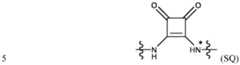IONIZABLE LIPID NANOPARTICLES FIELD OF INVENTION [0001] The present invention relates to ionizable lipid nanoparticles (LNPs) and more in particular to ionizable lipid nanoparticles comprising an ionizable lipid and a squaramide modified lipid, preferably a squaramide modified phospholipid. The provided ionizable LNPs are useful as a delivery system, particularly for use in gene therapy, cell targeted therapy or gene editing. BACKGROUND OF INVENTION [0002] The effective targeted delivery of biologically active substances such as small molecule drugs, proteins, and nucleic acids represents a continuing medical challenge. In particular, the ex vivo or in vivo delivery of nucleic acids to cells or living organisms is made difficult by the relative instability and low cell permeability of such nucleic acids. [0003] Gene therapy is thus based on the genetic modification of cells to produce a therapeutic effect by the delivery of nucleic acids ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^. Indeed, sometimes the whole or part of a gene is defective or missing from birth, or a gene can change or mutate during life. Any of these variations can disrupt how proteins are synthetized, which can contribute to health problems or diseases. By gene therapy, a defective gene or genetic sequence that causes a medical problem can be replaced with a healthy version that does not cause said problem; genes (or sequences) can also be added to help the body fight or treat disease; or genes (or sequences) that are causing problems can be knocked down or knocked out. Thereby, gene therapy can be used to treat inherited or acquired diseases. [0004] Gene editing, is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living cell or organism. Unlike early genetic engineering techniques that randomly inserts genetic material into a host genome, genome editing targets the insertions to site-specific locations. The basic mechanism involved in genetic manipulations through programmable endonucleases is the recognition of target genomic loci and binding of effector DNA-binding domain (DBD), double-strand breaks (DSBs) in target DNA by the restriction endonucleases (FokI and Cas), and the repair of DSBs through homology-directed recombination (HDR) or non-homologous end joining (NHEJ). There are a few major classes of endonucleases: meganucleases, zinc-finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated nucleases (Cas). Among these, CRISPR-Cas systems have emerged as the dominant gene editing tool because they recognize their target sequence via RNA-DNA hybridization, unlike other endonucleases that rely on protein-DNA interactions. Two different classes of gene editing therapeutics are currently being explored, ex vivo gene editing and in vivo gene editing. [0005] Lipid nanoparticles (LNPs) also referred as ionizable LNPs are often biodegradable and biocompatible and present low immunogenicity, in general. Ionizable ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ and have gained attention as mRNA delivery platforms for vaccination against COVID- 19. ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ lipophilic drugs and therapeutic nucleic acids. Usually, ionizable LNPs consists of a few components, plus the payload, which is typically a nucleic acid sequence. The main functional component is the ionizable cationic lipid, which usually represents around 50% of the entire structure. They facilitate entrapment of the nucleic acid during formation, help to maintain a neutral charge while in circulation, and improve intracellular delivery into the cells. Ionizable LNPs are a specific type of nanoparticle, which is clearly different from liposomes in terms of both composition and morphology. In that regard, ionizable lipids (ILs) ensure efficient intracellular delivery and promote cytoplasmic release of the nucleic acid entrapped. The presence of secondary or tertiary amine heads in the hydrophilic head group of ionizable lipids renders those lipids positively charged under acidic conditions while remaining neutral at physiological pH 7.4 and, as a consequence ionizable LNPs are considerably less toxic and less immunogenic, surpassing other nanoparticles in these aspects. The size and surface properties of these ionizable lipid nanoparticles can be easily ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ molecular weight, unsaturation degree, charge, and lipophilicity contrary to liposomes. Another attractive feature is the possibility of modifying the surface of the lipid nanoparticles with targeting moieties to achieve selective, cell- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ (Xu et al., ACS Nano 2022, 16, 7168-7196). [0006] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ to the surface of the lipid nanoparticle. This ligand molecule may for example, recognize ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ manner, or for example facilitate the entry of the nanoparticle into the target cell by other means, such as endocytosis or via perturbation of the cell lipidic bilayer. However, each ligand has its pros and cons and a particular ligand should be selected according to the targeted cell type, the lipid nanoparticle, and the range of molecules available (Xu et al., ACS Nano 2022, 16, 7168-7196). [0007] However, there is still a need to develop ionizable lipid nanoparticles in which the grafting of ligands onto lipid nanoparticles should: (i) maintain the integrity and functionality of the ligand as well as of the nanoparticle once said ligand is conjugated to said nanoparticle ; (ii) expose the ligand at the surface of the particle; (iii) stabilize the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ for its target. In addition, the grafting technique should have the following characteristics: ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ that the ligand is oriented outward; (ii) simplicity and reproducibility; (iii) easy scale-up and characterization; and (iv) a high yield to limit costs (Xu et al., ACS Nano 2022, 16, 7168-7196). [0008] Accordingly, there is still a need for developing ionizable lipid nanoparticles which solve some of the issues described above herein. SUMMARY [0009] The present invention relates to an ionizable lipid nanoparticle (LNP) comprising a functional moiety RL-NH-including a nitrogen containing group -NH- and a group RL, the group RL comprising a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof; and wherein said group RL is conjugated to the LNP via a squaramide moiety. [0010] The present invention also relates to an ionizable LNP comprising a squaramide modified lipid, and in particular comprising a squaramide modified phospholipid. In some aspects, the squaramide modified lipid, preferably phospholipid, is covalently linked to the group RL comprising a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0011] In some embodiments, the squaramide moiety of the squaramide modified lipid disclosed herein is represented by a formula (SQ):

wherein N is a nitrogen atom from the functional moiety NH- as defined in the present invention, and wherein N* is a nitrogen atom of an amino group of a lipid (preferably a phospholipid). [0012] In some aspects, the ionizable LNP comprises a compound of formula (I):

wherein Pl is a lipid moiety, and in particular a phospholipid moiety; E-*NH- is an extender moiety comprising an extender group E and a group -*NH-; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0013] According to the present disclosure an ionizable LNP comprises 1% or more of an ionizable lipid relative to the total weight of the LNP (w/w). In some embodiments the ionizable LNP comprises at least 5%, preferably at least 15%, more preferably at least 25%, even more preferably at least 35%, even yet more preferably at least 45% of an ionizable lipid relative to the total weight of the LNP (w/w). In some aspects, in addition to the compound of formula (I), the ionizable LNP further comprises a nucleic acid, an ionizable cationic lipid, a non-cationic lipid, a sterol and a PEGylated lipid. [0014] In some embodiments of any of the above aspects and embodiments, the ionizable LNP has a total lipid to nucleic acid ratio of about 2,5:1 to about 40:1, preferably 5:1 to 10:1. [0015] In some embodiments, the ionizable LNP has a diameter ranging from about 40 nm to about 200 nm. In some embodiments, the ionizable LNP has a diameter of less than about 120 nm. In some embodiments, the ionizable LNP has a diameter of about 70 nm to about 100 nm. [0016] In some aspects, the extender group E of the extender moiety E-*NH- comprises one or more groups selected from the group consisting of a polyethylene glycol (PEG) or a polypropylene glycol (PPG) and an aromatic moiety. [0017] In some aspects, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by group -(CH
2)q-C(O)-X-, or a bioisostere moiety thereof, and the compound of formula (I) is represented by formula (Ia1):
wherein p is 1 to 200, preferably 20 to 80; q is 0 or 1; X is O or NH when q is 1 and X is NH when q is 0 or 1, preferably X is NH; and Pl and RL-NH are as defined and described in classes and subclasses disclosed in the present invention. [0018] In some aspects, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH
2)q-C(O)-X-group, or a bioisostere moiety thereof, and the compound of formula (I) is represented by formula (Ia
2) or by formula (Ia3):
wherein p is 1 to 200, preferably 20 to 80; m
1 is 0, 1 or 2, m
2 is 0, 1 or 2; q is 0 or 1; X is O or NH when q is 1 and X is NH when q is 0 or 1; preferably X is NH; and Pl, Ar and RL-NH are as defined and described in classes and subclasses disclosed in the present invention. [0019] In some aspects, X is NH. In some aspects, p is 20 to 50. [0020] In some aspects, the content of the compound of formula (I) is between 0.01% to 2% of the total weight of the ionizable LNP, preferably 0.1% to 1%, more preferably about 0.5% of the total weight of the ionizable LNP. [0021] The functional moiety RL-NH- includes a group -NH- which forms part of the squaramide moiety of formula (SQ) as defined in the present invention, and a functional group RL comprising a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety and combinations thereof. Accordingly, in some aspects, RL-NH- is a functional moiety comprising or consisting of a group selected from a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety and combinations thereof. [0022] In some aspects, RL-NH- comprises a labeling agent. In some aspects, the labeling agent is a fluorescent dye such as fluorescein, rhodamine, boron-dipyrromethene (Bodipy®) dyes, and Alexa fluor®, or a quantum dot or a radionuclide. [0023] In some aspects, RL-NH- comprises a cell-type targeting ligand or a receptor targeting ligand selected from the group consisting of saccharides, hormones, peptides, glycosylated peptides, glycoproteins, proteins or functionally active fragments thereof, membrane receptors or functionally active fragments thereof, antibodies or functionally active fragments thereof, spiegelmers, nucleic acid or peptide aptamers, vitamins, drug moieties and combinations thereof. [0024] In some aspects, RL-NH- comprises a steric shielding agent selected from the group consisting of polyethylene glycol, pHPMA, and polysaccharides. [0025] In some aspects, the functional moiety RL-NH- comprises a group Z and one or more spacers L; wherein Z is H or a cell-type targeting ligand or a receptor targeting ligand selected from the group consisting of saccharides, hormones, peptides, glycosylated peptides, proteins, glycoproteins, or functionally active fragments thereof, membrane receptors or functionally active fragments thereof, antibodies or functionally active fragments thereof, spiegelmers, nucleic acid or peptide aptamers, vitamins, drug moieties and combinations thereof, and L comprises one or more groups selected from the group consisting of an aryl or a heteroaryl groups, an optionally substituted group comprising saturated or unsaturated, linear or branched C2-C40 hydrocarbon chains, a polyethylene glycol (PEG), a polypropylene glycol (PPG), pHPMA, PLGA, polymers of alkylene diamines, and combinations thereof. [0026] In some aspects, Z is H or comprises a cell-type targeting ligand or a receptor targeting ligand selected from the group consisting of saccharides, hormones, peptides, glycosylated peptides, proteins, glycoproteins, or functionally active fragments thereof, membrane receptors or functionally active fragments thereof, antibodies or functionally active fragments thereof, spiegelmers, nucleic acid or peptide aptamers, vitamins, drug moieties and combinations thereof. [0027] In some aspects, the functional moiety RL-NH- does not comprise one or more spacers L and the functional moiety RL-NH- consists of a group Z-NH-. [0028] In some aspects, Z is or comprises a saccharide selected from the group consisting of monosaccharides, oligosaccharides, polysaccharides and derivatives thereof. [0029] In some aspects, the saccharide is selected from the group consisting of mannose, galactose, N-acetylglucosamine, N-acetylgalactosamine, fucose, fructose, glucose, xylose, trehalose, desosamine, glucuronic acid, S6-galactose, S6-N-acetylgalactosamine, P6-mannose, P6-glucose, sialic acid, S1-fructose and P1-fructose. In some preferred aspects, the saccharide is selected from the group consisting of mannose, fructose, glucose, N-acetylglucosamine, N-acetylgalactosamine, trehalose, glucuronic acid, S6- galactose, S6-N-acetylgalactosamine, P6-mannose, P6-glucose, sialic acid and P1- fructose, more preferably mannose or glucose. [0030] In some aspects, Z is or comprises a linear peptide or a cyclic peptide, wherein said peptide may be a peptide featuring biological activity. For example, the peptide may be a peptide targeting transmembrane receptors, being said peptide targeting transmembrane receptors linked or not to cellular transcytose mechanisms allowing the crossing of a natural barrier, such as the blood brain barrier (BBB). In particular, the peptide is a blood brain barrier (BBB) shuttle peptide (or BBB-penetrating peptide) with an enhanced transport activity across the blood brain barrier. In some aspects, the BBB peptide targets the transferrin receptor TfR1. In some preferred aspects, the peptide is a peptide with a RGD motif, including a cyclic RGD peptide. In particular RGD-based peptides target an integrin subclass. Expression of TfR1 and integrins in different tissues is key for targeting CNS (via BBB) and muscle tissues. [0031] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from a muscle targeting peptide (MTP). [0032] In some aspects, the spacer group L comprises one or more groups selected from the group consisting of an arylene or a heteroarylene group Ar; an optionally substituted group comprising saturated or unsaturated, linear or branched C1-C40 hydrocarbon chains, an alkylene amine, an acyl group, a polyethylene glycol (PEG), a polypropylene glycol (PPG), pHPMA, PLGA, polymers of alkylene diamines, and combinations thereof. [0033] In some aspects, L comprises a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers. In some aspects, the polyethylene glycol (PEG) is PEG3, PEG4, or PEG5. [0034] In some aspects, L comprises one or more arylene or a heteroarylene groups Ar. [0035] In some aspects, the arylene or a heteroarylene group Ar is a bivalent aromatic radical (or bivalent aromatic moiety), i.e. which is linked to two different groups of the compound of formula (I) of the present invention (i.e. forming a bridge between two parts of the compound of formula (I)), and which may additionally comprise one or more optional substitutions. Preferably, said arylene or a heteroarylene group Ar is a 6- to 10- membered arylene group or a 5- or 12-membered heteroarylene group comprising one or more heteroatoms selected from the group consisting of N, O, S and Se. In some particular aspects, the arylene or the heteroarylene group Ar is a phenylene or pyridylene group, optionally comprising one or more substitutions. In some aspects, said arylene or heteroarylene group Ar comprises one or more substitutions selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. [0036] In some aspects, Z is a saccharide or a peptide, and L comprises a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers. In other aspects, Z is a saccharide and L comprises a polyethylene glycol (PEG) comprising 1 to 40 ethylene glycol monomers and an arylene or a heteroarylene group Ar, preferably wherein said PEG and Ar groups are covalently linked by an amide moiety or a bioisostere moiety thereof. In other aspects, Z is a saccharide and L comprises a polyethylene glycol (PEG) comprising 1 to 40 ethylene glycol monomers, one or more C
1-6 alkyl groups and an arylene or a heteroarylene group Ar, preferably wherein said PEG and Ar groups are covalently linked by an amide moiety or a bioisostere moiety thereof, or wherein said PEG and C
1-6 alkyl group are covalently linked by an amide moiety or a bioisostere moiety thereof. [0037] In some aspects, L comprises a polyethylene glycol (PEG) comprising 1 to 40 ethylene glycol monomers and an arylene or a heteroarylene group Ar, as defined in the present invention, wherein said PEG and Ar groups are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof; or L comprises a polyethylene glycol (PEG) comprising 1 to 40 ethylene glycol monomers, one or more C
1-6 alkyl groups and an arylene or a heteroarylene group Ar, as defined in the present invention, wherein said PEG and C alkyl group are covalently linke 1 1-6 d by an amide moiety -N(R )C(O)-, or a bioisostere moiety thereof; wherein R1 is selected from the group consisting of H, C1-6 alkyl, C
1-6 haloalkyl, Z-(OCH
2 ^CH
2)n ^, Z-NH-(CH
2)r ^(OCH
2-CH
2)n ^, and Z-C(O) ^ (CH2)r ^(OCH2-CH2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl and Z-(OCH ^CH )n ^,wherein n is selected f 1 2 2 rom 1 to 40 and more preferably R is H or Z-(OCH2 ^CH2)n ^,wherein n is selected from 1 to 40. [0038] In some aspects, the one or more spacer L is selected from the group consisting of L
1, L
2 and L
3 and said compound of formula (I) is selected from the group consisting of formula (Ib1), (Ib2), (Ib3), (Ib4) and (Ib5):
wherein E, Pl, and Z are as defined and described in classes and subclasses disclosed in the present invention and wherein L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L2 comprises one or more arylene or a heteroarylene groups Ar; and L
3 is a group C
1-6 alkylene. [0039] In some aspects, the polyethylene glycol (PEG) is PEG3, PEG4, or PEG5. In some aspects, L2 comprises a phenylene group or a pyridylene group. In some aspects, L3 is a C1-3 alkylene. [0040] In some aspects, L
1 and L
2 are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl, Z-(OCH2 ^CH2)n ^, Z-NH-(CH2)r ^(OCH2- CH
2)n ^, and Z-C(O) ^(CH
2)r ^(OCH
2-CH
2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl and Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40. [0041] In some aspects, L3 is covalently linked to L2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group. [0042] In some aspects, L1 and L3 are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl, Z-(OCH
2 ^CH
2)n ^, Z-NH-(CH
2)r ^(OCH
2- CH2)n ^, and Z-C(O) ^(CH2)r ^(OCH2-CH2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl and Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH2 ^CH2)n ^,wherein n is selected from 1 to 40. [0043] In some aspects, when L
2 comprises an arylene or a heteroarylene group Ar, L
1 and the squaramide moiety of formula (SQ) are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para, or when L2 comprises an arylene or a heteroarylene group Ar and L3 is present, L1 and L3 are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0044] In some aspects, when L2 comprises an arylene or a heteroarylene group Ar and L3 is a group C1-6 alkylene, L3 and the squaramide moiety of formula (SQ) are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0045] In some aspects, when L2 comprises an arylene or a heteroarylene group Ar and L3 is a group C1-6 alkylene, one or more groups L3 are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0046] In some aspects, L
1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L2 comprises one or more arylene or a heteroarylene groups; L3 is a C1-6 alkylene group, L3 is covalently linked to L2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L
1 and L
2 are covalently linked by an amide moiety or by a bioisostere moiety thereof. [0047] In some aspects, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L
2 comprises one or more arylene or a heteroarylene groups; L
3 is a C
1-6 alkylene group, L
3 is covalently linked to L
2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L1 and L3 are covalently linked by an amide moiety or by a bioisostere moiety thereof. [0048] In some aspects, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L2 comprises one or more arylene or a heteroarylene groups; L3 is a C
1-6 alkylene group, L
3 is covalently linked to L
2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L1 and L2 are covalently linked by an amide moiety or by a bioisostere moiety thereof or L1 and L3 are covalently linked by an amide moiety or by a bioisostere moiety thereof. [0049] In some aspects, the compound of formula (I) is selected from the group consisting of formula (Ic), (Id) and (Ie):
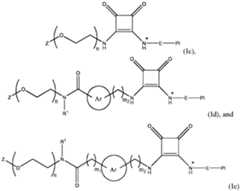
wherein n is selected from 1 to 40, m1 is 1 or 2, m2 is 0, 1 or 2, and E, Pl, Z and Ar are as defined and described in classes and subclasses disclosed in the present invention. In some particular aspects n is 3, 4 or 5; Z is or comprises a saccharide selected from the group consisting of monosaccharides, oligosaccharides, polysaccharides and derivatives thereof, preferably a saccharide selected from the group consisting of mannose, galactose, N-acetylglucosamine, N-acetylgalactosamine, fucose, fructose, glucose, xylose, trehalose, desosamine, glucuronic acid, S6-galactose, S6-N-acetylgalactosamine, P6- mannose, P6-glucose, sialic acid, S1-fructose and P1-fructose, preferably selected from the group consisting of mannose, fructose, glucose, N-acetylglucosamine, N- acetylgalactosamine, trehalose, glucuronic acid, S6-galactose, S6-N- acetylgalactosamine, P6-mannose, P6-glucose, sialic acid and P1-fructose, more preferably mannose; Ar is a 6- to 10-membered aromatic carbocyclic group or a 5- or 12- membered heterocyclic group comprising one or more heteroatoms selected from the group consisting of N, O, S and Se, preferably a phenylene or pyridylene group, optionally comprising one or more substitutions selected from the group consisting of halogen, C alkyl, C haloalkyl, C acyl and C al 1 1-6 1-6 1-6 1-6 koxy; and R is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl, Z-(OCH
2 ^CH
2)n ^, Z-NH-(CH
2)r ^ (OCH
2-CH
2)n ^, and Z-C(O) ^(CH
2)r ^(OCH
2-CH
2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl and Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH2 ^CH2)n ^,wherein n is selected from 1 to 40. [0050] In other aspects, the present invention refers to a pharmaceutical composition comprising an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, and at least one pharmaceutically acceptable vehicle. [0051] Another aspect of the invention refers to an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, or to a pharmaceutical composition comprising the same, for use as a medicament and, in particular, for use in gene therapy or gene editing. [0052] Another aspect of the invention refers to a non-therapeutic method for delivering an agent (e.g. a nucleic acid or a protein) to a target cell comprising: contacting the target cell with an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a group RL conjugated to the ionizable LNP via a squaramide moiety of formula (SQ):

wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a lipid, preferably a phospholipid, comprising the group RL a cell-type targeting ligand or a receptor targeting ligand of the target cell. [0053] Another aspect of the invention refers to an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, for use in a method for delivering an agent (e.g. a nucleic acid or a protein) to a target cell; wherein the ionizable LNP comprises an agent (e.g. the nucleic acid or the protein) to be delivered and a group RL conjugated to the ionizable LNP via a squaramide moiety of

wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a lipid, preferably a phospholipid, comprising the group RL a cell-type targeting ligand or a receptor targeting ligand of the target cell; and wherein said method comprises contacting the target cell with the ionizable LNP. [0054] In some aspects, the agent is selected from the group consisting of a nucleic acid, a chemotherapeutic agent, a small molecule drug, a protein and a peptide, or a combination thereof. [0055] In some aspects, the method for delivering an agent (e.g. a nucleic acid or a protein) comprises contacting the target cell with an ionizable LNP, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a group RL, conjugated to the LNP via a squaramide moiety of formula (SQ):

wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a phospholipid Pl, comprising the group RL a cell- type targeting ligand or a receptor targeting ligand of the target cell

wherein Pl is a phospholipid moiety; E-*NH- is an extender moiety comprising an extender group E and a group -*NH-; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein the functional moiety RL-NH- of the compound of formula (I) comprises a cell-type targeting ligand or a receptor targeting ligand of the target cell. [0056] Another aspect of the invention refers to a method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein said method comprises: reacting a surface exposed squarate moiety of an ionizable LNP comprising a squarate modified lipid, preferably a squarate modified phospholipid, with a compound of formula (IIb), or a pharmaceutically acceptable salt thereof, at a pH between 7 and 9.5, preferably between 8 and 9: RL-NH2 (IIb) so that the group RL is conjugated to the ionizable LNP via a squaramide moiety of formula (SQ) as disclosed herein; or reacting a surface exposed primary amine moiety of an ionizable LNP comprising a primary amine modified lipid, preferably a primary amine modified phospholipid, with a compound of formula (IIIb), or a pharmaceutically acceptable salt thereof, at a pH between 7 and 9.5, preferably between 8 and 9:

so that the group RL is conjugated to the ionizable LNP via a squaramide moiety of formula (SQ) as disclosed herein; wherein RL and R2 are as defined and described in classes and subclasses disclosed in the present invention. In some embodiments the squarate modified lipid is a compound of formula (IIa), or a pharmaceutically acceptable salt thereof,
[0057] Another aspect of the invention refers to a method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein said method comprises: putting into contact an ionizable lipid, a non-cationic lipid, a sterol, a PEGylated lipid and a compound of formula (IIa), or a pharmaceutically acceptable salt thereof,
with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention, to form an ionizable lipid nanoparticle; conjugating the compound of formula (IIa), or a pharmaceutically acceptable salt thereof, of the formed ionizable lipid nanoparticle with a compound of formula (IIb), or a pharmaceutically acceptable salt thereof, at a pH between 7 and 9.5, preferably between 8 and 9: RL-NH2 (IIb); wherein R2 is selected from the group consisting of linear C1-12 alkyl, branched C3-12 alkyl, linear C
1-12 haloalkyl, branched C
3-12 haloalkyl and benzyl; Pl is a phospholipid moiety; E-*NH- is an extender moiety; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0058] Accordingly, the compounds of formula (IIa) and (IIb), or their pharmaceutically acceptable salts thereof, are useful to obtain the compounds of formula (I), as defined and described in classes and subclasses in the present invention. [0059] In some aspects, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X- group, or a bioisostere moiety thereof and the compound of formula (IIa) is represented by a formula (IIa
1):
wherein Pl, X; p, q and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0060] In some aspects, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X-group, or a bioisostere moiety thereof and the compound of formula (IIa) is represented by a formula (IIa2) or formula (IIa3):
wherein Pl, X, p, m1, m2, q, Ar and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0061] In some aspects, the functional moiety RL-NH- comprises a group Z, one or more spacers L, and the compound of formula (IIb), or a pharmaceutically acceptable salt thereof, is represented by formula (IIb1): (IIb
1) [0062] In some aspects, the compound of formula (IIb
1), or a pharmaceutically acceptable salt thereof, comprises one or more than one spacer L is selected from the group consisting of L1, L2 and L3 and, is selected from the group consisting of formula (IIb2), (IIb3), (IIb4), (IIb5) and (IIb6): (IIb
2)
wherein Z, L1, L2 and L3 are as defined and described in classes and subclasses in the present invention. [0063] In other aspects, the compound of formula (IIb) is a compound selected from the group consisting of formula (IIc) (IId) and (IIe):
(IIc),
, or a pharmaceutically acceptable salt thereof, wherein n, m , m , 1 1 2 Z, Ar and R are as defined and described in classes and subclasses disclosed in the present invention. [0064] Yet another aspect of the present invention refers to a method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein said method comprises: putting into contact an ionizable lipid, a non-cationic lipid, a sterol, a PEGylated lipid and a compound of formula (IIIa), or a pharmaceutically acceptable salt thereof,
with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention, to form an ionizable lipid nanoparticle; conjugating the compound of formula (IIIa) of the formed ionizable lipid nanoparticle with a compound of formula (IIIb), at a pH between 7 and 9.5, preferably between 8 and

wherein R2 is selected from the group consisting of linear C1-12 alkyl, branched C3-12 alkyl, linear C1-12 haloalkyl, branched C3-12 haloalkyl and benzyl; Pl is a phospholipid moiety; E-*NH- is an extender moiety; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0065] Accordingly, the compounds of formula (IIIa) and (IIIb), or their pharmaceutically acceptable salts thereof, are useful to obtain the compounds of formula (I), as defined and described in classes and subclasses in the present invention. [0066] In some aspects the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a -(CH
2)q-C(O)-X- group, or a bioisostere moiety thereof and the compound of formula (IIIa) is represented by a formula (IIIa1):
wherein Pl, X, p and q are as defined and described in classes and subclasses disclosed in the present invention. [0067] In some aspects, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X-group, or a bioisostere moiety thereof and compound of formula (IIIa) is represented by a formula (IIIa2) or (IIIa3):
wherein Pl, X, Ar, p, m1, m2 and q are as defined and described in classes and subclasses disclosed in the present invention. [0068] In some aspects, the functional moiety RL-NH- comprises a group Z, one or more spacers L, and the compound of formula (IIIb), or a pharmaceutically acceptable salt thereof, is represented by formula (IIIb1):
(IIIb
1). wherein R2, Z and L are as defined and described in classes and subclasses in the present invention. [0069] In some aspects, the compound of formula (IIIb1), or a pharmaceutically acceptable salt thereof, comprises one or more than one spacer L is selected from the group consisting of L1, L2 and L3 and, is selected from the group consisting of formula (IIIb2), (IIIb3), (IIIb4), (IIIb5) and (IIIb6):
, and
wherein R2, Z, L
1, L
2 and L
3 are as defined and described in classes and subclasses in the present invention. [0070] In other aspects, the compound of formula (IIIb) is a compound selected from the group consisting of formula (IIIc) (IIId) and (IIIe):
or a pharmaceutically acceptable salt thereof, wherein n, m
1, m
2, Z, Ar, R1 and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0071] Yet another aspect of the present invention refers to a method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein said method comprises putting in contact a compound of formula (I), an ionizable cationic lipid, a non-cationic lipid, a sterol and a PEGylated lipid, with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention. DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS [0072] Various coupling chemistries that conjugate various ligands to lipid nanoparticles have been described. However, not all such coupling chemistries are compatible with and/or effective under particular conditions and/or with particular substrates. [0073] The present disclosure relates to ionizable lipid nanoparticles comprising a compound of formula (I), as previously defined, which couples different types of ligands using a squaramide moiety of formula (SQ) as disclosed herein. The present disclosure appreciates that not all ligands have the same characteristics and therefore not all coupling chemistries are effective for conjugation into a lipid nanoparticle. [0074] The present disclosure therefore recognizes a particular remaining need to provide suitable coupling chemistries that (i) maintain the integrity and functionality of the ligand as well as of the nanoparticle once the once said ligand is conjugated to said nanoparticle; (ii) expose the ligand at the surface of the particle; (iii) stabilize the ligand in the blood and/or biological medium; ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ target. [0075] In particular, the present disclosure recognizes the need that the grafting of a ligand to the ionizable ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ to control the localization of the conjugation on the ligand to ensure that the ligand is oriented outward; (ii) simplicity and reproducibility; (iii) easy scale-up and characterization; and (iv) a high yield to limit costs. [0076] The squaramide moiety is a conformationally rigid cyclobutene ring derived from squaric acid (diketoclyclobutenediol) which benefits from unique physical and chemical properties which make it surprisingly useful for developing ionizable lipid nanoparticles where a wide range of ligands may be grafted. Moreover, by selecting appropriate pH conditions the first and second substitution of the squarate can be controlled, allowing thus to provide a more selective substitution, resulting in a more flexible scaffold for developing lipid nanoparticles with a wide range of ligands when compared to other solutions (linkers) known in the prior art. General Description and Definitions [0077] ^ ^ ^ ^ ^ ^ ^ ^alkyl ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ saturated hydrocarbon chain, comprising 1-8 carbon atoms (also named (C1-C8)alkyl), such as methyl, ethyl, propyl, isopropyl, n-butyl, iso-butyl, sec-butyl, tert-butyl, tert-butyl- methyl, n-pentyl, n hexyl, n-heptyl, or n- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^alkylene group ^ corresponds to the bivalent group obtained by removal of a hydrogen atom from an alkyl group, as defined above herein, resulting in a moiety with two points of attachment. [0078] ^ ^ ^ ^ ^ ^ ^ ^acyl ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^C(O)R group, where R is an alkyl group as defined earlier or a phenyl group. An acyl group includes for example acetyl, ethylcarbonyl, or benzoyl group. [0079] ^ ^ ^ ^ ^ ^ ^ ^alkoxy ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ -O-Alk group wherein Alk is an alkyl group as defined above. An alkoxy group includes for example methoxy, ethoxy, n- propyloxy, or tert-butyloxy group. [0080] ^ ^ ^aryl group ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ an aromatic monocyclic (i.e. phenyl) or bicyclic system (i.e. phenyl) comprising 4-12 carbon atoms, preferably 6 to 10, it being understood that in the case of a bicyclic system, one of the cycles is aromatic and the other cycle is aromatic or unsaturated. Aryl groups include for example phenyl, naphthyl, indenyl, or benzocyclobutenyl groups, optionally substituted by one or more groups optionally comprising one or more substitutions selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. A preferred aryl group used herein is phenyl. ^ ^ ^ ^ ^ ^ ^ ^arylene group ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ by removal of a hydrogen atom from an aryl group, as defined above herein, resulting in a moiety with two points of attachment. A preferred arylene group used herein is phenylene optionally substituted by one or more groups optionally comprising one or more substitutions selected from the group consisting of halogen, C
1-6 alkyl, C
1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. [0081] ^ ^ ^heteroaryl group ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-atom aromatic ring or ring system containing 1 to 2 rings which are fused together or linked covalently, typically containing 5 to 6 atoms on each ring; at least one of which is aromatic and in which one or more carbon atoms in one or more of these rings is replaced by oxygen, nitrogen, sulfur or selenium atoms, where the nitrogen and sulfur heteroatoms may optionally be oxidized and the nitrogen heteroatoms may optionally be quaternized. Such rings may be fused to an aryl ring. Non-limiting examples of such heteroaryl groups include: triazolyl, pyrrolyl, furanyl, thiophenyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl, oxatriazolyl, thiatriazolyl, pyridinyl, pyrimidyl, pyrazinyl, pyridazinyl, oxazinyl, dioxinyl, thiazinyl, triazinyl, imidazo[2,1-b][1,3]thiazolyl, thieno[3,2-b]furanyl, thieno[3,2-b]thiophenyl, thieno[2,3-d][1,3]thiazolyl, thieno[2,3-d]imidazolyl, tetrazolo[1,5-a]pyridinyl, indolyl, indolizinyl, isoindolyl, benzofuranyl, isobenzofuranyl, benzothiophenyl, isobenzothiophenyl, indazolyl, benzimidazolyl, 1,3-benzoxazolyl, 1,2-benzisoxazolyl, 2,1-benzisoxazolyl, 1,3-benzothiazolyl, 1,2-benzoisothiazolyl, 2,1-benzoisothiazolyl, benzotriazolyl, 1,2,3-benzoxadiazolyl, 2,1,3-benzoxadiazolyl, 1,2,3-benzothiadiazolyl, 2,1,3-benzothiadiazolyl, thienopyridinyl, purinyl, imidazo[1,2-a]pyridinyl, 6-oxo-pyridazin-1(6H)-yl, 2-oxopyridin-1 (2H)-yl, 6-oxo-pyrudazin-1(6H)-yl, 2-oxopyridin-1(2H)-yl, 1,3-benzodioxolyl, quinolinyl, isoquinolinyl, cinnolinyl, quinazolinyl, quinoxalinyl, optionally substituted by one or more groups selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. A preferred heteroaryl group used herein is pyridyl. ^ ^ ^ ^ ^ ^ ^ ^heteroarylene group ^ corresponds to the bivalent group obtained by removal of a hydrogen atom from a heteroaryl group, as defined above herein, resulting in a moiety with two points of attachment. A preferred heteroarylene group used herein is pyridylene optionally substituted by one or more groups selected from the group consisting of halogen, C
1-6 alkyl, C
1-6 haloalkyl, C
1-6 acyl and C
1-6 alkoxy. [0082] ^ ^ ^ ^ ^ ^ ^ ^heteroatom ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ phosphorus, selenium, or silicon (including, any oxidized form of nitrogen, sulfur, phosphorus, selenium, or silicon; the quaternized form of any basic nitrogen or; a substitutable nitrogen of a heterocyclic ring, for example N (as in 3,4-dihydro-2H- pyrrolyl), NH (as in pyrrolidinyl) or NR+ (as in N-substituted pyrrolidinyl)). [0083] ^ ^ ^ ^ ^ ^ ^ ^unsaturated ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ units of unsaturation. [0084] ^ ^ ^ ^ ^ ^ ^ ^halogen ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ [0085] ^ ^ ^ ^ ^ ^ ^ ^arylalkyl ^ refers to a ^Alk-Ar group, wherein Alk represents an alkyl group as defined earlier, and Ar represents an aryl group as defined earlier. [0086] ^ ^ ^ ^ ^ ^ ^ ^heteroalkyl ^ refers to a linear or branched saturated hydrocarbon chain, comprising 1 to 5 carbon atoms and at least 1 or 2 heteroatoms, such as sulfur, nitrogen or oxygen atoms, in particular groups alkoxy, alkylamines, dialkylamines, thioethers, among others. Heteroalkyl groups, for example include -O(CH2)nOCH3, - (CH2)nOCH3, -N(CH2)n-N(CH2CH3)2, -N(CH2CH3)2, or -(CH2)n-S-(CH2)n-CH3, where n is selected from 1 to 4, among others. [0087] ^ ^ ^ ^ ^ ^ ^ ^cycloalkyl ^ refers to a saturated monocyclic or polycyclic system, such as a fused or bridged bicyclic system, comprising 3-12 carbon atoms, such as the cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, adamantly, decalinyl, or norbornyl groups. [0088] ^ ^ ^ ^ ^ ^ ^ ^haloalkyl ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ comprising 1-6 carbon atoms and substituted with one or more, and notably 1-6 halogen atoms, such as the trifluoromethyl or 2,2,2-trifluoroethyl groups. [0089] ^ ^ ^ ^ ^ ^ ^ ^O-Ra^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ a haloalkyl or an arylalkyl group, as defined earlier, is connected to the remainder of the molecule through an oxygen atom. O-cycloalkyl includes for example the O-cyclopentyl or O-cyclohexyl group. [0090] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^optionally substituted ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ means that one or more hydrogens of the designated moiety of compounds are replaced ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ explicit or implicit from the structure (e.g.,


^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ each substitutable position of the group, and when more than one position in any given structure may be substituted with more than one substituent selected from a specified group, the substituent may be either the same or different at every position. Combinations of substituents envisioned by this disclosure are preferably those that result in the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ refers to compounds that are not substantially altered when subjected to conditions to allow for their production, detection, and, in certain embodiments, their recovery, purification, and use for one or more of the purposes disclosed herein. [0091] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ alkyl, acyl, aryl, heteroaryl, arylalkyl, heteroalkyl, cycloalkyl, alkoxy, haloalkyl, haloalkoxy, or a group O-Ra, wherein Ra and each of the substituents are as defined above herein, among others. [0092] When the terms ^compounds ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^, ^compounds of ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ pharmaceutically acceptable salts that said moieties and compounds may form. As used ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^pharmaceutically acceptable salt ^ includes conventional salts formed from pharmaceutically acceptable inorganic or organic acids or bases as well as quaternary ammonium salts. More specific examples of suitable acid salts include hydrochloric, hydrobromic, sulfuric, phosphoric, nitric, perchloric, fumaric, acetic, propionic, succinic, glycolic, formic, lactic, maleic, tartaric, citric, palmoic, malonic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicylic, fumaric, toluenesulfonic, methanesulfonic, naphthalene-2-sulfonic, benzenesulfonic hydroxynaphthoic, hydroiodic, malic, steroic, tannic etc. More specific examples of suitable basic salts include sodium, lithium, potassium, magnesium, aluminium, calcium, zinc, N,N'- dibenzylethylenediamine, chloroprocaine, choline, diethanolamine, ethylenediamine, N- methylglucamine and procaine salts. For example, preferred salt forms include sodium salts of the compounds of formula (III) disclosed within the scope of the present description. [0093] Pharmaceutically acceptable salts are well known in the art. For example, S. M. Berge et al., describe pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences, 1977, 66, 1 ^19, incorporated herein by reference. [0094] Many organic compounds can form complexes with solvents in which they are reacted or from which they are precipitated or crystallized. These complexes are known as "solvates". For example, a complex with water is known as a "hydrate". Solvates of the compounds of formula (III) are within the scope of the present invention. [0095] ^ ^ ^ ^ ^ ^ ^ ^isomer ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ molecular formulae as identified herein but which differ by nature or in the binding sequence of their atoms or in the layout of their atoms in space. Isomers which differ in ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ stereoisomers which are non-superposable mirror images of each other are designated as ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ diastereoisomers. A pair of diastereoisomers is designated as epimers. Unless otherwise stated, structures depicted herein are also meant to include all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or conformational)) forms of the structure; for example, the R and S configurations for each asymmetric center, Z and E double bond isomers, and Z and E conformational isomers. Therefore, single stereochemical isomers as well as enantiomeric, diastereomeric, and geometric (or conformational) mixtures of the present compounds are within the scope of the present disclosure. Unless otherwise stated, all tautomeric forms are within the scope of the disclosure. [0096] ^ ^ ^ ^ ^ ^ ^ ^anomer ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ in the configuration of their C-1 carbon atom if said monosaccharide is an aldose, and in the configuration of their C-2 carbon atom if they are ketoses, wherein said C-1 or C-2 carbon atom ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ [0097] ^ ^ ^ ^ ^ ^ ^ ^bioisostere ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ particular to the groups amide, urethane and ester, included in the embodiments and aspects defined in the present invention, refers to other possible groups or moieties which are comparable in electronic and steric arrangement to said specific group, meaning that the bioisostere groups share some common biological properties in addition to their physicochemical analogy. [0098] Additionally, unless otherwise stated, the present disclosure also includes compounds that differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures including the replacement of hydrogen by deuterium or tritium, or the replacement of a carbon by a 13C- or 14C-enriched carbon are within the scope of this disclosure. Such compounds are useful, for example, as analytical tools, as probes in biological assays, or as therapeutic agents in accordance with the present disclosure. In some embodiments, compounds of this disclosure comprise one or more deuterium atoms. [0099] Combinations of substituents and variables envisioned by this disclosure are only ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ refers to compounds which possess stability sufficient to allow manufacture and which maintains the integrity of the compound for a sufficient period of time to be useful for the purposes detailed herein (e.g., therapeutic or prophylactic administration to a subject). Agents [0100] As used herein, the term "agent" (also referred to as "actives" or "active agents) refers to any compound or molecule that, when administered to a subject, has a therapeutic, diagnostic, and/or prophylactic effect and/or elicits a desired biological and/or pharmacological effect. Such agents include, but are not limited to, nucleic acids, chemotherapeutic agents, small molecule drugs, proteins and peptides, antibodies, antibody fragments, among others. Nucleic acids [0101] In some embodiments the agent is a therapeutic nucleic acid (TNA) is encapsulated in the LNP. [0102] As used herein, the phrases "nucleic acid therapeutic", "therapeutic nucleic acid" and "TNA" are used interchangeably and refer to any modality of therapeutic using nucleic acids as an active component of therapeutic agent to treat a disease or disorder. As used herein, these phrases refer to RNA-based therapeutics and DNA-based therapeutics. Non-limiting examples of RNA-based therapeutics include mRNA, antisense RNA and oligonucleotides, ribozymes, aptamers, interfering RNAs (RNAi), Dicer-substrate dsRNA, small hairpin RNA (shRNA), locked nucleic acids (LNAs), asymmetrical interfering RNA (aiRNA), microRNA (miRNA), CRISPR/Cas9 technology and single guide RNA (sgRNA). Non-limiting examples of DNA-based therapeutics include minicircle DNA, minigene, viral DNA (e.g., Lentiviral or AAV genome) or non-viral synthetic DNA vectors, closed-ended linear duplex DNA (ceDNA/CELiD), plasmids, bacmids, DOGGYBONETM DNA vectors, minimalistic immunological-defined gene expression (MIDGE)-vector, nonviral ministring DNA vector (linear-covalently closed DNA vector), or dumbbell-shaped DNA minimal vector ("dumbbell DNA"). [0103] Illustrative therapeutic nucleic acids of the present disclosure can include, but are not limited to, minigenes, plasmids, minicircles, small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASO), ribozymes, closed ended double stranded DNA (e.g., ceDNA, CELiD, linear covalently closed DNA ("ministring"), doggyboneTM, protelomere closed ended DNA, or dumbbell linear DNA), dicer-substrate dsRNA, small hairpin RNA (shRNA), LNAs, asymmetrical interfering RNA (aiRNA), microRNA (miRNA), mRNA, tRNA, rRNA, CRISPR/Cas9 technology and sgRNA, and DNA viral vectors, viral RNA vector, and any combination thereof. [0104] siRNA or miRNA that can downregulate the intracellular levels of specific proteins through a process called RNA interference (RNAi) are also contemplated by the present disclosure to be nucleic acid therapeutics. After siRNA or miRNA is introduced into the cytoplasm of a host cell, these double-stranded RNA constructs can bind to a protein called RISC. The sense strand of the siRNA or miRNA is removed by the RISC complex. The RISC complex, when combined with the complementary mRNA, can induce either a translation blockade or mRNA cleavage and release the cut strands. RNAi is by inducing specific destruction of mRNA that results in downregulation of a corresponding protein. [0105] Antisense oligonucleotides (ASO) and ribozymes that inhibit mRNA translation into protein can be nucleic acid therapeutics. For antisense constructs, these single stranded deoxy nucleic acids have a complementary sequence to the sequence of the target protein mRNA, and Watson - capable of binding to the mRNA by Crick base pairing. This binding prevents translation of a target mRNA, modulates splicing and/or triggers RNaseH degradation of the mRNA transcript. As a result, the antisense oligonucleotide has increased specificity of action (i.e., down-regulation of a specific disease-related protein). [0106] In any of the aspects and embodiments provided herein, the therapeutic nucleic acid can be a therapeutic RNA. Said therapeutic RNA can be an inhibitor of mRNA translation, agent of RNA interference (RNAi), catalytically active RNA molecule (ribozyme), transfer RNA (tRNA) or an RNA that binds an mRNA transcript (ASO), protein or other molecular ligand (aptamer). In any of the methods provided herein, the agent of RNAi can be a double-stranded RNA, single-stranded RNA, microRNA, short interfering RNA, short hairpin RNA, or a triplex-forming oligonucleotide. [0107] In one embodiment, the TNA is a denatured TNA. In one embodiment, the denatured TNA is a closed ended DNA (ceDNA). The term "denatured therapeutic nucleic acid" refers to a partially or fully TNA where the conformation has changed from the standard B-form structure. The conformational changes may include changes in the secondary structure (i.e., base pair interactions within a single nucleic acid molecule) and/or changes in the tertiary structure (i.e., double helix structure). [0108] In one embodiment, the TNA is one or more components of the CRISPR/Cas9 system. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system (CRISPR/Cas9) is used to edit the genome, wherein the enzyme Cas9 makes cuts in the DNA and allows new genetic sequences to be inserted. Single- guide RNAs are used to direct Cas9 to the specific spot in DNA where cuts are desired. [0109] In some embodiments, the TNA is encapsulated in the LNP. In some embodiments, the TNA is selected from the group consisting of minigenes, plasmids, minicircles, small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASO), ribozymes, closed-ended (ceDNA), ministring, doggyboneTM protelomere closed ended DNA (ceDNA), or dumbbell linear DNA, dicer-substrate dsRNA, small hairpin RNA (shRNA), asymmetrical interfering RNA (aiRNA), microRNA (miRNA), mRNA, tRNA, rRNA, DNA viral vectors, viral RNA vector, non- viral vector and any combination thereof. In some embodiments, the TNA is ceDNA. In some embodiments, the TNA is siRNA. In some embodiments, the TNA is a plasmid. In some embodiments, the TNA is mRNA. In some embodiments, the TNA is one or more components of the CRISPR/Cas9 system as detailed herein. Cationic lipids and ionizable lipids [0110] The ionizable LNPs disclosed herein comprise a ionizable lipid. In some embodiments, the ionizable LNPs disclosed herein may further comprise a cationic lipid. In some embodiments, the cationic lipid is, e.g., a non-fusogenic cationic lipid. By a "non- fusogenic cationic lipid" is meant a cationic lipid that can condense and/or encapsulate the nucleic acid cargo, but does not have, or has very little, fusogenic activity. The cationic lipid and ionizable lipid are typically employed to condense the nucleic acid cargo, at low pH and to drive membrane association and fusogenicity. [0111] Cationic lipids are lipids comprising at least one quaternary amino group that is permanently positively charged and ionizable lipids are lipids comprising a secondary or tertiary amine group which becomes protonated under acidic conditions, for example at pH of 6.5 or lower. [0112] In some embodiments, the ionizable LNP comprises 1% or more of an ionizable lipid relative to the total weight of the LNP (w/w). In some embodiments the ionizable LNP comprises at least 5%, preferably at least 15%, more preferably at least 25%, even more preferably at least 35%, even yet more preferably at least 45% of an ionizable lipid relative to the total weight of the LNP (w/w). In one embodiment, the ionizable lipid, or the addition of ionizable lipid and cationic lipid, represents 1-90% (mol), for example 20- 90% (mol) of the total lipid present in the lipid particles (e.g., lipid nanoparticles). For example, ionizable lipid molar content, or the addition of ionizable lipid and cationic lipid molar content, can be 20-70% (mol), 30-60% (mol), 40-60% (mol), 40-55% (mol) or 45- 55% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticles). In some embodiments, ionizable lipid comprises from about 40 mol % to about 60 mol % of the total lipid present in the lipid nanoparticles (LNPs). [0113] In some embodiments, the cationic lipid is selected from the group consisting of N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA); N-[1- (2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTAP); 1,2-dioleoyl- sn-glycero-3-ethylphosphocholine (DOEPC); 1,2-dilauroyl-sn-glycero-3- ethylphosphocholine (DLEPC); 1,2-dimyristoyl-sn-glycero-3-ethylphosphocholine (DMEPC); 1,2-dimyristoleoyl- sn-glycero-3-ethylphosphocholine (14:1), N1-[2-((1S)-1- [(3-aminopropyl)amino]-4-[di(3-amino-propyl)amino]butylcarboxamido)ethyl]-3,4 - di[oleyloxy]-benzamide (MVL5); Dioctadecylamido-glycylspermine (DOGS); 3b4N- (N',N'-dimethylaminoethyl)carbamoyl] cholesterol (DC- Chol);Dioctadecyldimethylammonium Bromide (DDAB); a Saint lipid (e.g., SAINT-2, N-methyl-4-(dioleyl)methylpyridinium); 1,2-dimyristyloxypropyl-3- dimethylhydroxyethylammonium bromide (DMRIE); 1,2-dioleoyl-3-dimethyl- hydroxyethyl ammonium bromide (DORIE); 1,2-dioleoyloxypropyl-3- dimethylhydroxyethyl ammonium chloride (DORI); Di-alkylated Amino Acid (DILA2) (e.g., C18:1-norArg-C16); Dioleyldimethylammonium chloride (DODAC); 1-palmitoyl- 2-oleoyl-sn-glycero-3-ethylphosphocholine (POEPC); Dioctadecyldimethylammonium bromide (DDAB), (R)-5-guanidinopentane-1,2-diy1 dioleate hydrochloride (DOPen-G), (R)-N,N,N-trimethy1-4,5-bis(oleoyloxy)pentan-l-aminium chloride (DOTAPen) and 1,2-dimyristoleoyl-sn-glycero-3-ethylphosphocholine (MOEPC). In some variations, the ionizable lipid is selected from the group consisting of 1,2-dilinoleyloxy-3- dimethylaminopropane (DLinDMA), 2,2-dilinoley1-4-(2dimethylaminoethyl)-[1,3]- dioxolane (DLin-KC2-DMA), heptatriaconta-6,9,28,31-tetraen-19-y1-4- (dimethylamino)butanoate (DLin-MC3-DMA), 1,2-Dioleoyloxy-3- dimethylaminopropane (DODAP), 1,2-Dioleyloxy-3-dimethylaminopropane (DODMA), Morpholinocholesterol (Mo-CHOL), and (R)-5-(dimethylamino)pentane-1,2-diyl dioleate hydrochloride (DODAPen-Cl). In some embodiments, the ionizable lipid is DLin-MC3-DMA. [0114] In some embodiments, the ionizable lipid is MC3 (6Z,9Z,28Z,31Z)- heptatriaconta-6,9,28,31-tetraen-19-y1-4-(dimethylamino)butanoate (DLin-MC3-DMA or MC3) having the following structure:

[0115] The lipid DLin-MC3-DMA is described in Jayaraman et al., Angew. Chem. Int. Ed Engl. (2012), 51(34): 8529-8533. [0116] In some embodiments, the ionizable lipid is the lipid ATX-002 as described in WO2015/074085:
[0117] In some embodiments, the ionizable lipid is (13Z,16Z)-N,N-dimethy1-3- nonyldocosa-13,16-dien-1-amine (Compound 32), as described in WO2012/040184. [0118] In some embodiments, the ionizable lipid is Compound 6 or Compound 22 as described in WO2015/199952:

Non-cationic lipids [0119] In one embodiment, the lipid particles (LNPs) may further comprise a non- cationic lipid. The non-cationic lipid can serve to increase fusogenicity and also increase stability of the LNP during formation. Non-cationic lipids include amphipathic lipids, neutral lipids and anionic lipids. Accordingly, the non-cationic lipid can be a neutral uncharged, zwitterionic, or anionic lipid. Non-cationic lipids are typically employed to enhance fusogenicity. [0120] Exemplary non-cationic lipids include, but are not limited to, distearoyl-sn- glycero-phosphoethanolamine, distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylglycerol (DOPG), dipalmitoylphosphatidylglycerol (DPPG), dioleoyl-phosphatidylethanolamine (DOPE), palmitoyloleoylphosphatidylcholine (POPC), palmitoyloleoylphosphatidylethanolamine (POPE),dioleoyl- phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-l-carboxylate (DOPE- mal), dipalmitoyl phosphatidyl ethanolamine (DPPE), dimyristoylphosphoethanolamine (DMPE), distearoyl-phosphatidyl-ethanolamine (DSPE), monomethyl- phosphatidylethanolamine (such as 16-O-monomethyl PE), dimethyl- phosphatidylethanolamine (such as 16-O-dimethyl PE), 18-1-trans PE, 1-stearoyl-2- oleoyl-phosphatidyethanolamine (SOPE), hydrogenated soy phosphatidylcholine (HSPC), egg phosphatidylcholine (EPC), dioleoylphosphatidylserine (DOPS), sphingomyelin (SM), dimyristoyl phosphatidylcholine (DMPC), dimyristoyl phosphatidylglycerol (DMPG), distearoylphosphatidylglycerol (DSPG), dierucoylphosphatidylcholine (DEPC), palmitoyloleyolphosphatidylglycerol (POPG), dielaidoyl-phosphatidylethanolamine (DEPE), 1,2-dilauroyl-sn-glycero-3 -pho sphoethanolamine (DLPE); 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine (DPHyPE); lecithin, phosphatidylethanolamine, lysolecithin, lysophosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, sphingomyelin, egg sphingomyelin (ESM), cephalin, cardiolipin, phosphatidicacid,cerebrosides, dicetylphosphate, lysophosphatidylcholine, dilinoleoylphosphatidylcholine, or mixtures thereof. It is to be understood that other diacylphosphatidylcholine and diacylphosphatidylethanolamine phospholipids can also be used. The acyl groups in these lipids are preferably acyl groups derived from fatty acids having C10-C24 carbon chains, e.g., lauroyl, myristoyl, palmitoyl, stearoyl, or oleoyl. Other examples of non-cationic lipids suitable for use in the LNPs include nonphosphorous lipids such as, e.g., stearylamine, dodecylamine, hexadecylamine, acetyl palmitate, glycerolricinoleate, hexadecyl stereate, isopropyl myristate, amphoteric acrylic polymers, triethanolamine-lauryl sulfate, alkyl-aryl sulfate polyethyloxylated fatty acid amides, dioctadecyldimethyl ammonium bromide, ceramide, sphingomyelin, and the like. [0121] In one embodiment, the non-cationic lipid is a phospholipid. In one embodiment, the non-cationic lipid is selected from the group consisting of DSPC, DPPC, DMPC, DOPC, POPC, DOPE, and SM. In some embodiments, the non-cationic lipid is DSPC. In other embodiments, the non-cationic lipid is DOPC. In other embodiments, the non- cationic lipid is DOPE. [0122] In some embodiments, the non-cationic lipid can comprise 0-30% (mol) of the total lipid present in the lipid nanoparticle. In some embodiments, the non-cationic lipid content is 0.5-15% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). In some embodiments, the non-cationic lipid content is 5-12% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). In some embodiments, the non-cationic lipid content is 5-10% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). In one embodiment, the non-cationic lipid content is about 10% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). [0123] Exemplary non-cationic lipids are described in International Patent Application Publication No. WO2017/099823 and US Patent Application Publication No. US2018/0028664. Sterols [0124] In one embodiment, the lipid particles (e.g., lipid nanoparticles) can further comprise a component, such as a sterol, to provide membrane integrity and stability of the lipid particle. In one embodiment, an exemplary sterol that can be used in the lipid particle is cholesterol, or a derivative thereof. Non-limiting examples of cholesterol ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-cholestanol, 5 ^-coprostanol, cholesteryl- (2'-hydroxy)-ethyl ether, cholesteryl-(4'-hydroxy)-butyl ether, and 6-ketocholestanol; non- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- cholestanone, and cholesteryl decanoate; and mixtures thereof. In some embodiments, the cholesterol derivative is a polar analogue such as cholesteryl-(4'-hydroxy)-butyl ether. In some embodiments, cholesterol derivative is cholestryl hemisuccinate (CHEMS). [0125] Exemplary cholesterol derivatives are described in International Patent Application Publication No. WO2009/127060 and U.S. Patent Application Publication No. US2010/0130588. [0126] In one embodiment, the component providing membrane integrity, such as a sterol, can comprise 0-50% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). In some embodiments, such a component is 20-50% (mol) of the total lipid content of the lipid particle (e.g., lipid nanoparticle). In some embodiments, such a component is 30-40% (mol) of the total lipid content of the lipid particle (e.g., lipid nanoparticle). In some embodiments, such a component is 35-45% (mol) of the total lipid content of the lipid particle (e.g., lipid nanoparticle). In some embodiments, such a component is 37-40% (mol) of the total lipid content of the lipid particle (e.g., lipid nanoparticle). In one embodiment, the sterol content is about 38% (mol) of the total lipid present in the lipid particle (e.g., lipid nanoparticle). PEGylated lipids [0127] In one embodiment, the lipid particle (e.g., lipid nanoparticle) can further comprise a polyethylene glycol (PEG) or a conjugated squaramide modified lipid molecule (for example, a squaramide modified compound of formula (I)). Generally, these are used to inhibit aggregation of lipid particle (e.g., lipid nanoparticle) and/or provide steric stabilization. Exemplary conjugated squaramide modified lipids include, but are not limited to, PEG-lipid conjugates, polyoxazoline (POZ)-lipid conjugates, polyamide -lipid conjugates (such as ATTA-lipid conjugates), cationic-polymer lipid (CPL) conjugates, and mixtures thereof. In some embodiments, the conjugated squaramide modified lipid molecule is a PEGylated lipid, for example, a (methoxy polyethylene glycol)-squaramide modified conjugated lipid. In some other embodiments, the PEGylated lipid is PEG2000-DMG (dimyristoylglycerol). [0128] Exemplary PEGylated lipids include, but are not limited to, PEG-diacylglycerol (DAG) (such as 1-(monomethoxy-polyethyleneglycol)-2,3-dimyristoylglycerol (PEG- DMG)), PEG-dialkyloxypropyl (DAA), PEG-phospholipid, PEG-ceramide (Cer), a pegylated phosphatidylethanoloamine (PEG-PE), PEG succinate diacylglycerol (PEGS- DAG) (such as 4-0-(2',3'-di(tetradecanoyloxy)propy1-1-0-(w- methoxy(polyethoxy)ethyl) butanedioate (PEG-S-DMG)), PEG dialkoxypropylcarbam, N-(carbonyl-methoxypoly ethylene glycol 2000)-1,2-distearoyl-sn-glycero-3- phosphoethanolamine sodium salt, or a mixture thereof. Additional exemplary PEG-lipid conjugates are described, for example, in US5,885,613, US6,287,591, US2003/0077829, US2003/0077829, US2005/0175682, US2008/0020058, US2011/0117125, US2010/0130588, US2016/0376224, and US2017/0119904. [0129] In one embodiment, the PEG-DAA PEGylated lipid can be, for example, PEG- dilauryloxypropyl, PEG-dimyristyloxypropyl, PEG-dipalmityloxypropyl, or PEG- distearyloxypropyl. The PEG-lipid can be one or more of PEG-DMG, PEG- dilaurylglycerol, PEG-dipalmitoylglycerol, PEG-disterylglycerol, PEG- dilaurylglycamide, PEG-dimyristylglycamide, PEG-dipalmitoylglycamide, PEG- disterylglycamide, PEG-cholesterol (1-[8'-(Cholest-5-en-3[beta]-oxy)carboxamido-3',6'- dioxaoctanyl]carbamoy1-[omega]-methyl-poly(ethyleneglycol), PEG-DMB (3,4Ditetradecoxylbenzyl-[omega]-methyl-poly(ethyleneglycol)ether), and 1,2- dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)- 2000]. In one embodiment, the PEG-lipid can be selected from the group consisting of PEG-DMG,1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N- [methoxy(polyethylene glycol)-2000], ,

[0130] In some embodiments, the PEGylated lipid is selected from the group consisting N-(Carbonyl-methoxypolyethyleneglycoln)-1,2-dimyristoyl-sn-glycero-3- phosphoethanolamine (DMPE-PEGn, where n is 350, 500, 750, 1000, 2000 or 5000), N- (Carbonyl-methoxypolyethyleneglycol.)-1,2-distearoyl-sn-glycero-3- phosphoethanolamine (DSPE-PEGn, where n is 350, 500, 750, 1000,2000 or 5000), DSPE-polyglycelin-cyclohexyl-carboxylic acid, DSPE-polyglycelin-2-methylglutar- carboxylic acid, 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine (DSPE) conjugated Polyethylene Glycol (DSPE-PEG-OH), polyethylene glycol-dimyristolglycerol (PEG- DMG), polyethylene glycol-distearoyl glycerol (PEG-DSG), or N-octanoyl-sphingosine- 1-{succinyl[methoxy(polyethylene glycol)200011 (C8 PEG2000 Ceramide). In some examples of DMPE-PEGn, where n is 350, 500, 750, 1000, 2000 or 5000, the PEG-lipid is N-(Carbonyl-methoxypolyethyleneglycol 2000)-1,2-dimyristoyl-sn-glycero-3- phosphoethanolamine (DMPE-PEG 2,000). In some examples of DSPE-PEGn. where n is 350, 500, 750, 1000 2000 or 5000, the PEG-lipid is N-(Carbonyl- methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-PEG 2,000). In some embodiments, the PEG-lipid is DSPE-PEG-O11. In some preferred embodiments, the PEG-lipid is PEG-DMG. [0131] In some embodiments, the conjugated squaramide modified lipid, e.g., PEGylated lipid, includes a tissue-specific targeting ligand (for example, a group RL), e.g., first or second targeting ligand. For example, PEG-DSPE conjugated with a mannose ligand. [0132] In one embodiment, lipids conjugated with a molecule other than a PEG can also be used in place of PEG-lipid. For example, polyoxazoline (POZ)-lipid conjugates, polyamide-lipid conjugates (such as ATTA-lipid conjugates), and cationic -polymer lipid (CPL) conjugates can be used in place of or in addition to the PEG-lipid. Exemplary conjugated lipids, i.e., PEG-lipids, (POZ)-lipid conjugates, ATTA-lipid conjugates and cationic polymer-lipids are described in the International Patent Application Publication Nos. WO1996/010392, WO1998/051278, WO20021087541, WO2005/026372, WO2008/147438, WO2009/086558, WO2012/000104, WO2017/117528, WO2017/099823, WO2015/199952, WO2017/004143, WO2015/095346, WO2012/000104, WO2012/000104, and WO2010/006282, U.S. Patent Application Publication Nos. US2003/0077829, US2005/0175682, US2008/0020058, US2011/0117125, US2013/0303587, US2018/0028664, US2015/0376115, US2016/0376224, US2016/0317458, US2013/0303587, US2013/0303587, and US20110123453, and U.S. Patent Nos. US5,885,613, US6,287,591, US6,320,017, and US6,586,559. [0133] In some embodiments, the PEGylated lipid can comprise 0,01-20% (mol) of the total lipid present in the lipid nanoparticle. In some embodiments, PEGylated lipid content is 0.5-10% (mol). In some embodiments, PEGylated lipid content is 1-5% (mol). In some embodiments, PEGylated lipid content is 1-3% (mol). In some embodiments, PEGylated lipid content is 1-2% (mol). In one embodiment, PEGylated lipid content is about 1% (mol). In one embodiment, PEGylated lipid content is about 1.5% (mol). In one embodiment, PEGylated lipid content is about 2% (mol). [0134] It is understood that molar ratios of the cationic lipid, e.g., ionizable cationic lipid, with the non-cationic-lipid, sterol, and PEGylated lipid (squaramide modified conjugated such as squaramide modified conjugated DSPE-PEG or not conjugated such as PEG-DMG) can be varied as needed. Functional moiety RL-NH- [0135] The functional moiety RL-NH- may be of any type and is typically selected depending on the biological effect which is sought when chemically modifying the LNP. [0136] In some embodiments, RL-NH- comprises a cell-type targeting ligand or a receptor targeting ligand, a labelling agent, a steric shielding agent, a drug moiety or combinations thereof. In some embodiments, the functional moiety RL-NH- may also comprise a (nano)-particle, including a magnetic (nano-) particle and a quantum dot. For instance, in some embodiments, RL-NH- may comprise an iron, stain, silicium, gold or carbon (nano)-particle. [0137] In some embodiments, RL-NH- is a functional moiety comprising, or consisting of, a labeling agent, e.g. a fluorescent dye such as fluorescein, rhodamine, boron- dipyrromethene (Bodipy®) dyes, and Alexa fluor®, a quantum dot or a radionuclide. [0138] In some embodiments, RL-NH- is a functional moiety comprising, or consisting of, a steric shielding agent, e.g. an agent able to mask certain epitopes of the capsid, thereby avoiding the binding of neutralizing antibodies. For instance, in some embodiments, RL-NH- may comprise a polyethylene glycol (PEG), pHPMA, or a polysaccharide. In some embodiments, RL-NH- comprises a polyethylene glycol (PEG), comprising from 1 to 40 ethylene glycol monomers, e.g. from 1 to 10, such as e.g. ^(OCH
2CH
2) ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH
2CH
2)
2 ^ (referred to herein as ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH2CH2)3 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH2CH2)4 ^ (referred to ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH2CH2)5 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ [0139] In some embodiments, the functional moiety RL-NH- comprises a group Z and optionally one or more spacers L. In other embodiments, the functional moiety RL-NH- consists of a group Z-NH- and does not comprise one or more spacers L. Group Z [0140] In some embodiments, Z is a functional moiety comprising, or consisting of a cell-type targeting ligand or a receptor targeting ligand, namely a ligand enabling targeting of a specific type of cell or a specific type of receptor. In some embodiments, such a ligand can enable modification of the tropism of the LNP, namely its capacity to selectively transfect a given cell line, tissue, and/or organ. For instance, in some embodiments, Z can comprise or consist of a ligand which specifically binds to a membrane biological entity (e.g. a membrane receptor) of the targeted cell. In some embodiments, such a ligand can be, for instance, a saccharide, a hormone, including a steroid hormone, a peptide such as RGD peptide, Angiopep-2 or muscle targeting peptides, a protein or a functionally active fragment thereof, a membrane receptor or a functionally active fragment thereof, CB1 and CB2 ligands, an antibody including heavy- chain antibody, or functionally active ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ScFv, a diabody, a spiegelmer, an aptamer including nucleic acid aptamer and peptide aptamer, a small chemical molecules known to bind to the targeted biological entity and the likes such as vitamins and drugs, and/or any suitable combination thereof. [0141] As used herein, ^ ^ ^ ^ ^ ^ ^ ^cell-type targeting ligand ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ (chemical or biological) that mediates binding and transduction or transfection of the target cell types, or increases transduction or transfection by different mechanisms, such as increased cell entry, and therefore can be used to increase efficiency and/or specificity of gene transfer into the targeted cell types, or to increase endosomal escape via conjugated ligands as a factor that enhances transduction/transfection, for improving the potency of the LNP. For example, the target cell is of a particular tissue type, and the cell- type targeting ligand binds to a marker protein, surface antigen, receptor protein, that is expressed by cells of the target tissue [0142] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^receptor targeting ligand ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ (chemical or biological) that is able to bind to a specific receptor and direct (or target) the LNP to this receptor and/or drive subsequent LNP-receptor internalization, increasing efficiency in LNP transport and/or transduction or transfection to/of the targeted cells or tissues. [0143] ^ ^ ^functionally active fragment ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ e.g., a protein, a membrane receptor or an antibody, which retains the functional activity of its full-length counterpart. [0144] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from a saccharide. Details on saccharides are provided hereafter. [0145] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from proteins such as transferrin or a peptide derived thereof (e.g. the THR peptide), Epidermal Growth Factor (EGF), and basic Fibroblast Growth Factor FGF. [0146] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from vitamins such as folic acid. [0147] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from a muscle targeting peptide (MTP). In certain embodiments, Z is a cancer cell targeting peptide and comprises a peptide such as RGD, including cyclic RGD. [0148] In some embodiments, Z comprises, or consists of, a cell-type targeting ligand or a receptor targeting ligand derived from small molecules or hormones such as naproxen, ibuprofen, cholesterol, progesterone, or estradiol. [0149] In some embodiments, Z comprises an antibody or antigen-binding portion thereof. In some such embodiments, an antibody may be or comprise, for example, a single chain antibody or variable domain, such as a camelid antibody, a heavy-chain antibody, a nanobody, a shark antibody, etc. In some embodiments, an antibody or antigen binding portion thereof may be or comprise a Fab, a ^ ^ ^ ^ ^ a VHH, a ScFv, a diabody, etc. In some particular embodiments, an antibody or antigen binding portion thereof may be characterized by having specific affinity for a particular cell-specific protein, membrane protein, and/or membrane protein receptor. [0150] In some embodiments, Z comprises or consists of a cell-type targeting ligand or a receptor targeting ligand selected from the group consisting of saccharides, hormones, peptides, glycosylated peptides, proteins, glycoproteins, or fragments thereof, membrane receptors or fragments thereof, antibodies or fragments thereof, spiegelmers, nucleic acid or peptide aptamers, vitamins, and drugs. [0151] In a specific embodiment, Z comprises or consists of a peptide or a protein, wherein the peptide may be a linear or a cyclic peptide. In some embodiments the peptide consists of proteinogenic amino acids. In some embodiments the peptide comprises non canonical, non-natural or modified amino acids. In some embodiments the peptide comprises a muscle targeting peptide (MTP) or a cancer cell targeting peptide and comprises a peptide such as RGD, including cyclic RGD. [0152] In a specific embodiment, Z comprises or consists of a saccharide selected from the group consisting of monosaccharides, oligosaccharides and polysaccharides; preferably the saccharide is a monosaccharide, wherein said monosaccharide is preferably selected from the group consisting of mannose, galactose, N-acetylglucosamine, N- acetylgalactosamine, fucose, fructose, glucose, xylose, trehalose, desosamine, glucuronic acid, S6-galactose, S6-N-acetylgalactosamine, P6-mannose, P6-glucose, sialic acidand P1-fructose, more preferably selected from the group consisting of mannose, fructose, glucose, N-acetylglucosamine, N-acetylgalactosamine, trehalose, glucuronic acid, S6- galactose, S6-N-acetylgalactosamine, P6-mannose, P6-glucose, sialic acid and P1- fructose, even more preferably mannose or glucose. [0153] In some particular embodiments, suitable examples of saccharides include, but are not limited to, monosaccharides, oligosaccharides, polysaccharides, and derivatives thereof; or a saccharide substituted by a peptide. [0154] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^derivatives ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ oligosaccharides or polysaccharides, is meant to encompass saccharides containing one or more non-hydroxyl group(s). Examples of such non-hydroxyl groups include, but are not limited to, a hydrogen, an alkyl, an amino group (such as e.g. NH2, an alkyl amino, a dialkyl amino), an N-acetylamino group and/or a thiol group. [0155] In some embodiments, the non-hydroxyl group is a negatively charged group such as a phosphate, a phosphonate, a sulfate, a sulfonate and a carboxyl group. [0156] ^Monosaccharides ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^s ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ and the most basic units of carbohydrates. Monosaccharides can be classified by the number of carbon atoms they contain, e.g., 3 (trioses), 4 (tetroses), 5 (pentoses), 6 (hexoses), 7 (heptoses), and so on. [0157] Examples of monosaccharides include, but are not limited to, glycolaldehyde, glyceraldehyde, dihydroxyacetone, erythrose, threose, erythrulose, arabinose, lyxose, ribose, xylose, ribulose, xylulose, allose, altrose, galactose, glucose, gulose, idose, mannose, talose, fructose, psicose, sorbose, tagatose, mannoheptulose, and sedoheptulose. [0158] Deoxymonosaccharides are common derivatives of monosaccharides encompassed in the present invention, i.e., monosaccharides that have had a hydroxyl group replaced with a hydrogen atom. [0159] Examples of deoxymonosaccharides include, but are not limited to, deoxyribose, fucose, fuculose, rhamnose, quinovose, pneumose. [0160] 2-amino-2-deoxymonosaccharides are also common derivatives of monosaccharides encompassed in the present invention, i.e., monosaccharides that have had a hydroxyl group replaced with an amino group. [0161] Examples of 2-amino-2-deoxymonosaccharides include, but are not limited to, glucosamine, galactosamine, and daunosamine, as well as their acetylated forms, including, but not limited to, N-acetylglucosamine, and N-acetylgalactosamine. [0162] In some embodiments, the monosaccharide contains a negatively charged group such as a phosphate group, a sulfate group or a carboxyl group. [0163] Examples of monosaccharides containing a phosphate group, include, but are not limited to, glucose-6-phosphate, mannose-6-phosphate and fructose-1-phosphate [0164] Examples of monosaccharides containing a sulfate group, include, but are not limited to, galactose-6-sulfate (S6-galactose), N-acetylgalactosamine-6-sulfate (S6-N-acetylgalactosamine). [0165] Examples of monosaccharides containing a carboxyl group, include, but are not limited to, glucuronic acid and sialic acid. [0166] It is to be understood that the monosaccharides and derivatives thereof mentioned herein also encompass acyclic (open-chain) forms and cyclic forms. [0167] It is also to be understood that the monosaccharides and derivatives thereof mentioned herein also encompass D-stereoisomers and L-stereoisomers, as well as mixtures of D- and L- stereoisomers (e.g., racemic mixtures). [0168] It is also to be understood that the monosaccharides and derivatives thereof ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-anomers, as well as racemic mixtures ^ ^ ^- ^ ^ ^ ^-anomers. [0169] ^Oligosaccharides ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ (typically from two to ten) of monosaccharides. [0170] In some embodiments, an oligosaccharide according to the present invention comprises at least two, three, four, five, six, seven, eight, nine or ten monosaccharides, e.g., selected from the monosaccharides disclosed hereinabove, including their derivatives. [0171] In some embodiments, such oligosaccharide(s) can be a homooligosaccharide (i.e., composed of units of the same monosaccharide, including their derivatives) or heterooligosaccharides (i.e., composed of units of at least two different monosaccharides, including their derivatives). [0172] In some embodiments, examples of oligosaccharides include, but are not limited to, disaccharides, trisaccharides, tetrasaccharides, pentasaccharides, hexasaccharides, heptasaccharides, octasaccharides, nonasaccharides, and decasaccharides. [0173] In some embodiments, specific examples of disaccharides include, but are not limited to, cellobiose, chitobiose, gentiobiose, gentiobiulose, isomaltose, kojibiose, lactose, lactulose, laminaribiose, maltose, maltulose, mannobiose, melibiose, melibiulose, nigerose, palatinose, rutinose, rutinulose, sophorose, sucrose, trehalose, turanose, and xylobiose. [0174] In some embodiments, specific examples of trisaccharides include, but are not limited to, kestose, maltotriose, maltotriulose, melezitose, nigerotriose, and raffinose. [0175] In some embodiments, specific examples of tetrasaccharides include, but are not limited to, lychnose, maltotetraose, nigerotetraose, nystose, sesamose, and stachyose. [0176] In some embodiments, specific examples of oligosaccharides include, but are not limited to, acarbose, fructooligosaccharide, galactooligosaccharide, isomaltooligosaccharide, and maltodextrin. [0177] In some embodiments, oligosaccharides can be multi-antennary structures whereby some or all monosaccharides in the oligosaccharide are not linked to one another through O-glycosidic bonds but with branched linker structures. An example of a multi- antennary saccharide is tri-antennary N-acetylgalactosamine, which is a ligand for asialoglycoprotein receptor ASGPR (see e.g., Zhou et al., Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins; ACS Cent. Sci.2021). [0178] ^Polysaccharides ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ (typically more than ten) of monosaccharides. They range in structure from linear to highly branched. [0179] In some embodiments, a polysaccharide comprises more than ten monosaccharides (such as, e.g., 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 or more), e.g., selected from monosaccharides disclosed hereinabove, including their derivatives. In a similar way as described above for oligosaccharides, polysaccharides can be homopolysaccharides or heteropolysaccharides. [0180] In some embodiments, examples of polysaccharides include, but are not limited to, beta-glucans, lentinan, sizofiran, zymosan, cellulose, hemicellulose, chitin, chitosan, ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ mannan, pectin, starch, amylopectin, amylose, and xanthan gum. [0181] In some embodiments, a saccharide or derivative thereof according to the present invention is a monosaccharide, preferably a hexose. In some embodiments, a preferential saccharide or derivative thereof according to the present invention is mannose, glucose, galactose, N-acetylglucosamine, N-acetylgalactosamine, S6-galactose, S6-N-acetylgalactosamine, glucuronic acid, P6-galactose or P1-galactose. In some embodiments, a preferential saccharide or derivative thereof according to the present invention is mannose, galactose, N-acetylglucosamine, or N-acetylgalactosamine. [0182] In some embodiments, a saccharide or derivative thereof is mannose. In some embodiments, a saccharide or derivative thereof is galactose. In some embodiments, a saccharide or derivative thereof is N-acetylglucosamine. In some embodiments, a saccharide or derivative thereof is N-acetylgalactosamine. [0183] In some embodiments, a saccharide or derivative thereof according to the present invention is a deoxymonosaccharide. In some preferential embodiments, a deoxymonosaccharide is preferably fucose. [0184] In some embodiments, a saccharide or derivative thereof is a saccharide containing a non-hydroxyl group which is a dialkyl amino group. In some preferential embodiments, a saccharide containing a non-hydroxyl group which is a dialkyl amino group is a desosamine. [0185] In some embodiments, a saccharide or derivative thereof is a saccharide containing a non-hydroxyl group which is a sulfate group. In some preferential embodiments, a saccharide containing a non-hydroxyl group which is sulfate group is S6-galactose, or S6-N-acetylgalactosamine. [0186] In some embodiments, a saccharide or derivative thereof is a saccharide containing a non-hydroxyl group which is a phosphate group. In some preferential embodiments, a saccharide containing a non-hydroxyl group which is phosphate group is P6-glucose, P6- mannose, or P1-fructose. [0187] In some embodiments, a saccharide or derivative thereof is a saccharide containing a non-hydroxyl group which is a carboxyl group. In some preferential embodiments, a saccharide containing a non-hydroxyl group which is carboxyl group is glucuronic acid or sialic acid. [0188] In some embodiments, the saccharide is selected from the group comprising, or consisting of mannose, galactose, N-acetylglucosamine, N-acetylgalactosamine, fucose, fructose, glucose, xylose, trehalose, desosamine, glucuronic acid, S6-galactose, S6-N- acetylgalactosamine, P6-mannose, P6-glucose, sialic acid and P1-fructose. In some preferred embodiments the saccharide is selected from the group consisting of mannose, fructose, glucose, N-acetylglucosamine, N-acetylgalactosamine, trehalose, glucuronic acid, S6-galactose, S6-N-acetylgalactosamine, P6-mannose, P6-glucose, sialic acid and P1-fructose, more preferably mannose or glucose. Spacer L [0189] In some embodiments, the functional moiety RL-NH- comprises a group Z and at least one spacer L. In particular, in some embodiments, one or more spacers L are present for linking the group Z to the squaramide moiety of formula (SQ). [0190] In some embodiments, L may be any chemical chain which can comprise heteroatoms as well as cyclic moieties such as aryl and/or heteroaryl groups. [0191] In some embodiments, L may comprise up to 1000 carbon atoms and even more. The length and the chemical nature of L may be optimized depending on the group Z which is intended to be coupled to the LNP and the biological effect which is sought. [0192] In some embodiments, L is a chemical chain group comprising from 2 to 1000 carbon atoms, preferably from 2 to 500 carbon atoms, from 2 to 300 carbon atoms, e.g. from 2 to 100 carbon atoms, 2 to 40 carbon atoms, from 4 to 30 carbon atoms, or from 4 to 20 carbon atoms. [0193] In some embodiments, L connects the group Z to the squaramide moiety of formula (SQ), as defined in the present disclosure, and preferably comprises up to 1000 carbon atoms and is preferably in the form of a chemical chain which optionally comprises heteroatoms (e.g. O, NH, S, Se or P) and/or cyclic moieties, such as aryl and/or heteroaryl groups. [0194] In some embodiments, L may be selected from alkyl (e.g., C
1-20, C
1-12, C
1-6 alkyl), ether, polyether, polyester, alkyl amide, or a combination thereof. As used ^ ^ ^ ^ ^ ^ ^ ^combination ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ L may comprise several hydrocarbon chains, oligomer chains or polymeric chains (e.g.2, 3, 4, 5 or 6) linked by any appropriate group, such as -O-, -S-, -NHC(O)-, -OC(O)-, -C(O)-O-C(O)-, -NH-, -NH-CO-NH-, -O-CO-, -NH-(CS)- NH-, -NH-CS- phosphodiester or phosphorothioate groups. The use of a variety of alkyls is contemplated, including, but not limited to, ^(CH2)n ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ about 20 or more. In some embodiments, L comprises a C
2-20 straight or branched alkyl chain. In some embodiments, L is a polyether (e.g., polyethylene or polypropylene glycol). The use of a variety of ethers and polyethers is contemplated, including, but not limited to, ^(OCH
2CH
2)
n ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ an integer from about 1 to about 40 or more representing n the average number of PEG units in a range of molecular weights and oligomeric forms. In some embodiments, L is a polyethylene glycol ^ ^ ^ ^ ^ ^ ^ of formula ^(OCH
2CH
2)
n ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-10, an integer from 1-6, and integer from 3-6, and integer from 3-5, or an integer selected from 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, L is a polypropylene glycol, e.g., of formula ^(OCH(CH3)CH2)n ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-10, an integer from 1-6, and integer from 3-6, and integer from 3-5, or an integer selected from 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, L is an alkyl amide. The use of a variety of alkyl amides is contemplated, including, but not limited to, ^(CH2)y ^C(O)NH ^(CH2)p ^ and ^(OCH
2CH
2)
y ^C(O)NH ^(OCH
2CH
2)
p ^ ^ ^ ^ ^ ^ ^ ^ ^ ^y ^ ^ ^ ^ ^p ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^y ^ ^ ^ ^ ^p ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ or more. In some embodiments, L is an alkyl amide of formula ^(CH2)y ^C(O)NH ^(CH2)p ^ or of formula ^(OCH
2CH
2)
y ^C(O)NH ^(OCH
2CH
2)
p ^ ^ ^ ^ ^ ^ ^ ^ ^ ^y ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ selected from an integer from 1-10, an integer from 1-6, and integer from 3-6, and integer from 3-5, or an integer independently selected from 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. The use of a variety of amides having the linking units of alkyl or ether bonds is contemplated, including, but not limited to, ^ R3^C(O)NH ^R4^ ^ ^ ^ ^ ^ ^ ^ ^ ^R3^ ^ ^ ^ ^ ^4^ ^ ^ ^ each independently selected from alkyls (e.g., C
1-20, C
1-12, C
1-6 alkyl), ethers, or polyethers (e.g., PEGs having a molecular weight between about 200 to 2,000 g/mol). [0195] In some embodiments, L may also comprise an alkylene diamine, e.g., ^NH-(CH
2)
r-NH ^, ^ ^ ^ ^ ^ ^r ^ is an integer from 2 to 20, for instance from 2 to 10, or an integer selected from 2, 3, 4, or 5. In some embodiments, L is a polymer of alkylene diamines (also known as polyamines), e.g., a compound of formula ^NH-[(CH2)r-NH]t-, ^ ^ ^ ^ ^ ^r ^ is as defined above and herein, and ^t ^ is an integer of at least 2, for example of at least 3, 4, 5, 10 or more. Polymers of alkyl diamines of interest are, for instance, spermidine, and spermine. [0196] In some embodiments, L may also comprise polyamides such as poly(N-(2-hydroxypropyl)methacrylamide) (pHPMA), (e.g., pHPMA having a molecular weight between about 200 and about 5000 g/mol). [0197] In some embodiments, L may also comprise polyesters such as polycaprolactone (e.g., polycaprolactone having a molecular weight between about 200 and about 5000 g/mol) or poly(D,L-lactic-co-glycolic acid) (PLGA) (e.g., PLGA having a molecular weight between about 200 and about 5000 g/mol). [0198] In some embodiments, L may be selected from an optionally substituted group comprising, or consisting of, a saturated or unsaturated, linear or branched C
2-C
40 hydrocarbon chains, polyethylene glycol, polypropylene glycol, pHPMA, PLGA, polymers of alkylene diamines, and combinations thereof. [0199] In some embodiments, L is a polyethylene glycol (PEG), comprising from 1 to 40 ethylene glycol monomers, e.g. from 2 to 10, such as e.g. ^(OCH2CH2)2 ^ (referred to ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH2CH2)3 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^(OCH
2CH
2)
3 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^, ^(OCH
2CH
2)
4 ^ (referred to herein as ^ ^ ^ ^ ^ ^ ^ ^ or ^(OCH
2CH
2)
5 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^. [0200] In some embodiments, L may comprise one or more arylene or a heteroarylene groups Ar. In some particular aspects, the arylene or a heteroarylene group Ar is a 6- to 10-membered aromatic carbocyclic group or a 5- or 12-membered heterocyclic group comprising one or more heteroatoms selected from the group consisting of N, O, S and Se. In some embodiments, the group Ar is substituted by an acyl or an amide moiety, or a bioisostere thereof. In some particular aspects, the arylene or a heteroarylene group Ar is selected from the group consisting of phenylene and pyridylene. For example, in some embodiments, L comprises an optionally substituted phenylene moiety. For example, in some embodiments, L comprises an optionally substituted pyridylene moiety. In other embodiments, said phenylene or pyridylene groups are substituted by one or more moieties selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. [0201] In some embodiments, L may comprise an alkylene, ether, polyether, alkylene amide, arylene group, heteroarylene group, an acyl group or a combination thereof. In a specific embodiment, L comprises a polyether, arylene group, heteroarylene group, acyl group or a combination thereof. In a particular embodiment, L comprises an arylene or a heteroarylene group Ar. Preferably, said arylene or a heteroarylene group Ar is a 6- to 10-membered aromatic carbocyclic group or a 5- or 12-membered heterocyclic group comprising one or more heteroatoms selected from the group consisting of N, O, S and Se. In some particular aspects, the arylene or a heteroarylene group Ar is selected from the group consisting of phenylene and pyridylene optionally substituted by one or more moieties selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C
1-6 alkoxy. [0202] In a specific embodiment, L comprises a PEG. In another specific embodiment, L comprises a PEG and one or more aromatic groups, such as an arylene group and/or heteroarylene group Ar. In another specific embodiment, L comprises a PEG, one or more groups C
1-6 alkyl and one or more aromatic groups, such as an arylene group and/or heteroarylene group Ar. In a specific embodiment, L comprises a PEG and an aryl or a heteroaryl group Ar selected from the group consisting of phenylene and pyridylene optionally substituted by one or more moieties selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-6 alkoxy. R1 and R2 [0203] In some embodiments, wherein R1 is selected from the group consisting of H, C1- 6 alkyl, C1-6 haloalkyl, Z-(OCH2 ^CH2)n ^, Z-NH-(CH2)r ^(OCH2-CH2)n ^, and Z-C(O) ^(CH
2)r ^(OCH
2-CH
2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention. In some embodiments, R1 is selected from the group consisting of H, C1-6 alkyl, C
1-6 haloalkyl and Z-(OCH
2 ^CH
2)n ^, wherein n is selected from 1 to 40. In a preferred embodiment, R1 is selected from the group consisting of H, C
1-6 alkyl and C
1-6 haloalkyl. [0204] In some embodiments, R2 is selected from the group consisting of linear C1-12 alkyl, branched C3-12 alkyl, linear C1-12 haloalkyl, branched C3-12 haloalkyl and benzyl. In some preferred embodiments R2 is methyl, ethyl or benzyl, more preferably ethyl. Arylene or a heteroarylene group Ar [0205] In some embodiments, the group Ar is an arylene group, wherein said arylene group is as defined and described in classes and subclasses disclosed in the present invention. In other embodiments, the group Ar is an heteroarylene group, wherein said heteroaryl group is as defined and described in classes and subclasses disclosed in the present invention. [0206] In some embodiments, said arylene or a heteroarylene group Ar is a 6- to 10- membered arylene group or a 5- or 12-membered heteroarylene group comprising one or more heteroatoms selected from the group consisting of N, O, S and Se. In some particular embodiments, the arylene or a heteroarylene group Ar is selected from the group consisting of phenylene and pyridylene optionally substituted by one or more moieties selected from the group consisting of halogen, C1-6 alkyl, C1-6 haloalkyl, C1-6 acyl and C1-
6 alkoxy. Pl [0207] In some embodiments, the group Pl is a phospholipid comprising two fatty acids selected independently from saturated, monounsaturated and polyunsaturated C
8-30 fatty acids, for example C10-28, C12-26, C14-24, C16-22 fatty acids. In some embodiments, the fatty acids are saturated fatty acids. In some embodiments, the fatty acids are C18 saturated fatty acids. [0208] In some embodiments, the phospholipid moiety Pl is a phosphatidylcholine, comprising two fatty acids as described above herein linked to the extender group E by the terminal nitrogen atom of the choline moiety. Extender moiety E-NH- and extender group E [0209] In some embodiments, the extender group E of the extender moiety E-*NH- comprises one or more groups selected from the group consisting of a polyethylene glycol (PEG) or a polypropylene glycol (PPG) and an aromatic moiety. [0210] In some embodiments, the extender group E comprises a group PEG1000 (p=21) to PEG5000 (p=118), and an aromatic group Ar, as defined herein. [0211] In some embodiments, the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof. [0212] In some aspects, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a -(CH
2)q-C(O)-X- group, or a bioisostere moiety thereof, wherein q and X are as defined in classes and subclasses disclosed in the present invention. [0213] In some embodiments, the extender group E of the extender moiety E-*NH- comprises a PEG with 1 to 200, preferably 20 to 80, ethylene glycol units (-OCH
2CH
2), i.e. the group E comprises a group (OCH2CH2)p, wherein p is 1 to 200, preferably 20 to 80, representing p the average number of PEG units in a range of molecular weights and oligomeric form. [0214] In some embodiments, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X-group, or a bioisostere moiety thereof, wherein q and X are as defined in classes and subclasses disclosed in the present invention. [0215] In some embodiments, the extender group E comprises a PEG and the phospholipid is a phosphatidylcholine, wherein the PEG is linked to the terminal nitrogen of the choline moiety of the phosphatidylcholine moiety by a -(CH
2)q-C(O)-X-group, wherein q and X are as defined in classes and subclasses disclosed in the present invention. [0216] In some embodiments, the extender group E comprises a PEG and an aromatic group Ar, and the phospholipid moiety Pl is a phosphatidylcholine moiety, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the terminal nitrogen of the choline moiety of the phosphatidylcholine moiety by a -(CH
2)q-C(O)-X-group, wherein q and X are as defined in classes and subclasses disclosed in the present invention. Compound of formula (I) [0217] Some embodiments refer to a compound of formula (I):
wherein Pl is a phospholipid moiety; E-*NH- is an extender moiety comprising an extender group E and a group -*NH-; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0218] In some aspects, the extender group E of the extender moiety E-*NH- comprises a polyethylene glycol (PEG) or a polypropylene glycol (PPG). [0219] In some aspects, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a -(CH
2)q-C(O)-X-group, or a bioisostere moiety thereof and the compound of formula (I) is represented by formula (Ia1):
p is 1 to 200; preferably 20 to 80; q is 0 or 1; X is O or NH when q is 1 and X is NH when q is 0 or 1; preferably X is NH; and Pl and RL-NH are as defined and described in classes and subclasses disclosed in the present invention. [0220] In some aspects, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere moiety thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH
2)q-C(O)-X-group, and the compound of formula (I) is represented by formula (Ia
2) or (Ia3):
wherein p is 1 to 200; preferably 20 to 80; q is 0 or 1; m1 is 0, 1 or 2, m2 is 0, 1 or 2; X is O or NH when q is 1 and X is NH when q is 0 or 1; preferably X is NH; and Pl, Ar and RL-NH are as defined and described in classes and subclasses disclosed in the present invention. [0221] In some embodiments, L is an optionally substituted group selected from the group consisting of saturated or unsaturated, linear or branched C
2-C
40 hydrocarbon chains, polyethylene glycol, polypropylene glycol, pHPMA, PLGA, polymers of alkyl diamines and combinations thereof; preferably L is polyethylene glycol. In some embodiments, L is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers. In some preferred embodiments, the polyethylene glycol (PEG) is PEG3, PEG4, or PEG5. [0222] In some particular embodiments, the compound of formula (I), as defined in the present disclosure, comprises more than one spacer L selected from the group consisting of L1, L2 and L3, and said compound of formula (I) is selected from the group consisting of formula (Ib1), (Ib2), (Ib3), (Ib4) and (Ib5):
wherein Pl, E, Z, L
1, L
2 and L
3 are as defined and described in classes and subclasses disclosed in the present invention. [0223] In some embodiments, L1 is an optionally substituted group selected from the group consisting of saturated or unsaturated, linear or branched C
2-C
40 hydrocarbon chains, polyethylene glycol, polypropylene glycol, pHPMA, PLGA, polymers of alkyl diamines and combinations thereof; preferably L1 is polyethylene glycol. In some embodiments, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers. In some preferred embodiments the polyethylene glycol (PEG) is PEG3, PEG4, or PEG5. [0224] In some embodiments, L2 comprises one or more arylene or a heteroarylene groups Ar, as defined herein. In some preferred embodiments embodiments, L
2 comprises a phenylene group or a pyridylene group. [0225] In some aspects, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L
2 comprises one or more arylene or a heteroarylene groups; L
3 is a C
1-6 alkylene group, L
3 is covalently linked to L
2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L1 and L2 or L1 and L3 are covalently linked by an amide moiety, or a bioisostere moiety thereof, preferably an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl, Z-(OCH2 ^CH2)n ^, Z- NH-(CH2)r ^(OCH2-CH2)n ^, and Z-C(O) ^(CH2)r ^(OCH2-CH2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; more preferably R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl and Z-(OCH2 ^CH2)n ^,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40. [0226] In some aspects, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L
2 comprises one or more arylene or a heteroarylene groups; L
3 is a C
1-6 alkylene group; L
3 is covalently linked to L
2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L1 and L3 are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl, Z- (OCH2 ^CH2)n ^, Z-NH-(CH2)r ^(OCH2-CH2)n ^, and Z-C(O) ^(CH2)r ^(OCH2-CH2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl and Z-(OCH2 ^CH2)n ^ ,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH2 ^CH2)n ^ ,wherein n is selected from 1 to 40. [0227] In some aspects, L
1 and L
2 are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl, Z-(OCH2 ^CH2)n ^, Z-NH-(CH2)r ^(OCH2- CH
2)n ^, and Z-C(O) ^(CH
2)r ^(OCH
2-CH
2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C
1-6 alkyl, C
1-6 haloalkyl and Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH
2 ^CH
2)n ^,wherein n is selected from 1 to 40. In some preferred embodiments, n is 3, 4 or 5, r is 2 and R1 is H. [0228] In some aspects, L
1 and L
3 are covalently linked by an amide moiety -N(R1)C(O)-, or a bioisostere moiety thereof, wherein R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl, Z-(OCH2 ^CH2)n ^, Z-NH-(CH2)r ^(OCH2- CH2)n ^, and Z-C(O) ^(CH2)r ^(OCH2-CH2)n ^, wherein r is selected from 1 to 3, n is selected from 0 to 40, and Z is as defined and described in classes and subclasses disclosed in the present invention; preferably R1 is selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl and Z-(OCH2 ^CH2)n ^, wherein n is selected from 1 to 40 and more preferably R1 is H or Z-(OCH
2 ^CH
2)n ^, wherein n is selected from 1 to 40. In some preferred embodiments, n is 3, 4 or 5, r is 2 and R1 is H. [0229] Examples of bioisostere moieties of the amide -N(R1)C(O)- may be selected from -C(O)N(R1)-, -N(R3)C(O)N(R1)-, -N(R1)C(O)N(R3)-, -N(R1)C(S)-, -C(S)N(R1)-, -N(R1)C(S)N(R3)-, -N(R3)C(S)N(R1)-, -S(O)
2-N(R1)-, -N(R1)-S(O)
2- and a triazolyl group, among others, wherein R3 and R1 are each independently selected from the group consisting of H, C1-6 alkyl, C1-6 haloalkyl, aryl, alkylaryl, Z-(OCH2 ^CH2)n ^, Z-NH-(CH
2)r ^(OCH
2-CH
2)n ^, and Z-C(O) ^(CH
2)r ^(OCH
2-CH
2)n ^, wherein r is selected from 1 to 3, n is selected from 1 to 40, and R3 may be the same or different from R1. [0230] In some embodiments, when L
2 comprises an arylene or a heteroarylene group Ar, and L1 and the squaramide moiety of formula (SQ), or L1 and L3, when present, are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0231] In some embodiments, when L
2 comprises an arylene or a heteroarylene group Ar and L3 is a group C1-6 alkylene, L3 and the squaramide moiety of formula (SQ) are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0232] In some embodiments, when L2 comprises an arylene or a heteroarylene group Ar and L3 is a group C1-6 alkylene, one or more groups L3 are covalently bonded to said arylene or a heteroarylene group Ar in positions orto, meta or para. [0233] In some embodiments, L
1 or L
3 may be selected from alkyl (e.g., C
1-20, C
1-12, C
1- 6 alkyl), ether, polyether, polyester, alkyl amide, or a combination thereof. As used herein, ^combination ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ L
1 or L
3 may comprise several hydrocarbon chains, oligomer chains or polymeric chains (e.g.2, 3, 4, 5 or 6) linked by any appropriate group, such as -O-, -S-, -NHC(O)-, -OC(O)-, -C(O)-O-C(O)-, -NH-, -NH-CO-NH-, -O-CO-, -NH-(CS)-NH-, -NH-CS- phosphodiester or phosphorothioate groups. The use of a variety of alkyls is contemplated, including, but not limited to, ^(CH2)m ^ ^ ^ ^ ^ ^ ^ ^ ^ ^m ^ ^ ^ from about 2 to about 20 or more. In some embodiments, L3 comprises a C2-20 straight or branched alkyl chain. [0234] In some embodiments, L
1 or L
3 may also comprise polyesters such as polycaprolactone (e.g., polycaprolactone having a molecular weight between about 200 and about 5000 g/mol) or poly(D,L-lactic-co-glycolic acid) (PLGA) (e.g., PLGA having a molecular weight between about 200 and about 5000 g/mol). [0235] In some embodiments, L1 or L3 may be selected from an optionally substituted group comprising, or consisting of, saturated or unsaturated, linear or branched C2-C40 hydrocarbon chains, polyethylene glycol, polypropylene glycol, pHPMA, PLGA, polymers of alkylene diamines, and combinations thereof. The use of a variety of alkyl amides is contemplated, including, but not limited to, ^(CH2)m3 ^C(O)NH ^(CH2)m4 ^ and ^(OCH
2CH
2)
m3 ^C(O)NH ^(OCH
2CH
2)
m4 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^
3 ^ ^ ^ ^ ^ ^
4 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^m3 ^ ^ ^ ^ ^m4 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ or more. In some embodiments, L1 or L3 is an alkyl amide of formula ^(CH2)m3 ^C(O)NH ^(CH2)m4 ^ or ^(OCH2CH2)m3 ^ C(O)NH ^(OCH
2CH
2)
m4 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^m
3 ^ ^ ^ ^ ^m
4 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ an integer from 1-10, an integer from 1-6, and integer from 3-6, and integer from 3-5, or an integer independently selected from 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. The use of a variety of amides having the linking units of alkyl or ether bonds is contemplated, including, but not limited to, ^R
5 ^C(O)NH ^R
6 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^
5 ^ ^ ^ ^ ^ ^
6 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ from alkyls (e.g., C1-20, C1-12, C1-6 alkyl), ethers, or polyethers. [0236] In some embodiments, L1 may also comprise an acyl group -C(O)-(CH2)r, or an alkylene amine, e.g., ^NH-(CH
2)
r-, or an alkylene diamine, e.g., ^NH-(CH
2)
r-NH ^, where ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ to 10, or an integer selected from 2, 3, 4, or 5. In some embodiments, L1 is a polymer of alkylene diamines (also known as polyamines), e.g., a compound of formula ^NH-[(CH
2)
r-NH]
t ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ more. Polymers of alkyl diamines of interest are, for instance, spermidine, and spermine. In some embodiments, L1 may also comprise polyamides such as poly(N-(2- hydroxypropyl)methacrylamide) (pHPMA), (e.g., pHPMA having a molecular weight between about 200 and about 5000 g/mol). [0237] In some embodiments, L3 is a group C1-6 alkylene. In some preferred embodiments, L
3 is a C
1-3 alkylene. [0238] In some particular embodiments, the compound of formula (I) is selected from the group consisting of formula (Ic), (Id) and (Ie):
wherein Pl, E, n, m1, m2, N*, Z, Ar and R1 are as defined and described in classes and subclasses disclosed in the present invention. [0239] In another preferred embodiment, RL comprises a group Z and one spacer L, wherein Z is a saccharide, L is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers and the compound of formula (I) is represented by formula (IIb), as defined herein. [0240] In a preferred embodiment, the compound of formula (IIb1) comprises a group Z and one spacer L1, wherein Z is a saccharide, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers and the compound of formula (I) is represented by formula (Ic), as defined herein. [0241] In a preferred embodiment, the compound of formula (I) is represented by formula (Ib2) or (Ib3) comprising a group Z and spacers L
1, L
2 and L
3, as defined herein, Z is a saccharide, L
1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers, L2 is an arylene or a heteroarylene group Ar, L3 is a group C1-6 alkylene, L1 and L
2 are covalently linked by an amide moiety -N(R1)C(O)-, wherein R1 is as defined herein and the compound of formula (I) is represented by formula (IId). [0242] In another preferred embodiment of the LNP has a moiety of formula (Ib4) or (Ib5) comprising a group Z and spacers L1, L2 and L3, as defined herein, Z is a saccharide, L
1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers, L
2 is an arylene or a heteroarylene group Ar, L3 is a group C1-6 alkylene, L1 and L3 are covalently linked by an amide moiety -N(R1)C(O)-, wherein R1 is as defined herein and the moiety of formula (II) is represented by formula (IIe). [0243] It will be appreciated that, in the case wherein L (or L1) comprises a PEG group directly linked to a saccharide Z, the terminal oxygen atom of the PEG group, when present on the side of Z, can be part of Z. This is the case for example when Z is a saccharide wherein the anomeric carbon bears the PEG linker. [0244] In an illustrative embodiment, the compound of formula (I) is selected from:

n° Structure 3B10C 5B10C 6B10C 7B10C 7B8C 7B9C [0245] For the purposes of the present disclosure, when the compound of formula (I) comprises a saccharide, the stereochemistry of the anomeric carbon of said saccharides, when present, has not been represented in the drawings and figures of the compound of formula (I) disclosed in the present invention. Intermediate compounds of formula (IIa) (IIb), (IIIa) and (IIIb) [0246] In some embodiments, the present invention provides a compound of formula (IIa) which is useful in obtaining a compound of formula (I), as disclosed in the present invention:
wherein Pl, E and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0247] In some embodiments, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a
-(CH2
)q-C(O)-X- group, or a bioisostere moiety thereof and the compound of formula (IIa) is represented by formula (IIa
1):
wherein Pl, X, p, q and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0248] In some embodiments, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X-group, and the compound of formula (IIa) is represented by formula (IIa
2) or formula (IIa3):
wherein Pl, X, p, m1, m2, q, Ar and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0249] In some embodiments, the present invention provides a compound of formula (IIb) which is useful in obtaining a compound of formula (I), as disclosed in the present invention: (IIb) [0250] In some embodiments, the compound of formula (IIb) comprises more than one spacer L selected from the group consisting of L
1, L
2 and L
3, and said compound of formula (IIb) is selected from the group consisting of formula (IIb1), (IIb
2), (IIb
3), (IIb
4), (IIb5), and (IIb6), (IIb1). (IIb2) (IIb3),
wherein Z, L
1, L
2 and L
3 are as defined and described in classes and subclasses disclosed in the present invention. [0251] In some embodiments, the compound of formula (IIb) is selected from the group consisting of (IIc), (IId) and (IIe):
, [0252] In some embodiments, the present invention provides a compound of formula (IIIa) which is useful in obtaining a compound of formula (I), as disclosed in the present invention: (IIIa) wherein Pl and E are as defined and described in classes and subclasses disclosed in the present invention. [0253] In some embodiments, the extender group E of the extender moiety E-*NH- comprises a PEG covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X- group, or a bioisostere moiety thereof and the compound of formula (IIIa) is represented

wherein Pl, X, p and q are as defined and described in classes and subclasses disclosed in the present invention. [0254] In some embodiments, the extender group E of the extender moiety E-*NH- comprises an aryl group Ar and a PEG, wherein the aromatic group Ar and the PEG are linked by an amide group or a bioisostere thereof, and wherein the PEG is covalently linked to the phospholipid moiety Pl by a -(CH2)q-C(O)-X-group, and the compound of formula (IIIa) is represented by formula (IIIa
2) or formula (IIIa3):
wherein N*, Pl, Ar, X, p, m1, m2 and q are as defined and described in classes and subclasses disclosed in the present invention. [0255] In some embodiments, the present invention provides a compound of formula (IIIb) which is useful in obtaining a compound of formula (I), as disclosed in the present invention:
or a pharmaceutically acceptable salt thereof, wherein R2 and RL-NH- are defined and described in classes and subclasses in the present invention. [0256] For example, in some embodiments, the functional moiety RL-NH- comprises a group Z, one or more spacers L, and the compound of formula (III) is represented by formula (IIIb1):
(IIIb
1). wherein R2 , Z and L are as defined and described in classes and subclasses in the present invention. [0257] In some embodiments, the functional moiety RL-NH- comprises a group Z, and one or more than one spacer L selected from the group consisting of L1, L2 and L3 and, the compound of formula (IIIb1) is selected from the group consisting of formula (IIIb2), (IIIb3), (IIIb4), (IIIb5) and (IIIb6):
wherein R2 , Z, L
1, L
2 and L
3 are as defined and described in classes and subclasses in the present invention. In some aspects, L1 is a polyethylene glycol (PEG), comprising 1 to 40 ethylene glycol monomers; L2 comprises one or more arylene or a heteroarylene groups; L
3 is a C
1-6 alkylene group, L
3 is covalently linked to L
2 by one carbon atom of the arylene group or by one carbon atom or one heteroatom of the heteroarylene group; and L
1 and L2 or L1 and L3 are covalently linked by an amide moiety or a bioisostere moiety thereof. [0258] In some particular embodiments, the compound of formula (IIIb), as disclosed herein, is selected from the group consisting of formula (IIIc), (IIId) and (IIIe):
(IIIe), or a pharmaceutically acceptable salt thereof, wherein n, m
1, m
2, Z, Ar, R1 and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0259] In some embodiments, a compound is selected from a compound of Table 2, or a salt thereof. Table 2 n° Structure Formula (II) or (III)B1 Formula (IIIb) B2 Formula (IIb) B1 Formula (IIIb3) n° Structure Formula (II) or (III)B2 Formula (IIb2) B1 Formula (IIIb3) B1 Formula (IIIb3) B1 Formula (IIIb3)
n° Structure Formula (II) or (III)B2 Formula (IIb2) C1 Formula (IIa2) C1 Formula (IIa
3) 0C1 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[square Formula (polyethylene glycol)-2000], DSPE-PEG2000-SQUA, CAS 2315262- (IIa
1) 14-5 0C2 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- Formula [amino(polyethylene glycol)-2000], DSPE-PEG2000-NH2, CAS (IIIa1) 474922-26-4 Method for obtaining the squaramide modified ionizable LNPs [0260] Another aspect of the present invention refers to a method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention, wherein said method comprises: reacting a surface exposed squarate moiety of an ionizable LNP comprising a squarate modified phospholipid with a compound of formula (IIb), or a pharmaceutical salt thereof, at a pH between 7 and 9.5, preferably between 8 and 9: RL-NH2 (IIb) so that the group RL is conjugated to the ionizable LNP via a squaramide moiety of formula (SQ) as defined in the present description; or reacting a surface exposed primary amine moiety of an ionizable LNP comprising a primary amine modified phospholipid with a compound of formula (IIIb), or a pharmaceutical salt thereof, at a pH between 7 and 9.5, preferably between 8 and 9:

so that the group RL is conjugated to the ionizable LNP via a squaramide moiety of formula (SQ) as defined in the present description; wherein RL and R2 are as defined and described in classes and subclasses disclosed in the present invention. [0261] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^surface-exposed ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ moiety or a squarate moiety that is at least partially exposed at the outer surface of the LNP. [0262] In some embodiments said method of manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention comprises: putting into contact an ionizable lipid, a non-cationic lipid, a sterol, a PEGylated lipid and a compound of formula (IIa), or a pharmaceutically acceptable salt thereof,

with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention, to form an ionizable lipid nanoparticle; conjugating the compound of formula (IIa), or a pharmaceutically acceptable salt thereof, of the formed ionizable lipid nanoparticle with a compound of formula (IIb), or a pharmaceutically acceptable salt thereof, at a pH between 8 and 9.5: RL-NH
2 (IIb); wherein R2 is selected from the group consisting of linear C1-12 alkyl, branched C3-12 alkyl, linear C1-12 haloalkyl, branched C3-12 haloalkyl and benzyl; Pl is a phospholipid moiety; E-*NH- is an extender moiety; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0263] In other embodiments, said method of manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention comprises: putting into contact an ionizable lipid, a non-cationic lipid, a sterol, a PEGylated lipid and a compound of formula (IIIa), or a pharmaceutically acceptable salt thereof, (IIIa) with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention, to form an ionizable lipid nanoparticle; conjugating the compound of formula (IIIa) of the formed lipid nanoparticle with a compound of formula (IIIb), at a pH between 8 and 9.5:

wherein R2 is selected from the group consisting of linear C
1-12 alkyl, branched C
3-12 alkyl, linear C
1-12 haloalkyl, branched C
3-12 haloalkyl and benzyl; Pl is a phospholipid moiety; E-*NH- is an extender moiety; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein said functional moiety RL-NH- comprises a steric shielding agent, a labelling agent, a cell-type targeting ligand or a receptor targeting ligand, a drug moiety or a combination thereof. [0264] In yet other embodiments, said method for manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention comprises putting in contact a compound of formula (I), an ionizable lipid, a non-cationic lipid, a sterol and a PEGylated lipid, with an agent (such as a nucleic acid), as defined and described in classes and subclasses disclosed in the present invention. [0265] In some embodiments, the methods herein disclosed include conditions suitable for reacting a squarate moiety of the compounds of formula (IIa) and (IIIb) as defined and described in classes and subclasses disclosed in the present invention, with the amino group of, respectively, a compound of formula (IIb) and (IIIa). [0266] In some embodiments, suitable conditions to obtain the compound of formula (I) as defined and described in classes and subclasses disclosed in the present invention include suitable conditions to promote the formation of a covalent bond between an amino group and the squarate moiety. [0267] In some embodiments, said suitable conditions include an aqueous buffer having a pH ranging from 5.5 to 10, preferably from 7 to 10, e.g. from 8 to 9.5, such as 9, 9.1 or 9.2. In some preferred embodiments, the pH is 9. [0268] In some embodiments, an incubation buffer can be selected from TRIS buffer, borate buffer, Hepes buffer, acetate buffer, phosphate buffer e.g. PBS, or Dulbecco's phosphate-buffered saline (dPBS). In some preferred embodiments, the buffer is TRIS buffer. [0269] In some embodiments, an incubation can last from several minutes to several hours, for instance from 5 min to 6 hours, e.g. from 3 to 5 hours. In some preferential embodiments, the incubation is about 4 hours. In some embodiments, an incubation can last from several hours to several days, for instance from 6 to 72 hours, e.g. from 12 to 48 hours or from 16 to 24 hours. In some embodiments, the incubation is ended when a sufficient yield of coupling is achieved. [0270] In some embodiments, the temperature of incubation is typically from 4°C to 50°C. In some preferential embodiments, the incubation is performed at room temperature, i.e. at a temperature from 18 °C to 30 °C, e.g. at around 20°C. In some embodiments, the incubating solution can be stirred. [0271] Generally, lipid nanoparticles (LNPs) can be formed by any method known in the art. For example, LNPs can be prepared by the methods described, for example, in US2013/0037977, US2010/0015218, US2013/0156845, US2013/0164400, US2012/0225129, and US2010/0130588. In some embodiments, LNPs can be prepared using a continuous mixing method, a direct dilution process, or an in-line dilution process. The processes and apparatuses for apparatuses for preparing lipid nanoparticles using direct dilution and in-line dilution processes are described in US2007/0042031. The processes and apparatuses for preparing lipid nanoparticles using step-wise dilution processes are described in US2004/0142025. In one embodiment, the lipid particles (e.g., lipid nanoparticles) can be prepared by an impinging jet process. [0272] Generally, the ionizable LNP particles are formed by mixing the lipids (ionizable lipid, a non-cationic lipid, a sterol and a PEGylated lipid, as defined and described in classes and subclasses disclosed in the present invention), including a squarate modified lipid of formula (IIa), or a pharmaceutically acceptable salt thereof:
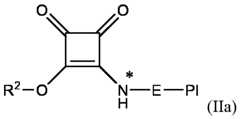
or an amine modified lipid of formula (IIIa), or a pharmaceutically acceptable salt thereof:
or a squaramide modified compound of formula (I)
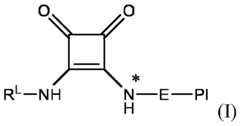
wherein Pl, E and R2 are as defined and described in classes and subclasses of the present description, dissolved in alcohol (e.g., ethanol) with a nucleic acid, or other agent (protein, small molecule drug, etc.), as defined herein, dissolved in a buffer, e.g., TRIS buffer, borate buffer, Hepes buffer, acetate buffer, citrate buffer, phosphate buffer e.g. PBS, or Dulbecco's phosphate-buffered saline (dPBS). The relative amounts of the nucleic acid and lipids in an ionizable LNP may vary. [0273] In some embodiments, the w/w ratio of the lipid component to the nucleic acid in an ionizable LNP may be from about 5: 1 to about 50: 1. In some embodiments, the wt/wt ratio of the lipid component to a therapeutic and/or prophylactic may be from about 10: 1 to about 20: 1. In one embodiment, the w/w ratio is about 10: 1. [0274] The lipid solution can contain an ionizable lipid, a non-cationic lipid (e.g., a phospholipid, such as DSPC, DOPE, and DOPC), PEG or PEG conjugated molecule (e.g., PEG-lipid), and a sterol (e.g., cholesterol) at a total lipid concentration of 5-30 mg/mL in an alcohol, e.g., in ethanol. In some embodiments the lipid solution further comprises a cationic lipid and optionally other lipids (anionic lipids for example). In the lipid solution, mol ratio of the lipids can range from about 25-60% for the ionizable lipid, or the addition of ionizable and cationic lipid, preferably about 35-65%; about 0-30% for the non- cationic lipid, preferably about 6-12%; about 0.01-20% for the PEG or PEG conjugated lipid molecule, preferably about 0.5-2%; and about 0-75% for the sterol, preferably about 30-50%. [0275] The nucleic acid solution can comprise the nucleic acid (such as a mRNA) at a concentration range from 0.3 to 1.0 mg/mL in buffered solution (e.g., a citrate buffer), with pH in the range of 3.5-5.LNPs can be prepared by combining the lipid solution with nucleic acid solution at wt:wt ratios between about 5:1 and about 50:1. The lipid solution is rapidly injected using a NanoAssemblr microfluidic based system at flow rates between about 10 ml/min and about 18 ml/min into the nucleic acid solution to produce a suspension with a water to ethanol ratio between about 1:1 and about 4:1. [0276] LNPs can be processed by dialysis to remove ethanol and achieve buffer exchange. Formulations are dialyzed against phosphate buffered saline (PBS) or Dulbecco's PBS (DPBS), pH 7.4, at 4°C for several hours. [0277] In some embodiments, a method of the invention may comprise one or several additional steps prior to, or after the step of incubation as described above. In particular, the compounds of formula (II) and formula (III), including all compounds selected from (IIa), (IIa
1), (IIb
1), (IIb
2), (IIb
3), (IIb
4), (IIb
5), (IIb
6), (IIIa), (IIIa1), (IIIb
1), (IIIb
2), (IIIb
3), (IIIb4), (IIIb5), (IIIb6), can be prepared by several methods. The starting products are commercial products or products prepared according to known synthesis from commercial compounds or known to one skilled in the art. [0278] In some examples the synthesis of a compound of formula (IIa):
comprises reacting an amine compound Pl-E-NH
2, wherein Pl and E are as defined and described in classes and subclasses of the present description, with a diester of squaric acid of formula (IV):
in suitable conditions to obtain a compound of formula (IIIb); wherein R2 is defined according to the classes and subclasses disclosed in the present invention. [0279] The compounds of formula (IIb), (IIb1), (IIb2), (IIb3), (IIb4), (IIb5), (IIb6), (IIc), IId) and (IIe), comprising an amino terminal group, may be obtained commercially ormay be prepared, for example, by reduction of the azide function, by methods and conditions for azide reduction well-known in the art, starting from the corresponding azide precursor. [0280] In some examples the synthesis of a compound of formula (IIIb) comprises reacting a functional moiety comprising an amino group RL-NH L 2, wherein R is as defined and described in classes and subclasses of the present description, with a diester of squaric acid of formula (IV):

in suitable conditions to obtain a compound of formula (IIIb); wherein R2 is defined according to the classes and subclasses disclosed in the present invention. [0281] In some examples, the synthesis of a compound of formula (IIIb) comprises: - providing a precursor compound of formula (V): (V), wherein Z, L1 are defined according to the classes and subclasses disclosed in the present invention; - reacting said compound of formula (V) with a diester of squaric acid of formula (IV), as defined herein, in suitable conditions to obtain a compound of formula (IIIb1):
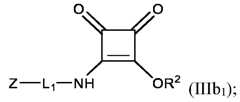
wherein R2 is defined according to the classes and subclasses disclosed in the present invention. [0282] The compounds of formula (V) may be prepared, for example, by reduction of the azide function, by methods and conditions for azide reduction well-known in the art, starting from the corresponding azide precursor disclosed in document WO2022096681. [0283] In other examples, the synthesis of a compound of formula (IIIb) comprises: - providing a precursor of formula (VIa) or a precursor of formula (VIIa):
wherein Z, L2, L3 and R2 are defined according to the classes and subclasses disclosed in the present invention; - reacting said compound of formula (VIa) or said compound of formula (VIIa) with a compound of formula (VIII),
in suitable conditions to obtain, respectively, a compound of formula (IIIb5) or a compound of formula (IIIb
6), wherein L
1 and L
3 are covalently linked by an amide moiety -N(R1)C(O)-, and wherein R1 is defined according to the classes and subclasses disclosed in the present invention. [0284] Suitable conditions for reacting a compound of formula (VIa) or a compound of formula (VIIa) with a compound of formula (VIII), as defined above herein are those well known in the art for peptidic coupling. [0285] Intermediates compounds of formula (VIa) and (VIIa) may be prepared from respectively the corresponding amino acid precursor of formulas (VIa ^ ^ ^ ^ ^ ^ ^ ^ ^a ^ ^ ^
with a compound of formula (IV) in presence of a base and an alcoholic solvent, wherein L2 and L3 and the compound of formula (IV) are defined according to the classes and subclasses disclosed in the present invention. [0286] In other examples, the synthesis of a compound of formula (IIIb) comprises: - providing a precursor of formula (VIb) or a precursor of formula (VIIb):
wherein Z, L2, L3 and R2 are defined according to the classes and subclasses disclosed in the present invention; - reacting said compound of formula (VIb) or said compound of formula (VIIb) with a compound of formula (VIII), (VIII), in suitable conditions to obtain, respectively, a compound of formula (IIIb
3) or a compound of formula (IIIb4), wherein L1 and L2 are covalently linked by an amide moiety -N(R1)C(O)-, and wherein R1, Z, L1 and L2 are defined according to the classes and subclasses disclosed in the present invention. [0287] Suitable conditions to react a compound of formula (VIb) or a compound of formula (VIIb) with a compound of formula (VIII), as defined above herein are those well known in the art for peptidic coupling. [0288] Intermediates compounds of formula (VIb) and (VIIb) may be prepared from respectively the corresponding amino acid precursor of formulas (VIb ^ ^ ^ ^ ^ ^ ^ ^ ^b ^ ^ ^
with a compound of formula (IV) in presence of a base and an alcoholic solvent, wherein L
2 and L
3 and the compound of formula (IV) are defined according to the classes and subclasses disclosed in the present invention. [0289] In yet other embodiments, said method of manufacturing an ionizable LNP as defined and described in classes and subclasses disclosed in the present invention comprises putting in contact a compound of formula (I), an ionizable lipid, a non-cationic lipid, a sterol and a PEGylated lipid; and wherein the functional moiety RL-NH- comprises a group Z and one or more spacers L, wherein Z is H or a cell-type specific ligand selected from the group consisting of monosaccharides, oligosaccharides, hormones, peptides, glycosylated peptides, vitamins, and drugs moieties, and L comprises one or more groups selected from the group consisting of an aryl or a heteroaryl groups, an optionally substituted group comprising saturated or unsaturated, linear or branched C
2-C
40 hydrocarbon chains, a polyethylene glycol (PEG), a polypropylene glycol (PPG), pHPMA, PLGA, polymers of alkylene diamines, and combinations thereof. Uses of provided LNPs [0290] Ionizable lipid nanoparticles (LNPs) of the present invention can be designed for one or more specific applications. Ionizable LNPs of the present invention can be used as enhanced delivery of agents (e.g., therapeutic agents). Ionizable LNPs of the present invention can be used to deliver a wide variety of different agents to target cells. Typically, the agent delivered by the ionizable LNPs is a nucleic acid (e.g. mRNAs, cDNAs, or gene editing tools such as CRISPR or alternative systems), a small molecule drug (e.g. antibiotics), a chemotherapy drug (e.g. HDAC inhibitors), a peptide, a protein (e.g. monoclonal antibodies or enzymes) and another biological molecule (e.g. a viral vectors such as rAAVs). In some embodiments, ionizable LNPs of the present invention can be used as a research tool. In some embodiments, ionizable LNPs of the present invention can be for use as a medicament, for instance for use in gene therapy or for use in protein replacement therapy as vectors for the delivery of therapeutic nucleic acids such as DNA or RNA or therapeutic proteins, delivering antibodies or antibody fragments, or for the delivery of other type of drugs, for example a chemotherapy drug. In some embodiments, ionizable LNPs of the present invention can be for use in a diagnostic method, e.g. as an imaging agent. In some embodiments, ionizable LNPs of the present invention can be used as a combination of both a therapeutic and diagnostic tool, e.g., theragnostic use. Modifications of biological functionalities and/or properties of LNPs [0291] In some embodiments, chemical modifications of the components of the ionizable LNPs may modify one, or several, of its biological functionalities and/or properties. In some embodiments, biological functionalities and/or properties can depend on the nature of functional moiety RL which is introduced to modify the ionizable LNPs in the present invention. In some embodiments, one or more biological properties of a modified ionizable LNPs can be altered compared to the unmodified ionizable LNPs, such as: - a modified selectivity of the ionizable LNPs towards a specific organ, tissue, and/or cell type (e.g. an increased selectivity or a shifted selectivity from one tissue/organ/cell to another); and/or - a modified immunoreactivity of the ionizable LNPs, e.g. a decreased immunogenicity of the ionizable LNPs and/or a decreased affinity for neutralizing antibodies, and/or said ionizable LNPs triggers an altered response when administered in vivo, e.g. does not generate ionizable LNPs-directed neutralizing antibodies; and/or - an increased efficiency of the ionizable LNPs; and/or - an increased transfection efficacy of the ionizable LNPs towards a specific cell, tissue, and/or organ; and/or - a reduced cellular toxicity when transfecting cells in culture; and/or - an induced cellular targeted mortality of cancer cells; and/or - enabling the visualization/monitoring of the ionizable LNPs upon in vivo administration or upon modification of cells in vitro; and/or - enabling theragnostic applications; e.g. combining a therapeutic agent and a diagnostic agent. [0292] In some embodiments, when the ionizable LNPs is used as a medicament, e.g. as a gene vector for gene therapy, such modified properties may result in an improvement in the therapeutic index of the ionizable LNPs. In some embodiments, an improvement in the therapeutic index of the ionizable LNPs can result from decreases in the relative dose of ionizable LNPs to administer to the subject in order to achieve the sought therapeutic effect, such a reduction in dosage can decrease the relative toxicity of the ionizable LNPs therapeutic regime. [0293] In some embodiments, the ionizable LNPs of the present invention show a preferential tropism for an organ or cell selected from liver, heart, brain, joints, retina, and/or skeletal muscle. In some embodiments, the ionizable LNPs of the invention show a preferential tropism for cultured cells selected from, but not limited to, hepatocytes, cardiomyocytes, myocytes, neurons, motor neurons, retinal pigmented cells, photoreceptors, chondrocytes, hematopoietic stem cells (HSC), and/or induced pluripotent stem cells (iPS). Uses and method for transducing/transfecting cells [0294] In some embodiments, the present invention relates to an ionizable LNPs according to the present invention, for use in transfecting a cell of a subject. [0295] ^ ^ ^transducing a cell ^ ^ ^ transfecting a cell ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ an agent, as defined herein, such as a nucleic acid, small molecule, or a protein (antibody or fragment thereof) into a cell. The transduced/transfected nucleic acid or protein of interest may be of any type and is selected depending on the sought effect. In some embodiments, when the ionizable LNP according to the present invention is used for transduced/transfecting a cell, it comprises a gene or a protein. [0296] In some embodiments, the ionizable LNP according to the invention can comprise any of the therapeutic nucleic acids as defined according to the classes and subclasses disclosed in the present invention, such as, for example, minigenes, plasmids, minicircles, small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASO), ribozymes, closed ended double stranded DNA (e.g., ceDNA, CELiD, linear covalently closed DNA ("ministring"), doggyboneTM, protelomere closed ended DNA, or dumbbell linear DNA), dicer-substrate dsRNA, small hairpin RNA (shRNA), LNAs, asymmetrical interfering RNA (aiRNA), microRNA (miRNA), mRNA, tRNA, rRNA, CRISPR/Cas9 technology and sgRNA, and DNA viral vectors, viral RNA vector, and any combination thereof. For example, the LNP according to the invention can comprise an exogenous gene expression cassette. In some embodiments, said cassette may comprise a promoter, a gene of interest, and a terminator. Nucleic acids useful in the disclosure typically include a first region of linked nucleosides encoding a polypeptide of ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-terminus of the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-terminus of the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-stabilizing region. In some embodiments, a nucleic acid further includes a poly-A region or a Kozak sequence ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-UTR). In some embodiments, a nucleic acid (e.g., an mRNA) may include ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ a polyadenylation signal. Any one of the regions of a nucleic acid may include one or more alternative components (e.g., an alternative nucleoside). In some embodiments, the ^ ^-stabilizing region may contain an alternative nucleoside such as an L-nucleoside, an ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-0-methyl nucleoside and/or the coding region, 5;- ^ ^ ^ ^ ^ ^- UTR, or cap region may include an alternative nucleoside such as a 5-substituted uridine (e.g., 5 -methoxy uridine), a 1-substituted pseudouridine (e.g., 1 -methyl-pseudouridine), and/or a 5-substituted cytidine (e.g., 5-methyl- cytidine). [0297] In some embodiments, as an additional or alternative example, the ionizable LNP of the invention may comprise a DNA template for homologous recombination in cells. In some embodiments, such ionizable LNP can be used in combination with gene editing tools, for promoting homologous recombination in targeted cells. In some embodiments, the gene editing tools can be a guide polynucleotide configured to specifically bind the target polynucleotide and direct an endonuclease or a fragment thereof. The endonuclease may be part of a clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) protein complex. The heterologous endonuclease may be a clustered regularly interspaced short palindromic repeats (CRISPR)-associated (Cas) endonuclease or any alternative system such as the OMEGA (Obligate Mobile Element-guided Activity) system or a system based on Fanzor (Fz), a eukaryotic programmable RNA-guided endonuclease encoded by transposable elements. [0298] In some embodiments, the gene editing tools can be of any type, and encompass, without being limited to, CRISPR and its associated systems (including without limitation a Cas protein such as a Cas9 protein, or fusion protein thereof, or a Cas mRNA such as a Cas9 mRNA, a crRNA and tracrRNA, the latter two being either separate or linked together in a single gRNA), TALEN, Zinc Finger Nuclease, meganuclease, as well as RNA and DNA encoding said gene editing proteins and their associated systems. [0299] Accordingly, the present invention further refers to a method for editing the genome of a target cell with the CRISPR/Cas9 system comprising: contacting the target cell with an ionizable LNP, as defined and described in classes and subclasses disclosed in the present invention, wherein the ionizable LNP comprises one or more components of the CRISPR/Cas9 system and a group RL conjugated to the LNP via a squaramide moiety of formula (SQ) as defined herein, wherein said group RL comprises a cell-type specific ligand of the target cell. For example, the Cas9 enzyme and single-guide RNA can be encapsulated in the ionizable LNP. [0300] In some embodiments, the present invention also refers to a non-therapeutic method for delivering an agent (e.g. a nucleic acid or a protein) to a target cell comprising: contacting the target cell with an ionizable LNP, as defined and described in classes and subclasses disclosed in the present invention, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a group RL, conjugated to the ionizable LNP via a squaramide moiety of formula (SQ):
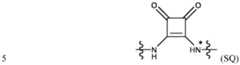
wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a phospholipid lipid Pl, comprising the group RL a cell-type targeting ligand or a receptor targeting ligand of the target cell. [0301] In some embodiments, the present invention also refers to an ionizable LNP for use in a non-therapeutic method for delivering an agent (e.g. a nucleic acid or a protein) to a target cell, wherein said method comprises: contacting the target cell with an ionizable LNP, as defined and described in classes and subclasses disclosed in the present invention, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a group RL, conjugated to the ionizable LNP via a squaramide

wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a phospholipid lipid Pl, comprising the group RL a cell-type targeting ligand or a receptor targeting ligand of the target cell. [0302] In some embodiments, the method for delivering an agent (e.g. a nucleic acid or a protein) comprises contacting the target cell with an ionizable LNP, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a group RL, conjugated to the ionizable LNP via a squaramide moiety of formula (SQ):
wherein N is a nitrogen atom from the functional moiety RL-NH-, and wherein N* is a nitrogen atom of an amino group of a phospholipid Pl, comprising the group RL a cell- type specific ligand of the target cell

wherein Pl is a phospholipid moiety; E-*NH- is an extender moiety comprising an extender group E and a group -*NH-; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein the functional moiety RL-NH- of the compound of formula (I) comprises a cell-type targeting ligand or a receptor targeting ligand of the target cell. [0303] In some embodiments, the present invention also relates to use of a LNP according to the present invention for transfecting a cell of a subject. [0304] In some embodiments, the present invention also relates to a method for transfecting a cell of a subject, comprising administering an ionizable LNP according to the present invention to said subject. [0305] In some embodiments, the present invention also relates to a method of delivering an agent (e.g. a gene or a protein, or small molecule) to a target cell, the method comprising contacting a cell with an ionizable LNP, as defined and described in classes and subclasses disclosed in the present, and an agent (e.g. a nucleic acid (a therapeutic nucleic acid) or a protein) to be expressed in the contacted cell, in particular the gene to be expressed in the contacted cell, wherein the ionizable LNP comprises the agent (e.g. the nucleic acid or the protein) to be delivered and a compound of formula (I):

wherein Pl is a phospholipid moiety; E-*NH- is an extender moiety comprising an extender group E and a group -*NH-; and RL-NH- is a functional moiety including a nitrogen containing group -NH- and a group RL, and wherein the functional moiety RL-NH- of the compound of formula (I) comprises a cell-type targeting ligand or receptor targeting ligand of the target cell. [0306] In some embodiments, the present invention also relates to a method for delivering an agent (e.g. a gene or a protein) into a cell of a subject, comprising administering an ionizable LNP, according to the present invention, comprising said agent (e.g. said gene or said protein), or a composition comprising the same, to said subject. [0307] In some embodiments, the present invention further relates to an in vitro or ex vivo method for transducing/transfecting a cell, comprising contacting said cell with an ionizable LNP according to the invention. In some embodiments, the cell may be from a subject (e.g., a patient). In some embodiments, after transduction/transfection, the cell may be transplanted to a subject in need thereof (e.g., the patient, and/or another subject). [0308] In some embodiments, an ionizable LNP can be administered to a cell in vivo, ex vivo, or in vitro. In some embodiments, the cell may be derived from a mammal (e.g., humans, non-human primates, cows, mice, sheep, goats, pigs, rats, etc.) In some embodiments, the cell may be derived from a human. In some embodiments, the cell may be, but is not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, lung cells, bone cells, stem cells, mesenchymal cells, neural cells, cardiac cells, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle cells, beta cells, pituitary cells, synovial lining cells, ovarian cells, testicular cells, fibroblasts, B cells, T cells, reticulocytes, leukocytes, granulocytes, tumor cellsand induced pluripotent stem cells (iPS). Use in gene therapy [0309] In some embodiments, LNPs described herein may be particularly useful in gene therapy, e.g., to deliver a therapeutic nucleic acid of interest to a subject. [0310] Accordingly, in some embodiments, the present invention also relates to a LNP according to the present invention, for use in gene therapy. [0311] In some embodiments, the present invention also relates to a method of gene therapy in a subject in need thereof, comprising administering a LNP according to the present invention to said subject. [0312] In some embodiments, a LNP of the invention can be delivered by any appropriate route to the subject. In some embodiments, appropriate administration routes encompass, without being limited to, inhalational, topical, intra-tissue (e.g. intramuscular, intracardiac, intrahepatic, intrarenal), conjunctival (e.g. intraretinal, subretinal), mucosal (e.g. buccal, nasal), intra-articular, intravitreal, intracranial, intracerebral, intravascular (e.g. intravenous), intra-arterial, intraventricular, intracisternal, intraperitoneal, and intralymphatic routes. In some embodiments, the route of administration is selected depending on the targeted tissue and/or organ, namely, depending on the tissue and/or organ in which transfection is sought. [0313] LNPs according to the present invention may be useful for preventing or treating a disease, disorder, or condition. In particular, such LNPs may be useful in treating a disease, disorder, or condition characterized by missing or aberrant protein or polypeptide activity. In some embodiments, the LNP includes an mRNA encoding a missing or aberrant polypeptide may be administered or delivered to a cell. Subsequent translation of the mRNA may produce the polypeptide, thereby reducing or eliminating an issue caused by the absence of or aberrant activity caused by the polypeptide. [0314] Diseases, disorders, and/or conditions characterized by dysfunctional or aberrant protein or polypeptide activity for which a LNP according to the present invention may be administered include, but are not limited to, rare diseases, infectious diseases (as both vaccines and therapeutics), cancer and proliferative diseases, genetic diseases (e.g ., cystic fibrosis), autoimmune diseases, diabetes, neurodegenerative diseases, cardiovascular diseases, and metabolic diseases. [0315] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^prevent ^ ^ ^preventing ^ ^ ^ ^ ^prevention ^ ^ ^ ^ ^ ^ ^ ^ prophylactic and preventative measures, wherein the object is to reduce the chances that a subject will develop a given disease over a given period of time. Such a reduction may be reflected, e.g., in a delayed onset of at least one symptom of the disease in the subject. [0316] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^treating ^ ^ ^ ^treatment ^ ^ ^ ^alleviation ^ ^ ^ ^ ^ ^ ^ ^ therapeutic treatment, excluding prophylactic or preventative measures; wherein the object is to slow down (lessen) a given disease. Those in need of treatment include those already with the disease as well those suspected to have the disease. A subject is ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^treated ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ an LNP according to the present invention, said subject shows observable and/or measurable reduction in or absence of one or more of the following: one or more of the symptoms associated with the disease; reduced morbidity and mortality; and/or improvement in quality of life issues. The above parameters for assessing successful treatment and improvement in the targeted disease are readily measurable by routine procedures familiar to a physician. [0317] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^subject ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ some embodiments ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^patient ^ ^ i.e., a warm-blooded animal, more preferably a human, who/which is awaiting the receipt of, or is receiving medical care or was/is/will be the object of a medical procedure, or is monitored for the development of ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^mammal ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ non-human primates, domestic and farm animals, and zoo, sports, or pet animals, such as dogs, cats, cattle, horses, sheep, pigs, goats, rabbits, etc. Preferably, the mammal is a primate, more preferably a human. Composition [0318] In some embodiments, the present invention further relates to a composition comprising a LNP according to the invention. In some embodiments, LNPs in the composition according to the present invention comprises at least one agent (e.g. a gene or a protein). [0319] In some embodiments, the composition is a pharmaceutical composition comprising a LNP according to the invention and at least one pharmaceutically acceptable vehicle. [0320] ^ ^ ^ ^ ^ ^ ^ ^pharmaceutically acceptable ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ excipients, carriers, and/or preservatives, is meant to define molecular entities and compositions that do not produce an allergic or similar untoward reaction when administered to a subject, preferably a human. For human administration, pharmaceutical compositions should meet sterility, pyrogenicity, and general safety and purity standards as required by regulatory offices, such as, for example, FDA Office or EMA. [0321] In some embodiments, pharmaceutically acceptable vehicles, excipients, carriers and preservatives that may be used in these compositions include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, proteins (such as, e.g., serum albumin, gelatin, immunoglobulins and the like), buffer substances (such as, e.g., phosphates, citrates or other organic acids, and the like), amino acids (such as, e.g., glycine, glutamine, asparagine, arginine, lysine and the like), antioxidants (such as, e.g., ascorbic acid and the like), chelating agents (such as, e.g., EDTA), sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes (such as, e.g., protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate and the like), hydrophilic polymers (such as, e.g., polyvinylpyrrolidone, polyethylene- polyoxypropylene block polymers and the like), cellulose-based substances (such as, e.g., sodium carboxymethylcellulose), polyacrylates, waxes, nonionic surfactants (such as, e.g., Tween, pluronics, polyethylene glycol and the like), wool fat, and suitable combinations thereof. [0322] In some embodiments, a pharmaceutical composition according to the present invention comprises vehicles which are pharmaceutically acceptable for a formulation intended for injection into a subject. In some embodiments, these may be in particular isotonic, sterile, saline solutions (monosodium or disodium phosphate, sodium, potassium, calcium or magnesium chloride and the like or mixtures of such salts), or dry, especially freeze-dried compositions which upon addition, depending on the case, of sterilized water or physiological saline, permit the constitution of injectable solutions. [0323] In some embodiments, a pharmaceutical composition according to the present invention comprise one or more agents that promote the entry of LNP described herein into a mammalian cell, such as, e.g., natural and/or synthetic polymers, such as poloxamer, chitosan, cyclodextrins, dendrimers, poly(lactic-co-glycolic acid) polymers, and the like. [0324] In some embodiments, LNPs comprising at least one agent (e.g. a gene or a protein) according to the present invention is comprised as part of a medicament. In some embodiments, the invention thus relates to a medicament comprising LNPs comprising at least one agent (e.g. a gene or protein) according to the present invention. Regimen [0325] In some embodiments, LNPs according to the present invention are to be administered to a subject in need thereof in a therapeutically effective amount. [0326] ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^about ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ that said numerical value is approximate and small variations would not significantly affect the practice of the disclosed embodiments. Such small variations are, e.g., of ± 1%, ± 2%, ± 3%, ± 4%, ± 5%, ± 6%, ± 7%, ± 8%, ± 9%, ± 10% or more. [0327] In some embodiments, the dose of LNPs required to achieve a desired effect or a therapeutic effect will vary based on several factors including, but not limited to, the specific route of administration, the level of gene, RNA or protein expression required to achieve a therapeutic effect, the specific disease being treated, and the stability of the gene, RNA, and/or protein product. A person skilled in the art can adjust dosing and/or determine a dose range to treat a particular subject and/or a particular disease based on the aforementioned factors, as well as other factors that are well known in the art. [0328] In some embodiments, the volume of LNPs administered to a subject will also depend, among other things, on the size of the subject, the dose of the LNP required to obtain therapeutic effect, the concentration of the LNP, and the proposed route of administration. [0329] In some embodiments, the rate of administration of LNPs delivered to a subject will also depend, among other things, on the size of the subject, the dose of the LNP required to obtain therapeutic effect, the concentration of the LNP, the volume of the LNP solution, and the proposed route of administration. [0330] In some embodiments, the total dose or total volume of LNPs may be administered continuously (i.e., wherein the total dose or total volume of modified LNPs is injected in a single shot or infusion); or discontinuously (i.e., wherein fractions of the total dose or total volume of LNPs are injected with intermittent periods between each shot, preferably with short intermittent periods such as periods of time of 15 seconds, 30 seconds, 45 seconds, 1 minute, 2 minutes, 3 minutes, 4 minutes, or 5 minutes between each shot or infusion). Kits [0331] The present invention also relates to kits and kits-of-parts, for: - transfecting a cell of a subject; and/or - delivering an agent (e.g. a gene or a protein) to a subject; and/or - preventing and/or treating a disease in a subject. [0332] In some embodiments, the kits or kits-of-parts comprise one or more LNPs and/or compositions according to the present invention. [0333] In some embodiments, the kits or kits-of-parts further comprise a device for delivery of one or more LNPs and/or compositions according to the present invention. [0334] In some embodiments, the kits further include instructions for delivery of one or more LNPs and/ or compositions according to the present invention. In some embodiments, kits comprise instructions for preventing and/or treating a targeted disease, using the compositions, and/or methods described herein. [0335] In some embodiments, kits described herein may further include other materials desirable from a commercial and user standpoint, including buffers, diluents, filters, needles, syringes, and/or package inserts with instructions for performing any methods described herein. BRIEF DESCRIPTION OF THE FIGURES [0336] Figure ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ [0337] Figure 2A ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ Figure 2B ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ [0338] Figure 3A ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ Figure 3B ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ EXAMPLES [0339] The starting products used are commercial products or products prepared according to known synthesis from commercial compounds or known to one skilled in the art. [0340] The structures of the compounds described in the examples were determined according to the usual spectrophotometric techniques (nuclear magnetic resonance (NMR), liquid chromatography-mass spectrometry (LC/MS) and purity was determined by high performance liquid chromatography (HPLC). [0341] Synthesis intermediates and compounds of the invention are named according to the IUPAC (The International Union of Pure and Applied Chemistry) nomenclature and described in their neutral form. [0342] The following abbreviations have been used: ACN: acetonitrile Boc: tert-Butyloxycarbonyl CH2Cl2 or DCM: dichloromethane DBU: 1,8-Diazabicyclo[5.4.0]undec-7-ene DIPEA: N,N-diisopropylethylamine DMF : dimethylformamide DMSO : dimethylsulfoxide DSPE-PEG2000-amine : 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino(polyethylene glycol)-2000] EtOAc: ethyl acetate EtOH: ethanol HATU: Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium HCl: hydrochloric acid H2O: water MeOH: methanol MgSO
4: magnesium sulfate Na2SO4: sodium sulfate NaHCO
3: sodium bicarbonate NEt
3 or TEA: tritethylamine NH3: ammoniac PEG: polyethylene glycol Pd/C: palladium on carbon SFC: SuperCritical Fluid Chromatography TMSOTf: Trimethylsilyl trifluoromethanesulfonate LC/MS: liquid chromatography-mass spectrometry HPLC: High Performance Liquid Chromatography NMR: Nuclear Magnetic Resonance [0343] HPLC/MS method for purity determination for saccharide end-group: Instruments: Shimadzu LCMS-2020 Single Quadrupole Liquid Chromatograph Mass Spectrometer, HPLC ^ Shimadzu Nexera-i LC-2040C 3D with DAD detector; ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^m C18, 4.6*50 mm, or equivalent; Column temperature: 30°C; Eluent: A = H2O, B = ACN; Flow: 0.5 mL/min Gradient conditions:

Analysis time: 12 min [0344] UV/UPLC method for purity determination for peptide end-group: Instruments: UPLC Acquity HClass; Column: BEHC18 (WATERS), 150*2.1 mm, or equivalent; Column temperature: 60°C; Eluent: A = H2O (0.1 TFA), B = ACN (0.1% TFA); Flow rate: 0.5 mL/min; Gradient conditions: 0 to 100% of B in 10 min; Detection: 214 nm [0345] SFC/MS method for purity determination for lipids: Instruments: Waters UPC2; Column: Waters Acquity UPC2 Torus 2-PIC (100x4.6mm, 5µm); Column temperature: 40°C: Pression (BPR): 170 bar; Eluent: A = CO
2, B = MeOH + 20 mM Ammonia; Flow rate: 2.5 mL/min; Gradient conditions: Time [min] Mobile phase A Mobile phase B [%] [%] 0.0 95 5 5.00 50 50 6.00 50 50 Analysis time: 12 min [0346] Preparation 1: preparation of 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1- yl)amino)benzoic acid
[0347] To a mixture of 4-aminobenzoic acid (5.0 g) and DIPEA (9.5 mL) in EtOH was added 3,4-diethoxycyclobut-3-ene-1,2-dione (6.8 g). The reaction mixture was stirred at room temperature for 3 h, and was next diluted with H
2O, and treated with 1M HCl until pH = 4. The resulting precipitate was filtered, washed with H2O, and dried. A sample of the crude material was purified by preparative Reverse Phase HPLC using ACN/H2O as eluent. The fractions containing the pure product were combined, concentrated under reduced pressure to deliver desired intermediate (0.249 g, 36.8% yield) as a yellow solid. LC/MS (6 min): RT = 2.1 min, found [M+H]+ = 261.75.1H NMR (300 MHz, DMSO- d6) ^ (ppm): 12.80 (s, 1H), 11.00 (s, 1H), 7.92 (d, J = 8.8 Hz, 2H), 7.49 (d, J = 8.8 Hz, 2H), 4.80 (q, J = 7.1 Hz, 2H), 1.44 (t, J = 7.1 Hz, 3H). [0348] Preparation 2: Preparation of 4-(((2-ethoxy-3,4-dioxocyclobut-1-en-1- yl)amino)methyl) benzoic acid
[0349] To a mixture of 4-(aminomethyl)benzoic acid (2.5 g) and DIPEA (4.3 mL) in EtOH was added 3,4-diethoxycyclobut-3-ene-1,2-dione (3.1 g). The reaction was stirred at room temperature for 3 h, next was diluted with H
2O, and treated with 1M HCl until the pH = 4. The resulting precipitate was filtered off, washed with H2O, and dried. The crude material was purified by preparative Reverse Phase HPLC using ACN/H2O as eluent. The fractions containing the pure product were combined, concentrated under reduced pressure to dryness to deliver desired intermediate (0.42 g, 43.7% yield) as a yellow solid. LC/MS (6 min): RT = 2.05 min, found [M+H]+ = 275.75.1H NMR (300 MHz, DMSO-d
6) ^ (ppm): 12.95 (s, 1H), 9.40 ^ 8.99 (m, 1H), 8.01 ^ 7.84 (m, 2H), 7.42
[0351] Compounds 10C1 and 10C2 are also commercially available: compound 10C1 refers to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[square (polyethylene glycol)- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-PEG2000- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-14-5) and compound 10C2 refers to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-PEG2000-NH2 », CAS 474922-26-4). Example 1.1 : Synthesis of compound (1B1): 3-((3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthen]-5-yl)amino)-4-ethoxy cyclobut-3-ene-1,2-dione
[0352] A mixture of commercially available of 5-aminofluorescein (50 mg) and 3,4- diethoxycyclobut-3-ene-1,2-dione (25 mg) in anhydrous EtOH under argon atmosphere was cooled down to 0°C. Then, DIPEA (28 µL) was added to the solution, and the reaction mixture was allowed to stir at room temperature overnight. Next, 1M HCl was added dropwise to bring the pH to 3. The resulting mixture was extracted with DCM (3x15 mL). The combined organic layers were dried over Na
2SO
4, filtered, and the solvent was evaporated under reduced pressure to obtain 46 mg of the crude product. [0353] The reaction was repeated using similar conditions, only replacing DIPEA with TEA (22 µL). The crude product (52 mg) was combined with the previous batch and the material was purified by preparative HPLC to obtain in total 57 mg of desired compound as an orange solid. LC/MS (6 min): RT = 2.82 min, [M+H]+ = 471.55. HPLC purity: 99.92 % (222 nm), 99.96 % (303 nm).1H NMR (300 MHz, DMSO-d6) ^ 10.17 (s, 1H), 7.96 (d, J = 2.1 Hz, 1H), 7.73 (dd, J = 8.4, 2.2 Hz, 1H), 7.31 ^ 7.21 (m, 1H), 6.70 ^ 6.48 (m, 6H), 4.81 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H). Example 1.2 – Synthesis of compound (4B2): (3S,4S,5S,6R)-2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)-6-(hydroxymethyl) tetrahydro- 2H-pyran-3,4,5-triol
[0354] To a solution of (3S,4S,5S,6R)-2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (1.0 g) (obtained as described in the international patent application published under WO 2022/096681) in MeOH was added Pd/C (0.16 g) under argon atmosphere. The reaction mixture was stirred under hydrogen atmosphere overnight, filtered through a pad of Celite, and the filtrate was concentrated under reduced pressure to dryness. The crude product was used for the next step without further purification (0.74 g, yield 80% yield). LC/MS (6 min): RT = 0.5 min, found [M+H]+ = 312.10 Example 1.3 – Synthesis of compound (3B1): 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1-yl)amino)-N-(2-(2-(2-(((3S,4S,5S,6R)-3,4,5- trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)ethoxy)ethoxy)ethyl) benzamide

[0355] To a mixture of intermediate 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1- yl)amino)benzoic acid described in preparation 1 (151 mg), HATU (220 mg) and DIPEA (0.25 mL) in DMF was added under argon atmosphere compound 4B2 obtained in example 1.2 (150 mg). The reaction mixture was stirred for 2 hours at room temperature. Next, the solvent was evaporated under reduced pressure. The residue was purified by preparative Reverse Phase HPLC using ACN/H2O as eluent. The fractions containing the pure product were combined and concentrated under reduced pressure. The resulting oil was dissolved in a small amount of H
2O, the solution was being frozen, and next freeze-dried to deliver the final material (66 mg, 40.3% yield) as a white powder. LC/MS (12 min): RT = 4.79 min, [M+H]+ = 555.1 ; [M-H]- = 553.1. HPLC purity: 99.9 % (200 nm), 99.8 % (315 nm).1H NMR (300 MHz, DMSO-d6) ^ (ppm): 10.86 (s, 1H), 8.44 (t, J = 5.6 Hz, 1H), 7.84 (d, 2H), 7.43 (d, J = 8.5 Hz, 2H), 4.79 (q, J = 7.1 Hz, 2H), 4.70 (dd, J = 5.8, 4.6 Hz, 2H), 4.63 (d, J = 1.6 Hz, 1H), 4.54 (d, J = 5.8 Hz, 1H), 4.43 (t, J = 6.0 Hz, 1H), 3.76 ^ 3.35 (m, 18H), 1.44 (t, J = 7.1 Hz, 3H) Example 1.4 – Synthesis of compound (5B1): 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1-yl)amino)-N-(2-(2-(2-(((3R,4S,5S,6R)-3,4,5- trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)ethoxy)ethoxy)ethyl) benzamide
iminoethoxy) tetrahydro-2H-pyran-3,4,5-triyl triacetate
[0357] To a solution of ^-D(+)-glucose pentaacetate (10 g) was added morpholine (8.8 mL), and the mixture was stirred at room temperature overnight. The reaction mixture was next washed with 2M HCl (2x200 ml), water (200 mL), and dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting yellow oil was dissolved in dry DCM under argon atmosphere, the solution was cooled down to 0 °C, and treated with trichloroacetonitrile (25.7 mL). After being stirred for 1 hour at 0 °C, DBU (0.76 mL) was added, the reaction mixture was stirred at 0 °C for 1 hour, and next at room temperature overnight. The solvents were removed under reduced pressure, and the residue was purified by column chromatography eluting with hexane and EtOAc mixture to deliver the desired product as a yellow oil (10.3 g, 82% yield). 1H NMR (300 MHz, DMSO-d6) ^ (ppm): 9.99 (s, 1H), 6.43 (s, 1H), 5.41 (t, J = 9.9 Hz, 1H), 5.19 ^ 5.05 (m, 2H), 4.25 ^ 4.07 (m, 3H), 2.01 ^ 1.94 (m, 12H) [0358] Step 2: synthesis of (2R,3R,4S,5R)-2-(acetoxymethyl)-6-(2-(2-(2-azidoethoxy) ethoxy)ethoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate

[0359] To a suspension of compound obtained at previous step (10.3 g) and molecular sieves in dry DCM was added 2-(2-(2-azidoethoxy)ethoxy)ethan-1-ol (6.9 g), synthesized as described in the application WO2022096681, at room temperature under argon atmosphere. The mixture was cooled down to -25oC, and TMSOTf (4.5 mL) was added. The reaction mixture was stirred at -25°C for 1 hour, and then was allowed to stir at room temperature overnight. The reaction was quenched with saturated NaHCO
3 solution, DCM was added, the phases were separated, and the organic phase was washed with water, brine, and dried over Na2SO4. The resulting suspension was filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by column chromatography eluting with hexane and EtOAc mixture to give the desired product as a yellow oil (3.8 g, 29% yield). 1H NMR (300 MHz, DMSO-d6) ^ (ppm): 5.25 (t, J = 9.4 Hz, 1H), 4.90 (t, J = 9.7 Hz, 1H), 4.85 ^ 4.72 (m, 2H), 4.21 ^ 4.12 (m, 1H), 4.05 ^ 3.99 (m, 1H), 3.99 ^ 3.92 (m, 1H), 3.84 ^ 3.75 (m, 1H), 3.61 ^ 3.52 (m, 9H), 3.40 ^ 3.37 (m, 2H), 2.02 (s, 3H), 2.00 ^ 1.96 (m, 6H), 1.94 (s, 3H) [0360] Step 3: synthesis of (3R,4S,5S,6R)-2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol

[0361] Compound obtained at previous step (3.8 g) was dissolved in 7N NH3 in MeOH and stirred at room temperature overnight. Next, the solvent was evaporated under reduced pressure, and the crude desired product was used for the next step without further purification (3.0 g, quantitative yield).1H NMR (300 MHz, DMSO-d6) ^ (ppm): 4.99 ^ 4.84 (m, 2H), 4.57 ^ 4.40 (m, 1H), 4.15 (d, J = 7.8 Hz, 1H), 3.92 ^ 3.81 (m, 1H), 3.70 ^ 3.22 (m, 18H) [0362] Step 4: synthesis of (3R,4S,5S,6R)-2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol

[0363] To a solution of compound obtained at previous step (500 mg) in MeOH was added Pd/C under argon atmosphere. The inert gas was evacuated and backfilled with hydrogen (in total three times). The reaction mixture was stirred under hydrogen atmosphere (balloon) overnight, filtered through a pad of Celite, and the filtrate was concentrated under reduced pressure to dryness. The crude product was used for the next step without further purification (460 mg, quantitative yield). LC/MS (6 min): RT = 0.5 min, found [M+H]+ = 312.05. [0364] Step 5: 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1-yl)amino)-N-(2-(2-(2- (((3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy) ethoxy)ethoxy)ethyl)benzamide (5B1) [0365] To a mixture of intermediate 4-((2-ethoxy-3,4-dioxocyclobut-1-en-1- yl)amino)benzoic acid described in preparation 1 (256 mg), HATU (372 mg) and DIPEA (0.43 mL) in DMF was added under argon atmosphere compound obtained in previous step (254 mg). The reaction mixture was stirred for 2 hours at room temperature. The solvent was evaporated under reduced pressure. The residue was purified by preparative Reverse Phase HPLC using ACN/H
2O as eluent. The fractions containing the pure product were combined and concentrated under reduced pressure. The resulting oil was dissolved in a small amount of H2O, the solution was being frozen, and next freeze- dried to deliver the final product as a white solid foam (74 mg, 16% yield). LC/MS (12 min): RT = 4.79 min, found [M+H]+ = 555.1 ; [M-H]- = 553.1. HPLC purity: 99.8% (200nm), 99.8% (315nm).1H NMR (300 MHz, DMSO-d6) ^ (ppm): 10.91 (s, 1H), 8.44 (t, J = 5.5 Hz, 1H), 7.90 ^ 7.79 (m, 2H), 7.44 (d, J = 8.5 Hz, 2H), 4.96 (d, J = 4.9 Hz, 1H), 4.89 (m, 2H), 4.79 (m, 2H), 4.48 (t, J = 5.9 Hz, 1H), 4.15 (d, J = 7.7 Hz, 1H), 3.92 ^ 3.78 (m, 1H), 3.71 ^ 3.61 (m, 1H), 3.61 ^ 3.49 (m, 9H), 3.43 (m, 3H), 3.08 (m, 3H), 2.95 (m, 1H), 1.42 (t, 3H) Example 1.5 – Synthesis of compound (6B1)

Step 1: Peptide GRGDSP synthesis on solid support [0366] Peptide GRGDSP (SEQ ID No.: 1) was synthesized on solid support (rink amide resin) at a scale of 0.25 mmol on a GYROS PROTEIN Symphony X peptide synthesizer. The syntheses were performed according to a standard protocol in Fmoc/tBu strategy using as activator HATU and a piperidine solution in DMF for deprotection of the Fmoc protecting group. Step 2: Cleavage and deprotection [0367] The cleavage from the resin and the deprotection of the side chains were carried out by treatment with a mixture TFA/H2O/TIPS/DTT (90: 5: 2.5: 2.5) for 90 min. The cleavage solution was filtered from resin, the peptide was precipitated in ice-cold diethyl ether, isolated by centrifugation, and freeze-dried in order to eliminate the maximum of residual TFA. Step 3: Procedure for grafting 3,4-diethoxycyclobut-3-ene-1,2-dione for compound (6B1) [0368] To a solution of peptide (0.1 mmol) in distilled water (0,5 mL), DIEA was added until a pH between 7 and 7.5 is reached. A solution of 3,4-diethoxycyclobut-3-ene-1,2- dione (1.2 eq.0.12 mmol) in EtOH (0,5mL) was added. The reaction was stirred for 2h at room temperature. Step 4: Purification and salt exchange [0369] The raw peptide was purified directly from the reaction mixture using a reverse phase preparative HPLC system (Waters Delta Prep 4000) using a reverse phase column (Vydac Denali prep C-18, 10 ^m, 120 Å, 50 300 mm) and a suitable gradient of ACN+TFA 0.1%/ H2O+TFA 0.1% as eluent. The fractions containing the purified target peptide were identified by UV measurement (UV/Visible Waters 2489 detector) at 214 nm and the selected fractions were then combined and freeze-dried. [0370] The exchange of trifluoroacetate salt into acetate was carried out during purification via a proprietary buffer. [0371] ESI+MS (m/z): [M+H]+ calcd for C42H60N12O16: 990.01 found: [M+H]+: 989.76, [M+2H]2+: 494.50, found: 495.29. UPLC purity: 96.7 % (214 nm), RT = 3.72 min Example 1.6 – Synthesis of compound (7B1)

[0372] The linear peptide DfKRG (SEQ ID No.: 2) was synthesized on solid support (2- chloro chloroTrityl resin) at a scale of 0.25 mmol on a GYROS PROTEIN Symphony X peptide synthesizer. The syntheses were performed according to a standard protocol in Fmoc/tBu strategy using as activator HATU and a piperidine solution in DMF for deprotection of the Fmoc protecting group. Step 1: Spacer Boc-Aryl-PEG2-COOH synthesis [0373] The peptidyl resin Boc-Aryl-PEG2-resin was synthesized using standard deprotection and coupling cycles. The cleavage from the resin of the protected spacer was performed under selective mild conditions by the treatment of HFIP/DCM (20:80) for 1h at RT, the resin was removed by filtration and the filtrate was evaporated to dryness under vacuum. The protected spacer was precipitated in ice-cold diethyl ether, isolated by centrifugation. Step 2: Peptide DfKRG synthesis and spacer introduction on solid support [0374] The peptidyl resin Fmoc-Asp(Otbu)-DPhe-Lys(Alloc)-Arg(Pbf)-Gly-resin was synthesized, followed by the deprotection of the Alloc group by using 0.35 eq. of Palladium (0) and 24 eq. of phenylsilane. Then the spacer from step 1 (1.5 eq.) was introduced using BOP (1.5 eq.) as activator and DIEA (3eq.) in DMF at RT for 3h. Step 3: Cleavage from the solid support [0375] The cleavage from the resin was performed under selective mild conditions by the treatment of HFIP/DCM (20:80) for 1h at RT, the resin was removed by filtration and the filtrate was evaporated to dryness under vacuum. Crude peptide was precipitated by addition of cold diethylether. Protected peptide was thus obtained by filtration. Step 4: Head to tail cyclization: [0376] Protected peptide H-Asp(OtBu)-DPhe-Lys(Boc-Spacer)-Arg(Pbf)-Gly-OH was solubilized in DMF (at 10 mM). To this solution were successively added DIEA (3 eq.) and BOP (1.1 eq.). After stirring at room temperature overnight, the solution was concentrated under vacuum, precipitated by added Et2O and dried under vacuum. Step 5: Acido labile protecting group removal [0377] Protected cyclic peptide was treated with TFA/H
2O/TIPS (92.5/5/2.5, v/v/v)) solution at room temperature for 2 hours. Mixture was evaporated to dryness under vacuum, and crude peptide was precipitated by addition of cold diethylether and placed in a freeze-dryer to remove as much residual TFA as possible prior to the coupling step of the diethyl squarate motif and used without further purification. Step 6: Procedure for grafting 3,4-diethoxycyclobut-3-ene-1,2-dione for compound (7B1) [0378] To a solution of crude peptide (0.1 mmol) in distilled water (0,5 mL), DIEA was added until a pH between 7 and 7.5 is reached. A solution of 3,4-diethoxycyclobut-3-ene- 1,2-dione (1.2 eq.0.12 mmol) in EtOH (0.5mL) was added. The reaction was stirred for 2h at room temperature. Step 7: Purification and salt exchange. [0379] The raw peptide was purified directly from the reaction mixture using a reverse phase preparative HPLC system (Waters Delta Prep 4000) using a reverse phase column (Vydac Denali prep C-18, 10 ^m, 120 Å, 50 300 mm) and a suitable gradient of ACN+TFA 0.1%/ H2O+TFA 0.1% as eluent. The fractions containing the purified target peptide were identified by UV measurement (UV/Visible Waters 2489 detector) at 214 nm and the selected fractions were then combined and freeze-dried. The exchange of trifluoroacetate salt into acetate was carried out during purification via a proprietary buffer. ESI+MS (m/z): [M+H]+ calcd for C47H63N11O14: 1007.08 found: [M+H]+: 1007.50, [M+2H]2+: 504.04, found: 503.87. UPLC purity: 94.0 % (214 nm), RT = 4.25 min

Step 1: Peptide synthesis [0380] Linear peptidic sequence GRKfD (SEQ ID No: 3) was synthesized on solid support [2-chloroTrityl resin] on an automated CS Bio peptide synthesizer, each amino acid bearing the adapted protecting group for its side chain. The synthesis was performed according to a standard protocol in Fmoc/tBu strategy using for example HATU as activator and a piperidine solution in DMF for deprotection of the Fmoc protecting group. Step 2: Solid support cleavage [0381] After drying in vacuo, the peptidyl resin was exposed to 20% HFIP in DCM solution several times to lead to the sequence cleaved from the resin. The filtrate was concentrated in vacuo, solubilized in ACN/H
2O followed by lyophilization. Step 3: Backbone cyclisation The protected peptide was dissolved in DMF and treated with DIPEA in the presence of PyBOP leading to the cyclization of the backbone intermediate. Once the conversion was complete, the reaction mixture was concentrated in vacuo, dissolved in ACN/H
2O and submitted to a new freeze-dry cycle. Step 4: Side chain of cyclic peptide and tBoc-PEG2 spacer deprotection [0382] The side chain protecting group of the peptide and tBoc-PEG2 spacer were cleaved using a mixture of TFA/H
2O/TIPS during 2 hours. Isolation of the cyclic backbone peptide was performed by precipitation using Et2O/Pentane and the material was washed several times with this mixture to remove protecting groups and maximum of residual TFA. The material was then solubilized in ACN/H
2O followed by freeze- drying step. Step 5: Peptide purification [0383] The cyclized crude material was dissolved in an appropriate co-mixture of ACN/Milli-Q H2O and injected on a C18 preparative HPLC column using acidic eluents (A: MilliQ-H O+0.1% AcOH ; B : ACN+0.05% + 2 AcOH). ESI-MS (m/z): [M+H] calcd for C34H54N10O10: 762.40, found: 763.82. [M+2H]2+: 381.20, found: 382.56. UPLC purity: 98.7 % (215 nm), RT = 0.67 min Example 1.8 – Synthesis of compound (8C1)

[0384] The building block from preparation 1 was solubilized in DMF (at 10 mM). To this solution are successively added HATU (0.6 mmol) and commercially available DSPE-PEG2000-amine (0.21 mmol). After stirring at room temperature overnight, the solvent was evaporated under reduced pressure. The residue was purified by preparative SFC. The fractions containing the pure product were combined and concentrated under reduced pressure. The resulting oil was dissolved in a small amount of H2O, the solution was being frozen, and next freeze-dried to deliver the final product as a light yellow solid (23.8 mg, 44 % yield). LC/MS (6 min): RT = 3.69 min, found [M+5H]5+ = 607.2;. HPLC purity: 97% (200 nm), 1H NMR (400 MHz, CDCl3 ^ ^ (ppm): 9.31 ^ 9.25 (m, 1H), 7.84 (d, J = 8.6 Hz, 2H), 7.51 ^ 7.45 (m, 2H), 7.12 ^ 7.05 (m, 1H), 5.26 ^ 5.22 (m, 1H), 4.89 (q, J = 7.1 Hz, 2H), 4.39 (dd, J = 11.9, 3.1 Hz, 1H), 4.17 (dd, J = 12.0, 6.8 Hz, 1H),

this solution are successively added HATU (0.62 mmol) and commercially available DSPE-PEG2000-amine (0.21 mmol). After stirring at room temperature overnight, the solvent was evaporated under reduced pressure. The residue was purified by preparative SFC. The fractions containing the pure product were combined and concentrated under reduced pressure. The resulting oil was dissolved in a small amount of H2O, the solution was being frozen, and next freeze-dried to deliver the final product as a white solid (13 mg, 16% yield). LC/MS (6 min): RT = 3.67 min, found [M+6H]6+ = 507.3;. HPLC purity: 98% (200 nm), 1H NMR (400 MHz, CDCl
3 ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ 9.16 (m, 1H), 7.85 (d, J = 8.1 Hz, 2H), 7.44 ^ 7.34 (m, 2H), 7.22 ^ 7.14 (m, 1H), 5.23 (dd, J = 6.7, 3.3 Hz, 1H), 4.94 ^ 4.83 (m, 1H), 4.82 ^ 4.72 (m, 2H), 4.69 ^ 4.59 (m, 1H), 4.37 (d, J = 3.2 Hz, 1H), 4.17 (dd, J = 12.0, 6.8 Hz, 1H), 4.07 ^ 3.94 (m, 6H), 3.89 ^ 3.39 (m, 186H), 2.28 (td, J = 7.4, 2.3 Hz, 4H), 1.59 (s, 4H), 1.44 (t, J = 7.1 Hz, 3H), 1.25 (s, 56H), 0.88 (t, J = 6.8 Hz, 3H). EXAMPLE 2: Production and coupling of LNPs [0386] Conjugated LNPs were generated by coupling the squarate moieties of the invention to at least one primary amine as described below. 2.1 Production of mRNA containing LNPs [0387] Lipid nanoparticles were prepared on a Nanoassemblr ^ microfluidic system (Precision NanoSystems) according to the manufacturer's instructions. Depending on the desired formulation, an ethanol solution consisting of an ionizable lipid (e.g. Dlin-MC3- DMA, CAS 1224606-06-7), a zwitterionic lipid (e.g., distearoylphosphatidylcholine (DSPC, CAS 816-94-4), a component to provide membrane integrity (such as a sterol, e.g., cholesterol, CAS 54-88-5), PEG-lipids molecule (e.g., 1-(monomethoxy- polyethyleneglycol)-2,3-dimyristoylglycerol, with an average PEG molecular weight of ^ ^ ^ ^ ^ ^ ^ ^ ^- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-62-4) and: a functionalized PEG-lipid molecule of formula (IIIa), in particular, the compound of formula (IIIa
1) 10C2, 1,2-distearoyl-sn- glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^- PEG2000- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-26-4,) for the LNP1 illustrated below in Table 3, or a functionalized PEG-lipid molecule of formula (IIa), in particular, the compound of formula (IIa1) 10C1, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[square (polyethylene glycol)- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-PEG2000- ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^-14-5) for the LNP2 illustrated below in Table 3, the compound of formula (IIa2) 8C1 for the LNP4 illustrated below in Table 3, or the compound of formula (IIa3) 9C1 for the LNP5 illustrated below in Table 3, at the appropriate molar ratio (e.g. 50:10:38:1.5:0.5), was prepared. [0388] LNP3 was used as a negative control and did not include a functionalized PEG- lipid molecule of formula (IIa) nor a functionalized PEG-lipid molecule of formula (IIIa). [0389] Furthermore, an aqueous solution with an mRNA encoding for eGFP protein was prepared in 50 mM citrate buffer at pH 3.0. LNP were prepared at a total lipid to mRNA weight ratio of approximately 10:1. Lipid and mRNA-containing solutions were mixed 1:3 (ethanol: citrate) at a constant ^ow rate of 12 ml/min to form LNPs. The product was then dialyzed against DPBS to remove the residual ethanol as well as to raise the pH to 7.4. Table 3 shows the LNPs prepared and tested. Table 3: Exemplary LNPs LNP No. Lipid mix Lipid Molar mRNA Ratio LNP1 MC3:DSPC: Chol:DMG-
50:10:38:1.5:0.5eGFP PEG2000:compound 10C2 LNP2 MC3:DSPC: Chol:DMG- 50:10:38:1.5:0.5 eGFP PEG2000:compound 10C1 LNP3; Negative MC3:DSPC: Chol:DMG- 50:10:38:2
eGFP control PEG2000 LNP4 MC3:DSPC: Chol:DMG- 50:10:38:1.5:0.5
eGFP PEG2000:compound 8C1 LNP5 MC3:DSPC: Chol:DMG-
50:10:38:1.5:0.5eGFP PEG2000:compound 9C1 [0390] Characterization of particle size of LNP (Z-average diameter), polydispersity index (PDI) and zeta-potential were determined by dynamic light scattering utilizing a Malvern Zetasizer ZS. The mRNA concentration and encapsulation of LNPs were evaluated by Ribogreen dye according to the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ guidelines. ±1% Triton was used to ascertain the fraction of encapsulated mRNA by comparison to a relevant free mRNA standard curve. 2.2. Production and purification of chemically conjugated LNPs [0391] Compounds (1B1), (3B1), (5B1), (6B1), (7B1) and (7B2) were obtained as detailed above in Example 1. Compound (1B2) was obtained commercially. [0392] The coupling of the squarate linkers on LNP1, or LNP3 as a control, were carried out with a solution of Tris buffer pH8.5 or pH9 containing compounds (1B1), (3B1), (5B1), (6B1) or (7B1) at a molar ratio of 1E2 to 1E3 equivalents and incubated for 4h at 20°C. At the end of the incubation period, unbound linkers were removed, and buffers were exchanged against formulation buffer (DPBS, Ca2+, Mg2+) using dialysis cassettes or desalting columns. [0393] The coupling of the amino linkers on LNP2, LNP4 or LNP5, or LNP3 as a control, were carried out with a solution of Tris buffer pH9 containing compound (1B2) or (7B2) at a molar ratio of 1E2 to 1E3 equivalents and incubated for 4h at 20°C. At the end of the incubation period, unbound linkers were removed, and buffers were exchanged against formulation buffer (DPBS, Ca2+, Mg2+) using dialysis cassettes or desalting columns. 2.3. Characterization of chemically conjugated LNPs 2.3.a. Physical characterization of conjugated LNPs [0394] The integrity of the different ligand-conjugated LNPs (LNP1-1B10C, LNP1- 3B10C, LNP1-5B10C, LNP1-6B10C, LNP1-7B10C, LNP2-1B10C, LNP4-7B8C and LNP5-7B9C) obtained at EXAMPLE 2.2 was evaluated by the characterization of particle size (Z-average diameter) and polydispersity index (PDI) utilizing a Malvern Zetasizer ZS. The mRNA concentration and encapsulation of LNPs were evaluated with Ribogreen dye according to the ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ guidelines. ±1% Triton was used to ascertain the fraction of encapsulated mRNA by comparison to a relevant free mRNA standard curve. [0395] Table 4 below shows that the mild coupling conditions maintain the integrity and high encapsulation efficiency of the LNPs after the conjugation and purification steps, allowing to produce functionalized LNPs comprising compounds of formula (I). The measurement of the zeta potential of the conjugated LNPs compared to the parental LNPs also demonstrates successful conjugation, indicated by the variation in zeta potential. The zeta potential serves as an indicator of changes in the surface charge of the particles, which depends on the nature of the ligands grafted onto the particles (e.g. density, charge of the ligands, etc.). Table 4 ^ Physical characterization LNPs before and after conjugation (N/A: not available) Encapsulation Zeta Coupling Size (nm) PdI Efficiency (%) Potential (mV) LNP1 82,0 ± 1,1 0,068 ± 0,021 92,6 -1,8 ± 2,6 LNP2 76,3 0,05 93 -1,4 LNP3 77,0 0,08 96 -1,4 LNP4 70,0 0,06 72 -4,7 LNP5 65,8 0,07 83 -2,8 LNP1-1B10C 73 0,05 N/A ND LNP2-1B10C 83,9 0,09 N/A ND LNP1-3B10C 80,6 ± 0,7 0,053 ± 0,045 93,4 -12,6 ± 2,0 LNP1-5B10C 81,2 ± 0,5 0,080 ± 0,069 91,7 -13,4 ± 2,7 LNP1-6B10C 81,9 ± 0,5 0,063 ± 0,039 85,7 -7,2 ± 2,7 LNP1-7B10C 79,4 ± 0,9 0,062 ± 0,030 89,7 -6,0 ± 1,6 LNP4-7B8C 85,8 ± 5,5 N/A 81,8 8,6 ± 1,6 LNP5-7B9C 75,9 ± 1.7 N/A 85,5 7,5 ± 1,3 2.3.b. Analysis of LNPs functionalization following ligand coupling by binding assay (End group detection) [0396] The efficacy of ligands coupling on LNPs was evaluated by DOT-Blot analysis and binding assay: a. The detection of fluorescent moieties (1B1 and 1B2) conjugated at the surface of the LNPs was performed by DOT blot analysis using fluorescein-HRP antibody (anti-FITC) (Figure 1). Successful coupling resulted in specific antibody detection. As expected, no coupling was observed when the reactive moiety-containing ligands (1B1) were incubated with LNP3 (Negative control), or when LNP1 including a lipid of formula (IIIa), (10C2), including an amine reactive end group was incubated with ligands lacking their reactive squarate moieties, i.e., compound of formula (IIb), (1B2), having as well an amine reactive end group, which demonstrates the specificity of the conjugation driven by the squarate moieties and the covalent nature of the bond created to form a squaramide moiety of formula (SQ). b. The detection of mannose moieties on the LNPs were performed by DOT blot analysis using concanavalin-HRP lectin (ConA), which binds selectively to mannose. (Figure 2A). As expected, no coupling was observed when the reactive squarate moiety-containing ligands of formula (IIIb), such as (3B1), were incubated with LNP3 (Negative control), or when LNP1 including a lipid of formula (IIIa), (10C2), including an amine reactive end group was incubated with ligands lacking their reactive squarate moieties, i.e., compound of formula (IIb), (4B2), having as well an amine reactive end group, which demonstrates the specificity of the conjugation driven by the squarate moieties and the covalent nature of the bond created to form a squaramide moiety of formula (SQ). The exposure of mannose moieties at the surface of the LNPs was evaluated using ConA binding assay. In this assay, the conjugated particles were incubated with the lectin ConA, and the changes in particle size (Z-average diameter) were monitored over time utilizing a Malvern Zetasizer ZS. The results demonstrated that the mannose conjugated LNPs were effectively recognized and bound by the ConA, which resulted in an increase of the particle size. This increase confirms the successful interaction of ConA with the mannose moieties exposed on the surface of the LNPs (Figure 2B) c. The functionalization of LNPs conjugated with RGD motif-containing peptides was performed by DOT-blot analysis, using biotinylated ^v ^3 integrin (which binds selectively to the ligands with a squarate end group of formula (IIIb) 6B1 and 7B1, and the ligand with an amine end group of formula (IIb), (7B2), followed by streptavidin-HRP detection (Figure 3A). As expected, no coupling was observed when the reactive moiety-containing ligands (6B, 7B1 or 7B2) were incubated with LNP3 (Negative control), indicating that the conjugation is specific and driven by the squarate moieties. The exposure of mannose moieties at the surface of the LNPs was evaluated using ^v ^3 integrin binding assay. In this assay, the conjugated particles were incubated with th ^ ^v ^3 integrin, and the changes in particle size (Z-average diameter) were monitored over time utilizing a Malvern Zetasizer ZS. The results demonstrated that the conjugated LNPs were effectively recognized and bound by the integrin, which resulted in an increase of the particle size. This increase confirms the successful interaction of the Integrin with the peptide moieties exposed on the surface of the LNPs (Figure 3B) [0397] FITC, conA or Integrin detection was observed for all conjugated LNPs, evidencing the successful coupling of (1) squarate linkers of formula (IIIb), such as 1B1, 3B1, 5B1, 6B1 and 7B1 on LNPs comprising amine functionalized lipids of formula (IIIa), such as LNP1 comprising the amine functionalized lipid 10C2; or of (2) amine derived linkers of formula (IIb), such as 1B2 or 7B2, on LNPs comprising a squarate functionalized lipid of formula (IIa), such as LNP2 comprising the squarate functionalized lipid 10C1, LNP4 comprising the squarate functionalized lipid 8C1 or LNP5 comprising the squarate functionalized lipid 9C1. The mild coupling conditions preserved the structural integrity of the ligands, ensuring their functionality and enabling effective interactions with their respective target. The preservation of ligand functionality is crucial to maintain their biological activity and binding specificity after conjugation. Consequently, the LNPs can effectively engage in their intended biological interactions, confirming the robustness and efficacy of the coupling process. These results validate that the mild coupling conditions are optimal for producing functionalized LNPs with retained ligands functionality, which is essential for their potential applications in targeted delivery and therapeutic interventions. 3.3.c. Analysis of the obtained LNPs by LC-MS [0398] The newly formed DSPE-PEG lipid conjugates (with various ligands) were characterized, using an analytical method that combines high-performance liquid chromatography (HPLC) with mass spectrometry (MS). This integrated method allows for the precise separation and identification of the compound formula (I) 3B10C, 5B10C, 6B10C, and 7B10C from the compound of formula IIIa110C2. [0399] HPLC was used to separate the DSPE-PEG lipid conjugates based on their chemical properties. This separation facilitates the isolation of individual lipid species from complex mixtures. [0400] HPLC/MS method for determination of conjugated DSPE-PEG2000-lipids: Instruments: Column: Zorbax Eclipse Plus Phenyl-Hexyl ^ Narrow Bore RR (150 x 2.1 mm, 3,5 µm) Eluent: A = MeOH at 5 mM of ammonium acetate, B = MeCN at 5 mM of ammonium acetate. Flow rate: 300 µL/min Sample preparation: dilution of LNPs in a 4-volume equivalent of MeOH Injection volume: 5 µL Gradient conditions: Time [min] Mobile phase A [%] Mobile phase B [%] 0 70 30 20 0 100 25 0 100 27 70 30 33 70 30 Analysis time: 33 min [0401] Following HPLC separation, the lipid conjugates were analyzed using mass spectrometry to determine their molecular weights. Due to the polydisperse nature of PEG, which results in a range of molecular weights and oligomeric forms, the analysis was performed on the most abundant oligomers. [0402] These analyses allowed for the identification of all newly formed entities, confirming the covalent nature of the bonds between the lipids and ligands (Table 5 below). Table 5 – Separation of lipid species from LNPs before and after conjugation through HPLC Compounds of formula (IIIb) Elution time attached to 10C (min) LNP1 N/A 14.12 LNP1-3B10C 3B1 10.15 LNP1-5B10C 5B1 10.13 LNP1-6B10C 6B1 9.60 LNP1-7B10C 7B1 9.97 3.3.d. Functional assay in vitro (U87-MG glioblastoma cells) [0403] The mannose-conjugated LNP1-3B10C was tested to assess if transfection of mRNA can be observed. For this purpose, U87-MG cells were transfected and analyzed 24h after transfection via monitoring of transfected (eGFP-positive) and non-transfected cell populations by fluorescence microscopy. [0404] The mannose-conjugated LNP1-3B10C successfully transfected U87-MG cells.