WO2025101738A1 - Brm targeting compounds and associated methods of use - Google Patents
Brm targeting compounds and associated methods of useDownload PDFInfo
- Publication number
- WO2025101738A1 WO2025101738A1PCT/US2024/054907US2024054907WWO2025101738A1WO 2025101738 A1WO2025101738 A1WO 2025101738A1US 2024054907 WUS2024054907 WUS 2024054907WWO 2025101738 A1WO2025101738 A1WO 2025101738A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- pyrazino
- pyridazin
- hexahydropyrrolo
- piperidine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
Definitions
- the bifunctional compoundsare useful as modulators of targeted ubiquitination, especially with respect to Switch/Sucrose Non-Fermentable (SWI/SNF)-Related, Matrix-Associated, Actin- Dependent Regulator of Chromatin, Subfamily A, Member 2 (SMARCA2) (i.e., BRAHMA or BRM), which are degraded and/or otherwise inhibited by bifunctional compounds according to the present disclosure.
- SMARCA2Switch/Sucrose Non-Fermentable
- BRAHMA or BRMActin- Dependent Regulator of Chromatin, Subfamily A, Member 2
- BACKGROUNDThe human SWItch/Sucrose Non-Fermentable (SWI/SNF) complexes are ATP- dependent chromatin remodelers. These large complexes play important roles in essential cellular processes, such as transcription, DNA repair and replication by regulating DNA accessibility.
- SMARCA2 and SMARCA4are the subunits containing catalytic ATPase domains and they are essential for the function of SWI/SNF in perturbation of histone- DNA contacts, thereby providing access points to transcription factors and cognate DNA elements that facilitate gene activation and repression.
- SMARCA2 and SMARCA4shares a high degree of homology (up to 75%).
- SMARCA4is frequently mutated in primary tumors (i.e., deleted or inactivated), particularly in lung cancer (12%), melanoma, liver cancer and pancreatic cancer.
- SMARCA2is one of the top essential genes in SMARCA4-mutant (deleted) cancer cell line.
- SMARCA4 deleted cancer cellsexclusively rely on SMARCA2 ATPase activity for their chromatin remodeling activity for cellular functions such as cell proliferation, survival and growth.
- SMARCA4-related or deficient cancersgenetic synthetic lethality.
- Previous studieshave demonstrated the strong synthetic lethality using gene expression manipulation such as RNAi; downregulating SMARCA2 gene expression in SMARCA4 mutated cancer cells results in suppression of cancer cell proliferation.
- SMARCA2/4 bromodomain inhibitorse.g., PFI-3 exhibit none to minor effects on cell proliferation inhibition [Vangamudi et al. Cancer Res 2015].
- SMARCA2is also reported to play roles in multiple myeloma expressing t(4;14) chromosomal translocation [Chooi et al. Cancer Res abstract 2018]. SMARCA2 interacts with NSD2 and regulates gene expression such as PRL3 and CCND1. SMARCA2 gene expression downregulation with shRNA reduces cell cycle S phase and suppresses cell proliferation of t(4;14) MM cells. [0009] Therapeutic compounds that inhibit SMARCA2 and/or SMARCA4 are needed.
- R 1is halo, C 1-6 alkyl, or C 1-6 haloalkyl
- each R2is independently H, D, or F
- each R 3is independently H, D, C 1-6 alkyl, C 1-6 haloalkyl, C 3-6 heterocycloalkyl or C 3-6 cycloalkyl
- sis 1, 2 or 3
- tis 1, 2, 3, or 4
- R 4is H, D, C 1-6 alkyl, C 1-6 alkoxyalkyl, C 3-6 cycloalkyl, or C 1-6 haloalkyl
- R5is H, D, or F
- L 1is absent, or is O, S, S(O), SO 2 , NR 3 , C(R 3 ) 2 or CO
- L 2is absent, or is O, S, S(O), SO 2 , NR 3 , C(R 3 ) 2 or CO
- co-administrationand “co-administering” or “combination therapy” refer to both concurrent administration (administration of two or more therapeutic agents at the same time) and time varied administration (administration of one or more therapeutic agents at a time different from that of the administration of an additional therapeutic agent or agents), as long as the therapeutic agents are present in the patient to some extent, preferably at effective amounts, at the same time.
- one or more of the present compounds described hereinare co-administered in combination with at least one additional bioactive agent, especially including an anticancer agent.
- the co-administration of compoundsresults in synergistic activity and/or therapy, including anticancer activity.
- compoundrefers to any specific chemical compound disclosed herein and includes tautomers, regioisomers, geometric isomers, and where applicable, stereoisomers, including optical isomers (enantiomers) and other stereoisomers (diastereomers) thereof, as well as pharmaceutically acceptable salts and derivatives, including prodrug and/or deuterated forms thereof where applicable, in context. - 4 - 4860-6199-1670.2 105807.001049 – PCT Application Deuterated small molecules contemplated are those in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by deuterium.
- the term compoundgenerally refers to a single compound, but also may include other compounds such as stereoisomers, regioisomers and/or optical isomers (including racemic mixtures) as well as specific enantiomers or enantiomerically enriched mixtures of disclosed compounds.
- the termalso refers, in context to prodrug forms of compounds which have been modified to facilitate the administration and delivery of compounds to a site of activity. It is noted that in describing the present compounds, numerous substituents and variables associated with same, among others, are described. It is understood by those of ordinary skill that molecules which are described herein are stable compounds as generally described hereunder.
- ubiquitin ligaserefers to a family of proteins that facilitate the transfer of ubiquitin to a specific substrate protein, targeting the substrate protein for degradation.
- an E3 ubiquitin ligase proteinthat alone or in combination with an E2 ubiquitin- conjugating enzyme causes the attachment of ubiquitin to a lysine on a target protein, and subsequently targets the specific protein substrates for degradation by the proteasome.
- E3 ubiquitin ligase alone or in complex with an E2 ubiquitin conjugating enzymeis responsible for the transfer of ubiquitin to targeted proteins.
- the ubiquitin ligaseis involved in polyubiquitination such that a second ubiquitin is attached to the first; a third is attached to the second, and so forth.
- Polyubiquitinationmarks proteins for degradation by the proteasome.
- mono-ubiquitinationin which only a single ubiquitin is added by the ubiquitin ligase to a substrate molecule.
- Mono- ubiquitinated proteinsare not targeted to the proteasome for degradation but may instead be altered in their cellular location or function, for example, via binding other proteins that have domains capable of binding ubiquitin. Further complicating matters, different lysines on ubiquitin can be targeted by an E3 to make chains.
- lysineis Lys48 on the ubiquitin chain. This is the lysine used to make polyubiquitin, which is recognized by the proteasome.
- Cyereblon (CRBN) E3 Ubiquitin Ligaserefers to the substrate recognition subunit of the Cullin RING E3 ubiquitin ligase complexes. CRBN are one of the most popular E3 ligases recruited by bifunctional Proteolysis-targeting chimeras (PROTACs) to induce ubiquitination and subsequent proteasomal degradation of a target protein (Maniaci C. et al., Bioorg Med Chem.2019, 27(12): 2466-2479).
- PROTACsProteolysis-targeting chimeras
- alkylby itself or as part of another substituent, means, unless otherwise stated, a straight or branched chain hydrocarbon radical having up to twelve - 5 - 4860-6199-1670.2 105807.001049 – PCT Application carbon atoms. In some embodiments, the number of carbon atoms is designated (i.e., C 1- C 8 means one to eight carbons).
- alkyl groupsinclude methyl, ethyl, n-propyl, iso- propyl, n-butyl, t-butyl, iso-butyl, sec-butyl, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like.
- Alkyl groupsmay be optionally substituted as provided herein.
- the alkyl groupis a C 1- C 6 alkyl; in some embodiments, it is a C 1- C 4 alkyl.
- C 1 -C 3includes C 1- C 3 , C 1- C 2 , C 2- C 3 , C 1 , C 2 , and C 3 .
- the term “optionally substituted”, as used in combination with a substituent defined herein,means that the substituent may, but is not required to, have one or more hydrogens replaced with one or more suitable functional groups or other substituents as provided herein.
- a substituentmay be optionally substituted with one or more of: halo, cyano, C1-6 alkyl, C3-6 cycloalkyl, C2-6 alkenyl, C2-6 alkynyl, halo(C1-6)alkyl, C1-6 alkoxy, halo(C 1-6 alkoxy), C 1-6 alkylthio, C 1-6 alkylamino, NH 2 , NH(C 1-6 alkyl), N(C 1-6 alkyl) 2 , NH(C 1- 6 alkoxy), N(C 1-6 alkoxy) 2 , —C(O)NHC 1-6 alkyl, —C(O)N(C 1-6 alkyl) 2 , —C(O)NH 2 , —C(O)C 1-6 alkyl, —C(O)2C1-6 alkyl, —NHCO(C1-6 alkyl), —N(C1-6 alkyl)CO(C1-6 alkyl), —S(S(
- each of the above optional substituentsare themselves optionally substituted by one or two groups.
- the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl groupis substituted, they can be optionally substituted.
- these groupscan be optionally substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, -C(O)NH(C 1 -C 6 alkyl), - C(O)N(C 1 -C 6 alkyl) 2 , -OC(O)NH(C 1 -C 6 alkyl), - OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and -S(O) 2 N(C 1 -C 6 alkyl) 2 .
- substituentsindependently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloal
- the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl groupis optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa , -SR a , -NR a R d , or NR c R d .
- the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl groupis optionally substituted by one or more R f groups.
- R f groups- 6 - 4860-6199-1670.2 105807.001049 – PCT Application
- the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl groupis optionally substituted by one or more R 7 or R 8 groups.
- cycloalkylrefers to a 3-12 membered cyclic alkyl group, and includes bridged and spirocycles (e.g., adamantine). Cycloalkyl groups may be fully saturated or partially unsaturated.
- cycloalkylalso includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) wherein a single cycloalkyl ring (as defined above) can be condensed with one or more groups selected from heterocycles, carbocycles, aryls, or heteroaryls to form the multiple condensed ring system.
- Such multiple condensed ring systemsmay be optionally substituted with one or more (e.g., 1, 2, 3 or 4) oxo groups on the carbocycle or heterocycle portions of the multiple condensed ring.
- the rings of the multiple condensed ring systemcan be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements.
- the individual rings of the multiple condensed ring systemmay be connected in any order relative to one another. It is also to be understood that the point of attachment of a multiple condensed ring system (as defined above for a cycloalkyl) can be at any position of the cycloalkylic ring.
- cycloalkyl groupsinclude cyclopropyl, cyclobutyl, cyclopentyl, cycloheptyl, cyclohexyl, cycloheptyl, cyclooctyl, indenyl, bicyclo[2.2.1]heptanyl, bicyclo[3.1.1]heptanyl, bicyclo[4.1.0]heptanyl, spiro[3.3] heptanyl, and spiro[3.4]octanyl.
- the cycloalkyl groupis a 3-7 membered cycloalkyl.
- cycloalkenylwhen used alone or as part of a substituent group refers to monocyclic or multicyclic, partially saturated ring structure having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C3-C6”).
- Cycloalkenyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups can share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- the term -C 3 -C 6 cycloalkenylrefers to a cycloalkenyl group having between three and six carbon atoms.
- the cycloalkenyl groupmay be attached at any carbon atom of the partially saturated ring such that the result is a stable structure.
- Cycloalkenyl groupsinclude groups in which the partially saturated ring is fused to an aryl group.
- cycloalkenyl groupsinclude, for - 7 - 4860-6199-1670.2 105807.001049 – PCT Application example, cyclopropenyl (C 3 ), cyclobutenyl (C 4 ), cyclopropenylmethyl (C 4 ), cyclopentenyl (C 5 ), cyclohexenyl (C6), 1-methylcyclopropenyl (C4), 2-methylcyclopentenyl (C4), adamantenyl (C10), spiro[3.3]heptenyl, bicyclo[3.3.0]octenyl, indanyl, and the like.
- cycloalkenyl groups of the disclosureare optionally substituted.
- the cycloalkenyl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1- C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, -C(O)NH(C 1 -C 6 alkyl), -C(O)N(C 1 - C 6 alkyl) 2 , -OC(O)NH(C 1 -C 6 alkyl), -OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and - S(O)2N(C1-C6alkyl)2.
- the cycloalkenyl groupis optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the cycloalkenyl group is optionally substituted by one or more R f groups.
- alkenylrefers to C2-C12 alkyl group that contains at least one carbon-carbon double bond.
- the alkenyl groupis optionally substituted.
- the alkenyl groupis a C 2- C 6 alkenyl.
- alkynylrefers to C 2- C 12 alkyl group that contains at least one carbon-carbon triple bond. In some embodiments, the alkenyl group is optionally substituted. In some embodiments, the alkynyl group is a C2-C6 alkynyl.
- alkoxyused in their conventional sense, and refer to those alkyl groups attached to the remainder of the molecule via an oxygen atom (“oxy”), an amino group (“amino”) or thio group.
- alkylaminoincludes mono- di- alkylamino groups, the alkyl portions can be the same or different.
- alkoxyalkylrefers to a linear monovalent hydrocarbon radical of one to six carbon atoms or a branched monovalent hydrocarbon radical of three to six carbons substituted with an alkoxy group, as defined above, e.g., 2-methoxyethyl, 1-, 2-, or 3- methoxypropyl, 2-ethoxyethyl, and the like.
- haloor “halogen”, by itself or as part of another substituent, means a fluorine, chlorine, bromine, or iodine atom.
- haloalkylrefers to any alkyl radical having one or more hydrogen atoms replaced by a halogen atom.
- heteroalkylrefers to an alkyl group in which one or more carbon atom has been replaced by a heteroatom selected from S, O, P and N.
- exemplary heteroalkylsinclude alkyl ethers, secondary and tertiary alkyl amines, alkyl amides, alkyl sulfides, and the like.
- the groupmay be a terminal group or a bridging group.
- arylrefers to a single, all carbon aromatic ring or a multiple condensed all carbon ring system wherein at least one of the rings is aromatic.
- an aryl grouphas 6 to 12 carbon atoms.
- Arylincludes a phenyl radical.
- Arylalso includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) having about 9 to 12 carbon atoms in which at least one ring is aromatic and wherein the other rings may be aromatic or not aromatic.
- Such multiple condensed ring systemsare optionally substituted with one or more (e.g., 1, 2 or 3) oxo groups on any carbocycle portion of the multiple condensed ring system.
- the rings of the multiple condensed ring systemcan be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements. It is to be understood that the point of attachment of a multiple condensed ring system, as defined above, can be at any position of the aromatic ring.
- aryl groupsinclude, but are not limited to, phenyl, indenyl, naphthyl, 1, 2, 3,4-tetrahydronaphth- yl, and the like.
- heteroarylrefers to a single aromatic ring that has at least one atom other than carbon in the ring, wherein the atoms are selected from the group consisting of oxygen, nitrogen and sulfur; “heteroaryl” also includes multiple condensed ring systems that have at least one such aromatic ring, which multiple condensed ring systems are further described below.
- heteroarylincludes single aromatic rings of from about 1 to 6 carbon atoms and about 1-4 heteroatoms selected from the group consisting of oxygen, nitrogen and sulfur.
- the sulfur and nitrogen atomsmay also be present in an oxidized form provided the ring is aromatic.
- Exemplary heteroaryl ring systemsinclude but are not limited to pyridyl, pyrimidinyl, oxazolyl or furyl.
- Heteroarylalso includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) wherein a heteroaryl group, as defined above, is condensed with one or more rings selected from heteroaryls (to form for example a naphthyridinyl such as 1,8-naphthyridinyl), heterocycles, (to form for example a 1, 2, 3, 4-tetra- hydronaphthyridinyl such as 1,2,3,4-tetrahydro-1,8-naphthyridinyl), carbocycles (to form for example 5,6,7,8-tetrahydroquinolyl) and aryls (to form for example indazolyl) to form the multiple condensed ring system.
- heteroarylsto form for example a naphthyridinyl such as 1,8-naphthyridinyl
- heterocyclesto form for example a 1, 2, 3, 4-tetra- hydron
- a heteroaryl(a single aromatic ring or multiple condensed ring system) has about 1-20 carbon atoms and about 1-6 heteroatoms within the heteroaryl ring.
- a heteroaryl(a single aromatic ring or multiple condensed ring system) can also have about 5 to 12 or about 5 to 10 members within the heteroaryl ring.
- Multiple condensed ring systemsmay be optionally substituted with one or more (e.g., 1, 2, 3 or 4) oxo groups on the carbocycle or heterocycle portions of the condensed ring.
- the rings of a multiple condensed ring systemcan be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements.
- the individual rings of the multiple condensed ring - 9 - 4860-6199-1670.2 105807.001049 – PCT Application systemmay be connected in any order relative to one another.
- the point of attachment of a multiple condensed ring system(as defined above for a heteroaryl) can be at any position of the heteroaryl ring.
- the point of attachment for a heteroaryl or heteroaryl multiple condensed ring systemcan be at any suitable atom of the heteroaryl ring including a carbon atom and a heteroatom (e.g., a nitrogen).
- heteroarylsinclude but are not limited to pyridyl, pyrrolyl, pyrazinyl, pyrimidinyl, pyridazinyl, pyrazolyl, thienyl, indolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl, furyl, oxadiazolyl, thiadiazolyl, quinolyl, isoquinolyl, benzothiazolyl, benzoxazolyl, indazolyl, quinoxalyl, quinazolyl, 5,6,7,8-tetrahydroisoquinolinyl benzofuranyl, benzimidazolyl, thianaphthenyl, pyrrolo[2,3-b]pyridinyl, quinazolinyl-4(3H)-one, triazolyl, 4,5,6,7-tetrahydro-1H-indazole and 3b,4,4
- heteroarylrefers to a single aromatic ring containing at least one heteroatom.
- the termincludes 5-membered and 6-membered monocyclic aromatic rings that include one or more heteroatoms.
- Non-limiting examples of heteroarylinclude but are not limited to pyridyl, furyl, thiazole, pyrimidine, oxazole, and thiadiazole.
- heterocycloalkylwhen used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, saturated ring structure containing at least one heteroatom selected from the group consisting of O, N, P, B and S.
- Heterocycloalkyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups.
- the cyclic groupscan share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- the term -C3-C6 heterocycloalkylrefers to a heterocycloalkyl group having between three and six carbon ring atoms.
- the heterocycloalkyl groupmay be attached at any heteroatom or carbon atom of the group such that the result is a stable structure.
- heterocycloalkyl groupsinclude, but are not limited to, azepanyl, aziridinyl, azetidinyl, pyrrolidinyl, dioxolanyl, imidazolidinyl, pyrazolidinyl, piperazinyl, piperidinyl, dioxanyl, morpholinyl, dithianyl, thiomorpholinyl, oxazepanyl, oxiranyl, oxetanyl, quinuclidinyl, tetrahydrofuranyl, tetrahydropyranyl, piperazinyl, azepanyl, diazepanyl, oxepanyl, dioxepanyl, azocanyl, diazocanyl, oxocanyl, dioxocanyl, azaspiro[2.2]pentanyl, oxaazaspiro[3.3]heptanyl, hept
- heterocycloalkyl - 10 - 4860-6199-1670.2 105807.001049 – PCT Application groups of the disclosureare optionally substituted.
- the heterocycloalkyl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, -C(O)NH(C 1 -C 6 alkyl), - C(O)N(C 1 -C 6 alkyl) 2 , -OC(O)NH(C 1 -C 6 alkyl), -OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and -S(O)
- the heterocycloalkyl groupis optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the heterocycloalkyl group is optionally substituted by one or more R f groups.
- heterocycloalkenylwhen used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, partially saturated ring structure containing at least one heteroatom selected from the group consisting of O, N, P, B and S.
- Heterocycloalkenyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups.
- the cyclic groupscan share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- the term -C3-C6 heterocycloalkenylrefers to a heterocycloalkenyl group having between three and six carbon atoms.
- heterocycloalkenyl groupmay be attached at any heteroatom or carbon atom of the partially saturated ring such that the result is a stable structure.
- Heterocycloalkenyl groupsinclude groups in which the partially saturated ring is fused to an aryl group, such as, for example isoindoline, , or in which the partially saturated ring is fused to a heteroaryl group, such as, for example, 6,7-dihydro-5H-pyrrolo[3,4-b] .
- heterocycloalkenyl groups of the disclosureare optionally substituted.
- the heterocycloalkenyl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 - C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2.
- the heterocycloalkenyl groupis optionally substituted by 1-6 groups selected from D, halogen, - OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the heterocycloalkenyl group is optionally substituted by one or more R f groups.
- the phrase “one or more R f groups”is meant to include 1, 2, 3, 4, 5, 6, 7 or 8 R f groups. In some embodiments, “one or more R f groups” is meant to include 1 R f group.
- R f groupsis meant to include up to 2 R f groups. In some embodiments, “one or more R f groups” is meant to include up to 3 R f groups. In some embodiments, “one or more R f groups” is meant to include up to 4 R f groups. In some embodiments, “one or more R f groups” is meant to include up to 5 R f groups. In some embodiments, “one or more R f groups” is meant to include up to 6 R f groups. In some embodiments, “one or more R f groups” is meant to include up to 7 R f groups.
- “one or more R f groups”is meant to include up to 8 R f groups.
- the term “heteroatom”is meant to include oxygen (O), nitrogen (N), sulfur (S), phosphorus (P), boron (B), and silicon (Si). The nitrogen and sulfur can be in an oxidized form when feasible.
- the term “chiral”refers to molecules which have the property of non- superimposability of the mirror image partner, while the term “achiral” refers to molecules which are superimposable on their mirror image partner.
- the term “stereoisomers”refers to compounds which have identical chemical constitution but differ with regard to the arrangement of the atoms or groups in space, e.g., enantiomers, diastereomers, tautomers.
- the term “patient” or “subject”is used throughout the specification to describe an animal, preferably a human or a domesticated animal, to whom treatment, including prophylactic treatment, with the compositions according to the present disclosure is provided.
- the term patientrefers to that specific animal, including a domesticated animal such as a dog or cat or a farm animal such as a horse, cow, sheep, etc.
- the term patientrefers to a human patient unless otherwise stated or implied from the context of the use of the term.
- the term “effective”is used to describe an amount of a compound, composition or component which, when used within the context of its intended use, effects an intended result. The term effective subsumes all other effective amount or effective concentration terms, which are otherwise described or used in the present application.
- “Pharmaceutically acceptable”means approved or approvable by a regulatory agency of the Federal or a state government or the corresponding agency in countries other than the United States, or that is listed in the U.S. Pharmacopoeia or other generally recognized pharmacopoeia for use in animals, e.g., in humans.
- “Pharmaceutically acceptable salt”refers to a salt of a compound of the disclosure that is pharmaceutically acceptable and that possesses the desired pharmacological activity of the parent compound.
- such saltsare non-toxic may be inorganic or organic acid - 12 - 4860-6199-1670.2 105807.001049 – PCT Application addition salts and base addition salts.
- such saltsinclude: (1) acid addition salts, formed with inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like; or formed with organic acids such as acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethane-disulfonic acid, 2-hydroxyethanesulfonic acid, benzenesulfonic acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-toluenesulfonic acid
- Saltsfurther include, by way of example only, sodium, potassium, calcium, magnesium, ammonium, tetraalkylammonium, and the like; and when the compound contains a basic functionality, salts of non-toxic organic or inorganic acids, such as hydrochloride, hydrobromide, tartrate, mesylate, acetate, maleate, oxalate and the like.
- a “pharmaceutically acceptable excipient”refers to a substance that is non-toxic, biologically tolerable, and otherwise biologically suitable for administration to a subject, such as an inert substance, added to a pharmacological composition or otherwise used as a vehicle, carrier, or diluent to facilitate administration of an agent and that is compatible therewith.
- excipientsinclude calcium carbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin, vegetable oils, and polyethylene glycols.
- a “solvate”refers to a physical association of a compound of Formula I with one or more solvent molecules.
- “Treating” or “treatment” of any disease or disorderrefers, in one embodiment, to ameliorating the disease or disorder (e.g., arresting or reducing the development of the disease or at least one of the clinical symptoms thereof). In another embodiment “treating” or “treatment” refers to ameliorating at least one physical parameter, which may not be discernible by the subject.
- treatingrefers to modulating the disease or disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both.
- treatingor “treatment” refers to delaying the onset of the disease or disorder.
- the disclosureis directed to a compound of Formula (I): - 13 - 4860-6199-1670.2 105807.001049 – PCT Application or a
- R 1is halo, C 1-6 alkyl, or C 1-6 haloalkyl
- each R2is independently H, D, or F
- each R3is independently H, D, C1-6 alkyl, C1-6 haloalkyl, C3-6 heterocycloalkyl or C3-6 cycloalkyl
- sis 1, 2 or 3
- tis 1, 2, 3, or 4
- R4is H, D, C1-6 alkyl, C1-6 alkoxyalkyl, C3-6 cycloalkyl, or C1-6 haloalkyl
- R 5is H, D, or F
- L1is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO
- L2is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO
- R 1 in Formula Iis halo, C 1-6 alkyl, or C 1-6 haloalkyl. In some embodiments, R 1 in Formula I is halo. In some embodiments, R 1 in Formula I is C1-6 alkyl. In some embodiments, R 1 in Formula I is C1-6 haloalkyl. [0055] In other embodiments, R1 in Formula I is F. In other embodiments, R1 in Formula I is Cl. In other embodiments, R 1 in Formula I is methyl. [0056] In some embodiments, each R2 in Formula I is independently H, D, or F. In some embodiments, each R2 in Formula I is H. In some embodiments, each R2 in Formula I is D.
- each R 2 in Formula Iis F.
- at least one R2 in Formula Iis H.
- at least one R2 in Formula Iis D.
- at least one R2 in Formula Iis F.
- s in Formula (I)is 1, 2 or 3.
- s in Formula (I)is 1.
- s in Formula (I)is 2.
- s in Formula (I)is 3.
- each R 3 in Formula Iis independently H, D, C 1-6 alkyl, C 1-6 haloalkyl, C 3-6 heterocycloalkyl or C 3-6 cycloalkyl.
- each R 3 in Formula Iis H. In some embodiments, each R3 in Formula I is D. In some embodiments, each R3 in Formula I is C1-6 alkyl. In some embodiments, each R3 in Formula I is C1-6 haloalkyl. In some embodiments, each R 3 in Formula I is C 3-6 heterocycloalkyl. In some embodiments, each R 3 in Formula I is C3-6 cycloalkyl. [0060] In other embodiments, at least one R3 in Formula I is H. In other embodiments, at least one R 3 in Formula I is D. In other embodiments, at least one R 3 in Formula I is C 1-6 alkyl. In other embodiments, at least one R 3 in Formula I is haloalkyl.
- At least one R3 in Formula Iis C3-6 cycloalkyl. - 15 - 4860-6199-1670.2 105807.001049 – PCT Application [0061]
- t in Formula (I)is 1, 2, 3 or 4.
- t in Formula (I)is 1.
- t in Formula (I)is 2.
- t in Formula (I)is 3.
- t in Formula (I)is 4.
- R 4 in Formula Iis independently H, D, C 1-6 alkyl, C 1-6 alkoxyalkyl, haloalkyl, or C 3-6 cycloalkyl. In some embodiments, R 4 in Formula I is H.
- R4 in Formula Iis D. In other embodiments, R4 in Formula I is C1-6 alkyl. In other embodiments, R 4 in Formula I is C 1-6 alkoxyalkyl. In other embodiments, R 4 in Formula I is C 1-6 haloalkyl. In yet other embodiments, R 4 in Formula I is C 3-6 cycloalkyl. [0063] In some embodiments, R5 in Formula I is independently H, D, or F. In some embodiments, R5 in Formula I is H. In other embodiments, R5 in Formula I is D. In other embodiments, R 5 in Formula I is F.
- L1 in Formula Iis absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO. In some embodiments, L1 in Formula (I) is absent. In some embodiments, L1 in Formula (I) is O. In some embodiments, L 1 in Formula (I) is S. In other embodiments, L 1 in Formula (I) is S(O). In other embodiments, L 1 in Formula (I) is SO 2 . In other embodiments, L 1 in Formula (I) is NR3. In yet other embodiments, L1 in Formula (I) is C(R3)2. In yet other embodiments, L1 in Formula (I) is CO. In yet other embodiments, L1 in Formula (I) is methylene.
- ring A 1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L1 is linked to ring A1 through a nitrogen atom in ring A1, L1 is absent.
- L1is not absent.
- L 1is absent, which means that ring A 1 is attached directly to the fused tricyclic group by a bond.
- fused tricyclic groupcan be taken to mean [1',2':4,5]pyrazino[2,3-c]pyridazine;” and having a core structure .
- L2 in Formula Iis absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO. In some embodiments, L2 in Formula (I) is absent. In some embodiments, L2 in Formula (I) is O. In some embodiments, L 2 in Formula (I) is S. In other embodiments, L 2 in Formula (I) is S(O). In other embodiments, L2 in Formula (I) is SO2. In other embodiments, L2 in Formula (I) - 16 - 4860-6199-1670.2 105807.001049 – PCT Application is NR 3 . In yet other embodiments, L 2 in Formula (I) is C(R 3 ) 2 .
- L 2 in Formula (I)is CO. In yet other embodiments, L2 in Formula (I) is methylene. [0069] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L 2 is linked to ring A 1 through a nitrogen atom in ring A 1 , L 2 is absent.
- ring A1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L 2 is linked to ring A 1 through a nitrogen atom in ring A 1 , L 2 is not O, S or NR3.
- L2is an alkylene spacer.
- ring A 1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a carbon atom in ring A1, L2 is not absent.
- ring A1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a carbon atom in ring A1, L2 is an alkylene spacer.
- ring A 1 in Formula (I)is a 9-10 membered heteroaryl group, a 5- 7 membered heterocycloalkyl group or a 9-10 membered heterocycloalkenyl group.
- ring A1 in Formula (I)is a 9-10 membered heteroaryl group.
- ring A 1is a 5-7 membered heterocycloalkyl group. In other embodiments, ring A 1 is a 9-10 membered heterocycloalkenyl group.
- ring A1 in Formula (I)is a quinoline, a naphthyridine, a quinoxaline, a tetrahydro-isoquinoline, a tetrahydro-naphthyridine, a tetrahydropyrido-pyrazine, a piperidine, an isoindoline, a dihydropyrrolo-pyridine, a dihydropyrrolo-pyrazine, a pyrrolo- pyridine, a pyrazolo-pyridine, a triazolo-pyridine, a tetrahydropyrazolo-pyridine, or a tetrahydropyrrolo-pyridine.
- ring A 1 in Formula (I)is a quinoline. In some embodiments, ring A 1 in Formula (I) is a naphthyridine. In some embodiments, ring A 1 in Formula (I) is a quinoxaline. In some embodiments, ring A1 in Formula (I) is a tetrahydro-isoquinoline. In some embodiments, ring A1 in Formula (I) is a tetrahydro-naphthyridine. In other embodiments, ring A 1 in Formula (I) is a tetrahydropyrido-pyrazine.
- ring A 1 in Formula (I)is - 17 - 4860-6199-1670.2 105807.001049 – PCT Application a piperidine.
- ring A 1 in Formula (I)is an isoindoline.
- ring A1 in Formula (I)is a dihydropyrrolo-pyridine.
- ring A1 in Formula (I)is a dihydropyrrolo-pyrazine.
- ring A1 in Formula (I)is a pyrrolo-pyridine.
- ring A 1 in Formula (I)is a pyrazolo-pyridine.
- ring A 1 in Formula (I)is a triazolo-pyridine. In yet other embodiments, ring A1 in Formula (I) is a tetrahydropyrazolo-pyridine. In yet other embodiments, ring A 1 in Formula (I) is a tetrahydropyrrolo-pyridine.
- ring A 1 in any of the formulae described hereinis selected from: , , , , , , , each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or OR a ; each R 8 is independently D, C 1-6 alkyl, C 1-6 haloalkyl, C 3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2.
- each R 8is independently D, C 1-6 alkyl, C 1-6 haloalkyl, C 3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2.
- ring A1 in any of the formulae described hereinis selected from ; wherein each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or OR a ; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2.
- ring A1 in any of the formulae described hereinis .
- ring A1 in any of the formulae described hereinis .
- ring A 1 in any of the formulae described hereinis ring A1 in any of the formulae described herein is ring A 1 in any of the formulae described herein is [0085] In some embodiments, ring A1 in any of the formulae described herein is ring A 1 in any of the formulae described herein is ring A1 in any of the formulae described herein is . In other ring A 1 in any of the formulae described herein is ring A1 in any of the formulae described herein is .
- ring A 1 in any of the formulae described hereinis ring A1 in any of the formulae described .
- ring A1 in any of the formulae described hereinis [0093]
- ring A1 in any of the formulae described hereinis [0094]
- ring A1 in any of the formulae described hereinis [0095]
- ring A1 in any of the formulae described hereinis .
- ring A 1 in any of the formulae described hereinis .
- ring A1 in any of the formulae described hereinis .
- each R7 in any of the formulae described hereinis independently D, halo, C 1-6 alkyl, C 1-6 haloalkyl, C 3-6 cycloalkyl, -CN or OR a .
- at least one R 7is D.
- at least one R 7is C 1-6 alkyl.
- at least one R7is C1-6 haloalkyl.
- At least one R7is C3-6 cycloalkyl. In yet other embodiments, at least one R 7 is -CN. In yet other embodiments, at least one R 7 is OR a .
- each R8 in any of the formulae described hereinis independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo. In some embodiments, at least one R 8 is D. In some embodiments, at least one R 8 is C 1-6 alkyl. In other embodiments, at least one R8 is C1-6 haloalkyl. In other embodiments, at least one R7 is C3-6 cycloalkyl.
- each R8is oxo.
- each m in any of the formulae described hereinis independently 0, 1, 2, 3, 4, 5, or 6. In some embodiments, m is 0. In some embodiments, m is 1. In some embodiments, m is 2. In other embodiments, m is 3. In other embodiments, m is 4. In yet other embodiments, m is 5. In yet other embodiments, m is 6. [00103] In some embodiments, each n in any of the formulae described herein is independently 0, 1, 2 or 3. In some embodiments, n is 0. In some embodiments, n is 1. In other embodiments, n is 2. In other embodiments, n is 3.
- each o in any of the formulae described hereinis independently 0, 1 or 2. In some embodiments, o is 0. In some embodiments, o is 1. In other embodiments, o is 2. [00105] The “ ” denotes points of attachment of ring A1 to L1 and to L2. The structures shown for ring A1 are intended to be spatially accurate.
- ring A2 in Formula (I)is a 3-7 membered cycloalkyl group or a 4-7-membered heterocycloalkyl group.
- ring A 2 in Formula (I)is a 3-7 membered cycloalkyl group. In some embodiments, ring A 2 in Formula (I) is a cyclohexyl group. In some embodiments, ring A2 is a 4-7-membered heterocycloalkyl group. In some embodiments, ring A 2 in Formula (I) is a piperazine group, a morpholine group, a piperidine group, a pyrrolidine group, an azetidine group or an azabicyclo-hexane group. [00107] In some embodiments, ring A2 in Formula (I) is a piperazine group.
- each R f in Formula Iis independently D, oxo, halogen, C 1 -C 8 alkoxy, C1-C8 alkyl, C1-6 haloalkyl, C1-6 haloalkoxy, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, C 6-10 aryl, C 5-12 heteroaryl, C 3-8 cycloalkyl, C 3-8 cycloalkenyl, C 3-8 heterocycloalkyl, C 3-8 heterocycloalkenyl, -OR a , -SR a , -NR c R d , -NR a R c , -C(O)R b , -OC(O)R b , -C(O)OR b , -C(O)NR c R d , - S(O)R b , -S(O)
- At least one R f in Formula Iis D. In some embodiments, at least one R f in Formula I is oxo. In some embodiments, at least one R f in Formula I is halogen. In some embodiments, at least one R f in Formula I is C 1 -C 8 alkoxy. In some embodiments, at least one R f in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d .
- At least one R f in Formula Iis haloalkyl. In some embodiments, at least one - 23 - 4860-6199-1670.2 105807.001049 – PCT Application R f in Formula I is -OH. In some embodiments, R f in Formula I is -CN. In some embodiments, at least one R f in Formula I is -NO2. In some embodiments, at least one R f in Formula I is -C2-C6 alkenyl. In some embodiments, at least one R f in Formula I is -C2-C6 alkynyl. In some embodiments, at least one R f in Formula I is C 6-10 aryl.
- At least one R f in Formula Iis C 5-12 heteroaryl. In some embodiments, at least one R f in Formula I is C 3-8 cycloalkyl. In other embodiments, at least one R f in Formula I is C3-8 cycloalkenyl. In other embodiments, at least one R f in Formula I is C 3-8 heterocycloalkyl. In other embodiments, at least one R f in Formula I is C 3-8 heterocycloalkenyl. In other embodiments, at least one R f in Formula I is -OR a . In other embodiments, at least one R f in Formula I is -SR a .
- At least one R f in Formula Iis -NR c R d . In other embodiments, at least one R f in Formula I is - NR a R c . In other embodiments, at least one R f in Formula I is -C(O)R b . In other embodiments, at least one R f in Formula I is -OC(O)R b . In other embodiments, at least one R f in Formula I is - C(O)OR b . In other embodiments, at least one R f in Formula I is -C(O)NR c R d . In yet other embodiments, at least one Rf in Formula I is -S(O)Rb.
- At least one R f in Formula Iis -S(O)2R b . In yet other embodiments, at least one R f in Formula I is -C(O)NR b OR b . In yet other embodiments, at least one Rf in Formula I is -S(O) 2 ORb. In yet other embodiments, at least one Rf in Formula I is - OS(O) 2 OR b . In yet other embodiments, at least one R f in Formula I is -OPO(OR b )(OR b ).
- each R a in Formula Iis independently H, D, -C(O)R b , - C 2 , - P - - - - - - - C 10 alkyl, - C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3- 8 heterocycloalkyl, or C3-8 heterocycloalkenyl.
- at least one R a in Formula Iis H.
- at least one R a in Formula Iis D.
- At least one R a in Formula Iis -P(OR c )2, -P(O)R c R b , - P(O)OR c OR b , -S(O)R b , -S(O)NR c R d , -S(O) 2 R b , -S(O) 2 NR c R d , SiR b 3 , and the like.
- At least one R a in Formula Iis -C 1 -C 10 alkyl, -C 2 -C 10 alkenyl, -C 2 -C 10 alkynyl, C 6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, and the like.
- each R b in Formula Iis independently H, D, -C 1 -C 6 alkyl, -C 2 - C 6 alkenyl, -C 2 -C 6 alkynyl, C 6-10 aryl, C 3-8 cycloalkyl, C 3-8 cycloalkenyl, C 5-12 heteroaryl, C 3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl.
- at least one R b in Formula Iis H.
- at least one R b in Formula Iis D.
- at least one R b in Formula Iis -C 1 -C 6 alkyl.
- At least one R b in Formula Iis -C2-C6 alkenyl. In some embodiments, at least one R b in Formula I is -C2-C6 alkynyl. In other embodiments, at least one R b in Formula I is C 6-10 aryl. In other embodiments, at least one R b in Formula I is C 3-8 cycloalkyl. In other embodiments, at least one R b in Formula I is C3-8 cycloalkenyl. In other embodiments, at least one R b in Formula I is C5-12 heteroaryl. In other embodiments, at least one R b in Formula I is C3-8 heterocycloalkyl.
- each R c or R d in Formula Iis independently H, D, -C 1 -C 6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl.
- R c or R d in Formula Iis H.
- R c or R d in Formula Iis D.
- R c or R d in Formula Iis -C1-C10 alkyl. In some embodiments, R c or R d in Formula I is -C2-C6 alkenyl. In some embodiments, R c or R d in Formula I is -C 2 -C 6 alkynyl. In other embodiments, Rc or Rd in Formula I is -OC 1 -C 6 alkyl. In other embodiments, R c or R d in Formula I is -O-cycloalkyl. In other embodiments, R c or R d in Formula I is C6-10 aryl. In other embodiments, R c or R d in Formula I is C3-8 cycloalkyl.
- R c or R d in Formula Iis C 3-8 cycloalkenyl. In other embodiments, R c or R d in Formula I is C 5-12 heteroaryl. In other embodiments, R c or R d in Formula I is C 3-8 heterocycloalkyl. In other embodiments, R c or R d in Formula I is C3-8 heterocycloalkenyl. [00119] In yet other embodiments, R c and R d in Formula I, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocycloalkenyl group.
- R c and R d in Formula Iform a monocyclic heterocycloalkyl. In yet other embodiments, R c and R d in Formula I form a multicyclic heterocycloalkyl. In yet other embodiments, R c and R d in Formula I form a monocyclic heterocycloalkenyl group. In yet other embodiments, R c and R d in Formula I form a multicyclic heterocycloalkenyl group. [00120] In some embodiments, the compounds of Formula (I) are the pharmaceutically acceptable salts. In some embodiments, the compounds of Formula (I) are solvates.
- the compounds of Formula (I)are N-oxides. In some embodiments, the compounds of Formula (I) are stereoisomers. [00121] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIa and Formula IIb: or a pharmaceutically acceptable salt thereof; wherein each R1, R2, R4, L1, Ring A1, L2, ring A2, X 1 , and X 2 are defined above with respect to Formula (I) or each R 1 , R 2 , R 4 , L 1 , Ring A 1 , L 2 , ring A 2 , X 1 , and X 2 are defined as described herein.
- the compounds of Formula (I)are represented by compounds of Formula IIa. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula IIb. [00123] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIa and Formula IIIb: (IIIb) - 26 - 4860-6199-1670.2 105807.001049 – PCT Application or a pharmaceutically acceptable salt thereof; wherein each R 1 , R 2 , R 4 , L 2 , and ring A 1 are defined above with respect to Formula (I) or each R1, R2, R4, L2, and ring A1 are defined as described herein; and wherein X is N or CR3. [00124] In some embodiments, X is N.
- Xis CR 3 . In some embodiments, X is N only when it forms a stable bond with L 2 or with ring A 1 when L 2 is absent. [00125] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIa. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIb.
- the compounds of Formula (I)are represented by compounds of Formula IVa and Formula IVb or a pharmaceutically acceptable salt thereof; wherein each R1, R2, R4, L2, and ring A1 are defined above with respect to Formula (I) or each R 1 , R 2 , R 4 , L 2 , and ring A 1 are defined as described herein; and wherein X is defined above with respect to Formula (IIIa) and Formula (IIIb).
- the compounds of Formula (I)are represented by compounds of Formula IVa.
- the compounds of Formula (I)are represented by compounds of Formula IVb.
- the compounds of Formula (I)are represented by compounds of Formula Va (Va) - 27 - 4860-6199-1670.2 105807.001049 – PCT Application or a A1 are defined above with respect to Formula (I) or each R 1 , R 2 , R 4 , L 2 , and ring A 1 are defined as described herein; and wherein X is defined above with respect to Formula (IIIa) and Formula (IIIb).
- the compounds of Formula (I)are represented by compounds of Formula Va.
- the compounds of Formula (I)are represented by compounds of Formula Vb.
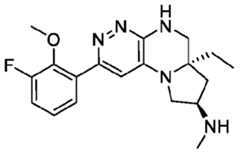
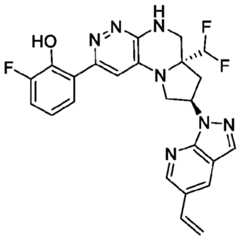
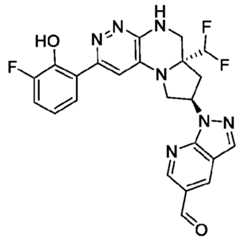
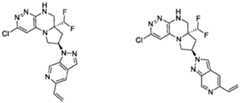
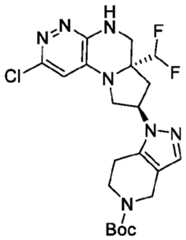
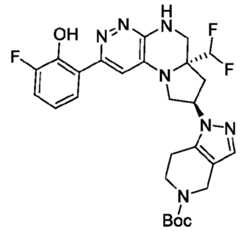
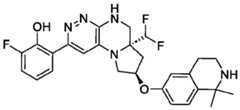
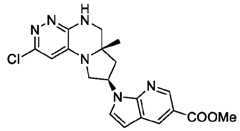
- the compounds of Formula (I)are: (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyr
- the compounds of Formula (I)are: - 32 - 4860-6199-1670.2 105807.001049 – PCT Application (S)-3-(6-(1-((1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridin-5- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-
- compositions and Methods of Administrationare typically formulated to provide a therapeutically effective amount of a compound of the present disclosure as the active ingredient, or a pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or derivative thereof.
- the pharmaceutical compositionscontain pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including inert solid diluents and fillers, diluents, including sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- compositionscan be administered alone or in combination with one or more other agents, which are also typically administered in the form of pharmaceutical compositions.
- the one or more compounds of the invention and other agent(s)may be mixed into a preparation or both components may be formulated into separate preparations to use them in combination separately or at the same time.
- the concentration of one or more compounds provided in the pharmaceutical compositions of the present inventionis less than 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%, 0.004%, 0.003%, 0.002%, 0.001%, 0.0009%, 0.0008%, 0.0007%, 0.0006%, 0.0005%, 0.0004%, 0.0003%, 0.0002%, or 0.0001% (or a number in the range defined by and including any two numbers above)
- the concentration of one or more compounds of the inventionis greater than 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19.75%, 19.50%, 19.25%, 19%, 18.75%, 18.50%, 18.25% 18%, 17.75%, 17.50%, 17.25% 17%, 16.75%, 16.50%, 16.25%, 16%, 15.75%, 15.50%, 15.25% 15%, 14.75%, 14.50%, 14.25% 14%, 13.75%, 13.50%, 13.25%, 13%, 12.75%, 12.50%, 12.25%, 12%, 11.75%, 11.50%, 11.25% 11%, 10.75%, 10.50%, 10.25% 10%, 9.75%, 9.50%, 9.25%, 9%, 8.75%, 8.50%, 8.25% 8%, 7.75%, 7.50%, 7.25%, 7%, 6.75%, 6.50%, 6.25%, 6%, 5.75%, 5.50%, 5.25%, 5%, 5%,
- the concentration of one or more compounds of the inventionis in the range from approximately 0.0001% to approximately 50%, approximately 0.001% to approximately 40%, approximately 0.01% to approximately 30%, approximately 0.02% to approximately 29%, approximately 0.03% to approximately 28%, approximately 0.04% to approximately 27%, approximately 0.05% to approximately 26%, approximately 0.06% to approximately 25%, approximately 0.07% to approximately 24%, approximately 0.08% to approximately 23%, approximately 0.09% to approximately 22%, approximately 0.1% to approximately 21%, approximately 0.2% to approximately 20%, approximately 0.3% to approximately 19%, approximately 0.4% to approximately 18%, approximately 0.5% to approximately 17%, approximately 0.6% to approximately 16%, approximately 0.7% to approximately 15%, approximately 0.8% to approximately 14%, approximately 0.9% to approximately 12%, approximately 1% to approximately 10% w/w, w/v or v/v.
- the concentration of one or more compounds of the inventionis in the range from approximately 0.001% to approximately 10%, approximately 0.01% to approximately 5%, approximately 0.02% to approximately 4.5%, approximately 0.03% to approximately 4%, approximately 0.04% to approximately 3.5%, approximately 0.05% to approximately 3%, approximately 0.06% to approximately 2.5%, approximately 0.07% to approximately 2%, approximately 0.08% to approximately 1.5%, approximately 0.09% to approximately 1%, approximately 0.1% to approximately 0.9% w/w, w/v or v/v.
- the amount of one or more compounds of the inventionis equal to or less than 10 g, 9.5 g, 9.0 g, 8.5 g, 8.0 g, 7.5 g, 7.0 g, 6.5 g, 6.0 g, 5.5 g, 5.0 g, 4.5 g, 4.0 g, 3.5 g, 3.0 g, 2.5 g, 2.0 g, 1.5 g, 1.0 g, 0.95 g, 0.9 g, 0.85 g, 0.8 g, 0.75 g, 0.7 g, 0.65 g, 0.6 g, 0.55 g, 0.5 g, 0.45 g, 0.4 g, 0.35 g, 0.3 g, 0.25 g, 0.2 g, 0.15 g, 0.1 g, 0.09 g, 0.08 g, 0.07 g, 0.06 g, 0.05 g, 0.04 g, 0.03 g, 0.02 g, 0.01 g, 0.009
- the amount of one or more compounds of the inventionis more than 0.0001 g, 0.0002 g, 0.0003 g, 0.0004 g, 0.0005 g, 0.0006 g, 0.0007 g, 0.0008 g, 0.0009 g, 0.001 g, 0.0015 g, 0.002 g, 0.0025 g, 0.003 g, 0.0035 g, 0.004 g, 0.0045 g, 0.005 g, 0.0055 g, 0.006 g, 0.0065 g, 0.007 g, 0.0075 g, 0.008 g, 0.0085 g, 0.009 g, 0.0095 g, 0.01 g, 0.015 g, 0.02 - 35 - 4860-6199-1670.2 105807.001049 – PCT Application g, 0.025 g, 0.03 g, 0.035 g, 0.04 g, 0.045 g, 0.05 g
- the amount of one or more compounds of the inventionis in the range of 0.0001-10 g, 0.0005-9 g, 0.001-8 g, 0.005-7 g, 0.01-6 g, 0.05-5 g, 0.1-4 g, 0.5-4 g, or 1- 3 g.
- the compounds according to the inventionare effective over a wide dosage range. For example, in the treatment of adult humans, dosages from 0.01 to 1000 mg, from 0.5 to 100 mg, from 1 to 50 mg per day, and from 5 to 40 mg per day are examples of dosages that may be used. An exemplary dosage is 10 to 30 mg per day.
- a pharmaceutical composition of the inventiontypically contains an active ingredient (e.g., a compound of the disclosure) of the present invention or a pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including but not limited to inert solid diluents and fillers, diluents, sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- active ingrediente.g., a compound of the disclosure
- a pharmaceutically acceptable salt and/or coordination complex thereofe.g., a pharmaceutically acceptable excipients, carriers, including but not limited to inert solid diluents and fillers, diluents, sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- the inventionprovides a pharmaceutical composition for oral administration containing a compound of the invention, and a pharmaceutical excipient suitable for oral administration.
- the inventionprovides a solid pharmaceutical composition for oral administration containing: (i) an effective amount of a compound of the invention; optionally (ii) an effective amount of a second agent; and (iii) a pharmaceutical excipient suitable for oral administration.
- the compositionfurther contains: (iv) an effective amount of a third agent.
- the pharmaceutical compositionmay be a liquid pharmaceutical composition suitable for oral consumption.
- compositions of the invention suitable for oral administrationcan be presented as discrete dosage forms, such as capsules, - 36 - 4860-6199-1670.2 105807.001049 – PCT Application cachets, or tablets, or liquids or aerosol sprays each containing a predetermined amount of an active ingredient as a powder or in granules, a solution, or a suspension in an aqueous or non- aqueous liquid, an oil-in- water emulsion, or a water-in-oil liquid emulsion.
- dosage formscan be prepared by any of the methods of pharmacy, but all methods include the step of bringing the active ingredient into association with the carrier, which constitutes one or more necessary ingredients.
- compositionsare prepared by uniformly and intimately admixing the active ingredient with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product into the desired presentation.
- a tabletcan be prepared by compression or molding, optionally with one or more accessory ingredients.
- Compressed tabletscan be prepared by compressing in a suitable machine the active ingredient in a free- flowing form such as powder or granules, optionally mixed with an excipient such as, but not limited to, a binder, a lubricant, an inert diluent, and/or a surface active or dispersing agent.
- Molded tabletscan be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
- This inventionfurther encompasses anhydrous pharmaceutical compositions and dosage forms comprising an active ingredient, since water can facilitate the degradation of some compounds.
- watermay be added (e.g., 5%) in the pharmaceutical arts as a means of simulating long-term storage in order to determine characteristics such as shelf- life or the stability of formulations over time.
- Anhydrous pharmaceutical compositions and dosage forms of the inventioncan be prepared using anhydrous or low moisture containing ingredients and low moisture or low humidity conditions.
- compositions and dosage forms of the invention which contain lactosecan be made anhydrous if substantial contact with moisture and/or humidity during manufacturing, packaging, and/or storage is expected.
- An anhydrous pharmaceutical compositionmay be prepared and stored such that its anhydrous nature is maintained. Accordingly, anhydrous compositions may be packaged using materials known to prevent exposure to water such that they can be included in suitable formulary kits. Examples of suitable packaging include, but are not limited to, hermetically sealed foils, plastic or the like, unit dose containers, blister packs, and strip packs.
- An active ingredientcan be combined in an intimate admixture with a pharmaceutical carrier according to conventional pharmaceutical compounding techniques.
- the carriercan take a wide variety of forms depending on the form of preparation desired for administration.
- any of the usual pharmaceutical mediacan be employed as carriers, such as, for example, water, glycols, oils, alcohols, flavoring agents, preservatives, coloring agents, and the like in the case of oral liquid preparations (such as suspensions, solutions, and elixirs) or aerosols; or carriers such as starches, sugars, micro- - 37 - 4860-6199-1670.2 105807.001049 – PCT Application crystalline cellulose, diluents, granulating agents, lubricants, binders, and disintegrating agents can be used in the case of oral solid preparations, in some embodiments without employing the use of lactose.
- carrierssuch as, for example, water, glycols, oils, alcohols, flavoring agents, preservatives, coloring agents, and the like in the case of oral liquid preparations (such as suspensions, solutions, and elixirs) or aerosols; or carriers such as starches, sugars, micro- - 37 - 4860-6199-1670.2
- suitable fillers for use in the pharmaceutical compositions and dosage forms disclosed hereininclude, but are not limited to, talc, calcium carbonate (e.g., granules or powder), microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch, and mixtures thereof.
- Disintegrantsmay be used in the compositions of the invention to provide tablets that disintegrate when exposed to an aqueous environment. Too much of a disintegrant may produce tablets which may disintegrate in the bottle. Too little may be insufficient for disintegration to occur and may thus alter the rate and extent of release of the active ingredient(s) from the dosage form.
- a sufficient amount of disintegrant that is neither too little nor too much to detrimentally alter the release of the active ingredient(s)may be used to form the dosage forms of the compounds disclosed herein.
- the amount of disintegrant usedmay vary based upon the type of formulation and mode of administration, and may be readily discernible to those of ordinary skill in the art. About 0.5 to about 15 weight percent of disintegrant, or about 1 to about 5 weight percent of disintegrant, may be used in the pharmaceutical composition.
- Disintegrantsthat can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, agar-agar, alginic acid, calcium carbonate, microcrystalline cellulose, croscarmellose sodium, crospovidone, polacrilin potassium, sodium starch glycolate, potato or tapioca starch, other starches, pre-gelatinized starch, other starches, clays, other algins, other celluloses, gums or mixtures thereof.
- Lubricantswhich can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, calcium stearate, magnesium stearate, mineral oil, light mineral oil, glycerin, sorbitol, mannitol, polyethylene glycol, other glycols, stearic acid, sodium lauryl sulfate, talc, hydrogenated vegetable oil (e.g., peanut oil, cottonseed oil, sunflower - 38 - 4860-6199-1670.2 105807.001049 – PCT Application oil, sesame oil, olive oil, corn oil, and soybean oil), zinc stearate, ethyl oleate, ethyl laureate, agar, or mixtures thereof.
- Additional lubricantsinclude, for example, a syloid silica gel, a coagulated aerosol of synthetic silica, or mixtures thereof.
- a lubricantcan optionally be added, in an amount of less than about 1 weight percent of the pharmaceutical composition.
- the active ingredient thereinmay be combined with various sweetening or flavoring agents, coloring matter or dyes and, if so desired, emulsifying and/or suspending agents, together with such diluents as water, ethanol, propylene glycol, glycerin and various combinations thereof.
- the tabletscan be uncoated or coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay materialsuch as glyceryl monostearate or glyceryl distearate can be employed.
- Formulations for oral usecan also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example, peanut oil, liquid paraffin or olive oil.
- Surfactantwhich can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, hydrophilic surfactants, lipophilic surfactants, and mixtures thereof. That is, a mixture of hydrophilic surfactants may be employed, a mixture of lipophilic surfactants may be employed, or a mixture of at least one hydrophilic surfactant and at least one lipophilic surfactant may be employed.
- a suitable hydrophilic surfactantmay generally have an HLB value of at least 10, while suitable lipophilic surfactants may generally have an HLB value of or less than about 10.
- HLBhydrophilic-lipophilic balance
- Surfactants with lower HLB valuesare more lipophilic or hydrophobic, and have greater solubility in oils, while surfactants with higher HLB values are more hydrophilic, and have greater solubility in aqueous solutions.
- Hydrophilic surfactantsare generally considered to be those compounds having an HLB value greater than about 10, as well as anionic, cationic, or zwitterionic compounds for which the HLB scale is not generally applicable.
- lipophilic (e.g., hydrophobic) surfactantsare compounds having an HLB value equal to or less than about 10.
- Hydrophilic surfactantsmay be either ionic or non-ionic. Suitable ionic surfactants include, but are not limited to, alkylammonium salts; fusidic acid salts; fatty acid derivatives of - 39 - 4860-6199-1670.2 105807.001049 – PCT Application amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; lecithins and hydrogenated lecithins; lysolecithins and hydrogenated lysolecithins; phospholipids and derivatives thereof; lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acyl lactylates
- ionic surfactantsinclude, by way of example: lecithins, lysolecithin, phospholipids, lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acylactylates; mono- and di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di- glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof.
- Ionic surfactantsmay be the ionized forms of lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, phosphatidylserine, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, lysophosphatidylserine, PEG- phosphatidylethanolamine, PVP -phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholylsarcosine, caproate, capry
- Hydrophilic non-ionic surfactantsmay include, but are not limited to, alkylglucosides; alkylmaltosides; alkylthioglucosides; lauryl macrogolglycerides; polyoxyalkylene alkyl ethers such as polyethylene glycol alkyl ethers; polyoxyalkylene alkylphenols such as polyethylene glycol alkyl phenols; polyoxyalkylene alkyl phenol fatty acid esters such as polyethylene glycol fatty acids monoesters and polyethylene glycol fatty acids diesters; polyethylene glycol glycerol fatty acid esters; polyglycerol fatty acid esters; polyoxyalkylene sorbitan fatty acid esters such as polyethylene glycol sorbitan fatty acid esters; hydrophilic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids, and sterols; polyoxyethylene stearoyl
- the polyolmay be - 40 - 4860-6199-1670.2 105807.001049 – PCT Application glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide.
- hydrophilic-non-ionic surfactantsinclude, without limitation, PEG- 10 laurate, PEG- 12 laurate, PEG-20 laurate, PEG-32 laurate, PEG-32 dilaurate, PEG- 12 oleate, PEG- 15 oleate, PEG-20 oleate, PEG-20 dioleate, PEG-32 oleate, PEG-200 oleate, PEG-400 oleate, PEG- 15 stearate, PEG-32 distearate, PEG-40 stearate, PEG- 100 stearate, PEG-20 dilaurate, PEG-25 glyceryl trioleate, PEG-32 dioleate, PEG-20 glyceryl laurate, PEG-30 glyceryl laurate, PEG-20 glyceryl stearate, PEG-20 glyceryl oleate, PEG-30 glyceryl oleate, PEG-30 glyce
- Suitable lipophilic surfactantsinclude, by way of example only: fatty alcohols; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; propylene glycol fatty acid esters; sorbitan fatty acid esters; polyethylene glycol sorbitan fatty acid esters; sterols and sterol derivatives; polyoxyethylated sterols and sterol derivatives; polyethylene glycol alkyl ethers; sugar esters; sugar ethers; lactic acid derivatives of mono- and di-glycerides; hydrophobic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids and sterols; oil-soluble vitamins/vitamin derivatives; and mixtures thereof.
- preferred lipophilic surfactantsinclude glycerol fatty acid esters, propylene glycol fatty acid esters, and mixtures thereof, or are hydrophobic transesterification products of a polyol with at least one member of the group consisting of vegetable oils, hydrogenated vegetable oils, and triglycerides.
- the compositionmay include a solubilizer to ensure good solubilization and/or dissolution of the compound of the present invention and to minimize precipitation of the compound of the present invention. This can be especially important for compositions for non-oral use, e.g., compositions for injection.
- a solubilizermay also be added - 41 - 4860-6199-1670.2 105807.001049 – PCT Application to increase the solubility of the hydrophilic drug and/or other components, such as surfactants, or to maintain the composition as a stable or homogeneous solution or dispersion.
- solubilizersinclude, but are not limited to, the following: alcohols and polyols, such as ethanol, isopropanol, butanol, benzyl alcohol, ethylene glycol, propylene glycol, butanediols and isomers thereof, glycerol, pentaerythritol, sorbitol, mannitol, transcutol, dimethyl isosorbide, polyethylene glycol, polypropylene glycol, polyvinylalcohol, hydroxypropyl methylcellulose and other cellulose derivatives, cyclodextrins and cyclodextrin derivatives; ethers of polyethylene glycols having an average molecular weight of about 200 to about 6000, such as tetrahydrofurfuryl alcohol PEG ether (glycofurol) or methoxy PEG ; amides and other nitrogen-containing compounds such as 2-pyrrolidone, 2-piperidone, ⁇
- solubilizersmay also be used. Examples include, but not limited to, triacetin, triethylcitrate, ethyl oleate, ethyl caprylate, dimethylacetamide, N-methylpyrrolidone, N-hydroxyethylpyrrolidone, polyvinylpyrrolidone, hydroxypropyl methylcellulose, hydroxypropyl cyclodextrins, ethanol, polyethylene glycol 200-100, glycofurol, transcutol, propylene glycol, and dimethyl isosorbide.
- solubilizersinclude sorbitol, glycerol, triacetin, ethyl alcohol, PEG-400, glycofurol and propylene glycol.
- the amount of solubilizer that can be includedis not particularly limited.
- the amount of a given solubilizermay be limited to a bioacceptable amount, which may be readily determined by one of skill in the art.
- the solubilizercan be in a weight ratio of 10%, 25%o, 50%), 100%o, or up to about 200%> by weight, based on the combined weight of the drug, and other excipients. If desired, very small amounts of solubilizer may also be used, such as 5%>, 2%>, 1%) or even less. Typically, the solubilizer may be present in an amount of about 1%> to about 100%, more typically about 5%> to about 25%> by weight. - 42 - 4860-6199-1670.2 105807.001049 – PCT Application [00171]
- the compositioncan further include one or more pharmaceutically acceptable additives and excipients.
- additives and excipientsinclude, without limitation, detackifiers, anti-foaming agents, buffering agents, polymers, antioxidants, preservatives, chelating agents, viscomodulators, tonicifiers, flavorants, colorants, odorants, opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof.
- an acid or a basemay be incorporated into the composition to facilitate processing, to enhance stability, or for other reasons.
- Examples of pharmaceutically acceptable basesinclude amino acids, amino acid esters, ammonium hydroxide, potassium hydroxide, sodium hydroxide, sodium hydrogen carbonate, aluminum hydroxide, calcium carbonate, magnesium hydroxide, magnesium aluminum silicate, synthetic aluminum silicate, synthetic hydrocalcite, magnesium aluminum hydroxide, diisopropylethylamine, ethanolamine, ethylenediamine, triethanolamine, triethylamine, triisopropanolamine, trimethylamine, tris(hydroxymethyl)aminomethane (TRIS) and the like.
- basesthat are salts of a pharmaceutically acceptable acid, such as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p-toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid, and the like.
- a pharmaceutically acceptable acidsuch as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids
- Salts of polyprotic acidssuch as sodium phosphate, disodium hydrogen phosphate, and sodium dihydrogen phosphate can also be used.
- the cationcan be any convenient and pharmaceutically acceptable cation, such as ammonium, alkali metals, alkaline earth metals, and the like.
- Examplemay include, but not limited to, sodium, potassium, lithium, magnesium, calcium and ammonium.
- Suitable acidsare pharmaceutically acceptable organic or inorganic acids. Examples of suitable inorganic acids include hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid, nitric acid, boric acid, phosphoric acid, and the like.
- suitable organic acidsinclude acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acids, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, methanesulfonic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p- toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid and the like.
- the inventionprovides a pharmaceutical composition for injection containing a compound of the present invention and a pharmaceutical excipient suitable for injection. Components and amounts of agents in the compositions are as described herein.
- the forms in which the novel compositions of the present invention may be incorporated for administration by injectioninclude aqueous or oil suspensions, or emulsions, with sesame oil, corn oil, cottonseed oil, or peanut oil, as well as elixirs, mannitol, dextrose, or a sterile aqueous solution, and similar pharmaceutical vehicles.
- Aqueous solutions in salineare also conventionally used for injection.
- Ethanol, glycerol, propylene glycol, liquid polyethylene glycol, and the like (and suitable mixtures thereof), cyclodextrin derivatives, and vegetable oilsmay also be employed.
- the proper fluiditycan be maintained, for example, by the use of a coating, such as lecithin, for the maintenance of the required particle size in the case of dispersion and by the use of surfactants.
- the prevention of the action of microorganismscan be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the like.
- Sterile injectable solutionsare prepared by incorporating the compound of the present invention in the required amount in the appropriate solvent with various other ingredients as enumerated above, as required, followed by filtered sterilization.
- dispersionsare prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above.
- certain desirable methods of preparationare vacuum-drying and freeze- drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile- filtered solution thereof.
- the inventionprovides a pharmaceutical composition for transdermal delivery containing a compound of the present invention and a pharmaceutical excipient suitable for transdermal delivery.
- Compositions of the present inventioncan be formulated into preparations in solid, semisolid, or liquid forms suitable for local or topical administration, such as gels, water soluble jellies, creams, lotions, suspensions, foams, powders, slurries, ointments, solutions, oils, pastes, suppositories, sprays, emulsions, saline solutions, dimethylsulfoxide (DMSO)-based solutions.
- DMSOdimethylsulfoxide
- carriers with higher densitiesare capable of providing an area with a prolonged exposure to the active ingredients.
- a solution formulationmay provide more immediate exposure of the active ingredient to the chosen area.
- the pharmaceutical compositionsalso may comprise suitable solid or gel phase carriers or excipients, which are compounds that allow increased penetration of, or assist in the delivery of, therapeutic molecules across the stratum corneum permeability barrier of the skin. There are many of these penetration- enhancing molecules known to those trained in the art of topical formulation.
- humectantse.g., urea
- glycolse.g., propylene glycol
- alcoholse.g., ethanol
- fatty acidse.g., oleic acid
- surfactantse.g., isopropyl myristate and sodium lauryl sulfate
- pyrrolidonese.g., isopropyl myristate and sodium lauryl sulfate
- pyrrolidonese.glycerol monolaurate, sulfoxides, terpenes (e.g., menthol)
- aminesamides, alkanes, alkanols, water, calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols.
- transdermal delivery devicesSuch transdermal patches may be used to provide continuous or discontinuous infusion of a compound of the present invention in controlled amounts, either with or without another agent.
- transdermal patchesfor the delivery of pharmaceutical agents is well known in the art. See, e.g., U.S. Pat. Nos.5,023,252, 4,992,445 and 5,001,139. Such patches may be constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents.
- compositions for inhalation or insufflationinclude solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders.
- the liquid or solid compositionsmay contain suitable pharmaceutically acceptable excipients as described supra.
- the compositionsare administered by the oral or nasal respiratory route for local or systemic effect.
- Compositions in preferably pharmaceutically acceptable solventsmay be nebulized by use of inert gases. Nebulized solutions may be inhaled directly from the nebulizing device or the nebulizing device may be attached to a face mask tent, or intermittent positive pressure breathing machine.
- compositionsmay be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner.
- Other Pharmaceutical Compositionsmay also be prepared from compositions described herein and one or more pharmaceutically acceptable excipients suitable for sublingual, buccal, - 45 - 4860-6199-1670.2 105807.001049 – PCT Application rectal, intraosseous, intraocular, intranasal, epidural, or intraspinal administration. Preparations for such pharmaceutical compositions are well-known in the art.
- Administration of the compounds or pharmaceutical composition of the present inventioncan be affected by any method that enables delivery of the compounds to the site of action. These methods include oral routes, intraduodenal routes, parenteral injection (including intravenous, intraarterial, subcutaneous, intramuscular, intravascular, intraperitoneal or infusion), topical (e.g., transdermal application), rectal administration, via local delivery by catheter or stent or through inhalation. Compounds can also be administered intraadiposally or intrathecally. [00187] In some embodiments, the compounds or pharmaceutical composition of the present invention are administered by intravenous injection.
- an effective dosageis in the range of about 0.001 to about 100 mg per kg body weight per day, preferably about 1 to about 35 mg/kg/day, in single or divided doses. For a 70 kg human, this would amount to about 0.05 to 7 g/day, preferably about 0.05 to about 2.5 g/day.
- dosage levels below the lower limit of the aforesaid rangemay be more than adequate, while in other cases still larger doses may be employed without causing any harmful side effect, e.g., by dividing such larger doses into several small doses for administration throughout the day.
- a compound of the inventionis administered in a single dose.
- such administrationwill be by injection, e.g., intravenous injection, in order to introduce the agent quickly.
- other routesmay be used as appropriate.
- a single dose of a compound of the inventionmay also be used for treatment of an acute condition.
- a compound of the inventionis administered in multiple doses.
- Dosingmay be about once, twice, three times, four times, five times, six times, or more than six times per day. Dosing may be about once a month, once every two weeks, once a week, or once every other day.
- a compound of the invention and another agentare - 46 - 4860-6199-1670.2 105807.001049 – PCT Application administered together about once per day to about 6 times per day.
- the administration of a compound of the invention and an agentcontinues for less than about 7 days.
- the administrationcontinues for more than about 6, 10, 14, 28 days, two months, six months, or one year. In some cases, continuous dosing is achieved and maintained as long as necessary. [00192] Administration of the compounds of the invention may continue as long as necessary.
- a compound of the inventionis administered for more than 1, 2, 3, 4, 5, 6, 7, 14, or 28 days. In some embodiments, a compound of the invention is administered for less than 28, 14, 7, 6, 5, 4, 3, 2, or 1 day. In some embodiments, a compound of the invention is administered chronically on an ongoing basis, e.g., for the treatment of chronic effects.
- An effective amount of a compound of the inventionmay be administered in either single or multiple doses by any of the accepted modes of administration of agents having similar utilities, including rectal, buccal, intranasal and transdermal routes, by intra-arterial injection, intravenously, intraperitoneally, parenterally, intramuscularly, subcutaneously, orally, topically, or as an inhalant.
- compositions of the inventionmay also be delivered via an impregnated or coated device such as a stent, for example, or an artery-inserted cylindrical polymer.
- a method of administrationmay, for example, aid in the prevention or amelioration of restenosis following procedures such as balloon angioplasty.
- compounds of the inventionmay slow or inhibit the migration and proliferation of smooth muscle cells in the arterial wall which contribute to restenosis.
- a compound of the inventionmay be administered, for example, by local delivery from the struts of a stent, from a stent graft, from grafts, or from the cover or sheath of a stent.
- a compound of the inventionis admixed with a matrix.
- Such a matrixmay be a polymeric matrix and may serve to bond the compound to the stent.
- Polymeric matrices suitable for such useinclude, for example, lactone-based polyesters or copolyesters such as polylactide, polycaprolactonglycolide, polyorthoesters, polyanhydrides, polyaminoacids, polysaccharides, polyphosphazenes, poly (ether-ester) copolymers (e.g. PEO- PLLA); polydimethylsiloxane, poly(ethylene-vinylacetate), acrylate-based polymers or copolymers (e.g.
- Compounds of the inventionmay be applied to the surface of the stent by various methods such as dip/spin coating, spray coating, dip-coating, and/or brush-coating. The compounds may be applied in a solvent and the solvent may be allowed to evaporate, thus forming a layer of compound onto the stent.
- the compoundmay be located in the body of the stent or graft, for example - 47 - 4860-6199-1670.2 105807.001049 – PCT Application in microchannels or micropores.
- the compounddiffuses out of the body of the stent to contact the arterial wall.
- Such stentsmay be prepared by dipping a stent manufactured to contain such micropores or microchannels into a solution of the compound of the invention in a suitable solvent, followed by evaporation of the solvent. Excess drug on the surface of the stent may be removed via an additional brief solvent wash.
- compounds of the inventionmay be covalently linked to a stent or graft.
- a covalent linkermay be used which degrades in vivo, leading to the release of the compound of the invention. Any bio-labile linkage may be used for such a purpose, such as ester, amide or anhydride linkages.
- Compounds of the inventionmay additionally be administered intravascularly from a balloon used during angioplasty. Extravascular administration of the compounds via the pericard or via advential application of formulations of the invention may also be performed to decrease restenosis.
- a variety of stent devices which may be used as describedare disclosed, for example, in the following references, all of which are hereby incorporated by reference: U.S. Pat. No. 5451233; U.S. Pat. No.5040548; U.S. Pat.
- the compounds of the inventionmay be administered in dosages. It is known in the art that due to intersubject variability in compound pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy.
- Dosing for a compound of the inventionmay be found by routine experimentation in light of the instant disclosure.
- a compound of the inventionis administered in a composition that comprises one or more agents, and the agent has a shorter half- life than the compound of the invention unit dose forms of the agent and the compound of the invention may be adjusted accordingly.
- the subject pharmaceutical compositionmay, for example, be in a form suitable for oral administration as a tablet, capsule, pill, powder, sustained release formulations, solution, suspension, for parenteral injection as a sterile solution, suspension or emulsion, for topical administration as an ointment or cream or for rectal administration as a suppository.
- the pharmaceutical compositionmay be in unit dosage forms suitable for single administration of precise dosages.
- the pharmaceutical compositionwill include a conventional pharmaceutical carrier or excipient and a compound according to the invention as an active ingredient. In addition, it may include other medicinal or pharmaceutical agents, carriers, adjuvants, etc.
- Exemplary parenteral administration formsinclude solutions or suspensions of active compound in sterile aqueous solutions, for example, aqueous propylene glycol or dextrose solutions. Such dosage forms can be suitably buffered, if desired. - 48 - 4860-6199-1670.2 105807.001049 – PCT Application [00200] [00201] Methods of Use [00202] The method typically comprises administering to a subject a therapeutically effective amount of a compound of the invention.
- the therapeutically effective amount of the subject combination of compoundsmay vary depending upon the intended application (in vitro or in vivo), or the subject and disease condition being treated, e.g., the weight and age of the subject, the severity of the disease condition, the manner of administration and the like, which can readily be determined by one of ordinary skill in the art.
- the termalso applies to a dose that will induce a particular response in target cells, e.g., reduction of proliferation or downregulation of activity of a target protein.
- the specific dosewill vary depending on the particular compounds chosen, the dosing regimen to be followed, whether it is administered in combination with other compounds, timing of administration, the tissue to which it is administered, and the physical delivery system in which it is carried.
- the present inventionprovides a pharmaceutical composition comprising a compound of bispecific formula, or pharmaceutically acceptable salt thereof.
- the present inventionprovides a pharmaceutical composition comprising a compound of bispecific formula for use in degrading a target protein in a cell.
- a method of degrading a target proteincomprising administering to a cell therapeutically effective amount of a bispecific compound, or pharmaceutically acceptable salt, wherein the compound is effective for degrading the target protein.
- the present inventionprovides a pharmaceutical composition comprising a compound of bispecific formula, for use in treating or preventing of a disease or disorder in which SMARCA2 and/or SMARCA4 plays a role.
- the present inventionprovides a pharmaceutical composition comprising a compound of bispecific formula, for use in treating or preventing of a disease or disorder in which SWI/SNF mutations plays a role.
- target proteinsare SMARCA2, SMARCA4 and/or PB1.
- target protein complexis SWI/SNF in a cell.
- diseases or disorders dependent on SMARCA2 or SMARCA4include cancers.
- diseases or disorders dependent on SWI/SNF complexinclude cancers.
- Exemplary cancers which may be treated by the present compounds either alone or in combination with at least one additional anti-cancer agentinclude squamous-cell carcinoma, - 49 - 4860-6199-1670.2 105807.001049 – PCT Application basal cell carcinoma, adenocarcinoma, hepatocellular carcinomas, and renal cell carcinomas, cancer of the bladder, bowel, breast, cervix, colon, esophagus, head, kidney, liver, lung, neck, ovary, pancreas, prostate, and stomach; leukemias; benign and malignant lymphomas, particularly Burkitt's lymphoma and Non-Hodgkin's lymphoma; benign and malignant melanomas; myeloproliferative diseases; sarcomas, including Ewing's sarcoma, hemangiosarcoma, Kaposi's sarcoma, liposarcoma, myosarcomas, peripheral neuroepithelioma
- the cancers which may be treated using compounds according to the present disclosureinclude, for example, T-lineage Acute lymphoblastic Leukemia (T- ALL), T-lineage lymphoblastic Lymphoma (T-LL), Peripheral T-cell lymphoma, Adult T-cell Leukemia, Pre-B ALL, Pre-B Lymphomas, Large B-cell Lymphoma, Burkitts Lymphoma, B-cell ALL, Philadelphia chromosome positive ALL and Philadelphia chromosome positive CML.
- the canceris a SMARCA2 and/or SMARAC4- dependent cancer.
- the present inventionprovides a pharmaceutical composition comprising a compound of bispecific formula for use in the diseases or disorders dependent upon SMARCA2 and/or SMARCA4 is cancer.
- Compounds of the disclosure, as well as pharmaceutical compositions comprising themcan be administered to treat any of the described diseases, alone or in combination with a medical therapy.
- Medical therapiesinclude, for example, surgery and radiotherapy (e.g., gamma- radiation, neutron beam radiotherapy, electron beam radiotherapy, proton therapy, brachytherapy, systemic radioactive isotopes).
- radiotherapye.g., gamma- radiation, neutron beam radiotherapy, electron beam radiotherapy, proton therapy, brachytherapy, systemic radioactive isotopes.
- compounds of the disclosure, as well as pharmaceutical compositions comprising themcan be administered to treat any of the described diseases, alone or in combination with one or more other agents.
- the compounds of the disclosure, as well as pharmaceutical compositions comprising themcan be administered in combination with agonists of nuclear receptors agents. - 50 - 4860-6199-1670.2 105807.001049 – PCT Application [00219] In other methods, the compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered in combination with antagonists of nuclear receptors agents. [00220] In other methods, the compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered in combination with an anti-proliferative agent.
- the compounds of the inventioncan be used in combination with chemotherapeutic agents, agonists or antagonists of nuclear receptors, or other anti-proliferative agents.
- the compounds of the inventioncan also be used in combination with a medical therapy such as surgery or radiotherapy, e.g., gamma-radiation, neutron beam radiotherapy, electron beam radiotherapy, proton therapy, brachytherapy, and systemic radioactive isotopes.
- chemotherapeutic agentsinclude any of: abarelix, aldesleukin, alemtuzumab, alitretinoin, allopurinol, all-trans retinoic acid, altretamine, anastrozole, arsenic trioxide, asparaginase, azacitidine, bendamustine, bevacizumab, bexarotene, bleomycin, bortezombi, bortezomib, busulfan intravenous, busulfan oral, calusterone, capecitabine, carboplatin, carmustine, cetuximab, chlorambucil, cisplatin, cladribine, clofarabine, cyclophosphamide, cytarabine, dacarbazine, dactinomycin, dalteparin sodium, dasatinib, daunorubicin, decitabine, denileukin, denileukin difti
- the compounds of the inventioncan be used in combination with a therapeutic agent that targets an epigenetic regulator.
- epigenetic regulators - 51 - 4860-6199-1670.2 105807.001049 – PCT Applicationinclude bromodomain inhibitors, the histone lysine methyltransferase inhibitors, histone arginine methyl transferase inhibitors, histone demethylase inhibitors, histone deacetylase inhibitors, histone acetylase inhibitors, and DNA methyltransferase inhibitors.
- Histone deacetylase inhibitorsinclude, e.g., vorinostat.
- Histone arginine methyl transferase inhibitorsinclude inhibitors of protein arginine methyltransferases (PRMTs) such as PRMT5, PRMT1 and PRMT4.
- PRMTsprotein arginine methyltransferases
- DNA methyltransferase inhibitorsinclude inhibitors of DNMT1 and DNMT3.
- RuxolitinibPI3 kinase inhibitors including PI3K-delta selective and broad spectrum PI3K inhibitors, MEK inhibitors, Cyclin Dependent kinase inhibitors, including CDK4/6 inhibitors and CDK9 inhibitors, BRAF inhibitors, mTOR inhibitors, proteasome inhibitors (e.g. Bortezomib, Carfilzomib), HDAC inhibitors (e.g. panobinostat, vorinostat), DNA methyl transferase inhibitors, dexamethasone, bromo and extra terminal family member (BET) inhibitors, BTK inhibitors (e.g.
- the inhibitor of an immune checkpoint moleculeis an inhibitor of PD-1, e.g., an anti-PD-1 monoclonal antibody.
- the anti-PD-1 monoclonal antibodyis nivolumab, pembrolizumab (also known as MK-3475), or PDR001.
- the anti-PD-1 monoclonal antibodyis nivolumab or pembrolizumab.
- the anti-PD1 antibodyis pembrolizumab.
- the inhibitor of an immune checkpoint moleculeis an inhibitor of PD-L1, e.g., an anti-PD-L1 monoclonal antibody.
- the anti-PD-L1 monoclonal antibodyis atezolizumab, durvalumab, or BMS-935559.
- the inhibitor of an immune checkpoint moleculeis an inhibitor of CTLA-4, e.g., an anti-CTLA-4 antibody.
- the anti-CTLA-4 antibodyis ipilimumab.
- the agentis an alkylating agent, a proteasome inhibitor, a corticosteroid, or an immunomodulatory agent.
- an alkylating agentinclude cyclophosphamide (CY), melphalan (MEL), and bendamustine.
- the proteasome inhibitoris carfilzomib.
- the corticosteroidis dexamethasone (DEX).
- the immunomodulatory agentis lenalidomide (LEN) or pomalidomide (POM).
- reaction mixturewas slowly warmed until reaching room temperature. Then the reaction mixture cooled to -78 °C.
- Difluoromethyl trifluoromethanesulfonate(5.06 mL, 39.9 mmol) was added dropwise to the reaction mixture at -78 °C.
- the reaction mixturewas allowed to slowly warm to room temperature and stirred overnight.
- the product mixturewas diluted with a saturated ammonium chloride aqueous solution (200 mL) and stirred for 10 minutes. The diluted product mixture was transferred to a separatory funnel and extracted with EtOAc (200 mL). The organic layer was washed with a saturated sodium chloride aqueous solution (2 x 200 mL).
- Step 5(6aS,8R)-8-(Benzyloxy)-2-chloro-6a-(difluoromethyl)-6a,7,8,9-tetrahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one and (6aR,8R)-8-(benzyloxy)-2-chloro-6a- (difluoromethyl)- 6(5H)-one [00237] Trifluoroacetic acid (7.36 mL, 96.1 mmol) was added to a stirring solution of tert- butyl (4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl)carbamoyl)-2-(difluoromethyl)- pyrrolidine-1-carboxylate (5.4 g, 9.61 mmol) at room temperature.
- the reaction mixturewas - 55 - 4860-6199-1670.2 105807.001049 – PCT Application stirred for 5 hours.
- the product mixturewas concentrated under reduced pressure.
- the residue obtainedwas dissolved in acetonitrile (15 mL).
- N,N-Diisopropylethylamine (8.37 mL, 48.1 mmol)was added to the stirring reaction mixture.
- the reaction mixturewas heated to 80 °C and stirred for 16 hours.
- the product mixturewas concentrated under reduced pressure.
- the reaction mixturewas heated to 55 °C and stirred for 1.5 hours.
- the reaction mixturewas cooled to room temperature and borane dimethyl sulfide complex (2.0 M in THF, 1.0 mL, 2.0 mmol) was added.
- the reaction mixturewas heated to 55 °C and stirred for 1.5 hours.
- the reaction mixturewas cooled to 0 °C and quenched by slow addition of MeOH (9 mL).
- the reaction mixturewas concentrated.
- the residue obtainedwas dissolved in EtOH (14.5 mL).
- Acetic acid (2.02 mL, 35.4 mmol) and sodium cyanoborohydride (270 mg, 4.3 mmol)were added in sequence to the reaction mixture.
- the reaction mixturewas heated to 70 °C and stirred overnight.
- the product mixturewas cooled to 0 °C and quenched by slow addition of a saturated sodium bicarbonate aqueous solution (60 mL).
- the diluted product mixturewas extracted with DCM (3 x 60 mL).
- the combined organic layerswere dried over Na2SO4, filtered, and concentrated under reduced pressure.
- the residue obtainedwas purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain (6aR,8R)-8-(benzyloxy)-2-chloro-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (607 mg, 93%).
- reaction mixturewas stirred for 10 minutes.
- the reaction mixture solutionwas added to a stirring solution of (6aR,8R)-2-chloro-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (1.0 g, 3.61 mmol) in a mixture of THF (9 mL) and DCM (16.5 mL).
- the combined reaction mixturewas stirred for 30 minutes at room temperature.
- the product mixturewas diluted with a saturated sodium bicarbonate aqueous solution (100 mL).
- the reaction mixturewas stirred for 30 minutes.
- the product mixturewas diluted with water (30 mL), a saturated sodium carbonate aqueous solution (30 mL), and a saturated sodium chloride aqueous solution (30 mL).
- the diluted product mixturewas extracted with a 3:1 chloroform:isopropanol solution (6 x 100 mL).
- the combined organic layerswere dried over MgSO4, filtered, and concentrated under reduced pressure.
- the solid obtainedwas suspended in a 10% MeOH/DCM solution (10 mL) and filtered.
- Step 1Methyl 5-bromo-2-(dibromomethyl)benzoate [00247] A suspension of methyl 5-bromo-2-methylbenzoate (6.08 g, 26.6 mmol), NBS (14.0 g, 79.7 mmol), and benzoyl peroxide (0.32 g, 1.33 mmol) in DCE (60.0 mL) was stirred at 80 °C for 18 h.
- the reaction mixturewas cooled to ambient temperature, diluted with DCM (140 - 58 - 4860-6199-1670.2 105807.001049 – PCT Application mL), washed with 10% Na 2 S 2 O 3 solution (100 mL), saturated NaHCO 3 solution (100 mL), water (100 mL), and brine (100 mL), then dried over Na2SO4, filtered and concentrated. The residue was dried under high vacuum to give the desired product, methyl 5-bromo-2-(dibromomethyl)- benzoate (9.875 g, 96.1%), as light yellow solid.
- Step 2Methyl 5-bromo-2-formylbenzoate
- Silver nitrate(6.57 g, 38.7 was in water (60.0 mL). This solution was added dropwise into the stirred solution of methyl 5-bromo-2-(dibromomethyl)benzoate (5.99 g, 15.5 mmol) in IPA (60.0 mL) at 0 °C.
- Step 3tert-Butyl 4-(4-formyl-3-methoxycarbonylphenyl)-3,6-dihydro-2H-pyridine-1- carboxylate
- N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester5.55 g, 17.9 mmol
- methyl 5-bromo-2-formylbenzoate(2.91 g, 12.0 mmol)
- Pd(dppf)Cl2(0.44 g, 0.6 mmol)
- CsOAc(9.18 g, 47.8 mmol) in 1,4-dioxane (40.0 mL) and water (10.0 mL) was heated at 95 °C under N 2 atmosphere for 1.5 h, when HPLC indicated the full conversion of the starting material.
- the reactionwas cooled to ambient temperature and partitioned between EtOAc and water (100 mL each). The organic layer was separated, and the aqueous layer extracted with EtOAc (50 mL ⁇ 3). The combined organic phase was washed with water and brine (100 mL each), dried over Na 2 SO 4 , filtered and concentrated.
- Step 4tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin- 5-yl)-3,6-dihydropyridine-1(2H)-carboxylate [00253] To a suspension tert-butyl 4-(4-formyl-3-methoxycarbonylphenyl)-3,6-dihydro-2H- pyridine-1-carboxylate (2.88 g, 8.34 mmol) and H-Glu(OMe)-NH 2 hydrochloride (2.05 g, 10.4 mmol) in DCM (40 mL) was slowly added DIPEA (4.36 mL, 25.0 mmol) and the resulting mixture was stirred at ambient temperature for 30 min.
- Step 5tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)-3,6-dihydro- pyridine-1(2H)-carboxylate [00255] tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin-5-yl)- 3,6-dihydropyridine-1(2H)-carboxylate (1.01 g, 2.20 mmol) in THF (15.0 mL) was added t- BuOK (1.0 M in THF,, 2.75 mmol, 2.75 mL) dropwise at -78 °C.
- the resulting mixturewas stirred at -78 °C for 2 h.
- the reactionwas quenched by the addition of 1 N HCl solution until pH 6 and extracted with DCM (50 mL ⁇ 3).
- the combined organic phasewas washed with brine (50 - 60 - 4860-6199-1670.2 105807.001049 – PCT Application mL), dried over Na 2 SO 4 , filtered and concentrated.
- the residuewas suspended in a minimum amount of DCM (2.00 mL) and MTBE (20.0 mL) was added at 0 °C.
- the white precipitatewas filtered off and washed with cold MTBE.
- Step 7(S)-3-(1-oxo-6-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6- dione;hydrochloride [00259] tert-Butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-1- carboxylate (809 mg, 1.89 mmol) was suspended in 1,4-dioxane (2.00 mL) and 4 N HCl in 1,4- dioxane (15.0 mL) was added at 0 °C. The reaction was stirred at ambient temperature for 18 h.
- Step 1tert-butyl 4-(4-cyano- piperazine-1-carboxylate [00261] To a solution of methyl 2-cyano-5-fluorobenzoate (20.0 g, 112 mmol) and tert-butyl 1- piperazinecarboxylate (31.2 g, 167 mmol) in NMP (20.0 mL) was added N,N-diisopropyl- ethylamine (0.01 mL, 0.04 mmol) and heated to 120 °C for 4 h.
- Step 2tert-butyl 4-(4-formyl-3-(methoxycarbonyl)phenyl)piperazine-1-carboxylate
- tert-butyl 4-(4-cyano-3-methoxycarbonylphenyl)piperazine-1- carboxylate8.10 g, 23.5 mmol
- pyridine11.4 mL, 141 mmol
- acetic acid8.05 mL, 141 mmol
- sodium phosphinate monohydrate4.97 g, 46.9 mmol
- water10.0 mL
- Step 3tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin- 5-yl)piperazine-1-carboxylate [00265] To a solution of tert-butyl 4-(4-formyl-3-methoxycarbonylphenyl)piperazine-1- carboxylate (4.20 g, 12.0 mmol) in DCM (50.0 mL) was added N,N-diisopropylethylamine (5.25 mL, 30.1 mmol) and methyl (4S)-4,5-diamino-5-oxopentanoate (2.32 g, 14.5 mmol).
- the reactionwas stirred at RT for 1 h and acetic acid (6.89 mL, 121 mmol) was added to this solution. The reaction was further stirred for another hour and sodium triacetoxyborohydride (7.66 g, 36.2 mmol) was added. The reaction was stirred overnight to see full conversion, monitored by HPLC. The reaction mixture was diluted with DCM (30.0 mL) and quenched with water (30.0 mL). Further extraction was done by DCM (30 mL x 2). The organic phase was dried over Na2SO4, filtered, and concentrated to obtain the crude.
- Step 4tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperazine- 1-carboxylate
- tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3- oxoisoindolin-5-yl)piperazine-1-carboxylate(3.80 g, 8.25 mmol) in THF (80.0 mL), was added potassium t-butoxide (1.0 M in THF, 9.90 mL, 9.90 mmol) at -78°C.
- the reactionwas - 63 - 4860-6199-1670.2 105807.001049 – PCT Application stirred at -78°C for 3 h.
- the reaction temperaturewas maintained at 0 °C and quenched by addition of 1N HCl to achieve pH 3.
- NaHCO3(sat. aq.) was added dropwise slowly to achieve pH ⁇ 6.
- the reaction mixturewas then diluted further by DCM and washed with water. Water phase was further extracted by DCM. Organic phase was collected, dried over Na 2 SO 4 , filtered and concentrated to obtain the crude.
- Step [00269]To a round bottomed flask containing tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3- oxoisoindolin-5-yl)piperazine-1-carboxylate (4.3 g, 10.0 mmol) was added 4N HCl in dioxane (34.0 mL, 136 mmol) at 0 °C. The solution was stirred at 0 °C to RT for 3 h to see full conversion.
- Step 5methyl (S)-5-amino-4-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin- 2-yl)-5-oxopentanoate [00279] To a solution of methyl 5-[4-(dimethoxymethyl)piperidin-1-yl]-2-formylbenzoate (450.0 mg, 1.4 mmol) in DCM (2 mL) and DMF (2 mL) was added N,N-diisopropylethylamine (0.61 mL, 3.5 mmol) and methyl (S)-4,5-diamino-5-oxopentanoate (269 mg, 1.68 mmol).
- Step 6(S)-3-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione
- a 1.0 M solution of potassium t-butoxide (8.30 mL, 8.30 mmol) in THFwas added to a solution of methyl (S)-5-amino-4-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)-5-oxopentanoate (3.00 g, 6.92 mmol) in THF (80.0 mL) at -78 °C and the reaction was stirred for 3 hours.
- Step 7(S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde
- 2,2,2-Trifluoroacetic acid(2.38 mL, 31.1 mmol) was added to a solution of (S)-3-(6- (4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (500 mg, 1.25 mmol) in a 3 to 1 mixture of DCM (9.3 mL) and acetone (3.1 mL).
- the reaction mixturewas stirred at 55 °C for ⁇ 48 h, after which additional BH3 ⁇ Me2S (2 M in THF; 2.5 mL, 5 mmol) was added.
- the reactionwas stirred at 55 °C for 3 h then cooled, quenched with MeOH, and concentrated.
- the residuewas dissolved in EtOH (40 mL) then cooled to 0 °C and treated with AcOH (6.3 mL, 105 mmol) then NaBH3CN (0.8 g, 12.7 mmol) in two portions.
- the reactionwas warmed to room temperature then stirred at 70 °C for 6 h, after which it was cooled to room temperature and concentrated.
- the residuewas diluted with DCM and sat.
- Step 2(6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-ol
- 6aR,8R-8-(benzyloxy)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]
- pyrazino[2,3-c]pyridazine(1.2 g, 3.5 mmol) in DCM (35 mL) at 0 °C was added BCl3 (1 M in - 68 - 4860-6199-1670.2 105807.001049 – PCT Application DCM; 10 mL, 10 mmol).
- Step 3(6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-yl 4-nitrobenzoate [00289] To a mixture of (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1’,2’:4,5] pyrazino[2,3-c]pyridazine-8-ol (200 mg, 0.79 mmol), 4-nitrobenzoic acid (262 mg, 1.57 mmol), and polymer-supported PPh 3 (100-200 mesh, ⁇ 1.6 mmol/g loading; 735 mg, 1.18 mmol)
- Step 4(6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-ol
- 6aR,8S-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-yl 4-nitrobenzoate (1.16 g, 2.86 mmol) in MeOH (8 mL) and THF (16 mL) was added NaOH (802 mg, 1.18 mmol) in water (9 mL).
- reaction mixturewas stirred at ⁇ 78 °C for 1 h, after which ethyl iodide (1.9 mL, 23.6 mmol) was added.
- the reactionwas stirred at ⁇ 78 °C for 5 min then allowed to warm to room temperature with stirring. After 3.5 h, the reaction was quenched with MeOH (55 mL).
- MeOH55 mL
- a solution of NaOH (1.91 g, 47.7 mmol) in water (55 mL)was added and the mixture was stirred at room temperature for 20 min then poured into water. The aqueous layer was extracted with EtOAc (3 x).
- Step 2(4R)-4-(Benzyloxy)-1-(tert-butoxycarbonyl)-2-ethylpyrrolidine-2-carboxylic acid
- 4-R4-(Benzyloxy)-1-(tert-butoxycarbonyl)-2-ethylpyrrolidine-2-carboxylic acid
- To 1-(tert-butyl) 2-methyl (4R)-4-(benzyloxy)-2-ethylpyrrolidine-1,2-dicarboxylate (2.72 g, 7.48 mmol) in 1:1 THF/MeOH (40 mL)was added a solution of NaOH (2.99 g, 74.8 mmol) in water (20 mL).
- the reactionwas stirred at 75 °C for 2.5 h then allowed to cool to room temperature.
- the mixturewas poured into water then extracted with MTBE (3 x).
- Step 3tert-Butyl (2R,4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3- yl)carbamoyl)-2-ethylpyrrolidine-1-carboxylate - 71 - 4860-6199-1670.2 105807.001049 – PCT Application [00298] To crude (4R)-4- - -2-ethylpyrrolidine-2-carboxylic acid (672 mg, 1.43 mmol) and N,N-diisopropylethylamine (1.21 mL, 6.92 mmol) in MeCN (10 mL) was added HATU (880 mg, 2.31 mmol).
- Step 4(6aR,8R)-8-(Benzyloxy)-2-chloro-6a-ethyl-6a,7,8,9- tetrahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-6(5H)-one [00300] To tert-butyl (2R,4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl)carbamoyl)- 2-ethylpyrrolidine-1-carboxylate (473 mg, 0.88 mmol) in DCM (17 mL) was added TFA (17 mL, 222 mmol).
- the reactionwas stirred at 65 °C and monitored by LC-MS. After 1.5 hours, the reaction was cooled and filtered to remove the triphenylphosphine, then rinsed with THF (20 mL) then methanol (40 mL). To the filtrate was added potassium carbonate (815 mg, 5.90 - 74 - 4860-6199-1670.2 105807.001049 – PCT Application mmol), and the suspension was allowed to stir for 2 hours. The reaction was diluted with DCM and sat. NaHCO3 (aq). The organic layer was separated, and the water layer was extracted with DCM (1 x) then EtOAc (1 x). The organic layers were combined, dried with sodium sulfate, filtered, and condensed.
- Step 2(6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-amine [00314] To (6aR,8R)-8-azido-2-chloro-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazine (117 mg, 0.42 mmol) in THF (5 mL) was added PPh 3 (220 mg, 0.84 mmol).
- the mixturewas dissolved in 1,4- dioxane (6.3 mL) and water (1.26 mL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 7 hours.
- the product mixturewas diluted with water (30 mL) and a saturated sodium chloride aqueous solution (40 mL). The diluted product mixture was extracted with DCM (3 x 60 mL). The combined organic layers were dried over Na 2 SO 4 , filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-5% MeOH/DCM to obtain the title compound (151 mg, 69%).
- the mixturewas dissolved in 1,4-dioxane (5 mL) and water (0.5 mL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 16 hours.
- the product mixturewas diluted with water (30 mL) and a saturated sodium chloride aqueous solution (40 mL). The diluted product mixture was extracted with DCM (3 x 60 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure.
- the reaction mixturewas warmed to room temperature and stirred for 1 hour.
- the reaction mixturewas cooled to 0 °C and triethylamine (202 ⁇ L, 1.45 mmol) and methanesulfonyl chloride (56 ⁇ L, 0.72 mmol) were added in sequence.
- the reaction mixturewas warmed to room temperature and stirred for 30 minutes.
- the product mixturewas diluted with a saturated sodium bicarbonate aqueous solution (30 mL).
- the diluted product mixturewas extracted with DCM (3x40 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure.
- the reaction mixturewas warmed to room temperature and stirred for 1 hour.
- the reaction mixturewas cooled to 0 °C and triethylamine (101 ⁇ L, 0.72 mmol) and methanesulfonyl chloride - 86 - 4860-6199-1670.2 105807.001049 – PCT Application (28 ⁇ L, 0.36 mmol) were added in sequence.
- the reaction mixturewas warmed to room temperature and stirred for 30 minutes.
- the product mixturewas diluted with a saturated sodium bicarbonate aqueous solution (30 mL).
- the diluted product mixturewas extracted with DCM (3x40 mL). The combined organic layers were dried over Na 2 SO 4 , filtered, and concentrated under reduced pressure.
- the reaction mixturewas heated to 60 °C and stirred for 16 hours.
- the product mixturewas diluted with a saturated sodium bicarbonate aqueous solution (35 mL).
- the diluted product mixturewas extracted with a 3:1 chloroform:isopropanol solution (3 x 35 mL).
- the combined organic layerswere dried over MgSO4, filtered, and concentrated under reduced pressure.
- reaction mixturestirred at room temperature for 30 minutes.
- Sodium triacetoxyborohydride(11.6 mg, 0.055 mmol) was added to the reaction mixture at room temperature.
- the reaction mixturewas heated to 35 °C and stirred for 2 hours.
- the reaction mixturewas cooled to room temperature and diluted with DMSO (4 mL).
- the diluted reaction mixturewas filtered and directly purified by prep-HPLC (Waters CSH-C18, 5 ⁇ M, 30x100mm, 8.5-28.5% MeCN/water (containing 0.2%TFA) over 5 min) to give the title compound (9.3 mg, 50%) as its TFA salt.
- Step 2(6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine
- Diisopropyl azodicarboxylate37.4 ⁇ L, 0.19 mmol
- 6aR,8S-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol
- Intermediate 135.0 mg, 0.13 mmol
- 6-vinylquinolin-2-ol(32.5 mg, 0.19 mmol)
- polymer-supported PPh3100-200 mesh
- Step 32-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)-5,6,6a,7,8,9 hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c] 2- -6-fluorophenol [00355] To (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (47 mg, 0.1 mmol) in 1,4-dioxane (1.6 mL) - 90 - 4860-6199-1670.2 105807.001049 – PCT Application and water (0.16 mL) was added 3-fluoro-2-hydroxyphen
- Step 42-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinoline-6-carbaldehyde
- Osmium tetroxide4% in water; 62.9 ⁇ L, 0.009 mmol
- 2,6-lutidine(22.9 ⁇ L, 0.19 mmol) were added to a solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl) oxy)-5,6,6a,7,8,9 he
- Step 4(S)-3-(6-(1-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6-yl)methyl) piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 91 - 4860-6199-1670.2 105807.001049 – PCT Application [00359] To 2-(( - - -5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinoline-6-carbaldehyde (50.0 mg, 0.09
- Examples 9-10 shown below in Table 4were prepared as TFA salts by the method used in preparing Example 8, utilizing the appropriate starting materials and intermediates. Table 4. Examples 9-10 d E ) + 2 oxoisoindolin-2-yl)piperidine-2,6-dione - 92 - 4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found E ) + Example 11.
- Step 2(S)-3-(6-(1-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-2-yl)methyl) piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 93 - 4860-6199-1670.2 105807.001049 – PCT Application [00364] This was in Example 8, using 2-vinylquinolin-6-ol instead of 6-vinylquinolin-2-ol.
- the reactionwas stirred at 85 °C overnight.
- the reactionwas cooled to room temperature, diluted with DCM, washed with a saturated solution of brine, then dried over sodium sulfate, filtered and concentrated under reduced pressure.
- the crude materialwas purified via SiO 2 FCC: 0-5% MeOH/DCM to obtain 6-vinyl-1,8-naphthyridin-2-ol (99 mg, 26% yield).
- Step 3(6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine
- Step 42-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo 2-yl)-6-fluorophenol [00372] To (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (30 mg, 0.07 mmol) in 1,4- - 95 - 4860-6199-1670.2 105807.001049 – PCT Application dioxane (1.6 mL) and water (0.16 mL) was added 3-fluoro-2-hydroxyphenyl
- Step 6(S)-3-(6-(1-((7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridin-3- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 96 - 4860-6199-1670.2 105807.001049 – PCT Application [00376] To 7-(( - - -5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-n
- Step 2tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroisoquinoline-2(1H)-carboxylate
- Diisopropyl azodicarboxylate64 ⁇ L, 0.33 mmol
- (6aR,8S)- 2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin- 8-olIntermediate 1; 60.0 mg, 0.22 mmol), tert-butyl 6-hydroxy-3,4-dihydr
- Step 3tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinoline-2(1H)-carboxylate [00382] To tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl
- Step 42-((6aR,8R)-6a-(difluoromethyl)-8-((1,2,3,4-tetrahydroisoquinolin-6-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00384] To a solution of tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro- isoquinoline-2(1H)-carboxylate (51.6 mg, 0.09 mmol) in DCM (1.0 m
- Step 4(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl) 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00386] 2-((6aR,8R)-6a-(difluoromethyl)
- Step 2tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4-dihydroisoquinoline-2(1H)- carboxylate
- Diisopropyl azodicarboxylate37.4 ⁇ L, 0.19 mmol
- (6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol(35.0 mg, 0.13 mmol), tert-butyl 6-hydroxy-1-methyl-3,4-d
- Step 3tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinoline-2(1H)-carboxylate [00394] To tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl
- Step 42-((6aR,8R)-6a-(difluoromethyl)-8-((1-methyl-1,2,3,4-tetrahydroisoquinolin-6- yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00396] To a solution of tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8
- Step 5(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00398] 2-((6aR,8R)-6a-(Difluoromethyl)-8-((1-methyl-1,2,3,4-tetrahydroisoquinolin-6- yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazin
- Step 2tert-butyl 2-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9 hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate - 104 - 4860-6199-1670.2 105807.001049 – PCT Application [00403] Diisopropyl azodicarboxylate (22 ⁇ L, 0.11 mmol) was added to a solution of (6aR,8S)- 2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin- 8-ol
- Step3tert-butyl 2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridine-6(5H)-carboxylate [00405] To tert-butyl 2-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9 hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6-naphthyridine- 6(5H)-carboxylate (35 mg,
- Step 5(S)-3-(6-(4-((2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00409] 2-Chloro-6-((6aR,8R)-6a-(difluoromethyl)-8-((5,6,7,8-tetrahydro-1,6-naphthyridin-2- yl) oxy)-5,6,6a,7,8,9-hexahydropyrrol
- Step 2(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro-2,7- naphthyridin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00414] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 6-hydroxy-3,4-dihydro-2,7-naphthyridine-2(1H)-carboxylate instead of tert- butyl 2-hydroxy-7,8-dihydro-1,6-
- Step 2(S)-3-(6-(4-((5-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)isoindolin-2- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00418] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 5-hydroxyisoindoline-2-carboxylate instead of tert-butyl 2-hydroxy-7,8- dihydro-1,6-naphthyridine-6(5H)-carboxy
- Step 2(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 109 - 4860-6199-1670.2 105807.001049 – PCT Application [00422] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 6-hydroxy-5-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-but
- Step 2(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 110 - 4860-6199-1670.2 105807.001049 – PCT Application [00426] This was in Example 18, using tert-butyl 6-hydroxy-7-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-butyl 2-hydroxy-7,8-dihydro
- Step 2(3S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,3-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00430] This example was synthesized by procedures analogous to that described in Example 16, using tert-butyl 6-hydroxy-1,3-dimethyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1
- the absolute configurationwas arbitrarily assigned.
- Step 2(S)-3-(6-(4-(((R)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2- hydroxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1- methyl-3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione [00434] This example was synthesized by procedures analogous to that described in Example 16, using tert-butyl (R)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of (racemic) tert-butyl 6-hydroxy-1-methyl-3,4
- Step 2tert-butyl (2-(6- [00439] A solution of tert-butyl (2-(6-((tert-butoxycarbonyl)oxy)pyridin-2-yl)ethyl)carbamate (4.0 g, 11.8 mmol) in 1:1 Methanol/THF (22 mL) was added 1N Sodium hydroxide (35.5 mL, 35.5 mmol) was stirred vigorously at RT for 2 hours.1N Hydrochloric acid (ca 35 mL) was added until pH turned 7.
- Step 3tert-butyl (2-(3-bromo-6-hydroxypyridin-2-yl)ethyl)carbamate
- a solution of tert-butyl carbamate (2.4 g, 10.1 mmol) in anhydrous acetonitrile (50.0 mL)was cooled to 0 °C.
- N-Bromosuccinimide (1.77 g, 10.1 mmol)was added and stirred for 10 minutes and then stirred at RT for 15 minutes. Reaction mixture was diluted with water and extracted with 3:1 chloroform/isopropanol (3x 100 mL).
- Step 45-methyl-5,6,7,8-tetrahydro-1,6-naphthyridin-2-ol
- Step 5tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate - 115 - 4860-6199-1670.2 105807.001049 – PCT Application [00445] To a solution of 5- 2-ol (0.5 g, 2.5 mmol) in anhydrous THF (25.0 mL) were added N,N-diisopropylethylamine (2.17 mL, 12.4 mmol), di- tert butyl dicarbonate (1.63 g, 7.5 mmol) and DMAP (30.4 mg, 0.25 mmol).
- Step 6(3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dion [00447] This compound was synthesized by procedures analogous to that described in Example 16, using tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate instead of tert-butyl 6-hydroxy-1-methyl-3,4-
- the reactionwas cooled to RT and neutralized using 1N hydrochloric acid (aq) until pH turned 8 and extracted into ethyl acetate.
- the aqueous layercontains product and organic impurities were in ethyl acetate layer.
- the aqueous layerwas basified to pH 10 using saturated sodium carbonate (aq) and extracted with 3:1 chloroform/iPrOH.
- the organic layerwas dried over anhydrous sodium sulfate, concentrated to give 1,1-dimethyl-3,4-dihydro-2H- isoquinolin-6-ol (320 mg, 69% yield) as pale brown solid.
- Step 2(6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4-tetrahydro- isoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine
- Diisopropylwas added to a solution of (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (Intermediate 1; 25.0 mg, 0.09 mmol), 1,1-dimethyl-1,2,3,4- tetrahydr
- Step 4(S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,1-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00456] To a solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4-tetrahydro- isoquinolin-6
- Methyl 1-[(4R,6S)- - 2,8,10,11-tetrazatricyclo- [7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carboxylate(65.0 mg, 0.14 mmol) was dissolved in THF (2 mL) and cooled in an ice bath.
- Step 26-vinyl-3H-[1,2,3]triazolo[4,5-b]pyridine
- Step 3(6aR,8R)-2-chloro-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine - 124 - 4860-6199-1670.2 105807.001049 – PCT Application [00479] A mixture of (6aR,8S)-2- -5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (30.0 mg, 0.12 mmol), triphenylphosphine (45.63 mg, 0.17 mmol), 6-vinyl-3H-[1,2,3]triazolo[4,5-b]pyridine (27.1 mg, 0.
- Step 42-fluoro-6-((6aR,8R)-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol [00481] A mixture of (6aR,8R)-2-chloro-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (40.0 mg, 0.1 mmol), 3-fluoro-2-hydroxyphenylboronic acid (48.4 mg, 0.31 mmol), 3-fluor
- Step 53-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo[4,5-b]pyridine-6- carbaldehyde - 125 - 4860-6199-1670.2 105807.001049 – PCT Application [00483] To a mixture of 2- - -8-(6-vinyl-3H-[1,2,3]triazolo- [4,5-b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2- yl)phenol (12.0 mg, 0.03 mmol) and sodium periodate (1
- Step 6(S)-3-(6-(4-((3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo[4,5- b]pyridin-6-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00485] To a mixture of (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2-yl)piperidine-2,6-dione hydrochloride (2.83 mg, 0.01 mmol) and 3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a
- Step 2(S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin-1-yl)methyl) piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 128 - 4860-6199-1670.2 105807.001049 – PCT Application [00491] A mixture of 2-((6aR,8R)-6a-ethyl-8-(methyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,
- the reactionwas stirred 1-2 h, at which point no conversion was observed.
- the reactionwas purified via prep-HPLCMS (Waters CSH-C18, 5 ⁇ m, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the starting material 2-((6aR,8R)-6a-ethyl-8-(methyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (14.7 mg, 0.019 mmol).
- Step 2(6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine [00499] To (6aR,8R)-8-azido-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (368 mg, 0.834 mmol) in THF (10 mL) was added PPh3 (437 mg, 1.67 mmol) The reaction was stirred at 70 °C for 1 h then treated with water (2 mL).
- reactionwas stirred for 13 h at 70 °C then allowed to cool slowly to room temperature and subsequently poured into water.
- the aqueous layerwas extracted with DCM (3 x) and the combined organic layers were washed with 2 N HCl (aq).
- Step 3tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate [00501] To a briefly sonicated mixture of (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (HCl salt; 26 mg, 0.063 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (21 mg, 0.11 mmol) and Et3N (26 ⁇ L
- Step 42-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00503] To tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (10 mg, 0.13 m
- Examples 39-40[00506] Examples 39 and 40 shown below in Table 5 were prepared as TFA salts by analogous methods used in preparing Example 38, utilizing the appropriate starting materials and intermediates.
- the asterisk (*) in Example 40indicates an arbitrarily assigned stereocenter (absolute configuration at the stereocenter denoted by * was not confirmed). Table 5. Examples 39-40 - 133 - 4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found ) + 4 4 y p p , Example 41.
- Step 2(S)-3-(6-(4-((4-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidin-1-yl)methyl) piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00510] To 2-chloro-6-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexa
- Step 2(S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00514] To 2-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo-[1',
- Step 5tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate - 137 - 4860-6199-1670.2 105807.001049 – PCT Application [00518] To a briefly - -5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (56 mg, 0.17 mmol), tert-butyl 4- oxopiperidine-1-carboxylate (57 mg,
- Step 6tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate [00520] To tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (113 mg, 0.17 mmol
- Step 72-((6aR,8R)-8-(ethyl(piperidin-4-yl)amino)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00522] A mixture of tert- - -5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate (79 mg,
- Step 8(S)-3-(6-(4-((4-(ethyl((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a- (fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8- yl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00524] To 2-((6aR,8R)-8-(ethyl(piperidin-4-yl)amino)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydr
- Examples 44-45 shown below in Table 6were prepared as TFA salts by analogous methods used in preparing Example 43, utilizing the appropriate starting materials and intermediates. Table 6. Examples 44-45 Calcd. Found ) + 4 4 yl)piperidine-2,6-dione Example 46.
- Step 2tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl) amino)piperidine-1-carboxylate - 142 - 4860-6199-1670.2 105807.001049 – PCT Application [00533] A mixture of tert- - hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl)amino)piperidine-1- carboxylate (11 mg, 0.021 mmol), (3-fluoro-2-hydroxyphenyl)boronic acid
- Step 32-((6aR,8R)-6a-ethyl-8-(piperidin-4-yl((tetrahydrofuran-2-yl)methyl)amino)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00535] A solution of tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2
- Step 4(3S)-3-(6-(4-((4-((((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)-piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00537] A mixture of 2-((6aR,8R)-6a-ethyl-8-(piperidin-4-yl((tetrahydrofuran-2-yl)methyl)- amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[
- the reactionwas stirred at room temperature for 30 min, after which additional AcOH (6.0 ⁇ L, 0.11 mmol) and NaBH(OAc)3 (13 mg, 0.063 mmol) were added.
- the reactionwas stirred for a further 20 min, then was diluted with MeCN and water, filtered through a 0.2 ⁇ m PTFE syringe filter, and purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 ⁇ m, 30x100mm, 7.5-27.5% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (0.9 mg, 3.6%) as a white solid.
- Examples 48-49[00538] Examples 48-49 shown below in Table 7 were prepared as TFA salts by analogous methods used in preparing Example 47, utilizing the appropriate starting materials and intermediates. Table 7. Examples 48-49 d ) + 4 , dione - 144 - 4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found ) + Example 50.
- Lithium aluminum mg, THF)was added dropwise to a solution of methyl 1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5- carboxy
- Examples 55-57[00552] Examples 55-57 shown below in Table 9 were prepared as TFA salts by analogous methods used in preparing Example 54, utilizing the appropriate starting materials and intermediates. Table 9. Examples 55-57 + yl)piperidine-2,6-dione - 149 - 4860-6199-1670.2 105807.001049 – PCT Application Example A.
- SMARCA2 HiBiT and SMARCA4 HiBiT Degradation AssayPreparation of SMARCA2/4-HiBiT knock-in cells
- HiBiT peptide knock-in of SMARCA2 in LgBiT expressing HEK293T cellswas performed by CRISPR-mediated tagging system as described Promega. The homozygous HiBiT knock-in on c-terminus SMARCA2 was confirmed by sanger sequence.
- SMARCA2-HiBiT knock-in Hela monoclonal cell (CS302366) and SMARCA4-HiBiT knock-in Hela monoclonal cell (CS3023226)were purchased from Promega.
- Compoundswere dissolved in DMSO to make 10 mM stock and 3-fold series dilutions were further conducted keeping the highest concentration 10 ⁇ M.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
105807.001048 BRM TARGETING COMPOUNDS AND ASSOCIATED METHODS OF USE CROSS REFERENCE TO RELATED APPLICATION [0001] This application which claims benefit under 35 U.S.C. § 119(e) of Provisional U.S. Patent Application No.63/596,637, filed November 7, 2023, the contents of which are hereby incorporated by reference in its entirety. TECHNICAL FIELD [0002] The description provides bifunctional compounds comprising a target protein binding moiety and a E3 ubiquitin ligase binding moiety, and associated methods of use. The bifunctional compounds are useful as modulators of targeted ubiquitination, especially with respect to Switch/Sucrose Non-Fermentable (SWI/SNF)-Related, Matrix-Associated, Actin- Dependent Regulator of Chromatin, Subfamily A, Member 2 (SMARCA2) (i.e., BRAHMA or BRM), which are degraded and/or otherwise inhibited by bifunctional compounds according to the present disclosure. BACKGROUND [0003] The human SWItch/Sucrose Non-Fermentable (SWI/SNF) complexes are ATP- dependent chromatin remodelers. These large complexes play important roles in essential cellular processes, such as transcription, DNA repair and replication by regulating DNA accessibility. [0004] Mutations in the genes encoding up to 20 canonical SWI/SNF subunits are observed in nearly 20% of all human cancers with the highest frequency of mutations observed in rhabdoid tumors, female cancers (including ovarian, uterine, cervical and endometrial), lung adenocarcinoma, gastric adenocarcinoma, melanoma, esophageal, and renal clear cell carcinoma. [0005] SMARCA2 (BRM) and SMARCA4 (BRG1) are the subunits containing catalytic ATPase domains and they are essential for the function of SWI/SNF in perturbation of histone- DNA contacts, thereby providing access points to transcription factors and cognate DNA elements that facilitate gene activation and repression. [0006] SMARCA2 and SMARCA4 shares a high degree of homology (up to 75%). SMARCA4 is frequently mutated in primary tumors (i.e., deleted or inactivated), particularly in lung cancer (12%), melanoma, liver cancer and pancreatic cancer. SMARCA2 is one of the top essential genes in SMARCA4-mutant (deleted) cancer cell line. This is because SMARCA4 deleted cancer cells exclusively rely on SMARCA2 ATPase activity for their chromatin remodeling activity for cellular functions such as cell proliferation, survival and growth. Thus, 105807.001049 – PCT Application targeting SMARCA2 may be promising therapeutic approach in SMARCA4-related or deficient cancers (genetic synthetic lethality). [0007] Previous studies have demonstrated the strong synthetic lethality using gene expression manipulation such as RNAi; downregulating SMARCA2 gene expression in SMARCA4 mutated cancer cells results in suppression of cancer cell proliferation. However, SMARCA2/4 bromodomain inhibitors (e.g., PFI-3) exhibit none to minor effects on cell proliferation inhibition [Vangamudi et al. Cancer Res 2015]. This phenotypic discrepancy between gene expression downregulation and small molecule-based approach lead us to investigating protein degradation bispecific molecules in SMARCA4 deficient cancers. [0008] SMARCA2 is also reported to play roles in multiple myeloma expressing t(4;14) chromosomal translocation [Chooi et al. Cancer Res abstract 2018]. SMARCA2 interacts with NSD2 and regulates gene expression such as PRL3 and CCND1. SMARCA2 gene expression downregulation with shRNA reduces cell cycle S phase and suppresses cell proliferation of t(4;14) MM cells. [0009] Therapeutic compounds that inhibit SMARCA2 and/or SMARCA4 are needed. SUMMARY [0010] The present disclosure is directed to compounds of Formula (I): or a pharmaceutically acceptable salt thereof; wherein R1 is halo, C1-6 alkyl, or C1-6 haloalkyl; each R2 is independently H, D, or F; each R3 is independently H, D, C1-6 alkyl, C1-6 haloalkyl, C3-6 heterocycloalkyl or C3-6 cycloalkyl; s is 1, 2 or 3; t is 1, 2, 3, or 4; R4 is H, D, C1-6 alkyl, C1-6 alkoxyalkyl, C3-6 cycloalkyl, or C1-6 haloalkyl; R5 is H, D, or F; L1 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; L2 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; - 2 -4860-6199-1670.2 105807.001049 – PCT Application ring A1 is a 9-10 membered heteroaryl group, 5-7 membered heterocycloalkyl, or a 9-10 membered heterocycloalkenyl group; ring A2 is a 3-7 membered cycloalkyl group or a 4-7-membered heterocycloalkyl group; X1 is CH2, CO, CH=CH (when X2 = CO), or N=CH (when X2 = CO); X2 is CH2, CO, CH=CH (when X1 = CO), or N=CH (when X1 = CO); wherein the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, heterocycloalkenyl group, heteroaryl group or heterocycloalkyl group is optionally substituted by one or more Rf groups; each Rf is independently D, oxo, halogen, C1-C8 alkoxy, C1-C8 alkyl, C1-6 haloalkyl, C1-6 haloalkoxy, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)RcRd, -P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; each Ra is independently H, D, -C(O)Rb, -C(O)ORc, -C(O)NRcRd, -C(=NRb)NRbRc, - C(=NORb)NRbRc, -C(=NCN)NRbRc, -P(ORc)2, -P(O)RcRb, -P(O)RcRd, -P(O)ORcORb, -S(O)Rb, - S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rb, is independently H, D, -C1-C6alkyl, -C2-C6alkenyl, -C2-C6alkynyl, C6-10aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rc or Rd is independently H, D, -C1-C10 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, -OC1- C6alkyl, -O-cycloalkyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; or Rc and Rd, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocycloalkenyl group. [0011] Stereoisomers of the compounds of Formula I, and the pharmaceutical salts and stereoisomers thereof, are also contemplated, described, and encompassed herein. Methods of using compounds of Formula I are described, as well as pharmaceutical compositions including the compounds of Formula I. - 3 -4860-6199-1670.2 105807.001049 – PCT Application DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS [0012] Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure pertains. The terminology used in the description is for describing particular embodiments only and is not intended to be limiting of the disclosure. [0013] Where a range of values is provided, it is understood that each intervening value, to the tenth of the unit of the lower limit unless the context clearly dictates otherwise (such as in the case of a group containing a number of carbon atoms in which case each carbon atom number falling within the range is provided), between the upper and lower limit of that range and any other stated or intervening value in that stated range is encompassed within the disclosure. The upper and lower limits of these smaller ranges may independently be included in the smaller ranges is also encompassed within the disclosure, subject to any specifically excluded limit in the stated range. Where the stated range includes one or both of the limits, ranges excluding either both of those included limits are also included in the disclosure. [0014] The following terms are used to describe the present disclosure. In instances where a term is not specifically defined herein, that term is given an art-recognized meaning by those of ordinary skill applying that term in context to its use in describing the present disclosure. [0015] The articles “a” and “an” as used herein and in the appended claims are used herein to refer to one or to more than one (e.g., to at least one) of the grammatical object of the article unless the context clearly indicates otherwise. By way of example, “an element” means one element or more than one element. [0016] The terms “co-administration” and “co-administering” or “combination therapy” refer to both concurrent administration (administration of two or more therapeutic agents at the same time) and time varied administration (administration of one or more therapeutic agents at a time different from that of the administration of an additional therapeutic agent or agents), as long as the therapeutic agents are present in the patient to some extent, preferably at effective amounts, at the same time. In certain preferred aspects, one or more of the present compounds described herein, are co-administered in combination with at least one additional bioactive agent, especially including an anticancer agent. In particularly preferred aspects, the co-administration of compounds results in synergistic activity and/or therapy, including anticancer activity. [0017] The term “compound”, as used herein, unless otherwise indicated, refers to any specific chemical compound disclosed herein and includes tautomers, regioisomers, geometric isomers, and where applicable, stereoisomers, including optical isomers (enantiomers) and other stereoisomers (diastereomers) thereof, as well as pharmaceutically acceptable salts and derivatives, including prodrug and/or deuterated forms thereof where applicable, in context. - 4 -4860-6199-1670.2 105807.001049 – PCT Application Deuterated small molecules contemplated are those in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by deuterium. [0018] Within its use in context, the term compound generally refers to a single compound, but also may include other compounds such as stereoisomers, regioisomers and/or optical isomers (including racemic mixtures) as well as specific enantiomers or enantiomerically enriched mixtures of disclosed compounds. The term also refers, in context to prodrug forms of compounds which have been modified to facilitate the administration and delivery of compounds to a site of activity. It is noted that in describing the present compounds, numerous substituents and variables associated with same, among others, are described. It is understood by those of ordinary skill that molecules which are described herein are stable compounds as generally described hereunder. [0019] The term “ubiquitin ligase” refers to a family of proteins that facilitate the transfer of ubiquitin to a specific substrate protein, targeting the substrate protein for degradation. For example, an E3 ubiquitin ligase protein that alone or in combination with an E2 ubiquitin- conjugating enzyme causes the attachment of ubiquitin to a lysine on a target protein, and subsequently targets the specific protein substrates for degradation by the proteasome. Thus, E3 ubiquitin ligase alone or in complex with an E2 ubiquitin conjugating enzyme is responsible for the transfer of ubiquitin to targeted proteins. In general, the ubiquitin ligase is involved in polyubiquitination such that a second ubiquitin is attached to the first; a third is attached to the second, and so forth. Polyubiquitination marks proteins for degradation by the proteasome. However, there are some ubiquitination events that are limited to mono-ubiquitination, in which only a single ubiquitin is added by the ubiquitin ligase to a substrate molecule. Mono- ubiquitinated proteins are not targeted to the proteasome for degradation but may instead be altered in their cellular location or function, for example, via binding other proteins that have domains capable of binding ubiquitin. Further complicating matters, different lysines on ubiquitin can be targeted by an E3 to make chains. The most common lysine is Lys48 on the ubiquitin chain. This is the lysine used to make polyubiquitin, which is recognized by the proteasome. [0020] As used herein, “Cereblon (CRBN) E3 Ubiquitin Ligase” refers to the substrate recognition subunit of the Cullin RING E3 ubiquitin ligase complexes. CRBN are one of the most popular E3 ligases recruited by bifunctional Proteolysis-targeting chimeras (PROTACs) to induce ubiquitination and subsequent proteasomal degradation of a target protein (Maniaci C. et al., Bioorg Med Chem.2019, 27(12): 2466-2479). [0021] As used herein, the term “alkyl”, by itself or as part of another substituent, means, unless otherwise stated, a straight or branched chain hydrocarbon radical having up to twelve - 5 -4860-6199-1670.2 105807.001049 – PCT Application carbon atoms. In some embodiments, the number of carbon atoms is designated (i.e., C1-C8 means one to eight carbons). Examples of alkyl groups include methyl, ethyl, n-propyl, iso- propyl, n-butyl, t-butyl, iso-butyl, sec-butyl, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like. Alkyl groups may be optionally substituted as provided herein. In some embodiments, the alkyl group is a C1-C6 alkyl; in some embodiments, it is a C1-C4 alkyl. [0022] When a range of carbon atoms is used herein, for example, C1-C6, all ranges, as well as individual numbers of carbon atoms are encompassed. For example, “C1-C3” includes C1-C3, C1- C2, C2-C3, C1, C2, and C3. [0023] Unless otherwise specified, the term “optionally substituted”, as used in combination with a substituent defined herein, means that the substituent may, but is not required to, have one or more hydrogens replaced with one or more suitable functional groups or other substituents as provided herein. For example, a substituent may be optionally substituted with one or more of: halo, cyano, C1-6 alkyl, C3-6 cycloalkyl, C2-6 alkenyl, C2-6 alkynyl, halo(C1-6)alkyl, C1-6 alkoxy,halo(C1-6alkoxy), C1-6alkylthio, C1-6alkylamino, NH2, NH(C1-6alkyl), N(C1-6alkyl)2, NH(C1-6alkoxy), N(C1-6 alkoxy)2, —C(O)NHC1-6 alkyl, —C(O)N(C1-6 alkyl)2, —C(O)NH2, —C(O)C1-6 alkyl, —C(O)2C1-6 alkyl, —NHCO(C1-6 alkyl), —N(C1-6 alkyl)CO(C1-6 alkyl), —S(O)C1-6 alkyl, —S(O)2C1-6 alkyl, oxo, 6-12 membered aryl, benzyl, pyridinyl, pyrazolyl, thiazolyl, isothiazolyl, or other 5 to 12 membered heteroaryl groups. In some embodiments, each of the above optional substituents are themselves optionally substituted by one or two groups.[0024] Unless otherwise specified, in those embodiments wherein the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl groupand/or heterocycloalkyl group is substituted, they can be optionally substituted. In some embodiments, these groups can be optionally substituted with 1, 2, or 3 substituentsindependently selected from -OH, -CN, amino,halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), - C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2.[0025] In some embodiments, the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl group isoptionally substituted by 1-6 groups selectedfrom D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd.[0026] In some embodiments, the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl group is optionally substituted by one or more Rf groups. - 6 -4860-6199-1670.2 105807.001049 – PCT Application[0027] In some embodiments, the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, aryl heterocycloalkenyl group, heteroaryl group and/or heterocycloalkyl group is optionally substituted by one or more R7 or R8 groups. [0028] The term “optionally substituted -CH2-,” refers to “-CH2-“ or substituted -CH2-.” A substituted -CH2- may also be referred to as -CH(substituent)- or -C(substituent)(substituent)-, wherein each substituent is independently selected from the optional substituents described herein. [0029] The term “cycloalkyl” as used herein refers to a 3-12 membered cyclic alkyl group, and includes bridged and spirocycles (e.g., adamantine). Cycloalkyl groups may be fully saturated or partially unsaturated. The term “cycloalkyl” also includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) wherein a single cycloalkyl ring (as defined above) can be condensed with one or more groups selected from heterocycles, carbocycles, aryls, or heteroaryls to form the multiple condensed ring system. Such multiple condensed ring systems may be optionally substituted with one or more (e.g., 1, 2, 3 or 4) oxo groups on the carbocycle or heterocycle portions of the multiple condensed ring. The rings of the multiple condensed ring system can be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements. It is to be understood that the individual rings of the multiple condensed ring system may be connected in any order relative to one another. It is also to be understood that the point of attachment of a multiple condensed ring system (as defined above for a cycloalkyl) can be at any position of the cycloalkylic ring. Examples of cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cycloheptyl, cyclohexyl, cycloheptyl, cyclooctyl, indenyl, bicyclo[2.2.1]heptanyl, bicyclo[3.1.1]heptanyl, bicyclo[4.1.0]heptanyl, spiro[3.3] heptanyl, and spiro[3.4]octanyl. In some embodiments, the cycloalkyl group is a 3-7 membered cycloalkyl. [0030] The term “cycloalkenyl” when used alone or as part of a substituent group refers to monocyclic or multicyclic, partially saturated ring structure having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C3-C6”). Cycloalkenyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 cycloalkenyl refers to a cycloalkenyl group having between three and six carbon atoms. The cycloalkenyl group may be attached at any carbon atom of the partially saturated ring such that the result is a stable structure. Cycloalkenyl groups include groups in which the partially saturated ring is fused to an aryl group. Examples of cycloalkenyl groups include, for - 7 -4860-6199-1670.2 105807.001049 – PCT Application example, cyclopropenyl (C3), cyclobutenyl (C4), cyclopropenylmethyl (C4), cyclopentenyl (C5), cyclohexenyl (C6), 1-methylcyclopropenyl (C4), 2-methylcyclopentenyl (C4), adamantenyl (C10), spiro[3.3]heptenyl, bicyclo[3.3.0]octenyl, indanyl, and the like. In some embodiments, cycloalkenyl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the cycloalkenyl group is substituted, the cycloalkenyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1- C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1- C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and - S(O)2N(C1-C6alkyl)2. In other embodiments, the cycloalkenyl group is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the cycloalkenyl group is optionally substituted by one or more Rf groups. [0031] The term “alkenyl” as used herein refers to C2-C12 alkyl group that contains at least one carbon-carbon double bond. In some embodiments, the alkenyl group is optionally substituted.In some embodiments, the alkenyl group is a C2-C6alkenyl. [0032] The term “akynyl” as used herein refers to C2-C12 alkyl group that contains at least one carbon-carbon triple bond. In some embodiments, the alkenyl group is optionally substituted. In some embodiments, the alkynyl group is a C2-C6 alkynyl. [0033] The terms “alkoxy,” “alkylamino” and “alkylthio”, are used in their conventional sense, and refer to those alkyl groups attached to the remainder of the molecule via an oxygen atom (“oxy”), an amino group (“amino”) or thio group. The term “alkylamino” includes mono- di- alkylamino groups, the alkyl portions can be the same or different. [0034] The term “alkoxyalkyl” as used herein refers to a linear monovalent hydrocarbon radical of one to six carbon atoms or a branched monovalent hydrocarbon radical of three to six carbons substituted with an alkoxy group, as defined above, e.g., 2-methoxyethyl, 1-, 2-, or 3- methoxypropyl, 2-ethoxyethyl, and the like. [0035] The terms “halo” or “halogen”, by itself or as part of another substituent, means a fluorine, chlorine, bromine, or iodine atom. [0036] The term “haloalkyl” as used herein refers to any alkyl radical having one or more hydrogen atoms replaced by a halogen atom. [0037] The term “heteroalkyl” refers to an alkyl group in which one or more carbon atom has been replaced by a heteroatom selected from S, O, P and N. Exemplary heteroalkyls include alkyl ethers, secondary and tertiary alkyl amines, alkyl amides, alkyl sulfides, and the like. The group may be a terminal group or a bridging group. As used herein reference to the normal chain when used in the context of a bridging group refers to the direct chain of atoms linking the two terminal positions of the bridging group. - 8 -4860-6199-1670.2 105807.001049 – PCT Application [0038] The term “aryl” as used herein refers to a single, all carbon aromatic ring or a multiple condensed all carbon ring system wherein at least one of the rings is aromatic. For example, in certain embodiments, an aryl group has 6 to 12 carbon atoms. Aryl includes a phenyl radical. Aryl also includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) having about 9 to 12 carbon atoms in which at least one ring is aromatic and wherein the other rings may be aromatic or not aromatic. Such multiple condensed ring systems are optionally substituted with one or more (e.g., 1, 2 or 3) oxo groups on any carbocycle portion of the multiple condensed ring system. The rings of the multiple condensed ring system can be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements. It is to be understood that the point of attachment of a multiple condensed ring system, as defined above, can be at any position of the aromatic ring. Non-limiting examples of aryl groups include, but are not limited to, phenyl, indenyl, naphthyl, 1, 2, 3,4-tetrahydronaphth- yl, and the like. [0039] The term “heteroaryl” as used herein refers to a single aromatic ring that has at least one atom other than carbon in the ring, wherein the atoms are selected from the group consisting of oxygen, nitrogen and sulfur; “heteroaryl” also includes multiple condensed ring systems that have at least one such aromatic ring, which multiple condensed ring systems are further described below. Thus, “heteroaryl” includes single aromatic rings of from about 1 to 6 carbon atoms and about 1-4 heteroatoms selected from the group consisting of oxygen, nitrogen and sulfur. The sulfur and nitrogen atoms may also be present in an oxidized form provided the ring is aromatic. Exemplary heteroaryl ring systems include but are not limited to pyridyl, pyrimidinyl, oxazolyl or furyl. “Heteroaryl” also includes multiple condensed ring systems (e.g., ring systems comprising 2, 3 or 4 rings) wherein a heteroaryl group, as defined above, is condensed with one or more rings selected from heteroaryls (to form for example a naphthyridinyl such as 1,8-naphthyridinyl), heterocycles, (to form for example a 1, 2, 3, 4-tetra- hydronaphthyridinyl such as 1,2,3,4-tetrahydro-1,8-naphthyridinyl), carbocycles (to form for example 5,6,7,8-tetrahydroquinolyl) and aryls (to form for example indazolyl) to form the multiple condensed ring system. Thus, a heteroaryl (a single aromatic ring or multiple condensed ring system) has about 1-20 carbon atoms and about 1-6 heteroatoms within the heteroaryl ring. A heteroaryl (a single aromatic ring or multiple condensed ring system) can also have about 5 to 12 or about 5 to 10 members within the heteroaryl ring. Multiple condensed ring systems may be optionally substituted with one or more (e.g., 1, 2, 3 or 4) oxo groups on the carbocycle or heterocycle portions of the condensed ring. The rings of a multiple condensed ring system can be connected to each other via fused, spiro and bridged bonds when allowed by valency requirements. It is to be understood that the individual rings of the multiple condensed ring - 9 -4860-6199-1670.2 105807.001049 – PCT Application system may be connected in any order relative to one another. It is also to be understood that the point of attachment of a multiple condensed ring system (as defined above for a heteroaryl) can be at any position of the heteroaryl ring. It is also to be understood that the point of attachment for a heteroaryl or heteroaryl multiple condensed ring system can be at any suitable atom of the heteroaryl ring including a carbon atom and a heteroatom (e.g., a nitrogen). Exemplary heteroaryls include but are not limited to pyridyl, pyrrolyl, pyrazinyl, pyrimidinyl, pyridazinyl, pyrazolyl, thienyl, indolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl, furyl, oxadiazolyl, thiadiazolyl, quinolyl, isoquinolyl, benzothiazolyl, benzoxazolyl, indazolyl, quinoxalyl, quinazolyl, 5,6,7,8-tetrahydroisoquinolinyl benzofuranyl, benzimidazolyl, thianaphthenyl, pyrrolo[2,3-b]pyridinyl, quinazolinyl-4(3H)-one, triazolyl, 4,5,6,7-tetrahydro-1H-indazole and 3b,4,4a,5-tetrahydro-1H-cyclopropa[3,4]cyclo-penta[1,2-c]pyrazole. In one embodiment the term “heteroaryl” refers to a single aromatic ring containing at least one heteroatom. For example, the term includes 5-membered and 6-membered monocyclic aromatic rings that include one or more heteroatoms. Non-limiting examples of heteroaryl include but are not limited to pyridyl, furyl, thiazole, pyrimidine, oxazole, and thiadiazole. [0040] The term “heterocycloalkyl” when used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, saturated ring structure containing at least one heteroatom selected from the group consisting of O, N, P, B and S. Heterocycloalkyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic heterocycloalkyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic heterocycloalkyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 heterocycloalkyl refers to a heterocycloalkyl group having between three and six carbon ring atoms. The heterocycloalkyl group may be attached at any heteroatom or carbon atom of the group such that the result is a stable structure. Examples of heterocycloalkyl groups include, but are not limited to, azepanyl, aziridinyl, azetidinyl, pyrrolidinyl, dioxolanyl, imidazolidinyl, pyrazolidinyl, piperazinyl, piperidinyl, dioxanyl, morpholinyl, dithianyl, thiomorpholinyl, oxazepanyl, oxiranyl, oxetanyl, quinuclidinyl, tetrahydrofuranyl, tetrahydropyranyl, piperazinyl, azepanyl, diazepanyl, oxepanyl, dioxepanyl, azocanyl, diazocanyl, oxocanyl, dioxocanyl, azaspiro[2.2]pentanyl, oxaazaspiro[3.3]heptanyl, heptanyl, dioxaspiro[3.3]heptanyl, 3- azabicyclo[3.1.0]hexanyl, , and the like. In some embodiments, heterocycloalkyl - 10 -4860-6199-1670.2 105807.001049 – PCT Application groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the heterocycloalkyl group is substituted, the heterocycloalkyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), - C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the heterocycloalkyl group is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the heterocycloalkyl group is optionally substituted by one or more Rf groups. [0041] The term “heterocycloalkenyl” when used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, partially saturated ring structure containing at least one heteroatom selected from the group consisting of O, N, P, B and S. Heterocycloalkenyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic heterocycloalkenyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic heterocycloalkenyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 heterocycloalkenyl refers to a heterocycloalkenyl group having between three and six carbon atoms. The heterocycloalkenyl group may be attached at any heteroatom or carbon atom of the partially saturated ring such that the result is a stable structure. Heterocycloalkenyl groups include groups in which the partially saturated ring is fused to an aryl group, such as, for example isoindoline, , or in which the partially saturated ring is fused to a heteroaryl group, such as, for example, 6,7-dihydro-5H-pyrrolo[3,4-b] . In some embodiments, heterocycloalkenyl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the heterocycloalkenyl group is substituted, the heterocycloalkenyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1- C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the heterocycloalkenyl group is optionally substituted by 1-6 groups selected from D, halogen, - OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the heterocycloalkenyl group is optionally substituted by one or more Rf groups. [0042] As used herein, the phrase “one or more Rf groups” is meant to include 1, 2, 3, 4, 5, 6, 7 or 8 Rf groups. In some embodiments, “one or more Rf groups” is meant to include 1 Rf group. - 11 -4860-6199-1670.2 105807.001049 – PCT Application In some embodiments, “one or more Rf groups” is meant to include up to 2 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 3 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 4 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 5 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 6 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 7 Rf groups. In some embodiments, “one or more Rf groups” is meant to include up to 8 Rf groups. [0043] As used herein, the term “heteroatom” is meant to include oxygen (O), nitrogen (N), sulfur (S), phosphorus (P), boron (B), and silicon (Si). The nitrogen and sulfur can be in an oxidized form when feasible. [0044] As used herein, the term “chiral” refers to molecules which have the property of non- superimposability of the mirror image partner, while the term “achiral” refers to molecules which are superimposable on their mirror image partner. [0045] As used herein, the term “stereoisomers” refers to compounds which have identical chemical constitution but differ with regard to the arrangement of the atoms or groups in space, e.g., enantiomers, diastereomers, tautomers. [0046] The term “patient” or “subject” is used throughout the specification to describe an animal, preferably a human or a domesticated animal, to whom treatment, including prophylactic treatment, with the compositions according to the present disclosure is provided. For treatment of those infections, conditions or disease states which are specific for a specific animal such as a human patient, the term patient refers to that specific animal, including a domesticated animal such as a dog or cat or a farm animal such as a horse, cow, sheep, etc. In general, in the present disclosure, the term patient refers to a human patient unless otherwise stated or implied from the context of the use of the term. [0047] The term “effective” is used to describe an amount of a compound, composition or component which, when used within the context of its intended use, effects an intended result. The term effective subsumes all other effective amount or effective concentration terms, which are otherwise described or used in the present application. [0048] “Pharmaceutically acceptable” means approved or approvable by a regulatory agency of the Federal or a state government or the corresponding agency in countries other than the United States, or that is listed in the U.S. Pharmacopoeia or other generally recognized pharmacopoeia for use in animals, e.g., in humans. [0049] “Pharmaceutically acceptable salt” refers to a salt of a compound of the disclosure that is pharmaceutically acceptable and that possesses the desired pharmacological activity of the parent compound. In particular, such salts are non-toxic may be inorganic or organic acid - 12 -4860-6199-1670.2 105807.001049 – PCT Application addition salts and base addition salts. Specifically, such salts include: (1) acid addition salts, formed with inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like; or formed with organic acids such as acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethane-disulfonic acid, 2-hydroxyethanesulfonic acid, benzenesulfonic acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-toluenesulfonic acid, camphorsulfonic acid, 4-methylbicyclo[2.2.2]-oct-2-ene-1-carboxylic acid, glucoheptonic acid, 3-phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid, gluconic acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, muconic acid, and the like; or (2) salts formed when an acidic proton present in the parent compound either is replaced by a metal ion, e.g., an alkali metal ion, an alkaline earth ion, or an aluminum ion; or coordinates with an organic base such as ethanolamine, diethanolamine, triethanolamine, N- methylglucamine and the like. Salts further include, by way of example only, sodium, potassium, calcium, magnesium, ammonium, tetraalkylammonium, and the like; and when the compound contains a basic functionality, salts of non-toxic organic or inorganic acids, such as hydrochloride, hydrobromide, tartrate, mesylate, acetate, maleate, oxalate and the like. [0050] A “pharmaceutically acceptable excipient” refers to a substance that is non-toxic, biologically tolerable, and otherwise biologically suitable for administration to a subject, such as an inert substance, added to a pharmacological composition or otherwise used as a vehicle, carrier, or diluent to facilitate administration of an agent and that is compatible therewith. Examples of excipients include calcium carbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin, vegetable oils, and polyethylene glycols. [0051] A “solvate” refers to a physical association of a compound of Formula I with one or more solvent molecules. [0052] “Treating” or “treatment” of any disease or disorder refers, in one embodiment, to ameliorating the disease or disorder (e.g., arresting or reducing the development of the disease or at least one of the clinical symptoms thereof). In another embodiment “treating” or “treatment” refers to ameliorating at least one physical parameter, which may not be discernible by the subject. In yet another embodiment, “treating” or “treatment” refers to modulating the disease or disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both. In yet another embodiment, “treating” or “treatment” refers to delaying the onset of the disease or disorder. [0053] In one aspect, the disclosure is directed to a compound of Formula (I): - 13 -4860-6199-1670.2 105807.001049 – PCT Application or a R1 is halo, C1-6 alkyl, or C1-6 haloalkyl; each R2 is independently H, D, or F; each R3 is independently H, D, C1-6 alkyl, C1-6 haloalkyl, C3-6 heterocycloalkyl or C3-6 cycloalkyl; s is 1, 2 or 3; t is 1, 2, 3, or 4; R4 is H, D, C1-6 alkyl, C1-6 alkoxyalkyl, C3-6 cycloalkyl, or C1-6 haloalkyl; R5 is H, D, or F; L1 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; L2 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; ring A1 is a 9-10 membered heteroaryl group, 5-7 membered heterocycloalkyl, or a 9-10 membered heterocycloalkenyl group; ring A2 is a 3-7 membered cycloalkyl group or a 4-7-membered heterocycloalkyl group; X1 is CH2, CO, CH=CH (when X2 = CO), or N=CH (when X2 = CO); X2 is CH2, CO, CH=CH (when X1 = CO), or N=CH (when X1 = CO); wherein the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, heterocycloalkenyl group, heteroaryl group or heterocycloalkyl group is optionally substituted by one or more Rf groups; each Rf is independently D, oxo, halogen, C1-C8 alkoxy, C1-C8 alkyl, C1-6 haloalkyl, C1-6 haloalkoxy, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)RcRd, -P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, - S(O)2ORb, -OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; each Ra is independently H, D, -C(O)Rb, -C(O)ORc, -C(O)NRcRd, -C(=NRb)NRbRc, - C(=NORb)NRbRc, -C(=NCN)NRbRc, -P(ORc)2, -P(O)RcRb, -P(O)RcRd, -P(O)ORcORb, -S(O)Rb, - S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 - 14 -4860-6199-1670.2 105807.001049 – PCT Application aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rb, is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rc or Rd is independently H, D, -C1-C10 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, -OC1- C6 alkyl, -O-cycloalkyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; or Rc and Rd, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocycloalkenyl group. [0054] In some embodiments, R1 in Formula I is halo, C1-6 alkyl, or C1-6 haloalkyl. In some embodiments, R1 in Formula I is halo. In some embodiments, R1 in Formula I is C1-6 alkyl. In some embodiments, R1 in Formula I is C1-6 haloalkyl.[0055] In other embodiments, R1 in Formula I is F. In other embodiments, R1 in Formula I is Cl. In other embodiments, R1 in Formula I is methyl. [0056] In some embodiments, each R2 in Formula I is independently H, D, or F. In some embodiments, each R2 in Formula I is H. In some embodiments, each R2 in Formula I is D. In some embodiments, each R2 in Formula I is F. [0057] In other embodiments, at least one R2 in Formula I is H. In other embodiments, at least one R2 in Formula I is D. In other embodiments, at least one R2 in Formula I is F. [0058] In some embodiments, s in Formula (I) is 1, 2 or 3. In some embodiments, s in Formula (I) is 1. In other embodiments, s in Formula (I) is 2. In yet other embodiments, s in Formula (I) is 3. [0059] In some embodiments, each R3 in Formula I is independently H, D, C1-6 alkyl, C1-6 haloalkyl, C3-6 heterocycloalkyl or C3-6 cycloalkyl. In some embodiments, each R3 in Formula I is H. In some embodiments, each R3 in Formula I is D. In some embodiments, each R3 in Formula I is C1-6 alkyl. In some embodiments, each R3 in Formula I is C1-6 haloalkyl. In some embodiments, each R3 in Formula I is C3-6 heterocycloalkyl. In some embodiments, each R3 in Formula I is C3-6 cycloalkyl. [0060] In other embodiments, at least one R3 in Formula I is H. In other embodiments, at least one R3 in Formula I is D. In other embodiments, at least one R3 in Formula I is C1-6 alkyl. In other embodiments, at least one R3 in Formula I is haloalkyl. In other embodiments, at least one R3 in Formula I is C3-6 cycloalkyl. - 15 -4860-6199-1670.2 105807.001049 – PCT Application [0061] In some embodiments, t in Formula (I) is 1, 2, 3 or 4. In some embodiments, t in Formula (I) is 1. In some embodiments, t in Formula (I) is 2. In other embodiments, t in Formula (I) is 3. In other embodiments, t in Formula (I) is 4. [0062] In some embodiments, R4 in Formula I is independently H, D, C1-6 alkyl, C1-6 alkoxyalkyl, haloalkyl, or C3-6 cycloalkyl. In some embodiments, R4 in Formula I is H. In some embodiments, R4 in Formula I is D. In other embodiments, R4 in Formula I is C1-6 alkyl. In other embodiments, R4 in Formula I is C1-6 alkoxyalkyl. In other embodiments, R4 in Formula I is C1-6 haloalkyl. In yet other embodiments, R4 in Formula I is C3-6 cycloalkyl. [0063] In some embodiments, R5 in Formula I is independently H, D, or F. In some embodiments, R5 in Formula I is H. In other embodiments, R5 in Formula I is D. In other embodiments, R5 in Formula I is F. [0064] In some embodiments, L1 in Formula I is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO. In some embodiments, L1 in Formula (I) is absent. In some embodiments, L1 in Formula (I)is O. In some embodiments, L1in Formula (I) is S. In other embodiments, L1in Formula (I) is S(O). In other embodiments, L1 in Formula (I) is SO2. In other embodiments, L1 in Formula (I) is NR3. In yet other embodiments, L1 in Formula (I) is C(R3)2. In yet other embodiments, L1 in Formula (I) is CO. In yet other embodiments, L1 in Formula (I) is methylene. [0065] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L1 is linked to ring A1 through a nitrogen atom in ring A1, L1 is absent.[0066] In those embodiments in which ring A1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L1 is linked to ring A1 through a carbon atom in ring A1, L1 is not absent. [0067] In some embodiments, L1 is absent, which means that ring A1 is attached directly to the fused tricyclic group by a bond. As used herein, “fused tricyclic group” can be taken to mean [1',2':4,5]pyrazino[2,3-c]pyridazine;” and having a core structure . [0068] In some embodiments, L2 in Formula I is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO. In some embodiments, L2 in Formula (I) is absent. In some embodiments, L2 in Formula (I) is O. In some embodiments, L2 in Formula (I) is S. In other embodiments, L2 in Formula (I) is S(O). In other embodiments, L2 in Formula (I) is SO2. In other embodiments, L2 in Formula (I) - 16 -4860-6199-1670.2 105807.001049 – PCT Application is NR3. In yet other embodiments, L2 in Formula (I) is C(R3)2. In yet other embodiments, L2 in Formula (I) is CO. In yet other embodiments, L2 in Formula (I) is methylene. [0069] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a nitrogen atom in ring A1, L2 is absent. [0070] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a nitrogen atom in ring A1, L2 is not O, S or NR3. [0071] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a nitrogen atom in ring A1, L2 is an alkylene spacer.[0072] In those embodiments in which ring A1is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a carbon atom in ring A1, L2 is not absent. [0073] In those embodiments in which ring A1 is a 5-7 membered heteroaryl, a 9-10 membered heteroaryl group, a 5-7 membered heterocycloalkyl group or a 9-10 membered heterocyclo- alkenyl group, in which L2 is linked to ring A1 through a carbon atom in ring A1, L2 is an alkylene spacer.[0074] In some embodiments, ring A1in Formula (I) is a 9-10 membered heteroaryl group, a 5- 7 membered heterocycloalkyl group or a 9-10 membered heterocycloalkenyl group. In some embodiments, ring A1 in Formula (I) is a 9-10 membered heteroaryl group. In other embodiments, ring A1 is a 5-7 membered heterocycloalkyl group. In other embodiments, ring A1 is a 9-10 membered heterocycloalkenyl group. [0075] In some embodiments, ring A1 in Formula (I) is a quinoline, a naphthyridine, a quinoxaline, a tetrahydro-isoquinoline, a tetrahydro-naphthyridine, a tetrahydropyrido-pyrazine, a piperidine, an isoindoline, a dihydropyrrolo-pyridine, a dihydropyrrolo-pyrazine, a pyrrolo- pyridine, a pyrazolo-pyridine, a triazolo-pyridine, a tetrahydropyrazolo-pyridine, or a tetrahydropyrrolo-pyridine. [0076] In some embodiments, ring A1 in Formula (I) is a quinoline. In some embodiments, ring A1 in Formula (I) is a naphthyridine. In some embodiments, ring A1 in Formula (I) is a quinoxaline. In some embodiments, ring A1 in Formula (I) is a tetrahydro-isoquinoline. In some embodiments, ring A1 in Formula (I) is a tetrahydro-naphthyridine. In other embodiments, ring A1 in Formula (I) is a tetrahydropyrido-pyrazine. In other embodiments, ring A1 in Formula (I) is - 17 -4860-6199-1670.2 105807.001049 – PCT Application a piperidine. In other embodiments, ring A1 in Formula (I) is an isoindoline. In other embodiments, ring A1 in Formula (I) is a dihydropyrrolo-pyridine. In other embodiments, ring A1 in Formula (I) is a dihydropyrrolo-pyrazine. In yet other embodiments, ring A1 in Formula (I) is a pyrrolo-pyridine. In yet other embodiments, ring A1 in Formula (I) is a pyrazolo-pyridine. In yet other embodiments, ring A1 in Formula (I) is a triazolo-pyridine. In yet other embodiments, ring A1 in Formula (I) is a tetrahydropyrazolo-pyridine. In yet other embodiments, ring A1 in Formula (I) is a tetrahydropyrrolo-pyridine. [0077] In some embodiments, ring A1 in any of the formulae described herein is selected from: , ,, , , , each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. from: , , , , - 18 -4860-6199-1670.2 105807.001049 – PCT Application , 6 6 6 - or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. [0079] In other embodiments, when L1 is absent, ring A1 in any of the formulae described herein is selected from; wherein each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. In ring A1 in any of the formulae described herein is . ring A1 in any of the formulae described herein is . - 19 -4860-6199-1670.2 105807.001049 – PCT Application [0082] In some embodiments, ring A1 in any of the formulae described herein is ring A1 in any of the formulae described herein is ring A1 in any of the formulae described herein is [0085] In some embodiments, ring A1 in any of the formulae described herein is ring A1 in any of the formulae described herein is ring A1 in any of the formulae described herein is . In other ring A1 in any of the formulae described herein is ring A1 in any of the formulae described herein is . - 20 -4860-6199-1670.2 105807.001049 – PCT Application [0090] In other embodiments, ring A1 in any of the formulae described herein is ring A1 in any of the formulae described . ring A1 in any of the formulae described [0093] In other embodiments, ring A1 in any of the formulae described herein is [0094] In yet other embodiments, ring A1 in any of the formulae described herein is [0095] In yet other embodiments, ring A1 in any of the formulae described herein is . [0096] In other embodiments, ring A1 in any of the formulae described herein is . other embodiments, ring A1 in any of the formulae described herein is . - 21 -4860-6199-1670.2 105807.001049 – PCT Application [0098] In yet other embodiments, ring A1 in any of the formulae described herein is embodiments, ring A1 in any of the formulae described herein is [00100] In some embodiments, each R7 in any of the formulae described herein is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa. In some embodiments, at least one R7 is D. In some embodiments, at least one R7 is C1-6 alkyl. In other embodiments, at least one R7 is C1-6 haloalkyl. In other embodiments, at least one R7 is C3-6cycloalkyl. In yet other embodiments, at least one R7is -CN. In yet other embodiments, at least one R7 is ORa. [00101] In some embodiments, each R8 in any of the formulae described herein is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo. In some embodiments, at least one R8 is D. In some embodiments, at least one R8 is C1-6 alkyl. In other embodiments, at least one R8 is C1-6 haloalkyl. In other embodiments, at least one R7 is C3-6 cycloalkyl. In yet other embodiments, at least one R8 is oxo. [00102] In some embodiments, each m in any of the formulae described herein is independently 0, 1, 2, 3, 4, 5, or 6. In some embodiments, m is 0. In some embodiments, m is 1. In some embodiments, m is 2. In other embodiments, m is 3. In other embodiments, m is 4. In yet other embodiments, m is 5. In yet other embodiments, m is 6. [00103] In some embodiments, each n in any of the formulae described herein is independently 0, 1, 2 or 3. In some embodiments, n is 0. In some embodiments, n is 1. In other embodiments, n is 2. In other embodiments, n is 3. [00104] In some embodiments, each o in any of the formulae described herein is independently 0, 1 or 2. In some embodiments, o is 0. In some embodiments, o is 1. In other embodiments, o is 2.[00105] The “ ” denotes points of attachment of ring A1 to L1 and to L2. The structuresshown for ring A1 are intended to be spatially accurate. In other words, the “ ” on the left side of a specific ring A1 structure denotes a point of attachment to L1; or if L1 is absent, the point ofattachment is to the fused tricyclic group; and the “ ” on the right side of a specific ring A1 - 22 -4860-6199-1670.2 105807.001049 – PCT Application structure denotes a point of attachment to L2; or if L2 is absent, the point of attachment is to ring A2. [00106] In some embodiments, ring A2 in Formula (I) is a 3-7 membered cycloalkyl group or a 4-7-membered heterocycloalkyl group. In some embodiments, ring A2 in Formula (I) is a 3-7 membered cycloalkyl group. In some embodiments, ring A2 in Formula (I) is a cyclohexyl group. In some embodiments, ring A2 is a 4-7-membered heterocycloalkyl group. In some embodiments, ring A2 in Formula (I) is a piperazine group, a morpholine group, a piperidine group, a pyrrolidine group, an azetidine group or an azabicyclo-hexane group. [00107] In some embodiments, ring A2 in Formula (I) is a piperazine group. In some embodiments, ring A2 in Formula (I) is a morpholine group. In other embodiments, ring A2 in Formula (I) is a piperidine group. In other embodiments, ring A2 in Formula (I) is a pyrrolidine group. In yet other embodiments, ring A2 in Formula (I) is an azetidine group. In yet other embodiments, ring A2 in Formula (I) is an azabicyclo-hexane group.[00108] In some embodiments, X1in Formula (I) is CH2, CO, CH=CH (when X2= CO), or N=CH (when X2 = CO). In some embodiments, X1 in Formula (I) is CH2. In some embodiments, X1 is CO. In other embodiments, X1 is CH=CH (when X2 = CO). In other embodiments, X1 is N=CH (when X2 = CO). [00109] In some embodiments, X2 in Formula (I) is CH2, CO, CH=CH (when X2 = CO), or N=CH (when X2 = CO). In some embodiments, X2 in Formula (I) is CH2. In some embodiments, X2 is CO. In other embodiments, X2 is CH=CH (when X1 = CO). In otherembodiments, X2is N=CH (when X1= CO). [00110] In some embodiments, each Rf in Formula I is independently D, oxo, halogen, C1-C8 alkoxy, C1-C8 alkyl, C1-6 haloalkyl, C1-6 haloalkoxy, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, - S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, -P(O)RcRd, -P(O)(ORb)(ORb), - B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, - ORa, -SRa, -NRaRd, or NRcRd. [00111] In some embodiments, at least one Rf in Formula I is D. In some embodiments, at least one Rf in Formula I is oxo. In some embodiments, at least one Rf in Formula I is halogen. In some embodiments, at least one Rf in Formula I is C1-C8 alkoxy. In some embodiments, at least one Rf in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. In some embodiments, at least one Rf in Formula I is haloalkyl. In some embodiments, at least one - 23 -4860-6199-1670.2 105807.001049 – PCT Application Rf in Formula I is -OH. In some embodiments, Rf in Formula I is -CN. In some embodiments, at least one Rf in Formula I is -NO2. In some embodiments, at least one Rf in Formula I is -C2-C6 alkenyl. In some embodiments, at least one Rf in Formula I is -C2-C6 alkynyl. In some embodiments, at least one Rf in Formula I is C6-10 aryl. In some embodiments, at least one Rf in Formula I is C5-12 heteroaryl. In some embodiments, at least one Rf in Formula I is C3-8 cycloalkyl. In other embodiments, at least one Rf in Formula I is C3-8 cycloalkenyl. In other embodiments, at least one Rf in Formula I is C3-8 heterocycloalkyl. In other embodiments, at least one Rf in Formula I is C3-8 heterocycloalkenyl. In other embodiments, at least one Rf in Formula I is -ORa. In other embodiments, at least one Rf in Formula I is -SRa. In other embodiments, at least one Rf in Formula I is -NRcRd. In other embodiments, at least one Rf in Formula I is - NRaRc. In other embodiments, at least one Rf in Formula I is -C(O)Rb. In other embodiments, at least one Rf in Formula I is -OC(O)Rb. In other embodiments, at least one Rf in Formula I is - C(O)ORb. In other embodiments, at least one Rf in Formula I is -C(O)NRcRd. In yet otherembodiments, at least one Rf in Formula I is -S(O)Rb. In yet other embodiments, at least one Rf in Formula I is -S(O)2NRcRd. In yet other embodiments, at least one Rf in Formula I is - S(O)(=NRb)Rb. In yet other embodiments, at least one Rf in Formula I is -SF5. In yet other embodiments, at least one Rf in Formula I is -P(O)RbRb. In yet other embodiments, at least one Rf in Formula I is -P(O)(ORb)(ORb). In yet other embodiments, at least one Rf in Formula I is - B(ORc)(ORd). In yet other embodiments, at least one Rf in Formula I is -S(O)2Rb. In yet other embodiments, at least one Rf in Formula I is -C(O)NRbORb. In yet other embodiments, at leastone Rf in Formula I is -S(O)2ORb. In yet other embodiments, at least one Rf in Formula I is - OS(O)2ORb. In yet other embodiments, at least one Rf in Formula I is -OPO(ORb)(ORb). [00112] In some embodiments, each Ra in Formula I is independently H, D, -C(O)Rb, - C2, - P - - - - - - C10alkyl, - C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3- 8 heterocycloalkyl, or C3-8 heterocycloalkenyl. [00113] In some embodiments, at least one Ra in Formula I is H. In some embodiments, at least one Ra in Formula I is D. In some embodiments, at least one Ra in Formula I is -C(O)Rb. In some embodiments, at least one Ra in Formula I is -C(O)ORc. In some embodiments, at least one Ra in Formula I is -C(O)NRcRd. In some embodiments, at least one Ra in Formula I is - C(=NRb)NRbRc. In some embodiments, at least one Ra in Formula I is C(=NORb)NRbRc. In some embodiments, at least one Ra in Formula I is -C(=NCN)NRbRc. [00114] In other embodiments, at least one Ra in Formula I is -P(ORc)2, -P(O)RcRb, - P(O)ORcORb, -S(O)Rb, -S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, and the like. In yet other - 24 -4860-6199-1670.2 105807.001049 – PCT Application embodiments, at least one Ra in Formula I is -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, and the like. [00115] In some embodiments, each Rb in Formula I is independently H, D, -C1-C6 alkyl, -C2- C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl. [00116] In some embodiments, at least one Rb in Formula I is H. In some embodiments, at least one Rb in Formula I is D. In some embodiments, at least one Rb in Formula I is -C1-C6 alkyl. In some embodiments, at least one Rb in Formula I is -C2-C6 alkenyl. In some embodiments, at least one Rb in Formula I is -C2-C6 alkynyl. In other embodiments, at least one Rb in Formula I is C6-10 aryl. In other embodiments, at least one Rb in Formula I is C3-8 cycloalkyl. In other embodiments, at least one Rb in Formula I is C3-8 cycloalkenyl. In other embodiments, at least one Rb in Formula I is C5-12 heteroaryl. In other embodiments, at least one Rb in Formula I is C3-8heterocycloalkyl. In other embodiments, at least one Rb in Formula I is C3-8heterocycloalkenyl. [00117] In some embodiments, each Rc or Rd in Formula I is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl. [00118] In some embodiments, Rc or Rd in Formula I is H. In some embodiments, Rc or Rd in Formula I is D. In some embodiments, Rc or Rd in Formula I is -C1-C10 alkyl. In some embodiments, Rc or Rd in Formula I is -C2-C6 alkenyl. In some embodiments, Rc or Rd inFormula I is -C2-C6alkynyl. In other embodiments, Rc or Rd in Formula I is -OC1-C6alkyl. In other embodiments, Rc or Rd in Formula I is -O-cycloalkyl. In other embodiments, Rc or Rd in Formula I is C6-10 aryl. In other embodiments, Rc or Rd in Formula I is C3-8 cycloalkyl. In other embodiments, Rc or Rd in Formula I is C3-8 cycloalkenyl. In other embodiments, Rc or Rd in Formula I is C5-12 heteroaryl. In other embodiments, Rc or Rd in Formula I is C3-8 heterocycloalkyl. In other embodiments, Rc or Rd in Formula I is C3-8 heterocycloalkenyl. [00119] In yet other embodiments, Rc and Rd in Formula I, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocycloalkenyl group. In yet other embodiments, Rc and Rd in Formula I form a monocyclic heterocycloalkyl. In yet other embodiments, Rc and Rd in Formula I form a multicyclic heterocycloalkyl. In yet other embodiments, Rc and Rd in Formula I form a monocyclic heterocycloalkenyl group. In yet other embodiments, Rc and Rd in Formula I form a multicyclic heterocycloalkenyl group. [00120] In some embodiments, the compounds of Formula (I) are the pharmaceutically acceptable salts. In some embodiments, the compounds of Formula (I) are solvates. In some - 25 -4860-6199-1670.2 105807.001049 – PCT Application embodiments, the compounds of Formula (I) are N-oxides. In some embodiments, the compounds of Formula (I) are stereoisomers. [00121] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIa and Formula IIb: or a pharmaceutically acceptable salt thereof; wherein each R1, R2, R4, L1, Ring A1, L2, ring A2, X1, and X2 are defined above with respect to Formula (I) or each R1, R2, R4, L1, Ring A1, L2, ring A2, X1, and X2 are defined as described herein. [00122] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIa. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula IIb. [00123] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIa and Formula IIIb: (IIIb) - 26 -4860-6199-1670.2 105807.001049 – PCT Application or a pharmaceutically acceptable salt thereof; wherein each R1, R2, R4, L2, and ring A1 are defined above with respect to Formula (I) or each R1, R2, R4, L2, and ring A1 are defined as described herein; and wherein X is N or CR3. [00124] In some embodiments, X is N. In other embodiments, X is CR3. In some embodiments, X is N only when it forms a stable bond with L2 or with ring A1 when L2 is absent. [00125] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIa. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula IIIb. [00126] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IVa and Formula IVb or a pharmaceutically acceptable salt thereof; wherein each R1, R2, R4, L2, and ring A1 are defined above with respect to Formula (I) or each R1, R2, R4, L2, and ring A1 are defined as described herein; and wherein X is defined above with respect to Formula (IIIa) and Formula (IIIb). [00127] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IVa. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula IVb. [00128] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula Va (Va) - 27 -4860-6199-1670.2 105807.001049 – PCT Application or a A1 are defined above with respect to Formula (I) or each R1, R2, R4, L2, and ring A1 are defined as described herein; and wherein X is defined above with respect to Formula (IIIa) and Formula (IIIb). [00129] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula Va. In other embodiments, the compounds of Formula (I) are represented by compounds of Formula Vb. [00130] In yet further embodiments, the compounds of Formula (I) are: (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl) piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl) piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- c]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[4,3- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-2H-pyrazolo[3,4- c]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 28 -4860-6199-1670.2 105807.001049 – PCT Application (S)-3-(6-(1-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((2-(((6aS,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxyquinolin-6- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-2- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridin-3- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((6-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4-dihydroisoquinolin- 2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 29 -4860-6199-1670.2 105807.001049 – PCT Application (S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro-2,7- naphthyridin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((5-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)isoindolin-2-yl)methyl)-piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,3-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((R)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((S)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy-phenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((S)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy-phenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-chloro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; - 30 -4860-6199-1670.2 105807.001049 – PCT Application (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,1-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-[5-[4-[[1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5- yl]methyl]piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione; (3S)-3-[5-[4-[[1-[(4R,6R)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5- yl]methyl]piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridin-5- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo-[4,5-b]pyridin-6- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3-methyl-1H-pyrazolo[3,4-b]pyridin- 5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1H-pyrazolo[3,4-b]pyridin- 5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2-dimethylpiperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1-yl)methyl)piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3-difluoropiperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 31 -4860-6199-1670.2 105807.001049 – PCT Application (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(2-hydroxy-3-methylphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1-yl)methyl)piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(ethyl((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aS,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3- difluoropiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-((4-((cyclobutylmethyl)((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(isopentyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; or a pharmaceutically acceptable salt thereof. [00131] In yet further embodiments, the compounds of Formula (I) are: - 32 -4860-6199-1670.2 105807.001049 – PCT Application (S)-3-(6-(1-((1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridin-5- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1,4,6,7- tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione; (3S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-7-methyl-1,4,6,7- tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione; (S)-3-(6-(4-(((R)-2-(((6aR,8R)-2-(3-Chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((R)-2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((S)-2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((S)-2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; or a pharmaceutically acceptable salt thereof. [00132] It will be apparent that the compounds of Formula I, including all subgenera described herein, may have multiple stereogenic centers. As a result, there exist multiple stereoisomers (enantiomers and diastereomers) of the compounds of Formula I (and subgenera described herein). The present disclosure contemplates and encompasses each stereoisomer of any - 33 -4860-6199-1670.2 105807.001049 – PCT Application compound of Formula I (and subgenera described herein), as well as mixtures of said stereoisomers. [00133] Pharmaceutically acceptable salts and solvates of the compounds of Formula I (including all subgenera described herein) are also within the scope of the disclosure. [00134] Isotopic variants of the compounds of Formula I (including all subgenera described herein) are also contemplated by the present disclosure. Pharmaceutical Compositions and Methods of Administration [00135] The subject pharmaceutical compositions are typically formulated to provide a therapeutically effective amount of a compound of the present disclosure as the active ingredient, or a pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or derivative thereof. Where desired, the pharmaceutical compositions contain pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including inert solid diluents and fillers, diluents, including sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants. [00136] The subject pharmaceutical compositions can be administered alone or in combination with one or more other agents, which are also typically administered in the form of pharmaceutical compositions. Where desired, the one or more compounds of the invention and other agent(s) may be mixed into a preparation or both components may be formulated into separate preparations to use them in combination separately or at the same time. [00137] In some embodiments, the concentration of one or more compounds provided in the pharmaceutical compositions of the present invention is less than 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%, 0.004%, 0.003%, 0.002%, 0.001%, 0.0009%, 0.0008%, 0.0007%, 0.0006%, 0.0005%, 0.0004%, 0.0003%, 0.0002%, or 0.0001% (or a number in the range defined by and including any two numbers above) w/w, w/v or v/v. [00138] In some embodiments, the concentration of one or more compounds of the invention is greater than 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19.75%, 19.50%, 19.25%, 19%, 18.75%, 18.50%, 18.25% 18%, 17.75%, 17.50%, 17.25% 17%, 16.75%, 16.50%, 16.25%, 16%, 15.75%, 15.50%, 15.25% 15%, 14.75%, 14.50%, 14.25% 14%, 13.75%, 13.50%, 13.25%, 13%, 12.75%, 12.50%, 12.25%, 12%, 11.75%, 11.50%, 11.25% 11%, 10.75%, 10.50%, 10.25% 10%, 9.75%, 9.50%, 9.25%, 9%, 8.75%, 8.50%, 8.25% 8%, 7.75%, 7.50%, 7.25%, 7%, 6.75%, 6.50%, 6.25%, 6%, 5.75%, 5.50%, 5.25%, 5%, 4.75%, 4.50%, 4.25%, 4%, 3.75%, 3.50%, 3.25%, 3%, - 34 -4860-6199-1670.2 105807.001049 – PCT Application 2.75%, 2.50%, 2.25%, 2%, 1.75%, 1.50%, 1.25% , 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%, 0.004%, 0.003%, 0.002%, 0.001%, 0.0009%, 0.0008%, 0.0007%, 0.0006%, 0.0005%, 0.0004%, 0.0003%, 0.0002%, or 0.0001% (or a number in the range defined by and including any two numbers above) w/w, w/v, or v/v. [00139] In some embodiments, the concentration of one or more compounds of the invention is in the range from approximately 0.0001% to approximately 50%, approximately 0.001% to approximately 40%, approximately 0.01% to approximately 30%, approximately 0.02% to approximately 29%, approximately 0.03% to approximately 28%, approximately 0.04% to approximately 27%, approximately 0.05% to approximately 26%, approximately 0.06% to approximately 25%, approximately 0.07% to approximately 24%, approximately 0.08% to approximately 23%, approximately 0.09% to approximately 22%, approximately 0.1% to approximately 21%, approximately 0.2% to approximately 20%, approximately 0.3% to approximately 19%, approximately 0.4% to approximately 18%, approximately 0.5% to approximately 17%, approximately 0.6% to approximately 16%, approximately 0.7% to approximately 15%, approximately 0.8% to approximately 14%, approximately 0.9% to approximately 12%, approximately 1% to approximately 10% w/w, w/v or v/v. [00140] In some embodiments, the concentration of one or more compounds of the invention is in the range from approximately 0.001% to approximately 10%, approximately 0.01% to approximately 5%, approximately 0.02% to approximately 4.5%, approximately 0.03% to approximately 4%, approximately 0.04% to approximately 3.5%, approximately 0.05% to approximately 3%, approximately 0.06% to approximately 2.5%, approximately 0.07% to approximately 2%, approximately 0.08% to approximately 1.5%, approximately 0.09% to approximately 1%, approximately 0.1% to approximately 0.9% w/w, w/v or v/v. [00141] In some embodiments, the amount of one or more compounds of the invention is equal to or less than 10 g, 9.5 g, 9.0 g, 8.5 g, 8.0 g, 7.5 g, 7.0 g, 6.5 g, 6.0 g, 5.5 g, 5.0 g, 4.5 g, 4.0 g, 3.5 g, 3.0 g, 2.5 g, 2.0 g, 1.5 g, 1.0 g, 0.95 g, 0.9 g, 0.85 g, 0.8 g, 0.75 g, 0.7 g, 0.65 g, 0.6 g, 0.55 g, 0.5 g, 0.45 g, 0.4 g, 0.35 g, 0.3 g, 0.25 g, 0.2 g, 0.15 g, 0.1 g, 0.09 g, 0.08 g, 0.07 g, 0.06 g, 0.05 g, 0.04 g, 0.03 g, 0.02 g, 0.01 g, 0.009 g, 0.008 g, 0.007 g, 0.006 g, 0.005 g, 0.004 g, 0.003 g, 0.002 g, 0.001 g, 0.0009 g, 0.0008 g, 0.0007 g, 0.0006 g, 0.0005 g, 0.0004 g, 0.0003 g, 0.0002 g, or 0.0001 g (or a number in the range defined by and including any two numbers above). [00142] In some embodiments, the amount of one or more compounds of the invention is more than 0.0001 g, 0.0002 g, 0.0003 g, 0.0004 g, 0.0005 g, 0.0006 g, 0.0007 g, 0.0008 g, 0.0009 g, 0.001 g, 0.0015 g, 0.002 g, 0.0025 g, 0.003 g, 0.0035 g, 0.004 g, 0.0045 g, 0.005 g, 0.0055 g, 0.006 g, 0.0065 g, 0.007 g, 0.0075 g, 0.008 g, 0.0085 g, 0.009 g, 0.0095 g, 0.01 g, 0.015 g, 0.02 - 35 -4860-6199-1670.2 105807.001049 – PCT Application g, 0.025 g, 0.03 g, 0.035 g, 0.04 g, 0.045 g, 0.05 g, 0.055 g, 0.06 g, 0.065 g, 0.07 g, 0.075 g, 0.08 g, 0.085 g, 0.09 g, 0.095 g, 0.1 g, , 0.15 g, 0.2 g, , 0.25 g, 0.3 g, , 0.35 g, 0.4 g, , 0.45 g, 0.5 g, 0.55 g, 0.6 g, , 0.65 g, 0.7 g, 0.75 g, 0.8 g, 0.85 g, 0.9 g, 0.95 g, 1 g, 1.5 g, 2 g, 2.5, 3 g, 3.5, 4 g, 4.5 g, 5 g, 5.5 g, 6 g, 6.5g, 7 g, 7.5g, 8 g, 8.5 g, 9 g, 9.5 g, or 10 g (or a number in the range defined by and including any two numbers above). [00143] In some embodiments, the amount of one or more compounds of the invention is in the range of 0.0001-10 g, 0.0005-9 g, 0.001-8 g, 0.005-7 g, 0.01-6 g, 0.05-5 g, 0.1-4 g, 0.5-4 g, or 1- 3 g. [00144] The compounds according to the invention are effective over a wide dosage range. For example, in the treatment of adult humans, dosages from 0.01 to 1000 mg, from 0.5 to 100 mg, from 1 to 50 mg per day, and from 5 to 40 mg per day are examples of dosages that may be used. An exemplary dosage is 10 to 30 mg per day. The exact dosage will depend upon the route of administration, the form in which the compound is administered, the subject to be treated, the body weight of the subject to be treated, and the preference and experience of the attending physician. [00145] A pharmaceutical composition of the invention typically contains an active ingredient (e.g., a compound of the disclosure) of the present invention or a pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including but not limited to inert solid diluents and fillers, diluents, sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants. [00146] Described below are non- limiting exemplary pharmaceutical compositions and methods for preparing the same. Pharmaceutical Compositions for Oral Administration [00147] In some embodiments, the invention provides a pharmaceutical composition for oral administration containing a compound of the invention, and a pharmaceutical excipient suitable for oral administration. [00148] In some embodiments, the invention provides a solid pharmaceutical composition for oral administration containing: (i) an effective amount of a compound of the invention; optionally (ii) an effective amount of a second agent; and (iii) a pharmaceutical excipient suitable for oral administration. In some embodiments, the composition further contains: (iv) an effective amount of a third agent. [00149] In some embodiments, the pharmaceutical composition may be a liquid pharmaceutical composition suitable for oral consumption. Pharmaceutical compositions of the invention suitable for oral administration can be presented as discrete dosage forms, such as capsules, - 36 -4860-6199-1670.2 105807.001049 – PCT Application cachets, or tablets, or liquids or aerosol sprays each containing a predetermined amount of an active ingredient as a powder or in granules, a solution, or a suspension in an aqueous or non- aqueous liquid, an oil-in- water emulsion, or a water-in-oil liquid emulsion. Such dosage forms can be prepared by any of the methods of pharmacy, but all methods include the step of bringing the active ingredient into association with the carrier, which constitutes one or more necessary ingredients. In general, the compositions are prepared by uniformly and intimately admixing the active ingredient with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product into the desired presentation. For example, a tablet can be prepared by compression or molding, optionally with one or more accessory ingredients. Compressed tablets can be prepared by compressing in a suitable machine the active ingredient in a free- flowing form such as powder or granules, optionally mixed with an excipient such as, but not limited to, a binder, a lubricant, an inert diluent, and/or a surface active or dispersing agent. Molded tablets can be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent. [00150] This invention further encompasses anhydrous pharmaceutical compositions and dosage forms comprising an active ingredient, since water can facilitate the degradation of some compounds. For example, water may be added (e.g., 5%) in the pharmaceutical arts as a means of simulating long-term storage in order to determine characteristics such as shelf- life or the stability of formulations over time. Anhydrous pharmaceutical compositions and dosage forms of the invention can be prepared using anhydrous or low moisture containing ingredients and low moisture or low humidity conditions. Pharmaceutical compositions and dosage forms of the invention which contain lactose can be made anhydrous if substantial contact with moisture and/or humidity during manufacturing, packaging, and/or storage is expected. An anhydrous pharmaceutical composition may be prepared and stored such that its anhydrous nature is maintained. Accordingly, anhydrous compositions may be packaged using materials known to prevent exposure to water such that they can be included in suitable formulary kits. Examples of suitable packaging include, but are not limited to, hermetically sealed foils, plastic or the like, unit dose containers, blister packs, and strip packs. [00151] An active ingredient can be combined in an intimate admixture with a pharmaceutical carrier according to conventional pharmaceutical compounding techniques. The carrier can take a wide variety of forms depending on the form of preparation desired for administration. In preparing the compositions for an oral dosage form, any of the usual pharmaceutical media can be employed as carriers, such as, for example, water, glycols, oils, alcohols, flavoring agents, preservatives, coloring agents, and the like in the case of oral liquid preparations (such as suspensions, solutions, and elixirs) or aerosols; or carriers such as starches, sugars, micro- - 37 -4860-6199-1670.2 105807.001049 – PCT Application crystalline cellulose, diluents, granulating agents, lubricants, binders, and disintegrating agents can be used in the case of oral solid preparations, in some embodiments without employing the use of lactose. For example, suitable carriers include powders, capsules, and tablets, with the solid oral preparations. If desired, tablets can be coated by standard aqueous or nonaqueous techniques. [00152] Binders suitable for use in pharmaceutical compositions and dosage forms include, but are not limited to, corn starch, potato starch, or other starches, gelatin, natural and synthetic gums such as acacia, sodium alginate, alginic acid, other alginates, powdered tragacanth, guar gum, cellulose and its derivatives (e.g., ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose), polyvinyl pyrrolidone, methyl cellulose, pre- gelatinized starch, hydroxypropyl methyl cellulose, microcrystalline cellulose, and mixtures thereof. [00153] Examples of suitable fillers for use in the pharmaceutical compositions and dosage forms disclosed herein include, but are not limited to, talc, calcium carbonate (e.g., granules or powder), microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch, and mixtures thereof. [00154] Disintegrants may be used in the compositions of the invention to provide tablets that disintegrate when exposed to an aqueous environment. Too much of a disintegrant may produce tablets which may disintegrate in the bottle. Too little may be insufficient for disintegration to occur and may thus alter the rate and extent of release of the active ingredient(s) from the dosage form. Thus, a sufficient amount of disintegrant that is neither too little nor too much to detrimentally alter the release of the active ingredient(s) may be used to form the dosage forms of the compounds disclosed herein. The amount of disintegrant used may vary based upon the type of formulation and mode of administration, and may be readily discernible to those of ordinary skill in the art. About 0.5 to about 15 weight percent of disintegrant, or about 1 to about 5 weight percent of disintegrant, may be used in the pharmaceutical composition. Disintegrants that can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, agar-agar, alginic acid, calcium carbonate, microcrystalline cellulose, croscarmellose sodium, crospovidone, polacrilin potassium, sodium starch glycolate, potato or tapioca starch, other starches, pre-gelatinized starch, other starches, clays, other algins, other celluloses, gums or mixtures thereof. [00155] Lubricants which can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, calcium stearate, magnesium stearate, mineral oil, light mineral oil, glycerin, sorbitol, mannitol, polyethylene glycol, other glycols, stearic acid, sodium lauryl sulfate, talc, hydrogenated vegetable oil (e.g., peanut oil, cottonseed oil, sunflower - 38 -4860-6199-1670.2 105807.001049 – PCT Application oil, sesame oil, olive oil, corn oil, and soybean oil), zinc stearate, ethyl oleate, ethyl laureate, agar, or mixtures thereof. Additional lubricants include, for example, a syloid silica gel, a coagulated aerosol of synthetic silica, or mixtures thereof. A lubricant can optionally be added, in an amount of less than about 1 weight percent of the pharmaceutical composition. [00156] When aqueous suspensions and/or elixirs are desired for oral administration, the active ingredient therein may be combined with various sweetening or flavoring agents, coloring matter or dyes and, if so desired, emulsifying and/or suspending agents, together with such diluents as water, ethanol, propylene glycol, glycerin and various combinations thereof. [00157] The tablets can be uncoated or coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a time delay material such as glyceryl monostearate or glyceryl distearate can be employed. Formulations for oral use can also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example, peanut oil, liquid paraffin or olive oil. [00158] Surfactant which can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, hydrophilic surfactants, lipophilic surfactants, and mixtures thereof. That is, a mixture of hydrophilic surfactants may be employed, a mixture of lipophilic surfactants may be employed, or a mixture of at least one hydrophilic surfactant and at least one lipophilic surfactant may be employed. [00159] A suitable hydrophilic surfactant may generally have an HLB value of at least 10, while suitable lipophilic surfactants may generally have an HLB value of or less than about 10. An empirical parameter used to characterize the relative hydrophilicity and hydrophobicity of non-ionic amphiphilic compounds is the hydrophilic-lipophilic balance (" HLB" value). Surfactants with lower HLB values are more lipophilic or hydrophobic, and have greater solubility in oils, while surfactants with higher HLB values are more hydrophilic, and have greater solubility in aqueous solutions. [00160] Hydrophilic surfactants are generally considered to be those compounds having an HLB value greater than about 10, as well as anionic, cationic, or zwitterionic compounds for which the HLB scale is not generally applicable. Similarly, lipophilic (e.g., hydrophobic) surfactants are compounds having an HLB value equal to or less than about 10. However, HLB value of a surfactant is merely a rough guide generally used to enable formulation of industrial, pharmaceutical and cosmetic emulsions. [00161] Hydrophilic surfactants may be either ionic or non-ionic. Suitable ionic surfactants include, but are not limited to, alkylammonium salts; fusidic acid salts; fatty acid derivatives of - 39 -4860-6199-1670.2 105807.001049 – PCT Application amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; lecithins and hydrogenated lecithins; lysolecithins and hydrogenated lysolecithins; phospholipids and derivatives thereof; lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acyl lactylates; mono- and di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di-glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof. [00162] Within the aforementioned group, ionic surfactants include, by way of example: lecithins, lysolecithin, phospholipids, lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acylactylates; mono- and di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di- glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof. [00163] Ionic surfactants may be the ionized forms of lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, phosphatidylserine, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, lysophosphatidylserine, PEG- phosphatidylethanolamine, PVP -phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholylsarcosine, caproate, caprylate, caprate, laurate, myristate, palmitate, oleate, ricinoleate, linoleate, linolenate, stearate, lauryl sulfate, teracecyl sulfate, docusate, lauroyl carnitines, palmitoyl carnitines, myristoyl carnitines, and salts and mixtures thereof. [00164] Hydrophilic non-ionic surfactants may include, but are not limited to, alkylglucosides; alkylmaltosides; alkylthioglucosides; lauryl macrogolglycerides; polyoxyalkylene alkyl ethers such as polyethylene glycol alkyl ethers; polyoxyalkylene alkylphenols such as polyethylene glycol alkyl phenols; polyoxyalkylene alkyl phenol fatty acid esters such as polyethylene glycol fatty acids monoesters and polyethylene glycol fatty acids diesters; polyethylene glycol glycerol fatty acid esters; polyglycerol fatty acid esters; polyoxyalkylene sorbitan fatty acid esters such as polyethylene glycol sorbitan fatty acid esters; hydrophilic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids, and sterols; polyoxyethylene sterols, derivatives, and analogues thereof; polyoxyethylated vitamins and derivatives thereof; polyoxyethylene-polyoxypropylene block copolymers; and mixtures thereof; polyethylene glycol sorbitan fatty acid esters and hydrophilic transesterification products of a polyol with at least one member of the group consisting of triglycerides, vegetable oils, and hydrogenated vegetable oils. The polyol may be - 40 -4860-6199-1670.2 105807.001049 – PCT Application glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide. [00165] Other hydrophilic-non-ionic surfactants include, without limitation, PEG- 10 laurate, PEG- 12 laurate, PEG-20 laurate, PEG-32 laurate, PEG-32 dilaurate, PEG- 12 oleate, PEG- 15 oleate, PEG-20 oleate, PEG-20 dioleate, PEG-32 oleate, PEG-200 oleate, PEG-400 oleate, PEG- 15 stearate, PEG-32 distearate, PEG-40 stearate, PEG- 100 stearate, PEG-20 dilaurate, PEG-25 glyceryl trioleate, PEG-32 dioleate, PEG-20 glyceryl laurate, PEG-30 glyceryl laurate, PEG-20 glyceryl stearate, PEG-20 glyceryl oleate, PEG-30 glyceryl oleate, PEG-30 glyceryl laurate, PEG-40 glyceryl laurate, PEG-40 palm kernel oil, PEG-50 hydrogenated castor oil, PEG-40 castor oil, PEG-35 castor oil, PEG-60 castor oil, PEG-40 hydrogenated castor oil, PEG-60 hydrogenated castor oil, PEG-60 corn oil, PEG-6 caprate/caprylate glycerides, PEG-8 caprate/caprylate glycerides, polyglyceryl-10 laurate, PEG-30 cholesterol, PEG-25 phyto sterol, PEG-30 soya sterol, PEG-20 trioleate, PEG-40 sorbitan oleate, PEG-80 sorbitan laurate, polysorbate 20, polysorbate 80, POE-9 lauryl ether, POE-23 lauryl ether, POE-10 oleyl ether, POE-20 oleyl ether, POE-20 stearyl ether, tocopheryl PEG- 100 succinate, PEG-24 cholesterol, polyglyceryl-lOoleate, Tween 40, Tween 60, sucrose monostearate, sucrose mono laurate, sucrose monopalmitate, PEG 10-100 nonyl phenol series, PEG 15-100 octyl phenol series, and poloxamers. [00166] Suitable lipophilic surfactants include, by way of example only: fatty alcohols; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; propylene glycol fatty acid esters; sorbitan fatty acid esters; polyethylene glycol sorbitan fatty acid esters; sterols and sterol derivatives; polyoxyethylated sterols and sterol derivatives; polyethylene glycol alkyl ethers; sugar esters; sugar ethers; lactic acid derivatives of mono- and di-glycerides; hydrophobic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids and sterols; oil-soluble vitamins/vitamin derivatives; and mixtures thereof. Within this group, preferred lipophilic surfactants include glycerol fatty acid esters, propylene glycol fatty acid esters, and mixtures thereof, or are hydrophobic transesterification products of a polyol with at least one member of the group consisting of vegetable oils, hydrogenated vegetable oils, and triglycerides. [00167] In one embodiment, the composition may include a solubilizer to ensure good solubilization and/or dissolution of the compound of the present invention and to minimize precipitation of the compound of the present invention. This can be especially important for compositions for non-oral use, e.g., compositions for injection. A solubilizer may also be added - 41 -4860-6199-1670.2 105807.001049 – PCT Application to increase the solubility of the hydrophilic drug and/or other components, such as surfactants, or to maintain the composition as a stable or homogeneous solution or dispersion. [00168] Examples of suitable solubilizers include, but are not limited to, the following: alcohols and polyols, such as ethanol, isopropanol, butanol, benzyl alcohol, ethylene glycol, propylene glycol, butanediols and isomers thereof, glycerol, pentaerythritol, sorbitol, mannitol, transcutol, dimethyl isosorbide, polyethylene glycol, polypropylene glycol, polyvinylalcohol, hydroxypropyl methylcellulose and other cellulose derivatives, cyclodextrins and cyclodextrin derivatives; ethers of polyethylene glycols having an average molecular weight of about 200 to about 6000, such as tetrahydrofurfuryl alcohol PEG ether (glycofurol) or methoxy PEG ; amides and other nitrogen-containing compounds such as 2-pyrrolidone, 2-piperidone, ε-caprolactam, N- alkylpyrrolidone, N-hydroxyalkylpyrrolidone, N-alkylpiperidone, N-alkylcaprolactam, dimethylacetamide and polyvinylpyrrolidone; esters such as ethyl propionate, tributylcitrate, acetyl triethylcitrate, acetyl tributyl citrate, triethylcitrate, ethyl oleate, ethyl caprylate, ethyl butyrate, triacetin, propylene glycol monoacetate, propylene glycol diacetate, ε-caprolactone and isomers thereof, δ-valerolactone and isomers thereof, β-butyrolactone and isomers thereof; and other solubilizers known in the art, such as dimethyl acetamide, dimethyl isosorbide, N-methyl pyrrolidones, monooctanoin, diethylene glycol monoethyl ether, and water. [00169] Mixtures of solubilizers may also be used. Examples include, but not limited to, triacetin, triethylcitrate, ethyl oleate, ethyl caprylate, dimethylacetamide, N-methylpyrrolidone, N-hydroxyethylpyrrolidone, polyvinylpyrrolidone, hydroxypropyl methylcellulose, hydroxypropyl cyclodextrins, ethanol, polyethylene glycol 200-100, glycofurol, transcutol, propylene glycol, and dimethyl isosorbide. Particularly preferred solubilizers include sorbitol, glycerol, triacetin, ethyl alcohol, PEG-400, glycofurol and propylene glycol. [00170] The amount of solubilizer that can be included is not particularly limited. The amount of a given solubilizer may be limited to a bioacceptable amount, which may be readily determined by one of skill in the art. In some circumstances, it may be advantageous to include amounts of solubilizers far in excess of bioacceptable amounts, for example to maximize the concentration of the drug, with excess solubilizer removed prior to providing the composition to a subject using conventional techniques, such as distillation or evaporation. Thus, if present, the solubilizer can be in a weight ratio of 10%, 25%o, 50%), 100%o, or up to about 200%> by weight, based on the combined weight of the drug, and other excipients. If desired, very small amounts of solubilizer may also be used, such as 5%>, 2%>, 1%) or even less. Typically, the solubilizer may be present in an amount of about 1%> to about 100%, more typically about 5%> to about 25%> by weight. - 42 -4860-6199-1670.2 105807.001049 – PCT Application [00171] The composition can further include one or more pharmaceutically acceptable additives and excipients. Such additives and excipients include, without limitation, detackifiers, anti-foaming agents, buffering agents, polymers, antioxidants, preservatives, chelating agents, viscomodulators, tonicifiers, flavorants, colorants, odorants, opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof. [00172] In addition, an acid or a base may be incorporated into the composition to facilitate processing, to enhance stability, or for other reasons. Examples of pharmaceutically acceptable bases include amino acids, amino acid esters, ammonium hydroxide, potassium hydroxide, sodium hydroxide, sodium hydrogen carbonate, aluminum hydroxide, calcium carbonate, magnesium hydroxide, magnesium aluminum silicate, synthetic aluminum silicate, synthetic hydrocalcite, magnesium aluminum hydroxide, diisopropylethylamine, ethanolamine, ethylenediamine, triethanolamine, triethylamine, triisopropanolamine, trimethylamine, tris(hydroxymethyl)aminomethane (TRIS) and the like. Also suitable are bases that are salts of a pharmaceutically acceptable acid, such as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p-toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid, and the like. Salts of polyprotic acids, such as sodium phosphate, disodium hydrogen phosphate, and sodium dihydrogen phosphate can also be used. When the base is a salt, the cation can be any convenient and pharmaceutically acceptable cation, such as ammonium, alkali metals, alkaline earth metals, and the like. Example may include, but not limited to, sodium, potassium, lithium, magnesium, calcium and ammonium. [00173] Suitable acids are pharmaceutically acceptable organic or inorganic acids. Examples of suitable inorganic acids include hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid, nitric acid, boric acid, phosphoric acid, and the like. Examples of suitable organic acids include acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acids, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, methanesulfonic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p- toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid and the like. Pharmaceutical compositions for injection - 43 -4860-6199-1670.2 105807.001049 – PCT Application [00174] In some embodiments, the invention provides a pharmaceutical composition for injection containing a compound of the present invention and a pharmaceutical excipient suitable for injection. Components and amounts of agents in the compositions are as described herein. [00175] The forms in which the novel compositions of the present invention may be incorporated for administration by injection include aqueous or oil suspensions, or emulsions, with sesame oil, corn oil, cottonseed oil, or peanut oil, as well as elixirs, mannitol, dextrose, or a sterile aqueous solution, and similar pharmaceutical vehicles. [00176] Aqueous solutions in saline are also conventionally used for injection. Ethanol, glycerol, propylene glycol, liquid polyethylene glycol, and the like (and suitable mixtures thereof), cyclodextrin derivatives, and vegetable oils may also be employed. The proper fluidity can be maintained, for example, by the use of a coating, such as lecithin, for the maintenance of the required particle size in the case of dispersion and by the use of surfactants. The prevention of the action of microorganisms can be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the like. [00177] Sterile injectable solutions are prepared by incorporating the compound of the present invention in the required amount in the appropriate solvent with various other ingredients as enumerated above, as required, followed by filtered sterilization. Generally, dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above. In the case of sterile powders for the preparation of sterile injectable solutions, certain desirable methods of preparation are vacuum-drying and freeze- drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile- filtered solution thereof. [00178] In some embodiments, the invention provides a pharmaceutical composition for transdermal delivery containing a compound of the present invention and a pharmaceutical excipient suitable for transdermal delivery. [00179] Compositions of the present invention can be formulated into preparations in solid, semisolid, or liquid forms suitable for local or topical administration, such as gels, water soluble jellies, creams, lotions, suspensions, foams, powders, slurries, ointments, solutions, oils, pastes, suppositories, sprays, emulsions, saline solutions, dimethylsulfoxide (DMSO)-based solutions. In general, carriers with higher densities are capable of providing an area with a prolonged exposure to the active ingredients. In contrast, a solution formulation may provide more immediate exposure of the active ingredient to the chosen area. - 44 -4860-6199-1670.2 105807.001049 – PCT Application [00180] The pharmaceutical compositions also may comprise suitable solid or gel phase carriers or excipients, which are compounds that allow increased penetration of, or assist in the delivery of, therapeutic molecules across the stratum corneum permeability barrier of the skin. There are many of these penetration- enhancing molecules known to those trained in the art of topical formulation. [00181] Examples of such carriers and excipients include, but are not limited to, humectants (e.g., urea), glycols (e.g., propylene glycol), alcohols (e.g., ethanol), fatty acids (e.g., oleic acid), surfactants (e.g., isopropyl myristate and sodium lauryl sulfate), pyrrolidones, glycerol monolaurate, sulfoxides, terpenes (e.g., menthol), amines, amides, alkanes, alkanols, water, calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols. [00182] Another exemplary formulation for use in the methods of the present invention employs transdermal delivery devices ("patches"). Such transdermal patches may be used to provide continuous or discontinuous infusion of a compound of the present invention in controlled amounts, either with or without another agent. [00183] The construction and use of transdermal patches for the delivery of pharmaceutical agents is well known in the art. See, e.g., U.S. Pat. Nos.5,023,252, 4,992,445 and 5,001,139. Such patches may be constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents. Pharmaceutical Compositions for Inhalation [00184] Compositions for inhalation or insufflation include solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders. The liquid or solid compositions may contain suitable pharmaceutically acceptable excipients as described supra. Preferably the compositions are administered by the oral or nasal respiratory route for local or systemic effect. Compositions in preferably pharmaceutically acceptable solvents may be nebulized by use of inert gases. Nebulized solutions may be inhaled directly from the nebulizing device or the nebulizing device may be attached to a face mask tent, or intermittent positive pressure breathing machine. Solution, suspension, or powder compositions may be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner. Other Pharmaceutical Compositions [00185] Pharmaceutical compositions may also be prepared from compositions described herein and one or more pharmaceutically acceptable excipients suitable for sublingual, buccal, - 45 -4860-6199-1670.2 105807.001049 – PCT Application rectal, intraosseous, intraocular, intranasal, epidural, or intraspinal administration. Preparations for such pharmaceutical compositions are well-known in the art. See, e.g., Anderson, Philip O.; Knoben, James E.; Troutman, William G, eds., Handbook of Clinical Drug Data, Tenth Edition, McGraw-Hill, 2002; Pratt and Taylor, eds., Principles of Drug Action, Third Edition, Churchill Livingston, New York, 1990; Katzung, ed., Basic and Clinical Pharmacology, Ninth Edition, McGraw Hill, 20037ybg; Goodman and Gilman, eds., The Pharmacological Basis of Therapeutics, Tenth Edition, McGraw Hill, 2001 ; Remingtons Pharmaceutical Sciences, 20th Ed., Lippincott Williams & Wilkins., 2000; Martindale, The Extra Pharmacopoeia, Thirty- Second Edition (The Pharmaceutical Press, London, 1999); all of which are incorporated by reference herein in their entirety. [00186] Administration of the compounds or pharmaceutical composition of the present invention can be affected by any method that enables delivery of the compounds to the site of action. These methods include oral routes, intraduodenal routes, parenteral injection (including intravenous, intraarterial, subcutaneous, intramuscular, intravascular, intraperitoneal or infusion), topical (e.g., transdermal application), rectal administration, via local delivery by catheter or stent or through inhalation. Compounds can also be administered intraadiposally or intrathecally. [00187] In some embodiments, the compounds or pharmaceutical composition of the present invention are administered by intravenous injection. [00188] The amount of the compound administered will be dependent on the subject being treated, the severity of the disorder or condition, the rate of administration, the disposition of the compound and the discretion of the prescribing physician. However, an effective dosage is in the range of about 0.001 to about 100 mg per kg body weight per day, preferably about 1 to about 35 mg/kg/day, in single or divided doses. For a 70 kg human, this would amount to about 0.05 to 7 g/day, preferably about 0.05 to about 2.5 g/day. In some instances, dosage levels below the lower limit of the aforesaid range may be more than adequate, while in other cases still larger doses may be employed without causing any harmful side effect, e.g., by dividing such larger doses into several small doses for administration throughout the day. [00189] In some embodiments, a compound of the invention is administered in a single dose. [00190] Typically, such administration will be by injection, e.g., intravenous injection, in order to introduce the agent quickly. However, other routes may be used as appropriate. A single dose of a compound of the invention may also be used for treatment of an acute condition. [00191] In some embodiments, a compound of the invention is administered in multiple doses. Dosing may be about once, twice, three times, four times, five times, six times, or more than six times per day. Dosing may be about once a month, once every two weeks, once a week, or once every other day. In another embodiment a compound of the invention and another agent are - 46 -4860-6199-1670.2 105807.001049 – PCT Application administered together about once per day to about 6 times per day. In another embodiment the administration of a compound of the invention and an agent continues for less than about 7 days. In yet another embodiment the administration continues for more than about 6, 10, 14, 28 days, two months, six months, or one year. In some cases, continuous dosing is achieved and maintained as long as necessary. [00192] Administration of the compounds of the invention may continue as long as necessary. In some embodiments, a compound of the invention is administered for more than 1, 2, 3, 4, 5, 6, 7, 14, or 28 days. In some embodiments, a compound of the invention is administered for less than 28, 14, 7, 6, 5, 4, 3, 2, or 1 day. In some embodiments, a compound of the invention is administered chronically on an ongoing basis, e.g., for the treatment of chronic effects. [00193] An effective amount of a compound of the invention may be administered in either single or multiple doses by any of the accepted modes of administration of agents having similar utilities, including rectal, buccal, intranasal and transdermal routes, by intra-arterial injection, intravenously, intraperitoneally, parenterally, intramuscularly, subcutaneously, orally, topically, or as an inhalant. [00194] The compositions of the invention may also be delivered via an impregnated or coated device such as a stent, for example, or an artery-inserted cylindrical polymer. Such a method of administration may, for example, aid in the prevention or amelioration of restenosis following procedures such as balloon angioplasty. Without being bound by theory, compounds of the invention may slow or inhibit the migration and proliferation of smooth muscle cells in the arterial wall which contribute to restenosis. A compound of the invention may be administered, for example, by local delivery from the struts of a stent, from a stent graft, from grafts, or from the cover or sheath of a stent. In some embodiments, a compound of the invention is admixed with a matrix. Such a matrix may be a polymeric matrix and may serve to bond the compound to the stent. Polymeric matrices suitable for such use, include, for example, lactone-based polyesters or copolyesters such as polylactide, polycaprolactonglycolide, polyorthoesters, polyanhydrides, polyaminoacids, polysaccharides, polyphosphazenes, poly (ether-ester) copolymers (e.g. PEO- PLLA); polydimethylsiloxane, poly(ethylene-vinylacetate), acrylate-based polymers or copolymers (e.g. polyhydroxyethyl methylmethacrylate, polyvinyl pyrrolidinone), fluorinated polymers such as polytetrafluoroethylene and cellulose esters. Suitable matrices may be nondegrading or may degrade with time, releasing the compound or compounds. Compounds of the invention may be applied to the surface of the stent by various methods such as dip/spin coating, spray coating, dip-coating, and/or brush-coating. The compounds may be applied in a solvent and the solvent may be allowed to evaporate, thus forming a layer of compound onto the stent. Alternatively, the compound may be located in the body of the stent or graft, for example - 47 -4860-6199-1670.2 105807.001049 – PCT Application in microchannels or micropores. When implanted, the compound diffuses out of the body of the stent to contact the arterial wall. Such stents may be prepared by dipping a stent manufactured to contain such micropores or microchannels into a solution of the compound of the invention in a suitable solvent, followed by evaporation of the solvent. Excess drug on the surface of the stent may be removed via an additional brief solvent wash. In yet other embodiments, compounds of the invention may be covalently linked to a stent or graft. A covalent linker may be used which degrades in vivo, leading to the release of the compound of the invention. Any bio-labile linkage may be used for such a purpose, such as ester, amide or anhydride linkages. Compounds of the invention may additionally be administered intravascularly from a balloon used during angioplasty. Extravascular administration of the compounds via the pericard or via advential application of formulations of the invention may also be performed to decrease restenosis. [00195] A variety of stent devices which may be used as described are disclosed, for example, in the following references, all of which are hereby incorporated by reference: U.S. Pat. No. 5451233; U.S. Pat. No.5040548; U.S. Pat. No.5061273; U.S. Pat. No.5496346; U.S. Pat. No. 5292331; U.S. Pat. No.5674278; U.S. Pat. No.3657744; U.S. Pat. No.4739762; U.S. Pat. No. 5195984; U.S. Pat. No.5292331 ; U.S. Pat. No.5674278; U.S. Pat. No.5879382; U.S. Pat. No. 6344053. [00196] The compounds of the invention may be administered in dosages. It is known in the art that due to intersubject variability in compound pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy. Dosing for a compound of the invention may be found by routine experimentation in light of the instant disclosure. [00197] When a compound of the invention is administered in a composition that comprises one or more agents, and the agent has a shorter half- life than the compound of the invention unit dose forms of the agent and the compound of the invention may be adjusted accordingly. [00198] The subject pharmaceutical composition may, for example, be in a form suitable for oral administration as a tablet, capsule, pill, powder, sustained release formulations, solution, suspension, for parenteral injection as a sterile solution, suspension or emulsion, for topical administration as an ointment or cream or for rectal administration as a suppository. The pharmaceutical composition may be in unit dosage forms suitable for single administration of precise dosages. The pharmaceutical composition will include a conventional pharmaceutical carrier or excipient and a compound according to the invention as an active ingredient. In addition, it may include other medicinal or pharmaceutical agents, carriers, adjuvants, etc. [00199] Exemplary parenteral administration forms include solutions or suspensions of active compound in sterile aqueous solutions, for example, aqueous propylene glycol or dextrose solutions. Such dosage forms can be suitably buffered, if desired. - 48 -4860-6199-1670.2 105807.001049 – PCT Application [00200] [00201] Methods of Use [00202] The method typically comprises administering to a subject a therapeutically effective amount of a compound of the invention. The therapeutically effective amount of the subject combination of compounds may vary depending upon the intended application (in vitro or in vivo), or the subject and disease condition being treated, e.g., the weight and age of the subject, the severity of the disease condition, the manner of administration and the like, which can readily be determined by one of ordinary skill in the art. The term also applies to a dose that will induce a particular response in target cells, e.g., reduction of proliferation or downregulation of activity of a target protein. The specific dose will vary depending on the particular compounds chosen, the dosing regimen to be followed, whether it is administered in combination with other compounds, timing of administration, the tissue to which it is administered, and the physical delivery system in which it is carried. [00203] In certain embodiment, the present invention provides a pharmaceutical composition comprising a compound of bispecific formula, or pharmaceutically acceptable salt thereof. [00204] In certain embodiment, the present invention provides a pharmaceutical composition comprising a compound of bispecific formula for use in degrading a target protein in a cell. [00205] In certain embodiment, a method of degrading a target protein comprising administering to a cell therapeutically effective amount of a bispecific compound, or pharmaceutically acceptable salt, wherein the compound is effective for degrading the target protein. [00206] In certain embodiment, the present invention provides a pharmaceutical composition comprising a compound of bispecific formula, for use in treating or preventing of a disease or disorder in which SMARCA2 and/or SMARCA4 plays a role. [00207] In certain embodiment, the present invention provides a pharmaceutical composition comprising a compound of bispecific formula, for use in treating or preventing of a disease or disorder in which SWI/SNF mutations plays a role. [00208] In certain embodiment, target proteins are SMARCA2, SMARCA4 and/or PB1. [00209] In certain embodiment, target protein complex is SWI/SNF in a cell. [00210] In certain embodiment, diseases or disorders dependent on SMARCA2 or SMARCA4 include cancers. [00211] In certain embodiment, diseases or disorders dependent on SWI/SNF complex include cancers. [00212] Exemplary cancers which may be treated by the present compounds either alone or in combination with at least one additional anti-cancer agent include squamous-cell carcinoma, - 49 -4860-6199-1670.2 105807.001049 – PCT Application basal cell carcinoma, adenocarcinoma, hepatocellular carcinomas, and renal cell carcinomas, cancer of the bladder, bowel, breast, cervix, colon, esophagus, head, kidney, liver, lung, neck, ovary, pancreas, prostate, and stomach; leukemias; benign and malignant lymphomas, particularly Burkitt's lymphoma and Non-Hodgkin's lymphoma; benign and malignant melanomas; myeloproliferative diseases; sarcomas, including Ewing's sarcoma, hemangiosarcoma, Kaposi's sarcoma, liposarcoma, myosarcomas, peripheral neuroepithelioma, synovial sarcoma, gliomas, astrocytomas, oligodendrogliomas, ependymomas, gliobastomas, neuroblastomas, ganglioneuromas, gangliogliomas, medulloblastomas, pineal cell tumors, meningiomas, meningeal sarcomas, neurofibromas, and Schwannomas; bowel cancer, breast cancer, prostate cancer, cervical cancer, uterine cancer, lung cancer, ovarian cancer, testicular cancer, thyroid cancer, astrocytoma, esophageal cancer, pancreatic cancer, stomach cancer, liver cancer, colon cancer, melanoma; carcinosarcoma, Hodgkin's disease, Wilms' tumor and teratocarcinomas. [00213] In certain embodiments, the cancers which may be treated using compounds according to the present disclosure include, for example, T-lineage Acute lymphoblastic Leukemia (T- ALL), T-lineage lymphoblastic Lymphoma (T-LL), Peripheral T-cell lymphoma, Adult T-cell Leukemia, Pre-B ALL, Pre-B Lymphomas, Large B-cell Lymphoma, Burkitts Lymphoma, B-cell ALL, Philadelphia chromosome positive ALL and Philadelphia chromosome positive CML. [00214] In certain further embodiment, the cancer is a SMARCA2 and/or SMARAC4- dependent cancer. [00215] In certain embodiment, the present invention provides a pharmaceutical composition comprising a compound of bispecific formula for use in the diseases or disorders dependent upon SMARCA2 and/or SMARCA4 is cancer. [00216] Compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered to treat any of the described diseases, alone or in combination with a medical therapy. Medical therapies include, for example, surgery and radiotherapy (e.g., gamma- radiation, neutron beam radiotherapy, electron beam radiotherapy, proton therapy, brachytherapy, systemic radioactive isotopes). [00217] In other aspects, compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered to treat any of the described diseases, alone or in combination with one or more other agents. [00218] In other methods, the compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered in combination with agonists of nuclear receptors agents. - 50 -4860-6199-1670.2 105807.001049 – PCT Application [00219] In other methods, the compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered in combination with antagonists of nuclear receptors agents. [00220] In other methods, the compounds of the disclosure, as well as pharmaceutical compositions comprising them, can be administered in combination with an anti-proliferative agent. Combination Therapies [00221] For treating cancer and other proliferative diseases, the compounds of the invention can be used in combination with chemotherapeutic agents, agonists or antagonists of nuclear receptors, or other anti-proliferative agents. The compounds of the invention can also be used in combination with a medical therapy such as surgery or radiotherapy, e.g., gamma-radiation, neutron beam radiotherapy, electron beam radiotherapy, proton therapy, brachytherapy, and systemic radioactive isotopes. Examples of suitable chemotherapeutic agents include any of: abarelix, aldesleukin, alemtuzumab, alitretinoin, allopurinol, all-trans retinoic acid, altretamine, anastrozole, arsenic trioxide, asparaginase, azacitidine, bendamustine, bevacizumab, bexarotene, bleomycin, bortezombi, bortezomib, busulfan intravenous, busulfan oral, calusterone, capecitabine, carboplatin, carmustine, cetuximab, chlorambucil, cisplatin, cladribine, clofarabine, cyclophosphamide, cytarabine, dacarbazine, dactinomycin, dalteparin sodium, dasatinib, daunorubicin, decitabine, denileukin, denileukin diftitox, dexrazoxane, docetaxel, doxorubicin, dromostanolone propionate, eculizumab, epirubicin, erlotinib, estramustine, etoposide phosphate, etoposide, exemestane, fentanyl citrate, filgrastim, floxuridine, fludarabine, fluorouracil, fulvestrant, gefitinib, gemcitabine, gemtuzumab ozogamicin, goserelin acetate, histrelin acetate, ibritumomab tiuxetan, idarubicin, ifosfamide, imatinib mesylate, interferon alfa 2a, irinotecan, lapatinib ditosylate, lenalidomide, letrozole, leucovorin, leuprolide acetate, levamisole, lomustine, meclorethamine, megestrol acetate, melphalan, mercaptopurine, methotrexate, methoxsalen, mitomycin C, mitotane, mitoxantrone, nandrolone phenpropionate, nelarabine, nofetumomab, oxaliplatin, paclitaxel, pamidronate, panobinostat, panitumumab, pegaspargase, pegfilgrastim, pemetrexed disodium, pentostatin, pipobroman, plicamycin, procarbazine, quinacrine, rasburicase, rituximab, ruxolitinib, sorafenib, streptozocin, sunitinib, sunitinib maleate, tamoxifen, temozolomide, teniposide, testolactone, thalidomide, thioguanine, thiotepa, topotecan, toremifene, tositumomab, trastuzumab, tretinoin, uracil mustard, valrubicin, vinblastine, vincristine, vinorelbine, vorinstat and zoledronate. [00222] In some embodiments, the compounds of the invention can be used in combination with a therapeutic agent that targets an epigenetic regulator. Examples of epigenetic regulators - 51 -4860-6199-1670.2 105807.001049 – PCT Application include bromodomain inhibitors, the histone lysine methyltransferase inhibitors, histone arginine methyl transferase inhibitors, histone demethylase inhibitors, histone deacetylase inhibitors, histone acetylase inhibitors, and DNA methyltransferase inhibitors. Histone deacetylase inhibitors include, e.g., vorinostat. Histone arginine methyl transferase inhibitors include inhibitors of protein arginine methyltransferases (PRMTs) such as PRMT5, PRMT1 and PRMT4. DNA methyltransferase inhibitors include inhibitors of DNMT1 and DNMT3. [00223] For treating cancer and other proliferative diseases, the compounds of the invention can be used in combination with targeted therapies, including JAK kinase inhibitors (e.g. Ruxolitinib), PI3 kinase inhibitors including PI3K-delta selective and broad spectrum PI3K inhibitors, MEK inhibitors, Cyclin Dependent kinase inhibitors, including CDK4/6 inhibitors and CDK9 inhibitors, BRAF inhibitors, mTOR inhibitors, proteasome inhibitors (e.g. Bortezomib, Carfilzomib), HDAC inhibitors (e.g. panobinostat, vorinostat), DNA methyl transferase inhibitors, dexamethasone, bromo and extra terminal family member (BET) inhibitors, BTK inhibitors (e.g. ibrutinib, acalabrutinib), BCL2 inhibitors (e.g. venetoclax), dual BCL2 family inhibitors (e.g. BCL2/BCLxL), PARP inhibitors, FLT3 inhibitors, or LSD1 inhibitors. [00224] In some embodiments, the inhibitor of an immune checkpoint molecule is an inhibitor of PD-1, e.g., an anti-PD-1 monoclonal antibody. In some embodiments, the anti-PD-1 monoclonal antibody is nivolumab, pembrolizumab (also known as MK-3475), or PDR001. In some embodiments, the anti-PD-1 monoclonal antibody is nivolumab or pembrolizumab. In some embodiments, the anti-PD1 antibody is pembrolizumab. In some embodiments, the inhibitor of an immune checkpoint molecule is an inhibitor of PD-L1, e.g., an anti-PD-L1 monoclonal antibody. In some embodiments, the anti-PD-L1 monoclonal antibody is atezolizumab, durvalumab, or BMS-935559. In some embodiments, the inhibitor of an immune checkpoint molecule is an inhibitor of CTLA-4, e.g., an anti-CTLA-4 antibody. In some embodiments, the anti-CTLA-4 antibody is ipilimumab. [00225] In some embodiments, the agent is an alkylating agent, a proteasome inhibitor, a corticosteroid, or an immunomodulatory agent. Examples of an alkylating agent include cyclophosphamide (CY), melphalan (MEL), and bendamustine. In some embodiments, the proteasome inhibitor is carfilzomib. In some embodiments, the corticosteroid is dexamethasone (DEX). In some embodiments, the immunomodulatory agent is lenalidomide (LEN) or pomalidomide (POM). [00226] Compounds of the invention can be prepared using numerous preparatory reactions known in the literature. The Schemes below provide general guidance in connection with preparing the compounds of the invention. One skilled in the art would understand that the preparations shown in the Schemes can be modified or optimized using general knowledge of - 52 -4860-6199-1670.2 105807.001049 – PCT Application organic chemistry to prepare various compounds of the invention. Example synthetic methods for preparing compounds of the invention are provided in the Schemes below. [00227] The following Examples are provided to illustrate some of the concepts described within this disclosure. While the Examples are considered to provide an embodiment, it should not be considered to limit the more general embodiments described herein. EXAMPLES Intermediate 1. (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol [00229] Iodomethane (3.0 mL, 48.2 mmol) was added to a stirring solution of (2S,4R)-4- (benzyloxy)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid (6.2 g, 19.3 mmol) and potassium carbonate (3.32 g, 24 mmol) in DMF (46.4 mL). The reaction was stirred at room temperature for 2 hours. The product mixture was diluted with EtOAc (200 mL). The diluted product mixture was washed with water (200 mL) and a saturated sodium chloride aqueous solution (2 x 200 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was used without further purification.1-(tert-butyl) 2- methyl (2S,4R)-4-(benzyloxy)pyrrolidine-1,2-dicarboxylate was obtained as a yellow oil (6.46 g, 99%). LCMS calcd for C13H18NO3 [M+H-C5H8O2]+: m/z = 236.1; Found: 236.1. [00230] Step 2.1-(tert-Butyl) 2-methyl (4R)-4-(benzyloxy)-2-(difluoromethyl)pyrrolidine-1,2- dicarboxylate - 53 -4860-6199-1670.2 105807.001049 – PCT Application [00231] Lithium bis(trimethylsilyl)amide (1 M in THF, 30 mL, 30 mmol) was added to a stirring solution of 1-(tert-butyl) 2-methyl (2S,4R)-4-(benzyloxy)pyrrolidine-1,2-dicarboxylate (6.7 g, 20 mmol) in THF (100 mL) at -78 °C. For 45 minutes, the reaction mixture was slowly warmed until reaching room temperature. Then the reaction mixture cooled to -78 °C. Difluoromethyl trifluoromethanesulfonate (5.06 mL, 39.9 mmol) was added dropwise to the reaction mixture at -78 °C. The reaction mixture was allowed to slowly warm to room temperature and stirred overnight. The product mixture was diluted with a saturated ammonium chloride aqueous solution (200 mL) and stirred for 10 minutes. The diluted product mixture was transferred to a separatory funnel and extracted with EtOAc (200 mL). The organic layer was washed with a saturated sodium chloride aqueous solution (2 x 200 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-50% EtOAc/hexanes to obtain 1-(tert-butyl) 2-methyl (4R)-4-(benzyloxy)-2-(difluoromethyl)pyrrolidine-1,2- dicarboxylate as an inseparable mixture of diastereomers (5.6 g, 73%). LCMS calcd for C14H18F2NO3 [M+H-C5H8O2]+: m/z = 286.1; Found: 286.0. [00232] Step 3. (4R)-4-(Benzyloxy)-1-(tert-butoxycarbonyl)-2-(difluoromethyl)pyrrolidine-2- carboxylic acid [00233] An aqueous solution of sodium hydroxide (2.5 M, 58.4 mL, 146 mmol) was added to a stirring solution of 1-(tert-butyl) 2-methyl (4R)-4-(benzyloxy)-2-(difluoromethyl)pyrrolidine-1,2- dicarboxylate (5.6 g, 14.5 mmol) in THF (27 mL) and MeOH (27 mL) at room temperature. The reaction mixture was heated to 65 °C and stirred for 2 hours. The product mixture was cooled to room temperature and acidified with an aqueous solution of hydrochloric acid (1 M, 160 mL, 160 mmol). The acidified product mixture was transferred to a separatory funnel and extracted with EtOAc (2 x 200 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was used without further purification. (4R)-4-(benzyloxy)-1-(tert-butoxycarbonyl)-2-(difluoromethyl)pyrrolidine-2-carboxylic acid was obtained as an orange oil (5.4 g, 100%). LCMS calcd for C13H16F2NO3 [M+H-C5H8O2]+: m/z = 272.1; Found: 271.9. [00234] Step 4. tert-Butyl (4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl)carbamoyl)- 2-(difluoromethyl)pyrrolidine-1-carboxylate - 54 -4860-6199-1670.2 105807.001049 – PCT Application [00235] 1-[Bis(dimethylamino)- - [4,5-b]pyridinium 3-oxid hexafluorophosphate (6.55 g, 17.2 mmol) was added to a stirring solution of (4R)-4-(benzyloxy)- 1-(tert-butoxycarbonyl)-2-(difluoromethyl)pyrrolidine-2-carboxylic acid (5.77 g, 15.5 mmol) and N,N-diisopropylethylamine (9.72 mL, 55.8 mmol) in acetonitrile (75 mL). The reaction mixture was stirred 40 minutes at room temperature. The product mixture was concentrated under reduced pressure. The resulting residue was dissolved in THF (62.4 mL).4-Bromo-6- chloropyridazin-3-amine (4.2 g, 20.2 mmol) was added to the solution and the resulting solution was stirred. Sodium hydride (2.2 g, 55.2 mmol, 60% dispersion in mineral oil) was added portion-wise to the stirring reaction mixture at room temperature. After 1 hour, the product mixture was cooled to 0 °C and quenched with a saturated ammonium chloride aqueous solution (100 mL). The quenched product mixture was transferred to a separatory funnel and was diluted with water (100 mL). The diluted product mixture was extracted with EtOAc (2 x 200 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-50% EtOAc/hexanes to obtain tert-butyl (4R)-4-(benzyloxy)-2-((4-bromo-6- chloropyridazin-3-yl)carbamoyl)-2-(difluoromethyl)pyrrolidine-1-carboxylate (5.4 g, 62%). LCMS calcd for C22H25BrClF2N4O4 [M+H]+: m/z = 561.1; Found: 561.9. [00236] Step 5. (6aS,8R)-8-(Benzyloxy)-2-chloro-6a-(difluoromethyl)-6a,7,8,9-tetrahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one and (6aR,8R)-8-(benzyloxy)-2-chloro-6a- (difluoromethyl)- 6(5H)-one [00237] Trifluoroacetic acid (7.36 mL, 96.1 mmol) was added to a stirring solution of tert- butyl (4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl)carbamoyl)-2-(difluoromethyl)- pyrrolidine-1-carboxylate (5.4 g, 9.61 mmol) at room temperature. The reaction mixture was - 55 -4860-6199-1670.2 105807.001049 – PCT Application stirred for 5 hours. The product mixture was concentrated under reduced pressure. The residue obtained was dissolved in acetonitrile (15 mL). N,N-Diisopropylethylamine (8.37 mL, 48.1 mmol) was added to the stirring reaction mixture. The reaction mixture was heated to 80 °C and stirred for 16 hours. The product mixture was concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-100% EtOAc/hexanes to obtain (6aS,8R)-8-(benzyloxy)-2-chloro-6a-(difluoromethyl)-6a,7,8,9- tetrahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (562 mg, 15%) and (6aR,8R)-8- (benzyloxy)-2-chloro-6a-(difluoromethyl)-6a,7,8,9-tetrahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-6(5H)-one (1.87 g, 51%). LCMS calcd for C17H16ClF2N4O2 [M+H]+: m/z = 381.1; Found: 381.0. [00238] Step 6. (6aR,8R)-8-(benzyloxy)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00239] Borane dimethyl sulfide complex (2.0 M in THF, 2.23 mL, 4.46 mmol) was added to a stirring solution of (6aR,8R)-8-(benzyloxy)-2-chloro-6a-(difluoromethyl)-6a,7,8,9-tetrahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (680 mg, 1.79 mmol) in THF (9 mL). The reaction mixture was heated to 55 °C and stirred for 1.5 hours. The reaction mixture was cooled to room temperature and borane dimethyl sulfide complex (2.0 M in THF, 1.0 mL, 2.0 mmol) was added. The reaction mixture was heated to 55 °C and stirred for 1.5 hours. The reaction mixture was cooled to 0 °C and quenched by slow addition of MeOH (9 mL). The reaction mixture was concentrated. The residue obtained was dissolved in EtOH (14.5 mL). Acetic acid (2.02 mL, 35.4 mmol) and sodium cyanoborohydride (270 mg, 4.3 mmol) were added in sequence to the reaction mixture. The reaction mixture was heated to 70 °C and stirred overnight. The product mixture was cooled to 0 °C and quenched by slow addition of a saturated sodium bicarbonate aqueous solution (60 mL). The diluted product mixture was extracted with DCM (3 x 60 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain (6aR,8R)-8-(benzyloxy)-2-chloro-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (607 mg, 93%). LCMS calcd for C17H18ClF2N4O [M+H]+: m/z = 367.1; Found: 367.0. - 56 -4860-6199-1670.2 105807.001049 – PCT Application [00240] Step 7. (6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-ol [00241] Boron trichloride (1.0 M was added dropwise to a stirring solution of (6aR,8R)-8-(benzyloxy)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazine (607 mg, 1.65 mmol) in DCM (15.6 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 30 minutes. The product mixture was quenched by slow addition of saturated sodium bicarbonate solution (100 mL) at 0 °C. The diluted product mixture was extracted with a 3:1 chloroform:isopropanol solution (6 x 100 mL).The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure to obtain the title compound (432 mg, 94%) as a white solid. LCMS calcd for C10H12ClF2N4O [M+H]+: m/z = 277.1; Found: 277.0. [00242] Step 8. (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-yl 4-nitrobenzoate [00243] Diisopropyl azodicarboxylate (1.42 mL, 7.23 mmol) was added dropwise to a stirring solution of triphenylphosphine (1.9 g, 7.23 mmol) in THF (10 mL) at room temperature. The reaction mixture was stirred for 10 minutes. Then 4-nitrobenzoic acid (1.21 g, 7.23 mmol) was added to the reaction mixture. The reaction mixture was stirred for 10 minutes. The reaction mixture solution was added to a stirring solution of (6aR,8R)-2-chloro-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (1.0 g, 3.61 mmol) in a mixture of THF (9 mL) and DCM (16.5 mL). The combined reaction mixture was stirred for 30 minutes at room temperature. The product mixture was diluted with a saturated sodium bicarbonate aqueous solution (100 mL). The diluted product mixture was extracted with a 3:1 chloroform:isopropanol solution (3 x 100 mL). The combined organic layers were dried over - 57 -4860-6199-1670.2 105807.001049 – PCT Application MgSO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-15% MeOH/DCM to obtain the title compound (1.46 g, 95%). LCMS calcd for C17H15ClF2N5O4 [M+H]+: m/z = 426.1; Found: 426.0. [00244] Step 9. (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-ol [00245] Potassium carbonate (2.5 g, 18.1 mmol) was added to a stirring solution of (6aR,8S)-2- chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl 4-nitrobenzoate (1.46 g, 3.43 mmol) in MeOH (16.5 mL) at room temperature. The reaction mixture was stirred for 30 minutes. The product mixture was diluted with water (30 mL), a saturated sodium carbonate aqueous solution (30 mL), and a saturated sodium chloride aqueous solution (30 mL). The diluted product mixture was extracted with a 3:1 chloroform:isopropanol solution (6 x 100 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure. The solid obtained was suspended in a 10% MeOH/DCM solution (10 mL) and filtered. The white solid was washed with 10% MeOH/DCM (10 mL) then collected to obtain (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol. The filtrate was concentrated and purified by silica gel flash column chromatography with a gradient of 0-20% MeOH/DCM to obtain the title compound (865 mg, 91%). LCMS calcd for C10H12ClF2N4O [M+H]+: m/z = 277.1; Found: 276.9. Intermediate 2. (S)-3-(1-oxo-6- 4- isoindolin-2- piperidine-2,6-dione [00246] Step 1: Methyl 5-bromo-2-(dibromomethyl)benzoate [00247] A suspension of methyl 5-bromo-2-methylbenzoate (6.08 g, 26.6 mmol), NBS (14.0 g, 79.7 mmol), and benzoyl peroxide (0.32 g, 1.33 mmol) in DCE (60.0 mL) was stirred at 80 °C for 18 h. The reaction mixture was cooled to ambient temperature, diluted with DCM (140 - 58 -4860-6199-1670.2 105807.001049 – PCT Application mL), washed with 10% Na2S2O3 solution (100 mL), saturated NaHCO3 solution (100 mL), water (100 mL), and brine (100 mL), then dried over Na2SO4, filtered and concentrated. The residue was dried under high vacuum to give the desired product, methyl 5-bromo-2-(dibromomethyl)- benzoate (9.875 g, 96.1%), as light yellow solid.1H NMR (300 MHz, CDCl3) δ 8.04 (dd, J = 5.4, 3.1 Hz, 2H), 7.97 (s, 1H), 7.74 (dd, J = 8.6, 2.1 Hz, 1H), 3.96 (s, 3H). [00248] Step 2: Methyl 5-bromo-2-formylbenzoate [00249] Silver nitrate (6.57 g, 38.7 was in water (60.0 mL). This solution was added dropwise into the stirred solution of methyl 5-bromo-2-(dibromomethyl)benzoate (5.99 g, 15.5 mmol) in IPA (60.0 mL) at 0 °C. The resulting mixture was stirred at ambient temperature in the dark for 4 h, when HPLC indicated the disappearance of the starting material. The precipitated silver salt was filtered off and washed with IPA in small portions. IPA was evaporated under reduced pressure and the remaining aqueous phase was extracted with EtOAc (50.0 mL × 3). The combined organic phase was washed with water (50.0 mL), brine (50.0 mL), then dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was dried under high vacuum to give the desired product, methyl 5-bromo-2-formylbenzoate (3.26 g, 13.4 86.8 %), as white solid. LCMS calculated for C9H8BrO3 (M+H)+: m/z = 242.9; found: 242.8.1H NMR (300 MHz, CDCl3) δ 10.58 (s, 1H), 8.13 (d, J = 1.5 Hz, 1H), 7.85 – 7.76 (m, 2H), 3.99 (s, 3H). [00250] Step 3: tert-Butyl 4-(4-formyl-3-methoxycarbonylphenyl)-3,6-dihydro-2H-pyridine-1- carboxylate [00251] A suspension of N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester (5.55 g, 17.9 mmol), methyl 5-bromo-2-formylbenzoate (2.91 g, 12.0 mmol), Pd(dppf)Cl2 (0.44 g, 0.6 mmol), and CsOAc (9.18 g, 47.8 mmol) in 1,4-dioxane (40.0 mL) and water (10.0 mL) was heated at 95 °C under N2 atmosphere for 1.5 h, when HPLC indicated the full conversion of the starting material. The reaction was cooled to ambient temperature and partitioned between EtOAc and water (100 mL each). The organic layer was separated, and the aqueous layer extracted with EtOAc (50 mL × 3). The combined organic phase was washed with water and brine (100 mL each), dried over Na2SO4, filtered and concentrated. The residue was purified by flash column chromatography (0~25% EtOAc in heptanes) to give the desired product, tert-butyl - 59 -4860-6199-1670.2 105807.001049 – PCT Application 4-(4-formyl-3-methoxycarbonylphenyl)-3,6-dihydro-2H-pyridine-1-carboxylate (3.30 g, 79.9 %), as yellow solid. Rf = 0.4 (25% EtOAc in heptanes).1H NMR (300 MHz, CDCl3) δ 10.57 (s, 1H), 7.93 (t, J = 4.8 Hz, 2H), 7.63 (dd, J = 8.1, 1.5 Hz, 1H), 6.25 (s, 1H), 4.12 (d, J = 2.8 Hz, 2H), 3.98 (s, 3H), 3.66 (t, J = 5.7 Hz, 2H), 2.56 (d, J = 1.4 Hz, 2H), 1.49 (s, 9H). [00252] Step 4: tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin- 5-yl)-3,6-dihydropyridine-1(2H)-carboxylate [00253] To a suspension tert-butyl 4-(4-formyl-3-methoxycarbonylphenyl)-3,6-dihydro-2H- pyridine-1-carboxylate (2.88 g, 8.34 mmol) and H-Glu(OMe)-NH2 hydrochloride (2.05 g, 10.4 mmol) in DCM (40 mL) was slowly added DIPEA (4.36 mL, 25.0 mmol) and the resulting mixture was stirred at ambient temperature for 30 min. AcOH (2.86 mL, 50.0 mol) was then added and the mixture was stirred for 1 h. NaBH(OAc)3 (5.30 g, 25.0 mmol) was added in portions and the reaction was stirred at ambient temperature for 18 h. The reaction was diluted with DCM (150 mL), washed with saturated NaHCO3 solution, dried over Na2SO4, filtered and concentrated. The residue was purified by flash column chromatography (0~5% MeOH in DCM) to give the desired product, tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3- oxoisoindolin-5-yl)-3,6-dihydropyridine-1(2H)-carboxylate (3.01 g, 78.9 %), as white solid. Rf = 0.3 (5% MeOH in DCM). LCMS calculated for C24H32N3O6 (M+H)+: m/z = 458.2; found: 458.1.1H NMR (300 MHz, DMSO) δ 7.75 – 7.67 (m, 2H), 7.57 (d, J = 8.3 Hz, 2H), 7.20 (s, 1H), 6.26 (s, 1H), 4.75 (dd, J = 10.2, 4.6 Hz, 1H), 4.52 (dd, J = 45.0, 17.7 Hz, 2H), 4.02 (d, J = 7.1 Hz, 2H), 3.56 (t, J = 5.5 Hz, 2H), 3.50 (s, 3H), 2.58 – 2.50 (m, 2H), 2.29 – 1.96 (m, 4H), 1.43 (s, 9H). [00254] Step 5: tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)-3,6-dihydro- pyridine-1(2H)-carboxylate [00255] tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin-5-yl)- 3,6-dihydropyridine-1(2H)-carboxylate (1.01 g, 2.20 mmol) in THF (15.0 mL) was added t- BuOK (1.0 M in THF,, 2.75 mmol, 2.75 mL) dropwise at -78 °C. The resulting mixture was stirred at -78 °C for 2 h. The reaction was quenched by the addition of 1 N HCl solution until pH 6 and extracted with DCM (50 mL × 3). The combined organic phase was washed with brine (50 - 60 -4860-6199-1670.2 105807.001049 – PCT Application mL), dried over Na2SO4, filtered and concentrated. The residue was suspended in a minimum amount of DCM (2.00 mL) and MTBE (20.0 mL) was added at 0 °C. The white precipitate was filtered off and washed with cold MTBE. The filter cake was dried under air flow to give tert- butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)-3,6-dihydropyridine-1(2H)- carboxylate (718 mg, 1.69 mmol, 76.7% yield) as white solid. LCMS calculated for C19H20N3O5 (M+2H-tBu)+: m/z = 370.14; found: 370.09.1H NMR (300 MHz, DMSO) δ 10.99 (s, 1H), 7.75 – 7.71 (m, 2H), 7.58 (d, J = 8.5 Hz, 1H), 6.28 (s, 1H), 5.13 (dd, J = 13.2, 5.1 Hz, 1H), 4.39 (dd, J = 41.6, 17.5 Hz, 2H), 4.02 (s, 2H), 3.56 (t, J = 5.6 Hz, 2H), 3.01 – 2.83 (m, 1H), 2.71 – 2.51 (m, 3H), 2.40 (qd, J = 13.3, 4.5 Hz, 1H), 2.01 (ddd, J = 10.2, 5.1, 3.1 Hz, 1H), 1.43 (s, 9H). [00256] Step 6. tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-1- carboxylate [00257] tert-Butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)-3,6- dihydropyridine-1(2H)-carboxylate (818 mg, 1.92 mmol) in MeOH (5.00 mL) and THF (5.00 mL) was hydrogenated under 30 psi for 2 h with 10% Pd/C. The solid materials were filtered off and washed with MeOH in small portions. The filtrate was concentrated and the residue was purified by flash column chromatography on silica gel (0~4% MeOH in DCM) to give tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-1-carboxylate (809 mg, 98.4 %) as white solid. Rf = 0.25 (5% MeOH in DCM). LCMS calculated for C19H22N3O5 (M+2H- tBu)+: m/z = 372.2; found: 372.0.1H NMR (300 MHz, DMSO) δ 10.99 (s, 1H), 7.55 (d, J = 11.0 Hz, 3H), 5.11 (dd, J = 13.3, 5.1 Hz, 1H), 4.35 (dd, J = 41.3, 17.1 Hz, 2H), 4.08 (d, J = 10.3 Hz, 2H), 2.99 – 2.74 (m, 4H), 2.59 (dd, J = 15.4, 2.2 Hz, 1H), 2.39 (qd, J = 13.1, 4.3 Hz, 1H), 2.10 – 1.92 (m, 1H), 1.79 (d, J = 12.1 Hz, 2H), 1.66 – 1.45 (m, 2H), 1.42 (s, 9H). [00258] Step 7: (S)-3-(1-oxo-6-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6- dione;hydrochloride [00259] tert-Butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-1- carboxylate (809 mg, 1.89 mmol) was suspended in 1,4-dioxane (2.00 mL) and 4 N HCl in 1,4- dioxane (15.0 mL) was added at 0 °C. The reaction was stirred at ambient temperature for 18 h. The white precipitate was filtered off and washed with cold 1,4-dioxane in small portions. The filter cake was dried under air flow to give (S)-3-(1-oxo-6-(piperidin-4-yl)isoindolin-2-yl) piperidine-2,6-dione hydrochloride (688 mg, quant.) as white solid. LCMS calculated for C18H22N3O3 (M+H)+: m/z = 328.2; found: 328.1. - 61 -4860-6199-1670.2 105807.001049 – PCT Application Intermediate 3. (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2-yl)piperidine-2,6-dione; hydrochloride [00260] Step 1: tert-butyl 4-(4-cyano- piperazine-1-carboxylate [00261] To a solution of methyl 2-cyano-5-fluorobenzoate (20.0 g, 112 mmol) and tert-butyl 1- piperazinecarboxylate (31.2 g, 167 mmol) in NMP (20.0 mL) was added N,N-diisopropyl- ethylamine (0.01 mL, 0.04 mmol) and heated to 120 °C for 4 h. HPLC monitoring after 4 h showed the consumption of starting material. The reaction mixture was cooled to -20°C overnight and product crashed out. The solid was filtered and washed with heptanes to obtain yellow solid as the desired product tert-butyl 4-(4-cyano-3-(methoxycarbonyl)phenyl)piperazine- 1-carboxylate (28.0 g, 72.6 %). LCMS calculated for C13H16N3O2 (M+2H-Boc)+: m/z = 246.17; found: 245.99.1H NMR (300 MHz, DMSO) δ 7.73 (d, J = 8.8 Hz, 1H), 7.47 (d, J = 2.7 Hz, 1H), 7.22 (dd, J = 8.8, 2.7 Hz, 1H), 3.89 (s, 3H), 3.51 – 3.38 (m, 8H), 1.42 (s, 9H). [00262] Step 2: tert-butyl 4-(4-formyl-3-(methoxycarbonyl)phenyl)piperazine-1-carboxylate [00263] To a solution of tert-butyl 4-(4-cyano-3-methoxycarbonylphenyl)piperazine-1- carboxylate (8.10 g, 23.5 mmol) in pyridine (11.4 mL, 141 mmol) and acetic acid (8.05 mL, 141 mmol) was added sodium phosphinate monohydrate (4.97 g, 46.9 mmol) and water (10.0 mL). To the stirring solution was added Raney Nickel (2.75 g, 46.9 mmol) in multiple portions to avoid the emulsion formation. The reaction was heated to 75°C for 7 h and monitored by HPLC to see only ~50% conversion. To the solution was added another 1.0 equiv of Raney Nickel and heated at 75°C overnight. HPLC monitoring showed ~94% conversion. The heating was stopped and the reaction was cooled down, diluted with MeOH and filtered through celite and washed with MeOH to remove Raney Nickel. The filtrate was concentrated to remove pyridine and MeOH. The concentrated solution was then washed with water. The organic phase was dried over Na2SO4, filtered and concentrated to obtain the crude. Purified by FCC (0% to 60% - 62 -4860-6199-1670.2 105807.001049 – PCT Application EA/Heptanes) to obtain yellowish solid as desired product tert-butyl 4-(4-formyl-3-methoxy- carbonylphenyl)piperazine-1-carboxylate (5.00 g, 61.1 %). LCMS calculated for C18H25N2O5 (M+H)+: m/z = 349.2; found: 348.9.1H NMR (300 MHz, CDCl3) δ 10.34 (s, 1H), 7.92 (d, J = 8.8 Hz, 1H), 7.26 (d, J = 1.6 Hz, 1H), 7.00 (dd, J = 8.8, 2.6 Hz, 1H), 3.96 (s, 3H), 3.66 – 3.55 (m, 4H), 3.39 (dd, J = 13.4, 8.6 Hz, 4H), 1.48 (s, 9H). [00264] Step 3: tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin- 5-yl)piperazine-1-carboxylate [00265] To a solution of tert-butyl 4-(4-formyl-3-methoxycarbonylphenyl)piperazine-1- carboxylate (4.20 g, 12.0 mmol) in DCM (50.0 mL) was added N,N-diisopropylethylamine (5.25 mL, 30.1 mmol) and methyl (4S)-4,5-diamino-5-oxopentanoate (2.32 g, 14.5 mmol). The reaction was stirred at RT for 1 h and acetic acid (6.89 mL, 121 mmol) was added to this solution. The reaction was further stirred for another hour and sodium triacetoxyborohydride (7.66 g, 36.2 mmol) was added. The reaction was stirred overnight to see full conversion, monitored by HPLC. The reaction mixture was diluted with DCM (30.0 mL) and quenched with water (30.0 mL). Further extraction was done by DCM (30 mL x 2). The organic phase was dried over Na2SO4, filtered, and concentrated to obtain the crude. Crude was further purified by FCC (0% to 7% MeOH/DCM) to obtain white solid as desired compound tert-butyl (S)-4-(2-(1-amino- 5-methoxy-1,5-dioxopentan-2-yl)-3-oxoisoindolin-5-yl)piperazine-1-carboxylate (3.80 g, 68.5 %). LCMS calculated for C23H33N4O6 (M+H)+: m/z = 461.2; found: 461.1.1H NMR (300 MHz, CDCl3) δ 7.33 (dd, J = 10.8, 5.3 Hz, 2H), 7.16 (dd, J = 8.4, 2.4 Hz, 1H), 6.40 (s, 1H), 5.58 (s, 1H), 5.00 – 4.83 (m, 1H), 4.39 (q, J = 16.8 Hz, 2H), 3.63 (s, 3H), 3.62 – 3.55 (m, 4H), 3.22 – 3.13 (m, 4H), 2.49 – 2.28 (m, 3H), 2.20 (tt, J = 11.4, 8.2 Hz, 1H), 1.49 (s, 9H). [00266] Step 4: tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperazine- 1-carboxylate [00267] To a solution of tert-butyl (S)-4-(2-(1-amino-5-methoxy-1,5-dioxopentan-2-yl)-3- oxoisoindolin-5-yl)piperazine-1-carboxylate (3.80 g, 8.25 mmol) in THF (80.0 mL), was added potassium t-butoxide (1.0 M in THF, 9.90 mL, 9.90 mmol) at -78°C. The reaction was - 63 -4860-6199-1670.2 105807.001049 – PCT Application stirred at -78°C for 3 h. The reaction temperature was maintained at 0 °C and quenched by addition of 1N HCl to achieve pH 3. To this solution NaHCO3 (sat. aq.) was added dropwise slowly to achieve pH ~6. The reaction mixture was then diluted further by DCM and washed with water. Water phase was further extracted by DCM. Organic phase was collected, dried over Na2SO4, filtered and concentrated to obtain the crude. tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3- yl)-3-oxoisoindolin-5-yl)piperazine-1-carboxylate (2.90 g, 82.0 %). The crude was about 96% pure (HPLC) which was taken to the next step without further purification. LCMS calculated for C18H21N4O5 (M+2H-tBu)+: m/z = 373.2; found: 373.0.1H NMR (300 MHz, CDCl3) δ 8.06 (s, 1H), 7.37 (dd, J = 9.3, 5.3 Hz, 2H), 7.17 (dd, J = 8.4, 2.3 Hz, 1H), 5.22 (dd, J = 13.1, 5.2 Hz, 1H), 4.35 (dd, J = 43.1, 15.6 Hz, 2H), 3.72 – 3.49 (m, 4H), 3.30 – 3.13 (m, 4H), 2.96 – 2.73 (m, 2H), 2.43 – 2.15 (m, 2H), 1.49 (s, 9H). [00268] Step [00269] To a round bottomed flask containing tert-butyl (S)-4-(2-(2,6-dioxopiperidin-3-yl)-3- oxoisoindolin-5-yl)piperazine-1-carboxylate (4.3 g, 10.0 mmol) was added 4N HCl in dioxane (34.0 mL, 136 mmol) at 0 °C. The solution was stirred at 0 °C to RT for 3 h to see full conversion. The precipitate was filtered and dried under air flow to yield (S)-3-(1-oxo-6- (piperazin-1-yl)isoindolin-2-yl)piperidine-2,6-dione;hydrochloride (3.30 g, 90.1 %). LCMS calculated for C17H21N4O3 (M+H)+: m/z = 329.2; found: 329.0.1H NMR (300 MHz, DMSO) δ 10.98 (s, 1H), 9.27 (s, 2H), 7.49 (d, J = 8.3 Hz, 1H), 7.39 – 7.19 (m, 2H), 5.10 (dd, J = 13.2, 5.1 Hz, 1H), 4.27 (dt, J = 27.7, 13.9 Hz, 2H), 3.49 – 3.40 (m, 4H), 3.22 (s, 4H), 2.98 – 2.83 (m, 1H), 2.59 (d, J = 16.3 Hz, 1H), 2.46 – 2.27 (m, 1H), 2.09 – 1.94 (m, 1H). Intermediate 4. (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde [00270] Step 1. methyl 2-cyano-5-[4-(hydroxymethyl)piperidin-1-yl]benzoate - 64 -4860-6199-1670.2 105807.001049 – PCT Application [00271] To a solution of methyl g, 27.9 mmol) and 4- piperidine-methanol (9.64 g, 83.7 mmol) in NMP (20 mL), N, N-diisopropylethylamine (7.29 mL, 41.9 mmol) was added and the reaction was heated at 120°C for 4 h. DIPEA was removed using rotary evaporation. The solution was further diluted by ethyl acetate and washed with H2O. The organic layer was collected and dried over Na2SO4, filtered and concentrated. The crude product was purified by FCC (0% to 40% EA/heptanes) to obtain the title compound as a yellowish solid (5.1 g, 18.6 mmol, 67% yield). LCMS: (M+H)+ = 275.1. [00273] To a solution of methyl 2-cyano-5-[4-(hydroxymethyl)piperidin-1-yl]benzoate (2.5 g, 9.1 mmol) in DCM (30 mL) was added Dess-Martin Periodinane (5.8 g, 13.7 mmol) at 0°C. The reaction was stirred for 15 mins at room temperature, and TLC monitoring showed full consumption of starting material (eluent: 100% EA). The reaction mixture was diluted with DCM and quenched by saturated NaHCO3 until effervescence stopped. The aqueous phase was extracted with DCM and the organic phase was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude was purified by FCC (10% to 85% EA/heptanes) to obtain the title compound that was taken to next step without further purification.1H NMR (300 MHz, CDCl3) δ 9.71 (s, 1H), 7.64 - 7.46 (m, 2H), 6.99 (dt, J = 10.4, 5.2 Hz, 1H), 3.98 (dt, J = 13.1, 4.1 Hz, 2H), 3.22 – 3.05 (m, 2H), 2.65 – 2.47 (m, 1H), 2.12 – 1.99 (m, 2H), 1.85 – 1.67 (m, 2H). [00275] To a solution of methyl 2-cyano-5-(4-formylpiperidin-1-yl)benzoate (2.1 g, 7.71 mmol) in methanol (30 mL) was added trimethyl orthoformate (2.54 mL, 23.1 mmol) at 0°C. The reaction was stirred for 7 h at room temperature. The reaction was quenched by addition of TEA (0.2 eq), solvent was concentrated under reduced pressure and the crude was dissolved in ethyl - 65 -4860-6199-1670.2 105807.001049 – PCT Application acetate. This solution was washed with water (30 mL x 2), organic phase was collected, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude was purified by FCC (0% to 45% EA/Heptanes) to obtain the title compound. (1.26 g, 3.9 mmol, 52% yield).1H NMR (300 MHz, CDCl3) δ 7.62 - 7.47 (m, 2H), 6.97 (dd, J = 8.8, 2.8 Hz, 1H), 4.06 (d, J = 6.5 Hz, 1H), 3.98 (s, 3H), 3.93 (d, J = 12.9 Hz, 2H), 3.43 – 3.30 (m, 6H), 2.89 (td, J = 12.9, 2.4 Hz, 2H), 1.95 – 1.79 (m, 3H), 1.39 (ddd, J = 16.0, 13.5, 3.6 Hz, 2H). 4. 5- 1- -2- [00277] To a solution of methyl 2-cyano-5-[4-(dimethoxymethyl)piperidin-1-yl]benzoate (1.2 g, 3.77 mmol) in pyridine (2.44 mL, 30.2 mmol) and acetic acid (2.16 mL, 37.7 mmol) was added sodium phosphinate hydrate (0.8 g, 7.54 mmol) and water (5 mL). To the stirring solution, Raney nickel slurry in water (663 mg, 11.3 mmol) was added in multiple portions to avoid emulsion formation. The reaction was heated to 75°C overnight. The heating was stopped and the reaction was cooled down, diluted with MeOH, filtered through celite, and washed with MeOH. The filtrate was concentrated to remove pyridine and methanol. The residue was dissolved in ethyl acetate and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The product was purified by FCC (0% to 35% EA/Heptanes) to obtain the title compound (742 mg, 2.31 mmol, 61% yield). [00278] Step 5: methyl (S)-5-amino-4-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin- 2-yl)-5-oxopentanoate [00279] To a solution of methyl 5-[4-(dimethoxymethyl)piperidin-1-yl]-2-formylbenzoate (450.0 mg, 1.4 mmol) in DCM (2 mL) and DMF (2 mL) was added N,N-diisopropylethylamine (0.61 mL, 3.5 mmol) and methyl (S)-4,5-diamino-5-oxopentanoate (269 mg, 1.68 mmol). The reaction was stirred at rt for 1h and acetic acid (0.8 mL, 14. mmol) was added to this solution. The reaction was further stirred for another hour and sodium triacetoxyborohydride (890 mg, 4.2 mmol) was added, and the reaction was stirred over the weekend. The reaction was stopped, - 66 -4860-6199-1670.2 105807.001049 – PCT Application diluted with DCM (50 mL), and quenched by saturated NaHCO3 solution dropwise until pH of 8- 9 was maintained. The organic phase was collected, dried over Na2SO4, filtered and concentrated. The residue was purified by FCC (0% to 100% EA/Heptanes) to obtain the title compound (400 mg, 0.93 mmol, 66% yield). [00280] Step 6: (S)-3-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione [00281] A 1.0 M solution of potassium t-butoxide (8.30 mL, 8.30 mmol) in THF was added to a solution of methyl (S)-5-amino-4-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)-5-oxopentanoate (3.00 g, 6.92 mmol) in THF (80.0 mL) at -78 °C and the reaction was stirred for 3 hours. The reaction was warmed to 0 °C and the pH was carefully adjusted to ~ 3 using a 1N aqueous HCl solution. To this solution, a saturated aqueous NaHCO3 was carefully added dropwise to adjust the pH ~ 6. DCM was added and the phases were separated. The combined organics were washed with water, dried over MgSO4, filtered and concentrated under reduced pressure. The crude material was triturated with DCM (5.00 mL) followed by MTBE (20.0 mL) upon which a white precipitate crashed out. The solids were filtered and dried via vacuum filtration to obtain 3-(6-(4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione (1.89 g, 68% yield).1H NMR (300 MHz, DMSO) δ 10.97 (s, 1H), 7.41 (d, J = 8.4 Hz, 1H), 7.25 (dd, J = 8.5, 2.3 Hz, 1H), 7.14 (d, J = 2.2 Hz, 1H), 5.10 (dd, J = 13.3, 5.1 Hz, 1H), 4.33 (d, J = 16.8 Hz, 1H), 4.19 (d, J = 16.7 Hz, 1H), 4.08 (d, J = 6.6 Hz, 1H), 3.77 (d, J = 12.3 Hz, 2H), 3.27 (s, 6H), 2.98 – 2.82 (m, 1H), 2.65 (dd, J = 29.4, 15.4 Hz, 3H), 2.38 (qd, J = 13.3, 4.4 Hz, 1H), 2.04 – 1.92 (m, 1H), 1.72 (d, J = 10.8 Hz, 3H), 1.34 (dt, J = 21.6, 10.9 Hz, 2H). [00282] Step 7: (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde [00283] 2,2,2-Trifluoroacetic acid (2.38 mL, 31.1 mmol) was added to a solution of (S)-3-(6- (4-(dimethoxymethyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (500 mg, 1.25 mmol) in a 3 to 1 mixture of DCM (9.3 mL) and acetone (3.1 mL). The reaction was stirred overnight and concentrated under reduced pressure to obtain the title compound (440 mg, quantitative yield) as the TFA salt. LCMS calcd for C19H22N3O4 (M+H)+ m/z = 356.2; Found: 356.2. - 67 -4860-6199-1670.2 105807.001049 – PCT Application Intermediate 5. (6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-ol [00284] Step 1: (6aR,8R)-8- - 5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazine [00285] To a solution of (6aR,8R)-8-(benzyloxy)-2-chloro-6a-ethyl-6a,7,8,9-tetrahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (1.9 g, 5.3 mmol, Intermediate 7) in THF (25 mL) at 0 °C was added BH3·Me2S (2 M in THF; 5 mL, 10 mmol). The reaction mixture was stirred at 55 °C for ~48 h, after which additional BH3·Me2S (2 M in THF; 2.5 mL, 5 mmol) was added. The reaction was stirred at 55 °C for 3 h then cooled, quenched with MeOH, and concentrated. The residue was dissolved in EtOH (40 mL) then cooled to 0 °C and treated with AcOH (6.3 mL, 105 mmol) then NaBH3CN (0.8 g, 12.7 mmol) in two portions. The reaction was warmed to room temperature then stirred at 70 °C for 6 h, after which it was cooled to room temperature and concentrated. The residue was diluted with DCM and sat. NaHCO3 (aq) and extracted with DCM (3 x). The combined organic layers were dried with Na2SO4, filtered and concentrated. The crude material was purified via SiO2 FCC (0-20% MeOH in DCM) to give (6aR,8R)-8-(benzyloxy)-2- chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (1.22 g, 60%). LCMS calcd for C18H22ClN4O [M+H]+: m/z = 345.1; Found: 345.0. [00286] Step 2: (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-ol [00287] To (6aR,8R)-8-(benzyloxy)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazine (1.2 g, 3.5 mmol) in DCM (35 mL) at 0 °C was added BCl3 (1 M in - 68 -4860-6199-1670.2 105807.001049 – PCT Application DCM; 10 mL, 10 mmol). The reaction was stirred at 0 °C for 1.5 h then quenched slowly with sat. NaHCO3 (aq). The mixture was poured into water, neutralized with additional sat. NaHCO3 (aq), then extracted with DCM (3 x). The combined organic layers were washed with brine (1 x), dried with MgSO4, filtered then concentrated. The residue was taken up in MTBE then sonicated and filtered to give (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-ol (740 mg, 84%) as a white solid. LCMS calcd for C11H16ClN4O [M+H]+: m/z = 255.1; Found: 255.0. [00288] Step 3: (6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-yl 4-nitrobenzoate [00289] To a mixture of (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1’,2’:4,5] pyrazino[2,3-c]pyridazine-8-ol (200 mg, 0.79 mmol), 4-nitrobenzoic acid (262 mg, 1.57 mmol), and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 735 mg, 1.18 mmol) in THF (10 mL) was added diisopropyl azodicarboxylate (0.23 mL, 1.18 mmol). The reaction was stirred at 65 °C 25 min then allowed to cool to room temperature. The mixture was filtered and the resulting filtrate was purified via SiO2 FCC (0-10% MeOH in DCM) to afford (6aR,8S)-2-chloro- 6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl 4-nitrobenzoate (assumed quantitative yield) as a yellow solid. LCMS calcd for C18H19ClN5O4 [M+H]+: m/z = 404.1; Found: 404.0. [00290] Step 4: (6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-ol [00291] To (6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-yl 4-nitrobenzoate (1.16 g, 2.86 mmol) in MeOH (8 mL) and THF (16 mL) was added NaOH (802 mg, 1.18 mmol) in water (9 mL). The reaction was stirred at room temperature for 15 min then diluted with a small amount of water and subsequently bulk-extracted with DCM (3 x). The combined organic layers were washed with brine (1 x) then dried with MgSO4, filtered and concentrated. The residue was taken up in MTBE and the resulting mixture was filtered through a 0.45 µm PTFE fritted filter. The collected solids were washed with MTBE and dried to - 69 -4860-6199-1670.2 105807.001049 – PCT Application give the title compound (548 mg, 75%) as a white solid. LCMS calcd for C11H16ClN4O [M+H]+: m/z = 255.1; Found: 255.0. Intermediate 6. (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[4,3- b]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00292] Intermediate 6 shown in Table 1 were made using procedures identical to those described for (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[3,4-c]pyridin-1-yl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine using the appropriate starting materials. Table 1. Intermediate 6 l F Intermediate 7. (6aR,8R)-8-(Benzyloxy)-2-chloro-6a-ethyl-6a,7,8,9-tetrahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one [00293] Step 1: 1-(tert-Butyl) 2-methyl (4R)-4-(benzyloxy)-2-ethylpyrrolidine-1,2- dicarboxylate - 70 -4860-6199-1670.2 105807.001049 – PCT Application [00294] To 1-(tert-butyl) 2-methyl (2R,4R)-4-(benzyloxy)pyrrolidine-1,2-dicarboxylate (2.00 g, 5.96 mmol) in THF (55 mL) was added LiHMDS (12 mL, 12 mmol) slowly at −78 °C. The reaction mixture was stirred at −78 °C for 1 h, after which ethyl iodide (1.9 mL, 23.6 mmol) was added. The reaction was stirred at −78 °C for 5 min then allowed to warm to room temperature with stirring. After 3.5 h, the reaction was quenched with MeOH (55 mL). A solution of NaOH (1.91 g, 47.7 mmol) in water (55 mL) was added and the mixture was stirred at room temperature for 20 min then poured into water. The aqueous layer was extracted with EtOAc (3 x). The combined organic layers were washed with brine (1 x) then concentrated and purified via SiO2 FCC (0-30% EtOAc in hexanes) to afford 1-(tert-butyl) 2-methyl (4R)-4-(benzyloxy)-2- ethylpyrrolidine-1,2-dicarboxylate (1.11 g, 51%). LCMS calcd for C15H22NO3 [M−Boc+2H]+: m/z = 264.2; Found: 264.0. [00295] Step 2: (4R)-4-(Benzyloxy)-1-(tert-butoxycarbonyl)-2-ethylpyrrolidine-2-carboxylic acid [00296] To 1-(tert-butyl) 2-methyl (4R)-4-(benzyloxy)-2-ethylpyrrolidine-1,2-dicarboxylate (2.72 g, 7.48 mmol) in 1:1 THF/MeOH (40 mL) was added a solution of NaOH (2.99 g, 74.8 mmol) in water (20 mL). The reaction was stirred at 75 °C for 2.5 h then allowed to cool to room temperature. The mixture was poured into water then extracted with MTBE (3 x). The combined organic layers were washed with water (2x). The combined aqueous layers were extracted once more with MTBE then acidified to pH < 2 with 2 N HCl (aq) and subsequently extracted with EtOAc (3x). The combined EtOAc layers were washed with brine, dried with MgSO4, filtered then concentrated to give crude (4R)-4-(benzyloxy)-1-(tert-butoxycarbonyl)-2-ethylpyrrolidine- 2-carboxylic acid (assumed quantitative yield) as a yellow oil. LCMS calcd for C14H20NO3 [M−Boc+2H]+: m/z = 250.1; Found: 250.0. [00297] Step 3: tert-Butyl (2R,4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3- yl)carbamoyl)-2-ethylpyrrolidine-1-carboxylate - 71 -4860-6199-1670.2 105807.001049 – PCT Application [00298] To crude (4R)-4- - -2-ethylpyrrolidine-2-carboxylic acid (672 mg, 1.43 mmol) and N,N-diisopropylethylamine (1.21 mL, 6.92 mmol) in MeCN (10 mL) was added HATU (880 mg, 2.31 mmol). The reaction was stirred at room temperature for 20 min then concentrated. The residue was taken up in THF (8 mL) then 4-bromo-6-chloro- pyridazin-3-amine (802 mg, 3.85 mmol) was added, followed by NaH (60% dispersion in mineral oil; 385 mg, 9.62 mmol). After rinsing the sides of the flask with THF (2 mL), the reaction was stirred at room temperature for 50 min then cooled to 0 °C and quenched with sat. aq. NH4Cl. The mixture was poured into water then extracted with EtOAc (3 x). The combined organic layers were washed with brine (1 x) then concentrated. The residue was purified via SiO2 FCC (0-100% EtOAc in hexanes) to afford “diastereomer A” tert-butyl (2R,4R)-4-(benzyloxy)-2- ((4-bromo-6-chloropyridazin-3-yl)carbamoyl)-2-ethylpyrrolidine-1-carboxylate (473 mg, 46%) and “diastereomer B” tert-butyl (2S,4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl) carbamoyl)-2-ethylpyrrolidine-1-carboxylate (286 mg, 28%). LCMS calcd for C23H29BrClN4O4 [M+H]+: m/z = 539.1; Found: 539.0 (diastereomer A) and 539.0 (diastereomer B). [00299] Step 4: (6aR,8R)-8-(Benzyloxy)-2-chloro-6a-ethyl-6a,7,8,9- tetrahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-6(5H)-one [00300] To tert-butyl (2R,4R)-4-(benzyloxy)-2-((4-bromo-6-chloropyridazin-3-yl)carbamoyl)- 2-ethylpyrrolidine-1-carboxylate (473 mg, 0.88 mmol) in DCM (17 mL) was added TFA (17 mL, 222 mmol). The reaction was stirred at room temperature for 3 h then concentrated. The residue was dissolved in MeCN (15 mL). N,N-Diisopropylethylamine (0.76 mL, 4.38 mmol) was added and the reaction was stirred at 80 °C for 2 h then allowed to cool to room temperature overnight. The reaction was concentrated to afford crude (6aR,8R)-8-(benzyloxy)-2-chloro-6a-ethyl- 6a,7,8,9-tetrahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (Intermediate 7, assumed quantitative yield) which was used directly without further purification. LCMS calcd for C18H20ClN4O2 [M +H]+: m/z = 359.1; Found: 359.1. - 72 -4860-6199-1670.2 105807.001049 – PCT Application [00301] Intermediate 8. (6aR,8S)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol [00302] Step 1: (6aR,8R)-8- - 2-methoxyphenyl)-6a,7,8,9- tetrahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one [00303] A mixture of (6aR,8R)-8-(benzyloxy)-2-chloro-6a-ethyl-6a,7,8,9-tetrahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (Intermediate 7; 314 mg, 0.88 mmol), 3-fluoro-2- methoxyphenylboronic acid (447 mg, 2.63 mmol), dichloro-1,1'-bisdiphenylphosphino)ferrocene palladium (II) dichloromethane (215 mg, 0.26 mmol) and K2CO3 (605 mg, 4.38 mmol) in 1,4- dioxane (8 mL) and water (2 mL) was sparged with N2 for 5 min then stirred at 100 °C for 2 h. The reaction was allowed to cool to room temperature and subsequently filtered through a 0.45 µm PTFE fritted filter which was then washed with EtOAc. The filtrate was concentrated and the resulting residue was purified via SiO2 FCC (0-10% MeOH in DCM) to give (6aR,8R)-8- (benzyloxy)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-6a,7,8,9-tetrahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-6(5H)-one as an orange solid (468 mg, 119%). LCMS calcd for C25H26FN4O3 [M +H]+: m/z = 449.2; Found: 449.1. [00304] Step 2: (6aR,8R)-8-(Benzyloxy)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5] [00305] To (6aR,8R)-8-(benzyloxy)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-6a,7,8,9- tetrahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-6(5H)-one (393 mg, 0.88 mmol) in THF (5 - 73 -4860-6199-1670.2 105807.001049 – PCT Application mL) was added LiAlH4 (1M in THF; 1.5 mL, 1.5 mmol) slowly at 0 °C. The reaction was allowed to warm to room temperature. After 100 min, additional LiAlH4 (1 M in THF; 1.5 mL, 1.5 mmol) was added. The reaction was stirred for 1 h then cooled to 0 °C. MeOH (8 mL) was added slowly, followed by AcOH (1.5 mL, 26 mmol). The reaction was allowed to warm to room temperature. Sodium cyanoborohydride (551 mg, 8.76 mmol) was added and the reaction was stirred at 80 °C for 17 h. After cooling to 0 °C, the mixture was neutralized with sat. aq. NaHCO3 then poured into water and extracted with EtOAc (3 x). The combined organic layers were washed with brine, dried with MgSO4, filtered then concentrated. The crude residue was purified via SiO2 FCC (0-10% MeOH in DCM) to afford (6aR,8R)-8-(benzyloxy)-6a-ethyl-2-(3-fluoro-2- methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine as a beige solid (347 mg, 91%). LCMS calcd for C25H28FN4O2 [M +H]+: m/z = 435.2; Found: 435.1. [00306] Step 3. (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol [00307] To (6aR,8R)-8-(benzyloxy)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (961 mg, 2.21 mmol) in DCM (25.0 mL) was added trichloroborane (6.63 mL, 6.63 mmol) slowly (down side of flask) at -78 °C. After complete addition, the reaction stirred for ~2 min then warmed to 0 °C (ice-water bath). After 20 min, the reaction was quenched with dropwise addition of water at 0 °C and neutralized with sat. NaHCO3 (aq) then extracted with DCM (3 x). The DCM layers were combined, dried with MgSO4, filtered then concentrated. The crude material was purified via SiO2 FCC: (0-20% MeOH in DCM) to yield the title compound (551 mg, 72%). LCMS calcd for C18H22FN4O2 [M+H]+: m/z = 345.2; Found: 345.1. [00308] Step 4. (6aR,8S)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol [00309] To a briefly sonicated mixture of 4-nitrobenzoic acid (485 mg, 2.90 mmol), (6aR,8R)- 6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c] pyridazin-8-ol (500.0 mg, 1.45 mmol) and triphenylphosphine (100-200 mesh, ~1.6 mmol/g loading; 2.72 g, 4.36 mmol) in THF (20.0 mL) was added diisopropyl azodicarboxylate (858 µL, 4.36 mmol). The reaction was stirred at 65 °C and monitored by LC-MS. After 1.5 hours, the reaction was cooled and filtered to remove the triphenylphosphine, then rinsed with THF (20 mL) then methanol (40 mL). To the filtrate was added potassium carbonate (815 mg, 5.90 - 74 -4860-6199-1670.2 105807.001049 – PCT Application mmol), and the suspension was allowed to stir for 2 hours. The reaction was diluted with DCM and sat. NaHCO3 (aq). The organic layer was separated, and the water layer was extracted with DCM (1 x) then EtOAc (1 x). The organic layers were combined, dried with sodium sulfate, filtered, and condensed. The residue was purified via SiO2 (0-20% MeOH in DCM) to yield the desired product as a white solid (Intermediate 8; 330 mg, 66% yield). LCMS calcd for C18H22FN4O2 [M+H]+: m/z = 345.2; Found: 345.0. Intermediate 9. (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-N-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine [00310] To (6aR,8S)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (Intermediate 8; 134 mg, 0.39 mmol), N-methyl-2- nitrobenzenesulfonamide (252 mg, 1.2 mmol) and PPh3 (153 mg, 0.58 mmol) in THF (3 mL) and DCM (3 mL) was added diisopropyl azodicarboxylate (115 µL, 0.58 mmol). The reaction was stirred at room temperature for 5 min then at 50 °C for 40 min. After allowing to cool to room temperature, additional PPh3 (153 mg, 0.58 mmol) and diisopropyl azodicarboxylate (328 µL, 1.67 mmol) were added sequentially and the reaction was stirred at 50 °C for 10 min then allowed to cool to room temperature. MeCN (6 mL) and thiophenol (320 µL, 3.1 mmol) were added, followed by Cs2CO3 (634 mg, 2.0 mmol). The mixture was stirred at 50 °C for 4 h, cooled briefly, then treated with additional thiophenol (320 µL, 3.1 mmol), MeCN (4 mL) and Cs2CO3 (634 mg, 2.0 mmol). The reaction was stirred at 50 °C overnight then concentrated to near dryness. The mixture was acidified with 2 N HCl (aq) then washed with DCM (3 x). The aqueous phase was basified with 1 N NaOH (aq) to pH > 12 then extracted with DCM (3 x). The combined, latter three DCM layers were combined and washed with brine (1 x), dried with MgSO4, filtered then concentrated to give (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-N- methyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (43 mg, 31%). LCMS calcd for C19H25FN5O [M+H]+: m/z = 358.2; Found: 358.2. Intermediate 10. (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-amine - 75 -4860-6199-1670.2 105807.001049 – PCT Application [00311] Step 1: (6aR,8R)-8-azido-2- hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazine [00312] To (6aR,8S)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (150 mg, 0.59 mmol) and PPh3 (185 mg, 0.71 mmol) in THF (5 mL) was added diisopropyl azodicarboxylate (139 µL, 0.71 mmol). After stirring briefly, diphenylphosphoryl azide (152 µL, 0.71 mmol) was added and the reaction was stirred at room temperature overnight. The reaction was concentrated, treated with a minimal amount of water then purified directly via SiO2 FCC (0-10% MeOH in DCM) to afford (6aR,8R)-8-azido-2-chloro-6a-ethyl- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (117 mg, 71%). LCMS calcd for C11H15ClN7 [M+H]+: m/z = 280.1; Found: 280.0. [00313] Step 2: (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazin-8-amine [00314] To (6aR,8R)-8-azido-2-chloro-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino [2,3-c]pyridazine (117 mg, 0.42 mmol) in THF (5 mL) was added PPh3 (220 mg, 0.84 mmol). The reaction was stirred at 70 °C for 50 min then treated with water (1 mL). The reaction was stirred at 70 °C overnight then allowed to cool to room temperature, at which point 6 N HCl (aq) was added until a pH < 2 was obtained. The reaction was poured into water and extracted with DCM (4 x) to remove impurities. The aqueous layer was concentrated to give the HCl salt of (6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (111 mg, 81%) as an off-white solid. LCMS calcd for C11H17ClN5 [M+H]+: m/z = 254.1; Found: 254.0. Intermediate 11. tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate - 76 -4860-6199-1670.2 105807.001049 – PCT Application [00315] To a briefly - ethyl-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (60 mg, 0.18 mmol), tert-butyl 4- oxopiperidine-1-carboxylate (62 mg, 0.31 mmol) and Et3N (77 µL, 0.55 mmol) in DCE (0.5 mL) was added Ti(OiPr)4 (92 µL, 0.31 mmol). The reaction was stirred at 60 °C for 2 h then cooled to 0 °C. MeOH (0.5 mL) was added, followed by NaBH4 (49 mg, 1.3 mmol). The reaction was allowed to warm to room temperature and stirred for 1 h. Water/MeCN (1:1) and TFA (141 µL, 1.8 mmol) were added, after which the mixture was filtered through a 0.2 µm PTFE syringe filter and purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 13.5-33.5% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (assumed quantitative yield). LCMS calcd for C21H34ClN6O2 [M+H]+: m/z = 437.2; Found: 437.2. Intermediate 12. tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate [00316] To tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (122 mg, 0.18 mmol), acetaldehyde (92 µL, 1.7 mmol) and AcOH (32 µL, 0.55 mmol) in MeOH (2 mL) was added NaBH3CN (35 mg, 0.55 mmol). The reaction was stirred at 50 °C overnight then neutralized with sat. NaHCO3 (aq). The aqueous layer was extracted with DCM (3 x). The combined organic layers were washed with brine (1 x), dried with MgSO4, filtered, then concentrated to afford the title compound (assumed quantitative yield) which was used without further purification. LCMS calcd for C23H38ClN6O2 [M+H]+: m/z = 465.3; Found: 465.2. Intermediate 13. (6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine - 77 -4860-6199-1670.2 105807.001049 – PCT Application [00317] This intermediate was using analogous procedures to those used in the synthesis of Intermediate 10. LCMS calcd for C10H13ClF2N5 [M+H]+: m/z = 276.1; Found: 276.0. Intermediate 14. (6aS,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine [00318] Step 1: (6aS,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-ol [00319] This compound was synthesized using analogous procedures to those described in the synthesis of Intermediate 1. LCMS calcd for C10H12ClF2N4O [M+H]+: m/z = 277.1; Found: 276.9. [00320] Step 2: (6aS,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-amine [00321] This intermediate was synthesized, as its HCl salt, using analogous procedures to those described in the synthesis of Intermediate 10. LCMS calcd for C10H13ClF2N5 [M+H]+: m/z = 276.1; Found: 276.0. Example 1. (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 78 -4860-6199-1670.2 105807.001049 – PCT Application [00323] A 20 mL scintillation vial was charged with 5-bromo-1H-pyrazolo[3,4-b]pyridine (300 mg, 1.51 mmol), vinylboronic acid pinacol ester (513 µL, 3.03 mmol), potassium carbonate (628 mg, 4.54 mmol), and XPhos Pd G2 (119 mg, 0.15 mmol). The mixture was dissolved in 1,4- dioxane (6.3 mL) and water (1.26 mL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 7 hours. The product mixture was diluted with water (30 mL) and a saturated sodium chloride aqueous solution (40 mL). The diluted product mixture was extracted with DCM (3 x 60 mL). Thecombined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-5% MeOH/DCM to obtain the title compound (151 mg, 69%). LCMS calcd for C8H8N3 [M+H]+: m/z = 146.1; Found: 146.0. [00324] Step 2. (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[3,4-b]pyridin- 1-yl)-5,6,6a,7,8,9-hexahydropyrrolo c]pyridazine [00325] Triphenylphosphine (50 mg, 0.19 mmol) and di-tert-butyl azodicarboxylate (50 mg, 0.215 mmol) were added in sequence to a stirring solution of (6aR,8S)-2-chloro-6a- - 79 -4860-6199-1670.2 105807.001049 – PCT Application (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (35 mg, 0.127 mmol) and 5-vinyl-1H-pyrazolo[3,4-b]pyridine (46 mg, 0.316 mmol) in THF (500 µL) and DCM (1 mL). The reaction mixture was heated to 35 °C. The reaction mixture was stirred for 1.5 hours at 35 °C. The product mixture was concentrated and purified directly by silica gel flash column chromatography with a gradient of 0-5% MeOH/DCM to obtain the title compound (42 mg, 82%). LCMS calcd for C18H17ClF2N7 [M+H]+: m/z = 404.1; Found: 404.1. [00326] Step 3.2-((6aR,8R)-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[3,4-b]pyridin-1-yl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00327] A 20 mL scintillation vial was charged with (6aR,8R)-2-chloro-6a-(difluoromethyl)-8- (5-vinyl-1H-pyrazolo[3,4-b]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazine (62 mg, 0.154 mmol), (3-fluoro-2-hydroxyphenyl)boronic acid (78 mg, 0.5 mmol), XPhos Pd G2 (24 mg, 0.03 mmol), and potassium carbonate (106 mg, 0.77 mmol). The mixture was dissolved in 1,4-dioxane (1.2 mL) and water (311 µL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 1 hour. The product mixture was diluted with DCM (3 mL) and MeOH (3 mL). The diluted product mixture was filtered and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain the title compound (42 mg, 57%). LCMS calcd for C24H21F3N7O [M+H]+: m/z = 480.2; Found: 480.0. [00328] Step 4.1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5- carbaldehyde - 80 -4860-6199-1670.2 105807.001049 – PCT Application [00329] Osmium tetroxide (4 wt. and sodium periodate (56 mg, 0.26 mmol) were added in sequence to a stirring solution of ((6aR,8R)-6a-(difluoromethyl)-8-(5- vinyl-1H-pyrazolo[3,4-b]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-2-yl)-6-fluorophenol (42 mg, 0.088 mmol) in THF (1 mL) and water (318 µL). The reaction mixture was stirred at room temperature for 2 hours. The product mixture was diluted with a saturated sodium sulfite aqueous solution (10 mL). The diluted product mixture was stirred for 30 minutes. The product mixture was diluted with a saturated sodium bicarbonate aqueous solution (20 mL). The diluted product mixture was extracted with a 3:1 chloroform:isopropanol solution (3 x 35 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure to obtain the title compound (32 mg, 76%). The residue obtained was used directly in the next step without purification. LCMS calcd for C23H19F3N7O2 [M+H]+: m/z = 482.2; Found: 482.0. [00330] Step 5. (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00331] N,N-Diisopropylethylamine (17.4 µL, 0.1 mmol) was added to a stirring solution of 1- ((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbaldehyde (32 mg, 0.033 mmol) and (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2-yl)piperidine-2,6-dione;hydro- chloride (24.2 mg, 0.066 mmol) in DMSO (1 mL). The reaction mixture stirred at room temperature for 30 minutes. Sodium triacetoxyborohydride (21 mg, 0.1 mmol) was added to the reaction mixture at room temperature. The reaction mixture was heated to 35 °C and stirred for 16 hours. The reaction mixture was cooled to room temperature and diluted with DMSO (4 mL). The diluted reaction mixture was filtered and directly purified by prep-HPLC (Waters CSH-C18, 5 µM, 30x100mm, 12.6-32.6% MeCN/water (containing 0.2%TFA) over 5 min) to give the title compound (7.8 mg, 23%) as its TFA salt. LCMS calcd for C40H39F3N11O4 [M+H]+: m/z = 794.3; Found: 794.2. - 81 -4860-6199-1670.2 105807.001049 – PCT Application Examples 2-3 [00332] The compounds in Table 2 were made using similar conditions to Example 1 using the appropriate starting materials. Table 2. Examples 2-3 Calcd. Found E )+ oxoisoindolin-2-yl)piperidine-2,6-dione - 82 -4860-6199-1670.2 105807.001049 – PCT Application Example 4. (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- c]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00334] A 20 mL scintillation vial was charged with 5-bromo-1H-pyrazolo[3,4-c]pyridine (233 mg, 1.18 mmol), vinylboronic acid pinacol ester (400 µL, 2.36 mmol), potassium carbonate (326 mg, 2.36 mmol), and [1,1’-bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane (96 mg, 0.12 mmol). The mixture was dissolved in 1,4-dioxane (5 mL) and water (0.5 mL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 16 hours. The product mixture was diluted with water (30 mL) and a saturated sodium chloride aqueous solution (40 mL). The diluted product mixture was extracted with DCM (3 x 60 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-5% MeOH/DCM to obtain 5-vinyl-1H-pyrazolo[3,4-c]pyridine (134 mg, 78%). LCMS calcd for C8H8N3 [M+H]+: m/z = 146.1; Found: 146.0. [00335] Step 2. (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8- - 83 -4860-6199-1670.2 105807.001049 – PCT Application [00336] Methanesulfonyl chloride (56 µL, 0.72 mmol) was added to a stirring solution of (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (80 mg, 0.29 mmol) and triethylamine (202 µL, 1.45 mmol) in DCM (3 mL) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 1 hour. The reaction mixture was cooled to 0 °C and triethylamine (202 µL, 1.45 mmol) and methanesulfonyl chloride (56 µL, 0.72 mmol) were added in sequence. The reaction mixture was warmed to room temperature and stirred for 30 minutes. The product mixture was diluted with a saturated sodium bicarbonate aqueous solution (30 mL). The diluted product mixture was extracted with DCM (3x40 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain the title compound (42 mg, 41%). LCMS calcd for C11H14ClF2N4O3S [M+H]+: m/z = 355.0; Found: 354.9. [00337] Step 3. (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[3,4-c]pyridin- 1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine and (6aR,8R)-2-chloro- 6a-(difluoromethyl)-8-(5-vinyl-2H-pyrazolo[3,4-c]pyridin-2-yl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00338] Cesium carbonate (116 mg, 0.36 mmol) was added to a stirring solution of (6aR,8S)-2- chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl methanesulfonate (42 mg, 0.12 mmol) and 5-vinyl-1H-pyrazolo[3,4-c]pyridine (43 mg, 0.30 mmol) in DMF (500 µL) at room temperature. The reaction mixture was heated to 80 °C for 1.5 hours. The product mixture was diluted with DCM (5 mL), filtered, and concentrated. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0- 10% MeOH/DCM to obtain (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-1H-pyrazolo[3,4- c]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (21 mg, 44%) and (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-(5-vinyl-2H-pyrazolo[3,4-c]pyridin-2-yl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (10 mg, 21%). LCMS calcd for C18H17ClF2N7 [M+H]+: m/z = 404.1; Found: 404.0 and LCMS calcd for C18H17ClF2N7 [M+H]+: m/z = 404.1; Found: 404.0. - 84 -4860-6199-1670.2 105807.001049 – PCT Application Examples 4-6 [00339] The compounds in Table 3 were made using similar conditions to Example 1 using the appropriate starting materials. Table 3. Examples 4-6 Calcd. Found E )+ 2,6-dione - 85 -4860-6199-1670.2 105807.001049 – PCT Application Example 7. (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro- 5H-pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00340] Step 1. (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-hydrazineyl-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c] [00341] Methanesulfonyl chloride (28 µL, 0.36 mmol) was added to a stirring solution of (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (40 mg, 0.15 mmol) and triethylamine (101 µL, 0.72 mmol) in DCM (3 mL) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 1 hour. The reaction mixture was cooled to 0 °C and triethylamine (101 µL, 0.72 mmol) and methanesulfonyl chloride - 86 -4860-6199-1670.2 105807.001049 – PCT Application (28 µL, 0.36 mmol) were added in sequence. The reaction mixture was warmed to room temperature and stirred for 30 minutes. The product mixture was diluted with a saturated sodium bicarbonate aqueous solution (30 mL). The diluted product mixture was extracted with DCM (3x40 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue obtained was dissolved in EtOH (2 mL). Hydrazine monohydrate (68 µL, 2.2 mmol) was added to the reaction mixture. The reaction mixture was heated to 80 °C and stirred for 16 hours. The product mixture was concentrated under reduced pressure. The residue obtained was purified by prep-HPLC (Waters CSH-C18, 5 µM, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to give the title compound (32 mg, 55%) as its TFA salt. LCMS calcd for C10H14ClF2N6 [M+H]+: m/z = 291.1; Found: 290.9. [00342] Step 2. tert-butyl 1-((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine- 5-carboxylate [00343] Triethylamine (101 µL, 0.72 mmol) was added to a stirring solution of tert-butyl 3- ((dimethylamino)methylene)-4-oxopiperidine-1-carboxylate (92 mg, 0.36 mmol) and the TFA salt of (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-hydrazineyl-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazine (32 mg, 0.79 mmol) in EtOH (2 mL). The reaction mixture was heated to 60 °C and stirred for 16 hours. The product mixture was diluted with a saturated sodium bicarbonate aqueous solution (35 mL). The diluted product mixture was extracted with a 3:1 chloroform:isopropanol solution (3 x 35 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain tert- butyl 1-((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxylate (22.3 mg, 58%). LCMS calcd for C21H27ClF2N7O2 [M+H]+: m/z = 482.2; Found: 482.1. - 87 -4860-6199-1670.2 105807.001049 – PCT Application [00344] Step 3. tert-butyl 1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridine-5-carboxylate [00345] A 20 mL scintillation vial was charged with tert-butyl 1-((6aR,8R)-2-chloro-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7- tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxylate (22 mg, 0.046 mmol), (3-fluoro-2- hydroxyphenyl)boronic acid (23 mg, 0.15 mmol), XPhos Pd G2 (7 mg, 0.009 mmol), and potassium carbonate (32 mg, 0.23 mmol). The mixture was dissolved in 1,4-dioxane (800 µL) and water (200 µL). The reaction mixture was sparged with N2 gas for 5 minutes, sealed, and heated to 100 °C. The reaction mixture was stirred at 100 °C for 1 hour. The product mixture was diluted with DCM (3 mL) and MeOH (3 mL). The diluted product mixture was filtered and concentrated under reduced pressure. The residue obtained was purified by silica gel flash column chromatography with a gradient of 0-10% MeOH/DCM to obtain the title compound (20.3 mg, 80%). LCMS calcd for C27H31F3N7O3 [M+H]+: m/z = 558.2; Found: 558.1. [00346] Step 4.2-((6aR,8R)-6a-(difluoromethyl)-8-(4,5,6,7-tetrahydro-1H-pyrazolo[4,3- c]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol [00347] Trifluoroacetic acid (400 µL, 5.2 mmol) was added to a stirring solution of tert-butyl 1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5- carboxylate (20.3 mg, 0.036 mmol) in DCM (2 mL) at room temperature. The reaction mixture was stirred for 30 minutes. The product mixture was concentrated under reduced pressure to - 88 -4860-6199-1670.2 105807.001049 – PCT Application obtain the title compound (25 mg, 100%) as its TFA salt. The residue was used without further purification. LCMS calcd for C22H23F3N7O [M+H]+: m/z = 458.2; Found: 458.1. [00348] Step 5. (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00349] N,N-Diisopropylethylamine (9.5 µL, 0.055 mmol) was added to a stirring solution of the TFA salt of 2-((6aR,8R)-6a-(difluoromethyl)-8-(4,5,6,7-tetrahydro-1H-pyrazolo[4,3- c]pyridin-1-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol (25 mg, 0.018 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5- yl)piperidine-4-carbaldehyde (13 mg, 0.037 mmol) in DMSO (1 mL). The reaction mixture stirred at room temperature for 30 minutes. Sodium triacetoxyborohydride (11.6 mg, 0.055 mmol) was added to the reaction mixture at room temperature. The reaction mixture was heated to 35 °C and stirred for 2 hours. The reaction mixture was cooled to room temperature and diluted with DMSO (4 mL). The diluted reaction mixture was filtered and directly purified by prep-HPLC (Waters CSH-C18, 5 µM, 30x100mm, 8.5-28.5% MeCN/water (containing 0.2%TFA) over 5 min) to give the title compound (9.3 mg, 50%) as its TFA salt. LCMS calcd for C41H44F3N10O4 [M+H]+: m/z = 797.4; Found: 797.2. Example 8. (S)-3-(6-(1-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00350] Step 1: 6-vinylquinolin-2-ol [00351] A mixture of 6-bromo-quinolin-2-ol (0.5 g, 2.2 mmol), potassium vinyltrifluoroborate (0.6 g, 4.46 mmol), cesium carbonate (2.18 g, 6.7 mmol) and chloro(2-dicyclohexylphosphino- 2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) (0.161 g, 0.22 mmol) in - 89 -4860-6199-1670.2 105807.001049 – PCT Application 1,4-dioxane (8.9 mL) and water (2.2 mL) was sparged with N2 for 5 minutes. The reaction was heated at 85 °C for 4 hours. The reaction was cooled to room temperature, diluted with DCM, washed with a saturated solution of brine, then dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude material was purified by triturating with 1:4 MTBE:Hexanes (50 mL) to obtain 6-vinylquinolin-2-ol (300 mg, 78% yield). LCMS calcd for C11H10NO [M+H]+: m/z = 172.1; Found: 171.9. [00352] Step 2: (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00353] Diisopropyl azodicarboxylate (37.4 µL, 0.19 mmol) was added to a solution of (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (Intermediate 1; 35.0 mg, 0.13 mmol), 6-vinylquinolin-2-ol (32.5 mg, 0.19 mmol) and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 119 mg, 0.19 mmol) in 1:2 THF:DCM (3 mL) . The reaction mixture was stirred at 30 °C overnight. then cooled toroom temperature and filtered. The filtrate was directly purified via SiO2FCC: 0-5% MeOH/DCM to obtain (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (47 mg, 86% yield) as a thick, viscous yellow oil. LCMS calcd for C21H19ClF2N5O [M+H]+: m/z = 430.1; Found: 429.9. [00354] Step 3: 2-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)-5,6,6a,7,8,9 hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c] 2- -6-fluorophenol [00355] To (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl)oxy)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (47 mg, 0.1 mmol) in 1,4-dioxane (1.6 mL) - 90 -4860-6199-1670.2 105807.001049 – PCT Application and water (0.16 mL) was added 3-fluoro-2-hydroxyphenylboronic acid (49.0 mg, 0.31 mmol) and potassium carbonate (57.9 mg, 0.42 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)] palladium(II) (7.5 mg, 0.01 mmol) was added. The mixture was sparged with N2 for ~2 min then stirred at 85 °C. After 1 h, the reaction was cooled to RT, dried over sodium sulfate, concentrated under reduced pressure and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (50.0 mg, 95%) as a pale yellow solid. LCMS calcd for C27H23F3N5O2 [M+H]+: m/z = 506.2; Found: 506.0. [00356] Step 4: 2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinoline-6-carbaldehyde [00357] Osmium tetroxide (4% in water; 62.9 µL, 0.009 mmol) and 2,6-lutidine (22.9 µL, 0.19 mmol) were added to a solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinylquinolin-2-yl) oxy)-5,6,6a,7,8,9 hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (50.0 mg, 0.09 mmol) in 1,4-dioxane (1.5 mL) and water (0.5 mL). Sodium periodate (63.5 mg, 0.29 mmol) was added, and the reaction was stirred at RT for 2 hours. The reaction was poured into brine and extracted with CHCl3:iPrOH (3:1). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to obtain the title compound (50 mg, assumed quant. yield). The material was used in the following step without additional purification. LCMS calcd for C26H21F3N5O3 [M+H]+: m/z = 508.2; Found: 508.0. [00358] Step 4: (S)-3-(6-(1-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6-yl)methyl) piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 91 -4860-6199-1670.2 105807.001049 – PCT Application [00359] To 2-(( - - -5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinoline-6-carbaldehyde (50.0 mg, 0.09 mmol) and (S)-3-(1-oxo-6-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6-dione (HCl salt; 89.0 mg, 0.2 mmol) in DMSO (1.0 mL) was added N,N-diisopropylethylamine (86 µL, 0.49 mmol). The reaction was stirred for 45 minutes at 35 °C then treated with sodium triacetoxyboro- hydride (104.0 mg, 0.49 mmol). The reaction was stirred at 35 °C overnight then cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Fluoro-Phenyl, 5 uM, 30x100mm, 10.9-30.9 % CH3CN in H2O with 0.1% TFA over 5 minutes) to yield the title compound (3.4 mg, 4% yield) as a white TFA salt. LCMS calcd for C44H42F3N8O5 [M+H]+: m/z = 819.3; Found: 819.2. Examples 9-10 [00360] Examples 9-10 shown below in Table 4 were prepared as TFA salts by the method used in preparing Example 8, utilizing the appropriate starting materials and intermediates. Table 4. Examples 9-10 d E )+ 2 oxoisoindolin-2-yl)piperidine-2,6-dione - 92 -4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found E )+ Example 11. (S)-3-(6-(1-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-2- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00362] A mixture of 2-bromo-quinolin-6-ol (0.5 g, 2.2 mmol), potassium vinyltrifluoroborate (0.6 g, 4.46 mmol), cesium carbonate (2.18 g, 6.7 mmol) and tetrakis(triphenylphosphine)- palladium(0) (0.26 g, 0.22 mmol) in 1,4-dioxane (8.9 mL) and water (2.2 mL) was sparged with N2 for 5 minutes. The reaction was stirred at 85 °C for 4 hours. The reaction was cooled to room temperature, diluted with DCM, washed with a saturated solution of brine, then dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude material was purified by triturating with 1:4 MTBE:Hexanes (50 mL) to obtain2-vinylquinolin-6-ol (220 mg, 58% yield). LCMS calcd for C11H10NO [M+H]+: m/z = 172.1; Found: 172.0. [00363] Step 2: (S)-3-(6-(1-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-2-yl)methyl) piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 93 -4860-6199-1670.2 105807.001049 – PCT Application [00364] This was in Example 8, using 2-vinylquinolin-6-ol instead of 6-vinylquinolin-2-ol. LCMS calcd for C44H42F3N8O5 [M+H]+: m/z = 819.3; Found: 819.2. Example 12. (S)-3-(6-(1-((7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8- naphthyridin-3-yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00366] A mixture of 6-bromo-1,8-naphthyridin-2-ol (0.5 g, 2.2 mmol), potassium vinyltrifluoro-borate (0.6 g, 4.46 mmol), cesium carbonate (2.18 g, 6.7 mmol) and chloro(2- dicyclohexyl-phosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) (0.160 g, 0.22 mmol) in 1,4-dioxane (8.9 mL) and water (2.2 mL) was sparged with N2 for 5 minutes. The reaction was stirred at 85 °C overnight. The reaction was cooled to room temperature, diluted with DCM, washed with a saturated solution of brine, then dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude material was purified via SiO2 FCC: 0-5% MeOH/DCM to obtain 6-vinyl-1,8-naphthyridin-2-ol (99 mg, 26% yield). - 94 -4860-6199-1670.2 105807.001049 – PCT Application [00368] To a solution of 6-vinyl-1,8-naphthyridin-2-ol (99 mg, 0.52 mmol) in phosphorous oxychloride (1.65 mL) was added N,N-diisopropylethylamine (0.14 mL, 0.79 mmol). The reaction was stirred at 80 °C overnight. Reaction was cooled to RT, excess phosphorous oxychloride was removed under rotavap and the residue was dissolved in ethyl acetate and washed with saturated solution of ammonium chloride. The combined organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure to give crude 2-chloro-6- vinyl-1,8-naphthyridine (61 mg, 60% yield) as a pale brown solid. This material was used in the following step without additional purification. LCMS calcd for C10H8ClN2 [M+H]+: m/z = 191.0; Found: 191.0. [00369] Step 3: (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00370] A solution of (6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (30 mg, 0.11 mmol) in anhydrous THF (1.0 mL) was cooled to 0 °C. Sodium hydride (60% dispersion in mineral oil; 13.0 mg, 0.33 mmol) was added, stirred for 5 minutes and then 2-chloro-6-vinyl-1,8-naphthyridine (31.0 mg, 0.16 mmol) was added and stirred at RT for 1 hour. The reaction was quenched with MeOH (0.2 mL) and solvent was removed under reduced pressure. The crude was directly purified via SiO2 FCC: 0 to 5% MeOH in DCM to obtain the title compound (30 mg, 64% yield) as pale brown solid. LCMS calcd for C20H18ClF2N6O [M+H]+: m/z = 431.1; Found: 431.1. [00371] Step 4: 2-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo 2-yl)-6-fluorophenol [00372] To (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((6-vinyl-1,8-naphthyridin-2-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (30 mg, 0.07 mmol) in 1,4- - 95 -4860-6199-1670.2 105807.001049 – PCT Application dioxane (1.6 mL) and water (0.16 mL) was added 3-fluoro-2-hydroxyphenylboronic acid (32.6 mg, 0.21 mmol) and potassium carbonate (38.5 mg, 0.28 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′- amino-1,1′-biphenyl)]palladium(II) (5.0 mg, 0.007 mmol) was added. The mixture was sparged with N2 for ~2 min then stirred at 85 °C. After 1 h, the reaction was cooled to RT, dried over sodium sulfate, concentrated under reduced pressure and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (24.6 mg, 71%) as a pale yellow solid. LCMS calcd for C26H22F3N6O2 [M+H]+: m/z = 507.2; Found: 507.1. [00373] Step 5: 7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridine-3- carbaldehyde [00374] Osmium tetroxide (4% in water; 37.6 µL, 0.006 mmol) and 2,6-lutidine (13.7 µL, 0.12 mmol) were added to a solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((6-vinyl-1,8- naphthyridin-2-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol (30.0 mg, 0.06 mmol) in 1,4-dioxane (1.5 mL) and water (0.5 mL). Sodium periodate (38.1 mg, 0.18 mmol) was added, and the reaction was stirred at RT for 2 hours. The reaction was poured into brine and extracted with CHCl3:iPrOH (3:1). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to obtain the title compound (30.0 mg, assumed quant. yield). The material was used in the following step without additional purification. LCMS calcd for C25H20F3N6O3 [M+H]+: m/z = 509.2; Found: 509.0. [00375] Step 6: (S)-3-(6-(1-((7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridin-3- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 96 -4860-6199-1670.2 105807.001049 – PCT Application [00376] To 7-(( - - -5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridine-3-carbaldehyde (30.0 mg, 0.06 mmol) and (S)-3-(1-oxo-6-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6-dione (HCl salt; 53.3 mg, 0.12 mmol) in DMSO (1.0 mL) was added N,N-diisopropylethylamine (51µL, 0.3 mmol). The reaction was stirred for 45 minutes at 35 °C then treated with sodium triacetoxyborohydride (62.5 mg, 0.3 mmol). The reaction was stirred at 35 °C overnight then cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Fluoro- Phenyl, 5 uM, 30x100mm, 12.2-32.2 % CH3CN in H2O with 0.1% TFA over 5 minutes) to yield the title compound (3.0 mg, 5% yield) as a white TFA salt. LCMS calcd for C43H41F3N9O5 [M+H]+: m/z = 820.3; Found: 820.7. Example 13. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro- isoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00377] Step 1: tert-butyl 6- 2(1H)-carboxylate [00378] To 1,2,3,4-tetrahydroisoquinolin-6-ol (300 mg, 2.01 mmol), DMAP (24.6 mg, 0.2 mmol) and N,N-diisopropylethylamine (525 µL, 3.02 mmol) were added to a solution of Boc2O (0.49 g, 2.2 mmol) in THF (8.0 mL). The resulting mixture was stirred at RT for 1 h then diluted - 97 -4860-6199-1670.2 105807.001049 – PCT Application with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-40% EtOAc in hexanes) to give tert-butyl 6-hydroxy-3,4-dihydroiso- quinoline-2(1H)-carboxylate (320 mg, 64%). LCMS calcd for C10H12NO3 [M-tBu+2H]+: m/z = 194.1; Found: 194.0. [00379] Step 2: tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroisoquinoline-2(1H)-carboxylate [00380] Diisopropyl azodicarboxylate (64 µL, 0.33 mmol) was added to a solution of (6aR,8S)- 2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin- 8-ol (Intermediate 1; 60.0 mg, 0.22 mmol), tert-butyl 6-hydroxy-3,4-dihydroisoquinoline-2(1H)- carboxylate (81.1 mg, 0.33 mmol) and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 203 mg, 0.33 mmol) in 1:2 THF/DCM (3.0 mL) and the reaction mixture was stirred at RT overnight, filtered, and the filtrate was directly purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (70 mg, 64%) as a thick viscous yellow oil. LCMS calcd for C24H29ClF2N5O3[M+H]+: m/z = 508.2; Found: 508.1. [00381] Step 3: tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinoline-2(1H)-carboxylate [00382] To tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroisoquinoline-2(1H)- carboxylate (70 mg, 0.14 mmol) in 1,4-dixane (1.6 mL) and water (0.16 mL) was added 3-fluoro- 2-hydroxyphenyl-boronic acid (65.0 mg, 0.41 mmol) and potassium carbonate (76.2 mg, 0.56 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexylphosphino- 2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) (9.9 mg, 0.014 mmol) was added, sparged with N2 for ~2 min and stirred at 85 °C. After 1 h, reaction was cooled to RT, - 98 -4860-6199-1670.2 105807.001049 – PCT Application dried over sodium sulfate, concentrated under reduced pressure and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (51.6 mg, 64%) as a pale yellow solid. LCMS calcd for C30H33F3N5O4 [M+H]+: m/z = 584.2; Found: 584.2. [00383] Step 4: 2-((6aR,8R)-6a-(difluoromethyl)-8-((1,2,3,4-tetrahydroisoquinolin-6-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00384] To a solution of tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro- isoquinoline-2(1H)-carboxylate (51.6 mg, 0.09 mmol) in DCM (1.0 mL) was added TFA (0.34 mL, 4.4 mmol) and stirred at RT for 1 h, and then concentrated to give crude 2-((6aR,8R)-6a- (difluoromethyl)-8-((1,2,3,4-tetrahydroisoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (42.7 mg, assumed quant. yield) as a thick viscous yellow oil. This material was used in the following step without additional purification. LCMS calcd for C25H25F3N5O2[M+H]+: m/z = 484.2; Found: 484.0. [00385] Step 4: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl) 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00386] 2-((6aR,8R)-6a-(difluoromethyl)-8-((1,2,3,4-tetrahydroisoquinolin-6-yl)oxy)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (43.0 mg, 0.089 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde (63.2 mg, 0.18 mmol) in DMSO (1.0 mL) was added acetic acid (25.4 µL, 0.45 mmol). The reaction was stirred for 45 minutes at 35 °C and then sodium triacetoxyborohydride (94.2 mg, 0.45 mmol) was added. The solution was stirred at 35 °C overnight. The reaction was cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Fluoro- Phenyl, 5 uM, 30x100mm, 15.0-25.0 % CH3CN in H2O with 0.2% TFA) over 5 minutes to yield title compound (9.2 mg, 10% yield) as a white TFA salt. LCMS calcd for C44H46F3N8O5 [M+H]+: m/z = 823.4; Found: 823.2. - 99 -4860-6199-1670.2 105807.001049 – PCT Application Example 14. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00387] This compound was synthesized by procedures analogous to that described in Example 13, using (2-hydroxy-3-methylphenyl)boronic acid instead of (3-fluoro-2-hydroxyphenyl)boronic acid in Example 14, Step 2. LCMS calcd for C45H49F2N8O5 [M+H]+: m/z = 819.4; Found: 819.3. Example 15. (S)-3-(6-(4-((6-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00388] This example was synthesized by procedures analogous to that described in Example 13, using (3-chloro-2-hydroxyphenyl)boronic acid instead of (3-fluoro-2-hydroxyphenyl)boronic acid. LCMS calcd for C44H46ClF2N8O5 [M+H]+: m/z = 839.3; Found: 839.3. Example 16. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 100 -4860-6199-1670.2 105807.001049 – PCT Application [00389] Step tert- -carboxylate [00390] To 6-hydroxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-2-ium bromide (250 mg, 1.0 mmol), DMAP (12.5 mg, 0.1 mmol) and N,N-diisopropylethylamine (0.44 mL, 2.6 mmol) were added to a solution of Boc2O (0.27 g, 1.2 mmol) in THF (5.8 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-40% EtOAc in hexanes) to give tert- butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (56 mg, 20%). LCMS calcd for C11H14NO3 [M-tBu+2H]+: m/z = 208.1; Found: 208.0. [00391] Step 2: tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4-dihydroisoquinoline-2(1H)- carboxylate [00392] Diisopropyl azodicarboxylate (37.4 µL, 0.19 mmol) was added to a solution of (6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (35.0 mg, 0.13 mmol), tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline- 2(1H)-carboxylate (40.0 mg, 0.15 mmol) and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 103 mg, 0.16 mmol) in 1:2 THF/DCM (3.0 mL) and the reaction mixture was stirred at RT overnight and then filtered and the filtrate was directly purified via SiO2 FCC: 0-5% MeOH/DCM to obtain tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexa- hydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4-dihydroisoquinoline- - 101 -4860-6199-1670.2 105807.001049 – PCT Application 2(1H)-carboxylate (65 mg, 98%) as a thick viscous yellow oil. LCMS calcd for C25H31ClF2N5O3[M+H]+: m/z = 522.2; Found: 522.1. [00393] Step 3: tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinoline-2(1H)-carboxylate [00394] To tert-butyl 6-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinoline-2(1H)-carboxylate (65 mg, 0.13 mmol) in 1,4-dioxane (1.6 mL) and water (0.16 mL) was added 3-fluoro-2-hydroxyphenylboronic acid (58.2 mg, 0.38 mmol) and potassium carbonate (69.0 mg, 0.5 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexyl-phosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′- biphenyl)]palladium(II) (9.0 mg, 0.013 mmol) was added, sparged with N2 for ~2 min and stirred at 85 °C. After 1 h, reaction was cooled to RT, dried over sodium sulfate, concentrated under reduced pressure and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (52.8 mg, 70%) as a pale yellow solid. LCMS calcd for C31H35F3N5O4 [M+H]+: m/z = 598.3; Found: 598.1. [00395] Step 4: 2-((6aR,8R)-6a-(difluoromethyl)-8-((1-methyl-1,2,3,4-tetrahydroisoquinolin-6- yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00396] To a solution of tert-butyl 6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl- 3,4-dihydroisoquinoline-2(1H)-carboxylate (52.8 mg, 0.09 mmol) in DCM (1.0 mL) was added TFA (0.34 mL, 4.4 mmol) and stirred at RT for 1 h, and then concentrated to give the title compound (43.9 mg, assumed quant. yield) as a thick, viscous brown oil. This material was used - 102 -4860-6199-1670.2 105807.001049 – PCT Application in the following step without additional purification. LCMS calcd for C26H27F3N5O2[M+H]+: m/z = 498.2; Found: 498.1. [00397] Step 5: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydroiso- quinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00398] 2-((6aR,8R)-6a-(Difluoromethyl)-8-((1-methyl-1,2,3,4-tetrahydroisoquinolin-6- yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (43.9 mg, 0.089 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde (62.7 mg, 0.18 mmol) in DMSO (1.0 mL) was added acetic acid (25.2 µL, 0.44 mmol). The reaction was stirred for 45 minutes at 35 °C and then sodium triacetoxyborohydride (93.5 mg, 0.44 mmol) was added. The solution was stirred at 35 °C overnight. The reaction was cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Phenyl- Hexyl, 5 uM, 30x100mm, 10.0-30.0 % CH3CN in H2O with 0.2% TFA) over 5 minutes to yieldtitle compound (3.6 mg, 4% yield) as a white TFA salt. LCMS calcd for C45H48F3N8O5[M+H]+: m/z = 837.4; Found: 837.4. Example 17. (3S)-3-(6-(4-((6-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00399] This example was synthesized by procedures analogous to that described in Example 16, using (6aR,8S)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5] pyrazino[2,3-c]pyridazin-8-ol instead of (6aR,8S)-2-chloro-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol. LCMS calcd for C46H52FN8O5 [M+H]+: m/z = 815.4; Found: 815.3. - 103 -4860-6199-1670.2 105807.001049 – PCT Application Example 18. (S)-3-(6-(4-((2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00400] Step 1: tert-butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate [00401] To 5,6,7,8-tetrahydro-1,6-naphthyridin-2-ol (150 mg, 0.99 mmol), DMAP (12.2 mg, 0.09 mmol) and N,N-diisopropylethylamine (261 µL, 1.5 mmol) were added to a solution of Boc2O (0.55 g, 2.5 mmol) in THF (5.0 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (0-5% methanol in DCM) to give tert-butyl 2-((tert- butoxycarbonyl)oxy)-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate (190 mg, 0.55 mmol) which was treated with 1N sodium hydroxide (aq) (3.95 mL, 3.95 mmol) in 1:1 THF:methanol (4 mL) and the resulting reaction mixture was stirred at RT for 2 hours. The reaction was treated with 1N HCl (aq) until the pH turned 7 and extracted with ethyl acetate, dried over sodium sulfate, concentrated under reduced pressure, and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (130 mg, 95% yield) as a white solid. LCMS calcd for C13H19N2O3 [M+H]+: m/z = 251.1; Found: 251.1. [00402] Step 2: tert-butyl 2-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9 hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate - 104 -4860-6199-1670.2 105807.001049 – PCT Application [00403] Diisopropyl azodicarboxylate (22 µL, 0.11 mmol) was added to a solution of (6aR,8S)- 2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin- 8-ol (Intermediate 1; 20.0 mg, 0.08 mmol), tert-butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine- 6(5H)-carboxylate (20.0 mg, 0.08 mmol) and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 68 mg, 0.11 mmol) in 1:2 THF:DCM (3 mL). The reaction mixture was stirred at RT overnight, filtered, and the filtrate was directly purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (35 mg, 95%) as a thick, viscous yellow oil. LCMS calcd for C23H28ClF2N6O3[M+H]+: m/z = 509.2; Found: 509.0. [00404] Step3: tert-butyl 2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridine-6(5H)-carboxylate [00405] To tert-butyl 2-(((6aR,8R)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9 hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6-naphthyridine- 6(5H)-carboxylate (35 mg, 0.07 mmol) in 1,4-dioxane (1.6 mL) and water (0.16 mL) was added 3-chloro-2-hydroxyphenylboronic acid (36 mg, 0.21 mmol) and potassium carbonate (38 mg, 0.28 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexyl- phosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) (5.0 mg, 0.007 mmol) was added, sparged with N2 for ~2 min and stirred at 85 °C. After 1 h, reaction was cooled to RT, dried over sodium sulfate, concentrated under reduced pressure and purified via SiO2 FCC: 0-5% MeOH/DCM to obtain the title compound (30.1 mg, 73%) as a pale yellow solid. LCMS calcd for C29H32ClF2N6O4 [M+H]+: m/z = 601.2; Found: 601.1. [00406] Step 4: 2-chloro-6-((6aR,8R)-6a-(difluoromethyl)-8-((5,6,7,8-tetrahydro-1,6- naphthyridin-2-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2- yl)phenol - 105 -4860-6199-1670.2 105807.001049 – PCT Application [00407] To a solution of tert- - -6a-(difluoro- methyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro- 1,6-naphthyridine-6(5H)-carboxylate (30.1 mg, 0.05 mmol) in DCM (1.0 mL) was added TFA (0.2 mL, 4.4 mmol) and stirred at RT for 1 h, and then concentrated to give 2-chloro-6- ((6aR,8R)-6a-(difluoromethyl)-8-((5,6,7,8-tetrahydro-1,6-naphthyridin-2-yl)oxy)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol (25.1 mg, assumed quant. yield) as a thick, viscous yellow oil. This material was used in the following step without additional purification. LCMS calcd for C24H24ClF2N6O2 [M+H]+: m/z = 501.2; Found: 501.0. [00408] Step 5: (S)-3-(6-(4-((2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00409] 2-Chloro-6-((6aR,8R)-6a-(difluoromethyl)-8-((5,6,7,8-tetrahydro-1,6-naphthyridin-2- yl) oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol (25.1 mg, 0.05 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde (36 mg, 0.1 mmol) in DMSO (1.0 mL) was added acetic acid (14.4 µL, 0.25 mmol). The reaction was stirred for 30 minutes at 35 °C and then sodium triacetoxyborohydride (52.9 mg, 0.25 mmol) was added and continued stirring at 35 °C overnight. The reaction was cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Fluoro- Phenyl, 5 uM, 30x100mm, 12.3-32.3 % CH3CN in H2O with 0.2% TFA) over 5 minutes to yield title compound (9.2.0 mg, 10% yield) as a white TFA salt.1H NMR (400 MHz, MeOD) δ 7.60 (d, J = 8.5 Hz, 2H), 7.47 (t, J = 6.6 Hz, 2H), 7.44 – 7.32 (m, 2H), 7.08 (d, J = 8.1 Hz, 2H), 6.79 (d, J = 8.5 Hz, 1H), 6.21 (t, J = 55.7 Hz, 1H), 5.88 (s, 1H), 5.22 – 5.09 (m, 2H), 4.50 – 4.37 (m, - 106 -4860-6199-1670.2 105807.001049 – PCT Application 2H), 4.00 (t, J = 14.4 Hz, 2H), 4.28 (m, 1H), 3.85 (d, J = 12.5 Hz, 2H), 3.17 – 3.03 (m, 2H), 2.99 – 2.74 (m, 5H), 2.52 (d, J = 12.6 Hz, 1H), 2.20 (d, J = 12.8 Hz, 3H), 1.99 (d, J = 12.7 Hz, 2H), 1.57 (d, J = 12.1 Hz, 3H). Note: some protons hidden due to overlap with water or NMR solvent peaks. LCMS calcd for C43H45ClF2N9O5 [M+H]+: m/z = 840.3; Found: 840.4. Example 19. (S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00410] This example was synthesized by procedures analogous to that described in Example 18, using (3-fluoro-2-hydroxyphenyl)boronic acid instead of (3-chloro-2-hydroxyphenyl)boronic acid. LCMS calcd for C43H45F3N9O5 [M+H]+: m/z = 824.3; Found: 824.3. Example 20. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro-2,7- naphthyridin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00411] Step 1: tert-butyl 6- 2(1H)-carboxylate [00412] To 6-hydroxy-1,2,3,4-tetrahydro-2,7-naphthyridin-2-ium bromide (250 mg, 1.08 mmol), DMAP (13.2 mg, 0.11 mmol) and N,N-diisopropylethylamine (471 µL, 2.7 mmol) were - 107 -4860-6199-1670.2 105807.001049 – PCT Application added to a solution of Boc2O (0.26 g, 1.2 mmol) in THF (4.3 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (0-5% methanol in DCM) to give the title compound (142 mg, 52% yield) as a white solid. LCMS calcd for C13H19N2O3 [M+H]+: m/z = 251.1; Found: 251.0. [00413] Step 2: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro-2,7- naphthyridin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00414] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 6-hydroxy-3,4-dihydro-2,7-naphthyridine-2(1H)-carboxylate instead of tert- butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate. LCMS calcd for C43H45F3N9O5 [M+H]+: m/z = 824.3; Found: 824.3. Example 21. (S)-3-(6-(4-((5-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)isoindolin-2- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00415] Step 1: tert-butyl 5- 2- [00416] To 5-hydroxyisoindolin-2-ium chloride (250 mg, 1.46 mmol), DMAP (17.8 mg, 0.1 mmol) and N,N-diisopropylethylamine (0.63 mL, 3.6 mmol) were added to a solution of Boc2O (0.35 g, 1.6 mmol) in THF (5.8 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-30% EtOAc in hexanes) to give tert-butyl 5-hydroxyisoindoline-2- carboxylate (143 mg, 42%) as a white solid. LCMS calcd for C9H10NO3 [M-tBu+2H]+: m/z = 180.1; Found: 179.9. - 108 -4860-6199-1670.2 105807.001049 – PCT Application [00417] Step 2: (S)-3-(6-(4-((5-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)isoindolin-2- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00418] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 5-hydroxyisoindoline-2-carboxylate instead of tert-butyl 2-hydroxy-7,8- dihydro-1,6-naphthyridine-6(5H)-carboxylate. LCMS calcd for C43H45F2N8O5 [M+H]+: m/z = 791.3; .: 791.3. Example 22. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00420] To 5-methyl-1,2,3,4-tetrahydroisoquinolin-6-ol;hydrobromide (250 mg, 1.02 mmol), DMAP (12.5 mg, 0.1 mmol) and N,N-diisopropylethylamine (0.45 mL, 2.6 mmol) were added to a solution of Boc2O (0.24 g, 1.1 mmol) in THF (4.3 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-30% EtOAc in hexanes) to give tert-butyl 6-hydroxy-5-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (90 mg, 33%) as a white solid. LCMS calcd for C11H14NO3 [M-tBu+2H]+: m/z = 208.1; Found: 208.0. [00421] Step 2: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 109 -4860-6199-1670.2 105807.001049 – PCT Application [00422] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl 6-hydroxy-5-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate. LCMS calcd for C45H48F3N8O5 [M+H]+: m/z = 837.4; Found: 837.4. Example 23. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00423] Step 1: tert-butyl 6-hydroxy-7-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate [00424] To 7-methyl-1,2,3,4-tetrahydroisoquinolin-6-ol;hydrobromide (250 mg, 1.02 mmol), DMAP (12.5 mg, 0.1 mmol) and N,N-diisopropylethylamine (0.45 mL, 2.6 mmol) were added to a solution of Boc2O (0.24 g, 1.1 mmol) in THF (4.3 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-30% EtOAc in hexanes) to give tert-butyl 6-hydroxy-7-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (121 mg, 45%) as a white solid. LCMS calcd for C11H14NO3 [M-tBu+2H]+: m/z = 208.1; Found: 208.0. [00425] Step 2: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 110 -4860-6199-1670.2 105807.001049 – PCT Application [00426] This was in Example 18, using tert-butyl 6-hydroxy-7-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate. LCMS calcd for C45H48F3N8O5 [M+H]+: m/z = 837.4; Found: 837.3. Example 24. (3S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,3-dimethyl- 3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00427] Step 1: tert-butyl 6-hydroxy-1,3-dimethyl-3,4-dihydroisoquinoline-2(1H)-carboxyla [00428] To 1,3-dimethyl-1,2,3,4-tetrahydroisoquinolin-6-ol;hydrobromide (250 mg, 0.97 mmol), DMAP (11.8 mg, 0.09 mmol) and N,N-diisopropylethylamine (0.42 mL, 2.4 mmol) were added to a solution of Boc2O (0.25 g, 1.2 mmol) in THF (5.8 mL). The resulting mixture was stirred at RT overnight then diluted with water and extracted with ethyl acetate, dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-50% EtOAc in hexanes) to give tert-butyl 6-((tert-butoxycarbonyl)oxy)-1,3-dimethyl-3,4-dihydroisoquinoline-2(1H)-carboxylate, which was treated with 1N sodium hydroxide (aq) (1.9 mL, 1.9 mmol) in 1:1 THF:Methanol (2 mL), and the resulting reaction mixture was stirred at RT for 2 hours. The reaction was treated with 1N - 111 -4860-6199-1670.2 105807.001049 – PCT Application HCl (aq) until the pH turned 7 and extracted with ethyl acetate, dried over sodium sulfate, concentrated under reduced pressure to obtain the title compound (60 mg, 69% yield) as a white solid. LCMS calcd for C12H16NO3 [M-tBu+2H]+: m/z = 222.1; Found: 221.9. [00429] Step 2: (3S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,3-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00430] This example was synthesized by procedures analogous to that described in Example 16, using tert-butyl 6-hydroxy-1,3-dimethyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate. LCMS calcd for C46H50F3N8O5 [M+H]+: m/z = 851.4; Found: 851.3. Example 25. (S)-3-(6-(4-(((R)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1- methyl-3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione [00431] Step 1: tert-butyl (R)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate [00432] To (racemic)-6-hydroxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-2-ium bromide (1.0 g, 4.1 mmol), DMAP (50.0 mg, 0.4 mmol) and N,N-diisopropylethylamine (1.78 mL, 10.2 mmol) were added to a solution of Boc2O (2.2 g, 10.2 mmol) in THF (20.0 mL). The resulting mixture was stirred at RT for 1 h then diluted with EtOAc and washed with brine. The organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-30% EtOAc in hexanes) to give tert-butyl 6-((tert-butoxycarbonyl)oxy)-1-methyl-3,4-dihydroisoquinoline-2(1H)- carboxylate (1.4 g, 94%), which was treated with 1N sodium hydroxide (aq) (15 mL, 15 mmol) in 1:1 THF:Methanol (5 mL) and the resulting reaction mixture was stirred at RT for 2 hours. The reaction was treated with 1N HCl (aq) until the pH turned 7 and extracted with ethyl acetate, - 112 -4860-6199-1670.2 105807.001049 – PCT Application dried over sodium sulfate, concentrated under reduced pressure to obtain (racemic) tert-butyl-6- hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (900 mg, 88% yield) as a white solid. LCMS calcd for C11H14NO3 [M-tBu+2H]+: m/z = 208.1; Found: 208.0. The isolated racemic material was separated into single enantiomers using prep-Chiral HPLC (Lux i- Amylose-3, 5% Isopropanol:Methanol in Hexanes, 1mL/min over 30 minutes) to yield tert-butyl (R)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (tR=4.60 min, Peak A, >99%ee) and tert-butyl (S)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (tR=5.20min, PeakB, >99%ee). The absolute configuration was arbitrarily assigned. [00433] Step 2: (S)-3-(6-(4-(((R)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2- hydroxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1- methyl-3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione [00434] This example was synthesized by procedures analogous to that described in Example 16, using tert-butyl (R)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of (racemic) tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate. LCMS calcd for C45H48F3N8O5 [M+H]+: m/z = 837.4; Found: 837.3. Example 26. (S)-3-(6-(4-(((S)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy- phenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1- methyl-3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione [00435] This example was synthesized by procedures analogous to that described in Example 18, using tert-butyl (S)-6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate instead of (racemic) tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate.1H NMR (400 MHz, MeOD) δ 7.36 (d, J = 8.4 Hz, 1H), 7.30 – 7.19 (m, 4H), 7.13 (d, J = 8.6 Hz, 1H), 7.02 (s, 1H), 6.92 (td, J = 8.1, 4.8 Hz, 1H), 6.88 – 6.76 (m, 2H), 6.09 (t, J = 55.8 Hz, 1H), 5.27 (s, 1H), 5.03 (dd, J = 13.3, 5.1 Hz, 1H), 4.47 – 4.16 (m, 2H), 4.09 (dd, J = 12.5, 5.4 Hz, 1H), 3.92 (dd, J = 27.0, 12.7 Hz, 2H), 3.72 (d, J = 12.2 Hz, 3H), 3.45 (d, J = 55.3 Hz, 1H), 3.07 (s, - 113 -4860-6199-1670.2 105807.001049 – PCT Application 3H), 2.92 (dd, J = 14.6, 6.4 Hz, 1H), 2.78 (dd, J = 13.1, 4.5 Hz, 2H), 2.68 (d, J = 17.7 Hz, 2H), 2.49 – 2.32 (m, 1H), 2.08 (dd, J = 12.1, 4.5 Hz, 3H), 1.85 (d, J = 13.0 Hz, 2H), 1.70 – 1.36 (m, 6H). Note: some protons hidden due to overlap with water or NMR solvent peaks. LCMS calcd for C45H48F3N8O5 [M+H]+: m/z = 837.4; Found: 837.3. Example 27. (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione [00436] Step 1: tert-butyl (2-(6-((tert-butoxycarbonyl)oxy)pyridin-2-yl)ethyl)carbamate [00437] To a solution of 6-(2-aminoethyl)pyridin-2-ol;hydrochloride (2.5g, 14.3 mmol) in anhydrous THF (72 mL) were added N,N-diisopropylethylamine (9.97 mL, 57.2 mmol), di-tert butyl dicarbonate (9.4 g, 42.9 mmol) and DMAP (175.0 mg, 1.43 mmol). The mixture was stirred at RT overnight then diluted with ethyl acetate (200 mL) and washed with 1N HCl (2x 50 mL) and brine (50 mL). The combined organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (10-40% ethyl acetate in hexanes) to give tert-butyl (2-(6-((tert- butoxycarbonyl)oxy)pyridin-2-yl)ethyl)carbamate (4.1 g, 85% yield) as a thick, colorless oil. LCMS calcd for C17H27N2O5 [M+H]+: m/z = 339.2; Found: 339.1. [00438] Step 2: tert-butyl (2-(6- [00439] A solution of tert-butyl (2-(6-((tert-butoxycarbonyl)oxy)pyridin-2-yl)ethyl)carbamate (4.0 g, 11.8 mmol) in 1:1 Methanol/THF (22 mL) was added 1N Sodium hydroxide (35.5 mL, 35.5 mmol) was stirred vigorously at RT for 2 hours.1N Hydrochloric acid (ca 35 mL) was added until pH turned 7. At pH 7 the mixture turned cloudy and extracted with 3:1 - 114 -4860-6199-1670.2 105807.001049 – PCT Application chloroform/isopropanol (3x 100 mL), dried, concentrated to give tert-butyl (2-(6- hydroxypyridin-2-yl)ethyl)carbamate (2.4 g, 10.0 mmol, 85% yield) as a white solid. LCMS calcd for C12H19N2O3 [M+H]+: m/z = 239.1; Found: 238.8. [00440] Step 3: tert-butyl (2-(3-bromo-6-hydroxypyridin-2-yl)ethyl)carbamate [00441] A solution of tert-butyl carbamate (2.4 g, 10.1 mmol) in anhydrous acetonitrile (50.0 mL) was cooled to 0 °C. N-Bromosuccinimide (1.77 g, 10.1 mmol) was added and stirred for 10 minutes and then stirred at RT for 15 minutes. Reaction mixture was diluted with water and extracted with 3:1 chloroform/isopropanol (3x 100 mL). The combined organic layer was dried with Na2SO4, concentrated, then purified via SiO2 FCC (0 to 5% methanol in DCM) to give 3:1 regioisomeric mixture of tert-butyl N-[2-(3-bromo-6-hydroxy- 2-pyridinyl)ethyl]carbamate (1.7 g, 53% yield) as a white solid. LCMS calcd for C12H18BrN2O3 [M+H]+: m/z = 317.0; Found: 316.8. [00442] Step 4: 5-methyl-5,6,7,8-tetrahydro-1,6-naphthyridin-2-ol [00443] A mixture of tert-butyl N-[2-(3-bromo-6-hydroxy-2-pyridinyl)ethyl]carbamate (1.0 g, 3.15 mmol), 1-ethoxyvinyltri-n-butyltin (1.6 mL, 4.73 mmol) and tetrakis(triphenylphosphine)- palladium(0) (0.36 g, 0.32 mmol) in anhydrous 1,4-dioxane (16.7ml) was sparged with nitrogen for 10 minutes and stirred at 100 °C overnight. The reaction was cooled to RT, 4M Hydrochloric acid (6.43 mL, 25.71 mmol) in dioxane was added and stirred at 50 °C for 30 minutes. A pale- yellow solid was precipitated during the reaction which was collected by vacuum filtration. The collected solid was dissolved in DCE (15.7 mL), and sodium triacetoxyborohydride (1.0 g, 4.73 mmol) was added and the reaction stirred at 50 °C for 45 minutes. The reaction mixture was cooled to RT and concentrated under reduced pressure to give 5-methyl-5,6,7,8-tetrahydro-1,6- naphthyridin-2-ol (assumed quantitative yield) as a viscous yellow oil, which was taken to the next step without further purification. LCMS calcd for C9H13N2O [M+H]+: m/z = 165.1; Found: 165.0. [00444] Step 5: tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate - 115 -4860-6199-1670.2 105807.001049 – PCT Application [00445] To a solution of 5- 2-ol (0.5 g, 2.5 mmol) in anhydrous THF (25.0 mL) were added N,N-diisopropylethylamine (2.17 mL, 12.4 mmol), di- tert butyl dicarbonate (1.63 g, 7.5 mmol) and DMAP (30.4 mg, 0.25 mmol). The mixture was stirred at rt for 2 hours and then diluted with 3:1 chloroform/isopropanol (100 mL) and washed with brine (3x50 ml). The combined organic layer was dried with Na2SO4, concentrated, to give tert-butyl 2-((tert-butoxycarbonyl)oxy)-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate (assumed quantitative yield), which was treated with 1N sodium hydroxide (aq) (10 mL, 10 mmol) in 1:1 THF:Methanol (10 mL) and the resulting reaction mixture was stirred at RT for 1 hour. The reaction was treated with 1N hydrochloric acid (aq) until the pH turned 7 and extracted with 3:1 chloroform/isopropanol (3x50 mL). The combined organic layer was dried over sodium sulfate, concentrated, then purified via SiO2 FCC (0-10% methanol in DCM) to give tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate (160 mg, 25% yield) as a pale-yellow solid. LCMS calcd for C14H21N2O3 [M+H]+: m/z = 265.2; Found: 264.9. [00446] Step 6: (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dion [00447] This compound was synthesized by procedures analogous to that described in Example 16, using tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate instead of tert-butyl 6-hydroxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate. LCMS calcd for C44H47F3N9O5 [M+H]+: m/z = 838.4; Found: 838.2. Example 28. (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-chloro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine- 2,6-dione - 116 -4860-6199-1670.2 105807.001049 – PCT Application [00448] This was in Example 18, using tert-butyl 2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate instead of tert-butyl 2-hydroxy-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate. LCMS calcd for C44H47ClF2N9O5 [M+H]+: m/z = 854.3; Found: 854.2. Example 29. (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,1-dimethyl- 3,4-dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00449] Step 1: 1,1-dimethyl-1,2,3,4-tetrahydroisoquinolin-6-ol [00450] A solution of 3-(2-aminoethyl)phenol;hydrochloride (450.0 mg, 2.59 mmol) in acetone (1.92 mL, 25.9 mmol) was added 12M sodium hydroxide (aq) (1.5 mL, 18.1 mmol) and stirred at 100 °C for 16 h. The reaction was cooled to RT and neutralized using 1N hydrochloric acid (aq) until pH turned 8 and extracted into ethyl acetate. The aqueous layer contains product and organic impurities were in ethyl acetate layer. The aqueous layer was basified to pH 10 using saturated sodium carbonate (aq) and extracted with 3:1 chloroform/iPrOH. The organic layer was dried over anhydrous sodium sulfate, concentrated to give 1,1-dimethyl-3,4-dihydro-2H- isoquinolin-6-ol (320 mg, 69% yield) as pale brown solid. LCMS calcd for C11H16NO [M+H]+: m/z = 178.1; Found: 178.0. - 117 -4860-6199-1670.2 105807.001049 – PCT Application [00451] Step 2: (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4-tetrahydro- isoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00452] Diisopropyl was added to a solution of (6aR,8S)-2-chloro-6a-(difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-8-ol (Intermediate 1; 25.0 mg, 0.09 mmol), 1,1-dimethyl-1,2,3,4- tetrahydroisoquinolin-6-ol (22.4 mg, 0.13 mmol) and polymer-supported PPh3 (100-200 mesh, ~1.6 mmol/g loading; 84.6 mg, 0.14 mmol) in 1:2 THF:DCM (3 mL) and the reaction mixture was stirred at 40 °C for 2h and then filtered and the filtrate was directly purified via SiO2 FCC: 0- 40% MeOH/DCM to obtain the title compound (30 mg, 78%) as a thick, colorless oil. LCMS calcd for C21H25ClF2N5O [M+H]+: m/z = 436.2; Found: 436.1. [00453] Step3: 2-((6aR,8R)-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4- tetrahydroisoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3- c]pyridazin-2-yl)-6-fluorophenol [00454] To (6aR,8R)-2-chloro-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4-tetrahydro- isoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (30 mg, 0.07 mmol) in 1,4-dixane (1.6 mL) and water (0.16 mL) was added 3-fluoro-2-hydroxy- phenylboronic acid (32.2 mg, 0.21 mmol) and potassium carbonate (38 mg, 0.28 mmol). The solution was sparged with N2 for ~2 min and then chloro(2-dicyclohexyl-phosphino-2′,6′- dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) (5.0 mg, 0.007 mmol) was added, sparged with N2 for ~2 min and stirred at 85 °C. After 1 h, reaction was cooled to RT, dried over sodium sulfate, concentrated under reduced pressure and purified via prep-LCMS (Waters CSH-C18, 5 uM, 30x100mm, 17.5-34.5 % CH3CN in H2O with 0.2% TFA) over 5 minutes to yield the title compound (10.0 mg, 28%) as a white TFA salt. LCMS calcd for C27H29F3N5O2 [M+H]+: m/z = 512.2; Found: 512.1. - 118 -4860-6199-1670.2 105807.001049 – PCT Application [00455] Step 4: (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,1-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00456] To a solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((1,1-dimethyl-1,2,3,4-tetrahydro- isoquinolin-6-yl)oxy)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol (10.0 mg, 0.02 mmol) in DMSO (1.0 mL) was added (S)-1-(2-(2,6-dioxopiperidin- 3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (13.9 mg, 0.04 mmol) and acetic acid (11.2 µL, 0.2 mmol). The reaction was stirred for 30 minutes at 35 °C and then sodium triacetoxy- borohydride (16.6 mg, 0.08 mmol) was added and continued stirring at 35 °C overnight. The reaction was cooled to room temperature, filtered, and directly purified via prep-LCMS (Waters CSH-Fluoro-Phenyl, 5 uM, 30x100mm, 12.0-32.0 % CH3CN in H2O with 0.2% TFA) over 5 minutes to yield title compound (5.0 mg, 21% yield) as a white TFA salt. LCMS calcd for C46H50F3N8O5 [M+H]+: m/z = 851.4; Found: 851.3. Example 30. (3S)-3-[5-[4-[[1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5-yl]methyl] piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione [00457] Step 1. (6aS,8S)-2-chloro-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino- 8-ol [00458] Prepared using procedures analogous to those described for Intermediate 1, utilizing methyl iodide instead of difluoromethyl trifluoromethanesulfonate in Step 2. LCMS calcd for C10H14ClN4O [M+H]+: m/z = 241.1; Found: 241.3. - 119 -4860-6199-1670.2 105807.001049 – PCT Application [00459] Step 2. Methyl 1-[(4R,6S)-12-chloro-6-methyl-2,8,10,11-tetrazatricyclo[7.4.0.02,6]- trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carboxylate [00460] Triphenylphosphine mg, 1H-pyrrolo[2,3-b]pyridine-5- carboxylate (190.31 mg, 1.08 mmol) were dissolved in THF (5 mL), flushed with nitrogen and cooled in an ice bath. After 10 minutes, (4S,6S)-12-chloro-6-methyl-2,8,10,11-tetrazatricyclo- [7.4.0.02,6]trideca-1(9),10,12-trien-4-ol (130.0 mg, 0.54 mmol) was added to it and stirred at 0 °C. Diisopropyl azodicarboxylate (0.32 mL, 1.62 mmol) was dissolved in THF and added dropwise to the reaction mixture. After stirring for about 10 minutes at 0 °C, it was stirred at 50 °C for four hours. The reaction was quenched with water and extracted with DCM. The crude was purified by prep HPLC on a C18 column (0.1% TFA in water and ACN). The fractions were concentrated, basified with aq. sodium carbonate solution (10%) and extracted with DCM to obtain the title compound (132.2 mg, 61% yield). LCMS calc. for C19H20ClN6O2 [M+H]+: m/z = 399.1; found 399.3. [00461] Step 3. Methyl 1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- [00462] Methyl 1-[(4R,6S)-12-chloro-6-methyl-2,8,10,11-tetrazatricyclo[7.4.0.02,6]trideca- 1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carboxylate (130.0 mg, 0.33 mmol), 3-fluoro-2- hydroxyphenylboronic acid (152.46 mg, 0.98 mmol), cesium acetate (187.69 mg, 0.98 mmol) and XPhos Pd G2 (25.64 mg, 0.03 mmol) were added to a vial with 1,4-dioxane (3 mL). Water (158 mL) was then added to it and purged with nitrogen for about a minute, then stirred overnight at 90 °C. After the completion of the reaction, it was quenched with water and basified with 10% aqueous sodium carbonate solution and extracted with chloroform/IPA. The organic pool was dried over anhydrous sodium sulfate, filtered, and concentrated to get the crude. The crude was purified by prep HPLC (0.1 % TFA in water and ACN). The fractions were concentrated, basified and extracted with chloroform/IPA to obtain the title compound (87.4 mg, 56% yield). LCMS calc. for C25H24FN6O3 [M+H]+ : m/z = 475.1; found 475.2. - 120 -4860-6199-1670.2 105807.001049 – PCT Application [00463] Step 4.2-fluoro-6-[(4R,6S)-4-[5-(hydroxymethyl)pyrrolo[2,3-b]pyridin-1-yl]-6- methyl-2,8,10,11-tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-12-yl]phenol [00464] Methyl 1-[(4R,6S)- - 2,8,10,11-tetrazatricyclo- [7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carboxylate (65.0 mg, 0.14 mmol) was dissolved in THF (2 mL) and cooled in an ice bath. LDBBA (1.37 mL, 0.34 mmol) was then added. One more equivalent of LDBBA was added and stirred for 30 minutes. One more equivalent of the LDBBA was then added and stirred for an additional 30 min. The reaction mixture was then quenched with methanol and basified with sodium carbonate solution. It was then extracted with chloroform/IPA to get the crude which was purified by prep HPLC (0.1 % TFA in water and ACN). The fractions were concentrated, basified and extracted with chloroform/IPA to obtain the title compound (22 mg, 36% yield). LCMS calc. for C24H24FN6O2 [M+H]+ : m/z = 447.19; found 447.3. [00465] Step 5.1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11-tetrazatricyclo- [7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carbaldehyde [00466] 2-Fluoro-6-[(4R,6S)-4-[5-(hydroxymethyl)pyrrolo[2,3-b]pyridin-1-yl]-6-methyl- 2,8,10,11-tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-12-yl]phenol (19.0 mg, 0.04 mmol) was dissolved in chloroform (2 mL) and manganese(IV) oxide (74 mg, 0.85 mmol) was added to it in one portion. The reaction was stirred vigorously overnight at 50 °C. After the completion of the reaction, it was diluted with chloroform/IPA and filtered through syringe filter. The filtrate was concentrated and then purified by prep HPLC (0.1% TFA in water and ACN). The fractions were concentrated, basified with 10% aq. sodium carbonate solution and extracted with chloroform/IPA to obtain the title compound (9.2 mg, 0.021 mmol, 49% yield). LCMS calc. for C24H22FN6O2 [M+H]+: m/z = 445.1; found 445.3. - 121 -4860-6199-1670.2 105807.001049 – PCT Application [00467] Step 6. (3S)-3-[5-[4-[[1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5-yl]methyl]- piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione [00468] The 1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11-tetrazatricyclo- [7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridine-5-carbaldehyde (9.0 mg, 0.02 mmol) was dissolved in DMSO (1 mL) and (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2- yl)piperidine-2,6-dione (7.98 mg, 0.02 mmol) was added to it. The mixture was stirred and then sodium triacetoxyborohydride (12.87 mg, 0.06 mmol) was added. The reaction mixture was stirred ovenight at 37 °C. One equivalent of (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2- yl)piperidine-2,6-dione (7.98 mg, 0.02 mmol) and sodium triacetoxyborohydride (12.87 mg, 0.06 mmol) were added, and the reaction was stirred at 37 °C for another 3 hours. The reaction was mixture was diluted with DMSO and a few drops of MeOH and purified by prep HPLC (0.01% TFA in water and pure ACN). The fractions were evaporated and additional TFA/water were added and evaporated again to obtain the title compound (8.6 mg, 55% yield). LCMS calc. for C41H42FN10O4 [M+H]+: m/z = 757.3; found 757.5. Example 31. (3S)-3-[5-[4-[[1-[(4R,6R)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5-yl]methyl] piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione [00469] Step 1. (6aR,8S)-2-chloro-6a-methyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-ol [00470] This compound was prepared using procedures analogous to those described for Intermediate 1, utilizing methyl iodide instead of difluoromethyl trifluoromethanesulfonate in Step 2. LCMS calcd for C10H14ClN4O [M+H]+: m/z = 241.1; Found: 241.3. - 122 -4860-6199-1670.2 105807.001049 – PCT Application [00471] Step 2. (3S)-3-[5-[4-[[1-[(4R,6R)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5-yl]methyl]- piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione [00472] This compound was prepared using procedures analogous to those described for Example 30. LCMS calc. for C41H42FN10O4 [M+H]+: m/z = 757.3; found 757.7. Example 32. (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00473] This compound was prepared by procedures analogous to those described for Example 30, utilizing methyl 1H-pyrazolo[5,4-b]pyridine-5-carboxylate instead of methyl 1H-pyrrolo[2,3- b]pyridine-5-carboxylate. LCMS calculated for C40H41FN11O4 (M+H)+: m/z = 758.33; found: 758.61. LCMS calculated for C40H39FN11O4 (M-H)-: m/z = 756.32; found: 756.77.1H NMR (300 MHz, MeOD) δ 8.76 (d, J = 2.0 Hz, 1H), 8.48 (d, J = 2.0 Hz, 1H), 8.28 (s, 1H), 7.50 (d, J = 8.3 Hz, 1H), 7.40 – 7.26 (m, 4H), 7.04 – 6.95 (m, 1H), 6.83 (s, 1H), 6.38 – 6.23 (m, 1H), 5.13 (dd, J = 13.3, 5.2 Hz, 1H), 4.65 (s, 2H), 4.49 – 4.31 (m, 3H), 4.15 (dd, J = 12.5, 6.5 Hz, 1H), 3.88 – 3.36 (m, 9H), 3.00 – 2.79 (m, 2H), 2.76 – 2.58 (m, 3H), 2.49 (qd, J = 13.1, 4.9 Hz, 1H), 2.22 – 2.12 (m, 1H), 1.57 (s, 3H). Example 33. (S)-3-(6-(4-((3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo- [4,5-b]pyridin-6-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 123 -4860-6199-1670.2 105807.001049 – PCT Application [00474] Step 1: (6aR,8S)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-ol [00475] This compound was synthesized by procedures analogous to that described for Intermediate 1, using fluoroiodomethane instead of difluoromethyl trifluoromethanesulfonate. LCMS calcd for C10H13ClFN4O [M+H]+: m/z = 259.1; Found: 259.2. [00476] Step 2: 6-vinyl-3H-[1,2,3]triazolo[4,5-b]pyridine [00477] A mixture of 6-bromo-3H-[1,2,3]triazolo[4,5-b]pyridine (300.0 mg, 1.51 mmol), 4,4,5,5-tetramethyl-2-vinyl-1,3,2-dioxaborolane (0.56 mL, 3.32 mmol), potassium carbonate (417 mg, 3.01 mmol) and dichloro 1,1'-bisdiphenylphosphino)ferrocene palladium (II) dichloromethane (123.41 mg, 0.15 mmol) in 1,4-dioxane (5 mL) and water (0.5 mL) was stirred at 100 °C overnight. The reaction was diluted with DCM, washed with water, concentrated and purified on FCC (0-100% DCM/EA) to give 6-vinyl-3H-[1,2,3]triazolo[4,5-b]pyridine (163 mg, 74% yield). LCMS calcd for C7H7N4 [M+H]+: m/z = 147.2; Found: 147.1. [00478] Step 3: (6aR,8R)-2-chloro-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine - 124 -4860-6199-1670.2 105807.001049 – PCT Application [00479] A mixture of (6aR,8S)-2- -5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (30.0 mg, 0.12 mmol), triphenylphosphine (45.63 mg, 0.17 mmol), 6-vinyl-3H-[1,2,3]triazolo[4,5-b]pyridine (27.1 mg, 0.19 mmol) and di-tert-butyl azodicarboxylate (40 mg, 0.17 mmol) in DCM (2 mL) and THF (1 mL) was stirred at 30 °C overnight. The mixture was concentrated and purified on FCC (0-10% MeOH/DCM) to give the title compound (40 mg, 0.10 mmol, 89% yield). LCMS calcd for C17H17ClFN8 [M+H]+: m/z = 387.1; Found: 387.1. [00480] Step 4: 2-fluoro-6-((6aR,8R)-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol [00481] A mixture of (6aR,8R)-2-chloro-6a-(fluoromethyl)-8-(6-vinyl-3H-[1,2,3]triazolo[4,5- b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (40.0 mg, 0.1 mmol), 3-fluoro-2-hydroxyphenylboronic acid (48.4 mg, 0.31 mmol), potassium carbonate (42.9 mg, 0.31 mmol) and XPhos Pd G2 (16.3 mg, 0.02 mmol) in 1,4-dioxane (1 mL) and water (0.1 mL) was sparged for 2 min with N2 and stirred at 100 °C for 1h. The mixture was purified via SiO2 FCC (0-10% MeOH/DCM) to give the title compound (12 mg, 0.03 mmol, 25% yield). LCMS calcd for C23H21F2N8O [M+H]+: m/z = 463.2; Found: 463.0. [00482] Step 5: 3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo[4,5-b]pyridine-6- carbaldehyde - 125 -4860-6199-1670.2 105807.001049 – PCT Application [00483] To a mixture of 2- - -8-(6-vinyl-3H-[1,2,3]triazolo- [4,5-b]pyridin-3-yl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2- yl)phenol (12.0 mg, 0.03 mmol) and sodium periodate (16.7 mg, 0.08 mmol) in THF (1 mL) and water (0.3 mL) was added osmium tetroxide (16.5 uL). The mixture was stirred at room temperature for 2h. The mixture was diluted with ethyl acetate, washed with water, concentrated and purified on FCC (0-5% MeOH/DCM) to give the title compound (3 mg, 25% yield). LCMS calcd for C22H19F2N8O2 [M+H]+: m/z = 464.2; Found: 464.2. [00484] Step 6:(S)-3-(6-(4-((3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo[4,5- b]pyridin-6-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00485] To a mixture of (S)-3-(1-oxo-6-(piperazin-1-yl)isoindolin-2-yl)piperidine-2,6-dione hydrochloride (2.83 mg, 0.01 mmol) and 3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a- (fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H- [1,2,3]triazolo[4,5-b]pyridine-6-carbaldehyde (3.0 mg, 0.01 mmol) in DMSO (1 mL) was added N,N-diisopropylethylamine (3.38 uL, 0.02 mmol). The mixture was stirred at r.t. for 30 min., then sodium triacetoxyborohydride (4.11 mg, 0.02 mmol) was added. The mixture was stirred at 35 °C overnight. The mixture was purified with Prep-LCMS (Waters CSH-Fluro- Phenyl, 5 uM, 30x100mm, 5-23% MeCN/water (containing 0.2%TFA) over 12 min) to give the title compound (1.4 mg, 28% yield). LCMS calcd for C39H39F2N12O4 [M+H]+: m/z = 777.3; Found: 777.1. Example 34. (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione - 126 -4860-6199-1670.2 105807.001049 – PCT Application [00486] This compound was synthesized by procedures analogous to that described in Example 33, using 5-bromo-3-methyl-1H-pyrazolo[3,4-b]pyridine instead of 6-bromo-3H-[1,2,3]triazolo- [4,5-b]pyridine. LCMS calcd for C41H42F2N11O4 [M+H]+: m/z = 790.3; Found: 790.2. Example 35. (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00487] This compound was synthesized by procedures analogous to that described in Example 33, using 5-bromo-6-methyl-1H-pyrazolo[3,4-b]pyridine instead of 6-bromo-3H-[1,2,3]triazolo- [4,5-b]pyridine. LCMS calcd for C41H42F2N11O4 [M+H]+: m/z = 790.3; Found: 790.2. Example 36. (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 127 -4860-6199-1670.2 105807.001049 – PCT Application [00488] Step 1: - - hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00489] To (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-N-methyl-5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (Intermediate 10; 13 mg, 0.037 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (15 mg, 0.074 mmol) and AcOH (11 µL, 0.19 mmol) in THF (1 mL) and MeOH (1 mL) was added NaBH3CN (7.0 mg, 0.11 mmol). The reaction was stirred at room temperature for 15 min then at 60 °C for 1 h. Additional tert-butyl 4- oxopiperidine-1-carboxylate (22 mg, 0.11 mmol) and NaBH3CN (4.7 mg, 0.074 mmol) were added, and the reaction was stirred at 80 °C for 2.5 h. Another portion of tert-butyl 4- oxopiperidine-1-carboxylate (22 mg, 0.11 mmol) and NaBH3CN (12 mg, 0.19 mmol) were added, and the reaction was stirred at 80 °C for an additional 22 h then allowed to cool to room temperature and concentrated. The residue was taken up in DCM (3 mL) then treated with BBr3 (0.15 mL, 1.6 mmol) and stirred for 35 min. Additional BBr3 (0.15 mL, 1.6 mmol) was added, and the reaction was stirred overnight at room temperature. The mixture was neutralized with sat. NaHCO3 (aq) then extracted with 3:1 CHCl3/IPA (4 x). The aqueous layer was acidified with conc. HCl and concentrated. The residue was taken up in MeOH then filtered through a 0.45 µm PTFE frit filter. The solids were washed with MeOH and the filtrate was subsequently concentrated to give the HCl/HBr salt of 2-((6aR,8R)-6a-ethyl-8-(methyl(piperidin-4-yl)amino)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (assumed quantitative yield). LCMS calcd for C23H32FN6O [M+H]+: m/z = 427.3; Found: 427.2. [00490] Step 2: (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin-1-yl)methyl) piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 128 -4860-6199-1670.2 105807.001049 – PCT Application [00491] A mixture of 2-((6aR,8R)-6a-ethyl-8-(methyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (20 mg, 0.047 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (20 mg, 0.056 mmol) in DMF (1 mL) was stirred for 5 min then treated with AcOH (13 µL, 0.23 mmol) and NaBH(OAc)3 (30 mg, 0.014 mmol). The reaction was stirred 1-2 h, at which point no conversion was observed. The reaction was purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the starting material 2-((6aR,8R)-6a-ethyl-8-(methyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (14.7 mg, 0.019 mmol). To this material was added (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5- yl)piperidine-4-carbaldehyde (8.2 mg, 0.023 mmol) and DMF (1 mL). The reaction was stirred for 40 min, then treated with AcOH (5.7 µL, 0.096 mmol) and NaBH(OAc)3 (12 mg, 0.057 mmol). The reaction was stirred for 1 h then diluted with MeOH, filtered through a 0.2 um PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (3.6 mg, 17%) as a white solid. LCMS calcd for C42H53FN9O4 [M+H]+: m/z = 766.4; Found: 766.4. Example 37. (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00492] Step 1: 2-((6aR,8R)-8-((2,2-dimethylpiperidin-4-yl)(methyl)amino)-6a-ethyl- 5,6,6a,7,8,9-hexahydropyrrolo 2-yl)-6-fluorophenol - 129 -4860-6199-1670.2 105807.001049 – PCT Application [00493] To (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-N-methyl-5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (14 mg, 0.040 mmol), tert-butyl 2,2-dimethyl-4-oxopiperidine-1-carboxylate (91 mg, 0.40 mmol) and AcOH (23 µL, 0.40 mmol) in THF (1 mL) and MeOH (1 mL) was added NaBH3CN (25 mg, 0.40 mmol). The reaction was stirred at 80 °C for 17 h then allowed to cool to room temperature and subsequently concentrated. The residue was taken up in DCM (3 mL) and treated with BCl3 (1 M in DCM; 4 mL, 4.0 mmol) dropwise. After stirring at room temperature for 1 h, the temperature was increased to 50 °C then allowed to cool to room temperature and stirred over the weekend. The reaction was quenched slowly with water then extracted with DCM (3 x) then purified via prep-HPCLMS (Waters CSH- C18, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.1%TFA) over 5 min) to give the TFA salt of the title compound (16 mg, 49%) as a clear oil. LCMS calcd for C25H36FN6O [M+H]+: m/z = 455.3; Found: 455.2. [00494] Step 2: (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00495] To 2-((6aR,8R)-8-((2,2-dimethylpiperidin-4-yl)(methyl)amino)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (16 mg, 0.020 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (9.7 mg, 0.027 mmol) in DMF (1 mL) was added Et3N (16 µL, 0.12 mmol). The reaction was stirred for 5 min then treated with NaBH(OAc)3 (12 mg, 0.058 mmol) and subsequently stirred at 35 °C for 20 h. Additional (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4- carbaldehyde (9.7 mg, 0.027 mmol) then NaBH(OAc)3 (12 mg, 0.058 mmol) were added and the reaction was allowed to stir for an additional 24 h at 35 °C. The reaction was allowed to cool to room temperature, diluted with MeCN/water and a small amount of TFA, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH Phenyl-Hexyl, 5 µm, 30x100mm, 5-23% MeCN/water (containing 0.1%TFA) over 12 min) to give the TFA salt of the title compound (2.0 mg, 9%) as a white solid. LCMS calcd for C44H57FN9O4 [M+H]+: m/z = 794.5; Found: 794.4. Example 38. (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 130 -4860-6199-1670.2 105807.001049 – PCT Application [00496] Step 1: - - hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine [00497] To (6aR,8S)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol (~78% purity; 368 mg, 0.833 mmol) and PPh3 (459 mg, 1.67 mmol) in THF (10 mL) was added diisopropyl azodicarboxylate (328 µL, 1.67 mmol), followed by diphenylphosphoryl azide (358 µL, 1.67 mmol). The reaction was stirred at room temperature for 1 h then quenched with a minimal amount of water. After concentrating to ~25% volume, the reaction was purified via SiO2 FCC (0-10% MeOH in DCM) to give (6aR,8R)-8- azido-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazine (assumed quantitative yield). LCMS calcd for C18H21FN7O [M+H]+: m/z = 370.2; Found: 370.1. [00498] Step 2: (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine [00499] To (6aR,8R)-8-azido-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazine (368 mg, 0.834 mmol) in THF (10 mL) was added PPh3 (437 mg, 1.67 mmol) The reaction was stirred at 70 °C for 1 h then treated with water (2 mL). The reaction was stirred for 13 h at 70 °C then allowed to cool slowly to room temperature and subsequently poured into water. The aqueous layer was extracted with DCM (3 x) and the combined organic layers were washed with 2 N HCl (aq). The combined aqueous layers were extracted with 3:1 CHCl3/IPA (1 x) then concentrated to give the HCl salt of (6aR,8R)-6a-ethyl- - 131 -4860-6199-1670.2 105807.001049 – PCT Application 2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]-pyrazino[2,3- c]pyridazin-8-amine (78 mg, 27%) as an off-white solid. LCMS calcd for C18H23FN5O [M+H]+: m/z = 344.2; Found: 344.1. [00500] Step 3: tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate [00501] To a briefly sonicated mixture of (6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (HCl salt; 26 mg, 0.063 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (21 mg, 0.11 mmol) and Et3N (26 µL, 0.19 mmol) in DCE (0.5 mL) was added Ti(OiPr)4 (31 µL, 0.11 mmol). The reaction was stirred at 60 °C for 3 h then cooled to 0 °C. MeOH (0.5 mL) was added, followed by NaBH4 (17 mg, 0.44 mmol). The reaction was allowed to warm to room temperature and stirred for 40 min. Water/MeCN (1:1) and TFA (48 µL, 0.63 mmol) were added, after which the mixture was briefly stirred, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 15.9-35.9% MeCN/water (containing 0.1%TFA) over 5 min) to give the TFA salt of tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (10 mg, 21%) as a clear film. LCMS calcd for C28H40FN6O3 [M+H]+: m/z = 527.3; Found: 527.2. [00502] Step 4: 2-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00503] To tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-methoxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (10 mg, 0.13 mmol) in MeOH (1 mL) was added acetaldehyde (2.2 µL, 0.040 mmol) and AcOH (2.3 µL, 0.040 mmol), followed by NaBH3CN (2.5 mg, 0.040 mmol). The reaction was stirred at room temperature for 1 h, at which point additional acetaldehyde (2.2 µL, 0.040 mmol) was added, followed by stirring at room temperature for an additional 2 h then stirring at 50 °C for 19 - 132 -4860-6199-1670.2 105807.001049 – PCT Application h. The reaction was allowed to cool to room temperature and concentrated. The residue was taken up in DCM (2 mL) then treated with BCl3 (1 M in DCM; 1.34 mL, 1.34 mmol) and stirred at room temperature for 72 h. Additional BCl3 (1 M in DCM; 1.00 mL, 1.00 mmol) was added and the reaction was stirred at 50 °C for 24 h, then cooled to 0 °C. Water (2 mL) was slowly added with stirring and the biphasic mixture was allowed to warm to room temperature. The DCM was evaporated and the resulting aqueous phase was filtered through a 0.2 µm PTFE syringe filter then purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of 2-((6aR,8R)-6a-ethyl-8- (ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2- yl)-6-fluorophenol (assumed quantitative yield). LCMS calcd for C24H34FN6O [M+H]+: m/z = 441.3; Found: 441.2. [00504] Step 5: (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00505] To 2-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[-1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (11 mg, 0.013 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (6.6 mg, 0.019 mmol) in DMF (1 mL) was added AcOH (3.8 µL, 0.067 mmol). The reaction was stirred for 5 min then treated with NaBH(OAc)3 (8.5 mg, 0.040 mmol) and subsequently stirred at room temperature for 50 min. The reaction was diluted with MeCN and water, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 6-26% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (2.5 mg, 17%) as a white solid. LCMS calcd for C43H55FN9O4 [M+H]+: m/z = 780.4; Found: 780.4. Examples 39-40 [00506] Examples 39 and 40 shown below in Table 5 were prepared as TFA salts by analogous methods used in preparing Example 38, utilizing the appropriate starting materials and intermediates. The asterisk (*) in Example 40 indicates an arbitrarily assigned stereocenter (absolute configuration at the stereocenter denoted by * was not confirmed). Table 5. Examples 39-40 - 133 -4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found )+ 4 4 y p p , Example 41. (S)-3-(6-(4-((4-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidin-1- yl)methyl) - - 134 -4860-6199-1670.2 105807.001049 – PCT Application [00507] Step 1: 2-chloro-6-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol [00508] A mixture of tert- - hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate (43 mg, 0.092 mmol), (3-chloro-2-hydroxyphenyl)boronic acid (48 mg, 0.28 mmol), XPhos Pd G2 (22 mg, 0.028 mmol) and K2CO3 (64 mg, 0.46 mmol) in 1,4-dioxane (1 mL) and water (0.25 mL) was sparged with N2 for 2 min. The reaction was stirred at 100 °C for 20 min then allowed to cool to room temperature and concentrated. The residue was taken up in DCM (2 mL) then treated with TFA (0.70 mL, 9.2 mmol). After stirring at room temperature until full conversion was observed by LC-MS, the reaction was concentrated to near dryness. The residue was taken up in water, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH- C18, 5 µm, 30x100mm, 6.8-26.8% MeCN/water (containing 0.2%TFA) over 5 min) to give 2- chloro-6-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol as its TFA salt (assumed quantitative yield). LCMS calcd for C24H34ClN6O [M+H]+: m/z = 457.2; Found: 457.2. [00509] Step 2: (S)-3-(6-(4-((4-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidin-1-yl)methyl) piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00510] To 2-chloro-6-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol (73 mg, 0.092 mmol) and (S)- 1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (32 mg, 0.090 mmol) in DMF (1 mL) was added AcOH (18 µL, 0.32 mmol). The reaction was stirred for 2 min then treated with NaBH(OAc)3 (41 mg, 0.19 mmol) and subsequently stirred at room temperature for 20 min. The reaction was filtered through a 0.2 µm PTFE syringe filter then purified via prep- HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 6.7-26.7% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (1.7 mg, 1.6%) as a white solid. LCMS calcd for C43H55ClN9O4 [M+H]+: m/z = 796.4; Found: 796.4. - 135 -4860-6199-1670.2 105807.001049 – PCT Application Example 42. (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00511] Step 1: 2-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-methylphenol [00512] A mixture of tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate (43 mg, 0.092 mmol), (2-hydroxy-3-methylphenyl)boronic acid (42 mg, 0.28 mmol), XPhos Pd G2 (22 mg, 0.028 mmol) and K2CO3 (64 mg, 0.46 mmol) in 1,4-dioxane (1 mL) and water (0.25 mL) was sparged with N2 for 2 min. The reaction was stirred at 100 °C for 20 min then allowed to cool to room temperature and concentrated. The residue was taken up in DCM (2 mL) then treated with TFA (0.70 mL, 9.2 mmol). After stirring at room temperature until full conversion was observed by LC-MS, the reaction was concentrated to near dryness. The residue was taken up in water, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH- C18, 5 µm, 30x100mm, 7.8-27.8% MeCN/water (containing 0.2%TFA) over 5 min) to give 2- ((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-2-yl)-6-methylphenol as its TFA salt (assumed quantitative yield). LCMS calcd for C25H37N6O [M+H]+: m/z = 437.3; Found: 437.3. [00513] Step 2: (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00514] To 2-((6aR,8R)-6a-ethyl-8-(ethyl(piperidin-4-yl)amino)-5,6,6a,7,8,9- hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-methylphenol (72 mg, 0.092 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (32 mg, - 136 -4860-6199-1670.2 105807.001049 – PCT Application 0.090 mmol) in DMF (1 mL) was added AcOH (18 µL, 0.32 mmol). The reaction was stirred for 2 min then treated with NaBH(OAc)3 (40 mg, 0.19 mmol) and subsequently stirred at room temperature for 20 min. The reaction was diluted with MeCN and water, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 5.8-25.8% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (4.9 mg, 4.8%) as a white solid. LCMS calcd for C44H58N9O4 [M+H]+: m/z = 776.5; Found: 776.4. Example 43. (S)-3-(6-(4-((4-(ethyl((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a- (fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8- yl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00515] Step 1: (6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-amine [00516] This intermediate was synthesized, as its HCl salt, using analogous procedures to those used in the synthesis of Intermediate 10, using (6aR,8S)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol instead of (6aR,8S)-2-chloro-6a-ethyl- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-ol. LCMS calcd for C10H14ClFN5 [M+H]+: m/z = 258.1; Found: 258.0. [00517] Step 5: tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate - 137 -4860-6199-1670.2 105807.001049 – PCT Application [00518] To a briefly - -5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-amine (56 mg, 0.17 mmol), tert-butyl 4- oxopiperidine-1-carboxylate (57 mg, 0.29 mmol) and Et3N (71 µL, 0.51 mmol) in DCE (0.5 mL) was added Ti(OiPr)4 (84 µL, 0.29 mmol). The reaction was stirred at 60 °C for 3 h then cooled to 0 °C. MeOH (0.5 mL) was added, followed by NaBH4 (49 mg, 1.3 mmol). The reaction was allowed to warm to room temperature and stirred for 40 min. Water/MeCN (1:1, 4 mL) was added, followed by TFA (129 µL, 1.7 mmol). After stirring briefly, the mixture was filtered through a 0.2 µm PTFE syringe filter and purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 15.9-35.9% MeCN/water (containing 0.1%TFA) over 5 min) to give the TFA salt of tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (assumed quantitative yield) as a clear film. LCMS calcd for C20H31ClFN6O2 [M+H]+: m/z = 441.2; Found: 441.1. [00519] Step 6: tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate [00520] To tert-butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (113 mg, 0.17 mmol), acetaldehyde (85 µL, 1.5 mmol) and AcOH (29 µL, 0.51 mmol) in MeOH (2 mL) was added NaBH3CN (32 mg, 0.51 mmol). The reaction was stirred at 50 °C overnight then neutralized with sat. NaHCO3 (aq). The aqueous layer was extracted with DCM (3 x). The combined organic layers were washed with brine (1 x), dried with MgSO4, filtered, then concentrated to afford tert- butyl 4-(((6aR,8R)-2-chloro-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate (assumed quantitative yield), which was used without further purification. LCMS calcd for C22H35ClFN6O2 [M+H]+: m/z = 469.2; Found: 469.2. - 138 -4860-6199-1670.2 105807.001049 – PCT Application [00521] Step 7: 2-((6aR,8R)-8-(ethyl(piperidin-4-yl)amino)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00522] A mixture of tert- - -5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidine-1-carboxylate (79 mg, 0.17 mmol), (3-fluoro-2-hydroxyphenyl)boronic acid (79 mg, 0.51 mmol), XPhos Pd G2 (46 mg, 0.069 mmol) and K2CO3 (117 mg, 0.84 mmol) in 1,4-dioxane (1 mL) and water (0.25 mL) was sparged with N2 for 2 min. The reaction was stirred at 100 °C for 20 min then allowed to cool briefly and concentrated. The residue was taken up in DCM (2 mL) then treated with TFA (1.29 mL, 16.9 mmol). After stirring at room temperature until full conversion was observed by LC-MS, the reaction was concentrated. The residue was purified via prep-HPLCMS (Waters CSH Phenyl-Hexyl, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.2%TFA) over 5 min) to afford 2-((6aR,8R)-8-(ethyl(piperidin-4-yl)amino)-6a-(fluoromethyl)-5,6,6a,7,8,9-hexahydro- pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol as its TFA salt (assumed quantitative yield). LCMS calcd for C23H31F2N6O [M+H]+: m/z = 445.3; Found: 445.3. [00523] Step 8: (S)-3-(6-(4-((4-(ethyl((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a- (fluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8- yl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00524] To 2-((6aR,8R)-8-(ethyl(piperidin-4-yl)amino)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (133 mg, 0.17 mmol) and (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)piperidine-4-carbaldehyde (61 mg, 0.17 mmol) in DMF (1 mL) was added AcOH (49 µL, 0.85 mmol). The reaction was stirred for 2 min then treated with NaBH(OAc)3 (108 mg, 0.51 mmol) and subsequently stirred at room temperature for 35 min. The reaction was diluted with MeCN and water, filtered through a 0.2 µm PTFE syringe filter, then purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 5-21% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (2.6 mg, 1.4%) as a white solid. LCMS calcd for C42H52F2N9O4 [M+H]+: m/z = 784.4; Found: 784.4. Examples 44-45 - 139 -4860-6199-1670.2 105807.001049 – PCT Application [00525] Examples 44-45 shown below in Table 6 were prepared as TFA salts by analogous methods used in preparing Example 43, utilizing the appropriate starting materials and intermediates. Table 6. Examples 44-45 Calcd. Found )+ 4 4 yl)piperidine-2,6-dione Example 46. (3S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione - 140 -4860-6199-1670.2 105807.001049 – PCT Application [00526] Step 1: - - 4- yl)(methyl)amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol [00527] This intermediate was synthesized, as its TFA salt, using analogous procedures to those described in Example 37. LCMS calcd for C24H32F3N6O [M+H]+: m/z = 477.3; Found: 477.2. [00528] Step 2: (3S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00529] To a stirring solution of 2-((6aR,8R)-6a-(difluoromethyl)-8-((2,2-dimethylpiperidin-4- yl)(methyl)amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- fluorophenol (TFA salt; 52 mg, 0.063 mmol), (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin- 5-yl)piperidine-4-carbaldehyde (31 mg, 0.088 mmol) and AcOH (18 µL, 0.32 mmol) in DMF (1 mL) was added NaBH(OAc)3 (40 mg, 0.19 mmol). The reaction was stirred at room temperature for 45 min then treated with Et3N (43 µL, 0.31 mmol) and stirred at 35 °C. After 15 min, additional Et3N (62 µL, 0.45 mmol) was added, and the reaction was stirred for another 2 h at 35 °C. The reaction was allowed to cool to room temperature then diluted with MeCN, water, and a minimal amount of TFA. Purification via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 5-25% MeCN/water (containing 0.1%TFA) over 5 min) gave the TFA salt of the title compound (10 mg, 14%) as a white solid. LCMS calcd for C43H53F3N9O4 [M+H]+: m/z = 816.4; Found: 816.5. - 141 -4860-6199-1670.2 105807.001049 – PCT Application Example 47. (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione [00530] Step 1: tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl)amino)piperidine-1- carboxylate [00531] To tert-butyl 4-(((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]- pyrazino[2,3-c]pyridazin-8-yl)amino)piperidine-1-carboxylate (14 mg, 0.021 mmol), tetrahydrofuran-2-carbaldehyde (6.3 mg, 0.063 mmol) and AcOH (3.6 µL, 0.063 mmol) in MeOH (2 mL) was added NaBH3CN (4.0 mg, 0.063 mmol). The reaction was stirred at 60 °C overnight, after which additional tetrahydrofuran-2-carbaldehyde (18 mg, 0.18 mmol) was added. The reaction was stirred for 5 h then allowed to cool to room temperature. The reaction was neutralized with sat. NaHCO3 (aq) then extracted with DCM (3 x). The combined DCM layers were washed with brine (1 x), dried with MgSO4, filtered then concentrated to give tert-butyl 4- (((6aR,8R)-2-chloro-6a-ethyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8- yl)((tetrahydrofuran-2-yl)methyl)amino)piperidine-1-carboxylate (assumed quantitative yield), which was used without further purification. LCMS calcd for C26H42ClN6O3 [M+H]+: m/z = 521.3; Found: 521.2. [00532] Step 2: tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl) amino)piperidine-1-carboxylate - 142 -4860-6199-1670.2 105807.001049 – PCT Application [00533] A mixture of tert- - hexahydropyrrolo- [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl)amino)piperidine-1- carboxylate (11 mg, 0.021 mmol), (3-fluoro-2-hydroxyphenyl)boronic acid (9.9 mg, 0.063 mmol), XPhos Pd G2 (5.0 mg, 0.0063 mmol) and K2CO3 (15 mg, 0.11 mmol) in 1,4-dioxane (1 mL) and water (0.25 mL) was sparged with N2 for 1 min. The reaction was stirred at 100 °C for 45 min then allowed to cool to room temperature. Additional (3-fluoro-2-hydroxyphenyl)boronic acid (9.9 mg, 0.063 mmol), XPhos Pd G2 (5.0 mg, 0.0063 mmol) and K2CO3 (15 mg, 0.11 mmol) were added. The mixture was sparged with N2 for 2 min then stirred at 100 °C for 1 h then allowed to cool to room temperature. The crude mixture was diluted with MeCN, water, and MeOH, then filtered through a 0.2 µm PTFE syringe filter and purified via prep-HPLCMS (Waters CSH-C18, 5 µm, 30x100mm, 19.2-39.2% MeCN/water (containing 0.2%TFA) over 5 min) to give tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2-yl)methyl)amino)- piperidine-1-carboxylate as its TFA salt (assumed quantitative yield). LCMS calcd for C32H46FN6O4 [M+H]+: m/z = 597.4; Found: 597.3. [00534] Step 3: 2-((6aR,8R)-6a-ethyl-8-(piperidin-4-yl((tetrahydrofuran-2-yl)methyl)amino)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol [00535] A solution of tert-butyl 4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)-piperidine-1-carboxylate (TFA salt; 17 mg, 0.021 mmol) in DCM (1 mL) was added TFA (0.16 mL, 2.1 mmol). The reaction was stirred at room temperature for 20 min then concentrated to give 2-((6aR,8R)-6a-ethyl-8-(piperidin-4-yl((tetrahydrofuran-2- yl)methyl)amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6- - 143 -4860-6199-1670.2 105807.001049 – PCT Application fluorophenol as its TFA salt (assumed quantitative yield). LCMS calcd for C27H38FN6O2 [M+H]+: m/z = 497.3; Found: 497.23. [00536] Step 4: (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)-piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00537] A mixture of 2-((6aR,8R)-6a-ethyl-8-(piperidin-4-yl((tetrahydrofuran-2-yl)methyl)- amino)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)-6-fluorophenol (TFA salt; 18 mg, 0.021 mmol), (S)-1-(2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5- yl)piperidine-4-carbaldehyde (7.5 mg, 0.021 mmol) and AcOH (6.0 µL, 0.11 mmol) in DMF (1 mL) was stirred for 2 min then treated with NaBH(OAc)3 (13 mg, 0.063 mmol). The reaction was stirred at room temperature for 30 min, after which additional AcOH (6.0 µL, 0.11 mmol) and NaBH(OAc)3 (13 mg, 0.063 mmol) were added. The reaction was stirred for a further 20 min, then was diluted with MeCN and water, filtered through a 0.2 µm PTFE syringe filter, and purified via prep-HPLCMS (Waters CSH Fluoro-Phenyl, 5 µm, 30x100mm, 7.5-27.5% MeCN/water (containing 0.2%TFA) over 5 min) to give the TFA salt of the title compound (0.9 mg, 3.6%) as a white solid. LCMS calcd for C46H59FN9O5 [M+H]+: m/z = 836.5; Found: 836.5. Examples 48-49 [00538] Examples 48-49 shown below in Table 7 were prepared as TFA salts by analogous methods used in preparing Example 47, utilizing the appropriate starting materials and intermediates. Table 7. Examples 48-49 d )+ 4 , dione - 144 -4860-6199-1670.2 105807.001049 – PCT Application Calcd. Found )+ Example 50. (S)-3-(6-(1-((1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00539] Step 1. Methyl 1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5- carboxylate [00540] The title compound was synthesized by procedures analogous to that described in Example 1, Steps 2-3 using appropriate intermediates and commercial reagents. LCMS calcd for C24H23FN7O3 [M+H]+: m/z = 476.2; Found: 476.2. - 145 -4860-6199-1670.2 105807.001049 – PCT Application [00541] Step 2.2-Fluoro-6-((6aR,8R)-8-(5-(hydroxymethyl)-1H-pyrazolo[3,4-b]pyridin-1-yl)- 6a-methyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2-yl)phenol [00542] Lithium aluminum mg, THF) was added dropwise to a solution of methyl 1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5- carboxylate (70 mg, 0.15 mmol) in THF (1 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min. The reaction was quenched with 15% NaOH solution and purified by prep-HPLC (15-45% MeCN in water, 0.1% TFA) to give the desired product (46 mg, 70% yield) as a light yellow solid. LCMS calcd for C23H23FN7O2 [M+H]+: m/z = 448.2; Found: 448.2. [00543] Step 3.1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridine-5- carbaldehyde [00544] A suspension of 2-fluoro-6-((6aR,8R)-8-(5-(hydroxymethyl)-1H-pyrazolo[3,4- b]pyridin-1-yl)-6a-methyl-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-2- yl)phenol (46 mg, 0.1 mmol) and manganese(IV) oxide (180 mg, 2.1 mmol) in chloroform (1 mL) was heated at 50°C for 18 h. The solid materials were filtered off and washed with chloroform (1 mL). The filtrate was concentrated and purified by prep-HPLC (15-50% MeCN in water, 0.1% TFA) to give the desired product (30 mg, 66% yield), as yellow solid. LCMS calcd for C23H21FN7O2 [M+H]+: m/z = 446.2; Found: 446.2. [00545] Step 4. (S)-3-(6-(1-((1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione [00546] The title compound, as the TFA salt, was synthesized by procedures analogous to that described in Example 1 Step 5 using appropriate intermediates and commercial reagents. LCMS calcd for C41H42FN10O4 [M+H]+: m/z = 757.3; Found: 757.6. - 146 -4860-6199-1670.2 105807.001049 – PCT Application Examples 51-53 [00547] Examples 51-53 shown below in Table 8 were prepared as TFA salts by analogous methods used in preparing Example 7, utilizing the appropriate starting materials and intermediates. Table 8. Examples 51-53 Calcd. Found + , , , , , pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6- - 147 -4860-6199-1670.2 105807.001049 – PCT Application methyl-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridin- Example 54. (S)-3-(6-(4-(((R)-2-(((6aR,8R)-2-(3-Chloro-2-hydroxyphenyl)-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8- yl)oxy)-5-methyl-7,8-dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1- oxoisoindolin-2-yl)piperidine-2,6-dione [00548] Step 1. tert-Butyl (R)-2-hydroxy-5-methyl-7,8-dihydro-1,6-naphthyridine-6(5H)- carboxylate - 148 -4860-6199-1670.2 105807.001049 – PCT Application [00549] The title compound was synthesized by procedures analogous to that described in Example 27 Step 1-5 using appropriate intermediates and commercial reagents. The isolated racemic material was separated into single enantiomers using prep-Chiral HPLC (Lux i- Amylose-3, 22% Isopropanol:Methanol in Hexanes, 30 mL/min over 30 minutes) to yield the title compound (tR = 4.94 min, Peak A, >99%ee) and it’s enantiomer (tR = 6.56 min, Peak B, >99%ee). The absolute configuration was arbitrarily assigned. LCMS calcd for C14H21N2O3 [M+H]+: m/z = 265.2; Found: 264.9. [00550] Step 2. (S)-3-(6-(4-(((R)-2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a- (difluoromethyl)-5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5- methyl-7,8-dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione [00551] The title compound, as the TFA salt, was synthesized by procedures analogous to that described in Example 16 using appropriate intermediates and commercial reagents. LCMS calcdfor C44H47ClF2N9O5[M+H]+: m/z = 854.3; Found: 853.7. Examples 55-57 [00552] Examples 55-57 shown below in Table 9 were prepared as TFA salts by analogous methods used in preparing Example 54, utilizing the appropriate starting materials and intermediates. Table 9. Examples 55-57 + yl)piperidine-2,6-dione - 149 -4860-6199-1670.2 105807.001049 – PCT Application Example A. SMARCA2 HiBiT and SMARCA4 HiBiT Degradation Assay Preparation of SMARCA2/4-HiBiT knock-in cells [00553] HiBiT peptide knock-in of SMARCA2 in LgBiT expressing HEK293T cells was performed by CRISPR-mediated tagging system as described Promega. The homozygous HiBiT knock-in on c-terminus SMARCA2 was confirmed by sanger sequence. SMARCA2-HiBiT knock-in Hela monoclonal cell (CS302366) and SMARCA4-HiBiT knock-in Hela monoclonal cell (CS3023226) were purchased from Promega. The heterozygous HiBiT-knock-in was confirmed by sanger sequence in both SMARCA2-HiBiT and SMARCA4-HiBiT monoclonal cells. SMARCA2 HiBiT and SMARCA4 HiBiT degradation assay in HeLa cells [00554] Dispense 10ul aliquot of prepared Hela-SMARCA2-HiBiT or Hela-SMARCA4-HiBiT cells (1:1 ratio of cells:Trypan Blue (#1450013, Bio-Rad)) onto cell counting slide (#145-0011, - 150 -4860-6199-1670.2 105807.001049 – PCT Application Bio-Rad) and obtain cell density and cell viability using cell counter (TC20, Bio-Rad). Remove appropriate volume of resuspended cells from culture flask to accommodate 2500 cells/well @ 20 ^L/well. Transfer Hela-HiBiT cells to 50 mL conical (#430290, Corning). Spin down at 1000 rpm for 5 min using tabletop centrifuge (SPINCHRON 15, Beckman). Discard supernatant and resuspend cell pellet in modified EMEM (#30-2003, ATCC) cell culture media containing 10% FBS (F2422-500ML, Sigma), and 1X Penicillin/Streptomycin (200g/L) (30-002-CI, Corning) to a cell density of 125,000 cells/mL. Dispense 20 ^Lof resuspended Hela-HiBit cells per well in 384-well TC treated plate (#12-565-343, Thermo Scientific) using standard cassette (#50950372, Thermo Scientific) on Multidrop Combi (#5840310, Thermo Scientific) inside laminar flow cabinet. [00555] Compounds were dissolved in DMSO to make 10 mM stock and 3-fold series dilutions were further conducted keeping the highest concentration 10 µM. Dispense test compounds onto plates using digital liquid dispenser (D300E, Tecan). Incubate plates in humidified tissue culture incubator @37̊ °C for 18 hours. Add 20 ^L of prepared Nano-Glo® HiBiT Lytic detection buffer (N3050, Promega) to each well of 384-well plate using small tube cassette (#24073295, Thermo Scientific) on Multidrop Combi, incubate @ RT for 30-60 min. Read plates on microplate reader (Envision 2105, PerkinElmer) using 384 well Ultra-Sensitive luminescence mode. Raw data files and compound information reports are swept into centralized data lake and deconvoluted using automated scripts designed by TetraScience, Inc. Data analysis, curve-fitting and reporting done in Dotmatics Informatics Suite using Screening Ultra module. [00556] Results are summarized below in Table 10. In Table 10, A = DC50 < 0.1 µM; B = 0.1 µM ≤ DC50 < 1 µM and C = DC50 > 1 µM. In Table 10, A = Dmax > 75% and B = 50% < Dmax ≤ 75% and C = Dmax < 50%. Table 10. Biological Data 11 B C B C - 151 -4860-6199-1670.2 105807.001049 – PCT Application Example SMARCA2 SMARCA2 SMARCA4 SMARCA4 57 A A C C - 152 -4860-6199-1670.2 105807.001049 – PCT Application [00557] While we have described a number of embodiments of this invention, it is apparent that our basic examples may be altered to provide other embodiments that utilize the compounds and methods of this invention. Therefore, it will be appreciated that the scope of this invention is to be defined by the appended claims rather than by the specific embodiments that have been represented by way of example. - 153 -4860-6199-1670.2
Claims
105807.001049 – PCT Application What is claimed: 1. A compound of Formula (I): or a R1 is halo, C1-6 alkyl, or C1-6 haloalkyl; each R2 is independently H, D, or F; each R3 is independently H, D, C1-6 alkyl, C1-6 haloalkyl, C3-6 heterocycloalkyl or C3-6 cycloalkyl; s is 1, 2 or 3; t is 1, 2, 3, or 4; R4 is H, D, C1-6 alkyl, C1-6 alkoxyalkyl, C3-6 cycloalkyl, or C1-6 haloalkyl; R5 is H, D, or F; L1 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; L2 is absent, or is O, S, S(O), SO2, NR3, C(R3)2 or CO; ring A1 is a 9-10 membered heteroaryl group, 5-7 membered heterocycloalkyl, or a 9-10 membered heterocycloalkenyl group; ring A2 is a 3-7 membered cycloalkyl group or a 4-7-membered heterocycloalkyl group; X1 is CH2, CO, CH=CH (when X2 = CO), or N=CH (when X2 = CO); X2 is CH2, CO, CH=CH (when X1 = CO), or N=CH (when X1 = CO); wherein the alkyl group, haloalkyl group, cycloalkyl group, alkoxyalkyl group, heterocycloalkenyl group, heteroaryl group or heterocycloalkyl group is optionally substituted by one or more Rf groups; each Rf is independently D, oxo, halogen, C1-C8 alkoxy, C1-C8 alkyl, C1-6 haloalkyl, C1-6 haloalkoxy, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, C3-8 heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)RcRd, -P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, - S(O)2ORb, -OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 groups selected from D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; - 154 -4860-6199-1670.2
105807.001049 – PCT Application each Ra is independently H, D, -C(O)Rb, -C(O)ORc, -C(O)NRcRd, -C(=NRb)NRbRc, - C(=NORb)NRbRc, -C(=NCN)NRbRc, -P(ORc)2, -P(O)RcRb, -P(O)RcRd, -P(O)ORcORb, -S(O)Rb, - S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rb, is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, C6-10 aryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C5-12 heteroaryl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; each Rc or Rd is independently H, D, -C1-C10 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, -OC1- C6alkyl, -O-cycloalkyl, C6-10 aryl, C5-12 heteroaryl, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 heterocycloalkyl, or C3-8 heterocycloalkenyl; or Rc and Rd, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocycloalkenyl group. 2. The compound according to claim 1 that is a compound of formula IIa or formula IIb: or a pharmaceutically acceptable salt thereof; 3. The compound according to claim 2 that is a compound of formula IIa. 4. The compound according to claim 2 that is a compound of formula IIb. 5. The compound according to claim 1 or claim 2 that is a compound of formula IIIa or formula IIIb: - 155 -4860-6199-1670.2
105807.001049 – PCT Application or a or 6. The compound according to claim 5 that is a compound of formula IIIa. 7. The compound according to claim 5 that is a compound of formula IIIb. 8. The compound according to claim 1 or claim 2 that is a compound of formula IVa or formula IVb: or a pharmaceutically acceptable salt thereof; wherein X is N or CR3. 9. The compound according to claim 8 that is a compound of formula IVa. 10. The compound according to claim 8 that is a compound of formula IVb. - 156 -4860-6199-1670.2
105807.001049 – PCT Application 11. The compound according to claim 1 or claim 2 that is a compound of formula Va or formula Vb: or a pharmaceutically acceptable salt thereof; wherein X is N or CR3. 12. The compound according to claim 11 that is a compound of formula Va. 13. The compound according to claim 11 that is a compound of formula Vb. 14. The compound according to any one of the preceding claims, wherein R1 is halo. 15. The compound according to claim 14, wherein R1 is F. 16. The compound according to claim 1, wherein s is 1. 17. The compound according to claim 1, wherein s is 2. 18. The compound according to claim 1, wherein s is 3. 19. The compound according to any one of the preceding claims, wherein each R2 is F. 20. The compound according to any one of claims 1-18, wherein each R2 is H. 21. The compound according to claim 1, wherein t is 1. 22. The compound according to claim 1, wherein t is 2. - 157 -4860-6199-1670.2
105807.001049 – PCT Application 23. The compound according to claim 1, wherein t is 3. 24. The compound according to claim 1, wherein t is 4. 25. The compound according to claim 1, wherein each R3 is H. 26. The compound according to any one of the preceding claims, wherein R4 is H. 27. The compound according to any one of claims 1-25, wherein R4 is C1-6 alkyl. 28. The compound according to any one of claims 1-25, wherein R4 is CHF2.29. The compound according to claim 1, wherein R5is H. 30. The compound according to any one of claims 1-4, wherein L1 is O. 31. The compound according to any one of claims 1-4, wherein L1 is NR3. 32. The compound according to any one of claims 1-4, wherein L1 is absent. 33. The compound according to any one of the preceding claims, wherein L2 is C(R3)2. 34. The compound according to claim 33, wherein L2 is a methylene group. 35. The compound according to any one of the preceding claims, wherein ring A1 is a 9-10 membered heteroaryl group. 36. The compound according to any one of claims 1-34, wherein ring A1 is a 9-10 membered heterocycloalkenyl group. 37. The compound according to any one of claims 1-34, wherein ring A1 is a 5-7 membered heterocycloalkyl group. 38. The compound according to any one of claims 1-34, wherein ring A1 is selected from - 158 -4860-6199-1670.2
105807.001049 – PCT Application , , wherein each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. 39. The to one of claims 1-34, wherein A1 is selected from , , , , , , - 159 -4860-6199-1670.2
105807.001049 – PCT Application ; each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. 40. The compound according to any one of claims 1-34, wherein when L1 is absent, ring A1 is selected from; wherein each R7 is independently D, halo, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, -CN or ORa; each R8 is independently D, C1-6 alkyl, C1-6 haloalkyl, C3-6 cycloalkyl, or oxo; each m is independently 0, 1, 2, 3, 4, 5, or 6; each n is independently 0, 1, 2 or 3; and each o is independently 0, 1 or 2. 41. The compound according to any one of claims 1-4, wherein ring A2 is a 4-7-membered heterocycloalkyl group. 42. The compound according claim 41, wherein ring A2 is a piperidine group or a piperazine group. 43. The compound according to any one of claims 1-4, wherein X1 is CH2. 44. The compound according to any one of claims 1-4, wherein X1 is CO. 45. The compound according to any one of claims 1-4, wherein X2 is CH2. - 160 -4860-6199-1670.2
105807.001049 – PCT Application 46. The compound according to any one of claims 1-4, wherein X2 is CO. 47. The compound according to any one of claims 5-13, wherein X is N. 48. The compound according to any one of claims 5-13, wherein X is CR3. 49. The compound according to claim 1 that is: (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl) piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1H- pyrazolo[3,4-b]pyridin-5-yl)methyl) piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4- c]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[4,3- b]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydro-pyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-2H-pyrazolo[3,4- c]pyridin-5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-6- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 161 -4860-6199-1670.2
105807.001049 – PCT Application (S)-3-(6-(1-((2-(((6aS,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo [1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxyquinolin-6- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)quinolin-2- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(1-((7-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,8-naphthyridin-3- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxy-3-methylphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((6-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4-dihydroisoquinolin- 2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7,8-dihydro-1,6- naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-3,4-dihydro-2,7- naphthyridin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 162 -4860-6199-1670.2
105807.001049 – PCT Application (S)-3-(6-(4-((5-(((6aR,8R)-6a-(difluoromethyl)-2-(2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)isoindolin-2-yl)methyl)-piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-7-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,3-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((R)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((S)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy-phenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-(((S)-6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxy-phenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1-methyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (3S)-3-(6-(4-((2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-chloro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-((6-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-1,1-dimethyl-3,4- dihydroisoquinolin-2(1H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-[5-[4-[[1-[(4R,6S)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5- yl]methyl]piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione; - 163 -4860-6199-1670.2
105807.001049 – PCT Application (3S)-3-[5-[4-[[1-[(4R,6R)-12-(3-fluoro-2-hydroxyphenyl)-6-methyl-2,8,10,11- tetrazatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]pyrrolo[2,3-b]pyridin-5- yl]methyl]piperazin-1-yl]-3-oxo-1H-isoindol-2-yl]piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridin-5- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((3-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3H-[1,2,3]triazolo-[4,5-b]pyridin-6- yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-3-methyl-1H-pyrazolo[3,4-b]pyridin- 5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1H-pyrazolo[3,4-b]pyridin- 5-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2-dimethylpiperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1-yl)methyl)piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3-difluoropiperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-ethyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(ethyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 164 -4860-6199-1670.2
105807.001049 – PCT Application (S)-3-(6-(4-((4-(ethyl((6aR,8R)-6a-ethyl-2-(2-hydroxy-3-methylphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1-yl)methyl)piperidin- 1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(ethyl((6aR,8R)-2-(3-fluoro-2-hydroxyphenyl)-6a-(fluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)piperidin- 1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aS,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-3,3- difluoropiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)(methyl)amino)-2,2- dimethylpiperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (3S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)((tetrahydrofuran-2- yl)methyl)amino)piperidin-1-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-((4-((cyclobutylmethyl)((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo-[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((4-(((6aR,8R)-6a-ethyl-2-(3-fluoro-2-hydroxyphenyl)-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]-pyrazino[2,3-c]pyridazin-8-yl)(isopentyl)amino)piperidin-1- yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; or a pharmaceutically acceptable salt thereof. 50. The compound according to claim 1 that is: (S)-3-(6-(1-((1-((6aR,8R)-2-(3-Fluoro-2-hydroxyphenyl)-6a-methyl-5,6,6a,7,8,9- hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1H-pyrazolo[3,4-b]pyridin-5- yl)methyl)piperidin-4-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; (S)-3-(6-(4-((1-((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-1,4,6,7-tetrahydro-5H- pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; - 165 -4860-6199-1670.2
105807.001049 – PCT Application (3S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-6-methyl-1,4,6,7- tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione; (3S)-3-(6-(4-((1-((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)-7-methyl-1,4,6,7- tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2- yl)piperidine-2,6-dione; (S)-3-(6-(4-(((R)-2-(((6aR,8R)-2-(3-Chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((R)-2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((S)-2-(((6aR,8R)-6a-(difluoromethyl)-2-(3-fluoro-2-hydroxyphenyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; (S)-3-(6-(4-(((S)-2-(((6aR,8R)-2-(3-chloro-2-hydroxyphenyl)-6a-(difluoromethyl)- 5,6,6a,7,8,9-hexahydropyrrolo[1',2':4,5]pyrazino[2,3-c]pyridazin-8-yl)oxy)-5-methyl-7,8- dihydro-1,6-naphthyridin-6(5H)-yl)methyl)piperidin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6- dione; or a pharmaceutically acceptable salt thereof. 51. The compound of any one of the preceding claims, in the form of a pharmaceutically acceptable salt. 52. A pharmaceutical composition comprising a compound according to any one of the preceding claims, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient. 53. A method of treating cancer in a subject in need thereof comprising administering to the subject a compound of any one of claims 1-51 or a pharmaceutical composition of claim 52. - 166 -4860-6199-1670.2
105807.001049 – PCT Application 54. The method of claim 53, wherein the cancer is SMARCA4 deleted cancer. 55. The method according claim 53 or claim 54, wherein the cancer is squamous-cell carcinoma, basal cell carcinoma, adenocarcinoma, hepatocellular carcinomas, and renal cell carcinomas, cancer of the bladder, bowel, breast, cervix, colon, esophagus, head, kidney, liver, lung, neck, ovary, pancreas, prostate, and stomach; leukemias; benign and malignant lymphomas, particularly Burkitt's lymphoma and Non-Hodgkin's lymphoma; benign and malignant melanomas; myeloproliferative diseases; sarcomas, including Ewing's sarcoma, hemangiosarcoma, Kaposi's sarcoma, liposarcoma, myosarcomas, peripheral neuroepithelioma, synovial sarcoma, gliomas, astrocytomas, oligodendrogliomas, ependymomas, gliobastomas, neuroblastomas, ganglioneuromas, gangliogliomas, medulloblastomas, pineal cell tumors, meningiomas, meningeal sarcomas, neurofibromas, and Schwannomas; bowel cancer, breast cancer, prostate cancer, cervical cancer, uterine cancer, lung cancer, ovarian cancer, testicular cancer, thyroid cancer, astrocytoma, esophageal cancer, pancreatic cancer, stomach cancer, liver cancer, colon cancer, melanoma; carcinosarcoma, Hodgkin's disease, Wilms' tumor and teratocarcinomas. 56. The method according to any one of claims 53-55, wherein the cancer is T-lineage Acute lymphoblastic Leukemia (T-ALL), T-lineage lymphoblastic Lymphoma (T-LL), Peripheral T- cell lymphoma, Adult T-cell Leukemia, Pre-B ALL, Pre-B Lymphomas, Large B-cell Lymphoma, Burkitts Lymphoma, B-cell ALL, Philadelphia chromosome positive ALL and Philadelphia chromosome positive CML. 57. The method of claim 55 wherein the lung cancer is SMARCA4 deficient non-small cell lung cancer. 58. A method of degrading a SMARCA protein comprising contacting the SMARCA protein with a compound of any one of claims 1-51 or a pharmaceutical composition of claim 52. - 167 -4860-6199-1670.2
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363596637P | 2023-11-07 | 2023-11-07 | |
| US63/596,637 | 2023-11-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2025101738A1true WO2025101738A1 (en) | 2025-05-15 |
Family
ID=93650461
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/054907PendingWO2025101738A1 (en) | 2023-11-07 | 2024-11-07 | Brm targeting compounds and associated methods of use |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2025101738A1 (en) |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3657744A (en) | 1970-05-08 | 1972-04-25 | Univ Minnesota | Method for fixing prosthetic implants in a living body |
| US4739762A (en) | 1985-11-07 | 1988-04-26 | Expandable Grafts Partnership | Expandable intraluminal graft, and method and apparatus for implanting an expandable intraluminal graft |
| US5040548A (en) | 1989-06-01 | 1991-08-20 | Yock Paul G | Angioplasty mehtod |
| US5061273A (en) | 1989-06-01 | 1991-10-29 | Yock Paul G | Angioplasty apparatus facilitating rapid exchanges |
| US5195984A (en) | 1988-10-04 | 1993-03-23 | Expandable Grafts Partnership | Expandable intraluminal graft |
| US5292331A (en) | 1989-08-24 | 1994-03-08 | Applied Vascular Engineering, Inc. | Endovascular support device |
| US5451233A (en) | 1986-04-15 | 1995-09-19 | Yock; Paul G. | Angioplasty apparatus facilitating rapid exchanges |
| US5496346A (en) | 1987-01-06 | 1996-03-05 | Advanced Cardiovascular Systems, Inc. | Reinforced balloon dilatation catheter with slitted exchange sleeve and method |
| US5674278A (en) | 1989-08-24 | 1997-10-07 | Arterial Vascular Engineering, Inc. | Endovascular support device |
| US6344053B1 (en) | 1993-12-22 | 2002-02-05 | Medtronic Ave, Inc. | Endovascular support device and method |
| WO2022099117A1 (en)* | 2020-11-06 | 2022-05-12 | Prelude Therapeutics Incorporated | Brm targeting compounds and associated methods of use |
| WO2024059806A1 (en)* | 2022-09-15 | 2024-03-21 | Prelude Therapeutics Incorporated | Brm targeting compounds and associated methods of use |
- 2024
- 2024-11-07WOPCT/US2024/054907patent/WO2025101738A1/enactivePending
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3657744A (en) | 1970-05-08 | 1972-04-25 | Univ Minnesota | Method for fixing prosthetic implants in a living body |
| US4739762A (en) | 1985-11-07 | 1988-04-26 | Expandable Grafts Partnership | Expandable intraluminal graft, and method and apparatus for implanting an expandable intraluminal graft |
| US4739762B1 (en) | 1985-11-07 | 1998-10-27 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| US5451233A (en) | 1986-04-15 | 1995-09-19 | Yock; Paul G. | Angioplasty apparatus facilitating rapid exchanges |
| US5496346A (en) | 1987-01-06 | 1996-03-05 | Advanced Cardiovascular Systems, Inc. | Reinforced balloon dilatation catheter with slitted exchange sleeve and method |
| US5195984A (en) | 1988-10-04 | 1993-03-23 | Expandable Grafts Partnership | Expandable intraluminal graft |
| US5061273A (en) | 1989-06-01 | 1991-10-29 | Yock Paul G | Angioplasty apparatus facilitating rapid exchanges |
| US5040548A (en) | 1989-06-01 | 1991-08-20 | Yock Paul G | Angioplasty mehtod |
| US5292331A (en) | 1989-08-24 | 1994-03-08 | Applied Vascular Engineering, Inc. | Endovascular support device |
| US5674278A (en) | 1989-08-24 | 1997-10-07 | Arterial Vascular Engineering, Inc. | Endovascular support device |
| US5879382A (en) | 1989-08-24 | 1999-03-09 | Boneau; Michael D. | Endovascular support device and method |
| US6344053B1 (en) | 1993-12-22 | 2002-02-05 | Medtronic Ave, Inc. | Endovascular support device and method |
| WO2022099117A1 (en)* | 2020-11-06 | 2022-05-12 | Prelude Therapeutics Incorporated | Brm targeting compounds and associated methods of use |
| WO2024059806A1 (en)* | 2022-09-15 | 2024-03-21 | Prelude Therapeutics Incorporated | Brm targeting compounds and associated methods of use |
Non-Patent Citations (8)
| Title |
|---|
| "Handbook of Clinical Drug Data", 2002, MCGRAW-HILL |
| "Principles of Drug Action", 1990 |
| "Remingtons Pharmaceutical Sciences", 2000, LIPPINCOTT WILLIAMS & WILKINS. |
| "The Pharmacological Basis of Therapeutics", 2001, MCGRAW HILL, pages: 20037 |
| CHOOI ET AL., CANCER RES, 2018 |
| MANIACI C. ET AL., BIOORG MED CHEM., vol. 27, no. 12, 2019, pages 2466 - 2479 |
| MARTINDALE: "The Extra Pharmacopoeia", 1999, THE PHARMACEUTICAL PRESS |
| VANGAMUDI ET AL., CANCER RES, 2015 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2022099117A1 (en) | Brm targeting compounds and associated methods of use | |
| US11685744B2 (en) | CDK inhibitors and their use as pharmaceuticals | |
| US12129262B2 (en) | CDK inhibitors and their use as pharmaceuticals | |
| WO2024059806A1 (en) | Brm targeting compounds and associated methods of use | |
| US20230365576A1 (en) | BRM Targeting Compounds And Associated Methods Of Use | |
| EP4619404A1 (en) | Brm targeting compounds and associated methods of use | |
| WO2023287787A1 (en) | Brm targeting compounds and associated methods of use | |
| WO2025101738A1 (en) | Brm targeting compounds and associated methods of use | |
| WO2025184459A1 (en) | Brm and brg1 targeting compounds and associated methods of use | |
| US20250074916A1 (en) | Brm targeting compounds and associated methods of use | |
| EA050130B1 (en) | CDK INHIBITORS AND THEIR USE AS MEDICINES |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24812655 Country of ref document:EP Kind code of ref document:A1 |