WO2024220930A2 - Mapt-modulating compositions and methods of use thereof - Google Patents
Mapt-modulating compositions and methods of use thereofDownload PDFInfo
- Publication number
- WO2024220930A2 WO2024220930A2PCT/US2024/025577US2024025577WWO2024220930A2WO 2024220930 A2WO2024220930 A2WO 2024220930A2US 2024025577 WUS2024025577 WUS 2024025577WWO 2024220930 A2WO2024220930 A2WO 2024220930A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- modified oligonucleotide
- certain embodiments
- positions
- ligands
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7125—Nucleic acids or oligonucleotides having modified internucleoside linkage, i.e. other than 3'-5' phosphodiesters
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/312—Phosphonates
- C12N2310/3125—Methylphosphonates
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/322—2'-R Modification
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/343—Spatial arrangement of the modifications having patterns, e.g. ==--==--==--
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
- C12N2310/3513—Protein; Peptide
Definitions
- the present disclosureprovides compounds, compositions, and methods for modulating the expression or activity of microtubule associated protein tau (MAPT).
- the compounds, compositions, and methodscan be used to reduce the expression of MAPT mRNA in a cell or animal.
- the compounds, compositions, and methodscan be used to reduce the amount of MAPT protein in a cell or animal.
- the animalhas a CNS related disease, disorder or condition.
- the disease, disorder or conditionis a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
- a neurodegenerative diseaseincluding a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
- Certain compounds, compositions and methods provided hereinare directed to reducing a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal.
- a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereofincluding a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal.
- the compounds and compositions provided hereinare potent and tolerable and inhibit MAPT expression, which can be used to treat, prevent, ameliorate, or slow progression of a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compounds and compositionscomprise one or more features that are effective for increasing potency.
- the compounds and compositionscomprise one or more features that are effective for increasing tolerability. In certain embodiments, compounds and compositions comprise one or more features that are effective for targeting the compound or composition to a cell or tissue. In certain embodiments, the compounds and compositions are more potent, have greater duration of action or have greater therapeutic value than compounds publicly disclosed.
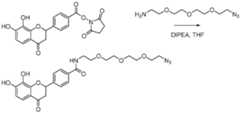
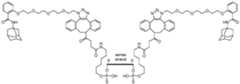
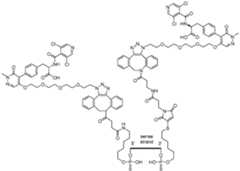
- FIG. 1shows exemplary compounds of the present disclosure comprising one or more ligands.
- FIG. 2shows exemplary compounds of the present disclosure comprising one or more Tropomyosin Receptor Kinase B (TrkB) ligands.
- TrkBTropomyosin Receptor Kinase B
- FIG. 3shows exemplary compounds of the present disclosure comprising one or more cannabinoid receptor type 1 (CB 1 ) ligands.
- FIG. 4shows exemplary compounds of the present disclosure comprising one or more ⁇ 4 ⁇ 1/7 integrin ligands.
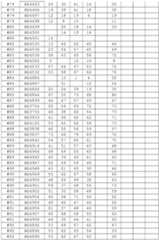
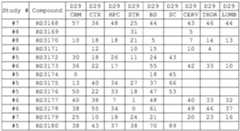
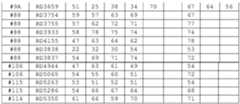
- Oligomeric compounds referenced by Compound Number or Ref ID NOindicate a combination of nucleobase sequence, chemical modification, and motif.
- the use of the singularincludes the plural unless specifically stated otherwise.
- the articles “a” and “an”are used herein to refer to one or to more than one (i.e., to at least one) of the grammatical object of the article.
- an elementmeans one element or more than one element, e.g., a plurality of elements.
- the use of “or”means “and/or” unless stated otherwise.
- MMTMicrotubule Associated Protein Tau
- SEQ ID NO: 1GenBank Accession No. NM_001377265.1
- SEQ ID NO: 2nucleotide sequence of MAPT
- “MAPT mRNA”means an mRNA encoding a MAPT protein.
- MAPTmay be referred to in either upper or lower case.
- MAPT specific inhibitorrefers to any agent capable of specifically inhibiting MAPT RNA and/or MAPT protein expression or activity at the molecular level.
- MAPT specific inhibitorsinclude nucleic acids (including oligonucleotide compounds), peptides, antibodies, small molecules, and other agents capable of inhibiting the expression of MAPT RNA and/or MAPT protein.
- “2’-O-methoxyethyl” or “2’-MOE”means a 2’-O(CH 2 ) 2 -OCH 3 modification.
- a 2’-O- methoxyethyl modified sugaris a modified sugar with 2’-O(CH 2 ) 2 -OCH 3 in the place of the 2’-OH group of a ribosyl ring.
- “5’ start site”means the nucleotide of the target nucleic acid or region which is aligned to the 3’-most nucleoside of an antisense oligonucleotide.
- 3’ stop sitemeans the nucleotide of the target nucleic acid or region which is aligned to the 5’-most nucleoside of an antisense oligonucleotide.
- “About”means within ⁇ 10% of a value.
- administeringrefers to routes of introducing a compound or composition provided herein to an individual to perform its intended function.
- routes of administrationinclude, but are not limited to, intrathecal (IT) administration, intracerebroventricular (ICV) administration, parenteral administration, such as subcutaneous administration, intravenous administration, intramuscular administration, intraarterial administration, intraperitoneal administration, or intracranial administration, e.g.
- “Ameliorate”refers to an improvement or lessening of at least one indicator, sign, or symptom of an associated disease, disorder, or condition. In certain embodiments, amelioration includes a delay or slowing in the progression or severity of one or more indicators of a condition or disease. The progression or severity of indicators may be determined by subjective or objective measures, which are known to those skilled in the art.
- “Animal”refers to a human or non-human animal, including, but not limited to, mice, rats, rabbits, dogs, cats, pigs, and non-human primates, including, but not limited to, monkeys and chimpanzees.
- Antisense oligonucleotideor “antisense strand” means an oligonucleotide which includes a region that is complementary to a target nucleic acid, e.g., a MAPT RNA or a region thereof.
- “Complementarity” in reference to an oligonucleotidemeans the nucleobase sequence of such oligonucleotide or one or more regions thereof that is complementary to the nucleobase sequence of another oligonucleotide or nucleic acid or one or more regions thereof when the two nucleobase sequences are aligned in opposing directions.
- Complementary nucleobasesare limited to the following pairs: adenine (A) and thymine ( ⁇ ), adenine (A) and uracil (U), and cytosine (C) and guanine (G) unless otherwise specified.
- Complementary oligonucleotides and/or nucleic acidsneed not have nucleobase complementarity at each nucleoside and may include one or more nucleobase mismatches.
- “fully complementary” or “100% complementary” in reference to oligonucleotidesmeans that such oligonucleotides have nucleobase matches at each nucleoside without any nucleobase mismatches.
- compositionor “pharmaceutical composition” means a mixture of substances suitable for administering to an individual.
- a compositionmay comprise one or more compounds or salt thereof and a sterile aqueous solution.
- Co-administrationmeans administration of two or more compounds in any manner in which the pharmacological effects of both are manifest in the patient. Co-administration does not require both compounds to be administered in a single pharmaceutical composition, in the same dosage form, by the same route of administration, or at the same time. The effects of both compounds need not manifest themselves at the same time. The effects need only be overlapping for a period of time and need not be coextensive. Co-administration includes parallel or sequential administration of the one or more compounds.
- Conjugate groupmeans a group of atoms that is attached to an oligonucleotide.
- a conjugate groupis optionally attached to an oligonucleotide through a conjugate linker.
- a conjugate groupmay, for example, alter the distribution, targeting, or half-life of a compound into which it is incorporated.
- Conjugate groupsinclude lipids (or lipophilic moieties), ligands, and other targeting moieties.
- Conjugate linkermeans a group of atoms comprising at least one bond that connects a linked moiety to an oligonucleotide.
- Identityin reference to an oligonucleotide means the nucleobase sequence of such oligonucleotide or one or more regions thereof that matches the nucleobase sequence of another oligonucleotide or nucleic acid or one or more regions thereof. Identity of an oligonucleotide to another oligonucleotide or nucleic acid need not require each nucleobase to match and may include one or more different nucleobases. By contrast, “fully identical” or “100% identity” in reference to oligonucleotides means that such oligonucleotides have the same nucleobase at each relative position over its length as the other oligonucleotide or nucleic acid.
- “Individual”means a human or non-human animal selected for treatment or therapy. “Inhibiting the expression or activity” with reference to a target nucleic acid or protein means to reduce or block the expression or activity of such target relative to the expression or activity in an untreated or control sample and does not necessarily indicate a total elimination of expression or activity.
- the term “internucleoside linkage”is the covalent linkage between adjacent nucleosides in an oligonucleotide.
- “modified internucleoside linkage”means any internucleoside linkage other than a phosphodiester internucleoside linkage.
- “Phosphorothioate internucleoside linkage”is a modified internucleoside linkage in which one of the non-bridging oxygen atoms of a phosphodiester internucleoside linkage is replaced with a sulfur atom.
- Representative internucleoside linkages having a chiral centerinclude but are not limited to alkylphosphonates and phosphorothioates.
- Modified oligonucleotides comprising internucleoside linkages having a chiral centercan be prepared as populations of modified oligonucleotides comprising stereorandom internucleoside linkages, or as populations of modified oligonucleotides comprising phosphorothioate linkages in particular stereochemical configurations as further described below.
- chiral internucleoside linkages of modified oligonucleotides described hereincan be stereorandom or in a particular stereochemical configuration.
- the compounds of the present disclosuremay also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds.
- the compoundsmay be radiolabeled with radioactive isotopes, such as for example tritium ( 3 H), iodine-125 ( 125 I), or carbon-14 ( 14 C). All isotopic variations of the compounds of the present disclosure, whether radioactive or not, are encompassed within the scope of the present disclosure.
- isotopic variantrefers to a therapeutic agent (e.g., a compound and/or modified oligonucleotide disclosed herein) that contains an unnatural proportion of an isotope at one or more of the atoms that constitute such a therapeutic agent.
- a therapeutic agente.g., a compound and/or modified oligonucleotide disclosed herein
- an “isotopic variant” of a therapeutic agentcontains unnatural proportions of one or more isotopes, including, but not limited to, hydrogen (H), deuterium ( 2 H), tritium ( 3 H), carbon-11 ( 11 C), carbon-12 ( 12 C), carbon-13 ( 13 C), carbon-14 ( 14 C), nitrogen-13 ( 13 N), nitrogen-14 ( 14 N), nitrogen-15 ( 15 N), oxygen-14 ( 14 O), oxygen-15 ( 15 O), oxygen-16 ( 16 O), oxygen-17 ( 17 O), oxygen-18 ( 18 O), fluorine-17 ( 17 F), fluorine-18 ( 18 F), phosphorus-31 ( 31 P), phosphorus-32 ( 32 P), phosphorus-33 ( 33 P), sulfur-32 ( 32 S), sulfur-33 ( 33 S), sulfur-34 ( 34 S), sulfur-35 ( 35 S), sulfur-36 ( 36 S), chlorine-35 ( 35 Cl), chlorine-36 ( 36 Cl), chlorine-37 ( 37 Cl), bromine-79 ( 79 Br), bromine-81 ( 81 Br), iodine 123 (
- an “isotopic variant” of a therapeutic agentcontains unnatural proportions of one or more isotopes, including, but not limited to, hydrogen (H), deuterium ( 2 H), tritium ( 3 H), carbon-11 ( 11 C), carbon-12 ( 12 C), carbon-13 ( 13 C), carbon-14 ( 14 C), nitrogen-13 ( 13 N), nitrogen-14 ( 14 N), nitrogen-15 ( 15 N), oxygen-14 ( 14 O), oxygen-15 ( 15 O), oxygen-16 ( 16 O), oxygen-17 ( 17 O), oxygen-18 ( 18 O), fluorine-17 ( 17 F), fluorine-18 ( 18 F), phosphorus-31 ( 31 P), phosphorus-32 ( 32 P), phosphorus-33 ( 33 P), sulfur-32 ( 32 S), sulfur-33 ( 33 S), sulfur-34 ( 34 S), sulfur-35 ( 35 S), sulfur-36 ( 36 S), chlorine-35 ( 35 Cl), chlorine-36 ( 36 Cl), chlorine-37 ( 37 Cl), bromine-79 ( 79 Br), bromine-81 ( 81 Br), iodine 123 (
- any hydrogencan be 2 H, for example, or any carbon can be 13 C, for example, or any nitrogen can be 15 N, for example, or any oxygen can be 18 O, for example, where feasible according to the judgment of one of skill.
- an “isotopic variant” of a therapeutic agentcontains unnatural proportions of deuterium (D).
- Lipidor “lipophilic moiety” refers to an aliphatic, cyclic (such as alicyclic), or polycyclic (such as polyalicyclic) compound, such as a steroid (e.g., sterol) or a linear or branched aliphatic hydrocarbon.
- a steroide.g., sterol
- a linear or branched aliphatic hydrocarbonsuch as a steroid (e.g., sterol) or a linear or branched aliphatic hydrocarbon.
- lipidincludes cholesterol, retinoic acid, cholic acid, adamantane acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-bis- O(hexadecyl)glycerol, geranyloxyhexyanol, hexadecylglycerol, borneol, menthol, 1,3- propanediol, heptadecyl group, palmitic acid, myristic acid, O3-(oleoyl)lithocholic acid, O3- (oleoyl)cholenic acid, ibuprofen, naproxen, dimethoxytrityl, or phenoxazine.
- lipidincludes a saturated or unsaturated C 4 -C 30 hydrocarbon chain (e.g., C 4 -C 30 alkyl or alkenyl).
- the lipophilic moietycontains a saturated or unsaturated C 5 -C 20 hydrocarbon chain (e.g., a linear C 5 -C 20 alkyl or alkenyl).
- the lipophilic moietycontains a saturated or unsaturated C 14 -C 20 hydrocarbon chain (e.g., a linear C 14 -C 20 alkyl or alkenyl).
- the lipophilic moietycontains a saturated or unsaturated C 6 -C 18 hydrocarbon chain (e.g., a linear C 6 -C 18 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C16 hydrocarbon chain (e.g., a linear C 16 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C 17 hydrocarbon chain (e.g., a linear C 17 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C18 hydrocarbon chain (e.g., a linear C 18 alkyl or alkenyl).
- the lipophilic moietycontains a saturated or unsaturated C 22 hydrocarbon chain (e.g., a linear C 22 alkyl or alkenyl).
- “Mismatch” or “non-complementary”means a nucleobase of a first oligonucleotide or nucleic acid that is not complementary to the corresponding nucleobase of a second oligonucleotide or nucleic acid when the first oligonucleotide/nucleic acid and second oligonucleotide/nucleic acid are aligned in an antiparallel orientation.
- nucleobasesincluding, but not limited to, a universal nucleobase, inosine, and hypoxanthine, are capable of hybridizing with at least one nucleobase but are still mismatched or non- complementary with respect to the nucleobase to which they are hybridized.
- a nucleobase of a first oligonucleotide/nucleic acid that is not capable of hybridizing to the corresponding nucleobase of a second oligonucleotide/nucleic acid when the first and second oligonucleotides are aligned in an antiparallel orientationis a mismatch or non-complementary nucleobase.
- Modified oligonucleotidemeans an oligonucleotide, wherein at least one sugar, nucleobase, or internucleoside linkage is modified. “Modulating” refers to changing or adjusting a feature in a cell, tissue, organ or organism. For example, modulating MAPT RNA can mean to increase or decrease the level of MAPT RNA and/or MAPT protein in a cell, tissue, organ or organism. A “modulator” effects the change in the cell, tissue, organ or organism. For example, a MAPT compound can be a modulator that decreases the amount of MAPT RNA and/or MAPT protein in a cell, tissue, organ or organism.
- “Motif”means the pattern of unmodified and modified sugar moieties, nucleobases, and/or internucleoside linkages, in an oligonucleotide.
- Nucleic acidrefers to molecules composed of monomeric nucleotides. A nucleic acid includes, but is not limited to, ribonucleic acids (RNA), deoxyribonucleic acids (DNA), single-stranded nucleic acids, and double-stranded nucleic acids.
- “Nucleobase”means a heterocyclic moiety capable of pairing with a base of another nucleic acid.
- nucleobase sequencemeans the order of contiguous nucleobases in a nucleic acid or oligonucleotide independent of any sugar or internucleoside linkage.
- Nucleosidemeans a compound comprising a nucleobase and a sugar moiety. The nucleobase and sugar moiety are each, independently, unmodified or modified.
- Modified nucleosidemeans a nucleoside comprising a modified nucleobase and/or a modified sugar moiety. Modified nucleosides include abasic nucleosides, which lack a nucleobase.
- Oligonucleotidesmeans a compound comprising one or more oligonucleotides and optionally one or more additional features, such as a conjugate group or terminal group.
- oligomeric compoundsinclude single-stranded and double- stranded compounds, such as, oligonucleotides, antisense oligonucleotides, interfering RNA compounds (RNAi compounds), microRNA targeting oligonucleotides, occupancy-based compounds (e.g., mRNA processing or translation blocking compounds and splicing compounds).
- RNAi compoundsinterfering RNA compounds
- microRNA targeting oligonucleotidese.g., occupancy-based compounds (e.g., mRNA processing or translation blocking compounds and splicing compounds).
- RNAi compoundsinclude double-stranded compounds (e.g., short-interfering RNA (siRNA) and double-stranded RNA (dsRNA)) and single-stranded compounds (e.g., single-stranded siRNA (ssRNA), single-stranded RNAi (ssRNAi), short hairpin RNA (shRNA) and microRNA mimics) which work at least in part through the RNA-induced silencing complex (RISC) pathway resulting in sequence specific degradation and/or sequestration of a target nucleic acid through a process known as RNA interference (RNAi).
- siRNAsingle-stranded siRNA
- ssRNAisingle-stranded RNAi
- shRNAshort hairpin RNA
- microRNA mimicsRNA-induced silencing complex
- RNAi compoundis meant to be equivalent to other terms used to describe nucleic acid compounds that are capable of mediating sequence-specific RNA interference, for example, interfering RNA (iRNA), iRNA agent, RNAi agent, short interfering oligonucleotide, short interfering nucleic acid, short interfering modified oligonucleotide, chemically modified siRNA, and others. Additionally, the term “RNAi” is meant to be equivalent to other terms used to describe sequence-specific RNA interference. “Oligomeric duplex” means a duplex formed by two oligomeric compounds having complementary nucleobase sequences.
- Each oligomeric compound of an oligomeric duplexmay be referred to as a “duplexed oligomeric compound.”
- the oligonucleotides of each oligomeric compound of an oligomeric duplexmay include non-complementary overhanging nucleosides.
- duplexed oligomeric compoundand “modified oligonucleotide” are used interchangeably.
- oligomeric duplexand “compound” are used interchangeably.
- “Oligonucleotide”means a polymer of linked nucleosides, each of which can be modified or unmodified, independent from one another.
- Parent administrationmeans administration through injection or infusion.
- Parenteral administrationincludes subcutaneous administration, intravenous administration, intramuscular administration, intraarterial administration, intraperitoneal administration, or intracranial administration, e.g. intrathecal or intracerebroventricular administration.
- “Pharmaceutically acceptable carrier or diluent”means any substance suitable for use in administering to an individual.
- a pharmaceutically acceptable carrier or diluentaids the administration of a compound to and absorption by an individual and can be included in the compositions of the present disclosure without causing a significant adverse toxicological effect on the patient.
- Non-limiting examples of pharmaceutically acceptable excipientsinclude water, NaCl, normal saline solutions, and the like.
- a pharmaceutically acceptable carriercan be a sterile aqueous solution, such as PBS or water-for-injection.
- a pharmaceutically acceptable saltmeans or refers to physiologically and pharmaceutically acceptable salts of compounds, such as oligomeric compounds or oligonucleotides, i.e., salts that retain the desired biological activity of the parent compound and do not impart undesired toxicological effects thereto.
- Tropomyosin Receptor Kinase B” or “TrkB,” as may be used interchangeably herein,means the receptor for brain-derived neurotrophic factor (BDNF) protein encoded by the NTRK2 gene.
- BDNFbrain-derived neurotrophic factor
- TrkBis also known as tyrosine receptor kinase B, BDNF/NT-3 growth factors receptor and neurotrophic tyrosine kinase, receptor, type 2.
- a pharmaceutically acceptable saltis any salt of a compound provided herein which retains its biological properties and which is not toxic or otherwise undesirable for pharmaceutical use.
- the pharmaceutically acceptable salts of the therapeutic agents disclosed hereininclude salts that are prepared with relatively nontoxic acids or bases, depending on the particular substituents found on the compounds or modified oligonucleotides described herein. When compounds of the present disclosure contain relatively acidic functionalities, base addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired base, either neat or in a suitable inert solvent.
- acid addition saltscan be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired acid, either neat or in a suitable inert solvent.
- the compounds of the present disclosuremay exist as salts, such as with pharmaceutically acceptable acids.
- Such saltsmay be derived from a variety of organic and inorganic counter-ions well known in the art.
- Such saltsinclude, but are not limited to: (1) acid addition salts formed with organic or inorganic acids such as hydrochloric, hydrobromic, sulfuric, nitric, phosphoric, sulfamic, acetic, trifluoroacetic, trichloroacetic, propionic, hexanoic, cyclopentylpropionic, glycolic, glutaric, pyruvic, lactic, malonic, succinic, sorbic, ascorbic, malic, maleic, fumaric, tartaric, citric, benzoic, 3-(4-hydroxybenzoyl)benzoic, picric, cinnamic, mandelic, phthalic, lauric, methanesulfonic, ethanesulfonic, 1,2-ethane- disulfonic, 2-hydroxyethanesulfonic, benzenesulfonic, 4-chlorobenzenesulfonic, 2- naphthalenesulfonic, 4-to
- Pharmaceutically acceptable saltsfurther include, by way of example only and without limitation, sodium, potassium, calcium, magnesium, ammonium, tetraalkylammonium, and the like, and when the compound contains a basic functionality, salts of non-toxic organic or inorganic acids, such as hydrohalides, e.g.
- the pharmaceutically acceptable salt of the compounds and modified oligonucleotides disclosed hereinis a sodium or a potassium salt. In some embodiments, the pharmaceutically acceptable salt of the compounds and modified oligonucleotides disclosed herein is a sodium salt.
- the neutral forms of the compoundsare preferably regenerated by contacting the salt with a base or acid and isolating the parent compound in the conventional manner. The parent form of the compound may differ from the various salt forms in certain physical properties, such as solubility in polar solvents.
- compounds of the present disclosurecontain both basic and acidic functionalities that allow the compounds to be converted into either base or acid addition salts.
- the neutral forms of the compoundsmay be regenerated by contacting the salt with a base or acid and isolating the parent compound in a conventional manner.
- the parent form of the compoundsdiffers from the various salt forms in certain physical properties, such as solubility in polar solvents, but, unless specifically indicated, the salts disclosed herein are equivalent to the parent form of the compound for the purposes of the present disclosure.
- “Pharmaceutical agent”means a compound that provides a therapeutic benefit when administered to an individual.
- “Phosphorothioate linkage”means a modified phosphate linkage in which one of the non-bridging oxygen atoms is replaced with a sulfur atom.
- “Portion”means a defined number of contiguous (i.e., linked) nucleobases of a nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of a target nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of an oligonucleotide. “Prevent” refers to delaying or forestalling the onset, development or progression of a disease, disorder, or condition for a period of time.
- RNA interference compoundor “RNAi compound” means a compound that acts, at least in part, through an RNA-induced silencing complex (RISC) pathway or Ago2, but not through RNase ⁇ , to modulate a target nucleic acid and/or protein encoded by a target nucleic acid.
- RNAi compoundsinclude, but are not limited to double-stranded siRNA, single- stranded siRNA, and microRNA, including microRNA mimics.
- Sense oligonucleotide” or “sense strand”means the strand of a double-stranded compound that includes a region that is substantially complementary to a region of the antisense strand of the compound.
- Specifically inhibitwith reference to a target nucleic acid or protein means to reduce or block expression or activity of the target nucleic acid or protein while minimizing or eliminating effects on non-target nucleic acids or proteins.
- “Subunit” with reference to an oligonucleotidemeans a nucleotide, nucleoside, nucleobase or sugar or a modified nucleotide, nucleoside, nucleobase or sugar as provided herein.
- Target nucleic acid“target RNA,” and “nucleic acid target” all mean a nucleic acid capable of being targeted by compounds described herein.
- “Target region”means a portion of a target nucleic acid to which one or more compounds is targeted.
- Targeting moietymeans a conjugate group that provides an enhanced affinity for a selected target, e.g., molecule, cell or cell type, compartment, e.g., a cellular or organ compartment, tissue, organ or region of the body, as, e.g., compared to a compound absent such a moiety.
- Terminal groupmeans a chemical group or group of atoms that is covalently linked to a terminus of an oligonucleotide.
- “Therapeutically effective amount” or “effective amount”means an amount of a compound, pharmaceutical agent, or composition that provides a therapeutic benefit to an individual.
- a “therapeutically effective amount” or “effective amount”is an amount sufficient for a compound to accomplish a stated purpose relative to the absence of the compound (e.g., achieve the effect for which it is administered, treat, prevent or ameliorate a disease or reduce one or more symptoms of a disease or condition).
- An example of a “therapeutically effective amount” or “effective amount”is an amount sufficient to contribute to the treatment, prevention, amelioration, or reduction of a symptom or symptoms of a disease.
- a “reduction” of a symptom or symptomsmeans decreasing of the severity or frequency of the symptom(s), or elimination of the symptom(s).
- a “prophylactically effective amount” of a drugis an amount of a drug that, when administered to a subject, will have the intended prophylactic effect, e.g., preventing or delaying the onset (or reoccurrence) of an injury, disease, pathology or condition, or reducing the likelihood of the onset (or reoccurrence) of an injury, disease, pathology, or condition, or their symptoms.
- a therapeutically effective amountwill show an increase or decrease of at least 5%, 10%, 15%, 20%, 25%, 40%, 50%, 60%, 75%, 80%, 90%, or at least 100%.
- Therapeutic efficacycan also be expressed as “-fold” increase or decrease.
- a therapeutically effective amountcan have at least a 1.2- fold, 1.5-fold, 2-fold, 5-fold, or more effect over a control.
- treatingrefers to any indicia of success in the therapy or amelioration of an injury, disease, pathology or condition, including any objective or subjective parameter such as abatement; remission; diminishing of symptoms or making the injury, pathology or condition more tolerable to the patient; slowing in the rate of degeneration or decline; making the final point of degeneration less debilitating; improving a patient's physical or mental well-being.
- the treatment or amelioration of symptomscan be based on objective or subjective parameters, including the results of a physical examination.
- the term “treating” and conjugations thereof,may include prevention of an injury, pathology, condition, or disease.
- treatingis preventing.
- treatingdoes not include preventing.
- Treating” or “treatment” as used hereinalso broadly includes any approach for obtaining beneficial or desired results in a subject's condition, including clinical results.
- Beneficial or desired clinical resultscan include, but are not limited to, alleviation or amelioration of one or more symptoms or conditions, diminishment of the extent of a disease, stabilizing (i.e., not worsening) the state of disease, prevention of a disease’s transmission or spread, delay or slowing of disease progression, amelioration or palliation of the disease state, diminishment of the reoccurrence of disease, and remission, whether partial or total and whether detectable or undetectable.
- treatmentas used herein includes any cure, amelioration, or prevention of a disease.
- Treatmentmay prevent the disease from occurring; inhibit the disease’s spread; relieve the disease’s symptoms, fully or partially remove the disease’s underlying cause, shorten a disease’s duration, or do a combination of these things.
- Treating” and “treatment” as used hereininclude prophylactic treatment.
- Treatment methodsinclude administering to a subject a therapeutically effective amount of a compound described herein.
- the administering stepmay consist of a single administration or may include a series of administrations.
- the length of the treatment perioddepends on a variety of factors, such as the severity of the condition, the age of the patient, the concentration of the compound, the activity of the compositions used in the treatment, or a combination thereof.
- the effective dosage of an agent used for the treatment or prophylaxismay increase or decrease over the course of a particular treatment or prophylaxis regime. In some instances, chronic administration may be required.
- the compositionsare administered to the subject in an amount and for a duration sufficient to treat the patient. “Treat” refers to administering a compound or pharmaceutical composition to an animal in order to effect an alteration or improvement of a disease, disorder, or condition in the animal.
- Certain compounds of the present disclosurepossess asymmetric carbon atoms (optical or chiral centers) or double bonds; the enantiomers, racemates, diastereomers, tautomers, geometric isomers, stereoisometric forms that may be defined, in terms of absolute stereochemistry, as (R)- or (S)- or, as (D)- or (L)-for amino acids, and individual isomers are encompassed within the scope of the present disclosure.
- the compounds of the present disclosuredo not include those that are known in art to be too unstable to synthesize and/or isolate.
- the present disclosureis meant to include compounds in racemic and optically pure forms.
- Optically active (R)- and (S)-, or (D)- and (L)-isomersmay be prepared using chiral synthons or chiral reagents or resolved using conventional techniques.
- the compounds described hereincontain olefinic bonds or other centers of geometric asymmetry, and unless specified otherwise, it is intended that the compounds include both E and Z geometric isomers.
- the term “isomers”refers to compounds having the same number and kind of atoms, and hence the same molecular weight, but differing in respect to the structural arrangement or configuration of the atoms.
- tautomerrefers to one of two or more structural isomers which exist in equilibrium and which are readily converted from one isomeric form to another.
- chirally enriched populationmeans a plurality of molecules of identical molecular formula, wherein the number or percentage of molecules within the population that contain a particular stereochemical configuration at a particular chiral center is greater than the number or percentage of molecules expected to contain the same particular stereochemical configuration at the same particular chiral center within the population if the particular chiral center were stereorandom. Chirally enriched populations of molecules having multiple chiral centers within each molecule may contain one or more stereorandom chiral centers.
- the moleculesare modified oligonucleotides. In certain embodiments, the molecules are compounds comprising modified oligonucleotides.
- structures depicted hereinare also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms.
- compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by 13 C- or 14 C-enriched carbonare within the scope of this disclosure.
- “stereorandom chiral center” in the context of a population of molecules of identical molecular formulameans a chiral center having a random stereochemical configuration.
- the number of molecules having the (S) configuration of the stereorandom chiral centermay be but is not necessarily the same as the number of molecules having the (R) configuration of the stereorandom chiral center.
- the stereochemical configuration of a chiral centeris considered random when it is the results of a synthetic method that is not designed to control the stereochemical configuration.
- a stereorandom chiral centeris a stereorandom phosphorothioate internucleoside linkage.
- MAPT expressionis inhibited.
- MAPT translationis inhibited.
- MAPT activityis inhibited.
- MAPT expression, translation, or activityis reduced by at least 10% relative to the expression, translation, or activity in an untreated or control sample.
- MAPT expression, translation, or activityis reduced by at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%, 10-50%, 25- 50%, 25-75%, 50-75%, 50-99%, or 75-99% relative to the expression, translation, or activity in an untreated or control sample.
- MAPT expression, translation, or activityis reduced as measured by any suitable assay, including but not limited to, an immunoassay, a hybridization-based assay, or a sequencing-based assay (e.g., RNA-Seq).
- the disclosurerelates to compounds targeted to a MAPT nucleic acid.
- the MAPT nucleic acidhas the sequence set forth in GenBank Accession No. NM_001377265.1 (incorporated herein as SEQ ID NO: 1), and nucleotides 2624000 to 2761000 of NT_010783.14 (incorporated herein as SEQ ID NO: 2).
- the compoundis an oligomeric compound.
- the compoundis single-stranded. In certain embodiments, the compound is double-stranded. Certain embodiments provide a compound comprising a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209- .
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any
- Certain embodimentsprovide a compound comprising a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprising a modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- the modified oligonucleotideis at least 80%, at least 85%, at least 90%, or at least 95% complementary to SEQ ID NO: 1 or 2.
- the modified oligonucleotidecomprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
- the compoundis double-stranded.
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- the compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence provided in Tables 2 and 3, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide comprising a 5′-phosphonate modification, where the first modified oligonucleotide is at least 80% complementary to a region of SEQ ID NO: 1 or 2, and a second modified oligonucleotide comprising one or more ligands described herein (e.g., one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB 1 ) ligands, or one or more ⁇ 4 ⁇ 1/7 integrin ligands).
- ligands described hereine.g., one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB 1 ) ligands, or one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the first modified oligonucleotidecomprises a 5′-terminal nucleoside comprising the 5′-phosphonate modification.
- the 5′-phosphonate modificationis a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the modified oligonucleotide or first modified oligonucleotide of any preceding compoundhas at least 80%, at least 85%, at least 90%, or at least 95% complementarity or identity to SEQ ID NO: 1 or 2 over its length.
- the modified oligonucleotide or first modified oligonucleotidehas at least 1, at least 2, at least 3 mismatches to a region of SEQ ID NO: 1 or 2.
- the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strandis 14 to 30 linked nucleosides in length.
- the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strandis 14 to 23 linked nucleosides in length. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 19 to 23 linked nucleosides in length. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 21 to 23 linked nucleosides in length.
- the first modified oligonucleotideis fully complementary to the second modified oligonucleotide.
- the modified oligonucleotide or first modified oligonucleotide of any preceding compoundcomprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
- the second modified oligonucleotide of any preceding compoundcomprises at least one modification selected from the group consisting of a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
- the modified internucleoside linkageis a phosphorothioate internucleoside linkage or a methylphosphonate internucleoside linkage. In certain embodiments, the phosphorothioate internucleoside linkage or methylphosphonate internucleoside linkage is at the 3’ terminus of the first or second modified oligonucleotide or at the 5’ terminus of the first modified oligonucleotide.
- the modified sugarcomprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar. In certain embodiments, the modified sugar comprises a 2’-F modification.
- the modified sugarcomprises a 2’-OMe modification.
- each nucleoside of the first modified oligonucleotidecomprises a modified sugar.
- each nucleoside of the second modified oligonucleotidecomprises a modified sugar.
- the modified sugarcomprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar or a combination thereof.
- the modified sugarcomprises a modification selected from the group consisting of 2’-MOE, 2’-F, and 2’-OMe or a combination thereof.
- the first modified oligonucleotidecomprises no more than ten 2’-F sugar modifications.
- the second modified oligonucleotidecomprises no more than five 2’-F sugar modifications.
- the compound of any preceding embodimentcomprises a conjugate group.
- the conjugate groupis attached to the 5’ end of the modified oligonucleotide.
- the conjugate groupis a targeting moiety.
- the targeting moietycomprises one or more ligands.
- the targeting moietycomprises one or more ligands selected from one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB 1 ) ligands, and one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the targeting moietycomprises one or more TrkB ligands. In certain embodiments, the targeting moiety comprises one or more CB 1 ligands. In certain embodiments, the targeting moiety comprises one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the one or more TrkB ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide.
- the one or more CB 1 ligandsis attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the one or more ⁇ 4 ⁇ 1/7 integrin ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide.
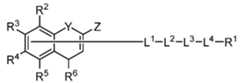
- R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , and R 22are each independently optionally substituted unsaturated or partially unsaturated alkyl.
- R 7 , R 8 , R 9 , and R 10are each independently alkenyl.
- R 7 , R 8 , R 9 , and R 10are each independently alkynyl.
- R 2is OR 7 .
- R 3is OR 11 .
- R 7 and R 11are each independently hydrogen, optionally substituted alkyl or optionally substituted alkenyl. In certain embodiments, one or both R 7 and R 11 are each independently hydrogen. In certain embodiments, one or both R 7 and R 11 are each independently optionally substituted alkyl. In certain embodiments, one or both R 7 and R 11 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, one or both R 7 and R 11 are each independently alkenyl. In certain embodiments, R 7 is optionally substituted alkyl and R 11 is hydrogen. In certain embodiments, R 7 is hydrogen and R 11 is optionally substituted alkyl. In certain embodiments, R 7 is alkenyl and R 11 is hydrogen.
- R 7is hydrogen and R 11 is optionally substituted alkenyl.
- the TrkB ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (II-C), wherein: R 1 is the modified oligonucleotide; L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein.
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXXVII) or a salt, solvate, or hydrate thereof: Formula (XXXXVII), wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 11 and R 13 are each independently absent, hydrogen, or optionally substituted alkyl; R 12 , R 14 , and R 15 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R 16 is hydrogen, halogen, –CN, –N3, –SOn16R 1A , –SOv16NR 16B R 16C , ⁇ NHNR 16B R 16C , ⁇ ONR 16B R 16C , ⁇ NHC(O)NHNR 16B
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXIX) or a salt, solvate, or hydrate thereof: Formula (XXXXIX), wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 17 , R 18 , and R 19 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; z4 is 0, 1, or 2; and z5 is 0, 1, 2, or 3.
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXX) or a salt, solvate, or hydrate thereof: Formula (XXXXX), wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 20 is hydrogen, halogen, –CN, –N 3 , –SO n20 R 1A , –SO v20 NR 20B R 20C , ⁇ NHNR 20B R 20C , ⁇ ONR 20B R 20C , ⁇ NHC(O)NHNR 20B R 20C , ⁇ NHC(O)NR 20B R 20C , –N(O)m20, –NR 20B R 20C , –C(O)R 20D , –C(O)OR 20D , –C(O)NR 20B R 20C , –OR 20A , -NR 20B SO2R
- the CB 1 ligand of a modified oligonucleotideis of the Formula (XXXXXI) or a salt, solvate, or hydrate thereof: , Formula (XXXXXI) wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein;
- X 1is NR 10 or CR 11 R 12 ;
- R 10 , R 11 , and R 12are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
- R 19is hydrogen, –SOn19R 19A , –SOv19NR 19B R 19C , ⁇ NHNR 19B R 19C , ⁇ ONR 19B R 19C , ⁇ NHC(O)NHNR 19B R 19C , ⁇ NHC(O)NR 19B R
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 is H, polyethylene glycol (PEG), optionally substituted heteroalkyl, or optionally substituted heteroaryl; and R 3 , and R 4 are each independently H, halogen, optionally substituted alkyl, or optionally substituted -O-alkyl.
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXIII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXIII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 , R 3 , R 4 , and R 5 are each independently H, halogen, optionally substituted alkyl, optionally substituted -O-alkyl, cycloalkyl, or absent; R 8 is optionally substituted C 1 -C 5 alkyl, optionally substituted C 1 -C 5 alkylene-(C 3 - C 6 )-cycloalkyl, or optionally substituted (C 1 -C 4 )-alkylene-(C 1 -C 4 )-alkoxy; and R 6 , and R 7 are each independently H, halogen, alkyl, or optionally
- R 2is H, -CONHR 4 , -CH 2 OR 4 , -(CH 2 ) 2 OR 4 , -CH 2 NHCOR 4 , or -OR 4 ;
- R 3is H, optionally substituted alkyl, or optionally substituted cycloalkyl;
- R 4is H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl;
- R 5is -OH or absent;
- Xis H, optionally substituted CH 2 , optionally substituted NH, or cycloalkyl.
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXXIII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXXXIII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 is H, -CONHR 3 , -CH 2 OR 3 , -(CH 2 ) 2 OR 3 , -CH 2 NHCOR 3 , or -OR 3 ; each instance of R 3 is independently H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; and X is H or halogen.
- L 1 , L 2 , L 3 , and L 4are each independently absent, a bond, an optionally substituted alkyl linker, an optionally substituted polyethylene glycol (PEG) linker, an optionally substituted heteroalkyl linker, or an optionally substituted heteroaryl linker.
- L 1is an optionally substituted heteroaryl linker.
- L 1is an optionally substituted unsaturated heteroaryl, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker.
- L 1comprises the structure: .
- L 1is an optionally substituted heteroalkyl linker.
- the optionally substituted heteroalkyl linkeris an optionally substituted heteroalkyl or optionally substituted C 1 -C 10 alkyl chain in which one or more carbon atoms are replaced with O, N, or S.
- L 1comprises the structure: or .
- L 1comprises the structure: or –N(CH 3 )–.
- L 2is an optionally substituted PEG linker.
- the PEG linkeris five PEG units in length.
- the PEG linkeris four PEG units in length.
- the PEG linkeris three PEG units in length.
- L 2is an optionally substituted alkyl linker.
- the heteroalkyl linkercomprises two substituents joined together to form an optionally substituted carbocyclyl ring.
- L 4comprises the structure: or a salt thereof, wherein X is O or S.
- L 4comprises the structure: or a salt thereof, wherein X is O or S.
- L 1 – L 2 –L 3 –L 4comprises the structure: ,
- TrkB ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof:
- Ris the modified oligonucleotide; and X is S or O.
- the CB 1 ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof:
- Formula (XXXXXXXVII)Formula (XXXXXXXVIII) wherein: R is the modified oligonucleotide; and X is S or O.
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXX)
- Ris the modified oligonucleotide; and X is S or O.
- the compound of any preceding embodimentcomprises a lipid.
- the lipidis attached to an internucleoside linkage of the modified oligonucleotide.
- the modified oligonucleotidecomprises one or more lipids.
- the one or more lipidsare attached to one or more internucleoside linkages of the modified oligonucleotide.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide.
- the compound of any preceding embodimentcomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide.
- the modified oligonucleotidecomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the one or more substituted or unsubstituted alkyl or alkenylare attached to one or more internucleoside linkages of the modified oligonucleotide.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 4 -C 30 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 5 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 14 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 22 hydrocarbon chain.
- a substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide).
- the internucleoside linkageis between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide.
- the internucleoside linkageis between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXIII-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXVI.
- the modified oligonucleotidecomprises Formula (XXXVI), or a salt, solvate, or hydrate thereof: Formula (XXXVI), wherein: R C is –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R 2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 6 , -N(R 6 ), or -SR 6 ; each R 3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubsti
- the modified oligonucleotidecomprises Formula (XXXVII), or a salt, solvate, or hydrate thereof: Formula (XXXVII), wherein: each R 2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 6 , -N(R 6 ), or -SR 6 ; each R 3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 7 , -N(R 7 ), or -SR 7 ; R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; each R 6 is independently hydrogen, substituted or unsubstit
- the modified oligonucleotidecomprises Formula (XXXVIII), or a salt, solvate, or hydrate thereof: Formula (XXXVIII), wherein: each R 2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 6 , -N(R 6 ), or -SR 6 ; each R 3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 7 , -N(R 7 ), or -SR 7 ; R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; each R 6 is independently hydrogen, substituted or unsubstit
- the modified oligonucleotidecomprises Formula (XXXXII), or a salt, solvate, or hydrate thereof: mA ⁇ mA Formula (XXXXII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXIII), or a salt, solvate, or hydrate thereof: mA ⁇ mU Formula (XXXXIII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXIV), or a salt, solvate, or hydrate thereof: mA ⁇ mG Formula (XXXXIV) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXV), or a salt, solvate, or hydrate thereof: mA ⁇ mC Formula (XXXXV) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXVI), or a salt, solvate, or hydrate thereof: mU ⁇ mA Formula (XXXXVI) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXVII), or a salt, solvate, or hydrate thereof: mU ⁇ mG Formula (XXXXVII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXVIII), or a salt, solvate, or hydrate thereof: mU ⁇ fC Formula (XXXXVIII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXIX), or a salt, solvate, or hydrate thereof: fG ⁇ mU Formula (XXXXIX) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXX), or a salt, solvate, or hydrate thereof: mG ⁇ fG Formula (XXXX) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXXI), or a salt, solvate, or hydrate thereof: mG ⁇ mC Formula (XXXXI) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXXII), or a salt, solvate, or hydrate thereof:
- the modified oligonucleotidecomprises an internucleoside linkage of one of the following Formulae: Formula (XXXXXV), Formula (XXXXXVI).
- the compound of any preceding embodimentcomprises a 5′- phosphonate modification.
- a modified oligonucleotidecomprises one or more sugars having a phosphonate modification at the 5′ position.
- the modified oligonucleotidecomprises a 5′-phosphonate modification. In certain embodiments, the modified oligonucleotide comprises a 5′-terminal nucleoside (e.g., 5′ terminus) comprising the 5′-phosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification or a 5′- ethylenephosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-ethylenephosphonate modification.
- the modified oligonucleotideis the first modified oligonucleotide or antisense oligonucleotide.
- Certain embodimentsprovide a compound comprising: a first modified oligonucleotide comprising a 5′-phosphonate modification, where the first modified oligonucleotide is at least 80% complementary to a region of SEQ ID NO: 1 or 2; and a second modified oligonucleotide comprising one or more ligands.
- the first modified oligonucleotidecomprises a 5′-terminal nucleoside comprising the 5′-phosphonate modification.
- the 5′- phosphonate modificationis a 5′-vinylphosphonate modification or a 5′-ethylenephosphonate modification. In some embodiments, the 5′-phosphonate modification is a 5′- vinylphosphonate modification. In some embodiments, the 5′-phosphonate modification is a 5′-ethylenephosphonate modification. In some embodiments, the second modified oligonucleotide comprises one or more ligands selected from one or more TrkB ligands, one or more CB 1 ligands, and one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the one or more TrkB ligands, the one or more CB 1 ligands, or the one or more ⁇ 4 ⁇ 1/7 integrin ligandsare attached to the 5’ end of the second modified oligonucleotide. In some embodiments, the one or more TrkB ligands, the one or more CB 1 ligands, or the one or more ⁇ 4 ⁇ 1/7 integrin ligands are attached to the 3’ end of the second modified oligonucleotide.
- the one or more TrkB ligands, the one or more CB 1 ligands, or the one or more ⁇ 4 ⁇ 1/7 integrin ligandsare attached to the 5’ end and the 3’ end of the second modified oligonucleotide.
- the one or more TrkB ligandsare selected from any one of Formulae I-XXXIV, XXXXVII, XXXXIX-XXXXXX, XXXXXV-XXXXXVII, and XXXXXIX-XXXXXXIII;
- the one or more CB 1 ligandsare selected from any one of Formulae XXXXXI and XXXXXIV-XXXXXXXVIII;
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsare selected from any one of Formulae XXXXXII-XXXXXIV and XXXXXXIX-XXXXXXXIV.
- the second modified oligonucleotidecomprises one or more TrkB ligands.
- the one or more TrkB ligandsare selected from any one of Formulae I-XXXIV, XXXXVII, XXXXIX-XXXXXX, XXXXXV- XXXXXVII, and XXXXXIX-XXXXXXIII.
- the one or more TrkB ligandsare selected from any one of Formulae XIX-XXXI and XXXXXV.
- the second modified oligonucleotidecomprises one TrkB ligand.
- the second modified oligonucleotidecomprises two TrkB ligands. In some embodiments, the second modified oligonucleotide comprises at least two TrkB ligands. In some embodiments, the at least two TrkB ligands are the same. In some embodiments, the at least two TrkB ligands are different. In some embodiments, the second modified oligonucleotide comprises one or more CB 1 ligands. In some embodiments, the one or more CB 1 ligands are selected from any one of Formulae XXXXXI and XXXXXIV-XXXXXXXVIII.
- the one or more CB 1 ligandsare selected from any one of Formulae XXXXXXIV- XXXXXXVI.
- the second modified oligonucleotidecomprises one CB 1 ligand. In some embodiments, the second modified oligonucleotide comprises two CB 1 ligands. In some embodiments, the second modified oligonucleotide comprises at least two CB 1 ligands. In some embodiments, the at least two CB 1 ligands are the same. In some embodiments, the at least two CB 1 ligands are different.
- the second modified oligonucleotidecomprises one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsare selected from any one of Formulae XXXXXII-XXXXXIV and XXXXXXIX- XXXXXXXIV.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsare selected from any one of Formulae XXXXXXI, XXXXXXXII, and XXXXXXIV.
- the second modified oligonucleotidecomprises one ⁇ 4 ⁇ 1/7 integrin ligand. In some embodiments, the second modified oligonucleotide comprises two ⁇ 4 ⁇ 1/7 integrin ligands. In some embodiments, the second modified oligonucleotide comprises at least two ⁇ 4 ⁇ 1/7 integrin ligands. In some embodiments, the at least two ⁇ 4 ⁇ 1/7 integrin ligands are the same. In some embodiments, the at least two ⁇ 4 ⁇ 1/7 integrin ligands are different. In some embodiments, the second modified oligonucleotide comprises one or more lipids.
- the second modified oligonucleotidecomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 4 -C 30 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 5 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 14 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C16 hydrocarbon chain, a saturated or unsaturated C 17 hydrocarbon chain, a saturated or unsaturated C 18 hydrocarbon chain, or a saturated or unsaturated C 22 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylare attached to an internucleoside linkage of the second modified oligonucleotide.
- the internucleoside linkageis between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the second modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the second modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the second modified oligonucleotide.
- the internucleoside linkage of the second modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI.
- the second modified oligonucleotidecomprises any one of Formulae XXXV- XXXXVI.
- the first modified oligonucleotideis 14 to 30 linked nucleosides in length.
- the second modified oligonucleotideis 14 to 30 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the first modified oligonucleotidehas a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200, and 209-217.
- the second modified oligonucleotidehas a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206.
- the first modified oligonucleotideis selected from any one of the IA Ref ID NOs in Table 3.
- the second modified oligonucleotideis selected from any one of the IS Ref ID NOs in Table 3.
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of Ref ID NOs listed in Table 3 and a second modified oligonucleotide 14 to 21 linked nucleosides in length fully complementary to the first modified oligonucleotide.
- Certain embodimentsprovide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3.
- the second modified oligonucleotidecomprises any one or more TrkB ligands provided herein.
- the TrkB ligandis selected from Formulae I-XXXIV, XXXXVII, XXXXIX-XXXXXX, XXXXXV-XXXXXVII, and XXXXXIX-XXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the TrkB ligandis selected from Formulae XIX-XXXI and XXXXXV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the TrkB ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the TrkB ligand through a phosphorothioate group. In certain embodiments, provided herein is a compound selected from the group consisting of any one of the compounds listed in Table 3. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3.
- the second modified oligonucleotidecomprises any one or more CB 1 ligands provided herein.
- the CB 1 ligandis selected from Formulae XXXXXI and XXXXXIV- XXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the CB 1 ligandis selected from Formulae XXXXXIV-XXXXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the CB 1 ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the CB 1 ligand through a phosphorothioate group. In certain embodiments, provided herein is a compound selected from the group consisting of any one of the compounds listed in Table 3. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3.
- the second modified oligonucleotidecomprises any one or more ⁇ 4 ⁇ 1/7 integrin ligands provided herein.
- the ⁇ 4 ⁇ 1/7 integrin ligandis selected from Formulae XXXXXII-XXXXXIV and XXXXXXIX-XXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the ⁇ 4 ⁇ 1/7 integrin ligandis selected from Formulae XXXXXXXI, XXXXXXII, and XXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the ⁇ 4 ⁇ 1/7 integrin ligand through a phosphodiester group.
- the modified oligonucleotideis attached to the ⁇ 4 ⁇ 1/7 integrin ligand through a phosphorothioate group.
- provided hereinis a compound selected from the group consisting of any one of the compounds listed in Table 3.
- the pharmaceutically acceptable salt of the modified oligonucleotides provided hereinis a sodium salt or a potassium salt.
- the pharmaceutically acceptable salt of the compounds provided hereinis a sodium salt or a potassium salt.
- provided hereinis a population of modified oligonucleotides, wherein all of the phosphorothioate internucleoside linkages of the modified oligonucleotide are stereorandom.
- provided hereinis a population of compounds, wherein all of the phosphorothioate internucleoside linkages of the modified oligonucleotide are stereorandom.
- the compound of any foregoing embodimentis in a pharmaceutically acceptable salt form.
- the pharmaceutically acceptable saltis a sodium salt.
- the pharmaceutically acceptable saltis a potassium salt.
- Certain embodimentsprovide a composition comprising the compound of any one of the foregoing embodiments and a pharmaceutically acceptable carrier.
- Certain embodimentsprovide a composition comprising a compound of any preceding embodiment, for use in therapy.
- Certain embodimentsprovide a method of treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT in an individual comprising administering to the individual a compound targeted to MAPT, thereby treating, preventing, or ameliorating the disease.
- the compound or composition of any foregoing embodimentis administered to an individual.
- the disease, disorder or conditionis a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereofincluding a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- administering the compoundinhibits or reduces or improves a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual in a therapeutically effective amount.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual at a dosage level sufficient to deliver about 1 to 100 mg/kg of body weight of the individual. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual at a fixed dose of about 25 mg to about 1,000 mg. In certain embodiments, the compound or composition is administered to the individual one or more times in a day up to the dosage level or fixed dose. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual daily, weekly, monthly, quarterly or yearly.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual about once per quarter (i.e., once every three months) to about once per year. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual about once per quarter, about once every six months or about once per year. Certain embodiments provide a method of inhibiting expression of MAPT in a cell comprising contacting the cell with a compound targeted to MAPT, thereby inhibiting expression of MAPT in the cell. In certain embodiments, the cell is in the liver of an individual.
- the individualhas, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereofincluding a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- Certain embodimentsprovide a method of reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an individual, comprising administering a compound targeted to MAPT to the individual, thereby reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive
- the individualhas, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundis a compound targeted to MAPT.
- the compoundis any of the foregoing compounds.
- the compound or compositionis administered parenterally.
- the compound or compositionis administered by intrathecal (IT) administration.
- the disease, disorder or conditionis a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundis a compound targeted to MAPT.
- the compoundis any of the foregoing compounds.
- Certain embodimentsprovide use of a compound targeted to MAPT in the manufacture of a medicament for treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT.
- the disease, disorder or conditionis a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairmentvv.
- the compoundis a compound targeted to MAPT.
- the compoundis any of the foregoing compounds. In certain embodiments, any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3. In certain embodiments, any one of the compounds shown in FIGs. 1-4 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds shown in FIGs. 1-4. In certain embodiments, any one of the compounds in Table 2 can be used in any aspect of the disclosure.
- the compoundis any one of the compounds in Table 2. In certain embodiments, any one of the compounds in Table 3 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds in Table 3. In certain embodiments, any one of the compounds in Table A can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds in Table A. In some embodiments of any of the aspects of the disclosure, the compound is selected from any one of compounds 1-285 in Table A.
- Table Ashown below, provides the following for each of compounds 1-285: antisense (“A”) and sense (“S”) strand nucleobase sequences (SEQ ID NO) shown with strand modifications (as defined in Table 1), and, where a sense strand comprises a ligand, the type of ligand (e.g., a TrkB ligand, a CB 1 ligand, or an ⁇ 4 ⁇ 1/7 integrin ligand as described herein) at the 5’ end (“5’-Ligand”) and/or the 3’ end (“3’-Ligand”) of the sense strand.
- the 5’-Ligand and/or the 3’-Ligandare attached to the sense strand via a linker.
- the linkercomprises polyethylene glycol (PEG). In some embodiments, the linker comprises a PEG chain of one, two, three, four, five, six, seven, or eight PEG units in length. In certain embodiments, the linker comprises a PEG chain one PEG unit in length. In certain embodiments, the linker comprises a PEG chain two PEG units in length. In certain embodiments, the linker comprises a PEG chain three PEG units in length. In certain embodiments, the linker comprises a PEG chain four PEG units in length. In certain embodiments, the linker comprises a PEG chain five PEG units in length. In certain embodiments, the linker comprises a PEG chain six PEG units in length.
- PEGpolyethylene glycol
- the linkercomprises a PEG chain seven PEG units in length. In certain embodiments, the linker comprises a PEG chain eight PEG units in length. In some embodiments, the linker comprises an optionally substituted heteroaryl or optionally substituted heterocyclyl group. In certain embodiments, the linker comprises PEG and an optionally substituted heteroaryl or optionally substituted heterocyclyl group. Table A. Exemplary Compounds
- the disclosurerelates to methods of inhibiting MAPT expression, which can be useful for treating, preventing, or ameliorating a disease associated with MAPT in an individual, by administration of a compound that targets MAPT.
- the compoundcan be a MAPT specific inhibitor.
- the compoundcan be an antisense oligonucleotide, an oligomeric compound, or an oligonucleotide targeted to MAPT (e.g., a compound of any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3).
- the disclosurerelates to treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT.
- diseases, disorders or conditions associated with MAPT treatable, preventable, and/or ameliorable with the methods provided hereininclude a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereofincluding a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- Certain compounds provided hereinare directed to compounds and compositions that reduce a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal.
- a method of treating, preventing, or ameliorating a disease associated with MAPT in an individualcomprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby treating, preventing, or ameliorating the disease.
- the individualis identified as having, or at risk of having, a disease associated with MAPT.
- the diseaseis a CNS related disease.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides) in length having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209- 217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- a single-stranded compoundcan be 14 to 30, 14 to 23, 14 to 20, 16 to 20, or 14 to 16, linked nucleosides in length. In certain embodiments, a single-stranded compound can be 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30, linked nucleosides in length. In certain embodiments, a double-stranded compound can comprise two oligonucleotides of the same or different lengths, as described elsewhere herein. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the compoundis administered to the individual parenterally.
- the compoundis administered to the individual by intrathecal (IT) administration.
- administering the compoundimproves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal.
- a method of treating, preventing, or ameliorating a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animalcomprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby treating, preventing, or ameliorating a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dr
- the compoundcomprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159- 168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199- 200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149- 155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length)
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotide
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- administering the compoundimproves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal.
- the individualis identified as having, or at risk of having, a disease associated with MAPT.
- a method of inhibiting expression of MAPT in an individual having, or at risk of having, a disease associated with MAPTcomprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby inhibiting expression of MAPT in the individual. In certain embodiments, administering the compound inhibits expression of MAPT in the liver. In certain embodiments, the disease is a CNS related disease.
- the individualhas, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT.
- the compoundcomprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188,
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159- 168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199- 200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149- 155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length)
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotide
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the compoundis administered to the individual parenterally.
- the compoundis administered to the individual by intrathecal (IT) administration.
- administering the compoundimproves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof, a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- ITintrathecal
- administering the compoundimproves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof, a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD
- a method of inhibiting expression of MAPT in a cellcomprises contacting the cell with a compound comprising a MAPT specific inhibitor, thereby inhibiting expression of MAPT in the cell.
- the cellis a hepatocyte. In certain embodiments, the cell is in the liver.
- the cellis in the liver of an individual who has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT.
- the compoundcomprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188,
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the individualhas, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT.
- the compoundcomprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188,
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the compoundis administered to the individual parenterally.
- the compoundis administered to the individual by intrathecal (IT) administration.
- the individualis identified as having, or at risk of having, a disease associated with MAPT.
- Certain embodimentsare drawn to a compound comprising a MAPT specific inhibitor for use in treating a disease, disorder or condition associated with MAPT.
- the disease, disorder or conditionis a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188,
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the compoundis administered to the individual parenterally.
- the compoundis administered to the individual by intrathecal (IT) administration.
- ITintrathecal
- Certain embodimentsare drawn to a compound comprising a MAPT specific inhibitor for use in reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT.
- the compoundcomprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotide
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- Certain embodimentsare drawn to the use of a compound comprising a MAPT specific inhibitor for the manufacture or preparation of a medicament for treating a disease associated with MAPT. Certain embodiments are drawn to the use of a compound comprising a MAPT specific inhibitor for the preparation of a medicament for treating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof.
- the disease, disorder or conditionis a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a nucleobase sequencecomprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188,
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190- 192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- Certain embodimentsare drawn to the use of a compound comprising a MAPT specific inhibitor for the manufacture or preparation of a medicament for reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof in an individual having, or at risk of having, a CNS related disease, disorder or condition or a symptom thereof associated with MAPT.
- the CNS related disease, disorder or conditionis a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- Certain embodimentsare drawn to use of a compound comprising a MAPT specific inhibitor for the preparation of a medicament for treating a disease, disorder or condition associated with MAPT.
- the disease, disorder or conditionis a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment.
- the compoundcomprises an antisense oligonucleotide targeted to MAPT.
- the compoundcomprises an oligonucleotide targeted to MAPT.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a compoundcomprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- a compoundcomprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcan be single-stranded or double-stranded.
- the compoundcan be an antisense oligonucleotide or oligomeric compound.
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having
- a compoundcomprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide.
- a first modified oligonucleotidee.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length
- a second modified oligonucleotidee.
- a compoundcomprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
- the compoundcan be an oligomeric compound.
- the compoundcan be single-stranded or double-stranded. In any of the foregoing methods or uses, the compound can be targeted to MAPT. In certain embodiments, the compound comprises or consists of a modified oligonucleotide. In certain embodiments, the compound comprises one or more modified oligonucleotides. In certain embodiments, the compound comprises a first modified oligonucleotide and a second modified oligonucleotide.
- a modified oligonucleotideis 8 to 80 linked nucleosides in length, 10 to 30 linked nucleosides in length, 14 to 30 linked nucleosides in length, 14 to 23 linked nucleosides in length, or 19 to 23 linked nucleosides in length.
- a modified oligonucleotideis at least 80%, at least 85%, at least 90%, at least 95% or 100% complementary to any of the nucleobase sequences recited in SEQ ID NOs: 1 and 3 over its length.
- a modified oligonucleotidecomprises at least one modified internucleoside linkage, at least one modified sugar and/or at least one modified nucleobase.
- the modified internucleoside linkageis a phosphorothioate internucleoside linkage.
- the modified sugaris a bicyclic sugar, 2’-MOE, 2’-F, or 2’-OMe.
- the modified nucleobaseis a 5-methylcytosine.
- each modified oligonucleotideis independently 12 to 30, 14 to 30, 14 to 25, 14 to 24, 14 to 23, 16 to 23, 17 to 23, 18 to 23, 19 to 23, 19 to 22, or 19 to 20 linked nucleosides in length.
- a modified oligonucleotidehas at least 1, at least 2, at least 3 mismatches to a region of SEQ ID NO: 1 and 2.
- the compoundcomprises a first and second modified oligonucleotide, wherein there is a region of complementarity between a first modified oligonucleotide and a second modified oligonucleotide.
- the region of complementarity between the first oligonucleotide and the second oligonucleotideis 14 to 23, 19 to 23, or 21 to 23 linked nucleosides in length.
- the first modified oligonucleotideis fully complementary to the second modified oligonucleotide.
- the first modified oligonucleotidecomprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
- the second modified oligonucleotidecomprises at least one modification selected from the group consisting of a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
- the modified internucleoside linkageis a phosphorothioate internucleoside linkage or a methylphosphonate internucleoside linkage.
- the modified internucleoside linkageis at the 3’ terminus of the first or second modified oligonucleotide or at the 5’ terminus of the first or second modified oligonucleotide.
- the first or second modified oligonucleotidecomprises one or more modified sugars.
- each nucleoside of the first or second modified oligonucleotidecomprises a modified sugar.
- the modified sugarcomprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar.
- the modified sugarcomprises a modification selected from group consisting of 2’-MOE, 2’-F, and 2’-OMe or a combination thereof.
- the first or second modified oligonucleotidecomprises no more than ten 2’-F sugar modifications. In certain embodiments, the first or second modified oligonucleotide comprises no more than five 2’-F sugar modifications.
- a compoundcomprises a conjugate group. In certain embodiments, the conjugate group is attached to the 5’ end of a modified oligonucleotide. In certain embodiments, the conjugate group is attached to the 3’ end of a modified oligonucleotide.
- a conjugate groupis attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide.
- the conjugate groupis a targeting moiety.
- the targeting moietycomprises one or more ligands.
- the targeting moietycomprises one or more TrkB ligands.
- the one or more TrkB ligandsis attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide.
- the TrkB ligandis selected from Formulae I- XXXIV, XXXXVII, XXXXIX-XXXXXX, XXXXXV-XXXXXVII, and XXXXXIX-XXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the TrkB ligand through a phosphodiester group.
- the modified oligonucleotideis attached to the TrkB ligand through a phosphorothioate group.
- the conjugate groupcomprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl).
- the modified oligonucleotidecomprises one or more ligands and one or more lipids.
- the modified oligonucleotideis the second modified oligonucleotide.
- the one or more lipidsare attached to an internucleoside linkage of the modified oligonucleotide.
- the internucleoside linkage of the modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotidecomprises any one of Formulae XXXV-XXXXVI.
- the modified oligonucleotidecomprises one or more TrkB ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl).
- the modified oligonucleotideis the second modified oligonucleotide.
- the one or more TrkB ligandsis attached to the 5’ end of the modified oligonucleotide.
- the one or more TrkB ligandsis attached to the 3’ end of the modified oligonucleotide.
- the one or more TrkB ligandsis attached to the 5’ end and the 3’ end of the modified oligonucleotide.
- the one or more lipidsare attached to an internucleoside linkage of the modified oligonucleotide.
- the one or more TrkB ligandsis selected from any one of Formulae I-XXXIV, XXXXVII, XXXXXIX-XXXXX, XXXXXV- XXXXXVII, and XXXXXIX-XXXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof.
- the one or more TrkB ligandsis selected from any one of Formulae I-XXXIV, XXXXVII, XXXXIX-XXXXXX, XXXXXV- XXXXVII, and XXXXXIX-XXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXV-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- a compoundcomprises a conjugate group.
- the conjugate groupis attached to the 5’ end of a modified oligonucleotide.
- the conjugate groupis attached to the 3’ end of a modified oligonucleotide.
- a conjugate groupis attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide.
- the conjugate groupis a targeting moiety.
- the targeting moietycomprises one or more ligands.
- the targeting moietycomprises one or more CB 1 ligands.
- the one or more CB 1 ligandsis attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide.
- the CB 1 ligandis selected from Formulae XXXXXI and XXXXXXIV- XXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the CB 1 ligand through a phosphodiester group.
- the modified oligonucleotideis attached to the CB 1 ligand through a phosphorothioate group.
- the conjugate groupcomprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl).
- the modified oligonucleotidecomprises one or more ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl).
- the modified oligonucleotideis the second modified oligonucleotide.
- the one or more lipidsare attached to an internucleoside linkage of the modified oligonucleotide.
- the internucleoside linkage of the modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotidecomprises any one of Formulae XXXV-XXXXVI.
- the modified oligonucleotidecomprises one or more CB 1 ligands, and one or more lipids.
- the modified oligonucleotideis the second modified oligonucleotide.
- the one or more CB 1 ligandsis attached to the 5’ end of the modified oligonucleotide.
- the one or more CB 1 ligandsis attached to the 3’ end of the modified oligonucleotide.
- the one or more CB 1 ligandsis attached to the 5’ end and the 3’ end of the modified oligonucleotide.
- the one or more lipidsare attached to an internucleoside linkage of the modified oligonucleotide.
- the one or more CB 1 ligandsis selected from any one of Formulae XXXXXI and XXXXXXIV-XXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof.
- the one or more CB 1 ligandsis selected from any one of Formulae XXXXXI and XXXXXXIV-XXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXXV-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- a compoundcomprises a conjugate group.
- the conjugate groupis attached to the 5’ end of a modified oligonucleotide. In certain embodiments, the conjugate group is attached to the 3’ end of a modified oligonucleotide. In certain embodiments, a conjugate group is attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more ligands. In certain embodiments, the targeting moiety comprises one or more ⁇ 4 ⁇ 1/7 integrin ligands.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsis attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide.
- the ⁇ 4 ⁇ 1/7 integrin ligandis selected from Formulae XXXXXII- XXXXXIV and XXXXXXIX-XXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotideis attached to the ⁇ 4 ⁇ 1/7 integrin ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the ⁇ 4 ⁇ 1/7 integrin ligand through a phosphorothioate group.
- the conjugate groupcomprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide comprises one or more ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl).
- the modified oligonucleotideis the second modified oligonucleotide.
- the one or more lipidse.g., one or more substituted or unsubstituted alkyl or alkenyl
- the internucleoside linkage of the modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- the modified oligonucleotidecomprises any one of Formulae XXXV-XXXXVI. In certain embodiments, the modified oligonucleotide comprises one or more ⁇ 4 ⁇ 1/7 integrin ligands and one or more lipids. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more ⁇ 4 ⁇ 1/7 integrin ligands is attached to the 5’ end of the modified oligonucleotide. In certain embodiments, the one or more ⁇ 4 ⁇ 1/7 integrin ligands is attached to the 3’ end of the modified oligonucleotide.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsis attached to the 5’ end and the 3’ end of the modified oligonucleotide.
- the one or more lipidsare attached to an internucleoside linkage of the modified oligonucleotide.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsis selected from any one of Formulae XXXXXII-XXXXXIV and XXXXXXIX-XXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof.
- the one or more ⁇ 4 ⁇ 1/7 integrin ligandsis selected from any one of Formulae XXXXXII-XXXXXIV and XXXXXXIX-XXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXXV-XXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide.
- a compoundcomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide.
- the modified oligonucleotidecomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the one or more substituted or unsubstituted alkyl or alkenylare attached to one or more internucleoside linkages of the modified oligonucleotide.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 4 -C 30 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 5 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 14 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 22 hydrocarbon chain.
- a substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide).
- a substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide).
- the internucleoside linkageis between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide.
- the internucleoside linkage of the modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI.
- the modified oligonucleotidecomprises any one of Formulae XXXV-XXXXVI.
- a compoundcomprises a 5′-phosphonate modification.
- a modified oligonucleotidecomprises one or more sugars having a phosphonate modification at the 5′ position.
- the modified oligonucleotidecomprises a 5′-phosphonate modification.
- the modified oligonucleotidecomprises a 5′-terminal nucleoside (e.g., 5′ terminus) comprising the 5′-phosphonate modification.
- the 5′-phosphonate modificationis a 5′-vinylphosphonate modification or a 5′- ethylenephosphonate modification.
- the 5′-phosphonate modificationis a 5′-vinylphosphonate modification.
- the 5′-phosphonate modificationis a 5′-ethylenephosphonate modification.
- the modified oligonucleotideis the first modified oligonucleotide or antisense oligonucleotide.
- the compoundcomprises a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs in Table 3 and a second modified oligonucleotide 14 to 23 linked nucleosides in length fully complementary to the first modified oligonucleotide.
- the compoundcomprises a first modified oligonucleotide selected from any one of the IA Ref ID NOs in Table 3 and a second modified oligonucleotide selected from any one of the IS Ref ID NOs in Table 3.
- the compoundis in a pharmaceutically acceptable salt form.
- the pharmaceutically acceptable saltis a sodium salt.
- the pharmaceutically acceptable saltis a potassium salt.
- a compositioncomprises the compound of any one of the foregoing embodiments and a pharmaceutically acceptable carrier.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual in a therapeutically effective amount.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual at a dosage level sufficient to deliver about 1 to 100 mg/kg of body weight of the individual.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual at a fixed dose of about 25 mg to about 1,000 mg.
- the compositionis administered to the individual one or more times in a day up to the dosage level or fixed dose.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual daily, weekly, monthly, quarterly or yearly.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual about once per quarter (i.e., once every three months) to about once per year.
- a compound or composition comprising a compound of any preceding embodimentis administered to an individual about once per quarter, about once every six months or about once per year.
- the oligomeric compoundcomprises a nucleobase sequence complementary to that of a target nucleic acid.
- the disclosurerelates to a compound that comprises or consists of a modified oligonucleotide.
- the modified oligonucleotidehas a nucleobase sequence complementary to that of a target nucleic acid.
- the disclosurerelates to a compound that comprises or consists of an antisense oligonucleotide.
- the antisense oligonucleotidehas a nucleobase sequence complementary to that of a target nucleic acid.
- the disclosurerelates to a compound that is a single-stranded compound.
- the single-stranded compoundcomprises or consists of an oligomeric compound.
- such an oligomeric compoundcomprises or consists of an oligonucleotide and optionally a conjugate group.
- the oligonucleotideis a modified oligonucleotide.
- the oligonucleotideis an antisense oligonucleotide.
- the oligonucleotide or modified oligonucleotide of a single-stranded compoundcomprises a self-complementary nucleobase sequence.
- the disclosurerelates to a compound that is a double-stranded compound.
- the double-stranded compoundcomprises or consists of an oligomeric compound.
- the double-stranded compoundcomprises a first oligonucleotide and a second oligonucleotide.
- the first oligonucleotidehas a region complementarity to a target nucleic acid and the second oligonucleotide has a region complementarity to the first modified oligonucleotide.
- the double-stranded compoundcomprises a modified oligonucleotide.
- the modified oligonucleotidehas a region complementarity to a target nucleic acid.
- the double-stranded compoundcomprises a first modified oligonucleotide and a second modified oligonucleotide.
- the first modified oligonucleotidehas a region complementarity to a target nucleic acid and the second modified oligonucleotide has a region complementarity to the first modified oligonucleotide.
- an oligonucleotide or modified oligonucleotide of a double-stranded compoundis an RNA oligonucleotide.
- the thymine nucleobase in the modified oligonucleotideis replaced by a uracil nucleobase.
- a compound described hereincomprises a conjugate group.
- the first oligonucleotide or first modified oligonucleotide of a double-stranded compoundcomprises a conjugate group.
- the second oligonucleotide or second modified oligonucleotide of a double-stranded compoundcomprises a conjugate group.
- a first oligonucleotide or first modified oligonucleotide and a second oligonucleotide or second modified oligonucleotide of a double-stranded compoundeach comprises a conjugate group.
- a compoundis 14-30 linked nucleosides in length.
- the first oligonucleotide or first modified oligonucleotide of a double-stranded compoundis 14-30 linked nucleosides in length.
- the second oligonucleotide or second modified oligonucleotideis 14-30 linked nucleosides in length.
- the oligonucleotides or modified oligonucleotides of a double-stranded compoundare blunt ended at one or both ends of the compound.
- the oligonucleotides or modified oligonucleotides of a double-stranded compoundinclude non- complementary overhanging nucleosides at one or both ends of the compound.
- a compoundhas a nucleobase sequence comprising at least 14 contiguous nucleobases of any of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217.
- one of the oligonucleotides or modified oligonucleotides of a double-stranded compoundhas a nucleobase sequence comprising at least 14 contiguous nucleobases of any of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- single-stranded and double-stranded compoundsinclude, but are not limited to, oligonucleotides, antisense oligonucleotides, siRNAs, microRNA targeting oligonucleotides, occupancy-based compounds (e.g., mRNA processing or translation blocking compounds and splicing compounds), and single-stranded RNAi compounds (e.g. small hairpin RNAs (shRNAs), single stranded siRNAs (ssRNAs) and microRNA mimics).
- a compound described hereinhas a nucleobase sequence that, when written in the 5’ to 3’ direction, comprises the reverse complement of the target region of a target nucleic acid to which it is targeted.
- a compound described hereincomprises an oligonucleotide 12 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 12 to 23 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 14 to 30 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 14 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 15 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 15 to 23 linked subunits in length.
- a compound described hereincomprises an oligonucleotide 16 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 16 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 18 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 18 to 23 linked subunits in length.
- a compound described hereincomprises an oligonucleotide 19 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 19 to 23 linked subunits in length.
- such oligonucleotidesare 12 to 30 linked subunits, 12 to 23 linked subunits, 14 to 30 linked subunits, 14 to 23 linked subunits, 15 to 30 linked subunits, 15 to 23 linked subunits, 16 to 30 linked subunits, 16 to 23 linked subunits, 17 to 30 linked subunits, 17 to 23 linked subunits, 18 to 30 linked subunits, 18 to 23 linked subunits, 19 to 30 linked subunits or 19 to 23 linked subunits, respectively.
- a compound described hereincomprises an oligonucleotide 14 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 16 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 18 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 19 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 20 linked subunits in length.
- a compound described hereincomprises an oligonucleotide 21 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 22 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 23 linked subunits in length. In other embodiments, a compound described herein comprises an oligonucleotide 8 to 80, 12 to 50, 13 to 30, 13 to 50, 14 to 30, 14 to 50, 15 to 30, 15 to 50, 16 to 30, 16 to 50, 17 to 30, 17 to 50, 18 to 23, 18 to 24, 18 to 25, 18 to 50, 19 to 23, 19 to 30, 19 to 50, 20 to 23 or 20 to 30 linked subunits.
- the compound described hereincomprises an oligonucleotide 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 linked subunits in length, or a range defined by any two of the above values.
- the compoundmay further comprise an additional moiety, such as a conjugate group or delivery moiety.
- such compoundsare oligomeric compounds, and the additional moiety is attached to an oligonucleotide.
- a conjugate groupis attached to a nucleoside of an oligonucleotide.
- compoundsmay be shortened or truncated.
- one or more subunitsmay be deleted from the 5’ end (5’ truncation), or alternatively from the 3’ end (3’ truncation) of an oligonucleotide.
- compoundsmay be lengthened.
- one or more subunitsmay be attached to the 3′ end or 5′ end of an oligonucleotide.
- At least one subunite.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits
- oligonucleotidee.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits
- At least one subunite.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits
- at least one or more subunitsmay be attached to the 3′ end or 5′ end of an oligonucleotide of a double-stranded compound creating a 3′ and/or 5′ end overhang.
- At least one subunite.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits
- at least one subunitis attached to the 5′ end of both oligonucleotides of a double-stranded compound.
- At least one subunite.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunit
- at least one subunitis attached to the 3′ end of both oligonucleotides of a double-stranded compound.
- subunitsare attached to both oligonucleotides of a double-stranded compound at the same end (e.g., that subunits are attached to the 3′ end of one of the oligonucleotides and subunits are attached to the 5′ end of the other oligonucleotide).
- the number of subunits attached to each oligonucleotidemay be the same or may be different.
- the number of subunits attached to each oligonucleotideis the same. In certain embodiments, when subunits are attached to both oligonucleotides of a double-stranded compound at the same end, the number of subunits attached to each oligonucleotide is different. This scenario, where subunits are attached to both oligonucleotides of a double-stranded compound at the same end, may occur at one or both ends of a double-stranded compound. In certain embodiments, the subunits attached to the 3′ and/or 5′ end are modified.
- compounds described hereinare oligonucleotides. In certain embodiments, compounds described herein are modified oligonucleotides. In certain embodiments, compounds described herein are antisense oligonucleotides. In certain embodiments, compounds described herein are oligomeric compounds. In certain embodiments, compounds described herein are RNAi compounds. In certain embodiments, compounds described herein are siRNA compounds. In certain embodiments, a compound described herein can comprise any of the oligonucleotide sequences targeted to MAPT described herein. In certain embodiments, the compound can be double-stranded.
- the compoundcomprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- the compoundcomprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
- the compoundcomprises a second oligonucleotide.
- the compoundcomprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
- the compoundcomprises ribonucleotides in which the oligonucleotide has uracil (U) in place of thymine ( ⁇ ) for any of the sequences provided here.
- the compoundcomprises deoxyribonucleotides in which the oligonucleotide has thymine ( ⁇ ) in place of uracil (U) for any of the sequences provided here.
- compounds described hereincomprise or consist of modified oligonucleotides.
- compounds described hereincomprise or consist of antisense oligonucleotides.
- compoundscomprise or consist of oligomeric compounds.
- compounds described hereinare capable of hybridizing to a target nucleic acid.
- compounds described hereinselectively affect one or more target nucleic acid.
- Such compoundscomprise a nucleobase sequence that hybridizes to one or more target nucleic acid, resulting in one or more desired activity and does not hybridize to one or more non-target nucleic acid or does not hybridize to one or more non-target nucleic acid in such a way that results in a significant undesired activity.
- hybridization of a compound described herein to a target nucleic acidresults in recruitment of one or more proteins that cause the cleavage of the target nucleic acid.
- certain compounds described herein or a portion of the compoundis loaded into an RNA-induced silencing complex (RISC), ultimately resulting in cleavage of the target nucleic acid.
- RISCRNA-induced silencing complex
- RNAi compoundsmay be double-stranded (siRNA) or single-stranded (ssRNA).
- hybridization of compounds described herein to a target nucleic aciddoes not result in recruitment of a protein that cleaves that target nucleic acid.
- hybridization of the compound to the target nucleic acidresults in the alteration of splicing of the target nucleic acid.
- hybridization of the compound to a target nucleic acidresults in inhibition of a binding interaction between the target nucleic acid and a protein or other nucleic acid.
- hybridization of the compound to the target nucleic acidresults in the alteration of RNA processing. In certain such embodiments, hybridization of the compound to a target nucleic acid results in alteration of translation of the target nucleic acid. Activities resulting from the hybridization of a compound to a target nucleic acid may be observed directly or indirectly. In certain embodiments, observation or detection of an activity involves observation or detection of a change in an amount of a target nucleic acid or protein encoded by such target nucleic acid, a change in the ratio of splice variants of a nucleic acid or protein, and/or a phenotypic change in a cell or animal.
- the disclosurerelates to compounds that comprise or consist of oligonucleotides.
- Oligonucleotidesconsist of linked nucleosides.
- oligonucleotidesmay be unmodified RNA or DNA or may be modified.
- the oligonucleotidesare modified oligonucleotides.
- the modified oligonucleotidescomprise at least one modified sugar, modified nucleobase or modified internucleoside linkage relative to an unmodified RNA or DNA.
- an oligonucleotidehas a modified nucleoside.
- a modified nucleosidemay comprise a modified sugar, a modified nucleobase or both a modified sugar and a modified nucleobase.
- Modified oligonucleotidesmay also include end modifications, e.g., 5’-end modifications and 3’-end modifications.
- Sugar Modifications and MotifsIn certain embodiments, a modified sugar is a substituted furanosyl sugar or non- bicyclic modified sugar. In certain embodiments, a modified sugar is a bicyclic or tricyclic modified sugar. In certain embodiments, a modified sugar is a sugar surrogate. A sugar surrogate may comprise one or more substitutions described herein. In certain embodiments, a modified sugar is a substituted furanosyl or non-bicyclic modified sugar.
- the furanosyl sugaris a ribosyl sugar.
- the furanosyl sugarcomprises one or more substituent groups, including, but not limited to, substituent groups at the 2’, 3’, 4’, and 5’ positions.
- substituents at the 2’ positioninclude, but are not limited to, F and OCH 3 (“OMe”, “O-methyl” or “methoxy”).
- substituent groups at the 2’ position suitable for non-bicyclic modified sugarsinclude, but are not limited to, halo, allyl, amino, azido, SH, CN, OCN, CF 3 , OCF 3 , F, Cl, Br, SCH 3 , SOCH 3 , SO 2 CH 3 , ⁇ 2 , ⁇ 2 , ⁇ 3 , and ⁇ H 2 .
- substituent groups at the 2’ positioninclude, but are not limited to, O-(C 1 -C 10 ) alkoxy, alkoxyalkyl, O-alkyl, S-alkyl, N-alkyl, O- alkenyl, S-alkenyl, N-alkenyl, O-alkynyl, S-alkynyl, N-alkynyl, O-alkyl-O-alkyl, alkynyl, wherein the alkyl, alkenyl and alkynyl can be substituted or unsubstitutedC 1 to C 10 alkyl or C 2 to C 10 alkenyl and alkynyl.
- substituent groups at the 2’ positioninclude, but are not limited to, alkaryl, aralkyl, O-alkaryl, and O-aralkyl.
- these 2’ substituent groupscan be further substituted with one or more substituent groups independently selected from hydroxyl, alkoxy, carboxy, benzyl, phenyl, nitro ( ⁇ 2 ), thiol, thioalkoxy, thioalkyl, halogen, alkyl, aryl, alkenyl, and alkynyl.
- substituent groups at the 2’ positioninclude, but are not limited to, O[(CH 2 )nO]mCH 3 , O(CH 2 )nOCH 3 , O(CH 2 )nCH 3 , O(CH2)nONH 2 , O(CH 2 )nNH 2 , O(CH 2 ) n SCH 3 , and O(CH 2 ) n ON[(CH 2 ) n CH 3 )] 2 , where n and m are independently from 1 to about 10.
- substituent groups at the 4’ position suitable for non-bicyclic modified sugarsinclude, but are not limited to, alkoxy (e.g., methoxy), alkyl, and those described in Manoharan et al., WO 2015/106128.
- substituent groups at the 5’ position suitable for non-bicyclic modified sugarsinclude, but are not limited to, methyl (“Me” or “CH 3 ”) (R or S), vinyl, and methoxy.
- the 5' modificationis a 5'-monophosphate ((HO)2(O)P-O-5'); 5'-diphosphate ((HO)2(O)P-O- P(HO)(O)-O-5'); 5'-triphosphate ((HO) 2 (O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-guanosine cap (7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O- 5'); 5'adenosine cap (Appp), and any modified or unmodified nucleotide cap structure (N-O- 5'(HO)
- one or more sugarscomprise a 5′- phosphonate modification.
- the 5′-phosphonate modificationis a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification.
- one or more sugarscomprise a 5′-vinylphosphonate modification.
- one or more sugarscomprise a 5′-ethylenephosphonate modification.
- the 5′ modificationis at the terminus of an oligonucleotide. In certain embodiments the 5′ modification is at the terminus of an antisense oligonucleotide.
- substituents described herein for the 2’, 4’ and 5’ positioncan be added to other specific positions on the sugar. In certain embodiments, such substituents may be added to the 3’ position of the sugar on the 3’ terminal nucleoside or the 5’ position of the 5’ terminal nucleoside.
- a non-bicyclic modified sugarmay comprise more than one non-bridging sugar substituent.
- non-bicyclic modified sugars substituentsinclude, but are not limited to, 5’-Me-2’-F, 5’-Me-2’-OMe (including both R and S isomers).
- modified sugar substituentsinclude those described in Migawa et al., WO 2008/101157 and Rajeev et al., US2013/0203836.
- a modified sugaris a bicyclic sugar.
- a bicyclic sugaris a modified sugar comprising two rings, wherein the second ring is formed via a bridge connecting two of the atoms in the first ring thereby forming a bicyclic structure.
- a bicyclic sugarcomprises a bridging substituent that bridges two atoms of the furanosyl ring to form a second ring. In certain embodiments, a bicyclic sugar does not comprise a furanosyl moiety.
- a “bicyclic nucleoside” (“BNA”)is a nucleoside having a bicyclic sugar.
- the bicyclic sugarcomprises a bridge between the 4’ and 2’ furanose ring atoms.
- the bicyclic sugarcomprises a bridge between the 5’ and 3’ furanose ring atoms.
- the furanose ringis a ribose ring.
- 4’ to 2’ bridging substituentsinclude, but are not limited to, 4'-CH 2 -2', 4'-(CH 2 ) 2 -2', 4'- (CH 2 ) 3 -2', 4'-CH 2 -O-2' (“LNA”), 4'-CH 2 -S-2', 4'- (CH 2 )2-O-2' (“ENA”), 4'-CH(CH 3 )-O-2' (“constrained ethyl” or “cEt” when in the S configuration), 4’-CH2-O-CH 2 -2’, 4’-CH 2 -N(R)-2’, 4'- CH(CH 2 OCH 3 )-O-2' (“constrained MOE” or “cMOE”) and analogs thereof (e.g., U.S.
- Patent No. 7,399,845)4'-C(CH 3 )(CH 3 )- O-2' and analogs thereof (e.g., U.S. Patent No. 8,278,283), 4'-CH 2 -N(OCH 3 )-2' and analogs thereof (e.g., U.S. Patent No. 8,278,425), 4'-CH 2 -O-N(CH 3 )-2' (e.g., U.S. Patent Publication No. 2004/0171570), 4'-CH 2 -N(R)-O-2', wherein R is ⁇ , C 1 -C 12 alkyl, or a protecting group (e.g., U.S. Patent No.
- bicyclic nucleosidescan be prepared having one or more stereochemical sugar configurations including for example ⁇ -L-ribofuranose and ⁇ -D-ribofuranose (see e.g., WO 99/14226). Specified bicyclic nucleosides herein are in the ⁇ -D configuration, unless otherwise specified.
- a modified sugaris a sugar surrogate.
- a sugar surrogatehas the oxygen atom replaced, e.g., with a sulfur, carbon or nitrogen atom.
- the sugar surrogatemay also comprise bridging and/or non-bridging substituents as described herein.
- sugar surrogatescomprise rings having other than 5 atoms.
- the sugar surrogatecomprises a cyclobutyl moiety in place of the pentofuranosyl sugar.
- the sugar surrogatecomprises a six membered ring in place of the pentofuranosyl sugar.
- the sugar surrogatecomprises a tetrahydropyran (“THP”) in place of the pentofuranosyl sugar.
- the sugar surrogatecomprises a morpholino in place of the pentofuranosyl sugar.
- sugar surrogatescomprise acyclic moieties.
- the sugar surrogateis an unlocked nucleic acid (“UNA”).
- UNAis unlocked acyclic nucleic acid, wherein any of the bonds of the sugar has been removed, forming an unlocked "sugar” residue.
- UNAalso encompasses a monomer where the bonds between C1’-C4’ have been removed (i.e. the covalent carbon-oxygen-carbon bond between the C1’ and C4’ carbons).
- the C2’-C3’ bondi.e. the covalent carbon-carbon bond between the C2’ and C3’ carbons
- sugar surrogatescomprise peptide nucleic acid (“PNA”), acyclic butyl nucleic acid (see, e.g., Kumar et al., Org. Biomol. Chem., 2013, 11, 5853-5865), and nucleosides and oligonucleotides described in Manoharan et al., US2013/130378, the entire contents of which is hereby incorporated herein by reference.
- PNApeptide nucleic acid
- acyclic butyl nucleic acidsee, e.g., Kumar et al., Org. Biomol. Chem., 2013, 11, 5853-5865
- nucleosides and oligonucleotidesdescribed in Manoharan et al., US2013/130378, the entire contents of which is hereby incorporated herein by reference.
- the disclosurerelates to compounds comprising at least one oligonucleotide wherein the nucleosides of such oligonucleotide comprise one or more types of modified sugars and/or unmodified sugars arranged along the oligonucleotide or region thereof in a defined pattern or “sugar motif”.
- such sugar motifsinclude, but are not limited to, any of the patterns of sugar modifications described herein.
- an oligonucleotidecomprises a gapmer sugar motif.
- a gapmer oligonucleotidecomprises or consists of a region having two external “wing” regions and a central or internal “gap” region.
- the gap and wing regionsform a contiguous sequence of nucleosides, wherein the majority of nucleoside sugars of each of the wings differ from the majority of nucleoside sugars of the gap.
- the wing regionscomprise a majority of modified sugars and the gap comprises a majority of unmodified sugars.
- the nucleosides of the gapare deoxynucleosides.
- one or both oligonucleotides of a double-stranded compoundcomprise a triplet sugar motif.
- An oligonucleotide with a triplet sugar motifcomprises three identical sugar modifications on three consecutive nucleosides.
- the tripletis at or near the cleavage site of the oligonucleotide.
- an oligonucleotide of a double-stranded compoundmay contain more than one triplet sugar motif.
- the identical sugar modification of the triplet sugar motifis a 2’-F modification.
- one or both oligonucleotides of a double-stranded compoundcomprise a quadruplet sugar motif.
- An oligonucleotide with a quadruplet sugar motifcomprises four identical sugar modifications on four consecutive nucleosides.
- the quadrupletis at or near the cleavage site.
- an oligonucleotide of a double-stranded compoundmay contain more than one quadruplet sugar motif.
- the identical sugar modification of the quadruplet sugar motifis a 2’-F modification.
- the cleavage site of the antisense oligonucleotideis typically around the 10, 11, and 12 positions from the 5’-end.
- the quadruplet sugar motifis at the 8, 9, 10, 11 positions; the 9, 10, 11, 12 positions; the 10, 11, 12, 13 positions; the 11, 12, 13, 14 positions; or the 12, 13, 14, 15 positions of the sense oligonucleotide, counting from the first nucleoside of the 5’-end of the sense oligonucleotide, or, the count starting from the first paired nucleotide within the duplex region from the 5’-end of the sense oligonucleotide.
- the quadruplet sugar motifis at the 8, 9, 10, 11 positions; the 9, 10, 11, 12 positions; the 10, 11, 12, 13 positions; the 11, 12, 13, 14 positions; or the 12, 13, 14, 15 positions of the antisense oligonucleotide, counting from the first nucleoside of the 5’-end of the antisense oligonucleotide, or, the count starting from the first paired nucleotide within the duplex region from the 5’- end of the antisense oligonucleotide.
- the cleavage sitemay change according to the length of the duplex region of the double- stranded compound and may change the position of the quadruplet accordingly.
- an oligonucleotidecomprises an alternating sugar motif.
- one or both oligonucleotides of a double-stranded compoundcomprise an alternating sugar motif.
- An oligonucleotide with an alternating sugar motifcomprises at least two different sugar modifications wherein one or more consecutive nucleosides comprising a first sugar modification alternates with one or more consecutive nucleosides comprising a second sugar modification and one or more consecutive nucleosides comprising a third sugar modification, etc.
- the alternating motifcan be “ABABABABABAB...,” “AABBAABBAABB...,” “AABAABAABAAB...,” “AAABAAABAAAB...,” “AAABBBAAABBB...,” or “ABCABCABCABC...” etc.
- the alternating sugar motifis repeated for at least 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, or 23 contiguous nucleobases along an oligonucleotide.
- the alternating sugar motifis comprised of two different sugar modifications.
- the alternating sugar motifcomprises 2’-OMe and 2’-F sugar modifications.
- each nucleoside of an oligonucleotideis independently modified with one or more sugar modifications provided herein.
- each oligonucleotide of a double-stranded compoundindependently has one or more sugar motifs provided herein.
- an oligonucleotide containing a sugar motifis fully modified in that each nucleoside other than the nucleosides comprising the sugar motif comprises a sugar modification.
- Nucleobase Modifications and MotifsIn certain embodiments, compounds described herein comprise modified oligonucleotides.
- modified oligonucleotidescomprise one or more nucleosides comprising a modified nucleobase.
- modified oligonucleotidescomprise one or more nucleosides that do not comprise a nucleobase, referred to as an abasic nucleoside.
- modified nucleobasesare selected from: 5-substituted pyrimidines, 6-azapyrimidines, alkyl or alkynyl substituted pyrimidines, alkyl substituted purines, and ⁇ -2, N-6 and O-6 substituted purines.
- modified nucleobasesare selected from: 2-aminopropyladenine, 5- hydroxymethyl cytosine, 5- methylcytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-N-methylguanine, 6-N- methyladenine, 2-propyladenine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-propynyl (C ⁇ C-CH 3 ) uracil, 5-propynylcytosine, 6-azouracil, 6-azocytosine, 6-azothymine, 5- ribosyluracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl, 8- aza and other 8-substituted purines, 5-halo, particularly, 5-bromo, 5-trifluoromethyl, 5- halouracil, and 5-halocytosine
- nucleobasesinclude tricyclic pyrimidines, such as 1,3-diazaphenoxazine-2-one, 1,3-diazaphenothiazine-2- one, and 9-(2-aminoethoxy)-1,3-diazaphenoxazine-2-one (G-clamp).
- Modified nucleobasesmay also include those in which the purine or pyrimidine base is replaced with other heterocycles, for example, 7-deaza-adenine, 7-deazaguanosine, 2-aminopyridine and 2- pyridone.
- Further nucleobasesinclude those disclosed in U.S.
- Patent 3,687,808Modified Nucleosides in Biochemistry, Biotechnology and Medicine, Herdewijn, ⁇ . ed. Wiley-VCH, 2008; The Concise Encyclopedia Of Polymer Science And Engineering, pages 858-859; Kroschwitz, J.L., Ed., John Wiley & Sons, 1990, 858-859; Englisch et al., Angewandte Chemie, International Edition, 1991, 30, 613; Sanghvi, Y.S., Chapter 15, dsRNA Research and Applications, pages 289-302; Antisense Research and Applications, Crooke, S.T.
- compounds described hereincomprise oligonucleotides.
- oligonucleotidescomprise modified and/or unmodified nucleobases arranged along the oligonucleotide or region thereof in a defined pattern or motif.
- each nucleobaseis modified.
- none of the nucleobasesare modified.
- each purine or each pyrimidineis modified.
- each adenineis modified.
- each guanineis modified.
- each thymineis modified.
- each uracilis modified.
- each cytosineis modified.
- some or all of the cytosine nucleobases in a modified oligonucleotideare 5-methylcytosines.
- modified oligonucleotidescomprise a block of modified nucleobases. In certain such embodiments, the block is at the 3’-end of the oligonucleotide.
- the blockis within 3 nucleosides of the 3’-end of the oligonucleotide. In certain embodiments, the block is at the 5’-end of the oligonucleotide. In certain embodiments, the block is within 3 nucleosides of the 5’-end of the oligonucleotide.
- Internucleoside Linkage Modifications and MotifsA 3' to 5' phosphodiester linkage is the naturally occurring internucleoside linkage of RNA and DNA. In certain embodiments, compounds described herein have one or more modified, i.e. non-naturally occurring, internucleoside linkages.
- internucleoside linkagesmay impart desirable properties such as, for example, enhanced cellular uptake, enhanced affinity for target nucleic acids, and increased stability in the presence of nucleases.
- Methods of preparation of phosphorous- containing and non-phosphorous-containing internucleoside linkagesare well known to those skilled in the art.
- Further neutral internucleoside linkagesinclude nonionic linkages comprising siloxane (dialkylsiloxane), carboxylate ester, carboxamide, sulfide, sulfonate ester and amides (See, for example: Carbohydrate Modifications in Antisense Research; Y.S. Sanghvi and P.D. Cook, Eds., ACS Symposium Series 580; Chapters 3 and 4, 40-65).
- Further neutral internucleoside linkagesinclude nonionic linkages comprising mixed ⁇ , O, S and CH 2 component parts.
- compounds provided hereincomprise at least one modified internucleoside linkage.
- a modified internucleoside linkagemay be placed at any position of an oligonucleotide.
- a modified internucleoside linkagemay be placed within the sense oligonucleotide, antisense oligonucleotide, or both oligonucleotides of the double-stranded compound.
- the internucleoside linkage modificationmay occur on every nucleoside of an oligonucleotide.
- internucleoside linkage modificationsmay occur in an alternating pattern along an oligonucleotide.
- a double-stranded compoundcomprises 6-8 modified internucleoside linkages.
- the 6-8 modified internucleoside linkagesare phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages.
- the sense oligonucleotidecomprises at least two modified internucleoside linkages at either or both the 5’-end and the 3’-end.
- the modified internucleoside linkagesare phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages.
- the antisense oligonucleotidecomprises at least two modified internucleoside linkages at either or both the 5’-end and the 3’-end.
- the modified internucleoside linkagesare phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages.
- a double-stranded compoundcomprises an overhang region.
- a double-stranded compoundcomprises a phosphorothioate or alkylphosphonate internucleoside linkage modification in the overhang region.
- a double-stranded compoundcomprises a phosphorothioate or alkylphosphonate internucleotide linkage linking the overhang nucleotide with a paired nucleotide that is next to the overhang nucleotide.
- a phosphorothioate or alkylphosphonate internucleotide linkagelinking the overhang nucleotide with a paired nucleotide that is next to the overhang nucleotide.
- modified oligonucleotidescomprise one or more internucleoside linkages having chiral centers. Representative chiral internucleoside linkages include, but are not limited to, alkylphosphonates and phosphorothioates.
- Modified oligonucleotides comprising internucleoside linkages having chiral centerscan be prepared as populations of modified oligonucleotides comprising stereorandom internucleoside linkages, or as populations of modified oligonucleotides comprising phosphorothioate linkages in particular stereochemical configurations.
- populations of modified oligonucleotidescomprise phosphorothioate internucleoside linkages wherein all of the phosphorothioate internucleoside linkages are stereorandom.
- Such modified oligonucleotidescan be generated using synthetic methods that result in random selection of the stereochemical configuration of each phosphorothioate linkage.
- each individual phosphorothioate of each individual oligonucleotide moleculehas a defined stereoconfiguration.
- populations of modified oligonucleotidesare enriched for modified oligonucleotides comprising one or more particular phosphorothioate internucleoside linkages in a particular, independently selected stereochemical configuration.
- the particular configuration of the particular phosphorothioate linkageis present in at least 65% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 70% of the molecules in the population.
- the particular configuration of the particular phosphorothioate linkageis present in at least 80% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 90% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 99% of the molecules in the population.
- Such enriched populations of modified oligonucleotidescan be generated using synthetic methods known in the art, e.g., methods described in Oka et al., JACS 125, 8307 (2003), Wan et al. Nuc. Acid. Res. 42, 13456 (2014), and WO 2017/015555.
- a population of modified oligonucleotidesis enriched for modified oligonucleotides having at least one indicated phosphorothioate in the (Sp) configuration. In certain embodiments, a population of modified oligonucleotides is enriched for modified oligonucleotides having at least one phosphorothioate in the (Rp) configuration.
- Conjugate GroupsIn certain embodiments, the compounds described herein comprise or consist of one or more oligonucleotides and, optionally, one or more conjugate groups. Conjugate groups may be attached to either or both ends of an oligonucleotide and/or at any internal position.
- a conjugate groupis attached at the 3’ end of an oligonucleotide. In certain embodiments, a conjugate group is attached at the 5’ end of an oligonucleotide. In certain embodiments, oligonucleotides are covalently attached to one or more conjugate groups. In certain embodiments, conjugate groups are terminal groups attached to either or both ends of an oligonucleotide. In certain such embodiments, terminal groups are attached at the 3’ end of an oligonucleotide. In certain such embodiments, terminal groups are attached at the 5’ end of an oligonucleotide.
- terminal groupsinclude, but are not limited to, capping groups, phosphate moieties, protecting groups, modified or unmodified nucleosides, and two or more nucleosides that are independently modified or unmodified, such as an overhang.
- conjugate groupsmodify one or more properties of the attached oligonucleotide, including, but not limited to, pharmacodynamics, pharmacokinetics, stability, activity, half-life, binding, absorption, tissue distribution, cellular distribution, cellular uptake, charge and clearance.
- conjugate groupsenhance the affinity of a compound for a selected target, e.g., molecule, cell or cell type, compartment, e.g., a cellular or organ compartment, tissue, organ or region of the body, as, e.g., compared to a compound absent such a conjugate group.
- conjugate groupsimpart a new property on the attached oligonucleotide, e.g., fluorophores or reporter groups that enable detection of the oligonucleotide.
- conjugate groupsinclude, but are not limited to, intercalators, reporter molecules, polyamines, polyamides, peptides, carbohydrates, vitamin moieties, polyethylene glycols, thioethers, polyethers, cholesterols, thiocholesterols, cholic acid moieties, folate, lipids, phospholipids, biotin, phenazine, phenanthridine, anthraquinone, adamantane, acridine, fluoresceins, rhodamines, coumarins, fluorophores, and dyes.
- conjugate groupsinclude an active drug substance, for example, aspirin, warfarin, phenylbutazone, ibuprofen, suprofen, fen-bufen, ketoprofen, (S)- (+)-pranoprofen, carprofen, dansylsarcosine, 2,3,5-triiodobenzoic acid, fingolimod, flufenamic acid, folinic acid, a benzothiadiazide, chlorothiazide, a diazepine, indo-methicin, a barbiturate, a cephalosporin, a sulfa drug, an antidiabetic, an antibacterial, or an antibiotic.
- active drug substancefor example, aspirin, warfarin, phenylbutazone, ibuprofen, suprofen, fen-bufen, ketoprofen, (S)- (+)-pranoprofen, carprofen, dans
- conjugate groupsare targeting moieties.
- a targeting moietyincludes, but is not limited to, a lectin, glycoprotein, lipid, protein, peptide, peptide mimetic, receptor ligand, antibody, thyrotropin, melanotropin, surfactant protein A, carbohydrate, carbohydrate derivative, modified carbohydrate, carbohydrate cluster, polysaccharide, modified polysaccharide, or polysaccharide derivative, mucin carbohydrate, multivalent lactose, multivalent galactose, N-acetyl-galactosamine (GalNAc), N-acetylglucosamine multivalent mannose, multivalent fucose, glycosylated polyaminoacids, multivalent galactose, transferrin, bisphosphonate, polyglutamate, polyaspartate, a lipid, cholesterol, a steroid, bile acid, folate, vitamin ⁇ 12, vitamin A, bio
- conjugate groupsmay include, but are not limited to, the conjugate groups described in the following references such as cholesterol (e.g., Letsinger et al., Proc. Natl. Acid. Sci. USA, 1989, 86: 6553-6556), cholic acid (e.g., Manoharan et al., Biorg. Med. Chem. Let., 1994, 4:1053-1060), thioether, e.g., hexyl-S-tritylthiol (e.g., Manoharan et al., ⁇ nn. NY. Acad. Sci., 1992, 660:306-309; Manoharan et al., Biorg. Med. Chem.
- cholesterole.g., Letsinger et al., Proc. Natl. Acid. Sci. USA, 1989, 86: 6553-6556
- cholic acide.g., Manoharan et al., Biorg. Med. Chem.
- thiocholesterole.g., Oberhauser et al., Nucl. Acids Res., 1992, 20:533-538
- aliphatic chainse.g., do-decan-diol or undecyl residues
- phospholipidse.g., di-hexadecyl-rac- glycerol or triethyl-ammonium 1,2-di-O-hexadecyl-rac-glycero-3-H-phosphonate (e.g, Manoharan et al., Tetrahedron Lett., 1995, 36:3651-365
- tocopherole.g., Nishina et al., Molecular Therapy Nucleic Acids, 2015, 4, e220 and Nishina et al., Molecular Therapy, 2008, 16:734-740
- GalNAcand other carbohydrates (e.g., Maier et al., Bioconjugate Chemistry, 2003, 14, 18-29; Rensen et al., J. Med. Chem.
- Conjugate groupsmay be attached to oligonucleotides through conjugate linkers.
- a conjugate linkercomprises a chain structure, such as a hydrocarbyl chain, or an oligomer of repeating units or combination of such repeating units.
- a conjugate linkercomprises one or more groups selected from alkyl, amino, ⁇ x ⁇ , amide, disulfide, polyethylene glycol, ether, thioether, and hydroxylamino. In certain embodiments, a conjugate linker comprises at least one phosphorus group. In certain embodiments, a conjugate linker comprises at least one phosphate group. In certain embodiments, a conjugate linker includes at least one neutral linking group.
- conjugate linkersinclude, but are not limited to, pyrrolidine, 8-amino-3,6- dioxaoctanoic acid (ADO), succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC) and 6-aminohexanoic acid ( ⁇ or AHA).
- ADO8-amino-3,6- dioxaoctanoic acid
- SMCCsuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate
- ⁇ or AHA6-aminohexanoic acid
- conjugate linkersinclude, but are not limited to, substituted or unsubstituted C 1 -C 10 alkyl, substituted or unsubstituted C 2 - C 10 alkenyl, or substituted or unsubstituted C 2 -C 10 alkynyl, wherein a nonlimiting list of preferred substituent groups includes hydroxyl, amino, alkoxy, carboxy, benzyl, phenyl, nitro, thiol, thioalkoxy, halogen, alkyl, aryl, alkenyl, and alkynyl.
- conjugate linkerscomprise 1-10 linker-nucleosides.
- linker- nucleosidesmay be modified or unmodified nucleosides. It is typically desirable for linker- nucleosides to be cleaved from the compound after it reaches a target tissue. Accordingly, linker-nucleosides herein can be linked to one another and to the remainder of the compound through cleavable bonds. Herein, linker-nucleosides are not considered to be part of the oligonucleotide.
- a compoundcomprises an oligonucleotide consisting of a specified number or range of linked nucleosides and/or a specified percent complementarity to a reference nucleic acid and the compound also comprises a conjugate group comprising a conjugate linker comprising linker-nucleosides
- those linker-nucleosidesare not counted toward the length of the oligonucleotide and are not used in determining the percent complementarity of the oligonucleotide for the reference nucleic acid.
- conjugate groups and conjugate linkers as well as other modificationsinclude, without limitation, those described in the following references: US 5,994,517; US 6,300,319; US 6,660,720; US 6,906,182; US 7,262,177; US 7,491,805; US 8,106,022; US 7,723,509; US 9,127,276; US 2006/0148740; US 2011/0123520; WO2013/033230; WO2012/037254, Biessen et al., J. Med. Chem. 1995, 38, 1846-1852; Lee et al., Bioorganic & Medicinal Chemistry 2011,19, 2494-2500; Rensen et al., J. Biol. Chem.
- a compound provided hereincomprises a conjugate group.
- an oligonucleotide provided hereincomprises a conjugate group.
- the conjugate groupis a targeting moiety.
- the targeting moietycomprises one or more TrkB ligands.
- R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , and R 22are each independently optionally substituted unsaturated or partially unsaturated alkyl.
- R 7 , R 8 , R 9 , and R 10are each independently alkenyl.
- R 7 , R 8 , R 9 , and R 10are each independently alkynyl.
- R 2is OR 7 .
- R 3is OR 11 .
- R 7 and R 11are each independently hydrogen, optionally substituted alkyl or optionally substituted alkenyl. In certain embodiments, one or both R 7 and R 11 are each independently hydrogen. In certain embodiments, one or both R 7 and R 11 are each independently optionally substituted alkyl. In certain embodiments, one or both R 7 and R 11 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, one or both R 7 and R 11 are each independently alkenyl. In certain embodiments, R 7 is optionally substituted alkyl and R 11 is hydrogen. In certain embodiments, R 7 is hydrogen and R 11 is optionally substituted alkyl. In certain embodiments, R 7 is alkenyl and R 11 is hydrogen.
- R 7is hydrogen and R 11 is optionally substituted alkenyl.
- the TrkB ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (II-A),
- R 1is the modified oligonucleotide
- L 1 , L 2 , L 3 , L 4 , and R 1are as described herein.
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXVII) or a salt, solvate, or hydrate thereof: wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein;
- R 11 and R 13are each independently absent, hydrogen, or optionally substituted alkyl;
- R 12 , R 14 , and R 15are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
- R 16is hydrogen, halogen, –CN, –N3, –SOn16R 1A , –SOv16NR 16
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXIX) or a salt, solvate, or hydrate thereof: Formula (XXXXIX), wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 17 , R 18 , and R 19 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; z4 is 0, 1, or 2; and z5 is 0, 1, 2, or 3.
- the TrkB ligand of a modified oligonucleotideis of the Formula (XXXXX) or a salt, solvate, or hydrate thereof: Formula (XXXXX), wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 20 is hydrogen, halogen, –CN, –N3, –SOn20R 1A , –SOv20NR 20B R 20C , ⁇ NHNR 20B R 20C , ⁇ ONR 20B R 20C , ⁇ NHC(O)NHNR 20B R 20C , ⁇ NHC(O)NR 20B R 20C , –N(O)m20, –NR 20B R 20C , –C(O)R 20D , –C(O)OR 20D , –C(O)NR 20B R 20C , –OR 20A , -NR 20B SO2R 20A ,
- the CB 1 ligand of a modified oligonucleotideis of the Formula (XXXXXI) or a salt, solvate, or hydrate thereof: , Formula (XXXXXI) wherein: L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein;
- X 1is NR 10 or CR 11 R 12 ;
- R 10 , R 11 , and R 12are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
- R 19is hydrogen, –SOn19R 19A , –SOv19NR 19B R 19C , ⁇ NHNR 19B R 19C , ⁇ ONR 19B R 19C , ⁇ NHC(O)NHNR 19B R 19C , ⁇ NHC(O)NR 19B R
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 is H, polyethylene glycol (PEG), optionally substituted heteroalkyl, or optionally substituted heteroaryl; and R 3 , and R 4 are each independently H, halogen, optionally substituted alkyl, or optionally substituted -O-alkyl.
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXIII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXIII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 , R 3 , R 4 , and R 5 are each independently H, halogen, optionally substituted alkyl, optionally substituted -O-alkyl, cycloalkyl, or absent; R 8 is optionally substituted C 1 -C 5 alkyl, optionally substituted C 1 -C 5 alkylene-(C 3 - C 6 )-cycloalkyl, or optionally substituted (C 1 -C 4 )-alkylene-(C 1 -C 4 )-alkoxy; and R 6 , and R 7 are each independently H, halogen, alkyl, or optionally
- R 2is H, -CONHR 4 , -CH 2 OR 4 , -(CH 2 ) 2 OR 4 , -CH 2 NHCOR 4 , or -OR 4 ;
- R 3is H, optionally substituted alkyl, or optionally substituted cycloalkyl;
- R 4is H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl;
- R 5is -OH or absent;
- Xis H, optionally substituted CH 2 , optionally substituted NH, or cycloalkyl.
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis of the Formula (XXXXXXXIII) or a salt, solvate, or hydrate thereof: .
- Formula (XXXXXXXIII)wherein L 1 , L 2 , L 3 , L 4 , and R 1 are as described herein; R 2 is H, -CONHR 3 , -CH 2 OR 3 , -(CH 2 ) 2 OR 3 , -CH 2 NHCOR 3 , or -OR 3 ; each instance of R 3 is independently H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; and X is H or halogen.
- L 1 , L 2 , L 3 , and L 4are each independently absent, a bond, an optionally substituted alkyl linker, an optionally substituted polyethylene glycol (PEG) linker, an optionally substituted heteroalkyl linker, or an optionally substituted heteroaryl linker.
- L 1is an optionally substituted heteroaryl linker.
- L 1is an optionally substituted unsaturated heteroaryl, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker.
- L 1comprises the structure: .
- L 1is an optionally substituted heteroalkyl linker.
- the optionally substituted heteroalkyl linkeris an optionally substituted heteroalkyl or optionally substituted C 1 -C 1 0 alkyl chain in which one or more carbon atoms are replaced with O, N, or S.
- L 1comprises the structure: o .
- L 1comprises the structure: –N(CH 3 )–.
- L 2is an optionally substituted PEG linker.
- the PEG linkeris five PEG units in length.
- the PEG linkeris four PEG units in length.
- the PEG linkeris three PEG units in length.
- L 2is an optionally substituted alkyl linker.
- the heteroalkyl linkercomprises two substituents joined together to form an optionally substituted carbocyclyl ring.
- L 4comprises the structure: salt thereof, wherein X is O or S.
- L 4comprises the structure: or a salt thereof, wherein X is O or S.
- L 1 – L 2 –L 3 –L 4comprises the structure: ,
- TrkB ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (IV),
- the CB 1 ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXXVI)
- the ⁇ 4 ⁇ 1/7 integrin ligand of a modified oligonucleotideis selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXXIX)
- a compound provided hereincomprises a conjugate group.
- an oligonucleotide provided hereincomprises a conjugate group.
- the conjugate groupis a lipid.
- an internucleoside linkage of a modified oligonucleotide provided hereincomprises one or more lipids.
- R Cis –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R 2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 6 , -N(R 6 ), or -SR 6 ; each R 3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR 7 , -N(R 7 ), or -SR 7 ; R 4 and
- the modified oligonucleotidecomprises Formula (XXXXII), or a salt, solvate, or hydrate thereof: mA ⁇ mA Formula (XXXXII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXIII), or a salt, solvate, or hydrate thereof: mA ⁇ mU Formula (XXXXIII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXIV), or a salt, solvate, or hydrate thereof: mA ⁇ mG Formula (XXXXIV) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXV), or a salt, solvate, or hydrate thereof: mA ⁇ mC Formula (XXXXV) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXVII), or a salt, solvate, or hydrate thereof: mU ⁇ mG Formula (XXXXVII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXVIII), or a salt, solvate, or hydrate thereof: mU ⁇ fC Formula (XXXXVIII) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXX), or a salt, solvate, or hydrate thereof: mG ⁇ fG Formula (XXXX) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXXI), or a salt, solvate, or hydrate thereof: mG ⁇ mC Formula (XXXXI) wherein: R 4 and R 5 are independently an oligonucleotide, or R 4 and R 5 are joined together to form a single oligonucleotide; and each X is independently O or S.
- the modified oligonucleotidecomprises Formula (XXXXXII), or a salt, solvate, or hydrate thereof:
- the modified oligonucleotidecomprises an internucleoside linkage of one of the following Formulae: Formula (XXXXXV), Formula (XXXXXVI).
- the compound of any preceding embodimentcomprises one or more lipid conjugate groups.
- the one or more lipid conjugate groupsare attached to one or more internucleoside linkages of the modified oligonucleotide.
- the one or more lipid conjugate groupsare attached to the 5’ or 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipid conjugate groups are attached to an internucleoside linkage and the 5’ or 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipid conjugate groups are attached to an internucleoside linkage and both the 5’ and 3’ ends of the modified oligonucleotide.
- the one or more ligandsare attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide.
- the one or more conjugate groupscomprise at least one ligand (e.g., at least one TrkB ligand, at least one CB 1 ligand, at least one ⁇ 4 ⁇ 1/7 integrin ligand) attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide and at least one lipid.
- at least one ligande.g., at least one TrkB ligand, at least one CB 1 ligand, at least one ⁇ 4 ⁇ 1/7 integrin ligand
- the one or more conjugate groupscomprise at least one ligand (e.g., at least one TrkB ligand, at least one CB 1 ligand, at least one ⁇ 4 ⁇ 1/7 integrin ligand) attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide and one or more lipid conjugate groups attached to one or more internucleoside linkages of the modified oligonucleotide.
- ligande.g., at least one TrkB ligand, at least one CB 1 ligand, at least one ⁇ 4 ⁇ 1/7 integrin ligand
- the modified oligonucleotidecomprises a ligand (e.g., a TrkB ligand, a CB 1 ligand, an ⁇ 4 ⁇ 1/7 integrin ligand) and a lipid.
- the modified oligonucleotidecomprises one or more ligands (e.g., one or more TrkB ligands, one or more CB 1 ligands, one or more ⁇ 4 ⁇ 1/7 integrin ligands) and one or more lipids.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide.
- the compound of any preceding embodimentcomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide.
- the modified oligonucleotidecomprises one or more substituted or unsubstituted alkyl or alkenyl.
- the one or more substituted or unsubstituted alkyl or alkenylare attached to one or more internucleoside linkages of the modified oligonucleotide.
- the modified oligonucleotideis the second modified oligonucleotide or sense oligonucleotide.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 4 -C 30 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 5 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 14 -C 20 hydrocarbon chain.
- the one or more substituted or unsubstituted alkyl or alkenylcomprise a saturated or unsaturated C 16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C 22 hydrocarbon chain.
- a substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide).
- a substituted or unsubstituted alkyl or alkenylis attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide).
- the internucleoside linkageis between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide.
- the internucleoside linkageis between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide.
- the internucleoside linkage of the modified oligonucleotideis selected from any one of Formulae XXXXIII-XXXXVI.
- the modified oligonucleotidecomprises any one of Formulae XXXV-XXXXVI.
- Target Nucleic Acids and Target Regionscompounds described herein comprise or consist of an oligonucleotide comprising a region that is complementary to a target nucleic acid.
- the target nucleic acidis an endogenous RNA molecule.
- the target nucleic acidencodes a protein.
- the target nucleic acidis non-coding.
- the target nucleic acidis selected from an mRNA and a pre-mRNA, including intronic, exonic and untranslated regions.
- the target RNAis an mRNA.
- the target nucleic acidis a pre-mRNA.
- the target regionis entirely within an exon.
- the target regionis entirely within an intron.
- the target regionspans an intron/exon junction.
- the target regionis at least 50% within an intron.
- compounds disclosed hereinhybridize with a MAPT nucleic acid. The most common mechanism of hybridization involves hydrogen bonding between complementary nucleobases of the nucleic acid molecules.
- Hybridizationcan occur under varying conditions. Hybridization conditions are sequence-dependent and are determined by the nature and composition of the nucleic acid molecules to be hybridized. Methods of determining whether a sequence hybridizes specifically to a target nucleic acid are well known in the art. In certain embodiments, the compounds provided herein specifically hybridize with a MAPT nucleic acid. Nucleotide sequences that encode MAPT include, without limitation, the following: GenBank Accession No. NM_001377265.1 (incorporated herein as SEQ ID NO: 1), and nucleotides 2624000 to 2761000 of NT_010783.14 (incorporated herein as SEQ ID NO: 2).
- Complementarity Oligonucleotides provided hereinmay have a defined percent complementarity to a particular nucleic acid, target region, oligonucleotide, or portion thereof. Non-complementary nucleobases may be tolerated provided that the oligonucleotide remains able to specifically hybridize to the nucleic acid, oligonucleotide, or portion thereof.
- the oligonucleotides provided herein, or a specified portion thereofare at least, or are up to 70%, 80%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% complementary to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof.
- the oligonucleotides provided herein, or a specified portion thereofare 70% to 75%, 75% to 80%, 80% to 85%, 85% to 90%, 90% to 95%, 95% to 100%, or any number in between these ranges, complementary to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof. Percent complementarity of an oligonucleotide with a target nucleic acid, a target region, an oligonucleotide or specified portion thereof can be determined using routine methods.
- an oligonucleotide in which 18 of 20 nucleobases of the oligonucleotide are complementary to a target region, and would therefore specifically hybridizewould represent 90 percent complementarity.
- the remaining non-complementary nucleobasesmay be clustered or interspersed with complementary nucleobases and need not be contiguous to each other or to complementary nucleobases.
- an oligonucleotide which is 18 nucleobases in length having four non-complementary nucleobases which are flanked by two regions of complete complementarity with the target nucleic acidwould have 77.8% overall complementarity with the target nucleic acid.
- Percent complementarity of an oligonucleotide with a region of a target nucleic acid, a target region, an oligonucleotide or specified portion thereofcan be determined routinely using BLAST programs (basic local alignment search tools) known in the art.
- oligonucleotides described herein, or specified portions thereofare fully complementary (i.e. 100% complementary) to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof.
- an oligonucleotidemay be fully complementary to a target nucleic acid, a target region, an oligonucleotide, or specified portion thereof.
- each nucleobase of an oligonucleotideis complementary to the corresponding nucleobase of a target nucleic acid, a target region, an oligonucleotide, or a specified portion thereof.
- a 20 nucleobase oligonucleotideis fully complementary to a target sequence that is 400 nucleobases long, so long as there is a corresponding 20 nucleobase portion of the target nucleic acid that is fully complementary to the compound.
- “Fully complementary”can also be used in reference to a specified portion of the first and/or the second nucleic acid.
- a 20 nucleobase portion of a 30 nucleobase oligonucleotidecan be “fully complementary” to a 20 nucleobase region of a target sequence that is 400 nucleobases long.
- the 20 nucleobase portion of the 30 nucleobase compoundis fully complementary to the target sequence if the target sequence has a corresponding 20 nucleobase portion wherein each nucleobase is complementary to the 20 nucleobase portion of the compound.
- the entire 30 nucleobase compoundmay or may not be fully complementary to the target sequence, depending on whether the remaining 10 nucleobases of the compound are also complementary to the target sequence.
- oligonucleotides described hereincomprise one or more mismatched nucleobases relative to a target nucleic acid, a target region, an oligonucleotide or a specified portion thereof.
- oligonucleotides described herein that are, or are up to 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, or 23 nucleobases in lengthcomprise no more than 4, no more than 3, no more than 2, or no more than 1 non- complementary nucleobase(s) relative to a target nucleic acid, or specified portion thereof.
- oligonucleotides described herein that are, or are up to 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 nucleobases in lengthcomprise no more than 6, no more than 5, no more than 4, no more than 3, no more than 2, or no more than 1 non-complementary nucleobase(s) relative to a target nucleic acid, a target region, an oligonucleotide, or specified portion thereof.
- the mismatchis at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 from the 5’-end of the oligonucleotide.
- the mismatchis at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12, 13 or 14 from the 3’-end of the oligonucleotide.
- the mismatchforms a wobble base pair with a corresponding nucleobase on the target nucleic acid.
- the mismatchforms a wobble base pair selected from hypoxanthine (nucleobase of inosine) and uracil (I:U base pair); guanine and uracil (G:U base pair); hypoxanthine and adenine (I:A base pair); and hypoxanthine and cytosine (I:C base pair).
- a mismatched nucleobase on an oligonucleotidecomprises hypoxanthine, guanine, or uracil.
- oligonucleotides described hereinmay be complementary to a portion of a nucleic acid.
- portionrefers to a defined number of contiguous nucleobases within a region of a nucleic acid.
- a “portion”can also refer to a defined number of contiguous nucleobases of an oligonucleotide.
- the oligonucleotidesare complementary to at least an 8 nucleobase portion of a nucleic acid.
- the oligonucleotidesare complementary to at least a 9 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 10 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least an 11 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 12 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 13 nucleobase portion of a nucleic acid.
- the oligonucleotidesare complementary to at least a 14 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 15 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 16 nucleobase portion of a nucleic acid. Also contemplated are oligonucleotides that are complementary to at least a 9, 10, 17, 18, 19, 20, 21, 22, 23 or more nucleobase portion of a nucleic acid, or a range defined by any two of these values. In certain embodiments, the oligonucleotide is an antisense oligonucleotide.
- a portion of the antisense oligonucleotideis compared to an equal length portion of the target nucleic acid. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion is compared to an equal length portion of the target nucleic acid.
- the oligonucleotideis a sense oligonucleotide. In certain embodiments, a portion of the sense oligonucleotide is compared to an equal length portion of an antisense oligonucleotide.
- an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion of a sense oligonucleotideis compared to an equal length portion of an antisense oligonucleotide.
- IdentityThe oligonucleotides provided herein may also have a defined percent identity to a particular nucleic acid, target region, oligonucleotide, or specified portion thereof. As used herein, an oligonucleotide is identical to a sequence disclosed herein if it has the same nucleobase pairing ability.
- RNA which contains thymidine in place of uracil in a disclosed RNA sequencewould be considered identical to the RNA sequence since both uracil and thymidine pair with adenine.
- Shortened and lengthened versions of the compounds described herein as well as compounds having non-identical bases relative to the compounds provided hereinalso are contemplated.
- the non-identical basesmay be adjacent to each other or dispersed throughout the compound. Percent identity of an oligonucleotide is calculated according to the number of bases that have identical base pairing relative to the sequence to which it is being compared.
- oligonucleotides described herein, or portions thereofare, or are at least, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100% identical to one or more of the nucleic acids, oligonucleotides, or a portion thereof, disclosed herein. In certain embodiments, oligonucleotides described herein are about 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical, or any percentage between such values, to a particular nucleic acid or oligonucleotide, or portion thereof.
- an oligonucleotidemay have one or more mismatched nucleobases.
- the mismatchis at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 from the 5’-end of the oligonucleotide.
- the mismatchis at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12, 13 or 14 from the 3’-end of the oligonucleotide.
- a portion of the oligonucleotideis compared to an equal length portion of the target nucleic acid.
- an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portionis compared to an equal length portion of the target nucleic acid.
- the oligonucleotideis a sense oligonucleotide. In certain embodiments, a portion of the sense oligonucleotide is compared to an equal length portion of the target nucleic acid. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion is compared to an equal length portion of the target nucleic acid.
- compositions and FormulationsCompounds described herein may be admixed with pharmaceutically acceptable active or inert substances for the preparation of pharmaceutical compositions or formulations. Compositions and methods for the formulation of pharmaceutical compositions are dependent upon a number of criteria, including, but not limited to, route of administration, extent of disease, or dose to be administered. Certain embodiments provide pharmaceutical compositions comprising one or more compounds or a salt thereof. In certain embodiments, the compounds are antisense oligonucleotides. In certain embodiments, the compounds are oligomeric compounds. In certain embodiments, the compounds comprise or consist of one or more modified oligonucleotides. In certain such embodiments, the pharmaceutical composition comprises one or more compound and a suitable pharmaceutically acceptable diluent or carrier.
- a pharmaceutical compositioncomprises one or more compound and a sterile saline solution. In certain embodiments, such pharmaceutical composition consists of one compound and a sterile saline solution. In certain embodiments, the sterile saline is pharmaceutical grade saline. In certain embodiments, a pharmaceutical composition comprises one or more compound and sterile water. In certain embodiments, a pharmaceutical composition consists of one compound and sterile water. In certain embodiments, the sterile water is pharmaceutical grade water. In certain embodiments, a pharmaceutical composition comprises one or more compounds and phosphate-buffered saline (PBS). In certain embodiments, a pharmaceutical composition consists of one compound and sterile PBS.
- PBSphosphate-buffered saline
- the sterile PBSis pharmaceutical grade PBS.
- a compound described herein targeted to MAPTcan be utilized in pharmaceutical compositions by combining the compound with a suitable pharmaceutically acceptable diluent or carrier.
- a pharmaceutically acceptable diluentis water, such as sterile water suitable for injection.
- employed in the methods described hereinis a pharmaceutical composition comprising a compound targeted to MAPT and a pharmaceutically acceptable diluent.
- the pharmaceutically acceptable diluentis water.
- the compoundcomprises or consists of one or more modified oligonucleotide provided herein.
- compositionscomprising compounds provided herein encompass any pharmaceutically acceptable salts, esters, or salts of such esters, or any other oligonucleotide which, upon administration to an animal, including a human, is capable of providing (directly or indirectly) the biologically active metabolite or residue thereof.
- the compoundsare antisense oligonucleotides.
- the compoundsare oligomeric compounds.
- the compoundcomprises or consists of one or more modified oligonucleotide. Accordingly, for example, the disclosure is also drawn to pharmaceutically acceptable salts of compounds, prodrugs, pharmaceutically acceptable salts of such prodrugs, and other bioequivalents.
- Suitable pharmaceutically acceptable saltsinclude, but are not limited to, sodium and potassium salts.
- a prodrugcan include the incorporation of additional nucleosides at one or both ends of a compound which are cleaved by endogenous nucleases within the body, to form the active compound.
- the compounds or compositionsfurther comprise a pharmaceutically acceptable carrier or diluent.
- RNARibonucleic acid
- DNADNA having a modified sugar (2’-OH for the natural 2’-H of DNA) or as an RNA having a modified base (methylated uracil for natural uracil of RNA).
- nucleic acid sequences provided hereinare intended to encompass nucleic acids containing any combination of natural or modified RNA and/or DNA, including, but not limited to, such nucleic acids having modified nucleobases.
- Each of the references recited in the present applicationis incorporated herein by reference in its entirety.
- Example 1Preparation of N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)oxy)-6-(4-methylpiperidin-1-yl)-1,3,5-triazin-2-amine (BA-120) STEP 1: 2-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)-4,6-dichloro-1,3,5-triazine To a suspension of 2,4,6-trichloro-1,3,5-triazine (1.0 g, 5.42 mmol) and NaHCO 3 (911 mg, 10.84 mmol) in acetone (8 mL) at 0°C was added 17-azido-3,6,9,12,15- pentaoxaheptadecan-1-ol (1.67 g, 5.42 mmol
- STEP 2N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)- 6-chloro-1,3,5-triazin-2-amine
- 2-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)-4,6-dichloro- 1,3,5-triazine500 mg, 1.1 mmol
- THF9.5 mL
- DIEA0.29 mL, 1.65 mmol
- STEP 3N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)- 6-(4-methylpiperidin-1-yl)-1,3,5-triazin-2-amine
- N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)oxy)-6-chloro-1,3,5-triazin-2-amine50 mg, 0.088 mmol
- 4-methylpiperidine(16 mL, 0.13 mmol
- DIEA18 mL, 0.10 mmol
- reaction mixturewas stirred at room temperature for 16 h then quenched by addition of water (10 ml) and extracted with DCM (3x10 ml). The organic layer was washed with a saturated solution of NaHCO 3 (10 ml) and brine (10 ml). The combined organic extracts were dried over anhydrous Na 2 SO 4 and concentrated to obtain the title compound as a brown oil (0.11 g, quant.).
- reaction mixturewas stirred at room temperature under inert atmosphere for 2 hours and then diluted with saturated ammonium chloride solution (15 ml) and extracted with ethyl acetate (3 x 15 ml). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na 2 SO 4 , filtered, concentrated and purified by column chromatography using 0-15 % MeOH in DCM to provide the title product as a clear oil (0.12 mg, 55%).
- reaction mixturewas stirred at 0°C for 2 hours and then diluted with saturated aqueous sodium bicarbonate (10 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na 2 SO 4 , filtered and concentrated under reduced pressure to obtain the title compound as a brown oil (0.11 g, 83%).
- reaction mixturewas heated at 65°C for 2 hours and then diluted with water (15 ml) and extracted with ethyl acetate (3 x 15 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na 2 SO 4, filtered, concentrated and purified by silica gel column chromatography using a gradient 0-15% MeOH in DCM to provide the title compound as a clear oil (45 mg, 44%).
- reactionwas neutralized and quenched with glacial acetic acid and then extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine (3 x 100 mL), dried over anhydrous Na 2 SO 4 , filtered, concentrated and purified by reversed-phase flash chromatography (column, C 1 8 silica gel; mobile phase, acetonitrile in water, 10 % to 100 % gradient in 20 min; detector, UV 254 nm.) to afford the title compound (484 mg, 26 %) as a light yellow solid.
- the reaction mixturewas diluted with ethyl acetate (50 mL) and washed with water (2 x 100 mL). The organic layer was washed with sat. NaHCO 3 (50 mL) and brine (50 mL), dried over Na 2 SO 4 , filtered, concentrated and purified and by silica gel chromatography (0 to 100% ethyl acetate in hexane, followed by 0 to 40% methanol in ethyl acetate, 12g Claricep column) to afford the title compound (231 mg, 90%) as a clear oil.
- STEP 43-hydroxy-1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy)naphthalen-1-yl)butan-1-one
- a solution of 1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy) naphthalen-1-yl)ethan-1-one (6.5 g, 11 mmol) in THF (260 mL)was treated with LiHMDS (15.9 mL, 0.796 mmol) for 10 min at -40°C under nitrogen atmosphere followed by the addition of CH 3 CHO (4.2 mL, 44 mmol) in THF dropwise in portions at -40 °C.
- reaction mixturewas stirred at room temperature under inert atmosphere for 3 hours, then diluted with water (20 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na 2 SO 4 , concentrated and purified by silica gel column chromatography using a gradient 50-100% EtOAc in hexane to afford the title compound (0.20 g, 57%) as a yellow oil.
- reaction mixturewas concentrated under reduced pressure, treated with citric acid solution (10%) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (5 ml), dried over anhydrous Na 2 SO 4 and concentrated to afford the title compound (0.19 g, 97%) as a white solid.
- Example 7Preparation of (S)-2-(1-(4-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl) ureido) phenyl)acetyl)morpholine-3-carbonyl)piperidin-4- yl)acetic acid (BA-215)
- STEP 1methyl (S)-2-(1-(4-(2-(4-(3-(4-(((tert-butoxycarbonyl)amino)methyl)phenyl)ureido) phenyl)acetyl) morpholine-3-carbonyl)piperidin-4-yl)acetate
- 2-(4-(3-(4-(((tert- butoxycarbonyl)amino)methyl)phenyl)ureido)phenyl)acetic acid(0.15 g, 0.36
- reaction mixturewas stirred at room temperature under inert atmosphere for 3 hours and then diluted with water (20 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (15 ml), dried over anhydrous Na 2 SO 4 , filtered, concentrated and purified by silica gel column chromatography using a gradient 0- 20% MeOH in DCM to afford the title compound (0.11 g, 48%) as a yellow oil.
- Example 12Preparation of N1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-N3,N5- bis(2-hydroxyethyl)benzene-1,3,5-tricarboxamide (BA-144)
- STEP 1tert-butyl (2-((2-oxoazepan-3-yl)carbamoyl)phenyl)carbamate
- 2-((tert-butoxycarbonyl)amino)benzoic acid(2.01 g, 8.4 mmol) and 3-aminoazepan-2-one (1.19 g, 9.27 mmol, 1.1 equiv.) in DMF (10 mL) was added DIPEA (3.0 mL, 16.9 mmol, 2 equiv.) and HATU (4.8 g, 12.6 mmol, 1.5 equiv.).
- the reaction mixturewas stirred at 20 °C for 18h during which time an off-white precipitate formed.
- the mixturewas poured into water (200 mL) and sonicated with swirling for 5-10 min.
- the precipitatewas isolated by filtration, washed with water and air-drying to afford the title product (340 mg, 33.5%) as a gray powder.
- STEP 55-(1-azido-3,6,9,12-tetraoxapentadecan-15-amido)-N-(2-((2-oxoazepan-3- yl)carbamoyl)phenyl)benzo[b]thiophene-2-carboxamide
- To a mixture of 5-amino-N-(2-((2-oxoazepan-3- yl)carbamoyl)phenyl)benzo[b]thiophene-2-carboxamide (0.16 g, 0.4 mmol, 1.5 eq) in dry DMA (2 mL)was added DIPEA (0.13 mL, 0.77 mmol, 3 eq) and 2,5-dioxopyrrolidin-1-yl 1- azido-3,6,9,12-tetraoxapentadecan-15-oate (0.1 g, 0.26 mmol).
- Example 15Preparation of N-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-4-(7,8- dihydroxy-4-oxochroman-2-yl)benzamide (BA-167)
- a suspension of 2,5-dioxopyrrolidin-1-yl 4-(7,8-dihydroxy-4-oxochroman-2- yl)benzoate(665 mg, 1.684 mmol, 1 eq) and 2-(2-(2-(2-azidoethoxy)ethoxy)ethan-1- amine (440 mg, 2 mmol, 1.2 eq) and DIPEA (0.5 ml, 3.7 mmol, 2.2 eq) in THF (20 mL) was stirred for 3h at 60°C.
- Example 17Preparation of (Z)-1-(3-((3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6- yl)amino)-4-methylphenyl)-3-(3-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)-2-fluoro- 5-(trifluoromethyl)phenyl)urea (BA-173)
- STEP 1methyl 3-(benzyloxy)-2-fluoro-5-(trifluoromethyl)benzoate
- To a solution of 3-(benzyloxy)-2-fluoro-5-(trifluoromethyl)benzoic acid(4.9 g, 15.593 mmol, 1 eq) in methanol (49.00 mL, 1210.243 mmol, 77.61 eq) was added two drop of H 2 SO 4 (305.85 mg, 3.119 mmol, 0.2 eq) at room temperature under nitrogen atmosphere.
- reactionwas quenched by the addition of water (5 mL) and extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with brine (3 x 50 mL), dried over anhydrous Na 2 SO 4 , filtered, concentrated and purified by silica gel column chromatography, eluted with EA/PE ( ⁇ 30%) to afford the title compound (740 mg) as a yellow oil.
- the filtratewas concentrated and purified by reversed-phase flash chromatography: column, C 1 8 silica gel; mobile phase, ACN in water, 10% to 95% gradient in 30 min; detector, UV 254 nm, to afford the title compound (1 g, 70.9%) as a yellow oil.
- Example 18Preparation of 2-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one (BA-183)
- STEP 1methyl 4-(benzyl(methyl)amino)benzoate
- bromomethylbenzene (12.42 g, 72.64 mmol, 8.63 mL, 1.2 eq) and K 2 CO 3 (25.10 g, 181.61 mmol, 3 eq) in DMF (100 mL)was stirred at 80°C for 2 hours.
- reaction mixturewas stirred at 25 °C for 8 hr and then quenched by addition of water (30mL) and partitioned between ethyl acetate (50 mL) and brine (20mL). The aqueous phase was further extracted with ethyl acetate (2 ⁇ 40 mL) and the combined organic extracts were washed with brine (2 ⁇ 50 mL), dried over Na 2 SO 4 , filtered and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent of 0 ⁇ 30% Ethyl acetate/Petroleum ether gradient @ 50 mL/min) to afford the title compound (960 mg,69.0%) as a yellow oil.
- ISCO®20 g SepaFlash® Silica Flash Column, Eluent of 0 ⁇ 30% Ethyl acetate/Petroleum ether gradient @ 50 mL/min
- STEP 71-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oic acid
- a solution of tert-butyl 1-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oate (640 mg, 1.40 mmol, 1 eq) in HCl/dioxane (3 mL)was stirred at 25°C for 0.5 hr.
- the reaction mixturewas concentrated under reduced pressure to afford the title compound (600 mg, crude) as a yellow oil which was used for next step without further purification.
- STEP 122-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one 2-(17-chloro-3,6,9,12,15-pentaoxaheptadecyl)-8-(4-(methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one (57 mg, 0.099 mmol, 1 eq), and sodium azide (32 mg, 0.497 mmol, 5 eq) in dry DMF (1 mL) were heated at 70°C for 12 h.
- Example 19Preparation of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(8-hydroxy- 4-methoxyquinolin-2-yl)benzamide (BA-225) STEP 1: methyl 4-(8-hydroxy-4-methoxyquinolin-2-yl)benzoate To a solution of 1-(2-amino-3-hydroxyphenyl)ethan-1-one (2.15g, 14.2 mmol) in methanol (30 mL), was added methyl 4-formylbenzoate (7.0 g, 43 mmol) followed by the sulfuric acid (650 mL, 12 mmol) and the mixture heated at reflux for 48 hours.
- HATU(1.03g, 2.72 mmol) and N 3 -PEG 5 -NH 2 (686 mg, 2.24 mmol) were added at RT, followed by the addition of DIPEA (0.9 mL, 4.8 mmol) and stirred for 30 minutes.
- the reaction mixturewas diluted with water and extracted with DCM.
- the combined organic extractswere dried over Na 2 SO 4 , filtered, concentrated and purified by flash column chromatography (column: Biotage sfar silica HCD Duo 5, 20 micron, 50g) eluted with MeOH:DCM (0 to 20%, v/v, 10CV) to afford the title compound (770 mg, 83%) as a brown solid.
- Example 21Preparation of 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one (BA- 169) STEPS 1-4 STEP 1: 1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl 4-methylbenzenesulfonate To a solution of 2-[2-[2-[2-[2-[2-(2- benzyloxyethoxy)ethoxy]ethoxy]ethoxy]ethanol (5 g, 13.42 mmol, 1 eq) in DCM (100 mL) was added TEA (2.72 g, 26.85 mmol, 3.74 mL, 2 eq), 4-toluenesulfonyl chloride (2.82 g, 14.77 mmol, 1.1 eq) and D
- STEP 2ethyl 4-((1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate
- 1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl 4- methylbenzenesulfonate (3.25 g, 6.17 mmol, 1 eq) and ethyl 4-aminobenzoate (1.02 g, 6.17 mmol, 1.29 mL, 1 eq) in DMF (10 mL)was added K 2 CO 3 (2.56 g, 18.51 mmol, 3 eq) and KI (1.02 g, 6.17 mmol, 1 eq).
- STEP 3ethyl 4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate
- ethyl 4-((1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)benzoate(1 g, 1.92 mmol, 1 eq) in THF (10 mL) was added NaH (115.46 mg, 2.89 mmol, 60% purity, 1.5 eq) at 0°C under nitrogen atmosphere.
- STEP 71-(4-amino-2-(benzyloxy)-3-nitrophenyl)ethan-1-one
- a solution of 1-(2-(benzyloxy)-4-fluoro-3-nitrophenyl)ethan-1-one(11.5 g, 39.76 mmol, 1 eq) in acetonitrile (100 mL) was added NH 3 ⁇ H 2 O (83.60 g, 596.35 mmol, 91.87 mL, 25% purity, 15 eq) dropwise at 25°C.
- the mixturewas heated at 50°C for 2 hours and then concentrated to afford the title compound (8.5 g, crude) as a yellow oil which was used for next step without further purification.
- STEP 11N-(3-hydroxy-4-(3-(4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)phenyl)-3-oxopropanoyl)-2-nitrophenyl)acetamide
- 3-acetamido-6-acetyl-2-nitrophenyl4-(methyl(1-phenyl- 2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate (1 g, 1.38 mmol, 1 eq) in pyridine (15 mL) was added KOH (386.52 mg, 6.89 mmol, 5 eq) and the mixture was stirred at 60°C for 2 hr.
- STEP 127-amino-2-(4-((17-hydroxy-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)-8-nitro-4H-chromen-4-one
- N-(3-hydroxy-4-(3-(4-(methyl(1-phenyl-2,5,8,11,14,17- hexaoxanonadecan-19-yl)amino)phenyl)-3-oxopropanoyl)-2-nitrophenyl)acetamide(1.3 g, 1.79 mmol, 1 eq) in acetic acid (20 mL) was added H 2 SO 4 (175.68 mg, 1.79 mmol, 95.48 ⁇ L, 1 eq).
- STEP 137,8-diamino-2-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)-4H-chromen-4-one
- 7-amino-2-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)-8-nitro-4H-chromen-4-one(1 g, 1.74 mmol, 1 eq) in methanol (30 mL) was added Pd/C (10%, 0.5 g) under nitrogen atmosphere. The suspension was degassed and purged with hydrogen (x 3).
- STEP 158-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino) phenyl)chromeno[7,8-d]imidazol-6(3H)-one
- 8-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one450 mg, 809.91 ⁇ mol, 1 eq) in DCM (20 mL) was added SOCl 2 (8.10 mmol, 587.53 ⁇ L, 10 eq).
- Example 22Preparation of 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)-2-methylchromeno[7,8-d]imidazol-6(3H)- one (BA-170 and tautomer BA-201)
- STEP 18-(4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one
- 7,8-diamino-2-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl) amino)phenyl)-4H-chromen-4-one700 mg, 1.28 mmol, 1 eq) in acetonitrile
- STEP 38-(4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one 8-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one (310 mg, 0.527 mmol, 1 eq), and sodium azide (171 mg, 2.63 mmol, 5 eq) in dry DMF (2mL) was heated at 70°C for 12 hours.
- EXAMPLE 23N-(18-azido-3,6,9,12,15-pentaoxaoctadecyl)-4-(7,8-bis(allyloxy)-4-oxo- 4H-chromen-2-yl)benzamide (BA-203) To a solution of N-(18-azido-3,6,9,12,15-pentaoxaoctadecyl)-4-(7,8-dihydroxy-4- oxo-4H-chromen-2-yl)benzamide (BA-118, 200 mg, 0.34 mmol, 1eq) in DMF (5mL), was added K 2 CO 3 (94mg, 0.68 mmol, 2eq) followed by allyl bromide (35.2uL, 0.408 mmol, 1.2eq) at room temperature and the mixture was then heated to 80°C for 3 hours.
- one Ligandis conjugated to the 3' end of an oligonucleotide.
- Example 24General Procedure I Type A - Ligand Conjugated to 5’ end of Sense Strand STEP 1: 5'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V, 1M, pH7) and acetonitrile (20%-50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile was then added and the reaction monitored by LCMS and HPLC.
- Example 25General Procedure I Type B - Ligand Conjugated to 5’ end of Sense Strand STEP 1: 5'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) is added to an aqueous solution of 5’- (C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) is added and the reaction monitored by HPLC and LCMS. Upon completion, excess TCEP is removed by molecular weight cut-off with sodium phosphate buffer (100 mM, pH7, 3x).
- TCEPTris(2-carboxyethyl)phosphine hydrochloride
- Example 26General Procedure II Type A - Bis-homo-3',5'-Ligand Conjugated Sense Strand STEP 1: 3’,5’-bis-DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20%-50% V/V) were added to an aqueous solution of 3’,5’ amine functionalized sense strand. A solution of DBCO-NHS (3 eq) in DMSO or CH 3 CN was then added and the reaction monitored by LCMS and HPLC.
- Example 27General Procedure II Type B - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 3’,5’-bis-DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) is added to an aqueous solution of 5’, 3’-Bis (C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) is added and the reaction monitored by HPLC and LCMS. Upon completion, excess TCEP is removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x).
- TCEPTris(2-carboxyethyl)phosphine hydrochloride
- Example 28General Procedure II Type C - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 5'-DBCO / 3'-(C6-SS-C6)-mC Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20% -50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand.
- Tris(2-carboxyethyl)phosphine hydrochloride (TCEP)25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x).
- a solution of DBCO-MAL (3 eq) in DMSOwas added and the reaction was monitored by LCMS and HPLC. Upon completion, any solids were removed via centrifugation and the solution was purified by reverse phase HPLC, dried by lyophilization and the dried bis-DBCO modified sense strand reconstituted in RNase free water for step 3.
- STEP 3Bis-homo-5',3'- Ligand Conjugated Sense Strand
- a solution of 5’-, 3’-Bis-DBCO functionalized sense strand(1 eq) was added a solution of Ligand A-N 3 (3 eq) in DMSO or THF and the reaction was monitored by HPLC and LCMS.
- the bis-homo-5’-, 3’conjugated sense strandwas purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). The product was confirmed by HPLC and LCMS.
- Example 29General Procedure II Type D - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 5'-(C6-SS-C6)-mC / 3'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20% -50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile was then added and the reaction was monitored by LCMS and HPLC. Upon completion, any precipitate was removed via centrifugation and the aqueous solution purified by reverse phase HPLC.
- DBCO- NHS1.5-3 eq
- STEP 3Bis-homo-5',3'- Ligand Conjugated Sense Strand
- a solution of 5’-, 3’-Bis-DBCO functionalized sense strand(1 eq) was added a solution of Ligand A-N 3 (3 eq) in DMSO or THF and the reaction was monitored by HPLC and LCMS.
- the bis-homo-5’-, 3’conjugated sense strandwas purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). The product was confirmed by HPLC and LCMS.
- Example 30General Procedure III Type A - Bis-hetero-3',5'- Ligand Conjugated Sense Strand STEP 1: 5’-conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand A solution of Ligand A—N 3 (2 eq) in DMSO was added to an aqueous solution of 5’- DBCO modified sense strand (1 eq, see above for preparation) and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon ® Ultra-15 Centrifugal filter (3K, 5 times).
- STEP 2The 5’-conjugated, 3’-DBCO modified sense strand Sodium phosphate buffer (10% V/V 1M, pH7) was added to a solution of 5’- conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand (1 eq) in water. Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction monitored by HPLC and LCMS.
- Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7)was added and the reaction was monitored by HPLC and LCMS.
- TCEPTris(2- carboxyeth
- STEP 2The 5’-DBCO, 3’-conjugated sense strand Sodium phosphate buffer (10% V/V 1M, pH7) was added to a solution of 5’- conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand (1 eq) in water. Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction monitored by HPLC and LCMS.
- Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7)was added and the reaction was monitored by HPLC and LCMS.
- TCEPTris(2- carboxyethy
- Example 32General Procedure IV Type A - Ligand Conjugated to 3’ end of Sense Strand STEP 1: 3’- DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) was added to an aqueous solution of 3’-(C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction monitored by HPLC and LCMS.
- TCEPTris(2-carboxyethyl)phosphine hydrochloride
- Example 33General Procedure IV Type B - Ligand Conjugated to 3’ end of Sense Strand STEP 1: 3'- DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V, 1M, pH7) and acetonitrile (20%-50% V/V) are added to an aqueous solution of 3’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile is then added and the reaction monitored by LCMS and HPLC. Upon completion, any precipitate is removed via centrifugation, the aqueous solution purified by reverse phase HPLC, dried by lyophilization and the dried DBCO modified sense strand reconstituted in RNase free water.
- 3'- DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V, 1M, pH7) and acetonitrile (20%-50% V/V)are added to an aqueous solution of 3’-amine functionalized sense strand.
- Example 33-AGeneral Procedure V STEP 1: 5’-conjugated sense strand A solution of Ligand A—N3 (2 eq) in DMSO was added to an aqueous solution of 5’- DBCO modified sense strand (1 eq), and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand was purified by reverse phase HPLC and desalted with molecular weight cut-off with Amicon ® Ultra-15 Centrifugal filter (3K, 5 times). Compounds RD3171-RD3188 were prepared using General Procedure V.
- Example 34Bis-5'-, 3'- conjugated sense strand RD3937 5' BA-120 / 3' BA-120 FXXXXXXIV+IS1525+FXXXXXXV SEQ: RD3181 / 5'EP BA-120 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9418.9 (calc. 9420.6g/mol)
- Example 35Mono 5' conjugated sense strand RD3666 5' BA-120 FXXXXXXIV+IS1402 SEQ: RD3181 / 5'VP BA-120 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z 8342.7 (calc. 8344.2 g/mol)
- Example 36Mono 5' conjugated sense strand RD3913 5' BA-168 FXXXXXXVI+IS1402 SEQ: RD3181 / 5'EP BA-168 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z: 8620.5 (calc. 8622.2 g/mol)
- Example 37Mono 5' conjugated sense strand RD3995 5' BA-177 FXXXXXXVII+IS1402 SEQ: RD3181 / 5'EP BA-177 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 97% purity, confirmed by HPLC.
- Example 39Bis 5'-, 3'- conjugated sense strand RD6054 5' BA-236 / 3' BA-236 FXXXXXXVIII+IS2460+LK0181 5'VP BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity and confirmed by HPLC. LCMS: m/z: 9401.6 (calc.
- Example 40Bis 5'-, 3'- conjugated sense strand RD6039 5' BA-236 / 3' BA-236 FXXXXXXVIII+IS2458+FXXXXXXVIII BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 95% purity and confirmed by HPLC. LCMS: m/z: 9045.3 (calc.
- Example 42Bis 5'-, 3'- conjugated sense strand RD6049 5' BA-236 / 3' BA-236 FXXXXXXVIII+IS2459+FXXXXXXVIII BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 92% purity and confirmed by HPLC. LCMS: m/z: 9082.3 (calc. 9084.0g/mol)
- Example 43Mono 5' conjugated sense strand RD4518 5' BA-236 FXXXXXXVIII+IS1640 SEQ: RD3953 / 5'EP BA-120 was conjugated to an oligo sense strand according to general procedure type I type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z: 8641.4 (calc. 8639.7 g/mol)
- Example 44Mono 5' conjugated sense strand RD3941 5' BA-128 / 3' BA-128 FXXXXXIX+IS1525+FXXXXXXXIV SEQ: RD3181 / 5'EP BA-128 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 96% purity, confirmed by HPLC. LCMS: m/z: 9482.4 (calc.
- Example 45Bis-5'-, 3'- conjugated sense strand RD6037 5' BA-128 / 3' BA-128 FXXXXXXIX+IS2458+FXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 97% purity and confirmed by HPLC. LCMS: m/z: 9429.1 (calc.
- Example 46Bis-5'-, 3'- conjugated sense strand RD6047 5' BA-128 / 3' BA-128 FXXXXXXIX+IS2459+FXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9466.1 (calc.
- Example 47Bis-5'-, 3'- conjugated sense strand RD6042 5' BA-128 / 3' BA-128 FXXXXXXIX+IS1664+FXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 98% purity and confirmed by HPLC. LCMS: m/z: 9785.3 (calc. 9485.8 g/mol) AND
- Example 48Bis-5'-, 3'- conjugated sense strand RD6052 5' BA-128 / 3' BA-128 FXXXXXXIX+IS2460+FXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9785.3 (calc. 9787.0 g/mol)
- Example 49Mono 5' conjugated sense strand RD3667 5' BA-128 FXXXXXXIX+IS1402 SEQ: RD3181 / 5'VP BA-128 was conjugated to an oligo sense strand according to general procedure I type A. The product was ma prepared de with 97% purity, confirmed by HPLC. LCMS: m/z: 8374.4 (calc. 8375.9 g/mol)
- Example 50Mono 5' conjugated sense strand RD3971 5' BA-171 FXXXXXX+IS1402 SEQ: RD3181 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 98% purity, confirmed by HPLC.
- Example 52Bis 5'-, 3'-conjugated sense strand RD4235 5' BA-171 /3' BA-171 FXXXXXX+IS1525+FXXXXXXXX SEQ: RD3181 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 89% purity, confirmed by HPLC. LCMS: m/z: 9664.9 (calc. 9666.9 g/mol)
- Example 53Bis 5'-, 3'- conjugated sense strand RD4420 5' BA-171 /3' BA-171 FXXXXXXX+IS1641+FXXXXXXXX SEQ: RD3953 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure II type B. The product was prepared with 98% purity, confirmed by HPLC. LCMS: m/z: 9794.2 (calc. 9795.9 g/mol)
- Example 54Mono 5' conjugated sense strand RD4336 5' BA-210 FXXXXXXXI+IS1402 SEQ: RD3181 / 5'EP BA-210 was conjugated to an oligo sense strand according to general procedure I type A. The prepared was made with 96% purity, confirmed by HPLC. LCMS: m/z: 8503.5 (calc. 8505.3 g/mol)
- Example 56Bis 5'-, 3'- conjugated sense strand RD6027 5' BA-135 /3' BA-135 FXXXXXV+IS2006+FXXXXXV SEQ: RD3181 / 5'VP BA-135 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9249.5, (calc.
- Example 57Bis 5'-, 3'- conjugated sense strand RD6028 5' BA-136 /3' BA-136 FXXXXXVI+IS2006+FXXXXXVI SEQ: RD3181 / 5'VP BA-136 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 97% purity, confirmed by HPLC. LCMS: m/z 9075.2, (calc.
- Example 58Bis 5'-, 3'- conjugated sense strand RD6029 5' BA-137 /3' BA-137 FXXXXXVII+IS2006+FXXXXXVII SEQ: RD3181 / 5'VP BA-137 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z 9169.2, (calc. 9171.0 g/mol)
- Example 59Bis 5'-, 3'- conjugated sense strand RD6030 5' BA-144 /3' BA-144 FXXXXXIX+IS2006+FXXXXXIX SEQ: RD3181 / 5'VP BA-144 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9567.7, (calc. 9569.5 g/mol)
- Example 60Bis 5'-, 3'- conjugated sense strand RD6031 5' BA-173 /3' BA-173 FXXIV+IS2006+FXXIV SEQ: RD3181 / 5'VP BA-173 was conjugated to an oligo sense strand according to general procedure II type A. The product was made with 81% purity, confirmed by HPLC. LCMS: m/z 9681.5, (calc. 9683.4 g/mol)
- Example 61Bis 5'-, 3'- conjugated sense strand RD6032 5' BA-183 /3' BA-183 FXXXXXXI+IS2006+FXXXXXXI SEQ: RD3181 / 5'VP BA-183 was conjugated to an oligo sense strand according to general procedure II type A. The product was made with 86% purity, confirmed by HPLC. LCMS: m/z9337.4, (calc.
- Example 62Bis 5'-, 3'- conjugated sense strand RD6034 5' BA-216 /3' BA-216 FXXXXXXII+IS2006+FXXXXXXII SEQ: RD3181 / 5'VP BA-216 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9373.3, (calc.
- Example 63Bis 5'-, 3'- conjugated sense strand RD6036 5' BA-198 /3' BA-198 FXVI+IS2458+FXVI SEQ: RD3175 / 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 98% purity, confirmed by HPLC. LCMS: m/z 9329.4, (calc.
- Example 64Bis 5'-, 3'- conjugated sense strand RD6041 5' BA-198 /3' BA-198 FXVI+ IS1664+FXVI SEQ: RD3176/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9384.4, (calc.
- Example 65Bis 5'-, 3'- conjugated sense strand RD6046 5' BA-198 /3' BA-198 FXVI+ IS2459+FXVI SEQ: RD3186/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity, confirmed by HPLC. LCMS: m/z 9366.4, (calc.
- Example 66Bis 5'-, 3'- conjugated sense strand RD6051 5' BA-198 /3' BA-198 FXVI+ IS2460+FXVI SEQ: RD3959/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9685.6, (calc. 9687.3 g/mol)
- Example 67Bis 5'-, 3'- conjugated sense strand RD6035 5' BA-225 /3' BA-225 FXXXIII+IS2006+FXXXIII BA-225 was conjugated to an oligo sense strand according to general procedure II type A.
- Example 69Mono 5'- conjugated sense strand RD4310 5' BA-197 FXV+IS1402 BA-197 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z 8310.4, (calc. 8312.0 g/mol)
- Example 70Mono 3'- conjugated sense strand RD4157 3' BA-118 IS1599+FIV BA-118 was conjugated to an oligo sense strand according to general procedure IV type A. The product was prepared with 86% purity, confirmed by HPLC. LCMS: m/z 8652.1, (calc. 8653.4 g/mol)
- Example 71Mono 3'- conjugated sense strand RD4269 3' BA-129 IS1599+FXXXXXXIII BA-129 was conjugated to an oligo sense strand according to general procedure IV type A. The product was prepared with 97% purity, confirmed by HPLC. LCMS: m/z 8582.1, (calc. 8583.8 g/mol)
- Example 72Bis 5'-, 3'- conjugated sense strand RD5470 5' BA-198 /3' BA-198 FXVI+IS2178+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 95% purity, confirmed by HPLC.
- Example 74Bis 5'-, 3'- conjugated sense strand RD5947 5' BA-198 /3' BA-198 FXVI+IS1681+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9215.3, (calc.
- Example 75Bis 5'-, 3'- conjugated sense strand RD5948 5' BA-198 /3' BA-198 FXVI+IS1681+FXVI SEQ: RD3953/ 5'EP IA1299 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9215.3, (calc.
- Example 76Bis 5'-, 3'- conjugated sense strand RD5954 5' BA-198 /3' BA-198 FXVI+IS1688+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9414.7, (calc.
- Example 77Bis 5'-, 3'- conjugated sense strand RD5955 5' BA-198 /3' BA-198 FXVI+IS1688+FXVI SEQ: RD3953/ 5'EP IA1299 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9414.7, (calc.
- Example 78Bis 5'-, 3'- conjugated sense strand RD5956 5' BA-198 /3' BA-198 FXVI+IS2422+FXVI SEQ: RD3953/ 5'VP IA1250 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9774.1, (calc.
- Example 79Bis 5'-, 3'- conjugated sense strand RD5967 5' BA-198 /3' BA-198 FXVI+IS2424+FXVI SEQ: RD3953/ 5'VP IA1250 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9774.1, (calc. 9775.7 g/mol)
- Example 80Synthesis of ligand-conjugated oligonucleotides using amide linkers Oligonucleotides conjugated to ligands using amide linkers may be synthesized using the following methods: The synthetic methods above may be used to synthesize, for example, the following oligonucleotides comprising ligands at the 5' and/or 3' ends:
- Example 81General Procedures for Synthesis of Oligonucleotides Strands were synthesized on solid phase using an oligonucleotide synthesizer Oligopilot100 (Cytiva Life Sciences). Solid support (CPG, 80-90 ⁇ mol/g, 500A, from LGC- Biosearch Technologies, Petaluma, CA) was loaded to 150-300 ⁇ mol scales. RNA and 2' modified RNA phosphoramidites were purchased from Hongene Biotech (Union City, CA).
- 2'-O-methyl phosphoramidites usedwere: 5'-O-(4,4'-Dimethoxytrityl)-N 6 -benzoyl-2'-O-methyl-adenosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N 4 -acetyl-2'-O-methyl-cytidine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N 2 -isobutyryl-2'-O-methyl-guanosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl
- 2'-Fluoro phosphoramidites usedwere: 5'-O-(4,4'-Dimethoxytrityl)-N 6 -benzoyl-2'-fluoroadenosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N 4 -acetyl-2'-fluorocytidine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N 2 -isobutyryl-2'-fluoroguanosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-2'-
- Coupling timeswere 6 minutes carried out at 3.0 equivalents for each step.
- the support bound oligonucleotidePrior to coupling the support bound oligonucleotide is treated with a solution of Dichloroacetic Acid in Dichloromethane (3% Deblock, Sigma Aldrich) and washed with Anhydrous Acetonitrile. Cleavage and deprotection of support bound oligomer After completion of the solid phase synthesis, the support was treated with AMA solution, a 1:1 volume solution of NH 4 OH:CH 3 NH 2 (Fisher Scientific, Spectrum Chemicals), for 20 minutes at 65°C. The solution was then evaporated. Prior to purification, in-process analysis is performed on analytical HPLC and LCMS to determine crude purity, identify the target mass and monitor the deprotection for completion.
- Buffer solution mixturesare 100 mM TEAA, 5% ACN at pH of 7.0 (buffer A) and 1:1 acetonitrile:methanol (buffer B). Gradient was set at 5-30% or 30-60% Buffer B over 5 minutes at 70°C with a flowrate of 1.0 mL/minute. The minimum spec of the purified pool is 85%. Desalting Once a pool has been established, the oligos are desalted using Pall Minimate EVO System (Product ID: OAPMPUNV). Cassette used is the Pall Minimate TFF capsule with 3k Omega membrane (Product ID: OA003C12). Retentate is collected for lyophilization or annealing directly.
- RNA antisense strandswere prepared according to procedures well known to those of skill in the art. The concentrations of both sense strand and antisense strands were determined by Nanodrop. The double-stranded siRNA was prepared by mixing equimolar amounts of sense stand and antisense strand. The annealing process was monitored by RP-HPLC, non- denaturing method. After annealing, no more that 5% of antisense strand was in the duplex mixture. Duplex concentration was determined by measuring the solution absorbance on Nanodrop.
- Example 83General procedure for preparation of Carbon chain containing dinucleotide amidites STEP 1: N,N-Diisopropylethylamine (DIPEA, 3 eq) is added to a stirred solution of optionally base protected 3-(5-["A/C/G/U”]-5-(DMTrO-methyl)-2-[F/OMe”]- tetrahydrofuran-3-yl)acetic acid (1 eq), optionally base protected 1-["A/C/G/U”]-2-[F/OMe”]- 5-((heptadecylamino)methyl) tetrahydrofuran-3-ol (1.15 eq) and HATU (1.5 eq) in DMF.
- DIPEAN,N-Diisopropylethylamine
- RNA Isolationwas performed according to the RNeasy Micro Kit (Qiagen Cat #74004) instructions. Following RNA isolation, a 96-well plate was placed on ice while the qRT-PCR reaction was prepared.
- RNA2 ⁇ l was added to the reaction mixture containing 5 ⁇ l TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher #44444432), 1 ⁇ l human MAPT TaqMan Gene Expression Assay (Thermo Fisher: Hs00213484_m1, FAM), 1 ⁇ l mouse GAPDH TaqMan Gene Expression Assay (Thermo Fisher: Mm99999915_g1, VIC) and 11 ⁇ l RT-PCR grade nuclease-free water in a MicroAmp Optical 96-well plate (0.2 mL).
- RNA Isolationwas performed according to the RNeasy Micro Kit (Qiagen Cat #74004) instructions. Following RNA isolation, a 96-well plate was placed on ice while the qRT-PCR reaction was prepared.
- RNA2 ⁇ l was added to the reaction mixture containing 5 ⁇ l TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher #44444432), 1 ⁇ l cyno MAPT TaqMan Gene Expression Assay (Thermo Fisher: Mf00902189_m1, FAM), 1 ⁇ l cyno ARL1 TaqMan Gene Expression Assay (Thermo Fisher: Mf02795431_m1, VIC) and 11 ⁇ l RT- PCR grade nuclease-free water in a MicroAmp Optical 96-well plate (0.2 mL).
- qPCRwas performed using a QuantStudio3 qPCR machine with the following cycles: 50°C for 1 minute, 95°C for 20 seconds, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. Results are presented in Table 5 below as percent inhibition of MAPT RNA, relative to control (pre-dosed level from the same animal). Due to intrathecal injection variation, certain animals may have received a lower dose and therefore may show lower inhibition as a result. The amount of test compound present in CSF was measured at 1h, 6h, and 24h post dose. Table 6 shows the CSF compound concentration ( ⁇ g/mL) for the two animals dosed with RD4845. Table 5 Percent MAPT RNA Inhibition in Brain and Spinal Cord
- any particular embodiment of this disclosure that falls within the prior artmay be explicitly excluded from any one or more of the claims. Because such embodiments are deemed to be known to one of ordinary skill in the art, they may be excluded even if the exclusion is not set forth explicitly herein. Any particular embodiment of the disclosure can be excluded from any claim, for any reason, whether or not related to the existence of prior art.
- the citation of any publicationis for its disclosure prior to the filing date and should not be construed as an admission that the present disclosure is not entitled to antedate such publication by virtue of prior disclosure. Further, the dates of publication provided could be different from the actual publication dates that may need to be independently confirmed.
- a pronoun in a gendere.g., masculine, feminine, neuter, other, etc.
- the pronounshall be construed as gender neutral (e.g., construed to refer to all genders equally) regardless of the implied gender unless the context clearly indicates or requires otherwise.
- words used in the singularinclude the plural, and words used in the plural includes the singular, unless the context clearly indicates or requires otherwise. Claims or descriptions that include “or” between one or more members of a group are considered satisfied if one, more than one, or all of the group members are present in, employed in, or otherwise relevant to a given product or process unless indicated to the contrary or otherwise evident from the context.
- the disclosureincludes embodiments in which exactly one member of the group is present in, employed in, or otherwise relevant to a given product or process.
- the disclosureincludes embodiments in which more than one, or all of the group members are present in, employed in, or otherwise relevant to a given product or process.
- the disclosureencompasses all variations, combinations, and permutations in which one or more limitations, elements, clauses, and descriptive terms from one or more of the listed claims is introduced into another claim.
- any claim that is dependent on another claimcan be modified to include one or more limitations found in any other claim that is dependent on the same base claim.
- elementsare presented as lists (e.g., in Markush group format), each subgroup of the elements is also disclosed, and any element(s) can be removed from the group.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Public Health (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Steroid Compounds (AREA)
Abstract
Description
MAPT-MODULATING COMPOSITIONS AND METHODS OF USE THEREOF CROSS-REFERENCE TO RELATED APPLICATIONS This application claims the benefit of priority under 35 U.S.C. § 119(e) of U.S. Provisional Application No. 63/460,874, filed April 20, 2023; U.S. Provisional Application No. 63/520,070, filed August 16, 2023; and U.S. Provisional Application No. 63/619,022, filed January 9, 2024. The disclosure of each of the prior applications is considered part of and is incorporated by reference in its entirety in the disclosure of this application. REFERENCE TO AN ELECTRONIC SEQUENCE LISTING The contents of the electronic sequence listing (A127870019WO00-SEQ-JIB.xml; Size: 210,445 bytes; and Date of Creation: April 18, 2024) is herein incorporated by reference in its entirety. SUMMARY The present disclosure provides compounds, compositions, and methods for modulating the expression or activity of microtubule associated protein tau (MAPT). In certain embodiments, the compounds, compositions, and methods can be used to reduce the expression of MAPT mRNA in a cell or animal. In certain embodiments, the compounds, compositions, and methods can be used to reduce the amount of MAPT protein in a cell or animal. In certain embodiments, the animal has a CNS related disease, disorder or condition. In certain embodiments, the disease, disorder or condition is a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome. Certain compounds, compositions and methods provided herein are directed to reducing a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal. In certain embodiments, the compounds and compositions provided herein are potent and tolerable and inhibit MAPT expression, which can be used to treat, prevent, ameliorate, or slow progression of a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compounds and compositions comprise one or more features that are effective for increasing potency. In certain embodiments, the compounds and compositions comprise one or more features that are effective for increasing tolerability. In certain embodiments, compounds and compositions comprise one or more features that are effective for targeting the compound or composition to a cell or tissue. In certain embodiments, the compounds and compositions are more potent, have greater duration of action or have greater therapeutic value than compounds publicly disclosed. BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 shows exemplary compounds of the present disclosure comprising one or more ligands. FIG. 2 shows exemplary compounds of the present disclosure comprising one or more Tropomyosin Receptor Kinase B (TrkB) ligands. FIG. 3 shows exemplary compounds of the present disclosure comprising one or more cannabinoid receptor type 1 (CB1) ligands. FIG. 4 shows exemplary compounds of the present disclosure comprising one or more α4β1/7 integrin ligands. DETAILED DESCRIPTION It is to be understood that both the foregoing summary and the following detailed description are exemplary and explanatory only and are not restrictive of the embodiments, as claimed. The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. All documents, or portions of documents, cited in this application, including, but not limited to, patents, patent applications, articles, books, treatises, and GenBank, NCBI and other sequence reference records are hereby expressly incorporated by reference for the portions of the document discussed herein, as well as in their entirety as of the date of filing this application. It is understood that the sequence set forth in each SEQ ID NO contained herein is independent of any modification to a sugar moiety, an internucleoside linkage, or a nucleobase even if shown in context with a modified compound. As such, compounds defined by a SEQ ID NO may comprise, independently, one or more modifications to a sugar moiety, an internucleoside linkage, or a nucleobase. Oligomeric compounds referenced by Compound Number or Ref ID NO indicate a combination of nucleobase sequence, chemical modification, and motif. Herein, the use of the singular includes the plural unless specifically stated otherwise. For example, the articles “a” and “an” are used herein to refer to one or to more than one (i.e., to at least one) of the grammatical object of the article. By way of example, “an element” means one element or more than one element, e.g., a plurality of elements. As used herein, the use of “or” means “and/or” unless stated otherwise. Furthermore, the use of the term “including” as well as other forms, such as “includes” and “included”, is not limiting and is used interchangeably with, the phrase "including but not limited to". Definitions Unless otherwise indicated, the following terms have the following meanings: “Microtubule Associated Protein Tau,” used interchangeably with the term “MAPT,” refers to any nucleic acid or protein of MAPT. Exemplary nucleotide and amino acid sequences of MAPT can be found, for example, at GenBank Accession No. NM_001377265.1 (incorporated herein as SEQ ID NO: 1), and nucleotides 2624000 to 2761000 of NT_010783.14 (incorporated herein as SEQ ID NO: 2). Additional examples of MAPT sequences are readily available through publicly available databases, e.g., GenBank, UniProt, and OMIM. Further information on MAPT can be found, for example, at www.ncbi.nlm.nih.gov/gene/?term=MAPT. MAPT, as used herein, also refers to variations of the MAPT gene including variants provided in the SNP database. Numerous sequence variations within the MAPT gene have been identified and may be found at, for example, NCBI dbSNP and UniProt (see, e.g., www.ncbi.nlm.nih.gov/snp/?term=MAPT). “MAPT mRNA” means an mRNA encoding a MAPT protein. MAPT may be referred to in either upper or lower case. “MAPT specific inhibitor” refers to any agent capable of specifically inhibiting MAPT RNA and/or MAPT protein expression or activity at the molecular level. For example, MAPT specific inhibitors include nucleic acids (including oligonucleotide compounds), peptides, antibodies, small molecules, and other agents capable of inhibiting the expression of MAPT RNA and/or MAPT protein. “2’-O-methoxyethyl” or “2’-MOE” means a 2’-O(CH2)2-OCH3 modification. A 2’-O- methoxyethyl modified sugar is a modified sugar with 2’-O(CH2)2-OCH3 in the place of the 2’-OH group of a ribosyl ring. “5’ start site” means the nucleotide of the target nucleic acid or region which is aligned to the 3’-most nucleoside of an antisense oligonucleotide. “3’ stop site” means the nucleotide of the target nucleic acid or region which is aligned to the 5’-most nucleoside of an antisense oligonucleotide. “About” means within ±10% of a value. For example, if it is stated, “a compound achieved about 70% inhibition of MAPT”, it is implied that MAPT levels are inhibited within a range of 60% and 80%. When about is present before a series of numbers or a range, it is understood that “about” can modify each of the numbers in the series or range. “Administer” or “administering” refers to routes of introducing a compound or composition provided herein to an individual to perform its intended function. An example, routes of administration that can be used include, but are not limited to, intrathecal (IT) administration, intracerebroventricular (ICV) administration, parenteral administration, such as subcutaneous administration, intravenous administration, intramuscular administration, intraarterial administration, intraperitoneal administration, or intracranial administration, e.g. intrathecal or intracerebroventricular administration. “Ameliorate” refers to an improvement or lessening of at least one indicator, sign, or symptom of an associated disease, disorder, or condition. In certain embodiments, amelioration includes a delay or slowing in the progression or severity of one or more indicators of a condition or disease. The progression or severity of indicators may be determined by subjective or objective measures, which are known to those skilled in the art. “Animal” refers to a human or non-human animal, including, but not limited to, mice, rats, rabbits, dogs, cats, pigs, and non-human primates, including, but not limited to, monkeys and chimpanzees. “Antisense oligonucleotide” or “antisense strand” means an oligonucleotide which includes a region that is complementary to a target nucleic acid, e.g., a MAPT RNA or a region thereof. “Complementarity” in reference to an oligonucleotide means the nucleobase sequence of such oligonucleotide or one or more regions thereof that is complementary to the nucleobase sequence of another oligonucleotide or nucleic acid or one or more regions thereof when the two nucleobase sequences are aligned in opposing directions. Complementary nucleobases, as described herein, are limited to the following pairs: adenine (A) and thymine (Τ), adenine (A) and uracil (U), and cytosine (C) and guanine (G) unless otherwise specified. Complementary oligonucleotides and/or nucleic acids need not have nucleobase complementarity at each nucleoside and may include one or more nucleobase mismatches. By contrast, “fully complementary” or “100% complementary” in reference to oligonucleotides means that such oligonucleotides have nucleobase matches at each nucleoside without any nucleobase mismatches. “Composition” or “pharmaceutical composition” means a mixture of substances suitable for administering to an individual. For example, a composition may comprise one or more compounds or salt thereof and a sterile aqueous solution. “Co-administration” means administration of two or more compounds in any manner in which the pharmacological effects of both are manifest in the patient. Co-administration does not require both compounds to be administered in a single pharmaceutical composition, in the same dosage form, by the same route of administration, or at the same time. The effects of both compounds need not manifest themselves at the same time. The effects need only be overlapping for a period of time and need not be coextensive. Co-administration includes parallel or sequential administration of the one or more compounds. “Conjugate group” means a group of atoms that is attached to an oligonucleotide. A conjugate group is optionally attached to an oligonucleotide through a conjugate linker. A conjugate group may, for example, alter the distribution, targeting, or half-life of a compound into which it is incorporated. Conjugate groups include lipids (or lipophilic moieties), ligands, and other targeting moieties. “Conjugate linker” means a group of atoms comprising at least one bond that connects a linked moiety to an oligonucleotide. “Identity” in reference to an oligonucleotide means the nucleobase sequence of such oligonucleotide or one or more regions thereof that matches the nucleobase sequence of another oligonucleotide or nucleic acid or one or more regions thereof. Identity of an oligonucleotide to another oligonucleotide or nucleic acid need not require each nucleobase to match and may include one or more different nucleobases. By contrast, “fully identical” or “100% identity” in reference to oligonucleotides means that such oligonucleotides have the same nucleobase at each relative position over its length as the other oligonucleotide or nucleic acid. “Individual” means a human or non-human animal selected for treatment or therapy. “Inhibiting the expression or activity” with reference to a target nucleic acid or protein means to reduce or block the expression or activity of such target relative to the expression or activity in an untreated or control sample and does not necessarily indicate a total elimination of expression or activity. As used herein, the term “internucleoside linkage” is the covalent linkage between adjacent nucleosides in an oligonucleotide. As used herein, “modified internucleoside linkage” means any internucleoside linkage other than a phosphodiester internucleoside linkage. “Phosphorothioate internucleoside linkage” is a modified internucleoside linkage in which one of the non-bridging oxygen atoms of a phosphodiester internucleoside linkage is replaced with a sulfur atom. Representative internucleoside linkages having a chiral center include but are not limited to alkylphosphonates and phosphorothioates. Modified oligonucleotides comprising internucleoside linkages having a chiral center can be prepared as populations of modified oligonucleotides comprising stereorandom internucleoside linkages, or as populations of modified oligonucleotides comprising phosphorothioate linkages in particular stereochemical configurations as further described below. Unless otherwise indicated, chiral internucleoside linkages of modified oligonucleotides described herein can be stereorandom or in a particular stereochemical configuration. The compounds of the present disclosure may also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds. For example, the compounds may be radiolabeled with radioactive isotopes, such as for example tritium (3H), iodine-125 (125I), or carbon-14 (14C). All isotopic variations of the compounds of the present disclosure, whether radioactive or not, are encompassed within the scope of the present disclosure. The term “isotopic variant” refers to a therapeutic agent (e.g., a compound and/or modified oligonucleotide disclosed herein) that contains an unnatural proportion of an isotope at one or more of the atoms that constitute such a therapeutic agent. In certain embodiments, an “isotopic variant” of a therapeutic agent contains unnatural proportions of one or more isotopes, including, but not limited to, hydrogen (H), deuterium (2H), tritium (3H), carbon-11 (11C), carbon-12 (12C), carbon-13 (13C), carbon-14 (14C), nitrogen-13 (13N), nitrogen-14 (14N), nitrogen-15 (15N), oxygen-14 (14O), oxygen-15 (15O), oxygen-16 (16O), oxygen-17 (17O), oxygen-18 (18O), fluorine-17 (17F), fluorine-18 (18F), phosphorus-31 (31P), phosphorus-32 (32P), phosphorus-33 (33P), sulfur-32 (32S), sulfur-33 (33S), sulfur-34 (34S), sulfur-35 (35S), sulfur-36 (36S), chlorine-35 (35Cl), chlorine-36 (36Cl), chlorine-37 (37Cl), bromine-79 (79Br), bromine-81 (81Br), iodine 123 (123I), iodine-125 (125I), iodine-127 (127I), iodine-129 (129I), and iodine-131 (131I). In certain embodiments, an “isotopic variant” of a therapeutic agent contains unnatural proportions of one or more isotopes, including, but not limited to, hydrogen (H), deuterium (2H), tritium (3H), carbon-11 (11C), carbon-12 (12C), carbon-13 (13C), carbon-14 (14C), nitrogen-13 (13N), nitrogen-14 (14N), nitrogen-15 (15N), oxygen-14 (14O), oxygen-15 (15O), oxygen-16 (16O), oxygen-17 (17O), oxygen-18 (18O), fluorine-17 (17F), fluorine-18 (18F), phosphorus-31 (31P), phosphorus-32 (32P), phosphorus-33 (33P), sulfur-32 (32S), sulfur-33 (33S), sulfur-34 (34S), sulfur-35 (35S), sulfur-36 (36S), chlorine-35 (35Cl), chlorine-36 (36Cl), chlorine-37 (37Cl), bromine-79 (79Br), bromine-81 (81Br), iodine 123 (123I), iodine-125 (125I), iodine-127 (127I), iodine-129 (129I), and iodine-131 (131I). It will be understood that, in a therapeutic agent (e.g., a compound and/or modified oligonucleotide disclosed herein), any hydrogen can be2H, for example, or any carbon can be13C, for example, or any nitrogen can be15N, for example, or any oxygen can be18O, for example, where feasible according to the judgment of one of skill. In certain embodiments, an “isotopic variant” of a therapeutic agent contains unnatural proportions of deuterium (D). “Lipid” or “lipophilic moiety” refers to an aliphatic, cyclic (such as alicyclic), or polycyclic (such as polyalicyclic) compound, such as a steroid (e.g., sterol) or a linear or branched aliphatic hydrocarbon. The term lipid includes cholesterol, retinoic acid, cholic acid, adamantane acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-bis- O(hexadecyl)glycerol, geranyloxyhexyanol, hexadecylglycerol, borneol, menthol, 1,3- propanediol, heptadecyl group, palmitic acid, myristic acid, O3-(oleoyl)lithocholic acid, O3- (oleoyl)cholenic acid, ibuprofen, naproxen, dimethoxytrityl, or phenoxazine. The term lipid includes a saturated or unsaturated C4-C30 hydrocarbon chain (e.g., C4-C30 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C5-C20 hydrocarbon chain (e.g., a linear C5-C20 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C14-C20 hydrocarbon chain (e.g., a linear C14-C20 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C6-C18 hydrocarbon chain (e.g., a linear C6-C18 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C16 hydrocarbon chain (e.g., a linear C16 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C17 hydrocarbon chain (e.g., a linear C17 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C18 hydrocarbon chain (e.g., a linear C18 alkyl or alkenyl). In certain embodiments, the lipophilic moiety contains a saturated or unsaturated C22 hydrocarbon chain (e.g., a linear C22 alkyl or alkenyl). “Mismatch” or “non-complementary” means a nucleobase of a first oligonucleotide or nucleic acid that is not complementary to the corresponding nucleobase of a second oligonucleotide or nucleic acid when the first oligonucleotide/nucleic acid and second oligonucleotide/nucleic acid are aligned in an antiparallel orientation. For example, nucleobases including, but not limited to, a universal nucleobase, inosine, and hypoxanthine, are capable of hybridizing with at least one nucleobase but are still mismatched or non- complementary with respect to the nucleobase to which they are hybridized. As another example, a nucleobase of a first oligonucleotide/nucleic acid that is not capable of hybridizing to the corresponding nucleobase of a second oligonucleotide/nucleic acid when the first and second oligonucleotides are aligned in an antiparallel orientation is a mismatch or non-complementary nucleobase. “Modified oligonucleotide” means an oligonucleotide, wherein at least one sugar, nucleobase, or internucleoside linkage is modified. “Modulating” refers to changing or adjusting a feature in a cell, tissue, organ or organism. For example, modulating MAPT RNA can mean to increase or decrease the level of MAPT RNA and/or MAPT protein in a cell, tissue, organ or organism. A “modulator” effects the change in the cell, tissue, organ or organism. For example, a MAPT compound can be a modulator that decreases the amount of MAPT RNA and/or MAPT protein in a cell, tissue, organ or organism. “Motif” means the pattern of unmodified and modified sugar moieties, nucleobases, and/or internucleoside linkages, in an oligonucleotide. “Nucleic acid” refers to molecules composed of monomeric nucleotides. A nucleic acid includes, but is not limited to, ribonucleic acids (RNA), deoxyribonucleic acids (DNA), single-stranded nucleic acids, and double-stranded nucleic acids. “Nucleobase” means a heterocyclic moiety capable of pairing with a base of another nucleic acid. As used herein a “naturally occurring nucleobase” is adenine (A), thymine (Τ), cytosine (C), uracil (U), and guanine (G). A “modified nucleobase” is a naturally occurring nucleobase that is chemically modified. A “universal base” or “universal nucleobase” is a nucleobase other than a naturally occurring nucleobase and modified nucleobase and is capable of pairing with any nucleobase. “Nucleobase sequence” means the order of contiguous nucleobases in a nucleic acid or oligonucleotide independent of any sugar or internucleoside linkage. “Nucleoside” means a compound comprising a nucleobase and a sugar moiety. The nucleobase and sugar moiety are each, independently, unmodified or modified. “Modified nucleoside” means a nucleoside comprising a modified nucleobase and/or a modified sugar moiety. Modified nucleosides include abasic nucleosides, which lack a nucleobase. “Oligomeric Compound” means a compound comprising one or more oligonucleotides and optionally one or more additional features, such as a conjugate group or terminal group. Examples of oligomeric compounds include single-stranded and double- stranded compounds, such as, oligonucleotides, antisense oligonucleotides, interfering RNA compounds (RNAi compounds), microRNA targeting oligonucleotides, occupancy-based compounds (e.g., mRNA processing or translation blocking compounds and splicing compounds). RNAi compounds include double-stranded compounds (e.g., short-interfering RNA (siRNA) and double-stranded RNA (dsRNA)) and single-stranded compounds (e.g., single-stranded siRNA (ssRNA), single-stranded RNAi (ssRNAi), short hairpin RNA (shRNA) and microRNA mimics) which work at least in part through the RNA-induced silencing complex (RISC) pathway resulting in sequence specific degradation and/or sequestration of a target nucleic acid through a process known as RNA interference (RNAi). The term “RNAi compound” is meant to be equivalent to other terms used to describe nucleic acid compounds that are capable of mediating sequence-specific RNA interference, for example, interfering RNA (iRNA), iRNA agent, RNAi agent, short interfering oligonucleotide, short interfering nucleic acid, short interfering modified oligonucleotide, chemically modified siRNA, and others. Additionally, the term “RNAi” is meant to be equivalent to other terms used to describe sequence-specific RNA interference. “Oligomeric duplex” means a duplex formed by two oligomeric compounds having complementary nucleobase sequences. Each oligomeric compound of an oligomeric duplex may be referred to as a “duplexed oligomeric compound.” The oligonucleotides of each oligomeric compound of an oligomeric duplex may include non-complementary overhanging nucleosides. In some embodiments, the terms “duplexed oligomeric compound” and “modified oligonucleotide” are used interchangeably. In other embodiments, the terms “oligomeric duplex” and “compound” are used interchangeably. “Oligonucleotide” means a polymer of linked nucleosides, each of which can be modified or unmodified, independent from one another. “Parenteral administration” means administration through injection or infusion. Parenteral administration includes subcutaneous administration, intravenous administration, intramuscular administration, intraarterial administration, intraperitoneal administration, or intracranial administration, e.g. intrathecal or intracerebroventricular administration. “Pharmaceutically acceptable carrier or diluent” means any substance suitable for use in administering to an individual. In certain embodiments, a pharmaceutically acceptable carrier or diluent aids the administration of a compound to and absorption by an individual and can be included in the compositions of the present disclosure without causing a significant adverse toxicological effect on the patient. Non-limiting examples of pharmaceutically acceptable excipients include water, NaCl, normal saline solutions, and the like. For example, a pharmaceutically acceptable carrier can be a sterile aqueous solution, such as PBS or water-for-injection. One of skill in the art will recognize that other pharmaceutical excipients are useful in the present disclosure. “Pharmaceutically acceptable salt” means or refers to physiologically and pharmaceutically acceptable salts of compounds, such as oligomeric compounds or oligonucleotides, i.e., salts that retain the desired biological activity of the parent compound and do not impart undesired toxicological effects thereto. “Tropomyosin Receptor Kinase B” or “TrkB,” as may be used interchangeably herein, means the receptor for brain-derived neurotrophic factor (BDNF) protein encoded by the NTRK2 gene. TrkB is also known as tyrosine receptor kinase B, BDNF/NT-3 growth factors receptor and neurotrophic tyrosine kinase, receptor, type 2. As used herein, a pharmaceutically acceptable salt is any salt of a compound provided herein which retains its biological properties and which is not toxic or otherwise undesirable for pharmaceutical use. The pharmaceutically acceptable salts of the therapeutic agents disclosed herein include salts that are prepared with relatively nontoxic acids or bases, depending on the particular substituents found on the compounds or modified oligonucleotides described herein. When compounds of the present disclosure contain relatively acidic functionalities, base addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired base, either neat or in a suitable inert solvent. When compounds of the present disclosure contain relatively basic functionalities, acid addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired acid, either neat or in a suitable inert solvent. Thus, the compounds of the present disclosure may exist as salts, such as with pharmaceutically acceptable acids. Such salts may be derived from a variety of organic and inorganic counter-ions well known in the art. Such salts include, but are not limited to: (1) acid addition salts formed with organic or inorganic acids such as hydrochloric, hydrobromic, sulfuric, nitric, phosphoric, sulfamic, acetic, trifluoroacetic, trichloroacetic, propionic, hexanoic, cyclopentylpropionic, glycolic, glutaric, pyruvic, lactic, malonic, succinic, sorbic, ascorbic, malic, maleic, fumaric, tartaric, citric, benzoic, 3-(4-hydroxybenzoyl)benzoic, picric, cinnamic, mandelic, phthalic, lauric, methanesulfonic, ethanesulfonic, 1,2-ethane- disulfonic, 2-hydroxyethanesulfonic, benzenesulfonic, 4-chlorobenzenesulfonic, 2- naphthalenesulfonic, 4-toluenesulfonic, camphoric, camphorsulfonic, 4-methylbicyclo[2.2.2]- oct-2-ene-1-carboxylic, glucoheptonic, 3-phenylpropionic, trimethylacetic, tert-butylacetic, lauryl sulfuric, gluconic, benzoic, glutamic, hydroxynaphthoic, salicylic, stearic, cyclohexylsulfamic, quinic, muconic acid and the like acids; or (2) salts formed when an acidic proton present in the parent compound either (a) is replaced by a metal ion, e.g., an alkali metal ion, an alkaline earth ion or an aluminum ion, or alkali metal or alkaline earth metal hydroxides, such as sodium, potassium, calcium, magnesium, aluminum, lithium, zinc, and barium hydroxide, ammonia, or (b) coordinates with an organic base, such as aliphatic, alicyclic, or aromatic organic amines, such as ammonia, methylamine, dimethylamine, diethylamine, picoline, ethanolamine, diethanolamine, triethanolamine, ethylenediamine, lysine, arginine, ornithine, choline, N,N′-dibenzylethylene-diamine, chloroprocaine, diethanolamine, procaine, N-benzylphenethylamine, N-methylglucamine piperazine, tris(hydroxymethyl)-aminomethane, tetramethylammonium hydroxide, and the like (see, for example, Berge et al., “Pharmaceutical Salts,” Journal of Pharmaceutical Science, 1977, 66, 1-19). Pharmaceutically acceptable salts further include, by way of example only and without limitation, sodium, potassium, calcium, magnesium, ammonium, tetraalkylammonium, and the like, and when the compound contains a basic functionality, salts of non-toxic organic or inorganic acids, such as hydrohalides, e.g. hydrochloride and hydrobromide, sulfate, phosphate, sulfamate, nitrate, acetate, trifluoroacetate, trichloroacetate, propionate, hexanoate, cyclopentylpropionate, glycolate, glutarate, pyruvate, lactate, malonate, succinate, sorbate, ascorbate, malate, maleate, fumarate, tartarate, citrate, benzoate, 3-(4-hydroxybenzoyl)benzoate, picrate, cinnamate, mandelate, phthalate, laurate, methanesulfonate (mesylate), ethanesulfonate, 1,2-ethane-disulfonate, 2- hydroxyethanesulfonate, benzenesulfonate (besylate), 4-chlorobenzenesulfonate, 2- naphthalenesulfonate, 4-toluenesulfonate, camphorate, camphorsulfonate, 4- methylbicyclo[2.2.2]-oct-2-ene-1-carboxylate, glucoheptonate, 3-phenylpropionate, trimethylacetate, tert-butylacetate, lauryl sulfate, gluconate, benzoate, glutamate, hydroxynaphthoate, salicylate, stearate, cyclohexylsulfamate, quinate, muconate, and the like. In some embodiments, the pharmaceutically acceptable salt of the compounds and modified oligonucleotides disclosed herein is a sodium or a potassium salt. In some embodiments, the pharmaceutically acceptable salt of the compounds and modified oligonucleotides disclosed herein is a sodium salt. The neutral forms of the compounds are preferably regenerated by contacting the salt with a base or acid and isolating the parent compound in the conventional manner. The parent form of the compound may differ from the various salt forms in certain physical properties, such as solubility in polar solvents. In embodiments, compounds of the present disclosure contain both basic and acidic functionalities that allow the compounds to be converted into either base or acid addition salts. The neutral forms of the compounds may be regenerated by contacting the salt with a base or acid and isolating the parent compound in a conventional manner. The parent form of the compounds differs from the various salt forms in certain physical properties, such as solubility in polar solvents, but, unless specifically indicated, the salts disclosed herein are equivalent to the parent form of the compound for the purposes of the present disclosure. “Pharmaceutical agent” means a compound that provides a therapeutic benefit when administered to an individual. “Phosphorothioate linkage” means a modified phosphate linkage in which one of the non-bridging oxygen atoms is replaced with a sulfur atom. “Portion” means a defined number of contiguous (i.e., linked) nucleobases of a nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of a target nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of an oligonucleotide. “Prevent” refers to delaying or forestalling the onset, development or progression of a disease, disorder, or condition for a period of time. “RNA interference compound” or “RNAi compound” means a compound that acts, at least in part, through an RNA-induced silencing complex (RISC) pathway or Ago2, but not through RNase Η, to modulate a target nucleic acid and/or protein encoded by a target nucleic acid. RNAi compounds include, but are not limited to double-stranded siRNA, single- stranded siRNA, and microRNA, including microRNA mimics. “Sense oligonucleotide” or “sense strand” means the strand of a double-stranded compound that includes a region that is substantially complementary to a region of the antisense strand of the compound. “Specifically inhibit” with reference to a target nucleic acid or protein means to reduce or block expression or activity of the target nucleic acid or protein while minimizing or eliminating effects on non-target nucleic acids or proteins. “Subunit” with reference to an oligonucleotide means a nucleotide, nucleoside, nucleobase or sugar or a modified nucleotide, nucleoside, nucleobase or sugar as provided herein. “Target nucleic acid,” “target RNA,” and “nucleic acid target” all mean a nucleic acid capable of being targeted by compounds described herein. “Target region” means a portion of a target nucleic acid to which one or more compounds is targeted. “Targeting moiety” means a conjugate group that provides an enhanced affinity for a selected target, e.g., molecule, cell or cell type, compartment, e.g., a cellular or organ compartment, tissue, organ or region of the body, as, e.g., compared to a compound absent such a moiety. “Terminal group” means a chemical group or group of atoms that is covalently linked to a terminus of an oligonucleotide. “Therapeutically effective amount” or “effective amount” means an amount of a compound, pharmaceutical agent, or composition that provides a therapeutic benefit to an individual. A “therapeutically effective amount” or “effective amount” is an amount sufficient for a compound to accomplish a stated purpose relative to the absence of the compound (e.g., achieve the effect for which it is administered, treat, prevent or ameliorate a disease or reduce one or more symptoms of a disease or condition). An example of a “therapeutically effective amount” or “effective amount” is an amount sufficient to contribute to the treatment, prevention, amelioration, or reduction of a symptom or symptoms of a disease. A “reduction” of a symptom or symptoms (and grammatical equivalents of this phrase) means decreasing of the severity or frequency of the symptom(s), or elimination of the symptom(s). A “prophylactically effective amount” of a drug is an amount of a drug that, when administered to a subject, will have the intended prophylactic effect, e.g., preventing or delaying the onset (or reoccurrence) of an injury, disease, pathology or condition, or reducing the likelihood of the onset (or reoccurrence) of an injury, disease, pathology, or condition, or their symptoms. The term “therapeutically effective amount,” as used herein, refers to that amount of the therapeutic agent sufficient to provide a therapeutic benefit to an individual, such as treating, preventing or ameliorating the disease or disorder or symptom thereof, as described above. For example, for the given parameter, a therapeutically effective amount will show an increase or decrease of at least 5%, 10%, 15%, 20%, 25%, 40%, 50%, 60%, 75%, 80%, 90%, or at least 100%. Therapeutic efficacy can also be expressed as “-fold” increase or decrease. For example, a therapeutically effective amount can have at least a 1.2- fold, 1.5-fold, 2-fold, 5-fold, or more effect over a control. The terms “treating” or “treatment” refer to any indicia of success in the therapy or amelioration of an injury, disease, pathology or condition, including any objective or subjective parameter such as abatement; remission; diminishing of symptoms or making the injury, pathology or condition more tolerable to the patient; slowing in the rate of degeneration or decline; making the final point of degeneration less debilitating; improving a patient's physical or mental well-being. The treatment or amelioration of symptoms can be based on objective or subjective parameters, including the results of a physical examination. The term “treating” and conjugations thereof, may include prevention of an injury, pathology, condition, or disease. In embodiments, treating is preventing. In embodiments, treating does not include preventing. “Treating” or “treatment” as used herein (and as well-understood in the art) also broadly includes any approach for obtaining beneficial or desired results in a subject's condition, including clinical results. Beneficial or desired clinical results can include, but are not limited to, alleviation or amelioration of one or more symptoms or conditions, diminishment of the extent of a disease, stabilizing (i.e., not worsening) the state of disease, prevention of a disease’s transmission or spread, delay or slowing of disease progression, amelioration or palliation of the disease state, diminishment of the reoccurrence of disease, and remission, whether partial or total and whether detectable or undetectable. In other words, “treatment” as used herein includes any cure, amelioration, or prevention of a disease. Treatment may prevent the disease from occurring; inhibit the disease’s spread; relieve the disease’s symptoms, fully or partially remove the disease’s underlying cause, shorten a disease’s duration, or do a combination of these things. “Treating” and “treatment” as used herein include prophylactic treatment. Treatment methods include administering to a subject a therapeutically effective amount of a compound described herein. The administering step may consist of a single administration or may include a series of administrations. The length of the treatment period depends on a variety of factors, such as the severity of the condition, the age of the patient, the concentration of the compound, the activity of the compositions used in the treatment, or a combination thereof. It will also be appreciated that the effective dosage of an agent used for the treatment or prophylaxis may increase or decrease over the course of a particular treatment or prophylaxis regime. In some instances, chronic administration may be required. For example, the compositions are administered to the subject in an amount and for a duration sufficient to treat the patient. “Treat” refers to administering a compound or pharmaceutical composition to an animal in order to effect an alteration or improvement of a disease, disorder, or condition in the animal. Certain compounds of the present disclosure possess asymmetric carbon atoms (optical or chiral centers) or double bonds; the enantiomers, racemates, diastereomers, tautomers, geometric isomers, stereoisometric forms that may be defined, in terms of absolute stereochemistry, as (R)- or (S)- or, as (D)- or (L)-for amino acids, and individual isomers are encompassed within the scope of the present disclosure. The compounds of the present disclosure do not include those that are known in art to be too unstable to synthesize and/or isolate. The present disclosure is meant to include compounds in racemic and optically pure forms. Optically active (R)- and (S)-, or (D)- and (L)-isomers may be prepared using chiral synthons or chiral reagents or resolved using conventional techniques. When the compounds described herein contain olefinic bonds or other centers of geometric asymmetry, and unless specified otherwise, it is intended that the compounds include both E and Z geometric isomers. As used herein, the term “isomers” refers to compounds having the same number and kind of atoms, and hence the same molecular weight, but differing in respect to the structural arrangement or configuration of the atoms. The term “tautomer,” as used herein, refers to one of two or more structural isomers which exist in equilibrium and which are readily converted from one isomeric form to another. It will be apparent to one skilled in the art that certain compounds of this disclosure may exist in tautomeric forms, all such tautomeric forms of the compounds being within the scope of the disclosure. Unless otherwise stated, structures depicted herein are also meant to include all stereochemical forms of the structure (i.e., the R and S configurations for each asymmetric center). Therefore, single stereochemical isomers as well as enantiomeric and diastereomeric mixtures of the present compounds are within the scope of the disclosure. As used herein, “chirally enriched population” means a plurality of molecules of identical molecular formula, wherein the number or percentage of molecules within the population that contain a particular stereochemical configuration at a particular chiral center is greater than the number or percentage of molecules expected to contain the same particular stereochemical configuration at the same particular chiral center within the population if the particular chiral center were stereorandom. Chirally enriched populations of molecules having multiple chiral centers within each molecule may contain one or more stereorandom chiral centers. In certain embodiments, the molecules are modified oligonucleotides. In certain embodiments, the molecules are compounds comprising modified oligonucleotides. Unless otherwise stated, structures depicted herein are also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by13C- or14C-enriched carbon are within the scope of this disclosure. As used herein, “stereorandom chiral center” in the context of a population of molecules of identical molecular formula means a chiral center having a random stereochemical configuration. For example, in a population of molecules comprising a stereorandom chiral center, the number of molecules having the (S) configuration of the stereorandom chiral center may be but is not necessarily the same as the number of molecules having the (R) configuration of the stereorandom chiral center. The stereochemical configuration of a chiral center is considered random when it is the results of a synthetic method that is not designed to control the stereochemical configuration. In certain embodiments, a stereorandom chiral center is a stereorandom phosphorothioate internucleoside linkage. Certain Embodiments In certain aspects, the disclosure relates to methods, compounds and compositions for inhibiting MAPT. In certain embodiments, MAPT is specifically inhibited. In certain embodiments, MAPT is specifically degraded. In certain embodiments, MAPT expression is inhibited. In certain embodiments, MAPT translation is inhibited. In certain embodiments, MAPT activity is inhibited. In certain embodiments, MAPT expression, translation, or activity is reduced by at least 10% relative to the expression, translation, or activity in an untreated or control sample. For example, in certain embodiments, MAPT expression, translation, or activity is reduced by at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%, 10-50%, 25- 50%, 25-75%, 50-75%, 50-99%, or 75-99% relative to the expression, translation, or activity in an untreated or control sample. In certain embodiments, MAPT expression, translation, or activity is reduced as measured by any suitable assay, including but not limited to, an immunoassay, a hybridization-based assay, or a sequencing-based assay (e.g., RNA-Seq). In certain aspects, the disclosure relates to compounds targeted to a MAPT nucleic acid. In certain embodiments, the MAPT nucleic acid has the sequence set forth in GenBank Accession No. NM_001377265.1 (incorporated herein as SEQ ID NO: 1), and nucleotides 2624000 to 2761000 of NT_010783.14 (incorporated herein as SEQ ID NO: 2). In certain embodiments, the compound is an oligomeric compound. In certain embodiments, the compound is single-stranded. In certain embodiments, the compound is double-stranded. Certain embodiments provide a compound comprising a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209- . Certain embodiments provide a compound comprising a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. Certain embodiments provide a compound comprising a modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, the modified oligonucleotide is at least 80%, at least 85%, at least 90%, or at least 95% complementary to SEQ ID NO: 1 or 2. In certain embodiments, the modified oligonucleotide comprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase. In certain embodiments, the compound is double-stranded. Certain embodiments provide a compound comprising a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, the compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence provided in Tables 2 and 3, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. Certain embodiments provide a compound comprising a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. Certain embodiments provide a compound comprising a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. Certain embodiments provide a compound comprising a first modified oligonucleotide comprising a 5′-phosphonate modification, where the first modified oligonucleotide is at least 80% complementary to a region of SEQ ID NO: 1 or 2, and a second modified oligonucleotide comprising one or more ligands described herein (e.g., one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB1) ligands, or one or more α4β1/7 integrin ligands). In certain embodiments, the first modified oligonucleotide comprises a 5′-terminal nucleoside comprising the 5′-phosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the modified oligonucleotide or first modified oligonucleotide of any preceding compound has at least 80%, at least 85%, at least 90%, or at least 95% complementarity or identity to SEQ ID NO: 1 or 2 over its length. In certain embodiments, the modified oligonucleotide or first modified oligonucleotide has at least 1, at least 2, at least 3 mismatches to a region of SEQ ID NO: 1 or 2. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 14 to 30 linked nucleosides in length. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 14 to 23 linked nucleosides in length. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 19 to 23 linked nucleosides in length. In certain embodiments, the region of complementarity between the first modified oligonucleotide or first strand and the second modified oligonucleotide or second strand is 21 to 23 linked nucleosides in length. In certain embodiments, the first modified oligonucleotide is fully complementary to the second modified oligonucleotide. In certain embodiments, the modified oligonucleotide or first modified oligonucleotide of any preceding compound comprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase. In certain embodiments, the second modified oligonucleotide of any preceding compound comprises at least one modification selected from the group consisting of a modified internucleoside linkage, a modified sugar, and a modified nucleobase. In certain embodiments, the modified internucleoside linkage is a phosphorothioate internucleoside linkage or a methylphosphonate internucleoside linkage. In certain embodiments, the phosphorothioate internucleoside linkage or methylphosphonate internucleoside linkage is at the 3’ terminus of the first or second modified oligonucleotide or at the 5’ terminus of the first modified oligonucleotide. In certain embodiments, the modified sugar comprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar. In certain embodiments, the modified sugar comprises a 2’-F modification. In certain embodiments, the modified sugar comprises a 2’-OMe modification. In certain embodiments, each nucleoside of the first modified oligonucleotide comprises a modified sugar. In certain embodiments, each nucleoside of the second modified oligonucleotide comprises a modified sugar. In certain embodiments, the modified sugar comprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar or a combination thereof. In certain embodiments, the modified sugar comprises a modification selected from the group consisting of 2’-MOE, 2’-F, and 2’-OMe or a combination thereof. In certain embodiments, the first modified oligonucleotide comprises no more than ten 2’-F sugar modifications. In certain embodiments, the second modified oligonucleotide comprises no more than five 2’-F sugar modifications. In certain embodiments, the compound of any preceding embodiment comprises a conjugate group. In certain embodiments, the conjugate group is attached to the 5’ end of the modified oligonucleotide. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more ligands. In certain embodiments, the targeting moiety comprises one or more ligands selected from one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB1) ligands, and one or more α4β1/7 integrin ligands. In certain embodiments, the targeting moiety comprises one or more TrkB ligands. In certain embodiments, the targeting moiety comprises one or more CB1 ligands. In certain embodiments, the targeting moiety comprises one or more α4β1/7 integrin ligands. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the one or more TrkB ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the one or more CB1 ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the one or more α4β1/7 integrin ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (I) or a salt, solvate, or hydrate thereof: Formula (I), wherein: R1 is the modified oligonucleotide; L1, L2, L3, and L4 are as described herein; R2 is hydrogen, -OR7, -SR8, or -NR9R10; R3 is hydrogen, -OR11, -SR12, or -NR13R14; R4 is hydrogen, -OR15, -SR16, or -NR17R18; R5 is hydrogen, -OR19, -SR20, or -NR21R22; R6 is hydrogen, -OH, optionally substituted -O-alkyl, optionally substituted -OAc, - NH2, optionally substituted -NHAc, -SH, or =O; R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, and R22 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, optionally substituted heteroaryl; Y is CH2, NH, S, or O; and Z is optionally substituted aryl or optionally substituted heteroaryl. In certain embodiments, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, and R22 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, R7, R8, R9, and R10 are each independently alkenyl. In certain embodiments, R7, R8, R9, and R10 are each independently alkynyl. In certain embodiments, R2 is OR7. In certain embodiments, R3 is OR11. In certain embodiments, R7 and R11 are each independently hydrogen, optionally substituted alkyl or optionally substituted alkenyl. In certain embodiments, one or both R7 and R11 are each independently hydrogen. In certain embodiments, one or both R7 and R11 are each independently optionally substituted alkyl. In certain embodiments, one or both R7 and R11 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, one or both R7 and R11 are each independently alkenyl. In certain embodiments, R7 is optionally substituted alkyl and R11 is hydrogen. In certain embodiments, R7 is hydrogen and R11 is optionally substituted alkyl. In certain embodiments, R7 is alkenyl and R11 is hydrogen. In certain embodiments, R7 is hydrogen and R11 is optionally substituted alkenyl. In certain embodiments, the TrkB ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (II-C), wherein: R1 is the modified oligonucleotide; L1, L2, L3, L4, and R1 are as described herein. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXVII) or a salt, solvate, or hydrate thereof: Formula (XXXXXVII), wherein: L1, L2, L3, L4, and R1 are as described herein; R11 and R13 are each independently absent, hydrogen, or optionally substituted alkyl; R12, R14, and R15 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R16 is hydrogen, halogen, –CN, –N3, –SOn16R1A, –SOv16NR16BR16C, −NHNR16BR16C, −ONR16BR16C, −NHC(O)NHNR16BR16C, −NHC(O)NR16BR16C, –N(O)m16, –NR16BR16C, –C(O)R16D, –C(O)OR16D, –C(O)NR16BR16C, –OR16A, -NR16BSO2R16A, -NR16BC(O)R16D, - NR16BC(O)OR16D, –NR16BOR16D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; and are each independently a single bond or a double bond, wherein if is a single bond, then is a double bond and R13 is absent; and further wherein if is a single bond, then is a double bond and R11 is absent; R16A, R16B, R16C, R16D are each independently hydrogen, halogen, –CF3, –CCI3, –CBr3, –Cl3, –COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; or R16B and R16C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; z3 is 0, 1, 2, 3, 4, or 5; n16 is 0, 1, 2, 3, or 4; and v16 and m16 are each independently 1 or 2. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXIX) or a salt, solvate, or hydrate thereof: Formula (XXXXXIX), wherein: L1, L2, L3, L4, and R1 are as described herein; R17, R18, and R19 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; z4 is 0, 1, or 2; and z5 is 0, 1, 2, or 3. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXX) or a salt, solvate, or hydrate thereof: Formula (XXXXXX), wherein: L1, L2, L3, L4, and R1 are as described herein; R20 is hydrogen, halogen, –CN, –N3, –SOn20R1A, –SOv20NR20BR20C, −NHNR20BR20C, −ONR20BR20C, −NHC(O)NHNR20BR20C, −NHC(O)NR20BR20C, –N(O)m20, –NR20BR20C, –C(O)R20D, –C(O)OR20D, –C(O)NR20BR20C, –OR20A, -NR20BSO2R20A, -NR20BC(O)R20D; -NR20BC(O)OR20D, –NR20BOR20D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R21 is hydrogen, halogen, –CN, –N3, –SOn21R1A, –SOv21NR21BR21C, −NHNR21BR21C, −ONR21BR21C, −NHC(O)NHNR21BR21C, −NHC(O)NR21BR21C, –N(O)m21, –NR21BR21C, –C(O)R21D, –C(O)OR21D, –C(O)NR21BR21C, –OR21A, -NR21BSO2R21A, -NR21BC(O)R21D; -NR21BC(O)OR21D, –NR21BOR21D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R22 and R23 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R24 is hydrogen, halogen, –CN, –N3, –SOn24R24A, –SOv24NR24BR24C, −NHNR24BR24C, −ONR24BR24C, −NHC(O)NHNR24BR24C, −NHC(O)NR24BR24C, –N(O)m24, –NR24BR24C, –C(O)R24D, –C(O)OR24D, –C(O)NR24BR24C, –OR24A, -NR24BSO2R24A, -NR24BC(O)R24D; -NR24BC(O)OR24D, –NR24BOR24D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R20A, R20B, R20C, R20D, R21A, R21B, R21C, R21D, R24A, R24B, R24C, and R24D are each independently hydrogen, halogen, –CF3, –CCI3, –CBr3, –Cl3,–COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R20B, R20C, R21B, R21C, R24B, R24C, R24B, and R24C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; n21, n22, n24, z6 and z8 are each independently 0, 1, 2, 3, or 4; v20, v21, v24, m20, m21, and m24 are each independently 1 or 2; and z7 is 0, 1, or 2. In certain embodiments, the CB1 ligand of a modified oligonucleotide is of the Formula (XXXXXXI) or a salt, solvate, or hydrate thereof: , Formula (XXXXXXI) wherein: L1, L2, L3, L4, and R1 are as described herein; X1 is NR10 or CR11R12; R10, R11, and R12 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R19 is hydrogen, –SOn19R19A, –SOv19NR19BR19C, −NHNR19BR19C, −ONR19BR19C, −NHC(O)NHNR19BR19C, −NHC(O)NR19BR19C, –NR19BR19C, –C(O)R19D, –C(O)OR19D, – C(O)NR19BR19C, –OR19A, –NR19BSO2R19A, –NR19BC(O)R19D, -NR19BC(O)OR19D, – NR19BOR19D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R19A, R19B, R19C, R19D are each independently hydrogen, halogen, –CF3, –CCl3, –CBr3, –CI3, –COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; wherein R19B and R19C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; n19 is 0, 1, 2, 3, or 4; and v19 is 1 or 2. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXII) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, polyethylene glycol (PEG), optionally substituted heteroalkyl, or optionally substituted heteroaryl; and R3, and R4 are each independently H, halogen, optionally substituted alkyl, or optionally substituted -O-alkyl. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXIII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXIII) wherein L1, L2, L3, L4, and R1 are as described herein; R2, R3, R4, and R5 are each independently H, halogen, optionally substituted alkyl, optionally substituted -O-alkyl, cycloalkyl, or absent; R8 is optionally substituted C1-C5 alkyl, optionally substituted C1-C5 alkylene-(C3- C6)-cycloalkyl, or optionally substituted (C1-C4)-alkylene-(C1-C4)-alkoxy; and R6, and R7 are each independently H, halogen, alkyl, or optionally substituted alkyl, optionally substituted heteroalkyl, In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXIV) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXIV) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, -CONHR4, -CH2OR4, -(CH2)2OR4, -CH2NHCOR4, or -OR4; R3 is H, optionally substituted alkyl, or optionally substituted cycloalkyl; R4 is H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; R5 is -OH or absent; and X is H, optionally substituted CH2, optionally substituted NH, or cycloalkyl. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXXXIII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXXXIII) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, -CONHR3, -CH2OR3, -(CH2)2OR3, -CH2NHCOR3, or -OR3; each instance of R3 is independently H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; and X is H or halogen. In certain embodiments, L1, L2, L3, and L4 are each independently absent, a bond, an optionally substituted alkyl linker, an optionally substituted polyethylene glycol (PEG) linker, an optionally substituted heteroalkyl linker, or an optionally substituted heteroaryl linker. In certain embodiments, L1 is an optionally substituted heteroaryl linker. In certain embodiments, L1 is an optionally substituted unsaturated heteroaryl, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker. In certain embodiments, L1 comprises the structure: . In certain embodiments, L1 is an optionally substituted heteroalkyl linker. In certain embodiments, the optionally substituted heteroalkyl linker is an optionally substituted heteroalkyl or optionally substituted C1-C10 alkyl chain in which one or more carbon atoms are replaced with O, N, or S. In certain embodiments, L1 comprises the structure: or . In certain embodiments, L1 comprises the structure: or –N(CH3)–. In certain embodiments, L2 is an optionally substituted PEG linker. In certain embodiments, the PEG linker is five PEG units in length. In certain embodiments, the PEG linker is four PEG units in length. In certain embodiments, the PEG linker is three PEG units in length. In certain embodiments, L2 is an optionally substituted alkyl linker. In certain embodiments, L2 is an optionally substituted C1-20 alkyl linker. In certain embodiments, L2 is an optionally substituted C8 alkyl linker. In certain embodiments, L3 is an optionally substituted heteroaryl linker. In certain embodiments, L3 is an optionally substituted partially unsaturated heteroaryl linker, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker. In certain embodiments, L3 comprises the structure: . In certain embodiments, L4 is an optionally substituted heteroalkyl linker. In certain embodiments, the heteroalkyl linker is substituted with one or more =O substituents. In certain embodiments, the heteroalkyl linker comprises two substituents joined together to form an optionally substituted carbocyclyl ring. In certain embodiments, L4 comprises the structure: or a salt thereof, wherein X is O or S. In certain embodiments, L4 comprises the structure: or a salt thereof, wherein X is O or S. In certain embodiments, L1– L2–L3–L4 comprises the structure: ,
, or a salt thereof, wherein X is O or S. In certain embodiments, the TrkB ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof:
Formula (XXXXXXXIII), wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, the CB1 ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof:
Formula (XXXXXXXVII) Formula (XXXXXXXVIII) wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXXX)
Formula (XXXXXXXXIV) wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, the compound of any preceding embodiment comprises a lipid. In certain embodiments, the lipid is attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises one or more lipids. In certain embodiments, the one or more lipids are attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the compound of any preceding embodiment comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl are attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C22 hydrocarbon chain. In certain embodiments, a substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide). In certain embodiments, the internucleoside linkage is between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises Formula (XXXV), or a salt, solvate, or hydrate thereof: Formula (XXXV), wherein: Y is –C(=O)N(RC)–, or –N(RC)C(=O)–; Q1 and Q3 are each independently –H, –OR4, a ligand, a linker, or a lipid; Q2 and Q4 are each independently a bond, a ligand, a linker, or a lipid; RC is independently –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl; each R9 is independently a substituted or unsubstituted heteroaryl; each instance of Z1 or Z2 is independently a bond, C1-C6 alkylene, or C2-C6 alkenylene; and each X is independently O or S; or a salt thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVI), or a salt, solvate, or hydrate thereof: Formula (XXXVI), wherein: RC is –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVII), or a salt, solvate, or hydrate thereof: Formula (XXXVII), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVIII), or a salt, solvate, or hydrate thereof: Formula (XXXVIII), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXIX), or a salt, solvate, or hydrate thereof:
Formula (XXXIX), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXX), or a salt solvate, or hydrate thereof:
Formula (XXXX), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXI), or a salt, solvate, or hydrate thereof:
mUëmU Formula (XXXXI) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXII), or a salt, solvate, or hydrate thereof: mAëmA Formula (XXXXII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIII), or a salt, solvate, or hydrate thereof: mAëmU Formula (XXXXIII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIV), or a salt, solvate, or hydrate thereof: mAëmG Formula (XXXXIV) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXV), or a salt, solvate, or hydrate thereof: mAëmC Formula (XXXXV) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVI), or a salt, solvate, or hydrate thereof: mUëmA Formula (XXXXVI) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVII), or a salt, solvate, or hydrate thereof: mUëmG Formula (XXXXVII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVIII), or a salt, solvate, or hydrate thereof: mUëfC Formula (XXXXVIII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIX), or a salt, solvate, or hydrate thereof: fGëmU Formula (XXXXIX) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXX), or a salt, solvate, or hydrate thereof: mGëfG Formula (XXXXX) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXXI), or a salt, solvate, or hydrate thereof: mGëmC Formula (XXXXXI) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXXII), or a salt, solvate, or hydrate thereof:
mCëmA Formula (XXXXXII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises an internucleoside linkage of one of the following Formulae: Formula (XXXXXV), Formula (XXXXXVI). In certain embodiments, the compound of any preceding embodiment comprises a 5′- phosphonate modification. For example, in certain embodiments, a modified oligonucleotide comprises one or more sugars having a phosphonate modification at the 5′ position. In certain embodiments, the modified oligonucleotide comprises a 5′-phosphonate modification. In certain embodiments, the modified oligonucleotide comprises a 5′-terminal nucleoside (e.g., 5′ terminus) comprising the 5′-phosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification or a 5′- ethylenephosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-ethylenephosphonate modification. In certain embodiments, the modified oligonucleotide is the first modified oligonucleotide or antisense oligonucleotide. Certain embodiments provide a compound comprising: a first modified oligonucleotide comprising a 5′-phosphonate modification, where the first modified oligonucleotide is at least 80% complementary to a region of SEQ ID NO: 1 or 2; and a second modified oligonucleotide comprising one or more ligands. In some embodiments, the first modified oligonucleotide comprises a 5′-terminal nucleoside comprising the 5′-phosphonate modification. In some embodiments, the 5′- phosphonate modification is a 5′-vinylphosphonate modification or a 5′-ethylenephosphonate modification. In some embodiments, the 5′-phosphonate modification is a 5′- vinylphosphonate modification. In some embodiments, the 5′-phosphonate modification is a 5′-ethylenephosphonate modification. In some embodiments, the second modified oligonucleotide comprises one or more ligands selected from one or more TrkB ligands, one or more CB1 ligands, and one or more α4β1/7 integrin ligands. In some embodiments, the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end of the second modified oligonucleotide. In some embodiments, the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 3’ end of the second modified oligonucleotide. In some embodiments, the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end and the 3’ end of the second modified oligonucleotide. In some embodiments, the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX-XXXXXXXIII; the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII; and the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV. In some embodiments, the second modified oligonucleotide comprises one or more TrkB ligands. In some embodiments, the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV- XXXXXXVII, and XXXXXXIX-XXXXXXXIII. In some embodiments, the one or more TrkB ligands are selected from any one of Formulae XXIX-XXXI and XXXXXXV. In some embodiments, the second modified oligonucleotide comprises one TrkB ligand. In some embodiments, the second modified oligonucleotide comprises two TrkB ligands. In some embodiments, the second modified oligonucleotide comprises at least two TrkB ligands. In some embodiments, the at least two TrkB ligands are the same. In some embodiments, the at least two TrkB ligands are different. In some embodiments, the second modified oligonucleotide comprises one or more CB1 ligands. In some embodiments, the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII. In some embodiments, the one or more CB1 ligands are selected from any one of Formulae XXXXXXXIV- XXXXXXXVI. In some embodiments, the second modified oligonucleotide comprises one CB1 ligand. In some embodiments, the second modified oligonucleotide comprises two CB1 ligands. In some embodiments, the second modified oligonucleotide comprises at least two CB1 ligands. In some embodiments, the at least two CB1 ligands are the same. In some embodiments, the at least two CB1 ligands are different. In some embodiments, the second modified oligonucleotide comprises one or more α4β1/7 integrin ligands. In some embodiments, the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX- XXXXXXXXIV. In some embodiments, the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXXXI, XXXXXXXXII, and XXXXXXXXIV. In some embodiments, the second modified oligonucleotide comprises one α4β1/7 integrin ligand. In some embodiments, the second modified oligonucleotide comprises two α4β1/7 integrin ligands. In some embodiments, the second modified oligonucleotide comprises at least two α4β1/7 integrin ligands. In some embodiments, the at least two α4β1/7 integrin ligands are the same. In some embodiments, the at least two α4β1/7 integrin ligands are different. In some embodiments, the second modified oligonucleotide comprises one or more lipids. In some embodiments, the second modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl. In some embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain. In some embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain. In some embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain. In some embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain, a saturated or unsaturated C17 hydrocarbon chain, a saturated or unsaturated C18 hydrocarbon chain, or a saturated or unsaturated C22 hydrocarbon chain. In some embodiments, the one or more substituted or unsubstituted alkyl or alkenyl are attached to an internucleoside linkage of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the second modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the second modified oligonucleotide. In some embodiments, the internucleoside linkage of the second modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI. In some embodiments, the second modified oligonucleotide comprises any one of Formulae XXXV- XXXXXVI. In some embodiments, the first modified oligonucleotide is 14 to 30 linked nucleosides in length. In some embodiments, the second modified oligonucleotide is 14 to 30 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In some embodiments, the first modified oligonucleotide has a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200, and 209-217. In some embodiments, the second modified oligonucleotide has a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206. In some embodiments, the first modified oligonucleotide is selected from any one of the IA Ref ID NOs in Table 3. In some embodiments, the second modified oligonucleotide is selected from any one of the IS Ref ID NOs in Table 3. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of Ref ID NOs listed in Table 3 and a second modified oligonucleotide 14 to 21 linked nucleosides in length fully complementary to the first modified oligonucleotide. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3. In certain embodiments, the second modified oligonucleotide comprises any one or more TrkB ligands provided herein. In certain embodiments, the TrkB ligand is selected from Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX-XXXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the TrkB ligand is selected from Formulae XXIX-XXXI and XXXXXXV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the TrkB ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the TrkB ligand through a phosphorothioate group. In certain embodiments, provided herein is a compound selected from the group consisting of any one of the compounds listed in Table 3. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3. In certain embodiments, the second modified oligonucleotide comprises any one or more CB1 ligands provided herein. In certain embodiments, the CB1 ligand is selected from Formulae XXXXXXI and XXXXXXXIV- XXXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the CB1 ligand is selected from Formulae XXXXXXXIV-XXXXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the CB1 ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the CB1 ligand through a phosphorothioate group. In certain embodiments, provided herein is a compound selected from the group consisting of any one of the compounds listed in Table 3. Certain embodiments provide a compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs listed in Table 3 and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs listed in Table 3. In certain embodiments, the second modified oligonucleotide comprises any one or more α4β1/7 integrin ligands provided herein. In certain embodiments, the α4β1/7 integrin ligand is selected from Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the α4β1/7 integrin ligand is selected from Formulae XXXXXXXXI, XXXXXXXXII, and XXXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the α4β1/7 integrin ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the α4β1/7 integrin ligand through a phosphorothioate group. In certain embodiments, provided herein is a compound selected from the group consisting of any one of the compounds listed in Table 3. In certain embodiments, the pharmaceutically acceptable salt of the modified oligonucleotides provided herein is a sodium salt or a potassium salt. In certain embodiments, the pharmaceutically acceptable salt of the compounds provided herein is a sodium salt or a potassium salt. In certain embodiments, provided herein is a population of modified oligonucleotides, wherein all of the phosphorothioate internucleoside linkages of the modified oligonucleotide are stereorandom. In certain embodiments, provided herein is a population of compounds, wherein all of the phosphorothioate internucleoside linkages of the modified oligonucleotide are stereorandom. In certain embodiments, the compound of any foregoing embodiment is in a pharmaceutically acceptable salt form. In certain embodiments, the pharmaceutically acceptable salt is a sodium salt. In certain embodiments, the pharmaceutically acceptable salt is a potassium salt. Certain embodiments provide a composition comprising the compound of any one of the foregoing embodiments and a pharmaceutically acceptable carrier. Certain embodiments provide a composition comprising a compound of any preceding embodiment, for use in therapy. Certain embodiments provide a method of treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT in an individual comprising administering to the individual a compound targeted to MAPT, thereby treating, preventing, or ameliorating the disease. In certain embodiments, the compound or composition of any foregoing embodiment is administered to an individual. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, administering the compound inhibits or reduces or improves a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual in a therapeutically effective amount. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual at a dosage level sufficient to deliver about 1 to 100 mg/kg of body weight of the individual. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual at a fixed dose of about 25 mg to about 1,000 mg. In certain embodiments, the compound or composition is administered to the individual one or more times in a day up to the dosage level or fixed dose. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual daily, weekly, monthly, quarterly or yearly. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual about once per quarter (i.e., once every three months) to about once per year. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual about once per quarter, about once every six months or about once per year. Certain embodiments provide a method of inhibiting expression of MAPT in a cell comprising contacting the cell with a compound targeted to MAPT, thereby inhibiting expression of MAPT in the cell. In certain embodiments, the cell is in the liver of an individual. In certain embodiments, the individual has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. Certain embodiments provide a method of reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an individual, comprising administering a compound targeted to MAPT to the individual, thereby reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in the individual. In certain embodiments, the individual has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound is a compound targeted to MAPT. In certain embodiments, the compound is any of the foregoing compounds. In certain embodiments, the compound or composition is administered parenterally. In certain embodiments, the compound or composition is administered by intrathecal (IT) administration. Certain embodiments provide use of a compound targeted to MAPT for treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound is a compound targeted to MAPT. In certain embodiments, the compound is any of the foregoing compounds. Certain embodiments provide use of a compound targeted to MAPT in the manufacture of a medicament for treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairmentvv. In certain embodiments, the compound is a compound targeted to MAPT. In certain embodiments, the compound is any of the foregoing compounds. In certain embodiments, any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3. In certain embodiments, any one of the compounds shown in FIGs. 1-4 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds shown in FIGs. 1-4. In certain embodiments, any one of the compounds in Table 2 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds in Table 2. In certain embodiments, any one of the compounds in Table 3 can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds in Table 3. In certain embodiments, any one of the compounds in Table A can be used in any aspect of the disclosure. In some embodiments of a compound, composition, method, or use of the disclosure, the compound is any one of the compounds in Table A. In some embodiments of any of the aspects of the disclosure, the compound is selected from any one of compounds 1-285 in Table A. Table A, shown below, provides the following for each of compounds 1-285: antisense (“A”) and sense (“S”) strand nucleobase sequences (SEQ ID NO) shown with strand modifications (as defined in Table 1), and, where a sense strand comprises a ligand, the type of ligand (e.g., a TrkB ligand, a CB1 ligand, or an α4β1/7 integrin ligand as described herein) at the 5’ end (“5’-Ligand”) and/or the 3’ end (“3’-Ligand”) of the sense strand. In some embodiments, the 5’-Ligand and/or the 3’-Ligand are attached to the sense strand via a linker. In some embodiments, the linker comprises polyethylene glycol (PEG). In some embodiments, the linker comprises a PEG chain of one, two, three, four, five, six, seven, or eight PEG units in length. In certain embodiments, the linker comprises a PEG chain one PEG unit in length. In certain embodiments, the linker comprises a PEG chain two PEG units in length. In certain embodiments, the linker comprises a PEG chain three PEG units in length. In certain embodiments, the linker comprises a PEG chain four PEG units in length. In certain embodiments, the linker comprises a PEG chain five PEG units in length. In certain embodiments, the linker comprises a PEG chain six PEG units in length. In certain embodiments, the linker comprises a PEG chain seven PEG units in length. In certain embodiments, the linker comprises a PEG chain eight PEG units in length. In some embodiments, the linker comprises an optionally substituted heteroaryl or optionally substituted heterocyclyl group. In certain embodiments, the linker comprises PEG and an optionally substituted heteroaryl or optionally substituted heterocyclyl group. Table A. Exemplary Compounds
Certain Indications In certain aspects, the disclosure relates to methods of inhibiting MAPT expression, which can be useful for treating, preventing, or ameliorating a disease associated with MAPT in an individual, by administration of a compound that targets MAPT. In certain embodiments, the compound can be a MAPT specific inhibitor. In certain embodiments, the compound can be an antisense oligonucleotide, an oligomeric compound, or an oligonucleotide targeted to MAPT (e.g., a compound of any one of the compounds shown in FIGs. 1-4, Table A, Table 2, and Table 3). In certain aspects, the disclosure relates to treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT. In certain embodiments, diseases, disorders or conditions associated with MAPT treatable, preventable, and/or ameliorable with the methods provided herein include a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. Certain compounds provided herein are directed to compounds and compositions that reduce a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal. In certain embodiments, a method of treating, preventing, or ameliorating a disease associated with MAPT in an individual comprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby treating, preventing, or ameliorating the disease. In certain embodiments, the individual is identified as having, or at risk of having, a disease associated with MAPT. In certain embodiments, the disease is a CNS related disease. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides) in length having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209- 217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In certain embodiments, a single-stranded compound can be 14 to 30, 14 to 23, 14 to 20, 16 to 20, or 14 to 16, linked nucleosides in length. In certain embodiments, a single-stranded compound can be 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30, linked nucleosides in length. In certain embodiments, a double-stranded compound can comprise two oligonucleotides of the same or different lengths, as described elsewhere herein. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, the compound is administered to the individual parenterally. In certain embodiments, the compound is administered to the individual by intrathecal (IT) administration. In certain embodiments, administering the compound improves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal. In certain embodiments, a method of treating, preventing, or ameliorating a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal comprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby treating, preventing, or ameliorating a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159- 168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199- 200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149- 155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, administering the compound improves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an animal. In certain embodiments, the individual is identified as having, or at risk of having, a disease associated with MAPT. In certain embodiments, a method of inhibiting expression of MAPT in an individual having, or at risk of having, a disease associated with MAPT comprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby inhibiting expression of MAPT in the individual. In certain embodiments, administering the compound inhibits expression of MAPT in the liver. In certain embodiments, the disease is a CNS related disease. In certain embodiments, the individual has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159- 168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171- 172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199- 200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149- 155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81- 143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, the compound is administered to the individual parenterally. In certain embodiments, the compound is administered to the individual by intrathecal (IT) administration. In certain embodiments, administering the compound improves, preserves, or prevents a CNS related disease, disorder or condition or a symptom thereof, a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, a method of inhibiting expression of MAPT in a cell comprises contacting the cell with a compound comprising a MAPT specific inhibitor, thereby inhibiting expression of MAPT in the cell. In certain embodiments, the cell is a hepatocyte. In certain embodiments, the cell is in the liver. In certain embodiments, the cell is in the liver of an individual who has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a method of reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in an individual having, or at risk of having, a disease associated with MAPT comprises administering to the individual a compound comprising a MAPT specific inhibitor, thereby reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment in the individual. In certain embodiments, the individual has, or is at risk of having, a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, the compound is administered to the individual parenterally. In certain embodiments, the compound is administered to the individual by intrathecal (IT) administration. In certain embodiments, the individual is identified as having, or at risk of having, a disease associated with MAPT. Certain embodiments are drawn to a compound comprising a MAPT specific inhibitor for use in treating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In certain embodiments, the compound is administered to the individual parenterally. In certain embodiments, the compound is administered to the individual by intrathecal (IT) administration. Certain embodiments are drawn to a compound comprising a MAPT specific inhibitor for use in reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201- 206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. Certain embodiments are drawn to the use of a compound comprising a MAPT specific inhibitor for the manufacture or preparation of a medicament for treating a disease associated with MAPT. Certain embodiments are drawn to the use of a compound comprising a MAPT specific inhibitor for the preparation of a medicament for treating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof. In certain embodiments, the disease, disorder or condition is a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP- 17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190- 192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. Certain embodiments are drawn to the use of a compound comprising a MAPT specific inhibitor for the manufacture or preparation of a medicament for reducing or inhibiting a CNS related disease, disorder or condition or a symptom thereof in an individual having, or at risk of having, a CNS related disease, disorder or condition or a symptom thereof associated with MAPT. In certain embodiments, the CNS related disease, disorder or condition is a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. Certain embodiments are drawn to use of a compound comprising a MAPT specific inhibitor for the preparation of a medicament for treating a disease, disorder or condition associated with MAPT. In certain embodiments, the disease, disorder or condition is a CNS related disease, disorder or condition or a symptom thereof or a neurodegenerative disease or a symptom thereof, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, Dravet’s Syndrome or cognitive impairment. In certain embodiments, the compound comprises an antisense oligonucleotide targeted to MAPT. In certain embodiments, the compound comprises an oligonucleotide targeted to MAPT. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) and having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequences of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, a compound comprises a modified oligonucleotide selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 11- 73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and a second modified oligonucleotide having a nucleobase sequence selected from the group consisting of the nucleobase sequence of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In any of the foregoing embodiments, the compound can be single-stranded or double-stranded. In any of the foregoing embodiments, the compound can be an antisense oligonucleotide or oligomeric compound. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide (e.g., of 14 to 30, for example, 14 to 23, linked nucleosides in length) having a region of complementarity to the first modified oligonucleotide. In certain embodiments, a compound comprises a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide. In any of the foregoing methods or uses, the compound can be an oligomeric compound. In any of the foregoing methods or uses, the compound can be single-stranded or double-stranded. In any of the foregoing methods or uses, the compound can be targeted to MAPT. In certain embodiments, the compound comprises or consists of a modified oligonucleotide. In certain embodiments, the compound comprises one or more modified oligonucleotides. In certain embodiments, the compound comprises a first modified oligonucleotide and a second modified oligonucleotide. In certain embodiments, a modified oligonucleotide is 8 to 80 linked nucleosides in length, 10 to 30 linked nucleosides in length, 14 to 30 linked nucleosides in length, 14 to 23 linked nucleosides in length, or 19 to 23 linked nucleosides in length. In certain embodiments, a modified oligonucleotide is at least 80%, at least 85%, at least 90%, at least 95% or 100% complementary to any of the nucleobase sequences recited in SEQ ID NOs: 1 and 3 over its length. In certain embodiments, a modified oligonucleotide comprises at least one modified internucleoside linkage, at least one modified sugar and/or at least one modified nucleobase. In certain embodiments, the modified internucleoside linkage is a phosphorothioate internucleoside linkage. In certain embodiments, the modified sugar is a bicyclic sugar, 2’-MOE, 2’-F, or 2’-OMe. In certain embodiments, the modified nucleobase is a 5-methylcytosine. In any of the foregoing embodiments, each modified oligonucleotide is independently 12 to 30, 14 to 30, 14 to 25, 14 to 24, 14 to 23, 16 to 23, 17 to 23, 18 to 23, 19 to 23, 19 to 22, or 19 to 20 linked nucleosides in length. In certain embodiments, a modified oligonucleotide has at least 1, at least 2, at least 3 mismatches to a region of SEQ ID NO: 1 and 2. In any of the foregoing methods or uses, the compound comprises a first and second modified oligonucleotide, wherein there is a region of complementarity between a first modified oligonucleotide and a second modified oligonucleotide. In certain embodiments, the region of complementarity between the first oligonucleotide and the second oligonucleotide is 14 to 23, 19 to 23, or 21 to 23 linked nucleosides in length. In certain embodiments, the first modified oligonucleotide is fully complementary to the second modified oligonucleotide. In certain embodiments, the first modified oligonucleotide comprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase. In certain embodiments, the second modified oligonucleotide comprises at least one modification selected from the group consisting of a modified internucleoside linkage, a modified sugar, and a modified nucleobase. In certain embodiments, the modified internucleoside linkage is a phosphorothioate internucleoside linkage or a methylphosphonate internucleoside linkage. In certain embodiments, the modified internucleoside linkage is at the 3’ terminus of the first or second modified oligonucleotide or at the 5’ terminus of the first or second modified oligonucleotide. In certain embodiments, the first or second modified oligonucleotide comprises one or more modified sugars. In certain embodiments, each nucleoside of the first or second modified oligonucleotide comprises a modified sugar. In certain embodiments, the modified sugar comprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar. In certain embodiments, the modified sugar comprises a modification selected from group consisting of 2’-MOE, 2’-F, and 2’-OMe or a combination thereof. In certain embodiments, the first or second modified oligonucleotide comprises no more than ten 2’-F sugar modifications. In certain embodiments, the first or second modified oligonucleotide comprises no more than five 2’-F sugar modifications. In any of the foregoing methods or uses, in certain embodiments, a compound comprises a conjugate group. In certain embodiments, the conjugate group is attached to the 5’ end of a modified oligonucleotide. In certain embodiments, the conjugate group is attached to the 3’ end of a modified oligonucleotide. In certain embodiments, a conjugate group is attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more ligands. In certain embodiments, the targeting moiety comprises one or more TrkB ligands. In certain embodiments, the one or more TrkB ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the TrkB ligand is selected from Formulae I- XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX-XXXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the TrkB ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the TrkB ligand through a phosphorothioate group. In certain embodiments, the conjugate group comprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide comprises one or more ligands and one or more lipids. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more lipids are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises one or more TrkB ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more TrkB ligands is attached to the 5’ end of the modified oligonucleotide. In certain embodiments, the one or more TrkB ligands is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more TrkB ligands is attached to the 5’ end and the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl) are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the one or more TrkB ligands is selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV- XXXXXXVII, and XXXXXXIX-XXXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof. In certain embodiments, the one or more TrkB ligands is selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV- XXXXXXVII, and XXXXXXIX-XXXXXXXIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXXV-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In any of the foregoing methods or uses, in certain embodiments, a compound comprises a conjugate group. In certain embodiments, the conjugate group is attached to the 5’ end of a modified oligonucleotide. In certain embodiments, the conjugate group is attached to the 3’ end of a modified oligonucleotide. In certain embodiments, a conjugate group is attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more ligands. In certain embodiments, the targeting moiety comprises one or more CB1 ligands. In certain embodiments, the one or more CB1 ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the CB1 ligand is selected from Formulae XXXXXXI and XXXXXXXIV- XXXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the CB1 ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the CB1 ligand through a phosphorothioate group. In certain embodiments, the conjugate group comprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide comprises one or more ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl) are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises one or more CB1 ligands, and one or more lipids. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more CB1 ligands is attached to the 5’ end of the modified oligonucleotide. In certain embodiments, the one or more CB1 ligands is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more CB1 ligands is attached to the 5’ end and the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipids are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the one or more CB1 ligands is selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof. In certain embodiments, the one or more CB1 ligands is selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXXV-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In any of the foregoing methods or uses, in certain embodiments, a compound comprises a conjugate group. In certain embodiments, the conjugate group is attached to the 5’ end of a modified oligonucleotide. In certain embodiments, the conjugate group is attached to the 3’ end of a modified oligonucleotide. In certain embodiments, a conjugate group is attached to the 5’ end of a modified oligonucleotide, and a conjugate group is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more ligands. In certain embodiments, the targeting moiety comprises one or more α4β1/7 integrin ligands. In certain embodiments, the one or more α4β1/7 integrin ligands is attached at the 5’ or 3’ end of the oligonucleotide or both the 5’ and 3’ ends of the oligonucleotide. In certain embodiments, the α4β1/7 integrin ligand is selected from Formulae XXXXXXII- XXXXXXIV and XXXXXXXIX-XXXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is attached to the α4β1/7 integrin ligand through a phosphodiester group. In certain embodiments, the modified oligonucleotide is attached to the α4β1/7 integrin ligand through a phosphorothioate group. In certain embodiments, the conjugate group comprises one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide comprises one or more ligands and one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl). In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more lipids (e.g., one or more substituted or unsubstituted alkyl or alkenyl) are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises one or more α4β1/7 integrin ligands and one or more lipids. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide. In certain embodiments, the one or more α4β1/7 integrin ligands is attached to the 5’ end of the modified oligonucleotide. In certain embodiments, the one or more α4β1/7 integrin ligands is attached to the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more α4β1/7 integrin ligands is attached to the 5’ end and the 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipids are attached to an internucleoside linkage of the modified oligonucleotide. In certain embodiments, the one or more α4β1/7 integrin ligands is selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI, or a salt, solvate, or hydrate thereof. In certain embodiments, the one or more α4β1/7 integrin ligands is selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide, and the modified oligonucleotide also comprises any one of Formulae XXXV-XXXXXVI, or a salt, solvate, or hydrate thereof, wherein R is the modified oligonucleotide. In any of the foregoing methods or uses, in certain embodiments, a compound comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl are attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C22 hydrocarbon chain. In certain embodiments, a substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide). In certain embodiments, a substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide). In certain embodiments, the internucleoside linkage is between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. In any of the foregoing methods or uses, in certain embodiments, a compound comprises a 5′-phosphonate modification. For example, in certain embodiments, a modified oligonucleotide comprises one or more sugars having a phosphonate modification at the 5′ position. In certain embodiments, the modified oligonucleotide comprises a 5′-phosphonate modification. In certain embodiments, the modified oligonucleotide comprises a 5′-terminal nucleoside (e.g., 5′ terminus) comprising the 5′-phosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification or a 5′- ethylenephosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-vinylphosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′-ethylenephosphonate modification. In certain embodiments, the modified oligonucleotide is the first modified oligonucleotide or antisense oligonucleotide. In any of the foregoing methods or uses, the compound comprises a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs in Table 3 and a second modified oligonucleotide 14 to 23 linked nucleosides in length fully complementary to the first modified oligonucleotide. In certain embodiments, the compound comprises a first modified oligonucleotide selected from any one of the IA Ref ID NOs in Table 3 and a second modified oligonucleotide selected from any one of the IS Ref ID NOs in Table 3. In certain embodiments, the compound is in a pharmaceutically acceptable salt form. In certain embodiments, the pharmaceutically acceptable salt is a sodium salt. In certain embodiments, the pharmaceutically acceptable salt is a potassium salt. In certain embodiments, a composition comprises the compound of any one of the foregoing embodiments and a pharmaceutically acceptable carrier. In any of the foregoing methods or uses, a compound or composition comprising a compound of any preceding embodiment is administered to an individual in a therapeutically effective amount. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual at a dosage level sufficient to deliver about 1 to 100 mg/kg of body weight of the individual. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual at a fixed dose of about 25 mg to about 1,000 mg. In certain embodiments, the composition is administered to the individual one or more times in a day up to the dosage level or fixed dose. In any of the foregoing methods or uses, a compound or composition comprising a compound of any preceding embodiment is administered to an individual daily, weekly, monthly, quarterly or yearly. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual about once per quarter (i.e., once every three months) to about once per year. In certain embodiments, a compound or composition comprising a compound of any preceding embodiment is administered to an individual about once per quarter, about once every six months or about once per year. Certain Compounds In certain aspects, the disclosure relates to a compound that comprises or consists of an oligomeric compound. In certain embodiments, the oligomeric compound comprises a nucleobase sequence complementary to that of a target nucleic acid. In certain aspects, the disclosure relates to a compound that comprises or consists of a modified oligonucleotide. In certain embodiments, the modified oligonucleotide has a nucleobase sequence complementary to that of a target nucleic acid. In certain aspects, the disclosure relates to a compound that comprises or consists of an antisense oligonucleotide. In certain embodiments, the antisense oligonucleotide has a nucleobase sequence complementary to that of a target nucleic acid. In certain aspects, the disclosure relates to a compound that is a single-stranded compound. In certain embodiments, the single-stranded compound comprises or consists of an oligomeric compound. In certain embodiments, such an oligomeric compound comprises or consists of an oligonucleotide and optionally a conjugate group. In certain embodiments, the oligonucleotide is a modified oligonucleotide. In certain embodiments, the oligonucleotide is an antisense oligonucleotide. In certain embodiments, the oligonucleotide or modified oligonucleotide of a single-stranded compound comprises a self-complementary nucleobase sequence. In certain aspects, the disclosure relates to a compound that is a double-stranded compound. In certain embodiments, the double-stranded compound comprises or consists of an oligomeric compound. In certain embodiments, the double-stranded compound comprises a first oligonucleotide and a second oligonucleotide. In certain embodiments, the first oligonucleotide has a region complementarity to a target nucleic acid and the second oligonucleotide has a region complementarity to the first modified oligonucleotide. In certain embodiments, the double-stranded compound comprises a modified oligonucleotide. In certain embodiments, the modified oligonucleotide has a region complementarity to a target nucleic acid. In certain embodiments, the double-stranded compound comprises a first modified oligonucleotide and a second modified oligonucleotide. In certain embodiments, the first modified oligonucleotide has a region complementarity to a target nucleic acid and the second modified oligonucleotide has a region complementarity to the first modified oligonucleotide. In certain embodiments, an oligonucleotide or modified oligonucleotide of a double-stranded compound is an RNA oligonucleotide. In such embodiments, the thymine nucleobase in the modified oligonucleotide is replaced by a uracil nucleobase. In certain embodiments, a compound described herein comprises a conjugate group. In certain embodiments, the first oligonucleotide or first modified oligonucleotide of a double-stranded compound comprises a conjugate group. In certain embodiments, the second oligonucleotide or second modified oligonucleotide of a double-stranded compound comprises a conjugate group. In certain embodiments, a first oligonucleotide or first modified oligonucleotide and a second oligonucleotide or second modified oligonucleotide of a double-stranded compound each comprises a conjugate group. In certain embodiments, a compound is 14-30 linked nucleosides in length. In certain embodiments, the first oligonucleotide or first modified oligonucleotide of a double-stranded compound is 14-30 linked nucleosides in length. In certain embodiments, the second oligonucleotide or second modified oligonucleotide is 14-30 linked nucleosides in length. In certain embodiments, the oligonucleotides or modified oligonucleotides of a double-stranded compound are blunt ended at one or both ends of the compound. In certain embodiments, the oligonucleotides or modified oligonucleotides of a double-stranded compound include non- complementary overhanging nucleosides at one or both ends of the compound. In certain embodiments, a compound has a nucleobase sequence comprising at least 14 contiguous nucleobases of any of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194- 197, 199-200 and 209-217. In certain embodiments, one of the oligonucleotides or modified oligonucleotides of a double-stranded compound has a nucleobase sequence comprising at least 14 contiguous nucleobases of any of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. Examples of single-stranded and double-stranded compounds include, but are not limited to, oligonucleotides, antisense oligonucleotides, siRNAs, microRNA targeting oligonucleotides, occupancy-based compounds (e.g., mRNA processing or translation blocking compounds and splicing compounds), and single-stranded RNAi compounds (e.g. small hairpin RNAs (shRNAs), single stranded siRNAs (ssRNAs) and microRNA mimics). In certain embodiments, a compound described herein has a nucleobase sequence that, when written in the 5’ to 3’ direction, comprises the reverse complement of the target region of a target nucleic acid to which it is targeted. In certain embodiments, a compound described herein comprises an oligonucleotide 12 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 12 to 23 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 14 to 30 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 14 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 15 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 15 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 16 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 16 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 18 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 18 to 23 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 19 to 30 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 19 to 23 linked subunits in length. In other words, such oligonucleotides are 12 to 30 linked subunits, 12 to 23 linked subunits, 14 to 30 linked subunits, 14 to 23 linked subunits, 15 to 30 linked subunits, 15 to 23 linked subunits, 16 to 30 linked subunits, 16 to 23 linked subunits, 17 to 30 linked subunits, 17 to 23 linked subunits, 18 to 30 linked subunits, 18 to 23 linked subunits, 19 to 30 linked subunits or 19 to 23 linked subunits, respectively. In certain embodiments, a compound described herein comprises an oligonucleotide 14 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 16 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 17 linked subunits in length. In certain embodiments, compound described herein comprises an oligonucleotide 18 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 19 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 20 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 21 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 22 linked subunits in length. In certain embodiments, a compound described herein comprises an oligonucleotide 23 linked subunits in length. In other embodiments, a compound described herein comprises an oligonucleotide 8 to 80, 12 to 50, 13 to 30, 13 to 50, 14 to 30, 14 to 50, 15 to 30, 15 to 50, 16 to 30, 16 to 50, 17 to 30, 17 to 50, 18 to 23, 18 to 24, 18 to 25, 18 to 50, 19 to 23, 19 to 30, 19 to 50, 20 to 23 or 20 to 30 linked subunits. In certain such embodiments, the compound described herein comprises an oligonucleotide 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 linked subunits in length, or a range defined by any two of the above values. In certain embodiments, the compound may further comprise an additional moiety, such as a conjugate group or delivery moiety. In certain embodiments, such compounds are oligomeric compounds, and the additional moiety is attached to an oligonucleotide. In certain embodiments, a conjugate group is attached to a nucleoside of an oligonucleotide. In certain embodiments, compounds may be shortened or truncated. For example, one or more subunits may be deleted from the 5’ end (5’ truncation), or alternatively from the 3’ end (3’ truncation) of an oligonucleotide. In certain embodiments, compounds may be lengthened. For example, one or more subunits may be attached to the 3′ end or 5′ end of an oligonucleotide. In certain embodiments, at least one subunit (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits) is attached to the 5′ end of an oligonucleotide. In certain embodiments, at least one subunit (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits) is attached to the 3′ end of an oligonucleotide. In certain embodiments, at least one or more subunits may be attached to the 3′ end or 5′ end of an oligonucleotide of a double-stranded compound creating a 3′ and/or 5′ end overhang. In certain embodiments, at least one subunit (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunits) is attached to the 5′ end of both oligonucleotides of a double-stranded compound. In certain embodiments, at least one subunit (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, or more subunit) is attached to the 3′ end of both oligonucleotides of a double-stranded compound. In certain embodiments, subunits are attached to both oligonucleotides of a double-stranded compound at the same end (e.g., that subunits are attached to the 3′ end of one of the oligonucleotides and subunits are attached to the 5′ end of the other oligonucleotide). In certain embodiments, when subunits are attached to both oligonucleotides of a double-stranded compound at the same end, the number of subunits attached to each oligonucleotide may be the same or may be different. In certain embodiments, when subunits are attached to both oligonucleotides of a double-stranded compound at the same end, the number of subunits attached to each oligonucleotide is the same. In certain embodiments, when subunits are attached to both oligonucleotides of a double-stranded compound at the same end, the number of subunits attached to each oligonucleotide is different. This scenario, where subunits are attached to both oligonucleotides of a double-stranded compound at the same end, may occur at one or both ends of a double-stranded compound. In certain embodiments, the subunits attached to the 3′ and/or 5′ end are modified. In certain embodiments, compounds described herein are oligonucleotides. In certain embodiments, compounds described herein are modified oligonucleotides. In certain embodiments, compounds described herein are antisense oligonucleotides. In certain embodiments, compounds described herein are oligomeric compounds. In certain embodiments, compounds described herein are RNAi compounds. In certain embodiments, compounds described herein are siRNA compounds. In certain embodiments, a compound described herein can comprise any of the oligonucleotide sequences targeted to MAPT described herein. In certain embodiments, the compound can be double-stranded. In certain embodiments, the compound comprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, the compound comprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217. In certain embodiments, the compound comprises a second oligonucleotide. In certain embodiments, the compound comprises an oligonucleotide comprising at least an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 or 23 contiguous nucleobase portion of any one of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206. In certain embodiments, the compound comprises ribonucleotides in which the oligonucleotide has uracil (U) in place of thymine (Τ) for any of the sequences provided here. In certain embodiments, the compound comprises deoxyribonucleotides in which the oligonucleotide has thymine (Τ) in place of uracil (U) for any of the sequences provided here. Certain Mechanisms In certain embodiments, compounds described herein comprise or consist of modified oligonucleotides. In certain embodiments, compounds described herein comprise or consist of antisense oligonucleotides. In certain embodiments, compounds comprise or consist of oligomeric compounds. In certain embodiments, compounds described herein are capable of hybridizing to a target nucleic acid. In certain embodiments, compounds described herein selectively affect one or more target nucleic acid. Such compounds comprise a nucleobase sequence that hybridizes to one or more target nucleic acid, resulting in one or more desired activity and does not hybridize to one or more non-target nucleic acid or does not hybridize to one or more non-target nucleic acid in such a way that results in a significant undesired activity. In certain embodiments, hybridization of a compound described herein to a target nucleic acid results in recruitment of one or more proteins that cause the cleavage of the target nucleic acid. For example, certain compounds described herein or a portion of the compound is loaded into an RNA-induced silencing complex (RISC), ultimately resulting in cleavage of the target nucleic acid. For example, certain compounds described herein result in cleavage of the target nucleic acid by Argonaute. Compounds that are loaded into RISC are RNAi compounds. RNAi compounds may be double-stranded (siRNA) or single-stranded (ssRNA). In certain embodiments, hybridization of compounds described herein to a target nucleic acid does not result in recruitment of a protein that cleaves that target nucleic acid. In certain such embodiments, hybridization of the compound to the target nucleic acid results in the alteration of splicing of the target nucleic acid. In certain embodiments, hybridization of the compound to a target nucleic acid results in inhibition of a binding interaction between the target nucleic acid and a protein or other nucleic acid. In certain such embodiments, hybridization of the compound to the target nucleic acid results in the alteration of RNA processing. In certain such embodiments, hybridization of the compound to a target nucleic acid results in alteration of translation of the target nucleic acid. Activities resulting from the hybridization of a compound to a target nucleic acid may be observed directly or indirectly. In certain embodiments, observation or detection of an activity involves observation or detection of a change in an amount of a target nucleic acid or protein encoded by such target nucleic acid, a change in the ratio of splice variants of a nucleic acid or protein, and/or a phenotypic change in a cell or animal. Certain Modifications In certain aspects, the disclosure relates to compounds that comprise or consist of oligonucleotides. Oligonucleotides consist of linked nucleosides. In certain embodiments, oligonucleotides may be unmodified RNA or DNA or may be modified. In certain embodiments, the oligonucleotides are modified oligonucleotides. In certain embodiments, the modified oligonucleotides comprise at least one modified sugar, modified nucleobase or modified internucleoside linkage relative to an unmodified RNA or DNA. In certain embodiments, an oligonucleotide has a modified nucleoside. A modified nucleoside may comprise a modified sugar, a modified nucleobase or both a modified sugar and a modified nucleobase. Modified oligonucleotides may also include end modifications, e.g., 5’-end modifications and 3’-end modifications. Sugar Modifications and Motifs In certain embodiments, a modified sugar is a substituted furanosyl sugar or non- bicyclic modified sugar. In certain embodiments, a modified sugar is a bicyclic or tricyclic modified sugar. In certain embodiments, a modified sugar is a sugar surrogate. A sugar surrogate may comprise one or more substitutions described herein. In certain embodiments, a modified sugar is a substituted furanosyl or non-bicyclic modified sugar. In certain embodiments, the furanosyl sugar is a ribosyl sugar. In certain embodiments, the furanosyl sugar comprises one or more substituent groups, including, but not limited to, substituent groups at the 2’, 3’, 4’, and 5’ positions. In certain embodiments, substituents at the 2’ position include, but are not limited to, F and OCH3 (“OMe”, “O-methyl” or “methoxy”). In certain embodiments, substituent groups at the 2’ position suitable for non-bicyclic modified sugars include, but are not limited to, halo, allyl, amino, azido, SH, CN, OCN, CF3, OCF3, F, Cl, Br, SCH3, SOCH3, SO2CH3, ΟΝΟ2, ΝΟ2, Ν3, and ΝH2. In certain embodiments, substituent groups at the 2’ position include, but are not limited to, O-(C1-C10) alkoxy, alkoxyalkyl, O-alkyl, S-alkyl, N-alkyl, O- alkenyl, S-alkenyl, N-alkenyl, O-alkynyl, S-alkynyl, N-alkynyl, O-alkyl-O-alkyl, alkynyl, wherein the alkyl, alkenyl and alkynyl can be substituted or unsubstitutedC1 to C10 alkyl or C2 to C10 alkenyl and alkynyl. In certain embodiments, substituent groups at the 2’ position include, but are not limited to, alkaryl, aralkyl, O-alkaryl, and O-aralkyl. In certain embodiments, these 2’ substituent groups can be further substituted with one or more substituent groups independently selected from hydroxyl, alkoxy, carboxy, benzyl, phenyl, nitro (ΝΟ2), thiol, thioalkoxy, thioalkyl, halogen, alkyl, aryl, alkenyl, and alkynyl. In certain embodiments, substituent groups at the 2’ position include, but are not limited to, O[(CH2)nO]mCH3, O(CH2)nOCH3, O(CH2)nCH3, O(CH2)nONH2, O(CH2)nNH2, O(CH2)nSCH3, and O(CH2)nON[(CH2)nCH3)]2, where n and m are independently from 1 to about 10. In certain embodiments, substituent groups at the 2’ position include, but are not limited to, OCH2CH2OCH3 (“MOE”), O(CH2)2ON(CH3)2 (“DMAOE”), O(CH2)2O(CH2)2N(CH3)2 (“DMAEOE”), and OCH2C(=O)-N(H)CH3 (“NMA”). In certain embodiments, substituent groups at the 4’ position suitable for non-bicyclic modified sugars include, but are not limited to, alkoxy (e.g., methoxy), alkyl, and those described in Manoharan et al., WO 2015/106128. In certain embodiments, substituent groups at the 5’ position suitable for non-bicyclic modified sugars include, but are not limited to, methyl (“Me” or “CH3”) (R or S), vinyl, and methoxy. In certain embodiments, the 5' modification is a 5'-monophosphate ((HO)2(O)P-O-5'); 5'-diphosphate ((HO)2(O)P-O- P(HO)(O)-O-5'); 5'-triphosphate ((HO)2(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-guanosine cap (7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O- 5'); 5'adenosine cap (Appp), and any modified or unmodified nucleotide cap structure (N-O- 5'(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-monothiophosphate (phosphorothioate; (HO)2(S)P-O-5'); 5'-monodithiophosphate (phosphorodithioate; (HO)(HS)(S)P-O-5'), 5'phosphorothiolate ((HO)2(O)P-S-5'); any additional combination of oxygen/sulfur replaced monophosphate, diphosphate and triphosphates (e.g. 5'-alpha-thiotriphosphate, 5'- gammathiotriphosphate, etc.), 5'-phosphoramidates ((HO)2(O)P-NH-5', (HO)(NH2)(O)P-O- 5'), 5'alkylphosphonates (R=alkyl=methyl, ethyl, isopropyl, propyl, etc., e.g. RP(OH)(O)-O- 5'-, 5'alkenylphosphonates (i.e. vinyl, substituted vinyl), (OH)2(O)P-5'-CH2-), 5'alkyletherphosphonates (R=alkylether=methoxymethyl (MeOCH2-), ethoxymethyl, etc., e.g. RP(OH)(O)-O-5'-). In certain embodiments, one or more sugars comprise a 5′- phosphonate modification. In certain embodiments, the 5′-phosphonate modification is a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification. In certain embodiments, one or more sugars comprise a 5′-vinylphosphonate modification. In certain embodiments, one or more sugars comprise a 5′-ethylenephosphonate modification. In certain embodiments the 5′ modification is at the terminus of an oligonucleotide. In certain embodiments the 5′ modification is at the terminus of an antisense oligonucleotide. In certain embodiments, substituents described herein for the 2’, 4’ and 5’ position can be added to other specific positions on the sugar. In certain embodiments, such substituents may be added to the 3’ position of the sugar on the 3’ terminal nucleoside or the 5’ position of the 5’ terminal nucleoside. In certain embodiments, a non-bicyclic modified sugar may comprise more than one non-bridging sugar substituent. In certain such embodiments, non-bicyclic modified sugars substituents include, but are not limited to, 5’-Me-2’-F, 5’-Me-2’-OMe (including both R and S isomers). In certain embodiments, modified sugar substituents include those described in Migawa et al., WO 2008/101157 and Rajeev et al., US2013/0203836. In certain embodiments, a modified sugar is a bicyclic sugar. A bicyclic sugar is a modified sugar comprising two rings, wherein the second ring is formed via a bridge connecting two of the atoms in the first ring thereby forming a bicyclic structure. In certain embodiments, a bicyclic sugar comprises a bridging substituent that bridges two atoms of the furanosyl ring to form a second ring. In certain embodiments, a bicyclic sugar does not comprise a furanosyl moiety. A “bicyclic nucleoside” (“BNA”) is a nucleoside having a bicyclic sugar. In certain embodiments, the bicyclic sugar comprises a bridge between the 4’ and 2’ furanose ring atoms. In certain embodiments, the bicyclic sugar comprises a bridge between the 5’ and 3’ furanose ring atoms. In certain such embodiments, the furanose ring is a ribose ring. In certain embodiments, 4’ to 2’ bridging substituents include, but are not limited to, 4'-CH2-2', 4'-(CH2)2-2', 4'- (CH2)3-2', 4'-CH2-O-2' (“LNA”), 4'-CH2-S-2', 4'- (CH2)2-O-2' (“ENA”), 4'-CH(CH3)-O-2' (“constrained ethyl” or “cEt” when in the S configuration), 4’-CH2-O-CH2-2’, 4’-CH2-N(R)-2’, 4'- CH(CH2OCH3)-O-2' (“constrained MOE” or “cMOE”) and analogs thereof (e.g., U.S. Patent No. 7,399,845), 4'-C(CH3)(CH3)- O-2' and analogs thereof (e.g., U.S. Patent No. 8,278,283), 4'-CH2-N(OCH3)-2' and analogs thereof (e.g., U.S. Patent No. 8,278,425), 4'-CH2-O-N(CH3)-2' (e.g., U.S. Patent Publication No. 2004/0171570), 4'-CH2-N(R)-O-2', wherein R is Η, C1-C12 alkyl, or a protecting group (e.g., U.S. Patent No. 7,427,672), 4'-CH2-C(H)(CH3)-2' (e.g., Chattopadhyaya el al., J. Org. Chem., 2009, 74, 118- 134), and 4'-CH2-C(=CH2)-2' and analogs thereof (e.g., U.S. Patent No. 8,278,426). The entire contents of each of the foregoing are hereby incorporated herein by reference. Additional representative U.S. Patents and U.S. Patent Publications that teach the preparation of bicyclic nucleic acid nucleotides include, but are not limited to, the following: U.S. Patent Nos. 6,268,490; 6,525,191; 6,670,461; 6,770,748; 6,794,499; 6,998,484; 7,053,207; 7,034,133; 7,084,125; 7,399,845; 7,427,672; 7,569,686; 7,741,457; 8,022,193; 8,030,467; 8,278,425; 8,278,426; 8,278,283; US 2008/0039618; and US 2009/0012281, US 2013/0190383; and WO 2013/036868, the entire contents of each of which are hereby incorporated herein by reference. Any of the foregoing bicyclic nucleosides can be prepared having one or more stereochemical sugar configurations including for example α-L-ribofuranose and β-D-ribofuranose (see e.g., WO 99/14226). Specified bicyclic nucleosides herein are in the β-D configuration, unless otherwise specified. In certain embodiments, a modified sugar is a sugar surrogate. In certain embodiments, a sugar surrogate has the oxygen atom replaced, e.g., with a sulfur, carbon or nitrogen atom. In certain such embodiments, the sugar surrogate may also comprise bridging and/or non-bridging substituents as described herein. In certain embodiments, sugar surrogates comprise rings having other than 5 atoms. In certain such embodiments, the sugar surrogate comprises a cyclobutyl moiety in place of the pentofuranosyl sugar. In certain embodiments, the sugar surrogate comprises a six membered ring in place of the pentofuranosyl sugar. In certain embodiments, the sugar surrogate comprises a tetrahydropyran (“THP”) in place of the pentofuranosyl sugar. In certain embodiments, the sugar surrogate comprises a morpholino in place of the pentofuranosyl sugar. Representative US patents that teach the preparation of such modified sugar structures include, but are not limited to, U.S. Patent Nos. 4,981,957; 5,118,800; 5,166,315; 5,185,444; 5,319,080; 5,359,044; 5,393,878; 5,446,137; 5,466,786; 5,514,785; 5,519,134; 5,567,811; 5,576,427; 5,591,722; 5,597,909; 5,610,300; 5,627,053; 5,639,873; 5,646,265; 5,658,873; 5,670,633; 5,700,920; 7,875,733; 7,939,677, 8,088,904; 8,440,803; and 9,005,906, the entire contents of each of the foregoing are hereby incorporated herein by reference. In some embodiments, sugar surrogates comprise acyclic moieties. In certain embodiments, the sugar surrogate is an unlocked nucleic acid (“UNA”). A UNA is unlocked acyclic nucleic acid, wherein any of the bonds of the sugar has been removed, forming an unlocked "sugar" residue. In one example, UNA also encompasses a monomer where the bonds between C1’-C4’ have been removed (i.e. the covalent carbon-oxygen-carbon bond between the C1’ and C4’ carbons). In another example, the C2’-C3’ bond (i.e. the covalent carbon-carbon bond between the C2’ and C3’ carbons) of the sugar has been removed. Representative U.S. publications that teach the preparation of UNA include, but are not limited to, U.S. Patent No. 8,314,227; and U.S. Patent Publication Nos. 2013/0096289; 2013/0011922; and 2011/0313020, the entire contents of each of which are hereby incorporated herein by reference. In certain embodiments, sugar surrogates comprise peptide nucleic acid (“PNA”), acyclic butyl nucleic acid (see, e.g., Kumar et al., Org. Biomol. Chem., 2013, 11, 5853-5865), and nucleosides and oligonucleotides described in Manoharan et al., US2013/130378, the entire contents of which is hereby incorporated herein by reference. Many other bicyclic and tricyclic sugar and sugar surrogate ring systems are known in the art that can be used in modified nucleosides. In certain aspects, the disclosure relates to compounds comprising at least one oligonucleotide wherein the nucleosides of such oligonucleotide comprise one or more types of modified sugars and/or unmodified sugars arranged along the oligonucleotide or region thereof in a defined pattern or “sugar motif”. In certain instances, such sugar motifs include, but are not limited to, any of the patterns of sugar modifications described herein. In certain embodiments, an oligonucleotide comprises a gapmer sugar motif. A gapmer oligonucleotide comprises or consists of a region having two external “wing” regions and a central or internal “gap” region. The gap and wing regions form a contiguous sequence of nucleosides, wherein the majority of nucleoside sugars of each of the wings differ from the majority of nucleoside sugars of the gap. In certain embodiments, the wing regions comprise a majority of modified sugars and the gap comprises a majority of unmodified sugars. In certain embodiments, the nucleosides of the gap are deoxynucleosides. Compounds with a gapmer sugar motif are described in, for example US Patent 8,790,919, the entire contents of which is hereby incorporated herein by reference. In certain embodiments, one or both oligonucleotides of a double-stranded compound comprise a triplet sugar motif. An oligonucleotide with a triplet sugar motif comprises three identical sugar modifications on three consecutive nucleosides. In certain embodiments, the triplet is at or near the cleavage site of the oligonucleotide. In certain embodiments, an oligonucleotide of a double-stranded compound may contain more than one triplet sugar motif. In certain embodiments, the identical sugar modification of the triplet sugar motif is a 2’-F modification. Compounds with a triplet sugar motif are disclosed, for example, in US Patent 10,668,170, the entire contents of which is incorporated herein by reference. In certain embodiments, one or both oligonucleotides of a double-stranded compound comprise a quadruplet sugar motif. An oligonucleotide with a quadruplet sugar motif comprises four identical sugar modifications on four consecutive nucleosides. In certain embodiments, the quadruplet is at or near the cleavage site. In certain embodiments, an oligonucleotide of a double-stranded compound may contain more than one quadruplet sugar motif. In certain embodiments, the identical sugar modification of the quadruplet sugar motif is a 2’-F modification. For a double-stranded compound having a duplex region of 19-23 nucleotides in length, the cleavage site of the antisense oligonucleotide is typically around the 10, 11, and 12 positions from the 5’-end. In certain embodiments, the quadruplet sugar motif is at the 8, 9, 10, 11 positions; the 9, 10, 11, 12 positions; the 10, 11, 12, 13 positions; the 11, 12, 13, 14 positions; or the 12, 13, 14, 15 positions of the sense oligonucleotide, counting from the first nucleoside of the 5’-end of the sense oligonucleotide, or, the count starting from the first paired nucleotide within the duplex region from the 5’-end of the sense oligonucleotide. In certain embodiments, the quadruplet sugar motif is at the 8, 9, 10, 11 positions; the 9, 10, 11, 12 positions; the 10, 11, 12, 13 positions; the 11, 12, 13, 14 positions; or the 12, 13, 14, 15 positions of the antisense oligonucleotide, counting from the first nucleoside of the 5’-end of the antisense oligonucleotide, or, the count starting from the first paired nucleotide within the duplex region from the 5’- end of the antisense oligonucleotide. The cleavage site may change according to the length of the duplex region of the double- stranded compound and may change the position of the quadruplet accordingly. In certain embodiments, an oligonucleotide comprises an alternating sugar motif. In certain embodiments, one or both oligonucleotides of a double-stranded compound comprise an alternating sugar motif. An oligonucleotide with an alternating sugar motif comprises at least two different sugar modifications wherein one or more consecutive nucleosides comprising a first sugar modification alternates with one or more consecutive nucleosides comprising a second sugar modification and one or more consecutive nucleosides comprising a third sugar modification, etc. For example, if A, Β and C each represent one type of modification to the nucleoside, the alternating motif can be “ABABABABABAB...,” “AABBAABBAABB...,” “AABAABAABAAB...,” “AAABAAABAAAB...,” “AAABBBAAABBB...,” or “ABCABCABCABC...” etc. In certain embodiments, the alternating sugar motif is repeated for at least 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, or 23 contiguous nucleobases along an oligonucleotide. In certain embodiments, the alternating sugar motif is comprised of two different sugar modifications. In certain embodiments, the alternating sugar motif comprises 2’-OMe and 2’-F sugar modifications. In certain embodiments, each nucleoside of an oligonucleotide is independently modified with one or more sugar modifications provided herein. In certain embodiments, each oligonucleotide of a double-stranded compound independently has one or more sugar motifs provided herein. In certain embodiments, an oligonucleotide containing a sugar motif, is fully modified in that each nucleoside other than the nucleosides comprising the sugar motif comprises a sugar modification. Nucleobase Modifications and Motifs In certain embodiments, compounds described herein comprise modified oligonucleotides. In certain embodiments, modified oligonucleotides comprise one or more nucleosides comprising a modified nucleobase. In certain embodiments, modified oligonucleotides comprise one or more nucleosides that do not comprise a nucleobase, referred to as an abasic nucleoside. In certain embodiments, modified nucleobases are selected from: 5-substituted pyrimidines, 6-azapyrimidines, alkyl or alkynyl substituted pyrimidines, alkyl substituted purines, and Ν-2, N-6 and O-6 substituted purines. In certain embodiments, modified nucleobases are selected from: 2-aminopropyladenine, 5- hydroxymethyl cytosine, 5- methylcytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-N-methylguanine, 6-N- methyladenine, 2-propyladenine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-propynyl (C≡C-CH3) uracil, 5-propynylcytosine, 6-azouracil, 6-azocytosine, 6-azothymine, 5- ribosyluracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl, 8- aza and other 8-substituted purines, 5-halo, particularly, 5-bromo, 5-trifluoromethyl, 5- halouracil, and 5-halocytosine, 7-methylguanine, 7-methyladenine, 2-F-adenine, 2- aminoadenine, 7-deazaguanine, 7-deazaadenine, 3-deazaguanine, 3-deazaadenine, 6-Ν- benzoyladenine, 2-N-isobutyrylguanine, 4-N-benzoylcytosine, 4-N-benzoyluracil, 5-methyl 4-Ν-benzoylcytosine, 5-methyl 4-N-benzoyluracil, universal bases, hydrophobic bases, promiscuous bases, size expanded bases, and fluorinated bases. Further modified nucleobases include tricyclic pyrimidines, such as 1,3-diazaphenoxazine-2-one, 1,3-diazaphenothiazine-2- one, and 9-(2-aminoethoxy)-1,3-diazaphenoxazine-2-one (G-clamp). Modified nucleobases may also include those in which the purine or pyrimidine base is replaced with other heterocycles, for example, 7-deaza-adenine, 7-deazaguanosine, 2-aminopyridine and 2- pyridone. Further nucleobases include those disclosed in U.S. Patent 3,687,808; Modified Nucleosides in Biochemistry, Biotechnology and Medicine, Herdewijn, Ρ. ed. Wiley-VCH, 2008; The Concise Encyclopedia Of Polymer Science And Engineering, pages 858-859; Kroschwitz, J.L., Ed., John Wiley & Sons, 1990, 858-859; Englisch et al., Angewandte Chemie, International Edition, 1991, 30, 613; Sanghvi, Y.S., Chapter 15, dsRNA Research and Applications, pages 289-302; Antisense Research and Applications, Crooke, S.T. and Lebleu, Β., Eds., CRC Press, 1993, 273-288; Antisense Drug Technology, Crooke S.T., Ed., CRC Press, 2008, 163-166 and 442-443 (Chapters 6 and 15), each of which are hereby incorporated herein by reference. Publications that teach the preparation of certain of the above noted modified nucleobases, as well as other modified nucleobases include without limitation, US Application Publication Nos. 2003/0158403 and 2003/0175906; U.S. Patents 4,845,205; 5,130,302; 5,134,066; 5,175,273; 5,367,066; 5,432,272; 5,434,257; 5,457,187; 5,459,255; 5,484,908; 5,502,177; 5,525,711; 5,552,540; 5,587,469; 5,594,121; 5,596,091; 5,614,617; 5,645,985; 5,681,941; 5,811,534; 5,750,692; 5,948,903; 5,587,470; 5,457,191; 5,763,588; 5,830,653; 5,808,027; 6,005,096. 6,015,886; 6,147,200; 6,166,197; 6,166,199; 6,222,025; 6,235,887; 6,380,368; 6,528,640; 6,639,062; 6,617,438; 7,045,610; 7,427,672; and 7,495,088, the entire contents of each of which are hereby incorporated herein by reference. In certain embodiments, compounds described herein comprise oligonucleotides. In certain embodiments, oligonucleotides comprise modified and/or unmodified nucleobases arranged along the oligonucleotide or region thereof in a defined pattern or motif. In certain embodiments, each nucleobase is modified. In certain embodiments, none of the nucleobases are modified. In certain embodiments, each purine or each pyrimidine is modified. In certain embodiments, each adenine is modified. In certain embodiments, each guanine is modified. In certain embodiments, each thymine is modified. In certain embodiments, each uracil is modified. In certain embodiments, each cytosine is modified. In certain embodiments, some or all of the cytosine nucleobases in a modified oligonucleotide are 5-methylcytosines. In certain embodiments, modified oligonucleotides comprise a block of modified nucleobases. In certain such embodiments, the block is at the 3’-end of the oligonucleotide. In certain embodiments, the block is within 3 nucleosides of the 3’-end of the oligonucleotide. In certain embodiments, the block is at the 5’-end of the oligonucleotide. In certain embodiments, the block is within 3 nucleosides of the 5’-end of the oligonucleotide. Internucleoside Linkage Modifications and Motifs A 3' to 5' phosphodiester linkage is the naturally occurring internucleoside linkage of RNA and DNA. In certain embodiments, compounds described herein have one or more modified, i.e. non-naturally occurring, internucleoside linkages. Certain non-naturally occurring internucleoside linkages may impart desirable properties such as, for example, enhanced cellular uptake, enhanced affinity for target nucleic acids, and increased stability in the presence of nucleases. Representative phosphorus-containing modified internucleoside linkages include, but are not limited to, phosphotriesters, alkylphosphonates (e.g. methylphosphonates), phosphoramidates, and phosphorothioates (“P=S”), and phosphorodithioates (“HS-P=S”). Representative non-phosphorus containing internucleoside linking groups include, but are not limited to, methylenemethylimino (-CH2-N(CH3)-O-CH2), thiodiester, thionocarbamate (-O-C(=O)(NH)-S-); siloxane (-O-SiH2-O-); and N,N'- dimethylhydrazine (-CH2-Ν((CΗ3)-Ν((CΗ3)-). Methods of preparation of phosphorous- containing and non-phosphorous-containing internucleoside linkages are well known to those skilled in the art. Neutral internucleoside linkages include, without limitation, phosphotriesters, methylphosphonates, MMI (3'-CH2-N(CH3)-O-5'), amide-3 (3'-CH2-C(=O)- N(H)-5'), amide-4 (3'-CH2-N(H)-C(=O)-5'), formacetal (3'-O-CH2-O-5'), methoxypropyl, and thioformacetal (3'-S-CH2-O-5'). Further neutral internucleoside linkages include nonionic linkages comprising siloxane (dialkylsiloxane), carboxylate ester, carboxamide, sulfide, sulfonate ester and amides (See, for example: Carbohydrate Modifications in Antisense Research; Y.S. Sanghvi and P.D. Cook, Eds., ACS Symposium Series 580; Chapters 3 and 4, 40-65). Further neutral internucleoside linkages include nonionic linkages comprising mixed Ν, O, S and CH2 component parts. In certain embodiments, compounds provided herein comprise at least one modified internucleoside linkage. A modified internucleoside linkage may be placed at any position of an oligonucleotide. For double-stranded compounds, a modified internucleoside linkage may be placed within the sense oligonucleotide, antisense oligonucleotide, or both oligonucleotides of the double-stranded compound. In certain embodiments, the internucleoside linkage modification may occur on every nucleoside of an oligonucleotide. In certain embodiments, internucleoside linkage modifications may occur in an alternating pattern along an oligonucleotide. In certain embodiments, essentially each internucleoside linking group is a phosphate internucleoside linkage (Ρ=O). In certain embodiments, each internucleoside linking group of a modified oligonucleotide is a phosphorothioate (P=S). In certain embodiments, each internucleoside linking group of a modified oligonucleotide is independently selected from a phosphorothioate and phosphate internucleoside linkage. In certain embodiments, the pattern of the internucleoside linkage modification on each oligonucleotide of a double-stranded compound is the same. In certain embodiments, the pattern of the internucleoside linkage modification on each oligonucleotide of a double-stranded compound is different. In certain embodiments, a double-stranded compound comprises 6-8 modified internucleoside linkages. In certain embodiments, the 6-8 modified internucleoside linkages are phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages. In certain embodiments, the sense oligonucleotide comprises at least two modified internucleoside linkages at either or both the 5’-end and the 3’-end. In certain such embodiments, the modified internucleoside linkages are phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages. In certain embodiments, the antisense oligonucleotide comprises at least two modified internucleoside linkages at either or both the 5’-end and the 3’-end. In certain such embodiments, the modified internucleoside linkages are phosphorothioate internucleoside linkages or alkylphosphonate internucleoside linkages. In certain embodiments, a double-stranded compound comprises an overhang region. In certain embodiments, a double-stranded compound comprises a phosphorothioate or alkylphosphonate internucleoside linkage modification in the overhang region. In certain embodiments, a double-stranded compound comprises a phosphorothioate or alkylphosphonate internucleotide linkage linking the overhang nucleotide with a paired nucleotide that is next to the overhang nucleotide. For instance, there may be at least two phosphorothioate internucleoside linkages between the terminal three nucleosides, in which two of the three nucleosides are overhang nucleosides, and the third is a paired nucleoside next to the overhang nucleoside. These terminal three nucleosides may be at the 3’-end of the antisense oligonucleotide, the 3’-end of the sense oligonucleotide, the 5’-end of the antisense oligonucleotide, or the 5’end of the antisense oligonucleotide. In certain embodiments, modified oligonucleotides comprise one or more internucleoside linkages having chiral centers. Representative chiral internucleoside linkages include, but are not limited to, alkylphosphonates and phosphorothioates. Modified oligonucleotides comprising internucleoside linkages having chiral centers can be prepared as populations of modified oligonucleotides comprising stereorandom internucleoside linkages, or as populations of modified oligonucleotides comprising phosphorothioate linkages in particular stereochemical configurations. In certain embodiments, populations of modified oligonucleotides comprise phosphorothioate internucleoside linkages wherein all of the phosphorothioate internucleoside linkages are stereorandom. Such modified oligonucleotides can be generated using synthetic methods that result in random selection of the stereochemical configuration of each phosphorothioate linkage. As is well understood by those of skill in the art, each individual phosphorothioate of each individual oligonucleotide molecule has a defined stereoconfiguration. In certain embodiments, populations of modified oligonucleotides are enriched for modified oligonucleotides comprising one or more particular phosphorothioate internucleoside linkages in a particular, independently selected stereochemical configuration. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 65% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 70% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 80% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 90% of the molecules in the population. In certain embodiments, the particular configuration of the particular phosphorothioate linkage is present in at least 99% of the molecules in the population. Such enriched populations of modified oligonucleotides can be generated using synthetic methods known in the art, e.g., methods described in Oka et al., JACS 125, 8307 (2003), Wan et al. Nuc. Acid. Res. 42, 13456 (2014), and WO 2017/015555. In certain embodiments, a population of modified oligonucleotides is enriched for modified oligonucleotides having at least one indicated phosphorothioate in the (Sp) configuration. In certain embodiments, a population of modified oligonucleotides is enriched for modified oligonucleotides having at least one phosphorothioate in the (Rp) configuration. Conjugate Groups In certain embodiments, the compounds described herein comprise or consist of one or more oligonucleotides and, optionally, one or more conjugate groups. Conjugate groups may be attached to either or both ends of an oligonucleotide and/or at any internal position. In certain embodiments, a conjugate group is attached at the 3’ end of an oligonucleotide. In certain embodiments, a conjugate group is attached at the 5’ end of an oligonucleotide. In certain embodiments, oligonucleotides are covalently attached to one or more conjugate groups. In certain embodiments, conjugate groups are terminal groups attached to either or both ends of an oligonucleotide. In certain such embodiments, terminal groups are attached at the 3’ end of an oligonucleotide. In certain such embodiments, terminal groups are attached at the 5’ end of an oligonucleotide. In certain embodiments, terminal groups include, but are not limited to, capping groups, phosphate moieties, protecting groups, modified or unmodified nucleosides, and two or more nucleosides that are independently modified or unmodified, such as an overhang. In certain embodiments, conjugate groups modify one or more properties of the attached oligonucleotide, including, but not limited to, pharmacodynamics, pharmacokinetics, stability, activity, half-life, binding, absorption, tissue distribution, cellular distribution, cellular uptake, charge and clearance. In certain embodiments, conjugate groups enhance the affinity of a compound for a selected target, e.g., molecule, cell or cell type, compartment, e.g., a cellular or organ compartment, tissue, organ or region of the body, as, e.g., compared to a compound absent such a conjugate group. In certain embodiments, conjugate groups impart a new property on the attached oligonucleotide, e.g., fluorophores or reporter groups that enable detection of the oligonucleotide. In certain embodiments, conjugate groups include, but are not limited to, intercalators, reporter molecules, polyamines, polyamides, peptides, carbohydrates, vitamin moieties, polyethylene glycols, thioethers, polyethers, cholesterols, thiocholesterols, cholic acid moieties, folate, lipids, phospholipids, biotin, phenazine, phenanthridine, anthraquinone, adamantane, acridine, fluoresceins, rhodamines, coumarins, fluorophores, and dyes. In certain embodiments, conjugate groups include an active drug substance, for example, aspirin, warfarin, phenylbutazone, ibuprofen, suprofen, fen-bufen, ketoprofen, (S)- (+)-pranoprofen, carprofen, dansylsarcosine, 2,3,5-triiodobenzoic acid, fingolimod, flufenamic acid, folinic acid, a benzothiadiazide, chlorothiazide, a diazepine, indo-methicin, a barbiturate, a cephalosporin, a sulfa drug, an antidiabetic, an antibacterial, or an antibiotic. In certain embodiments, conjugate groups are targeting moieties. In certain embodiments, a targeting moiety includes, but is not limited to, a lectin, glycoprotein, lipid, protein, peptide, peptide mimetic, receptor ligand, antibody, thyrotropin, melanotropin, surfactant protein A, carbohydrate, carbohydrate derivative, modified carbohydrate, carbohydrate cluster, polysaccharide, modified polysaccharide, or polysaccharide derivative, mucin carbohydrate, multivalent lactose, multivalent galactose, N-acetyl-galactosamine (GalNAc), N-acetylglucosamine multivalent mannose, multivalent fucose, glycosylated polyaminoacids, multivalent galactose, transferrin, bisphosphonate, polyglutamate, polyaspartate, a lipid, cholesterol, a steroid, bile acid, folate, vitamin Β12, vitamin A, biotin, or an RGD peptide or RGD peptide mimetic. In certain embodiments, conjugate groups may include, but are not limited to, the conjugate groups described in the following references such as cholesterol (e.g., Letsinger et al., Proc. Natl. Acid. Sci. USA, 1989, 86: 6553-6556), cholic acid (e.g., Manoharan et al., Biorg. Med. Chem. Let., 1994, 4:1053-1060), thioether, e.g., hexyl-S-tritylthiol (e.g., Manoharan et al., Αnn. NY. Acad. Sci., 1992, 660:306-309; Manoharan et al., Biorg. Med. Chem. Let., 1993, 3:2765-2770), thiocholesterol (e.g., Oberhauser et al., Nucl. Acids Res., 1992, 20:533-538), aliphatic chains, e.g., do-decan-diol or undecyl residues (e.g., Saison- Behmoaras et al., ΕΜΒΟ J, 1991, 10:1111-1118; Kabanov et al., FEBS Lett., 1990, 259:327- 330; Svinarchuk et al., Biochimie, 1993, 75:49-54), phospholipids, e.g., di-hexadecyl-rac- glycerol or triethyl-ammonium 1,2-di-O-hexadecyl-rac-glycero-3-H-phosphonate (e.g, Manoharan et al., Tetrahedron Lett., 1995, 36:3651-3654; Shea et al., Nucl. Acids Res., 1990, 18:3777-3783), polyamines or a polyethylene glycol chains (e.g., Manoharan et al., Nucleosides & Nucleotides, 1995, 14:969-973), adamantane acetic acid (e.g., Manoharan et al., Tetrahedron Lett., 1995, 36:3651-3654), palmityl (e.g., Mishra et al., Biochim. Biophys. Acta, 1995, 1264:229-237), octadecylamine or hexylamino-carbonyloxychole sterol moiety (e.g., Crooke et al. J. Pharmacol. Exp. Ther., 1996, 277:923-937), tocopherol (e.g., Nishina et al., Molecular Therapy Nucleic Acids, 2015, 4, e220 and Nishina et al., Molecular Therapy, 2008, 16:734-740), GalNAc and other carbohydrates (e.g., Maier et al., Bioconjugate Chemistry, 2003, 14, 18-29; Rensen et al., J. Med. Chem. 2004, 47, 5798-5808; WO2009/073809 and US Patents 8,106,022; 8,450,467 and 8,828,957; and WO2014/179445; WO2014/179620 and US Patents 9,127,276; 9,181,549 and 10,844,379) each of which is incorporated herein by reference in its entirety. Conjugate groups may be attached to oligonucleotides through conjugate linkers. In certain embodiments, a conjugate linker comprises a chain structure, such as a hydrocarbyl chain, or an oligomer of repeating units or combination of such repeating units. In certain embodiments, a conjugate linker comprises one or more groups selected from alkyl, amino, οxο, amide, disulfide, polyethylene glycol, ether, thioether, and hydroxylamino. In certain embodiments, a conjugate linker comprises at least one phosphorus group. In certain embodiments, a conjugate linker comprises at least one phosphate group. In certain embodiments, a conjugate linker includes at least one neutral linking group. In certain embodiments, conjugate linkers include, but are not limited to, pyrrolidine, 8-amino-3,6- dioxaoctanoic acid (ADO), succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC) and 6-aminohexanoic acid (ΑΗΕΧ or AHA). Other conjugate linkers include, but are not limited to, substituted or unsubstituted C1-C10 alkyl, substituted or unsubstituted C2- C10 alkenyl, or substituted or unsubstituted C2-C10 alkynyl, wherein a nonlimiting list of preferred substituent groups includes hydroxyl, amino, alkoxy, carboxy, benzyl, phenyl, nitro, thiol, thioalkoxy, halogen, alkyl, aryl, alkenyl, and alkynyl. In certain embodiments, conjugate linkers comprise 1-10 linker-nucleosides. In certain embodiments, such linker- nucleosides may be modified or unmodified nucleosides. It is typically desirable for linker- nucleosides to be cleaved from the compound after it reaches a target tissue. Accordingly, linker-nucleosides herein can be linked to one another and to the remainder of the compound through cleavable bonds. Herein, linker-nucleosides are not considered to be part of the oligonucleotide. Accordingly, in embodiments in which a compound comprises an oligonucleotide consisting of a specified number or range of linked nucleosides and/or a specified percent complementarity to a reference nucleic acid and the compound also comprises a conjugate group comprising a conjugate linker comprising linker-nucleosides, those linker-nucleosides are not counted toward the length of the oligonucleotide and are not used in determining the percent complementarity of the oligonucleotide for the reference nucleic acid. In certain embodiments, conjugate groups and conjugate linkers as well as other modifications include, without limitation, those described in the following references: US 5,994,517; US 6,300,319; US 6,660,720; US 6,906,182; US 7,262,177; US 7,491,805; US 8,106,022; US 7,723,509; US 9,127,276; US 2006/0148740; US 2011/0123520; WO2013/033230; WO2012/037254, Biessen et al., J. Med. Chem. 1995, 38, 1846-1852; Lee et al., Bioorganic & Medicinal Chemistry 2011,19, 2494-2500; Rensen et al., J. Biol. Chem. 2001, 276, 37577-37584; Rensen et al., J. Med. Chem. 2004, 47, 5798-5808; Sliedregt et al., J. Med. Chem. 1999, 42, 609-618; Valentijn et al., Tetrahedron, 1997, 53, 759-770; Lee, Carhohydr Res, 1978, 67, 509-514; Connolly et al., J Biol Chem, 1982, 257, 939-945; Pavia et al., Int J Pep Protein Res, 1983, 22, 539-548; Lee et al., Biochem, 1984, 23, 4255-4261; Lee et al., Glycoconjugate J, 1987, 4, 317-328; Toyokuni et al., Tetrahedron Lett, 1990, 31, 2673-2676; Biessen et al., J Med Chem, 1995, 38, 1538-1546; Valentijn et al., Tetrahedron, 1997, 53, 759-770; Kim et al., Tetrahedron Lett, 1997, 38, 3487-3490; Lee et al., Bioconjug Chem, 1997, 8, 762-765; Kato et al., Glycohiol, 2001, 11, 821-829; Rensen et al., J Biol Chem, 2001, 276, 37577-37584; Lee et al., Methods Enzymol, 2003, 362, 38-43; Westerlind et al., Glycoconj J, 2004, 21, 227-241; Lee et al., Bioorg Med Chem Lett, 2006, 16(19), 5132-5135; Maierhofer et al., Bioorg Med Chem, 2007, 15, 7661-7676; Khorev et al., Bioorg Med Chem, 2008, 16, 5216-5231; Lee et al., Bioorg Med Chem, 2011, 19, 2494-2500; Kornilova et al., Analyt Biochem, 2012, 425, 43-46; Pujol et al., Angew Chemie Int Ed Engl, 2012, 51, 7445-7448; Biessen et al., J Med Chem, 1995, 38, 1846-1852; Sliedregt et al., J Med Chem, 1999, 42, 609-618; Rensen et al., J Med Chem, 2004, 47, 5798-5808; Rensen et al., Arterioscler Thromh Vase Biol, 2006, 26, 169-175; van Rossenberg et al., Gene Ther, 2004, 11, 457-464; Sato et al., JAm Chem Soc, 2004, 126, 14013-14022; Lee et al., J Org Chem, 2012, 77, 7564-7571; Biessen et al., FASEB J, 2000, 14, 1784-1792; Rajur et al., Bioconjug Chem, 1997, 8, 935-940; Duff et al., Methods Enzymol, 2000, 313, 297-321; Maier et al., Bioconjug Chem, 2003, 14, 18-29; Jayaprakash et al., Org Lett, 2010, 12, 5410- 5413; Manoharan, Antisense Nucleic Acid Drug Dev, 2002, 12, 103-128; Merwin et al., Bioconjug Chem, 1994, 5, 612-620; Tomiya et al., Bioorg Med Chem, 2013, 21, 5275-5281; International applications WO1998/013381; WO2011/038356; WO1997/046098; W02008/098788; W02004/101619; WO2012/037254; WO2011/120053; WO2011/100131; WO2011/163121; WO2012/177947; W02013/033230; W02013/075035; WO2012/083185; WO2012/083046; W02009/082607; WO2009/134487; W02010/144740; W02010/148013; WO1997/020563; W02010/088537; W02002/043771; W02010/129709; WO2012/068187; WO2009/126933; W02004/024757; WO2010/054406; WO2012/089352; WO2012/089602; WO2013/166121; WO2013/165816; U.S. Patents 4,751,219; 7,582,744; 8,552,163; 8,137,695; 6,908,903; 6,383,812; 7,262,177; 6,525,031; 5,994,517; 6,660,720; 6,300,319; 7,723,509; 8,106,022; 7,491,805; 7,491,805; 8,541,548; 8,344,125; 8,313,772; 8,349,308; 8,450,467; 8,501,930; 8,158,601; 7,262,177; 6,906,182; 6,620,916; 8,435,491; 8,404,862; 7,851,615; Published U.S. Patent Application Publications US2011/0097264; US2011/0097265; US2013/0004427; US2003/0119724; US2011/0207799; US2012/0035115; US2012/0230938; US2005/0164235; US2006/0183886; US2012/0136042; US2012/0095075; US2013/0109817; US2006/0148740; US2008/0206869; US2012/0165393; US2012/0101148; US2013/0121954; US2011/0123520; US2003/0077829; US2008/0108801; and US2009/0203132; each of which is incorporated herein by reference in its entirety. Certain Conjugate Groups In certain embodiments, a compound provided herein comprises a conjugate group. In certain embodiments, an oligonucleotide provided herein comprises a conjugate group. In certain embodiments, the conjugate group is a targeting moiety. In certain embodiments, the targeting moiety comprises one or more TrkB ligands. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (I) or a salt, solvate, or hydrate thereof: Formula (I), wherein: R1 is the modified oligonucleotide; L1, L2, L3, and L4 are as described herein; R2 is hydrogen, -OR7, -SR8, or -NR9R10; R3 is hydrogen, -OR11, -SR12, or -NR13R14; R4 is hydrogen, -OR15, -SR16, or -NR17R18; R5 is hydrogen, -OR19, -SR20, or -NR21R22; R6 is hydrogen, -OH, optionally substituted -O-alkyl, optionally substituted -OAc, - NH2, optionally substituted -NHAc, -SH, or =O; R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, and R22 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, optionally substituted heteroaryl; Y is CH2, NH, S, or O; and Z is optionally substituted aryl or optionally substituted heteroaryl. In certain embodiments, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, and R22 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, R7, R8, R9, and R10 are each independently alkenyl. In certain embodiments, R7, R8, R9, and R10 are each independently alkynyl. In certain embodiments, R2 is OR7. In certain embodiments, R3 is OR11. In certain embodiments, R7 and R11 are each independently hydrogen, optionally substituted alkyl or optionally substituted alkenyl. In certain embodiments, one or both R7 and R11 are each independently hydrogen. In certain embodiments, one or both R7 and R11 are each independently optionally substituted alkyl. In certain embodiments, one or both R7 and R11 are each independently optionally substituted unsaturated or partially unsaturated alkyl. In certain embodiments, one or both R7 and R11 are each independently alkenyl. In certain embodiments, R7 is optionally substituted alkyl and R11 is hydrogen. In certain embodiments, R7 is hydrogen and R11 is optionally substituted alkyl. In certain embodiments, R7 is alkenyl and R11 is hydrogen. In certain embodiments, R7 is hydrogen and R11 is optionally substituted alkenyl. In certain embodiments, the TrkB ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (II-A),
Formula (II-C), wherein: R1 is the modified oligonucleotide; L1, L2, L3, L4, and R1 are as described herein. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXVII) or a salt, solvate, or hydrate thereof: wherein: L1, L2, L3, L4, and R1 are as described herein; R11 and R13 are each independently absent, hydrogen, or optionally substituted alkyl; R12, R14, and R15 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R16 is hydrogen, halogen, –CN, –N3, –SOn16R1A, –SOv16NR16BR16C, −NHNR16BR16C, −ONR16BR16C, −NHC(O)NHNR16BR16C, −NHC(O)NR16BR16C, –N(O)m16, –NR16BR16C, –C(O)R16D, –C(O)OR16D, –C(O)NR16BR16C, –OR16A, -NR16BSO2R16A, -NR16BC(O)R16D, - NR16BC(O)OR16D, –NR16BOR16D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; and are each independently a single bond or a double bond, wherein if is a single bond, then is a double bond and R13 is absent; and further wherein if is a single bond, then is a double bond and R11 is absent; R16A, R16B, R16C, R16D are each independently hydrogen, halogen, –CF3, –CCl3, –CBr3, –Cl3, –COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R16B and R16C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; z3 is 0, 1, 2, 3, 4, or 5; n16 is 0, 1, 2, 3, or 4; and v16 and m16 are each independently 1 or 2. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXIX) or a salt, solvate, or hydrate thereof: Formula (XXXXXIX), wherein: L1, L2, L3, L4, and R1 are as described herein; R17, R18, and R19 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; z4 is 0, 1, or 2; and z5 is 0, 1, 2, or 3. In certain embodiments, the TrkB ligand of a modified oligonucleotide is of the Formula (XXXXXX) or a salt, solvate, or hydrate thereof: Formula (XXXXXX), wherein: L1, L2, L3, L4, and R1 are as described herein; R20 is hydrogen, halogen, –CN, –N3, –SOn20R1A, –SOv20NR20BR20C, −NHNR20BR20C, −ONR20BR20C, −NHC(O)NHNR20BR20C, −NHC(O)NR20BR20C, –N(O)m20, –NR20BR20C, –C(O)R20D, –C(O)OR20D, –C(O)NR20BR20C, –OR20A, -NR20BSO2R20A, -NR20BC(O)R20D; -NR20BC(O)OR20D, –NR20BOR20D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R21 is hydrogen, halogen, –CN, –N3, –SOn21R1A, –SOv21NR21BR21C, −NHNR21BR21C, −ONR21BR21C, −NHC(O)NHNR21BR21C, −NHC(O)NR21BR21C, –N(O)m21, –NR21BR21C, –C(O)R21D, –C(O)OR21D, –C(O)NR21BR21C, –OR21A, -NR21BSO2R21A, -NR21BC(O)R21D; -NR21BC(O)OR21D, –NR21BOR21D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R22 and R23 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R24 is hydrogen, halogen, –CN, –N3, –SOn24R24A, –SOv24NR24BR24C, −NHNR24BR24C, −ONR24BR24C, −NHC(O)NHNR24BR24C, −NHC(O)NR24BR24C, –N(O)m24, –NR24BR24C, –C(O)R24D, –C(O)OR24D, –C(O)NR24BR24C, –OR24A, -NR24BSO2R24A, -NR24BC(O)R24D; -NR24BC(O)OR24D, –NR24BOR24D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R20A, R20B, R20C, R20D, R21A, R21B, R21C, R21D, R24A, R24B, R24C, and R24D are each independently hydrogen, halogen, –CF3, –CCl3, –CBr3, –Cl3,–COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R20B, R20C, R21B, R21C, R24B, R24C, R24B, and R24C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; n21, n22, n24, z6 and z8 are each independently 0, 1, 2, 3, or 4; v20, v21, v24, m20, m21, and m24 are each independently 1 or 2; and z7 is 0, 1, or 2. In certain embodiments, the CB1 ligand of a modified oligonucleotide is of the Formula (XXXXXXI) or a salt, solvate, or hydrate thereof: , Formula (XXXXXXI) wherein: L1, L2, L3, L4, and R1 are as described herein; X1 is NR10 or CR11R12; R10, R11, and R12 are each independently hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R19 is hydrogen, –SOn19R19A, –SOv19NR19BR19C, −NHNR19BR19C, −ONR19BR19C, −NHC(O)NHNR19BR19C, −NHC(O)NR19BR19C, –NR19BR19C, –C(O)R19D, –C(O)OR19D, – C(O)NR19BR19C, –OR19A, –NR19BSO2R19A, –NR19BC(O)R19D, -NR19BC(O)OR19D, – NR19BOR19D, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R19A, R19B, R19C, R19D are each independently hydrogen, halogen, –CF3, –CCI3, –CBr3, –CI3, –COOH, –CONH2, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; wherein R19B and R19C substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; n19 is 0, 1, 2, 3, or 4; and v19 is 1 or 2. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXII) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, polyethylene glycol (PEG), optionally substituted heteroalkyl, or optionally substituted heteroaryl; and R3, and R4 are each independently H, halogen, optionally substituted alkyl, or optionally substituted -O-alkyl. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXIII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXIII) wherein L1, L2, L3, L4, and R1 are as described herein; R2, R3, R4, and R5 are each independently H, halogen, optionally substituted alkyl, optionally substituted -O-alkyl, cycloalkyl, or absent; R8 is optionally substituted C1-C5 alkyl, optionally substituted C1-C5 alkylene-(C3- C6)-cycloalkyl, or optionally substituted (C1-C4)-alkylene-(C1-C4)-alkoxy; and R6, and R7 are each independently H, halogen, alkyl, or optionally substituted alkyl, optionally substituted heteroalkyl, , , , In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXIV) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXIV) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, -CONHR4, -CH2OR4, -(CH2)2OR4, -CH2NHCOR4, or -OR4; R3 is H, optionally substituted alkyl, or optionally substituted cycloalkyl; R4 is H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; R5 is -OH or absent; and X is H, optionally substituted CH2, optionally substituted NH, or cycloalkyl. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is of the Formula (XXXXXXXXIII) or a salt, solvate, or hydrate thereof: . Formula (XXXXXXXXIII) wherein L1, L2, L3, L4, and R1 are as described herein; R2 is H, -CONHR3, -CH2OR3, -(CH2)2OR3, -CH2NHCOR3, or -OR3; each instance of R3 is independently H, polyethylene glycol, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted cycloalkyl, or optionally substituted heteroaryl; and X is H or halogen. In certain embodiments, L1, L2, L3, and L4 are each independently absent, a bond, an optionally substituted alkyl linker, an optionally substituted polyethylene glycol (PEG) linker, an optionally substituted heteroalkyl linker, or an optionally substituted heteroaryl linker. In certain embodiments, L1 is an optionally substituted heteroaryl linker. In certain embodiments, L1 is an optionally substituted unsaturated heteroaryl, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker. In certain embodiments, L1 comprises the structure: . In certain embodiments, L1 is an optionally substituted heteroalkyl linker. In certain embodiments, the optionally substituted heteroalkyl linker is an optionally substituted heteroalkyl or optionally substituted C1-C10 alkyl chain in which one or more carbon atoms are replaced with O, N, or S. In certain embodiments, L1 comprises the structure: o . In certain embodiments, L1 comprises the structure: –N(CH3)–. In certain embodiments, L2 is an optionally substituted PEG linker. In certain embodiments, the PEG linker is five PEG units in length. In certain embodiments, the PEG linker is four PEG units in length. In certain embodiments, the PEG linker is three PEG units in length. In certain embodiments, L2 is an optionally substituted alkyl linker. In certain embodiments, L2 is an optionally substituted C1-20 alkyl linker. In certain embodiments, L2 is an optionally substituted C8 alkyl linker. In certain embodiments, L3 is an optionally substituted heteroaryl linker. In certain embodiments, L3 is an optionally substituted partially unsaturated heteroaryl linker, an optionally substituted heteroaryl or an optionally substituted saturated or partially unsaturated heterocycloalkyl linker. In certain embodiments, L3 comprises the structure: . In certain embodiments, L4 is an optionally substituted heteroalkyl linker. In certain embodiments, the heteroalkyl linker is substituted with one or more =O substituents. In certain embodiments, the heteroalkyl linker comprises two substituents joined together to form an optionally substituted carbocyclyl ring. In certain embodiments, L4 comprises the structure: salt thereof, wherein X is O or S. In certain embodiments, L4 comprises the structure: or a salt thereof, wherein X is O or S. In certain embodiments, L1– L2–L3–L4 comprises the structure: ,
, , , wherein X is O or S. In certain embodiments, the TrkB ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (IV),
Formula (XXXXXXXXV) wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, the CB1 ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXXVI)
Formula (XXXXXXXVIII) wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, the α4β1/7 integrin ligand of a modified oligonucleotide is selected from the following Formulae or a salt, solvate, or hydrate thereof: Formula (XXXXXXXIX)
Formula (XXXXXXXXIV) wherein: R is the modified oligonucleotide; and X is S or O. In certain embodiments, a compound provided herein comprises a conjugate group. In certain embodiments, an oligonucleotide provided herein comprises a conjugate group. In certain embodiments, the conjugate group is a lipid. In certain embodiments, an internucleoside linkage of a modified oligonucleotide provided herein comprises one or more lipids. In certain embodiments, the modified oligonucleotide comprises Formula (XXXV), or a salt solvate, or hydrate thereof: Formula (XXXV), wherein: Y is –C(=O)N(RC)–, or –N(RC)C(=O)–; Q1 and Q3 are each independently –H, –OR4, a ligand, a linker, or a lipid; Q2 and Q4 are each independently a bond, , a ligand, a linker, or a lipid; RC is independently –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently an substituted or unsubstituted heteroaryl; each R9 is independently an substituted or unsubstituted heteroaryl; each instance of Z1 or Z2 is independently a bond, C1-C6 alkylene, or C2-C6 alkenylene; and each X is independently O or S; or a salt thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVI), or a salt solvate, or hydrate thereof:
Formula (XXXVI), wherein: RC is –H, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVII), or a salt solvate, or hydrate thereof:
Formula (XXXVI), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXVIII), or a salt, solvate, or hydrate thereof:
Formula (XXXVIII), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXIX), or a salt, solvate, or hydrate thereof:
Formula (XXXIX), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXX), or a salt solvate, or hydrate thereof:
Formula (XXXX), wherein: each R2 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR6, -N(R6), or -SR6; each R3 is independently –H, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, –OR7, -N(R7), or -SR7; R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; each R6 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R7 is independently hydrogen, substituted or unsubstituted alkyl, or substituted or unsubstituted heteroalkyl; each R8 is independently a substituted or unsubstituted heteroaryl ring; each R9 is independently a substituted or unsubstituted heteroaryl ring; and each X is independently O or S; or a salt or prodrug thereof. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXI), or a salt solvate, or hydrate thereof:
mUëmU Formula (XXXXI). wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXII), or a salt, solvate, or hydrate thereof: mAëmA Formula (XXXXII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIII), or a salt, solvate, or hydrate thereof: mAëmU Formula (XXXXIII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIV), or a salt, solvate, or hydrate thereof: mAëmG Formula (XXXXIV) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXV), or a salt, solvate, or hydrate thereof: mAëmC Formula (XXXXV) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVI), or a salt, solvate, or hydrate thereof: mUëmA Formula (XXXXVI) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVII), or a salt, solvate, or hydrate thereof: mUëmG Formula (XXXXVII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXVIII), or a salt, solvate, or hydrate thereof: mUëfC Formula (XXXXVIII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXIX), or a salt, solvate, or hydrate thereof: fGëmU Formula (XXXXIX) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXX), or a salt, solvate, or hydrate thereof: mGëfG Formula (XXXXX) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXXI), or a salt, solvate, or hydrate thereof: mGëmC Formula (XXXXXI) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises Formula (XXXXXII), or a salt, solvate, or hydrate thereof:
mCëmA Formula (XXXXXII) wherein: R4 and R5 are independently an oligonucleotide, or R4 and R5 are joined together to form a single oligonucleotide; and each X is independently O or S. In certain embodiments, the modified oligonucleotide comprises an internucleoside linkage of one of the following Formulae: Formula (XXXXXV), Formula (XXXXXVI). In certain embodiments, the compound of any preceding embodiment comprises one or more lipid conjugate groups. In certain embodiments, the one or more lipid conjugate groups are attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the one or more lipid conjugate groups are attached to the 5’ or 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipid conjugate groups are attached to an internucleoside linkage and the 5’ or 3’ end of the modified oligonucleotide. In certain embodiments, the one or more lipid conjugate groups are attached to an internucleoside linkage and both the 5’ and 3’ ends of the modified oligonucleotide. In certain embodiments, the one or more ligands (e.g., one or more TrkB ligands, one or more CB1 ligands, one or more α4β1/7 integrin ligands) are attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide. In certain embodiments, the one or more conjugate groups comprise at least one ligand (e.g., at least one TrkB ligand, at least one CB1 ligand, at least one α4β1/7 integrin ligand) attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide and at least one lipid. In certain embodiments, the one or more conjugate groups comprise at least one ligand (e.g., at least one TrkB ligand, at least one CB1 ligand, at least one α4β1/7 integrin ligand) attached to the 5’ or 3’ end of the modified oligonucleotide or both the 5’ and 3’ ends of the modified oligonucleotide and one or more lipid conjugate groups attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises a ligand (e.g., a TrkB ligand, a CB1 ligand, an α4β1/7 integrin ligand) and a lipid. In certain embodiments, the modified oligonucleotide comprises one or more ligands (e.g., one or more TrkB ligands, one or more CB1 ligands, one or more α4β1/7 integrin ligands) and one or more lipids. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the compound of any preceding embodiment comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide. In certain embodiments, the modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl are attached to one or more internucleoside linkages of the modified oligonucleotide. In certain embodiments, the modified oligonucleotide is the second modified oligonucleotide or sense oligonucleotide. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C17 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C18 hydrocarbon chain. In certain embodiments, the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C22 hydrocarbon chain. In certain embodiments, a substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide). In certain embodiments, a substituted or unsubstituted alkyl or alkenyl is attached to an internucleoside linkage of a modified oligonucleotide (e.g., a second modified oligonucleotide or sense oligonucleotide). In certain embodiments, the internucleoside linkage is between nucleosides that are within 10 positions (e.g., within 8 positions, within 6 positions, within 5 positions, within 4 positions, within 3 positions, within 2 positions) from a terminal end (e.g., the 5′ and/or 3′ end) of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between nucleosides that are within 5 positions from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 5′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage is between positions 2 and 3 from the 3′ end of the modified oligonucleotide. In certain embodiments, the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI. In certain embodiments, the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI. Target Nucleic Acids and Target Regions In certain embodiments, compounds described herein comprise or consist of an oligonucleotide comprising a region that is complementary to a target nucleic acid. In certain embodiments, the target nucleic acid is an endogenous RNA molecule. In certain embodiments, the target nucleic acid encodes a protein. In certain embodiments, the target nucleic acid is non-coding. In certain such embodiments, the target nucleic acid is selected from an mRNA and a pre-mRNA, including intronic, exonic and untranslated regions. In certain embodiments, the target RNA is an mRNA. In certain embodiments, the target nucleic acid is a pre-mRNA. In certain such embodiments, the target region is entirely within an exon. In certain such embodiments, the target region is entirely within an intron. In certain embodiments, the target region spans an intron/exon junction. In certain embodiments, the target region is at least 50% within an intron. In certain embodiments, compounds disclosed herein hybridize with a MAPT nucleic acid. The most common mechanism of hybridization involves hydrogen bonding between complementary nucleobases of the nucleic acid molecules. Hybridization can occur under varying conditions. Hybridization conditions are sequence-dependent and are determined by the nature and composition of the nucleic acid molecules to be hybridized. Methods of determining whether a sequence hybridizes specifically to a target nucleic acid are well known in the art. In certain embodiments, the compounds provided herein specifically hybridize with a MAPT nucleic acid. Nucleotide sequences that encode MAPT include, without limitation, the following: GenBank Accession No. NM_001377265.1 (incorporated herein as SEQ ID NO: 1), and nucleotides 2624000 to 2761000 of NT_010783.14 (incorporated herein as SEQ ID NO: 2). Complementarity Oligonucleotides provided herein may have a defined percent complementarity to a particular nucleic acid, target region, oligonucleotide, or portion thereof. Non-complementary nucleobases may be tolerated provided that the oligonucleotide remains able to specifically hybridize to the nucleic acid, oligonucleotide, or portion thereof. In certain embodiments, the oligonucleotides provided herein, or a specified portion thereof are at least, or are up to 70%, 80%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% complementary to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof. In certain embodiments, the oligonucleotides provided herein, or a specified portion thereof, are 70% to 75%, 75% to 80%, 80% to 85%, 85% to 90%, 90% to 95%, 95% to 100%, or any number in between these ranges, complementary to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof. Percent complementarity of an oligonucleotide with a target nucleic acid, a target region, an oligonucleotide or specified portion thereof can be determined using routine methods. For example, an oligonucleotide in which 18 of 20 nucleobases of the oligonucleotide are complementary to a target region, and would therefore specifically hybridize, would represent 90 percent complementarity. In this example, the remaining non-complementary nucleobases may be clustered or interspersed with complementary nucleobases and need not be contiguous to each other or to complementary nucleobases. As such, an oligonucleotide which is 18 nucleobases in length having four non-complementary nucleobases which are flanked by two regions of complete complementarity with the target nucleic acid would have 77.8% overall complementarity with the target nucleic acid. Percent complementarity of an oligonucleotide with a region of a target nucleic acid, a target region, an oligonucleotide or specified portion thereof can be determined routinely using BLAST programs (basic local alignment search tools) known in the art. In certain embodiments, oligonucleotides described herein, or specified portions thereof, are fully complementary (i.e. 100% complementary) to a target nucleic acid, a target region, an oligonucleotide or specified portion thereof. For example, an oligonucleotide may be fully complementary to a target nucleic acid, a target region, an oligonucleotide, or specified portion thereof. As used herein, “fully complementary” means each nucleobase of an oligonucleotide is complementary to the corresponding nucleobase of a target nucleic acid, a target region, an oligonucleotide, or a specified portion thereof. For example, a 20 nucleobase oligonucleotide is fully complementary to a target sequence that is 400 nucleobases long, so long as there is a corresponding 20 nucleobase portion of the target nucleic acid that is fully complementary to the compound. “Fully complementary” can also be used in reference to a specified portion of the first and/or the second nucleic acid. For example, a 20 nucleobase portion of a 30 nucleobase oligonucleotide can be “fully complementary” to a 20 nucleobase region of a target sequence that is 400 nucleobases long. The 20 nucleobase portion of the 30 nucleobase compound is fully complementary to the target sequence if the target sequence has a corresponding 20 nucleobase portion wherein each nucleobase is complementary to the 20 nucleobase portion of the compound. At the same time, the entire 30 nucleobase compound may or may not be fully complementary to the target sequence, depending on whether the remaining 10 nucleobases of the compound are also complementary to the target sequence. In certain embodiments, oligonucleotides described herein comprise one or more mismatched nucleobases relative to a target nucleic acid, a target region, an oligonucleotide or a specified portion thereof. In certain embodiments, oligonucleotides described herein that are, or are up to 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, or 23 nucleobases in length comprise no more than 4, no more than 3, no more than 2, or no more than 1 non- complementary nucleobase(s) relative to a target nucleic acid, or specified portion thereof. In certain embodiments, oligonucleotides described herein that are, or are up to 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 nucleobases in length comprise no more than 6, no more than 5, no more than 4, no more than 3, no more than 2, or no more than 1 non-complementary nucleobase(s) relative to a target nucleic acid, a target region, an oligonucleotide, or specified portion thereof. In certain embodiments, the mismatch is at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 from the 5’-end of the oligonucleotide. In certain embodiments, the mismatch is at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12, 13 or 14 from the 3’-end of the oligonucleotide. In certain embodiments, the mismatch forms a wobble base pair with a corresponding nucleobase on the target nucleic acid. For example, in certain embodiments, the mismatch forms a wobble base pair selected from hypoxanthine (nucleobase of inosine) and uracil (I:U base pair); guanine and uracil (G:U base pair); hypoxanthine and adenine (I:A base pair); and hypoxanthine and cytosine (I:C base pair). Accordingly, in certain embodiments, a mismatched nucleobase on an oligonucleotide comprises hypoxanthine, guanine, or uracil. In certain embodiments, oligonucleotides described herein may be complementary to a portion of a nucleic acid. As used herein, “portion” refers to a defined number of contiguous nucleobases within a region of a nucleic acid. A “portion” can also refer to a defined number of contiguous nucleobases of an oligonucleotide. In certain embodiments, the oligonucleotides are complementary to at least an 8 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 9 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 10 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least an 11 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 12 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 13 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 14 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 15 nucleobase portion of a nucleic acid. In certain embodiments, the oligonucleotides are complementary to at least a 16 nucleobase portion of a nucleic acid. Also contemplated are oligonucleotides that are complementary to at least a 9, 10, 17, 18, 19, 20, 21, 22, 23 or more nucleobase portion of a nucleic acid, or a range defined by any two of these values. In certain embodiments, the oligonucleotide is an antisense oligonucleotide. In certain embodiments, a portion of the antisense oligonucleotide is compared to an equal length portion of the target nucleic acid. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion is compared to an equal length portion of the target nucleic acid. In certain embodiments, the oligonucleotide is a sense oligonucleotide. In certain embodiments, a portion of the sense oligonucleotide is compared to an equal length portion of an antisense oligonucleotide. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion of a sense oligonucleotide is compared to an equal length portion of an antisense oligonucleotide. Identity The oligonucleotides provided herein may also have a defined percent identity to a particular nucleic acid, target region, oligonucleotide, or specified portion thereof. As used herein, an oligonucleotide is identical to a sequence disclosed herein if it has the same nucleobase pairing ability. For example, a DNA which contains thymidine in place of uracil in a disclosed RNA sequence would be considered identical to the RNA sequence since both uracil and thymidine pair with adenine. Shortened and lengthened versions of the compounds described herein as well as compounds having non-identical bases relative to the compounds provided herein also are contemplated. The non-identical bases may be adjacent to each other or dispersed throughout the compound. Percent identity of an oligonucleotide is calculated according to the number of bases that have identical base pairing relative to the sequence to which it is being compared. In certain embodiments, oligonucleotides described herein, or portions thereof, are, or are at least, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100% identical to one or more of the nucleic acids, oligonucleotides, or a portion thereof, disclosed herein. In certain embodiments, oligonucleotides described herein are about 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical, or any percentage between such values, to a particular nucleic acid or oligonucleotide, or portion thereof. In certain embodiments, an oligonucleotide may have one or more mismatched nucleobases. In certain such embodiments, the mismatch is at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 from the 5’-end of the oligonucleotide. In certain such embodiments, the mismatch is at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12, 13 or 14 from the 3’-end of the oligonucleotide. In certain embodiments, a portion of the oligonucleotide is compared to an equal length portion of the target nucleic acid. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion is compared to an equal length portion of the target nucleic acid. In certain embodiments, the oligonucleotide is a sense oligonucleotide. In certain embodiments, a portion of the sense oligonucleotide is compared to an equal length portion of the target nucleic acid. In certain embodiments, an 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 nucleobase portion is compared to an equal length portion of the target nucleic acid. Pharmaceutical Compositions and Formulations Compounds described herein may be admixed with pharmaceutically acceptable active or inert substances for the preparation of pharmaceutical compositions or formulations. Compositions and methods for the formulation of pharmaceutical compositions are dependent upon a number of criteria, including, but not limited to, route of administration, extent of disease, or dose to be administered. Certain embodiments provide pharmaceutical compositions comprising one or more compounds or a salt thereof. In certain embodiments, the compounds are antisense oligonucleotides. In certain embodiments, the compounds are oligomeric compounds. In certain embodiments, the compounds comprise or consist of one or more modified oligonucleotides. In certain such embodiments, the pharmaceutical composition comprises one or more compound and a suitable pharmaceutically acceptable diluent or carrier. In certain embodiments, a pharmaceutical composition comprises one or more compound and a sterile saline solution. In certain embodiments, such pharmaceutical composition consists of one compound and a sterile saline solution. In certain embodiments, the sterile saline is pharmaceutical grade saline. In certain embodiments, a pharmaceutical composition comprises one or more compound and sterile water. In certain embodiments, a pharmaceutical composition consists of one compound and sterile water. In certain embodiments, the sterile water is pharmaceutical grade water. In certain embodiments, a pharmaceutical composition comprises one or more compounds and phosphate-buffered saline (PBS). In certain embodiments, a pharmaceutical composition consists of one compound and sterile PBS. In certain embodiments, the sterile PBS is pharmaceutical grade PBS. A compound described herein targeted to MAPT can be utilized in pharmaceutical compositions by combining the compound with a suitable pharmaceutically acceptable diluent or carrier. In certain embodiments, a pharmaceutically acceptable diluent is water, such as sterile water suitable for injection. Accordingly, in one embodiment, employed in the methods described herein is a pharmaceutical composition comprising a compound targeted to MAPT and a pharmaceutically acceptable diluent. In certain embodiments, the pharmaceutically acceptable diluent is water. In certain embodiments, the compound comprises or consists of one or more modified oligonucleotide provided herein. Pharmaceutical compositions comprising compounds provided herein encompass any pharmaceutically acceptable salts, esters, or salts of such esters, or any other oligonucleotide which, upon administration to an animal, including a human, is capable of providing (directly or indirectly) the biologically active metabolite or residue thereof. In certain embodiments, the compounds are antisense oligonucleotides. In certain embodiments, the compounds are oligomeric compounds. In certain embodiments, the compound comprises or consists of one or more modified oligonucleotide. Accordingly, for example, the disclosure is also drawn to pharmaceutically acceptable salts of compounds, prodrugs, pharmaceutically acceptable salts of such prodrugs, and other bioequivalents. Suitable pharmaceutically acceptable salts include, but are not limited to, sodium and potassium salts. A prodrug can include the incorporation of additional nucleosides at one or both ends of a compound which are cleaved by endogenous nucleases within the body, to form the active compound. In certain embodiments, the compounds or compositions further comprise a pharmaceutically acceptable carrier or diluent. EXAMPLES The following examples describe the process to identify lead compounds targeted to MAPT. Certain compounds are distinguished as having high potency and tolerability. The following examples serve only to illustrate the compounds described herein and are not intended to limit the same. The following examples and related sequence listing accompanying this filing may identify sequence as either “RNA” or “DNA”; however, as disclosed herein, those sequences may be modified with any combination of chemical modifications. One of skill in the art will readily appreciate that the designation of a sequence as “RNA” or “DNA” is, in certain instances, arbitrary. For example, an oligonucleotide comprising a nucleoside comprising a 2’-OH sugar moiety and a thymine base could be described as a DNA having a modified sugar (2’-OH for the natural 2’-H of DNA) or as an RNA having a modified base (methylated uracil for natural uracil of RNA). Accordingly, nucleic acid sequences provided herein, including, but not limited to, those in the sequence listing, are intended to encompass nucleic acids containing any combination of natural or modified RNA and/or DNA, including, but not limited to, such nucleic acids having modified nucleobases. Each of the references recited in the present application is incorporated herein by reference in its entirety. Table 1 Chemical Nomenclature
Table 2 Compound Sequence and Targeting Position on SEQ ID NO: 1 Table 3 Compound Chemistry FXX mC*mG.mC.mA.mU.mG.fG.mU.fC.fA.fG.fU.mA.mA.mA.mA.mG.mC.mAëmA*mA*dQ IS1502 94 XI IA124 vU*fU*mG.fA.mU.fG.mC.fA.mG.fG.mA.fG.mU.fU.mG.fU.mA.fA.mG.fC.mC*mU*mC 59 9 RD5019 FXX FXX mA*mG.mG.mC.mU.mU.mA.fC.mA.fA.fC.fU.fC.mC.mU.mG.mC.mA.mU.mC.mA.mA*dQ IS1648129 XI XI IA162 vU*fU*mG.fA.mU.fG.mC.fA.mG.fG.mA.fG.mU.fU.mG.fU.mA.fA.mG.fU.mC*mU*mC 209 5 RD5020 FXX FXX mA*mG.mA.mC.mU.mU.mA.fC.mA.fA.fC.fU.fC.mC.mU.mG.mC.mA.mU.mC.mA.mA*dQ IS2016201 XI XI IA162 vU*fU*mG.fA.mU.fG.mC.fA.mG.fG.mA.fG.mU.fU.mG.fU.mA.fA.mG.fC.mU*mU*mC 210 6 RD5021 FXX FXX mA*mA.mG.mC.mU.mU.mA.fC.mA.fA.fC.fU.fC.mC.mU.mG.mC.mA.mU.mC.mA.mA*dQ IS2017202 XI XI IA162 vU*fU*mG.fA.mU.fG.mC.fA.mG.fG.mA.fG.mU.fU.mG.fU.mA.fA.mG*fU*mC 211 7 RD5022 FXX FXX mG*mA.mC.mU.mU.mA.fC.mA.fA.fC.fU.fC.mC.mU.mG.mC.mA.mU.mC.mA.mA*dQ IS2018203 XI XI IA162 vU*fU*mG.fA.mU.fG.mC.fA.mG.fG.mA.fG.mU.fU.mG.fU.mA.fA.mG*fC*mU 212 8 RD5023 FXX FXX mA*mG.mC.mU.mU.mA.fC.mA.fA.fC.fU.fC.mC.mU.mG.mC.mA.mU.mC.mA.mA*dQ IS2019204 XI XI IA162 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA.fA.mG*mC*mC 177 9 RD5024 FXX FXX mG*mC.mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS1649154 XI XI IA163 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA.fA.mG*mU*mC 213 0 RD5025 FXX FXX mA*mC.mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS2020205 XI XI IA163 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA.fA.mG*mC 214 1 RD5026 FXX FXX mG*mC.mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS1649154 XI XI IA163 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA.fA*mG*mU 215 2 RD5027 FXX FXX mA*mC.mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS2020205 XI XI IA163 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA*fA*mG 216 3 RD5028 FXX FXX mC*mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS2021206 XI XI IA163 vU*fU*mG.fU.mG.fA.mU.fG.mC.fA.mG.fG.mA.fU.mU.fU.mG.fU.mA.fA.mG*mC*mU 217 4 RD5029 FXX FXX mG*mC.mU.mU.mA.mC.mA.fA.mA.fU.fC.fC.fU.mG.mC.mA.mU.mC.mA.mC.mA.mA*dQ IS1649154 XI XI IA160 vU*fU*mU.fG.mC.fU.mU.fU.mU.fA.mC.fU.mG.fA.mC.fC.mA.fU.mG.fU.mG*mA*mG 188 5 RD5065 FXX FXX mU*mC.mA.mC.mA.mU.mG.fG.mU.fC.fA.fG.fU.mA.mA.mA.mA.mG.mC.mAëmA.mA*dQ IS1997164 XI XI IA160 vU*fU*mU.fG.mC.fU.mU.fU.mU.fA.mC.fU.mG.fA.mC.fC.mA.fU.mG.fU.mG*mA*mG 188 5 RD5262 FXX FXX mU*mC.mA.mC.mA.mU.mG.fG.mU.fC.fA.fG.fU.mA.mA.mA.mA.mG.mC.mA.mA.mA*dQ IS2123164 XI XI IA161 vU*fU*mU.fG.mC.fU.mU.fU.mU.fA.mC.fU.mG.fA.mC.fC.mA.fU.mG*fC*mC 196 2 RD5263 FXX FXX mG*mG.mC.mA.mU.mG.fG.mU.fC.fA.fG.fU.mA.mA.mA.mA.mG.mC.mA.mA.mA*dQ IS2124172 XI XI
PREPARATION OF LIGANDS Ligands BA-120, BA-128, BA-171, BA-168, BA-177, BA-210, BA-215, BA-236, BA-135, BA-136, BA-137, BA-144, BA-118, BA-196, BA-197, BA-198, BA-167, BA-216, BA-173, BA-183, BA-225, BA-129, BA-169, BA-170, BA-201, and BA-203 were prepared as described below. Example 1: Preparation of N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)oxy)-6-(4-methylpiperidin-1-yl)-1,3,5-triazin-2-amine (BA-120) STEP 1: 2-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)-4,6-dichloro-1,3,5-triazine To a suspension of 2,4,6-trichloro-1,3,5-triazine (1.0 g, 5.42 mmol) and NaHCO3 (911 mg, 10.84 mmol) in acetone (8 mL) at 0°C was added 17-azido-3,6,9,12,15- pentaoxaheptadecan-1-ol (1.67 g, 5.42 mmol) over 10 mins. The cooling bath was removed, and the mixture stirred at room temperature for 20 hours. The solvent was evaporated, and the resulting residue was partitioned between water and DCM to form an emulsion. Brine was added and the organic phase was isolated, washed with brine, dried, filtered, concentrated and purified by silica-gel column chromatography using a gradient 0-100% ethyl acetate in hexanes to afford the title compound (1.48 g, 60%). MS (ESI): m/z = 455.3 [M+H]+. STEP 2: N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)- 6-chloro-1,3,5-triazin-2-amine To a mixture of 2-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)-4,6-dichloro- 1,3,5-triazine (500 mg, 1.1 mmol) in THF (9.5 mL) at 0°C was added a mixture of adamantylamine (166 mg, 0.151 ml, 1.1 mmol) and DIEA (0.29 mL, 1.65 mmol) in THF (1.2 mL) dropwise. The cooling bath was removed, and the mixture stirred overnight at rt and then concentrated and purified by silica-gel column chromatography using a gradient 0-85% ethyl acetate in hexanes to afford the title compound (282 mg, 90%). MS (ESI) m/z = 570.6 [M+H]+. STEP 3: N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)- 6-(4-methylpiperidin-1-yl)-1,3,5-triazin-2-amine To a N-((3s,5s,7s)-adamantan-1-yl)-4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)oxy)-6-chloro-1,3,5-triazin-2-amine (50 mg, 0.088 mmol) in anhydrous THF (0.76 ml) at 0°C was added a mixture of 4-methylpiperidine (16 mL, 0.13 mmol) and DIEA (18 mL, 0.10 mmol) in THF (0.1 mL) dropwise. The mixture was stirred at 60°C for 3 hours then concentrated and the residue purified by reverse-phase column chromatography using a gradient 0-100 % acetonitrile in water (+0.1% formic acid) to afford the title compound (36 mg, 65%). MS (ESI): m/z = 633.2 [M+H]+, 655.2 [M+Na]+.
Example 2: Preparation of (S)-3-(4-(5-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2- methyl-3-oxo-2,3-dihydropyridazin-4-yl)phenyl)-2-(3,5- dichloroisonicotinamido)propanoic acid (BA-128) STEP 1: tert-butyl (S)-2-(3,5-dichloroisonicotinamido)-3-(4-(5-(2-(2-(2-(2-hydroxyethoxy) ethoxy)ethoxy)ethoxy)-2-methyl-3-oxo-2,3-dihydropyridazin-4-yl)phenyl)propanoate A solution of tert-butyl (S)-2-(3,5-dichloroisonicotinamido)-3-(4-(2-methyl-3-oxo-5- ((1-phenyl-2,5,8,11-tetraoxatridecan-13-yl)oxy)-2,3-dihydropyridazin-4- yl)phenyl)propanoate (prepared as described in Gong et. al., " Synthesis and Biological Evaluation of Novel Pyridazinone-Based α4 Integrin Receptor Antagonists", J. Med. Chem., 2006, 49, 11, 3402-3411, 0.81 g, 1.02 mmol) in methanol (17 ml) was stirred under Hydrogen atmosphere (1atm), in the presence of Pd/C (0.11 g, 1.02 mmol) for 2 hours. The catalyst was removed by filtration, and the filtrate concentrated and purified by silica gel column chromatography using a 0-5% MeOH in DCM to obtain the titled compound (0.47 g, 66%). MS (ESI) m/z 696.6 [M+H]+ STEP 2: tert-butyl (S)-2-(3,5-dichloroisonicotinamido)-3-(4-(2-methyl-5-(2-(2-(2-(2- ((methylsulfonyl)oxy)ethoxy)ethoxy)ethoxy)ethoxy)-3-oxo-2,3-dihydropyridazin-4- yl)phenyl)propanoate Methanesulfonyl chloride (17 ul, 0.216 mmol) in DCM (1.4 ml) was added dropwise to a solution of tert-butyl (S)-2-(3,5-dichloroisonicotinamido)-3-(4-(5-(2-(2-(2-(2- hydroxyethoxy) ethoxy)ethoxy)ethoxy)-2-methyl-3-oxo-2,3-dihydropyridazin-4- yl)phenyl)propanoate (0.10 g, 0.144 mmol) in pyridine (1.4 ml) at 0°C. The reaction mixture was stirred at room temperature for 16 h then quenched by addition of water (10 ml) and extracted with DCM (3x10 ml). The organic layer was washed with a saturated solution of NaHCO3 (10 ml) and brine (10 ml). The combined organic extracts were dried over anhydrous Na2SO4 and concentrated to obtain the title compound as a brown oil (0.11 g, quant.). MS (ESI) m/z 773.5 [M+H]+ STEP 3: tert-butyl (S)-3-(4-(5-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2-methyl-3- oxo-2,3-dihydropyridazin-4-yl)phenyl)-2-(3,5-dichloroisonicotinamido)propanoate To a solution of tert-butyl (S)-2-(3,5-dichloroisonicotinamido)-3-(4-(2-methyl-5-(2- (2-(2-(2-((methylsulfonyl)oxy)ethoxy)ethoxy)ethoxy)ethoxy)-3-oxo-2,3-dihydropyridazin-4- yl)phenyl)propanoate (0.11 mg, 0.143 mmol) in DMF (2.3 ml) was added NaN3 (21 mg, 0.316 mmol) followed by 1 drop of water. The mixture was stirred for 6 hours at 80°C then cooled to room temperature and partitioned between ethyl acetate (20 ml) and water (20 ml). The organic phase was separated, and the aqueous phase was extracted twice with ethyl acetate (2x15 ml). The combined organic extracts were washed with brine (x2), dried, filtered, concentrated and purified by silica-gel column chromatography using a gradient 0- 100% ethyl acetate in hexane to afford the titled compound as a clear oil (85 mg, 82%). MS (ESI) m/z 721.6 [M+H]+ STEP 4: (S)-3-(4-(5-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2-methyl-3-oxo-2,3- dihydropyridazin-4-yl)phenyl)-2-(3,5-dichloroisonicotinamido)propanoic acid TFA (0.14 ml) was added dropwise at 0°C to a solution of tert-butyl (S)-3-(4-(5-(2-(2- (2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2-methyl-3-oxo-2,3-dihydropyridazin-4- yl)phenyl)-2-(3,5-dichloroisonicotinamido)propanoate (25 mg, 0.035 mmol) in DCM (0.3 ml). The cooling bath was removed, and the mixture stirred at room temperature for 17 hours and then concentrated and purified by silica-gel column chromatography using a gradient 0- 100% methanol in DCM to afford the title product (16 mg, 69%). MS (ESI) m/z 687.8 [M+Na]+ 1H NMR (500 MHz, DMSO-d6) δ 12.95 (s, 1H), 9.31 (d, J = 10 Hz, 1H), 8.63 (s, 2H), 8.20 (s, 1H), 7.42 (d, J = 10 Hz, 2H), 7.29 (d, J = 10 Hz, 2H), 4.78-4.72 (m, 1H), 4.36-4.32 (m, 2H), 3.66 (s, 5H), 3.57 (t, J = 5 Hz, 2H), 3.36 (t, J = 5 Hz, 2H), 3.53-3.47 (m, 8H), 3.20 (dd, J = 10, 5 Hz, 1H), 2.93 (q, J = 10 Hz, 1H) Example 3: Preparation of (3S)-3-(2-(4-(14-azido-3-methyl-6,9,12-trioxa-3- azatetradecyl)-2-oxopyridin-1(2H)-yl)-5-methylhexanamido)-3-(2’,4’,6’-trimethyl-[1,1’- biphenyl]-3-yl)propanoic acid (BA-171) STEP 1: ethyl (E)-2-(4-(2-ethoxyvinyl)-2-oxopyridin-1(2H)-yl)-5-methylhexanoate To a stirred solution of ethyl 2-(4-bromo-2-oxopyridin-1-yl)-5-methylhexanoate (4.8 g, 14.54 mmol) and 2-[(E)-2-ethoxyethenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (5.76 g, 29.081 mmol) in dioxane (50 ml) and water (5 ml) were added K2CO3 (4.02 g, 29.10 mmol) and Pd(PPh3)4 (1.68 g, 1.45 mmol) at room temperature under nitrogen atmosphere. The resulting mixture was stirred overnight at 70°C under nitrogen atmosphere. The mixture was cooled to room temperature and then quenched by the addition of water. The resulting mixture was extracted with ethyl acetate and the combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with PE / EA (1:1) to afford the title product (Pinacol containing). The residue was further purified by reversed-phase flash chromatography with the following conditions: column, C18 silica gel; mobile phase, acetonitrile in water, 10-100% gradient in 25 min; detector, UV 254 nm. This resulted in ethyl 2-(4-[(E)-2-ethoxyethenyl]-2- oxopyridin-1-yl-5-methylhexanoate (4 g, 86%) as a brown oil. MS (ESI) m/z 322.1 [M+H]+ 1H NMR (300 MHz, CDCl3) δ 7.16 (m, J = 19.4, 10.1 Hz, 2H), 6.37 (s, 1H), 6.19 (d, J = 7.4 Hz, 1H), 5.60 (d, J = 13.0 Hz, 1H), 5.54 (m, J = 10.2, 5.6 Hz, 1H), 4.19 (q, J = 7.2 Hz, 2H), 3.94 (q, J = 7.1 Hz, 2H), 2.29 – 2.03 (m, 1H), 1.86 (d, J = 12.3 Hz, 1H), 1.57 (m, J = 13.3, 6.8 Hz, 1H), 1.35 (t, J = 7.0 Hz, 3H), 1.24 (m, J = 8.5, 7.8 Hz, 4H), 1.14 – 1.04 (m, 1H), 0.87 (m, J = 6.8, 3.0 Hz, 6H) STEP 2: ethyl 5-methyl-2-(2-oxo-4-(2-oxoethyl)pyridin-1(2H)-yl)hexanoate Ethyl 2-(4-[(E)-2-ethoxyethenyl]-2-oxopyridin-1-yl-5-methylhexanoate (3.9 g, 12.134 mmol) and TFA (40 ml) were stirred overnight at room temperature. The resulting mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography, eluted with PE / EA (1:2) to afford the title compound (1.9 g, 53%) as a yellow oil. MS (ESI) m/z 294.2 1H NMR (300 MHz, Chloroform-d) δ 9.76 (t, J = 2.0 Hz, 1H), 7.35 (d, J = 7.1 Hz, 1H), 6.54 – 6.47 (m, 1H), 6.14 (m, J = 7.2, 2.0 Hz, 1H), 5.56 (m, J = 10.1, 5.6 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 3.56 (d, J = 2.0, 0.8 Hz, 2H), 2.19 (m, J = 14.0, 11.1, 5.5 Hz, 1H), 1.88 (m, J = 18.8, 10.4, 4.8 Hz, 1H), 1.59 (m, J = 13.3, 6.7 Hz, 1H), 1.34 – 1.16 (m, 4H), 1.16 – 0.98 (m, 1H), 0.88 (q, J = 6.6, 3.1 Hz, 6H) STEP 3: ethyl 5-methyl-2-(2-oxo-4-(2,2,3,3,16-pentamethyl-4,7,10,13-tetraoxa-16-aza-3- silaoctadecan-18-yl)pyridin-1(2H)-yl)hexanoate To a stirred solution of ethyl 5-methyl-2-[2-oxo-4-(2-oxoethyl)pyridin-1-yl]hexanoate (1.8 g, 6.136 mmol) in DCM (27 ml) was added 15,15,16,16-tetramethyl-5,8,11,14-tetraoxa- 2-aza-15-silaheptadecane (1.97 g, 6.127 mmol), STAB (2.60 g, 12.268 mmol). The resulting mixture was stirred for 2 hours at room temperature, quenched by the addition of water (100 ml) and extracted with DCM (3 x 120ml). The combined organic extracts were washed with brine (2 x 50 ml), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with DCM / MeOH (10:1) to afford ethyl 5-methyl-2-[2- oxo-4-(2,2,3,3,16-pentamethyl-4,7,10,13-tetraoxa-16-aza-3-silaoctadecan-18-yl)pyridin-1- yl]hexanoate (1.6 g, 44%) as a yellow-brown oil. MS(ESI) m/z 599.3 [M+H]+ 1HNMR (300 MHz, Acetonitrile-d3) δ 7.25 (d, J = 7.1 Hz, 1H), 6.20 (s, 1H), 6.11 (m, J = 7.1, 2.0 Hz, 1H), 5.08 (m, J = 10.2, 5.4 Hz, 1H), 4.08 (q, J = 7.1 Hz, 2H), 3.66 (m, J = 5.7, 4.4 Hz, 2H), 3.57 – 3.47 (m, 11H), 3.47 – 3.37 (m, 3H), 2.68 – 2.48 (m, 4H), 2.24 (s, 3H), 2.15 – 1.99 (m, 1H), 1.90 (m, 1H), 1.48 (m, J = 13.4, 6.8 Hz, 1H), 1.25 – 1.11 (m, 5H),1.07-0.88 (m, 1H), 0.83 (s, 9H), 0.79 (m, J = 6.6, 3.0 Hz, 6H), 0.02 (s, 6H) STEP 4: 5-methyl-2-(2-oxo-4-(2,2,3,3,16-pentamethyl-4,7,10,13-tetraoxa-16-aza-3- silaoctadecan-18-yl)pyridin-1(2H)-yl)hexanoic acid To a stirred solution of ethyl 5-methyl-2-[2-oxo-4-(2,2,3,3,16-pentamethyl-4,7,10,13- tetraoxa-16-aza-3-silaoctadecan-18-yl)pyridin-1-yl]hexanoate (1.4 g, 2.338 mmol) in THF (14 ml) and water (2.8 ml) was added lithium hydroxide (223.95 mg, 9.352 mmol) and the resulting mixture was stirred for 2 hours at room temperature. Acetic acid (0.71 g, 11.688 mmol) in water (14 ml) was then added dropwise at 0°C. The resulting mixture was extracted with CH3Cl (3 x 100 ml). The combined organic extracts were washed with brine (2 x 40 ml), dried over anhydrous MgSO4, filtered, and concentrated to provide the title compound (0.90 g, 67%) as a yellow semi-solid, which was used in the next step without further purification. MS(ESI) m/z 571.3 [M+H]+ STEP 5: methyl (3S)-3-(5-methyl-2-(2-oxo-4-(2,2,3,3,16-pentamethyl-4,7,10,13-tetraoxa-16- aza-3-silaoctadecan-18-yl)pyridin-1(2H)-yl)hexanamido)-3-(2',4',6'-trimethyl-[1,1'-biphenyl]- 3-yl)propanoate To a stirred mixture of methyl (3S)-3-amino-3-(2',4',6'-trimethyl-[1,1'-biphenyl]-3- ylpropanoate (600 mg, 2.017 mmol) and 5-methyl-2-[2-oxo-4-(2,2,3,3,16-pentamethyl- 4,7,10,13-tetraoxa-16-aza-3-silaoctadecan-18-yl)pyridin-1-yl]hexanoic acid (1.15 g, 2.017 mmol) in DMF (12 ml) were added DIEA (782.27 mg, 6.051 mmol), HBTU (765.13 mg, 2.017 mmol) and HOBT (27.26 mg, 0.202 mmol) and the resulting mixture was stirred for 2h at room temperature under nitrogen atmosphere and then purified by reversed-phase flash chromatography with the following conditions: column, C18 silica gel; mobile phase, acetonitrile in water, 40-100% gradient in 10 min; 100% gradient in 20 min; detector, UV 220 nm/305nm to provide the title compound (509.0 mg, 29%) as a brown oil. MS(ESI) m/z 850.6 [M+H]+ 1H NMR (300 MHz, DMSO-d6) δ 8.95 (m, J = 27.0, 8.5, 5.6 Hz, 1H), 7.57 (m, J = 12.3, 7.3, 5.2 Hz, 1H), 7.45 – 7.19 (m, 2H), 7.12 – 6.86 (m, 4H), 6.18 (m, J = 21.3, 11.5, 5.6 Hz, 2H), 5.77 (d, J = 5.1 Hz, 1H), 5.34 (m, J = 61.7, 13.7, 5.9 Hz, 2H), 3.68 (q, J = 5.2 Hz, 2H), 3.63 – 3.39 (m, 15H), 3.32 (s, 2H), 2.83 (t, J = 6.5 Hz, 2H), 2.24 (m, J = 12.7, 5.5 Hz, 6H), 1.95 – 1.80 (m, 7H), 1.70 – 1.57 (m, 1H), 1.57 – 1.47 (m, 1H), 1.47-1.34 (m, 1H), 1.24 (d, J = 5.5 Hz, 2H), 1.15-0.96 (m, 1H), 0.93 – 0.79 (m, 13H), 0.72 (t, J = 6.0 Hz, 4H), 0.08 – 0.01 (m, 6H) STEP 6: methyl (3S)-3-(2-(4-(14-hydroxy-3-methyl-6,9,12-trioxa-3-azatetradecyl)-2- oxopyridin-1(2H)-yl)-5-methylhexanamido)-3-(2',4',6'-trimethyl-[1,1'-biphenyl]-3- yl)propanoate To a solution of methyl (3S)-3-(5-methyl-2-(2-oxo-4-(2,2,3,3,16-pentamethyl- 4,7,10,13-tetraoxa-16-aza-3-silaoctadecan-18-yl)pyridin-1(2H)-yl)hexanamido)-3-(2',4',6'- trimethyl-[1,1'-biphenyl]-3-yl)propanoate (0.25 g, 0.294 mmol) in THF (2 ml), TBAF (0.35 ml, 0.35 mmol, 1M in THF) was added dropwise. The reaction mixture was stirred at room temperature under inert atmosphere for 2 hours and then diluted with saturated ammonium chloride solution (15 ml) and extracted with ethyl acetate (3 x 15 ml). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by column chromatography using 0-15 % MeOH in DCM to provide the title product as a clear oil (0.12 mg, 55%). MS (ESI) m/z 759.1 [M+Na]+ STEP 7: methyl (3S)-3-(5-methyl-2-(4-(3-methyl-14-((methylsulfonyl)oxy)-6,9,12-trioxa-3- azatetradecyl)-2-oxopyridin-1(2H)-yl)hexanamido)-3-(2',4',6'-trimethyl-[1,1'-biphenyl]-3- yl)propanoate To a solution of methyl (3S)-3-(2-(4-(14-hydroxy-3-methyl-6,9,12-trioxa-3- azatetradecyl)-2-oxopyridin-1(2H)-yl)-5-methylhexanamido)-3-(2',4',6'-trimethyl-[1,1'- biphenyl]-3-yl)propanoate (0.12 g, 0.163 mmol) in DCM (1 ml), methanesulfonyl chloride (0.016 ml, 0.212 mmol) and triethylamine (0.045 ml, 0.33 mmol) were added. The reaction mixture was stirred at 0°C for 2 hours and then diluted with saturated aqueous sodium bicarbonate (10 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to obtain the title compound as a brown oil (0.11 g, 83%). MS (ESI) m/z 815.1 [M+H]+ STEP 8: methyl (3S)-3-(2-(4-(14-azido-3-methyl-6,9,12-trioxa-3-azatetradecyl)-2- oxopyridin-1(2H)-yl)-5-methylhexanamido)-3-(2',4',6'-trimethyl-[1,1'-biphenyl]-3- yl)propanoate To a solution of methyl (3S)-3-(5-methyl-2-(4-(3-methyl-14-((methylsulfonyl)oxy)- 6,9,12-trioxa-3-azatetradecyl)-2-oxopyridin-1(2H)-yl)hexanamido)-3-(2',4',6'-trimethyl-[1,1'- biphenyl]-3-yl)propanoate (0.11 g, 0.135 mmol) in DMF (1 ml), sodium azide (24 mg, 0.37 mmol) was added. The reaction mixture was heated at 65°C for 2 hours and then diluted with water (15 ml) and extracted with ethyl acetate (3 x 15 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography using a gradient 0-15% MeOH in DCM to provide the title compound as a clear oil (45 mg, 44%). MS (ESI) m/z 762.2 [M+H]+ STEP 9: (3S)-3-(2-(4-(14-azido-3-methyl-6,9,12-trioxa-3-azatetradecyl)-2-oxopyridin- 1(2H)-yl)-5-methylhexanamido)-3-(2',4',6'-trimethyl-[1,1'-biphenyl]-3-yl)propanoic acid To a solution of methyl (3S)-3-(2-(4-(14-azido-3-methyl-6,9,12-trioxa-3- azatetradecyl)-2-oxopyridin-1(2H)-yl)-5-methylhexanamido)-3-(2',4',6'-trimethyl-[1,1'- biphenyl]-3-yl)propanoate (45 mg, 0.059 mmol) in a mixture of methanol/water/dioxane (1.5 ml, 1:1:1), lithium hydroxide (4 mg, 0.177 mmol) was added and the mixture stirred at room temperature for 2 hours. The solvents were evaporated in vacuo and the resulting residue was treated with 10% citric acid solution and extracted with ethyl acetate (3 x 15 ml). The combined organic extracts were washed with brine (5 ml), dried over anhydrous Na2SO4, filtered and concentrated to provide the title compound as a clear oil (30 mg, 75%). MS (ESI) m/z 748.1 [M+H]+ 1H NMR (500 MHz, DMSO-d6) δ 12.35 (bs, 1H), 9.00 (d, J = 10 Hz, 1H), 7.69 (d, J = 10 Hz, 1H), 7.37 (t, J = 10 Hz, 1H), 7.27 (d, J = 5 Hz, 1H), 7.06 (s, 1H), 6.99 (d, J = 5 Hz, 1H), 6.91 (s, 2H), 6.29 (s, 1H), 6.18 (d, J = 5 Hz, 1H), 5.47 (q, J = 5 Hz, 1H), 5.17 (q, J = 5 Hz, 1H), 4.09 (m, 3H), 2.64 (t, 5 Hz, 2H), 3.61-3.57 (m, 13H), 3.16 (s, 6H), 2.26 (s, 3H), 1.90 (s, 6H), 1.87-1.79 (m, 1H), 1.68-1.64 (m, 1H), 1.41-1.35 (m, 1H), 0.95-0.82 (m, 3H), 6.60 (d, J = 10 Hz, 6H) Example 4: Preparation of N-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-5-((6-(bis(4- chlorophenyl)methyl)-4-((1-((trifluoromethyl)sulfonyl)piperidin-4-yl)amino)quinolin-8- yl)oxy)pentanamide (BA-168) STEP 1: Ethyl 5-((6-(bis(4-chlorophenyl)methyl)-4-((1-((trifluoromethyl)sulfonyl)piperidin- 4-yl)amino)quinolin-8-yl)oxy)pentanoate To a mixture of 6-[bis(4-chlorophenyl)methyl]-4-[(1-trifluoromethanesulfonyl piperidin-4-yl)amino] quinolin-8-ol (2.0 g, 3.3 mmol, prepared as described in J. Med. Chem., 2018, 61, 22, 10276-10298) and ethyl 5-bromopentanoate (0.75 g, 3.6 mmol) in DMF (5 mL) was added Cs2CO3 (3.20 g, 9.8 mmol) and the resulting mixture was stirred overnight at 60°C under nitrogen atmosphere. Ethyl acetate (100 mL) was added and the mixture washed with water (3 x 100 mL). The organic layer was dried Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with DCM: methanol (10: 1, V/V) to afford the title compound (1.9 g, 78 %) as a yellow solid. MS (ESI): m/z = 738.2 [M+H]+ 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 5.9 Hz, 1H), 7.82 (s, 1H), 7.57 – 7.47 (m, 1H), 7.45 – 7.32 (m, 4H), 7.31 (s, OH), 7.25 – 7.17 (m, 4H), 6.93 (s, 1H), 6.76 (d, J = 5.9 Hz, 1H), 5.71 (s, 1H), 4.08 – 3.98 (m, 4H), 3.98 – 3.86 (m, 4H), 3.40 (t, J = 12.7 Hz, 2H), 2.36 (t, J = 7.2 Hz, 2H), 2.10 (d, J = 12.7 Hz, 2H), 1.74 (s, 4H), 1.71 (s, 1H), 1.72 – 1.65 (m, 2H), 1.24 (s, 1H), 1.16 (t, J = 7.1 Hz, 3H) STEP 2: 5-((6-(bis(4-chlorophenyl)methyl)-4-((1-((trifluoromethyl)sulfonyl)piperidin-4- yl)amino)quinolin-8-yl)oxy)pentanoic acid Sodium hydroxide (1M aq, 7.8 mL, 25 mmol) was added to a (ethyl 5-((6-(bis(4- chlorophenyl)methyl)-4-((1-((trifluoromethyl)sulfonyl)piperidin-4-yl)amino)quinolin-8- yl)oxy)pentanoate (1.9 g, 2.6 mmol) in THF (15 mL), and the mixture stirred for overnight at room temperature under nitrogen atmosphere. The reaction was neutralized and quenched with glacial acetic acid and then extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine (3 x 100 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by reversed-phase flash chromatography (column, C18 silica gel; mobile phase, acetonitrile in water, 10 % to 100 % gradient in 20 min; detector, UV 254 nm.) to afford the title compound (484 mg, 26 %) as a light yellow solid. MS (ESI): m/z = 710.0 [M+H]+ 1H NMR (400 MHz, DMSO-d6) δ 8.35 (d, J = 5.3 Hz, 1H), 7.73 (s, 1H), 7.40 (d, J = 8.2 Hz, 4H), 7.21 (d, J = 8.2 Hz, 4H), 6.78 (d, J = 10.1 Hz, 2H), 6.65 (d, J = 5.6 Hz, 1H), 5.77 (s, 1H), 5.68 (s, 1H), 3.98 (t, J = 5.8 Hz, 2H), 3.88 (d, J = 13.4 Hz, 3H), 3.40 (t, J = 12.4 Hz, 2H), 2.31 (t, J = 7.0 Hz, 2H), 2.15 – 2.06 (m, 2H), 1.79 – 1.57 (m, 6H) STEP 3: N-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-5-((6-(bis(4-chlorophenyl)methyl)- 4-((1-((trifluoromethyl)sulfonyl)piperidin-4-yl)amino)quinolin-8-yl)oxy)pentanamide A solution of the 5-((6-(bis(4-chlorophenyl)methyl)-4-((1-((trifluoromethyl)sulfonyl) piperidin-4-yl)amino)quinolin-8-yl)oxy)pentanoic acid (0.20 g, 0.44 mmol), DIPEA (0.15 mL, 0.90 mmol) and amine (0.12 g, 0.55 mmol, 1.25 equiv.) in DMA (3 mL) was treated with HATU (0.34 g, 0.88 mmol) and stirred for 3 hours. The reaction mixture was diluted with ethyl acetate (50 mL) and washed with water (2 x 100 mL). The organic layer was washed with sat. NaHCO3 (50 mL) and brine (50 mL), dried over Na2SO4, filtered, concentrated and purified and by silica gel chromatography (0 to 100% ethyl acetate in hexane, followed by 0 to 40% methanol in ethyl acetate, 12g Claricep column) to afford the title compound (231 mg, 90%) as a clear oil. MS (ESI): m/z = 910.6 [M+H]+1H NMR (499 MHz, DMSO-d6) δ 8.45 (s, 1H), 8.38 (d, J = 5.9 Hz, 1H), 7.82 (s, 1H), 7.48 – 7.29 (m, 4H), 7.25 – 7.17 (m, 4H), 6.92 (s, 1H), 6.78 (d, J = 6.0 Hz, 1H), 5.73 (d, J = 20.1 Hz, 2H), 4.07 – 3.85 (m, 6H), 3.59 – 3.53 (m, 2H), 3.53 – 3.45 (m, 4H), 3.38 (dt, J = 21.8, 5.4 Hz, 5H), 3.22 (q, J = 5.9 Hz, 2H), 2.19 (t, J = 6.8 Hz, 2H), 2.10 (dd, J = 13.5, 3.8 Hz, 2H), 1.70 (tt, J = 12.7, 7.9 Hz, 7H) Example 5: Preparation of (R)-(6-((17-azido-3,6,9,12,15-pentaoxaheptadecyl) oxy)naphthalen-1-yl)(5-methyl-3-(morpholinomethyl)-2,3-dihydro-[1,4]oxazino[2,3,4- hi]indol-6-yl)methanone (BA-177) STEP 1: methyl 6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy)-1-naphthoate To a stirred solution of methyl 6-hydroxynaphthalene-1-carboxylate (5.0 g, 25 mmol) in acetonitrile (150 mL) were added K2CO3 (5.13 g, 37.1 mmol) and 2,2,3,3-tetramethyl- 4,7,10, 13,16,19-hexaoxa-3-silahenicosan-21-yl 4-methylbenzenesulfonate (15.0 g, 27.2 mmol) at room temperature. The resulting mixture was stirred overnight at 70°C under nitrogen atmosphere and then allowed to cool to room temperature. The mixture was diluted with ethyl acetate (200mL), washed with water (2 x 50 mL) and brine (2 x 50 mL), dried over anhydrous MgSO4, filtered, and concentrated to afford the title compound as a yellow brown oil. MS (ESI): m/z = 581 [M+H]+ STEP 2: N-methoxy-N-methyl-6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3- silahenicosan-21-yl)oxy)-1-naphthamide To a stirred mixture of methyl 6-[(2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3- silahenicosan-21-yl)oxy]naphthalene-1-carboxylate (15 g, 26 mmol) and N,O- dimethylhydroxyl amine hydrochloride (3.78 g, 38.7 mmol) in THF (300 mL) were added i- PrMgCl (99 mL, 129 mmol) dropwise at 0°C under argon atmosphere. The resulting mixture was stirred for 1 hour at 0°C under argon atmosphere. The reaction was quenched by the addition of sat. NH4Cl (aq.) (60 mL) at room temperature and extracted with ethyl acetate (3 x 60 mL). The combined organic extracts were washed with brine (2 x 20 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with CH2Cl2 / EA (1:1) to afford the title compound (9.6 g, 52 %) as a yellow oil. MS (ESI): m/z = 610 [M+H]+ STEP 3: 1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21-yl)oxy) naphthalen-1-yl)ethan-1-one To a stirred solution of N-methoxy-N-methyl-6-[(2,2,3,3-tetramethyl-4,7,10,13,16,19- hexaoxa-3-silahenicosan-21-yl)oxy]naphthalene-1-carboxamide (9.6 g, 16 mmol) in THF (38.4 mL) was added MeMgBr (157 mL, 157 mmol) at room temperature under argon atmosphere. The resulting mixture was stirred for 1h at room temperature, quenched by the addition of aqueous NH4Cl (sat.) (50 mL) and extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine (2 x 60 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with CH2Cl2 / EA (1:1) to afford the title compound (6.5 g, 72 %) as a light brown oil. 1H NMR (300 MHz, DMSO-d6) δ 8.54 (d, J = 9.4 Hz, 1H), 8.05 – 7.92 (m, 2H), 7.53 (dd, J = 8.2, 7.3 Hz, 1H), 7.41 (d, J = 2.7 Hz, 1H), 7.26 (dd, J = 9.4, 2.7 Hz, 1H), 4.26 – 4.16 (m, 2H), 3.84 – 3.75 (m, 2H), 3.68 – 3.36 (m, 20H), 2.68 (s, 3H), 0.82 (s, 9H) MS (ESI): m/z= 565 (M+H)+. STEP 4: 3-hydroxy-1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy)naphthalen-1-yl)butan-1-one A solution of 1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy) naphthalen-1-yl)ethan-1-one (6.5 g, 11 mmol) in THF (260 mL) was treated with LiHMDS (15.9 mL, 0.796 mmol) for 10 min at -40°C under nitrogen atmosphere followed by the addition of CH3CHO (4.2 mL, 44 mmol) in THF dropwise in portions at -40 °C. The resulting mixture was stirred for 30 min at -40°C under nitrogen atmosphere, quenched with aqueous NH4Cl (sat.) at -40°C and extracted with ethyl acetate (3 x 20 mL). The combined organic extracts were washed with brine (3 x 20 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by reversed-phase flash chromatography: column, C18 silica gel; mobile phase, ACN in H2O, 10% to 95% gradient in 30 min; detector, UV 254 nm to afford the title compound (1.65 g, 24 %) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 9.4 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.88 (dd, J = 7.2, 1.2 Hz, 1H), 7.55 (t, J = 7.7 Hz, 1H), 7.43 (d, J = 2.7 Hz, 1H), 7.27 (dd, J = 9.4, 2.6 Hz, 1H), 4.72 (d, J = 5.0 Hz, 1H), 4.23 (dd, J = 5.7, 3.6 Hz, 2H), 4.23 – 4.11 (m, 1H), 3.85 – 3.79 (m, 2H), 3.74 (s, 1H), 3.70 – 3.59 (m, 4H), 3.63 – 3.53 (m, 2H), 3.57 – 3.47 (m, 13H), 3.43 (t, J = 5.2 Hz, 2H), 1.16 (d, J = 6.2 Hz, 3H), 0.85 (s, 9H) MS (ESI): m/z = 609 [M+H]+ STEP 5: 1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan-21- yl)oxy)naphthalen-1-yl)butane-1,3-dione A mixture of 3-hydroxy-1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3- silahenicosan-21-yl)oxy)naphthalen-1-yl)butan-1-one (1.5 g, 2.464 mmol) and Dess-Martin periodinane (1.36 g, 3.20 mmol) in CH3Cl (20 mL) was stirred for 1hour at room temperature under nitrogen atmosphere. The resulting mixture was filtered and the filter cake washed with DCM (3 x 20 mL). The filtrate was concentrated and purified by reversed phase flash chromatography: column, C18 silica gel; mobile phase, ACN in H2O, 10% to 90% gradient in 30 min; detector, UV 254 nm to afford the title compound (1.1 g, 74 %) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 9.3 Hz, 1H), 8.00 (d, J = 8.2 Hz, 1H), 7.65 (d, J = 1.2 Hz, 1H), 7.59 – 7.52 (m, 1H), 7.45 (d, J = 2.7 Hz, 1H), 7.28 (dd, J = 9.4, 2.7 Hz, 1H), 4.29 – 4.16 (m, 2H), 3.83 (dd, J = 3.8, 2.2 Hz, 2H), 3.67 (d, J = 5.0 Hz, 2H), 3.62 (dd, J = 4.6, 1.9 Hz, 2H), 3.58 – 3.54 (m, 2H), 3.53 – 3.49 (m, 14H), 3.42 (dd, J = 6.0, 4.3 Hz, 2H), 2.21 (s, 3H), 0.85 (s, 12H) MS (ESI): m/z = 607 [M+H]+ STEP 6: (R)-(6-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl)oxy)naphthalen-1-yl)(5- methyl-3-(morpholinomethyl)-2,3-dihydro-[1,4]oxazino[2,3,4-hi]indol-6-yl)methanone A mixture of (3R)-3-(morpholin-4-ylmethyl)-2,3-dihydro-1,4-benzoxazin-4-amine (900 mg, 3.610 mmol), 1-(6-((2,2,3,3-tetramethyl-4,7,10,13,16,19-hexaoxa-3-silahenicosan- 21-yl)oxy)naphthalen-1-yl)butane-1,3-dione (1.10 g, 1.81 mmol) in ethanol (18 mL) was stirred for 20 min at room temperature under nitrogen atmosphere, followed by the addition of H2SO4 (5% in ethanol, 3 mL) dropwise at room temperature. The resulting mixture was stirred for 2 hours at 78°C under nitrogen atmosphere, quenched by the addition of Na2HCO3(sat) at 0°C and extracted with ethyl acetate (3 x 30 mL). The combined organic extracts were washed with brine (3 x 30 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by reversed-phase flash chromatography: column, C18 silica gel; mobile phase, ACN in H2O, 10% to 95% gradient in 30 min; detector, UV 254 nm to afford the title compound (704.6 mg, 27 %) as a yellow oil. 1H NMR (300 MHz, DMSO-d6) δ 7.98 (d, J = 8.5 Hz, 1H), 7.72 (d, J = 9.4 Hz, 1H), 7.57 (t, J = 7.7 Hz, 1H), 7.47 (s, 1H), 7.39 – 7.30 (m, 1H), 7.15 (dd, J = 9.2, 3.0 Hz, 1H), 6.85 – 6.73 (m, 1H), 6.64 – 6.55 (m, 1H), 6.32 (d, J = 7.9 Hz, 1H), 4.88 (s, 1H), 4.73 (d, J = 11.6 Hz, 1H), 4.59-4.56 (m, 1H), 4.27 – 4.13 (m, 3H), 3.82 (s, 2H), 3.67 –3.50 (m, 22H), 3.48 – 3.36 (m, 2H), 2.61 (dd, J = 12.7, 7.6 Hz, 1H), 2.49 – 2.18 (m, 8H) MS (ESI): m/z = 707 [M+H]+ STEP 7: (R)-(6-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)naphthalen-1-yl)(5-methyl- 3-(morpholinomethyl)-2,3-dihydro-[1,4]oxazino[2,3,4-hi]indol-6-yl)methanone To a solution of (R)-(6-((17-hydroxy-3,6,9,12,15- pentaoxaheptadecyl)oxy)naphthalen-1-yl)(5-methyl-3-(morpholinomethyl)-2,3-dihydro- [1,4]oxazino[2,3,4-hi]indol-6-yl)methanone (0.25 g, 0.35 mmol) in anhydrous dichloromethane (3.5 mL), Et3N (74 mL, 0.53 mmol) was added followed by the MsCl (30 mL, 0.3 mmol) at 0°C. The mixture was slowly warmed to room temperature and after 1 hour the reaction was quenched with saturated NaHCO3 and extracted with dichloromethane (3 x 50 mL). The combined organic extracts were washed with brine, dried over Na2SO4, filtered and concentrated to afford the crude mesylate which was used in the next step without further purification. To a solution of the mesylate in DMF (3.5 mL), NaN3 (69 mg, 1.06 mmol) was added at room temperature and the reaction mixture was then heated to 90°C for 16 hours, and then cooled to room temperature. The mixture was diluted with water and extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine, dried over Na2SO4, filtered, concentrated and purified by flash chromatography (25g, 20 micron Biotage column) using hexanes /EtOAc, 0-20% to afford the title compound (232 mg, 67.4%) as a brown oil. 1H NMR (500 MHz, DMSO-d6) δ 7.97 (d, J = 10Hz, 1 H), 7.71 (d, J = 5Hz, 1 H), 7.57-7.54 (m, 1 H), 7.46 (d, J = 5Hz, 1 H), 7.34-7.32 (m, 1 H), 6.78 (t, J = 10Hz, 1 H), 6.58(d, J = 10 Hz, 1 H), 6.32 (d, J= 10Hz, 1 H), 4.88-4.85 (br, 1 H), 4.72 (d, J =10 Hz,1 H), 4.24-4.16 (m, 3 H), 3.82-3.80 (m, H), 4.01-3.99 (m, 2 H), 3.61-3.49 (m, 23 H), 3.37 – 3.34 (m, 18 H), 2.49-2.39 (m, 8 H) MS (ESI): m/z = 754.2 [M+Na]+ Example 6: 4-(((2S,4S)-1-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl) ureido)phenyl)acetyl)-4-fluoropyrrolidin-2-yl)methoxy)benzoic acid (BA-210) STEP 1: methyl 4-(((2S,4S)-1-(2-(4-(3-(4-(((tert- butoxycarbonyl)amino)methyl)phenyl)ureido) phenyl) acetyl)-4-fluoropyrrolidin-2- yl)methoxy)benzoate To a solution of 2-(4-(3-(4-(((tert- butoxycarbonyl)amino)methyl)phenyl)ureido)phenyl) acetic acid (prepared as described byTolomelli et. al., "Dehydro-β-proline Containing α4β1 Integrin Antagonists: Stereochemical Recognition in Ligand–Receptor Interplay", ACS Med. Chem. Lett. 2015, 6, 6, 701–706; 0.25 g, 0.626 mmol) in anhydrous DMF (3 ml), methyl 4-(((2S,4S)-4-fluoropyrrolidin-2- yl)methoxy)benzoate (prepared as described by Muro et. al., "A novel and potent VLA-4 antagonist based on trans-4-substituted cyclohexanecarboxylic acid", Bioorg. Med. Chem. 2009, 17(3), 1232-430.23 g, 0.626 mmol), HATU (0.31 g, 0.814 mmol) and triethylamine (0.20 ml, 1.25 mmol) were added. The reaction mixture was stirred at room temperature under inert atmosphere for 3 hours. Water (15 ml) was added and the mixture extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na2SO4, concentrated and purified by silica gel column chromatography using a gradient 50-100% EtOAc in hexane to afford the title compound (0.30 g, 76%) as a yellow oil. MS (ESI) m/z 657.8 [M+Na]+ STEP 2: methyl 4-(((2S,4S)-1-(2-(4-(3-(4-(aminomethyl)phenyl)ureido)phenyl)acetyl)-4- fluoropyrrolidin-2-yl)methoxy)benzoate TFA (0.3 ml) was added dropwise at 0°C to a solution of methyl 4-(((2S,4S)-1-(2-(4- (3-(4-(((tert-butoxycarbonyl)amino)methyl)phenyl)ureido) phenyl) acetyl)-4- fluoropyrrolidin-2-yl)methoxy)benzoate (0.30 g, 0.473 mmol) in DCM (3 ml). The cooling bath was removed, and the mixture was stirred for 2 hours at room temperature, then concentrated to quantitatively afford the title compound as a yellow oil. MS (ESI) m/z 1069.3 [2M]+ STEP 3: methyl 4-(((2S,4S)-1-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl) phenyl)ureido)phenyl)acetyl)-4-fluoropyrrolidin-2-yl)methoxy)benzoate To a solution of methyl 4-(((2S,4S)-1-(2-(4-(3-(4-(aminomethyl)phenyl)ureido) phenyl)acetyl)-4-fluoropyrrolidin-2-yl)methoxy)benzoate (0.28 g, 0.43 mmol) in anhydrous DMF (3 ml), 1-azido-3,6,9,12-tetraoxapentadecan-15-oic acid (0.125 g, 0.43 mmol), HATU (0.21 g, 0.56 mmol) and triethylamine (0.18 ml, 1.29 mmol) were added. The reaction mixture was stirred at room temperature under inert atmosphere for 3 hours, then diluted with water (20 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (10 ml), dried over anhydrous Na2SO4, concentrated and purified by silica gel column chromatography using a gradient 50-100% EtOAc in hexane to afford the title compound (0.20 g, 57%) as a yellow oil. MS (ESI) m/z 808.9 [M+H]+ STEP 4: 4-(((2S,4S)-1-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl)ureido) phenyl)acetyl)-4-fluoropyrrolidin-2-yl)methoxy)benzoic acid To a solution of methyl 4-(((2S,4S)-1-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa- 2-azaheptadecyl) phenyl)ureido)phenyl)acetyl)-4-fluoropyrrolidin-2-yl)methoxy)benzoate (0.20 g, 0.248 mmol) in methanol/water/dioxane (3 ml, 1:1:1), lithium hydroxide (20 mg, 0.744 mmol) was added and stirred at room temperature for 18 hours. The reaction mixture was concentrated under reduced pressure, treated with citric acid solution (10%) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (5 ml), dried over anhydrous Na2SO4 and concentrated to afford the title compound (0.19 g, 97%) as a white solid. MS (ESI) m/z 816.9 [M+Na]+ 1H NMR (500 MHz, DMSO-d6) δ 12.32 (s, 1H), 8.60 (s, 1H), 8.59 (s, 1H), 8.28 (t, J = 5.9 Hz, 1H), 7.89 – 7.85 (m, 1H), 7.38 (d, J = 8.5 Hz, 4H), 7.15 (d, J = 8.4 Hz, 2H), 7.10 – 7.06 (m, 1H), 4.44 – 4.35 (m, 1H), 4.20 (d, J = 5.9 Hz, 2H), 3.94 – 3.76 (m, 3H), 3.67 – 3.46 (m, 17H), 3.40 – 3.35 (m, 3H), 2.75 (d, J = 15.4 Hz, 2H), 2.65 (d, J = 15.4 Hz, 2H), 2.37 (t, J = 6.4 Hz, 2H), 2.32 – 2.22 (m, 2H), 1.91 (s, 2H)
Example 7: Preparation of (S)-2-(1-(4-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl) ureido) phenyl)acetyl)morpholine-3-carbonyl)piperidin-4- yl)acetic acid (BA-215) STEP 1: methyl (S)-2-(1-(4-(2-(4-(3-(4-(((tert-butoxycarbonyl)amino)methyl)phenyl)ureido) phenyl)acetyl) morpholine-3-carbonyl)piperidin-4-yl)acetate To a solution of 2-(4-(3-(4-(((tert- butoxycarbonyl)amino)methyl)phenyl)ureido)phenyl)acetic acid (0.15 g, 0.36 mmol) in anhydrous DMF (3 ml), methyl (S)-2-(1-(morpholine-3-carbonyl)piperidin-4-yl)acetate (prepared as described by Chiba et. al., "Identified a morpholinyl-4-piperidinylacetic acid derivative as a potent oral active VLA-4 antagonist", Bioorg. & Med. Chem. Lett., 15, 1, 41- 45; 97 mg, 0.36 mmol), HATU (0.18 g, 0.47 mmol) and triethyl amine (0.10 ml) were added. The reaction mixture was stirred at room temperature under inert atmosphere for 3 hours and then diluted with water (20 ml) and extracted with ethyl acetate (3 x 20 ml). The combined organic extracts were washed with brine (25 ml), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography using a gradient 0-20% MeOH in DCM to afford the title compound (0.18 g, 57%) as a yellow oil. MS (ESI) m/z 652.6 [M+H]+. STEP 2: methyl (S)-2-(1-(4-(2-(4-(3-(4- (aminomethyl)phenyl)ureido)phenyl)acetyl)morpholine-3-carbonyl)piperidin-4-yl)acetate TFA (0.3 ml) was added dropwise at 0°C to a solution of methyl (S)-2-(1-(4-(2-(4-(3- (4-(((tert-butoxycarbonyl)amino)methyl)phenyl)ureido)phenyl)acetyl) morpholine-3- carbonyl)piperidin-4-yl)acetate (0.18 g, 0.276 mmol) in DCM (2 ml). The cooling bath was removed, and the mixture was stirred for 5 hours at room temperature, then concentrated to quantitatively afford the title compound as a yellow oil. MS (ESI) m/z 552.6 [M+H]+ STEP 3: methyl (S)-2-(1-(4-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl) ureido)phenyl)acetyl)morpholine-3-carbonyl)piperidin-4-yl)acetate To a solution of methyl (S)-2-(1-(4-(2-(4-(3-(4- (aminomethyl)phenyl)ureido)phenyl)acetyl) morpholine-3-carbonyl)piperidin-4-yl)acetate (0.18 g, 0.276 mmol) in anhydrous DMF (1.5 ml), 1-azido-3,6,9,12-tetraoxapentadecan-15- oic acid (80 mg, 0.276 mmol), HATU (0.14 g, 0.36 mmol) and triethylamine (0.20 ml) were added. The reaction mixture was stirred at room temperature under inert atmosphere for 3 hours and then diluted with water (20 ml) and extracted with DCM (3 x 20 ml). The combined organic extracts were washed with brine (15 ml), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography using a gradient 0- 20% MeOH in DCM to afford the title compound (0.11 g, 48%) as a yellow oil. MS (ESI) m/z 825.5 [M+H]+ STEP 4: (S)-2-(1-(4-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl)phenyl)ureido) phenyl)acetyl)morpholine-3-carbonyl)piperidin-4-yl)acetic acid To a solution of methyl (S)-2-(1-(4-(2-(4-(3-(4-(17-azido-3-oxo-6,9,12,15-tetraoxa-2- azaheptadecyl) phenyl)ureido)phenyl)acetyl)morpholine-3-carbonyl)piperidin-4-yl)acetate (0.11 g, 0.248 mmol) in methanol/water/dioxane (1.5 ml, 1:1:1), lithium hydroxide (10 mg, 0.40 mmol) was added and stirred at room temperature for 5 hours. The solvents were evaporated in vacuo and the resulting residue was treated with 10% citric acid solution and purified by reverse-phase column chromatography (C18) using a gradient 0-40% ACN in water (+0.1% formic acid) to afford the title compound as a white solid (56 mg, 51%). MS (ESI) m/z 811.9 [M+H]+1H NMR (500 MHz, DMSO-d6) δ 12.13 (s, 1H) 8.64 (s, 1H), 8.61 (s, 1H), 8.28 (t, J = 5.9 Hz, 1H), 7.41 – 7.32 (m, 4H), 7.18 – 7.09 (m, 4H), 5.12 (s, 1H) 4.20 (d, J = 5.8 Hz, 2H), 3.87 – 3.72 (m, 4H), 3.68 – 3.52 (m, 13H), 3.52 – 3.46 (m, 10H), 3.38 (dd, J = 5.6, 4.3 Hz, 3H), 2.57 (s, 1H), 2.37 (t, J = 6.4 Hz, 2H), 2.21-2.08 (m, 2H), 2.15 (s, 2H), 1.68 (s, 2H) Example 8: Preparation of N-((3s,5s,7s)-adamantan-1-yl)-2-(2-(2-(2-(2- azidoethoxy)ethoxy)ethoxy)ethoxy)- benzamide (BA-236) STEP 1: N-((3s,5s,7s)-adamantan-1-yl)-2-hydroxybenzamide To a stirred solution of 2-hydroxybenzoic acid (0.50 g, 3.620 mmol) in DCM (5 mL) and DMF (5 mL) were added HOBT (0.73 g, 5.430 mmol), EDCI (1.04 g, 5.430 mmol), tricyclo[3.3.1.13,7]decan-1-amine (0.55 g, 3.620 mmol) and DIEA (1.40 g, 10.860 mmol) at room temperature. The resulting mixture was stirred for 4hr at room temperature under nitrogen atmosphere. The reaction was quenched with water and extracted with ethyl acetate. The combined organic extracts were washed with brine, dried over Na2SO4, filtered, concentrated and purified by chromatography C18(ACN/H2O) to afford the title compound (280 mg, 28.5%) as a yellow oil. MS(ESI) m/z= 272.2 [M+H]+ STEP 2: N-((3s,5s,7s)-adamantan-1-yl)-2-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)- benzamide To a stirred solution of 2-hydroxy-N-(tricyclo[3.3.1.13,7]decan-3-yl)benzamide (0.28 g, 1.032 mmol) in acetonitrile (2.8 mL) were added Cs2CO3 (0.67 g, 2.064 mmol) and 11- azido-1-iodo-3,6,9-trioxaundecane (0.34 g, 1.032 mmol) at room temperature. The resulting mixture was stirred for 16 h at RT and then for 4 hours at 50°C under nitrogen. The reaction was quenched with water and extracted with ethyl acetate. The combined extracts were dried over sodium sulfate, filtered, concentrated and purified by chromatography C18(ACN/H2O) to afford the title compound (252.1 mg, 51.2%) as c colorless oil. MS(ESI) m/z=473.3, [M+H]+ 1H NMR (300 MHz, DMSO-d6) δ 7.94 (s, 1H), 7.87 – 7.86 (m, 1H), 7.46 – 7.44 (m, 1H), 7.13 (d, J = 8.3 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 4.25 (t, J = 4.4 Hz, 2H), 3.83 (t, J = 4.3 Hz, 2H), 3.59 – 3.56 (m, 6H), 3.54 (s, 4H), 3.42 – 3.34 (m, 2H), 2.06 (s, 9H), 1.67 (s, 6H) Example 9: Preparation of 1-Azido-N-(3-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5- ylidene)propyl)-N-methyl-3,6,9,12-tetraoxapentadecan-15-amide (BA-135) To a stirred solution of 3-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N- methylpropan-1-amineHCl salt (200 mg, 0.669 mmol, 1 eq) and DIPEA (0.23 mL, 1.338 mmol, 2 eq) in DMF (2 mL) was added 2,5-dioxopyrrolidin-1-yl 1-azido-3,6,9,12- tetraoxapentadecan-15-oate (253 mg, 0.736 mmol, 1.1 eq) and the mixture stirred for 30 min at RT. LCMS showed amide formation. Water (20 mL) was added and the mixture extracted with DCM (2x50 m). The combined organic extracts were dried, concentrated and purified by column chromatography (0-10% MeOH/DCM) to afford the title compound (160 mg, 45%, 95% purity) as pale-yellow oil. LCMS: m/z 537 [M+1] 1H NMR (499 MHz, DMSO- d6) δ 7.30 – 6.97 (m, 8H), 5.90 – 5.71 (m, 1H), 3.58 (ddd, J = 6.7, 4.0, 1.6 Hz, 3H), 3.56 – 3.42 (m, 10H), 3.42 – 3.35 (m, 4H), 3.32 (m, 2H)2.84 (s, 2H), 2.65 (s, 2H), 2.40 (d, J = 7.7 Hz, 2H), 2.26 (br.d, J = 35.2 Hz, 4H) Example 10: Preparation of 1-azido-N-(2-(5-hydroxy-1H-indol-2-yl)ethyl)-3,6,9,12- tetraoxapentadecan-15-amide (BA-136) To a stirred solution of 2-(2-aminoethyl)-1H-indol-5-ol (55 mg, 0.313 mmol, 1 eq) and DIPEA (0.1 mL, 0.625 mmol, 2 eq) in DMF (1 mL) was added 2,5-dioxopyrrolidin-1-yl 1-azido-3,6,9,12-tetraoxapentadecan-15-oate (118 mg, 0.344 mmol, 1.1 eq) and the mixture stirred for 30 min. at RT. LCMS showed amide formation. Water was added (20 mL) and the mixture extracted with DCM (2x50 mL) The combined organic extracts were dried, concentrated, and purified by column chromatography (0-20% MeOH/DCM) to afford the title compound (133 mg, 94%, 95% purity) as a clear oil. LCMS, m/z 450 (M+1) 1H NMR (499 MHz, DMSO- d6) δ 10.46 (d, J = 2.5 Hz, 1H), 8.56 (s, 1H), 7.91 (t, J = 5.7 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.81 (d, J = 2.3 Hz, 1H), 6.58 (dd, J = 8.6, 2.3 Hz, 1H), 3.63 – 3.56 (m, 4H), 3.56 – 3.44 (m, 12H), 3.37 (dd, J = 5.6, 4.3 Hz, 2H), 3.30 – 3.28 (m, 2H), 2.71 (t, J = 7.6 Hz, 2H), 2.31 (t, J = 7.6 Hz, 2H) Example 11: Preparation of N1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-N3,N5- bis(2-hydroxyethyl)benzene-1,3,5-tricarboxamide (BA-137) Trimethyl benzene-1,3,5-tricarboxylate (0.5 g, 2.3 mmol, 1 eq) and 2-(2-(2-(2- azidoethoxy)ethoxy)ethoxy)ethan-1-amine (578 mg, 2.3 mmol, 1 eq) were stirred for 2h at 150°C. The reaction was monitored by LCMS which showed the mono amide as the major product with ~20% diamide. The mixture was cooled and 2-aminoethan-1-ol (1.4 mL, 22.9 mmol, 10 eq) was added and the mixture stirred at 150°C for 3h. LCMS showed the tris- amide as the major product. Saturated sodium chloride solution (20 mL) was added, and the mixture extracted with DCM (5x100 m). The combined organic extracts were dried concentrated and purified by column chromatography (0-20% MeOH/DCM) to provide the title compound (70 mg, 6%, 95% purity) as a pale-yellow oil. LCMS, m/z 450 [M+1] 1H NMR (499 MHz, DMSO- d6) δ 8.70 (t, J = 5.6 Hz, 1H), 8.61 (t, J = 5.6 Hz, 2H), 8.44 – 8.39 (m, 3H), 4.76 (t, J = 5.6 Hz, 2H), 3.60 – 3.50 (m, 14H), 3.45 (q, J = 5.8 Hz, 2H), 3.41 – 3.33 (m, 6H). Example 12: Preparation of N1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-N3,N5- bis(2-hydroxyethyl)benzene-1,3,5-tricarboxamide (BA-144) STEP 1: tert-butyl (2-((2-oxoazepan-3-yl)carbamoyl)phenyl)carbamate To a solution of 2-((tert-butoxycarbonyl)amino)benzoic acid (2.01 g, 8.4 mmol) and 3-aminoazepan-2-one (1.19 g, 9.27 mmol, 1.1 equiv.) in DMF (10 mL) was added DIPEA (3.0 mL, 16.9 mmol, 2 equiv.) and HATU (4.8 g, 12.6 mmol, 1.5 equiv.). The mixture was stirred at 20 °C for 3h. LCMS showed the desired product. The mixture was poured into water (260 mL) and the resulting slurry was briefly sonicated and aged at room temp for 30 min. Precipitated solids were isolated by filtration, washed with water (2x 50 mL) and air- dried to afford the title compound (2.71g, 92%) as a greyish-white solid. LCMS: MS(+) m/z: 348.3 [M+H]+ 1H NMR (499 MHz, DMSO- d6) δ 10.52 (s, 1H), 8.51 (d, J = 7.0 Hz, 1H), 8.18 (dd, J = 8.4, 1.2 Hz, 1H), 7.86 (dd, J = 7.4, 4.9 Hz, 1H), 7.76 (dd, J = 7.9, 1.5 Hz, 1H), 7.47 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.08 (td, J = 7.6, 1.2 Hz, 1H), 4.60 (ddd, J = 11.3, 7.1, 1.7 Hz, 1H), 3.25 (ddd, J = 15.7, 11.2, 4.9 Hz, 1H), 3.10 (dt, J = 13.8, 6.3 Hz, 1H), 1.97 – 1.83 (m, 2H), 1.79 (dd, J = 14.2, 4.3 Hz, 1H), 1.71 (qt, J = 12.7, 3.5 Hz, 1H), 1.58 (qd, J = 13.1, 2.6 Hz, 1H), 1.46 (s, 9H), 1.32 – 1.18 (m, 1H). STEP 2: 2-amino-N-(2-oxoazepan-3-yl)benzamide (TFA salt) TFA (6.0 mL, 78 mmol, 10 eq) was added to a solution of tert-butyl (2-((2- oxoazepan-3-yl)carbamoyl)phenyl)carbamate (2.71 g, 7.8 mmol) in DCM (20 mL) and the mixture stirred at 20°C for 18h. LCMS data showed the desired product. The mixture was concentrated to dryness, redissolved in DCM/EtOAc (1:1) and reconcentrated to yield the title compound (2.74g, 102%) as a thick brown oil. LCMS: MS (+) m/z: 248.9 [M+H]+ 1H NMR (499 MHz, DMSO- d6) δ 8.31 (d, J = 6.9 Hz, 1H), 7.91 (dd, J = 7.4, 4.9 Hz, 1H), 7.65 (dd, J = 7.8, 1.5 Hz, 1H), 7.36 (ddd, J = 8.5, 7.2, 1.5 Hz, 1H), 7.03 (dd, J = 8.2, 1.2 Hz, 1H), 6.97 (td, J = 7.5, 1.2 Hz, 1H), 4.60 (dt, J = 10.4, 3.9 Hz, 1H), 3.23 (ddd, J = 15.7, 11.2, 4.9 Hz, 1H), 3.16 – 3.05 (m, 1H), 1.91 (tt, J = 16.0, 3.0 Hz, 2H), 1.78 (dt, J = 13.5, 4.2 Hz, 1H), 1.69 (qt, J = 15.4, 4.2 Hz, 1H), 1.62 – 1.51 (m, 1H), 1.32 – 1.18 (m, 1H). STEP 3: 5-nitro-N-(2-((2-oxoazepan-3-yl)carbamoyl)phenyl)benzo[b]thiophene-2- carboxamide To a solution of 5-nitrobenzo[b]thiophene-2-carboxylic acid (0.5 g, 2.1 mmol) and 2- amino-N-(2-oxoazepan-3-yl)benzamide (1 g, 3.2 mmol, 1.5 eq) in DMF (10 mL) was added DIPEA (1.6 mL, 9 mmol, 4eq) and HATU (1.3 g, 3.4 mmol, 1.5 eq). The reaction mixture was stirred at 20 °C for 18h during which time an off-white precipitate formed. The mixture was poured into water (200 mL) and sonicated with swirling for 5-10 min. The precipitate was isolated by filtration, washed with water and air-drying to afford the title product (340 mg, 33.5%) as a gray powder. LCMS: MS(+) m/z: 453.3 [M+H]+ 1H NMR (499 MHz, DMSO- d6) δ 12.41 (s, 1H), 9.07 (d, J = 2.3 Hz, 1H), 8.69 (d, J = 7.1 Hz, 1H), 8.44 (dd, J = 8.3, 1.2 Hz, 1H), 8.41 – 8.34 (m, 2H), 8.30 (dd, J = 8.9, 2.3 Hz, 1H), 7.98 – 7.88 (m, 2H), 7.60 (ddd, J = 8.5, 7.3, 1.5 Hz, 1H), 7.28 (td, J = 7.6, 1.2 Hz, 1H), 4.69 (ddd, J = 11.3, 7.1, 1.7 Hz, 1H), 3.30 – 3.21 (m, 1H), 3.17 – 3.08 (m, 1H), 1.98 – 1.88 (m, 2H), 1.84 – 1.75 (m, 1H), 1.80 – 1.67 (m, 1H), 1.71 – 1.54 (m, 1H), 1.33 – 1.20 (m, 1H). STEP 4: 5-amino-N-(2-((2-oxoazepan-3-yl)carbamoyl)phenyl)benzo[b]thiophene-2- carboxamide To a suspension of 5-nitro-N-(2-((2-oxoazepan-3- yl)carbamoyl)phenyl)benzo[b]thiophene-2-carboxamide (0.2 g, 0.44 mmol) and zinc metal (0.057 g, 0.9 mmol, 2 eq) in methanol (9 mL) and water (1 mL) was added ammonium chloride (0.059 g, 1.1 mmol, 2.5 eq). The reaction mixture was stirred at 20 °C overnight. The mixture was treated with sat. NaHCO3 (5 mL) and sonicated with swirling until relatively homogenous (zinc metal is still present on the bottom of the reaction vessel). Filtration, washing with water and air-drying yielded the title compound (178 mg, 95%) as a beige solid. LCMS: MS(+) m/z: 423.4 [M+H]+ 1H NMR (499 MHz, DMSO- d6) δ 12.25 (s, 1H), 8.65 (d, J = 6.8 Hz, 1H), 8.46 (d, J = 8.4 Hz, 1H), 7.90 (d, J = 7.8 Hz, 2H), 7.78 (s, 1H), 7.67 (d, J = 8.7 Hz, 1H), 7.58 (s, 1H), 7.24 (t, J = 7.4 Hz, 1H), 7.07 (s, 1H), 6.87 (d, J = 8.5 Hz, 1H), 5.25 (s, 2H), 4.94 (s, 1H), 4.80 (s, 1H), 4.71 (d, J = 7.7 Hz, 1H), 3.14 – 3.06 (m, 1H), 1.92 (s, 2H), 1.78 (s, 2H), 1.60 (d, J = 12.9 Hz, 1H), 1.34 – 1.16 (m, 1H). STEP 5: 5-(1-azido-3,6,9,12-tetraoxapentadecan-15-amido)-N-(2-((2-oxoazepan-3- yl)carbamoyl)phenyl)benzo[b]thiophene-2-carboxamide To a mixture of 5-amino-N-(2-((2-oxoazepan-3- yl)carbamoyl)phenyl)benzo[b]thiophene-2-carboxamide (0.16 g, 0.4 mmol, 1.5 eq) in dry DMA (2 mL) was added DIPEA (0.13 mL, 0.77 mmol, 3 eq) and 2,5-dioxopyrrolidin-1-yl 1- azido-3,6,9,12-tetraoxapentadecan-15-oate (0.1 g, 0.26 mmol). The reaction mixture was stirred at 20 °C over the weekend, after which LCMS showed partial conversion. HATU (0.5 g) was added, and the mixture stirred overnight. The mixture was diluted with DCM (60 mL) and washed with water (50 mL), sat. NaHCO3 and sat. NaCl, dried over Na2SO4, concentrated, purified on Silicagel (0%-25% MeOH in EtOAc) and dried under high vacuum to afford the title compound (68 mg, 15%) as a beige solid. LCMS: MS(+) m/z: 696.6 [M+H]+ 1H NMR (499 MHz, DMSO- d6) δ 12.37 (s, 1H), 10.16 (s, 1H), 8.66 (d, J = 7.1 Hz, 1H), 8.50 – 8.38 (m, 2H), 8.03 – 7.95 (m, 2H), 7.93 – 7.88 (m, 2H), 7.59 (ddd, J = 10.9, 7.3, 1.8 Hz, 2H), 7.26 (td, J = 7.6, 1.2 Hz, 1H), 4.76 – 4.66 (m, 1H), 3.74 (t, J = 6.2 Hz, 2H), 3.57 – 3.48 (m, 12H), 3.37 (d, J = 4.9 Hz, 1H), 3.11 (dd, J = 14.4, 7.5 Hz, 1H), 2.61 (t, J = 6.4 Hz, 2H), 1.97 – 1.88 (m, 2H), 1.83 – 1.68 (m, 1H), 1.60 (q, J = 11.9, 11.5 Hz, 1H), 1.27 (t, J = 13.1 Hz, 1H), 1.19 – 1.01 (m, 1H) Example 13: Preparation of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7,8- dihydroxy-4-oxochroman-2-yl)benzamide (BA-118) A solution of 2,5-dioxopyrrolidin-1-yl 4-(7,8-dihydroxy-4-oxochroman-2-yl)benzoate (144 mg, 0.365 mmol, 1 eq), and 17-azido-3,6,9,12,15-pentaoxaheptadecan-1-amine (223 mg, 0.73 mmol, 2eq.) in DMF (2 mL) was stirred for 20 min at 60°C. The mixture was then concentrated, and the crude material purified by flash chromatography using Ethyl acetate/MeOH, 0-5% as an eluent, to afford the title compound (160 mg, 75%) as a brown solid. LCMS m/z 587 (M+1) 1H NMR (499 MHz, DMSO- d6) δ 8.71 (t, J = 5.6 Hz, 1H), 8.25 (d, J = 8.6 Hz, 2H), 8.02 (d, J = 8.6 Hz, 2H),, 7.41 (d, J = 8.6 Hz, 1H), 6.99 (s, 1H), 6.96 (d, J = 8.6 Hz, 1H), 3.60 – 3.48 (m, 20H), 3.45 (q, J = 5.8 Hz, 2H), 3.40 – 3.34 (m, 2H) Example 14: Preparation of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7-hydroxy- 8-methoxy-4-oxochroman-2-yl)benzamide (BA-196), N-(17-azido-3,6,9,12,15- pentaoxaheptadecyl)-4-(8-hydroxy-7-methoxy-4-oxochroman-2-yl)benzamide (BA-197), and N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7,8-dimethoxy-4-oxochroman-2- yl)benzamide (BA-198) To a stirred suspension of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7,8- dihydroxy-4-oxochroman-2-yl)benzamide (400 mg, 0.683 mmol, 1 eq) and K2CO3 (188 mg, 1.365 mmol, 2 eq) in DMF (10 mL) was added Iodomethane (0.043 mL, 0.683 mmol, 1 eq), and the mixture stirred overnight at RT. LCMS showed a mixture of starting material, mono and di-methylation. The mixture was concentrated and purified by reverse phase column chromatography using 0-60% Water/MeCN with 0.1% formic acid as eluent, to afford three products, as yellow sticky solids: N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7-hydroxy-8-methoxy-4-oxochroman-2- yl)benzamide (BA-196) (50 mg, 12% yield) 85% purity by NMR (contains 15% regioisomer) LCMS: m/z = 601 [M+1] 1H NMR (500 MHz, DMSO- d6) δ 10.66 (s, 1H), 8.71 (t, J = 5.5 Hz, 1H), 8.16 (d, J = 8.38 Hz, 2H), 8.04 (d, J = 8.38 Hz, 2H) 7.65 (d, J = 8.84 Hz, 1H), 7.03 (s, 1H), 7.02 (d, J = 8.84 Hz, 1H), 3.96 (s, 1H), 3.60 – 3.42 (m, 20H), 3.47 – 3.42 (m, 2H), 3.40 – 3.34 (m, 2H). N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(8-hydroxy-7-methoxy-4-oxochroman-2- yl)benzamide (BA-197) (15 mg, 4% yield) 65% pure by NMR (contains 35% regioisomer and di-methylated product) LCMS: m/z = 601 [M+1] 1H NMR (500 MHz, DMSO- d6) δ 9.75 (s, 1H), 8.71 (q, J = 5.8 Hz, 1H), 8.23 (d, J = 8.6 Hz, 2H), 8.02 (d, J = 8.6 Hz, 2H), 7.53 (d, J = 8.9 Hz, 1H), 7.22 (d, J = 8.9 Hz, 1H), 7.03 (s, 1H), 3.95 (s, 3H), 3.62 – 3.42 (m, 24H), 3.39 – 3.35 (m, 2H). N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7,8-dimethoxy-4-oxochroman-2- yl)benzamide (BA-198) (100 mg, 24% yield) 100% pure LCMS: m/z = 615 [M+1] 1H NMR (500 MHz, DMSO- d6) δ 8.71 (t, J = 5.6 Hz, 1H), 8.16 (d, J = 8.5 Hz, 2H), 8.04 (d, J = 8.5 Hz, 2H), 7.80 (d, J = 9.0 Hz, 1H), 7.30 (d, J = 9.0 Hz, 1H), 7.07 (s, 1H), 3.97 (s, 3H), 3.96 (s, 3H), 3.60 – 3.42 (m, 22H), 3.40 – 3.34 (m, 2H). Example 15: Preparation of N-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-4-(7,8- dihydroxy-4-oxochroman-2-yl)benzamide (BA-167) A suspension of 2,5-dioxopyrrolidin-1-yl 4-(7,8-dihydroxy-4-oxochroman-2- yl)benzoate (665 mg, 1.684 mmol, 1 eq) and 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethan-1- amine (440 mg, 2 mmol, 1.2 eq) and DIPEA (0.5 ml, 3.7 mmol, 2.2 eq) in THF (20 mL) was stirred for 3h at 60°C. The mixture was concentrated, and the resulting solids were triturated with methanol, and filtered to afford the title compound (595 mg, 71%) as a beige solid, with >95% purity by HPLC. LCMS m/z 521 (M+Na) 1H NMR (499 MHz, DMSO- d6) δ 8.71 (t, J = 5.6 Hz, 1H), 8.25 (d, 8.6 Hz, 2H), 8.02 (d, 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 1H), 6.99 (s, 1H), 6.96 (d, J = 8.7 Hz, 1H), 3.61 – 3.51 (m, 12H), 3.45 (q, J = 5.8 Hz, 2H), 3.39 – 3.35 (m, 2H)
Example 16: Preparation of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(6-oxo-6H- [1,3]dioxolo[4,5-h]chromen-8-yl)benzamide (BA-216) To a solution of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(7,8-dihydroxy-4- oxo-4H-chromen-2-yl)benzamide (0.2g, 0.341 mmol) in DMF (5 mL), was added K2CO3 (94 mg, 0.682 mmol) followed by the diiodomethane(50 mL, 0.511mmol) at RT and then heated to 90°C for 12h. The mixture was then cooled, extracted with DCM and water, dried over Na2SO4, filtered, concentrated and purified by flash chromatography (column: Biotage sfar silica HCD Duo 5, 20 micron, 25g) eluted with 0 to 15% DCM:MeOH (15 CV) to afford the title compound (128 mg, 64%) as tan solid. MS(ESI) m/z= 621.1 [M+Na]+ 1H NMR (500 MHz, DMSO-d6) δ 8.72 (t, J = 5.5 Hz, 1H), 8.10 (d, J = 10.0 Hz, 2H), 8.02 (d, J = 10.0Hz, 2H), 7.61 (d, J = 5.0 Hz,1H), 7.16 (d, J = 10 Hz, 1H), 7.05 (s, 1H), 3.59 – 3.36 (m, 24H), 3.33(s, 2H)
Example 17: Preparation of (Z)-1-(3-((3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6- yl)amino)-4-methylphenyl)-3-(3-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2-fluoro- 5-(trifluoromethyl)phenyl)urea (BA-173) STEP 1: methyl 3-(benzyloxy)-2-fluoro-5-(trifluoromethyl)benzoate To a solution of 3-(benzyloxy)-2-fluoro-5-(trifluoromethyl)benzoic acid (4.9 g, 15.593 mmol, 1 eq) in methanol (49.00 mL, 1210.243 mmol, 77.61 eq) was added two drop of H2SO4 (305.85 mg, 3.119 mmol, 0.2 eq) at room temperature under nitrogen atmosphere. The resulting mixture was stirred overnight at room temperature under nitrogen atmosphere. The reaction was quenched by the addition of NaHCO3 (sat) and the resulting mixture was extracted with ethyl acetate (3 x 20mL). The combined organic extracts were washed with brine (3 x 20mL), dried over anhydrous Na2SO4, filtered, and concentrated to afford the crude title compound (5 g, crude) was used in the next step directly without further purification. LCMS-(M+H)+ : 329 1H NMR (400 MHz, Chloroform-d) δ 7.79 (dd, J = 5.5, 2.3, 0.9 Hz, 1H), 7.48 – 7.32 (m, 6H), 5.19 (s, 2H), 3.96 (s, 3H). STEP 2: methyl 2-fluoro-3-hydroxy-5-(trifluoromethyl)benzoate A mixture of methyl 3-(benzyloxy)-2-fluoro-5-(trifluoromethyl)benzoate (5 g, 15.232 mmol, 1 eq) and Pd/C (1.51 g, 14.189 mmol, 0.93 eq) in methanol (150 mL) was stirred for 3 hours at room temperature under hydrogen atmosphere. The mixture was then filtered, and the filter cake washed with methanol (3x30 mL). The filtrate was concentrated to afford the title compound (3.7 g, crude) as a white solid. LCMS: (M+H)+ 239 STEP 3: methyl 2-fluoro-3-((2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan-15- yl)oxy)-5-(trifluoromethyl)benzoate A mixture of methyl 2-fluoro-3-hydroxy-5-(trifluoromethyl)benzoate (3.7 g, 15.537 mmol, 1 eq), K2CO3 (3.22g, 23.306 mmol, 1.5 eq) and TBSO-PEG3-OTs (7.18 g, 15.537 mmol, 1.0 eq) in acetonitrile (111 mL) was stirred overnight at 70°C under nitrogen atmosphere. The reaction was quenched with water and the resulting mixture extracted with ethyl acetate (3 x 50mL). The combined organic extracts were washed with brine (3 x 50mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with EA/PE (30%) to afford the title compound (6 g, 58.4%) as a yellow oil. LCMS: (M+H)+ 529 1H NMR (400 MHz, Chloroform-d) δ 7.83 – 7.77 (m, 1H), 7.43 (dd, J = 7.0, 2.3 Hz, 1H), 4.32 – 4.25 (m, 2H), 3.98 (s, 3H), 3.93 (dd, J = 5.5, 3.8 Hz, 2H), 3.82 – 3.68 (m, 4H), 3.72 – 3.65 (m, 6H), 3.57 (t, J = 5.5 Hz, 2H), 0.91 (s, 9H), 0.08 (s, 6H) STEP 4: 2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan-15-yl)oxy]-5- (trifluoromethyl)benzoic acid To a solution of methyl 2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3- silapentadecan-15-yl)oxy]-5-(trifluoromethyl)benzoate (5.59 g, 10.575 mmol, 1 eq) in THF (55 mL) was added lithium hydroxide in water (26.44 mL, 52.875 mmol, 5 eq) dropwise under nitrogen atmosphere. The resulting mixture was stirred for 2hr at under nitrogen atmosphere and then acetic acid (3.81 g, 63.450 mmol, 6 eq) was added dropwise at room temperature. The resulting mixture was extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine (2 x 50mL), dried over anhydrous Na2SO4, filtered, the concentrated and purified by silica gel column chromatography, eluted with EA/PE (75%) to afford the title compound (4.0 g, 73.5%) as a yellow oil. LCMS: (M+H)+ 515 STEP 5: 15-[2-fluoro-3-isocyanato-5-(trifluoromethyl)phenoxy]-2,2,3,3-tetramethyl- 4,7,10,13-tetraoxa-3-silapentadecane A solution/mixture of 2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3- silapentadecan-15-yl)oxy]-5-(trifluoromethyl)benzoic acid (1.5 g, 2.915 mmol, 1 eq) ,TEA (324.47 mg, 3.207 mmol, 1.1 eq) and DPPA (882.43 mg, 3.207 mmol, 1.1 eq) in toluene (30 mL) was stirred overnight at room temperature under nitrogen atmosphere. The reaction was quenched by the addition of water (5 mL) and extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with brine (3 x 50 mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with EA/PE (~30%) to afford the title compound (740 mg) as a yellow oil. LCMS: (M+H)+ 512 STEP 6: 2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan-15-yl)oxy]-5- (trifluoromethyl) aniline 15-[2-fluoro-3-isocyanato-5-(trifluoromethyl) phenoxy]-2,2,3,3-tetramethyl- 4,7,10,13-tetraoxa-3-silapentadecane (750 mg, 1.466 mmol, 1 eq) in toluene (20.45 mL, 192.237 mmol, 131.13 eq) was heated at 80°C for 2 hours under nitrogen atmosphere and then concentrated to afford the title compound (720 mg, crude) as a yellow oil. LCMS: (M+H)+ 486 STEP 7: 1,3-dimethyl 2-(4-bromo-2-nitrophenyl) propanedioate To 4-bromo-1-fluoro-2-nitrobenzene (4 g, 18.182 mmol, 1 eq) and 1,3-dimethyl propanedioate (3.60 g, 27.249 mmol, 1.50 eq) in DMSO- d6 (40 mL) was added NaH (872.67 mg, 21.818 mmol, 1.2 eq, 60%) in portions at room temperature under nitrogen atmosphere. The resulting mixture was heated at 60°C for 2h under nitrogen atmosphere and then quenched with sat. NH4Cl (aq.)/ice at 0°C and extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with water (4x40 mL) and brine (2x40 mL), dried over anhydrous MgSO4, filtered, and concentrated to afford the title compound (5.68 g, crude). LCMS: (M+H)+ 332 STEP 8: (4-bromo-2-nitrophenyl) acetic acid A solution of 1,3-dimethyl 2-(4-bromo-2-nitrophenyl)propanedioate (5.68 g, 17.103 mmol) in AcOH (57 mL) and HCl (57 mL) was heated at 110°C overnight under argon atmosphere. The mixture was allowed to cool to room temperature and then concentrated. The product was isolated by precipitation by the addition of CH2Cl2 to afford the title compound (2.9 g, 60.5%) as a grey solid. LCMS: (M+H)+ 260 STEP 9: ethyl 2-(4-bromo-2-nitrophenyl) acetate A solution/mixture of (4-bromo-2-nitrophenyl) acetic acid (2.9 g, 10.729 mmol, 1 eq) and H2SO4 (0.42 mL, 7.939 mmol, 0.74 eq) in ethanol was stirred for 2h at 85 °C under argon atmosphere. The reaction was quenched with NaHCO3 at 0°C and extracted with ethyl acetate. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered filtration, and concentrated to afford the title compound (2.9 g, 87.3%) as a brown oil. LCMS: (M+H)+ 288 STEP 10: ethyl 2-{4-[(2-methyl-5-nitrophenyl) amino]-2-nitrophenyl}acetate To a solution of ethyl 2-(4-bromo-2-nitrophenyl)acetate (2.9 g, 10.066 mmol, 1 eq) and 2-amino-4-nitrotoluene (2.30 g, 15.099 mmol, 1.5 eq) in toluene was added with Pd(OAc)2 (0.23 g, 1.007 mmol, 0.1 eq) and xantphos (1.16 g, 2.013 mmol, 0.2 eq) under nitrogen atmosphere followed by the addition of Cs2CO3 (8.20 g, 25.165 mmol, 2.5 eq) in portions at room temperature. The resulting mixture was heated at 80°C for 3 h under nitrogen atmosphere and then cooled to room temperature and extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with brine (3 x 50mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with EA/PE to afford the title compound (2.4 g, 66.35%) as a yellow oil. LCMS: (M+H)+ 360 STEP 11: 6-[(5-amino-2-methylphenyl)amino]-1,3-dihydroindol-2-one A solution/mixture of ethyl 2-{4-[(2-methyl-5-nitrophenyl)amino]-2- nitrophenyl}acetate (2.4 g, 5.566 mmol, 1 eq) and Pd/C (598.23 mg, 5.622 mmol, 1.01 eq) in acetic acid (40 mL) and THF (20 mL) was stirred for overnight at room temperature under hydrogen atmosphere. The resulting mixture was filtered, and the filter cake washed with THF (3x30 mL). The filtrate was concentrated and purified by reversed-phase flash chromatography: column, C18 silica gel; mobile phase, ACN in water, 10% to 95% gradient in 30 min; detector, UV 254 nm, to afford the title compound (1 g, 70.9%) as a yellow oil. LCMS: (M+H)+ 254 1H NMR (400 MHz, Chloroform-d) δ 7.69 (s, 1H), 7.06 (d, J = 7.9 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 6.57 (dd, J = 6.2, 2.2 Hz, 2H), 6.50 (d, J = 2.1 Hz, 1H), 6.33 (dd, J = 8.0, 2.4 Hz, 1H), 5.33 (s, 1H), 3.47 (s, 2H), 2.13 (s, 3H), 2.01 (d, J = 1.8 Hz, 2H) STEP 12: 1-{2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan-15- yl)oxy]-5-(trifluoromethyl)phenyl}-3-{4-methyl-3-[(2-oxo-1,3-dihydroindol-6- yl)amino]phenyl}urea To a solution of 2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan- 15-yl)oxy]-5-(trifluoromethyl)aniline (720 mg, 1.483 mmol, 1.00 eq) and triphosgene (175.99 mg, 0.593 mmol, 0.4 eq) in THF (4 mL, 49.371 mmol, 239.74 eq) was added DIEA (574.91 mg, 4.449 mmol, 3 eq) at 0°C under nitrogen atmosphere and then stirred for 15 min at 0°C. 6-[(5-amino-2-methylphenyl)amino]-1,3-dihydroindol-2-one (751.16 mg, 2.966 mmol, 2.00 eq in THF) was added dropwise/ in portions at 0 °C and the mixture stirred for 30 min at 0 °C under nitrogen atmosphere. The reaction was quenched with NaHCO3 at 0°C and extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with brine (3 x 50mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by silica gel column chromatography, eluted with EA/PE (70%) to afford the title compound (700 mg, 61.7%) as a yellow oil. LCMS: (M+H)+ 765 STEP 13: 1-[2-fluoro-3-(2-{2-[2-(2-hydroxyethoxy)ethoxy]ethoxy}ethoxy)-5- (trifluoromethyl)phenyl]-3-(4-methyl-3-{[(3Z)-2-oxo-3-(1H-pyrrol-2-ylmethylidene)-1H- indol-6- l]amino}phenyl)urea A solution of 1-{2-fluoro-3-[(2,2,3,3-tetramethyl-4,7,10,13-tetraoxa-3-silapentadecan- 15-yl)oxy]-5-(trifluoromethyl)phenyl}-3-{4-methyl-3-[(2-oxo-1,3-dihydroindol-6- yl)amino]phenyl}urea (700 mg, 0.915 mmol, 1 eq), pyrrole-2-carboxaldehyde (104.44 mg, 1.098 mmol, 1.2 eq), and piperidine (155.85 mg, 1.830 mmol, 2.0 eq) in ethanol (7 mL) was heated at 80°C for 2hr under nitrogen atmosphere. The resulting mixture was concentrated under reduced pressure and diluted with THF (7 mL). TBAF (358.92 mg, 1.373 mmol, 1.5 eq) was added and the resulting mixture stirred for 2hr at room temperature under nitrogen atmosphere. The mixture was then extracted with ethyl acetate (3 x 20mL) and the combined organic extracts were washed with brine (3 x 20mL), dried over anhydrous Na2SO4, filtered, concentrated and purified by reversed-phase flash chromatography: column, C18 silica gel; mobile phase, ACN in water, 10% to 95% gradient in 30 min; detector, UV 254 nm, to afford the title compound (545.4 mg, 80.3%) as a red solid. LCMS: (M+H)+ 728 1H NMR (300 MHz, DMSO- d6) δ 13.15 (s, 1H), 10.70 (s, 1H), 9.09 (s, 1H), 8.72 (d, J = 3.0 Hz, 1H), 8.19 (dd, J = 6.3, 1.9 Hz, 1H), 7.60 (s, 1H), 7.48 – 7.39 (m, 2H), 7.35 (d, J = 2.1 Hz, 1H), 7.25 (s, 1H), 7.14 (dd, J = 8.2, 5.6 Hz, 2H), 7.04 (dd, J = 8.1, 2.1 Hz, 1H), 6.73 – 6.66 (m, 1H), 6.57 (dd, J = 8.4, 2.0 Hz, 1H), 6.47 (d, J = 2.0 Hz, 1H), 6.30 (q, J = 2.7 Hz, 1H), 4.56 (t, J = 5.4 Hz, 1H), 4.28 (t, J = 4.4 Hz, 2H), 3.77 (dd, J = 5.5, 3.4 Hz, 2H), 3.60 (dd, J = 5.8, 3.1 Hz, 2H), 3.57 – 3.50 (m, 2H), 3.55 – 3.43 (m, 2H), 3.43 – 3.35 (m, 2H), 3.33 (s, 4H), 2.15 (s, 3H) STEP 14: (Z)-2-(2-(2-(2-(3-(3-(3-((3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)amino)- 4-methylphenyl)ureido)-2-fluoro-5-(trifluoromethyl)phenoxy)ethoxy)ethoxy)ethoxy)ethyl methanesulfonate To a mixture of 1-[2-fluoro-3-(2-{2-[2-(2-hydroxyethoxy)ethoxy]ethoxy}ethoxy)-5- (trifluoromethyl)phenyl]-3-(4-methyl-3-{[(3Z)-2-oxo-3-(1H-pyrrol-2-ylmethylidene)-1H- indol-6- l]amino}phenyl)urea (200 mg, 0.275 mmol, 1 eq) and triethylamine (0.1 ml, 0.7 mmol, 2.6 eq) in dry DCM (2 mL) was added methanesulfonyl chloride (0.025 mL, 0.33 mmol, 1.2 eq). The solution was stirred for 30 min at RT. LCMS showed formation of desired product with about 20% di-mesyl. The reaction was quenched with aq. saturated NaHCO3 (5 mL), extracted with DCM 2x50 mL, concentrated, and purified by column chromatography using 0-20% MeOH/DCM, to afford the title compound (170 mg) as a brown gum. LCMS (m/z = 806 M+1) 1H NMR (499 MHz, DMSO- d6) δ 13.15 (s, 1H), 10.70 (s, 1H), 9.09 (d, J = 4.0 Hz, 1H), 8.71 (d, J = 2.9 Hz, 1H), 8.18 (dd, J = 6.2, 2.2 Hz, 1H), 7.59 (s, 1H), 7.43 (d, J = 8.5 Hz, 2H), 7.38 – 7.33 (m, 1H), 7.25 (td, J = 2.6, 1.5 Hz, 1H), 7.13 (dd, J = 9.8, 6.9 Hz, 2H), 7.01 (ddd, J = 25.8, 8.2, 2.2 Hz, 1H), 6.77 – 6.67 (m, 1H), 6.57 (dd, J = 8.3, 2.0 Hz, 1H), 6.47 (d, J = 2.0 Hz, 1H), 6.29 (dt, J = 3.6, 2.4 Hz, 1H), 4.32 – 4.25 (m, 4H), 3.80 – 3.74 (m, 2H), 3.68 – 3.63 (m, 2H), 3.62 – 3.54 (m, 5H), 3.54 (dd, J = 5.1, 2.0 Hz, 4H), 3.16 (s, 3H), 2.15 (d, J = 2.7 Hz, 3H) STEP 15: (Z)-1-(3-((3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)amino)-4- methylphenyl)-3-(3-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethoxy)-2-fluoro-5- (trifluoromethyl)phenyl)urea (Z)-2-(2-(2-(2-(3-(3-(3-((3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)amino)-4- methylphenyl)ureido)-2-fluoro-5-(trifluoromethyl)phenoxy)ethoxy)ethoxy)ethoxy)ethyl methanesulfonate (170 mg, 0.211 mmol, 1 eq), and sodium azide (41 mg, 0.634 mmol, 3 eq) in dry DMF (1 mL) was heated at 50°C for 12 h. The mixture was cooled and diluted with water 5mL, extracted with DCM (20 mL), dried over Na2SO4, filtered, concentrated and purified by column chromatography using 0-20% MeOH/DCM, to afford the title compound (60 mg, 34%) as a brick red solid (98% HPLC purity). LCMS m/z-775 (M++Na) 1H NMR (499 MHz, DMSO- d6) δ 13.15 (s, 1H), 10.70 (s, 1H), 9.09 (s, 1H), 8.72 (d, J = 2.8 Hz, 1H), 8.18 (dd, J = 6.3, 2.1 Hz, 1H), 7.59 (s, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 2.2 Hz, 1H), 7.25 (td, J = 2.6, 1.4 Hz, 1H), 7.13 (dd, J = 10.7, 7.9 Hz, 2H), 7.04 (dd, J = 8.2, 2.2 Hz, 1H), 6.70 (dt, J = 3.7, 1.7 Hz, 1H), 6.57 (dd, J = 8.3, 2.0 Hz, 1H), 6.47 (d, J = 2.0 Hz, 1H), 6.29 (dt, J = 3.6, 2.4 Hz, 1H), 4.30 – 4.25 (m, 2H), 3.80 – 3.74 (m, 2H), 3.62 – 3.57 (m, 4H), 3.57 – 3.49 (m, 6H), 3.37 (dd, J = 5.6, 4.3 Hz, 3H), 2.15 (s, 3H). Example 18: Preparation of 2-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one (BA-183) STEP 1: methyl 4-(benzyl(methyl)amino)benzoate A solution of methyl 4-(methylamino) benzoate (10 g, 60.54 mmol, 1 eq), bromomethylbenzene (12.42 g, 72.64 mmol, 8.63 mL, 1.2 eq) and K2CO3 (25.10 g, 181.61 mmol, 3 eq) in DMF (100 mL) was stirred at 80°C for 2 hours. The mixture was then concentrated, water (600mL) was added and extracted with DCM (800 mL × 3). The combined organic extracts were dried over Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 120 g SepaFlash® Silica Flash Column), using 0~10% Ethyl acetate/Petroleum ether gradient @ 80 mL/min) to afford the title compound (14.3 g, 92.5%) as a yellow solid. MS ES+: 256.0 1H NMR (400 MHz, DMSO- d6) δ = 7.77 - 7.70 (m, 2H), 7.35 - 7.29 (m, 2H), 7.27 - 7.16 (m, 3H), 6.75 (d, J = 9.1 Hz, 2H), 4.68 (s, 2H), 3.74 (s, 3H), 3.11 (s, 3H) STEP 2: 4-(benzyl(methyl)amino)benzoic acid To a solution of methyl 4-[benzyl(methyl)amino] benzoate (14.3 g, 56.01 mmol, 1 eq) in THF (100 mL) was added lithium hydroxide (1 M in water, 560 mL, 10 eq) and the mixture was stirred at 25°C for 0.5 hr. The pH was adjusted with 1M HCl until a white solid precipitated out at 0 ℃. The mixture was diluted with water (200 mL) and filtered, and the filter cake dried under reduced pressure to afford the title compound (13.49 g, 99.8%) as a white solid which was used without further purification. MS ES+: 241.9 1H NMR (400 MHz, DMSO- d6) δ = 7.73 (d, J = 8.8 Hz, 2H), 7.37 - 7.28 (m, 2H), 7.28 - 7.12 (m, 3H), 6.73 (d, J = 8.9 Hz, 2H), 4.67 (s, 2H), 3.10 (s, 3H). STEP 3: 3-acetamido-6-acetyl-2-nitrophenyl 4-(benzyl(methyl)amino)benzoate To a mixture of 4-[benzyl(methyl)amino] benzoic acid (1.5 g, 6.22 mmol, 1 eq) and N-(4-acetyl-3-hydroxy-2-nitro-phenyl) acetamide (1.48 g, 6.22 mmol, 1 eq) in DCM (15 mL) was added EDCI (2.38 g, 12.43 mmol, 2 eq) and DMAP (151.90 mg, 1.24 mmol, 0.2 eq) in one portion at 25°C. The mixture was stirred at 25 °C for 2 hours and then concentrated under reduced pressure and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent of 0~35% Ethyl acetate/Petroleum ether gradient @ 50 mL/min) to afford the title compound (1.2 g, 41.8%) as a yellow solid. MS ES+: 462.2 STEP 4: 7-amino-2-(4-(benzyl(methyl)amino)phenyl)-8-nitro-4H-chromen-4-one To (3-acetamido-6-acetyl-2-nitro-phenyl) 4-[benzyl(methyl)amino] benzoate (1.2 g, 2.60 mmol, 1 eq) in THF (15 mL) was added NaH (312.02 mg, 7.80 mmol, 60% purity, 3 eq) in one portion at 0 °C under nitrogen atmosphere. The mixture was stirred at 0-25°C for 1hr and then poured into aq. NH4Cl (600 mL) and extracted with ethyl acetate (800 mL × 3). The combined organic layers were concentrated to afford the crude product (1.2 g, crude) which was used in next step without further purification. To a mixture of the crude (1.2 g, 2.60 mmol, 1 eq) in acetic acid (12 mL) was added H2SO4 (0.36 mL) in one portion at 25°C. The mixture was heated to 110°C for 30 minutes and then quenched by addition aq. The pH was adjusted to pH=7 by addition of NaHCO3 (800 ml) at 0 °C, and then extracted with ethyl acetate (400 mL × 3). The combined organic extracts were dried over Na2SO4, filtered, and concentrated under reduced pressure to afford the title compound (600 mg, crude) as a yellow solid which was used for the next step without further purification. MS ES+: 402.0 STEP 5: 7,8-diamino-2-(4-(methylamino)phenyl)-4H-chromen-4-one A mixture of 7-amino-2-[4-[benzyl(methyl)amino]phenyl]-8-nitro-chromen-4-one (300 mg, 747.36 μmol, 1 eq), Pd/C (300 mg, 10% purity) and Pd(OH)2 (300 mg, 20% purity) in methanol (5 mL) was degassed and purged with H2 (x3), and then the mixture was stirred at 25°C for 1 hour under H2 atmosphere (15 Psi). The reaction mixture was filtered and concentrated under reduced pressure to afford the title compound the diamine 60 (200 mg, 95.1%) as a yellow solid which was used for next step without further purification. MS ES+: 282.1. STEP 6: tert-butyl 1-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oate To a solution of tert-butyl prop-2-enoate (585.42 mg, 4.57 mmol, 662.99 uL, 1.5 eq) in THF (10 mL) under nitrogen atmosphere were added NaOMe (5.4 M, 5.64 uL, 0.01 eq) followed by 2-[2-[2-[2-(2-benzyloxyethoxy) ethoxy] ethoxy] ethoxy] ethanol (1 g, 3.05 mmol, 1 eq). The reaction mixture was stirred at 25 °C for 8 hr and then quenched by addition of water (30mL) and partitioned between ethyl acetate (50 mL) and brine (20mL). The aqueous phase was further extracted with ethyl acetate (2×40 mL) and the combined organic extracts were washed with brine (2×50 mL), dried over Na2SO4, filtered and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent of 0~30% Ethyl acetate/Petroleum ether gradient @ 50 mL/min) to afford the title compound (960 mg,69.0%) as a yellow oil. MS ES+: 456. 1H NMR (400 MHz, DMSO- d6) δ = 7.41 - 7.19 (m, 5H), 4.48 (s, 2H), 3.59 - 3.48 (m, 22H), 2.41 (t, J = 6.3 Hz, 2H), 1.39 (s, 9H). STEP 7: 1-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oic acid A solution of tert-butyl 1-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oate (640 mg, 1.40 mmol, 1 eq) in HCl/dioxane (3 mL) was stirred at 25°C for 0.5 hr. The reaction mixture was concentrated under reduced pressure to afford the title compound (600 mg, crude) as a yellow oil which was used for next step without further purification. MS ES+: 401.2 STEP 8: N-(8-amino-2-(4-(methylamino)phenyl)-4-oxo-4H-chromen-7-yl)-1-phenyl- 2,5,8,11,14,17-hexaoxaicosan-20-amide To a mixture of 7,8-diamino-2-[4-(methylamino)phenyl]chromen-4-one (290 mg, 1.03 mmol, 1 eq) and 1-phenyl-2,5,8,11,14,17-hexaoxaicosan-20-oic acid (412.83 mg, 1.03 mmol, 1 eq) in DMF (2 mL) was added DIPEA (399.71 mg, 3.09 mmol, 538.69 μL, 3 eq) and HATU (587.97 mg, 1.55 mmol, 1.5 eq) in one portion at 0 °C. The mixture was stirred at 25°C for 0.5 hr and then concentrated and purified by flash silica gel chromatography (ISCO®; 12 g SepaFlash® Silica Flash Column, Eluent of 0~10% Ethyl acetate/Petroleum ether gradient @ 40 mL/min) to afford the title compound (490 mg, 71.6%) as a yellow oil. MS ES+: 664.4 STEP 9: 8-(4-(methylamino)phenyl)-2-(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)chromeno[7,8-d]imidazol-6(3H)-one N-(8-amino-2-(4-(methylamino)phenyl)-4-oxo-4H-chromen-7-yl)-1-phenyl- 2,5,8,11,14,17-hexaoxaicosan-20-amide (490 mg, 738.22 μmol, 1 eq) in acetic acid (10 mL) was heated to 110°C for 30 minutes. The reaction was quenched by addition aq. NaHCO3 (300 mL) to pH = 7-8 at 0 °C, and then extracted with ethyl acetate (3x200 mL). The combined organic extracts were dried over Na2SO4, filtered, and concentrated to afford the title compound (300 mg, crude) as a yellow oil which was used for next step without further purification. MS ES+: 646.2 STEP 10: 2-(17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one A mixture of 2-[2-[2-[2-[2-[2-(2- benzyloxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethyl]-8-[4-(methylamino)phenyl]-3H- pyrano[2,3-e]benzimidazol-6-one (300 mg, 464.58 umol, 1 eq) and Pd(OH)2 (255.32 mg, 363.61 μmol, 20% purity) in methanol (10 mL) was degassed and purged with H2 gas (x3), and then the mixture was stirred at 25°C for 1 hour under H2 atmosphere (15 Psi). The mixture was then filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 12 g SepaFlash® Silica Flash Column, Eluent of 0~10% Ethyl acetate/Petroleum ether gradient @ 40 mL/min) to afford the title compound (185 mg, 71.7%) as a yellow oil. MS ES+: 556.2 STEP 11: 2-(17-chloro-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one To a solution of 2-[2-[2-[2-[2-[2-(2- hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethyl]-8-[4-(methylamino)phenyl]-3H- pyrano[2,3-e]benzimidazol-6-one (240 mg, 431.95 μmol, 1 eq) in CHCI3 (0.5 mL) was added SOCl2 (513.89 mg, 4.32 mmol, 313.35 μL, 10 eq) dropwise at 0°C. The mixture was heated at 60°C for 10 min and the reaction was then quenched by addition aq. NaHCO3 (5 ml) to pH = 7-8 at 0 °C, and then extracted with ethyl acetate (30 mL × 3). The combined organic extracts were dried over Na2SO4, filtered, concentrated and was purified by prep. HPLC (Welch Xtimate C18150×30mm×5um); Mobile Phase A [water(NH3H2O+NH4HCO3)- ACN]; Mobile Phase B: acetonitrile, Flow rate: 25 mL/min, gradient condition from 14% B to 54%). The isolated residue was partitioned between acetonitrile (2 mL) and water (10 mL) and the solution lyophilized to dryness to afford the title compound (25.02 mg, 9.6%, 95.5% purity) as a yellow oil. MS ES+: 574.5 1H NMR (400 MHz, DMSO- d6) δ = 8.02 - 7.86 (m, 2H), 7.77 (d, J = 8.5 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 6.76 (s, 1H), 6.70 (d, J = 8.9 Hz, 2H), 6.55 (q, J =4.6 Hz, 1H), 3.91 (t, J = 6.6 Hz, 2H), 3.71 - 3.61 (m, 4H), 3.59 - 3.55 (m, 2H), 3.52 (dd, J = 2.9, 5.4 Hz, 4H), 3.48 - 3.43 (m, 10H), 3.17 (br t, J = 6.6 Hz, 2H),2.78 (d, J = 4.9 Hz, 3H). STEP 12: 2-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-8-(4- (methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one 2-(17-chloro-3,6,9,12,15-pentaoxaheptadecyl)-8-(4-(methylamino)phenyl)chromeno [7,8-d]imidazol-6(3H)-one (57 mg, 0.099 mmol, 1 eq), and sodium azide (32 mg, 0.497 mmol, 5 eq) in dry DMF (1 mL) were heated at 70°C for 12 h. The mixture was cooled and diluted with brine (20 mL), extracted with DCM (50 mL), dried over Na2SO4, filtered, concentrated and purified by column chromatography using 0-10% MeOH/DCM, to afford the title compound (29:71 tautomeric mixture) (51 mg, 88%) as a yellow gum, >95% LCMS and HPLC purity. LCMS m/z 580, 603 (M+Na) 1H NMR (499 MHz, DMSO- d6) δ 12.83 (s, 1H), 7.92 (s, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.52 (s, 1H), 6.76 (s, 1H), 6.73 – 6.67 (m, 2H), 6.56 (q, J = 4.9 Hz, 1H), 3.91 (t, J = 6.6 Hz, 2H), 3.60 – 3.40 (m, 18H), 3.36 (dd, J = 5.6, 4.3 Hz, 2H), 3.17 (t, J = 6.6 Hz, 2H), 2.78 (d, J = 4.9 Hz, 3H). Example 19: Preparation of N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(8-hydroxy- 4-methoxyquinolin-2-yl)benzamide (BA-225) STEP 1: methyl 4-(8-hydroxy-4-methoxyquinolin-2-yl)benzoate To a solution of 1-(2-amino-3-hydroxyphenyl)ethan-1-one (2.15g, 14.2 mmol) in methanol (30 mL), was added methyl 4-formylbenzoate (7.0 g, 43 mmol) followed by the sulfuric acid (650 mL, 12 mmol) and the mixture heated at reflux for 48 hours. The mixture was then concentrated and purified by flash column chromatography (column: Biotage sfar silica HCD Duo 5, 20 micron,100g) eluted with 0 to 100% EA in hexane (15 CV) to afford the title compound (495 mg, 11%) as yellow solid. MS(ESI) m/z= 310 [M+H]+. 1H NMR (500 MHz, DMSO-d6) δ 9.67 (br, 1H), 8.01 (d, J = 10.0Hz, 2H), 7.66 (s, 1H), 7.54 (d, J = 10 Hz, 2H), 7.39 (t, J = 10 Hz,1H), 7.12 (d, J = 10 Hz, 1H), 4.19(s, 3H), 3.89(s, 3H). STEP 2: N-(17-azido-3,6,9,12,15-pentaoxaheptadecyl)-4-(8-hydroxy-4-methoxyquinolin-2- yl)benzamide To a solution of methyl 4-(8-hydroxy-4-methoxyquinolin-2-yl)benzoate (495 mg, 1.6 mmol) in THF:H2O (6 mL:1 mL, v/v), lithium hydroxide (192 mg, 8.35 mmol) was added and the mixture stirred at room temperature for 3hours. The mixture was then concentrated and dissolved in DMF (8 mL). HATU (1.03g, 2.72 mmol) and N3-PEG5-NH2 (686 mg, 2.24 mmol) were added at RT, followed by the addition of DIPEA (0.9 mL, 4.8 mmol) and stirred for 30 minutes. The reaction mixture was diluted with water and extracted with DCM. The combined organic extracts were dried over Na2SO4, filtered, concentrated and purified by flash column chromatography (column: Biotage sfar silica HCD Duo 5, 20 micron, 50g) eluted with MeOH:DCM (0 to 20%, v/v, 10CV) to afford the title compound (770 mg, 83%) as a brown solid. MS(ESI) m/z= 584.0 [M+H]+ 1H NMR (500 MHz, DMSO-d6) δ 9.55 (br, 1H), 8.68 (t, J = 5 Hz, 1H), 8.01 (d, J = 10.0 Hz, 2H), 7.66 (s,1H), 7.54 (d, J = 10 Hz, 1H), 7.39 (t, J = 10 Hz,1H), 7.12 (d, J = 10 Hz, 1H), 4.19(s, 3H), 3.59-3.38 (m, 24H) Example 20: Preparation of 8-((1-(10-azidodecyl)-1H-1,2,3-triazol-4-yl)methoxy)-7- hydroxy-2-phenyl-4H-chromen-4-one (BA-129) STEP 1: 7-hydroxy-2-phenyl-8-(prop-2-yn-1-yloxy)-4H-chromen-4-one To a stirred suspension of 7,8-dihydroxy-2-phenyl-4H-chromen-4-one (1.5 g, 5.906mmol, 1 eq) and K2CO3 (0.815 g, 5.906 mmol, 1 eq) in DMF (20 mL) was added propargyl bromide (0.447 mL, 5.906 mmol, 1 eq) and the mixture stirred overnight at room temperature. Water (100 mL) was added resulting in the formation of a precipitate, which was removed by filtration. The filtrate was extracted with DCM (2x100 mL), and the combined extracts were dried over Na2SO4, filtered, concentrated, and the resulting solid was triturated with methanol, isolated by filtration, and dried under high vacuum to obtain the title compound (500 mg, 29%) as a white solid. LCMS: m/z = 293 M+11H NMR (499 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.17 – 8.09 (m, 2H), 7.68 (d, J = 8.8 Hz, 1H), 7.65 – 7.55 (m, 3H), 7.03 (d, J = 8.8 Hz, 1H), 6.94 (s, 1H), 4.90 (d, J = 2.5 Hz, 2H), 3.54 (t, J = 2.4 Hz, 1H).1H NMR (499 MHz, CD3OD) δ 8.15 – 8.07 (m, 2H), 7.80 (d, J = 8.9 Hz, 1H), 7.63 – 7.53 (m, 3H), 7.03 (d, J = 8.9 Hz, 1H), 6.85 (s, 1H), 4.95 (d, J = 2.5 Hz, 2H), 2.91 (t, J = 2.4 Hz, 1H) STEP 2: 8-((1-(10-bromodecyl)-1H-1,2,3-triazol-4-yl)methoxy)-7-hydroxy-2-phenyl-4H- chromen-4-one To a solution of 7-hydroxy-2-phenyl-8-(prop-2-yn-1-yloxy)-4H-chromen-4-one (0.15 g, 0.514 mmol, 1 eq) and 1-azido-10-bromodecane (0.162 g, 0.616 mmol, 1.2 eq) in THF (5 mL) at room temperature was added a solution of CuSO4·5H2O (64 mg, 0.257 mmol, 0.5 eq) in water (1 mL) followed by a solution of sodium ascorbate (76 mg, 0.385 mmol, 0.75 eq) in water (1 mL). The reaction was stirred for 2 hours at room temperature and then diluted with DCM (50 mL) and washed with NaHCO3 (50 mL) The aqueous phase was extracted with DCM (2x100 mL) and the organic extracts were dried over Na2SO4, filtered, concentrated and purified by chromatography, 0-100% EtOAc/Hexane, to afford the title compound (280 mg, 98%) as a gum. LCMS: m/z = 554 (M+) STEP 3: 8-((1-(10-azidodecyl)-1H-1,2,3-triazol-4-yl)methoxy)-7-hydroxy-2-phenyl-4H- chromen-4-one A solution of 8-((1-(10-bromodecyl)-1H-1,2,3-triazol-4-yl)methoxy)-7-hydroxy-2- phenyl-4H-chromen-4-one (0.275 g, 0.496 mmol, 1eq) and sodium azide (74 mg, 2.482 mmol, 5 eq) in dry DMF (5 mL) under argon was heated to 60°C for 2 hours. The mixture was cooled to room temperature, diluted with aqueous saturated bicarbonate (50 mL) and extracted with ethyl acetate (100 mL). The combined organic extracts were washed with brine (100 mL), dried over Na2SO4, filtered, concentrated and purified by silica gel chromatography, eluting with 0-100% EtOAc/Hexane to afford the title compound (220 mg, 84%) as a beige solid. 99% purity by HPLC. LCMS m/z 517 (M+1) 1H NMR (499 MHz, DMSO-d6) δ 10.80 (s, 1H), 8.10 (s, 1H), 8.01 – 7.94 (m, 2H), 7.65 (d, J = 8.8 Hz, 1H), 7.61 – 7.52 (m, 3H), 7.04 (d, J = 8.7 Hz, 1H), 6.87 (s, 1H), 5.26 (s, 2H), 4.20 (t, J = 7.0 Hz, 2H), 3.30 (t, J = 7.0 Hz, 2H), 1.59 (p, J = 7.1 Hz, 2H), 1.51 (dq, J = 8.3, 6.8 Hz, 2H), 1.32 – 1.07 (m, 10H), 1.04 (q, J = 6.9 Hz, 2H). Example 21: Preparation of 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one (BA- 169) STEPS 1-4 STEP 1: 1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl 4-methylbenzenesulfonate To a solution of 2-[2-[2-[2-[2-(2- benzyloxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethanol (5 g, 13.42 mmol, 1 eq) in DCM (100 mL) was added TEA (2.72 g, 26.85 mmol, 3.74 mL, 2 eq), 4-toluenesulfonyl chloride (2.82 g, 14.77 mmol, 1.1 eq) and DMAP (164.01 mg, 1.34 mmol, 0.1 eq). The mixture was stirred at 30°C for 5 hours, concentrated under reduced pressure and the residue purified by flash silica gel chromatography (ISCO®; 40 g SepaFlash® Silica Flash Column, Eluent of 0~50% Ethyl acetate/Petroleum ether gradient @ 100 mL/min) to afford the title compound (6.5 g, 92% yield) as a colorless oil. MS ES+: 527.1. 1H NMR (400 MHz, CDCI3) δ = 7.80 (d, J = 8.3 Hz, 2H), 7.38 - 7.32 (m, 6H), 7.31 - 7.28 (m, 1H), 4.57 (s, 2H), 4.18 - 4.14 (m, 2H), 3.69 - 3.62 (m, 18H), 3.58 (s, 4H), 2.45 (s, 3H). STEP 2: ethyl 4-((1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate To a solution of 1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl 4- methylbenzenesulfonate (3.25 g, 6.17 mmol, 1 eq) and ethyl 4-aminobenzoate (1.02 g, 6.17 mmol, 1.29 mL, 1 eq) in DMF (10 mL) was added K2CO3 (2.56 g, 18.51 mmol, 3 eq) and KI (1.02 g, 6.17 mmol, 1 eq). The mixture was stirred at 180°C for 3 hours under microwave. The mixture was cooled to room temperature, concentrated and the residue purified by flash silica gel chromatography (ISCO®; 80 g SepaFlash® Silica Flash Column, Eluent of 0~60% Ethyl acetate/Petroleum ether gradient @ 100 mL/min) to afford the title compound (2 g, 31%) as a yellow oil. MS ES+: 520.2. 1H NMR (400 MHz, CDCl3) δ = 7.91 - 7.82 (m, 2H), 7.34 (d, J = 4.4 Hz, 4H), 7.32 - 7.28 (m, 1H), 6.62 (d, J = 8.6 Hz, 2H), 4.56 (s, 2H), 4.32 (q, J = 7.1 Hz, 2H), 3.72 (t, J = 5.1 Hz, 2H), 3.67 - 3.64 (m, 18H), 3.64 - 3.61 (m, 2H), 3.35 (t, J = 5.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). STEP 3: ethyl 4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate To a solution of ethyl 4-((1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)benzoate (1 g, 1.92 mmol, 1 eq) in THF (10 mL) was added NaH (115.46 mg, 2.89 mmol, 60% purity, 1.5 eq) at 0°C under nitrogen atmosphere. After stirring for 0.5 hour, methyl iodide (1.37 g, 9.62 mmol, 599.02 uL, 5 eq) was added and the mixture stirred at 25°C for 4 hours under nitrogen. The above reaction was repeated and the two reactions were worked up together. The reaction mixtures were quenched by addition of aq. NH4Cl (20 mL) at 0°C, and then diluted with water (50 mL) and extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with brine (100 mL x 1), dried over anhydrous Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 40 g SepaFlash® Silica Flash Column, Eluent of 0~60% Ethyl acetate/Petroleum ether gradient @ 100 mL/min) to afford the title compound (1.7 g, 83%) as a yellow oil. MS ES+: 534.7 1H NMR (400 MHz, CDCI3) δ = 7.94 - 7.87 (m, 2H), 7.34 (d, J = 4.4 Hz, 4H), 7.31 - 7.28 (m, 1H), 6.70 (d, J = 9.0 Hz, 2H), 4.57 (s, 2H), 4.33 (q, J = 7.1 Hz, 2H), 3.70 - 3.59 (m, 24H), 3.07 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H) STEP 4: 4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoic acid To a solution of ethyl 4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)benzoate (1.2 g, 2.25 mmol, 1 eq) in THF (12 mL) was added a solution of NaOH (269.82 mg, 6.75 mmol, 3 eq) in water (4 mL). The mixture stirred at 65°C for 2 hours and then cooled to room temperature, concentrated, diluted with water (10 mL), acidified with 1N HCl to pH=5-6 and extracted with ethyl acetate (20 mL x 3). The combined organic extracts were washed with brine (40 mL x 1), dried over anhydrous Na2SO4, filtered, and concentrated to afford the title compound (1.1 g, crude) as a yellow oil which was used into the next step without further purification. MS ES+: 506.1. STEPS 5-9 STEP 5: 1-(4-fluoro-2-hydroxy-3-nitrophenyl)ethan-1-one To a solution of H2SO4 (80 mL) was added 1-(4-fluoro-2-hydroxy-phenyl) ethanone (20 g, 129.75 mmol, 1 eq) at 0°C, HNO3 (15.63 g, 168.68 mmol, 11.16 mL, 68% purity, 1.3 eq) was added dropwise over 30 min. The mixture was stirred at 25°C for 1 hour and then ice-water (800 ml) was added and then mixture extracted with DCM (800 mL × 3). The combined organic extracts were washed with brine (500 mL), dried over Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 120 g SepaFlash® Silica Flash Column, Eluent of 0~10% Ethyl acetate/Petroleum ether gradient @ 80 mL/min) to afford the title compound (13.4 g, crude) as a yellow solid. STEP 6: 1-(2-(benzyloxy)-4-fluoro-3-nitrophenyl)ethan-1-one A solution of 1-(4-fluoro-2-hydroxy-3-nitro-phenyl) ethanone (13.4 g, 67.29 mmol, 1 eq), K2CO3 (18.60 g, 134.58 mmol, 2 eq) and bromomethylbenzene (12.66 g, 74.02 mmol, 8.79 mL, 1.1 eq) in acetonitrile (130 mL) was heated at 70°C for 8 hours. The mixture was concentrated and purified by flash silica gel chromatography (ISCO®; 80 g SepaFlash® Silica Flash Column, Eluent of 0~10% Ethyl acetate/Petroleum ether gradient @ 65 mL/min) to afford the title compound (11.5 g, 59%) as a yellow solid.1H NMR (400 MHz, DMSO- d6) δ = 8.07 (dd, J = 6.4, 8.9 Hz, 1H), 7.54 (t, J = 9.0 Hz, 1H), 7.45 - 7.33 (m, 5H), 5.06 (s, 2H), 2.61 (s, 3H). STEP 7: 1-(4-amino-2-(benzyloxy)-3-nitrophenyl)ethan-1-one To a solution of 1-(2-(benzyloxy)-4-fluoro-3-nitrophenyl)ethan-1-one (11.5 g, 39.76 mmol, 1 eq) in acetonitrile (100 mL) was added NH3·H2O (83.60 g, 596.35 mmol, 91.87 mL, 25% purity, 15 eq) dropwise at 25°C. The mixture was heated at 50°C for 2 hours and then concentrated to afford the title compound (8.5 g, crude) as a yellow oil which was used for next step without further purification. MS ES+: 286.9 1H NMR (400 MHz, DMSO- d6) δ = 7.69 (d, J = 9.0 Hz, 1H), 7.48 - 7.30 (m, 5H), 6.82 (s, 2H), 6.70 (d, J = 9.1 Hz, 1H), 4.94 (s, 2H), 2.46 (s, 3H) STEP 8: N-(4-acetyl-3-(benzyloxy)-2-nitrophenyl)acetamide To a solution of 1-(4-amino-2-benzyloxy-3-nitro-phenyl)ethanone (8.5 g, 29.69 mmol, 1 eq) and acetyl chloride (2.56 g, 32.66 mmol, 2.33 mL, 1.1 eq) was added toluene (80 mL) and the mixture stirred at 120°C for 8 hours and then concentrated to afford the title compound (9g, crude) as a yellow oil which was used for next step without further purification. MS ES+: 329.3 1H NMR (400 MHz, CDCI3) δ = 13.74 (s, 1H), 8.94 (br s, 1H), 8.09 (d, J = 9.1 Hz, 1H), 7.88 (d, J = 9.1 Hz, 1H), 7.43 - 7.30 (m, 3H), 7.09 (s,3H), 2.66 (s, 3H), 2.27 (s, 3H). STEP 9: N-(4-acetyl-3-hydroxy-2-nitrophenyl)acetamide To a solution of N-(4-acetyl-3-benzyloxy-2-nitro-phenyl)acetamide (9 g, 27.41 mmol, 1 eq) in DCM (90 mL) was added a solution of BBr3 (1 M, 32.89 mL, 1.2 eq) dropwise at - 78°C under nitrogen atmosphere and the mixture then stirred at -78°C for 1 hr. Ice-water (1500 ml) was added and the mixture was extracted with DCM (1000 mL × 3). The combined organic extracts were washed with brine (500 mL), dried over Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 80 g SepaFlash® Silica Flash Column, Eluent of 0~20% Ethyl acetate/Petroleum ether gradient @ 80 mL/min) to afford the title compound (5.5 g, 84%) as a yellow solid. MS ES+: 239.0 1H NMR (400 MHz, DMSO- d6) δ = 13.30 (s, 1H), 10.40 (s, 1H), 8.21 (d, J = 8.9 Hz, 1H), 7.36 (d, J = 8.9 Hz, 1H), 2.74 (s, 3H), 2.16 (s, 3H) STEPS 10-16 STEP 10: 3-acetamido-6-acetyl-2-nitrophenyl 4-(methyl(1-phenyl-2,5,8,11,14,17- hexaoxanonadecan-19-yl)amino)benzoate To a solution of 4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)benzoic acid (1.4 g, 2.77 mmol, 1 eq) in DCM (10 mL) was added EDCI (1.06 g, 5.54 mmol, 2 eq), DMAP (67.66 mg, 553.80 μmol, 0.2 eq) and N-(4-acetyl-3-hydroxy-2- nitrophenyl)acetamide (659.56 mg, 2.77 mmol, 1 eq). The mixture was stirred at 25°C for 2 hours and then concentrated and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent of 0~100% Ethyl acetate/Petroleum ether gradient @ 100 mL/min) to afford the title compound (1 g, 50%) as a yellow oil. MS ES+: 726.6 1H NMR (400 MHz, CDCI3) δ = 8.75 (s, 1H), 8.46 (d, J = 9.0 Hz, 1H), 8.01 (dd, J = 6.2, 8.9 Hz, 3H), 7.34 (d, J = 4.5 Hz, 4H), 6.75 - 6.71 (m, 2H), 4.57 (s, 2H), 3.67 - 3.62 (m, 24H), 3.11 (s, 3H), 2.53 (s, 3H), 2.26 (s, 3H). STEP 11: N-(3-hydroxy-4-(3-(4-(methyl(1-phenyl-2,5,8,11,14,17-hexaoxanonadecan-19- yl)amino)phenyl)-3-oxopropanoyl)-2-nitrophenyl)acetamide To a solution of 3-acetamido-6-acetyl-2-nitrophenyl 4-(methyl(1-phenyl- 2,5,8,11,14,17-hexaoxanonadecan-19-yl)amino)benzoate (1 g, 1.38 mmol, 1 eq) in pyridine (15 mL) was added KOH (386.52 mg, 6.89 mmol, 5 eq) and the mixture was stirred at 60°C for 2 hr. After cooling to room temperature, the mixture was acidified with 1N HCl to pH=5- 6, and then diluted with water (20 mL) and extracted with ethyl acetate (20 mL x 3). The combined organic extracts were washed with brine (40 mL x 1), dried over anhydrous Na2SO4, filtered, and concentrated to afford the title compound (1.3 g, crude) as a yellow oil which was used into the next step without further purification. MS ES+: 726.2. STEP 12: 7-amino-2-(4-((17-hydroxy-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)-8-nitro-4H-chromen-4-one To a solution of N-(3-hydroxy-4-(3-(4-(methyl(1-phenyl-2,5,8,11,14,17- hexaoxanonadecan-19-yl)amino)phenyl)-3-oxopropanoyl)-2-nitrophenyl)acetamide (1.3 g, 1.79 mmol, 1 eq) in acetic acid (20 mL) was added H2SO4 (175.68 mg, 1.79 mmol, 95.48 μL, 1 eq). The mixture was heated at 110°C for 0.5 hour and then cooled to room temperature. The reaction was neutralized with aq. NaHCO3 to pH=7-8 and then extracted with ethyl acetate (20 mL x 3). The combined organic extracts were washed with brine (40 mL x 1), dried over anhydrous Na2SO4, filtered, concentrated, and the resulting residue was dissolved in dioxane (20 mL) and HCl (20 mL). The mixture was heated at 110 °C for 1 hour and then concentrated, to afford the title compound (1 g, crude) as a brown gum which was used into the next step without further purification. MS ES+: 576.3. STEP 13: 7,8-diamino-2-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)-4H-chromen-4-one To a solution of 7-amino-2-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)-8-nitro-4H-chromen-4-one (1 g, 1.74 mmol, 1 eq) in methanol (30 mL) was added Pd/C (10%, 0.5 g) under nitrogen atmosphere. The suspension was degassed and purged with hydrogen (x 3). The mixture was stirred under hydrogen (15 Psi) at 25°C for 2 hours and then filtered and the filtrate concentrated to afford the title compound (950 mg, crude) as a yellow gum which was used into the next step without further purification. MS ES+: 546.2 STEP 14: 8-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl) chromeno[7,8-d]imidazol-6(3H)-one To a solution of 77,8-diamino-2-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)-4H-chromen-4-one (850 mg, 1.56 mmol, 1 eq) in acetonitrile (20 mL) was added trimethoxymethane (181.85 mg, 1.71 mmol, 187.86 uL, 1.1 eq) and iodine (39.54 mg, 155.79 μmol, 0.1 eq). The mixture was stirred at 25°C for 1 hour and then quenched by addition aq. NaHSO3 (20 mL), and extracted with ethyl acetate (20 mL x 3). The combined organic extracts were washed with brine (40 mL x 1), dried over anhydrous Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent of 0~15% MeOH/DCM gradient @ 100 mL/min) to afford the title compound (500 mg, 58% yield) as a red gum. MS ES+: 556.3 1H NMR (400 MHz, DMSO- d6) δ = 8.71 (br s, 1H), 8.04 (br d, J = 6.9 Hz, 2H), 7.87 (br d, J = 4.5 Hz, 1H), 7.66 (br s, 1H), 7.55 - 7.00 (m, 1H), 6.86 (br d, J = 8.1 Hz, 3H), 3.62 (br s, 4H), 3.47 (br s, 18H), 3.38 (br s, 2H), 3.16 (s, 1H), 3.05 (br s, 3H). STEP 15: 8-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino) phenyl)chromeno[7,8-d]imidazol-6(3H)-one To a solution of 8-(4-((17-hydroxy-3,6,9,12,15-pentaoxaheptadecyl) (methyl)amino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one (450 mg, 809.91 μmol, 1 eq) in DCM (20 mL) was added SOCl2 (8.10 mmol, 587.53 μL, 10 eq). The mixture was stirred at 25°C for 1 hour and then poured into saturated NaHCO3 aq. (30 mL) and extracted with ethyl acetate (30 mL x 3). The combined organic extracts were washed with brine (60 mL x 1), dried over anhydrous Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 12 g SepaFlash®), using 0-10% MeOH/DCM gradient @ 80 mL/min) to afford the title compound as a mixture of tautomers (350 mg, 69%) as a brown gum which was 91.7% pure. MS ES+: 574.1 1H NMR (400 MHz, DMSO- d6) δ = 9.56 (s, 1H), 8.16 (d, J = 8.9 Hz, 2H), 8.03 (d, J = 8.6 Hz, 1H), 7.79 (d, J = 8.6 Hz, 1H), 6.93 (s, 1H), 6.84 (d, J = 9.0 Hz, 2H), 3.70 - 3.67 (m, 2H), 3.66 - 3.58 (m, 6H), 3.55 - 3.46 (m, 16H), 3.06 (s, 3H) STEP 16: 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)chromeno[7,8-d]imidazol-6(3H)-one 8-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino) phenyl)chromeno[7,8-d]imidazol-6(3H)-one (180mg, 0.314 mmol, 1 eq), and sodium azide (102 mg, 1.568 mmol, 5 eq) in dry DMF (1 mL) was heated for 12 hours at 70°C. The mixture was cooled, diluted with water (10 mL), extracted with DCM (50 mL), dried over Na2SO4, filtered, concentrated and purified by column chromatography using 0-20% MeOH/DCM, to afford the title compound as a tautomeric mixture (28:72) (50 mg, 25%) as a yellow gum, which was >95% purity by LCMS. LCMS m/z = 681 (M++1) 1H NMR (499 MHz, DMSO- d6) δ 13.09 (s, 1H), 8.43 (s, 1H), 8.12 (d, J = 8.9 Hz, 1H), 7.96 (d, J = 9.1 Hz, 2H), 7.85 (d, J = 8.6 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 6.91 – 6.81 (m, 2H), 6.83 (s, 1H), 3.67 – 3.60 (m, 3H), 3.58 – 3.54 (m, 2H), 3.54 – 3.47 (m, 18H), 3.36 (m, 2H), 3.05 (s, 3H)
Example 22: Preparation of 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)-2-methylchromeno[7,8-d]imidazol-6(3H)- one (BA-170 and tautomer BA-201) STEP 1: 8-(4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one To a solution of 7,8-diamino-2-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl) amino)phenyl)-4H-chromen-4-one (700 mg, 1.28 mmol, 1 eq) in acetonitrile (15 mL) was added 1,1,1-trimethoxyethane (169.55 mg, 1.41 mmol, 1.1 eq) and iodine (32.56 mg, 128.29 μmol, 25.84 μL, 0.1 eq). The mixture was stirred at 25°C for 1 hour and then quenched by addition aq. NaHSO3 (20 mL) and extracted with ethyl acetate (20 mL x 3). The combined organic extracts were washed with brine (40 mL x 1), dried over anhydrous Na2SO4, filtered, concentrated and purified by flash silica gel chromatography (ISCO®; 20 g SepaFlash® Silica Flash Column, Eluent 0~15% MeOH/DCM gradient @ 100 mL/min) to afford the title compound (470 mg, 64%) as a brown gum. MS ES+: 570.2 STEP 2: 8-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one To a solution of 8-(4-((17-azido-3,6,9,12,15- pentaoxaheptadecyl)(methyl)amino)phenyl)-2-methylchromeno[7,8-d]imidazol-6(3H)- one(400 mg, 702.19 umol, 1 eq) in DCM (6 mL) was added SOCl2 (835.40 mg, 7.02 mmol, 509.39 μL, 10 eq). The mixture was stirred at 25 °C for 4 hours and then poured into saturated aq. NaHCO3 (40 mL) and extracted with ethyl acetate (40 mL x 3). The combined organic extracts were washed with brine (80 mL x 1), dried over anhydrous Na2SO4, filtered, and concentrated to afford the title compound as a mixture of tautomers (320 mg, 71%) as a brown gum. MS ES+: 588.3 1H NMR (400 MHz, DMSO- d6) δ = 8.08 - 7.85 (m, 2H), 7.76 (d, J = 8.5 Hz, 1H), 7.50 (br d, J = 8.3 Hz, 1H), 6.87 (br d, J = 8.9 Hz, 2H), 6.79 (s, 1H), 3.70 - 3.66 (m, 2H), 3.66 - 3.60 (m, 6H), 3.54 - 3.47 (m, 16H), 3.05 (s, 3H), 2.61 (s, 3H). STEP 3: 8-(4-((17-azido-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one 8-(4-((17-chloro-3,6,9,12,15-pentaoxaheptadecyl)(methyl)amino)phenyl)-2- methylchromeno[7,8-d]imidazol-6(3H)-one (310 mg, 0.527 mmol, 1 eq), and sodium azide (171 mg, 2.63 mmol, 5 eq) in dry DMF (2mL) was heated at 70°C for 12 hours. The mixture was cooled, diluted with water (20 mL) and extracted with DCM (100 mL). The organic extract was dried over Na2SO4, filtered, concentrated and purified by column chromatography (0-20% MeOH/DCM), to afford the title compound as a mixture of tautomers: BA-170:BA-201 (30:70) (120 mg, 38%) as a dark orange gum with >95% purity. LCMS m/z = 595 (M+1) Tautomeric structures were confirmed by1H NMR: BA-170 isomer, wherein the 7-NH proton appears further down field at δ 13.38; and BA-201 isomer, wherein the 9-NH proton appears upfield at δ 12.98 The BA-170 tautomeric mixture (80 mg) was further purified by flash column chromatography to obtain pure BA-201 (50 mg) as a dark orange gum. BA-170:1H NMR: (499 MHz, DMSO- d6) δ 13.38, 12.85 (2s, 1H), 8.09, 7.94 (d, J = 8.7 Hz, 2H), 7.77, 7.72 (d, J = 8.5 Hz, 1H), 7.54, 7.47 (d, J = 8.5 Hz, 1H), 6.88, 6.85 (d, J = 9.0 Hz, 2H), 6.81, 6.79 (s, 1H), 3.67 – 3.62 (m, 2H), 3.58 – 3.54 (m, 2H), 3.54 – 3.45 (m, 16H), 3.38 – 3.35 (m, 2H), 3.06, 3.05 (s, 3H), 2.63, 2.60 (s, 3H). BA-201:1H NMR (499 MHz, DMSO- d6) δ 12.98 (s, 1H), 7.98 (d, J = 8.7 Hz, 2H), 7.76 (d, J = 8.5 Hz, 1H), 7.50 (d, J = 8.5 Hz, 1H), 6.87 (d, J = 9.0 Hz, 2H), 6.80 (s, 1H), 3.62 (m, 4H), 3.58 – 3.54 (m, 2H), 3.54 – 3.45 (m, 16H), 3.40 – 3.34 (m, 2H), 3.05 (s, 3H), 2.61 (s, 3H). EXAMPLE 23: N-(18-azido-3,6,9,12,15-pentaoxaoctadecyl)-4-(7,8-bis(allyloxy)-4-oxo- 4H-chromen-2-yl)benzamide (BA-203) To a solution of N-(18-azido-3,6,9,12,15-pentaoxaoctadecyl)-4-(7,8-dihydroxy-4- oxo-4H-chromen-2-yl)benzamide (BA-118, 200 mg, 0.34 mmol, 1eq) in DMF (5mL), was added K2CO3 (94mg, 0.68 mmol, 2eq) followed by allyl bromide (35.2uL, 0.408 mmol, 1.2eq) at room temperature and the mixture was then heated to 80°C for 3 hours. The mixture was then filtered, excess DMF was azeotroped with heptane. Purified by flash chromatography (25g biotage 20micron) 0 to 10% DCM/MeOH 10CV to afford the title compound (140 mg, 62%) as yellow oil. LCMS: m/z 689 (M++Na) 1H NMR (499 MHz, DMSO-d6) δ 8.71 (t, J = 5.6 Hz, 1H), 8.16 (d, J = 8.2 Hz, 2H), 8.04 (d, J = 8.3 Hz, 2H), 7.76 (d, J = 8.9 Hz, 1H), 7.28 (d, J = 9.0 Hz, 1H), 7.07 (s, 1H), 6.13 (dddt, J = 24.8, 16.0, 10.4, 5.4 Hz, 2H), 5.51 – 5.39 (m, 2H), 5.32 (d, J = 10.6 Hz, 1H), 5.27 (d, J = 10.4 Hz, 1H), 4.79 (d, J = 5.0 Hz, 2H), 4.70 (d, J = 5.9 Hz, 2H), 3.60 – 3.48 (m, 20H), 3.45 (q, J = 6.0 Hz, 2H), 3.37 (t, J = 4.9 Hz, 2H). PREPARATION OF LIGAND-CONJUGATED OLIGONUCLEOTIDES Exemplary ligand-conjugated oligonucleotides falling within the scope of the present disclosure, may be synthesized according to the following procedures. In some instances, one Ligand (Ligand A in the general procedures described below) is conjugated to the 5' end of an oligonucleotide. In some instances, two identical Ligands (Ligand A and Ligand A) are conjugated to the 5' and 3' ends of an oligonucleotide. In some instances, two different Ligands (Ligand A and Ligand B) are conjugated to the 5' and 3' ends of an oligonucleotide. In some instances, one Ligand (Ligand A) is conjugated to the 3' end of an oligonucleotide. Example 24: General Procedure I Type A - Ligand Conjugated to 5’ end of Sense Strand STEP 1: 5'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V, 1M, pH7) and acetonitrile (20%-50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile was then added and the reaction monitored by LCMS and HPLC. Upon completion, any precipitate was removed via centrifugation and the aqueous solution purified by reverse phase HPLC, dried by lyophilization and the dried 5'- DBCO functionalized sense strand reconstituted in RNase free water. STEP 2: 5’- Ligand Conjugated Sense Strand A solution of Ligand A—N3 (2 eq) in DMSO or THF was added to a solution of 5’- DBCO modified sense strand (1 eq) and the reaction monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Example 25: General Procedure I Type B - Ligand Conjugated to 5’ end of Sense Strand STEP 1: 5'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) is added to an aqueous solution of 5’- (C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) is added and the reaction monitored by HPLC and LCMS. Upon completion, excess TCEP is removed by molecular weight cut-off with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO is added and the reaction monitored by LCMS and HPLC. Upon completion, any solids are removed via centrifugation, the solution purified by reverse phase HPLC, dried by lyophilization and the dried product reconstituted in RNase free water. STEP 2: 5'- Ligand Conjugated Sense Strand To a solution of 5’-DBCO functionalized sense strand (1 eq) is added a solution of Ligand A—N3 (3 eq) in DMSO or THF and the reaction monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand is purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Example 26: General Procedure II Type A - Bis-homo-3',5'-Ligand Conjugated Sense Strand STEP 1: 3’,5’-bis-DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20%-50% V/V) were added to an aqueous solution of 3’,5’ amine functionalized sense strand. A solution of DBCO-NHS (3 eq) in DMSO or CH3CN was then added and the reaction monitored by LCMS and HPLC. Upon completion, the product was purified by reverse phase HPLC, dried by lyophilization and reconstituted in RNase free water. STEP 2: 3’,5’-bis-conjugated sense strand A solution of Ligand A—N3 (3 eq) in DMSO or CH3CN was added to a solution of 3’,5’-bis-DBCO modified sense strand (1 eq) and the reaction monitored by HPLC and LCMS. Upon completion, the 3’,5’-bis conjugated sense strand was purified by reverse phase HPLC, dried by lyophilization, reconstituted in RNase free water and desalted using Amicon® Ultra-15 Centrifugal filter (3K, 5 times).
Example 27: General Procedure II Type B - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 3’,5’-bis-DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) is added to an aqueous solution of 5’, 3’-Bis (C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) is added and the reaction monitored by HPLC and LCMS. Upon completion, excess TCEP is removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO is added and the reaction monitored by LCMS and HPLC. Upon completion, any solids are removed via centrifugation and the solution purified by reverse phase HPLC, dried by lyophilization and the dried bis-DBCO modified sense strand reconstituted in RNase free water. STEP 2: 3’,5’-bis-conjugated sense strand To a solution of 5’,3’-Bis-DBCO functionalized sense strand (1 eq) is added a solution of Ligand A-N3 (3 eq) in DMSO or THF and the reaction monitored by HPLC and LCMS. Upon completion, the bis-homo-5’-, 3’conjugated sense strand is purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Example 28: General Procedure II Type C - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 5'-DBCO / 3'-(C6-SS-C6)-mC Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20% -50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile was then added and the reaction was monitored by LCMS and HPLC. Upon completion, any precipitate was removed via centrifugation and the aqueous solution purified by reverse phase HPLC. The product fractions were combined, dried by lyophilization and the dried N-DBCO modified sense strand reconstituted in RNase free water for step 2. STEP 2: 5',3'-Bis DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) was added to an aqueous solution of 5’-DBCO / 3’-(C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction was monitored by LCMS and HPLC. Upon completion, any solids were removed via centrifugation and the solution was purified by reverse phase HPLC, dried by lyophilization and the dried bis-DBCO modified sense strand reconstituted in RNase free water for step 3. STEP 3: Bis-homo-5',3'- Ligand Conjugated Sense Strand To a solution of 5’-, 3’-Bis-DBCO functionalized sense strand (1 eq) was added a solution of Ligand A-N3 (3 eq) in DMSO or THF and the reaction was monitored by HPLC and LCMS. Upon completion, the bis-homo-5’-, 3’conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). The product was confirmed by HPLC and LCMS.
Example 29: General Procedure II Type D - Bis-homo-5',3'- Ligand Conjugated Sense Strand STEP 1: 5'-(C6-SS-C6)-mC / 3'-DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) and acetonitrile (20% -50% V/V) were added to an aqueous solution of 5’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile was then added and the reaction was monitored by LCMS and HPLC. Upon completion, any precipitate was removed via centrifugation and the aqueous solution purified by reverse phase HPLC. The product fractions were combined, dried by lyophilization and the dried N-DBCO modified sense strand reconstituted in RNase free water for step 2. STEP 2: 5',3'-Bis DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V 1M, pH7) was added to an aqueous solution of 5’-DBCO / 3’-(C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction was monitored by LCMS and HPLC. Upon completion, any solids were removed via centrifugation and the solution was purified by reverse phase HPLC, dried by lyophilization and the dried bis-DBCO modified sense strand reconstituted in RNase free water for step 3. STEP 3: Bis-homo-5',3'- Ligand Conjugated Sense Strand To a solution of 5’-, 3’-Bis-DBCO functionalized sense strand (1 eq) was added a solution of Ligand A-N3 (3 eq) in DMSO or THF and the reaction was monitored by HPLC and LCMS. Upon completion, the bis-homo-5’-, 3’conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). The product was confirmed by HPLC and LCMS.
Example 30: General Procedure III Type A - Bis-hetero-3',5'- Ligand Conjugated Sense Strand STEP 1: 5’-conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand A solution of Ligand A—N3 (2 eq) in DMSO was added to an aqueous solution of 5’- DBCO modified sense strand (1 eq, see above for preparation) and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). STEP 2: The 5’-conjugated, 3’-DBCO modified sense strand Sodium phosphate buffer (10% V/V 1M, pH7) was added to a solution of 5’- conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand (1 eq) in water. Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction monitored by HPLC and LCMS. Upon completion, the aqueous solution was purified by reverse phase HPLC, dried by lyophilization and the dried 5’-conjugated, 3’-DBCO modified sense strand was reconstituted in Sodium Phosphate buffer (100mM) for step 3. STEP 3: Bis-hetero-3',5'- Ligand Conjugated Sense Strand A solution of Ligand B—N3 (2 eq) in DMSO was added to an aqueous solution of 5’- conjugated, 3’-DBCO functionalized sense strand (1 eq) and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-, 3’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times).
Example 31: General Procedure III Type B - Bis-hetero-3',5'- Ligand Conjugated Sense Strand STEP 1: 5'-(C6-SS-C6)-mC, 3’-conjugated sense strand A solution of Ligand A—N3 (2 eq) in DMSO was added to an aqueous solution of 3'- DBCO modified sense strand (1 eq, see above for preparation) and the reaction was monitored by HPLC and LCMS. Upon completion, the 3’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). STEP 2: The 5’-DBCO, 3’-conjugated sense strand Sodium phosphate buffer (10% V/V 1M, pH7) was added to a solution of 5’- conjugated, 3’-(C6-SS-C6)-mC functionalized sense strand (1 eq) in water. Tris(2- carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction was monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction monitored by HPLC and LCMS. Upon completion, the aqueous solution was purified by reverse phase HPLC, dried by lyophilization and the dried 5’-conjugated, 3’-DBCO modified sense strand was reconstituted in Sodium Phosphate buffer (100mM) for step 3. STEP 3: Bis-hetero-3',5'- Ligand Conjugated Sense Strand A solution of Ligand B—N3 (2 eq) in DMSO was added to an aqueous solution of 5’- conjugated, 3’-DBCO functionalized sense strand (1 eq) and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-, 3’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Example 32: General Procedure IV Type A - Ligand Conjugated to 3’ end of Sense Strand STEP 1: 3’- DBCO modified sense strand Sodium Phosphate buffer (10% V/V 1M, pH7) was added to an aqueous solution of 3’-(C6-SS-C6)-mC functionalized sense strand. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP, 25 eq) in water (pH7) was added and the reaction monitored by HPLC and LCMS. Upon completion, excess TCEP was removed by MWCO with sodium phosphate buffer (100 mM, pH=7, 3x). A solution of DBCO-MAL (3 eq) in DMSO was added and the reaction was monitored by HPLC and LCMS. Upon completion, any solids were removed via centrifugation and the solution was purified by reverse phase HPLC, dried by lyophilization and the dried 3’-DBCO modified sense strand was reconstituted in 100mM Sodium Phosphate buffer for step 2. STEP 2: 3'- Ligand Conjugated Sense Strand A solution of Ligand A—N3 (3 eq) in DMSO was added to an aqueous solution of 3’- DBCO functionalized sense strand (1 eq) and the reaction was monitored by HPLC and LCMS. Upon completion, the 3’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5x). Example 33: General Procedure IV Type B - Ligand Conjugated to 3’ end of Sense Strand STEP 1: 3'- DBCO Functionalized Sense Strand Sodium Phosphate buffer (10% V/V, 1M, pH7) and acetonitrile (20%-50% V/V) are added to an aqueous solution of 3’-amine functionalized sense strand. A solution of DBCO- NHS (1.5-3 eq) in DMSO or acetonitrile is then added and the reaction monitored by LCMS and HPLC. Upon completion, any precipitate is removed via centrifugation, the aqueous solution purified by reverse phase HPLC, dried by lyophilization and the dried DBCO modified sense strand reconstituted in RNase free water. STEP 2: 3’- Ligand Conjugated Sense Strand A solution of Ligand A—N3 (2 eq) in DMSO or THF is added to a solution of 3’- DBCO modified sense strand (1 eq) and the reaction monitored by HPLC and LCMS. Upon completion, the 3’-conjugated sense strand was purified by reverse phase HPLC or molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Example 33-A: General Procedure V STEP 1: 5’-conjugated sense strand A solution of Ligand A—N3 (2 eq) in DMSO was added to an aqueous solution of 5’- DBCO modified sense strand (1 eq), and the reaction was monitored by HPLC and LCMS. Upon completion, the 5’-conjugated sense strand was purified by reverse phase HPLC and desalted with molecular weight cut-off with Amicon® Ultra-15 Centrifugal filter (3K, 5 times). Compounds RD3171-RD3188 were prepared using General Procedure V. Example 34: Bis-5'-, 3'- conjugated sense strand RD3937 5' BA-120 / 3' BA-120 FXXXXXXXIV+IS1525+FXXXXXXXV SEQ: RD3181 / 5'EP BA-120 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9418.9 (calc. 9420.6g/mol)
Example 35: Mono 5' conjugated sense strand RD3666 5' BA-120 FXXXXXXXIV+IS1402 SEQ: RD3181 / 5'VP BA-120 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z 8342.7 (calc. 8344.2 g/mol)
Example 36: Mono 5' conjugated sense strand RD3913 5' BA-168 FXXXXXXXVI+IS1402 SEQ: RD3181 / 5'EP BA-168 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z: 8620.5 (calc. 8622.2 g/mol) Example 37: Mono 5' conjugated sense strand RD3995 5' BA-177 FXXXXXXXVII+IS1402 SEQ: RD3181 / 5'EP BA-177 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 97% purity, confirmed by HPLC. LCMS: m/z: 8441.8 (calc. 8443.3 g/mol) Example 38: Mono 5' conjugated sense strand RD4389 5' BA-177 FXXXXXXXVII+IS1640 SEQ: RD3953 / 5'EP BA-177 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z: 8900.7 (calc. 8898.9 g/mol) Example 39: Bis 5'-, 3'- conjugated sense strand RD6054 5' BA-236 / 3' BA-236 FXXXXXXXVIII+IS2460+LK0181 5'VP BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity and confirmed by HPLC. LCMS: m/z: 9401.6 (calc. 9403.2 g/mol) AND Example 40: Bis 5'-, 3'- conjugated sense strand RD6039 5' BA-236 / 3' BA-236 FXXXXXXXVIII+IS2458+FXXXXXXXVIII BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 95% purity and confirmed by HPLC. LCMS: m/z: 9045.3 (calc. 9046.9g/mol) AND Example 41: Bis 5'-, 3'- conjugated sense strand RD6044 5' BA-236 / 3' BA-236 FXXXXXXXVIII+ IS1664+FXXXXXXXVIII BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 97% purity and confirmed by HPLC. LCMS: m/z: 9100.4 (calc. 9102.0g/mol) AND Example 42: Bis 5'-, 3'- conjugated sense strand RD6049 5' BA-236 / 3' BA-236 FXXXXXXXVIII+IS2459+FXXXXXXXVIII BA-236 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 92% purity and confirmed by HPLC. LCMS: m/z: 9082.3 (calc. 9084.0g/mol) Example 43: Mono 5' conjugated sense strand RD4518 5' BA-236 FXXXXXXXVIII+IS1640 SEQ: RD3953 / 5'EP BA-120 was conjugated to an oligo sense strand according to general procedure type I type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z: 8641.4 (calc. 8639.7 g/mol)
Example 44: Mono 5' conjugated sense strand RD3941 5' BA-128 / 3' BA-128 FXXXXXXXIX+IS1525+FXXXXXXXXIV SEQ: RD3181 / 5'EP BA-128 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 96% purity, confirmed by HPLC. LCMS: m/z: 9482.4 (calc. 9483.4 g/mol) Example 45: Bis-5'-, 3'- conjugated sense strand RD6037 5' BA-128 / 3' BA-128 FXXXXXXXIX+IS2458+FXXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 97% purity and confirmed by HPLC. LCMS: m/z: 9429.1 (calc. 9430.7 g/mol) AND Example 46: Bis-5'-, 3'- conjugated sense strand RD6047 5' BA-128 / 3' BA-128 FXXXXXXXIX+IS2459+FXXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9466.1 (calc. 9467.8 g/mol) AND Example 47: Bis-5'-, 3'- conjugated sense strand RD6042 5' BA-128 / 3' BA-128 FXXXXXXXIX+IS1664+FXXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 98% purity and confirmed by HPLC. LCMS: m/z: 9785.3 (calc. 9485.8 g/mol) AND
Example 48: Bis-5'-, 3'- conjugated sense strand RD6052 5' BA-128 / 3' BA-128 FXXXXXXXIX+IS2460+FXXXXXXXIX BA-128 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity and confirmed by HPLC. LCMS: m/z: 9785.3 (calc. 9787.0 g/mol)
Example 49: Mono 5' conjugated sense strand RD3667 5' BA-128 FXXXXXXXIX+IS1402 SEQ: RD3181 / 5'VP BA-128 was conjugated to an oligo sense strand according to general procedure I type A. The product was ma prepared de with 97% purity, confirmed by HPLC. LCMS: m/z: 8374.4 (calc. 8375.9 g/mol) Example 50: Mono 5' conjugated sense strand RD3971 5' BA-171 FXXXXXXX+IS1402 SEQ: RD3181 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 98% purity, confirmed by HPLC. LCMS: m/z: 8456.9 (calc. 8458.4 g/mol) AND Example 51: Mono 5' conjugated sense strand RD4387 5' BA-171 FXXXXXXX+IS1640 SEQ: RD3953 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 89% purity, confirmed by HPLC. LCMS: m/z: 8915.8 (calc. 8914.0 g/mol)
Example 52: Bis 5'-, 3'-conjugated sense strand RD4235 5' BA-171 /3' BA-171 FXXXXXXX+IS1525+FXXXXXXXX SEQ: RD3181 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure II type C. The product was prepared with 89% purity, confirmed by HPLC. LCMS: m/z: 9664.9 (calc. 9666.9 g/mol)
Example 53: Bis 5'-, 3'- conjugated sense strand RD4420 5' BA-171 /3' BA-171 FXXXXXXXX+IS1641+FXXXXXXXX SEQ: RD3953 / 5'EP BA-171 was conjugated to an oligo sense strand according to general procedure II type B. The product was prepared with 98% purity, confirmed by HPLC. LCMS: m/z: 9794.2 (calc. 9795.9 g/mol)
Example 54: Mono 5' conjugated sense strand RD4336 5' BA-210 FXXXXXXXXI+IS1402 SEQ: RD3181 / 5'EP BA-210 was conjugated to an oligo sense strand according to general procedure I type A. The prepared was made with 96% purity, confirmed by HPLC. LCMS: m/z: 8503.5 (calc. 8505.3 g/mol)
Example 55: Mono 5' conjugated sense strand RD4394 5' BA-215 FXXXXXXXXII+IS1640 SEQ: RD3953 / 5'EP BA-215 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z: 8977.9 (calc. 8979.7 g/mol)
Example 56: Bis 5'-, 3'- conjugated sense strand RD6027 5' BA-135 /3' BA-135 FXXXXXXV+IS2006+FXXXXXXV SEQ: RD3181 / 5'VP BA-135 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9249.5, (calc. 9251.3g/mol) Example 57: Bis 5'-, 3'- conjugated sense strand RD6028 5' BA-136 /3' BA-136 FXXXXXXVI+IS2006+FXXXXXXVI SEQ: RD3181 / 5'VP BA-136 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 97% purity, confirmed by HPLC. LCMS: m/z 9075.2, (calc. 9077.0 g/mol) Example 58: Bis 5'-, 3'- conjugated sense strand RD6029 5' BA-137 /3' BA-137 FXXXXXXVII+IS2006+FXXXXXXVII SEQ: RD3181 / 5'VP BA-137 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 92% purity, confirmed by HPLC. LCMS: m/z 9169.2, (calc. 9171.0 g/mol)
Example 59: Bis 5'-, 3'- conjugated sense strand RD6030 5' BA-144 /3' BA-144 FXXXXXXIX+IS2006+FXXXXXXIX SEQ: RD3181 / 5'VP BA-144 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9567.7, (calc. 9569.5 g/mol)
Example 60: Bis 5'-, 3'- conjugated sense strand RD6031 5' BA-173 /3' BA-173 FXXIV+IS2006+FXXIV SEQ: RD3181 / 5'VP BA-173 was conjugated to an oligo sense strand according to general procedure II type A. The product was made with 81% purity, confirmed by HPLC. LCMS: m/z 9681.5, (calc. 9683.4 g/mol)
Example 61: Bis 5'-, 3'- conjugated sense strand RD6032 5' BA-183 /3' BA-183 FXXXXXXXI+IS2006+FXXXXXXXI SEQ: RD3181 / 5'VP BA-183 was conjugated to an oligo sense strand according to general procedure II type A. The product was made with 86% purity, confirmed by HPLC. LCMS: m/z9337.4, (calc. 9339.2 g/mol) Example 62: Bis 5'-, 3'- conjugated sense strand RD6034 5' BA-216 /3' BA-216 FXXXXXXXII+IS2006+FXXXXXXXII SEQ: RD3181 / 5'VP BA-216 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9373.3, (calc. 9375.2 g/mol) Example 63: Bis 5'-, 3'- conjugated sense strand RD6036 5' BA-198 /3' BA-198 FXVI+IS2458+FXVI SEQ: RD3175 / 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 98% purity, confirmed by HPLC. LCMS: m/z 9329.4, (calc. 9331.0 g/mol) AND Example 64: Bis 5'-, 3'- conjugated sense strand RD6041 5' BA-198 /3' BA-198 FXVI+ IS1664+FXVI SEQ: RD3176/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9384.4, (calc. 9386.1 g/mol) AND Example 65: Bis 5'-, 3'- conjugated sense strand RD6046 5' BA-198 /3' BA-198 FXVI+ IS2459+FXVI SEQ: RD3186/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 90% purity, confirmed by HPLC. LCMS: m/z 9366.4, (calc. 9368.1 g/mol) AND Example 66: Bis 5'-, 3'- conjugated sense strand RD6051 5' BA-198 /3' BA-198 FXVI+ IS2460+FXVI SEQ: RD3959/ 5'VP BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9685.6, (calc. 9687.3 g/mol) Example 67: Bis 5'-, 3'- conjugated sense strand RD6035 5' BA-225 /3' BA-225 FXXXIII+IS2006+FXXXIII BA-225 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 80% purity, confirmed by HPLC. LCMS: m/z 9343.4, (calc. 9345.2 g/mol) Example 68: Mono 5'- conjugated sense strand RD4267 5' BA-196 FXIV+IS1402 BA-196 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z 8310.5, (calc. 8311.4 g/mol)
Example 69: Mono 5'- conjugated sense strand RD4310 5' BA-197 FXV+IS1402 BA-197 was conjugated to an oligo sense strand according to general procedure I type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z 8310.4, (calc. 8312.0 g/mol)
Example 70: Mono 3'- conjugated sense strand RD4157 3' BA-118 IS1599+FIV BA-118 was conjugated to an oligo sense strand according to general procedure IV type A. The product was prepared with 86% purity, confirmed by HPLC. LCMS: m/z 8652.1, (calc. 8653.4 g/mol)
Example 71: Mono 3'- conjugated sense strand RD4269 3' BA-129 IS1599+FXXXXXXXIII BA-129 was conjugated to an oligo sense strand according to general procedure IV type A. The product was prepared with 97% purity, confirmed by HPLC. LCMS: m/z 8582.1, (calc. 8583.8 g/mol) Example 72: Bis 5'-, 3'- conjugated sense strand RD5470 5' BA-198 /3' BA-198 FXVI+IS2178+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z 9414.8, (calc. 9416.5 g/mol) Example 73: Bis 5'-, 3'- conjugated sense strand RD5946 5' BA-198 /3' BA-198 FXVI+IS2178+FXVI SEQ: RD3953/ 5'EP IA1299 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 95% purity, confirmed by HPLC. LCMS: m/z 9414.8, (calc. 9416.5 g/mol) Example 74: Bis 5'-, 3'- conjugated sense strand RD5947 5' BA-198 /3' BA-198 FXVI+IS1681+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9215.3, (calc. 9217.0 g/mol) Example 75: Bis 5'-, 3'- conjugated sense strand RD5948 5' BA-198 /3' BA-198 FXVI+IS1681+FXVI SEQ: RD3953/ 5'EP IA1299 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 94% purity, confirmed by HPLC. LCMS: m/z 9215.3, (calc. 9217.0 g/mol) Example 76: Bis 5'-, 3'- conjugated sense strand RD5954 5' BA-198 /3' BA-198 FXVI+IS1688+FXVI SEQ: RD3953/ 5'VP IA1603 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9414.7, (calc. 9415.5 g/mol) Example 77: Bis 5'-, 3'- conjugated sense strand RD5955 5' BA-198 /3' BA-198 FXVI+IS1688+FXVI SEQ: RD3953/ 5'EP IA1299 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 93% purity, confirmed by HPLC. LCMS: m/z 9414.7, (calc. 9415.5 g/mol) Example 78: Bis 5'-, 3'- conjugated sense strand RD5956 5' BA-198 /3' BA-198 FXVI+IS2422+FXVI SEQ: RD3953/ 5'VP IA1250 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9774.1, (calc. 9775.7 g/mol) Example 79: Bis 5'-, 3'- conjugated sense strand RD5967 5' BA-198 /3' BA-198 FXVI+IS2424+FXVI SEQ: RD3953/ 5'VP IA1250 BA-198 was conjugated to an oligo sense strand according to general procedure II type A. The product was prepared with 87% purity, confirmed by HPLC. LCMS: m/z 9774.1, (calc. 9775.7 g/mol)
Example 80: Synthesis of ligand-conjugated oligonucleotides using amide linkers Oligonucleotides conjugated to ligands using amide linkers may be synthesized using the following methods: The synthetic methods above may be used to synthesize, for example, the following oligonucleotides comprising ligands at the 5' and/or 3' ends:
Example 81: General Procedures for Synthesis of Oligonucleotides Strands were synthesized on solid phase using an oligonucleotide synthesizer Oligopilot100 (Cytiva Life Sciences). Solid support (CPG, 80-90 µmol/g, 500A, from LGC- Biosearch Technologies, Petaluma, CA) was loaded to 150-300 µmol scales. RNA and 2' modified RNA phosphoramidites were purchased from Hongene Biotech (Union City, CA). 2'-O-methyl phosphoramidites used were: 5'-O-(4,4'-Dimethoxytrityl)-N6-benzoyl-2'-O-methyl-adenosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N4-acetyl-2'-O-methyl-cytidine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N2-isobutyryl-2'-O-methyl-guanosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-2'-O-methyl-uridine-3'-O-[(2-cyanoethyl)-(N,N-diisopropyl)]- phosphoramidite. 2'-Fluoro phosphoramidites used were: 5'-O-(4,4'-Dimethoxytrityl)-N6-benzoyl-2'-fluoroadenosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N4-acetyl-2'-fluorocytidine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-N2-isobutyryl-2'-fluoroguanosine-3'-O-[(2-cyanoethyl)-(N,N- diisopropyl)]-phosphoramidite 5'-O-(4,4'-Dimethoxytrityl)-2'-fluorouridine-3'-O-[(2-cyanoethyl)-(N,N-diisopropyl)]- phosphoramidite. To create phosphorohioate linkages 3-((Dimethylamino-methylidene)amino)-3H- 1,2,4-dithiazole-3-thione (DDTT. 0.1M solution from Chemgenes, Wilmington, MA) was used for 4-6 minutes. To create phosphodiester linkage a solution of I2O in Pyridine/Water (0.05M from Sigma Aldrich, St Louis, MO) was used. Following the oxidation/sulfurization a mixture of 20% n-Methylimidazole in Acetonitrile, and 40% Acetic Anhydride in 60% Lutidine in Acetonitrile (Sigma Aldrich, St Louis, MO) were used to acetylate any unreacted chain attached to the CPG. Phosphoramidites were dissolved in anhydrous acetonitrile (0.2M) and molecular sieves (4A) were added and set overnight (Sigma Aldrich, St. Louis, MO). For the oligonucleotide chain 5-(Ethylthio)-1H-Tetrazole (ETT, 0.6M in acetonitrile, from Sigma Aldrich) was used as activator solution. Coupling times were 6 minutes carried out at 3.0 equivalents for each step. Prior to coupling the support bound oligonucleotide is treated with a solution of Dichloroacetic Acid in Dichloromethane (3% Deblock, Sigma Aldrich) and washed with Anhydrous Acetonitrile. Cleavage and deprotection of support bound oligomer After completion of the solid phase synthesis, the support was treated with AMA solution, a 1:1 volume solution of NH4OH:CH3NH2 (Fisher Scientific, Spectrum Chemicals), for 20 minutes at 65°C. The solution was then evaporated. Prior to purification, in-process analysis is performed on analytical HPLC and LCMS to determine crude purity, identify the target mass and monitor the deprotection for completion. LCMS method A Waters XBridge Oligonucleotide BEH C18 Column, 130Å, 2.5 µm, 2.1 mm x 50 mm (P/N 186003952) column was used with buffer solutions :400 mM HFIP + 15 mM TEA (buffer A) and 100% methanol (buffer B) at a gradient of 15-40% or 50-75% Buffer B over 15 CV at 70°C with a flowrate of 1 mL/minute.. Concentration by tangential flow filtration (TFF) The crude oligos are concentrated using Pall Minimate EVO System (Product ID: OAPMPUNV), using a Pall Minimate TFF cassette capsule with 3k Omega membrane. Purification Purification was performed using reverse phase HPLC. Waters XBridge Prep C185 µm OBD, 250 x 19 mm (P/N: 186004021). Buffer solution mixtures are 100 mM TEAA, 5% ACN at pH of 7.0 (buffer A) and 1:1 acetonitrile:methanol (buffer B). Gradient was 5-30% or 30-60% Buffer B over 60 minutes at 60°C with a flowrate of 20 mL/minute. After purification, fractions are analyzed by reverse phase UPLC. The column used is a Waters ACQUITY UPLC Oligonucleotide BEH C181.7 µm, 2.1 x 50 mm (P/N: 186003949). Buffer solution mixtures are 100 mM TEAA, 5% ACN at pH of 7.0 (buffer A) and 1:1 acetonitrile:methanol (buffer B). Gradient was set at 5-30% or 30-60% Buffer B over 5 minutes at 70°C with a flowrate of 1.0 mL/minute. The minimum spec of the purified pool is 85%. Desalting Once a pool has been established, the oligos are desalted using Pall Minimate EVO System (Product ID: OAPMPUNV). Cassette used is the Pall Minimate TFF capsule with 3k Omega membrane (Product ID: OA003C12). Retentate is collected for lyophilization or annealing directly. PREPARATION OF DOUBLE-STRANDED siRNA Example 82: General Procedure for Annealing Ligand Conjugated RNA sense strands were prepared as described herein. RNA antisense strands were prepared according to procedures well known to those of skill in the art. The concentrations of both sense strand and antisense strands were determined by Nanodrop. The double-stranded siRNA was prepared by mixing equimolar amounts of sense stand and antisense strand. The annealing process was monitored by RP-HPLC, non- denaturing method. After annealing, no more that 5% of antisense strand was in the duplex mixture. Duplex concentration was determined by measuring the solution absorbance on Nanodrop. Example 83: General procedure for preparation of Carbon chain containing dinucleotide amidites STEP 1: N,N-Diisopropylethylamine (DIPEA, 3 eq) is added to a stirred solution of optionally base protected 3-(5-["A/C/G/U"]-5-(DMTrO-methyl)-2-[F/OMe"]- tetrahydrofuran-3-yl)acetic acid (1 eq), optionally base protected 1-["A/C/G/U"]-2-[F/OMe"]- 5-((heptadecylamino)methyl) tetrahydrofuran-3-ol (1.15 eq) and HATU (1.5 eq) in DMF. The mixture is stirred for 2h at room temperature, and then added dropwise to water or saturated aqueous sodium bicarbonate solution. The resulting precipitated solids are isolated by filtration, washed with water, dried, redissolved in ethyl acetate and purified by chromatography. STEP 2: N, N-diisopropyl chlorophosphoramidite (3 eq) is added dropwise to a stirred solution of alcohol (1 eq) and diisopropylethylamine (6 eq) in DCM. The reaction mixture is stirred at room temperature for 3 hours, then quenched with saturated aqueous sodium bicarbonate solution, and extracted with DCM. The combined extracts are washed with brine, dried over Na2SO4, concentrated, purified by chromatography and, dried under high vacuum. The Carbon chain containing dinucleotide amidites in the table below, were prepared according to the general procedure outlined above. Analytical data for prepared dinucleotide amidites comprising a C chain: *Purity determined by HPLC Example 84: Effect of modified oligonucleotides targeting human MAPT in hMAPT transgenic mice Compounds were evaluated in an in vivo human MAPT transgenic mice PD study. The animals received a single vehicle or 0.2 mg (10 mg/kg) dose by intracisterna magna (ICM) or IT or ICV on day 1 (n=3/group). Animals were observed every day for behavioral changes. Brain regions were collected on day 15 or day 29, and tissue was immediately placed in homogenizing tube, snap frozen, then kept at -80°C for gene expression analysis. RNA Isolation was performed according to the RNeasy Micro Kit (Qiagen Cat #74004) instructions. Following RNA isolation, a 96-well plate was placed on ice while the qRT-PCR reaction was prepared. 2 µl of RNA was added to the reaction mixture containing 5 µl TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher #44444432), 1 µl human MAPT TaqMan Gene Expression Assay (Thermo Fisher: Hs00213484_m1, FAM), 1 µl mouse GAPDH TaqMan Gene Expression Assay (Thermo Fisher: Mm99999915_g1, VIC) and 11 µl RT-PCR grade nuclease-free water in a MicroAmp Optical 96-well plate (0.2 mL). qPCR was performed using a QuantStudio3 qPCR machine with the following cycles: 50℃ for 1 minute, 95℃ for 20 seconds, 40 cycles at 95℃ for 15 seconds, and 60℃ for 1 minute. Results are presented in the tables below as percent inhibition of CTNNB1, relative to vehicle control. Results are presented in Tables 4 below as percent inhibition of hMAPT, relative to vehicle control. Table 4 Average MAPT Inhibition Example 85: Effect of modified oligonucleotides targeting human MAPT in non-human primates Certain Compounds were evaluated in an in vivo cynomolgus monkey PD study. The animals received a single 60 mg dose in a volume of 2 mL in artificial cerebrospinal fluid (aCSF) via intrathecal lumbar injection on Day 1. Animals were observed every day for behavioral changes. Brain regions were collected post dosing as indicated below for each animal, and tissue was immediately placed in homogenizing tubes, snap frozen, and then kept at -80°C until analysis. RNA Isolation was performed according to the RNeasy Micro Kit (Qiagen Cat #74004) instructions. Following RNA isolation, a 96-well plate was placed on ice while the qRT-PCR reaction was prepared. 2 µl of RNA was added to the reaction mixture containing 5 µl TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher #44444432), 1 µl cyno MAPT TaqMan Gene Expression Assay (Thermo Fisher: Mf00902189_m1, FAM), 1 µl cyno ARL1 TaqMan Gene Expression Assay (Thermo Fisher: Mf02795431_m1, VIC) and 11 µl RT- PCR grade nuclease-free water in a MicroAmp Optical 96-well plate (0.2 mL). qPCR was performed using a QuantStudio3 qPCR machine with the following cycles: 50℃ for 1 minute, 95℃ for 20 seconds, 40 cycles at 95℃ for 15 seconds, and 60℃ for 1 minute. Results are presented in Table 5 below as percent inhibition of MAPT RNA, relative to control (pre-dosed level from the same animal). Due to intrathecal injection variation, certain animals may have received a lower dose and therefore may show lower inhibition as a result. The amount of test compound present in CSF was measured at 1h, 6h, and 24h post dose. Table 6 shows the CSF compound concentration (µg/mL) for the two animals dosed with RD4845. Table 5 Percent MAPT RNA Inhibition in Brain and Spinal Cord
Table 6 CSF Compound Concentration SEQ ID NO: 1 1 gcagtcaccg ccacccacca gctccggcac caacagcagc gccgctgcca ccgcccacct 61 tctgccgccg ccaccacagc caccttctcc tcctccgctg tcctctcccg tcctcgcctc 121 tgtcgactat caggtgaact ttgaaccagg atggctgagc cccgccagga gttcgaagtg 181 atggaagatc acgctgggac gtacgggttg ggggacagga aagatcaggg gggctacacc 241 atgcaccaag accaagaggg tgacacggac gctggcctga aagaatctcc cctgcagacc 301 cccactgagg acggatctga ggaaccgggc tctgaaacct ctgatgctaa gagcactcca 361 acagcggaag ctgaagaagc aggcattgga gacaccccca gcctggaaga cgaagctgct 421 ggtcacgtga cccaagagga gttgagagtt ccgggccggc agaggaaggc gcctgaaagg 481 cccctggcca atgagattag cgcccacgtc cagcctggac cctgcggaga ggcctctggg 541 gtctctgggc cgtgcctcgg ggagaaagag ccagaagctc ccgtcccgct gaccgcgagc 601 cttcctcagc accgtcccgt ttgcccagcg cctcctccaa caggaggccc tcaggagccc 661 tccctggagt ggggacaaaa aggcggggac tgggccgaga agggtccggc ctttccgaag 721 cccgccacca ctgcgtatct ccacacagag cctgaaagtg gtaaggtggt ccaggaaggc 781 ttcctccgag agccaggccc cccaggtctg agccaccagc tcatgtccgg catgcctggg 841 gctcccctcc tgcctgaggg ccccagagag gccacacgcc aaccttcggg gacaggacct 901 gaggacacag agggcggccg ccacgcccct gagctgctca agcaccagct tctaggagac 961 ctgcaccagg aggggccgcc gctgaagggg gcagggggca aagagaggcc ggggagcaag 1021 gaggaggtgg atgaagaccg cgacgtcgat gagtcctccc cccaagactc ccctccctcc 1081 aaggcctccc cagcccaaga tgggcggcct ccccagacag ccgccagaga agccaccagc 1141 atcccaggct tcccagcgga gggtgccatc cccctccctg tggatttcct ctccaaagtt 1201 tccacagaga tcccagcctc agagcccgac gggcccagtg tagggcgggc caaagggcag 1261 gatgcccccc tggagttcac gtttcacgtg gaaatcacac ccaacgtgca gaaggagcag 1321 gcgcactcgg aggagcattt gggaagggct gcatttccag gggcccctgg agaggggcca 1381 gaggcccggg gcccctcttt gggagaggac acaaaagagg ctgaccttcc agagccctct 1441 gaaaagcagc ctgctgctgc tccgcggggg aagcccgtca gccgggtccc tcaactcaaa 1501 gctcgcatgg tcagtaaaag caaagacggg actggaagcg atgacaaaaa agccaagaca 1561 tccacacgtt cctctgctaa aaccttgaaa aataggcctt gccttagccc caaacacccc 1621 actcctggta gctcagaccc tctgatccaa ccctccagcc ctgctgtgtg cccagagcca 1681 ccttcctctc ctaaatacgt ctcttctgtc acttcccgaa ctggcagttc tggagcaaag 1741 gagatgaaac tcaagggggc tgatggtaaa acgaagatcg ccacaccgcg gggagcagcc 1801 cctccaggcc agaagggcca ggccaacgcc accaggattc cagcaaaaac cccgcccgct 1861 ccaaagacac cacccagctc tggtgaacct ccaaaatcag gggatcgcag cggctacagc 1921 agccccggct ccccaggcac tcccggcagc cgctcccgca ccccgtccct tccaacccca 1981 cccacccggg agcccaagaa ggtggcagtg gtccgtactc cacccaagtc gccgtcttcc 2041 gccaagagcc gcctgcagac agcccccgtg cccatgccag acctgaagaa tgtcaagtcc 2101 aagatcggct ccactgagaa cctgaagcac cagccgggag gcgggaaggt gcagataatt 2161 aataagaagc tggatcttag caacgtccag tccaagtgtg gctcaaagga taatatcaaa 2221 cacgtcccgg gaggcggcag tgtgcaaata gtctacaaac cagttgacct gagcaaggtg 2281 acctccaagt gtggctcatt aggcaacatc catcataaac caggaggtgg ccaggtggaa 2341 gtaaaatctg agaagcttga cttcaaggac agagtccagt cgaagattgg gtccctggac 2401 aatatcaccc acgtccctgg cggaggaaat aaaaagattg aaacccacaa gctgaccttc 2461 cgcgagaacg ccaaagccaa gacagaccac ggggcggaga tcgtgtacaa gtcgccagtg 2521 gtgtctgggg acacgtctcc acggcatctc agcaatgtct cctccaccgg cagcatcgac 2581 atggtagact cgccccagct cgccacgcta gctgacgagg tgtctgcctc cctggccaag 2641 cagggtttgt gatcaggccc ctggggcggt caataattgt ggagaggaga gaatgagaga 2701 gtgtggaaaa aaaaagaata atgacccggc ccccgccctc tgcccccagc tgctcctcgc 2761 agttcggtta attggttaat cacttaacct gcttttgtca ctcggctttg gctcgggact 2821 tcaaaatcag tgatgggagt aagagcaaat ttcatctttc caaattgatg ggtgggctag 2881 taataaaata tttaaaaaaa aacattcaaa aacatggcca catccaacat ttcctcaggc 2941 aattcctttt gattcttttt tcttccccct ccatgtagaa gagggagaag gagaggctct 3001 gaaagctgct tctgggggat ttcaagggac tgggggtgcc aaccacctct ggccctgttg 3061 tgggggtgtc acagaggcag tggcagcaac aaaggatttg aaacttggtg tgttcgtgga 3121 gccacaggca gacgatgtca accttgtgtg agtgtgacgg gggttggggt ggggcgggag 3181 gccacggggg aggccgaggc aggggctggg cagaggggag aggaagcaca agaagtggga 3241 gtgggagagg aagccacgtg ctggagagta gacatccccc tccttgccgc tgggagagcc 3301 aaggcctatg ccacctgcag cgtctgagcg gccgcctgtc cttggtggcc gggggtgggg 3361 gcctgctgtg ggtcagtgtg ccaccctctg cagggcagcc tgtgggagaa gggacagcgg 3421 gtaaaaagag aaggcaagct ggcaggaggg tggcacttcg tggatgacct ccttagaaaa 3481 gactgacctt gatgtcttga gagcgctggc ctcttcctcc ctccctgcag ggtagggggc 3541 ctgagttgag gggcttccct ctgctccaca gaaaccctgt tttattgagt tctgaaggtt 3601 ggaactgctg ccatgatttt ggccactttg cagacctggg actttagggc taaccagttc 3661 tctttgtaag gacttgtgcc tcttgggaga cgtccacccg tttccaagcc tgggccactg 3721 gcatctctgg agtgtgtggg ggtctgggag gcaggtcccg agccccctgt ccttcccacg 3781 gccactgcag tcaccccgtc tgcgccgctg tgctgttgtc tgccgtgaga gcccaatcac 3841 tgcctatacc cctcatcaca cgtcacaatg tcccgaattc ccagcctcac caccccttct 3901 cagtaatgac cctggttggt tgcaggaggt acctactcca tactgagggt gaaattaagg 3961 gaaggcaaag tccaggcaca agagtgggac cccagcctct cactctcagt tccactcatc 4021 caactgggac cctcaccacg aatctcatga tctgattcgg ttccctgtct cctcctcccg 4081 tcacagatgt gagccagggc actgctcagc tgtgacccta ggtgtttctg ccttgttgac 4141 atggagagag ccctttcccc tgagaaggcc tggccccttc ctgtgctgag cccacagcag 4201 caggctgggt gtcttggttg tcagtggtgg caccaggatg gaagggcaag gcacccaggg 4261 caggcccaca gtcccgctgt cccccacttg caccctagct tgtagctgcc aacctcccag 4321 acagcccagc ccgctgctca gctccacatg catagtatca gccctccaca cccgacaaag 4381 gggaacacac ccccttggaa atggttcttt tcccccagtc ccagctggaa gccatgctgt 4441 ctgttctgct ggagcagctg aacatataca tagatgttgc cctgccctcc ccatctgcac 4501 cctgttgagt tgtagttgga tttgtctgtt tatgcttgga ttcaccagag tgactatgat 4561 agtgaaaaga aaaaaaaaaa aaaaaaagga cgcatgtatc ttgaaatgct tgtaaagagg 4621 tttctaaccc accctcacga ggtgtctctc acccccacac tgggactcgt gtggcctgtg 4681 tggtgccacc ctgctggggc ctcccaagtt ttgaaaggct ttcctcagca cctgggaccc 4741 aacagagacc agcttctagc agctaaggag gccgttcagc tgtgacgaag gcctgaagca 4801 caggattagg actgaagcga tgatgtcccc ttccctactt ccccttgggg ctccctgtgt 4861 cagggcacag actaggtctt gtggctggtc tggcttgcgg cgcgaggatg gttctctctg 4921 gtcatagccc gaagtctcat ggcagtccca aaggaggctt acaactcctg catcacaaga 4981 aaaaggaagc cactgccagc tggggggatc tgcagctccc agaagctccg tgagcctcag 5041 ccacccctca gactgggttc ctctccaagc tcgccctctg gaggggcagc gcagcctccc 5101 accaagggcc ctgcgaccac agcagggatt gggatgaatt gcctgtcctg gatctgctct 5161 agaggcccaa gctgcctgcc tgaggaagga tgacttgaca agtcaggaga cactgttccc 5221 aaagccttga ccagagcacc tcagcccgct gaccttgcac aaactccatc tgctgccatg 5281 agaaaaggga agccgccttt gcaaaacatt gctgcctaaa gaaactcagc agcctcaggc 5341 ccaattctgc cacttctggt ttgggtacag ttaaaggcaa ccctgaggga cttggcagta 5401 gaaatccagg gcctcccctg gggctggcag cttcgtgtgc agctagagct ttacctgaaa 5461 ggaagtctct gggcccagaa ctctccacca agagcctccc tgccgttcgc tgagtcccag 5521 caattctcct aagttgaagg gatctgagaa ggagaaggaa atgtggggta gatttggtgg 5581 tggttagaga tatgcccccc tcattactgc caacagtttc ggctgcattt cttcacgcac 5641 ctcggttcct cttcctgaag ttcttgtgcc ctgctcttca gcaccatggg ccttcttata 5701 cggaaggctc tgggatctcc cccttgtggg gcaggctctt ggggccagcc taagatcatg 5761 gtttagggtg atcagtgctg gcagataaat tgaaaaggca cgctggcttg tgatcttaaa 5821 tgaggacaat ccccccaggg ctgggcactc ctcccctccc ctcacttctc ccacctgcag 5881 agccagtgtc cttgggtggg ctagatagga tatactgtat gccggctcct tcaagctgct 5941 gactcacttt atcaatagtt ccatttaaat tgacttcagt ggtgagactg tatcctgttt 6001 gctattgctt gttgtgctat ggggggaggg gggaggaatg tgtaagatag ttaacatggg 6061 caaagggaga tcttggggtg cagcacttaa actgcctcgt aacccttttc atgatttcaa 6121 ccacatttgc tagagggagg gagcagccac ggagttagag gcccttgggg tttctctttt 6181 ccactgacag gctttcccag gcagctggct agttcattcc ctccccagcc aggtgcaggc 6241 gtaggaatat ggacatctgg ttgctttggc ctgctgccct ctttcagggg tcctaagccc 6301 acaatcatgc ctccctaaga ccttggcatc cttccctcta agccgttggc acctctgtgc 6361 cacctctcac actggctcca gacacacagc ctgtgctttt ggagctgaga tcactcgctt 6421 caccctcctc atctttgttc tccaagtaaa gccacgaggt cggggcgagg gcagaggtga 6481 tcacctgcgt gtcccatcta cagacctgca gcttcataaa acttctgatt tctcttcagc 6541 tttgaaaagg gttaccctgg gcactggcct agagcctcac ctcctaatag acttagcccc 6601 atgagtttgc catgttgagc aggactattt ctggcacttg caagtcccat gatttcttcg 6661 gtaattctga gggtgggggg agggacatga aatcatctta gcttagcttt ctgtctgtga 6721 atgtctatat agtgtattgt gtgttttaac aaatgattta cactgactgt tgctgtaaaa 6781 gtgaatttgg aaataaagtt attactctga ttaaa EQUIVALENTS AND SCOPE It is to be understood that this disclosure is not limited to any or all of the particular embodiments described expressly herein, and as such may, of course, vary. It is also to be understood that the terminology used herein is for the purpose of describing particular embodiments only and is not intended to be limiting. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure belongs. Although any methods and materials similar or equivalent to those described herein can also be used in the practice or testing of the present disclosure, the preferred methods and materials are now described. As will be readily apparent to the skilled artisan, and should be understood from the terms used herein, where words or terms are defined herein, their applicability should not be limited to the embodiments immediately preceding or following the definition and should be used where context permits throughout the disclosure. All publications and patents cited in this specification are cited to disclose and describe the methods and/or materials in connection with which the publications are cited. All such publications and patents are herein incorporated by references as if each individual publication or patent were specifically and individually indicated to be incorporated by reference. Such incorporation by reference is expressly limited to the methods and/or materials described in the cited publications and patents and does not extend to any lexicographical definitions from the cited publications and patents (i.e., any lexicographical definition in the publications and patents cited that is not also expressly repeated in the disclosure should not be treated as such and should not be read as defining any terms appearing in the accompanying claims). If there is a conflict between any of the incorporated references and this disclosure, this disclosure shall control. In addition, any particular embodiment of this disclosure that falls within the prior art may be explicitly excluded from any one or more of the claims. Because such embodiments are deemed to be known to one of ordinary skill in the art, they may be excluded even if the exclusion is not set forth explicitly herein. Any particular embodiment of the disclosure can be excluded from any claim, for any reason, whether or not related to the existence of prior art. The citation of any publication is for its disclosure prior to the filing date and should not be construed as an admission that the present disclosure is not entitled to antedate such publication by virtue of prior disclosure. Further, the dates of publication provided could be different from the actual publication dates that may need to be independently confirmed. As will be apparent to those of skill in the art upon reading this disclosure, each of the individual embodiments described and illustrated herein has discrete components and features which may be readily separated from or combined with the features of any of the other several embodiments without departing from the scope or spirit of the present disclosure. Any recited method can be carried out in the order of events recited or in any other order that is logically possible. In the claims articles such as “a,” “an,” and “the” may mean one or more than one unless indicated to the contrary or otherwise evident from the context. Wherever used herein, a pronoun in a gender (e.g., masculine, feminine, neuter, other, etc.) the pronoun shall be construed as gender neutral (e.g., construed to refer to all genders equally) regardless of the implied gender unless the context clearly indicates or requires otherwise. Wherever used herein, words used in the singular include the plural, and words used in the plural includes the singular, unless the context clearly indicates or requires otherwise. Claims or descriptions that include “or” between one or more members of a group are considered satisfied if one, more than one, or all of the group members are present in, employed in, or otherwise relevant to a given product or process unless indicated to the contrary or otherwise evident from the context. The disclosure includes embodiments in which exactly one member of the group is present in, employed in, or otherwise relevant to a given product or process. The disclosure includes embodiments in which more than one, or all of the group members are present in, employed in, or otherwise relevant to a given product or process. Furthermore, the disclosure encompasses all variations, combinations, and permutations in which one or more limitations, elements, clauses, and descriptive terms from one or more of the listed claims is introduced into another claim. For example, any claim that is dependent on another claim can be modified to include one or more limitations found in any other claim that is dependent on the same base claim. Where elements are presented as lists (e.g., in Markush group format), each subgroup of the elements is also disclosed, and any element(s) can be removed from the group. It should it be understood that, in general, where the disclosure, or aspects of the disclosure, is/are referred to as comprising particular elements and/or features, certain embodiments of the disclosure or aspects of the disclosure consist, or consist essentially of, such elements and/or features. For purposes of simplicity, those embodiments have not been specifically set forth in haec verba herein. It is also noted that the terms “comprising” and “containing” are intended to be open and permits the inclusion of additional elements or steps. Where ranges are given, endpoints are included in such ranges unless otherwise specified. Furthermore, unless otherwise indicated or otherwise evident from the context and understanding of one of ordinary skill in the art, values that are expressed as ranges can assume any specific value or sub–range within the stated ranges in different embodiments of the disclosure, to the tenth of the unit of the lower limit of the range, unless the context clearly dictates otherwise. Those skilled in the art will recognize or be able to ascertain using no more than routine experimentation many equivalents to the specific embodiments described herein. The scope of the present embodiments described herein is not intended to be limited to the above Description, but rather is as set forth in the appended claims. Those of ordinary skill in the art will appreciate that various changes and modifications to this description may be made without departing from the spirit or scope of the disclosure, as defined in the following claims.
Claims
CLAIMS What is claimed is: 1. A compound comprising a modified oligonucleotide 14 to 30 linked nucleosides in length having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209- 217.
2. A compound comprising a modified oligonucleotide 14 to 23 linked nucleosides in length having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
3. A compound comprising a modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217.
4. The compound of any one of claims 1-3, wherein the modified oligonucleotide is at least 80%, at least 85%, at least 90%, or at least 95% complementary to SEQ ID NO: 1 or 2.
5. The compound of any one of claims 1-4, wherein the modified oligonucleotide comprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
6. The compound of any one of claims 1-5, wherein the compound is double-stranded.
7. A compound comprising a first modified oligonucleotide 14 to 23 linked nucleosides in length having a nucleobase sequence comprising at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23 contiguous nucleobases of any of the nucleobase sequence of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 14 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
8. A compound comprising a first modified oligonucleotide 14 to 23 linked nucleosides in length having a nucleobase sequence comprising the nucleobase sequence of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 or SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206, and a second modified oligonucleotide 14 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
9. A compound comprising a first modified oligonucleotide having a nucleobase sequence selected from the group consisting of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200 and 209-217 and SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206 and a second modified oligonucleotide 19 to 23 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
10. The compound of any one of claims 7-9, wherein the first modified oligonucleotide has at least 80%, at least 85%, at least 90%, or at least 95% complementarity or identity to SEQ ID NO: 1 or 2 over its length.
11. The compound of any one of claims 7-10, wherein the first modified oligonucleotide has at least 1, at least 2, at least 3 mismatches to a region of SEQ ID NO: 1 or 2 that is the same length as the first modified oligonucleotide.
12. The compound of any one of claims 7-11, wherein the region of complementarity between the first modified oligonucleotide and the second modified oligonucleotide is 14 to 23 linked nucleosides in length.
13. The compound of any one of claims 7-11, wherein the region of complementarity between the first modified oligonucleotide and the second modified oligonucleotide is 19 to 23 linked nucleosides in length.
14. The compound of any one of claims 7-11, wherein the region of complementarity between the first modified oligonucleotide and the second modified oligonucleotide is 21 to 23 linked nucleosides in length.
15. The compound of any one of claims 7-11, wherein the first modified oligonucleotide is fully complementary to the second modified oligonucleotide.
16. The compound of any one of claims 7-15, wherein the first modified oligonucleotide comprises at least one modification selected from a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
17. The compound of any one of claims 7-16, wherein the second modified oligonucleotide comprises at least one modification selected from the group consisting of a modified internucleoside linkage, a modified sugar, and a modified nucleobase.
18. The compound of any one of claims 5, 16 and 17, wherein the modified internucleoside linkage is a phosphorothioate internucleoside linkage or a methylphosphonate internucleoside linkage.
19. The compound of claim 18, wherein the phosphorothioate internucleoside linkage or methylphosphonate internucleoside linkage is at the 3’ terminus of the first or second modified oligonucleotide or at the 5’ terminus of the first modified oligonucleotide.
20. The compound of any one of claims 5, 16 and 17, wherein the modified sugar comprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar.
21. The compound of claim 20, wherein the modified sugar comprises a 2’-F modification.
22. The compound of claim 20, wherein the modified sugar comprises a 2’-OMe modification.
23. The compound of any one of claims 7-15, wherein each nucleoside of the first modified oligonucleotide comprises a modified sugar.
24. The compound of any one of claims 7-15, wherein each nucleoside of the second modified oligonucleotide comprises a modified sugar.
25. The compound of claim 23 or 24, wherein the modified sugar comprises a modification selected from the group consisting of a halogen, an alkoxy group and a bicyclic sugar, or a combination thereof.
26. The compound of claim 25, wherein the modified sugar comprises a modification selected from group consisting of LNA, cEt, 2’-MOE, 2’-F, 2’-OMe, and 2’-deoxy, or a combination thereof.
27. The compound of claim 26, wherein the first modified oligonucleotide comprises no more than ten 2’-F sugar modifications.
28. The compound of claim 27, wherein the second modified oligonucleotide comprises no more than five 2’-F sugar modifications.
29. The compound of any preceding claim, comprising a conjugate group.
30. The compound of claim 29, wherein the conjugate group is attached to the 5’ end of the modified oligonucleotide.
31. The compound of claim 29 or 30, wherein the conjugate group comprises a targeting moiety.
32. The compound of claim 31, wherein the targeting moiety comprises one or more ligands selected from one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB1) ligands, and one or more α4β1/7 integrin ligands.
33. The compound of claim 32, wherein the modified oligonucleotide is the second modified oligonucleotide.
34. The compound of claim 33, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end of the modified oligonucleotide.
35. The compound of claim 33, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 3’ end of the modified oligonucleotide.
36. The compound of claim 33, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end and the 3’ end of the modified oligonucleotide.
37. The compound of claim 33, wherein: the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX- XXXXXXXIII; the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII; and the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV.
38. The compound of any one of claims 29-37, wherein the conjugate group comprises one or more lipids.
39. The compound of claim 38, wherein the modified oligonucleotide is the second modified oligonucleotide.
40. The compound of claim 38 or 39, wherein the one or more lipids are attached to an internucleoside linkage of the modified oligonucleotide.
41. The compound of claim 40, wherein the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI.
42. The compound of any one of claims 38-40, wherein the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI.
43. The compound of any one of claims 32-42, wherein the modified oligonucleotide comprises one or more TrkB ligands, one or more CB1 ligands, or one or more α4β1/7 integrin ligands.
44. The compound of claim 43, wherein the modified oligonucleotide comprises one or more TrkB ligands.
45. The compound of claim 44, wherein the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV- XXXXXXVII, and XXXXXXIX-XXXXXXXIII.
46. The compound of claim 45, wherein the one or more TrkB ligands are selected from any one of Formulae XXIX-XXXI and XXXXXXV.
47. The compound of any one of claims 44-46, wherein the modified oligonucleotide comprises one or two TrkB ligands.
48. The compound of any one of claims 44-46, wherein the modified oligonucleotide comprises at least two TrkB ligands.
49. The compound of claim 48, wherein the at least two TrkB ligands are the same.
50. The compound of claim 48, wherein the at least two TrkB ligands are different.
51. The compound of claim 43, wherein the modified oligonucleotide comprises one or more CB1 ligands.
52. The compound of claim 51, wherein the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII.
53. The compound of claim 52, wherein the one or more CB1 ligands are selected from any one of Formulae XXXXXXXIV-XXXXXXXVI.
54. The compound of any one of claims 51-53, wherein the modified oligonucleotide comprises one or two CB1 ligands.
55. The compound of any one of claims 51-53, wherein the modified oligonucleotide comprises at least two CB1 ligands.
56. The compound of claim 55, wherein the at least two CB1 ligands are the same.
57. The compound of claim 55, wherein the at least two CB1 ligands are different.
58. The compound of claim 43, wherein the modified oligonucleotide comprises one or more α4β1/7 integrin ligands.
59. The compound of claim 58, wherein the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX- XXXXXXXXIV.
60. The compound of claim 59, wherein the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXXXI, XXXXXXXXII, and XXXXXXXXIV.
61. The compound of any one of claims 58-60, wherein the modified oligonucleotide comprises one or two α4β1/7 integrin ligands.
62. The compound of any one of claims 58-60, wherein the modified oligonucleotide comprises at least two α4β1/7 integrin ligands.
63. The compound of claim 62, wherein the at least two α4β1/7 integrin ligands are the same.
64. The compound of claim 62, wherein the at least two α4β1/7 integrin ligands are different.
65. The compound of any one of claims 44-64, wherein the modified oligonucleotide comprises one or more lipids.
66. The compound of claim 65, wherein the modified oligonucleotide is the second modified oligonucleotide.
67. The compound of any one of claims 44-66, wherein the modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl.
68. The compound of claim 67, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain.
69. The compound of claim 68, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain, optionally wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain.
70. The compound of claim 69, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain, a saturated or unsaturated C17 hydrocarbon chain, a saturated or unsaturated C18 hydrocarbon chain, or a saturated or unsaturated C22 hydrocarbon chain.
71. The compound of any one of claims 67-70, wherein the one or more substituted or unsubstituted alkyl or alkenyl are attached to an internucleoside linkage of the modified oligonucleotide.
72. The compound of claim 71, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the modified oligonucleotide.
73. The compound of claim 72, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the modified oligonucleotide.
74. The compound of claim 71, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the modified oligonucleotide.
75. The compound of claim 74, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the modified oligonucleotide.
76. The compound of any one of claims 71-75, wherein the internucleoside linkage of the modified oligonucleotide is selected from any one of Formulae XXXXXIII-XXXXXVI.
77. The compound of any one of claims 65-76, wherein the modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI.
78. The compound of any one of claims 1-6, wherein the modified oligonucleotide comprises a 5′-phosphonate modification.
79. The compound of any one of claims 7-77, wherein the first modified oligonucleotide comprises a 5′-phosphonate modification.
80. The compound of claim 78 or 79, wherein the 5′-phosphonate modification is a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification.
81. A compound comprising: a first modified oligonucleotide comprising a 5′-phosphonate modification, wherein the first modified oligonucleotide is at least 80% complementary to a region of SEQ ID NO: 1 or 2; and a second modified oligonucleotide comprising one or more ligands.
82. The compound of claim 81, wherein the first modified oligonucleotide comprises a 5′- terminal nucleoside comprising the 5′-phosphonate modification.
83. The compound of claim 81 or 82, wherein the 5′-phosphonate modification is a 5′- vinylphosphonate modification or a 5′-ethylenephosphonate modification.
84. The compound of any one of claims 81-83, wherein the 5′-phosphonate modification is a 5′-vinylphosphonate modification.
85. The compound of any one of claims 81-83, wherein the 5′-phosphonate modification is a 5′-ethylenephosphonate modification.
86. The compound of any one of claims 81-85, wherein the second modified oligonucleotide comprises one or more ligands selected from one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB1) ligands, and one or more α4β1/7 integrin ligands.
87. The compound of claim 86, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end of the second modified oligonucleotide.
88. The compound of claim 86, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 3’ end of the second modified oligonucleotide.
89. The compound of claim 86, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end and the 3’ end of the second modified oligonucleotide.
90. The compound of claim 86, wherein: the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX- XXXXXXXIII; the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII; and the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV.
91. The compound of any one of claims 81-90, wherein the second modified oligonucleotide comprises one or more TrkB ligands.
92. The compound of claim 91, wherein the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV- XXXXXXVII, and XXXXXXIX-XXXXXXXIII.
93. The compound of claim 92, wherein the one or more TrkB ligands are selected from any one of Formulae XXIX-XXXI and XXXXXXV.
94. The compound of any one of claims 91-93, wherein the second modified oligonucleotide comprises one or two TrkB ligands.
95. The compound of any one of claims 91-93, wherein the second modified oligonucleotide comprises at least two TrkB ligands.
96. The compound of claim 95, wherein the at least two TrkB ligands are the same.
97. The compound of claim 95, wherein the at least two TrkB ligands are different.
98. The compound of any one of claims 81-90, wherein the second modified oligonucleotide comprises one or more CB1 ligands.
99. The compound of claim 98, wherein the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII.
100. The compound of claim 99, wherein the one or more CB1 ligands are selected from any one of Formulae XXXXXXXIV-XXXXXXXVI.
101. The compound of any one of claims 98-100, wherein the second modified oligonucleotide comprises one or two CB1 ligands.
102. The compound of any one of claims 98-100, wherein the second modified oligonucleotide comprises at least two CB1 ligands.
103. The compound of claim 102, wherein the at least two CB1 ligands are the same.
104. The compound of claim 102, wherein the at least two CB1 ligands are different.
105. The compound of any one of claims 81-90, wherein the second modified oligonucleotide comprises one or more α4β1/7 integrin ligands.
106. The compound of claim 105, wherein the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX- XXXXXXXXIV.
107. The compound of claim 106, wherein the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXXXI, XXXXXXXXII, and XXXXXXXXIV.
108. The compound of any one of claims 105-107, wherein the second modified oligonucleotide comprises one or two α4β1/7 integrin ligands.
109. The compound of any one of claims 105-107, wherein the second modified oligonucleotide comprises at least two α4β1/7 integrin ligands.
110. The compound of claim 109, wherein the at least two α4β1/7 integrin ligands are the same.
111. The compound of claim 109, wherein the at least two α4β1/7 integrin ligands are different.
112. The compound of any one of claims 81-111, wherein the second modified oligonucleotide comprises one or more lipids.
113. The compound of any one of claims 81-111, wherein the second modified oligonucleotide comprises one or more substituted or unsubstituted alkyl or alkenyl.
114. The compound of claim 113, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C4-C30 hydrocarbon chain.
115. The compound of claim 114, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C5-C20 hydrocarbon chain, optionally wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C14-C20 hydrocarbon chain.
116. The compound of claim 115, wherein the one or more substituted or unsubstituted alkyl or alkenyl comprise a saturated or unsaturated C16 hydrocarbon chain, a saturated or unsaturated C17 hydrocarbon chain, a saturated or unsaturated C18 hydrocarbon chain, or a saturated or unsaturated C22 hydrocarbon chain.
117. The compound of any one of claims 113-116, wherein the one or more substituted or unsubstituted alkyl or alkenyl are attached to an internucleoside linkage of the second modified oligonucleotide.
118. The compound of claim 117, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 5′ end of the second modified oligonucleotide.
119. The compound of claim 118, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 5′ end of the second modified oligonucleotide.
120. The compound of claim 117, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, positions 7 and 8, positions 8 and 9, positions 9 and 10, positions 10 and 11, positions 11 and 12, positions 12 and 13, or positions 13 and 14 from the 3′ end of the second modified oligonucleotide.
121. The compound of claim 120, wherein the internucleoside linkage is between positions 1 and 2, positions 2 and 3, positions 3 and 4, positions 4 and 5, positions 5 and 6, positions 6 and 7, or positions 7 and 8 from the 3′ end of the second modified oligonucleotide.
122. The compound of any one of claims 117-121, wherein the internucleoside linkage of the second modified oligonucleotide is selected from any one of Formulae XXXXXIII- XXXXXVI.
123. The compound of any one of claims 112-122, wherein the second modified oligonucleotide comprises any one of Formulae XXXV-XXXXXVI.
124. The compound of any one of claims 81-123, wherein the first modified oligonucleotide is 14 to 30 linked nucleosides in length.
125. The compound of any one of claims 81-124, wherein the second modified oligonucleotide is 14 to 30 linked nucleosides in length having a region of complementarity to the first modified oligonucleotide.
126. The compound of any one of claims 81-125, wherein the first modified oligonucleotide has a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 11-73, 144-147, 177-188, 190-192, 194-197, 199-200, and 209-217.
127. The compound of any one of claims 81-126, wherein the second modified oligonucleotide has a nucleobase sequence comprising at least 14 contiguous nucleobases of any one of SEQ ID NOs: 81-143, 149-155, 157, 159-168, 171-172, 174, 176 and 201-206.
128. The compound of any one of claims 81-127, wherein the first modified oligonucleotide is selected from any one of the IA Ref ID NOs in Table 3.
129. The compound of any one of claims 81-128, wherein the second modified oligonucleotide is selected from any one of the IS Ref ID NOs in Table 3.
130. A compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs in Table 3, and a second modified oligonucleotide 14 to 21 linked nucleosides in length fully complementary to the first modified oligonucleotide.
131. A compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs in Table 3, and a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs in Table 3.
132. A compound comprising a first modified oligonucleotide selected from the group consisting of any one of the IA Ref ID NOs in Table 3 and/or a second modified oligonucleotide selected from the group consisting of any one of the IS Ref ID NOs in Table 3.
133. The compound of claim 130, 131, or 132, comprising a conjugate group.
134. The compound of claim 133, wherein the conjugate group is attached to the 5’ end of the first or second modified oligonucleotide.
135. The compound of claim 133 or 134, wherein the conjugate group comprises a targeting moiety.
136. The compound of claim 135, wherein the targeting moiety comprises one or more ligands selected from one or more Tropomyosin receptor B (TrkB) ligands, one or more cannabinoid receptor type 1 (CB1) ligands, and one or more α4β1/7 integrin ligands.
137. The compound of claim 136, wherein the modified oligonucleotide is the second modified oligonucleotide.
138. The compound of claim 137, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end of the modified oligonucleotide.
139. The compound of claim 137, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 3’ end of the modified oligonucleotide.
140. The compound of claim 137, wherein the one or more TrkB ligands, the one or more CB1 ligands, or the one or more α4β1/7 integrin ligands are attached to the 5’ end and the 3’ end of the modified oligonucleotide.
141. The compound of claim 137, wherein: the one or more TrkB ligands are selected from any one of Formulae I-XXXIV, XXXXXVII, XXXXXIX-XXXXXX, XXXXXXV-XXXXXXVII, and XXXXXXIX- XXXXXXXIII; the one or more CB1 ligands are selected from any one of Formulae XXXXXXI and XXXXXXXIV-XXXXXXXVIII; and the one or more α4β1/7 integrin ligands are selected from any one of Formulae XXXXXXII-XXXXXXIV and XXXXXXXIX-XXXXXXXXIV.
142. A compound of any one of the preceding claims, wherein the compound is in a pharmaceutically acceptable salt form.
143. The compound of claim 142, wherein the pharmaceutically acceptable salt is a sodium salt.
144. The compound of claim 142, wherein the pharmaceutically acceptable salt is a potassium salt.
145. A composition comprising the compound or modified oligonucleotide of any preceding claim and a pharmaceutically acceptable carrier.
146. A composition comprising a compound or modified oligonucleotide of any preceding claim, for use in therapy.
147. A method of treating, preventing or ameliorating a disease, disorder or condition associated with MAPT in an individual comprising administering to the individual a compound targeted to MAPT, thereby treating, preventing, or ameliorating the disease, disorder or condition.
148. A method of administering the compound or modified oligonucleotide of any one of claims 1-144 or composition of claim 146 to an individual.
149. The method of claim 147 or 148, wherein the disease, disorder or condition is a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
150. The method of any one of claims 147-149, wherein administering the compound inhibits or reduces or improves a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
151. A method of inhibiting expression of MAPT in a cell comprising contacting the cell with a compound targeted to MAPT, thereby inhibiting expression of MAPT in the cell.
152. The method of claim 151, wherein the cell is in the brain of an individual.
153. The method of claim 152, wherein the individual has, or is at risk of having, a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
154. A method of reducing or inhibiting a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome in an individual, comprising administering a compound targeted to MAPT to the individual, thereby reducing or inhibiting a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome in the individual.
155. The method of claim 154, wherein the individual has, or is at risk of having, a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
156. The method of any one of claims 147-155, wherein the compound is a compound targeted to MAPT.
157. The method of any one of claims 147-156, wherein the compound or modified oligonucleotide is the compound or modified oligonucleotide of any one of claims 1-144 or composition of claim 146.
158. The method of claim 157, wherein the compound or composition is administered parenterally.
159. The method of claim 157, wherein the compound or composition is administered by intrathecal administration.
160. Use of a compound targeted to MAPT for treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT.
161. The use of claim 160, wherein the disease, disorder or condition is a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
162. The use of claim 160 or 161, wherein the compound is a compound targeted to MAPT.
163. The use of any one of claims 160-162, wherein the compound or modified oligonucleotide is the compound or modified oligonucleotide of any one of claims 1-144 or composition of claim 146.
164. Use of a compound targeted to MAPT in the manufacture of a medicament for treating, preventing, or ameliorating a disease, disorder or condition associated with MAPT.
165. The use of claim 164, wherein the disease is a neurodegenerative disease, including a tauopathy, Alzheimer’s disease, frontotemporal dementia (FTD), FTDP-17, progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), corticobasal ganglionic degeneration (CBD), epilepsy, or Dravet’s Syndrome.
166. The use of claim 164 or 165, wherein the compound is a compound targeted to MAPT.
167. The use of any one of claims 164-166, wherein the compound or modified oligonucleotide is the compound or modified oligonucleotide of any one of claims 1-144 or composition of claim 146.
168. The method or use of any preceding claim, wherein the compound or composition is administered to an individual about once every three months to about once every year.
169. The method or use of any preceding claim, wherein the compound or composition is administered to an individual about once every three months, about once every six months, or about once every year.
170. A method for delivering a therapeutic oligonucleotide to the brain of a subject, comprising administration of a compound, or a stereoisomer, tautomer, prodrug, or salt thereof, of any one of claims 1-144 or composition of claim 146 to the subject.
171. The method of claim 170, wherein the therapeutic oligonucleotide is delivered to one or more brain regions selected from the group consisting of the striatum, the cerebellum, the brain stem, the hippocampus, the frontal cortex, and the spinal cord.
172. A method for treating or ameliorating a disease, disorder, or symptom thereof in a subject, comprising administration of a compound, or a stereoisomer, tautomer, prodrug, or salt thereof, of any one of claims 1-144 or composition of claim 146 to the subject.
173. The method of claim 172, wherein the disease, disorder, or symptom thereof is a central nervous system (CNS) disease, disorder, or symptom thereof.
174. The method of claim 172 or 173, wherein the disease, disorder, or symptom thereof is Alzheimer’s disease, or a symptom thereof.
175. The method of any one of claims 170-174, wherein the administration is intrathecal administration or intracerebroventricular (ICV) administration.
176. A method of delivering one or more cargo molecules to a cell or tissue of a subject in vivo, comprising administering to the subject a compound of any one of claims 1-144 or composition of claim 146.
177. The method of claim 176, wherein the cell or tissue is CNS cell or tissue.
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363460874P | 2023-04-20 | 2023-04-20 | |
| US63/460,874 | 2023-04-20 | ||
| US202363520070P | 2023-08-16 | 2023-08-16 | |
| US63/520,070 | 2023-08-16 | ||
| US202463619022P | 2024-01-09 | 2024-01-09 | |
| US63/619,022 | 2024-01-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2024220930A2true WO2024220930A2 (en) | 2024-10-24 |
| WO2024220930A3 WO2024220930A3 (en) | 2024-12-26 |
Family
ID=91277045
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/025577PendingWO2024220930A2 (en) | 2023-04-20 | 2024-04-19 | Mapt-modulating compositions and methods of use thereof |
Country Status (2)
| Country | Link |
|---|---|
| TW (1) | TW202448484A (en) |
| WO (1) | WO2024220930A2 (en) |
Citations (142)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US4751219A (en) | 1985-02-05 | 1988-06-14 | Nederlandse Centrale Organisatie Voor Toegepast-Natuur-Wetenschappelijk Onderzoek | Synthetic glycolipides, a process for the preparation thereof and several uses for these synthetic glycolipides |
| US4845205A (en) | 1985-01-08 | 1989-07-04 | Institut Pasteur | 2,N6 -disubstituted and 2,N6 -trisubstituted adenosine-3'-phosphoramidites |
| US4981957A (en) | 1984-07-19 | 1991-01-01 | Centre National De La Recherche Scientifique | Oligonucleotides with modified phosphate and modified carbohydrate moieties at the respective chain termini |
| US5118800A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | Oligonucleotides possessing a primary amino group in the terminal nucleotide |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5134066A (en) | 1989-08-29 | 1992-07-28 | Monsanto Company | Improved probes using nucleosides containing 3-dezauracil analogs |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5175273A (en) | 1988-07-01 | 1992-12-29 | Genentech, Inc. | Nucleic acid intercalating agents |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5319080A (en) | 1991-10-17 | 1994-06-07 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5367066A (en) | 1984-10-16 | 1994-11-22 | Chiron Corporation | Oligonucleotides with selectably cleavable and/or abasic sites |
| US5432272A (en) | 1990-10-09 | 1995-07-11 | Benner; Steven A. | Method for incorporating into a DNA or RNA oligonucleotide using nucleotides bearing heterocyclic bases |
| US5434257A (en) | 1992-06-01 | 1995-07-18 | Gilead Sciences, Inc. | Binding compentent oligomers containing unsaturated 3',5' and 2',5' linkages |
| US5446137A (en) | 1993-12-09 | 1995-08-29 | Syntex (U.S.A.) Inc. | Oligonucleotides containing 4'-substituted nucleotides |
| US5457191A (en) | 1990-01-11 | 1995-10-10 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5457187A (en) | 1993-12-08 | 1995-10-10 | Board Of Regents University Of Nebraska | Oligonucleotides containing 5-fluorouracil |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5466786A (en) | 1989-10-24 | 1995-11-14 | Gilead Sciences | 2'modified nucleoside and nucleotide compounds |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5502177A (en) | 1993-09-17 | 1996-03-26 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| US5552540A (en) | 1987-06-24 | 1996-09-03 | Howard Florey Institute Of Experimental Physiology And Medicine | Nucleoside derivatives |
| US5567811A (en) | 1990-05-03 | 1996-10-22 | Amersham International Plc | Phosphoramidite derivatives, their preparation and the use thereof in the incorporation of reporter groups on synthetic oligonucleotides |
| US5576427A (en) | 1993-03-30 | 1996-11-19 | Sterling Winthrop, Inc. | Acyclic nucleoside analogs and oligonucleotide sequences containing them |
| US5587470A (en) | 1990-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5591722A (en) | 1989-09-15 | 1997-01-07 | Southern Research Institute | 2'-deoxy-4'-thioribonucleosides and their antiviral activity |
| US5594121A (en) | 1991-11-07 | 1997-01-14 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified purines |
| US5596091A (en) | 1994-03-18 | 1997-01-21 | The Regents Of The University Of California | Antisense oligonucleotides comprising 5-aminoalkyl pyrimidine nucleotides |
| US5597909A (en) | 1994-08-25 | 1997-01-28 | Chiron Corporation | Polynucleotide reagents containing modified deoxyribose moieties, and associated methods of synthesis and use |
| US5610300A (en) | 1992-07-01 | 1997-03-11 | Ciba-Geigy Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| WO1997020563A1 (en) | 1995-11-22 | 1997-06-12 | The Johns-Hopkins University | Ligands to enhance cellular uptake of biomolecules |
| US5639873A (en) | 1992-02-05 | 1997-06-17 | Centre National De La Recherche Scientifique (Cnrs) | Oligothionucleotides |
| US5645985A (en) | 1991-11-26 | 1997-07-08 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified pyrimidines |
| US5646265A (en) | 1990-01-11 | 1997-07-08 | Isis Pharmceuticals, Inc. | Process for the preparation of 2'-O-alkyl purine phosphoramidites |
| US5658873A (en) | 1993-04-10 | 1997-08-19 | Degussa Aktiengesellschaft | Coated sodium percarbonate particles, a process for their production and detergent, cleaning and bleaching compositions containing them |
| US5670633A (en) | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5681941A (en) | 1990-01-11 | 1997-10-28 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| WO1997046098A1 (en) | 1996-06-06 | 1997-12-11 | Neorx Corporation | Cluster clearing agents |
| WO1998013381A1 (en) | 1996-09-26 | 1998-04-02 | Ajinomoto Co., Inc. | Modified physiologically active proteins and medicinal compositions containing the same |
| US5830653A (en) | 1991-11-26 | 1998-11-03 | Gilead Sciences, Inc. | Methods of using oligomers containing modified pyrimidines |
| WO1999014226A2 (en) | 1997-09-12 | 1999-03-25 | Exiqon A/S | Bi- and tri-cyclic nucleoside, nucleotide and oligonucleotide analogues |
| US5948903A (en) | 1991-01-11 | 1999-09-07 | Isis Pharmaceuticals, Inc. | Synthesis of 3-deazapurines |
| US6015886A (en) | 1993-05-24 | 2000-01-18 | Chemgenes Corporation | Oligonucleotide phosphate esters |
| US6147200A (en) | 1999-08-19 | 2000-11-14 | Isis Pharmaceuticals, Inc. | 2'-O-acetamido modified monomers and oligomers |
| US6166197A (en) | 1995-03-06 | 2000-12-26 | Isis Pharmaceuticals, Inc. | Oligomeric compounds having pyrimidine nucleotide (S) with 2'and 5 substitutions |
| US6222025B1 (en) | 1995-03-06 | 2001-04-24 | Isis Pharmaceuticals, Inc. | Process for the synthesis of 2′-O-substituted pyrimidines and oligomeric compounds therefrom |
| US6235887B1 (en) | 1991-11-26 | 2001-05-22 | Isis Pharmaceuticals, Inc. | Enhanced triple-helix and double-helix formation directed by oligonucleotides containing modified pyrimidines |
| US6268490B1 (en) | 1997-03-07 | 2001-07-31 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogues |
| US6300319B1 (en) | 1998-06-16 | 2001-10-09 | Isis Pharmaceuticals, Inc. | Targeted oligonucleotide conjugates |
| US6383812B1 (en) | 1999-05-28 | 2002-05-07 | Academia Sinica | Anti liver disease drug R-YEEE and method of synthesizing branched galactose-terminal glycoproteins |
| WO2002043771A2 (en) | 2000-12-01 | 2002-06-06 | Cell Works Inc. | Conjugates of glycosylated/galactosylated peptide |
| US6525191B1 (en) | 1999-05-11 | 2003-02-25 | Kanda S. Ramasamy | Conformationally constrained L-nucleosides |
| US6528640B1 (en) | 1997-11-05 | 2003-03-04 | Ribozyme Pharmaceuticals, Incorporated | Synthetic ribonucleic acids with RNAse activity |
| US20030077829A1 (en) | 2001-04-30 | 2003-04-24 | Protiva Biotherapeutics Inc.. | Lipid-based formulations |
| US20030119724A1 (en) | 1995-11-22 | 2003-06-26 | Ts`O Paul O.P. | Ligands to enhance cellular uptake of biomolecules |
| US20030158403A1 (en) | 2001-07-03 | 2003-08-21 | Isis Pharmaceuticals, Inc. | Nuclease resistant chimeric oligonucleotides |
| US6617438B1 (en) | 1997-11-05 | 2003-09-09 | Sirna Therapeutics, Inc. | Oligoribonucleotides with enzymatic activity |
| US20030175906A1 (en) | 2001-07-03 | 2003-09-18 | Muthiah Manoharan | Nuclease resistant chimeric oligonucleotides |
| US6639062B2 (en) | 1997-02-14 | 2003-10-28 | Isis Pharmaceuticals, Inc. | Aminooxy-modified nucleosidic compounds and oligomeric compounds prepared therefrom |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| WO2004024757A2 (en) | 2002-09-11 | 2004-03-25 | Santaris Pharma A/S | Modified pna molecules |
| US6770748B2 (en) | 1997-03-07 | 2004-08-03 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogue |
| US20040171570A1 (en) | 2002-11-05 | 2004-09-02 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| WO2004101619A1 (en) | 2003-05-15 | 2004-11-25 | Shionogi Co., Ltd. | Rational design and synthesis of functional glycopeptide |
| US6908903B1 (en) | 1994-12-07 | 2005-06-21 | Aletheon Pharmaceuticals, Inc. | Cluster clearing agents |
| US20050164235A1 (en) | 2003-04-17 | 2005-07-28 | Muthiah Manoharan | Modified iRNA agents |
| US6998484B2 (en) | 2000-10-04 | 2006-02-14 | Santaris Pharma A/S | Synthesis of purine locked nucleic acid analogues |
| US7045610B2 (en) | 1998-04-03 | 2006-05-16 | Epoch Biosciences, Inc. | Modified oligonucleotides for mismatch discrimination |
| US7053207B2 (en) | 1999-05-04 | 2006-05-30 | Exiqon A/S | L-ribo-LNA analogues |
| US20060148740A1 (en) | 2005-01-05 | 2006-07-06 | Prosensa B.V. | Mannose-6-phosphate receptor mediated gene transfer into muscle cells |
| US7084125B2 (en) | 1999-03-18 | 2006-08-01 | Exiqon A/S | Xylo-LNA analogues |
| US20080039618A1 (en) | 2002-11-05 | 2008-02-14 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20080108801A1 (en) | 2003-04-17 | 2008-05-08 | Muthiah Manoharan | Lipophilic Conjugated iRNA Agents |
| US7399845B2 (en) | 2006-01-27 | 2008-07-15 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| WO2008098788A2 (en) | 2007-02-16 | 2008-08-21 | Ktb Tumorforschungsgesellschaft Mbh | Receptor and antigen targeted prodrug |
| WO2008101157A1 (en) | 2007-02-15 | 2008-08-21 | Isis Pharmaceuticals, Inc. | 5'-substituted-2'-f modified nucleosides and oligomeric compounds prepared therefrom |
| US20080206869A1 (en) | 2005-01-24 | 2008-08-28 | Avaris Ab | Nucleic Acid Complex |
| US7427672B2 (en) | 2003-08-28 | 2008-09-23 | Takeshi Imanishi | Artificial nucleic acids of n-o bond crosslinkage type |
| US7491805B2 (en) | 2001-05-18 | 2009-02-17 | Sirna Therapeutics, Inc. | Conjugates and compositions for cellular delivery |
| US7495088B1 (en) | 1989-12-04 | 2009-02-24 | Enzo Life Sciences, Inc. | Modified nucleotide compounds |
| WO2009073809A2 (en) | 2007-12-04 | 2009-06-11 | Alnylam Pharmaceuticals, Inc. | Carbohydrate conjugates as delivery agents for oligonucleotides |
| US7569686B1 (en) | 2006-01-27 | 2009-08-04 | Isis Pharmaceuticals, Inc. | Compounds and methods for synthesis of bicyclic nucleic acid analogs |
| US20090203132A1 (en) | 2004-09-09 | 2009-08-13 | Swayze Eric E | Pyrrolidinyl groups for attaching conjugates to oligomeric compounds |
| US7582744B2 (en) | 2004-08-10 | 2009-09-01 | Alnylam Pharmaceuticals, Inc. | Chemically modified oligonucleotides |
| WO2009126933A2 (en) | 2008-04-11 | 2009-10-15 | Alnylam Pharmaceuticals, Inc. | Site-specific delivery of nucleic acids by combining targeting ligands with endosomolytic components |
| WO2009134487A2 (en) | 2008-01-31 | 2009-11-05 | Alnylam Pharmaceuticals, Inc. | Optimized methods for delivery of dsrna targeting the pcsk9 gene |
| WO2010054406A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| US7723509B2 (en) | 2003-04-17 | 2010-05-25 | Alnylam Pharmaceuticals | IRNA agents with biocleavable tethers |
| WO2010088537A2 (en) | 2009-01-29 | 2010-08-05 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| WO2010129709A1 (en) | 2009-05-05 | 2010-11-11 | Alnylam Pharmaceuticals, Inc. | Lipid compositions |
| WO2010144740A1 (en) | 2009-06-10 | 2010-12-16 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| WO2010148013A2 (en) | 2009-06-15 | 2010-12-23 | Alnylam Pharmaceuticals, Inc. | Lipid formulated dsrna targeting the pcsk9 gene |
| US7875733B2 (en) | 2003-09-18 | 2011-01-25 | Isis Pharmaceuticals, Inc. | Oligomeric compounds comprising 4′-thionucleosides for use in gene modulation |
| WO2011038356A2 (en) | 2009-09-25 | 2011-03-31 | Johns Hopkins University | Novel liver-targeting agents and their synthesis |
| US20110097265A1 (en) | 2009-10-26 | 2011-04-28 | Institute Of Nuclear Energy Research Atomic Energy Council, Executive Yuan | Quantification method for remaining liver function and novel liver receptor imaging agent |
| US20110097264A1 (en) | 2009-10-26 | 2011-04-28 | Institute Of Nuclear Energy Research Atomic Energy Council, Executive Yuan | Radiolabeling method using multivalent glycoligands as hepatic receptor imaging agent |
| WO2011100131A2 (en) | 2010-01-28 | 2011-08-18 | Alnylam Pharmacuticals, Inc. | Monomers and oligonucleotides comprising cycloaddition adduct(s) |
| US20110207799A1 (en) | 2010-02-24 | 2011-08-25 | Roche Madison Inc. | Compositions for Targeted Delivery of siRNA |
| WO2011120053A1 (en) | 2010-03-26 | 2011-09-29 | Mersana Therapeutics, Inc. | Modified polymers for delivery of polynucleotides, method of manufacture, and methods of use thereof |
| US8030467B2 (en) | 2006-05-11 | 2011-10-04 | Isis Pharmaceuticals, Inc. | 5′-modified bicyclic nucleic acid analogs |
| US20110313020A1 (en) | 2008-12-03 | 2011-12-22 | Marina Biotech, Inc. | UsiRNA Complexes |
| WO2011163121A1 (en) | 2010-06-21 | 2011-12-29 | Alnylam Pharmaceuticals, Inc. | Multifunctional copolymers for nucleic acid delivery |
| US8088904B2 (en) | 2007-08-15 | 2012-01-03 | Isis Pharmaceuticals, Inc. | Tetrahydropyran nucleic acid analogs |
| US20120035115A1 (en) | 2008-09-23 | 2012-02-09 | Alnylam Pharmaceuticals, Inc. | Chemical modifications of monomers and oligonucleotides with cycloaddition |
| US8137695B2 (en) | 2006-08-18 | 2012-03-20 | Arrowhead Madison Inc. | Polyconjugates for in vivo delivery of polynucleotides |
| WO2012037254A1 (en) | 2010-09-15 | 2012-03-22 | Alnylam Pharmaceuticals, Inc. | MODIFIED iRNA AGENTS |
| WO2012068187A1 (en) | 2010-11-19 | 2012-05-24 | Merck Sharp & Dohme Corp. | Poly(amide) polymers for the delivery of oligonucleotides |
| WO2012083046A2 (en) | 2010-12-17 | 2012-06-21 | Arrowhead Research Corporation | Galactose cluster-pharmacokinetic modulator targeting moiety for sirna |
| WO2012083185A2 (en) | 2010-12-17 | 2012-06-21 | Arrowhead Research Corporations | Peptide-based in vivo sirna delivery system |
| WO2012089602A1 (en) | 2010-12-29 | 2012-07-05 | F. Hoffmann-La Roche Ag | Small molecule conjugates for intracellular delivery of biologically active compounds |
| US20120230938A1 (en) | 2006-08-18 | 2012-09-13 | Arrowhead Madison Inc. | Polyconjugates for In Vivo Delivery of Polynucleotides |
| US8278283B2 (en) | 2007-07-05 | 2012-10-02 | Isis Pharmaceuticals, Inc. | 6-disubstituted or unsaturated bicyclic nucleic acid analogs |
| US8278426B2 (en) | 2007-06-08 | 2012-10-02 | Isis Pharmaceuticals, Inc. | Carbocyclic bicyclic nucleic acid analogs |
| US8278425B2 (en) | 2007-05-30 | 2012-10-02 | Isis Pharmaceuticals, Inc. | N-substituted-aminomethylene bridged bicyclic nucleic acid analogs |
| US8314227B2 (en) | 2007-05-22 | 2012-11-20 | Marina Biotech, Inc. | Hydroxymethyl substituted RNA oligonucleotides and RNA complexes |
| WO2012177947A2 (en) | 2011-06-21 | 2012-12-27 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of apolipoprotein c-iii (apoc3) genes |
| US20130004427A1 (en) | 2009-12-11 | 2013-01-03 | The Regents Of The University Of Michigan | Targeted dendrimer-drug conjugates |
| US20130011922A1 (en) | 2007-03-02 | 2013-01-10 | F/K/A Mdrna, Inc. | Nucleic acid compounds for inhibiting gene expression and uses thereof |
| WO2013033230A1 (en) | 2011-08-29 | 2013-03-07 | Isis Pharmaceuticals, Inc. | Oligomer-conjugate complexes and their use |
| WO2013036868A1 (en) | 2011-09-07 | 2013-03-14 | Marina Biotech Inc. | Synthesis and uses of nucleic acid compounds with conformationally restricted monomers |
| US20130109817A1 (en) | 2010-03-26 | 2013-05-02 | Mersana Therapeutics, Inc. | Modified Polymers for Delivery of Polynucleotides, Method of Manufacture, and Methods of Use Thereof |
| US20130121954A1 (en) | 2011-08-26 | 2013-05-16 | Arrowhead Madison Inc. | Poly(vinyl ester) Polymers for In Vivo Nucleic Acid Delivery |
| WO2013075035A1 (en) | 2011-11-18 | 2013-05-23 | Alnylam Pharmaceuticals | Rnai agents, compositions and methods of use thereof for treating transthyretin (ttr) associated diseases |
| US20130130378A1 (en) | 2010-04-22 | 2013-05-23 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising acyclic and abasic nucleosides and analogs |
| US20130190383A1 (en) | 2010-04-26 | 2013-07-25 | Marina Biotech, Inc. | Nucleic acid compounds with conformationally restricted monomers and uses thereof |
| US20130203836A1 (en) | 2010-04-01 | 2013-08-08 | Isis Pharmaceuticals, Inc. | 2' and 5' modified monomers and oligonucleotides |
| US8541548B2 (en) | 1999-06-07 | 2013-09-24 | Arrowhead Madison Inc. | Compounds and methods for reversible modification of biologically active molecules |
| WO2013166121A1 (en) | 2012-05-02 | 2013-11-07 | Merck Sharp & Dohme Corp. | Novel tetragalnac containing conjugates and methods for delivery of oligonucleotides |
| WO2013165816A2 (en) | 2012-05-02 | 2013-11-07 | Merck Sharp & Dohme Corp. | SHORT INTERFERING NUCLEIC ACID (siNA) COMPOSITIONS |
| US8790919B2 (en) | 2004-03-15 | 2014-07-29 | Isis Pharmaceuticals, Inc. | Compositions and methods for optimizing cleavage of RNA by RNase H |
| US8828957B2 (en) | 2003-12-11 | 2014-09-09 | Microvax, Llc | Methods for generating immunity to antigen |
| WO2014179445A1 (en) | 2013-05-01 | 2014-11-06 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| WO2014179620A1 (en) | 2013-05-01 | 2014-11-06 | Isis Pharmaceuticals, Inc. | Conjugated antisense compounds and their use |
| WO2015106128A2 (en) | 2014-01-09 | 2015-07-16 | Alnylam Pharmaceuticals, Inc. | MODIFIED RNAi AGENTS |
| WO2017015555A1 (en) | 2015-07-22 | 2017-01-26 | Wave Life Sciences Ltd. | Oligonucleotide compositions and methods thereof |
| US10668170B2 (en) | 2011-11-18 | 2020-06-02 | Alnylam Pharmaceuticals, Inc. | Modified RNAi agents |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100280098A1 (en)* | 2007-10-05 | 2010-11-04 | Juliano Rudolph L | Receptor targeted oligonucleotides |
| TWI702046B (en)* | 2013-07-19 | 2020-08-21 | 美商Ionis製藥公司 | Compositions for modulating tau expression |
| JOP20200228A1 (en)* | 2015-12-21 | 2017-06-16 | Novartis Ag | Compositions and methods for decreasing tau expression |
| EP4121537A4 (en)* | 2020-03-18 | 2024-07-31 | University Of Massachusetts | OLIGONUCLEOTIDES FOR MAPT MODULATION |
| JP2023521604A (en)* | 2020-03-30 | 2023-05-25 | アルナイラム ファーマシューティカルズ, インコーポレイテッド | Microtubule-associated protein tau (MAPT) iRNA agent compositions and methods of use thereof |
| CA3233101A1 (en)* | 2021-09-24 | 2023-03-30 | Alnylam Pharmaceuticals, Inc. | Microtubule associated protein tau (mapt) irna agent compositions and methods of use thereof |
| AU2023218398A1 (en)* | 2022-02-11 | 2024-08-29 | Adarx Pharmaceuticals, Inc. | Trkb ligand conjugated compounds and uses thereof |
| EP4486336A2 (en)* | 2022-03-01 | 2025-01-08 | Adarx Pharmaceuticals, Inc. | Cb1 ligand conjugated compounds and uses thereof |
| CN119486739A (en)* | 2022-04-04 | 2025-02-18 | 阿达尔克斯制药有限公司 | α4β1/7 integrin ligand conjugated compounds and uses thereof |
- 2024
- 2024-04-19TWTW113114826Apatent/TW202448484A/enunknown
- 2024-04-19WOPCT/US2024/025577patent/WO2024220930A2/enactivePending
Patent Citations (191)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US5118800A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | Oligonucleotides possessing a primary amino group in the terminal nucleotide |
| US4981957A (en) | 1984-07-19 | 1991-01-01 | Centre National De La Recherche Scientifique | Oligonucleotides with modified phosphate and modified carbohydrate moieties at the respective chain termini |
| US5367066A (en) | 1984-10-16 | 1994-11-22 | Chiron Corporation | Oligonucleotides with selectably cleavable and/or abasic sites |
| US4845205A (en) | 1985-01-08 | 1989-07-04 | Institut Pasteur | 2,N6 -disubstituted and 2,N6 -trisubstituted adenosine-3'-phosphoramidites |
| US4751219A (en) | 1985-02-05 | 1988-06-14 | Nederlandse Centrale Organisatie Voor Toegepast-Natuur-Wetenschappelijk Onderzoek | Synthetic glycolipides, a process for the preparation thereof and several uses for these synthetic glycolipides |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5552540A (en) | 1987-06-24 | 1996-09-03 | Howard Florey Institute Of Experimental Physiology And Medicine | Nucleoside derivatives |
| US5175273A (en) | 1988-07-01 | 1992-12-29 | Genentech, Inc. | Nucleic acid intercalating agents |
| US5134066A (en) | 1989-08-29 | 1992-07-28 | Monsanto Company | Improved probes using nucleosides containing 3-dezauracil analogs |
| US5591722A (en) | 1989-09-15 | 1997-01-07 | Southern Research Institute | 2'-deoxy-4'-thioribonucleosides and their antiviral activity |
| US5466786B1 (en) | 1989-10-24 | 1998-04-07 | Gilead Sciences | 2' Modified nucleoside and nucleotide compounds |
| US5466786A (en) | 1989-10-24 | 1995-11-14 | Gilead Sciences | 2'modified nucleoside and nucleotide compounds |
| US7495088B1 (en) | 1989-12-04 | 2009-02-24 | Enzo Life Sciences, Inc. | Modified nucleotide compounds |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5750692A (en) | 1990-01-11 | 1998-05-12 | Isis Pharmaceuticals, Inc. | Synthesis of 3-deazapurines |
| US5681941A (en) | 1990-01-11 | 1997-10-28 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| US5646265A (en) | 1990-01-11 | 1997-07-08 | Isis Pharmceuticals, Inc. | Process for the preparation of 2'-O-alkyl purine phosphoramidites |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US6166199A (en) | 1990-01-11 | 2000-12-26 | Isis Pharmaceuticals, Inc | N-2 substituted purines |
| US5808027A (en) | 1990-01-11 | 1998-09-15 | Isis Pharmaceuticals, Inc. | N-2 substituted purines in oligonucleotides |
| US5587470A (en) | 1990-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5587469A (en) | 1990-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Oligonucleotides containing N-2 substituted purines |
| US5670633A (en) | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5457191A (en) | 1990-01-11 | 1995-10-10 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5567811A (en) | 1990-05-03 | 1996-10-22 | Amersham International Plc | Phosphoramidite derivatives, their preparation and the use thereof in the incorporation of reporter groups on synthetic oligonucleotides |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5432272A (en) | 1990-10-09 | 1995-07-11 | Benner; Steven A. | Method for incorporating into a DNA or RNA oligonucleotide using nucleotides bearing heterocyclic bases |
| US5948903A (en) | 1991-01-11 | 1999-09-07 | Isis Pharmaceuticals, Inc. | Synthesis of 3-deazapurines |
| US5319080A (en) | 1991-10-17 | 1994-06-07 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5393878A (en) | 1991-10-17 | 1995-02-28 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5594121A (en) | 1991-11-07 | 1997-01-14 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified purines |
| US5645985A (en) | 1991-11-26 | 1997-07-08 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified pyrimidines |
| US6380368B1 (en) | 1991-11-26 | 2002-04-30 | Isis Pharmaceuticals, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified pyrimidines |
| US6235887B1 (en) | 1991-11-26 | 2001-05-22 | Isis Pharmaceuticals, Inc. | Enhanced triple-helix and double-helix formation directed by oligonucleotides containing modified pyrimidines |
| US5830653A (en) | 1991-11-26 | 1998-11-03 | Gilead Sciences, Inc. | Methods of using oligomers containing modified pyrimidines |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5639873A (en) | 1992-02-05 | 1997-06-17 | Centre National De La Recherche Scientifique (Cnrs) | Oligothionucleotides |
| US5434257A (en) | 1992-06-01 | 1995-07-18 | Gilead Sciences, Inc. | Binding compentent oligomers containing unsaturated 3',5' and 2',5' linkages |
| US5700920A (en) | 1992-07-01 | 1997-12-23 | Novartis Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5610300A (en) | 1992-07-01 | 1997-03-11 | Ciba-Geigy Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5576427A (en) | 1993-03-30 | 1996-11-19 | Sterling Winthrop, Inc. | Acyclic nucleoside analogs and oligonucleotide sequences containing them |
| US5658873A (en) | 1993-04-10 | 1997-08-19 | Degussa Aktiengesellschaft | Coated sodium percarbonate particles, a process for their production and detergent, cleaning and bleaching compositions containing them |
| US6015886A (en) | 1993-05-24 | 2000-01-18 | Chemgenes Corporation | Oligonucleotide phosphate esters |
| US5763588A (en) | 1993-09-17 | 1998-06-09 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US6005096A (en) | 1993-09-17 | 1999-12-21 | Gilead Sciences, Inc. | Pyrimidine derivatives |
| US5502177A (en) | 1993-09-17 | 1996-03-26 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US5457187A (en) | 1993-12-08 | 1995-10-10 | Board Of Regents University Of Nebraska | Oligonucleotides containing 5-fluorouracil |
| US5446137B1 (en) | 1993-12-09 | 1998-10-06 | Behringwerke Ag | Oligonucleotides containing 4'-substituted nucleotides |
| US5446137A (en) | 1993-12-09 | 1995-08-29 | Syntex (U.S.A.) Inc. | Oligonucleotides containing 4'-substituted nucleotides |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5811534A (en) | 1994-02-01 | 1998-09-22 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| US5596091A (en) | 1994-03-18 | 1997-01-21 | The Regents Of The University Of California | Antisense oligonucleotides comprising 5-aminoalkyl pyrimidine nucleotides |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| US5597909A (en) | 1994-08-25 | 1997-01-28 | Chiron Corporation | Polynucleotide reagents containing modified deoxyribose moieties, and associated methods of synthesis and use |
| US6908903B1 (en) | 1994-12-07 | 2005-06-21 | Aletheon Pharmaceuticals, Inc. | Cluster clearing agents |
| US6222025B1 (en) | 1995-03-06 | 2001-04-24 | Isis Pharmaceuticals, Inc. | Process for the synthesis of 2′-O-substituted pyrimidines and oligomeric compounds therefrom |
| US6166197A (en) | 1995-03-06 | 2000-12-26 | Isis Pharmaceuticals, Inc. | Oligomeric compounds having pyrimidine nucleotide (S) with 2'and 5 substitutions |
| US20030119724A1 (en) | 1995-11-22 | 2003-06-26 | Ts`O Paul O.P. | Ligands to enhance cellular uptake of biomolecules |
| US5994517A (en) | 1995-11-22 | 1999-11-30 | Paul O. P. Ts'o | Ligands to enhance cellular uptake of biomolecules |
| WO1997020563A1 (en) | 1995-11-22 | 1997-06-12 | The Johns-Hopkins University | Ligands to enhance cellular uptake of biomolecules |
| US20060183886A1 (en) | 1995-11-22 | 2006-08-17 | Cell Works Therapeutics, Inc., A Delaware Corporation | Ligands to enhance cellular uptake of biomolecules |
| WO1997046098A1 (en) | 1996-06-06 | 1997-12-11 | Neorx Corporation | Cluster clearing agents |
| WO1998013381A1 (en) | 1996-09-26 | 1998-04-02 | Ajinomoto Co., Inc. | Modified physiologically active proteins and medicinal compositions containing the same |
| US6620916B1 (en) | 1996-09-26 | 2003-09-16 | Ajinomoto Co., Inc. | Modified physiologically active proteins and medicinal compositions containing the same |
| US6639062B2 (en) | 1997-02-14 | 2003-10-28 | Isis Pharmaceuticals, Inc. | Aminooxy-modified nucleosidic compounds and oligomeric compounds prepared therefrom |
| US6268490B1 (en) | 1997-03-07 | 2001-07-31 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogues |
| US6770748B2 (en) | 1997-03-07 | 2004-08-03 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogue |
| WO1999014226A2 (en) | 1997-09-12 | 1999-03-25 | Exiqon A/S | Bi- and tri-cyclic nucleoside, nucleotide and oligonucleotide analogues |
| US7034133B2 (en) | 1997-09-12 | 2006-04-25 | Exiqon A/S | Oligonucleotide analogues |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| US6794499B2 (en) | 1997-09-12 | 2004-09-21 | Exiqon A/S | Oligonucleotide analogues |
| US6528640B1 (en) | 1997-11-05 | 2003-03-04 | Ribozyme Pharmaceuticals, Incorporated | Synthetic ribonucleic acids with RNAse activity |
| US6617438B1 (en) | 1997-11-05 | 2003-09-09 | Sirna Therapeutics, Inc. | Oligoribonucleotides with enzymatic activity |
| US7045610B2 (en) | 1998-04-03 | 2006-05-16 | Epoch Biosciences, Inc. | Modified oligonucleotides for mismatch discrimination |
| US6300319B1 (en) | 1998-06-16 | 2001-10-09 | Isis Pharmaceuticals, Inc. | Targeted oligonucleotide conjugates |
| US6525031B2 (en) | 1998-06-16 | 2003-02-25 | Isis Pharmaceuticals, Inc. | Targeted Oligonucleotide conjugates |
| US6660720B2 (en) | 1998-06-16 | 2003-12-09 | Isis Pharmaceuticals, Inc. | Targeted oligonucleotide conjugates |
| US7084125B2 (en) | 1999-03-18 | 2006-08-01 | Exiqon A/S | Xylo-LNA analogues |
| US7053207B2 (en) | 1999-05-04 | 2006-05-30 | Exiqon A/S | L-ribo-LNA analogues |
| US6525191B1 (en) | 1999-05-11 | 2003-02-25 | Kanda S. Ramasamy | Conformationally constrained L-nucleosides |
| US6383812B1 (en) | 1999-05-28 | 2002-05-07 | Academia Sinica | Anti liver disease drug R-YEEE and method of synthesizing branched galactose-terminal glycoproteins |
| US8541548B2 (en) | 1999-06-07 | 2013-09-24 | Arrowhead Madison Inc. | Compounds and methods for reversible modification of biologically active molecules |
| US6147200A (en) | 1999-08-19 | 2000-11-14 | Isis Pharmaceuticals, Inc. | 2'-O-acetamido modified monomers and oligomers |
| US6998484B2 (en) | 2000-10-04 | 2006-02-14 | Santaris Pharma A/S | Synthesis of purine locked nucleic acid analogues |
| US7262177B2 (en) | 2000-12-01 | 2007-08-28 | Cell Works Therapeutics, Inc. | Conjugates of glycosylated/galactosylated peptide, bifunctional linker, and nucleotidic monomers/polymers, and related compositions and methods of use |
| US6906182B2 (en) | 2000-12-01 | 2005-06-14 | Cell Works Therapeutics, Inc. | Conjugates of glycosylated/galactosylated peptide, bifunctional linker, and nucleotidic monomers/polymers, and related compositions and method of use |
| WO2002043771A2 (en) | 2000-12-01 | 2002-06-06 | Cell Works Inc. | Conjugates of glycosylated/galactosylated peptide |
| US20030077829A1 (en) | 2001-04-30 | 2003-04-24 | Protiva Biotherapeutics Inc.. | Lipid-based formulations |
| US7491805B2 (en) | 2001-05-18 | 2009-02-17 | Sirna Therapeutics, Inc. | Conjugates and compositions for cellular delivery |
| US20030158403A1 (en) | 2001-07-03 | 2003-08-21 | Isis Pharmaceuticals, Inc. | Nuclease resistant chimeric oligonucleotides |
| US20030175906A1 (en) | 2001-07-03 | 2003-09-18 | Muthiah Manoharan | Nuclease resistant chimeric oligonucleotides |
| WO2004024757A2 (en) | 2002-09-11 | 2004-03-25 | Santaris Pharma A/S | Modified pna molecules |
| US20080039618A1 (en) | 2002-11-05 | 2008-02-14 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20040171570A1 (en) | 2002-11-05 | 2004-09-02 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20080108801A1 (en) | 2003-04-17 | 2008-05-08 | Muthiah Manoharan | Lipophilic Conjugated iRNA Agents |
| US8344125B2 (en) | 2003-04-17 | 2013-01-01 | Alnylam Pharmaceuticals, Inc. | Modified iRNA agents |
| US7851615B2 (en) | 2003-04-17 | 2010-12-14 | Alnylam Pharmaceuticals, Inc. | Lipophilic conjugated iRNA agents |
| US7723509B2 (en) | 2003-04-17 | 2010-05-25 | Alnylam Pharmaceuticals | IRNA agents with biocleavable tethers |
| US20050164235A1 (en) | 2003-04-17 | 2005-07-28 | Muthiah Manoharan | Modified iRNA agents |
| WO2004101619A1 (en) | 2003-05-15 | 2004-11-25 | Shionogi Co., Ltd. | Rational design and synthesis of functional glycopeptide |
| US7427672B2 (en) | 2003-08-28 | 2008-09-23 | Takeshi Imanishi | Artificial nucleic acids of n-o bond crosslinkage type |
| US7939677B2 (en) | 2003-09-18 | 2011-05-10 | Isis Pharmaceuticals, Inc. | Oligomeric compounds comprising 4′-thionucleosides for use in gene modulation |
| US7875733B2 (en) | 2003-09-18 | 2011-01-25 | Isis Pharmaceuticals, Inc. | Oligomeric compounds comprising 4′-thionucleosides for use in gene modulation |
| US8828957B2 (en) | 2003-12-11 | 2014-09-09 | Microvax, Llc | Methods for generating immunity to antigen |
| US8790919B2 (en) | 2004-03-15 | 2014-07-29 | Isis Pharmaceuticals, Inc. | Compositions and methods for optimizing cleavage of RNA by RNase H |
| US7582744B2 (en) | 2004-08-10 | 2009-09-01 | Alnylam Pharmaceuticals, Inc. | Chemically modified oligonucleotides |
| US8404862B2 (en) | 2004-08-10 | 2013-03-26 | Alnylam Pharmaceuticals, Inc. | Ligand-conjugated monomers |
| US20090203132A1 (en) | 2004-09-09 | 2009-08-13 | Swayze Eric E | Pyrrolidinyl groups for attaching conjugates to oligomeric compounds |
| US20060148740A1 (en) | 2005-01-05 | 2006-07-06 | Prosensa B.V. | Mannose-6-phosphate receptor mediated gene transfer into muscle cells |
| US20080206869A1 (en) | 2005-01-24 | 2008-08-28 | Avaris Ab | Nucleic Acid Complex |
| US7741457B2 (en) | 2006-01-27 | 2010-06-22 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| US20090012281A1 (en) | 2006-01-27 | 2009-01-08 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| US7399845B2 (en) | 2006-01-27 | 2008-07-15 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| US8022193B2 (en) | 2006-01-27 | 2011-09-20 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| US7569686B1 (en) | 2006-01-27 | 2009-08-04 | Isis Pharmaceuticals, Inc. | Compounds and methods for synthesis of bicyclic nucleic acid analogs |
| US8030467B2 (en) | 2006-05-11 | 2011-10-04 | Isis Pharmaceuticals, Inc. | 5′-modified bicyclic nucleic acid analogs |
| US8137695B2 (en) | 2006-08-18 | 2012-03-20 | Arrowhead Madison Inc. | Polyconjugates for in vivo delivery of polynucleotides |
| US20120230938A1 (en) | 2006-08-18 | 2012-09-13 | Arrowhead Madison Inc. | Polyconjugates for In Vivo Delivery of Polynucleotides |
| WO2008101157A1 (en) | 2007-02-15 | 2008-08-21 | Isis Pharmaceuticals, Inc. | 5'-substituted-2'-f modified nucleosides and oligomeric compounds prepared therefrom |
| WO2008098788A2 (en) | 2007-02-16 | 2008-08-21 | Ktb Tumorforschungsgesellschaft Mbh | Receptor and antigen targeted prodrug |
| US20130011922A1 (en) | 2007-03-02 | 2013-01-10 | F/K/A Mdrna, Inc. | Nucleic acid compounds for inhibiting gene expression and uses thereof |
| US8314227B2 (en) | 2007-05-22 | 2012-11-20 | Marina Biotech, Inc. | Hydroxymethyl substituted RNA oligonucleotides and RNA complexes |
| US20130096289A1 (en) | 2007-05-22 | 2013-04-18 | Marina Biotech, Inc. | Hydroxymethyl substituted rna oligonucleotides and rna complexes |
| US8278425B2 (en) | 2007-05-30 | 2012-10-02 | Isis Pharmaceuticals, Inc. | N-substituted-aminomethylene bridged bicyclic nucleic acid analogs |
| US8278426B2 (en) | 2007-06-08 | 2012-10-02 | Isis Pharmaceuticals, Inc. | Carbocyclic bicyclic nucleic acid analogs |
| US8278283B2 (en) | 2007-07-05 | 2012-10-02 | Isis Pharmaceuticals, Inc. | 6-disubstituted or unsaturated bicyclic nucleic acid analogs |
| US8088904B2 (en) | 2007-08-15 | 2012-01-03 | Isis Pharmaceuticals, Inc. | Tetrahydropyran nucleic acid analogs |
| US8440803B2 (en) | 2007-08-15 | 2013-05-14 | Isis Pharmaceuticals, Inc. | Tetrahydropyran nucleic acid analogs |
| US9005906B2 (en) | 2007-08-15 | 2015-04-14 | Isis Pharmaceuticals, Inc. | Tetrahydropyran nucleic acid analogs |
| US8106022B2 (en) | 2007-12-04 | 2012-01-31 | Alnylam Pharmaceuticals, Inc. | Carbohydrate conjugates as delivery agents for oligonucleotides |
| US8450467B2 (en) | 2007-12-04 | 2013-05-28 | Alnylam Pharmaceuticals, Inc. | Carbohydrate conjugates as delivery agents for oligonucleotides |
| WO2009082607A2 (en) | 2007-12-04 | 2009-07-02 | Alnylam Pharmaceuticals, Inc. | Targeting lipids |
| WO2009073809A2 (en) | 2007-12-04 | 2009-06-11 | Alnylam Pharmaceuticals, Inc. | Carbohydrate conjugates as delivery agents for oligonucleotides |
| US20120136042A1 (en) | 2007-12-04 | 2012-05-31 | Alnylam Pharmaceuticals, Inc | Carbohydrate conjugates as delivery agents for oligonucleotides |
| WO2009134487A2 (en) | 2008-01-31 | 2009-11-05 | Alnylam Pharmaceuticals, Inc. | Optimized methods for delivery of dsrna targeting the pcsk9 gene |
| WO2009126933A2 (en) | 2008-04-11 | 2009-10-15 | Alnylam Pharmaceuticals, Inc. | Site-specific delivery of nucleic acids by combining targeting ligands with endosomolytic components |
| US20110123520A1 (en) | 2008-04-11 | 2011-05-26 | Alnylam Pharmaceuticals, Inc. | Site-specific delivery of nucleic acids by combining targeting ligands with endosomolytic components |
| US20120035115A1 (en) | 2008-09-23 | 2012-02-09 | Alnylam Pharmaceuticals, Inc. | Chemical modifications of monomers and oligonucleotides with cycloaddition |
| WO2010054406A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| US20120095075A1 (en) | 2008-11-10 | 2012-04-19 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| US20110313020A1 (en) | 2008-12-03 | 2011-12-22 | Marina Biotech, Inc. | UsiRNA Complexes |
| US20120101148A1 (en) | 2009-01-29 | 2012-04-26 | Alnylam Pharmaceuticals, Inc. | lipid formulation |
| WO2010088537A2 (en) | 2009-01-29 | 2010-08-05 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| WO2010129709A1 (en) | 2009-05-05 | 2010-11-11 | Alnylam Pharmaceuticals, Inc. | Lipid compositions |
| WO2010144740A1 (en) | 2009-06-10 | 2010-12-16 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| US8158601B2 (en) | 2009-06-10 | 2012-04-17 | Alnylam Pharmaceuticals, Inc. | Lipid formulation |
| WO2010148013A2 (en) | 2009-06-15 | 2010-12-23 | Alnylam Pharmaceuticals, Inc. | Lipid formulated dsrna targeting the pcsk9 gene |
| US8552163B2 (en) | 2009-09-25 | 2013-10-08 | Johns Hopkins University | Liver-targeting agents and their synthesis |
| WO2011038356A2 (en) | 2009-09-25 | 2011-03-31 | Johns Hopkins University | Novel liver-targeting agents and their synthesis |
| US20110097264A1 (en) | 2009-10-26 | 2011-04-28 | Institute Of Nuclear Energy Research Atomic Energy Council, Executive Yuan | Radiolabeling method using multivalent glycoligands as hepatic receptor imaging agent |
| US8435491B2 (en) | 2009-10-26 | 2013-05-07 | Institute Of Nuclear Energy Research Atomic Energy Council, Executive Yuan | Quantification method for remaining liver function and novel liver receptor imaging agent |
| US20110097265A1 (en) | 2009-10-26 | 2011-04-28 | Institute Of Nuclear Energy Research Atomic Energy Council, Executive Yuan | Quantification method for remaining liver function and novel liver receptor imaging agent |
| US20130004427A1 (en) | 2009-12-11 | 2013-01-03 | The Regents Of The University Of Michigan | Targeted dendrimer-drug conjugates |
| WO2011100131A2 (en) | 2010-01-28 | 2011-08-18 | Alnylam Pharmacuticals, Inc. | Monomers and oligonucleotides comprising cycloaddition adduct(s) |
| US8313772B2 (en) | 2010-02-24 | 2012-11-20 | Arrowhead Madison Inc. | Compositions for targeted delivery of siRNA |
| US20110207799A1 (en) | 2010-02-24 | 2011-08-25 | Roche Madison Inc. | Compositions for Targeted Delivery of siRNA |
| US20130109817A1 (en) | 2010-03-26 | 2013-05-02 | Mersana Therapeutics, Inc. | Modified Polymers for Delivery of Polynucleotides, Method of Manufacture, and Methods of Use Thereof |
| US8349308B2 (en) | 2010-03-26 | 2013-01-08 | Mersana Therapeutics, Inc. | Modified polymers for delivery of polynucleotides, method of manufacture, and methods of use thereof |
| WO2011120053A1 (en) | 2010-03-26 | 2011-09-29 | Mersana Therapeutics, Inc. | Modified polymers for delivery of polynucleotides, method of manufacture, and methods of use thereof |
| US20130203836A1 (en) | 2010-04-01 | 2013-08-08 | Isis Pharmaceuticals, Inc. | 2' and 5' modified monomers and oligonucleotides |
| US20130130378A1 (en) | 2010-04-22 | 2013-05-23 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising acyclic and abasic nucleosides and analogs |
| US20130190383A1 (en) | 2010-04-26 | 2013-07-25 | Marina Biotech, Inc. | Nucleic acid compounds with conformationally restricted monomers and uses thereof |
| WO2011163121A1 (en) | 2010-06-21 | 2011-12-29 | Alnylam Pharmaceuticals, Inc. | Multifunctional copolymers for nucleic acid delivery |
| WO2012037254A1 (en) | 2010-09-15 | 2012-03-22 | Alnylam Pharmaceuticals, Inc. | MODIFIED iRNA AGENTS |
| WO2012068187A1 (en) | 2010-11-19 | 2012-05-24 | Merck Sharp & Dohme Corp. | Poly(amide) polymers for the delivery of oligonucleotides |
| WO2012083046A2 (en) | 2010-12-17 | 2012-06-21 | Arrowhead Research Corporation | Galactose cluster-pharmacokinetic modulator targeting moiety for sirna |
| US8501930B2 (en) | 2010-12-17 | 2013-08-06 | Arrowhead Madison Inc. | Peptide-based in vivo siRNA delivery system |
| WO2012083185A2 (en) | 2010-12-17 | 2012-06-21 | Arrowhead Research Corporations | Peptide-based in vivo sirna delivery system |
| US20120165393A1 (en) | 2010-12-17 | 2012-06-28 | Arrowhead Madison Inc. | Peptide-Based In Vivo siRNA Delivery System |
| WO2012089602A1 (en) | 2010-12-29 | 2012-07-05 | F. Hoffmann-La Roche Ag | Small molecule conjugates for intracellular delivery of biologically active compounds |
| WO2012089352A1 (en) | 2010-12-29 | 2012-07-05 | F. Hoffmann-La Roche Ag | Small molecule conjugates for intracellular delivery of nucleic acids |
| WO2012177947A2 (en) | 2011-06-21 | 2012-12-27 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of apolipoprotein c-iii (apoc3) genes |
| US20130121954A1 (en) | 2011-08-26 | 2013-05-16 | Arrowhead Madison Inc. | Poly(vinyl ester) Polymers for In Vivo Nucleic Acid Delivery |
| WO2013033230A1 (en) | 2011-08-29 | 2013-03-07 | Isis Pharmaceuticals, Inc. | Oligomer-conjugate complexes and their use |
| WO2013036868A1 (en) | 2011-09-07 | 2013-03-14 | Marina Biotech Inc. | Synthesis and uses of nucleic acid compounds with conformationally restricted monomers |
| US10668170B2 (en) | 2011-11-18 | 2020-06-02 | Alnylam Pharmaceuticals, Inc. | Modified RNAi agents |
| WO2013075035A1 (en) | 2011-11-18 | 2013-05-23 | Alnylam Pharmaceuticals | Rnai agents, compositions and methods of use thereof for treating transthyretin (ttr) associated diseases |
| WO2013166121A1 (en) | 2012-05-02 | 2013-11-07 | Merck Sharp & Dohme Corp. | Novel tetragalnac containing conjugates and methods for delivery of oligonucleotides |
| WO2013165816A2 (en) | 2012-05-02 | 2013-11-07 | Merck Sharp & Dohme Corp. | SHORT INTERFERING NUCLEIC ACID (siNA) COMPOSITIONS |
| WO2014179620A1 (en) | 2013-05-01 | 2014-11-06 | Isis Pharmaceuticals, Inc. | Conjugated antisense compounds and their use |
| US9127276B2 (en) | 2013-05-01 | 2015-09-08 | Isis Pharmaceuticals, Inc. | Conjugated antisense compounds and their use |
| US9181549B2 (en) | 2013-05-01 | 2015-11-10 | Isis Pharmaceuticals, Inc. | Conjugated antisense compounds and their use |
| WO2014179445A1 (en) | 2013-05-01 | 2014-11-06 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| US10844379B2 (en) | 2013-05-01 | 2020-11-24 | Ionis Pharmaceuticals, Inc. | Conjugated antisense compounds and their use |
| WO2015106128A2 (en) | 2014-01-09 | 2015-07-16 | Alnylam Pharmaceuticals, Inc. | MODIFIED RNAi AGENTS |
| WO2017015555A1 (en) | 2015-07-22 | 2017-01-26 | Wave Life Sciences Ltd. | Oligonucleotide compositions and methods thereof |
Non-Patent Citations (66)
| Title |
|---|
| "Biotechnology and Medicine", 2008, WILEY-VCH, article "Modified Nucleosides in Biochemistry" |
| "GenBank", Database accession no. NM_001377265.1 |
| BERGE ET AL.: "Pharmaceutical Salts", JOURNAL OF PHARMACEUTICAL SCIENCE, vol. 66, 1977, pages 1 - 19, XP002675560, DOI: 10.1002/jps.2600660104 |
| BIESSEN ET AL., FASEB, vol. 14, 2000, pages 1784 - 1792 |
| BIESSEN ET AL., J MED CHEM, vol. 38, 1995, pages 1846 - 1852 |
| BIESSEN ET AL., J. MED. CHEM., vol. 38, 1995, pages 1846 - 1852 |
| CHATTOPADHYAYA, J. ORG. CHEM., vol. 74, 2009, pages 118 - 134 |
| CHIBA: "Identified a morpholinyl-4-piperidinylacetic acid derivative as a potent oral active VLA-4 antagonist", BIOORG. & MED. CHEM. LETT., vol. 15, no. 1, pages 41 - 45, XP025313466, DOI: 10.1016/j.bmcl.2004.10.041 |
| CONNOLLY ET AL., J BIOL CHEM, vol. 257, 1982, pages 939 - 945 |
| CROOKE ET AL., J. PHARMACOL. EXP. THER., vol. 277, 1996, pages 923 - 937 |
| DUFF ET AL., METHODS ENZYMOL, vol. 313, 2000, pages 297 - 321 |
| ENGLISCH ET AL., ANGEWANDTE CHEMIE, vol. 30, 1991, pages 613 |
| GONG: "Synthesis and Biological Evaluation of Novel Pyridazinone-Based α4 Integrin Receptor Antagonists", J. MED. CHEM, vol. 49, no. 11, 2006, pages 3402 - 3411 |
| J. MED. CHEM., vol. 61, no. 22, 2018, pages 10276 - 10298 |
| JAYAPRAKASH ET AL., ORG LETT, vol. 12, 2010, pages 5410 - 5413 |
| KABANOV ET AL., FEBS LETT., vol. 259, 1990, pages 327 - 330 |
| KATO ET AL., GLYCOHIOL, vol. 11, 2001, pages 821 - 829 |
| KHOREV ET AL., BIOORG MED CHEM, vol. 16, 2008, pages 5216 - 5231 |
| KIM ET AL., TETRAHEDRON LETT, vol. 38, 1997, pages 3487 - 3490 |
| KORNILOVA ET AL., ANALYT BIOCHEM, vol. 425, 2012, pages 43 - 46 |
| KUMAR ET AL., ORG. BIOMOL. CHEM., vol. 11, 2013, pages 5853 - 5865 |
| LEE ET AL., BIOCHEM, vol. 23, 1984, pages 4255 - 4261 |
| LEE ET AL., BIOORG MED CHEM LETT, vol. 16, no. 19, 2006, pages 5132 - 5135 |
| LEE ET AL., BIOORG MED CHEM, vol. 19, 2011, pages 2494 - 2500 |
| LEE ET AL., BIOORGANIC & MEDICINAL CHEMISTRY, vol. 19, 2011, pages 2494 - 2500 |
| LEE ET AL., GLYCOCONJUGATE J, vol. 4, 1987, pages 317 - 328 |
| LEE ET AL., J ORG CHEM, vol. 77, 2012, pages 7564 - 7571 |
| LEE ET AL., METHODS ENZYMOL, vol. 362, 2003, pages 38 - 43 |
| LEE, CARHOHYDR RES, vol. 67, 1978, pages 509 - 514 |
| LETSINGER ET AL., PROC. NATL. ACID. SCI. USA, vol. 86, 1989, pages 6553 - 6556 |
| MAIER ET AL., BIOCONJUG CHEM, vol. 14, 2003, pages 18 - 29 |
| MAIER ET AL., BIOCONJUGATE CHEMISTRY, vol. 14, 2003, pages 18 - 29 |
| MAIERHOFER ET AL., BIOORG MED CHEM, vol. 15, 2007, pages 7661 - 7676 |
| MANOHARAN ET AL., ANN. NY. ACAD. SCI., vol. 660, 1992, pages 306 - 309 |
| MANOHARAN ET AL., BIORG. MED. CHEM. LET., vol. 3, 1993, pages 2765 - 2770 |
| MANOHARAN ET AL., BIORG. MED. CHEM. LET., vol. 4, 1994, pages 1053 - 1060 |
| MANOHARAN ET AL., NUCLEOSIDES & NUCLEOTIDES, vol. 14, 1995, pages 969 - 973 |
| MANOHARAN ET AL., TETRAHEDRON LETT., vol. 36, 1995, pages 3651 - 3654 |
| MANOHARAN, ANTISENSE NUCLEIC ACID DRUG DEV, vol. 12, 2002, pages 103 - 128 |
| MERWIN ET AL., BIOCONJUG CHEM, vol. 5, 1994, pages 612 - 620 |
| MISHRA ET AL., BIOCHIM. BIOPHYS. ACTA, vol. 1264, 1995, pages 229 - 237 |
| NISHINA ET AL., MOLECULAR THERAPY NUCLEIC ACIDS, vol. 4, 2015, pages e220 |
| NISHINA ET AL., MOLECULAR THERAPY, vol. 16, 2008, pages 734 - 166,442-443 |
| OBERHAUSER ET AL., NUCL. ACIDS RES., vol. 20, 1992, pages 533 - 538 |
| OKA ET AL., JACS, vol. 125, 2003, pages 8307 - 65 |
| PAVIA ET AL., INT J PEP PROTEIN RES, vol. 22, 1983, pages 539 - 548 |
| PUJOL ET AL., ANGEW CHEMIE INT ED ENGL, vol. 51, 2012, pages 7445 - 7448 |
| RAJUR ET AL., BIOCONJUG CHEM, vol. 8, 1997, pages 935 - 940 |
| RENSEN ET AL., ARTERIOSCLER THROMH VASE BIOL, vol. 26, 2006, pages 169 - 175 |
| RENSEN ET AL., J BIOL CHEM, vol. 276, 2001, pages 37577 - 37584 |
| RENSEN ET AL., J MED CHEM, vol. 47, 2004, pages 5798 - 5808 |
| RENSEN ET AL., J. BIOL. CHEM., vol. 276, 2001, pages 37577 - 37584 |
| RENSEN ET AL., J. MED. CHEM., vol. 47, 2004, pages 5798 - 5808 |
| SAISON-BEHMOARAS ET AL., EMBO J, vol. 10, 1991, pages 1111 - 1118 |
| SATO ET AL., JAM CHEM SOC, vol. 126, 2004, pages 14013 - 14022 |
| SHEA ET AL., NUCL. ACIDS RES., vol. 18, 1990, pages 3777 - 3783 |
| SLIEDREGT ET AL., J MED CHEM, vol. 42, 1999, pages 609 - 618 |
| SLIEDREGT ET AL., J. MED. CHEM., vol. 42, 1999, pages 609 - 618 |
| SVINARCHUK ET AL., BIOCHIMIE, vol. 75, 1993, pages 49 - 54 |
| TOLOMELLI: "Dehydro-β-proline Containing aa(3i Integrin Antagonists: Stereochemical Recognition in Ligand-Receptor Interplay", ACS MED. CHEM. LETT., vol. 6, no. 6, 2015, pages 701 - 706 |
| TOMIYA ET AL., BIOORG MED CHEM, vol. 21, 2013, pages 5275 - 5281 |
| TOYOKUNI ET AL., TETRAHEDRON LETT, vol. 31, 1990, pages 2673 - 2676 |
| VALENTIJN ET AL., TETRAHEDRON, vol. 53, 1997, pages 759 - 770 |
| VAN ROSSENBERG ET AL., GENE THER, vol. 11, 2004, pages 457 - 464 |
| WAN ET AL., NUC. ACID. RES., vol. 42, 2014, pages 13456 |
| WESTERLIND ET AL., GLYCOCONJ J, vol. 21, 2004, pages 227 - 241 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW202448484A (en) | 2024-12-16 |
| WO2024220930A3 (en) | 2024-12-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TW202146650A (en) | Oligonucleotide compositions and methods thereof | |
| TW202016301A (en) | Extrahepatic delivery | |
| EP3359523A2 (en) | Oligonucleotide compositions and methods thereof | |
| TW201707711A (en) | Oligonucleotide composition and method therefor | |
| KR20240027745A (en) | Methods for synthesizing linked modified oligomeric compounds | |
| CA2902571A1 (en) | Bridged bicyclic nucleosides | |
| WO2019182037A1 (en) | Antisense oligonucleotide having reduced toxicity | |
| CN119256083A (en) | Compositions for regulating complement factor B and methods of use thereof | |
| CN111902537A (en) | Modulators of DNM2 expression | |
| KR20240129626A (en) | Splice switcher antisense oligonucleotides with modified backbone chemistry | |
| KR20240099244A (en) | Angiotensinogen regulating composition | |
| WO2021054370A1 (en) | Nucleic acid complex | |
| WO2024220930A2 (en) | Mapt-modulating compositions and methods of use thereof | |
| KR20250125967A (en) | Advanced RNA Targeting (ARNATAR) | |
| JP6029147B2 (en) | Oligonucleotide derivatives for miRNA function suppression | |
| KR20250024534A (en) | Compositions and methods for inhibiting SNCA expression | |
| JP6934695B2 (en) | Nucleic acid medicine and its use | |
| WO2024249328A2 (en) | Sod1-modulating compositions and methods of use thereof | |
| TW202506137A (en) | Lrrk2-modulating compositions and methods of use thereof | |
| TW202521694A (en) | Coagulation factor xi-modulating compositions and methods of use thereof | |
| CN119137272A (en) | Angiotensinogen modulating compositions and methods of use thereof | |
| CN118786133A (en) | Angiotensinogen regulating compositions and methods of use thereof | |
| CN118922540A (en) | Prekallikrein modulation compositions and methods of use thereof | |
| TW202335674A (en) | Lipid conjugation for targeting astrocytes of the central nervous system | |
| TW202308662A (en) | Lipid conjugation for targeting neurons of the central nervous system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24729118 Country of ref document:EP Kind code of ref document:A2 |