WO2025059508A1 - Compositions and methods of reducing immunogenicity and improving stability of antibodies - Google Patents
Compositions and methods of reducing immunogenicity and improving stability of antibodiesDownload PDFInfo
- Publication number
- WO2025059508A1 WO2025059508A1PCT/US2024/046679US2024046679WWO2025059508A1WO 2025059508 A1WO2025059508 A1WO 2025059508A1US 2024046679 WUS2024046679 WUS 2024046679WWO 2025059508 A1WO2025059508 A1WO 2025059508A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amino acid
- acid position
- human igg1
- position corresponding
- chothia canonical
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/567—Framework region [FR]
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
Definitions
- BACKGROUND Antibodiesare natural proteins that the vertebrate immune system forms in response to foreign substances (antigens), primarily for defense against infection.
- antibodieshave been induced in animals under artificial conditions and harvested for use in therapy or diagnosis of disease conditions, or for biological research.
- Each individual antibody producing-cellproduces a single type of antibody with a chemically defined composition, however, antibodies obtained directly from animal serum in response to antigen inoculation actually comprise an ensemble of non-identical molecules (i.e., polyclonal antibodies) made from an ensemble of individual antibody-producing cells.
- rodent (specifically murine) monoclonal antibodies in human therapyis limited because of problems associated with their non-human origin, in particular their immunogenicity in a human host.
- monoclonal antibody technologyhas evolved from full mouse antibodies to chimeric antibodies (mouse variable domains grafted on a human IgG backbone), and to humanized antibodies (mouse CDRs grafted on a human IgG backbone).
- Humanization of mouse monoclonal antibodieswas initially achieved by combining mouse variable domains with human constant domains, creating so called chimeric antibodies having about 70% of Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT human content.
- CDRscomplementarity-determining regions
- Loss of stabilityin turn, can lead to a number of problems, including low expression yield, reduced processability, shorter shelf-life, and unacceptable pharmacokinetic parameters.
- SUMMARYThe present disclosure relates to antibodies and antigen-binding antibody fragments that reduce immunogenicity and improve stability of antibodies.
- compositionscomprising these antibodies or antigen-binding antibody fragments, and methods of using these antibodies and antigen-binding antibody fragments to reduce immunogenicity.
- a humanized antibodycomprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis a positively charged amino acid
- the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis a negatively charged amino acid
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acidis a basic amino acid.
- the negatively charged amino acidis an acidic amino acid. In some instances, the positively charged amino acid is an arginine. In some instances, the negatively charged amino acid is an aspartic acid. In some instances, the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with at least one of: an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivativecomprises a hydroxy- substituted phenyl group.
- the substituted phenylalanine derivativeis a tyrosine residue.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chainis a glutamine
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chainis a glutamine
- the humanized antibodyfurther comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: a serine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and a serine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT chain.
- the humanized antibodyfurther comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- a humanized antibodycomprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, and a light chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with at least one of: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with both: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivativecomprises a hydroxy- substituted phenyl group.
- the substituted phenylalanine derivativeis a tyrosine.
- the polar uncharged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chainis a glutamine.
- the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain variable domainis a glutamine.
- the humanized antibodyfurther comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the humanized antibodyfurther Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acidis a basic amino acid.
- the negatively charged amino acidis an acidic amino acid.
- the positively charged amino acidis an arginine. In some instances, the negatively charged amino acid is an aspartic acid.
- the humanized antibodyfurther comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain.
- a humanized antibodycomprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a positively Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged residue at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis an arginine.
- the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis an aspartic acid.
- the humanized antibodyfurther comprises one or both of: an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 49 of a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT human IgG1 heavy chain; and an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- a humanized antibodycomprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- a humanized antibodycomprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the humanized antibodyfurther comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis an arginine.
- the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis an aspartic acid.
- the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chainis a serine.
- the humanized antibodyfurther comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain.
- the humanized antibodyfurther comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond interaction with at least one of an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond interaction with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chaincomprises a hydroxy-substituted phenyl group.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainis a tyrosine.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chainis a glutamine.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chainis a glutamine.
- the antibodyis an antigen-binding antibody fragment.
- the antigen-binding antibody fragmentis a Fab fragment, a Fab’2 fragment, or a single chain Fv.
- the humanized antibodyis a full antibody, a chimeric antibody, a veneered antibody, a CDR-grafted antibody, or a recombinant antibody.
- the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT humanized antibodyis a human IgG antibody.
- the human IgG antibodyhas a human IgG1 isotype.
- the antibodyis conjugated to a therapeutic, cytotoxic, cytostatic, immunomodulatory, neurotrophic, or neuroprotective agent.
- the heavy chain variable domainis fused to a heavy chain constant region and the light chain variable domain is fused to a light chain constant region.
- the heavy chain constant regionis a mutant form of a natural human heavy chain constant region which has reduced binding to an Fc ⁇ receptor relative to a natural heavy chain constant region.
- the heavy chain constant regionis of IgG1 isotype.
- the antibodyhas at least one mutation in a constant region. In some instances, the at least one mutation reduces complement fixation or activation by the constant region. In some instances, the at least one mutation is at one or more positions of: 241, 264, 265, 270, 296, 297, 318, 320, 322, 329, and 331 by EU numbering. In some instances, the humanized antibody has an alanine at positions 318, 320, and 322 by EU numbering.
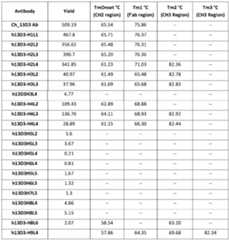
- the humanized antibodyhas increased thermal stability as compared to a reference antibody. In some instances, the increased thermal stability comprises an increase in expression yield compared to the reference antibody. In some instances, the increased thermal stability comprises an increase in expression yield of at least 100 mg/L compared to the reference antibody. In some instances, the increased thermal stability comprises an increase in melting point as compared to the reference antibody. In some instances, the increase in melting point is an increase of at least 2°C compared to the reference antibody. In some instances, the increase in melting point is an increase of at least 5°C compared to the reference antibody. In some instances, the humanized antibody has a melting temperature of 55°C or greater. In some instances, the humanized antibody has a melting temperature of 65°C or greater.
- nucleic acidencoding the heavy chain variable domain and/or the light chain variable domain of an antibodies disclosed herein.
- a vectorcomprising a nucleic acid encoding a heavy chain variable domain and a light chain variable domain operably linked to one or more regulatory sequences to effect expression in a mammalian cell of the humanized antibodies disclosed herein.
- the one or more regulatory sequencescomprises one or more of a enhancer, ribosome binding site, transcription termination signal, and promoter, optionally, wherein the promoter is a eukaryotic promoter.
- the nucleic acidis codon- optimized for expression in a host cell.
- Also disclosed hereinis a host cell comprising any nucleic acid described herein. Also disclosed herein is a method of reducing immunogenicity of a parent antibody by introducing amino acid substitution(s) into the parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91
- Also disclosed hereinis a method of increasing thermal stability of an antibody by introducing amino acid substitution(s) into a parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position
- the methodfurther comprises introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- the methodfurther comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain.
- the modified antibodyhas a melting temperature of 65°C or greater. Also disclosed herein is a method of increasing thermal stability of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has increase in melting temperature of 2°C or greater compared to the parent antibody as measured by differential scanning calorimetry.
- the methodfurther comprises introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- the methodfurther comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain.
- the modified antibodyhas increase in melting temperature of 5°C or greater compared to the parent antibody as measured by differential scanning calorimetry. In some instances, the modified antibody has a melting temperature of 65°C or greater.
- FIGs. 1A-1Cshow Chothia canonical amino acid sequences for heavy chain variable regions disclosed herein. VH/VL interfaces are shown in shaded gray, and Vernier zone residues are shown with stippled shading.
- FIGs. 2A-2Cshow Chothia canonical amino acid sequences for light chain variable regions disclosed herein. VH/VL interfaces are shown in shaded gray, and Vernier zone residues are shown with stippled shading. DETAILED DESCRIPTION A.
- antibodyincludes intact antibodies and binding fragments thereof. Typically, fragments compete with the intact antibody from which they were derived for specific binding to the target including separate heavy chains, light chains Fab, Fab', F(ab')2, F(ab)c, Dabs, nanobodies, and Fv. Fragments can be produced by recombinant DNA techniques, or by enzymatic or chemical separation of intact immunoglobulins.
- antibodyalso includes a bispecific antibody and/or a humanized antibody.
- a bispecific or bifunctional antibodyis an Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT artificial hybrid antibody having two different heavy/light chain pairs and two different binding sites (see, e.g., Songsivilai and Lachmann, Clin. Exp. Immunol., 79:315-321 (1990); Kostelny et al., J. Immunol., 148:1547-53 (1992)).
- the nine amino acids that have hydrophobic side chainsare glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp).
- Six amino acidshave side chains that are polar but not charged: serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr).
- Three amino acidshave basic side chains at neutral pH: arginine (Arg), lysine (Lys), and histidine (His).
- an antibody to its target antigenmeans an affinity and/or avidity of at least 10 6 , 10 7 , 10 8 , 10 9 , 10 10 , 10 11 , or 10 12 M -1 . Specific binding is detectably higher in magnitude and distinguishable from non-specific binding occurring to at least one unrelated target. Specific binding can be the result of formation of bonds between particular functional groups or particular spatial fit (e.g., lock and key type) whereas nonspecific binding is usually the result of van der Waals forces. Specific binding does not however necessarily imply that an antibody binds one and only one target.
- the basic antibody structural unitis a tetramer of subunits.
- Each tetramerincludes two identical pairs of polypeptide chains, each pair having one “light” (about 25 kDa) and one “heavy” chain (about 50-70 kDa).
- the amino-terminal portion of each chainincludes a variable region of about 100 to 110 or more amino acids primarily responsible for antigen recognition. This variable region is initially expressed linked to a cleavable signal peptide.
- the variable region without the signal peptideis sometimes referred to as a mature variable region.
- a light chain mature variable regionmeans a light chain variable region without the light chain signal peptide.
- the carboxy-terminal portion of each chaindefines a constant region primarily responsible for effector function.
- Light chainsare classified as either kappa or lambda.
- Heavy chainsare classified as gamma, mu, alpha, delta, or epsilon, and define the antibody's isotype as IgG, IgM, IgA, IgD and IgE, respectively.
- the variable and constant regionsare joined by a “J” region of about 12 or more amino acids, with the heavy chain also including a “D” region of about 10 or more amino acids. See generally, Fundamental Immunology, Paul, W., ed., 2nd ed. Raven Press, N.Y., 1989, Ch. 7 (incorporated by reference in its entirety for all purposes).
- An immunoglobulin light or heavy chain variable region(also referred to herein as a “light chain variable domain” (“VL domain”) or “heavy chain variable domain” (“VH domain”), respectively) consists of a “framework” region interrupted by three “complementarity determining regions” or “CDRs.”
- the framework regionsserve to align the CDRs for specific binding to an epitope of an antigen.
- the CDRsinclude the amino acid residues of an antibody that are primarily responsible for antigen binding. From amino-terminus to carboxyl-terminus, both VL and VH domains comprise the following framework (FR) and CDR regions: FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4.
- CDRs 1, 2, and 3 of a VL domainare also referred to herein, respectively, as CDR-L1, CDR-L2, and CDR-L3; CDRs 1, 2, and 3 of a VH domain are also referred to herein, respectively, as CDR-H1, CDR-H2, and CDR-H3.
- Rthe R
- CDR-H1the N-terminal residue of the light chain constant region.
- the applicationshould also be understood as disclosing the VL sequence without the C-terminal R.
- the assignment of amino acids to each VL and VH domainis in accordance with any conventional definition of CDRs.
- Kabatprovides a widely used numbering convention (Kabat numbering) in which corresponding residues between different heavy chains or between different light chains are assigned the same number.
- Kabata certain definition of CDRs (e.g., Kabat) that definition specifies the minimum number of CDR residues present in the antibody (i.e., the Kabat CDRs). It does not exclude that other residues falling within another Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT conventional CDR definition but outside the specified definition are also present.
- an antibody comprising CDRs defined by Kabatincludes among other possibilities, an antibody in which the CDRs contain Kabat CDR residues and no other CDR residues, and an antibody in which CDR H1 is a composite Chothia-Kabat CDR H1 and other CDRs contain Kabat CDR residues and no additional CDR residues based on other definitions.
- Table 1Conventional Definitions of CDRs using Kabat Numbering Composite of This is because the Kabat numbering scheme places insertions of extra residues at 35A and 35B, whereas Chothia numbering places them at 31A and 31B. If neither H35A nor H35B (Kabat numbering) is present, the Chothia CDR-H1 loop ends at H32.
- An epitopetypically includes at least 3, and more usually, at least 5 or 8-10 amino acids in a unique spatial conformation.
- Methods of determining spatial conformation of epitopesinclude, for example, x-ray crystallography and 2-dimensional nuclear magnetic resonance. See, e.g., Epitope Mapping Protocols, in Methods in Molecular Biology, Vol. 66, Glenn E. Morris, Ed. (1996).
- Antibodies that recognize the same or overlapping epitopescan be identified in a simple immunoassay showing the ability of one antibody to compete with the binding of another antibody to a target antigen.
- the epitope of an antibodycan also be defined by X-ray crystallography of the antibody bound to its antigen to identify contact residues.
- two antibodieshave the same epitope if all amino acid mutations in the antigen that reduce or eliminate binding of one antibody reduce or eliminate binding of the other.
- Two antibodieshave overlapping epitopes if some amino acid mutations that reduce or eliminate binding of one antibody reduce or eliminate binding of the other.
- Competition between antibodiesis determined by an assay in which an antibody under test inhibits specific binding of a reference antibody to a common antigen (see, e.g., Junghans et al., Cancer Res. 50:1495, 1990).
- a test antibodycompetes with a reference antibody if an excess of a test antibody (e.g., at least 2x, 5x, 10x, 20x or 100x) inhibits binding of the reference antibody by at least 50% as measured in a competitive binding assay.
- test antibodiesinhibit binding of the reference antibody by at least 75%, 90% or 99%.
- Antibodies identified by competition assayinclude antibodies binding to the same epitope as the reference antibody and antibodies binding to an adjacent epitope sufficiently proximal to the epitope bound by the reference antibody for steric hindrance to occur.
- pharmaceutically acceptablemeans that the carrier, diluent, excipient, or auxiliary is compatible with the other ingredients of the formulation and not substantially deleterious to the recipient thereof.
- patientor “subject” includes human and other mammalian subjects that receive either prophylactic or therapeutic treatment.
- biological samplerefers to a sample of biological material within or obtainable from a biological source, for example a human or mammalian subject. Such samples can be organs, organelles, tissues, sections of tissues, bodily fluids, peripheral blood, blood plasma, blood serum, cells, molecules such as proteins and peptides, and any parts or combinations derived therefrom.
- a biological sample and a control samplecan both be obtained from the same tissue.
- control samplesconsist essentially or entirely of normal, healthy tissues and can be used in comparison to a biological sample thought to comprise immunogenic disease-affected regions.
- the tissue in the control sampleis the same type as the tissue in the biological sample.
- the immunogenic disease-affected cells thought to be in the biological samplearise from the same cell type as the type of cells in the control sample.
- the term “disease”refers to any abnormal condition that impairs physiological function. The term is used broadly to encompass any disorder, illness, abnormality, pathology, sickness, condition, or syndrome in which physiological function is impaired, irrespective of the nature of the etiology.
- Immunogenicityrefers to the ability of a foreign molecule or substance to provoke or to stimulate an immune response in a cell or a subject.
- An immune responsecan include activation of a non-specific innate response and/or activation of an adaptive response that includes a cell-mediated immune response, which is carried out by T cells, and a humoral immune response, which is controlled by activated B cells and endogenous antibodies.
- the humanized antibodies disclosed hereinreduce immunogenicity in the cell or the subject.
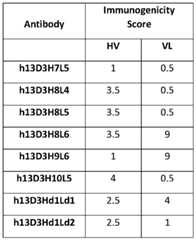
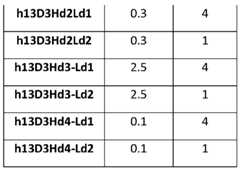
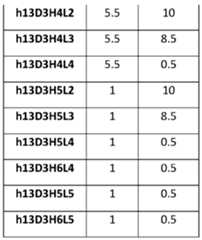
- An “immunogenicity score”refers to a value assigned to immunogenicity or reduction thereof caused by an antibody (e.g., humanized antibody) disclosed herein.
- Immunogenicity Scoreis a predictor of immunogenicity. The higher the score, the greater the predicted immunogenicity.
- the scorecan be an absolute number based on predicted potential binding of the antibody (e.g., humanized antibody) within a protein sequence of an endogenous protein (e.g., a major histocompatibility class (MHC) class II protein).
- immunogenicity scorecan be calculated using a publicly available program such as Immune Epitope Database (IEDB) and EpiQuest.
- IEDBImmune Epitope Database
- symptomrefers to a subjective evidence of a disease, such as altered gait, as perceived by the subject.
- a “sign”refers to objective evidence of a disease as observed by a physician.
- positive response to treatmentrefers to a more favorable response in an individual patient or average response in a population of patients relative to an average response in a control population not receiving treatment.
- amino acidsare grouped as follows: Group I (hydrophobic side chains): met, ala, val, leu, ile; Group II (neutral hydrophilic side chains): cys, ser, thr; Group III (acidic side chains): asp, glu; Group IV (basic side chains): asn, gln, his, lys, arg; Group V (residues influencing chain orientation): gly, pro; and Group VI (aromatic side chains): trp, tyr, phe. Conservative substitutions involve substitutions between amino acids in the same class.
- Non-conservative substitutionsconstitute exchanging a member of one of these classes for a member of another.
- Percentage sequence identitiesare determined with antibody sequences maximally aligned by the Kabat numbering convention. After alignment, if a subject antibody region (e.g., the entire mature variable region of a heavy or light chain) is being compared with the same region of a reference antibody, the percentage sequence identity between the subject and reference antibody regions is the number of positions occupied by the same amino acid in both the subject and reference antibody region divided by the total number of aligned positions of the two regions, with gaps not counted, multiplied by 100 to convert to percentage.
- a “reference Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT antibody” as used hereinrefers to an antibody or fragment thereof that competes for binding with a test antibody (e.g., an antibody having one or more substitutions described herein) to the same epitope.
- the reference antibodydoes not include the substitution in its sequence and can also be referred to as a parent antibody.
- the reference antibodycomprises the same sequence as the humanized antibody without the one or more amino acid substitutions as described herein.
- Compositions or methods “comprising” or “including” one or more recited elementsmay include other elements not specifically recited.
- a humanized antibodyis a genetically engineered antibody in which CDRs from a non- human “donor” antibody are grafted into human “acceptor” antibody sequences (see, e.g., Queen, US 5,530,101 and 5,585,089; Winter, US 5,225,539; Carter, US 6,407,213; Adair, US 5,859,205; and Foote, US 6,881,557).
- the acceptor antibody sequencescan be, for example, a mature human antibody sequence, a composite of such sequences, a consensus sequence of human antibody sequences, or a germline region sequence.
- a humanized antibodyis an antibody having at least three, four, five or all CDRs entirely or substantially from a donor antibody and variable region framework sequences and constant regions, if present, entirely or substantially from human antibody sequences.
- a humanized heavy chainhas at least one, two and usually all three CDRs entirely or substantially from a donor antibody heavy chain, and a heavy chain variable region framework sequence and heavy chain constant region, if Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT present, substantially from human heavy chain variable region framework and constant region sequences.
- a humanized light chainhas at least one, two and usually all three CDRs entirely or substantially from a donor antibody light chain, and a light chain variable region framework sequence and light chain constant region, if present, substantially from human light chain variable region framework and constant region sequences.
- a humanized antibodycomprises a humanized heavy chain and a humanized light chain.
- a CDR in a humanized antibodyis substantially from a corresponding CDR in a non-human antibody when at least 85%, 90%, 95% or 100% of corresponding residues (as defined by any conventional definition but preferably defined by Kabat) are identical between the respective CDRs.
- variable region framework sequences of an antibody chain or the constant region of an antibody chainare substantially from a human variable region framework sequence or human constant region respectively when at least 85%, 90%, 95% or 100% of corresponding residues defined by Kabat are identical.
- WHOWorld Health Organization
- INNInternational non-proprietary names
- an antibodymust have at least 85% identity to human germline antibody sequences (i.e., prior to somatic hypermutation).
- Mixed antibodiesare antibodies for which one antibody chain (e.g., heavy chain) meets the threshold but the other chain (e.g., light chain) does not meet the threshold.
- humanized antibodies provided hereinhave at least 85% sequence identity to human germline sequences and some of the humanized antibodies provided herein have less than 85% sequence identity to human germline sequences.
- Some of the heavy chains of the humanized antibodies provided hereinhave from about 60% to 100% sequence identity to human germ line sequences, such as, for example, in the range of about 60% to 69%, 70% to 79%, 80% to 84%, or 85% to 89%.
- Some heavy chainsfall below the 2014 WHO INN Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT definition and have, for example, about 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, or 82%, 83%, or 84% sequence identity to human germ line sequences, while other heavy chains meet the 2014 WHO INN definition and have about 85%, 86%, 87%, 88%, 89% or greater sequence identity to human germ line sequences.
- Some of the light chains of the humanized antibodies provided hereinhave from about 60% to 100% sequence identity to human germ line sequences, such as, for example, in the range of about 80% to 84% or 85% to 89%. Some light chains fall below the 2014 WHO INN definition and have, for example, about 81%, 82%, 83% or 84% sequence identity to human germ line sequences, while other light chains meet the 2014 WHO INN definition and have about 85%, 86%, 87%, 88%, 89% or greater sequence identity to human germ line sequences. Some humanized antibodies provided herein that are "chimeric" under the 2014 WHO INN definition have heavy chains with less than 85% identity to human germ line sequences paired with light chains having less than 85% identity to human germ line sequences.
- Some humanized antibodies provided hereinare "mixed" under the 2014 WHO INN definition, for example, having a heavy chain with at least 85% sequence identity to human germ line sequences paired with a light chain having less than 85% sequence identity to human germ line sequences, or vice versa.
- Some humanized antibodies provided hereinmeet the 2014 WHO INN definition of "humanized” and have a heavy chain with at least 85% sequence identity to human germ line sequences paired with a light chain having at least 85% sequence identity to human germ line sequences.
- humanized antibodiesoften incorporate all six CDRs (defined by any conventional definition but preferably as defined by Kabat) from a mouse antibody, they can also be made with less than all CDRs (e.g., at least 3, 4, or 5 CDRs) from a mouse antibody (e.g., Pascalis et al., J. Immunol. 169:3076, 2002; Vajdos et al., J. of Mol. Biol., 320: 415-428, 2002; Iwahashi et al., Mol. Immunol. 36:1079-1091, 1999; Tamura et al, J. Immunol., 164:1432-1441, 2000).
- CDRsdefined by any conventional definition but preferably as defined by Kabat
- CDR residues not contacting antigen and not in the SDRscan be identified based on previous studies (for example residues H60-H65 in CDR H2 are often not required), from regions of Kabat CDRs lying outside Chothia hypervariable loops (Chothia, J. Mol. Biol. 196:901, 1987), by molecular Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT modeling and/or empirically, or as described in Gonzales et al., Mol. Immunol. 41: 863, 2004.
- the amino acid occupying the positioncan be an amino acid occupying the corresponding position (by Kabat numbering) in the acceptor antibody sequence.
- the number of such substitutions of acceptor for donor amino acids in the CDRs to includereflects a balance of competing considerations.
- Such substitutionsare potentially advantageous in decreasing the number of mouse amino acids in a humanized antibody and consequently decreasing potential immunogenicity and/or for meeting the WHO INN definition of “humanized”.
- substitutionscan also cause changes of affinity, and significant reductions in affinity are preferably avoided.
- Positions for substitution within CDRs and amino acids to substitutecan also be selected empirically.
- the human acceptor antibody sequencescan optionally be selected from among the many known human antibody sequences to provide a high degree of sequence identity (e.g., 65-85% identity) between a human acceptor sequence variable region frameworks and corresponding variable region frameworks of a donor antibody chain.
- a human acceptor sequence variable region frameworkse.g., 65-85% identity
- disclosed hereinare humanized antibodies having a salt bridge between an amino acid in the heavy chain variable domain and an amino acid in the light chain variable domain.
- one or more amino acid substitutionsare introduced into the humanized antibody.
- the one or more amino acid substitutionsare introduced into a Chothia canonical structure.
- the one or more amino acid substitutionsare introduced into a VH/VL interface, including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) of Chothia canonical amino acid positions 35, 37, 39, 45, 47, 91, 93, 95, and/or 103 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 27F, 34, 36, 38, 44, 46, 87, 89, 91, 96 and/or 98 of the human IgG1 light chain.
- Exemplary amino acid substitutions that can be introduced into a VH/VL interfaceare shown in FIGs.
- one or more amino acid substitutionsare introduced into a Vernier zone residue, including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30) of Chothia canonical amino acid positions 2, 27-30, 47-49, 67, 69, 71, 73, 78, 93, 94, and/or 103 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 2, 4, 35, 36, 46-49, 64, 66, 68, 69, 71, and/or 98 of the human IgG1 light chain.
- substitutions at one or moree.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,
- Vernier zone residue positions and substitutionsare shown in FIGs. 1A-1C and 2A-2C, and it is appreciated that any combination of substitutions at each position is contemplated herein.
- one or more amino acid substitutionsare introduced to generate a backmutation, including substitutions at one or more (e.g., 2, 3, or 4) of Chothia canonical amino acid positions 19, 44, and/or 83 of the human IgG1 heavy chain and/or at Chothia canonical amino acid position 18 of the human IgG1 light chain. It is appreciated that any combination of substitutions at each position is contemplated herein.
- one or more amino acid substitutionsare deimmunizing mutation(s), including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, or 8) of Chothia canonical amino acid positions 43, 44, 49, 74, 77, and/or 91 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 3 and/or 100 of the human IgG1 light chain. It is appreciated that any combination of substitutions at each position is contemplated herein.
- the humanized antibodyincludes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis a positively charged amino acid.
- the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis a negatively charged amino acid.
- the positively and negatively charged amino acidsform an interchain salt bridge.
- the positively charged amino acidis a basic amino acid.
- the negatively charged amino acidis an acidic amino acid.
- the positively charged amino acidis an arginine.
- the negatively charged amino acidis an aspartic acid.
- the humanized antibodyfurther comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with at least one of: an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivativecomprises a hydroxy-substituted phenyl group.
- the substituted phenylalanine derivativeis a tyrosine residue.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chainis a glutamine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine.
- the humanized antibodyfurther comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: a serine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT chain; and a serine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain.
- the humanized antibodyfurther comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- the heavy chain variable domainincludes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the light chain variable domainincludes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the heavy chain variable domainincludes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain
- a light chain variable domainincludes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain
- the humanized antibodyincludes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, and a light chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with at least one of: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond with both: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivativecomprises a hydroxy-substituted phenyl group.
- the substituted phenylalanine derivativeis a tyrosine.
- the polar uncharged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chainis a glutamine.
- the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain variable domainis a glutamine.
- the humanized antibodyfurther comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acidis a basic amino acid.
- the negatively charged amino acidis an acidic amino acid.
- the positively charged amino acidis an arginine.
- the negatively charged amino acidis an aspartic acid.
- the humanized antibodyfurther comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain.
- the humanized antibodyincludes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged residue at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis an arginine.
- the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis an aspartic acid.
- the humanized antibodyfurther comprises one or both of: an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the humanized antibodyincludes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the light chain variable domaincomprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the humanized antibodyfurther comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis an arginine.
- the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis an aspartic acid.
- the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chainis a serine.
- the humanized antibodyincludes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human
- the light chain variable domaincomprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain.
- the humanized antibodyfurther comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain.
- the heavy chain variable regioncomprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainform an interchain salt bridge.
- the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chainis an arginine.
- the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chainis an aspartic acid.
- the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chainis a serine.
- the humanized antibodyfurther comprises at least one (e.g., two or three) of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- a tryptophanat an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain
- a phenylalanineat an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain
- an alanineat an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain.
- the humanized antibodyfurther comprises at least one (e.g., two, three, or four) of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain.
- a glutamineat an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain
- a tyrosineat an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain
- an arginineat an amino acid position corresponding to Chothia can
- the humanized antibodyfurther comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond interaction with at least one of an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainforms a hydrogen bond interaction with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chaincomprises a hydroxy-substituted phenyl group.
- the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chainis a tyrosine.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chainis a glutamine.
- the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chainis a glutamine.
- the antibodyis an antigen-binding antibody fragment.
- the antigen-binding antibody fragmentis a Fab fragment, a Fab’2 fragment, or a single chain Fv.
- the humanized antibodyis a full antibody, a chimeric antibody, a veneered antibody, a CDR-grafted antibody, or a recombinant antibody.
- the isotype of the antibody from which the Fc region is originatedis not particularly limited, and an Fc region originated from an IgG1, IgG2, IgG3, or IgG4 monoclonal antibody can be appropriately used.
- the humanized antibodyis a human IgG antibody.
- the human IgG antibodyhas a human IgG1 isotype.
- the antibodyis conjugated to a therapeutic, cytotoxic, cytostatic, immunomodulatory, neurotrophic, or neuroprotective agent.
- the heavy chain variable domainis fused to a heavy chain constant region and the light chain variable domain is fused to a light chain constant region.
- the heavy chain constant regionis a mutant form of a natural human heavy chain constant region which has reduced binding to an Fc ⁇ receptor relative to the natural heavy chain constant region.
- the heavy chain constant regionis of IgG1 isotype.
- the antibodyhas at least one mutation in a constant region. In some instances, the at least one mutation reduces complement fixation or activation by the constant region.
- the at least one mutationis at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, or 11) positions of: 241, 264, 265, 270, 296, 297, 318, 320, 322, 329, and 331 by EU numbering.
- the humanized antibodyhas an alanine at positions 318, 320, and 322 by EU numbering. Additional representative heavy and light chain variable domain sequences are provided in Tables 2 and 3, respectively. Table 2.
- Heavy Chain Variable Domain SequencesAttorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT SEQ ID NO Name Sequence EVQLVESGGGLVQPGGSLKLSCAASGFTFSNYFMSWVRQTPEKRLEWVAYIST S Q Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT EVQLVESGGGLVQPGGSLRLSCAASGFTFSNYFMGWVRQAPGEDL 16 h13D3VHv13 EWVAYISTGGDSANYADNVKGRFTISRDNAKNTGYLQMNSLRAED abe 3.
- the humanized antibodyhas increased thermal stability as compared to a reference antibody.
- the increased thermal stabilitycomprises an increase (e.g., at least a 5% increase, at least a 10% increase, at least a 15% increase, at least a 20% increase, at least a 25% increase, at least a 30% increase, at least a 35% increase, at least a 40% increase, at least a 45% increase, or at least a 50% increase, or a 1% increase to a 50% increase, a 1% increase to a 40% increase, a 1% increase to a 30% increase, a 1% increase to a 20% increase, a 1% increase to a 10% increase, a 1% increase to a 5% increase, a 5% increase to a 50% increase, a 5% increase to a 40% increase, a 5% increase to a 30% increase, a 5% increase to a 20% increase, a 5% increase to a 10% increase, a 10% increase to a 50% increase, a 10% increase to a 40% increase, a 10% increase to a 50% increase, a 10% increase to a 40% increase, a 10% increase
- the increased thermal stabilitycomprises an increase in expression yield of at least 100 mg/L compared to the reference antibody.
- the increase in thermal stabilitycomprises an increase in melting point as compared to the reference antibody (e.g., a similar antibody not including one or more of the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT amino acid substitutions described herein).
- the increase in melting pointis an increase of at least 2 °C, at least 5 °C, or at least 10 °C as compared to the reference antibody.
- the humanized antibodyhas a melting temperature of 55 °C or greater. In some instances, the humanized antibody has a melting temperature of 65°C or greater.
- the humanized antibodyhas a melting temperature of 70°C or greater. In some embodiments, the humanized antibody has a melting temperature of about 55 °C to about 75 °C, about 55 °C to about 70 °C, about 55 °C to about 65 °C, about 55 °C to about 60 °C, about 60 °C to about 75 °C, about 60 °C to about 70 °C, about 60 °C to about 65 °C, about 65 °C to about 75 °C, about 65 °C to about 70 °C, about 70 °C to about 75 °C, or about 70 °C to about 80 °C. In some instances, the melting temperature is determined by differential scanning calorimetry.
- the humanized antibodyhas a decreased immunogenicity score as compared to a reference antibody. In some instances, the humanized antibody has a decreased immunogenicity as compared to a reference antibody.
- C. Generation of Parent AntibodiesMethods for generating a parent antibody that can be used as the starting point for further humanization using any of the methods described herein, are known in the art. For example, an animal can be immunized with an antigen and optionally, a heterologous carrier and/or adjuvant.
- Suitable carriers that can be mixed with the antigen prior to immunizationinclude serum albumins, keyhole limpet hemocyanin, immunoglobulin molecules, thyroglobulin, ovalbumin, tetanus toxoid, or a toxoid from other pathogenic bacteria, such as diphtheria (e.g., CRM197), E. coli, cholera, or H. pylori, or an attenuated toxin derivative.
- T cell epitopesare also suitable carrier molecules.
- conjugatescan be formed by linking an antigen to an immunostimulatory polymer molecule (e.g., tripalmitoyl-S-glycerine cysteine (Pam 3 Cys), mannan (a mannose polymer), or glucan (a ⁇ 1 ⁇ 2 polymer)), cytokines (e.g., IL-1, IL-1 alpha and ⁇ peptides, IL-2, ⁇ -INF, IL-10, GM-CSF), and chemokines (e.g., MIP1- ⁇ and ⁇ , and RANTES).
- Immunogensmay be linked to the carriers with or without spacers amino acids (e.g., gly-gly). Additional carriers include virus-like particles.
- VLPsVirus-like particles
- pseudovirionsrepresent subunit structures composed of multiple copies of a viral capsid and/or envelope protein capable of self-assembly into VLPs of defined spherical symmetry in vivo.
- a viral capsid and/or envelope proteincapable of self-assembly into VLPs of defined spherical symmetry in vivo.
- Attorney Docket No.: 50887-0046WO1// Client Ref: 791-PCT peptide immunogens can be linked to at least one artificial T-cell epitope capable of binding a large proportion of MHC Class II molecules., such as the pan DR epitope ("PADRE").
- PADREpan DR epitope
- PADREis described in US 5,736,142, WO 95/07707, and Alexander et al., Immunity 1:751-761, 1994.
- Active immunogenscan be presented in multimeric form in which multiple copies of an immunogen and/or its carrier are presented as a single covalent molecule.
- Fragmentsare often administered with pharmaceutically acceptable adjuvants.
- the adjuvantincreases the titer of induced antibodies and/or the binding affinity of induced antibodies relative to the situation if the peptide were used alone.
- a variety of adjuvantscan be used in combination with an immunogenic fragment to elicit an immune response. Some adjuvants augment the intrinsic response to an immunogen without causing conformational changes in the immunogen that affect the qualitative form of the response.
- Some adjuvantsinclude aluminum salts, such as aluminum hydroxide and aluminum phosphate, 3 De-O-acylated monophosphoryl lipid A (MPL TM ) (see GB 2220211 (RIBI ImmunoChem Research Inc., Hamilton, Montana, now part of Corixa).
- MPL TM3 De-O-acylated monophosphoryl lipid A
- Stimulon TM QS-21is a triterpene glycoside or saponin isolated from the bark of the Quillaja Saponaria Molina tree found in South America (see Kensil et al., in Vaccine Design: The Subunit and Adjuvant Approach (eds.
- adjuvantsare oil in water emulsions (such as squalene or peanut oil), optionally in combination with immune stimulants, such as monophosphoryl lipid A (see Stoute et al., N. Engl. J. Med. 336:86-91 (1997)), pluronic polymers, and killed mycobacteria.
- Ribi adjuvantsare oil-in-water emulsions. Ribi contains a metabolizable oil (squalene) emulsified with saline containing Tween 80.
- Ribialso contains refined mycobacterial products which act as immunostimulants and bacterial monophosphoryl lipid A.
- Another adjuvantis CpG (WO 98/40100).
- Adjuvantscan be administered as a component of a therapeutic composition with an active agent or can be administered separately, before, concurrently with, or after administration of the therapeutic agent.
- Peptidesand optionally a carrier fused to the peptide
- Peptidescan also be administered in the form of a nucleic acid encoding the peptide and expressed in situ in a subject.
- a nucleic acid segment encoding an immunogenis typically linked to regulatory elements, such as a promoter and enhancer that allow expression of the DNA segment in the intended target cells of a subject.
- the linked regulatory elements and coding sequencesare often cloned into a vector.
- Antibodiescan also be administered in the form of nucleic acids encoding the antibody heavy and/or light chains. If both heavy and light chains are present, the chains are preferably linked as a single chain antibody.
- the DNAcan be delivered in naked form (i.e., without colloidal or encapsulating materials).
- retroviral systemssee, e.g., Lawrie and Tumin, Cur. Opin. Genet. Develop. 3:102-109 (1993)
- retroviral systemsincluding retrovirus derived vectors such MMLV, HIV-1, and ALV
- retrovirus derived vectorssuch MMLV, HIV-1, and ALV
- adenoviral vectorssee, e.g., Bett et al., J. Virol. 67, 5911 (1993)
- adeno-associated virus vectorssee, e.g., Zhou et al., J. Exp. Med.
- lentiviral vectorssuch as those based on HIV or FIV gag sequences, viral vectors from the pox family including vaccinia virus and the avian pox viruses, viral vectors from the alpha virus genus such as those derived from Sindbis and Semliki Forest Viruses (see, e.g., Dubensky et al., J. Virol.
- Vectors and DNA encoding an immunogen or encoding the antibody heavy and/or light chainscan also be adsorbed to or associated with particulate carriers, examples of which include polymethyl methacrylate polymers and polylactides and poly(lactide-co-glycolides), (see, e.g., McGee et al., J. Micro Encap. 1996).
- Vectors or segments therefrom encoding the antibody heavy and/or light chainscan be incorporated in cells ex vivo, for example to cells explanted from an individual patient (e.g., lymphocytes, bone marrow aspirates, tissue biopsy) or universal donor hematopoietic stem cells, followed by reimplantation of the cells into a patient, usually after selection for cells which have incorporated the transgenes. (See, e.g., WO 2017/091512).
- Exemplary patient-derived cellsAttorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT include patient derived induced pluripotent stem cells (iPSCs) or other types of stem cells (embryonic, hematopoietic, neural, or mesenchymal).
- iPSCspatient derived induced pluripotent stem cells
- a vector or segment therefrom encoding the antibody heavy and/or light chainscan be introduced into any region of interest in cells ex vivo, such as an albumin gene or other safe harbor gene.
- Cells incorporating the vectorcan be implanted with or without prior differentiation. Cells can be implanted into a specific tissue, such as a secretory tissue or a location of pathology, or systemically, such as by infusion into the blood.
- cellscan be implanted into a secretory tissue of a patient, such as the liver, optionally with prior differentiation to cells present in that tissue, such as hepatocytes in the case of a liver. Expression of the antibody in the liver results in secretion of the antibody to the blood.
- Humanized Antibody Screening AssaysHumanized antibodies can be initially screened for the intended binding specificity as described above. Active immunogens can likewise be screened for capacity to induce antibodies with such binding specificity. In this case, an active immunogen is used to immunize a laboratory animal and the resulting sera tested for the appropriate binding specificity. Antibodies having the desired binding specificity can then be tested in cellular and animal models. Active immunogens can also be tested for induction of antibodies in the sera.
- Both passive and active immunogenscan be tested for passage of antibodies across the blood brain barrier into the brain of a wild type or transgenic animal.
- Antibodies or fragments inducing an antibodycan also be tested in non-human primates without disease or that naturally or through induction develop symptoms of diseases characterized by changes in antigen levels.
- Tests on an antibody or active agentare usually performed in conjunction with a control in which a parallel experiment is conduct except that the antibody or active agent is absent (e.g., replaced by vehicle). Reduction, delay or inhibition of signs or symptoms disease attributable to an antibody or active agent under test can then be assessed relative to the control.
- the present regimencan also be used in treatment or prophylaxis of any of these diseases associated with the antigen specifically bound by the humanized antibodies provided herein.
- Patients amenable to treatmentinclude individuals at risk of disease but not showing symptoms, as well as patients presently showing symptoms.
- Patients at risk of diseaseinclude those having a known genetic risk of disease. Such individuals include those having relatives who have experienced this disease, and those whose risk is determined by analysis of genetic or biochemical markers.
- treatmentcan begin at any age (e.g., 10, 20, 30). Usually, however, it is not necessary to begin treatment until a patient reaches 40, 50, 60 or 70 years of age.
- Treatmenttypically entails multiple dosages over a period of time. Treatment can be monitored by assaying antibody levels over time. If the response falls, a booster dosage is indicated.
- F. Nucleic Acids, Host Cells, and Methods of Producing the SameThe disclosure further provides nucleic acids encoding any of the heavy and light chains described in Tables 2 and 3 (e.g., encoding any one of SEQ ID NOs: 1-35). Optionally, such nucleic acids further encode a signal peptide and can be expressed with the signal peptide linked to the variable region.
- Coding sequences of nucleic acidscan be operably linked with regulatory sequences to ensure expression of the coding sequences, such as a promoter, enhancer, ribosome binding site, transcription termination signal, and the like.
- the regulatory sequencescan include a promoter, for example, a prokaryotic promoter or a eukaryotic promoter.
- the nucleic acids encoding heavy or light chainscan be codon-optimized for expression in a host cell.
- the nucleic acids encoding heavy and light chainscan encode a selectable gene.
- the nucleic acids encoding heavy and light chainscan occur in isolated form or can be cloned into one or more vectors.
- the nucleic acidscan be synthesized by, for example, solid state synthesis or PCR of overlapping oligonucleotides. Nucleic acids encoding heavy and light chains can be joined as one contiguous nucleic acid, e.g., within an expression vector, or can be separate, e.g., each cloned into its own expression vector. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT In some instances, the nucleic acid encodes for any of the heavy chain variable domain sequences and/or light chain variable domain humanized sequences described herein. Also disclosed herein are vectors. In some instances, the vector includes a nucleic acid encoding for any of the heavy chain variable domain sequences disclosed herein.
- the vectorincludes a nucleic acid encoding for any of the light chain variable domain sequences disclosed herein.
- a vectorcan be operably linked to one or more regulatory sequences to effect expression in a mammalian cell of the humanized antibody disclosed herein.
- the vectorcan include one or more regulatory sequences that include one or more of an enhancer, a ribosome binding site, a transcription termination signal, and a promoter.
- a promotercan be a eukaryotic promoter. Examples of the vectors include M13 series vectors, pUC series vectors, pBR322, pBluescript, and pCR-Script.
- pGEM-TpDIRECT
- pT7can also be used for the purpose of cDNA subcloning and excision.
- the nucleic acidis codon-optimized for expression in a host cell.
- animal cells or fungus cellscan be appropriately used.
- examples of the animal cellscan include the following cells: (1) mammalian cells such as CHO (Chinese hamster ovary cell line), COS (monkey kidney cell line), myeloma cells (Sp2/O, NS0, etc.), BHK (baby hamster kidney cell line), HEK293 (human embryonic kidney cell line with sheared adenovirus (Ad)5 DNA), PER.C6 cell (human embryonic retinal cell line transformed with the adenovirus type 5 (Ad5) E1A and E1B genes), Hela, and Vero (Current Protocols in Protein Science (May, 2001, Unit 5.9, Table 5.9.1)); (2) amphibian cells such as Xenopus oocytes; and (3) insect cells such as sf9, sf21, and Tn5.
- mammalian cellssuch as CHO (Chinese hamster ovary cell line), COS (monkey kidney cell line), myeloma cells (Sp2/O, NS
- the antibodycan also be prepared using E. coli (mAbs 2012 Mar-Apr; 4 (2): 217-225) or yeast (WO2000023579).
- the antibody prepared using E. coliis not glycosylated.
- the antibody prepared using yeastis glycosylated.
- the DNA encoding all or part of the humanized antibody of interestis integrated to expression vectors so as to be expressed under the control of expression control regions, for example, an enhancer and a promoter.
- host cellsare transformed with the resulting expression vectors and allowed to express antibodies. In this case, appropriate hosts and expression vectors can be used in combination.
- the humanized antibodycan be recovered, for example, by culturing the transformed cells and then separating the antibody from within the molecule-transformed cells or from the culture solution thereof.
- the antibodycan be separated and purified by appropriately using in combination methods such as centrifugation, ammonium sulfate fractionation, salting out, ultrafiltration, C1q, FcRn, protein A and protein G columns, affinity chromatography, ion- exchanged chromatography, and gel filtration chromatography.
- a production methodcan include the steps of: (i) preparing a library of humanized antibodies; (ii) selecting, from the prepared library, a humanized antibody having one or more substitutions as described herein; (iii) culturing a host cell comprising a nucleic acid encoding all or part of the humanized antibody selected in the step (ii); and (iv) recovering the humanized antibody from the host cell culture.
- the humanized antibodymay be antibody fragments such as Fv, Fab, or Fab' or may be Fc region-containing antibodies.
- humanized antibodiescan be conjugated with other therapeutic moieties, other proteins, other antibodies, and/or detectable labels.
- Such therapeutic moietiescan be any agent that can be used to treat, combat, ameliorate, prevent, or improve an unwanted condition or disease in a patient, such as disease or disorder associated with the antigen recognized by the humanized antibody.
- the methodsfurther comprise detecting, measuring, and/or monitoring immunogenicity levels and/or the antigen levels in a subject.
- Conjugated therapeutic moietiescan include, neurotrophic agents, neuroprotective agents, radiotherapeutic agents, radioactive (radiopharmaceuticals), fluorescent, paramagnetic tracers, ultrasound contrast agents, immunomodulators, or any biologically active agents that facilitate or enhance the activity of the antibody, or modify bioavailability, and distribution in the body or within organs.
- a neurotrophic agentcan be any agent, including chemical or proteinaceous agents, which promotes neuron maintenance, growth, or differentiation.
- a neuroprotective agentcan be agent, including chemical or proteinaceous agents, which protects neurons from acute insult or degenerative processes.
- An immunomodulatorcan be any agent that stimulates or inhibits the development or maintenance of an immunologic response.
- a radiotherapeutic agentcan be any molecule or compound that emits radiation.
- ricina cellular toxin derived from plants
- S-acetylmercaptosuccinic anhydridefor the antibody
- succinimidyl 3-(2-pyridyldithio)propionatefor ricin.
- saporinan inhibitor of ribosomal assembly
- radioisotopesexamples include, for example, yttrium 90 (90Y), indium 111 (111In), 131 I, 99 mTc, radiosilver-111, radiosilver-199, and Bismuth 213 .
- Linkage of radioisotopes to antibodiesmay be performed with conventional bifunction chelates.
- sulfur-based linkersmay be used for radiosilver-111 and radiosilver-199 linkage. See Hazra et al., Cell Biophys. 24-25:1-7 (1994).
- antibodiescan be conjugated with toxic chemotherapeutic drugs such as maytansine, geldanamycin, tubulin inhibitors such as tubulin binding agents (e.g., auristatins), or minor groove binding agents such as calicheamicin.
- toxic chemotherapeutic drugssuch as maytansine, geldanamycin
- tubulin inhibitorssuch as tubulin binding agents (e.g., auristatins), or minor groove binding agents such as calicheamicin.
- Other representative therapeutic moietiesinclude agents known to be useful for treatment, management, or amelioration of a disease or disorder associated with changes in immunogenicity.
- Antibodiescan also be coupled with other proteins.
- antibodiescan be coupled with Fynomers.
- Fynomersare small binding proteins (e.g., 7 kDa) derived from the human Fyn SH3 domain. They can be stable and soluble, and they can lack cysteine residues and disulfide bonds.
- Fynomerscan be engineered to bind to target molecules with the same affinity and specificity as antibodies. They are suitable for creating multi-specific fusion proteins based on antibodies. For example, Fynomers can be fused to N-terminal and/or C- Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT terminal ends of antibodies to create bi- and tri-specific FynomAbs with different architectures. Fynomers can be selected using Fynomer libraries through screening technologies using FACS, Biacore, and cell-based assays that allow efficient selection of Fynomers with optimal properties. Examples of Fynomers are disclosed in Grabulovski et al., J. Biol. Chem.
- the antibodies disclosed hereincan also be coupled or conjugated to one or more other antibodies (e.g., to form antibody heteroconjugates). Antibodies can also be coupled with a detectable label. Such antibodies can be used in a subject having or being treated for a disease or disorder associated with changes in immunogenicity.
- Such antibodiesare particularly useful for performing such determinations in subjects having or being susceptible to of a disease or disorder associated with changes in immunogenicity.
- Representative detectable labels that may be coupled or linked to an antibodyinclude various enzymes, such as horseradish peroxidase, alkaline phosphatase, beta- galactosidase, or acetylcholinesterase; prosthetic groups, such streptavidin/biotin and avidin/biotin; fluorescent materials, such as umbelliferone, fluorescein, fluorescein isothiocyanate, rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride or phycoerythrin; luminescent materials, such as luminol; bioluminescent materials, such as luciferase, luciferin, and aequorin; radioactive materials, such as radiosilver-111, radiosilver-199, Bismuth 213 , iodine ( 131 I, 125
- Linkage of radioisotopes to antibodiesmay be performed with conventional bifunction chelates.
- sulfur-based linkersmay be used for radiosilver-111 and radiosilver-199 linkage. See Hazra et al., Cell Biophys. 24-25:1-7 (1994).
- Linkage of silver radioisotopesmay involve reducing the immunoglobulin with ascorbic acid.
- Radioisotopessuch as 111In and 90Y
- Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT ibritumomab tiuxetancan be used and will react with such isotopes to form 111In-ibritumomab tiuxetan and 90Y-ibritumomab tiuxetan, respectively.
- Therapeutic moieties, other proteins, other antibodies, and/or detectable labelsmay be coupled or conjugated, directly or indirectly through an intermediate (e.g., a linker), to an antibody of the disclosure.
- Suitable linkersinclude, for example, cleavable and non- cleavable linkers. Different linkers that release the coupled therapeutic moieties, proteins, antibodies, and/or detectable labels under acidic or reducing conditions, on exposure to specific proteases, or under other defined conditions can be employed. H.
- compositionsthat include a humanized antibody disclosed herein and a pharmaceutically acceptable carrier.
- a humanized antibody or agent for inducing an antibody or a pharmaceutical composition the sameis administered to a patient susceptible to, or otherwise at risk of a disease in regimen (dose, frequency and route of administration) effective to reduce the risk, lessen the severity, or delay the onset of at least one sign or symptom of the disease (e.g., a disease associated with the antigen specifically bound by the humanized antibody).
- a humanized antibody or agent to induce an antibodyis administered to a patient suspected of, or already suffering from a disease (e.g., a disease associated with the antigen specifically bound by the humanized antibody) in a regimen (dose, frequency and route of administration) effective to Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT ameliorate or at least inhibit further deterioration of at least one sign or symptom of the disease.
- the regimenis preferably effective to decrease the level of antigen in the subject or treat a subject having a disease associated with the antigen specifically bound by the humanized antibody.
- the modified antibodyincludes one or more of (i) a heavy chain variable domain comprising one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14) of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to
- a heavy chain variable domaincomprising one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14) of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted
- the methodfurther includes introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- the methodfurther includes introducing at least one (e.g., 2, 3, 4, or 5) of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain.
- at least onee.g., 2, 3, 4, or 5
- the modified antibodyhas a melting temperature of 65 °C or greater (e.g., 75 °C or greater, or 80 °C or greater).
- the methods described hereincan further include introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has increase in melting temperature of 2 °C or greater (e.g., 5 °C or greater, or 10 °C or greater) compared to the parent antibody as measured by differential scanning calorimetry.
- the methodfurther includes introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain.
- the methodfurther includes introducing at least one (e.g., 2, 3, 4, or 5) of the following amino acid substitutions into a parent antibody: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain.
- at least onee.g., 2, 3, 4, or 5
- the modified antibodyhas increase in melting temperature of 5°C or greater (e.g., 10 °C or greater, or 15 °C or greater) compared to the parent antibody as measured by differential scanning calorimetry In some instances, the modified antibody has a melting temperature of 65°C or greater (e.g., 70 °C or greater, or 75 °C or greater). In some instances, the modified antibody has an immunogenicity score of 5 or less, 4 or less, 3 or less, 2 or less, or 1 or less.
- a regimenis considered therapeutically or prophylactically effective if an individual treated patient achieves an outcome more favorable than the mean outcome in a control population of comparable patients not treated by methods of the disclosure, or if a more favorable outcome is demonstrated in treated patients versus control patients in a controlled clinical trial (e.g., a phase II, phase II/III or phase III trial) at the p ⁇ 0.05 or 0.01 or even 0.001 level.
- Effective dosesvary depending on many different factors, such as means of administration, target site, physiological state of the patient, whether the patient is human or an animal, other medications administered, and whether treatment is prophylactic or therapeutic.
- Exemplary dosage ranges for humanized antibodiesare from about 0.01 to 60 mg/kg, or from about 0.1 to 3 mg/kg or 0.15-2 mg/kg or 0.15-1.5 mg/kg, of patient body weight.
- Antibodycan be administered such doses daily, on alternative days, weekly, fortnightly, monthly, quarterly, or according to any other schedule determined by empirical analysis.
- An exemplary treatmententails administration in multiple dosages over a prolonged period, for example, of at least six months. Additional exemplary treatment regimen entail administration once per every two weeks or once a month or once every 3 to 6 months.
- the amount of an agent for active administrationvaries from 0.1-500 ⁇ g per patient and more usually from 1-100 or 1-10 ⁇ g per injection for human administration.
- a typical regimenconsists of an immunization followed by booster injections at time intervals, such as 6 week intervals or two months.
- Another regimenconsists of an immunization followed by Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT booster injections 1, 2 and 12 months later.
- Another regimenentails an injection every two months for life.
- booster injectionscan be on an irregular basis as indicated by monitoring of immune response.
- Antibodies or agents for inducing antibodiesare preferably administered via a peripheral route (i.e., one in which an administered or induced antibody crosses the blood brain barrier to reach an intended site in the brain.)
- Routes of administrationinclude topical, intravenous, oral, subcutaneous, intraarterial, intracranial, intrathecal, intraperitoneal, intranasal, intraocular, intradermal, or intramuscular.
- Some routes for administration of antibodiesare intravenous and subcutaneous.
- Some routes for active immunizationare subcutaneous and intramuscular. This type of injection is most typically performed in the arm or leg muscles.
- agentsare injected directly into a particular tissue where deposits have accumulated, for example intracranial injection.
- compositions for parenteral administrationare preferably sterile and substantially isotonic and manufactured under GMP conditions.
- Pharmaceutical compositionscan be provided in unit dosage form (i.e., the dosage for a single administration).
- Pharmaceutical compositionscan be formulated using one or more physiologically acceptable carriers, diluents, excipients or auxiliaries. The formulation depends on the route of administration chosen.
- antibodiescan be formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hank’s solution, Ringer’s solution, or physiological saline or acetate buffer (to reduce discomfort at the site of injection).
- the solutioncan contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- antibodiescan be in lyophilized form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- a suitable vehiclee.g., sterile pyrogen-free water
- the present regimencan be administered in combination with another agent effective in treatment or prophylaxis of the disease being treated.
- Antibodiesare administered in an effective regimen meaning a dosage, route of administration and frequency of administration that delays the onset, reduces the severity, inhibits further deterioration, and/or ameliorates at least one sign or symptom of a disorder being treated. If a patient is already suffering from a disorder, the regimen can be referred to as a therapeutically effective regimen.
- the regimencan be referred to as a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT prophylactically effective regimen.
- therapeutic or prophylactic efficacycan be observed in an individual patient relative to historical controls or past experience in the same patient.
- therapeutic or prophylactic efficacycan be demonstrated in a preclinical or clinical trial in a population of treated patients relative to a control population of untreated patients.
- Administrationcan be parenteral, intravenous, oral, subcutaneous, intra-arterial, intracranial, intrathecal, intraperitoneal, topical, intranasal or intramuscular.
- Intravenous administrationcan be, for example, by infusion over a period such as 30-90 min.
- the frequency of administrationdepends on the half-life of the antibody in the circulation, the condition of the patient and the route of administration among other factors.
- the frequencycan be daily, weekly, monthly, quarterly, or at irregular intervals in response to changes in the patient’s condition or progression of the disorder being treated.
- An exemplary frequency for intravenous administrationis between weekly and quarterly over a continuous cause of treatment, although more or less frequent dosing is also possible.
- an exemplary dosing frequencyis daily to monthly, although more or less frequent dosing is also possible. I.
- Diagnostics and Monitoring MethodsAlso provided are methods of detecting the presence of an antigen specifically bound by the humanized antibody in a subject. Such methods are useful to diagnose or confirm diagnosis of diseases associated with the antigen recognized by the antibody specifically bound by the humanized antibody. The methods can also be used on asymptomatic subjects. The methods are also useful for monitoring disease progression and/or response to treatment in subjects who have been previously diagnosed with a disease associated with the antigen specifically bound by the humanized antibody. Diagnostic reagents can be administered by intravenous injection into the body of the patient, or directly into the brain by intracranial injection or by drilling a hole through the skull. The dosage of reagent should be within the same ranges as for treatment methods.
- the reagentis labeled, although in some methods, the primary reagent is unlabeled and a secondary labeling agent is used to bind to the primary reagent.
- the choice of labeldepends on the means Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT of detection.
- a fluorescent labelis suitable for optical detection.
- Use of paramagnetic labelsis suitable for tomographic detection without surgical intervention.
- Radioactive labelscan also be detected using positron emission tomography (PET) or single- photon emission computed tomography (SPECT). Diagnosis is performed by comparing the number, size, and/or intensity of labeled loci to corresponding base line values.
- PETpositron emission tomography
- SPECTsingle- photon emission computed tomography
- the base line valuescan represent the mean levels in a population of normal individuals. Base line values can also represent previous levels determined in the same subject. For example, base line values can be determined in a subject before beginning treatment, and measured values thereafter compared with the base line values.
- diagnosis of a disease or disorder associated with changes in antigen levelsmay be aided by performing a PET scan.
- a PET scancan be performed using, for example, a conventional PET imager and auxiliary equipment.
- the scantypically includes one or more regions of the tissue known in general to be associated with a disease or disorder associated with changes in antigen levels and one or more regions in which to serve as controls.
- a PET scanis performed concurrent with or in the same patient visit as an MRI or CAT scan.
- An MRI or CAT scanprovides more anatomical detail of the tissue than a PET scan.
- the image from a PET scancan be superimposed on an MRI or CAT scan image more precisely indicating the location of PET ligand.
- Some machinescan perform both PET scanning and MRI or CAT scanning without the patient changing positions between the scans facilitating superimposition of images.
- Suitable PET ligandsinclude radiolabeled antibodies of the disclosure.
- the radioisotope usedcan be, for example, C 11 , N 13 , O 15 , F 18 , or I 123 .
- the interval between administering the PET ligand and performing the scancan depend on the PET ligand and particularly its rate of uptake and clearing into the brain, and the half- life of its radiolabel.
- PET scanscan also be performed as a prophylactic measure in asymptomatic patients or in patients who have mild symptoms but have not yet been diagnosed with a disease or disorder associated with changes in antigen levels but are at elevated risk of developing a disease or disorder associated with the antigen specifically bound by the humanized antibody.
- scansare particularly useful for individuals considered at elevated risk of a disease or disorder associated with the antigen specifically bound by the humanized antibody because of a family history, genetic or biochemical risk factors, or mature age.
- Prophylactic Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT scanscan commence for example, at a patient age between 45 and 75 years. In some patients, a first scan is performed at age 50 years.
- Prophylactic scanscan be performed at intervals of for example, between six months and ten years, preferably between 1-5 years. In some patients, prophylactic scans are performed annually.
- the methodscan be used to monitor a course of therapeutic and prophylactic treatment with the agents provided herein.
- a baseline measurement of antibody in the subjectis made before administration, a second measurement is made soon thereafter to determine the peak antibody level, and one or more further measurements are made at intervals to monitor decay of antibody levels. When the level of antibody has declined to baseline or a predetermined percentage of the peak less baseline (e.g., 50%, 25% or 10%), administration of a further dose of antibody is administered.
- kitscomprising any of the humanized antibodies disclosed herein and related materials, such as instructions for use (e.g., package insert).
- the instructions for usemay contain, for example, instructions for administration of the humanized antibody and optionally one or more additional agents.
- the containers of humanized antibodymay be unit doses, bulk packages (e.g., multi-dose packages), or sub-unit doses.
- Package insertrefers to instructions customarily included in commercial packages of therapeutic products that contain information about the indications, usage, dosage, administration, contraindications and/or warnings concerning the use of such therapeutic products
- Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Kitscan also include a second container comprising a pharmaceutically-acceptable buffer, such as bacteriostatic water for injection (BWFI), phosphate-buffered saline, Ringer's solution and dextrose solution.
- BWFIbacteriostatic water for injection
- phosphate-buffered salinephosphate-buffered saline
- Ringer's solutionRinger's solution
- dextrose solutiondextrose solution
- the humanized antibodiescan be used for detecting the antigen specifically bound by the humanized antibody in the context of clinical diagnosis or treatment or in research. Binding of the humanized antibodies to the biological sample can be compared to binding of the antibodies to a control sample.
- the control sample and the biological samplecan comprise cells of the same tissue origin. Control samples and biological samples can be obtained from the same individual or different individuals and on the same occasion or on different occasions. If desired, multiple biological samples and multiple control samples are evaluated on multiple occasions to protect against random variation independent of the differences between the samples.
- a direct comparisoncan then be made between the biological sample(s) and the control sample(s) to determine whether antibody binding the biological sample(s) is increased, decreased, or the same relative to antibody binding to the control sample(s).
- Increased binding of the antibody to the biological sample(s) relative to the control sample(s)indicates the presence of the antigen specifically bound by the antibody in the biological sample(s).
- the increased antibody bindingis statistically significant.
- antibody binding to the biological sampleis at least 1.5-fold, 2-fold, 3-fold, 4-fold, 5-fold, 10-fold, 20-fold, or 100-fold higher than antibody binding to the control sample.
- multiple biological samples from the patientare evaluated on multiple occasions to establish both a baseline and measure of random variation independent of treatment.
- a therapeutic agentis then administered in a regimen.
- the regimenmay include multiple administrations of the agent over a period of time.
- binding of the antibodiesis evaluated on multiple occasions in multiple biological samples from the patient, both to establish a measure of random variation and to show a trend in response to immunotherapy.
- the various assessments of antibody binding to the biological samplesare then compared. If only two assessments are made, a direct comparison can be made between the two assessments to determine whether antibody binding has increased, decreased, or remained the same between the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT two assessments. If more than two measurements are made, the measurements can be analyzed as a time course starting before treatment with the therapeutic agent and proceeding through the course of therapy.
- the antibodiescan also be used as research reagents for laboratory research in detecting an antigen, or fragments thereof.
- antibodiescan be labeled with fluorescent molecules, spin-labeled molecules, enzymes, or radioisotopes, and can be provided in the form of kit with all the necessary reagents to perform the detection assay.
- the antibodiescan also be used to purify the antigen, or binding partners of the antigen, e.g., by affinity chromatography.
- the version associated with the accession number at the effective filing date of this applicationis meant.
- the effective filing datemeans the earlier of the actual filing date or filing date of a priority application referring to the accession number if applicable.
- the version most recently published at the effective filing date of the applicationis meant unless otherwise indicated. Any feature, step, element, embodiment, or aspect of the disclosure can be used in combination with any other unless specifically indicated otherwise.
- Example 1Methods of Selecting and Producing making anti-TDP43 monoclonal antibody 13D3 Beginning with a murine sequence for monoclonal antibody 13D3 humanization, a search was made over the protein sequences in the Protein Data Bank (PDB) database (Deshpande et al, Nucleic Acids Res. 2005 Jan 1; 33(Database Issue): D233–D237) to find structures, which would provide a rough structural model of 13D3. The crystal structure of an antibody fab PDB code 5BK5 (Scally, et al., Nat Commun.
- PDBProtein Data Bank
- the 5BK5 antibodyis a human germline-derived antibody and has same canonical classes and belonging to human germline IGHV3-48’03 for heavy chain and IGKV2-30*02 for light chain. Therefore, 5BK5 variable heavy and variable light chain sequences were also used as human acceptor framework.
- the framework regions of 5BK5 VH and 5BK5 VLwere chosen as the acceptor sequences for the CDRs of 13D3.
- a model of the 13D3 CDRs grafted onto the respective human frameworks for VH and VLwas built and used as a guidance for further backmutations.
- the amino acid sequences consisting of 5BK5 VH human frameworks and 13D3 CDRswere designated hu13D3VHvd1 and the amino acid sequences consisting of 5BK5 VL human framework and 13D3 VL CDRs were designated hu13D3VLvd1.
- hu13D3-VH and hu13D3-VLwere designed to enable assessment of various framework residues for their contributions to antigen binding, thermostability, developability (deamination, oxidation, N-glycosylation, proteolysis and aggregation) and immunogenicity.
- the positions considered for mutationinclude those under the following groups. ⁇ Positions that define the canonical CDR conformations (Martin (2010) Protein sequence and structure analysis of antibody variable domains. In: Kontermann R and Dübel S (eds). Antibody Engineering. Heidelberg, Germany: Springer International Publishing AG), ⁇ Positions that are within the Vernier zone (Foote and Winter (1992) Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol.
- hu13D3-VH_vd1includes the CDR-H1, H2, and H3 loops of 13D3-VH grafted onto the framework of 5BK5 VH.
- hu13D3-VH_vd1reverts all framework substitutions at positions that are key for defining the Chothia canonical classes, are part of the Vernier zone, or localize to the VH/VL domain interface or contribute to structural stability.
- substitutions designed for this heavy chainincluded one or more of L5V, T77S, R19K. G44R, S77T, L78A, L78G, L80A, L80G, L82cG, and R83M.
- hu13D3-VH_vd2included L78A.
- Hu13D3-VH_vd3includes G44R, S49A, and S74A.
- Hu13D3-VH_vd4included various changes made in versions 1, 2 and 3 are tried in combination.
- hu13D3-VL_vd1includes the CDR-L1, L2, and L3 loops of 13D3-VL grafted onto the framework of 5BK5 VL along with reverting all framework substitutions at positions that are key for defining the Chothia canonical classes, are part of the Vernier zone, or locate to the VH/VL domain interface.
- the following substitutionswere introduced: V3Q, L9S, I2V, P15L, P18Q, E17Q, L38Q, K39R, R46L, A80S, L92G, V94I, V94A, Q100D, and L104V.
- Affinitywas analyzed using a bivalent analyte model.
- Hu13D3 binding to pTDP43 peptidewas maintained among all tested antibodies, compared to the Ch_13D3 Ab and Ch_13D3, all variants show sufficient binding with approximately 0.5-1nM Affinity (Bivalent analyte model), demonstrating that each antibody possessed affinity to TDP43.
- each of h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2demonstrated superior binding as shown by KD1 (M) values that are about 2 orders of magnitude better binder than antibodies made using conventional methods (e.g., h13D3-H9L7).
- KD1 (M) valuesthat are about 2 orders of magnitude better binder than antibodies made using conventional methods (e.g., h13D3-H9L7).
- Phospho-TDP43_392-414 bindingwas determined using a Biacore TM system.
- these antibodieshave KD values of single digit nanomolar to sub-nanomolar values, which are similar to or lower than KD values for antibodies that lack humanizing mutations (e.g., chimeric 13D3 antibodies).
- humanizing mutationse.g., chimeric 13D3 antibodies.
- antibodies comprising humanizing mutations of the present disclosureretain high-affinity binding to their target epitope.
- Example 3. Thermal Stability and Expression Yield of Humanized AntibodiesAttorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT
- thermal stability (TM) of the humanize antibodieswas evaluated. 3.33 mM/0.5 mg/mL of test concentration was added in 1xPBS Buffer pH 7.4.
- antibody variantse.g., h13D3-H3L4, h13D3-H5L2, h13D3-H5L3, h13D3- H5L4, h13D3-H6L4, h13D3-H5L5, h13D3-H6L5, h13D3-H7L5, h13D3-H8L4, h13D3-H8L5, and h13D3-H8L6) exhibited extremely low expression yields, which are indicative of antibody instability. As a result of the low yields, many of these variants were not assessed for melting temperatures.
- humanized antibodiescomprising the humanization mutations of the present disclosure (e.g., h13D3-Hd1Ld1, h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2) were expressed in high yield, demonstrating the stability of these antibodies.
- Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCTThe melting temperature data in Table 5 further demonstrate the stability of antibodies comprising humanization mutations of the present disclosure.
- humanized antibodies comprising the humanization mutations of the present disclosuree.g., h13D3-Hd1Ld1, h13D3- Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2
- Tm Onset values of 70 degrees Celsius or higher for the CH2 regiondemonstrating the high stability of these antibodies.
- the data in Table 5demonstrate that antibodies comprising humanizing mutations of the present disclosure exhibit high thermal stability, resulting in stable antibodies that can be expressed in high yield.
- Immune Epitope DatabaseIEDB
- EpiQuestIEDB immunogenicity analysis tool is hosted by the National Institute of Allergy and Infectious Diseases.
- the immunogenicity prediction method usedis based on predicted potential binding of a peptide within a protein sequence to major histocompatibility class (MHC) class II.
- MHCmajor histocompatibility class
- the programidentifies potential immunogenic regions within the protein sequence.
- the MHC class II toolpredicts immunogenic regions using broad spectrum alleles (i.e., 26 reference alleles) for MHC class II in the human population.
- the softwaregenerates a series of 15 residue peptides which overlap in 10 residues.
- Each generated 15 residue peptideis predicted for binding to the 26 reference alleles.
- a percentile rank for each of the three methods(combinatorial library, SMM_align and Sturniolo) was generated by comparing each peptide's score against the scores of five million random 15 residue peptides selected from the SWISSPROT database.
- the adjusted percentile rankis the percentile rank adjusted based on the frequency of peptide lengths. A low number percentile rank indicates high affinity.
- the median percentile rank of the three methodswere then used to generate the rank for consensus method.
- prediction resultAttorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT is collapsed to show only the Percentile Rank and Adjusted Rank.
- the maximum median percentile rank thresholdis set at 20.
- the EpiQuest tool, T-Scannerranges cytotoxic T lymphocyte (CTL) epitopes according to their predicted immunodominance.
- the immunodominance of epitopesis defined as their relative strength in functional assays related to the target kill or release of respective cytokines. These parameters indicate functionality of the T-epitope.
- This programis designed to analyze and sort according to their immunodominance the CTL peptide epitopes eluted from target cells. Generally, only a low number of peptide epitopes that bind to MHC class I have actual functional activity.
- Relative strength of a CTL (T) epitopeis defined by the strength of its binding to MHC class I and T cell receptor (TCR) (in the context of MHCI).
- TCRT cell receptor
- the algorithmdetects the structural and compositional features of the peptide epitope that allow it to elicit a high-affinity for TCR. This analysis is haplotype-specific and the EpiQuest T-scanner has matrix for analysis of HLAA2 and H2kB haplotypes-binding peptides.
- Tables 6show some humanized antibodies comprising humanizing mutations of the present disclosure have lower predicted immunogenicity scores relative to the chimeric 13D3 antibody and many antibodies humanized using traditional humanization strategies. Table 6.
- antibody variantse.g., h13D3-H1L1, h13D3-H2L2, h13D3- H2L3, h13D3-H2L4, h13D3-H3L2, h13D3-H3L3, h13D3-H3L4, h13D3-H4L2, h13D3-H4L3, h13D3-H4L4, h13D3-H5L2, h13D3-H5L3, and h13D3-H8L6) exhibited high predicted immunogenicity values, which are indicative of actual immunogenicity in human subjects.
- humanized antibodies comprising the humanization mutations of the present disclosuredemonstrated low predicted immunogenicity values, demonstrating that these antibodies are unlikely to be immunogenic in human subjects.
- Tables 4, 5, and 6demonstrate that antibodies comprising humanizing mutations of the present disclosure retain strong antigen binding, exhibit high thermal stability, can be expressed in high yield, and have significantly reduced immunogenicity, even when compared to antibodies that are humanized using traditional humanization methods.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Abstract
Description
Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT COMPOSITIONS AND METHODS OF REDUCING IMMUNOGENICITY AND IMPROVING STABILITY OF ANTIBODIES CROSS-REFERENCE TO RELATED APPLICATIONS This application claims the benefit of priority to U.S. Provisional Patent Application No. 63/538,598, filed on September 15, 2023, the content of which is hereby incorporated by reference in its entirety. SEQUENCE LISTING This application contains a Sequence Listing that has been submitted electronically as an XML file named 50887-0046WO1_ST26_SL.XML.” The XML file, created on August 30, 2024, is 35,169 bytes in size. The material in the XML file is hereby incorporated by reference in its entirety. BACKGROUND Antibodies are natural proteins that the vertebrate immune system forms in response to foreign substances (antigens), primarily for defense against infection. For over a century, antibodies have been induced in animals under artificial conditions and harvested for use in therapy or diagnosis of disease conditions, or for biological research. Each individual antibody producing-cell produces a single type of antibody with a chemically defined composition, however, antibodies obtained directly from animal serum in response to antigen inoculation actually comprise an ensemble of non-identical molecules (i.e., polyclonal antibodies) made from an ensemble of individual antibody-producing cells. The utility of rodent (specifically murine) monoclonal antibodies in human therapy is limited because of problems associated with their non-human origin, in particular their immunogenicity in a human host. In order to minimize the human immune response against therapeutic antibody drugs, monoclonal antibody technology has evolved from full mouse antibodies to chimeric antibodies (mouse variable domains grafted on a human IgG backbone), and to humanized antibodies (mouse CDRs grafted on a human IgG backbone). Humanization of mouse monoclonal antibodies was initially achieved by combining mouse variable domains with human constant domains, creating so called chimeric antibodies having about 70% of Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT human content. A further degree of humanization was subsequently achieved by grafting the complementarity-determining regions (CDRs) of mouse monoclonal antibodies onto human framework regions of the variable antibody domains of human antibodies. In addition, several amino acid residues present in those framework regions were identified as interacting with the CDRs or antigen and were back mutated in the humanized antibody to improve binding. (Almagro et al., Frontiers Bioscience 13:1619-1633 (2008)). Immunogenicity is the result of a complex series of responses to a substance that is perceived as foreign, and may include production of neutralizing and non-neutralizing antibodies, formation of immune complexes, complement activation, mast cell activation, inflammation, hypersensitivity responses, and anaphylaxis. Several factors can contribute to protein immunogenicity, including but not limited to protein sequence, route and frequency of administration, and patient population. Several humanization methods known in the art, primarily consisting of introducing mutations in regions of the antibody sequence predicted to be immunogenic. However, there are drawback to these methods, including a reduction in the stability of the resulting antibody. Often, the introduction of a deimmunizing mutation during the humanization process impacts antibody conformation (e.g., by disrupting the network of molecular interactions that hold the antibody in the correct conformation for antigen recognition). For example, a deimmunizing mutation might interfere with the intrachain interactions between the light chain and heavy chain, thereby causing significant loss of molecular stability. Loss of stability, in turn, can lead to a number of problems, including low expression yield, reduced processability, shorter shelf-life, and unacceptable pharmacokinetic parameters. As there is a need to develop improved humanized antibodies that have reduced immunogenicity and/or improved stability, there is a need for methods of reducing immunogenicity and/or increasing the stability of humanized antibodies. SUMMARY The present disclosure relates to antibodies and antigen-binding antibody fragments that reduce immunogenicity and improve stability of antibodies. Also disclosed herein are compositions comprising these antibodies or antigen-binding antibody fragments, and methods of using these antibodies and antigen-binding antibody fragments to reduce immunogenicity. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Thus, disclosed herein is a humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is a positively charged amino acid, and the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is a negatively charged amino acid. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid is a basic amino acid. In some instances, the negatively charged amino acid is an acidic amino acid. In some instances, the positively charged amino acid is an arginine. In some instances, the negatively charged amino acid is an aspartic acid. In some instances, the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative comprises a hydroxy- substituted phenyl group. In some instances, the substituted phenylalanine derivative is a tyrosine residue. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain is a glutamine, and/or wherein the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. In some instances, the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: a serine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and a serine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT chain. In some instances, the humanized antibody further comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. Also disclosed herein is a humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, and a light chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative comprises a hydroxy- substituted phenyl group. In some instances, the substituted phenylalanine derivative is a tyrosine. In some instances, the polar uncharged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. In some instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain variable domain, is a glutamine. In some instances, the humanized antibody further comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the humanized antibody further Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid is a basic amino acid. In some instances, the negatively charged amino acid is an acidic amino acid. In some instances, the positively charged amino acid is an arginine. In some instances, the negatively charged amino acid is an aspartic acid. In some instances, the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. Also disclosed herein is a humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a positively Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged residue at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is an aspartic acid. In some instances, the humanized antibody further comprises one or both of: an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 49 of a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT human IgG1 heavy chain; and an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In another embodiment, discloses herein is a humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises a positively charged amino acid at an Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. In some instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, is a serine. In yet another embodiment, disclosed herein is a humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. In some instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain is a serine. In some instances, the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. In some instances, the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, forms a hydrogen bond interaction with at least one of an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond interaction with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, comprises a hydroxy-substituted phenyl group. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, is a tyrosine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, is a glutamine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, is a glutamine. In some instances, the antibody is an antigen-binding antibody fragment. In some instances, the antigen-binding antibody fragment is a Fab fragment, a Fab’2 fragment, or a single chain Fv. In some instances, the humanized antibody is a full antibody, a chimeric antibody, a veneered antibody, a CDR-grafted antibody, or a recombinant antibody. In some instances, the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT humanized antibody is a human IgG antibody. In some instances, the human IgG antibody has a human IgG1 isotype. In some instances, the antibody is conjugated to a therapeutic, cytotoxic, cytostatic, immunomodulatory, neurotrophic, or neuroprotective agent. In some instances, the heavy chain variable domain is fused to a heavy chain constant region and the light chain variable domain is fused to a light chain constant region. In some instances, the heavy chain constant region is a mutant form of a natural human heavy chain constant region which has reduced binding to an Fcγ receptor relative to a natural heavy chain constant region. In some instances, the heavy chain constant region is of IgG1 isotype. In some instances, the antibody has at least one mutation in a constant region. In some instances, the at least one mutation reduces complement fixation or activation by the constant region. In some instances, the at least one mutation is at one or more positions of: 241, 264, 265, 270, 296, 297, 318, 320, 322, 329, and 331 by EU numbering. In some instances, the humanized antibody has an alanine at positions 318, 320, and 322 by EU numbering. In some instances, the humanized antibody has increased thermal stability as compared to a reference antibody. In some instances, the increased thermal stability comprises an increase in expression yield compared to the reference antibody. In some instances, the increased thermal stability comprises an increase in expression yield of at least 100 mg/L compared to the reference antibody. In some instances, the increased thermal stability comprises an increase in melting point as compared to the reference antibody. In some instances, the increase in melting point is an increase of at least 2°C compared to the reference antibody. In some instances, the increase in melting point is an increase of at least 5°C compared to the reference antibody. In some instances, the humanized antibody has a melting temperature of 55°C or greater. In some instances, the humanized antibody has a melting temperature of 65°C or greater. In some instances, the humanized antibody has a melting temperature of 70°C or greater. In some instances, the melting temperature is determined by differential scanning calorimetry. In some instances, the humanized antibody has a decreased immunogenicity score as compared to a reference antibody. In some instances, the immunogenicity score is determined using Formula I: Ig Score = (-KDHI +4.5) X Pt (average) X Tail Bonus (Formula I). In some instances, the reference antibody comprises the same sequence as the humanized antibody without the one or more amino acid substitutions. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Also disclosed herein is a pharmaceutical composition comprising any one of the humanized antibodies disclosed herein and a pharmaceutically acceptable carrier. Also disclosed herein is a nucleic acid encoding the heavy chain variable domain and/or the light chain variable domain of an antibodies disclosed herein. Also disclosed herein is a vector comprising a nucleic acid encoding a heavy chain variable domain and a light chain variable domain operably linked to one or more regulatory sequences to effect expression in a mammalian cell of the humanized antibodies disclosed herein. In some instances, the one or more regulatory sequences comprises one or more of a enhancer, ribosome binding site, transcription termination signal, and promoter, optionally, wherein the promoter is a eukaryotic promoter. In some instances, the nucleic acid is codon- optimized for expression in a host cell. Also disclosed herein is a host cell transformed with any vector described herein. Also disclosed herein is a host cell comprising any nucleic acid described herein. Also disclosed herein is a method of reducing immunogenicity of a parent antibody by introducing amino acid substitution(s) into the parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. Also disclosed herein is a method of increasing thermal stability of an antibody by introducing amino acid substitution(s) into a parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Also disclosed herein is a method of reducing immunogenicity of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has a reduction in immunogenicity compared to the parent antibody. In some instances, the method further comprises introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the method further comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the modified antibody has a melting temperature of 65°C or greater. Also disclosed herein is a method of increasing thermal stability of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has increase in melting temperature of 2°C or greater compared to the parent antibody as measured by differential scanning calorimetry. In some instances, the method further comprises introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the method further comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the modified antibody has increase in melting temperature of 5°C or greater compared to the parent antibody as measured by differential scanning calorimetry. In some instances, the modified antibody has a melting temperature of 65°C or greater. BRIEF DESCRIPTION OF THE DRAWINGS FIGs. 1A-1C show Chothia canonical amino acid sequences for heavy chain variable regions disclosed herein. VH/VL interfaces are shown in shaded gray, and Vernier zone residues are shown with stippled shading. FIGs. 2A-2C show Chothia canonical amino acid sequences for light chain variable regions disclosed herein. VH/VL interfaces are shown in shaded gray, and Vernier zone residues are shown with stippled shading. DETAILED DESCRIPTION A. Definitions The term “antibody” includes intact antibodies and binding fragments thereof. Typically, fragments compete with the intact antibody from which they were derived for specific binding to the target including separate heavy chains, light chains Fab, Fab', F(ab')2, F(ab)c, Dabs, nanobodies, and Fv. Fragments can be produced by recombinant DNA techniques, or by enzymatic or chemical separation of intact immunoglobulins. The term “antibody” also includes a bispecific antibody and/or a humanized antibody. A bispecific or bifunctional antibody is an Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT artificial hybrid antibody having two different heavy/light chain pairs and two different binding sites (see, e.g., Songsivilai and Lachmann, Clin. Exp. Immunol., 79:315-321 (1990); Kostelny et al., J. Immunol., 148:1547-53 (1992)). Neutral amino acid residue refers to an alanine residue, an asparagine residue, an isoleucine residue, a glycine residue, a glutamine residue, a cysteine residue, a threonine residue, a serine residue, a tyrosine residue, a phenylalanine residue, a proline residue, a valine residue, a methionine residue, a leucine residue, and a tryptophan residue. The nine amino acids that have hydrophobic side chains are glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). Six amino acids have side chains that are polar but not charged: serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr). Three amino acids have basic side chains at neutral pH: arginine (Arg), lysine (Lys), and histidine (His). Two amino acids have acidic side chains at neutral pH: aspartic acid or aspartate (Asp) and glutamic acid or glutamate (Glu). Specific binding of an antibody to its target antigen means an affinity and/or avidity of at least 106, 107, 108, 109, 1010, 1011, or 1012 M-1. Specific binding is detectably higher in magnitude and distinguishable from non-specific binding occurring to at least one unrelated target. Specific binding can be the result of formation of bonds between particular functional groups or particular spatial fit (e.g., lock and key type) whereas nonspecific binding is usually the result of van der Waals forces. Specific binding does not however necessarily imply that an antibody binds one and only one target. The basic antibody structural unit is a tetramer of subunits. Each tetramer includes two identical pairs of polypeptide chains, each pair having one “light” (about 25 kDa) and one “heavy” chain (about 50-70 kDa). The amino-terminal portion of each chain includes a variable region of about 100 to 110 or more amino acids primarily responsible for antigen recognition. This variable region is initially expressed linked to a cleavable signal peptide. The variable region without the signal peptide is sometimes referred to as a mature variable region. Thus, for example, a light chain mature variable region means a light chain variable region without the light chain signal peptide. The carboxy-terminal portion of each chain defines a constant region primarily responsible for effector function. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Light chains are classified as either kappa or lambda. Heavy chains are classified as gamma, mu, alpha, delta, or epsilon, and define the antibody's isotype as IgG, IgM, IgA, IgD and IgE, respectively. Within light and heavy chains, the variable and constant regions are joined by a “J” region of about 12 or more amino acids, with the heavy chain also including a “D” region of about 10 or more amino acids. See generally, Fundamental Immunology, Paul, W., ed., 2nd ed. Raven Press, N.Y., 1989, Ch. 7 (incorporated by reference in its entirety for all purposes). An immunoglobulin light or heavy chain variable region (also referred to herein as a “light chain variable domain” (“VL domain”) or “heavy chain variable domain” (“VH domain”), respectively) consists of a “framework” region interrupted by three “complementarity determining regions” or “CDRs.” The framework regions serve to align the CDRs for specific binding to an epitope of an antigen. The CDRs include the amino acid residues of an antibody that are primarily responsible for antigen binding. From amino-terminus to carboxyl-terminus, both VL and VH domains comprise the following framework (FR) and CDR regions: FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4. CDRs 1, 2, and 3 of a VL domain are also referred to herein, respectively, as CDR-L1, CDR-L2, and CDR-L3; CDRs 1, 2, and 3 of a VH domain are also referred to herein, respectively, as CDR-H1, CDR-H2, and CDR-H3. When the application discloses a VL sequence with R as the C-terminal residue, the R can alternatively be considered as being the N-terminal residue of the light chain constant region. Thus, the application should also be understood as disclosing the VL sequence without the C-terminal R. The assignment of amino acids to each VL and VH domain is in accordance with any conventional definition of CDRs. Conventional definitions include, the Kabat definition (Kabat, Sequences of Proteins of Immunological Interest (National Institutes of Health, Bethesda, MD, 1987 and 1991), the Chothia definition (Chothia & Lesk, J. Mol. Biol. 196:901-917, 1987; Chothia et al., Nature 342:878-883, 1989); a composite of Chothia Kabat CDR in which CDR- H1 is a composite of Chothia and Kabat CDRs; the AbM definition used by Oxford Molecular’s antibody modelling software; and, the contact definition of Martin et al (bioinfo.org.uk/abs) (see Table 1). Kabat provides a widely used numbering convention (Kabat numbering) in which corresponding residues between different heavy chains or between different light chains are assigned the same number. When an antibody is said to comprise CDRs by a certain definition of CDRs (e.g., Kabat) that definition specifies the minimum number of CDR residues present in the antibody (i.e., the Kabat CDRs). It does not exclude that other residues falling within another Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT conventional CDR definition but outside the specified definition are also present. For example, an antibody comprising CDRs defined by Kabat includes among other possibilities, an antibody in which the CDRs contain Kabat CDR residues and no other CDR residues, and an antibody in which CDR H1 is a composite Chothia-Kabat CDR H1 and other CDRs contain Kabat CDR residues and no additional CDR residues based on other definitions. Table 1. Conventional Definitions of CDRs using Kabat Numbering Composite of This is because the Kabat numbering scheme places insertions of extra residues at 35A and 35B, whereas Chothia numbering places them at 31A and 31B. If neither H35A nor H35B (Kabat numbering) is present, the Chothia CDR-H1 loop ends at H32. If only H35A is present, it ends at H33. If both H35A and H35B are present, it ends at H34. The term “epitope” refers to a site on an antigen to which an antibody binds. An epitope can be formed from contiguous amino acids or noncontiguous amino acids juxtaposed by tertiary folding of one or more proteins. Epitopes formed from contiguous amino acids (also known as linear epitopes) are typically retained on exposure to denaturing solvents whereas epitopes formed by tertiary folding (also known as Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT conformational epitopes) are typically lost on treatment with denaturing solvents. An epitope typically includes at least 3, and more usually, at least 5 or 8-10 amino acids in a unique spatial conformation. Methods of determining spatial conformation of epitopes include, for example, x-ray crystallography and 2-dimensional nuclear magnetic resonance. See, e.g., Epitope Mapping Protocols, in Methods in Molecular Biology, Vol. 66, Glenn E. Morris, Ed. (1996). Antibodies that recognize the same or overlapping epitopes can be identified in a simple immunoassay showing the ability of one antibody to compete with the binding of another antibody to a target antigen. The epitope of an antibody can also be defined by X-ray crystallography of the antibody bound to its antigen to identify contact residues. Alternatively, two antibodies have the same epitope if all amino acid mutations in the antigen that reduce or eliminate binding of one antibody reduce or eliminate binding of the other. Two antibodies have overlapping epitopes if some amino acid mutations that reduce or eliminate binding of one antibody reduce or eliminate binding of the other. Competition between antibodies is determined by an assay in which an antibody under test inhibits specific binding of a reference antibody to a common antigen (see, e.g., Junghans et al., Cancer Res. 50:1495, 1990). A test antibody competes with a reference antibody if an excess of a test antibody (e.g., at least 2x, 5x, 10x, 20x or 100x) inhibits binding of the reference antibody by at least 50% as measured in a competitive binding assay. Some test antibodies inhibit binding of the reference antibody by at least 75%, 90% or 99%. Antibodies identified by competition assay (competing antibodies) include antibodies binding to the same epitope as the reference antibody and antibodies binding to an adjacent epitope sufficiently proximal to the epitope bound by the reference antibody for steric hindrance to occur. The term “pharmaceutically acceptable” means that the carrier, diluent, excipient, or auxiliary is compatible with the other ingredients of the formulation and not substantially deleterious to the recipient thereof. The term “patient” or “subject” includes human and other mammalian subjects that receive either prophylactic or therapeutic treatment. An individual is at increased risk of a disease if the subject has at least one known risk- factor (e.g., genetic, biochemical, family history, and situational exposure) placing individuals Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT with that risk factor at a statistically significant greater risk of developing the disease than individuals without the risk factor. The term “biological sample” refers to a sample of biological material within or obtainable from a biological source, for example a human or mammalian subject. Such samples can be organs, organelles, tissues, sections of tissues, bodily fluids, peripheral blood, blood plasma, blood serum, cells, molecules such as proteins and peptides, and any parts or combinations derived therefrom. The term biological sample can also encompass any material derived by processing the sample. Derived material can include cells or their progeny. Processing of the biological sample may involve one or more of filtration, distillation, extraction, concentration, fixation, inactivation of interfering components, and the like. The term “control sample” refers to a biological sample not known or suspected to include a tissue with no or little increased immunogenicity, or at least not known or suspect to include diseased tissues of a given type. Control samples can be obtained from individuals not afflicted with an immunogenic disease. Alternatively, control samples can be obtained from patients afflicted with an immunogenic disease. Such samples can be obtained at the same time as a biological sample thought to comprise an immunogenic disease or on a different occasion. A biological sample and a control sample can both be obtained from the same tissue. Preferably, control samples consist essentially or entirely of normal, healthy tissues and can be used in comparison to a biological sample thought to comprise immunogenic disease-affected regions. In some instances, the tissue in the control sample is the same type as the tissue in the biological sample. In some instances, the immunogenic disease-affected cells thought to be in the biological sample arise from the same cell type as the type of cells in the control sample. The term “disease” refers to any abnormal condition that impairs physiological function. The term is used broadly to encompass any disorder, illness, abnormality, pathology, sickness, condition, or syndrome in which physiological function is impaired, irrespective of the nature of the etiology. “Immunogenicity” refers to the ability of a foreign molecule or substance to provoke or to stimulate an immune response in a cell or a subject. An immune response can include activation of a non-specific innate response and/or activation of an adaptive response that includes a cell-mediated immune response, which is carried out by T cells, and a humoral immune response, which is controlled by activated B cells and endogenous antibodies. In some Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT instances, the humanized antibodies disclosed herein reduce immunogenicity in the cell or the subject. An “immunogenicity score” refers to a value assigned to immunogenicity or reduction thereof caused by an antibody (e.g., humanized antibody) disclosed herein. Immunogenicity Score is a predictor of immunogenicity. The higher the score, the greater the predicted immunogenicity. The score can be an absolute number based on predicted potential binding of the antibody (e.g., humanized antibody) within a protein sequence of an endogenous protein (e.g., a major histocompatibility class (MHC) class II protein). In some instances, immunogenicity score can be calculated using a publicly available program such as Immune Epitope Database (IEDB) and EpiQuest. In some instances, an immunogenicity score is calculated using Formula I: Ig Score = (-KDHI +4.5) X Pt (average) X Tail Bonus (Formula I). The term “symptom” refers to a subjective evidence of a disease, such as altered gait, as perceived by the subject. A “sign” refers to objective evidence of a disease as observed by a physician. The term “positive response to treatment” refers to a more favorable response in an individual patient or average response in a population of patients relative to an average response in a control population not receiving treatment. For purposes of classifying amino acids substitutions as conservative or nonconservative, amino acids are grouped as follows: Group I (hydrophobic side chains): met, ala, val, leu, ile; Group II (neutral hydrophilic side chains): cys, ser, thr; Group III (acidic side chains): asp, glu; Group IV (basic side chains): asn, gln, his, lys, arg; Group V (residues influencing chain orientation): gly, pro; and Group VI (aromatic side chains): trp, tyr, phe. Conservative substitutions involve substitutions between amino acids in the same class. Non-conservative substitutions constitute exchanging a member of one of these classes for a member of another. Percentage sequence identities are determined with antibody sequences maximally aligned by the Kabat numbering convention. After alignment, if a subject antibody region (e.g., the entire mature variable region of a heavy or light chain) is being compared with the same region of a reference antibody, the percentage sequence identity between the subject and reference antibody regions is the number of positions occupied by the same amino acid in both the subject and reference antibody region divided by the total number of aligned positions of the two regions, with gaps not counted, multiplied by 100 to convert to percentage. A “reference Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT antibody” as used herein refers to an antibody or fragment thereof that competes for binding with a test antibody (e.g., an antibody having one or more substitutions described herein) to the same epitope. The reference antibody does not include the substitution in its sequence and can also be referred to as a parent antibody. In some instances, the reference antibody comprises the same sequence as the humanized antibody without the one or more amino acid substitutions as described herein. Compositions or methods “comprising” or “including” one or more recited elements may include other elements not specifically recited. For example, a composition that “comprises” or “includes” an antibody may contain the antibody alone or in combination with other ingredients. Designation of a range of values includes all integers within or defining the range, and all subranges defined by integers within the range. Unless otherwise apparent from the context, the term “about” encompasses insubstantial variations, such as values within a standard margin of error of measurement (e.g., SEM) of a stated value. Statistical significance means p ^0.05. The singular forms of the articles “a,” “an,” and “the” include plural references unless the context clearly dictates otherwise. For example, the term “a compound” or “at least one compound” can include a plurality of compounds, including mixtures thereof. B. Humanized Antibodies A humanized antibody is a genetically engineered antibody in which CDRs from a non- human “donor” antibody are grafted into human “acceptor” antibody sequences (see, e.g., Queen, US 5,530,101 and 5,585,089; Winter, US 5,225,539; Carter, US 6,407,213; Adair, US 5,859,205; and Foote, US 6,881,557). The acceptor antibody sequences can be, for example, a mature human antibody sequence, a composite of such sequences, a consensus sequence of human antibody sequences, or a germline region sequence. Thus, a humanized antibody is an antibody having at least three, four, five or all CDRs entirely or substantially from a donor antibody and variable region framework sequences and constant regions, if present, entirely or substantially from human antibody sequences. Similarly, a humanized heavy chain has at least one, two and usually all three CDRs entirely or substantially from a donor antibody heavy chain, and a heavy chain variable region framework sequence and heavy chain constant region, if Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT present, substantially from human heavy chain variable region framework and constant region sequences. Similarly, a humanized light chain has at least one, two and usually all three CDRs entirely or substantially from a donor antibody light chain, and a light chain variable region framework sequence and light chain constant region, if present, substantially from human light chain variable region framework and constant region sequences. Other than nanobodies and dAbs, a humanized antibody comprises a humanized heavy chain and a humanized light chain. A CDR in a humanized antibody is substantially from a corresponding CDR in a non-human antibody when at least 85%, 90%, 95% or 100% of corresponding residues (as defined by any conventional definition but preferably defined by Kabat) are identical between the respective CDRs. The variable region framework sequences of an antibody chain or the constant region of an antibody chain are substantially from a human variable region framework sequence or human constant region respectively when at least 85%, 90%, 95% or 100% of corresponding residues defined by Kabat are identical. To be classified as humanized under the 2014 World Health Organization (WHO) International non-proprietary names (INN) definition of humanized antibodies, an antibody must have at least 85% identity to human germline antibody sequences (i.e., prior to somatic hypermutation). Mixed antibodies are antibodies for which one antibody chain (e.g., heavy chain) meets the threshold but the other chain (e.g., light chain) does not meet the threshold. An antibody is classified as chimeric if neither chain meets the threshold, even though the variable framework regions for both chains were substantially human with some murine backmutations. See, Jones et al. (2016) The INNs and outs of antibody nonproprietary names, mAbs 8:1, 1-9, DOI: 10.1080/19420862.2015.1114320. See also “WHO-INN: International nonproprietary names (INN) for biological and biotechnological substances (a review)” (Internet) 2014. Available from: www.who.int/medicines/services/inn/ BioRev2014.pdf), incorporated herein by reference. For the avoidance of doubt, the term “humanized” as used herein is not intended to be limited to the 2014 WHO INN definition of humanized antibodies. Some of the humanized antibodies provided herein have at least 85% sequence identity to human germline sequences and some of the humanized antibodies provided herein have less than 85% sequence identity to human germline sequences. Some of the heavy chains of the humanized antibodies provided herein have from about 60% to 100% sequence identity to human germ line sequences, such as, for example, in the range of about 60% to 69%, 70% to 79%, 80% to 84%, or 85% to 89%. Some heavy chains fall below the 2014 WHO INN Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT definition and have, for example, about 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, or 82%, 83%, or 84% sequence identity to human germ line sequences, while other heavy chains meet the 2014 WHO INN definition and have about 85%, 86%, 87%, 88%, 89% or greater sequence identity to human germ line sequences. Some of the light chains of the humanized antibodies provided herein have from about 60% to 100% sequence identity to human germ line sequences, such as, for example, in the range of about 80% to 84% or 85% to 89%. Some light chains fall below the 2014 WHO INN definition and have, for example, about 81%, 82%, 83% or 84% sequence identity to human germ line sequences, while other light chains meet the 2014 WHO INN definition and have about 85%, 86%, 87%, 88%, 89% or greater sequence identity to human germ line sequences. Some humanized antibodies provided herein that are "chimeric" under the 2014 WHO INN definition have heavy chains with less than 85% identity to human germ line sequences paired with light chains having less than 85% identity to human germ line sequences. Some humanized antibodies provided herein are "mixed" under the 2014 WHO INN definition, for example, having a heavy chain with at least 85% sequence identity to human germ line sequences paired with a light chain having less than 85% sequence identity to human germ line sequences, or vice versa. Some humanized antibodies provided herein meet the 2014 WHO INN definition of "humanized" and have a heavy chain with at least 85% sequence identity to human germ line sequences paired with a light chain having at least 85% sequence identity to human germ line sequences. Although humanized antibodies often incorporate all six CDRs (defined by any conventional definition but preferably as defined by Kabat) from a mouse antibody, they can also be made with less than all CDRs (e.g., at least 3, 4, or 5 CDRs) from a mouse antibody (e.g., Pascalis et al., J. Immunol. 169:3076, 2002; Vajdos et al., J. of Mol. Biol., 320: 415-428, 2002; Iwahashi et al., Mol. Immunol. 36:1079-1091, 1999; Tamura et al, J. Immunol., 164:1432-1441, 2000). In some antibodies only part of the CDRs, namely the subset of CDR residues required for binding, termed the SDRs, are needed to retain binding in a humanized antibody. CDR residues not contacting antigen and not in the SDRs can be identified based on previous studies (for example residues H60-H65 in CDR H2 are often not required), from regions of Kabat CDRs lying outside Chothia hypervariable loops (Chothia, J. Mol. Biol. 196:901, 1987), by molecular Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT modeling and/or empirically, or as described in Gonzales et al., Mol. Immunol. 41: 863, 2004. In such humanized antibodies at positions in which one or more donor CDR residues is absent or in which an entire donor CDR is omitted, the amino acid occupying the position can be an amino acid occupying the corresponding position (by Kabat numbering) in the acceptor antibody sequence. The number of such substitutions of acceptor for donor amino acids in the CDRs to include reflects a balance of competing considerations. Such substitutions are potentially advantageous in decreasing the number of mouse amino acids in a humanized antibody and consequently decreasing potential immunogenicity and/or for meeting the WHO INN definition of “humanized”. However, substitutions can also cause changes of affinity, and significant reductions in affinity are preferably avoided. Positions for substitution within CDRs and amino acids to substitute can also be selected empirically. The human acceptor antibody sequences can optionally be selected from among the many known human antibody sequences to provide a high degree of sequence identity (e.g., 65-85% identity) between a human acceptor sequence variable region frameworks and corresponding variable region frameworks of a donor antibody chain. In some instances, disclosed herein are humanized antibodies having a salt bridge between an amino acid in the heavy chain variable domain and an amino acid in the light chain variable domain. In some instances, one or more amino acid substitutions are introduced into the humanized antibody. In some instances, the one or more amino acid substitutions are introduced into a Chothia canonical structure. In some instances, the one or more amino acid substitutions are introduced into a VH/VL interface, including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20) of Chothia canonical amino acid positions 35, 37, 39, 45, 47, 91, 93, 95, and/or 103 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 27F, 34, 36, 38, 44, 46, 87, 89, 91, 96 and/or 98 of the human IgG1 light chain. Exemplary amino acid substitutions that can be introduced into a VH/VL interface are shown in FIGs. 1A- 1C and 2A-2C, and it is appreciated that any combination of substitutions at each position is contemplated herein. In some instances, one or more amino acid substitutions are introduced into a Vernier zone residue, including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30) of Chothia canonical amino acid positions 2, 27-30, 47-49, 67, 69, 71, 73, 78, 93, 94, and/or 103 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 2, 4, 35, 36, 46-49, 64, 66, 68, 69, 71, and/or 98 of the human IgG1 light chain. Exemplary Vernier zone residue positions and substitutions are shown in FIGs. 1A-1C and 2A-2C, and it is appreciated that any combination of substitutions at each position is contemplated herein. In some instances, one or more amino acid substitutions are introduced to generate a backmutation, including substitutions at one or more (e.g., 2, 3, or 4) of Chothia canonical amino acid positions 19, 44, and/or 83 of the human IgG1 heavy chain and/or at Chothia canonical amino acid position 18 of the human IgG1 light chain. It is appreciated that any combination of substitutions at each position is contemplated herein. In some instances, one or more amino acid substitutions are deimmunizing mutation(s), including substitutions at one or more (e.g., 2, 3, 4, 5, 6, 7, or 8) of Chothia canonical amino acid positions 43, 44, 49, 74, 77, and/or 91 of the human IgG1 heavy chain and/or at Chothia canonical amino acid positions 3 and/or 100 of the human IgG1 light chain. It is appreciated that any combination of substitutions at each position is contemplated herein. In some instances, the humanized antibody includes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some embodiments, the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is a positively charged amino acid. In some instances, the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is a negatively charged amino acid. The positively and negatively charged amino acids form an interchain salt bridge. In some instances, the positively charged amino acid is a basic amino acid. In some instances, the negatively charged amino acid is an acidic amino acid. In some instances, the positively charged amino acid is an arginine. In some instances, the negatively charged amino acid is an aspartic acid. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT In some instances, the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative comprises a hydroxy-substituted phenyl group. In some instances, the substituted phenylalanine derivative is a tyrosine residue. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain is a glutamine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. In some instances, the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: a serine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT chain; and a serine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. In some instances, the humanized antibody further comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the heavy chain variable domain includes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the light chain variable domain includes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the heavy chain variable domain includes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain includes a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the humanized antibody includes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, and a light chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative comprises a hydroxy-substituted phenyl group. In some instances, the substituted phenylalanine derivative is a tyrosine. In some instances, the polar uncharged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. In some instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain variable domain, is a glutamine. In some instances, the humanized antibody further comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid is a basic amino acid. In some instances, the negatively charged amino acid is an acidic amino acid. In some instances, the positively charged amino acid is an arginine. In some instances, the negatively charged amino acid is an aspartic acid. In some instances, the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. In some instances, the humanized antibody includes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged residue at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is an aspartic acid. In some instances, the humanized antibody further comprises one or both of: an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the humanized antibody includes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. In some instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, is a serine. In some instances, the humanized antibody includes a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the light chain variable domain comprises an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. In some instances, the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. In some instances, the heavy chain variable region comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. In some instances, the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. In some instances, the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. In some Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT instances, the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain is a serine. In some instances, the humanized antibody further comprises at least one (e.g., two or three) of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. In some instances, the humanized antibody further comprises at least one (e.g., two, three, or four) of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. In some instances, the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, forms a hydrogen bond interaction with at least one of an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond interaction with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. In some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain comprises a hydroxy-substituted phenyl group. In Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT some instances, the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, is a tyrosine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain is a glutamine. In some instances, the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. In some instances, the antibody is an antigen-binding antibody fragment. In some instances, the antigen-binding antibody fragment is a Fab fragment, a Fab’2 fragment, or a single chain Fv. In some instances, the humanized antibody is a full antibody, a chimeric antibody, a veneered antibody, a CDR-grafted antibody, or a recombinant antibody. The isotype of the antibody from which the Fc region is originated is not particularly limited, and an Fc region originated from an IgG1, IgG2, IgG3, or IgG4 monoclonal antibody can be appropriately used. In some instances, the humanized antibody is a human IgG antibody. In some instances, the human IgG antibody has a human IgG1 isotype. In some instances, the antibody is conjugated to a therapeutic, cytotoxic, cytostatic, immunomodulatory, neurotrophic, or neuroprotective agent. In some instances, the heavy chain variable domain is fused to a heavy chain constant region and the light chain variable domain is fused to a light chain constant region. In some instances, the heavy chain constant region is a mutant form of a natural human heavy chain constant region which has reduced binding to an Fcγ receptor relative to the natural heavy chain constant region. In some instances, the heavy chain constant region is of IgG1 isotype. In some instances, the antibody has at least one mutation in a constant region. In some instances, the at least one mutation reduces complement fixation or activation by the constant region. In some instances, the at least one mutation is at one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, or 11) positions of: 241, 264, 265, 270, 296, 297, 318, 320, 322, 329, and 331 by EU numbering. In some instances, the humanized antibody has an alanine at positions 318, 320, and 322 by EU numbering. Additional representative heavy and light chain variable domain sequences are provided in Tables 2 and 3, respectively. Table 2. Heavy Chain Variable Domain Sequences Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT SEQ ID NO Name Sequence EVQLVESGGGLVQPGGSLKLSCAASGFTFSNYFMSWVRQTPEKRLEWVAYIST S Q Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT EVQLVESGGGLVQPGGSLRLSCAASGFTFSNYFMGWVRQAPGEDL 16 h13D3VHv13 EWVAYISTGGDSANYADNVKGRFTISRDNAKNTGYLQMNSLRAED abe 3. g t C an Varabe oman Sequences SEQ ID NO Name Sequence Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT DVVMTQSPSSLPVTLGQPASISCRSSQSLVHSNGKTYLHWYQQRP 30 h13D3VLv7 GQSPRLLIYKVSDRYSGVPDRFSGSGSGTDFTLKISRVESEDVGV In some instances, the humanized antibody has increased thermal stability as compared to a reference antibody. The increased thermal stability comprises an increase (e.g., at least a 5% increase, at least a 10% increase, at least a 15% increase, at least a 20% increase, at least a 25% increase, at least a 30% increase, at least a 35% increase, at least a 40% increase, at least a 45% increase, or at least a 50% increase, or a 1% increase to a 50% increase, a 1% increase to a 40% increase, a 1% increase to a 30% increase, a 1% increase to a 20% increase, a 1% increase to a 10% increase, a 1% increase to a 5% increase, a 5% increase to a 50% increase, a 5% increase to a 40% increase, a 5% increase to a 30% increase, a 5% increase to a 20% increase, a 5% increase to a 10% increase, a 10% increase to a 50% increase, a 10% increase to a 40% increase, a 10% increase to a 30% increase, a 10% increase to a 20% increase, a 20% increase to a 50% increase, a 20% increase to a 40% increase, a 20% increase to a 30% increase, a 30% increase to a 50% increase, a 30% increase to a 40% increase, or a 40% increase to a 50% increase) in expression yield compared to the reference antibody (e.g., a similar antibody not including one or more of the amino acid substitutions described herein). In some instances, the increased thermal stability comprises an increase in expression yield of at least 100 mg/L compared to the reference antibody. In some instances, the increase in thermal stability comprises an increase in melting point as compared to the reference antibody (e.g., a similar antibody not including one or more of the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT amino acid substitutions described herein). In some instances, the increase in melting point is an increase of at least 2 °C, at least 5 °C, or at least 10 °C as compared to the reference antibody. In some instances, the humanized antibody has a melting temperature of 55 °C or greater. In some instances, the humanized antibody has a melting temperature of 65°C or greater. In some instances, the humanized antibody has a melting temperature of 70°C or greater. In some embodiments, the humanized antibody has a melting temperature of about 55 °C to about 75 °C, about 55 °C to about 70 °C, about 55 °C to about 65 °C, about 55 °C to about 60 °C, about 60 °C to about 75 °C, about 60 °C to about 70 °C, about 60 °C to about 65 °C, about 65 °C to about 75 °C, about 65 °C to about 70 °C, about 70 °C to about 75 °C, or about 70 °C to about 80 °C. In some instances, the melting temperature is determined by differential scanning calorimetry. In some instances, the humanized antibody has a decreased immunogenicity score as compared to a reference antibody. In some instances, the humanized antibody has a decreased immunogenicity as compared to a reference antibody. C. Generation of Parent Antibodies Methods for generating a parent antibody that can be used as the starting point for further humanization using any of the methods described herein, are known in the art. For example, an animal can be immunized with an antigen and optionally, a heterologous carrier and/or adjuvant. Suitable carriers that can be mixed with the antigen prior to immunization include serum albumins, keyhole limpet hemocyanin, immunoglobulin molecules, thyroglobulin, ovalbumin, tetanus toxoid, or a toxoid from other pathogenic bacteria, such as diphtheria (e.g., CRM197), E. coli, cholera, or H. pylori, or an attenuated toxin derivative. T cell epitopes are also suitable carrier molecules. Some conjugates can be formed by linking an antigen to an immunostimulatory polymer molecule (e.g., tripalmitoyl-S-glycerine cysteine (Pam3Cys), mannan (a mannose polymer), or glucan (a β 1 ^2 polymer)), cytokines (e.g., IL-1, IL-1 alpha and β peptides, IL-2, γ-INF, IL-10, GM-CSF), and chemokines (e.g., MIP1-α and β, and RANTES). Immunogens may be linked to the carriers with or without spacers amino acids (e.g., gly-gly). Additional carriers include virus-like particles. Virus-like particles (VLPs), also called pseudovirions or virus-derived particles, represent subunit structures composed of multiple copies of a viral capsid and/or envelope protein capable of self-assembly into VLPs of defined spherical symmetry in vivo. (Powilleit et al., (2007) PLoS ONE 2(5):e415.) Alternatively, Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT peptide immunogens can be linked to at least one artificial T-cell epitope capable of binding a large proportion of MHC Class II molecules., such as the pan DR epitope ("PADRE"). PADRE is described in US 5,736,142, WO 95/07707, and Alexander et al., Immunity 1:751-761, 1994. Active immunogens can be presented in multimeric form in which multiple copies of an immunogen and/or its carrier are presented as a single covalent molecule. [0001] Fragments are often administered with pharmaceutically acceptable adjuvants. The adjuvant increases the titer of induced antibodies and/or the binding affinity of induced antibodies relative to the situation if the peptide were used alone. A variety of adjuvants can be used in combination with an immunogenic fragment to elicit an immune response. Some adjuvants augment the intrinsic response to an immunogen without causing conformational changes in the immunogen that affect the qualitative form of the response. Some adjuvants include aluminum salts, such as aluminum hydroxide and aluminum phosphate, 3 De-O-acylated monophosphoryl lipid A (MPLTM) (see GB 2220211 (RIBI ImmunoChem Research Inc., Hamilton, Montana, now part of Corixa). StimulonTM QS-21 is a triterpene glycoside or saponin isolated from the bark of the Quillaja Saponaria Molina tree found in South America (see Kensil et al., in Vaccine Design: The Subunit and Adjuvant Approach (eds. Powell & Newman, Plenum Press, NY, 1995); US 5,057,540), (Aquila BioPharmaceuticals, Framingham, MA; now Antigenics, Inc., New York, NY). Other adjuvants are oil in water emulsions (such as squalene or peanut oil), optionally in combination with immune stimulants, such as monophosphoryl lipid A (see Stoute et al., N. Engl. J. Med. 336:86-91 (1997)), pluronic polymers, and killed mycobacteria. Ribi adjuvants are oil-in-water emulsions. Ribi contains a metabolizable oil (squalene) emulsified with saline containing Tween 80. Ribi also contains refined mycobacterial products which act as immunostimulants and bacterial monophosphoryl lipid A. Another adjuvant is CpG (WO 98/40100). Adjuvants can be administered as a component of a therapeutic composition with an active agent or can be administered separately, before, concurrently with, or after administration of the therapeutic agent. Peptides (and optionally a carrier fused to the peptide) can also be administered in the form of a nucleic acid encoding the peptide and expressed in situ in a subject. A nucleic acid segment encoding an immunogen is typically linked to regulatory elements, such as a promoter and enhancer that allow expression of the DNA segment in the intended target cells of a subject. For expression in blood cells, as is desirable for induction of an immune response, promoter and Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT enhancer elements from light or heavy chain immunoglobulin genes or the CMV major intermediate early promoter and enhancer are suitable to direct expression. The linked regulatory elements and coding sequences are often cloned into a vector. Antibodies can also be administered in the form of nucleic acids encoding the antibody heavy and/or light chains. If both heavy and light chains are present, the chains are preferably linked as a single chain antibody. The DNA can be delivered in naked form (i.e., without colloidal or encapsulating materials). Alternatively, a number of viral vector systems can be used including retroviral systems (see, e.g., Lawrie and Tumin, Cur. Opin. Genet. Develop. 3:102-109 (1993)) including retrovirus derived vectors such MMLV, HIV-1, and ALV; adenoviral vectors (see, e.g., Bett et al., J. Virol. 67, 5911 (1993)); adeno-associated virus vectors (see, e.g., Zhou et al., J. Exp. Med. 179:1867 (1994)), lentiviral vectors such as those based on HIV or FIV gag sequences, viral vectors from the pox family including vaccinia virus and the avian pox viruses, viral vectors from the alpha virus genus such as those derived from Sindbis and Semliki Forest Viruses (see, e.g., Dubensky et al., J. Virol. 70:508-519 (1996)), Venezuelan equine encephalitis virus (see US 5,643,576) and rhabdoviruses, such as vesicular stomatitis virus (see WO 96/34625) and papillomaviruses (Ohe et al., Human Gene Therapy 6:325-333 (1995); Woo et al., WO 94/12629 and Xiao & Brandsma, Nucleic Acids. Res. 24:2630-2622 (1996)). DNA encoding an immunogen, or encoding the antibody heavy and/or light chains, or a vector containing the same, can be packaged into liposomes. Suitable lipids and related analogs are described by US 5,208,036, US 5,264,618, US 5,279,833, and US 5,283,185. Vectors and DNA encoding an immunogen or encoding the antibody heavy and/or light chains can also be adsorbed to or associated with particulate carriers, examples of which include polymethyl methacrylate polymers and polylactides and poly(lactide-co-glycolides), (see, e.g., McGee et al., J. Micro Encap. 1996). Vectors or segments therefrom encoding the antibody heavy and/or light chains can be incorporated in cells ex vivo, for example to cells explanted from an individual patient (e.g., lymphocytes, bone marrow aspirates, tissue biopsy) or universal donor hematopoietic stem cells, followed by reimplantation of the cells into a patient, usually after selection for cells which have incorporated the transgenes. (See, e.g., WO 2017/091512). Exemplary patient-derived cells Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT include patient derived induced pluripotent stem cells (iPSCs) or other types of stem cells (embryonic, hematopoietic, neural, or mesenchymal). A vector or segment therefrom encoding the antibody heavy and/or light chains can be introduced into any region of interest in cells ex vivo, such as an albumin gene or other safe harbor gene. Cells incorporating the vector can be implanted with or without prior differentiation. Cells can be implanted into a specific tissue, such as a secretory tissue or a location of pathology, or systemically, such as by infusion into the blood. For example, cells can be implanted into a secretory tissue of a patient, such as the liver, optionally with prior differentiation to cells present in that tissue, such as hepatocytes in the case of a liver. Expression of the antibody in the liver results in secretion of the antibody to the blood. D. Humanized Antibody Screening Assays Humanized antibodies can be initially screened for the intended binding specificity as described above. Active immunogens can likewise be screened for capacity to induce antibodies with such binding specificity. In this case, an active immunogen is used to immunize a laboratory animal and the resulting sera tested for the appropriate binding specificity. Antibodies having the desired binding specificity can then be tested in cellular and animal models. Active immunogens can also be tested for induction of antibodies in the sera. Both passive and active immunogens can be tested for passage of antibodies across the blood brain barrier into the brain of a wild type or transgenic animal. Antibodies or fragments inducing an antibody can also be tested in non-human primates without disease or that naturally or through induction develop symptoms of diseases characterized by changes in antigen levels. Tests on an antibody or active agent are usually performed in conjunction with a control in which a parallel experiment is conduct except that the antibody or active agent is absent (e.g., replaced by vehicle). Reduction, delay or inhibition of signs or symptoms disease attributable to an antibody or active agent under test can then be assessed relative to the control. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT E. Patients Amenable to Treatment Changes in antigen levels have been found in several diseases. The present regimen can also be used in treatment or prophylaxis of any of these diseases associated with the antigen specifically bound by the humanized antibodies provided herein. Patients amenable to treatment include individuals at risk of disease but not showing symptoms, as well as patients presently showing symptoms. Patients at risk of disease include those having a known genetic risk of disease. Such individuals include those having relatives who have experienced this disease, and those whose risk is determined by analysis of genetic or biochemical markers. In asymptomatic patients, treatment can begin at any age (e.g., 10, 20, 30). Usually, however, it is not necessary to begin treatment until a patient reaches 40, 50, 60 or 70 years of age. Treatment typically entails multiple dosages over a period of time. Treatment can be monitored by assaying antibody levels over time. If the response falls, a booster dosage is indicated. F. Nucleic Acids, Host Cells, and Methods of Producing the Same The disclosure further provides nucleic acids encoding any of the heavy and light chains described in Tables 2 and 3 (e.g., encoding any one of SEQ ID NOs: 1-35). Optionally, such nucleic acids further encode a signal peptide and can be expressed with the signal peptide linked to the variable region. Coding sequences of nucleic acids can be operably linked with regulatory sequences to ensure expression of the coding sequences, such as a promoter, enhancer, ribosome binding site, transcription termination signal, and the like. The regulatory sequences can include a promoter, for example, a prokaryotic promoter or a eukaryotic promoter. The nucleic acids encoding heavy or light chains can be codon-optimized for expression in a host cell. The nucleic acids encoding heavy and light chains can encode a selectable gene. The nucleic acids encoding heavy and light chains can occur in isolated form or can be cloned into one or more vectors. The nucleic acids can be synthesized by, for example, solid state synthesis or PCR of overlapping oligonucleotides. Nucleic acids encoding heavy and light chains can be joined as one contiguous nucleic acid, e.g., within an expression vector, or can be separate, e.g., each cloned into its own expression vector. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT In some instances, the nucleic acid encodes for any of the heavy chain variable domain sequences and/or light chain variable domain humanized sequences described herein. Also disclosed herein are vectors. In some instances, the vector includes a nucleic acid encoding for any of the heavy chain variable domain sequences disclosed herein. In some instances, the vector includes a nucleic acid encoding for any of the light chain variable domain sequences disclosed herein. A vector can be operably linked to one or more regulatory sequences to effect expression in a mammalian cell of the humanized antibody disclosed herein. In some instances, the vector can include one or more regulatory sequences that include one or more of an enhancer, a ribosome binding site, a transcription termination signal, and a promoter. A promoter can be a eukaryotic promoter. Examples of the vectors include M13 series vectors, pUC series vectors, pBR322, pBluescript, and pCR-Script. In addition to these vectors, for example, pGEM-T, pDIRECT, or pT7 can also be used for the purpose of cDNA subcloning and excision. In some instances, the nucleic acid is codon-optimized for expression in a host cell. In the case of using eukaryotic cells as the host cells, animal cells or fungus cells can be appropriately used. Specifically, examples of the animal cells can include the following cells: (1) mammalian cells such as CHO (Chinese hamster ovary cell line), COS (monkey kidney cell line), myeloma cells (Sp2/O, NS0, etc.), BHK (baby hamster kidney cell line), HEK293 (human embryonic kidney cell line with sheared adenovirus (Ad)5 DNA), PER.C6 cell (human embryonic retinal cell line transformed with the adenovirus type 5 (Ad5) E1A and E1B genes), Hela, and Vero (Current Protocols in Protein Science (May, 2001, Unit 5.9, Table 5.9.1)); (2) amphibian cells such as Xenopus oocytes; and (3) insect cells such as sf9, sf21, and Tn5. The antibody can also be prepared using E. coli (mAbs 2012 Mar-Apr; 4 (2): 217-225) or yeast (WO2000023579). The antibody prepared using E. coli is not glycosylated. On the other hand, the antibody prepared using yeast is glycosylated. In some instances, the DNA encoding all or part of the humanized antibody of interest is integrated to expression vectors so as to be expressed under the control of expression control regions, for example, an enhancer and a promoter. Next, host cells are transformed with the resulting expression vectors and allowed to express antibodies. In this case, appropriate hosts and expression vectors can be used in combination. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT The humanized antibody can be recovered, for example, by culturing the transformed cells and then separating the antibody from within the molecule-transformed cells or from the culture solution thereof. The antibody can be separated and purified by appropriately using in combination methods such as centrifugation, ammonium sulfate fractionation, salting out, ultrafiltration, C1q, FcRn, protein A and protein G columns, affinity chromatography, ion- exchanged chromatography, and gel filtration chromatography. For example, a production method can include the steps of: (i) preparing a library of humanized antibodies; (ii) selecting, from the prepared library, a humanized antibody having one or more substitutions as described herein; (iii) culturing a host cell comprising a nucleic acid encoding all or part of the humanized antibody selected in the step (ii); and (iv) recovering the humanized antibody from the host cell culture. The humanized antibody may be antibody fragments such as Fv, Fab, or Fab' or may be Fc region-containing antibodies. G. Conjugated Antibodies In some embodiments, humanized antibodies can be conjugated with other therapeutic moieties, other proteins, other antibodies, and/or detectable labels. See WO 03/057838; US 8,455,622. Such therapeutic moieties can be any agent that can be used to treat, combat, ameliorate, prevent, or improve an unwanted condition or disease in a patient, such as disease or disorder associated with the antigen recognized by the humanized antibody. In some embodiments, the methods further comprise detecting, measuring, and/or monitoring immunogenicity levels and/or the antigen levels in a subject. Conjugated therapeutic moieties can include, neurotrophic agents, neuroprotective agents, radiotherapeutic agents, radioactive (radiopharmaceuticals), fluorescent, paramagnetic tracers, ultrasound contrast agents, immunomodulators, or any biologically active agents that facilitate or enhance the activity of the antibody, or modify bioavailability, and distribution in the body or within organs. A neurotrophic agent can be any agent, including chemical or proteinaceous agents, which promotes neuron maintenance, growth, or differentiation. A neuroprotective agent can be agent, including chemical or proteinaceous agents, which protects neurons from acute insult or degenerative processes. An immunomodulator can be any agent that stimulates or inhibits the development or maintenance of an immunologic response. A radiotherapeutic agent can be any molecule or compound that emits radiation. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Some such antibodies can be modified to act as immunotoxins. See, e.g., U.S. Patent No. 5,194,594. For example, ricin, a cellular toxin derived from plants, can be coupled to antibodies by using the bifunctional reagents S-acetylmercaptosuccinic anhydride for the antibody and succinimidyl 3-(2-pyridyldithio)propionate for ricin. See Pietersz et al., Cancer Res. 48(16):4469-4476 (1998). The coupling results in loss of B-chain binding activity of ricin, while impairing neither the toxic potential of the A-chain of ricin nor the activity of the antibody. Similarly, saporin, an inhibitor of ribosomal assembly, can be coupled to antibodies via a disulfide bond between chemically inserted sulfhydryl groups. See Polito et al., Leukemia 18:1215-1222 (2004). Some such antibodies can be linked to radioisotopes. Examples of radioisotopes include, for example, yttrium90 (90Y), indium111 (111In),131I,99mTc, radiosilver-111, radiosilver-199, and Bismuth213. Linkage of radioisotopes to antibodies may be performed with conventional bifunction chelates. For radiosilver-111 and radiosilver-199 linkage, sulfur-based linkers may be used. See Hazra et al., Cell Biophys. 24-25:1-7 (1994). Linkage of silver radioisotopes may involve reducing the immunoglobulin with ascorbic acid. For radioisotopes such as 111In and 90Y, ibritumomab tiuxetan can be used and will react with such isotopes to form 111In- ibritumomab tiuxetan and 90Y-ibritumomab tiuxetan, respectively. See Witzig, Cancer Chemother. Pharmacol., 48 Suppl 1:S91-S95 (2001). Some such antibodies can be linked to other therapeutic moieties. Such therapeutic moieties can be, for example, cytotoxic, cytostatic, neurotrophic, or neuroprotective. For example, antibodies can be conjugated with toxic chemotherapeutic drugs such as maytansine, geldanamycin, tubulin inhibitors such as tubulin binding agents (e.g., auristatins), or minor groove binding agents such as calicheamicin. Other representative therapeutic moieties include agents known to be useful for treatment, management, or amelioration of a disease or disorder associated with changes in immunogenicity. Antibodies can also be coupled with other proteins. For example, antibodies can be coupled with Fynomers. Fynomers are small binding proteins (e.g., 7 kDa) derived from the human Fyn SH3 domain. They can be stable and soluble, and they can lack cysteine residues and disulfide bonds. Fynomers can be engineered to bind to target molecules with the same affinity and specificity as antibodies. They are suitable for creating multi-specific fusion proteins based on antibodies. For example, Fynomers can be fused to N-terminal and/or C- Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT terminal ends of antibodies to create bi- and tri-specific FynomAbs with different architectures. Fynomers can be selected using Fynomer libraries through screening technologies using FACS, Biacore, and cell-based assays that allow efficient selection of Fynomers with optimal properties. Examples of Fynomers are disclosed in Grabulovski et al., J. Biol. Chem. 282:3196-3204 (2007); Bertschinger et al., Protein Eng. Des. Sel. 20:57-68 (2007); Schlatter et al., MAbs. 4:497-508 (2011); Banner et al., Acta. Crystallogr. D. Biol. Crystallogr. 69(Pt6):1124-1137 (2013); and Brack et al., Mol. Cancer Ther. 13:2030-2039 (2014). The antibodies disclosed herein can also be coupled or conjugated to one or more other antibodies (e.g., to form antibody heteroconjugates). Antibodies can also be coupled with a detectable label. Such antibodies can be used in a subject having or being treated for a disease or disorder associated with changes in immunogenicity. Such antibodies are particularly useful for performing such determinations in subjects having or being susceptible to of a disease or disorder associated with changes in immunogenicity. Representative detectable labels that may be coupled or linked to an antibody include various enzymes, such as horseradish peroxidase, alkaline phosphatase, beta- galactosidase, or acetylcholinesterase; prosthetic groups, such streptavidin/biotin and avidin/biotin; fluorescent materials, such as umbelliferone, fluorescein, fluorescein isothiocyanate, rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride or phycoerythrin; luminescent materials, such as luminol; bioluminescent materials, such as luciferase, luciferin, and aequorin; radioactive materials, such as radiosilver-111, radiosilver-199, Bismuth213, iodine (131I,125I,123I,121I,), carbon (14C), sulfur (5S), tritium (3H), indium (115In,113In,112In,111In,), technetium (99Tc), thallium (201Ti), gallium (68Ga,67Ga), palladium (103Pd), molybdenum (99Mo), xenon (133Xe), fluorine (18F),153Sm,177Lu,159Gd,149Pm,140La,175Yb,166Ho,90Y,47Sc,186Re,188Re,142Pr,105Rh,97Ru,68Ge,57Co,65Zn,85Sr,32P,153Gd,169Yb,51Cr,54Mn,75Se,113Sn, and paramagnetic metal ions; and molecules that are radiolabelled or conjugated to specific radioisotopes. Linkage of radioisotopes to antibodies may be performed with conventional bifunction chelates. For radiosilver-111 and radiosilver-199 linkage, sulfur-based linkers may be used. See Hazra et al., Cell Biophys. 24-25:1-7 (1994). Linkage of silver radioisotopes may involve reducing the immunoglobulin with ascorbic acid. For radioisotopes such as 111In and 90Y, Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT ibritumomab tiuxetan can be used and will react with such isotopes to form 111In-ibritumomab tiuxetan and 90Y-ibritumomab tiuxetan, respectively. See Witzig, Cancer Chemother. Pharmacol., 48 Suppl 1:S91-S95 (2001). Therapeutic moieties, other proteins, other antibodies, and/or detectable labels may be coupled or conjugated, directly or indirectly through an intermediate (e.g., a linker), to an antibody of the disclosure. See, e.g., Arnon et al., “Monoclonal Antibodies For Immunotargeting Of Drugs In Cancer Therapy,” in Monoclonal Antibodies And Cancer Therapy, Reisfeld et al. (eds.), pp. 243-56 (Alan R. Liss, Inc. 1985); Hellstrom et al., “Antibodies For Drug Delivery,” in Controlled Drug Delivery (2nd Ed.), Robinson et al. (eds.), pp. 623-53 (Marcel Dekker, Inc. 1987); Thorpe, “Antibody Carriers Of Cytotoxic Agents In Cancer Therapy: A Review,” in Monoclonal Antibodies 84: Biological And Clinical Applications, Pinchera et al. (eds.), pp. 475- 506 (1985); “Analysis, Results, And Future Prospective Of The Therapeutic Use Of Radiolabeled Antibody In Cancer Therapy,” in Monoclonal Antibodies For Cancer Detection And Therapy, Baldwin et al. (eds.), pp. 303-16 (Academic Press 1985); and Thorpe et al., Immunol. Rev., 62:119-58 (1982). Suitable linkers include, for example, cleavable and non- cleavable linkers. Different linkers that release the coupled therapeutic moieties, proteins, antibodies, and/or detectable labels under acidic or reducing conditions, on exposure to specific proteases, or under other defined conditions can be employed. H. Pharmaceutical Compositions, Methods of Use, and Methods of Reducing Immunogenicity and/or Increasing Thermostability of a Parent Antibody Disclosed herein are pharmaceutical compositions that include a humanized antibody disclosed herein and a pharmaceutically acceptable carrier. In some instances, a humanized antibody or agent for inducing an antibody or a pharmaceutical composition the same is administered to a patient susceptible to, or otherwise at risk of a disease in regimen (dose, frequency and route of administration) effective to reduce the risk, lessen the severity, or delay the onset of at least one sign or symptom of the disease (e.g., a disease associated with the antigen specifically bound by the humanized antibody). In therapeutic applications, a humanized antibody or agent to induce an antibody is administered to a patient suspected of, or already suffering from a disease (e.g., a disease associated with the antigen specifically bound by the humanized antibody) in a regimen (dose, frequency and route of administration) effective to Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT ameliorate or at least inhibit further deterioration of at least one sign or symptom of the disease. In particular, the regimen is preferably effective to decrease the level of antigen in the subject or treat a subject having a disease associated with the antigen specifically bound by the humanized antibody. In some instances, disclosed herein are methods of reducing immunogenicity of a parent antibody by introducing amino acid substitution(s) into the parent antibody to produce a modified antibody. In some instances, the modified antibody includes one or more of (i) a heavy chain variable domain comprising one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14) of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. In some instances, disclosed herein are methods of increasing thermal stability of an antibody by introducing by introducing amino acid substitution(s) into the parent antibody to Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14) of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. Also provided herein are methods of reducing immunogenicity of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has a reduction in immunogenicity. In some instances, the method further includes introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the method further includes introducing at least one (e.g., 2, 3, 4, or 5) of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the modified antibody has a melting temperature of 65 °C or greater (e.g., 75 °C or greater, or 80 °C or greater). In some instances, the methods described herein can further include introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has increase in melting temperature of 2 °C or greater (e.g., 5 °C or greater, or 10 °C or greater) compared to the parent antibody as measured by differential scanning calorimetry. In some instances, the method further includes introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. In some instances, the method further includes introducing at least one (e.g., 2, 3, 4, or 5) of the following amino acid substitutions into a parent antibody: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. In some instances, the modified antibody has increase in melting temperature of 5°C or greater (e.g., 10 °C or greater, or 15 °C or greater) compared to the parent antibody as measured by differential scanning calorimetry In some instances, the modified antibody has a melting temperature of 65°C or greater (e.g., 70 °C or greater, or 75 °C or greater). In some instances, the modified antibody has an immunogenicity score of 5 or less, 4 or less, 3 or less, 2 or less, or 1 or less. A regimen is considered therapeutically or prophylactically effective if an individual treated patient achieves an outcome more favorable than the mean outcome in a control population of comparable patients not treated by methods of the disclosure, or if a more favorable outcome is demonstrated in treated patients versus control patients in a controlled clinical trial (e.g., a phase II, phase II/III or phase III trial) at the p < 0.05 or 0.01 or even 0.001 level. Effective doses of vary depending on many different factors, such as means of administration, target site, physiological state of the patient, whether the patient is human or an animal, other medications administered, and whether treatment is prophylactic or therapeutic. Exemplary dosage ranges for humanized antibodies are from about 0.01 to 60 mg/kg, or from about 0.1 to 3 mg/kg or 0.15-2 mg/kg or 0.15-1.5 mg/kg, of patient body weight. Antibody can be administered such doses daily, on alternative days, weekly, fortnightly, monthly, quarterly, or according to any other schedule determined by empirical analysis. An exemplary treatment entails administration in multiple dosages over a prolonged period, for example, of at least six months. Additional exemplary treatment regimen entail administration once per every two weeks or once a month or once every 3 to 6 months. The amount of an agent for active administration varies from 0.1-500 ^g per patient and more usually from 1-100 or 1-10 ^g per injection for human administration. The timing of injections can vary significantly from once a day, to once a year, to once a decade. A typical regimen consists of an immunization followed by booster injections at time intervals, such as 6 week intervals or two months. Another regimen consists of an immunization followed by Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT booster injections 1, 2 and 12 months later. Another regimen entails an injection every two months for life. Alternatively, booster injections can be on an irregular basis as indicated by monitoring of immune response. Antibodies or agents for inducing antibodies are preferably administered via a peripheral route (i.e., one in which an administered or induced antibody crosses the blood brain barrier to reach an intended site in the brain.) Routes of administration include topical, intravenous, oral, subcutaneous, intraarterial, intracranial, intrathecal, intraperitoneal, intranasal, intraocular, intradermal, or intramuscular. Some routes for administration of antibodies are intravenous and subcutaneous. Some routes for active immunization are subcutaneous and intramuscular. This type of injection is most typically performed in the arm or leg muscles. In some methods, agents are injected directly into a particular tissue where deposits have accumulated, for example intracranial injection. Pharmaceutical compositions for parenteral administration are preferably sterile and substantially isotonic and manufactured under GMP conditions. Pharmaceutical compositions can be provided in unit dosage form (i.e., the dosage for a single administration). Pharmaceutical compositions can be formulated using one or more physiologically acceptable carriers, diluents, excipients or auxiliaries. The formulation depends on the route of administration chosen. For injection, antibodies can be formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hank’s solution, Ringer’s solution, or physiological saline or acetate buffer (to reduce discomfort at the site of injection). The solution can contain formulatory agents such as suspending, stabilizing and/or dispersing agents. Alternatively, antibodies can be in lyophilized form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use. The present regimen can be administered in combination with another agent effective in treatment or prophylaxis of the disease being treated. Antibodies are administered in an effective regimen meaning a dosage, route of administration and frequency of administration that delays the onset, reduces the severity, inhibits further deterioration, and/or ameliorates at least one sign or symptom of a disorder being treated. If a patient is already suffering from a disorder, the regimen can be referred to as a therapeutically effective regimen. If the patient is at elevated risk of the disorder relative to the general population but is not yet experiencing symptoms, the regimen can be referred to as a Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT prophylactically effective regimen. In some instances, therapeutic or prophylactic efficacy can be observed in an individual patient relative to historical controls or past experience in the same patient. In other instances, therapeutic or prophylactic efficacy can be demonstrated in a preclinical or clinical trial in a population of treated patients relative to a control population of untreated patients. Administration can be parenteral, intravenous, oral, subcutaneous, intra-arterial, intracranial, intrathecal, intraperitoneal, topical, intranasal or intramuscular. Some antibodies can be administered into the systemic circulation by intravenous or subcutaneous administration. Intravenous administration can be, for example, by infusion over a period such as 30-90 min. The frequency of administration depends on the half-life of the antibody in the circulation, the condition of the patient and the route of administration among other factors. The frequency can be daily, weekly, monthly, quarterly, or at irregular intervals in response to changes in the patient’s condition or progression of the disorder being treated. An exemplary frequency for intravenous administration is between weekly and quarterly over a continuous cause of treatment, although more or less frequent dosing is also possible. For subcutaneous administration, an exemplary dosing frequency is daily to monthly, although more or less frequent dosing is also possible. I. Diagnostics and Monitoring Methods Also provided are methods of detecting the presence of an antigen specifically bound by the humanized antibody in a subject. Such methods are useful to diagnose or confirm diagnosis of diseases associated with the antigen recognized by the antibody specifically bound by the humanized antibody. The methods can also be used on asymptomatic subjects. The methods are also useful for monitoring disease progression and/or response to treatment in subjects who have been previously diagnosed with a disease associated with the antigen specifically bound by the humanized antibody. Diagnostic reagents can be administered by intravenous injection into the body of the patient, or directly into the brain by intracranial injection or by drilling a hole through the skull. The dosage of reagent should be within the same ranges as for treatment methods. Typically, the reagent is labeled, although in some methods, the primary reagent is unlabeled and a secondary labeling agent is used to bind to the primary reagent. The choice of label depends on the means Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT of detection. For example, a fluorescent label is suitable for optical detection. Use of paramagnetic labels is suitable for tomographic detection without surgical intervention. Radioactive labels can also be detected using positron emission tomography (PET) or single- photon emission computed tomography (SPECT). Diagnosis is performed by comparing the number, size, and/or intensity of labeled loci to corresponding base line values. The base line values can represent the mean levels in a population of normal individuals. Base line values can also represent previous levels determined in the same subject. For example, base line values can be determined in a subject before beginning treatment, and measured values thereafter compared with the base line values. In some patients, diagnosis of a disease or disorder associated with changes in antigen levels may be aided by performing a PET scan. A PET scan can be performed using, for example, a conventional PET imager and auxiliary equipment. The scan typically includes one or more regions of the tissue known in general to be associated with a disease or disorder associated with changes in antigen levels and one or more regions in which to serve as controls. In some methods, a PET scan is performed concurrent with or in the same patient visit as an MRI or CAT scan. An MRI or CAT scan provides more anatomical detail of the tissue than a PET scan. However, the image from a PET scan can be superimposed on an MRI or CAT scan image more precisely indicating the location of PET ligand. Some machines can perform both PET scanning and MRI or CAT scanning without the patient changing positions between the scans facilitating superimposition of images. Suitable PET ligands include radiolabeled antibodies of the disclosure. The radioisotope used can be, for example, C11, N13, O15, F18, or I123. The interval between administering the PET ligand and performing the scan can depend on the PET ligand and particularly its rate of uptake and clearing into the brain, and the half- life of its radiolabel. PET scans can also be performed as a prophylactic measure in asymptomatic patients or in patients who have mild symptoms but have not yet been diagnosed with a disease or disorder associated with changes in antigen levels but are at elevated risk of developing a disease or disorder associated with the antigen specifically bound by the humanized antibody. For asymptomatic patients, scans are particularly useful for individuals considered at elevated risk of a disease or disorder associated with the antigen specifically bound by the humanized antibody because of a family history, genetic or biochemical risk factors, or mature age. Prophylactic Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT scans can commence for example, at a patient age between 45 and 75 years. In some patients, a first scan is performed at age 50 years. Prophylactic scans can be performed at intervals of for example, between six months and ten years, preferably between 1-5 years. In some patients, prophylactic scans are performed annually. The methods can be used to monitor a course of therapeutic and prophylactic treatment with the agents provided herein. In some methods, a baseline measurement of antibody in the subject is made before administration, a second measurement is made soon thereafter to determine the peak antibody level, and one or more further measurements are made at intervals to monitor decay of antibody levels. When the level of antibody has declined to baseline or a predetermined percentage of the peak less baseline (e.g., 50%, 25% or 10%), administration of a further dose of antibody is administered. In some methods, peak or subsequent measured levels less background are compared with reference levels previously determined to constitute a beneficial prophylactic or therapeutic treatment regimen in other subjects. If the measured antibody level is significantly less than a reference level (e.g., less than the mean minus one or, preferably, two standard deviations of the reference value in a population of subjects benefiting from treatment) administration of an additional dose of antibody is indicated. J. Kits [0002] The disclosure further provides kits (e.g., containers) comprising any of the humanized antibodies disclosed herein and related materials, such as instructions for use (e.g., package insert). The instructions for use may contain, for example, instructions for administration of the humanized antibody and optionally one or more additional agents. The containers of humanized antibody may be unit doses, bulk packages (e.g., multi-dose packages), or sub-unit doses. Package insert refers to instructions customarily included in commercial packages of therapeutic products that contain information about the indications, usage, dosage, administration, contraindications and/or warnings concerning the use of such therapeutic products Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Kits can also include a second container comprising a pharmaceutically-acceptable buffer, such as bacteriostatic water for injection (BWFI), phosphate-buffered saline, Ringer's solution and dextrose solution. It can also include other materials desirable from a commercial and user standpoint, including other buffers, diluents, filters, needles, and syringes. K. Other Applications The humanized antibodies can be used for detecting the antigen specifically bound by the humanized antibody in the context of clinical diagnosis or treatment or in research. Binding of the humanized antibodies to the biological sample can be compared to binding of the antibodies to a control sample. The control sample and the biological sample can comprise cells of the same tissue origin. Control samples and biological samples can be obtained from the same individual or different individuals and on the same occasion or on different occasions. If desired, multiple biological samples and multiple control samples are evaluated on multiple occasions to protect against random variation independent of the differences between the samples. A direct comparison can then be made between the biological sample(s) and the control sample(s) to determine whether antibody binding the biological sample(s) is increased, decreased, or the same relative to antibody binding to the control sample(s). Increased binding of the antibody to the biological sample(s) relative to the control sample(s) indicates the presence of the antigen specifically bound by the antibody in the biological sample(s). In some instances, the increased antibody binding is statistically significant. Optionally, antibody binding to the biological sample is at least 1.5-fold, 2-fold, 3-fold, 4-fold, 5-fold, 10-fold, 20-fold, or 100-fold higher than antibody binding to the control sample. In some instances, multiple biological samples from the patient are evaluated on multiple occasions to establish both a baseline and measure of random variation independent of treatment. A therapeutic agent is then administered in a regimen. The regimen may include multiple administrations of the agent over a period of time. Optionally, binding of the antibodies is evaluated on multiple occasions in multiple biological samples from the patient, both to establish a measure of random variation and to show a trend in response to immunotherapy. The various assessments of antibody binding to the biological samples are then compared. If only two assessments are made, a direct comparison can be made between the two assessments to determine whether antibody binding has increased, decreased, or remained the same between the Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT two assessments. If more than two measurements are made, the measurements can be analyzed as a time course starting before treatment with the therapeutic agent and proceeding through the course of therapy. The antibodies can also be used as research reagents for laboratory research in detecting an antigen, or fragments thereof. In such uses, antibodies can be labeled with fluorescent molecules, spin-labeled molecules, enzymes, or radioisotopes, and can be provided in the form of kit with all the necessary reagents to perform the detection assay. The antibodies can also be used to purify the antigen, or binding partners of the antigen, e.g., by affinity chromatography. All patent filings, websites, other publications, accession numbers and the like cited above or below are incorporated by reference in their entirety for all purposes to the same extent as if each individual item were specifically and individually indicated to be so incorporated by reference. If different versions of a sequence are associated with an accession number at different times, the version associated with the accession number at the effective filing date of this application is meant. The effective filing date means the earlier of the actual filing date or filing date of a priority application referring to the accession number if applicable. Likewise, if different versions of a publication, website or the like are published at different times, the version most recently published at the effective filing date of the application is meant unless otherwise indicated. Any feature, step, element, embodiment, or aspect of the disclosure can be used in combination with any other unless specifically indicated otherwise. Although the present disclosure has been described in some detail by way of illustration and example for purposes of clarity and understanding, it will be apparent that certain changes and modifications may be practiced within the scope of the appended claims. EXAMPLES Example 1: Methods of Selecting and Producing making anti-TDP43 monoclonal antibody 13D3 Beginning with a murine sequence for monoclonal antibody 13D3 humanization, a search was made over the protein sequences in the Protein Data Bank (PDB) database (Deshpande et al, Nucleic Acids Res. 2005 Jan 1; 33(Database Issue): D233–D237) to find structures, which would provide a rough structural model of 13D3. The crystal structure of an antibody fab PDB code 5BK5 (Scally, et al., Nat Commun. 2017 Nov 16;8(1):1568), a human germline antibody, was Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT used for both Vh and Vk (i.e., VL) structure since it had good resolution (3.0 Å) and overall sequence similarity to 13D3 Vh and Vk, retaining the same canonical structures for the loops. The 5BK5 antibody is a human germline-derived antibody and has same canonical classes and belonging to human germline IGHV3-48’03 for heavy chain and IGKV2-30*02 for light chain. Therefore, 5BK5 variable heavy and variable light chain sequences were also used as human acceptor framework. Accordingly, the framework regions of 5BK5 VH and 5BK5 VL were chosen as the acceptor sequences for the CDRs of 13D3. A model of the 13D3 CDRs grafted onto the respective human frameworks for VH and VL was built and used as a guidance for further backmutations. The amino acid sequences consisting of 5BK5 VH human frameworks and 13D3 CDRs were designated hu13D3VHvd1 and the amino acid sequences consisting of 5BK5 VL human framework and 13D3 VL CDRs were designated hu13D3VLvd1. Additional versions of hu13D3-VH and hu13D3-VL were designed to enable assessment of various framework residues for their contributions to antigen binding, thermostability, developability (deamination, oxidation, N-glycosylation, proteolysis and aggregation) and immunogenicity. The positions considered for mutation include those under the following groups. ^ Positions that define the canonical CDR conformations (Martin (2010) Protein sequence and structure analysis of antibody variable domains. In: Kontermann R and Dübel S (eds). Antibody Engineering. Heidelberg, Germany: Springer International Publishing AG), ^ Positions that are within the Vernier zone (Foote and Winter (1992) Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol. 224(2):487- 99), ^ Positions that localize to the VH/VL domain interface (summarized in Léger and Saldanha (2000) Preparation of recombinant antibodies from immune rodent spleens and the design of their humanization by CDR grafting. In: Shepherd P and Dean C (eds). Monoclonal Antibodies: A Practical Approach. Oxford, UK: Oxford University Press), ^ Positions that are susceptible to post-translational modifications, such as glycosylation or pyroglutamination, ^ Positions that are occupied by residues that are predicted to clash with CDRs, according to the model of 13D3 CDRs grafted onto VH and VL frameworks, and/or Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT ^ Positions that are occupied by residues that are rare among sequenced human antibodies, where either the parental mouse 13D3 residue or some other residue is much more prevalent within human antibody repertoire. The goal of identifying mutations in the antibody sequences is to allow the antibodies to be stabilized in such a manner to allow for these improved properties such as improved Tm, improved yield, improved binding, and reduced predicted immunogenicity. Based on the above criteria, the following heavy and light variable regions were generated. hu13D3-VH_vd1 includes the CDR-H1, H2, and H3 loops of 13D3-VH grafted onto the framework of 5BK5 VH. hu13D3-VH_vd1 reverts all framework substitutions at positions that are key for defining the Chothia canonical classes, are part of the Vernier zone, or localize to the VH/VL domain interface or contribute to structural stability. The substitutions designed for this heavy chain included one or more of L5V, T77S, R19K. G44R, S77T, L78A, L78G, L80A, L80G, L82cG, and R83M. hu13D3-VH_vd2 included L78A. Hu13D3-VH_vd3 includes G44R, S49A, and S74A. Hu13D3-VH_vd4 included various changes made in versions 1, 2 and 3 are tried in combination. For the light chain variable region, hu13D3-VL_vd1 includes the CDR-L1, L2, and L3 loops of 13D3-VL grafted onto the framework of 5BK5 VL along with reverting all framework substitutions at positions that are key for defining the Chothia canonical classes, are part of the Vernier zone, or locate to the VH/VL domain interface. The following substitutions were introduced: V3Q, L9S, I2V, P15L, P18Q, E17Q, L38Q, K39R, R46L, A80S, L92G, V94I, V94A, Q100D, and L104V. For hu13D3-VL_vd2, the mutations from vld1 were introduced and also included L92A. Complete sequences of heavy and light variable domains are provided in Tables 2 and 3. Example 2. Binding Data of Humanized Antibodies A binding screening assay was established to determine whether 13D3 and humanized variants thereof would selectively bind to phosphorylated TDP-43 found in cytoplasmic aggregates. To test binding affinity, a CAP Chip assay was performed in which binding to TDP43 was determined. 0.25ug/mL bio-TDP43-phospho-peptide was used and standard CAP chip protocol (Cytiva) was followed. Regeneration between each run cycle for improved baseline binding levels was introduced. Affinity was analyzed using a bivalent analyte model. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT As shown in Table 4, Hu13D3 binding to pTDP43 peptide was maintained among all tested antibodies, compared to the Ch_13D3 Ab and Ch_13D3, all variants show sufficient binding with approximately 0.5-1nM Affinity (Bivalent analyte model), demonstrating that each antibody possessed affinity to TDP43. In addition, each of h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2 demonstrated superior binding as shown by KD1 (M) values that are about 2 orders of magnitude better binder than antibodies made using conventional methods (e.g., h13D3-H9L7). In a second experiment, Phospho-TDP43_392-414 binding was determined using a BiacoreTM system. This assay produced a measured affinity of about 2 nM and can be used for humanization screening of 13D3 antibodies. Table 4. Affinity Binding Data Antibodyka1 (1/Ms) kd1 (1/s) KD1(M) RU (max) Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT h13D3-H8L5 1.14E+09 2.40E+00 2.11E-09 16.3 h13D3-H8L6 1.74E+08 1.45E-01 8.31E-10 16 The results set forth in Table 4, above, demonstrate that antibodies comprising the humanizing mutations of the present disclosure (including, for example, h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2) retain the strong binding to phospho-TDP-43. For example, as shown in Table 4, these antibodies have KD values of single digit nanomolar to sub-nanomolar values, which are similar to or lower than KD values for antibodies that lack humanizing mutations (e.g., chimeric 13D3 antibodies). Thus, antibodies comprising humanizing mutations of the present disclosure retain high-affinity binding to their target epitope. Example 3. Thermal Stability and Expression Yield of Humanized Antibodies Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT Next, thermal stability (TM) of the humanize antibodies was evaluated. 3.33 mM/0.5 mg/mL of test concentration was added in 1xPBS Buffer pH 7.4. Temperature ranged from about 25°C -110 °C, and onset TM was determined for the CH2 region, the F’ab region, and the CH3 regions as shown in Tables 5. Expression yields for the humanized variants are also included in Table 5. Table 5. Yield and Tm Data TmOnset °C Tm1 °C Tm2 °C Tm3 °C Antibody Yield (CH2 re ion) (f'ab re ion) (CH3 Re ion) (CH3 Re ion) Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT h13D3-H9L5 57.84 *64.5 69.32 82.48 h13D3-H9L6 214.07 61.18 -- 68.17 -- Table 5 demonstrates that several attempts at humanizing the 13D3 chimeric antibody using traditional humanization methods resulted in significantly reduced antibody stability. For example, many antibody variants (e.g., h13D3-H3L4, h13D3-H5L2, h13D3-H5L3, h13D3- H5L4, h13D3-H6L4, h13D3-H5L5, h13D3-H6L5, h13D3-H7L5, h13D3-H8L4, h13D3-H8L5, and h13D3-H8L6) exhibited extremely low expression yields, which are indicative of antibody instability. As a result of the low yields, many of these variants were not assessed for melting temperatures. In contrast, humanized antibodies comprising the humanization mutations of the present disclosure (e.g., h13D3-Hd1Ld1, h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2) were expressed in high yield, demonstrating the stability of these antibodies. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT The melting temperature data in Table 5 further demonstrate the stability of antibodies comprising humanization mutations of the present disclosure. Many antibody variants (e.g., h13D3-H8L6, h13D3-H9L4, h13D3-H9L5, h13D3-H9L6, h13D3-H9L7, h13D3-H9L8, h13D3- H10L5, h13D3-H10L6, h13D3-H10L7, and h13D3-H10L8) exhibited low Tm Onset values for the CH2 region (e.g., 5 to 10 degrees Celsius below the Tm Onset for PB-0344, a reference antibody), which are indicative of antibody instability. In contrast, humanized antibodies comprising the humanization mutations of the present disclosure (e.g., h13D3-Hd1Ld1, h13D3- Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld1, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2) demonstrated high Tm Onset values of 70 degrees Celsius or higher for the CH2 region, demonstrating the high stability of these antibodies. Thus, the data in Table 5 demonstrate that antibodies comprising humanizing mutations of the present disclosure exhibit high thermal stability, resulting in stable antibodies that can be expressed in high yield. Example 4. Predicted Immunogenicity of Humanized Antibodies Immunogenicity scores are predicted values of immunogenicity calculated from two different software programs: Immune Epitope Database (IEDB) and EpiQuest. IEDB immunogenicity analysis tool is hosted by the National Institute of Allergy and Infectious Diseases. For IEDB, the immunogenicity prediction method used is based on predicted potential binding of a peptide within a protein sequence to major histocompatibility class (MHC) class II. The program identifies potential immunogenic regions within the protein sequence. The MHC class II tool predicts immunogenic regions using broad spectrum alleles (i.e., 26 reference alleles) for MHC class II in the human population. The software generates a series of 15 residue peptides which overlap in 10 residues. Each generated 15 residue peptide is predicted for binding to the 26 reference alleles. For each peptide, a percentile rank for each of the three methods (combinatorial library, SMM_align and Sturniolo) was generated by comparing each peptide's score against the scores of five million random 15 residue peptides selected from the SWISSPROT database. The adjusted percentile rank is the percentile rank adjusted based on the frequency of peptide lengths. A low number percentile rank indicates high affinity. The median percentile rank of the three methods were then used to generate the rank for consensus method. By default, prediction result Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT is collapsed to show only the Percentile Rank and Adjusted Rank. The maximum median percentile rank threshold is set at 20. The EpiQuest tool, T-Scanner, ranges cytotoxic T lymphocyte (CTL) epitopes according to their predicted immunodominance. The immunodominance of epitopes is defined as their relative strength in functional assays related to the target kill or release of respective cytokines. These parameters indicate functionality of the T-epitope. This program is designed to analyze and sort according to their immunodominance the CTL peptide epitopes eluted from target cells. Generally, only a low number of peptide epitopes that bind to MHC class I have actual functional activity. Relative strength of a CTL (T) epitope is defined by the strength of its binding to MHC class I and T cell receptor (TCR) (in the context of MHCI). The algorithm detects the structural and compositional features of the peptide epitope that allow it to elicit a high-affinity for TCR. This analysis is haplotype-specific and the EpiQuest T-scanner has matrix for analysis of HLAA2 and H2kB haplotypes-binding peptides. Collectively, the data shown in Tables 6 show some humanized antibodies comprising humanizing mutations of the present disclosure have lower predicted immunogenicity scores relative to the chimeric 13D3 antibody and many antibodies humanized using traditional humanization strategies. Table 6. Antibody Immunogenicity Score Immunogenicity Immunogenicity Antib d S r Antib d S r Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT h13D3H4L2 5.5 10 h13D3Hd2Ld1 0.3 4 h13D3H4L3 55 85 h13D3Hd2Ld2 03 1 e pred cted mmunogen c ty data in Table 6 demonstrate that antibodies comprising humanization mutations of the present disclosure have significantly reduced immunogenicity compared to chimeric 13D3 antibody and many antibodies humanized using traditional humanization strategies. Many antibody variants (e.g., h13D3-H1L1, h13D3-H2L2, h13D3- H2L3, h13D3-H2L4, h13D3-H3L2, h13D3-H3L3, h13D3-H3L4, h13D3-H4L2, h13D3-H4L3, h13D3-H4L4, h13D3-H5L2, h13D3-H5L3, and h13D3-H8L6) exhibited high predicted immunogenicity values, which are indicative of actual immunogenicity in human subjects. In contrast, humanized antibodies comprising the humanization mutations of the present disclosure (e.g., h13D3-Hd1Ld2, h13D3-Hd2Ld1, h13D3-Hd2Ld2, h13D3-Hd3Ld2, h13D3-Hd4Ld1, and h13D3-Hd4Ld2) demonstrated low predicted immunogenicity values, demonstrating that these antibodies are unlikely to be immunogenic in human subjects. Collectively, the data in Tables 4, 5, and 6 demonstrate that antibodies comprising humanizing mutations of the present disclosure retain strong antigen binding, exhibit high thermal stability, can be expressed in high yield, and have significantly reduced immunogenicity, even when compared to antibodies that are humanized using traditional humanization methods.
Claims
Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT What is claimed is: 1. A humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising a charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 2. The humanized antibody of claim 1, wherein the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is a positively charged amino acid, and the charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is a negatively charged amino acid. 3. The humanized antibody of claim 2, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. 4. The humanized antibody of any one of claims 1-3, wherein the positively charged amino acid is a basic amino acid. 5. The humanized antibody of any one of claims 1-4, wherein the negatively charged amino acid is an acidic amino acid. 6. The humanized antibody of any one of claims 1-5, wherein the positively charged amino acid is an arginine. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 7. The humanized antibody of any one of claims 1-6, wherein the negatively charged amino acid is an aspartic acid. 8. The humanized antibody of any one of claims 1-7, wherein the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. 9. The humanized antibody of claim 8, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 10. The humanized antibody of claim 8 or 9, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 11. The humanized antibody of any one of claim 8-10, wherein the substituted phenylalanine derivative comprises a hydroxy-substituted phenyl group. 12. The humanized antibody of claim 11, wherein the substituted phenylalanine derivative is a tyrosine residue. 13. The humanized antibody of any one of claims 9-12, wherein the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain is a glutamine, and/or wherein the amino acid at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. 14. The humanized antibody of any one of claims 1-13, wherein the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. 15. The humanized antibody of any one of claims 1-14, wherein the humanized antibody further comprises at least one of following: an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 16. The humanized antibody of any one of claims 1-14, wherein the humanized antibody further comprises at least one of following: a serine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and a serine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 17. The humanized antibody of any one of claims 1-16, wherein the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. 18. The humanized antibody of any one of claims 1-17, wherein the humanized antibody further comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. 19. A humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. 20. The humanized antibody of claim 19, wherein the humanized antibody further comprises a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, and a light chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain. 21. The humanized antibody of claim 20, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with at least one of: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 22. The humanized antibody of claim 21, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond with both: the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 23. The humanized antibody of any one of claim 20-22, wherein the substituted phenylalanine derivative comprises a hydroxy-substituted phenyl group. 24. The humanized antibody of claim 23, wherein the substituted phenylalanine derivative is a tyrosine. 25. The humanized antibody of any one of claims 20-24, wherein the polar uncharged amino acid residue at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain is a glutamine. 26. The humanized antibody of any one of claims 20-25, wherein the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain variable domain, is a glutamine. 27. The humanized antibody of any one of claims 19-26, wherein the humanized antibody further comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. 28. The humanized antibody of any one of claims 19-27, wherein the humanized antibody further comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 29. The humanized antibody of claim 27 or 28, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. 30. The humanized antibody of any one of claims 27-29, wherein the positively charged amino acid is a basic amino acid. 31. The humanized antibody of any one of claims 27-30, wherein the negatively charged amino acid is an acidic amino acid. 32. The humanized antibody of any one of claim 27-31 wherein the positively charged amino acid is an arginine. 33. The humanized antibody of any one of claims 27-32, wherein the negatively charged amino acid is an aspartic acid. 34. The humanized antibody of any one of claims 19-33, wherein the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. 35. The humanized antibody of any one of claims 19-34, wherein the humanized antibody further comprises at least one of following: Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 36. The humanized antibody of any one of claims 19-35, wherein the humanized antibody further comprises at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. 37. A humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 38. The humanized antibody of claim 37, wherein the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. 39. The humanized antibody of claim 37 or 38, wherein the light chain variable comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. 40. The humanized antibody of any one of claims 37-39, wherein the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 41. The humanized antibody of any one of claims 37-40, wherein the light chain variable domain comprises each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged residue at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 42. The humanized antibody of claim 40 or 41, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. 43. The humanized antibody of any one of claims 37-42, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain is an arginine. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 44. The humanized antibody of any one of claims 37-43, wherein the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain is an aspartic acid. 45. The humanized antibody of any one of claims 37-44, wherein the humanized antibody further comprises one or both of: an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; and an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 46. A humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 47. The humanized antibody of claim 46, wherein the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. 48. The humanized antibody of claim 46 or 47, wherein the light chain variable domain comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 49. The humanized antibody of any one of claims 46-48, wherein the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 50. The humanized antibody of any one of claims 46-49, wherein the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 51. The humanized antibody of any one of claims 46-50, wherein the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 52. The humanized antibody of claim 51, wherein the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 53. The humanized antibody of any one of claims 46-52, wherein the heavy chain variable region comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. 54. The humanized antibody of any one of claims 46-53, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. 55. The humanized antibody of any one of claims 51-54, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. 56. The humanized antibody of any one of claims 46-55, wherein the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. 57. The humanized antibody of any one of claims 46-56, wherein the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain, is a serine. 58. A humanized antibody comprising a heavy chain variable domain and a light chain variable domain, the heavy chain variable domain comprising a polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain, and a light chain variable domain comprising at least one of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 59. The humanized antibody of claim 58, wherein the light chain variable domain comprises a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 60. The humanized antibody of claim 58 or 59, wherein the light chain variable domain comprises an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. 61. The humanized antibody of any one of claims 58-60, wherein the light chain variable domain comprises a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 62. The humanized antibody of any one of claims 58-61, wherein the light chain variable domain comprising each of the following: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; an alanine residue at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; and a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain. 63. The humanized antibody of any one of claims 58-62, wherein the humanized antibody further comprises one or both of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 64. The humanized antibody of claim 63, wherein the heavy chain variable region comprises an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain. 65. The humanized antibody of any one of claims 58-64, wherein the heavy chain variable region comprises a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 66. The humanized antibody of any one of claims 58-65, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, and the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain form an interchain salt bridge. 67. The humanized antibody of any one of claims 63-66, wherein the positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain, is an arginine. 68. The humanized antibody of any one of claims 58-67, wherein the negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain, is an aspartic acid. 69. The humanized antibody of any one of claims 58-68, wherein the polar uncharged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain is a serine. 70. The humanized antibody of any one of claims 37-69, wherein the humanized antibody further comprises at least one of following: a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain. 71. The humanized antibody of any one of claims 37-70, wherein the humanized antibody further comprises at least one of the following: Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. 72. The humanized antibody of any one of claims 37-71, wherein the humanized antibody further comprises a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain. 73. The humanized antibody of claim 72, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, forms a hydrogen bond interaction with at least one of an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and an amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 74. The humanized antibody of claim 71 or 72, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain forms a hydrogen bond interaction with both the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, and the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain. 75. The humanized antibody of any one of claims 72-74, wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, comprises a hydroxy-substituted phenyl group. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 76. The humanized antibody of claim 75 wherein the substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain, is a tyrosine. 77. The humanized antibody of any one of claims 73-76, wherein the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 38 of a human IgG1 light chain, is a glutamine. 78. The humanized antibody of any one of claims 73-77, wherein the amino acid at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 heavy chain, is a glutamine. 79. The humanized antibody of any one of claims 1-78, wherein the antibody is an antigen-binding antibody fragment. 80. The humanized antibody of claim 79, wherein the antigen-binding antibody fragment is a Fab fragment, a Fab’2 fragment, or a single chain Fv. 81. The humanized antibody of any one of claims 1-78, wherein the humanized antibody is a full antibody, a chimeric antibody, a veneered antibody, a CDR-grafted antibody, or a recombinant antibody. 82. The humanized antibody any one of claims 1-78 or 81, wherein the humanized antibody is a human IgG antibody. 83. The humanized antibody of claim 79, wherein the human IgG antibody has a human IgG1 isotype. 84. The humanized antibody of any one of claims 1-83, wherein the antibody is conjugated to a therapeutic, cytotoxic, cytostatic, immunomodulatory, neurotrophic, or neuroprotective agent. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 85. The humanized antibody of any one of claims 1-78 or 81-83, wherein the heavy chain variable domain is fused to a heavy chain constant region and the light chain variable domain is fused to a light chain constant region. 86. The humanized antibody of claim 85, wherein the heavy chain constant region is a mutant form of a natural human heavy chain constant region which has reduced binding to an Fcγ receptor relative to a natural heavy chain constant region. 87. The humanized antibody of claim 85 or 86, wherein the heavy chain constant region is of IgG1 isotype. 88. The humanized antibody of any one of claims 1-86, wherein the antibody has at least one mutation in a constant region. 89. The humanized antibody of claim 88, wherein the at least one mutation reduces complement fixation or activation by the constant region. 90. The humanized antibody of claim 89, where the at least one mutation is at one or more positions of: 241, 264, 265, 270, 296, 297, 318, 320, 322, 329, and 331 by EU numbering. 91. The humanized antibody of claim 90, wherein the humanized antibody has an alanine at positions 318, 320, and 322 by EU numbering. 92. The humanized antibody of any one of claims 1-91, wherein the humanized antibody has increased thermal stability as compared to a reference antibody. 93. The humanized antibody of claim 92, wherein the increased thermal stability comprises an increase in expression yield compared to the reference antibody. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 94. The humanized antibody of claim 93, wherein the increased thermal stability comprises an increase in expression yield of at least 100 mg/L compared to the reference antibody. 95. The humanized antibody of any one of claims 92-94, wherein the increased thermal stability comprises an increase in melting point as compared to the reference antibody. 96. The humanized antibody of claim 95, wherein the increase in melting point is an increase of at least 2°C compared to the reference antibody. 97. The humanized antibody of claim 95, wherein the increase in melting point is an increase of at least 5°C compared to the reference antibody. 98. The humanized antibody of claim 92, wherein the humanized antibody has a melting temperature of 55°C or greater. 99. The humanized antibody of claim 98, wherein the humanized antibody has a melting temperature of 65°C or greater. 100. The humanized antibody of claim 98, wherein the humanized antibody has a melting temperature of 70°C or greater. 101. The antibody of any one of claims 95-100, wherein the melting temperature is determined by differential scanning calorimetry. 102. The humanized antibody of any one of claims 1-101, wherein the humanized antibody has a decreased immunogenicity score as compared to a reference antibody. 103. The humanized antibody of claim 102, wherein the immunogenicity score is determined using Formula I: Ig Score = (-KDHI +4.5) X Pt (average) X Tail Bonus (Formula I). Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 104. The humanized antibody of any one of claims 92-103, wherein the reference antibody comprises the same sequence as the humanized antibody without the one or more amino acid substitutions. 105. A pharmaceutical composition comprising the humanized antibody of any one of claims 1-104 and a pharmaceutically acceptable carrier. 106. A nucleic acid encoding the heavy chain variable domain and/or the light chain variable domain of an antibody of any one of claims 1-104. 107. A vector comprising a nucleic acid encoding a heavy chain variable domain and a light chain variable domain operably linked to one or more regulatory sequences to effect expression in a mammalian cell of the humanized antibody of any one of claims 1-104. 108. The vector of claim 107, wherein the one or more regulatory sequences comprises one or more of a enhancer, ribosome binding site, transcription termination signal, and promoter, optionally, wherein the promoter is a eukaryotic promoter. 109. The vector of claim 107 or 108, wherein the nucleic acid is codon-optimized for expression in a host cell. 110. A host cell transformed with the vector of any one of claims 107-109. 111. A host cell comprising the nucleic acid of claim 106. 112. A method of reducing immunogenicity of a parent antibody by introducing amino acid substitution(s) into the parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. 113. A method of increasing thermal stability of an antibody by introducing amino acid substitution(s) into a parent antibody to produce a modified antibody comprising: (i) a heavy chain variable domain comprising one or more of: Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT a positively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; a leucine substituted with an alanine at an amino acid position corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; a substituted phenylalanine derivative at an amino acid position corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; a tryptophan at an amino acid position corresponding to Chothia canonical amino acid position 103 of a human IgG1 heavy chain; and a phenylalanine at an amino acid position corresponding to Chothia canonical amino acid position 100C of a human IgG1 heavy chain; and/or (ii) a light chain variable domain comprising one or more of: a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain; a glutamine at an amino acid position corresponding to Chothia canonical amino acid position 17 of a human IgG1 light chain; a tyrosine at an amino acid position corresponding to Chothia canonical amino acid position 36 of a human IgG1 light chain; an arginine at an amino acid position corresponding to Chothia canonical amino acid position 39 of a human IgG1 light chain; an alanine at an amino acid position corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain; a negatively charged amino acid at an amino acid position corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; and a valine at an amino acid position corresponding to Chothia canonical amino acid position 104 of a human IgG1 light chain. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 114. A method of reducing immunogenicity of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has a reduction in immunogenicity compared to the parent antibody. 115. The method of claim 114, the method further comprising introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. 116. The method of claim 114 or 115, wherein the method further comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. 117. The method of any one of claims 114-116, wherein the modified antibody has a melting temperature of 65°C or greater. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 118. A method of increasing thermal stability of a parent antibody by introducing a first amino acid substitution and a second amino acid substitution into the parent antibody to generate a modified antibody, wherein the first amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 44 of a human IgG1 heavy chain; and the second amino acid substitution is at an amino acid corresponding to Chothia canonical amino acid position 100 of a human IgG1 light chain; wherein the modified antibody has increase in melting temperature of 2°C or greater compared to the parent antibody as measured by differential scanning calorimetry. 119. The method of claim 118, the method further comprising introducing a third amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 91 of a human IgG1 heavy chain; and a fourth amino acid substitution into the parent antibody at an amino acid corresponding to Chothia canonical amino acid position 3 of a human IgG1 light chain. 120. The method of claim 118 or 119, wherein the method further comprises introducing at least one of the following amino acid substitutions: an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 49 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 74 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 77 of a human IgG1 heavy chain; an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 78 of a human IgG1 heavy chain; and an amino acid substitution at an amino acid corresponding to Chothia canonical amino acid position 92 of a human IgG1 light chain. 121. The method of any one of claims 118-120, wherein the modified antibody has increase in melting temperature of 5°C or greater compared to the parent antibody as measured by differential scanning calorimetry. Attorney Docket No.: 50887-0046WO1 // Client Ref: 791-PCT 122. The method of any one of claims 118-121, wherein the modified antibody has a melting temperature of 65°C or greater.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363538598P | 2023-09-15 | 2023-09-15 | |
| US63/538,598 | 2023-09-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2025059508A1true WO2025059508A1 (en) | 2025-03-20 |
Family
ID=92932839
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/046679PendingWO2025059508A1 (en) | 2023-09-15 | 2024-09-13 | Compositions and methods of reducing immunogenicity and improving stability of antibodies |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2025059508A1 (en) |
Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2220211A (en) | 1988-06-29 | 1990-01-04 | Ribi Immunochem Research Inc | Modified lipopolysaccharides |
| US5057540A (en) | 1987-05-29 | 1991-10-15 | Cambridge Biotech Corporation | Saponin adjuvant |
| US5194594A (en) | 1990-09-07 | 1993-03-16 | Techniclone, Inc. | Modified antibodies |
| US5208036A (en) | 1985-01-07 | 1993-05-04 | Syntex (U.S.A.) Inc. | N-(ω, (ω-1)-dialkyloxy)- and N-(ω, (ω-1)-dialkenyloxy)-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5283185A (en) | 1991-08-28 | 1994-02-01 | University Of Tennessee Research Corporation | Method for delivering nucleic acids into cells |
| WO1994012629A1 (en) | 1992-12-02 | 1994-06-09 | Baylor College Of Medicine | Episomal vectors for gene therapy |
| WO1995007707A1 (en) | 1993-09-14 | 1995-03-23 | Cytel Corporation | Alteration of immune response using pan dr-binding peptides |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| WO1996034625A1 (en) | 1995-05-04 | 1996-11-07 | Yale University | Recombinant vesiculoviruses and their uses |
| US5643576A (en) | 1994-05-27 | 1997-07-01 | The University Of North Carolina At Chapel Hill | Method of inducing an immune response with a live Venezuelan Equine Encephalitis virus expressing a heterologous immunogen |
| WO1998040100A1 (en) | 1997-03-10 | 1998-09-17 | Ottawa Civic Loeb Research Institute | USE OF NUCLEIC ACIDS CONTAINING UNMETHYLATED CpG DINUCLEOTIDE AS AN ADJUVANT |
| US5859205A (en) | 1989-12-21 | 1999-01-12 | Celltech Limited | Humanised antibodies |
| WO2000023579A1 (en) | 1998-10-22 | 2000-04-27 | The Regents Of The University Of California | Functionally assembled antigen-specific intact recombinant antibody and a method for production thereof |
| US6407213B1 (en) | 1991-06-14 | 2002-06-18 | Genentech, Inc. | Method for making humanized antibodies |
| WO2003057838A2 (en) | 2001-12-28 | 2003-07-17 | Abgenix, Inc. | Antibodies against the muc18 antigen |
| US6881557B2 (en) | 2001-07-12 | 2005-04-19 | Arrowsmith Technologies Llp | Super humanized antibodies |
| US8455622B2 (en) | 2006-12-01 | 2013-06-04 | Seattle Genetics, Inc. | Variant target binding agents and uses thereof |
| WO2017091512A1 (en) | 2015-11-23 | 2017-06-01 | Sangamo Biosciences, Inc. | Methods and compositions for engineering immunity |
| WO2023156549A1 (en)* | 2022-02-16 | 2023-08-24 | Ac Immune Sa | Humanized anti-tdp-43 binding molecules and uses thereof |
- 2024
- 2024-09-13WOPCT/US2024/046679patent/WO2025059508A1/enactivePending
Patent Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5208036A (en) | 1985-01-07 | 1993-05-04 | Syntex (U.S.A.) Inc. | N-(ω, (ω-1)-dialkyloxy)- and N-(ω, (ω-1)-dialkenyloxy)-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US5057540A (en) | 1987-05-29 | 1991-10-15 | Cambridge Biotech Corporation | Saponin adjuvant |
| GB2220211A (en) | 1988-06-29 | 1990-01-04 | Ribi Immunochem Research Inc | Modified lipopolysaccharides |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5585089A (en) | 1988-12-28 | 1996-12-17 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5859205A (en) | 1989-12-21 | 1999-01-12 | Celltech Limited | Humanised antibodies |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US5194594A (en) | 1990-09-07 | 1993-03-16 | Techniclone, Inc. | Modified antibodies |
| US6407213B1 (en) | 1991-06-14 | 2002-06-18 | Genentech, Inc. | Method for making humanized antibodies |
| US5283185A (en) | 1991-08-28 | 1994-02-01 | University Of Tennessee Research Corporation | Method for delivering nucleic acids into cells |
| WO1994012629A1 (en) | 1992-12-02 | 1994-06-09 | Baylor College Of Medicine | Episomal vectors for gene therapy |
| WO1995007707A1 (en) | 1993-09-14 | 1995-03-23 | Cytel Corporation | Alteration of immune response using pan dr-binding peptides |
| US5736142A (en) | 1993-09-14 | 1998-04-07 | Cytel Corporation | Alteration of immune response using pan DR-binding peptides |
| US5643576A (en) | 1994-05-27 | 1997-07-01 | The University Of North Carolina At Chapel Hill | Method of inducing an immune response with a live Venezuelan Equine Encephalitis virus expressing a heterologous immunogen |
| WO1996034625A1 (en) | 1995-05-04 | 1996-11-07 | Yale University | Recombinant vesiculoviruses and their uses |
| WO1998040100A1 (en) | 1997-03-10 | 1998-09-17 | Ottawa Civic Loeb Research Institute | USE OF NUCLEIC ACIDS CONTAINING UNMETHYLATED CpG DINUCLEOTIDE AS AN ADJUVANT |
| WO2000023579A1 (en) | 1998-10-22 | 2000-04-27 | The Regents Of The University Of California | Functionally assembled antigen-specific intact recombinant antibody and a method for production thereof |
| US6881557B2 (en) | 2001-07-12 | 2005-04-19 | Arrowsmith Technologies Llp | Super humanized antibodies |
| WO2003057838A2 (en) | 2001-12-28 | 2003-07-17 | Abgenix, Inc. | Antibodies against the muc18 antigen |
| US8455622B2 (en) | 2006-12-01 | 2013-06-04 | Seattle Genetics, Inc. | Variant target binding agents and uses thereof |
| WO2017091512A1 (en) | 2015-11-23 | 2017-06-01 | Sangamo Biosciences, Inc. | Methods and compositions for engineering immunity |
| WO2023156549A1 (en)* | 2022-02-16 | 2023-08-24 | Ac Immune Sa | Humanized anti-tdp-43 binding molecules and uses thereof |
Non-Patent Citations (43)
| Title |
|---|
| "Monoclonal Antibodies For Cancer Detection And Therapy", 1985, ACADEMIC PRESS, article "Analysis, Results, And Future Prospective Of The Therapeutic Use Of Radiolabeled Antibody In Cancer Therapy", pages: 303 - 16 |
| "Monoclonal Antibodies: A Practical Approach", OXFORD UNIVERSITY PRESS |
| ALEXANDER ET AL., IMMUNITY, vol. 1, 1994, pages 751 - 761 |
| ALMAGRO ET AL., FRONTIERS BIOSCIENCE, vol. 13, 2008, pages 1619 - 1633 |
| ARNON ET AL.: "Monoclonal Antibodies And Cancer Therapy", ALAN R. LISS, INC., article "Monoclonal Antibodies For Immunotargeting Of Drugs In Cancer Therapy", pages: 243 - 56 |
| BANNER ET AL., ACTA. CRYSTALLOGR. D. BIOL. CRYSTALLOGR., vol. 69, no. Pt6, 2013, pages 1124 - 1137 |
| BERTSCHINGER ET AL., , PROTEIN ENG. DES. SEL., vol. 20, 2007, pages 57 - 68 |
| BETT ET AL., J. VIROL., vol. 67, 1993, pages 5911 |
| BRACK ET AL., MOL. CANCER THER., vol. 13, 2014, pages 2030 - 2039 |
| CHOTHIA ET AL., NATURE, vol. 342, 1989, pages 878 - 883 |
| CHOTHIALESK, J. MOL. BIOL., vol. 196, 1987, pages 901 - 917 |
| CURRENT PROTOCOLS IN PROTEIN SCIENCE, May 2001 (2001-05-01) |
| DESHPANDE ET AL., NUCLEIC ACIDS RES, vol. 33, 1 January 2005 (2005-01-01), pages 233 - 237 |
| DUBENSKY ET AL., J. VIROL., vol. 70, 1996, pages 508 - 519 |
| FOOTEWINTER: "Antibody framework residues affecting the conformation of the hypervariable loops", J MOL BIOL., vol. 224, no. 2, 1992, pages 487 - 99, XP024011188, DOI: 10.1016/0022-2836(92)91010-M |
| GONZALES ET AL., MOL. IMMUNOL., vol. 41, 2004, pages 863 |
| GRABULOVSKI ET AL., J. BIOL. CHEM., vol. 282, 2007, pages 3196 - 3204 |
| HAZRA ET AL., CELL BIOPHYS., vol. 24-25, 1994, pages 1 - 7 |
| HELLSTROM ET AL.: "Controlled Drug Delivery", 1987, MARCEL DEKKER, INC, article "Antibodies For Drug Delivery", pages: 623 - 53 |
| IWAHASHI ET AL., MOL. IMMUNOL., vol. 36, 1999, pages 1079 - 1091 |
| JONES ET AL.: "The INNs and outs of antibody nonproprietary names", MABS, vol. 8, no. 1, 2016, pages 1 - 9, XP055813029, DOI: 10.1080/19420862.2015.1114320 |
| JUNGHANS ET AL., CANCER RES., vol. 50, 1990, pages 1495 |
| KOSTELNY ET AL., J. IMMUNOL., vol. 148, 1992, pages 1547 - 53 |
| LAWRIETUMIN, CUR. OPIN. GENET. DEVELOP., vol. 3, 1993, pages 102 - 109 |
| MABS, vol. 4, no. 2, March 2012 (2012-03-01), pages 217 - 225 |
| MCGEE ET AL., J. MICRO ENCAP., 1996 |
| OHE ET AL., HUMAN GENE THERAPY, vol. 6, 1995, pages 325 - 333 |
| PASCALIS ET AL., J. IMMUNOL., vol. 169, 2002, pages 3076 |
| PIETERSZ ET AL., CANCER RES., vol. 48, no. 16, 1998, pages 4469 - 4476 |
| POLITO ET AL., LEUKEMIA, vol. 18, 2004, pages 1215 - 1222 |
| POWILLEIT ET AL., PLOS ONE, vol. 2, no. 5, 2007, pages e415 |
| SCALLY ET AL., NAT COMMUN., vol. 8, no. 1, 16 November 2017 (2017-11-16), pages 1568 |
| SCHLATTER ET AL., MABS, vol. 4, 2011, pages 497 - 508 |
| SONGSIVILAILACHMANN, CLIN. EXP. IMMUNOL., vol. 79, 1990, pages 315 - 321 |
| STOUTE ET AL., N. ENGL. J. MED., vol. 336, 1997, pages 86 - 91 |
| TAMURA ET AL., J. IMMUNOL., vol. 164, 2000, pages 1432 - 1441 |
| THORPE ET AL., IMMUNOL. RE, vol. 62, 1982, pages 119 - 58 |
| THORPE ET AL.: "Monoclonal Antibodies 84: Biological And Clinical Applications", 1985, article "Antibody Carriers Of Cytotoxic Agents In Cancer Therapy: A Review", pages: 475 - 506 |
| VAJDOS ET AL., J. OF MOL. BIOL., vol. 320, 2002, pages 415 - 428 |
| WHO-INN: INTERNATIONAL NONPROPRIETARY NAMES (INN) FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES (A REVIEW, 2014, Retrieved from the Internet <URL:www.who.int/medicines/services/inn/BioRev2014.pdf> |
| WITZIG, CANCER CHEMOTHER. PHARMACOL., vol. 48, 2001, pages S91 - S95 |
| XIAOBRANDSMA, NUCLEIC ACIDS. RES., vol. 24, 1996, pages 2630 - 2622 |
| ZHOU ET AL., J. EXP. MED., vol. 179, 1994, pages 1867 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11584791B2 (en) | Antibodies recognizing tau | |
| US20240309078A1 (en) | Antibodies recognizing tau | |
| JP7681316B2 (en) | Antibodies that recognize tau | |
| US10906964B2 (en) | Antibodies recognizing tau | |
| TWI859197B (en) | Antibodies recognizing tau | |
| US12049498B2 (en) | Humanized antibodies recognizing sortilin | |
| AU2018448903A1 (en) | Antibodies recognizing tau | |
| US20230348588A1 (en) | Antibodies recognizing sortilin | |
| WO2025059508A1 (en) | Compositions and methods of reducing immunogenicity and improving stability of antibodies | |
| TW202530252A (en) | Compositions and methods of reducing immunogenicity and improving stability of antibodies | |
| JP7753264B2 (en) | Antibodies that recognize sortilin | |
| EA047155B1 (en) | ANTIBODIES RECOGNIZING TAU |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24783065 Country of ref document:EP Kind code of ref document:A1 |