WO2024229356A2 - Artificial solemoviridae satellite rnas - Google Patents
Artificial solemoviridae satellite rnasDownload PDFInfo
- Publication number
- WO2024229356A2 WO2024229356A2PCT/US2024/027683US2024027683WWO2024229356A2WO 2024229356 A2WO2024229356 A2WO 2024229356A2US 2024027683 WUS2024027683 WUS 2024027683WWO 2024229356 A2WO2024229356 A2WO 2024229356A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- rna
- virus
- hrv
- sequence
- dna
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8201—Methods for introducing genetic material into plant cells, e.g. DNA, RNA, stable or transient incorporation, tissue culture methods adapted for transformation
- C12N15/8202—Methods for introducing genetic material into plant cells, e.g. DNA, RNA, stable or transient incorporation, tissue culture methods adapted for transformation by biological means, e.g. cell mediated or natural vector
- C12N15/8203—Virus mediated transformation
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8241—Phenotypically and genetically modified plants via recombinant DNA technology
- C12N15/8261—Phenotypically and genetically modified plants via recombinant DNA technology with agronomic (input) traits, e.g. crop yield
- C12N15/8271—Phenotypically and genetically modified plants via recombinant DNA technology with agronomic (input) traits, e.g. crop yield for stress resistance, e.g. heavy metal resistance
Definitions
- RNA moleculescomprising from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule (cargo RNA sequence); and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the cargo RNA molecule is heterologous to the 5’ RNA replication element and the 3’ RNA replication element.
- RdRPSolemoviridae RNA-dependent RNA polymerase
- the 5’ RNA replication element and the 3’ RNA replication elementare obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related;
- the 5’ RNA replication element, the 3’ RNA replication element, and the RdRPare obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; or
- the 5’ RNA replication element, the 3’ RNA replication element, and/or the RdRP coding regionare obtained from different Solemoviridae genomes, and the members of each respective set of the 5’ RNA replication elements, 3’ RNA replication elements, and/or RdRP coding regions have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another.
- the RNA moleculecomprises: at least one heterologous RNA virus (HRV) amplicon in sense or antisense orientation to the first 5’ RNA replication element comprising: I. (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV Agent Ref: P14419WO00 - 2 - 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked; or II.
- HRVheterologous RNA virus
- heterologous RNA virus (HRV) subgenomic promoteroperably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP).
- HRVheterologous RNA virus RNA-dependent RNA polymerase
- Agricultural formulations as well as bacterial, fungal, plant, insect, and invertebrate animal cellscomprising the herein disclosed recombinant RNAs are also provided.
- RNA moleculescomprising the herein disclosed recombinant RNA molecules; and a cell containing the recombinant RNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements of the recombinant RNA molecule.
- the cellfurther comprises one or more of: (i) a viral capsid protein (CP); (ii) an RNA-binding protein (RBP) that binds to the RNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; (iv) a second RNA-dependent RNA polymerase (2 nd RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the RNA molecule; (v) a viral movement protein (MP); (vi) a heterologous RNA virus (HRV); or (vii) an hrvRdRP, optionally wherein the hrvRdRP recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter.
- CPa viral capsid protein
- RBPRNA-binding protein
- RBPRNA-binding protein
- methods of establishing a synthetic Solemoviridae satellite RNA in a plant cellcomprising: providing to a plant cell any of the herein disclosed recombinant RNA molecules, wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element, wherein the RNA molecule optionally comprises an encapsidation recognition element (ERE) and is or can be encapsidated by a capsid protein, whereby the RdRP protein catalyzes synthesis of the synthetic Solemoviridae satellite RNA from the recombinant RNA molecule.
- ERPencapsidation recognition element
- Also provided are methods of obtaining a phenotypic change in a plant or plant cellcomprising: providing to a plant or plant cell any of the herein disclosed recombinant RNA molecules, wherein the cargo RNA molecule comprises RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element and catalyzes synthesis of a synthetic Solemoviridae RNA from the recombinant RNA molecule, and wherein and the cargo RNA molecule effects the phenotypic change.
- the methodsfurther comprise providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof.
- Also provided are methods of manufacturing a synthetic Solemoviridae satellite particlecomprising combining any of the herein disclosed recombinant RNA molecules with a viral capsid Agent Ref: P14419WO00 - 3 - protein, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein.
- EREencapsidation recognition element
- plant propagulescomprising any of the herein disclosed recombinant RNA molecules and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- the plant propagulefurther comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA
- the heterologous RNA virus RdRPis an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP.
- plantscomprising any of the herein disclosed recombinant RNA molecules and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- Solemoviridae satellite systemsthat are self-replicating when introduced into a plant or plant cell, comprising: (1) any of the herein disclosed recombinant Solemoviridae satellite RNAs (e.g., recombinant RNA molecules); and (2) an exogenous Solemoviridae that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA, optionally wherein the Solemoviridae satellite system further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- Solemoviridae satellite systemfurther comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the
- Figure 1shows a non-limiting embodiment of a structure of a Solemoviridae satellite construct.
- the 5’ RNA replication elementis labelled “5’ RRE” and the 3’ RNA replication element is labeled “3’ RRE.”
- Figure 2shows non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising: (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR).
- HRVheterologous RNA virus
- cleavable sequenceis located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element.
- the cleavable sequenceis optionally a self-cleaving ribozyme, a self-cleaving inducible ribozyme, or an Agent Ref: P14419WO00 - 4 - siRNA or miRNA recognition site.
- a subgenomic promoter and/or an IRESis/are operably linked to the cargo RNA.
- Figure 3shows non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising a heterologous RNA virus (HRV) subgenomic promoter (HRV sgp) which is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP) and which is operably linked to the cargo RNA molecule.
- HRV sgp and cargo RNAare in sense and antisense orientation relative to the Solemoviridae 5’ RRE are shown.
- Figure 4depicts a Solemoviridae satellite construct comprising heterologous RNA virus (HRV) subgenomic promoters (HRV sgp) with: (i) one HRV sgp operably linked to a cargo RNA; and (ii) one HRV sgp operably linked to RNA encoding an hrvRdRP which recognizes both of the HRV sgp (i.e., can drive expression of the operably linked hrvRdRP and cargo RNA).
- HRV sgpheterologous RNA virus
- HRV sgpheterologous RNA virus subgenomic promoters
- an IRESis operably linked to the cargo RNA and/or an IRES is operably linked to the RNA encoding the hrvRdRP.
- FIG. 5depicts non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising a heterologous RNA virus (HRV) subgenomic promoter (HRV sgp) which is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP) and which is operably linked to the cargo RNA molecule flanked by HRV 5’RR and HRV 3’ RR.
- HRV sgp and cargo RNAare in sense or antisense orientation relative to the Solemoviridae 5’ RRE are shown.
- the HRV 5’ RR and 3’ RR which flank the cargo RNAprovide for hrvRdRP-mediated replication of an RNA comprising from 5’ to 3’ the HRV 5’ RR, cargo RNA, and HRV 3’ RR.
- the HRV 5’ RR and 3’ RRare flanked by ribozymes.
- Figure 6depicts a commensal satellite with a cargo RNA molecule including an HRV (“HRV1”, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV (“HRV1”, e.g., TMV) RdRP binding to either the replication regions or to the subgenomic promoter, where the commensal satellite is a Solemoviridae satellite.
- HRV1tobacco mosaic virus
- RdRPRNA encoding the HRV RdRP
- solid squareswhich can further amplify the HRV amplicon, as well as RNA encoding another cargo (solid circles). In the absence of the commensal virus, no amplification of the commensal satellite occurs.
- Figure 7depicts a commensal satellite with a cargo RNA molecule including an HRV (HRV1, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV RdRP (“HRV1 RdRP”, solid squares) binding to the HRV1 replication regions, where the commensal satellite is a Solemoviridae satellite.
- HRVtobacco mosaic virus
- the resulting transcriptsinclude RNA encoding the HRV (HRV1) RdRP which can further amplify the HRV amplicon.
- the HRV1 ampliconincludes sequence for a HRV2 amplicon (indicated in italicized text), encoding a coding and/or noncoding cargo (solid circles) and designed to be amplified in the presence of a second acute viral RdRP (“HRV2 RdRP”, hexagonal symbol), which can be provided, Agent Ref: P14419WO00 - 5 - e.g., by introduction of a second acute virus (“HRV2”, e.g., cowpea mosaic virus, CPMV) into the plant. In the absence of the commensal virus, no amplification of the commensal satellite occurs.
- HRV2 RdRPhexagonal symbol
- Figure 8depicts a commensal satellite with a cargo RNA molecule including an HRV (“HRV1”, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV (“HRV1”, e.g., TMV) RdRP binding to either of two subgenomic promoters, where the commensal satellite is a Solemoviridae satellite.
- HRV1tobacco mosaic virus
- TMVtobacco mosaic virus
- the resulting transcriptsinclude RNA encoding the HRV RdRP (“HRV1 RdRP”, solid squares), which can further amplify the HRV amplicon, as well as RNA encoding a noncoding RNAi cargo, the sense and antisense strands of which are formed during the amplification process to yield a double-stranded RNA molecule (dsRNA) for silencing of a target gene.
- HRV1 RdRPsolid squares
- RNA encoding a noncoding RNAi cargothe sense and antisense strands of which are formed during the amplification process to yield a double-stranded RNA molecule (dsRNA) for silencing of a target gene.
- dsRNAdouble-stranded RNA molecule
- the term “and/or” as used in a phrase such as “A and/or B” hereinis intended to include “A and B,” “A or B,” “A” (alone), and “B” (alone).
- the term “and/or” as used in a phrase such as “A, B, and/or C”is intended to encompass each of the following embodiments: A, B, and C; A, B, or C; A or C; A or B; B or C; A and C; A and B; B and C; A (alone); B (alone); and C (alone).
- F 1the terms “F 1 ,” “F 2 ,” and the like refer to filial generations of plants or seed obtained from a parent plant which has been selfed or that has been crossed to another plant.
- heterologouswhen used to describe a first element in reference to a second element means that the first element and second element do not exist in nature disposed as described.
- a heterologous nucleic acid molecule or sequenceis a nucleic acid molecule or sequence that (a) is not native to a cell in which it is expressed, (b) is linked or fused to a nucleic acid molecule or sequence with which it is not linked to or fused to in nature, or with which it is not linked to or fused to in nature in the same way, (c) has been altered or mutated by the hand of man relative to its native state, or (d) has altered expression as compared to its native expression levels under similar conditions.
- a “heterologous promoter”is used to drive transcription of a sequence that is not one that is natively transcribed by that promoter (e.g., a eukaryote promoter used to drive transcription of a DNA molecule encoding a Solemoviridae RNA sequence); thus, a “heterologous promoter” sequence can be included in an expression construct by a recombinant nucleic acid technique.
- a recombinant polynucleotidesuch as those provided by this disclosure includes genetic sequences of two or more different viruses of the family Solemoviridae, which genetic sequences are “heterologous” in that they would not naturally occur together.
- heterologousrefers to a molecule or to a discrete part of a molecule; for example, referring to a cargo RNA molecule (e.g., a nucleic acid such as a protein-encoding RNA, an ssRNA, a regulatory RNA, an interfering RNA, or a guide RNA), which can be part of a larger molecule, or referring to a structure (e.g., structures Agent Ref: P14419WO00 - 6 - including a promoter (e.g., for a DNA dependent RNA polymerase) or subgenomic promoter (e.g., for an RNA-dependent RNA polymerase), an RNA effecter, RNA cleavage agent recognition site, or a polynucleotide comprising or encoding an expression-enhancing element, encapsidation recognition element (ERE), selectable or scoreable marker, DNA aptamer, RNA aptamer; a transcription factor binding
- IRESinternal ribosome entry site
- An IRES elementis generally between 100-800 nucleotides.
- IRESencephalomyocarditis virus IRES
- ECMVencephalomyocarditis virus
- maize hsp101 IRES 5’UTRcrucifer infecting tobamovirus crTMV CR-CP 148 IRES
- tobacco etch virus (TEV) IRES 5’UTRhibiscus chlorotic ringspot virus (HCRSV) IRES.
- HCRSVhibiscus chlorotic ringspot virus
- an IRES sequenceis derived from non-plant eukaryotic virus sequences that include but are not limited to: acute bee paralysis virus (ABPV), classical swine fever virus (CSFV), coxsackievirus B3 virus (CVB3), encephalomyocarditis virus (ECMV), enterovirus 71 (E71), hepatitis A virus (HAV), human rhinovirus (HRV2), human rhinovirus (HRV2), human lymphotropic virus (HTLV), polyoma virus (PV), and Zea mays (ZmHSP101).
- a virusA virus
- HRV2human rhinovirus
- HRV2human rhinovirus
- HTLVhuman lymphotropic virus
- PVpolyoma virus
- ZmHSP101Zea mays
- the phrase “operably linked”refers to a juxtaposition wherein the components so described are in a relationship permitting them to function in their intended manner.
- a promoteris operably linked to a coding sequence if the promoter provides for transcription or expression of the coding sequence.
- percent identityrefers to percent (%) sequence identity with respect to a reference polynucleotide or polypeptide sequence following alignment by standard techniques. Alignment for purposes of determining percent nucleic acid or amino acid sequence identity can be achieved in various ways that are within the capabilities of one of skill in the art, for example, using publicly available computer software such as BLAST, BLAST-2, PSI-BLAST, or Megalign software.
- the softwareis MUSCLE (Edgar, Nucleic Acids Res., 32(5): 1792-1797, 2004).
- MUSCLENucleic Acids Res., 32(5): 1792-1797, 2004.
- percent sequence identity valuesare generated using the sequence comparison computer program BLAST (Altschul et al. (1990) J. Mol. Biol., 215:403-410).
- the percent sequence identity of a given nucleic acid or amino acid sequence, A, to, with, or against a given nucleic acid or amino acid sequence, B,is calculated as follows: 100 multiplied by (the fraction X/Y), where X is the number of nucleotides or amino acids scored as identical matches by a sequence alignment program (e.g., BLAST) in that program’s alignment of A and B, and where Y is the total number of nucleotides or amino acids in B.
- the term “plant”includes a whole plant and any descendant, cell, tissue, or part of a plant.
- plant partsinclude any part(s) of a plant, including, for example and without limitation: seed (including mature seed and immature seed); a plant cutting; a plant cell; a plant cell culture; or a plant organ (e.g., pollen, mature or immature embryos, flowers, fruits, shoots, leaves, roots, stems, and explants).
- a plant tissue or plant organis or includes a seed, protoplast, callus, or any other group of plant cells that is organized into a structural or functional unit.
- a plant cell or tissue cultureis capable of regenerating a plant having the physiological and morphological characteristics of the plant from which the cell or tissue was obtained, and of regenerating a plant having substantially the same genotype as the plant.
- Regenerable cells in a plant cell or tissue culturecan include embryos, protoplasts, meristematic cells, callus, pollen, leaves, anthers, roots, root tips, flowers, and/or stalks.
- some plant cellsare not capable of being regenerated to produce plants and are referred to herein as “non-regenerable” plant cells.
- the term “transcriptome”refers to the sum total of all RNA molecules expressed in a cell.
- RNA moleculesinclude mRNAs, tRNAs, ribosomal RNAs, miRNAs, viral RNAs (both genomic and sub-genomic), and long non-coding RNAs.
- RNA moleculesinclude mRNAs, tRNAs, ribosomal RNAs, miRNAs, viral RNAs (both genomic and sub-genomic), and long non-coding RNAs.
- any of the preceding definitionsis inconsistent with definitions provided in any patent or non-patent reference incorporated herein by reference, any patent or non-patent reference cited herein, or in any patent or non-patent reference found elsewhere, it is understood that the preceding definition will be used herein.
- nucleic acid sequences described hereinare given, when read from left to right, in the 5’ to 3’ direction. Nucleic acid sequences may be provided as DNA or as RNA, as specified.
- nucleic acid sequencescan encode the same polypeptide sequence, and such modified nucleic acid sequences (e.g., for the purposes of codon optimization for a given species) are within the scope of the present disclosure.
- modified nucleic acid sequencese.g., for the purposes of codon optimization for a given species
- recombinant polynucleotidese.g., recombinant DNA, recombinant RNA, recombinant ssRNAs, recombinant dsRNAs, recombinant vectors, etc.
- recombinant polynucleotidesincluding one or more sequences of or derived from a virus of the family Solemoviridae; in particular, a 5’ or 3’ RNA replication element that is recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP).
- RdRPSolemoviridae RNA-dependent RNA polymerase
- This disclosureis further related to methods of making and using such recombinant polynucleotides, for Agent Ref: P14419WO00 - 8 - example, by employing such recombinant polynucleotides to express a heterologous cargo sequence in a plant and optionally thereby modifying expression of an endogenous target sequence and/or genotype or phenotype of the plant.
- the virus of the family Solemoviridaeis a commensal virus of the family Solemoviridae, that is, a virus of the family Solemoviridae that is endemic or native to a given eukaryote host (such as a host plant) without causing apparent negative effects on the host (i.e., is considered non-pathogenic), is often present at a persistent but low population (i.e., low viral titer), and is often vertically transmitted to succeeding generations of the host.
- this disclosureis related to a recombinant DNA molecule that includes a promoter that is functional in a cell and is operably linked to a DNA sequence encoding an RNA molecule.
- the RNA moleculeincludes, in 5’ to 3’ order: (a) a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA sequence; and (c) a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP.
- RdRPSolemoviridae RNA-dependent RNA polymerase
- Figure 1shows an embodiment of a generalized structure of a DNA polynucleotide encoding a Solemoviridae satellite, where in certain embodiments the 5’ RNA replication element corresponds to the 5’ untranslated region (UTR) of a virus of the family Solemoviridae and where the 3’ RNA replication element corresponds to the 3’ untranslated region (UTR) of a virus of the family Solemoviridae.
- the 5’ RNA replication element and/or the 3’ RNA replication elementinclude nucleotides that extend into the predicted coding sequence or open reading frame of the virus of the family Solemoviridae.
- Recombinant DNA molecules provided hereincan include a promoter that is functional in a cell (e.g., a bacterial cell, a plant cell, a fungal cell, or an animal cell) and is operably linked to a DNA sequence encoding an RNA molecule (e.g. a 5’ RNA replication element, a cargo RNA sequence; and a 3’ RNA replication element; a ribozyme, an intron, or a RNA encoding a protein (e.g., a capsid, movement, RdRP, or an RdRP protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter).
- a promoterthat is functional in a cell (e.g., a bacterial cell, a plant cell, a fungal cell, or an animal cell) and is operably linked to a DNA sequence encoding an RNA molecule (e.g. a 5’ RNA replication element, a cargo RNA sequence; and a
- a promoter functional in a plant cellprovides for systemic gene expression, or alternatively for cell-, tissue-, or organ-specific gene expression, or expression that is inducible by external signals or agents (for example, light-, pathogen-, wound-, stress-, or hormone-inducible elements, or chemical inducers) or elements that are capable of cell-cycle regulated gene transcription; such elements may be located in the 5’ or 3’ regions of the native gene or engineered into a polynucleotide.
- Promotersinclude those from viruses, bacteria, fungi, animals, and plants.
- RNA polymerasee.g., RNA pol I, pol II, or pol III
- RNA polymerasee.g., RNA pol I, pol II, or pol III
- Embodiments of promotersinclude those from cauliflower mosaic virus (e.g., p35S), bacteriophage (e.g., pT7), and plants (e.g., pATUBQ10).
- the promoteris operably linked to nucleotide sequences encoding multiple guide RNAs, wherein the sequences encoding guide RNAs are separated by a cleavage site such as a nucleotide sequence encoding a microRNA recognition/cleavage Agent Ref: P14419WO00 - 9 - site or a self-cleaving ribozyme (see, e.g., Ferré-D’Amaré and Scott (2010) Cold Spring Harbor Perspectives Biol., 2:a003574).
- the promoteris a pol II promoter operably linked to a nucleotide sequence encoding the RNA.
- the promoter operably linked to one or more polynucleotides encoding elements of a genome-editing systemis a constitutive promoter that drives DNA expression in plant cells.
- the promoterdrives DNA expression in the nucleus or in an organelle such as a chloroplast or mitochondrion.
- constitutive promoters active in plant cellsinclude a CaMV 35S promoter as disclosed in U.S. Pat. Nos.5,858,742 and 5,322,938, a rice actin promoter as disclosed in U.S. Pat. No.5,641,876, a maize chloroplast aldolase promoter as disclosed in U.S. Pat.
- the promoter operably linked to one or more polynucleotides encoding elements of a genome-editing systemis a promoter from figwort mosaic virus (FMV), a RUBISCO promoter, or a pyruvate phosphate dikinase (PDK) promoter, which is active in the chloroplasts of mesophyll cells.
- FMVfigwort mosaic virus
- RUBISCORUBISCO promoter
- PDKpyruvate phosphate dikinase
- the promoteris heterologous to the cell it is functional in and/or to the other elements to which the promoter is operably linked.
- Embodiments of recombinant polynucleotides provided hereincomprise or encode RNA molecules containing 5’ and 3’ RNA replication elements recognized by a Solemoviridae RNA- dependent RNA polymerase (RdRP).
- RdRPSolemoviridae RNA-dependent RNA polymerase
- recognition by a Solemoviridae RdRPis identified in an in vitro RdRP assay (e.g., an assay adapted from Horiuchi et al. Plant Cell Physiol. 42(2):197-203, 2001).
- recognition by a Solemoviridae RdRPis identified by an in vivo RdRP assay wherein an RNA comprising 5’ and 3’ RNA replication elements is introduced into a cell comprising the RdRP, and replication of the RNA is assayed (e.g., by an RT-PCR assay or an assay for a reporter gene encoded by a cargo RNA located in the RNA comprising 5’ and 3’ RNA replication elements).
- cells comprising the RdRPare engineered by introducing a gene or RNA molecule encoding the RdRP into the cell.
- the cell comprising the RdRPis a cell which contains a virus of the family Solemoviridae which expresses the RdRP; in such embodiments the Solemoviridae virus can be one that is native to or is known to naturally occur in the cell, or it can be a non-native Solemoviridae virus, or alternatively a recombinant virus of any suitable viral family that is engineered to express the Solemoviridae RdRP.
- the recombinant polynucleotidescomprise a 5’ RNA replication element and a 3’ RNA replication element obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related.
- the Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one anotherare taxonomically related, e.g., genomes classified as belonging to the same genus, family, and/or order.
- the recombinant polynucleotidescomprise a 5’ RNA replication element, a 3’ RNA replication Agent Ref: P14419WO00 - 10 - element, and an RdRP are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally taxonomically related, e.g., genomes classified as belonging to the same genus, family, and/or order.
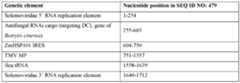
- Non-limiting examples of a 5’ RNA replication element and a 3’ RNA replication element from the same Solemoviridae genome and the corresponding RdRP protein that recognizes that 5’ RNA replication element and 3’ RNA replication elementinclude those set forth in each row of Table 18.
- the recombinant polynucleotidescomprise a 5’ RNA replication element, a 3’ RNA replication element, and/or an RdRP coding region are obtained from two Solemoviridae genomes wherein the members of each pair of the 5’ RNA replication elements, 3’ RNA replication elements, and RdRP coding regions of the two Solemoviridae genomes have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another.
- the recombinant polynucleotidescomprise a 5’ RNA replication element and a 3’ RNA replication element obtained from distinct Solemoviridae genomes.
- the recombinant polynucleotidese.g., recombinant DNAs or recombinant RNAs
- the distinct Solemoviridae genomeswill have less than 85%, 80%, 75%, or 70% sequence identity to one another.
- the distinct Solemoviridae genomeswill have 50%, 60%, or 65% to any one of 70%, 75%, 80%, or 84% sequence identity to one another.
- the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a single row of Table 18, or variants thereof having at least 85%, 90%, 95%, 98%, or 99% sequence identity to the 5’ RNA replication element, 3’ RNA replication elements, and RdRP, or variants thereof wherein the secondary structures of the RNA replication elements are conserved,are used together in an expression system, plant cell, plant propagule, plant, or method provided herein.
- the 5’ RNA replication elements and 3’ RNA replication elements in a given row of Table 18 or variants thereofare operably linked to a cargo RNA and replicated by the corresponding RdRP or variant thereof in the row.
- the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a given row of Table 18 (i.e., SEQ ID NO: 467, 468, 469, respectively) or aforementioned or otherwise disclosed variants thereofare used in a dicot plant cell-based expression system, dicot plant cell, dicot plant propagule, dicot plant, or related dicot plant-based method provided herein.
- the aforementioned dicotis a member of the genus Brassica, Capsicum, Cucumis, Cucurbita, Euphorbia, Gossypium, Macroptilium, Nicotiana, Solanum, or Glycine.
- the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a given row of Table 18 (i.e., SEQ ID NO: 467, 468, 469, respectively) or aforementioned or otherwise disclosed variants thereofare used in a monocot plant cell-based expression system, monocot plant cell, monocot plant propagule, monocot plant, or related monocot plant-based method provided herein.
- the aforementioned monocotis a member of the genus Avena, Hordeum, Oryza, Secale, Triticum, Sorghum, or Zea.
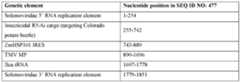
- Examples of DNA molecules which encode RNA molecules comprising or containing 5’ and 3’ RNA replication elements recognized by a Solemoviridae RdRPare set forth in Table 1.
- DNA molecules which encode RNAs comprising or containing 5’ RNA replication elements recognized by a Solemoviridae RdRPinclude SEQ ID NO: 467, 531, and 532.
- DNA molecules which encode RNAs comprising or containing 3’ RNA replication elements recognized by a Solemoviridae RdRPinclude SEQ ID NO: 468, 533, and 534.
- Structural featurese.g., dsRNA hairpins and ssRNA loops
- Table 1Structural features (e.g., dsRNA hairpins and ssRNA loops) identified in Solemoviridae 5’ and 3’ RNA replication elements are shown in Table 1 by way of dot bracket notation.
- the dot bracket notation provided in Table 1was generated using RNA Fold software for predicting RNA secondary structure based on minimum free energy predictions of base pair probabilities.
- a dot ‘.’signifies an unpaired base and a bracket ‘(’ or ‘)’ represents a paired base.
- RNA secondary structure set forth in Table 1 or in equivalent RNAsare substituted with distinct nucleotides which maintain the RNA secondary structure (e.g., presence or absence of base pairing).
- the RNA secondary structure set forth in Table 1 or in equivalent RNAsis maintained by making substitutions in the nucleotide sequence that result in no changes in the position of base-paired nucleotides or non-base-paired nucleotides.
- the RNA secondary structure set forth in Table 1 or in equivalent RNAsis maintained by substituting nucleotides in the secondary structure which are not base paired with nucleotides which will not base pair, and/or by substituting nucleotides in the secondary structure which are base paired with nucleotides which will base pair.
- maintaining the RNA secondary structureneed not be absolute (e.g., the structure is partially maintained).
- a dsRNA structureis partially maintained when one, two, three or more nucleotides, particularly at the 5’ end and/or 3’ end of a hairpin-forming structure are substituted with nucleotides which do not base pair and thus reduce the total length of dsRNA in the structure.
- an unpaired RNA structureis partially maintained when one, two, three or more nucleotides, particularly at the 5’ end and/or 3’ end of a loop structure are substituted with nucleotides which base pair and thus reduce the total length of ssRNA in the loop structure.
- Solemoviridae satellite RNAsinclude those where the 5’ RNA replication element includes one or more of these 5’ structural features and/or wherein the 3’ RNA replication element includes one or more of these 3’ structural features.
- the 5’ RNA replication elementscomprise an RNA encoded by a DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%, or 100% sequence identity to SEQ ID NO: 467, 531, or 532, optionally wherein the encoded RNA maintains or partially maintains a corresponding structural feature set forth in Table 1.
- the 3’ RNA replication elementscomprise an RNA encoded by a DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%, or 100% sequence identity to SEQ ID NO: 468, 533, or 534, wherein the encoded RNA optionally maintains or partially maintains a corresponding structural feature set forth in Table 1.
- Recombinant polynucleotidese.g., recombinant DNA, recombinant RNA, recombinant ssRNAs, recombinant dsRNAs, recombinant vectors, etc.
- additional RNA elementsinclude RNAs encoding a Solemoviridae capsid or coat protein (CP). Examples of DNA sequences encoding Solemoviridae CP include the corresponding sequences of Solemoviridae genomes set forth in Table 1.
- DNA sequences encoding Solemoviridae CPalso include the sequence of the SEQ ID NO: 472 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- DNA sequences encoding a Solemoviridae CPalso include the sequence of the DNA encoding the CP disclosed in SEQ ID NO: 471 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- DNA sequences encoding a Solemoviridae CP and Solemoviridae CP sequencesalso include the sequences set forth in Table 17 as well as the DNA and protein sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- Embodiments of additional RNA elementsinclude RNAs encoding a Solemoviridae RdRP.
- DNA sequences encoding Solemoviridae RDRPinclude the corresponding sequences of Solemoviridae genomes set forth in Table 1.
- Examples of DNA sequences encoding Solemoviridae RdRPalso include the sequence of SEQ ID NO: 470, 537, and 538 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- Examples of DNA sequences encoding Solemoviridae RdRPalso include the sequence of the DNA encoding the Solemoviridae RdRP disclosed in SEQ ID NO: 469, 535, and 536 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- RNAs encoding Solemoviridae RdRP and Solemoviridae RdRP protein sequencesalso include the sequences set forth in Table 17 as well as the DNA and protein sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto.
- Embodiments of additional RNA elementsinclude RNAs encoding a viral movement protein (MP).
- the cargo RNAcomprises an RNA encoding a viral MP.
- the viral movement proteinis believed to bind to the RNA and to assist its Agent Ref: P14419WO00 - 13 - movement (and thus the movement of the cargo RNA) throughout the plant, e.g., via the plasmodesmata.
- Viral MPsinclude movement proteins identified from tobacco mosaic virus (TMV), cowpea mosaic virus, potato leafroll virus, tomato spotted wilt virus, and tomato mosaic virus. MPs from a variety of viruses are described in Table 3.
- Embodiments of additional RNA elementsinclude tRNA-like sequences (TLS). TLS can trigger mobility of otherwise nonmobile RNAs, assisting to increase systemic delivery of the RNA molecule. TLS include tRNAs and tRNA-like sequences identified from other genetic elements, e.g., mRNAs. An isoleucine tRNA encoded by SEQ ID NO: 466 is an example of a useful tRNA-like sequence.
- RNA sequencesincluding TLS identified in Arabidopsis which are useful for building polynucleotides are described in Table 4.
- Table 4mobile mRNA sequences were downloaded from the PLAMOM database for Arabidopsis.
- the tRNA “seed alignment” from the RFAM databasewas downloaded in stockholm format (multiple sequence alignment + secondary structure).
- a covariance modelwas created with INFERNAL for the tRNA stockholm alignment.
- PLAMOM mRNA sequenceswere scanned for significant similarity to tRNAs based on primary and secondary structure features. mRNA sequences with significant hits (E-val ⁇ 1) were then saved to a fasta file.
- such a tRNA-like sequenceincludes a tRNA-like sequence from an Arabidopsis Flowering Time T (FT) mRNA.
- the RNA moleculeincludes at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition element (ERE) comprising TMV-OAS.
- the RNA moleculeincludes a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466.
- the RNA moleculeincludes a modified tRNA-like sequence that has at least 90%, 95%, 98%, or 99% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence.
- RNAs encoding a viral capsid proteininclude RNAs encoding a viral capsid protein (CP).
- CPviral capsid protein
- Such capsid proteinsare also sometimes referred to as coat proteins, with both capsid and coat proteins being referred to as “CP.”.
- the capsid proteinis heterologous to the virus of the family Solemoviridae.
- the capsid proteinis a Solemoviridae capsid protein.
- the cargo RNAcomprises an RNA encoding a viral CP.
- the CPcan be provided, e.g., by co-expression of a recombinant construct encoding the CP or by native expression by a virus endogenous to or introduced into a plant cell. Encapsidation of an RNA molecule by the CP is achieved provided it contains an encapsidation recognition element (ERE), e.g., an origin of assembly sequence (OAS). Table 2 describes several OAS and CP sequences from a variety of viruses useful in engineering constructs which provide for RNA encapsidation.
- ERPencapsidation recognition element
- OASorigin of assembly sequence
- the OASis positioned near the 3’ end of a construct, e.g., within the 3’ region of a cargo RNA or 3’ to a cargo RNA.
- the OASis found 5’ to the 3’ RNA replication elements (e.g., the 3’ RNA replication elements set forth in Table 1).
- a TMV-OAS positioned at the 3’ end of the RNA Agent Ref: P14419WO00 - 14 - moleculeis recognized by the TMV capsid protein, leading to assembly of a TMV virion around the RNA.
- Embodiments wherein the recombinant RNAs are complexed with RNA binding proteins (RBPs)are also provided herein.
- Embodiments of RBPsinclude RNA recognition motifs (RRMs) such as: (i) Lys/Arg-Gly-Phe/Tyr-Gly/Ala-Phe/Tyr-Val/Ile/Leu-X-Phe/Tyr, where X can be any amino acid (SEQ ID NO: 464); (ii) Ile/Val/Leu-Phe/Tyr-Ile/Val/Leu-X-Asn-Leu, where X can be any amino acid (SEQ ID NO: 465).
- RBP and RRMinclude those disclosed in Maris et al.2005, doi.org/10.1111/j.1742-4658.2005.04653.x.
- Embodiments of additional RNA elementsinclude at least one ribozyme.
- Ribozymesinclude self-cleaving ribozyme, a ligand-responsive ribozyme (aptazyme), a trans-cleaving ribozyme designed to cleave a target sequence (e.g., a trans-cleaving hammerhead ribozyme designed to cleave the pepper phytoene desaturase (PDS) sequence (the RNA encoded by SEQ ID NO: 421), a hepatitis delta virus (HDV) ribozyme (the RNA encoded by SEQ ID NO: 423), or a hammerhead ribozyme (the RNA encoded by SEQ ID NO: 420).
- PDSpepper phytoene desaturase
- HDVhepatitis delta virus
- multiple ribozymesare included in a polynucleotide.
- Useful ribozymesinclude Twister, Hammerhead, Hairpin, and other ribozymes.
- Non- limiting examples of useful ribozymesinclude those provided in Table 14.
- such a ribozymee.g., a self-cleaving ribozyme
- such a ribozymee.g., a self-cleaving ribozyme
- a ribozymeis located 5’ to the HRV 5’ RNA replication region and/or 3’ to the HRV 3’ RNA replication region in a recombinant RNA comprising an imbedded heterologous RNA virus (HRV) amplicon.
- HRVheterologous RNA virus
- intronic sequencesare placed in a 5’UTR downstream of a promoter (e.g., a promoter active in plant cells) used to drive expression of a recombinant RNA.
- a promotere.g., a promoter active in plant cells
- intronic sequencesare placed 5’ to a 5’ RNA replication element, in a cargo RNA, or 3’ to a 3’ RNA replication element.
- Embodiments of recombinant polynucleotides and additional RNA elementsinclude subgenomic promoters recognized by an RNA-dependent RNA polymerase (RdRP) and/or RNA molecules encoding an RNA-dependent RNA polymerase (RdRP).
- RdRPRNA-dependent RNA polymerase
- RdRPRNA-dependent RNA polymerase
- Examples of such subgenomic promoters and RdRPinclude a Brome Mosaic Virus subgenomic promoter and RdRP (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 subgenomic promoters and RdRP (Koev and Miller; J Virol.2000 Jul;74(13):5988-96.
- Brome Mosaic Virus subgenomic promoter and RdRP(Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 subgenomic promoters and RdRP (Koev and Miller; J Virol.2000 Jul;74(13):5988-96.
- such subgenomic promotersare placed either 5’ and/or 3’ to an RNA Agent Ref: P14419WO00 - 15 - molecule comprising a 5’ RNA replication element, a cargo RNA, and a 3’ RNA replication element to permit production of either or both + and – strands of the RNA molecule when the RdRP is provided.
- such subgenomic promotersare operably linked to a cargo RNA molecule and/or to any additional RNA element to permit production of the corresponding cargo and/or additional RNA when the RdRP is provided.
- the subgenomic promotersare operably linked to a cargo RNA comprising an HRV-inhibitory RNA or to a cargo RNA that encodes a protein which inhibits infection, movement, transmission, and/or replication of the HRV.
- the subgenomic promotersare operably linked to a cargo RNA comprising an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV.
- the subgenomic promotersare operably linked to a cargo RNA comprising an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV which does not encode the hrvRdRP.
- Embodiments of other optional elements in the recombinant polynucleotides provided hereininclude: a) a discrete expression cassette including a second promoter operably linked to a DNA sequence to be transcribed, and optionally a terminator element (see, e.g., a NOS or CaMV35S terminator); (b) an expression-enhancing element (e.g., a DNA encoding an expression-enhancing intronic sequence); (c) a DNA or RNA sequence encoding a marker (e.g., a selectable marker such as DNA or RNA encoding an antibiotic resistance or herbicide resistance sequence; DNA encoding a scorable marker or detectable label (e.g., a beta-glucuronidase, fluorescent protein, luciferase, etc.); (d) a DNA aptamer; (e) a DNA or RNA sequence encoding an RNA aptamer; (f) T-DNA left and right border DNA sequences; (g) a
- recombinant polynucleotidescomprising a cargo RNA molecule or comprising DNA encoding a cargo RNA molecule.
- the recombinant polynucleotideincludes a single cargo RNA molecule.
- the recombinant polynucleotideincludes at least two cargo RNA molecules, e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 cargo RNA molecules; in embodiments, the at least two cargo RNA molecules are the same (e.g., multiple copies of a non-coding RNA sequence or multiple copies of a RNA sequence encoding a Agent Ref: P14419WO00 - 16 - polypeptide) or are different (e.g., two or more different non-coding RNA sequences, or two or more different coding RNA sequences, or combinations of non-coding and coding cargo RNA sequences).
- the at least two cargo RNA moleculesare the same (e.g., multiple copies of a non-coding RNA sequence or multiple copies of a RNA sequence encoding a Agent Ref: P14419WO00 - 16 - polypeptide) or are different (e.g., two or more different non-coding RNA sequences, or two or more different coding RNA sequences, or
- a cargo RNA moleculeis up to about 5 kilobases (kb) in length.
- Cargo RNA moleculescan range in length from any one of about 20 nucleotides (nt), 100nt, 200nt, 300nt, 400nt, 500nt, 600nt, 700nt, 800nt, or 900nt to any one of about 1kb, 2kb, 3kb, 4kb, 5kb, 6kb, 7kb, 8kb, 9kb, 10kb, 11kb, 12kb, 13kb, or 14 kb in length.
- RNA lengths of the cargo RNA moleculeare less than or equal to 100 nucleotides (nt) can range in length from any one of about 20nt, 30nt, or 40nt to any one of about 50nt, 60nt, 70nt, 80nt, 90nt, or 100nt in length.
- Recombinant RNAs comprising a cargo RNA of up to about 5 kb in lengthcan in certain embodiments be encapsidated by a Solemoviridae capsid protein.
- cargo RNAscan range in length from any one of about 20nt, 100nt, 200nt, 300nt, 400nt, 500nt, 600nt, 700nt, 800nt, or 900nt to any one of about 1kb, 2kb, 3kb, 4kb, or 5kb in length.
- Recombinant RNAscomprising a cargo RNA of up to about 3kb, 4kb, 5kb, 6kb, 7kb, 8kb, 9kb, 10kb, 11kb, 12kb, 13kb, or 14kb in length can in certain embodiments be encapsidated by a heterologous viral capsid protein set forth in Table 2.
- recombinant RNAscomprising a cargo RNA of up to about 14kb and encapsidated by a heterologous viral capsid protein can comprise an OAS element set forth in Table 2 and be encapsidated by a corresponding capsid protein set forth in Table 2.
- the cargo RNA moleculeis greater than 14kb, for example, 15kb, 16kb, 17kb, 18kb, 19kb, or even 20kb.
- the cargo RNA moleculeincludes: (a) at least one coding sequence, (b) at least one non-coding sequence, or (c) both at least one coding sequence and at least one non-coding sequence.
- the cargo RNA moleculesinclude combinations of coding/non-coding sequence; multiple non-coding/coding sequences; as well as aptamers, ribozymes, and other elements as is described herein.
- the cargo RNA moleculeincludes (a) a coding sequence to be expressed in a plant, and (b) at least one non-coding sequence that modifies expression or translation of the coding sequence, such as a recognition and cleavage sequence for an siRNA or miRNA that is endogenously expressed in the plant (see, e.g., US Patent Nos.8,334,430, 9,139,838, 9,976,152, 10,793,869, 10,876,126) and can bind to and cleave an RNA transcript containing the recognition and cleavage sequence; in such embodiments, it is possible to achieve spatially or temporally or developmentally specific expression of the coding sequence in the plant.
- a coding or non-coding cargo RNAcan be optimized for expression in plants by methods which include using codons which occur more frequently in plant genes and/or by eliminating polyadenylation sites in the cargo RNA (e.g., as described in at least US Pat. Nos.5380831, 5689052, and 7741118, which are each incorporated herein by reference in their entireties).
- the cargo RNA moleculeincludes at least one coding sequence (e.g., a translatable sequence).
- the coding sequenceis accordingly a protein or a polypeptide such as those described in this disclosure’s working examples.
- a cargo RNAcomprises a selectable marker RNA encoding an antibiotic resistance or herbicide resistance polypeptide sequence or a scorable marker RNA encoding a scorable marker protein (e.g., a beta- Agent Ref: P14419WO00 - 17 - glucuronidase, fluorescent protein, luciferase, etc.).
- a scorable marker proteine.g., a beta- Agent Ref: P14419WO00 - 17 - glucuronidase, fluorescent protein, luciferase, etc.
- selectable marker/selection agent combinationsinclude glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzyme/glufosinate, or a neomycin phosphotransferase (npt)/neomycin or kanamycin.
- scorable markersinclude ⁇ -glucuronidase (GUS), luciferase, and fluorescent proteins such as green fluorescent protein (GFP), yellow fluorescent protein (YFP), and cyan fluorescent protein (CFP).
- the cargo RNA sequenceencodes at least one protein or polypeptide that provides a desirable trait in a plant in which the protein or polypeptide is expressed.
- polypeptides useful in agricultural applicationsinclude, for example, bacteriocins, lysins, antimicrobial peptides, nodule C-rich peptides, and bacteriocyte regulatory peptides.
- Such polypeptidescan be used to alter the level, activity, or metabolism of target microorganisms for increasing the fitness of beneficial insects (such as honeybees and silkworms) or for decreasing the fitness of pest invertebrates (such as aphids, caterpillars, beetle larvae, and mites).
- Embodiments of agriculturally useful polypeptidesinclude peptide toxins, such as those naturally produced by entomopathogenic bacteria (e.g., Bacillus thuringiensis, Photorhabdus luminescens, Serratia entomophila, or Xenorhabdus nematophila), as is known in the art.
- entomopathogenic bacteriae.g., Bacillus thuringiensis, Photorhabdus luminescens, Serratia entomophila, or Xenorhabdus nematophila
- Embodiments of agriculturally useful polypeptidesinclude polypeptides (including small peptides such as cyclodipeptides or diketopiperazines) for controlling agriculturally important pests or pathogens, e.g., antimicrobial polypeptides or antifungal polypeptides for controlling diseases in plants, or pesticidal polypeptides (e.g., insecticidal polypeptides and/or nematicidal polypeptides) for controlling invertebrate pests such as insects or nematodes.
- polypeptidesincluding small peptides such as cyclodipeptides or diketopiperazines
- antimicrobial polypeptides or antifungal polypeptidesfor controlling diseases in plants
- pesticidal polypeptidese.g., insecticidal polypeptides and/or nematicidal polypeptides
- invertebrate pestssuch as insects or nematodes.
- Embodiments of antimicrobial polypeptidesinclude cathelicidins, cecropins, beta-defensins, amphibian antimicrobial peptides (e.g., aurein-like peptides, esculentin, gaegurin, brevinin, rugosin, ranatuerin, ranacyclin, uperin, ocellatin, grahamin, nigrocin, dermoseptin, temporin, bombinin, maximin), enterocins, ponicerins, megourins, apidaecins, abaecins, attacin, bacteriocins and lantibiotics, dermcidin, formaecin, halocidins, lactocin, tachystatins, and some insecticidal toxins produced by spiders and scorpions.
- amphibian antimicrobial peptidese.g., aurein-like peptides, esculentin
- Embodiments of agriculturally useful polypeptidesinclude antibodies, nanobodies, and fragments thereof, e.g., antibody or nanobody fragments that retain at least some (e.g., at least 10%) of the specific binding activity of the intact antibody or nanobody.
- Embodiments of agriculturally useful polypeptidesinclude transcription factors, e.g., plant transcription factors; see., e.g., the “AtTFDB” database listing the transcription factor families identified in the model plant Arabidopsis thaliana), publicly available at agris- knowledgebase[dot]org/AtTFDB/.
- Embodiments of agriculturally useful polypeptidesinclude nucleases, for example, exonucleases or endonucleases (e.g., Cas nucleases such as Cas9 or Cas12a).
- Embodiments of agriculturally useful polypeptidesfurther include cell-penetrating peptides, enzymes (e.g., amylases, cellulases, peptidases, lipases, chitinases), peptide pheromones (for example, yeast or fungal mating pheromones, invertebrate reproductive and larval signaling pheromones, see, e.g., Altstein (2004) Peptides, 25:1373–1376).
- enzymese.g., amylases, cellulases, peptidases, lipases, chitinases
- peptide pheromonesfor example, yeast or fungal mating pheromones, invertebrate reproductive and
- Embodiments of agriculturally useful polypeptidesconfer a beneficial agronomic trait, e.g., herbicide tolerance, insect control, modified yield, increased fungal or oomycte Agent Ref: P14419WO00 - 18 - disease resistance, increased virus resistance, increased nematode resistance, increased bacterial disease resistance, plant growth and development, modified starch production, modified oils production, high oil production, modified fatty acid content, high protein production, fruit ripening, enhanced animal and human nutrition, production of biopolymers, environmental stress resistance, pharmaceutical peptides (e.g., hormones, enzymes, transcription factors, antigens, antibodies, or antibody fragments) and secretable peptides, improved processing traits, improved digestibility (e.g., reduced levels of toxins or reduced levels of compounds with “anti-nutritive” qualities such as lignins, lectins, and phytates), enzyme production, flavor, nitrogen fixation, hybrid seed production, fiber production, and biofuel production.
- beneficial agronomic trait
- Non-limiting examples of agriculturally useful polypeptidesinclude polypeptides that confer herbicide resistance (US Patent Nos.6,803,501; 6,448,476; 6,248,876; 6,225,114; 6,107,549; 5,866,775; 5,804,425; 5,633,435; and 5,463,175), increased yield (US Patent Nos.
- the cargo RNAencodes one or more small signaling peptides (SSPs), also called peptide hormones, which are an attractive option for use as cargoes in RNA commensal satellites due to their small size (5-75 amino acids) and potency.
- SSPssmall signaling peptides
- SSPsresult from processing longer precursor polypeptides (derived from ORF regions).
- SSPsoriginate from a wider range of sources including intergenic/intronic regions, long non-coding RNAs, pri-miRNAs, and 5′ and 3′ UTRs of mRNAs.
- Non-limiting examples of SSPsinclude miPEP172c, miPEP171d, BomiPEP397a, AtmiPEP397a, BvmiPEP164b, and AtmiPEP164b peptides set forth in Table 13.
- the RNA moleculefurther includes an internal ribosome entry site (IRES) located 5’ and immediately adjacent to the at least one coding sequence.
- IRSinternal ribosome entry site
- the cargo RNA moleculeincludes multiple coding sequences, and the RNA molecule further includes an IRES located 5’ and immediately adjacent to each of the coding sequences (e.g., open translational reading frames encoding a protein of interest.
- IRES sequencesinclude those depicted in Table 5.
- the cargo RNA moleculeincludes a non-coding sequence such as those described in this disclosure’s working examples.
- non-coding sequencesinclude a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA sequence that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA).
- hpRNAhairpin RNA
- siRNAsmall interfering RNA
- miRNAmicroRNA
- aptazymea ligand-responsive ribozyme
- RNA aptameror a long noncoding RNA (lncRNA).
- the cargo RNAincludes a selectable or scorable RNA marker, such as an RNA aptamer or a regulatory RNA, such as an siRNA or siRNA precursor (see, e.g., US Patent No.8,404,927, 8,455,716, 9,777,288, 10,378,012), a miRNA or a miRNA precursor (see, e.g., US Patent Nos.8,410,334, 8,395,023, 9,708,620), a trans-acting siRNA or trans-acting siRNA precursor (see, e.g., US Patent Nos.8,030,473, 8,476,422, 8,816,061, 9,018,002), a phased sRNA or phased sRNA precursor (see, e.g., US Patent No.8,404,928), an siRNA or miRNA decoy (see, e.g., US Patent Nos.8,946,511, 9,873,888), an siRNA or miRNA cleavage blocker (see, e
- RNA aptamersinclude those that exhibit fluorescence upon binding a molecule.
- the fluorescent RNA aptamercan be the Broccoli RNA aptamer.
- RNA aptamersthat can be used include, but are not limited to, Spinach, Spinach2, Carrot, Radish, Corn, Red Broccoli, Orange Broccoli, and Broccoli Fluorets.
- Other useful RNA aptamersthat can be used include those provided in Table 15.
- Suitable regulatory RNAscan be used to down-regulate (i.e., silence) the expression of a marker gene.
- PDSphytoene desaturase
- silencing of the gene yields a photobleached phenotypeis widely used as a marker gene because silencing of the gene yields a photobleached phenotype.
- RNAssuch as decoys or cleavage blockers can also be used to interfere with endogenous small RNA-regulated pathways, resulting in a visible phenotype; see, e.g., US Patent Nos.8,946,511, 9,873,888, 9,040,774).
- Antiviral cargo RNAs, and in particular antiviral cargo RNAs directed against viral pathogensare provided herein.
- the antiviral cargo RNAscomprise a heterologous RNA Virus (HRV)-inhibitory RNA or encode an HRV-inhibitory protein, wherein the HRV-inhibitory RNA or protein inhibits infection, movement, transmission, and/or replication of the HRV.
- HRVRNA Virus
- Target viral pathogensinclude an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae.
- the target viral pathogenis Cucumber Mosaic Virus, Brome mosaic virus, Citrus tristeza virus, Beet yellows virus, Cowpea mosaic virus, Potato virus X; Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, sugarcane mosaic virus, Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, Pepper mild mottle virus, Turnip crinkle virus, Tomato bushy stunt virus, Tomato spotted wilt virus, watermelon bud necrosis virus, Turnip yellow mosaic virus, Spinach latent virus, Olive latent virus 2, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, Citrus leaf blotch virus, Apple latent spherical virus, Soybean latent spherical virus, Celery latent virus, Black grass varicosavirus
- the targeted viral pathogenis a heterologous RNA virus disclosed in Table 7.
- antiviral inhibitory RNAs (RNAi sequences) used as cargo RNAsare obtained for a chosen target gene of a viral pathogen using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/).
- siRNA/miRNA prediction toolssee, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/).
- Other examples of non-coding RNA sequences having antiviral activitye.g., dsRNA molecules which produce miRNA or siRNA
- examplesinclude those disclosed in US Patent No. 8,455,716, which is incorporated herein by reference in its entirety.
- Non-limiting examples of viral targets for antiviral cargo RNA moleculesinclude the viral genes and genomes provided in Table 7 as well as other variants of those viral sequences.
- cargo RNAs encoding antiviral proteinsare provided.
- Non-limiting examples of antiviral proteinsinclude the N protein (Whitham, S. et al. Cell 78, 1101–1115 (1994)) and endogenous plant viral resistance proteins provided in Table 8.
- Antifungal cargo RNAs, and in particular antifungal cargo RNAs directed against plant fungal pathogens,are provided herein.
- Target fungal pathogensinclude Botrytis, Fusarium, Magnaporthe, Phytophthora, Rhizoctonia, Sclerotinia, and Verticillium sp.
- the antifungal cargo RNAcomprises a non-coding RNA sequence having antifungal activity (e.g., dsRNA molecules which produce miRNA or siRNA) and in particular a dsRNA directed against a fungal pathogen target gene.
- such antifungal cargo RNAscomprising dsRNA-mediated control of fungal pathogens are modeled after those described in Qiao et al., 2021, doi: 10.1111/pbi.13589; Duanis-Assaf, et al., 2022, DOI: 10.1111/pbi.13708; Yang et al., 2022, doi: 10.3389/fmicb.2021.660976; Sundaresha et al., 2021, doi: 10.20944/preprints202102.0280.v1; and Gaffar et al., 2019, doi: 10.3389/fmicb.2019.01662.
- Non-limiting examples of antifungal cargo RNAi targetsare provided in Agent Ref: P14419WO00 - 21 - Table 10.
- antifungal inhibitory RNAs (RNAi sequences) used as cargo RNAsare obtained for a chosen target gene of a fungal pathogen (e.g., a fungal pathogen gene set forth in Table 10) using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/).
- antifungal cargo RNAsencode antifungal proteins.
- Useful antifungal proteinsinclude nodule-specific cysteine-rich antimicrobial peptides (Vellivelli et al., 2020, doi: 10.1073/pnas.2003526117), defensins (Asano et al., 2013, doi: 10.1371/journal.ppat.1003581), the conidial germination-inhibiting antifungal peptides disclosed in International Patent Application publication WO2023/004435, which is incorporated by reference herein, including their homodimers, heterodimers, and fusions with signal or cell-penetrating peptides (e.g., the sequences provided in Tables 4 and 5 of WO2023/004435); the various antifungal antimicrobial peptides disclosed in De Cesare et al.
- Insecticidal cargo RNAsand in particular insecticidal or insect inhibitory cargo RNAs directed against insects are provided herein.
- Target insectsinclude sucking insects (e.g., heteropteran and homopteran insects including aphids, whiteflies, and plant bugs), caterpillars (e.g., lepidopteran insects including fall army, black cutworm, corn earworm, soybean looper, and velvetbean caterpillar), beetles (e.g., coleopteran insects including Colorado Potato Beetle and corn rootworms), and flies (e.g., dipteran insects including Ceratitis capitata).
- Insecticidal or insect inhibitory cargo RNAs provided hereincan be directed against insects at various stages of their development (e.g., embryonic, larval, pupal, or adult stages).
- the insecticidal or insect inhibitory cargo RNAcomprises a non-coding RNA sequence having insecticidal or insect inhibitory activity (e.g., dsRNA molecules which produce miRNA or siRNA) and in particular a dsRNA directed against an insect target gene.
- insecticidal cargo RNAs comprising dsRNA-mediated control of insectscomprise or are modeled after those described in US Patent Nos.11,091,770 and 11,186,837, which are each incorporated herein by reference in their entireties.
- Non-limiting examples of insecticidal or insect- inhibitory cargo RNAi targetsare provided in Table 9.
- Non-limiting examples of insecticidal cargo RNAi targetsinclude insect Actin, SNF7, Tyrosine hydroxylase, C002, Hunchback, V-ATPase subunit A, COPI coatomer beta prime subunit, ribosomal protein L19, and ubiquitin C genes.
- insecticidal or insect inhibitory RNAs (RNAi sequences) used as cargoare obtained for a chosen target gene of an insect (e.g., an insect gene set forth in Table 9 or US Patent Nos.11,091,770 and 11,186,837) using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/).
- insecticidal cargo RNAsencode insecticidal proteins.
- Useful insecticidal proteins encoded by insecticidal cargo RNAsinclude native and modified Bacillus thuringiensis Cry, vegetative insecticidal proteins (VIP), and Cyt proteins (Palma et al.2014, doi: 10.3390/toxins6123296; US Patent No.11,267,849, incorporated herein by reference in its entirety) as well as insecticidal or insect-inhibitory proteins provided in Table 9.
- Cargo RNAscan also encode “resistance” or “R” genes which confer resistance to certain arthropods, bacteria such as Pseudomonas sp., Xanthomonas sp., and Erwinia sp., and fungal pathogens including Cochliobolus, Blumeria, Fusarium, Melampsora, and Magnaporthe sp.
- R genes encoded by cargo RNAsinclude those provided in Table 12.
- the cargo RNA moleculecomprises a CRISPR guide RNA, e.g., a crRNA, gRNA, or sgRNA.
- CRISPR-associated endonucleasessuch as Cas9, Cas12, and Cas13 endonucleases are used as genome editing tools in different plants; see, e.g., Wolter et al. (2019) BMC Plant Biol., 19:176-183); Aman et al. (2016) Genome Biol., 19:1-10.
- CRISPR/Cas9requires a two- component crRNA:tracrRNA “guide RNA” (“gRNA”) that contains a targeting sequence (the “CRISPR RNA” or “crRNA” sequence) and a Cas9 nuclease-recruiting sequence (tracrRNA).
- gRNAguide RNA
- sgRNAsingle guide RNA
- sgRNAsingle guide RNA
- tracrRNAfor binding the nuclease
- crRNAto guide the nuclease to the sequence targeted for editing
- CRISPR nucleases and guide RNAsprovide algorithms for designing guide RNA sequences; see, e.g., guide design tools provided by Integrated DNA Technologies at www[dot]idtdna[dot]com/pages/products/crispr-genome-editing/alt-r-crispr-cas9-system.
- RNA replication elements of the virus of the family Solemoviridaeflank an internal sequence wherein the cargo RNA is operably linked to one or more elements of a heterologous RNA virus (HRV).
- HRVheterologous RNA virus
- cargo RNAsare imbedded within a heterologous RNA virus (HRV) amplicon comprising; (i) an HRV 5’ replication region (HRV 5’ RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR), wherein (i), (ii), and (iii) are operably linked.
- HRVheterologous RNA virus
- Figure 2An illustrative example of a Solemoviridae satellite construct with such an HRV amplicon is shown in Figure 2.
- an HRV ampliconin plants comprising a Solemoviridae satellite construct, the virus of the family Solemoviridae, and an HRV RdRP is illustrated in Figure 6.
- HRV 5’ replication regions(5’RR), 3’ replication regions (3’RR), and corresponding HRV RNA-dependent RNA Polymerases (RdRP) that recognize such replication regions are set forth in Table 7.
- an internal ribosome entry site(IRES; e.g. an IRES in Table 5) is typically operably linked to the coding cargo RNA.
- one or more self-cleaving or inducible ribozymesare operably linked to the 5’ end of the HRV 5’ RR and to the 3’ end of the HRV 3’ RR.
- the HRV ampliconfurther comprises a subgenomic promoter which is operably linked to the cargo RNA molecule.
- subgenomic promotersinclude a subgenomic promoter of the HRV and/or a Brome Mosaic Agent Ref: P14419WO00 - 23 - Virus subgenomic promoter (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 subgenomic promoters (Koev and Miller; J Virol.2000 Jul;74(13):5988-96, doi: 10.1128/jvi.74.13.5988-5996.2000), and an Alternanthera mosaic virus (AltMV-MU) sgp1, sgp2, or sgp3 subgenomic promoter (Putlyaev et al., Biochemistry (Mosc).;80(8):1039-46, doi: 10.1134/S000629791508009X).
- AltMV-MUAlternant
- HRV ampliconscan be in the sense or antisense orientation with respect to the Solemoviridae 5’ RNA replication element.
- HRV ampliconWhen the HRV amplicon is oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element, the HRV 5’ RR and 3’ RR are present in the recombinant RNA molecule in the sense orientation, as in the corresponding sequences found in the plus (+) strand of the HRV genomic RNA.
- the HRV 5’ RR and 3’ RRare present in the recombinant RNA molecule in antisense orientation, as in the corresponding sequences found in the negative (-) strand of the HRV genomic RNA.
- a plant cell or plant containing the recombinant RNA containing the HRV ampliconare provided with an RNA-dependent RNA polymerase that recognizes the HRV 5’ RR and 3’RR (hrvRdRP)
- the HRV ampliconundergoes amplification (e.g., hrvRdRP-mediated replication).
- Such hrvRdRPcan be provided by sources that include: (i) infection by the HRV; (ii) introduction by vector-mediated delivery of a polynucleotide encoding the hrvRdRP (e.g., Agrobacterium-mediated delivery or viral vector mediated delivery); or (iii) introduction of a nucleic acid encoding the hrvRdRP.
- sourcesthat include: (i) infection by the HRV; (ii) introduction by vector-mediated delivery of a polynucleotide encoding the hrvRdRP (e.g., Agrobacterium-mediated delivery or viral vector mediated delivery); or (iii) introduction of a nucleic acid encoding the hrvRdRP.
- HRV1 RdRPand “HRV2 RdRP”
- Amplificatione.g., an increase in copy number of the HRV amplicon
- the recombinant nucleotides provided hereincomprise Solemoviridae 5’ and 3’ RNA replication elements flanking a heterologous RNA virus (HRV) subgenomic promoter operably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP).
- HRVheterologous RNA virus
- hrvRdRPheterologous RNA virus RNA-dependent RNA polymerase
- FIG. 8Another illustrative example where subgenomic promoters drive expression of an HRV RdRP and a dsRNA cargo in plants comprising a Solemoviridae satellite construct is shown in Figure 8.
- an internal ribosome entry siteIRES; e.g. an IRES in Table 5
- IRESinternal ribosome entry site
- Embodiments of subgenomic promotersinclude a subgenomic promoter of the HRV and/or a Brome Mosaic Virus subgenomic promoter (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 Agent Ref: P14419WO00 - 24 - subgenomic promoters (Koev and Miller; J Virol.2000 Jul;74(13):5988-96.
- a Brome Mosaic Virus subgenomic promoter(Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 Agent Ref: P14419WO00 - 24 - subgenomic promoters (Koev and Miller; J Virol.2000 Jul;74(13):59
- RNAs from subgenomic promotersprovides for additional copies of the cargo RNA and an enhancement of desirable phenotypes conferred by the cargo RNA (e.g., increased antiviral, antifungal, or insecticidal activity in comparison to control plants lacking the additional expressed cargo RNA or lacking the cargo RNA).
- the subgenomic promoters and operably linked cargo RNAsare oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element, the subgenomic promoters and operably linked cargo RNAs are present in the recombinant RNA molecule as a sense strand where the subgenomic promoter is recognized by the hrvRdRP to produce the desired cargo RNA.
- the subgenomic promoters and operably linked cargo RNAsare oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element in the recombinant RNA molecule (positive strand)
- the subgenomic promotercan be recognized by the hrvRdRP to produce the desired cargo RNA.
- the HRV ampliconis oriented in the antisense orientation relative to the Solemoviridae 5’ RNA replication element in the recombinant RNA molecule, the subgenomic promoter cannot be recognized by the hrvRdRP to produce the desired cargo RNA.
- the negative strand of the recombinant RNA molecule produced by the Solemoviridae RdRPwould contain the subgenomic promoters and operably linked cargo RNA in a sense orientation where the subgenomic promoter can be recognized by the hrvRdRP to produce the desired cargo RNA.
- the HRV ampliconsfurther comprise a HRV 5’ RR and 3’ RR which flank the cargo RNA and provide for hrvRdRP-mediated replication of an RNA comprising from 5’ to 3’ the HRV 5’ RR, cargo RNA, and HRV 3’ RR (e.g., as illustrated in the non-limiting example of Figure 5).
- an RNA encoding the cargo moleculecan be produced (e.g., via hrvRdRP-mediated synthesis of the cargo RNA from the subgenomic promoter).
- Such hrvRdRPcan be provided by sources that include: (i) infection by the HRV; (ii) introduction by vector-mediated delivery (e.g., Agrobacterium-mediated delivery or viral vector mediated delivery); (iii) introduction of a nucleic acid encoding the hrvRdRP; or (iv) inclusion of a cargo RNA in the recombinant nucleotides comprising Solemoviridae 5’ and 3’ RNA replication elements.
- vector-mediated deliverye.g., Agrobacterium-mediated delivery or viral vector mediated delivery
- the subgenomic promoter and operably linked cargo RNAare present in the recombinant RNA molecule as the antisense strand, and the cargo RNA encodes both an hrvRdRP and a second coding or non-coding RNA where both the hrvRdRP and a second coding or non-coding RNA are operably linked to a subgenomic promoter recognized by the hrvRdRP.
- an IRESis operably linked to the RNA encoding the hrvRdRP.
- RNA replication elementsresults in an RNA where the subgenomic promoters recognized by the hrvRdRP can drive expression of the HRV RdRP and a second coding or non-coding RNA.
- An illustrative example of a Solemoviridae satellite construct with subgenomic promoters in antisense orientation relative to the 5’ RRE and driving expression of both an hrvRdRP that recognizes the subgenomic promoters and a second cargo RNAis shown in Figure 4.
- an RNA molecule including at least one HRV ampliconis amplified directly by the hrvRdRP (e.g., without initial or further amplification by the Solemoviridae RdRP).
- the HRV ampliconincludes, in 5’ to 3’ order, (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) a cargo RNA molecule; and (iii) a heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked.
- HRV 5’RRheterologous RNA virus
- HRV 3’RRRNA replication region
- the HRV ampliconincludes, in 5’ to 3’ order, a subgenomic promoter that is operably linked to (v) a cargo RNA molecule, wherein the subgenomic promoter is recognized by the hrvRdRP.
- HRV ampliconscan be provided in either an isolated form or in a composition.
- the RNA molecule including the HRV ampliconcan be provided to a plant, for example, by transcription in the plant from a recombinant DNA molecule encoding the RNA molecule that is transiently expressed in the plant or that is stably integrated into the plant’s genome, or by delivery to the plant of an exogenous RNA molecule including the HRV amplicon, for example, by contacting a surface of the plant with the exogenous RNA molecule including the HRV amplicon, or by introducing the exogenous RNA molecule including the HRV amplicon into the plant’s vascular system (e.g., by injection, infusion, petiole uptake, root uptake).
- a plantfor example, by transcription in the plant from a recombinant DNA molecule encoding the RNA molecule that is transiently expressed in the plant or that is stably integrated into the plant’s genome, or by delivery to the plant of an exogenous RNA molecule including the HRV amplicon, for example, by
- the cargo RNA moleculeincludes at least one antiviral RNA (e.g., an antiviral inhibitory RNA or an RNA encoding an antiviral polypeptide) that provides the plant with resistance to at least one viral pathogen (which in some instances can be the heterologous RNA virus itself).
- antiviral RNAe.g., an antiviral inhibitory RNA or an RNA encoding an antiviral polypeptide
- Such embodimentsare useful as antiviral treatments for plants, to prevent or decrease the severity of infection of a plant by a viral pathogen.
- plants including poinsettia (Euphorbia pulcherrima) that comprise Solemoviridae satellite RNAs containing HRV amplicons disclosed hereincan exhibit control of HRV that include Poinsettia mosaic virus and Euphorbia mosaic virus.
- plantssuch as poinsettia (Euphorbia pulcherrima) that comprise a Poinsettia latent virus satellite RNA containing an HRV amplicon disclosed herein can exhibit control of HRV that include Poinsettia mosaic virus and Euphorbia mosaic virus.
- RNA molecules that contain HRV amplification sequences(such as the HRV amplicons described herein which contain either (1) a pair of HRV 5’ and 3’ RNA replication regions, or (2) a subgenomic promoter that is recognized by the hrvRdRP) potentially also serve as a “sponge” or “decoy” that reduces the corresponding hrvRdRP’s Agent Ref: P14419WO00 - 26 - efficiency in recognizing and amplifying the HRV viral genome itself, thus potentially decreasing a pathogenic HRV’s deleterious effects on an infected plant.
- RNA polynucleotidescomprising at least one cleavable sequence are provided.
- the at least one cleavable sequenceis located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element.
- the cleavable sequenceis a self-cleaving ribozyme (e.g., a hammerhead ribozyme; Tang and Breaker. Proc Natl Acad Sci USA.2000 May 23;97(11):5784-9.
- a cargo RNA molecule that is integrated into a polynucleotideincludes at least one CRISPR guide RNA; release of the guide RNA is mediated, e.g., by flanking DR sequences, ribozyme sequences, or other self-cleaving or trans-cleaving RNAs, or by cleavage by an endogenous ribonuclease.
- the corresponding Cas nucleasecan be provided by separate or concurrent delivery, e.g., by co-delivery with a vector or polynucleotide, or by transient or stable expression of the corresponding Cas nuclease in the cell to which the polynucleotide is delivered.
- guide sequence designsare constrained by the requirement that the DNA target sequence (to which the crRNA is designed to be complementary) must be adjacent to a proto-spacer adjacent motif (“PAM”) sequence that is recognized by the specific Cas nuclease to be employed.
- PAMproto-spacer adjacent motif
- Cas nucleasesrecognize specific PAM sequences and there is a diversity of nucleases and corresponding PAM sequences; see, e.g., Smakov et al. (2017) Nature Reviews Microbiol., doi:10.1038/nrmicro.2016.184.
- Cas9 nucleasescleave dsDNA, require a GC-rich PAM sequence located 3’ to the DNA target sequence to be targeted by the crRNA component of the guide RNA, and cleave leaving blunt ends.
- Cas12a nucleases cleave dsDNArequire a T-rich PAM sequence located 5’ to the DNA target sequence to be targeted by the crRNA component of the guide RNA, and cleave leaving staggered ends with a 5’ overhang.
- Cas13 nucleasescleave single-stranded RNAs and do not require a PAM sequence; instead, Cas13 nuclease are guided to their targets by a single crRNA with a direct repeat (“DR”).
- DRdirect repeat
- the crRNA component of a guide RNAis generally designed to have a length of between 17 – 24 nucleotides (frequently 19, 20, or 21 nucleotides) and exact complementarity (i.
- Non-limiting examples of effective guide RNA designare found, e.g., in US Patent Application Publications US 2019/0032131, 2015/0082478, and 2019/0352655, which are each incorporated by reference in their entirety herein.
- CRISPR “arrays”can be designed to include one or multiple guide RNA sequences corresponding to one or more desired target DNA Agent Ref: P14419WO00 - 27 - sequence(s); see, for example, Cong et al. (2013) Science, 339:819-823; Ran et al. (2013) Nature Protocols, 8:2281-2308.
- the 5’ RNA replication elementincludes a 5’ UTR element of a Solemoviridae genome (e.g., a Solemoviridae genome including a Solemoviridae 5’ UTR set forth in Table 1).
- the 5’ RNA replication elementfurther includes a genomic sequence of the Solemoviridae that is natively located 3’ to and optionally adjacent or immediately adjacent to the 5’ UTR sequence.
- the 3’ RNA replication elementincludes a 3’ UTR sequence of a Solemoviridae genome (e.g., a Solemoviridae genome including a Solemoviridae 3’ UTR set forth in Table 1).
- the 3’ RNA replication elementfurther includes a genomic sequence of the Solemoviridae that is natively located 5’ to and optionally adjacent or immediately adjacent to the 3’ UTR sequence.
- the RNA moleculefurther includes at least one RNA molecule encoding a viral MP.
- the at least one RNA molecule encoding an MPis located (a) before the cargo RNA molecule, (b) after the cargo RNA molecule, or (c) both before and after the cargo RNA molecule.
- the at least one RNA sequence encoding an MPincludes at least two RNA sequences encoding different MPs or a single RNA sequence encoding multiple copies of MPs.
- the recombinant DNA moleculefurther includes a discrete expression cassette including a second promoter that is functional in the cell and is operably linked to a DNA sequence encoding at least one viral movement protein, and optionally a terminator element.
- the RNA moleculefurther includes an encapsidation recognition element (ERE), where the ERE is located close to or adjacent to the 3’ RNA replication element, and optionally wherein the 3’ RNA replication element includes a 3’ UTR sequence of the virus of the family Solemoviridae.
- the EREincludes a viral OAS such as a tobacco mosaic virus OAS (TMV-OAS) or an OAS set forth in Table 2.
- the RNA moleculefurther includes at least one tRNA-like sequence (TLS), and wherein the at least one tRNA-like sequence includes a tRNA-like sequence from an Arabidopsis FT mRNA (e.g. a TLS in an Arabidopsis FT mRNA of Table 4).
- the RNA moleculeincludes a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466.
- the RNA moleculeincludes a modified tRNA-like sequence that has at least 90% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence.
- the RNA moleculefurther includes at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition sequence including TMV-OAS.
- the cargo RNA moleculeis up to about 5kb in length.
- the cargo RNA moleculeincludes: (a) at least one coding sequence, (b) at least one non-coding sequence, or (c) both at least one coding sequence and at least one non-coding sequence.
- the Agent Ref: P14419WO00 - 28 - cargo RNA moleculeincludes at least one coding sequence, and wherein the RNA molecule further includes an internal ribosome entry site (IRES) located 5’ and immediately adjacent to the at least one coding sequence.
- the cargo RNA moleculeincludes multiple coding sequences, and wherein the RNA molecule further includes an IRES located 5’ and immediately adjacent to each of the coding sequences.
- the cargo RNA moleculeincludes at least one non-coding sequence, and wherein the at least one non-coding sequence is selected from the group consisting of a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA sequence that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; and a long noncoding RNA (lncRNA).
- hpRNAhairpin RNA
- RNA pseudoknotan RNA sequence that forms at least partially double-stranded RNA
- siRNAsmall interfering RNA
- miRNAmicroRNA
- miRNAmiRNA
- a ribozymea ligand-responsive ribozyme
- RNA aptamera long noncoding RNA (l
- a DNA sequence encoding at least one ribozymeis provided. In embodiments, the at least one ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. In embodiments, a DNA sequence encoding at least one ligand-responsive ribozyme (aptazyme) is provided. In embodiments, the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element.
- RNA moleculescomprising the aforementioned or otherwise disclosed 5’ RNA replication elements, a cargo RNA molecule(s), and 3’ RNA replication elements, as well as additional aforementioned or otherwise disclosed elements are also provided herein.
- the recombinant RNA moleculesare produced by a recombinant DNA molecule provided herein.
- the recombinant RNA moleculesare produced by an in vivo or in vitro (e.g., cell free) RNA replication process through the action of a RdRP acting on: (i) 5’ and 3’ RNA replication elements; and/or (ii) a subgenomic promoter.
- Expression systemscomprising the recombinant polynucleotides are also provided. Such expression systems include both cell-based and cell free expression systems.
- cell-free expression systemcan include (a) an RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or other element.
- Embodiments of additional elementsinclude at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter.
- the RdRP proteinis provided by a virus of the family Solemoviridae, e.g. a virus of the family Solemoviridae that is endogenous to a cell in which expression is desired.
- cell-based expression systemcan include (a) a recombinant DNA molecule including a heterologous promoter that is functional in a cell and is operably linked to a DNA sequence encoding an RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or Agent Ref: P14419WO00 - 29 - other element.
- Embodiments of additional elementsinclude at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes the subgenomic promoter.
- cell-based expression systemcan include (a) a recombinant RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or other element.
- Embodiments of additional RNA elementsinclude at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter.
- the RdRP proteinis provided by a virus of the family Solemoviridae.
- an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoteris provided by a heterologous RNA virus (HRV) or by another nucleic acid introduced into the cell (e.g., by a vector or other recombinant nucleic acid).
- HRVheterologous RNA virus
- the 5’ RNA replication element and the 3’ RNA replication elementare obtained from the same virus of the family Solemoviridae and/or from the same Solemoviridae genome (e.g., both obtained from the same Solemoviridae capsid genome or both obtained from the same Solemoviridae genome) or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related.
- a cell used in the expression systemis a bacterial cell, a plant cell, a fungal cell, or an animal cell (e.g., an insect cell).
- a cell used in the expression systemendogenously contains a virus of the family Solemoviridae having a genome that encodes an RdRP that recognizes the 5’ and 3’ RNA replication elements.
- the expression systemfurther includes a viral capsid protein that is recognized by the encapsidation recognition element and encapsidates the RNA molecule.
- the viral capsid proteinis: (a) expressed by the recombinant DNA molecule in the cell (e.g., where the recombinant DNA molecule further includes a discrete expression cassette comprising a second promoter operably linked to a DNA sequence encoding the viral capsid protein, and optionally a terminator element), (b) co-expressed by a second recombinant DNA molecule in the cell; (c) provided exogenously to the cell; or (d) expressed by a virus in the cell.
- the RdRP proteinis heterologous to the cell. In embodiments, the RdRP protein is provided exogenously to the cell.
- the RdRP protein that recognizes the 5’ and 3’ RNA replication elementsis endogenously expressed in the plant cell by the virus of the family Solemoviridae (e.g., where the virus of the family Solemoviridae occurs naturally in the plant cell).
- the virus of the family Solemoviridaeis native to or endemic to the plant cell.
- the virus of the family Solemoviridae that is endemic to the plant cellis non-pathogenic.
- the virus of the family Solemoviridae that is endemic to the plant cellis non-pathogenic and commensal.
- the virus of the family Solemoviridaeis an exogenously introduced virus of the family Solemoviridae (i.e., not endemic or native to the host, but artificially introduced).
- a virus of the family Solemoviridae natively found in one plant Agent Ref: P14419WO00 - 30 - species, variety, or germplasmcan be introduced, with or without a corresponding recombinant Solemoviridae satellite RNA, into a different plant species, variety, or germplasm.
- a complete self-replicating Solemoviridae satellite systemis introduced into a plant or plant cells, wherein the self-replicating Solemoviridae satellite system includes: (1) a recombinant Solemoviridae satellite RNA comprising, from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the promoter and the cargo RNA molecule are heterologous to the 5’ RNA replication element and the 3’ RNA replication element; and (2) an exogenous virus of the family Solemoviridae (e.g., a virus of the family Solemoviridae that is not endemic or
- the Solemoviridae RdRPcomprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536.
- the recombinant DNA molecule or recombinant RNA moleculefurther comprises at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition sequence including a TMV-OAS.
- Cells comprising any of the aforementioned or otherwise disclosed recombinant polynucleotidesare provided herein.
- Cells comprising the recombinant polynucleotidesinclude prokaryotic (e.g., a bacterium, such as a bacterium capable of transforming a eukaryotic cell) or eukaryotic (e.g., a plant cell, fungal cell, or animal cell such as an insect cell) cells.
- the cellsare bacterial cells capable of transforming a plant cell (e.g., an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell.
- Bacterial cells capable of transforming a plant cell suitable for use with the recombinant polynucleotides provided hereininclude Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp.
- Vectors suitable for maintenance, propagation, and/or expression of the recombinant polynucleotides in the aforementioned prokaryotic or eukaryotic cellsare also provided herein.
- Such vectorscan comprise any of the aforementioned or otherwise disclosed recombinant polynucleotides, recombinant DNA molecules, and recombinant RNA molecules as well as those polynucleotides molecules described in the Examples.
- the bacterium that mediates the plant transformationis an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp.
- the vectorincludes T-DNAs flanking the recombinant DNA molecule encoding the recombinant RNA molecule (e.g., as described in US patent application publications US20170369898 and US20180312854, each incorporated Agent Ref: P14419WO00 - 31 - herein by reference in their entireties).
- the vectoris contained within a plant cell or within a bacterial cell (Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell).
- a bacterial cellAgrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell.
- Viral particlescomprising any of the aforementioned or otherwise disclosed recombinant RNA molecules are also provided.
- the recombinant RNAis introduced into a host or production plant by using Agrobacterium-mediated transformation with a polynucleotide comprising (5’ to 3’): (i) a promoter which is operably linked to a viral MP coding sequence and a TLS element, flanked by Solemoviridae 5’ and 3’ RNA replication elements; (ii) a promoter which is operably linked to a cargo RNA molecule and a TLS element, flanked by Solemoviridae 5’ and 3’ RNA replication elements; (iii) a promoter operably linked to an RdRP coding sequence; and (iv) a promoter operably linked to a CP encoding sequence.
- a polynucleotidecomprising (5’ to 3’): (i) a promoter which is operably linked to a viral MP coding sequence and a TLS element, flanked by Solemoviridae 5’ and 3’ RNA replication elements; (
- Heterologous promotersindependently drive expression of the capsid protein and the cargo as depicted.
- the RNA expressed from the polynucleotideincludes an OAS.
- a host plantis transformed for production of the synthetic Solemoviridae satellite RNA and satellite particles comprising the encapsidated RNA.
- the expressed and encapsidated Solemoviridae satellite RNAis subsequently isolated from leaf material or other tissue of the host plant, purified (and, if desired, formulated) for high pressure spraying onto plants that endogenously contain the corresponding virus of the family Solemoviridae or a recombinant (e.g., stably transformed or transiently expressed in the plant) source of the Solemoviridae RdRP for subsequent expression and replication of the Solemoviridae satellite RNA and satellite particles comprising the same in encapsidated form.
- spraying with the encapsidated satellite particles with certain cargo RNA moleculescan be used to modify the plant as desired.
- plants without a systemic virus of the family Solemoviridae which provides the Solemoviridae RdRPcan further comprise a recombinant DNA or RNA molecule which encodes and provides the RdRP, e.g., stably integrated into the plant’s genome or transiently expressed in the plant.
- a plant that transgenically or transiently expresses a Solemoviridae RdRPis also useful for evaluating recombinant Solemoviridae satellites in planta.
- Target plants and plant cells used as hosts for synthetic Solemoviridae satellite RNAsinclude both monocot and dicot plants and plant cells which can support Solemoviridae replication.
- the virus of the family Solemoviridaeis endogenous to (endemic to or natively found in) the plant or plant cell.
- the virus of the family Solemoviridaeis introduced to and becomes established in the plant or plant cell.
- Embodimentsinclude row crop plants, fruit-producing plants and trees, vegetables, trees, and ornamental plants including ornamental flowers, shrubs, trees, groundcovers, and turf grasses.
- the host plants and plant cells for synthetic Solemoviridae satellite RNAsinclude row crop plants, fruit-producing plants and trees, vegetables, trees, and ornamental plants including ornamental flowers, shrubs, trees, Agent Ref: P14419WO00 - 32 - groundcovers, and turf grasses.
- the host plants and plant cells for synthetic Solemoviridae satellite RNAsinclude commercially important cultivated crops, trees, and plants, including: alfalfa (Medicago sativa), almonds (Prunus dulcis), apples (Malus x domestica), apricots (Prunus armeniaca, P. brigantine, P. mandshurica, P. mume, P.
- sibiricaasparagus (Asparagus officinalis), bananas (Musa spp.), barley (Hordeum vulgare), beans (Phaseolus spp.), blueberries and cranberries (Vaccinium spp.), cacao (Theobroma cacao), canola and rapeseed or oilseed rape, (Brassica napus), Polish canola (Brassica rapa), and related cruciferous vegetables including broccoli, kale, cabbage, and turnips (Brassica carinata, B. juncea, B. oleracea, B. napus, B. nigra, and B.
- Coffea arabica, Coffea canephora, and Coffea libericacotton (Gossypium hirsutum L.), cowpea (Vigna unguiculata and other Vigna spp.), fava beans (Vicia faba), cucumber (Cucumis sativus), currants and gooseberries (Ribes spp.), date (Phoenix dactylifera), duckweeds (family Lemnoideae), eggplant or aubergine (Solanum melongena), eucalyptus (Eucalyptus spp.), flax (Linum usitatissumum L.), geraniums (Pelargonium spp.), grapefruit (Citrus x paradisi), grapes (Vitus spp.) including wine grapes (Vitus vinifera and hybrids thereof), guava (Psidium guajava), hops (Humulus lupulus), hemp and cannabis
- the host plant or plant cells for synthetic Solemoviridae satellite RNAsis a dicot plant or plant cell selected from the genera Brassica, Capsicum, Cucumis, Cucurbita, Gossypium, Nicotiana, Solanum, or Glycine.
- the host plant Agent Ref: P14419WO00 - 33 - or plant cells for synthetic Solemoviridae satellite RNAsis a monocot plant or plant cell selected from the genera Avena, Hordeum, Oryza, Secale, Triticum, Sorghum, or Zea.
- monocot target plants and plant cells used as hosts for synthetic Solemoviridae satellite RNAsinclude oats (Avena sativa), barley (Hordeum vulgare), rice (Oryza sativa, Oryza glaberrima, Oryza rufipogen), rye (Secale cereale), wheat (Triticum aestivum), sorghum (Sorghum bicolor), and maize (Zea mays) plants and plant cells.
- the recombinant RNA molecule or a formulation thereofis provided by contacting the plant or plant cell with the recombinant RNA molecule or formulation thereof.
- the recombinant RNA moleculeis provided by expressing in the plant or plant cell a DNA molecule that encodes the recombinant RNA molecule or a formulation.
- the recombinant RNA moleculeis provided by contacting the plant or plant cell with cells (such as bacterial cells) which comprise a DNA molecule that encodes the recombinant RNA molecule and are capable of transforming the plant or plant cell.
- the recombinant RNA moleculeis provided by contacting the plant or plant cell with a satellite particle comprising an encapsidated recombinant RNA molecule or a formulation thereof.
- the 5’ RNA replication elementhas a nucleotide sequence obtained or derived from a Solemoviridae genomic sequence; and/or the 3’ RNA replication element has a nucleotide sequence obtained or derived from a Solemoviridae genomic sequence.
- the 5’ and/or 3’ RNA replication elementcan be obtained from the corresponding Solemoviridae genomic sequence by synthesizing or cloning a copy of the corresponding Solemoviridae genomic sequence.
- the 5’ and/or 3’ RNA replication elementcan be derived from the corresponding Solemoviridae genomic sequence by synthesizing a copy of a modified Solemoviridae genomic sequence or sequences.
- modifications of Solemoviridae genomic sequences present in a derived sequenceinclude: (i) substitutions of nucleotides which maintain the RNA secondary structure; (ii) substitution of nucleotides based on a consensus obtained by alignment of 5’ or 3’ RNA replication elements; (iii) insertions, deletions, and/or substitution of nucleotides to facilitate assembly and/or operable linkage to other elements in the satellite RNA which include cargo RNA molecules, tRNA-like elements, encapsidation recognition element (ERE), RNA encoding a viral movement protein (MP), IRES elements, an HRV 5’RR, HRV 3’RR, and/or HRV subgenomic promoter; or (iv) any combination of (i), (ii),
- the plant cellincludes the virus of the family Solemoviridae
- the RdRP proteinis provided to the plant cell by the virus of the family Solemoviridae.
- the virus of the family Solemoviridaeis endemic to the plant cell.
- the virus of the family Solemoviridae that is endemic to the plant cellis non- Agent Ref: P14419WO00 - 34 - pathogenic and/or commensal to the plant cell.
- the virus of the family Solemoviridaeis exogenously provided to the plant cell.
- the RdRP proteinis exogenously provided to the plant cell.
- the recombinant RNA moleculeis produced in a fermentation system.
- the recombinant RNA moleculeis provided to the plant cell by transcribing in the plant cell a recombinant DNA construct including a promoter functional in the plant cell and operably linked to a DNA sequence encoding the recombinant RNA molecule.
- the recombinant RNA moleculefurther includes an encapsidation recognition element (ERE), and the plant cell further includes a viral capsid protein (CP) capable of encapsidating the synthetic Solemoviridae satellite RNA.
- the viral capsid proteinis exogenously provided to the plant cell.
- the recombinant DNA constructfurther includes a DNA sequence encoding a viral capsid protein. Still in other embodiments, the recombinant DNA construct further includes a second promoter functional in the plant cell and operably linked to the DNA sequence encoding the viral capsid protein. In embodiments, the viral capsid protein is expressed in the plant cell and encapsidates the synthetic Solemoviridae satellite RNA. In yet other embodiments, the plant cell includes the virus of the family Solemoviridae, and the virus of the family Solemoviridae provides to the plant cell: (a) the RdRP protein, (b) the viral capsid protein, or (c) both the RdRP protein and the viral capsid protein.
- the methodscan further comprise a first step of providing a population of plants comprising the plant cells comprising: (i) the virus of the family Solemoviridae which provides the RdRP; or (ii) recombinant polynucleotide molecule that encodes the RdRP; and then providing the recombinant RNA molecule to the plants comprising the plant cells.
- the methodscan further comprise the step of determining if the plant cell comprises a virus of the family Solemoviridae which can provide the RdRP.
- the plant cellcomprises the virus of the family Solemoviridae which can provide the RdRP
- the virus of the family Solemoviridae, the RdRP protein, and/or the recombinant polynucleotide encoding the RdRPis optionally not exogenously provided to the plant cell.
- the plant celldoes not comprise the virus of the family Solemoviridae which can provide the RdRP and the virus of the family Solemoviridae is exogenously provided to the cell
- the RdRP protein or the recombinant polynucleotide encoding the RdRPis exogenously provided to the plant cell, or a combination of the virus of the family Solemoviridae, RdRP protein, or polynucleotide encoding the RdRP is exogenously provided to the plant cell.
- a complete self-replicating Solemoviridae satellite systemis introduced into a plant or plant cells, wherein the self-replicating Solemoviridae satellite system includes: (1) a recombinant Solemoviridae satellite RNA comprising, from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA- dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the promoter and the cargo RNA molecule are heterologous to the 5’ RNA replication element and the 3’ RNA replication element; and (2) an Agent Ref: P14419WO00 - 35 - exogenous virus of the family Solemoviridae (e.g., a virus of the family
- the Solemoviridae RdRPcomprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536.
- the presence or absence of a virus of the family Solemoviridae in a target plantcan be determined by an RNA detection assay (e.g., an RT-PCR assay) using nucleic acid probes and/or primers which can detect any part of a Solemoviridae genome including a 5’ RNA replication element, a CP and/or RdRP coding region, and/or a 3’ RNA replication element.
- Such probes and primersinclude those which detect any of the 5’ or 3’ RNA replication elements set forth in Table 1 or having significant sequence identity thereto (e.g., at least about 80%, 85%, 90%, 95%, 98%, or 99% of a length of at least about 18, 20, 30, 40 or 50 nt).
- the presence or absence of a virus of the family Solemoviridae in a target plantcan be determined by a protein detection assay (e.g., an immunoassay) directed to a Solemoviridae CP or RdRP (e.g., a CP or RdRP encoded by or homologous to a CP or RdRP encoded by a Solemoviridae genome disclosed in Table 1).
- Target plants and plant cells used in the methodsinclude all aforementioned target plants and plant cell hosts for synthetic Solemoviridae satellite RNAs (e.g., recombinant RNAs).
- the recombinant RNA that effects: (i) a phenotypic change in the plant or plant cell; (ii) increases a plant’s resistance to a pest or pathogen; or (iii) increases a plant’s resistance to stresscan include an RNA for modulating a target gene’s expression relative to the target gene’s expression in a control plant or plant cell not provided with the recombinant RNA molecule, and the phenotypic change, increased resistance to the pest or pathogen, or increased resistance to stress is a result of the modulation.
- the modulationis (a) an increase of the target gene’s expression; or (b) a decrease of the target gene’s expression.
- expression of the target geneis increased by up to about 1%, 2%, 3%, %, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61%, 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%
- expression of the target geneis increased by up to about 2-, 3-, 4-, 5-, 6-, 8-, 9-, 10-fold, or more relative to a reference level (e.g., a level found in a control plant or plant cell lacking the recombinant RNA molecule).
- a reference levele.g., a level found in a control plant or plant cell lacking the recombinant RNA molecule.
- expression of the target geneis decreased by at least about 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61%, Agent Ref: P14419WO00 - 36 - 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%,
- RNAs for modifying the genomeinclude gRNAs recognized by CAS nucleases, RNAs encoding TALENs or artificial zinc finger proteins (aZFN).
- RNAs for modifying the epigenomeinclude RNAs which provide RNA directed DNA methylation such as in promoter regions of target genes (Matzke and Mosher (2014), doi: 10.1038/nrg3683).
- Embodiments of an RNA for modifying the transcriptomeinclude one or more RNAs that comprise any of: a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA molecule that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a phased siRNA or phased siRNA precursor (see, e.g., US Patent No.8,404,928); a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA).
- hpRNAhairpin RNA
- the cargo RNA moleculecan comprise an RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA.
- phenotypes that are changedinclude developmental rate, growth rate, size, yield (e.g., intrinsic yield), vigor, photosynthetic capability, flavor, starch production, protein content, carbohydrate content, oil content, fatty acid content, lipid content, digestibility, biomass, shoot length, root length, root architecture, seed set, seed weight, seed quality (e.g., nutritional content), germination, fruit set, rate of fruit ripening, production of biopolymers, production of fibers, production of biofuels, production of pharmaceutical peptides (e.g., hormones, enzymes, transcription factors, antigens, antibodies, or antibody fragments), production of secretable peptides, enzyme production, improved processing traits, or amount of harvestable produce.
- developmental ratee.g., growth rate, size, yield (e.g., intrinsic yield), vigor, photosynthetic capability, flavor, starch production, protein content, carbohydrate content, oil content, fatty acid content, lipid content, digestibility, biomass, shoot length, root length
- phenotypes that are changedinclude taste, appearance, or shelf-life of a product harvested from the plant.
- phenotypes that are changedinclude flower size, flower color, flower patterning, flower morphology including presence or absence of stamens, flower number, flower longevity, flower fragrance, leaf size, leaf color, leaf patterning, leaf morphology, plant height, or plant architecture.
- the recombinant RNAcan comprise an RNA that inhibits expression of a gene of the pest or pathogen and/or inhibits replication of the genome of the pest or pathogen.
- the pest or pathogenis selected from the group comprising: a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate (e.g., an arthropod or a Agent Ref: P14419WO00 - 37 - nematode).
- Target viruses other than a virus of the family Solemoviridaeinclude; (i) positive-strand RNA viruses in the Bromoviridae, Closteroviridae, Luteoviridae, or Potyviridae family; (ii) negative strand RNA viruses in the Bunyaviridae and Rhabdoviridae family; (iii) dsDNA viruses in the family Caulimoviridae; and (iv) ssDNA viruses in the family Geminiviridae.
- Target arthropods pestsinclude coleopteran and lepidopteran insects.
- Target fungal pathogensinclude Magnaporthe spp., Botrytis spp., Puccinia spp.; Fusarium spp., Blumeria spp., Mycosphaerella spp., Colletotrichum spp., Ustilago spp., Melampsora spp., Phakopsora spp., Phytophthora spp., and Rhizoctonia spp.
- the cargo RNA moleculeeffects an increase in the plant’s resistance to a pest or pathogen, relative to that in a plant not provided with the recombinant RNA molecule.
- the recombinant RNAcan comprise an RNA that targets a plant gene which provides such resistance.
- the RNA that effects an increase in the plant’s resistance to stress in the plant or plant cellincludes an RNA for modulating the target gene’s expression relative to the target gene’s expression in a control plant or plant cell not provided with the recombinant RNA molecule, and wherein the increase stress resistance is a result of the modulation.
- the modulationis (a) an increase of the target gene’s expression; or (b) a decrease of the target gene’s expression.
- the RNA that effects an increase in the plant’s resistance to stress in the plant or plant cellcomprises a messenger RNA encoding a protein which confers the stress resistance.
- the messenger RNAincludes an RNA sequence absent in the transcriptome of the plant or plant cell lacking the recombinant RNA.
- the stressincludes at least one abiotic stress selected from the group including: nutrient stress, light stress, drought stress, heat stress, and cold stress.
- the stressincludes at least one biotic stress selected from the group including: crowding, shading, and allelopathy (e.g., resulting from allelopathic chemicals including a juglone produced by walnut trees).
- the cargo RNAcan encode the exogenous polypeptide.
- the polypeptideis isolated (e.g., separated from at least one other cellular components such as a carbohydrate, a lipid, or another protein) or polypeptide is purified.
- manufacturecan occur in either a cell-based system or a cell-free system.
- Cell-based methods of manufacturing a synthetic Solemoviridae satellite particlecan comprise: (a) providing to a plant cell the recombinant RNA molecule, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule and a viral capsid protein, wherein the ERE provides for encapsidation of the RNA by the Agent Ref: P14419WO00 - 38 - viral capsid protein; and optionally isolating the synthetic Solemoviridae satellite particle from the plant cell, a plant comprising the plant cell, or from media in which the plant cell or the plant had been grown.
- ERPencapsidation recognition element
- Cell-free methods of manufacturing a synthetic Solemoviridae satellite particleinclude methods where the recombinant RNA molecule is combined with a viral capsid protein in a vessel, wherein the recombinant RNA molecule comprises an ERE, and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein in the vessel; optionally wherein the method further comprises isolating the synthetic Solemoviridae satellite particle from uncombined RNA and/or viral capsid protein in the vessel.
- the synthetic satellite particleis isolated (e.g., separated from at least one other cellular components such as an organelle, a membrane, a carbohydrate, a lipid, or another protein) or is purified.
- the methodscan further comprise formulating the synthetic Solemoviridae satellite particle.
- Synthetic Solemoviridae satellite particlescomprising the recombinant RNA, including those made by the aforementioned methods are also provided.
- Methods of providing any of the aforementioned synthetic Solemoviridae satellite particles to a plantincluding contacting (e.g., spraying, dusting, injecting, soaking, etc.) the plant with the synthetic Solemoviridae satellite particle or a formulation thereof are also provided.
- the recombinant polynucleotides, cells comprising the same, and synthetic Solemoviridae satellite particles described hereincan be formulated either in pure form (e.g., the composition contains only the recombinant polynucleotide) or together with one or more additional formulation components to facilitate application or delivery of the compositions.
- the additional formulation componentincludes, e.g., a carrier (i.e., a component that has an active role in delivering the active agent (e.g., recombinant polynucleotide); for example, a carrier can encapsulate, covalently or non-covalently modify, or otherwise associate with the active agent in a manner that improves delivery of the active agent) or an excipient (e.g., a delivery vehicle, adjuvant, diluent, surfactant, stabilizer, or tonicity agent).
- the compositionis formulated for delivery to a plant.
- the disclosureprovides a formulation comprising any of the compositions described herein.
- the formulationis a liquid, a gel, or a powder.
- the formulationis configured to be sprayed on plants, to be injected into plants (see, e.g., US Patent No.11,844,318) or otherwise introduced into the vascular system of a plant, to be rubbed on leaves, to be soaked into plants, to be coated onto plants, or be coated on seeds, or to be delivered through root uptake (e.g., in a hydroponic system or via soil).
- the compositioncan be formulated into emulsifiable concentrates, suspension concentrates, directly sprayable or dilutable solutions, coatable pastes, diluted emulsions, spray powders, soluble powders, dispersible powders, wettable powders, dusts, granules, encapsulations in polymeric substances, microcapsules, foams, aerosols, carbon dioxide gas preparations, tablets, resin preparations, paper preparations, nonwoven fabric preparations, or knitted or woven fabric preparations.
- the compositionis a Agent Ref: P14419WO00 - 39 - liquid.
- the compositionis a solid.
- the compositionis an aerosol, such as in a pressurized aerosol can.
- the recombinant polynucleotidemakes up about 0.1% to about 100% of the composition, such as any one of about 0.01% to about 100%, about 1% to about 99.9%, about 0.1% to about 10%, about 1% to about 25%, about 10% to about 50%, about 50% to about 99%, or about 0.1% to about 90% of active ingredients (e.g., recombinant polynucleotides).
- the compositionincludes at least any of 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or more active ingredients (e.g., recombinant polynucleotides).
- active ingredientse.g., recombinant polynucleotides
- the concentrated agentsare preferred as commercial products, the final user normally uses diluted agents, which have a substantially lower concentration of active ingredient.
- the compositionis formulated for topical delivery to a plant.
- the topical deliveryis spraying, leaf rubbing (e.g., with or without an abrasive), soaking, coating (e.g., coating using micro-particulates or nano-particulates), or delivery through root uptake (e.g., delivery in a hydroponic system or by a root drench).
- the compositionfurther comprises a carrier and/or an excipient.
- the compositiondoes not comprise a carrier or excipient, e.g., comprises a naked polynucleotide (e.g., a naked RNA).
- the recombinant polynucleotideis delivered at a concentration of at least 0.1 grams per acre, e.g., at least 0.1, 1, 2, 5, 10, 15, 20, 25, 30, 35, 40, 45, or 50 grams per acre. In some embodiments, less than 120 liters per acre is delivered, e.g., less than 110, 100, 90, 80, 70, 60, 50, 40, 30, 20, 15, 10, 5, or 2 liters per acre or less than 1 liter per acre.
- the formulationcomprises a carrier. In some embodiments the formulation is an emulsion or a reverse emulsion, a liquid, or a gel.
- the formulationincludes a carrier that serves as a physical support (e.g., solid or semi-solid surfaces or matrices, powders, or particles or nanoparticles).
- the active agentis encapsulated or enclosed in or attached to or complexed with a carrier including a liposome, vesicle, micelle, or other fluid compartment.
- the active agentis encapsulated or enclosed in or attached to or complexed with a carrier including a naturally occurring or synthetic, branched or linear polymer (e.g., pectin, agarose, chitin, chitosan, DEAE-dextran, polyvinylpyrrolidone (“PVP”), or polyethylenimine (“PEI”).
- PVPpolyvinylpyrrolidone
- PEIpolyethylenimine
- the carrierincludes cations or a cationic charge, such as cationic liposomes or cationic polymers such as polyamines (e.g., spermine, spermidine, putrescine).
- the carrierincludes a polypeptide such as an enzyme, (e.g., cellulase, pectolyase, maceroenzyme, pectinase), a cell penetrating or pore- forming peptide (e.g., poly-lysine, poly-arginine, or polyhomoarginine peptides).
- an enzymee.g., cellulase, pectolyase, maceroenzyme, pectinase
- a cell penetrating or pore- forming peptidee.g., poly-lysine, poly-arginine, or polyhomoarginine peptides.
- Non-limiting examples of carriersinclude cationic liposomes and poly
- the carrierincludes a nanomaterial, such as carbon or Agent Ref: P14419WO00 - 40 - silica nanoparticles, carbon nanotubes, carbon nanofibers, or carbon quantum dots.
- Non-limiting examples of carriersinclude particles or nanoparticles (e.g., particles or nanoparticles made of materials such as carbon, silicon, silicon carbide, gold, tungsten, polymers, or ceramics) in various size ranges and shapes, magnetic particles or nanoparticles (e.g., silenceMag Magnetotransfection TM agent, OZ Biosciences, San Diego, CA), abrasive or scarifying agents, needles or microneedles, matrices, and grids.
- particulates and nanoparticulatesare useful in delivery of the polynucleotide composition or the nuclease or both.
- Useful particulates and nanoparticlesinclude those made of metals (e.g., gold, silver, tungsten, iron, cerium), ceramics (e.g., aluminum oxide, silicon carbide, silicon nitride, tungsten carbide), polymers (e.g., polystyrene, polydiacetylene, and poly(3,4- ethylenedioxythiophene) hydrate), semiconductors (e.g., quantum dots), silicon (e.g., silicon carbide), carbon (e.g., graphite, graphene, graphene oxide, or carbon nanosheets, nanocomplexes, or nanotubes), and composites (e.g., polyvinylcarbazole/graphene, polystyrene/graphene, platinum/graphene, palladium/graphene nanocomposites).
- metalse.g., gold, silver, tungsten, iron, cerium
- ceramicse.g., aluminum oxide, silicon carbide,
- such particulates and nanoparticulatesare further covalently or non-covalently functionalized, or further include modifiers or cross-linked materials such as polymers (e.g., linear or branched polyethylenimine, poly-lysine), polynucleotides (e.g., DNA or RNA), polysaccharides, lipids, polyglycols (e.g., polyethylene glycol, thiolated polyethylene glycol), polypeptides or proteins, and detectable labels (e.g., a fluorophore, an antigen, an antibody, or a quantum dot).
- polymerse.g., linear or branched polyethylenimine, poly-lysine
- polynucleotidese.g., DNA or RNA
- polysaccharidese.g., DNA or RNA
- lipidslipids
- polyglycolse.g., polyethylene glycol, thiolated polyethylene glycol
- Embodiments of compositions including particulatesinclude those formulated, e.g., as liquids, colloids, dispersions, suspensions, aerosols, gels, and solids.
- Embodimentsinclude nanoparticles affixed to a surface or support, e.g., an array of carbon nanotubes vertically aligned on a silicon or copper wafer substrate.
- Embodimentsinclude polynucleotide compositions including particulates (e.g., gold or tungsten or magnetic particles) delivered by a Biolistic-type technique or with magnetic force.
- the size of the particles used in Biolisticsis generally in the “microparticle” range, for example, gold microcarriers in the 0.6, 1.0, and 1.6 micrometer size ranges (see, e.g., instruction manual for the Helios® Gene Gun System, Bio-Rad, Hercules, CA; Randolph-Anderson et al. (2015) "Submicron gold particles are superior to larger particles for efficient Biolistic® transformation of organelles and some cell types", Bio-Rad US/EG Bulletin 2015), but successful Biolistics delivery using larger (40 - 48 nanometer) nanoparticles has been reported in cultured animal cells; see O'Brian and Lummis (2011) BMC Biotechnol., 11 :66 - 71.
- nanoparticleswhich are generally in the nanometer (nm) size range or less than 1 micrometer, e.g., with a diameter of less than about 1 nm, less than about 3 nm, less than about 5 nm, less than about 10 nm, less than about 20 nm, less than about 40 nm, less than about 60 nm, less than about 80 nm, and less than about 100 nm.
- nanoparticlescommercially available (all from Sigma-Aldrich Corp., St.
- Embodimentsinclude polynucleotide compositions including materials such as gold, silicon, cerium, or carbon, e.g., gold or gold-coated nanoparticles, silicon carbide whiskers, carborundum, porous silica nanoparticles, gelatin/silica nanoparticles, nanoceria or cerium oxide nanoparticles (CNPs), carbon nanotubes (CNTs) such as single-, double-, or multi-walled carbon nanotubes and their chemically functionalized versions (e.g., carbon nanotubes functionalized with amide, amino, carboxylic acid, sulfonic acid, or polyethylene glycol moieties), and graphene or graphene oxide or graphene complexes; see, for example, Wong et al.
- materialssuch as gold, silicon, cerium, or carbon, e.g., gold or gold-coated nanoparticles, silicon carbide whiskers, carborundum, porous silica nanoparticles, gelatin/silica nanoparticle
- the compositionincludes an excipient, e.g., a delivery vehicle, adjuvant, diluent, surfactant, stabilizer, or tonicity agent or a combination thereof.
- the excipientis a crop oil concentrate, a vegetable oil concentrate, a modified vegetable oil, a nitrogen source, a deposition (drift control) and/or retention agent (with or without ammonium sulfate and/or defoamer), a compatibility agent, a buffering agent and/or acidifier, a water conditioning agent, a basic blend, a spreader-sticker and/or extender, an adjuvant plus foliar fertilizer, an antifoam agent, a foam marker, a scent, or a tank cleaner and/or neutralizer.
- the excipientis an adjuvant described in the Compendium of Herbicide Adjuvants (Young et al. (2016).
- Examples of delivery vehicles and diluentsinclude, but are not limited to, lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia, calcium phosphate, alginates, tragacanth, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, water, saline solution, syrup, methylcellulose, methyl- and propylhydroxybenzoates, talc, magnesium stearate, and mineral oil.
- delivery vehiclesinclude, but are not limited to, solid or liquid excipient materials, solvents, stabilizers, slow-release excipients, colorings, and surface-active substances (surfactants).
- the excipiente.g., delivery vehicle
- the stabilizing vehicleincludes, e.g., an epoxidized vegetable oil, an antifoaming agent, e.g. silicone oil, a preservative, a viscosity regulator, a binding agent, or a tackifier.
- the stabilizing Agent Ref: P14419WO00 - 42 - vehicleis a buffer suitable for the recombinant polynucleotide.
- the compositionis microencapsulated in a polymer bead delivery vehicle.
- the stabilizing vehicleprotects the recombinant polynucleotide against UV and/or acidic conditions.
- the delivery vehiclecontains a pH buffer.
- the compositionis formulated to have a pH in the range of about 4.5 to about 9.0, including for example pH ranges of about any one of 5.0 to about 8.0, about 6.5 to about 7.5, or about 6.5 to about 7.0.
- the composition provided hereinincludes an adjuvant.
- Adjuvantsare agents that do not possess the polynucleotide activity, but impart beneficial properties to a formulation.
- adjuvantsare either pre-mixed in the formulation or added to a spray tank to improve mixing or application or to enhance performance. They are used extensively in products designed for foliar applications. Adjuvants can be used to customize the formulation to specific needs and compensate for local conditions. Adjuvants can be designed to perform specific functions, including wetting, spreading, sticking, reducing evaporation, reducing volatilization, buffering, emulsifying, dispersing, reducing spray drift, and reducing foaming. No single adjuvant can perform all these functions, but compatible adjuvants often can be combined to perform multiple functions simultaneously.
- adjuvants included in the formulationare binders, dispersants and stabilizers, specifically, for example, casein, gelatin, polysaccharides (e.g., starch, gum arabic, cellulose derivatives, alginic acid, etc.), lignin derivatives, bentonite, sugars, synthetic water-soluble polymers (e.g., polyvinyl alcohol, polyvinylpyrrolidone, polyacrylic acid, etc.), PAP (acidic isopropyl phosphate), BHT (2,6-di-t-butyl-4-methylphenol), BHA (a mixture of 2-t-butyl-4-methoxyphenol and 3- t-butyl-4-methoxyphenol), vegetable oils, mineral oils, fatty acids and fatty acid esters.
- bindersspecifically, for example, casein, gelatin, polysaccharides (e.g., starch, gum arabic, cellulose derivatives, alginic acid, etc.), lignin derivatives, benton
- the compositions provided hereinare in a liquid formulation.
- Liquid formulationsare generally mixed with water, but in some instances are used with crop oil, diesel fuel, kerosene, or other light oil as an excipient.
- the amount of active ingrediente.g., recombinant polynucleotides
- an emulsifiable concentrate formulationcontains a liquid active ingredient, one or more petroleum-based solvents, and an agent that allows the formulation to be mixed with water to form an emulsion.
- Such concentratescan be used in agricultural, ornamental and turf, forestry, structural, food processing, livestock, and public health pest formulations.
- theseare adaptable to application equipment from small portable sprayers to hydraulic sprayers, low-volume ground sprayers, mist blowers, and low-volume aircraft sprayers.
- Some active ingredientsreadily dissolve in a liquid excipient. When mixed with an excipient, they form a solution that does not settle out or separate, e.g., a homogenous solution.
- formulations of these typesinclude an active ingredient, a carrier and/or an excipient, and one or more other ingredients. Solutions can be used in any type of sprayer, indoors and outdoors.
- the compositionis formulated as an invert emulsion.
- An invert emulsionis a water-soluble active ingredient dispersed in an oil excipient.
- Invert emulsionsrequire an emulsifier that Agent Ref: P14419WO00 - 43 - allows the active ingredient to be mixed with a large volume of petroleum-based excipient, usually fuel oil. Invert emulsions aid in reducing drift. With other formulations, some spray drift results when water droplets begin to evaporate before reaching target surfaces; as a result, the droplets become very small and lightweight. Because oil evaporates more slowly than water, invert emulsion droplets shrink less, and more active ingredient reaches the target. Oil further helps to reduce runoff and improve rain resistance. It further serves as a sticker-spreader by improving surface coverage and absorption. Because droplets are relatively large and heavy, it is difficult to get thorough coverage on the undersides of foliage.
- a flowable or liquid formulationcombines many of the characteristics of emulsifiable concentrates and wettable powders. Manufacturers use these formulations when the active ingredient is a solid that does not dissolve in either water or oil. The active ingredient, impregnated on a substance such as clay, is ground to a very fine powder. The powder is then suspended in a small amount of liquid. The resulting liquid product is quite thick. Flowables and liquids share many of the features of emulsifiable concentrates, and they have similar disadvantages. They require moderate agitation to keep them in suspension and leave visible residues, similar to those of wettable powders.
- Aerosol formulationscontain one or more active ingredients and a solvent. Most aerosols contain a low percentage of active ingredients. There are two types of aerosol formulations—the ready- to-use type commonly available in pressurized sealed containers and those products used in electrical or gasoline-powered aerosol generators that release the formulation as a smoke or fog.
- Ready to use aerosol formulationsare usually small, self-contained units that release the formulation when the nozzle valve is triggered. The formulation is driven through a fine opening by an inert gas under pressure, creating fine droplets. These products are used in greenhouses, in small areas inside buildings, or in localized outdoor areas. Commercial models, which hold five to 5 pounds of active ingredient, are usually refillable.
- Smoke or fog aerosol formulationsare not under pressure. They are used in machines that break the liquid formulation into a fine mist or fog (aerosol) using a rapidly whirling disk or heated surface.
- the compositioncomprises a liquid excipient.
- a liquid excipientincludes, for example, aromatic or aliphatic hydrocarbons (e.g., xylene, toluene, alkylnaphthalene, phenylxylylethane, kerosene, gas oil, hexane, cyclohexane, etc.), halogenated hydrocarbons (e.g., chlorobenzene, dichloromethane, dichloroethane, trichloroethane, etc.), alcohols (e.g., methanol, ethanol, isopropyl alcohol, butanol, hexanol, benzyl alcohol, ethylene glycol, etc.), ethers Agent Ref: P14419WO00 - 44 - (e.g., diethyl ether, ethylene glycol dimethyl ether, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, propylene glycol monomethyl ether, tetrahydro
- the compositioncomprises a gaseous excipient.
- Gaseous excipientsinclude, for example, butane gas, floron gas, liquefied petroleum gas (LPG), dimethyl ether, and carbon dioxide gas.
- LPGliquefied petroleum gas
- the compositionsare provided as a dry formulation.
- Dry formulationscan be divided into two types: ready-to-use and concentrates that must be mixed with water to be applied as a spray. Most dust formulations are ready to use and contain a low percentage of active ingredients (less than about 10 percent by weight), plus a very fine, dry inert excipient (e.g., talc, chalk, clay, nut hulls, or volcanic ash). The size of individual dust particles varies. A few dust formulations are concentrates and contain a high percentage of active ingredients. In some embodiments, these are mixed with dry inert excipients before applying. In some embodiments, dusts are used dry and can easily drift to non-target sites. [0120] In some instances, the composition is formulated as a powder.
- the compositionis formulated as a wettable powder.
- Wettable powdersare dry, finely ground formulations that look like dusts. They usually must be mixed with water for application as a spray. A few products, however, can be applied either as a dust or as a wettable powder—the choice is left to the applicator. Wettable powders have about 1 to about 95 percent active ingredient by weight; in some cases, more than about 50 percent. The particles do not dissolve in water. They settle out quickly unless constantly agitated to keep them suspended. They can be used for most pest problems and in most types of spray equipment where agitation is possible. Wettable powders have excellent residual activity. Because of their physical properties, most of the formulation remains on the surface of treated porous materials such as concrete, plaster, and untreated wood.
- the compositionis formulated as a soluble powder. Soluble powder formulations look like wettable powders. However, when mixed with water, soluble powders dissolve readily and form a true solution. After they are mixed thoroughly, no additional agitation is necessary. The amount of active ingredient in soluble powders ranges from about 15 to about 95 percent by weight; in some cases, more than about 50 percent. Soluble powders have all the advantages of wettable powders and none of the disadvantages, except the inhalation hazard during mixing. [0122] In some instances, the composition is formulated as a water-dispersible granule.
- Water- dispersible granulesalso known as dry flowables, are like wettable powders, except instead of being dust-like, they are formulated as small, easily measured granules.
- Water-dispersible granulesmust be Agent Ref: P14419WO00 - 45 - mixed with water to be applied. Once in water, the granules break apart into fine particles similar to wettable powders. The formulation requires constant agitation to keep it suspended in water. The percentage of active ingredient is high, often as much as 90 percent by weight. Water-dispersible granules share many of the same advantages and disadvantages of wettable powders, except they are more easily measured and mixed.
- the compositioncomprises a solid excipient.
- Solid excipientsinclude finely-divided powder or granules of clay (e.g.
- kaolin claydiatomaceous earth, bentonite, Fubasami clay, acid clay, etc.
- synthetic hydrated silicon oxidetalc, ceramics, other inorganic minerals (e.g., sericite, quartz, sulfur, activated carbon, calcium carbonate, hydrated silica, etc.), a substance which can be sublimated and is in the solid form at room temperature (e.g., 2,4,6-triisopropyl-1,3,5-trioxane, naphthalene, p-dichlorobenzene, camphor, adamantan, etc.); wool; silk; cotton; hemp; pulp; synthetic resins (e.g., polyethylene resins such as low-density polyethylene, straight low-density polyethylene and high-density polyethylene; ethylene-vinyl ester copolymers such as ethylene-vinyl acetate copolymers; ethylene-methacrylic acid ester copolymers such as ethylene-methyl methacrylate copo
- the compositionis provided in a microencapsulated formulation (e.g., a nanocapsule). Microencapsulated formulations are mixed with water and sprayed in the same manner as other sprayable formulations. After spraying, the encapsulation shell or coating breaks down and slowly releases the active ingredient. [0125] In some instances, the composition is provided in a liposome. In some instances, the composition is provided in a vesicle. [0126] In some instances, a composition provided herein includes a surfactant. Surfactants, also called wetting agents and spreaders, physically alter the surface tension of a spray droplet.
- surfactantsalso called wetting agents and spreaders
- SurfactantsFor a Agent Ref: P14419WO00 - 46 - formulation to perform its function properly, a spray droplet must be able to wet the foliage and spread out evenly over a leaf. Surfactants enlarge the area of formulation coverage, thereby increasing exposure to the active agent. Surfactants are particularly important when applying a formulation to waxy or hairy leaves. Without proper wetting and spreading, spray droplets often run off or fail to cover leaf surfaces adequately. Too much surfactant, however, can cause excessive runoff and reduce efficacy. [0127] Surfactants can be classified as anionic, cationic, or nonionic. Formulation activity in the presence of a nonionic surfactant can be quite different from activity in the presence of a cationic or anionic surfactant.
- anionic surfactantsare most effective when used with contact pesticides (pesticides that control a pest by direct contact rather than being absorbed systemically).
- Cationic surfactantsare not typically used as stand-alone surfactants because they usually are phytotoxic.
- Nonionic surfactantsoften used with systemic pesticides, help sprays to penetrate plant cuticles.
- Nonionic surfactantsare compatible with most pesticides, and most EPA-registered pesticides that require a surfactant recommend a nonionic type.
- Adjuvantsinclude, but are not limited to, stickers, extenders, plant penetrants, compatibility agents, buffers or pH modifiers, drift control additives, defoaming agents, and thickeners.
- surfactants included in the compositions described hereinare alkyl sulfate ester salts, alkyl sulfonates, alkyl aryl sulfonates, alkyl aryl ethers and polyoxyethylenated products thereof, polyethylene glycol ethers, polyvalent alcohol esters and sugar alcohol derivatives.
- the surfactantis a nonionic surfactant, a surfactant plus nitrogen source, an organo- silicone surfactant, or a high surfactant oil concentrate.
- the recombinant polynucleotidecan, in embodiments, be in a mixture with other active compounds, such as pesticidal agents (e.g., insecticides, sterilants, acaricides, nematicides, molluscicides, or fungicides, attractants, growth-regulating substances, or herbicides).
- pesticidal agentse.g., insecticides, sterilants, acaricides, nematicides, molluscicides, or fungicides, attractants, growth-regulating substances, or herbicides.
- the term “pesticidal agent”refers to any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest.
- a pesticidecan be a chemical substance or biological agent used against pests including insects, mollusks, pathogens, weeds, nematodes, and microbes that compete with humans for food, destroy property, spread disease, or are a nuisance.
- the term “pesticidal agent”further encompasses other bioactive molecules such as antibiotics, antivirals pesticides, antifungals, antihelminthics, nutrients, pollen, sucrose, and/or agents that stun or slow insect movement.
- this disclosureis related to a method of producing a modified plant propagule that comprises at least one plant cell comprising a recombinant RNA molecule.
- the methodincludes the steps of: isolating a plant propagule comprising at least one plant cell comprising a Agent Ref: P14419WO00 - 47 - recombinant RNA molecule and a Solemoviridae RNA-dependent RNA polymerase (RdRP) from a mixed population of plant cells comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule, wherein the recombinant RNA molecule comprises, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP; a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP.
- a plant propagulecomprising at least one plant cell comprising a Agent Ref: P14419WO00 - 47 - recombinant RNA molecule and a Solemoviridae RNA-
- the isolated plant propagule comprising at least one plant cell comprising a recombinant RNA moleculewill be free or substantially free of plant cells lacking the recombinant RNA.
- Such isolated plant propagules which are substantially free of plant cells lacking the recombinant RNAcan in certain embodiments comprise plant propagules where at least 75%, 80%, 85%, 90%, 95%, 98%, or 99% of the plant cells in the plant propagule contain the recombinant DNA molecule.
- the mixed population of plant cellscomprise a population of protoplasts or a population of cells in callus, an explant, a plant part, or whole plant.
- the mixed population of plant cellscan comprise a population of plant cells where less than 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5%, or 1% of the plant cells in the population contain the recombinant RNA molecule.
- the mixed population of plant cellscomprise plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP.
- the plant cells lacking the Solemoviridae RdRPwill also lack the recombinant RNA.
- the mixed population of plant cellscomprise plant cells comprising the Solemoviridae RdRP.
- the plant cells comprising the Solemoviridae RdRPcan further comprise the recombinant RNA.
- the cargo RNA sequencecomprises RNA that encodes a selectable or scorable marker.
- the mixed population of plant cellsis screened or selected for the presence of the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule.
- the mixed population of cells or a portion thereofare subjected to an assay for a screenable marker for the presence of the recombinant RNA molecule (e.g., an RNA sequence diagnostic for presence of the recombinant RNA molecule or a polypeptide encoded by the recombinant RNA molecule) and separated from plant cells lacking the recombinant RNA molecule.
- the isolationcomprises selecting for the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule.
- Examples of such selections in instances where the recombinant RNA encodes a selectable markercan comprise exposing the mixed population of plant cells to a selection agent (e.g., an herbicide or antibiotic) and isolating plant cells which survive exposure to the selection agent.
- a selectable markere.g., a protein which confers resistance to a selection agent such as an herbicide or antibiotic
- selectable marker/selection agent combinationsinclude glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzyme/glufosinate, or a neomycin phosphotransferase (npt)/neomycin or kanamycin.
- the selectable or scorable markeris an RNA aptamer (e.g., a Broccoli aptamer) or a regulatory RNA (e.g., an siRNA, siRNA precursor, miRNA, or miRNA precursor, or a phased siRNA or phased siRNA precursor that downregulates expression of an endogenous gene in Agent Ref: P14419WO00 - 48 - the plant, resulting in a detectable phenotype, e.g., bleaching caused by downregulation of a pigment- producing gene).
- the mixed populationis located within a plant or a plant part.
- the plant or plant partis screened or selected for presence of the recombinant RNA molecule prior to isolating the plant propagule. In other embodiments, the plant or plant part is screened or selected for systemic presence of the recombinant RNA molecule prior to isolating the plant propagule. In some embodiments, the plant cells, plant, or plant part in the mixed population or that are isolated lack DNA that encodes the recombinant RNA molecule.
- the plant propagule comprising the recombinant RNA moleculeis isolated by detecting the RNA molecule in one or more plant cells comprising the recombinant RNA molecule and separating the one or more plant cells comprising the recombinant RNA molecule from the plant cells lacking the recombinant DNA molecule.
- the plant propaguleis a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule.
- the modified plant propaguleis a mosaic comprising both plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP.
- the plant propagulelacks DNA that encodes the recombinant RNA molecule.
- the modified plant propagulecomprises the cell comprising the recombinant RNA molecule, or a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the cell comprising the recombinant RNA molecule.
- any of the aforementioned methodscan further comprise multiplying the cell, seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus to obtain progeny, wherein the progeny comprise the recombinant RNA molecule.
- the multiplying of the cellsconsists of culturing a plurality of explants obtained from the cell, seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus.
- the isolated propagulecomprises the cell and the aforementioned methods can further comprise regenerating a plant, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the recombinant RNA from the cell.
- the isolated propagulecomprises callus and the aforementioned methods can further comprise regenerating a plant, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, or explant comprising the recombinant RNA from said callus.
- a plantis regenerated and the aforementioned methods can further comprise recovering F 1 seed or F 1 progeny or clonal progeny comprising the recombinant RNA from the plant.
- this disclosureis related to a method of providing a synthetic Solemoviridae satellite RNA to a plant or plant part by grafting one plant part to another plant part.
- the methodscan comprise grafting a scion onto a rootstock comprising any of the aforementioned or otherwise disclosed recombinant DNA molecules and/or recombinant RNA molecules (e.g., a recombinant RNA comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP), wherein at least one cell of the rootstock and/or the scion comprises the Solemoviridae RdRP.
- a recombinant RNAcomprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable
- the scioncan comprise a plant shoot, an apical or other meristem, a leaf attached to a petiole, or other plant part and the rootstock can comprise roots and aerial portions of the plant including the main stem, secondary stems, leaves, and/or reproductive structures of the plant,
- DNA that encodes the recombinant RNA moleculeis absent in the scion and/or the rootstock.
- the scionlacks the recombinant RNA molecule prior to grafting.
- the rootstockcomprises the Solemoviridae RdRP.
- the Solemoviridae RdRPis provided by a virus of the family Solemoviridae endemic to the rootstock (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal).
- the Solemoviridae RdRPis exogenously provided to the rootstock (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the rootstock or via a recombinant viral vector comprising DNA or RNA encoding the RdRP).
- the scioncomprises the Solemoviridae RdRP.
- the RdRPis provided by a virus of the family Solemoviridae endemic to the scion (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal).
- the RdRPis exogenously provided to the scion (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the scion or via a recombinant viral vector comprising DNA or RNA encoding the RdRP).
- the Solemoviridae RdRPcomprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536.
- the rootstock and/or the scioncomprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule.
- the rootstock and/or the scioncomprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein.
- this disclosureis related to a method of producing a grafted plant comprising a recombinant RNA molecule comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by an the Solemoviridae RdRP.
- RdRPSolemoviridae RNA-dependent RNA polymerase
- the recombinant RNA moleculeis provided by contacting the scion, the rootstock, or both the scion and the rootstock with a composition comprising the recombinant RNA molecule prior to grafting the scion onto the rootstock to produce the grafted plant.
- at least one cell of the rootstock and/or the scioncomprises a Solemoviridae RdRP prior to contacting the scion, the rootstock, or both the scion and the rootstock with the composition.
- the rootstockcomprises the Solemoviridae RdRP.
- the Solemoviridae RdRPis provided by a virus of the family Solemoviridae endemic to the rootstock (e.g., a Agent Ref: P14419WO00 - 50 - virus of the family Solemoviridae which is non-pathogenic and/or commensal).
- the Solemoviridae RdRPis exogenously provided to the rootstock (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the rootstock or via a recombinant viral vector comprising DNA or RNA encoding the RdRP).
- the scioncomprises the Solemoviridae RdRP.
- the RdRPis provided by a virus of the family Solemoviridae endemic to the scion (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal).
- the RdRPis exogenously provided to the scion (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the scion or via a recombinant viral vector comprising DNA or RNA encoding the RdRP).
- the Solemoviridae RdRPcomprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536.
- DNA that encodes the recombinant RNA moleculeis absent in the scion, the rootstock, and/or the grafted plant.
- the compositioncan be provided to the scion, the rootstock, or both the scion and the rootstock according to any of the formulations disclosed herein.
- the formulationis a liquid, a gel, or a powder.
- the formulationis configured to be sprayed on to the scion, the rootstock, or both the scion and the rootstock; to be injected into the scion, the rootstock, or both the scion and the rootstock; to be soaked into the scion, the rootstock, or both the scion and the rootstock; or to be coated onto the scion, the rootstock, or both the scion and the rootstock.
- the contactingcomprises dipping the scion, the rootstock, or both the scion and the rootstock into the composition prior to grafting.
- this disclosureis related to a method for producing a plant that transmits any of the aforementioned or otherwise disclosed recombinant RNA molecules provided herein to progeny plants or seed.
- the methodsinclude the steps of: isolating an F 1 progeny plant or seed comprising at least one cell comprising a Solemoviridae RNA-dependent RNA polymerase (RdRP) and the recombinant RNA molecule comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP from a population of F 1 plants or seed obtained from at least one parent plant comprising the recombinant RNA molecule.
- RdRPSolemoviridae RNA-dependent RNA polymerase
- the parent plant or a part thereof comprising the plant cellis screened or selected for presence of the recombinant RNA molecule prior to isolating the F 1 progeny plant or seed. In some embodiments, the parent plant or one or more parts thereof are screened for systemic presence of the recombinant RNA molecule prior to isolating the F 1 progeny plants.
- floral tissuee.g., whole flowers or buds, sepal, calyx, or petal
- male reproductive tissuee.g., stamen, anther, or pollen
- female reproductive tissuee.g., whole fruit, ovary, pericarp, ovule, seed coat, endosperm, or embryo
- F 1 seedsare obtained from a parent plant selected for presence of the recombinant RNA molecule in pericarp tissue.
- the F 1 progeny plant or seed comprising the cellis isolated by screening the population of F 1 plants or seed obtained from a parent plant for the presence of the Agent Ref: P14419WO00 - 51 - recombinant RNA molecule and propagating the F 1 progeny plant or seed comprising the recombinant RNA molecule.
- the progeny plants or seed thereofare subjected to an assay for a screenable marker for the presence of the recombinant RNA (e.g., an RNA sequence diagnostic for presence of the recombinant RNA molecule or a polypeptide encoded by the recombinant RNA molecule) and separated from progeny plants and seed lacking the recombinant RNA progeny plants and seed lacking the recombinant RNA.
- Such screening assayscan be non-destructive assays wherein a portion of the progeny seed or plant is removed and assayed without loss of the viability or ability to propagate the seed or plant tissue comprising the recombinant RNA.
- an F 1 seed of the parent plantis non-destructively screened for presence of the recombinant RNA molecule.
- the F 1 seed of the parent plantis non-destructively screened by assaying maternally derived or endosperm tissue of the seed for the presence of the recombinant RNA molecule.
- Methods for non-destructive assays of seed or other plant tissuewhich can be adapted for such screens include but are not limited to those disclosed in US patent applications US20220221377 and US20210259176, both incorporated herein by reference in their entireties.
- the cargo RNA sequencecomprises RNA that encodes a selectable or scorable marker.
- the recombinant RNA moleculeencodes a selectable marker and the F 1 progeny plant or seed comprising the recombinant RNA molecule is isolated by selecting the F 1 progeny plant or seed comprising the recombinant RNA molecule for presence of the selectable marker.
- selectable markere.g., a protein which confers resistance to a selection agent such as an herbicide or antibiotic
- Examples of such selections in instances where the recombinant RNA encodes a selectable markercan comprise exposing the progeny seeds or plants to a selection agent (e.g., an herbicide or antibiotic) and isolating progeny seeds or plants which survive exposure to the selection agent.
- selectable marker/selection agent combinationsinclude glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzymes/glufosinate, and neomycin phosphotransferase (npt)/neomycin or kanamycin.
- the selectable or scorable markeris an RNA aptamer or a regulatory RNA.
- the F 1 progeny plant or seedlacks DNA that encodes the recombinant RNA molecule.
- the parent plantlacks DNA that encodes the recombinant RNA molecule.
- the selected F 1 progeny planttransmits the recombinant RNA molecule to at least F 2 progeny.
- the F 1 progeny plant or seed populationis obtained from a parent plant used as a pollen recipient.
- the F 1 progeny plant or seed populationis obtained from a parent plant used as a pollen donor.
- the F 1 progeny plant or seed populationis obtained by selfing the parent plant.
- the F 1 progeny plant or seed populationis obtained from the sexual crossing of two parent plants.
- the parent plant that comprises the recombinant RNA moleculeis the female parent plant. In other embodiments, the parent plant that comprises the recombinant RNA molecule is the male parent plant, and the recombinant RNA molecule is transmitted in pollen of the male parent plant.
- the methodscan further comprise introducing the recombinant RNA molecule or a polynucleotide encoding the recombinant RNA molecule into a plant cell and obtaining the parent plant comprising the recombinant RNA molecule from the plant cell.
- the recombinant RNA moleculefurther comprises at least one additional element selected from the group consisting of: (a) at least one RNA encoding a viral movement protein (MP); (b) at least one tRNA-like sequence; and c) an origin-of-assembly sequence (OAS).
- a parent and/or plantcomprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule.
- the parent and/or progeny plantcomprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein.
- this disclosureis related to a method of barcoding a plant, plant cell, progeny thereof, or part thereof.
- the methodscomprise providing to the plant or plant cell any of the aforementioned or otherwise disclosed recombinant RNA molecules provided herein, wherein the cargo RNA of the recombinant RNA molecule comprises a barcode RNA molecule, and wherein the plant or plant cell comprises a Solemoviridae RdRP.
- the barcode RNA moleculecomprises a sequence that uniquely identifies the plant, plant cell, progeny thereof, or part thereof.
- the barcode RNAcan be a randomly generated sequence.
- the barcode RNA moleculecomprises a sequence that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. In some embodiments, the barcode RNA molecule comprises a forward primer binding site and a reverse primer binding site for detection of the barcode RNA molecule. In embodiments, the barcode RNA molecule comprises a non- protein coding sequence. In some embodiments, the barcode RNA sequence is up to about 5 kb in length. In some embodiments, the barcode RNA has a length of 10 to 5000 nucleotides, 20 to 1000 nucleotides, or 50 to 500 nucleotides.
- the barcode RNA moleculehas a length of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, 800, 850, 900, 950, 1000, 1500, 2000, 2500, 3000, 3500, 4000, 4500, or 5000 nucleotides.
- the planttransmits the recombinant RNA molecule comprising the barcode RNA to progeny.
- the plant, plant cell, progeny thereof, or part thereoflacks DNA that encodes the recombinant RNA molecule.
- the methodscan further comprise isolating an F 1 progeny plant or seed comprising at least one cell comprising the Solemoviridae RdRP and the recombinant RNA molecule.
- the F 1 progeny plant or seedis obtained from the plant used as a pollen recipient.
- the F 1 progeny plant or seedis obtained from the plant used as a pollen donor.
- the F 1 progeny plant or seedis obtained by selfing the parent plant.
- the methodscan further comprise propagating the plant or plant cell to obtain a plant part or a plant propagule comprising the barcode RNA molecule.
- this disclosureis related to a method of identifying a barcoded plant, plant part, or plant cell.
- the methodscomprise screening for the presence of a barcode RNA molecule in the plant, plant part, or plant cell, wherein the plant, plant part, or plant cell comprises any of the Agent Ref: P14419WO00 - 53 - aforementioned or otherwise disclosed recombinant RNA molecules provided herein, and wherein the cargo RNA of the recombinant RNA molecule comprises the barcode RNA molecule.
- the methodscomprise obtaining a nucleic acid sample from the plant, plant part, or plant cell; and detecting the presence of the barcode RNA molecule in the sample.
- Assays for detection of a barcode RNAinclude RNA detection assays (e.g., an RT-PCR assay) using nucleic acid probes and/or primers which can detect the barcode RNA and/or sequencing of the barcode RNA.
- Such screening assayscan be non-destructive assays wherein a portion of the seed or plant is removed and assayed without loss of the viability or ability to propagate the seed or plant tissue comprising the barcode RNA.
- Methods for non-destructive assays of seed or other plant tissuewhich can be adapted for such screens include but are not limited to those disclosed in US patent applications US20220221377 and US20210259176, both incorporated herein by reference in their entireties.
- a seed of the plantis non-destructively screened for presence of the barcode RNA molecule.
- a seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callusis screened for the presence of the barcode RNA molecule.
- the methods disclosed hereinare not processes for modifying the germ line or genetic identity of human beings.
- the methods disclosed hereinare not processes for modifying the genetic identity of animals which are likely to cause them suffering without any substantial medical benefit to man or animal, and are also not drawn to animals resulting from such processes.
- the methods disclosed hereinare not methods for treatment of the human or animal body by surgery or therapy.
- the cells disclosed hereinare not human embryos.
- the cells disclosed hereinare not the human body or its parts, at the various stages of its formation and development.
- the plant cells, plant propagulese.g., a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus
- plants provided hereinare not produced by an exclusively biological process.
- the methods for producing plant cells, plant propagulese.g., a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus
- plants provided hereinare not exclusively biological processes.
- a recombinant RNA moleculecomprising from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; Agent Ref: P14419WO00 - 54 - wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked and wherein the cargo RNA molecule is heterologous to the 5’ RNA replication element and the 3’ RNA replication element, optionally wherein: (i) the 5’ RNA replication element and the 3’ RNA replication element are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; (ii) the 5’ RNA replication element, the 3’
- RNA replication elementcomprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; and/or (b) the 3’ RNA replication element comprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 468, 533, or 534.
- the 5’ RNA replication elementcomprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 467, 531, or 532
- the 3’ RNA replication elementcomprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 468, 533, or 534.
- the 5’ RNA replication elementcomprises an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 467, 531, or 532; or a variant thereof wherein one or more nucleotides in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure; and/or (b) the 3’ RNA replication element comprises an RNA molecule encoded by SEQ ID NO: 468, 533, or 534, or; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 468, 533, or 534; or a variant thereof wherein one of more base-paired residues in the
- RNA secondary structureis maintained by substituting nucleotides in the secondary structure which are not base paired with nucleotides which will not base pair, and/or by substituting nucleotides in the secondary structure which are base paired with nucleotides which will base pair. [0146] 5.
- RNA replication elementcomprises at least a segment of the 5’ untranslated region (UTR) of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 5’ Agent Ref: P14419WO00 - 55 - RNA replication element further comprises a genomic sequence of the virus of the family Solemoviridae that is natively located 3’ to and adjacent to the 5’ UTR sequence; and/or (b) the 3’ RNA replication element comprises at least a segment of the 3’ UTR of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 3’ RNA replication element further comprises a genomic sequence of the virus
- RNA moleculeof any one of embodiments 1 to 5, wherein the RNA molecule further comprises at least one of: (i) a tRNA-like element, optionally wherein the tRNA-like element provides for intercellular movement of the RNA and optionally wherein the intercellular movement is mediated by a viral movement protein (MP); (ii) an encapsidation recognition element (ERE), optionally wherein the ERE provides for encapsidation of the RNA by a Solemoviridae capsid protein or optionally wherein the ERE provides for encapsidation of the RNA by a non-Solemoviridae capsid protein; (iii) an RNA effecter; and/or (iv) RNA encoding a viral movement protein (MP), optionally wherein an internal ribosome entry site (IRES) is operably linked to the RNA encoding the MP.
- MPviral movement protein
- IVSinternal ribosome entry site
- RNA-like elementcomprises a tRNA-like molecule from an Arabidopsis FT mRNA or is a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 or is a modified tRNA-like sequence that has at least 90% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence, and/or wherein the ERE is a tobacco mosaic virus (TMV) OAS.
- TMVtobacco mosaic virus
- the recombinant RNA molecule of embodiment 1, wherein the cargo RNA moleculeis up to about 5kb in length.
- the cargo RNA moleculecomprises: (a) at least one coding sequence, optionally wherein the coding sequence encodes a selectable or scoreable marker; (b) at least one non-coding sequence; or (c) both at least one coding sequence and at least one non-coding sequence. [0151] 10.
- RNA molecule of embodiment 1wherein the cargo RNA molecule comprises at least one coding sequence, and wherein the RNA molecule further comprises an internal ribosome entry site (IRES) which is operably linked to at least one coding sequence, optionally wherein the operably linked IRES is located 5’ and immediately adjacent to the coding sequence.
- IRESinternal ribosome entry site
- hpRNAhairpin RNA
- RNAhairpin RNA
- RNAhairpin RNA
- RNARNA that forms multiple stem-loops
- an RNA pseudoknotan RNA molecule Agent Ref: P14419WO00 -
- RNA molecule of embodiment 1further comprising an RNA comprising encoding at least one ribozyme, optionally wherein the at least one ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element.
- RNA molecule of embodiment 1further comprising an RNA molecule comprising at least one ligand-responsive ribozyme (aptazyme), optionally wherein the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element.
- aptazymeoptionally wherein the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element.
- RNA binding proteinscomprise an RNA recognition motif.
- RNA moleculecomprises: at least one heterologous RNA virus (HRV) amplicon in sense or antisense orientation to the first 5’ RNA replication element comprising: I. (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked; or II.
- HRVheterologous RNA virus
- heterologous RNA virus (HRV) subgenomic promoteroperably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP).
- RNA moleculecomprises from 5’ terminus to 3’ terminus: (a) the 5’ RNA replication element; (b) the HRV amplicon in antisense orientation to the first 5’ RNA replication element; optionally wherein the HRV amplicon further comprises: (i) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to the HRV 5’RR and HRV 3’RR, wherein the RNA molecule encoding the HRV RNA- dependent RNA polymerase (hrvRdRP) is optionally operably linked to a subgenomic promoter recognized by the hrvRdRP; or Agent Ref: P14419WO00 - 57 - (ii) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to linked to a subgenomic promoter recognized by the hrvRdRP; or Agent Ref: P14419WO
- a virusselected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae.
- the Bromovirusis a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus
- the Closterovirusis a Citrus tristeza virus or Beet yellows virus
- the Comovirusis Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus
- the Potexvirusis Potato virus X or Citrus yellow vein clearing virus
- the Potyvirusis a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus
- the Tobamovirusis a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus
- the Tombusis a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing
- the HRV 5’ RRcomprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 161 to 185, or 186, or comprises the RNA sequence encoded by SEQ ID NO: 161 to 185, or 186, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 161 to 185, or 186; and/or (b) the HRV 3’ RR comprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 187 to 210, or 211, or comprises the RNA sequence of the RNA encoded by SEQ ID NO: 187 to 210, or 211, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 187 to 210, or 211.
- the HRV 3’ RRcomprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 3’ RR or the variant is recognized by the hrvRdRP, optionally wherein the RNA comprising the HRV 3’ RR further comprises a genomic sequence of the HRV that is natively located 5’ to and adjacent to the 3’ UTR sequence; and/or (b) the HRV 5’ RR comprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 5’ RR or the variant is recognized by the hrvRdRP, optionally wherein the RNA comprising the HRV 3’ RR or the variant is recognized by the hr
- the cargo RNAcomprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV.
- RNAcomprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV which does not encode the hrvRdRP.
- RNA encoded by the Cucumber Mosaic Virus and the HRV 5’RRcomprises an RNA encoded by the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7,
- the HRV 3’ RRcomprises an RNA encoded by the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7;
- the HRVis a Brome mosaic virus and the HRV 5’RR comprises an RNA encoded by the Brome mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded
- the cleavable sequenceis optionally a self-cleaving ribozyme, a self-cleaving inducible ribozyme, or an siRNA or miRNA recognition site.
- 35The cell of embodiment 34, wherein the cell is a plant cell and DNA which encodes the recombinant RNA molecule is absent from the cell.
- RNA moleculecomprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein, and wherein the cell contains the viral capsid protein.
- EEEoperably linked encapsidation recognition element
- the encapsidation recognition element (ERE)is a Solemoviridae ERE, wherein the viral capsid protein in the cell is a Solemoviridae capsid protein, and wherein the RNA molecule is encapsidated by the Solemoviridae capsid protein.
- invention 37, 38, or 39further comprising the reverse complement of the recombinant RNA molecule.
- 41The expression system of any one of embodiments 37 to 40, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell.
- 42The expression system of any one of embodiments 37 to 40, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell.
- the cellfurther comprises: (i) a viral capsid protein (CP), (ii) an RNA-binding protein (RBP) that can bind to the RNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; (iv) a second RNA-dependent RNA polymerase (RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the RNA molecule (2 nd RdRP); (v) a viral movement protein (MP); (v) a heterologous RNA virus (HRV); or (vi) an hrvRdRP, optionally wherein the hrvRdRP recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter.
- CPviral capsid protein
- RBPRNA-binding protein
- RBPRNA-binding protein
- the expression system of embodiment 42 or 44wherein the RdRP, 2 nd RdRP, or hrvRdRP protein or a polynucleotide encoding the RdRP, 2 nd RdRP, or hrvRdRP protein is (a) expressed by a recombinant DNA molecule in the cell; (b) provided exogenously to the cell; (c) expressed by a recombinant RNA molecule in the cell; or (d) expressed by a virus in the cell. [0187] 46. The expression system of any one of embodiments 37 to 45, wherein the cell is a plant cell. [0188] 47.
- the expression system of embodiment 46wherein the plant cell contains a virus of the family Solemoviridae which expresses the RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element and/or wherein the plant cell contains an HRV that expresses the 2 nd RdRP or hrvRdRP protein.
- 48The expression system of embodiment 47, wherein the virus of the family Solemoviridae occurs naturally in the plant cell.
- a method of providing a synthetic Solemoviridae satellite RNA to a plantcomprising contacting the plant with the recombinant RNA molecule of any one of embodiments 1 to 28.
- contactingcomprises spraying, dusting, injecting, or soaking the plant or a part thereof with the recombinant RNA molecule or the formulation.
- 51The method of embodiment 49 or 50, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof.
- a method of establishing a synthetic Solemoviridae satellite RNA in a plant cellcomprising: providing to a plant cell the recombinant RNA molecule of any one of embodiments 1 to 28; wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element, wherein the RNA molecule is optionally comprises an ERE and is encapsidated by a capsid protein, whereby the RdRP protein catalyzes synthesis of the synthetic Solemoviridae satellite RNA from the recombinant RNA molecule. [0194] 53.
- the method of embodiment 52wherein the plant cell comprises a virus of the family Solemoviridae and wherein the RdRP protein is provided to the plant cell by the virus of the family Solemoviridae.
- 54The method of embodiment 52 or 53, wherein the virus of the family Solemoviridae is endemic to the plant cell, optionally wherein the virus of the family Solemoviridae which is endemic to the plant cell is non-pathogenic and/or commensal.
- 5555.
- the capsid proteincomprises a Solemoviridae capsid protein and the ERE is recognized by the Solemoviridae capsid protein, optionally wherein the recombinant RNA molecule comprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein.
- EREoperably linked encapsidation recognition element
- any one of embodiments 52 to 55further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0198] 57.
- a method of obtaining a phenotypic change in a plant or plant cellcomprising: providing to a plant or plant cell a recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA molecule comprises RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element and catalyzes synthesis of a synthetic Solemoviridae RNA from the recombinant RNA molecule, and wherein the cargo RNA molecule effects the phenotypic change.
- RNA that effects a phenotypic change in the plant or plant cellcomprises at least one RNA selected from an siRNA or siRNA precursor, a miRNA or miRNA precursor, and a phased siRNA or phased siRNA precursor.
- RNA that effects a phenotypic change in the plant or plant cellcomprises a messenger RNA.
- the messenger RNAcomprises an RNA molecule absent in the genome of the plant or plant cell.
- RNA that effects a phenotypic change in the plant or plant cellcomprises an RNA for modifying the genome of the plant or plant cell.
- RNA that effects a phenotypic change in the plant or plant cellcomprises an RNA for modifying the transcriptome and/or epigenome of the plant or plant cell, optionally wherein the RNA for modifying the epigenome targets an endogenous plant gene for RNA-induced transcriptional silencing.
- RNA for modifying the epigenometargets an endogenous plant gene for RNA-induced transcriptional silencing.
- phenotypic changecomprises an increase in the plant’s resistance to a pest or pathogen, optionally wherein the pest or pathogen is selected from the group comprising a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate.
- the pest or pathogenis selected from the group comprising a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate.
- the pathogenis a heterologous RNA virus (HRV), optionally wherein the HRV is a virus selected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae.
- HRVheterologous RNA virus
- the Bromovirusis a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus;
- the Closterovirusis a Citrus tristeza virus or Beet yellow virus;
- the Comovirusis Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus;
- the Potexvirusis Potato virus X or Citrus yellow vein clearing virus;
- the Potyvirusis a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus;
- the Tobamovirusis a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus;
- the Tombusvirusis a Turni
- 66The method of any one of embodiments 57 to 62, wherein the phenotypic change comprises an increase in the plant’s resistance to stress, optionally wherein the stress comprises at least one abiotic stress comprising nutrient stress, light stress, water stress, heat stress, and/or cold stress, or Agent Ref: P14419WO00 - 70 - optionally wherein the stress comprises at least one biotic stress comprising crowding, shading, or allelopathy.
- 67The method of any one of embodiments 57 to 66, wherein the recombinant RNA molecule is provided to the plant of plant cell in the form of an RNA, an encapsidated RNA, or a formulation thereof.
- 68The method of any one of embodiments 57 to 62, wherein the phenotypic change comprises an increase in the plant’s resistance to stress, optionally wherein the stress comprises at least one abiotic stress comprising nutrient stress, light stress, water stress, heat stress, and/or cold stress, or Agent Ref
- RNAcomprises a synthetic Solemoviridae satellite particle.
- the providingcomprises contacting the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof, optionally wherein contacting comprises spraying, dusting, injecting, or soaking the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof.
- the recombinant RNAfurther comprises its reverse complementary RNA molecule.
- any one of embodiments 57 to 70further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0213] 72.
- a method of manufacturing a synthetic Solemoviridae satellite particlecomprising combining the recombinant RNA molecule of any one of embodiments 1 to 28 with a viral capsid protein, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein.
- EREencapsidation recognition element
- the combiningcomprises (a) providing to a plant cell the recombinant RNA molecule, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule and a viral capsid protein, wherein the ERE provides for encapsidation of the RNA by the viral capsid protein; and optionally (b) isolating the synthetic Solemoviridae satellite particle from the plant cell, a plant comprising the plant cell, or from media in which the plant cell or the plant had been grown.
- EREencapsidation recognition element
- a plant propagulecomprising the recombinant RNA molecule of any one of embodiments 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a Agent Ref: P14419WO00 - 71 - heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- the plant propagule of embodiment 76 or 77wherein the plant propagule is a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule.
- 79.The plant propagule of embodiment 76, 77, or 78, wherein the plant propagule lacks DNA encoding the recombinant RNA molecule.
- a plantcomprising the recombinant RNA molecule of any one of embodiments 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- 82.The plant of embodiment 81, wherein the plant is a monocot or a dicot plant.
- 83The plant of embodiment 81, wherein the plant is of the family Asteraceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Poaceae, or Solanaceae.
- 85.The plant of any one of embodiments 81 to 84, wherein the plant comprises a virus of the family Solemoviridae, and wherein the Solemoviridae RdRP is provided to the plant cell by the virus of the family Solemoviridae, optionally wherein the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. [0227] 86.
- the plant of any one of embodiments 81 to 86, wherein the Solemoviridae RdRP, the 5’ RNA replication element, and/or the 3’ RNA replication elementare derived from a virus of the family Solemoviridae comprising one or both of the Solemoviridae RdRP, 5’ RNA replication element, and/or 3’ RNA replication elements.
- the Solemoviridae RdRP, the 5’ RNA replication element, and/or the 3’ RNA replication elementare derived from a virus of the family Solemoviridae comprising one or both of the Solemoviridae RdRP, 5’ RNA replication element, and/or 3’ RNA replication elements.
- RNA replication Agent Ref: P14419WO00 - 72 - elementhas at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 467, 531, or 532, and/or wherein the 3’ RNA replication element has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 468, 533, or 534.
- RNA virusRdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA
- the heterologous RNA virus RdRPis an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. [0233] 92.
- a Solemoviridae satellite systemthat is self-replicating when introduced into a plant or plant cell, comprising: (a) a recombinant Solemoviridae satellite RNA of any one of embodiments 1 to 28; and (b) an exogenous virus of the family Solemoviridae that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA, optionally wherein the Solemoviridae satellite system further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA.
- the Solemoviridae satellite systemfurther comprises a heterologous RNA virus (HRV) RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA
- the heterologous RNA virus RdRPis optionally an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7.
- a recombinant DNA moleculecomprising a first promoter which is operably linked to DNA encoding the RNA molecule of any one of embodiments 1-28.
- 96.A cell comprising the recombinant DNA molecule of embodiment 95, wherein the cell is a bacterial cell, a fungal cell, a plant cell, an insect cell, or an invertebrate animal cell.
- 97.A vector for bacterially mediated plant transformation, comprising the recombinant DNA molecule of embodiment 95. Agent Ref: P14419WO00 - 73 - [0239] 98.
- the vector of embodiment 97wherein the bacterium that mediates the plant transformation is an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., a Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. and the vector is adapted for transformation with the bacterium.
- the vector of embodiment 97 or 98, wherein the bacterium that mediates the plant transformationis an Agrobacterium sp., and wherein the vector further comprises T-DNAs flanking the DNA molecule encoding the recombinant RNA molecule. [0241] 100.
- An expression systemcomprising: (a) the recombinant DNA molecule of embodiment 95; and(b) a cell containing the recombinant DNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements encoded by the DNA molecule.
- the recombinant DNA moleculefurther comprises at least one additional element comprising: (i) DNA encoding at least one RNA encoding a viral movement protein (MP); (ii) DNA encoding at least one tRNA-like molecule; (iii) DNA encoding an encapsidation recognition element (ERE); (iv) DNA encoding an RNA comprising, from 5’ to 3’ and operably linked, a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP), the RNA of (i) and optionally an operably linked RNA of (ii) and/or (iii), and a 3’ RNA replication element; (v) DNA encoding an RNA promoter; (vi) DNA encoding an RNA- dependent RNA polymerase (RdRP) that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter; and/or (vii
- 103The expression system of embodiments 101 or 102, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell, optionally wherein the bacterial cell is an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell. [0245] 104.
- RNA-binding proteinthat can bind to the RNA molecule encoded by the DNA molecule, optionally wherein the RBP binds to an RNA effecter;
- RBPRNA-binding protein
- an RNA cleavage agentthat cleaves the RNA molecule; and/or
- an RNA promoter dependent RNA polymerase (RdRP) proteinthat recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter recognizes an RNA promoter in the RNA molecule.
- invention 104 or 105wherein: (i) the capsid protein, viral movement protein (MP), RdRP protein, and/or the RdRP protein that recognizes an HRV 5’ or 3’ Agent Ref: P14419WO00 - 74 - replication region and/or a subgenomic promoter is heterologous to the cell and/or (ii) wherein the RdRP protein or a polynucleotide encoding the RdRP protein is provided exogenously to the cell. [0248] 107.
- 108The expression system of any one of embodiments 101 to 106, wherein the cell is a plant cell.
- the expression system of 107wherein the plant cell contains a virus of the family Solemoviridae which expresses the RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element.
- 109The expression system of embodiment 108, wherein the virus of the family Solemoviridae occurs naturally in the plant cell.
- 110The expression system of any one of embodiments 101 to 109, wherein the recombinant DNA molecule further comprises at least one RNA encoding a viral MP, a tRNA-like molecule from an Arabidopsis FT mRNA, and an encapsidation recognition element comprising a TMV-OAS.
- 111The expression system of any one of embodiments 101 to 109, wherein the recombinant DNA molecule further comprises at least one RNA encoding a viral MP, a tRNA-like molecule from an Arabidopsis FT mRNA, and an encapsidation recognition element comprising a T
- An agricultural formulationcomprising the expression system of any one of embodiments 101 to 110. [0253] 112. The agricultural formulation of embodiment 111, wherein the formulation comprises the expression system and a carrier, an excipient and/or an adjuvant. [0254] 113. An agricultural formulation comprising the recombinant DNA molecule of embodiment 95. [0255] 114. The agricultural formulation of embodiment 113, wherein the formulation comprises the recombinant DNA molecule and a carrier, an excipient and/or an adjuvant. [0256] 115.
- a method of producing an exogenous polypeptide in a plant or plant cellcomprising: providing a plant or plant cell comprising the recombinant RNA molecule of any one of embodiments 1 to 28 or the recombinant DNA molecule of embodiment 95, wherein the cargo RNA molecule encoded by the RNA or DNA molecule comprises a translatable messenger RNA encoding the exogenous polypeptide, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element of the recombinant RNA and that catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule, and wherein the exogenous polypeptide is translated from the translatable messenger; optionally wherein the plant or plant cell further comprises a heterologous RNA virus (HRV) RNA promoter dependent RNA polymerase (hrvRdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or
- 121The method of any one of embodiments 115 to 120, further comprising the step of determining if the plant cell comprises a virus of the family Solemoviridae which can provide the RdRP.
- 122The method of any one of embodiments 115 to 120, further comprising the step of determining if the plant cell comprises a virus of the family Solemoviridae which can provide the RdRP.
- RdRPSolemoviridae RNA- dependent RNA polymerase
- the method of embodiment 122 or 123, wherein the mixed population of plant cellscomprise plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP.
- 125The method of embodiment 122, 123, or 124, wherein the mixed population of plant cells comprise plant cells comprising the Solemoviridae RdRP.
- 126The method of any one of embodiments 122 to 125, wherein the mixed population of plant cells is screened or selected for the presence of the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule.
- 127The method of any one of embodiments 122 to 125, wherein the mixed population of plant cells is screened or selected for the presence of the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule.
- the method of any one of embodiments 122 to 126, wherein the isolationcomprises selecting for the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule.
- 128The method of any one of embodiments 122 to 127, wherein the mixed population is located within a plant or a plant part.
- 129The method of any one of embodiments 122 to 128, wherein the plant or plant part is screened or selected for presence of the recombinant RNA molecule prior to isolating the plant propagule.
- Agent RefP14419WO00 - 76 - [0271] 130.
- any one of embodiments 122 to 132wherein the plant propagule comprising the recombinant RNA molecule is isolated by detecting the RNA molecule in one or more plant cells comprising the recombinant RNA molecule and separating the one or more plant cells comprising the recombinant RNA molecule from the plant cells lacking the recombinant DNA molecule.
- the plant propaguleis a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule.
- any one of embodiments 122 to 140wherein the isolated propagule comprises callus and the method further comprises regenerating a plant, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, or explant comprising the recombinant RNA from said callus.
- Agent RefP14419WO00 - 77 - [0284] 143.
- a method of providing a synthetic Solemoviridae satellite RNA to a plantcomprising: grafting a scion onto a rootstock comprising recombinant RNA molecule of any one of embodiments 1 to 28, wherein at least one cell of the rootstock and/or the scion comprises the Solemoviridae RdRP.
- a sciononto a rootstock comprising recombinant RNA molecule of any one of embodiments 1 to 28, wherein at least one cell of the rootstock and/or the scion comprises the Solemoviridae RdRP.
- a method for producing a plant that transmits a recombinant RNA molecule to progeny plants or seedcomprising isolating an F 1 progeny plant or seed comprising at least one cell comprising a Solemoviridae RNA-dependent RNA polymerase (RdRP) and the recombinant RNA molecule of any one of embodiments 1 to 28 from a population of F 1 plants or seed obtained from a parent plant comprising the recombinant RNA molecule.
- RdRPSolemoviridae RNA-dependent RNA polymerase
- the method of embodiment 151, wherein the F 1 progeny plant or seed comprising the cellis isolated by screening the population of F 1 plants or seed obtained from a parent plant for the presence of the recombinant RNA molecule and propagating the F 1 progeny plant or seed comprising the recombinant RNA molecule.
- the recombinant RNA moleculeencodes a selectable marker and the F 1 progeny plant or seed comprising the recombinant RNA molecule is isolated by selecting the F 1 progeny plant or seed comprising the recombinant RNA molecule for presence of the selectable marker.
- any one of embodiments 151 to 160wherein the parent plant or one or more parts thereof are screened for systemic presence of the recombinant RNA molecule prior to isolating the F 1 progeny plants, optionally wherein the part comprises floral tissue or male or female reproductive tissue.
- the partcomprises floral tissue or male or female reproductive tissue.
- 162The method of any one of embodiments 151 to 161, wherein the pericarp of the parent plant is screened or selected for presence of the recombinant RNA molecule.
- F 1 seedsare obtained from a parent plant selected for presence of the recombinant RNA molecule in pericarp tissue.
- any one of embodiments 151 to 165further comprising introducing the recombinant RNA molecule or a polynucleotide encoding the recombinant RNA molecule into a plant cell and obtaining the parent plant comprising the recombinant RNA molecule from the plant cell.
- 167The method of any one of embodiments 151 to 166, wherein the propagule, plant, plant part, scion, and/or rootstock comprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule and/or comprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein.
- the propagule, plant, plant part, scion, and/or rootstockcomprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule and/or comprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein.
- a method of barcoding a plant, plant cell, progeny thereof, or part thereofcomprising providing to the plant or plant cell the recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA of the recombinant RNA molecule comprises a barcode RNA molecule, and wherein the plant or plant cell comprises a Solemoviridae RNA-dependent RNA polymerase (RdRP).
- the method of embodiment 168, wherein the barcode RNA moleculecomprises a sequence that uniquely identifies the plant, plant cell, progeny thereof, or part thereof. [0311] 170.
- the barcode RNA moleculecomprises a sequence that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. [0312] 171. The method of any one of embodiments 168 to 170, wherein the barcode RNA molecule comprises a random sequence. [0313] 172. The method of any one of embodiments 168 to 171, wherein the barcode RNA molecule comprises a forward primer binding site and a reverse primer binding site that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. [0314] 173.
- any one of embodiments 168 to 172, wherein the barcode RNA moleculeis up to about 5kb in length. [0315] 174.
- any one of embodiments 168 to 176further comprising isolating an F 1 progeny plant or seed comprising at least one cell comprising the Solemoviridae RdRP and the recombinant RNA molecule.
- 179The method of embodiment 178, wherein the F 1 progeny plant or seed is obtained from the plant used as a pollen recipient.
- 180The method of embodiment 178, wherein the F 1 progeny plant or seed is obtained from the plant used as a pollen donor.
- 181. The method of embodiment 178, wherein the F 1 progeny plant or seedis obtained by selfing the plant. [0323] 182.
- a method of identifying a barcoded plant, plant part, or plant cellcomprising screening for the presence of a barcode RNA molecule in the plant, plant part, or plant cell, wherein the plant, plant part, or plant cell comprises the recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA of the recombinant RNA molecule comprises the barcode RNA molecule.
- RNA equivalents of the DNA sequencesare also contemplated and can be obtained from the DNA sequences provided. Table 2.
- Viral capsid protein and origin of assembly sequencesV irus 1 Origin of assembly s equence2 Capsid Protein
- Agent RefP14419WO00 - 81 - V irus 1 Origin of assembly s equence2 Capsid Protein NC0096421Bll l b i l database accession number for entries in the world wide web internet database “ncbi[dot]nlm[dot]nih.gov/nuccore.”
- Agent RefP14419WO00 - 82 - 2 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. Table 3. Movement proteins.
- V irus Movement protein( MP) Agent Ref: P14419WO00 - 83 - V irus Movement protein ( MP) “ncbi[dot]nlm[dot]nih.gov/nuccore.” Table 4.
- tRNA-like sequencesName 1 of Arabidopsis gene containing tRNA-like s equence that can confer cell-to-cell mobility Sequence2 Agent Ref: P14419WO00 - 84 - Name 1 of Arabidopsis gene containing tRNA-like Se 2 s equence that can confer cell-to-cell mobility quence esource (TAIR) database accession number for entries in the world wide web internet database “arabidopsis.org.” Table 5.
- TAIRcell-to-cell mobility quence esource
- Intron sequencesIntron number Sequence Agent Ref: P14419WO00 - 86 - Intron number Sequence 12 SEQ ID NO: 158 d target viral pathogens RDRP or RDRP or V irus name NCBI 5’ RR 3’ RR Replicase Replicase A i ID1 S 2 S 2 NT AA Agent Ref: P14419WO00 - 87 - RDRP or RDRP or V irus name NCBI 5’ RR 3’ RR Replicase Replicase A ccession ID1 Sequence 2 Sequence 2 NT AA Agent Ref: P14419WO00 - 88 - RDRP or RDRP or V irus name NCBI 5’ RR 3’ RR Replicase Replicase A ccession ID1 Sequence 2 Sequence 2 NT AA ase “ncbi[dot]nlm[dot]nih.gov/nuccore.” 2 RNA equivalents of the DNA sequences are also contemplated and can
- Antifungal and antibacterial cargo protein Cargo NCBI Accession NT AA
- I D1 Sequence Sequence Target pathogen target cropAgent Ref: P14419WO00 - 96 - C argo NCBI Accession NT AA Targe
- I D1 Sequence Sequence t pathogen target cropR. solani o, o, s s s o o o, o, Agent Ref: P14419WO00 - 97 - C argo NCBI Accession NT AA
- I D1 Sequence Sequence Target pathogen target cropS EQ ID SEQ ID P. syringae Citrus tomato, o, o, o, o, o, er Table 12.
- Agent RefP14419WO00 - 101 - NCBI or TAIR Gene Source Effector Accession NT AA accession number for entries in the world wide web internet database “arabidopsis.org.” The other descriptors refer to the National Center for Biotechnology Information database accession number for entries in the world wide web internet database “ncbi[dot]nlm[dot]nih.gov/nuccore.” Table 13 Bioactive Plant peptides Peptide name Sequence Function Agent Ref: P14419WO00 - 102 - Peptide name Sequence Function SEQ ID NO: miPEP171d 409 Increase adventitious root formation in grapevine Ribozymes Ribozyme NCBI Accession ID Rfam ID Sequence 1 KV767472.1 Agent Ref: P14419WO00 - 103 - Ribozyme NCBI Accession ID Rfam ID Sequence 1 Group II self- ASAF01074251.1 splicing intron (11469-11396 RF00029 SEQ
- Subgenomic promotersSubgenomic promoter Sequence 1 Pea earl -brownin virus CP SEQ ID NO: 453 Agent Ref: P14419WO00 - 104 - 1 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. *See Goulden et al. (1990) Nucleic Acids Res., 18:4507 – 4512, DOI:10.1093/NAR/18.15.4507. Table 17.
- a Solemoviridae satellite (COMSAT) that carries an antiviral inhibitory RNA (RNAi) cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (iii) an antiviral RNA molecule which induces an RNAi response; (iv) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); and (v) and the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking a TMV amplicon containing a cargo RNA containing a Pepper mild mottle virus (PMMoV) RNAi inducing Agent Ref: P14419WO00 - 105 - sequence (e.g., a PMMoV sequence which can form a dsRNA) is provided as SEQ ID NO: 473.
- the elements of the satelliteare set forth in Table 19. Table 19.
- a Solemoviridae satellite (COMSAT) with an imbedded HRV amplicon that carries an antiviral protein cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (iii) an antiviral protein cargo; (iv) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); and (v) the 3’ RNA replication element from a virus of the family Solemoviridae.
- COMSATSolemoviridae satellite
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking a tobacco mosaic virus (TMV) amplicon containing an antiviral cargo protein containing the coding sequence of the N gene of tobacco is provided as SEQ ID NO: 474.
- TMVtobacco mosaic virus
- This satelliteincludes TMV 5’ and 3’ replication region sequences (HRV 5’ and 3’ RR sequences) to promote secondary amplification by TMV, which is a pathogenic tobamovirus.
- the elements of this satelliteare set forth in Table 20.
- Similar antiviral Solemoviridae satellitesare designed for secondary amplification by tomato mosaic virus or tomato brown rugose fruit virus (which are also pathogenic tobamoviruses), and include an N protein (SEQ ID NO:254), L4 protein (SEQ ID NO:256), or a combination of N and L4 protein as the antiviral cargo sequence(s), and replacing the HRV 5’ and 3’ RR sequences of TMV with the HRV 5’ and 3’ RR sequences of either tomato mosaic virus or tomato brown rugose fruit virus as provided in Table 7, to promote secondary amplification by tomato mosaic virus or tomato brown rugose fruit virus, respectively.
- N proteinSEQ ID NO:254
- L4 proteinSEQ ID NO:256
- a combination of N and L4 proteinas the antiviral cargo sequence(s)
- replacing the HRV 5’ and 3’ RR sequences of TMVwith the HRV 5’ and 3’ RR sequences of either tomato mosaic virus or tomato brown rugose fruit virus as provided in
- Solanaceous plants containing the virus of the family Solemoviridae (or that otherwise are provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression) and that are provided with these antiviral Solemoviridae satellitesare expected to exhibit resistance to tobacco mosaic virus, tomato mosaic virus, or tomato brown rugose fruit virus, respectively, [0329] Table 20.
- a Solemoviridae satellite (COMSAT) that carries an antiviral RNAi cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antiviral RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- COMSATSolemoviridae satellite
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing a Pepper mild mottle virus (PMMoV) RNAi inducing sequence (e.g., a PMMoV sequence which can form a dsRNA) is provided as SEQ ID NO: 475.
- PMMoVPepper mild mottle virus
- SEQ ID NO: 475The elements of the satellite are set forth in Table 21. Table 21. Genetic element Nucleotide position in SEQ ID NO: 475 ’ Example 4.
- a Solemoviridae satellite (COMSAT) that carries an antiviral protein cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antiviral protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking an antiviral protein cargo containing the coding sequence of the N gene of tobacco is provided as SEQ ID NO: 476.
- RNA replication element 1-254 antiviral activityRNA replication element 1-254 antiviral activity
- Antiviral COMSATse.g., of Examples 1 to 4
- control RNAse.g., RNAs lacking the antiviral cargo
- target host plantssuch as pepper or tomato plants via micro- bombardment (“biolistic” delivery) using a “gene gun” or by bacterially mediated (e.g., by Agrobacterium) transient expression.
- Host plants lacking the appropriate endogenous Solemoviridae viruscan otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression.
- Plants, e.g., pepper and tomato plantsare grown in growth chambers using a long- day photoperiod (16 h light/8 h dark) with light intensity 100-150 micromole, until seedlings are about 2 weeks old.
- Antiviral COMSATsare prepared either as in vitro transcribed (IVT) products (capped or uncapped), or as Agrobacterium binary vectors.
- the IVT productis coated onto the surface of gold nanoparticles which are precipitated on inner surface of bullet tubes; these are accelerated at the abaxial surface of the seedlings’ leaves with helium pressure from 100 to 180 psi.
- COMSAT-carrying clones in binary vectorare transformed into Agrobacterium GV 2260, and the transformed Agrobacterium is selected by growing at 28°C under appropriate antibiotic selection. The positive transformants are verified by PCR and then grown at 28°C overnight in liquid LB medium with appropriate antibiotics plus rifamycin to maintain the Agrobacterium and prevent contamination.
- Agrobacterium cellsare collected by centrifugation and resuspended in MMA buffer at an OD600 of 0.2, incubated at 28°C on a shaker for 2 hours, and then infiltrated into the abaxial sides of the leaves.
- Control plantsare treated in the same way but with non-COMSAT control IVT transcripts, or empty buffers.
- tissues from systemic leaves(leaves distal to the treated leaves) are collected and subjected to RNA extraction, followed by cDNA syntheses.
- COMSAT titersare monitored in systemic tissue by qRT-PCR with COMSAT-specific primers.
- agent RefP14419WO00 - 108 - [0335]
- the efficacy of an antiviral COMSATcan be tested by challenging the COMSAT-treated plants with the viral pathogen of interest (e.g., cucumber mosaic virus, CMV, or tobacco mosaic virus, TMV), for example, by mechanical infection.
- the viral pathogen of intereste.g., cucumber mosaic virus, CMV, or tobacco mosaic virus, TMV
- Infectious preparations of such acute viral pathogensare prepared in planta (e.g., in Nicotiana benthamiana) or in the form of GFP-fused infectious clones in a binary vector or T7-based vector.
- Suitable viral inoculumscan be prepared as infectious sap extracted from an infected plant, as an Agrobacterium-based inoculum, or as IVT products.
- the inoculumis introduced into leaves of the COMSAT-treated plants by rub-inoculation (for infectious sap or IVT product), by agroinfiltration (for GFP-fused infectious clone in binary vector), or by micro-bombardment (for IVT product or plasmid of infectious clone in binary vector).
- the infectious sap or IVT productsare respectively diluted 3 times or to 50ng/microliter in phosphate buffer 0.05M, pH 7.4 and dropped onto leaf adaxial surfaces which is pre-dusted with an abrasive such as carborundum or bentonite.
- the inoculumis gently spread on the abrasive-dusted leaf surface by gloved fingers or cotton buds; after 30 seconds the inoculated leaf is washed with water.
- Micro-bombardment of infectious clonesis conducted following previously described methods (see, e.g., delivery techniques described in PCT/US22/78963; see also Bio-Rad Tech Note 2531 “Inoculation of Viral RNA and cDNA to Potato and Tobacco Plants Using the HeliosTM Gene Gun”).
- Infiltration with an Agrobacterium-based viral pathogen inoculumis performed using methods similar to that used for COMSAT infiltration, but the Agrobacterium suspension is diluted at OD600 of 0.1.
- Symptoms and titre of the acute viral pathogensare monitored in the plants, typically over time to confirm progress or decline of viral infection. Effectiveness of antiviral COMSATs is evaluated by comparing relative titres of systemic infected viruses in antiviral COMSAT-treated plants and the control plants. Tissues from inoculated leaves and systemic leaves (distal to the inoculated leaves) are collected, total RNA is extracted, optionally followed by cDNA synthesis. The viral titre can be quantitatively measured by qRT-PCR with virus-specific primers.
- Viral titre or presencecan be measured by immunoassay methods; for example, Tobacco mosaic virus (TMV) is routinely detected in plants using a commercially available strip assay (Agdia ImmunoStrip ® for TMV Agdia, Inc., Elkhart, IN, USA).
- TMVTobacco mosaic virus
- strip assayAgdia ImmunoStrip ® for TMV Agdia, Inc., Elkhart, IN, USA.
- viral titreis qualitatively evaluated by viral disease symptoms, or by proxy measurement (e.g., measuring GFP expressed by GFP-fused viral constructs).
- qRT-PCR measurementsare normalized to endogenous reference gene controls (e.g., actin, tubulin, ubiquitin-3, GADPH, or translation elongation factor EF1a, used individually or preferably in multiples, e.g., at least 3 reference genes or at least three deltaCt values).
- endogenous reference gene controlse.g., actin, tubulin, ubiquitin-3, GADPH, or translation elongation factor EF1a, used individually or preferably in multiples, e.g., at least 3 reference genes or at least three deltaCt values.
- the viral titreis compared to that of controls.
- a Solemoviridae satellite (COMSAT) that carries an insecticidal RNAi cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an insecticidal RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing an RNAi inducing sequence that targets Colorado potato beetle is provided as SEQ ID NO: 477.
- the elements of the satelliteare set forth in Table 23. Table 23.
- RNA replication element 1-254provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an insecticidal protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking an insecticidal cargo protein containing the coding sequence of the Vip3Aa gene is provided as SEQ ID NO: 478.
- the elements of the satelliteare set forth in Table 24. Table 24.
- COMSATS of Table 23 and Table 24are established in host plants essentially as set forth in Example 5. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression.
- the efficacy of an insecticidal or insect inhibitory COMSATcan be tested by challenging the COMSAT and control treated plants with the insect of interest (e.g., Colorado Potato Beetle for plants treated with the Agent Ref: P14419WO00 - 110 - Table 23 COMSAT or Fall Armyworm, Corn Earworm, or Black Cutworm for plants treated with the Table 24 COMSAT).
- insect of intereste.g., Colorado Potato Beetle for plants treated with the Agent Ref: P14419WO00 - 110 - Table 23 COMSAT or Fall Armyworm, Corn Earworm, or Black Cutworm for plants treated with the Table 24 COMSAT.
- a Solemoviridae satellite (COMSAT) that carries an antifungal RNAi cargois generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antifungal RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing an RNAi inducing sequence that targets a DCL gene of Botrytis cinerea is provided as SEQ ID NO: 479.
- the elements of the satelliteare set forth in Table 25. Table 25.
- RNA replication element 1-254o e ov ae sa e e a ca es a a u ga p oe ca go s ge e a y provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antifungal protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae.
- a COMSATcomprising Solemoviridae 5’ and 3’ RNA replication elements flanking an antifungal cargo protein containing the coding sequence of the CaAMP1 gene is provided as SEQ ID NO: 480.
- the elements of the satelliteare set forth in Table 26. Table 26. Genetic element Nucleotide position in SEQ ID NO: 480 Agent Ref: P14419WO00 - 111 - Solemoviridae 3’ RNA replication element 2048-2120 COMSAT components synthesized from a commercial vendor.
- the plasmidis used as a template for a PCR reaction to amplify the expression cassette with a T7 promoter.
- This PCR productis used as the template for an in vitro transcription reaction using a MEGAscriptTM T7 Transcription Kit from Thermo Fisher Scientific.
- the in vitro synthesized RNA or protein cargois then transformed into leaves of the target crop using gold nanoparticles from Bio-Rad according to the guidelines specified in the Helios gene gun manual or via Agrobacterium mediated transformation.
- Host plants lacking the appropriate endogenous Solemoviridae viruscan otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression.
- Replication and persistence of delivered RNA or expression of protein in local leavescan be determined by RT-PCR from local tissues at 2-weeks post transformation.
- RNA/proteinSystemic movement of RNA/protein is determined by qRT-PCR/ELISA in the sample collected from distal untreated leaf samples after 1-, 2-, and 3-months post transformation.
- An antifungal in vitro assayis performed according to Duanis-Assaf et. al. (Plant Biotechnol J.2022 Jan;20(1):226-237).
- An in vivo detached leaf assayis performed according to Li et. al. (Mol Plant Microbe Interact.2019 Dec;32(12):1649-1664).
- RNA quantityis measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific; 840274200) and the intactness is quality controlled through gel electrophoresis.
- the synthesized and quality- controlled RNA moleculeis precipitated onto gold nanoparticles and fired into 4-week-old Solanum lycopersicum ‘Early Girl’ leaves using a gene gun.
- RNAis extracted with the MagMax Plant RNA Isolation kit (Thermo Fisher Scientific; A33899) and quality controlled through Nanodrop spectrophotometer (Thermo Fisher Scientific; Agent Ref: P14419WO00 - 112 - 840274200) and RNA ScreenTape Analysis (Agilent; 5067-5576).
- RNAQuality controlled RNA is used for cDNA synthesis and qRT-PCR is used to amplify the Dicer-like 1 and Dicer-like 2 fragments and reference gene fragment RPL2.
- Delta-delta Ct analysisis applied to determine LogFold change of the Solemoviridae COMSAT in the tissues compared to non-Solemoviridae COMSAT containing Solanum lycopersicum ‘Early Girl’ plant tissues.
- Solemoviridae COMSAT with cDNA-based cargo expression amplificationA DNA molecule encoding an RNA molecule containing a 5' replicase recognition sequence from a virus of the family Solemoviridae; Cricket paralysis virus IRES; Avian leukosis virus reverse transcriptase; tRNA primer binding site (tRNA-trp); CaMV 35s promoter; a protein cargo (Superfolder GFP); ZmHSP101 IRES; Tobacco mosaic virus movement protein; and a 3' replicase recognition site from a virus of the family Solemoviridae is cloned into a pUC19 plasmid backbone and is transformed into E.
- RNA quantityis measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific; 840274200) and the intactness is quality controlled through gel electrophoresis.
- the synthesized and quality-controlled RNA moleculeis precipitated onto gold nanoparticles and fired into the 4-week-old Solanum lycopersicum ‘Early Girl’ leaves using a gene gun.
- Host plants lacking the appropriate endogenous Solemoviridae viruscan otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression.
- the following leaf tissue samplesare collected at 2-week and 4-week timepoints: fired leaf # 1; fired leaf # 2; nearest adjacent leaflet; opposite leaf, terminal leaflet; and apical leaf growth.
- Protein (GFP) expressionis quantified. Total protein is extracted from collected tissue samples using the Pierce Plant Total Protein Extraction Kit (Thermo Fisher Scientific; A44056) according to manufacturer specifications, and quality controlled by Nanodrop spectrophotometry paired with SDS-PAGE to verify extraction yield and integrity.
- GFP expressionis quantified relative to other protein expressing COMSAT designs as well as non-transfected tissue using the Abcam GFP ELISA Kit (ab171581) according to manufacturer specifications.
- Example 10. Detection of Satellite RNA Negative-Sense Strandwhere only the positive-sense strand of a satellite RNA is provided to a plant or plant cell (e.g., by delivery of the RNA itself or by delivery of DNA encoding the satellite RNA’s positive-sense strand), replication of the satellite RNA in a plant or plant cell can be verified by detection of the satellite RNA’s negative-sense strand, for example, by using a negative-sense-strand-specific Taqman® assay.
- a bell pepper endornavirus (BPEV) satellite of 2357 nucleotideswas constructed with the following elements in 5’ to 3’ orientation: (1) BPEV 5’ RNA replication element; (2) a first IRES sequence (PSVI IRES, with the addition of spacer nucleotides on the 5’ end); (3) RNA encoding a reporter protein (GFP); (4) a second IRES sequence (ZmHSP IRES); (5) a viral movement protein; (6) a zebrafish sequence (as a non-plant heterologous sequence for detection Agent Ref: P14419WO00 - 113 - purposes); (7) an isoleucine tRNA sequence; (8) BPEV 3’ RNA replication element.
- Similar satellitescan be designed and constructed using alternative genetic elements such as those provided elsewhere in this specification, such as pairs of 5’ and 3’ RNA replication elements from a virus of the family Solemoviridae, other pairs of HRV 5’ and 3’ replication region sequences, and/or other cargo sequences.
- pairs of 5’ and 3’ RNA replication elementsfrom a virus of the family Solemoviridae, other pairs of HRV 5’ and 3’ replication region sequences, and/or other cargo sequences.
- RNA extraction, library prep, and Illumina® sequencingusing a stranded kit (NEBNext® UltraTM II Directional RNA Library Prep Kit for Illumina®, catalogue numbers E7760S or E7760L, New England Biolabs, Ipswich, MA) with added DMSO treatment and plant rRNA depletion.
- Readswere mapped against the BPEV satellite RNA’s sequence and negative-sense reads were identified using the Integrative Genomics Viewer (IGV) browser (Robinson et al., Bioinformatics, 39(1) (2023), btac830). Negative-sense reads mapping to the BPEV satellite were present, confirming that replication of the BPEV satellite had occurred.
- IGVIntegrative Genomics Viewer
- BPEV negative-sense readswere also identified in BPEV infected plants that served as a positive control for the analysis.
- the above-described negative strand detection methodcan be adapted for use in the detection of negative strand RNA produced by Solemoviridae satellite RNAs disclosed herein. Example 11.
- RNA Negative-Sense Strand in a Tomato Plantwhere only the positive-sense strand of a satellite RNA is provided to a plant or plant cell (e.g., by delivery of the RNA itself or by delivery of DNA encoding the satellite RNA’s positive-sense strand), replication of the satellite RNA in a plant or plant cell can be verified by detection of the satellite RNA’s negative-sense strand, for example, by using a negative-sense-strand-specific Taqman® assay.
- Negative sense GFP produced by a satellite RNA containing a GFP protein encoding cargo sequencesis detected using Taqman® qPCR.
- the reference sequence that can be usedis an endogenous tomato gene, “Tom06” (NCBI Gene ID 101249087, ubiquitin-conjugating enzyme E2-17 kDa, from Solanum lycopersicum).
- Taqman® qPCR primers and probeswere ordered from Integrated DNA Technology, Coralville, IA.
- the negative sense GFP probeis labeled with FAM520 dye with ZEN/Iowa Black FQ quencher.
- the Tom06 probeis labeled with SUN5544 with ZEN/Iowa Black FQ quencher. Primers and probes are used at a 2:1 ratio.
- cDNAis synthesized via reverse transcription (30 minutes at 60 degrees C, then 10 minutes at 85 degrees C) using Maxima H Minus First Strand cDNA synthesis Kit (K1652, Thermo Fisher Scientific, Waltham MA) using the RT primers in Table 27. This was followed by treatment (30 minutes at 37 degrees C, then 1 minute at 80 degrees C) with thermolabile exonuclease I (E1050 New England Biolabs, Ipswich MA) to remove excess RT primers. The synthesized cDNA was cleaned with RNAClean XP beads (A63987 Beckman Coulter, Brea CA).
- Taqman® qPCRis carried out using Taqman® Advanced Fast MasterMix (444965, Thermo Fisher Scientific, Waltham MA) and the primer and probes in Table 28.
- Table 27Description of Reverse Transcriptase Primers Agent Ref: P14419WO00 - 114 - RT Primer Name RT Primer Sequence negative GFP reverse transcription primer ccactggattcagcgaagttATGCGTAAAGGCGAAGAG
- Agent RefP14419WO00 - 115 - Table 29. Description of qPCR primers and probes qPCR Primer/Probe Name qPCR Primer/Probe Sequence negative GFP forward primer CCACTGGATTCAGCGAAGT (SEQ ID NO: 630) k Q/ [0355] Although the foregoing disclosure has been described in some detail by way of illustration and example for purposes of clarity of understanding, the descriptions and examples should not be construed as limiting the scope of the disclosure. [0356] Other embodiments are within the claims.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Virology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Agent Ref: P14419WO00 - 1 - ARTIFICIAL SOLEMOVIRIDAE SATELLITE RNAs CROSS-REFERENCE TO RELATED APPLICATIONS [0001] This application claims priority to provisional application U.S. Serial No.63/499,755, filed May 3, 2023, which is hereby incorporated herein by reference in its entirety. INCORPORATION OF SEQUENCE LISTING [0002] The instant application contains a Sequence Listing which has been submitted electronically in XML file format and is hereby incorporated by reference in its entirety. The XML file, created on April 24, 2024, is named P14419WO00.xml and is 1,473,398 bytes in size. The XML file, created on May 1, 2023, is named P14419US000.xml, which is 1,030,587 bytes in size and was filed in provisional application U.S. Serial No.63/499,755 on May 3, 2023, is also incorporated herein by reference in its entirety. BACKGROUND [0003] There is need in the art for modifying polynucleotides for improving phenotypes and genotypes of organisms; in particular, for agricultural applications to improve plants such as crop plants. [0004] Members of the family Solemoviridae are RNA viruses having a monopartite, linear, single- stranded genome of about 4 to about 5 kilobases (kb). SUMMARY [0005] This disclosure provides recombinant RNA molecules comprising from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule (cargo RNA sequence); and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the cargo RNA molecule is heterologous to the 5’ RNA replication element and the 3’ RNA replication element. In certain embodiments, (i) the 5’ RNA replication element and the 3’ RNA replication element are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; (ii) the 5’ RNA replication element, the 3’ RNA replication element, and the RdRP are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; or (iii) the 5’ RNA replication element, the 3’ RNA replication element, and/or the RdRP coding region are obtained from different Solemoviridae genomes, and the members of each respective set of the 5’ RNA replication elements, 3’ RNA replication elements, and/or RdRP coding regions have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another. In certain embodiments, the RNA molecule comprises: at least one heterologous RNA virus (HRV) amplicon in sense or antisense orientation to the first 5’ RNA replication element comprising: I. (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV Agent Ref: P14419WO00 - 2 - 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked; or II. a heterologous RNA virus (HRV) subgenomic promoter operably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP). Agricultural formulations as well as bacterial, fungal, plant, insect, and invertebrate animal cells comprising the herein disclosed recombinant RNAs are also provided. [0006] Also provided are expression systems comprising: (a) an RNA molecule comprising the herein disclosed recombinant RNA molecules; and (b) a cell containing the recombinant RNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements of the recombinant RNA molecule. In certain embodiments, the cell further comprises one or more of: (i) a viral capsid protein (CP); (ii) an RNA-binding protein (RBP) that binds to the RNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; (iv) a second RNA-dependent RNA polymerase (2nd RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the RNA molecule; (v) a viral movement protein (MP); (vi) a heterologous RNA virus (HRV); or (vii) an hrvRdRP, optionally wherein the hrvRdRP recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter. [0007] Also provided are methods of providing a synthetic Solemoviridae satellite RNA to a plant, comprising contacting the plant with any of the herein disclosed recombinant RNA molecules. [0008] Also provided are methods of establishing a synthetic Solemoviridae satellite RNA in a plant cell, comprising: providing to a plant cell any of the herein disclosed recombinant RNA molecules, wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element, wherein the RNA molecule optionally comprises an encapsidation recognition element (ERE) and is or can be encapsidated by a capsid protein, whereby the RdRP protein catalyzes synthesis of the synthetic Solemoviridae satellite RNA from the recombinant RNA molecule. [0009] Also provided are methods of obtaining a phenotypic change in a plant or plant cell, comprising: providing to a plant or plant cell any of the herein disclosed recombinant RNA molecules, wherein the cargo RNA molecule comprises RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element and catalyzes synthesis of a synthetic Solemoviridae RNA from the recombinant RNA molecule, and wherein and the cargo RNA molecule effects the phenotypic change. In certain embodiments, the methods further comprise providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0010] Also provided are methods of manufacturing a synthetic Solemoviridae satellite particle, comprising combining any of the herein disclosed recombinant RNA molecules with a viral capsid Agent Ref: P14419WO00 - 3 - protein, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein. [0011] Also provided are plant propagules comprising any of the herein disclosed recombinant RNA molecules and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA. In certain embodiments, the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP. [0012] Also provided are plants comprising any of the herein disclosed recombinant RNA molecules and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA. [0013] Solemoviridae satellite systems that are self-replicating when introduced into a plant or plant cell, comprising: (1) any of the herein disclosed recombinant Solemoviridae satellite RNAs (e.g., recombinant RNA molecules); and (2) an exogenous Solemoviridae that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA, optionally wherein the Solemoviridae satellite system further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the synthetic Solemoviridae satellite RNA. BRIEF DESCRIPTION OF THE DRAWINGS [0014] Figure 1 shows a non-limiting embodiment of a structure of a Solemoviridae satellite construct. In this embodiment, the 5’ RNA replication element is labelled “5’ RRE” and the 3’ RNA replication element is labeled “3’ RRE.” [0015] Figure 2 shows non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising: (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR). Both sense and antisense orientations of the HRV amplicon relative to the Solemoviridae 5’ RNA replication element are shown. In certain embodiments, at least one cleavable sequence is located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element. Wherein the cleavable sequence is optionally a self-cleaving ribozyme, a self-cleaving inducible ribozyme, or an Agent Ref: P14419WO00 - 4 - siRNA or miRNA recognition site. In certain embodiments, a subgenomic promoter and/or an IRES is/are operably linked to the cargo RNA. [0016] Figure 3 shows non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising a heterologous RNA virus (HRV) subgenomic promoter (HRV sgp) which is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP) and which is operably linked to the cargo RNA molecule. Embodiments where the HRV sgp and cargo RNA are in sense and antisense orientation relative to the Solemoviridae 5’ RRE are shown. [0017] Figure 4 depicts a Solemoviridae satellite construct comprising heterologous RNA virus (HRV) subgenomic promoters (HRV sgp) with: (i) one HRV sgp operably linked to a cargo RNA; and (ii) one HRV sgp operably linked to RNA encoding an hrvRdRP which recognizes both of the HRV sgp (i.e., can drive expression of the operably linked hrvRdRP and cargo RNA). In certain embodiments, an IRES is operably linked to the cargo RNA and/or an IRES is operably linked to the RNA encoding the hrvRdRP. [0018] Figure 5 depicts non-limiting embodiments of a Solemoviridae satellite construct containing a heterologous RNA virus (HRV) amplicon comprising a heterologous RNA virus (HRV) subgenomic promoter (HRV sgp) which is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP) and which is operably linked to the cargo RNA molecule flanked by HRV 5’RR and HRV 3’ RR. Embodiments where the HRV sgp and cargo RNA are in sense or antisense orientation relative to the Solemoviridae 5’ RRE are shown. The HRV 5’ RR and 3’ RR which flank the cargo RNA provide for hrvRdRP-mediated replication of an RNA comprising from 5’ to 3’ the HRV 5’ RR, cargo RNA, and HRV 3’ RR. In certain embodiments, the HRV 5’ RR and 3’ RR are flanked by ribozymes. [0019] Figure 6 depicts a commensal satellite with a cargo RNA molecule including an HRV (“HRV1”, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV (“HRV1”, e.g., TMV) RdRP binding to either the replication regions or to the subgenomic promoter, where the commensal satellite is a Solemoviridae satellite. The resulting transcripts include RNA encoding the HRV RdRP (“HRV1 RdRP”, solid squares), which can further amplify the HRV amplicon, as well as RNA encoding another cargo (solid circles). In the absence of the commensal virus, no amplification of the commensal satellite occurs. [0020] Figure 7 depicts a commensal satellite with a cargo RNA molecule including an HRV (HRV1, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV RdRP (“HRV1 RdRP”, solid squares) binding to the HRV1 replication regions, where the commensal satellite is a Solemoviridae satellite. The resulting transcripts include RNA encoding the HRV (HRV1) RdRP which can further amplify the HRV amplicon. The HRV1 amplicon includes sequence for a HRV2 amplicon (indicated in italicized text), encoding a coding and/or noncoding cargo (solid circles) and designed to be amplified in the presence of a second acute viral RdRP (“HRV2 RdRP”, hexagonal symbol), which can be provided, Agent Ref: P14419WO00 - 5 - e.g., by introduction of a second acute virus (“HRV2”, e.g., cowpea mosaic virus, CPMV) into the plant. In the absence of the commensal virus, no amplification of the commensal satellite occurs. [0021] Figure 8 depicts a commensal satellite with a cargo RNA molecule including an HRV (“HRV1”, e.g., tobacco mosaic virus, TMV) amplicon designed to be amplified by the HRV (“HRV1”, e.g., TMV) RdRP binding to either of two subgenomic promoters, where the commensal satellite is a Solemoviridae satellite. The resulting transcripts include RNA encoding the HRV RdRP (“HRV1 RdRP”, solid squares), which can further amplify the HRV amplicon, as well as RNA encoding a noncoding RNAi cargo, the sense and antisense strands of which are formed during the amplification process to yield a double-stranded RNA molecule (dsRNA) for silencing of a target gene. In the absence of the commensal virus, no amplification of the commensal satellite occurs. DETAILED DESCRIPTION Definitions [0022] The term “and/or” where used herein is to be taken as specific disclosure of each of the two specified features or components with or without the other. Thus, the term “and/or” as used in a phrase such as “A and/or B” herein is intended to include “A and B,” “A or B,” “A” (alone), and “B” (alone). Likewise, the term “and/or” as used in a phrase such as “A, B, and/or C” is intended to encompass each of the following embodiments: A, B, and C; A, B, or C; A or C; A or B; B or C; A and C; A and B; B and C; A (alone); B (alone); and C (alone). [0023] As used herein, the terms “F1,” “F2,” and the like refer to filial generations of plants or seed obtained from a parent plant which has been selfed or that has been crossed to another plant. [0024] As used herein, the term “heterologous”, when used to describe a first element in reference to a second element means that the first element and second element do not exist in nature disposed as described. For example, a heterologous nucleic acid molecule or sequence is a nucleic acid molecule or sequence that (a) is not native to a cell in which it is expressed, (b) is linked or fused to a nucleic acid molecule or sequence with which it is not linked to or fused to in nature, or with which it is not linked to or fused to in nature in the same way, (c) has been altered or mutated by the hand of man relative to its native state, or (d) has altered expression as compared to its native expression levels under similar conditions. For example, a “heterologous promoter” is used to drive transcription of a sequence that is not one that is natively transcribed by that promoter (e.g., a eukaryote promoter used to drive transcription of a DNA molecule encoding a Solemoviridae RNA sequence); thus, a “heterologous promoter” sequence can be included in an expression construct by a recombinant nucleic acid technique. In embodiments, a recombinant polynucleotide such as those provided by this disclosure includes genetic sequences of two or more different viruses of the family Solemoviridae, which genetic sequences are “heterologous” in that they would not naturally occur together. In some embodiments “heterologous” refers to a molecule or to a discrete part of a molecule; for example, referring to a cargo RNA molecule (e.g., a nucleic acid such as a protein-encoding RNA, an ssRNA, a regulatory RNA, an interfering RNA, or a guide RNA), which can be part of a larger molecule, or referring to a structure (e.g., structures Agent Ref: P14419WO00 - 6 - including a promoter (e.g., for a DNA dependent RNA polymerase) or subgenomic promoter (e.g., for an RNA-dependent RNA polymerase), an RNA effecter, RNA cleavage agent recognition site, or a polynucleotide comprising or encoding an expression-enhancing element, encapsidation recognition element (ERE), selectable or scoreable marker, DNA aptamer, RNA aptamer; a transcription factor binding site, internal ribosome entry site (IRES), DNA spacer, an RNA cleavage agent recognition site, tRNA-like element, or a transcript-stabilizing or transcript-destabilizing RNA sequence) that is not found naturally in a plant virus of the family Solemoviridae. [0025] As used herein, the terms “include,” “includes,” and “including” are to be construed as at least having the features to which they refer while not excluding any additional unspecified features. The terms “comprise”, “comprises”, and “comprising” mean “include”, “includes”, and “including”, respectively. [0026] As used herein, the term “internal ribosome entry site” or “IRES” refers to a sequence (e.g., an RNA sequence) capable of recruiting a ribosome and translation machinery to initiate translation from an RNA sequence. An IRES element is generally between 100-800 nucleotides. An appropriate IRES can be obtained from plant and plant viral IRES sequences such as encephalomyocarditis virus IRES (ECMV), maize hsp101 IRES 5’UTR, crucifer infecting tobamovirus crTMV CR-CP 148 IRES, tobacco etch virus (TEV) IRES 5’UTR and hibiscus chlorotic ringspot virus (HCRSV) IRES. In addition, in embodiments, an IRES sequence is derived from non-plant eukaryotic virus sequences that include but are not limited to: acute bee paralysis virus (ABPV), classical swine fever virus (CSFV), coxsackievirus B3 virus (CVB3), encephalomyocarditis virus (ECMV), enterovirus 71 (E71), hepatitis A virus (HAV), human rhinovirus (HRV2), human rhinovirus (HRV2), human lymphotropic virus (HTLV), polyoma virus (PV), and Zea mays (ZmHSP101). Examples of IRES sequences useful in the compositions and methods described herein are shown in Table 5. [0027] As used herein, the phrase “operably linked” refers to a juxtaposition wherein the components so described are in a relationship permitting them to function in their intended manner. For instance, a promoter is operably linked to a coding sequence if the promoter provides for transcription or expression of the coding sequence. [0028] As used herein the term “percent identity” refers to percent (%) sequence identity with respect to a reference polynucleotide or polypeptide sequence following alignment by standard techniques. Alignment for purposes of determining percent nucleic acid or amino acid sequence identity can be achieved in various ways that are within the capabilities of one of skill in the art, for example, using publicly available computer software such as BLAST, BLAST-2, PSI-BLAST, or Megalign software. In some embodiments, the software is MUSCLE (Edgar, Nucleic Acids Res., 32(5): 1792-1797, 2004). Those skilled in the art can determine appropriate parameters for aligning sequences, including any algorithms needed to achieve maximal alignment over the full length of the sequences being compared. For example, in embodiments, percent sequence identity values are generated using the sequence comparison computer program BLAST (Altschul et al. (1990) J. Mol. Biol., 215:403-410). As an Agent Ref: P14419WO00 - 7 - illustration, the percent sequence identity of a given nucleic acid or amino acid sequence, A, to, with, or against a given nucleic acid or amino acid sequence, B, (which can alternatively be phrased as a given nucleic acid or amino acid sequence, A that has a certain percent sequence identity to, with, or against a given nucleic acid or amino acid sequence, B) is calculated as follows: 100 multiplied by (the fraction X/Y), where X is the number of nucleotides or amino acids scored as identical matches by a sequence alignment program (e.g., BLAST) in that program’s alignment of A and B, and where Y is the total number of nucleotides or amino acids in B. [0029] As used herein, the term “plant” includes a whole plant and any descendant, cell, tissue, or part of a plant. The term “plant parts” include any part(s) of a plant, including, for example and without limitation: seed (including mature seed and immature seed); a plant cutting; a plant cell; a plant cell culture; or a plant organ (e.g., pollen, mature or immature embryos, flowers, fruits, shoots, leaves, roots, stems, and explants). In embodiments, a plant tissue or plant organ is or includes a seed, protoplast, callus, or any other group of plant cells that is organized into a structural or functional unit. In embodiments, a plant cell or tissue culture is capable of regenerating a plant having the physiological and morphological characteristics of the plant from which the cell or tissue was obtained, and of regenerating a plant having substantially the same genotype as the plant. Regenerable cells in a plant cell or tissue culture can include embryos, protoplasts, meristematic cells, callus, pollen, leaves, anthers, roots, root tips, flowers, and/or stalks. In contrast, some plant cells are not capable of being regenerated to produce plants and are referred to herein as “non-regenerable” plant cells. [0030] As used herein, the term “transcriptome” refers to the sum total of all RNA molecules expressed in a cell. Such RNA molecules include mRNAs, tRNAs, ribosomal RNAs, miRNAs, viral RNAs (both genomic and sub-genomic), and long non-coding RNAs. [0031] To the extent to which any of the preceding definitions is inconsistent with definitions provided in any patent or non-patent reference incorporated herein by reference, any patent or non-patent reference cited herein, or in any patent or non-patent reference found elsewhere, it is understood that the preceding definition will be used herein. [0032] Unless otherwise stated, nucleic acid sequences described herein are given, when read from left to right, in the 5’ to 3’ direction. Nucleic acid sequences may be provided as DNA or as RNA, as specified. Furthermore, because of known codon degeneracy, different nucleic acid sequences can encode the same polypeptide sequence, and such modified nucleic acid sequences (e.g., for the purposes of codon optimization for a given species) are within the scope of the present disclosure. Where a term is provided in the singular, it also contemplates aspects of the invention described by the plural of that term. [0033] This disclosure provides, inter alia, recombinant polynucleotides (e.g., recombinant DNA, recombinant RNA, recombinant ssRNAs, recombinant dsRNAs, recombinant vectors, etc.) including one or more sequences of or derived from a virus of the family Solemoviridae; in particular, a 5’ or 3’ RNA replication element that is recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP). This disclosure is further related to methods of making and using such recombinant polynucleotides, for Agent Ref: P14419WO00 - 8 - example, by employing such recombinant polynucleotides to express a heterologous cargo sequence in a plant and optionally thereby modifying expression of an endogenous target sequence and/or genotype or phenotype of the plant. In embodiments, the virus of the family Solemoviridae is a commensal virus of the family Solemoviridae, that is, a virus of the family Solemoviridae that is endemic or native to a given eukaryote host (such as a host plant) without causing apparent negative effects on the host (i.e., is considered non-pathogenic), is often present at a persistent but low population (i.e., low viral titer), and is often vertically transmitted to succeeding generations of the host. [0034] In one aspect, this disclosure is related to a recombinant DNA molecule that includes a promoter that is functional in a cell and is operably linked to a DNA sequence encoding an RNA molecule. The RNA molecule includes, in 5’ to 3’ order: (a) a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA sequence; and (c) a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP. Figure 1 shows an embodiment of a generalized structure of a DNA polynucleotide encoding a Solemoviridae satellite, where in certain embodiments the 5’ RNA replication element corresponds to the 5’ untranslated region (UTR) of a virus of the family Solemoviridae and where the 3’ RNA replication element corresponds to the 3’ untranslated region (UTR) of a virus of the family Solemoviridae. In some embodiments, the 5’ RNA replication element and/or the 3’ RNA replication element include nucleotides that extend into the predicted coding sequence or open reading frame of the virus of the family Solemoviridae. [0035] Recombinant DNA molecules provided herein can include a promoter that is functional in a cell (e.g., a bacterial cell, a plant cell, a fungal cell, or an animal cell) and is operably linked to a DNA sequence encoding an RNA molecule (e.g. a 5’ RNA replication element, a cargo RNA sequence; and a 3’ RNA replication element; a ribozyme, an intron, or a RNA encoding a protein (e.g., a capsid, movement, RdRP, or an RdRP protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter). [0036] In embodiments, a promoter functional in a plant cell provides for systemic gene expression, or alternatively for cell-, tissue-, or organ-specific gene expression, or expression that is inducible by external signals or agents (for example, light-, pathogen-, wound-, stress-, or hormone-inducible elements, or chemical inducers) or elements that are capable of cell-cycle regulated gene transcription; such elements may be located in the 5’ or 3’ regions of the native gene or engineered into a polynucleotide. [0037] Promoters include those from viruses, bacteria, fungi, animals, and plants. Suitable promoters can be used to drive expression by any RNA polymerase (e.g., RNA pol I, pol II, or pol III) Embodiments of promoters include those from cauliflower mosaic virus (e.g., p35S), bacteriophage (e.g., pT7), and plants (e.g., pATUBQ10). In certain embodiments, the promoter is operably linked to nucleotide sequences encoding multiple guide RNAs, wherein the sequences encoding guide RNAs are separated by a cleavage site such as a nucleotide sequence encoding a microRNA recognition/cleavage Agent Ref: P14419WO00 - 9 - site or a self-cleaving ribozyme (see, e.g., Ferré-D’Amaré and Scott (2010) Cold Spring Harbor Perspectives Biol., 2:a003574). In certain embodiments, the promoter is a pol II promoter operably linked to a nucleotide sequence encoding the RNA. In certain embodiments, the promoter operably linked to one or more polynucleotides encoding elements of a genome-editing system is a constitutive promoter that drives DNA expression in plant cells. In certain embodiments, the promoter drives DNA expression in the nucleus or in an organelle such as a chloroplast or mitochondrion. Examples of constitutive promoters active in plant cells include a CaMV 35S promoter as disclosed in U.S. Pat. Nos.5,858,742 and 5,322,938, a rice actin promoter as disclosed in U.S. Pat. No.5,641,876, a maize chloroplast aldolase promoter as disclosed in U.S. Pat. No.7,151,204, and a nopaline synthase (NOS) and octopine synthase (OCS) promoter from Agrobacterium tumefaciens. In certain embodiments, the promoter operably linked to one or more polynucleotides encoding elements of a genome-editing system is a promoter from figwort mosaic virus (FMV), a RUBISCO promoter, or a pyruvate phosphate dikinase (PDK) promoter, which is active in the chloroplasts of mesophyll cells. In some embodiments, the promoter is heterologous to the cell it is functional in and/or to the other elements to which the promoter is operably linked. All of the patent publications referenced in this paragraph are incorporated herein by reference in their entirety. [0038] Embodiments of recombinant polynucleotides provided herein comprise or encode RNA molecules containing 5’ and 3’ RNA replication elements recognized by a Solemoviridae RNA- dependent RNA polymerase (RdRP). In certain embodiments, recognition by a Solemoviridae RdRP is identified in an in vitro RdRP assay (e.g., an assay adapted from Horiuchi et al. Plant Cell Physiol. 42(2):197-203, 2001). In certain embodiments, recognition by a Solemoviridae RdRP is identified by an in vivo RdRP assay wherein an RNA comprising 5’ and 3’ RNA replication elements is introduced into a cell comprising the RdRP, and replication of the RNA is assayed (e.g., by an RT-PCR assay or an assay for a reporter gene encoded by a cargo RNA located in the RNA comprising 5’ and 3’ RNA replication elements). In certain embodiments, cells comprising the RdRP are engineered by introducing a gene or RNA molecule encoding the RdRP into the cell. In other embodiments, the cell comprising the RdRP is a cell which contains a virus of the family Solemoviridae which expresses the RdRP; in such embodiments the Solemoviridae virus can be one that is native to or is known to naturally occur in the cell, or it can be a non-native Solemoviridae virus, or alternatively a recombinant virus of any suitable viral family that is engineered to express the Solemoviridae RdRP. In certain embodiments, the recombinant polynucleotides (e.g., recombinant DNAs or recombinant RNAs) comprise a 5’ RNA replication element and a 3’ RNA replication element obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related. In embodiments, the Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another are taxonomically related, e.g., genomes classified as belonging to the same genus, family, and/or order. In certain embodiments, the recombinant polynucleotides (e.g., recombinant DNAs or recombinant RNAs) comprise a 5’ RNA replication element, a 3’ RNA replication Agent Ref: P14419WO00 - 10 - element, and an RdRP are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally taxonomically related, e.g., genomes classified as belonging to the same genus, family, and/or order. Non-limiting examples of a 5’ RNA replication element and a 3’ RNA replication element from the same Solemoviridae genome and the corresponding RdRP protein that recognizes that 5’ RNA replication element and 3’ RNA replication element include those set forth in each row of Table 18. In certain embodiments, the recombinant polynucleotides (e.g., recombinant DNAs or recombinant RNAs) comprise a 5’ RNA replication element, a 3’ RNA replication element, and/or an RdRP coding region are obtained from two Solemoviridae genomes wherein the members of each pair of the 5’ RNA replication elements, 3’ RNA replication elements, and RdRP coding regions of the two Solemoviridae genomes have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another. In certain embodiments, the recombinant polynucleotides (e.g., recombinant DNAs or recombinant RNAs) comprise a 5’ RNA replication element and a 3’ RNA replication element obtained from distinct Solemoviridae genomes. In certain embodiments, the recombinant polynucleotides (e.g., recombinant DNAs or recombinant RNAs) comprise a 5’ RNA replication element, a 3’ RNA replication element, and an RdRP are obtained from distinct Solemoviridae genomes. In certain embodiments, the distinct Solemoviridae genomes will have less than 85%, 80%, 75%, or 70% sequence identity to one another. In certain embodiments, the distinct Solemoviridae genomes will have 50%, 60%, or 65% to any one of 70%, 75%, 80%, or 84% sequence identity to one another. [0039] In certain embodiments, the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a single row of Table 18, or variants thereof having at least 85%, 90%, 95%, 98%, or 99% sequence identity to the 5’ RNA replication element, 3’ RNA replication elements, and RdRP, or variants thereof wherein the secondary structures of the RNA replication elements are conserved, are used together in an expression system, plant cell, plant propagule, plant, or method provided herein. In certain embodiments, the 5’ RNA replication elements and 3’ RNA replication elements in a given row of Table 18 or variants thereof are operably linked to a cargo RNA and replicated by the corresponding RdRP or variant thereof in the row. In certain embodiments, the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a given row of Table 18 (i.e., SEQ ID NO: 467, 468, 469, respectively) or aforementioned or otherwise disclosed variants thereof are used in a dicot plant cell-based expression system, dicot plant cell, dicot plant propagule, dicot plant, or related dicot plant-based method provided herein. In certain embodiments, the aforementioned dicot is a member of the genus Brassica, Capsicum, Cucumis, Cucurbita, Euphorbia, Gossypium, Macroptilium, Nicotiana, Solanum, or Glycine. In certain embodiments, the combination of 5’ RNA replication elements, 3’ RNA replication elements, and RdRP set forth in a given row of Table 18 (i.e., SEQ ID NO: 467, 468, 469, respectively) or aforementioned or otherwise disclosed variants thereof are used in a monocot plant cell-based expression system, monocot plant cell, monocot plant propagule, monocot plant, or related monocot plant-based method provided herein. In certain Agent Ref: P14419WO00 - 11 - embodiments, the aforementioned monocot is a member of the genus Avena, Hordeum, Oryza, Secale, Triticum, Sorghum, or Zea. [0040] Examples of DNA molecules which encode RNA molecules comprising or containing 5’ and 3’ RNA replication elements recognized by a Solemoviridae RdRP are set forth in Table 1. DNA molecules which encode RNAs comprising or containing 5’ RNA replication elements recognized by a Solemoviridae RdRP include SEQ ID NO: 467, 531, and 532. DNA molecules which encode RNAs comprising or containing 3’ RNA replication elements recognized by a Solemoviridae RdRP include SEQ ID NO: 468, 533, and 534. [0041] Structural features (e.g., dsRNA hairpins and ssRNA loops) identified in Solemoviridae 5’ and 3’ RNA replication elements are shown in Table 1 by way of dot bracket notation. The dot bracket notation provided in Table 1 was generated using RNA Fold software for predicting RNA secondary structure based on minimum free energy predictions of base pair probabilities. A dot ‘.’ signifies an unpaired base and a bracket ‘(’ or ‘)’ represents a paired base. Dot bracket notation is further described in Mattei et al., Nucleic Acids Research, 42(10): 6146-6157, 2014; Ramlan and Zauner In: International Workshop on Computing With Biomolecules, E. Csuhaj-Varju, R. Freund, M. Oswald and K. Salomaa (Eds.), 27 August 2008, Wien, Austria, pp.75–86, From: Austrian Computer Society, 2008; and Hofacker et al., Monatshefte für Chemie Chem. Monthly, 125: 167–188, 1994. Such structural features can range in size from 20, 30, or 40 to about 500 nucleotides (nt). These structural features are useful for designing engineered polynucleotide sequences that function as Solemoviridae RNA replication elements and/or for constructing variants of the sequences set forth in SEQ ID NO: 467, 468, and 531-534 that function as 5’ and 3’ RNA replication elements. In certain embodiments, one of more residues in the RNA secondary structure set forth in Table 1 or in equivalent RNAs are substituted with distinct nucleotides which maintain the RNA secondary structure (e.g., presence or absence of base pairing). In certain embodiments the RNA secondary structure set forth in Table 1 or in equivalent RNAs, the RNA secondary structure is maintained by making substitutions in the nucleotide sequence that result in no changes in the position of base-paired nucleotides or non-base-paired nucleotides. In certain embodiments the RNA secondary structure set forth in Table 1 or in equivalent RNAs is maintained by substituting nucleotides in the secondary structure which are not base paired with nucleotides which will not base pair, and/or by substituting nucleotides in the secondary structure which are base paired with nucleotides which will base pair. In this context, it is understood that, in some embodiments, maintaining the RNA secondary structure need not be absolute (e.g., the structure is partially maintained). In certain embodiments, a dsRNA structure is partially maintained when one, two, three or more nucleotides, particularly at the 5’ end and/or 3’ end of a hairpin-forming structure are substituted with nucleotides which do not base pair and thus reduce the total length of dsRNA in the structure. In certain embodiments, an unpaired RNA structure is partially maintained when one, two, three or more nucleotides, particularly at the 5’ end and/or 3’ end of a loop structure are substituted with nucleotides which base pair and thus reduce the total length of ssRNA in the loop structure. The ability of such Agent Ref: P14419WO00 - 12 - partially maintained secondary structures to be recognized by the corresponding Solemoviridae RdRP is monitored by in vitro or in vivo assays. Embodiments of Solemoviridae satellite RNAs include those where the 5’ RNA replication element includes one or more of these 5’ structural features and/or wherein the 3’ RNA replication element includes one or more of these 3’ structural features. In certain embodiments, the 5’ RNA replication elements comprise an RNA encoded by a DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%, or 100% sequence identity to SEQ ID NO: 467, 531, or 532, optionally wherein the encoded RNA maintains or partially maintains a corresponding structural feature set forth in Table 1. In certain embodiments, the 3’ RNA replication elements comprise an RNA encoded by a DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%, or 100% sequence identity to SEQ ID NO: 468, 533, or 534, wherein the encoded RNA optionally maintains or partially maintains a corresponding structural feature set forth in Table 1. [0042] Recombinant polynucleotides (e.g., recombinant DNA, recombinant RNA, recombinant ssRNAs, recombinant dsRNAs, recombinant vectors, etc.) provided herein can also comprise or encode additional RNA elements. [0043] Embodiments of additional RNA elements include RNAs encoding a Solemoviridae capsid or coat protein (CP). Examples of DNA sequences encoding Solemoviridae CP include the corresponding sequences of Solemoviridae genomes set forth in Table 1. Examples of DNA sequences encoding Solemoviridae CP also include the sequence of the SEQ ID NO: 472 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. Examples of DNA sequences encoding a Solemoviridae CP also include the sequence of the DNA encoding the CP disclosed in SEQ ID NO: 471 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. Examples of DNA sequences encoding a Solemoviridae CP and Solemoviridae CP sequences also include the sequences set forth in Table 17 as well as the DNA and protein sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. [0044] Embodiments of additional RNA elements include RNAs encoding a Solemoviridae RdRP. Examples of DNA sequences encoding Solemoviridae RDRP include the corresponding sequences of Solemoviridae genomes set forth in Table 1. Examples of DNA sequences encoding Solemoviridae RdRP also include the sequence of SEQ ID NO: 470, 537, and 538 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. Examples of DNA sequences encoding Solemoviridae RdRP also include the sequence of the DNA encoding the Solemoviridae RdRP disclosed in SEQ ID NO: 469, 535, and 536 as well as DNA sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. Examples of DNA sequences encoding Solemoviridae RdRP and Solemoviridae RdRP protein sequences also include the sequences set forth in Table 17 as well as the DNA and protein sequences having at least 85%, 90%, 95%, 98%, or 99% identity thereto. [0045] Embodiments of additional RNA elements include RNAs encoding a viral movement protein (MP). In certain embodiments, the cargo RNA comprises an RNA encoding a viral MP. Without being bound to hypothesis or theory, the viral movement protein is believed to bind to the RNA and to assist its Agent Ref: P14419WO00 - 13 - movement (and thus the movement of the cargo RNA) throughout the plant, e.g., via the plasmodesmata. Viral MPs include movement proteins identified from tobacco mosaic virus (TMV), cowpea mosaic virus, potato leafroll virus, tomato spotted wilt virus, and tomato mosaic virus. MPs from a variety of viruses are described in Table 3. [0046] Embodiments of additional RNA elements include tRNA-like sequences (TLS). TLS can trigger mobility of otherwise nonmobile RNAs, assisting to increase systemic delivery of the RNA molecule. TLS include tRNAs and tRNA-like sequences identified from other genetic elements, e.g., mRNAs. An isoleucine tRNA encoded by SEQ ID NO: 466 is an example of a useful tRNA-like sequence. Other mobile RNAs including TLS identified in Arabidopsis which are useful for building polynucleotides are described in Table 4. In assembling Table 4, mobile mRNA sequences were downloaded from the PLAMOM database for Arabidopsis. The tRNA “seed alignment” from the RFAM database was downloaded in stockholm format (multiple sequence alignment + secondary structure). A covariance model was created with INFERNAL for the tRNA stockholm alignment. PLAMOM mRNA sequences were scanned for significant similarity to tRNAs based on primary and secondary structure features. mRNA sequences with significant hits (E-val < 1) were then saved to a fasta file. In one embodiment, such a tRNA-like sequence includes a tRNA-like sequence from an Arabidopsis Flowering Time T (FT) mRNA. In some embodiments, the RNA molecule includes at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition element (ERE) comprising TMV-OAS. In some embodiments, the RNA molecule includes a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466. In some embodiments, the RNA molecule includes a modified tRNA-like sequence that has at least 90%, 95%, 98%, or 99% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence. [0047] Embodiments of additional RNA elements include RNAs encoding a viral capsid protein (CP). Such capsid proteins are also sometimes referred to as coat proteins, with both capsid and coat proteins being referred to as “CP.”. In embodiments, the capsid protein is heterologous to the virus of the family Solemoviridae. In embodiments, the capsid protein is a Solemoviridae capsid protein. In certain embodiments, the cargo RNA comprises an RNA encoding a viral CP. The CP can be provided, e.g., by co-expression of a recombinant construct encoding the CP or by native expression by a virus endogenous to or introduced into a plant cell. Encapsidation of an RNA molecule by the CP is achieved provided it contains an encapsidation recognition element (ERE), e.g., an origin of assembly sequence (OAS). Table 2 describes several OAS and CP sequences from a variety of viruses useful in engineering constructs which provide for RNA encapsidation. In embodiments, the OAS is positioned near the 3’ end of a construct, e.g., within the 3’ region of a cargo RNA or 3’ to a cargo RNA. For example, in some embodiments, the OAS is found 5’ to the 3’ RNA replication elements (e.g., the 3’ RNA replication elements set forth in Table 1). In embodiments, a TMV-OAS positioned at the 3’ end of the RNA Agent Ref: P14419WO00 - 14 - molecule is recognized by the TMV capsid protein, leading to assembly of a TMV virion around the RNA. [0048] Embodiments wherein the recombinant RNAs are complexed with RNA binding proteins (RBPs) are also provided herein. Embodiments of RBPs include RNA recognition motifs (RRMs) such as: (i) Lys/Arg-Gly-Phe/Tyr-Gly/Ala-Phe/Tyr-Val/Ile/Leu-X-Phe/Tyr, where X can be any amino acid (SEQ ID NO: 464); (ii) Ile/Val/Leu-Phe/Tyr-Ile/Val/Leu-X-Asn-Leu, where X can be any amino acid (SEQ ID NO: 465). Such RBP and RRM include those disclosed in Maris et al.2005, doi.org/10.1111/j.1742-4658.2005.04653.x. [0049] Embodiments of additional RNA elements include at least one ribozyme. Ribozymes include self-cleaving ribozyme, a ligand-responsive ribozyme (aptazyme), a trans-cleaving ribozyme designed to cleave a target sequence (e.g., a trans-cleaving hammerhead ribozyme designed to cleave the pepper phytoene desaturase (PDS) sequence (the RNA encoded by SEQ ID NO: 421), a hepatitis delta virus (HDV) ribozyme (the RNA encoded by SEQ ID NO: 423), or a hammerhead ribozyme (the RNA encoded by SEQ ID NO: 420). In various embodiments, multiple ribozymes are included in a polynucleotide. Useful ribozymes include Twister, Hammerhead, Hairpin, and other ribozymes. Non- limiting examples of useful ribozymes include those provided in Table 14. In certain embodiments, such a ribozyme (e.g., a self-cleaving ribozyme) is located 5’ to the 5’ RNA replication element and/or 3’ to the 3’ RNA replication element in the recombinant RNA. In certain embodiments, such a ribozyme (e.g., a self-cleaving ribozyme) is located 5’ to the HRV 5’ RNA replication region and/or 3’ to the HRV 3’ RNA replication region in a recombinant RNA comprising an imbedded heterologous RNA virus (HRV) amplicon. [0050] Embodiments of additional RNA elements include intronic sequences. Examples of intronic sequences that can be included in the recombinant polynucleotides provided herein are described in Table 6. In certain embodiments, intronic sequences are placed in a 5’UTR downstream of a promoter (e.g., a promoter active in plant cells) used to drive expression of a recombinant RNA. In certain embodiments, intronic sequences are placed 5’ to a 5’ RNA replication element, in a cargo RNA, or 3’ to a 3’ RNA replication element. [0051] Embodiments of recombinant polynucleotides and additional RNA elements include subgenomic promoters recognized by an RNA-dependent RNA polymerase (RdRP) and/or RNA molecules encoding an RNA-dependent RNA polymerase (RdRP). Examples of such subgenomic promoters and RdRP include a Brome Mosaic Virus subgenomic promoter and RdRP (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 subgenomic promoters and RdRP (Koev and Miller; J Virol.2000 Jul;74(13):5988-96. Doi: 10.1128/jvi.74.13.5988-5996.2000), Alternanthera mosaic virus (AltMV-MU) sgp1, sgp2, and sgp3 subgenomic promoters and RdRP (Putlyaev et al., Biochemistry (Mosc).;80(8):1039- 46DOI: 10.1134/S000629791508009X). Additional examples of subgenomic promoters are provided in Table 16. In certain embodiments, such subgenomic promoters are placed either 5’ and/or 3’ to an RNA Agent Ref: P14419WO00 - 15 - molecule comprising a 5’ RNA replication element, a cargo RNA, and a 3’ RNA replication element to permit production of either or both + and – strands of the RNA molecule when the RdRP is provided. In certain embodiments, such subgenomic promoters are operably linked to a cargo RNA molecule and/or to any additional RNA element to permit production of the corresponding cargo and/or additional RNA when the RdRP is provided. In certain embodiments, the subgenomic promoters are operably linked to a cargo RNA comprising an HRV-inhibitory RNA or to a cargo RNA that encodes a protein which inhibits infection, movement, transmission, and/or replication of the HRV. In certain embodiments, the subgenomic promoters are operably linked to a cargo RNA comprising an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV. In certain embodiments, the subgenomic promoters are operably linked to a cargo RNA comprising an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV which does not encode the hrvRdRP. [0052] Embodiments of other optional elements in the recombinant polynucleotides provided herein include: a) a discrete expression cassette including a second promoter operably linked to a DNA sequence to be transcribed, and optionally a terminator element (see, e.g., a NOS or CaMV35S terminator); (b) an expression-enhancing element (e.g., a DNA encoding an expression-enhancing intronic sequence); (c) a DNA or RNA sequence encoding a marker (e.g., a selectable marker such as DNA or RNA encoding an antibiotic resistance or herbicide resistance sequence; DNA encoding a scorable marker or detectable label (e.g., a beta-glucuronidase, fluorescent protein, luciferase, etc.); (d) a DNA aptamer; (e) a DNA or RNA sequence encoding an RNA aptamer; (f) T-DNA left and right border DNA sequences; (g) a spacer DNA sequence; (h) a DNA sequence encoding a transcription factor binding site; (i) a DNA sequence encoding a localization sequence (e.g., DNA encoding a targeting peptide, such as a nuclear localization signal (NLS), a mitochondrial localization signal, or a plastid localization signal); or (j) a DNA sequence encoding at least one sequence-specific recombinase recognition site (SSRRS: e.g., a pair of sequence-specific recombinase recognition sites that are recognized by a given recombinase, such as LOX sites recognized by a CRE recombinase); and (k) a DNA sequence encoding a transcript-stabilizing or transcript-destabilizing sequence (see, e.g., US Published Patent Application 2007/0011761, incorporated herein by reference in its entirety; Geisberg et al. (2014) Cell, 156: 812-824). [0053] Provided herein are recombinant polynucleotides comprising a cargo RNA molecule or comprising DNA encoding a cargo RNA molecule. In some embodiments, the recombinant polynucleotide includes a single cargo RNA molecule. In other embodiments, the recombinant polynucleotide includes at least two cargo RNA molecules, e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 cargo RNA molecules; in embodiments, the at least two cargo RNA molecules are the same (e.g., multiple copies of a non-coding RNA sequence or multiple copies of a RNA sequence encoding a Agent Ref: P14419WO00 - 16 - polypeptide) or are different (e.g., two or more different non-coding RNA sequences, or two or more different coding RNA sequences, or combinations of non-coding and coding cargo RNA sequences). [0054] In certain embodiments, a cargo RNA molecule is up to about 5 kilobases (kb) in length. Cargo RNA molecules can range in length from any one of about 20 nucleotides (nt), 100nt, 200nt, 300nt, 400nt, 500nt, 600nt, 700nt, 800nt, or 900nt to any one of about 1kb, 2kb, 3kb, 4kb, 5kb, 6kb, 7kb, 8kb, 9kb, 10kb, 11kb, 12kb, 13kb, or 14 kb in length. Other lengths of the cargo RNA molecule are less than or equal to 100 nucleotides (nt) can range in length from any one of about 20nt, 30nt, or 40nt to any one of about 50nt, 60nt, 70nt, 80nt, 90nt, or 100nt in length. Recombinant RNAs comprising a cargo RNA of up to about 5 kb in length can in certain embodiments be encapsidated by a Solemoviridae capsid protein. In such embodiments, cargo RNAs can range in length from any one of about 20nt, 100nt, 200nt, 300nt, 400nt, 500nt, 600nt, 700nt, 800nt, or 900nt to any one of about 1kb, 2kb, 3kb, 4kb, or 5kb in length. Recombinant RNAs comprising a cargo RNA of up to about 3kb, 4kb, 5kb, 6kb, 7kb, 8kb, 9kb, 10kb, 11kb, 12kb, 13kb, or 14kb in length can in certain embodiments be encapsidated by a heterologous viral capsid protein set forth in Table 2. In certain embodiments, recombinant RNAs comprising a cargo RNA of up to about 14kb and encapsidated by a heterologous viral capsid protein can comprise an OAS element set forth in Table 2 and be encapsidated by a corresponding capsid protein set forth in Table 2. In some embodiments, the cargo RNA molecule is greater than 14kb, for example, 15kb, 16kb, 17kb, 18kb, 19kb, or even 20kb. In embodiments, the cargo RNA molecule includes: (a) at least one coding sequence, (b) at least one non-coding sequence, or (c) both at least one coding sequence and at least one non-coding sequence. Such cargo RNA molecules include combinations of coding/non-coding sequence; multiple non-coding/coding sequences; as well as aptamers, ribozymes, and other elements as is described herein. In embodiments, the cargo RNA molecule includes (a) a coding sequence to be expressed in a plant, and (b) at least one non-coding sequence that modifies expression or translation of the coding sequence, such as a recognition and cleavage sequence for an siRNA or miRNA that is endogenously expressed in the plant (see, e.g., US Patent Nos.8,334,430, 9,139,838, 9,976,152, 10,793,869, 10,876,126) and can bind to and cleave an RNA transcript containing the recognition and cleavage sequence; in such embodiments, it is possible to achieve spatially or temporally or developmentally specific expression of the coding sequence in the plant. In embodiments, a coding or non-coding cargo RNA can be optimized for expression in plants by methods which include using codons which occur more frequently in plant genes and/or by eliminating polyadenylation sites in the cargo RNA (e.g., as described in at least US Pat. Nos.5380831, 5689052, and 7741118, which are each incorporated herein by reference in their entireties). [0055] In embodiments, the cargo RNA molecule includes at least one coding sequence (e.g., a translatable sequence). In some embodiments, the coding sequence is accordingly a protein or a polypeptide such as those described in this disclosure’s working examples. In some embodiments, a cargo RNA comprises a selectable marker RNA encoding an antibiotic resistance or herbicide resistance polypeptide sequence or a scorable marker RNA encoding a scorable marker protein (e.g., a beta- Agent Ref: P14419WO00 - 17 - glucuronidase, fluorescent protein, luciferase, etc.). Examples of selectable marker/selection agent combinations include glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzyme/glufosinate, or a neomycin phosphotransferase (npt)/neomycin or kanamycin. Examples of scorable markers include β-glucuronidase (GUS), luciferase, and fluorescent proteins such as green fluorescent protein (GFP), yellow fluorescent protein (YFP), and cyan fluorescent protein (CFP). In embodiments, the cargo RNA sequence encodes at least one protein or polypeptide that provides a desirable trait in a plant in which the protein or polypeptide is expressed. Non-limiting examples of polypeptides useful in agricultural applications include, for example, bacteriocins, lysins, antimicrobial peptides, nodule C-rich peptides, and bacteriocyte regulatory peptides. Such polypeptides can be used to alter the level, activity, or metabolism of target microorganisms for increasing the fitness of beneficial insects (such as honeybees and silkworms) or for decreasing the fitness of pest invertebrates (such as aphids, caterpillars, beetle larvae, and mites). Embodiments of agriculturally useful polypeptides include peptide toxins, such as those naturally produced by entomopathogenic bacteria (e.g., Bacillus thuringiensis, Photorhabdus luminescens, Serratia entomophila, or Xenorhabdus nematophila), as is known in the art. Embodiments of agriculturally useful polypeptides include polypeptides (including small peptides such as cyclodipeptides or diketopiperazines) for controlling agriculturally important pests or pathogens, e.g., antimicrobial polypeptides or antifungal polypeptides for controlling diseases in plants, or pesticidal polypeptides (e.g., insecticidal polypeptides and/or nematicidal polypeptides) for controlling invertebrate pests such as insects or nematodes. Embodiments of antimicrobial polypeptides include cathelicidins, cecropins, beta-defensins, amphibian antimicrobial peptides (e.g., aurein-like peptides, esculentin, gaegurin, brevinin, rugosin, ranatuerin, ranacyclin, uperin, ocellatin, grahamin, nigrocin, dermoseptin, temporin, bombinin, maximin), enterocins, ponicerins, megourins, apidaecins, abaecins, attacin, bacteriocins and lantibiotics, dermcidin, formaecin, halocidins, lactocin, tachystatins, and some insecticidal toxins produced by spiders and scorpions. Embodiments of agriculturally useful polypeptides include antibodies, nanobodies, and fragments thereof, e.g., antibody or nanobody fragments that retain at least some (e.g., at least 10%) of the specific binding activity of the intact antibody or nanobody. Embodiments of agriculturally useful polypeptides include transcription factors, e.g., plant transcription factors; see., e.g., the “AtTFDB” database listing the transcription factor families identified in the model plant Arabidopsis thaliana), publicly available at agris- knowledgebase[dot]org/AtTFDB/. Embodiments of agriculturally useful polypeptides include nucleases, for example, exonucleases or endonucleases (e.g., Cas nucleases such as Cas9 or Cas12a). Embodiments of agriculturally useful polypeptides further include cell-penetrating peptides, enzymes (e.g., amylases, cellulases, peptidases, lipases, chitinases), peptide pheromones (for example, yeast or fungal mating pheromones, invertebrate reproductive and larval signaling pheromones, see, e.g., Altstein (2004) Peptides, 25:1373–1376). Embodiments of agriculturally useful polypeptides confer a beneficial agronomic trait, e.g., herbicide tolerance, insect control, modified yield, increased fungal or oomycte Agent Ref: P14419WO00 - 18 - disease resistance, increased virus resistance, increased nematode resistance, increased bacterial disease resistance, plant growth and development, modified starch production, modified oils production, high oil production, modified fatty acid content, high protein production, fruit ripening, enhanced animal and human nutrition, production of biopolymers, environmental stress resistance, pharmaceutical peptides (e.g., hormones, enzymes, transcription factors, antigens, antibodies, or antibody fragments) and secretable peptides, improved processing traits, improved digestibility (e.g., reduced levels of toxins or reduced levels of compounds with “anti-nutritive” qualities such as lignins, lectins, and phytates), enzyme production, flavor, nitrogen fixation, hybrid seed production, fiber production, and biofuel production. Non-limiting examples of agriculturally useful polypeptides include polypeptides that confer herbicide resistance (US Patent Nos.6,803,501; 6,448,476; 6,248,876; 6,225,114; 6,107,549; 5,866,775; 5,804,425; 5,633,435; and 5,463,175), increased yield (US Patent Nos. RE38,446; 6,716,474; 6,663,906; 6,476,295; 6,441,277; 6,423,828; 6,399,330; 6,372,211; 6,235,971; 6,222,098; and 5,716,837), insect control (US Patent Nos.6,809,078; 6,713,063; 6,686,452; 6,657,046; 6,645,497; 6,642,030; 6,639,054; 6,620,988; 6,593,293; 6,555,655; 6,538,109; 6,537,756; 6,521,442; 6,501,009; 6,468,523; 6,326,351; 6,313,378; 6,284,949; 6,281,016; 6,248,536; 6,242,241; 6,221,649; 6,177,615; 6,156,573; 6,153,814; 6,110,464; 6,093,695; 6,063,756; 6,063,597; 6,023,013; 5,959,091; 5,942,664; 5,942,658, 5,880,275; 5,763,245; 5,763,241; 10,017,549; 10,233,217; 10,487,123; 10,494,408; 10,494,409; 10,611,806; 10,612,037; 10,669,317; 10,827,755; 11,254,950; 11,267,849; 11,130,965; 11,136,593; and 11,180,774), fungal disease resistance (US Patent Nos.6,653,280; 6,573,361; 6,506,962; 6,316,407; 6,215,048; 5,516,671; 5,773,696; 6,121,436; 6,316,407; and 6,506,962), virus resistance (US Patent Nos.6,617,496; 6,608,241; 6,015,940; 6,013,864; 5,850,023; and 5,304,730), nematode resistance (US Patent No. 6,228,992), bacterial disease resistance (US Patent No.5,516,671), plant growth and development (US Patent Nos.6,723,897 and 6,518,488), starch production (US Patent Nos.6,538,181; 6,538,179; 6,538,178; 5,750,876; 6,476,295), modified oils production (US Patent Nos.6,444,876; 6,426,447; and 6,380,462), high oil production (US Patent Nos.6,495,739; 5,608,149; 6,483,008; and 6,476,295), modified fatty acid content (US Patent Nos.6,828,475; 6,822,141; 6,770,465; 6,706,950; 6,660,849; 6,596,538; 6,589,767; 6,537,750; 6,489,461; and 6,459,018), high protein production (US Patent No. 6,380,466), fruit ripening (US Patent No.5,512,466), enhanced animal and human nutrition (US Patent Nos.6,723,837; 6,653,530; 6,5412,59; 5,985,605; and 6,171,640), biopolymers (US Patent Nos. RE37,543; 6,228,623; 5,958,745; and 6,946,588), environmental stress resistance (US Patent No. 6,072,103), pharmaceutical peptides (e.g., hormones, enzymes, transcription factors, antigens, antibodies, or antibody fragments) and secretable peptides (US Patent Nos.6,812,379; 6,774,283; 6,140,075; and 6,080,560), improved processing traits (US Patent No.6,476,295), improved digestibility (US Patent No. 6,531,648) low raffinose (US Patent No.6,166,292), industrial enzyme production (US Patent No. 5,543,576), improved flavor (US Patent No.6,011,199), nitrogen fixation (US Patent No.5,229,114), hybrid seed production (US Patent No.5,689,041), fiber production (US Patent Nos.6,576,818; 6,271,443; 5,981,834; and 5,869,720) and biofuel production (US Patent No.5,998,700). In certain Agent Ref: P14419WO00 - 19 - embodiments, the cargo RNA encodes one or more small signaling peptides (SSPs), also called peptide hormones, which are an attractive option for use as cargoes in RNA commensal satellites due to their small size (5-75 amino acids) and potency. In certain embodiments, SSPs result from processing longer precursor polypeptides (derived from ORF regions). In other embodiments, SSPs originate from a wider range of sources including intergenic/intronic regions, long non-coding RNAs, pri-miRNAs, and 5′ and 3′ UTRs of mRNAs. Non-limiting examples of SSPs include miPEP172c, miPEP171d, BomiPEP397a, AtmiPEP397a, BvmiPEP164b, and AtmiPEP164b peptides set forth in Table 13. [0056] In some embodiments, the RNA molecule further includes an internal ribosome entry site (IRES) located 5’ and immediately adjacent to the at least one coding sequence. In yet other embodiments, the cargo RNA molecule includes multiple coding sequences, and the RNA molecule further includes an IRES located 5’ and immediately adjacent to each of the coding sequences (e.g., open translational reading frames encoding a protein of interest. Useful IRES sequences include those depicted in Table 5. [0057] In certain embodiments, the cargo RNA molecule includes a non-coding sequence such as those described in this disclosure’s working examples. Such non-coding sequences include a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA sequence that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA). In certain embodiments, the cargo RNA includes a selectable or scorable RNA marker, such as an RNA aptamer or a regulatory RNA, such as an siRNA or siRNA precursor (see, e.g., US Patent No.8,404,927, 8,455,716, 9,777,288, 10,378,012), a miRNA or a miRNA precursor (see, e.g., US Patent Nos.8,410,334, 8,395,023, 9,708,620), a trans-acting siRNA or trans-acting siRNA precursor (see, e.g., US Patent Nos.8,030,473, 8,476,422, 8,816,061, 9,018,002), a phased sRNA or phased sRNA precursor (see, e.g., US Patent No.8,404,928), an siRNA or miRNA decoy (see, e.g., US Patent Nos.8,946,511, 9,873,888), an siRNA or miRNA cleavage blocker (see, e.g., US Patent 9,040,774), an siRNA or miRNA recognition and cleavage sequence (see, e.g., US Patent Nos. 8,334,430, 9,139,838, 9,976,152, 10,793,869, 10,876,126), a riboswitch (see, e.g. U.S. Patent Application Public. No.20130102651; U.S. Patent No.6,630,306; U.S. Patent No.6,949,379), or a ribozyme. Suitable RNA aptamers include those that exhibit fluorescence upon binding a molecule. For example, the fluorescent RNA aptamer can be the Broccoli RNA aptamer. Other fluorescent RNA aptamers that can be used include, but are not limited to, Spinach, Spinach2, Carrot, Radish, Corn, Red Broccoli, Orange Broccoli, and Broccoli Fluorets. Other useful RNA aptamers that can be used include those provided in Table 15. Suitable regulatory RNAs can be used to down-regulate (i.e., silence) the expression of a marker gene. For example, phytoene desaturase (PDS) is widely used as a marker gene because silencing of the gene yields a photobleached phenotype. Regulatory RNAs such as decoys or cleavage blockers can also be used to interfere with endogenous small RNA-regulated pathways, resulting in a visible phenotype; see, e.g., US Patent Nos.8,946,511, 9,873,888, 9,040,774). Agent Ref: P14419WO00 - 20 - [0058] Antiviral cargo RNAs, and in particular antiviral cargo RNAs directed against viral pathogens are provided herein. In certain embodiments, the antiviral cargo RNAs comprise a heterologous RNA Virus (HRV)-inhibitory RNA or encode an HRV-inhibitory protein, wherein the HRV-inhibitory RNA or protein inhibits infection, movement, transmission, and/or replication of the HRV. Target viral pathogens include an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae. In certain embodiments the target viral pathogen is Cucumber Mosaic Virus, Brome mosaic virus, Citrus tristeza virus, Beet yellows virus, Cowpea mosaic virus, Potato virus X; Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, sugarcane mosaic virus, Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, Pepper mild mottle virus, Turnip crinkle virus, Tomato bushy stunt virus, Tomato spotted wilt virus, watermelon bud necrosis virus, Turnip yellow mosaic virus, Spinach latent virus, Olive latent virus 2, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, Citrus leaf blotch virus, Apple latent spherical virus, Soybean latent spherical virus, Celery latent virus, Black grass varicosavirus-like virus, Maize suscal virus, Horseradish latent virus, Bean latent virus, Rice latent virus 1, or Rice latent virus 2. In certain embodiments, the targeted viral pathogen is a heterologous RNA virus disclosed in Table 7. In certain embodiments, antiviral inhibitory RNAs (RNAi sequences) used as cargo RNAs are obtained for a chosen target gene of a viral pathogen using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/). Other examples of non-coding RNA sequences having antiviral activity (e.g., dsRNA molecules which produce miRNA or siRNA) that can be used as antiviral cargo RNAs include those disclosed in US Patent No. 8,455,716, which is incorporated herein by reference in its entirety. Non-limiting examples of viral targets for antiviral cargo RNA molecules include the viral genes and genomes provided in Table 7 as well as other variants of those viral sequences. In certain embodiments, cargo RNAs encoding antiviral proteins are provided. Non-limiting examples of antiviral proteins include the N protein (Whitham, S. et al. Cell 78, 1101–1115 (1994)) and endogenous plant viral resistance proteins provided in Table 8. [0059] Antifungal cargo RNAs, and in particular antifungal cargo RNAs directed against plant fungal pathogens, are provided herein. Target fungal pathogens include Botrytis, Fusarium, Magnaporthe, Phytophthora, Rhizoctonia, Sclerotinia, and Verticillium sp. In certain embodiments, the antifungal cargo RNA comprises a non-coding RNA sequence having antifungal activity (e.g., dsRNA molecules which produce miRNA or siRNA) and in particular a dsRNA directed against a fungal pathogen target gene. In certain embodiments, such antifungal cargo RNAs comprising dsRNA-mediated control of fungal pathogens are modeled after those described in Qiao et al., 2021, doi: 10.1111/pbi.13589; Duanis-Assaf, et al., 2022, DOI: 10.1111/pbi.13708; Yang et al., 2022, doi: 10.3389/fmicb.2021.660976; Sundaresha et al., 2021, doi: 10.20944/preprints202102.0280.v1; and Gaffar et al., 2019, doi: 10.3389/fmicb.2019.01662. Non-limiting examples of antifungal cargo RNAi targets are provided in Agent Ref: P14419WO00 - 21 - Table 10. In certain embodiments, antifungal inhibitory RNAs (RNAi sequences) used as cargo RNAs are obtained for a chosen target gene of a fungal pathogen (e.g., a fungal pathogen gene set forth in Table 10) using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/). In other embodiments, antifungal cargo RNAs encode antifungal proteins. Useful antifungal proteins include nodule-specific cysteine-rich antimicrobial peptides (Vellivelli et al., 2020, doi: 10.1073/pnas.2003526117), defensins (Asano et al., 2013, doi: 10.1371/journal.ppat.1003581), the conidial germination-inhibiting antifungal peptides disclosed in International Patent Application publication WO2023/004435, which is incorporated by reference herein, including their homodimers, heterodimers, and fusions with signal or cell-penetrating peptides (e.g., the sequences provided in Tables 4 and 5 of WO2023/004435); the various antifungal antimicrobial peptides disclosed in De Cesare et al. (2020) mBio 11:e02123-20; doi.org/10.1128/mBio.02123-20, including Table 1 (supplemental material, journals[dot]asm[dot]org/doi/suppl/10.1128/mbio.02123-20/suppl_file/mbio.02123-20-st001[dot]pdf), and other antifungal proteins provided in Table 11. [0060] Insecticidal cargo RNAs, and in particular insecticidal or insect inhibitory cargo RNAs directed against insects are provided herein. Target insects include sucking insects (e.g., heteropteran and homopteran insects including aphids, whiteflies, and plant bugs), caterpillars (e.g., lepidopteran insects including fall army, black cutworm, corn earworm, soybean looper, and velvetbean caterpillar), beetles (e.g., coleopteran insects including Colorado Potato Beetle and corn rootworms), and flies (e.g., dipteran insects including Ceratitis capitata). Insecticidal or insect inhibitory cargo RNAs provided herein can be directed against insects at various stages of their development (e.g., embryonic, larval, pupal, or adult stages). In certain embodiments, the insecticidal or insect inhibitory cargo RNA comprises a non-coding RNA sequence having insecticidal or insect inhibitory activity (e.g., dsRNA molecules which produce miRNA or siRNA) and in particular a dsRNA directed against an insect target gene. In certain embodiments, such insecticidal cargo RNAs comprising dsRNA-mediated control of insects comprise or are modeled after those described in US Patent Nos.11,091,770 and 11,186,837, which are each incorporated herein by reference in their entireties. Non-limiting examples of insecticidal or insect- inhibitory cargo RNAi targets are provided in Table 9. Non-limiting examples of insecticidal cargo RNAi targets include insect Actin, SNF7, Tyrosine hydroxylase, C002, Hunchback, V-ATPase subunit A, COPI coatomer beta prime subunit, ribosomal protein L19, and ubiquitin C genes. In certain embodiments, insecticidal or insect inhibitory RNAs (RNAi sequences) used as cargo are obtained for a chosen target gene of an insect (e.g., an insect gene set forth in Table 9 or US Patent Nos.11,091,770 and 11,186,837) using siRNA/miRNA prediction tools (see, e.g., on the world wide web internet site “zhaolab[dot]org/pssRNAit/). In other embodiments, insecticidal cargo RNAs encode insecticidal proteins. Useful insecticidal proteins encoded by insecticidal cargo RNAs include native and modified Bacillus thuringiensis Cry, vegetative insecticidal proteins (VIP), and Cyt proteins (Palma et al.2014, doi: 10.3390/toxins6123296; US Patent No.11,267,849, incorporated herein by reference in its entirety) as well as insecticidal or insect-inhibitory proteins provided in Table 9. Agent Ref: P14419WO00 - 22 - [0061] Cargo RNAs can also encode “resistance” or “R” genes which confer resistance to certain arthropods, bacteria such as Pseudomonas sp., Xanthomonas sp., and Erwinia sp., and fungal pathogens including Cochliobolus, Blumeria, Fusarium, Melampsora, and Magnaporthe sp. Non-limiting examples of R genes encoded by cargo RNAs include those provided in Table 12. [0062] In some embodiments, the cargo RNA molecule comprises a CRISPR guide RNA, e.g., a crRNA, gRNA, or sgRNA. CRISPR-associated endonucleases such as Cas9, Cas12, and Cas13 endonucleases are used as genome editing tools in different plants; see, e.g., Wolter et al. (2019) BMC Plant Biol., 19:176-183); Aman et al. (2018) Genome Biol., 19:1-10. CRISPR/Cas9 requires a two- component crRNA:tracrRNA “guide RNA” (“gRNA”) that contains a targeting sequence (the “CRISPR RNA” or “crRNA” sequence) and a Cas9 nuclease-recruiting sequence (tracrRNA). Efficient Cas9 gene editing is also achieved with the use of a chimeric “single guide RNA” (“sgRNA”), an engineered (synthetic) single RNA molecule that mimics a naturally occurring crRNA-tracrRNA complex and contains both a tracrRNA (for binding the nuclease) and at least one crRNA (to guide the nuclease to the sequence targeted for editing); see, for example, Cong et al. (2013) Science, 339:819-823; Xing et al. (2014) BMC Plant Biol., 14:327-340. Chemically modified sgRNAs have been demonstrated to be effective in genome editing; see, for example, Hendel et al. (2015) Nature Biotechnol., 985-991. Commercial manufacturers of CRISPR nucleases and guide RNAs provide algorithms for designing guide RNA sequences; see, e.g., guide design tools provided by Integrated DNA Technologies at www[dot]idtdna[dot]com/pages/products/crispr-genome-editing/alt-r-crispr-cas9-system. Some Cas nucleases, including Cas12a and Cas13, do not require a tracrRNA. [0063] Also provided herein are recombinant polynucleotides wherein the 5’ and 3’ RNA replication elements of the virus of the family Solemoviridae flank an internal sequence wherein the cargo RNA is operably linked to one or more elements of a heterologous RNA virus (HRV). [0064] In certain embodiments, cargo RNAs are imbedded within a heterologous RNA virus (HRV) amplicon comprising; (i) an HRV 5’ replication region (HRV 5’ RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR), wherein (i), (ii), and (iii) are operably linked. An illustrative example of a Solemoviridae satellite construct with such an HRV amplicon is shown in Figure 2. Amplification of such an HRV amplicon in plants comprising a Solemoviridae satellite construct, the virus of the family Solemoviridae, and an HRV RdRP is illustrated in Figure 6. Examples of HRV 5’ replication regions (5’RR), 3’ replication regions (3’RR), and corresponding HRV RNA-dependent RNA Polymerases (RdRP) that recognize such replication regions are set forth in Table 7. In certain embodiments where the cargo RNA encodes a protein, an internal ribosome entry site (IRES; e.g. an IRES in Table 5) is typically operably linked to the coding cargo RNA. In certain embodiments one or more self-cleaving or inducible ribozymes are operably linked to the 5’ end of the HRV 5’ RR and to the 3’ end of the HRV 3’ RR. In certain embodiments, the HRV amplicon further comprises a subgenomic promoter which is operably linked to the cargo RNA molecule. Examples of subgenomic promoters include a subgenomic promoter of the HRV and/or a Brome Mosaic Agent Ref: P14419WO00 - 23 - Virus subgenomic promoter (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 subgenomic promoters (Koev and Miller; J Virol.2000 Jul;74(13):5988-96, doi: 10.1128/jvi.74.13.5988-5996.2000), and an Alternanthera mosaic virus (AltMV-MU) sgp1, sgp2, or sgp3 subgenomic promoter (Putlyaev et al., Biochemistry (Mosc).;80(8):1039-46, doi: 10.1134/S000629791508009X). Further examples of subgenomic promoters include those in Table 16. Such HRV amplicons can be in the sense or antisense orientation with respect to the Solemoviridae 5’ RNA replication element. When the HRV amplicon is oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element, the HRV 5’ RR and 3’ RR are present in the recombinant RNA molecule in the sense orientation, as in the corresponding sequences found in the plus (+) strand of the HRV genomic RNA. When the HRV amplicon is oriented in the antisense orientation relative to the Solemoviridae 5’ RNA replication element, the HRV 5’ RR and 3’ RR are present in the recombinant RNA molecule in antisense orientation, as in the corresponding sequences found in the negative (-) strand of the HRV genomic RNA. Under certain conditions where a plant cell or plant containing the recombinant RNA containing the HRV amplicon are provided with an RNA-dependent RNA polymerase that recognizes the HRV 5’ RR and 3’RR (hrvRdRP), the HRV amplicon undergoes amplification (e.g., hrvRdRP-mediated replication). Such hrvRdRP can be provided by sources that include: (i) infection by the HRV; (ii) introduction by vector-mediated delivery of a polynucleotide encoding the hrvRdRP (e.g., Agrobacterium-mediated delivery or viral vector mediated delivery); or (iii) introduction of a nucleic acid encoding the hrvRdRP. A non-limiting example of a recombinant satellite RNA that contains HRV 5’RR and 3’RR sequence pairs that are respectively recognized by two distinct HRV RdRP (“HRV1 RdRP” and “HRV2 RdRP”) is illustrated in Figure 7. Amplification (e.g., an increase in copy number of the HRV amplicon) provides for additional copies of the cargo RNA and an enhancement of desirable phenotypes conferred by the cargo RNA (e.g., increased antiviral, antifungal, or insecticidal activity in comparison to control plants lacking the amplified cargo RNA or lacking the cargo RNA). [0065] In other embodiments, the recombinant nucleotides provided herein comprise Solemoviridae 5’ and 3’ RNA replication elements flanking a heterologous RNA virus (HRV) subgenomic promoter operably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP). An illustrative example of a Solemoviridae satellite construct with subgenomic promoters in sense or antisense orientation relative to the 5’ RRE is shown in Figure 3. Another illustrative example where subgenomic promoters drive expression of an HRV RdRP and a dsRNA cargo in plants comprising a Solemoviridae satellite construct is shown in Figure 8. In certain embodiments where the cargo RNA linked to the subgenomic promoter encodes a protein, an internal ribosome entry site (IRES; e.g. an IRES in Table 5) is typically operably linked to the cargo RNA. Embodiments of subgenomic promoters include a subgenomic promoter of the HRV and/or a Brome Mosaic Virus subgenomic promoter (Siegal et al.1998, doi: 10.1073/pnas.95.20.11613), barley yellow dwarf virus (BYDV) sgRNA1, sgRNA2, and sgRNA3 Agent Ref: P14419WO00 - 24 - subgenomic promoters (Koev and Miller; J Virol.2000 Jul;74(13):5988-96. Doi: 10.1128/jvi.74.13.5988- 5996.2000), and an Alternanthera mosaic virus (AltMV-MU) sgp1, sgp2, or sgp3 subgenomic promoter (Putlyaev et al., Biochemistry (Mosc).;80(8):1039-46DOI: 10.1134/S000629791508009X). Further subgenomic promoters include those in Table 16. Such subgenomic promoters and operably linked cargo RNAs can be in the sense or antisense orientation with respect to the Solemoviridae 5’ RNA replication element. Expression of cargo RNAs from subgenomic promoters provides for additional copies of the cargo RNA and an enhancement of desirable phenotypes conferred by the cargo RNA (e.g., increased antiviral, antifungal, or insecticidal activity in comparison to control plants lacking the additional expressed cargo RNA or lacking the cargo RNA). When the subgenomic promoters and operably linked cargo RNAs are oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element, the subgenomic promoters and operably linked cargo RNAs are present in the recombinant RNA molecule as a sense strand where the subgenomic promoter is recognized by the hrvRdRP to produce the desired cargo RNA. When the subgenomic promoters and operably linked cargo RNAs are oriented in the sense orientation relative to the Solemoviridae 5’ RNA replication element in the recombinant RNA molecule (positive strand), the subgenomic promoter can be recognized by the hrvRdRP to produce the desired cargo RNA. When the HRV amplicon is oriented in the antisense orientation relative to the Solemoviridae 5’ RNA replication element in the recombinant RNA molecule, the subgenomic promoter cannot be recognized by the hrvRdRP to produce the desired cargo RNA. However, the negative strand of the recombinant RNA molecule produced by the Solemoviridae RdRP would contain the subgenomic promoters and operably linked cargo RNA in a sense orientation where the subgenomic promoter can be recognized by the hrvRdRP to produce the desired cargo RNA. In certain embodiments, the HRV amplicons further comprise a HRV 5’ RR and 3’ RR which flank the cargo RNA and provide for hrvRdRP-mediated replication of an RNA comprising from 5’ to 3’ the HRV 5’ RR, cargo RNA, and HRV 3’ RR (e.g., as illustrated in the non-limiting example of Figure 5). Under certain conditions where a plant cell or plant containing the recombinant RNA containing the subgenomic promoters and operably linked cargo RNA are provided with an RNA-dependent RNA polymerase that recognizes the subgenomic promoter (hrvRdRP), an RNA encoding the cargo molecule can be produced (e.g., via hrvRdRP-mediated synthesis of the cargo RNA from the subgenomic promoter). Such hrvRdRP can be provided by sources that include: (i) infection by the HRV; (ii) introduction by vector-mediated delivery (e.g., Agrobacterium-mediated delivery or viral vector mediated delivery); (iii) introduction of a nucleic acid encoding the hrvRdRP; or (iv) inclusion of a cargo RNA in the recombinant nucleotides comprising Solemoviridae 5’ and 3’ RNA replication elements. In one particular embodiment, the subgenomic promoter and operably linked cargo RNA are present in the recombinant RNA molecule as the antisense strand, and the cargo RNA encodes both an hrvRdRP and a second coding or non-coding RNA where both the hrvRdRP and a second coding or non-coding RNA are operably linked to a subgenomic promoter recognized by the hrvRdRP. In certain embodiments, an IRES is operably linked to the RNA encoding the hrvRdRP. Production of the negative strand of the Agent Ref: P14419WO00 - 25 - recombinant nucleotides comprising Solemoviridae 5’ and 3’ RNA replication elements results in an RNA where the subgenomic promoters recognized by the hrvRdRP can drive expression of the HRV RdRP and a second coding or non-coding RNA. An illustrative example of a Solemoviridae satellite construct with subgenomic promoters in antisense orientation relative to the 5’ RRE and driving expression of both an hrvRdRP that recognizes the subgenomic promoters and a second cargo RNA is shown in Figure 4. [0066] In a related aspect, an RNA molecule including at least one HRV amplicon is amplified directly by the hrvRdRP (e.g., without initial or further amplification by the Solemoviridae RdRP). In one embodiment, the HRV amplicon includes, in 5’ to 3’ order, (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) a cargo RNA molecule; and (iii) a heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked. In another embodiment, the HRV amplicon includes, in 5’ to 3’ order, a subgenomic promoter that is operably linked to (v) a cargo RNA molecule, wherein the subgenomic promoter is recognized by the hrvRdRP. Such HRV amplicons can be provided in either an isolated form or in a composition. In embodiments, the RNA molecule including the HRV amplicon can be provided to a plant, for example, by transcription in the plant from a recombinant DNA molecule encoding the RNA molecule that is transiently expressed in the plant or that is stably integrated into the plant’s genome, or by delivery to the plant of an exogenous RNA molecule including the HRV amplicon, for example, by contacting a surface of the plant with the exogenous RNA molecule including the HRV amplicon, or by introducing the exogenous RNA molecule including the HRV amplicon into the plant’s vascular system (e.g., by injection, infusion, petiole uptake, root uptake). In embodiments, the cargo RNA molecule includes at least one antiviral RNA (e.g., an antiviral inhibitory RNA or an RNA encoding an antiviral polypeptide) that provides the plant with resistance to at least one viral pathogen (which in some instances can be the heterologous RNA virus itself). Such embodiments are useful as antiviral treatments for plants, to prevent or decrease the severity of infection of a plant by a viral pathogen. [0067] In certain embodiments, plants including poinsettia (Euphorbia pulcherrima) that comprise Solemoviridae satellite RNAs containing HRV amplicons disclosed herein can exhibit control of HRV that include Poinsettia mosaic virus and Euphorbia mosaic virus. In other embodiments, plants such as poinsettia (Euphorbia pulcherrima) that comprise a Poinsettia latent virus satellite RNA containing an HRV amplicon disclosed herein can exhibit control of HRV that include Poinsettia mosaic virus and Euphorbia mosaic virus. Without seeking to be limited by theory, RNA molecules that contain HRV amplification sequences (such as the HRV amplicons described herein which contain either (1) a pair of HRV 5’ and 3’ RNA replication regions, or (2) a subgenomic promoter that is recognized by the hrvRdRP) potentially also serve as a “sponge” or “decoy” that reduces the corresponding hrvRdRP’s Agent Ref: P14419WO00 - 26 - efficiency in recognizing and amplifying the HRV viral genome itself, thus potentially decreasing a pathogenic HRV’s deleterious effects on an infected plant. [0068] RNA polynucleotides comprising at least one cleavable sequence are provided. In certain embodiments, the at least one cleavable sequence is located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element. In certain embodiments, the cleavable sequence is a self-cleaving ribozyme (e.g., a hammerhead ribozyme; Tang and Breaker. Proc Natl Acad Sci USA.2000 May 23;97(11):5784-9. doi: 10.1073/pnas.97.11.5784), a self-cleaving inducible ribozyme, or an siRNA or miRNA recognition site. [0069] In an embodiment, a cargo RNA molecule that is integrated into a polynucleotide includes at least one CRISPR guide RNA; release of the guide RNA is mediated, e.g., by flanking DR sequences, ribozyme sequences, or other self-cleaving or trans-cleaving RNAs, or by cleavage by an endogenous ribonuclease. The corresponding Cas nuclease can be provided by separate or concurrent delivery, e.g., by co-delivery with a vector or polynucleotide, or by transient or stable expression of the corresponding Cas nuclease in the cell to which the polynucleotide is delivered. For many Cas nucleases, guide sequence designs are constrained by the requirement that the DNA target sequence (to which the crRNA is designed to be complementary) must be adjacent to a proto-spacer adjacent motif (“PAM”) sequence that is recognized by the specific Cas nuclease to be employed. Cas nucleases recognize specific PAM sequences and there is a diversity of nucleases and corresponding PAM sequences; see, e.g., Smakov et al. (2017) Nature Reviews Microbiol., doi:10.1038/nrmicro.2016.184. For example, Cas9 nucleases cleave dsDNA, require a GC-rich PAM sequence located 3’ to the DNA target sequence to be targeted by the crRNA component of the guide RNA, and cleave leaving blunt ends. Cas12a nucleases cleave dsDNA, require a T-rich PAM sequence located 5’ to the DNA target sequence to be targeted by the crRNA component of the guide RNA, and cleave leaving staggered ends with a 5’ overhang. Cas13 nucleases cleave single-stranded RNAs and do not require a PAM sequence; instead, Cas13 nuclease are guided to their targets by a single crRNA with a direct repeat (“DR”). In practice, the crRNA component of a guide RNA is generally designed to have a length of between 17 – 24 nucleotides (frequently 19, 20, or 21 nucleotides) and exact complementarity (i. e., perfect base-pairing) to the targeted gene or nucleic acid sequence that is itself adjacent to a PAM motif (when required by the Cas nuclease). A crRNA component having less than 100% complementarity to the target sequence can be used (e. g., a crRNA with a length of 20 nucleotides and between 1 – 4 mismatches to the target sequence) but this increases the potential for off-target effects. [0070] Non-limiting examples of effective guide RNA design are found, e.g., in US Patent Application Publications US 2019/0032131, 2015/0082478, and 2019/0352655, which are each incorporated by reference in their entirety herein. For the purposes of gene editing, CRISPR “arrays” can be designed to include one or multiple guide RNA sequences corresponding to one or more desired target DNA Agent Ref: P14419WO00 - 27 - sequence(s); see, for example, Cong et al. (2013) Science, 339:819-823; Ran et al. (2013) Nature Protocols, 8:2281-2308. [0071] In certain embodiments, the 5’ RNA replication element includes a 5’ UTR element of a Solemoviridae genome (e.g., a Solemoviridae genome including a Solemoviridae 5’ UTR set forth in Table 1). In embodiments, the 5’ RNA replication element further includes a genomic sequence of the Solemoviridae that is natively located 3’ to and optionally adjacent or immediately adjacent to the 5’ UTR sequence. In embodiments, the 3’ RNA replication element includes a 3’ UTR sequence of a Solemoviridae genome (e.g., a Solemoviridae genome including a Solemoviridae 3’ UTR set forth in Table 1). In embodiments, the 3’ RNA replication element further includes a genomic sequence of the Solemoviridae that is natively located 5’ to and optionally adjacent or immediately adjacent to the 3’ UTR sequence. [0072] In other embodiments, the RNA molecule further includes at least one RNA molecule encoding a viral MP. In certain embodiments, the at least one RNA molecule encoding an MP is located (a) before the cargo RNA molecule, (b) after the cargo RNA molecule, or (c) both before and after the cargo RNA molecule. In embodiments, the at least one RNA sequence encoding an MP includes at least two RNA sequences encoding different MPs or a single RNA sequence encoding multiple copies of MPs. [0073] In some embodiments, the recombinant DNA molecule further includes a discrete expression cassette including a second promoter that is functional in the cell and is operably linked to a DNA sequence encoding at least one viral movement protein, and optionally a terminator element. [0074] In some embodiments, the RNA molecule further includes an encapsidation recognition element (ERE), where the ERE is located close to or adjacent to the 3’ RNA replication element, and optionally wherein the 3’ RNA replication element includes a 3’ UTR sequence of the virus of the family Solemoviridae. In embodiments, the ERE includes a viral OAS such as a tobacco mosaic virus OAS (TMV-OAS) or an OAS set forth in Table 2. [0075] In embodiments, the RNA molecule further includes at least one tRNA-like sequence (TLS), and wherein the at least one tRNA-like sequence includes a tRNA-like sequence from an Arabidopsis FT mRNA (e.g. a TLS in an Arabidopsis FT mRNA of Table 4). In some embodiments, the RNA molecule includes a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466. In some embodiments, the RNA molecule includes a modified tRNA-like sequence that has at least 90% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence. In still other embodiments, the RNA molecule further includes at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition sequence including TMV-OAS. [0076] In some embodiments, the cargo RNA molecule is up to about 5kb in length. In embodiments, the cargo RNA molecule includes: (a) at least one coding sequence, (b) at least one non-coding sequence, or (c) both at least one coding sequence and at least one non-coding sequence. In other embodiments, the Agent Ref: P14419WO00 - 28 - cargo RNA molecule includes at least one coding sequence, and wherein the RNA molecule further includes an internal ribosome entry site (IRES) located 5’ and immediately adjacent to the at least one coding sequence. In other embodiments, the cargo RNA molecule includes multiple coding sequences, and wherein the RNA molecule further includes an IRES located 5’ and immediately adjacent to each of the coding sequences. [0077] In other embodiments, the cargo RNA molecule includes at least one non-coding sequence, and wherein the at least one non-coding sequence is selected from the group consisting of a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA sequence that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; and a long noncoding RNA (lncRNA). [0078] In embodiments, a DNA sequence encoding at least one ribozyme is provided. In embodiments, the at least one ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. In embodiments, a DNA sequence encoding at least one ligand-responsive ribozyme (aptazyme) is provided. In embodiments, the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. [0079] Recombinant RNA molecules comprising the aforementioned or otherwise disclosed 5’ RNA replication elements, a cargo RNA molecule(s), and 3’ RNA replication elements, as well as additional aforementioned or otherwise disclosed elements are also provided herein. In certain embodiments, the recombinant RNA molecules are produced by a recombinant DNA molecule provided herein. In certain embodiments, the recombinant RNA molecules are produced by an in vivo or in vitro (e.g., cell free) RNA replication process through the action of a RdRP acting on: (i) 5’ and 3’ RNA replication elements; and/or (ii) a subgenomic promoter. [0080] Expression systems comprising the recombinant polynucleotides are also provided. Such expression systems include both cell-based and cell free expression systems. In certain embodiments, cell-free expression system can include (a) an RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or other element. Embodiments of additional elements include at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter. In some embodiments, the RdRP protein is provided by a virus of the family Solemoviridae, e.g. a virus of the family Solemoviridae that is endogenous to a cell in which expression is desired. In certain embodiments, cell-based expression system can include (a) a recombinant DNA molecule including a heterologous promoter that is functional in a cell and is operably linked to a DNA sequence encoding an RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or Agent Ref: P14419WO00 - 29 - other element. Embodiments of additional elements include at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes the subgenomic promoter. In certain embodiments, cell-based expression system can include (a) a recombinant RNA molecule comprising, in 5’ to 3’ order: (i) a 5’ RNA replication element; (ii) a cargo RNA molecule; and (iii) a 3’ RNA replication element; and, optionally, further comprising at least one additional RNA or other element. Embodiments of additional RNA elements include at least one RNA encoding a viral MP, at least one tRNA-like sequence, an OAS, an RdRP protein that recognizes the 5’ and 3’ RNA replication elements, a subgenomic promoter, and/or an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter. In some embodiments, the RdRP protein is provided by a virus of the family Solemoviridae. In some embodiments, an RdRP that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter is provided by a heterologous RNA virus (HRV) or by another nucleic acid introduced into the cell (e.g., by a vector or other recombinant nucleic acid). In still other embodiments, the 5’ RNA replication element and the 3’ RNA replication element are obtained from the same virus of the family Solemoviridae and/or from the same Solemoviridae genome (e.g., both obtained from the same Solemoviridae capsid genome or both obtained from the same Solemoviridae genome) or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related. In some embodiments, a cell used in the expression system is a bacterial cell, a plant cell, a fungal cell, or an animal cell (e.g., an insect cell). In embodiments, a cell used in the expression system endogenously contains a virus of the family Solemoviridae having a genome that encodes an RdRP that recognizes the 5’ and 3’ RNA replication elements. In some embodiments, the expression system further includes a viral capsid protein that is recognized by the encapsidation recognition element and encapsidates the RNA molecule. In some embodiments, the viral capsid protein is: (a) expressed by the recombinant DNA molecule in the cell (e.g., where the recombinant DNA molecule further includes a discrete expression cassette comprising a second promoter operably linked to a DNA sequence encoding the viral capsid protein, and optionally a terminator element), (b) co-expressed by a second recombinant DNA molecule in the cell; (c) provided exogenously to the cell; or (d) expressed by a virus in the cell. In embodiments, the RdRP protein is heterologous to the cell. In embodiments, the RdRP protein is provided exogenously to the cell. In certain embodiments, the RdRP protein that recognizes the 5’ and 3’ RNA replication elements is endogenously expressed in the plant cell by the virus of the family Solemoviridae (e.g., where the virus of the family Solemoviridae occurs naturally in the plant cell). In embodiments, the virus of the family Solemoviridae is native to or endemic to the plant cell. In embodiments, the virus of the family Solemoviridae that is endemic to the plant cell is non-pathogenic. In embodiments, the virus of the family Solemoviridae that is endemic to the plant cell is non-pathogenic and commensal. In embodiments, the virus of the family Solemoviridae is an exogenously introduced virus of the family Solemoviridae (i.e., not endemic or native to the host, but artificially introduced). For example, a virus of the family Solemoviridae natively found in one plant Agent Ref: P14419WO00 - 30 - species, variety, or germplasm can be introduced, with or without a corresponding recombinant Solemoviridae satellite RNA, into a different plant species, variety, or germplasm. In embodiments, a complete self-replicating Solemoviridae satellite system is introduced into a plant or plant cells, wherein the self-replicating Solemoviridae satellite system includes: (1) a recombinant Solemoviridae satellite RNA comprising, from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the promoter and the cargo RNA molecule are heterologous to the 5’ RNA replication element and the 3’ RNA replication element; and (2) an exogenous virus of the family Solemoviridae (e.g., a virus of the family Solemoviridae that is not endemic or native to the plant or plant cells) that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA. In embodiments, the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. In embodiments, the recombinant DNA molecule or recombinant RNA molecule further comprises at least one RNA encoding a viral MP, a tRNA-like sequence from an Arabidopsis FT mRNA, and an encapsidation recognition sequence including a TMV-OAS. [0081] Cells comprising any of the aforementioned or otherwise disclosed recombinant polynucleotides (e.g., recombinant DNAs, recombinant RNAs, or vectors comprising or encoding the same) are provided herein. Cells comprising the recombinant polynucleotides include prokaryotic (e.g., a bacterium, such as a bacterium capable of transforming a eukaryotic cell) or eukaryotic (e.g., a plant cell, fungal cell, or animal cell such as an insect cell) cells. In certain embodiments, the cells are bacterial cells capable of transforming a plant cell (e.g., an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell. Bacterial cells capable of transforming a plant cell suitable for use with the recombinant polynucleotides provided herein include Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell are disclosed in US patent application publications US20170369898 and US20180312854, each incorporated herein by reference in their entireties. [0082] Vectors suitable for maintenance, propagation, and/or expression of the recombinant polynucleotides in the aforementioned prokaryotic or eukaryotic cells are also provided herein. Such vectors can comprise any of the aforementioned or otherwise disclosed recombinant polynucleotides, recombinant DNA molecules, and recombinant RNA molecules as well as those polynucleotides molecules described in the Examples. In some embodiments, the bacterium that mediates the plant transformation is an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. In embodiments where an Agrobacterium sp. is used, the vector includes T-DNAs flanking the recombinant DNA molecule encoding the recombinant RNA molecule (e.g., as described in US patent application publications US20170369898 and US20180312854, each incorporated Agent Ref: P14419WO00 - 31 - herein by reference in their entireties). In some embodiments, the vector is contained within a plant cell or within a bacterial cell (Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell). [0083] Viral particles comprising any of the aforementioned or otherwise disclosed recombinant RNA molecules are also provided. In one embodiment, the recombinant RNA is introduced into a host or production plant by using Agrobacterium-mediated transformation with a polynucleotide comprising (5’ to 3’): (i) a promoter which is operably linked to a viral MP coding sequence and a TLS element, flanked by Solemoviridae 5’ and 3’ RNA replication elements; (ii) a promoter which is operably linked to a cargo RNA molecule and a TLS element, flanked by Solemoviridae 5’ and 3’ RNA replication elements; (iii) a promoter operably linked to an RdRP coding sequence; and (iv) a promoter operably linked to a CP encoding sequence. Heterologous promoters independently drive expression of the capsid protein and the cargo as depicted. The RNA expressed from the polynucleotide includes an OAS. A host plant is transformed for production of the synthetic Solemoviridae satellite RNA and satellite particles comprising the encapsidated RNA. The expressed and encapsidated Solemoviridae satellite RNA is subsequently isolated from leaf material or other tissue of the host plant, purified (and, if desired, formulated) for high pressure spraying onto plants that endogenously contain the corresponding virus of the family Solemoviridae or a recombinant (e.g., stably transformed or transiently expressed in the plant) source of the Solemoviridae RdRP for subsequent expression and replication of the Solemoviridae satellite RNA and satellite particles comprising the same in encapsidated form. In certain embodiments, spraying with the encapsidated satellite particles with certain cargo RNA molecules can be used to modify the plant as desired. The presence of a movement protein and/or tRNA-like sequences facilitates systemic movement throughout the plant receiving the high-pressure spray satellite particles comprising the desired recombinant RNA molecules comprising the encoded MP and cargo RNA. In certain embodiments, plants without a systemic virus of the family Solemoviridae which provides the Solemoviridae RdRP can further comprise a recombinant DNA or RNA molecule which encodes and provides the RdRP, e.g., stably integrated into the plant’s genome or transiently expressed in the plant. A plant that transgenically or transiently expresses a Solemoviridae RdRP is also useful for evaluating recombinant Solemoviridae satellites in planta. [0084] Target plants and plant cells used as hosts for synthetic Solemoviridae satellite RNAs (e.g., recombinant RNAs) provided herein include both monocot and dicot plants and plant cells which can support Solemoviridae replication. In embodiments, the virus of the family Solemoviridae is endogenous to (endemic to or natively found in) the plant or plant cell. In other embodiments, the virus of the family Solemoviridae is introduced to and becomes established in the plant or plant cell. Embodiments include row crop plants, fruit-producing plants and trees, vegetables, trees, and ornamental plants including ornamental flowers, shrubs, trees, groundcovers, and turf grasses. In certain embodiments, the host plants and plant cells for synthetic Solemoviridae satellite RNAs include row crop plants, fruit-producing plants and trees, vegetables, trees, and ornamental plants including ornamental flowers, shrubs, trees, Agent Ref: P14419WO00 - 32 - groundcovers, and turf grasses. In embodiments, the host plants and plant cells for synthetic Solemoviridae satellite RNAs include commercially important cultivated crops, trees, and plants, including: alfalfa (Medicago sativa), almonds (Prunus dulcis), apples (Malus x domestica), apricots (Prunus armeniaca, P. brigantine, P. mandshurica, P. mume, P. sibirica), asparagus (Asparagus officinalis), bananas (Musa spp.), barley (Hordeum vulgare), beans (Phaseolus spp.), blueberries and cranberries (Vaccinium spp.), cacao (Theobroma cacao), canola and rapeseed or oilseed rape, (Brassica napus), Polish canola (Brassica rapa), and related cruciferous vegetables including broccoli, kale, cabbage, and turnips (Brassica carinata, B. juncea, B. oleracea, B. napus, B. nigra, and B. rapa, and hybrids of these), carnation (Dianthus caryophyllus), carrots (Daucus carota sativus), cassava (Manihot esculentum), cherry (Prunus avium), chickpea (Cicer arietinum), chicory (Cichorium intybus), chili peppers and other capsicum peppers (Capsicum annuum, C. frutescens, C. chinense, C. pubescens, C. baccatum), chrysanthemums (Chrysanthemum spp.), coconut (Cocos nucifera), coffee (wild and domesticated Coffea spp. including Coffea arabica, Coffea canephora, and Coffea liberica), cotton (Gossypium hirsutum L.), cowpea (Vigna unguiculata and other Vigna spp.), fava beans (Vicia faba), cucumber (Cucumis sativus), currants and gooseberries (Ribes spp.), date (Phoenix dactylifera), duckweeds (family Lemnoideae), eggplant or aubergine (Solanum melongena), eucalyptus (Eucalyptus spp.), flax (Linum usitatissumum L.), geraniums (Pelargonium spp.), grapefruit (Citrus x paradisi), grapes (Vitus spp.) including wine grapes (Vitus vinifera and hybrids thereof), guava (Psidium guajava), hops (Humulus lupulus), hemp and cannabis (Cannabis sativa and Cannabis spp.), irises (Iris spp.), lemon (Citrus limon), lettuce (Lactuca sativa), limes (Citrus spp.), maize (Zea mays L.), mango (Mangifera indica), mangosteen (Garcinia mangostana), melon (Cucumis melo), millets (Setaria spp., Echinochloa spp., Eleusine spp., Panicum spp., Pennisetum spp.), oats (Avena sativa), oil palm (Ellis quineensis), olive (Olea europaea), onion (Allium cepa) and other alliums (Allium spp.), orange (Citrus sinensis), papaya (Carica papaya), peaches and nectarines (Prunus persica), pear (Pyrus spp.), pea (Pisa sativum), peanut (Arachis hypogaea), peonies (Paeonia spp.), petunias (Petunia spp.), pineapple (Ananas comosus), plantains (Musa spp.), plum (Prunus domestica), poinsettia (Euphorbia pulcherrima), poplar (Populus spp.), potato (Solanum tuberosum), pumpkins and squashes (Cucurbita pepo, C. maxima, C. moschata), rice (Oryza sativa L.), roses (Rosa spp.), rubber (Hevea brasiliensis), rye (Secale cereale), safflower (Carthamus tinctorius L), sesame seed (Sesame indium), sorghum (Sorghum bicolor), soybean (Glycine max L.), strawberries (Fragaria spp., Fragaria x ananassa), sugar beet (Beta vulgaris), sugarcanes (Saccharum spp.), sunflower (Helianthus annuus), sweet potato (Ipomoea batatas), tangerine (Citrus tangerina), tea (Camellia sinensis), tobacco (Nicotiana tabacum L.), tomato (Solanum lycopersicum or Lycopersicon esculentum), tulips (Tulipa spp.), walnuts (Juglans spp. L.), watermelon (Citrullus lanatus), wheat (Triticum aestivum), and yams (Discorea spp.). Wild relatives of domesticated plants are also of interest. In certain embodiments, the host plant or plant cells for synthetic Solemoviridae satellite RNAs is a dicot plant or plant cell selected from the genera Brassica, Capsicum, Cucumis, Cucurbita, Gossypium, Nicotiana, Solanum, or Glycine. In certain embodiments, the host plant Agent Ref: P14419WO00 - 33 - or plant cells for synthetic Solemoviridae satellite RNAs is a monocot plant or plant cell selected from the genera Avena, Hordeum, Oryza, Secale, Triticum, Sorghum, or Zea. In certain embodiments, monocot target plants and plant cells used as hosts for synthetic Solemoviridae satellite RNAs (e.g., recombinant RNAs) provided herein include oats (Avena sativa), barley (Hordeum vulgare), rice (Oryza sativa, Oryza glaberrima, Oryza rufipogen), rye (Secale cereale), wheat (Triticum aestivum), sorghum (Sorghum bicolor), and maize (Zea mays) plants and plant cells. [0085] Methods of using or use of any of the aforementioned or otherwise disclosed recombinant polynucleotides, expression systems, cells, and /or vectors to: (i) provide a synthetic Solemoviridae satellite RNA to a plant cell, (ii) obtain a phenotypic change in a plant or plant cell; (ii) increase a plant’s resistance of a pest or pathogen, (iii) increase a plant’s resistance to stress, (iv) express a polypeptide in a plant or plant cell, and/or (v) manufacture a synthetic Solemoviridae virus particle are also provided. In certain embodiments of the methods, the recombinant RNA molecule or a formulation thereof is provided by contacting the plant or plant cell with the recombinant RNA molecule or formulation thereof. In other embodiments, the recombinant RNA molecule is provided by expressing in the plant or plant cell a DNA molecule that encodes the recombinant RNA molecule or a formulation. In other embodiments, the recombinant RNA molecule is provided by contacting the plant or plant cell with cells (such as bacterial cells) which comprise a DNA molecule that encodes the recombinant RNA molecule and are capable of transforming the plant or plant cell. In other embodiments, the recombinant RNA molecule is provided by contacting the plant or plant cell with a satellite particle comprising an encapsidated recombinant RNA molecule or a formulation thereof. In embodiments, the 5’ RNA replication element has a nucleotide sequence obtained or derived from a Solemoviridae genomic sequence; and/or the 3’ RNA replication element has a nucleotide sequence obtained or derived from a Solemoviridae genomic sequence. In certain embodiments, the 5’ and/or 3’ RNA replication element can be obtained from the corresponding Solemoviridae genomic sequence by synthesizing or cloning a copy of the corresponding Solemoviridae genomic sequence. In certain embodiments, the 5’ and/or 3’ RNA replication element can be derived from the corresponding Solemoviridae genomic sequence by synthesizing a copy of a modified Solemoviridae genomic sequence or sequences. Such modifications of Solemoviridae genomic sequences present in a derived sequence include: (i) substitutions of nucleotides which maintain the RNA secondary structure; (ii) substitution of nucleotides based on a consensus obtained by alignment of 5’ or 3’ RNA replication elements; (iii) insertions, deletions, and/or substitution of nucleotides to facilitate assembly and/or operable linkage to other elements in the satellite RNA which include cargo RNA molecules, tRNA-like elements, encapsidation recognition element (ERE), RNA encoding a viral movement protein (MP), IRES elements, an HRV 5’RR, HRV 3’RR, and/or HRV subgenomic promoter; or (iv) any combination of (i), (ii), or (iii). In certain embodiments, the plant cell includes the virus of the family Solemoviridae, and the RdRP protein is provided to the plant cell by the virus of the family Solemoviridae. In embodiments, the virus of the family Solemoviridae is endemic to the plant cell. In embodiments, the virus of the family Solemoviridae that is endemic to the plant cell is non- Agent Ref: P14419WO00 - 34 - pathogenic and/or commensal to the plant cell. In other embodiments, the virus of the family Solemoviridae is exogenously provided to the plant cell. In some embodiments, the RdRP protein is exogenously provided to the plant cell. In embodiments, the recombinant RNA molecule is produced in a fermentation system. In embodiments, the recombinant RNA molecule is provided to the plant cell by transcribing in the plant cell a recombinant DNA construct including a promoter functional in the plant cell and operably linked to a DNA sequence encoding the recombinant RNA molecule. In embodiments, the recombinant RNA molecule further includes an encapsidation recognition element (ERE), and the plant cell further includes a viral capsid protein (CP) capable of encapsidating the synthetic Solemoviridae satellite RNA. In embodiments, wherein the viral capsid protein is exogenously provided to the plant cell. In other embodiments, the recombinant DNA construct further includes a DNA sequence encoding a viral capsid protein. Still in other embodiments, the recombinant DNA construct further includes a second promoter functional in the plant cell and operably linked to the DNA sequence encoding the viral capsid protein. In embodiments, the viral capsid protein is expressed in the plant cell and encapsidates the synthetic Solemoviridae satellite RNA. In yet other embodiments, the plant cell includes the virus of the family Solemoviridae, and the virus of the family Solemoviridae provides to the plant cell: (a) the RdRP protein, (b) the viral capsid protein, or (c) both the RdRP protein and the viral capsid protein. In certain embodiments, the methods can further comprise a first step of providing a population of plants comprising the plant cells comprising: (i) the virus of the family Solemoviridae which provides the RdRP; or (ii) recombinant polynucleotide molecule that encodes the RdRP; and then providing the recombinant RNA molecule to the plants comprising the plant cells. In certain embodiments, the methods can further comprise the step of determining if the plant cell comprises a virus of the family Solemoviridae which can provide the RdRP. In embodiments wherein it is determined that the plant cell comprises the virus of the family Solemoviridae which can provide the RdRP, the virus of the family Solemoviridae, the RdRP protein, and/or the recombinant polynucleotide encoding the RdRP is optionally not exogenously provided to the plant cell. In other embodiments wherein it is determined that the plant cell does not comprise the virus of the family Solemoviridae which can provide the RdRP and the virus of the family Solemoviridae is exogenously provided to the cell, the RdRP protein or the recombinant polynucleotide encoding the RdRP is exogenously provided to the plant cell, or a combination of the virus of the family Solemoviridae, RdRP protein, or polynucleotide encoding the RdRP is exogenously provided to the plant cell. In embodiments, a complete self-replicating Solemoviridae satellite system is introduced into a plant or plant cells, wherein the self-replicating Solemoviridae satellite system includes: (1) a recombinant Solemoviridae satellite RNA comprising, from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA- dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked, and wherein the promoter and the cargo RNA molecule are heterologous to the 5’ RNA replication element and the 3’ RNA replication element; and (2) an Agent Ref: P14419WO00 - 35 - exogenous virus of the family Solemoviridae (e.g., a virus of the family Solemoviridae that is not endemic or native to the plant or plant cells) that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA. In embodiments, the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. The presence or absence of a virus of the family Solemoviridae in a target plant can be determined by an RNA detection assay (e.g., an RT-PCR assay) using nucleic acid probes and/or primers which can detect any part of a Solemoviridae genome including a 5’ RNA replication element, a CP and/or RdRP coding region, and/or a 3’ RNA replication element. Such probes and primers include those which detect any of the 5’ or 3’ RNA replication elements set forth in Table 1 or having significant sequence identity thereto (e.g., at least about 80%, 85%, 90%, 95%, 98%, or 99% of a length of at least about 18, 20, 30, 40 or 50 nt). The presence or absence of a virus of the family Solemoviridae in a target plant can be determined by a protein detection assay (e.g., an immunoassay) directed to a Solemoviridae CP or RdRP (e.g., a CP or RdRP encoded by or homologous to a CP or RdRP encoded by a Solemoviridae genome disclosed in Table 1). Target plants and plant cells used in the methods include all aforementioned target plants and plant cell hosts for synthetic Solemoviridae satellite RNAs (e.g., recombinant RNAs). [0086] In certain embodiments of any of the aforementioned or otherwise disclosed methods, the recombinant RNA that effects: (i) a phenotypic change in the plant or plant cell; (ii) increases a plant’s resistance to a pest or pathogen; or (iii) increases a plant’s resistance to stress can include an RNA for modulating a target gene’s expression relative to the target gene’s expression in a control plant or plant cell not provided with the recombinant RNA molecule, and the phenotypic change, increased resistance to the pest or pathogen, or increased resistance to stress is a result of the modulation. In embodiments, the modulation is (a) an increase of the target gene’s expression; or (b) a decrease of the target gene’s expression. In some embodiments, expression of the target gene is increased by up to about 1%, 2%, 3%, %, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61%, 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, 100%, or more than 100% relative to a reference level (e.g., a level found in a control plant or plant cell lacking the recombinant RNA). In certain embodiments, expression of the target gene is increased by up to about 2-, 3-, 4-, 5-, 6-, 8-, 9-, 10-fold, or more relative to a reference level (e.g., a level found in a control plant or plant cell lacking the recombinant RNA molecule). In some embodiments, expression of the target gene is decreased by at least about 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61%, Agent Ref: P14419WO00 - 36 - 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, 100%, or more than 100% relative to a reference level (e.g., a level found in a level found in a control plant or plant cell lacking the recombinant RNA). In certain embodiments, expression of the target gene is decreased by up to about 2-, 3-, 4-, 5-, 6-, 8-, 9-, 10-fold, or more relative to a reference level (e.g., a level found in a control plant or plant cell lacking the recombinant RNA molecule). Modulation of target gene expression can be effected by one or more RNAs for modifying the genome, epigenome, and/or transcriptome of the plant or plant cell. RNAs for modifying the genome include gRNAs recognized by CAS nucleases, RNAs encoding TALENs or artificial zinc finger proteins (aZFN). RNAs for modifying the epigenome include RNAs which provide RNA directed DNA methylation such as in promoter regions of target genes (Matzke and Mosher (2014), doi: 10.1038/nrg3683). Embodiments of an RNA for modifying the transcriptome include one or more RNAs that comprise any of: a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA molecule that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a phased siRNA or phased siRNA precursor (see, e.g., US Patent No.8,404,928); a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA). [0087] In any of the aforementioned or otherwise disclosed methods wherein a phenotypic change is obtained or effected in a plant or plant cell, the cargo RNA molecule can comprise an RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA. In certain embodiments, phenotypes that are changed include developmental rate, growth rate, size, yield (e.g., intrinsic yield), vigor, photosynthetic capability, flavor, starch production, protein content, carbohydrate content, oil content, fatty acid content, lipid content, digestibility, biomass, shoot length, root length, root architecture, seed set, seed weight, seed quality (e.g., nutritional content), germination, fruit set, rate of fruit ripening, production of biopolymers, production of fibers, production of biofuels, production of pharmaceutical peptides (e.g., hormones, enzymes, transcription factors, antigens, antibodies, or antibody fragments), production of secretable peptides, enzyme production, improved processing traits, or amount of harvestable produce. In some embodiments, phenotypes that are changed include taste, appearance, or shelf-life of a product harvested from the plant. In other embodiments, phenotypes that are changed include flower size, flower color, flower patterning, flower morphology including presence or absence of stamens, flower number, flower longevity, flower fragrance, leaf size, leaf color, leaf patterning, leaf morphology, plant height, or plant architecture. [0088] In any of the aforementioned or otherwise disclosed methods wherein a plant’s resistance to a pest or pathogen is increased, the recombinant RNA can comprise an RNA that inhibits expression of a gene of the pest or pathogen and/or inhibits replication of the genome of the pest or pathogen. In certain embodiments, the pest or pathogen is selected from the group comprising: a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate (e.g., an arthropod or a Agent Ref: P14419WO00 - 37 - nematode). Target viruses other than a virus of the family Solemoviridae include; (i) positive-strand RNA viruses in the Bromoviridae, Closteroviridae, Luteoviridae, or Potyviridae family; (ii) negative strand RNA viruses in the Bunyaviridae and Rhabdoviridae family; (iii) dsDNA viruses in the family Caulimoviridae; and (iv) ssDNA viruses in the family Geminiviridae. Target arthropods pests include coleopteran and lepidopteran insects. Target fungal pathogens include Magnaporthe spp., Botrytis spp., Puccinia spp.; Fusarium spp., Blumeria spp., Mycosphaerella spp., Colletotrichum spp., Ustilago spp., Melampsora spp., Phakopsora spp., Phytophthora spp., and Rhizoctonia spp. In embodiments, the cargo RNA molecule effects an increase in the plant’s resistance to a pest or pathogen, relative to that in a plant not provided with the recombinant RNA molecule. [0089] In any of the aforementioned or otherwise disclosed methods wherein a plant’s resistance to stress is increased, the recombinant RNA can comprise an RNA that targets a plant gene which provides such resistance. In embodiments, the RNA that effects an increase in the plant’s resistance to stress in the plant or plant cell includes an RNA for modulating the target gene’s expression relative to the target gene’s expression in a control plant or plant cell not provided with the recombinant RNA molecule, and wherein the increase stress resistance is a result of the modulation. In embodiments, the modulation is (a) an increase of the target gene’s expression; or (b) a decrease of the target gene’s expression. In still other embodiments, the RNA that effects an increase in the plant’s resistance to stress in the plant or plant cell comprises a messenger RNA encoding a protein which confers the stress resistance. In still other embodiments, the messenger RNA includes an RNA sequence absent in the transcriptome of the plant or plant cell lacking the recombinant RNA. In embodiments, the stress includes at least one abiotic stress selected from the group including: nutrient stress, light stress, drought stress, heat stress, and cold stress. In other embodiments, the stress includes at least one biotic stress selected from the group including: crowding, shading, and allelopathy (e.g., resulting from allelopathic chemicals including a juglone produced by walnut trees). [0090] In any of the aforementioned or otherwise disclosed methods wherein an exogenous polypeptide (e.g., an exogenous polypeptide) is expressed in a plant or plant cell, the cargo RNA can encode the exogenous polypeptide. In embodiments, the polypeptide is isolated (e.g., separated from at least one other cellular components such as a carbohydrate, a lipid, or another protein) or polypeptide is purified. [0091] In any of the aforementioned or otherwise disclosed methods wherein a synthetic Solemoviridae satellite particle is manufactured, such manufacture can occur in either a cell-based system or a cell-free system. Cell-based methods of manufacturing a synthetic Solemoviridae satellite particle can comprise: (a) providing to a plant cell the recombinant RNA molecule, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule and a viral capsid protein, wherein the ERE provides for encapsidation of the RNA by the Agent Ref: P14419WO00 - 38 - viral capsid protein; and optionally isolating the synthetic Solemoviridae satellite particle from the plant cell, a plant comprising the plant cell, or from media in which the plant cell or the plant had been grown. Cell-free methods of manufacturing a synthetic Solemoviridae satellite particle include methods where the recombinant RNA molecule is combined with a viral capsid protein in a vessel, wherein the recombinant RNA molecule comprises an ERE, and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein in the vessel; optionally wherein the method further comprises isolating the synthetic Solemoviridae satellite particle from uncombined RNA and/or viral capsid protein in the vessel. In certain embodiments of either cell-based or cell-free methods, the synthetic satellite particle is isolated (e.g., separated from at least one other cellular components such as an organelle, a membrane, a carbohydrate, a lipid, or another protein) or is purified. In certain embodiments, the methods can further comprise formulating the synthetic Solemoviridae satellite particle. [0092] Synthetic Solemoviridae satellite particles comprising the recombinant RNA, including those made by the aforementioned methods are also provided. Methods of providing any of the aforementioned synthetic Solemoviridae satellite particles to a plant, including contacting (e.g., spraying, dusting, injecting, soaking, etc.) the plant with the synthetic Solemoviridae satellite particle or a formulation thereof are also provided. [0093] The recombinant polynucleotides, cells comprising the same, and synthetic Solemoviridae satellite particles described herein can be formulated either in pure form (e.g., the composition contains only the recombinant polynucleotide) or together with one or more additional formulation components to facilitate application or delivery of the compositions. In embodiments, the additional formulation component includes, e.g., a carrier (i.e., a component that has an active role in delivering the active agent (e.g., recombinant polynucleotide); for example, a carrier can encapsulate, covalently or non-covalently modify, or otherwise associate with the active agent in a manner that improves delivery of the active agent) or an excipient (e.g., a delivery vehicle, adjuvant, diluent, surfactant, stabilizer, or tonicity agent). In some embodiments, the composition is formulated for delivery to a plant. [0094] In some aspects, the disclosure provides a formulation comprising any of the compositions described herein. In some embodiments, the formulation is a liquid, a gel, or a powder. In some embodiments, the formulation is configured to be sprayed on plants, to be injected into plants (see, e.g., US Patent No.11,844,318) or otherwise introduced into the vascular system of a plant, to be rubbed on leaves, to be soaked into plants, to be coated onto plants, or be coated on seeds, or to be delivered through root uptake (e.g., in a hydroponic system or via soil). [0095] Depending on the intended objectives and prevailing circumstances, the composition can be formulated into emulsifiable concentrates, suspension concentrates, directly sprayable or dilutable solutions, coatable pastes, diluted emulsions, spray powders, soluble powders, dispersible powders, wettable powders, dusts, granules, encapsulations in polymeric substances, microcapsules, foams, aerosols, carbon dioxide gas preparations, tablets, resin preparations, paper preparations, nonwoven fabric preparations, or knitted or woven fabric preparations. In some instances, the composition is a Agent Ref: P14419WO00 - 39 - liquid. In some instances, the composition is a solid. In some instances, the composition is an aerosol, such as in a pressurized aerosol can. [0096] In some instances, the recombinant polynucleotide makes up about 0.1% to about 100% of the composition, such as any one of about 0.01% to about 100%, about 1% to about 99.9%, about 0.1% to about 10%, about 1% to about 25%, about 10% to about 50%, about 50% to about 99%, or about 0.1% to about 90% of active ingredients (e.g., recombinant polynucleotides). In some instances, the composition includes at least any of 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or more active ingredients (e.g., recombinant polynucleotides). In some instances, the concentrated agents are preferred as commercial products, the final user normally uses diluted agents, which have a substantially lower concentration of active ingredient. [0097] In some embodiments, the composition is formulated for topical delivery to a plant. In some embodiments, the topical delivery is spraying, leaf rubbing (e.g., with or without an abrasive), soaking, coating (e.g., coating using micro-particulates or nano-particulates), or delivery through root uptake (e.g., delivery in a hydroponic system or by a root drench). [0098] In some embodiments, the composition further comprises a carrier and/or an excipient. In other embodiments, the composition does not comprise a carrier or excipient, e.g., comprises a naked polynucleotide (e.g., a naked RNA). [0099] In some embodiments, the recombinant polynucleotide is delivered at a concentration of at least 0.1 grams per acre, e.g., at least 0.1, 1, 2, 5, 10, 15, 20, 25, 30, 35, 40, 45, or 50 grams per acre. In some embodiments, less than 120 liters per acre is delivered, e.g., less than 110, 100, 90, 80, 70, 60, 50, 40, 30, 20, 15, 10, 5, or 2 liters per acre or less than 1 liter per acre. [0100] In some aspects, the formulation comprises a carrier. In some embodiments the formulation is an emulsion or a reverse emulsion, a liquid, or a gel. In embodiments, the formulation includes a carrier that serves as a physical support (e.g., solid or semi-solid surfaces or matrices, powders, or particles or nanoparticles). In embodiments, the active agent is encapsulated or enclosed in or attached to or complexed with a carrier including a liposome, vesicle, micelle, or other fluid compartment. In embodiments, the active agent is encapsulated or enclosed in or attached to or complexed with a carrier including a naturally occurring or synthetic, branched or linear polymer (e.g., pectin, agarose, chitin, chitosan, DEAE-dextran, polyvinylpyrrolidone (“PVP”), or polyethylenimine (“PEI”). In embodiments the carrier includes cations or a cationic charge, such as cationic liposomes or cationic polymers such as polyamines (e.g., spermine, spermidine, putrescine). In embodiments, the carrier includes a polypeptide such as an enzyme, (e.g., cellulase, pectolyase, maceroenzyme, pectinase), a cell penetrating or pore- forming peptide (e.g., poly-lysine, poly-arginine, or polyhomoarginine peptides). [0101] Non-limiting examples of carriers include cationic liposomes and polymer nanoparticles such as those reviewed by Zhang et al. (2007) J. Controlled Release, 123:1 - 10, and the cross-linked multilamellar liposomes described in US Patent Application Publication 2014/0356414 Al, incorporated by reference in its entirety herein. In embodiments, the carrier includes a nanomaterial, such as carbon or Agent Ref: P14419WO00 - 40 - silica nanoparticles, carbon nanotubes, carbon nanofibers, or carbon quantum dots. Non-limiting examples of carriers include particles or nanoparticles (e.g., particles or nanoparticles made of materials such as carbon, silicon, silicon carbide, gold, tungsten, polymers, or ceramics) in various size ranges and shapes, magnetic particles or nanoparticles (e.g., silenceMag MagnetotransfectionTM agent, OZ Biosciences, San Diego, CA), abrasive or scarifying agents, needles or microneedles, matrices, and grids. [0102] In certain embodiments, particulates and nanoparticulates are useful in delivery of the polynucleotide composition or the nuclease or both. Useful particulates and nanoparticles include those made of metals (e.g., gold, silver, tungsten, iron, cerium), ceramics (e.g., aluminum oxide, silicon carbide, silicon nitride, tungsten carbide), polymers (e.g., polystyrene, polydiacetylene, and poly(3,4- ethylenedioxythiophene) hydrate), semiconductors (e.g., quantum dots), silicon (e.g., silicon carbide), carbon (e.g., graphite, graphene, graphene oxide, or carbon nanosheets, nanocomplexes, or nanotubes), and composites (e.g., polyvinylcarbazole/graphene, polystyrene/graphene, platinum/graphene, palladium/graphene nanocomposites). In certain embodiments, such particulates and nanoparticulates are further covalently or non-covalently functionalized, or further include modifiers or cross-linked materials such as polymers (e.g., linear or branched polyethylenimine, poly-lysine), polynucleotides (e.g., DNA or RNA), polysaccharides, lipids, polyglycols (e.g., polyethylene glycol, thiolated polyethylene glycol), polypeptides or proteins, and detectable labels (e.g., a fluorophore, an antigen, an antibody, or a quantum dot). In various embodiments, such particulates and nanoparticles are neutral, or carry a positive charge, or carry a negative charge. [0103] Embodiments of compositions including particulates include those formulated, e.g., as liquids, colloids, dispersions, suspensions, aerosols, gels, and solids. Embodiments include nanoparticles affixed to a surface or support, e.g., an array of carbon nanotubes vertically aligned on a silicon or copper wafer substrate. Embodiments include polynucleotide compositions including particulates (e.g., gold or tungsten or magnetic particles) delivered by a Biolistic-type technique or with magnetic force. The size of the particles used in Biolistics is generally in the “microparticle” range, for example, gold microcarriers in the 0.6, 1.0, and 1.6 micrometer size ranges (see, e.g., instruction manual for the Helios® Gene Gun System, Bio-Rad, Hercules, CA; Randolph-Anderson et al. (2015) "Submicron gold particles are superior to larger particles for efficient Biolistic® transformation of organelles and some cell types", Bio-Rad US/EG Bulletin 2015), but successful Biolistics delivery using larger (40 - 48 nanometer) nanoparticles has been reported in cultured animal cells; see O'Brian and Lummis (2011) BMC Biotechnol., 11 :66 - 71. [0104] Other embodiments of useful particulates are nanoparticles, which are generally in the nanometer (nm) size range or less than 1 micrometer, e.g., with a diameter of less than about 1 nm, less than about 3 nm, less than about 5 nm, less than about 10 nm, less than about 20 nm, less than about 40 nm, less than about 60 nm, less than about 80 nm, and less than about 100 nm. Specific, non-limiting embodiments of nanoparticles commercially available (all from Sigma-Aldrich Corp., St. Louis, MO) include gold nanoparticles with diameters of 5, 10, or 15 nm; silver nanoparticles with particle sizes of Agent Ref: P14419WO00 - 41 - 10, 20, 40, 60, or 100 nm; palladium “nanopowder” of less than 25 nm particle size; single-, double-, and multi-walled carbon nanotubes, e.g., with diameters of 0.7 - 1.1, 1.3 - 2.3, 0.7 - 0.9, or 0.7 - 1.3 nm, or with nano tube bundle dimensions of 2 - 10 nm by 1- 5 micrometers, 6 - 9 nm by 5 micrometers, 7 - 15 nm by 0.5 - 10 micrometers, 7 - 12 nm by 0.5 - 10 micrometers, 110 - 170 nm by 5 - 9 micrometers, 6 - 13 nm by 2.5 - 20 micrometers. Embodiments include polynucleotide compositions including materials such as gold, silicon, cerium, or carbon, e.g., gold or gold-coated nanoparticles, silicon carbide whiskers, carborundum, porous silica nanoparticles, gelatin/silica nanoparticles, nanoceria or cerium oxide nanoparticles (CNPs), carbon nanotubes (CNTs) such as single-, double-, or multi-walled carbon nanotubes and their chemically functionalized versions (e.g., carbon nanotubes functionalized with amide, amino, carboxylic acid, sulfonic acid, or polyethylene glycol moieties), and graphene or graphene oxide or graphene complexes; see, for example, Wong et al. (2016) Nano Lett., 16: 1161 - 1172; Giraldo et al. (2014) Nature Materials, 13:400-409; Shen et al. (2012) Theranostics, 2:283 - 294; Kim et al. (2011) Bioconjugate Chem., 22:2558 - 2567; Wang et al. (2010) J. Am. Chem. Soc. Comm., 132:9274 - 9276; Zhao et al. (2016) Nanoscale Res. Lett., 11: 195 - 203; and Choi et al. (2016) J. Controlled Release, 235:222 - 235. See also, for example, the various types of particles and nanoparticles, their preparation, and methods for their use, e.g., in delivering polynucleotides and polypeptides to cells, disclosed in US Patent Application Publications 2010/0311168, 2012/0023619, 2012/0244569, 2013/0145488, 2013/0185823, 2014/0096284, 2015/0040268, 2015/0047074, and 2015/0208663, all of which are incorporated herein by reference in their entirety. [0105] In some aspects, the composition includes an excipient, e.g., a delivery vehicle, adjuvant, diluent, surfactant, stabilizer, or tonicity agent or a combination thereof. In some embodiments, the excipient is a crop oil concentrate, a vegetable oil concentrate, a modified vegetable oil, a nitrogen source, a deposition (drift control) and/or retention agent (with or without ammonium sulfate and/or defoamer), a compatibility agent, a buffering agent and/or acidifier, a water conditioning agent, a basic blend, a spreader-sticker and/or extender, an adjuvant plus foliar fertilizer, an antifoam agent, a foam marker, a scent, or a tank cleaner and/or neutralizer. In some embodiments, the excipient is an adjuvant described in the Compendium of Herbicide Adjuvants (Young et al. (2016). Compendium of Herbicide Adjuvants (13th ed.), Purdue University). [0106] Examples of delivery vehicles and diluents include, but are not limited to, lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia, calcium phosphate, alginates, tragacanth, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, water, saline solution, syrup, methylcellulose, methyl- and propylhydroxybenzoates, talc, magnesium stearate, and mineral oil. Further examples of delivery vehicles include, but are not limited to, solid or liquid excipient materials, solvents, stabilizers, slow-release excipients, colorings, and surface-active substances (surfactants). In some instances, the excipient (e.g., delivery vehicle) is a stabilizing vehicle. In embodiments, the stabilizing vehicle includes, e.g., an epoxidized vegetable oil, an antifoaming agent, e.g. silicone oil, a preservative, a viscosity regulator, a binding agent, or a tackifier. In some instances, the stabilizing Agent Ref: P14419WO00 - 42 - vehicle is a buffer suitable for the recombinant polynucleotide. In some instances, the composition is microencapsulated in a polymer bead delivery vehicle. In some instances, the stabilizing vehicle protects the recombinant polynucleotide against UV and/or acidic conditions. In some instances, the delivery vehicle contains a pH buffer. In some instances, the composition is formulated to have a pH in the range of about 4.5 to about 9.0, including for example pH ranges of about any one of 5.0 to about 8.0, about 6.5 to about 7.5, or about 6.5 to about 7.0. [0107] In some instances, the composition provided herein includes an adjuvant. Adjuvants are agents that do not possess the polynucleotide activity, but impart beneficial properties to a formulation. For example, adjuvants are either pre-mixed in the formulation or added to a spray tank to improve mixing or application or to enhance performance. They are used extensively in products designed for foliar applications. Adjuvants can be used to customize the formulation to specific needs and compensate for local conditions. Adjuvants can be designed to perform specific functions, including wetting, spreading, sticking, reducing evaporation, reducing volatilization, buffering, emulsifying, dispersing, reducing spray drift, and reducing foaming. No single adjuvant can perform all these functions, but compatible adjuvants often can be combined to perform multiple functions simultaneously. [0108] Among nonlimiting examples of adjuvants included in the formulation are binders, dispersants and stabilizers, specifically, for example, casein, gelatin, polysaccharides (e.g., starch, gum arabic, cellulose derivatives, alginic acid, etc.), lignin derivatives, bentonite, sugars, synthetic water-soluble polymers (e.g., polyvinyl alcohol, polyvinylpyrrolidone, polyacrylic acid, etc.), PAP (acidic isopropyl phosphate), BHT (2,6-di-t-butyl-4-methylphenol), BHA (a mixture of 2-t-butyl-4-methoxyphenol and 3- t-butyl-4-methoxyphenol), vegetable oils, mineral oils, fatty acids and fatty acid esters. [0109] In embodiments, the compositions provided herein are in a liquid formulation. Liquid formulations are generally mixed with water, but in some instances are used with crop oil, diesel fuel, kerosene, or other light oil as an excipient. The amount of active ingredient (e.g., recombinant polynucleotides) often ranges from about 0.5 to about 80 percent by weight. [0110] In embodiments, an emulsifiable concentrate formulation contains a liquid active ingredient, one or more petroleum-based solvents, and an agent that allows the formulation to be mixed with water to form an emulsion. Such concentrates can be used in agricultural, ornamental and turf, forestry, structural, food processing, livestock, and public health pest formulations. In embodiments, these are adaptable to application equipment from small portable sprayers to hydraulic sprayers, low-volume ground sprayers, mist blowers, and low-volume aircraft sprayers. Some active ingredients readily dissolve in a liquid excipient. When mixed with an excipient, they form a solution that does not settle out or separate, e.g., a homogenous solution. In embodiments, formulations of these types include an active ingredient, a carrier and/or an excipient, and one or more other ingredients. Solutions can be used in any type of sprayer, indoors and outdoors. [0111] In some instances, the composition is formulated as an invert emulsion. An invert emulsion is a water-soluble active ingredient dispersed in an oil excipient. Invert emulsions require an emulsifier that Agent Ref: P14419WO00 - 43 - allows the active ingredient to be mixed with a large volume of petroleum-based excipient, usually fuel oil. Invert emulsions aid in reducing drift. With other formulations, some spray drift results when water droplets begin to evaporate before reaching target surfaces; as a result, the droplets become very small and lightweight. Because oil evaporates more slowly than water, invert emulsion droplets shrink less, and more active ingredient reaches the target. Oil further helps to reduce runoff and improve rain resistance. It further serves as a sticker-spreader by improving surface coverage and absorption. Because droplets are relatively large and heavy, it is difficult to get thorough coverage on the undersides of foliage. Invert emulsions are most commonly used along rights-of-way where drift to susceptible non- target areas can be a problem. [0112] A flowable or liquid formulation combines many of the characteristics of emulsifiable concentrates and wettable powders. Manufacturers use these formulations when the active ingredient is a solid that does not dissolve in either water or oil. The active ingredient, impregnated on a substance such as clay, is ground to a very fine powder. The powder is then suspended in a small amount of liquid. The resulting liquid product is quite thick. Flowables and liquids share many of the features of emulsifiable concentrates, and they have similar disadvantages. They require moderate agitation to keep them in suspension and leave visible residues, similar to those of wettable powders. [0113] Flowables/liquids are easy to handle and apply. Because they are liquids, they are subject to spilling and splashing. They contain solid particles, so they contribute to abrasive wear of nozzles and pumps. Flowable and liquid suspensions settle out in their containers. Because flowable and liquid formulations tend to settle, packaging in containers of five gallons or less makes remixing easier. [0114] Aerosol formulations contain one or more active ingredients and a solvent. Most aerosols contain a low percentage of active ingredients. There are two types of aerosol formulations—the ready- to-use type commonly available in pressurized sealed containers and those products used in electrical or gasoline-powered aerosol generators that release the formulation as a smoke or fog. [0115] Ready to use (RTU) aerosol formulations are usually small, self-contained units that release the formulation when the nozzle valve is triggered. The formulation is driven through a fine opening by an inert gas under pressure, creating fine droplets. These products are used in greenhouses, in small areas inside buildings, or in localized outdoor areas. Commercial models, which hold five to 5 pounds of active ingredient, are usually refillable. [0116] Smoke or fog aerosol formulations are not under pressure. They are used in machines that break the liquid formulation into a fine mist or fog (aerosol) using a rapidly whirling disk or heated surface. [0117] In some embodiments, the composition comprises a liquid excipient. In embodiments, a liquid excipient includes, for example, aromatic or aliphatic hydrocarbons (e.g., xylene, toluene, alkylnaphthalene, phenylxylylethane, kerosene, gas oil, hexane, cyclohexane, etc.), halogenated hydrocarbons (e.g., chlorobenzene, dichloromethane, dichloroethane, trichloroethane, etc.), alcohols (e.g., methanol, ethanol, isopropyl alcohol, butanol, hexanol, benzyl alcohol, ethylene glycol, etc.), ethers Agent Ref: P14419WO00 - 44 - (e.g., diethyl ether, ethylene glycol dimethyl ether, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, propylene glycol monomethyl ether, tetrahydrofuran, dioxane, etc.), esters (e.g., ethyl acetate, butyl acetate, etc.), ketones (e.g., acetone, methyl ethyl ketone, methyl isobutyl ketone, cyclohexanone, etc.), nitriles (e.g., acetonitrile, isobutyronitrile, etc.), sulfoxides (e.g., dimethyl sulfoxide, etc.), amides (e.g., N,N-dimethylformamide, N,N-dimethylacetamide, cyclic imides (e.g. N- methylpyrrolidone) alkylidene carbonates (e.g., propylene carbonate, etc.), vegetable oil (e.g., soybean oil, cottonseed oil, etc.), vegetable essential oils (e.g., orange oil, hyssop oil, lemon oil, etc.), or water. [0118] In some embodiments, the composition comprises a gaseous excipient. Gaseous excipients include, for example, butane gas, floron gas, liquefied petroleum gas (LPG), dimethyl ether, and carbon dioxide gas. [0119] In some embodiments, the compositions are provided as a dry formulation. Dry formulations can be divided into two types: ready-to-use and concentrates that must be mixed with water to be applied as a spray. Most dust formulations are ready to use and contain a low percentage of active ingredients (less than about 10 percent by weight), plus a very fine, dry inert excipient (e.g., talc, chalk, clay, nut hulls, or volcanic ash). The size of individual dust particles varies. A few dust formulations are concentrates and contain a high percentage of active ingredients. In some embodiments, these are mixed with dry inert excipients before applying. In some embodiments, dusts are used dry and can easily drift to non-target sites. [0120] In some instances, the composition is formulated as a powder. In some instances, the composition is formulated as a wettable powder. Wettable powders are dry, finely ground formulations that look like dusts. They usually must be mixed with water for application as a spray. A few products, however, can be applied either as a dust or as a wettable powder—the choice is left to the applicator. Wettable powders have about 1 to about 95 percent active ingredient by weight; in some cases, more than about 50 percent. The particles do not dissolve in water. They settle out quickly unless constantly agitated to keep them suspended. They can be used for most pest problems and in most types of spray equipment where agitation is possible. Wettable powders have excellent residual activity. Because of their physical properties, most of the formulation remains on the surface of treated porous materials such as concrete, plaster, and untreated wood. In such cases, only the water penetrates the material. [0121] In some instances, the composition is formulated as a soluble powder. Soluble powder formulations look like wettable powders. However, when mixed with water, soluble powders dissolve readily and form a true solution. After they are mixed thoroughly, no additional agitation is necessary. The amount of active ingredient in soluble powders ranges from about 15 to about 95 percent by weight; in some cases, more than about 50 percent. Soluble powders have all the advantages of wettable powders and none of the disadvantages, except the inhalation hazard during mixing. [0122] In some instances, the composition is formulated as a water-dispersible granule. Water- dispersible granules, also known as dry flowables, are like wettable powders, except instead of being dust-like, they are formulated as small, easily measured granules. Water-dispersible granules must be Agent Ref: P14419WO00 - 45 - mixed with water to be applied. Once in water, the granules break apart into fine particles similar to wettable powders. The formulation requires constant agitation to keep it suspended in water. The percentage of active ingredient is high, often as much as 90 percent by weight. Water-dispersible granules share many of the same advantages and disadvantages of wettable powders, except they are more easily measured and mixed. Because of low dust, they cause less inhalation hazard to the applicator during handling. [0123] In some embodiments, the composition comprises a solid excipient. Solid excipients include finely-divided powder or granules of clay (e.g. kaolin clay, diatomaceous earth, bentonite, Fubasami clay, acid clay, etc.), synthetic hydrated silicon oxide, talc, ceramics, other inorganic minerals (e.g., sericite, quartz, sulfur, activated carbon, calcium carbonate, hydrated silica, etc.), a substance which can be sublimated and is in the solid form at room temperature (e.g., 2,4,6-triisopropyl-1,3,5-trioxane, naphthalene, p-dichlorobenzene, camphor, adamantan, etc.); wool; silk; cotton; hemp; pulp; synthetic resins (e.g., polyethylene resins such as low-density polyethylene, straight low-density polyethylene and high-density polyethylene; ethylene-vinyl ester copolymers such as ethylene-vinyl acetate copolymers; ethylene-methacrylic acid ester copolymers such as ethylene-methyl methacrylate copolymers and ethylene-ethyl methacrylate copolymers; ethylene-acrylic acid ester copolymers such as ethylene-methyl acrylate copolymers and ethylene-ethyl acrylate copolymers; ethylene-vinylcarboxylic acid copolymers such as ethylene-acrylic acid copolymers; ethylene-tetracyclododecene copolymers; polypropylene resins such as propylene homopolymers and propylene-ethylene copolymers; poly-4-methylpentene-1, polybutene-1, polybutadiene, polystyrene; acrylonitrile-styrene resins; styrene elastomers such as acrylonitrile-butadiene-styrene resins, styrene-conjugated diene block copolymers, and styrene- conjugated diene block copolymer hydrides; fluororesins; acrylic resins such as poly(methyl methacrylate); polyamide resins such as nylon 6 and nylon 66; polyester resins such as polyethylene terephthalate, polyethylene naphthalate, polybutylene terephthalate, and polycyclohexylenedimethylene terephthalate; polycarbonates, polyacetals, polyacrylsulfones, polyarylates, polyacrylates, hydroxybenzoic acid polyesters, polyetherimides, polyester carbonates, polyphenylene ether resins, polyvinyl chloride, polyvinylidene chloride, polyurethane, and porous resins such as foamed polyurethane, foamed polypropylene, or foamed ethylene, etc.), glasses, metals, ceramics, fibers, cloths, knitted fabrics, sheets, papers, yarn, foam, porous substances, and multifilaments. [0124] In some instances, the composition is provided in a microencapsulated formulation (e.g., a nanocapsule). Microencapsulated formulations are mixed with water and sprayed in the same manner as other sprayable formulations. After spraying, the encapsulation shell or coating breaks down and slowly releases the active ingredient. [0125] In some instances, the composition is provided in a liposome. In some instances, the composition is provided in a vesicle. [0126] In some instances, a composition provided herein includes a surfactant. Surfactants, also called wetting agents and spreaders, physically alter the surface tension of a spray droplet. For a Agent Ref: P14419WO00 - 46 - formulation to perform its function properly, a spray droplet must be able to wet the foliage and spread out evenly over a leaf. Surfactants enlarge the area of formulation coverage, thereby increasing exposure to the active agent. Surfactants are particularly important when applying a formulation to waxy or hairy leaves. Without proper wetting and spreading, spray droplets often run off or fail to cover leaf surfaces adequately. Too much surfactant, however, can cause excessive runoff and reduce efficacy. [0127] Surfactants can be classified as anionic, cationic, or nonionic. Formulation activity in the presence of a nonionic surfactant can be quite different from activity in the presence of a cationic or anionic surfactant. Selecting the wrong surfactant can reduce the efficacy of a product and injure the target plant. For example, anionic surfactants are most effective when used with contact pesticides (pesticides that control a pest by direct contact rather than being absorbed systemically). Cationic surfactants are not typically used as stand-alone surfactants because they usually are phytotoxic. [0128] Nonionic surfactants, often used with systemic pesticides, help sprays to penetrate plant cuticles. Nonionic surfactants are compatible with most pesticides, and most EPA-registered pesticides that require a surfactant recommend a nonionic type. Adjuvants include, but are not limited to, stickers, extenders, plant penetrants, compatibility agents, buffers or pH modifiers, drift control additives, defoaming agents, and thickeners. [0129] Among nonlimiting examples of surfactants included in the compositions described herein are alkyl sulfate ester salts, alkyl sulfonates, alkyl aryl sulfonates, alkyl aryl ethers and polyoxyethylenated products thereof, polyethylene glycol ethers, polyvalent alcohol esters and sugar alcohol derivatives. In some embodiments, the surfactant is a nonionic surfactant, a surfactant plus nitrogen source, an organo- silicone surfactant, or a high surfactant oil concentrate. [0130] In formulations and in the use forms prepared from these formulations, the recombinant polynucleotide can, in embodiments, be in a mixture with other active compounds, such as pesticidal agents (e.g., insecticides, sterilants, acaricides, nematicides, molluscicides, or fungicides, attractants, growth-regulating substances, or herbicides). As used herein, the term “pesticidal agent” refers to any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest. A pesticide can be a chemical substance or biological agent used against pests including insects, mollusks, pathogens, weeds, nematodes, and microbes that compete with humans for food, destroy property, spread disease, or are a nuisance. The term “pesticidal agent” further encompasses other bioactive molecules such as antibiotics, antivirals pesticides, antifungals, antihelminthics, nutrients, pollen, sucrose, and/or agents that stun or slow insect movement. [0131] In instances where the recombinant polynucleotide is applied to plants, a mixture with other known compounds, such as herbicides, fertilizers, growth regulators, safeners, semiochemicals, or else with agents for improving plant properties is also possible. [0132] In another aspect, this disclosure is related to a method of producing a modified plant propagule that comprises at least one plant cell comprising a recombinant RNA molecule. The method, in general, includes the steps of: isolating a plant propagule comprising at least one plant cell comprising a Agent Ref: P14419WO00 - 47 - recombinant RNA molecule and a Solemoviridae RNA-dependent RNA polymerase (RdRP) from a mixed population of plant cells comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule, wherein the recombinant RNA molecule comprises, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP; a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP. In certain embodiments, the isolated plant propagule comprising at least one plant cell comprising a recombinant RNA molecule will be free or substantially free of plant cells lacking the recombinant RNA. Such isolated plant propagules which are substantially free of plant cells lacking the recombinant RNA can in certain embodiments comprise plant propagules where at least 75%, 80%, 85%, 90%, 95%, 98%, or 99% of the plant cells in the plant propagule contain the recombinant DNA molecule. In embodiments, the mixed population of plant cells comprise a population of protoplasts or a population of cells in callus, an explant, a plant part, or whole plant. In embodiments, the mixed population of plant cells can comprise a population of plant cells where less than 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5%, or 1% of the plant cells in the population contain the recombinant RNA molecule. In some embodiments, the mixed population of plant cells comprise plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP. In certain embodiments, the plant cells lacking the Solemoviridae RdRP will also lack the recombinant RNA. In other embodiments, the mixed population of plant cells comprise plant cells comprising the Solemoviridae RdRP. In certain embodiments, the plant cells comprising the Solemoviridae RdRP can further comprise the recombinant RNA. In some embodiments, the cargo RNA sequence comprises RNA that encodes a selectable or scorable marker. In embodiments, the mixed population of plant cells is screened or selected for the presence of the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule. In such screens, the mixed population of cells or a portion thereof are subjected to an assay for a screenable marker for the presence of the recombinant RNA molecule (e.g., an RNA sequence diagnostic for presence of the recombinant RNA molecule or a polypeptide encoded by the recombinant RNA molecule) and separated from plant cells lacking the recombinant RNA molecule. In some embodiments, the isolation comprises selecting for the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule. Examples of such selections in instances where the recombinant RNA encodes a selectable marker (e.g., a protein which confers resistance to a selection agent such as an herbicide or antibiotic) can comprise exposing the mixed population of plant cells to a selection agent (e.g., an herbicide or antibiotic) and isolating plant cells which survive exposure to the selection agent. Examples of selectable marker/selection agent combinations include glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzyme/glufosinate, or a neomycin phosphotransferase (npt)/neomycin or kanamycin. In certain embodiments, the selectable or scorable marker is an RNA aptamer (e.g., a Broccoli aptamer) or a regulatory RNA (e.g., an siRNA, siRNA precursor, miRNA, or miRNA precursor, or a phased siRNA or phased siRNA precursor that downregulates expression of an endogenous gene in Agent Ref: P14419WO00 - 48 - the plant, resulting in a detectable phenotype, e.g., bleaching caused by downregulation of a pigment- producing gene). In embodiments, the mixed population is located within a plant or a plant part. In some embodiments, the plant or plant part is screened or selected for presence of the recombinant RNA molecule prior to isolating the plant propagule. In other embodiments, the plant or plant part is screened or selected for systemic presence of the recombinant RNA molecule prior to isolating the plant propagule. In some embodiments, the plant cells, plant, or plant part in the mixed population or that are isolated lack DNA that encodes the recombinant RNA molecule. In embodiments, the plant propagule comprising the recombinant RNA molecule is isolated by detecting the RNA molecule in one or more plant cells comprising the recombinant RNA molecule and separating the one or more plant cells comprising the recombinant RNA molecule from the plant cells lacking the recombinant DNA molecule. In some embodiments, the plant propagule is a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule. In other embodiments, the modified plant propagule is a mosaic comprising both plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP. In certain embodiments, at least 99%, 98%, 95%, 90%, 85%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5%, or 1% of the plant cells in the mosaic can comprise the recombinant RNA molecule. In certain embodiments that are particularly advantageous for at least regulatory reasons, the plant propagule lacks DNA that encodes the recombinant RNA molecule. In embodiments, the modified plant propagule comprises the cell comprising the recombinant RNA molecule, or a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the cell comprising the recombinant RNA molecule. Plant propagules made by any of the aforementioned methods and/or incorporating any of the aforementioned features are also provided herein. [0133] In some embodiments, any of the aforementioned methods can further comprise multiplying the cell, seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus to obtain progeny, wherein the progeny comprise the recombinant RNA molecule. In other embodiments, the multiplying of the cells consists of culturing a plurality of explants obtained from the cell, seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus. In yet other embodiments, the isolated propagule comprises the cell and the aforementioned methods can further comprise regenerating a plant, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the recombinant RNA from the cell. In still other embodiments, the isolated propagule comprises callus and the aforementioned methods can further comprise regenerating a plant, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, or explant comprising the recombinant RNA from said callus. In embodiments, a plant is regenerated and the aforementioned methods can further comprise recovering F1 seed or F1 progeny or clonal progeny comprising the recombinant RNA from the plant. [0134] In another aspect, this disclosure is related to a method of providing a synthetic Solemoviridae satellite RNA to a plant or plant part by grafting one plant part to another plant part. In certain Agent Ref: P14419WO00 - 49 - embodiments, the methods can comprise grafting a scion onto a rootstock comprising any of the aforementioned or otherwise disclosed recombinant DNA molecules and/or recombinant RNA molecules (e.g., a recombinant RNA comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP), wherein at least one cell of the rootstock and/or the scion comprises the Solemoviridae RdRP. In certain embodiments, the scion can comprise a plant shoot, an apical or other meristem, a leaf attached to a petiole, or other plant part and the rootstock can comprise roots and aerial portions of the plant including the main stem, secondary stems, leaves, and/or reproductive structures of the plant, In embodiments, DNA that encodes the recombinant RNA molecule is absent in the scion and/or the rootstock. In embodiments, the scion lacks the recombinant RNA molecule prior to grafting. In embodiments, the rootstock comprises the Solemoviridae RdRP. In some embodiments, the Solemoviridae RdRP is provided by a virus of the family Solemoviridae endemic to the rootstock (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal). In other embodiments, the Solemoviridae RdRP is exogenously provided to the rootstock (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the rootstock or via a recombinant viral vector comprising DNA or RNA encoding the RdRP). In embodiments, the scion comprises the Solemoviridae RdRP. In some embodiments, the RdRP is provided by a virus of the family Solemoviridae endemic to the scion (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal). In other embodiments, the RdRP is exogenously provided to the scion (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the scion or via a recombinant viral vector comprising DNA or RNA encoding the RdRP). In embodiments, the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. In embodiments, the rootstock and/or the scion comprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule. In some embodiments, the rootstock and/or the scion comprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein. [0135] In another aspect, this disclosure is related to a method of producing a grafted plant comprising a recombinant RNA molecule comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by an the Solemoviridae RdRP. In certain embodiments, the recombinant RNA molecule is provided by contacting the scion, the rootstock, or both the scion and the rootstock with a composition comprising the recombinant RNA molecule prior to grafting the scion onto the rootstock to produce the grafted plant. In certain embodiments, at least one cell of the rootstock and/or the scion comprises a Solemoviridae RdRP prior to contacting the scion, the rootstock, or both the scion and the rootstock with the composition. In embodiments, the rootstock comprises the Solemoviridae RdRP. In some embodiments, the Solemoviridae RdRP is provided by a virus of the family Solemoviridae endemic to the rootstock (e.g., a Agent Ref: P14419WO00 - 50 - virus of the family Solemoviridae which is non-pathogenic and/or commensal). In other embodiments, the Solemoviridae RdRP is exogenously provided to the rootstock (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the rootstock or via a recombinant viral vector comprising DNA or RNA encoding the RdRP). In embodiments, the scion comprises the Solemoviridae RdRP. In some embodiments, the RdRP is provided by a virus of the family Solemoviridae endemic to the scion (e.g., a virus of the family Solemoviridae which is non-pathogenic and/or commensal). In other embodiments, the RdRP is exogenously provided to the scion (e.g., via a DNA expression cassette which is integrated into the chromosomal or plastidic DNA of the scion or via a recombinant viral vector comprising DNA or RNA encoding the RdRP). In embodiments, the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. In embodiments, DNA that encodes the recombinant RNA molecule is absent in the scion, the rootstock, and/or the grafted plant. The composition can be provided to the scion, the rootstock, or both the scion and the rootstock according to any of the formulations disclosed herein. In some embodiments, the formulation is a liquid, a gel, or a powder. In some embodiments, the formulation is configured to be sprayed on to the scion, the rootstock, or both the scion and the rootstock; to be injected into the scion, the rootstock, or both the scion and the rootstock; to be soaked into the scion, the rootstock, or both the scion and the rootstock; or to be coated onto the scion, the rootstock, or both the scion and the rootstock. In certain embodiments, the contacting comprises dipping the scion, the rootstock, or both the scion and the rootstock into the composition prior to grafting. [0136] In another aspect, this disclosure is related to a method for producing a plant that transmits any of the aforementioned or otherwise disclosed recombinant RNA molecules provided herein to progeny plants or seed. In certain embodiments, the methods include the steps of: isolating an F1 progeny plant or seed comprising at least one cell comprising a Solemoviridae RNA-dependent RNA polymerase (RdRP) and the recombinant RNA molecule comprising, in 5’ to 3’ order, a 5’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP); a cargo RNA sequence; and a 3’ RNA replication element that is capable of being recognized by the Solemoviridae RdRP from a population of F1 plants or seed obtained from at least one parent plant comprising the recombinant RNA molecule. In embodiments, the parent plant or a part thereof comprising the plant cell is screened or selected for presence of the recombinant RNA molecule prior to isolating the F1 progeny plant or seed. In some embodiments, the parent plant or one or more parts thereof are screened for systemic presence of the recombinant RNA molecule prior to isolating the F1 progeny plants. In other embodiments, floral tissue (e.g., whole flowers or buds, sepal, calyx, or petal), male reproductive tissue (e.g., stamen, anther, or pollen), or female reproductive tissue (e.g., whole fruit, ovary, pericarp, ovule, seed coat, endosperm, or embryo) of the parent plant is screened or selected for presence of the recombinant RNA molecule. In embodiments, F1 seeds are obtained from a parent plant selected for presence of the recombinant RNA molecule in pericarp tissue. In embodiments, the F1 progeny plant or seed comprising the cell is isolated by screening the population of F1 plants or seed obtained from a parent plant for the presence of the Agent Ref: P14419WO00 - 51 - recombinant RNA molecule and propagating the F1 progeny plant or seed comprising the recombinant RNA molecule. In such screens, the progeny plants or seed thereof are subjected to an assay for a screenable marker for the presence of the recombinant RNA (e.g., an RNA sequence diagnostic for presence of the recombinant RNA molecule or a polypeptide encoded by the recombinant RNA molecule) and separated from progeny plants and seed lacking the recombinant RNA progeny plants and seed lacking the recombinant RNA. Such screening assays can be non-destructive assays wherein a portion of the progeny seed or plant is removed and assayed without loss of the viability or ability to propagate the seed or plant tissue comprising the recombinant RNA. In some embodiments, an F1 seed of the parent plant is non-destructively screened for presence of the recombinant RNA molecule. In other embodiments, the F1 seed of the parent plant is non-destructively screened by assaying maternally derived or endosperm tissue of the seed for the presence of the recombinant RNA molecule. Methods for non-destructive assays of seed or other plant tissue which can be adapted for such screens include but are not limited to those disclosed in US patent applications US20220221377 and US20210259176, both incorporated herein by reference in their entireties. In some embodiments, the cargo RNA sequence comprises RNA that encodes a selectable or scorable marker. In some embodiments, the recombinant RNA molecule encodes a selectable marker and the F1 progeny plant or seed comprising the recombinant RNA molecule is isolated by selecting the F1 progeny plant or seed comprising the recombinant RNA molecule for presence of the selectable marker. Examples of such selections in instances where the recombinant RNA encodes a selectable marker (e.g., a protein which confers resistance to a selection agent such as an herbicide or antibiotic) can comprise exposing the progeny seeds or plants to a selection agent (e.g., an herbicide or antibiotic) and isolating progeny seeds or plants which survive exposure to the selection agent. Examples of selectable marker/selection agent combinations include glyphosate-resistant EPSPS enzymes and/or glyphosate oxidases/glyphosate, a bialaphos resistance (bar) or phosphinothricin acyl transferase (pat) enzymes/glufosinate, and neomycin phosphotransferase (npt)/neomycin or kanamycin. In certain embodiments, the selectable or scorable marker is an RNA aptamer or a regulatory RNA. In some embodiments, the F1 progeny plant or seed lacks DNA that encodes the recombinant RNA molecule. In other embodiments, the parent plant lacks DNA that encodes the recombinant RNA molecule. In embodiments, the selected F1 progeny plant transmits the recombinant RNA molecule to at least F2 progeny. In some embodiments, the F1 progeny plant or seed population is obtained from a parent plant used as a pollen recipient. In other embodiments, the F1 progeny plant or seed population is obtained from a parent plant used as a pollen donor. In embodiments, the F1 progeny plant or seed population is obtained by selfing the parent plant. In other embodiments, the F1 progeny plant or seed population is obtained from the sexual crossing of two parent plants. In some embodiments, the parent plant that comprises the recombinant RNA molecule is the female parent plant. In other embodiments, the parent plant that comprises the recombinant RNA molecule is the male parent plant, and the recombinant RNA molecule is transmitted in pollen of the male parent plant. Agent Ref: P14419WO00 - 52 - [0137] In certain embodiments, the methods can further comprise introducing the recombinant RNA molecule or a polynucleotide encoding the recombinant RNA molecule into a plant cell and obtaining the parent plant comprising the recombinant RNA molecule from the plant cell. In embodiments, the recombinant RNA molecule further comprises at least one additional element selected from the group consisting of: (a) at least one RNA encoding a viral movement protein (MP); (b) at least one tRNA-like sequence; and c) an origin-of-assembly sequence (OAS). In embodiments, a parent and/or plant comprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule. In some embodiments, the parent and/or progeny plant comprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein. [0138] In another aspect, this disclosure is related to a method of barcoding a plant, plant cell, progeny thereof, or part thereof. The methods comprise providing to the plant or plant cell any of the aforementioned or otherwise disclosed recombinant RNA molecules provided herein, wherein the cargo RNA of the recombinant RNA molecule comprises a barcode RNA molecule, and wherein the plant or plant cell comprises a Solemoviridae RdRP. In embodiments, the barcode RNA molecule comprises a sequence that uniquely identifies the plant, plant cell, progeny thereof, or part thereof. In some embodiments, the barcode RNA can be a randomly generated sequence. In some embodiments, the barcode RNA molecule comprises a sequence that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. In some embodiments, the barcode RNA molecule comprises a forward primer binding site and a reverse primer binding site for detection of the barcode RNA molecule. In embodiments, the barcode RNA molecule comprises a non- protein coding sequence. In some embodiments, the barcode RNA sequence is up to about 5 kb in length. In some embodiments, the barcode RNA has a length of 10 to 5000 nucleotides, 20 to 1000 nucleotides, or 50 to 500 nucleotides. In certain embodiments, the barcode RNA molecule has a length of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, 800, 850, 900, 950, 1000, 1500, 2000, 2500, 3000, 3500, 4000, 4500, or 5000 nucleotides. In embodiments, the plant transmits the recombinant RNA molecule comprising the barcode RNA to progeny. In some embodiments, the plant, plant cell, progeny thereof, or part thereof lacks DNA that encodes the recombinant RNA molecule. In some embodiments, the methods can further comprise isolating an F1 progeny plant or seed comprising at least one cell comprising the Solemoviridae RdRP and the recombinant RNA molecule. In some embodiments, the F1 progeny plant or seed is obtained from the plant used as a pollen recipient. In other embodiments, the F1 progeny plant or seed is obtained from the plant used as a pollen donor. In embodiments, the F1 progeny plant or seed is obtained by selfing the parent plant. In embodiments, the methods can further comprise propagating the plant or plant cell to obtain a plant part or a plant propagule comprising the barcode RNA molecule. [0139] In another aspect, this disclosure is related to a method of identifying a barcoded plant, plant part, or plant cell. The methods comprise screening for the presence of a barcode RNA molecule in the plant, plant part, or plant cell, wherein the plant, plant part, or plant cell comprises any of the Agent Ref: P14419WO00 - 53 - aforementioned or otherwise disclosed recombinant RNA molecules provided herein, and wherein the cargo RNA of the recombinant RNA molecule comprises the barcode RNA molecule. In certain embodiments, the methods comprise obtaining a nucleic acid sample from the plant, plant part, or plant cell; and detecting the presence of the barcode RNA molecule in the sample. Assays for detection of a barcode RNA include RNA detection assays (e.g., an RT-PCR assay) using nucleic acid probes and/or primers which can detect the barcode RNA and/or sequencing of the barcode RNA. Such screening assays can be non-destructive assays wherein a portion of the seed or plant is removed and assayed without loss of the viability or ability to propagate the seed or plant tissue comprising the barcode RNA. Methods for non-destructive assays of seed or other plant tissue which can be adapted for such screens include but are not limited to those disclosed in US patent applications US20220221377 and US20210259176, both incorporated herein by reference in their entireties. In some embodiments, a seed of the plant is non-destructively screened for presence of the barcode RNA molecule. In other embodiments, a seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus is screened for the presence of the barcode RNA molecule. [0140] In certain optional embodiments, the methods disclosed herein are not processes for modifying the germ line or genetic identity of human beings. In certain optional embodiments, the methods disclosed herein are not processes for modifying the genetic identity of animals which are likely to cause them suffering without any substantial medical benefit to man or animal, and are also not drawn to animals resulting from such processes. In certain optional embodiments, the methods disclosed herein are not methods for treatment of the human or animal body by surgery or therapy. In certain optional embodiments, the cells disclosed herein are not human embryos. In certain optional embodiments, the cells disclosed herein are not the human body or its parts, at the various stages of its formation and development. In certain optional embodiments provided herein, the plant cells, plant propagules (e.g., a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus), and plants provided herein are not produced by an exclusively biological process. In certain optional embodiments provided herein, the methods for producing plant cells, plant propagules (e.g., a seed, seedling, ovule, embryo, pollen, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus), and plants provided herein are not exclusively biological processes. Embodiments [0141] Various embodiments of the compositions, systems, and methods described herein are set forth in the following set of numbered embodiments. [0142] 1. A recombinant RNA molecule comprising from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; Agent Ref: P14419WO00 - 54 - wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked and wherein the cargo RNA molecule is heterologous to the 5’ RNA replication element and the 3’ RNA replication element, optionally wherein: (i) the 5’ RNA replication element and the 3’ RNA replication element are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; (ii) the 5’ RNA replication element, the 3’ RNA replication element, and the RdRP are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another and which are optionally related; or (iii) the 5’ RNA replication element, the 3’ RNA replication element, and/or the RdRP coding region are obtained from different Solemoviridae genomes, and wherein the members of each respective set of the 5’ RNA replication elements, 3’ RNA replication elements, and/or RdRP coding regions have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another. [0143] 2. The recombinant RNA molecule of embodiment 1, wherein: (a) the 5’ RNA replication element comprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; and/or (b) the 3’ RNA replication element comprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 468, 533, or 534. [0144] 3. The recombinant RNA molecule of embodiment 1, wherein: (a) the 5’ RNA replication element comprises an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 467, 531, or 532; or a variant thereof wherein one or more nucleotides in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure; and/or (b) the 3’ RNA replication element comprises an RNA molecule encoded by SEQ ID NO: 468, 533, or 534, or; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 468, 533, or 534; or a variant thereof wherein one of more base-paired residues in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure. [0145] 4. The recombinant RNA molecule of embodiment 3, wherein the RNA secondary structure is maintained by substituting nucleotides in the secondary structure which are not base paired with nucleotides which will not base pair, and/or by substituting nucleotides in the secondary structure which are base paired with nucleotides which will base pair. [0146] 5. The recombinant RNA molecule of any one of embodiments 1 to 4, wherein: (a) the 5’ RNA replication element comprises at least a segment of the 5’ untranslated region (UTR) of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 5’ Agent Ref: P14419WO00 - 55 - RNA replication element further comprises a genomic sequence of the virus of the family Solemoviridae that is natively located 3’ to and adjacent to the 5’ UTR sequence; and/or (b) the 3’ RNA replication element comprises at least a segment of the 3’ UTR of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 3’ RNA replication element further comprises a genomic sequence of the virus of the family Solemoviridae that is natively located 5’ to and adjacent to the 3’ UTR sequence, and optionally wherein the Solemoviridae genome of (a) and (b) are the same. [0147] 6. The recombinant RNA molecule of any one of embodiments 1 to 5, wherein the RNA molecule further comprises at least one of: (i) a tRNA-like element, optionally wherein the tRNA-like element provides for intercellular movement of the RNA and optionally wherein the intercellular movement is mediated by a viral movement protein (MP); (ii) an encapsidation recognition element (ERE), optionally wherein the ERE provides for encapsidation of the RNA by a Solemoviridae capsid protein or optionally wherein the ERE provides for encapsidation of the RNA by a non-Solemoviridae capsid protein; (iii) an RNA effecter; and/or (iv) RNA encoding a viral movement protein (MP), optionally wherein an internal ribosome entry site (IRES) is operably linked to the RNA encoding the MP. [0148] 7. The recombinant RNA molecule of embodiment 6, wherein the tRNA-like element comprises a tRNA-like molecule from an Arabidopsis FT mRNA or is a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 or is a modified tRNA-like sequence that has at least 90% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence, and/or wherein the ERE is a tobacco mosaic virus (TMV) OAS. [0149] 8. The recombinant RNA molecule of embodiment 1, wherein the cargo RNA molecule is up to about 5kb in length. [0150] 9. The recombinant RNA molecule of embodiment 1, wherein the cargo RNA molecule comprises: (a) at least one coding sequence, optionally wherein the coding sequence encodes a selectable or scoreable marker; (b) at least one non-coding sequence; or (c) both at least one coding sequence and at least one non-coding sequence. [0151] 10. The recombinant RNA molecule of embodiment 1, wherein the cargo RNA molecule comprises at least one coding sequence, and wherein the RNA molecule further comprises an internal ribosome entry site (IRES) which is operably linked to at least one coding sequence, optionally wherein the operably linked IRES is located 5’ and immediately adjacent to the coding sequence. [0152] 11. The recombinant RNA molecule of embodiment 1, wherein the cargo RNA molecule comprises at least one non-coding sequence, and wherein the at least one non-coding sequence is a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA molecule Agent Ref: P14419WO00 - 56 - that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a phased RNA or phased RNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA). [0153] 12. The recombinant RNA molecule of embodiment 1, further comprising an RNA comprising encoding at least one ribozyme, optionally wherein the at least one ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. [0154] 13. The recombinant RNA molecule of embodiment 1, further comprising an RNA molecule comprising at least one ligand-responsive ribozyme (aptazyme), optionally wherein the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. [0155] 14. The recombinant RNA molecule of embodiment 1, wherein: (i) the RNA further comprises at least a segment of its reverse complementary RNA molecule; and/or (ii) the recombinant RNA molecule is complexed with one or more RNA binding proteins or encapsidated by a viral capsid protein, optionally wherein the viral capsid protein is a Solemoviridae capsid protein, optionally wherein the RNA comprises an ERE which provides for encapsidation of the RNA by the Solemoviridae capsid protein, and/or optionally wherein the RNA binding proteins comprise an RNA recognition motif. [0156] 15. The recombinant RNA molecule of embodiment 1, wherein the RNA molecule comprises: at least one heterologous RNA virus (HRV) amplicon in sense or antisense orientation to the first 5’ RNA replication element comprising: I. (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked; or II. a heterologous RNA virus (HRV) subgenomic promoter operably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP). [0157] 16. The recombinant RNA of embodiment 15, wherein the RNA molecule comprises from 5’ terminus to 3’ terminus: (a) the 5’ RNA replication element; (b) the HRV amplicon in antisense orientation to the first 5’ RNA replication element; optionally wherein the HRV amplicon further comprises: (i) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to the HRV 5’RR and HRV 3’RR, wherein the RNA molecule encoding the HRV RNA- dependent RNA polymerase (hrvRdRP) is optionally operably linked to a subgenomic promoter recognized by the hrvRdRP; or Agent Ref: P14419WO00 - 57 - (ii) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to linked to a subgenomic promoter recognized by the hrvRdRP; and (c) the 3’ RNA replication element. [0158] 17. The recombinant RNA of embodiment 15 or 16, wherein the HRV 5’RR, HRV 3’RR, and hrvRdRP comprise an HRV 5’RR, HRV 3’RR, and hrvRdRP from a virus selected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae. [0159] 18. The recombinant RNA of embodiment 17, wherein: (i) the Bromovirus is a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus; (ii) the Closterovirus is a Citrus tristeza virus or Beet yellows virus; (iii) the Comovirus is Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus; (iv) the Potexvirus is Potato virus X or Citrus yellow vein clearing virus; (v) the Potyvirus is a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus; (vi) the Tobamovirus is a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus; (vii) the Tombusvirus is a Turnip crinkle virus or Tomato bushy stunt virus; (iv) the Tospoviridae is a Tomato spotted wilt virus or watermelon bud necrosis virus; or (viii) the Tymovirus is a Turnip yellow mosaic virus, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, or Citrus leaf blotch virus. [0160] 19. The recombinant RNA of any one of embodiments 1 to 18, wherein the HRV 5’ RR and the HRV 3’ RR are obtained from the same HRV genome. [0161] 20. The recombinant RNA of any one of embodiments 1 to 18, wherein the HRV 5’ RR and the HRV 3’ RR are obtained from distinct HRV genomes. [0162] 21. The recombinant RNA molecule of any one of embodiments 1 to 20, wherein: a) the HRV 5’ RR comprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 161 to 185, or 186, or comprises the RNA sequence encoded by SEQ ID NO: 161 to 185, or 186, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 161 to 185, or 186; and/or (b) the HRV 3’ RR comprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 187 to 210, or 211, or comprises the RNA sequence of the RNA encoded by SEQ ID NO: 187 to 210, or 211, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 187 to 210, or 211. [0163] 22. The recombinant RNA molecule of any one of embodiments 1 to 20, wherein: (a) the HRV 5’ RR is encoded by a DNA molecule comprising SEQ ID NO: 161 to 185, or 186; or a variant thereof comprising DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 161 to 185, or 186; or a variant thereof wherein one of more residues in Agent Ref: P14419WO00 - 58 - the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure; and/or (b) the HRV 3’ RR is encoded by a DNA molecule comprising SEQ ID NO: 187 to 210, or 211; or a variant thereof comprising DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 187 to 210, or 211; or a variant thereof wherein one of more residues in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure [0164] 23. The recombinant RNA molecule of embodiment 15, wherein: (a) the HRV 3’ RR comprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 3’ RR or the variant is recognized by the hrvRdRP, optionally wherein the RNA comprising the HRV 3’ RR further comprises a genomic sequence of the HRV that is natively located 5’ to and adjacent to the 3’ UTR sequence; and/or (b) the HRV 5’ RR comprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 5’ RR or the variant is recognized by the hrvRdRP, optionally wherein the RNA comprising the HRV 5’ RR further comprises a genomic sequence of the HRV that is natively located 3’ to and adjacent to the 5’ UTR sequence. [0165] 24 The recombinant RNA molecule of any one of embodiments 1 to 23, wherein the cargo RNA comprises an HRV-inhibitory RNA or encodes an HRV-inhibitory protein, wherein the HRV- inhibitory RNA or HRV-inhibitory protein inhibits infection, movement, transmission, and/or replication of the HRV. [0166] 25. The recombinant RNA molecule of any one of embodiments 1 to 24, wherein the cargo RNA comprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV. [0167] 26. The recombinant RNA molecule of any one of embodiments 1 to 24, wherein the cargo RNA comprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV which does not encode the hrvRdRP. [0168] 27. The recombinant RNA molecule of any one of embodiments 1 to 26, wherein: (i) the HRV is a Cucumber Mosaic Virus and the HRV 5’RR comprises an RNA encoded by the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7; Agent Ref: P14419WO00 - 59 - (ii) the HRV is a Brome mosaic virus and the HRV 5’RR comprises an RNA encoded by the Brome mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Brome mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Brome mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Brome mosaic virus 3’ RR DNA sequence in Table 7; (iii) the HRV is a Citrus tristeza virus and the HRV 5’RR comprises an RNA encoded by the Citrus tristeza virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus tristeza virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus RdRP sequence in Table 7; (iv) the HRV is Beet yellows virus and the HRV 5’RR comprises an RNA encoded by the Beet yellows virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Beet yellows virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus RdRP sequence in Table 7; (v) the HRV is Cowpea mosaic virus and the HRV 5’RR comprises an RNA encoded by the Cowpea mosaic virus 5’ RR DNA sequence in Table 7 or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cowpea mosaic virus 5’ RR DNA sequence in Table 7; (vi) the HRV is Potato virus X and the HRV 5’RR comprises an RNA encoded by the Potato virus X 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Potato virus X 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X RdRP sequence in Table 7; (vii) the HRV is Pepper mottle virus and the HRV 5’RR comprises an RNA encoded by the Pepper mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Pepper mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus RdRP sequence in Table 7; Agent Ref: P14419WO00 - 60 - (viii) the HRV is Bean yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Bean yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Bean yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus RdRP sequence in Table 7; (ix) the HRV is Barley stripe mosaic virus and the HRV 5’RR comprises an RNA encoded by the Barley stripe mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Barley stripe mosaic virus 5’ RR DNA sequence in Table 7, and the HRV 3’ RR comprises an RNA encoded by the Barley stripe mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Barley stripe mosaic virus 3’ RR DNA sequence in Table 7; (x) the HRV is Wheat stripe mosaic virus and the HRV 5’RR comprises an RNA encoded by the Wheat stripe mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Wheat stripe mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus RdRP sequence in Table 7; (xi) the HRV Rice yellow mottle virus and the HRV 5’RR comprises an RNA encoded by the Rice yellow mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Rice yellow mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus RdRP sequence in Table 7; (xii) the HRV is Maize dwarf mosaic virus and the HRV 5’RR comprises an RNA encoded by the Maize dwarf mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Maize dwarf mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a Agent Ref: P14419WO00 - 61 - protein having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus RdRP sequence in Table 7; (xiii) the HRV is zucchini yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Zucchini yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Zucchini yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus RdRP sequence in Table 7; (xiv) the HRV is watermelon mosaic virus and the HRV 5’RR comprises an RNA encoded by the Watermelon mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Watermelon mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus RdRP sequence in Table 7; (xv) the HRV is sugarcane mosaic virus and the HRV 5’RR comprises an RNA encoded by the Sugarcane mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Sugarcane mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus RdRP sequence in Table 7; (xvi) the HRV is Tobacco mosaic virus and the HRV 5’RR comprises an RNA encoded by the Tobacco mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tobacco mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus RdRP sequence in Table 7; (xvii) the HRV is Tomato mosaic virus and the HRV 5’RR comprises an RNA encoded by the Tomato mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato mosaic virus 3’ RR DNA sequence in Table 7, or Agent Ref: P14419WO00 - 62 - an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus RdRP sequence in Table 7; (xviii) the HRV is Tomato brown rugose fruit virus and the HRV 5’RR comprises an RNA encoded by the Tomato brown rugose fruit virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato brown rugose fruit virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus RdRP sequence in Table 7; (xix) the HRV is Turnip vein-clearing virus and the HRV 5’RR comprises an RNA encoded by the Turnip vein-clearing virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip vein-clearing virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus RdRP sequence in Table 7; (xx) the HRV is Pepper mild mottle virus and the HRV 5’RR comprises an RNA encoded by the Pepper mild mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Pepper mild mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus RdRP sequence in Table 7; (xxi) the HRV is Turnip crinkle virus and the HRV 5’RR comprises an RNA encoded by the Turnip crinkle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip crinkle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus RdRP sequence in Table 7; (xxii) the HRV is Tomato bushy stunt virus and the HRV 5’RR comprises an RNA encoded by the Tomato bushy stunt virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus 5’ RR DNA sequence in Agent Ref: P14419WO00 - 63 - Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato bushy stunt virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus RdRP sequence in Table 7; (xxiii) the HRV is Tomato spotted wilt virus and the HRV 5’RR comprises an RNA encoded by the Tomato spotted wilt virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato spotted wilt virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus RdRP sequence in Table 7; (xxiv) the HRV is watermelon bud necrosis virus and the HRV 5’RR comprises an RNA encoded by the Watermelon bud necrosis virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Watermelon bud necrosis virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus RdRP sequence in Table 7; (xxv) the HRV is Turnip yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Turnip yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus RdRP sequence in Table 7; (xxvi) the HRV is Spinach latent virus and the HRV 5’RR comprises an RNA encoded by the Spinach latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Spinach latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus RdRP sequence of SEQ ID NO: 510; Agent Ref: P14419WO00 - 64 - (xxvii) the HRV is Spinach latent virus and the HRV 5’RR comprises an RNA encoded by the Spinach latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Spinach latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus RdRP sequence of SEQ ID NO: 511; (xxviii) the HRV is Olive latent virus 2 and the HRV 5’RR comprises an RNA encoded by the Olive latent virus 25’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 25’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Olive latent virus 23’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 23’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 2 RdRP sequence in Table 7; (xxix) the HRV is Citrus yellow vein clearing virus and the HRV 5’RR comprises an RNA encoded by the Citrus yellow vein clearing virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus yellow vein clearing virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus RdRP sequence in Table 7; (xxx) the HRV is Potato latent virus and the HRV 5’RR comprises an RNA encoded by the Potato latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Potato latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus RdRP sequence in Table 7; (xxxi) the HRV is Apple stem grooving virus and the HRV 5’RR comprises an RNA encoded by the Apple stem grooving virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Apple stem grooving virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus RdRP sequence in Table 7; Agent Ref: P14419WO00 - 65 - (xxxii) the HRV is Citrus leaf blotch virus and the HRV 5’RR comprises an RNA encoded by the Citrus leaf blotch virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus leaf blotch virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus RdRP sequence in Table 7; (xxxiii) the HRV is Apple latent spherical virus and the HRV 5’RR comprises an RNA encoded by the Apple latent spherical virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Apple latent spherical virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus RdRP sequence in Table 7; (xxxiv) the HRV is Soybean latent spherical virus and the HRV 5’RR comprises an RNA encoded by the Soybean latent spherical virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Soybean latent spherical virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus RdRP sequence in Table 7; (xxxv) the HRV is Celery latent virus and the HRV 5’RR comprises an RNA encoded by the Celery latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Celery latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus RdRP sequence in Table 7; (xxxvi) the HRV is Black grass varicosavirus-like virus and the HRV 5’RR comprises an RNA encoded by the Black grass varicosavirus-like virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Black grass varicosavirus-like virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus 3’ RR DNA Agent Ref: P14419WO00 - 66 - sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus sequence in Table 7; or (xxxvii) the HRV is Maize suscal virus and the HRV 5’RR comprises an RNA encoded by the Maize suscal virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Maize suscal virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus sequence in Table 7. [0169] 28. The recombinant RNA molecule of any one of embodiments 1 to 27, further comprising an RNA comprising encoding at least one cleavable sequence, optionally wherein the at least one cleavable sequence is located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element. wherein the cleavable sequence is optionally a self-cleaving ribozyme, a self-cleaving inducible ribozyme, or an siRNA or miRNA recognition site. [0170] 29. An agricultural formulation comprising the recombinant RNA molecule of any one of embodiments 1 to 28. [0171] 30. The agricultural formulation of embodiment 29, wherein the recombinant RNA molecule is complexed with one or more RNA binding proteins or encapsidated by a viral capsid protein, optionally wherein the viral capsid protein is a Solemoviridae capsid protein and optionally wherein the RNA comprises an ERE which provides for encapsidation of the RNA by the Solemoviridae capsid protein. [0172] 31. The agricultural formulation of embodiment 30, wherein the RNA binding proteins comprise an RNA recognition motif. [0173] 32. The agricultural formulation of embodiment 30 or 31, wherein the viral capsid protein is heterologous to the virus of the family Solemoviridae. [0174] 33. The agricultural formulation of anyone of embodiments 29 to 32, wherein the formulation comprises the recombinant RNA molecule and a carrier, an excipient, and/or an adjuvant. [0175] 34. A cell comprising the recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cell is a bacterial cell, a fungal cell, a plant cell, an insect cell, or an invertebrate animal cell. [0176] 35. The cell of embodiment 34, wherein the cell is a plant cell and DNA which encodes the recombinant RNA molecule is absent from the cell. [0177] 36. The cell of embodiment 34, wherein the cell comprises a recombinant DNA molecule which encodes the recombinant RNA molecule. [0178] 37. An expression system comprising: (a) an RNA molecule comprising the recombinant RNA molecule of any one of embodiments 1 to 28; and Agent Ref: P14419WO00 - 67 - (b) a cell containing the recombinant RNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements of the recombinant RNA molecule. [0179] 38. The expression system of embodiment 37, wherein the recombinant RNA molecule comprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein, and wherein the cell contains the viral capsid protein. [0180] 39. The expression system of embodiment 37, wherein the encapsidation recognition element (ERE) is a Solemoviridae ERE, wherein the viral capsid protein in the cell is a Solemoviridae capsid protein, and wherein the RNA molecule is encapsidated by the Solemoviridae capsid protein. [0181] 40. The expression system of embodiment 37, 38, or 39, further comprising the reverse complement of the recombinant RNA molecule. [0182] 41. The expression system of any one of embodiments 37 to 40, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell. [0183] 42. The expression system of any one of embodiments 37 to 41, wherein the cell further comprises: (i) a viral capsid protein (CP), (ii) an RNA-binding protein (RBP) that can bind to the RNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; (iv) a second RNA-dependent RNA polymerase (RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the RNA molecule (2nd RdRP); (v) a viral movement protein (MP); (v) a heterologous RNA virus (HRV); or (vi) an hrvRdRP, optionally wherein the hrvRdRP recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter. [0184] 43. The expression system of embodiment 42, wherein the CP, RBP, RdRP, 2nd RdRP, hrvRdRP, and/or the MP is: (a) expressed by a recombinant DNA molecule in the cell; (b) provided exogenously to the cell; (c) expressed by a recombinant RNA molecule in the cell; or (d) expressed by a virus in the cell. [0185] 44. The expression system of embodiment 42, wherein the RdRP, CP, RBP, 2nd RdRP, hrvRdRP, and/or the MP protein is heterologous to the cell. [0186] 45. The expression system of embodiment 42 or 44, wherein the RdRP, 2nd RdRP, or hrvRdRP protein or a polynucleotide encoding the RdRP, 2nd RdRP, or hrvRdRP protein is (a) expressed by a recombinant DNA molecule in the cell; (b) provided exogenously to the cell; (c) expressed by a recombinant RNA molecule in the cell; or (d) expressed by a virus in the cell. [0187] 46. The expression system of any one of embodiments 37 to 45, wherein the cell is a plant cell. [0188] 47. The expression system of embodiment 46, wherein the plant cell contains a virus of the family Solemoviridae which expresses the RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element and/or wherein the plant cell contains an HRV that expresses the 2nd RdRP or hrvRdRP protein. [0189] 48. The expression system of embodiment 47, wherein the virus of the family Solemoviridae occurs naturally in the plant cell. Agent Ref: P14419WO00 - 68 - [0190] 49. A method of providing a synthetic Solemoviridae satellite RNA to a plant, comprising contacting the plant with the recombinant RNA molecule of any one of embodiments 1 to 28. [0191] 50. The method of embodiment 49, wherein contacting comprises spraying, dusting, injecting, or soaking the plant or a part thereof with the recombinant RNA molecule or the formulation. [0192] 51. The method of embodiment 49 or 50, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0193] 52. A method of establishing a synthetic Solemoviridae satellite RNA in a plant cell, comprising: providing to a plant cell the recombinant RNA molecule of any one of embodiments 1 to 28; wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element, wherein the RNA molecule is optionally comprises an ERE and is encapsidated by a capsid protein, whereby the RdRP protein catalyzes synthesis of the synthetic Solemoviridae satellite RNA from the recombinant RNA molecule. [0194] 53. The method of embodiment 52, wherein the plant cell comprises a virus of the family Solemoviridae and wherein the RdRP protein is provided to the plant cell by the virus of the family Solemoviridae. [0195] 54. The method of embodiment 52 or 53, wherein the virus of the family Solemoviridae is endemic to the plant cell, optionally wherein the virus of the family Solemoviridae which is endemic to the plant cell is non-pathogenic and/or commensal. [0196] 55. The method of embodiment 52, 53, or 54, wherein the capsid protein comprises a Solemoviridae capsid protein and the ERE is recognized by the Solemoviridae capsid protein, optionally wherein the recombinant RNA molecule comprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein. [0197] 56. The method of any one of embodiments 52 to 55, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0198] 57. A method of obtaining a phenotypic change in a plant or plant cell, comprising: providing to a plant or plant cell a recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA molecule comprises RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element and catalyzes synthesis of a synthetic Solemoviridae RNA from the recombinant RNA molecule, and wherein the cargo RNA molecule effects the phenotypic change. Agent Ref: P14419WO00 - 69 - [0199] 58. The method of embodiment 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises at least one RNA selected from an siRNA or siRNA precursor, a miRNA or miRNA precursor, and a phased siRNA or phased siRNA precursor. [0200] 59. The method of embodiment 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises a messenger RNA. [0201] 60. The method of embodiment 59, wherein the messenger RNA comprises an RNA molecule absent in the genome of the plant or plant cell. [0202] 61. The method of embodiment 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises an RNA for modifying the genome of the plant or plant cell. [0203] 62. The method of embodiment 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises an RNA for modifying the transcriptome and/or epigenome of the plant or plant cell, optionally wherein the RNA for modifying the epigenome targets an endogenous plant gene for RNA-induced transcriptional silencing. [0204] 63. The method of any one of embodiments 57 to 62, wherein the phenotypic change comprises an increase in the plant’s resistance to a pest or pathogen, optionally wherein the pest or pathogen is selected from the group comprising a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate. [0205] 64. The method of embodiment 63, wherein the pathogen is a heterologous RNA virus (HRV), optionally wherein the HRV is a virus selected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae. [0206] 65. The method of embodiment 64, wherein: (i) the Bromovirus is a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus; (ii) the Closterovirus is a Citrus tristeza virus or Beet yellow virus; (iii) the Comovirus is Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus; (iv) the Potexvirus is Potato virus X or Citrus yellow vein clearing virus; (v) the Potyvirus is a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus; (vi) the Tobamovirus is a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus; (vii) the Tombusvirus is a Turnip crinkle virus or Tomato bushy stunt virus; (iv) the Tospoviridae is a Tomato spotted wilt virus or watermelon bud necrosis virus; or (viii) the Tymovirus is a Turnip yellow mosaic virus, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, or Citrus leaf blotch virus. [0207] 66. The method of any one of embodiments 57 to 62, wherein the phenotypic change comprises an increase in the plant’s resistance to stress, optionally wherein the stress comprises at least one abiotic stress comprising nutrient stress, light stress, water stress, heat stress, and/or cold stress, or Agent Ref: P14419WO00 - 70 - optionally wherein the stress comprises at least one biotic stress comprising crowding, shading, or allelopathy. [0208] 67. The method of any one of embodiments 57 to 66, wherein the recombinant RNA molecule is provided to the plant of plant cell in the form of an RNA, an encapsidated RNA, or a formulation thereof. [0209] 68. The method of embodiment 67, wherein the encapsidated RNA comprises a synthetic Solemoviridae satellite particle. [0210] 69. The method of any one of embodiments 57 to 68, wherein the providing comprises contacting the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof, optionally wherein contacting comprises spraying, dusting, injecting, or soaking the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof. [0211] 70. The method of any one of embodiments 57 to 69, wherein the recombinant RNA further comprises its reverse complementary RNA molecule. [0212] 71. The method of any one of embodiments 57 to 70, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. [0213] 72. A method of manufacturing a synthetic Solemoviridae satellite particle, comprising combining the recombinant RNA molecule of any one of embodiments 1 to 28 with a viral capsid protein, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein. [0214] 73. The method of embodiment 72, wherein the recombinant RNA molecule is combined with a viral capsid protein in a vessel. [0215] 74. The method of embodiment 73, wherein the combining comprises (a) providing to a plant cell the recombinant RNA molecule, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule and a viral capsid protein, wherein the ERE provides for encapsidation of the RNA by the viral capsid protein; and optionally (b) isolating the synthetic Solemoviridae satellite particle from the plant cell, a plant comprising the plant cell, or from media in which the plant cell or the plant had been grown. [0216] 75. The method of embodiment 72, 73, or 74, further comprising the step of formulating the synthetic Solemoviridae satellite particle wherein the formulating comprises combining the synthetic Solemoviridae satellite particle with a carrier, an excipient, and/or an adjuvant. [0217] 76. A plant propagule comprising the recombinant RNA molecule of any one of embodiments 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a Agent Ref: P14419WO00 - 71 - heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. [0218] 77. The plant propagule of embodiment 76, wherein the plant propagule is a seed, a seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, embryo, or callus. [0219] 78. The plant propagule of embodiment 76 or 77, wherein the plant propagule is a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule. [0220] 79. The plant propagule of embodiment 76, 77, or 78, wherein the plant propagule lacks DNA encoding the recombinant RNA molecule. [0221] 80. The plant propagule of any one of embodiments 76 to 79, wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. [0222] 81. A plant comprising the recombinant RNA molecule of any one of embodiments 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. [0223] 82. The plant of embodiment 81, wherein the plant is a monocot or a dicot plant. [0224] 83. The plant of embodiment 81, wherein the plant is of the family Asteraceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Poaceae, or Solanaceae. [0225] 84. The plant of embodiment 81, 82, or 83, wherein the plant lacks DNA that encodes the recombinant RNA molecule. [0226] 85. The plant of any one of embodiments 81 to 84, wherein the plant comprises a virus of the family Solemoviridae, and wherein the Solemoviridae RdRP is provided to the plant cell by the virus of the family Solemoviridae, optionally wherein the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. [0227] 86. The plant of any one of embodiments 81 to 85, wherein the virus of the family Solemoviridae is endemic to the plant, optionally wherein the endemic virus of the family Solemoviridae is non-pathogenic and/or commensal. [0228] 87. The plant of any one of embodiments 81 to 86, wherein the Solemoviridae RdRP, the 5’ RNA replication element, and/or the 3’ RNA replication element are derived from a virus of the family Solemoviridae comprising one or both of the Solemoviridae RdRP, 5’ RNA replication element, and/or 3’ RNA replication elements. [0229] 88. The plant of any one of embodiments 81 to 87, wherein the RdRP has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to SEQ ID NO: 469, 535, or 536, wherein the 5’ RNA replication Agent Ref: P14419WO00 - 72 - element has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 467, 531, or 532, and/or wherein the 3’ RNA replication element has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 468, 533, or 534. [0230] 89. The plant of any one of embodiments 81 to 88, wherein the plant is a grafted plant and wherein the rootstock and/or scion of the grafted plant comprise at least one cell comprising the recombinant RNA and the Solemoviridae RdRP. [0231] 90. The plant of any one of embodiments 81 to 89, wherein the plant is not produced by an essentially biological process. [0232] 91. The plant of any one of embodiments 81 to 90, wherein the plant further comprises a heterologous RNA virus (HRV) RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. [0233] 92. A Solemoviridae satellite system that is self-replicating when introduced into a plant or plant cell, comprising: (a) a recombinant Solemoviridae satellite RNA of any one of embodiments 1 to 28; and (b) an exogenous virus of the family Solemoviridae that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA, optionally wherein the Solemoviridae satellite system further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. [0234] 93. The self-replicating Solemoviridae satellite system of embodiment 92, wherein the exogenous virus of the family Solemoviridae is endemic or native to a different species, variety, or germplasm of plant. [0235] 94. The self-replicating Solemoviridae satellite system of embodiment 92, wherein the Solemoviridae satellite system further comprises a heterologous RNA virus (HRV) RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is optionally an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. [0236] 95. A recombinant DNA molecule comprising a first promoter which is operably linked to DNA encoding the RNA molecule of any one of embodiments 1-28. [0237] 96. A cell comprising the recombinant DNA molecule of embodiment 95, wherein the cell is a bacterial cell, a fungal cell, a plant cell, an insect cell, or an invertebrate animal cell. [0238] 97. A vector for bacterially mediated plant transformation, comprising the recombinant DNA molecule of embodiment 95. Agent Ref: P14419WO00 - 73 - [0239] 98. The vector of embodiment 97, wherein the bacterium that mediates the plant transformation is an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., a Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. and the vector is adapted for transformation with the bacterium. [0240] 99. The vector of embodiment 97 or 98, wherein the bacterium that mediates the plant transformation is an Agrobacterium sp., and wherein the vector further comprises T-DNAs flanking the DNA molecule encoding the recombinant RNA molecule. [0241] 100. The vector of embodiment 97, 98, or 99, contained within a bacterial or plant cell. [0242] 101. An expression system comprising: (a) the recombinant DNA molecule of embodiment 95; and(b) a cell containing the recombinant DNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements encoded by the DNA molecule. [0243] 102. The expression system of embodiment 101, wherein the recombinant DNA molecule further comprises at least one additional element comprising: (i) DNA encoding at least one RNA encoding a viral movement protein (MP); (ii) DNA encoding at least one tRNA-like molecule; (iii) DNA encoding an encapsidation recognition element (ERE); (iv) DNA encoding an RNA comprising, from 5’ to 3’ and operably linked, a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP), the RNA of (i) and optionally an operably linked RNA of (ii) and/or (iii), and a 3’ RNA replication element; (v) DNA encoding an RNA promoter; (vi) DNA encoding an RNA- dependent RNA polymerase (RdRP) that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter; and/or (vii) a DNA molecule comprising a promoter which is operably linked to the DNA encoding the RNA of at least one of (i), (ii), (iii), (iv), (v), or (vi). [0244] 103. The expression system of embodiments 101 or 102, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell, optionally wherein the bacterial cell is an Agrobacterium sp., a Sinorhizobium sp., a Mesorhizobium sp., Bradyrhizobium sp., Rhizobium sp., or an Ensifer sp. cell. [0245] 104. The expression system of any one of embodiment 101, 102, or 103, further comprising: (i) a viral capsid protein that can encapsidate an RNA molecule comprising the encapsidation recognition element (ERE); (ii) an RNA-binding protein (RBP) that can bind to the RNA molecule encoded by the DNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; and/or (iv) an RNA promoter dependent RNA polymerase (RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter recognizes an RNA promoter in the RNA molecule. [0246] 105. The expression system of embodiment 104, wherein the viral capsid protein is: (a) expressed by the recombinant DNA molecule in the cell, (b) co-expressed by a second recombinant DNA molecule in the cell; (c) provided exogenously to the cell; or (d) expressed by a virus in the cell. [0247] 106. The expression system of embodiment 104 or 105, wherein: (i) the capsid protein, viral movement protein (MP), RdRP protein, and/or the RdRP protein that recognizes an HRV 5’ or 3’ Agent Ref: P14419WO00 - 74 - replication region and/or a subgenomic promoter is heterologous to the cell and/or (ii) wherein the RdRP protein or a polynucleotide encoding the RdRP protein is provided exogenously to the cell. [0248] 107. The expression system of any one of embodiments 101 to 106, wherein the cell is a plant cell. [0249] 108. The expression system of 107, wherein the plant cell contains a virus of the family Solemoviridae which expresses the RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element. [0250] 109. The expression system of embodiment 108, wherein the virus of the family Solemoviridae occurs naturally in the plant cell. [0251] 110. The expression system of any one of embodiments 101 to 109, wherein the recombinant DNA molecule further comprises at least one RNA encoding a viral MP, a tRNA-like molecule from an Arabidopsis FT mRNA, and an encapsidation recognition element comprising a TMV-OAS. [0252] 111. An agricultural formulation comprising the expression system of any one of embodiments 101 to 110. [0253] 112. The agricultural formulation of embodiment 111, wherein the formulation comprises the expression system and a carrier, an excipient and/or an adjuvant. [0254] 113. An agricultural formulation comprising the recombinant DNA molecule of embodiment 95. [0255] 114. The agricultural formulation of embodiment 113, wherein the formulation comprises the recombinant DNA molecule and a carrier, an excipient and/or an adjuvant. [0256] 115. A method of producing an exogenous polypeptide in a plant or plant cell, comprising: providing a plant or plant cell comprising the recombinant RNA molecule of any one of embodiments 1 to 28 or the recombinant DNA molecule of embodiment 95, wherein the cargo RNA molecule encoded by the RNA or DNA molecule comprises a translatable messenger RNA encoding the exogenous polypeptide, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element of the recombinant RNA and that catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule, and wherein the exogenous polypeptide is translated from the translatable messenger; optionally wherein the plant or plant cell further comprises a heterologous RNA virus (HRV) RNA promoter dependent RNA polymerase (hrvRdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter recognizes an RNA promoter in the RNA molecule. [0257] 116. The method of embodiment 115, further comprising a first step of providing a population of plants comprising the plant cells comprising the virus of the family Solemoviridae which provides the RdRP protein; and then providing the recombinant RNA or DNA molecule, the cell, the vector. the recombinant RNA or DNA molecule, or formulation thereof to the plants comprising the plant cells and optionally further comprising providing the hrvRdRP to the plants comprising the plant cells. Agent Ref: P14419WO00 - 75 - [0258] 117. The method of embodiment 115 or 111, wherein the virus of the family Solemoviridae is exogenously provided to the plant cell and/or wherein an HRV encoding the hrvRdRP is provided to the plant cell. [0259] 118. The method of embodiment 115, 116, or 117, wherein the RdRP and/or hrvRdRP protein or a recombinant polynucleotide encoding the RdRP and/or hrvRdRP protein is exogenously provided to the plant cell. [0260] 119. The method of any one of embodiments 115 to 118, further comprising a first step of providing a population of plants comprising the plant cells comprising the RdRP protein or the recombinant polynucleotide encoding the RdRP; and then providing the recombinant RNA molecule to the plants comprising the plant cells. [0261] 120. The method of any one of embodiments 115 to 119, wherein the recombinant RNA molecule has been produced in a fermentation system. [0262] 121. The method of any one of embodiments 115 to 120, further comprising the step of determining if the plant cell comprises a virus of the family Solemoviridae which can provide the RdRP. [0263] 122. A method of producing a modified plant propagule that comprises at least one plant cell comprising a recombinant RNA molecule of embodiment 1 to 28, comprising isolating a plant propagule comprising at least one plant cell comprising the recombinant RNA molecule and a Solemoviridae RNA- dependent RNA polymerase (RdRP) from a mixed population of plant cells comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule. [0264] 123. The method of embodiment 122, wherein the mixed population of plant cells comprise a population of protoplasts or a population of cells in callus or an explant. [0265] 124. The method of embodiment 122 or 123, wherein the mixed population of plant cells comprise plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP. [0266] 125. The method of embodiment 122, 123, or 124, wherein the mixed population of plant cells comprise plant cells comprising the Solemoviridae RdRP. [0267] 126. The method of any one of embodiments 122 to 125, wherein the mixed population of plant cells is screened or selected for the presence of the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule. [0268] 127. The method of any one of embodiments 122 to 126, wherein the isolation comprises selecting for the plant cell comprising the recombinant RNA molecule prior to isolating the plant propagule. [0269] 128. The method of any one of embodiments 122 to 127, wherein the mixed population is located within a plant or a plant part. [0270] 129. The method of any one of embodiments 122 to 128, wherein the plant or plant part is screened or selected for presence of the recombinant RNA molecule prior to isolating the plant propagule. Agent Ref: P14419WO00 - 76 - [0271] 130. The method of any one of embodiments 122 to 128, wherein the plant or plant part is screened or selected for systemic presence of the recombinant RNA molecule prior to isolating the plant propagule. [0272] 131. The method of any one of embodiments 122 to 130, wherein the recombinant RNA molecule encodes a selectable marker and the plant propagule comprising the recombinant RNA molecule is isolated by selecting for presence of the selectable marker. [0273] 132. The method of embodiment 131, further comprising selecting a plant propagule comprising a recombinant RNA molecule wherein the selectable marker has been removed. [0274] 133. The method of any one of embodiments 122 to 132, wherein the plant propagule comprising the recombinant RNA molecule is isolated by detecting the RNA molecule in one or more plant cells comprising the recombinant RNA molecule and separating the one or more plant cells comprising the recombinant RNA molecule from the plant cells lacking the recombinant DNA molecule. [0275] 134. The method of any one of embodiments 122 to 133, wherein the plant propagule is a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule. [0276] 135. The method of any one of embodiments 122 to 134, wherein the plant propagule is a mosaic comprising both plant cells comprising the Solemoviridae RdRP and plant cells lacking the Solemoviridae RdRP. [0277] 136. The method of any one of embodiments 122 to 134, wherein the plant propagule lacks DNA that encodes the recombinant RNA molecule. [0278] 137. The method of any one of embodiments 122 to 136, wherein the plant or plant part lacks DNA that encodes the recombinant RNA molecule. [0279] 138. The method of any one of embodiments 122 to 137, wherein the plant propagule comprises the cell, or a seed, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the cell. [0280] 139. The method of embodiment 138, further comprising multiplying the cell, seed, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus to obtain progeny, wherein the progeny comprise the recombinant RNA molecule. [0281] 140. The method of embodiment 139, wherein the multiplying comprises culturing a plurality of explants obtained from the cell, seed, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus. [0282] 141. The method of any one of embodiments 122 to 140, wherein the isolated propagule comprises the cell and the method further comprises regenerating a plant, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, or callus comprising the recombinant RNA from said cell. [0283] 142. The method of any one of embodiments 122 to 140, wherein the isolated propagule comprises callus and the method further comprises regenerating a plant, seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, or explant comprising the recombinant RNA from said callus. Agent Ref: P14419WO00 - 77 - [0284] 143. The method of embodiment 141 or 142, wherein a plant is regenerated and wherein the method further comprises recovering F1 seed or F1 progeny comprising the recombinant RNA from the plant. [0285] 144. A method of providing a synthetic Solemoviridae satellite RNA to a plant comprising: grafting a scion onto a rootstock comprising recombinant RNA molecule of any one of embodiments 1 to 28, wherein at least one cell of the rootstock and/or the scion comprises the Solemoviridae RdRP. [0286] 145. The method of embodiment 144, wherein the scion lacks the recombinant RNA molecule prior to grafting. [0287] 146. The method of embodiment 144 or 145, wherein the rootstock comprises the Solemoviridae RdRP. [0288] 147. The method of any one of embodiments 144 to 146, wherein the RdRP is provided by a virus of the family Solemoviridae endemic to the rootstock, optionally wherein the virus of the family Solemoviridae endemic to the rootstock is non-pathogenic and/or commensal. [0289] 148. The method of any one of embodiments 144 to 146, wherein the RdRP is exogenously provided to the rootstock. [0290] 149. The method of any one of embodiments 144 to 148, wherein the scion comprises the Solemoviridae RdRP. [0291] 150. The method of any one of embodiments 144 to 149, wherein the RdRP is provided by a virus of the family Solemoviridae endemic to the scion and/or wherein the RdRP is exogenously provided to the scion, optionally wherein the virus of the family Solemoviridae endemic to the scion is non-pathogenic and/or commensal. [0292] 151. A method for producing a plant that transmits a recombinant RNA molecule to progeny plants or seed comprising isolating an F1 progeny plant or seed comprising at least one cell comprising a Solemoviridae RNA-dependent RNA polymerase (RdRP) and the recombinant RNA molecule of any one of embodiments 1 to 28 from a population of F1 plants or seed obtained from a parent plant comprising the recombinant RNA molecule. [0293] 152. The method of embodiment 151, wherein the F1 progeny plant or seed comprising the cell is isolated by screening the population of F1 plants or seed obtained from a parent plant for the presence of the recombinant RNA molecule and propagating the F1 progeny plant or seed comprising the recombinant RNA molecule. [0294] 153. The method of embodiment 151, wherein the recombinant RNA molecule encodes a selectable marker and the F1 progeny plant or seed comprising the recombinant RNA molecule is isolated by selecting the F1 progeny plant or seed comprising the recombinant RNA molecule for presence of the selectable marker. [0295] 154. The method of any one of embodiments 151 to 153, wherein the F1 progeny plant or seed lack DNA that encodes the recombinant RNA molecule. Agent Ref: P14419WO00 - 78 - [0296] 155. The method of any one of embodiments 151 to 154, wherein the parent plant lacks DNA that encodes the recombinant RNA molecule. [0297] 156. The method of embodiment 153, wherein the selected F1 progeny plant transmits the recombinant RNA molecule to at least F2 progeny. [0298] 157. The method of any one of embodiments 151 to 156, wherein the F1 progeny plant or seed population is obtained from a parent plant used as a pollen recipient. [0299] 158. The method of any one of embodiments 151 to 156, wherein the F1 progeny plant or seed population is obtained from a parent plant used as a pollen donor. [0300] 159. The method of any one of embodiments 151 to 156, wherein the F1 progeny plant or seed population is obtained by selfing the parent plant. [0301] 160. The method of any one of embodiments 151 to 159, wherein the parent plant or a part thereof comprising the plant cell is screened or selected for presence of the recombinant RNA molecule prior to isolating the F1 progeny plant or seed. [0302] 161. The method of any one of embodiments 151 to 160, wherein the parent plant or one or more parts thereof are screened for systemic presence of the recombinant RNA molecule prior to isolating the F1 progeny plants, optionally wherein the part comprises floral tissue or male or female reproductive tissue. [0303] 162. The method of any one of embodiments 151 to 161, wherein the pericarp of the parent plant is screened or selected for presence of the recombinant RNA molecule. [0304] 163. The method of any one of embodiments 151 to 162, wherein F1 seeds are obtained from a parent plant selected for presence of the recombinant RNA molecule in pericarp tissue. [0305] 164. The method of any one of embodiments 151 to 163, wherein an F1 seed of the parent plant is non-destructively screened for presence of the recombinant RNA molecule. [0306] 165. The method of embodiment 164, wherein the F1 seed of the parent plant is non- destructively screened by assaying maternally derived or endosperm tissue of the seed for the presence of the recombinant RNA molecule. [0307] 166. The method of any one of embodiments 151 to 165, further comprising introducing the recombinant RNA molecule or a polynucleotide encoding the recombinant RNA molecule into a plant cell and obtaining the parent plant comprising the recombinant RNA molecule from the plant cell. [0308] 167. The method of any one of embodiments 151 to 166, wherein the propagule, plant, plant part, scion, and/or rootstock comprises a heterologous viral coat protein which can encapsidate the recombinant RNA molecule and/or comprises the recombinant RNA molecule encapsidated by a heterologous viral coat protein. [0309] 168. A method of barcoding a plant, plant cell, progeny thereof, or part thereof comprising providing to the plant or plant cell the recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA of the recombinant RNA molecule comprises a barcode RNA molecule, and wherein the plant or plant cell comprises a Solemoviridae RNA-dependent RNA polymerase (RdRP). Agent Ref: P14419WO00 - 79 - [0310] 169. The method of embodiment 168, wherein the barcode RNA molecule comprises a sequence that uniquely identifies the plant, plant cell, progeny thereof, or part thereof. [0311] 170. The method of embodiment 168 or 169, wherein the barcode RNA molecule comprises a sequence that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. [0312] 171. The method of any one of embodiments 168 to 170, wherein the barcode RNA molecule comprises a random sequence. [0313] 172. The method of any one of embodiments 168 to 171, wherein the barcode RNA molecule comprises a forward primer binding site and a reverse primer binding site that is not present in the genome and/or transcriptome of a wild-type plant of the same species, a pathogen thereof, or a symbiont thereof. [0314] 173. The method of any one of embodiments 168 to 172, wherein the barcode RNA molecule is up to about 5kb in length. [0315] 174. The method of any one of embodiments 168 to 172, wherein the barcode RNA molecule has a length of 10 to 5000 nucleotides, 20 to 1000 nucleotides, or 50 to 500 nucleotides, optionally wherein the barcode RNA molecule has a length of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, 800, 850, 900, 950, 1000, 1500, 2000, 2500, 3000, 3500, 4000, 4500, or 5000 nucleotides. [0316] 175. The method of any one of embodiments 168 to 174, wherein the barcode RNA molecule comprises a non-protein coding sequence. [0317] 176. The method of any one of embodiments 168 to 174, wherein the plant transmits the recombinant RNA molecule to progeny. [0318] 177. The method of any one of embodiments 168 to 176, wherein the plant, plant cell, progeny thereof, or part thereof lacks DNA that encodes the recombinant RNA molecule. [0319] 178. The method of any one of embodiments 168 to 176, further comprising isolating an F1 progeny plant or seed comprising at least one cell comprising the Solemoviridae RdRP and the recombinant RNA molecule. [0320] 179. The method of embodiment 178, wherein the F1 progeny plant or seed is obtained from the plant used as a pollen recipient. [0321] 180. The method of embodiment 178, wherein the F1 progeny plant or seed is obtained from the plant used as a pollen donor. [0322] 181. The method of embodiment 178, wherein the F1 progeny plant or seed is obtained by selfing the plant. [0323] 182. The method of any one of embodiments 168 to 181, further comprising propagating the plant or plant cell to obtain a plant part or a plant propagule comprising the barcode RNA molecule, optionally wherein said propagule comprises callus, tubers, and/or rootstock. Agent Ref: P14419WO00 - 80 - [0324] 183. A method of identifying a barcoded plant, plant part, or plant cell, the method comprising screening for the presence of a barcode RNA molecule in the plant, plant part, or plant cell, wherein the plant, plant part, or plant cell comprises the recombinant RNA molecule of any one of embodiments 1 to 28, wherein the cargo RNA of the recombinant RNA molecule comprises the barcode RNA molecule. Summary of Tables Table 1. Solemoviridae 5’ and 3’ RNA Replication Element DNA Coding Sequences Description1 SEQ ID NO2 Dot-Bracket structure of encoded RNA Poinsettia latent virus (PnLV) (((((((....)))))))........((((((.(((((.(((..((.....)))))))))).)))))).( SE ID O ... ... .) (( .)) (( .. ... .)) ... .)) u e o e es e wo w e we e e a a ase c o o .gov ucco e.2 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. Table 2. Viral capsid protein and origin of assembly sequences Virus1 Origin of assembly sequence2 Capsid Protein Agent Ref: P14419WO00 - 81 - Virus1 Origin of assembly sequence2 Capsid Protein NC0096421Bll l b i l database accession number for entries in the world wide web internet database “ncbi[dot]nlm[dot]nih.gov/nuccore.” Agent Ref: P14419WO00 - 82 -2 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. Table 3. Movement proteins. VirusMovement protein (MP) Agent Ref: P14419WO00 - 83 - VirusMovement protein (MP) “ncbi[dot]nlm[dot]nih.gov/nuccore.” Table 4. tRNA-like sequences Name1 of Arabidopsis gene containing tRNA-like sequence that can confer cell-to-cell mobilitySequence2 Agent Ref: P14419WO00 - 84 - Name1 of Arabidopsis gene containing tRNA-likeSe 2 sequence that can confer cell-to-cell mobilityquence esource (TAIR) database accession number for entries in the world wide web internet database “arabidopsis.org.” Table 5. IRES sequences IRES source NCBI Accession ID1 Sequence2 Agent Ref: P14419WO00 - 85 - IRES source NCBI Accession ID1 Sequence2 Tobacco mosaic virus SEQ ID NO: 125 y Information database “ncbi[dot]nlm[dot]nih.gov/nuccore.”2 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. Table 6. Intron sequences Intron number Sequence Agent Ref: P14419WO00 - 86 - Intron number Sequence 12 SEQ ID NO: 158 d target viral pathogens RDRP or RDRP or Virus name NCBI 5’ RR 3’ RR Replicase Replicase A i ID1 S2 S2 NT AA Agent Ref: P14419WO00 - 87 - RDRP or RDRP or Virus name NCBI 5’ RR 3’ RR Replicase Replicase Accession ID1 Sequence2 Sequence2 NT AA Agent Ref: P14419WO00 - 88 - RDRP or RDRP or Virus name NCBI 5’ RR 3’ RR Replicase Replicase Accession ID1 Sequence2 Sequence2 NT AA ase “ncbi[dot]nlm[dot]nih.gov/nuccore.”2 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. Table 8. Antiviral Cargo Molecules GeneNCBI or TAIR1 NT AAT Examples of Accession ID SequenceSequencearget Target Crops Agent Ref: P14419WO00 - 89 - GeneNCBI or TAIR1 NT AA Examples of Accession ID SequenceSequenceTarget Target Crops SEQ ID Tobacco etch r accession number for entries in the world wide web internet database “arabidopsis.org.” The other descriptors refer to the National Center for Biotechnology Information database accession number for entries in the world wide web internet database “ncbi[dot]nlm[dot]nih.gov/nuccore.” Agent Ref: P14419WO00 - 90 - Table 9 Insecticidal or Insect Inhibitory and Nematocidal or Nematode Inhibitory Cargo Molecules e name N Examples GenNCBI Accession T AA Cargo ID Sequence SequencetypeTarget of Target Crops Agent Ref: P14419WO00 - 91 - NCBI Accessio NT AA Cargo Examples Gene namen ID Sequence SequencetypeTarget of Target Crops s, Agent Ref: P14419WO00 - 92 - ne nameN NT AA Cargo Examples GeCBI Accession ID Sequence SequencetypeTarget of Target Crops s, s, , , s Agent Ref: P14419WO00 - 93 - meNCBI NT AA Cargo Examples Gene naAccession ID Sequence SequencetypeTarget of Target Crops s, s, er Table 10 Antifungal and Antibacterial Cargo RNAi Targets Cargo (RNAi NCBI AccessionSequence Tar Examples of target target gene)ID1get pathogen crop Agent Ref: P14419WO00 - 94 - Cargo (RNAi NCBI Accession Examples of targ t gene)IDSequence Targe et targe1t pathogen crop SEQ ID tomato tobacco Agent Ref: P14419WO00 - 95 - Cargo (RNAi NCBI Accession Examples of target target gene)ID1Sequence Target pathogen crop PBS2 SEQ ID tomato tobacco er Table 11. Antifungal and antibacterial cargo protein CargoNCBI Accession NT AA Examples of ID1 SequenceSequenceTarget pathogen target crop , Agent Ref: P14419WO00 - 96 - CargoNCBI Accession NT AATarge Examples of ID1 SequenceSequencet pathogen target crop R. solani o, o, s s s o o o o, o, Agent Ref: P14419WO00 - 97 - CargoNCBI Accession NT AA Examples of ID1 SequenceSequenceTarget pathogen target crop SEQ ID SEQ ID P. syringae Citrus tomato, o, o, o, o, o, er Table 12. Resistance Gene NCBI or TAIR Agent Ref: P14419WO00 - 98 - NCBI or TAIR Gene Source Effector Accession NT AA c . ce , ce ce Agent Ref: P14419WO00 - 99 - NCBI or TAIR Gene Source Effector Accession NT AA ce ce , m , m , m Agent Ref: P14419WO00 - 100 - NCBI or TAIR Gene Source Effector Accession NT AA , m , m da da . . . . . . Agent Ref: P14419WO00 - 101 - NCBI or TAIR Gene Source Effector Accession NT AA accession number for entries in the world wide web internet database “arabidopsis.org.” The other descriptors refer to the National Center for Biotechnology Information database accession number for entries in the world wide web internet database “ncbi[dot]nlm[dot]nih.gov/nuccore.” Table 13 Bioactive Plant peptides Peptide name Sequence Function Agent Ref: P14419WO00 - 102 - Peptide name Sequence Function SEQ ID NO: miPEP171d 409 Increase adventitious root formation in grapevine Ribozymes Ribozyme NCBI Accession ID Rfam ID Sequence1 KV767472.1 Agent Ref: P14419WO00 - 103 - Ribozyme NCBI Accession ID Rfam ID Sequence1 Group II self- ASAF01074251.1 splicing intron (11469-11396 RF00029 SEQ ID NO: 434 Table 15 RNA Aptamers RNA aptamer Sequence1 Pepper SEQ ID NO: 439 plated and can be obtained from the RNA sequences prov e . Table 16 Subgenomic promoters Subgenomic promoter Sequence1 Pea earl -brownin virus CP SEQ ID NO: 453 Agent Ref: P14419WO00 - 104 -1 RNA equivalents of the DNA sequences are also contemplated and can be obtained from the DNA sequences provided. *See Goulden et al. (1990) Nucleic Acids Res., 18:4507 – 4512, DOI:10.1093/NAR/18.15.4507. Table 17. Solemoviridae RdRP and CP sequences 5’ RNA Replica RdRP RdRP DNA CP CP DNA Solemoviridae Genome tion Element Protein Coding Protein Coding ce 2 5’ RNA 3’ RNA Solemoviridae Replication Replication RdRP Protein Plant Host EXAMPLES Example 1. An antiviral Solemoviridae satellite with an imbedded HRV amplicon and RNAi cargo [0325] A Solemoviridae satellite (COMSAT) that carries an antiviral inhibitory RNA (RNAi) cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (iii) an antiviral RNA molecule which induces an RNAi response; (iv) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); and (v) and the 3’ RNA replication element from a virus of the family Solemoviridae. [0326] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking a TMV amplicon containing a cargo RNA containing a Pepper mild mottle virus (PMMoV) RNAi inducing Agent Ref: P14419WO00 - 105 - sequence (e.g., a PMMoV sequence which can form a dsRNA) is provided as SEQ ID NO: 473. The elements of the satellite are set forth in Table 19. Table 19. Genetic element Nucleotide position in SEQ ID NO: 473 Solemoviridae 5’ RNA replication element 1-254 protein cargo [0327] A Solemoviridae satellite (COMSAT) with an imbedded HRV amplicon that carries an antiviral protein cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (iii) an antiviral protein cargo; (iv) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); and (v) the 3’ RNA replication element from a virus of the family Solemoviridae. [0328] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking a tobacco mosaic virus (TMV) amplicon containing an antiviral cargo protein containing the coding sequence of the N gene of tobacco is provided as SEQ ID NO: 474. This satellite includes TMV 5’ and 3’ replication region sequences (HRV 5’ and 3’ RR sequences) to promote secondary amplification by TMV, which is a pathogenic tobamovirus. The elements of this satellite are set forth in Table 20. Similar antiviral Solemoviridae satellites are designed for secondary amplification by tomato mosaic virus or tomato brown rugose fruit virus (which are also pathogenic tobamoviruses), and include an N protein (SEQ ID NO:254), L4 protein (SEQ ID NO:256), or a combination of N and L4 protein as the antiviral cargo sequence(s), and replacing the HRV 5’ and 3’ RR sequences of TMV with the HRV 5’ and 3’ RR sequences of either tomato mosaic virus or tomato brown rugose fruit virus as provided in Table 7, to promote secondary amplification by tomato mosaic virus or tomato brown rugose fruit virus, respectively. Solanaceous plants containing the virus of the family Solemoviridae (or that otherwise are provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression) and that are provided with these antiviral Solemoviridae satellites are expected to exhibit resistance to tobacco mosaic virus, tomato mosaic virus, or tomato brown rugose fruit virus, respectively, [0329] Table 20. Agent Ref: P14419WO00 - 106 - Genetic element Nucleotide position in SEQ ID NO: 474 Solemoviridae 5’ RNA replication element 1-254 [0330] A Solemoviridae satellite (COMSAT) that carries an antiviral RNAi cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antiviral RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. [0331] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing a Pepper mild mottle virus (PMMoV) RNAi inducing sequence (e.g., a PMMoV sequence which can form a dsRNA) is provided as SEQ ID NO: 475. The elements of the satellite are set forth in Table 21. Table 21. Genetic element Nucleotide position in SEQ ID NO: 475 ’ Example 4. An antiviral Solemoviridae satellite with antiviral protein cargo [0332] A Solemoviridae satellite (COMSAT) that carries an antiviral protein cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antiviral protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. Agent Ref: P14419WO00 - 107 - [0333] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking an antiviral protein cargo containing the coding sequence of the N gene of tobacco is provided as SEQ ID NO: 476. The elements of the satellite are set forth in Table 22. Table 22. Genetic element Nucleotide position in SEQ ID NO: 476 Solemoviridae 5’ RNA replication element 1-254 antiviral activity [0334] Antiviral COMSATs (e.g., of Examples 1 to 4) or control RNAs (e.g., RNAs lacking the antiviral cargo) are delivered into target host plants, such as pepper or tomato plants via micro- bombardment (“biolistic” delivery) using a “gene gun” or by bacterially mediated (e.g., by Agrobacterium) transient expression. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression. Plants, e.g., pepper and tomato plants, are grown in growth chambers using a long- day photoperiod (16 h light/8 h dark) with light intensity 100-150 micromole, until seedlings are about 2 weeks old. Antiviral COMSATs are prepared either as in vitro transcribed (IVT) products (capped or uncapped), or as Agrobacterium binary vectors. For micro-bombardment, the IVT product is coated onto the surface of gold nanoparticles which are precipitated on inner surface of bullet tubes; these are accelerated at the abaxial surface of the seedlings’ leaves with helium pressure from 100 to 180 psi. For agroinfiltration, COMSAT-carrying clones in binary vector are transformed into Agrobacterium GV 2260, and the transformed Agrobacterium is selected by growing at 28°C under appropriate antibiotic selection. The positive transformants are verified by PCR and then grown at 28°C overnight in liquid LB medium with appropriate antibiotics plus rifamycin to maintain the Agrobacterium and prevent contamination. Agrobacterium cells are collected by centrifugation and resuspended in MMA buffer at an OD600 of 0.2, incubated at 28°C on a shaker for 2 hours, and then infiltrated into the abaxial sides of the leaves. Control plants are treated in the same way but with non-COMSAT control IVT transcripts, or empty buffers. At different timepoints after COMSAT delivery, tissues from systemic leaves (leaves distal to the treated leaves) are collected and subjected to RNA extraction, followed by cDNA syntheses. COMSAT titers are monitored in systemic tissue by qRT-PCR with COMSAT-specific primers. Agent Ref: P14419WO00 - 108 - [0335] The efficacy of an antiviral COMSAT can be tested by challenging the COMSAT-treated plants with the viral pathogen of interest (e.g., cucumber mosaic virus, CMV, or tobacco mosaic virus, TMV), for example, by mechanical infection. Infectious preparations of such acute viral pathogens are prepared in planta (e.g., in Nicotiana benthamiana) or in the form of GFP-fused infectious clones in a binary vector or T7-based vector. Suitable viral inoculums can be prepared as infectious sap extracted from an infected plant, as an Agrobacterium-based inoculum, or as IVT products. The inoculum is introduced into leaves of the COMSAT-treated plants by rub-inoculation (for infectious sap or IVT product), by agroinfiltration (for GFP-fused infectious clone in binary vector), or by micro-bombardment (for IVT product or plasmid of infectious clone in binary vector). For rub-inoculation, the infectious sap or IVT products are respectively diluted 3 times or to 50ng/microliter in phosphate buffer 0.05M, pH 7.4 and dropped onto leaf adaxial surfaces which is pre-dusted with an abrasive such as carborundum or bentonite. The inoculum is gently spread on the abrasive-dusted leaf surface by gloved fingers or cotton buds; after 30 seconds the inoculated leaf is washed with water. Micro-bombardment of infectious clones is conducted following previously described methods (see, e.g., delivery techniques described in PCT/US22/78963; see also Bio-Rad Tech Note 2531 “Inoculation of Viral RNA and cDNA to Potato and Tobacco Plants Using the Helios™ Gene Gun”). Infiltration with an Agrobacterium-based viral pathogen inoculum is performed using methods similar to that used for COMSAT infiltration, but the Agrobacterium suspension is diluted at OD600 of 0.1. [0336] Symptoms and titre of the acute viral pathogens are monitored in the plants, typically over time to confirm progress or decline of viral infection. Effectiveness of antiviral COMSATs is evaluated by comparing relative titres of systemic infected viruses in antiviral COMSAT-treated plants and the control plants. Tissues from inoculated leaves and systemic leaves (distal to the inoculated leaves) are collected, total RNA is extracted, optionally followed by cDNA synthesis. The viral titre can be quantitatively measured by qRT-PCR with virus-specific primers. Viral titre or presence can be measured by immunoassay methods; for example, Tobacco mosaic virus (TMV) is routinely detected in plants using a commercially available strip assay (Agdia ImmunoStrip® for TMV Agdia, Inc., Elkhart, IN, USA). Alternatively, viral titre is qualitatively evaluated by viral disease symptoms, or by proxy measurement (e.g., measuring GFP expressed by GFP-fused viral constructs). Generally, qRT-PCR measurements are normalized to endogenous reference gene controls (e.g., actin, tubulin, ubiquitin-3, GADPH, or translation elongation factor EF1a, used individually or preferably in multiples, e.g., at least 3 reference genes or at least three deltaCt values). The viral titre (as fold changed, relative to an endogenous reference gene) is compared to that of controls. Example 6. An insect inhibitory Solemoviridae satellite with insecticidal protein or RNAi cargo [0337] A Solemoviridae satellite (COMSAT) that carries an insecticidal RNAi cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an insecticidal RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. Agent Ref: P14419WO00 - 109 - [0338] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing an RNAi inducing sequence that targets Colorado potato beetle is provided as SEQ ID NO: 477. The elements of the satellite are set forth in Table 23. Table 23. Genetic element Nucleotide position in SEQ ID NO: 477 Solemoviridae 5’ RNA replication element 1-254 provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an insecticidal protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. [0340] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking an insecticidal cargo protein containing the coding sequence of the Vip3Aa gene is provided as SEQ ID NO: 478. The elements of the satellite are set forth in Table 24. Table 24. Genetic element Nucleotide position in SEQ ID NO: 478 ’ [0341] COMSATS of Table 23 and Table 24 are established in host plants essentially as set forth in Example 5. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression. The efficacy of an insecticidal or insect inhibitory COMSAT can be tested by challenging the COMSAT and control treated plants with the insect of interest (e.g., Colorado Potato Beetle for plants treated with the Agent Ref: P14419WO00 - 110 - Table 23 COMSAT or Fall Armyworm, Corn Earworm, or Black Cutworm for plants treated with the Table 24 COMSAT). Example 7. An antifungal Solemoviridae satellite with antifungal protein or RNAi cargo [0342] A Solemoviridae satellite (COMSAT) that carries an antifungal RNAi cargo is generally provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antifungal RNA molecule which induces an RNAi response; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. [0343] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking a cargo RNA containing an RNAi inducing sequence that targets a DCL gene of Botrytis cinerea is provided as SEQ ID NO: 479. The elements of the satellite are set forth in Table 25. Table 25. Genetic element Nucleotide position in SEQ ID NO: 479 Solemoviridae 5’ RNA replication element 1-254 o e ov ae sa e e a ca es a a u ga p oe ca go s ge e a y provided in 5’ to 3’ orientation as follows: (i) 5' replication element from a virus of the family Solemoviridae; (ii) an antifungal protein cargo; and (iii) the 3’ RNA replication element from a virus of the family Solemoviridae. [0345] A COMSAT comprising Solemoviridae 5’ and 3’ RNA replication elements flanking an antifungal cargo protein containing the coding sequence of the CaAMP1 gene is provided as SEQ ID NO: 480. The elements of the satellite are set forth in Table 26. Table 26. Genetic element Nucleotide position in SEQ ID NO: 480 Agent Ref: P14419WO00 - 111 - Solemoviridae 3’ RNA replication element 2048-2120 COMSAT components synthesized from a commercial vendor. The plasmid is used as a template for a PCR reaction to amplify the expression cassette with a T7 promoter. This PCR product is used as the template for an in vitro transcription reaction using a MEGAscript™ T7 Transcription Kit from Thermo Fisher Scientific. The in vitro synthesized RNA or protein cargo is then transformed into leaves of the target crop using gold nanoparticles from Bio-Rad according to the guidelines specified in the Helios gene gun manual or via Agrobacterium mediated transformation. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression. Replication and persistence of delivered RNA or expression of protein in local leaves can be determined by RT-PCR from local tissues at 2-weeks post transformation. Systemic movement of RNA/protein is determined by qRT-PCR/ELISA in the sample collected from distal untreated leaf samples after 1-, 2-, and 3-months post transformation. [0347] To validate the efficacy of an antifungal COMSAT, in vitro and in vivo fungal bioassays are performed. An antifungal in vitro assay is performed according to Duanis-Assaf et. al. (Plant Biotechnol J.2022 Jan;20(1):226-237). An in vivo detached leaf assay is performed according to Li et. al. (Mol Plant Microbe Interact.2019 Dec;32(12):1649-1664). Disease phenotypes are evaluated in COMSAT and control plants by measuring a lesion area developed by the fungus in inoculated leaves at 3- and 6-days post inoculation. Example 8. Replication of a Solemoviridae COMSAT in tomato [0348] A DNA molecule encoding an RNA molecule containing a 5’ replicase recognition sequence from a virus of the family Solemoviridae; Dicer-like 1 and Dicer-like 2 fragments; ZmHSP101 IRES; TMV movement protein; Flowering Locus T from Arabidopsis thaliana; and a 3’ replicase recognition sequence from a virus of the family Solemoviridae is cloned into a pUC19 plasmid backbone and transformed into E. coli DH5a (New England Biolabs; C2987I). The plasmid is extracted, and PCR is used to add a T7 promotor sequence. In-vitro transcription is used to synthesize the RNA molecule, which is then purified using a Monarch® RNA Cleanup Kit (50 µg) (New England Biolabs; T2040L). RNA quantity is measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific; 840274200) and the intactness is quality controlled through gel electrophoresis. The synthesized and quality- controlled RNA molecule is precipitated onto gold nanoparticles and fired into 4-week-old Solanum lycopersicum ‘Early Girl’ leaves using a gene gun. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression. The following leaf tissue samples are collected at 2-week and 4-week timepoints: fired leaf # 1; fired leaf # 2; nearest adjacent leaflet; opposite leaf, terminal leaflet; and apical leaf growth. RNA is extracted with the MagMax Plant RNA Isolation kit (Thermo Fisher Scientific; A33899) and quality controlled through Nanodrop spectrophotometer (Thermo Fisher Scientific; Agent Ref: P14419WO00 - 112 - 840274200) and RNA ScreenTape Analysis (Agilent; 5067-5576). Quality controlled RNA is used for cDNA synthesis and qRT-PCR is used to amplify the Dicer-like 1 and Dicer-like 2 fragments and reference gene fragment RPL2. Delta-delta Ct analysis is applied to determine LogFold change of the Solemoviridae COMSAT in the tissues compared to non-Solemoviridae COMSAT containing Solanum lycopersicum ‘Early Girl’ plant tissues. Example 9. Solemoviridae COMSAT with cDNA-based cargo expression amplification [0349] A DNA molecule encoding an RNA molecule containing a 5' replicase recognition sequence from a virus of the family Solemoviridae; Cricket paralysis virus IRES; Avian leukosis virus reverse transcriptase; tRNA primer binding site (tRNA-trp); CaMV 35s promoter; a protein cargo (Superfolder GFP); ZmHSP101 IRES; Tobacco mosaic virus movement protein; and a 3' replicase recognition site from a virus of the family Solemoviridae is cloned into a pUC19 plasmid backbone and is transformed into E. coli DH5a (New England Biolabs; C2987I). The plasmid is extracted, and PCR is used to add a T7 promotor sequence. In-vitro transcription is used to synthesize the RNA molecule, which is then purified using a Monarch® RNA Cleanup Kit (50 µg) (New England Biolabs; T2040L). RNA quantity is measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific; 840274200) and the intactness is quality controlled through gel electrophoresis. The synthesized and quality-controlled RNA molecule is precipitated onto gold nanoparticles and fired into the 4-week-old Solanum lycopersicum ‘Early Girl’ leaves using a gene gun. Host plants lacking the appropriate endogenous Solemoviridae virus can otherwise be provided with the appropriate Solemoviridae RdRP, e.g., through transient or transgenic expression. The following leaf tissue samples are collected at 2-week and 4-week timepoints: fired leaf # 1; fired leaf # 2; nearest adjacent leaflet; opposite leaf, terminal leaflet; and apical leaf growth. Protein (GFP) expression is quantified. Total protein is extracted from collected tissue samples using the Pierce Plant Total Protein Extraction Kit (Thermo Fisher Scientific; A44056) according to manufacturer specifications, and quality controlled by Nanodrop spectrophotometry paired with SDS-PAGE to verify extraction yield and integrity. GFP expression is quantified relative to other protein expressing COMSAT designs as well as non-transfected tissue using the Abcam GFP ELISA Kit (ab171581) according to manufacturer specifications. Example 10. Detection of Satellite RNA Negative-Sense Strand [0350] Where only the positive-sense strand of a satellite RNA is provided to a plant or plant cell (e.g., by delivery of the RNA itself or by delivery of DNA encoding the satellite RNA’s positive-sense strand), replication of the satellite RNA in a plant or plant cell can be verified by detection of the satellite RNA’s negative-sense strand, for example, by using a negative-sense-strand-specific Taqman® assay. [0351] In one non-limiting example, a bell pepper endornavirus (BPEV) satellite of 2357 nucleotides was constructed with the following elements in 5’ to 3’ orientation: (1) BPEV 5’ RNA replication element; (2) a first IRES sequence (PSVI IRES, with the addition of spacer nucleotides on the 5’ end); (3) RNA encoding a reporter protein (GFP); (4) a second IRES sequence (ZmHSP IRES); (5) a viral movement protein; (6) a zebrafish sequence (as a non-plant heterologous sequence for detection Agent Ref: P14419WO00 - 113 - purposes); (7) an isoleucine tRNA sequence; (8) BPEV 3’ RNA replication element. Similar satellites can be designed and constructed using alternative genetic elements such as those provided elsewhere in this specification, such as pairs of 5’ and 3’ RNA replication elements from a virus of the family Solemoviridae, other pairs of HRV 5’ and 3’ replication region sequences, and/or other cargo sequences. [0352] The positive-sense strand of this BPEV satellite RNA was produced through in vitro transcription (IVT), and biolistically delivered with a gene gun into bell pepper plant leaves. Samples were collected 4 weeks post gene gun firing from a local leaf. Samples underwent RNA extraction, library prep, and Illumina® sequencing using a stranded kit (NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina®, catalogue numbers E7760S or E7760L, New England Biolabs, Ipswich, MA) with added DMSO treatment and plant rRNA depletion. Reads were mapped against the BPEV satellite RNA’s sequence and negative-sense reads were identified using the Integrative Genomics Viewer (IGV) browser (Robinson et al., Bioinformatics, 39(1) (2023), btac830). Negative-sense reads mapping to the BPEV satellite were present, confirming that replication of the BPEV satellite had occurred. BPEV negative-sense reads were also identified in BPEV infected plants that served as a positive control for the analysis. The above-described negative strand detection method can be adapted for use in the detection of negative strand RNA produced by Solemoviridae satellite RNAs disclosed herein. Example 11. Detection of Satellite RNA Negative-Sense Strand in a Tomato Plant [0353] Where only the positive-sense strand of a satellite RNA is provided to a plant or plant cell (e.g., by delivery of the RNA itself or by delivery of DNA encoding the satellite RNA’s positive-sense strand), replication of the satellite RNA in a plant or plant cell can be verified by detection of the satellite RNA’s negative-sense strand, for example, by using a negative-sense-strand-specific Taqman® assay. [0354] Negative sense GFP produced by a satellite RNA containing a GFP protein encoding cargo sequences is detected using Taqman® qPCR. The reference sequence that can be used is an endogenous tomato gene, “Tom06” (NCBI Gene ID 101249087, ubiquitin-conjugating enzyme E2-17 kDa, from Solanum lycopersicum). Taqman® qPCR primers and probes were ordered from Integrated DNA Technology, Coralville, IA. The negative sense GFP probe is labeled with FAM520 dye with ZEN/Iowa Black FQ quencher. The Tom06 probe is labeled with SUN5544 with ZEN/Iowa Black FQ quencher. Primers and probes are used at a 2:1 ratio. cDNA is synthesized via reverse transcription (30 minutes at 60 degrees C, then 10 minutes at 85 degrees C) using Maxima H Minus First Strand cDNA synthesis Kit (K1652, Thermo Fisher Scientific, Waltham MA) using the RT primers in Table 27. This was followed by treatment (30 minutes at 37 degrees C, then 1 minute at 80 degrees C) with thermolabile exonuclease I (E1050 New England Biolabs, Ipswich MA) to remove excess RT primers. The synthesized cDNA was cleaned with RNAClean XP beads (A63987 Beckman Coulter, Brea CA). Taqman® qPCR is carried out using Taqman® Advanced Fast MasterMix (444965, Thermo Fisher Scientific, Waltham MA) and the primer and probes in Table 28. Table 27. Description of Reverse Transcriptase Primers Agent Ref: P14419WO00 - 114 - RT Primer Name RT Primer Sequence negative GFP reverse transcription primer ccactggattcagcgaagttATGCGTAAAGGCGAAGAG
Agent Ref: P14419WO00 - 115 - Table 29. Description of qPCR primers and probes qPCR Primer/Probe Name qPCR Primer/Probe Sequence negative GFP forward primer CCACTGGATTCAGCGAAGT (SEQ ID NO: 630) k Q/ [0355] Although the foregoing disclosure has been described in some detail by way of illustration and example for purposes of clarity of understanding, the descriptions and examples should not be construed as limiting the scope of the disclosure. [0356] Other embodiments are within the claims.
Claims
Agent Ref: P14419WO00 - 116 - WHAT IS CLAIMED IS: 1. A recombinant RNA molecule comprising from 5’ terminus to 3’ terminus: (a) a 5’ RNA replication element recognized by a Solemoviridae RNA-dependent RNA polymerase (RdRP); (b) a cargo RNA molecule; and (c) a 3’ RNA replication element recognized by the RdRP; wherein the 5’ RNA replication element, the cargo RNA molecule, and the 3’ RNA replication element are operably linked; wherein the cargo RNA molecule is heterologous to the 5’ RNA replication element and the 3’ RNA replication element; and, optionally, wherein: (i) the 5’ RNA replication element and the 3’ RNA replication element are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another; (ii) the 5’ RNA replication element, the 3’ RNA replication element, and the RdRP are obtained from the same Solemoviridae genome or from Solemoviridae genomes having at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another; or (iii) the 5’ RNA replication element, the 3’ RNA replication element, and/or the RdRP coding region are obtained from different Solemoviridae genomes, and wherein the members of each respective set of the 5’ RNA replication elements, 3’ RNA replication elements, and/or the RdRP coding regions have at least 85%, 90%, 95%, 98%, or 99% sequence identity to one another. 2. The recombinant RNA molecule of claim 1, wherein: (a) the 5’ RNA replication element comprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; and/or (b) the 3’ RNA replication element comprises at least one RNA secondary structure provided in Table 1 or adopted by an RNA molecule encoded by SEQ ID NO: 468, 533, or 534. 3. The recombinant RNA molecule of claim 1, wherein: (a) the 5’ RNA replication element comprises an RNA molecule encoded by SEQ ID NO: 467, 531, or 532; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 467, 531, or 532; or a variant thereof wherein one or more nucleotides in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure; and/or (b) the 3’ RNA replication element comprises an RNA molecule encoded by SEQ ID NO: 468, 533, or 534, or; a variant thereof encoded by a DNA molecule having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 468, 533, or 534; or a variant thereof wherein one of more base-paired residues in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure. Agent Ref: P14419WO00 - 117 - 4. The recombinant RNA molecule of claim 3, wherein the RNA secondary structure is maintained by substituting nucleotides in the secondary structure which are not base paired with nucleotides which will not base pair, and/or by substituting nucleotides in the secondary structure which are base paired with nucleotides which will base pair. 5. The recombinant RNA molecule of claim 1, wherein: (a) the 5’ RNA replication element comprises at least a segment of the 5’ untranslated region (UTR) of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 5’ RNA replication element further comprises a genomic sequence of the virus of the family Solemoviridae that is natively located 3’ to and adjacent to the 5’ UTR sequence; and/or (b) the 3’ RNA replication element comprises at least a segment of the 3’ UTR of the Solemoviridae genome or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the variant is recognized by the RdRP, optionally wherein the 3’ RNA replication element further comprises a genomic sequence of the virus of the family Solemoviridae that is natively located 5’ to and adjacent to the 3’ UTR sequence, and optionally wherein the Solemoviridae genome of (a) and (b) are the same. 6. The recombinant RNA molecule of claim 1, wherein the RNA molecule further comprises at least one of: (i) a tRNA-like element, optionally wherein the tRNA-like element provides for intercellular movement of the RNA and optionally wherein the intercellular movement is mediated by a viral movement protein (MP); (ii) an encapsidation recognition element (ERE), optionally wherein the ERE provides for encapsidation of the RNA by a Solemoviridae capsid protein or optionally wherein the ERE provides for encapsidation of the RNA by a non-Solemoviridae capsid protein; (iii) an RNA effecter; and/or (iv) RNA encoding a viral movement protein (MP), optionally wherein an internal ribosome entry site (IRES) is operably linked to the RNA encoding the MP. 7. The recombinant RNA molecule of claim 6, wherein the tRNA-like element comprises a tRNA-like molecule from an Arabidopsis FT mRNA or is a tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 or is a modified tRNA-like sequence that has at least 90% sequence identity to a scaffold tRNA-like sequence encoded by a DNA sequence selected from the group consisting of SEQ ID NOs: 76-123, and 466 and that maintains the secondary structure of the scaffold tRNA-like sequence, and/or wherein the ERE is a tobacco mosaic virus (TMV) OAS. 8. The recombinant RNA molecule of claim 1, wherein the cargo RNA molecule is up to about 5kb in length. Agent Ref: P14419WO00 - 118 - 9. The recombinant RNA molecule of claim 1, wherein the cargo RNA molecule comprises: (a) at least one coding sequence, optionally wherein the coding sequence encodes a selectable or scoreable marker; (b) at least one non-coding sequence; or (c) both at least one coding sequence and at least one non-coding sequence. 10. The recombinant RNA molecule of claim 1, wherein the cargo RNA molecule comprises at least one coding sequence, and wherein the RNA molecule further comprises an internal ribosome entry site (IRES) which is operably linked to at least one coding sequence, optionally wherein the operably linked IRES is located 5’ and immediately adjacent to the coding sequence. 11. The recombinant RNA molecule of claim 1, wherein the cargo RNA molecule comprises at least one non-coding sequence, and wherein the at least one non-coding sequence is a hairpin RNA (hpRNA); an RNA that forms multiple stem-loops; an RNA pseudoknot; an RNA molecule that forms at least partially double-stranded RNA; a small interfering RNA (siRNA) or siRNA precursor; a microRNA (miRNA) or miRNA precursor; a phased RNA or phased RNA precursor; a ribozyme; a ligand-responsive ribozyme (aptazyme); an RNA aptamer; or a long noncoding RNA (lncRNA). 12. The recombinant RNA molecule of claim 1, further comprising an RNA comprising encoding at least one ribozyme, optionally wherein the at least one ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. 13. The recombinant RNA molecule of claim 1, further comprising an RNA molecule comprising at least one ligand-responsive ribozyme (aptazyme), optionally wherein the at least one ligand-responsive ribozyme is located 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element. 14. The recombinant RNA molecule of claim 1, wherein: (i) the RNA further comprises at least a segment of its reverse complementary RNA molecule; and/or (ii) the recombinant RNA molecule is complexed with one or more RNA binding proteins or encapsidated by a viral capsid protein, optionally wherein the viral capsid protein is a Solemoviridae capsid protein, optionally wherein the RNA comprises an ERE which provides for encapsidation of the RNA by the Solemoviridae capsid protein, and/or optionally wherein the RNA binding proteins comprise an RNA recognition motif. 15. The recombinant RNA molecule of claim 1, wherein the RNA molecule comprises: at least one heterologous RNA virus (HRV) amplicon in sense or antisense orientation to the first 5’ RNA replication element comprising: Agent Ref: P14419WO00 - 119 - I. (i) a heterologous RNA virus (HRV) 5’ replication region (HRV 5’RR); (ii) the cargo RNA molecule; and (iii) the heterologous RNA virus (HRV) 3’ RNA replication region (HRV 3’RR); wherein the HRV 5’ RR and HRV 3’ RR HRV are recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP); and wherein the HRV 5’RR, cargo RNA molecule, and HRV 3’RR are operably linked; or II. a heterologous RNA virus (HRV) subgenomic promoter operably linked to the cargo RNA molecule; wherein the subgenomic promoter is recognized by a heterologous RNA virus RNA-dependent RNA polymerase (hrvRdRP). 16. The recombinant RNA of claim 15, wherein the RNA molecule comprises from 5’ terminus to 3’ terminus: (a) the 5’ RNA replication element; (b) the HRV amplicon in antisense orientation to the first 5’ RNA replication element; optionally wherein the HRV amplicon further comprises: (i) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to the HRV 5’RR and HRV 3’RR, wherein the RNA molecule encoding the HRV RNA-dependent RNA polymerase (hrvRdRP) is optionally operably linked to a subgenomic promoter recognized by the hrvRdRP; or (ii) an RNA molecule encoding an HRV RNA-dependent RNA polymerase (hrvRdRP) which is operably linked to linked to a subgenomic promoter recognized by the hrvRdRP; and (c) the 3’ RNA replication element. 17. The recombinant RNA of claim 15, wherein the HRV 5’RR, HRV 3’RR, and hrvRdRP comprise an HRV 5’RR, HRV 3’RR, and hrvRdRP from a virus selected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae. 18. The recombinant RNA of claim 17, wherein: (i) the Bromovirus is a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus; (ii) the Closterovirus is a Citrus tristeza virus or Beet yellow virus; (iii) the Comovirus is Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus; (iv) the Potexvirus is Potato virus X or Citrus yellow vein clearing virus; (v) the Potyvirus is a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus; (vi) the Tobamovirus is a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus; (vii) the Tombusvirus is a Turnip crinkle virus or Tomato bushy stunt virus; (iv) the Tospoviridae Agent Ref: P14419WO00 - 120 - is a Tomato spotted wilt virus or watermelon bud necrosis virus; or (viii) the Tymovirus is a Turnip yellow mosaic virus, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, or Citrus leaf blotch virus. 19. The recombinant RNA of claim 15, wherein the HRV 5’ RR and the HRV 3’ RR are obtained from the same HRV genome. 20. The recombinant RNA of claim 15, wherein the HRV 5’ RR and the HRV 3’ RR are obtained from distinct HRV genomes. 21. The recombinant RNA molecule of claim 15, wherein: (a) the HRV 5’ RR comprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 161 to 185, or 186, or comprises the RNA sequence encoded by SEQ ID NO: 161 to 185, or 186, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 161 to 185, or 186; and/or (b) the HRV 3’ RR comprises at least one RNA secondary structure adopted by an RNA encoded by SEQ ID NO: 187 to 210, or 211, or comprises the RNA sequence encoded by SEQ ID NO: 187 to 210, or 211, or comprises a contiguous fragment of at least 80%, 85%, 90%, or 95% of the full sequence of the RNA encoded by SEQ ID NO: 187 to 210, or 211. 22. The recombinant RNA molecule of claim 15, wherein: (a) the HRV 5’ RR is encoded by a DNA molecule comprising SEQ ID NO: 161 to 185, or 186 ; or a variant thereof comprising DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 161 to 185, or 186 ; or a variant thereof wherein one of more residues in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure; and/or (b) the HRV 3’ RR is encoded by a DNA molecule comprising SEQ ID NO: 187 to 210, or 211; or a variant thereof comprising DNA having at least 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% sequence identity to SEQ ID NO: 187 to 210, or 211; or a variant thereof wherein one of more residues in the RNA secondary structure are substituted with distinct nucleotides which maintain the RNA secondary structure. 23. The recombinant RNA molecule of claim 15, wherein: (a) the HRV 3’ RR comprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 3’ RR or the variant is recognized by the Agent Ref: P14419WO00 - 121 - hrvRdRP, optionally wherein the RNA comprising the HRV 3’ RR further comprises a genomic sequence of the HRV that is natively located 5’ to and adjacent to the 3’ UTR sequence; and/or (d) the HRV 5’ RR comprises an RNA molecule containing at least a segment of the 3’ untranslated region (UTR) of the HRV genome, or a variant thereof having one or more nucleotide substitutions, insertions, and/or deletions, wherein the HRV 5’ RR or the variant is recognized by the hrvRdRP, optionally wherein the RNA comprising the HRV 5’ RR further comprises a genomic sequence of the HRV that is natively located 3’ to and adjacent to the 5’ UTR sequence. 24 The recombinant RNA molecule of claim 15, wherein the cargo RNA comprises an HRV-inhibitory RNA or encodes an HRV-inhibitory protein, wherein the HRV-inhibitory RNA or HRV-inhibitory protein inhibits infection, movement, transmission, and/or replication of the HRV. 25. The recombinant RNA molecule of claim 15, wherein the cargo RNA comprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV. 26. The recombinant RNA molecule of claim 15, wherein the cargo RNA comprises an RNA having at least 20 contiguous nucleotides having an identical or complementary sequence to a segment of equivalent length of the genomic RNA of the HRV which does not encode the hrvRdRP. 27. The recombinant RNA molecule of claim 15, wherein: (i) the HRV is a Cucumber Mosaic Virus and the HRV 5’RR comprises an RNA encoded by the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cucumber Mosaic Virus 3’ RR DNA sequence in Table 7; (ii) the HRV is a Brome mosaic virus and the HRV 5’RR comprises an RNA encoded by the Brome mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Brome mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Brome mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Brome mosaic virus 3’ RR DNA sequence in Table 7; (iii) the HRV is a Citrus tristeza virus and the HRV 5’RR comprises an RNA encoded by the Citrus tristeza virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus tristeza virus 3’ RR DNA sequence in Table 7, or Agent Ref: P14419WO00 - 122 - an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus tristeza virus RdRP sequence in Table 7; (iv) the HRV is Beet yellows virus and the HRV 5’RR comprises an RNA encoded by the Beet yellows virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Beet yellows virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Beet yellows virus RdRP sequence in Table 7; (v) the HRV is Cowpea mosaic virus and the HRV 5’RR comprises an RNA encoded by the Cowpea mosaic virus 5’ RR DNA sequence in Table 7 or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Cowpea mosaic virus 5’ RR DNA sequence in Table 7; (vi) the HRV is Potato virus X and the HRV 5’RR comprises an RNA encoded by the Potato virus X 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Potato virus X 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Potato virus X RdRP sequence in Table 7; (vii) the HRV is Pepper mottle virus and the HRV 5’RR comprises an RNA encoded by the Pepper mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Pepper mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mottle virus RdRP sequence in Table 7; (viii) the HRV is Bean yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Bean yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Bean yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Bean yellow mosaic virus RdRP sequence in Table 7; (ix) the HRV is Barley stripe mosaic virus and the HRV 5’RR comprises an RNA encoded by the Barley stripe mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least Agent Ref: P14419WO00 - 123 - 85%, 90%, 95%, or 99% sequence identity to the Barley stripe mosaic virus 5’ RR DNA sequence in Table 7, and the HRV 3’ RR comprises an RNA encoded by the Barley stripe mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Barley stripe mosaic virus 3’ RR DNA sequence in Table 7; (x) the HRV is Wheat stripe mosaic virus and the HRV 5’RR comprises an RNA encoded by the Wheat stripe mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Wheat stripe mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Wheat stripe mosaic virus RdRP sequence in Table 7; (xi) the HRV Rice yellow mottle virus and the HRV 5’RR comprises an RNA encoded by the Rice yellow mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Rice yellow mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Rice yellow mottle virus RdRP sequence in Table 7; (xii) the HRV is Maize dwarf mosaic virus and the HRV 5’RR comprises an RNA encoded by the Maize dwarf mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Maize dwarf mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Maize dwarf mosaic virus RdRP sequence in Table 7; (xiii) the HRV is zucchini yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Zucchini yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Zucchini yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Zucchini yellow mosaic virus RdRP sequence in Table 7; Agent Ref: P14419WO00 - 124 - (xiv) the HRV is watermelon mosaic virus and the HRV 5’RR comprises an RNA encoded by the Watermelon mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Watermelon mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon mosaic virus RdRP sequence in Table 7; (xv) the HRV is sugarcane mosaic virus and the HRV 5’RR comprises an RNA encoded by the Sugarcane mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Sugarcane mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Sugarcane mosaic virus RdRP sequence in Table 7; (xvi) the HRV is Tobacco mosaic virus and the HRV 5’RR comprises an RNA encoded by the Tobacco mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tobacco mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tobacco mosaic virus RdRP sequence in Table 7; (xvii) the HRV is Tomato mosaic virus and the HRV 5’RR comprises an RNA encoded by the Tomato mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato mosaic virus RdRP sequence in Table 7; (xviii) the HRV is Tomato brown rugose fruit virus and the HRV 5’RR comprises an RNA encoded by the Tomato brown rugose fruit virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato brown rugose fruit virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus 3’ RR DNA sequence in Agent Ref: P14419WO00 - 125 - Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato brown rugose fruit virus RdRP sequence in Table 7; (xix) the HRV is Turnip vein-clearing virus and the HRV 5’RR comprises an RNA encoded by the Turnip vein-clearing virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip vein-clearing virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip vein-clearing virus RdRP sequence in Table 7; (xx) the HRV is Pepper mild mottle virus and the HRV 5’RR comprises an RNA encoded by the Pepper mild mottle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Pepper mild mottle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Pepper mild mottle virus RdRP sequence in Table 7; (xxi) the HRV is Turnip crinkle virus and the HRV 5’RR comprises an RNA encoded by the Turnip crinkle virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip crinkle virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip crinkle virus RdRP sequence in Table 7; (xxii) the HRV is Tomato bushy stunt virus and the HRV 5’RR comprises an RNA encoded by the Tomato bushy stunt virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato bushy stunt virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato bushy stunt virus RdRP sequence in Table 7; (xxiii) the HRV is Tomato spotted wilt virus and the HRV 5’RR comprises an RNA encoded by the Tomato spotted wilt virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Tomato spotted wilt virus 3’ RR DNA Agent Ref: P14419WO00 - 126 - sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Tomato spotted wilt virus RdRP sequence in Table 7; (xxiv) the HRV is watermelon bud necrosis virus and the HRV 5’RR comprises an RNA encoded by the Watermelon bud necrosis virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Watermelon bud necrosis virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Watermelon bud necrosis virus RdRP sequence in Table 7; (xxv) the HRV is Turnip yellow mosaic virus and the HRV 5’RR comprises an RNA encoded by the Turnip yellow mosaic virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Turnip yellow mosaic virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Turnip yellow mosaic virus RdRP sequence in Table 7; (xxvi) the HRV is Spinach latent virus and the HRV 5’RR comprises an RNA encoded by the Spinach latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Spinach latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus RdRP sequence of SEQ ID NO: 510; (xxvii) the HRV is Spinach latent virus and the HRV 5’RR comprises an RNA encoded by the Spinach latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Spinach latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Spinach latent virus RdRP sequence of SEQ ID NO: 511; (xxviii) the HRV is Olive latent virus 2 and the HRV 5’RR comprises an RNA encoded by the Olive latent virus 25’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 25’ RR DNA sequence in Table 7, the Agent Ref: P14419WO00 - 127 - HRV 3’ RR comprises an RNA encoded by the Olive latent virus 23’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 23’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Olive latent virus 2 RdRP sequence in Table 7; (xxix) the HRV is Citrus yellow vein clearing virus and the HRV 5’RR comprises an RNA encoded by the Citrus yellow vein clearing virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus yellow vein clearing virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus yellow vein clearing virus RdRP sequence in Table 7; (xxx) the HRV is Potato latent virus and the HRV 5’RR comprises an RNA encoded by the Potato latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Potato latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Potato latent virus RdRP sequence in Table 7; (xxxi) the HRV is Apple stem grooving virus and the HRV 5’RR comprises an RNA encoded by the Apple stem grooving virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Apple stem grooving virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Apple stem grooving virus RdRP sequence in Table 7; (xxxii) the HRV is Citrus leaf blotch virus and the HRV 5’RR comprises an RNA encoded by the Citrus leaf blotch virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Citrus leaf blotch virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Citrus leaf blotch virus RdRP sequence in Table 7; (xxxiii) the HRV is Apple latent spherical virus and the HRV 5’RR comprises an RNA encoded by the Apple latent spherical virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus 5’ RR DNA Agent Ref: P14419WO00 - 128 - sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Apple latent spherical virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Apple latent spherical virus RdRP sequence in Table 7; (xxxiv) the HRV is Soybean latent spherical virus and the HRV 5’RR comprises an RNA encoded by the Soybean latent spherical virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Soybean latent spherical virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Soybean latent spherical virus RdRP sequence in Table 7; (xxxv) the HRV is Celery latent virus and the HRV 5’RR comprises an RNA encoded by the Celery latent virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Celery latent virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Celery latent virus RdRP sequence in Table 7; (xxxvi) the HRV is Black grass varicosavirus-like virus and the HRV 5’RR comprises an RNA encoded by the Black grass varicosavirus-like virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Black grass varicosavirus-like virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Black grass varicosavirus-like virus sequence in Table 7; or (xxxvii) the HRV is Maize suscal virus and the HRV 5’RR comprises an RNA encoded by the Maize suscal virus 5’ RR DNA sequence in Table 7, an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus 5’ RR DNA sequence in Table 7, the HRV 3’ RR comprises an RNA encoded by the Maize suscal virus 3’ RR DNA sequence in Table 7, or an RNA encoded by a DNA having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus 3’ RR DNA sequence in Table 7, and the RdRP comprises a protein having at least 85%, 90%, 95%, or 99% sequence identity to the Maize suscal virus sequence in Table 7. Agent Ref: P14419WO00 - 129 - 28. The recombinant RNA molecule of claim 15, further comprising an RNA comprising encoding at least one cleavable sequence, optionally wherein the at least one cleavable sequence is located: (i) 5’ to the 5’ RNA replication element or 3’ to the 3’ RNA replication element; and/or (ii) between the 3’ end of the 5’ RNA replication element and the HRV amplicon and/or between the HRV amplicon and the 5’ end of the 3’ RNA replication element. wherein the cleavable sequence is optionally a self-cleaving ribozyme, a self-cleaving inducible ribozyme, or an siRNA or miRNA recognition site. 29. An agricultural formulation comprising the recombinant RNA molecule of any one of claims 1 to 28. 30. The agricultural formulation of claim 29, wherein the recombinant RNA molecule is complexed with one or more RNA binding proteins or encapsidated by a viral capsid protein, optionally wherein the viral capsid protein is a Solemoviridae capsid protein and optionally wherein the RNA comprises an ERE which provides for encapsidation of the RNA by the Solemoviridae capsid protein. 31. The agricultural formulation of claim 30, wherein the RNA binding proteins comprise an RNA recognition motif. 32. The agricultural formulation of claim 30, wherein the viral capsid protein is heterologous to the Solemoviridae. 33. The agricultural formulation of claim 29, wherein the formulation comprises the recombinant RNA molecule and a carrier, an excipient, and/or an adjuvant. 34. A cell comprising the recombinant RNA molecule of any one of claims 1 to 28, wherein the cell is a bacterial cell, a fungal cell, a plant cell, an insect cell, or an invertebrate animal cell. 35. The cell of claim 34, wherein the cell is a plant cell and DNA which encodes the recombinant RNA molecule is absent from the cell. 36. The cell of claim 34, wherein the cell comprises a recombinant DNA molecule which encodes the recombinant RNA molecule. 37. An expression system comprising: (a) an RNA molecule comprising the recombinant RNA molecule of any one of claims 1 to 28; and Agent Ref: P14419WO00 - 130 - (b) a cell containing the recombinant RNA molecule and an RdRP protein that recognizes the 5’ and 3’ RNA replication elements of the recombinant RNA molecule. 38. The expression system of claim 37, wherein the recombinant RNA molecule comprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein, and wherein the cell contains the viral capsid protein. 39. The expression system of claim 37, wherein the encapsidation recognition element (ERE) is a Solemoviridae ERE, wherein the viral capsid protein in the cell is a Solemoviridae capsid protein, and wherein the RNA molecule is encapsidated by the Solemoviridae capsid protein. 40. The expression system of claim 37, further comprising the reverse complement of the recombinant RNA molecule. 41. The expression system of claim 37, wherein the cell is a bacterial cell, a plant cell, a fungal cell, an insect cell, or an invertebrate animal cell. 42. The expression system of claim 37, wherein the cell further comprises: (i) a viral capsid protein (CP), (ii) an RNA-binding protein (RBP) that can bind to the RNA molecule, optionally wherein the RBP binds to an RNA effecter; (iii) an RNA cleavage agent that cleaves the RNA molecule; (iv) a second RNA- dependent RNA polymerase (RdRP) protein that recognizes an HRV 5’ or 3’ replication region and/or a subgenomic promoter in the RNA molecule (2nd RdRP); (v) a viral movement protein (MP); (v) a heterologous RNA virus (HRV); or (vi) an hrvRdRP, optionally wherein the hrvRdRP recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter. 43. The expression system of claim 42, wherein the CP, RBP, RdRP, 2nd RdRP, hrvRdRP, and/or the MP is: (a) expressed by a recombinant DNA molecule in the cell; (b) provided exogenously to the cell; (c) expressed by a recombinant RNA molecule in the cell; or (d) expressed by a virus in the cell. 44. The expression system of claim 42, wherein the RdRP, CP, RBP, 2nd RdRP, hrvRdRP, and/or the MP protein is heterologous to the cell. 45. The expression system of claim 42, wherein the RdRP, 2nd RdRP, or hrvRdRP protein or a polynucleotide encoding the RdRP, 2nd RdRP, or hrvRdRP protein is (a) expressed by a recombinant DNA molecule in the cell; (b) provided exogenously to the cell; (c) expressed by a recombinant RNA molecule in the cell; or (d) expressed by a virus in the cell. Agent Ref: P14419WO00 - 131 - 46. The expression system of claim 37, wherein the cell is a plant cell. 47. The expression system of claim 46, wherein the plant cell contains a virus of the family Solemoviridae which expresses the RdRP protein that recognizes the 5’ RNA replication element and the 3’ RNA replication element and/or wherein the plant cell contains an HRV that expresses the 2nd RdRP or hrvRdRP protein. 48. The expression system of claim 47, wherein the virus of the family Solemoviridae occurs naturally in the plant cell. 49. A method of providing a synthetic Solemoviridae satellite RNA to a plant, comprising contacting the plant with the recombinant RNA molecule of any one of claims 1 to 28. 50. The method of claim 49, wherein contacting comprises spraying, dusting, injecting, or soaking the plant or a part thereof with the recombinant RNA molecule or the formulation. 51. The method of claim 49, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. 52. A method of establishing a synthetic Solemoviridae satellite RNA in a plant cell, comprising: providing to a plant cell the recombinant RNA molecule of any one of claims 1 to 28; wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element, wherein the RNA molecule is optionally comprises an ERE and is encapsidated by a capsid protein, whereby the RdRP protein catalyzes synthesis of the synthetic Solemoviridae satellite RNA from the recombinant RNA molecule. 53. The method of claim 52, wherein the plant cell comprises a virus of the family Solemoviridae and wherein the RdRP protein is provided to the plant cell by the virus of the family Solemoviridae. 54. The method of claim 52, wherein the virus of the family Solemoviridae is endemic to the plant cell, optionally wherein the virus of the family Solemoviridae which is endemic to the plant cell is non- pathogenic and/or commensal. Agent Ref: P14419WO00 - 132 - 55. The method of claim 52, wherein the capsid protein comprises a Solemoviridae capsid protein and the ERE is recognized by the Solemoviridae capsid protein, optionally wherein the recombinant RNA molecule comprises an operably linked encapsidation recognition element (ERE) recognized by a viral capsid protein. 56. The method of claim 52, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. 57. A method of obtaining a phenotypic change in a plant or plant cell, comprising: providing to a plant or plant cell a recombinant RNA molecule of any one of claims 1 to 28, wherein the cargo RNA molecule comprises RNA that effects a phenotypic change in the plant or plant cell in comparison to a plant or plant cell lacking the recombinant RNA, wherein the plant or plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element and catalyzes synthesis of a synthetic Solemoviridae RNA from the recombinant RNA molecule, and wherein the cargo RNA molecule effects the phenotypic change. 58. The method of claim 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises at least one RNA selected from an siRNA or siRNA precursor, a miRNA or miRNA precursor, and a phased siRNA or phased siRNA precursor. 59. The method of claim 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises a messenger RNA. 60. The method of claim 59, wherein the messenger RNA comprises an RNA molecule absent in the genome of the plant or plant cell. 61. The method of claim 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises an RNA for modifying the genome of the plant or plant cell. 62. The method of claim 57, wherein the RNA that effects a phenotypic change in the plant or plant cell comprises an RNA for modifying the transcriptome and/or epigenome of the plant or plant cell, optionally wherein the RNA for modifying the epigenome targets an endogenous plant gene for RNA- induced transcriptional silencing. Agent Ref: P14419WO00 - 133 - 63. The method of claim 57, wherein the phenotypic change comprises an increase in the plant’s resistance to a pest or pathogen, optionally wherein the pest or pathogen is selected from the group comprising a bacterium, a virus other than a virus of the family Solemoviridae, a fungus, an oomycete, and an invertebrate. 64. The method of claim 63, wherein the pathogen is a heterologous RNA virus (HRV), optionally wherein the HRV is a virus selected from the group consisting of an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, and Secoviridae. 65. The method of claim 64, wherein: (i) the Bromovirus is a Cucumber Mosaic Virus, Spinach latent virus, Olive latent virus, or Brome mosaic virus; (ii) the Closterovirus is a Citrus tristeza virus or Beet yellow virus; (iii) the Comovirus is Cowpea mosaic virus, Apple latent spherical virus, or Soybean latent spherical virus; (iv) the Potexvirus is Potato virus X or Citrus yellow vein clearing virus; (v) the Potyvirus is a Pepper mottle virus, Bean yellow mosaic virus, Barley stripe mosaic virus, Wheat stripe mosaic virus, Rice yellow mottle virus, Maize dwarf mosaic virus, zucchini yellow mosaic virus, watermelon mosaic virus, or sugar cane mosaic virus; (vi) the Tobamovirus is a Tobacco mosaic virus, Tomato mosaic virus, Tomato brown rugose fruit virus, Turnip vein-clearing virus, or Pepper mild mottle virus; (vii) the Tombusvirus is a Turnip crinkle virus or Tomato bushy stunt virus; (iv) the Tospoviridae is a Tomato spotted wilt virus or watermelon bud necrosis virus; or (viii) the Tymovirus is a Turnip yellow mosaic virus, Citrus yellow vein clearing virus, Potato latent virus, Apple stem grooving virus, or Citrus leaf blotch virus. 66. The method of claim 57, wherein the phenotypic change comprises an increase in the plant’s resistance to stress, optionally wherein the stress comprises at least one abiotic stress comprising nutrient stress, light stress, water stress, heat stress, and/or cold stress, or optionally wherein the stress comprises at least one biotic stress comprising crowding, shading, or allelopathy. 67. The method of claim 57, wherein the recombinant RNA molecule is provided to the plant of plant cell in the form of an RNA, an encapsidated RNA, or a formulation thereof. 68. The method of claim 67, wherein the encapsidated RNA comprises a synthetic Solemoviridae satellite particle. 69. The method of claim 57, wherein the providing comprises contacting the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof, optionally wherein contacting comprises spraying, Agent Ref: P14419WO00 - 134 - dusting, injecting, or soaking the plant or plant cell with the RNA, encapsidated RNA, or formulation thereof. 70. The method of claim 57, wherein the recombinant RNA further comprises its reverse complementary RNA molecule. 71. The method of claim 57, further comprising providing an hrvRdRP to the plant which recognizes the HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, optionally wherein the hrvRdRP is provided by introducing a recombinant DNA or RNA encoding the hrvRdRP into the plant or a part thereof. 72. A method of manufacturing a synthetic Solemoviridae satellite particle, comprising combining the recombinant RNA molecule of any one of claims 1 to 28 with a viral capsid protein, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the ERE provides for encapsidation of the RNA by the viral capsid protein. 73. The method of claim 72, wherein the recombinant RNA molecule is combined with a viral capsid protein in a vessel. 74. The method of claim 73, wherein the combining comprises (a) providing to a plant cell the recombinant RNA molecule, wherein the recombinant RNA molecule comprises an encapsidation recognition element (ERE), and wherein the plant cell comprises an RdRP protein that recognizes the 5’ RNA replication element and 3’ RNA replication element catalyzes synthesis of a synthetic Solemoviridae satellite RNA from the recombinant RNA molecule and a viral capsid protein, wherein the ERE provides for encapsidation of the RNA by the viral capsid protein; and optionally (b) isolating the synthetic Solemoviridae satellite particle from the plant cell, a plant comprising the plant cell, or from media in which the plant cell or the plant had been grown. 75. The method of claim 72, further comprising the step of formulating the synthetic Solemoviridae satellite particle wherein the formulating comprises combining the synthetic Solemoviridae satellite particle with a carrier, an excipient, and/or an adjuvant. 76. A plant propagule comprising the recombinant RNA molecule of any one of claims 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. Agent Ref: P14419WO00 - 135 - 77. The plant propagule of claim 76, wherein the plant propagule is a seed, a seedling, root, stem, leaf, shoot, tuber, rhizome, stolon, bulb, explant, embryo, or callus. 78. The plant propagule of claim 76, wherein the plant propagule is a mosaic comprising both plant cells comprising the recombinant RNA molecule and plant cells lacking the recombinant RNA molecule. 79. The plant propagule of claim 76, wherein the plant propagule lacks DNA encoding the recombinant RNA molecule. 80. The plant propagule of claim 76, wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. 81. A plant comprising the recombinant RNA molecule of any one of claims 1 to 28 and a Solemoviridae RdRP, optionally wherein the plant propagule further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. 82. The plant of claim 81, wherein the plant is a monocot or a dicot plant. 83. The plant of claim 81, wherein the plant is of the family Asteraceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Poaceae, or Solanaceae. 84. The plant of claim 81, wherein the plant lacks DNA that encodes the recombinant RNA molecule. 85. The plant of claim 81, wherein the plant comprises a virus of the family Solemoviridae, and wherein the Solemoviridae RdRP is provided to the plant cell by the virus of the family Solemoviridae, optionally wherein the Solemoviridae RdRP comprises a protein having at least 85%, 90%, 95%, 97%, or 99% sequence identity to SEQ ID NO: 469, 535, or 536. 86. The plant of claim 81, wherein the virus of the family Solemoviridae is endemic to the plant, optionally wherein the endemic virus of the family Solemoviridae is non-pathogenic and/or commensal. Agent Ref: P14419WO00 - 136 - 87. The plant of claim 81, wherein the Solemoviridae RdRP, the 5’ RNA replication element, and/or the 3’ RNA replication element are derived from a virus of the family Solemoviridae comprising one or both of the Solemoviridae RdRP, 5’ RNA replication element, and/or 3’ RNA replication elements. 88. The plant of claim 81, wherein the RdRP has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to SEQ ID NO: 469, 535, or 536, wherein the 5’ RNA replication element has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 467, 531, or 532, and/or wherein the 3’ RNA replication element has at least 85%, 90%, 95%, 97%, 98%, or 99% identity to the RNA encoded by SEQ ID NO: 468, 533, or 534. 89. The plant of claim 81, wherein the plant is a grafted plant and wherein the rootstock and/or scion of the grafted plant comprise at least one cell comprising the recombinant RNA and the Solemoviridae RdRP. 90. The plant of claim 81, wherein the plant is not produced by an essentially biological process. 91. The plant of claim 81, wherein the plant further comprises a heterologous RNA virus (HRV) RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the heterologous RNA virus RdRP is an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, a Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7. 92. A Solemoviridae satellite system that is self-replicating when introduced into a plant or plant cell, comprising: (a) a recombinant Solemoviridae satellite RNA of any one of claims 1 to 28; and (b) an exogenous virus of the family Solemoviridae that is capable of replication in the plant or plant cells and that encodes the Solemoviridae RdRP that recognizes the 5’ and 3’ replicase recognition sequences in the recombinant Solemoviridae satellite RNA, optionally wherein the Solemoviridae satellite system further comprises a heterologous RNA virus RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA. 93. The self-replicating Solemoviridae satellite system of claim 92, wherein the exogenous virus of the family Solemoviridae is endemic or native to a different species, variety, or germplasm of plant. 94. The self-replicating Solemoviridae satellite system of claim 92, wherein the Solemoviridae satellite system further comprises a heterologous RNA virus (HRV) RdRP which recognizes an HRV 5’ or 3’ replication region and/or the subgenomic promoter in the synthetic Solemoviridae satellite RNA, and the Agent Ref: P14419WO00 - 137 - heterologous RNA virus RdRP is optionally an Alphaflexivirus, Betaflexivirus, Bromovirus, Celavirus, Closterovirus, Comovirus, Potexvirus, Potyvirus, Tobamovirus, Tombusvirus, Tospoviridae, Trivirinae, Tymovirus, Varicosavirus, or Secoviridae RdRP or an RdRP set forth in Table 7.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363499755P | 2023-05-03 | 2023-05-03 | |
| US63/499,755 | 2023-05-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2024229356A2true WO2024229356A2 (en) | 2024-11-07 |
| WO2024229356A3 WO2024229356A3 (en) | 2024-12-05 |
Family
ID=91302004
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/027683PendingWO2024229356A2 (en) | 2023-05-03 | 2024-05-03 | Artificial solemoviridae satellite rnas |
Country Status (2)
| Country | Link |
|---|---|
| AR (1) | AR132589A1 (en) |
| WO (1) | WO2024229356A2 (en) |
Citations (143)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5229114A (en) | 1987-08-20 | 1993-07-20 | The United States Of America As Represented By The Secretary Of Agriculture | Approaches useful for the control of root nodulation of leguminous plants |
| US5304730A (en) | 1991-09-03 | 1994-04-19 | Monsanto Company | Virus resistant plants and method therefore |
| US5322938A (en) | 1987-01-13 | 1994-06-21 | Monsanto Company | DNA sequence for enhancing the efficiency of transcription |
| US5380831A (en) | 1986-04-04 | 1995-01-10 | Mycogen Plant Science, Inc. | Synthetic insecticidal crystal protein gene |
| US5463175A (en) | 1990-06-25 | 1995-10-31 | Monsanto Company | Glyphosate tolerant plants |
| US5512466A (en) | 1990-12-26 | 1996-04-30 | Monsanto Company | Control of fruit ripening and senescence in plants |
| US5516671A (en) | 1993-11-24 | 1996-05-14 | Monsanto Company | Method of controlling plant pathogens |
| US5543576A (en) | 1990-03-23 | 1996-08-06 | Mogen International | Production of enzymes in seeds and their use |
| US5608149A (en) | 1990-06-18 | 1997-03-04 | Monsanto Company | Enhanced starch biosynthesis in tomatoes |
| US5633435A (en) | 1990-08-31 | 1997-05-27 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases |
| US5641876A (en) | 1990-01-05 | 1997-06-24 | Cornell Research Foundation, Inc. | Rice actin gene and promoter |
| US5689052A (en) | 1993-12-22 | 1997-11-18 | Monsanto Company | Synthetic DNA sequences having enhanced expression in monocotyledonous plants and method for preparation thereof |
| US5689041A (en) | 1989-08-10 | 1997-11-18 | Plant Gentic Systems N.V. | Plants modified with barstar for fertility restoration |
| US5716837A (en) | 1995-02-10 | 1998-02-10 | Monsanto Company | Expression of sucrose phosphorylase in plants |
| US5750876A (en) | 1994-07-28 | 1998-05-12 | Monsanto Company | Isoamylase gene, compositions containing it, and methods of using isoamylases |
| US5763245A (en) | 1991-09-23 | 1998-06-09 | Monsanto Company | Method of controlling insects |
| US5763241A (en) | 1987-04-29 | 1998-06-09 | Monsanto Company | Insect resistant plants |
| US5773696A (en) | 1996-03-29 | 1998-06-30 | Monsanto Company | Antifungal polypeptide and methods for controlling plant pathogenic fungi |
| US5850023A (en) | 1992-11-30 | 1998-12-15 | Monsanto Company | Modified plant viral replicase genes |
| US5858742A (en) | 1983-01-17 | 1999-01-12 | Monsanto Company | Chimeric genes for transforming plant cells using viral promoters |
| US5866775A (en) | 1990-09-28 | 1999-02-02 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvyl-3-phosphoshikimate synthases |
| US5869720A (en) | 1993-09-30 | 1999-02-09 | Monsanto Company | Transgenic cotton plants producing heterologous peroxidase |
| US5880275A (en) | 1989-02-24 | 1999-03-09 | Monsanto Company | Synthetic plant genes from BT kurstaki and method for preparation |
| US5942664A (en) | 1996-11-27 | 1999-08-24 | Ecogen, Inc. | Bacillus thuringiensis Cry1C compositions toxic to lepidopteran insects and methods for making Cry1C mutants |
| US5942658A (en) | 1993-07-29 | 1999-08-24 | Monsanto Company | Transformed plant with Bacillus thuringiensis toxin gene |
| US5959091A (en) | 1984-12-10 | 1999-09-28 | Monsanto Company | Truncated gene of Bacillus thuringiensis encoding a polypeptide toxin |
| US5958745A (en) | 1996-03-13 | 1999-09-28 | Monsanto Company | Methods of optimizing substrate pools and biosynthesis of poly-β-hydroxybutyrate-co-poly-β-hydroxyvalerate in bacteria and plants |
| US5981834A (en) | 1988-10-04 | 1999-11-09 | Monsanto Company | Genetically engineering cotton plants for altered fiber |
| US5985605A (en) | 1996-06-14 | 1999-11-16 | Her Majesty The Queen In Right Of Canada, As Represented By The Dept. Of Agriculture & Agri-Food Canada | DNA sequences encoding phytases of ruminal microorganisms |
| US5998700A (en) | 1996-07-02 | 1999-12-07 | The Board Of Trustees Of Southern Illinois University | Plants containing a bacterial Gdha gene and methods of use thereof |
| US6011199A (en) | 1992-12-15 | 2000-01-04 | Commonwealth Scientific | Method for producing fruiting plants with improved fruit flavour |
| US6013864A (en) | 1993-02-03 | 2000-01-11 | Monsanto Company | Plants resistant to infection by luteoviruses |
| US6015940A (en) | 1992-04-07 | 2000-01-18 | Monsanto Company | Virus resistant potato plants |
| US6023013A (en) | 1997-12-18 | 2000-02-08 | Monsanto Company | Insect-resistant transgenic plants |
| US6063756A (en) | 1996-09-24 | 2000-05-16 | Monsanto Company | Bacillus thuringiensis cryET33 and cryET34 compositions and uses therefor |
| US6063597A (en) | 1997-12-18 | 2000-05-16 | Monsanto Company | Polypeptide compositions toxic to coleopteran insects |
| US6072103A (en) | 1997-11-21 | 2000-06-06 | Calgene Llc | Pathogen and stress-responsive promoter for gene expression |
| US6080560A (en) | 1994-07-25 | 2000-06-27 | Monsanto Company | Method for producing antibodies in plant cells |
| US6093695A (en) | 1996-09-26 | 2000-07-25 | Monsanto Company | Bacillus thuringiensis CryET29 compositions toxic to coleopteran insects and ctenocephalides SPP |
| US6107549A (en) | 1998-03-10 | 2000-08-22 | Monsanto Company | Genetically engineered plant resistance to thiazopyr and other pyridine herbicides |
| US6110464A (en) | 1996-11-20 | 2000-08-29 | Monsanto Company | Broad-spectrum δ-endotoxins |
| US6121436A (en) | 1996-12-13 | 2000-09-19 | Monsanto Company | Antifungal polypeptide and methods for controlling plant pathogenic fungi |
| US6140075A (en) | 1994-07-25 | 2000-10-31 | Monsanto Company | Method for producing antibodies and protein toxins in plant cells |
| US6166292A (en) | 1996-04-26 | 2000-12-26 | Ajinomoto Co., Inc. | Raffinose synthetase gene, method of producing raffinose and transgenic plant |
| US6171640B1 (en) | 1997-04-04 | 2001-01-09 | Monsanto Company | High beta-conglycinin products and their use |
| US6228992B1 (en) | 1998-05-18 | 2001-05-08 | Pioneer Hi-Bred International, Inc. | Proteins for control of nematodes in plants |
| US6228623B1 (en) | 1996-03-13 | 2001-05-08 | Monsanto Company | Polyhydroxyalkanoates of narrow molecular weight distribution prepared in transgenic plants |
| US6242241B1 (en) | 1996-11-20 | 2001-06-05 | Monsanto Company | Polynucleotide compositions encoding broad-spectrum δ-endotoxins |
| US6271443B1 (en) | 1996-10-29 | 2001-08-07 | Calgene Llc | Cotton and rice cellulose synthase DNA sequences |
| USRE37543E1 (en) | 1996-08-13 | 2002-02-05 | Monsanto Company | DNA sequence useful for the production of polyhydroxyalkanoates |
| US6372211B1 (en) | 1997-04-21 | 2002-04-16 | Monsanto Technolgy Llc | Methods and compositions for controlling insects |
| US6380462B1 (en) | 1998-08-14 | 2002-04-30 | Calgene Llc | Method for increasing stearate content in soybean oil |
| US6380466B1 (en) | 1997-05-08 | 2002-04-30 | Calgene Llc | Production of improved rapeseed exhibiting yellow-seed coat |
| US6426447B1 (en) | 1990-11-14 | 2002-07-30 | Monsanto Technology Llc | Plant seed oils |
| US6441277B1 (en) | 1997-06-17 | 2002-08-27 | Monsanto Technology Llc | Expression of fructose 1,6 bisphosphate aldolase in transgenic plants |
| US6444876B1 (en) | 1998-06-05 | 2002-09-03 | Calgene Llc | Acyl CoA: cholesterol acyltransferase related nucleic acid sequences |
| US6448476B1 (en) | 1998-11-17 | 2002-09-10 | Monsanto Technology Llc | Plants and plant cells transformation to express an AMPA-N-acetyltransferase |
| US6459018B1 (en) | 1998-06-12 | 2002-10-01 | Monsanto Technology Llc | Polyunsaturated fatty acids in plants |
| US6468523B1 (en) | 1998-11-02 | 2002-10-22 | Monsanto Technology Llc | Polypeptide compositions toxic to diabrotic insects, and methods of use |
| US6483008B1 (en) | 1990-08-15 | 2002-11-19 | Calgene Llc | Methods for producing plants with elevated oleic acid content |
| US6489461B1 (en) | 1999-06-08 | 2002-12-03 | Calgene Llc | Nucleic acid sequences encoding proteins involved in fatty acid beta-oxidation and methods of use |
| US6495739B1 (en) | 1998-07-24 | 2002-12-17 | Calgene Llc | Plant phosphatidic acid phosphatases |
| US6501009B1 (en) | 1999-08-19 | 2002-12-31 | Monsanto Technology Llc | Expression of Cry3B insecticidal protein in plants |
| US6506962B1 (en) | 1999-05-13 | 2003-01-14 | Monsanto Technology Llc | Acquired resistance genes in plants |
| US6518488B1 (en) | 2000-07-21 | 2003-02-11 | Monsanto Technology Llc | Nucleic acid molecules and other molecules associated with the β-oxidation pathway |
| US6531648B1 (en) | 1998-12-17 | 2003-03-11 | Syngenta Participations Ag | Grain processing method and transgenic plants useful therein |
| US6537750B1 (en) | 1998-08-04 | 2003-03-25 | Cargill Incorporated | Plant fatty acid desaturase promoters |
| US6538181B1 (en) | 1990-06-11 | 2003-03-25 | Calgene Llc | Glycogen biosynthetic enzymes in plants |
| US6538178B1 (en) | 1990-06-18 | 2003-03-25 | Monsanto Technology Llc | Increased starch content in plants |
| US6541259B1 (en) | 1999-04-15 | 2003-04-01 | Calgene Llc | Nucleic acid sequences to proteins involved in isoprenoid synthesis |
| US6555655B1 (en) | 1999-05-04 | 2003-04-29 | Monsanto Technology, Llc | Coleopteran-toxic polypeptide compositions and insect-resistant transgenic plants |
| US6573361B1 (en) | 1999-12-06 | 2003-06-03 | Monsanto Technology Llc | Antifungal proteins and methods for their use |
| US6589767B1 (en) | 1997-04-11 | 2003-07-08 | Abbott Laboratories | Methods and compositions for synthesis of long chain polyunsaturated fatty acids |
| US6593293B1 (en) | 1999-09-15 | 2003-07-15 | Monsanto Technology, Llc | Lepidopteran-active Bacillus thuringiensis δ-endotoxin compositions and methods of use |
| US6596538B1 (en) | 1997-06-05 | 2003-07-22 | Calgene Llc | Fatty acyl-CoA: fatty alcohol acyltransferases |
| US6608241B1 (en) | 1985-10-29 | 2003-08-19 | Monsanto Technology Llc | Protection of plants against viral infection |
| US6617496B1 (en) | 1985-10-16 | 2003-09-09 | Monsanto Company | Effecting virus resistance in plants through the use of negative strand RNAs |
| US6620988B1 (en) | 1997-12-18 | 2003-09-16 | Monsanto Technology, Llc | Coleopteran-resistant transgenic plants and methods of their production using modified Bacillus thuringiensis Cry3Bb nucleic acids |
| US6630306B1 (en) | 1996-12-19 | 2003-10-07 | Yale University | Bioreactive allosteric polynucleotides |
| US6639054B1 (en) | 2000-01-06 | 2003-10-28 | Monsanto Technology Llc | Preparation of deallergenized proteins and permuteins |
| US6653530B1 (en) | 1998-02-13 | 2003-11-25 | Calgene Llc | Methods for producing carotenoid compounds, tocopherol compounds, and specialty oils in plant seeds |
| US6657046B1 (en) | 2000-01-06 | 2003-12-02 | Monsanto Technology Llc | Insect inhibitory lipid acyl hydrolases |
| US6660849B1 (en) | 1997-04-11 | 2003-12-09 | Calgene Llc | Plant fatty acid synthases and use in improved methods for production of medium-chain fatty acids |
| US6663906B2 (en) | 1997-06-17 | 2003-12-16 | Monsanto Technology Llc | Expression of fructose 1,6 bisphosphate aldolase in transgenic plants |
| USRE38446E1 (en) | 1990-07-20 | 2004-02-24 | Calgene, Llc. | Sucrose phosphate synthase (SPS), its process for preparation its cDNA, and utilization of cDNA to modify the expression of SPS in plant cells |
| US6706950B2 (en) | 2000-07-25 | 2004-03-16 | Calgene Llc | Nucleic acid sequences encoding β-ketoacyl-ACP synthase and uses thereof |
| US6713063B1 (en) | 1996-11-20 | 2004-03-30 | Monsanto Technology, Llc | Broad-spectrum δ-endotoxins |
| US6723837B1 (en) | 1999-07-12 | 2004-04-20 | Monsanto Technology Llc | Nucleic acid molecule and encoded protein associated with sterol synthesis and metabolism |
| US6723897B2 (en) | 1998-08-10 | 2004-04-20 | Monsanto Technology, Llc | Methods for controlling gibberellin levels |
| US6770465B1 (en) | 1999-06-09 | 2004-08-03 | Calgene Llc | Engineering B-ketoacyl ACP synthase for novel substrate specificity |
| US6774283B1 (en) | 1985-07-29 | 2004-08-10 | Calgene Llc | Molecular farming |
| US6803501B2 (en) | 2000-03-09 | 2004-10-12 | Monsanto Technology, Llc | Methods for making plants tolerant to glyphosate and compositions thereof using a DNA encoding an EPSPS enzyme from Eleusine indica |
| US6812379B2 (en) | 1998-07-10 | 2004-11-02 | Calgene Llc | Expression of eukaryotic peptides in plant plastids |
| US6822141B2 (en) | 1998-07-02 | 2004-11-23 | Calgene Llc | Diacylglycerol acyl transferase proteins |
| US6828475B1 (en) | 1994-06-23 | 2004-12-07 | Calgene Llc | Nucleic acid sequences encoding a plant cytoplasmic protein involved in fatty acyl-CoA metabolism |
| US6946588B2 (en) | 1996-03-13 | 2005-09-20 | Monsanto Technology Llc | Nucleic acid encoding a modified threonine deaminase and methods of use |
| US6949379B2 (en) | 2000-10-20 | 2005-09-27 | Canji, Inc. | Aptamer-mediated regulation of gene expression |
| US7151204B2 (en) | 2001-01-09 | 2006-12-19 | Monsanto Technology Llc | Maize chloroplast aldolase promoter compositions and methods for use thereof |
| US20070011761A1 (en) | 2005-05-19 | 2007-01-11 | Monsanto Technology, L.L.C. | Post-transcriptional regulation of gene expression |
| US20100311168A1 (en) | 2009-04-07 | 2010-12-09 | Dow Agrosciences Llc | Nanoparticle mediated delivery of sequence specific nucleases |
| US8030473B2 (en) | 2005-01-07 | 2011-10-04 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Method to trigger RNA interference |
| US20120023619A1 (en) | 2010-07-07 | 2012-01-26 | Dow Agrosciences Llc | Linear dna molecule delivery using pegylated quantum dots for stable trasformation in plants |
| US20120244569A1 (en) | 2011-03-23 | 2012-09-27 | Dow Agrosciences Llc | Quantum dot carrier peptide conjugates suitable for imaging and delivery applications in plants |
| US8334430B2 (en) | 2005-10-13 | 2012-12-18 | Monsanto Technology Llc | Methods for producing hybrid seed |
| US8395023B2 (en) | 2004-12-21 | 2013-03-12 | Monsanto Technology Llc | Recombinant DNA constructs and methods for controlling gene expression |
| US8404927B2 (en) | 2004-12-21 | 2013-03-26 | Monsanto Technology Llc | Double-stranded RNA stabilized in planta |
| US8404928B2 (en) | 2006-08-31 | 2013-03-26 | Monsanto Technology Llc | Phased small RNAs |
| US8410334B2 (en) | 2007-02-20 | 2013-04-02 | Monsanto Technology Llc | Invertebrate microRNAs |
| US20130102651A1 (en) | 2007-08-28 | 2013-04-25 | California Institute Of Technology | General composition framework for ligand-controlled rna regulatory systems |
| US8455716B2 (en) | 2009-04-20 | 2013-06-04 | Monsanto Technology Llc | Multiple virus resistance in plants |
| US20130145488A1 (en) | 2011-12-06 | 2013-06-06 | Iowa State University Research Foundation, Inc. | Mesoporous silica nanoparticles suitable for co-delivery |
| US20130185823A1 (en) | 2012-01-16 | 2013-07-18 | Academia Sinica | Mesoporous silica nanoparticle-mediated delivery of dna into arabidopsis root |
| US20140096284A1 (en) | 2012-10-01 | 2014-04-03 | Iowa State University Research Foundation, Inc. | Method for the delivery of molecules lyophilized onto microparticles to plant tissues |
| US20140356414A1 (en) | 2013-06-03 | 2014-12-04 | University Of Southern California | Targeted Crosslinked Multilamellar Liposomes |
| US8946511B2 (en) | 2006-10-12 | 2015-02-03 | Monsanto Technology Llc | Plant microRNAs and methods of use thereof |
| US20150040268A1 (en) | 2013-04-25 | 2015-02-05 | Morflora Israel Ltd | Methods and compositions for the delivery of nucleic acids to seeds |
| US20150047074A1 (en) | 2013-08-09 | 2015-02-12 | Massachusetts Institute Of Technology | Nanobionic engineering of organelles and photosynthetic organisms |
| US20150082478A1 (en) | 2013-08-22 | 2015-03-19 | E I Du Pont De Nemours And Company | Plant genome modification using guide rna/cas endonuclease systems and methods of use |
| US9040774B2 (en) | 2008-07-01 | 2015-05-26 | Monsanto Technology Llc | Recombinant DNA constructs encoding ribonuclease cleavage blockers and methods for modulating expression of a target gene |
| US20150208663A1 (en) | 2013-10-15 | 2015-07-30 | Board Of Trustees Of The University Of Arkansas | Method of delivery of bio-active agents to plant cells by using nano-sized materials as carriers |
| US9139838B2 (en) | 2011-07-01 | 2015-09-22 | Monsanto Technology Llc | Methods and compositions for selective regulation of protein expression |
| US9777288B2 (en) | 2013-07-19 | 2017-10-03 | Monsanto Technology Llc | Compositions and methods for controlling leptinotarsa |
| US20170369898A1 (en) | 2014-12-19 | 2017-12-28 | AgBiome, Inc. | Sequences to facilitate incorporation of dna into the genome of an organism |
| US9873888B2 (en) | 2009-10-23 | 2018-01-23 | Monsanto Technology Llc | Transgenic soybean plants and chromosomes |
| US9976152B2 (en) | 2007-06-26 | 2018-05-22 | Monsanto Technology Llc | Temporal regulation of gene expression by microRNAs |
| US10017549B2 (en) | 2008-08-29 | 2018-07-10 | Monsanto Technology Llc | Hemipteran and coleopteran active toxin proteins from Bacillus thuringiensis |
| US20180312854A1 (en) | 2006-05-16 | 2018-11-01 | Monsanto Technology Llc | Use of non-agrobacterium bacterial species for plant transformation |
| US20190032131A1 (en) | 2016-12-12 | 2019-01-31 | Integrated Dna Technologies, Inc. | Genome editing detection |
| US10233217B2 (en) | 2014-10-16 | 2019-03-19 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to Lepidopteran pests |
| US10378012B2 (en) | 2014-07-29 | 2019-08-13 | Monsanto Technology Llc | Compositions and methods for controlling insect pests |
| US20190352655A1 (en) | 2017-01-28 | 2019-11-21 | Inari Agriculture, Inc. | Novel plant cells, plants, and seeds |
| US10487123B2 (en) | 2014-10-16 | 2019-11-26 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US10612037B2 (en) | 2016-06-20 | 2020-04-07 | Monsanto Technology Llc | Insecticidal proteins toxic or inhibitory to hemipteran pests |
| US10827755B2 (en) | 2015-11-18 | 2020-11-10 | Monsanto Technology Llc | Insecticidal compositions and methods |
| US11091770B2 (en) | 2014-04-01 | 2021-08-17 | Monsanto Technology Llc | Compositions and methods for controlling insect pests |
| US20210259176A1 (en) | 2004-08-26 | 2021-08-26 | Monsanto Technology Llc | Methods of Seed Breeding Using High Throughput Nondestructive Seed Sampling |
| US11130965B2 (en) | 2016-10-27 | 2021-09-28 | Syngenta Participations Ag | Insecticidal proteins |
| US11136593B2 (en) | 2016-09-09 | 2021-10-05 | Syngenta Participations Ag | Insecticidal proteins |
| US11180774B2 (en) | 2017-01-12 | 2021-11-23 | Syngenta Participations Ag | Insecticidal proteins |
| US11186837B2 (en) | 2004-04-09 | 2021-11-30 | Monsanto Technology Llc | Compositions and methods for controlling Diabrotica |
| US20220221377A1 (en) | 2006-03-02 | 2022-07-14 | Monsanto Technology Llc | Automated Contamination-Free Seed Sampler And Methods Of Sampling, Testing And Bulking Seeds |
| WO2023004435A1 (en) | 2021-07-23 | 2023-01-26 | Flagship Pioneering Innovations Vii, Llc | Compositions for fungal control and related methods |
| US11844318B2 (en) | 2018-07-25 | 2023-12-19 | Invaio Sciences International Gmbh | Injection systems, injection tools and methods for same |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10225066A1 (en)* | 2002-06-06 | 2003-12-18 | Basf Plant Science Gmbh | New transgenic double-stranded RNA, useful for expressing proteins or suppressing genes in plants, contains sequences that allow replication through RNA polymerase |
| ATE469231T1 (en)* | 2003-11-10 | 2010-06-15 | Icon Genetics Gmbh | PLANT EXPRESSION SYSTEM DERIVED FROM RNA VIRUS |
| US20230227830A1 (en)* | 2020-06-20 | 2023-07-20 | Halo-Bio Rnai Therapeutics, Inc. | Methods and compositions of rna nanostructures for replication and sub-genomic expression by rna-directed rna polymerase |
- 2024
- 2024-05-03ARARP240101124Apatent/AR132589A1/enunknown
- 2024-05-03WOPCT/US2024/027683patent/WO2024229356A2/enactivePending
Patent Citations (185)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5858742A (en) | 1983-01-17 | 1999-01-12 | Monsanto Company | Chimeric genes for transforming plant cells using viral promoters |
| US5959091A (en) | 1984-12-10 | 1999-09-28 | Monsanto Company | Truncated gene of Bacillus thuringiensis encoding a polypeptide toxin |
| US6774283B1 (en) | 1985-07-29 | 2004-08-10 | Calgene Llc | Molecular farming |
| US6617496B1 (en) | 1985-10-16 | 2003-09-09 | Monsanto Company | Effecting virus resistance in plants through the use of negative strand RNAs |
| US6608241B1 (en) | 1985-10-29 | 2003-08-19 | Monsanto Technology Llc | Protection of plants against viral infection |
| US5380831A (en) | 1986-04-04 | 1995-01-10 | Mycogen Plant Science, Inc. | Synthetic insecticidal crystal protein gene |
| US5322938A (en) | 1987-01-13 | 1994-06-21 | Monsanto Company | DNA sequence for enhancing the efficiency of transcription |
| US6284949B1 (en) | 1987-04-29 | 2001-09-04 | Monsanto Company | Insect-resistant plants comprising a Bacillus thuringiensis gene |
| US5763241A (en) | 1987-04-29 | 1998-06-09 | Monsanto Company | Insect resistant plants |
| US5229114A (en) | 1987-08-20 | 1993-07-20 | The United States Of America As Represented By The Secretary Of Agriculture | Approaches useful for the control of root nodulation of leguminous plants |
| US5981834A (en) | 1988-10-04 | 1999-11-09 | Monsanto Company | Genetically engineering cotton plants for altered fiber |
| US7741118B1 (en) | 1989-02-24 | 2010-06-22 | Monsanto Technology Llc | Synthetic plant genes and method for preparation |
| US5880275A (en) | 1989-02-24 | 1999-03-09 | Monsanto Company | Synthetic plant genes from BT kurstaki and method for preparation |
| US5689041A (en) | 1989-08-10 | 1997-11-18 | Plant Gentic Systems N.V. | Plants modified with barstar for fertility restoration |
| US5641876A (en) | 1990-01-05 | 1997-06-24 | Cornell Research Foundation, Inc. | Rice actin gene and promoter |
| US5543576A (en) | 1990-03-23 | 1996-08-06 | Mogen International | Production of enzymes in seeds and their use |
| US6538181B1 (en) | 1990-06-11 | 2003-03-25 | Calgene Llc | Glycogen biosynthetic enzymes in plants |
| US6538179B1 (en) | 1990-06-18 | 2003-03-25 | Monsanto Technology Llc | Enhanced starch biosynthesis in seeds |
| US5608149A (en) | 1990-06-18 | 1997-03-04 | Monsanto Company | Enhanced starch biosynthesis in tomatoes |
| US6538178B1 (en) | 1990-06-18 | 2003-03-25 | Monsanto Technology Llc | Increased starch content in plants |
| US5463175A (en) | 1990-06-25 | 1995-10-31 | Monsanto Company | Glyphosate tolerant plants |
| USRE38446E1 (en) | 1990-07-20 | 2004-02-24 | Calgene, Llc. | Sucrose phosphate synthase (SPS), its process for preparation its cDNA, and utilization of cDNA to modify the expression of SPS in plant cells |
| US6483008B1 (en) | 1990-08-15 | 2002-11-19 | Calgene Llc | Methods for producing plants with elevated oleic acid content |
| US5633435A (en) | 1990-08-31 | 1997-05-27 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases |
| US5804425A (en) | 1990-08-31 | 1998-09-08 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases |
| US6248876B1 (en) | 1990-08-31 | 2001-06-19 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases |
| US6225114B1 (en) | 1990-09-28 | 2001-05-01 | Monsanto Company | Modified gene encoding glyphosate-tolerant 5-enolpruvyl-3-phosphoshikimate synthase |
| US5866775A (en) | 1990-09-28 | 1999-02-02 | Monsanto Company | Glyphosate-tolerant 5-enolpyruvyl-3-phosphoshikimate synthases |
| US6426447B1 (en) | 1990-11-14 | 2002-07-30 | Monsanto Technology Llc | Plant seed oils |
| US5512466A (en) | 1990-12-26 | 1996-04-30 | Monsanto Company | Control of fruit ripening and senescence in plants |
| US5304730A (en) | 1991-09-03 | 1994-04-19 | Monsanto Company | Virus resistant plants and method therefore |
| US5763245A (en) | 1991-09-23 | 1998-06-09 | Monsanto Company | Method of controlling insects |
| US6015940A (en) | 1992-04-07 | 2000-01-18 | Monsanto Company | Virus resistant potato plants |
| US5850023A (en) | 1992-11-30 | 1998-12-15 | Monsanto Company | Modified plant viral replicase genes |
| US6011199A (en) | 1992-12-15 | 2000-01-04 | Commonwealth Scientific | Method for producing fruiting plants with improved fruit flavour |
| US6013864A (en) | 1993-02-03 | 2000-01-11 | Monsanto Company | Plants resistant to infection by luteoviruses |
| US5942658A (en) | 1993-07-29 | 1999-08-24 | Monsanto Company | Transformed plant with Bacillus thuringiensis toxin gene |
| US5869720A (en) | 1993-09-30 | 1999-02-09 | Monsanto Company | Transgenic cotton plants producing heterologous peroxidase |
| US5516671A (en) | 1993-11-24 | 1996-05-14 | Monsanto Company | Method of controlling plant pathogens |
| US5689052A (en) | 1993-12-22 | 1997-11-18 | Monsanto Company | Synthetic DNA sequences having enhanced expression in monocotyledonous plants and method for preparation thereof |
| US6828475B1 (en) | 1994-06-23 | 2004-12-07 | Calgene Llc | Nucleic acid sequences encoding a plant cytoplasmic protein involved in fatty acyl-CoA metabolism |
| US6140075A (en) | 1994-07-25 | 2000-10-31 | Monsanto Company | Method for producing antibodies and protein toxins in plant cells |
| US6080560A (en) | 1994-07-25 | 2000-06-27 | Monsanto Company | Method for producing antibodies in plant cells |
| US5750876A (en) | 1994-07-28 | 1998-05-12 | Monsanto Company | Isoamylase gene, compositions containing it, and methods of using isoamylases |
| US6476295B2 (en) | 1995-02-10 | 2002-11-05 | Monsanto Technology, Llc | Expression of sucrose phosphorylase in plants |
| US5716837A (en) | 1995-02-10 | 1998-02-10 | Monsanto Company | Expression of sucrose phosphorylase in plants |
| US6235971B1 (en) | 1995-02-10 | 2001-05-22 | Monsanto Company | Expression of sucrose phoshorylase in plants |
| US6222098B1 (en) | 1995-02-10 | 2001-04-24 | Monsanto Company | Expression of sucrose phosphorylase in plants |
| US6946588B2 (en) | 1996-03-13 | 2005-09-20 | Monsanto Technology Llc | Nucleic acid encoding a modified threonine deaminase and methods of use |
| US6228623B1 (en) | 1996-03-13 | 2001-05-08 | Monsanto Company | Polyhydroxyalkanoates of narrow molecular weight distribution prepared in transgenic plants |
| US5958745A (en) | 1996-03-13 | 1999-09-28 | Monsanto Company | Methods of optimizing substrate pools and biosynthesis of poly-β-hydroxybutyrate-co-poly-β-hydroxyvalerate in bacteria and plants |
| US5773696A (en) | 1996-03-29 | 1998-06-30 | Monsanto Company | Antifungal polypeptide and methods for controlling plant pathogenic fungi |
| US6215048B1 (en) | 1996-03-29 | 2001-04-10 | Monsanto Company | Nucleic acid sequences encoding an antifungal polypeptide, aly AFP from alyssum and methods for their use |
| US6653280B2 (en) | 1996-03-29 | 2003-11-25 | Monsanto Technology Llc | Antifungal polypeptide AlyAFP from Alyssum and methods for controlling plant pathogenic fungi |
| US6166292A (en) | 1996-04-26 | 2000-12-26 | Ajinomoto Co., Inc. | Raffinose synthetase gene, method of producing raffinose and transgenic plant |
| US5985605A (en) | 1996-06-14 | 1999-11-16 | Her Majesty The Queen In Right Of Canada, As Represented By The Dept. Of Agriculture & Agri-Food Canada | DNA sequences encoding phytases of ruminal microorganisms |
| US5998700A (en) | 1996-07-02 | 1999-12-07 | The Board Of Trustees Of Southern Illinois University | Plants containing a bacterial Gdha gene and methods of use thereof |
| USRE37543E1 (en) | 1996-08-13 | 2002-02-05 | Monsanto Company | DNA sequence useful for the production of polyhydroxyalkanoates |
| US6248536B1 (en) | 1996-09-24 | 2001-06-19 | Monsanto Company | Bacillus thuringiensis CryET33 and CryET34 compositions and uses thereof |
| US6399330B1 (en) | 1996-09-24 | 2002-06-04 | Monsanto Technology Llc | Bacillus thuringiensis cryet33 and cryet34 compositions and uses thereof |
| US6326351B1 (en) | 1996-09-24 | 2001-12-04 | Monsanto Technology Llc | Bacillus thuringiensis CryET33 and CryET34 compositions and uses therefor |
| US6063756A (en) | 1996-09-24 | 2000-05-16 | Monsanto Company | Bacillus thuringiensis cryET33 and cryET34 compositions and uses therefor |
| US6537756B1 (en) | 1996-09-26 | 2003-03-25 | Monsanto Technology, Llc | Bacillus thuringiensis CryET29 compositions toxic to coleopteran insects and Ctenocephalides SPP |
| US6686452B2 (en) | 1996-09-26 | 2004-02-03 | Monsanto Technology Llc | Bacillus thuringiensis CryET29 compositions toxic to coleopteran insects and ctenocephalides SPP |
| US6093695A (en) | 1996-09-26 | 2000-07-25 | Monsanto Company | Bacillus thuringiensis CryET29 compositions toxic to coleopteran insects and ctenocephalides SPP |
| US6271443B1 (en) | 1996-10-29 | 2001-08-07 | Calgene Llc | Cotton and rice cellulose synthase DNA sequences |
| US6576818B1 (en) | 1996-10-29 | 2003-06-10 | Calgene Llc | Plant cellulose synthase and promoter sequences |
| US6221649B1 (en) | 1996-11-20 | 2001-04-24 | Monsanto Company | Chimeric bacillus thuringiensis-endotoxins and host cells expressing same |
| US6713063B1 (en) | 1996-11-20 | 2004-03-30 | Monsanto Technology, Llc | Broad-spectrum δ-endotoxins |
| US6156573A (en) | 1996-11-20 | 2000-12-05 | Monsanto Company | Hybrid Bacillus thuringiensis δ-endotoxins with novel broad-spectrum insecticidal activity |
| US6521442B2 (en) | 1996-11-20 | 2003-02-18 | Monsanto Technology Llc | Polynucleotide compositions encoding broad spectrum δ-endotoxins |
| US6645497B2 (en) | 1996-11-20 | 2003-11-11 | Monsanto Technology, Llc | Polynucleotide compositions encoding broad-spectrum δ endotoxins |
| US6242241B1 (en) | 1996-11-20 | 2001-06-05 | Monsanto Company | Polynucleotide compositions encoding broad-spectrum δ-endotoxins |
| US6281016B1 (en) | 1996-11-20 | 2001-08-28 | Monsanto Company | Broad-spectrum insect resistant transgenic plants |
| US6110464A (en) | 1996-11-20 | 2000-08-29 | Monsanto Company | Broad-spectrum δ-endotoxins |
| US6538109B2 (en) | 1996-11-20 | 2003-03-25 | Monsanto Technology, Llc | Polynucleotide compositions encoding broad spectrum delta-endotoxins |
| US5942664A (en) | 1996-11-27 | 1999-08-24 | Ecogen, Inc. | Bacillus thuringiensis Cry1C compositions toxic to lepidopteran insects and methods for making Cry1C mutants |
| US6313378B1 (en) | 1996-11-27 | 2001-11-06 | Monsanto Technology Llc | Lepidopteran-resistent transgenic plants |
| US6177615B1 (en) | 1996-11-27 | 2001-01-23 | Monsanto Company | Lepidopteran-toxic polypeptide and polynucleotide compositions and methods for making and using same |
| US6809078B2 (en) | 1996-11-27 | 2004-10-26 | Monsanto Technology Llc | Compositions encoding lepidopteran-toxic polypeptides and methods of use |
| US6423828B1 (en) | 1996-11-27 | 2002-07-23 | Monsanto Technology Llc | Nuclei acid and polypeptide compositions encoding lepidopteran-toxic polypeptides |
| US6153814A (en) | 1996-11-27 | 2000-11-28 | Monsanto Company | Polypeptide compositions toxic to lepidopteran insects and methods for making same |
| US6121436A (en) | 1996-12-13 | 2000-09-19 | Monsanto Company | Antifungal polypeptide and methods for controlling plant pathogenic fungi |
| US6316407B1 (en) | 1996-12-13 | 2001-11-13 | Monsanto Company | Antifungal polypeptide from alfalfa and methods for controlling plant pathogenic fungi |
| US6630306B1 (en) | 1996-12-19 | 2003-10-07 | Yale University | Bioreactive allosteric polynucleotides |
| US6171640B1 (en) | 1997-04-04 | 2001-01-09 | Monsanto Company | High beta-conglycinin products and their use |
| US6589767B1 (en) | 1997-04-11 | 2003-07-08 | Abbott Laboratories | Methods and compositions for synthesis of long chain polyunsaturated fatty acids |
| US6660849B1 (en) | 1997-04-11 | 2003-12-09 | Calgene Llc | Plant fatty acid synthases and use in improved methods for production of medium-chain fatty acids |
| US6372211B1 (en) | 1997-04-21 | 2002-04-16 | Monsanto Technolgy Llc | Methods and compositions for controlling insects |
| US6380466B1 (en) | 1997-05-08 | 2002-04-30 | Calgene Llc | Production of improved rapeseed exhibiting yellow-seed coat |
| US6596538B1 (en) | 1997-06-05 | 2003-07-22 | Calgene Llc | Fatty acyl-CoA: fatty alcohol acyltransferases |
| US6716474B2 (en) | 1997-06-17 | 2004-04-06 | Monsanto Technology Llc | Expression of fructose 1,6 bisphosphate aldolase in transgenic plants |
| US6441277B1 (en) | 1997-06-17 | 2002-08-27 | Monsanto Technology Llc | Expression of fructose 1,6 bisphosphate aldolase in transgenic plants |
| US6663906B2 (en) | 1997-06-17 | 2003-12-16 | Monsanto Technology Llc | Expression of fructose 1,6 bisphosphate aldolase in transgenic plants |
| US6072103A (en) | 1997-11-21 | 2000-06-06 | Calgene Llc | Pathogen and stress-responsive promoter for gene expression |
| US6620988B1 (en) | 1997-12-18 | 2003-09-16 | Monsanto Technology, Llc | Coleopteran-resistant transgenic plants and methods of their production using modified Bacillus thuringiensis Cry3Bb nucleic acids |
| US6642030B1 (en) | 1997-12-18 | 2003-11-04 | Monsanto Technology, Llc | Nucleic acid compositions encoding modified Bacillus thuringiensis coleopteran-toxic crystal proteins |
| US6063597A (en) | 1997-12-18 | 2000-05-16 | Monsanto Company | Polypeptide compositions toxic to coleopteran insects |
| US6023013A (en) | 1997-12-18 | 2000-02-08 | Monsanto Company | Insect-resistant transgenic plants |
| US6653530B1 (en) | 1998-02-13 | 2003-11-25 | Calgene Llc | Methods for producing carotenoid compounds, tocopherol compounds, and specialty oils in plant seeds |
| US6107549A (en) | 1998-03-10 | 2000-08-22 | Monsanto Company | Genetically engineered plant resistance to thiazopyr and other pyridine herbicides |
| US6228992B1 (en) | 1998-05-18 | 2001-05-08 | Pioneer Hi-Bred International, Inc. | Proteins for control of nematodes in plants |
| US6444876B1 (en) | 1998-06-05 | 2002-09-03 | Calgene Llc | Acyl CoA: cholesterol acyltransferase related nucleic acid sequences |
| US6459018B1 (en) | 1998-06-12 | 2002-10-01 | Monsanto Technology Llc | Polyunsaturated fatty acids in plants |
| US6822141B2 (en) | 1998-07-02 | 2004-11-23 | Calgene Llc | Diacylglycerol acyl transferase proteins |
| US6812379B2 (en) | 1998-07-10 | 2004-11-02 | Calgene Llc | Expression of eukaryotic peptides in plant plastids |
| US6495739B1 (en) | 1998-07-24 | 2002-12-17 | Calgene Llc | Plant phosphatidic acid phosphatases |
| US6537750B1 (en) | 1998-08-04 | 2003-03-25 | Cargill Incorporated | Plant fatty acid desaturase promoters |
| US6723897B2 (en) | 1998-08-10 | 2004-04-20 | Monsanto Technology, Llc | Methods for controlling gibberellin levels |
| US6380462B1 (en) | 1998-08-14 | 2002-04-30 | Calgene Llc | Method for increasing stearate content in soybean oil |
| US6468523B1 (en) | 1998-11-02 | 2002-10-22 | Monsanto Technology Llc | Polypeptide compositions toxic to diabrotic insects, and methods of use |
| US6448476B1 (en) | 1998-11-17 | 2002-09-10 | Monsanto Technology Llc | Plants and plant cells transformation to express an AMPA-N-acetyltransferase |
| US6531648B1 (en) | 1998-12-17 | 2003-03-11 | Syngenta Participations Ag | Grain processing method and transgenic plants useful therein |
| US6541259B1 (en) | 1999-04-15 | 2003-04-01 | Calgene Llc | Nucleic acid sequences to proteins involved in isoprenoid synthesis |
| US6555655B1 (en) | 1999-05-04 | 2003-04-29 | Monsanto Technology, Llc | Coleopteran-toxic polypeptide compositions and insect-resistant transgenic plants |
| US6506962B1 (en) | 1999-05-13 | 2003-01-14 | Monsanto Technology Llc | Acquired resistance genes in plants |
| US6489461B1 (en) | 1999-06-08 | 2002-12-03 | Calgene Llc | Nucleic acid sequences encoding proteins involved in fatty acid beta-oxidation and methods of use |
| US6770465B1 (en) | 1999-06-09 | 2004-08-03 | Calgene Llc | Engineering B-ketoacyl ACP synthase for novel substrate specificity |
| US6723837B1 (en) | 1999-07-12 | 2004-04-20 | Monsanto Technology Llc | Nucleic acid molecule and encoded protein associated with sterol synthesis and metabolism |
| US6501009B1 (en) | 1999-08-19 | 2002-12-31 | Monsanto Technology Llc | Expression of Cry3B insecticidal protein in plants |
| US6593293B1 (en) | 1999-09-15 | 2003-07-15 | Monsanto Technology, Llc | Lepidopteran-active Bacillus thuringiensis δ-endotoxin compositions and methods of use |
| US6573361B1 (en) | 1999-12-06 | 2003-06-03 | Monsanto Technology Llc | Antifungal proteins and methods for their use |
| US6639054B1 (en) | 2000-01-06 | 2003-10-28 | Monsanto Technology Llc | Preparation of deallergenized proteins and permuteins |
| US6657046B1 (en) | 2000-01-06 | 2003-12-02 | Monsanto Technology Llc | Insect inhibitory lipid acyl hydrolases |
| US6803501B2 (en) | 2000-03-09 | 2004-10-12 | Monsanto Technology, Llc | Methods for making plants tolerant to glyphosate and compositions thereof using a DNA encoding an EPSPS enzyme from Eleusine indica |
| US6518488B1 (en) | 2000-07-21 | 2003-02-11 | Monsanto Technology Llc | Nucleic acid molecules and other molecules associated with the β-oxidation pathway |
| US6706950B2 (en) | 2000-07-25 | 2004-03-16 | Calgene Llc | Nucleic acid sequences encoding β-ketoacyl-ACP synthase and uses thereof |
| US6949379B2 (en) | 2000-10-20 | 2005-09-27 | Canji, Inc. | Aptamer-mediated regulation of gene expression |
| US7151204B2 (en) | 2001-01-09 | 2006-12-19 | Monsanto Technology Llc | Maize chloroplast aldolase promoter compositions and methods for use thereof |
| US11186837B2 (en) | 2004-04-09 | 2021-11-30 | Monsanto Technology Llc | Compositions and methods for controlling Diabrotica |
| US20210259176A1 (en) | 2004-08-26 | 2021-08-26 | Monsanto Technology Llc | Methods of Seed Breeding Using High Throughput Nondestructive Seed Sampling |
| US8404927B2 (en) | 2004-12-21 | 2013-03-26 | Monsanto Technology Llc | Double-stranded RNA stabilized in planta |
| US9708620B2 (en) | 2004-12-21 | 2017-07-18 | Monsanto Technology Llc | Recombinant DNA constructs and methods for controlling gene expression |
| US10793869B2 (en) | 2004-12-21 | 2020-10-06 | Monsanto Technology Llc | Recombinant DNA constructs and methods for controlling gene expression |
| US8395023B2 (en) | 2004-12-21 | 2013-03-12 | Monsanto Technology Llc | Recombinant DNA constructs and methods for controlling gene expression |
| US8816061B2 (en) | 2005-01-07 | 2014-08-26 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Method to trigger RNA interference |
| US8030473B2 (en) | 2005-01-07 | 2011-10-04 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Method to trigger RNA interference |
| US8476422B2 (en) | 2005-01-07 | 2013-07-02 | State of Oregon acting by and through the State Board of Higher Eduction on behalf of Oregon State University | Method to trigger RNA interference |
| US20070011761A1 (en) | 2005-05-19 | 2007-01-11 | Monsanto Technology, L.L.C. | Post-transcriptional regulation of gene expression |
| US8334430B2 (en) | 2005-10-13 | 2012-12-18 | Monsanto Technology Llc | Methods for producing hybrid seed |
| US10876126B2 (en) | 2005-10-13 | 2020-12-29 | Monsanto Technology Llc | Methods for producing hybrid seed |
| US20220221377A1 (en) | 2006-03-02 | 2022-07-14 | Monsanto Technology Llc | Automated Contamination-Free Seed Sampler And Methods Of Sampling, Testing And Bulking Seeds |
| US20180312854A1 (en) | 2006-05-16 | 2018-11-01 | Monsanto Technology Llc | Use of non-agrobacterium bacterial species for plant transformation |
| US8404928B2 (en) | 2006-08-31 | 2013-03-26 | Monsanto Technology Llc | Phased small RNAs |
| US8946511B2 (en) | 2006-10-12 | 2015-02-03 | Monsanto Technology Llc | Plant microRNAs and methods of use thereof |
| US8410334B2 (en) | 2007-02-20 | 2013-04-02 | Monsanto Technology Llc | Invertebrate microRNAs |
| US9976152B2 (en) | 2007-06-26 | 2018-05-22 | Monsanto Technology Llc | Temporal regulation of gene expression by microRNAs |
| US20130102651A1 (en) | 2007-08-28 | 2013-04-25 | California Institute Of Technology | General composition framework for ligand-controlled rna regulatory systems |
| US9040774B2 (en) | 2008-07-01 | 2015-05-26 | Monsanto Technology Llc | Recombinant DNA constructs encoding ribonuclease cleavage blockers and methods for modulating expression of a target gene |
| US10017549B2 (en) | 2008-08-29 | 2018-07-10 | Monsanto Technology Llc | Hemipteran and coleopteran active toxin proteins from Bacillus thuringiensis |
| US20100311168A1 (en) | 2009-04-07 | 2010-12-09 | Dow Agrosciences Llc | Nanoparticle mediated delivery of sequence specific nucleases |
| US8455716B2 (en) | 2009-04-20 | 2013-06-04 | Monsanto Technology Llc | Multiple virus resistance in plants |
| US9873888B2 (en) | 2009-10-23 | 2018-01-23 | Monsanto Technology Llc | Transgenic soybean plants and chromosomes |
| US20120023619A1 (en) | 2010-07-07 | 2012-01-26 | Dow Agrosciences Llc | Linear dna molecule delivery using pegylated quantum dots for stable trasformation in plants |
| US20120244569A1 (en) | 2011-03-23 | 2012-09-27 | Dow Agrosciences Llc | Quantum dot carrier peptide conjugates suitable for imaging and delivery applications in plants |
| US9139838B2 (en) | 2011-07-01 | 2015-09-22 | Monsanto Technology Llc | Methods and compositions for selective regulation of protein expression |
| US20130145488A1 (en) | 2011-12-06 | 2013-06-06 | Iowa State University Research Foundation, Inc. | Mesoporous silica nanoparticles suitable for co-delivery |
| US20130185823A1 (en) | 2012-01-16 | 2013-07-18 | Academia Sinica | Mesoporous silica nanoparticle-mediated delivery of dna into arabidopsis root |
| US20140096284A1 (en) | 2012-10-01 | 2014-04-03 | Iowa State University Research Foundation, Inc. | Method for the delivery of molecules lyophilized onto microparticles to plant tissues |
| US20150040268A1 (en) | 2013-04-25 | 2015-02-05 | Morflora Israel Ltd | Methods and compositions for the delivery of nucleic acids to seeds |
| US20140356414A1 (en) | 2013-06-03 | 2014-12-04 | University Of Southern California | Targeted Crosslinked Multilamellar Liposomes |
| US9777288B2 (en) | 2013-07-19 | 2017-10-03 | Monsanto Technology Llc | Compositions and methods for controlling leptinotarsa |
| US20150047074A1 (en) | 2013-08-09 | 2015-02-12 | Massachusetts Institute Of Technology | Nanobionic engineering of organelles and photosynthetic organisms |
| US20150082478A1 (en) | 2013-08-22 | 2015-03-19 | E I Du Pont De Nemours And Company | Plant genome modification using guide rna/cas endonuclease systems and methods of use |
| US20150208663A1 (en) | 2013-10-15 | 2015-07-30 | Board Of Trustees Of The University Of Arkansas | Method of delivery of bio-active agents to plant cells by using nano-sized materials as carriers |
| US11091770B2 (en) | 2014-04-01 | 2021-08-17 | Monsanto Technology Llc | Compositions and methods for controlling insect pests |
| US10378012B2 (en) | 2014-07-29 | 2019-08-13 | Monsanto Technology Llc | Compositions and methods for controlling insect pests |
| US10494408B2 (en) | 2014-10-16 | 2019-12-03 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US10611806B2 (en) | 2014-10-16 | 2020-04-07 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to Lepidopteran pests |
| US10494409B2 (en) | 2014-10-16 | 2019-12-03 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US10669317B2 (en) | 2014-10-16 | 2020-06-02 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US10487123B2 (en) | 2014-10-16 | 2019-11-26 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US11267849B2 (en) | 2014-10-16 | 2022-03-08 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to lepidopteran pests |
| US10233217B2 (en) | 2014-10-16 | 2019-03-19 | Monsanto Technology Llc | Chimeric insecticidal proteins toxic or inhibitory to Lepidopteran pests |
| US20170369898A1 (en) | 2014-12-19 | 2017-12-28 | AgBiome, Inc. | Sequences to facilitate incorporation of dna into the genome of an organism |
| US10827755B2 (en) | 2015-11-18 | 2020-11-10 | Monsanto Technology Llc | Insecticidal compositions and methods |
| US10612037B2 (en) | 2016-06-20 | 2020-04-07 | Monsanto Technology Llc | Insecticidal proteins toxic or inhibitory to hemipteran pests |
| US11254950B2 (en) | 2016-06-20 | 2022-02-22 | Monsanto Technology Llc | Insecticidal proteins toxic or inhibitory to hemtpteran pests |
| US11136593B2 (en) | 2016-09-09 | 2021-10-05 | Syngenta Participations Ag | Insecticidal proteins |
| US11130965B2 (en) | 2016-10-27 | 2021-09-28 | Syngenta Participations Ag | Insecticidal proteins |
| US20190032131A1 (en) | 2016-12-12 | 2019-01-31 | Integrated Dna Technologies, Inc. | Genome editing detection |
| US11180774B2 (en) | 2017-01-12 | 2021-11-23 | Syngenta Participations Ag | Insecticidal proteins |
| US20190352655A1 (en) | 2017-01-28 | 2019-11-21 | Inari Agriculture, Inc. | Novel plant cells, plants, and seeds |
| US11844318B2 (en) | 2018-07-25 | 2023-12-19 | Invaio Sciences International Gmbh | Injection systems, injection tools and methods for same |
| WO2023004435A1 (en) | 2021-07-23 | 2023-01-26 | Flagship Pioneering Innovations Vii, Llc | Compositions for fungal control and related methods |
Non-Patent Citations (35)
| Title |
|---|
| "NCBI Gene ID", Database accession no. 101249087 |
| ALTSCHUL ET AL., J. MOL. BIOL., vol. 215, 1990, pages 403 - 410 |
| ALTSTEIN, PEPTIDES, vol. 25, 2004, pages 1373 - 1376 |
| AMAN ET AL., GENOME BIOL., vol. 19, 2018, pages 1 - 10 |
| CHOI ET AL., J. CONTROLLED RELEASE, vol. 235, 2016, pages 222 - 235 |
| CONG ET AL., SCIENCE, vol. 339, 2013, pages 819 - 823 |
| DE CESARE ET AL., MBIO, vol. 11, 2020, pages 02123 - 20 |
| DUANIS-ASSAF, PLANT BIOTECHNOL J, vol. 20, no. 1, January 2022 (2022-01-01), pages 226 - 237 |
| EDGAR, NUCLEIC ACIDS RES., vol. 32, no. 5, 2004, pages 1792 - 1797 |
| GEISBERG ET AL., CELL, vol. 156, 2014, pages 812 - 824 |
| GIRALDO ET AL., NATURE MATERIALS, vol. 13, 2014, pages 400 - 409 |
| HENDEL ET AL., NATURE BIOTECHNOL., 2015, pages 985 - 991 |
| HOFACKER ET AL., MONATSHEFTE FIIR CHEMIE CHEM. MONTHLY, vol. 125, 1994, pages 167 - 188 |
| HORIUCHI ET AL., PLANT CELL PHYSIOL., vol. 42, no. 2, 2001, pages 197 - 203 |
| KIM, BIOCONJUGATE CHEM., vol. 22, 2011, pages 2558 - 2567 |
| KOEVMILLER, J VIROL, vol. 74, no. 13, July 2000 (2000-07-01), pages 5988 - 96 |
| LI, MOL PLANT MICROBE INTERACT, vol. 32, no. 12, December 2019 (2019-12-01), pages 1649 - 1664 |
| MATTEI ET AL., NUCLEIC ACIDS RESEARCH, vol. 42, no. 10, 2014, pages 6146 - 6157 |
| O'BRIANLUMMIS, BMC BIOTECHNOL., vol. 11, 2011, pages 66 - 71 |
| PUTLYAEV ET AL., BIOCHEMISTRY, vol. 80, no. 8, pages 1039 - 46 |
| RAMLANZAUNER: "International Workshop on Computing With Biomolecules", 27 August 2008, AUSTRIAN COMPUTER SOCIETY, pages: 75 - 86 |
| RAN ET AL., NATURE PROTOCOLS, vol. 8, 2013, pages 2281 - 2308 |
| RANDOLPH-ANDERSON ET AL.: "Submicron gold particles are superior to larger particles for efficient Biolistic® transformation of organelles and some cell types", BIO-RAD US/EG BULLETIN, 2015 |
| ROBINSON ET AL., BIOINFORMATICS, vol. 39, no. 1, 2023 |
| SHEN ET AL., THERANOSTICS, vol. 2, 2012, pages 283 - 294 |
| SMAKOV ET AL., NATURE REVIEWS MICROBIOL., 2017 |
| TANGBREAKER, PROC NATL ACAD SCI USA., vol. 97, no. 11, 23 May 2000 (2000-05-23), pages 5784 - 9 |
| WANG ET AL., J. AM. CHEM. SOC. COMM.., vol. 132, 2010, pages 9274 - 9276 |
| WHITHAM, S. ET AL., CELL, vol. 78, 1994, pages 1101 - 1115 |
| WOLTER ET AL., BMC PLANT BIOL., vol. 19, 2019, pages 176 - 183 |
| WONG ET AL., NANO LETT., vol. 16, 2016, pages 1161 - 1172 |
| XING ET AL., BMC PLANT BIOL.., vol. 14, 2014, pages 327 - 340 |
| YOUNG ET AL.: "Compendium of Herbicide Adjuvants", 2016, PURDUC UNIVERSITY |
| ZHANG ET AL., J. CONTROLLED RELEASE, vol. 123, 2007, pages 1 - 10 |
| ZHAO ET AL., NANOSCALE RES. LETT., vol. 11, 2016, pages 195 - 203 |
Also Published As
| Publication number | Publication date |
|---|---|
| AR132589A1 (en) | 2025-07-16 |
| WO2024229356A3 (en) | 2024-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12102091B2 (en) | Control of plant pests using RNA molecules | |
| CN103282499A (en) | Process of transfecting plants | |
| US20250270582A1 (en) | Methods for modifying plants with a recombinant rna molecule | |
| CN103763916A (en) | Pesticidal nucleic acids and proteins and uses thereof | |
| BR112020007465A2 (en) | pest control of hemiptera using RNA molecules | |
| US20250230452A1 (en) | Polynucleotides for modifying organisms | |
| US11578338B2 (en) | Hemipteran active insecticidal protein | |
| US20150203866A1 (en) | Methods and compositions for plant pest control | |
| WO2024229356A2 (en) | Artificial solemoviridae satellite rnas | |
| US20230189818A1 (en) | Rna-based control of botrytis | |
| WO2024229398A1 (en) | Artificial amalgavirus satellite rnas | |
| WO2024229362A1 (en) | ARTIFICIAL MARTELLIVIRALES SATELLITE RNAs | |
| WO2024229347A1 (en) | Artificial tombusviridae satellite rnas | |
| WO2024229359A2 (en) | Artificial tymovirales satellite rnas | |
| WO2024229351A1 (en) | Artificial secoviridae satellite rnas | |
| WO2024229385A1 (en) | Artificial ghabrivirales satellite rnas | |
| WO2024229395A1 (en) | Partitiviral satellite rna amplification systems for plants | |
| WO2024229403A1 (en) | Endornaviral satellite rna amplification systems for plants | |
| CN118922549A (en) | Polynucleotides for modifying organisms | |
| CN118556127A (en) | Polynucleotides for modifying organisms |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24729529 Country of ref document:EP Kind code of ref document:A2 |