used to normalize for target gene expression. Reactions were prepared by adding 1 ⁇ L of cDNA, 5 ⁇ L 2X Master Mix, 0.5 ⁇ L 20X Taqman Gene Expression Assay and 3.5 ⁇ L nuclease-free H2O. Control reactions without cDNA template were included to detect contamination.
- CIEA NOG mice6-8 week old female CIEA NOG mice (NOD.Cg-Prkdc scid Il2rg tm1Sug /JicTac from Taconic) or female athymic homozygous nude mice (CrTac:NCr-Foxn1 nu from Taconic or Crl:NU(NCr)-Foxn1 nu from Charles River Laboratories) were used.
- miceFor models originating from cell culture (CAL-27, CAL- 33, MIA PaCa-2, 135848, 455876, 848979, CN0375), the mice were inoculated subcutaneously in the right axilla with 1 x 10 6 to 5 x 10 6 cells suspended in 100 ⁇ l of 1:1 ratio of serum-free media to Matrigel.
- tumor fragmentsU-CRC 14-929, 245127, 354836, 944545
- the micewere implanted with tumor fragments 2-3 mm in diameter into the right axilla via trocar.
- female inbred FVB mice(6-8 weeks of age) were obtained from Taconic (FVB/NTac).
- micewere inoculated subcutaneously in the right axilla with 1 x 10 6 of 65671 (FVB/N strain) cells suspended in 100 ⁇ l of 1:1 serum-free media:Matrigel.
- Compound 2Rwas prepared as a suspension in 1:2 propylene glycol:1% Tween80/Na3PO4 or as a solution in 50% propylene glycol/35% PEG400/10% TPGS in water/5% 1N NaOH and administered via oral gavage.
- Alpelisib and buparlisibwere prepared as suspensions in 1% carboxymethylcellulose with 0.2% Tween80 and administered via oral gavage.
- Copanlisibwas prepared as a solution in 0.5% mannitol in water and administered through tail vein injection.
- Erlotinibwas prepared as a solution in 5% DMSO/95% PEG300 and administered via oral gavage.
- Omipalisibwas prepared as a solution in 2% DMSO/40% PEG300/2% Tween80/56% ddH20 and administered via oral gavage.
- Sotorasibwas prepared as a suspension in 0.5% carboxymethylcellulose with 1% Tween80 and administered via oral gavage.
- Taselisibwas prepared as a suspension in 0.5% methylcellulose with 0.2% Tween80 and administered via oral gavage.
- Trametinibwas prepared as a suspension in 0.5% hydroxypropyl methylcellulose with 0.2% Tween80 and administered via oral gavage.
- mice were randomized into treatment arms and treatment was initiated (Day 1 of study). Subcutaneous tumor volume and body weights were measured two to three times a week. Tumor volumes were calculated by measuring two perpendicular diameters with calipers and using the formula: tumor volume(length ⁇ width2)/2. Mice were treated and monitored daily until individual mouse tumor burdens reached IACUC approved limitations (either 500 for ulcer-prone models or 1000 mm3).
- Percent A Treated/A Controlwas calculated on the day the median control mouse was euthanized as follows: [(Tfinal - Tinitial)/(Cfinal – Cinitial)] x 100, where Cinitial and Cfinal are the median tumor volumes on the first day of treatment and the day indicated for the vehicle control group and Tinitial and Tfinal are the median tumor volumes on the first day of treatment and the day indicated for the treated group. Increase is lifespan was calculated as [(T day – C day )/C day ] x 100, where Tday is the day the median treated mouse was euthanized and Cday is the day the median control mouse was euthanized.
- CRcomplete response
- PRpartial response
- SDStable disease
- miceFemale athymic nude mice (8-10 weeks of age) were acclimated for a minimum of three days prior to being placed on study. At study initiation, mice were administered vehicle control or test article based upon individual mouse body weight (0.2mL/20g). For assessment of blood glucose levels, 1-2 drops of blood were taken from the tail of the mice and measured using an ACCU-CHEK Aviva glucometer before treatment (time 0) and at 0.5, 1, 2, and 4 hours following treatment. For assessment of blood insulin levels, ⁇ 30-40 ⁇ L of blood was collected into EDTA microvette tubes (Sarstedt, cat# 16.444.100) immediately following the blood glucose measurement.
- Log-transformed tumor volumewas used for the analysis to better satisfy the assumption of linearity in growth over time.
- ⁇ 0can be interpreted as the overall average logarithmic tumor volume at first time measurement for all mice.
- ⁇ 1represents the rate change in logarithmic scale tumor-volume across time for the vehicle group, and ⁇ 2 is defined analogously for the Compound 2R treatment group.
- the ⁇ 2 – ⁇ 1represents the difference of logarithmic scale tumor growth ratio between Compound 2R treatment vs. vehicle group.
- a Wald testwas used for the significance comparisons between different groups.
- ⁇ 0can still be interpreted as the overall average logarithmic tumor volume at first time measurement for all mice.
- ⁇ 1represents the linear change in log scale tumor-volume across time for the vehicle group
- ⁇ kwere the parameters for the linear change of tumor log-volume for the compound treatment groups given by the respective indicator variable.
- the ⁇ k – ⁇ 1represent the difference between log scale tumor growth ratio between compound treatments vs. vehicle group
- e.g. ⁇ k – ⁇ k,-1represents the differences of rates of log scale tumor growth ratio between their respective treatment groups.
- a Wald testwas used for the significance comparisons among different groups.
- the 1-position of the quinazoline ring in Compound 2Rforms a key hydrogen bond with the backbone amide of MET793 of EGFR (Fig.1C).
- the substitution at the 4-positionfits into a hydrophobic pocket formed by the aliphatic sidechains of LYS745 and THR790, whereas the hydrophilic group at the 6-position faces outward to a solvent accessible area of EGFR.
- the 1-position of Compound 2Rforms a hydrogen bond with the backbone amide of VAL882 of PI3K ⁇ (Fig.1E).
- Compound 2Rexhibits low nanomolar potency against EGFR and PI3K ⁇ with IC 50 ’s of 15 nM and 6.4 nM, respectively (Fig.17A and Fig.18A).
- Figure 1Dshows the crystal structure of PI3K ⁇ co-complexed with COMPOUND 2R.
- MELKwas the only off target protein kinase inhibited by Compound 2R with potency within 50-fold of that observed against PI3K ⁇ .
- Cellular evidence for the ability of Compound 2R to co-target EGFR and PI3Kwas generated in the CAL-33 HNSCC model, which is known to possess a PIK3CA mutation (H1047R), see Fig.2N.
- Compound 2Rdemonstrated concentration-dependent inhibition of EGFR, PI3K, and mTOR as measured by lower levels of phosphorylated EGFR, AKT, and 4E-BP1 (Fig.1F, & Figs.21A-21E).
- Compound 2Ralso tolerated dosing at 150 mg/kg, leading to a further boost in efficacy in the 848979-319-R model and a 3-fold improvement in median survival compared to mice treated at 100 mg/kg (Fig.14C). Therefore, collective efficacy data generated against the HNSCC PDX panel at the lower dose is a conservative indicator of the monotherapy potential of Compound 2R. Head-to-head evaluation of Compound 2R versus the combination of erlotinib and alpelisib was carried out in the 944545-341-R PDX model (Figs.2R-2T).
- Compound 2Rsingle agent treatment resulted in a mean tumor regression of 70%, no regressions were observed in the erlotinib/alpelisib combination arm. Higher doses of alpelisib were not tolerated in the combination regimen (Fig.23). Compound 2R improves response of KRAS and BRAF mutant colorectal tumors to MEK inhibition [00709] Triple combinations directed against EGFR (cetuximab), PI3K (alpelisib), and the MAPK pathway (encorafenib or trametinib), while scientifically sound, have proven challenging due in part to poor tolerability.
- PPAR ⁇ agonismis a unique feature of Compound 2R [00716]
- TR-FRETTime-resolved fluorescence resonance energy transfer
- Compound 2RThe intracellular effects of Compound 2R on PPAR ⁇ activity was evaluated in reporter gene assays carried out in 293H cells.
- Expression of PPAR ⁇ target genes involved in the induction of adipocyte differentiationwere measured by RT-PCR in treated 3T3-L1 cells and provided further evidence for a functional interaction between Compound 2R and PPAR ⁇ .
- 3T3-L1 preadipocyteswere cultured in differentiation induction medium containing Compound 2R (10 ⁇ M) or rosiglitazone (1 ⁇ M) for 8 or 24h followed by assessment of gene expression of the adipocyte markers PPAR ⁇ , lipoprotein lipase (LPL) and adiponectin (Fig.6C). These markers are known to be upregulated in response to rosiglitazone. Maximal induction of PPAR ⁇ ranged from 2- to 4-fold in response to both compounds, providing further support for the ability of Compound 2R to act as an agonist of PPAR ⁇ . The one log difference in concentration of Compound 2R and rosiglitazone further suggest that Compound 2R is acting as a weak PPAR ⁇ agonist.
- the final solved structurecontains two molecules of PPAR ⁇ (chains A and B) and one molecule of Compound 2R bound to chain B.
- the PPAR ⁇ ligand binding regionis known to be a large and mostly hydrophobic cavity that can bind a wide variety of small molecules.
- the quinazoline core of Compound 2Rappears to sit in a pocket of PPAR ⁇ formed by stacking of the aliphatic sidechains of Leu330 and Arg288 (Fig.6F).
- the functional groups of the 6-position core quinazoline groups in Compound 2Rbinds in a site distinct from the orthosteric pocket that binds rosiglitazone (Fig.16A).
- PI3K/mTOR pathwaydrives resistance to a broad assortment of targeted therapies, including highly selective allosteric MEK inhibitors and covalent inhibitors of KRASG12C.
- the design of Compound 2Rwas driven by the concept of developing a single molecule that could selectively target the PI3K/mTOR pathway in addition to EGFR. Such a molecule would ostensibly be useful to deliver single agent therapy to selected patient populations, for example, patients diagnosed with squamous tumors, where EGFR and PI3K and/or mTOR are genomic drivers.
- Compound 2Rwas rationally designed to inhibit EGFR and PI3K, selectively and concurrently.
- the reversed binding mode of the quinazoline core between EGFR and PI3K ⁇was an important element in the design of Compound 2R, which shows low nanomolar potency against both of its intended targets.
- Other studieshave previously reported on the feasibility of designing dual inhibitors of tyrosine and phosphoinositide kinases. Through iterative medicinal chemistry and x-ray crystallography, they identified molecules that adopt a single binding mode to inhibit PI3 kinases and multiple tyrosine kinases with a high degree of potency.
- Compound 2Ris a remarkably selective ATP-competitive protein kinase inhibitor. Only four of 432 kinases tested were found to exhibit an IC 50 ⁇ 1 ⁇ M and none came within 10-fold of Compound 2R’s on target potency against EGFR (IC 50 ⁇ 15 nM). Erlotinib, which is roughly one log more potent against EGFR than Compound 2R, has been reported to exhibit an IC 50 ⁇ 1 ⁇ M against a minimum of 20 off-target kinases. [00720] Preclinical proof-of-concept supporting advanced development of Compound 2R comes in part from monotherapy studies conducted against HNSCC models, where objective responses were observed in all of the models tested.
- Compound 2Rhas the potential to challenge the widely accepted view that dual PI3K/mTOR inhibitors are inherently more toxic than isoform-specific PI3K inhibitors. Treatment with Compound 2R led to complete regressions of CAL-33 xenografts over a wide dose range spanning 25 to 100 mg/kg. In contrast, alpelisib (BYL- 719/PiqrayTM), which is the only clinically approved PI3K ⁇ -selective inhibitor, was reported to result in stasis but not regressions of CAL-33 tumors (See Mizrachi, A., et al.
- PI3K ⁇ -selective inhibitorshave been reported to possess an improved preclinical therapeutic window compared to pan-PI3K inhibitors on the basis of their reduced propensity for hyperglycemia.
- the inventorsfind that PI3K inhibitors from both classes lead to an elevation in blood glucose levels, a property, surprisingly, not shared with Compound 2R, which uniquely does not cause hyperglycemia.
- the agonistic activity of Compound 2R against the PPAR ⁇ receptorcounteracts PI3K-driven hyperglycemia.
- PPAR ⁇ agonistsare commonly used in the treatment of type 2 diabetes to increase sensitivity to insulin.
- Compound 2Rmay be less susceptible to insulin-mediated reactivation of PI3K signaling reported for alpelisib.
- the inventorscannot rule out the possibility that immunomodulatory effects of Compound 2R contribute to its antitumor efficacy in this syngeneic model.
- Other targets that could be envisioned to possibly be a viable targetmay include KRASG12D cancers where the EGFR/HER family and the S6 pathway impact response to KRASG12D inhibitor.
- Compound 2Rhas a role in the treatment of not only HSNCC, but also squamous lung cancers, as well as a subset of triple negative breast cancers, specifically those driven by EGFR and PI3K, including but not limited to: all PIK3CA mutant cancers, including certain breast cancers, GI cancers of the stomach or colon, esophagus, cervix, head & neck, squamous lung, and certain gynecologic cancers of the ovary and uterus.
- Compound 2Rwhich has the unique capability of concurrently and selectively inhibiting EGFR, PI3K, and mTOR, illustrates the power of rational computational drug design to target multiple adaptive resistance mechanisms in a single molecule.
- Example 58Blood and Oral Glucose and Adiponectin responses/tolerance with Compound 2R and Alpelisib
- MTX- 531refers to a compound of Formula (I) referred to herein as "Compound 2R” or “COMPOUND 2R” and is used interchangeably herein in the present specification.
- MTX-531(Compound 2R) is found in Table 1 and Fig.1, and is known as N-(2-chloro-5-(4- ((1R-phenylethyl)amino)quinazolin-6-yl)pyridin-3-yl)methanesulfonamide.
- Table 1 and Fig.1The structure of MTX-531 (Compound 2R) is found in Table 1 and Fig.1, and is known as N-(2-chloro-5-(4- ((1R-phenylethyl)amino)quinazolin-6-yl)pyridin-3-yl)methanesulfonamide.
- miceAthymic female nude mice were fasted three hours prior to receiving a single oral dose of Compound 2R or alpelisib at the indicated dose. One hour after drug dosing, mice were administered an oral bolus of glucose (time zero). Blood glucose readings were obtained at the indicated time points (time zero and 0.25, 0.5, 1, 2, and 4 hours after receiving the glucose bolus). For assessment of blood glucose levels, 1-2 drops of blood were taken from the tails of mice and measured using a glucometer. Statistical analysis of differences in glucose levels relative to the vehicle control values were carried out by ordinary one-way ANOVA.
- Oral glucose tolerance testing in fasted mice after five days of PI3K inhibitor dosing[00731] Athymic female nude mice were treated daily for five days with an oral dose of Compound 2R or alpelisib at the indicated dose. On the fifth day, mice were fasted three hours prior to drug dosing. One hour later, mice were administered an oral bolus of glucose (time zero). Blood glucose readings were obtained at the indicated time points (time zero, 0.25, 0.5, 1, 2, and 4 hours after receiving glucose bolus).
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
SUBSTITUTED AMINOBENZYL HETEROARYL COMPOUNDS AS EGFR AND/OR PI3K INHIBITORS HAVING IMPROVED THERAPEUTIC INDEX AGAINST SOLID TUMORS CROSS REFERENCE TO RELATED APPLICATIONS [001] This patent application claims the priority benefit under 35 U.S.C. §l19(e) of U.S. Provisional Application No.63/597,924 filed on November 10, 2023, the contents of the aforementioned application is incorporated herein by reference in its entirety. STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT [002] This invention was made with government support under CA220199, CA213715, CA241764, CA261407 and CA267412 awarded by the National Institutes of Health. The government has certain rights in the invention. SEQUENCE LISTING [003] This application incorporates by reference in its entirety, the Sequence Listing entitled “262340-557381_SL.xml” (12,083 bytes in size), which was created on November 8, 2024, using MS-DOS compatible software and computer and is filed electronically herewith. TECHNICAL FIELD OF THE DISCLOSURE [004] This disclosure is in the field of medicinal chemistry. In particular, the disclosure relates to a new class of small molecules having a substituted aminobenzyl quinazoline structure or a substituted aminobenzyl quinoline structure, which function as dual inhibitors of EGFR proteins and PI3K proteins. The disclosure further relates to the use of the compounds described herein as therapeutics for the treatment of diseases and conditions mediated by EGFR proteins and/or PI3K proteins, such as cancer and other diseases. BACKGROUND [005] Every year more than 830,000 patients are diagnosed with head and neck cancer worldwide and at least 430,000 patients die from this disease (see e.g., Cramer, J.D., et al.,Nat Rev Clin Oncol, 2019.16(11): p.669-683). Ninety percent of all head and neck cancers are squamous cell carcinomas (HNSCCs) and are characterized by significant heterogeneity at both the clinical and molecular level (see e.g., Psyrri, A., T.Y. Seiwert, and A. Jimeno, American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting, 2013: p.246-255). EGFR overexpression is an early and frequent molecular change in HNSCC, a change that has been shown to be associated with reduced survival (see e.g., Grandis, J.R. and D.J. Tweardy, Cancer Research, 1993.53(15): p.3579-3584). Cetuximab remains the only U.S. FDA-approved EGFR-targeted therapy available for HNSCC. A fundamental problem in EGFR-targeted therapy in HNSCC is patient selection, since a consistent mechanism for resistance has not been identified. PI3K mutations, which are particularly common in HPV+ head and neck cancers, confer increased resistance to EGFR inhibition (see e.g., Simpson, D.R., L.K. Mell, and E.E.W. Cohen, Oral Oncology, 2015.51(4): p.291-298; Young, N.R., et al., Molecular Oncology, 2013.7(3): p.359-368). PIK3CA has therefore emerged as a candidate biomarker of EGFR resistance. The PI3K/AKT/mTOR pathway, which supports tumor cell survival and progression, is aberrantly activated in a large percentage of human tumors (see e.g., Yap, T.A., et al., Current Opinion in Pharmacology, 2015.23: p.98-107; Liu, P., et al., Nature Reviews Drug Discovery, 2009.8: p.627-644; Janku, F., T.A. Yap, and F. Meric-Bernstam, Nature Reviews Clinical Oncology, 2018.15: p.273-291). Squamous cancers show a particularly high incidence of genomic alterations in this pathway, encompassing PIK3CA mutations and other alterations, for instance in PIK3R1, PTEN, and AKT, that result in activation of this pathway. Despite intensive efforts, the only PI3K inhibitors that have received regulatory approval for the treatment of solid tumors are copanlisib (panPI3K) and alpelisib (PI3Kα-selective). Lack of progress can largely be attributed to unacceptable toxicities, which are in part driven by the need for high exposures to elicit monotherapy activity. The rationale for a dual EGFR/PI3K inhibitor program is driven by the central premise that 1) a panPI3K/mTOR inhibitor approach is superior to isoform-selective approaches, since molecular alterations of downstream players in the PI3K pathway, e.g. PTEN, can obviate the need for PI3K activity (see e.g., Tao, J.J., et al., Science Signaling, 2014.7(318): p. ra29-ra29), and 2) EGFR and PI3K play reciprocal roles in tumor adaptation when the other kinase is targeted. Anti-EGFR treatment has been shown to reverse acquired and intrinsic resistance to PI3Kα inhibition in HNSCC (see e.g., Elkabets, M., et al., Cancer Cell, 2015.27(4): p.533-546). AXL was shown to interact with EGFR to activate PLCγ and PKC, leading to activation of mTOR in a PI3K-independent manner. [006] EGFR and PI3K emerge as potential co-targeting candidates to test this concept, since these oncogenic kinases drive adaptive resistance across a broad spectrum of human cancers, as exemplified by squamous head and neck cancers (HNSCC). In HNSCC, EGFR and PI3K are each known to mediate resistance to inhibition of the other. The clinical activity of cetuximab, which is the only approved kinase-targeted therapy for this disease, is modest. Molecular aberrations in the PI3K/mTOR pathway leading to its dysregulation are found in up to 80% of HNSCC and confer increased resistance to EGFR inhibition. Treatment failures in the PI3K field have been attributed to unacceptable toxicities, in part driven by the need for high exposures to elicit monotherapy activity. The PI3Kα inhibitor alpelisib (PiqrayTM) is the only approved clinical agent in this target class, based on its activity against PIK3CA mutated advanced breast cancer. Both alpelisib and the pan-PI3K inhibitor copanlisib have been shown to restore sensitivity to cetuximab in preclinical models of HNSCC. [007] However, the benefits did not outweigh the risks when these PI3K inhibitors were combined with cetuximab in the clinic. The design of an efficacious PI3K inhibitor with an improved therapeutic index that additionally is not prone to EGFR-mediated adaptive resistance presents an area of high unmet medical need. [008] EGFR and PI3K pathway signaling have also been implicated in the adaptive resistance of KRAS and BRAF mutant colorectal cancer (CRC) to RAS/MAP kinase pathway intervention. Whereas EGFR inhibitors alone are not indicated for the treatment of KRAS mutant disease, preclinical findings support their use in the combination setting with PI3K/mTOR or MEK inhibitors to treat KRAS mutant CRC. In the case of BRAF mutant colorectal tumors, the presence of a negative feedback activation loop that activates EGFR upon inhibition of BRAF also leads to reactivation of the MAPK and PI3K pathways. The combination of encorafenib and cetuximab to target BRAF and EGFR, respectively, has become the new standard of care for the treatment of BRAFV633 metastatic CRC. Further addition of the PI3K inhibitor alpelisib to this regimen provided added efficacy but came at the cost of an increase in toxicity. [009] Hyperglycemia, hyperinsulinemia, insulin resistance and body weight loss are a dose limiting toxicity observed in subjects treated with PI3K inhibitors. Some of the problems associated with the use of PI3K inhibitors include: hyperinsulinemia antagonizes PI3K inhibition by stimulating tumor IR and IGF1 R hybrid receptor signaling; hyperglycemia overrides the metabolic action of PI3K inhibitors in tumors by increasing glucose utilization and aerobic glycolysis, thus promoting tumor glucose uptake which drives cell growth and replication irrespective of PI3K inhibition, and the T1 D-like symptoms induced by PI3K inhibitors are dose limiting and severely limit tumor target coverage. In some studies, it has been shown that the toxicology observed with PI3K inhibitors is not normalized by drugs used to manage diabetes (e.g., treatment of CD-17 scid mice with GSK690693 potently induces hyperglycemia. This negative side effect was not prevented or reduced by several drug used to treat clinical diabetes including rosiglitazone maleate, vildagliptin, metformin, and Exendin-4; see for example, Crouthamel et al., Clin Cancer Res, 15:217-225, 2009). Hyperglycemia induced by AKT inhibitors (a direct PI3K target) can be partially resolved by fasting in rodents, but hyperinsulinemia is not reduced. High PI3K coverage would thus appear to be unachievable in oncology since it is assumed that glucose cannot be regulated without insulin signaling. [0010] Circulating glucose and metabolic homeostasis are regulated by the, equally important, opposing actions of insulin and glucagon (Unger and Cherrington, J Clin Invest., 122:4-12, 2012). In view of the fact that several prominent features of type 2 diabetes and type 1 diabetes, including hyperglycemia, hyperinsulinemia, body weight loss, increased insulin resistance and decreased insulin production, are associated with treatment of cancers using P13K pathway inhibitors, research efforts to develop novel treatments for cancer, could benefit greatly from studies evaluating the effects of such antagonistic antigen binding proteins to effectively treat subjects who have, or who have been diagnosed with cancer. [0011] The disclosures of all publications, patents, patent applications and published patent applications referred to herein are hereby incorporated herein by reference in their entirety. SUMMARY [0012] The compounds of Formula I are substituted benzylamino compounds and may have advantages over the analogous unsubstituted benzylamino compounds or analogous phenylamino compounds. For example, the compounds of Formula I may possess properties selected from one or more of increased solubility in pH 7.4 aqueous buffer solution, increased solubility in simulated intestinal fluid (SIF), and increased solubility in simulated gastric fluid (SGF), as compared to the analogous unsubstituted benzylamino compounds or analogous phenylamino compounds. Increased solubility in the mediums described above can be indicative of increased bioavailability in a biological system, such as a human subject. Further, an increase in bioavailability may allow for equivalent biological activity of smaller doses, compared to the required dose of a less soluble active pharmaceutical ingredient (API). [0013] In one aspect, the disclosure includes a compound of Formula I Formula I or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring A is selected from phenyl or a 5 or 6 membered heteroaryl; X is N or C-R5; R1a is selected from the group consisting of H or C1-6 alkyl; R1b is selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, halo, CN, and NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with one or more R” substituents; or, R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with one or more R” substituents; each R2 is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; or one R2 substituent and R1b, together with the phenyl group to which R2 is attached and the carbon atom to which R1b is attached, form a bicyclic group having the general structure , wherein Ring B is a C3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, OC(O)OR’, OC(O)N(R’)2, NR’C(O)N(R’)2, SOR’, SON(R’)2, SO2R’, SO2N(R’)2, NR’SOR’, NR’SON(R’)2, NR’SO2R’, and NR’SO2N(R’)2, wherein the C1-6 alkyl, hetercycloalky, and heteroaryl are each optionally and independently substituted with one or more R” substituents; or two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heterocycloalkyl, a fused bicyclic cycloalkyl, a fused bicyclic aryl, or a fused bicyclic heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R4 is selected from halo, OH, NH2, CN, C1-6 alkyl, and OC1-6 alkyl; each R5 is selected from hydrogen, halo, OH, NH2, CN, and C1-6 alkyl; each R’ is independently selected from hydrogen, OH, CN, C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R” is independently selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or substituents selected from halo, oxo, alkoxy, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, and C(O)NHC1-6 alkyl; and m, n, and p are each an integer selected from 0-4. [0014] In another aspect, the disclosure includes a pharmaceutical composition comprising a compound or salt according to Formula (I) described herein and a pharmaceutically acceptable excipient. [0015] In another aspect, the disclosure includes a method of modulating the activity of an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound, salt or a composition described herein. [0016] In another aspect, the disclosure includes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound, salt or a composition described herein. [0017] In one embodiment of this aspect, the EGFR and/or PI3K mediated disease is a cancer. BRIEF DESCRIPTION OF THE FIGURES [0018] Reference to MTX-531 in the figures refers to COMPOUND 2R as shown in the present disclosure. [0019] Figure 1A is a chemical structure of COMPOUND 2R ((R)-N-(2-chloro-5-(4-((1- phenylethyl)amino)quinazolin-6-yl)pyridin-3-yl)methane-sulfonamide). [0020] Figure 1B is a crystal structure of EGFR co-complexed with COMPOUND 2R (PDB Code 8SC7) solved at 2.0 Angstroms. [0021] Figure 1C shows the secondary structure of the crystal structure displayed in Fig.1B with a view of COMPOUND 2R bound to EGFR from the ATP binding site. Graphics were generated by Molegro Virtual Docker 5.557. [0022] Figure 1D is a crystal structure of PI3Kγ co-complexed with COMPOUND 2R (PDB Code 8SC8) solved at 2.7 Angstroms. [0023] Figure 1E shows the secondary structure of the crystal structure displayed in Fig.1D with a view of COMPOUND 2R bound to PI3Kγ from the ATP binding site. Graphics were generated by Molegro Virtual Docker 5.5. [0024] Figure 1F is a table of biochemical potency of COMPOUND 2R and approved PI3K inhibitors against purified HER and PI3K family members. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. Enzyme inhibition curves are shown in Figures 17, 18, and 19. [0025] Figure 1G depicts kinase selectivity of COMPOUND 2R. Selectivity was determined against a panel of 482 protein and lipid kinases by carrying out single point testing at a final concentration of 10 µM COMPOUND 2R. Kinases inhibited by >80% at 10 µM were re-tested in dose response assays to determine IC50 values. Kinases inhibited by 50% or more at concentrations < 1 µM are depicted here. Illustration reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com). [0026] Figure 1H depicts a western immunoblot of EGFR and PI3K/mTOR pathway targets in EGF-stimulated CAL-33 cells treated for 2 hours with COMPOUND 2R over an eight-point dose response. Data are representative of two independent experiments. Quantification of phosphorylated kinase expression was carried out by densitometry analysis to determine EC50 values. [0027] Figure 2A depicts a western immunoblot showing protein expression from a related to the pharmacodynamic modulation of EGFR and PI3K/mTOR pathway expression in subcutaneous CAL-33 tumors excised from mice treated with a single oral dose of 100 mg/kg COMPOUND 2R. Tumors were harvested at the indicated time point (3 mice/time point) followed by immunoblot analysis. [0028] Figure 2B shows quantification of phosphorylated kinase expression of the individual bands shown in Fig.2A. by densitometry analysis. [0029] Figure 2C depicts antitumor efficacy of COMPOUND 2R against subcutaneous CAL-33 xenografts. COMPOUND 2R was dosed daily by oral gavage at the indicated dosage (n = 5-6 per group). Mice were treated for 134 days except for the 100 mg/kg arm of the CAL-33 study, where dosing was stopped after 37 days of treatment to monitor durability of the complete responders. Data are shown as mean tumor volume + s.e.m. [0030] Figure 2D depicts antitumor efficacy of COMPOUND 2R against subcutaneous CAL-27 xenografts. COMPOUND 2R was dosed daily by oral gavage at the indicated dosage (n = 5-6 per group). Mice were treated with CAL-27 for 145 days. Data are shown as mean tumor volume + s.e.m. [0031] Figure 2E shows the effects of CAL-33 treatment model on survival quantitated by sacrificing individual mice when tumor burden reached an equivalent size of 1000 mm3. A one- way ANOVA comparison between all groups was carried out to determine statistical significance. Tumor volumes on day 31 were statistically significant between both the 25 and 100 mg/kg treatment groups and the vehicle control (**** = p-value <0.0001). There was no statistical difference in tumor volume between the two doses. All groups showed no weight loss and steadily gained weight during the duration of the study. [0032] Figure 2F shows the effects of CAL-27 treatment model on survival quantitated by sacrificing individual mice when tumor burden reached an equivalent size of 500 mm3. A one- way ANOVA comparison between all groups was carried out to determine statistical significance. Tumor volumes on day 45 were statistically significant between both the 10 and 100 mg/kg treatment groups and the vehicle control (**** = p-value <0.0001) as well as between the two doses (** = p-value <0.01). All groups showed no weight loss and steadily gained weight during the duration of the study. [0033] Figure 2G depicts a western immunoblot showing pharmacodynamic modulation of protein expression in the pEGFR and PI3K/mTOR pathway after a single oral dose of 100 mg/kg COMPOUND 2R in mice with subcutaneous NCI 848979-319-R patient-derived xenografts. Tumors were excised two hours after treatment with a single oral dose of 100 mg/kg COMPOUND 2R followed by immunoblot analysis (5 mice/group). [0034] Figure 2H shows protein expression as percent of control for the phosphorylated kinase expression from the individual immunoblot bands in Fig.2G as quantified by densitometry analysis. [0035] Figure 2I shows percent expression as percent of control for cleaved PARP from the individual immunoblot band in Fig.2G as quantified by densitometry analysis. [0036] Figure 2J depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CAE545K mutation (245127-232-R). The HNSCC PDX graft was obtained from the NCI PDX biorepository. Mice were treated daily by oral gavage with vehicle or 100 mg/kg COMPOUND 2R (n = 5-8 per group). Data are shown as mean tumor volume + s.e.m. Statistical differences in tumor growth rates for COMPOUND 2R vs. vehicle treatment were determined using a linear mixed model with treatment as fixed effect and time as random effect (* = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001, **** = p-value <0.0001). [0037] Figure 2K depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CAE545A mutation (354836-022-R). The HNSCC PDX graft was obtained from the NCI PDX biorepository. Mice were treated daily by oral gavage with vehicle or 100 mg/kg COMPOUND 2R (n = 5-8 per group). Data are shown as mean tumor volume + s.e.m. Statistical differences in tumor growth rates for COMPOUND 2R vs. vehicle treatment were determined using a linear mixed model with treatment as fixed effect and time as random effect (* = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001, **** = p-value <0.0001). [0038] Figure 2L depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CAG364R mutation (455876-151-R). The HNSCC PDX graft was obtained from the NCI PDX biorepository. Mice were treated daily by oral gavage with vehicle or 100 mg/kg COMPOUND 2R (n = 5-8 per group). Data are shown as mean tumor volume + s.e.m. Statistical differences in tumor growth rates for COMPOUND 2R vs. vehicle treatment were determined using a linear mixed model with treatment as fixed effect and time as random effect (* = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001, **** = p-value <0.0001). [0039] Figure 2M depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CAE726K mutation (848979-319-R). The HNSCC PDX graft was obtained from the NCI PDX biorepository. Mice were treated daily by oral gavage with vehicle or 100 mg/kg COMPOUND 2R (n = 5-8 per group). Data are shown as mean tumor volume + s.e.m. Statistical differences in tumor growth rates for COMPOUND 2R vs. vehicle treatment were determined using a linear mixed model with treatment as fixed effect and time as random effect (* = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001, **** = p-value <0.0001). [0040] Figure 2N depicts tumor growth inhibition after oral daily administration of 100 mg/kg COMPOUND 2R to mice bearing subcutaneous patient-derived squamous head and neck carcinomas xenografts with the PIK3CAH1047L mutation (944545-341-R). The HNSCC PDX graft was obtained from the NCI PDX biorepository. Mice were treated daily by oral gavage with vehicle or 100 mg/kg COMPOUND 2R (n = 5-8 per group). Data are shown as mean tumor volume + s.e.m. Statistical differences in tumor growth rates for COMPOUND 2R vs. vehicle treatment were determined using a linear mixed model with treatment as fixed effect and time as random effect (* = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001, **** = p- value <0.0001). [0041] Figure 2O depicts Waterfall plot of the best individual response of HNSCC PDX- implanted mice treated with COMPOUND 2R in Figs.2J-2N. The percent increase in tumor burden observed in the vehicle treated mice during these studies ranged between 773% and 912% for all five models. [0042] Figure 2P depicts line graphs representing extension in survival of mice implanted with 944545-341-R xenografts and treated daily with 100 mg/kg COMPOUND 2R. Mice were treated daily for 155 days or until tumor burden reached 1000 mm3. Statistical differences in survival between the vehicle and COMPOUND 2R-treated groups was determined using the Log-rank (Mantel-Cox) test (*** = p-value <0.001). [0043] Figure 2Q shows days of treatment study for six different mice. The letters “SD” indicates stable disease, meaning that it does not qualify for tumor shrinkage or tumor growth. The letters “CR” indicates complete response and requires disappearance of the tumor and lack of palpable tumor. The letters “PD” indicates progressive disease and is defined as > 2-fold increase in tumor volume. The letters “PR” indicates a partial response and designated with a ≥30% decrease in tumor volume. [0044] Figure 2R depicts a comparative assessment of antitumor efficacy of COMPOUND 2R versus the combination of erlotinib and alpelisib against subcutaneous 944845-341-R xenografts. Mice (n = 8 per group) were treated daily for 27 days at the indicated doses. Data are shown as mean tumor volume + s.e.m. Statistical significance was determined by carrying out a one-way ANOVA comparison between all treatment groups. [0045] Figure 2S shows tumor volumes on day 14 were statistically significant between both drug-treated groups and the vehicle control group (**** = p-value <0.0001) and between COMPOUND 2R and the combination of erlotinib and alpelisib (*** = p-value <0.001). [0046] Figure 2T shows the mean body weight change in mice between both drug-treated groups and the vehicle control group over 14 days of treatment. [0047] Figure 3A depicts tumor growth inhibition after treatment with COMPOUND 2R, trametinib or the combination administered orally to mice bearing KRASA146T CN0375-F275 patient-derived xenografts (n = 5 per group). Data are shown as mean tumor volume ± s.e.m. [0048] Figure 3B shows the best antitumor response seen in individual animals from each group in Fig.3A is shown in the waterfall plot. The percent increase in tumor burden observed in the vehicle treated mice was 887 %. [0049] Figure 3C shows the extension in survival conferred by each of the single agents and by the combination of agents shown in Fig.3A. A linear mixed model fit was used to determine statistical significance of tumor growth rate differences (**** = p-value <0.0001). [0050] Figure 3D depicts tumor growth inhibition after treatment with COMPOUND 2R, trametinib or the combination administered orally to mice bearing BRAFV600E UM-CRC 14- 929 patient-derived xenografts (n = 5 per group). Data are shown as mean tumor volume ± s.e.m. [0051] Figure 3E shows the best antitumor response seen in individual animals from each group in Fig.3D is shown in the waterfall plot. The percent increase in tumor burden observed in the vehicle treated mice was 844%. [0052] Figure 3F shows the extension in survival conferred by each of the single agents and combination of agents from treatment groups depicted in Fig.3D. A linear mixed model fit was used to determine statistical significance of tumor growth rate differences (* = p-value <0.05, *** = p-value <0.001, **** = p-value <0.0001). [0053] Figure 4A depicts tumor growth inhibition after treatment with COMPOUND 2R, sotorasib, or the combination administered orally to mice bearing KRASG12C patient-derived xenografts. The study was carried out in the colorectal PDX model NCI 135848-042T (n = 5 per group). Data are shown as mean tumor volume ± s.e.m. [0054] Figure 4B depicts tumor growth inhibition after treatment with COMPOUND 2R, sotorasib, or the combination administered orally to mice bearing KRASG12C patient-derived xenografts. The study was carried out in the MiaPaCa pancreatic model (n = 5 per group). Data are shown as mean tumor volume ± s.e.m. [0055] Figure 4C shows the best antitumor response seen in individual animals from each group is shown in the waterfall plots at the bottom. The percent increase in tumor burden observed in the vehicle treated mice in the colorectal model NCI 135848-042T during this study was 434% (Fig.4A). A linear mixed model fit was used to determine statistical significance of tumor growth rate differences (* = p-value <0.05, **** = p-value <0.0001). [0056] Figure 4D shows the best antitumor response seen in individual animals from each group is shown in the waterfall plots at the bottom. The percent increase in tumor burden observed in the vehicle treated mice in the MIA PaCa-2 pancreatic model during this study was 970% (Fig. 4B). A linear mixed model fit was used to determine statistical significance of tumor growth rate differences (* = p-value <0.05, **** = p-value <0.0001). [0057] Figure 5A depicts line graphs of mean (+ SEM) blood glucose in mice treated with the indicated PI3K inhibitor (n = 5-6 per group). [0058] Figure 5B depicts line graphs of mean (+ SEM) insulin levels in mice treated with the indicated PI3K inhibitor (n = 5-6 per group). [0059] Figure 5C depicts line graphs of mean (+ SEM) blood glucose levels in mice treated with a panel of PI3K inhibitors (n = 3-5 per group). [0060] Figure 5D depicts comparative antitumor efficacy of COMPOUND 2R vs alpelisib against subcutaneously implanted KPC tumors. Data are shown as mean tumor volume ± SEM (n = 8 per group). Statistical significance of differences in tumor growth were determined using a linear mixed model (**** = p-value <0.0001). [0061] Figure 5E shows the best antitumor response seen in individual animals from each group from Fig.5D is shown in the waterfall plots. The percent increase in tumor burden observed in the vehicle treated mice was 679%. [0062] Figure 5F shows extension of survival of KPC-implanted mice treated daily with COMPOUND 2R or alpelisib as described in Fig.5D. Mice were treated daily for 65 days or until tumor burden reached 500 mm3. Statistical differences in survival between the vehicle and COMPOUND 2R-treated groups was determined using the Log-rank (Mantel-Cox) test (**** = p-value <0.0001). [0063] Figure 5G depicts bar graphs representing mean (+ SEM) insulin concentrations in CAL-33 tumors excised from mice treated daily for 5 days with alpelisib (50 mg/kg) or COMPOUND 2R (100 mg/kg) (n = 5/arm). [0064] Figure 5H depicts bar graphs representing mean (+ SEM) insulin concentrations in KPC tumors excised from mice treated daily for 5 days with alpelisib (50 mg/kg) or COMPOUND 2R (100 mg/kg) (n = 5/arm). [0065] Figure 6A depicts COMPOUND 2R competitive binding and transactivation of PPARγ. A LanthaScreenTM TR-FRET Assay was used to determine the percent displacement of fluorescein-labeled PPARγ agonist, GW1929, from terbium-labeled PPARγ. Curve fitting resulted in an EC50 of 2.5 µM for COMPOUND 2R (R2 value = 0.9712). [0066] Figure 6B COMPOUND 2R is a weak activator of PPARγ in a cell-based transcription assay. PPAR-gamma-UAS-bla HEK 293H cells were treated in duplicate over a range of concentrations of COMPOUND 2R. Curve fitting resulted in an EC50 of 3.4 µM for COMPOUND 2R (R2 value = 0.9898). [0067] Figure 6C depicts the effects of COMPOUND 2R on PPARγ target gene expression. The indicated PPARγ target gene expression levels were analyzed in quadruplicate by quantitative RT-PCR analysis of total RNA extracts from 3T3-L1 cells treated with differentiation medium containing rosiglitazone or COMPOUND 2R for 8 or 24 hours. Data are represented as the mean and upper/lower limits of relative fold gene quantitation (RQ). Student’s t-test was used to determine statistical differences between treatment groups (** = p- value <0.01, *** = p-value <0.001, **** = p-value <0.0001). [0068] Figure 6D shows a western immunoblot of PPARγ expression was carried out in total cell lysates from 3T3-L1 adipocytes at 7 days post induction of differentiationThe two bands representing PPARγ1 (53 kDa) and PPARγ2 (57 kDa) were upregulated in the 3T3-L1 cells differentiated in the presence of 10 µM COMPOUND 2R and the reference compound rosiglitazone at 1µM. [0069] Figure 6E depicts the crystal structure of PPARγ co-complexed with COMPOUND 2R (PDB Code 8SC9) solved at 1.9 Angstroms. [0070] Figure 6F shows the secondary structure of the crystal structured displayed in Fig.6E and a view of COMPOUND 2R bound to PPARγ from the ligand binding pocket is shown in the right panel. Graphics were generated by Molegro Virtual Docker 5.5. [0071] Figure 7A depicts the structural features of NVP-AEE788 bound to EGFR used in the design of COMPOUND 2R. [0072] Figure 7B depicts the structural features omipalisib bound to PI3Kγ used in the design of COMPOUND 2R. The computational design of COMPOUND 2R was based on analysis of x- ray crystal structures of NVP-AEE788 (Fig.7A) and omipalisib kinase inhibitors bound to their respective targets. The flipped binding mode of the uinazoline core between EGFR (Fig.7A) and PI3Kγ was leveraged to design a potent and selective inhibitor of both enzyme families. [0073] Figure 8A depicts the pharmacokinetic analysis of COMPOUND 2R in mice. COMPOUND 2R exhibits high oral bioavailability and a dose-dependent increase in plasma concentrations in mice. Mice received a single treatment of COMPOUND 2R at 5 mg/kg (IV) or 25 and 100 mg/kg (PO), n = 3 mice per treatment arm. Blood was collected at the indicated time points, and the plasma concentration of COMPOUND 2R was determined using liquid chromatography-mass spectrometry. [0074] Figure 8B shows data of pharmacokinetic parameters after single treatment of 5 mg/kg COMPOUND 2R (IV). [0075] Figure 8C shows data of pharmacokinetic parameters after single treatment of 25 mg/kg COMPOUND 2R (PO). [0076] Figure 8D shows data of pharmacokinetic parameters after single treatment of 100 mg/kg COMPOUND 2R (PO). [0077] Figure 9 depicts tumor response rates and survival data for CAL-33 and CAL-27 xenografts. COMPOUND 2R was dosed daily by oral gavage at the indicated dosage (n = 5-6 per group). Mice were treated for 134 days (CAL-33) or 145 days (CAL-27) except for the 100 mg/kg arm of the CAL-33 study, where dosing was stopped after 37 days of treatment to monitor durability of the complete responders. Treatment with COMPOUND 2R generated statistically significant increases in median survival rates across multiple dosages in mice bearing CAL-33 and CAL-27 xenografts. Treatment also resulted in complete response rates of > 80% at doses of 25 mg/kg or higher. Tumor responses were durable, as > 60% of these responder mice remained tumor-free at study termination (>300 days post-tumor implantation). Abbreviations: ILS, increase in life span; TFS, tumor-free survivor [0078] Figure 10A depicts a western immunoblot showing baseline protein expression in five HNSCC PDX tumor models. Tumors from five HNSCC models from the NCI Patient-Derived Models Repository (PDMR) were lysed and baseline protein expression was analyzed for proteins related to EGFR and PI3K/mTOR signaling through immunoblotting. Lysates were normalized for total protein concentration and equivalents amounts of protein were added to each lane for expression analysis of a given kinase. [0079] Figure 10B shows data summarizing the genomic alterations in the tumor models as summarized in the NCI PDMR database. [0080] Figure 11 depicts a western immunoblot showing pharmacodynamic modulation of EGFR and PI3K/mTOR pathway expression in subcutaneous NCI 944545-341-R patient-derived xenografts. Mice were treated daily with COMPOUND 2R orally at 100 mg/kg for five days. At two hours after the fifth dose, tumors were excised and immunoblot analysis was carried out (3 mice/group). [0081] Figure 12A depicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models. Mice bearing subcutaneous HNSCC PDX tumors (NCI 245127-232-R) were dosed daily with COMPOUND 2R at 100 mg/kg (PO). Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5-8 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, **** = p-value ≤0.0001). [0082] Figure 12B depicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models. Mice bearing subcutaneous HNSCC PDX tumors (NCI 354836-022-R) were dosed daily with COMPOUND 2R at 100 mg/kg (PO). Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5-8 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, **** = p-value ≤0.0001). [0083] Figure 12C depicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models. Mice bearing subcutaneous HNSCC PDX tumors (NCI 455876-151-R) were dosed daily with COMPOUND 2R at 100 mg/kg (PO). Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5-8 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, **** = p-value ≤0.0001). [0084] Figure 12D depicts Kaplan-Meier survival plots for a panel of PIK3CA mutant HNSCC PDX models. Mice bearing subcutaneous HNSCC PDX tumors (NCI 848979-319-R) were dosed daily with COMPOUND 2R at 100 mg/kg (PO). Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5-8 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, **** = p-value ≤0.0001). [0085] Figure 13A depicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R. Mice bearing subcutaneous HNSCC PDX tumors (NCI 245127-232-R) were dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group). Mean group body weight changes (± SEM) were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [0086] Figure 13B depicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R. Mice bearing subcutaneous HNSCC PDX tumors (NCI 354836-022-R) were dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group). Mean group body weight changes (± SEM) were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [0087] Figure 13C depicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R. Mice bearing subcutaneous HNSCC PDX tumors (NCI 455876-151-R) were dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group). Mean group body weight changes (± SEM) were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [0088] Figure 13D depicts body weight change of tumor-bearing mice in response to daily oral treatment with COMPOUND 2R. Mice bearing subcutaneous HNSCC PDX tumors (NCI 848979-319-R) were dosed with COMPOUND 2R at 100 mg/kg (PO) daily (5-8 mice/group). Mean group body weight changes (± SEM) were calculated when body weights were recorded (2-3 times weekly). Change in body weight was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [0089] Figure 14A depicts body weight change in mice dosed daily (PO) with COMPOUND 2R at 150 mg/kg or vehicle control in a PDX model of PIK3CA mutant HNSCC bearing subcutaneous NCI 848979-319-R xenografts. Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, *** = p-value ≤0.001). [0090] Figure 14B depicts tumor volume in mice dosed daily (PO) with COMPOUND 2R at 150 mg/kg or vehicle control in a PDX model of PIK3CA mutant HNSCC bearing subcutaneous NCI 848979-319-R xenografts were dosed daily. Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, *** = p-value ≤0.001). [0091] Figure 14C depicts percent survival in mice dosed daily (PO) with COMPOUND 2R at 150 mg/kg or vehicle controrl in a PDX model of PIK3CA mutant HNSCC bearing subcutaneous NCI 848979-319-R xenografts. Dosing occurred until individual tumors reached 1000 mm3 to facilitate comparative survival analysis (5 mice/group). A comparison of the survival curves was performed in GraphPad Prism using the Log-rank (Mantel-Cox) test (** = p-value ≤0.01, *** = p-value ≤0.001). [0092] Figure 15A depicts a western immunoblot showing pharmacodynamic modulation of protein expression in the EGFR and PI3K/mTOR pathway in subcutaneous UM CRC 14-929 patient-derived xenografts. Mice were treated orally with COMPOUND 2R at 100 mg/kg, trametinib at 1 mg/kg or the combination. At two hours after a single dose, tumors were excised and immunoblot analysis was carried out (3 mice/group). [0093] Figure 15B depicts the quantification of phosphorylated kinase expression was carried out by densitometry analysis of the individual bands in Fig.15A. [0094] Figure 16A depicts an overlay of COMPOUND 2R x-ray binding modes with commercial PPARγ agonist rosiglitazone and pioglitazone. This figure shows an overlay of x- ray crystal binding modes of COMPOUND 2R, rosiglitazone (PDB Code 4EMA) and pioglitazone (PDB Code 2XKW). [0095] Figure 16B depicts the compound structure of COMPOUND 2R (MTX-531) for comparison with the structural overlay in Fig.16A. [0096] Figure 16C depicts the compound struct of rosiglitazone for comparison with the structural overlay in Fig.16A. [0097] Figure 16D depicts the compound structure or pioglitazone for comparison with the structural overlay in Fig.16A. [0098] Figure 17A depicts line graph of the inhibitory activity of COMPOUND 2R against EGFR (ErbB1). The biochemical potency of COMPOUND 2R was evaluated against purified HER family members. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [0099] Figure 17B depicts line graph of the inhibitory activity of COMPOUND 2R against HER2 (ErbB2). The biochemical potency of COMPOUND 2R was evaluated against purified HER family members. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00100] Figure 17C depicts line graph of the inhibitory activity of COMPOUND 2R against HER4 (ErbB4). The biochemical potency of COMPOUND 2R was evaluated against purified HER family members. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme [00101] Figure 18A depicts the line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3Cα/PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00102] Figure 18B depicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3Cβ/PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00103] Figure 18C depicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3Cδ/PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00104] Figure 18D depicts line graph of the inhibitory activity of COMPOUND 2R against PI3K family member PIK3Cγ/PIK3R1. The biochemical potency of COMPOUND 2R was evaluated against the purified PI3K. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00105] Figure 19A depicts the inhibitory activity of COMPOUND 2R against FRAP (mTOR). The biochemical potency of COMPOUND 2R was evaluated against purified FRAP1. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00106] Figure 19B depicts the inhibitory activity of COMPOUND 2R against DNA-PK. The biochemical potency of COMPOUND 2R was evaluated against purified DNA-PK. Nanomolar IC50 values were determined at a concentration of ATP corresponding to the Km apparent for that enzyme. [00107] Figure 20A depicts the broad kinome screening of COMPOUND 2R. The inhibitory potency of COMPOUND 2R was determined against a panel of 482 protein and lipid kinases by carrying out single point testing at a final concentration of 10 µM COMPOUND 2R. [00108] Figure 20B depicts COMPOUND 2R IC50 values for nine kinases identified in a broad kinome screening as inhibited by >80% at 10 µM (shown in bold font in Fig.20A). [00109] Figure 21A depicts a line graph representing the potency of COMPOUND 2R on the downstream effector pEGFRγ1068 within the EGFR and PI3K pathways in CAL-33 cells. Densitometry using ImageJ software was performed for immunoblots of pEGFRγ1068 in CAL- 33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 µM. Pixel intensity of the band of interest was normalized to the loading control. The ratio of the normalized pixel intensity of treated to control values was multiplied by 100. The IC50 value of 3.131e-007 was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad. [00110] Figure 21B depicts a line graph representing the potency of COMPOUND 2R on the downstream effector pAKTS473 within the EGFR and PI3K pathways in CAL-33 cells. Densitometry using ImageJ software was performed for immunoblots of pAKTS473 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 µM. Pixel intensity of the band of interest was normalized to the loading control. The ratio of the normalized pixel intensity of treated to control values was multiplied by 100. The IC50 value of 4.279e-007 was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad. [00111] Figure 21C depicts a line graph representing the potency of COMPOUND 2R on the downstream effector pAKTT308 within the EGFR and PI3K pathways in CAL-33 cells. Densitometry using ImageJ software was performed for immunoblots of pAKTT308 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 µM. Pixel intensity of the band of interest was normalized to the loading control. The ratio of the normalized pixel intensity of treated to control values was multiplied by 100. The IC50 value of 2.979e-007 was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad. [00112] Figure 21D depicts a line graph representing the potency of COMPOUND 2R on the downstream effector pS6S235/236 in the EGFR and PI3K pathways in CAL-33 cells. Densitometry using ImageJ software was performed for immunoblots of pS6S235/236 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 µM. Pixel intensity of the band of interest was normalized to the loading control. The ratio of the normalized pixel intensity of treated to control values was multiplied by 100. The IC50 value of 5.970e-006 was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad. [00113] Figure 21E depicts a line graph representing the potency of COMPOUND 2R on the downstream effector p4E-BP1S65 of the EGFR and PI3K pathways in CAL-33 cells. Densitometry using ImageJ software was performed for immunoblots of p4E-BP1S65 in CAL-33 lysates following a 2-hour treatment with concentrations of COMPOUND 2R ranging from 10 nM to 10 µM. Pixel intensity of the band of interest was normalized to the loading control. The ratio of the normalized pixel intensity of treated to control values was multiplied by 100. The IC50 value of 3.390e-007 was determined by fitting data to a variable slope (four parameters) equation using Prism GraphPad. [00114] Figure 22A depicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 245127 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas. [00115] Figure 22B depicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 354836 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas. [00116] Figure 22C depicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 455876 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas. [00117] Figure 22D depicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 848979 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas. [00118] Figure 22E depicts spaghetti plots for the single agent activity of MTX-531 in the HNSCC PDX 944545 model. Compound efficacy was determined from differences in HNSCC PDX growth in response to treatment with COMPOUND 2R compared to control using linear mixed regression analysis. The expected log mass trajectories for vehicle versus COMPOUND 2R treatment are depicted in the shaded areas. [00119] Figure 23 depicts a line graph showing the selection of a combination dosage of erlotinib and alpelisib in mice. Mice were treated with the combination of erlotinib and alpelisib daily via oral gavage for 5 weeks (8 mice/group). The dosage of erlotinib was held constant at 50 mg/kg and the dosage of alpelisib was varied (12.5, 25 and 50 mg/kg). Body weights were recorded 2-3 times per week. Mean group body weight change (± SEM) was calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. Body weight loss of > 20% and/or > 10% treatment-related deaths in a group are considered unacceptable toxicity parameters. [00120] Figure 24A depicts body weight change of CRC NCI CN0375-F725 PDX subcutaneous tumor-bearing mice in response to daily oral treatment with COMPOUND 2R in combination with the MEK inhibitor trametinib. Mice were dosed with COMPOUND 2R at 100 mg/kg, trametinib at 1 mg/kg or the combination (PO) daily (5 mice/group). Body weights were recorded 2-3 times per week. Mean group body weight change (± SEM) were calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [00121] Figure 24B depicts body weight change of CRC UM-CRC 14-929 PDX subcutaneous tumor-bearing mice in response to daily oral treatment with COMPOUND 2R in combination with the MEK inhibitor trametinib. Mice were dosed with COMPOUND 2R at 100 mg/kg, trametinib at 1 mg/kg or the combination (PO) daily (5 mice/group). Body weights were recorded 2-3 times per week. Mean group body weight change (± SEM) were calculated as follows: [(BWDAY – BWINIT)/BWINIT]*100, where BWINIT is the body weight of the animal on the first day of treatment and BWDAY is the body weight of the animal on a given day of treatment. [00122] Figure 25A depicts COMPOUND 2R activity in PPARα time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays. COMPOUND 2R was titrated across a 10-point concentration range to test for competitive binding to PPARα against GW7647 in a TR-FRET assay. COMPOUND 2R failed to displace the reference ligands demonstrating specificity for PPARγ. [00123] Figure 25B depicts COMPOUND 2R activity in PPARδ time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays. COMPOUND 2R was titrated across a 10-point concentration range to test for competitive binding PPARδ against GW0742 in a TR-FRET assay. COMPOUND 2R failed to displace the reference ligands demonstrating specificity for PPARγ. [00124] Figure 26A depicts electron density maps of the COMPOUND 2R binding site of PPARγ in the initial Fo-Fc difference electron density map of the model (contoured at 3 σ) resulting from refinement of the initial model prior to modelling of the compound with BUSTER. Shown is the region of compound binding site in chain B. [00125] Figure 26B depicts the final 2Fo-Fc electron density map (contoured at 1.0 σ) of the COMPOUND 2R binding site of PPARγ resulting from refinement of the final model with BUSTER. Shown is the region of the compound 2R binding site. [00126] Figure 27 depicts a line graph showing blood glucose levels in athymic mice following a single dose of alpelisib or MTX-531(Compound 2R). [00127] Figure 28 depicts a line graph showing oral glucose tolerance testing in fasted mice following a single oral dose of alpelisib or MTX-531(Compound 2R). [00128] Figure 29 depicts a line graph showing oral glucose tolerance testing in fasted mice after five days of PI3K inhibitor dosing. [00129] Figure 30 depicts a bar graph representing adiponectin levels in blood from C57/BL6 mice treated with MTX-531 (Compound 2R) versus the PPAR gamma agonist rosiglitazone. [00130] Figure 31 depicts a bar graph representing adiponectin levels in blood from athymic nude mice treated with MTX-531 (Compound 2R) versus comparator PI3K and PPAR gamma targeted agents. DETAILED DESCRIPTION OF THE DISCLOSURE [00131] The present disclosure provides a method for preventing, treating, reducing, inhibiting or controlling a neoplasia, tumor or cancer and/or the establishment of metastases in a subject involving administering a compound of Formula I or a pharmaceutically acceptable salt thereof. [00132] For purposes of this disclosure, the chemical elements are identified in accordance with the Periodic Table of the Elements, CAS version, Handbook of Chemistry and Physics, 98th Ed. Additionally, general principles of organic chemistry are described in "Organic Chemistry", Second Ed., Thomas Sorrell, University Science Books, Sausolito: 2006, and "March’s Advanced Organic Chemistry", 7th Ed., Ed.: Smith, M.B. and March, J., John Wiley & Sons, New York: 2015, the entire contents of which are hereby incorporated by reference. Definitions [00133] As used herein, "about" means within acceptable error range for the particular value as determined by one of ordinary skill in the art, which will depend in part on how the value is measured or determined, i.e., the limitations of the measurement system. For example, "about" can mean within 1 or more than 1 standard deviation per the practice in the art. Alternatively, the term "about" can mean a range of up to 10%. For example, if the specification recites the term "about 5", this term refers to all integers and fractional amounts ranging from 4.5 to 5.5. When particular values are provided in the application and claims, unless otherwise stated, the meaning of "about" should be assumed to be within acceptable error range for that particular value. [00134] As used herein, an "effective amount" is defined as the amount required to confer a therapeutic effect on the treated patient, and is typically determined based on age, surface area, weight, and condition of the patient. The interrelationship of dosages for animals and humans (based on milligrams per meter squared of body surface) is described by Freireich et al., Cancer Chemother. Rep., 50: 219 (1966). Body surface area may be approximately determined from height and weight of the patient. See, e.g., Scientific Tables, Geigy Pharmaceuticals, Ardsley, New York, 537 (1970). As used herein, "patient" refers to a mammal, including a human. [00135] The term “pharmaceutically acceptable salt” as used herein, refers to any salt (e.g., obtained by reaction with an acid or a base) of a compound of the present disclosure that is physiologically tolerated in the target patient (e.g., a mammal). Salts of the compounds of the present disclosure may be derived from inorganic or organic acids and bases. Examples of acids include, but are not limited to, hydrochloric, hydrobromic, sulfuric, nitric, perchloric, fumaric, maleic, phosphoric, glycolic, lactic, salicylic, succinic, toluene-p-sulfonic, tartaric, acetic, citric, methanesulfonic, ethanesulfonic, formic, benzoic, malonic, sulfonic, naphthalene-2-sulfonic, benzenesulfonic acid, and the like. Other acids, such as oxalic, while not in themselves pharmaceutically acceptable, may be employed in the preparation of salts useful as intermediates in obtaining the compounds of the disclosure and their pharmaceutically acceptable acid addition salts. [00136] As used herein, "pharmaceutically acceptable carrier" includes any and all solvents, dispersion media, coatings, surfactants, antioxidants, preservatives {e.g., antibacterial agents, antifungal agents), isotonic agents, absorption delaying agents, salts, preservatives, drugs, drug stabilizers, gels, binders, excipients, disintegration agents, lubricants, sweetening agents, flavoring agents, dyes, such like materials and combinations thereof, as would be known to one of ordinary skill in the art (see, for example, Remington's Pharmaceutical Sciences, 18th Ed. Mack Printing Company, 1990, pp.1289-1329). [00137] As used herein, "pharmaceutically acceptable carrier" or "pharmaceutical acceptable excipient" includes any material which, when combined with an active ingredient, allows the ingredient to retain biological activity and is non-reactive with the subject's immune system, and can include any and all solvents, diluents, carriers, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents, and the like that are physiologically compatible, non-toxic, and does not interfere with the mechanism of action of the compound of Formula I or a pharmaceutically acceptable salt thereof. Preferably, the pharmaceutical acceptable excipient is suitable for intravenous, intramuscular, subcutaneous, parenteral, spinal or epidermal administration (e.g., by injection or infusion). Depending on the route of administration, a compound of Formula I or a pharmaceutically acceptable salt thereof, may be coated in a material to protect the compound from the action of acids and other natural conditions that may inactivate the compound. Pharmaceutically acceptable excipients include sterile aqueous solutions or dispersions and sterile powders for the extemporaneous preparation of sterile injectable solutions or dispersion. The use of such media and agents for pharmaceutically active substances is known in the art. Except insofar as any conventional media or agent is incompatible with the active compound, use thereof in the pharmaceutical compositions of the disclosure is contemplated. Supplementary active compounds can also be incorporated into the compositions. [00138] The phrase "stable or chemically feasible," as used herein, refers to compounds that are not substantially altered when subjected to conditions to allow for their production, detection, and preferably their recovery, purification, and use for one or more of the purposes disclosed herein. In some embodiments, a stable compound or chemically feasible compound is one that is not substantially altered when kept at a temperature of 40 °C or less, in the absence of moisture or other chemically reactive conditions, for at least a week. [00139] The methods of treatment of the disclosure comprise administering a safe and effective amount of a compound described herein or a pharmaceutically-acceptable salt thereof to a patient in need thereof. [00140] As used herein, the term "subject" is intended to include human and non-human animals, and is used synonymously with the term "patient". Preferred subjects include human patients in need of enhancement of an immune response that may be beneficial in the patient’s treatment of cancer and/or cancer metastasis. The methods are particularly suitable for treating human patients having a disorder that can be treated by augmenting the T-cell mediated immune response. In a particular embodiment, the methods are particularly suitable for treatment of cancer cells in vivo. [00141] “Administering” refers to the physical introduction of a therapeutic agent to a subject (e.g., a composition or formulation comprising the therapeutic agent), using any of the various methods and delivery systems known to those skilled in the art. Exemplary routes of administration include intravenous, intramuscular, subcutaneous, intraperitoneal, spinal or other parenteral routes of administration, for example by injection or infusion. The phrase “parenteral administration” as used herein means modes of administration other than enteral and topical administration, usually by injection, and includes, without limitation, intravenous, intramuscular, intraarterial, intrathecal, intralymphatic, intralesional, intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal, epidural and intrasternal injection and infusion, as well as in vivo electroporation. In some aspects, the therapeutic agent is administered via a non- parenteral route, in some aspects, orally. Other non-parenteral routes include a topical, epidermal or mucosal route of administration, for example, intranasally, vaginally, rectally, sublingually or topically. Administering can also be performed, for example, once, a plurality of times, and/or over one or more extended periods. [00142] “Treatment” or “therapy” of a subject refers to any type of intervention or process performed on, or the administration of an active agent to, the subject with the objective of reversing, alleviating, ameliorating, inhibiting, slowing down progression, development, severity or recurrence of a symptom, complication or condition, or biochemical indicia associated with a disease. Response Evaluation Criteria In Solid Tumors (RECIST) is a measure for treatment efficacy and are established rules that define when tumors respond, stabilize, or progress during treatment. RECIST 1.1 is the current guideline to solid tumor measurement and definitions for objective assessment of change in tumor size for use in adult and pediatric cancer clinical trials. [00143] As used herein, “effective treatment” refers to treatment producing a beneficial effect, e.g., amelioration of at least one symptom of a disease or disorder. A beneficial effect can take the form of an improvement over baseline, i.e., an improvement over a measurement or observation made prior to initiation of therapy according to the method. A beneficial effect can also take the form of arresting, slowing, retarding, or stabilizing of a deleterious progression of a marker of solid tumor. Effective treatment can refer to alleviation of at least one symptom of a solid tumor. Such effective treatment can, e.g., reduce patient pain, reduce the size and/or number of lesions, reduce the size of the tumor mass, can reduce or prevent metastasis of a tumor, and/or can slow tumor growth. By “reduce” or other forms of the word, such as “reducing” or “reduction,” is meant lowering of an event or characteristic (e.g., tumor growth). It is understood that this is typically in relation to some standard or expected value, in other words it is relative, but that it is not always necessary for the standard or relative value to be referred to. For example, “reduces tumor growth” means reducing the rate of growth of a tumor relative to a standard or a control. [00144] The term “effective amount” refers to an amount of an agent that provides the desired biological, therapeutic, and/or prophylactic result. That result can be reduction, amelioration, palliation, lessening, delaying, and/or alleviation of one or more of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system. In reference to solid tumors, an effective amount comprises an amount sufficient to cause a tumor to shrink and/or to decrease the growth rate of the tumor (such as to suppress tumor growth) or to delay other unwanted cell proliferation. In some aspects, an effective amount is an amount sufficient to prevent or delay tumor recurrence. An effective amount can be administered in one or more administrations. The effective amount of the drug or composition can: (i) reduce the number of cancer cells; (ii) reduce tumor size; (iii) inhibit, retard, slow to some extent and can stop cancer cell infiltration into peripheral organs; (iv) inhibit (i.e., slow to some extent and can stop tumor metastasis; (v) inhibit tumor growth; (vi) prevent or delay occurrence and/or recurrence of tumor; and/or (vii) relieve to some extent one or more of the symptoms associated with the cancer. In one example, an “effective amount” is the amount of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, alone or the amount of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and the amount an additional therapeutic agent (e.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein), in combination, clinically proven to affect a significant decrease in cancer or slowing of progression of cancer, such as an advanced solid tumor. [00145] As used herein, the terms “fixed dose,” “flat dose,” and “flat-fixed dose” are used interchangeably and refer to a dose that is administered to a patient without regard for the weight or body surface area (BSA) of the patient. The fixed or flat dose is therefore not provided as a mg/kg dose, but rather as an absolute amount of the agent (e.g., an amount in μg or mg). [00146] The use of the term “fixed dose combination” with regard to a composition of the invention means that two or more different inhibitors as described herein (e.g., a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof (e.g a first inhibitor), and the amount an additional therapeutic agent (e.g., an inhibitor of RAS, KRAS, BRAF and other secondary agents disclosed herein; i.e. a second inhibitor) in a single composition are present in the composition in particular (fixed) ratios with each other. In some aspects, the fixed dose is based on the weight (e.g., mg) of the first and second inhibitors. In certain aspects, the fixed dose is based on the concentration (e.g., mg/mL) of the first and second inhibitors. In some aspects, the ratio is at least about 1:1, about 1:2, about 1:3, about 1:4, about 1:5, about 1:6, about 1:7, about 1:8, about 1:9, about 1:10, about 1:15, about 1:20, about 1:30, about 1:40, about 1:50, about 1:60, about 1:70, about 1:80, about 1:90, about 1:100, about 1:120, about 1:140, about 1:160, about 1:180, about 1:200, about 200:1, about 180:1, about 160:1, about 140:1, about 120:1, about 100:1, about 90:1, about 80:1, about 70:1, about 60:1, about 50:1, about 40:1, about 30:1, about 20:1, about 15:1, about 10:1, about 9:1, about 8:1, about 7:1, about 6:1, about 5:1, about 4:1, about 3:1, or about 2:1 mg first inhibitor to mg second inhibitor. [00147] The term “weight based dose” as referred to herein means that a dose that is administered to a patient is calculated based on the weight of the patient. [00148] “Dosing interval,” as used herein, means the amount of time that elapses between multiple doses of a formulation disclosed herein being administered to a subject. Dosing interval can thus be indicated as ranges. [00149] The term “dosing frequency” as used herein refers to the frequency of administering doses of a formulation disclosed herein in a given time. Dosing frequency can be indicated as the number of doses per a given time, e.g., once a week or once in two weeks, etc. [00150] The terms “about once a week,” “once about every week,” “once about every two weeks,” or any other similar dosing interval terms as used herein means approximate number, and “about once a week” or “once about every week” can include every seven days ±two days, i.e., every five days to every nine days. The dosing frequency of “once a week” thus can be every five days, every six days, every seven days, every eight days, or every nine days. “Once about every three weeks” can include every 21 days±3 days, i.e., every 25 days to every 31 days. Similar approximations apply, for example, to once about every two weeks, once about every four weeks, once about every five weeks, once about every six weeks, once about every seven weeks, once about every eight weeks, once about every nine weeks, once about every ten weeks, once about every eleven weeks, and once about every twelve weeks. In some aspects, a dosing interval of once about every six weeks or once about every twelve weeks means that the first dose can be administered any day in the first week, and then the next dose can be administered any day in the sixth or twelfth week, respectively. In other aspects, a dosing interval of once about every six weeks or once about every twelve weeks means that the first dose is administered on a particular day of the first week (e.g., Monday) and then the next dose is administered on the same day of the sixth or twelfth weeks (i.e., Monday), respectively. [00151] “Such as” has the same meaning as "such as but not limited to." Similarly, "include" has the same meaning as “include but not limited to,” while “including” has the same meaning as “including but not limited to.” [00152] Terms of degree such as “about”, “substantially”, and “approximately” as used herein mean a reasonable amount of deviation of the modified term such that the end result is not significantly changed. These terms of degree should be construed as including a deviation of at least +5% of the modified term if this deviation would not negate the meaning of the word it modifies. All ranges disclosed herein are inclusive of the endpoints, and the endpoints are independently combinable with each other. [00153] The terms "tumor," "cancer" and "neoplasia" are used interchangeably and refer to a cell or population of cells whose growth, proliferation or survival is greater than growth, proliferation or survival of a normal counterpart cell, e.g. a cell proliferative or differentiative disorder. Typically, the growth is uncontrolled. The term "malignancy" refers to invasion of nearby tissue. The term "metastasis" refers to spread or dissemination of a tumor, cancer or neoplasia to other sites, locations or regions within the subject, in which the sites, locations or regions are distinct from the primary tumor or cancer. [00154] As used herein, the term "MTX-531" described in the specification herein, for example in the Examples, and Figures; refers to a compound of Formula (I) referred to herein as "Compound 2R" or "COMPOUND 2R". The structure of MTX-531 (Compound 2R) is found in Table 1 and and Fig.1 is known as N-(2-chloro-5-(4-((1R-phenylethyl)amino)quinazolin-6- yl)pyridin-3-yl)methanesulfonamide. [00155] “Effective amount” or “therapeutically effective amount” as disclosed in the present invention refers to the amount or dose of the compound or a pharmaceutically acceptable salt thereof according to the present invention which, upon single or multiple dose administration to the subject, provides the desired effect in the subject under diagnosis or treatment. For example, it may include an amount of a compound of the present disclosure that is sufficient to treat, prevent or inhibit a disease or condition such as cancer, in a subject that has in addition to cancer, a disease or condition associated with type II diabetes and/or hyperglycemia, (as used herein, the term “hyperglycemia” refers to higher than normal fasting blood glucose concentration, optionally at least 125 mg/dL) and/or insulin resistance. The amount of a given compound of the present disclosure that will correspond to such an amount will vary depending upon various factors, such as the given compound, the composition, the route of administration, the type of disease or disorder, the identity of the subject or host being treated, and the like, but can nevertheless be routinely determined by one skilled in the art. In one embodiment, a “therapeutically effective amount” is an amount sufficient to have a desired effect on a subject, such as have a therapeutic benefit with respect to the treatment of cancer. [00156] “Minimum effective dose” as disclosed in the present invention refers to the minimum administration dose at which the drug effect is produced. Specifically, as the drug effect experiment of multiple administrations on db/db mice in the present invention is concerned, it refers to the minimum dose at which the blood glucose AUC of the group taken the test compound is significantly different (p<0.05) from that of the Vehicle group. [00157] “Efficacy dose” as disclosed in the present invention refers to the administration dose at which the drug effect is produced. Specifically, as the drug effect experiment of multiple administrations on db/db mice in the present invention is concerned, it refers to the dose at which the blood glucose AUC of the group taken the test compound is significantly different (p<0.05) from that of the Vehicle group. [00158] “Vehicle group” as disclosed in the present invention refers to the vehicle control group. [00159] “Treatment” as disclosed in the present invention includes attenuating, inhibiting, reversing, slowing, delaying or halting the progression or severity of an existing condition, disease, disorder or symptom, for example, cancer. “Prevention” as disclosed in the present invention includes reducing the risk of acquiring a particular disease, disease condition or disorder. [00160] The term “primate” includes humans (male or female) and non-human primates. The term “non-human” primate includes monkeys, such as a Cynomolgus Monkey. [00161] The term “subject” includes humans and animals, which includes pets. The terms “pet” and “companion animal” are interchangeable and include non-human primates, rodent animals (especially rodent mammals), and non-human and non-rodent animals (especially non- human and non-rodent mammals). The term “rodent animal” or “rodent mammal” includes mice and rats. The term “non-human and non-rodent animal” or “non-human and non-rodent mammal” means an animal or a mammal that is neither human nor rodent. The term “non-human and non-rodent animal” or “non-human and non-rodent mammal” includes, but is not limited to, dogs, cats, rabbits, pigs, alpacas, horses, sheep and bovines. [00162] The term “EGFR” is an acronym for epidermal growth factor receptor, a protein that helps cells grow and is involved in cell signaling pathways that control cell division and survival. EGFR is also involved in various processes related to cancer, such as cell proliferation, apoptosis, angiogenesis, and metastasis. The term EGFR can include human and animal variants of EGFR. In one embodiment, the term EGFR relates to human EGFR, which is illustratively exemplified as UniProt P00533, and has an amino acid sequence as provided in SEQ ID NO: 1. [00163] The term PI3K is an acronymm for the term “Phosphatidylinositol-3 kinase”. PI3K is a family of enzymes that send signals in cells and help control cell growth. In one embodiment, the term PI3K includes all of the class I PI3K human enzymes, including, human PI3K-alpha, PI3K-beta, PI3K-delta, and PI3K-gamma. For example, compounds of the present disclosure inhibit a PI3K enzyme, for example, human PI3K-gamma which is illustratively exemplified as UniProt P48736, and has an amino acid sequence as provided in SEQ ID NO: 2. For example, compounds of the present disclosure inhibit a PI3K enzyme, for example, human PI3K-alpha which is illustratively exemplified as UniProt P42336, and has an amino acid sequence as provided in SEQ ID NO: 4. For example, compounds of the present disclosure inhibit a PI3K enzyme, for example, human PI3K-beta, which is illustratively exemplified as UniProt P42338, and has an amino acid sequence as provided in SEQ ID NO: 5. For example, compounds of the present disclosure inhibit a PI3K enzyme, for example, human PI3K-delta which is illustratively exemplified as UniProt O00329, and has an amino acid sequence as provided in SEQ ID NO: 6. [00164] The term PPAR-gamma is an acronym for the term “Peroxisome proliferator- activated receptor gamma (PPAR-γ or PPARG. PPAR-gamma is a transcription factor and nuclear receptor that regulates gene expression and controls how the body uses glucose and lipids. In one embodiment, PI3K is human PI3K-gamma which is illustratively exemplified as UniProt P37231, and has an amino acid sequence as provided in SEQ ID NO: 3. [00165] As used herein, an "alkyl" group refers to a saturated aliphatic hydrocarbon group containing 1-12 (e.g., 1-8, 1-6, or 1-4) carbon atoms. An alkyl group can be straight or branched. Examples of alkyl groups include, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, n-heptyl, or 2-ethylhexyl. [00166] As used herein, an "alkenyl" group refers to an aliphatic carbon group that contains 2-8 (e.g., 2-12, 2-6, or 2-4) carbon atoms and at least one double bond. Like an alkyl group, an alkenyl group can be straight or branched. Examples of an alkenyl group include, but are not limited to allyl, isoprenyl, 2-butenyl, and 2-hexenyl. [00167] As used herein, an "alkynyl" group refers to an aliphatic carbon group that contains 2-8 (e.g., 2-12, 2-6, or 2-4) carbon atoms and has at least one triple bond. An alkynyl group can be straight or branched. Examples of an alkynyl group include, but are not limited to, propargyl and butynyl. [00168] As used herein, an “alkylene” group refers to a bivalent alkyl group that connects to two attachment points simultaneously, wherein the alkylene unit can be bivalent on the same carbon or two different carbons of the alkyl moiety. Examples of alkylene groups are, without limitation, methylene, ethylene, propylene, and butylene, as well as branched structures, such as –CH2(CH2)- (1,1-ethylene) and – CH2CH2(CH2)- (1,2-propylene). [00169] As used herein an “aryl” group refers to a mono-, bi-, or tri-cyclic ring system wherein all rings in the system are aromatic and contain no heteroatoms in the ring. Examples of aryl groups include, but are not limited to phenyl, naphthyl, anthracenyl, and tetracenyl. [00170] As used herein, a "carbocycle" or “carbocyclyl” group refers to a mono-, bi-, or tricyclic (fused or bridged) hydrocarbon ring system that contains no heteroatoms in the ring structures, wherein at least one of the rings in the system is non-aromatic, and can be completely saturated or partially unsaturated. The terms "carbocycle" or “carbocyclyl” encompass a "cycloalkyl" group and a "cycloalkenyl" group, each of which is set forth below. [00171] As used herein, a "cycloalkyl" group refers to a saturated carbocyclic mono-, bi-, or tricyclic (fused or bridged) ring system of 3-20 (e.g., 5-10) carbon atoms. Examples of cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, adamantyl, norbornyl, cubyl, octahydro-indenyl, decahydro-naphthyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl, bicyclo[3.3.1]nonyl, bicyclo[3.3.2.]decyl, bicyclo[2.2.2]octyl, adamantyl, or ((aminocarbonyl)cycloalkyl)cycloalkyl. [00172] A "cycloalkenyl" group, as used herein, refers to a non-aromatic carbocyclic mono-, bi, or tricyclic (fused or bridged) ring system of 3-20 (e.g., 4-8) carbon atoms, wherein at least one ring in the system has one or more double bonds. Examples of cycloalkenyl groups include cyclopentenyl, 1,4-cyclohexa-di-enyl, cycloheptenyl, cyclooctenyl, hexahydro-indenyl, octahydro-naphthyl, cyclohexenyl, cyclopentenyl, bicyclo[2.2.2]octenyl, or bicyclo[3.3.1]nonenyl. [00173] As used herein, the terms "heterocycle" and "heterocyclyl" are used interchangeably and refer to a mono-, bi-, or tricyclic (fused or bridged) non-aromatic hydrocarbon ring system that contains at least one heteroatom in the ring structure and can be completely saturated or partially unsaturated. The terms "heterocycle" and "heterocyclyl" encompass a “heterocycloalkyl” group and a “heterocycloalkenyl” group, each of which is set forth below. [00174] As used herein, a "heterocycloalkyl" group refers to a 3-20 membered mono-, di-, or tricylic (fused or bridged) (e.g., 5- to 10-membered) saturated ring structure, in which one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof). Examples of a heterocycloalkyl group include piperidyl, piperazyl, tetrahydropyranyl, tetrahydrofuryl, 1,4- dioxolanyl, 1,4-dithianyl, 1,3-dioxolanyl, oxazolidyl, isoxazolidyl, morpholinyl, thiomorpholyl, octahydrobenzofuryl, octahydrochromenyl, octahydrothiochromenyl, octahydroindolyl, octahydropyrindinyl, decahydroquinolinyl, octahydrobenzo[b]thiopheneyl, 2-oxa- bicyclo[2.2.2]octyl, 1-aza-bicyclo[2.2.2]octyl, 3-aza-bicyclo[3.2.1]octyl, and 2,6-dioxa- tricyclo[3.3.1.0.3.7]nonyl. [00175] A "heterocycloalkenyl" group, as used herein, refers to a 3-20 membered mono-, di-, or tricylic (fused or bridged) (e.g., 5- to 10-membered) non-aromatic ring structure, in which one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof), and wherein at least one of the ring structures has one or more double bonds. [00176] A "heteroaryl" group, as used herein, refers to a monocyclic, bicyclic, or tricyclic ring system having 4 to 15 ring atoms wherein one or more of the ring atoms is a heteroatom (e.g., N, O, S, or combinations thereof) and in which the monocyclic ring system is aromatic or at least one of the rings in the bicyclic or tricyclic ring systems is aromatic. A heteroaryl group includes a benzofused ring system having 2 to 3 rings. For example, a benzofused group includes benzo fused with one or two 4 to 8 membered heterocycloaliphatic moieties (e.g., indolizyl, indolyl, isoindolyl, 3H-indolyl, indolinyl, benzo[b]furyl, benzo[b]thiophenyl, quinolinyl, or isoquinolinyl). Some examples of heteroaryl are azetidinyl, pyridyl, 1H-indazolyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, tetrazolyl, benzofuryl, isoquinolinyl, benzothiazolyl, xanthene, thioxanthene, phenothiazine, dihydroindole, benzo[1,3]dioxole, benzo[b]furyl, benzo[b]thiophenyl, indazolyl, benzimidazolyl, benzthiazolyl, puryl, cinnolyl, quinolyl, quinazolyl,cinnolyl, phthalazyl, quinazolyl, quinoxalyl, isoquinolyl, 4H-quinolizyl, benzo-1,2,5-thiadiazolyl, or 1,8-naphthyridyl. [00177] Without limitation, monocyclic heteroaryls include furyl, thiophenyl, 2H- pyrrolyl, pyrrolyl, oxazolyl, thazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,3,4- thiadiazolyl, 2H-pyranyl, 4-H-pranyl, pyridyl, pyridazyl, pyrimidyl, pyrazolyl, pyrazyl, or 1,3,5- triazyl. [00178] Without limitation, bicyclic heteroaryls include indolizyl, indolyl, isoindolyl, 3H- indolyl, indolinyl, benzo[b]furyl, benzo[b]thiophenyl, quinolinyl, isoquinolinyl, indolizinyl, isoindolyl, indolyl, benzo[b]furyl, bexo[b]thiophenyl, indazolyl, benzimidazyl, benzthiazolyl, purinyl, 4H-quinolizyl, quinolyl, isoquinolyl, cinnolyl, phthalazyl, quinazolyl, quinoxalyl, 1,8- naphthyridyl, or pteridyl. [00179] As used herein, "cyclic moiety" and "cyclic group" refer to mono-, bi-, and tri- cyclic ring systems including cycloaliphatic, heterocycloaliphatic, aryl, or heteroaryl, each of which has been previously defined. [00180] As used herein, a "bridged bicyclic ring system" refers to a bicyclic heterocyclicaliphatic ring system or bicyclic cycloaliphatic ring system in which the rings are bridged. Examples of bridged bicyclic ring systems include, but are not limited to, adamantanyl, norbornanyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl, bicyclo[3.3.1]nonyl, bicyclo[3.2.3]nonyl, 2-oxabicyclo[2.2.2]octyl, 1-azabicyclo[2.2.2]octyl, 3-azabicyclo[3.2.1]octyl, and 2,6-dioxa- tricyclo[3.3.1.0.3.7]nonyl. [00181] As used herein, an "alkoxy" group refers to an alkyl-O- group where "alkyl" has been defined previously. [00182] As used herein, a "carbonyl" refers to -C(O)-. [00183] As used herein, an "oxo" refers to =O. [00184] As used herein a “carboxy” refers to -C(O)OH. [00185] As used herein an “ester” refers to –C(O)O-W, in which W is, for example, alkyl, carbocyclyl, or heterocyclyl. [00186] As described herein, compounds of the disclosure may optionally be substituted with one or more substituents, such as are illustrated generally herein, or as exemplified by particular classes, subclasses, and species of the disclosure. [00187] The phrase "optionally substituted" is used interchangeably with the phrase "substituted or unsubstituted." As described herein, compounds of the disclosure can optionally be substituted with one or more substituents, as exemplified by particular classes, subclasses, and species of the disclosure. [00188] In general, the term "substituted," whether preceded by the term "optionally" or not, refers to the replacement of hydrogen radicals in a given structure with the radical of a specified substituent. Unless otherwise indicated, an optionally substituted group can have a substituent at each substitutable position of the group, and when more than one position in any given structure can be substituted with more than one substituent selected from a specified group, the substituent can be either the same or different at every position. A ring substituent, such as a heterocycloalkyl, can be bound to another ring, such as a cycloalkyl, to form a spiro- bicyclic ring system, e.g., both rings share one common atom. As one of ordinary skill in the art will recognize, combinations of substituents envisioned by this disclosure are those combinations that result in the formation of stable or chemically feasible compounds. [00189] As used herein, "treat" in reference to a condition means: (1) to ameliorate, diminish or counteract the condition or one or more of the biological manifestations of the condition, (2) to interfere with (a) one or more points in the biological cascade that leads to or is responsible for the condition or (b) one or more of the biological manifestations of the condition, (3) to alleviate one or more of the symptoms or effects associated with the condition, or (4) to slow the progression of the condition or one or more of the biological manifestations of the condition. [00190] Additionally, unless otherwise stated, structures depicted herein are also meant to include compounds that differ only in the presence of one or more isotopically enriched atoms. Examples of isotopes that can be incorporated into compounds of the disclosure and pharmaceutically acceptable salts thereof include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, sulphur, fluorine, iodine, and chlorine, such as2H,3H,11C,13C,14C,15N,170,180,31P,32P,35S,18F,36Cl,123I and125I. [00191] Compounds of the present disclosure and pharmaceutically acceptable salts of said compounds that contain the aforementioned isotopes and/or other isotopes of other atoms are within the scope of the present disclosure. Isotopically-labelled compounds of the present disclosure, for example those into which radioactive isotopes, such as3H and14C, are incorporated, are useful in drug and/or substrate tissue distribution assays. Tritiated hydrogen (3H) and carbon-14 (14C) isotopes are particularly preferred for their ease of preparation and detectability.11C and18F isotopes are particularly useful in PET (positron emission tomography), and125l isotopes are particularly useful in SPECT (single photon emission computerized tomography), all useful in brain imaging. Further, substitution with heavier isotopes such as deuterium (2H) can afford certain therapeutic advantages resulting from greater metabolic stability, for example increased in vivo half-life or reduced dosage requirements and may be preferred in some circumstances. Isotopically labeled compounds of the disclosure can generally be prepared by carrying out the procedures disclosed in the schemes and/or in the examples below, and substituting a readily available isotopically labeled reagent for a non-isotopically labeled reagent. [00192] Unless otherwise stated, structures depicted herein are also meant to include all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or conformational)) forms of the structure. "Isomer" refers to compounds that have the same composition and molecular weight but differ in physical and/or chemical properties; for example (Z) and (E) double bond isomers, and (Z) and (E) conformational isomers. The structural difference may be in constitution (geometric isomers) or in the ability to rotate the plane of polarized light (stereoisomers); for example, the R and S configurations for each asymmetric center. The compounds of the disclosure may contain one or more asymmetric centers, also referred to as chiral centers, and may, therefore, exist as individual enantiomers, diastereomers, or other stereoisomeric forms, or as mixtures thereof. All such isomeric forms are included within the present disclosure, including mixtures thereof. Chiral centers may also be present in a substituent such as an alkyl group. [00193] Where the stereochemistry of a chiral center present in a compound of the disclosure, or in any chemical structure illustrated herein, is not specified the structure is intended to encompass any stereoisomer and all mixtures thereof. Thus, compounds of the disclosure containing one or more chiral centers may be used as racemic mixtures, enantiomerically enriched mixtures, or as enantiomerically pure individual stereoisomers. [00194] Individual stereoisomers of a compound of the disclosure which contain one or more asymmetric centers may be resolved by methods known to those skilled in the art. For example, such resolution may be carried out (1) by formation of diastereoisomeric salts, complexes or other derivatives; (2) by selective reaction with a stereoisomer-specific reagent, for example by enzymatic oxidation or reduction; or (3) by gas-liquid or liquid chromatography in a chiral environment, for example, on a chiral support such as silica with a bound chiral ligand or in the presence of a chiral solvent. The skilled artisan will appreciate that where the desired stereoisomer is converted into another chemical entity by one of the separation procedures described above, a further step is required to liberate the desired form. Alternatively, specific stereoisomers may be synthesized by asymmetric synthesis using optically active reagents, substrates, catalysts or solvents, or by converting one enantiomer to the other by asymmetric transformation. [00195] Unless otherwise stated, all tautomeric forms of the compounds of the disclosure are within the scope of the disclosure. [00196] Any numerical range disclosed herein encompasses the and lower limits and each intervening value, unless otherwise specified. Other than in the working examples, or where otherwise indicated, numerical values (such as numbers expressing quantities of ingredients, reaction conditions) as used in the specification and claims are modified by the term "about". Accordingly, unless indicated to the contrary, such numbers are approximations that may vary depending upon the desired properties sought to be obtained. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should be construed in light of the number of significant digits and ordinary rounding techniques. [00197] While the numerical parameters setting forth the scope of the disclosed subject matter are approximations, the numerical values set forth in the working examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in its respective testing measurements. [00198] Unless defined otherwise, the meanings of technical and scientific terms as used herein are those commonly understood by one of ordinary skill in the art to which the disclosed subject matter belongs. [00199] Embodiments [00200] In one aspect, the disclosure includes a compound of Formula I or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring A is selected from phenyl or a 5 or 6 membered heteroaryl; X is N or C-R5; R1a is selected from the group consisting of H or C1-6 alkyl; R1b is selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, halo, CN, and NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with one or more R” substituents; or, R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with one or more R” substituents; each R2 is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; or one R2 substituent and R1b, together with the phenyl group to which R2 is attached and the carbon atom to which R1b is attached, form a bicyclic group having the general structure , wherein Ring B is a C3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, OC(O)OR’, OC(O)N(R’)2, NR’C(O)N(R’)2, SOR’, SON(R’)2, SO2R’, SO2N(R’)2, NR’SOR’, NR’SON(R’)2, NR’SO2R’, and NR’SO2N(R’)2, wherein the C1-6 alkyl, hetercycloalky, and heteroaryl are each optionally and independently substituted with one or more R” substituents; or two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heterocycloalkyl, a fused bicyclic cycloalkyl, a fused bicyclic aryl, or a fused bicyclic heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R4 is selected from halo, OH, NH2, CN, C1-6 alkyl, and OC1-6 alkyl; each R5 is selected from hydrogen, halo, OH, NH2, CN, and C1-6 alkyl; each R’ is independently selected from hydrogen, OH, CN, C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R” is independently selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or substituents selected from halo, oxo, alkoxy, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, and C(O)NHC1-6 alkyl; and m, n, and p are each an integer selected from 0-4. [00201] In one embodiment, R1a is H. [00202] In another embodiment, R1b is C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, OH, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, C(O)N(C1-6 alkyl)2, halo, CN, or NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, C(O)N(C1-6 alkyl)2. [00203] In another embodiment, R1b is C1-6 alkyl, OC1-6 alkyl, OH, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, C(O)N(C1-6 alkyl)2, halo, or CN, wherein each C1-6 alkyl is optionally and independently substituted with OC1-6 alkyl, OH, halo, or CN. [00204] In a further embodiment, R1b is C1-6 alkyl, OC1-6 alkyl, OH, halo, or CN, wherein each C1-6 alkyl is optionally and independently substituted with OH, CN, or halo. [00205] In still a further embodiment, R1b is C1-6 alkyl, C1-6 alkyl-OH, or CN. [00206] In still a further embodiment, R1b is methyl, CN, or CH2OH. [00207] In another embodiment, R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with C1-6 alkyl, OC1-6 alkyl, oxo, OH, halo, CN, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, or C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl is optionally and independently substituted with one or substituents selected from halo, alkoxy, CN, or NH2. [00208] In another embodiment, R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with oxo, OH, halo, or CN. [00209] In a further embodiment, R1a and R1b, together with the methylene moiety to which they are attached, form an unsubstituted spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl. [00210] In still a further embodiment, R1a and R1b, together with the methylene moiety to which they are attached, form a [00211] In another embodiment, each R2 substituent is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, C(O)C1-6 alkyl, and C(O)NH2. [00212] In a further embodiment, each R2 substituent is independently selected from halo and OH. [00213] In one embodiment, n is 0 or 1. [00214] In a further embodiment, n is 0. [00215] In one embodiment, one R2 substituent and R1b, together with the phenyl group to which R2 is attached and the carbon atom to which R1b is attached, form a bicyclic group having the general structure wherein Ring B is a C3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, or C(O)NH2. [00216] In one embodiment, Ring B is a C4-6 cycloalkyl or 4-6 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, OC1-6 alkyl, CN, or NH2, C(O)C1-6 alkyl. [00217] In a further embodiment, Ring B is a C4-5 cycloalkyl or 5 membered heterocycloalkyl, each of which is optionally substituted with OH. [00218] In another embodiment, the general structure is selected from [00219] In one embodiment, each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OH, OC1-6 alkyl, N(C1-6 alkyl)2, NH(C1-6 alkyl), NH2, C(O)H, C(O)C1-6 alkyl, COOH, C(O)OC1-6 alkyl, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, OC(O)OC1-6 alkyl, OC(O)NH2, OC(O)NH(C1-6 alkyl), OC(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO(C1-6 alkyl), SONH2, SONH(C1-6 alkyl), SON(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO(C1-6 alkyl), NHSO(C1-6 alkyl), N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), NHSO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2NH2, N(C1-6 alkyl)SO2NH(C1-6 alkyl), and N(C1-6 alkyl)SO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl are each optionally and independently substituted with one or more R” substituents. [00220] In another embodiment, each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OH, OC1-6 alkyl, N(C1-6 alkyl)2, NH(C1-6 alkyl), NH2, C(O)H, C(O)C1-6 alkyl, COOH, C(O)OC1-6 alkyl, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, OC(O)OC1-6 alkyl, OC(O)NH2, OC(O)NH(C1-6 alkyl), OC(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, NHSO2(C1-6 alkyl), N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), NHSO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2NH2, N(C1-6 alkyl)SO2NH(C1-6 alkyl), and N(C1-6 alkyl)SO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl are each optionally and independently substituted with one or more R” substituents. [00221] In one embodiment, each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), NHSO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl are each optionally and independently substituted with one or more R” substituents. [00222] In another embodiment, each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH(C1-6 alkyl), SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl are each optionally and independently substituted with one or more R” substituents. [00223] In a further embodiment, each R3 is independently selected from C1-6 alkyl, triazolyl, oxadiazolyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH(C1-6 alkyl), SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), wherein each C1-6 alkyl, triazolyl and oxadiazolyl, are each optionally and independently substituted with one or more substituents independently selected from oxo, OH, halo, C1-6 alkyl, NH2, NHC1-6 alkyl, and N(C1-6 alkyl)2. [00224] In still a further embodiment, each R3 substituent is independently selected from halo, CN, NH2, OH, C(O)H, C(O)N(CH3)2, C(O)NH(CH3), C(O)NH(Et), C(O)NH(isopropyl), C(O)NH(tert-butyl), C(O)NH(cyclopropyl), C(O)NCH3(CN), C(O)NH(OH), C(O)NCH3(OH), C(O)NH2, NHC(O)NHCH3, NHS(O)2CH3, S(O)2CH3, methyl, methoxy, NHS(O)2CH2CH2N(CH3)2, CF3, CH2OH, C(CH3)2OH, , , , [00225] In one embodiment, the compound of Formula I is a compound of Formula Ia or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein each X’ is independently CH, C-R3, or N. [00226] In one embodiment, the ring is a phenyl, pyridyl, pyrimidyl, pyrazyl, or triazyl, which is optionally substituted by R3. [00227] In a further embodiment, the ring [00228] In one embodiment, p is 0, 1, or 2. [00229] In a further embodiment, p is 1 or 2. [00230] In another further embodiment, the ring is selected from
[00231] In one embodiment, two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more R” substituents. [00232] In a further embodiment, two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C1-6 alkyl, halo, OH, OC1-6 alkyl, oxo, CN, NH2, NH(C1-6 alkyl), and N(C1-6 alkyl)2. [00233] In still a further embodiment, two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C1-6 alkyl, oxo, and NH2. [00234] In one embodiment, the compound of Formula I is a compound of Formula Ib or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein Ring C is a phenyl, a 5-6 membered heteroaryl, or a 5-6 membered heterocyclic ring; and X’ is CH, C-R”, or N. [00235] In one embodiment, Ring C of Formula Ib is a phenyl ring. [00236] In one embodiment, Ring C of Formula Ib is a 5 membered heteroaryl or a 5 membered heterocyclic ring. [00237] In a further embodiment, Ring C of Formula Ib is pyrrole, pyrazole, imidazole, triazole, oxazole, hydrofuran, dihydrofuran, hydropyrrole, dihydropyrrole, hydroimidazole, dihydroimidazole, hydrooxazole, dihydrooxazole, or [00238] In one embodiment, the compound of Formula I is a compound of Formula Ic-1, Formula Ic-2, or Formula Ic-3: or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein each X’ is independently CH or N. [00239] In another embodiment, the ring of Formula Ic-1 is [00240] In another embodiment, the ring of Formula Ic-2 is or [00241] In another embodiment, the ring of Formula Ic-3 is [00242] In a further embodiment, Ring A is a bicyclic group selected from [00243] In one embodiment, m is 0. [00244] In another embodiment, X is N. [00245] In another embodiment, X is C-H or C-CN. [00246] In a further embodiment, X is C-CN. [00247] In another aspect, the disclosure includes a pharmaceutical composition comprising a compound of Formula I, Ia, Ib, Ic-1, Ic-2, or Ic-3, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof described herein and a pharmaceutically acceptable excipient. [00248] In another aspect, the disclosure includes a method of modulating the activity of an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound, salt or a composition described herein. [00249] In another aspect, the disclosure includes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound, salt or a composition described herein. [00250] In one embodiment, the EGFR and/or PI3K mediated disease is a cancer. [00251] In a further embodiment, the cancer is selected from neoplasm, giant and spindle cell carcinoma; small cell carcinoma; papillary carcinoma; squamous cell carcinoma; lymphoepithelial carcinoma; basal cell carcinoma; pilomatrix carcinoma; transitional cell carcinoma; papillary transitional cell carcinoma; adenocarcinoma, gastrinoma, cholangiocarcinoma, hepatocellular carcinoma; combined hepatocellular carcinoma and cholangiocarcinoma, trabecular adenocarcinoma, adenoid cystic carcinoma; adenocarcinoma in adenomatous polyp; adenocarcinoma, familial polyposis coli, solid carcinoma; carcinoid tumor, bronchiolo-alveolar adenocarcinoma, papillary adenocarcinoma, chromophobe carcinoma; acidophil carcinoma; oxyphilic adenocarcinoma, basophil carcinoma; clear cell adenocarcinoma, granular cell carcinoma; follicular adenocarcinoma, papillary and follicular adenocarcinoma, nonencapsulating sclerosing carcinoma; adrenal cortical carcinoma; endometroid carcinoma; skin appendage carcinoma; apocrine adenocarcinoma, sebaceous adenocarcinoma, ceruminous adenocarcinoma, mucoepidermoid carcinoma; cystadenocarcinoma, papillary cystadenocarcinoma, papillary serous cystadenocarcinoma, mucinous cystadenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma; infiltrating duct carcinoma; medullary carcinoma; lobular carcinoma; inflammatory carcinoma; Paget's disease, mammary; acinar cell carcinoma; adenosquamous carcinoma; adenocarcinoma with squamous metaplasia, thymoma, malignant; ovarian stromal tumor, thecoma, granulosa cell tumor, androblastoma, Sertoli cell carcinoma; Leydig cell tumor, lipid cell tumor, paraganglioma, extra-mammary paraganglioma, pheochromocytoma, glomangiosarcoma, amelanotic melanoma; superficial spreading melanoma; melanoma in giant pigmented nevus; epithelioid cell melanoma; blue nevus, sarcoma; fibrosarcoma, fibrous histiocytoma, myxosarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, stromal sarcoma; mixed tumor; Mullerian mixed tumor; nephroblastoma, hepatoblastoma, carcinosarcoma, mesenchymoma, Brenner tumor, phyllodes tumor, synovial sarcoma; mesothelioma, dysgerminoma, embryonal carcinoma; teratoma, struma ovarii, choriocarcinoma, mesonephroma, hemangiosarcoma, hemangioendothelioma, Kaposi's sarcoma; hemangiopericytoma, lymphangiosarcoma, osteosarcoma, juxtacortical osteosarcoma, chondrosarcoma, chondroblastoma, mesenchymal chondrosarcoma, giant cell tumor of bone; Ewing's sarcoma; odontogenic tumor, ameloblastic odontosarcoma, ameloblastoma, ameloblastic fibrosarcoma, pinealoma, chordoma, glioma, ependymoma, astrocytoma, protoplasmic astrocytoma, fibrillary astrocytoma, astroblastoma, glioblastoma, oligodendroglioma, oligodendroblastoma, primitive neuroectodermal, cerebellar sarcoma; ganglioneuroblastoma, neuroblastoma, retinoblastoma, olfactory neurogenic tumor; meningioma, neurofibrosarcoma, neurilemmoma, granular cell tumor, lymphoma; Hodgkin's disease; Hodgkin's; paragranuloma, lymphoma – small lymphocytic, lymphoma – large cell, diffuse; lymphoma, follicular; mycosis fungoides, other specified non-Hodgkin's lymphomas; histiocytosis, multiple myeloma, mast cell sarcoma; immunoproliferative small intestinal disease; leukemia; lymphoid leukemia; plasma cell leukemia; erythroleukemia, lymphosarcoma cell leukemia; myeloid leukemia; basophilic leukemia; eosinophilic leukemia; monocytic leukemia; mast cell leukemia; megakaryoblastic leukemia; myeloid sarcoma; and hairy cell leukemia. [00252] In still a further embodiment, the cancer is selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, (cancers of the ovaries and uterus), thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, breast cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma. [00253] In yet a further embodiment, the cancer is selected from breast cancer, carcinomas of the head and neck, head and neck cancer, and skin cancer. [00254] In one embodiment, the head and neck cancer is squamous head and neck cancer. In another embodiment, the breast cancer is triple negative breast cancer. [00255] In another embodiment, the cancer is a metastatic or a malignant cancer. [00256] In some embodiments, the compound of Formula I is a compound selected from the compounds listed in Table 1, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof. [00257] In one embodiment, a compound or salt described herein has a solubility that is at least 0.5 times more soluble that a compound of Formula X or X’: wherein R2, R3, R4, X, m, n, p, and Ring A are defined as for Formula I, as measured in simulated intestinal fluid pH of 6.8. [00258] In a further embodiment, the compound or salt is at least 2 times more soluble that a compound of Formula X or X’. [00259] In another further embodiment, the compound or salt is at least 5 times more soluble that a compound of Formula X or X’. [00260] In still a further embodiment, the compound or salt is at least 10 times more soluble that a compound of Formula X or X’. [00261] In one embodiment, the compound of Formula X is Comparative Compound 2, and the compound of Formula X’ is Comparative Compound 1 or Comparative Compound 2: [00262] Table 1: Compounds of the disclosure listed by name and structure Docket No.262340-557381 Docket No.262340-557381 Docket No.262340-557381 [00263] In another aspect, the disclosure provides a compound of Formula Ia-1 or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein, each X’ is N, C-R3, or CH; R1a is selected from the group consisting of H or C1-6 alkyl; R1b is selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, halo, CN, and NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with one or more R” substituents; each R2 is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, OC(O)OR’, OC(O)N(R’)2, NR’C(O)N(R’)2, SOR’, SON(R’)2, SO2R’, SO2N(R’)2, NR’SOR’, NR’SON(R’)2, NR’SO2R’, and NR’SO2N(R’)2, wherein the C1-6 alkyl, hetercycloalky, and heteroaryl are each optionally and independently substituted with one or more R” substituents; each R4 is selected from halo, OH, NH2, CN, C1-6 alkyl, and OC1-6 alkyl; each R’ is independently selected from hydrogen, OH, CN, C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R” is independently selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or substituents selected from halo, oxo, alkoxy, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, and C(O)NHC1-6 alkyl; and m, n, and p are each an integer selected from 0-4. [00264] In one embodiment, R1a is H. [00265] In another embodiment, R1b is C1-6 alkyl, C1-6 alkyl-OH, or CN. [00266] In a further embodiment, R1b is methyl, CN, or CH2OH. [00267] In one embodiment, each R2 substituent is independently selected from halo and OH. [00268] In another embodiment, n is 0. [00269] In one embodiment, each R3 substituent is independently selected from halo, CN, NH2, OH, C(O)H, C(O)N(CH3)2, C(O)NH(CH3), C(O)NH(Et), C(O)NH(isopropyl), C(O)NH(tert-butyl), C(O)NH(cyclopropyl), C(O)NCH3(CN), C(O)NH(OH), C(O)NCH3(OH), C(O)NH2, NHC(O)NHCH3, NHS(O)2CH3, S(O)2CH3, methyl, methoxy, NHS(O)2CH2CH2N(CH3)2, CF3, CH2OH, C(CH3)2OH, [00270] In one embodiment, the ring is a phenyl, pyridyl, pyrimidyl, pyrazyl, or triazyl, which is optionally substituted by R3. [00271] In a further embodiment, the ring [00272] In one embodiment, p is 1 or 2. [00273] In one embodiment, the compound of Formula Ia-1 is a compound of Formula Ia- 2 [00274] In another embodiment, the ring of Formula Ia-2 is selected from
[00275] In one embodiment, m is 0. [00276] In another embodiment, the compound of Formula Ia-1 is a compound selected from Compounds 1, 2, 2R, 2S, 3-6, 11, 14-16, 21, 22, 29-33, 36, 37, 45, 107-111, 111R, 111S, 112, 113, 118, 132-135, 138, 141, 142, and 148. [00277] In one aspect, the disclosure includes a pharmaceutical composition comprising a compound or salt described herein, and a pharmaceutically acceptable excipient. [00278] In another aspect, the disclosure includes a method of modulating an EGFR and/or PI3K enzyme in a biological sample, said method comprising contacting the biological sample with a compound or salt described herein. [00279] In still another aspect, the disclosure includes a method of preventing or treating an EGFR and/or PI3K mediated disease in a subject, said method comprising administering to the subject a compound or salt described herein. [00280] In one embodiment of this aspect, the EGFR and/or PI3K mediated disease is a cancer. [00281] In another embodiment, the cancer is selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma. [00282] In a further embodiment, the cancer is a metastatic or a malignant cancer. [00283] In another embodiment, the cancer is selected from breast cancer, carcinomas of the head and neck, head and neck cancer, and skin cancer. [00284] In a further embodiment, the head and neck cancer is squamous head and neck cancer. [00285] In another further embodiment, the breast cancer is triple negative breast cancer. [00286] In another aspect, the disclosure provides a compound of Formula I, wherein the compound of Formula I has an increased solubility compared to a compound of Formula X or a compound of Formula X’ Wherein R1a, R1b, R2, R3, R4, X, m, n, p, and Ring A are all defined herein. [00287] One embodiment of this aspect, R1a is hydrogen and R1b is C1-6 alkyl. In a further embodiment, R1a is hydrogen and R1b is methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, t- butyl, pentyl, or hexyl. In still a further embodiment, R1a is hydrogen and R1b is methyl. [00288] In one embodiment of this aspect, the compound of Formula I is selected from a compound listed in Table 1. In a further embodiment of this aspect, the compound of Formula I is selected from Compound 2, 2R, 2S, 8, 10, 22, 48, 48R, 48S, 111, 111R, and 111S. In a further embodiment of this aspect, the compound of Formula I is selected from Compound 2R, 48R, and 111R. [00289] In one embodiment of this aspect, the compound of Formula X’ is Comparative Compound 1 or Comparative Compound 3, which have the structures below. Comparative Compound 1 Comparative Compound 3 [00290] In another embodiment of this aspect, the compound of Formula X is Comparative Compound 2, which has the structure Comparative Compound 2 [00291] In one embodiment, the compound of Formula I is at least 0.5 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least twice as soluble as a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 5.0 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 10 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 50 times more soluble than a compound of Formula X or a compound of Formula X’. In another embodiment, the compound of Formula I is at least 100 times more soluble than a compound of Formula X or a compound of Formula X’. [00292] In one embodiment, the compound of Formula I has increased solubility over a compound of Formula X or a compound of Formula X’ in phosphate buffered saline (PBS) at about pH 7.4. [00293] In another embodiment, the compound of Formula I has increased solubility over a compound of Formula X or a compound of Formula X’ in simulated intestinal fluid (SIF). [00294] In another embodiment, the compound of Formula I has increased solubility over a compound of Formula X or a compound of Formula X’ in simulated gastric fluid (SGF). [00295] Pharmaceutically acceptable salts, compositions, and formulations [00296] It also will be appreciated that certain of the compounds of the present disclosure can exist in free form for treatment, or where appropriate, as a pharmaceutically acceptable derivative (e.g., a salt) thereof. According to the present disclosure, a pharmaceutically acceptable derivative includes, but is not limited to, pharmaceutically acceptable prodrugs, salts, esters, salts of such esters, or any other adduct or derivative that upon administration to a patient in need is capable of providing, directly or indirectly, a compound as otherwise described herein, or a metabolite or residue thereof. [00297] As used herein, the term "pharmaceutically acceptable salt" refers to those salts that are, within the scope of sound medical judgement, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like. [00298] Pharmaceutically acceptable salts are well known in the art. For example, S. M. Berge et al., describe pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences, 1977, 66, 1-19, incorporated herein by reference. Pharmaceutically acceptable salts of the compounds of this disclosure include those derived from suitable inorganic and organic acids and bases. Examples of pharmaceutically acceptable, nontoxic acid addition salts include salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange. Other pharmaceutically acceptable salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate, persulfate, 3-phenylpropionate, phosphate, picrate, pivalate, propionate, stearate, succinate, sulfate, tartrate, thiocyanate, p-toluenesulfonate, undecanoate, valerate salts, and the like. Salts derived from appropriate bases include alkali metal, alkaline earth metal, ammonium and N+(C1-4alkyl)4 salts. This disclosure also envisions the quaternization of any basic nitrogen-containing groups of the compounds disclosed herein. Water or oil-soluble or dispersable products may be obtained by such quaternization. Representative alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like. Further pharmaceutically acceptable salts include, when appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed using counterions such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, lower alkyl sulfonate and aryl sulfonate. [00299] A compound of Formula I, or a pharmaceutically acceptable salt thereof, can be formulated as pharmaceutical compositions comprising a therapeutically or prophylactically effective amount of the compound or salt, and one or more pharmaceutically compatible (acceptable) ingredients. In some aspects, pharmaceutical compositions of a compound of Formula I, or a pharmaceutically acceptable salt thereof, and pharmaceutical excipients are provided in which an effective amount of the compound or salt, is in admixture with the excipients, suitable for administration to a mammal. In preferred aspects, a compound of Formula I, or a pharmaceutically acceptable salt thereof, is formulated for administration to a human. The present disclosure provides a pharmaceutical composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, formulated for administration to a human subject in need thereof. The formulated composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, will generally comprise one or more pharmaceutically compatible (acceptable) ingredients. [00300] Exemplary pharmaceutical or non-pharmaceutical compositions typically include one or more carriers (e.g., sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like). Water is a more typical carrier when the pharmaceutical composition is administered intravenously. Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions. Suitable excipients include, for example, amino acids, starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol, and the like. The composition, if desired, can also contain minor amounts of wetting or emulsifying agents, or pH buffering agents. These compositions can take the form of solutions, suspensions, emulsion, tablets, pills, capsules, powders, sustained-release formulations and the like. Examples of suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences" by E. W. Martin. Such compositions will typically contain a therapeutically effective amount of a compound of Formula I, or a pharmaceutically acceptable salt thereof, in purified form, together with a suitable amount of carrier so as to provide the form for proper administration to the subject. The formulations correspond to the mode of administration. [00301] The pharmaceutically acceptable carrier or vehicle can be particulate, so that the compositions are, for example, in tablet or powder form. The carrier(s) can be liquid, with the compositions being, for example, an oral syrup, flavored water, or injectable liquid. [00302] When intended for oral administration, the composition is preferably in solid or liquid form, where semi-solid, semi-liquid, suspension and gel forms are included within the forms considered herein as either solid or liquid. [00303] As a solid composition for oral administration, the composition can be formulated into a powder, granule, compressed tablet, pill, capsule, chewing gum, wafer or the like form. Such a solid composition typically contains one or more inert diluents. In addition, one or more of the following can be present: binders such as carboxymethylcellulose, ethyl cellulose, microcrystalline cellulose, or gelatin; excipients such as starch, lactose or dextrins, disintegrating agents such as alginic acid, sodium alginate, Primogel, corn starch and the like; lubricants such as magnesium stearate or Sterotex; glidants such as colloidal silicon dioxide; sweetening agents such as sucrose or saccharin, a flavoring agent such as peppermint, methyl salicylate or orange flavoring, and a coloring agent. [00304] When the composition is in the form of a capsule, e.g., a gelatin capsule, it can contain, in addition to materials of the above type, a liquid carrier such as polyethylene glycol, cyclodextrin or fatty oil. [00305] The composition can be in the form of a liquid, e.g., an elixir, syrup, solution, emulsion or suspension. The liquid can be useful for oral administration or for delivery by injection. When intended for oral administration, a composition can comprise one or more of a sweetening agent, preservatives, dye/colorant, and flavor enhancer. In some aspects, the composition is formulated into a powder and the end user mixes the power in aqueous solution for oral administration. In a composition for administration by injection (as described above), one or more of a surfactant, preservative, wetting agent, dispersing agent, suspending agent, buffer, stabilizer and isotonic agent can also be included. [00306] The composition and preparation of capsules are well known in the art. For example, capsules may be prepared from gelatin (e.g., Type A, Type B), carrageenan (e.g., kappa, iota, lambda) and/or modified cellulose (e.g., hydroxypropyl methyl cellulose, methyl cellulose, hydroxypropyl methyl cellulose acetate succinate, hydroxypropyl methyl cellulose phthalate, cellulose acetate phthalate), and optionally one or more excipients such as oils (e.g., fish oil, olive oil, corn oil, soybean oil, coconut oil, tri-, di- and monoglycerides), plasticizers (e.g., glycerol, glycerin, sorbitol, polyethylene glycol, citric acid, citric acid esters such as triethylcitrate, polyalcohols), co-solvents (e.g., triacetin, propylene carbonate, ethyl lactate, propylene glycol, oleic acid, dimethylisosorbide, stearyl alcohol, cetyl alcohol, cetostearyl alcohol, glyceryl behenate, glyceryl palmitostearate), surfactants, buffering agents, lubricating agents, humectants, preservatives, colorants and flavorants. Capsules may be hard or soft. Examples of hard capsules include ConiSnap®, DRcaps®, OceanCaps®, Pearlcaps®, Plantcaps®, DUOCAP®, Vcaps®, and Vcaps®. Plus capsules available from Capsugel®. Hard capsules may be prepared, for example, by forming two telescoping capsule halves, filling one of the halves with a fill comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, and sealing the capsule halves together. The fill may be in any suitable form, such as dry powder, granulation, suspension or liquid. Examples of soft capsules include soft gelatin (also called softgel or soft elastic) capsules, such as SGcaps®. Soft capsules may be prepared, for example, by rotary die, plate, reciprocating die or Accogel® machine method. In embodiments, the capsule may be a liquid-filled hard capsule or a soft-gelatin capsule. [00307] Tablets can be made by compression or molding, optionally with one or more accessory ingredients. Compressed tablets can be prepared by compressing in a suitable machine a compound of formula (I) or pharmaceutically acceptable salt thereof in a free-flowing form such as a powder or granules, optionally mixed with a binder, lubricant, inert diluent, preservative, surface-active or dispersing agent. Molded tablets can be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent. The tablets can be optionally coated or scored and can be formulated so as to provide sustained, extended, delayed or controlled release. Methods of formulating such sustained, extended, delayed or controlled release compositions are known in the art and disclosed in issued U.S. patents, including but not limited to U.S. Pat. Nos.4,369,174, 4,842,866, and the references cited therein. Coatings, for example enteric coatings, can be used for delivery of compounds to the intestine (see, e.g., U.S. Pat. Nos.6,638,534, 5,217,720, 6,569,457, and the references cited therein). In addition to tablets, other dosage forms, such as capsules, granulations and gel-caps, can be formulated to provide sustained, extended, delayed or controlled release. [00308] In one embodiment, the pharmaceutical composition is formulated for parenteral administration. Examples of a pharmaceutical composition suitable for parenteral administration include aqueous sterile injection solutions and non-aqueous sterile injection solutions, each containing, for example, anti-oxidants, buffers, bacteriostatic agents and/or solutes that render the formulation isotonic with the blood of the intended recipient; and aqueous sterile suspensions and non-aqueous sterile suspensions, each containing, for example, suspending agents and/or thickening agents. The formulations can be presented in unit-dose or multi-dose containers, for example, sealed ampules or vials, and can be stored in a freeze dried (lyophilized) condition requiting only the addition of a sterile liquid carrier, such as water, immediately prior to use. In one embodiment, the pharmaceutical composition is formulated for intravenous administration. [00309] In some embodiments, the pharmaceutical composition further includes a pharmaceutically acceptable excipient. A pharmaceutically acceptable excipient may be any substance, not itself a therapeutic agent, used as a carrier, diluent, adjuvant, binder, and/or vehicle for delivery of a therapeutic agent to a patient, or added to a pharmaceutical composition to improve its handling or storage properties or to permit or facilitate formation of a compound or pharmaceutical composition into a unit dosage form for administration. Pharmaceutically acceptable excipients are known in the pharmaceutical arts and are disclosed, for example, in Remington: The Science and Practice of Pharmacy, 21.sup.st Ed. (Lippincott Williams & Wilkins, Baltimore, Md., 2005). As will be known to those in the art, pharmaceutically acceptable excipients can provide a variety of functions and can be described as wetting agents, buffering agents, suspending agents, lubricating agents, emulsifiers, disintegrants, absorbents, preservatives, surfactants, colorants, flavorants, and sweeteners. Examples of pharmaceutically acceptable excipients include without limitation: (1) sugars, such as lactose, glucose and sucrose; (2) starches, such as corn starch and potato starch; (3) cellulose and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose, cellulose acetate, hydroxypropyl methylcellulose, and hydroxypropylcellulose; (4) powdered tragacanth; (5) malt; (6) gelatin; (7) talc; (8) excipients, such as cocoa butter and suppository waxes; (9) oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10) glycols, such as propylene glycol; (11) polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; (12) esters, such as ethyl oleate and ethyl laurate; (13) agar; (14) buffering agents, such as magnesium hydroxide and aluminum hydroxide; (15) alginic acid; (16) pyrogen-free water; (17) isotonic saline; (18) Ringer's solution; (19) ethyl alcohol; (20) pH buffered solutions; (21) polyesters, polycarbonates and/or polyanhydrides; and (22) other non-toxic compatible substances employed in pharmaceutical formulations. [00310] Materials used in preparing the pharmaceutical compositions can be non-toxic in the amounts used. It will be evident to those of ordinary skill in the art that the optimal dosage of the active ingredient(s) in the pharmaceutical composition will depend on a variety of factors. Relevant factors include, without limitation, the type of animal (e.g., human), the particular form of a compound of Formula I, or a pharmaceutically acceptable salt thereof, the manner of administration, the composition employed, and the severity of the disease or condition being treated. [00311] In addition to administering the compound as a raw chemical, the compounds of the disclosure may be administered as part of a pharmaceutical preparation containing suitable pharmaceutically acceptable carriers comprising excipients and auxiliaries which facilitate processing of the compounds into preparations which can be used pharmaceutically. The preparations, particularly those preparations which can be administered orally or topically and which can be used for one type of administration, such as tablets, dragees, slow release lozenges and capsules, mouth rinses and mouth washes, gels, liquid suspensions, hair rinses, hair gels, shampoos and also preparations which can be administered rectally, such as suppositories, as well as suitable solutions for administration by intravenous infusion, injection, topically or orally, contain from about 0.01 to 99 percent, in one embodiment from about 0.25 to 75 percent of active compound(s), together with the excipient. [00312] The pharmaceutical compositions of the disclosure may be administered to any patient which may experience the beneficial effects of the compounds of the disclosure. Foremost among such patients are mammals, e.g., humans, although the disclosure is not intended to be so limited. Other patients include veterinary animals (cows, sheep, pigs, horses, dogs, cats and the like). [00313] The compounds and pharmaceutical compositions thereof may be administered by any means that achieve their intended purpose. For example, administration may be by parenteral, subcutaneous, intravenous, intramuscular, intraperitoneal, transdermal, buccal, intrathecal, intracranial, intranasal or topical routes. Alternatively, or concurrently, administration may be by the oral route. The dosage administered will be dependent upon the age, health, and weight of the recipient, kind of concurrent treatment, if any, frequency of treatment, and the nature of the effect desired. [00314] The pharmaceutical preparations of the present disclosure are manufactured in a manner which is itself known, for example, by means of conventional mixing, granulating, dragee-making, dissolving, or lyophilizing processes. Thus, pharmaceutical preparations for oral use can be obtained by combining the active compounds with solid excipients, optionally grinding the resulting mixture and processing the mixture of granules, after adding suitable auxiliaries, if desired or necessary, to obtain tablets or dragee cores. [00315] Suitable excipients are, in particular, fillers such as saccharides, for example lactose or sucrose, mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example tricalcium phosphate or calcium hydrogen phosphate, as well as binders such as starch paste, using, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, tragacanth, methyl cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, and/or polyvinyl pyrrolidone. If desired, disintegrating agents may be added such as the above- mentioned starches and also carboxymethyl-starch, cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof, such as sodium alginate. Auxiliaries are, above all, flow-regulating agents and lubricants, for example, silica, talc, stearic acid or salts thereof, such as magnesium stearate or calcium stearate, and/or polyethylene glycol. Dragee cores are provided with suitable coatings which, if desired, are resistant to gastric juices. For this purpose, concentrated saccharide solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, polyethylene glycol and/or titanium dioxide, lacquer solutions and suitable organic solvents or solvent mixtures. In order to produce coatings resistant to gastric juices, solutions of suitable cellulose preparations such as acetylcellulose phthalate or hydroxypropylmethyl- cellulose phthalate, are used. Dye stuffs or pigments may be added to the tablets or dragee coatings, for example, for identification or in order to characterize combinations of active compound doses. [00316] Other pharmaceutical preparations which can be used orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer such as glycerol or sorbitol. The push-fit capsules can contain the active compounds in the form of granules which may be mixed with fillers such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the active compounds are in one embodiment dissolved or suspended in suitable liquids, such as fatty oils, or liquid paraffin. In addition, stabilizers may be added. [00317] Possible pharmaceutical preparations which can be used rectally include, for example, suppositories, which consist of a combination of one or more of the active compounds with a suppository base. Suitable suppository bases are, for example, natural or synthetic triglycerides, or paraffin hydrocarbons. In addition, it is also possible to use gelatin rectal capsules which consist of a combination of the active compounds with a base. Possible base materials include, for example, liquid triglycerides, polyethylene glycols, or paraffin hydrocarbons. [00318] Suitable formulations for parenteral administration include aqueous solutions of the active compounds in water-soluble form, for example, water-soluble salts and alkaline solutions. In addition, suspensions of the active compounds as appropriate oily injection suspensions may be administered. Suitable lipophilic solvents or vehicles include fatty oils, for example, sesame oil, or synthetic fatty acid esters, for example, ethyl oleate or triglycerides or polyethylene glycol-400. Aqueous injection suspensions may contain substances which increase the viscosity of the suspension include, for example, sodium carboxymethyl cellulose, sorbitol, and/or dextran. Optionally, the suspension may also contain stabilizers. [00319] The topical compositions of this disclosure are formulated in one embodiment as oils, creams, lotions, ointments and the like by choice of appropriate carriers. Suitable carriers include vegetable or mineral oils, white petrolatum (white soft paraffin), branched chain fats or oils, animal fats and high molecular weight alcohol (greater than C12). The carriers may be those in which the active ingredient is soluble. Emulsifiers, stabilizers, humectants and antioxidants may also be included as well as agents imparting color or fragrance, if desired. Additionally, transdermal penetration enhancers can be employed in these topical formulations. Examples of such enhancers can be found in U.S. Pat. Nos.3,989,816 and 4,444,762; each herein incorporated by reference in its entirety. [00320] Ointments may be formulated by mixing a solution of the active ingredient in a vegetable oil such as almond oil with warm soft paraffin and allowing the mixture to cool. A typical example of such an ointment is one which includes about 30% almond oil and about 70% white soft paraffin by weight. Lotions may be conveniently prepared by dissolving the active ingredient, in a suitable high molecular weight alcohol such as propylene glycol or polyethylene glycol. [00321] One of ordinary skill in the art will readily recognize that the foregoing represents merely a detailed description of certain preferred embodiments of the present disclosure. Various modifications and alterations of the compositions and methods described above can readily be achieved using expertise available in the art and are within the scope of the disclosure. [00322] Methods of treatment [00323] In some embodiments of the present disclosure, the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, can be employed under a variety of conditions and therapeutic uses to treat a variety of diseases or conditions related to the aberrant expression of PI3K and/or EGFR activity, including cancer. [00324] In some embodiments, the compound of Formula I, a compound of Table 1, for example, Compound 2R, or a pharmaceutically acceptable salt thereof, or a combination comprising a compound of Formula I, a compound of Table 1, for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, NRAS, panRAS, MAPK, ALK, BRAF, or MEK inhibitor, is administered to a subject with cancer, and whrein the subject also has a disease or condition associated with diabetes type I or II, or impaired glucose metabolism, or hyperglycemia, or insulin resistance, or is overweight, or is obese, or has any form of metabolic syndrome associated with defective glucose utilization and/or glucose metabolism, or requires medication to provide glycemic control. When administered to such a subject, the administration of the compound of Formula I, Compound 2R, or a pharmaceutically acceptable salt thereof, or a combination disclosed herein, the subject does not significantly elevate any one of: blood glucose, blood insulin or hyperglycemia in the subject, after one or more administrations of the compound or combination. [00325] In some embodiments of the present disclosure, the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, or Compound 2R or a salt thereof, can be employed under a variety of conditions and therapeutic uses to treat a variety of diseases or conditions related to the aberrant expression of PI3K and/or EGFR activity, for example, cancer, or the treatment of diseases associated with a tumor, cancer or a neoplastic growth. Exemplary cancers that can be treated with the compounds of Formula I, for example, Compound 2R are illustrated herein. In some embodiments, the subject with cancer, or likely to develop cancer may be a subject that does not metabolize glucose normally, for example may have a metabolic disease that is characterized by hyperglycemia caused by insulin resistance. In some embodiments, the subject to be treated is one that may respond to inhibition of PI3K and/or EGFR and which also has diabetes type I or diabetes type II. The subject to be treated, may also include subjects that have cancer that is sensitive to a PI3K and/or EGFR inhibitor and also has dyslipidaemia, obesity and/or hepatic steatosis associated with insulin resistance and diabetes mellitus. The subject to be treated, may also include subjects that have cancer that is sensitive to a PI3K and/or EGFR inhibitor and also has hyperglycemia, insulin intolerance and/or elevated glucose levels above normal healthy levels when tested after fasting, for example the subject may have diabetes, and/or obesity, and/or a condition associated with improper glucose metabolism, for example, glucose tolerance disorder, diabetes mellitus (especially Type II diabetes mellitus, obesity diabetes mellitus), abnormal lipid metabolism (synonymous with hyperlipidemia above), hypertension, heart failure, hyperuricemia, fatty liver (including non- alcoholic hepatitis), coronary heart disease (myocardial infarction, angina pectoris), cerebral infarction (cerebral thrombosis, transient ischemic attack), bone/joint disease (knee osteoarthritis, hip osteoarthritis, deformed spondylitis, low back pain), sleep apnea syndrome/Pickwick syndrome, menstrual disorders (abnormal menstrual cycle, abnormal menstruation and cycle, amenorrhea, abnormal menstrual symptoms), metabolic syndrome, and the like. [00326] In some embodiments, the subject with cancer, is a subject with diabetes type 1, diabetes type 2, diabetic retinopathy, metabolic syndrome, insulin resistance, pancreatic dysfunction, and wherein administration of the compound or pharmaceutically acceptable salt thereof of Formula I, does not significantly elevate blood glucose or cause hyperglycemia in the subject, after one or more administrations of the compound or pharmaceutically acceptable salt thereof, wherein hyperglycemia is defined as a plasma or whole blood glucose measurement greater than 125 mg/dL while fasting and greater than 200 mg/dL, 2 hours postprandial, during a 75-g oral glucose tolerance test (OGTT) using a blood glucose meter employing strips comprising glucose oxidase, hexokinase, or combinations thereof. [00327] The exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular agent, its mode of administration, and the like. The compounds of the disclosure are preferably formulated in dosage unit form for ease of administration and uniformity of dosage. The expression “dosage unit form” as used herein refers to a physically discrete unit of agent appropriate for the patient to be treated. It will be understood, however, that the total daily usage of the compounds and compositions of the present disclosure will be decided by the attending physician within the scope of sound medical judgment. The specific effective dose level for any particular patient or organism will depend upon a variety of factors including the disorder being treated and the severity of the disorder; the activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed, and like factors well known in the medical arts. The term “patient”, as used herein, means an animal, preferably a mammal, and most preferably a human. The term "patient" and "subject" are used interchangeably. [00328] The pharmaceutically acceptable compositions of this disclosure can be administered to humans and other animals orally, rectally, parenterally, intracisternally, intravaginally, intraperitoneally, topically (as by powders, ointments, drops or patch), bucally, as an oral or nasal spray, or the like, depending on the severity of the disease, for example, a cancer, being treated. [00329] In an aspect, a disclosed method of inhibiting replication of cancer cells in a subject can comprise repeating the administration of the compounds of the present disclosure or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure . In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure can be administered systemically to the subject. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, can be administered to the subject at least two times. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure can be administered to the subject two or more times. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure can be administered at routine or regular intervals. For example, in an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure can be administered to the subject one time per day, or two times per day, or three or more times per day. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure can be administered to the subject daily, or one time per week, or two times per week, or three or more times per week, etc. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, can be administered to the subject weekly, or every other week, or every third week, or every fourth week, etc. In an aspect, a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, can be administered to the subject monthly, or every other month, or every third month, or every fourth month, etc. In an aspect, the repeated administration of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure occurs over a pre-determined or definite duration of time. In an aspect, the repeated administration of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure or a composition comprising an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure, occurs over an indefinite period of time. [00330] In certain embodiments, the compounds or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure may be administered orally or parenterally to the subject in need thereof, at therapeutically effective amounts or prophylactically effective amounts, of about wherein the therapeutically effective amount of the compound according to Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is selected from the group consisting of 0.01 mg/kg to about 1,000 mg/kg, 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 20 mg/kg, 0.05 mg/kg to 10 mg/kg, 0.1 mg/kg to 75 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 50 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 20 mg/kg, 0.1 mg/kg to 10 mg/kg, 0.5 mg/kg to 150 mg/kg, 0.5 mg/kg to 100 mg/kg, 0.5 mg/kg to 90 mg/kg, 0.5 mg/kg to 80 mg/kg, 0.5 mg/kg to 70 mg/kg, 0.5 mg/kg to 60 mg/kg, 0.5 mg/kg to 50 mg/kg, 0.5 mg/kg to 40 mg/kg, 0.5 mg/kg to 30 mg/kg, 0.5 mg/kg to 20 mg/kg, 0.5 mg/kg to 10 mg/kg, 1 mg/kg to 500 mg/kg, 1 mg/kg to 400 mg/kg, 1 mg/kg to 300 mg/kg, 1 mg/kg to 200 mg/kg, 1 mg/kg to 100 mg/kg, 1 mg/kg to 90 mg/kg, 1 mg/kg to 80 mg/kg, 1 mg/kg to 70 mg/kg, 1 mg/kg to 60 mg/kg, 1 mg/kg to 50 mg/kg, 1 mg/kg to 40 mg/kg, 1 mg/kg to 30 mg/kg, 1 mg/kg to 20 mg/kg, 1 mg/kg to 10 mg/kg, 5 mg/kg to 75 mg/kg, 5 mg/kg to 70 mg/kg, 5 mg/kg to 60 mg/kg, 5 mg/kg to 50 mg/kg, 5 mg/kg to 40 mg/kg, 5 mg/kg to 30 mg/kg, 5 mg/kg to 20 mg/kg, and 5 mg/kg to 10 mg/kg body weight of the recipient per day as a daily dose. Therapeutically effective daily doses range from 1 mg to about 1,000 mg, and all integers and values therebetween. Therapeutically effective weekly doses range from about 7mg to about 7,000 mg, and all integers and values therebetween. [00331] In related embodiments, the method of treating a cancer, or an advanced cancer, or a metastatic cancer comprises oral or parenteral administration of a compound according to Formula I, for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient, wherein the daily dose ranges from about 1 mg per day to about 1,000 mg per day, and all values and integers therebetween, dosed according to a daily dosing regimen or intermittently. In some embodiments, the daily dose is administered as fractionated oral or parenteral dosages twice or three, or four times per day. In various embodiments, daily doses or unit doses may include therapeutically effective doses. In the various methods described herein, in some embodiments, the subject to be treated has a cancer that can be treated with the compounds of the present disclosure, and also have impaired glucose metabolism, such as hyperglycemia, and/or insulin resistance, and/or is overweight, and/or is obese, and/or or has any form of metabolic syndrome associated with defective glucose utilization and glucose metabolism, and/or requires medication to provide glycemic control [00332] While treatment regimen and therapeutically effective doses may vary according to the subject’s age, weight, health condition, severity of disease and past treatments administered to treat the cancer, or an advanced cancer, or a metastatic cancer, in some embodiments, the daily dose is administered 1, 2, 3, or 4 times per 28-day treatment cycle, on an intermittent schedule for 3 weeks on and one week off. Alternatively, the daily dose is administered on day 1, 8, and 15 of a 28-day treatment cycle. [00333] In various embodiments, wherein treatment of the subject with cancer in need thereof, the daily dose or therapeutic doses may be administered as multiple cycles of treatment which may be administered for a time period sufficient to effect at least a partial tumor response. In some related embodiments, the patient is administered a daily dose for a time period of 28 day cycles, until the patient no longer shows therapeutic benefit or clinical signs of toxicity preclude continuation of therapy. Some illustrative time periods may include one or more of the following: wherein the time period is at least 6 months; wherein the time period is at least 12 months; wherein the time period is sufficient to effect a complete tumor response is effected in the patient. In some illustrative treatment regimens, the patient is administered a treatment cycle comprising administering a daily dose of a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient for 21 days followed by no administration for seven days, wherein the patient is administered one to twelve treatment cycles per year. [00334] In certain embodiments, the compounds of Formula (I), for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure may be administered orally or parenterally to the subject in need thereof, at therapeutically effective amounts or prophylactically effective amounts, of about 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 20 mg/kg, 0.05 mg/kg to 10 mg/kg, 0.1 mg/kg to 75 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 50 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 20 mg/kg, 0.1 mg/kg to 10 mg/kg, 0.5 mg/kg to 150 mg/kg, 0.5 mg/kg to 100 mg/kg, 0.5 mg/kg to 90 mg/kg, 0.5 mg/kg to 80 mg/kg, 0.5 mg/kg to 70 mg/kg, 0.5 mg/kg to 60 mg/kg, 0.5 mg/kg to 50 mg/kg, 0.5 mg/kg to 40 mg/kg, 0.5 mg/kg to 30 mg/kg, 0.5 mg/kg to 20 mg/kg, 0.5 mg/kg to 10 mg/kg, 1 mg/kg to 500 mg/kg, 1 mg/kg to 400 mg/kg, 1 mg/kg to 300 mg/kg, 1 mg/kg to 200 mg/kg, 1 mg/kg to 100 mg/kg, 1 mg/kg to 90 mg/kg, 1 mg/kg to 80 mg/kg, 1 mg/kg to 70 mg/kg, 1 mg/kg to 60 mg/kg, 1 mg/kg to 50 mg/kg, 1 mg/kg to 40 mg/kg, 1 mg/kg to 30 mg/kg, 1 mg/kg to 20 mg/kg, 1 mg/kg to 10 mg/kg, 5 mg/kg to 75 mg/kg, 5 mg/kg to 70 mg/kg, 5 mg/kg to 60 mg/kg, 5 mg/kg to 50 mg/kg, 5 mg/kg to 40 mg/kg, 5 mg/kg to 30 mg/kg, 5 mg/kg to 20 mg/kg, and 5 mg/kg to 10 mg/kg body weight of the recipient per day, one or more times a day, or one or more times per week, or one or more times per month, or one or more times per year, to obtain the desired therapeutic effect. [00335] The pharmaceutical compositions of the present disclosure can be tested in clinical studies. Suitable clinical studies can be, for example, open label, dose escalation studies in patients with cancer. Such studies prove in particular the synergism of the active ingredients of the combination of the invention. The beneficial effects on cancer can be determined directly through the results of these studies which are known as such to a person skilled in the art. Such studies can be, in particular, suitable to compare the effects of a monotherapy using the active ingredients and a combination of the invention. In various embodiments, the dose of a compound or pharmaceutically acceptable salt thereof of Formula I, is escalated until the Maximum Tolerated Dosage is reached. [00336] In various embodiments, a compound of Formula (I), for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure is administered in a fixed dose and the dose of a secondary active agent is escalated. In other embodiments, the dose of a secondary active agent is a fixed dose, and the dose of a compound of Formula (I), for example, Compound 2R, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of the disclosure is escalated. Each patient can receive doses of the compounds either daily or intermittently. The efficacy of the treatment can be determined in such studies, e.g., after 12, 18 or 24 weeks of treatment, by evaluation of symptom scores every 4 to 6 weeks. It is envisioned that the administration of a combination therapy of the present disclosure will result not only in a beneficial effect, e.g. a synergistic therapeutic effect, e.g. with regard to alleviating, delaying progression of or inhibiting the symptoms, but also in further surprising beneficial effects, e.g. fewer side-effects, an improved quality of life or a decreased morbidity, compared with a monotherapy applying only one of the pharmaceutically active ingredients used in the combination of the invention. A further benefit can be that lower and/or less frequent doses of one or both of the active ingredients of the combination of the invention can be used, which can diminish the incidence or severity of side- effects. This is in accordance with the desires and requirements of the patients to be treated. [00337] Dosage amounts and dosing regimens for the pharmaceutical compositions of the present disclosure can be adjusted to provide the optimum desired response (e.g., a therapeutic response). For example, a single bolus can be administered, or several divided doses (multiple or repeat or maintenance) can be administered over time and the dose can be proportionally reduced or increased as indicated by the exigencies of the therapeutic situation. It is especially advantageous to formulate parenteral compositions in dosage unit form for ease of administration and uniformity of dosage. Dosage unit form as used herein refers to physically discrete units suited as unitary dosages for the mammalian patients to be treated; each unit containing a predetermined quantity of active compound calculated to produce the desired therapeutic effect in association with the required pharmaceutical carrier. The specification for the dosage unit forms of the present disclosure will be dictated primarily by the unique characteristics of the of the compounds of Formula I, as it pertains to their dual inhibitory action on pan-PI3K and/mTOR and EGFR targets, and the particular therapeutic effect to be achieved. [00338] Thus, the skilled artisan would appreciate, based upon the disclosures provided herein, that the dose and dosing regimen is adjusted in accordance with methods well-known in the therapeutic arts as it pertains to oncotherapy. That is, the maximum tolerable dose can be readily established, and the effective amount providing a detectable therapeutic benefit to a patient may also be determined, as can the temporal requirements for administering each agent to provide a detectable therapeutic benefit to the patient. Accordingly, while various dose and administration regimens are exemplified herein, these examples in no way limit the dose and administration regimen that may be provided to a patient in practicing the present invention. [00339] It is to be noted that dosage values may vary with the type and severity of the condition to be alleviated, and may include single or multiple doses. It is to be further understood that for any particular subject, specific dosage regimens should be adjusted over time according to the patient need and the professional judgment of the person administering or supervising the administration of the compositions, and that dosage ranges set forth herein are exemplary only and are not intended to limit the scope or practice of the claimed composition. Further, the dosage regimen with the compositions of this invention may be based on a variety of factors, including the type of disease, the age, weight, sex, medical condition of the patient, the severity of the condition, the route of administration, and the particular compound or mixtures of compounds disclosed herein, and in some embodiments, combinations of the compounds disclosed herein in combination with a secondary anti -cancer agents employed. Thus, the dosage regimen can vary widely, but can be determined routinely using standard methods. For example, doses may be adjusted based on pharmacokinetic or pharmacodynamic parameters, which may include clinical effects such as toxic effects and/or laboratory values. Thus, the present invention encompasses intra-patient dose-escalation as determined by the skilled artisan. Determining appropriate dosages and regimens are well-known in the relevant art and would be understood to be encompassed by the skilled artisan once provided the teachings disclosed herein. [00340] Liquid dosage forms for oral administration include, but are not limited to, pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In addition to the active compounds, the liquid dosage forms may contain inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof. Besides inert diluents, the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents. [00341] Injectable preparations, for example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents. The sterile injectable preparation may also be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that may be employed are water, Ringer’s solution, U.S.P. and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose any bland fixed oil can be employed including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid are used in the preparation of injectables. [00342] The injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use. [00343] In order to prolong the effect of a compound of the present disclosure, it is often desirable to slow the absorption of the compound from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material with poor water solubility. The rate of absorption of the compound then depends upon its rate of dissolution that, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally administered compound form is accomplished by dissolving or suspending the compound in an oil vehicle. Injectable depot forms are made by forming microencapsule matrices of the compound in biodegradable polymers such as polylactide- polyglycolide. Depending upon the ratio of compound to polymer and the nature of the particular polymer employed, the rate of compound release can be controlled. Examples of other biodegradable polymers include poly(orthoesters) and poly(anhydrides). Depot injectable formulations are also prepared by entrapping the compound in liposomes or microemulsions that are compatible with body tissues. [00344] Compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this disclosure with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound. [00345] Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules. In such solid dosage forms, the active compound is mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar--agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cetyl alcohol and glycerol monostearate, h) absorbents such as kaolin and bentonite clay, and i) lubricants such as talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, and mixtures thereof. In the case of capsules, tablets and pills, the dosage form may also comprise buffering agents. [00346] Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like. The solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions that can be used include polymeric substances and waxes. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polethylene glycols and the like. [00347] The active compounds can also be in microencapsulated form with one or more excipients as noted above. The solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings, release controlling coatings and other coatings well known in the pharmaceutical formulating art. In such solid dosage forms the active compound may be admixed with at least one inert diluent such as sucrose, lactose or starch. Such dosage forms may also comprise, as is normal practice, additional substances other than inert diluents, e.g., tableting lubricants and other tableting aids such a magnesium stearate and microcrystalline cellulose. In the case of capsules, tablets and pills, the dosage forms may also comprise buffering agents. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions that can be used include polymeric substances and waxes. [00348] Dosage forms for topical or transdermal administration of a compound of this disclosure include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants or patches. The active component is admixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives or buffers as may be required. Ophthalmic formulation, eardrops, and eye drops are also contemplated as being within the scope of this disclosure. Additionally, the present disclosure contemplates the use of transdermal patches, which have the added advantage of providing controlled delivery of a compound to the body. Such dosage forms are prepared by dissolving or dispensing the compound in the proper medium. Absorption enhancers can also be used to increase the flux of the compound across the skin. The rate can be controlled by either providing a rate controlling membrane or by dispersing the compound in a polymer matrix or gel. [00349] The present disclosure may be used to treat a neoplastic disease, such as solid or non-solid cancers. As used herein, "treatment" encompasses the reduction, control and/or inhibition of a neoplastic disease. Such diseases include a sarcoma, carcinoma, adenocarcinoma, melanoma, myeloma, blastoma, glioma, lymphoma or leukemia. Exemplary cancers include, for example, carcinoma, sarcoma, adenocarcinoma, melanoma, neural (blastoma, glioma), mesothelioma and reticuloendothelial, lymphatic or hematopoietic neoplastic disorders (e.g., myeloma, lymphoma or leukemia). In particular aspects, a neoplasm, tumor or cancer includes a lung adenocarcinoma, lung carcinoma, diffuse or interstitial gastric carcinoma, colorectal, adenocarcinoma, prostate adenocarcinoma, esophagus carcinoma, breast carcinoma, for example, triple negative breast cancer, pancreatic cancer, e.g. pancreatic adenocarcinoma, carcinomas of the cervix, ovarian adenocarcinoma, adenocarcinoma of the adrenal gland, adenocarcinoma of the endometrium or uterine adenocarcinoma, and carcinomas of the head and neck. [00350] Neoplasia, tumors and cancers include benign, malignant, metastatic and non- metastatic types, and include any stage (I, II, III, IV or V) or grade (G1, G2, G3, etc.) of neoplasia, tumor, or cancer, or a neoplasia, tumor, cancer or metastasis that is progressing, worsening, stabilized or in remission. Cancers that may be treated according to the disclosure include but are not limited to cells or neoplasms of the bladder, blood, bone, bone marrow, brain, breast, colon, esophagus, gastrointestinal system, gum, head, kidney, liver, lung, nasopharynx, neck, ovary, prostate, skin, stomach, testis, tongue, or uterus. In addition, the cancer may specifically be of the following histological type, though it is not limited to the following: neoplasm, malignant; carcinoma; carcinoma, undifferentiated; giant and spindle cell carcinoma; small cell carcinoma; papillary carcinoma; squamous cell carcinoma; lymphoepithelial carcinoma; basal cell carcinoma; pilomatrix carcinoma; transitional cell carcinoma; papillary transitional cell carcinoma; adenocarcinoma, gastrinoma, malignant; cholangiocarcinoma, hepatocellular carcinoma; combined hepatocellular carcinoma and cholangiocarcinoma, trabecular adenocarcinoma, adenoid cystic carcinoma; adenocarcinoma in adenomatous polyp; adenocarcinoma, familial polyposis coli, solid carcinoma; carcinoid tumor, malignant; bronchiolo-alveolar adenocarcinoma, papillary adenocarcinoma, chromophobe carcinoma; acidophil carcinoma; oxyphilic adenocarcinoma, basophil carcinoma; clear cell adenocarcinoma, granular cell carcinoma; follicular adenocarcinoma, papillary and follicular adenocarcinoma, nonencapsulating sclerosing carcinoma; adrenal cortical carcinoma; endometroid carcinoma; skin appendage carcinoma; apocrine adenocarcinoma, sebaceous adenocarcinoma, ceruminous adenocarcinoma, mucoepidermoid carcinoma; cystadenocarcinoma, papillary cystadenocarcinoma, papillary serous cystadenocarcinoma, mucinous cystadenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma; infiltrating duct carcinoma; medullary carcinoma; lobular carcinoma; inflammatory carcinoma; Paget's disease, mammary; acinar cell carcinoma; adenosquamous carcinoma; adenocarcinoma with squamous metaplasia, thymoma, malignant; ovarian stromal tumor, malignant; thecoma, malignant; granulosa cell tumor, malignant; androblastoma, malignant; Sertoli cell carcinoma; Leydig cell tumor, malignant; lipid cell tumor, malignant; paraganglioma, malignant; extra-mammary paraganglioma, malignant; pheochromocytoma, glomangiosarcoma, malignant melanoma; amelanotic melanoma; superficial spreading melanoma; malignant melanoma in giant pigmented nevus; epithelioid cell melanoma; blue nevus, malignant; sarcoma; fibrosarcoma, fibrous histiocytoma, malignant; myxosarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, stromal sarcoma; mixed tumor; Mullerian mixed tumor; nephroblastoma, hepatoblastoma, carcinosarcoma, mesenchymoma, malignant; Brenner tumor, malignant; phyllodes tumor, malignant; synovial sarcoma; mesothelioma, malignant; dysgerminoma, embryonal carcinoma; teratoma, malignant; struma ovarii, malignant; choriocarcinoma, mesonephroma, malignant; hemangiosarcoma, hemangioendothelioma, malignant; Kaposi's sarcoma; hemangiopericytoma, malignant; lymphangiosarcoma, osteosarcoma, juxtacortical osteosarcoma, chondrosarcoma, chondroblastoma, malignant; mesenchymal chondrosarcoma, giant cell tumor of bone; Ewing's sarcoma; odontogenic tumor, malignant; ameloblastic odontosarcoma, ameloblastoma, malignant; ameloblastic fibrosarcoma, pinealoma, malignant; chordoma, glioma, malignant; ependymoma, astrocytoma, protoplasmic astrocytoma, fibrillary astrocytoma, astroblastoma, glioblastoma, oligodendroglioma, oligodendroblastoma, primitive neuroectodermal, cerebellar sarcoma; ganglioneuroblastoma, neuroblastoma, retinoblastoma, olfactory neurogenic tumor; meningioma, malignant; neurofibrosarcoma, neurilemmoma, malignant; granular cell tumor, malignant; malignant lymphoma; Hodgkin's disease; Hodgkin's; paragranuloma, malignant lymphoma, small lymphocytic, malignant lymphoma, large cell, diffuse; malignant lymphoma, follicular; mycosis fungoides, other specified non-Hodgkin's lymphomas; malignant histiocytosis, multiple myeloma, mast cell sarcoma; immunoproliferative small intestinal disease; leukemia; lymphoid leukemia; plasma cell leukemia; erythroleukemia, lymphosarcoma cell leukemia; myeloid leukemia; basophilic leukemia; eosinophilic leukemia; monocytic leukemia; mast cell leukemia; megakaryoblastic leukemia; myeloid sarcoma; and hairy cell leukemia. Preferably, the neoplastic disease may be tumors associated with a cancer selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, ovarian cancer, thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma. The tumor may be metastatic or a malignant tumor. [00351] In some embodiments, the neoplastic disease to be treated using the compounds of Formula I, or their pharmaceutically acceptable salts, solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, as a single agent treatment, or when combined with a secondary anti-cancer active agent, for example, an inhibitor of KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK, is pancreatic cancer, breast cancer, for example, triple negative breast cancer, lung cancer, prostate cancer and skin cancer. Most preferably, the neoplastic disease to be treated is pancreatic cancer, colorectal cancer and/or carcinomas of the head and neck. In some embodiments the cancer comprises colorectal cancer, pancreatic cancer, breast cancer. In related embodiments, the compounds of the present invention, or their pharmaceutically acceptable salts, solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof are effective against cancers which harbor at least one mutation selected from: a BRAF mutation, a KRAS mutation, and a NRAS mutation, for example, wherein the cancer harbors a KRASG12 mutation, and/or a KRASA136 mutation. In various embodiments, the cancer harbors a BRAFV600 mutation. [00352] Combination Therapies [00353] Some embodiments of the present disclosure provide methods for administering an effective amount of a compound of the disclosure and at least one additional therapeutic agent (including, but not limited to, chemotherapeutic antineoplastics, apoptosis-modulating agents, antimicrobials, antivirals, antifungals, and anti-inflammatory agents) and/or therapeutic technique (e.g., surgical intervention, and/or radiotherapies). In a particular embodiment, the additional therapeutic agent(s) is an anticancer agent. [00354] In some embodiments, the present disclosure provides for the treatment of certain cancers to a subject in need, by administering a therapeutically effective combination comprising a compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, pan-RAS, NRAS, MAPK, ALK, BRAF or MEK inhibitor. These various combination therapies may provide a "synergistic effect", i.e., the effect achieved when the active ingredients used together is greater than the sum of the effects that results from using the compounds separately. These combination therapy methods are particularly effective against a cancer that is resistant or refractory to treatment using the P13K and EGFR inhibitor (i.e. a compound or a pharmaceutically acceptable salt thereof of Formula I) alone, another anti-cancer agent alone, or the P13K and EGFR inhibitor in combination with another anti-cancer agent. [00355] A number of suitable secondary anticancer agents are contemplated for use in the methods of the present disclosure. Indeed, the present disclosure contemplates, but is not limited to, administration of numerous anticancer agents such as: agents that induce apoptosis; polynucleotides (e.g., anti-sense, ribozymes, siRNA); polypeptides (e.g., enzymes and antibodies); biological mimetics; alkaloids; alkylating agents; antitumor antibiotics; antimetabolites; hormones; platinum compounds; monoclonal or polyclonal antibodies (e.g., antibodies conjugated with anticancer drugs, toxins, defensins), toxins; radionuclides; biological response modifiers (e.g., interferons (e.g., IFN-α) and interleukins (e.g., IL-2)); adoptive immunotherapy agents; hematopoietic growth factors; agents that induce tumor cell differentiation (e.g., all-trans-retinoic acid); gene therapy reagents (e.g., antisense therapy reagents and nucleotides); tumor vaccines; angiogenesis inhibitors; proteosome inhibitors: NF- КB modulators; anti-CDK compounds; HDAC inhibitors; and the like. Numerous other examples of chemotherapeutic compounds and anticancer therapies suitable for co- administration with the disclosed compounds are known to those skilled in the art. In some preferred embodiments, the secondary anticancer agent to be used in therapeutically effective combinations may include one or more KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, and MEK inhibitors. [00356] In certain embodiments, secondary, anticancer agents comprise agents that induce or stimulate apoptosis. Agents that induce apoptosis include, but are not limited to, radiation (e.g., X-rays, gamma rays, UV); tumor necrosis factor (TNF)-related factors (e.g., TNF family receptor proteins, TNF family ligands, TRAIL, antibodies to TRAIL-R1 or TRAIL-R2); kinase inhibitors (e.g., epidermal growth factor receptor (EGFR) kinase inhibitor, vascular growth factor receptor (VGFR) kinase inhibitor, fibroblast growth factor receptor (FGFR) kinase inhibitor, platelet-derived growth factor receptor (PDGFR) kinase inhibitor, and Bcr-Abl kinase inhibitors (such as GLEEVEC)); antisense molecules; antibodies (e.g., HERCEPTIN, RITUXAN, ZEVALIN, and AVASTIN); anti-estrogens (e.g., raloxifene and tamoxifen); anti- androgens (e.g., flutamide, bicalutamide, finasteride, aminoglutethamide, ketoconazole, and corticosteroids); cyclooxygenase 2 (COX-2) inhibitors (e.g., celecoxib, meloxicam, NS-398, and non-steroidal anti-inflammatory drugs (NSAIDs)); anti-inflammatory drugs (e.g., butazolidin, DECADRON, DELTASONE, dexamethasone, dexamethasone intensol, DEXONE, HEXADROL, hydroxychloroquine, METICORTEN, ORADEXON, ORASONE, oxyphenbutazone, PEDIAPRED, phenylbutazone, PLAQUENIL, prednisolone, prednisone, PRELONE, and TANDEARIL); and cancer chemotherapeutic drugs (e.g., irinotecan (CAMPTOSAR), CPT-11, fludarabine (FLUDARA), dacarbazine (DTIC), dexamethasone, mitoxantrone, MYLOTARG, VP-16, cisplatin, carboplatin, oxaliplatin, 5-FU, doxorubicin, gemcitabine, bortezomib, gefitinib, bevacizumab, TAXOTERE or TAXOL); cellular signaling molecules; ceramides and cytokines; staurosporine, and the like. [00357] In still other embodiments, the compositions and methods of the present disclosure provide a compound of the disclosure and at least one anti-hyperproliferative or antineoplastic agent selected from alkylating agents, antimetabolites, and natural products (e.g., herbs and other plant and/or animal derived compounds). [00358] Alkylating agents suitable for use in the present compositions and methods include, but are not limited to: 1) nitrogen mustards (e.g., mechlorethamine, cyclophosphamide, ifosfamide, melphalan (L-sarcolysin); and chlorambucil); 2) ethylenimines and methylmelamines (e.g., hexamethylmelamine and thiotepa); 3) alkyl sulfonates (e.g., busulfan); 4) nitrosoureas (e.g., carmustine (BCNU); lomustine (CCNU); semustine (methyl-CCNU); and streptozocin (streptozotocin)); and 5) triazenes (e.g., dacarbazine (DTIC; dimethyltriazenoimid- azolecarboxamide). [00359] In some embodiments, antimetabolites suitable for use in the present compositions and methods include, but are not limited to: 1) folic acid analogs (e.g., methotrexate (amethopterin)); 2) pyrimidine analogs (e.g., fluorouracil (5-fluorouracil; 5-FU), floxuridine (fluorode-oxyuridine; FudR), and cytarabine (cytosine arabinoside)); and 3) purine analogs (e.g., mercaptopurine (6-mercaptopurine; 6-MP), thioguanine (6-thioguanine; TG), and pentostatin (2’-deoxycoformycin)). [00360] In still further embodiments, chemotherapeutic agents suitable for use in the compositions and methods of the present disclosure include, but are not limited to: 1) vinca alkaloids (e.g., vinblastine (VLB), vincristine); 2) epipodophyllotoxins (e.g., etoposide and teniposide); 3) antibiotics (e.g., dactinomycin (actinomycin D), daunorubicin (daunomycin; rubidomycin), doxorubicin, bleomycin, plicamycin (mithramycin), and mitomycin (mitomycin C)); 4) enzymes (e.g., L-asparaginase); 5) biological response modifiers (e.g., interferon-alfa); 6) platinum coordinating complexes (e.g., cisplatin (cis-DDP) and carboplatin); 7) anthracenediones (e.g., mitoxantrone); 8) substituted ureas (e.g., hydroxyurea); 9) methylhydrazine derivatives (e.g., procarbazine (N-methylhydrazine; MIH)); 10) adrenocortical suppressants (e.g., mitotane (o,p’–DDD) and aminoglutethimide); 11) adrenocorticosteroids (e.g., prednisone); 12) progestins (e.g., hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate); 13) estrogens (e.g., diethylstilbestrol and ethinyl estradiol); 14) antiestrogens (e.g., tamoxifen); 15) androgens (e.g., testosterone propionate and fluoxymesterone); 16) antiandrogens (e.g., flutamide): and 17) gonadotropin-releasing hormone analogs (e.g., leuprolide). [00361] Any oncolytic agent that is routinely used in a cancer therapy context finds use in the compositions and methods of the present disclosure. For example, the U.S. Food and Drug Administration maintains a formulary of oncolytic agents approved for use in the United States. International counterpart agencies to the U.S.F.D.A. maintain similar formularies. Table 2 provides a list of exemplary antineoplastic agents approved for use in the U.S. Those skilled in the art will appreciate that the “product labels” required on all U.S. approved chemotherapeutics describe approved indications, dosing information, toxicity data, and the like, for the exemplary agents. [00362] Table 2.
[00363] Anticancer agents further include compounds which have been identified to have anticancer activity. In some embodiments, the additional active agent that finds utility when combined with a compound of Formula I, or a pharmaceutically acceptable salt thereof or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, is an inhibitor of KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEK. Examples include, but are not limited to, 3-AP, 12-O- tetradecanoylphorbol-13-acetate, 17AAG, 852A, ABI-007, ABR-217620, ABT-751, ADI-PEG 20, AE-941, AG-013736, AGRO100, alanosine, AMG 706, antibody G250, antineoplastons, AP23573, apaziquone, APC8015, atiprimod, ATN-161, atrasenten, azacitidine, BB-10901, BCX-1777, bevacizumab, BG00001, bicalutamide, BMS 247550, bortezomib, bryostatin-1, buserelin, calcitriol, CCI-779, CDB-2914, cefixime, cetuximab, CG0070, cilengitide, clofarabine, combretastatin A4 phosphate, CP-675,206, CP-724,714, CpG 7909, curcumin, decitabine, DENSPM, doxercalciferol, E7070, E7389, ecteinascidin 743, efaproxiral, eflornithine, EKB-569, enzastaurin, erlotinib, exisulind, fenretinide, flavopiridol, fludarabine, flutamide, fotemustine, FR901228, G17DT, galiximab, gefitinib, genistein, glufosfamide, GTI- 2040, histrelin, HKI-272, homoharringtonine, HSPPC-96, hu14.18-interleukin-2 fusion protein, HuMax-CD4, iloprost, imiquimod, infliximab, interleukin-12, IPI-504, irofulven, ixabepilone, lapatinib, lenalidomide, lestaurtinib, leuprolide, LMB-9 immunotoxin, lonafarnib, luniliximab, mafosfamide, MB07133, MDX-010, MLN2704, monoclonal antibody 3F8, monoclonal antibody J591, motexafin, MS-275, MVA-MUC1-IL2, nilutamide, nitrocamptothecin, nolatrexed dihydrochloride, nolvadex, NS-9, O6-benzylguanine, oblimersen sodium, ONYX- 015, oregovomab, OSI-774, panitumumab, paraplatin, PD-0325901, pemetrexed, PHY906, pioglitazone, pirfenidone, pixantrone, PS-341, PSC 833, PXD101, pyrazoloacridine, R115777, RAD001, ranpirnase, rebeccamycin analogue, rhuAngiostatin protein, rhuMab 2C4, rosiglitazone, rubitecan, S-1, S-8184,satraplatin, SB-, 15992, SGN-0010, SGN-40, sorafenib, SR31747A, ST1571, SU011248,suberoylanilide hydroxamic acid, suramin, talabostat, talampanel, tariquidar, temsirolimus, TGFa-PE38 immunotoxin, thalidomide, thymalfasin, tipifarnib, tirapazamine, TLK286,trabectedin, trimetrexate glucuronate, TroVax, UCN-1, valproic acid, vinflunine, VNP40101M, volociximab, vorinostat, VX-680, ZD1839, ZD6474, zileuton, and zosuquidar trihydrochloride, sotorasib, sorafenib, panitumumab, encorafenib, copanlisib trametinib, cobimetinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, ERAS-007, ulixertinib, ASTX029, ravoxertinib, PLX4720 (Plexxikon), PD184352(CI-1040- Pfizer), E6201 (Esai Co Ltd), GDC-0623 (RG 7421 Genentech Inc), CH5126766 (RO5126766 - Chugai Pharmaceutical Co.), HL-085(Shanghai Kechow Pharma, Inc.), SHR7390 (Hengrui Medicine), TQ-B3234 (Chiatai Tianqing), CS-3006 (Cstone Pharmaceuticals) and FCN-159 (Fosun Pharma). [00364] For a more detailed description of anticancer agents and other therapeutic agents, those skilled in the art are referred to any number of instructive manuals including, but not limited to, the Physician's Desk Reference and to Goodman and Gilman's "Pharmaceutical Basis of Therapeutics" tenth edition, Eds. Hardman et al., 2002. [00365] The amounts of each of the active agents in the combinations described herein can include amounts of each agent that are found to be clinically relevant amounts that provide therapeutic benefit in the aggregate when dosed in combination. For example, the additional active agent may be dosed or provided in compositions which are dosed with the compounds of the present disclosure in amounts that do cause adverse effects and that may be titrated when studied in a clinical trial of the combination. In each case, the combination may be provided in compositions that may be administered separately or formulated in a single composition and may be dosed in amounts that are either therapeutically effective amounts individually, or when one or both of the active agents of the combination are dosed at sub-optimal or sub-therapeutic levels, if the combination as a whole is therapeutically effective. In some embodiments, the amount of the additional active agent when used in a therapeutically effective combination with the compounds of Formula I, (for example, any compound as provided in Table 1 herein) or their pharmaceutically acceptable salts or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, may range from about 0.1 mg/kg to about 500 mg/kg body weight of the patient, or preferably, ranging from about 1 mg/kg to about 200 mg/kg body weight of the patient, and all ranges therebetween. [00366] In various embodiments, the additional active agent that is an inhibitor of KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEK, can include one or more of: sotorasib, sorafenib, panitumumab, encorafenib, trametinib, cobimetinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, divarasib, adagrasib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, glecirasib, ERAS-007, ulixertinib, ASTX029, ravoxertinib, PLX4720 (Plexxikon), ERAS-007, LY3537982, ASTX029, PD184352(CI-1040- Pfizer), E6201 (Esai Co Ltd), GDC-0623 (RG 7421 Genentech Inc), CH5126766 (RO5126766 - Chugai Pharmaceutical Co.), HL-085(Shanghai Kechow Pharma, Inc.), SHR7390 (Hengrui Medicine), TQ-B3234 (Chiatai Tianqing), CS-3006 (Cstone Pharmaceuticals) and FCN-159 (Fosun Pharma), each of which are used in amounts that are clinically therapeutic, or dosed in combination at their FDA approved dosages for the treatment of a cancer, for example at dosages, or daily doses, or therapeutically effective daily doses that range from about 0.1 mg/kg to about 500 mg/kg body weight of the patient, or preferably, ranging from about 1 mg/kg to about 200 mg/kg body weight of the patient, and all ranges therebetween, or may be dosed in a therapeutically effective combination with the compounds of Formula I, (for example, any compound as provided in Table 1 herein) or their pharmaceutically acceptable salts or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, in an amount that may range from 0.1 mg/kg to about 500 mg/kg (body weight of the patient), or preferably, ranging from about 1 mg/kg to about 200 mg/kg (body weight of the patient), and all ranges therebetween. [00367] The present disclosure provides methods for administering a compound of the disclosure with radiation therapy. The disclosure is not limited by the types, amounts, or delivery and administration systems used to deliver the therapeutic dose of radiation to an animal. For example, the animal may receive photon radiotherapy, particle beam radiation therapy, other types of radiotherapies, and combinations thereof. In some embodiments, the radiation is delivered to the animal using a linear accelerator. In still other embodiments, the radiation is delivered using a gamma knife. [00368] The source of radiation can be external or internal to the animal. External radiation therapy is most common and involves directing a beam of high-energy radiation to a tumor site through the skin using, for instance, a linear accelerator. While the beam of radiation is localized to the tumor site, it is nearly impossible to avoid exposure of normal, healthy tissue. However, external radiation is usually well tolerated by animals. Internal radiation therapy involves implanting a radiation-emitting source, such as beads, wires, pellets, capsules, particles, and the like, inside the body at or near the tumor site including the use of delivery systems that specifically target cancer cells (e.g., using particles attached to cancer cell binding ligands). Such implants can be removed following treatment, or left in the body inactive. Types of internal radiation therapy include, but are not limited to, brachytherapy, interstitial irradiation, intracavity irradiation, radioimmunotherapy, and the like. [00369] The animal may optionally receive radiosensitizers (e.g., metronidazole, misonidazole, intra-arterial Budr, intravenous iododeoxyuridine (IudR), nitroimidazole, 5- substituted-4-nitroimidazoles, 2H-isoindolediones, [[(2-bromoethyl)-amino]methyl]-nitro-1H- imidazole-1-ethanol, nitroaniline derivatives, DNA-affinic hypoxia selective cytotoxins, halogenated DNA ligand, 1,2,4 benzotriazine oxides, 2-nitroimidazole derivatives, fluorine- containing nitroazole derivatives, benzamide, nicotinamide, acridine-intercalator, 5- thiotretrazole derivative, 3-nitro-1,2,4-triazole, 4,5-dinitroimidazole derivative, hydroxylated texaphrins, cisplatin, mitomycin, tiripazamine, nitrosourea, mercaptopurine, methotrexate, fluorouracil, bleomycin, vincristine, carboplatin, epirubicin, doxorubicin, cyclophosphamide, vindesine, etoposide, paclitaxel, heat (hyperthermia), and the like), radioprotectors (e.g., cysteamine, aminoalkyl dihydrogen phosphorothioates, amifostine (WR 2721), IL-1, IL-6, and the like). Radiosensitizers enhance the killing of tumor cells. Radioprotectors protect healthy tissue from the harmful effects of radiation. [00370] Any type of radiation can be administered to an animal, so long as the dose of radiation is tolerated by the animal without unacceptable negative side-effects. Suitable types of radiotherapy include, for example, ionizing (electromagnetic) radiotherapy (e.g., X-rays or gamma rays) or particle beam radiation therapy (e.g., high linear energy radiation). Ionizing radiation is defined as radiation comprising particles or photons that have sufficient energy to produce ionization, i.e., gain or loss of electrons (as described in, for example, U.S.5,770,581 incorporated herein by reference in its entirety). The effects of radiation can be at least partially controlled by the clinician. In one embodiment, the dose of radiation is fractionated for maximal target cell exposure and reduced toxicity. [00371] In one embodiment, the total dose of radiation administered to an animal is about .01 Gray (Gy) to about 100 Gy. In another embodiment, about 10 Gy to about 65 Gy (e.g., about 15 Gy, 20 Gy, 25 Gy, 30 Gy, 35 Gy, 40 Gy, 45 Gy, 50 Gy, 55 Gy, or 60 Gy) are administered over the course of treatment. While in some embodiments a complete dose of radiation can be administered over the course of one day, the total dose is ideally fractionated and administered over several days. Desirably, radiotherapy is administered over the course of at least about 3 days, e.g., at least 5, 7, 10, 14, 17, 21, 25, 28, 32, 35, 38, 42, 46, 52, or 56 days (about 1-8 weeks). Accordingly, a daily dose of radiation will comprise approximately 1-5 Gy (e.g., about 1 Gy, 1.5 Gy, 1.8 Gy, 2 Gy, 2.5 Gy, 2.8 Gy, 3 Gy, 3.2 Gy, 3.5 Gy, 3.8 Gy, 4 Gy, 4.2 Gy, or 4.5 Gy), or 1-2 Gy (e.g., 1.5-2 Gy). The daily dose of radiation should be sufficient to induce destruction of the targeted cells. If stretched over a period, in one embodiment, radiation is not administered every day, thereby allowing the animal to rest and the effects of the therapy to be realized. For example, radiation desirably is administered on 5 consecutive days, and not administered on 2 days, for each week of treatment, thereby allowing 2 days of rest per week. However, radiation can be administered 1 day/week, 2 days/week, 3 days/week, 4 days/week, 5 days/week, 6 days/week, or all 7 days/week, depending on the animal’s responsiveness and any potential side effects. Radiation therapy can be initiated at any time in the therapeutic period. In one embodiment, radiation is initiated in week 1 or week 2, and is administered for the remaining duration of the therapeutic period. For example, radiation is administered in weeks 1- 6 or in weeks 2-6 of a therapeutic period comprising 6 weeks for treating, for instance, a solid tumor. Alternatively, radiation is administered in weeks 1-5 or weeks 2-5 of a therapeutic period comprising 5 weeks. These exemplary radiotherapy administration schedules are not intended, however, to limit the present disclosure. [00372] Antimicrobial therapeutic agents may also be used as therapeutic agents in the present disclosure. Any agent that can kill, inhibit, or otherwise attenuate the function of microbial organisms may be used, as well as any agent contemplated to have such activities. Antimicrobial agents include, but are not limited to, natural and synthetic antibiotics, antibodies, inhibitory proteins (e.g., defensins), antisense nucleic acids, membrane disruptive agents and the like, used alone or in combination. Indeed, any type of antibiotic may be used including, but not limited to, antibacterial agents, antiviral agents, antifungal agents, and the like. [00373] In some embodiments, the present disclosure provides a method for treating a cancer in a subject in need thereof, comprising, administering to the subject, a therapeutically effective combination comprising a compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, NRAS, MAPK, ALK, BRAF, or MEK inhibitor. In some embodiments, the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is found in table 1, as provided above. In some preferred embodiments, the the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is selected from Compound 1, 2R or 2S, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof. In some more preferred embodiments, the compound or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is Compound 2R, which can be used in a method of treating cancer, by combining said compound with at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor to form a therapeutically effective combination for the treatment of cancer. [00374] In some embodiments, the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor comprises: sotorasib, sorafenib, panitumumab, encorafenib, trametinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, PLX4720 (company ?), PD0325901 (Company ?), PD184352(Company ?), E6201 (Esai Co Ltd), GDC-0623 (RG 7421 Genentech Inc), CH5126766 (RO5126766 - Chugai Pharmaceutical Co.), HL-085(Shanghai Kechow Pharma, Inc.), SHR7390 (Hengrui Medicine), TQ-B3234 (Chiatai Tianqing), CS-3006 (Cstone Pharmaceuticals) and FCN-159 (Fosun Pharma) and combinations thereof. [00375] In some embodiments, the amount of the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor for use in the combination can be any amount that has therapeutic benefit to the subject in need of the combination treatment, which can include a daily dose, a therapeutically effective daily dose selected from about 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 20 mg/kg, 0.05 mg/kg to 10 mg/kg, 0.1 mg/kg to 75 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 50 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 20 mg/kg, 0.1 mg/kg to 10 mg/kg, 0.5 mg/kg to 150 mg/kg, 0.5 mg/kg to 100 mg/kg, 0.5 mg/kg to 90 mg/kg, 0.5 mg/kg to 80 mg/kg, 0.5 mg/kg to 70 mg/kg, 0.5 mg/kg to 60 mg/kg, 0.5 mg/kg to 50 mg/kg, 0.5 mg/kg to 40 mg/kg, 0.5 mg/kg to 30 mg/kg, 0.5 mg/kg to 20 mg/kg, 0.5 mg/kg to 10 mg/kg, 1 mg/kg to 500 mg/kg, 1 mg/kg to 400 mg/kg, 1 mg/kg to 300 mg/kg, 1 mg/kg to 200 mg/kg, 1 mg/kg to 100 mg/kg, 1 mg/kg to 90 mg/kg, 1 mg/kg to 80 mg/kg, 1 mg/kg to 70 mg/kg, 1 mg/kg to 60 mg/kg, 1 mg/kg to 50 mg/kg, 1 mg/kg to 40 mg/kg, 1 mg/kg to 30 mg/kg, 1 mg/kg to 20 mg/kg, 1 mg/kg to 10 mg/kg, 5 mg/kg to 75 mg/kg, 5 mg/kg to 70 mg/kg, 5 mg/kg to 60 mg/kg, 5 mg/kg to 50 mg/kg, 5 mg/kg to 40 mg/kg, 5 mg/kg to 30 mg/kg, 5 mg/kg to 20 mg/kg, and 5 mg/kg to 10 mg/kg body weight of the recipient per day, one or more times a day, or one or more times per week, or one or more times per month, or one or more times per year, to obtain the desired therapeutic effect. [00376] In some embodiments of the present disclosure, a compound of the disclosure and one or more therapeutic agents or anticancer agents are administered to an animal under one or more of the following conditions: at different periodicities, at different durations, at different concentrations, by different administration routes, etc. In some embodiments, the compound is administered prior to the therapeutic or anticancer agent, e.g., 0.5, 1, 2, 3, 4, 5, 10, 12, or 18 hours, 1, 2, 3, 4, 5, or 6 days, or 1, 2, 3, or 4 weeks prior to the administration of the therapeutic or anticancer agent. In some embodiments, the compound is administered after the therapeutic or anticancer agent, e.g., 0.5, 1, 2, 3, 4, 5, 10, 12, or 18 hours, 1, 2, 3, 4, 5, or 6 days, or 1, 2, 3, or 4 weeks after the administration of the anticancer agent. In some embodiments, the compound and the therapeutic or anticancer agent are administered concurrently but on different schedules, e.g., the compound is administered daily while the therapeutic or anticancer agent is administered once a week, once every two weeks, once every three weeks, or once every four weeks. In other embodiments, the compound is administered once a week while the therapeutic or anticancer agent is administered daily, once a week, once every two weeks, once every three weeks, or once every four weeks. [00377] In various embodiments, single or multiple administrations of the pharmaceutical compositions are administered depending on the dosage and frequency as required and tolerated by the patient. The dosage can be administered once but may be applied periodically until either a therapeutic result is achieved or until side effects warrant discontinuation of therapy. [00378] In various embodiments, the combination therapy comprises administering to the subject with a cancer, a therapeutically effective combination comprising a compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I, and at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor simultaneously, either in the same pharmaceutical composition or in separate pharmaceutical compositions. In various embodiments, the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I, and at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor are administered sequentially, i.e., the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor is administered either prior to or after the administration of the compound, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I. [00379] In various embodiments, the administrations of the combination, i.e. a compound of Formula I of the present invention, or a pharmaceutically acceptable salt thereof and at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor are concurrent, i.e., the administration period of the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor and the compound of Formula I, of the present invention, or a pharmaceutically acceptable salt thereof overlap with each other. [00380] In various embodiments, the administrations of the at least one KRAS, NRAS, MAPK, ALK, BRAF, or MEK inhibitor and the compound of Formula I, are non-concurrent. For example, in various embodiments, the administration of the at least one KRAS, NRAS, pan- RAS, MAPK, ALK, or MEK inhibitor is terminated before the compound of Formula I is administered. In various embodiments, the administration of the compound of Formula I is terminated before the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor is administered. [00381] Toxicity and therapeutic index of the pharmaceutical compositions of the invention can be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., for determining the LD50 (the dose lethal to 50% of the population) and the ED50 (the dose therapeutically effective in 50% of the population). The dose ratio between toxic and therapeutic effective dose is the therapeutic index and it can be expressed as the ratio LD50/ED50. Compositions that exhibit large therapeutic indices are generally preferred. [00382] In various embodiments, the methods described herein may be used in combination with other conventional anti-cancer therapeutic approaches directed to treatment or prevention of proliferative disorders, such approaches including, but not limited to immunotherapy, chemotherapy, small molecule kinase inhibitor targeted therapy, surgery, radiation therapy, and stem cell transplantation. For example, such methods can be used in prophylactic cancer prevention, prevention of cancer recurrence and metastases after surgery, and as an adjuvant of other conventional cancer therapy. The present disclosure recognizes that the effectiveness of conventional cancer therapies (e.g., chemotherapy, radiation therapy, phototherapy, immunotherapy, and surgery) can be enhanced through the use of the combination methods described herein. [00383] A wide array of chemotherapeutic compounds has been shown to have anti- neoplastic activities. These compounds have been used as pharmaceutical agents in chemotherapy to shrink solid tumors, prevent metastases and further growth, or decrease the number of malignant T-cells in leukemic or bone marrow malignancies. Although chemotherapy has been effective in treating various types of malignancies, many anti-neoplastic compounds induce undesirable side effects, for example, known αPI3K inhibitors, for example, Alpelisib have been known to induce adverse side effects such as hyperglycemia. The hyperinsulinemia that occurs in patients with Alpelisib-induced hyperglycemia may provide breast cancer cells with a survival mechanism and reduce the efficacy of Alpelisib as demonstrated in preclinical studies. Patients with an absolute or relative deficiency in insulin, and those with insulin resistance or pancreatic dysfunction, may experience exacerbated or prolonged hyperglycemia. Furthermore, if not successfully managed, hyperglycemia can necessitate α- selective PI3K inhibitor dose reductions, treatment interruptions, or treatment discontinuation. Optimal hyperglycemia management is therefore required to maintain Alpelisib dose intensity and maximize treatment benefit. [00384] It has been unexpected and surprising that the compounds of Formula I, for example, compounds, or their pharmaceutical acceptable salts, such as those exemplified in Table 1, herein, do not substantially increase hyperglycemia, blood glucose or blood insulin when administered in therapeutically effective amounts to subjects with cancer, as shown in the examples and experiments of the present disclosure. It has been shown that when two or more different treatments are combined, the treatments may work synergistically and allow reduction of dosage of each of the treatments, thereby reducing the detrimental side effects exerted by each compound at higher dosages. In other instances, malignancies that are refractory to a treatment may respond to a combination therapy of compounds or their pharmaceutically acceptable salts, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof of Formula I, (for example, the compounds or their salts of Table 1) in combination with at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor. [00385] As used herein, the term hyperglycemia generally refers to a blood glucose greater than 125 mg/dL while fasting and greater than 180 mg/dL 2 hours postprandial. For example, as used herein, hyperglycemia is defined as a plasma or whole blood glucose measurement greater than 125 mg/dL while fasting and greater than 200 mg/dL, 2 hours postprandial, during a 75-g oral glucose tolerance test (OGTT) using a blood glucose meter employing strips comprising glucose oxidase, hexokinase, or combinations thereof. [00386] A patient has impaired glucose tolerance, or pre-diabetes, with a fasting plasma glucose of 100 mg/dL to 125 mg/dL. A patient is termed diabetic with a fasting blood glucose of greater than 125 mg/dL. It has been unexpectedly found, that administration of one or more doses (daily or weekly, or monthly) of the compounds of Formula I, or a pharmaceutically acceptable salts, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof does not significantly increase the blood glucose, or insulin or cause hyperglycemia in a patient, when administered the compounds of Formula I, or a pharmaceutically acceptable salts, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof as part of a cancer treatment regimen. As used herein, not significantly increasing blood glucose levels, or blood insulin levels after one or more administrations of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor, means that the blood or plasma glucose levels or the blood or plasma insulin levels in the patient do not rise more than about 30%, or about 29%, or about 28%, or about 27%, or about 26%, or about 25%, or about 24%, or about 23%, or about 22%, or about 21%, or about 20%, or about 19%, or about 18%, or about 17%, or about 16%, or about 15%, or about 14%, or about 13%, or about 12%, or about 11%, or about 10%, or about 5%, or about 1% of the value of blood or plasma glucose or blood or plasma insulin as a result of the administration alone, when measured around one to two hours after administration, when compared to a measurement of blood or plasma glucose or insulin taken just prior (e.g.1 minute to about 30 minutes) to the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor. [00387] In some embodiments, after a single or multiple administrations of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, or MEK inhibitor, the blood or plasma glucose levels or the blood or plasma insulin levels does not significantly rise as a result of the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor alone, i.e. the blood or plasma glucose levels or the blood or plasma insulin levels does not rise more than about 0.1% to about 20% of the levels of the patient's blood or plasma glucose levels or the blood or plasma insulin levels measured just immediately (1-60 mins) prior to the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF,or MEK inhibitor. In the case of hyperglycemic patients, for example, diabetic patients, the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor, the blood or plasma glucose levels or the blood or plasma insulin levels does not significantly rise as a result of the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor alone, i.e. the blood or plasma glucose levels or the blood or plasma insulin levels does not rise more than about 0.1% to about 20% of the levels of the patient's blood or plasma glucose levels or the blood or plasma insulin levels measured just immediately (1-60 mins) prior to the administration of the compounds of Formula I, or in combination with an at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor. [00388] Methods for the measurement of blood glucose and insulin are well known in the art. A patient's blood or plasma glucose level or amount, can be measured using any glucose sensor known in the art. The term “glucose sensor” as used herein is a broad term, and is to be given its ordinary and customary meaning to a person of ordinary skill in the art (and is not to be limited to a special or customized meaning), and refers without limitation to any mechanism (e.g., enzymatic or non-enzymatic) by which glucose can be quantified. For example, some embodiments utilize a membrane that contains glucose oxidase that catalyzes the conversion of oxygen and glucose to hydrogen peroxide and gluconate, as illustrated by the following chemical reaction: Glucose + O2 Gluconate + H2O2. Because for each glucose molecule metabolized, there is a proportional change in the co-reactant O2 and the product H2O2, one can use an electrode to monitor the current change in either the co-reactant or the product to determine glucose concentration. In some embodiments, in the detection of glucose where glucose oxidase produces hydrogen peroxide as a byproduct, hydrogen peroxide reacts with the surface of the working electrode producing two protons (2H+), two electrons (2e) and one molecule of oxygen (O2), which produces the electronic current being detected by a sensor relative and proportional to the amount of glucose present at the time of sensing. [00389] In some embodiments, measuring a patient's (for example, a human patient) glucose (whether using the patient's whole blood or plasma) level can be typically accomplished using two generally established methods. One method uses an electronic blood glucose meter. A sample of blood is obtained by piercing the skin of a patient with a lancet. The sample of blood is then placed on a chemically-active test-strip, which interfaces with the blood glucose meter. Within several seconds of inserting the test-strip into the blood glucose meter, the blood glucose level of the user is read and shown on the digital display of the blood glucose meter. Another method can involve the measurement of whole blood or plasma glucose concentration using a colorimetric method based on enzymatic oxidation of glucose in the presence of glucose oxidase. The produced hydrogen peroxide reacts with phenol and 4-aminophenazone in a reaction catalyzed by peroxidase to form a red-violet quinoneimine dye as indicator. The intensity of the final color is directly proportional to the glucose concentration in the sample, and can be measured at 505 nm. The obtained absorbance measurements can then be determined and compared using a blood/plasma glucose standard curve. [00390] The blood glucose meter method provides an accurate snapshot of a user's blood glucose level at a single moment in time. However, the blood glucose meter method does not indicate whether the user's glucose level is rising, falling, or steady. Additionally, the blood glucose meter method fails to capture a user's changing blood sugar levels after meals, between meals, and during the night. An alternative improved method for measuring a user's blood glucose level in a continuous or real-time basis is to use a continuous glucose monitor (CGM) system. A CGM system generally includes a sensor, a transmitter, and a receiver (such as a handheld receiver). Some CGM systems use a glucose oxidase sensor. Some CGM devices use glucose binding protein (GBP) sensors. GBP CGM sensors may be preferred because they may be able to provide faster response times and better stability at lower cost than glucose oxidase based sensors. More information on glucose binding proteins and continuous glucose monitors can be found in U.S. Pat. Nos.6,855,556, 7,496,392, 7,787,923, 7,792,561, 7,851,593, and 8,623,639, the entire contents of each of which are hereby incorporated by reference. [00391] In some embodiments, blood glucose levels in a patient can be measured using a handheld device, for example, a CONTOUR®Next (Bayer HealthCare LLC, Mishawaka, IN USA) blood glucose monitor, using blood glucose strips (CONTOUR®NEXT Test Strips - Bayer HealthCare LLC, Mishawaka, IN USA). Other blood glucose measurements can employ the glucose-oxidase method for example, (Infinity™ Glucose (Ox) TR15221 Thermo Scientific, USA) as described in, for example, Christiansen M et. al. A new, wireless-enabled blood glucose monitoring system that links to a smart mobile device: accuracy and user performance evaluation. J. Diabetes Sci Technol.2017; 11:567-573, 3. Bernstein, et al. A New Test Strip Technology Platform for Self-Monitoring of Blood Glucose. J Diabetes Sci Technol 2013;7(5):1388-1399 the disclosures of which are incorporated by reference herein in their entireties. EXAMPLES [00392] General synthetic schemes [00393] Compounds of Formula I can be synthesized using the general methods provided in Scheme 1. In accordance with Scheme 1, a bromide compound of formula G1 can be converted to a borane compound of formula G2 by reacting with an agent such as 4,4,5,5- tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolane, or the like. A compound of formula G2 can then be coupled to a compound of formula G5 to provide a compound of Formula I, using coupling chemistry known to those having skill in the art, such as palladium catalyzed coupling conditions. The compound of formula G5 can be synthesized by reacting a compound of formula G3 with a compound of formula G4 under nucleophilic aromatic substitution conditions. It will be also understood that the order of reactions as specified in Scheme 1 can also be reversed, so that a compound of formula G2 reacts with a compound of formula G3 to form an intermediate product, followed by reacting the intermediate product with a compound of formula G4 to provide a compound of Formula I. It will also be understood that the compounds represented by formula G4 can possess a stereocenter, wherein R1a and R1b are different. Further, a compound of G4 can be a single enantiomer or a racemic mixture of enantiomers, which will provide an enantiomeric or racemic compound of Formula I, respectively. It will be generally understood to one having skill in the art that reaction conditions that are successful when using one enantiomer of a compound of formula G4 as starting material will most likely be equally successful for the other enantiomer of the compound of formula G4. [00394] Scheme 1: Synthesis of compounds of Formula I
[00395] Compounds of Formula I can also be synthesized according to Scheme 2. Following the scheme, a compound of Formula G6 first undergoes nucleophilic substitution with an amine of Formula G7. The resulting compound of Formula G8, where Xa is a group appropriate for functional group interconversion to the tetramethyl-1,3,2-dioxaborolane, is converted to a compound of Formula G9. Exposure of the compound of Formula of G9 to a compound of Formula G10 under coupling conditions, for example palladium catalyzed coupling conditions provides a compound of Formula I. [00396] Scheme 2: Alternative route to compounds of Formula I [00397] Synthetic examples [00398] Example 1: N-[5-[4-(1-phenylethylamino)quinazolin-6-yl]-3- pyridyl]methanesulfonamide (Compound 1) [00399] Step 1: Synthesis of (5-amino-3-pyridyl) boronic acid (2a) [00400] To a stirred solution of BPD (1.76 g, 6.94 mmol, 1.2 eq) in dioxane (5 mL) was added 5-bromopyridin-3-amine, 1a, (1 g, 5.78 mmol, 1 eq), AcOK (1.70 g, 17.34 mmol, 3 eq) and Pd(dppf)Cl2.CH2Cl2 (472.02 mg, 578.00 μmol, 0.1 eq), the reaction was purged with Ar 3 times, and stirred at 100 °C for 16 h under Ar. LCMS showed starting material was consumed completely and the MS of desired product was detected. The crude product was triturated with EtOAc (6mL) at 25oC for 60 min. Then filtered and filtrate was concentrate in vacuum. Compound 2a (5-amino-3-pyridyl) boronic acid (2 g, crude) was obtained as a black solid. [00401] Step 2: Synthesis of 6-(5-amino-3-pyridyl)-N-(1-phenylethyl)quinazolin-4- amine (3a) [00402] To a stirred solution of (5-amino-3-pyridyl) boronic acid, 2a, (402.32 mg, 1.83 mmol, 2 eq) in DMF (4 mL) and H2O (1.5 mL) was added 6-bromo-N-(1-phenylethyl) quinazolin-4-amine (300 mg, 914.06 μmol, 1 eq), K3PO4 (582.07 mg, 2.74 mmol, 3 eq) and Pd(PPh3)4 (105.63 mg, 91.41 μmol, 0.1 eq), the reaction was stirred at 100oC for 4 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was purified by prep-HPLC (column: C18-1 150*30mm*5um;mobile phase: [water(TFA)-ACN];B%: 1%- 45%,8min). Compound 3a, 6-(5- amino-3-pyridyl)-N-(1-phenylethyl)quinazolin-4-amine (80 mg, 175.66 μmol, 19.22% yield, TFA) was obtained as a white solid. [00403] Step 3: Synthesis of N-methylsulfonyl-N-[5-[4-(1- phenylethylamino)quinazolin-6-yl]-3-pyridyl]methanesulfonamide (4a) [00404] To a stirred solution of 6-(5-amino-3-pyridyl)-N-(1-phenylethyl)quinazolin-4- amine, 3a, (40 mg, 117.16 μmol, 1 eq) in dry DCM (2 mL) was added TEA (59.28 mg, 585.81 μmol, 81.54 μL, 5 eq), the reaction was degassed with N2 and cooled to 0 °C. Then MsCl (50 mg, 436.49 μmol, 33.78 μL, 3.73 eq) was dropwise added to the reaction at 0 °C. The reaction was stirred at 0 °C for 1 h under N2. TLC (PE: EtOAc =1:1, Rf = 0.53) showed the starting material remained and a new spot formed. Then MsCl (50 mg, 436.49 μmol, 33.78 μL, 3.73 eq) was added, and the mixture was stirred at 0 °C for 1 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was quenched by adding 5 mL water and extracted with DCM (3*5 mL). The organic layer was washed with brine (5mL), dried over anhydrous Na2SO4, then filtered and concentrated in vacuum. Compound 4a, N-methylsulfonyl-N-[5-[4-(1-phenylethylamino) quinazolin-6-yl]-3-pyridyl] methanesulfonamide (40 mg, 80.39 μmol, 68.61% yield) was obtained as a yellow oil. [00405] Step 4: Synthesis of N-[5-[4-(1-phenylethylamino)quinazolin-6-yl]-3- pyridyl]methanesulfonamide (Compound 1) [00406] To a stirred solution of N-methylsulfonyl-N-[5-[4-(1-phenylethylamino) quinazolin-6-yl]-3-pyridyl] methanesulfonamide, 4a, (40 mg, 80.39 μmol, 1 eq) in MeOH (2 mL) was added K2CO3 (22.22 mg, 160.78 μmol, 2 eq), the reaction was stirred at 60 °C for 2 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was purified by prep-HPLC (column: Waters Xbridge BEH C18100*30mm*10um; mobile phase: [water (NH4HCO3)-ACN]; B%: 20%-50%, 10min). Compound 1, N-[5-[4-(1-phenylethylamino) quinazolin-6-yl]-3-pyridyl] methanesulfonamide (5.64 mg, 13.33 μmol, 16.58% yield, 99.13% purity) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 8.82 (s, 2 H), 8.66 (br d, J=7.88 Hz, 1 H), 8.47 (d, J=2.25 Hz, 1 H), 8.44 (s, 1 H), 8.07 (dd, J=8.63, 1.63 Hz, 1 H), 7.93 (t, J=2.06 Hz, 1 H), 7.80 (d, J=8.63 Hz, 1 H), 7.45 (d, J=7.50 Hz, 2 H), 7.32 (t, J=7.57 Hz, 2 H), 7.19 - 7.25 (m, 1 H), 5.65 (t, J=7.25 Hz, 1 H), 3.12 (s, 3 H), 1.62 (d, J=7.13 Hz, 3 H). MS (M + H)+ =420.1 [00407] Example 2: Synthesis of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (3b) [00408] Step 1: Synthesis of 2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)pyridin-3-amine (2c) [00409] To a stirred solution of 5-bromo-2-chloro-pyridin-3-amine, 1c, (20 g, 96.41 mmol, 1 eq) in dioxane (250 mL) was added 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)-1,3,2-dioxaborolane (29.38 g, 115.69 mmol, 1.2 eq), KOAc (23.65 g, 241.01 mmol, 2.5 eq), and Pd(dppf)Cl2 (3.53 g, 4.82 mmol, 0.05 eq), and the mixture was purged with N23 times, and then stirred at 100 °C for 16 h. TLC (Petroleum ether/Ethyl acetate=5:1, Rf=0.25) showed a little starting material was remaining and a new spot was formed. The reaction mixture was poured into water (150 mL). The aqueous phase was extracted with ethyl acetate (300 mL*3). The combined organic phase was dried with anhydrous Na2SO4, filtered and concentrated in vacuum. The residue was purified by flash column (ISCO 120 g silica, 10-15% Ethyl acetate in Petroleum ether, gradient over 15 min). Compound 2c, 2-chloro-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl) pyridin-3-amine (6.75 g, 26.52 mmol, 30% yield) was obtained as a yellow solid. [00410] Step 2: Synthesis of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (3b) [00411] To a stirred solution of 2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)pyridin-3-amine, 2c, (27 g, 106.08 mmol, 1 eq) in DCM (200 mL) was added TEA (42.94 g, 424.33 mmol, 59.06 mL, 4 eq), MsCl (31.170 g, 272.11 mmol, 21.06 mL, 2.57 eq) at 0oC. The mixture was stirred at 0oC for 1 h. TLC (Petroleum ether/Ethyl acetate=3:1, Rf=0.84) showed starting material was consumed completely and new spot was formed. The reaction mixture was concentrate in vacuum. The residue was poured into MeOH (10 mL). The mixture was stirred at 20 °C for 1 h, filtered, and the filter cake was concentrate in vacuum to give the crude product. Compound 3b, N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3-pyridyl]-N- methylsulfonyl-methanesulfonamide (20 g, 48.70 mmol, 46% yield) was obtained as a white solid.1H NMR (400 MHz, CHLOROFORM-d) δ = 8.77 (d, J = 1.8 Hz, 1H), 8.04 (d, J = 1.6 Hz, 1H), 3.53 (s, 6H), 1.45 - 1.32 (m, 12H) [00412] Example 3: Synthesis of N-[2-chloro-5-[4-(1-phenylethylamino)quinazolin-6- yl]-3-pyridyl]methanesulfonamide (Compound 2) [00413] Step 1: Synthesis of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine (2b) [00414] To a stirred solution of 6-bromo-4-chloro-quinazoline, 1b, (3 g, 12.32 mmol, 1 eq) in i-PrOH (30 mL) was added 1-phenylethanamine (1.49 g, 12.32 mmol, 1.57 mL, 1 eq) and TEA (1.99 g, 19.71 mmol, 2.74 mL, 1.6 eq), the reaction was stirred at 80 °C for 16 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was cooled to ambient temperature, quenched with water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. Compound 2b, 6-bromo-N- (1-phenylethyl) quinazolin-4-amine (3 g, 9.14 mmol, 74.19% yield) was obtained as white solid. [00415] Step 2: Synthesis of N-[2-chloro-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]methanesulfonamide (Compound 2) [00416] To a stirred solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine, 2b, (50 mg, 152.34 μmol, 1 eq) in DMF (2 mL) and H2O (0.5 mL) was added N-[2-chloro-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (62.57 mg, 152.34 μmol, 1 eq), K3PO4 (97.01 mg, 457.03 μmol, 3 eq) and Pd(PPh3)4 (17.60 mg, 15.23 μmol, 0.1 eq), the reaction was stirred at 80 ℃ for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was evaporated to obtain the product. The crude product was purified by prep-HPLC (column: Phenomenex C1875*30mm*3um; mobile phase: [water (NH4HCO3)- ACN]; B%: 10%-40%, 8min). Compound 2 N-[2-chloro-5-[4-(1-phenylethylamino) quinazolin- 6-yl]-3-pyridyl] methanesulfonamide (8.53 mg, 18.79 μmol, 12.33% yield, 100% purity) was obtained as white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.98 (br s, 1 H), 8.85 (d, J=1.63 Hz, 1 H), 8.79 (d, J=2.25 Hz, 1 H), 8.67 (br d, J=7.88 Hz, 1 H), 8.44 (s, 1 H), 8.24 (d, J=2.38 Hz, 1 H), 8.14 (dd, J=8.76, 1.75 Hz, 1 H), 7.80 (d, J=8.63 Hz, 1 H), 7.45 (d, J=7.38 Hz, 2 H), 7.33 (t, J=7.57 Hz, 2 H), 7.16 - 7.27 (m, 1 H), 5.65 (t, J=7.25 Hz, 1 H), 3.17 (s, 3 H), 1.63 (d, J=7.00 Hz, 3 H). MS (M + H)+ =454.0 [00417] Compounds 2R and 2S were synthesized analogously to Compound 2 in Example 3 using the appropriate chiral version of Intermediate 2b. [00418] Compound 2R:1H NMR (400MHz, DMSO-d6) δ = 10.97 (br d, J=7.6 Hz, 1H), 9.97 (br s, 1H), 9.45 (s, 1H), 8.94 - 8.86 (m, 2H), 8.43 (dd, J=1.6, 8.8 Hz, 1H), 8.36 (d, J=2.4 Hz, 1H), 8.01 (d, J=8.8 Hz, 1H), 7.57 (d, J=7.4 Hz, 2H), 7.40 - 7.33 (m, 2H), 7.31 - 7.24 (m, 1H), 5.85 (t, J=7.2 Hz, 1H), 3.25 (s, 3H), 1.75 (d, J=7.0 Hz, 3H). MS (M + H)+ =454.0 [00419] Compound 2S:1H NMR (400MHz, DMSO-d6) δ = 9.98 (br s, 1H), 8.85 (s, 1H), 8.79 (d, J=2.2 Hz, 1H), 8.67 (br d, J=7.7 Hz, 1H), 8.44 (s, 1H), 8.24 (d, J=2.2 Hz, 1H), 8.14 (dd, J=1.6, 8.7 Hz, 1H), 7.80 (d, J=8.7 Hz, 1H), 7.45 (d, J=7.5 Hz, 2H), 7.33 (t, J=7.6 Hz, 2H), 7.26 - 7.19 (m, 1H), 5.65 (quin, J=7.1 Hz, 1H), 3.17 (s, 3H), 1.63 (d, J=7.0 Hz, 3H). MS (M + H)+ =454.1 [00420] Example 4: Synthesis of N-[2-methoxy-5-[4-(1-phenylethylamino)quinazolin- 6-yl]-3-pyridyl]methanesulfonamide (Compound 3) [00421] To a stirred solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine (50 mg, 152.34 μmol, 1 eq) in DMF (2 mL) and H2O (0.5 mL) was added N-[2-methoxy-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-3-pyridyl]methanesulfonamide (50.00 mg, 152.34 μmol, 1 eq), K3PO4 (97.01 mg, 457.03 μmol, 3 eq) and Pd(PPh3)4 (17.60 mg, 15.23 μmol, 0.1 eq), the reaction was stirred at 80 °C for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was purified by prep-HPLC (column: Phenomenex Luna C18150*30mm*5um; mobile phase: [water (TFA)-ACN]; B%: 10%-40%, 8min). Compound 3, N-[2-methoxy-5-[4-(1- phenylethylamino) quinazolin-6-yl]-3-pyridyl]methanesulfonamide (47.45 mg, 82.94 μmol, 54.44% yield, 98.50% purity, TFA) was obtained as white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.18 (br d, J=7.50 Hz, 1 H), 9.52 (s, 1 H), 8.94 (d, J=1.38 Hz, 1 H), 8.88 (s, 1 H), 8.53 (d, J=2.25 Hz, 1 H), 8.35 (dd, J=8.76, 1.63 Hz, 1 H), 8.07 (d, J=2.25 Hz, 1 H), 7.88 (d, J=8.75 Hz, 1 H), 7.49 (d, J=7.38 Hz, 2 H), 7.38 (t, J=7.57 Hz, 2 H), 7.25 - 7.33 (m, 1 H), 5.83 (t, J=7.25 Hz, 1 H), 4.00 (s, 3 H), 3.09 (s, 3 H), 1.70 (d, J=7.00 Hz, 3 H). MS (M + H)+ =450.1 [00422] Example 5:
[00423] Step 1: Synthesis of (5-amino-6-methyl-3-pyridyl) boronic acid (2) [00424] To a stirred solution of 5-bromo-2-methyl-pyridin-3-amine, 1d, (1 g, 5.35 mmol, 1 eq) in dioxane (5 mL) was added BPD (1.63 g, 6.42 mmol, 1.2 eq), AcOK (1.57 g, 16.04 mmol, 3 eq) and Pd(dppf)Cl2.CH2Cl2 (436.62 mg, 534.65 μmol, 0.1 eq), the reaction was purged with Ar 3 times, and stirred at 100 °C for 16 h under Ar. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was poured into water (50 mL). The aqueous phase was extracted with ethyl acetate (50 mL*2). The combined organic phase was dried with anhydrous Na2SO4, filtered and concentrated in vacuum. Compound 2d, (5-amino-6-methyl-3-pyridyl) boronic acid (2.8 g, crude) was obtained as black oil. [00425] Step 2: Synthesis of 6-(5-amino-6-methyl-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (3d) [00426] To a stirred solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine, 2d, (420.59 mg, 1.28 mmol, 1 eq) in DMF (4 mL) and H2O (1.5 mL) was added (5-amino-6-methyl- 3-pyridyl)boronic acid (194.51 mg, 1.28 mmol, 1 eq), K3PO4 (815.12 mg, 3.84 mmol, 3 eq) and Pd(PPh3)4 (147.91 mg, 128.00 μmol, 0.1 eq), the reaction was stirred at 100 °C for 4 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was purified by prep-HPLC (column: C18-1 150*30mm*5um; mobile phase: [water (TFA)-ACN]; B%: 1%-45%, 8min). Compound 3d, 6- (5-amino-6-methyl-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (70 mg, 144.70 μmol, 10.78% yield, TFA) was obtained as yellow solid. [00427] Step 3: Synthesis of N-[2-methyl-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]-N-methylsulfonyl-methanesulfonamide (4d) [00428] To a stirred solution of 6-(5-amino-6-methyl-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (40 mg, 112.54 μmol, 1 eq) in DCM (2 mL) was added TEA (56.94 mg, 562.69 μmol, 78.32 μL, 5 eq), the reaction was degassed with N2 and cooled to 0 °C. Then MsCl (55 mg, 1.57 mmol, 37.16 μL, 4 eq) was dropwise added to the reaction at 0 °C. The reaction was stirred at 0 °C for 1 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was quenched by adding 5 mL water and extracted with DCM (3*5 mL). The organic layer was washed with brine (5mL), dried over anhydrous Na2SO4, then filtered and concentrated in vacuum. Compound 4d, N-[2-methyl-5-[4- (1-phenylethylamino) quinazolin-6-yl]-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (40 mg, 78.18 μmol, 69.47% yield) was obtained as a yellow oil. [00429] Step 4: Synthesis of N-[2-methyl-5-[4-(1-phenylethylamino)quinazolin-6-yl]- 3-pyridyl]methanesulfonamide (Compound 4) [00430] To a stirred solution of N-[2-methyl-5-[4-(1-phenylethylamino) quinazolin-6-yl]- 3-pyridyl]-N-methylsulfonyl-methanesulfonamide, 4d, (40 mg, 78.18 μmol, 1 eq) in MeOH (2 mL) was added K2CO3 (21.61 mg, 156.37 μmol, 2 eq), the reaction was stirred at 60 °C for 2 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was purified by prep-HPLC (column: Waters Xbridge BEH C18100*30mm*10um; mobile phase: [water (NH4HCO3)-ACN]; B%: 25%-50%, 10 min). Compound 4, N-[2-methyl-5-[4-(1-phenylethylamino) quinazolin-6-yl]-3- pyridyl]methanesulfonamide (3.5 mg, 8.91 μmol, 11.40% yield, 96.59% purity) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 8.79 (s, 1 H), 8.76 (s, 1 H), 8.64 (br d, J=7.88 Hz, 1 H), 8.42 (s, 1 H), 8.08 (dd, J=8.63, 1.63 Hz, 1 H), 7.99 (d, J=1.88 Hz, 1 H), 7.78 (d, J=8.63 Hz, 1 H), 7.45 (d, J=7.50 Hz, 2 H), 7.32 (t, J=7.57 Hz, 2 H), 7.18 - 7.25 (m, 1 H), 5.65 (br t, J=7.25 Hz, 1 H), 3.05 (s, 3 H), 2.56 (s, 3 H), 1.62 (d, J=7.13 Hz, 3 H). MS (M + H)+ =434.2 [00431] Example 6: Synthesis of 6-(2-aminopyrimidin-5-yl)-N-(1-phenylethyl) quinazolin-4-amine (Compound 7) [00432] To a solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine (100 mg, 304.69 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (297.82 mg, 914.06 μmol, 3 eq), Pd(dppf)Cl2 (22.29 mg, 30.47 μmol, 0.1 eq) and 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan- 2-yl) pyrimidin-2-amine (67.36 mg, 304.69 μmol, 1 eq), the mixture was bubbled with N2 for 1 minute and stirred at 100oC for 2 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was filtered, and filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase:[water (0.04%HCl)-ACN];B%: 10%-40%,7min) Compound 7, 6-(2-aminopyrimidin-5-yl)-N-(1-phenylethyl)quinazolin-4-amine (13.84 mg, 36.53 μmol, 11.99% yield, 100% purity, HCl) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.67 (br d, J=5.63 Hz, 1 H), 9.20 (s, 1 H) 8.98 (s, 2 H), 8.90 (s, 1 H), 8.40 (dd, J=8.76, 1.75 Hz, 1 H), 7.91 (d, J=8.75 Hz, 1 H), 7.55 (d, J=7.38 Hz, 2 H), 7.35 - 7.40 (m, 2 H), 7.26 - 7.31 (m, 1 H), 5.85 (quin, J=7.29 Hz, 1 H), 1.74 (d, J=7.00 Hz, 3 H). MS (M + H)+ =343.1 [00433] Example 7: Synthesis of N-(1-phenylethyl)-6-(1H-pyrazolo [3, 4-b] pyridin-5- yl) quinazolin-4-amine (Compound 8) [00434] To a stirred solution of N-(1-phenylethyl)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (100 mg, 266.47 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (260.47 mg, 799.41 μmol, 3 eq), Pd(dppf)Cl2 (19.50 mg, 26.65 μmol, 0.1 eq) and 5-bromo-2H-pyrazolo[3,4-b]pyridine (52.77 mg, 266.47 μmol, 1 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water (0.04%HCl)-ACN];B%: 19%-39%,7min). Compound N-(1-phenylethyl)-6-(1H-pyrazolo[3,4-b]pyridin-5-yl)quinazolin- 4-amine (5.33 mg, 12.39 μmol, 4.65% yield, 93.63% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.50 (br s, 1 H), 9.17 (s, 1 H), 9.09 (d, J=2.13 Hz, 1 H), 8.95 (s, 1 H), 8.73 (d, J=2.13 Hz, 1 H), 8.52 (dd, J=8.75, 1.75 Hz, 1 H), 8.31 (s, 1 H), 7.96 (d, J=8.63 Hz, 1 H), 7.53 (d, J=7.50 Hz, 2 H), 7.39 (t, J=7.50 Hz, 2 H), 7.26 - 7.34 (m, 1 H), 5.79 - 5.99 (m, 1 H), 1.74 (d, J=7.00 Hz, 3 H). MS (M + H)+ =367.1 [00435] Example 8: Synthesis of 6-(2-methyl-1H-imidazo [4, 5-b] pyridin-6-yl)-N-(1- phenylethyl) quinazolin-4-amine (Compound 10) [00436] To a solution of N-(1-phenylethyl)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan- 2-yl) quinazolin-4-amine (100 mg, 266.47 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (260.47 mg, 799.42 μmol, 3 eq), Pd(dppf)Cl2 (19.50 mg, 26.65 μmol, 0.1 eq) and 6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (56.50 mg, 266.47 μmol, 1 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of the desired product was detected. The reaction mixture was concentrated in vacuum, and the crude product was purified by prep-HPLC (column: Phenomenex Luna C18100*30mm*5um; mobile phase: [water (0.04%HCl)-ACN]; B%: 1%- 35%,10min). Compound 10, 6-(2-methyl-1H-imidazo[4,5-b]pyridin-6-yl)-N-(1- phenylethyl)quinazolin-4-amine (7.09 mg, 17.01 μmol, 6.38% yield, 100% purity, HCl) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.98 (br s, 1 H), 9.47 (br s, 1 H), 9.09 (s, 1 H), 8.94 (s, 1 H), 8.76 (br s, 1 H), 8.56 (dd, J=8.76, 1.63 Hz, 1 H), 8.02 (d, J=8.63 Hz, 1 H), 7.59 (d, J=7.50 Hz, 2 H), 7.34 - 7.42 (m, 2 H), 7.25 - 7.32 (m, 1 H), 5.87 (quin, J=7.10 Hz, 1 H), 2.79 (s, 3 H), 1.77 (d, J=7.00 Hz, 3 H). MS (M + H)+ =381.1 [00437] Example 9: Synthesis of 2-[5-[4-(1-phenylethylamino)quinazolin-6-yl]-3- pyridyl]propan-2-ol (Compound 21) [00438] To a stirred solution of N-(1-phenylethyl)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (100 mg, 266.47 μmol, 1 eq) in DMF (1 mL) and H2O (0.2 mL) was added Cs2CO3 (260.47 mg, 799.42 μmol, 3 eq), Pd(dppf)Cl2 (19.50 mg, 26.65 μmol, 0.1 eq) and 2-(5-bromo-3-pyridyl)propan-2-ol (57.58 mg, 266.47 μmol, 1 eq), the mixture was bubbled with N2, the mixture was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Welch Xtimate C18100*25mm*3um;mobile phase: [water(0.04%HCl)-ACN];B%: 5%-35%,8min). Compound 2-[5-[4-(1-phenylethylamino)quinazolin-6-yl]-3-pyridyl]propan-2-ol (61.61 mg, 141.46 μmol, 53.09% yield 96.65% purity, HCl) was obtained as a brown solid.1H NMR (400 MHz, DMSO-d6) δ ppm 11.55 (br d, J=7.75 Hz, 1 H) 9.95 (d, J=1.25 Hz, 1 H) 9.48 (d, J=1.75 Hz, 1 H) 9.26 (s, 1 H) 8.90 - 8.95 (m, 2 H) 8.62 (dd, J=8.82, 1.69 Hz, 1 H) 8.05 (d, J=8.75 Hz, 1 H) 7.63 (d, J=7.38 Hz, 2 H) 7.32 - 7.39 (m, 2 H)7.23 - 7.29 (m, 1 H) 5.86 (quin, J=7.13 Hz, 1 H) 1.80 (d, J=7.00 Hz, 3 H) 1.65 (s, 6 H). MS (M + H)+ =385.1 [00439] Example 10: Synthesis of 6-(5, 6-dimethoxy-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (Compound 22) [00440] To a solution of N-(1-phenylethyl)-6-(4,4,5,5-tetramethyl-1, 3, 2-dioxaborolan-2- yl) quinazolin-4-amine (200 mg, 532.95 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added Cs2CO3 (520.93 mg, 1.60 mmol, 3 eq), 5-bromo-2,3-dimethoxy-pyridine (116.21 mg, 532.95 μmol, 1 eq), Pd(dppf)Cl2 (39.00 mg, 53.29 μmol, 0.1 eq), the mixture was bubbled with N2 for 1 minute, and then stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Welch Xtimate C18100*25mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%: 25%-45%, 8min). Compound 22, 6-(5, 6-dimethoxy-3-pyridyl)-N-(1-phenylethyl) quinazolin-4-amine (18.27 mg, 41.49 μmol, 7.79% yield, 96.04% purity, HCl) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.77 (br d, J=6.97 Hz, 1 H), 9.25 (s, 1 H), 8.90 (s, 1 H), 8.40 - 8.46 (m, 1 H), 8.26 (d, J=1.96 Hz, 1 H), 7.84 - 7.93 (m, 2 H), 7.56 (d, J=7.58 Hz, 2 H), 7.34 - 7.39 (m, 2 H), 7.29 (d, J=7.34 Hz, 1 H), 5.85 (quin, J=7.18 Hz, 1 H), 3.95 (d, J=9.05 Hz, 6 H), 1.75 (d, J=6.97 Hz, 3 H). MS (M + H)+ =387.1 [00441] Example 11: Synthesis of N-(1-phenylethyl)-6-(3H-triazolo [4,5-b]pyridin-6- yl)quinazolin-4-amine (Compound 24) [00442] Step 1: Synthesis of N-(1-phenylethyl)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinazolin-4-amine (2e) [00443] To a stirred solution of 6-bromo-N-(1-phenylethyl) quinazolin-4-amine, 1e, (4 g, 12.19 mmol, 1 eq) in dioxane (8 mL) was added AcOK (3.60 g, 36.68 mmol, 3.01 eq), Pd(dppf)Cl2.CH2Cl2 (995.27 mg, 1.22 mmol, 0.1 eq), BPD (3.71 g, 14.62 mmol, 1.2 eq), the mixture was purged with Ar, the mixture was stirred at 110 °C for 4 h under Ar. LCMS showed starting material was consumed completely and the MS of desired product was detected. TLC (PE : EtOAc = 1 : 1, Rf = 0.35) showed the starting material was consumed completely and new spot was formed. The residue was partitioned between ethyl acetate (100 mL) and H2O (50 mL). The separated organic layer was washed with water, dried over Na2SO4 and evaporated to dryness. The residue was purified by flash column (ISCO 10 g silica, 50-60% Ethyl acetate in Petroleum ether, gradient over 20 min). Compound 2e, N-(1-phenylethyl)-6-(4, 4, 5, 5- tetramethyl-1, 3, 2-dioxaborolan-2-yl) quinazolin-4-amine (1.5g, 4.00 mmol, 32.80% yield) was obtained as brown oil.1H NMR (400 MHz, DMSO-d6) δ ppm 8.85 (d, J=7.75 Hz, 1 H), 8.80 (s, 1 H), 8.42 (s, 1 H), 7.98 (dd, J=8.32, 1.06 Hz, 1 H), 7.63 (d, J=8.25 Hz, 1 H), 7.45 (d, J=7.38 Hz, 2 H), 7.31 (t, J=7.63 Hz, 2 H), 7.13 - 7.25 (m, 1 H), 5.64 (quin, J=7.19 Hz, 1 H), 1.56 - 1.66 (m, 3 H), 1.08 (s, 12 H). MS (M + H)+ =376.3 [00444] Step 2: Synthesis of N-(1-phenylethyl)-6-(3H-triazolo [4,5-b]pyridin-6- yl)quinazolin-4-amine (Compound 24) [00445] To a stirred solution of N-(1-phenylethyl)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinazolin-4-amine, 2e, (150 mg, 399.71 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added K3PO4 (254.54 mg, 1.20 mmol, 3 eq), palladium; triphenylphosphane (46.19 mg, 39.97 μmol, 0.1 eq) and 6-bromo-3H-trazolo[4,5-b]pyridine (63.64 mg, 319.77 μmol, 0.8 eq), the mixture was bubbled with N2, and then stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Waters Xbridge BEH C18100*30mm*10um; mobile phase:[water (10mM NH4HCO3)-ACN];B%: 15%-40%,8min). Compound 24, N-(1-phenylethyl)-6-(3H-triazolo[4,5- b]pyridin-6-yl)quinazolin-4-amine (3.47 mg, 9.36 μmol, 2.34% yield, 99.14% purity) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.25 (d, J=2.00 Hz, 1 H), 8.95 (d, J=1.75 Hz, 1 H), 8.83 (s, 1 H), 8.66 (br d, J=7.63 Hz, 1 H), 8.45 (s, 1 H), 8.31 (dd, J=8.63, 1.88 Hz, 1 H), 7.82 (d, J=8.63 Hz, 1 H), 7.48 (d, J=7.63 Hz, 2 H), 7.34 (t, J=7.57 Hz, 2 H), 7.19 - 7.27 (m, 1 H), 5.67 (t, J=7.25 Hz, 1 H), 1.64 (d, J=7.13 Hz, 3 H). MS (M + H)+ =368.1 [00446] Example 12: Synthesis of 6-(1-methylpyrazolo[4,3-b]pyridin-6-yl)-N-(1- phenylethyl)quinazolin-4-amine (Compound 27) [00447] To a stirred solution of N-(1-phenylethyl)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (100 mg, 266.47 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (260.47 mg, 799.42 μmol, 3 eq), Pd(dppf)Cl2 (19.50 mg, 26.65 μmol, 0.1 eq) and 6-bromo-1-methyl-pyrazolo[4,3-b]pyridine (56.50 mg, 266.47 μmol, 1 eq), the mixture was bubbled with N2, and then stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water(0.04%HCl)-ACN];B%: 15%- 35%,7min) Compound 27, 6-(1-methylpyrazolo[4,3-b]pyridin-6-yl)-N-(1- phenylethyl)quinazolin-4-amine (42.87 mg, 99.21 μmol, 37.23% yield, 96.48% purity, HCl) was obtained as yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.91 (br d, J=7.46 Hz, 1 H), 9.50 (br s, 1 H), 9.15 (d, J=1.83 Hz,1 H), 8.86 - 8.97 (m, 2 H), 8.60 (dd, J=8.68, 1.71 Hz, 1 H), 8.38 (d, J=0.61 Hz, 1 H), 8.01 (d, J=8.80 Hz, 1 H), 7.59 (d, J=8.07 Hz, 2H), 7.38 (t, J=7.52 Hz, 2 H), 7.24 - 7.33 (m, 1 H), 5.80 - 5.97 (m, 1 H), 4.21 (s, 3 H), 1.77 (d, J=6.97 Hz, 3 H). MS (M + H)+ =381.1 [00448] Example 13: Synthesis of 2-amino-N, N-dimethyl-5-[4-(1-phenylethylamino) quinazolin-6-yl] pyridine-3-carboxamide (Compound 36) [00449] To a stirred solution of 6-bromo-N-(1-phenylethyl)quinazolin-4-amine (150 mg, 457.03 μmol, 1 eq) in DMF (3 mL) and H2O(0.5 mL) was added 2-amino-N, N-dimethyl-5-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan-2-yl)pyridine-3-carboxamide (133.07mg, 457.03 μmol, 1 eq) Cs2CO3 (446.73 mg, 1.37 mmol, 3 eq) and Pd(dppf)Cl2 (33.44 mg, 45.70 μmol, 0.1 eq), the mixture was bubbled with N2 for 1 minute, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was filtered, and filtrate was purified by prep-HPLC (column: Welch Xtimate C18 100*25mm*3um; mobile phase: [water (0.04%HCl)-ACN];B%: 10%-40%,8min). Compound 36 2-amino-N, N-dimethyl-5-[4-(1-phenylethylamino) quinazolin-6-yl] pyridine-3-carboxamide (80.96 mg, 174.17 μmol, 38.11% yield, 96.58% purity, HCl) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.87 (br d, J=7.13 Hz, 1 H), 9.29 (s, 1 H), 8.89 (s, 1 H), 8.78 (d, J=2.25 Hz, 1 H), 8.40 - 8.47 (m, 2 H), 7.91 (d, J=8.88 Hz, 1 H), 7.56 (d, J=7.38 Hz, 2 H), 7.33 - 7.40 (m, 2 H), 7.24 - 7.31 (m, 1 H), 5.85 (quin, J=7.13 Hz, 1 H), 3.00 (br d, J=11.01 Hz, 6 H), 1.75 (d, J=7.00 Hz, 3 H). MS (M + H)+ =413.1 [00450] Example 14: Synthesis of 5-[4-(1-phenylethylamino)quinazolin-6-yl]-1,3- benzoxazol-2-amine (Compound 41) [00451] To a solution of N-(1-phenylethyl)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan- 2-yl)quinazolin-4-amine (100 mg, 266.47 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (260.47 mg, 799.42 μmol, 3 eq), Pd(dppf)Cl2 (19.50 mg, 26.65 μmol, 0.1 eq) and 5-bromo-1,3-benzoxazol-2-amine (56.77 mg, 266.47 μmol, 1 eq), the mixture was bubbled with N2, and then stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna C18 100*30mm*5um; mobile phase: [water (0.04%HCl)-ACN]; B%: 10%-40%,10min) Compound 415-[4-(1-phenylethylamino)quinazolin-6-yl]-1,3-benzoxazol-2-amine (23.95 mg, 62.28 μmol, 23.37% yield, 99.18% purity) was obtained as a off-white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.55 (br d, J=7.38 Hz, 1 H), 9.07 (s, 1 H), 8.92 (s, 1 H), 8.42 (dd, J=8.76, 1.75 Hz, 1 H), 7.93 (br d, J=8.63 Hz, 2 H), 7.78 (s, 1 H), 7.49 - 7.59 (m, 4 H), 7.35 - 7.42 (m, 2 H), 7.25 - 7.33 (m, 1 H), 5.87 (quin, J=7.13 Hz, 1 H), 1.74 (d, J=7.13 Hz, 3 H). MS (M + H)+ =382.1 [00452] Example 15:
[00453] Step 1: Synthesis of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (2f) [00454] A solution of 4-bromoaniline (184.88 g, 1.07 mol, 1 eq) in toluene (1.5 L) was added ethyl (E)-2-cyano-3-ethoxy-prop-2-enoate (200 g, 1.18 mol, 1.1 eq), the mixture was stirred at 110 °C for 6 h. TLC (Petroleum ether/Ethyl acetate=3:1, Rf=0.88) showed starting material was consumed completely and a new spot was formed. The reaction mixture was filtered, and filter cake was concentrate in vacuum to give a crude product. Compound 2f, ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (143 g, 484.53 mmol, 45.08% yield) was obtained as a white solid.1H NMR (400MHz, CHLOROFORM-d) δ = 10.74 (br d, J=13.0 Hz, 1H), 8.68 - 7.78 (m, 1H), 7.54 - 7.46 (m, 2H), 7.10 - 6.96 (m, 2H), 4.35 - 4.21 (m, 2H), 1.35 (td, J=7.1, 9.5 Hz, 3H) [00455] Step 2: Synthesis of 6-bromo-4-hydroxy-quinoline-3-carbonitrile (3f) [00456] A solution of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (23 g, 77.93 mmol, 1 eq) in Ph2O (200 mL) was stirred at 270 °C for 8 h. TLC (Petroleum ether/Ethyl acetate=3:1, Rf=0.43) showed a little starting material remained and a new spot was formed. The reaction mixture was poured into MTBE (200 mL). The reaction mixture was filtered, and filter cake was concentrate in vacuum to give a crude product. Compound 3f, 6-bromo-4-hydroxy- quinoline-3-carbonitrile (38.16 g, crude) was obtained as a brown solid.1H NMR (400MHz, DMSO-d6) δ = 12.96 (br s, 1H), 8.76 (s, 1H), 8.16 (br s, 1H), 7.99 - 7.85 (m, 1H), 7.58 (br d, J=8.8 Hz, 1H). [00457] Step 3: Synthesis of N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3- pyridyl]methanesulfonamide (4f) [00458] To a stirred solution of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (4 g, 9.74 mmol, 1 eq) in dioxane (30 mL) and H2O (6 mL) was added 6-bromo-4-hydroxy-quinoline-3-carbonitrile, 3f, (3.15 g, 12.66 mmol, 1.3 eq), Cs2CO3 (9.52 g, 29.22 mmol, 3 eq) ,Pd(dppf)Cl2 (712.64 mg, 973.94 μmol, 0.1 eq), the mixture was bubbled with N23 times, and stirred at 100 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was filtered and the filtrate was purified by prep-HPLC(column: Agela DuraShell C18 250*70mm*10um;mobile phase: [water(10mM NH4HCO3)-ACN];B%: 1%-20%,20min). Compound 4f N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3-pyridyl]methanesulfonamide (2.52 g, 6.72 mmol, 69.03% yield) was obtained as a yellow solid. MS (M + H)+ =375.0 [00459] Step 4: Synthesis of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (5f) [00460] To a solution of POCl3 (18 mL) was added N-[2-chloro-5-(3-cyano-4-hydroxy-6- quinolyl)-3-pyridyl]methanesulfonamide (2.5 g, 6.67 mmol, 1 eq), the mixture was purged with N23 times, and stirred at 130 °C for 16 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrate in vacuum. The residue was dissolved with ethyl acetate (10 mL). The mixture was poured into water (20 mL), and the aqueous phase was extracted with ethyl acetate (20 mL*2). The combined organic phase was dried with anhydrous Na2SO4, filtered and concentrated in vacuum. The crude product was purified by flash column (ISCO 20 g silica, 70 % ethyl acetate in petroleum ether, gradient over 20 min). Based on TLC(Petroleum ether/Ethyl acetate=0:1, Rf=0.78). Compound 5f, N-[2- chloro-5-(4-chloro-3-cyano-6-quinolyl)-3-pyridyl] methanesulfonamide (1.2 g, 3.05 mmol, 45.75% yield) was obtained as a yellow solid.1H NMR (400MHz, DMSO-d6) δ = 9.95 (s, 1H), 9.26 (s, 1H), 8.82 (d, J=2.3 Hz, 1H), 8.57 (d, J=1.9 Hz, 1H), 8.45 - 8.42 (m, 1H), 8.36 - 8.32 (m, 1H), 8.31 (d, J=2.3 Hz, 1H), 3.21 (s, 3H). MS (M + H)+ =393.0 [00461] Step 5: Synthesis of N-[2-chloro-5-[3-cyano-4-(1-phenylethylamino)-6- quinolyl]-3-pyridyl]methanesulfonamide (Compound 48) [00462] To a stirred solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (80 mg, 203.43 μmol, 1 eq) in MeCN (3 mL) was added 1- phenylethanamine (24.65 mg, 203.43 μmol, 25.95 μL, 1 eq), and TEA (32.94 mg, 325.50 μmol, 45.30 μL, 1.6 eq), and the mixture was stirred at 90 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum, and the crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water (0.04%HCl) -ACN]; B%: 23%- 50%,7min). Compound 48, N-[2-chloro-5-[3-cyano-4-(1-phenylethylamino)-6-quinolyl]-3- pyridyl] methanesulfonamide (41.7 mg, 81.06 μmol, 39.85% yield, 100% purity, HCl) was obtained as an off-white solid.1H NMR (400MHz, DMSO-d6) δ = 10.00 (s, 1H), 9.38 (br s, 1H), 9.13 (s, 1H), 8.96 (br s, 1H), 8.87 (d, J=2.3 Hz, 1H), 8.37 - 8.27 (m, 2H), 8.07 (d, J=8.6 Hz, 1H), 7.51 - 7.45 (m, 2H), 7.39 (t, J=7.6 Hz, 2H), 7.33 - 7.27 (m, 1H), 6.10 - 5.95 (m, 1H), 3.20 (s, 3H), 1.81 (d, J=6.6 Hz, 3H). MS (M + H)+ =478.0. [00463] Compounds 48R and 48S were synthesized using a procedure analogous to Compound 48 in Example 15, substituting (1R)-1-phenylethanamine or (1S)-1- phenylethanamine for racemic 1-phenylethanamine. [00464] Compound 48R:1H NMR (400 MHz, DMSO-d6) δ = 10.00 (s, 1H), 9.03 (s, 1H), 8.98 (br d, J = 6.9 Hz, 1H), 8.87 - 8.80 (m, 2H), 8.31 - 8.25 (m, 2H), 8.02 (d, J = 8.8 Hz, 1H), 7.49 - 7.44 (m, 2H), 7.38 (t, J = 7.6 Hz, 2H), 7.32 - 7.26 (m, 1H), 5.98 (br t, J = 7.2 Hz, 1H), 3.20 (s, 3H), 1.78 (d, J = 6.8 Hz, 3H). MS (M + H)+ = 478.0. [00465] Compound 48S:1H NMR (400 MHz, DMSO-d6) δ = 10.00 (br s, 1H), 9.04 (s, 1H), 9.00 (br s, 1H), 8.88 - 8.80 (m, 2H), 8.32 - 8.25 (m, 2H), 8.02 (d, J = 8.8 Hz, 1H), 7.50 - 7.43 (m, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.35 - 7.26 (m, 1H), 5.98 (br t, J = 7.2 Hz, 1H), 3.20 (s, 3H), 1.78 (d, J = 6.7 Hz, 3H). MS (M + H)+ = 478.0. [00466] Example 16: Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 53) [00467] Step 1: Synthesis of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (2g) [00468] To a stirred solution of 6-bromo-4-chloro-quinoline-3-carbonitrile (1 g, 3.74 mmol, 1 eq) in i-PrOH (15 mL) was added TEA (605.22 mg, 5.98 mmol, 832.49 μL, 1.6 eq) 1- phenylethanamine (543.59 mg, 4.49 mmol, 572.20 μL, 1.2 eq), the mixture was stirred at 80 °C for 12 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. Compound 2g, 6-bromo-4-(1- phenylethylamino)quinoline-3-carbonitrile (1.1 g, 3.12 mmol, 83.54% yield) was obtained as a brown solid. MS (M + H)+ = 354.1. [00469] Step 2: Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 53) [00470] To a stirred solution of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (500 mg, 1.42 mmol, 1 eq) in DMF (5 mL) and H2O (1 mL) was added (2-aminopyrimidin-5- yl)boronic acid (197.20 mg, 1.42 mmol, 1 eq), Pd(PPh3)4 (1.64 g, 1.42 mmol, 1 eq) and K3PO4 (903.96 mg, 4.26 mmol, 3 eq), the mixture was bubbled with N2 for 1 minute, and stirred at 100 °C for 3 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was filtered, and filtrate was purified by prep-HPLC (column: Phenomenex luna C18250*50mm*10 um;mobile phase: [water(0.04%HCl)-ACN];B%: 10%- 40%,10min). Compound 536-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino)quinoline-3- carbonitrile (157.87 mg, 379.75 μmol, 26.75% yield, 96.91% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ = 10.09 (br d, J = 8.3 Hz, 1H), 9.36 (s, 1H), 9.18 (s, 2H), 9.08 (s, 1H), 8.41 (dd, J = 1.6, 8.8 Hz, 1H), 8.14 (d, J = 8.9 Hz, 1H), 7.55 (d, J = 7.4 Hz, 2H), 7.43 - 7.35 (m, 2H), 7.33 - 7.28 (m, 1H), 6.14 - 6.03 (m, 1H), 1.87 (d, J = 6.8 Hz, 3H). MS (M + H)+ = 367.1. [00471] Compound 53R was synthesized using the analogous procedure for Compound 53 in Example 16 with chiral starting material. [00472] Compound 53R:1H NMR (400 MHz, DMSO-d6) δ = 10.00 (br s, 1H), 9.30 (br s, 1H), 9.12 (br s, 1H), 9.07 (s, 1H), 8.40 (br d, J = 8.8 Hz, 1H), 8.13 (br d, J = 8.7 Hz, 1H), 7.54 (br d, J = 7.6 Hz, 2H), 7.42 - 7.37 (m, 2H), 7.34 - 7.27 (m, 1H), 6.19 - 5.86 (m, 1H), 1.86 (br d, J = 6.6 Hz, 3H). MS (M + H)+ =367.1 [00473] Example 17: [00474] Step 1: Synthesis of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (2h) [00475] To a stirred solution of 6-bromo-4-chloro-quinoline-3-carbonitrile (1 g, 3.74 mmol, 1 eq) in i-PrOH (15 mL) was added TEA (605.22 mg, 5.98 mmol, 832.49 μL, 1.6 eq), 1- phenylethanamine (543.59 mg, 4.49 mmol, 572.20 μL, 1.2 eq), the mixture was stirred at 80 °C for 12 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. Compound 2h, 6-bromo-4-(1- phenylethylamino)quinoline-3-carbonitrile (1.1 g, 3.12 mmol, 83.54% yield) was obtained as a brown solid. MS (M + H)+ = 354.1. [00476] Step 2: Synthesis of 6-(2-aminopyrimidin-5-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 54) [00477] To a stirred solution of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile (500 mg, 1.42 mmol, 1 eq) in DMF (5 mL) and H2O (1 mL) was added 5-(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine (348.02 mg, 1.42 mmol, 1 eq), Pd(PPh3)4 (164.04 mg, 142.00 μmol, 0.1 eq) and K3PO4 (903.96 mg, 4.26 mmol, 3 eq), the mixture was bubbled with N2 for 1 minute, and stirred at 100oC for 3 h. LCMS showed the starting material was concentrate in vacuum. The reaction mixture was filtered, and filtrate was concentrated to afford 20 mg crude product. The crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water(0.1%TFA)-ACN];B%: 17%- 30%,7min). Compound 54, 4-(1-phenylethylamino)-6-(1H-pyrazolo[3,4-b]pyridin-5- yl)quinoline-3-carbonitrile (10.40 mg, 20.62 μmol, 1.45% yield, 100% purity, TFA) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ = 9.18 - 9.00 (m, 2H), 8.97 - 8.86 (m, 1H), 8.78 (s, 1H), 8.71 (d, J = 1.8 Hz, 1H), 8.35 (br d, J = 8.8 Hz, 1H), 8.30 (s, 1H), 8.00 (d, J = 8.7 Hz, 1H), 7.47 (br d, J = 7.6 Hz, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.32 - 7.23 (m, 1H), 6.08 - 5.88 (m, 1H), 1.79 (d, J = 6.7 Hz, 3H). MS (M + H)+ = 391.1. [00478] Example 18: Synthesis of 4-[[(1R)-1-phenylethyl] amino]-6-(1H- pyrazolo [3, 4-b] pyridin-5-yl) quinoline-3-carbonitrile (Compound 54R) [00479] Step 1: Synthesis of 6-bromo-4-chloro-quinoline-3-carbonitrile (2i) [00480] To a solution of 6-bromo-4-hydroxy-quinoline-3-carbonitrile (10 g, 40.15 mmol, 1 eq) in SOCl2 (50 mL) was added DMF (293.47 mg, 4.02 mmol, 308.92 μL, 0.1 eq), the mixture was purged with N2, the reaction was stirred at 20 °C for 12 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. Compound 2i 6-bromo-4-chloro-quinoline-3-carbonitrile (10.3 g, 38.50 mmol, 95.90% yield) was obtained as a black solid. MS (M + H)+ =269.1 [00481] Step 2: Synthesis of 6-bromo-4-[[(1R)-1-phenylethyl] amino] quinoline-3- carbonitrile (3i) [00482] To a solution of 6-bromo-4-chloro-quinoline-3-carbonitrile, 2i, (2 g, 7.48 mmol, 1 eq) in i-PrOH (15 mL) was added (1R)-1-phenylethanamine (996.58 mg, 8.22 mmol, 1.06 mL, 1.1 eq) and TEA (1.21 g, 11.96 mmol, 1.66 mL, 1.6 eq), the mixture was purged with N2, the reaction was stirred at 80 °C for 12 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was filtered and filtrate was concentrated in vacuum. Compound 3i, 6-bromo-4-[[(1R)-1-phenylethyl] amino] quinoline-3-carbonitrile (2.4 g, 6.81 mmol, 91.14% yield) was obtained as black oil. MS (M + H)+ =352.1 [00483] Step 3: Synthesis of 4-[[(1R)-1-phenylethyl] amino]-6-(1H- pyrazolo [3, 4-b] pyridin-5-yl) quinoline-3-carbonitrile (Compound 54R) [00484] To a solution of 6-bromo-4-[[(1R)-1-phenylethyl] amino] quinoline-3-carbonitrile (1.57 g, 4.46 mmol, 1 eq) in DMF (15 mL) and H2O (3 mL) was added Cs2CO3 (4.36 g, 13.37 mmol, 3 eq), Pd(dppf)Cl2 (326.15 mg, 445.73 μmol, 0.1 eq) and 5-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl) -1H-pyrazolo [3,4-b]pyridine (1.09 g, 4.46 mmol, 1 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LC-MS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filter caked was concentrated in vacuum. The filer cake was purified by prep- HPLC(column: Phenomenex luna C18250*50mm*10 um;mobile phase: [water(0.04%HCl)- ACN];B%: 15%-45%,10min). Compound 54R, 4-[[(1R)-1-phenylethyl]amino]-6-(1H- pyrazolo[3, 4-b]pyridin-5-yl) quinoline-3-carbonitrile (70.43 mg, 159.40 μmol,3.58% yield, 96.62% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.79 (br s, 1 H), 9.25 (br s, 1 H), 9.13 (br d, J=9.17 Hz, 2 H), 8.78 (br d, J=1.59 Hz, 1 H), 8.50 (br d, J=8.80 Hz, 1 H), 8.32 (s, 1 H), 8.10 - 8.21 (m, 1 H), 7.52 (br d, J=7.46 Hz, 2 H), 7.41 (t, J=7.64 Hz, 2 H), 7.28 - 7.35 (m,1 H), 6.10 (br t, J=6.97 Hz, 1 H), 1.85 (br d, J=6.60 Hz, 3 H). MS (M + H)+ =391.1 [00485] Example 19: Synthesis of 4-(1-phenylethylamino)-6-(1H-pyrrolo [2, 3-b] pyridin-5-yl) quinoline-3-carbonitrile (Compound 55) [00486] To a solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (80 mg, 200.35 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (195.84 mg, 601.06 μmol, 3 eq), Pd(dppf)Cl2 (14.66 mg, 20.04 μmol, 0.1 eq) and 5-bromo-1H-pyrrolo[2,3-b]pyridine (39.48 mg, 200.35 μmol, 1 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water(0.04%HCl)-ACN];B%: 20%-40%,7min). Compound 55, 4-(1-phenylethylamino)- 6-(1H-pyrrolo[2,3-b]pyridin-5-yl)quinoline-3-carbonitrile (12.19 mg, 28.28 μmol, 14.11% yield, 98.80% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 12.00 (br s, 1 H) 9.81 (br d, J=7.82 Hz, 1 H) 9.20 (s, 1 H) 9.13 (s, 1 H) 8.84 (d, J=1.96 Hz, 1 H) 8.54 (s, 1 H) 8.49 (dd, J=8.80, 1.34 Hz, 1 H) 8.14 (d, J=8.80 Hz, 1 H) 7.59 - 7.64 (m, 1 H) 7.52 (d, J=7.46 Hz, 2 H) 7.41 (t, J=7.64 Hz, 2 H) 7.28 - 7.35 (m, 1 H) 6.62 (dd, J=3.30, 1.71 Hz, 1 H) 6.00 - 6.18 (m, 1 H) 1.85 (d, J=6.60 Hz, 3 H). MS (M + H)+ = 390.0 [00487] Example 20: Synthesis of 6-(2-methyl-3H-imidazo[4,5-b]pyridin-6-yl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 56) [00488] To a stirred solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl)quinoline-3-carbonitrile (120 mg, 300.53 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (293.76 mg, 901.60 μmol, 3 eq), Pd(dppf)Cl2 (21.99 mg, 30.05 μmol, 0.1 eq) and 6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (82.84 mg, 390.69 μmol, 1.3 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was filtered, the filtrate was concentrated in vacuum to afford crude product (20 mg), the crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water (0.04%HCl)-ACN]; B%: 12%-28%, 7min). Compound 566-(2-methyl- 3H-imidazo[4, 5-b]pyridin-6-yl)-4-(1-phenylethylamino)quinoline-3-carbonitrile (9 mg, 18.76 μmol, 6.24% yield, 91.93% purity, HCl) was obtained as a brown solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.26 (br d, J=8.07 Hz, 1 H) 9.51 (s, 1 H) 9.17 (s, 1 H) 9.09 (s, 1 H) 8.92 (s, 1 H) 8.52 (br d, J=8.80 Hz, 1 H) 8.20 (d, J=8.68 Hz, 1 H) 7.57 (br d, J=7.70 Hz, 2 H) 7.35 - 7.43 (m, 2 H) 7.26 - 7.33 (m, 1 H) 6.01 - 6.15 (m, 1 H) 2.85 (s, 3 H) 1.89 (br d, J=6.60 Hz, 3 H). MS (M + H)+ =405.1 [00489] Example 21: Synthesis of 6-(5-hydroxy-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 61) [00490] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 5-bromopyridin-3-ol (43.58 mg, 250.44 μmol, 1 eq), the mixture was bubbled with N2, and stirred at 100°C for 3h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filter cake was washed by DMSO then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna C18150*30mm*5um;mobile phase: [water(0.1%TFA)-ACN];B%: 1%-35%,8min). Compound 61, 6-(5-hydroxy-3-pyridyl)-4-(1- phenylethylamino)quinoline-3-carbonitrile (30.91 mg, 62.70 μmol, 25.03% yield, 97.45% purity, TFA) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.24 (br d, J=8.19 Hz, 1 H), 9.06 (d, J=1.22 Hz, 1 H), 8.92 (s, 1 H), 8.68 (d, J=1.59 Hz, 1 H), 8.26 - 8.35 (m, 2 H), 8.01 (d, J=8.68 Hz, 1 H), 7.86 (s, 1 H), 7.43 - 7.49 (m, 2 H), 7.38 (t, J=7.64 Hz, 2 H), 7.25 - 7.33 (m, 1 H), 5.94 - 6.06 (m, 1 H), 1.79 (d, J=6.72 Hz, 3 H). MS (M + H)+ =367.1 [00491] Example 22: Synthesis of 6-(5-cyano-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 64) [00492] To a solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 5-bromopyridine-3-carbonitrile (45.83 mg, 250.44 μmol, 1 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filtrate was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (HCl)-ACN]; B%: 15%-45%,8min). Compound 64, 6-(5- cyano-3-pyridyl)-4-(1-phenylethylamino)quinoline-3-carbonitrile (12.41 mg, 28.52 μmol, 11.39% yield, 94.65% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO- d6) δ ppm 10.04 (br d, J=7.82 Hz, 1 H), 9.51 (d, J=2.20 Hz, 1 H), 9.41 (s, 1 H), 9.14 (s, 2 H), 9.01 (t, J=1.96 Hz, 1 H), 8.52 (dd, J=8.74, 1.41 Hz, 1 H), 8.20 (d, J=8.68 Hz, 1 H), 7.54 (d, J=7.46 Hz, 2 H), 7.40 (t, J=7.52 Hz, 2 H), 7.27 - 7.35 (m, 1 H), 6.10 (quin, J=7.06 Hz, 1 H), 1.87 (d, J=6.60 Hz, 3 H). MS (M + H)+ = 376.2 [00493] Example 23: Synthesis of 6-(6-amino-5-cyano-3-pyridyl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 65) [00494] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.4 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 2-amino-5-bromo-pyridine-3-carbonitrile (49.59 mg, 250.44 μmol, 1 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filtrate was purified by prep-HPLC (column: Phenomenex luna C18 80*40mm*3 um;mobile phase: [water(HCl)-ACN]; B%: 17%-43%, 7min). Compound 65, 6-(6- amino-5-cyano-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (26.28 mg, 59.16 μmol, 23.62% yield, 96.10% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.86 (br d, J=7.46 Hz, 1 H) 9.05 - 9.19 (m, 2 H) 8.83 - 8.96 (m, 1 H) 8.60 (d, J=2.20 Hz, 1 H) 8.35 - 8.43 (m, 1 H) 8.01 - 8.15 (m, 1 H) 7.52 (d, J=7.46 Hz, 2 H) 7.38 - 7.42 (m, 2 H) 7.30 - 7.34 (m, 1 H) 5.85 - 6.27 (m, 1 H) 1.86 (d, J=6.72 Hz, 3 H). MS (M + H)+ = 391.1 [00495] Example 24: Synthesis of 6-[5-(1-hydroxy-1-methyl-ethyl)-3-pyridyl]-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 67) [00496] To a stirred solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (120 mg, 300.53 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (293.76 mg, 901.60 μmol, 3 eq), Pd(dppf)Cl2 (21.99 mg, 30.05 μmol, 0.1 eq) and 2-(5-bromo-3-pyridyl)propan-2-ol (84.42 mg, 390.69 μmol, 1.3 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was filtered, filter cake washed by MeOH, the filter liquor was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)- ACN];B%: 5%-25%,8min). Compound 67, 6-[5-(1-hydroxy-1-methyl- ethyl)-3-pyridyl]-4-(1-phenylethylamino) quinoline-3-carbonitrile (36.18 mg, 77.82 μmol, 25.89% yield, 95.70% purity, HCl) was obtained as a brown solid.1H NMR (400 MHz, DMSO- d6) δ ppm 10.72 (br d, J=8.07 Hz, 1 H) 9.91 (s, 1 H) 9.62 (s, 1 H) 9.39 (s, 1 H) 9.11 (s, 1 H) 8.94 (s, 1 H) 8.60 (br d, J=8.68 Hz, 1 H) 8.25 (d, J=8.80 Hz, 1 H) 7.62 (d, J=7.58 Hz, 2 H) 7.37 (t, J=7.52 Hz, 2 H) 7.24 - 7.33 (m, 1 H) 6.02 - 6.15 (m, 1 H) 1.93 (d, J=6.60 Hz, 3 H) 1.64 (s, 6 H). MS (M + H)+ =409.1 [00497] Example 25: Synthesis of 6-(5,6-dimethoxy-3-pyridyl)-4-(1- phenylethylamino)quinoline-3-carbonitrile (Compound 68) [00498] Step 1: Synthesis of 6-bromo-4-(1-phenylethylamino) quinoline-3- carbonitrile (2j) [00499] To a solution of 6-bromo-4-chloro-quinoline-3-carbonitrile (8 g, 29.91 mmol, 1 eq) i-PrOH (30 mL) was added 1-phenylethanamine (3.99 g, 32.90 mmol, 4.20 mL, 1.1 eq) and TEA (4.84 g, 47.85 mmol, 6.66 mL, 1.6 eq), the reaction was stirred at 80 °C for 12 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. Compound 2j, 6-bromo-4-(1- phenylethylamino) quinoline-3-carbonitrile (9 g, 25.55 mmol, 85.44% yield) was obtained as a gray solid. MS (M + H)+ =352.1 [00500] Step 2: Synthesis of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinoline-3-carbonitrile (3j) [00501] To a solution of 6-bromo-4-(1-phenylethylamino)quinoline-3-carbonitrile, 2j, (7 g, 19.87 mmol, 1 eq) in dioxane (80 mL) was added AcOK (5.85 g, 59.61 mmol, 3 eq), Pd(dppf)Cl2.CH2Cl2 (1.62 g, 1.99 mmol, 0.1 eq), BPD (6.06 g, 23.84 mmol, 1.2 eq), the mixture was purged with Ar then the reaction was stirred at 110 °C for 8 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. TLC (PE: EtOAc = 3: 1, Rf = 0.24) showed the starting material was consumed completely and new spot was formed. The reaction mixture was cooled to room temperature and quenched by water (50 mL), extracted with ethyl acetate (40 mL *2). The combined organics were washed with brine (30 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue. The residue was purified by flash column (ISCO 40 g silica, 50-70 % ethyl acetate in petroleum ether, gradient over 40 min). Compound 2j, 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)quinoline-3-carbonitrile (5.5 g, 13.77 mmol, 69.32% yield) was obtained as brown oil. MS (M + H)+ =400.3 [00502] Step 3: Synthesis of 6-(5,6-dimethoxy-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (Compound 68) [00503] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile, 3j, (230 mg, 576.02 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (563.04 mg, 1.73 mmol, 3 eq), Pd(dppf)Cl2 (42.15 mg, 57.60 μmol, 0.1 eq) and 5-bromo-2,3-dimethoxy-pyridine (163.28 mg, 748.83 μmol, 1.3 eq), the mixture was bubbled with N2, and the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um;mobile phase: [water(0.04%HCl)- ACN];B%: 10%-30%,8min). Compound 68, 6-(5,6-dimethoxy-3-pyridyl)-4-(1-phenylethylamino)quinoline- 3-carbonitrile (39.53 mg, 88.45 μmol, 15.36% yield, 100% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.05 (br s, 1 H) 8.89 (br s, 1 H) 8.25 - 8.39 (m, 2 H) 8.00 (br d, J=8.68 Hz, 1 H) 7.70 - 7.82 (m, 1 H) 7.45 - 7.52 (m, 2 H) 7.39 (t, J=7.58 Hz, 2 H) 7.23 - 7.34 (m, 1 H) 6.00 (br d, J=5.99 Hz, 1 H) 3.83 - 4.05 (m, 6 H) 1.80 (br d, J=6.60 Hz, 3 H). MS (M + H)+ =411.1 [00504] Example 26: Synthesis of 4-(1-phenylethylamino)-6-(3H- triazolo [4, 5-b] pyridin-6-yl) quinoline-3-carbonitrile (Compound 70) [00505] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl)quinoline-3-carbonitrile (150 mg, 375.66 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (367.20 mg, 1.13 mmol, 3 eq), Pd(dppf)Cl2 (27.49 mg, 37.57 μmol, 0.1 eq) and 6-bromo-3H-triazolo[4,5-b]pyridine (97.19 mg, 488.36 μmol, 1.3 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna C1875*30mm*3um; mobile phase: [water(0.04%HCl)-ACN];B%: 5%-35%,8min). Compound 70, 4-(1-phenylethylamino)-6-(3H-triazolo[4, 5-b]pyridin-6-yl)quinoline-3- carbonitrile (21.49 mg, 49.03 μmol, 13.05% yield, 97.63% purity, HCl) was obtained as off- white solid.1H NMR (400 MHz, DMSO-d6) δ = 9.36 - 9.29 (m, 1H), 9.23 - 9.15 (m, 1H), 9.07 - 8.82 (m, 2H), 8.56 - 8.41 (m, 1H), 8.14 - 7.97 (m, 1H), 7.49 (br d, J = 6.9 Hz, 2H), 7.43 - 7.35 (m, 2H), 7.33 - 7.25 (m, 1H), 6.10 - 5.90 (m, 1H), 1.80 (br d, J = 6.4 Hz, 3H). MS (M + H)+ =392.1 [00506] Example 27: Synthesis of 6-(1-methyl pyrazolo [4, 3-b] pyridin-6-yl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 73) [00507] To a stirred solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (200 mg, 500.89 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (489.59 mg, 1.50 mmol, 3 eq), Pd(dppf)Cl2 (36.65 mg, 50.09 μmol, 0.1 eq) and 6-bromo-1-methyl-pyrazolo[4,3-b]pyridine (138.07 mg, 651.15 μmol, 1.3 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prp-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%: 10%- 30%,8min). Compound 73, 6-(1-methylpyrazolo[4, 3-b]pyridin-6-yl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (70.96 mg, 175.44 μmol, 35.03% yield, 100% purity) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.94 (br s, 1 H), 9.42 (br s, 1 H), 9.17 (s, 1 H), 9.10 (s, 1 H), 8.88 (br s, 1 H), 8.55 (br d, J=8.75 Hz, 1 H), 8.38 (s, 1 H), 8.15 (br d, J=8.75 Hz, 1 H), 7.54 (br d, J=7.75 Hz, 2 H), 7.40 (t, J=7.63 Hz, 2 H), 7.27 - 7.34 (m, 1 H), 6.10 (br t, J=7.07 Hz, 1 H), 4.20 (s, 3 H), 1.86 (br d, J=6.38 Hz, 3 H). MS (M + H)+ =405.1 [00508] Example 28: Synthesis of 6-(2-amino-1, 3-benzoxazol-5-yl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 87) [00509] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinoline-3-carbonitrile (120 mg, 300.53 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added Cs2CO3 (293.76 mg, 901.60 μmol, 3 eq), Pd(dppf)Cl2 (21.99 mg, 30.05 μmol, 0.1 eq) and 5-bromo-1,3-benzoxazol-2-amine (83.23 mg, 390.69 μmol, 1.3 eq), the mixture was bubbled with N2, and stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water(0.04%HCl)-ACN]; B%: 5%-25%,8min). Compound 87, 6-(2-amino-1,3-benzoxazol-5-yl)-4-(1-phenylethylamino)quinoline-3-carbonitrile (15.31 mg, 34.64 μmol, 11.53% yield, 100% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.83 (br s, 1 H), 9.11 (s, 2 H), 8.40 (dd, J=8.82, 1.31 Hz, 1 H), 8.11 (br d, J=8.75 Hz, 1 H), 8.02 (br s, 1 H), 7.82 (s, 1 H), 7.57 (s, 2 H), 7.51 (d, J=7.50 Hz, 2 H), 7.41 (t, J=7.57 Hz, 2 H), 7.27 - 7.35 (m, 1 H), 5.95 - 6.20 (m, 1 H), 1.84 (d, J=6.63 Hz, 3 H). MS (M + H)+ =406.1 [00510] Example 29: Synthesis of N-[2-chloro-5-[3-cyano-4-[[(1R)-1-(4- fluorophenyl)ethyl]amino]-6-quinolyl]-3-pyridyl]methanesulfonamide (Compound 97R)
[00511] Step 1: Synthesis of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate (2k) [00512] A solution of 4-bromoaniline (184.88 g, 1.07 mol, 1 eq) in toluene (1.5 L) was added ethyl (E)-2-cyano-3-ethoxy-prop-2-enoate (200 g, 1.18 mol, 1.1 eq), the mixture was stirred at 110oC for 6 h. TLC (Petroleum ether/Ethyl acetate=3:1, Rf=0.88) showed starting material was consumed completely and new spot was formed. The reaction mixture was filtered, and filter caked was concentrate in vacuum. Compound 2k, ethyl (Z)-3-(4-bromoanilino)-2- cyano-prop-2-enoate (150 g, 508.25 mmol, 47.29% yield) was obtained as a white solid.1H NMR (400 MHz, CHLOROFORM-d) δ = 10.75 (br d, J = 13.0 Hz, 1H), 8.42 - 7.78 (m, 1H), 7.52 (br d, J = 8.6 Hz, 2H), 7.07 - 6.94 (m, 2H), 4.36 - 4.24 (m, 2H), 1.43 - 1.30 (m, 3H). [00513] Step 2: Synthesis of 6-bromo-4-hydroxy-quinoline-3-carbonitrile (3k) [00514] A solution of ethyl (Z)-3-(4-bromoanilino)-2-cyano-prop-2-enoate, 2k, (40 g, 135.53 mmol, 1 eq) in Ph2O (400 mL) was stirred at 270oC for 8 h. TLC (Petroleum ether/Ethyl acetate=3:1, Rf=0.43) showed a little starting material was remained and new spot was formed. The reaction mixture was poured into MTBE (200 mL). The reaction mixture was filtered, and filter cake was concentrated in vacuum. Compound 3k, 6-bromo-4-hydroxy-quinoline-3- carbonitrile (2 g, 8.03 mmol, 5.92% yield) was obtained as a yellow solid. [00515] Step 3: Synthesis of N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3- pyridyl]methanesulfonamide (4k) [00516] To a stirred solution of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (1.7 g, 4.14 mmol, 1 eq) in dioxane (30 mL), H2O (5 mL) was added 6-bromo-4-hydroxy-quinoline-3-carbonitrile, 3k, (1.55 g, 6.21 mmol, 1.5 eq), Pd(dppf)Cl2 (302.87 mg, 413.93 μmol, 0.1 eq), Cs2CO3 (4.05 g, 12.42 mmol, 3 eq) the mixture was bubbled with N2 for 1 minute, and the mixture was stirred at 100 °C for 16 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. The crude residue was purified by prep-HPLC (Agela DuraShell C18250*80mm*10um column; 1-30 % acetonitrile in a 10mM ammonium bicarbonate solution in water, 20 min gradient). Compound 4k, N-[2-chloro-5-(3-cyano-4- hydroxy-6-quinolyl)-3-pyridyl]methanesulfonamide (1 g, 2.67 mmol, 64.46% yield) was obtained as a white solid. MS (M + H)+ = 375.0. [00517] Step 4: Synthesis of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (5k) [00518] A solution of N-[2-chloro-5-(3-cyano-4-hydroxy-6-quinolyl)-3- pyridyl]methanesulfonamide, 4k, (1 g, 2.67 mmol, 1 eq) in POCl3 (16.50 g, 107.61 mmol, 10 mL, 40.33 eq) was stirred at 120 °C for 16 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. The reaction mixture was dissolved with DCM (10 mL), and poured into water (30 mL). The aqueous phase was extracted with ethyl acetate (30 mL*2). The combined organic phase was dried with anhydrous Na2SO4, filtered and concentrated in vacuum. Compound 5k, N-[2-chloro- 5-(4-chloro-3-cyano-6-quinolyl)-3-pyridyl]methanesulfonamide (600 mg, 1.53 mmol, 57.19% yield) was obtained as a yellow solid. MS (M + H)+ = 393.0. [00519] Step 5: Synthesis of N-[2-chloro-5-[3-cyano-4-[[(1R)-1-(4-fluorophenyl) ethyl]amino]-6-quinolyl]-3-pyridyl]methanesulfonamide (Compound 97R) [00520] To a stirred solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide, 5k, (100 mg, 254.29 μmol, 1 eq) in i-PrOH (3 mL) was added (1R)-1-(4-fluorophenyl)ethanamine (35.39 mg, 254.29 μmol, 1 eq), TEA (41.17 mg, 406.87 μmol, 56.63 μL, 1.6 eq), the mixture was stirred at 80 °C for 16 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrated, and the crude residue was purified by prep-HPLC (Phenomenex Gemini-NX 150*30mm*5um column; 20-50 % acetonitrile in a 0.1% trifluoroacetic acid solution in water, 9 min gradient). Compound 97R, N-[2-chloro-5-[3-cyano-4-[[(1R)-1-(4- fluorophenyl)ethyl]amino] -6-quinolyl]-3-pyridyl]methanesulfonamide (14.70 mg, 23.93 μmol, 9.41% yield, 99.31% purity, TFA) was obtained as a yellow solid.1H NMR (400 MHz, DMSO- d6) δ = 9.98 (br s, 1H), 9.01 (s, 1H), 8.90 - 8.81 (m, 2H), 8.78 (br s, 1H), 8.32 - 8.22 (m, 2H), 8.01 (d, J = 8.8 Hz, 1H), 7.50 (dd, J = 5.4, 8.4 Hz, 2H), 7.20 (t, J = 8.8 Hz, 2H), 5.94 (br t, J = 7.1 Hz, 1H), 3.20 (s, 3H), 1.76 (d, J = 6.6 Hz, 3H). MS (M + H)+ = 496.0. [00521] Compound 97S was synthesized using the same procedure as Compound 97R in Example 29, but substituting (1S)-1-(4-fluorophenyl)ethanamine for (1R)-1-(4- fluorophenyl) ethanamine. [00522] Compound 97S:1H NMR (400 MHz, DMSO-d6) δ = 9.98 (br s, 1H), 9.00 (s, 1H), 8.90 - 8.75 (m, 3H), 8.31 - 8.23 (m, 2H), 8.01 (d, J = 8.6 Hz, 1H), 7.50 (dd, J = 5.4, 8.4 Hz, 2H), 7.20 (t, J = 8.8 Hz, 2H), 5.94 (br t, J = 6.9 Hz, 1H), 3.20 (s, 3H), 1.76 (d, J = 6.6 Hz, 3H). MS (M + H)+ = 496.0. [00523] Example 30: Synthesis of N-[2-chloro-5-[3-cyano-4-(indan-1-ylamino)-6- quinolyl]-3-pyridyl]methanesulfonamide (Compound 101) [00524] To a solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (70 mg, 178.01 μmol, 1 eq) in i-PrOH (1 mL) was added TEA (54.04 mg, 534.02 μmol, 74.33 μL, 3 eq) and indan-1-amine (23.71 mg, 178.01 μmol, 22.80 μL, 1 eq). The mixture was stirred at 80 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C18 80*40mm*3 um;mobile phase:[water(0.04%HCl)- ACN];B%: 25%-45%,7min). Compound 101, N-[2-chloro-5-[3-cyano-4-(indan-1-ylamino)-6-quinolyl]-3-pyridyl] methanesulfonamide (20.04 mg, 38.07 μmol, 21.39% yield, 100% purity, HCl) was obtained as a pale yellow solid.1H NMR (400MHz, DMSO-d6) δ = 9.99 (s, 1H), 9.86 (br d, J=8.4 Hz, 1H), 9.19 (s, 1H), 9.03 (d, J=1.3 Hz, 1H), 8.79 (d, J=2.4 Hz, 1H), 8.39 (dd, J=1.6, 8.8 Hz, 1H), 8.27 (d, J=2.4 Hz, 1H), 8.20 (d, J=8.8 Hz, 1H), 7.43 (d, J=7.4 Hz, 1H), 7.41 - 7.32 (m, 2H), 7.31 - 7.25 (m, 1H), 6.30 (q, J=8.0 Hz, 1H), 3.17 (s, 3H), 3.16 - 3.09 (m, 1H), 3.06 - 2.95 (m, 1H), 2.76 (dtd, J=2.8, 7.9, 12.8 Hz, 1H), 2.46 - 2.38 (m, 1H). MS (M + H)+ = 490.0. [00525] Example 31: Synthesis of N-[2-chloro-5-[3-cyano-4-[(2-hydroxyindan-1- yl)amino]-6-quinolyl]-3- pyridyl]methanesulfonamide (Compound 103) [00526] To a solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3-pyridyl]methane sulfonamide (60 mg, 152.58 μmol, 1 eq) in MeCN (1 mL) was added 1-aminoindan-2-ol (22.76 mg, 152.58 μmol, 1 eq) and TEA (46.32 mg, 457.73 μmol, 63.71 μL, 3 eq). The mixture was stirred at 80 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum, and the crude product was purified by prep-HPLC (column: Waters Xbridge BEH C18 100*30mm*10um;mobile phase:[water(10mM NH4HCO3)-ACN];B%: 18%-48%,8min). Compound 103, N-[2-chloro-5-[3-cyano-4-[(2-hydroxyindan- 1-yl)amino]-6-quinolyl]-3- pyridyl]methanesulfonamide (10.94 mg, 21.62 μmol, 14.17% yield, 100% purity) was obtained as a pale yellow solid.1H NMR (400MHz, DMSO-d6) δ = 9.90 (br s, 1H), 8.86 (d, J=1.6 Hz, 1H), 8.79 (d, J=2.3 Hz, 1H), 8.62 (s, 1H), 8.26 (d, J=2.3 Hz, 1H), 8.20 (br d, J=9.1 Hz, 1H), 8.14 (dd, J=1.7, 8.7 Hz, 1H), 7.99 (d, J=8.6 Hz, 1H), 7.38 (d, J=7.0 Hz, 1H), 7.35 - 7.24 (m, 3H), 5.92 (dd, J=4.9, 9.0 Hz, 1H), 5.48 (d, J=4.6 Hz, 1H), 4.79 - 4.73 (m, 1H), 3.23 - 3.16 (m, 1H), 3.15 (s, 3H), 2.98 (br d, J=15.4 Hz, 1H). MS (M + H)+ = 506.2. [00527] Example 32: Synthesis of N-[2-chloro-5-[3-cyano-4-[[(1S)-2-hydroxy-1- phenyl-ethyl]amino]-6-quinolyl]-3-pyridyl]methanesulfonamide (Compound 104S) [00528] To a stirred solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methanesulfonamide (80 mg, 203.43 μmol, 1 eq) in MeCN (3 mL) was added (2S)-2- amino-2-phenyl-ethanol (33.49 mg, 244.12 μmol, 1.2 eq), TEA (32.94 mg, 325.50 μmol, 45.30 μL, 1.6 eq), and the mixture was stirred at 90 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Waters Xbridge BEH C18 100*30mm*10um;mobile phase: [water (10mM NH4HCO3) -ACN]; B%: 5%-30%,8min). Compound 104S, N-[2-chloro-5-[3-cyano-4-[[(1S)-2-hydroxy-1-phenyl -ethyl]amino]-6- quinolyl]-3-pyridyl]methane sulfonamide (28 mg, 56.68 μmol, 27.86% yield, 100% purity) was obtained as a white solid.1H NMR (400MHz, DMSO-d6) δ = 9.96 (br s, 1H), 8.98 (s, 1H), 8.85 (d, J=2.0 Hz, 1H), 8.46 (s, 1H), 8.27 (d, J=2.1 Hz, 1H), 8.21 - 8.12 (m, 2H), 7.97 (d, J=8.6 Hz, 1H), 7.46 (d, J=7.5 Hz, 2H), 7.36 (t, J=7.6 Hz, 2H), 7.30 - 7.24 (m, 1H), 5.79 - 5.71 (m, 1H), 5.33 (t, J=5.9 Hz, 1H), 3.97 - 3.88 (m, 1H), 3.86 - 3.78 (m, 1H), 3.19 (s, 3H). MS (M + H)+ =494.2. [00529] Compound 104R was synthesized using the same procedure as Compound 104S in Example 32, but substituting (2R)-2-amino-2-phenyl-ethanol for (2S)-2-amino-2- phenyl-ethanol. [00530] Compound 104R:1H NMR (400MHz, DMSO-d6) δ = 9.97 (br s, 1H), 8.99 (s, 1H), 8.86 (d, J=2.0 Hz, 1H), 8.47 (s, 1H), 8.28 (d, J=2.3 Hz, 1H), 8.22 - 8.12 (m, 2H), 7.98 (d, J=8.6 Hz, 1H), 7.47 (d, J=7.4 Hz, 2H), 7.37 (t, J=7.5 Hz, 2H), 7.32 - 7.25 (m, 1H), 5.80 - 5.72 (m, 1H), 5.34 (t, J=5.9 Hz, 1H), 3.98 - 3.89 (m, 1H), 3.83 (td, J=5.2, 10.9 Hz, 1H), 3.20 (s, 3H). MS (M + H)+ =494.2 [00531] Example 33: Synthesis of N-[2-chloro-5-[3-cyano-4-[(1- phenylcyclopropyl)amino]-6-quinolyl]-3-pyridyl]methanesulfonamide (Compound 106) [00532] To a stirred solution of N-[2-chloro-5-(4-chloro-3-cyano-6-quinolyl)-3- pyridyl]methane sulfonamide (100 mg, 254.29 μmol, 1 eq) in MeCN (2 mL) was added 1- phenylcyclopropanamine hydrochloride (43.14 mg, 254.29 μmol, 1 eq), pyridine hydrochloride (47.02 mg, 406.87 μmol, 1.6 eq), and the mixture was stirred at 90 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture filtered to give a filtrate, and the filtrate was purified by prep-HPLC (column: Phenomenex Gemini-NX 80*40mm*3um;mobile phase: [water(10mM NH4HCO3)-ACN];B%:10%-40%, 8 min). Compound 106, N-[2-chloro-5-[3-cyano-4-[(1-phenylcyclopropyl)amino]-6-quinolyl]-3- pyridyl]methane sulfonamide (19.4 mg, 39.21 μmol, 15.42% yield, 99.04% purity) was obtained as a off-white solid.1H NMR (400MHz, DMSO-d6) δ= 9.93 (br s, 1H), 9.15 (s, 1H), 8.92 (d, J=1.6 Hz, 1H), 8.83 (d, J=2.3 Hz, 1H), 8.48 (s, 1H), 8.30 (d, J=2.4 Hz, 1H), 8.18 (dd, J=1.8, 8.7 Hz, 1H), 7.99 (d, J=8.6 Hz, 1H), 7.34 - 7.28 (m, 2H), 7.21 - 7.15 (m, 3H), 3.17 (s, 3H), 1.78 - 1.69 (m, 2H), 1.59 (br s, 2H). MS (M + H)+ =490.2. [00533] Example 34: Synthesis of N-[2-chloro-5-[4-[[(1S)-1-(4- fluorophenyl)ethyl]amino]quinazolin-6-yl]-3-pyridyl]methanesulfonamide (Compound 111S)
[00534] Step 1: Synthesis of N-[(1S)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4- amine (2l) [00535] A solution of 4-chloro-6-iodo-quinazoline (2 g, 6.88 mmol, 1 eq) in i-PrOH (20 mL) was added (1S)-1-(4-fluorophenyl)ethanamine (1.05 g, 7.57 mmol, 1.1 eq), TEA (1.11 g, 11.02 mmol, 1.53 mL, 1.6 eq), the mixture was stirred at 80 °C for 16 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. Compound 2l, N-[(1S)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin- 4-amine (2.5 g, 6.36 mmol, 92.35% yield) was obtained as a yellow oil. MS (M + H)+ = 394.1. [00536] Step 2: Synthesis of N-[2-chloro-5-[4-[[(1S)-1-(4-fluorophenyl)ethyl]amino] quinazolin-6-yl]-3-pyridyl]methanesulfonamide (Compound 111S) [00537] To a stirred solution of N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)-3-pyridyl]-N-methylsulfonylmethanesulfonamide, 3b, (2.09 g, 5.09 mmol, 1 eq) in DMF (15 mL) /H2O (3 mL) was added N-[(1S)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4-amine, 2l, (2 g, 5.09 mmol, 1 eq), Pd(PPh3)4 (587.78 mg, 508.65 μmol, 0.1 eq), K3PO4 (3.24 g, 15.26 mmol, 3eq) the mixture was purged with N2 for three times, and the mixture was stirred at 100 °C for 5 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was filtered, and the filtrate was purified by prep-HPLC (Agela DuraShell C18250*80mm*10um column; 15-45 % acetonitrile in a 10 mM ammonium bicarbonate solution in water, 20 min gradient). Compound 111S, N-[2-chloro-5-[4-[[(1S)-1-(4- fluorophenyl)ethyl]amino]quinazolin-6-yl]-3-pyridyl]methanesulfonamide (993.30 mg, 2.10 mmol, 41.38% yield, 100% purity) was obtained as a yellow solid.1H NMR (400 MHz, DMSO- d6+D2O) δ = 8.78 (d, J = 2.0 Hz, 1H), 8.74 (d, J = 2.2 Hz, 1H), 8.51 (s, 1H), 8.21 (d, J = 2.2 Hz, 1H), 8.13 (dd, J = 2.0, 8.6 Hz, 1H), 7.83 (d, J = 8.8 Hz, 1H), 7.50 (dd, J = 5.5, 8.6 Hz, 2H), 7.15 - 7.08 (m, 2H), 5.68 (d, J = 6.8 Hz, 1H), 3.18 (s, 3H), 1.64 (d, J = 7.1 Hz, 3H). MS (M + H)+ = 472.1. [00538] Compound 111R was synthesized using the same procedure as Compound 111S in Example 34, but substituting (1R)-1-(4-fluorophenyl)ethanamine for (1S)-1-(4- fluorophenyl)ethanamine. [00539] Compound 111R:1H NMR (400 MHz, DMSO-d6, T=273+80K) δ = 8.73 (d, J = 2.0 Hz, 1H), 8.71 (d, J = 2.2 Hz, 1H), 8.43 (s, 1H), 8.19 (d, J = 2.2 Hz, 1H), 8.08 (dd, J = 2.0, 8.6 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.48 (dd, J = 5.7, 8.4 Hz, 2H), 7.10 (t, J = 8.8 Hz, 2H), 5.63 (q, J = 7.0 Hz, 1H), 3.16 (s, 3H), 1.62 (d, J = 6.8 Hz, 3H)). MS (M + H)+ = 472.1. [00540] Example 35: Synthesis of 6-(2-aminopyrimidin-5-yl)-N-[(1R)-1-(4- fluorophenyl)ethyl]quinazolin -4-amine (Compound 121R) [00541] Step 1: Synthesis of N-(3-ethynylphenyl)-6-iodo-quinazolin-4-amine (2m) [00542] To a stirred solution of 4-chloro-6-iodo-quinazoline, 1m, (300 mg, 1.03 mmol, 1 eq) in i-PrOH (5 mL) was added (1R)-1-(4-fluorophenyl)ethanamine (143.73 mg, 1.03 mmol, 1 eq), TEA (167.20 mg, 1.65 mmol, 229.99 μL, 1.6 eq), and the mixture was stirred at 80 °C for 15 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was concentrate in vacuum. Compound 2m, N-[(1R)-1-(4- fluorophenyl)ethyl]-6-iodo -quinazolin-4-amine (300 mg, 762.98 μmol, 73.88% yield) was obtained as yellow solid. MS (M + H)+ = 394.1. [00543] Step 2: Synthesis of 6-(2-aminopyrimidin-5-yl)-N-[(1R)-1-(4-fluorophenyl) ethyl]quinazolin-4-amine (Compound 121R) [00544] To a stirred solution of N-[(1R)-1-(4-fluorophenyl)ethyl]-6-iodo-quinazolin-4- amine, 2m, (300 mg, 762.98 μmol, 1 eq) in DMF (5 mL), H2O (1 mL) was added 5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-amine (168.67 mg, 762.98 μmol, 1eq), K3PO4 (485.86 mg, 2.29 mmol, 3 eq), Pd(PPh3)4 (88.17 mg, 76.30 μmol, 0.1 eq), the mixture was bubbled with N2 for 1 minute, and the mixture was stirred at 120oC for 16 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was filtered, and filtrate was purified directly. The crude residue was purified by prep-HPLC (Phenomenex luna C18250*50mm*10 um; 10-40 % acetonitrile in a 0.05% hydrochloric acid solution in water, 10 min gradient). Compound 121R, 6-(2-aminopyrimidin-5-yl)-N-[(1R)-1-(4- fluorophenyl)ethyl]quinazolin-4-amine (283.62 mg, 707.18 μmol, 92.69% yield, 98.95% purity, HCl) was obtained as pale yellow solid.1H NMR (400 MHz, DMSO-d6, T=273+80K) δ = 10.78 (br s, 1H), 9.30 (br s, 1H), 9.00 (s, 2H), 8.83 (s, 1H), 8.37 (dd, J = 1.8, 8.8 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 7.64 (dd, J = 5.5, 8.6 Hz, 2H), 7.17 (t, J = 8.8 Hz, 2H), 5.88 (t, J = 7.2 Hz, 1H), 1.78 (d, J = 7.0 Hz, 3H). MS (M + H)+ = 361.2. [00545] Example 36: Synthesis of 4-(1-phenylethylamino)-6-(3-quinolyl)quinoline-3- carbonitrile (Compound 122) [00546] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 3-bromoquinoline (52.11 mg, 250.44 μmol, 33.62 μL, 1 eq), the mixture was bubbled with N2, and stirred at 100°C for 3h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Waters Xbridge Prep OBD C18150*40mm*10um;mobile phase: [water(10mM NH4HCO3)-ACN];B%: 35%-65%,8min). Compound 122, 4-(1-phenylethylamino)-6-(3-quinolyl)quinoline-3-carbonitrile (19.89 mg, 49.67 μmol, 19.83% yield, 100% purity) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.53 (s, 1 H), 9.12 (s, 1 H), 8.83 (s, 1 H), 8.47 (s, 1 H), 8.31 (br dd, J=14.43, 8.56 Hz, 2 H), 8.08 - 8.16 (m, 2 H), 8.01 (d, J=8.44 Hz, 1 H), 7.82 (br t, J=7.58 Hz, 1 H), 7.64 - 7.74 (m, 1 H), 7.46 (br d, J=7.58 Hz, 2 H), 7.35 (br t, J=7.46 Hz, 2 H), 7.20 - 7.29 (m, 1 H), 5.89 (br t, J=7.15 Hz, 1 H), 1.75 (br d, J=6.60 Hz, 3 H). MS (M + H)+ = 401.1 [00547] Example 37: Synthesis of 4-(1-phenylethylamino)-6-(3-pyridyl) quinoline-3- carbonitrile (Compound 123) [00548] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (0.5 mL) and H2O (0.1 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 3-bromopyridine (39.57 mg, 250.44 μmol, 24.13 μL, 1 eq), the mixture was bubbled with N2, and stirred at 100oC for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C18 100*40mm*5 um;mobile phase: [water(0.1%TFA)-ACN];B%: 1%-35%,8min). Compound 123, 4-(1-phenylethylamino)-6-(3-pyridyl) quinoline-3-carbonitrile (28.25 mg, 60.83 μmol, 24.29% yield, 100% purity, TFA) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ = 9.30 (br s, 1H), 9.22 (d, J = 1.3 Hz, 1H), 9.11 (s, 1H), 8.95 (s, 1H), 8.76 (d, J = 4.6 Hz, 1H), 8.47 (br d, J = 7.7 Hz, 1H), 8.39 (dd, J = 1.4, 8.7 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.75 (dd, J = 5.2, 7.8 Hz, 1H), 7.51 - 7.45 (m, 2H), 7.39 (t, J = 7.6 Hz, 2H), 7.33 - 7.25 (m, 1H), 6.03 (quin, J = 6.9 Hz, 1H), 1.80 (d, J = 6.7 Hz, 3H). MS (M + H)+ = 351.1. [00549] Example 38: Synthesis of 4-(1-phenylethylamino)-6-(1H-pyrazolo [4, 3-c] pyridin-3-yl) quinoline-3-carbonitrile (Compound 124) [00550] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 3-bromo-1H-pyrazolo[4,3-c]pyridine (49.59 mg, 250.44 μmol, 49.59 μL, 1 eq), the mixture was bubbled with N2, and stirred at 100oC for 3 h. LCMS showed starting material was consumed completely and the Ms of desired product was detected. The reaction mixture was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C18100*40mm*5 um;mobile phase: [water(0.1%TFA)-ACN];B%: 1%- 35%,8min) Compound 124, 4-(1-phenylethylamino)-6-(1H-pyrazolo[4,3-c]pyridin-3- yl)quinoline-3-carbonitrile (7.96 mg, 15.17 μmol, 6.06% yield, 96.13% purity, TFA) was obtained as a gray solid.1H NMR (400 MHz, DMSO-d6) δ = 15.06 - 14.65 (m, 1H), 9.92 (s, 1H), 9.30 (d, J = 1.3 Hz, 1H), 8.84 (br d, J = 6.8 Hz, 1H), 8.71 (s, 1H), 8.62 (d, J = 6.7 Hz, 1H), 8.53 (dd, J = 1.6, 8.7 Hz, 1H), 8.15 (d, J = 6.7 Hz, 1H), 8.06 (d, J = 8.6 Hz, 1H), 7.46 (d, J = 7.5 Hz, 2H), 7.35 (t, J = 7.6 Hz, 2H), 7.30 - 7.20 (m, 1H), 5.98 - 5.83 (m, 1H), 1.76 (d, J = 6.6 Hz, 3H). MS (M + H)+ = 391.1 [00551] Example 39: Synthesis of 4-(1-phenylethylamino)-6-(1H-pyrazolo [3,4- c]pyridin-3-yl)quinoline-3-carbonitrile (Compound 125) [00552] To a stirred solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.5 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 3-bromo-1H-pyrazolo[3,4-c]pyridine (59.51 mg, 300.53 μmol, 59.51 μL, 1.2 eq), the mixture was bubbled with N2 for 1 minute, and stirred at 100oC for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filter cake was washed by DMSO then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Waters Xbridge BEH C18 100*30mm*10um;mobile phase: [water( NH4HCO3)-ACN];B%: 35%-60%,8min). Compound 125, 4-(1-phenylethylamino)-6-(1H-pyrazolo[3,4-c]pyridin-3-yl)quinoline-3-carbonitrile (7.28 mg, 18.65 μmol, 7.45% yield, 100% purity) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 14.02 (br s, 1 H), 9.15 (br d, J=9.41 Hz, 2 H), 8.46 (s, 1 H), 8.40 (br d, J=7.21 Hz, 2 H), 8.13 (br d, J=5.13 Hz, 1 H), 7.99 (br d, J=8.31 Hz, 1 H), 7.46 (br d, J=7.70 Hz, 2 H), 7.35 (br t, J=7.15 Hz, 2 H), 7.26 (br d, J=7.34 Hz, 1 H), 5.84 (br s, 1 H), 1.75 (br d, J=5.99 Hz, 3 H).MS (M + H)+ = 391.0 [00553] Example 40: Synthesis of 6-(5-cyano-6-methoxy-3-pyridyl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 126) [00554] To a solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.4 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 5-bromo-2-methoxy-pyridine-3-carbonitrile (53.35 mg, 250.44 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%: 20%-40%, 8min). Compound 126, 6-(5-cyano-6- methoxy-3-pyridyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (22.91 mg, 50.74 μmol, 20.26% yield, 97.87% purity, HCl) was obtained as a white solid.1H NMR (400 MHz, DMSO- d6) δ ppm 9.50 (br s, 1 H), 9.15 (br s, 1 H), 9.10 (d, J=2.20 Hz, 1 H), 9.00 (s, 1 H), 8.90 (d, J=2.32Hz, 1 H), 8.40 (d, J=8.56 Hz, 1 H), 8.02 - 8.10 (m, 1 H), 7.50 (d, J=7.46 Hz, 2 H), 7.39 (t, J=7.58 Hz, 2 H), 7.27 - 7.34 (m, 1 H), 6.05 (br t, J=7.34 Hz, 1 H) 4.09 (s, 3 H) 1.83 (d, J=6.72 Hz, 3 H). MS (M + H)+ = 406.1 [00555] Example 41: Synthesis of 6-[3-(2-oxo-3H-1, 3, 4-oxadiazol-5-yl) phenyl]-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 127) [00556] To a solution of 5-(3-bromophenyl)-3H-1, 3, 4-oxadiazol-2-one (50 mg, 207.43 μmol, 1 eq) in DMF (1 mL) and H2O (0.2 mL) was added Cs2CO3 (202.76 mg, 622.30 μmol, 3 eq), Pd(dppf)Cl2 (15.18 mg, 20.74 μmol, 0.1 eq) and 4-(1-phenylethylamino)-6-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline-3-carbonitrile (82.83 mg, 207.43 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%: 15%-40%, 8min). Compound 127, 6-[3-(2-oxo-3H-1, 3, 4- oxadiazol-5-yl) phenyl]-4-(1-phenylethylamino)quinoline-3-carbonitrile (10.32 mg, 21.96 μmol, 10.59% yield, 100% purity, HCl) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 12.73 (s, 1 H), 9.45 (br s, 1 H), 9.06 (s, 1 H), 8.96 (s, 1 H), 8.34 (d, J=8.80 Hz, 1 H), 8.17 (s, 1 H), 7.99 - 8.09 (m, 2 H), 7.88 (d, J=7.82 Hz, 1 H), 7.69 - 7.77 (m, 1 H), 7.41 - 7.48 (m, 2 H), 7.35 (t, J=7.64 Hz, 2 H), 7.22 -7.30 (m, 1 H), 6.00 (br t, J=7.34 Hz, 1 H), 1.78 (d, J=6.72 Hz, 3 H). MS (M + H)+ = 434.0 [00557] Example 42: Synthesis of 4-(1-phenylethylamino)-6-[3-(4H-1, 2, 4-triazol-3- yl) phenyl] quinoline-3-carbonitrile (Compound 128) [00558] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinoline-3-carbonitrile (100 mg, 250.44 μmol, 1 eq) in DMF (2.5 mL) and H2O (0.4 mL) was added Cs2CO3 (244.80 mg, 751.33 μmol, 3 eq), Pd(dppf)Cl2 (18.33 mg, 25.04 μmol, 0.1 eq) and 3-(3-bromophenyl)-1H-1,2,4-triazole (56.11 mg, 250.44 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered and filtrate was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (HCl)-ACN]; B%: 10%-35%, 8min). Compound 128, 4- (1-phenylethylamino)-6-[3-(4H-1, 2, 4-triazol-3-yl) phenyl] quinoline-3-carbonitrile (12.89 mg, 28.46 μmol, 11.36% yield, 100% purity, HCl) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.82 (br s, 1 H), 9.15 - 9.23 (m, 1 H), 9.12 (s, 1 H),8.53 (br s, 2 H), 8.43 (d, J=8.31 Hz, 1 H), 8.05 - 8.19 (m, 2 H), 7.97 (br d, J=7.82 Hz, 1 H), 7.71 (t, J=7.76 Hz, 1 H), 7.50 (d, J=7.58 Hz, 2 H), 7.40 (t, J=7.58 Hz, 2 H), 7.27 - 7.34 (m, 1 H), 6.09 (br t, J=7.40 Hz, 1 H), 1.84 (d, J=6.60 Hz, 3 H). MS (M + H)+ = 417.1 [00559] Example 43: Synthesis of 6-[5-(2-oxo-3H-1, 3, 4-oxadiazol-5-yl)-3-pyridyl]-4- (1-phenylethylamino) quinoline-3-carbonitrile (Compound 129) [00560] To a solution of 5-(5-bromo-3-pyridyl)-3H-1, 3, 4-oxadiazol-2-one (181.84 mg, 751.33 μmol, 1 eq) in DMF (1 mL) and H2O (0.2 mL) was added Cs2CO3 (734.39 mg, 2.25 mmol, 3 eq), Pd(dppf)Cl2 (54.98 mg, 75.13 μmol, 0.1 eq) and 4-(1-phenylethylamino)-6- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline-3-carbonitrile (300 mg, 751.33 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water(HCl)-ACN]; B%: 22%-45%,7min). Compound 129, 6-[5-(2-oxo-3H-1,3,4-oxadiazol-5- yl)-3-pyridyl]-4-(1-phenylethylamino)quinoline-3-carbonitrile (46.87 mg, 99.53 μmol, 13.25% yield, 100% purity, HCl) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 12.97 (s, 1 H), 9.82 (br d, J=7.63 Hz, 1 H), 9.35 (d, J=2.13 Hz, 1 H), 9.27 (s, 1 H), 9.13 (s, 1 H), 9.08 (d, J=2.00 Hz, 1 H), 8.62 (t, J=2.06 Hz, 1 H), 8.53 (dd, J=8.76, 1.50 Hz, 1 H) 8.16 (d, J=8.75 Hz, 1 H) 7.51 (d, J=7.38 Hz, 2 H) 7.41 (t, J=7.57 Hz, 2 H) 7.29 - 7.35 (m, 1 H) 6.09 (quin, J=7.07 Hz, 1 H) 1.85 (d, J=6.63 Hz, 3 H). MS (M + H)+ = 435.0 [00561] Example 44: Synthesis of 6-(3, 5-difluoro-4-hydroxy-phenyl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 130) [00562] To a solution of 4-(1-phenylethylamino)-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinoline-3-carbonitrile (80 mg, 200.35 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (195.84 mg, 601.06 μmol, 3 eq), Pd(dppf)Cl2 (14.66 mg, 20.04 μmol, 0.1 eq) and 4-bromo-2,6-difluoro-phenol (41.87 mg, 200.35 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, then the filtrate was purified by prep-HPLC (column: Phenomenex Luna C18150*30mm*5um; mobile phase: [water (0.04%TFA)-ACN]; B%: 1%-40%, 8min). Compound 130, 6-(3, 5- difluoro-4-hydroxy-phenyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (11.49 mg, 22.29 μmol, 11.13% yield, 100% purity, TFA) was obtained as off-white solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.60 (br s, 1 H), 8.86 (s, 2 H) 8.75 (br s, 1 H), 8.25 (br d, J=8.88 Hz, 1 H), 7.88 - 7.95 (m, 1 H) 7.66 - 7.74 (m, 2 H), 7.43 - 7.48 (m, 2 H), 7.38 (t, J=7.63 Hz, 2 H), 7.25 - 7.32 (m, 1 H), 5.97 (br t, J=6.82 Hz, 1 H), 1.79 (d, J=6.63 Hz, 3 H). MS (M + H)+ = 402.1 [00563] Example 45: Synthesis of 6-(3, 5-dichloro-4-hydroxy-phenyl)-4-(1- phenylethylamino) quinoline-3-carbonitrile (Compound 131) [00564] To a solution of 4-(1-phenylethylamino)-6-(4, 4, 5, 5-tetramethyl-1, 3, 2- dioxaborolan-2-yl) quinoline-3-carbonitrile (80 mg, 200.35 μmol, 1 eq) in DMF (2 mL) and H2O (0.4 mL) was added Cs2CO3 (195.84 mg, 601.06 μmol, 3 eq), Pd(dppf)Cl2 (14.66 mg, 20.04 μmol, 0.1 eq) and 4-bromo-2,6-dichloro-phenol (48.47 mg, 200.35 μmol, 1 eq), the mixture was bubbled with N2, the reaction was stirred at 100 °C for 3 h. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%: 20%-40%, 8min). Compound 131, 6-(3, 5-dichloro-4-hydroxy-phenyl)-4-(1-phenylethylamino) quinoline-3-carbonitrile (15.23 mg, 32.35 μmol, 16.15% yield, 100% purity, HCl) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.59 (br s, 1 H), 9.53 (br s, 1 H), 8.99 (br s, 2 H), 8.34 (d, J=8.68 Hz, 1 H), 8.02 (br d, J=8.80 Hz, 1 H), 7.98 (s, 2 H), 7.48 (d, J=7.58 Hz, 2 H), 7.39 (t, J=7.58 Hz, 2 H), 7.26 - 7.33 (m, 1 H), 6.04 (br t, J=7.15 Hz, 1 H), 1.83 (d, J=6.60 Hz, 3 H). MS (M + H)+ = 434.0 [00565] Example 46: Synthesis of 2-methoxy-5-[4-[[(1R)-1-phenylethyl]amino] quinazolin-6-yl]pyridine-3-carbonitrile (Compound 132) [00566] To a stirred solution of 5-bromo-2-methoxy-pyridine-3-carbonitrile (45.41 mg, 213.18 μmol, 1 eq) in H2O (0.2 mL) and DMF (1 mL) was added N-[(1R)-1-phenylethyl]-6- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine (80 mg, 213.18 μmol, 1 eq), Cs2CO3 (208.37 mg, 639.54 μmol, 3 eq) and Pd(dppf)Cl2 (15.60 mg, 21.32 μmol, 0.1 eq), the reaction was stirred at 100 °C for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.04%HCl)-ACN]; B%:10%-35%, 8min). Compound 132, 2-methoxy-5-[4-[[(1R)-1-phenylethyl] amino] quinazolin-6-yl] pyridine- 3-carbonitrile (3.4 mg, 8.91 μmol, 4.18% yield, 100% purity) was obtained as a pale solid.1H NMR (400 MHz, DMSO-d6) δ ppm 9.75 - 9.94 (m, 1 H), 8.99 (d, J=2.57 Hz, 1 H), 8.93 (d, J=0.98 Hz, 1 H), 8.75 - 8.83 (m, 2 H), 8.34 - 8.43 (m, 1 H), 7.85 (d, J=8.80 Hz, 1 H), 7.49 (d, J=7.58 Hz, 2 H), 7.37 (t, J=7.52 Hz, 2 H), 7.23 - 7.33 (m, 1 H), 5.72 - 5.87 (m, 1 H), 4.08 (s, 3 H), 1.69 (d, J=6.97 Hz, 3 H). MS (M + H)+ =382.0 [00567] Example 47: Synthesis of methyl 5-[4-[[(1R)-1-phenylethyl]amino]quinazolin -6-yl]pyridine-3-carboxylate (Compound 135) [00568] Step 1: Synthesis of 6-bromo-N-[(1R)-1-phenylethyl]quinazolin-4-amine (2n) [00569] To a stirred solution of 6-bromo-4-chloro-quinazoline, 1n, (4 g, 16.43 mmol, 1 eq) in i-PrOH (40 mL) was added (1R)-1-phenylethanamine (1.99 g, 16.43 mmol, 2.12 mL, 1 eq) and TEA (2.66 g, 26.28 mmol, 3.66 mL, 1.6 eq) , the reaction was stirred at 80 ℃ for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was cooled to ambient temperature, quenched with water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. Compound 2n, 6-bromo-N- [(1R)-1-phenylethyl] quinazolin-4-amine (5.6 g, crude) was obtained as yellow solid. [00570] Step 2: Synthesis of N-[(1R)-1-phenylethyl]-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (3n) [00571] To a stirred solution of 6-bromo-N-[(1R)-1-phenylethyl]quinazolin-4-amine, 2n, (5.6 g, 17.06 mmol, 1 eq) in dioxane (60 mL) was added BPD (5.20 g, 20.47 mmol, 1.2 eq), Pd(dppf)Cl2.CH2Cl2 (1.39 g, 1.71 mmol, 0.1 eq) and AcOK (5.02 g, 51.19 mmol, 3 eq), the reaction was stirred at 110 °C for 12 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was cooled to ambient temperature, quenched with water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by flash column (ISCO 40 g silica, 40-60 % ethyl acetate in petroleum ether, gradient over 20 min). Based on TLC (PE: EtOAc = 2: 1, Rf = 0.30). Compound 3n, N-[(1R)-1-phenylethyl]-6-(4, 4, 5, 5-tetramethyl-1,3,2-dioxaborolan-2- yl)quinazolin-4-amine (1.8 g, 4.80 mmol, 28.11% yield) was obtained as yellow oil . [00572] Step 3: Synthesis of methyl 5-[4-[[(1R)-1-phenylethyl]amino]quinazolin-6- yl]pyridine-3-carboxylate (Compound 135) [00573] To a stirred solution of methyl 5-bromopyridine-3-carboxylate, 3n, (41.45 mg, 191.86 μmol, 1.2 eq) in DMF (1.5 mL) and H2O (0.3 mL) was added N-[(1R)-1-phenylethyl]-6- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine (60 mg, 159.88 μmol, 1 eq), K3PO4 (101.81 mg, 479.65 μmol, 3 eq) and Pd(dppf)Cl2 (11.70 mg, 15.99 μmol, 0.1 eq), the reaction was stirred at 80 °C for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, and filtrate was used for purified directly. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (TFA)-ACN]; B%: 20%-45%, 8min). Compound 135, methyl 5-[4-[[(1R)-1-phenylethyl] amino] quinazolin-6-yl] pyridine-3- carboxylate (9.16 mg, 23.19 μmol, 14.50% yield, 97.326% purity) was obtained as a brown solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.22 - 10.35 (m, 1 H), 9.34 (d, J=2.20 Hz, 1 H), 9.19 (d, J=1.71 Hz, 1 H), 9.08 (s, 1 H), 8.92 (s, 1 H), 8.67 - 8.72 (m, 1 H), 8.51 (d, J=9.05 Hz, 1 H), 7.91 (d, J=8.80 Hz, 1 H), 7.50 (d, J=7.34 Hz, 2 H), 7.38 (t, J=7.52 Hz, 2 H), 7.25 - 7.34 (m, 1 H), 5.75 - 5.96 (m, 1 H), 3.96 (s, 3 H), 1.72 (d, J=6.97 Hz, 3 H). MS (M + H)+ =385.0 [00574] Example 48: Synthesis of 6-[4-[[(1R)-1-phenylethyl]amino]quinazolin-6-yl]- 3H-isobenzofuran-1-one (Compound 136) [00575] To a stirred solution of 6-bromo-3H-isobenzofuran-1-one (40.87 mg, 191.86 μmol, 1.2 eq) in DMF (1.5 mL) and H2O (0.3 mL) was added N-[(1R)-1-phenylethyl]-6- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine (60 mg, 159.88 μmol, 1 eq), K3PO4 (101.81 mg, 479.65 μmol, 3 eq) and Pd(dppf)Cl2 (11.70 mg, 15.99 μmol, 0.1 eq), the reaction was stirred at 80 °C for 3 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, the filtrate was concentrated, and the crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (TFA)-ACN]; B%: 20%-50%,8min). Compound 136, 6-[4-[[(1R)-1-phenylethyl]amino]quinazolin-6-yl]-3H-isobenzofuran-1-one (23.53 mg, 60.08 μmol, 37.57% yield, 97.385% purity) was obtained as a pale yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.33 (br d, J=7.70 Hz, 1 H), 9.07 (d, J=1.47 Hz, 1 H), 8.91 (s, 1 H), 8.49 (dd, J=8.80, 1.59 Hz, 1 H), 8.37 (s, 1 H), 8.28 (dd, J=8.01, 1.53 Hz, 1 H), 7.83 - 7.96 (m, 2 H), 7.50 (d, J=7.46 Hz, 2 H), 7.39 (t, J=7.52 Hz, 2 H), 7.25 - 7.33 (m, 1 H), 5.77 - 5.94 (m, 1 H), 5.53 (s, 2 H), 1.72 (d, J=6.97 Hz, 3 H). MS (M + H)+ =382.0 [00576] Example 49: Synthesis of N-[(1R)-1-phenylethyl]-6-[3-(4H-1,2,4-triazol-3- yl)phenyl]quinazolin-4-amine (Compound 137) [00577] Step 1: Synthesis of 6-iodo-N-[(1R)-1-phenylethyl]quinazolin-4-amine (2p) [00578] To a stirred solution of 4-chloro-6-iodo-quinazoline, 1p, (3 g, 10.33 mmol, 1 eq) in i-PrOH (30 mL) was added (1R)-1-phenylethanamine (1.25 g, 10.33 mmol, 1.33 mL, 1 eq) and TEA (1.67 g, 16.52 mmol, 2.30 mL, 1.6 eq), the reaction was stirred at 80°C for 3h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was cooled to ambient temperature, quenched with water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. Compound 2p, 6-iodo-N- [(1R)-1-phenylethyl] quinazolin-4-amine (3.2 g, 8.53 mmol, 82.58% yield) was obtained as yellow oil [00579] Step 2: Synthesis of N-[(1R)-1-phenylethyl]-6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)quinazolin-4-amine (3p) [00580] To a stirred solution of 6-iodo-N-[(1R)-1-phenylethyl]quinazolin-4-amine, 2p, (3.20 g, 8.53 mmol, 1 eq) in dioxane (30 mL) was added BPD (2.17 g, 8.53 mmol, 1 eq) Pd(dppf)Cl2 (624.05 mg, 853.00 μmol, 0.1 eq) and AcOK (2.51 g, 25.59 mmol, 3 eq), the reaction was stirred at 110 °C for 12 h under Ar. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was cooled to ambient temperature, quenched with water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by flash column (ISCO 80 g silica, 40-60 % ethyl acetate in petroleum ether, gradient over 20 min). Based on TLC (PE: EtOAc = 1:1, Rf = 0.30). Compound 3p, N-[(1R)-1-phenylethyl]-6-(4, 4, 5,5-tetramethyl-1,3,2-dioxaborolan-2- yl)quinazolin-4-amine (2.4 g, 6.40 mmol, 74.98% yield) was obtained as a yellow oil. [00581] Step 3: Synthesis of N-[(1R)-1-phenylethyl]-6-[3-(4H-1,2,4-triazol-3- yl)phenyl]quinazolin-4-amine (Compound 137) [00582] To a stirred solution of 3-(3-bromophenyl)-1H-1,2,4-triazole (35.82 mg, 159.88 μmol, 1 eq) in DMF (1 mL) and H2O (0.2 mL) was added N-[(1R)-1-phenylethyl]-6-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)quinazolin-4-amine, 3p, (60mg, 159.88 μmol, 1 eq), Cs2CO3 (156.28 mg, 479.65 μmol, 3 eq) and Pd(dppf)Cl2 (11.70 mg, 15.99 μmol, 0.1 eq), the reaction was stirred at 100 °C for 12 h under N2. LCMS showed starting material was consumed completely and the MS of desired product was detected. The reaction was filtered, then the filtrate was concentrated in vacuum. The crude product was purified by prep-HPLC (column: Phenomenex Luna 80*30mm*3um; mobile phase: [water (0.1% TFA)-ACN]; B%: 10%-30%, 8min). Compound 137, N-[(1R)-1-phenylethyl]-6-[3-(4H-1, 2, 4-triazol-3-yl) phenyl] quinazolin-4-amine (7.9 mg, 20.13 μmol, 12.59% yield, 100% purity) was obtained as a yellow solid.1H NMR (400 MHz, DMSO-d6) δ ppm 10.09 - 10.48 (m, 1 H), 9.02 (s, 1 H), 8.89 (s, 1 H), 8.45 (s, 1 H), 8.41 (d, J=8.92 Hz, 1 H), 8.13 (br d, J=8.19 Hz, 1 H), 7.90 (d, J=8.68 Hz, 2 H), 7.65 - 7.78 (m, 1 H), 7.50 (d, J=7.34 Hz, 2 H), 7.38 (t, J=7.46 Hz, 2 H), 7.26 - 7.32 (m, 1 H), 5.71 - 5.98 (m, 1 H), 1.72 (d, J=7.09 Hz, 3 H). MS (M + H)+ =393.1 [00583] Example 50: Synthesis of 6-imidazo[1,2-a]pyrazin-3-yl-N-(1- phenylethyl)quinazolin-4-amine (Compound 139) [00584] Step 1: Synthesis of 6-iodo-N-(1-phenylethyl)quinazolin-4-amine (2q) [00585] To a solution of 4-chloro-6-iodo-quinazoline, 1q, (500 mg, 1.72 mmol, 1 eq) in i- PrOH (5 mL) was added 1-phenylethanamine (208.58 mg, 1.72 mmol, 219.56 μL, 1 eq) and TEA (522.51 mg, 5.16 mmol, 718.72 μL, 3 eq), the mixture was stirred at 80 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum to give a crude product. Compound 2q, 6-iodo-N- (1-phenylethyl)quinazolin-4-amine (800 mg, crude) was obtained as a brown solid. MS (M + H)+ = 376.1. [00586] Synthesis of 6-imidazo[1,2-a]pyrazin-3-yl-N-(1-phenylethyl)quinazolin-4- amine (Compound 139) [00587] To a stirred solution of 6-iodo-N-(1-phenylethyl)quinazolin-4-amine, 2q, (200 mg, 533.04 μmol, 1 eq) in dioxane (2 mL) was added imidazo[1,2-a]pyrazine (127.00 mg, 1.07 mmol, 2 eq), Pd(OAc)2 (5.98 mg, 26.65 μmol, 0.05 eq), PPh3 (13.98 mg, 53.30 μmol, 0.1 eq), Cs2CO3 (347.35 mg, 1.07 mmol, 2 eq), the mixture was bubbled with N2 for 1 minute, and the mixture was stirred at 100 °C for 16 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture filtered to give a filtrate, and the filtrate was purified by prep-HPLC (column: Phenomenex luna C1880*40mm*3 um;mobile phase: [water(0.04%HCl)- ACN];B%: 18%-42%,7min). Compound 139, 6-imidazo[1,2- a]pyrazin-3-yl-N-(1-phenylethyl)quinazolin-4-amine (60.6 mg, 150.42 μmol, 28.22% yield, 100% purity, HCl) was obtained as a yellow solid.1H NMR (400MHz, DMSO-d6) δ = 10.77 (br d, J=7.6 Hz, 1H), 9.28 (dd, J=1.4, 8.5 Hz, 2H), 8.96 (s, 1H), 8.83 (dd, J=1.4, 4.8 Hz, 1H), 8.44 (dd, J=1.7, 8.7 Hz, 1H), 8.37 (s, 1H), 8.13 - 8.04 (m, 2H), 7.54 (d, J=7.4 Hz, 2H), 7.37 (t, J=7.4 Hz, 2H), 7.32 -7.25 (m, 1H), 5.86 (quin, J=7.1 Hz, 1H), 1.73 (d, J=7.0 Hz, 3H). MS (M + H)+ = 367.1. [00588] Example 51: Synthesis of 2-[(6-imidazo[1,2-a]pyrazin-3-ylquinazolin-4- yl)amino]-2-phenyl-ethanol (Compound 140) [00589] Step 1: Synthesis of 2-[(6-iodoquinazolin-4-yl)amino]-2-phenyl-ethanol (2r) [00590] To a solution of 4-chloro-6-iodo-quinazoline, 1r, (200 mg, 688.50 μmol, 1 eq) in i-PrOH (2 mL) was added TEA (209.00 mg, 2.07 mmol, 287.49 μL, 3 eq) and 2-amino-2- phenyl-ethanol (94.45 mg, 688.50 μmol, 1 eq), the mixture was stirred at 80 °C for 2 h. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture was concentrated in vacuum to give a crude product. Compound 2R, 2-[(6- iodoquinazolin-4-yl) amino]-2-phenyl-ethanol (300 mg, crude) was obtained as a yellow solid. MS (M + H)+ = 392.0. [00591] Step 2: Synthesis of 2-[(6-imidazo[1,2-a]pyrazin-3-ylquinazolin-4-yl)amino]- 2-phenyl-ethanol (Compound 140) [00592] To a solution of 2-[(6-iodoquinazolin-4-yl)amino]-2-phenyl-ethanol, 2r, (200 mg, 511.24 μmol, 1 eq) in dioxane (2 mL) was added imidazo[1,2-a]pyrazine (121.80 mg, 1.02 mmol, 2 eq), PPh3 (13.41 mg, 51.12 μmol, 0.1 eq), Cs2CO3 (333.14 mg, 1.02 mmol, 2 eq) and Pd(OAc)2 (5.74 mg, 25.56 μmol, 0.05 eq). The mixture was purged with N2 for 1 minute, and then the mixture was stirred at 100 °C for 16 h under N2 atmosphere. LCMS showed the starting material was consumed completely, and desired MS was detected. The reaction mixture filtered to give a filtrate, and the filtrate was purified by prep-HPLC(column: Phenomenex luna C18 250*50mm*10 um;mobile phase: [water(0.04%HCl)-ACN];B%: 10%-40%,10min). Compound 140, 2-[(6-imidazo[1,2-a]pyrazin -3-ylquinazolin-4-yl)amino]-2-phenyl-ethanol (36.29 mg, 82.84 μmol, 16.20% yield, 95.62% purity, HCl) was obtained as a yellow solid.1H NMR (400MHz, DMSO-d6) δ = 10.96 (br d, J=7.5 Hz, 1H), 9.58 - 9.47 (m, 1H), 9.36 (d, J=1.3 Hz, 1H), 9.00 - 8.91 (m, 2H), 8.51 (s, 1H), 8.46 (dd, J=1.7, 8.7 Hz, 1H), 8.19 - 8.07 (m, 2H), 7.55 (d, J=7.3 Hz, 2H), 7.40 - 7.32 (m, 2H), 7.31 - 7.25 (m, 1H), 5.78 - 5.67 (m, 1H), 4.08 (dd, J=8.8, 11.4 Hz, 1H), 3.86 (dd, J=4.6, 11.4 Hz, 1H). MS (M + H)+ = 383.1. [00593] Example 52: Synthesis of N-[5-[4-(benzylamino)quinazolin-6-yl]-2-chloro-3- pyridyl]methanesulfonamide (Comparative Compound 1) [00594] To a stirred solution of N-benzyl-6-iodo-quinazolin-4-amine (1.8 g, 4.98 mmol, 1 eq) in DMF (25 mL) and H2O (5 mL) was added N-[2-chloro-5-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)-3-pyridyl]-N-methylsulfonyl-methanesulfonamide (2.05 g, 4.98 mmol, 1 eq), K3PO4 (3.17 g, 14.95 mmol, 3 eq), Pd(PPh3)4 (575.89 mg, 498.37 μmol, 0.1 eq), the mixture was bubbled with N2 for 1 minute, and the mixture was stirred at 100oC for 4 h. LCMS showed the starting material was consumed completely and desired MS was detected. The reaction mixture was filtered and filtrate was concentrated and purified by prep-HPLC (Phenomenex luna c18 250mm*100mm*15um column; 15-45 % acetonitrile in a 0.1% trifluoroacetic acid solution in water, 42 min gradient). Comparative Compound 1, N-[5-[4-(benzylamino)quinazolin-6-yl]-2- chloro-3-pyridyl]methanesulfonamide (738.80 mg, 1.30 mmol, 26.09% yield, 97.46% purity, TFA) was obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ = 10.63 - 10.61 (m, 1H), 10.05 (s, 1H), 8.92 (s, 2H), 8.77 (s, 1H), 8.43 - 8.40 (m, 1H), 8.26 (s, 1H), 7.98 - 7.94 (m, 1H), 7.45 -7.43 (m, 2H), 7.39 -7.35 (m, 2H), 7.32 -7.30 (m, 1H), 5.01 (d, J = 5.2 Hz, 2H), 3.19 (s, 3H). MS (M + H)+ =440.1. [00595] Assay Examples [00596] In the following assay examples, the activity of the compounds of the disclosure can be compared to the following three compounds: [00597] Comparative compound 2 is a dual enzyme inhibitor against EGFR and PI3K. The description and synthesis of Comparative Compound 2 and Comparative Compound 3 can be found in International Application No. PCT/US2015/065827 filed on December 15, 2015 and is referenced as Mol 211 and Mol 167 respectively, the disclosure of which is incorporated herein by reference in its entirety. [00598] Example 53: Affinity of selected compounds of the disclosure for PI3Ka, EGFR, and DNA-PK enzymes. [00599] The Z´-LYTE® biochemical assay employs a fluorescence-based, coupled- enzyme format and is based on the differential sensitivity of phosphorylated and non- phosphorylated peptides to proteolytic cleavage (Fig.6A and 6B). The peptide substrate is labeled with two fluorophores—one at each end—that make up a FRET pair. In the primary reaction, the kinase transfers the gamma-phosphate of ATP to a single tyrosine, serine or threonine residue in a synthetic FRET-peptide. In the secondary reaction, a site-specific protease recognizes and cleaves non-phosphorylated FRET-peptides. Phosphorylation of FRET-peptides suppresses cleavage by the Development Reagent. Cleavage disrupts FRET between the donor (i.e., coumarin) and acceptor (i.e., fluorescein) fluorophores on the FRET-peptide, whereas uncleaved, phosphorylated FRET-peptides maintain FRET. A ratiometric method, which calculates the ratio (the Emission Ratio) of donor emission to acceptor emission after excitation of the donor fluorophore at 400 nm, is used to quantitate reaction progress. [00600] A significant benefit of this ratiometric method for quantitating reaction progress is the elimination of well-to-well variations in FRET-peptide concentration and signal intensities. As a result, the assay yields very high Z´-factor values (>0.7) at a low percent phosphorylation. [00601] Both cleaved and uncleaved FRET-peptides contribute to the fluorescence signals and therefore to the Emission Ratio. The extent of phosphorylation of the FRET-peptide can be calculated from the Emission Ratio. The Emission Ratio will remain low if the FRET-peptide is phosphorylated (i.e., no kinase inhibition) and will be high if the FRET-peptide is non- phosphorylated (i.e., kinase inhibition). [00602] Enzyme: The ADAPTA universal kinase assay is a homogenous, fluorescent based immunoassay for the detection of ADP. In contrast to ATP depletion assays, the ADAPTA assay is extremely sensitive to ADP formation such that a majority of the signal change occurs in the first 10-20% conversion of ATP to ADP. This makes the ADAPTA universal kinase assay ideally suited for use with low activity kinases. [00603] The principle of the ADAPTA universal kinase assay is outlined below. The assay itself can be divided into two phases: a kinase reaction phase, and an ADP detection phase. In the kinase reaction phase, all components required for the kinase reaction are added to the well, and the reaction is allowed to incubate for 60 minutes. After the reaction, a detection solution consisting of a europium labeled anti-ADP antibody, an Alexa Fluor® 647 labeled ADP tracer, and EDTA (to stop the kinase reaction) is added to the assay well. ADP formed by the kinase reaction (in the absence of an inhibitor) will displace the Alexa Fluor® 647 labeled ADP tracer from the antibody, resulting in a decrease in the TR-FRET signal. In the presence of an inhibitor, the amount of ADP formed by the kinase reaction is reduced, and the resulting intact antibody-tracer interaction results in a high TR-FRET signal. [00604] Z′-LYTE® Assay Conditions: [00605] Test Compounds: The Test Compounds are screened in 1% DMSO (final) in the well. For 10 point titrations, 3-fold serial dilutions are conducted from the starting concentration. [00606] Peptide/Kinase Mixtures: All Peptide/Kinase Mixtures are diluted to a 2X working concentration in the appropriate Kinase Buffer. [00607] ATP Solution: All ATP Solutions are diluted to a 4X working concentration in Kinase Buffer (50 mM HEPES pH 7.5, 0.01% BRIJ-35, 10 mM MgCl2, 1 mM EGTA). ATP Km apparent is previously determined using a Z´-LYTE® assay. [00608] Development Reagent Solution: The Development Reagent is diluted in Development Buffer. [00609] 10X Novel PKC Lipid Mix: 2 mg/mL Phosphatidyl Serine, 0.2 mg/mL DAG in 20 mM HEPES, pH 7.4, 0.3% CHAPS. For 5 mL 10X Novel PKC Lipid Mix: 1. Add 10 mgs Phosphatidyl Serine (Avanti Polar Lipids Part# 8400032C or 840039C) and 1 mg DAG (Avanti Polar Lipids Part# 800811C) to a glass tube.2. Remove the chloroform from lipid mixture by evaporating to a clear, thin film under a stream of nitrogen. Continuous rotation of the tube, at an angle to ensure maximum surface area of the lipid solution, will promote the thinnest film.3. Add 5 mLs resuspension buffer, 20 mM HEPES, 0.3% CHAPS, pH 7.4, to the dried lipid mix 4. Heat gently to 50-60 °C for 1-2 minutes and vortex in short intervals until the lipids are dissolved to a clear or slightly hazy solution. The lipids are typically in solution after 2-3 heat/vortex cycles.5. Cool to room temperature, aliquot into single use volumes and store at –20 °C. [00610] Assay Protocol: Bar-coded Corning, low volume NBS, black 384-well plate (Corning Cat. #4514) 1.2.5 μL – 4X Test Compound or 100 nL 100X plus 2.4 μL kinase buffer. 2.5 μL – 2X Peptide/Kinase Mixture.3.2.5 μL – 4X ATP Solution.4.30-second plate shake.5. 60-minute Kinase Reaction incubation at room temperature.6.5 μL – Development Reagent Solution.7.30-second plate shake.8.60-minute Development Reaction incubation at room temperature.9. Read on fluorescence plate reader and analyze the data. [00611] In a typical experiment, each data point uses 100 nL – 100X test compound in 100% DMSO. Commonly, 100 nL of a 10 μM solution of test compound is used for each experiment, which is equivalent to 1 picomole of test compound. Accordingly, a 10 μM single- point assay uses 100 picomoles of test compound, and a 10-point titration uses about 200 picomoles of test compound – 100 picomoles for the initial test and another 100 picomoles for the serial dilution. [00612] ADP formation is determined by calculating the emission ratio from the assay well. The emission ratio is calculated by dividing the intensity of the tracer (acceptor) emission by the intensity of the Eu (donor) emission at 615 nm as shown in the equation below. [00613] Since the ADAPTA technology measures ADP formation (i.e. conversion of ATP to ADP) it can be used to measure any type of ATP hydrolysis, including intrinsic ATPase activity of kinases. In this case, the substrate is water, not a lipid or peptide. The SelectScreen® service screens CHUK in this way, so a substrate is not included in the kinase reaction. A reference for using intrinsic ATPase activity to screen for kinase inhibitors is provided below. [00614] Adapta® Assay Conditions [00615] Test Compounds: The Test Compounds are screened in 1% DMSO (final) in the well. For 10 point titrations, 3-fold serial dilutions are conducted from the starting concentration. [00616] Substrate/Kinase Mixtures: All Substrate/Kinase Mixtures are diluted to a 2X working concentration in the appropriate Kinase Buffer (see section Kinase Specific Assay Conditions for a complete description). [00617] ATP Solution: All ATP Solutions are diluted to a 4X working concentration in water. ATP Km apparent is previously determined using a radiometric assay except when no substrate is available in which case an Adapta® assay is conducted. [00618] Detection Mix: The Detection Mix is prepared in TR-FRET Dilution Buffer. The Detection mix consists of EDTA (30 mM), Eu-anti-ADP antibody (6 nM) and ADP tracer. The detection mix contains the EC60 concentration of tracer for 5-150 ^M ATP. [00619] Assay Protocol: Bar-coded Corning, low volume, white 384-well plate (Corning Cat. #4512)1.2.5 μL – 4X Test Compound in 30 mM HEPES or 100 nL 100X in 100% DMSO plus 2.4 μL 30 mM HEPES.2.2.5 μL – 4X ATP Solution.3.5 μL – 2X Substrate/Kinase Mixture.4.30-second plate shake.5.1-minute centrifuge at 1000 x g.6.60-minute Kinase Reaction incubation at room temperature.7.5 μL – Detection Mix.8.30-second plate shake.9. 1-minute centrifuge at 1000 x g.10.60-minute Detection Mix equilibration at room temperature. 11. Read on fluorescence plate reader and analyze the data. [00620] In a typical experiment, each data point uses 100 nL – 100X test compound in 100% DMSO. Commonly, 100 nL of a 10 μM solution of test compound is used for each experiment, which is equivalent to 1 picomole of test compound. Accordingly, a 10 μM single- point assay uses 100 picomoles of test compound, and a 10-point titration uses about 200 picomoles of test compound – 100 picomoles for the initial test and another 100 picomoles for the serial dilution. [00621] The affinity for PI3Ka, EGFR, and DNA-PK enzymes of selected compounds of the disclosure vs. Comparative Compound 1 and Comparative Compound 2 is presented as the 50% inhibitory concentration (IC50) in Table 3 below. The IC50 data in Table 3 is presented as “++++” (value is 2 nM or less), “+++” (value is greater than 2 nM and less than or equal to 20 nM), “++” (value is greater than 20 nM and less than or equal to 200 nM) and “+” (value is greater than 200 nM). NT is “not tested.” [00622] Table 3: IC50 values for selected compounds of the disclosure.
[00623] The affinity for PI3Ka, EGFR, and DNA-PK enzymes of selected compounds of the disclosure vs. Comparative Compound 1 and Comparative Compound 2 is presented as the percent inhibition at 100 nM, in Table 4 below. The % inhibition at 100 nM data in Table 4 is presented as “*” (value is 10% or less), “**” (value is greater than 10% and less than or equal to 80%), “***” (value is greater than 80% and less than or equal to 90%) and “****” (value is greater than 90%). [00624] Table 4: Percent inhibition values for selected compounds of the disclosure.
[00625] Example 54: Effect of Compound 2 on tumor volumes in xenograft mice. [00626] Xenograft Studies. Female NCR nude mice (CrTac:NCr-Foxn1nu from Taconic), 6-7 weeks old, were implanted subcutaneously with 5x106 cells in a 1:1 serum-free media/Matrigel® mixture into the region of the right axilla. Mice were randomized into treatment groups and treatments initiated when tumors reached 100 to 200 mg. Compound 2R was administered daily by oral gavage as a fine suspension in 1:2 propylene glycol in 1% Tween80/Na3PO4 based upon individual animal body weight (0.2 mL/20 g). Subcutaneous tumor volume and body weights were measured two to three times a week. Tumor volumes were calculated by measuring two perpendicular diameters with calipers and using the formula: tumor volume = (length x width2)/2. Individual mice were dosed daily until their tumor burden reached 500 mg for the CAL-27 and HCC-70 models or 1000 mg for the CAL-33 model, to allow for calculation of increase in progression-free survival. Percent increase in survival was calculated by comparing the median time to reach either 500 mg or 1000 mg in the treated group versus the vehicle control group. A complete response (CR) is defined as a tumor below the limits of palpation (<40 mg). [00627] Figures 2C-2F show the performance of Compound 2R in controlling tumor size. Single agent activity of Compound 2R was demonstrated in models of squamous head and neck cancer (PIK3CA mutant human CAL-33, PI3K gamma mutant human CAL-27) as well as a model of triple negative breast cancer (human HCC-70). Tumor growth curves as well as survival and objective response data are summarized in the accompanying figures 2C-F and the table below. Compound 2R was well tolerated in these studies at the highest dose tested (100 mg/kg) with no signs of body weight loss or other clinical signs despite daily dosing over >50 days. [00628] Example 55: Assessment of metabolic stability in liver microsomes. [00629] Working solution: 5 μL of compound and control stock solution (10 mM in dimethyl sulfoxide (DMSO)) were diluted with 495 μL of acetonitrile (ACN) (intermediate solution concentration: 100 μM, 99% ACN) [00630] NADPH Cofactor Preparation: NADPH powder: β-Nicotinamide adenine dinucleotide phosphate reduced form, tetrasodium salt; NADPH^4Na. The appropriate amount of NADPH powder was weighed and diluted into a 10 mM MgCl2 solution (working solution concentration: 10 unit/mL; final concentration in reaction system: 1 unit/mL) [00631] Liver Microsomes Preparation: The appropriate concentrations of microsome working solutions were prepared in 100 mM potassium phosphate buffer. Cold (4°C) acetonitrile (ACN) containing 200 ng/mL tolbutamide and 200 ng/mL labetalol as internal standards (IS) was used as the stop solution [00632] Assay Procedure: Pre-warm empty 'Incubation' plates T60 and NCF60 for 10 minutes. Dilute liver microsomes to 0.56 mg/mL in 100 mM phosphate buffer. Transfer 445 μL microsome working solutions (0.56 mg/mL) into pre-warmed 'Incubation' plates T60 and NCF60, Then pre-incubate 'Incubation' plates T60 and NCF60 for 10 min at 37°C with constant shaking. Transfer 54 µL liver microsomes to blank plate, then add 6 µL NAPDH cofactor to blank plate, and then add 180 µL quenching solution to blank plate. Add 5 µL compound working solution (100 μM) into 'incubation' plates (T60 and NCF60) containing microsomes and mix 3 times thoroughly. [00633] For the NCF60 plate, add 50 μL of buffer and mix 3 times thoroughly. Start timing; plate will be incubated at 37°C for 60 min while shaking. In 'Quenching' plate T0, add 180 µL quenching solution and 6 µL NAPDH cofactor. Ensure the plate is chilled to prevent evaporation. For the T60 plate, mix 3 times thoroughly, and immediately remove 54 µL mixture for the 0-min time point to 'Quenching' plate. Then add 44 µL NAPDH cofactor to incubation plate (T60). Start timing; plate will be incubated at 37°C for 60 min while shaking. At 5, 10, 20, 30, and 60 min, add 180 µL quenching solution to 'Quenching' plates, mix once, and serially transfer 60 µL sample from T60 plate per time point to 'Quenching' plates. For NCF60: mix once, and transfer 60 µL sample from the NCF60 incubation to 'Quenching' plate containing quenching solution at the 60-min time point. All sampling plates are shaken for 10 min, then centrifuged at 4000 rpm for 20 minutes at 4°C. Transfer 80 µL supernatant into 240 µL HPLC water, and mix by plate shaker for 10 min. Each bioanalysis plate was sealed and shaken for 10 minutes prior to LC-MS/MS analysis. [00634] The equation of first order kinetics was used to calculate T1/2 and CLint(mic) (μL/min/mg). Equation of first order kinetics:
[00635] The biological stability of the compounds of the disclosure can be measured by determining its half-life in the presence microsomes. Presented in Table 5, is the half-life of selected compounds of the disclosure in the presence of human liver microsomes (HLM) or mouse liver microsomes (MLM) as described above. In Table 5, half-life is presented as “++++” (value is greater than 60 minutes), “+++” (value is greater than 30 minutes and less than or equal to 60 minutes), “++” (value is greater than 15 minutes and less than or equal to 30 minutes) and “+” (value is 15 minutes or less). [00636] Table 5: Half-life of selected compounds of the disclosure in HLM or MLM. [00637] The results in Table 5 suggest that the compounds of the present disclosure would possess robust biological stability in vivo, as most of the compounds tested have a half-life greater than 60 minutes in the presence of both human liver microsomes and mouse liver microsomes. [00638] Example 56: Assessment of the solubility of the compounds of the disclosure. [00639] Preparation of stock solutions: The stock solutions of test compounds and control compound diclofenac were prepared in DMSO at the concentrations of 10 mM. [00640] Simulated Gastric Fluid (SGF): An aqueous mixture including hydrochloric acid, sodium chloride, and pepsin (pH = 1.2). [00641] Simulated Intestinal Fluid (SIF): Prepared by dissolving 6.8 g of KH2PO4 into about 500 mL ultrapure water and adjust the solution to a pH 6.8 with 0.1 M NaOH.10 g trypsin is then dissolved into ultrapure water. The two solutions are mixed well and diluted with ultrapure water to a final volume of 1000 mL. [00642] Procedure for solubility determination: 15 µL of stock solution (10 mM) of each sample was placed in order into their proper 96-well rack.485 µL of (SIF, SGF, PBS 7.4, FESSIF, or FESSGF) was added into each vial of the cap-less Solubility Sample plate. The assay was performed in duplicate. Add one stir stick to each vial and seal using a molded PTFE/Silicone plug. Then the solubility sample plate was transferred to the Eppendorf Thermomixer Comfort plate shaker and shaken at 25°C at 1100 rpm for 2 hours. After completion of the 2 hours, plugs were removed and the stir sticks were removed using a big magnet, the samples from the Solubility Sample plate were transferred into the filter plate. Using the Vacuum Manifold, all the samples were filtered. Aliquot of 5 µL and 5 µL DMSO were taken from the filtrate followed by addition of 490 µL of a mixture of H2O and acetonitrile containing internal standard (1:1). A certain proportion of ultrapure water was used to dilute the diluent according to the peak shape. The dilution factor was changed according to the solubility values and the LC-MS signal response. [00643] Preparation of 300 µM standards (STD): From the 10 mM DMSO STD plate, 6 µL was transferred into the remaining empty plate, and then 194 µL of DMSO was added to that plate to have a STD concentration of 300 µM. From the 300 µM DMSO STD plate, 5 µL DMSO STD and 5 µL SIF was transferred into the remaining empty plate, and then 490 µL of a mixture of H2O and acetonitrile containing internal standard (1:1) was added to that plate to have a final STD concentration of 3 µM. A certain proportion of ultrapure water was used to dilute the diluent according to the peak shape. The concentrations of the standard samples were changed according to the LC-MS signal response. [00644] Procedure for sample analysis: The plate was placed into the well plate autosampler. The samples were evaluated by LC-MS/MS analysis. [00645] Data analysis: All calculations were carried out using Microsoft Excel. The filtrate was analyzed and quantified against a standard of known concentration using LC coupled with mass spectral peak identification and quantitation. Solubility values of the test compound and control compound were calculated as follows: [00646] Any value of the compounds that was not within the specified limits was rejected and the experiment was repeated. [00647] The solubility data for selected compounds of the disclosure is provided in Table 6. The solubility data in Table 6 is presented as “****” (value is greater than 200 μM), “***” (value is greater than 100 μM and less than or equal to 200 μM), “**” (value is greater than 20 μM and less than or equal to 100 μM) and “*” (value is 20 μM or less). NT is “not tested.” [00648] Table 6: Solubility data for selected compounds of the disclosure. [00649] The results in Table 6 show that the solubility of the compounds of the present disclosure are significantly improved compared to Comparative Compounds 1-3. Seven out of ten compounds tested show an improvement in solubility in simulated intestinal fluid, with Compounds 2R and 2S being at least 5 times as soluble. Compound 2R also shows the same improvement in solubility in PBS buffer. As in known to those skilled in the art, increased solubility in an active pharmaceutical ingredient (API) can suggest an increase in bioavailability in, for example, an orally administered composition. [00650] Example 57: Assessment of the pharmacokinetics of Compound 2R [00651] The pharmacokinetics of Compound 2R (HCl salt) and Comparative Compound 2 were studied following a single intravenous and/or oral administration to mice, dogs, and monkeys (n = 3 for all species). The IV and PO solution formulations for Comparative Compound 2 contained 20% (w/v) propylene glycol, 75% (w/v) 50 mM trisodium phosphate (pH 12), and 5% (w/v) Kolliphor EL. The formulation for Compound 2R contained 1:2 propylene glycol: 1%Tween 80/Na3PO4 (50 mM). Subsequent pharmacokinetic data analysis was performed using non-compartmental analysis modules in Phoenix/WINNONLIN 6.3 (Pharsight, St. Louis, Missouri, USA), and the linear trapezoidal rule was used for AUC calculation. Terminal elimination half-life (t½) was calculated based on data points (≥ 3) in the terminal phase; Tmax = Time of maximum observed concentration; AUClast/D = Area under the concentration-time curve from time zero to the last observed concentration/divided by dose; Cl_obs = Total body clearance; Cmax = maximum observed concentration; F = bioavailability of oral dose form. Blood samples were assayed for compounds using protein precipitation with acetonitrile followed by HPLC/MS/MS analysis. Blood concentration-time data were analyzed by non-compartmental methods. [00652] Table 7. IV PK parameters for Comparative Compound 2 and Compound 2R [00653] Table 8. Oral PK parameters for Comparative Compound 2 and Compound 2R [00654] The enhanced solubility of Compound 2R led to improved exposure when compared to Comparative Compound 2 as expressed by the PK parameter AUClast/D. IV pharmacokinetic analysis demonstrated about a 3-fold increase in exposure for mice dosed with Compound 2R (AUClast/D = 14,240 vs 5094 h*mg/ml) and monkeys (AUClast/D = 3783 vs 1067). Dogs showed approximately a 15-fold increase in exposure when dosed intravenously with Compound 2R compared to Comparative Compound 2 (AUClast/D = 28655 vs 1975). Oral PK analysis revealed a similar trend in mice as reflected by 3-fold enhanced exposure with Compound 2R relative to Comparative Compound 2. However, improvement in exposure in response to oral treatment with Compound 2R was especially strong in both dogs (>28-fold) and monkeys (>74-fold) as shown by AUClast/D values of 13,808 vs 487 h*mg/ml (dogs) and 2222 vs 30 h*mg/ml (monkeys). [00655] Example 58. Potent and selective dual inhibitor of EGFR and PI3K [00656] The present study was undertaken to design a PI3K inhibitor that would be better tolerated than previously reported molecules in this target class, and also be highly selective for both PI3K and EGFR. The combinatorial potential of such an agent for the treatment of KRAS mutant cancers is significant. In this study, the inventors provide evidence for the feasibility of computationally designing a dual inhibitor of EGFR and PI3K that is highly selective for its intended targets. Preclinical proof-of-concept studies have been carried out with Compound 2R, which is shown to be highly efficacious as a single agent to treat HNSCC, in addition to the other exemplified compoiunds or their pharmaceutically acceptable salts thereof.. Furthermore, the present disclosure shows that Compound 2R dramatically improves therapeutic outcomes in combination with RAS pathway intervention in BRAF and KRAS mutated colorectal and pancreatic cancers. Compound 2R regimens were well tolerated and efficacious over a wide range of doses in preclinical models. In addition, Compound 2R regimens surprisingly and unexpectedly, did not produce a hyperglycemic response as commonly seen with PI3K inhibitors. Collectively, the preclinical data suggest that Compound 2R may be better tolerated in the clinic compared to predecessor PI3K inhibitors for the treatment of cancer [00657] EGFR crystallization [00658] The EGFR construct used for crystallization (G696-G1022) was produced as described previously (PDB Code 2GS2)58. Crystals of apo-EGFR were obtained using hanging- drop vapor diffusion set-ups and EGFR at a concentration of 5.6 mg/mL (25 mM Tris/HCl, 50 mM NaCl, 2 mM DTT, pH 7.5). The protein solution (2 pL) was mixed with 0.6 pL of reservoir solution (0.10 M HEPES/NaOH pH 6.70, 1.05 M Na-Succinate pH 7.00) and equilibrated at 12°C over 0.4 mL of reservoir solution. Well diffracting crystals were selected for data collection after 13 days. Crystals were soaked for 3.5 hours with 1 mM Compound 2R in 30% solubilizing mix 4 from the Molecular Dimensions CryoSol kit. [00659] PI3Kγ crystallization [00660] The PIK3CG construct used for crystallization (S144-A1102) was produced as described previously (PDB Code 1E8Y)59. Apo crystals of PI3Ky were obtained using hanging- drop vapor diffusion set-ups. PI3Ky was used at a concentration of 7.1 mg/mL (20 mM Tris/HCl, 0.5 mM ammonium sulfate, 5 mM DTT, 0.02 % (v/v) CHAPS, 1 % ethylene glycol, pH 7.2).1 pL of the protein solution was mixed with 1 pL of reservoir solution (100 mM Tris/HCl pH8.2, 0.2 M lithium sulfate, 15 % (w/v) PEG 4000) and equilibrated at 20°C over 300 pL of reservoir solution. Well diffracting crystals were selected for soaking after 20 days. Crystals were soaked with 5 mM Compound 2R for 16 hours in 30% ligand solubilization mix SM1 from molecular dimensions (MD1-90) diluted in reservoir solution. [00661] PPARγ crystallization [00662] The PPARγ construct used for crystallization (L232-Y505 of UniProt entry P37231) was produced as described previously (PDB entry 3SZ1)60. Crystals of apo-PPARy were obtained using sitting-drop vapour diffusion set-ups. An aliquot (0.2 pL) of the PPARy protein solution at a concentration of 8.9 mg/mL (20mM Tris-HCl, 100mM NaCl, 5% Glycerol, 1mM TCEP, pH 8.0) was mixed with 0.1 pL of reservoir solution (0.10 M BIS-TRIS-Propane pH 9.00, 2.20 M (NH4)2-Sulfate) and equilibrated at 20°C over 70 μL of reservoir solution. Well diffracting crystals were selected for data collection after 11 days. Crystals were soaked for 20 hours with 2.5 mM Compound 2R. [00663] Crystallography Data Collection Complete datasets of 2.0 Å, 2.7 Å, and 1.9 Å were collected for EGFR/Compound 2R and PI3Kγ/Compound 2R at the European Synchrotron Radiation Facility (Grenoble, France, beamline ID30a1). A complete 1.9 Å data set of a PPARγ/Compound 2R crystal was collected at SLS (PSI, Villigen, CH, beamline PXI). The data were integrated, analyzed and scaled by the programs XDS, Pointless, Aimless and Staraniso from within the autoPROC pipeline, respectively. [00664] Structure Determination and Refinement for EGFR [00665] Molecular replacement was carried out using a previously solved structure of EGFR as a starting model. Several rounds of alternating manual re-building and refinement with REFMAC5 resulted in the final model (Table 9). [00666] Table 9- Refinement statistics for EGFR, PI3Kγ, and PPARγ x-ray crystal structures. The numbers in parentheses refer to the highest resolution bin.
[00667] [00668] Structure Determination and Refinement for PI3Kγ [00669] Molecular replacement was carried out using a published structure of PI3Kγ (PDB accession code 4ANV) as a starting model. Several rounds of alternating manual re- building and refinement with BUSTER and REFMAC resulted in the final model (Table 9). Atomic displacement factors were modelled with a single isotropic B-factor per atom and a TLS group per protein chain. The restraints for the compound were generated by the program GRADE from Global Phasing with the bigplanes option. [00670] Structure Determination and Refinement for PPARγ [00671] Molecular replacement was carried out using a published structure of PPARγ (PDB accession code 3SZ1) as a starting model. The sequence used in the structure determination and refinement is according to isoform 1 of PPARγ (residues 204 to 478). Several rounds of alternating manual re-building and refinement with BUSTER resulted in the final model (Table 9). Atomic displacement factors were modelled with a single isotropic B-factor per atom and a TLS group per protein chain. The restraints for the compound Compound 2R were generated by the program GRADE from Global Phasing with the bigplanes option. [00672] Biochemical kinase assays [00673] All biochemical kinase profiling was carried out at ThermoFisher Scientific. Most kinases were screened using Z-Lyte or LanthaScreenTM assays. Z-Lyte assays, employ a fluorescence-based, coupled-enzyme format, which is based on the differential sensitivity of phosphorylated and non-phosphorylated peptides to proteolytic cleavage. This radiometric method yields very high Z’-factor values (>0.7) at a low percent phosphorylation. The LanthaScreen format is another FRET approach that detects binding of a fluorescent tracer to a kinase with a terbium-labeled GST antibody. Screening of lipid kinases was carried out using the Adapta universal kinase assay format, which is a fluorescent based immunoassay for the detection of ADP. A known inhibitor, usually staurosporine for protein kinases and PI-103 for the PIK3CA/PIK3R isoforms, was titrated on the same plate as Compound 2R, to ensure that it was inhibited within the expected IC50 range previously determined. All assays were carried out at the ATP Km apparent, which was previously determined for that kinase. Dose response curves were curve fit to model number 205 (sigmoidal dose response model) using XLfit graphing software from IDBS. [00674] Nuclear receptor assays [00675] LanthaScreen time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays were carried out at Thermo-Fisher Scientific for screening Compound 2R against the family of PPAR nuclear hormone receptors. Compounds were screened in 1% DMSO (final concentration) and 3-fold serial dilutions were conducted for 10- point titrations. A known inhibitor was titrated on each plate: GW1929 (PPARγ), GW7647 (PPARα), and GW0742 (PPAR(β), to ensure that reference compounds were displaced within an expected IC50 range previously determined. Dose response curves were curve fit to model number 205 (sigmoidal dose-response model) using XLfit graphing software from IDBS. Intracellular effects on the PPARγ nuclear hormone receptor were determined at Thermo-Fisher Scientific using GeneBLAzerTM Beta-lactamase reporter technology. PPAR-gamma-UAS-bla HEK 293T cells were thawed and resuspended in Assay Media (DMEM phenol red free, 2% CD-treated FBS, 0.1 mM NEAA, 1 mM sodium pyruvate, 100 U/mL/100pg/mL Pen/Strep) to a concentration of 9.4 x 105 cells/mL.4 pL (picoliters) of a 10x serial dilution of rosiglitazone (control agonist starting concentration, 316 nM) or Compound 2R was added to appropriate wells of a 384-well poly-D-lysine assay plate.32 pL of cell suspension (30,000) cells was added to each well followed by 4 pL of assay media to bring the final assay volume to 40 pL. Plates were incubated for 16-24 hours at 37°C/5% CO2 in a humidified incubator. Plates were incubated at room temperature for two hours followed by determination of fluorescence. A Z’- factor of 0.5 or greater was required to meet QC criteria. [00676] Reagents, cell lines and PDX models [00677] Compound 2R was synthesized as described herein, the compound was stored protected from light at -20°C in powder form. For in vitro assays, Compound 2R was formulated in 100% DMSO. Alpelisib (A-4477), erlotinib (E-4997) and trametinib (T-8123) were purchased from LC Labs. Sotorasib (C-1499) was purchased from Chemgood. Omipalisib (6792) was purchased from Tocris. Buparlisib (HY-70063), copanlisib (HY-15346) and taselisib (HY- 13898) were purchased from MedChemExpress. Rosiglitazone (NC9560589) was purchased from Cayman Chemical. Bovine insulin (I0516) and methylisobutylxanthine (IBMX; I5879) were purchased from MilliporeSigma. Dexamethasone (API-04) was purchased from G Biosciences. Human epidermal growth factor, hEGF, (PHG0313) was purchased from Gibco/ThermoFisher Scientific. [00678] CAL-27 and CAL-33 cell lines were obtained from Leibniz Institute DSMZ- German Collection of Microorganisms and Cell Cultures. MIA PaCa-2 and 3T3-L1 cell lines were obtained from the American Type Culture Collection. KPC tumors originated from 65671 cells (FVB/N strain), which were kindly provided by Marina Pasca di Magliano, University of Michigan61. The following models were received from the NCI Patient-Derived Models Repository (PDMR) as cryopreserved cells: 135848-042-T (lot# CK3998), 455876-151-R (lot# CK9041), 848979-319-R (lot# CK9558), and CN0375-F725-PDC (lot# CK5838). The following models were received from the NCI PDMR as cryopreserved tumor fragments: 245127-232-R (lot# JC2149), 354836-022-R (lot# JK0388) and 944545-341-R (lot# MA1086). The PDX model CRC 14-929 was established at the University of Michigan (Leopold lab)62.. [00679] All patients provided informed written consent and samples were procured with approval of the University of Michigan Institutional Review Board (HUM00065489). Demographic information on all the PDX models used in this study are provided in Table 10. [00680] Table 10. Demographic Data for Patient-Derived Xenograft Models. [00681] Cell culture [00682] RPMI 1640 medium (11875-093), DMEM (11965-092), penicillin streptomycin (15150-122), GlutaMax (35050-061), sodium pyruvate (11360-070) and PBS (10010-023) were all obtained from Gibco/Thermo Fisher Scientific. FBS was obtained from Cytivia (SH3039603). Bovine Calf Serum (CBS) was purchased from ATCC (30-2022). Cells were grown in the appropriate medium and maintained at 37°C in a humidified incubator with 5-10% CO2. SOPs issued by the NCI PDMR were used to thaw, expand, and maintain the PDC lines obtained from there. All cell lines were determined to be Mycoplasma free using Lonza’s MycoAlert Mycoplasma Detection kit. Cell lines were authenticated by STR analysis (American Type Culture Collection, human and mouse STR profiling service). [00683] Adipocyte Differentiation [00684] 3T3-L1 cells were differentiated according to the methods published previously. Cells were seeded at a density of 6.0 x 105 cells per T-75 flask (Corning) in DMEM supplemented with 10% CBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate and grown to confluency. The culture medium was refreshed and the cells were grown for 48 hours. To induce differentiation, the cells were cultured for 48 hours in differentiation induction medium containing DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate, 1.0 µM dexamethasone, 0.5 mM IBMX, 1.0 µg/mL insulin containing DMSO, Rosiglitazone 1 mM or Compound 2R 10 mM. Thereafter, the medium was changed to DMEM, 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 1mM sodium pyruvate, 1.0 mg/mL insulin every 48 hrs until 7 days post-induction. [00685] Immunoblot Analysis [00686] To generate cell lysates for immunoblot analysis, cells were seeded 6.0 x 105 cells per dish in 60 mm tissue culture plates with the appropriate growth media, including all supplements. The following day, the media was removed from the dishes and replaced with the appropriate serum-free growth media. The next day, the cells were treated with DMSO or Compound 2R at the indicated concentrations. At 15 minutes prior to lysis, the cells were stimulated with hEGF. Cells were lysed at 2 hours following treatment. Cells were washed with cold PBS and lysed with NP-40 lysis buffer (50 mM Tris pH 7.5, 1% NP-40, 150 mM NaCl, 10% glycerol, 1 mM EDTA) supplemented with protease and phosphatase inhibitors (Roche). Tumors were minced and manually homogenized in NP-40 lysis buffer. Lysates rocked at 4°C for 30-60 minutes and cleared by centrifugation. Equal amounts of protein (10–20 μg) in normalized lysates were resolved by SDS–PAGE, transferred to 0.2 µm or 0.45µm polyvinyldifluoride (PVDF) membrane (Millipore Sigma), probed with specific primary and secondary antibodies, and detected by chemiluminescence with ECL detection reagents (Cytiva). The following primary antibodies were used at 1:1000 dilution, unless otherwise noted: anti-p- EGFR (tyr1068) #3777, anti-EGFR #2646 (1:10,000), anti-p-AKT (thr308) #13038, anti-p-AKT (ser473) #4060, anti-AKT #9272 (1:5000), anti-pS6 (ser235/236) # 4857, anti-S6 #2217 (1:10,000), anti-p-PRAS40 (thr246) #2997, anti-PRAS40 #2691 (1:10,000), anti-p-4E-BP1 (ser65) #9451), anti-4E-BP1 #9644, anti-p-p70 S6K (thr389) #97596, anti-p70 S6K #9202, anti- PPARγ #2443, anti-cleaved PARP #9541, all from Cell Signaling Technologies; anti-ii-actin HRP conjugated #197277 (1:10,000) and anti-vinculin #129002 (1:10,000) from Abcam. For the secondary antibody, peroxidase-conjugated AffiniPure goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, 111-035-003) was used at a 1:10,000 dilution. To quantify pixel intensity for each selected protein band, images were converted to grayscale and saved as .tif files. ImageJ software (https://imagej.nih.gov/ij/) was used to create a rectangular ROI around the widest band for each protein on a given blot. The same ROI was applied to each band for a given protein and histograms were generated indicating the intensity of each band. This signal intensity was determined for both the phosphorylated protein and the housekeeping protein for each target protein of interest. Data was exported to Microsoft Excel. The level of phosphorylation for a given protein for each sample was determined by dividing the signal intensity of the phosphorylated protein band by the signal intensity of the housekeeping protein band. The level of phosphorylation for each target protein was averaged within the control treatment group. Percent inhibition of phosphorylated protein expression for compound treated samples were compared to the average of the control sample by taking the signal intensity of the treated sample, dividing it by the average signal intensity of the control sample and multiplying by 100. Standard error of the mean was calculated for each replicate within a given treatment condition. GraphPad Prism was used to plot the results. [00687] qRT-PCR Analysis of PPARγ Target Genes [00688] Total RNA was extracted from 3T3-L1 cells with 1 mL Trizol (Invitrogen) according to the manufacturer’s instructions at 8 and 24 hours following the addition of differentiation induction medium containing DMSO, Rosiglitazone 1µM or Compund 2R 10 µM. Genomic DNA was removed from the RNA extracts using the RNAse-Free DNase Kit and purified using the RNeasy MinElute Cleanup Kit (Qiagen). RNA extracts were quantified and assessed for quality with the RNA Broad Range Assay Kit and the RNA IQ kit respectively, using a Qubit 4 Flourometer (Invitrogen). First strand cDNA was synthesized using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s instructions. Briefly, 20 µL 5X VILO Reaction Mix, 10 µL 10X SuperScript Enzyme Mix and 65 µL of DEPC-treated dH2O were added to 5 µg of RNA in a 100 µL reaction volume. PCR reactions were amplified in the VeritiTM 96-well Thermal Cycler (Applied Biosystems) under the following conditions: 10 min at 25°C, 90 min at 42°C, 5 min at 85°C. PCR reactions without reverse transcriptase were included to detect genomic DNA contamination. Quantitative PCR reactions were performed in quadruplicate using mouse-specific Taqman Gene Expression Single Tube Assays (Applied Biosystems) for lipoprotein lipase (LPL) (Mm00434764_m1), PPARγ (Mm00440940_m1) and adiponectin (Mm04933656_m1). The housekeeping gene, RLPL0 (Mm00725448_s1), was used to normalize for target gene expression. Reactions were prepared by adding 1 µL of cDNA, 5 µL 2X Master Mix, 0.5 µL 20X Taqman Gene Expression Assay and 3.5 µL nuclease-free H2O. Control reactions without cDNA template were included to detect contamination. Amplification and analysis of PCR reactions were performed using the QuantStudio 5 Real-Time PCR System (Applied Biosystems) programmed as follows: 20 sec at 95°C followed by 40 cycles at 95°C for 1 sec and annealing/extending for 20 seconds at 60°C. Fold gene expression (RQ) was calculated using the formula, RQ = 2(-AACt) where AACt equals ACt (treated sample) – ACt (DMSO-treated sample) and ACt equals (target gene Ct – RLPL0 reference gene Ct). Data are represented as the mean RQ +SEM. The analysis of statistical differences between treatment groups was performed using the Student’s t-test. [00689] In vivo xenograft studies [00690] All procedures related to animal handling, care and treatment were performed under an approved protocol (PRO00010150) according to the guidelines set forth by the University of Michigan Institutional Animal Care and Use Committee (IACUC) and following the guidance of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals were housed per institutional guidelines as determined by the affiliated IACUC, consisting of a typical 12/12 light/dark cycles, ambient temperatures of 68-75°C with 30-70% humidity. Mice were maintained under pathogen-free conditions and food and water was provided ad libitum. For human-derived cell lines and PDX models, 6-8 week old female CIEA NOG mice (NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac from Taconic) or female athymic homozygous nude mice (CrTac:NCr-Foxn1nu from Taconic or Crl:NU(NCr)-Foxn1nu from Charles River Laboratories) were used. For models originating from cell culture (CAL-27, CAL- 33, MIA PaCa-2, 135848, 455876, 848979, CN0375), the mice were inoculated subcutaneously in the right axilla with 1 x 106 to 5 x 106 cells suspended in 100 µl of 1:1 ratio of serum-free media to Matrigel. For models originating from tumor fragments (UM-CRC 14-929, 245127, 354836, 944545), the mice were implanted with tumor fragments 2-3 mm in diameter into the right axilla via trocar. For the KPC model, female inbred FVB mice (6-8 weeks of age) were obtained from Taconic (FVB/NTac). The mice were inoculated subcutaneously in the right axilla with 1 x 106 of 65671 (FVB/N strain) cells suspended in 100 µl of 1:1 serum-free media:Matrigel. [00691] Compound 2R was prepared as a suspension in 1:2 propylene glycol:1% Tween80/Na3PO4 or as a solution in 50% propylene glycol/35% PEG400/10% TPGS in water/5% 1N NaOH and administered via oral gavage. Alpelisib and buparlisib were prepared as suspensions in 1% carboxymethylcellulose with 0.2% Tween80 and administered via oral gavage. Copanlisib was prepared as a solution in 0.5% mannitol in water and administered through tail vein injection. Erlotinib was prepared as a solution in 5% DMSO/95% PEG300 and administered via oral gavage. Omipalisib was prepared as a solution in 2% DMSO/40% PEG300/2% Tween80/56% ddH20 and administered via oral gavage. Sotorasib was prepared as a suspension in 0.5% carboxymethylcellulose with 1% Tween80 and administered via oral gavage. Taselisib was prepared as a suspension in 0.5% methylcellulose with 0.2% Tween80 and administered via oral gavage. Trametinib was prepared as a suspension in 0.5% hydroxypropyl methylcellulose with 0.2% Tween80 and administered via oral gavage. All compounds were prepared fresh daily and administered based upon individual mouse body weight (0.2 mL/20 g). On reaching a mean tumor volume of ~100 to 200 mm3, mice were randomized into treatment arms and treatment was initiated (Day 1 of study). Subcutaneous tumor volume and body weights were measured two to three times a week. Tumor volumes were calculated by measuring two perpendicular diameters with calipers and using the formula: tumor volume = (length × width2)/2. Mice were treated and monitored daily until individual mouse tumor burdens reached IACUC approved limitations (either 500 for ulcer-prone models or 1000 mm3). Percent A Treated/A Control (% AT/AC) was calculated on the day the median control mouse was euthanized as follows: [(Tfinal - Tinitial)/(Cfinal – Cinitial)] x 100, where Cinitial and Cfinal are the median tumor volumes on the first day of treatment and the day indicated for the vehicle control group and Tinitial and Tfinal are the median tumor volumes on the first day of treatment and the day indicated for the treated group. Increase is lifespan was calculated as [(Tday – Cday)/Cday] x 100, where Tday is the day the median treated mouse was euthanized and Cday is the day the median control mouse was euthanized. [00692] Modified RECIST (Response Evaluation in Solid Tumor) Criteria in Preclinical Efficacy Studies: [00693] For the control animals, the % tumor volume change was calculated when the tumor volume reached a predetermined size of either 500 mm3 or 1000 mm3, depending on the tumor model. This % change in tumor volume was calculated from baseline: ΔT = (Tfinal – Tinitital) / Tinitial x 100. For the treated animals, the response was determined by comparing tumor volume change at time t to its baseline: % tumor volume change = ΔT = (Tfinal – Tinitital) / Tinitial x 100. The best overall response for each animal was defined as the minimum % tumor volume change, occurring after the first seven days of treatment. The preclinical criteria for response were adapted from RECIST 1.164,65 and defined as follows: a complete response (CR) is the disappearance of the subcutaneous tumor (tumor no longer palpable. A partial response (PR) is z 30% decrease in tumor volume. Progressive disease (PD) was defined as an increase in tumor volume of >one doubling. Stable disease (SD) is defined as neither sufficient shrinkage of the tumor to qualify as a PR nor sufficient growth to qualify as PD. For pharmacodynamic studies, when tumors reached a mean tumor volume of ~150 – 300 mm3, mice were randomized into treatment arms and treated with vehicle or test article as indicated. At the indicated timepoints, the mice were euthanized, and the tumors were harvested and snap frozen in liquid nitrogen. Tumors were stored at -80°C. [00694] Glucose and insulin measurements [00695] Female athymic nude mice (8-10 weeks of age) were acclimated for a minimum of three days prior to being placed on study. At study initiation, mice were administered vehicle control or test article based upon individual mouse body weight (0.2mL/20g). For assessment of blood glucose levels, 1-2 drops of blood were taken from the tail of the mice and measured using an ACCU-CHEK Aviva glucometer before treatment (time 0) and at 0.5, 1, 2, and 4 hours following treatment. For assessment of blood insulin levels, ~30-40 µL of blood was collected into EDTA microvette tubes (Sarstedt, cat# 16.444.100) immediately following the blood glucose measurement. Blood was centrifuged at 4°C for 20 minutes at 2,000 x g and plasma was collected and stored at -80°C. Insulin levels in plasma and tumor samples were determined by ELISA (Crystal Chem Ultra-Sensitive Mouse Insulin ELISA kit, cat# 90080), according to the manufacturer’s instructions. Tumor samples were lysed as described above and diluted in the sample dilution buffer provided in the kit. [00696] Statistical analyses [00697] For two-arm comparisons: Quantitative data are presented as mean ± standard error of the mean (SEM). For animal studies, animals [00698] 603 were randomized prior to treatment and all animals treated were included for the analyses. A Linear Mixed model was used to evaluate the single agent activity of Compound 2R. Log tumor size was modeled as: Log Yij = β0 + β1I(Groupi = control)tij + β2 I(Groupi = treatment) tij + bi + εij, Where Yij represents the tumor volume of the i-th mouse for i ^ {1, …, n} at the j-th measurement j ^ {1, …, m}; I(Groupi = control) and I(Groupi = treatment) are indicator variables that equal 1 when mouse i is in the specified group, and 0 otherwise; tij represents the time elapsed between measurement time 1 and measurement j for the ith mouse; ɛij represents a normally distributed residual for the j-th measurement of the i-th mouse with expectation zero and variance σ2 , i.e., ɛij ~ N(0, σ2); and bi represents the random intercept for mouse i, with bi ~ N(0, τ2). Log-transformed tumor volume was used for the analysis to better satisfy the assumption of linearity in growth over time. Under this model, β0 can be interpreted as the overall average logarithmic tumor volume at first time measurement for all mice. β1 represents the rate change in logarithmic scale tumor-volume across time for the vehicle group, and β2 is defined analogously for the Compound 2R treatment group. Thus, the β2 – β1 represents the difference of logarithmic scale tumor growth ratio between Compound 2R treatment vs. vehicle group. A Wald test was used for the significance comparisons between different groups. [00699] For multiple treatment comparisons: [00700] The Linear Mixed model was analogously used to evaluate the single agent activity of COMPOUND 2R and its combination with either MEK or KRASG12C inhibitors. For these data, log tumor size was modeled as: Where Yij represents the tumor volume of the i-th mouse i ∈ {1, …, n} at the j-th measurement j ^ {1, …, m}; I(Groupi = T) is an indicator random variable that equals 1 when the treatment of mouse i is T, and 0 otherwise; tij represents the time elapsed between measurement time 1 and measurement j for the ith mouse; ɛij represents a normally distributed residual for the j-th measurement of the i-th mouse with expectation zero and variance σ2, i.e., ɛij ~ N(0, σ2); and bi represents the random intercept for mouse i, with bi ~ N(0, τ2). Log-transformed tumor volume was used for the analysis to better satisfy the assumption of linearity in growth over time. Under this model, β0 can still be interpreted as the overall average logarithmic tumor volume at first time measurement for all mice. β1 represents the linear change in log scale tumor-volume across time for the vehicle group, whereas βk were the parameters for the linear change of tumor log-volume for the compound treatment groups given by the respective indicator variable. Thus, the βk – β1, represent the difference between log scale tumor growth ratio between compound treatments vs. vehicle group, whereas e.g. βk– βk,-1 represents the differences of rates of log scale tumor growth ratio between their respective treatment groups. A Wald test was used for the significance comparisons among different groups. [00701] Pharmacokinetic studies [00702] Plasma samples were collected in CD1 mice from the dorsal metatarsal vein at all timepoints except 24h. For the 24h timepoint, heart puncture was used to collect samples. A volume of 0.05-0.1 mL was collected at each time point. K2-EDTA was used as the anticoagulant. Blood samples collected at each time point were transferred into plastic micro centrifuge tubes containing K2-EDTA. Collection tubes with blood samples and anticoagulant were inverted several times for proper mixing of the tube contents and placed on wet ice. The samples were centrifuged at 4000 g for 5 minutes at 4°C to obtain plasma. The samples were stored in a freezer at -75 ± 15°C prior to analysis. WinNonlin (PhoenixTM, version 6.1) or other similar software was used for pharmacokinetic calculations. Results: Compound 2R, and its pharmaceutically acceptable salts, are potent and selective dual inhibitors of EGFR and PI3K [00703] A structure-based drug design strategy was implemented to identify Compound 2R (Fig.1). Small molecules were rationally designed to inhibit both EGFR and PI3K in a selective and concurrent manner based upon analysis of known structural features of NVP- AEE78823 and omipalisib bound to EGFR and PI3Kγ, respectively (Figs.7A and 7B). Whereas the 6-position of the fused ring of NVP-AEE788 points out towards solvent, this position in omipalisib points towards the back of the ATP pocket of PI3Kγ towards the specificity pocket. The inventors leveraged this flipped binding mode of the quinazoline core between EGFR and PI3Kγ to computationally desgn potent and selective dual inhibitors of both enzyme families. X- ray crystal structures of EGFR and PI3Kγ co-complexed with Compound 2R confirmed the postulated reversed binding mode of Compound 2R to each of these targets. The 1-position of the quinazoline ring in Compound 2R forms a key hydrogen bond with the backbone amide of MET793 of EGFR (Fig.1C). The substitution at the 4-position fits into a hydrophobic pocket formed by the aliphatic sidechains of LYS745 and THR790, whereas the hydrophilic group at the 6-position faces outward to a solvent accessible area of EGFR. The 1-position of Compound 2R forms a hydrogen bond with the backbone amide of VAL882 of PI3Kγ (Fig.1E). Unlike what is seen with EGFR, groups at the 6-position of Compound 2R bind within a hydrophilic region of PI3K that is created by hydrophilic hydroxyl and amine groups of TYR867 and LYS833, respectively. The 4-position, which binds within a solvent exposed area of PI3Kγ, is especially important as it contains a chiral center. All studies described here have been carried out with the R-isomer, which is ~100-fold more potent against EGFR and ~10-fold less potent against PI3K than the S-isomer. Collectively, the observed binding mode is consistent with these isomeric potency differences. The methyl substituent of Compound 2R in the S-isomer is oriented toward a sterically restricted area of EGFR, thus hindering binding. [00704] In biochemical assays with purified kinases, Compound 2R exhibits low nanomolar potency against EGFR and PI3Kα with IC50’s of 15 nM and 6.4 nM, respectively (Fig.17A and Fig.18A). Figure 1D shows the crystal structure of PI3Kγ co-complexed with COMPOUND 2R. The strong potency of Compound 2R against multiple PI3K family members (Figs.18A-D) and mTOR (Fig.19A) reveals a biochemical profile distinctly different from clinically approved alpelisib/PIQRAYTM and copanlisib/ALIQOPATM. Copanlisib and Compound 2R share a pan-PI3K inhibitory profile, whereas alpelisib is one to three logs more potent against PI3Kα compared to the other PI3K isoforms. Compound 2R is only ~15-fold less potent against mTOR compared to PI3Kα, a feature that distinguishes it from both alpelisib and copanlisib. Subsequent evaluation of kinome selectivity against a broad panel of >400 protein and lipid kinases revealed that Compound 2R is exquisitely selective for HER and PI3K family members (Fig.1G). At 10 µM, only nine protein kinases outside of the HER family were inhibited by >80% (Fig.20A and 20B). Titration of Compound 2R against these non-targeted kinases revealed insignificant inhibition (IC50 >1 μM) against all but MELK (IC50178 nM), TTK (IC50695 nM), CDK8 (IC50949 nM), and CDK11 (IC50739 nM). MELK was the only off target protein kinase inhibited by Compound 2R with potency within 50-fold of that observed against PI3Kα. [00705] Cellular evidence for the ability of Compound 2R to co-target EGFR and PI3K was generated in the CAL-33 HNSCC model, which is known to possess a PIK3CA mutation (H1047R), see Fig.2N. After 2-hour treatment of cultured CAL-33 cells, Compound 2R demonstrated concentration-dependent inhibition of EGFR, PI3K, and mTOR as measured by lower levels of phosphorylated EGFR, AKT, and 4E-BP1 (Fig.1F, & Figs.21A-21E). Balanced cellular inhibition of these targets is reflected in comparable IC50 values (~300 nM), demonstrating that Compound 2R is equipotent at inhibiting both EGFR and PI3K/mTOR signaling. Furthermore, significant induction of apoptosis as measured by expression of cleaved PARP was observed when concentrations of Compound 2R approached 1 µM. [00706] Compound 2R monotherapy inhibits EGFR and PI3K signaling and leads to regression of squamous head and neck cancer xenografts [00707] Early single agent in vivo evaluation of Compound 2R focused on determination of its therapeutic activity against high passage HNSCC xenografts. The rationale for selection of HNSCC to demonstrate monotherapy activity was driven by the prominent roles played by both EGFR and PI3K in progression of this disease. EGFR is overexpressed in roughly 90% of HNSCC, while the PIK3CA pathway is the most frequently mutated oncogenic pathway in this disease (~30% incidence). Compound 2R exhibited high (~80%) oral bioavailability in mice and is therefore conducive to oral dosing studies (Figs.8A-8D). Pharmacodynamic evaluation of CAL-33 tumors excised from mice treated with a single oral dose of Compound 2R showed time-dependent suppression of EGFR and PI3K/mTOR pathway signaling (Fig.2A and 2B). A >50% reduction in levels of pEGFR and pAKT was maintained for 24 hours when mice were dosed at 100 mg/kg. At 48 hours after dosing, control levels of pEGFR and pS6 had returned, while 50% suppression of pAKTT308 expression remained. Consistent with its pharmacodynamic activity, Compound 2R was highly efficacious against both CAL-33 and CAL-27 xenografts resulting in a 33 to 100% incidence of complete responses across a broad range of doses (Fig.2B, and Fig.9). Dosing occurred daily until individual tumors reached the same size (500 or 1000 mm3 for CAL-27 and CAL-33, respectively) to facilitate comparative survival analysis. Studies were terminated >300 days post tumor implantation at which time the median increase in survival exceeded 1000% with >50% of the mice remaining tumor free in all groups dosed at 25 mg/kg or greater (Fig.9). The applicability of these findings to patient- derived tumors was explored by testing Compound 2R against a heterogeneous panel of PIK3CA mutant HNSCC PDX models obtained from the NCI Patient-Derived Models Repository (PDMR) (Figs.2J-2N and Figs.22A-22E). Baseline pathway expression analysis showed consistently high levels of pEGFR in 4 of the 5 models and highly variable expression of downstream kinases in the PI3K/mTOR pathway (Figs.10A and 10B). Representative pharmacodynamic data generated for two of these models showed that Compound 2R completely blocked activation of AKT (Figs.21B and 21C) and S6 (Fig.21D) within two hours of dosing Fig.11). Compound 2R was highly efficacious against the PDX panel, resulting in regressions in every model evaluated (Fig.2d, Fig.2e). One model, 944545-341-R, was an exceptional responder, as reflected by a 50% complete response rate and a >500% increase in median survival (Fig.2N). Regressions of 944545-341-R tumors were durable as 3 of 6 mice remained tumor free after 172 days of dosing. While Compound 2R did not elicit as high an incidence of complete regressions in the other four HNSCC PDX models, they nonetheless were highly sensitive as evidenced by a mean overall response rate of 66% and an increase in median survival ranging from 62 to 542% (Figs.12A-12D). [00708] Compound 2R, which was administered daily at 100 mg/kg, was well tolerated throughout these studies. Mice continued to gain weight throughout dosing regimens that in one study exceeded 10 weeks (Figs.13A- 13E and Fig.14A). Compound 2R also tolerated dosing at 150 mg/kg, leading to a further boost in efficacy in the 848979-319-R model and a 3-fold improvement in median survival compared to mice treated at 100 mg/kg (Fig.14C). Therefore, collective efficacy data generated against the HNSCC PDX panel at the lower dose is a conservative indicator of the monotherapy potential of Compound 2R. Head-to-head evaluation of Compound 2R versus the combination of erlotinib and alpelisib was carried out in the 944545-341-R PDX model (Figs.2R-2T). Whereas Compound 2R single agent treatment resulted in a mean tumor regression of 70%, no regressions were observed in the erlotinib/alpelisib combination arm. Higher doses of alpelisib were not tolerated in the combination regimen (Fig.23). Compound 2R improves response of KRAS and BRAF mutant colorectal tumors to MEK inhibition [00709] Triple combinations directed against EGFR (cetuximab), PI3K (alpelisib), and the MAPK pathway (encorafenib or trametinib), while scientifically sound, have proven challenging due in part to poor tolerability. Since Compound 2R targets two of the three critical signaling nodes targeted in these trials, the inventors investigated its potential to improve therapeutic outcome when combined with a MEK inhibitor to treat KRAS and BRAF mutant colorectal CRC PDX models. NCI CN0375-F725 (KRASA136T) xenografts did not respond to single agent treatment with the MEK inhibitor trametinib or Compound 2R, as neither agent resulted in regressions or stasis (Figs.3A-3C). However, an 80% objective response rate was achieved in the combination arm where animals exhibited a median increase in survival of 467%. [00710] The combination of Compound 2R and trametinib was also efficacious in a BRAFV600E mutant CRC PDX model (UM CRC 14-929) (Figs.3D-3F). Whereas trametinib was inactive, treatment with Compound 2R alone led to tumor stasis in most animals. Combination of Compound 2R with trametinib led to further improvement in efficacy, reflected by a 40% partial response rate. Pharmacodynamic assessment of kinase expression in excised tumors showed that combination treatment led to a striking 97% reduction in pS6 levels consistent with improved efficacy over single agent performance (Figs.15A and 15B). [00711] In both combination studies, Compound 2R was dosed at 100 mg/kg, corresponding to the same dose used in the monotherapy studies with HNSCC models. Addition of trametinib to the Compound 2R daily dosing regimen was well tolerated and did not lead to body weight loss (Fig.24B). [00712] Compound 2R in combination with a KRASG12C inhibitor improves therapeutic outcome in KRASG12C tumor-bearing mice [00713] Agents that directly target KRASG12C have limited activity against KRASG12C CRC in part due to EGFR-mediated reactivation of ERK signaling. This finding led to combination trials with agents directed against KRASG12C and EGFR. However, secondary resistance mechanisms driven by other RTK’s or upregulation of mTOR signaling limit durability of response to this combination strategy. Based on its EGFR/PI3K/mTOR inhibitory profile, the inventors anticipated that Compound 2R would prove efficacious in combination with KRASG12C inhibitors. This hypothesis was supported by data generated from combination of the KRASG12C inhibitor sotorasib with Compound 2R, in mice bearing KRASG12C CRC or pancreatic tumors. Dosing of the NCI 135848-042-T CRC model, which additionally harbors mutations in mTOR (S2215F) and ERBB2 (S310F), was curtailed to 10 days due to steady body weight loss exceeding 10% in the control animals (Fig.4A and 4C). Nonetheless, on the last day of treatment, tumor stasis was observed in the combination arm in contrast to inactivity seen in response to either single agent (Fig.4A and 4C). The combination of sotorasib and Compound 2R was most efficacious against MIA PaCa-2 xenografts, where all mice showed complete regressions, surpassing the 40% incidence of CR’s seen in response to single agent sotorasib (Fig.4B and 4D). [00714] Surprisingly, Compound 2R does not lead to hyperglycemia in mice. [00715] Hyperglycemia is the most common on-target side effect of PI3K inhibitors due to the central role of PI3Kα in insulin signaling. Since Compound 2R is a potent inhibitor of PI3Kα, the observation that mice dosed at therapeutic levels did not show a significant rise in blood glucose levels mice was unexpected and surprising (Fig.5A). Consistent with absence of hyperglycemia, Compound 2R treatment also unexpectedly, had no effect on blood insulin levels, unlike alpelisib, which elicited a significant rise in both plasma glucose and insulin levels (Fig.5B). This result was confirmed in subsequent testing of a large panel of PI3K inhibitors that included both clinically approved and failed agents in the clinic (Fig.5C). An escalation in dose of Compound 2R to 150 mg/kg also did not lead to hyperglycemia. It has been reported that systemic glucose-insulin feedback plays a role in mediating reactivation of PI3K signaling in tumors treated with a PI3K inhibitor. Prior studies have shown that mice bearing syngeneic Kras-Tp53-Pdx-Cre (KPC) pancreatic tumors failed to respond to alpelisib unless they were placed on a ketogenic diet. Since mice treated with Compound 2R are not subject to hyperglycemia, the inventors directly compared the efficacy of this molecule to alpelisib in KPC tumor-bearing mice. Before two weeks of daily dosing could be completed, all control and alpelisib-treated mice were sacrificed, reflecting the highly aggressive nature of this tumor model (Figs.5D-5F). In contrast, tumor stasis was observed in the Compound 2R-treated mice. The impact of Compound 2R on median survival (215% increase) is comparable to that shown for mice treated with alpelisib and placed on a ketogenic diet. Furthermore, insulin concentrations in CAL-33 and KPC tumors were significantly elevated in response to treatment with alplelisib, but not Compound 2R, consistent with differences in circulating levels of insulin (Figs.5G and 5H). Collectively, these studies suggest for the first time, that Compound 2R appears to be less prone to insulin-mediated feedback mechanisms that compromise the antitumor activity of PI3K inhibitors. PPARγ agonism is a unique feature of Compound 2R [00716] The inventors hypothesized that the absence of hyperglycemia in mice treated with Compound 2R stems from the molecule’s unique ability to act as an agonist of the nuclear hormone receptor PPARγ. Time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays showed Compound 2R to be a weak agonist of PPARγ (IC50 = 2.5 μM) (Fig.6A). Compound 2R was inactive against PPARα and PPARδ at concentrations as high as 100 μM (Fig.25A and Fig.25B). The intracellular effects of Compound 2R on PPARγ activity was evaluated in reporter gene assays carried out in 293H cells. Compound 2R exhibited cellular agonist activity against PPARγ with an EC50 of 3.4 μM (Fig.6B) and was significantly less potent than the reference compound rosiglitazone (EC50 = 14 nM; data not shown). Expression of PPARγ target genes involved in the induction of adipocyte differentiation were measured by RT-PCR in treated 3T3-L1 cells and provided further evidence for a functional interaction between Compound 2R and PPARγ. 3T3-L1 preadipocytes were cultured in differentiation induction medium containing Compound 2R (10 μM) or rosiglitazone (1 μM) for 8 or 24h followed by assessment of gene expression of the adipocyte markers PPARγ, lipoprotein lipase (LPL) and adiponectin (Fig.6C). These markers are known to be upregulated in response to rosiglitazone. Maximal induction of PPARγ ranged from 2- to 4-fold in response to both compounds, providing further support for the ability of Compound 2R to act as an agonist of PPARγ. The one log difference in concentration of Compound 2R and rosiglitazone further suggest that Compound 2R is acting as a weak PPARγ agonist. At the protein level, the extent of upregulation in expression levels of both PPARγ 1 and PPARγ 2 were again consistent with significant potency differences between rosiglitazone and Compound 2R (Fig.6D). [00717] The inventors next sought to generate an x-ray crystal structure of Compound 2R bound to PPARγ to provide structural evidence for their interaction. The 3-dimensional complex of PPARγ and Compound 2R was solved from crystals diffracted to 1.9 Å resolution (Fig.6E). Clear electron density at the compound binding site revealed the binding of the entire compound and allowed an unambiguous placement of the ligand (Fig.26A and 26B). The final solved structure contains two molecules of PPARγ (chains A and B) and one molecule of Compound 2R bound to chain B. The PPARγ ligand binding region is known to be a large and mostly hydrophobic cavity that can bind a wide variety of small molecules. The quinazoline core of Compound 2R appears to sit in a pocket of PPARγ formed by stacking of the aliphatic sidechains of Leu330 and Arg288 (Fig.6F). The functional groups of the 6-position core quinazoline groups in Compound 2R binds in a site distinct from the orthosteric pocket that binds rosiglitazone (Fig.16A). Discussion [00718] There are few examples of approved kinase-targeted drugs that lead to durable single agent activity. The PI3K/mTOR pathway drives resistance to a broad assortment of targeted therapies, including highly selective allosteric MEK inhibitors and covalent inhibitors of KRASG12C. The design of Compound 2R was driven by the concept of developing a single molecule that could selectively target the PI3K/mTOR pathway in addition to EGFR. Such a molecule would ostensibly be useful to deliver single agent therapy to selected patient populations, for example, patients diagnosed with squamous tumors, where EGFR and PI3K and/or mTOR are genomic drivers. Furthermore, this approach could also provide an attractive candidate for combination with RAS pathway inhibitors, which are subverted by these resistance drivers. The combination of cetuximab and adagrasib nearly doubled the RECIST response rate compared to adagrasib monotherapy in KRASG12 colorectal cancer patients. Nonetheless, secondary resistance to co-targeting EGFR and KRASG12C, in some cases driven by upregulation of mTOR signaling, has been reported in colorectal cancer. Compound 2R, by virtue of its dual inhibitory activity against EGFR and the PI3K/mTOR pathway, offers a single molecule strategy for overcoming two key resistance mechanisms found in KRASG12C CRC. [00719] Compound 2R was rationally designed to inhibit EGFR and PI3K, selectively and concurrently. The reversed binding mode of the quinazoline core between EGFR and PI3Kγ was an important element in the design of Compound 2R, which shows low nanomolar potency against both of its intended targets. Other studies have previously reported on the feasibility of designing dual inhibitors of tyrosine and phosphoinositide kinases. Through iterative medicinal chemistry and x-ray crystallography, they identified molecules that adopt a single binding mode to inhibit PI3 kinases and multiple tyrosine kinases with a high degree of potency. In contrast, Compound 2R is a remarkably selective ATP-competitive protein kinase inhibitor. Only four of 432 kinases tested were found to exhibit an IC50 < 1 μM and none came within 10-fold of Compound 2R’s on target potency against EGFR (IC50 ~15 nM). Erlotinib, which is roughly one log more potent against EGFR than Compound 2R, has been reported to exhibit an IC50 <1 μM against a minimum of 20 off-target kinases. [00720] Preclinical proof-of-concept supporting advanced development of Compound 2R comes in part from monotherapy studies conducted against HNSCC models, where objective responses were observed in all of the models tested. While every HNSCC PDX model studied here contained a hot spot mutation in PIK3CA, it remains unclear whether this marker is a prerequisite for efficacy. The inventors find this unlikely, since a number of kinases in the PI3K/mTOR pathway are found to be mutated in the HNSCC patient population, including other PIK3C isoforms, AKT, PTEN, MTOR, and RICTOR8. NCI 944545-341-R is not known to harbor any additional mutations to explain the durable complete regressions seen in response to Compound 2R that were not seen in the other PDX models. However, it is noteworthy that both pPRAS40 and p4E-BP1 expression were significantly upregulated in 944545-341-R (Fig.10A). Interestingly, phosphorylated PRAS40 has been reported to correlate with insulin-like growth factor-1 receptor induced resistance to EGFR inhibition in head and neck cancer. PRAS40 is an inhibitor of mTOR that upon phosphorylation by AKT leads to increased mTOR signaling. Given the panPI3K/mTOR inhibitory profile of Compound 2R, its striking activity against 944545-341-R tumors is perhaps not surprising. mTOR has been reported to restrain the tumor suppressor function of 4E-BP1 via phosphorylation, which can be reactivated in response to mTOR inhibition. [00721] In the combination setting, the effectiveness of Compound 2R at inhibiting both EGFR and PI3K/mTOR signaling is likely a major factor in its strong efficacy against KRASG12C CRC. The seminal study of Misale and colleagues identified EGFR signaling as the dominant mechanism of colorectal cancer resistance to KRASG12C targeted agents (See Amodio, V., et al. EGFR Blockade Reverts Resistance to KRAS(G12C) Inhibition in Colorectal Cancer. Cancer discovery 10, 1129-1139 (2020). The later discovery that co-targeting of EGFR and KRASG12C leads to upregulation of mTOR signaling illustrates the robust adaptive capacity of these tumors to rely on bypass signaling routes to survive. A potential role for mTOR signaling intervention in addressing adaptive resistance to KRAS inhibitors has also been reported for NSCLC and pancreatic cancer model. The importance of sustained mTOR inhibition to prevent resistance also extends to therapeutics targeting EGFR or PI3K46. [00722] Since the PI3K/AKT/mTOR pathway is the most frequently dysregulated pathway in human cancers, it has received intense scrutiny from the drug discovery community. Consequently, a multitude of drug candidates targeting this pathway have advanced to the clinic. Unfortunately, most of these trials have failed due to poor tolerability, drug resistance, and the inability to deliver single agent efficacy. Compound 2R has the potential to challenge the widely accepted view that dual PI3K/mTOR inhibitors are inherently more toxic than isoform-specific PI3K inhibitors. Treatment with Compound 2R led to complete regressions of CAL-33 xenografts over a wide dose range spanning 25 to 100 mg/kg. In contrast, alpelisib (BYL- 719/Piqray™), which is the only clinically approved PI3Kα-selective inhibitor, was reported to result in stasis but not regressions of CAL-33 tumors (See Mizrachi, A., et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nature Communications 8, 14292 (2017). [00723] Without wishing to be bound by any particular theory, it is surprising and unexpected to find that Compound 2R does not lead to PI3K inhibitor-induced hyperglycemia in mice, since that side effect is common to both mice and humans. In clinical trials, hyperglycemia has become a surrogate biomarker for demonstration of effective PI3K inhibition resulting from disruption of systemic glucose homeostasis. Interestingly, PI3Kα-selective inhibitors have been reported to possess an improved preclinical therapeutic window compared to pan-PI3K inhibitors on the basis of their reduced propensity for hyperglycemia. However, the inventors find that PI3K inhibitors from both classes lead to an elevation in blood glucose levels, a property, surprisingly, not shared with Compound 2R, which uniquely does not cause hyperglycemia. Without wishing to be limited to any particular theory, it is believed that the agonistic activity of Compound 2R against the PPARγ receptor counteracts PI3K-driven hyperglycemia. PPARγ agonists are commonly used in the treatment of type 2 diabetes to increase sensitivity to insulin. Consistent with the data presented infra, others have reported that the thiazolidinedione pioglitazone prevents hyperglycemia caused by the PI3K inhibitor PX- 86651. The inventors find it unlikely that PPARγ-mediated differentiation is contributing to the antitumor efficacy from Compound 2R treatment. Oncology clinical trials carried out with PPARγ agonists more potent than Compound 2R have largely been disappointing. Compound 2R is believed to be the first reported inhibitor of wild type PI3Kα that does not cause hyperglycemia. Consistent with this observation, mice treated with Compound 2R do not need to be placed on a ketogenic diet to be active against highly aggressive KPC tumors as reported for alpelisib. Based on this surprising and unexpected feature, Compound 2R may be less susceptible to insulin-mediated reactivation of PI3K signaling reported for alpelisib. However, the inventors cannot rule out the possibility that immunomodulatory effects of Compound 2R contribute to its antitumor efficacy in this syngeneic model. [00724] Other targets that could be envisioned to possibly be a viable target may include KRASG12D cancers where the EGFR/HER family and the S6 pathway impact response to KRASG12D inhibitor. As a single agent, Compound 2R has a role in the treatment of not only HSNCC, but also squamous lung cancers, as well as a subset of triple negative breast cancers, specifically those driven by EGFR and PI3K, including but not limited to: all PIK3CA mutant cancers, including certain breast cancers, GI cancers of the stomach or colon, esophagus, cervix, head & neck, squamous lung, and certain gynecologic cancers of the ovary and uterus. Compound 2R, which has the unique capability of concurrently and selectively inhibiting EGFR, PI3K, and mTOR, illustrates the power of rational computational drug design to target multiple adaptive resistance mechanisms in a single molecule. [00725] Example 58. Blood and Oral Glucose and Adiponectin responses/tolerance with Compound 2R and Alpelisib [00726] As depicted and used in the figures in the present examples, the term "MTX- 531"; refers to a compound of Formula (I) referred to herein as "Compound 2R" or "COMPOUND 2R" and is used interchangeably herein in the present specification. The structure of MTX-531 (Compound 2R) is found in Table 1 and Fig.1, and is known as N-(2-chloro-5-(4- ((1R-phenylethyl)amino)quinazolin-6-yl)pyridin-3-yl)methanesulfonamide. [00727] Blood glucose levels in athymic mice following a single dose of alpelisib or Compound 2R. A follow-up study to that depicted in Figure 5A was carried out comparing effects of Compound 2R and clinically approved alpelisib on blood glucose levels in non-fasted mice of both genders. Mice were administered the vehicle control or PI3K inhibitor at the indicated dose. For assessment of blood glucose levels, 1-2 drops of blood were taken from the tails of the mice and measured using a glucometer at the indicated time point before (time zero) or after treatment. A statistical analysis was carried out for both genders comparing the hyperglycemic effects of each PI3K inhibitor. Data shown in Fig.27 depicts a statistically significant elevation in blood glucose levels in both genders in response to alpelisib versus Compound 2R at both the 30 minute and 1 hour time point post dosing. (**** p < 0.0001). [00728] Oral glucose tolerance testing in fasted mice following a single oral dose of alpelisib or Compound 2R. [00729] Athymic female nude mice were fasted three hours prior to receiving a single oral dose of Compound 2R or alpelisib at the indicated dose. One hour after drug dosing, mice were administered an oral bolus of glucose (time zero). Blood glucose readings were obtained at the indicated time points (time zero and 0.25, 0.5, 1, 2, and 4 hours after receiving the glucose bolus). For assessment of blood glucose levels, 1-2 drops of blood were taken from the tails of mice and measured using a glucometer. Statistical analysis of differences in glucose levels relative to the vehicle control values were carried out by ordinary one-way ANOVA. Data shown in Fig.28 depict a statistically significant elevation in blood glucose levels in response to alpelisib (*p = 0.0129; **p =0.0025), but no statistically significant elevation in response to Compound 2R. [00730] Oral glucose tolerance testing in fasted mice after five days of PI3K inhibitor dosing. [00731] Athymic female nude mice were treated daily for five days with an oral dose of Compound 2R or alpelisib at the indicated dose. On the fifth day, mice were fasted three hours prior to drug dosing. One hour later, mice were administered an oral bolus of glucose (time zero). Blood glucose readings were obtained at the indicated time points (time zero, 0.25, 0.5, 1, 2, and 4 hours after receiving glucose bolus). For assessment of blood glucose levels, 1-2 drops of blood were taken from the tails of mice and measured using a glucometer. Statistical analysis of differences in glucose levels relative to the vehicle control values were carried out by ordinary one-way ANOVA. Data shown in Fig.29 depict a statistically significant elevation in blood glucose levels in response to alpelisib (**p = 0.0029; ***p =0.0001;**** p<0.0001), but no statistically significant elevation in response to Compound 2R. [00732] Adiponectin levels in blood from C57/BL6 mice treated with Compound 2R versus the PPAR gamma agonist rosiglitazone (RGZ). [00733] An vivo study was conducted to follow up on the in vitro findings depicted in Figure 6C, specifically focusing on comparison of levels of adiponectin, a marker for PPAR gamma activity, in mice treated daily for five days with Compound 2R versus the known PPAR gamma agonist rosiglitazone. C57BL/6 mice were treated for five days with Compound 2R or rosiglitazone at the indicated doses. Blood samples were collected 2 hours following the last treatment and analyzed for adiponectin levels by an ELISA assay. As shown in Fig.30, a statistically significant elevation in adiponectin levels was observed for both compounds (59% and 36% increase in response to Compound 2R and RGZ, respectively, relative to vehicle control values). *p=0.0137; ***p=0.0003; ****p<0.0001. This observation provides further support for the PPAR gamma agonist attribute of Compound 2R. [00734] Adiponectin levels in blood from athymic nude mice treated with Compound 2R versus comparator PI3K and PPAR gamma targeted agents. [00735] An expanded in vivo study was conducted in athymic nude mice to compare levels of adiponectin in response to five days of daily oral treatment with Compound 2R versus a comparator PI3K inhibitor (alpelisib) or comparator PPAR gamma agonists (rosiglitazone/RGZ or pioglitazone/PGZ). Mice were treated for five days with each of these agents at the indicated doses. Blood samples were collected 1 hour following the last treatment and analyzed for adiponectin levels by an ELISA assay. As shown in Fig.31, the data shows that relative to the vehicle control samples, a statistically significant elevation in adiponectin levels was observed for Compound 2R and both comparator PPAR gamma agonists, but not for alpelisib (**p=0.0014; ***p=0.0004; ****p<0.0001). These observations provide further support for the PPAR gamma agonist attribute of Compound 2R. OTHER EMBODIMENTS [001] All publications and patents referred to in this disclosure are incorporated herein by reference to the same extent as if each individual publication or patent application were specifically and individually indicated to be incorporated by reference. Should the meaning of the terms in any of the patents or publications incorporated by reference conflict with the meaning of the terms used in this disclosure, the meaning of the terms in this disclosure are intended to be controlling. Furthermore, the foregoing discussion discloses and describes merely exemplary embodiments of the present disclosure. One skilled in the art will readily recognize from such discussion and from the accompanying drawings and claims, that various changes, modifications and variations can be made therein without departing from the spirit and scope of the disclosure as defined in the following claims.
Claims
We Claim: 1. A method for treating cancer in a subject in need thereof, comprising, administering to the subject, a therapeutically effective amount of a pharmaceutical composition comprising a compound according to Formula I: or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein, Ring A is selected from phenyl and a 5 or 6 membered heteroaryl; X is N or C-R5; R1a is selected from the group consisting of H and C1-6 alkyl; R1b is selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, halo, CN, and NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with one or more R” substituents; or, R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl and a 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with one or more R” substituents; each R2 is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; or one R2 substituent and R1b, together with the phenyl group to which R2 is attached and the carbon atom to which R1b is attached, form a bicyclic group having the general structure , wherein Ring B is a C3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2; each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OR’, N(R’)2, C(O)R’, C(O)OR’, C(O)N(R’)2, OC(O)OR’, OC(O)N(R’)2, NR’C(O)N(R’)2, SOR’, SON(R’)2, SO2R’, SO2N(R’)2, NR’SOR’, NR’SON(R’)2, NR’SO2R’, and NR’SO2N(R’)2, wherein the C1-6 alkyl, hetercycloalky, and heteroaryl are each optionally and independently substituted with one or more R” substituents; or two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heterocycloalkyl, a fused bicyclic cycloalkyl, a fused bicyclic aryl, or a fused bicyclic heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R4 is selected from halo, OH, NH2, CN, C1-6 alkyl, and OC1-6 alkyl; each R5 is selected from hydrogen, halo, OH, NH2, CN, and C1-6 alkyl; each R’ is independently selected from hydrogen, OH, CN, C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl, each of which is optionally and independently substituted with one or more R” substituents; each R” is independently selected from the group consisting of C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, and C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or substituents selected from halo, oxo, alkoxy, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, and C(O)NHC1-6 alkyl; and m, n, and p are each an integer selected from 0-4, wherein the compound or pharmaceutically acceptable salt thereof is a dual inhibitor of PI3K and EGFR, and wherein administration of the compound or pharmaceutically acceptable salt, or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, does not significantly elevate any one of: blood glucose, blood insulin, or hyperglycemia in the subject, after one or more administrations of the compound or pharmaceutically acceptable salt thereof.
2. The method of claim 1, wherein R1a is H.
3. The method of claim 1 or claim 2, wherein R1b is C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, heteroaryl, OC1-6 alkyl, OH, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, C(O)N(C1-6 alkyl)2, halo, CN, or NO2, wherein each C1-6 alkyl, cycloalkyl, hetercycloalkyl, aryl, or heteroaryl is optionally and independently substituted with OC1-6 alkyl, oxo, OH, halo, CN, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, or C(O)N(C1-6 alkyl)2.
4. The method of any one of claims 1-3, wherein R1b is C1-6 alkyl, OC1-6 alkyl, OH, NH2, NHC1-6 alkyl, N(C1-6 alkyl)2, C(O)C1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, C(O)N(C1-6 alkyl)2, halo, or CN, wherein each C1-6 alkyl is optionally and independently substituted with OC1-6 alkyl, OH, halo, or CN.
5. The method of any one of claims 1-4, wherein R1b is C1-6 alkyl, OC1-6 alkyl, OH, halo, or CN, wherein each C1-6 alkyl is optionally and independently substituted with OH, CN, or halo.
6. The method of any one of claims 1-5, wherein R1b is C1-6 alkyl, C1-6 alkyl-OH, or CN.
7. The method of any one of claims 1-6, wherein R1b is methyl, CN, or CH2OH. The compound or salt of claim 1, wherein R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl or 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with C1-6 alkyl, OC1-6 alkyl, oxo, OH, halo, CN, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, C(O)NH2, C(O)NHC1-6 alkyl, or C(O)N(C1-6 alkyl)2, wherein each C1-6 alkyl is optionally and independently substituted with one or substituents selected from halo, alkoxy, CN, and NH2.
8. The method of claim 8, wherein R1a and R1b, together with the methylene moiety to which they are attached, form a spirocyclic ring selected from a C3-7 cycloalkyl and a 3-7 membered heterocycloalkyl, each of which is optionally and independently substituted with oxo, OH, halo, or CN.
9. The method of any one of claims 8-9, wherein R1a and R1b, together with the methylene moiety to which they are attached, form an unsubstituted spirocyclic ring selected from a C3-7 cycloalkyl and a 3-7 membered heterocycloalkyl.
11. The method of any one of claims 1-11, wherein each R2 substituent is independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, C(O)C1-6 alkyl, and C(O)NH2.
12. The method of any one of claims 1-12, wherein each R2 substituent is independently selected from halo and OH.
13. The method of any one of claims 1-13, wherein n is 0 or 1.
14. The method of any one of claims 1-14, wherein n is 0.
15. The method of claim 1, wherein one R2 substituent and R1b, together with the phenyl group to which R2 is attached and the carbon atom to which R1b is attached, form a bicyclic group having the general structure , wherein Ring B is a C3-7 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, C1-6 alkyl, haloalkyl, OC1-6 alkyl, CN, NH2, C(O)C1-6 alkyl, C(O)OC1-6 alkyl, and C(O)NH2. The compound or salt of claim 16, wherein Ring B is a C4-6 cycloalkyl or 4-6 membered heterocycloalkyl, each of which is optionally substituted with one or more substituents independently selected from halo, OH, OC1-6 alkyl, CN, NH2, and C(O)C1-6 alkyl. The compound or salt of claim 16 or claim 17, wherein Ring B is a C4-5 cycloalkyl or 5 membered heterocycloalkyl, each of which is optionally substituted with OH.
17. The method of any one of claims 1-19, wherein each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OH, OC1-6 alkyl, N(C1-6 alkyl)2, NH(C1-6 alkyl), NH2, C(O)H, C(O)C1-6 alkyl, COOH, C(O)OC1-6 alkyl, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, OC(O)OC1-6 alkyl, OC(O)NH2, OC(O)NH(C1-6 alkyl), OC(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO(C1-6 alkyl), SONH2, SONH(C1-6 alkyl), SON(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO(C1-6 alkyl), NHSO(C1-6 alkyl), N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), NHSO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2NH2, N(C1-6 alkyl)SO2NH(C1-6 alkyl), and N(C1-6 alkyl)SO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl is optionally and independently substituted with one or more R” substituents.
18. The method of any one of claims 1-20, wherein each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, NO2, OH, OC1-6 alkyl, N(C1-6 alkyl)2, NH(C1-6 alkyl), NH2, C(O)H, C(O)C1-6 alkyl, COOH, C(O)OC1-6 alkyl, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, OC(O)OC1-6 alkyl, OC(O)NH2, OC(O)NH(C1-6 alkyl), OC(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, NHSO2(C1-6 alkyl), N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), NHSO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2NH2, N(C1-6 alkyl)SO2NH(C1-6 alkyl), and N(C1-6 alkyl)SO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl is optionally and independently substituted with one or more R” substituents.
19. The method of any one of claims 1-21, wherein each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH2, NHC(O)NH(C1-6 alkyl), NHC(O)N(C1-6 alkyl)2, N(C1-6 alkyl)C(O)NH2, N(C1-6 alkyl)C(O)NH(C1-6 alkyl), N(C1-6 alkyl)C(O)N(C1-6 alkyl)2, SO2(C1-6 alkyl), SO2NH2, SO2NH(C1-6 alkyl), SO2N(C1-6 alkyl)2, N(C1-6 alkyl)SO2(C1-6 alkyl), NHSO2(C1-6 alkyl), NHSO2NH2, NHSO2NH(C1-6 alkyl), and NHSO2N(C1-6 alkyl)2, wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl is optionally and independently substituted with one or more R” substituents.
20. The method of any one of claims 1-22, wherein each R3 is independently selected from C1-6 alkyl, a 5-6 membered heteroaryl, a 5-6 membered heterocycloalkyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH(C1-6 alkyl), SO2(C1-6 alkyl), and NHSO2(C1-6 alkyl), wherein each C1-6 alkyl, heteroaryl, and heterocycloalkyl is optionally and independently substituted with one or more R” substituents.
21. The method of any one of claims 1-23, wherein each R3 is independently selected from C1-6 alkyl, triazolyl, oxadiazolyl, halo, CN, OH, OC1-6 alkyl, NH2, C(O)H, C(O)NH2, C(O)NH(C1-6 alkyl), C(O)NH(C3-6 cycloalkyl), C(O)NH(CN), C(O)NH(OH), C(O)N(C1-6 alkyl)(OH), C(O)N(C1-6 alkyl)2, NHC(O)NH(C1-6 alkyl), SO2(C1-6 alkyl), and NHSO2(C1-6 alkyl), wherein each C1-6 alkyl, triazolyl and oxadiazolyl, is optionally and independently substituted with one or more substituents independently selected from oxo, OH, halo, C1-6 alkyl, NH2, NHC1-6 alkyl, and N(C1-6 alkyl)2.
22. The method of any one of claims 1-24, wherein each R3 substituent is independently selected from halo, CN, NH2, OH, C(O)H, C(O)N(CH3)2, C(O)NH(CH3), C(O)NH(Et), C(O)NH(isopropyl), C(O)NH(tert-butyl), C(O)NH(cyclopropyl), C(O)NCH3(CN), C(O)NH(OH), C(O)NCH3(OH), C(O)NH2, NHC(O)NHCH3, NHS(O)2CH3, S(O)2CH3, methyl, methoxy, NHS(O)2CH2CH2N(CH3)2, CF3, CH2OH, C(CH3)2OH,
23. The method of any one of claims 1-22, wherein the compound of Formula I is a compound of Formula Ia, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, wherein each X’ is independently CH, C-R3, or N.
26. The method of any one of claims 1-28, wherein p is 0, 1, or 2.
27. The method of any one of claims 1-29, wherein p is 1 or 2.
29. The method of claim 1, wherein two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more R” substituents.
30. The method of claim 32, wherein two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C1-6 alkyl, halo, OH, OC1-6 alkyl, oxo, CN, NH2, NH(C1-6 alkyl), and N(C1-6 alkyl)2.
31. The method of claim 30 or claim 31, wherein two R3 substituents, together with Ring A, to which they are attached, form a fused bicyclic heteroaryl, which is optionally and independently substituted with one or more substituents independently selected from C1-6 alkyl, oxo, and NH2.
36. The method of claim 1, wherein the cancer is selected from neoplasm, giant and spindle cell carcinoma; small cell carcinoma; papillary carcinoma; squamous cell carcinoma; lymphoepithelial carcinoma; basal cell carcinoma; pilomatrix carcinoma; transitional cell carcinoma; papillary transitional cell carcinoma; adenocarcinoma, gastrinoma, cholangiocarcinoma, hepatocellular carcinoma; combined hepatocellular carcinoma and cholangiocarcinoma, trabecular adenocarcinoma, adenoid cystic carcinoma; adenocarcinoma in adenomatous polyp; adenocarcinoma, familial polyposis coli, solid carcinoma; carcinoid tumor, bronchiolo-alveolar adenocarcinoma, papillary adenocarcinoma, chromophobe carcinoma; acidophil carcinoma; oxyphilic adenocarcinoma, basophil carcinoma; clear cell adenocarcinoma, granular cell carcinoma; follicular adenocarcinoma, papillary and follicular adenocarcinoma, nonencapsulating sclerosing carcinoma; adrenal cortical carcinoma; endometroid carcinoma; skin appendage carcinoma; apocrine adenocarcinoma, sebaceous adenocarcinoma, ceruminous adenocarcinoma, mucoepidermoid carcinoma; cystadenocarcinoma, papillary cystadenocarcinoma, papillary serous cystadenocarcinoma, mucinous cystadenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma; infiltrating duct carcinoma; medullary carcinoma; lobular carcinoma; inflammatory carcinoma; Paget's disease, mammary; acinar cell carcinoma; adenosquamous carcinoma; adenocarcinoma with squamous metaplasia, thymoma, malignant; ovarian stromal tumor, thecoma, granulosa cell tumor, androblastoma, Sertoli cell carcinoma; Leydig cell tumor, lipid cell tumor, paraganglioma, extra-mammary paraganglioma, pheochromocytoma, glomangiosarcoma, amelanotic melanoma; superficial spreading melanoma; melanoma in giant pigmented nevus; epithelioid cell melanoma; blue nevus, sarcoma; fibrosarcoma, fibrous histiocytoma, myxosarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, stromal sarcoma; mixed tumor; Mullerian mixed tumor; nephroblastoma, hepatoblastoma, carcinosarcoma, mesenchymoma, Brenner tumor, phyllodes tumor, synovial sarcoma; mesothelioma, dysgerminoma, embryonal carcinoma; teratoma, struma ovarii, choriocarcinoma, mesonephroma, hemangiosarcoma, hemangioendothelioma, Kaposi's sarcoma; hemangiopericytoma, lymphangiosarcoma, osteosarcoma, juxtacortical osteosarcoma, chondrosarcoma, chondroblastoma, mesenchymal chondrosarcoma, giant cell tumor of bone; Ewing's sarcoma; odontogenic tumor, ameloblastic odontosarcoma, ameloblastoma, ameloblastic fibrosarcoma, pinealoma, chordoma, glioma, ependymoma, astrocytoma, protoplasmic astrocytoma, fibrillary astrocytoma, astroblastoma, glioblastoma, oligodendroglioma, oligodendroblastoma, primitive neuroectodermal, cerebellar sarcoma; ganglioneuroblastoma, neuroblastoma, retinoblastoma, olfactory neurogenic tumor; meningioma, neurofibrosarcoma, neurilemmoma, granular cell tumor, lymphoma; Hodgkin's disease; Hodgkin's; paragranuloma, lymphoma – small lymphocytic, lymphoma – large cell, diffuse; lymphoma, follicular; mycosis fungoides, other specified non-Hodgkin's lymphomas; histiocytosis, multiple myeloma, mast cell sarcoma; immunoproliferative small intestinal disease; leukemia; lymphoid leukemia; plasma cell leukemia; erythroleukemia, lymphosarcoma cell leukemia; myeloid leukemia; basophilic leukemia; eosinophilic leukemia; monocytic leukemia; mast cell leukemia; megakaryoblastic leukemia; myeloid sarcoma; and hairy cell leukemia.
37. The method of claim 36, wherein the cancer is selected from prostate cancer, liver cancer, renal cancer, lung cancer, breast cancer, colorectal cancer, pancreatic cancer, brain cancer, hepatocellular cancer, lymphoma, leukemia, gastric cancer, cervical cancer, cholangial cancer, ovarian cancer, endometrial cancer, thyroid cancer, melanoma, carcinomas of the head and neck, head and neck cancer, skin cancer and soft tissue sarcoma and/or other forms of carcinoma.
38. The method of claim 37, wherein the cancer is selected from breast cancer, pancreatic, colorectal, lung, cholangial, ovarian, endometrial cancers, carcinomas of the head and neck, head and neck cancer, and skin cancer.
39. The method of claim 38, wherein the head and neck cancer is squamous head and neck cancer.
40. The method of claim 38, wherein the breast cancer is triple negative breast cancer.
41. The method of any one of claims 36 to 40, wherein the cancer is a metastatic or a malignant cancer.
42. The method of any one of claims 1 to 41, wherein the therapeutically effective amount of the compound according to Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is selected from the group consisting of 0.05 mg/kg to 500 mg/kg, 0.05 mg/kg to 400 mg/kg, 0.05 mg/kg to 300 mg/kg, 0.05 mg/kg to 200 mg/kg, 0.05 mg/kg to 100 mg/kg, 0.05 mg/kg to 90 mg/kg, 0.05 mg/kg to 80 mg/kg, 0.05 mg/kg to 75 mg/kg, 0.05 mg/kg to 70 mg/kg, 0.05 mg/kg to 60 mg/kg, 0.05 mg/kg to 50 mg/kg, 0.05 mg/kg to 40 mg/kg, 0.05 mg/kg to 30 mg/kg, 0.05 mg/kg to 20 mg/kg, 0.05 mg/kg to 10 mg/kg, 0.1 mg/kg to 75 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 50 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.1 mg/kg to 30 mg/kg, 0.1 mg/kg to 20 mg/kg, 0.1 mg/kg to 10 mg/kg, 0.5 mg/kg to 150 mg/kg, 0.5 mg/kg to 100 mg/kg, 0.5 mg/kg to 90 mg/kg, 0.5 mg/kg to 80 mg/kg, 0.5 mg/kg to 70 mg/kg, 0.5 mg/kg to 60 mg/kg, 0.5 mg/kg to 50 mg/kg, 0.5 mg/kg to 40 mg/kg, 0.5 mg/kg to 30 mg/kg, 0.5 mg/kg to 20 mg/kg, 0.5 mg/kg to 10 mg/kg, 1 mg/kg to 500 mg/kg, 1 mg/kg to 400 mg/kg, 1 mg/kg to 300 mg/kg, 1 mg/kg to 200 mg/kg, 1 mg/kg to 100 mg/kg, 1 mg/kg to 90 mg/kg, 1 mg/kg to 80 mg/kg, 1 mg/kg to 70 mg/kg, 1 mg/kg to 60 mg/kg, 1 mg/kg to 50 mg/kg, 1 mg/kg to 40 mg/kg, 1 mg/kg to 30 mg/kg, 1 mg/kg to 20 mg/kg, 1 mg/kg to 10 mg/kg, 5 mg/kg to 75 mg/kg, 5 mg/kg to 70 mg/kg, 5 mg/kg to 60 mg/kg, 5 mg/kg to 50 mg/kg, 5 mg/kg to 40 mg/kg, 5 mg/kg to 30 mg/kg, 5 mg/kg to 20 mg/kg, and 5 mg/kg to 10 mg/kg body weight of the recipient per day as a daily dose.
43. The method of claim 42, wherein the method comprises oral or parenteral administration of the compound according to Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof to the patient, wherein the daily dose ranges from about 1 mg per day to about 1,000 mg per day, and all values and integers therebetween, dosed according to a daily dosing regimen or intermittently.
44. The method of claim 43, wherein the daily dose is administered as fractionated oral or parenteral dosages twice or three or four times per day.
45. The method of claim 43, wherein the daily dose is administered 1, 2, 3, or 4 times per 28-day treatment cycle, on an intermittent schedule for 3 weeks on and one week off.
46. The method of claim 45, wherein the daily dose is administered on day 1, 8, and 15 of a 28-day treatment cycle.
47. The method of claim 43, wherein multiple cycles of treatment are administered to the patient for a time period sufficient to effect at least a partial tumor response.
48. The method of claim 43, wherein the patient is administered a daily dose for a time period of 28 day cycles, until the patient no longer shows therapeutic benefit or clinical signs of toxicity preclude continuation of therapy.
49. The method of claim 48, wherein the time period is at least 6 months.
50. The method of claim 48, wherein the time period is at least 12 months.
51. The method of any one of claims 48 to 50, wherein a complete tumor response is effected in the patient.
52. The method of claim 43, wherein the patient is administered a treatment cycle comprising administering a daily dose for 21 days followed by no administration for seven days, wherein the patient is administered one to twelve treatment cycles per year.
53. A method of treating a cancer in a subject in need thereof, comprising, administering to the subject, a therapeutically effective combination comprising a compound according to of claim 1, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, NRAS, panRAS, MAPK, ALK, BRAF, or MEK inhibitor.
54. The method of claim 53, wherein the cancer comprises colorectal cancer, pancreatic cancer, or breast cancer.
55. The method of claim 53, wherein the cancer harbors at least one mutation selected from: a BRAF mutation, a KRAS mutation, and a NRAS mutation.
56. The method of any one of claims 53 to 55, wherein the cancer harbors a KRASG12 mutation, and/or a KRASA136 mutation.
57. The method of any one of claims 53 to 55, wherein the cancer harbors a BRAFV600 mutation.
58. The method of any one of claims 53 to 57, wherein the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and the at least one KRAS, NRAS, panRAS, MAPK, ALK, BRAF, or MEK inhibitor are administered at substantially the same time.
59. The method of any one of claims 53 to 57, wherein the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, or MEK inhibitor are administered at different times.
60. The method of any one of claims 53 to 57, wherein the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, and MEK inhibitor are in a single pharmaceutical composition or unit dosage form.
61. The method of any one of claims 43 to 60, wherein the at least one KRAS, NRAS, pan-RAS, MAPK, ALK, BRAF, and MEK inhibitor comprises: sotorasib, sorafenib, panitumumab, encorafenib, trametinib, cobimetinib, binimetinib, vemurafenib, lapatinib, crizotinib, dabrafenib, divarasib, adagrasib, selumetinib, ulixertinib, pimasertib, mirdametinib, refametinib, glecirasib, ERAS-007, ulixertinib, ASTX029, ravoxertinib, PLX4720 (Plexxikon), ERAS-007, LY3537982, ASTX029, PD184352(CI-1040- Pfizer), E6201 (Esai Co Ltd), GDC- 0623 (RG 7421 Genentech Inc), CH5126766 (RO5126766 - Chugai Pharmaceutical Co.), HL- 085(Shanghai Kechow Pharma, Inc.), SHR7390 (Hengrui Medicine), TQ-B3234 (Chiatai Tianqing), CS-3006 (Cstone Pharmaceuticals) and FCN-159 (Fosun Pharma) … 62. The method of any one of claims 1 to 61, wherein the compound of Formula I, Compound 2R, or a pharmaceutically acceptable salt thereof, or a combination comprising a compound according to of claim 1, a compound of Table 1, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof, and at least one KRAS, NRAS, panRAS, MAPK, ALK, BRAF, or MEK inhibitor is administered to a subject with cancer, and a disease or condition selected from one or more of: diabetes type I or II, impaired glucose metabolism, hyperglycemia, insulin resistance, diabetic retinopathy, metabolic syndrome, pancreatic dysfunction, overweight, obesity, has any form of metabolic syndrome associated with defective glucose utilization and glucose metabolism, requires medication to provide glycemic control, and wherein the administration of the compound of Formula I, Compound 2R, or a pharmaceutically acceptable salt thereof, or the combination to the subject does not significantly elevate any one of: blood glucose, blood insulin or hyperglycemia in the subject, after one or more administrations of the compound or combination. 63. The method of any one of claims 1 to 62, wherein administration of the compound according to Formula I, a compound of Table 1, Compound 2R, or a pharmaceutically acceptable salt thereof, weakly agonizes PPARγ in the subject. 64. The method according to any one of claim 1 to 63, wherein the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof comprises one or more of: Compound 1, Compound 2R, and Compound 2S. 65. The method of claim 64, wherein the compound of Formula I, or a pharmaceutically acceptable salt or solvate thereof, or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof is Compound 2R: N-(2-chloro-5-(4-((1R-phenylethyl)amino)quinazolin-6-yl)pyridin-3- yl)methanesulfonamide. 66. The method of any one of claims 1-65, wherein the subject with cancer, is a subject with any one or more of the following conditions: diabetes type 1, diabetes type 2, diabetic retinopathy, metabolic syndrome, insulin resistance, pancreatic dysfunction, and wherein administration of the compound or pharmaceutically acceptable salt thereof of Formula I, does not significantly elevate blood glucose or cause hyperglycemia in the subject, after one or more administrations of the compound or pharmaceutically acceptable salt thereof, wherein hyperglycemia is defined as a plasma or whole blood glucose measurement greater than 125 mg/dL while fasting and greater than 200 mg/dL, 2 hours postprandial, during a 75-g oral glucose tolerance test (OGTT) using a blood glucose meter employing strips comprising glucose oxidase, hexokinase, or combinations thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363597924P | 2023-11-10 | 2023-11-10 | |
| US63/597,924 | 2023-11-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2025101914A1true WO2025101914A1 (en) | 2025-05-15 |
Family
ID=93650462
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/055148PendingWO2025101914A1 (en) | 2023-11-10 | 2024-11-08 | Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors having improved therapeutic index against solid tumors |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2025101914A1 (en) |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3989816A (en) | 1975-06-19 | 1976-11-02 | Nelson Research & Development Company | Vehicle composition containing 1-substituted azacycloheptan-2-ones |
| US4369174A (en) | 1979-06-28 | 1983-01-18 | Sansho Pharmaceutical Co., Ltd. | Cosmetic composition containing kojic acid ester |
| US4444762A (en) | 1980-04-04 | 1984-04-24 | Nelson Research & Development Company | Vehicle composition containing 1-substituted azacyclopentan-2-ones |
| US4842866A (en) | 1985-01-11 | 1989-06-27 | Abbott Laboratories Ltd. | Slow release solid preparation |
| US5217720A (en) | 1990-07-10 | 1993-06-08 | Shin-Etsu Chemical Co., Ltd. | Coated solid medicament form having releasability in large intestine |
| US5770581A (en) | 1990-12-20 | 1998-06-23 | Arch Development Corp. | Gene transcription and ionizing radiation: methods and compositions |
| US6569457B2 (en) | 1998-07-17 | 2003-05-27 | Bristol-Myers Squibb Company | Enteric coated pharmaceutical tablet and method of manufacturing |
| US6638534B1 (en) | 1998-07-28 | 2003-10-28 | Tanabe Seiyaku Co., Ltd. | Preparation capable of releasing drug at target site in intestine |
| US6855556B2 (en) | 2002-01-04 | 2005-02-15 | Becton, Dickinson And Company | Binding protein as biosensors |
| US7496392B2 (en) | 2003-11-26 | 2009-02-24 | Becton, Dickinson And Company | Fiber optic device for sensing analytes |
| US7787923B2 (en) | 2003-11-26 | 2010-08-31 | Becton, Dickinson And Company | Fiber optic device for sensing analytes and method of making same |
| US7851593B2 (en) | 2002-01-04 | 2010-12-14 | Becton, Dickinson And Company | Binding proteins as biosensors |
| US8623639B2 (en) | 2006-04-20 | 2014-01-07 | Becton, Dickinson And Company | Thermostable proteins and methods of making and using thereof |
| WO2022140456A1 (en)* | 2020-12-22 | 2022-06-30 | Mekanistic Therapeutics Llc | Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors |
- 2024
- 2024-11-08WOPCT/US2024/055148patent/WO2025101914A1/enactivePending
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3989816A (en) | 1975-06-19 | 1976-11-02 | Nelson Research & Development Company | Vehicle composition containing 1-substituted azacycloheptan-2-ones |
| US4369174A (en) | 1979-06-28 | 1983-01-18 | Sansho Pharmaceutical Co., Ltd. | Cosmetic composition containing kojic acid ester |
| US4444762A (en) | 1980-04-04 | 1984-04-24 | Nelson Research & Development Company | Vehicle composition containing 1-substituted azacyclopentan-2-ones |
| US4842866A (en) | 1985-01-11 | 1989-06-27 | Abbott Laboratories Ltd. | Slow release solid preparation |
| US5217720A (en) | 1990-07-10 | 1993-06-08 | Shin-Etsu Chemical Co., Ltd. | Coated solid medicament form having releasability in large intestine |
| US5770581A (en) | 1990-12-20 | 1998-06-23 | Arch Development Corp. | Gene transcription and ionizing radiation: methods and compositions |
| US6569457B2 (en) | 1998-07-17 | 2003-05-27 | Bristol-Myers Squibb Company | Enteric coated pharmaceutical tablet and method of manufacturing |
| US6638534B1 (en) | 1998-07-28 | 2003-10-28 | Tanabe Seiyaku Co., Ltd. | Preparation capable of releasing drug at target site in intestine |
| US6855556B2 (en) | 2002-01-04 | 2005-02-15 | Becton, Dickinson And Company | Binding protein as biosensors |
| US7851593B2 (en) | 2002-01-04 | 2010-12-14 | Becton, Dickinson And Company | Binding proteins as biosensors |
| US7496392B2 (en) | 2003-11-26 | 2009-02-24 | Becton, Dickinson And Company | Fiber optic device for sensing analytes |
| US7787923B2 (en) | 2003-11-26 | 2010-08-31 | Becton, Dickinson And Company | Fiber optic device for sensing analytes and method of making same |
| US7792561B2 (en) | 2003-11-26 | 2010-09-07 | Becton, Dickinson And Company | Fiber optic device for sensing analytes |
| US8623639B2 (en) | 2006-04-20 | 2014-01-07 | Becton, Dickinson And Company | Thermostable proteins and methods of making and using thereof |
| WO2022140456A1 (en)* | 2020-12-22 | 2022-06-30 | Mekanistic Therapeutics Llc | Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors |
Non-Patent Citations (26)
| Title |
|---|
| "Handbook of Chemistry and Physics", 2006, UNIVERSITY SCIENCE BOOKS, article "Additionally, general principles of organic chemistry are described in ''Organic Chemistry" |
| "March's Advanced Organic Chemistry", 2015, JOHN WILEY & SONS |
| "Remington's Pharmaceutical Sciences", 1990, MACK PRINTING COMPANY, pages: 1289 - 1329 |
| "UniProt", Database accession no. P37231 |
| AMODIO, V. ET AL.: "EGFR Blockade Reverts Resistance to KRAS(G12C) Inhibition in Colorectal Cancer", CANCER DISCOVERY, vol. 10, 2020, pages 1129 - 1139 |
| BERNSTEIN ET AL.: "A New Test Strip Technology Platform for Self-Monitoring of Blood Glucose", J DIABETES SCI TECHNOL, vol. 7, no. 5, 2013, pages 1388 - 1399 |
| CHRISTIANSEN M: "A new, wireless-enabled blood glucose monitoring system that links to a smart mobile device: accuracy and user performance evaluation", J. DIABETES SCI TECHNOL., vol. 11, 2017, pages 567 - 573 |
| CRAMER, J.D. ET AL., NAT REV CLIN ONCOL, vol. 16, no. 11, 2019, pages 669 - 683 |
| CROUTHAMEL ET AL., CLIN CANCER RES, vol. 15, 2009, pages 217 - 225 |
| ELKABETS, M. ET AL., CANCER CELL, vol. 27, no. 4, 2015, pages 533 - 546 |
| FREIREICH ET AL., CANCER CHEMOTHER. REP., vol. 50, 1966, pages 219 |
| GOODMANGILMAN ET AL.: "Pharmaceutical Basis of Therapeutics", 2002 |
| GRANDIS, J.R.D.J. TWEARDY, CANCER RESEARCH, vol. 53, no. 15, 1993, pages 3579 - 3584 |
| HOU JU ET AL: "Design, synthesis, anti-tumor activity, and molecular modeling of quinazoline and pyrido[2,3-d]pyrimidine derivatives targeting epidermal growth factor receptor", EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY, ELSEVIER MASSON, AMSTERDAM, NL, vol. 118, 20 April 2016 (2016-04-20), pages 276 - 289, XP029562551, ISSN: 0223-5234, DOI: 10.1016/J.EJMECH.2016.04.026* |
| JANKU, F.T.A. YAPF. MERIC-BERNSTAM, NATURE REVIEWS CLINICAL ONCOLOGY, vol. 15, 2018, pages 273 - 291 |
| KATERJI MEGHRI ET AL: "Dual inhibition of EGFR and PI3K with a single drug", NATURE CANCER, vol. 5, no. 8, 16 August 2024 (2024-08-16), pages 1131 - 1133, XP093239013, ISSN: 2662-1347, DOI: 10.1038/s43018-024-00806-0* |
| LIU, P. ET AL., NATURE REVIEWS DRUG DISCOVERY, vol. 8, 2009, pages 627 - 644 |
| MIZRACHI, A. ET AL.: "Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma", NATURE COMMUNICATIONS, vol. 8, 2017, pages 14292, XP055824013, DOI: 10.1038/ncomms14292 |
| PSYRRI, A.T.Y. SEIWERTA. JIMENO: "American Society of Clinical Oncology educational book", AMERICAN SOCIETY OF CLINICAL ONCOLOGY. ANNUAL MEETING, 2013, pages 246 - 255 |
| REMINGTON: "The Science and Practice of Pharmacy", 2005, LIPPINCOTT WILLIAMS & WILKINS |
| S. M. BERGE ET AL., J. PHARMACEUTICAL SCIENCES, vol. 66, 1977, pages 1 - 19 |
| SIMPSON, D.R.L.K. MELLE.E.W. COHEN, ORAL ONCOLOGY, vol. 51, no. 4, 2015, pages 291 - 298 |
| TAO, J.J. ET AL., SCIENCE SIGNALING, vol. 7, no. 318, 2014 |
| UNGERCHERRINGTON, J CLIN INVEST., vol. 122, 2012, pages 4 - 12 |
| YAP, T.A. ET AL., CURRENT OPINION IN PHARMACOLOGY, vol. 23, 2015, pages 98 - 107 |
| YOUNG, N.R. ET AL., MOLECULAR ONCOLOGY, vol. 7, no. 3, 2013, pages 359 - 368 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12264155B2 (en) | Compounds, pharmaceutical compositions, and methods of preparing compounds and of their use as ATR kinase inhibitors | |
| US20230122909A1 (en) | Compounds, pharmaceutical compositions, and methods of preparing compounds and of their use | |
| US20220304984A1 (en) | Amino acid transport inhibitors and the uses thereof | |
| US9636333B2 (en) | Methyl/fluoro-pyridinyl-methoxy substituted pyridinone-pyridinyl compounds and fluoro-pyrimidinyl-methoxy substituted pyridinone-pyridinyl compounds | |
| RU2701193C2 (en) | Arylquinazolines | |
| CN104159901B (en) | Triazolopyridine oxazine derivatives | |
| US20220265590A1 (en) | Dibenzylamines as amino acid transport inhibitors | |
| KR20220035925A (en) | Substituted 2-morpholinopyridine derivatives as ATR kinase inhibitors | |
| US11673876B2 (en) | Substituted aminobenzyl heteroaryl compounds as EGFR and/or PI3K inhibitors | |
| TW201014849A (en) | 1,2-disubstituted heterocyclic compounds | |
| AU2015362670A1 (en) | Small molecule inhibitors of EGFR and PI3K | |
| CN105228983A (en) | The compound regulated for kinases and indication thereof | |
| US20250026748A1 (en) | N-(5-substituted-[(1,3,4-thiadiazolyl) or (thiazolyl)])(substituted)carboxamide compounds and use thereof for inhibiting human polymerase theta | |
| US20150105367A1 (en) | Raf inhibitor compounds | |
| WO2016025650A1 (en) | Combinations of an erk inhibitor and a cdk4/6 inhibitor and related methods | |
| TW201306842A (en) | Combination therapies for treating hematologic malignancies using pyridopyrimidinone inhibitors of PI3K/MTOR with bendamustine and/or rituximab | |
| TW200817410A (en) | Triazolotriazines as kinase inhibitors | |
| TW201900648A (en) | Therapeutic compounds and compositions and methods of use thereof | |
| US20240197729A1 (en) | ALK-5 Inhibitors and Uses Thereof | |
| WO2014082085A1 (en) | Use of itk inhibitors for the treatment of cancer | |
| WO2024197401A1 (en) | 1,3,4-thiadiazol-2-yl carboxamide and 1,3-thiazol-2-yl carboxamide compounds and uses thereof as inhibitors of human polymerase theta | |
| WO2020132459A1 (en) | Quinolinyl-pyrazine-carboxamide compounds and uses thereof | |
| WO2025101914A1 (en) | Substituted aminobenzyl heteroaryl compounds as egfr and/or pi3k inhibitors having improved therapeutic index against solid tumors | |
| US20230133958A1 (en) | Compounds useful in modulating egfr and pi3k | |
| AU2023365344A1 (en) | Compounds useful in modulating egfr and pi3k |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24812669 Country of ref document:EP Kind code of ref document:A1 |