WO2024258967A1 - Anti-cd5 antibodies and their uses - Google Patents
Anti-cd5 antibodies and their usesDownload PDFInfo
- Publication number
- WO2024258967A1 WO2024258967A1PCT/US2024/033613US2024033613WWO2024258967A1WO 2024258967 A1WO2024258967 A1WO 2024258967A1US 2024033613 WUS2024033613 WUS 2024033613WWO 2024258967 A1WO2024258967 A1WO 2024258967A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cdr
- seq
- antibody
- amino acid
- nos
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C07K2317/524—CH2 domain
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C07K2317/53—Hinge
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/71—Decreased effector function due to an Fc-modification
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Definitions
- CD5is a cluster of differentiation protein expressed on the surface of T cells.
- WO 2020/256751describes the use of anti-CD5 antibodies conjugated to ALK5 inhibitors to direct ALK5 inhibitors to T cells, where they can exert a therapeutic benefit, while limiting toxicity of ALK5 inhibitors in non-target tissue.
- Anti-CD5 antibody-ALK5 inhibitor conjugatesare described in WO 2020/256751 as being useful for the treatment of cancer.
- Anti-CD5 antibodieshave also been described as useful for treating CD5+ B or T cell related diseases such as B or T cell malignancies, autoimmune diseases, transplantation diseases, and graft rejections (US 8,679,500). 4.
- the present disclosureprovides anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen-binding fragments thereof.
- the present disclosurefurther provides conjugates comprising ALK5 inhibitors and anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen-binding fragments thereof, nucleic acids encoding the anti-CD5 antibodies and antigen-binding fragments thereof, and cells engineered to express the nucleic acids.
- a conjugate comprising an ALK5 inhibitor and an anti-CD5 antibody or antigen binding fragment thereofis sometimes referred to herein simply as a “conjugate” for convenience.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and/or light chain variable sequences set forth in Tables 1A through 1K.
- an anti-CD5 antibody or antigen-binding fragment of the disclosuree.g., humanized anti-CD5 antibody or antigen-binding fragment thereof
- Tables 1A-1Kfurther include CDR sequences defined by the combined regions of overlap for the CDR sequences defined by the IMGT, Kabat, and Chothia schemes, and further include CDR sequences defined by the common regions of overlap for the CDR sequences defined by the IMGT, Kabat, and Chothia schemes.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 11, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:3, 8, and 15, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:4, 8, and 15, and light chain CDRs of SEQ ID NO:17, ATS, and SEQ ID NO:20.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 11, and 13, and light chain CDRs of SEQ ID NOs:17, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 7, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 7, and 13, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:1, 6, and 16, and light chain CDRs of SEQ ID NO:17, ATS, and SEQ ID NO:20.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 9, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 9, and 16, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 10, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 12, and 13, and light chain CDRs of SEQ ID NOs:18, 19, and 21.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:1, 88, and 89, and light chain CDRs of SEQ ID NO:94, WT, and SEQ ID NO:95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 90, and 91, and light chain CDRs of SEQ ID NOs:96, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 90, and 91, and light chain CDRs of SEQ ID NOs:99, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:3, 8, 91, and light chain CDRs of SEQ ID NOs:96, 97, 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:3, 8, 91, and light chain CDRs of SEQ ID NOs:99, 97, 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:4, 8, and 91, and light chain CDRs of SEQ ID NO:94, WT, and SEQ ID NO:95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 90, and 89, and light chain CDRs of SEQ ID NOs:96, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 90, and 89, and light chain CDRs of SEQ ID NOs:99, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:2, 102, and 91, and light chain CDRs of SEQ ID NOs:103, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:3, 8, and 91, and light chain CDRs of SEQ ID NOs:103, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:5, 102, and 89, and light chain CDRs of SEQ ID NOs:103, 97, and 95.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy chain CDRs of SEQ ID NOs:104, 8, and 91, and light chain CDRs of SEQ ID NOs:94, 97, and 105.
- references to “heavy chain CDRs of SEQ ID NOs:X, Y, and Z”refer to CDR-H1, CDR-H2, and CDR-H3 sequences, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:24 and 25, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:24 and 25, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:26 and 27, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:26 and 27, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:28 and 29, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:28 and 29, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:32 and 33, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:49 and 50, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:49 and 50, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:84 and 92, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:84 and 93, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:84 and 98, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:85 and 92, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:85 and 93, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:85 and 98, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:86 and 92, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:86 and 93, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:86 and 98, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:87 and 92, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:87 and 93, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:87 and 98, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively.
- an anti-CD5 antibody or antigen-binding fragment of the disclosurecomprises heavy and light chain variable regions of SEQ ID NOS:100 and 101, respectively.
- the disclosureprovides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:100 and 101, respectively.
- an anti-CD5 antibodycomprises a light chain and heavy chain of any one of AB-1 to AB-10 as set forth in Table 1L.
- an anti-CD5 antibodycomprises a light chain and heavy chain of SEQ ID NOs:69 and 70, respectively.
- an anti-CD5 antibodycomprises a light chain and heavy chain of SEQ ID NOs:71 and 72, respectively.
- an anti-CD5 antibodycomprises a light chain and heavy chain of SEQ ID NOs:73 and 74, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:75 and 76, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:77 and 78, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:79 and 80, respectively. [0058] In certain aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure is cross- reactive with human and cynomolgus and/or rhesus monkey CD5.
- anti-CD5 antibodies and antigen-binding fragments thereof of the disclosureare described in Section 6.1 and specific embodiments 1 to 197, infra.
- Further exemplary features of conjugates of the disclosure and conjugate componentsare described in Sections 6.2 to 6.5 and specific embodiments 203 to 261, infra.
- exemplary ALK5 inhibitors that can be used in conjugates of the disclosureare described in Section 6.3, including in Tables 2, 3A, 3B, and 4.
- the ALK5 inhibitorcan be directly conjugated to the antibody component or linked to the antibody component by a linker.
- the linkercan be a non- cleavable linker or, preferably, a cleavable linker (e.g., a protease-sensitive linker).
- a cleavable linkere.g., a protease-sensitive linker
- Exemplary non- cleavable and cleavable linkersare described in Section 6.4.
- the average number of ALK5 inhibitor molecules attached per antibody or antigen binding fragmentcan vary, and generally ranges from 2 to 8 ALK5 inhibitor molecules per antibody or antigen binding fragment.
- Drug loadingis described in detail in Section 6.5.
- Exemplary processes for making conjugates and exemplary kits useful for making conjugatesare described in specific embodiments 474 to 482, infra.
- Yet another aspect of the disclosureis a process for making an antibody-ALK5 inhibitor conjugate, where the antibody component is an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure (e.g., a humanized anti-CD5 antibody or antigen-binding fragment thereof).
- the antibody componentis an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure (e.g., a humanized anti-CD5 antibody or antigen-binding fragment thereof).
- a kitcomprising an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure (e.g., a humanized anti-CD5 antibody or antigen-binding fragment thereof) and an ALK5 inhibitor.
- the kitscan be used, for example, to make a conjugate of the disclosure.
- Other aspects of the disclosureare drawn to nucleic acids encoding the anti-CD5 antibodies and antibody-binding fragments of the disclosure.
- the portion of the nucleic acid nucleic acid encoding an anti-CD5 antibody or antigen-binding fragmentis codon-optimized for expression in a human cell.
- Vectorse.g., plasmids
- host cellscomprising the nucleic acids are also within the scope of the disclosure.
- the heavy and light chains coding sequencescan be present on a single vector or on separate vectors.
- Still another aspect of the disclosureis a method of making an anti-CD5 antibody or antigen- binding fragment thereof comprising incubating a cell comprising a nucleic acid or a vector according to the disclosure, under conditions suitable for expression of the coding region(s) and collecting the anti- CD5 antibody or antigen-binding fragment thereof.
- Nucleic acids, vectors, host cells, and uses thereofare further described in Section 6.6 and specific embodiments 198 to 202, infra.
- Yet another aspect of the disclosureis a pharmaceutical composition comprising an anti-CD5 antibody, antigen-binding fragment, conjugate, nucleic acid (or pair of nucleic acids), vector (or pair of vectors) or host cell according to the disclosure, and a physiologically suitable buffer, adjuvant, or diluent.
- Exemplary pharmaceutical excipients that can be used to formulate a pharmaceutical composition and exemplary pharmaceutical compositionsare described in Section 6.7 and specific embodiments 262 to 291, infra.
- Yet another aspect of the disclosureis a method of treating cancer comprising administering a prophylactically or therapeutically effective amount of an anti-CD5 antibody (e.g., a humanized anti-CD5 antibody), antigen-binding fragment thereof, conjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure to a subject in need thereof.
- an anti-CD5 antibodye.g., a humanized anti-CD5 antibody
- the methods of treating cancercomprise administering a conjugate of the disclosure or a pharmaceutical composition comprising the conjugate to a subject in need thereof.
- the conjugates and pharmaceutical compositionscan be administered as monotherapy or as part of a combination therapy, for example in combination with an immune checkpoint modulator (e.g., a checkpoint inhibitor).
- an anti-CD5 antibodye.g., a humanized anti-CD5 antibody
- antigen-binding fragment thereofconjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure for use in the treatment of cancer.
- use of an anti-CD5 antibodye.g., a humanized anti-CD5 antibody
- antigen-binding fragment thereofconjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure for the manufacture of a medicament for the treatment of cancer. 5.
- FIGS.1A-1Cshow amino acid sequence alignments between parental mouse anti-CD5 antibody (Antibody B) and human germline Ig alleles.
- FIG.1Ais the amino acid sequence alignment of the parental mouse anti-CD5 antibody light chain and Ig alleles immunoglobulin kappa variable 3-11 allele 1 (IGKV3-11*01), immunoglobulin kappa variable 3-11 allele 2 (IGKV3-11*02), and immunoglobulin kappa variable 1-16 allele 1 (IGKV1-16*01).
- Figurediscloses SEQ ID NOS 107-110, respectively, in order of appearance.
- FIG.1Bis the amino acid sequence alignment of the parental mouse anti-CD5 antibody heavy chain and Ig alleles IGKV3-11*01, IGKV3-11*02, and IGKV1-16*01.
- Figurediscloses SEQ ID NOS 111-114, respectively, in order of appearance.
- FIG.1Cis the tabular representation of the percent mismatch between the human Ig alleles and parental mouse anti-CD5 antibody light and heavy chains.
- FIGS.2A-2Bshow amino acid sequence alignments between parental mouse anti-CD5 antibody (Antibody B), the humanized anti-CD5 antibody AB-5, an exemplary human antibody 4JLR.pdb, and three human germline Ig alleles.
- FIG.2Adisplays the light chain amino acid sequence alignments of parental anti-CD5 antibody, AB-5, 4JLR.pdb, and Ig alleles IGKV1-16*01, IGKV1-16*02, and IGKV1-39*01.
- Figurediscloses SEQ ID NOS 115-120, respectively, in order of appearance.
- FIG.2Bdisplays the heavy chain amino acid sequence alignments of parental anti-CD5 antibody, AB-5, 4JLR.pdb, and Ig alleles IGKV1- 16*01, IGKV1-16*02, and IGKV1-39*01.
- Figurediscloses SEQ ID NOS 121-126, respectively, in order of appearance.
- FIGS.3A-3Eshow exemplary SDS-PAGE and endotoxin analyses of purified negative control and anti-CD5 antibodies of the disclosure.
- FIG.3Ais an SDS-PAGE image of the antibody AB-4.
- FIG.3Bis an SDS-PAGE image of a negative control antibody, NC-AB.
- FIG.3Cis an SDS-PAGE image of the antibody AB-6.
- FIG.3Dis an SDS-PAGE image of the antibody AN-8.
- Non-reduced antibody samples in FIGS.3A-3Ddisplay a single high MW band and reduced antibody samples are associated with only two bands at 55 kDa and 25 kDa, corresponding to the IgG heavy chain and IgG light chain, respectively.
- FIG.3Eis a table that displays the purified concentrations of the antibodies shown in FIGS.3A-3D, as well as their optical density (OD) values at 545 nm and endotoxin units (EU) per mL and per mg antibody sample.
- FIGS.4A-4Fshow the results of anti-CD5 antibody cell binding assays.
- FIG.4Ais a table that displays the antibody concentrations that were used in cell binding assays.
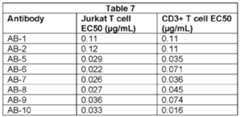
- FIG.4Bis a Jurkat T cell binding concentration-response graph of the antibodies AB-1, AB-2, AB-3, AB-4, and the negative control antibody NC-AB.
- FIG.4Cis a Jurkat T cell binding concentration-response graph of the antibodies AB-1, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10.
- FIG.4Dis a CD3+ T cell binding concentration-response graph of the antibodies AB-1, AB-2, AB-4, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10.
- FIG.4Eis a Jurkat T cell binding concentration-response graph of the parental mouse anti-human CD5 ( ⁇ -hCD5) antibody.
- FIG.4Fshows the raw data of a cell binding assay of ⁇ -hCD5 on mouse EL4 cells.
- FIGS.5A-5Fdisplay the internalization profiles of exemplary humanized anti-CD5 antibodies of the disclosure.
- FIG.5Ais a graph showing the change in signal intensity over the course of 6 hours for antibodies AB-1, AB-2, AB-3, AB-4, and NC-AB.
- FIG.5Bis a graph that shows the percent internalization values over time for the same antibodies in FIG.5A.
- FIG.5Cis a graph displaying the change in signal intensity the course of 6 hours for antibodies AB-1, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10.
- FIG.5Dis a graph displaying the percent internalization values over time for the same antibodies in FIG.5C.
- FIG.5Ais a graph showing the change in signal intensity over the course of 6 hours for antibodies AB-1, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10.
- FIG.5Eis a bar graph that displays the percent reduction of CD5 binding of the antibody, AB-4.
- FIG.5Fis a bar graph that displays the percent reduction of CD5 binding of the antibody, AB-8.
- FIG.6is a bar graph that shows the percent cytotoxicity associated with the control and exemplary humanized anti-CD5 antibodies of the disclosure.
- FIGS.7A-7Cshow binding of parental CD5 antibodies Antibody B and Antibody C to HEK293 cells (FIG.7A), human CD5 transfected HEK293 cells (FIG.7B) and rhesus monkey CD5 transfected HEK293 cells (FIG.7C) analyzed by flow cytometry.
- Antibodies and antibody fragments of the disclosureare described in detail in Section 4 and Section 6.1.
- the disclosurefurther provides conjugates useful for treating cancer comprising an antibody component covalently bonded to an ALK5 inhibitor, either directly or through a linker.
- An overview of the conjugates of the disclosureis presented in Section 6.2.
- the antibody component of the conjugatescan be an intact antibody or a fragment thereof.
- ALK5 inhibitors that can be used in the conjugates of the disclosureare described in Section 6.3.
- the conjugates of the disclosuretypically contain a linker between the antibody and ALK5 inhibitor. Exemplary linkers that can be used in conjugates of the disclosure are described in Section 6.4.
- the conjugates of the disclosurecan contain varying numbers of ALK5 inhibitor moieties per antibody. Drug loading is discussed in detail in Section 6.5.
- mAbmonoclonal antibody
- mAbmonoclonal antibody
- Fab and F(ab′)2 fragmentslack the Fc fragment of intact antibody, clear more rapidly from the circulation of the animal or plant, and may have less non-specific tissue binding than an intact antibody (Wahl et al., 1983, J. Nucl. Med.24:316).
- scFvrefers to a single chain Fv antibody in which the variable domains of the heavy chain and the light chain from a traditional antibody have been joined to form one chain.
- Native antibodies and immunoglobulinsare usually heterotetrameric glycoproteins of about 150,000 Daltons, composed of two identical light (L) chains and two identical heavy (H) chains. Each heavy chain has at the amino terminus a variable domain (VH) followed by a number of constant domains. Each light chain has a variable domain at the amino terminus (VL) and a constant domain at the carboxy terminus.
- VHvariable domain
- VLvariable domain at the amino terminus
- the antibodiesare preferably internalizing. Internalizing antibodies, after binding to their target molecules on cellular surface, are internalized by the cells as a result of the binding. The effect of this is that the conjugate is taken up by cells.
- antibody fragmentrefers to a portion of a full-length antibody, generally the target binding or variable region. Examples of antibody fragments include Fab, Fab′, F(ab′)2 and Fv fragments.
- an “Fv” fragmentis the minimum antibody fragment which contains a complete target recognition and binding site. This region consists of a dimer of one heavy and one light chain variable domain in a tight, noncovalent association (VH-VL dimer). It is in this configuration that the three CDRs of each variable domain interact to define a target binding site on the surface of the VH-VL dimer. Often, the six CDRs confer target binding specificity to the antibody. However, in some instances even a single variable domain (or half of an Fv comprising only three CDRs specific for a target) can have the ability to recognize and bind target. “Single chain Fv” or “scFv” antibody fragments comprise the VH and VL domains of an antibody in a single polypeptide chain.
- the Fv polypeptidefurther comprises a polypeptide linker between the VH and VL domain that enables the scFv to form the desired structure for target binding.
- the Fab fragmentcontains the constant domain of the light chain and the first constant domain (CH1) of the heavy chain.
- Fab′ fragmentsdiffer from Fab fragments by the addition of a few residues at the carboxyl terminus of the heavy chain CH1 domain including one or more cysteines from the antibody hinge region.
- F(ab′) fragmentsare produced by cleavage of the disulfide bond at the hinge cysteines of the F(ab′) 2 pepsin digestion product. Additional chemical couplings of antibody fragments are known to those of ordinary skill in the art.
- the antibodies of the disclosureare monoclonal antibodies.
- the term “monoclonal antibody” as used hereinis not limited to antibodies produced through hybridoma technology.
- the term “monoclonal antibody”refers to an antibody that is derived from a single clone, including any eukaryotic, prokaryotic, or phage clone and not the method by which it is produced.
- Monoclonal antibodies useful in connection with the present disclosurecan be prepared using a wide variety of techniques known in the art including the use of hybridoma, recombinant, and phage display technologies or a combination thereof.
- “Humanized” forms of non-human (e.g., murine) antibodiesare chimeric immunoglobulins, immunoglobulin chains or fragments thereof (such as Fv, Fab, Fab′, F(ab′) 2 or other target-binding subdomains of antibodies) which contain minimal sequences derived from non-human immunoglobulin.
- the humanized antibodywill comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the CDR regions correspond to those of a non-human immunoglobulin and all or substantially all of the FR regions are those of a human immunoglobulin sequence.
- the humanized antibodycan also comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin consensus sequence.
- Fcimmunoglobulin constant region
- Methods of antibody humanizationare known in the art. See, e.g., Riechmann et al., 1988, Nature 332:323-7; U.S. patent nos. 5,530,101; 5,585,089; 5,693,761; 5,693,762; and 6,180,370 to Queen et al.; European patent publication no. EP239400; PCT publication WO 91/09967; U.S. patent no.5,225,539; European patent publication no. EP592106; European patent publication no. EP519596; Padlan, 1991, Mol.
- the antibodies of the disclosureinclude derivatized antibodies.
- derivatized antibodiesare typically modified by glycosylation, acetylation, pegylation, phosphorylation, amidation, derivatization by known protecting/blocking groups, proteolytic cleavage, linkage to a cellular ligand or other protein (see Section 6.1 for a discussion of antibody conjugates), etc. Any of numerous chemical modifications can be carried out by known techniques, including, but not limited to, specific chemical cleavage, acetylation, formylation, metabolic synthesis of tunicamycin, etc. Additionally, the derivative can contain one or more non-natural amino acids, e.g., using ambrx technology (See, e.g., Wolfson, 2006, Chem.
- the antibodies or fragments thereofcan be antibodies or antibody fragments whose sequence has been modified to alter at least one constant region-mediated biological effector function relative to the corresponding wild type sequence.
- an antibody of the disclosurecan be modified to reduce at least one constant region-mediated biological effector function relative to an unmodified antibody, e.g., reduced binding to the Fc receptor (Fc ⁇ R) or to C1q.
- Fc ⁇ R and C1q bindingcan be reduced by mutating the immunoglobulin constant region segment of the antibody at particular regions necessary for Fc ⁇ R or C1q interactions (See, e.g., Canfield and Morrison, 1991, J. Exp.
- Reduction in Fc ⁇ R binding ability of the antibodycan also reduce other effector functions which rely on Fc ⁇ R interactions, such as opsonization, phagocytosis and antibody-dependent cellular cytotoxicity (“ADCC”), while reduction of C1q binding can reduce complement-dependent cytotoxicity (“CDCC). Reduction or elimination of effector function can thus prevent T cells targeted by an conjugate of the disclosure from being destroyed via ADCC or CDC.
- ADCCantibody-dependent cellular cytotoxicity
- effector function of an antibodyis modified by selective mutation of the Fc portion of the antibody, so that it maintains antigen specificity and internalization capacity but eliminates ADCC/CDC function.
- Numerous mutationshave been described in the art for reducing Fc ⁇ R and C1q binding and such mutations can be included in an antibody of the disclosure. For example, U.S. Pat.
- No.6,737,056discloses that single position Fc region amino acid modifications at positions 238, 265, 269, 270, 292, 294, 295, 298, 303, 324, 327, 329, 333, 335, 338, 373, 376, 414, 416, 419, 435, 438 or 439 result in reduced binding to Fc ⁇ RII and Fc ⁇ RII.
- numbering of amino acid residues in an Fc domain or constant regionis according to the EU numbering system, also called the EU index, as described in Kabat et al., 1991, Sequences of Proteins of Immunological Interest, 5th Ed. Public Health Service, National Institutes of Health, Bethesda, MD. U.S.
- patent no.9,790,268discloses that an asparagine residue at amino acid position 298 and a serine or threonine residue at amino acid position 300 reduce Fc ⁇ R binding.
- PCT publication no. WO 2014/190441describes modified Fc domains with reduced Fc ⁇ R binding having L234D/L235E : L234R/L235R/E233K, L234D/L235E/D265S : E233K/L234R/L235R/D265S, L234D/L235E/E269K : E233K/L234R/L235R/E269K, L234D/L235E/K322A : E233K/L234R/L235R/K322A, L234D/L235E/P329W : E233K/L234R/L235R/P329W, L234D/L235E/E269K/D265S/K322A
- antibodies or antigen binding fragments of the disclosurecomprise a first Fc region and a second Fc region that dimerize to form an Fc domain.
- sequences derived from human IgG1include the following: APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLV KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT QKSLSLSPGK (SEQ ID NO:51) (WT sequence) APEAAGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLV K
- an anti-CD5 antibody or antigen binding fragment thereof of the disclosurehas a first Fc region and/or a second Fc region comprising a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:54.
- an anti-CD5 antibody or antigen binding fragment thereof of the disclosurehas a first Fc region and/or a second Fc region comprising a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:81.
- Anti-CD5 antibodies and antigen binding fragmentscan comprise a hinge region, for example connecting a CH1 domain to a Fc region comprising a CH2 and CH3 domain.
- a human IgG1, IgG2, or IgG4 hingecan be used.
- Exemplary hinge sequencesinclude EPKSCDKTHTCPPCP (SEQ ID NO:66) (human IgG1), ESKYGPPCPSCP (SEQ ID NO:67) (human IgG4), ESKYGPPCPPCP (SEQ ID NO:68) (human IgG4 with a S228P substitution), and ERKCCVECPPCP (SEQ ID NO:83) (human IgG2).
- Exemplary amino acid sequences comprising a CH1 domain (which can be C-terminal to a VH, for example), hinge, CH2 domain, and CH3 domaininclude the following: ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVS NKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSPGK (SEQ ID NO:62) (derived from
- an antibody or fragment thereofcan be modified to acquire or improve at least one constant region-mediated biological effector function relative to an unmodified antibody, e.g., to enhance Fc ⁇ R interactions (See, e.g., US 2006/0134709).
- an antibody of the disclosurecan have a constant region that binds Fc ⁇ RIIA, Fc ⁇ RIIB and/or Fc ⁇ RIIIA with greater affinity than the corresponding wild type constant region.
- antibodies of the disclosurecan have alterations in biological activity that result in decreased opsonization, phagocytosis, or ADCC. Such alterations are known in the art. For example, modifications in antibodies that reduce ADCC activity are described in U.S. patent no.
- the antibodies or fragments thereofcan be antibodies or antibody fragments that have been modified to increase or reduce their binding affinities to the fetal Fc receptor, FcRn, for example, by mutating the immunoglobulin constant region segment at particular regions involved in FcRn interactions (See, e.g., WO 2005/123780). Such mutations can increase the antibody’s binding to FcRn, which protects the antibody from degradation and increases its half-life.
- an antibodyhas one or more amino acids inserted into one or more of its hypervariable regions, for example as described in Jung and Plückthun, 1997, Protein Engineering 10(9):959-966; Yazaki et al., 2004, Protein Eng. Des Sel.17(5):481-9; and U.S. patent publication no. 2007/0280931.
- the conjugates of the disclosureare generally composed of an ALK5 inhibitor covalently attached to an anti-CD5 antibody (e.g., a humanized anti-CD5) or antigen binding fragment thereof, typically via a linker, such that covalent attachment does not interfere with binding to CD5.
- an ALK5 inhibitorcan be conjugated to the antibody component via one or more native or engineered cysteine, lysine, or glutamine residues, one or more unnatural amino acids (e.g., p-acetylphenylalanine (pAcF), p-azidomethyl-L- phenylalanine (pAMF), or selenocysteine (Sec)), one or more glycans (e.g., fucose, 6-thiofucose, galactose, N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), or sialic acid (SA)), or one or more short peptide tags of four to six amino acids.
- pAcFp-acetylphenylalanine
- pAMFp-azidomethyl-L- phenylalanine
- Secsialic acid
- the antibody or fragment thereofis fused via a covalent bond (e.g., a peptide bond), through the antibody’s N-terminus or the C-terminus or internally, to an amino acid sequence of another protein (or portion thereof; for example, at least a 10, 20 or 50 amino acid portion of the protein).
- the antibody, or fragment thereofcan linked to the other protein at the N-terminus of the constant domain of the antibody. Recombinant DNA procedures can be used to create such fusions, for example as described in WO 86/01533 and EP0392745.
- the effector moleculecan increase half-life in vivo, and/or enhance the delivery of an antibody across an epithelial barrier to the immune system.
- suitable effector molecules of this typeinclude polymers, albumin, albumin binding proteins or albumin binding compounds such as those described in PCT publication no. WO 2005/117984.
- the metabolic process or reactionmay be an enzymatic process, such as proteolytic cleavage of a peptide linker of the conjugate, or hydrolysis of a functional group such as a hydrazone, ester, or amide.
- Intracellular metabolitesinclude, but are not limited to, antibodies and free drug which have undergone intracellular cleavage after entry, diffusion, uptake or transport into a cell.
- the terms “intracellularly cleaved” and “intracellular cleavage”refer to a metabolic process or reaction inside a cell on a conjugate whereby the covalent attachment, i.e. linker, between the drug moiety (D) and the antibody (Ab) is broken, resulting in the free drug dissociated from the antibody inside the cell.
- the cleaved moieties of the conjugateare thus intracellular metabolites.
- the ALK5 Inhibitor[0114]
- the ALK5 inhibitors of the disclosureare preferably small molecules that competitively and reversibly bind to ATP binding site in the cytoplasmic kinase domain of the ALK5 receptor, preventing downstream R-Smad phosphorylation.
- the ALK5 inhibitorsmay but not need be specific or selective for ALK5 vs. other TGF- ⁇ family receptors, such as ALK4 and/or ALK7 and/or TGF- ⁇ receptor II.
- the ALK5 inhibitorshave activity towards both ALK5 and TGF- ⁇ receptor II. While it is preferable that the ALK5 inhibitor have limited inhibitory activity towards the BMP II receptor, this is not necessary because the conjugates of the disclosure are targeted to T cells, in which BMP II activity is minimal or absent.
- the ALK5 inhibitors of the disclosurepreferably have an IC50 of 100 nM or less, more preferably 50 nM or less, and most preferably 20 nM or less when measured in an in vitro cellular assay using T cells from at least 3 subjects, at least 5 subjects or at least 10 subjects.
- ALK5 inhibitors suitable for use in the antibody-drug conjugates of the present disclosureinclude imidazole-benzodioxol compounds, imidazole-quinoxaline compounds, pyrazole-pyrrolo compounds and thiazole type compounds.
- imidazole-benzodioxol type ALK5 inhibitorshave the formula
- R 2is hydrogen or methyl
- halogensinclude fluorine or chlorine
- Ahas 1 carbon atom and B is a direct bond to the benzyl group and R 3 is an amide.
- R 2is hydrogen or methyl
- Ahas 1 carbon atom and B is a direct bond to the benzyl group.
- pyrazole type ALK5 inhibitorshave the formula to about 5 carbon atoms
- R 4is hydrogen, halogen, lower alkyl having from 1 to about 5 carbon atoms, alkoxy having from 1 to about 5 carbon atoms, haloalkyl, carboxyl, carboxyalkylester, nitrile, alkylamine or a group having the formula
- R 5is lower alkyl having from 1 to about 5 carbon atoms, halogen or morpholino
- R 6is pyrole, cyclohexyl, morpholino, pyrazole, pyran, imidazole, oxane, pyrrolidinyl or alkylamine
- Ais a direct bond or an alkyl having from 1 to about 5 carbon atoms.
- pyrazole-pyrrolo type ALK5 inhibitorshave the formula 1 to about 5 carbon atoms, alkanol, morpholino or alkylamine, R 2 is hydrogen, halogen or lower alkyl having from 1 to about 5 carbon atoms and R 8 is hydrogen, hydroxyl, amino, halogen or a group having the formula
- R 5is piperazinyl
- R 6is morpholino, piperidinyl, piperazinyl, alkoxy, hydroxyl, oxane, halogen, thioalkyl or alkylamine
- Ais a lower alkyl having from 1 to about 5 carbon atoms.
- thiazole type ALK5 inhibitorshave the formula or lower alkyl having from 1 to about 5 carbon atoms, and R 10 is or about 5 carbon atoms.
- the ALK5 inhibitorhas the formula a halogen, -OR 10 , -SR 10 , -N(R 10 )2, -C(O)R 10 , -C(O)N(R 10 )2, -N(R 10 )C(O)R 10 - C(O)OR 10 , -OC(O)R 10 , - S(O)R 10 , -S(O)2R 10 , -S(O)2N(R 10 )2, -P(O)(OR 10 )2, -OP(O)(OR 10 )2, -N02, and -CN; -C1-C10 alkyl, -C2- C l0 alkenyl, and -C 2 -C l0 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents independently selected from a halogen, -OR 10 , -SR 10 , -N(R 10 )2, -C
- the ALK5 inhibitorhas the formula , -NO2, -CN, or - - one or more independently selected from halogen, -OR 11 , -SR 11 , -S(O)R 10 , -S(O) 2 R 11 , -S(O) 2 N(R 11 ) 2 -N(R 11 ) 2 , - C(O)R 10 , -C(O)N(R 11 )2, -N(R 11 )C(O)R 10 , -C(O)OR 11 , -OC(O)R 10 , -NO2, and -CN;
- R 3is, at each occurrence, independently halogen, -C 1 -C 3 alkyl, -C 1 -C 3 haloalkyl, -OH, -NO 2 , -CN, -O-C 1
- the ALK5 inhibitoris an ALK5 inhibitor described in Table 16 of WO 2021/011834.
- the ALK5 inhibitorhas the formula L’ is absent, ⁇ S ⁇ , ⁇ O ⁇ , or ⁇ NH ⁇ ; A is absent, carbocycle, or heterocycle; Q 3 is N or CR 3 ; Q 4 is N or CR 4 ; Q 5 is N or CR 5 ; Q 6 is N or CR 6 ; Q 7 is C or N; R 1 is hydrogen, halo, C1–3 alkyl, or C1–3 haloalkyl; R 2 is, at each occurrence, independently halo, C1–3 alkyl, C1–3 alkoxy, C1–3 haloalkyl, or C1-3 haloalkoxy; R 3 is H, halo, C 1–3 alkyl, C 1–3 hal
- the ALK5 inhibitorhas the formula Q 4 is N or CR 4 ; Q 5 is N or CR 5 ; Q 6 is N or CR 6 ; R a and R b are each H, or R a and R b , together with the atoms to which they are attached, form a heterocyclic ring;
- R 1is H, C 1-3 alkyl, or C 1-3 haloalkyl;
- R 2is, at each occurrence, independently halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy;
- R 3is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy;
- R 4is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy;
- R 5is H, halo, C1-3 alkyl, C
- the ALK5 inhibitorhas the formula R 1 is H, C 1-3 alkyl, or C 1-3 haloalkyl; R 2 is, at each occurrence, independently halo, C 1-3 alkyl, C 1-3 alkoxy, C 1-3 haloalkyl, or C 1-3 haloalkoxy; R 6 is H, halo, C 1-3 alkyl, C 1-3 alkoxy, C 1-3 haloalkyl, or C 1-3 haloalkoxy; wherein R 1 , R 2 , and R 6 are, at each occurrence, independently substituted with 0-3 R 10 ; R 7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody; R 10 is, at each occurrence, independently C 1-3 alkoxy or C 1-3 haloalkoxy; and m is 0-3;
- the ALK5 inhibitorhas the formula R 1 is H, C 1-3 alkyl, or C 1-3 haloalkyl; R 2 is, at each occurrence, independently halo, C 1-3 alkyl, C 1-3 alkoxy, C 1-3 haloalkyl, or C 1-3 haloalkoxy; R 6 is H, halo, C 1-3 alkyl, C 1-3 alkoxy, C 1-3 haloalkyl, or C 1-3 haloalkoxy; wherein R 1 , R 2 , and R 6 are each substituted with 0-3 R 10 ; R 7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody, an antibody construct, or a targeting moiety; R 10 is, at each occurrence, independently C 1-3 alkoxy or C 1-3 haloalkoxy; and m
- the ALK5 inhibitorhas the formula ring B is carbocycle or heterocycle;
- R 1is H, C 1-3 alkyl, or C 1-3 haloalkyl;
- R 2is, at each occurrence, independently halo, C 1-3 alkyl, C 1-3 alkoxy, C 1-3 haloalkyl, or C 1-3 haloalkoxy; wherein R 1 and R 2 are, at each occurrence, independently substituted with 0-3 R 10 ;
- R 7is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody, an antibody construct, or a targeting moiety;
- R 9is, at each occurrence, independently halo, C 1-3 alkyl, C 1-3 haloalkyl, C 1-3 alkoxy, or C 1-3 haloalkoxy;
- R 10is, at each occurrence, independently C 1-3 al 1-3 al
- PCT publication no. WO 2000/61576 and U.S. patent publication no. US 2003/0149277disclose triarylimidazole derivatives and their use as ALK5 inhibitors.
- PCT publication no. WO 2001/62756discloses pyridinylimidazole derivatives and their use as ALK5 inhibitors.
- PCT publication no. WO 2002/055077discloses use of imidazolyl cyclic acetal derivatives as ALK5 inhibitors.
- PCT publication no. WO 2003/087304discloses tri-substituted heteroaryls and their use as ALK5 and/or ALK4 inhibitors.
- ALK5 inhibitorsare commercially available, including SB-525334 (CAS 356559-20-1), SB-505124 (CAS 694433-59-5), SB-431542 (CAS 301836-41-9), SB-202474 (EMD4 Biosciences Merck KGaA, Darmstadt, Germany), LY-364947 (CAS 396129-53-6), IN-1130, GW-788388 and D4476 (EMD4 Biosciences Merck KGaA, Darmstadt, Germany). [0143] The structures and names of ALK5 inhibitors described herein refer to the molecule prior to the attachment to the antibody and/or linker.
- Preferred ALK5 inhibitorsare those which can be attached to a linker via a free NH or NH 2 group, preferably an NH or NH2 group attached to or part of an alkyl, heteroaryl, or aryl group (e.g., as in Compounds 1-23, 26-29, 31, 35, 37, 39, 40, 42, 43, 45-48, 50-85, 87-90, 93, 96, 98-104, 106, 108, 109, 111, 112, 114, 116-120, 132, 146, 149, 156, 184, 187, 193, 218, 260-277, 282, and 283 shown in Table 3A).
- ALK5 inhibitorscan be derivatized to add a free NH or NH 2 group. Design of derivatized ALK5 inhibitors should preferably take into account the inhibitors’ structure activity relationships (SAR) to reduce the likelihood of abolishing inhibitory activity when adding an NH or NH2 group, although the activity may also be determined empirically. Exemplary derivatized counterparts of several compounds shown in Table 2 are shown below in Table 4. Table 4 Table 2 Derivative 1 Derivative 2
- the conjugatescomprise a linker between the ALK5 inhibitor and the antibody.
- Linkersare moieties comprising a covalent bond or a chain of atoms that covalently attaches an antibody to a drug moiety.
- linkersinclude a divalent radical such as an alkyldiyl, an aryldiyl, a heteroaryldiyl, moieties such as:-(CR 2 ) n O(CR 2 ) n -, repeating units of alkyloxy (e.g., polyethylenoxy, PEG, polymethyleneoxy) and alkylamino (e.g., polyethyleneamino, JeffamineTM); and diacid ester and amides including succinate, succinamide, diglycolate, malonate, and caproamide.
- PEG containing linkersare known in the art and commercially available (e.g., from BroadPharm (broadpharm.com).
- Exemplary PEG containing linkersinclude Mal-PEG2-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23203), Mal-PEG4-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23204), Mal-PEG4-Val-Cit- PAB-PNP (BroadPharm cat. no. BP-23668), Mal-amido-PEG2-Val-Cit-PAB-PNP (BroadPharm cat. no. BP-23675), Azido-PEG3-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23206), Azido-PEG4-Val-Cit-PAB- OH (BroadPharm cat.
- a linkermay comprise one or more linker components, such as stretcher and spacer moieties.
- a peptidyl linkercan comprise a peptidyl component of two or more amino acids and, optionally, one or more stretcher and/or spacer moieties.
- linker componentsare known in the art, some of which are described below.
- a linkermay be a “cleavable linker,” facilitating release of a drug in the cell.
- an acid- labile linkere.g., hydrazone
- protease-sensitive linkere.g., peptidase-sensitive
- photolabile linkere.g., dimethyl linker or disulfide-containing linker
- linkers and linker components known in the artinclude aleimidocaproyl (mc); maleimidocaproyl-p-aminobenzylcarbamate; maleimidocaproyl-peptide-aminobenzylcarbamate linkers, e.g., maleimidocaproyl-L-phenylalanine-L-lysine-p-aminobenzylcarbamate and maleimidocaproyl-L- valine-L-citrulline-p-aminobenzylcarbamate (vc); N-succinimidyl 3-(2-pyridyldithio)proprionate (also known as N-succinimidyl 4-(2-pyridyldithio)pentanoate or SPP); 4-succinimidyl-oxycarbonyl-2-methyl-2-(2- pyridyldithio)-toluene (SMPT); N-succinimidyl-oxycarbony
- the linkeris cleavable under intracellular or extracellular conditions, such that cleavage of the linker releases the ALK5 inhibitor from the antibody in the appropriate environment.
- the linkeris not cleavable and the drug is released, for example, by antibody degradation in lysosomes (see U.S. patent publication 2005/0238649 incorporated by reference herein in its entirety and for all purposes).
- Examples of non-cleavable linkers that can be used in the conjugates of the disclosureinclude N- maleimidomethylcyclohexane1-carboxylate, maleimidocaproyl or mercaptoacetamidocaproyl linkers.
- the linkeris cleavable by a cleaving agent that is present in the intracellular environment (for example, within a lysosome or endosome or caveolea).
- the linkercan be, for example, a peptidyl linker that is cleaved by an intracellular peptidase or protease enzyme, including, but not limited to, a lysosomal or endosomal protease.
- the peptidyl linkercomprises a peptidyl component that is at least two amino acids long or at least three amino acids long or more.
- Cleaving agentscan include, without limitation, cathepsins B and D and plasmin, all of which are known to hydrolyze dipeptide drug derivatives resulting in the release of active drug inside target cells (see, e.g., Dubowchik and Walker, 1999, Pharm. Therapeutics 83:67-123).
- a peptidyl linker that is cleavable by the thiol-dependent protease cathepsin-Be.g., a Phe-Leu or a Gly-Phe-Leu-Gly linker (SEQ ID NO: 106).
- Other examples of such linkersare described, e.g., in U.S. patent no.
- the peptidyl linker cleavable by an intracellular proteaseis a Val-Cit linker or a Phe-Lys linker (see, e.g., U.S. patent no.6,214,345, which describes the synthesis of doxorubicin with the val-cit linker).
- the cleavable linkeris pH-sensitive, that is, sensitive to hydrolysis at certain pH values. Typically, the pH-sensitive linker hydrolyzable under acidic conditions.
- an acid-labile linker that is hydrolyzable in the lysosomefor example, a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the like
- a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the likemay be used.
- a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the likemay be used.
- the hydrolyzable linkeris a thioether linker (such as, e.g., a thioether attached to the therapeutic agent via an acylhydrazone bond (see, e.g., U.S. patent no. 5,622,929).
- the linkeris cleavable under reducing conditions (for example, a disulfide linker).
- disulfide linkersare known in the art, including, for example, those that can be formed using SATA (N-succinimidyl-5-acetylthioacetate), SPDP (N-succinimidyl-3-(2- pyridyldithio)propionate), SPDB (N-succinimidyl-3-(2-pyridyldithio)butyrate) and SMPT (N-succinimidyl- oxycarbonyl-alpha-methyl-alpha-(2-pyridyl-dithio)toluene)-, SPDB and SMPT.
- SATAN-succinimidyl-5-acetylthioacetate
- SPDPN-succinimidyl-3-(2- pyridyldithio)propionate
- SPDBN-succinimidyl-3-(2-pyridyldithio)butyrate
- SMPTN
- the linkeris a malonate linker (Johnson et al., 1995, Anticancer Res.
- the linkeris a polyvalent linker that can be used to link many drug molecules to a single antibody molecule.
- the Fleximer linker technology developed by Mersanais based on incorporating drug molecules into a solubilizing poly-acetal backbone via a sequence of ester bonds.
- the methodologyenables highly-loaded conjugates (e.g., having a drug antibody ratio (DAR) up to 20) while maintaining good physicochemical properties.
- DARdrug antibody ratio
- Exemplary polyvalent linkerare described, for example, in WO 2009/073445; WO 2010/068795; WO 2010/138719; WO 2011/120053; WO 2011/171020; WO 2013/096901; WO 2014/008375; WO 2014/093379; WO 2014/093394; and WO 2014/093640, the contents of which are incorporated herein by reference in their entireties. [0158] Often the linker is not substantially sensitive to the extracellular environment.
- linkerin the context of a linker, means that no more than about 20%, 15%, 10%, 5%, 3%, or no more than about 1% of the linkers, in a sample of conjugate, are cleaved when the conjugate presents in an extracellular environment (for example, in plasma).
- Whether a linker is not substantially sensitive to the extracellular environmentcan be determined, for example, by incubating with plasma the conjugate for a predetermined time period (for example, 2, 4, 8, 16, or 24 hours) and then quantitating the amount of free drug present in the plasma.
- the linkercan promote cellular internalization.
- An conjugate of the disclosuremay be of Formula I, below, wherein an antibody (Ab) is conjugated to one or more ALK5 inhibitor drug moieties (D) through an optional linker (L) A b-(L-D) p I [0164] Accordingly, the antibody may be conjugated to the drug either directly or via a linker.
- pis the average number of drug (i.e., ALK5 inhibitor) moieties per antibody, which can range, e.g., from about 1 to about 20 drug moieties per antibody, and in certain embodiments, from 2 to about 8 drug moieties per antibody. Further details of drug loading are described in Section 6.5 below.
- a linker componentmay comprise a “stretcher” that links an antibody e.g., via a cysteine residue, to another linker component or to a drug moiety. Exemplary stretchers are shown below (wherein the left wavy line indicates the site of covalent attachment to an antibody and the right wavy line indicates the site of covalent attachment to another linker component or drug moiety):
- a linker componentmay comprise an amino acid unit.
- the amino acid unitallows for cleavage of the linker by a protease, thereby facilitating release of the drug from the conjugate upon exposure to intracellular proteases, such as lysosomal enzymes. See, e.g., Doronina et al., 2003, Nat. Biotechnol.21:778-784.
- Exemplary amino acid unitsinclude, but are not limited to, a dipeptide, a tripeptide, a tetrapeptide, and a pentapeptide.
- Exemplary dipeptidesinclude: valine-citrulline (VC or val-cit), alanine-phenylalanine (AF or ala-phe); phenylalanine- lysine (FK or phe-lys); or N-methyl-valine-citrulline (Me-val-cit).
- Exemplary tripeptidesinclude: glycine- valine-citrulline (gly-val-cit) and glycine-glycine-glycine (gly-gly-gly).
- a spacer unitmay be “self-immolative” or a “non-self-immolative.”
- a “non-self-immolative” spacer unitis one in which part or all of the spacer unit remains bound to the drug moiety upon enzymatic (e.g., proteolytic) cleavage of the conjugate.
- Examples of non-self-immolative spacer unitsinclude, but are not limited to, a glycine spacer unit and a glycine-glycine spacer unit.
- a “self-immolative” spacer unitallows for release of the drug moiety without a separate hydrolysis step.
- a spacer unit of a linkercomprises a p-aminobenzyl unit.
- a p-aminobenzyl alcoholis attached to an amino acid unit via an amide bond, and a carbamate, methylcarbamate, or carbonate is made between the benzyl alcohol and a cytotoxic agent.
- the spacer unitis p-aminobenzyloxycarbonyl (PAB).
- a spacer unitis a branched bis(hydroxymethyl)styrene (BHMS) unit as depicted below, which can be used to incorporate and release multiple drugs.
- BHMSbranched bis(hydroxymethyl)styrene
- a linkermay comprise any one or more of the above linker components.
- a linkeris as shown in brackets in the following conjugate formula: Ab–(–[Aa-Ww-Yy]-D) p II wherein Ab, A, a, W, w, D, and p are as defined in the preceding paragraph; Y is a spacer unit, and y is 0, 1, or 2; and. Exemplary embodiments of such linkers are described in U.S. patent publication no. 2005/0238649 A1, which is incorporated herein by reference. [0170] Exemplary linker components and combinations thereof are shown below in the context of conjugates of Formula II: O H N Y y D PAB
- Linkers componentsincluding stretcher, spacer, and amino acid units, may be synthesized by methods known in the art, such as those described in U.S. patent publication no.2005/0238649. 6.5.
- Drug loadingis represented by p and is the average number of ALK5 inhibitor moieties per antibody in a molecule. Drug loading (“p”) may be 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 or more moieties (D) per antibody, although frequently the average number is a fraction or a decimal. Generally, ALK5 inhibitor loading averages from 2 to 8 drug moieties per antibody, more preferably 2 to 4 drug moieties per antibody or 5 to 7 drug moieties per antibody.
- conjugate molecules(sometimes in the context of a pharmaceutical composition), each molecule composed of an antibody covalently attached to one or more ALK5 inhibitor moieties, with the drug loading ratio representing the average drug loading in the population or collection, although the ratio on an individual molecule basis may vary from one conjugate molecule to another in the population.
- the population or collectioncontains conjugate molecules comprising an antibody covalently attached to anywhere between 1 and 30 drug moieties, and in some embodiments anywhere between 1 and 20, between 1 and 15, between 2 and 12, between 2 and 8, between 4 and 15, or between 6 and 12 drug moieties.
- the loading (drug/antibody ratio) of an conjugatemay be controlled in different ways, e.g., by:(i) limiting the molar excess of drug-linker intermediate or linker reagent relative to antibody, (ii) limiting the conjugation reaction time or temperature, (iii) partial or limiting reductive conditions for cysteine thiol modification, (iv) engineering by recombinant techniques the amino acid sequence of the antibody such that the number and position of cysteine residues is modified for control of the number and/or position of linker-drug attachments (such as thioMab or thioFab prepared as disclosed in PCT publication no. WO 2006/034488 (herein incorporated by reference in its entirety)).
- the resulting productis a mixture of conjugate compounds with a distribution of one or more drug moieties attached to an antibody.
- the average number of drugs per antibodymay be calculated from the mixture by a dual ELISA antibody assay, which is specific for antibody and specific for the drug.
- Individual conjugate moleculesmay be identified in the mixture by mass spectroscopy and separated by HPLC, e.g. hydrophobic interaction chromatography.
- HPLCe.g. hydrophobic interaction chromatography.
- a homogeneous conjugate with a single loading valuemay be isolated from the conjugation mixture by electrophoresis or chromatography. 6.6.
- a host cellis transfected with one or more recombinant expression vectors carrying DNA fragments encoding the immunoglobulin light and heavy chains of the antibody such that the light and heavy chains are expressed in the host cell and, optionally, secreted into the medium in which the host cells are cultured, from which medium the antibodies can be recovered.
- Standard recombinant DNA methodologiesare used to obtain antibody heavy and light chain genes, incorporate these genes into recombinant expression vectors and introduce the vectors into host cells, such as those described in Molecular Cloning; A Laboratory Manual, Second Edition (Sambrook, Fritsch and Maniatis (eds), Cold Spring Harbor, N. Y., 1989), Current Protocols in Molecular Biology (Ausubel, F.
- DNA encoding a V H regioncan be converted to a full-length heavy chain gene by operatively linking the VH-encoding DNA to another DNA molecule encoding heavy chain constant regions (CH1, CH2, CH3 and, optionally, CH4).
- heavy chain constant regionsCH1, CH2, CH3 and, optionally, CH4.
- the sequences of human heavy chain constant region genesare known in the art (See, e.g., Kabat et al., 1991, Sequences of Proteins of Immunological Interest, Fifth Edition, U.S. Department of Health and Human Services, NIH Publication No.91-3242) and DNA fragments encompassing these regions can be obtained by standard PCR amplification.
- the heavy chain constant regioncan be an IgG1, IgG2, IgG3, IgG4, IgA, IgE, IgM or IgD constant region, but in certain embodiments is an IgG 1 or IgG 4 constant region.
- DNA encoding a VL regioncan be converted to a full-length light chain gene (as well as a Fab light chain gene) by operatively linking the V L -encoding DNA to another DNA molecule encoding the light chain constant region, CL.
- the sequences of human light chain constant region genesare known in the art (See, e.g., Kabat et al., 1991, Sequences of Proteins of Immunological Interest, Fifth Edition, U.S.
- DNAs encoding partial or full-length light and heavy chainsobtained as described above, are inserted into expression vectors such that the genes are operatively linked to transcriptional and translational control sequences.
- operatively linkedis intended to mean that an antibody gene is ligated into a vector such that transcriptional and translational control sequences within the vector serve their intended function of regulating the transcription and translation of the antibody gene.
- the expression vector and expression control sequencesare chosen to be compatible with the expression host cell used.
- the antibody light chain gene and the antibody heavy chain genecan be inserted into separate vectors or, more typically, both genes are inserted into the same expression vector.
- the antibody genesare inserted into the expression vector by standard methods (e.g., ligation of complementary restriction sites on the antibody gene fragment and vector, or blunt end ligation if no restriction sites are present).
- the expression vectorPrior to insertion of the anti-CD5 antibody-related light or heavy chain sequences, the expression vector can already carry antibody constant region sequences.
- one approach to converting the anti-CD5 monoclonal antibody-related VH and VL sequences to full-length antibody genesis to insert them into expression vectors already encoding heavy chain constant and light chain constant regions, respectively, such that the V H segment is operatively linked to the CH segment(s) within the vector and the V L segment is operatively linked to the CL segment within the vector.
- the recombinant expression vectorcan encode a signal peptide that facilitates secretion of the antibody chain from a host cell.
- the antibody chain genecan be cloned into the vector such that the signal peptide is linked in-frame to the amino terminus of the antibody chain gene.
- the signal peptidecan be an immunoglobulin signal peptide or a heterologous signal peptide (i.e., a signal peptide from a non-immunoglobulin protein).
- exemplary signal peptidesinclude MGSTAILGLLLAVLQGGRA (SEQ ID NO:60) and METDTLLLWVLLLWVPGSTGAS (SEQ ID NO:61).
- the recombinant expression vectors of the disclosurecarry regulatory sequences that control the expression of the antibody chain genes in a host cell.
- regulatory sequenceis intended to include promoters, enhancers and other expression control elements (e.g., polyadenylation signals) that control the transcription or translation of the antibody chain genes.
- promoterse.g., promoters, enhancers and other expression control elements (e.g., polyadenylation signals) that control the transcription or translation of the antibody chain genes.
- expression control elementse.g., polyadenylation signals
- Such regulatory sequencesare described, for example, in Goeddel, Gene Expression Technology: Methods in Enzymology 185, Academic Press, San Diego, Calif., 1990. It will be appreciated by those skilled in the art that the design of the expression vector, including the selection of regulatory sequences may depend on such factors as the choice of the host cell to be transformed, the level of expression of protein desired, etc.
- Suitable regulatory sequences for mammalian host cell expressioninclude viral elements that direct high levels of protein expression in mammalian cells, such as promoters and/or enhancers derived from cytomegalovirus (CMV) (such as the CMV promoter/enhancer), Simian Virus 40 (SV40) (such as the SV40 promoter/enhancer), adenovirus, (e.g., the adenovirus major late promoter (AdMLP)) and polyoma.
- CMVcytomegalovirus
- SV40Simian Virus 40
- AdMLPadenovirus major late promoter
- DHFR selectable markere.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- NSO myeloma cellse.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621
- the host cellcan be co- transfected with two expression vectors of the disclosure, the first vector encoding a heavy chain derived polypeptide and the second vector encoding a light chain derived polypeptide.
- the two vectorscan contain identical selectable markers, or they can each contain a separate selectable marker.
- a single vectorcan be used which encodes both heavy and light chain polypeptides.
- nucleic acid encoding one or more portions of an anti-CD5 antibodyfurther alterations or mutations can be introduced into the coding sequence, for example to generate nucleic acids encoding antibodies with different CDR sequences, antibodies with reduced affinity to the Fc receptor, or antibodies of different subclasses.
- the anti-CD5 antibodies of the disclosurecan also be produced by chemical synthesis (e.g., by the methods described in Solid Phase Peptide Synthesis, 2nd ed., 1984 The Pierce Chemical Co., Rockford, Ill.).
- Variant antibodiescan also be generated using a cell-free platform (See, e.g., Chu et al., Biochemia No.2, 2001 (Roche Molecular Biologicals) and Murray et al., 2013, Current Opinion in Chemical Biology, 17:420-426).
- a cell-free platformSee, e.g., Chu et al., Biochemia No.2, 2001 (Roche Molecular Biologicals) and Murray et al., 2013, Current Opinion in Chemical Biology, 17:420-426).
- an anti-CD5 antibody or antigen-binding fragment thereof of the disclosureOnce an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure has been produced by recombinant expression, it can be purified by any method known in the art for purification of an immunoglobulin molecule, for example, by chromatography (e.g., ion exchange, affinity, and sizing column chromatography), centrifugation, differential solubility, or by any other standard technique for the purification of proteins.
- the anti-CD5 antibodies of the present disclosure and/or binding fragmentscan be fused to heterologous polypeptide sequences described herein or otherwise known in the art to facilitate purification.
- the anti-CD5 antibody or antigen-binding fragmentcan, if desired, be further purified, e.g., by high performance liquid chromatography (see, e.g., Fisher, Laboratory Techniques In Biochemistry And Molecular Biology, Work and Burdon, eds., Elsevier, 1980), or by gel filtration chromatography on a SuperdexTM 75 column (Pharmacia Biotech AB, Uppsala, Sweden). 6.7.
- Suitable routes of administration of the conjugates, the antibodies, and antigen binding fragments of the disclosureinclude, without limitation, oral, parenteral, rectal, transmucosal, intestinal administration, intramedullary, intrathecal, direct intraventricular, intravenous, intravitreal, intracavitary, intraperitoneal, or intratumoral injections.
- the preferred routes of administrationare parenteral, more preferably intravenous.
- compositions of the disclosurecan be formulated according to known methods to prepare pharmaceutically useful compositions, for example whereby a conjugate is combined in a mixture with a pharmaceutically suitable excipient.
- a conjugateis combined in a mixture with a pharmaceutically suitable excipient.
- Sterile phosphate-buffered salineis one example of a pharmaceutically suitable excipient.
- Other suitable excipientsare well-known to those in the art. See, for example, Ansel et al., Pharmaceutical Dosage Forms And Drug Delivery Systems, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition (Mack Publishing Company 1990), and revised editions thereof.
- a conjugateis formulated in Good’s biological buffer (pH 6-7), using a buffer selected from the group consisting of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES); N-(2- acetamido)iminodiacetic acid (ADA); N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES); 4-(2- hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES); 2-(N-morpholino)ethanesulfonic acid (MES); 3- (N-morpholino)propanesulfonic acid (MOPS); 3-(N-morpholinyl)-2-hydroxypropanesulfonic acid (MOPSO); and piperazine-N,N'-bis(2-ethanesulfonic acid) [Pipes].
- a bufferselected from the group consisting of N-(2-acetamido)-2-aminoethan
- More preferred buffersare MES or MOPS, preferably in the concentration range of 20 to 100 mM, more preferably about 25 mM. Most preferred is 25 mM MES, pH 6.5.
- the formulationmay further comprise 25 mM trehalose and 0.01% v/v polysorbate 80 as excipients, with the final buffer concentration modified to 22.25 mM as a result of added excipients.
- the preferred method of storageis as a lyophilized formulation of the conjugates, stored in the temperature range of -20°C to 2°C, with the most preferred storage at 2°C to 8°C. [0198]
- a conjugatecan be formulated for intravenous administration via, for example, bolus injection, slow infusion or continuous infusion.
- the conjugateis infused over a period of less than about 4 hours, and more preferably, over a period of less than about 3 hours.
- the first 25-50 mgcould be infused within 30 minutes, preferably even 15 min, and the remainder infused over the next 2-3 hrs.
- Formulations for injectioncan be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative.
- the compositionscan take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- the rate of release of an conjugate from such a matrixdepends upon the molecular weight of the conjugate, the amount of conjugate within the matrix, and the size of dispersed particles. Saltzman et al., 1989, Biophys. J.55:163; Sherwood et al., supra. Other solid dosage forms are described in Ansel et al., Pharmaceutical Dosage Forms And Drug Delivery Systems, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition (Mack Publishing Company 1990), and revised editions thereof. [0200] Generally, the dosage of an administered conjugate for humans will vary depending upon such factors as the patient’s age, weight, height, sex, general medical condition and previous medical history.
- a dosage of 0.3-5 mg/kg for a 70 kg patientis 21- 350 mg, or 12-20 6 mg/m2 for a 1.7-m patient.
- the dosagemay be repeated as needed, for example, once per week for 2-10 weeks, once per week for 8 weeks, or once per week for 4 weeks. It may also be given less frequently, such as every other week for several months, or monthly or quarterly for many months, as needed in a maintenance therapy.
- Preferred dosagesmay include, but are not limited to, 0.3 mg/kg, 0.5 mg/kg, 0.7 mg/kg, 1.0 mg/kg, 1.2 mg/kg, 1.5 mg/kg, 2.0 mg/kg, 2.5 mg/kg, 3.0 mg/kg, 3.5 mg/kg, 4.0 mg/kg, 4.5 mg/kg, and 5.0 mg/kg. More preferred dosages are 0.6 mg/kg for weekly administration and 1.2 mg/kg for less frequent dosing. Any amount in the range of 0.3 to 5 mg/kg may be used. The dosage is preferably administered multiple times, once a week.
- a minimum dosage schedule of 4 weeks, more preferably 8 weeks, more preferably 16 weeks or longermay be used, with the dose frequency dependent on toxic side-effects and recovery therefrom, mostly related to hematological toxicities.
- the schedule of administrationmay comprise administration once or twice a week, on a cycle selected from the group consisting of:(i) weekly; (ii) every other week; (iii) one week of therapy followed by two, three or four weeks off; (iv) two weeks of therapy followed by one, two, three or four weeks off; (v) three weeks of therapy followed by one, two, three, four or five week off; (vi) four weeks of therapy followed by one, two, three, four or five week off; (vii) five weeks of therapy followed by one, two, three, four or five week off; and (viii) monthly.
- a conjugatemay be administered as one dosage every 2 or 3 weeks, repeated for a total of at least 3 dosages. Or, twice per week for 4-6 weeks. The dosage may be administered once every other week or even less frequently, so the patient can recover from any drug-related toxicities. Alternatively, the dosage schedule may be decreased, namely every 2 or 3 weeks for 2-3 months. The dosing schedule can optionally be repeated at other intervals and dosage may be given through various parenteral routes, with appropriate adjustment of the dose and schedule. 6.8. Methods of Treatment Using Conjugates of the Disclosure [0202] The conjugates of the disclosure can be used for the treatment of various cancers.
- the conjugatescan be used as monotherapy or as part of a combination therapy regimen, for example with a standard of care agent or regimen.
- the combination therapycomprises administering an conjugate in combination with immunotherapy, for example, checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy (e.g., autologous T cell therapy), oncolytic virus therapy, dendritic cell vaccine therapy, stimulator of interferon genes (STING) agonist therapy, toll-like receptor (TLR) agonist therapy, intratumoral CpG therapy, cytokine therapy (e.g., IL2, IL12, IFN- ⁇ , or INF- ⁇ therapy), or a combination thereof.
- immunotherapyfor example, checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy (e.g., autologous T cell therapy), oncolytic virus therapy, dendritic cell vaccine therapy, stimulator of inter
- the combination therapycomprises administering an conjugate in combination with immune preserving chemotherapy (e.g., an antimetabolite, such as 5-fluorouracil, gemcitabine, or methotrexate, an alkylating agent such as cyclophosphamide, dacarbazine, mechlorethamine, diaziquone, or temozolomide, an anthracycline such as doxorubicin or epirubicin, an antimicrotubule agent such as vinblastine, a platinum compound such as cisplatin or oxaliplatin, a taxane such as paclitaxel or docetaxel, or a topoisomerase inhibitor such as etoposide or mitoxantrone, or a vinca alkaloid such as vincristine).
- immune preserving chemotherapye.g., an antimetabolite, such as 5-fluorouracil, gemcitabine, or methotrexate, an alkylating agent such as cyclophosp
- Conjugates of the disclosurecan be used in combination with a checkpoint modulator (e.g., a checkpoint inhibitor), for example an agent targeting PD1, PDL1, CTLA4, TIGIT, LAG3, OX40, ICOS, GITR, CD40 or VISTA.
- a checkpoint modulatore.g., a checkpoint inhibitor
- Exemplary checkpoint modulators targeting TIGITinclude etigilimab, tiragolumab, and AB154.
- Exemplary checkpoint modulators targeting LAG3include LAG525, Sym022, relatlimab, and TSR-033.
- Exemplary checkpoint modulators targeting OX40include MEDI6469, PF-04518600, and BMS 986178.
- Exemplary checkpoint modulators targeting ICOSinclude MEDI-570, feladilimab, and BMS 986226.
- Exemplary checkpoint modulators targeting GITRinclude TRX-518, AMG 228, MK-4166, MEDI1873, INCAGN01876, and GWN323.
- Exemplary checkpoint modulators targeting CD40include selicrelumab, CP-870,893, and APX005M.
- An exemplary checkpoint modulator targeting VISTAis HMBD-002.
- the conjugates of the disclosurecan be used in combination with drugs that specifically target the BRAF mutations, such as venurafenibm, dabrafenib and trametinib.
- the conjugates of the disclosurecan be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab, nivolumab, pembrolizumab, cemiplimab, or avelumab.
- a checkpoint modulatore.g., inhibitor
- the conjugates of the disclosurecan be used in combination with standard of care chemotherapy treatments such as cisplatin, carboplatin, paclitaxel, gemcitabine, vinorelbin, irinotecan, etoposide, or vinblastine would be included.
- conjugatescan be used in combination with targeted therapies, such as bevacizumab or Erbitux.
- conjugatescan be used in combination with a checkpoint modulator (e.g., inhibitor), such as pembrolizumab, nivolumab, cemiplimab, dostarlimab, atezolizumab, avelumab, durvalumab, or ipilimumab.
- a checkpoint modulatore.g., inhibitor
- pembrolizumabnivolumab, cemiplimab, dostarlimab, atezolizumab, avelumab, durvalumab, or ipilimumab.
- the conjugates of the disclosurecan be used in combination with standard of care treatments, including but not limited to cisplatin, mitomycin-C, carboplatin, docetaxel, paclitaxel, doxorubicin, 5-FU, methotrexate, vinblastine, ifosfamide, and pemetrexed.
- the conjugatescan be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab.
- the conjugates of the disclosurecan be used in combination with standard of care treatments, for example agents that block angiogenesis and/or specific tyrosine kinases, such as sorafenib, sunitinib, temsirolimus, everolimus, pazopanib, and axitinib.
- agents that block angiogenesis and/or specific tyrosine kinasessuch as sorafenib, sunitinib, temsirolimus, everolimus, pazopanib, and axitinib.
- the conjugatescan be used in combination with a checkpoint modulator (e.g., inhibitor), such as nivolumab.
- the conjugates of the disclosurecan be used in combination with standard of care chemotherapeutic agents, such as the anthracyclines (doxorubicin or epirubicin) and the taxanes (paclitaxel or docetaxel), as well as fluorouracil, cyclophosphamide and carboplatin.
- chemotherapeutic agentssuch as the anthracyclines (doxorubicin or epirubicin) and the taxanes (paclitaxel or docetaxel), as well as fluorouracil, cyclophosphamide and carboplatin.
- the conjugates of the disclosurecan be used in combination with targeted therapies.
- Targeted therapies for HER2/neu positive tumorsinclude trastuzumab and pertuzumab and for estrogen receptor (ER) positive tumors include tamoxifen, toremifene and fulvestrant.
- the conjugatescan be used in combination with a checkpoint modulator (e.g., inhibitor), such as atezolizumab.
- a checkpoint modulatore.g., inhibitor
- the conjugates of the disclosurecan be used in combination with standard of care chemotherapeutic agents, such as gemcitabine, 5-fluouracil, irinotecan, oxaliplatin, paclitaxel, capecitabine, cisplatin, or docetaxel.
- conjugatescan be used in combination with targeted therapies, such as erlotinib, which inhibits EGFR.
- the conjugates of the disclosurecan be used in combination with standard of care chemotherapeutic agents, such as carboplatin, cyclophosphamide, etoposide, lomustine, methotrexate or procarbazine.
- standard of care chemotherapeutic agentssuch as carboplatin, cyclophosphamide, etoposide, lomustine, methotrexate or procarbazine.
- the conjugates of the disclosurecan be used in combination with standard of care chemotherapeutic agents, including docetaxel, optionally with the steroid prednisone, or cabazitaxel.
- the conjugatescan be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab.
- an conjugate of the disclosurein combination with one or more therapies does not restrict the order in which the therapies are administered.
- the conjugate of the disclosurecan be administered before, during or after a subject is treated with one or more therapies.
- an conjugate of the disclosureis administered prior to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks before), concomitantly with, or subsequent to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks after) treatment of a patient with another therapy (e.g., a second therapeutic agent as described above).
- another therapye.g., a second
- the conjugate of the disclosureis incorporated into the same regimen as a second therapeutic agent.
- Example 1Generation of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.1.1. Overview [0216] Antibodies against human proteins or peptides isolated from non-human animal cells (e.g., mouse anti-human CD5 antibodies) often elicit immune responses when used as therapeutics. Although humanized antibodies are considered to be less immunogenic than their parental counterparts, they may still be associated with antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), triggered by the interactions of the constant Fc regions of these antibodies with the Fc receptors on immune cells.
- ADCCantibody-dependent cellular cytotoxicity
- CDCcomplement-dependent cytotoxicity
- the first parental antibodywas a humanized anti-CD5 antibody (referred to herein as “Antibody A”)
- the second parental antibodywas a mouse anti-human CD5 antibody (referred to herein as “Antibody B”).
- Antibody Ahumanized anti-CD5 antibody
- Antibody Bmouse anti-human CD5 antibody
- the variable region sequences of the first parental antibody (Antibody A)(VH of SEQ ID NO:49 and VL of SEQ ID NO:50) were integrated into human IgG backbones.
- the heavy chain of the parental antibodywas integrated into either wildtype human IgG1, an IgG1 with the mutations L234A and L235A (IgG1(LALA)), an IgG1 with an N297A mutation (IgG1(N297A), or an IgG4 with an S228P mutation (IgG4(S228P).
- the heavy chains with the human IgG backboneswere then combined with the light chains integrated into human IgKappa.
- Full light chain and heavy chain sequences for the antibodies, referred to as AB-1 to AB-4,are set forth in Table 1L. 7.1.2.2.
- the parental mouse anti-human CD5 antibodywas humanized as follows: The amino acid sequences of the parental light and heavy chains were aligned with the human germline Ig alleles, IGKV3-11*01, IGKV3-11*02, and IGKV1-16*01.
- FIGS.1A-1Cdisplay amino acid residue alignment mismatches between the parental mouse antibody and the germline Ig frameworks.
- the light and heavy chain complementarity-determining region (CDR) amino acid sequences of the parental antibodywere grafted onto a human Ig framework sequence. The resulting sequence was further modified, such as by introducing back-mutations of various residues in order to retain the antigen-binding activity of the parent antibody.
- FIGS 2A and 2Billustrate the humanization of the parental mouse anti- human CD5 antibody VH and VL amino acid sequences into AB-5 VH and VL amino acid sequences, by using the antibody 4JLR.pdb as a reference framework.
- Resulting sequenceswere checked for confounding issues, such as N-glycosylation, interface changes, and potentially problematic proline residues.
- Six humanized anti-CD5 antibody VH-VL sequenceswere selected for further analyses. Full light chain and heavy chain sequences for the humanized antibodies, referred to as AB-5 to AB-10, are shown in Table 1L. The VH and VL were subsequently cloned into human IgG1(LALA) Fc backbones. 7.1.2.3.
- Anti-CD5 Construct VH/VL Isot es[0225] Antibody constructs were cloned into Expi293 cells and antibodies were produced and purified as described in Section 7.1.2.3. Exemplary SDS-PAGE images of three anti-CD5 antibodies, AB-4, AB-6, and AB-8, and the negative control antibody, NC-AB, are shown in FIGS.3A-3D. Furthermore, the purified antibodies had low endotoxin levels, which was below 1 EU/mg for all samples at the highest concentration evaluated (FIG.3E). 7.2.
- Example 2Target Cell Binding of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.2.1.
- CD5is a surface glycoprotein expressed on T cells.
- a cell binding assay using Jurkat T cellswas utilized. 7.2.2. Methods 7.2.2.1. Cell Culture and Maintenance [0227] Jurkat T cells were maintained in RPMI medium supplemented with 10% FBS. EL4 cells were maintained in DMEM medium supplemented with 10% FBS. CD3+ T cells were maintained in K5 medium supplemented with 10% FBS, 1X non-essential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 55 ⁇ M 2-mercaptoethanol.
- AB-4was associated with a 44% and AB-8 was associated with a 53% reduction of surface CD5 binding at 6 hours, further supporting efficient internalization of humanized anti-CD5 antibodies derived from both parental antibodies.
- Example 4ADCC Assessment of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.4.1. Overview [0239] Fc regions of humanized antibodies are often associated with antibody-dependent cellular cytotoxicity (ADCC). Therefore, humanized anti-CD5 antibodies comprising human IgG chains with low ADCC activity, such as IgG1-LALA, can help overcome this limitation. 7.4.2. Methods [0240] An ADCC assay was developed to evaluate ADCC effects of anti-CD5 antibodies.
- NK cellswere isolated from PBMCs and co-cultured with Jurkat T cells (4 x 10 4 cells/well) at an effector: target cell ratio (E:T) of 5:1.
- Humanized anti-CD5 antibodies, as well as the parental mouse anti-hCD5 antibody, a positive control antibody (Anti-human MHC Class I antibody) and a negative control antibody (NC-AB)were diluted to a concentration of 30 ⁇ g/mL and added to wells.
- the plateswere incubated for 4 hours at 37 °C, 5% CO2. Cells were incubated with CytoTox 96® reagent for 30 minutes at room temperature before data collection on a plate reader at 490nm. 7.4.3.
- the mixturewas incubated for 30 minutes at room temperature and then added drop-wise to the HEK-293 cells. After 48 hours, the transfected cells were gently detached from the plates with Accutase® cell detachment solution, washed 2X in ice-cold stain buffer (1X dPBS, 1% FBS, 0.05% sodium azide) and counted.1.5 x 10 5 cells were incubated with either 7.5 ⁇ g/mL Antibody C-PE (phycoerythrin) conjugate or Antibody B-PE in 100 ⁇ L stain buffer. After 60 minutes on ice, the cells were washed 2X in stain buffer to completely remove unbound antibody.
- Example 6Internalization of AB-4, AB-6, and AB-8 [0246] Internalization of humanized antibodies AB-4, AB-6, and AB-8 following binding to CD5 expressing Jurkat cells was studied. Briefly, antibodies were incubated with Jurkat cells for two to six hours, and antibody internalization was measured by FACS staining. [0247] Results are shown in FIGS.8A-8C. AB-4, AB-6, and AB-8 were each internalized. 7.7.
- Example 7Humanized Antibodies with Human IgG4 Fc 7.7.1.
- Fc regions of humanized anti-human CD5 antibodies AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10, and mouse anti-human CD5 Antibody Bwere substituted with human IgG4 Fc regions having a S228P substitution. Binding to Jurkat cells was assessed. 7.7.2. Methods [0249] Serial dilutions of anti-CD5 antibodies and isotype control antibody were prepared and incubated with Jurkat cells at 4 °C for 60 minutes. Cells were then washed three times. PE-goat anti-human IgG secondary antibody was added to the cells, and incubated for 30 minutes at 4 °C. Cells were washed and analyzed by FACS. 7.7.3.
- Log phase Jurkat cells(2E5/sample) were washed in cell culture medium, and plated in a 96-well U-bottom plate in cell culture medium. Antibodies at 1 ⁇ g/ml were incubated with the cells at 4 °C for 60 minutes. Cells were then washed two time and resuspended in growth medium (RPMI1640 + 10% FBS). Cells were incubated at 37 °C for 0 hours, 4 hours, or 6 hours. Cells were then washed two times in staining buffer. Secondary antibody in staining buffer (PE-goat anti-human IgG) was added and incubated for 30 minutes at 4°C.
- Primary antibody in staining bufferPE-goat anti-human IgG
- Resultsare shown in FIGS.10A-10B. All antibodies showed internalization at 4 hours and 6 hours. Percent internalization at 4 hours and 6 hours is summarized in Table 11. Table 11 Antibody Time (hours) Percent 7.9. Exam agonist-Conjugates (ATAC s) [0254] Antibodies AB-4, AB-8, and Antibody B were conjugated to ALK5 inhibitor compound C (see Table 2) and evaluated in a HEK-CD5 SBE luciferase assay to monitory the activity of the TGF ⁇ /SMAD signaling pathway following exposure to the antibody-ALK5 inhibitor conjugates. 7.9.1.
- HEK-CD5 cellswere plated in triplicate on a 96-well plate. After the cells attached, the conjugated antibodies were titrated and incubated for 18 hours in a 37°C/5% CO 2 incubator. TGF ⁇ was added to the plate and incubated for an additional 3 hours. The assay was developed using a commercially available luciferase detection reagent, the data was acquired on a luminometer and plotted using GraphPad Prism TM software. 7.9.2. Results [0256] Results are shown in FIG.11A-11B. Each conjugate was active in the assay. EC50 values are reported in Table 12. Table 12 Antibody-ALK5 inhibitor conjugate EC50 . . .
- T cellswere isolated from human PBMCs and plated in triplicate on round-bottom 96-well plates. Antibody was added to the cells and incubated on ice for 0.5 hours. Complement from rabbit sera was then added to a final concentration of 5%. The cells were incubated for 2 hours in a 37°C/5% CO 2 incubator. Propidium iodide was added to the cells and the data was acquired on a FACS instrument, analyzed using FlowJo TM software and plotted using GraphPad Prism TM . 7.10.3. Results [0259] Results are shown in FIG.12.
- An anti-CD5 antibody or antigen binding fragment thereofcomprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 11, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c
- An anti-CD5 antibody or antigen binding fragment thereofwhich is optionally an anti- CD5 antibody or antigen binding fragment according to embodiment 1, comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and
- VHcomprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively
- VLcomprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively.
- An anti-CD5 antibody or antigen binding fragment thereofwhich is optionally an antibody or antigen binding fragment according to any one of embodiments 1 to 17, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:22 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:23; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:24 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:25; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at
- the VHhas a sequence at least 95% identical to SEQ ID NO:22 and the VL has a sequence at least 95% identical to SEQ ID NO:23. 21.
- the VHhas a sequence at least 95% identical to SEQ ID NO:26 and the VL has a sequence at least 95% identical to SEQ ID NO:27. 31.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 34wherein the VH has a sequence at least 97% identical to SEQ ID NO:28 and the VL has a sequence at least 97% identical to SEQ ID NO:29. 37.
- the VHhas a sequence at least 95% identical to SEQ ID NO:30 and the VL has a sequence at least 95% identical to SEQ ID NO:31.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 39wherein the VH has a sequence at least 97% identical to SEQ ID NO:30 and the VL has a sequence at least 97% identical to SEQ ID NO:31. 42.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 39wherein the VH has a sequence at least 99% identical to SEQ ID NO:30 and the VL has a sequence at least 99% identical to SEQ ID NO:31.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 39wherein the VH has a sequence identical to SEQ ID NO:30 and the VL has a sequence identical to SEQ ID NO:31. 44.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 44wherein the VH has a sequence at least 97% identical to SEQ ID NO:32 and the VL has a sequence at least 97% identical to SEQ ID NO:33. 47.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 44wherein the VH has a sequence at least 99% identical to SEQ ID NO:32 and the VL has a sequence at least 99% identical to SEQ ID NO:33.
- An anti-CD5 antibody or antigen binding fragment thereofcomprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:34, 39, and 43, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:35, 40, 44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:46, 47, and 48, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:36, 41, and 44, respectively, and a V
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49which is a humanized anti-CD5 antibody or antigen binding fragment thereof.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:35, 40, 44, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:46, 47, and 48, respectively. 53.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:36, 41, and 44, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 54.
- VHcomprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of NYG, SEQ ID NO:3741, and SEQ ID NO:44, respectively
- VLcomprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively. 55.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:38, 42, and 43, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOS:46, 47, and 48, respectively 56.
- An anti-CD5 antibody or antigen binding fragment thereofwhich is optionally an antibody or antigen binding fragment according to any one of embodiments 49 to 55, comprising a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:49 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:50.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 56wherein the VH has a sequence at least 97% identical to SEQ ID NO:49 and the VL has a sequence at least 97% identical to SEQ ID NO:50. 59.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 56wherein the VH has a sequence at least 99% identical to SEQ ID NO:49 and the VL has a sequence at least 99% identical to SEQ ID NO:50. 60.
- An anti-CD5 antibody or antigen binding fragment thereofcomprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 88, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and
- VHcomprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 88, and 89, respectively
- VLcomprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively. 64.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively. 65.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, 95, respectively. 66.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, 95, respectively. 67.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively.
- the VHcomprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 91, respectively
- the VLcomprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively. 69.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively. 70.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 102, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 71.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 72.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 102, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 73.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 61wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:104, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:94, 97, and 105, respectively.
- the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 61 to 73which is a humanized anti-CD5 antibody or antigen binding fragment thereof.
- An anti-CD5 antibody or antigen binding fragment thereofwhich is optionally an antibody or antigen binding fragment according to any one of embodiments 61 to 74, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 9
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 76wherein the VH has a sequence at least 97% identical to SEQ ID NO:84 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 79.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 81wherein the VH has a sequence at least 97% identical to SEQ ID NO:84 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 84.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 87.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 92.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 91wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 94.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 97.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 96wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 99.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 102.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 101wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 104.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 101wherein the VH has a sequence at least 99% identical to SEQ ID NO:85 and the VL has a sequence at least 99% identical to SEQ ID NO:98. 105.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 107.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 106wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 109.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 112.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 111wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 114.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98.
- the VHhas a sequence at least 95% identical to SEQ ID NO:86 and the VL has a sequence at least 95% identical to SEQ ID NO:98.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 116wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 119.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 122.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 121wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 124.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 126wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 129.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 132.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 131wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 134.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 75wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:100 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:101. 137.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 136wherein the VH has a sequence at least 97% identical to SEQ ID NO:100 and the VL has a sequence at least 97% identical to SEQ ID NO:101.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 136wherein the VH has a sequence at least 99% identical to SEQ ID NO:100 and the VL has a sequence at least 99% identical to SEQ ID NO:101.
- 140.The anti-CD5 antibody or antigen binding fragment thereof of embodiment 136, wherein the VH has a sequence identical to SEQ ID NO:100 and the VL has a sequence identical to SEQ ID NO:101. 141.
- the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 146which comprises an antigen binding fragment. 149.
- the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 148which comprises a first Fc region and a second Fc region forming an Fc domain, optionally wherein the Fc domain is a human Fc domain.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 150wherein the one or more substitutions comprise N297A, N297Q, N297G, D265A/N297A, D265A/N297G, L235E, L234A/L235A, L234A/L235A/P329A, L234D/L235E : L234R/L235R/E233K, L234D/L235E/D265S : E233K/L234R/L235R/D265S, L234D/L235E/E269K : E233K/L234R/L235R/E269K, L234D/L235E/K322A : E233K/L234R/L235R/K322A, L234D/L235E/P329W : E233K/L234R/L235R/P329W, L234D/L235E/E269K
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutionscomprise N297A. 153.
- the anti-CD5 antibody or antigen binding fragment thereof embodiment 150 or embodiment 151, wherein the one or more substitutionscomprise N297Q.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutionscomprise N297G.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutionscomprise D265A/N297A. 156.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 169wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:51, SEQ ID NO:52, or SEQ ID NO:53. 171.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 170wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:52. 173.
- 176The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 149 to 168, wherein the Fc domain comprises a human IgG4 Fc domain or a variant thereof. 177.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 176wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:54.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 178, wherein the hinge regioncomprises one or more amino acid substitutions that reduce half-antibody exchange. 180.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 183, wherein the hinge regioncomprises the amino acid sequence of SEQ ID NO:83. 185.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 1which comprises a light chain having the amino acid sequence of SEQ ID NO:77 and a heavy chain having the amino acid sequence of SEQ ID NO:78. 193.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:56. 195.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:57. 196.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:58.
- the anti-CD5 antibody or antigen binding fragment thereof of embodiment 49which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:59. 198.
- the nucleic acid of embodiment 198which is codon optimized for expression in a mammalian cell, optionally wherein the mammalian cell is a human cell.
- a method of producing an anti-CD5 antibody or antigen binding fragment thereofcomprising culturing the cell of embodiment 201 in conditions under which the anti-CD5 antibody or antigen binding fragment thereof is expressed; and recovering the anti-CD5 antibody or antigen binding fragment thereof from the cell culture.
- An antibody-ALK5 inhibitor conjugatecomprising the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 conjugated to an ALK5 inhibitor.
- the antibody-ALK5 inhibitor conjugate of embodiment 205wherein the ALK5 inhibitor is an imidazole type compound which is an imidazole-benzodioxol compound or an imidazole-quinoxaline compound.
- the antibody-ALK5 inhibitor conjugate of embodiment 209wherein the ALK5 inhibitor is an imidazole-benzodioxol compound.
- the antibody-ALK5 inhibitor conjugate of embodiment 209wherein the ALK5 inhibitor is an imidazole-quinoxaline compound.
- the antibody-ALK5 inhibitor conjugate of embodiment 205wherein the ALK5 inhibitor is an imidazole-benzodioxol compound, an imidazole-quinoxaline compound, a pyrazole-pyrrolo compound, or a thiazole type compound.
- the antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitoris an ALK5 inhibitor identified in Table 2.
- 216The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 3A. 217.
- the antibody-ALK5 inhibitor conjugate of embodiment 203wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 3B. 218.
- the antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitoris an ALK5 inhibitor identified in Table 4. 219.
- the antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitorhas the structure . has the structure 221.
- the antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitorhas the structure . conjugate of embodiment 203, wherein the ALK5 inhibitor has the . inhibitor conjugate of any one of embodiments 203 to 222, wherein the antibody or antigen binding fragment via a linker. 224.
- the antibody-ALK5 inhibitor conjugate of embodiment 240, wherein the ALK5 inhibitoris conjugated via one or more lysine residues on the antibody or antigen binding fragment.
- the antibody-ALK5 inhibitor conjugate of embodiment 244, wherein the one or more unnatural amino acid residuescomprise p-azidomethyl-L-phenylalanine (pAMF) 247.
- the antibody-ALK5 inhibitor conjugate of embodiment 244, wherein the one or more unnatural amino acid residuescomprise selenocysteine (Sec). 248.
- the antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycanscomprise galactose. 252.
- the antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycanscomprise N-acetylgalactosamine (GalNAc). 253.
- SAsialic acid
- An antibody-ALK5 inhibitor conjugatecomprising the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 conjugated to a means for inhibiting ALK5, optionally wherein the conjugate further comprises a means for linking the anti-CD5 antibody or antigen binding fragment thereof to the means for inhibiting ALK5. 263.
- a pharmaceutical compositioncomprising the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 and a pharmaceutically acceptable carrier. 264.
- the pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical compositionhave a DAR between 1 and 15. 267.
- a method of treating cancercomprising administering to a subject in need thereof an antibody-ALK5 inhibitor conjugate according to any one of embodiments 203 to 262 or a pharmaceutical composition according to any one of embodiments 263 to 291. 293.
- the method of embodiment 292, wherein the canceris an immunogenic cancer.
- the method of embodiment 293, wherein the canceris a solid tumor that expresses a tumor antigen. 295.
- the method of embodiment 294, wherein the tumor antigenis gp100, melanA or MAGE A1. 296.
- the method of embodiment 295, wherein the tumor antigenis gp100.
- the tumor antigenis melanA. 298.
- the method of embodiment 295, wherein the tumor antigenis MAGE A1. 299.
- the method of embodiment 292, wherein the canceris a solid tumor comprising immune infiltrates.
- the immunotherapyis cytokine therapy, adoptive T cell therapy, chimeric antigen receptor (CAR) therapy, checkpoint modulator (e.g., checkpoint inhibitor) therapy, oncolytic virus therapy, dendritic cell vaccine therapy, STING agonist therapy, TLR agonist therapy, or intratumoral CpG therapy.
- CARchimeric antigen receptor
- checkpoint modulatore.g., checkpoint inhibitor
- the method of embodiment 300, wherein the immunotherapyis cytokine therapy, adoptive T cell therapy, chimeric antigen receptor (CAR) therapy or checkpoint modulator (e.g., checkpoint inhibitor) therapy.
- 303. The method of embodiment 302, wherein the immunotherapyis cytokine therapy.
- 304. The method of embodiment 303, wherein the cytokine therapyis IL2 therapy.
- 305. The method of embodiment 303, wherein the cytokine therapyis IL12 therapy.
- the method of embodiment 303, wherein the cytokine therapyis IFN- ⁇ therapy. 307.
- the method of embodiment 303, wherein the cytokine therapyis IFN- ⁇ therapy. 308.
- the method of embodiment 302, wherein the immunotherapyis adoptive T cell therapy. 309.
- the method of embodiment 308, wherein the adoptive T cell therapyis autologous T cell therapy.
- the immunotherapyis chimeric antigen receptor (CAR) therapy.
- CARchimeric antigen receptor
- the immunotherapyis checkpoint modulator (e.g., checkpoint inhibitor) therapy.
- the checkpoint modulatoris an antibody.
- the method of embodiment 302, embodiment 311, or embodiment 312, wherein the checkpoint modulatoris an inhibitor of PD1, PDL1, or CTLA4. 314.
- the method of embodiment 313, wherein the checkpoint modulatoris an inhibitor of PD1. 315.
- the method of embodiment 314, wherein the inhibitor of PD1is an antibody. 316.
- the method of embodiment 315, wherein the inhibitor of PD1is pembrolizumab, nivolumab, cemiplimab, or dostarlimab. 317.
- the method of embodiment 316, wherein the inhibitor of PD1is pembrolizumab. 318.
- the method of embodiment 316, wherein the inhibitor of PD1is nivolumab. 319.
- the method of embodiment 316, wherein the inhibitor of PD1is cemiplimab. 320.
- the method of embodiment 316, wherein the inhibitor of PD1is dostarlimab. 321.
- the checkpoint modulatoris an inhibitor of PDL1. 322.
- the method of embodiment 321, wherein the inhibitor of PDL1is an antibody. 323.
- the method of embodiment 322, wherein the inhibitor of PDL1is atezolizumab, avelumab, or durvalumab. 324.
- the method of embodiment 323, wherein the inhibitor of PDL1is atezolizumab. 325.
- the method of embodiment 323, wherein the inhibitor of PDL1is avelumab. 326.
- the method of embodiment 323, wherein the inhibitor of PDL1is durvalumab. 327.
- the method of embodiment 313, wherein the checkpoint modulatoris an inhibitor of CTLA4. 328.
- the method of embodiment 327, wherein the inhibitor of CTLA4is an antibody. 329.
- the method of embodiment 328, wherein the inhibitor of CTLA4is ipilimumab. 330.
- the method of embodiment 337, wherein the canceris non-small cell lung cancer (NSCLC), liver cancer, urothelial cancer, renal cancer, breast cancer, or melanoma. 342.
- the method of embodiment 337, wherein the canceris lung cancer. 343.
- the method of embodiment 342, wherein the canceris NSCLC. 344.
- the method of embodiment 343, wherein the NSCLCis adenocarcinoma. 345.
- the method of embodiment 343, wherein the NSCLCis squamous cell carcinoma. 346.
- the method of embodiment 343, wherein the NSCLCis large cell carcinoma. 347.
- the method of embodiment 342, wherein the canceris small cell lung cancer. 348.
- the method of embodiment 337, wherein the canceris liver cancer. 349.
- the method of embodiment 348, wherein the liver canceris hepatocellular carcinoma. 350.
- the method of embodiment 337, wherein the canceris urothelial cancer. 351.
- the method of embodiment 350, wherein the canceris bladder cancer. 352.
- the method of embodiment 350, wherein the canceris urethral cancer. 353.
- the method of embodiment 350, wherein the canceris ureteral cancer. 354.
- the method of embodiment 337, wherein the canceris renal cancer. 355.
- the method of embodiment 354, wherein the renal canceris renal cell carcinoma. 356.
- method of embodiment 354, wherein the renal canceris urothelial carcinoma. 357.
- the method of embodiment 337, wherein the canceris breast cancer. 358.
- the method of embodiment 337, wherein the canceris melanoma. 359.
- the method of embodiment 337, wherein the canceris pancreatic cancer. 360. The method of embodiment 337, wherein the cancer is glioblastoma. 361. The method of embodiment 337, wherein the cancer is a myelodysplastic syndrome. 362. The method of embodiment 337, wherein the cancer is prostate cancer. 363. The method of embodiment 337, wherein the cancer is colorectal cancer. 364. The method of embodiment 363, wherein the colorectal cancer is adenocarcinoma. 365. The method of embodiment 363, wherein the colorectal cancer is a carcinoid tumor. 366. The method of embodiment 363, wherein the colorectal cancer is a gastrointestinal stromal tumor. 367.
- any one of embodiments 292 to 369wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered as part of a combination therapy regimen which optionally comprises administering one or more agents which are not an antibody-ALK5 inhibitor conjugate according to any one of embodiments 203 to 261 (each a “second therapeutic agent”). 372.
- the method of embodiment 371, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical compositionis administered in combination with a standard of care therapy or therapeutic regimen. 373.
- the combination therapycomprises administering at least one second therapeutic agent to the subject. 374.
- the combination therapy regimencomprises immunotherapy, optionally wherein the immunotherapy is checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy, oncolytic virus therapy, dendritic cell vaccine therapy, STING agonist therapy, TLR agonist therapy, intratumoral CpG therapy, or cytokine therapy.
- the combination therapycomprises checkpoint modulator (e.g., checkpoint inhibitor) therapy.
- the checkpoint modulator therapycomprises T cell checkpoint modulator (e.g., checkpoint inhibitor) therapy.
- the T cell checkpoint modulator therapycomprises an antibody or an antigen-binding fragment thereof.
- the checkpoint modulator therapytargets PD1, PDL1, CTLA4, TIGIT, LAG3, OX40, ICOS, GITR, CD40, VISTA, or a combination thereof.
- the checkpoint modulator therapytargets PD1.
- a second therapeutic agentcomprises a means for targeting PD1. 381.
- the method of any one of embodiments 371 to 379, wherein a second therapeutic agentis pembrolizumab. 382.
- a second therapeutic agentis nivolumab. 383.
- the method of any one of embodiments 371 to 379, wherein a second therapeutic agentis cemiplimab. 384.
- the method of any one of embodiments 371 to 379, wherein a second therapeutic agentis dostarlimab. 385.
- the method of any one of embodiments 378 to 384, wherein the checkpoint modulator therapytargets PDL1.
- the method of any one of embodiments 371 to 385, wherein a second therapeutic agentcomprises a means for targeting PDL1. 387.
- the method of any one of embodiments 371 to 385, wherein a second therapeutic agentis atezolizumab. 388.
- a second therapeutic agentis avelumab. 389.
- the method of any one of embodiments 371 to 385, wherein a second therapeutic agentis durvalumab. 390.
- the method of any one of embodiments 378 to 389 wherein the checkpoint modulator therapytargets CTLA4. 391.
- the method of any one of embodiments 371 to 390, wherein a second therapeutic agentcomprises a means for targeting CTLA4. 392.
- the method of any one of embodiments 371 to 390, wherein a second therapeutic agentis ipilimumab. 393.
- the method of any one of embodiments 378 to 391, wherein the checkpoint modulator therapytargets TIGIT. 394.
- a second therapeutic agentcomprises a means for targeting TIGIT. 395.
- the method of any one of embodiments 371 to 393, wherein a second therapeutic agentis etigilimab. 396.
- the method of any one of embodiments 371 to 393, wherein a second therapeutic agentis tiragolumab. 397.
- the method of any one of embodiments 371 to 393, wherein a second therapeutic agentis AB154. 398.
- the method of any one of embodiments 378 to 397, wherein the checkpoint modulator therapytargets LAG3. 399.
- the method of any one of embodiments 371 to 398, wherein a second therapeutic agentcomprises a means for targeting LAG3. 400.
- a second therapeutic agentis LAG525.
- 401The method of any one of embodiments 371 to 398, wherein a second therapeutic agent is Sym022. 402.
- the method of any one of embodiments 371 to 398, wherein a second therapeutic agentis relatlimab. 403.
- a second therapeutic agentis TSR-033.
- 404The method of any one of embodiments 378 to 403, wherein the checkpoint modulator therapy targets OX40. 405.
- a second therapeutic agentis a means for targeting OX40. 406.
- a second therapeutic agentis MEDI6469. 407.
- the method of any one of embodiments 371 to 404, wherein a second therapeutic agentis PF-04518600. 408.
- the method of any one of embodiments 371 to 404, wherein a second therapeutic agentis BMS 986178. 409.
- the method of any one of embodiments 378 to 408, wherein the checkpoint modulator therapytargets CD40.
- the method of any one of embodiments 371 to 409, wherein a second therapeutic agentcomprises a means for targeting CD40. 411.
- the method of any one of embodiments 371 to 409, wherein a second therapeutic agentis selicrelumab. 412.
- a second therapeutic agentis CP-870,893. 413.
- the method of any one of embodiments 371 to 409, wherein a second therapeutic agentis APX005M. 414.
- the method of any one of embodiments 378 to 413, wherein the checkpoint modulator therapytargets ICOS. 415.
- the method of any one of embodiments 371 to 414, wherein a second therapeutic agentcomprises a means for targeting ICOS. 416.
- a second therapeutic agentis MEDI-570. 417.
- the method of any one of embodiments 371 to 414, wherein a second therapeutic agentis feladilimab. 418.
- a second therapeutic agentis BMS 986226. 419.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentcomprises a means for targeting GITR. 421.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentis TRX-518. 422.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentis AMG 228. 423.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentis MK-4166. 424.
- a second therapeutic agentis MEDI1873. 425.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentis INCAGN01876. 426.
- the method of any one of embodiments 371 to 419, wherein a second therapeutic agentis GWN323. 427.
- the method of any one of embodiments 378 to 426, wherein the checkpoint modulator therapytargets VISTA. 428.
- the method of any one of embodiments 371 to 427, wherein a second therapeutic agentcomprises a means for targeting VISTA. 429.
- the method of any one of embodiments 371 to 427, wherein a second therapeutic agentis HMBD-002. 430.
- a second therapeutic agentis a chimeric antigen receptor (CAR). 431.
- the method of any one of embodiments 371 to 430 wherein the combination therapycomprises adoptive T cell therapy.
- the method of embodiment 431, wherein the adoptive T cell therapyis autologous T cell therapy. 433.
- the method of any one of embodiments 371 to 432, wherein the combination therapycomprises oncolytic virus therapy.
- the method of any one of embodiments 371 to 433, wherein the combination therapycomprises dendritic cell vaccine therapy. 435.
- the method of any one of embodiments 371 to 434, wherein the combination therapycomprises STING agonist therapy. 436.
- the method of any one of embodiments 371 to 435, wherein the combination therapycomprises TLR agonist therapy. 437.
- the method of any one of embodiments 371 to 436, wherein the combination therapycomprises chemotherapy. 438.
- a second therapeutic agentis an antimetabolite, an alkylating agent, an anthracycline, an antimicrotubule agent, a platinum compound, a taxane, a topoisomerase inhibitor, or a vinca alkaloid.
- a second therapeutic agentis an antimetabolite.
- the antimetaboliteis 5-fluorouracil. 441.
- the method of embodiment 439, wherein the antimetaboliteis gemcitabine. 442.
- the method of embodiment 439, wherein the antimetaboliteis methotrexate. 443.
- a second therapeutic agentis an alkylating agent. 444.
- the method of embodiment 443, wherein the alkylating agentis cyclophosphamide. 445.
- the method of embodiment 443, wherein the alkylating agentis dacarbazine. 446.
- the method of embodiment 443, wherein the alkylating agentis mechlorethamine. 447.
- the method of embodiment 443, wherein the alkylating agentis diaziquone. 448.
- the method of embodiment 443, wherein the alkylating agentis temozolomide.
- a second therapeutic agentis an anthracycline. 450.
- the method of embodiment 449, wherein the anthracyclineis doxorubicin. 451.
- the method of embodiment 449, wherein the anthracyclineis epirubicin. 452.
- the method of embodiment 438, wherein a second therapeutic agentis an antimicrotubule agent. 453.
- the method of embodiment 452, wherein the antimicrotubule agentis vinblastine. 454.
- the method of embodiment 438, wherein a second therapeutic agentis a platinum compound. 455.
- the method of embodiment 454, wherein the platinum compoundis cisplatin. 456.
- the method of embodiment 454, wherein the platinum compoundis oxaliplatin. 457.
- a second therapeutic agentis a taxane. 458.
- the method of embodiment 457, wherein the taxaneis paclitaxel. 459.
- the method of embodiment 457, wherein the taxaneis docetaxel.
- a second therapeutic agentis a topoisomerase inhibitor. 461.
- the method of embodiment 460, wherein the topoisomerase inhibitoris etoposide. 462.
- the method of embodiment 460, wherein the topoisomerase inhibitoris mitoxantrone. 463.
- the method of embodiment 438, wherein a second therapeutic agentis a vinca alkaloid. 464.
- a second therapeutic agentis a cytokine. 467.
- the method of embodiment 466, wherein the cytokineis IL2. 468.
- the method of embodiment 466, wherein the cytokineis IL12. 469.
- the method of embodiment 466, wherein the cytokineis IFN- ⁇ . 470.
- the method of embodiment 466, wherein the cytokineis IFN- ⁇ . 471.
- the method of any one of embodiments 371 to 470which comprises treating the subject with the combination therapy. 472.
- a method of treating a subject having a disease or disorder associated with elevated CD5 expressioncomprising administering the anti-CD5 antibody or antigen binding fragment of any one of embodiments 1 to 197 to the subject, optionally wherein the disease or disorder is a B or T cell malignancy, an autoimmune disease, a transplantation disease, or a graft rejection. 474.
- a process for making antibody-ALK5 inhibitor conjugatecomprising conjugating the antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 to an ALK5 inhibitor, optionally wherein the antibody or antigen binding fragment thereof is conjugated to the ALK5 inhibitor via a linker.
- the ALK5 inhibitoris an ALK5 inhibitor described in any one of embodiments 203 to 261.
- the linkeris a linker described in any one of embodiments 223 to 238. 477.
- a kitcomprising the antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 and an ALK5 inhibitor. 479.
- the kit of embodiment 478, wherein the ALK5 inhibitoris an ALK5 inhibitor as described in any one of embodiments 203 to 261.
- 480.The kit of embodiment 478 or embodiment 479, further comprising a linker. 481.
- the kit of embodiment 478 or embodiment 479, wherein the ALK5 inhibitoris conjugated to a linker. 482.
- a method of delivering an ALK5 inhibitor to a T cellcomprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to a subject, e.g., a subject as described in any one of embodiments 292 to 472.
- a method of inducing an immune response in a subjectcomprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to the subject, e.g., a subject as described in any one of embodiments 292 to 472. 485.
- a method of stimulating T cells in a subjectcomprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to the subject, e.g., a subject as described in any one of embodiments 292 to 472.
- All publications, patents, patent applications and other documents cited in this applicationare hereby incorporated by reference in their entireties for all purposes to the same extent as if each individual publication, patent, patent application or other document were individually indicated to be incorporated by reference for all purposes. In the event that there is an inconsistency between the teachings of one or more of the references incorporated herein and the present disclosure, the teachings of the present specification are intended.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Dispersion Chemistry (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
ANTI-CD5 ANTIBODIES AND THEIR USES 1. CROSS-REFERENCE TO RELATED APPLICATIONS [0001] This application claims the priority benefit of U.S. provisional application no.63/507,751, filed June 13, 2023, the contents of which are incorporated herein in their entireties by reference thereto. 2. SEQUENCE LISTING [0002] The instant application contains a Sequence Listing which has been submitted electronically in XML format and is hereby incorporated by reference in its entirety. Said XML Sequence Listing, created on May 30, 2024 is named SYN-003WO_SL.XML and is 121,893 bytes in size. 3. BACKGROUND [0003] CD5 is a cluster of differentiation protein expressed on the surface of T cells. WO 2020/256751 describes the use of anti-CD5 antibodies conjugated to ALK5 inhibitors to direct ALK5 inhibitors to T cells, where they can exert a therapeutic benefit, while limiting toxicity of ALK5 inhibitors in non-target tissue. Anti-CD5 antibody-ALK5 inhibitor conjugates are described in WO 2020/256751 as being useful for the treatment of cancer. Anti-CD5 antibodies have also been described as useful for treating CD5+ B or T cell related diseases such as B or T cell malignancies, autoimmune diseases, transplantation diseases, and graft rejections (US 8,679,500). 4. SUMMARY [0004] The present disclosure provides anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen-binding fragments thereof. [0005] The present disclosure further provides conjugates comprising ALK5 inhibitors and anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen-binding fragments thereof, nucleic acids encoding the anti-CD5 antibodies and antigen-binding fragments thereof, and cells engineered to express the nucleic acids. A conjugate comprising an ALK5 inhibitor and an anti-CD5 antibody or antigen binding fragment thereof is sometimes referred to herein simply as a “conjugate” for convenience. [0006] The present disclosure further provides methods of using the anti-CD5 antibodies, antigen- binding fragments, and conjugates for cancer and cancer immunotherapies. [0007] In certain aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and/or light chain variable sequences set forth in Tables 1A through 1K. [0008] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy and/or light chain CDR sequences set forth in Tables 1A-1K. The CDR sequences set forth in Tables 1A-1K include CDR sequences defined according to the IMGT (Lefranc et al., 2003, Dev Comparat Immunol 27:55-77), Kabat (Kabat et al., 1991, Sequences of Proteins of Immunological Interest, 5th Ed. Public Health Service, National Institutes of Health, Bethesda, Md.), and Chothia (Al-Lazikani et al., 1997, J. Mol. Biol 273:927- 948) schemes for defining CDR boundaries. Tables 1A-1K further include CDR sequences defined by the combined regions of overlap for the CDR sequences defined by the IMGT, Kabat, and Chothia schemes, and further include CDR sequences defined by the common regions of overlap for the CDR sequences defined by the IMGT, Kabat, and Chothia schemes. TABLE 1A AB-1 to AB-4 Sequences E ID 49 50 34 39 43 45 48 35 40 44 46 47 48 36 41 44 46 47 48 41 44 45 48 38 42 43 46 47 48 TABLE 1B AB-5 Sequences SEQ ID 22 23 1 6 13 17 20 2 7 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 7 13 18 19 21 TABLE 1C AB-6 Sequences SEQ ID 24 25 1 6 16 17 20 2 9 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 9 16 18 19 21 TABLE 1D AB-7 Sequences SEQ ID 26 27 1 6 13 17 20 2 10 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 10 13 18 19 21 TABLE 1E AB-8 Sequences SEQ ID 28 29 1 6 13 17 20 2 11 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 11 13 18 19 21 TABLE 1F AB-9 Sequences SEQ ID 30 31 1 6 13 17 20 2 12 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 12 13 18 19 21 TABLE 1G AB-10 Sequences SEQ ID 32 33 1 6 13 17 20 2 11 14 18 19 21 3 8 15 18 19 21 4 8 15 17 20 5 11 13 18 19 21 TABLE 1H AB-11 Sequences (VH1, VH2, VH3, VH4) SEQ : 84 85 86 87 1 88 89 2 90 91 3 8 91 4 8 91 5 90 TABLE 1H AB-11 Sequences (VH1, VH2, VH3, VH4) SEQ : 89 TABLE 1I AB-11 Sequences (VL1, VL2) ID O: 92 93 94 95 96 97 95 96 97 95 94 95 96 97 95 TABLE 1J ID O: 98 94 TABLE 1J AB-11 Sequences (VL3) SEQ ID O: 95 99 97 95 99 97 95 94 95 99 97 95 TABLE 1K AB-11 Sequences (murine) ID O: 00 01 1 88 89 94 95 2 02 91 03 97 95 3 8 91 CDR-L1 amino acid sequence (Chothia definition) KASQDVGTAVA 103 CDR-L2 amino acid sequence (Chothia definition) WTSTRHT 97 95 4 8 91 94 95 5 02 89 03 97 95 04 8 91 94 97 05 [0009] n certan aspects, an ant-C 5 antbody or antgen-bndng ragment o t e dscosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOS: 1, 6, and 13, and light chain CDRs of SEQ ID NO:17, ATS, and SEQ ID NO:20. [0010] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 11, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0011] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:3, 8, and 15, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0012] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:4, 8, and 15, and light chain CDRs of SEQ ID NO:17, ATS, and SEQ ID NO:20. [0013] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 11, and 13, and light chain CDRs of SEQ ID NOs:17, 19, and 21. [0014] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 7, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0015] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 7, and 13, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0016] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:1, 6, and 16, and light chain CDRs of SEQ ID NO:17, ATS, and SEQ ID NO:20. [0017] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 9, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0018] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 9, and 16, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0019] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 10, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0020] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 10, and 13, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0021] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 12, and 14, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0022] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 12, and 13, and light chain CDRs of SEQ ID NOs:18, 19, and 21. [0023] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:1, 88, and 89, and light chain CDRs of SEQ ID NO:94, WT, and SEQ ID NO:95. [0024] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 90, and 91, and light chain CDRs of SEQ ID NOs:96, 97, and 95. [0025] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 90, and 91, and light chain CDRs of SEQ ID NOs:99, 97, and 95. [0026] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:3, 8, 91, and light chain CDRs of SEQ ID NOs:96, 97, 95. [0027] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:3, 8, 91, and light chain CDRs of SEQ ID NOs:99, 97, 95. [0028] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:4, 8, and 91, and light chain CDRs of SEQ ID NO:94, WT, and SEQ ID NO:95. [0029] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 90, and 89, and light chain CDRs of SEQ ID NOs:96, 97, and 95. [0030] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 90, and 89, and light chain CDRs of SEQ ID NOs:99, 97, and 95. [0031] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:2, 102, and 91, and light chain CDRs of SEQ ID NOs:103, 97, and 95. [0032] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:3, 8, and 91, and light chain CDRs of SEQ ID NOs:103, 97, and 95. [0033] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:5, 102, and 89, and light chain CDRs of SEQ ID NOs:103, 97, and 95. [0034] In other aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure (e.g., humanized anti-CD5 antibody or antigen-binding fragment thereof) comprises heavy chain CDRs of SEQ ID NOs:104, 8, and 91, and light chain CDRs of SEQ ID NOs:94, 97, and 105. [0035] As used herein, references to “heavy chain CDRs of SEQ ID NOs:X, Y, and Z” refer to CDR-H1, CDR-H2, and CDR-H3 sequences, respectively. Similarly, references to “light chain CDRs of SEQ ID NOs:X, Y, and Z” refer to CDR-L1, CDR-L2, and CDR-L3 sequences, respectively. [0036] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:22 and 23, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:22 and 23, respectively. [0037] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:24 and 25, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:24 and 25, respectively. [0038] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:26 and 27, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:26 and 27, respectively. [0039] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:28 and 29, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:28 and 29, respectively. [0040] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:30 and 31, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:30 and 31, respectively. [0041] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:32 and 33, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:32 and 33, respectively. [0042] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:49 and 50, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:49 and 50, respectively. [0043] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:84 and 92, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively. [0044] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:84 and 93, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively. [0045] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:84 and 98, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively. [0046] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:85 and 92, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively. [0047] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:85 and 93, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively. [0048] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:85 and 98, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively. [0049] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:86 and 92, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively. [0050] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:86 and 93, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively. [0051] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:86 and 98, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively. [0052] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:87 and 92, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 92, respectively. [0053] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:87 and 93, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 93, respectively. [0054] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:87 and 98, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:84 and 98, respectively. [0055] In further aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure comprises heavy and light chain variable regions of SEQ ID NOS:100 and 101, respectively. In yet other aspects, the disclosure provides an anti-CD5 antibody or antigen-binding fragment having heavy and light chain variable regions having at least 90%, at least 95%, at least 98%, at least 99%, or 100% sequence identity to SEQ ID NOS:100 and 101, respectively. [0056] In further aspects, an anti-CD5 antibody comprises a light chain and heavy chain of any one of AB-1 to AB-10 as set forth in Table 1L. TABLE 1L AB-1 to AB-10 light chain and heavy chain sequences ID 55 56 TABLE 1L AB-1 to AB-10 light chain and heavy chain sequences SEQ ID 57 58 59 69 70 TABLE 1L AB-1 to AB-10 light chain and heavy chain sequences SEQ ID 71 72 73 74 75 76 TABLE 1L AB-1 to AB-10 light chain and heavy chain sequences SEQ ID 77 78 79 80 [0057] In one aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:69 and 70, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:71 and 72, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:73 and 74, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:75 and 76, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:77 and 78, respectively. In another aspect, an anti-CD5 antibody comprises a light chain and heavy chain of SEQ ID NOs:79 and 80, respectively. [0058] In certain aspects, an anti-CD5 antibody or antigen-binding fragment of the disclosure is cross- reactive with human and cynomolgus and/or rhesus monkey CD5. [0059] Further exemplary features of the anti-CD5 antibodies and antigen-binding fragments thereof of the disclosure are described in Section 6.1 and specific embodiments 1 to 197, infra. [0060] Further exemplary features of conjugates of the disclosure and conjugate components are described in Sections 6.2 to 6.5 and specific embodiments 203 to 261, infra. For example, exemplary ALK5 inhibitors that can be used in conjugates of the disclosure are described in Section 6.3, including in Tables 2, 3A, 3B, and 4. In conjugates of the disclosure, the ALK5 inhibitor can be directly conjugated to the antibody component or linked to the antibody component by a linker. The linker can be a non- cleavable linker or, preferably, a cleavable linker (e.g., a protease-sensitive linker). Exemplary non- cleavable and cleavable linkers are described in Section 6.4. The average number of ALK5 inhibitor molecules attached per antibody or antigen binding fragment can vary, and generally ranges from 2 to 8 ALK5 inhibitor molecules per antibody or antigen binding fragment. Drug loading is described in detail in Section 6.5. Exemplary processes for making conjugates and exemplary kits useful for making conjugates are described in specific embodiments 474 to 482, infra. [0061] Yet another aspect of the disclosure is a process for making an antibody-ALK5 inhibitor conjugate, where the antibody component is an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure (e.g., a humanized anti-CD5 antibody or antigen-binding fragment thereof). [0062] Yet another aspect of the disclosure is a kit comprising an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure (e.g., a humanized anti-CD5 antibody or antigen-binding fragment thereof) and an ALK5 inhibitor. The kits can be used, for example, to make a conjugate of the disclosure. [0063] Other aspects of the disclosure are drawn to nucleic acids encoding the anti-CD5 antibodies and antibody-binding fragments of the disclosure. In some embodiments, the portion of the nucleic acid nucleic acid encoding an anti-CD5 antibody or antigen-binding fragment is codon-optimized for expression in a human cell. Vectors (e.g., plasmids) and host cells comprising the nucleic acids are also within the scope of the disclosure. The heavy and light chains coding sequences can be present on a single vector or on separate vectors. [0064] Still another aspect of the disclosure is a method of making an anti-CD5 antibody or antigen- binding fragment thereof comprising incubating a cell comprising a nucleic acid or a vector according to the disclosure, under conditions suitable for expression of the coding region(s) and collecting the anti- CD5 antibody or antigen-binding fragment thereof. [0065] Nucleic acids, vectors, host cells, and uses thereof are further described in Section 6.6 and specific embodiments 198 to 202, infra. [0066] Yet another aspect of the disclosure is a pharmaceutical composition comprising an anti-CD5 antibody, antigen-binding fragment, conjugate, nucleic acid (or pair of nucleic acids), vector (or pair of vectors) or host cell according to the disclosure, and a physiologically suitable buffer, adjuvant, or diluent. Exemplary pharmaceutical excipients that can be used to formulate a pharmaceutical composition and exemplary pharmaceutical compositions are described in Section 6.7 and specific embodiments 262 to 291, infra. [0067] Yet another aspect of the disclosure is a method of treating cancer comprising administering a prophylactically or therapeutically effective amount of an anti-CD5 antibody (e.g., a humanized anti-CD5 antibody), antigen-binding fragment thereof, conjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure to a subject in need thereof. [0068] In some embodiments, the methods of treating cancer comprise administering a conjugate of the disclosure or a pharmaceutical composition comprising the conjugate to a subject in need thereof. The conjugates and pharmaceutical compositions can be administered as monotherapy or as part of a combination therapy, for example in combination with an immune checkpoint modulator (e.g., a checkpoint inhibitor). Exemplary cancers that can be treated with the conjugates and pharmaceutical compositions of the disclosure and exemplary combination therapies are described in Section 6.8 and specific embodiments 292 to 473. [0069] Yet another aspect of the disclosure is an anti-CD5 antibody (e.g., a humanized anti-CD5 antibody), antigen-binding fragment thereof, conjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure for use in the treatment of cancer. [0070] Yet another aspect of the disclosure is use of an anti-CD5 antibody (e.g., a humanized anti-CD5 antibody), antigen-binding fragment thereof, conjugate, nucleic acid, vector, host cell or pharmaceutical composition according to the disclosure for the manufacture of a medicament for the treatment of cancer. 5. BRIEF DESCRIPTION OF THE FIGURES [0071] FIGS.1A-1C show amino acid sequence alignments between parental mouse anti-CD5 antibody (Antibody B) and human germline Ig alleles. FIG.1A is the amino acid sequence alignment of the parental mouse anti-CD5 antibody light chain and Ig alleles immunoglobulin kappa variable 3-11 allele 1 (IGKV3-11*01), immunoglobulin kappa variable 3-11 allele 2 (IGKV3-11*02), and immunoglobulin kappa variable 1-16 allele 1 (IGKV1-16*01). Figure discloses SEQ ID NOS 107-110, respectively, in order of appearance. FIG.1B is the amino acid sequence alignment of the parental mouse anti-CD5 antibody heavy chain and Ig alleles IGKV3-11*01, IGKV3-11*02, and IGKV1-16*01. Figure discloses SEQ ID NOS 111-114, respectively, in order of appearance. FIG.1C is the tabular representation of the percent mismatch between the human Ig alleles and parental mouse anti-CD5 antibody light and heavy chains. [0072] FIGS.2A-2B show amino acid sequence alignments between parental mouse anti-CD5 antibody (Antibody B), the humanized anti-CD5 antibody AB-5, an exemplary human antibody 4JLR.pdb, and three human germline Ig alleles. FIG.2A displays the light chain amino acid sequence alignments of parental anti-CD5 antibody, AB-5, 4JLR.pdb, and Ig alleles IGKV1-16*01, IGKV1-16*02, and IGKV1-39*01. Figure discloses SEQ ID NOS 115-120, respectively, in order of appearance. FIG.2B displays the heavy chain amino acid sequence alignments of parental anti-CD5 antibody, AB-5, 4JLR.pdb, and Ig alleles IGKV1- 16*01, IGKV1-16*02, and IGKV1-39*01. Figure discloses SEQ ID NOS 121-126, respectively, in order of appearance. [0073] FIGS.3A-3E show exemplary SDS-PAGE and endotoxin analyses of purified negative control and anti-CD5 antibodies of the disclosure. FIG.3A is an SDS-PAGE image of the antibody AB-4. FIG.3B is an SDS-PAGE image of a negative control antibody, NC-AB. FIG.3C is an SDS-PAGE image of the antibody AB-6. FIG.3D is an SDS-PAGE image of the antibody AN-8. Non-reduced antibody samples in FIGS.3A-3D display a single high MW band and reduced antibody samples are associated with only two bands at 55 kDa and 25 kDa, corresponding to the IgG heavy chain and IgG light chain, respectively. FIG.3E is a table that displays the purified concentrations of the antibodies shown in FIGS.3A-3D, as well as their optical density (OD) values at 545 nm and endotoxin units (EU) per mL and per mg antibody sample. [0074] FIGS.4A-4F show the results of anti-CD5 antibody cell binding assays. FIG.4A is a table that displays the antibody concentrations that were used in cell binding assays. FIG.4B is a Jurkat T cell binding concentration-response graph of the antibodies AB-1, AB-2, AB-3, AB-4, and the negative control antibody NC-AB. FIG.4C is a Jurkat T cell binding concentration-response graph of the antibodies AB-1, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10. FIG.4D is a CD3+ T cell binding concentration-response graph of the antibodies AB-1, AB-2, AB-4, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10. FIG.4E is a Jurkat T cell binding concentration-response graph of the parental mouse anti-human CD5 (α-hCD5) antibody. FIG.4F shows the raw data of a cell binding assay of α-hCD5 on mouse EL4 cells. [0075] FIGS.5A-5F display the internalization profiles of exemplary humanized anti-CD5 antibodies of the disclosure. FIG.5A is a graph showing the change in signal intensity over the course of 6 hours for antibodies AB-1, AB-2, AB-3, AB-4, and NC-AB. FIG.5B is a graph that shows the percent internalization values over time for the same antibodies in FIG.5A. FIG.5C is a graph displaying the change in signal intensity the course of 6 hours for antibodies AB-1, AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10. FIG.5D is a graph displaying the percent internalization values over time for the same antibodies in FIG.5C. FIG. 5E is a bar graph that displays the percent reduction of CD5 binding of the antibody, AB-4. FIG.5F is a bar graph that displays the percent reduction of CD5 binding of the antibody, AB-8. [0076] FIG.6 is a bar graph that shows the percent cytotoxicity associated with the control and exemplary humanized anti-CD5 antibodies of the disclosure. [0077] FIGS.7A-7C show binding of parental CD5 antibodies Antibody B and Antibody C to HEK293 cells (FIG.7A), human CD5 transfected HEK293 cells (FIG.7B) and rhesus monkey CD5 transfected HEK293 cells (FIG.7C) analyzed by flow cytometry. [0078] FIGS.8A-8C show internalization of AB-4 (FIG.8A), AB-6 (FIG.8B), and AB-8 (FIG.8C) after binding CD5 on the surface of Jurkat cells as measured by FACS. [0079] FIG.9 shows a dose response curve for binding of various exemplary antibodies of the disclosure having IgG4 Fc regions to Jurkat cells. [0080] FIGS.10A-10B show mean fluorescence intensity (MFI) (FIG.10A) and percent internalization (FIG.10B) for various exemplary antibodies of the disclosure following binding to Jurkat cells as measured by FACS. [0081] FIGS.11A-11B show percent SBE activity in HEK cells following exposure to exemplary antibody-TGFβ-antagonist-conjugates (ATACs) having the following antibody components: Antibody B, AB-4, and AB-8. FIG.11A: bar graph; FIG.11B: logarithmic plot. [0082] FIG.12 shows CDC activity of exemplary antibodies and ATACs of the disclosure. 6. DETAILED DESCRIPTION [0083] The disclosure provides anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen binding fragments thereof. Antibodies and antibody fragments of the disclosure are described in detail in Section 4 and Section 6.1.The disclosure further provides conjugates useful for treating cancer comprising an antibody component covalently bonded to an ALK5 inhibitor, either directly or through a linker. An overview of the conjugates of the disclosure is presented in Section 6.2. The antibody component of the conjugates can be an intact antibody or a fragment thereof. ALK5 inhibitors that can be used in the conjugates of the disclosure are described in Section 6.3. The conjugates of the disclosure typically contain a linker between the antibody and ALK5 inhibitor. Exemplary linkers that can be used in conjugates of the disclosure are described in Section 6.4. The conjugates of the disclosure can contain varying numbers of ALK5 inhibitor moieties per antibody. Drug loading is discussed in detail in Section 6.5. The disclosure further provides nucleic acids, vectors, and host cells useful for expressing the anti- CD5 antibodies and antigen binding fragments of the disclosure. Nucleic acids, vectors, and host cells are described in Section 6.6. The disclosure further provides pharmaceutical formulations comprising a conjugate of the disclosure. Pharmaceutical formulations comprising conjugates are described in Section 6.7. The disclosure further provides methods of treating various cancers using the conjugates of the disclosure. Methods of using the conjugates of the disclosure as monotherapy or as part of a combination therapy for the treatment of cancer are described in Section 6.8. 6.1. Anti-CD5 antibodies and antigen binding fragments [0084] The present disclosure provides anti-CD5 antibodies (e.g., humanized anti-CD5 antibodies) and antigen binding fragments thereof. Unless indicated otherwise, the term “anti-CD5 antibody” (Ab) refers to an immunoglobulin molecule that specifically binds to, or is immunologically reactive with, human CD5, and includes polyclonal, monoclonal, genetically engineered and otherwise modified forms of antibodies, including but not limited to chimeric antibodies, humanized antibodies, heteroconjugate antibodies (e.g., bispecific antibodies, diabodies, triabodies, and tetrabodies), and antigen binding fragments of antibodies, including, e.g., Fab′, F(ab′)2, Fab, Fv, rIgG, and scFv fragments. Moreover, unless otherwise indicated, the term “monoclonal antibody” (mAb) is meant to include both intact molecules, as well as, antibody fragments (such as, for example, Fab and F(ab′)2 fragments) which are capable of specifically binding to a protein. Fab and F(ab′)2 fragments lack the Fc fragment of intact antibody, clear more rapidly from the circulation of the animal or plant, and may have less non-specific tissue binding than an intact antibody (Wahl et al., 1983, J. Nucl. Med.24:316). [0085] The term “scFv” refers to a single chain Fv antibody in which the variable domains of the heavy chain and the light chain from a traditional antibody have been joined to form one chain. [0086] References to “VH” refer to the variable region of an immunoglobulin heavy chain of an antibody, including the heavy chain of an Fv, scFv, or Fab. References to “VL” refer to the variable region of an immunoglobulin light chain, including the light chain of an Fv, scFv, dsFv or Fab. Antibodies (Abs) and immunoglobulins (Igs) are glycoproteins having the same structural characteristics. While antibodies exhibit binding specificity to a specific target, immunoglobulins include both antibodies and other antibody-like molecules which lack target specificity. Native antibodies and immunoglobulins are usually heterotetrameric glycoproteins of about 150,000 Daltons, composed of two identical light (L) chains and two identical heavy (H) chains. Each heavy chain has at the amino terminus a variable domain (VH) followed by a number of constant domains. Each light chain has a variable domain at the amino terminus (VL) and a constant domain at the carboxy terminus. [0087] For optimal delivery of an ALK5 inhibitor conjugated to an antibody within a cell, the antibodies are preferably internalizing. Internalizing antibodies, after binding to their target molecules on cellular surface, are internalized by the cells as a result of the binding. The effect of this is that the conjugate is taken up by cells. Processes which allow the determination of the internalization of an antibody after binding to its antigen are known to the skilled person and are described for example on page 80 of PCT publication no. WO 2007/070538 and in Section 6.11 of WO 2020/256751. Once internalized, if a cleavable linker is used to attach the ALK5 inhibitor to the antibody, for example as described in Section 6.4, the ALK5 inhibitor can be released from the antibody by cleavage in the lysosome or by other cellular mechanism. [0088] The term “antibody fragment” refers to a portion of a full-length antibody, generally the target binding or variable region. Examples of antibody fragments include Fab, Fab′, F(ab′)2 and Fv fragments. An “Fv” fragment is the minimum antibody fragment which contains a complete target recognition and binding site. This region consists of a dimer of one heavy and one light chain variable domain in a tight, noncovalent association (VH-VL dimer). It is in this configuration that the three CDRs of each variable domain interact to define a target binding site on the surface of the VH-VL dimer. Often, the six CDRs confer target binding specificity to the antibody. However, in some instances even a single variable domain (or half of an Fv comprising only three CDRs specific for a target) can have the ability to recognize and bind target. “Single chain Fv” or “scFv” antibody fragments comprise the VH and VL domains of an antibody in a single polypeptide chain. Generally, the Fv polypeptide further comprises a polypeptide linker between the VH and VL domain that enables the scFv to form the desired structure for target binding. [0089] The Fab fragment contains the constant domain of the light chain and the first constant domain (CH1) of the heavy chain. Fab′ fragments differ from Fab fragments by the addition of a few residues at the carboxyl terminus of the heavy chain CH1 domain including one or more cysteines from the antibody hinge region. F(ab′) fragments are produced by cleavage of the disulfide bond at the hinge cysteines of the F(ab′)2 pepsin digestion product. Additional chemical couplings of antibody fragments are known to those of ordinary skill in the art. [0090] In certain embodiments, the antibodies of the disclosure are monoclonal antibodies. The term “monoclonal antibody” as used herein is not limited to antibodies produced through hybridoma technology. The term “monoclonal antibody” refers to an antibody that is derived from a single clone, including any eukaryotic, prokaryotic, or phage clone and not the method by which it is produced. Monoclonal antibodies useful in connection with the present disclosure can be prepared using a wide variety of techniques known in the art including the use of hybridoma, recombinant, and phage display technologies or a combination thereof. [0091] “Humanized” forms of non-human (e.g., murine) antibodies are chimeric immunoglobulins, immunoglobulin chains or fragments thereof (such as Fv, Fab, Fab′, F(ab′)2 or other target-binding subdomains of antibodies) which contain minimal sequences derived from non-human immunoglobulin. In general, the humanized antibody will comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the CDR regions correspond to those of a non-human immunoglobulin and all or substantially all of the FR regions are those of a human immunoglobulin sequence. The humanized antibody can also comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin consensus sequence. Methods of antibody humanization are known in the art. See, e.g., Riechmann et al., 1988, Nature 332:323-7; U.S. patent nos. 5,530,101; 5,585,089; 5,693,761; 5,693,762; and 6,180,370 to Queen et al.; European patent publication no. EP239400; PCT publication WO 91/09967; U.S. patent no.5,225,539; European patent publication no. EP592106; European patent publication no. EP519596; Padlan, 1991, Mol. Immunol., 28:489-498; Studnicka et al., 1994, Prot. Eng.7:805-814; Roguska et al., 1994, Proc. Natl. Acad. Sci.91:969-973; and U.S. patent no.5,565,332, all of which are hereby incorporated by reference in their entireties. [0092] The antibodies of the disclosure include derivatized antibodies. For example, but not by way of limitation, derivatized antibodies are typically modified by glycosylation, acetylation, pegylation, phosphorylation, amidation, derivatization by known protecting/blocking groups, proteolytic cleavage, linkage to a cellular ligand or other protein (see Section 6.1 for a discussion of antibody conjugates), etc. Any of numerous chemical modifications can be carried out by known techniques, including, but not limited to, specific chemical cleavage, acetylation, formylation, metabolic synthesis of tunicamycin, etc. Additionally, the derivative can contain one or more non-natural amino acids, e.g., using ambrx technology (See, e.g., Wolfson, 2006, Chem. Biol.13(10):1011-2). [0093] In yet another embodiment of the disclosure, the antibodies or fragments thereof can be antibodies or antibody fragments whose sequence has been modified to alter at least one constant region-mediated biological effector function relative to the corresponding wild type sequence. For example, in some embodiments, an antibody of the disclosure can be modified to reduce at least one constant region-mediated biological effector function relative to an unmodified antibody, e.g., reduced binding to the Fc receptor (FcγR) or to C1q. FcγR and C1q binding can be reduced by mutating the immunoglobulin constant region segment of the antibody at particular regions necessary for FcγR or C1q interactions (See, e.g., Canfield and Morrison, 1991, J. Exp. Med.173:1483-1491; Lund et al., 1991, J. Immunol.147:2657-2662; Lo. et al., 2017, J Biol Chem 292: 3900-08; Wang et al., 2018, Protein Cell 9:63-73). [0094] Reduction in FcγR binding ability of the antibody can also reduce other effector functions which rely on FcγR interactions, such as opsonization, phagocytosis and antibody-dependent cellular cytotoxicity (“ADCC”), while reduction of C1q binding can reduce complement-dependent cytotoxicity (“CDCC). Reduction or elimination of effector function can thus prevent T cells targeted by an conjugate of the disclosure from being destroyed via ADCC or CDC. Accordingly, in some embodiments, effector function of an antibody is modified by selective mutation of the Fc portion of the antibody, so that it maintains antigen specificity and internalization capacity but eliminates ADCC/CDC function. [0095] Numerous mutations have been described in the art for reducing FcγR and C1q binding and such mutations can be included in an antibody of the disclosure. For example, U.S. Pat. No.6,737,056 discloses that single position Fc region amino acid modifications at positions 238, 265, 269, 270, 292, 294, 295, 298, 303, 324, 327, 329, 333, 335, 338, 373, 376, 414, 416, 419, 435, 438 or 439 result in reduced binding to FcγRII and FcγRII. Unless otherwise specified herein, numbering of amino acid residues in an Fc domain or constant region is according to the EU numbering system, also called the EU index, as described in Kabat et al., 1991, Sequences of Proteins of Immunological Interest, 5th Ed. Public Health Service, National Institutes of Health, Bethesda, MD. U.S. patent no.9,790,268 discloses that an asparagine residue at amino acid position 298 and a serine or threonine residue at amino acid position 300 reduce FcγR binding. PCT publication no. WO 2014/190441 describes modified Fc domains with reduced FcγR binding having L234D/L235E : L234R/L235R/E233K, L234D/L235E/D265S : E233K/L234R/L235R/D265S, L234D/L235E/E269K : E233K/L234R/L235R/E269K, L234D/L235E/K322A : E233K/L234R/L235R/K322A, L234D/L235E/P329W : E233K/L234R/L235R/P329W, L234D/L235E/E269K/D265S/K322A : E233K/L234R/L235R/E269K/D265S/K322A, L234D/L235E/E269K/D265S/K322E/E333K: E233K/L234R/L235R/E269K/D265S/K322E/E333K mutations, where the set of mutations preceding a semicolon is in a first Fc polypeptide and the mutations following the semicolon are in a second Fc polypeptide of an Fc dimer. Mutations that can reduce FcγR receptor binding as well as C1q binding include N297A, N297Q, N297G, D265A/N297A, D265A/N297G, L235E, L234A/L235A, and L234A/L235A/P329A (Lo. et al., 2017, J Biol Chem 292: 3900-08; Wang et al., 2018, Protein Cell 9:63-73). [0096] In some embodiments, antibodies or antigen binding fragments of the disclosure comprise a first Fc region and a second Fc region that dimerize to form an Fc domain. For example, the Fc domain can comprise CH2 and CH3 domains derived from a human IgG1 domain (for example, having one of more of the amino acid substitutions described in this Section) or CH2 and CH3 domains derived from a human IgG2 domain or human IgG4 domain. Exemplary sequences derived from human IgG1 that can be used include the following: APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLV KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT QKSLSLSPGK (SEQ ID NO:51) (WT sequence) APEAAGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLV KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT QKSLSLSPGK (SEQ ID NO:52) (variant having L234A and L235A substitutions) APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYAST YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLV KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT QKSLSLSPGK (SEQ ID NO:53) (variant having a N297A substitution) [0097] In some embodiments, an anti-CD5 antibody (e.g., a humanized anti-CD5 antibody) or antigen binding fragment thereof of the disclosure has a first Fc region and/or a second Fc region comprising a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:51, SEQ ID NO:52, or SEQ ID NO:53. [0098] An exemplary sequence derived from human IgG4 that can be used is the following: APEFLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNST YRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLV KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYT QKSLSLSLGK (SEQ ID NO:54) [0099] In some embodiments, an anti-CD5 antibody or antigen binding fragment thereof of the disclosure has a first Fc region and/or a second Fc region comprising a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:54. [0100] An exemplary sequence derived from human IgG2 that can be used is the following: APPVAGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTF RVVSVLTVVHQDWLNGKEYKCKVSNKGLPAPIEKTISKTKGQPREPQVYTLPPSREEMTKNQVSLTCLVK GFYPSDISVEWESNGQPENNYKTTPPMLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQ KSLSLSPGK (SEQ ID NO:81) [0101] In some embodiments, an anti-CD5 antibody or antigen binding fragment thereof of the disclosure has a first Fc region and/or a second Fc region comprising a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:81. [0102] Anti-CD5 antibodies and antigen binding fragments can comprise a hinge region, for example connecting a CH1 domain to a Fc region comprising a CH2 and CH3 domain. For example, a human IgG1, IgG2, or IgG4 hinge can be used. Exemplary hinge sequences include EPKSCDKTHTCPPCP (SEQ ID NO:66) (human IgG1), ESKYGPPCPSCP (SEQ ID NO:67) (human IgG4), ESKYGPPCPPCP (SEQ ID NO:68) (human IgG4 with a S228P substitution), and ERKCCVECPPCP (SEQ ID NO:83) (human IgG2). [0103] Exemplary amino acid sequences comprising a CH1 domain (which can be C-terminal to a VH, for example), hinge, CH2 domain, and CH3 domain include the following: ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVS NKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO:62) (derived from human IgG1) ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVS NKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO:63) (derived from human IgG1, with L234A and L235A substitutions) ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYASTYRVVSVLTVLHQDWLNGKEYKCKVS NKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO:64) (derived from human IgG1, with N297A substitution) ASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVT VPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPCPPCPAPEFLGGPSVFLFPPKPKDTLMISRTPEVT CVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKG LPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV LDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLGK (SEQ ID NO:65) (derived from human IgG4, with S228P substitution) ASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVT VPSSNFGTQTYTCNVDHKPSNTKVDKTVERKCCVECPPCPAPPVAGPSVFLFPPKPKDTLMISRTPEVT CVVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTFRVVSVLTVVHQDWLNGKEYKCKVSNKG LPAPIEKTISKTKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDISVEWESNGQPENNYKTTPPM LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO:82) (derived from human IgG2) [0104] As an alternative to mutating a constant region to reduce effector function, e.g., mutating an Fc domain as described above, effector function can be eliminated by utilizing an antibody fragment (e.g., a Fab, Fab′, or F(ab′)2 fragment). [0105] In other embodiments of the disclosure, an antibody or fragment thereof can be modified to acquire or improve at least one constant region-mediated biological effector function relative to an unmodified antibody, e.g., to enhance FcγR interactions (See, e.g., US 2006/0134709). For example, an antibody of the disclosure can have a constant region that binds FcγRIIA, FcγRIIB and/or FcγRIIIA with greater affinity than the corresponding wild type constant region. [0106] Thus, antibodies of the disclosure can have alterations in biological activity that result in decreased opsonization, phagocytosis, or ADCC. Such alterations are known in the art. For example, modifications in antibodies that reduce ADCC activity are described in U.S. patent no. 5,834,597. [0107] In yet another aspect, the antibodies or fragments thereof can be antibodies or antibody fragments that have been modified to increase or reduce their binding affinities to the fetal Fc receptor, FcRn, for example, by mutating the immunoglobulin constant region segment at particular regions involved in FcRn interactions (See, e.g., WO 2005/123780). Such mutations can increase the antibody’s binding to FcRn, which protects the antibody from degradation and increases its half-life. [0108] In yet other aspects, an antibody has one or more amino acids inserted into one or more of its hypervariable regions, for example as described in Jung and Plückthun, 1997, Protein Engineering 10(9):959-966; Yazaki et al., 2004, Protein Eng. Des Sel.17(5):481-9; and U.S. patent publication no. 2007/0280931. 6.2. Antibody ALK5 inhibitor Conjugates [0109] The conjugates of the disclosure are generally composed of an ALK5 inhibitor covalently attached to an anti-CD5 antibody (e.g., a humanized anti-CD5) or antigen binding fragment thereof, typically via a linker, such that covalent attachment does not interfere with binding to CD5. [0110] Techniques for conjugating drugs to antibodies are well known in the art (See, e.g., Hellstrom et al., Controlled Drug Delivery, 2nd Ed., at pp.623-53 (Robinson et al., eds., 1987)); Thorpe et al., 1982, Immunol. Rev.62:119-58; Dubowchik et al., 1999, Pharmacology and Therapeutics 83:67-123; and Zhou, 2017, Biomedicines 5(4):E64). The ALK5 inhibitors are preferably attached to an antibody component in the conjugates of the disclosure via site-specific conjugation. For example, an ALK5 inhibitor can be conjugated to the antibody component via one or more native or engineered cysteine, lysine, or glutamine residues, one or more unnatural amino acids (e.g., p-acetylphenylalanine (pAcF), p-azidomethyl-L- phenylalanine (pAMF), or selenocysteine (Sec)), one or more glycans (e.g., fucose, 6-thiofucose, galactose, N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), or sialic acid (SA)), or one or more short peptide tags of four to six amino acids. See, e.g., Zhou, 2017, Biomedicines 5(4):E64, the contents of which are incorporated herein by reference in their entireties. [0111] In one example, the antibody or fragment thereof is fused via a covalent bond (e.g., a peptide bond), through the antibody’s N-terminus or the C-terminus or internally, to an amino acid sequence of another protein (or portion thereof; for example, at least a 10, 20 or 50 amino acid portion of the protein). The antibody, or fragment thereof, can linked to the other protein at the N-terminus of the constant domain of the antibody. Recombinant DNA procedures can be used to create such fusions, for example as described in WO 86/01533 and EP0392745. In another example the effector molecule can increase half-life in vivo, and/or enhance the delivery of an antibody across an epithelial barrier to the immune system. Examples of suitable effector molecules of this type include polymers, albumin, albumin binding proteins or albumin binding compounds such as those described in PCT publication no. WO 2005/117984. [0112] The metabolic process or reaction may be an enzymatic process, such as proteolytic cleavage of a peptide linker of the conjugate, or hydrolysis of a functional group such as a hydrazone, ester, or amide. Intracellular metabolites include, but are not limited to, antibodies and free drug which have undergone intracellular cleavage after entry, diffusion, uptake or transport into a cell. [0113] The terms “intracellularly cleaved” and “intracellular cleavage” refer to a metabolic process or reaction inside a cell on a conjugate whereby the covalent attachment, i.e. linker, between the drug moiety (D) and the antibody (Ab) is broken, resulting in the free drug dissociated from the antibody inside the cell. The cleaved moieties of the conjugate are thus intracellular metabolites. 6.3. The ALK5 Inhibitor [0114] The ALK5 inhibitors of the disclosure are preferably small molecules that competitively and reversibly bind to ATP binding site in the cytoplasmic kinase domain of the ALK5 receptor, preventing downstream R-Smad phosphorylation. [0115] The ALK5 inhibitors may but not need be specific or selective for ALK5 vs. other TGF-β family receptors, such as ALK4 and/or ALK7 and/or TGF-β receptor II. In some embodiments, the ALK5 inhibitors have activity towards both ALK5 and TGF-β receptor II. While it is preferable that the ALK5 inhibitor have limited inhibitory activity towards the BMP II receptor, this is not necessary because the conjugates of the disclosure are targeted to T cells, in which BMP II activity is minimal or absent. [0116] The ALK5 inhibitors of the disclosure preferably have an IC50 of 100 nM or less, more preferably 50 nM or less, and most preferably 20 nM or less when measured in an in vitro cellular assay using T cells from at least 3 subjects, at least 5 subjects or at least 10 subjects. An exemplary cellular assay set forth in Section 6.6 of WO 2020/256751. Human instead of mouse cells as well as antibodies recognizing human instead of mouse CD28 and CD3 can be used for conjugates targeting human rather than mouse CD5. [0117] Illustrative examples of ALK5 inhibitors suitable for use in the antibody-drug conjugates of the present disclosure include imidazole-benzodioxol compounds, imidazole-quinoxaline compounds, pyrazole-pyrrolo compounds and thiazole type compounds. [0118] In accordance with one aspect of the present disclosure, imidazole-benzodioxol type ALK5 inhibitors have the formula
R2 is hydrogen or lower alkyl having from 1 to about 5 carbon atoms and R3 is an amide, nitrile, alkynyl having from 1 to about 3 carbon atoms, carboxyl or alkanol group having from 1 to about 5 carbon atoms, A is a direct bond or an alkyl having from 1 to about 5 carbon atoms and B is a direct bond or an alkyl having from 1 to about 5 carbon atoms. In separate preferred embodiments of the present disclosure, R2 is hydrogen or methyl, A has 1 carbon atom and B is a direct bond to the benzyl group and R3 is an amide. In a combined preferred embodiment of the present disclosure, R2 is hydrogen or methyl, A has 1 carbon atom and B is a direct bond to the benzyl group. [0120] In accordance with another aspect of the present disclosure, imidazole-quinoxaline type ALK5 inhibitors have the formula is hydrogen, halogen or lower alkyl having from 1 to about 5 carbon atoms and R3 is an amide, nitrile, alkynyl having from 1 to about 3 carbon atoms, carboxyl or alkanol group having from 1 to about 5 carbon atoms, A is a direct bond or an alkyl having from 1 to about 5 carbon atoms and B is a direct bond or an alkyl having from 1 to about 5 carbon atoms. In separate preferred embodiments of the present disclosure, R2 is hydrogen or methyl, halogens include fluorine or chlorine, A has 1 carbon atom and B is a direct bond to the benzyl group and R3 is an amide. In a combined preferred embodiment of the present disclosure, R2 is hydrogen or methyl, A has 1 carbon atom and B is a direct bond to the benzyl group. [0122] In accordance with another aspect of the present disclosure, pyrazole type ALK5 inhibitors have the formula to about 5 carbon atoms, R4 is hydrogen, halogen, lower alkyl having from 1 to about 5 carbon atoms, alkoxy having from 1 to about 5 carbon atoms, haloalkyl, carboxyl, carboxyalkylester, nitrile, alkylamine or a group having the formula
[0124] where R5 is lower alkyl having from 1 to about 5 carbon atoms, halogen or morpholino, and R6 is pyrole, cyclohexyl, morpholino, pyrazole, pyran, imidazole, oxane, pyrrolidinyl or alkylamine, and A is a direct bond or an alkyl having from 1 to about 5 carbon atoms. [0125] In accordance with another aspect of the present disclosure, pyrazole-pyrrolo type ALK5 inhibitors have the formula 1 to about 5 carbon atoms, alkanol, morpholino or alkylamine, R2 is hydrogen, halogen or lower alkyl having from 1 to about 5 carbon atoms and R8 is hydrogen, hydroxyl, amino, halogen or a group having the formula
[0127] where R5 is piperazinyl, R6 is morpholino, piperidinyl, piperazinyl, alkoxy, hydroxyl, oxane, halogen, thioalkyl or alkylamine, and A is a lower alkyl having from 1 to about 5 carbon atoms. [0128] In accordance with another aspect of the present disclosure, thiazole type ALK5 inhibitors have the formula or lower alkyl having from 1 to about 5 carbon atoms, and R10 is or about 5 carbon atoms. [0130] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula a halogen, -OR10, -SR10, -N(R10)2, -C(O)R10, -C(O)N(R10)2, -N(R10)C(O)R10 - C(O)OR10, -OC(O)R10, - S(O)R10, -S(O)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, -OP(O)(OR10)2, -N02, and -CN; -C1-C10 alkyl, -C2- Cl0 alkenyl, and -C2-Cl0 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents independently selected from a halogen, -OR10, -SR10, -N(R10)2, -C(0)R10, -C(O)N(R10)2, -N(R10)C(O)R10 -C(0)OR10, -OC(0)R10, -S(0)R10, -S(0)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, -OP(O)(OR10)2, - N02, =0, =S, =N(R10), -CN, a C3-C10 carbocycle, and a 3- to 10membered heterocycle; and a C3-C10 carbocycle and a 3- to 10-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from a halogen, -OR10, -SR10, -N(R10)2, -C(0)R10, - C(O)N(R10)2,-N(R10)C(O)R10 -C(O)OR10, -OC(O)R10, -S(O)R10, -S(O)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, - OP(O)(OR10)2, -NO2, =O, =S, =N(R10), -CN, -C1-C6 alkyl, -C2-C6 alkenyl, and -C2-C6 alkynyl; R3 is selected from hydrogen; and -C1-C10 alkyl optionally substituted with one or more substituents independently selected from a halogen, -NO2, =O, =S, =N(R10), -CN, -OR10, -SR10, -N(R10)2, -C(O)R10, - C(O)N(R10)2, -N(R10)C(O)R10 -C(O)OR10, and -OC(O)R10; n and m are independently selected from 0, 1, 2, 3, and 4; Q is selected from a bond, -(CR102)p-, -(CR102)qC(=O)(CR102)q-, -(CR102)qC(=S)(CR102)q-, -(CR102)qC(=NR10)(CR102)q, -(CR102)qO(CR102)qO(CR102)q-, -(CR102)qS(CR102)q-, -(CR102)qN(R10)(CR102)q-, -(CR102)qOC(=O)O(CR102)q-, -(CR102)qC(=O)N(R10)(CR102)q-, -(CR102)qN(R10)C(=O)(CR102)q-, and - q is independently selected at each occurrence from 0, 1, 2, 3, 4, and 5; T is selected from an optionally substituted saturated C3-C7 carbocycle, an optionally substituted Cs-o bicyclic carbocycle, and an optionally substituted 4- to l2-membered heterocycle, wherein T is optionally substituted with one or more substituents independently selected at each occurrence from R13; R13 is independently selected at each occurrence from: a halogen, -OR10, -SR10, -N(R10)2, -C(0)R10, -C(O)N(R10)2, -N(R10)C(O)R10 - C(0)OR10, -OC(0)R10, - S(0)R10, -S(0)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, - OP(O)(OR10)2, -N02, =0, =S, =N(R10), and -CN; -C1-C10 alkyl, -C2-Cl0 alkenyl, and -C2-Cl0 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents independently selected from a halogen, -OR10, -SR10, -N(R10)2, -C(O)R10, - C(O)N(R10)2, -N(R10)C(O)R10 -C(O)OR10, -OC(O)R10, -S(O)R10, -S(O)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, - OP(O)(OR10)2, -NO2, =O, =S, =N(R10), -CN, a C3-C10 carbocycle, and a 3- to 10-membered heterocycle; and a C3-C10 carbocycle and a 3- to 10-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from a halogen, -OR10, -SR10, -N(R10)2, -C(O)R10, - C(O)N(R10)2, -N(R10)C(O)R10 -C(O)OR10, - OC(O)R10, -S(O)R10, -S(O)2R10, -S(O)2N(R10)2, -P(O)(OR10)2, - OP(O)(OR10)2, -NO2, =O, =S, =N(R10), -CN, -C1-C6 alkyl, -C2-C6 alkenyl, and -C2-C6 alkynyl; and R10 is independently selected at each occurrence from: hydrogen; -C1-C10 alkyl, -C2-Cl0 alkenyl, and -C2-Cl0 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents independently selected from a halogen, -OH, -CN, -N02, - NH2, =O, =S, -O-C1-C10 alkyl, C3-C12 carbocycle, and a 3- to 12-membered heterocycle; and a C3-C12 carbocycle and a 3- to 12-membered heterocycle, each of which is optionally substituted at each occurrence with one or more substituents independently selected from a halogen, -OH, -CN, -NO2, -NH2, =O, =S, -C1-C10 alkyl, -O-C1-C10 alkyl, and -C1-C10 haloalkyl. Exemplary compounds of the foregoing formula are described in WO 2019/195278. [0131] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula , -NO2, -CN, or - - one or more independently selected from halogen, -OR11, -SR11, -S(O)R10, -S(O)2R11, -S(O)2N(R11)2 -N(R11)2, - C(O)R10, -C(O)N(R11)2, -N(R11)C(O)R10, -C(O)OR11, -OC(O)R10, -NO2, and -CN; R3 is, at each occurrence, independently halogen, -C1-C3 alkyl, -C1-C3 haloalkyl, -OH, -NO2, -CN, -O-C1- C3 alkyl, or -O-C1-C3 haloalkyl; R4 is, at each occurrence, independently hydrogen or C1-C3 alkyl, or two R4 join together with atoms to which they are attached to form a 5- or 6-membered heterocycle optionally substituted with one or more substituents independently selected from halogen, -C1-C3 alkyl, -OH, -O-C1-C3 alkyl, and -O-C1-C3 haloalkyl; R5 is hydrogen, halogen, -OR61, -SR61, -N(R61)2, -NO2, -CN, and -C1-C6 alkyl, wherein said -C1-C6 alkyl is optionally substituted with one or more substituents independently selected from halogen, -OR61, -SR61, - N(R61)2, -NO2, and -CN; R6 is, at each occurrence, independently: halogen, -OR21, -SR21, -N(R21)2, -C(O)R20, -C(O)N(R21)2, - N(R21)C(O)R20, -C(O)OR21, -OC(O)R21, -S(O)R20, -S(O)2R21, -S(O)2N(R21)2, -OC(O)OR21, - OC(O)N(R21)2, -NR21C(=O)OR21, -N(R21)C(O)N(R21)2, -NO2, -CN; C1-C10 alkyl, C2-C10 alkenyl, and C2-C10 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, -OR21, -SR21, -N(R21)2, -C(O)R20, -C(O)N(R21)2, -N(R21)C(O)R20, -C(O)OR21, -OC(O)R21, - S(O)R20, -S(O)2R21, -S(O)2N(R21)2, -OC(O)OR21, -OC(O)N(R21)2, -NR21C(=O)OR21,-N(R21)C(O)N(R21)2, - NO2, =O, =S, =N(R21), -CN, a C3-C10 carbocycle, and a 3- to 10- membered heterocycle wherein said C3- C10 carbocycle and said 3- to 10-membered heterocycle are optionally substituted with one or more RX; and a C3-C10 carbocycle and a 3- to 10-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from halogen, -OR20, -OH, -SR20, -SH, -N(R21)2, - C(O)R20, -C(O)N(R21)2, -N(R21)C(O)R20, -C(O)OR21, -OC(O)R21, -S(O)R20, -S(O)2R21, -S(O)2N(R21)2, - OC(O)OR21, -OC(O)N(R21)2, -NR21C(=O)OR21, -N(R21)C(O)N(R21)2, -NO2, =O, =S, =N(R21), -CN, -C2- C6 alkenyl, -C2-C6 alkynyl and C1-C6 alkyl wherein said C1-C6 alkyl is optionally substituted with one or more substituents independently selected from RY; R7 and R8 are independently selected from hydrogen, halogen, C1-C3 alkyl, -OH, -O-C1-C3 alkyl, and -O- C1-C3 haloalkyl, or R7 and R8 join together with the atoms to which they are attached to form a C5-C6 carbocycle or 5- or 6- membered heterocycle each of which is optionally substituted with one or more substituents independently selected from halogen, -OR31, -SR31, -N(R31)2, -NO2, -CN and -C1-C6 alkyl wherein said C1-C6 alkyl is optionally substituted with one or more substituents independently selected from halogen, -OR31, -SR31, -N(R31)2, -NO2, and–CN; Y is selected from–O- and -N(R9)- and R9 is, at each occurrence, independently selected from: hydrogen; and -C1-C6 alkyl optionally substituted with one or more substituents independently selected from halogen, -OR41, -SR41, -S(O)R40, -S(O)2R41, -S(O)2N(R41)2 -N(R41)2, -C(O)R40, -C(O)N(R41)2, - N(R41)C(O)R40, -C(O)OR41, -OC(O)R40, -NO2, and -CN; each R10, R20, and R40 is independently selected at each occurrence from: -C1-C10 alkyl, -C2-C10 alkenyl, and -C2-C10 alkynyl, each of which is optionally substituted with one or more substituents independently selected from RY; and a C3-C12 carbocycle and a 3- to 12-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from RX; each R11, R21, R31, R41, and R61 is independently selected at each occurrence from: hydrogen; -C1-C10 alkyl, -C2-C10 alkenyl, and -C2-C10 alkynyl, each of which is optionally substituted with one or more substituents independently selected from RY; and a C3-C12 carbocycle and a 3- to 12- membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from RX, or two R11, R21, R31, R41, or R61 on the same N atom are taken together with the N atom to which they are attached to form a N-containing heterocycle optionally substituted with RX; each RX is independently selected at each occurrence from: halogen, -OR51, -SR51, -N(R51)2, -C(O)R50, -C(O)N(R51)2, -N(R51)C(O)R50, -C(O)OR51, -OC(O)R51, - S(O)R50, -S(O)2R51, -S(O)2N(R51)2, -OC(O)OR51, -OC(O)N(R51)2, -NR51C(=O)OR51, -N(R51)C(O)N(R51)2, - NO2, =O, =S, =N(R51), -CN, -C2-C6 alkenyl, -C2-C6 alkynyl and C1-C6 alkyl wherein said C1-C6 alkyl is optionally substituted with one or more substituents independently selected from -OR51, -SR51, -N(R51)2, - C(O)R50, -C(O)N(R51)2, -N(R51)C(O)R50, -C(O)OR51, -OC(O)R51, -S(O)R50, -S(O)2R51, -S(O)2N(R51)2, - OC(O)OR51, -OC(O)N(R51)2, -NR51C(=O)OR51, -N(R51)C(O)N(R51)2, and =O; each RY is independently selected at each occurrence from: halogen, -OR51, -SR51, -N(R51)2, -C(O)R50, -C(O)N(R51)2, -N(R51)C(O)R50, -C(O)OR51, -OC(O)R51, - S(O)R50, -S(O)2R51, -S(O)2N(R51)2, -OC(O)OR51, -OC(O)N(R51)2, -NR51C(=O)OR51, -N(R51)C(O)N(R51)2, - NO2, =O, =S, =N(R51), and -CN; each R50 is independently selected at each occurrence from: -C1-C10 alkyl, -C2-C10 alkenyl, and -C2-C10 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, -OH, -CN, -NO2, -NH2, =O, =S, -O-C1-C10 alkyl, C3- C12 carbocycle, and a 3- to 12-membered heterocycle; and a C3-C12 carbocycle and a 3- to 12-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from halogen, -OH, -CN, -NO2, -NH2, =O, =S, -C1-C10 alkyl, -O-C1-C10 alkyl, and -C1-C10 haloalkyl; each R51 is independently selected at each occurrence from: hydrogen; -C1-C10 alkyl, -C2-C10 alkenyl, and -C2-C10 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, -OH, -CN, -NO2, -NH2, =O, =S, -O-C1- C10 alkyl, C3-C12 carbocycle, and a 3- to 12-membered heterocycle; and a C3-C12 carbocycle and a 3- to 12-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from halogen, -OH, -CN, -NO2, -NH2, =O, =S, -C1-C10 alkyl, -O-C1-C10 alkyl, and - C1-C10 haloalkyl; Z1, Z2, Z3, and Z4 are independently selected from N or C(H); n is selected from 1, 2, and 3; m is 0, 1, or 2; s is selected from 0 and 1; and w is selected from 0, 1, 2, 3, 4, and 5. Exemplary compounds of the foregoing formula are described in WO 2021/011834. In some embodiments, the ALK5 inhibitor is an ALK5 inhibitor described in Table 16 of WO 2021/011834. [0132] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula L’ is absent, −S−, −O−, or −NH−; A is absent, carbocycle, or heterocycle; Q3 is N or CR3; Q4 is N or CR4; Q5 is N or CR5; Q6 is N or CR6; Q7 is C or N; R1 is hydrogen, halo, C1–3 alkyl, or C1–3 haloalkyl; R2 is, at each occurrence, independently halo, C1–3 alkyl, C1–3 alkoxy, C1–3 haloalkyl, or C1-3 haloalkoxy; R3 is H, halo, C1–3alkyl, C1–3haloalkyl, or C1–3 alkoxy; R4 is H, halo, C1–3 alkyl, C1–3haloalkyl, or C1–3 alkoxy; R5 is H, halo, C1–3 alkyl, C1–3haloalkyl, or C1–3 alkoxy; R6 is H, halo, C1–3 alkyl, C1–3haloalkyl, or C1–3 alkoxy; R7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody; R9 is, at each occurrence, independently halo, −OR′, −SR′, −N(R′)2, −C(O)R′, −C(O)O−C1–6 hydroxylalkyl, −C(O)N(R′) N(R′)C(O)R′, −C(O)OR′, −OC(O)R′, −S(O)R′, −S(O)2R′, −S(O)2OR′, −C(O)NHS(O)2−C1–6 alkyl, −C(O)NHS(O)2−C3–8 cycloalkyl, −P(O)(OR′)2, −OP(O)(OR′)2, −NO2, =O, −CN, C1–4 alkyl, C2– 5 alkenyl, C2–5 alkynyl, C1-4 haloalkyl, C2–5 haloalkenyl, C2–5 haloalkynyl, carbocycle, or heterocycle; wherein R1, R2, R3, R4, R5, R6, and R9 are each substituted with 0–3 R10; R10 is, at each occurrence, independently C1–3 alkoxy or C1–3 haloalkoxy; R′ is, at each occurrence, independently hydrogen, C1–6 alkyl, C2–6 alkenyl, C2–6 alkynyl, or C1–6 haloalkyl; m is 0–3; n is 0–5; p is 1-3; and q is 0-3. Exemplary compounds of the foregoing formula are described in WO 2022/006340. In some embodiments, the ALK5 inhibitor is an ALK5 inhibitor described in Table 15, Table 16, Table 17, Table 18, or Table 19 of WO 2022/006340.
[0133] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula Q4 is N or CR4; Q5 is N or CR5; Q6 is N or CR6; Ra and Rb are each H, or Ra and Rb, together with the atoms to which they are attached, form a heterocyclic ring; R1 is H, C1-3 alkyl, or C1-3 haloalkyl; R2 is, at each occurrence, independently halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R3 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R4 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R5 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R6 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; wherein R1, R2, R3, R4, R5, and R6 are, at each occurrence, independently substituted with 0-3 R10; R7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody, an antibody construct, or a targeting moiety; R10 is, at each occurrence, independently C1-3 alkoxy or C1-3 haloalkoxy; and m is 0-3; wherein Q6 is CR6 when R1 is methyl and Ra and Rb, together with the atoms to which they are attached, form a heterocyclic ring. Exemplary compounds of the foregoing formula are described in WO 2022/076905. [0134] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula R1 is H, C1-3 alkyl, or C1-3 haloalkyl; R2 is, at each occurrence, independently halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R6 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; wherein R1, R2, and R6 are, at each occurrence, independently substituted with 0-3 R10; R7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody; R10 is, at each occurrence, independently C1-3 alkoxy or C1-3 haloalkoxy; and m is 0-3; wherein Q6 is CR6 when R1 is methyl. Exemplary compounds of the foregoing formula are described in WO 2022/076905. [0135] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula R1 is H, C1-3 alkyl, or C1-3 haloalkyl; R2 is, at each occurrence, independently halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; R6 is H, halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; wherein R1, R2, and R6 are each substituted with 0-3 R10; R7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody, an antibody construct, or a targeting moiety; R10 is, at each occurrence, independently C1-3 alkoxy or C1-3 haloalkoxy; and m is 0-3. Exemplary compounds of the foregoing formula are described in WO 2022/076905. [0136] In accordance with another aspect of the present disclosure, the ALK5 inhibitor has the formula ring B is carbocycle or heterocycle; R1 is H, C1-3 alkyl, or C1-3 haloalkyl; R2 is, at each occurrence, independently halo, C1-3 alkyl, C1-3 alkoxy, C1-3 haloalkyl, or C1-3 haloalkoxy; wherein R1 and R2 are, at each occurrence, independently substituted with 0-3 R10; R7 is a reactive moiety capable of attachment to a linker or a reactive moiety capable of attachment to an antibody, an antibody construct, or a targeting moiety; R9 is, at each occurrence, independently halo, C1-3 alkyl, C1-3 haloalkyl, C1-3 alkoxy, or C1-3 haloalkoxy; R10 is, at each occurrence, independently C1-3 alkoxy or C1-3 haloalkoxy; m is 0-3; and n is 0-5. Exemplary compounds of the foregoing formula are described in WO 2022/076905. [0137] In some embodiments, the ALK5 inhibitor is an ALK5 inhibitor described in Table 17, Table 18, Table 19, or Table 20 of WO 2022/076905. [0138] In certain embodiments, the ALK5 inhibitor is selected from any of the compounds designated A to N in Table 2 below: Table 2 Designation Structure Name - - a hy - Table 2 Designation Structure Name 3- - - Table 2 Designation Structure Name [0139] n urt er spec c embodments, t e 5n btors seected rom any o t e compounds designated 1 to 283 in Table 3A below: Table 3A Table 3A Designation Compound Name ) )- yl) yl) Table 3A Designation Compound Name ) ne Table 3A Designation Compound Name de - - e Table 3A Designation Compound Name Table 3A Designation Compound Name Table 3A Designation Compound Name - - - Table 3A Designation Compound Name - - le - - - - - - Table 3A Designation Compound Name - - - Table 3A Designation Compound Name 7- ol- - Table 3A Designation Compound Name e e e Table 3A Designation Compound Name u e spec c e o e s, e o s seece o a y o e co pou s designated 1 to 463 in Table 3B below: Table 3B Table 3B Designation Compound 4- 3- 2- Table 3B Designation Compound ol- l- Table 3B Designation Compound 2- Table 3B Designation Compound - 2- - 5- - - 3- 4- Table 3B Designation Compound 3- 4- 2- 2- 2- 2- 2- 2- Table 3B Designation Compound 2- 2- 2- 2- 2- Table 3B Designation Compound 2- 2- 2- 3- 3- 3- 2- 2- 2- 3- 3- 3- Table 3B Designation Compound - e Table 3B Designation Compound - l- - e Table 3B Designation Compound - l- Table 3B Designation Compound - )- Table 3B Designation Compound l- - l- l- l- Table 3B Designation Compound - - - Table 3B Designation Compound 2- - Table 3B Designation Compound 2- 2- 2- l- Table 3B Designation Compound 2- H- l- l- l- l- Table 3B Designation Compound l- l- H- Table 3B Designation Compound l- 2- Table 3B Designation Compound - ol- ol- ol- ol- ol- l- Table 3B Designation Compound ol- ol- ol- ol- H- - - - l- - - Table 3B Designation Compound ol- ol- H- H- - l- Table 3B Designation Compound ol- - - - Table 3B Designation Compound 2- 2- - Table 3B Designation Compound - - Table 3B Designation Compound - - Table 3B Designation Compound H- H- - - H- [0141] The preparation and use of ALK5 inhibitors is well-known and well-documented in the scientific and patent literature. PCT publication no. WO 2000/61576 and U.S. patent publication no. US 2003/0149277 disclose triarylimidazole derivatives and their use as ALK5 inhibitors. PCT publication no. WO 2001/62756 discloses pyridinylimidazole derivatives and their use as ALK5 inhibitors. PCT publication no. WO 2002/055077 discloses use of imidazolyl cyclic acetal derivatives as ALK5 inhibitors. PCT publication no. WO 2003/087304 discloses tri-substituted heteroaryls and their use as ALK5 and/or ALK4 inhibitors. WO 2005/103028, U.S. patent publication no. US 2008/0319012 and U.S. patent no. 7,407,958 disclose 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors. One of the representative compounds, IN-1130, shows ALK5 and/or ALK4 inhibitor activity in several animal models. The following patents and patent publications provide additional examples of ALK5 inhibitors and provide illustrative synthesis schemes and methods of using ALK5 inhibitors: U.S. patent nos.6,465,493; 6,906,089; 7,365,066; 7,087,626; 7,368,445; 7,265,225; 7,405,299; 7,407,958; 7,511,056; 7,612,094; 7,691,865; 7,863,288; 8,410,146; 8,410,146; 8,420,685; 8,513,2228,614,226; 8,791,113; 8,815,893; 8,846,931;8,912,216; 8,987,301; 9,051,307; 9,051,318; 9,073,918 and PCT publication nos. WO 2004/065392; WO 2009/050183; WO 2009/133070; WO 2011/146287; WO 2013/009140; WO 2019/195278, WO 2021/011834, WO 2022/006340, and WO 2022/076905. The foregoing patents and patent publications are incorporated by reference in their entirety. [0142] Several ALK5 inhibitors are commercially available, including SB-525334 (CAS 356559-20-1), SB-505124 (CAS 694433-59-5), SB-431542 (CAS 301836-41-9), SB-202474 (EMD4 Biosciences Merck KGaA, Darmstadt, Germany), LY-364947 (CAS 396129-53-6), IN-1130, GW-788388 and D4476 (EMD4 Biosciences Merck KGaA, Darmstadt, Germany). [0143] The structures and names of ALK5 inhibitors described herein refer to the molecule prior to the attachment to the antibody and/or linker. [0144] Preferred ALK5 inhibitors are those which can be attached to a linker via a free NH or NH2 group, preferably an NH or NH2 group attached to or part of an alkyl, heteroaryl, or aryl group (e.g., as in Compounds 1-23, 26-29, 31, 35, 37, 39, 40, 42, 43, 45-48, 50-85, 87-90, 93, 96, 98-104, 106, 108, 109, 111, 112, 114, 116-120, 132, 146, 149, 156, 184, 187, 193, 218, 260-277, 282, and 283 shown in Table 3A). ALK5 inhibitors can be derivatized to add a free NH or NH2 group. Design of derivatized ALK5 inhibitors should preferably take into account the inhibitors’ structure activity relationships (SAR) to reduce the likelihood of abolishing inhibitory activity when adding an NH or NH2 group, although the activity may also be determined empirically. Exemplary derivatized counterparts of several compounds shown in Table 2 are shown below in Table 4. Table 4 Table 2 Derivative 1 Derivative 2
6.4. Linkers [0145] Typically, the conjugates comprise a linker between the ALK5 inhibitor and the antibody. Linkers are moieties comprising a covalent bond or a chain of atoms that covalently attaches an antibody to a drug moiety. In various embodiments, linkers include a divalent radical such as an alkyldiyl, an aryldiyl, a heteroaryldiyl, moieties such as:-(CR2)nO(CR2)n-, repeating units of alkyloxy (e.g., polyethylenoxy, PEG, polymethyleneoxy) and alkylamino (e.g., polyethyleneamino, Jeffamine™); and diacid ester and amides including succinate, succinamide, diglycolate, malonate, and caproamide. For example, various PEG containing linkers are known in the art and commercially available (e.g., from BroadPharm (broadpharm.com). Exemplary PEG containing linkers include Mal-PEG2-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23203), Mal-PEG4-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23204), Mal-PEG4-Val-Cit- PAB-PNP (BroadPharm cat. no. BP-23668), Mal-amido-PEG2-Val-Cit-PAB-PNP (BroadPharm cat. no. BP-23675), Azido-PEG3-Val-Cit-PAB-OH (BroadPharm cat. no. BP-23206), Azido-PEG4-Val-Cit-PAB- OH (BroadPharm cat. no. BP-23207), Azido-PEG3-Val-Cit-PAB-PNP (BroadPharm cat. no. BP-23368), Fmoc-PEG4-Ala-Ala-Asn-PAB (BP-23328), Azido-PEG5-Ala-Ala-Asn-PAB (BroadPharm cat. no. BP- 23329), Fmoc-PEG3-Ala-Ala-Asn(Trt)-PAB (BroadPharm cat no. BP-23285), Azido-PEG4-Ala-Ala- Asn(Trt)-PAB (BroadPharm cat no. BP-23284), and Fmoc-PEG3-Ala-Ala-Asn(Trt)-PAB-PNP (BroadPharm cat no. BP-23297). [0146] A linker may comprise one or more linker components, such as stretcher and spacer moieties. For example, a peptidyl linker can comprise a peptidyl component of two or more amino acids and, optionally, one or more stretcher and/or spacer moieties. Various linker components are known in the art, some of which are described below. [0147] A linker may be a “cleavable linker,” facilitating release of a drug in the cell. For example, an acid- labile linker (e.g., hydrazone), protease-sensitive (e.g., peptidase-sensitive) linker, photolabile linker, dimethyl linker or disulfide-containing linker (Chari et al., 1992, Cancer Research 52:127-131; U.S. patent no.5,208,020) may be used. [0148] Examples of linkers and linker components known in the art include aleimidocaproyl (mc); maleimidocaproyl-p-aminobenzylcarbamate; maleimidocaproyl-peptide-aminobenzylcarbamate linkers, e.g., maleimidocaproyl-L-phenylalanine-L-lysine-p-aminobenzylcarbamate and maleimidocaproyl-L- valine-L-citrulline-p-aminobenzylcarbamate (vc); N-succinimidyl 3-(2-pyridyldithio)proprionate (also known as N-succinimidyl 4-(2-pyridyldithio)pentanoate or SPP); 4-succinimidyl-oxycarbonyl-2-methyl-2-(2- pyridyldithio)-toluene (SMPT); N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP); N-succinimidyl 4-(2- pyridyldithio)butyrate (SPDB); 2-iminothiolane; S-acetylsuccinic anhydride; disulfide benzyl carbamate; carbonate; hydrazone linkers; N-(α-Maleimidoacetoxy)succinimide ester; N-[4-(p- Azidosalicylamido)butyl]-3'-(2'-pyridyldithio)propionamide (AMAS); N[β-Maleimidopropyloxy]succinimide ester (BMPS); [N-ε-Maleimidocaproyloxy]succinimide ester (EMCS); N-[γ- Maleimidobutyryloxy]succinimide ester (GMBS); Succinimidyl-4-[N-Maleimidomethyl]cyclohexane-1- carboxy-[6-amidocaproate] (LC-SMCC); Succinimidyl 6-(3-[2-pyridyldithio]-propionamido)hexanoate (LC- SPDP); m-Maleimidobenzoyl-N-hydroxysuccinimide ester (MBS); N-Succinimidyl[4- iodoacetyl]aminobenzoate (SIAB); Succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC); N-Succinimidyl 3-[2-pyridyldithio]-propionamido (SPDP); [N-ε- Maleimidocaproyloxy]sulfosuccinimide ester (Sulfo-EMCS); N-[γ-Maleimidobutyryloxy]sulfosuccinimide ester (Sulfo-GMBS); 4-Sulfosuccinimidyl-6-methyl-α-(2-pyridyldithio)toluamido]hexanoate-) (Sulfo-LC- SMPT); Sulfosuccinimidyl 6-(3'-[2-pyridyldithio]-propionamido)hexanoate (Sulfo-LC-SPDP); m- Maleimidobenzoyl-N-hydroxysulfosuccinimide ester (Sulfo-MBS); N-Sulfosuccinimidyl[4- iodoacetyl]aminobenzoate (Sulfo-SIAB); Sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1- carboxylate (Sulfo-SMCC); Sulfosuccinimidyl 4-[p-maleimidophenyl]butyrate (Sulfo-SMPB); ethylene glycol-bis(succinic acid N-hydroxysuccinimide ester) (EGS); disuccinimidyl tartrate (DST); 1,4,7,10- tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA); diethylenetriamine-pentaacetic acid (DTPA); thiourea linkers; and oxime containing linkers. [0149] In some embodiments, the linker is cleavable under intracellular or extracellular conditions, such that cleavage of the linker releases the ALK5 inhibitor from the antibody in the appropriate environment. In yet other embodiments, the linker is not cleavable and the drug is released, for example, by antibody degradation in lysosomes (see U.S. patent publication 2005/0238649 incorporated by reference herein in its entirety and for all purposes). [0150] Examples of non-cleavable linkers that can be used in the conjugates of the disclosure include N- maleimidomethylcyclohexane1-carboxylate, maleimidocaproyl or mercaptoacetamidocaproyl linkers. [0151] In some embodiments, the linker is cleavable by a cleaving agent that is present in the intracellular environment (for example, within a lysosome or endosome or caveolea). The linker can be, for example, a peptidyl linker that is cleaved by an intracellular peptidase or protease enzyme, including, but not limited to, a lysosomal or endosomal protease. In some embodiments, the peptidyl linker comprises a peptidyl component that is at least two amino acids long or at least three amino acids long or more. [0152] Cleaving agents can include, without limitation, cathepsins B and D and plasmin, all of which are known to hydrolyze dipeptide drug derivatives resulting in the release of active drug inside target cells (see, e.g., Dubowchik and Walker, 1999, Pharm. Therapeutics 83:67-123). For example, a peptidyl linker that is cleavable by the thiol-dependent protease cathepsin-B (e.g., a Phe-Leu or a Gly-Phe-Leu-Gly linker (SEQ ID NO: 106)). Other examples of such linkers are described, e.g., in U.S. patent no. 6,214,345, incorporated herein by reference in its entirety and for all purposes. [0153] In some embodiments, the peptidyl linker cleavable by an intracellular protease is a Val-Cit linker or a Phe-Lys linker (see, e.g., U.S. patent no.6,214,345, which describes the synthesis of doxorubicin with the val-cit linker). [0154] In other embodiments, the cleavable linker is pH-sensitive, that is, sensitive to hydrolysis at certain pH values. Typically, the pH-sensitive linker hydrolyzable under acidic conditions. For example, an acid-labile linker that is hydrolyzable in the lysosome (for example, a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the like) may be used. (See, e.g., U.S. patent nos.5,122,368; 5,824,805; 5,622,929; Dubowchik and Walker, 1999, Pharm. Therapeutics 83:67- 123; Neville et al., 1989, Biol. Chem.264:14653-14661.) Such linkers are relatively stable under neutral pH conditions, such as those in the blood, but are unstable at below pH 5.5 or 5.0, the approximate pH of the lysosome. In certain embodiments, the hydrolyzable linker is a thioether linker (such as, e.g., a thioether attached to the therapeutic agent via an acylhydrazone bond (see, e.g., U.S. patent no. 5,622,929). [0155] In yet other embodiments, the linker is cleavable under reducing conditions (for example, a disulfide linker). A variety of disulfide linkers are known in the art, including, for example, those that can be formed using SATA (N-succinimidyl-5-acetylthioacetate), SPDP (N-succinimidyl-3-(2- pyridyldithio)propionate), SPDB (N-succinimidyl-3-(2-pyridyldithio)butyrate) and SMPT (N-succinimidyl- oxycarbonyl-alpha-methyl-alpha-(2-pyridyl-dithio)toluene)-, SPDB and SMPT. (See, e.g., Thorpe et al., 1987, Cancer Res.47:5924-5931; Wawrzynczak et al., In Immunoconjugates: Antibody Conjugates in Radioimagery and Therapy of Cancer (C. W. Vogel ed., Oxford U. Press, 1987. See also U.S. patent no. 4,880,935.) [0156] In other embodiments, the linker is a malonate linker (Johnson et al., 1995, Anticancer Res. 15:1387-93), a maleimidobenzoyl linker (Lau et al., 1995, Bioorg-Med-Chem.3(10):1299-1304), or a 3'-N- amide analog (Lau et al., 1995, Bioorg-Med-Chem.3(10):1305-12). [0157] In some embodiments, the linker is a polyvalent linker that can be used to link many drug molecules to a single antibody molecule. For example, the Fleximer linker technology developed by Mersana is based on incorporating drug molecules into a solubilizing poly-acetal backbone via a sequence of ester bonds. The methodology enables highly-loaded conjugates (e.g., having a drug antibody ratio (DAR) up to 20) while maintaining good physicochemical properties. Exemplary polyvalent linker are described, for example, in WO 2009/073445; WO 2010/068795; WO 2010/138719; WO 2011/120053; WO 2011/171020; WO 2013/096901; WO 2014/008375; WO 2014/093379; WO 2014/093394; and WO 2014/093640, the contents of which are incorporated herein by reference in their entireties. [0158] Often the linker is not substantially sensitive to the extracellular environment. As used herein, “not substantially sensitive to the extracellular environment,” in the context of a linker, means that no more than about 20%, 15%, 10%, 5%, 3%, or no more than about 1% of the linkers, in a sample of conjugate, are cleaved when the conjugate presents in an extracellular environment (for example, in plasma). [0159] Whether a linker is not substantially sensitive to the extracellular environment can be determined, for example, by incubating with plasma the conjugate for a predetermined time period (for example, 2, 4, 8, 16, or 24 hours) and then quantitating the amount of free drug present in the plasma. [0160] In other, non-mutually exclusive embodiments, the linker can promote cellular internalization. In certain embodiments, the linker promotes cellular internalization when conjugated to the therapeutic agent (that is, in the milieu of the linker-therapeutic agent moiety of the conjugate as described herein). In yet other embodiments, the linker promotes cellular internalization when conjugated to both the ALK5 inhibitor and the antibody. [0161] In many embodiments, the linker is self-immolative. As used herein, the term “self-immolative” refers to a bifunctional chemical moiety that is capable of covalently linking together two spaced chemical moieties into a stable tripartite molecule. It will spontaneously separate from the second chemical moiety if its bond to the first moiety is cleaved. See for example, PCT publication nos. WO 2007/059404, WO 2006/110476, WO 2005/112919, WO 2010/062171, WO 2009/017394, WO 2007/089149, WO 2007/018431, WO 2004/043493 and WO 2002/083180, which are directed to drug-cleavable substrate conjugates where the drug and cleavable substrate are optionally linked through a self-immolative linker and which are all expressly incorporated by reference. Examples of self-immolative spacer units that can be used to generated self-immolative linkers are described under Formula I below. [0162] A variety of exemplary linkers that can be used with the present compositions and methods are described in PCT publication no. WO 2004/010957, U.S. patent publication no. US 2006/0074008, U.S. patent publication no. US 2005/0238649, and U.S. patent publication no. US 2006/0024317 (each of which is incorporated by reference herein in its entirety and for all purposes). [0163] An conjugate of the disclosure may be of Formula I, below, wherein an antibody (Ab) is conjugated to one or more ALK5 inhibitor drug moieties (D) through an optional linker (L) Ab-(L-D)pI [0164] Accordingly, the antibody may be conjugated to the drug either directly or via a linker. In Formula I, p is the average number of drug (i.e., ALK5 inhibitor) moieties per antibody, which can range, e.g., from about 1 to about 20 drug moieties per antibody, and in certain embodiments, from 2 to about 8 drug moieties per antibody. Further details of drug loading are described in Section 6.5 below. [0165] In some embodiments, a linker component may comprise a “stretcher” that links an antibody e.g., via a cysteine residue, to another linker component or to a drug moiety. Exemplary stretchers are shown below (wherein the left wavy line indicates the site of covalent attachment to an antibody and the right wavy line indicates the site of covalent attachment to another linker component or drug moiety):
e Chem.21:5-13. [0166] In some embodiments, a linker component may comprise an amino acid unit. In one such embodiment, the amino acid unit allows for cleavage of the linker by a protease, thereby facilitating release of the drug from the conjugate upon exposure to intracellular proteases, such as lysosomal enzymes. See, e.g., Doronina et al., 2003, Nat. Biotechnol.21:778-784. Exemplary amino acid units include, but are not limited to, a dipeptide, a tripeptide, a tetrapeptide, and a pentapeptide. Exemplary dipeptides include: valine-citrulline (VC or val-cit), alanine-phenylalanine (AF or ala-phe); phenylalanine- lysine (FK or phe-lys); or N-methyl-valine-citrulline (Me-val-cit). Exemplary tripeptides include: glycine- valine-citrulline (gly-val-cit) and glycine-glycine-glycine (gly-gly-gly). An amino acid unit may comprise amino acid residues that occur naturally, as well as minor amino acids and non-naturally occurring amino acid analogs, such as citrulline amino acid units can be designed and optimized in their selectivity for enzymatic cleavage by a particular enzyme, for example, cathepsin B, C and D, or a plasmin protease. [0167] In some embodiments, a linker component may comprise a “spacer” unit that links the antibody to a drug moiety, either directly or by way of a stretcher and/or an amino acid unit. A spacer unit may be “self-immolative” or a “non-self-immolative.” A “non-self-immolative” spacer unit is one in which part or all of the spacer unit remains bound to the drug moiety upon enzymatic (e.g., proteolytic) cleavage of the conjugate. Examples of non-self-immolative spacer units include, but are not limited to, a glycine spacer unit and a glycine-glycine spacer unit. A “self-immolative” spacer unit allows for release of the drug moiety without a separate hydrolysis step. In certain embodiments, a spacer unit of a linker comprises a p-aminobenzyl unit. In one such embodiment, a p-aminobenzyl alcohol is attached to an amino acid unit via an amide bond, and a carbamate, methylcarbamate, or carbonate is made between the benzyl alcohol and a cytotoxic agent. See, e.g., Hamann et al., 2005, Expert Opin. Ther. Patents 15:1087-1103. In one embodiment, the spacer unit is p-aminobenzyloxycarbonyl (PAB). In certain embodiments, the phenylene portion of a p-amino benzyl unit is substituted with Qm, wherein Q is --C1-C8 alkyl, --O--(C1-C8 alkyl), -halogen, -nitro or -cyano; and m is an integer ranging from 0-4. Examples of self-immolative spacer units further include, but are not limited to, aromatic compounds that are electronically similar to p- aminobenzyl alcohol (see, e.g., U.S. patent publication no. US 2005/0256030), such as 2-aminoimidazol- 5-methanol derivatives (Hay et al., 1999, Bioorg. Med. Chem. Lett.9:2237) and ortho- or para- aminobenzylacetals. Spacers can be used that undergo cyclization upon amide bond hydrolysis, such as substituted and unsubstituted 4-aminobutyric acid amides (Rodrigues et al., 1995, Chemistry Biology 2:223); appropriately substituted bicyclo[2.2.1] and bicyclo[2.2.2] ring systems (Storm et al., 1972, Amer. Chem. Soc.94:5815); and 2-aminophenylpropionic acid amides (Amsberry et al., 1990, J. Org. Chem. 55:5867). Elimination of amine-containing drugs that are substituted at the a-position of glycine (Kingsbury et al., 1984, J. Med. Chem.27:1447) are also examples of self-immolative spacers useful in conjugates. [0168] In one embodiment, a spacer unit is a branched bis(hydroxymethyl)styrene (BHMS) unit as depicted below, which can be used to incorporate and release multiple drugs. wherein Ab and D are defined as above for Formula I; A is a stretcher, and a is an integer from 0 to 1; W is an amino acid unit, and w is an integer from 0 to 12; Q is --C1-C8 alkyl, --O--(C1-C8 alkyl), -halogen, - nitro or -cyano; m is an integer ranging from 0-4; n is 0 or 1; and p ranges ranging from 1 to about 20. [0169] A linker may comprise any one or more of the above linker components. In certain embodiments, a linker is as shown in brackets in the following conjugate formula:Ab–(–[Aa-Ww-Yy]-D)pII wherein Ab, A, a, W, w, D, and p are as defined in the preceding paragraph; Y is a spacer unit, and y is 0, 1, or 2; and. Exemplary embodiments of such linkers are described in U.S. patent publication no. 2005/0238649 A1, which is incorporated herein by reference. [0170] Exemplary linker components and combinations thereof are shown below in the context of conjugates of Formula II: O H NYy D PAB
[0171] Linkers components, including stretcher, spacer, and amino acid units, may be synthesized by methods known in the art, such as those described in U.S. patent publication no.2005/0238649. 6.5. Drug Loading [0172] Drug loading is represented by p and is the average number of ALK5 inhibitor moieties per antibody in a molecule. Drug loading (“p”) may be 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 or more moieties (D) per antibody, although frequently the average number is a fraction or a decimal. Generally, ALK5 inhibitor loading averages from 2 to 8 drug moieties per antibody, more preferably 2 to 4 drug moieties per antibody or 5 to 7 drug moieties per antibody. [0173] As would be understood by one of skill in the art, in many instances references to an conjugate is shorthand for a population or collection of conjugate molecules (sometimes in the context of a pharmaceutical composition), each molecule composed of an antibody covalently attached to one or more ALK5 inhibitor moieties, with the drug loading ratio representing the average drug loading in the population or collection, although the ratio on an individual molecule basis may vary from one conjugate molecule to another in the population. In some embodiments, the population or collection contains conjugate molecules comprising an antibody covalently attached to anywhere between 1 and 30 drug moieties, and in some embodiments anywhere between 1 and 20, between 1 and 15, between 2 and 12, between 2 and 8, between 4 and 15, or between 6 and 12 drug moieties. Preferably, the average in the population is as described in the preceding paragraph, e.g., 2 to 8 drug moieties per antibody, more preferably 4 to 8 drug moieties per antibody or 5 to 7 drug moieties per antibody. [0174] Some conjugate populations can be in the form of compositions comprising conjugates as described herein and antibody molecules lacking drug moieties, e.g., antibodies to which attachment of the ALK5 antibody was unsuccessful. [0175] The average number of ALK5 inhibitor moieties per antibody in preparations of conjugate from conjugation reactions may be characterized by conventional means such as mass spectroscopy and, ELISA assay. [0176] The quantitative distribution of conjugate in terms of p may also be determined. In some instances, separation, purification, and characterization of homogeneous conjugate where p is a certain value from conjugate with other ALK5 inhibitor loadings may be achieved by means such as electrophoresis. [0177] For some antibody-drug conjugates, p may be limited by the number of attachment sites on the antibody. For example, where the attachment is a cysteine thiol, as in the exemplary embodiments above, an antibody may have only one or several cysteine thiol groups, or may have only one or several sufficiently reactive thiol groups through which a linker may be attached. In certain embodiments, higher drug loading, e.g., p>5, may cause aggregation, insolubility, toxicity, or loss of cellular permeability of certain antibody-drug conjugates. In certain embodiments, the drug loading for an conjugate of the disclosure ranges from 1 to about 8; from about 2 to about 6; from about 3 to about 5; from about 3 to about 4; from about 3.1 to about 3.9; from about 3.2 to about 3.8; from about 3.2 to about 3.7; from about 3.2 to about 3.6; from about 3.3 to about 3.8; or from about 3.3 to about 3.7. Indeed, it has been shown that for certain conjugates, the optimal ratio of drug moieties per antibody may be less than 8, and may be about 2 to about 5. See U.S. patent publication no. US 2005/0238649 (herein incorporated by reference in its entirety). [0178] In certain embodiments, less than the theoretical maximum of drug moieties are conjugated to an antibody during a conjugation reaction. An antibody may contain, for example, lysine residues that do not react with the drug-linker intermediate or linker reagent, as discussed below. Generally, antibodies do not contain many free and reactive cysteine thiol groups which may be linked to a drug moiety; indeed most cysteine thiol residues in antibodies exist as disulfide bridges. In certain embodiments, an antibody may be reduced with a reducing agent such as dithiothreitol (DTT) or tricarbonylethylphosphine (TCEP), under partial or total reducing conditions, to generate reactive cysteine thiol groups. In certain embodiments, an antibody is subjected to denaturing conditions to reveal reactive nucleophilic groups such as lysine or cysteine. [0179] The loading (drug/antibody ratio) of an conjugate may be controlled in different ways, e.g., by:(i) limiting the molar excess of drug-linker intermediate or linker reagent relative to antibody, (ii) limiting the conjugation reaction time or temperature, (iii) partial or limiting reductive conditions for cysteine thiol modification, (iv) engineering by recombinant techniques the amino acid sequence of the antibody such that the number and position of cysteine residues is modified for control of the number and/or position of linker-drug attachments (such as thioMab or thioFab prepared as disclosed in PCT publication no. WO 2006/034488 (herein incorporated by reference in its entirety)). [0180] It is to be understood that where more than one nucleophilic group reacts with a drug-linker intermediate or linker reagent followed by drug moiety reagent, then the resulting product is a mixture of conjugate compounds with a distribution of one or more drug moieties attached to an antibody. The average number of drugs per antibody may be calculated from the mixture by a dual ELISA antibody assay, which is specific for antibody and specific for the drug. Individual conjugate molecules may be identified in the mixture by mass spectroscopy and separated by HPLC, e.g. hydrophobic interaction chromatography. [0181] In some embodiments, a homogeneous conjugate with a single loading value may be isolated from the conjugation mixture by electrophoresis or chromatography. 6.6. Nucleic Acids, Recombinant Vectors and Host Cells [0182] The present disclosure provides nucleic acid molecules encoding immunoglobulin light and heavy chain genes for anti-CD5 antibodies and antigen binding fragments thereof (e.g., a VL and VL pair), vectors comprising such nucleic acids, and host cells capable of producing the anti-CD5 antibodies and antigen binding fragments thereof of the disclosure. In certain aspects, the nucleic acid molecules encode, and the host cells are capable of expressing, the anti-CD5 antibodies and antibody-binding fragments of the disclosure. [0183] An anti-CD5 antibody of the disclosure can be prepared by recombinant expression of immunoglobulin light and heavy chain genes in a host cell. To express an antibody recombinantly, a host cell is transfected with one or more recombinant expression vectors carrying DNA fragments encoding the immunoglobulin light and heavy chains of the antibody such that the light and heavy chains are expressed in the host cell and, optionally, secreted into the medium in which the host cells are cultured, from which medium the antibodies can be recovered. Standard recombinant DNA methodologies are used to obtain antibody heavy and light chain genes, incorporate these genes into recombinant expression vectors and introduce the vectors into host cells, such as those described in Molecular Cloning; A Laboratory Manual, Second Edition (Sambrook, Fritsch and Maniatis (eds), Cold Spring Harbor, N. Y., 1989), Current Protocols in Molecular Biology (Ausubel, F. M. et al., eds., Greene Publishing Associates, 1989) and in U.S. Pat. No.4,816,397. [0184] DNA encoding a VH region can be converted to a full-length heavy chain gene by operatively linking the VH-encoding DNA to another DNA molecule encoding heavy chain constant regions (CH1, CH2, CH3 and, optionally, CH4). The sequences of human heavy chain constant region genes are known in the art (See, e.g., Kabat et al., 1991, Sequences of Proteins of Immunological Interest, Fifth Edition, U.S. Department of Health and Human Services, NIH Publication No.91-3242) and DNA fragments encompassing these regions can be obtained by standard PCR amplification. The heavy chain constant region can be an IgG1, IgG2, IgG3, IgG4, IgA, IgE, IgM or IgD constant region, but in certain embodiments is an IgG1 or IgG4 constant region. [0185] DNA encoding a VL region can be converted to a full-length light chain gene (as well as a Fab light chain gene) by operatively linking the VL-encoding DNA to another DNA molecule encoding the light chain constant region, CL. The sequences of human light chain constant region genes are known in the art (See, e.g., Kabat et al., 1991, Sequences of Proteins of Immunological Interest, Fifth Edition, U.S. Department of Health and Human Services, NIH Publication No.91-3242) and DNA fragments encompassing these regions can be obtained by standard PCR amplification. The light chain constant region can be a kappa or lambda constant region, but in certain embodiments is a kappa constant region. [0186] To express the anti-CD5 antibodies of the disclosure, DNAs encoding partial or full-length light and heavy chains, obtained as described above, are inserted into expression vectors such that the genes are operatively linked to transcriptional and translational control sequences. In this context, the term "operatively linked" is intended to mean that an antibody gene is ligated into a vector such that transcriptional and translational control sequences within the vector serve their intended function of regulating the transcription and translation of the antibody gene. The expression vector and expression control sequences are chosen to be compatible with the expression host cell used. The antibody light chain gene and the antibody heavy chain gene can be inserted into separate vectors or, more typically, both genes are inserted into the same expression vector. [0187] The antibody genes are inserted into the expression vector by standard methods (e.g., ligation of complementary restriction sites on the antibody gene fragment and vector, or blunt end ligation if no restriction sites are present). Prior to insertion of the anti-CD5 antibody-related light or heavy chain sequences, the expression vector can already carry antibody constant region sequences. For example, one approach to converting the anti-CD5 monoclonal antibody-related VH and VL sequences to full-length antibody genes is to insert them into expression vectors already encoding heavy chain constant and light chain constant regions, respectively, such that the VH segment is operatively linked to the CH segment(s) within the vector and the VL segment is operatively linked to the CL segment within the vector. Additionally or alternatively, the recombinant expression vector can encode a signal peptide that facilitates secretion of the antibody chain from a host cell. The antibody chain gene can be cloned into the vector such that the signal peptide is linked in-frame to the amino terminus of the antibody chain gene. The signal peptide can be an immunoglobulin signal peptide or a heterologous signal peptide (i.e., a signal peptide from a non-immunoglobulin protein). Exemplary signal peptides include MGSTAILGLLLAVLQGGRA (SEQ ID NO:60) and METDTLLLWVLLLWVPGSTGAS (SEQ ID NO:61). [0188] In addition to the antibody chain genes, the recombinant expression vectors of the disclosure carry regulatory sequences that control the expression of the antibody chain genes in a host cell. The term “regulatory sequence” is intended to include promoters, enhancers and other expression control elements (e.g., polyadenylation signals) that control the transcription or translation of the antibody chain genes. Such regulatory sequences are described, for example, in Goeddel, Gene Expression Technology: Methods in Enzymology 185, Academic Press, San Diego, Calif., 1990. It will be appreciated by those skilled in the art that the design of the expression vector, including the selection of regulatory sequences may depend on such factors as the choice of the host cell to be transformed, the level of expression of protein desired, etc. Suitable regulatory sequences for mammalian host cell expression include viral elements that direct high levels of protein expression in mammalian cells, such as promoters and/or enhancers derived from cytomegalovirus (CMV) (such as the CMV promoter/enhancer), Simian Virus 40 (SV40) (such as the SV40 promoter/enhancer), adenovirus, (e.g., the adenovirus major late promoter (AdMLP)) and polyoma. For further description of viral regulatory elements, and sequences thereof, see, e.g., U.S. Pat. No.5,168,062 by Stinski, U.S. Pat. No.4,510,245 by Bell et al., and U.S. Pat. No.4,968,615 by Schaffner et al. [0189] It is possible to express the antibodies of the disclosure in either prokaryotic or eukaryotic host cells. In certain embodiments, expression of antibodies is performed in eukaryotic cells, e.g., mammalian host cells, of optimal secretion of a properly folded and immunologically active antibody. Exemplary mammalian host cells for expressing the recombinant antibodies of the disclosure include Chinese Hamster Ovary (CHO cells) (including DHFR- CHO cells, described in Urlaub and Chasin, 1980, Proc. Natl. Acad. Sci. USA 77:4216-4220, used with a DHFR selectable marker, e.g., as described in Kaufman and Sharp, 1982, Mol. Biol.159:601-621), NSO myeloma cells, COS cells and SP2 cells. When recombinant expression vectors encoding antibody genes are introduced into mammalian host cells, the antibodies are produced by culturing the host cells for a period of time sufficient to allow for expression of the antibody in the host cells or secretion of the antibody into the culture medium in which the host cells are grown. Antibodies can be recovered from the culture medium using standard protein purification methods. Host cells can also be used to produce portions of intact antibodies, such as Fab fragments or scFv molecules. It is understood that variations on the above procedure are within the scope of the present disclosure. For example, it can be desirable to transfect a host cell with DNA encoding either the light chain or the heavy chain (but not both) of an anti-CD5 antibody of this disclosure. [0190] For recombinant expression of an anti-CD5 antibody of the disclosure, the host cell can be co- transfected with two expression vectors of the disclosure, the first vector encoding a heavy chain derived polypeptide and the second vector encoding a light chain derived polypeptide. The two vectors can contain identical selectable markers, or they can each contain a separate selectable marker. Alternatively, a single vector can be used which encodes both heavy and light chain polypeptides. [0191] Once a nucleic acid encoding one or more portions of an anti-CD5 antibody, further alterations or mutations can be introduced into the coding sequence, for example to generate nucleic acids encoding antibodies with different CDR sequences, antibodies with reduced affinity to the Fc receptor, or antibodies of different subclasses. [0192] The anti-CD5 antibodies of the disclosure can also be produced by chemical synthesis (e.g., by the methods described in Solid Phase Peptide Synthesis, 2nd ed., 1984 The Pierce Chemical Co., Rockford, Ill.). Variant antibodies can also be generated using a cell-free platform (See, e.g., Chu et al., Biochemia No.2, 2001 (Roche Molecular Biologicals) and Murray et al., 2013, Current Opinion in Chemical Biology, 17:420-426). [0193] Once an anti-CD5 antibody or antigen-binding fragment thereof of the disclosure has been produced by recombinant expression, it can be purified by any method known in the art for purification of an immunoglobulin molecule, for example, by chromatography (e.g., ion exchange, affinity, and sizing column chromatography), centrifugation, differential solubility, or by any other standard technique for the purification of proteins. Further, the anti-CD5 antibodies of the present disclosure and/or binding fragments can be fused to heterologous polypeptide sequences described herein or otherwise known in the art to facilitate purification. [0194] Once isolated, the anti-CD5 antibody or antigen-binding fragment can, if desired, be further purified, e.g., by high performance liquid chromatography (see, e.g., Fisher, Laboratory Techniques In Biochemistry And Molecular Biology, Work and Burdon, eds., Elsevier, 1980), or by gel filtration chromatography on a Superdex™ 75 column (Pharmacia Biotech AB, Uppsala, Sweden). 6.7. Formulations and Administration [0195] Suitable routes of administration of the conjugates, the antibodies, and antigen binding fragments of the disclosure include, without limitation, oral, parenteral, rectal, transmucosal, intestinal administration, intramedullary, intrathecal, direct intraventricular, intravenous, intravitreal, intracavitary, intraperitoneal, or intratumoral injections. The preferred routes of administration are parenteral, more preferably intravenous. Alternatively, one may administer the conjugate, antibody, or antibody fragment in a local rather than systemic manner, for example, via injection of the conjugate, antibody, or antibody fragment directly into a solid or hematological tumor. [0196] Compositions of the disclosure can be formulated according to known methods to prepare pharmaceutically useful compositions, for example whereby a conjugate is combined in a mixture with a pharmaceutically suitable excipient. Sterile phosphate-buffered saline is one example of a pharmaceutically suitable excipient. Other suitable excipients are well-known to those in the art. See, for example, Ansel et al., Pharmaceutical Dosage Forms And Drug Delivery Systems, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition (Mack Publishing Company 1990), and revised editions thereof. [0197] In a preferred embodiment, a conjugate is formulated in Good’s biological buffer (pH 6-7), using a buffer selected from the group consisting of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES); N-(2- acetamido)iminodiacetic acid (ADA); N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES); 4-(2- hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES); 2-(N-morpholino)ethanesulfonic acid (MES); 3- (N-morpholino)propanesulfonic acid (MOPS); 3-(N-morpholinyl)-2-hydroxypropanesulfonic acid (MOPSO); and piperazine-N,N'-bis(2-ethanesulfonic acid) [Pipes]. More preferred buffers are MES or MOPS, preferably in the concentration range of 20 to 100 mM, more preferably about 25 mM. Most preferred is 25 mM MES, pH 6.5. The formulation may further comprise 25 mM trehalose and 0.01% v/v polysorbate 80 as excipients, with the final buffer concentration modified to 22.25 mM as a result of added excipients. The preferred method of storage is as a lyophilized formulation of the conjugates, stored in the temperature range of -20°C to 2°C, with the most preferred storage at 2°C to 8°C. [0198] A conjugate can be formulated for intravenous administration via, for example, bolus injection, slow infusion or continuous infusion. Preferably, the conjugate is infused over a period of less than about 4 hours, and more preferably, over a period of less than about 3 hours. For example, the first 25-50 mg could be infused within 30 minutes, preferably even 15 min, and the remainder infused over the next 2-3 hrs. Formulations for injection can be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative. The compositions can take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing and/or dispersing agents. Alternatively, the active ingredient can be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use. [0199] Additional pharmaceutical methods may be employed to control the duration of action of the conjugate. Control release preparations can be prepared through the use of polymers to complex or adsorb the conjugate. For example, biocompatible polymers include matrices of poly(ethylene-co-vinyl acetate) and matrices of a polyanhydride copolymer of a stearic acid dimer and sebacic acid. Sherwood et al., 1992, Bio/Technology 10:1446. The rate of release of an conjugate from such a matrix depends upon the molecular weight of the conjugate, the amount of conjugate within the matrix, and the size of dispersed particles. Saltzman et al., 1989, Biophys. J.55:163; Sherwood et al., supra. Other solid dosage forms are described in Ansel et al., Pharmaceutical Dosage Forms And Drug Delivery Systems, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition (Mack Publishing Company 1990), and revised editions thereof. [0200] Generally, the dosage of an administered conjugate for humans will vary depending upon such factors as the patient’s age, weight, height, sex, general medical condition and previous medical history. It may be desirable to provide the recipient with a dosage of conjugate that is in the range of from about 0.3 mg/kg to 5 mg/kg as a single intravenous infusion, although a lower or higher dosage also may be administered as circumstances dictate. A dosage of 0.3-5 mg/kg for a 70 kg patient, for example, is 21- 350 mg, or 12-206 mg/m2 for a 1.7-m patient. The dosage may be repeated as needed, for example, once per week for 2-10 weeks, once per week for 8 weeks, or once per week for 4 weeks. It may also be given less frequently, such as every other week for several months, or monthly or quarterly for many months, as needed in a maintenance therapy. Preferred dosages may include, but are not limited to, 0.3 mg/kg, 0.5 mg/kg, 0.7 mg/kg, 1.0 mg/kg, 1.2 mg/kg, 1.5 mg/kg, 2.0 mg/kg, 2.5 mg/kg, 3.0 mg/kg, 3.5 mg/kg, 4.0 mg/kg, 4.5 mg/kg, and 5.0 mg/kg. More preferred dosages are 0.6 mg/kg for weekly administration and 1.2 mg/kg for less frequent dosing. Any amount in the range of 0.3 to 5 mg/kg may be used. The dosage is preferably administered multiple times, once a week. A minimum dosage schedule of 4 weeks, more preferably 8 weeks, more preferably 16 weeks or longer may be used, with the dose frequency dependent on toxic side-effects and recovery therefrom, mostly related to hematological toxicities. The schedule of administration may comprise administration once or twice a week, on a cycle selected from the group consisting of:(i) weekly; (ii) every other week; (iii) one week of therapy followed by two, three or four weeks off; (iv) two weeks of therapy followed by one, two, three or four weeks off; (v) three weeks of therapy followed by one, two, three, four or five week off; (vi) four weeks of therapy followed by one, two, three, four or five week off; (vii) five weeks of therapy followed by one, two, three, four or five week off; and (viii) monthly. The cycle may be repeated 2, 4, 6, 8, 10, or 12 times or more. [0201] Alternatively, a conjugate may be administered as one dosage every 2 or 3 weeks, repeated for a total of at least 3 dosages. Or, twice per week for 4-6 weeks. The dosage may be administered once every other week or even less frequently, so the patient can recover from any drug-related toxicities. Alternatively, the dosage schedule may be decreased, namely every 2 or 3 weeks for 2-3 months. The dosing schedule can optionally be repeated at other intervals and dosage may be given through various parenteral routes, with appropriate adjustment of the dose and schedule. 6.8. Methods of Treatment Using Conjugates of the Disclosure [0202] The conjugates of the disclosure can be used for the treatment of various cancers. The conjugates can be used as monotherapy or as part of a combination therapy regimen, for example with a standard of care agent or regimen. In some embodiments, the combination therapy comprises administering an conjugate in combination with immunotherapy, for example, checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy (e.g., autologous T cell therapy), oncolytic virus therapy, dendritic cell vaccine therapy, stimulator of interferon genes (STING) agonist therapy, toll-like receptor (TLR) agonist therapy, intratumoral CpG therapy, cytokine therapy (e.g., IL2, IL12, IFN-α, or INF-γ therapy), or a combination thereof. In some embodiments, the combination therapy comprises administering an conjugate in combination with immune preserving chemotherapy (e.g., an antimetabolite, such as 5-fluorouracil, gemcitabine, or methotrexate, an alkylating agent such as cyclophosphamide, dacarbazine, mechlorethamine, diaziquone, or temozolomide, an anthracycline such as doxorubicin or epirubicin, an antimicrotubule agent such as vinblastine, a platinum compound such as cisplatin or oxaliplatin, a taxane such as paclitaxel or docetaxel, or a topoisomerase inhibitor such as etoposide or mitoxantrone, or a vinca alkaloid such as vincristine). [0203] Examples of cancers which can be treated using the conjugates of the disclosure include but not limited to pancreatic cancer, glioblastoma, myelodysplastic syndromes, prostate cancer (e.g., castrate resistant prostate cancer), liver cancer (e.g., hepatocellular carcinoma), melanoma, breast cancers, urothelial cancers (e.g., bladder cancer, urethral cancer, and ureteral cancer), renal cancers (e.g., renal cell carcinoma and urothelial carcinoma), lung cancers (e.g., non-small cell lung cancers (NSCLC) such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, and small cell lung cancer) and colorectal cancers (e.g., adenocarcinoma, carcinoid tumors, gastrointestinal stromal tumors, and colorectal lymphoma). Additional examples of cancers which can be treated using the conjugates of the disclosure include head and neck cancers (e.g., head and neck squamous cell carcinoma (HNSCC)) and ovarian cancer. [0204] Conjugates of the disclosure can be used in combination with a checkpoint modulator (e.g., a checkpoint inhibitor), for example an agent targeting PD1, PDL1, CTLA4, TIGIT, LAG3, OX40, ICOS, GITR, CD40 or VISTA. Checkpoint modulators include antibodies and small molecules, and include inhibitors (e.g., inhibitory antibodies), for example targeting PD1, PDL1, CTLA4, TIGIT, LAG3, ICOS, or VISTA, and agonists (e.g., agonist antibodies), for example targeting OX40, GITR, or CD40. [0205] Exemplary checkpoint modulators targeting PD1 include pembrolizumab, nivolumab, cemiplimab, and dostarlimab. Exemplary checkpoint modulatorstargeting PDL1 include atezolizumab, avelumab, durvalumab, BMS-1001, and BMS-1166. An exemplary checkpoint modulator targeting CTLA4 is ipilimumab. Exemplary checkpoint modulators targeting TIGIT include etigilimab, tiragolumab, and AB154. Exemplary checkpoint modulators targeting LAG3 include LAG525, Sym022, relatlimab, and TSR-033. Exemplary checkpoint modulators targeting OX40 include MEDI6469, PF-04518600, and BMS 986178. Exemplary checkpoint modulators targeting ICOS include MEDI-570, feladilimab, and BMS 986226. Exemplary checkpoint modulators targeting GITR include TRX-518, AMG 228, MK-4166, MEDI1873, INCAGN01876, and GWN323. Exemplary checkpoint modulators targeting CD40 include selicrelumab, CP-870,893, and APX005M. An exemplary checkpoint modulator targeting VISTA is HMBD-002. [0206] For treatment of melanomas carrying a BRAF mutation, the conjugates of the disclosure can be used in combination with drugs that specifically target the BRAF mutations, such as venurafenibm, dabrafenib and trametinib. [0207] For treatment of malignant melanomas, the conjugates of the disclosure can be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab, nivolumab, pembrolizumab, cemiplimab, or avelumab. [0208] For treatment of non-small-cell lung carcinoma (NSCLC), the conjugates of the disclosure can be used in combination with standard of care chemotherapy treatments such as cisplatin, carboplatin, paclitaxel, gemcitabine, vinorelbin, irinotecan, etoposide, or vinblastine would be included. In addition, the conjugates can be used in combination with targeted therapies, such as bevacizumab or Erbitux. In addition, the conjugates can be used in combination with a checkpoint modulator (e.g., inhibitor), such as pembrolizumab, nivolumab, cemiplimab, dostarlimab, atezolizumab, avelumab, durvalumab, or ipilimumab. [0209] For treatment of bladder cancer, the conjugates of the disclosure can be used in combination with standard of care treatments, including but not limited to cisplatin, mitomycin-C, carboplatin, docetaxel, paclitaxel, doxorubicin, 5-FU, methotrexate, vinblastine, ifosfamide, and pemetrexed. In addition, the conjugates can be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab. [0210] For treatment of renal cancer, the conjugates of the disclosure can be used in combination with standard of care treatments, for example agents that block angiogenesis and/or specific tyrosine kinases, such as sorafenib, sunitinib, temsirolimus, everolimus, pazopanib, and axitinib. In addition, the conjugates can be used in combination with a checkpoint modulator (e.g., inhibitor), such as nivolumab. [0211] For treatment of breast cancer, the conjugates of the disclosure can be used in combination with standard of care chemotherapeutic agents, such as the anthracyclines (doxorubicin or epirubicin) and the taxanes (paclitaxel or docetaxel), as well as fluorouracil, cyclophosphamide and carboplatin. In addition, the conjugates of the disclosure can be used in combination with targeted therapies. Targeted therapies for HER2/neu positive tumors include trastuzumab and pertuzumab and for estrogen receptor (ER) positive tumors include tamoxifen, toremifene and fulvestrant. In addition, the conjugates can be used in combination with a checkpoint modulator (e.g., inhibitor), such as atezolizumab. [0212] For pancreatic cancer, the conjugates of the disclosure can be used in combination with standard of care chemotherapeutic agents, such as gemcitabine, 5-fluouracil, irinotecan, oxaliplatin, paclitaxel, capecitabine, cisplatin, or docetaxel. In addition, conjugates can be used in combination with targeted therapies, such as erlotinib, which inhibits EGFR. [0213] For glioblastoma, the conjugates of the disclosure can be used in combination with standard of care chemotherapeutic agents, such as carboplatin, cyclophosphamide, etoposide, lomustine, methotrexate or procarbazine. [0214] For prostate cancer, the conjugates of the disclosure can be used in combination with standard of care chemotherapeutic agents, including docetaxel, optionally with the steroid prednisone, or cabazitaxel. In addition, the conjugates can be used in combination with a checkpoint modulator (e.g., inhibitor), such as ipilimumab. [0215] The use of an conjugate of the disclosure in combination with one or more therapies does not restrict the order in which the therapies are administered. For example, the conjugate of the disclosure can be administered before, during or after a subject is treated with one or more therapies. In some embodiments, an conjugate of the disclosure is administered prior to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks before), concomitantly with, or subsequent to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks after) treatment of a patient with another therapy (e.g., a second therapeutic agent as described above). In some embodiments, the conjugate of the disclosure is incorporated into the same regimen as a second therapeutic agent. 7. EXAMPLES 7.1. Example 1: Generation of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.1.1. Overview [0216] Antibodies against human proteins or peptides isolated from non-human animal cells (e.g., mouse anti-human CD5 antibodies) often elicit immune responses when used as therapeutics. Although humanized antibodies are considered to be less immunogenic than their parental counterparts, they may still be associated with antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), triggered by the interactions of the constant Fc regions of these antibodies with the Fc receptors on immune cells. [0217] In order to engineer humanized CD5 antibodies with low ADCC and CDC, two parental antibodies were used. The first parental antibody was a humanized anti-CD5 antibody (referred to herein as “Antibody A”)), and the second parental antibody was a mouse anti-human CD5 antibody (referred to herein as “Antibody B”). 7.1.2. Methods 7.1.2.1. Design of Constructs AB-1 to AB-4 Using Antibody A [0218] The variable region sequences of the first parental antibody (Antibody A) (VH of SEQ ID NO:49 and VL of SEQ ID NO:50) were integrated into human IgG backbones. More specifically, the heavy chain of the parental antibody was integrated into either wildtype human IgG1, an IgG1 with the mutations L234A and L235A (IgG1(LALA)), an IgG1 with an N297A mutation (IgG1(N297A), or an IgG4 with an S228P mutation (IgG4(S228P). The heavy chains with the human IgG backbones were then combined with the light chains integrated into human IgKappa. Full light chain and heavy chain sequences for the antibodies, referred to as AB-1 to AB-4, are set forth in Table 1L. 7.1.2.2. Humanization of Antibody B [0219] The parental mouse anti-human CD5 antibody (Antibody B) was humanized as follows: The amino acid sequences of the parental light and heavy chains were aligned with the human germline Ig alleles, IGKV3-11*01, IGKV3-11*02, and IGKV1-16*01. FIGS.1A-1C display amino acid residue alignment mismatches between the parental mouse antibody and the germline Ig frameworks. Next, the light and heavy chain complementarity-determining region (CDR) amino acid sequences of the parental antibody were grafted onto a human Ig framework sequence. The resulting sequence was further modified, such as by introducing back-mutations of various residues in order to retain the antigen-binding activity of the parent antibody. FIGS 2A and 2B illustrate the humanization of the parental mouse anti- human CD5 antibody VH and VL amino acid sequences into AB-5 VH and VL amino acid sequences, by using the antibody 4JLR.pdb as a reference framework. [0220] Resulting sequences were checked for confounding issues, such as N-glycosylation, interface changes, and potentially problematic proline residues. Six humanized anti-CD5 antibody VH-VL sequences were selected for further analyses. Full light chain and heavy chain sequences for the humanized antibodies, referred to as AB-5 to AB-10, are shown in Table 1L. The VH and VL were subsequently cloned into human IgG1(LALA) Fc backbones. 7.1.2.3. Production and Purification of the Humanized Anti-CD5 Antibodies [0221] All antibody constructs were expressed in Expi293 cells by transient transfection. Supernatant from transfected cells was collected and centrifuged at 8,500 rpm for 20 minutes at 4 °C and filtered via vacuum filtration using a 0.22 µm filter membrane. [0222] The antibodies in the filtered protein suspensions were purified using Protein A resin system. Briefly, each resin column was pre-equilibrated with buffer A (PBS, pH 7.4). Samples were loaded into the columns and washed with buffer D (1% Triton 114 in PBS, pH 7.4). Next, the columns were washed with buffer A and the target antibody was eluted with buffer B (0.1 M glycine, pH 3.0). Columns were neutralized using buffer C (1M Tris-HCl, pH 8.0). A small portion of solutions containing antibodies were run on a 12% SDS-PAGE. The antibodies in the remaining portion were dialyzed overnight against buffer A. The next day, the antibodies were concentrated by ultra-filtration at a 30KDa molecular cutoff at 4 °C. [0223] Endotoxin levels of the purified proteins were analyzed with the ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit (Genescript; cat. no. L00350) using antibodies that were diluted between 5- and 250- fold. 7.1.3. Results [0224] A total of four anti-CD5 antibody constructs were generated using the humanized parental antibody (Antibody A) and six anti-CD5 humanized antibody constructs were generated using the mouse anti-human CD5 parental antibody (Antibody B) as described in Sections 7.1.2.1 and 7.1.2.2. In addition, a negative control antibody, NC-AB, was designed and generated by integrating its VH and VL sequences into human IgG1(LALA) and human IgKappa, respectively. All anti-CD5 antibody constructs generated in this example are set forth in Table 5 below. Table 5 Anti-CD5 Construct VH/VL Isot es [0225] Antibody constructs were cloned into Expi293 cells and antibodies were produced and purified as described in Section 7.1.2.3. Exemplary SDS-PAGE images of three anti-CD5 antibodies, AB-4, AB-6, and AB-8, and the negative control antibody, NC-AB, are shown in FIGS.3A-3D. Furthermore, the purified antibodies had low endotoxin levels, which was below 1 EU/mg for all samples at the highest concentration evaluated (FIG.3E). 7.2. Example 2: Target Cell Binding of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.2.1. Overview [0226] CD5 is a surface glycoprotein expressed on T cells. In order to evaluate the antibody binding efficacy of humanized anti-CD5 antibodies, a cell binding assay using Jurkat T cells was utilized. 7.2.2. Methods 7.2.2.1. Cell Culture and Maintenance [0227] Jurkat T cells were maintained in RPMI medium supplemented with 10% FBS. EL4 cells were maintained in DMEM medium supplemented with 10% FBS. CD3+ T cells were maintained in K5 medium supplemented with 10% FBS, 1X non-essential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 55 μM 2-mercaptoethanol. [0228] All cell culture media were supplemented with 1X streptomycin-penicillin (100 μg/mL streptomycin-100 units/mL penicillin), cultured at 37°C, 5% CO2 in a humidified incubator and passaged 2 to 3 times a week. 7.2.2.2. Flow Cytometry Analysis [0229] Jurkat T cells were washed two times in FACS buffer (PBS + 1% BSA) and incubated with humanized anti-CD5 antibodies or the isotype control NC-AB diluted according to the table set forth in FIG.4A for 30 minutes on ice. After washing two times in FACS buffer, the samples were further incubated with a flurophore-conjugated secondary antibody, 30 minutes on ice, washed and acquired on a flow cytometer. Data was analyzed to yield EC50 values for each antibody evaluated. 7.2.3. Results [0230] In the first assessment, humanized anti-CD5 antibodies derived from the first parental antibody Antibody A (AB-1, AB-2, AB-3, and AB-4) were evaluated against the isotype control antibody (NC-AB). The humanized anti-CD5 antibodies AB-1, AB-2, and AB-3 displayed overlapping concentration-response curves, indicating comparable target binding kinetics (FIG.4B). The maximum MFI of AB-4 was lower than that of the other three anti-CD5 antibodies; yet, the EC50 values of all four antibodies were similar (FIG.4B and Table 6). Table 6 Antibody EC50 (µg/mL) [0231] Next, Jurkat T cell binding of humanized anti-CD5 antibodies derived from the parental mouse anti-hCD5 antibody Antibody B was compared to the Jurkat T cell binding of AB-1. The concentration- response curves for the antibodies AB-5 to AB-10 indicated that they displayed higher target binding than AB-1 (FIG.4C). [0232] Similar results were observed when the assessment was repeated with CD3+ T cells, wherein the binding of AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10 were compared to those of AB-1 and AB-2 (FIG. 4D). In sum, humanized anti-CD5 antibodies (AB-5 – AB-10) derived from the Antibody B displayed EC50 values nearly a magnitude lower than the EC50 values of AB-1 and AB-2 (Table 7). Table 7 Antibody Jurkat T cell CD3+ T cell EC50 EC50 (µg/mL) (µg/mL) [0233] Next, the spec c y o e parena mouse an - an o y n o y was assessed using Jurkat T cells and mouse EL4 cells. The parental antibody bound to Jurkat T cells with an EC50 value of 0.08 but it did not bind to mouse EL4 cells (FIGS.4E and 4F), indicating high specificity for the human CD5. 7.3. Example 3: Internalization of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.3.1. Overview [0234] Identifying antibodies that internalize into cells efficiently, rather than just binding to antigens on the cell surface, is essential to develop therapeutic antibodies that can mediate receptor endocytosis. 7.3.2. Methods [0235] A flow cytometry-based antibody internalization assay was used to evaluate endocytosis of the antibodies. Briefly, Jurkat cells were incubated with 1 µg/mL of an antibody, washed, and incubated with a secondary antibody, R-Phycoerythrin AffiniPure F(ab’)2 Fragment goat anti-human IgG (Jackson). Fluorescence intensity was measured at the start of the assessment (0hr) and at 4 and 6 hours. 7.3.3. Results [0236] In the first assessment, internalization of the humanized anti-CD5 antibodies AB-1, AB-2, AB-3, and AB-4 were evaluated against the isotype control antibody, NC-AB. All four anti-CD5 antibodies displayed similar decreases in signal intensity (FIG.5A), which was associated with comparable internalization rates (FIG.5B). [0237] Next, internalization of the humanized anti-CD5 antibodies AB-5, AB-6, AB-7, AB-8, AB-9, AB-10, and AB-1 was evaluated. The changes in signal intensity and internalization rates were similar for AB-1 in both assessments (FIGS.5A and 5C and FIGS.5B and 5D, respectively), thus enabling the comparison of internalization kinetics of the antibodies derived from the first parental antibody (Antibody A) to those derived from the parental mouse anti-hCD5 antibody (Antibody B). Similar to the findings of the first assessment, most antibodies displayed similar changes in signal intensity (FIG.5C) and internalization rates (FIG.5D). Overall, humanized anti-CD5 antibodies displayed a median internalization of 38% (Table 8). Table 8 Antibody Percent Antibody Percent Internalization Internalization ating fluorescence signal intensity at 0, 2 and 6 hours. This time, AB-4 was associated with a 44% and AB-8 was associated with a 53% reduction of surface CD5 binding at 6 hours, further supporting efficient internalization of humanized anti-CD5 antibodies derived from both parental antibodies. 7.4. Example 4: ADCC Assessment of Humanized Anti-CD5 Antibodies with Human IgG Backbones 7.4.1. Overview [0239] Fc regions of humanized antibodies are often associated with antibody-dependent cellular cytotoxicity (ADCC). Therefore, humanized anti-CD5 antibodies comprising human IgG chains with low ADCC activity, such as IgG1-LALA, can help overcome this limitation. 7.4.2. Methods [0240] An ADCC assay was developed to evaluate ADCC effects of anti-CD5 antibodies. Briefly, NK cells were isolated from PBMCs and co-cultured with Jurkat T cells (4 x 104 cells/well) at an effector: target cell ratio (E:T) of 5:1. Humanized anti-CD5 antibodies, as well as the parental mouse anti-hCD5 antibody, a positive control antibody (Anti-human MHC Class I antibody) and a negative control antibody (NC-AB) were diluted to a concentration of 30 µg/mL and added to wells. The plates were incubated for 4 hours at 37 °C, 5% CO2. Cells were incubated with CytoTox 96® reagent for 30 minutes at room temperature before data collection on a plate reader at 490nm. 7.4.3. Results [0241] As expected, the positive control antibody, anti-human MHC Class I antibody, was associated with strong cytotoxicity (FIG.6). AB-3 was the only antibody with a wildtype IgG1 backbone included in this assessment and none of the humanized anti-CD5 antibodies except AB-3 displayed measurable ADCC, as the percent cytotoxicity levels associated with these antibodies were indistinguishable from the cell death rates in no antibody controls (FIG.6). These results indicate that, relative to wildtype IgG1 backbone, IgG1-LALA, IgG1-N297A and IgG4 backbones protected against ADCC. 7.5. Example 5: Anti-CD5 Antibody Binding to Human or Rhesus CD5-HEK293 Cells 7.5.1. Overview [0242] Binding of anti-CD5 antibodies Antibody B and Antibody C (mouse parental antibody to AB-11 antibody sequences) to HEK cells expressing human or rhesus monkey CD5 was evaluated. 7.5.2. Methods [0243] Human embryonic kidney cells (HEK-293, ATCC), were plated on 10 cm2 tissue culture dishes. When the cells reached 70% confluency, they were transiently transfected with either a human or rhesus monkey CD5-containing DNA plasmid. In brief, Mirus Transit®-LT1, a broad-spectrum, low toxicity transfection reagent was combined with 1 μg plasmid DNA in a low serum cell culture medium (Opti- MEMTM). The mixture was incubated for 30 minutes at room temperature and then added drop-wise to the HEK-293 cells. After 48 hours, the transfected cells were gently detached from the plates with Accutase® cell detachment solution, washed 2X in ice-cold stain buffer (1X dPBS, 1% FBS, 0.05% sodium azide) and counted.1.5 x 105 cells were incubated with either 7.5 μg/mL Antibody C-PE (phycoerythrin) conjugate or Antibody B-PE in 100 μL stain buffer. After 60 minutes on ice, the cells were washed 2X in stain buffer to completely remove unbound antibody. The cells were acquired on an AttuneTM NxT acoustic cytometer and analysis was completed using FlowJoTM software. 7.5.3. Results [0244] Flow cytometry plots are shown in FIG.7A-7C, while percent positive CD5+ cells as measured by antibody binding and mean fluorescence intensities are shown in Tables 9A and 9B, respectively. Table 9A Antibody HEK293 HEK293-Human CD5 HEK293-Rhesus CD5 Table 9B A ib d HEK2 HEK2 H CD HEK2 Rh CD [0245] Antibody B bound to human CD5 expressing HEK293 cells, while Antibody C bound to both human and rhesus CD5 expressing cells. 7.6. Example 6: Internalization of AB-4, AB-6, and AB-8 [0246] Internalization of humanized antibodies AB-4, AB-6, and AB-8 following binding to CD5 expressing Jurkat cells was studied. Briefly, antibodies were incubated with Jurkat cells for two to six hours, and antibody internalization was measured by FACS staining. [0247] Results are shown in FIGS.8A-8C. AB-4, AB-6, and AB-8 were each internalized. 7.7. Example 7: Humanized Antibodies with Human IgG4 Fc 7.7.1. Overview [0248] Fc regions of humanized anti-human CD5 antibodies AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10, and mouse anti-human CD5 Antibody B were substituted with human IgG4 Fc regions having a S228P substitution. Binding to Jurkat cells was assessed. 7.7.2. Methods [0249] Serial dilutions of anti-CD5 antibodies and isotype control antibody were prepared and incubated with Jurkat cells at 4 °C for 60 minutes. Cells were then washed three times. PE-goat anti-human IgG secondary antibody was added to the cells, and incubated for 30 minutes at 4 °C. Cells were washed and analyzed by FACS. 7.7.3. Results [0250] Dose response curves for antibody binding to Jurkat cells are shown in FIG.9. EC50 values are shown in Table 10. Table 10 Antibody EC50 (nM) 7.8. Example 8: Int s with Human IgG1 or IgG4 Fc 7.8.1. Overview [0251] Internalization of (1) AB-5, AB-6, AB-7, AB-8, AB-9, and AB-10 and (2) AB-5, AB-6, AB-7, AB-8, AB-9, AB-10, and mouse anti-human CD5 Antibody B having human IgG4 Fc regions having a S228P substitution following binding to Jurkat cells was assessed. 7.8.2. Methods [0252] Log phase Jurkat cells (2E5/sample) were washed in cell culture medium, and plated in a 96-well U-bottom plate in cell culture medium. Antibodies at 1 μg/ml were incubated with the cells at 4 °C for 60 minutes. Cells were then washed two time and resuspended in growth medium (RPMI1640 + 10% FBS). Cells were incubated at 37 °C for 0 hours, 4 hours, or 6 hours. Cells were then washed two times in staining buffer. Secondary antibody in staining buffer (PE-goat anti-human IgG) was added and incubated for 30 minutes at 4°C. Cells were washed twice, resuspended in staining buffer and analyzed by FACS. 7.8.3. Results [0253] Results are shown in FIGS.10A-10B. All antibodies showed internalization at 4 hours and 6 hours. Percent internalization at 4 hours and 6 hours is summarized in Table 11. Table 11 Antibody Time (hours) Percent 7.9. Exam agonist-Conjugates (ATACs) [0254] Antibodies AB-4, AB-8, and Antibody B were conjugated to ALK5 inhibitor compound C (see Table 2) and evaluated in a HEK-CD5 SBE luciferase assay to monitory the activity of the TGFβ/SMAD signaling pathway following exposure to the antibody-ALK5 inhibitor conjugates. 7.9.1. Methods [0255] HEK-CD5 cells were plated in triplicate on a 96-well plate. After the cells attached, the conjugated antibodies were titrated and incubated for 18 hours in a 37°C/5% CO2 incubator. TGFβ was added to the plate and incubated for an additional 3 hours. The assay was developed using a commercially available luciferase detection reagent, the data was acquired on a luminometer and plotted using GraphPad PrismTM software. 7.9.2. Results [0256] Results are shown in FIG.11A-11B. Each conjugate was active in the assay. EC50 values are reported in Table 12. Table 12 Antibody-ALK5 inhibitor conjugate EC50 . . . [0257] Complement-dependent cytotoxicity (CDC) of antibodies and antibody-ALK5 inhibitor conjugates of the disclosure were assessed. 7.10.2. Methods [0258] T cells were isolated from human PBMCs and plated in triplicate on round-bottom 96-well plates. Antibody was added to the cells and incubated on ice for 0.5 hours. Complement from rabbit sera was then added to a final concentration of 5%. The cells were incubated for 2 hours in a 37°C/5% CO2 incubator. Propidium iodide was added to the cells and the data was acquired on a FACS instrument, analyzed using FlowJoTM software and plotted using GraphPad PrismTM. 7.10.3. Results [0259] Results are shown in FIG.12. CDC was only observed for AB-3, which has a wild-type IgG1 Fc domain. 8. SPECIFIC EMBODIMENTS [0260] The present disclosure is exemplified by the specific embodiments below. 1. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 11, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 15, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 15, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 11, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:17, 19, and 21, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 9, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (j) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 9, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (k) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 10, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (l) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 10, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (m) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 12, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; or (n) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 12, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 2. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an anti- CD5 antibody or antigen binding fragment according to embodiment 1, comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 9, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 9, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 10, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 10, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 12, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; or (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 12, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 3. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively. 4. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 11, and 14, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 5. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 15, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 6. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 15, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively. 7. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 11, and 13, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:17, 19, and 21, respectively. 8. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 9. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 10. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 16, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively. 11. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 9, and 14, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 12. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 9, and 16, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 13. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 10, and 14, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 14. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 10, and 13, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 15. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 12, and 14, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 16. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 12, and 13, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 17. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 16, which is a humanized anti-CD5 antibody or antigen binding fragment thereof. 18. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to any one of embodiments 1 to 17, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:22 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:23; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:24 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:25; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:26 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:27; (d) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:28 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:29; (e) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:30 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:31; or (f) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:32 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:33. 19. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:22 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:23. 20. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 19, wherein the VH has a sequence at least 95% identical to SEQ ID NO:22 and the VL has a sequence at least 95% identical to SEQ ID NO:23. 21. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 19, wherein the VH has a sequence at least 97% identical to SEQ ID NO:22 and the VL has a sequence at least 97% identical to SEQ ID NO:23. 22. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 19, wherein the VH has a sequence at least 99% identical to SEQ ID NO:22 and the VL has a sequence at least 99% identical to SEQ ID NO:23. 23. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 19, wherein the VH has a sequence identical to SEQ ID NO:22 and the VL has a sequence identical to SEQ ID NO:23. 24. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:24 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:25. 25. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 24, wherein the VH has a sequence at least 95% identical to SEQ ID NO:24 and the VL has a sequence at least 95% identical to SEQ ID NO:25. 26. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 24, wherein the VH has a sequence at least 97% identical to SEQ ID NO:24 and the VL has a sequence at least 97% identical to SEQ ID NO:25. 27. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 24, wherein the VH has a sequence at least 99% identical to SEQ ID NO:24 and the VL has a sequence at least 99% identical to SEQ ID NO:25. 28. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 24, wherein the VH has a sequence identical to SEQ ID NO:24 and the VL has a sequence identical to SEQ ID NO:25. 29. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:26 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:27. 30. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 29, wherein the VH has a sequence at least 95% identical to SEQ ID NO:26 and the VL has a sequence at least 95% identical to SEQ ID NO:27. 31. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 29, wherein the VH has a sequence at least 97% identical to SEQ ID NO:26 and the VL has a sequence at least 97% identical to SEQ ID NO:27. 32. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 29, wherein the VH has a sequence at least 99% identical to SEQ ID NO:26 and the VL has a sequence at least 99% identical to SEQ ID NO:27. 33. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 29, wherein the VH has a sequence identical to SEQ ID NO:26 and the VL has a sequence identical to SEQ ID NO:27. 34. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:28 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:29. 35. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 34, wherein the VH has a sequence at least 95% identical to SEQ ID NO:28 and the VL has a sequence at least 95% identical to SEQ ID NO:29. 36. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 34, wherein the VH has a sequence at least 97% identical to SEQ ID NO:28 and the VL has a sequence at least 97% identical to SEQ ID NO:29. 37. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 34, wherein the VH has a sequence at least 99% identical to SEQ ID NO:28 and the VL has a sequence at least 99% identical to SEQ ID NO:29. 38. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 34, wherein the VH has a sequence identical to SEQ ID NO:28 and the VL has a sequence identical to SEQ ID NO:29. 39. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:30 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:31. 40. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 39, wherein the VH has a sequence at least 95% identical to SEQ ID NO:30 and the VL has a sequence at least 95% identical to SEQ ID NO:31. 41. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 39, wherein the VH has a sequence at least 97% identical to SEQ ID NO:30 and the VL has a sequence at least 97% identical to SEQ ID NO:31. 42. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 39, wherein the VH has a sequence at least 99% identical to SEQ ID NO:30 and the VL has a sequence at least 99% identical to SEQ ID NO:31. 43. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 39, wherein the VH has a sequence identical to SEQ ID NO:30 and the VL has a sequence identical to SEQ ID NO:31. 44. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 18, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:32 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:33. 45. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 44, wherein the VH has a sequence at least 95% identical to SEQ ID NO:32 and the VL has a sequence at least 95% identical to SEQ ID NO:33. 46. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 44, wherein the VH has a sequence at least 97% identical to SEQ ID NO:32 and the VL has a sequence at least 97% identical to SEQ ID NO:33. 47. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 44, wherein the VH has a sequence at least 99% identical to SEQ ID NO:32 and the VL has a sequence at least 99% identical to SEQ ID NO:33. 48. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 44, wherein the VH has a sequence identical to SEQ ID NO:32 and the VL has a sequence identical to SEQ ID NO:33. 49. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:34, 39, and 43, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:35, 40, 44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:46, 47, and 48, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:36, 41, and 44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of NYG, SEQ ID NO41, and SEQ ID NO:44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively; or (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:38, 42, and 43, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOS:46, 47, and 48, respectively. 50. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49, which is a humanized anti-CD5 antibody or antigen binding fragment thereof. 51. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:34, 39, and 43, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively. 52. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:35, 40, 44, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:46, 47, and 48, respectively. 53. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:36, 41, and 44, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively. 54. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of NYG, SEQ ID NO:3741, and SEQ ID NO:44, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively. 55. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49 or embodiment 50, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:38, 42, and 43, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOS:46, 47, and 48, respectively 56. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to any one of embodiments 49 to 55, comprising a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:49 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:50. 57. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 56, wherein the VH has a sequence at least 95% identical to SEQ ID NO:49 and the VL has a sequence at least 95% identical to SEQ ID NO:50. 58. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 56, wherein the VH has a sequence at least 97% identical to SEQ ID NO:49 and the VL has a sequence at least 97% identical to SEQ ID NO:50. 59. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 56, wherein the VH has a sequence at least 99% identical to SEQ ID NO:49 and the VL has a sequence at least 99% identical to SEQ ID NO:50. 60. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 56, wherein the VH has a sequence identical to SEQ ID NO:49 and the VL has a sequence identical to SEQ ID NO:50. 61. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 88, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, 95, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, 95, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively; (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 102, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; (j) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; (k) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 102, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; or (l) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:104, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:94, 97, and 105, respectively. 62. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 88, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively. 63. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively. 64. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively. 65. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, 95, respectively. 66. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, 95, respectively. 67. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively. 68. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively. 69. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively. 70. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 102, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 71. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 72. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 102, and 89, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively. 73. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 61, wherein the VH comprises CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:104, 8, and 91, respectively, and the VL comprises CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:94, 97, and 105, respectively. 74. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 61 to 73, which is a humanized anti-CD5 antibody or antigen binding fragment thereof. 75. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to any one of embodiments 61 to 74, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (d) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (e) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (f) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (g) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (h) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (i) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (j) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (k) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (l) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; or (m) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:100 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:101. 76. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 77. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 76, wherein the VH has a sequence at least 95% identical to SEQ ID NO:84 and the VL has a sequence at least 95% identical to SEQ ID NO:92. 78. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 76, wherein the VH has a sequence at least 97% identical to SEQ ID NO:84 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 79. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 76, wherein the VH has a sequence at least 99% identical to SEQ ID NO:84 and the VL has a sequence at least 99% identical to SEQ ID NO:92. 80. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 76, wherein the VH has a sequence identical to SEQ ID NO:84 and the VL has a sequence identical to SEQ ID NO:92. 81. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 82. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 81, wherein the VH has a sequence at least 95% identical to SEQ ID NO:84 and the VL has a sequence at least 95% identical to SEQ ID NO:93. 83. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 81, wherein the VH has a sequence at least 97% identical to SEQ ID NO:84 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 84. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 81, wherein the VH has a sequence at least 99% identical to SEQ ID NO:84 and the VL has a sequence at least 99% identical to SEQ ID NO:93. 85. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 81, wherein the VH has a sequence identical to SEQ ID NO:84 and the VL has a sequence identical to SEQ ID NO:93. 86. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 87. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 86, wherein the VH has a sequence at least 95% identical to SEQ ID NO:98 and the VL has a sequence at least 95% identical to SEQ ID NO:98. 88. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 86, wherein the VH has a sequence at least 97% identical to SEQ ID NO:84 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 89. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 86, wherein the VH has a sequence at least 99% identical to SEQ ID NO:84 and the VL has a sequence at least 99% identical to SEQ ID NO:98. 90. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 86, wherein the VH has a sequence identical to SEQ ID NO:84 and the VL has a sequence identical to SEQ ID NO:98. 91. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 92. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 91, wherein the VH has a sequence at least 95% identical to SEQ ID NO:85 and the VL has a sequence at least 95% identical to SEQ ID NO:92. 93. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 91, wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 94. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 91, wherein the VH has a sequence at least 99% identical to SEQ ID NO:85 and the VL has a sequence at least 99% identical to SEQ ID NO:92. 95. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 91, wherein the VH has a sequence identical to SEQ ID NO:85 and the VL has a sequence identical to SEQ ID NO:92. 96. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 97. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 96, wherein the VH has a sequence at least 95% identical to SEQ ID NO:85 and the VL has a sequence at least 95% identical to SEQ ID NO:93. 98. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 96, wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 99. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 96, wherein the VH has a sequence at least 99% identical to SEQ ID NO:85 and the VL has a sequence at least 99% identical to SEQ ID NO:93. 100. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 96, wherein the VH has a sequence identical to SEQ ID NO:85 and the VL has a sequence identical to SEQ ID NO:93. 101. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 102. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 101, wherein the VH has a sequence at least 95% identical to SEQ ID NO:85 and the VL has a sequence at least 95% identical to SEQ ID NO:98. 103. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 101, wherein the VH has a sequence at least 97% identical to SEQ ID NO:85 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 104. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 101, wherein the VH has a sequence at least 99% identical to SEQ ID NO:85 and the VL has a sequence at least 99% identical to SEQ ID NO:98. 105. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 101, wherein the VH has a sequence identical to SEQ ID NO:85 and the VL has a sequence identical to SEQ ID NO:98. 106. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 107. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 106, wherein the VH has a sequence at least 95% identical to SEQ ID NO:86 and the VL has a sequence at least 95% identical to SEQ ID NO:92. 108. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 106, wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 109. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 106, wherein the VH has a sequence at least 99% identical to SEQ ID NO:86 and the VL has a sequence at least 99% identical to SEQ ID NO:92. 110. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 106, wherein the VH has a sequence identical to SEQ ID NO:86 and the VL has a sequence identical to SEQ ID NO:92. 111. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 112. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 111, wherein the VH has a sequence at least 95% identical to SEQ ID NO:86 and the VL has a sequence at least 95% identical to SEQ ID NO:83. 113. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 111, wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 114. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 111, wherein the VH has a sequence at least 99% identical to SEQ ID NO:86 and the VL has a sequence at least 99% identical to SEQ ID NO:93. 115. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 111, wherein the VH has a sequence identical to SEQ ID NO:86 and the VL has a sequence identical to SEQ ID NO:93. 116. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 117. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 116, wherein the VH has a sequence at least 95% identical to SEQ ID NO:86 and the VL has a sequence at least 95% identical to SEQ ID NO:98. 118. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 116, wherein the VH has a sequence at least 97% identical to SEQ ID NO:86 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 119. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 116, wherein the VH has a sequence at least 99% identical to SEQ ID NO:86 and the VL has a sequence at least 99% identical to SEQ ID NO:98. 120. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 116, wherein the VH has a sequence identical to SEQ ID NO:86 and the VL has a sequence identical to SEQ ID NO:98. 121. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92. 122. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 121, wherein the VH has a sequence at least 95% identical to SEQ ID NO:87 and the VL has a sequence at least 95% identical to SEQ ID NO:92. 123. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 121, wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:92. 124. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 121, wherein the VH has a sequence at least 99% identical to SEQ ID NO:87 and the VL has a sequence at least 99% identical to SEQ ID NO:92. 125. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 121, wherein the VH has a sequence identical to SEQ ID NO:87 and the VL has a sequence identical to SEQ ID NO:92. 126. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93. 127. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 126, wherein the VH has a sequence at least 95% identical to SEQ ID NO:87 and the VL has a sequence at least 95% identical to SEQ ID NO:93. 128. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 126, wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:93. 129. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 126, wherein the VH has a sequence at least 99% identical to SEQ ID NO:87 and the VL has a sequence at least 99% identical to SEQ ID NO:93. 130. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 126, wherein the VH has a sequence identical to SEQ ID NO:87 and the VL has a sequence identical to SEQ ID NO:93. 131. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98. 132. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 131, wherein the VH has a sequence at least 95% identical to SEQ ID NO:87 and the VL has a sequence at least 95% identical to SEQ ID NO:98. 133. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 131, wherein the VH has a sequence at least 97% identical to SEQ ID NO:87 and the VL has a sequence at least 97% identical to SEQ ID NO:98. 134. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 131, wherein the VH has a sequence at least 99% identical to SEQ ID NO:87 and the VL has a sequence at least 99% identical to SEQ ID NO:98. 135. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 131, wherein the VH has a sequence identical to SEQ ID NO:87 and the VL has a sequence identical to SEQ ID NO:98. 136. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 75, wherein the VH has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:100 and the VL has a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:101. 137. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 136, wherein the VH has a sequence at least 95% identical to SEQ ID NO:100 and the VL has a sequence at least 95% identical to SEQ ID NO:101. 138. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 136, wherein the VH has a sequence at least 97% identical to SEQ ID NO:100 and the VL has a sequence at least 97% identical to SEQ ID NO:101. 139. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 136, wherein the VH has a sequence at least 99% identical to SEQ ID NO:100 and the VL has a sequence at least 99% identical to SEQ ID NO:101. 140. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 136, wherein the VH has a sequence identical to SEQ ID NO:100 and the VL has a sequence identical to SEQ ID NO:101. 141. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 140, wherein the antigen binding fragment is a Fab, Fab', F(ab')2, scFv, or Fv fragment. 142. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 141, wherein the antigen binding fragment is a Fab. 143. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 141, wherein the antigen binding fragment is a Fab'. 144. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 141, wherein the antigen binding fragment is a F(ab')2. 145. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 141, wherein the antigen binding fragment is a scFv. 146. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 141, wherein the antigen binding fragment is a Fv fragment. 147. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 146, which comprises an antibody. 148. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 146, which comprises an antigen binding fragment. 149. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 148, which comprises a first Fc region and a second Fc region forming an Fc domain, optionally wherein the Fc domain is a human Fc domain. 150. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 149, wherein the first Fc region and/or second Fc region comprise one or more amino acid substitutions that reduce effector function. 151. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150, wherein the one or more substitutions comprise N297A, N297Q, N297G, D265A/N297A, D265A/N297G, L235E, L234A/L235A, L234A/L235A/P329A, L234D/L235E : L234R/L235R/E233K, L234D/L235E/D265S : E233K/L234R/L235R/D265S, L234D/L235E/E269K : E233K/L234R/L235R/E269K, L234D/L235E/K322A : E233K/L234R/L235R/K322A, L234D/L235E/P329W : E233K/L234R/L235R/P329W, L234D/L235E/E269K/D265S/K322A : E233K/L234R/L235R/E269K/D265S/K322A, or L234D/L235E/E269K/D265S/K322E/E333K: E233K/L234R/L235R/E269K/D265S/K322E/E333K. 152. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise N297A. 153. The anti-CD5 antibody or antigen binding fragment thereof embodiment 150 or embodiment 151, wherein the one or more substitutions comprise N297Q. 154. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise N297G. 155. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise D265A/N297A. 156. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise D265A/N297G. 157. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L235E. 158. The anti-CD5 antibody or antigen binding fragment thereof embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234A/L235A. 159. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234A/L235A/P329A. 160. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E : L234R/L235R/E233K. 161. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/D265S : E233K/L234R/L235R/D265S. 162. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/E269K : E233K/L234R/L235R/E269K. 163. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/K322A : E233K/L234R/L235R/K322A. 164. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/P329W : E233K/L234R/L235R/P329W. 165. The anti-CD5 antibody or antigen binding fragment thereof embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/E269K/D265S/K322A : E233K/L234R/L235R/E269K/D265S/K322A. 166. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 150 or embodiment 151, wherein the one or more substitutions comprise L234D/L235E/E269K/D265S/K322E/E333K: E233K/L234R/L235R/E269K/D265S/K322E/E333K. 167. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 150 to 166, wherein the first Fc region and/or second Fc region comprise L234A and L235A substitutions. 168. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 150 to 167, wherein the first Fc region and/or second Fc region comprise a N297A substitution. 169. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 149 to 168, wherein the Fc domain comprises a human IgG1 Fc domain or a variant thereof. 170. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 169, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:51, SEQ ID NO:52, or SEQ ID NO:53. 171. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 170, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:51. 172. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 170, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:52. 173. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 170, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:53. 174. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 149 to 168, wherein the Fc domain comprises a human IgG2 Fc domain or a variant thereof. 175. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 174, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:81. 176. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 149 to 168, wherein the Fc domain comprises a human IgG4 Fc domain or a variant thereof. 177. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 176, wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:54. 178. The anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 149 to 177, wherein the Fc domain comprises a hinge region. 179. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 178, wherein the hinge region comprises one or more amino acid substitutions that reduce half-antibody exchange. 180. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 179, wherein the Fc domain is an IgG4 Fc domain or variant thereof and the hinge region comprises a S228P substitution. 181. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 178, wherein the hinge region comprises an IgG1 hinge region. 182. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 181, wherein the hinge region comprises the amino acid sequence of SEQ ID NO:66. 183. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 178, wherein the hinge region comprises an IgG2 hinge region. 184. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 183, wherein the hinge region comprises the amino acid sequence of SEQ ID NO:83. 185. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 178, wherein the hinge region comprises an IgG4 hinge region. 186. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 185, wherein the hinge region comprises the amino acid sequence of SEQ ID NO:67. 187. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 185, wherein the hinge region comprises the amino acid sequence of SEQ ID NO:68. 188. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:69 and a heavy chain having the amino acid sequence of SEQ ID NO:70. 189. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:71 and a heavy chain having the amino acid sequence of SEQ ID NO:72. 190. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:73 and a heavy chain having the amino acid sequence of SEQ ID NO:74. 191. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:75 and a heavy chain having the amino acid sequence of SEQ ID NO:76. 192. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:77 and a heavy chain having the amino acid sequence of SEQ ID NO:78. 193. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 1, which comprises a light chain having the amino acid sequence of SEQ ID NO:79 and a heavy chain having the amino acid sequence of SEQ ID NO:80. 194. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49, which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:56. 195. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49, which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:57. 196. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49, which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:58. 197. The anti-CD5 antibody or antigen binding fragment thereof of embodiment 49, which comprises a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:59. 198. A nucleic acid comprising a coding region for the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 197. 199. The nucleic acid of embodiment 198, which is codon optimized for expression in a mammalian cell, optionally wherein the mammalian cell is a human cell. 200. A vector comprising the nucleic acid of embodiment 198 or embodiment 199, optionally wherein the vector is a plasmid. 201. A cell engineered to express the nucleic acid of any one of embodiments 198 to 199 or comprising the vector of embodiment 200 202. A method of producing an anti-CD5 antibody or antigen binding fragment thereof, comprising culturing the cell of embodiment 201 in conditions under which the anti-CD5 antibody or antigen binding fragment thereof is expressed; and recovering the anti-CD5 antibody or antigen binding fragment thereof from the cell culture. 203. An antibody-ALK5 inhibitor conjugate comprising the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 conjugated to an ALK5 inhibitor. 204. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor has an IC50 of 20 nM or lower. 205. The antibody-ALK5 inhibitor conjugate of embodiment 203 or embodiment 204, wherein the ALK5 inhibitor is an imidazole type compound, a pyrazole type compound, or a thiazole type compound. 206. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is an imidazole type compound. 207. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is a pyrazole type compound. 208. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is a thiazole type compound. 209. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is an imidazole type compound which is an imidazole-benzodioxol compound or an imidazole-quinoxaline compound. 210. The antibody-ALK5 inhibitor conjugate of embodiment 209, wherein the ALK5 inhibitor is an imidazole-benzodioxol compound. 211. The antibody-ALK5 inhibitor conjugate of embodiment 209, wherein the ALK5 inhibitor is an imidazole-quinoxaline compound. 212. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is pyrazole type compound which is a pyrazole-pyrrolo compound. 213. The antibody-ALK5 inhibitor conjugate of embodiment 205, wherein the ALK5 inhibitor is an imidazole-benzodioxol compound, an imidazole-quinoxaline compound, a pyrazole-pyrrolo compound, or a thiazole type compound. 214. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Section 6.3. 215. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 2. 216. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 3A. 217. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 3B. 218. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor is an ALK5 inhibitor identified in Table 4. 219. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor has the structure . has the structure 221. The antibody-ALK5 inhibitor conjugate of embodiment 203, wherein the ALK5 inhibitor has the structure . conjugate of embodiment 203, wherein the ALK5 inhibitor has the . inhibitor conjugate of any one of embodiments 203 to 222, wherein the antibody or antigen binding fragment via a linker. 224. The antibody-ALK5 inhibitor conjugate of embodiment 223, wherein the linker is a PEG containing linker. 225. The antibody-ALK5 inhibitor conjugate of embodiment 223 or embodiment 224, wherein the linker is a polyvalent linker. 226. The antibody-ALK5 inhibitor conjugate of any one of embodiments 223 to 225, wherein the linker is a non-cleavable linker. 227. The antibody-ALK5 inhibitor conjugate of embodiment 226, wherein the non-cleavable linker is an N-maleimidomethylcyclohexane1-carboxylate, maleimidocaproyl or mercaptoacetamidocaproyl linker. 228. The antibody-ALK5 inhibitor conjugate of embodiment 227, wherein the non-cleavable linker is an N-maleimidomethylcyclohexane1-carboxylate linker. 229. The antibody-ALK5 inhibitor conjugate of embodiment 227, wherein the non-cleavable linker is a maleimidocaproyl linker. 230. The antibody-ALK5 inhibitor conjugate of embodiment 227, wherein the non-cleavable linker is a mercaptoacetamidocaproyl linker. 231. The antibody-ALK5 inhibitor conjugate of any one of embodiments 223 to 225, wherein the linker is a cleavable linker. 232. The antibody-ALK5 inhibitor conjugate of embodiment 231, wherein the cleavable linker is a dipeptide linker, a disulfide linker, or a hydrazone linker. 233. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the cleavable linker is a dipeptide linker. 234. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the cleavable linker is a disulfide linker. 235. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the cleavable linker is a hydrazone linker. 236. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the linker is a protease-sensitive linker, e.g., a valine-citrulline dipeptide linker. 237. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the linker is a glutathione-sensitive disulfide linker. 238. The antibody-ALK5 inhibitor conjugate of embodiment 232, wherein the linker is an acid- sensitive disulfide linker. 239. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 238, wherein the ALK5 inhibitor is conjugated to the antigen or antigen binding fragment via site-specific conjugation. 240. The antibody-ALK5 inhibitor conjugate of embodiment 239, wherein the ALK5 inhibitor is conjugated via one or more cysteine, lysine, or glutamine residues on the antibody or antigen binding fragment. 241. The antibody-ALK5 inhibitor conjugate of embodiment 240, wherein the ALK5 inhibitor is conjugated via one or more cysteine residues on the antibody or antigen binding fragment. 242. The antibody-ALK5 inhibitor conjugate of embodiment 240, wherein the ALK5 inhibitor is conjugated via one or more lysine residues on the antibody or antigen binding fragment. 243. The antibody-ALK5 inhibitor conjugate of embodiment 240, wherein the ALK5 inhibitor is conjugated via one or more glutamine residues on the antibody or antigen binding fragment. 244. The antibody-ALK5 inhibitor conjugate of embodiment 239, wherein the ALK5 inhibitor is conjugated via one or more unnatural amino acid residues on the antibody or antigen binding fragment. 245. The antibody-ALK5 inhibitor conjugate of embodiment 244, wherein the one or more unnatural amino acid residues comprise p-acetylphenylalanine (pAcF). 246. The antibody-ALK5 inhibitor conjugate of embodiment 244, wherein the one or more unnatural amino acid residues comprise p-azidomethyl-L-phenylalanine (pAMF) 247. The antibody-ALK5 inhibitor conjugate of embodiment 244, wherein the one or more unnatural amino acid residues comprise selenocysteine (Sec). 248. The antibody-ALK5 inhibitor conjugate of embodiment 239, wherein the ALK5 inhibitor is conjugated via one or more glycans on the antibody or antigen binding fragment. 249. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise fucose. 250. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise 6-thiofucose. 251. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise galactose. 252. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise N-acetylgalactosamine (GalNAc). 253. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise N-acetylglucosamine (GlcNAc). 254. The antibody-ALK5 inhibitor conjugate of embodiment 248, wherein the one or more glycans comprise sialic acid (SA). 255. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 1 and 30. 256. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 1 and 20. 257. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 1 and 15. 258. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 2 and 12. 259. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 4 and 15. 260. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 6 and 12. 261. The antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 254, wherein the average number of ALK5 inhibitor molecules per antibody or antigen binding fragment molecule ranges between 2 and 8. 262. An antibody-ALK5 inhibitor conjugate comprising the anti-CD5 antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 conjugated to a means for inhibiting ALK5, optionally wherein the conjugate further comprises a means for linking the anti-CD5 antibody or antigen binding fragment thereof to the means for inhibiting ALK5. 263. A pharmaceutical composition comprising the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 and a pharmaceutically acceptable carrier. 264. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a drug antibody ratio (DAR) between 1 and 30. 265. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 20. 266. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 15. 267. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 12. 268. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 4 and 15. 269. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 6 and 12. 270. The pharmaceutical composition of embodiment 263, wherein at least 30% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 8. 271. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 30. 272. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 20. 273. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 15. 274. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 12. 275. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 4 and 15. 276. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 6 and 12. 277. The pharmaceutical composition of embodiment 263, wherein at least 40% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 8. 278. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 30. 279. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 20. 280. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 15. 281. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 12. 282. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 4 and 15. 283. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 6 and 12. 284. The pharmaceutical composition of embodiment 263, wherein at least 50% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 8. 285. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 30. 286. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 20. 287. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 1 and 15. 288. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 12. 289. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 4 and 15. 290. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 6 and 12. 291. The pharmaceutical composition of embodiment 263, wherein at least 60% of the antibody-ALK5 inhibitor conjugate molecules in the pharmaceutical composition have a DAR between 2 and 8. 292. A method of treating cancer, comprising administering to a subject in need thereof an antibody-ALK5 inhibitor conjugate according to any one of embodiments 203 to 262 or a pharmaceutical composition according to any one of embodiments 263 to 291. 293. The method of embodiment 292, wherein the cancer is an immunogenic cancer. 294. The method of embodiment 293, wherein the cancer is a solid tumor that expresses a tumor antigen. 295. The method of embodiment 294, wherein the tumor antigen is gp100, melanA or MAGE A1. 296. The method of embodiment 295, wherein the tumor antigen is gp100. 297. The method of embodiment 295, wherein the tumor antigen is melanA. 298. The method of embodiment 295, wherein the tumor antigen is MAGE A1. 299. The method of embodiment 292, wherein the cancer is a solid tumor comprising immune infiltrates. 300. The method of any one of embodiments 292 to 299, wherein the cancer is treatable by immunotherapy. 301. The method of embodiment 300, wherein the immunotherapy is cytokine therapy, adoptive T cell therapy, chimeric antigen receptor (CAR) therapy, checkpoint modulator (e.g., checkpoint inhibitor) therapy, oncolytic virus therapy, dendritic cell vaccine therapy, STING agonist therapy, TLR agonist therapy, or intratumoral CpG therapy. 302. The method of embodiment 300, wherein the immunotherapy is cytokine therapy, adoptive T cell therapy, chimeric antigen receptor (CAR) therapy or checkpoint modulator (e.g., checkpoint inhibitor) therapy. 303. The method of embodiment 302, wherein the immunotherapy is cytokine therapy. 304. The method of embodiment 303, wherein the cytokine therapy is IL2 therapy. 305. The method of embodiment 303, wherein the cytokine therapy is IL12 therapy. 306. The method of embodiment 303, wherein the cytokine therapy is IFN-α therapy. 307. The method of embodiment 303, wherein the cytokine therapy is IFN-γ therapy. 308. The method of embodiment 302, wherein the immunotherapy is adoptive T cell therapy. 309. The method of embodiment 308, wherein the adoptive T cell therapy is autologous T cell therapy. 310. The method of embodiment 302, wherein the immunotherapy is chimeric antigen receptor (CAR) therapy. 311. The method of embodiment 302, wherein the immunotherapy is checkpoint modulator (e.g., checkpoint inhibitor) therapy. 312. The method of embodiment 311, wherein the checkpoint modulator is an antibody. 313. The method of embodiment 302, embodiment 311, or embodiment 312, wherein the checkpoint modulator is an inhibitor of PD1, PDL1, or CTLA4. 314. The method of embodiment 313, wherein the checkpoint modulator is an inhibitor of PD1. 315. The method of embodiment 314, wherein the inhibitor of PD1 is an antibody. 316. The method of embodiment 315, wherein the inhibitor of PD1 is pembrolizumab, nivolumab, cemiplimab, or dostarlimab. 317. The method of embodiment 316, wherein the inhibitor of PD1 is pembrolizumab. 318. The method of embodiment 316, wherein the inhibitor of PD1 is nivolumab. 319. The method of embodiment 316, wherein the inhibitor of PD1 is cemiplimab. 320. The method of embodiment 316, wherein the inhibitor of PD1 is dostarlimab. 321. The method of embodiment 313, wherein the checkpoint modulator is an inhibitor of PDL1. 322. The method of embodiment 321, wherein the inhibitor of PDL1 is an antibody. 323. The method of embodiment 322, wherein the inhibitor of PDL1 is atezolizumab, avelumab, or durvalumab. 324. The method of embodiment 323, wherein the inhibitor of PDL1 is atezolizumab. 325. The method of embodiment 323, wherein the inhibitor of PDL1 is avelumab. 326. The method of embodiment 323, wherein the inhibitor of PDL1 is durvalumab. 327. The method of embodiment 313, wherein the checkpoint modulator is an inhibitor of CTLA4. 328. The method of embodiment 327, wherein the inhibitor of CTLA4 is an antibody. 329. The method of embodiment 328, wherein the inhibitor of CTLA4 is ipilimumab. 330. The method of embodiment 311 or 312, wherein the checkpoint modulator targets TIGIT. 331. The method of embodiment 311 or 312, wherein the checkpoint modulator targets LAG3. 332. The method of embodiment 311 or 312, wherein the checkpoint modulator targets OX40. 333. The method of embodiment 311 or 312, wherein the checkpoint modulator targets ICOS. 334. The method of embodiment 311 or 312, wherein the checkpoint modulator targets GITR. 335. The method of embodiment 311 or 312, wherein the checkpoint modulator targets CD40. 336. The method of embodiment 311 or 312, wherein the checkpoint modulator targets VISTA. 337. The method of any one of embodiments 292 to 336 wherein the cancer is lung cancer, liver cancer, urothelial cancer, renal cancer, breast cancer, melanoma, pancreatic cancer, glioblastoma, a myelodysplastic syndrome, prostate cancer, or colorectal cancer. 338. The method of any one of embodiments 292 to 336 wherein the cancer is head and neck cancer. 339. The method of embodiment 338, wherein the head and neck cancer is head and neck squamous cell carcinoma (HNSCC). 340. The method of any one of embodiments 292 to 336 wherein the cancer is ovarian cancer. 341. The method of embodiment 337, wherein the cancer is non-small cell lung cancer (NSCLC), liver cancer, urothelial cancer, renal cancer, breast cancer, or melanoma. 342. The method of embodiment 337, wherein the cancer is lung cancer. 343. The method of embodiment 342, wherein the cancer is NSCLC. 344. The method of embodiment 343, wherein the NSCLC is adenocarcinoma. 345. The method of embodiment 343, wherein the NSCLC is squamous cell carcinoma. 346. The method of embodiment 343, wherein the NSCLC is large cell carcinoma. 347. The method of embodiment 342, wherein the cancer is small cell lung cancer. 348. The method of embodiment 337, wherein the cancer is liver cancer. 349. The method of embodiment 348, wherein the liver cancer is hepatocellular carcinoma. 350. The method of embodiment 337, wherein the cancer is urothelial cancer. 351. The method of embodiment 350, wherein the cancer is bladder cancer. 352. The method of embodiment 350, wherein the cancer is urethral cancer. 353. The method of embodiment 350, wherein the cancer is ureteral cancer. 354. The method of embodiment 337, wherein the cancer is renal cancer. 355. The method of embodiment 354, wherein the renal cancer is renal cell carcinoma. 356. method of embodiment 354, wherein the renal cancer is urothelial carcinoma. 357. The method of embodiment 337, wherein the cancer is breast cancer. 358. The method of embodiment 337, wherein the cancer is melanoma. 359. The method of embodiment 337, wherein the cancer is pancreatic cancer. 360. The method of embodiment 337, wherein the cancer is glioblastoma. 361. The method of embodiment 337, wherein the cancer is a myelodysplastic syndrome. 362. The method of embodiment 337, wherein the cancer is prostate cancer. 363. The method of embodiment 337, wherein the cancer is colorectal cancer. 364. The method of embodiment 363, wherein the colorectal cancer is adenocarcinoma. 365. The method of embodiment 363, wherein the colorectal cancer is a carcinoid tumor. 366. The method of embodiment 363, wherein the colorectal cancer is a gastrointestinal stromal tumor. 367. The method of embodiment 363, wherein the colorectal cancer is colorectal lymphoma. 368. The method of any one of embodiments 292 to 367, wherein the cancer is treatable by ALK5 inhibitors. 369. The method of any one of embodiments 292 to 368, wherein the cancer is treatable by chemotherapy. 370. The method of any one of embodiments 292 to 369 wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered as monotherapy. 371. The method of any one of embodiments 292 to 369, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered as part of a combination therapy regimen which optionally comprises administering one or more agents which are not an antibody-ALK5 inhibitor conjugate according to any one of embodiments 203 to 261 (each a “second therapeutic agent”). 372. The method of embodiment 371, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered in combination with a standard of care therapy or therapeutic regimen. 373. The method of embodiment 371 or 372, wherein the combination therapy comprises administering at least one second therapeutic agent to the subject. 374. The method of any one of embodiments 371 to 373, wherein the combination therapy regimen comprises immunotherapy, optionally wherein the immunotherapy is checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy, oncolytic virus therapy, dendritic cell vaccine therapy, STING agonist therapy, TLR agonist therapy, intratumoral CpG therapy, or cytokine therapy. 375. The method of any one of embodiments 371 to 374, wherein the combination therapy comprises checkpoint modulator (e.g., checkpoint inhibitor) therapy. 376. The method of embodiment 375, wherein the checkpoint modulator therapy comprises T cell checkpoint modulator (e.g., checkpoint inhibitor) therapy. 377. The method of embodiment 376, wherein the T cell checkpoint modulator therapy comprises an antibody or an antigen-binding fragment thereof. 378. The method of any one of embodiments 375 to 377, wherein the checkpoint modulator therapy targets PD1, PDL1, CTLA4, TIGIT, LAG3, OX40, ICOS, GITR, CD40, VISTA, or a combination thereof. 379. The method of embodiment 378, wherein the checkpoint modulator therapy targets PD1. 380. The method of any one of embodiments 371 to 379, wherein a second therapeutic agent comprises a means for targeting PD1. 381. The method of any one of embodiments 371 to 379, wherein a second therapeutic agent is pembrolizumab. 382. The method of any one of embodiments 371 to 379, wherein a second therapeutic agent is nivolumab. 383. The method of any one of embodiments 371 to 379, wherein a second therapeutic agent is cemiplimab. 384. The method of any one of embodiments 371 to 379, wherein a second therapeutic agent is dostarlimab. 385. The method of any one of embodiments 378 to 384, wherein the checkpoint modulator therapy targets PDL1. 386. The method of any one of embodiments 371 to 385, wherein a second therapeutic agent comprises a means for targeting PDL1. 387. The method of any one of embodiments 371 to 385, wherein a second therapeutic agent is atezolizumab. 388. The method of any one of embodiments 371 to 385, wherein a second therapeutic agent is avelumab. 389. The method of any one of embodiments 371 to 385, wherein a second therapeutic agent is durvalumab. 390. The method of any one of embodiments 378 to 389 wherein the checkpoint modulator therapy targets CTLA4. 391. The method of any one of embodiments 371 to 390, wherein a second therapeutic agent comprises a means for targeting CTLA4. 392. The method of any one of embodiments 371 to 390, wherein a second therapeutic agent is ipilimumab. 393. The method of any one of embodiments 378 to 391, wherein the checkpoint modulator therapy targets TIGIT. 394. The method of any one of embodiments 371 to 393, wherein a second therapeutic agent comprises a means for targeting TIGIT. 395. The method of any one of embodiments 371 to 393, wherein a second therapeutic agent is etigilimab. 396. The method of any one of embodiments 371 to 393, wherein a second therapeutic agent is tiragolumab. 397. The method of any one of embodiments 371 to 393, wherein a second therapeutic agent is AB154. 398. The method of any one of embodiments 378 to 397, wherein the checkpoint modulator therapy targets LAG3. 399. The method of any one of embodiments 371 to 398, wherein a second therapeutic agent comprises a means for targeting LAG3. 400. The method of any one of embodiments 371 to 398, wherein a second therapeutic agent is LAG525. 401. The method of any one of embodiments 371 to 398, wherein a second therapeutic agent is Sym022. 402. The method of any one of embodiments 371 to 398, wherein a second therapeutic agent is relatlimab. 403. The method of any one of embodiments 371 to 398, wherein a second therapeutic agent is TSR-033. 404. The method of any one of embodiments 378 to 403, wherein the checkpoint modulator therapy targets OX40. 405. The method of any one of embodiments 371 to 404, wherein a second therapeutic agent is a means for targeting OX40. 406. The method of any one of embodiments 371 to 404, wherein a second therapeutic agent is MEDI6469. 407. The method of any one of embodiments 371 to 404, wherein a second therapeutic agent is PF-04518600. 408. The method of any one of embodiments 371 to 404, wherein a second therapeutic agent is BMS 986178. 409. The method of any one of embodiments 378 to 408, wherein the checkpoint modulator therapy targets CD40. 410. The method of any one of embodiments 371 to 409, wherein a second therapeutic agent comprises a means for targeting CD40. 411. The method of any one of embodiments 371 to 409, wherein a second therapeutic agent is selicrelumab. 412. The method of any one of embodiments 371 to 409, wherein a second therapeutic agent is CP-870,893. 413. The method of any one of embodiments 371 to 409, wherein a second therapeutic agent is APX005M. 414. The method of any one of embodiments 378 to 413, wherein the checkpoint modulator therapy targets ICOS. 415. The method of any one of embodiments 371 to 414, wherein a second therapeutic agent comprises a means for targeting ICOS. 416. The method of any one of embodiments 371 to 414, wherein a second therapeutic agent is MEDI-570. 417. The method of any one of embodiments 371 to 414, wherein a second therapeutic agent is feladilimab. 418. The method of any one of embodiments 371 to 414, wherein a second therapeutic agent is BMS 986226. 419. The method of any one of embodiments 378 to 418, wherein the checkpoint modulator therapy targets GITR. 420. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent comprises a means for targeting GITR. 421. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is TRX-518. 422. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is AMG 228. 423. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is MK-4166. 424. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is MEDI1873. 425. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is INCAGN01876. 426. The method of any one of embodiments 371 to 419, wherein a second therapeutic agent is GWN323. 427. The method of any one of embodiments 378 to 426, wherein the checkpoint modulator therapy targets VISTA. 428. The method of any one of embodiments 371 to 427, wherein a second therapeutic agent comprises a means for targeting VISTA. 429. The method of any one of embodiments 371 to 427, wherein a second therapeutic agent is HMBD-002. 430. The method of any one of embodiments 371 to 428, wherein a second therapeutic agent is a chimeric antigen receptor (CAR). 431. The method of any one of embodiments 371 to 430 wherein the combination therapy comprises adoptive T cell therapy. 432. The method of embodiment 431, wherein the adoptive T cell therapy is autologous T cell therapy. 433. The method of any one of embodiments 371 to 432, wherein the combination therapy comprises oncolytic virus therapy. 434. The method of any one of embodiments 371 to 433, wherein the combination therapy comprises dendritic cell vaccine therapy. 435. The method of any one of embodiments 371 to 434, wherein the combination therapy comprises STING agonist therapy. 436. The method of any one of embodiments 371 to 435, wherein the combination therapy comprises TLR agonist therapy. 437. The method of any one of embodiments 371 to 436, wherein the combination therapy comprises chemotherapy. 438. The method of embodiment 437, wherein a second therapeutic agent is an antimetabolite, an alkylating agent, an anthracycline, an antimicrotubule agent, a platinum compound, a taxane, a topoisomerase inhibitor, or a vinca alkaloid. 439. The method of embodiment 438, wherein a second therapeutic agent is an antimetabolite. 440. The method of embodiment 439, wherein the antimetabolite is 5-fluorouracil. 441. The method of embodiment 439, wherein the antimetabolite is gemcitabine. 442. The method of embodiment 439, wherein the antimetabolite is methotrexate. 443. The method of embodiment 438, wherein a second therapeutic agent is an alkylating agent. 444. The method of embodiment 443, wherein the alkylating agent is cyclophosphamide. 445. The method of embodiment 443, wherein the alkylating agent is dacarbazine. 446. The method of embodiment 443, wherein the alkylating agent is mechlorethamine. 447. The method of embodiment 443, wherein the alkylating agent is diaziquone. 448. The method of embodiment 443, wherein the alkylating agent is temozolomide. 449. The method of embodiment 438, wherein a second therapeutic agent is an anthracycline. 450. The method of embodiment 449, wherein the anthracycline is doxorubicin. 451. The method of embodiment 449, wherein the anthracycline is epirubicin. 452. The method of embodiment 438, wherein a second therapeutic agent is an antimicrotubule agent. 453. The method of embodiment 452, wherein the antimicrotubule agent is vinblastine. 454. The method of embodiment 438, wherein a second therapeutic agent is a platinum compound. 455. The method of embodiment 454, wherein the platinum compound is cisplatin. 456. The method of embodiment 454, wherein the platinum compound is oxaliplatin. 457. The method of embodiment 438, wherein a second therapeutic agent is a taxane. 458. The method of embodiment 457, wherein the taxane is paclitaxel. 459. The method of embodiment 457, wherein the taxane is docetaxel. 460. The method of embodiment 438, wherein a second therapeutic agent is a topoisomerase inhibitor. 461. The method of embodiment 460, wherein the topoisomerase inhibitor is etoposide. 462. The method of embodiment 460, wherein the topoisomerase inhibitor is mitoxantrone. 463. The method of embodiment 438, wherein a second therapeutic agent is a vinca alkaloid. 464. The method of embodiment 463, wherein the vinca alkaloid is vincristine. 465. The method of any one of embodiments 371 to 464, wherein the combination therapy comprises intratumoral CpG therapy. 466. The method of any one of embodiments 371 to 465, wherein a second therapeutic agent is a cytokine. 467. The method of embodiment 466, wherein the cytokine is IL2. 468. The method of embodiment 466, wherein the cytokine is IL12. 469. The method of embodiment 466, wherein the cytokine is IFN-α. 470. The method of embodiment 466, wherein the cytokine is IFN-γ. 471. The method of any one of embodiments 371 to 470, which comprises treating the subject with the combination therapy. 472. The method of any one of embodiments 371 to 471, which comprises administering the second therapeutic agent(s) to the subject. 473. A method of treating a subject having a disease or disorder associated with elevated CD5 expression, comprising administering the anti-CD5 antibody or antigen binding fragment of any one of embodiments 1 to 197 to the subject, optionally wherein the disease or disorder is a B or T cell malignancy, an autoimmune disease, a transplantation disease, or a graft rejection. 474. A process for making antibody-ALK5 inhibitor conjugate, comprising conjugating the antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 to an ALK5 inhibitor, optionally wherein the antibody or antigen binding fragment thereof is conjugated to the ALK5 inhibitor via a linker. 475. The process of embodiment 474, wherein the ALK5 inhibitor is an ALK5 inhibitor described in any one of embodiments 203 to 261. 476. The process of embodiment 474 or embodiment 475, wherein the linker is a linker described in any one of embodiments 223 to 238. 477. The process of any one of embodiments 474 to 476, wherein the antibody or antigen binding fragment thereof is conjugated to the ALK5 inhibitor via a linker, and wherein the process further comprises a step of conjugating the ALK5 inhibitor to the linker. 478. A kit comprising the antibody or antigen binding fragment thereof of any one of embodiments 1 to 197 and an ALK5 inhibitor. 479. The kit of embodiment 478, wherein the ALK5 inhibitor is an ALK5 inhibitor as described in any one of embodiments 203 to 261. 480. The kit of embodiment 478 or embodiment 479, further comprising a linker. 481. The kit of embodiment 478 or embodiment 479, wherein the ALK5 inhibitor is conjugated to a linker. 482. The kit of embodiment 480 or embodiment 481, wherein the linker is a linker as described in any one of embodiments 223 to 238. 483. A method of delivering an ALK5 inhibitor to a T cell, comprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to a subject, e.g., a subject as described in any one of embodiments 292 to 472. 484. A method of inducing an immune response in a subject, comprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to the subject, e.g., a subject as described in any one of embodiments 292 to 472. 485. A method of stimulating T cells in a subject, comprising administering the antibody-ALK5 inhibitor conjugate of any one of embodiments 203 to 262 to the subject, e.g., a subject as described in any one of embodiments 292 to 472. [0261] While various specific embodiments have been illustrated and described, it will be appreciated that various changes can be made without departing from the spirit and scope of the disclosure(s). 9. CITATION OF REFERENCES [0262] All publications, patents, patent applications and other documents cited in this application are hereby incorporated by reference in their entireties for all purposes to the same extent as if each individual publication, patent, patent application or other document were individually indicated to be incorporated by reference for all purposes. In the event that there is an inconsistency between the teachings of one or more of the references incorporated herein and the present disclosure, the teachings of the present specification are intended.
Claims
WHAT IS CLAIMED IS: 1. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 11, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 15, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 15, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 11, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:17, 19, and 21, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 9, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (j) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 9, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (k) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 10, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (l) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 10, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (m) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 12, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; or (n) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 12, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively.
2. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an anti- CD5 antibody or antigen binding fragment according to claim 1, comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 7, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 7, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 6, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:17, ATS, and SEQ ID NO:20, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 9, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 9, and 16, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 10, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 10, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 12, and 14, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; or (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 12, and 13, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively.
3. The anti-CD5 antibody or antigen binding fragment thereof of claim 1 or claim 2, which is a humanized anti-CD5 antibody or antigen binding fragment thereof.
4. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to any one of claims 1 to 3, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:22 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:23; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:24 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:25; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:26 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:27; (d) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:28 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:29; (e) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:30 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:31; or (f) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:32 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:33.
5. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:34, 39, and 43, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:35, 40, 44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:46, 47, and 48, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:36, 41, and 44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:18, 19, and 21, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of NYG, SEQ ID NO:41, and SEQ ID NO:44, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NO:45, RAN, and SEQ ID NO:48, respectively; or (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:38, 42, and 43, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOS:46, 47, and 48, respectively.
6. The anti-CD5 antibody or antigen binding fragment thereof of claim 5, which is a humanized anti-CD5 antibody or antigen binding fragment thereof.
7. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to claim 5 or claim 6, comprising a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:49 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:50.
8. An anti-CD5 antibody or antigen binding fragment thereof comprising: (a) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:1, 88, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively; (b) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively; (c) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 90, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively; (d) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:96, 97, 95, respectively; (e) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:99, 97, 95, respectively; (f) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:4, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NO:94, WT, and SEQ ID NO:95, respectively; (g) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:96, 97, and 95, respectively; (h) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 90, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:99, 97, and 95, respectively; (i) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:2, 102, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; (j) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:3, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR- L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; (k) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:5, 102, and 89, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:103, 97, and 95, respectively; or (l) a VH comprising CDR-H1, CDR-H2, and CDR-H3 having the amino acid sequences of SEQ ID NOs:104, 8, and 91, respectively, and a VL comprising CDR-L1, CDR-L2, and CDR-L3 having the amino acid sequences of SEQ ID NOs:94, 97, and 105, respectively.
9. The anti-CD5 antibody or antigen binding fragment thereof of claim 8, which is a humanized anti-CD5 antibody or antigen binding fragment thereof.
10. An anti-CD5 antibody or antigen binding fragment thereof, which is optionally an antibody or antigen binding fragment according to claim 8 or claim 9, comprising: (a) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (b) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (c) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:84 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (d) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (e) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (f) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:85 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (g) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (h) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (i) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:86 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; (j) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:92; (k) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:93; (l) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:87 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:98; or (m) a VH having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:100 and a VL having a sequence at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:101.
11. The anti-CD5 antibody or antigen binding fragment thereof of any one of claims 1 to 10, wherein the antigen binding fragment is a Fab, Fab', F(ab')2, scFv, or Fv fragment.
12. The anti-CD5 antibody or antigen binding fragment thereof of any one of claims 1 to 11, which comprises a first Fc region and a second Fc region forming an Fc domain, optionally wherein the Fc domain is a human Fc domain, optionally wherein the first Fc region and/or second Fc region comprise one or more amino acid substitutions that reduce effector function, optionally wherein the one or more substitutions comprise N297A, N297Q, N297G, D265A/N297A, D265A/N297G, L235E, L234A/L235A, L234A/L235A/P329A, L234D/L235E : L234R/L235R/E233K, L234D/L235E/D265S : E233K/L234R/L235R/D265S, L234D/L235E/E269K : E233K/L234R/L235R/E269K, L234D/L235E/K322A : E233K/L234R/L235R/K322A, L234D/L235E/P329W : E233K/L234R/L235R/P329W, L234D/L235E/E269K/D265S/K322A : E233K/L234R/L235R/E269K/D265S/K322A, or L234D/L235E/E269K/D265S/K322E/E333K: E233K/L234R/L235R/E269K/D265S/K322E/E333K.
13. The anti-CD5 antibody or antigen binding fragment thereof of claim 12, wherein the Fc domain comprises (a) a human IgG1 Fc domain or a variant thereof, optionally wherein wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:51, SEQ ID NO:52, or SEQ ID NO:53; (b) a human IgG2 Fc domain or a variant thereof, optionally wherein wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:81; or (c) a human IgG4 Fc domain or a variant thereof, optionally wherein the first Fc region and/or second Fc region comprise an amino acid sequence that is at least 90% identical (e.g., at least 95%, at least 97%, at least 99%, or 100% identical) to SEQ ID NO:54.
14. The anti-CD5 antibody or antigen binding fragment thereof of claim 1, which comprises: (a) a light chain having the amino acid sequence of SEQ ID NO:69 and a heavy chain having the amino acid sequence of SEQ ID NO:70; (b) a light chain having the amino acid sequence of SEQ ID NO:71 and a heavy chain having the amino acid sequence of SEQ ID NO:72; (c) a light chain having the amino acid sequence of SEQ ID NO:73 and a heavy chain having the amino acid sequence of SEQ ID NO:74; (d) a light chain having the amino acid sequence of SEQ ID NO:75 and a heavy chain having the amino acid sequence of SEQ ID NO:76; (e) a light chain having the amino acid sequence of SEQ ID NO:77 and a heavy chain having the amino acid sequence of SEQ ID NO:78; or (f) a light chain having the amino acid sequence of SEQ ID NO:79 and a heavy chain having the amino acid sequence of SEQ ID NO:80.
15. The anti-CD5 antibody or antigen binding fragment thereof of claim 5, which comprises: (a) a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:56; (b) a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:57; (c) a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:58; or (d) a light chain having the amino acid sequence of SEQ ID NO:55 and a heavy chain having the amino acid sequence of SEQ ID NO:59.
16. A nucleic acid comprising a coding region for the anti-CD5 antibody or antigen binding fragment thereof of any one of claims 1 to 15, optionally which is codon optimized for expression in a mammalian cell, optionally wherein the mammalian cell is a human cell.
17. A vector comprising the nucleic acid of claim 16, optionally wherein the vector is a plasmid.
18. A cell engineered to express the nucleic acid of claim 16 or comprising the vector of claim 17.
19. A method of producing an anti-CD5 antibody or antigen binding fragment thereof, comprising culturing the cell of claim 18 in conditions under which the anti-CD5 antibody or antigen binding fragment thereof is expressed; and recovering the anti-CD5 antibody or antigen binding fragment thereof from the cell culture.
20. An antibody-ALK5 inhibitor conjugate comprising the anti-CD5 antibody or antigen binding fragment thereof of any one of claims 1 to 15 conjugated to an ALK5 inhibitor.
21. The antibody-ALK5 inhibitor conjugate of claim 20, wherein the ALK5 inhibitor has the structure
. 5 inhibitor is linked 23. A pharmaceutical composition comprising the antibody-ALK5 inhibitor conjugate of any one of claims 20 to 22 and a pharmaceutically acceptable carrier. 24. A method of treating cancer, comprising administering to a subject in need thereof an antibody-ALK5 inhibitor conjugate according to any one of claims 20 to 22 or a pharmaceutical composition according to claim 23. 25. The method of claim 24, wherein the cancer is lung cancer, liver cancer, urothelial cancer, renal cancer, breast cancer, melanoma, pancreatic cancer, glioblastoma, a myelodysplastic syndrome, prostate cancer, or colorectal cancer. 26. The method of claim 24 or claim 25, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered as monotherapy. 27. The method of claim 24 or claim 25, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered as part of a combination therapy regimen which optionally comprises administering one or more agents which are not an antibody-ALK5 inhibitor conjugate (each a “second therapeutic agent”). 28. The method of claim 27, wherein the antibody-ALK5 inhibitor conjugate or pharmaceutical composition is administered in combination with a standard of care therapy or therapeutic regimen. 29. The method of claim 27 or 28, wherein the combination therapy comprises administering at least one second therapeutic agent to the subject. 30. The method of any one of claims 27 to 29, wherein the combination therapy regimen comprises immunotherapy, optionally wherein the immunotherapy is checkpoint modulator (e.g., checkpoint inhibitor) therapy, chimeric antigen receptor (CAR) therapy, adoptive T cell therapy, oncolytic virus therapy, dendritic cell vaccine therapy, STING agonist therapy, TLR agonist therapy, intratumoral CpG therapy, or cytokine therapy. 31. A method of treating a subject having a disease or disorder associated with elevated CD5 expression, comprising administering the anti-CD5 antibody or antigen binding fragment of any one of claims 1 to 15 to the subject, optionally wherein the disease or disorder is a B or T cell malignancy, an autoimmune disease, a transplantation disease, or a graft rejection. 32. A process for making antibody-ALK5 inhibitor conjugate, comprising conjugating the antibody or antigen binding fragment thereof of any one of claims 1 to 15 to an ALK5 inhibitor, optionally wherein the antibody or antigen binding fragment thereof is conjugated to the ALK5 inhibitor via a linker. 33. The process of claim 32, wherein the antibody or antigen binding fragment thereof is conjugated to the ALK5 inhibitor via a linker, and wherein the process further comprises a step of conjugating the ALK5 inhibitor to the linker. 34. A kit comprising the antibody or antigen binding fragment thereof of any one of claims 1 to 15 and an ALK5 inhibitor. 35. The kit of claim 34, further comprising a linker. 36. The kit of claim 34, wherein the ALK5 inhibitor is conjugated to a linker.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363507751P | 2023-06-13 | 2023-06-13 | |
| US63/507,751 | 2023-06-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024258967A1true WO2024258967A1 (en) | 2024-12-19 |
Family
ID=91856048
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/033613PendingWO2024258967A1 (en) | 2023-06-13 | 2024-06-12 | Anti-cd5 antibodies and their uses |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024258967A1 (en) |
Citations (95)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US614226A (en) | 1898-11-15 | Implement for remodeling valve-seats | ||
| US4510245A (en) | 1982-11-18 | 1985-04-09 | Chiron Corporation | Adenovirus promoter system |
| WO1986001533A1 (en) | 1984-09-03 | 1986-03-13 | Celltech Limited | Production of chimeric antibodies |
| EP0239400A2 (en) | 1986-03-27 | 1987-09-30 | Medical Research Council | Recombinant antibodies and methods for their production |
| US4816397A (en) | 1983-03-25 | 1989-03-28 | Celltech, Limited | Multichain polypeptides or proteins and processes for their production |
| US4880935A (en) | 1986-07-11 | 1989-11-14 | Icrf (Patents) Limited | Heterobifunctional linking agents derived from N-succinimido-dithio-alpha methyl-methylene-benzoates |
| EP0392745A2 (en) | 1989-04-05 | 1990-10-17 | Celltech Limited | Immunoconjugates and prodrugs and their use in association for drug delivery |
| US4968615A (en) | 1985-12-18 | 1990-11-06 | Ciba-Geigy Corporation | Deoxyribonucleic acid segment from a virus |
| WO1991009967A1 (en) | 1989-12-21 | 1991-07-11 | Celltech Limited | Humanised antibodies |
| US5122368A (en) | 1988-02-11 | 1992-06-16 | Bristol-Myers Squibb Company | Anthracycline conjugates having a novel linker and methods for their production |
| US5168062A (en) | 1985-01-30 | 1992-12-01 | University Of Iowa Research Foundation | Transfer vectors and microorganisms containing human cytomegalovirus immediate-early promoter-regulatory DNA sequence |
| EP0519596A1 (en) | 1991-05-17 | 1992-12-23 | Merck & Co. Inc. | A method for reducing the immunogenicity of antibody variable domains |
| US5208020A (en) | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| EP0592106A1 (en) | 1992-09-09 | 1994-04-13 | Immunogen Inc | Resurfacing of rodent antibodies |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US5622929A (en) | 1992-01-23 | 1997-04-22 | Bristol-Myers Squibb Company | Thioether conjugates |
| US5824805A (en) | 1995-12-22 | 1998-10-20 | King; Dalton | Branched hydrazone linkers |
| US5834597A (en) | 1996-05-20 | 1998-11-10 | Protein Design Labs, Inc. | Mutated nonactivating IgG2 domains and anti CD3 antibodies incorporating the same |
| WO2000061576A1 (en) | 1999-04-09 | 2000-10-19 | Smithkline Beecham Corporation | Triarylimidazoles |
| US6214345B1 (en) | 1993-05-14 | 2001-04-10 | Bristol-Myers Squibb Co. | Lysosomal enzyme-cleavable antitumor drug conjugates |
| WO2001062756A1 (en) | 2000-02-21 | 2001-08-30 | Smithkline Beecham P.L.C. | Pyridinylimidazoles |
| WO2002055077A1 (en) | 2001-01-11 | 2002-07-18 | Smithkline Beecham Corporation | Use of imidazolyl cyclic acetal derivatives in the manufacture of a medicament for the treatment of diseases mediated by the alk5 receptors |
| WO2002083180A1 (en) | 2001-03-23 | 2002-10-24 | Syntarga B.V. | Elongated and multiple spacers in activatible prodrugs |
| EP1291360A1 (en)* | 1991-12-13 | 2003-03-12 | Xoma Corporation | Methods and materials for preparation of modified antibody variable domains and therapeutic uses thereof |
| US20030149277A1 (en) | 2000-03-27 | 2003-08-07 | Gaster Laramie Mary | Triarylimidazole derivatives as cytokine inhibitors |
| WO2003087304A2 (en) | 2002-04-04 | 2003-10-23 | Biogen, Inc. | Tri-substituted heteroaryls and methods of making and using the same |
| WO2004010957A2 (en) | 2002-07-31 | 2004-02-05 | Seattle Genetics, Inc. | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| WO2004043493A1 (en) | 2002-11-14 | 2004-05-27 | Syntarga B.V. | Prodrugs built as multiple self-elimination-release spacers |
| WO2004065392A1 (en) | 2003-01-24 | 2004-08-05 | Smithkline Beecham Corporation | Condensed pyridines and pyrimidines and their use as alk-5 receptor ligands |
| US20050238649A1 (en) | 2003-11-06 | 2005-10-27 | Seattle Genetics, Inc. | Monomethylvaline compounds capable of conjugation to ligands |
| WO2005103028A1 (en) | 2004-04-21 | 2005-11-03 | In2Gen Co., Ltd | 2-pyridyl substituted imidazoles as alk5 and/or alk4 inhibitors |
| US20050256030A1 (en) | 2004-02-23 | 2005-11-17 | Bainian Feng | Heterocyclic self-immolative linkers and conjugates |
| WO2005112919A2 (en) | 2004-05-19 | 2005-12-01 | Medarex, Inc. | Self-immolative linkers and drug conjugates |
| WO2005117984A2 (en) | 2004-06-01 | 2005-12-15 | Celltech R & D Limited | Albumin-binding conjugates comprising a fatty acid and peg |
| WO2005123780A2 (en) | 2004-04-09 | 2005-12-29 | Protein Design Labs, Inc. | Alteration of fcrn binding affinities or serum half-lives of antibodies by mutagenesis |
| US20060024317A1 (en) | 2004-05-19 | 2006-02-02 | Medarex, Inc | Chemical linkers and conjugates thereof |
| WO2006034488A2 (en) | 2004-09-23 | 2006-03-30 | Genentech, Inc. | Cysteine engineered antibodies and conjugates |
| US20060134709A1 (en) | 2004-11-10 | 2006-06-22 | Jeffery Stavenhagen | Engineering Fc antibody regions to confer effector function |
| US7087626B2 (en) | 2001-05-24 | 2006-08-08 | Eli Lilly And Company | Pyrrole derivatives as pharmaceutical agents |
| WO2006110476A2 (en) | 2005-04-08 | 2006-10-19 | Medarex, Inc. | Cytotoxic compounds and conjugates comprising duocarmycins with cleavable substrates |
| WO2007018431A2 (en) | 2005-08-05 | 2007-02-15 | Syntarga B.V. | Triazole-containing releasable linkers and conjugates comprising the same |
| WO2007059404A2 (en) | 2005-11-10 | 2007-05-24 | Medarex, Inc. | Duocarmycin derivatives as novel cytotoxic compounds and conjugates |
| WO2007070538A2 (en) | 2005-12-12 | 2007-06-21 | Bayer Healthcare Llc | Anti-mn antibodies and methods of using same |
| WO2007089149A2 (en) | 2006-02-02 | 2007-08-09 | Syntarga B.V. | Water-soluble cc-1065 analogs and their conjugates |
| US7265225B2 (en) | 2002-11-22 | 2007-09-04 | Eli Lilly And Company | Quinolinyl-pyrrolopyrazoles |
| US20070280931A1 (en) | 1998-11-18 | 2007-12-06 | Chen Yvonne M | Antibody variants |
| US7365066B2 (en) | 2002-09-17 | 2008-04-29 | Eli Lilly And Company | Pyrazolopyridine derivatives as pharmaceutical agents |
| US7368445B2 (en) | 2004-03-01 | 2008-05-06 | Eli Lilly And Company | Fused pyrazole derivatives as TGF-β signal transduction inhibitors for the treatment of fibrosis and neoplasms |
| US7405299B2 (en) | 2002-11-27 | 2008-07-29 | Eli Lilly And Company | Compounds as pharmaceutical agents |
| US20080319012A1 (en) | 2004-04-21 | 2008-12-25 | In2Gen Co., Ltd. | 2-Pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| WO2009017394A1 (en) | 2007-08-01 | 2009-02-05 | Syntarga B.V. | Substituted cc-1065 analogs and their conjugates |
| US7511056B2 (en) | 2004-11-10 | 2009-03-31 | Eli Lilly And Company | TGF-β inhibitors |
| WO2009050183A2 (en) | 2007-10-17 | 2009-04-23 | Novartis Ag | Imidazo [1, 2-a] pyridine derivatives useful as alk inhibitors |
| WO2009073445A2 (en) | 2007-11-28 | 2009-06-11 | Mersana Therapeutics, Inc. | Biocompatible biodegradable fumagillin analog conjugates |
| WO2009133070A1 (en) | 2008-04-29 | 2009-11-05 | Novartis Ag | Imidazo-pyridine derivatives as activin-like receptor kinase (alk4 or alk5) inhibitors |
| WO2010022737A1 (en)* | 2008-08-29 | 2010-03-04 | Symphogen A/S | Anti-cd5 antibodies |
| US7691865B2 (en) | 2002-09-06 | 2010-04-06 | Biogen Idec Ma Inc. | Pyrazolopyridines and methods of making and using the same |
| WO2010062171A2 (en) | 2008-11-03 | 2010-06-03 | Syntarga B.V. | Novel cc-1065 analogs and their conjugates |
| WO2010068795A2 (en) | 2008-12-12 | 2010-06-17 | Pearson Robert M | Method and system for tissue treatment utilizing irreversible electroporation and thermal track coagulation |
| WO2010138719A1 (en) | 2009-05-28 | 2010-12-02 | Mersana Therapeutics, Inc. | Polyal drug conjugates comprising variable rate-releasing linkers |
| US7863288B2 (en) | 2005-06-22 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| WO2011120053A1 (en) | 2010-03-26 | 2011-09-29 | Mersana Therapeutics, Inc. | Modified polymers for delivery of polynucleotides, method of manufacture, and methods of use thereof |
| WO2011146287A1 (en) | 2010-05-20 | 2011-11-24 | Takeda Pharmaceutical Company Limited | Pyrazolo[4,3-b]pyridine-7-amine inhibitors of alk5 |
| WO2013009140A2 (en) | 2011-07-13 | 2013-01-17 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as alk5 and/or alk4 inhibitors |
| US8410146B2 (en) | 2004-04-21 | 2013-04-02 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| US8420685B2 (en) | 2004-04-21 | 2013-04-16 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| WO2013096901A1 (en) | 2011-12-23 | 2013-06-27 | Mersana Therapeutics, Inc. | Pharmaceutical formulations for fumagillin derivative-phf conjugates |
| US8513228B2 (en) | 2009-02-20 | 2013-08-20 | Richter Gedeon Nyrt. | Crystalline form of antiprogestin CDB-4124 |
| WO2014008375A1 (en) | 2012-07-05 | 2014-01-09 | Mersana Therapeutics, Inc. | Terminally modified polymers and conjugates thereof |
| US8679500B2 (en) | 2009-05-14 | 2014-03-25 | Institute National De La Sante Et De La Recherche Medicale (Inserm) | Compositions containing antibodies for treating CD5+ HLA-DR+ B or T cell related diseases |
| WO2014093379A1 (en) | 2012-12-10 | 2014-06-19 | Mersana Therapeutics, Inc. | Auristatin compounds and conjugates thereof |
| WO2014093640A1 (en) | 2012-12-12 | 2014-06-19 | Mersana Therapeutics,Inc. | Hydroxy-polmer-drug-protein conjugates |
| WO2014093394A1 (en) | 2012-12-10 | 2014-06-19 | Mersana Therapeutics, Inc. | Protein-polymer-drug conjugates |
| US8791113B2 (en) | 2010-06-28 | 2014-07-29 | Merck Patent Gmbh | 2,4-diaryl-substituted [1,8] naphthyridines as kinase inhibitors for use against cancer |
| US8815893B2 (en) | 2010-02-22 | 2014-08-26 | Merck Patent Gmbh | Hetarylaminonaphthyridines |
| US8846931B2 (en) | 2010-07-05 | 2014-09-30 | Merck Patent Gmbh | Bipyridyl derivatives |
| WO2014190441A1 (en) | 2013-05-31 | 2014-12-04 | Zymeworks Inc. | Heteromultimers with reduced or silenced effector function |
| US8912216B2 (en) | 2010-02-05 | 2014-12-16 | Merck Patent Gmbh | Hetaryl-[1,8]naphthyridine derivatives |
| US8987301B2 (en) | 2009-11-07 | 2015-03-24 | Merck Patent Gmbh | Heteroarylaminoquinolines as TGF-beta receptor kinase inhibitors |
| US9051318B2 (en) | 2011-03-09 | 2015-06-09 | Merck Patent Gmbh | Pyrido [2, 3-B] pyrazine compounds and their therapeutical uses such as for inhibiting ATP consuming proteins and treating diseases associated therewith |
| US9109035B2 (en) | 2006-03-17 | 2015-08-18 | Genentech, Inc. | Anti-TAT226 antibodies and immunoconjugates |
| US9790268B2 (en) | 2012-09-12 | 2017-10-17 | Genzyme Corporation | Fc containing polypeptides with altered glycosylation and reduced effector function |
| WO2019108863A1 (en)* | 2017-11-29 | 2019-06-06 | Magenta Therapeutics, Inc. | Compositions and methods for the depletion of cd5+ cells |
| WO2019195278A1 (en) | 2018-04-02 | 2019-10-10 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2020013803A1 (en)* | 2018-07-09 | 2020-01-16 | Synthis, Llc | Antibody-alk5 inhibitor conjugates and their uses |
| WO2020086776A1 (en)* | 2018-10-23 | 2020-04-30 | Magenta Therapeutics, Inc. | Fc silenced antibody drug conjugates (adcs) and uses thereof |
| WO2020256751A1 (en) | 2019-06-19 | 2020-12-24 | Synthis, Llc | Antib0dy-alk5 inhibitor conjugates and their uses |
| WO2021011834A1 (en) | 2019-07-16 | 2021-01-21 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2021142086A1 (en)* | 2020-01-08 | 2021-07-15 | Synthis Therapeutics, Inc. | Alk5 inhibitor conjugates and uses thereof |
| EP3915641A1 (en)* | 2020-05-27 | 2021-12-01 | International-Drug-Development-Biotech | Anti-cd5 antibodies and methods of use thereof |
| WO2022006340A1 (en) | 2020-07-01 | 2022-01-06 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2022076905A1 (en) | 2020-10-09 | 2022-04-14 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
- 2024
- 2024-06-12WOPCT/US2024/033613patent/WO2024258967A1/enactivePending
Patent Citations (106)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US614226A (en) | 1898-11-15 | Implement for remodeling valve-seats | ||
| US4510245A (en) | 1982-11-18 | 1985-04-09 | Chiron Corporation | Adenovirus promoter system |
| US4816397A (en) | 1983-03-25 | 1989-03-28 | Celltech, Limited | Multichain polypeptides or proteins and processes for their production |
| WO1986001533A1 (en) | 1984-09-03 | 1986-03-13 | Celltech Limited | Production of chimeric antibodies |
| US5168062A (en) | 1985-01-30 | 1992-12-01 | University Of Iowa Research Foundation | Transfer vectors and microorganisms containing human cytomegalovirus immediate-early promoter-regulatory DNA sequence |
| US4968615A (en) | 1985-12-18 | 1990-11-06 | Ciba-Geigy Corporation | Deoxyribonucleic acid segment from a virus |
| EP0239400A2 (en) | 1986-03-27 | 1987-09-30 | Medical Research Council | Recombinant antibodies and methods for their production |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US4880935A (en) | 1986-07-11 | 1989-11-14 | Icrf (Patents) Limited | Heterobifunctional linking agents derived from N-succinimido-dithio-alpha methyl-methylene-benzoates |
| US5122368A (en) | 1988-02-11 | 1992-06-16 | Bristol-Myers Squibb Company | Anthracycline conjugates having a novel linker and methods for their production |
| US6180370B1 (en) | 1988-12-28 | 2001-01-30 | Protein Design Labs, Inc. | Humanized immunoglobulins and methods of making the same |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5585089A (en) | 1988-12-28 | 1996-12-17 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5693761A (en) | 1988-12-28 | 1997-12-02 | Protein Design Labs, Inc. | Polynucleotides encoding improved humanized immunoglobulins |
| US5693762A (en) | 1988-12-28 | 1997-12-02 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| EP0392745A2 (en) | 1989-04-05 | 1990-10-17 | Celltech Limited | Immunoconjugates and prodrugs and their use in association for drug delivery |
| US5208020A (en) | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| WO1991009967A1 (en) | 1989-12-21 | 1991-07-11 | Celltech Limited | Humanised antibodies |
| EP0519596A1 (en) | 1991-05-17 | 1992-12-23 | Merck & Co. Inc. | A method for reducing the immunogenicity of antibody variable domains |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| EP1291360A1 (en)* | 1991-12-13 | 2003-03-12 | Xoma Corporation | Methods and materials for preparation of modified antibody variable domains and therapeutic uses thereof |
| US5622929A (en) | 1992-01-23 | 1997-04-22 | Bristol-Myers Squibb Company | Thioether conjugates |
| EP0592106A1 (en) | 1992-09-09 | 1994-04-13 | Immunogen Inc | Resurfacing of rodent antibodies |
| US6214345B1 (en) | 1993-05-14 | 2001-04-10 | Bristol-Myers Squibb Co. | Lysosomal enzyme-cleavable antitumor drug conjugates |
| US5824805A (en) | 1995-12-22 | 1998-10-20 | King; Dalton | Branched hydrazone linkers |
| US5834597A (en) | 1996-05-20 | 1998-11-10 | Protein Design Labs, Inc. | Mutated nonactivating IgG2 domains and anti CD3 antibodies incorporating the same |
| US20070280931A1 (en) | 1998-11-18 | 2007-12-06 | Chen Yvonne M | Antibody variants |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| US6465493B1 (en) | 1999-04-09 | 2002-10-15 | Smithkline Beecham Corporation | Triarylimidazoles |
| WO2000061576A1 (en) | 1999-04-09 | 2000-10-19 | Smithkline Beecham Corporation | Triarylimidazoles |
| WO2001062756A1 (en) | 2000-02-21 | 2001-08-30 | Smithkline Beecham P.L.C. | Pyridinylimidazoles |
| US6906089B2 (en) | 2000-03-27 | 2005-06-14 | Smithkline Beecham Corporation | Triarylimidazole derivatives as cytokine inhibitors |
| US20030149277A1 (en) | 2000-03-27 | 2003-08-07 | Gaster Laramie Mary | Triarylimidazole derivatives as cytokine inhibitors |
| WO2002055077A1 (en) | 2001-01-11 | 2002-07-18 | Smithkline Beecham Corporation | Use of imidazolyl cyclic acetal derivatives in the manufacture of a medicament for the treatment of diseases mediated by the alk5 receptors |
| WO2002083180A1 (en) | 2001-03-23 | 2002-10-24 | Syntarga B.V. | Elongated and multiple spacers in activatible prodrugs |
| US7087626B2 (en) | 2001-05-24 | 2006-08-08 | Eli Lilly And Company | Pyrrole derivatives as pharmaceutical agents |
| WO2003087304A2 (en) | 2002-04-04 | 2003-10-23 | Biogen, Inc. | Tri-substituted heteroaryls and methods of making and using the same |
| US7612094B2 (en) | 2002-04-04 | 2009-11-03 | Biogen Idec Ma Inc. | Tri-substituted heteroaryls and methods of making and using the same |
| US20060074008A1 (en) | 2002-07-31 | 2006-04-06 | Senter Peter D | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| WO2004010957A2 (en) | 2002-07-31 | 2004-02-05 | Seattle Genetics, Inc. | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| US7691865B2 (en) | 2002-09-06 | 2010-04-06 | Biogen Idec Ma Inc. | Pyrazolopyridines and methods of making and using the same |
| US7365066B2 (en) | 2002-09-17 | 2008-04-29 | Eli Lilly And Company | Pyrazolopyridine derivatives as pharmaceutical agents |
| WO2004043493A1 (en) | 2002-11-14 | 2004-05-27 | Syntarga B.V. | Prodrugs built as multiple self-elimination-release spacers |
| US7265225B2 (en) | 2002-11-22 | 2007-09-04 | Eli Lilly And Company | Quinolinyl-pyrrolopyrazoles |
| US7405299B2 (en) | 2002-11-27 | 2008-07-29 | Eli Lilly And Company | Compounds as pharmaceutical agents |
| WO2004065392A1 (en) | 2003-01-24 | 2004-08-05 | Smithkline Beecham Corporation | Condensed pyridines and pyrimidines and their use as alk-5 receptor ligands |
| US20050238649A1 (en) | 2003-11-06 | 2005-10-27 | Seattle Genetics, Inc. | Monomethylvaline compounds capable of conjugation to ligands |
| US20050256030A1 (en) | 2004-02-23 | 2005-11-17 | Bainian Feng | Heterocyclic self-immolative linkers and conjugates |
| US7368445B2 (en) | 2004-03-01 | 2008-05-06 | Eli Lilly And Company | Fused pyrazole derivatives as TGF-β signal transduction inhibitors for the treatment of fibrosis and neoplasms |
| WO2005123780A2 (en) | 2004-04-09 | 2005-12-29 | Protein Design Labs, Inc. | Alteration of fcrn binding affinities or serum half-lives of antibodies by mutagenesis |
| US20080319012A1 (en) | 2004-04-21 | 2008-12-25 | In2Gen Co., Ltd. | 2-Pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| US7407958B2 (en) | 2004-04-21 | 2008-08-05 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| US8410146B2 (en) | 2004-04-21 | 2013-04-02 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| WO2005103028A1 (en) | 2004-04-21 | 2005-11-03 | In2Gen Co., Ltd | 2-pyridyl substituted imidazoles as alk5 and/or alk4 inhibitors |
| US8420685B2 (en) | 2004-04-21 | 2013-04-16 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors |
| US20060024317A1 (en) | 2004-05-19 | 2006-02-02 | Medarex, Inc | Chemical linkers and conjugates thereof |
| WO2005112919A2 (en) | 2004-05-19 | 2005-12-01 | Medarex, Inc. | Self-immolative linkers and drug conjugates |
| WO2005117984A2 (en) | 2004-06-01 | 2005-12-15 | Celltech R & D Limited | Albumin-binding conjugates comprising a fatty acid and peg |
| WO2006034488A2 (en) | 2004-09-23 | 2006-03-30 | Genentech, Inc. | Cysteine engineered antibodies and conjugates |
| US20060134709A1 (en) | 2004-11-10 | 2006-06-22 | Jeffery Stavenhagen | Engineering Fc antibody regions to confer effector function |
| US7511056B2 (en) | 2004-11-10 | 2009-03-31 | Eli Lilly And Company | TGF-β inhibitors |
| WO2006110476A2 (en) | 2005-04-08 | 2006-10-19 | Medarex, Inc. | Cytotoxic compounds and conjugates comprising duocarmycins with cleavable substrates |
| US7863288B2 (en) | 2005-06-22 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| WO2007018431A2 (en) | 2005-08-05 | 2007-02-15 | Syntarga B.V. | Triazole-containing releasable linkers and conjugates comprising the same |
| WO2007059404A2 (en) | 2005-11-10 | 2007-05-24 | Medarex, Inc. | Duocarmycin derivatives as novel cytotoxic compounds and conjugates |
| WO2007070538A2 (en) | 2005-12-12 | 2007-06-21 | Bayer Healthcare Llc | Anti-mn antibodies and methods of using same |
| WO2007089149A2 (en) | 2006-02-02 | 2007-08-09 | Syntarga B.V. | Water-soluble cc-1065 analogs and their conjugates |
| US9109035B2 (en) | 2006-03-17 | 2015-08-18 | Genentech, Inc. | Anti-TAT226 antibodies and immunoconjugates |
| WO2009017394A1 (en) | 2007-08-01 | 2009-02-05 | Syntarga B.V. | Substituted cc-1065 analogs and their conjugates |
| WO2009050183A2 (en) | 2007-10-17 | 2009-04-23 | Novartis Ag | Imidazo [1, 2-a] pyridine derivatives useful as alk inhibitors |
| WO2009073445A2 (en) | 2007-11-28 | 2009-06-11 | Mersana Therapeutics, Inc. | Biocompatible biodegradable fumagillin analog conjugates |
| WO2009133070A1 (en) | 2008-04-29 | 2009-11-05 | Novartis Ag | Imidazo-pyridine derivatives as activin-like receptor kinase (alk4 or alk5) inhibitors |
| WO2010022737A1 (en)* | 2008-08-29 | 2010-03-04 | Symphogen A/S | Anti-cd5 antibodies |
| WO2010062171A2 (en) | 2008-11-03 | 2010-06-03 | Syntarga B.V. | Novel cc-1065 analogs and their conjugates |
| WO2010068795A2 (en) | 2008-12-12 | 2010-06-17 | Pearson Robert M | Method and system for tissue treatment utilizing irreversible electroporation and thermal track coagulation |
| US8513228B2 (en) | 2009-02-20 | 2013-08-20 | Richter Gedeon Nyrt. | Crystalline form of antiprogestin CDB-4124 |
| US8679500B2 (en) | 2009-05-14 | 2014-03-25 | Institute National De La Sante Et De La Recherche Medicale (Inserm) | Compositions containing antibodies for treating CD5+ HLA-DR+ B or T cell related diseases |
| WO2010138719A1 (en) | 2009-05-28 | 2010-12-02 | Mersana Therapeutics, Inc. | Polyal drug conjugates comprising variable rate-releasing linkers |
| US8987301B2 (en) | 2009-11-07 | 2015-03-24 | Merck Patent Gmbh | Heteroarylaminoquinolines as TGF-beta receptor kinase inhibitors |
| US8912216B2 (en) | 2010-02-05 | 2014-12-16 | Merck Patent Gmbh | Hetaryl-[1,8]naphthyridine derivatives |
| US8815893B2 (en) | 2010-02-22 | 2014-08-26 | Merck Patent Gmbh | Hetarylaminonaphthyridines |
| WO2011120053A1 (en) | 2010-03-26 | 2011-09-29 | Mersana Therapeutics, Inc. | Modified polymers for delivery of polynucleotides, method of manufacture, and methods of use thereof |
| US9073918B2 (en) | 2010-05-20 | 2015-07-07 | Takeda Pharmaceutical Company Limited | Pyrazolo[4,3-b]pyridine-7-amine inhibitors of ALK5 |
| WO2011146287A1 (en) | 2010-05-20 | 2011-11-24 | Takeda Pharmaceutical Company Limited | Pyrazolo[4,3-b]pyridine-7-amine inhibitors of alk5 |
| US8791113B2 (en) | 2010-06-28 | 2014-07-29 | Merck Patent Gmbh | 2,4-diaryl-substituted [1,8] naphthyridines as kinase inhibitors for use against cancer |
| US8846931B2 (en) | 2010-07-05 | 2014-09-30 | Merck Patent Gmbh | Bipyridyl derivatives |
| US9051307B2 (en) | 2010-07-05 | 2015-06-09 | Merck Patent Gmbh | Bipyridyl derivatives |
| US9051318B2 (en) | 2011-03-09 | 2015-06-09 | Merck Patent Gmbh | Pyrido [2, 3-B] pyrazine compounds and their therapeutical uses such as for inhibiting ATP consuming proteins and treating diseases associated therewith |
| WO2013009140A2 (en) | 2011-07-13 | 2013-01-17 | Sk Chemicals Co., Ltd. | 2-pyridyl substituted imidazoles as alk5 and/or alk4 inhibitors |
| WO2013096901A1 (en) | 2011-12-23 | 2013-06-27 | Mersana Therapeutics, Inc. | Pharmaceutical formulations for fumagillin derivative-phf conjugates |
| WO2014008375A1 (en) | 2012-07-05 | 2014-01-09 | Mersana Therapeutics, Inc. | Terminally modified polymers and conjugates thereof |
| US9790268B2 (en) | 2012-09-12 | 2017-10-17 | Genzyme Corporation | Fc containing polypeptides with altered glycosylation and reduced effector function |
| WO2014093394A1 (en) | 2012-12-10 | 2014-06-19 | Mersana Therapeutics, Inc. | Protein-polymer-drug conjugates |
| WO2014093379A1 (en) | 2012-12-10 | 2014-06-19 | Mersana Therapeutics, Inc. | Auristatin compounds and conjugates thereof |
| WO2014093640A1 (en) | 2012-12-12 | 2014-06-19 | Mersana Therapeutics,Inc. | Hydroxy-polmer-drug-protein conjugates |
| WO2014190441A1 (en) | 2013-05-31 | 2014-12-04 | Zymeworks Inc. | Heteromultimers with reduced or silenced effector function |
| WO2019108863A1 (en)* | 2017-11-29 | 2019-06-06 | Magenta Therapeutics, Inc. | Compositions and methods for the depletion of cd5+ cells |
| WO2019195278A1 (en) | 2018-04-02 | 2019-10-10 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2020013803A1 (en)* | 2018-07-09 | 2020-01-16 | Synthis, Llc | Antibody-alk5 inhibitor conjugates and their uses |
| WO2020086776A1 (en)* | 2018-10-23 | 2020-04-30 | Magenta Therapeutics, Inc. | Fc silenced antibody drug conjugates (adcs) and uses thereof |
| WO2020256751A1 (en) | 2019-06-19 | 2020-12-24 | Synthis, Llc | Antib0dy-alk5 inhibitor conjugates and their uses |
| WO2021011834A1 (en) | 2019-07-16 | 2021-01-21 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2021142086A1 (en)* | 2020-01-08 | 2021-07-15 | Synthis Therapeutics, Inc. | Alk5 inhibitor conjugates and uses thereof |
| EP3915641A1 (en)* | 2020-05-27 | 2021-12-01 | International-Drug-Development-Biotech | Anti-cd5 antibodies and methods of use thereof |
| WO2022006340A1 (en) | 2020-07-01 | 2022-01-06 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
| WO2022076905A1 (en) | 2020-10-09 | 2022-04-14 | Silverback Therapeutics, Inc. | Alk5 inhibitors, conjugates, and uses thereof |
Non-Patent Citations (40)
| Title |
|---|
| AL-LAZIKANI ET AL., J. MOL. BIOL, vol. 273, 1997, pages 927 - 948 |
| AMSBERRY ET AL., J. ORG. CHEM., vol. 55, 1990, pages 5867 |
| ANSEL ET AL.: "Pharmaceutical Dosage Forms And Drug Delivery Systems", 1990, MACK PUBLISHING COMPANY |
| CANFIELDMORRISON, J EXP. MED., vol. 173, 1991, pages 1483 - 1491 |
| CHARI ET AL., CANCER RESEARCH, vol. 52, no. 396129-53-6, 1992, pages 127 - 131 |
| CHU ET AL., BIOCHEMIA, no. 2, 2001 |
| CURRENT OPINION IN CHEMICAL BIOLOGY, vol. 17, pages 420 - 426 |
| DORONINA ET AL., NAT. BIOTECHNOL., vol. 21, 2003, pages 778 - 784 |
| DUBOWCHIK ET AL., PHARMACOLOGY AND THERAPEUTICS, vol. 83, 1999, pages 67 - 123 |
| DUBOWCHIKWALKER, PHARM. THERAPEUTICS, vol. 83, 1999, pages 67 - 123 |
| DUCRY ET AL., BIOCONJUGATE CHEM, vol. 21, 2010, pages 5 - 13 |
| FISHER: "Laboratory Techniques In Biochemistry And Molecular Biology", 1980, ELSEVIER |
| HAMANN ET AL., EXPERT OPIN. THER. PATENTS, vol. 15, 2005, pages 1087 - 1103 |
| HAY ET AL., BIOORG. MED. CHEM. LETT, vol. 9, 1999, pages 2237 |
| JOHNSON ET AL., ANTICANCER RES, vol. 15, 1995, pages 1387 - 93 |
| JUNGPLUCKTHUN, PROTEIN ENGINEERING, vol. 10, no. 9, 1997, pages 959 - 966 |
| KABAT ET AL.: "Sequences of Proteins of Immunological Interest", 1991, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES |
| KAUFMANSHARP, MOL. BIOL, vol. 159, 1982, pages 601 - 621 |
| KINGSBURY ET AL., J. MED. CHEM., vol. 27, 1984, pages 1447 |
| LAU ET AL., BIOORG-MED-CHEM, vol. 3, no. 10, 1995, pages 1305 - 1304 |
| LEFRANC ET AL., DEV COMPARAT IMMUNOL, vol. 27, 2003, pages 55 - 77 |
| LO. ET AL., J BIOL CHEM, vol. 292, 2017, pages 3900 - 08 |
| LUND ET AL., J. IMMUNOL., vol. 147, 1991, pages 2657 - 2662 |
| MURRAY ET AL., ROCHE MOLECULAR BIOLOGICALS, 2013 |
| NEVILLE ET AL., BIOL. CHEM., vol. 264, 1989, pages 14653 - 14661 |
| PADLAN, MOL. IMMUNOL, vol. 28, 1991, pages 489 - 498 |
| RIECHMANN ET AL., NATURE, vol. 332, 1988, pages 323 - 7 |
| RODRIGUES ET AL., CHEMISTRY BIOLOGY, vol. 2, 1995, pages 223 |
| ROGUSKA ET AL., PROC. NATL. ACAD. SCI., vol. 91, 1994, pages 969 - 973 |
| SALTZMAN ET AL., BIOPHYS. J, vol. 55, 1989, pages 163 |
| SHERWOOD ET AL., BIOΓΓECHNOLOGY, vol. 10, 1992, pages 1446 |
| STORM ET AL., AMER. CHEM. SOC, vol. 94, 1972, pages 5815 |
| STUDNICKA ET AL., PROT. ENG, vol. 7, 1994, pages 805 - 814 |
| THORPE ET AL., CANCER RES., vol. 47, 1987, pages 5924 - 5931 |
| THORPE ET AL., IMMUNOL. REV, vol. 62, 1982, pages 119 - 58 |
| URLAUBCHASIN, PROC. NATL. ACAD. SCI. USA, vol. 77, 1980, pages 4216 - 4220 |
| WANG ET AL., PROTEIN CELL, vol. 9, 2018, pages 63 - 73 |
| WAWRZYNCZAK ET AL.: "In Immunoconjugates: Antibody Conjugates in Radioimagery and Therapy of Cancer", 1987, OXFORD U. PRESS |
| WOLFSON, CHEM. BIOL., vol. 13, no. 10, 2006, pages 1011 - 2 |
| YAZAKI ET AL., PROTEIN ENG. DES SEL, vol. 17, no. 5, 2004, pages 481 - 9 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230218776A1 (en) | Anti-ntb-a antibodies and related compositions and methods | |
| US20210128742A1 (en) | CD123 Antibodies and Conjugates Thereof | |
| US20210047404A1 (en) | Cd33 antibodies and use of same to treat cancer | |
| JP7573685B2 (en) | Antibody-ALK5 inhibitor conjugates and uses thereof | |
| JP6449777B2 (en) | Anti-NTB-A antibodies and related compositions and methods | |
| TWI872065B (en) | Biparatopic fr-alpha antibodies and immunoconjugates | |
| TW202122421A (en) | Anti-pd-l1 antibodies and antibody-drug conjugates | |
| JP2024517776A (en) | Antibody-drug conjugates targeting nectin-4 and methods for preparing and using same | |
| KR20220110231A (en) | Anti-αvβ6 Antibodies and Antibody-Drug Conjugates | |
| JP2024059856A (en) | Antibody-alk5 inhibitor conjugates and their uses | |
| WO2023141714A1 (en) | Methods of using anti-her2 biparatopic antibody-drug conjugates in the treatment of cancer | |
| WO2024258967A1 (en) | Anti-cd5 antibodies and their uses | |
| RU2832083C2 (en) | Biparatopic fr-alpha antibodies and immunoconjugates | |
| HK1253305B (en) | Anti-cd123 antibodies and conjugates thereof | |
| HK1206750B (en) | Cd33 antibodies and use of same to treat cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24740275 Country of ref document:EP Kind code of ref document:A1 |