WO2024211346A1 - Mutant pi3k-alpha inhibitors and their use as pharmaceuticals - Google Patents
Mutant pi3k-alpha inhibitors and their use as pharmaceuticalsDownload PDFInfo
- Publication number
- WO2024211346A1 WO2024211346A1PCT/US2024/022742US2024022742WWO2024211346A1WO 2024211346 A1WO2024211346 A1WO 2024211346A1US 2024022742 WUS2024022742 WUS 2024022742WWO 2024211346 A1WO2024211346 A1WO 2024211346A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ethyl
- amino
- chloro
- oxo
- dimethyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
Definitions
- PI3Ksconsist of a p85 regulatory subunit in complex with a p110 catalytic subunit (p110 ⁇ , ⁇ , ⁇ or ⁇ ) (1).
- p110 ⁇coded by the PIK3CA gene shows a broad tissue distribution and the binding of a phosphorylated receptor tyrosine kinase (RTK) activates p110a through the release of a subset of inhibitory contacts with p85.
- RTKphosphorylated receptor tyrosine kinase
- P110 ⁇generate phosphatidylinositol3,4,5-trisphosphate (PtdIns(3,4,5)P3; also known as PIP3), which interact with 3-phosphoinositide-binding Pleckstrin homology (PH) domains found in diverse proteins, including protein kinases such as AKT resulting in its phosphorylation at Thr308 and Ser473 triggering a cascade of mitogenic signaling (2).
- This signalingresults in a multitude of cellular effects including proliferation, survival, chemotaxis, cellular trafficking, motility, metabolism, inflammatory and allergic responses, transcription and translation (3).
- PIK3CA hotspot mutationsare one of the most frequent oncogenic mutations in cancer.
- PIK3CA helical (E542K, E545K) and kinase (H1047R) domainsfunction by perturbing local interfaces between p85 and p110 ⁇ and increasing dynamic events required for catalysis on membranes (1,4).
- Oncogenic mutations in the PIK3CA geneincrease lipid kinase activity and transform cells and are the drivers of the pathology. These mutations are observed in a broad range of cancers including breast, colon, uterine, bladder, cervical, and lung cancer (5,6,7). [0004] Given its key role in cancer PI3Ks have been the focus of extensive drug development.
- pan-class I PI3K inhibitor copanlisib(Aliqopa/BAY 80-6946; Bayer) was approved for follicular lymphoma and in 2019, the PI3K ⁇ inhibitor alpelisib (Piqray/NVP-BYL719; Novartis) was approved for the treatment of advanced breast cancer, in combination with the estrogen receptor (ER) downregulator fulvestrant (1,8).
- ERestrogen receptor
- pan-class I PI3K and dual PI3K ⁇ /PI3K ⁇ , or even PI3K ⁇ selective inhibitorshave impacted the realization of full clinical utility of these compounds.
- the toxicity of PI3K inhibitorsis dependent on their isoform 105807.005004– PCT Application selectivity profile. Inhibition of PI3K ⁇ is associated with hyperglycemia and rash, whereas inhibition of PI3K ⁇ or PI3K ⁇ is associated with diarrhea, myelosuppression, and transaminitis (1).
- a recent studyreported that while progression of disease is the largest contributor to alpelisib discontinuation, adverse events are the leading cause for early drug cessation (10). Shorter alpelisib exposure is associated with greater cancer progression.
- ring Ais: W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, or a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C 2 -C 6 alkenyl, -C 2 -C 6 alkynyl, aryl, heteroaryl, cycloalkyl, - 3 - 4877-0794-3771.1 105807.005004– PCT Application cycloalkenyl, heterocycloalkenyl, NR c R d , OR b , or SR b ; wherein each group is optionally substituted by 1-6 R f groups; Y is CR 2 or N; n is
- R eis C 3 -C 8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C 1 - C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C 3 -C 8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 R f groups; each R f is independently H, D, oxo, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -
- substituents of compounds of the inventionare disclosed in groups or in ranges. It is specifically intended that the invention include each and every individual subcombination of the members of such groups and ranges.
- C1-C6 alkylis specifically intended to individually disclose methyl, ethyl, C3 alkyl, C4 alkyl, C5 alkyl, and C6 alkyl.
- C0 alkylrefers to a covalent bond. - 5 - 4877-0794-3771.1 105807.005004– PCT Application [0021] It is further intended that the compounds of the invention are stable.
- stablerefers to a compound that is sufficiently robust to survive isolation to a useful degree of purity from a reaction mixture, and preferably capable of formulation into an efficacious therapeutic agent.
- alkylwhen used alone or as part of a substituent group, refers to a straight- or branched-chain hydrocarbon group having from 1 to 12 carbon atoms (“C1-C12”), preferably 1 to 6 carbons atoms (“C 1 -C 6 ”), in the group.
- alkyl groupsinclude methyl (Me, C 1 alkyl), ethyl (Et, C 2 alkyl), n-propyl (C 3 alkyl), isopropyl (C 3 alkyl), butyl (C 4 alkyl), isobutyl (C4alkyl), sec-butyl (C4alkyl), tert-butyl (C4alkyl), pentyl (C5alkyl), isopentyl (C5alkyl), tert- pentyl (C5alkyl), hexyl (C6alkyl), isohexyl (C6alkyl), and the like. Alkyl groups of the are optionally substituted.
- the alkyl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1- C 6 haloalkoxy, -C(O)NH(C 1 -C 6 alkyl), -C(O)N(C 1 -C 6 alkyl) 2 , -OC(O)NH(C 1 -C 6 alkyl), - OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and -S(O) 2 N(C 1 -C 6 alkyl) 2 .
- substituentsindependently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1- C
- the alkyl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the alkyl group is optionally substituted by 1-6 R f groups.
- haloor halogen refers to chloro, fluoro, bromo, or iodo.
- cycloalkylwhen used alone or as part of a substituent group refers to cyclic- containing, non-aromatic hydrocarbon groups having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C 3- C 6 ”).
- Cycloalkyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkyl group, the cyclic groups can share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- cycloalkyl groupsinclude, for example, cyclopropyl (C3), cyclobutyl (C4), cyclopropylmethyl (C4), cyclopentyl (C5), cyclohexyl (C6), 1-methylcyclopropyl (C4), 2-methylcyclopentyl (C4), adamantanyl (C 10 ), spiro[3.3]heptanyl, bicyclo[3.3.0]octanyl, and the like.
- the cycloalkyl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the cycloalkyl group is optionally substituted by 1-6 R f groups.
- cycloalkenylwhen used alone or as part of a substituent group refers to monocyclic or multicyclic, partially saturated ring structure having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C3-C6”).
- Cycloalkenyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups share two common atoms (e.g., fused or bridged).
- the term -C 3 -C 6 cycloalkenylrefers to a cycloalkenyl group having between three and six carbon atoms.
- the cycloalkenyl groupmay be attached at any carbon atom of the partially saturated ring such that the result is a stable structure.
- Cycloalkenyl groupsinclude groups in which the partially saturated ring is fused to an aryl group. Examples of cycloalkenyl groups include, for example, cyclopropenyl (C 3 ), cyclobutenyl (C 4 ), cyclopropenylmethyl (C 4 ), cyclopentenyl (C5), cyclohexenyl (C6), 1-methylcyclopropenyl (C4), 2-methylcyclopentenyl (C4), adamantenyl (C10), spiro[3.3]heptenyl, bicyclo[3.3.0]octenyl, indanyl, and the like.
- Cycloalkenyl groups of the disclosureare optionally substituted. Unless otherwise specified, in those embodiments wherein the cycloalkenyl group is substituted, the cycloalkenyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1- C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, -C(O)NH(C 1 -C 6 alkyl), -C(O)N(C 1 - C 6 alkyl) 2 , -OC(O)NH(C 1 -C 6 alkyl), -OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and - S(O)2N(C1-C6alkyl)2.
- the cycloalkenyl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the cycloalkenyl group is optionally substituted by 1-6 R f groups.
- 1-6 R groupsselected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the cycloalkenyl group is optionally substituted by 1-6 R f groups.
- heterocycloalkylwhen used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, saturated ring structure containing at least one heteroatom selected from the group consisting of O, N and S.
- Heterocycloalkyl groups - 7 - 4877-0794-3771.1 105807.005004– PCT Application of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups.
- the cyclic groupscan share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- the term -C3-C6 heterocycloalkylrefers to a heterocycloalkyl group having between three and six carbon ring atoms.
- heterocycloalkyl groupmay be attached at any heteroatom or carbon atom of the group such that the result is a stable structure.
- heterocycloalkyl groupsinclude, but are not limited to, azepanyl, aziridinyl, azetidinyl, pyrrolidinyl, dioxolanyl, imidazolidinyl, pyrazolidinyl, piperazinyl, piperidinyl, dioxanyl, morpholinyl, dithianyl, thiomorpholinyl, oxazepanyl, oxiranyl, oxetanyl, quinuclidinyl, tetrahydrofuranyl, tetrahydropyranyl, piperazinyl, azepanyl, diazepanyl, oxepanyl, dioxepanyl, azocanyl diazocanyl, oxocanyl, dioxocanyl, di
- Heteroycloalkyl groups of the disclosureare optionally substituted. Unless otherwise specified, in those embodiments wherein the heterocycloalkyl group is substituted, the heterocycloalkyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, - C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, - S(O) 2 NH(C 1 -C 6 alkyl), and -S(O) 2 N(C 1 -C 6 alkyl) 2 .
- substituentsindependently selected from -OH, -CN,
- the heterocycloalkyl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , - SR a , -NR a R d , or NR c R d ; or the heterocycloalkyl group is optionally substituted by 1-6 R f groups.
- 1-6 R groupsselected from H, D, halogen, -OH, -CN, -OR a , - SR a , -NR a R d , or NR c R d ; or the heterocycloalkyl group is optionally substituted by 1-6 R f groups.
- heterocycloalkenylwhen used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, partially saturated ring structure containing at least one heteroatom selected from the group consisting of O, N and S.
- Heterocycloalkenyl groups of the disclosureinclude monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups.
- the cyclic groupscan share one common atom (i.e., spirocyclic).
- the cyclic groupsshare two common atoms (e.g., fused or bridged).
- the term -C3-C6 heterocycloalkenylrefers to a heterocycloalkenyl group having between three and six carbon atoms.
- heterocycloalkenyl groupmay be attached at any heteroatom or carbon atom of the - 8 - 4877-0794-3771.1 105807.005004– PCT Application partially saturated ring such that the result is a stable structure.
- Heterocycloalkenyl groupsinclude groups in which the partially saturated ring is fused to an aryl group, such as, for example isoindoline, , or in which the partially saturated ring is fused to a heteroaryl group, such as, for example, 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine, .
- Heteroycloalkenyl groups of the disclosureare optionally substituted.
- the heterocycloalkenyl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 -C 6 haloalkoxy, - C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, - S(O) 2 NH(C 1 -C 6 alkyl), and -S(O) 2 N(C 1 -C 6 alkyl) 2 .
- substituentsindependently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and
- the heterocycloalkenyl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , - SR a , -NR a R d , or NR c R d ; or the heterocycloalkenyl group is optionally substituted by 1-6 R f groups.
- 1-6 R groupsselected from H, D, halogen, -OH, -CN, -OR a , - SR a , -NR a R d , or NR c R d ; or the heterocycloalkenyl group is optionally substituted by 1-6 R f groups.
- heteroarylwhen used alone or as part of a substituent group refers to a mono- or bicyclic- aromatic ring structure including carbon atoms as well as up to five heteroatoms selected from nitrogen, oxygen, and sulfur. Heteroaryl rings can include a total of 5, 6, 7, 8, 9, or 10 ring atoms.
- heteroaryl groupsinclude but are not limited to, pyrrolyl, furyl, thiophenyl (thienyl), oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, triazolyl, thiadiazolyl, pyrazolyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, pyranyl, furazanyl, indolizinyl, indolyl, and the like.
- Heteroaryl groups of the disclosureare optionally substituted.
- the heteroaryl groupcan be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C 1 - C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C 1 -C 6 alkyl) 2 , -S(O) 2 NH(C 1 -C 6 alkyl), and -S(O) 2 N(C 1 -C 6 alkyl) 2 .
- substituentsindependently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, and C
- the heteroaryl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the heteroaryl group is optionally substituted by 1-6 R f groups.
- 1-6 R groupsselected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the heteroaryl group is optionally substituted by 1-6 R f groups.
- arylwhen used alone or as part of a substituent group refers to a mono- or bicyclic- aromatic carbon ring structure.
- Aryl ringscan include a total of 5, 6, 7, 8, 9, or 10 ring atoms.
- aryl groupsinclude but are not limited to, phenyl, napthyl, and the like.
- Aryl groups of the disclosureare optionally substituted. Unless otherwise specified, in those embodiments wherein the aryl group is substituted, the aryl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 - C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1- C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2.
- the aryl groupis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the aryl group is optionally substituted by 1-6 R f groups.
- 1-6 R groupsselected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d ; or the aryl group is optionally substituted by 1-6 R f groups.
- C1-6alkrefers to an aliphatic linker having 1, 2, 3, 4, 5, or 6 carbon atoms and includes, for example, –CH2-, –CH(CH3)-, -CH(CH3)-CH2-, and –C(CH3)2-.
- -C 0 alk-refers to a bond.
- C 0 -C 6 alkwhen used alone or as part of a substituent group refers to an aliphatic linker having 0, 1, 2, 3, 4, 5 or 6 carbon atoms.
- -C1alk-for example, refers to a -CH 2 -.
- -C 0 alk-refers to a bond.
- the -C 1 -C 6 alkyl, -C 1 - C 10 alkyl, -C 1 -C 8 alkoxide, -C 2 -C 6 alkenyl, -C 2 -C 10 alkenyl, -C 2 -C 6 alkynyl, -C 2 -C 10 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, and heterocycloalkyl groupsare optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , - NR a R d , or NR c R d ; or the -C 1 -C 6 alkyl, -C 1 -C 10 alkyl, -C 1 -C 8 alkoxide, -C 2 -C 6 alkenyl, -C 2
- alkoxyrefers to an –O-alkyl group.
- Example alkoxy groupsinclude methoxy, ethoxy, propoxy (e.g., n-propoxy and isopropoxy), t-butoxy, and the like.
- hydroxylalkylrefers to an alkyl group substituted by OH.
- the compounds described hereincan be asymmetric (e.g., having one or more stereocenters). All stereoisomers, such as enantiomers and diastereomers, are intended unless otherwise indicated.
- the compounds of the present inventionmay exist as rotational isomers. In some embodiments, the compounds of the present invention exist as mixtures of rotational isomers in any proportion. In other embodiments, the compounds of the present invention exist as particular rotational isomers, substantially free of other rotational isomers.
- Compounds of the inventioncan also include all isotopes of atoms occurring in the intermediates or final compounds. Isotopes include those atoms having the same atomic number but different mass numbers. For example, isotopes of hydrogen include tritium and deuterium.
- the compounds of the invention, and salts thereofare substantially isolated.
- substantially isolatedis meant that the compound is at least partially or substantially separated from the environment in which it was formed or detected.
- Partial separationcan include, for example, a composition enriched in the compound of the invention.
- Substantial separationcan include compositions containing at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 97%, or at least about 99% by weight of the compound of the invention, or salt thereof. Methods for isolating compounds and their salts are routine in the art.
- the present inventionalso includes pharmaceutically acceptable salts of the compounds described herein.
- pharmaceutically acceptable saltsrefers to derivatives of the disclosed compounds wherein the parent compound is modified by converting an existing acid or - 11 - 4877-0794-3771.1 105807.005004– PCT Application base moiety to its salt form.
- pharmaceutically acceptable saltsinclude, but are not limited to, mineral or organic acid salts of basic residues such as amines; alkali or organic salts of acidic residues such as carboxylic acids; and the like.
- the pharmaceutically acceptable salts of the present inventioninclude the conventional non-toxic salts of the parent compound formed, for example, from non-toxic inorganic or organic acids.
- the pharmaceutically acceptable salts of the present inventioncan be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods.
- such saltscan be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropriate base or acid in water or in an organic solvent, or in a mixture of the two; generally, nonaqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile are preferred.
- a “pharmaceutically acceptable excipient”refers to a substance that is non-toxic, biologically tolerable, and otherwise biologically suitable for administration to a subject, such as an inert substance, added to a pharmacological composition or otherwise used as a vehicle, carrier, or diluent to facilitate administration of an agent and that is compatible therewith.
- excipientsinclude calcium carbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin, vegetable oils, and polyethylene glycols.
- a “solvate”refers to a physical association of a compound of Formula I with one or more solvent molecules.
- Subjectincludes humans.
- Treating” or “treatment” of any disease or disorderrefers, in one embodiment, to ameliorating the disease or disorder (i.e., arresting or reducing the development of the disease or at least one of the clinical symptoms thereof). In another embodiment “treating” or “treatment” refers to ameliorating at least one physical parameter, which may not be discernible by the subject.
- “treating” or “treatment”refers to modulating the disease or - 12 - 4877-0794-3771.1 105807.005004– PCT Application disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both.
- “treating” or “treatment”refers to delaying the onset of the disease or disorder.
- “Compounds of the present disclosure,” and equivalent expressions,are meant to embrace compounds of Formula I as described herein, as well as its subgenera, which expression includes the stereoisomers (e.g., entaniomers, diastereomers) and constitutional isomers (e.g., tautomers) of compounds of Formula I as well as the pharmaceutically acceptable salts, where the context so permits.
- the term “isotopic variant”refers to a compound that contains proportions of isotopes at one or more of the atoms that constitute such compound that is greater than natural abundance.
- an “isotopic variant” of a compoundcan be radiolabeled, that is, contain one or more radioactive isotopes, or can be labeled with non-radioactive isotopes such as for example, deuterium ( 2 H or D), carbon-13 ( 13 C), nitrogen-15 ( 15 N), or the like.
- non-radioactive isotopessuch as for example, deuterium ( 2 H or D), carbon-13 ( 13 C), nitrogen-15 ( 15 N), or the like.
- the following atoms, where presentmay vary, so that for example, any hydrogen may be 2 H/D, any carbon may be 13 C, or any nitrogen may be 15 N, and that the presence and placement of such atoms may be determined within the skill of the art.
- stereomerscompounds that have the same molecular formula but differ in the nature or sequence of bonding of their atoms or the arrangement of their atoms in space are termed “isomers.” Isomers that differ in the arrangement of their atoms in space are termed “stereoisomers,” for example, diastereomers, enantiomers, and atropisomers.
- the compounds of this disclosuremay possess one or more asymmetric centers; such compounds can therefore be produced as individual (R)- or (S)-stereoisomers at each asymmetric center, or as mixtures thereof. Unless indicated otherwise, the description or naming of a particular compound in the specification and claims is intended to include all stereoisomers and mixtures, racemic or otherwise, thereof.
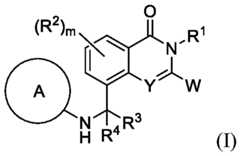
- the disclosureis directed to compounds of Formula I: - 13 - 4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, wherein ring A is: W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C 1 -C 8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, NR c R d , OR b , or SR b ; wherein each group is optionally substituted by 1-6 R f groups; Y is CR 2 or N; n is 1, 2, or 3; m is 1, 2 or 3;
- R eis C 3 -C 8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C 1 - C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C 1 -C 6 -alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C 3 -C 8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 R f groups; each R f is independently H, D, oxo, halogen, C 1 -C 8 alkoxide, C 1 -C 8 alkyl, haloalkyl, -OH, -
- ring A in Formula - 15- 4877-0794-3771.1 105807.005004– PCT Application
- ring A in Formula [0054]ring A in Formula [0055]
- R 6 in Formula (I)is -F.
- R 6 in Formula (I)is -Cl.
- R 6 in Formula (I)is -Br.
- R 6 in Formula (I)is -I.
- R 6 in Formula (I)is -CN.
- W in Formula (I)is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C 2 -C 6 alkenyl, -C 2 -C 6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, NR c R d , OR b , or SR b , wherein each group is optionally substituted by 1-6 R f groups.
- W in Formula (I)is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S.
- the 5-12 membered heterocyclic groupis an isoindoline group or a piperidine group.
- the 5-12 membered heterocyclic groupis an isoindoline group.
- the 5-12 membered heterocyclic groupis a piperidine group.
- the 5-12 membered heterocyclic groupis a 3-azabicyclo[3.1.0]hexane, pyrrolidine, azetidine, piperazine, 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine or 1,4,5,6- tetrahydropyrrolo[3,4-c]pyrazole group.
- the 5-12 membered heterocyclic groupis a 3-azabicyclo[3.1.0]hexane.
- the 5-12 membered heterocyclic groupis a pyrrolidine group.
- the 5-12 membered heterocyclic groupis an azetidine group.
- the 5-12 membered heterocyclic groupis a piperazine group. In some embodiments, the 5-12 membered heterocyclic group is 6,7-dihydro-5H- pyrrolo[3,4-b]pyridine group. In some embodiments, the 5-12 membered heterocyclic group is a 1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole group. In some embodiments, W in Formula (I) is NR c R d . In some embodiments, W in Formula (I) is substituted by 1-6 R f groups. [0058] In other embodiments, W in Formula (I) is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S.
- W in Formula (I)is C 1 -C 8 alkyl. In other embodiments, W in Formula (I) is haloalkyl. In other embodiments, W in - 16 - 4877-0794-3771.1 105807.005004– PCT Application Formula (I) is -C 2 -C 6 alkenyl. In other embodiments, W in Formula (I) is -C 2 -C 6 alkynyl. In other embodiments, W in Formula (I) is aryl. In other embodiments, W in Formula (I) is heteroaryl. In other embodiments, W in Formula (I) is cycloalkyl. In other embodiments, W in Formula (I) is cycloalkenyl.
- W in Formula (I)is heterocycloalkenyl. In other embodiments, W in Formula (I) is NR c R d . In other embodiments, W in Formula (I) is NR c R d . In other embodiments, W in Formula (I) is OR b . In other embodiments, W in Formula (I) is SR b . In some embodiments, W in Formula (I) is substituted by 1 or 2 R f groups. [0059] In some embodiments, Y in Formula (I) is CR 2 or N. In some embodiments, Y in Formula (I) is N. In other embodiments, Y in Formula (I) is CR 2 . In other embodiments, Y in Formula (I) is CH.
- n in Formula (I)is 1, 2, or 3. In some embodiments, n in Formula (I) is 1. In other embodiments, n in Formula (I) is 2. In yet other embodiments, n in Formula (I) is 3. [0061] In some embodiments, m in Formula (I) is 1, 2 or 3. In some embodiments, m in Formula (I) is 1. In other embodiments, m in Formula (I) is 2. In yet other embodiments, m in Formula (I) is 3.
- R 1 in Formula Iis H, D, OR a , C 1 -C 8 alkoxide, C 1 -C 8 alkyl, haloalkyl, -C3-C8 cycloalkyl, -C3-C8 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, or (C 1 -C 6 -alkyl)-R e ; wherein said C 1 -C 8 alkoxide, C 1 -C 8 alkyl, haloalkyl, C 3 - C 8 cycloalkyl, -C 3 -C 10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and (C1-C6-alkyl)-R e are optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis H. In some embodiments, R 1 in Formula I is D. In some embodiments, R 1 in Formula I is OR a . In some embodiments, R 1 in Formula I is C 1 - C8 alkoxide. In some embodiments, R 1 in Formula I is C1-C8 alkyl. In some embodiments, R 1 in Formula I is haloalkyl. In some embodiments, R 1 in Formula I is C3-C8 cycloalkyl. In some embodiments, R 1 in Formula I is C 3 -C 8 cycloalkenyl. In some embodiments, R 1 in Formula I is aryl. In some embodiments, R 1 in Formula I is heteroaryl.
- the C 1 -C 8 alkoxide, C1-C8 alkyl, haloalkyl, C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, and heteroarylare optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis H.
- R 1 in Formula Iis C1-C8alkyl.
- R 1 in Formula Iis methyl.
- R 1 in Formula Iis heterocycloalkyl optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis tetrahydro-2H-pyran optionally substituted - 17 - 4877-0794-3771.1 105807.005004– PCT Application by 1-6 R f groups.
- R 1 in Formula Iis tetrahydrothiophene optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis tetrahydrothiophene-1,1- dioxide optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis tetrahydrofuran optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis oxetane optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis pyrrolidine optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis heterocycloalkenyl optionally substituted by 1-6 R f groups.
- R 1 in Formula Iis (C1-C6-alkyl)-R e optionally substituted by 1-6 R f groups.
- R eis azetidine optionally substituted by 1-6 R f groups, pyrazole optionally substituted by 1-6 R f groups, p-methoxybenzene or propyl.
- R eis pyrazole optionally substituted by 1-6 R f groups.
- each R 2 in Formula Iis independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO 2 , -C 2 -C 6 alkenyl, -C 2 -C 6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -OR a , -SR a , -NR c R d , -NR a R c , -C(O)R b , -OC(O)R b , -C(O)OR b , -C(O)NR c R d , -S(O)R b ,
- At least one R 2 in Formula Iis H. In some embodiments, at least one R 2 in Formula I is D. In some embodiments, at least one R 2 in Formula I is halogen. In some embodiments, at least one R 2 in Formula I is C 1 -C 8 alkoxide. In some embodiments, at least one R 2 in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d .
- At least one R 2 in Formula Iis haloalkyl. In other embodiments, at least one R 2 in Formula I is -OH. In other embodiments, at least one R 2 in Formula I is -CN. In other embodiments, at least one R 2 in Formula I is -NO 2 . In other embodiments, at least one R 2 in Formula I is -C2-C6 alkenyl. In other embodiments, at least one R 2 in Formula I is -C2-C6 alkynyl. In other embodiments, at least one R 2 in Formula I is aryl. In other embodiments, at least one R 2 in Formula I is hetereoaryl.
- At least one R 2 in Formula Iis cycloalkyl. In other embodiments, at least one R 2 in Formula I is cycloalkenyl. In other - 18 - 4877-0794-3771.1 105807.005004– PCT Application embodiments, at least one R 2 in Formula I is heterocycloalkyl. In some embodiments, at least one R 2 in Formula I is heterocycloalkenyl. In other embodiments, at least one R 2 in Formula I is -OR a . In other embodiments, at least one R 2 in Formula I is -SR a . In other embodiments, at least one R 2 in Formula I is -NR c R d .
- At least one R 2 in Formula Iis -NR a R c . In other embodiments, at least one R 2 in Formula I is -C(O)R b . In other embodiments, at least one R 2 in Formula I is -OC(O)R b . In other embodiments, at least one R 2 in Formula I is -C(O)OR b . In other embodiments, at least one R 2 in Formula I is -C(O)NR c R d . In other embodiments, at least one R 2 in Formula I is -S(O)R b . In other embodiments, at least one R 2 in Formula I is - S(O) 2 NR c R d .
- At least one R 2 in Formula Iis - C(O)NR b OR b . In other embodiments, at least one R 2 in Formula I is -S(O)2OR b . In other embodiments, at least one R 2 in Formula I is -OS(O)2OR b . In other embodiments, at least one R 2 in Formula I is -OPO(OR b )(OR b ). [0071] In some embodiments, at least one R 2 in Formula I is H, C 1 -C 8 alkyl, CF 3 , Br, F, CN or CHF2. In some embodiments, at least one R 2 in Formula I is H.
- At least one R 2 in Formula Iis C 1 -C 8 alkyl. In some embodiments, at least one R 2 in Formula I is methyl. In some embodiments, at least one R 2 in Formula I is CF 3 . In some embodiments, at least one R 2 in Formula I is Br. In some embodiments, at least one R 2 in Formula I is F. In some embodiments, at least one R 2 in Formula I is CN. In some embodiments, at least one R 2 in Formula I is CHF 2 .
- R 3 in Formula Iis H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, - CN, -OR a , -SR a , -NR a R d , or NR c R d .
- R 3 in Formula Iis H.
- R 3 in Formula Iis D.
- R 3 in Formula Iis C1-C8 alkyl.
- the C1-C8 alkylis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , - NR a R d , or NR c R d .
- R 3 in Formula Iis haloalkyl.
- R 3 in Formula Iis CN. - 19 - 4877-0794-3771.1 105807.005004– PCT Application [0074]
- R 3 in Formula Iis H or C1-C8 alkyl.
- R 3 in Formula Iis H.
- R 3 in Formula Iis C1-C8alkyl.
- R 3 in Formula Iis methyl.
- R 4 in Formula Iis H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, - CN, -OR a , -SR a , -NR a R d , or NR c R d .
- R 4 in Formula Iis H.
- R 4 in Formula Iis D.
- R 4 in Formula Iis C1-C8 alkyl.
- the C1-C8 alkylis optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , - NR a R d , or NR c R d .
- R 4 in Formula Iis haloalkyl.
- R 4 in Formula Iis CN.
- R 4 in Formula Iis H or C 1 -C 8 alkyl.
- R 4 in Formula Iis H.
- R 4 in Formula Iis C 1 -C 8 alkyl.
- R 4 in Formula Iis methyl.
- R 3 and R 4 in Formula I together with the atom to which they are attachedare combined to form a C 3 -C 7 cycloalkyl or C 4 -C 8 heterocycloalkyl. In some embodiments, R 3 and R 4 in Formula I together with the atom to which they are attached are combined to form a C3-C7 cycloalkyl. In some embodiments, R 3 and R 4 in Formula I together with the atom to which they are attached are combined to form a C 4 -C 8 heterocycloalkyl.
- At least one R 5 in Formula Iis H. In some embodiments, at least one R 5 in Formula I is D. In some embodiments, at least one R 5 in Formula I is halogen. In some embodiments, at least one R 5 in Formula I is C1-C8 alkoxide. In some embodiments, at least one R 5 in Formula I is C 1 -C 8 alkyl. In some embodiments, the C 1 -C 8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d .
- At least one R 5 in Formula Iis haloalkyl. In other embodiments, at least one R 5 in Formula I is -OH. In other embodiments, at least one R 5 in Formula I is -CN. In other embodiments, at least one R 5 in Formula I is -NO2. In other embodiments, at least one R 5 in Formula I is -C 2 -C 6 alkenyl. In other embodiments, at least one R 5 in Formula I is -C 2 -C 6 alkynyl. In other embodiments, at least one R 5 in Formula I is aryl.
- At least one R 5 in Formula Iis hetereoaryl. In other embodiments, at least one R 5 in Formula I is cycloalkyl. In other embodiments, at least one R 5 in Formula I is cycloalkenyl. In other embodiments, at least one R 5 in Formula I is heterocycloalkyl. In some embodiments, at least one R 5 in Formula I is heterocycloalkenyl. In other embodiments, at least one R 5 in Formula I is -OR a . In other embodiments, at least one R 5 in Formula I is -SR a . In other embodiments, at least one R 5 in Formula I is -NR c R d .
- At least one R 5 in Formula Iis -NR a R c . In other embodiments, at least one R 5 in Formula I is -C(O)R b . In other embodiments, at least one R 5 in Formula I is -OC(O)R b . In other embodiments, at least one R 5 in Formula I is -C(O)OR b . In other embodiments, at least one R 5 in Formula I is -C(O)NR c R d . In other embodiments, at least one R 5 in Formula I is -S(O)R b . In other embodiments, at least one R 5 in Formula I is - S(O)2NR c R d .
- At least one R 5 in Formula Iis - C(O)NR b OR b . In other embodiments, at least one R 5 in Formula I is -S(O)2OR b . In other embodiments, at least one R 5 in Formula I is -OS(O)2OR b . In other embodiments, at least one R 5 in Formula I is -OPO(OR b )(OR b ). [0082] In some embodiments, at least one R 5 in Formula I is a carboxylic acid group or isostere thereof. In some embodiments, at least one R 5 in Formula I is a carboxylic acid group. In some embodiments, at least one R 5 in Formula I is -CO 2 H.
- each R a in Formula Iis independently H, D, -C(O)R b , - 2, - C 10 alkyl, - C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl.
- R a in Formula Iis H.
- R a in Formula Iis D.
- R a in Formula Iis -P(OR c ) 2 , -P(O)R c R b , -P(O)OR c OR b , -S(O)R b , -S(O)NR c R d , -S(O)2R b , -S(O)2NR c R d , SiR b 3, and the like.
- R a in Formula Iis -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and the like.
- each R b in Formula Iis independently H, D, -C 1 -C 6 alkyl, -C 2 -C 6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl.
- R b in Formula Iis H. In some embodiments, R b in Formula I is D. In some embodiments, R b in Formula I is -C1-C6 alkyl. In some embodiments, R b in Formula I is -C2-C6 alkenyl. In some embodiments, R b in Formula I is -C2-C6 alkynyl. In other embodiments, R b in Formula I is aryl. In other embodiments, R b in Formula I is cycloalkyl. In other embodiments, R b in Formula I is cycloalkenyl. In other embodiments, R b in Formula I is heteroaryl. In other embodiments, R b in Formula I is heterocycloalkyl.
- R b in Formula Iis heterocycloalkenyl.
- each R c or R d in Formula Iis independently H, D, -C 1 -C 6 alkyl, - C2-C6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl.
- R c or R d in Formula Iis H.
- R c or R d in Formula Iis D.
- R c or R d in Formula Iis -C1-C10 alkyl.
- R c or R d in Formula Iis -C2-C6 alkenyl. In some embodiments, R c or R d in Formula I is -C 2 -C 6 alkynyl. In other embodiments, R c or R d in Formula I is -OC 1 -C 6 alkyl. In other embodiments, R c or R d in Formula I is -O-cycloalkyl. In other embodiments, R c or R d in Formula I is aryl. In other embodiments, R c or R d in Formula I is cycloalkyl. In other embodiments, R c or R d in Formula I is cycloalkenyl.
- R c or R d in Formula Iis heteroaryl. In other embodiments, R c or R d in Formula I is heterocycloalkyl. In other embodiments, R c or R d in Formula I is heterocycloalkenyl. [0090] In yet other embodiments, R c and R d in Formula I, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or - 22 - 4877-0794-3771.1 105807.005004– PCT Application multicyclic heterocyclo-alkenyl group. In yet other embodiments, R c and R d in Formula I form a monocyclic heterocycloalkyl.
- R c and R d in Formula Iform a multicyclic heterocycloalkyl. In yet other embodiments, R c and R d in Formula I form a monocyclic heterocyclo-alkenyl group. In yet other embodiments, R c and R d in Formula I form a multicyclic heterocyclo-alkenyl group.
- R e in Formula Iis C 3 -C 8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C 3 -C 8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 R f groups.
- R e in Formula Iis C 3 -C 8 cycloalkyl optionally substituted by 1-6 R f groups. In some embodiments, R e in Formula I is heterocycloalkyl optionally substituted by 1-6 R f groups. In some embodiments, the heterocycloalkyl is attached to (C1-C6-alkyl) through a carbon atom of the heterocycloalkyl group. In some embodiments, the heterocycloalkyl is attached to (C 1 -C 6 -alkyl) through a sulfur atom of the heterocycloalkyl group. In other embodiments, R e in Formula I is cycloalkenyl optionally substituted by 1-6 R f groups.
- R e in Formula Iis heterocycloalkenyl optionally substituted by 1-6 R f groups.
- the heterocycloalkenylis attached to (C 1 -C 6 -alkyl) through a carbon atom of the heterocycloalkenyl group.
- the heterocycloalkenylis attached to (C 1 - C6-alkyl) through a sulfur atom of the heterocycloalkenyl group.
- R e in Formula Iis aryl optionally substituted by 1-6 R f groups.
- R e in Formula Iis heteroaryl optionally substituted by 1-6 R f groups.
- R e in Formula Iis azetidine or piperidine optionally substituted by 1-6 R f groups, pyrazole optionally substituted by 1-6 R f groups, phenyl optionally substituted by 1-6 R f groups or cycloalkyl optionally substituted by 1-6 R f groups.
- R e in Formula Iis azetidine optionally substituted by 1-6 R f groups.
- R e in Formula Iis piperidine optionally substituted by 1-6 R f groups.
- R e in Formula Iis phenyl optionally substituted by 1-6 R f groups.
- R e in Formula Iis cycloalkyl optionally substituted by 1-6 R f groups.
- each R f in Formula Iis independently H, D, oxo, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO 2 , -C 2 -C 6 alkenyl, -C 2 -C 6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -OR a , -SR a , -NR c R d , - 23 - 4877-0794-3771.1 105807.005004– PCT Application -NR a R c , -C(O)R b , -OC(O)R b , -C(O)OR b
- R f in Formula Iis H. In some embodiments, R f in Formula I is D. In some embodiments, R f in Formula I is oxo. In some embodiments, R f in Formula I is halogen. In some embodiments, R f in Formula I is C1-C8 alkoxide. In some embodiments, R f in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -OR a , -SR a , -NR a R d , or NR c R d .
- R f in Formula Iis haloalkyl. In some embodiments, R f in Formula I is -OH. In some embodiments, R f in Formula I is -CN. In some embodiments, R f in Formula I is -NO 2 . In some embodiments, R f in Formula I is -C2-C6 alkenyl. In some embodiments, R f in Formula I is - C2-C6 alkynyl. In some embodiments, R f in Formula I is aryl. In some embodiments, R f in Formula I is heteroaryl. In some embodiments, R f in Formula I is cycloalkyl. In other embodiments, R f in Formula I is cycloalkenyl.
- R f in Formula Iis heterocycloalkyl. In other embodiments, R f in Formula I is heterocycloalkenyl. In other embodiments, R f in Formula I is -OR a . In other embodiments, R f in Formula I is -SR a . In other embodiments, R f in Formula I is -NR c R d . In other embodiments, R f in Formula I is -NR a R c . In other embodiments, R f in Formula I is -C(O)R b . In other embodiments, R f in Formula I is - OC(O)R b . In other embodiments, R f in Formula I is -C(O)OR b .
- R f in Formula Iis -B(OR c )(OR d ). In yet other embodiments, R f in Formula I is -S(O)2R b . In yet other embodiments, R f in Formula I is -C(O)NR b OR b . In yet other embodiments, R f in Formula I is -S(O) 2 OR b . In yet other embodiments, R f in Formula I is - OS(O)2OR b . In yet other embodiments, R f in Formula I is -OPO(OR b )(OR b ). [0096] In some embodiments, the compounds of Formula (I) are the pharmaceutically acceptable salts.
- the compounds of Formula (I)are solvates. In some embodiments, the compounds of Formula (I) are N-oxides. In some embodiments, the compounds of Formula (I) are stereoisomers. - 24 - 4877-0794-3771.1 105807.005004– PCT Application [0097] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula II or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , m, n, W, and Y are as defined with respect to Formula (I).
- the compounds of Formula (I)are represented by compounds of Formula III or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , m, W and Y are as defined with respect to Formula (I).
- each R 5is H.
- R 6is -F.
- R 6is -Cl.
- R 6is -Br.
- R 6is -I.
- R 6is -CN.
- the compounds of Formula (I)are represented by compounds of Formula IV - 25 - 4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R c , R d ,m, and Y are defined with respect to Formula (I).
- each R 5is H.
- R 6is -F.
- R 6is -Cl.
- R 6is -Br.
- R 6is -I.
- R 6is -CN.
- the compounds of Formula (I)are represented by compounds of Formula IV-1, Formula IV-2, Formula IV-3, or Formula IV-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 2 , R c , R d , and m are defined with respect to Formula (I).
- the compounds of Formula (I)are represented by compounds of Formula V or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , and m are as defined with respect to Formula (I), and W a is a 5-10 membered - 26 - 4877-0794-3771.1 105807.005004– PCT Application heteroaryl group comprising 1-4 heteroatoms selected from N, O, and S, or an aryl group, wherein each group is optionally substituted by 1-6 R f groups.
- each R 5is H.
- R 6is -F.
- R 6is -Cl.
- R 6is -Br.
- R 6is -I.
- R 6is -CN.
- the compounds of Formula (I)are represented by compounds of Formula V-1, Formula V-2, Formula V-3, or Formula V-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 2 and m are as defined with respect to Formula (I), and W a is a 5-10 membered heteroaryl group comprising 1-4 heteroatoms selected from N, O, and S, or an aryl group, wherein each group is optionally substituted by 1-6 R f groups.
- the compounds of Formula (I)are represented by compounds of Formula VI - 27 - 4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , m, are defined with respect to Formula (I), and W b is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, or a cycloalkyl group, wherein each group is optionally substituted by 1-6 R f groups.
- each R 5is H.
- R 6is -F.
- R 6is -Cl.
- R 6is -Br.
- R 6is -I.
- R 6is -CN.
- the compounds of Formula (I)are represented by compounds of Formula VI-1, Formula VI-2, Formula VI-3, or Formula VI-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R 2 and m are as defined with respect to Formula (I), and W b is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, or a cycloalkyl group, wherein each group is optionally substituted by 1-6 R f groups.
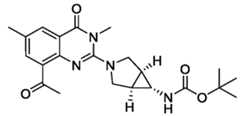
- the compounds of Formula (I)are: 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6s)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 28 - 4877-0794-3771.1 105807.005004– PCT Application 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl
- the compounds of Formula (I)are: 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6S)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((methoxycarbony
- the compounds of the disclosureare pharmaceutically acceptable salts of the compounds of Formula I (including all subgenera described herein). In other embodiments, the compounds of the disclosure are not salts. In some embodiments, the compounds of the disclosure are N-oxides of the compounds of Formula I (including all subgenera described herein). In other embodiments, the compounds of the disclosure are not N-oxides of the compounds of Formula I (including all subgenera described herein). In some embodiments, the compounds of the disclosure are solvates of the compounds of Formula I (including all subgenera described herein).
- the compounds - 44 - 4877-0794-3771.1 105807.005004– PCT Application of the disclosureare not solvates of the compounds of Formula I (including all subgenera described herein).
- Isotopic variants of the compounds of Formula Iare also contemplated by the present disclosure.
- the compounds of the disclosureinclude a carboxylic acid moiety. In some aspects, the present disclosure also encompasses carboxylic acid prodrugs of these embodiments.
- Carboxylic acid prodrugsinclude, but are not limited to, C1-C6 alkyl esters (e.g., methyl, ethyl, isopropyl, butyl, and isoamyl), 2-aminoethyl esters (e.g., 2- morpholinoethyl), C6-C10 aryl esters (e.g. phenyl, indanyl, and guaiacol), (acyloxy)alkyl esters, [(alkoxycarbonyl)oxy]alkyl esters, and (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl esters. See, e.g., Maag, H. (2007).
- compositions and Methods of Administration[00135]
- the disclosureis directed to pharmaceutical compositions comprising compounds of Formula I, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof.
- the subject pharmaceutical compositionsare typically formulated to provide a therapeutically effective amount of a compound of the present disclosure as the active ingredient, or a pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or derivative thereof.
- the pharmaceutical compositionscontain pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including inert solid diluents and fillers, diluents, including sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- the subject pharmaceutical compositionscan be administered alone or in combination with one or more other agents, which are also typically administered in the form of pharmaceutical compositions.
- the one or more compounds of the invention and other agent(s)may be mixed into a preparation or both components may be formulated into separate preparations to use them in combination separately or at the same time.
- the concentration of one or more compounds provided in the pharmaceutical compositions of the present inventionis less than 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, - 45 - 4877-0794-3771.1 105807.005004– PCT Application 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%
- the concentration of one or more compounds of the inventionis greater than 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19.75%, 19.50%, 19.25%, 19%, 18.75%, 18.50%, 18.25% 18%, 17.75%, 17.50%, 17.25% 17%, 16.75%, 16.50%, 16.25%, 16%, 15.75%, 15.50%, 15.25% 15%, 14.75%, 14.50%, 14.25% 14%, 13.75%, 13.50%, 13.25%, 13%, 12.75%, 12.50%, 12.25%, 12%, 11.75%, 11.50%, 11.25% 11%, 10.75%, 10.50%, 10.25% 10%, 9.75%, 9.50%, 9.25%, 9%, 8.75%, 8.50%, 8.25% 8%, 7.75%, 7.50%, 7.25%, 7%, 6.75%, 6.50%, 6.25%, 6%, 5.75%, 5.50%, 5.25%, 5%, 5%,
- the concentration of one or more compounds of the inventionis in the range from approximately 0.0001% to approximately 50%, approximately 0.001% to approximately 40%, approximately 0.01% to approximately 30%, approximately 0.02% to approximately 29%, approximately 0.03% to approximately 28%, approximately 0.04% to approximately 27%, approximately 0.05% to approximately 26%, approximately 0.06% to approximately 25%, approximately 0.07% to approximately 24%, approximately 0.08% to approximately 23%, approximately 0.09% to approximately 22%, approximately 0.1% to approximately 21%, approximately 0.2% to approximately 20%, approximately 0.3% to approximately 19%, approximately 0.4% to approximately 18%, approximately 0.5% to approximately 17%, approximately 0.6% to approximately 16%, approximately 0.7% to approximately 15%, approximately 0.8% to approximately 14%, approximately 0.9% to approximately 12%, approximately 1% to approximately 10% w/w, w/v or v/v.
- the concentration of one or more compounds of the inventionis in the range from approximately 0.001% to approximately 10%, approximately 0.01% to approximately 5%, approximately 0.02% to approximately 4.5%, approximately 0.03% to approximately 4%, approximately 0.04% to approximately 3.5%, approximately 0.05% to - 46 - 4877-0794-3771.1 105807.005004– PCT Application approximately 3%, approximately 0.06% to approximately 2.5%, approximately 0.07% to approximately 2%, approximately 0.08% to approximately 1.5%, approximately 0.09% to approximately 1%, approximately 0.1% to approximately 0.9% w/w, w/v or v/v.
- the amount of one or more compounds of the inventionis equal to or less than 10 g, 9.5 g, 9.0 g, 8.5 g, 8.0 g, 7.5 g, 7.0 g, 6.5 g, 6.0 g, 5.5 g, 5.0 g, 4.5 g, 4.0 g, 3.5 g, 3.0 g, 2.5 g, 2.0 g, 1.5 g, 1.0 g, 0.95 g, 0.9 g, 0.85 g, 0.8 g, 0.75 g, 0.7 g, 0.65 g, 0.6 g, 0.55 g, 0.5 g, 0.45 g, 0.4 g, 0.35 g, 0.3 g, 0.25 g, 0.2 g, 0.15 g, 0.1 g, 0.09 g, 0.08 g, 0.07 g, 0.06 g, 0.05 g, 0.04 g, 0.03 g, 0.02 g, 0.01 g, 0.009
- the amount of one or more compounds of the inventionis more than 0.0001 g, 0.0002 g, 0.0003 g, 0.0004 g, 0.0005 g, 0.0006 g, 0.0007 g, 0.0008 g, 0.0009 g, 0.001 g, 0.0015 g, 0.002 g, 0.0025 g, 0.003 g, 0.0035 g, 0.004 g, 0.0045 g, 0.005 g, 0.0055 g, 0.006 g, 0.0065 g, 0.007 g, 0.0075 g, 0.008 g, 0.0085 g, 0.009 g, 0.0095 g, 0.01 g, 0.015 g, 0.02 g, 0.025 g, 0.03 g, 0.035 g, 0.04 g, 0.045 g, 0.05 g, 0.055 g, 0.06 g, 0.065 g, 0.07 g,
- the amount of one or more compounds of the inventionis in the range of 0.0001-10 g, 0.0005-9 g, 0.001-8 g, 0.005-7 g, 0.01-6 g, 0.05-5 g, 0.1-4 g, 0.5-4 g, or 1-3 g.
- the compounds according to the inventionare effective over a wide dosage range. For example, in the treatment of adult humans, dosages from 0.01 to 1000 mg, from 0.5 to 100 mg, from 1 to 50 mg per day, and from 5 to 40 mg per day are examples of dosages that may be used. An exemplary dosage is 10 to 30 mg per day.
- a pharmaceutical composition of the inventiontypically contains an active ingredient (i.e., a compound of the disclosure) of the present invention or a pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, - 47 - 4877-0794-3771.1 105807.005004– PCT Application carriers, including but not limited to inert solid diluents and fillers, diluents, sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- the inventionprovides a pharmaceutical composition for oral administration containing a compound of the invention, and a pharmaceutical excipient suitable for oral administration.
- the inventionprovides a solid pharmaceutical composition for oral administration containing: (i) an effective amount of a compound of the invention; optionally (ii) an effective amount of a second agent; and (iii) a pharmaceutical excipient suitable for oral administration.
- the compositionfurther contains: (iv) an effective amount of a third agent.
- the pharmaceutical compositionmay be a liquid pharmaceutical composition suitable for oral consumption.
- compositions of the invention suitable for oral administrationcan be presented as discrete dosage forms, such as capsules, cachets, or tablets, or liquids or aerosol sprays each containing a predetermined amount of an active ingredient as a powder or in granules, a solution, or a suspension in an aqueous or non-aqueous liquid, an oil-in- water emulsion, or a water-in-oil liquid emulsion.
- dosage formscan be prepared by any of the methods of pharmacy, but all methods include the step of bringing the active ingredient into association with the carrier, which constitutes one or more necessary ingredients.
- compositionsare prepared by uniformly and intimately admixing the active ingredient with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product into the desired presentation.
- a tabletcan be prepared by compression or molding, optionally with one or more accessory ingredients.
- Compressed tabletscan be prepared by compressing in a suitable machine the active ingredient in a free- flowing form such as powder or granules, optionally mixed with an excipient such as, but not limited to, a binder, a lubricant, an inert diluent, and/or a surface active or dispersing agent.
- Molded tabletscan be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
- This inventionfurther encompasses anhydrous pharmaceutical compositions and dosage forms comprising an active ingredient, since water can facilitate the degradation of some - 48 - 4877-0794-3771.1 105807.005004– PCT Application compounds.
- watermay be added (e.g., 5%) in the pharmaceutical arts as a means of simulating long-term storage in order to determine characteristics such as shelf- life or the stability of formulations over time.
- Anhydrous pharmaceutical compositions and dosage forms of the inventioncan be prepared using anhydrous or low moisture containing ingredients and low moisture or low humidity conditions.
- compositions and dosage forms of the invention which contain lactosecan be made anhydrous if substantial contact with moisture and/or humidity during manufacturing, packaging, and/or storage is expected.
- An anhydrous pharmaceutical compositionmay be prepared and stored such that its anhydrous nature is maintained. Accordingly, anhydrous compositions may be packaged using materials known to prevent exposure to water such that they can be included in suitable formulary kits. Examples of suitable packaging include, but are not limited to, hermetically sealed foils, plastic or the like, unit dose containers, blister packs, and strip packs.
- An active ingredientcan be combined in an intimate admixture with a pharmaceutical carrier according to conventional pharmaceutical compounding techniques.
- the carriercan take a wide variety of forms depending on the form of preparation desired for administration.
- any of the usual pharmaceutical mediacan be employed as carriers, such as, for example, water, glycols, oils, alcohols, flavoring agents, preservatives, coloring agents, and the like in the case of oral liquid preparations (such as suspensions, solutions, and elixirs) or aerosols; or carriers such as starches, sugars, micro- crystalline cellulose, diluents, granulating agents, lubricants, binders, and disintegrating agents can be used in the case of oral solid preparations, in some embodiments without employing the use of lactose.
- suitable carriersinclude powders, capsules, and tablets, with the solid oral preparations.
- Binders suitable for use in pharmaceutical compositions and dosage formsinclude, but are not limited to, corn starch, potato starch, or other starches, gelatin, natural and synthetic gums such as acacia, sodium alginate, alginic acid, other alginates, powdered tragacanth, guar gum, cellulose and its derivatives (e.g., ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose), polyvinyl pyrrolidone, methyl cellulose, pre-gelatinized starch, hydroxy-propyl methyl cellulose, microcrystalline cellulose, and mixtures thereof.
- natural and synthetic gumssuch as acacia, sodium alginate, alginic acid, other alginates, powdered tragacanth, guar gum, cellulose and its derivatives (e.g., ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose), polyvinyl pyr
- suitable fillers for use in the pharmaceutical compositions and dosage forms disclosed hereininclude, but are not limited to, talc, calcium carbonate (e.g., granules or - 49 - 4877-0794-3771.1 105807.005004– PCT Application powder), microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch, and mixtures thereof.
- Disintegrantsmay be used in the compositions of the invention to provide tablets that disintegrate when exposed to an aqueous environment. Too much of a disintegrant may produce tablets which may disintegrate in the bottle.
- Too littlemay be insufficient for disintegration to occur and may thus alter the rate and extent of release of the active ingredient(s) from the dosage form.
- a sufficient amount of disintegrantthat is neither too little nor too much to detrimentally alter the release of the active ingredient(s) may be used to form the dosage forms of the compounds disclosed herein.
- the amount of disintegrant usedmay vary based upon the type of formulation and mode of administration, and may be readily discernible to those of ordinary skill in the art. About 0.5 to about 15 weight percent of disintegrant, or about 1 to about 5 weight percent of disintegrant, may be used in the pharmaceutical composition.
- Disintegrantsthat can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, agar-agar, alginic acid, calcium carbonate, microcrystalline cellulose, croscarmellose sodium, crospovidone, polacrilin potassium, sodium starch glycolate, potato or tapioca starch, other starches, pre-gelatinized starch, other starches, clays, other algins, other celluloses, gums or mixtures thereof.
- Lubricantswhich can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, calcium stearate, magnesium stearate, mineral oil, light mineral oil, glycerin, sorbitol, mannitol, polyethylene glycol, other glycols, stearic acid, sodium lauryl sulfate, talc, hydrogenated vegetable oil (e.g., peanut oil, cottonseed oil, sunflower oil, sesame oil, olive oil, corn oil, and soybean oil), zinc stearate, ethyl oleate, ethyl laureate, agar, or mixtures thereof.
- Additional lubricantsinclude, for example, a syloid silica gel, a coagulated aerosol of synthetic silica, or mixtures thereof.
- a lubricantcan optionally be added, in an amount of less than about 1 weight percent of the pharmaceutical composition.
- the active ingredient thereinmay be combined with various sweetening or flavoring agents, coloring matter or dyes and, if so desired, emulsifying and/or suspending agents, together with such diluents as water, ethanol, propylene glycol, glycerin and various combinations thereof.
- the tabletscan be uncoated or coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay materialsuch as glyceryl monostearate or glyceryl distearate can be employed.
- Formulations for oral usecan also be presented as hard gelatin capsules - 50 - 4877-0794-3771.1 105807.005004– PCT Application wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example, peanut oil, liquid paraffin or olive oil.
- Surfactant which can be used to form pharmaceutical compositions and dosage forms of the inventioninclude, but are not limited to, hydrophilic surfactants, lipophilic surfactants, and mixtures thereof. That is, a mixture of hydrophilic surfactants may be employed, a mixture of lipophilic surfactants may be employed, or a mixture of at least one hydrophilic surfactant and at least one lipophilic surfactant may be employed.
- a suitable hydrophilic surfactantmay generally have an HLB value of at least 10, while suitable lipophilic surfactants may generally have an HLB value of or less than about 10.
- HLBhydrophilic-lipophilic balance
- Surfactants with lower HLB valuesare more lipophilic or hydrophobic, and have greater solubility in oils, while surfactants with higher HLB values are more hydrophilic, and have greater solubility in aqueous solutions.
- Hydrophilic surfactantsare generally considered to be those compounds having an HLB value greater than about 10, as well as anionic, cationic, or zwitterionic compounds for which the HLB scale is not generally applicable.
- lipophilic (i.e., hydrophobic) surfactantsare compounds having an HLB value equal to or less than about 10.
- Hydrophilic surfactantsmay be either ionic or non-ionic. Suitable ionic surfactants include, but are not limited to, alkylammonium salts; fusidic acid salts; fatty acid derivatives of amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; lecithins and hydrogenated lecithins; lysolecithins and hydrogenated lysolecithins; phospholipids and derivatives thereof; lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acyl lactylates; mono- and di-acetylated tartaric acid esters of mono- and di-
- ionic surfactantsinclude, by way of example: lecithins, lysolecithin, phospholipids, lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acylactylates; mono- and - 51 - 4877-0794-3771.1 105807.005004– PCT Application di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di- glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof.
- Ionic surfactantsmay be the ionized forms of lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, phosphatidylserine, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, lysophosphatidylserine, PEG- phosphatidylethanolamine, PVP -phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholylsarcosine, caproate, capry
- Hydrophilic non-ionic surfactantsmay include, but are not limited to, alkylglucosides; alkylmaltosides; alkylthioglucosides; lauryl macrogolglycerides; polyoxyalkylene alkyl ethers such as polyethylene glycol alkyl ethers; polyoxyalkylene alkylphenols such as polyethylene glycol alkyl phenols; polyoxyalkylene alkyl phenol fatty acid esters such as polyethylene glycol fatty acids monoesters and polyethylene glycol fatty acids diesters; polyethylene glycol glycerol fatty acid esters; polyglycerol fatty acid esters; polyoxyalkylene sorbitan fatty acid esters such as polyethylene glycol sorbitan fatty acid esters; hydrophilic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids, and sterols; polyoxyethylene stea,
- the polyolmay be glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide.
- Other hydrophilic-non-ionic surfactantsinclude, without limitation, PEG-10 laurate, PEG- 12 laurate, PEG-20 laurate, PEG-32 laurate, PEG-32 dilaurate, PEG-12 oleate, PEG-15 oleate, PEG-20 oleate, PEG-20 dioleate, PEG-32 oleate, PEG-200 oleate, PEG-400 oleate, PEG- 15 stearate, PEG-32 distearate, PEG-40 stearate, PEG- 100 stearate, PEG-20 dilaurate, PEG-25 glyceryl trioleate, PEG-32 dioleate, PEG-20 glyceryl laurate, PEG-30 gly
- Suitable lipophilic surfactantsinclude, by way of example only: fatty alcohols; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; propylene glycol fatty acid esters; sorbitan fatty acid esters; polyethylene glycol sorbitan fatty acid esters; sterols and sterol derivatives; polyoxyethylated sterols and sterol derivatives; polyethylene glycol alkyl ethers; sugar esters; sugar ethers; lactic acid derivatives of mono- and di-glycerides; hydrophobic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids and sterols; oil-soluble vitamins/vitamin derivatives; and mixtures thereof.
- preferred lipophilic surfactantsinclude glycerol fatty acid esters, propylene glycol fatty acid esters, and mixtures thereof, or are hydrophobic transesterification products of a polyol with at least one member of the group consisting of vegetable oils, hydrogenated vegetable oils, and triglycerides.
- the compositionmay include a solubilizer to ensure good solubilization and/or dissolution of the compound of the present invention and to minimize precipitation of the compound of the present invention. This can be especially important for compositions for non-oral use, e.g., compositions for injection.
- a solubilizermay also be added to increase the solubility of the hydrophilic drug and/or other components, such as surfactants, or to maintain the composition as a stable or homogeneous solution or dispersion.
- suitable solubilizersinclude, but are not limited to, the following: alcohols and polyols, such as ethanol, isopropanol, butanol, benzyl alcohol, ethylene glycol, propylene glycol, butanediols and isomers thereof, glycerol, pentaerythritol, sorbitol, mannitol, transcutol, dimethyl isosorbide, polyethylene glycol, polypropylene glycol, polyvinylalcohol, hydroxypropyl methylcellulose and other cellulose derivatives, cyclodextrins and cyclodextrin - 53 - 4877-0794-3771.1 105807.005004– PCT Application derivatives; ethers of polyethylene
- solubilizersmay also be used. Examples include, but not limited to, triacetin, triethylcitrate, ethyl oleate, ethyl caprylate, dimethylacetamide, N-methylpyrrolidone, N-hydroxy-ethylpyrrolidone, polyvinylpyrrolidone, hydroxypropyl methylcellulose, hydroxypropyl cyclodextrins, ethanol, polyethylene glycol 200-100, glycofurol, transcutol, propylene glycol, and dimethyl isosorbide.
- solubilizersinclude sorbitol, glycerol, triacetin, ethyl alcohol, PEG-400, glycofurol and propylene glycol.
- the amount of solubilizer that can be includedis not particularly limited.
- the amount of a given solubilizermay be limited to a bioacceptable amount, which may be readily determined by one of skill in the art.
- the solubilizercan be in a weight ratio of 10%, 25%o, 50%), 100%o, or up to about 200%> by weight, based on the combined weight of the drug, and other excipients. If desired, very small amounts of solubilizer may also be used, such as 5%>, 2%>, 1%) or even less. Typically, the solubilizer may be present in an amount of about 1%> to about 100%, more typically about 5%> to about 25%> by weight.
- the compositioncan further include one or more pharmaceutically acceptable additives and excipients.
- additives and excipientsinclude, without limitation, detackifiers, anti-foaming agents, buffering agents, polymers, antioxidants, preservatives, chelating agents, viscomodulators, tonicifiers, flavorants, colorants, odorants, opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof.
- detackifiersanti-foaming agents
- buffering agentsbuffering agents
- polymersantioxidants
- preservativeschelating agents
- viscomodulatorstonicifiers
- flavorantscolorants
- odorantsopacifiers
- suspending agentsbinders
- fillersfillers
- plasticizersplasticizers
- lubricantsand mixtures thereof.
- an acid or a basemay be incorporated into the composition to facilitate processing, to enhance stability, or for other reasons.
- Examples of pharmaceutically acceptable basesinclude amino acids, amino acid esters, ammonium hydroxide, potassium hydroxide, sodium hydroxide, sodium hydrogen carbonate, aluminum hydroxide, calcium carbonate, magnesium hydroxide, magnesium aluminum silicate, synthetic aluminum silicate, synthetic hydrocalcite, magnesium aluminum hydroxide, diisopropylethylamine, ethanolamine, ethylenediamine, triethanolamine, triethylamine, triisopropanolamine, trimethylamine, tris(hydroxymethyl)-aminomethane (TRIS) and the like.
- amino acidsamino acid esters, ammonium hydroxide, potassium hydroxide, sodium hydroxide, sodium hydrogen carbonate, aluminum hydroxide, calcium carbonate, magnesium hydroxide, magnesium aluminum silicate, synthetic aluminum silicate, synthetic hydrocalcite, magnesium aluminum hydroxide, diisopropylethylamine, ethanolamine, ethylenediamine, triethanolamine, triethylamine, triisopropanolamine, trimethylamine, tris(hydroxymethyl)
- basesthat are salts of a pharmaceutically acceptable acid, such as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p-toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid, and the like.
- a pharmaceutically acceptable acidsuch as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids
- Salts of polyprotic acidssuch as sodium phosphate, disodium hydrogen phosphate, and sodium dihydrogen phosphate can also be used.
- the cationcan be any convenient and pharmaceutically acceptable cation, such as ammonium, alkali metals, alkaline earth metals, and the like.
- Examplemay include, but not limited to, sodium, potassium, lithium, magnesium, calcium and ammonium.
- Suitable acidsare pharmaceutically acceptable organic or inorganic acids. Examples of suitable inorganic acids include hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid, nitric acid, boric acid, phosphoric acid, and the like.
- suitable organic acidsinclude acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acids, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, methanesulfonic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p- toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid and the like.
- compositions for Injection[00175]
- the inventionprovides a pharmaceutical composition for injection containing a compound of the present invention and a pharmaceutical excipient suitable for injection.
- Components and amounts of agents in the compositionsare as described herein. - 55 - 4877-0794-3771.1 105807.005004– PCT Application
- the forms in which the novel compositions of the present invention may be incorporated for administration by injectioninclude aqueous or oil suspensions, or emulsions, with sesame oil, corn oil, cottonseed oil, or peanut oil, as well as elixirs, mannitol, dextrose, or a sterile aqueous solution, and similar pharmaceutical vehicles.
- Aqueous solutions in salineare also conventionally used for injection.
- Ethanol, glycerol, propylene glycol, liquid polyethylene glycol, and the like (and suitable mixtures thereof), cyclodextrin derivatives, and vegetable oilsmay also be employed.
- the proper fluiditycan be maintained, for example, by the use of a coating, such as lecithin, for the maintenance of the required particle size in the case of dispersion and by the use of surfactants.
- the prevention of the action of microorganismscan be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the like.
- Sterile injectable solutionsare prepared by incorporating the compound of the present invention in the required amount in the appropriate solvent with various other ingredients as enumerated above, as required, followed by filtered sterilization.
- dispersionsare prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above.
- certain desirable methods of preparationare vacuum-drying and freeze- drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile- filtered solution thereof.
- Pharmaceutical Compositions for Topical (e.g., Transdermal) Deliveryare prepared by incorporating the compound of the present invention in the required amount in the appropriate solvent with various other ingredients as enumerated above, as required, followed by filtered sterilization.
- dispersionsare prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above.
- certain desirable methods of preparationare vacuum-dry
- the inventionprovides a pharmaceutical composition for transdermal delivery containing a compound of the present invention and a pharmaceutical excipient suitable for transdermal delivery.
- Compositions of the present inventioncan be formulated into preparations in solid, semisolid, or liquid forms suitable for local or topical administration, such as gels, water soluble jellies, creams, lotions, suspensions, foams, powders, slurries, ointments, solutions, oils, pastes, suppositories, sprays, emulsions, saline solutions, dimethylsulfoxide (DMSO)-based solutions.
- DMSOdimethylsulfoxide
- carriers with higher densitiesare capable of providing an area with a prolonged exposure to the active ingredients.
- a solution formulationmay provide more immediate exposure of the active ingredient to the chosen area.
- the pharmaceutical compositionsalso may comprise suitable solid or gel phase carriers or excipients, which are compounds that allow increased penetration of, or assist in the delivery of, therapeutic molecules across the stratum corneum permeability barrier of the skin. There are many of these penetration- enhancing molecules known to those trained in the art of topical formulation.
- humectantse.g., urea
- glycolse.g., propylene glycol
- alcoholse.g., ethanol
- fatty acidse.g., oleic acid
- surfactantse.g., isopropyl myristate and sodium lauryl sulfate
- pyrrolidonese.g., isopropyl myristate and sodium lauryl sulfate
- pyrrolidonese.glycerol monolaurate, sulfoxides, terpenes (e.g., menthol)
- aminesamides, alkanes, alkanols, water, calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols.
- transdermal delivery devicesSuch transdermal patches may be used to provide continuous or discontinuous infusion of a compound of the present invention in controlled amounts, either with or without another agent.
- transdermal patchesfor the delivery of pharmaceutical agents is well known in the art. See, e.g., U.S. Pat. Nos.5,023,252, 4,992,445 and 5,001,139. Such patches may be constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents.
- Pharmaceutical Compositions for Inhalationare well known in the art.
- compositions for inhalation or insufflationinclude solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders.
- the liquid or solid compositionsmay contain suitable pharmaceutically acceptable excipients as described supra.
- the compositionsare administered by the oral or nasal respiratory route for local or systemic effect.
- Compositions in preferably pharmaceutically acceptable solventsmay be nebulized by use of inert gases. Nebulized solutions may be inhaled directly from the nebulizing device or the nebulizing device may be attached to a face mask tent, or intermittent positive pressure breathing machine. Solution, suspension, or powder compositions may be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner.
- Other Pharmaceutical Compositionsmay be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner.
- compositionsmay also be prepared from compositions described herein and one or more pharmaceutically acceptable excipients suitable for sublingual, buccal, rectal, intraosseous, intraocular, intranasal, epidural, or intraspinal administration. Preparations for such pharmaceutical compositions are well-known in the art.
- Administration of the compounds or pharmaceutical composition of the present inventioncan be affected by any method that enables delivery of the compounds to the site of action. These methods include oral routes, intraduodenal routes, parenteral injection (including intravenous, intraarterial, subcutaneous, intramuscular, intravascular, intraperitoneal or infusion), topical (e.g., transdermal application), rectal administration, via local delivery by catheter or stent or through inhalation. Compounds can also be administered intraadiposally or intrathecally. [00188] The amount of the compound administered will be dependent on the subject being treated, the severity of the disorder or condition, the rate of administration, the disposition of the compound and the discretion of the prescribing physician.
- an effective dosageis in the range of about 0.001 to about 100 mg per kg body weight per day, preferably about 1 to about 35 mg/kg/day, in single or divided doses. For a 70 kg human, this would amount to about 0.05 to 7 g/day, preferably about 0.05 to about 2.5 g/day. In some instances, dosage levels below the lower limit of the aforesaid range may be more than adequate, while in other cases still larger doses may be employed without causing any harmful side effect, e.g., by dividing such larger doses into several small doses for administration throughout the day. [00189] In some embodiments, a compound of the invention is administered in a single dose.
- a compound of the inventionis administered in multiple doses. Dosing may be about once, twice, three times, four times, five times, six times, or more than six - 58 - 4877-0794-3771.1 105807.005004– PCT Application times per day. Dosing may be about once a month, once every two weeks, once a week, or once every other day.
- a compound of the invention and another agentare administered together about once per day to about 6 times per day.
- the administration of a compound of the invention and an agentcontinues for less than about 7 days.
- the administrationcontinues for more than about 6, 10, 14, 28 days, two months, six months, or one year. In some cases, continuous dosing is achieved and maintained as long as necessary.
- Administration of the compounds of the inventionmay continue as long as necessary.
- a compound of the inventionis administered for more than 1, 2, 3, 4, 5, 6, 7, 14, or 28 days.
- a compound of the inventionis administered for less than 28, 14, 7, 6, 5, 4, 3, 2, or 1 day.
- a compound of the inventionis administered chronically on an ongoing basis, e.g., for the treatment of chronic effects.
- An effective amount of a compound of the inventionmay be administered in either single or multiple doses by any of the accepted modes of administration of agents having similar utilities, including rectal, buccal, intranasal and transdermal routes, by intra-arterial injection, intravenously, intraperitoneally, parenterally, intramuscularly, subcutaneously, orally, topically, or as an inhalant.
- the compositions of the inventionmay also be delivered via an impregnated or coated device such as a stent, for example, or an artery-inserted cylindrical polymer.
- Such a method of administrationmay, for example, aid in the prevention or amelioration of restenosis following procedures such as balloon angioplasty.
- compounds of the inventionmay slow or inhibit the migration and proliferation of smooth muscle cells in the arterial wall which contribute to restenosis.
- a compound of the inventionmay be administered, for example, by local delivery from the struts of a stent, from a stent graft, from grafts, or from the cover or sheath of a stent.
- a compound of the inventionis admixed with a matrix.
- a matrixmay be a polymeric matrix and may serve to bond the compound to the stent.
- Polymeric matrices suitable for such useinclude, for example, lactone-based polyesters or copolyesters such as polylactide, polycaprolactonglycolide, polyorthoesters, polyanhydrides, polyaminoacids, polysaccharides, polyphosphazenes, poly (ether-ester) copolymers (e.g. PEO-PLLA); polydimethylsiloxane, poly(ethylene-vinylacetate), acrylate-based polymers or copolymers (e.g. polyhydroxyethyl methylmethacrylate, polyvinyl pyrrolidinone), fluorinated polymers such as polytetrafluoroethylene and cellulose esters.
- lactone-based polyesters or copolyesterssuch as polylactide, polycaprolactonglycolide, polyorthoesters, polyanhydrides, polyaminoacids, polysaccharides, polyphosphazenes, poly (ether-ester) copo
- Suitable matricesmay be nondegrading or may degrade with time, releasing the compound or compounds.
- Application of the inventionmay be applied to the surface of the stent by various methods such as dip/spin coating, spray coating, dip-coating, and/or brush-coating.
- the compoundsmay be applied in a solvent and the solvent may be allowed to evaporate, thus forming a layer of compound onto the stent.
- the compoundmay be located in the body of the stent or graft, for example in microchannels or micropores. When implanted, the compound diffuses out of the body of the stent to contact the arterial wall.
- Such stentsmay be prepared by dipping a stent manufactured to contain such micropores or microchannels into a solution of the compound of the invention in a suitable solvent, followed by evaporation of the solvent. Excess drug on the surface of the stent may be removed via an additional brief solvent wash.
- compounds of the inventionmay be covalently linked to a stent or graft.
- a covalent linkermay be used which degrades in vivo, leading to the release of the compound of the invention. Any bio-labile linkage may be used for such a purpose, such as ester, amide or anhydride linkages.
- Compounds of the inventionmay additionally be administered intravascularly from a balloon used during angioplasty.
- Extravascular administration of the compounds via the pericard or via advential application of formulations of the inventionmay also be performed to decrease restenosis.
- a variety of stent devices which may be used as describedare disclosed, for example, in the following references, all of which are hereby incorporated by reference: U.S. Pat. No. 5451233; U.S. Pat. No.5040548; U.S. Pat. No.5061273; U.S. Pat. No.5496346; U.S. Pat. No. 5292331; U.S. Pat. No.5674278; U.S. Pat. No.3657744; U.S. Pat. No.4739762; U.S. Pat. No. 5195984; U.S.
- the compounds of the inventionmay be administered in dosages. It is known in the art that due to intersubject variability in compound pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy. Dosing for a compound of the invention may be found by routine experimentation in light of the instant disclosure.
- a compound of the inventionis administered in a composition that comprises one or more agents, and the agent has a shorter half- life than the compound of the invention unit dose forms of the agent and the compound of the invention may be adjusted accordingly.
- the subject pharmaceutical compositionmay, for example, be in a form suitable for oral administration as a tablet, capsule, pill, powder, sustained release formulations, solution, suspension, for parenteral injection as a sterile solution, suspension or emulsion, for topical administration as an ointment or cream or for rectal administration as a suppository.
- the pharmaceutical compositionmay be in unit dosage forms suitable for single administration of - 60 - 4877-0794-3771.1 105807.005004– PCT Application precise dosages.
- the pharmaceutical compositionwill include a conventional pharmaceutical carrier or excipient and a compound according to the invention as an active ingredient. In addition, it may include other medicinal or pharmaceutical agents, carriers, adjuvants, etc.
- Exemplary parenteral administration formsinclude solutions or suspensions of active compound in sterile aqueous solutions, for example, aqueous propylene glycol or dextrose solutions. Such dosage forms can be suitably buffered, if desired.
- Methods of UseThe method typically comprises administering to a subject a therapeutically effective amount of a compound of the invention.
- the therapeutically effective amount of the subject combination of compoundsmay vary depending upon the intended application (in vitro or in vivo), or the subject and disease condition being treated, e.g., the weight and age of the subject, the severity of the disease condition, the manner of administration and the like, which can readily be determined by one of ordinary skill in the art.
- IC 50refers to the half maximal inhibitory concentration of an inhibitor in inhibiting biological or biochemical function. This quantitative measure indicates how much of a particular inhibitor is needed to inhibit a given biological process (or component of a process, i.e., an enzyme, cell, cell receptor or microorganism) by half.
- the present disclosureprovides a method of modulating PI3K (e.g., PI3K ⁇ ) activity (e.g., in vitro or in vivo), comprising contacting a cell with a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N- oxide, or stereoisomer thereof.
- PI3Ke.g., PI3K ⁇
- a method of modulating PI3K activitye.g., PI3K ⁇ activity (e.g., in vitro or in vivo), comprising contacting a cell with a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N- oxide, or stereoisomer thereof.
- the present disclosureprovides a method of treating or preventing a disease or disorder disclosed herein in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a - 61 - 4877-0794-3771.1 105807.005004– PCT Application pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating a disease or disorder disclosed herein in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the disease or disorderis associated with an implicated PI3K activity.
- the disease or disorderis a disease or disorder in which PI3K activity is implicated.
- the disease or disorderis a cancer.
- the canceris selected from acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), adrenocortical carcinoma, aids-related cancers, aids- related lymphoma, anal cancer, astrocytoma, basal cell carcinoma, bile duct cancer, bladder cancer, bone cancer, osteosarcoma, malignant fibrous histiocytoma, brain tumors, breast cancer, bronchial tumors, Burkitt lymphoma, carcinoid tumor, cancer of unknown primary, cardiac (heart) tumors, atypical teratoid/rhabdoid tumor, primary CNS lymphoma, cervical cancer, cholangiocarcinoma, chordoma, chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), colorectal cancer, craniopharyngioma, cutaneous t-cell lymphoma, mycosis fungoides, Sezary syndrome
- ALLacute lymphoblastic
- the canceris Endometrial cancer, Breast cancer, oesophageal squamous-cell cancer, Cervical squamous-cell carcinoma, Cervical adenocarcinoma, Colorectal adenocarcinoma, Bladder Urothelial Carcinoma, Glioblastoma, Ovarian cancer, Non-small-cell Lung cancer, Esophagogastric cancer, Nerve-sheath tumor, Head and neck squamous-cell carcinoma, Melanoma, Esophagogastric adenocarcinoma, Soft-tissue sarcoma, Prostate cancer, Fibrolamellar carcinoma, Hepatocellular carcinoma, Diffuse glioma, Colorectal cancer, Pancreatic cancer, Cholangiocarcinoma, B-cell lymphoma, Mesothelioma, Adrenocortical carcinoma, Renal non-clear-cell carcinoma, Renal clear-cell carcinoma,
- the canceris endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, head and neck cancer, breast cancer, brain cancer, cervical cancer, bladder cancer, esophageal cancer, pancreatic cancer, bone cancer, hepatobiliary cancer, medulloblastoma, kidney cancer or prostate cancer.
- the canceris a breast cancer, a prostate cancer, or a brain cancer.
- the canceris a breast cancer.
- the canceris a prostate cancer.
- the canceris a brain cancer.
- the breast canceris metastatic breast cancer.
- the breast canceris ductal carcinoma in situ (DCIS).
- the breast canceris invasive ductal carcinoma.
- the breast canceris triple - 63 - 4877-0794-3771.1 105807.005004– PCT Application negative breast cancer.
- the breast canceris medullary carcinoma.
- the breast canceris tubular carcinoma.
- the breast canceris mucinous carcinoma.
- the breast canceris Paget disease of the breast or nipple.
- the breast canceris inflammatory breast cancer (IBC).
- the prostate canceris an adenocarcinoma. In some embodiments, the prostate cancer is a small cell carcinoma. In some embodiments, the prostate cancer is a neuroendocrine tumor. In some embodiments, the prostate cancer is a transitional cell carcinoma. In some embodiments, the prostate cancer is a sarcoma. [00213] In some embodiments, the brain cancer is an acoustic neuroma. In some embodiments, the brain cancer is an astrocytoma. In some embodiments, the brain cancer is a brain metastasis. In some embodiments, the brain cancer is choroid plexus carcinoma. In some embodiments, the brain cancer is craniopharyngioma.
- the brain canceris an embryonal tumor. In some embodiments, the brain cancer is an ependymoma. In some embodiments, the brain cancer is a glioblastoma. In some embodiments, the brain cancer is a glioma. In some embodiments, the brain cancer is a medulloblastoma. In some embodiments, the brain cancer is a meningioma. In some embodiments, the brain cancer is an oligodendroglioma. In some embodiments, the brain cancer is a pediatric brain tumor. In some embodiments, the brain cancer is a pineoblastoma. In some embodiments, the brain cancer is a pituitary tumor.
- the disease or disorder associated with PI3Kincludes, but is not limited to, CLOVES syndrome (congenial lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome), PIK3CA-related overgrowth syndrome (PROS), breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer.
- CLOVES syndromecongenial lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome
- PROSPIK3CA-related overgrowth syndrome
- the diseases or disorder associated with PI3Kis CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome).
- CLOVES syndromecongenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome.
- the disease or disorder associated with PI3Kis PIK3CA-related overgrowth syndrome (PROS).
- the disease or disorder associated with PI3Kis breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer.
- the disease or disorder associated with PI3Kis breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer.
- the disease or disorder associated with PI3Kis leukemia, lymphoma, or sarcoma.
- the canceris endometrial cancer, head and neck cancer, or a sarcoma.
- the canceris endometrial cancer. In some embodiments the cancer is head and neck cancer.
- the canceris a sarcoma.
- the sarcomais soft tissue sarcoma, osteosarcoma, chondrosarcoma, Ewing sarcoma, hemangioendothelioma, angiosarcoma, fibrosarcoma, myofibrosarcoma, chordoma, adamantinoma, liposarcoma, leiomyosarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, synovial sarcoma, or malignant solitary fibrous tumor.
- the sarcomais soft tissue sarcoma.
- the soft tissue sarcomais liposarcoma, atypical lipomatous tumor, dermatofibrosarcoma protuberans, malignant solitary fibrous tumor, inflammatory myofibroblastic tumor, low-grade myofibroblastic sarcoma, fibrosarcoma, myxofibrosarcoma, low-grade fibromyxoid sarcoma, giant cell tumor of soft tissues, leiomyosarcoma, malignant glomus tumor, rhabdomyosarcoma, hemangioendothelioma, angiosarcoma of soft tissue, extraskeletal osteosarcoma, gastrointestinal stromal tumor, malignant gastrointestinal stromal tumor (GIST), malignant peripheral nerve sheath tumor, malignant Triton tumor, malignant granular cell tumor, malignant ossifying fibromyxoid tumor, stromal sarcoma, myoepithelial carcinoma, malignant phosphaturic mesenchy
- the present disclosureprovides a method of treating or preventing a cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N- oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereofor a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating or preventing a breast cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating a breast cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating or preventing a prostate cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating a prostate cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating or preventing a brain cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a method of treating a brain cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof thereof for use in modulating PI3K (e.g., PI3K ⁇ ) activity (e.g., in vitro or in vivo).
- PI3Ke.g., PI3K ⁇
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a disease or disorder disclosed herein.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a disease or disorder disclosed herein.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a breast cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a breast cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a prostate cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a prostate cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a brain cancer in a subject in need thereof.
- the present disclosureprovides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a brain cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described oerein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for modulating PI3K (e.g., PI3K ⁇ ) activity (e.g., in vitro or in vivo).
- PI3Ke.g., PI3K ⁇
- the present disclosureprovides use of a compound as described oerein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a disease or disorder disclosed herein.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a disease or disorder disclosed herein.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a breast cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a breast cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a prostate cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a prostate cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a brain cancer in a subject in need thereof.
- the present disclosureprovides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a brain cancer in a subject in need thereof.
- the present disclosureprovides compounds that function as modulators of PI3K activity.
- the present disclosuretherefore provides a method of modulating PI3K activity in vitro or in vivo, said method comprising contacting a cell with a therapeutically effective amount of a compound, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, as defined herein.
- PI3Kis modulation is inhibition of PI3K.
- the PI3K inhibitoris a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof.
- the PI3K inhibitoris a PI3K ⁇ inhibitor. In some embodiments, the PI3K inhibitor is a PI3K ⁇ H1047R mutant inhibitor. In some embodiments, the PI3K inhibitor is alpha/beta non-selective. In some embodiments, the PI3K inhibitor is alpha selective. In some embodiments, the PI3K inhibitor is beta selective. [00257] Effectiveness of compounds of the disclosure can be determined by industry-accepted assays/disease models according to standard practices of elucidating the same as described in the art and are found in the current general knowledge.
- the present disclosurealso provides a method of treating a disease or disorder in which PI3K activity is implicated in a patient in need of such treatment, said method comprising administering to said patient a therapeutically effective amount of a compound, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition as defined herein.
- the disclosureprovides a method of modulating the activity of the PI3K ⁇ allosteric active site, wherein the modulation is induced through peripheral site targeting.
- the peripheral siteis targeted with an agent selected from a small molecule, a peptide, a peptidomimetic, a protein, a protein mimetic, a nucleic acid, an antibody, an antibody- drug conjugate, a nucleoprotein complex, an immunotherapy, or a combination thereof.
- an agentselected from a small molecule, a peptide, a peptidomimetic, a protein, a protein mimetic, a nucleic acid, an antibody, an antibody- drug conjugate, a nucleoprotein complex, an immunotherapy, or a combination thereof.
- Suitable solventscan be substantially nonreactive with the starting materials (reactants), the - 69 - 4877-0794-3771.1 105807.005004– PCT Application intermediates, or products at the temperatures at which the reactions are carried out, e.g., temperatures which can range from the solvent's freezing temperature to the solvent's boiling temperature.
- a given reactioncan be carried out in one solvent or a mixture of more than one solvent.
- suitable solvents for a particular reaction stepcan be selected by the skilled artisan.
- Preparation of compounds of the inventioncan involve the protection and deprotection of various chemical groups. The need for protection and deprotection, and the selection of appropriate protecting groups, can be readily determined by one skilled in the art.
- Reactionscan be monitored according to any suitable method known in the art.
- product formationcan be monitored by spectroscopic means, such as nuclear magnetic resonance spectroscopy (e.g., 1 H or 13 C), infrared spectroscopy, spectrophotometry (e.g., UV- visible), or mass spectrometry, or by chromatography such as high performance liquid chromatography (HPLC) or thin layer chromatography.
- spectroscopic meanssuch as nuclear magnetic resonance spectroscopy (e.g., 1 H or 13 C), infrared spectroscopy, spectrophotometry (e.g., UV- visible), or mass spectrometry
- HPLChigh performance liquid chromatography
- Lgis halogen (e.g., Cl, Br, or I) or pseudohalogen (e.g., OTf, OTs, or OMs)
- halogene.g., Cl, Br, or I
- pseudohalogene.g., OTf, OTs, or OMs
- palladium(0) catalystsuch as bis(triphenylphosphine)palladium(II) dichloride and an appropriate stannane, such as tributyl(1-ethoxyvinyl)tin
- Heck conditionse.g., palladium catalyst, such as palladium(II) acetate, a ligand, such as 1,3-bis(diphenylphosphino)propane, and an appropriate olefin, such as butyl vinyl ether
- carbonylation coupling conditionse.g., palladium catalyst, such as tetrakis(triphenylphosphine)palladium(0),
- Compounds 1-2can be converted to compounds 1-4 under either reductive conditions (e.g., in the presence of a suitable reducing agent, such as sodium borohydride) or nucleophilic addition conditions with nucleophiles 1-3 where M 1 is a metal (e.g., Li, MgCl, MgBr, ZnCl, or ZnBr).
- a suitable reducing agentsuch as sodium borohydride
- nucleophiles 1-3 where M 1 is a metale.g., Li, MgCl, MgBr, ZnCl, or ZnBr.
- Alcohols 1-4can be transformed to compounds 1-5 where Lg 1 is halogen (e.g., Cl, Br, or I) under standard deoxygenative halogenation conditions (e.g., thionyl chloride, phosphorous tribromide, or triphenylphosphine and iodine) or a pseudohalogen (e.g., OTf, OTs, or OMs) under standard sulfonylation conditions (e.g., in the presence of a sulfonylating agent, such as methanesulfonyl chloride, p-toluenesulfonyl chloride, or trifluoromethanesulfonic anhydride, and a base, such as triethylamine).
- halogene.g., Cl, Br, or I
- deoxygenative halogenation conditionse.g., thionyl chloride, phosphorous tribromide, or triphenylpho
- Compounds 2-1 where Lg 3 is halogen (e.g., Cl, Br, or I) or pseudohalogen (e.g., OTf or OMs)can be coupled with an amine 2-2 under standard amide bond formation conditions such as in the presence of an amide coupling reagent (e.g., N,N’-cabonyldiimidazole or HATU) and a base (e.g., diisopropylethylamine) to provide compounds 2-3.
- an amide coupling reagente.g., N,N’-cabonyldiimidazole or HATU
- a basee.g., diisopropylethylamine
- Anilines 2-3can be converted to heterocycles 2-4 under standard acylation conditions (e.g., in the presence of N,N’- cabonyldiimidazole or triphosgene and a suitable base, such as 1,8-diazabicyclo[5.4.0]undec-7- ene).
- Diones 2-4can be halogenated with suitable reagents, such as phosphoryl chloride or phosphoryl bromide, to provide compounds 2-5 where X 2 is halogen (e.g., Cl or Br).
- Reaction of heterocycles 2-4 with amines 2-6 in the presence of an amide coupling reagente.g., (benzotriazol-1-yl-oxy)tri-pyrrolidinophosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate
- an amide coupling reagente.g., (benzotriazol-1-yl-oxy)tri-pyrrolidinophosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate
- a basee.g., 1,8- diazabicyclo[5.4.0] undec-7-ene or triethylamine
- reaction of heterocycles 3-1 with amines 2-6 in the presence of an amide coupling reagente.g., (benzotriazol-1-yl-oxy)tri-pyrrolidino-phosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate
- an amide coupling reagente.g., (benzotriazol-1-yl-oxy)tri-pyrrolidino-phosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate
- a basee.g., 1,8-diazabicyclo[5.4.0] undec-7-ene or triethylamine
- Compounds 2-4can be transformed into compounds 3-1 under standard Stille conditions (e.g., palladium(0) catalyst, such as bis(triphenylphosphine)palladium(II) dichloride and an appropriate stannane, such as tri-n-butyl(1-ethoxyvinyl)tin), or Heck conditions (e.g., palladium catalyst, such as palladium(II) acetate, a ligand, such as 1,3-bis(diphenylphosphino)propane, and an appropriate olefin, such as butyl vinyl ether), or carbonylation coupling conditions (e.g., palladium catalyst, such as tetrakis(triphenylphosphine)palladium(0), a carbonyl source, such as carbon monoxide, and suitable boronic acid, such as phenylboronic acid).
- palladium(0) catalystsuch as bis(triphenylphosphine)palladium(II) dich
- Compounds 3-1can be converted to compounds 4-1 under reductive conditions (e.g., in the presence of a suitable reducing agent, such as sodium borohydride).
- Alcohols 4-1can be transformed to compounds 4-2 where Lg 2 is halogen (e.g., Cl, Br, or I) under standard deoxygenative halogenation conditions (e.g., thionyl chloride, phosphorous tribromide, or triphenylphosphine and iodine) or a pseudohalogen (e.g., OTf, OTs, or OMs) under standard sulfonylation conditions (e.g., in the presence of a sulfonylating agent, such as methanesulfonyl chloride, p-toluenesulfonyl chloride, or trifluoromethanesulfonic anhydride, and a base, such as triethylamine).
- halogene.g., Cl
- Scheme IV- 73 - 4877-0794-3771.1 105807.005004– PCT Application [00271]
- Compounds of Formula (I)can be prepared from as shown in Scheme V. Annulation of amino acids 5-1 where Z is CR 5 or N can afford bicycles 5-2. Reaction of compounds 5-2 with alcohols 5-3, which can be prepared as described in Scheme I, under standard Mitsunobu conditions, such as in the presence of an azodicarboxylate (e.g., diisopropyl azodicarboxylate) and a phosphine (e.g., triphenylphosphine) can afford compounds 5-4.
- an azodicarboxylatee.g., diisopropyl azodicarboxylate
- a phosphinee.g., triphenylphosphine
- a Lewis acid and an oxidante.g., FeCl3 or TFA and air
- amide coupling of anilines 2-3 with carboxylic acids 7-3 where W 1 is W and wherein this group is connected to the carboxylic acid functional group through a carbon atom of the W group under standard conditions, such as in the presence of an amide coupling reagent (e.g., EDC or HATU) and a base (e.g., 4- dimethylaminopyridine or triethylamine)can provide amides 7-4.
- an amide coupling reagente.g., EDC or HATU
- a basee.g., 4- dimethylaminopyridine or triethylamine
- Cyclization of compounds 7-4 under various conditionssuch as in the presence of an acid (e.g., p-toluenesulfonic acid or sulfuric acid), in the presence of a base (e.g., cesium carbonate or sodium hydroxide), in the presence of a dehydrating agent (e.g., acetic anhydride), or in the presence of I 2 and hexamethyldisilazane, can provide quinazolin-4(3H)-ones 7-2.
- an acide.g., p-toluenesulfonic acid or sulfuric acid
- a basee.g., cesium carbonate or sodium hydroxide
- a dehydrating agente.g., acetic anhydride
- I 2 and hexamethyldisilazanecan provide quinazolin-4(3H)-ones 7-2.
- Step 1.2-Amino-3-bromo-N,5-dimethylbenzamideTo a solution of 2-amino-3-bromo-5-methylbenzoic acid (2.00 g, 8.69 mmol) in THF (20.0 mL) was added 1,1'-carbonyldiimidazole (1.55 g, 9.56 mmol), and the resulting reaction mixture was stirred at room temperature for 12 h.
- Step 3.8-Bromo-2-chloro-3,6-dimethylquinazolin-4(3H)-oneN,N-Diisopropylethylamine (0.64 mL, 3.68 mmol) was added dropwise to a mixture of 8-bromo-3,6-dimethylquinazoline-2,4(1H,3H)-dione (495 mg, 1.84 mmol) in phosphorous oxychloride (12.4 mL, 132 mmol) at room temperature. The heterogeneous mixture was heated at 95 °C for 16 h. The reaction was cooled to room temperature and then concentrated.
- 3-Azabicyclo[3.1.0]hexane hydrochloride620 mg, 5.2 mmol
- 8-bromo-2-chloro-3,6-dimethylquinazolin-4(3H)-one750 mg, 2.6 mmol
- triethylamine - 78 - 4877-0794-3771.1 105807.005004– PCT Application(1.1 mL, 7.8 mmol) in THF (26 mL) and the reaction mixture was heated to 70 oC for 18 h.
- Sodium borohydride200 mg, 5.2 mmol
- 8-acetyl-2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethylquinazolin-4(3H)-one780 mg, 2.6 mmol
- the mixturewas diluted with DCM and water.
- Phosphorous tribromide(0.35 mL, 3.8 mmol) was added to a solution of crude 2-(3- azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)-one (450 mg) in DCM (7.5 mL), and the solution was stirred for 1 h.
- the reaction mixturewas cooled to room temperature, diluted with MeCN, and filtered.
- the crude solutionwas purified by prep-HPLC on a CSH-FP column (60-80% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (32 mg, 36 ⁇ mol, 16% yield).
- reaction mixturewas diluted with MeCN, filtered, and purified by prep-HPLC on a C18 column (60-80% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (8.2 mg, 12 ⁇ mol, 40% yield).
- tert-butyl N-[rac-(1R,5S)-3-(8-acetyl-3,6-dimethyl-4-oxoquinazolin- 2-yl)-3-azabicyclo[3.1.0]hexan-6-yl]carbamate140 mg, 0.339 mmol
- DCM3 mL
- TFA0.519 mL, 6.79 mmol
- 6-chloro-1H-pyrido[3,2-d][1,3]oxazine-2,4-dione(21.3 mg, 0.107 mmol) in THF (0.5 mL) was added triphenylphosphine (28.2 mg, 0.107 mmol) and diisopropyl azodicarboxylate (21.1 ⁇ L, 0.107 mmol).
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 6.
- LC-MS calc. for C16H17F3N3O2 [M+H] + : m/z340.1; Found: 340.0.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 7.
- LC-MS calc. for C22H22F3N5O3 [M+H] + : m/z497.1; Found: 496.9.
- Example 5Example 5
- tert-butyl dimethylchlorosilane208 mg, 1.38 mmol
- Step 4.8-Acetyl-6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methylquinazolin-4(3H)-one [00321] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 5. LC-MS calc. for C16H17FN3O3 [M+H] + : m/z318.1; Found: 318.0.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 3, Step 5.
- the reaction mixturewas bubbled with nitrogen for 1 min and stirred at 100 °C overnight.
- the reactionwas quenched with KF solution and stirred at room temperature for 1 h.
- the solidwas removed by filtration.
- the solutionwas diluted with DCM (10 mL), washed with water (10 mL) and brine (10 mL), dried over Na 2 SO 4 , filtered, and concentrated.
- the residuewas dissolved in THF (10 mL) and HCl (1 N, 5.0 mL, 5.0 mmol) was added.
- the resulting mixturewas stirred for 0.5 h.
- the reactionwas diluted with water (2 mL) and extracted with DCM (2 mL x 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 6.
- LC-MS calc. for C 23 H 36 N 3 O 3 Si [M+H] + m/z430.3; found 430.4.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 2.
- LC-MS calc. for C16H17ClN3O2 [M+H]+ m/z318.1; Found 318.3.
- Step 42-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-8-(1-hydroxyethyl)-3- methylquinazolin-4(3H)-one [00351]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 6.
- LC-MS calc. for C16H19ClN3O2 [M+H] + : m/z320.1; Found 320.3.
- Step 53-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00353]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 7.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 2 and Example 1, Step 6.
- LC-MS calc. for C18H24N3O3 [M+H] + m/z330.2; Found 330.4.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 5.
- Step 3.2,3-Bis(bromomethyl)pyrazineA mixture of 2,3-dimethylpyrazine (3.00 g, 27.7 mmol), N-bromosuccinimide (9.38 g, 52.7 mmol) and azobisisobutyronitrile (0.460 g, 2.77 mmol) in benzotrifluoride (30 mL) was stirred at 90 °C for 2 h. The mixture was filtered, and the organic layer was concentrated. The residue was purified by silica gel chromatography (0–5% EtOAc/hexane) to afford the title compound (400 mg, 1.3 mmol, 5.0% yield), a brown oil. LC-MS calc.
- Step 4.6-Trityl-5,7-dihydropyrrolo[3,4-b]pyrazineA mixture of 2,3-bis(bromomethyl)pyrazine (400 mg, 1.5 mmol), triphenylmethanamine (351 mg, 1.35 mmol) and N,N-diisopropylethylamine (583 mg, 4.51 mmol) in DMF (5 mL) was stirred at 60 °C for 12 h. The mixture was cooled to room temperature, and water (30 mL) was added.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 2, Step 3.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 2, Step 3.
- Step 2Methyl (1R,5S,6r)-3-(8-(1-hydroxyethyl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6-carboxylate [00389] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2, and Example 1, Step 6.
- Step 36-Chloro-3-((1-(2-((1R,5S,6r)-6-(methoxycarbonyl)-3-azabicyclo[3.1.0] hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00391]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 7.
- LC-MS calc. for C 25 H 27 ClN 5 O 5 [M+H] + m/z512.1; Found 512.3.
- Step 4Methyl (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylate [00393] To a solution of 6-chloro-3-((1-(2-((1R,5S,6r)-6-(methoxycarbonyl)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid (260 mg, 0.51 mmol) in toluene (3 mL) was added 1,1-di-tert- butoxytriamino)pic
- Step 5(1R,5S,6r)-3-(8-(1-((2-(tert-Butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid [00395] To a solution of methyl (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin- 3-yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo [3.1.0]hexane- 6-carboxylate (180 mg, 0.32 mmol) in THF (2 mL) was added aq.
- Step 66-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6r)-6-((2,2,2- trifluoroacetoxy)methyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl) amino)picolinic acid [00397] To a solution of (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid (25.3 mg, 0.0457 mmol) in THF (1 mL) was added borane dimethyl sulfide - 102
- Step 76-Chloro-3-((1-(2-((1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo [3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00399] To a solution of 6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6r)-6-((2,2,2- trifluoroacetoxy)methyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid (10.0 mg, 0.0172 mmol) in methanol (1 mL) was added potassium carbonate (7.15 mg, 0.0517 mmol).
- Step 36-Chloro-3-[1-[3,6-dimethyl-2-[1-[(2-methylpropan-2- yl)oxycarbonyl]pyrrolidin-3-yl]-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00405]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 5.
- Step 3Tert-Butyl (1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexane-3-carboxylate
- Step 35-Bromo-7-methyl-3-phenyl-2H-isoquinolin-1-one - 108 - 4877-0794-3771.1 105807.005004– PCT Application [00426] To a solution of [(3-bromo-5-methylbenzoyl)amino] 2,2-dimethylpropanoate (388 mg, 1.23 mmol) in methanol (4 mL) was added phenylacetylene (0.176 mL, 1.61 mmol), bis[(pentamethylcyclopentadienyl)dichloro-rhodium] (153 mg, 0.247 mmol), and cesium acetate (593 mg, 3.09 mmol).
- Step 45-Bromo-2,7-dimethyl-3-phenylisoquinolin-1-one
- CMFcesium carbonate
- iodomethane0.0594 mL, 0.955 mmol
- Step 55-(1-Hydroxyethyl)-2,7-dimethyl-3-phenylisoquinolin-1-one - 109 - 4877-0794-3771.1 105807.005004– PCT Application [00430]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 1 and Example 1, Step 6.
- Step 66-Chloro-3-[1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethyl- amino]pyridine-2-carboxylic acid and 1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethyl 3- amino-6-chloropyridine-2-carboxylate [00432]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Step 7.
- Step 28-(1-Hydroxyethyl)-3,6-dimethyl-2-(1-methylpyrazol-4-yl)quinazolin-4-one
- the title compoundwas synthesized by procedures analogous to those outlined in Example 8, Step 2 and Example 1, Step 6.
- Step 36-Chloro-3-[1-[3,6-dimethyl-2-(1-methylpyrazol-4-yl)-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid [00438] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 3, Step 5.
- the crude reaction mixturewas purified by prep-HPLC on a C18 column (15–20% MeCN/0.1% NH3 (aq.)) to afford the title compound as an ammonium salt.
- the reaction mixturewas quenched with ice-cold water (10 mL) and diluted with DCM (10 mL). The layers were separated, and the aqueous layer was extracted with DCM (2 x 10 mL). The combined organic layers were washed with brine (20 mL), dried over Na 2 SO 4 , filtered, and concentrated. The concentrated crude material was redissolved in DCM (3 mL), and to this were added tert-butyl 3- amino-6-chloropyridine-2-carboxylate (138 mg, 0.604 mmol), N,N-diisopropylethylamine (351 ⁇ L, 2.01 mmol). The reaction mixture was stirred at 40 °C overnight and then cooled to room temperature.
- reaction mixturewas concentrated, diluted with MeCN and water, and purified by prep-HPLC on a C18 column (5–17% MeCN/0.1% NH3 (aq.)) to afford the title compound as an ammonium salt (1.81 mg, 0.004 mmol, 9.90% yield), a white solid.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 24, Step 2.
- the crude materialwas purified by prep-HPLC on a fluoro-phenyl column (24–36% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 3, Step 5.
- the crude reaction mixturewas purified by prep-HPLC on a C18 column (12.3–32.3% MeCN/0.15% NH3 (aq.)) to afford the title compound as an ammonium salt.
- Examples 27 – 29[00474] Examples 27 – 29 listed in Tables 1 and 2 were synthesized according to procedures analogous to Example 26. All examples in this table were prepared as ammonium salts. Table 1. Examples 27 – 29 Table 2. Examples 27 – 29 - 119 - 4877-0794-3771.1 105807.005004– PCT Application Example 30.
- LiOH22 ⁇ L, 22 ⁇ mol, 1M (aq.)
- TFA salt2.0 mg, 3.5 ⁇ mol
- Ethyl 3-amino-6-fluoropicolinate[00485] 6-Fluoro-2-iodopyridin-3-amine (200 mg, 0.84 mmol), palladium(II) acetate (38 mg, 0.17 mmol), and 1,1’-ferrocenediyl-bis(diphenylphosphine) (140 mg, 0.25 mmol) were dissolved in EtOH (2.1 mL) and sealed in a Schlenk flask. The flask was degassed with CO and cooled to -78 oC under an atmosphere of CO. The flask was sealed and heated to 100 oC for 72 h. The reaction was cooled to room temperature and diluted with DCM and water.
- Step 3.3((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-fluoropicolinic acid [00489]
- the title compoundwas synthesized by procedures analogous to those outlined in Example 38, Step 3.
- Bromotripyrrolidinophosphonium hexafluorophosphate(240 mg, 0.51 mmol) (CAS: 132705-51-2), 8-(1-hydroxyethyl)-3,6-dimethylquinazoline-2,4(1H,3H)-dione (Isomer 1; Example 40, Step 2) (100 mg, 0.43 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (0.19 mL, 1.3 mmol) were dissolved in MeCN (2.1 mL) and the resulting mixture was stirred at room temperature for 1 hr.
- Example 416-Chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydro-quinazolin-8-yl)ethyl)amino)picolinic acid [00498] Step 1.2-(4,4-Dimethylpiperidin-1-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin- 4(3H)-one [00499] The title compound was synthesized from rac-8-(1-hydroxyethyl)-3,6- dimethylquinazoline-2,4(1H,3H)-dione (Example 40, Step 2) by procedure analogous to those outlined in Example 40, Step 3.
- the reactionwas diluted with water (2.0 mL) and DCM (1.0 mL) and the resulting two phases were separated. The aqueous layer was extracted with DCM (2 x 2 mL) and the combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude product was purified by prep-HPLC on a C18 column (54.6-74.6% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (2.4 mg, 4.2 ⁇ mol, 50% yield).
- Step 2.8-Acetyl-3-methoxy-6-methylquinazoline-2,4(1H,3H)-dione[00510] To a solution of 2-amino-3-bromo-N-ethyl-5-methylbenzamide (500 mg, 1.94 mmol) in THF (10.0 mL) was added 1,1’-carbonyldiimidazole (631 mg, 3.89 mmol) and 1,8- diazabicyclo [5.4.0]undec-7-ene (0.871 mL, 5.83 mmol). The mixture was refluxed for 16 h. The reaction mixture was cooled to room temperature and poured into a mixture of ice water (5.0 mL) and sat.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Steps 3-7.
- the title compoundwas synthesized by procedures analogous to those outlined in Example 1, Steps 6-7.
- LC-MS calc. for C23H25ClN5O4 [M+H] + : m/z470.2; Found: 470.1.
- Example 52Example 52.
- Examples 53 - 70 listed in Tables 7 and 8were synthesized according to procedures analogous to Example 9, [Step 1-3] (Method A), Example 13, [Step 9-10] (Method B), Example 3, Steps 4–5 (method C), or Example 40 (Method D).
- the appropriate starting materials for Methods A and Bcan be prepared according to procedures analogous to those reported herein. All examples in this table were prepared as the TFA salt unless otherwise noted.
- Examples 80 – 83Table 12. Examples 80 – 83 - 145 - 4877-0794-3771.1 105807.005004– PCT Application Example 84.6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino] pyridine-2-carboxylic acid - 146 - 4877-0794-3771.1 105807.005004– PCT Application [00526] The title compound was synthesized by procedures analogous to those outlined in Example 19, Steps 1–6.
- PI3K Pathway Activation Assay[00527] The inhibitory activity of compounds was evaluated by measuring phosphorylation of AKT on Ser473 as a readout of the PI3K pathway using HTRF (CisBio catalog number: 64AKSPE). These studies were conducted in the T-47D (heterozygous PIK3CA H1047R) and SKBR3 (PIK3CA WT) cell lines.
- T-47DRPMI 1640, ATCC ⁇ Modification (Gibco, A10491-01) supplemented with 10% v/v FBS (Gibco, 26140-079), 1% penicillin streptomycin (Gibco, 15140-122), and 7.4 ug/mL insulin (MilliporeSigma, I9278); SKBR3: McCoy’s 5a (Modified) Medium (Gibco, 16600-082) supplemented with 10% v/v FBS (Gibco, 26140-079). Cells were seeded in 384- well plates at a density of 4,000 cells/well.
- a “+”denotes an IC50 value of > 10000 nM
- a “++”denotes an IC50 value of 500 nM ⁇ IC50 ⁇ 10000 nM
- a “+++”denotes an IC50 value of ⁇ 500 nM.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
105807.005004– PCT Application MUTANT PI3K-ALPHA INHIBITORS AND THEIR USE AS PHARMACEUTICALS TECHNICAL FIELD [0001] The disclosure is directed to mutant PI3Kα inhibitors and methods of their use. BACKGROUND [0002] Class I PI3Ks consist of a p85 regulatory subunit in complex with a p110 catalytic subunit (p110α, β, γ or δ) (1). p110α, coded by the PIK3CA gene shows a broad tissue distribution and the binding of a phosphorylated receptor tyrosine kinase (RTK) activates p110a through the release of a subset of inhibitory contacts with p85. P110α generate phosphatidylinositol3,4,5-trisphosphate (PtdIns(3,4,5)P3; also known as PIP3), which interact with 3-phosphoinositide-binding Pleckstrin homology (PH) domains found in diverse proteins, including protein kinases such as AKT resulting in its phosphorylation at Thr308 and Ser473 triggering a cascade of mitogenic signaling (2). This signaling results in a multitude of cellular effects including proliferation, survival, chemotaxis, cellular trafficking, motility, metabolism, inflammatory and allergic responses, transcription and translation (3). [0003] PIK3CA hotspot mutations are one of the most frequent oncogenic mutations in cancer. Common hotspot mutations in PIK3CA helical (E542K, E545K) and kinase (H1047R) domains function by perturbing local interfaces between p85 and p110α and increasing dynamic events required for catalysis on membranes (1,4). Oncogenic mutations in the PIK3CA gene increase lipid kinase activity and transform cells and are the drivers of the pathology. These mutations are observed in a broad range of cancers including breast, colon, uterine, bladder, cervical, and lung cancer (5,6,7). [0004] Given its key role in cancer PI3Ks have been the focus of extensive drug development. In 2017, the pan-class I PI3K inhibitor copanlisib (Aliqopa/BAY 80-6946; Bayer) was approved for follicular lymphoma and in 2019, the PI3Kα inhibitor alpelisib (Piqray/NVP-BYL719; Novartis) was approved for the treatment of advanced breast cancer, in combination with the estrogen receptor (ER) downregulator fulvestrant (1,8). There are a number of PI3Kα selective inhibitors that are in late stage clinical trials (1). [0005] Although these approvals and early clinical data have validated the pathway as a viable drug target, the development of PI3K inhibitors has proved challenging, with progress hampered by poor drug tolerance (9). The lack of clinical benefit and poor tolerability of pan-class I PI3K and dual PI3Kα/PI3Kδ, or even PI3Kα selective inhibitors have impacted the realization of full clinical utility of these compounds. The toxicity of PI3K inhibitors is dependent on their isoform 105807.005004– PCT Application selectivity profile. Inhibition of PI3Kα is associated with hyperglycemia and rash, whereas inhibition of PI3Kδ or PI3Kγ is associated with diarrhea, myelosuppression, and transaminitis (1). A recent study reported that while progression of disease is the largest contributor to alpelisib discontinuation, adverse events are the leading cause for early drug cessation (10). Shorter alpelisib exposure is associated with greater cancer progression. Therefore, selective inhibitors of PI3Kα mutants H1047R and/or E545K/E543K while sparing wild type PI3Kα, β, γ or δ could enhance the therapeutic margin and result in therapies that benefit cancer patients that carry these mutations. [0006] Additional small molecule mutant PI3Kα selective inhibitors are needed. [0007] (1) PI3K inhibitors are finally coming of age. Vanhaesebroeck B, Perry MWD, Brown JR, André F, Okkenhaug K. Nat Rev Drug Discov.2021 Oct;20(10):741-769. doi: 10.1038/s41573-021-00209-1. Epub 2021 Jun 14. PMID: 34127844. [0008] (2) AKT/PKB Signaling: Navigating the Network. Manning BD, Toker A. Cell.2017 Apr 20;169(3):381-405. doi: 10.1016/j.cell.2017.04.001. PMID: 28431241. [0009] (3) Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017). PMID: 28802037 PMCID: PMC5726441 DOI: 10.1016/j.cell.2017.07.029. [0010] (4) PIK3CA mutations in advanced cancers: characteristics and outcomes. Janku F, Wheler JJ, Naing A, Stepanek VM, Falchook GS, Fu S, Garrido-Laguna I, Tsimberidou AM, Piha-Paul SA, Moulder SL, Lee JJ, Luthra R, Hong DS, Kurzrock R. Oncotarget.2012 Dec;3(12):1566-75. doi: 10.18632/oncotarget.716. PMID: 23248156. [0011] (5) Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Martínez-Sáez O, Chic N, Pascual T, Adamo B, Vidal M, González-Farré B, Sanfeliu E, Schettini F, Conte B, Brasó-Maristany F, Rodríguez A, Martínez D, Galván P, Rodríguez AB, Martinez A, Muñoz M, Prat A. Breast Cancer Res.2020 May 13;22(1):45. doi: 10.1186/s13058- 020-01284-9. PMID: 32404150. [0012] (6) Cancer-specific mutations in PIK3CA are oncogenic in vivo. Bader AG, Kang S, Vogt PK. Proc Natl Acad Sci U S A.2006 Jan 31;103(5):1475-9. doi: 10.1073/pnas.0510857103. Epub 2006 Jan 23. PMID: 16432179. [0013] (7) Oncogenic Signaling Pathways in The Cancer Genome Atlas. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, Chakravarty D, Daian F, Gao Q, Bailey MH, Liang WW, Foltz SM, Shmulevich I, Ding L, Heins Z, Ochoa A, Gross B, Gao J, Zhang H, Kundra R, Kandoth C, Bahceci I, Dervishi L, Dogrusoz U, Zhou W, Shen H, Laird PW, Way GP, Greene CS, Liang H, Xiao Y, Wang C, - 2 -4877-0794-3771.1 105807.005004– PCT Application Iavarone A, Berger AH, Bivona TG, Lazar AJ, Hammer GD, Giordano T, Kwong LN, McArthur G, Huang C, Tward AD, Frederick MJ, McCormick F, Meyerson M; Cancer Genome Atlas Research Network, Van Allen EM, Cherniack AD, Ciriello G, Sander C, Schultz N. Cell.2018 Apr 5;173(2):321-337.e10. doi: 10.1016/j.cell.2018.03.035. PMID: 29625050. [0014] (8) Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Pápai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D; SOLAR-1 Study Group. N Engl J Med.2019 May 16;380(20):1929-1940. doi: 10.1056/NEJMoa1813904. PMID: 31091374. [0015] (9) A multidisciplinary approach to optimizing care of patients treated with alpelisib. Rugo HS, Lacouture ME, Goncalves MD, Masharani U, Aapro MS, O'Shaughnessy JA. Breast. 2022 Feb;61:156-167. doi: 10.1016/j.breast.2021.12.016. Epub 2021 Dec 27. PMID: 35016012. [0016] (10) Factors leading to alpelisib discontinuation in patients with hormone receptor positive, human epidermal growth factor receptor-2 negative breast cancer. Cheung YM, Cromwell GE, Tolaney SM, Min L, McDonnell ME. Breast Cancer Res Treat.2022 Apr;192(2):303-311. doi: 10.1007/s10549-021-06476-1. Epub 2022 Jan 9. PMID: 35000092. SUMMARY OF THE INVENTION [0017] The disclosure is directed to compounds of Formula I: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, wherein ring A is: W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, or a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, - 3 -4877-0794-3771.1 105807.005004– PCT Application cycloalkenyl, heterocycloalkenyl, NRcRd, ORb, or SRb; wherein each group is optionally substituted by 1-6 Rf groups; Y is CR2 or N; n is 1, 2, or 3; m is 1, 2 or 3; R6 is -F, -Cl, -Br, -I or -CN; R1 is H, D, ORa, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl or (C1-C6-alkyl)-Re; wherein said C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and (C1-C6-alkyl)-Re are optionally substituted by 1-6 Rf groups; each R2 and R5 is independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, - OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, - C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, - P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or - OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; each R3 and R4 is independently H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, - SRa, -NRaRd, or NRcRd; or R3 and R4 together with the atom to which they are attached are combined to form a C3-C7 cycloalkyl or C4-C8 heterocycloalkyl; each Ra is independently H, D, -C(O)Rb, -C(O)ORc, -C(O)NRcRd, -C(=NRb)NRbRc, - C(=NORb)NRbRc, -C(=NCN)NRbRc, -P(ORc)2, -P(O)RcRb, -P(O)ORcORb, -S(O)Rb, -S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl; each Rb, is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl; each Rc or Rd is independently H, D, -C1-C10 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, -OC1- C6alkyl, -O-cycloalkyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, or heterocycloalkenyl; - 4 -4877-0794-3771.1 105807.005004– PCT Application or Rc and Rd, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocyclo-alkenyl group. Re is C3-C8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C1- C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C3-C8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 Rf groups; each Rf is independently H, D, oxo, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, - C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, - P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or - OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0018] Stereoisomers of the compounds of Formula I, and the pharmaceutical salts and stereoisomers thereof, are also contemplated, described, and encompassed herein. Methods of using compounds of Formula I are described, as well as pharmaceutical compositions including the compounds of Formula I. DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS [0019] The disclosure may be more fully appreciated by reference to the following description, including the following definitions and examples. Certain features of the disclosed compositions and methods which are described herein in the context of separate aspects, may also be provided in combination in a single aspect. Alternatively, various features of the disclosed compositions and methods that are, for brevity, described in the context of a single aspect, may also be provided separately or in any subcombination. [0020] At various places in the present specification, substituents of compounds of the invention are disclosed in groups or in ranges. It is specifically intended that the invention include each and every individual subcombination of the members of such groups and ranges. For example, the term “C1-C6 alkyl” is specifically intended to individually disclose methyl, ethyl, C3 alkyl, C4 alkyl, C5 alkyl, and C6 alkyl. “C0 alkyl” refers to a covalent bond. - 5 -4877-0794-3771.1 105807.005004– PCT Application [0021] It is further intended that the compounds of the invention are stable. As used herein “stable” refers to a compound that is sufficiently robust to survive isolation to a useful degree of purity from a reaction mixture, and preferably capable of formulation into an efficacious therapeutic agent. [0022] It is further appreciated that certain features of the invention, which are, for clarity, described in the context of separate embodiments, can also be provided in combination in a single embodiment. Conversely, various features of the invention which are, for brevity, described in the context of a single embodiment, can also be provided separately or in any suitable sub-combination. [0023] The term “alkyl,” when used alone or as part of a substituent group, refers to a straight- or branched-chain hydrocarbon group having from 1 to 12 carbon atoms (“C1-C12”), preferably 1 to 6 carbons atoms (“C1-C6”), in the group. Examples of alkyl groups include methyl (Me, C1alkyl), ethyl (Et, C2alkyl), n-propyl (C3alkyl), isopropyl (C3alkyl), butyl (C4alkyl), isobutyl (C4alkyl), sec-butyl (C4alkyl), tert-butyl (C4alkyl), pentyl (C5alkyl), isopentyl (C5alkyl), tert- pentyl (C5alkyl), hexyl (C6alkyl), isohexyl (C6alkyl), and the like. Alkyl groups of the are optionally substituted. Unless otherwise specified, in those embodiments wherein the alkyl group is substituted, the alkyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1- C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the alkyl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the alkyl group is optionally substituted by 1-6 Rf groups. [0024] The term “halo” or halogen refers to chloro, fluoro, bromo, or iodo. [0025] The term “cycloalkyl” when used alone or as part of a substituent group refers to cyclic- containing, non-aromatic hydrocarbon groups having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C3-C6”). Cycloalkyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic cycloalkyl group, the cyclic groups share two common atoms (e.g., fused or bridged). Examples of cycloalkyl groups include, for example, cyclopropyl (C3), cyclobutyl (C4), cyclopropylmethyl (C4), cyclopentyl (C5), cyclohexyl (C6), 1-methylcyclopropyl (C4), 2-methylcyclopentyl (C4), adamantanyl (C10), spiro[3.3]heptanyl, bicyclo[3.3.0]octanyl, and the like. Cycloalkyl groups of - 6 -4877-0794-3771.1 105807.005004– PCT Application the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the cycloalkyl group is substituted, the cycloalkyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1- C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1- C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the cycloalkyl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the cycloalkyl group is optionally substituted by 1-6 Rf groups. [0026] The term “cycloalkenyl” when used alone or as part of a substituent group refers to monocyclic or multicyclic, partially saturated ring structure having from 3 to 10 carbon atoms (“C3-C10”), preferably from 3 to 6 carbon atoms (“C3-C6”). Cycloalkenyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic cycloalkenyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 cycloalkenyl refers to a cycloalkenyl group having between three and six carbon atoms. The cycloalkenyl group may be attached at any carbon atom of the partially saturated ring such that the result is a stable structure. Cycloalkenyl groups include groups in which the partially saturated ring is fused to an aryl group. Examples of cycloalkenyl groups include, for example, cyclopropenyl (C3), cyclobutenyl (C4), cyclopropenylmethyl (C4), cyclopentenyl (C5), cyclohexenyl (C6), 1-methylcyclopropenyl (C4), 2-methylcyclopentenyl (C4), adamantenyl (C10), spiro[3.3]heptenyl, bicyclo[3.3.0]octenyl, indanyl, and the like. Cycloalkenyl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the cycloalkenyl group is substituted, the cycloalkenyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1- C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1- C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and - S(O)2N(C1-C6alkyl)2. In other embodiments, the cycloalkenyl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the cycloalkenyl group is optionally substituted by 1-6 Rf groups. [0027] The term “heterocycloalkyl” when used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, saturated ring structure containing at least one heteroatom selected from the group consisting of O, N and S. Heterocycloalkyl groups - 7 -4877-0794-3771.1 105807.005004– PCT Application of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic heterocycloalkyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic heterocycloalkyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 heterocycloalkyl refers to a heterocycloalkyl group having between three and six carbon ring atoms. The heterocycloalkyl group may be attached at any heteroatom or carbon atom of the group such that the result is a stable structure. Examples of heterocycloalkyl groups include, but are not limited to, azepanyl, aziridinyl, azetidinyl, pyrrolidinyl, dioxolanyl, imidazolidinyl, pyrazolidinyl, piperazinyl, piperidinyl, dioxanyl, morpholinyl, dithianyl, thiomorpholinyl, oxazepanyl, oxiranyl, oxetanyl, quinuclidinyl, tetrahydrofuranyl, tetrahydropyranyl, piperazinyl, azepanyl, diazepanyl, oxepanyl, dioxepanyl, azocanyl diazocanyl, oxocanyl, dioxocanyl, azaspiro[2.2]pentanyl, oxaazaspiro[3.3]heptanyl, oxaspiro[3.3]heptanyl, dioxaspiro[3.3]heptanyl, 3-azabicyclo[3.1.0]hexanyl, , and the like. Heteroycloalkyl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the heterocycloalkyl group is substituted, the heterocycloalkyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, - C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, - S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the heterocycloalkyl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, - SRa, -NRaRd, or NRcRd; or the heterocycloalkyl group is optionally substituted by 1-6 Rf groups. [0028] The term “heterocycloalkenyl” when used alone or as part of a substituent group refers to any three to twelve membered monocyclic or multicyclic, partially saturated ring structure containing at least one heteroatom selected from the group consisting of O, N and S. Heterocycloalkenyl groups of the disclosure include monocyclic groups, as well as multicyclic groups such as bicyclic and tricyclic groups. In those embodiments having at least one multicyclic heterocycloalkyenyl group, the cyclic groups can share one common atom (i.e., spirocyclic). In other embodiments having at least one multicyclic heterocycloalkenyl group, the cyclic groups share two common atoms (e.g., fused or bridged). The term -C3-C6 heterocycloalkenyl refers to a heterocycloalkenyl group having between three and six carbon atoms. The heterocycloalkenyl group may be attached at any heteroatom or carbon atom of the - 8 -4877-0794-3771.1 105807.005004– PCT Application partially saturated ring such that the result is a stable structure. Heterocycloalkenyl groups include groups in which the partially saturated ring is fused to an aryl group, such as, for example isoindoline, , or in which the partially saturated ring is fused to a heteroaryl group, such as, for example, 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine, . Heteroycloalkenyl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the heterocycloalkenyl group is substituted, the heterocycloalkenyl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, - C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, - S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the heterocycloalkenyl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, - SRa, -NRaRd, or NRcRd; or the heterocycloalkenyl group is optionally substituted by 1-6 Rf groups. [0029] The term “heterocyclic group,” when used alone or as part of a substituent group, refers to a heterocycloalkyl group or a heterocycloalkenyl group. [0030] The term “heteroaryl” when used alone or as part of a substituent group refers to a mono- or bicyclic- aromatic ring structure including carbon atoms as well as up to five heteroatoms selected from nitrogen, oxygen, and sulfur. Heteroaryl rings can include a total of 5, 6, 7, 8, 9, or 10 ring atoms. Examples of heteroaryl groups include but are not limited to, pyrrolyl, furyl, thiophenyl (thienyl), oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, triazolyl, thiadiazolyl, pyrazolyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, pyranyl, furazanyl, indolizinyl, indolyl, and the like. Heteroaryl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the heteroaryl group is substituted, the heteroaryl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1- C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), - OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the heteroaryl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the heteroaryl group is optionally substituted by 1-6 Rf groups. - 9 -4877-0794-3771.1 105807.005004– PCT Application [0031] The term “aryl” when used alone or as part of a substituent group refers to a mono- or bicyclic- aromatic carbon ring structure. Aryl rings can include a total of 5, 6, 7, 8, 9, or 10 ring atoms. Examples of aryl groups include but are not limited to, phenyl, napthyl, and the like. Aryl groups of the disclosure are optionally substituted. Unless otherwise specified, in those embodiments wherein the aryl group is substituted, the aryl group can be substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1- C6haloalkyl, and C1-C6haloalkoxy, -C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1- C6alkyl), -OC(O)N(C1-C6alkyl)2, -S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the aryl group is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; or the aryl group is optionally substituted by 1-6 Rf groups. [0032] When a range of carbon atoms is used herein, for example, C1-C6, all ranges, as well as individual numbers of carbon atoms are encompassed, for example, “C1-3” includes C1-3, C1-2, C2- 3, C1, C2, and C3. The term “C1-6alk” refers to an aliphatic linker having 1, 2, 3, 4, 5, or 6 carbon atoms and includes, for example, –CH2-, –CH(CH3)-, -CH(CH3)-CH2-, and –C(CH3)2-. The term “-C0alk-” refers to a bond. [0033] The term “C0-C6alk” when used alone or as part of a substituent group refers to an aliphatic linker having 0, 1, 2, 3, 4, 5 or 6 carbon atoms. The term “-C1alk-”, for example, refers to a -CH2-. The term “-C0alk-” refers to a bond. [0034] Unless otherwise specified, in those embodiments wherein the -C1-C6alkyl, -C1-C10 alkyl, -C1-C8 alkoxide, -C2-C6alkenyl, -C2-C10alkenyl, -C2-C6alkynyl, -C2-C10alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, and heterocycloalkyl groups are substituted, they can be optionally substituted with 1, 2, or 3 substituents independently selected from -OH, -CN, amino, halo, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, and C1-C6haloalkoxy, - C(O)NH(C1-C6alkyl), -C(O)N(C1-C6alkyl)2, -OC(O)NH(C1-C6alkyl), -OC(O)N(C1-C6alkyl)2, - S(O)2NH(C1-C6alkyl), and -S(O)2N(C1-C6alkyl)2. In other embodiments, the -C1-C6alkyl, -C1- C10 alkyl, -C1-C8 alkoxide, -C2-C6alkenyl, -C2-C10alkenyl, -C2-C6alkynyl, -C2-C10alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, and heterocycloalkyl groups are optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, - NRaRd, or NRcRd; or the -C1-C6alkyl, -C1-C10 alkyl, -C1-C8 alkoxide, -C2-C6alkenyl, -C2- C10alkenyl, -C2-C6alkynyl, -C2-C10alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, and heterocycloalkyl groups are optionally substituted by 1-6 Rf groups. - 10 -4877-0794-3771.1 105807.005004– PCT Application [0035] As used herein, “alkoxy” refers to an –O-alkyl group. Example alkoxy groups include methoxy, ethoxy, propoxy (e.g., n-propoxy and isopropoxy), t-butoxy, and the like. [0036] As used herein, “hydroxylalkyl” refers to an alkyl group substituted by OH. [0037] The compounds described herein can be asymmetric (e.g., having one or more stereocenters). All stereoisomers, such as enantiomers and diastereomers, are intended unless otherwise indicated. Compounds of the present invention that contain asymmetrically substituted carbon atoms can be isolated in optically active or racemic forms. Methods on how to prepare optically active forms from optically active starting materials are known in the art, such as by resolution of racemic mixtures or by stereoselective synthesis. Geometric isomers of olefins, C=N double bonds, and the like can also be present in the compounds described herein, and all such stable isomers are contemplated in the present invention. Geometric isomers of the compounds of the present invention are described and may be isolated as a mixture of isomers or as separated isomeric forms. [0038] Compounds of the invention may also include tautomeric forms. All tautomeric forms are encompassed. [0039] In some embodiments, the compounds of the present invention may exist as rotational isomers. In some embodiments, the compounds of the present invention exist as mixtures of rotational isomers in any proportion. In other embodiments, the compounds of the present invention exist as particular rotational isomers, substantially free of other rotational isomers. [0040] Compounds of the invention can also include all isotopes of atoms occurring in the intermediates or final compounds. Isotopes include those atoms having the same atomic number but different mass numbers. For example, isotopes of hydrogen include tritium and deuterium. [0041] In some embodiments, the compounds of the invention, and salts thereof, are substantially isolated. By “substantially isolated” is meant that the compound is at least partially or substantially separated from the environment in which it was formed or detected. Partial separation can include, for example, a composition enriched in the compound of the invention. Substantial separation can include compositions containing at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 97%, or at least about 99% by weight of the compound of the invention, or salt thereof. Methods for isolating compounds and their salts are routine in the art. [0042] The present invention also includes pharmaceutically acceptable salts of the compounds described herein. As used herein, “pharmaceutically acceptable salts” refers to derivatives of the disclosed compounds wherein the parent compound is modified by converting an existing acid or - 11 -4877-0794-3771.1 105807.005004– PCT Application base moiety to its salt form. Examples of pharmaceutically acceptable salts include, but are not limited to, mineral or organic acid salts of basic residues such as amines; alkali or organic salts of acidic residues such as carboxylic acids; and the like. The pharmaceutically acceptable salts of the present invention include the conventional non-toxic salts of the parent compound formed, for example, from non-toxic inorganic or organic acids. The pharmaceutically acceptable salts of the present invention can be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods. Generally, such salts can be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropriate base or acid in water or in an organic solvent, or in a mixture of the two; generally, nonaqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile are preferred. Lists of suitable salts are found in Remington’s Pharmaceutical Sciences, 17th ed., Mack Publishing Company, Easton, Pa., 1985, p.1418 and Journal of Pharmaceutical Science, 66, 1 (1977) p.1–19, each of which is incorporated herein by reference in its entirety. [0043] The phrase “pharmaceutically acceptable” is employed herein to refer to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio. [0044] A “pharmaceutically acceptable excipient” refers to a substance that is non-toxic, biologically tolerable, and otherwise biologically suitable for administration to a subject, such as an inert substance, added to a pharmacological composition or otherwise used as a vehicle, carrier, or diluent to facilitate administration of an agent and that is compatible therewith. Examples of excipients include calcium carbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin, vegetable oils, and polyethylene glycols. [0045] A “solvate” refers to a physical association of a compound of Formula I with one or more solvent molecules. [0046] “Subject” includes humans. The terms “human,” “patient,” and “subject” are used interchangeably herein. [0047] “Treating” or “treatment” of any disease or disorder refers, in one embodiment, to ameliorating the disease or disorder (i.e., arresting or reducing the development of the disease or at least one of the clinical symptoms thereof). In another embodiment “treating” or “treatment” refers to ameliorating at least one physical parameter, which may not be discernible by the subject. In yet another embodiment, “treating” or “treatment” refers to modulating the disease or - 12 -4877-0794-3771.1 105807.005004– PCT Application disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both. In yet another embodiment, “treating” or “treatment” refers to delaying the onset of the disease or disorder. [0048] “Compounds of the present disclosure,” and equivalent expressions, are meant to embrace compounds of Formula I as described herein, as well as its subgenera, which expression includes the stereoisomers (e.g., entaniomers, diastereomers) and constitutional isomers (e.g., tautomers) of compounds of Formula I as well as the pharmaceutically acceptable salts, where the context so permits. [0049] As used herein, the term “isotopic variant” refers to a compound that contains proportions of isotopes at one or more of the atoms that constitute such compound that is greater than natural abundance. For example, an “isotopic variant” of a compound can be radiolabeled, that is, contain one or more radioactive isotopes, or can be labeled with non-radioactive isotopes such as for example, deuterium (2H or D), carbon-13 (13C), nitrogen-15 (15N), or the like. It will be understood that, in a compound where such isotopic substitution is made, the following atoms, where present, may vary, so that for example, any hydrogen may be2H/D, any carbon may be13C, or any nitrogen may be15N, and that the presence and placement of such atoms may be determined within the skill of the art. [0050] It is also to be understood that compounds that have the same molecular formula but differ in the nature or sequence of bonding of their atoms or the arrangement of their atoms in space are termed “isomers.” Isomers that differ in the arrangement of their atoms in space are termed “stereoisomers,” for example, diastereomers, enantiomers, and atropisomers. The compounds of this disclosure may possess one or more asymmetric centers; such compounds can therefore be produced as individual (R)- or (S)-stereoisomers at each asymmetric center, or as mixtures thereof. Unless indicated otherwise, the description or naming of a particular compound in the specification and claims is intended to include all stereoisomers and mixtures, racemic or otherwise, thereof. Where one chiral center exists in a structure, but no specific stereochemistry is shown for that center, both enantiomers, individually or as a mixture of enantiomers, are encompassed by that structure. Where more than one chiral center exists in a structure, but no specific stereochemistry is shown for the centers, all enantiomers and diastereomers, individually or as a mixture, are encompassed by that structure. The methods for the determination of stereochemistry and the separation of stereoisomers are well-known in the art. [0051] The disclosure is directed to compounds of Formula I: - 13 -4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, wherein ring A is: W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, NRcRd, ORb, or SRb; wherein each group is optionally substituted by 1-6 Rf groups; Y is CR2 or N; n is 1, 2, or 3; m is 1, 2 or 3; R6 is -F, -Cl, -Br, -I or -CN; R1 is H, D, ORa, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, or (C1-C6-alkyl)-Re; wherein said C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and (C1-C6-alkyl)-Re are optionally substituted by 1-6 Rf groups; each R2 and R5 is independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, - OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, - C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, - P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or - OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; - 14 -4877-0794-3771.1 105807.005004– PCT Application each R3 and R4 is independently H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, - SRa, -NRaRd, or NRcRd; or R3 and R4 together with the atom to which they are attached are combined to form a C3-C7 cycloalkyl or C4-C8 heterocycloalkyl; each Ra is independently H, D, -C(O)Rb, -C(O)ORc, -C(O)NRcRd, -C(=NRb)NRbRc, - C(=NORb)NRbRc, -C(=NCN)NRbRc, -P(ORc)2, -P(O)RcRb, -P(O)ORcORb, -S(O)Rb, -S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl; each Rb, is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl; each Rc or Rd is independently H, D, -C1-C10 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, -OC1- C6alkyl, -O-cycloalkyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, or heterocycloalkenyl; or Rc and Rd, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or multicyclic heterocyclo-alkenyl group. Re is C3-C8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C1- C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C3-C8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 Rf groups; each Rf is independently H, D, oxo, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, - C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, - P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or - OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0052] In some embodiments, ring A in Formula - 15 -4877-0794-3771.1 105807.005004– PCT Application [0053] In other embodiments, ring A in Formula [0054] In some embodiments, ring A in Formula [0055] In some embodiments, R6 in Formula (I) is -F. In some embodiments, R6 in Formula (I) is -Cl. In some embodiments, R6 in Formula (I) is -Br. In some embodiments, R6 in Formula (I) is -I. In some embodiments, R6 in Formula (I) is -CN. [0056] In some embodiments, W in Formula (I) is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, NRcRd, ORb, or SRb, wherein each group is optionally substituted by 1-6 Rf groups. [0057] In some embodiments, W in Formula (I) is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S. In some embodiments, the 5-12 membered heterocyclic group is an isoindoline group or a piperidine group. In some embodiments, the 5-12 membered heterocyclic group is an isoindoline group. In some embodiments, the 5-12 membered heterocyclic group is a piperidine group. In some embodiments, the 5-12 membered heterocyclic group is a 3-azabicyclo[3.1.0]hexane, pyrrolidine, azetidine, piperazine, 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine or 1,4,5,6- tetrahydropyrrolo[3,4-c]pyrazole group. In some embodiments, the 5-12 membered heterocyclic group is a 3-azabicyclo[3.1.0]hexane. In some embodiments, the 5-12 membered heterocyclic group is a pyrrolidine group. In some embodiments, the 5-12 membered heterocyclic group is an azetidine group. In some embodiments, the 5-12 membered heterocyclic group is a piperazine group. In some embodiments, the 5-12 membered heterocyclic group is 6,7-dihydro-5H- pyrrolo[3,4-b]pyridine group. In some embodiments, the 5-12 membered heterocyclic group is a 1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole group. In some embodiments, W in Formula (I) is NRcRd. In some embodiments, W in Formula (I) is substituted by 1-6 Rf groups. [0058] In other embodiments, W in Formula (I) is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S. In other embodiments, W in Formula (I) is C1-C8 alkyl. In other embodiments, W in Formula (I) is haloalkyl. In other embodiments, W in - 16 -4877-0794-3771.1 105807.005004– PCT Application Formula (I) is -C2-C6 alkenyl. In other embodiments, W in Formula (I) is -C2-C6 alkynyl. In other embodiments, W in Formula (I) is aryl. In other embodiments, W in Formula (I) is heteroaryl. In other embodiments, W in Formula (I) is cycloalkyl. In other embodiments, W in Formula (I) is cycloalkenyl. In other embodiments, W in Formula (I) is heterocycloalkenyl. In other embodiments, W in Formula (I) is NRcRd. In other embodiments, W in Formula (I) is NRcRd. In other embodiments, W in Formula (I) is ORb. In other embodiments, W in Formula (I) is SRb. In some embodiments, W in Formula (I) is substituted by 1 or 2 Rf groups. [0059] In some embodiments, Y in Formula (I) is CR2 or N. In some embodiments, Y in Formula (I) is N. In other embodiments, Y in Formula (I) is CR2. In other embodiments, Y in Formula (I) is CH. [0060] In some embodiments, n in Formula (I) is 1, 2, or 3. In some embodiments, n in Formula (I) is 1. In other embodiments, n in Formula (I) is 2. In yet other embodiments, n in Formula (I) is 3. [0061] In some embodiments, m in Formula (I) is 1, 2 or 3. In some embodiments, m in Formula (I) is 1. In other embodiments, m in Formula (I) is 2. In yet other embodiments, m in Formula (I) is 3. [0062] In some embodiments, R1 in Formula I is H, D, ORa, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -C3-C8 cycloalkyl, -C3-C8 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, or (C1-C6-alkyl)-Re; wherein said C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, C3- C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and (C1-C6-alkyl)-Re are optionally substituted by 1-6 Rf groups. [0063] In some embodiments, R1 in Formula I is H. In some embodiments, R1 in Formula I is D. In some embodiments, R1 in Formula I is ORa. In some embodiments, R1 in Formula I is C1- C8 alkoxide. In some embodiments, R1 in Formula I is C1-C8 alkyl. In some embodiments, R1 in Formula I is haloalkyl. In some embodiments, R1 in Formula I is C3-C8 cycloalkyl. In some embodiments, R1 in Formula I is C3-C8 cycloalkenyl. In some embodiments, R1 in Formula I is aryl. In some embodiments, R1 in Formula I is heteroaryl. In some embodiments, the C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, and heteroaryl are optionally substituted by 1-6 Rf groups. [0064] In other embodiments, R1 in Formula I is H. In other embodiments, R1 in Formula I is C1-C8alkyl. In other embodiments, R1 in Formula I is methyl. [0065] In some embodiments, R1 in Formula I is heterocycloalkyl optionally substituted by 1-6 Rf groups. In some embodiments, R1 in Formula I is tetrahydro-2H-pyran optionally substituted - 17 -4877-0794-3771.1 105807.005004– PCT Application by 1-6 Rf groups. In other embodiments, R1 in Formula I is tetrahydrothiophene optionally substituted by 1-6 Rf groups. In other embodiments, R1 in Formula I is tetrahydrothiophene-1,1- dioxide optionally substituted by 1-6 Rf groups. In yet other embodiments, R1 in Formula I is tetrahydrofuran optionally substituted by 1-6 Rf groups. In yet other embodiments, R1 in Formula I is oxetane optionally substituted by 1-6 Rf groups. In yet other embodiments, R1 in Formula I is pyrrolidine optionally substituted by 1-6 Rf groups. [0066] In some embodiments, R1 in Formula I is heterocycloalkenyl optionally substituted by 1-6 Rf groups. [0067] In some embodiments, R1 in Formula I is (C1-C6-alkyl)-Re optionally substituted by 1-6 Rf groups. In some embodiments, Re is azetidine optionally substituted by 1-6 Rf groups, pyrazole optionally substituted by 1-6 Rf groups, p-methoxybenzene or propyl. In some embodiments, Re is pyrazole optionally substituted by 1-6 Rf groups. In other embodiments, Re is p-methoxybenzene. In other embodiments, Re is propyl. [0068] In some embodiments, each R2 in Formula I is independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)(ORb)(ORb), -B(ORc)(ORd) or -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, - OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0069] In some embodiments, at least one R2 in Formula I is H. In some embodiments, at least one R2 in Formula I is D. In some embodiments, at least one R2 in Formula I is halogen. In some embodiments, at least one R2 in Formula I is C1-C8 alkoxide. In some embodiments, at least one R2 in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0070] In other embodiments, at least one R2 in Formula I is haloalkyl. In other embodiments, at least one R2 in Formula I is -OH. In other embodiments, at least one R2 in Formula I is -CN. In other embodiments, at least one R2 in Formula I is -NO2. In other embodiments, at least one R2 in Formula I is -C2-C6 alkenyl. In other embodiments, at least one R2 in Formula I is -C2-C6 alkynyl. In other embodiments, at least one R2 in Formula I is aryl. In other embodiments, at least one R2 in Formula I is hetereoaryl. In other embodiments, at least one R2 in Formula I is cycloalkyl. In other embodiments, at least one R2 in Formula I is cycloalkenyl. In other - 18 -4877-0794-3771.1 105807.005004– PCT Application embodiments, at least one R2 in Formula I is heterocycloalkyl. In some embodiments, at least one R2 in Formula I is heterocycloalkenyl. In other embodiments, at least one R2 in Formula I is -ORa. In other embodiments, at least one R2 in Formula I is -SRa. In other embodiments, at least one R2 in Formula I is -NRcRd. In other embodiments, at least one R2 in Formula I is -NRaRc. In other embodiments, at least one R2 in Formula I is -C(O)Rb. In other embodiments, at least one R2 in Formula I is -OC(O)Rb. In other embodiments, at least one R2 in Formula I is -C(O)ORb. In other embodiments, at least one R2 in Formula I is -C(O)NRcRd. In other embodiments, at least one R2 in Formula I is -S(O)Rb. In other embodiments, at least one R2 in Formula I is - S(O)2NRcRd. In other embodiments, at least one R2 in Formula I is -S(O)(=NRb)Rb. In other embodiments, at least one R2 in Formula I is -SF5. In other embodiments, at least one R2 in Formula I is -P(O)RbRb. In other embodiments, at least one R2 in Formula I is -P(O)(ORb)(ORb). In other embodiments, at least one R2 in Formula I is -B(ORc)(ORd). In other embodiments, at least one R2 in Formula I is -S(O)2Rb. In other embodiments, at least one R2 in Formula I is - C(O)NRbORb. In other embodiments, at least one R2 in Formula I is -S(O)2ORb. In other embodiments, at least one R2 in Formula I is -OS(O)2ORb. In other embodiments, at least one R2 in Formula I is -OPO(ORb)(ORb). [0071] In some embodiments, at least one R2 in Formula I is H, C1-C8 alkyl, CF3, Br, F, CN or CHF2. In some embodiments, at least one R2 in Formula I is H. In some embodiments, at least one R2 in Formula I is C1-C8alkyl. In some embodiments, at least one R2 in Formula I is methyl. In some embodiments, at least one R2 in Formula I is CF3. In some embodiments, at least one R2 in Formula I is Br. In some embodiments, at least one R2 in Formula I is F. In some embodiments, at least one R2 in Formula I is CN. In some embodiments, at least one R2 in Formula I is CHF2. [0072] In some embodiments, R3 in Formula I is H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, - CN, -ORa, -SRa, -NRaRd, or NRcRd. [0073] In some embodiments, R3 in Formula I is H. In some embodiments, R3 in Formula I is D. In some embodiments, R3 in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, - NRaRd, or NRcRd. In some embodiments, R3 in Formula I is haloalkyl. In some embodiments, R3 in Formula I is CN. - 19 -4877-0794-3771.1 105807.005004– PCT Application [0074] In other embodiments, R3 in Formula I is H or C1-C8 alkyl. In other embodiments, R3 in Formula I is H. In other embodiments, R3 in Formula I is C1-C8alkyl. In other embodiments, R3 in Formula I is methyl. [0075] In some embodiments, R4 in Formula I is H, D, C1-C8 alkyl, haloalkyl, or CN; wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, - CN, -ORa, -SRa, -NRaRd, or NRcRd. [0076] In some embodiments, R4 in Formula I is H. In some embodiments, R4 in Formula I is D. In some embodiments, R4 in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, - NRaRd, or NRcRd. In some embodiments, R4 in Formula I is haloalkyl. In some embodiments, R4 in Formula I is CN. [0077] In other embodiments, R4 in Formula I is H or C1-C8 alkyl. In other embodiments, R4 in Formula I is H. In other embodiments, R4 in Formula I is C1-C8alkyl. In other embodiments, R4 in Formula I is methyl. [0078] In some embodiments, R3 and R4 in Formula I together with the atom to which they are attached are combined to form a C3-C7 cycloalkyl or C4-C8 heterocycloalkyl. In some embodiments, R3 and R4 in Formula I together with the atom to which they are attached are combined to form a C3-C7 cycloalkyl. In some embodiments, R3 and R4 in Formula I together with the atom to which they are attached are combined to form a C4-C8 heterocycloalkyl. [0079] In some embodiments, each R5 in Formula I is independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)(ORb)(ORb), -B(ORc)(ORd) or -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, - OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0080] In some embodiments, at least one R5 in Formula I is H. In some embodiments, at least one R5 in Formula I is D. In some embodiments, at least one R5 in Formula I is halogen. In some embodiments, at least one R5 in Formula I is C1-C8 alkoxide. In some embodiments, at least one R5 in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. - 20 -4877-0794-3771.1 105807.005004– PCT Application [0081] In other embodiments, at least one R5 in Formula I is haloalkyl. In other embodiments, at least one R5 in Formula I is -OH. In other embodiments, at least one R5 in Formula I is -CN. In other embodiments, at least one R5 in Formula I is -NO2. In other embodiments, at least one R5 in Formula I is -C2-C6 alkenyl. In other embodiments, at least one R5 in Formula I is -C2-C6 alkynyl. In other embodiments, at least one R5 in Formula I is aryl. In other embodiments, at least one R5 in Formula I is hetereoaryl. In other embodiments, at least one R5 in Formula I is cycloalkyl. In other embodiments, at least one R5 in Formula I is cycloalkenyl. In other embodiments, at least one R5 in Formula I is heterocycloalkyl. In some embodiments, at least one R5 in Formula I is heterocycloalkenyl. In other embodiments, at least one R5 in Formula I is -ORa. In other embodiments, at least one R5 in Formula I is -SRa. In other embodiments, at least one R5 in Formula I is -NRcRd. In other embodiments, at least one R5 in Formula I is -NRaRc. In other embodiments, at least one R5 in Formula I is -C(O)Rb. In other embodiments, at least one R5 in Formula I is -OC(O)Rb. In other embodiments, at least one R5 in Formula I is -C(O)ORb. In other embodiments, at least one R5 in Formula I is -C(O)NRcRd. In other embodiments, at least one R5 in Formula I is -S(O)Rb. In other embodiments, at least one R5 in Formula I is - S(O)2NRcRd. In other embodiments, at least one R5 in Formula I is -S(O)(=NRb)Rb. In other embodiments, at least one R5 in Formula I is -SF5. In other embodiments, at least one R5 in Formula I is -P(O)RbRb. In other embodiments, at least one R5 in Formula I is -P(O)(ORb)(ORb). In other embodiments, at least one R5 in Formula I is -B(ORc)(ORd). In other embodiments, at least one R5 in Formula I is -S(O)2Rb. In other embodiments, at least one R5 in Formula I is - C(O)NRbORb. In other embodiments, at least one R5 in Formula I is -S(O)2ORb. In other embodiments, at least one R5 in Formula I is -OS(O)2ORb. In other embodiments, at least one R5 in Formula I is -OPO(ORb)(ORb). [0082] In some embodiments, at least one R5 in Formula I is a carboxylic acid group or isostere thereof. In some embodiments, at least one R5 in Formula I is a carboxylic acid group. In some embodiments, at least one R5 in Formula I is -CO2H. In other embodiments, at least one R5 in Formula I is an isostere of a carboxylic acid group. [0083] In some embodiments, each Ra in Formula I is independently H, D, -C(O)Rb, - 2, - C10alkyl, - C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl. - 21 -4877-0794-3771.1 105807.005004– PCT Application [0084] In some embodiments, Ra in Formula I is H. In some embodiments, Ra in Formula I is D. In some embodiments, Ra in Formula I is -C(O)Rb. In some embodiments, Ra in Formula I is -C(O)ORc. In some embodiments, Ra in Formula I is -C(O)NRcRd. In some embodiments, Ra in Formula I is -C(=NRb)NRbRc. In some embodiments, Ra in Formula I is C(=NORb)NRbRc. In some embodiments, Ra in Formula I is -C(=NCN)NRbRc. [0085] In other embodiments, Ra in Formula I is -P(ORc)2, -P(O)RcRb, -P(O)ORcORb, -S(O)Rb, -S(O)NRcRd, -S(O)2Rb, -S(O)2NRcRd, SiRb3, and the like. In yet other embodiments, Ra in Formula I is -C1-C10alkyl, -C2-C10 alkenyl, -C2-C10 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, and the like. [0086] In some embodiments, each Rb in Formula I is independently H, D, -C1-C6 alkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl. [0087] In some embodiments, Rb in Formula I is H. In some embodiments, Rb in Formula I is D. In some embodiments, Rb in Formula I is -C1-C6 alkyl. In some embodiments, Rb in Formula I is -C2-C6 alkenyl. In some embodiments, Rb in Formula I is -C2-C6 alkynyl. In other embodiments, Rb in Formula I is aryl. In other embodiments, Rb in Formula I is cycloalkyl. In other embodiments, Rb in Formula I is cycloalkenyl. In other embodiments, Rb in Formula I is heteroaryl. In other embodiments, Rb in Formula I is heterocycloalkyl. In other embodiments, Rb in Formula I is heterocycloalkenyl. [0088] In some embodiments, each Rc or Rd in Formula I is independently H, D, -C1-C6 alkyl, - C2-C6 alkenyl, -C2-C6 alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, heterocycloalkyl, or heterocycloalkenyl. [0089] In some embodiments, Rc or Rd in Formula I is H. In some embodiments, Rc or Rd in Formula I is D. In some embodiments, Rc or Rd in Formula I is -C1-C10 alkyl. In some embodiments, Rc or Rd in Formula I is -C2-C6 alkenyl. In some embodiments, Rc or Rd in Formula I is -C2-C6 alkynyl. In other embodiments, Rc or Rd in Formula I is -OC1-C6alkyl. In other embodiments, Rc or Rd in Formula I is -O-cycloalkyl. In other embodiments, Rc or Rd in Formula I is aryl. In other embodiments, Rc or Rd in Formula I is cycloalkyl. In other embodiments, Rc or Rd in Formula I is cycloalkenyl. In other embodiments, Rc or Rd in Formula I is heteroaryl. In other embodiments, Rc or Rd in Formula I is heterocycloalkyl. In other embodiments, Rc or Rd in Formula I is heterocycloalkenyl. [0090] In yet other embodiments, Rc and Rd in Formula I, together with the atom to which they are both attached, form a monocyclic or multicyclic heterocycloalkyl, or a monocyclic or - 22 -4877-0794-3771.1 105807.005004– PCT Application multicyclic heterocyclo-alkenyl group. In yet other embodiments, Rc and Rd in Formula I form a monocyclic heterocycloalkyl. In yet other embodiments, Rc and Rd in Formula I form a multicyclic heterocycloalkyl. In yet other embodiments, Rc and Rd in Formula I form a monocyclic heterocyclo-alkenyl group. In yet other embodiments, Rc and Rd in Formula I form a multicyclic heterocyclo-alkenyl group. [0091] In some embodiments, Re in Formula I is C3-C8 cycloalkyl, heterocycloalkyl wherein the heterocycloalkyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkyl group, cycloalkenyl, heterocycloalkenyl wherein the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom or a sulfur atom of the heterocycloalkenyl group, aryl, or heteroaryl, and each C3-C8 cycloalkyl, heterocycloalkyl, cycloalkenyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted by 1-6 Rf groups. [0092] In some embodiments, Re in Formula I is C3-C8 cycloalkyl optionally substituted by 1-6 Rf groups. In some embodiments, Re in Formula I is heterocycloalkyl optionally substituted by 1-6 Rf groups. In some embodiments, the heterocycloalkyl is attached to (C1-C6-alkyl) through a carbon atom of the heterocycloalkyl group. In some embodiments, the heterocycloalkyl is attached to (C1-C6-alkyl) through a sulfur atom of the heterocycloalkyl group. In other embodiments, Re in Formula I is cycloalkenyl optionally substituted by 1-6 Rf groups. In other embodiments, Re in Formula I is heterocycloalkenyl optionally substituted by 1-6 Rf groups. In other embodiments, the heterocycloalkenyl is attached to (C1-C6-alkyl) through a carbon atom of the heterocycloalkenyl group. In other embodiments, the heterocycloalkenyl is attached to (C1- C6-alkyl) through a sulfur atom of the heterocycloalkenyl group. In yet other embodiments, Re in Formula I is aryl optionally substituted by 1-6 Rf groups. In yet other embodiments, Re in Formula I is heteroaryl optionally substituted by 1-6 Rf groups. [0093] In some embodiments, Re in Formula I is azetidine or piperidine optionally substituted by 1-6 Rf groups, pyrazole optionally substituted by 1-6 Rf groups, phenyl optionally substituted by 1-6 Rf groups or cycloalkyl optionally substituted by 1-6 Rf groups. In some embodiments, Re in Formula I is azetidine optionally substituted by 1-6 Rf groups. In some embodiments, Re in Formula I is piperidine optionally substituted by 1-6 Rf groups. In some embodiments, Re in Formula I is phenyl optionally substituted by 1-6 Rf groups. In some embodiments, Re in Formula I is cycloalkyl optionally substituted by 1-6 Rf groups. [0094] In some embodiments, each Rf in Formula I is independently H, D, oxo, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, - 23 -4877-0794-3771.1 105807.005004– PCT Application -NRaRc, -C(O)Rb, -OC(O)Rb, -C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, - SF5, -P(O)RbRb, -P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, - OS(O)2ORb, or -OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. [0095] In some embodiments, Rf in Formula I is H. In some embodiments, Rf in Formula I is D. In some embodiments, Rf in Formula I is oxo. In some embodiments, Rf in Formula I is halogen. In some embodiments, Rf in Formula I is C1-C8 alkoxide. In some embodiments, Rf in Formula I is C1-C8 alkyl. In some embodiments, the C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd. In some embodiments, Rf in Formula I is haloalkyl. In some embodiments, Rf in Formula I is -OH. In some embodiments, Rf in Formula I is -CN. In some embodiments, Rf in Formula I is -NO2. In some embodiments, Rf in Formula I is -C2-C6 alkenyl. In some embodiments, Rf in Formula I is - C2-C6 alkynyl. In some embodiments, Rf in Formula I is aryl. In some embodiments, Rf in Formula I is heteroaryl. In some embodiments, Rf in Formula I is cycloalkyl. In other embodiments, Rf in Formula I is cycloalkenyl. In other embodiments, Rf in Formula I is heterocycloalkyl. In other embodiments, Rf in Formula I is heterocycloalkenyl. In other embodiments, Rf in Formula I is -ORa. In other embodiments, Rf in Formula I is -SRa. In other embodiments, Rf in Formula I is -NRcRd. In other embodiments, Rf in Formula I is -NRaRc. In other embodiments, Rf in Formula I is -C(O)Rb. In other embodiments, Rf in Formula I is - OC(O)Rb. In other embodiments, Rf in Formula I is -C(O)ORb. In other embodiments, Rf in Formula I is -C(O)NRcRd. In yet other embodiments, Rf in Formula I is -S(O)Rb. In yet other embodiments, Rf in Formula I is -S(O)2NRcRd. In yet other embodiments, Rf in Formula I is - S(O)(=NRb)Rb. In yet other embodiments, Rf in Formula I is -SF5. In yet other embodiments, Rf in Formula I is -P(O)RbRb. In yet other embodiments, Rf in Formula I is -P(O)(ORb)(ORb). In yet other embodiments, Rf in Formula I is -B(ORc)(ORd). In yet other embodiments, Rf in Formula I is -S(O)2Rb. In yet other embodiments, Rf in Formula I is -C(O)NRbORb. In yet other embodiments, Rf in Formula I is -S(O)2ORb. In yet other embodiments, Rf in Formula I is - OS(O)2ORb. In yet other embodiments, Rf in Formula I is -OPO(ORb)(ORb). [0096] In some embodiments, the compounds of Formula (I) are the pharmaceutically acceptable salts. In some embodiments, the compounds of Formula (I) are solvates. In some embodiments, the compounds of Formula (I) are N-oxides. In some embodiments, the compounds of Formula (I) are stereoisomers. - 24 -4877-0794-3771.1 105807.005004– PCT Application [0097] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula II or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R1, R2, R3, R4, R5, R6, m, n, W, and Y are as defined with respect to Formula (I). [0098] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula III or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R1, R2, R3, R4, R5, R6, m, W and Y are as defined with respect to Formula (I). [0099] In some embodiments of the compounds of Formula (III), each R5 is H. [00100] In some embodiments of the compounds of Formula (III), R6 is -F. [00101] In some embodiments of the compounds of Formula (III), R6 is -Cl. [00102] In some embodiments of the compounds of Formula (III), R6 is -Br. [00103] In some embodiments of the compounds of Formula (III), R6 is -I. [00104] In some embodiments of the compounds of Formula (III), R6 is -CN. [00105] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IV - 25 -4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R1, R2, R3, R4, R5, R6, Rc, Rd,m, and Y are defined with respect to Formula (I). [00106] In some embodiments of the compounds of Formula (IV), each R5 is H. [00107] In some embodiments of the compounds of Formula (IV), R6 is -F. [00108] In some embodiments of the compounds of Formula (IV), R6 is -Cl. [00109] In some embodiments of the compounds of Formula (IV), R6 is -Br. [00110] In some embodiments of the compounds of Formula (IV), R6 is -I. [00111] In some embodiments of the compounds of Formula (IV), R6 is -CN. [00112] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula IV-1, Formula IV-2, Formula IV-3, or Formula IV-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R2, Rc, Rd, and m are defined with respect to Formula (I). [00113] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula V or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R1, R2, R3, R4, R5, R6, and m are as defined with respect to Formula (I), and Wa is a 5-10 membered - 26 -4877-0794-3771.1 105807.005004– PCT Application heteroaryl group comprising 1-4 heteroatoms selected from N, O, and S, or an aryl group, wherein each group is optionally substituted by 1-6 Rf groups. [00114] In some embodiments of the compounds of Formula (V), each R5 is H. [00115] In some embodiments of the compounds of Formula (V), R6 is -F. [00116] In some embodiments of the compounds of Formula (V), R6 is -Cl. [00117] In some embodiments of the compounds of Formula (V), R6 is -Br. [00118] In some embodiments of the compounds of Formula (V), R6 is -I. [00119] In some embodiments of the compounds of Formula (V), R6 is -CN. [00120] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula V-1, Formula V-2, Formula V-3, or Formula V-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R2 and m are as defined with respect to Formula (I), and Wa is a 5-10 membered heteroaryl group comprising 1-4 heteroatoms selected from N, O, and S, or an aryl group, wherein each group is optionally substituted by 1-6 Rf groups. [00121] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula VI - 27 -4877-0794-3771.1 105807.005004– PCT Application or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R1, R2, R3, R4, R5, R6, m, are defined with respect to Formula (I), and Wb is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, or a cycloalkyl group, wherein each group is optionally substituted by 1-6 Rf groups. [00122] In some embodiments of the compounds of Formula (VI), each R5 is H. [00123] In some embodiments of the compounds of Formula (VI), R6 is -F. [00124] In some embodiments of the compounds of Formula (VI), R6 is -Cl. [00125] In some embodiments of the compounds of Formula (VI), R6 is -Br. [00126] In some embodiments of the compounds of Formula (VI), R6 is -I. [00127] In some embodiments of the compounds of Formula (VI), R6 is -CN. [00128] In some embodiments, the compounds of Formula (I) are represented by compounds of Formula VI-1, Formula VI-2, Formula VI-3, or Formula VI-4: or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof; wherein each R2 and m are as defined with respect to Formula (I), and Wb is a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, or a cycloalkyl group, wherein each group is optionally substituted by 1-6 Rf groups. [00129] In yet further embodiments, the compounds of Formula (I) are: 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6s)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 28 -4877-0794-3771.1 105807.005004– PCT Application 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-[1-[2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethylamino]-6-chloropyridine-2-carboxylic acid; 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4-oxoquinazolin- 8-yl]ethylamino]pyridine-2-carboxylic acid; 6-Chloro-3-((1-(2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(dimethylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(cyclobutyl(methyl)carbamoyl)-3-azabicyclo[3.1.0] hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyrazin-6-yl)-3,6-dimethyl-4-oxoquinazolin- 8-yl]ethylamino]pyridine-2-carboxylic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6s)-6-((pyrrolidine-1-carbonyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-[3,6-dimethyl-2-[1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidin-3-yl]-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(methoxy(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; - 29 -4877-0794-3771.1 105807.005004– PCT Application 6-Chloro-3-[1-[3,6-dimethyl-2-(1-methylpyrazol-4-yl)-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4-(cyclobutanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(4-Acetylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4-(cyclopropanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-2-(4-(methylsulfonyl)piperazin-1-yl)-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-bromopicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropyrazine-2-carboxylic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinamide; Methyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinaldehyde; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinonitrile; - 30 -4877-0794-3771.1 105807.005004– PCT Application 2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-(1-((6-chloro-2-(trifluoromethyl)pyridin-3- yl)amino)ethyl)-3,6-dimethylquinazolin-4(3H)-one; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-cyanopicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-fluoropicolinic acid; 6-Chloro-3-((1-(2-((trans-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 5-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid; 2-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(difluoromethyl)-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)benzoic acid; 6-Chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3-ethyl-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-6- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-fluoro-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(tert-butoxy)-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; - 31 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-[1-[2-[4-(difluoromethyl)piperidin-1-yl]-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 3-[1-[2-(5-azaspiro[2.4]heptan-5-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethylamino]-6- chloropyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-[2-[(2-methylpropan-2-yl)oxycarbonyl]-2,7- diazaspiro[4.4]nonan-7-yl]-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-(2-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3-hydroxy-3-methylazetidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoroazetidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-[4-(dimethylcarbamoyl)piperidin-1-yl]-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3-fluoro-5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(5-fluoro-1,3-dihydroisoindol-2-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-((1-(2-(isoindolin-2-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(6-azaspiro[2.5]octan-6-yl)-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; - 32 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-((1-(2-((3S,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; 6-chloro-3-[1-[2-(3,3-difluoroazetidin-1-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-fluoro-3-methyl-2-(2-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-fluoro-3-methyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-chloro-2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-chloro-2-(3,3-difluoropyrrolidin-1-yl)-3-methyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropyrrolidin-1-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-((1-(6-chloro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-((1R,5S,6s)-6-(carbamoyloxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((isopropylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((ethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((dimethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; or a pharmaceutically acceptable salt, N-oxide, solvate, or stereoisomer thereof thereof. - 33 -4877-0794-3771.1 105807.005004– PCT Application [00130] In other embodiments, the compounds of Formula (I) are: 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6S)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-(((R)-1-(6-fluoro-2-((1R,5S,6S)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(6-fluoro-2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(5,7-dihydro-6H-pyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(5,7-dihydro-6H-pyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6S)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-(dimethylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-(dimethylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 34 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-(cyclobutyl(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-(cyclobutyl(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6S)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(5,7-dihydro-6H-pyrrolo[3,4-b]pyrazin-6-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(5,7-dihydro-6H-pyrrolo[3,4-b]pyrazin-6-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(3,6-dimethyl-4-oxo-2-((1R,5S,6S)-6-((pyrrolidine-1-carbonyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(3,6-dimethyl-4-oxo-2-((1R,5S,6S)-6-((pyrrolidine-1-carbonyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-(((R)-1-(2-((S)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((R)-1-(2-((R)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((S)-1-(2-((S)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((S)-1-(2-((R)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; - 35 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-(methoxy(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-(methoxy(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(6-fluoro-2-((1R,5S,6S)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(6-fluoro-2-((1R,5S,6S)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2,7-dimethyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-5- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2,7-dimethyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-5- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(3,6-dimethyl-2-(1-methyl-1H-pyrazol-4-yl)-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(3,6-dimethyl-2-(1-methyl-1H-pyrazol-4-yl)-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (S)-3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (R)-6-Chloro-3-((1-(2-(4-(cyclobutanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(2-(4-(cyclobutanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; - 36 -4877-0794-3771.1 105807.005004– PCT Application (R)-6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-3-((1-(2-(4-Acetylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (S)-3-((1-(2-(4-Acetylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (R)-6-Chloro-3-((1-(2-(4-(cyclopropanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(2-(4-(cyclopropanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-Chloro-3-((1-(3,6-dimethyl-2-(4-(methylsulfonyl)piperazin-1-yl)-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-Chloro-3-((1-(3,6-dimethyl-2-(4-(methylsulfonyl)piperazin-1-yl)-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-bromopicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-bromopicolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropyrazine-2-carboxylic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropyrazine-2-carboxylic acid; - 37 -4877-0794-3771.1 105807.005004– PCT Application 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinamide; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinamide; methyl 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate; methyl 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinaldehyde; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinaldehyde; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinonitrile; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinonitrile; 2-(3-azabicyclo[3.1.0]hexan-3-yl)-8-((R)-1-((6-chloro-2-(trifluoromethyl)pyridin-3- yl)amino)ethyl)-3,6-dimethylquinazolin-4(3H)-one; 2-(3-azabicyclo[3.1.0]hexan-3-yl)-8-((S)-1-((6-chloro-2-(trifluoromethyl)pyridin-3- yl)amino)ethyl)-3,6-dimethylquinazolin-4(3H)-one; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-cyanopicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-cyanopicolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-fluoropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-fluoropicolinic acid; 6-chloro-3-(((R)-1-(2-((3R,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((3R,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 38 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-(((R)-1-(2-((3S,4S)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((3S,4S)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(6-azaspiro[2.5]octan-6-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(6-azaspiro[2.5]octan-6-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((3S,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((3S,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((3R,4S)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((3R,4S)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 39 -4877-0794-3771.1 105807.005004– PCT Application 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (R)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1R)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1S)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3- 1-(3-ethyl-2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-6-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(3-ethyl-2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-6-methyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinic acid; (R)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; - 40 -4877-0794-3771.1 105807.005004– PCT Application 3-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-(tert-butoxy)-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3-(tert-butoxy)-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((dimethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-((dimethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((ethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-((ethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((1R,5S,6S)-6-((isopropylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((1R,5S,6R)-6-((isopropylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((R)-1-(2-((1R,5S,6S)-6-(carbamoyloxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-((S)-1-(2-((1R,5S,6R)-6-(carbamoyloxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-(((R)-1-(6-chloro-2-((1R,5S,6S)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(6-chloro-2-((1R,5S,6R)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((R)-3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((R)-1-(2-((S)-3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((R)-3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((S)-1-(2-((S)-3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 41 -4877-0794-3771.1 105807.005004– PCT Application 6-chloro-3-(((1R)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1S)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(6-chloro-2-(3,3-difluoropyrrolidin-1-yl)-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(6-chloro-2-(3,3-difluoropyrrolidin-1-yl)-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1R)-1-(6-chloro-2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1S)-1-(6-chloro-2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(6-fluoro-3-methyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(6-fluoro-3-methyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(6-fluoro-3-methyl-2-(2-methyl-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(6-fluoro-3-methyl-2-(2-methyl-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)- 4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(3,3-difluoroazetidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoroazetidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(3,6-dimethyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(3,6-dimethyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 42 -4877-0794-3771.1 105807.005004– PCT Application (R)-6-chloro-3-((1-(2-(4-(dimethylcarbamoyl)piperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4-(dimethylcarbamoyl)piperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(3,3-difluoroazetidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoroazetidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(3-hydroxy-3-methylazetidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3-hydroxy-3-methylazetidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(3,6-dimethyl-2-(2-methyl-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(3,6-dimethyl-2-(2-methyl-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(5-azaspiro[2.4]heptan-5-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(5-azaspiro[2.4]heptan-5-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1R)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-(((1S)-1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; - 43 -4877-0794-3771.1 105807.005004– PCT Application (R)-6-chloro-3-((1-(2-(3,3-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(3,3-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid; (R)-6-chloro-3-((1-(2-(4-(difluoromethyl)piperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; (S)-6-chloro-3-((1-(2-(4-(difluoromethyl)piperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 5-(((1R)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid; 5-(((1S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid; (R)-6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; (S)-6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; or a pharmaceutically acceptable salt, N-oxide, solvate, or stereoisomer thereof thereof. [00131] It will be apparent that the compounds of Formula I, including all subgenera described herein, may have multiple stereogenic centers. As a result, there exist multiple stereoisomers (enantiomers and diastereomers) of the compounds of Formula I (and subgenera described herein). The present disclosure contemplates and encompasses each stereoisomer of any compound of Formula I (and subgenera described herein), as well as mixtures of said stereoisomers. [00132] Pharmaceutically acceptable salts, N-oxides, solvates, or stereoisomers of the compounds of Formula I (including all subgenera described herein) are also within the scope of the disclosure. Thus, in some embodiments, the compounds of the disclosure are pharmaceutically acceptable salts of the compounds of Formula I (including all subgenera described herein). In other embodiments, the compounds of the disclosure are not salts. In some embodiments, the compounds of the disclosure are N-oxides of the compounds of Formula I (including all subgenera described herein). In other embodiments, the compounds of the disclosure are not N-oxides of the compounds of Formula I (including all subgenera described herein). In some embodiments, the compounds of the disclosure are solvates of the compounds of Formula I (including all subgenera described herein). In other embodiments, the compounds - 44 -4877-0794-3771.1 105807.005004– PCT Application of the disclosure are not solvates of the compounds of Formula I (including all subgenera described herein). [00133] Isotopic variants of the compounds of Formula I (including all subgenera described herein) are also contemplated by the present disclosure. [00134] In some embodiments, the compounds of the disclosure include a carboxylic acid moiety. In some aspects, the present disclosure also encompasses carboxylic acid prodrugs of these embodiments. Carboxylic acid prodrugs include, but are not limited to, C1-C6 alkyl esters (e.g., methyl, ethyl, isopropyl, butyl, and isoamyl), 2-aminoethyl esters (e.g., 2- morpholinoethyl), C6-C10 aryl esters (e.g. phenyl, indanyl, and guaiacol), (acyloxy)alkyl esters, [(alkoxycarbonyl)oxy]alkyl esters, and (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl esters. See, e.g., Maag, H. (2007). Prodrugs of Carboxylic Acids. In: Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W. (eds) Prodrugs. Biotechnology: Pharmaceutical Aspects, vol V. Springer, New York, NY. https://doi.org/10.1007/978-0-387-49785-3_20. Pharmaceutical Compositions and Methods of Administration [00135] In some embodiments, the disclosure is directed to pharmaceutical compositions comprising compounds of Formula I, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. [00136] The subject pharmaceutical compositions are typically formulated to provide a therapeutically effective amount of a compound of the present disclosure as the active ingredient, or a pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or derivative thereof. Where desired, the pharmaceutical compositions contain pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, carriers, including inert solid diluents and fillers, diluents, including sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants. [00137] The subject pharmaceutical compositions can be administered alone or in combination with one or more other agents, which are also typically administered in the form of pharmaceutical compositions. Where desired, the one or more compounds of the invention and other agent(s) may be mixed into a preparation or both components may be formulated into separate preparations to use them in combination separately or at the same time. [00138] In some embodiments, the concentration of one or more compounds provided in the pharmaceutical compositions of the present invention is less than 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, - 45 -4877-0794-3771.1 105807.005004– PCT Application 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%, 0.004%, 0.003%, 0.002%, 0.001%, 0.0009%, 0.0008%, 0.0007%, 0.0006%, 0.0005%, 0.0004%, 0.0003%, 0.0002%, or 0.0001% (or a number in the range defined by and including any two numbers above) w/w, w/v or v/v. [00139] In some embodiments, the concentration of one or more compounds of the invention is greater than 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 19.75%, 19.50%, 19.25%, 19%, 18.75%, 18.50%, 18.25% 18%, 17.75%, 17.50%, 17.25% 17%, 16.75%, 16.50%, 16.25%, 16%, 15.75%, 15.50%, 15.25% 15%, 14.75%, 14.50%, 14.25% 14%, 13.75%, 13.50%, 13.25%, 13%, 12.75%, 12.50%, 12.25%, 12%, 11.75%, 11.50%, 11.25% 11%, 10.75%, 10.50%, 10.25% 10%, 9.75%, 9.50%, 9.25%, 9%, 8.75%, 8.50%, 8.25% 8%, 7.75%, 7.50%, 7.25%, 7%, 6.75%, 6.50%, 6.25%, 6%, 5.75%, 5.50%, 5.25%, 5%, 4.75%, 4.50%, 4.25%, 4%, 3.75%, 3.50%, 3.25%, 3%, 2.75%, 2.50%, 2.25%, 2%, 1.75%, 1.50%, 1.25% , 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%, 0.09%, 0.08%, 0.07%, 0.06%, 0.05%, 0.04%, 0.03%, 0.02%, 0.01%, 0.009%, 0.008%, 0.007%, 0.006%, 0.005%, 0.004%, 0.003%, 0.002%, 0.001%, 0.0009%, 0.0008%, 0.0007%, 0.0006%, 0.0005%, 0.0004%, 0.0003%, 0.0002%, or 0.0001% (or a number in the range defined by and including any two numbers above) w/w, w/v, or v/v. [00140] In some embodiments, the concentration of one or more compounds of the invention is in the range from approximately 0.0001% to approximately 50%, approximately 0.001% to approximately 40%, approximately 0.01% to approximately 30%, approximately 0.02% to approximately 29%, approximately 0.03% to approximately 28%, approximately 0.04% to approximately 27%, approximately 0.05% to approximately 26%, approximately 0.06% to approximately 25%, approximately 0.07% to approximately 24%, approximately 0.08% to approximately 23%, approximately 0.09% to approximately 22%, approximately 0.1% to approximately 21%, approximately 0.2% to approximately 20%, approximately 0.3% to approximately 19%, approximately 0.4% to approximately 18%, approximately 0.5% to approximately 17%, approximately 0.6% to approximately 16%, approximately 0.7% to approximately 15%, approximately 0.8% to approximately 14%, approximately 0.9% to approximately 12%, approximately 1% to approximately 10% w/w, w/v or v/v. [00141] In some embodiments, the concentration of one or more compounds of the invention is in the range from approximately 0.001% to approximately 10%, approximately 0.01% to approximately 5%, approximately 0.02% to approximately 4.5%, approximately 0.03% to approximately 4%, approximately 0.04% to approximately 3.5%, approximately 0.05% to - 46 -4877-0794-3771.1 105807.005004– PCT Application approximately 3%, approximately 0.06% to approximately 2.5%, approximately 0.07% to approximately 2%, approximately 0.08% to approximately 1.5%, approximately 0.09% to approximately 1%, approximately 0.1% to approximately 0.9% w/w, w/v or v/v. [00142] In some embodiments, the amount of one or more compounds of the invention is equal to or less than 10 g, 9.5 g, 9.0 g, 8.5 g, 8.0 g, 7.5 g, 7.0 g, 6.5 g, 6.0 g, 5.5 g, 5.0 g, 4.5 g, 4.0 g, 3.5 g, 3.0 g, 2.5 g, 2.0 g, 1.5 g, 1.0 g, 0.95 g, 0.9 g, 0.85 g, 0.8 g, 0.75 g, 0.7 g, 0.65 g, 0.6 g, 0.55 g, 0.5 g, 0.45 g, 0.4 g, 0.35 g, 0.3 g, 0.25 g, 0.2 g, 0.15 g, 0.1 g, 0.09 g, 0.08 g, 0.07 g, 0.06 g, 0.05 g, 0.04 g, 0.03 g, 0.02 g, 0.01 g, 0.009 g, 0.008 g, 0.007 g, 0.006 g, 0.005 g, 0.004 g, 0.003 g, 0.002 g, 0.001 g, 0.0009 g, 0.0008 g, 0.0007 g, 0.0006 g, 0.0005 g, 0.0004 g, 0.0003 g, 0.0002 g, or 0.0001 g (or a number in the range defined by and including any two numbers above). [00143] In some embodiments, the amount of one or more compounds of the invention is more than 0.0001 g, 0.0002 g, 0.0003 g, 0.0004 g, 0.0005 g, 0.0006 g, 0.0007 g, 0.0008 g, 0.0009 g, 0.001 g, 0.0015 g, 0.002 g, 0.0025 g, 0.003 g, 0.0035 g, 0.004 g, 0.0045 g, 0.005 g, 0.0055 g, 0.006 g, 0.0065 g, 0.007 g, 0.0075 g, 0.008 g, 0.0085 g, 0.009 g, 0.0095 g, 0.01 g, 0.015 g, 0.02 g, 0.025 g, 0.03 g, 0.035 g, 0.04 g, 0.045 g, 0.05 g, 0.055 g, 0.06 g, 0.065 g, 0.07 g, 0.075 g, 0.08 g, 0.085 g, 0.09 g, 0.095 g, 0.1 g, , 0.15 g, 0.2 g, , 0.25 g, 0.3 g, , 0.35 g, 0.4 g, , 0.45 g, 0.5 g, 0.55 g, 0.6 g, , 0.65 g, 0.7 g, 0.75 g, 0.8 g, 0.85 g, 0.9 g, 0.95 g, 1 g, 1.5 g, 2 g, 2.5, 3 g, 3.5, 4 g, 4.5 g, 5 g, 5.5 g, 6 g, 6.5g, 7 g, 7.5g, 8 g, 8.5 g, 9 g, 9.5 g, or 10 g (or a number in the range defined by and including any two numbers above). [00144] In some embodiments, the amount of one or more compounds of the invention is in the range of 0.0001-10 g, 0.0005-9 g, 0.001-8 g, 0.005-7 g, 0.01-6 g, 0.05-5 g, 0.1-4 g, 0.5-4 g, or 1-3 g. [00145] The compounds according to the invention are effective over a wide dosage range. For example, in the treatment of adult humans, dosages from 0.01 to 1000 mg, from 0.5 to 100 mg, from 1 to 50 mg per day, and from 5 to 40 mg per day are examples of dosages that may be used. An exemplary dosage is 10 to 30 mg per day. The exact dosage will depend upon the route of administration, the form in which the compound is administered, the subject to be treated, the body weight of the subject to be treated, and the preference and experience of the attending physician. [00146] A pharmaceutical composition of the invention typically contains an active ingredient (i.e., a compound of the disclosure) of the present invention or a pharmaceutically acceptable salt and/or coordination complex thereof, and one or more pharmaceutically acceptable excipients, - 47 -4877-0794-3771.1 105807.005004– PCT Application carriers, including but not limited to inert solid diluents and fillers, diluents, sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants. [00147] Described below are non- limiting exemplary pharmaceutical compositions and methods for preparing the same. Pharmaceutical Compositions for Oral Administration. [00148] In some embodiments, the invention provides a pharmaceutical composition for oral administration containing a compound of the invention, and a pharmaceutical excipient suitable for oral administration. [00149] In some embodiments, the invention provides a solid pharmaceutical composition for oral administration containing: (i) an effective amount of a compound of the invention; optionally (ii) an effective amount of a second agent; and (iii) a pharmaceutical excipient suitable for oral administration. In some embodiments, the composition further contains: (iv) an effective amount of a third agent. [00150] In some embodiments, the pharmaceutical composition may be a liquid pharmaceutical composition suitable for oral consumption. Pharmaceutical compositions of the invention suitable for oral administration can be presented as discrete dosage forms, such as capsules, cachets, or tablets, or liquids or aerosol sprays each containing a predetermined amount of an active ingredient as a powder or in granules, a solution, or a suspension in an aqueous or non-aqueous liquid, an oil-in- water emulsion, or a water-in-oil liquid emulsion. Such dosage forms can be prepared by any of the methods of pharmacy, but all methods include the step of bringing the active ingredient into association with the carrier, which constitutes one or more necessary ingredients. In general, the compositions are prepared by uniformly and intimately admixing the active ingredient with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product into the desired presentation. For example, a tablet can be prepared by compression or molding, optionally with one or more accessory ingredients. Compressed tablets can be prepared by compressing in a suitable machine the active ingredient in a free- flowing form such as powder or granules, optionally mixed with an excipient such as, but not limited to, a binder, a lubricant, an inert diluent, and/or a surface active or dispersing agent. Molded tablets can be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent. [00151] This invention further encompasses anhydrous pharmaceutical compositions and dosage forms comprising an active ingredient, since water can facilitate the degradation of some - 48 -4877-0794-3771.1 105807.005004– PCT Application compounds. For example, water may be added (e.g., 5%) in the pharmaceutical arts as a means of simulating long-term storage in order to determine characteristics such as shelf- life or the stability of formulations over time. Anhydrous pharmaceutical compositions and dosage forms of the invention can be prepared using anhydrous or low moisture containing ingredients and low moisture or low humidity conditions. Pharmaceutical compositions and dosage forms of the invention which contain lactose can be made anhydrous if substantial contact with moisture and/or humidity during manufacturing, packaging, and/or storage is expected. An anhydrous pharmaceutical composition may be prepared and stored such that its anhydrous nature is maintained. Accordingly, anhydrous compositions may be packaged using materials known to prevent exposure to water such that they can be included in suitable formulary kits. Examples of suitable packaging include, but are not limited to, hermetically sealed foils, plastic or the like, unit dose containers, blister packs, and strip packs. [00152] An active ingredient can be combined in an intimate admixture with a pharmaceutical carrier according to conventional pharmaceutical compounding techniques. The carrier can take a wide variety of forms depending on the form of preparation desired for administration. In preparing the compositions for an oral dosage form, any of the usual pharmaceutical media can be employed as carriers, such as, for example, water, glycols, oils, alcohols, flavoring agents, preservatives, coloring agents, and the like in the case of oral liquid preparations (such as suspensions, solutions, and elixirs) or aerosols; or carriers such as starches, sugars, micro- crystalline cellulose, diluents, granulating agents, lubricants, binders, and disintegrating agents can be used in the case of oral solid preparations, in some embodiments without employing the use of lactose. For example, suitable carriers include powders, capsules, and tablets, with the solid oral preparations. If desired, tablets can be coated by standard aqueous or nonaqueous techniques. [00153] Binders suitable for use in pharmaceutical compositions and dosage forms include, but are not limited to, corn starch, potato starch, or other starches, gelatin, natural and synthetic gums such as acacia, sodium alginate, alginic acid, other alginates, powdered tragacanth, guar gum, cellulose and its derivatives (e.g., ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose), polyvinyl pyrrolidone, methyl cellulose, pre-gelatinized starch, hydroxy-propyl methyl cellulose, microcrystalline cellulose, and mixtures thereof. [00154] Examples of suitable fillers for use in the pharmaceutical compositions and dosage forms disclosed herein include, but are not limited to, talc, calcium carbonate (e.g., granules or - 49 -4877-0794-3771.1 105807.005004– PCT Application powder), microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch, and mixtures thereof. [00155] Disintegrants may be used in the compositions of the invention to provide tablets that disintegrate when exposed to an aqueous environment. Too much of a disintegrant may produce tablets which may disintegrate in the bottle. Too little may be insufficient for disintegration to occur and may thus alter the rate and extent of release of the active ingredient(s) from the dosage form. Thus, a sufficient amount of disintegrant that is neither too little nor too much to detrimentally alter the release of the active ingredient(s) may be used to form the dosage forms of the compounds disclosed herein. The amount of disintegrant used may vary based upon the type of formulation and mode of administration, and may be readily discernible to those of ordinary skill in the art. About 0.5 to about 15 weight percent of disintegrant, or about 1 to about 5 weight percent of disintegrant, may be used in the pharmaceutical composition. Disintegrants that can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, agar-agar, alginic acid, calcium carbonate, microcrystalline cellulose, croscarmellose sodium, crospovidone, polacrilin potassium, sodium starch glycolate, potato or tapioca starch, other starches, pre-gelatinized starch, other starches, clays, other algins, other celluloses, gums or mixtures thereof. [00156] Lubricants which can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, calcium stearate, magnesium stearate, mineral oil, light mineral oil, glycerin, sorbitol, mannitol, polyethylene glycol, other glycols, stearic acid, sodium lauryl sulfate, talc, hydrogenated vegetable oil (e.g., peanut oil, cottonseed oil, sunflower oil, sesame oil, olive oil, corn oil, and soybean oil), zinc stearate, ethyl oleate, ethyl laureate, agar, or mixtures thereof. Additional lubricants include, for example, a syloid silica gel, a coagulated aerosol of synthetic silica, or mixtures thereof. A lubricant can optionally be added, in an amount of less than about 1 weight percent of the pharmaceutical composition. [00157] When aqueous suspensions and/or elixirs are desired for oral administration, the active ingredient therein may be combined with various sweetening or flavoring agents, coloring matter or dyes and, if so desired, emulsifying and/or suspending agents, together with such diluents as water, ethanol, propylene glycol, glycerin and various combinations thereof. [00158] The tablets can be uncoated or coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a time delay material such as glyceryl monostearate or glyceryl distearate can be employed. Formulations for oral use can also be presented as hard gelatin capsules - 50 -4877-0794-3771.1 105807.005004– PCT Application wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example, peanut oil, liquid paraffin or olive oil. [00159] Surfactant which can be used to form pharmaceutical compositions and dosage forms of the invention include, but are not limited to, hydrophilic surfactants, lipophilic surfactants, and mixtures thereof. That is, a mixture of hydrophilic surfactants may be employed, a mixture of lipophilic surfactants may be employed, or a mixture of at least one hydrophilic surfactant and at least one lipophilic surfactant may be employed. [00160] A suitable hydrophilic surfactant may generally have an HLB value of at least 10, while suitable lipophilic surfactants may generally have an HLB value of or less than about 10. An empirical parameter used to characterize the relative hydrophilicity and hydrophobicity of non-ionic amphiphilic compounds is the hydrophilic-lipophilic balance (“HLB” value). Surfactants with lower HLB values are more lipophilic or hydrophobic, and have greater solubility in oils, while surfactants with higher HLB values are more hydrophilic, and have greater solubility in aqueous solutions. [00161] Hydrophilic surfactants are generally considered to be those compounds having an HLB value greater than about 10, as well as anionic, cationic, or zwitterionic compounds for which the HLB scale is not generally applicable. Similarly, lipophilic (i.e., hydrophobic) surfactants are compounds having an HLB value equal to or less than about 10. However, HLB value of a surfactant is merely a rough guide generally used to enable formulation of industrial, pharmaceutical and cosmetic emulsions. [00162] Hydrophilic surfactants may be either ionic or non-ionic. Suitable ionic surfactants include, but are not limited to, alkylammonium salts; fusidic acid salts; fatty acid derivatives of amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; lecithins and hydrogenated lecithins; lysolecithins and hydrogenated lysolecithins; phospholipids and derivatives thereof; lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acyl lactylates; mono- and di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di-glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof. [00163] Within the aforementioned group, ionic surfactants include, by way of example: lecithins, lysolecithin, phospholipids, lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkylsulfates; fatty acid salts; sodium docusate; acylactylates; mono- and - 51 -4877-0794-3771.1 105807.005004– PCT Application di-acetylated tartaric acid esters of mono- and di-glycerides; succinylated mono- and di- glycerides; citric acid esters of mono- and di-glycerides; and mixtures thereof. [00164] Ionic surfactants may be the ionized forms of lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, phosphatidylserine, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, lysophosphatidylserine, PEG- phosphatidylethanolamine, PVP -phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholylsarcosine, caproate, caprylate, caprate, laurate, myristate, palmitate, oleate, ricinoleate, linoleate, linolenate, stearate, lauryl sulfate, teracecyl sulfate, docusate, lauroyl carnitines, palmitoyl carnitines, myristoyl carnitines, and salts and mixtures thereof. [00165] Hydrophilic non-ionic surfactants may include, but are not limited to, alkylglucosides; alkylmaltosides; alkylthioglucosides; lauryl macrogolglycerides; polyoxyalkylene alkyl ethers such as polyethylene glycol alkyl ethers; polyoxyalkylene alkylphenols such as polyethylene glycol alkyl phenols; polyoxyalkylene alkyl phenol fatty acid esters such as polyethylene glycol fatty acids monoesters and polyethylene glycol fatty acids diesters; polyethylene glycol glycerol fatty acid esters; polyglycerol fatty acid esters; polyoxyalkylene sorbitan fatty acid esters such as polyethylene glycol sorbitan fatty acid esters; hydrophilic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids, and sterols; polyoxyethylene sterols, derivatives, and analogues thereof; polyoxyethylated vitamins and derivatives thereof; polyoxyethylene-polyoxypropylene block copolymers; and mixtures thereof; polyethylene glycol sorbitan fatty acid esters and hydrophilic transesterification products of a polyol with at least one member of the group consisting of triglycerides, vegetable oils, and hydrogenated vegetable oils. The polyol may be glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide. [00166] Other hydrophilic-non-ionic surfactants include, without limitation, PEG-10 laurate, PEG- 12 laurate, PEG-20 laurate, PEG-32 laurate, PEG-32 dilaurate, PEG-12 oleate, PEG-15 oleate, PEG-20 oleate, PEG-20 dioleate, PEG-32 oleate, PEG-200 oleate, PEG-400 oleate, PEG- 15 stearate, PEG-32 distearate, PEG-40 stearate, PEG- 100 stearate, PEG-20 dilaurate, PEG-25 glyceryl trioleate, PEG-32 dioleate, PEG-20 glyceryl laurate, PEG-30 glyceryl laurate, PEG-20 glyceryl stearate, PEG-20 glyceryl oleate, PEG-30 glyceryl oleate, PEG-30 glyceryl laurate, - 52 -4877-0794-3771.1 105807.005004– PCT Application PEG-40 glyceryl laurate, PEG-40 palm kernel oil, PEG-50 hydrogenated castor oil, PEG-40 castor oil, PEG-35 castor oil, PEG-60 castor oil, PEG-40 hydrogenated castor oil, PEG-60 hydrogenated castor oil, PEG-60 corn oil, PEG-6 caprate/caprylate glycerides, PEG-8 caprate/caprylate glycerides, polyglyceryl-10 laurate, PEG-30 cholesterol, PEG-25 phyto sterol, PEG-30 soya sterol, PEG-20 trioleate, PEG-40 sorbitan oleate, PEG-80 sorbitan laurate, polysorbate 20, polysorbate 80, POE-9 lauryl ether, POE-23 lauryl ether, POE-10 oleyl ether, POE-20 oleyl ether, POE-20 stearyl ether, tocopheryl PEG- 100 succinate, PEG-24 cholesterol, polyglyceryl-lOoleate, Tween 40, Tween 60, sucrose monostearate, sucrose mono laurate, sucrose monopalmitate, PEG 10-100 nonyl phenol series, PEG 15-100 octyl phenol series, and poloxamers. [00167] Suitable lipophilic surfactants include, by way of example only: fatty alcohols; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; propylene glycol fatty acid esters; sorbitan fatty acid esters; polyethylene glycol sorbitan fatty acid esters; sterols and sterol derivatives; polyoxyethylated sterols and sterol derivatives; polyethylene glycol alkyl ethers; sugar esters; sugar ethers; lactic acid derivatives of mono- and di-glycerides; hydrophobic transesterification products of a polyol with at least one member of the group consisting of glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids and sterols; oil-soluble vitamins/vitamin derivatives; and mixtures thereof. Within this group, preferred lipophilic surfactants include glycerol fatty acid esters, propylene glycol fatty acid esters, and mixtures thereof, or are hydrophobic transesterification products of a polyol with at least one member of the group consisting of vegetable oils, hydrogenated vegetable oils, and triglycerides. [00168] In one embodiment, the composition may include a solubilizer to ensure good solubilization and/or dissolution of the compound of the present invention and to minimize precipitation of the compound of the present invention. This can be especially important for compositions for non-oral use, e.g., compositions for injection. A solubilizer may also be added to increase the solubility of the hydrophilic drug and/or other components, such as surfactants, or to maintain the composition as a stable or homogeneous solution or dispersion. [00169] Examples of suitable solubilizers include, but are not limited to, the following: alcohols and polyols, such as ethanol, isopropanol, butanol, benzyl alcohol, ethylene glycol, propylene glycol, butanediols and isomers thereof, glycerol, pentaerythritol, sorbitol, mannitol, transcutol, dimethyl isosorbide, polyethylene glycol, polypropylene glycol, polyvinylalcohol, hydroxypropyl methylcellulose and other cellulose derivatives, cyclodextrins and cyclodextrin - 53 -4877-0794-3771.1 105807.005004– PCT Application derivatives; ethers of polyethylene glycols having an average molecular weight of about 200 to about 6000, such as tetrahydrofurfuryl alcohol PEG ether (glycofurol) or methoxy PEG ; amides and other nitrogen-containing compounds such as 2-pyrrolidone, 2-piperidone, ε-caprolactam, N- alkylpyrrolidone, N-hydroxyalkylpyrrolidone, N-alkylpiperidone, N-alkylcaprolactam, dimethylacetamide and polyvinylpyrrolidone; esters such as ethyl propionate, tributylcitrate, acetyl triethylcitrate, acetyl tributyl citrate, triethylcitrate, ethyl oleate, ethyl caprylate, ethyl butyrate, triacetin, propylene glycol monoacetate, propylene glycol diacetate, ε-caprolactone and isomers thereof, δ-valerolactone and isomers thereof, β-butyrolactone and isomers thereof; and other solubilizers known in the art, such as dimethyl acetamide, dimethyl isosorbide, N-methyl pyrrolidones, monooctanoin, diethylene glycol monoethyl ether, and water. [00170] Mixtures of solubilizers may also be used. Examples include, but not limited to, triacetin, triethylcitrate, ethyl oleate, ethyl caprylate, dimethylacetamide, N-methylpyrrolidone, N-hydroxy-ethylpyrrolidone, polyvinylpyrrolidone, hydroxypropyl methylcellulose, hydroxypropyl cyclodextrins, ethanol, polyethylene glycol 200-100, glycofurol, transcutol, propylene glycol, and dimethyl isosorbide. Particularly preferred solubilizers include sorbitol, glycerol, triacetin, ethyl alcohol, PEG-400, glycofurol and propylene glycol. [00171] The amount of solubilizer that can be included is not particularly limited. The amount of a given solubilizer may be limited to a bioacceptable amount, which may be readily determined by one of skill in the art. In some circumstances, it may be advantageous to include amounts of solubilizers far in excess of bioacceptable amounts, for example to maximize the concentration of the drug, with excess solubilizer removed prior to providing the composition to a subject using conventional techniques, such as distillation or evaporation. Thus, if present, the solubilizer can be in a weight ratio of 10%, 25%o, 50%), 100%o, or up to about 200%> by weight, based on the combined weight of the drug, and other excipients. If desired, very small amounts of solubilizer may also be used, such as 5%>, 2%>, 1%) or even less. Typically, the solubilizer may be present in an amount of about 1%> to about 100%, more typically about 5%> to about 25%> by weight. [00172] The composition can further include one or more pharmaceutically acceptable additives and excipients. Such additives and excipients include, without limitation, detackifiers, anti-foaming agents, buffering agents, polymers, antioxidants, preservatives, chelating agents, viscomodulators, tonicifiers, flavorants, colorants, odorants, opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof. - 54 -4877-0794-3771.1 105807.005004– PCT Application [00173] In addition, an acid or a base may be incorporated into the composition to facilitate processing, to enhance stability, or for other reasons. Examples of pharmaceutically acceptable bases include amino acids, amino acid esters, ammonium hydroxide, potassium hydroxide, sodium hydroxide, sodium hydrogen carbonate, aluminum hydroxide, calcium carbonate, magnesium hydroxide, magnesium aluminum silicate, synthetic aluminum silicate, synthetic hydrocalcite, magnesium aluminum hydroxide, diisopropylethylamine, ethanolamine, ethylenediamine, triethanolamine, triethylamine, triisopropanolamine, trimethylamine, tris(hydroxymethyl)-aminomethane (TRIS) and the like. Also suitable are bases that are salts of a pharmaceutically acceptable acid, such as acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p-toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid, and the like. Salts of polyprotic acids, such as sodium phosphate, disodium hydrogen phosphate, and sodium dihydrogen phosphate can also be used. When the base is a salt, the cation can be any convenient and pharmaceutically acceptable cation, such as ammonium, alkali metals, alkaline earth metals, and the like. Example may include, but not limited to, sodium, potassium, lithium, magnesium, calcium and ammonium. [00174] Suitable acids are pharmaceutically acceptable organic or inorganic acids. Examples of suitable inorganic acids include hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid, nitric acid, boric acid, phosphoric acid, and the like. Examples of suitable organic acids include acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acids, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, methanesulfonic acid, oxalic acid, para-bromophenylsulfonic acid, propionic acid, p- toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid, uric acid and the like. Pharmaceutical Compositions for Injection. [00175] In some embodiments, the invention provides a pharmaceutical composition for injection containing a compound of the present invention and a pharmaceutical excipient suitable for injection. Components and amounts of agents in the compositions are as described herein. - 55 -4877-0794-3771.1 105807.005004– PCT Application [00176] The forms in which the novel compositions of the present invention may be incorporated for administration by injection include aqueous or oil suspensions, or emulsions, with sesame oil, corn oil, cottonseed oil, or peanut oil, as well as elixirs, mannitol, dextrose, or a sterile aqueous solution, and similar pharmaceutical vehicles. [00177] Aqueous solutions in saline are also conventionally used for injection. Ethanol, glycerol, propylene glycol, liquid polyethylene glycol, and the like (and suitable mixtures thereof), cyclodextrin derivatives, and vegetable oils may also be employed. The proper fluidity can be maintained, for example, by the use of a coating, such as lecithin, for the maintenance of the required particle size in the case of dispersion and by the use of surfactants. The prevention of the action of microorganisms can be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the like. [00178] Sterile injectable solutions are prepared by incorporating the compound of the present invention in the required amount in the appropriate solvent with various other ingredients as enumerated above, as required, followed by filtered sterilization. Generally, dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above. In the case of sterile powders for the preparation of sterile injectable solutions, certain desirable methods of preparation are vacuum-drying and freeze- drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile- filtered solution thereof. Pharmaceutical Compositions for Topical (e.g., Transdermal) Delivery. [00179] In some embodiments, the invention provides a pharmaceutical composition for transdermal delivery containing a compound of the present invention and a pharmaceutical excipient suitable for transdermal delivery. [00180] Compositions of the present invention can be formulated into preparations in solid, semisolid, or liquid forms suitable for local or topical administration, such as gels, water soluble jellies, creams, lotions, suspensions, foams, powders, slurries, ointments, solutions, oils, pastes, suppositories, sprays, emulsions, saline solutions, dimethylsulfoxide (DMSO)-based solutions. In general, carriers with higher densities are capable of providing an area with a prolonged exposure to the active ingredients. In contrast, a solution formulation may provide more immediate exposure of the active ingredient to the chosen area. - 56 -4877-0794-3771.1 105807.005004– PCT Application [00181] The pharmaceutical compositions also may comprise suitable solid or gel phase carriers or excipients, which are compounds that allow increased penetration of, or assist in the delivery of, therapeutic molecules across the stratum corneum permeability barrier of the skin. There are many of these penetration- enhancing molecules known to those trained in the art of topical formulation. [00182] Examples of such carriers and excipients include, but are not limited to, humectants (e.g., urea), glycols (e.g., propylene glycol), alcohols (e.g., ethanol), fatty acids (e.g., oleic acid), surfactants (e.g., isopropyl myristate and sodium lauryl sulfate), pyrrolidones, glycerol monolaurate, sulfoxides, terpenes (e.g., menthol), amines, amides, alkanes, alkanols, water, calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols. [00183] Another exemplary formulation for use in the methods of the present invention employs transdermal delivery devices ("patches"). Such transdermal patches may be used to provide continuous or discontinuous infusion of a compound of the present invention in controlled amounts, either with or without another agent. [00184] The construction and use of transdermal patches for the delivery of pharmaceutical agents is well known in the art. See, e.g., U.S. Pat. Nos.5,023,252, 4,992,445 and 5,001,139. Such patches may be constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents. Pharmaceutical Compositions for Inhalation. [00185] Compositions for inhalation or insufflation include solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders. The liquid or solid compositions may contain suitable pharmaceutically acceptable excipients as described supra. Preferably the compositions are administered by the oral or nasal respiratory route for local or systemic effect. Compositions in preferably pharmaceutically acceptable solvents may be nebulized by use of inert gases. Nebulized solutions may be inhaled directly from the nebulizing device or the nebulizing device may be attached to a face mask tent, or intermittent positive pressure breathing machine. Solution, suspension, or powder compositions may be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner. Other Pharmaceutical Compositions. - 57 -4877-0794-3771.1 105807.005004– PCT Application [00186] Pharmaceutical compositions may also be prepared from compositions described herein and one or more pharmaceutically acceptable excipients suitable for sublingual, buccal, rectal, intraosseous, intraocular, intranasal, epidural, or intraspinal administration. Preparations for such pharmaceutical compositions are well-known in the art. See, e.g., Anderson, Philip O.; Knoben, James E.; Troutman, William G, eds., Handbook of Clinical Drug Data, Tenth Edition, McGraw-Hill, 2002; Pratt and Taylor, eds., Principles of Drug Action, Third Edition, Churchill Livingston, New York, 1990; Katzung, ed., Basic and Clinical Pharmacology, Ninth Edition, McGraw Hill, 20037ybg; Goodman and Gilman, eds., The Pharmacological Basis of Therapeutics, Tenth Edition, McGraw Hill, 2001 ; Remingtons Pharmaceutical Sciences, 20th Ed., Lippincott Williams & Wilkins., 2000; Martindale, The Extra Pharmacopoeia, Thirty- Second Edition (The Pharmaceutical Press, London, 1999); all of which are incorporated by reference herein in their entirety. [00187] Administration of the compounds or pharmaceutical composition of the present invention can be affected by any method that enables delivery of the compounds to the site of action. These methods include oral routes, intraduodenal routes, parenteral injection (including intravenous, intraarterial, subcutaneous, intramuscular, intravascular, intraperitoneal or infusion), topical (e.g., transdermal application), rectal administration, via local delivery by catheter or stent or through inhalation. Compounds can also be administered intraadiposally or intrathecally. [00188] The amount of the compound administered will be dependent on the subject being treated, the severity of the disorder or condition, the rate of administration, the disposition of the compound and the discretion of the prescribing physician. However, an effective dosage is in the range of about 0.001 to about 100 mg per kg body weight per day, preferably about 1 to about 35 mg/kg/day, in single or divided doses. For a 70 kg human, this would amount to about 0.05 to 7 g/day, preferably about 0.05 to about 2.5 g/day. In some instances, dosage levels below the lower limit of the aforesaid range may be more than adequate, while in other cases still larger doses may be employed without causing any harmful side effect, e.g., by dividing such larger doses into several small doses for administration throughout the day. [00189] In some embodiments, a compound of the invention is administered in a single dose. [00190] Typically, such administration will be by injection, e.g., intravenous injection, in order to introduce the agent quickly. However, other routes may be used as appropriate. A single dose of a compound of the invention may also be used for treatment of an acute condition. [00191] In some embodiments, a compound of the invention is administered in multiple doses. Dosing may be about once, twice, three times, four times, five times, six times, or more than six - 58 -4877-0794-3771.1 105807.005004– PCT Application times per day. Dosing may be about once a month, once every two weeks, once a week, or once every other day. In another embodiment a compound of the invention and another agent are administered together about once per day to about 6 times per day. In another embodiment the administration of a compound of the invention and an agent continues for less than about 7 days. In yet another embodiment the administration continues for more than about 6, 10, 14, 28 days, two months, six months, or one year. In some cases, continuous dosing is achieved and maintained as long as necessary. [00192] Administration of the compounds of the invention may continue as long as necessary. In some embodiments, a compound of the invention is administered for more than 1, 2, 3, 4, 5, 6, 7, 14, or 28 days. In some embodiments, a compound of the invention is administered for less than 28, 14, 7, 6, 5, 4, 3, 2, or 1 day. In some embodiments, a compound of the invention is administered chronically on an ongoing basis, e.g., for the treatment of chronic effects. [00193] An effective amount of a compound of the invention may be administered in either single or multiple doses by any of the accepted modes of administration of agents having similar utilities, including rectal, buccal, intranasal and transdermal routes, by intra-arterial injection, intravenously, intraperitoneally, parenterally, intramuscularly, subcutaneously, orally, topically, or as an inhalant. [00194] The compositions of the invention may also be delivered via an impregnated or coated device such as a stent, for example, or an artery-inserted cylindrical polymer. Such a method of administration may, for example, aid in the prevention or amelioration of restenosis following procedures such as balloon angioplasty. Without being bound by theory, compounds of the invention may slow or inhibit the migration and proliferation of smooth muscle cells in the arterial wall which contribute to restenosis. A compound of the invention may be administered, for example, by local delivery from the struts of a stent, from a stent graft, from grafts, or from the cover or sheath of a stent. In some embodiments, a compound of the invention is admixed with a matrix. Such a matrix may be a polymeric matrix and may serve to bond the compound to the stent. Polymeric matrices suitable for such use, include, for example, lactone-based polyesters or copolyesters such as polylactide, polycaprolactonglycolide, polyorthoesters, polyanhydrides, polyaminoacids, polysaccharides, polyphosphazenes, poly (ether-ester) copolymers (e.g. PEO-PLLA); polydimethylsiloxane, poly(ethylene-vinylacetate), acrylate-based polymers or copolymers (e.g. polyhydroxyethyl methylmethacrylate, polyvinyl pyrrolidinone), fluorinated polymers such as polytetrafluoroethylene and cellulose esters. Suitable matrices may be nondegrading or may degrade with time, releasing the compound or compounds. Compounds - 59 -4877-0794-3771.1 105807.005004– PCT Application of the invention may be applied to the surface of the stent by various methods such as dip/spin coating, spray coating, dip-coating, and/or brush-coating. The compounds may be applied in a solvent and the solvent may be allowed to evaporate, thus forming a layer of compound onto the stent. Alternatively, the compound may be located in the body of the stent or graft, for example in microchannels or micropores. When implanted, the compound diffuses out of the body of the stent to contact the arterial wall. Such stents may be prepared by dipping a stent manufactured to contain such micropores or microchannels into a solution of the compound of the invention in a suitable solvent, followed by evaporation of the solvent. Excess drug on the surface of the stent may be removed via an additional brief solvent wash. In yet other embodiments, compounds of the invention may be covalently linked to a stent or graft. A covalent linker may be used which degrades in vivo, leading to the release of the compound of the invention. Any bio-labile linkage may be used for such a purpose, such as ester, amide or anhydride linkages. Compounds of the invention may additionally be administered intravascularly from a balloon used during angioplasty. Extravascular administration of the compounds via the pericard or via advential application of formulations of the invention may also be performed to decrease restenosis. [00195] A variety of stent devices which may be used as described are disclosed, for example, in the following references, all of which are hereby incorporated by reference: U.S. Pat. No. 5451233; U.S. Pat. No.5040548; U.S. Pat. No.5061273; U.S. Pat. No.5496346; U.S. Pat. No. 5292331; U.S. Pat. No.5674278; U.S. Pat. No.3657744; U.S. Pat. No.4739762; U.S. Pat. No. 5195984; U.S. Pat. No.5292331 ; U.S. Pat. No.5674278; U.S. Pat. No.5879382; U.S. Pat. No. 6344053. [00196] The compounds of the invention may be administered in dosages. It is known in the art that due to intersubject variability in compound pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy. Dosing for a compound of the invention may be found by routine experimentation in light of the instant disclosure. [00197] When a compound of the invention is administered in a composition that comprises one or more agents, and the agent has a shorter half- life than the compound of the invention unit dose forms of the agent and the compound of the invention may be adjusted accordingly. [00198] The subject pharmaceutical composition may, for example, be in a form suitable for oral administration as a tablet, capsule, pill, powder, sustained release formulations, solution, suspension, for parenteral injection as a sterile solution, suspension or emulsion, for topical administration as an ointment or cream or for rectal administration as a suppository. The pharmaceutical composition may be in unit dosage forms suitable for single administration of - 60 -4877-0794-3771.1 105807.005004– PCT Application precise dosages. The pharmaceutical composition will include a conventional pharmaceutical carrier or excipient and a compound according to the invention as an active ingredient. In addition, it may include other medicinal or pharmaceutical agents, carriers, adjuvants, etc. [00199] Exemplary parenteral administration forms include solutions or suspensions of active compound in sterile aqueous solutions, for example, aqueous propylene glycol or dextrose solutions. Such dosage forms can be suitably buffered, if desired. Methods of Use [00200] The method typically comprises administering to a subject a therapeutically effective amount of a compound of the invention. The therapeutically effective amount of the subject combination of compounds may vary depending upon the intended application (in vitro or in vivo), or the subject and disease condition being treated, e.g., the weight and age of the subject, the severity of the disease condition, the manner of administration and the like, which can readily be determined by one of ordinary skill in the art. The term also applies to a dose that will induce a particular response in target cells, e.g., reduction of proliferation or downregulation of activity of a target protein. The specific dose will vary depending on the particular compounds chosen, the dosing regimen to be followed, whether it is administered in combination with other compounds, timing of administration, the tissue to which it is administered, and the physical delivery system in which it is carried. [00201] As used herein, the term "IC50" refers to the half maximal inhibitory concentration of an inhibitor in inhibiting biological or biochemical function. This quantitative measure indicates how much of a particular inhibitor is needed to inhibit a given biological process (or component of a process, i.e., an enzyme, cell, cell receptor or microorganism) by half. In other words, it is the half maximal (50%) inhibitory concentration (IC) of a substance (50% IC, or IC50). EC50 refers to the plasma concentration required for obtaining 50% of a maximum effect in vivo. [00202] In some aspects, the present disclosure provides a method of modulating PI3K (e.g., PI3Kα) activity (e.g., in vitro or in vivo), comprising contacting a cell with a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N- oxide, or stereoisomer thereof. [00203] In some aspects, the present disclosure provides a method of treating or preventing a disease or disorder disclosed herein in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a - 61 -4877-0794-3771.1 105807.005004– PCT Application pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00204] In some aspects, the present disclosure provides a method of treating a disease or disorder disclosed herein in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00205] In some embodiments, the disease or disorder is associated with an implicated PI3K activity. In some embodiments, the disease or disorder is a disease or disorder in which PI3K activity is implicated. [00206] In some embodiments, the disease or disorder is a cancer. [00207] In some embodiments, the cancer is selected from acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), adrenocortical carcinoma, aids-related cancers, aids- related lymphoma, anal cancer, astrocytoma, basal cell carcinoma, bile duct cancer, bladder cancer, bone cancer, osteosarcoma, malignant fibrous histiocytoma, brain tumors, breast cancer, bronchial tumors, Burkitt lymphoma, carcinoid tumor, cancer of unknown primary, cardiac (heart) tumors, atypical teratoid/rhabdoid tumor, primary CNS lymphoma, cervical cancer, cholangiocarcinoma, chordoma, chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), colorectal cancer, craniopharyngioma, cutaneous t-cell lymphoma, mycosis fungoides, Sezary syndrome, ductal carcinoma in situ (DCIS), embryonal tumors, medulloblastoma, endometrial cancer, ependymoma, esophageal cancer, esthesioneuroblastoma, Ewing sarcoma, extracranial germ cell tumor, extragonadal germ cell tumor, fallopian tube cancer, gallbladder cancer, gastric cancer, gastrointestinal carcinoid tumor, malignant gastrointestinal stromal tumors (GIST), germ cell tumors, gestational trophoblastic disease, hairy cell leukemia, head and neck cancer, hepatocellular cancer, Langerhans cell histiocytosis, Hodgkin lymphoma, islet cell tumors, pancreatic neuroendocrine tumors, Kaposi sarcoma, kidney cancer, laryngeal cancer, leukemia, liver cancer, lung cancer, lymphoma, male breast cancer, intraocular melanoma, Merkel cell carcinoma, malignant mesothelioma, metastatic cancer, metastatic squamous neck cancer, midline tract carcinoma with nut gene changes, mouth cancer, multiple endocrine neoplasia syndromes, multiple myeloma/plasma cell neoplasms, myelodysplastic syndromes, myelodysplastic neoplasms, myeloproliferative neoplasms, chronic myeloproliferative neoplasm, nasal cavity and paranasal sinus cancer, nasopharyngeal cancer, neuroblastoma, non-Hodgkin lymphoma, non-small cell lung cancer, oral cancer, lip and oral - 62 -4877-0794-3771.1 105807.005004– PCT Application cavity cancer, oropharyngeal cancer, malignant fibrous histiocytoma of bone, ovarian cancer, pancreatic cancer, pancreatic neuroendocrine tumors (islet cell tumors), papillomatosis, paraganglioma, paranasal sinus and nasal cavity cancer, parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma, pituitary tumor, plasma cell neoplasm, multiple myeloma, pleuropulmonary blastoma, primary central nervous system (CNS) lymphoma, primary peritoneal cancer, prostate cancer, rectal cancer, recurrent cancer, renal cell (kidney) cancer, retinoblastoma, rhabdomyosarcoma, salivary gland cancer, sarcoma, childhood vascular tumors, skin cancer, small cell lung cancer, small intestine cancer, soft tissue sarcoma, squamous cell carcinoma of the skin, testicular cancer, oropharyngeal cancer, hypopharyngeal cancer, thymoma, thymic carcinoma, thyroid cancer, tracheobronchial tumors, transitional cell cancer of the renal pelvis and ureter, urethral cancer, uterine sarcoma, vaginal cancer, vascular tumors, vulvar cancer, and Wilms tumor. [00208] In some embodiments, the cancer is Endometrial cancer, Breast cancer, oesophageal squamous-cell cancer, Cervical squamous-cell carcinoma, Cervical adenocarcinoma, Colorectal adenocarcinoma, Bladder Urothelial Carcinoma, Glioblastoma, Ovarian cancer, Non-small-cell Lung cancer, Esophagogastric cancer, Nerve-sheath tumor, Head and neck squamous-cell carcinoma, Melanoma, Esophagogastric adenocarcinoma, Soft-tissue sarcoma, Prostate cancer, Fibrolamellar carcinoma, Hepatocellular carcinoma, Diffuse glioma, Colorectal cancer, Pancreatic cancer, Cholangiocarcinoma, B-cell lymphoma, Mesothelioma, Adrenocortical carcinoma, Renal non-clear-cell carcinoma, Renal clear-cell carcinoma, Germ-cell carcinoma, Thymic tumor, Pheochromocytoma, Miscellaneous neuroepithelial tumor, thyroid cancer, leukemia, or encapsulated glioma. [00209] In some embodiments, the cancer is endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, head and neck cancer, breast cancer, brain cancer, cervical cancer, bladder cancer, esophageal cancer, pancreatic cancer, bone cancer, hepatobiliary cancer, medulloblastoma, kidney cancer or prostate cancer. [00210] In some embodiments, the cancer is a breast cancer, a prostate cancer, or a brain cancer. In some embodiments, the cancer is a breast cancer. In some embodiments, the cancer is a prostate cancer. In some embodiments, the cancer is a brain cancer. [00211] In some embodiments, the breast cancer is metastatic breast cancer. In some embodiments, the breast cancer is ductal carcinoma in situ (DCIS). In some embodiments, the breast cancer is invasive ductal carcinoma. In some embodiments, the breast cancer is triple - 63 -4877-0794-3771.1 105807.005004– PCT Application negative breast cancer. In some embodiments, the breast cancer is medullary carcinoma. In some embodiments, the breast cancer is tubular carcinoma. In some embodiments, the breast cancer is mucinous carcinoma. In some embodiments, the breast cancer is Paget disease of the breast or nipple. In some embodiments, the breast cancer is inflammatory breast cancer (IBC). [00212] In some embodiments, the prostate cancer is an adenocarcinoma. In some embodiments, the prostate cancer is a small cell carcinoma. In some embodiments, the prostate cancer is a neuroendocrine tumor. In some embodiments, the prostate cancer is a transitional cell carcinoma. In some embodiments, the prostate cancer is a sarcoma. [00213] In some embodiments, the brain cancer is an acoustic neuroma. In some embodiments, the brain cancer is an astrocytoma. In some embodiments, the brain cancer is a brain metastasis. In some embodiments, the brain cancer is choroid plexus carcinoma. In some embodiments, the brain cancer is craniopharyngioma. In some embodiments, the brain cancer is an embryonal tumor. In some embodiments, the brain cancer is an ependymoma. In some embodiments, the brain cancer is a glioblastoma. In some embodiments, the brain cancer is a glioma. In some embodiments, the brain cancer is a medulloblastoma. In some embodiments, the brain cancer is a meningioma. In some embodiments, the brain cancer is an oligodendroglioma. In some embodiments, the brain cancer is a pediatric brain tumor. In some embodiments, the brain cancer is a pineoblastoma. In some embodiments, the brain cancer is a pituitary tumor. [00214] In some embodiments, the disease or disorder associated with PI3K includes, but is not limited to, CLOVES syndrome (congenial lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome), PIK3CA-related overgrowth syndrome (PROS), breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer. [00215] In some embodiments, the diseases or disorder associated with PI3K is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome). [00216] In some embodiments, the disease or disorder associated with PI3K is PIK3CA-related overgrowth syndrome (PROS). [00217] In some embodiments, the disease or disorder associated with PI3K is breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer. - 64 -4877-0794-3771.1 105807.005004– PCT Application [00218] In some embodiments, the disease or disorder associated with PI3K is breast cancer, brain cancer, prostate cancer, endometrial cancer, gastric cancer, colorectal cancer, lung cancer, ovarian cancer, skin cancer, or head and neck cancer. [00219] In some embodiments, the disease or disorder associated with PI3K is leukemia, lymphoma, or sarcoma. [00220] In some embodiments, the cancer is endometrial cancer, head and neck cancer, or a sarcoma. [00221] In some embodiments, the cancer is endometrial cancer. In some embodiments the cancer is head and neck cancer. In some embodiments, the cancer is a sarcoma. [00222] In some embodiments, the sarcoma is soft tissue sarcoma, osteosarcoma, chondrosarcoma, Ewing sarcoma, hemangioendothelioma, angiosarcoma, fibrosarcoma, myofibrosarcoma, chordoma, adamantinoma, liposarcoma, leiomyosarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, synovial sarcoma, or malignant solitary fibrous tumor. [00223] In some embodiments, the sarcoma is soft tissue sarcoma. In some embodiments the soft tissue sarcoma is liposarcoma, atypical lipomatous tumor, dermatofibrosarcoma protuberans, malignant solitary fibrous tumor, inflammatory myofibroblastic tumor, low-grade myofibroblastic sarcoma, fibrosarcoma, myxofibrosarcoma, low-grade fibromyxoid sarcoma, giant cell tumor of soft tissues, leiomyosarcoma, malignant glomus tumor, rhabdomyosarcoma, hemangioendothelioma, angiosarcoma of soft tissue, extraskeletal osteosarcoma, gastrointestinal stromal tumor, malignant gastrointestinal stromal tumor (GIST), malignant peripheral nerve sheath tumor, malignant Triton tumor, malignant granular cell tumor, malignant ossifying fibromyxoid tumor, stromal sarcoma, myoepithelial carcinoma, malignant phosphaturic mesenchymal tumor, synovial sarcoma, epithelioid sarcoma, alveolar soft part sarcoma, clear cell sarcoma of soft tissue, extraskeletal myxoid chondrosarcoma, extraskeletal Ewing sarcoma, desmoplastic small round cell tumor, extrarenal rhabdoid tumor, perivascular epithelioid cell tumor, intimal sarcoma, undifferentiated spindle cell sarcoma, undifferentiated pleomorphic sarcoma, undifferentiated round cell sarcoma, undifferentiated epithelioid sarcoma, or undifferentiated sarcoma, not otherwise specified. [00224] In some aspects, the present disclosure provides a method of treating or preventing a cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N- oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. - 65 -4877-0794-3771.1 105807.005004– PCT Application [00225] In some aspects, the present disclosure provides a method of treating a cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00226] In some aspects, the present disclosure provides a method of treating or preventing a breast cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00227] In some aspects, the present disclosure provides a method of treating a breast cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00228] In some aspects, the present disclosure provides a method of treating or preventing a prostate cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00229] In some aspects, the present disclosure provides a method of treating a prostate cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00230] In some aspects, the present disclosure provides a method of treating or preventing a brain cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. [00231] In some aspects, the present disclosure provides a method of treating a brain cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition of the present disclosure. - 66 -4877-0794-3771.1 105807.005004– PCT Application [00232] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof thereof for use in modulating PI3K (e.g., PI3Kα) activity (e.g., in vitro or in vivo). [00233] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a disease or disorder disclosed herein. [00234] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a disease or disorder disclosed herein. [00235] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a cancer in a subject in need thereof. [00236] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a cancer in a subject in need thereof. [00237] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a breast cancer in a subject in need thereof. [00238] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a breast cancer in a subject in need thereof. [00239] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a prostate cancer in a subject in need thereof. [00240] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a prostate cancer in a subject in need thereof. [00241] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating or preventing a brain cancer in a subject in need thereof. [00242] In some aspects, the present disclosure provides a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof for use in treating a brain cancer in a subject in need thereof. - 67 -4877-0794-3771.1 105807.005004– PCT Application [00243] In some aspects, the present disclosure provides use of a compound as described oerein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for modulating PI3K (e.g., PI3Kα) activity (e.g., in vitro or in vivo). [00244] In some aspects, the present disclosure provides use of a compound as described oerein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a disease or disorder disclosed herein. [00245] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a disease or disorder disclosed herein. [00246] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a cancer in a subject in need thereof. [00247] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a cancer in a subject in need thereof. [00248] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a breast cancer in a subject in need thereof. [00249] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a breast cancer in a subject in need thereof. [00250] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a prostate cancer in a subject in need thereof. [00251] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a prostate cancer in a subject in need thereof. [00252] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating or preventing a brain cancer in a subject in need thereof. [00253] In some aspects, the present disclosure provides use of a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof in the manufacture of a medicament for treating a brain cancer in a subject in need thereof. - 68 -4877-0794-3771.1 105807.005004– PCT Application [00254] The present disclosure provides compounds that function as modulators of PI3K activity. The present disclosure therefore provides a method of modulating PI3K activity in vitro or in vivo, said method comprising contacting a cell with a therapeutically effective amount of a compound, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, as defined herein. [00255] In some embodiments, PI3K is modulation is inhibition of PI3K. [00256] In some embodiments, the PI3K inhibitor is a compound as described herein or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. In some embodiments, the PI3K inhibitor is a PI3Kα inhibitor. In some embodiments, the PI3K inhibitor is a PI3Kα H1047R mutant inhibitor. In some embodiments, the PI3K inhibitor is alpha/beta non-selective. In some embodiments, the PI3K inhibitor is alpha selective. In some embodiments, the PI3K inhibitor is beta selective. [00257] Effectiveness of compounds of the disclosure can be determined by industry-accepted assays/disease models according to standard practices of elucidating the same as described in the art and are found in the current general knowledge. [00258] The present disclosure also provides a method of treating a disease or disorder in which PI3K activity is implicated in a patient in need of such treatment, said method comprising administering to said patient a therapeutically effective amount of a compound, or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, or a pharmaceutical composition as defined herein. [00259] The disclosure provides a method of modulating the activity of the PI3Kα allosteric active site, wherein the modulation is induced through peripheral site targeting. In some embodiments, the peripheral site is targeted with an agent selected from a small molecule, a peptide, a peptidomimetic, a protein, a protein mimetic, a nucleic acid, an antibody, an antibody- drug conjugate, a nucleoprotein complex, an immunotherapy, or a combination thereof. Synthesis [00260] Compounds of the invention, including salts thereof, can be prepared using known organic synthesis techniques and can be synthesized according to any of numerous possible synthetic routes. [00261] The reactions for preparing compounds of the invention can be carried out in suitable solvents which can be readily selected by one of skill in the art of organic synthesis. Suitable solvents can be substantially nonreactive with the starting materials (reactants), the - 69 -4877-0794-3771.1 105807.005004– PCT Application intermediates, or products at the temperatures at which the reactions are carried out, e.g., temperatures which can range from the solvent's freezing temperature to the solvent's boiling temperature. A given reaction can be carried out in one solvent or a mixture of more than one solvent. Depending on the particular reaction step, suitable solvents for a particular reaction step can be selected by the skilled artisan. [00262] Preparation of compounds of the invention can involve the protection and deprotection of various chemical groups. The need for protection and deprotection, and the selection of appropriate protecting groups, can be readily determined by one skilled in the art. The chemistry of protecting groups can be found, for example, in T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd. Ed., Wiley & Sons, Inc., New York (1999), which is incorporated herein by reference in its entirety. [00263] Reactions can be monitored according to any suitable method known in the art. For example, product formation can be monitored by spectroscopic means, such as nuclear magnetic resonance spectroscopy (e.g.,1H or13C), infrared spectroscopy, spectrophotometry (e.g., UV- visible), or mass spectrometry, or by chromatography such as high performance liquid chromatography (HPLC) or thin layer chromatography. [00264] The expressions, “ambient temperature,” “room temperature,” and “r.t.” as used herein, are understood in the art, and refer generally to a temperature, e.g., a reaction temperature, that is about the temperature of the room in which the reaction is carried out, for example, a temperature from about 20oC to about 30oC. [00265] Compounds of the invention can be prepared using numerous preparatory rreactions known in the literature. The Schemes below provide general guidance in connection with preparing the compounds of the invention. One skilled in the art would understand that the preparations shown in the Schemes can be modified or optimized using general knowledge of organic chemistry to prepare various compounds of the invention. Example synthetic methods for preparing compounds of the invention are provided in the Schemes below. [00266] The following Examples are provided to illustrate some of the concepts described within this disclosure. While the Examples are considered to provide an embodiment, it should not be considered to limit the more general embodiments described herein. EXAMPLES General Synthetic Procedures - 70 -4877-0794-3771.1 105807.005004– PCT Application [00267] Compounds of Formula (I) can be prepared from optionally protected 1-1 as shown in Scheme I. Compounds 1-1 where Lg is halogen (e.g., Cl, Br, or I) or pseudohalogen (e.g., OTf, OTs, or OMs) can be transformed into compounds 1-2 under standard Stille conditions (e.g., palladium(0) catalyst, such as bis(triphenylphosphine)palladium(II) dichloride and an appropriate stannane, such as tributyl(1-ethoxyvinyl)tin), or Heck conditions (e.g., palladium catalyst, such as palladium(II) acetate, a ligand, such as 1,3-bis(diphenylphosphino)propane, and an appropriate olefin, such as butyl vinyl ether), or carbonylation coupling conditions (e.g., palladium catalyst, such as tetrakis(triphenylphosphine)palladium(0), a carbonyl source, such as carbon monoxide, and suitable boronic acid, such as phenylboronic acid). Compounds 1-2 can be converted to compounds 1-4 under either reductive conditions (e.g., in the presence of a suitable reducing agent, such as sodium borohydride) or nucleophilic addition conditions with nucleophiles 1-3 where M1 is a metal (e.g., Li, MgCl, MgBr, ZnCl, or ZnBr). Alcohols 1-4 can be transformed to compounds 1-5 where Lg1 is halogen (e.g., Cl, Br, or I) under standard deoxygenative halogenation conditions (e.g., thionyl chloride, phosphorous tribromide, or triphenylphosphine and iodine) or a pseudohalogen (e.g., OTf, OTs, or OMs) under standard sulfonylation conditions (e.g., in the presence of a sulfonylating agent, such as methanesulfonyl chloride, p-toluenesulfonyl chloride, or trifluoromethanesulfonic anhydride, and a base, such as triethylamine). Reaction of compounds 1-5 with optionally protected amines 1-6 optionally in the presence of a base (e.g., triethylamine) can provide compounds of Formula (I). In some instances, subsequent optional global or selective deprotection can also afford compounds of Formula (I). Scheme I - 71 -4877-0794-3771.1 105807.005004– PCT Application [00268] Intermediates for the synthesis of compounds of Formula (I) can be prepared as shown in Scheme II. Compounds 2-1 where Lg3 is halogen (e.g., Cl, Br, or I) or pseudohalogen (e.g., OTf or OMs) can be coupled with an amine 2-2 under standard amide bond formation conditions such as in the presence of an amide coupling reagent (e.g., N,N’-cabonyldiimidazole or HATU) and a base (e.g., diisopropylethylamine) to provide compounds 2-3. Anilines 2-3 can be converted to heterocycles 2-4 under standard acylation conditions (e.g., in the presence of N,N’- cabonyldiimidazole or triphosgene and a suitable base, such as 1,8-diazabicyclo[5.4.0]undec-7- ene). Diones 2-4 can be halogenated with suitable reagents, such as phosphoryl chloride or phosphoryl bromide, to provide compounds 2-5 where X2 is halogen (e.g., Cl or Br). Reaction of halides 2-5 with a compounds 2-6 under standard nucleophilic aromatic substitution conditions, such as in the presence of a suitable base (e.g., N,N-diisoproylethylamine), or under standard Buchwald-Hartwig coupling conditions (e.g., palladium catalyst such as, XPhos Pd G3, and a base, such as Cs2CO3 or K3PO4) can afford compounds 2-7. Scheme II [00269] Intermediates for the synthesis of compounds of Formula (I) can be prepared as shown in Scheme III. Reaction of heterocycles 2-4 with amines 2-6 in the presence of an amide coupling reagent (e.g., (benzotriazol-1-yl-oxy)tri-pyrrolidinophosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate) and a base (e.g., 1,8- diazabicyclo[5.4.0] undec-7-ene or triethylamine) can provide compounds 2-7. Alternatively, reaction of heterocycles 3-1 with amines 2-6 in the presence of an amide coupling reagent (e.g., (benzotriazol-1-yl-oxy)tri-pyrrolidino-phosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate) and a base (e.g., 1,8-diazabicyclo[5.4.0] undec-7-ene or triethylamine) can provide compound 3-2. Scheme III - 72 -4877-0794-3771.1 105807.005004– PCT Application [00270] Compounds of Formula (I) can be prepared from as shown in Scheme IV. Compounds 2-4 can be transformed into compounds 3-1 under standard Stille conditions (e.g., palladium(0) catalyst, such as bis(triphenylphosphine)palladium(II) dichloride and an appropriate stannane, such as tri-n-butyl(1-ethoxyvinyl)tin), or Heck conditions (e.g., palladium catalyst, such as palladium(II) acetate, a ligand, such as 1,3-bis(diphenylphosphino)propane, and an appropriate olefin, such as butyl vinyl ether), or carbonylation coupling conditions (e.g., palladium catalyst, such as tetrakis(triphenylphosphine)palladium(0), a carbonyl source, such as carbon monoxide, and suitable boronic acid, such as phenylboronic acid). Compounds 3-1 can be converted to compounds 4-1 under reductive conditions (e.g., in the presence of a suitable reducing agent, such as sodium borohydride). Alcohols 4-1 can be transformed to compounds 4-2 where Lg2 is halogen (e.g., Cl, Br, or I) under standard deoxygenative halogenation conditions (e.g., thionyl chloride, phosphorous tribromide, or triphenylphosphine and iodine) or a pseudohalogen (e.g., OTf, OTs, or OMs) under standard sulfonylation conditions (e.g., in the presence of a sulfonylating agent, such as methanesulfonyl chloride, p-toluenesulfonyl chloride, or trifluoromethanesulfonic anhydride, and a base, such as triethylamine). Reacton of compounds 4-2 with amines 1-6 optionally in the presence of a base (e.g., triethylamine) can provide compounds 4-3. Reaction of heterocycles 4-3 with amines 2-6 in the presence of an amide coupling reagent (e.g., (benzotriazol-1-yl-oxy)tri-pyrrolidinophosphonium hexafluorophosphate or bromotripyrrolidinophosphonium hexafluorophosphate) and a base (e.g., 1,8- diazabicyclo[5.4.0] undec-7-ene or triethylamine) can provide compounds 4-4. Scheme IV - 73 -4877-0794-3771.1 105807.005004– PCT Application [00271] Compounds of Formula (I) can be prepared from as shown in Scheme V. Annulation of amino acids 5-1 where Z is CR5 or N can afford bicycles 5-2. Reaction of compounds 5-2 with alcohols 5-3, which can be prepared as described in Scheme I, under standard Mitsunobu conditions, such as in the presence of an azodicarboxylate (e.g., diisopropyl azodicarboxylate) and a phosphine (e.g., triphenylphosphine) can afford compounds 5-4. Hydrolysis under standard conditions, such as in the presence of a base (e.g., K2CO3 or NaOH) or an acid (e.g., HCl) or upon workup, can afford compounds 5-5. Scheme V - 74 -4877-0794-3771.1 105807.005004– PCT Application [00272] Intermediates for the synthesis of compounds of Formula (I) can be prepared as from benzoic acids 6-1 as shown in Scheme VI. Benzoic acids 6-1 can be transformed into hydroxamic acids 6-2 by acid chloride formation under standard conditions, such as in the presence of thionyl chloride, and subsequent acylation of hydroxyl amine in the presence of a suitable base (e.g., triethylamine). Acylation of hydroxamic acids 6-2 with acid chlorides 6-3 where Rz is a C1-C6 alkyl group in the presence of suitable base (e.g., triethylamine) can afford compounds 6-4. Compounds 6-4 can react with an alkyne 6-5 where Ry is C1-C8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, or heterocycloalkenyl wherein each group is optionally substituted by 1-6 Rf groups via metal- catalyzed C–H activation in the presence of a metal catalyst (e.g., pentmathylcyclopentadienylrhodium(III) chloride dimer) and a base (e.g., cesium acetate) to provide bicyclic heterocycles 6-6. Selective alkylation of compounds 6-6 with electrophiles 6-7 where Lg6 is halogen (e.g., Cl, Br, or I) or pseudohalogen (e.g., OTf or OMs) in the presence of base (e.g., cesium carbonate) can provide compounds 6-8. Scheme VI - 75 -4877-0794-3771.1 105807.005004– PCT Application [00273] Intermediates for the synthesis of compounds of Formula (I) can be prepared as shown in Scheme VII. Reaction of compounds 2-3 with aldehydes 7-1 where W1 is W and wherein this group is connected to the aldehyde functional group through a carbon atom of the W group under standard conditions, such as in the presence of a Lewis acid and an oxidant (e.g., FeCl3 or TFA and air), can afford quinazolin-4(3H)-ones 7-2. Alternatively, amide coupling of anilines 2-3 with carboxylic acids 7-3 where W1 is W and wherein this group is connected to the carboxylic acid functional group through a carbon atom of the W group under standard conditions, such as in the presence of an amide coupling reagent (e.g., EDC or HATU) and a base (e.g., 4- dimethylaminopyridine or triethylamine) can provide amides 7-4. Cyclization of compounds 7-4 under various conditions, such as in the presence of an acid (e.g., p-toluenesulfonic acid or sulfuric acid), in the presence of a base (e.g., cesium carbonate or sodium hydroxide), in the presence of a dehydrating agent (e.g., acetic anhydride), or in the presence of I2 and hexamethyldisilazane, can provide quinazolin-4(3H)-ones 7-2. Scheme VII - 76 -4877-0794-3771.1 105807.005004– PCT Application Example 1. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00274] Step 1.2-Amino-3-bromo-N,5-dimethylbenzamide [00275] To a solution of 2-amino-3-bromo-5-methylbenzoic acid (2.00 g, 8.69 mmol) in THF (20.0 mL) was added 1,1'-carbonyldiimidazole (1.55 g, 9.56 mmol), and the resulting reaction mixture was stirred at room temperature for 12 h. Then methylamine (6.50 mL, 13 mmol, 2.0 M in THF) was added, and the reaction mixture was stirred for 4 h. The reaction was diluted with ethyl acetate (20.0 mL), and the organic layer was washed with water and brine, dried over Na2SO4, and concentrated. The crude material, which contained the title compound, was carried forward without further purification. LC-MS calc. for^C9H12BrN2O [M+H]+: m/z = 243.0, 245.0; Found: 242.9, 244.9. [00276] Step 2.8-Bromo-3,6-dimethylquinazoline-2,4(1H,3H)-dione - 77 -4877-0794-3771.1 105807.005004– PCT Application [00277] To a solution of 2-amino-3-bromo-N,5-dimethylbenzamide (500 mg, 2.06 mmol) in THF (10.0 mL) was added 1,1'-carbonyldiimidazole (500 mg, 3.09 mmol) and 1,8-diazabicyclo [5.4.0]undec-7-ene (0.614 mL, 4.11 mmol). The mixture was refluxed for 16 h. The reaction mixture was cooled to room temperature and poured into a mixture of ice water (5.0 mL) and sat. NH4Cl (aq.) (5.0 ml). The suspension was stirred for 10 min, and then the solid was collected by filtration to afford the title compound (495 mg, 1.84 mmol, 89.4% yield) as a white solid. LC- MS calc. for^C10H10BrN2O2 [M+H]+: m/z = 269.0, 271.0; Found: 269.0, 271.0. [00278] Step 3.8-Bromo-2-chloro-3,6-dimethylquinazolin-4(3H)-one [00279] N,N-Diisopropylethylamine (0.64 mL, 3.68 mmol) was added dropwise to a mixture of 8-bromo-3,6-dimethylquinazoline-2,4(1H,3H)-dione (495 mg, 1.84 mmol) in phosphorous oxychloride (12.4 mL, 132 mmol) at room temperature. The heterogeneous mixture was heated at 95 °C for 16 h. The reaction was cooled to room temperature and then concentrated. The brown sticky solution was dissolved with toluene (2.0 mL) and concentrated. The brown residue was cooled with an ice bath, and ice was added to the flask.5 N NaOH (aq.) was added until the mixture was basic (pH > 9). The aqueous mixture was extracted with EtOAc (3.0 x 5 mL). The combined organic layers were washed with brine (2.0 x 5 mL), dried over Na2SO4, filtered, and concentrated. The crude material, which contained the title compound, was carried forward without further purification. LC-MS calc. for^C10H9BrClN2O [M+H]+: m/z = 287.0, 289.0; Found: 286.8, 288.8. [00280] Step 4.2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-bromo-3,6-dimethylquinazolin-4(3H)-one [00281] 3-Azabicyclo[3.1.0]hexane hydrochloride (620 mg, 5.2 mmol) was added to a solution of 8-bromo-2-chloro-3,6-dimethylquinazolin-4(3H)-one (750 mg, 2.6 mmol) and triethylamine - 78 -4877-0794-3771.1 105807.005004– PCT Application (1.1 mL, 7.8 mmol) in THF (26 mL) and the reaction mixture was heated to 70 ºC for 18 h. The mixture was cooled to room temperature, diluted with water (20 mL), and extracted with DCM (20 mL × 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated. The crude residue containing the title compound was used without further purification. LC-MS calc. for C15H17BrN3O [M+H]+ m/z= 334.1, 336.1; Found 334.0, 335.9. [00282] Step 5.8-Acetyl-2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethylquinazolin-4(3H)-one [00283] Crude 2-(3-azabicyclo[3.1.0]hexan-3-yl)-8-bromo-3,6-dimethylquinazolin-4(3H)-one, 1-ethoxyvinyltri-n-butyltin (1.1 mL, 3.1 mmol), and tetrakis(triphenylphosphine) palladium(0) (300 mg, 0.26 mmol) were suspended in dioxane (10 mL) and the reaction mixture was heated to 100 ºC for 18 h. The reaction mixture was cooled to room temperature, conc. HCl (~1.0 mL) was added and the mixture was stirred at room temperature for 1 h. The mixture was diluted with DCM and water. The resulting two phases were separated and the aqueous layer was extracted with DCM (20 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue was purified by silica gel chromatography (0-100% EtOAc/hexanes) to afford the title compound (790 mg, 2.7 mmol, 102% yield). LC-MS calc. for C17H20N3O2 [M+H]+: m/z = 298.2; Found = 298.0. [00284] Step 6.2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin- 4(3H)-one [00285] Sodium borohydride (200 mg, 5.2 mmol) was added to a solution of 8-acetyl-2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethylquinazolin-4(3H)-one (780 mg, 2.6 mmol) in methanol (26 mL) and the reaction mixture was stirred at room temperature for 1 h. . The mixture was diluted with DCM and water. The resulting two phases were separated, and the aqueous layer was extracted with DCM (20 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue containing the title compound - 79 -4877-0794-3771.1 105807.005004– PCT Application was used without further purification. LC-MS calc. for C17H22N3O2 [M+H]+: m/z = 300.2; Found = 300.0. [00286] Step 7.3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00287] Phosphorous tribromide (0.35 mL, 3.8 mmol) was added to a solution of crude 2-(3- azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)-one (450 mg) in DCM (7.5 mL), and the solution was stirred for 1 h. The reaction was poured into sat. NaHCO3 (aq.) and diluted with DCM. The resulting two phases were separated, and the aqueous layer was extracted with DCM (20 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue was used without further purification. [00288] The crude residue dissolved in DMF (6.8 mL), divided, and one quarter was combined with 3-amino-6-chloropicolinic acid (120 mg, 0.72 mmol), and N,N-diisopropylethyl-amine (0.19 mL, 1.1 mmol). The resulting solution was heated at 100 ºC for 1 h. The reaction mixture was cooled to room temperature, diluted with MeCN, and filtered. The crude solution was purified by prep-HPLC on a CSH-FP column (60-80% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (32 mg, 36 µmol, 16% yield).1H NMR (400 MHz, DMSO-d6) δ 8.41 (bs, 1H), 7.68 (dd, J = 2.1, 1.0 Hz, 1H), 7.45 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 8.9 Hz, 1H), 7.05 (d, J = 9.0 Hz, 1H), 5.39 – 5.25 (m, 1H), 3.94 (d, J = 10.5 Hz, 1H), 3.81 (d, J = 10.4 Hz, 1H), 3.56 – 3.44 (m, 2H), 3.41 (s, 3H), 2.30 (s, 3H), 1.65 – 1.53 (m, J = 6.6 Hz, 5H), 0.62 – 0.49 (m, 1H), 0.38 – 0.31 (m, 1H). LC-MS calc. for C23H25ClN5O3 [M+H]+: m/z = 454.2; Found = 454.1. Example 2. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid (Isomer 1) [00289] Step 1. tert-Butyl 3-amino-6-chloropicolinate - 80 -4877-0794-3771.1 105807.005004– PCT Application [00290] To a solution of 3-amino-6-chloropyridine-2-carboxylic acid (2.00 g, 11.6 mmol) in toluene (20 mL) was added tert-butyl N,N'-diisopropylcarbamimidate (9.29 g, 46.4 mmol) dropwise. The reaction mixture was stirred at 65 °C for 15 min. The mixture was cooled to room temperature, diluted with water (20 mL), and extracted with EtOAc (20 mL × 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (EtOAc/heptane 0–20%) to afford the title compound (1.70 g, 7.43 mmol, 64.1% yield), a yellow solid. LC-MS calc. for C10H14ClN2O2 [M+H]+ m/z= 229.1; Found 229.4. [00291] Step 2. tert-Butyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate (Isomer 1) and tert-Butyl 3-((1-(2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- chloropicolinate (Isomer 2) [00292] The title compound was synthesized by procedures analogous to those outlines in Example 1, Step 1-7 substituting tert-butyl 3-amino-6-chloropicolinate for 3-amino-6- chloropicolinic acid. LC-MS calc. for C27H33ClN5O3 [M+H]+: m/z = 510.2; Found = 510.1. The isomers were separated using chiral prep-HPLC on a Lux iA3 column (30 mL/min, 95:5 hexane/EtOH) to afford two isomers: isomer 1 (tR= 4.62) and isomer 2 (tR= 5.32). [00293] Step 3.3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid (Isomer 1) [00294] TFA (35 µL, 0.45 mmol) was added to a solution of tert-butyl 3-((1-(2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- chloropicolinate (Isomer 1) (23 mg, 45 µmol) in DCM (0.45 mL), and the solution was stirred - 81 -4877-0794-3771.1 105807.005004– PCT Application for 18 h. The reaction mixture was diluted with MeCN, filtered, and purified by prep-HPLC on a C18 column (60-80% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (8.2 mg, 12 µmol, 40% yield).1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H), 7.68 (s, 1H), 7.44 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 9.0 Hz, 1H), 7.04 (d, J = 9.1 Hz, 1H), 5.36 – 5.23 (s, 1H), 3.94 (d, J = 10.4 Hz, 1H), 3.80 (d, J = 10.4 Hz, 1H), 3.54 – 3.44 (m, 2H), 3.41 (s, 3H), 2.30 (s, 3H), 1.64 – 1.54 (m, 5H), 0.62 – 0.52 (m, 1H), 0.38 – 0.30 (m, 1H). LC-MS calc. for C23H25ClN5O3 [M+H]+: m/z = 454.2; Found = 454.1. Example 3. 6-Chloro-3-((1-(2-((1R,5S,6s)-6-((methoxycarbonyl)amino)-3-azabicyclo[3.1.0] hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00295] Step 1. tert-Butyl ((1R,5S,6s)-3-(8-acetyl-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2- yl)-3-azabicyclo[3.1.0]hexan-6-yl)carbamate [00296] To a solution of 8-acetyl-3,6-dimethyl-1H-quinazoline-2,4-dione (150 mg, 0.649 mmol) in MeCN (4.5 mL) was added 1,8-diazabicyclo[5.4.0]undec-7-ene (0.290 mL, 1.94 mmol) and bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (361 mg, 0.775 mmol). The reaction was stirred for 1 h.2-methyl-2-propanyl (1R,5S)-3-azabicyclo[3.1.0]hex-6- ylcarbamate (192 mg, 0.969 mmol) was added and the reaction was stirred at 60 °C overnight. The reaction was cooled to room temperature and diluted with water (5 mL) and EtOAc (5 mL). The aqueous layer was extracted with EtOAc (2 x 10 mL). The combined organic layers were washed with brine, dried over MgSO4, and concentrated. The crude material was purified by silica gel chromatography (0–100% EtOAc/hexanes) to afford the title compound (138 mg, 0.3346 mmol, 51.798% yield) as a light yellow solid. LC-MS calc. for C22H29N4O4 [M+H]+ m/z= 413.2; Found 413.1. - 82 -4877-0794-3771.1 105807.005004– PCT Application [00297] Step 2.8-Acetyl-3,6-dimethyl-2-[rac-(1R,5S)-6-amino-3-azabicyclo[3.1.0]hexan-3- yl]quinazolin-4-one [00298] To a solution of tert-butyl N-[rac-(1R,5S)-3-(8-acetyl-3,6-dimethyl-4-oxoquinazolin- 2-yl)-3-azabicyclo[3.1.0]hexan-6-yl]carbamate (140 mg, 0.339 mmol) in DCM (3 mL) was added TFA (0.519 mL, 6.79 mmol). The reaction was stirred for 1 h. The reaction was concentrated and carried forward without further purification. LC-MS calc. for C17H21N4O2 [M+H]+ m/z= 313.2; Found 313.0. [00299] Step 3. Methyl ((1R,5S,6s)-3-(8-acetyl-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2- yl)-3-azabicyclo[3.1.0]hexan-6-yl)carbamate [00300] To a solution of 8-acetyl-3,6-dimethyl-2-[rac-(1R,5S)-6-amino-3- azabicyclo[3.1.0]hexan-3-yl]quinazolin-4-one (106 mg, 0.339 mmol) in THF (3 mL) was added N,N-diisopropylethylamine (0.296 mL, 1.70 mmol) and methyl chloroformate (0.0288 mL, 0.373 mmol). The reaction was stirred for 1 h. The reaction mixture was diluted with EtOAc (3 mL) and water (3 mL). The aqueous layer was extracted with EtOAc (3 x 5 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated. The crude material was carried forward without further purification. LC-MS calc. for C19H23N4O4 [M+H]+ m/z= 371.2; Found 371.1. [00301] Step 4. Methyl ((1R,5S,6s)-3-(8-(1-hydroxyethyl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexan-6-yl)carbamate - 83 -4877-0794-3771.1 105807.005004– PCT Application [00302] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C19H25N4O4 [M+H]+: m/z = 373.2; Found: 373.1. [00303] Step 5.6-Chloro-3-((1-(2-((1R,5S,6s)-6-((methoxycarbonyl)amino)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid [00304] To a solution of 6-chloro-1H-pyrido[3,2-d][1,3]oxazine-2,4-dione (21.3 mg, 0.107 mmol) in THF (0.5 mL) was added triphenylphosphine (28.2 mg, 0.107 mmol) and diisopropyl azodicarboxylate (21.1 ^L, 0.107 mmol). The reaction was stirred for 5 min. A solution of methyl N-[rac-(1R,5S)-3-[8-(1-hydroxyethyl)-3,6-dimethyl-4-oxoquinazolin-2-yl]-3-azabicyclo [3.1.0]hexan-6-yl]carbamate (20.0 mg, 0.0537 mmol) in THF (0.5 mL) was added and the reaction was stirred for 10 min. NaOH (aq.) (1 N, 0.269 mL, 0.269 mmol) was added and the reaction was stirred for 20 min. The reaction mixture was acidified to pH~2 with TFA and stirred for 30 min. The reaction was diluted with MeCN, filtered, and purified by prep-HPLC on a C18 column (42.6–62.6% MeCN/0.1% TFA (aq)) to afford the title compound (7.8 mg, 0.012 mmol, 22% yield) as a white solid. LC-MS calc. for C25H28ClN6O5 [M+H]+: m/z = 527.2; Found: 527.1. Example 4. 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00305] Step 1.8-Bromo-6-fluoro-3-methylquinazoline-2,4(1H,3H)-dione [00306] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 1–2. LC-MS calc. for C9H7BrFN2O2 [M+H]+: m/z = 273.0; Found: 273.5. [00307] Step 2.8-Acetyl-2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methylquinazolin-4(3H)-one - 84 -4877-0794-3771.1 105807.005004– PCT Application [00308] The title compound was synthesized by procedures analogous to those outlined in Example 40, Step 3. LC-MS calc. for C16H19F3N3O2 [M+H]+: m/z = 342.1; Found: 342.0. [00309] Step 3.2-(4,4-Difluoropiperidin-1-yl)-6-fluoro-8-(1-hydroxyethyl)-3- methylquinazolin-4(3H)-one [00310] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C16H17F3N3O2 [M+H]+: m/z = 340.1; Found: 340.0. [00311] Step 4.1-[2-(4,4-Difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8-yl]ethyl 3-amino-6-chloropyridine-2-carboxylate [00312] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7. LC-MS calc. for C22H22F3N5O3 [M+H]+: m/z = 497.1; Found: 496.9. Example 5. 3-[1-[2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethylamino]-6-chloropyridine-2-carboxylic acid [00313] The title compound was synthesized by procedures analogous to those outlined in Example 4, Steps 1–4. LC-MS calc. for C22H22ClFN5O3 [M+H]+: m/z = 458.1; Found: 458.0. Example 6. 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3- yl)-3-methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid - 85 -4877-0794-3771.1 105807.005004– PCT Application [00314] Step 1.8-Bromo-2-chloro-6-fluoro-3-methylquinazolin-4-one [00315] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 3. LC-MS calc. for C9H6BrClFN2O [M+H]+: m/z = 291.9, 293.9; Found: 291.4, 293.4. [00316] Step 2.8-Bromo-6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methylquinazolin-4(3H)-one [00317] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 4. LC-MS calc. for C14H14BrFN3O2 [M+H]+: m/z = 353.0, 355.0; Found: 353.9, 355.9. [00318] Step 3.8-Bromo-2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methylquinazolin-4(3H)-one [00319] To a solution of 8-bromo-6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan- 3-yl)-3-methylquinazolin-4(3H)-one (305 mg, 0.861 mmol) in DCM (4 mL) was added tert-butyl dimethylchlorosilane (208 mg, 1.38 mmol). The reaction was stirred for 10 min. Imidazole (235 mg, 3.44 mmol) was added, and the reaction was stirred 0.5 h. The reaction solution was washed - 86 -4877-0794-3771.1 105807.005004– PCT Application with water (5 mL) and brine (5 mL), dried over MgSO4, filtered, and concentrated. The crude material was purified by silica gel chromatography (0–40% EtOAc/hexanes) to afford the title compound (354 mg, 0.756 mmol, 87.8% yield) as a clear oil. LC-MS calc. for C20H28BrFN3O2Si [M+H]+: m/z = 468.1, 470.1; Found: 467.9, 469.9. [00320] Step 4.8-Acetyl-6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methylquinazolin-4(3H)-one [00321] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 5. LC-MS calc. for C16H17FN3O3 [M+H]+: m/z = 318.1; Found: 318.0. [00322] Step 5.8-Acetyl-2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methylquinazolin-4(3H)-one [00323] The title compound was synthesized by procedures analogous to those outlined in Example 6, Step 5. LC-MS calc. for C22H31FN3O3Si [M+H]+: m/z = 432.2; Found: 432.1. [00324] Step 6.6-Fluoro-8-(1-hydroxyethyl)-3-methyl-2-[rac-(1R,5S)-6-[tert- butyl(dimethyl)silyl]oxy-3-azabicyclo[3.1.0]hexan-3-yl]quinazolin-4-one [00325] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C22H33FN3O3Si [M+H]+: m/z = 434.2; Found: 434.1. [00326] Step 7.6-chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0 ]hexan-3- yl)-3-methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00327] The title compound was synthesized by procedures analogous to those outlined in Example 3, Step 5.1H NMR (400 MHz, DMSO-d6) δ 8.41 (d, J = 7.1 Hz, 1H), 7.63 – 7.46 (m, - 87 -4877-0794-3771.1 105807.005004– PCT Application 2H), 7.34 (d, J = 8.9 Hz, 1H), 7.03 (d, J = 9.0 Hz, 1H), 5.44 – 5.17 (m, 1H), 3.93 (d, J = 10.6 Hz, 1H), 3.80 (d, J = 10.6 Hz, 1H), 3.65 – 3.44 (m, 2H), 3.42 (s, 3H), 1.70 – 1.65 (m, 2H), 1.62 (d, J = 6.6 Hz, 3H). LC-MS calc. for C22H22ClFN5O4 [M+H]+: m/z = 474.1; Found: 474.0. Example 7. 6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00328] Step 1.2-(3-Fluoro-5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-8-(1-hydroxyethyl)-3,6- dimethylquinazolin-4-one [00329] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 1–6. LC-MS calc. for C19H20FN4O2 [M+H]+: m/z = 355.2; Found: 355.1. [00330] Step 2.6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00331] The title compound was synthesized by procedures analogous to those outlined in Example 3, Step 5. LC-MS calc. for C25H24ClN6O3 [M+H] +: m/z = 491.2; Found: 491.1. Example 8. 6-Chloro-3-((1-(2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid - 88 -4877-0794-3771.1 105807.005004– PCT Application [00332] Step 1.8-Bromo-2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethylquinazolin-4(3H)-one [00333] The title compound was synthesized by procedures analogous to those outlined in Example 6, Steps 2–3. LC-MS calc. for C21H31BrN3O2Si [M+H]+ m/z= 464.14/466.13; found 464.4/466.4. [00334] Step 2.8-Acetyl-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethylquinazolin-4(3H)-one [00335] To a solution of 8-bromo-2-[(1R,5S)-6-[tert-butyl(dimethyl)silyl]oxy-3- azabicyclo[3.1.0]hexan-3-yl]-3,6-dimethylquinazolin-4-one (1.40 g, 3.01 mmol) and 1- ethoxyvinyltri-n-butyltin (1.53 mL, 4.52 mmol) in 1,4-dioxane (30 mL) was added bis(triphenylphosphine)palladium(II) dichloride (212 mg, 0.301 mmol). The reaction mixture was bubbled with nitrogen for 1 min and stirred at 100 °C overnight. The reaction was quenched with KF solution and stirred at room temperature for 1 h. The solid was removed by filtration. The solution was diluted with DCM (10 mL), washed with water (10 mL) and brine (10 mL), dried over Na2SO4, filtered, and concentrated. The residue was dissolved in THF (10 mL) and HCl (1 N, 5.0 mL, 5.0 mmol) was added. The resulting mixture was stirred for 0.5 h. The reaction was diluted with water (2 mL) and extracted with DCM (2 mL x 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0–50% EtOAc/heptane) to yield the title compound (496 mg, 1.58 mmol, 52.5% yield). C17H20N3O3 [M+H]+ m/z=314.1; found 314.4. [00336] Step 3.8-Acetyl-2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethylquinazolin-4(3H)-one - 89 -4877-0794-3771.1 105807.005004– PCT Application [00337] The title compound was synthesized by procedures analogous to those outlined in Example 6, Step 3. LC-MS calc. for C23H34N3O3Si [M+H]+ m/z= 428.2; found 428.5. [00338] Step 4.2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)-one [00339] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C23H36N3O3Si [M+H]+ m/z= 430.3; found 430.4. [00340] Step 5.3-((1-(2-((1R,5S,6s)-6-((tert-Butyldimethylsilyl)oxy)-3-azabicyclo[3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00341] To a solution of 2-[(1R,5S)-6-[tert-butyl(dimethyl)silyl]oxy-3-azabicyclo[3.1.0]hexan- 3-yl]-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4-one (38.0 mg, 0.0884 mmol) and N,N- diisopropylethylamine (0.0462 mL, 0.265 mmol) in DCM (1 mL) was added methanesulfonyl chloride (0.0103 mL, 0.133 mmol) at 0 °C. The reaction mixture was stirred for 1 h.3-amino-6- chloropyridine-2-carboxylic acid (31 mg, 0.18 mmol) was added, and the reaction mixture was stirred at 40 °C overnight. The reaction was quenched with water (1 mL) and extracted with DCM (1 mL x 3). The combined organic layers were washed with brine (2 mL), dried over Na2SO4, filtered, and concentrated to afford the title compound (49 mg, 0.084 mmol, 95% yield). LC-MS calc. for C29H39ClN5O4Si [M+H]+: m/z = 584.2; found 584.5. [00342] Step 6.6-Chloro-3-[1-[2-[(1R,5S)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl]-3,6- dimethyl-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid - 90 -4877-0794-3771.1 105807.005004– PCT Application [00343] To a solution of 3-((1-(2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- chloropicolinic acid (49 mg, 0.084 mmol) in THF (1 mL) was added tetrabutylammonium fluoride solution (0.13 mL, 0.13 mmol). The reaction mixture was stirred for 0.5 h. The reaction was concentrated, and the crude material was purified by prep-HPLC on a C18 column (20–60% MeCN/0.1% TFA (aq)) to afford the title compound (21 mg, 0.039 mmol, 47% yield).1H NMR (300 MHz, DMSO-d6) δ 8.46 (s, 1H), 7.69 (d, J = 1.1 Hz, 1H), 7.47 (d, J = 1.9 Hz, 1H), 7.35 (d, J = 8.9 Hz, 1H), 7.07 (d, J = 9.1 Hz, 1H), 5.34 (s, 1H), 3.93 (d, J = 10.5 Hz, 1H), 3.79 (d, J = 10.5 Hz, 1H), 3.53 (dd, J = 19.1, 8.0 Hz, 2H), 3.42 (d, J = 4.3 Hz, 3H), 3.19 (s, 1H), 2.32 (s, 3H), 1.66 (d, J = 6.2 Hz, 2H), 1.61 (d, J = 6.6 Hz, 3H). LC-MS calc. for C23H25ClN5O4 [M+H]+: m/z =470.2 ; found 470.4. Example 9. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00344] Step 1.8-Bromo-6-chloro-3-methylquinazoline-2,4(1H,3H)-dione [00345] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 1–2. LC-MS calc. for C9H7BrClN2O2 [M+H]+ m/z= 288.9; Found 289.3. [00346] Step 2.2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-bromo-6-chloro-3-methylquinazolin- 4(3H)-one [00347] The title compound was synthesized by procedures analogous to those outlined in Example 40, Step 3. LC-MS calc. for C14H14BrClN3O [M+H]+ m/z= 354.0; Found 354.2. - 91 -4877-0794-3771.1 105807.005004– PCT Application [00348] Step 3.8-Acetyl-2-(3-azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methylquinazolin- 4(3H)-one [00349] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2. LC-MS calc. for C16H17ClN3O2 [M+H]+ m/z= 318.1; Found 318.3. [00350] Step 4: 2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-8-(1-hydroxyethyl)-3- methylquinazolin-4(3H)-one [00351] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C16H19ClN3O2 [M+H]+: m/z= 320.1; Found 320.3. [00352] Step 5: 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-chloro-3-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00353] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7.1H NMR (300 MHz, DMSO-d6) δ 12.85 (br, 1H), 8.42 (d, J = 7.1 Hz, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.63 (d, J = 2.5 Hz, 1H), 7.35 (d, J = 8.9 Hz, 1H), 7.05 (d, J = 9.0 Hz, 1H), 5.31 (p, J = 6.6 Hz, 1H), 4.03 (d, J = 10.6 Hz, 1H), 3.89 (d, J = 10.6 Hz, 1H), 3.60 – 3.50 (m, 2H), 3.44 (s, 3H), 1.67 – 1.59 (m, 5H), 0.61 (td, J = 7.6, 4.7 Hz, 1H), 0.32 (q, J = 4.2 Hz, 1H). LC-MS calc. for C22H22Cl2N5O3 [M+H]+ m/z= 474.1; Found 474.2. Example 10. 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(dimethylcarbamoyl)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid - 92 -4877-0794-3771.1 105807.005004– PCT Application [00354] To a solution of (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid (22 mg, 0.040 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3- triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (22.7 mg, 0.0596 mmol) in DMF (1 mL) was added triethylamine (17 mL, 0.12 mmol). The mixture was stirred for 5 min. To the mixture was added dimethylamine (2 N in THF, 39.7 mL, 0.0794 mmol). The mixture was stirred for 2 h, filtered, and purified by prep-HPLC on a C18 column (10–80% MeCN/0.1% TFA (aq)). The material was dissolved in TFA/DCM (2 mL, 1:1, v/v). The solution was stirred overnight, concentrated, and purified by prep-HPLC on a C18 column (10–70% MeCN/0.1% TFA (aq)) to afford the title compound (2.2 mg, 0.0042 mmol, 11% yield), a colorless solid. LC- MS calc. for C26H30ClN6O4 [M+H]+ m/z= 525.2; Found 525.3. Example 11. 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(cyclobutyl(methyl)carbamoyl)-3-azabicyclo [3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00355] To a solution of (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid (23.7 mg, 0.0428 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3- triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (24.4 mg, 0.0642 mmol) in DMF (1 mL) was added triethylamine (21 mL, 0.15 mmol). The mixture was stirred for 5 min and N- methylcyclobutanamine (8.67 mL, 0.0856 mmol) was added. The mixture was stirred for 2 h, filtered, and purified by prep-HPLC on a C18 column (10–80% MeCN/0.1% TFA (aq)). The material was dissolved in TFA (1 mL) and DCM (1 mL) and stirred overnight. The reaction - 93 -4877-0794-3771.1 105807.005004– PCT Application solution was concentrated, and purified by prep-HPLC on a C18 column (10–70% MeCN/0.1% TFA (aq)) to afford the title compound (2.6 mg, 0.0046 mmol, 11% yield), a colorless solid.1H NMR (300 MHz, DMSO-d6) Rotamer 1: δ 8.44 (d, J = 7.0 Hz, 1H), 7.71 (d, J = 2.0 Hz, 1H), 7.47 (d, J = 2.1 Hz, 1H), 7.34 (d, J = 9.0 Hz, 1H), 7.04 (dd, J = 9.2, 4.8 Hz, 1H), 5.46 – 5.21 (m, 1H), 4.83 (t, J = 8.7 Hz, 1H), 4.09 – 3.99 (m, 1H), 3.96 (d, J = 10.8 Hz, 1H), 3.68 – 3.56 (m, 2H), 3.47 (s, 3H), 3.00 (s, 3H), 2.32 (s, 3H), 2.26 – 2.12 (m, 1H), 2.11 – 1.88 (m, 6H), 1.67 – 1.57 (m, 4H), 1.55 – 1.43 (m, 1H).1H NMR (300 MHz, DMSO-d6) Rotamer 2: δ 8.44 (d, J = 7.0 Hz, 1H), 7.71 (d, J = 2.0 Hz, 1H), 7.47 (d, J = 2.1 Hz, 1H), 7.34 (d, J = 9.0 Hz, 1H), 7.04 (dd, J = 9.2, 4.8 Hz, 1H), 5.46 – 5.21 (m, 1H), 4.58 (t, J = 8.6 Hz, 1H), 4.09 – 3.99 (m, 1H), 3.96 (d, J = 10.8 Hz, 1H), 3.68 – 3.56 (m, 2H), 3.47 (s, 3H), 2.80 (s, 3H), 2.32 (s, 3H), 2.26 – 2.12 (m, 1H), 2.11 – 1.88 (m, 6H), 1.67 – 1.57 (m, 4H), 1.55 – 1.43 (m, 1H). LC-MS calc. for C29H34ClN6O4 [M+H]+ m/z= 565.2; Found 565.3. Example 12. 6-Chloro-3-((1-(2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00356] Step 1.8-Bromo-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethylquinazolin-4(3H)-one [00357] To a solution of 8-bromo-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethylquinazolin-4(3H)-one (240 mg, 0.685 mmol) in DMF (3 mL) was added sodium hydride (41.1 mg, 1.03 mmol) at 0 °C. The reaction was stirred at 0 °C for 10 min, then iodomethane (0.0512 mL, 0.822 mmol) was added. The resulting mixture was stirred for 1.5 h. The reaction was quenched with sat. aq. NH4Cl solution (2 mL) and extracted with DCM (2 mL × 3). The combined organic layers were washed with brine (3 mL), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (EtOAc/heptane 0– - 94 -4877-0794-3771.1 105807.005004– PCT Application 30%) to afford the title compound (196 mg, 0.538 mmol, 78.5% yield), a white solid. LC-MS calc. for C16H19BrN3O2 [M+H]+ m/z= 364.07/366.06; Found 364.3/366.3. [00358] Step 2.8-(1-Hydroxyethyl)-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethylquinazolin-4(3H)-one [00359] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2 and Example 1, Step 6. LC-MS calc. for C18H24N3O3 [M+H]+ m/z= 330.2; Found 330.4. [00360] Step 3.6-Chloro-3-((1-(2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00361] The title compound was synthesized by procedures analogous to those outlined inExample 8, Step 5. 1H NMR (300 MHz, DMSO-d6) δ 13.01 (s, 1H), 8.46 (d, J = 6.5 Hz, 1H), 7.70 (d, J = 1.1 Hz, 1H), 7.47 (d, J = 1.8 Hz, 1H), 7.35 (d, J = 8.9 Hz, 1H), 7.07 (d, J = 9.1 Hz, 1H), 5.34 (t, J = 6.2 Hz, 1H), 3.94 (d, J = 10.6 Hz, 1H), 3.83 (d, J = 10.6 Hz, 1H), 3.56 (s, 2H), 3.43 (s, 3H), 3.27 (s, 3H), 3.19 (s, 1H), 2.32 (s, 3H), 1.81 (s, 2H), 1.61 (d, J = 6.6 Hz, 3H). LC- MS calc. for C24H27ClN5O4 [M+H]+ m/z= 484.2; Found 484.4. Example 13. 6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyrazin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00362] Step 1.2-Hydroxy-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4-one - 95 -4877-0794-3771.1 105807.005004– PCT Application [00363] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C12H15N2O3 [M+H]+ m/z= 235.1; Found 235.2. [00364] Step 2. Tert-Butyl 6-chloro-3-[1-(2-hydroxy-3,6-dimethyl-4-oxoquinazolin-8- yl)ethylamino]pyridine-2-carboxylate [00365] The title compound was synthesized by procedures analogous to those outlined in Example 2, Step 2. LC-MS calc. for C22H26ClN4O4 [M+H]+ m/z = 445.2; Found 445.3. [00366] Step 3.2,3-Bis(bromomethyl)pyrazine [00367] A mixture of 2,3-dimethylpyrazine (3.00 g, 27.7 mmol), N-bromosuccinimide (9.38 g, 52.7 mmol) and azobisisobutyronitrile (0.460 g, 2.77 mmol) in benzotrifluoride (30 mL) was stirred at 90 °C for 2 h. The mixture was filtered, and the organic layer was concentrated. The residue was purified by silica gel chromatography (0–5% EtOAc/hexane) to afford the title compound (400 mg, 1.3 mmol, 5.0% yield), a brown oil. LC-MS calc. for C6H7Br2N2 [M+H]+ m/z= 264.9; Found 265.1. [00368] Step 4.6-Trityl-5,7-dihydropyrrolo[3,4-b]pyrazine [00369] A mixture of 2,3-bis(bromomethyl)pyrazine (400 mg, 1.5 mmol), triphenylmethanamine (351 mg, 1.35 mmol) and N,N-diisopropylethylamine (583 mg, 4.51 mmol) in DMF (5 mL) was stirred at 60 °C for 12 h. The mixture was cooled to room temperature, and water (30 mL) was added. The mixture was extracted with EtOAc (30 mL x 3), washed with water (30 mL x 3), dried over Na2SO4, and concentrated. The residue was purified by prep-HPLC on a C18 column (5–100% MeCN/0.1% TFA (aq)) to afford the title compound (67 mg, 0.18mmol, 12% yield) as a brown oil. LC-MS calc. for C25H22N3 [M+H-CPh3]+ m/z= 364.2; Found 243.4.1H NMR (400 MHz, DMSO-d6) δ 8.28 (s, 2H), 7.52–7.48 (m, 6H), 7.34- 7.30 (m, 6H), 7.21-7.17 (m, 3H), 3.98 (s, 4H). - 96 -4877-0794-3771.1 105807.005004– PCT Application [00370] Step 5.6,7-Dihydro-5H-pyrrolo[3,4-b]pyrazine [00371] 6-Trityl-5,7-dihydropyrrolo[3,4-b]pyrazine (40 mg, 0.11 mmol) was dissolved in HCl (4 N in dioxane, 1.0 mL, 4.0 mmol). The mixture was stirred for 0.5 h, then concentrated. The residue was slurred in MTBE (2 mL) and the solid was collected by filtration to afford the title compound (17.1 mg, 0.0881 mmol, 80.1% yield) as its HCl salt. LC-MS calc. for C6H8N3 [M+H]+ m/z = 122.1; Found 122.2. [00372] Step 6. Tert-Butyl 6-chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyrazin-6-yl)-3,6- dimethyl-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylate [00373] The title compound was synthesized by procedures analogous to those outlined in Example 40, Step 3. LC-MS calc. for C28H31ClN7O3 [M+H]+ m/z = 548.2; Found 548.3. [00374] Step 7.6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyrazin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00375] The title compound was synthesized by procedures analogous to those outlined in Example 2, Step 3.1H NMR (300 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.59 (s, 2H), 8.52 (d, J = 7.4 Hz, 1H), 7.75 (s, 1H), 7.50 (d, J = 1.9 Hz, 1H), 7.33 (d, J = 8.9 Hz, 1H), 7.11 (d, J = 9.1 Hz, 1H), 5.45 – 5.37 (m, 1H), 5.23 – 5.09 (m, 4H), 3.65 (s, 3H), 2.34 (s, 3H), 1.65 (d, J = 6.6 Hz, 3H). LC-MS calc. for C24H23ClN7O3 [M+H]+ m/z= 492.2/494.2; Found 492.3/494.4. Example 14. 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6s)-6-((pyrrolidine-1-carbonyl) oxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid - 97 -4877-0794-3771.1 105807.005004– PCT Application [00376] Step 1. Tert-butyl 3-((1-(2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- chloropicolinate [00377] To a solution of 1-(2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl methanesulfonate (236 mg, 0.465 mmol) and tert-butyl 3-amino-6-chloropyridine-2-carboxylate (213 mg, 0.930 mmol) in DCM (4 mL) was added N,N-diisopropylethylamine (0.324 mL, 1.86 mmol). The reaction mixture was stirred at 40 °C for 48 h. The reaction was quenched with water (3 mL) and extracted with DCM (3 mL × 3). The combined organic layers were washed with brine (5 mL), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0–20% EtOAc/heptane) to afford the title compound (196 mg, 0.306 mmol, 65.9% yield). LC-MS calc. for C33H47ClN5O4Si [M+H]+ m/z= 640.3; Found 640.4. [00378] Step 2. Tert-Butyl 6-chloro-3-((1-(2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0] hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinate [00379] To a solution of tert-butyl 3-((1-(2-((1R,5S,6s)-6-((tert-butyldimethylsilyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- chloropicolinate (196 mg, 0.306 mmol) in THF (3 mL) was added tetrabutylammonium fluoride (1 N in THF, 0.4 mL, 0.4 mmol). The reaction was stirred for 0.25 h. The reaction was diluted with DCM (5 mL), washed with water (3 mL) and brine (3 mL), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0–30% EtOAc/heptane) to - 98 -4877-0794-3771.1 105807.005004– PCT Application afford the title compound (115 mg, 0.219 mmol, 71.4% yield). LC-MS calc. for C27H33ClN5O4 [M+H]+ m/z= 526.2; Found 526.4. [00380] Step 3. Tert-Butyl 6-chloro-3-[1-[3,6-dimethyl-2-[(1R,5S)-6-(4- nitrophenoxy)carbonyloxy-3-azabicyclo[3.1.0]hexan-3-yl]-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylate [00381] To a solution of tert-butyl 6-chloro-3-((1-(2-((1R,5S,6s)-6-hydroxy-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinate (115 mg, 0.219 mmol) and N,N-diisopropylethylamine (0.114 mL, 0.656 mmol) in DCM (2 mL) was added 4-nitrophenyl chloroformate (52.9 mg, 0.262 mmol). The reaction mixture was stirred for 1 h. The reaction was quenched with water (2 mL) and extracted with DCM (2 mL × 2). The combined organic layers were dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0–60% EtOAc/heptane) to afford the title compound (110 mg, 0.159 mmol, 72.8% yield). LC-MS calc. for C34H36ClN6O8 [M+H]+ m/z= 691.2; found 691.3. [00382] Step 4. Tert-Butyl 6-chloro-3-((1-(3,6-dimethyl-2-((1R,5S,6s)-6-(((4- nitrophenoxy)carbonyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinate [00383] To a solution of tert-butyl 6-chloro-3-[1-[3,6-dimethyl-2-[(1R,5S)-6-(4- nitrophenoxy)carbonyloxy-3-azabicyclo[3.1.0]hexan-3-yl]-4-oxoquinazolin-8-yl]ethylamino] pyridine-2-carboxylate (27.0 mg, 0.0391 mmol) and N,N-diisopropylethylamine (0.0204 mL, 0.117 mmol) in MeCN (1 mL) was added pyrrolidine (4.17 mg, 0.0586 mmol). The reaction - 99 -4877-0794-3771.1 105807.005004– PCT Application mixture was stirred at 70 °C for 30 min. The reaction mixture was concentrated to afford the title compound, which was used in the next step without further purification. LC-MS calc. for C32H40ClN6O5 [M+H]+ m/z = 623.3; Found 623.4. [00384] Step 5.6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6s)-6-((pyrrolidine-1- carbonyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00385] The title compound was synthesized by procedures analogous to those outlined in Example 2, Step 3.1H NMR (300 MHz, DMSO-d6) δ 13.03 (s, 1H), 8.45 (d, J = 6.9 Hz, 1H), 7.71 (d, J = 1.2 Hz, 1H), 7.48 (d, J = 1.9 Hz, 1H), 7.31 (d, J = 9.0 Hz, 1H), 7.06 (d, J = 9.1 Hz, 1H), 5.37 (dd, J = 13.6, 6.9 Hz, 1H), 4.11 (d, J = 10.7 Hz, 1H), 3.92 – 3.81 (m, 2H), 3.60 (dd, J = 10.8, 3.2 Hz, 2H), 3.44 (d, J = 5.5 Hz, 3H), 3.23 (q, J = 5.6 Hz, 4H), 2.32 (s, 3H), 1.94 (s, 2H), 1.86 – 1.73 (m, 4H), 1.60 (d, J = 6.6 Hz, 3H). LC-MS calc. for C28H32ClN6O5 [M+H]+ m/z= 567.2; Found 567.3. Example 15. 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00386] Step 1: Methyl (1R,5S,6r)-3-(8-bromo-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2- yl)-3-azabicyclo[3.1.0]hexane-6-carboxylate [00387] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 4.1H NMR (300 MHz, CDCl3) δ 7.91 (dd, J = 2.0, 0.9 Hz, 1H), 7.78 – 7.71 (m, 1H), 3.97 – 3.93 (m, 1H), 3.70 (s, 3H), 3.68 – 3.62 (m, 2H), 3.49 (s, 3H), 2.41 (s, 3H), 2.23 (ddd, J = 3.1, 2.0, 1.0 Hz, 2H), 1.79 (t, J = 3.1 Hz, 1H). LC-MS calc. for C17H19BrN3O3 [M+H]+ m/z= 392.0/394.0; Found 392.0/393.9. - 100 -4877-0794-3771.1 105807.005004– PCT Application [00388] Step 2: Methyl (1R,5S,6r)-3-(8-(1-hydroxyethyl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6-carboxylate [00389] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2, and Example 1, Step 6.1H NMR (300 MHz, CDCl3) δ 7.87 (d, J = 2.0 Hz, 1H), 7.35 (d, J = 2.1 Hz, 1H), 5.32 – 5.05 (m, 2H), 3.85 (t, J = 10.8 Hz, 2H), 3.69 (s, 3H), 3.64 – 3.52 (m, 2H), 3.51 (s, 3H), 2.42 (s, 3H), 2.23 (d, J = 2.7 Hz, 2H), 1.82 (t, J = 3.0 Hz, 1H), 1.62 (d, J = 6.2 Hz, 3H). LC-MS calc. for C19H24N3O4 [M+H]+ m/z= 358.2; Found 358.6. [00390] Step 3: 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(methoxycarbonyl)-3-azabicyclo[3.1.0] hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00391] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7. LC-MS calc. for C25H27ClN5O5 [M+H]+ m/z= 512.1; Found 512.3. [00392] Step 4: Methyl (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylate [00393] To a solution of 6-chloro-3-((1-(2-((1R,5S,6r)-6-(methoxycarbonyl)-3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid (260 mg, 0.51 mmol) in toluene (3 mL) was added 1,1-di-tert- butoxytrimethylamine (1.22 mL, 5.12 mmol). The mixture was heated to 80 °C for 3 h. The - 101 -4877-0794-3771.1 105807.005004– PCT Application mixture was cooled to room temperature, diluted with EtOAc (5 mL), washed with water (1 mL) and saturated aqueous NaHCO3 (3 mL). The organic layer was dried over Na2SO4, concentrated, and purified by silica gel chromatography (0–40% EtOAc/heptane) to afford the title compound (220 mg, 0.39 mmol, 76% yield), a yellow oil. LC-MS calc. for C29H35ClN5O5 [M+H]+ m/z= 568.2; Found 568.3. [00394] Step 5: (1R,5S,6r)-3-(8-(1-((2-(tert-Butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid [00395] To a solution of methyl (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin- 3-yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo [3.1.0]hexane- 6-carboxylate (180 mg, 0.32 mmol) in THF (2 mL) was added aq. LiOH (1 N, 1.58 mL, 1.58 mmol). The mixture was stirred at 50 °C for 3 h. The mixture was cooled to room temperature and acidified to pH ~ 5 with 1 N HCl. The mixture was extracted with EtOAc (3 x 3 mL) and the combined organic phases were dried over Na2SO4 and concentrated to afford the title compound (173 mg, 0.312 mmol, 99% yield), an off-white solid. The crude product was used directly in the next step without any further purification. LC-MS calc. for C28H33ClN5O5 [M+H]+ m/z= 554.2; Found 554.4. [00396] Step 6: 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6r)-6-((2,2,2- trifluoroacetoxy)methyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl) amino)picolinic acid [00397] To a solution of (1R,5S,6r)-3-(8-(1-((2-(tert-butoxycarbonyl)-6-chloropyridin-3- yl)amino)ethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)-3-azabicyclo[3.1.0]hexane-6- carboxylic acid (25.3 mg, 0.0457 mmol) in THF (1 mL) was added borane dimethyl sulfide - 102 -4877-0794-3771.1 105807.005004– PCT Application complex (13 mL, 0.14 mmol). The mixture was stirred for 2 h. MeOH (0.5 mL) was added and the solution was concentrated and purified by prep-HPLC on a C18 column (10–80% MeCN/0.1% TFA (aq)). The material was dissolved in DCM (1 mL) and TFA (1 mL) and stirred for 16 h. The reaction solution was concentrated and purified by prep-HPLC on a C18 column (10–70% MeCN/0.1% TFA (aq)) to afford the title compound (10 mg, 0.017 mmol, 38% yield), a colorless solid. LC-MS calc. for C26H26ClF3N5O5 [M+H]+ m/z= 580.1; Found 580.4. [00398] Step 7: 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo [3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00399] To a solution of 6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6r)-6-((2,2,2- trifluoroacetoxy)methyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid (10.0 mg, 0.0172 mmol) in methanol (1 mL) was added potassium carbonate (7.15 mg, 0.0517 mmol). The mixture was stirred for 1 h. The mixture was filtered and purified by prep-HPLC on a C18 column (10–70% MeCN/0.1% TFA (aq)) to afford the title compound (2.7 mg, 0.0056 mmol, 32% yield), a colorless solid.1H NMR (300 MHz, DMSO-d6) δ 12.97 (br, 1H), 8.46 (d, J = 7.1 Hz, 1H), 7.73 – 7.67 (m, 1H), 7.47 (d, J = 2.1 Hz, 1H), 7.36 (d, J = 9.0 Hz, 1H), 7.07 (d, J = 9.1 Hz, 1H), 5.44 – 5.24 (m, 1H), 3.99 (d, J = 10.6 Hz, 1H), 3.81 (d, J = 10.5 Hz, 1H), 3.53 – 3.46 (m, 3H), 3.44 (s, 3H), 3.33 (dd, J = 6.5, 2.2 Hz, 2H), 2.32 (s, 3H), 1.60 (d, J = 6.6 Hz, 3H), 1.54 (t, J = 2.7 Hz, 2H), 1.07 – 0.98 (m, 1H). LC-MS calc. for C24H27ClN5O4 [M+H]+ m/z= 484.1; Found 484.2 Example 16. 6-Chloro-3-[1-[3,6-dimethyl-2-[1-[(2-methylpropan-2-yl)oxycarbonyl] pyrrolidin-3-yl]-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00400] Step 1. Tert-Butyl 3-(8-bromo-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2- yl)pyrrolidine-1-carboxylate - 103 -4877-0794-3771.1 105807.005004– PCT Application [00401] To a solution of 2-amino-3-bromo-N,5-dimethylbenzamide (700 mg, 2.88 mmol) in DCM (14 mL) was added 1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-3-carboxylic acid (682 mg, 3.17 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.10 g, 5.76 mmol) and 4-(dimethylamino)pyridine (1.40 g, 11.5 mmol). The resulting mixture was stirred over the weekend. The reaction was quenched with water (50 mL) and diluted with EtOAc (50 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (50 mL x 2). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated. After 5 months, the crude material was further purified by silica gel chromatography (0–50% EtOAc/heptane) to afford the title compound (103 mg, 0.244 mmol, 8.47% yield) as a white solid.1H NMR (300 MHz, CDCl3) δ 7.99 (dd, J = 1.9, 1.0 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 3.98 – 3.71 (m, 3H), 3.65 (s, 3H), 3.62 – 3.40 (m, 2H), 2.58 (dd, J = 13.0, 6.7 Hz, 1H), 2.45 (s, 3H), 2.27 (dq, J = 13.9, 6.9 Hz, 1H), 1.47 (s, 9H). LC-MS calc. for C19H25BrN3O3 [M+H]+: m/z= 422.1/424.1; Found 422.2/424.2. [00402] Step 2. Tert-Butyl 3-[8-(1-hydroxyethyl)-3,6-dimethyl-4-oxoquinazolin-2- yl]pyrrolidine-1-carboxylate [00403] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2, and Example 1, Step 6.1H NMR (300 MHz, CDCl3) δ 8.04 – 7.90 (m, 1H), 7.52 – 7.39 (m, 1H), 5.34 – 5.23 (m, 1H), 4.79 – 4.29 (m, 1H), 3.92 – 3.46 (m, 8H), 2.47 – 2.46 (m, 3H), 2.38 – 2.27 (m, 2H), 1.65 – 1.56 (m, 3H), 1.49 – 1.48 (m, 9H). LC-MS calc. for C21H30N3O4 [M+H]+: m/z= 388.2; Found 388.4. [00404] Step 3: 6-Chloro-3-[1-[3,6-dimethyl-2-[1-[(2-methylpropan-2- yl)oxycarbonyl]pyrrolidin-3-yl]-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid [00405] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 5.1H NMR (300 MHz, DMSO-d6) δ 12.96 (br, 1H), 8.43 – 8.40 (m, 1H), 7.87 – 7.82 (m, 1H), 7.60 – 7.58 (m, 1H), 7.33 – 7.28 (m, 1H), 7.11 – 6.90 (m, 1H), 5.51 – 5.46 (m, 1H), 4.02 – 3.81 (m, 2H), 3.63 – 3.61 (m, 4H), 3.44 – 3.39(m, 2H), 2.41 – 2.29 (m, 5H), 1.59 – 1.55 (m, 3H), 1.38 – 1.29 (m, 9H). LC-MS calc. for C27H33ClN5O5 [M+H]+ m/z= 542.2; Found 542.4. - 104 -4877-0794-3771.1 105807.005004– PCT Application Example 17.6-Chloro-3-((1-(2-((1R,5S,6r)-6-(methoxy(methyl)carbamoyl)-3- azabicyclo[3.1.0] hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid [00406] The title compound was synthesized by procedures analogous to those outlined in Example 10.1H NMR (300 MHz, DMSO-d6) δ 8.45 (d, J = 7.1 Hz, 1H), 7.71 (d, J = 2.1 Hz, 1H), 7.46 (d, J = 2.1 Hz, 1H), 7.34 (d, J = 8.9 Hz, 1H), 7.02 (d, J = 9.1 Hz, 1H), 5.41 – 5.24 (m, 1H), 4.01 (dd, J = 21.1, 10.9 Hz, 2H), 3.69 – 3.60 (m, 5H), 3.45 (s, 3H), 3.12 (s, 3H), 2.32 (s, 3H), 2.19 – 2.13 (m, 1H), 2.12 – 2.06 (m, 2H), 1.61 (d, J = 6.6 Hz, 3H). LC-MS calc. for C26H30ClN6O5 [M+H]+: m/z= 541.2; Found 541.4. Example 18. 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3- yl)-3-methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00407] Step 1.6-Fluoro-2-hydroxy-8-(1-hydroxyethyl)-3-methylquinazolin-4-one [00408] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2, and Example 1, Step 6. LC-MS calc. for C11H12FN2O3 [M+H]+: m/z = 239.1; Found 239.3. [00409] Step 2. Tert-Butyl 6-chloro-3-[1-(6-fluoro-2-hydroxy-3-methyl-4-oxoquinazolin-8- yl)ethylamino]pyridine-2-carboxylate - 105 -4877-0794-3771.1 105807.005004– PCT Application [00410] The title compound was synthesized by procedures analogous to those outlined in Example 2, Step 2. LC-MS calc. for C21H23ClFN4O4 [M+H]+: m/z= 449.1; Found 449.2. [00411] Step 3. Tert-Butyl (1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexane-3-carboxylate [00412] To a solution of (1R,5S,6s)-3-azabicyclo[3.1.0]hexan-6-ol hydrochloride (160 mg, 1.18 mmol) and triethylamine (0.493 mL, 3.54 mmol) in DCM (2 mL) was added di-tert-butyl dicarbonate (386 mg, 1.77 mmol). The reaction mixture was stirred for 30 min. The mixture was diluted with DCM (3 mL), washed with water (3 mL) and brine (3 mL), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (0– 80% EtOAc/heptane) to afford the title compound (220 mg, 1.1 mmol, 95% yield). LC-MS calc. for C10H18NO3 [M+H]+: m/z= 200.1; Found 200.2. [00413] Step 4. Tert-Butyl (1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexane-3-carboxylate [00414] To a solution of tert-butyl (1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexane-3- carboxylate (223 mg, 1.12 mmol) in DMF (2 mL) was added sodium hydride (89.5 mg, 2.24 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for 10 min, then iodomethane (191 mg, 1.34 mmol) was added. The mixture was stirred at room temperature for 30 min. The reaction solution was diluted with water (3 mL) and extracted with EtOAc (3 mL × 3). The combined organic layers were washed with brine (3 ml), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0–30% EtOAc/heptane) to afford the title compound (185 mg, 0.867 mmol, 78% yield). LC-MS calc. for C11H20NO3 [M+H]+: m/z= 214.1; Found 214.2. [00415] Step 5. (1R,5S,6s)-6-Methoxy-3-azabicyclo[3.1.0]hexane hydrochloride - 106 -4877-0794-3771.1 105807.005004– PCT Application [00416] A solution of tert-butyl (1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexane-3- carboxylate (185 mg, 0.867 mmol) in 2 N HCl in isopropyl acetate (2.0 mL, 4 mmol) was stirred for 1 h, then concentrated to afford the title compound (110 mg, 0.735 mmol, 85% yield). The crude product was used in the next reaction without further purification. LC-MS calc. for C6H12NO [M+H]+: m/z= 114.1; Found 114.1. [00417] Step 6. Tert-Butyl 6-chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-methoxy-3- azabicyclo[3.1.0]hexan-3-yl)-3-methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinate [00418] The title compound was synthesized by procedures analogous to those outlined in Example 40, Step 3. LC-MS calc. for C27H32ClFN5O4 [M+H]+: m/z= 544.2; Found 544.3. [00419] Step 7.6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan- 3-yl)-3-methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00420] The title compound was synthesized by procedures analogous to those outlined in Example 2, Step 3.1H NMR (300 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.43 (d, J = 7.0 Hz, 1H), 7.65 – 7.49 (m, 2H), 7.35 (d, J = 8.9 Hz, 1H), 7.03 (d, J = 9.1 Hz, 1H), 5.35 (t, J = 6.6 Hz, 1H), 3.97 (d, J = 10.6 Hz, 1H), 3.85 (d, J = 10.6 Hz, 1H), 3.59 (s, 2H), 3.44 (s, 3H), 3.27 (s, 3H), 3.18 (s, 1H), 1.82 (s, 2H), 1.64 (d, J = 6.6 Hz, 3H). LC-MS calc. for C23H24ClFN5O4 [M+H]+: m/z= 488.2; Found 488.3. Example 19. 6-Chloro-3-[1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino] pyridine-2-carboxylic acid - 107 -4877-0794-3771.1 105807.005004– PCT Application [00421] Step 1: 3-Bromo-N-hydroxy-5-methylbenzamide [00422] To a solution of 3-bromo-5-methylbenzoic acid (1.00 g, 4.65 mmol) in DCM (20 mL) was added oxalyl chloride (0.479 mL, 5.58 mmol) and N,N-dimethylformamide (17.0 mg, 0.233 mmol). The resulting mixture was stirred for 1 h, then concentrated. To a solution of hydroxylamine (50% in water, w/w) (0.922 g, 14.0 mmol) in ethyl acetate (15 mL) and water (15 mL) was added sodium bicarbonate (1.17 g, 14.0 mmol). The resulting mixture was stirred for 5 min. The previously prepared acyl chloride was added as a solution in EtOAc (15 mL) and stirred overnight. The reaction mixture was diluted with EtOAc (15 mL) and acidified to pH~2 with 1 N HCl. The layers were separated, and the organic layer was washed with 1 N HCl (30 mL), water (30 mL) and brine (30 mL). The organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford the title compound (1.0 g, 4.5 mmol, 97% yield), a white solid. The crude product was used in the next step directly without further purification.1H NMR (300 MHz, DMSO-d6) δ 11.28 (s, 1H), 9.13 (s, 1H), 7.69 (d, J = 1.8 Hz, 1H), 7.60 – 7.55 (m, 2H), 2.34 (d, J = 0.8 Hz, 3H). [00424] To a solution of 3-bromo-N-hydroxy-5-methylbenzamide (180 mg, 0.782 mmol) in THF (4 mL) was added triethylamine (0.273 mL, 1.96 mmol) and trimethylacetyl chloride (0.106 mL, 0.861 mmol). The resulting mixture was stirred for 3 h. To the mixture was added sat. aq. sodium bicarbonate (20 mL) and EtOAc (20 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (20 mL x 2). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated. The crude material was purified by silica gel chromatography (0–30% EtOAc/heptane) to afford the title compound (218 mg, 0.694 mmol, 89% yield), a colorless oil.1H NMR (300 MHz, CDCl3) δ 9.45 (br, 1H), 7.76 (s, 1H), 7.56 (s, 1H), 7.54 (s, 1H), 2.40 (s, 3H), 1.39 (s, 9H). LC-MS calc. for C13H17BrNO3 [M+H]+: m/z= 314.0/316.0; Found 314.2/316.2. [00425] Step 3: 5-Bromo-7-methyl-3-phenyl-2H-isoquinolin-1-one - 108 -4877-0794-3771.1 105807.005004– PCT Application [00426] To a solution of [(3-bromo-5-methylbenzoyl)amino] 2,2-dimethylpropanoate (388 mg, 1.23 mmol) in methanol (4 mL) was added phenylacetylene (0.176 mL, 1.61 mmol), bis[(pentamethylcyclopentadienyl)dichloro-rhodium] (153 mg, 0.247 mmol), and cesium acetate (593 mg, 3.09 mmol). The resulting mixture was stirred overnight. To the mixture was added water (30 mL) and EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (30 mL x 2). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (0–50% EtOAc/heptane) to afford the title compound (310 mg, 0.98 mmol, 79% yield), a white solid.1H NMR (300 MHz, CDCl3) δ 9.27 (br, 1H), 8.19 (s, 1H), 7.79 (s, 1H), 7.72 – 7.66 (m, 2H), 7.56 – 7.49 (m, 3H), 7.09 (s, 1H), 2.48 (s, 3H). LC-MS calc. for C16H13BrNO [M+H]+: m/z= 314.0/316.0; Found 314.2/316.2. [00427] Step 4: 5-Bromo-2,7-dimethyl-3-phenylisoquinolin-1-one [00428] To a solution of 5-bromo-7-methyl-3-phenyl-2H-isoquinolin-1-one (200 mg, 0.637 mmol) in DMF (6 mL) was added cesium carbonate (311 mg, 0.955 mmol) and iodomethane (0.0594 mL, 0.955 mmol). The resulting mixture was stirred for 2 h. To the mixture was added water (30 mL) and EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (30 mL). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was further purified by silica gel chromatography (0–40% EtOAc/heptane) to afford the title compound (41 mg, 0.13 mmol, 20% yield), a white solid.1H NMR (300 MHz, CDCl3) δ 8.23 (s, 1H), 7.73 (s, 1H), 7.54 – 7.46 (m, 3H), 7.46 – 7.40 (m, 2H), 6.73 (s, 1H), 3.42 (s, 3H), 2.48 (s, 3H). LC-MS calc. for C17H15BrNO [M+H]+: m/z= 328.0/330.0; Found 328.2/330.2. [00429] Step 5: 5-(1-Hydroxyethyl)-2,7-dimethyl-3-phenylisoquinolin-1-one - 109 -4877-0794-3771.1 105807.005004– PCT Application [00430] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 1 and Example 1, Step 6.1H NMR (300 MHz, CDCl3) δ 8.22 (s, 1H), 7.66 (s, 1H), 7.53 – 7.45 (m, 3H), 7.44 – 7.38 (m, 2H), 7.02 – 6.95 (m, 1H), 6.63 (s, 1H), 5.37 (q, J = 6.5 Hz, 1H), 3.42 (s, 3H), 2.50 (s, 3H), 1.56 (d, J = 6.5 Hz, 3H). LC-MS calc. for C19H20NO2 [M+H]+: m/z= 294.1; Found 294.3. [00431] Step 6: 6-Chloro-3-[1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethyl- amino]pyridine-2-carboxylic acid and 1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethyl 3- amino-6-chloropyridine-2-carboxylate [00432] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7.1H NMR (300 MHz, DMSO-d6) δ 12.80 (br, 1H), 8.35 (d, J = 6.3 Hz, 1H), 8.04 (s, 1H), 7.62 – 7.51 (m, 5H), 7.49 (d, J = 1.9 Hz, 1H), 7.34 (d, J = 8.9 Hz, 1H), 6.94 (d, J = 9.0 Hz, 1H), 6.82 (s, 1H), 5.38 – 5.25 (m, 1H), 3.35 (s, 3H), 2.40 (s, 3H), 1.55 (d, J = 6.5 Hz, 3H). LCMS calc. for C25H23ClN3O3 [M+H]+: m/z= 448.1/450.1; Found 448.4/450.4 Example 20. 6-Chloro-3-[1-[3,6-dimethyl-2-(1-methylpyrazol-4-yl)-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid [00433] Step 1: 8-Bromo-3,6-dimethyl-2-(1-methylpyrazol-4-yl)quinazolin-4-one [00434] To a suspension of 2-amino-3-bromo-N,5-dimethylbenzamide (200 mg, 0.82 mmol) in water (6 mL) was added 1-methyl-1H-pyrazole-4-carbaldehyde (91 mg, 0.82 mmol) and iron(III) chloride (89 mg, 0.55 mmol). The resulting mixture was heated to 100 °C for 48 h. - 110 -4877-0794-3771.1 105807.005004– PCT Application The mixture was cooled to room temperature, EtOAc (5 mL) was added, and the mixture was stirred until all the solid was dissolved. The layers were separated, and the aqueous layer was extracted by EtOAc (2 x 5 mL). The combined organic layers were dried over MgSO4 and concentrated. The crude material was purified by silica gel chromatography (0–100% EtOAc/heptane) to afford the title compound (243 mg, 0.729 mmol, 89.0% yield), a beige solid. LC-MS calc. for C14H14BrN4O [M+H]+: m/z= 333.0/335.0; Found 333.2/335.2. [00435] Step 2: 8-(1-Hydroxyethyl)-3,6-dimethyl-2-(1-methylpyrazol-4-yl)quinazolin-4-one [00436] The title compound was synthesized by procedures analogous to those outlined in Example 8, Step 2 and Example 1, Step 6. LC-MS calc. for C16H19N4O2 [M+H]+: m/z= 299.1; Found 299.3. [00437] Step 3: 6-Chloro-3-[1-[3,6-dimethyl-2-(1-methylpyrazol-4-yl)-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid [00438] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7.1H NMR (300 MHz, DMSO-d6) δ 8.48 (s, 2H), 8.14 (s, 1H), 7.83 (d, J = 9.3 Hz, 1H), 7.59 (d, J = 1.8 Hz, 1H), 7.32 (d, J = 8.9 Hz, 1H), 7.09 (d, J = 9.1 Hz, 1H), 5.53 (s, 1H), 3.97 (s, 3H), 3.77 – 3.69 (m, 3H), 2.40 (s, 3H), 1.63 (d, J = 6.6 Hz, 3H). LC-MS calc. for C22H22ClN6O3 [M+H]+: m/z= 453.1/455.1; Found 453.3/455.2. Example 21. 3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid - 111 -4877-0794-3771.1 105807.005004– PCT Application [00439] Step 1.8-Acetyl-3,6-dimethyl-1H-quinazoline-2,4-dione [00440] The title compound was synthesized from 8-bromo-3,6-dimethylquinazoline- 2,4(1H,3H)-dione by procedures analogous to those outlines in Example 1, Step 5. LC-MS calc. for C12H13N2O3 [M+H]+: m/z = 233.1; Found = 232.9. [00441] Step 2.8-(1-Hydroxyethyl)-3,6-dimethylquinazoline-2,4(1H,3H)-dione [00442] To a solution of 8-acetyl-3,6-dimethyl-1H-quinazoline-2,4-dione (600 mg, 2.58 mmol) in MeOH (50 mL) was added sodium borohydride (489 mg, 12.9 mmol) at 0 °C. The mixture was warmed to room temperature and stirred for 5h. The reaction was quenched with 1 M HCl (aq.) (10 mL), and the reaction mixture was extracted with EtOAc (3 x 20 mL). The combined organic layers were washed with brine (10 mL), dried over Na2SO4, filtered, and concentrated. The crude material was purified by silica gel chromatography (0–100% EtOAc/hexane) to afford the title compound (230 mg, 0.982 mmol, 38.0% yield) as an off-white solid. LC-MS calc. for C12H15N2O3 [M+H]+: m/z= 235.1; Found 235.1 [00443] Step 3.8-(1-Hydroxyethyl)-3,6-dimethyl-2-(piperazin-1-yl)quinazolin-4(3H)-one [00444] To a solution of 8-(1-hydroxyethyl)-3,6-dimethylquinazoline-2,4(1H,3H)-dione (230 mg, 0.982 mmol) in MeCN (5 mL) was added 1,8-diazabicyclo[5.4.0]undec-7-ene (0.734 mL, - 112 -4877-0794-3771.1 105807.005004– PCT Application 4.91 mmol) and bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (549 mg, 1.18 mmol). The reaction was stirred for 30 min. Piperazine (169 mg, 1.96 mmol) was added, and the reaction mixture was stirred at 60 °C for 3 h. The reaction mixture was cooled to room temperature and diluted with water and EtOAc. The aqueous layer was extracted with EtOAc (2 x 10 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated. The crude material was purified by silica gel chromatography (0–100% EtOAc/hexanes) to afford the title compound (110 mg, 0.364 mmol, 37.1% yield) as an off-white solid. LC-MS calc. for C16H23N4O2 [M+H]+: m/z = 303.1; Found: 303.1. [00445] Step 4.2-(4-Benzoylpiperazin-1-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)- one [00446] To a solution of benzoic acid (12.1 mg, 0.099 mmol) in DCM (1 mL) was added triethylamine (55.3 µL, 0.397 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3- triazolo[4,5-b] pyridinium 3-oxid hexafluorophosphate (41.5 mg, 0.109 mmol). The resulting mixture was stirred for 15 min.2-((1-(3,6-Dimethyl-4-oxo-2-(piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino) benzoic acid (30.0 mg, 0.071 mmol) was added, and the reaction was stirred for another 30 min. The reaction mixture was diluted with water (10 mL) and the layers were separated. The aqueous layer was extracted with DCM (2 x 10 mL). The combined organic layers were washed with brine (20 mL), dried over Na2SO4, filtered, and concentrated. The crude material was purified by silica gel column chromatography (0–100% EtOAc/hexane) to afford the title compound (25.1 mg, 0.061 mmol, 62.1% yield), a white solid. LC-MS calc. for C23H27N4O3 [M+H]+: m/z= 407.2; Found 407.1. [00447] Step 5.3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid [00448] The title compound was synthesized according to procedures analogous to those outlined in Example 1, Step 7 and was obtained as a hydrochloric acid salt.1H NMR (400 MHz, DMSO) δ 8.45 (d, J = 7.2 Hz, 1H), 7.74 (s, 1H), 7.52 (d, J = 2.1 Hz, 1H), 7.47 (m, 5H), 7.33 (d, J = 9.0 Hz, 1H), 7.11 (d, J = 9.1 Hz, 1H), 5.35 (m, 1H), 3.83 – 3.79 (m, 4H), 3.51 (s, 3H), 3.30 – - 113 -4877-0794-3771.1 105807.005004– PCT Application 3.26 (m, 4H) 2.34 (s, 3H), 1.60 (d, J = 6.6 Hz, 3H). LC-MS calc. for C29H30ClN6O4 [M+H]+: m/z = 561.2; Found: 561.0. Example 22. 6-Chloro-3-((1-(2-(4-(cyclobutanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00449] The title compound was synthesized according to procedures analogous to those outlined in Example 21, Steps 4-5. LC-MS calc. for C27H32ClN6O4 [M+H]+: m/z = 539.2; Found: 539.1. Example 23. 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00450] Step 1.8-Acetyl-3,6-dimethyl-2-(4-(pyridin-2-yl)piperazin-1-yl)quinazolin-4(3H)-one [00451] The title compound was synthesized according to procedures analogous to those outlined in Example 3, Step 1. LC-MS calc. for C21H24N5O2 [M+H]+: m/z = 378.2; Found: 378.1. [00452] Step 2.8-(1-Hydroxyethyl)-3,6-dimethyl-2-(4-(pyridin-2-yl)piperazin-1-yl)quinazolin- 4(3H)-one - 114 -4877-0794-3771.1 105807.005004– PCT Application [00453] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6. LC-MS calc. for C21H26N5O2 [M+H]+: m/z = 380.2; Found: 380.1. [00454] Step 3.6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00455] The title compound was synthesized by procedures analogous to those outlined in Example 3, Step 5. The crude reaction mixture was purified by prep-HPLC on a C18 column (15–20% MeCN/0.1% NH3 (aq.)) to afford the title compound as an ammonium salt.1H NMR (400 MHz, DMSO) δ 9.90 (d, J = 7.1 Hz, 1H), 8.15 (dd, J = 5.1, 1.9 Hz, 1H), 7.70 (t, J = 1.5 Hz, 1H), 7.57 (ddd, J = 8.9, 7.1, 2.0 Hz, 1H), 7.44 (d, J = 2.1 Hz, 1H), 6.90 (dd, J = 8.6, 3.8 Hz, 2H), 6.68 (dd, J = 7.1, 4.9 Hz, 1H), 6.61 (d, J = 8.7 Hz, 1H), 5.30 (m, 1H), 3.71 – 3.69 (m, 4H), 3.54 (s, 3H), 3.38 – 3.34 (m, 4H), 2.31 (s, 3H), 1.50 (d, J = 6.6 Hz, 3H). LC-MS calc. for C27H29ClN7O3 [M+H]+: m/z= 534.2; Found 534.0. Example 24. 6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid [00456] Step 1. Tert-Butyl 6-chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinate - 115 -4877-0794-3771.1 105807.005004– PCT Application [00457] To a solution of 8-(1-hydroxyethyl)-3,6-dimethyl-1H-quinazoline-2,4-dione (118 mg, 0.503 mmol) in DCM (3 mL) was added phosphorus tribromide (94.6 µL, 1.01 mmol) at 0 °C. The reaction was allowed to warm to room temperature and stirred for 1 h. The reaction mixture was quenched with ice-cold water (10 mL) and diluted with DCM (10 mL). The layers were separated, and the aqueous layer was extracted with DCM (2 x 10 mL). The combined organic layers were washed with brine (20 mL), dried over Na2SO4, filtered, and concentrated. The concentrated crude material was redissolved in DCM (3 mL), and to this were added tert-butyl 3- amino-6-chloropyridine-2-carboxylate (138 mg, 0.604 mmol), N,N-diisopropylethylamine (351 µL, 2.01 mmol). The reaction mixture was stirred at 40 °C overnight and then cooled to room temperature. Water (10 mL) was added, and the layers were separated. The aqueous layer was extracted with DCM (10 mL) and the combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated. The crude material was purified by silica gel column chromatography (0–20% MeOH/DCM) to afford the title compound as an off-white solid (40.1 mg, 0.089 mmol, 17.9% yield). LC-MS calc. for C22H26ClN4O4 [M+H]+: m/z = 445.2; Found: 445.1. [00458] Step 2.6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)picolinic acid [00459] To a solution of tert-butyl 6-chloro-3-[1-(2-hydroxy-3,6-dimethyl-4-oxoquinazolin-8- yl)ethylamino]pyridine-2-carboxylate (20.0 mg, 0.045 mmol) in DCM (1 mL) was added trifluoroacetic acid (138 µL, 1.80 mmol). The reaction mixture was stirred for 1 h. The reaction mixture was concentrated, diluted with MeCN and water, and purified by prep-HPLC on a C18 column (5–17% MeCN/0.1% NH3 (aq.)) to afford the title compound as an ammonium salt (1.81 mg, 0.004 mmol, 9.90% yield), a white solid.1H NMR (400 MHz, DMSO) δ 9.88 (broad s, 1H), 7.66 (s, 1H), 7.41 (s, 1H), 6.98 (d, J = 8.5 Hz, 1H), 6.63 (s, 1H), 6.55 (d, J = 8.7 Hz, 1H), 5.06 (m, 1H), 3.28 (s, 3H), 2.24 (s, 3H), 1.36 (d, J = 6.4 Hz, 3H). LC-MS calc. for C18H18ClN4O4 [M+H]+: m/z= 389.1; Found 389.1. Example 25. 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid - 116 -4877-0794-3771.1 105807.005004– PCT Application [00460] Step 1.8-(1-Hydroxyethyl)-3,6-dimethyl-2-(4-(pyridin-4-yl)piperazin-1-yl)quinazolin- 4(3H)-one [00461] The title compound was synthesized by procedures analogous to those outlined in Example 23, Steps 1 and 2. LC-MS calc. for C21H26N5O2 [M+H]+: m/z = 380.2; Found: 380.1. [00462] Step 2. Tert-Butyl 6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1- yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinate [00463] The title compound was synthesized by procedures analogous to those outlined in Example 24, Step 1. LC-MS calc. for C31H37ClN7O3 [M+H]+: m/z = 590.3; Found: 591.1. [00464] Step 3.6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00465] The title compound was synthesized by procedures analogous to those outlined in Example 24, Step 2. The crude material was purified by prep-HPLC on a fluoro-phenyl column (24–36% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt. LC-MS calc. for C27H29ClN7O3 [M+H]+: m/z= 534.2; Found 534.0. Example 26. 6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid - 117 -4877-0794-3771.1 105807.005004– PCT Application [00466] Step 1.8-Bromo-3,6-dimethyl-2-(piperazin-1-yl)quinazolin-4(3H)-one [00467] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 4. LC-MS calc. for C14H18BrN4O [M+H]+: m/z = 337.1, 339.1; Found: 337.1, 339.1. [00468] Step 2. Methyl 4-(8-bromo-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)piperazine- 1-carboxylate [00469] The title compound was synthesized by procedures analogous to those outlined in Example 3, Step 3. LC-MS calc. for C16H20BrN4O3 [M+H]+: m/z = 395.1, 397.1; Found: 395.1, 397.1. [00470] Step 3. Methyl 4-(8-(1-hydroxyethyl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2- yl)piperazine-1-carboxylate [00471] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 5–6. LC-MS calc. for C18H25N4O4 [M+H]+: m/z = 361.1; Found: 361.1. - 118 -4877-0794-3771.1 105807.005004– PCT Application [00472] Step 4.6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00473] The title compound was synthesized by procedures analogous to those outlined in Example 3, Step 5. The crude reaction mixture was purified by prep-HPLC on a C18 column (12.3–32.3% MeCN/0.15% NH3 (aq.)) to afford the title compound as an ammonium salt. LC- MS calc. for C24H28ClN6O5 [M+H]+: m/z= 515.1; Found 515.1. Examples 27 – 29 [00474] Examples 27 – 29 listed in Tables 1 and 2 were synthesized according to procedures analogous to Example 26. All examples in this table were prepared as ammonium salts. Table 1. Examples 27 – 29 Table 2. Examples 27 – 29 - 119 -4877-0794-3771.1 105807.005004– PCT Application Example 30. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid (Isomer 2) [00475] The title compound was synthesized by procedures analogous to those outlines in Example 2, Step 2, utilizing tert-butyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate (Isomer 2) (from Example 2, Step 2).1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H), 7.69 (d, J = 2.1 Hz, 1H), 7.45 (d, J = 2.1 Hz, 1H), 7.33 (d, J = 9.0 Hz, 1H), 7.06 (d, J = 9.0 Hz, 1H), 5.37 – 5.26 (m, 1H), 3.95 (d, J = 10.5 Hz, 1H), 3.82 (d, J = 10.4 Hz, 1H), 3.54 – 3.44 (m, 2H), 3.42 (s, 3H), 2.31 (s, 3H), 1.66 – 1.55 (m, 5H), 0.64 – 0.54 (m, 1H), 0.39 – 0.31 (m, 1H). LC-MS calc. for C23H25ClN5O3 [M+H]+: m/z = 454.2; Found = 454.1. Examples 31 – 37 - 120 -4877-0794-3771.1 105807.005004– PCT Application [00476] Examples 31- 37 listed in Tables 3 and 4 were synthesized according to procedures analogous to Example 1. All examples in this table were prepared as the TFA salt unless otherwise noted. Table 3. Examples 31 – 37 - 121 -4877-0794-3771.1 105807.005004– PCT Application Table 4. Examples 31 – 37 - 122 -4877-0794-3771.1 105807.005004– PCT Application Example 38. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-cyanopicolinic acid [00477] Step 1. Methyl 3-amino-6-cyanopicolinate - 123 -4877-0794-3771.1 105807.005004– PCT Application [00478] Methyl 3-amino-6-chloropyridine-2-carboxylate (50 mg, 0.27 mmol) and CuCN (48 mg, 0.54 mmol) were suspended in DMF (1.3 mL) and sealed into a microwave reaction tube. The reaction mixture was stirred in a microwave reactor for 5 min at 80 ºC, 5 min at 150 ºC, and 5 min at 220 ºC. The reaction was cooled to room temperature, diluted with DCM, and filtered through a pad of Celite. The organic solution was concentrated and purified by silica gel chromatography (0-100% EtOAc/hexanes). Further purification by prep-HPLC on a C18 column (15-35% MeCN/0.1% TFA (aq.)) afforded the title compound as a TFA salt (5.0 mg, 17 µmol, 6.4% yield). LC-MS calc. for C8H8N3O2 [M+H]+: m/z = 178.1; Found = 178.0. [00479] Step 2. Methyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-cyanopicolinate [00480] Phosphorous tribromide (47 µL, 0.50 mmol) was added to a solution of 2-(3- azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)-one (Example 1, Step 6) (60 mg, 0.20 mmol) in DCM (2.0 mL), and the solution was stirred for 1 h. The reaction was poured into sat. NaHCO3 (aq.) and diluted with DCM. The resulting two phases were separated, and the aqueous layer was extracted with DCM (10 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue was used without further purification. [00481] The crude residue dissolved in DMF (2.0 mL), divided in half, and one half was combined with methyl 3-amino-6-cyanopicolinate, TFA salt (5.0 mg, 17 µmol), and N,N- diisopropylethylamine (33 µL, 0.19 mmol). The resulting solution was heated at 100 ºC for 1 h. The reaction mixture was cooled to room temperature, diluted with MeCN, and filtered. The crude solution was purified by prep-HPLC on a CSH-FP column (45-59% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (2.0 mg, 3.5 µmol, 3.5% yield). LC-MS calc. for C25H27N6O3 [M+H]+: m/z = 459.2; Found = 459.2. - 124 -4877-0794-3771.1 105807.005004– PCT Application [00482] Step 3.3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-cyanopicolinic acid [00483] LiOH (22 µL, 22 µmol, 1M (aq.)) was added to a solution of methyl 3-((1-(2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6- cyanopicolinate, TFA salt (2.0 mg, 3.5 µmol) in dioxane (0.4 mL) and water (0.4 mL). Then resulting solution was stirred for 1 h. The solution was diluted with MeCN, TFA (50 µL) was added, and the mixture was filtered. The crude solution was purified by prep-HPLC using on a C18 column (55-75% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (0.7 mg, 1.3 µmol, 36% yield). LC-MS calc. for C24H25N6O3 [M+H]+: m/z = 445.2; Found = 445.1. Example 39. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-fluoropicolinic acid [00484] Step 1. Ethyl 3-amino-6-fluoropicolinate [00485] 6-Fluoro-2-iodopyridin-3-amine (200 mg, 0.84 mmol), palladium(II) acetate (38 mg, 0.17 mmol), and 1,1’-ferrocenediyl-bis(diphenylphosphine) (140 mg, 0.25 mmol) were dissolved in EtOH (2.1 mL) and sealed in a Schlenk flask. The flask was degassed with CO and cooled to -78 ºC under an atmosphere of CO. The flask was sealed and heated to 100 ºC for 72 h. The reaction was cooled to room temperature and diluted with DCM and water. The resulting two phases were separated. The aqueous phase was extracted with DCM (10 mL x 2), and the combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue was purified by prep-HPLC on a C18 column (19-39% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (8.2 mg, 28 µmol, 3.2% yield).1H NMR (400 MHz, CDCl3) δ 7.18 (dd, J = 8.8, 6.6 Hz, 1H), 6.93 (dd, J = 8.9, 3.8 Hz, 1H), 5.73 (s, 2H), 4.43 (q, J = 7.1 Hz, - 125 -4877-0794-3771.1 105807.005004– PCT Application 2H), 1.42 (t, J = 7.1 Hz, 3H).19F NMR (376 MHz, CDCl3) δ -76.95. LC-MS calc. for C8H10FN2O2 [M+H]+: m/z = 185.1; Found = 185.0. [00486] Step 2. Ethyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-fluoropicolinate [00487] The title compound was synthesized by procedures analogous to those outlined in Example 38, Step 2 by substituting ethyl 3-amino-6-fluoropicolinate for methyl 3-amino-6- cyanopicolinate. LC-MS calc. for C25H29FN5O3 [M+H]+: m/z = 466.2; Found = 466.1. [00488] Step 3.3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-fluoropicolinic acid [00489] The title compound was synthesized by procedures analogous to those outlined in Example 38, Step 3.1H NMR (400 MHz, DMSO-d6) δ 8.31 (bs, 1H), 7.68 (dd, J = 2.1, 0.9 Hz, 1H), 7.45 (d, J = 2.1 Hz, 1H), 7.18 (dd, J = 9.3, 6.3 Hz, 1H), 7.12 (dd, J = 9.1, 3.3 Hz, 1H), 5.38 – 5.28 (m, 1H), 3.96 (d, J = 10.5 Hz, 1H), 3.81 (d, J = 10.5 Hz, 1H), 3.55 – 3.43 (m, 2H), 3.42 (s, 3H), 2.30 (s, 3H), 1.65 – 1.54 (m, 5H), 0.63 – 0.54 (m, 1H), 0.38 – 0.31 (m, 1H). LC-MS calc.for C24H26FN5O3[M+H]+: m/z = 438.2; Found = 438.1. Example 40. 6-Chloro-3-((1-(2-((trans-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00490] Step 1.8-Acetyl-3,6-dimethylquinazoline-2,4(1H,3H)-dione - 126 -4877-0794-3771.1 105807.005004– PCT Application [00491] The title compound was synthesized from 8-bromo-3,6-dimethylquinazoline- 2,4(1H,3H)-dione (Example 1, Step 2) by procedures analogous to those outlines in Example 1, Step 5. LC-MS calc. for C12H13N2O3 [M+H]+: m/z = 233.1; Found = 232.9. [00492] Step 2.8-(1-Hydroxyethyl)-3,6-dimethylquinazoline-2,4(1H,3H)-dione (Isomer 1 and Isomer 2) [00493] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 6 to afford a mixture of isomers 1 and 2. LC-MS calc. for C12H15N2O3 [M+H]+: m/z = 235.1; Found = 235.0. The isomers were separated using chiral prep-HPLC on a Lux iA1 column (30 mL/min, 93:7 hexane/IPA) to afford two isomers: isomer 1 (tR= 6.78) and isomer 2 (tR= 7.52). [00494] Step 3.2-(trans-3,4-Difluoropyrrolidin-1-yl)-8-(1-hydroxyethyl)-3,6- dimethylquinazolin-4(3H)-one [00495] Bromotripyrrolidinophosphonium hexafluorophosphate (240 mg, 0.51 mmol) (CAS: 132705-51-2), 8-(1-hydroxyethyl)-3,6-dimethylquinazoline-2,4(1H,3H)-dione (Isomer 1; Example 40, Step 2) (100 mg, 0.43 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (0.19 mL, 1.3 mmol) were dissolved in MeCN (2.1 mL) and the resulting mixture was stirred at room temperature for 1 hr. Then rac-(3R,4R)-3,4-difluoropyrrolidine hydrochloride (74 mg, 0.51 mmol) (CAS#: 869481-92-5) was added and the reaction mixture was heated to 60 ºC for 18 h. The reaction mixture was cooled to room temperature then diluted with water and DCM. The resulting two phases were separated, and the aqueous layer was extracted with DCM (10 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The - 127 -4877-0794-3771.1 105807.005004– PCT Application crude residue was purified by silica gel chromatography (EtOAc/hexanes 0–100%) to afford the title compounds as a mixture of two isomers (52 mg, 0.16 mmol, 38% yield). LC-MS calc. for C16H20F2N3O2 [M+H]+: m/z = 324.1; Found = 324.1. [00496] Step 4. 6-Chloro-3-((1-(2-((trans-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00497] The title compound was synthesized from a mixture of 2-(trans-3,4- Difluoropyrrolidin-1-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin-4(3H)-one (Isomer 1 and Isomer 2; Example 40, Step 3)) by procedures analogous to those outlined in Example 1, Step 7 to afford a mixture of four isomers. LC-MS calc. for C22H23ClF2N5O3 [M+H]+: m/z = 478.1; Found = 478.0. Example 41. 6-Chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydro-quinazolin-8-yl)ethyl)amino)picolinic acid [00498] Step 1.2-(4,4-Dimethylpiperidin-1-yl)-8-(1-hydroxyethyl)-3,6-dimethylquinazolin- 4(3H)-one [00499] The title compound was synthesized from rac-8-(1-hydroxyethyl)-3,6- dimethylquinazoline-2,4(1H,3H)-dione (Example 40, Step 2) by procedure analogous to those outlined in Example 40, Step 3. LC-MS calc. for C19H28N3O2 [M+H]+: m/z = 330.2; Found = 330.1 [00500] Step 2.6-Chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00501] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7.1H NMR (400 MHz, DMSO-d6) δ 8.50 (bs, 1H), 7.74 (d, J = 2.0 Hz, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.35 (d, J = 8.9 Hz, 1H), 7.14 (d, J = 9.1 Hz, 1H), 5.42 – 5.27 (m, 1H), - 128 -4877-0794-3771.1 105807.005004– PCT Application 3.47 (s, 3H), 3.31 – 3.10 (m, 4H), 2.34 (s, 3H), 1.62 (d, J = 6.6 Hz, 3H), 1.56 – 1.45 (m, 4H), 1.02 (s, 6H). LC-MS calc. for C25H31ClN5O3 [M+H]+: m/z = 484.2; Found = 484.1. Example 42. 6-Chloro-3-((1-(2-((1R,5S,6r)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid [00502] Diisopropyl azodicarboxylate (82 µL, 0.42 mmol) was added to a solution of triphenylphosphine and 3-fluoro-4-nitrobenzoic acid (77 mg, 0.42 mmol) in THF (1.5 mL), and the resulting mixture was stirred for 30 min. Then a solution of 6-chloro-3-((1-(2-((1R,5S,6s)-6- hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl) amino)picolinic acid, TFA salt (Example 8) (9.8 mg, 21 µmol) in THF (0.50 mL) was added, and the reaction mixture was stirred for 18 h. The mixture was diluted with water and DCM. The resulting two phases were separated, and the aqueous layer was extracted with DCM (5.0 mL x 2). The combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude residue was purified by prep-HPLC on a C18 column (40-60% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (0.8 mg, 1.4 µmol, 8.1% yield). LC-MS calc. for C23H25ClN5O4 [M+H]+: m/z = 470.2; Found = 470.1. Example 43. 5-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid [00503] Step 1. Ethyl 5-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-2-chlorothiazole-4-carboxylate - 129 -4877-0794-3771.1 105807.005004– PCT Application [00504] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 7, substituting ethyl 5-amino-2-chlorothiazole-4-carboxylate for 3-amino-6- chloropicolinic acid for. LC-MS calc. for C23H27ClN5O3S [M+H]+: m/z = 488.1; Found = 488.0. [00505] Step 2.5-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid [00506] Boron tribromide (2.9 µL, 31 µmol) was added to a solution of ethyl 5-((1-(2-(3- azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-2- chlorothiazole-4-carboxylate, TFA salt (5.0 mg, 8.3 µmol) in DCM (0.50 mL) at RT and the reaction as stirred for 1 h. The reaction was diluted with water (2.0 mL) and DCM (1.0 mL) and the resulting two phases were separated. The aqueous layer was extracted with DCM (2 x 2 mL) and the combined organic layers were dried over sodium sulfate, filtered, and concentrated. The crude product was purified by prep-HPLC on a C18 column (54.6-74.6% MeCN/0.1% TFA (aq.)) to afford the title compound as a TFA salt (2.4 mg, 4.2 µmol, 50% yield).1H NMR (400 MHz, DMSO-d6) δ 8.33 (d, J = 8.4 Hz, 1H), 7.73 (s, 1H), 7.55 (d, J = 2.1 Hz, 1H), 4.96 – 4.77 (m, 1H), 3.96 (d, J = 10.5 Hz, 1H), 3.80 (d, J = 10.3 Hz, 1H), 3.50 (d, J = 10.3 Hz, 2H), 3.41 (s, 3H), 2.36 (s, 3H), 1.69 (d, J = 6.7 Hz, 3H), 1.65 – 1.56 (m, 2H), 0.65 – 0.53 (m, 1H), 0.41 – 0.33 (m, 1H). LC-MS calc. for C21H23ClN5O3S [M+H]+: m/z = 460.1; Found = 460.0. Example 44. 2-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(difluoromethyl)-6-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)benzoic acid [00507] Step 1.2-Amino-3-bromo-N-ethyl-5-methylbenzamide - 130 -4877-0794-3771.1 105807.005004– PCT Application [00508] To a solution of 2-amino-3-bromo-5-methylbenzoic acid (2.00 g, 8.69 mmol) in THF (20.0 mL) was added 1,1’-carbonyldiimidazole (1.55 g, 9.56 mmol), and the resulting reaction mixture was stirred at room temperature for 12 h. Then ethylamine (6.50 mL, 13 mmol, 2.0 M in THF) was added, and the reaction mixture was stirred for 4 h. The reaction was diluted with ethyl acetate (20.0 mL), and the organic layer was washed with water and brine, dried over Na2SO4, and concentrated. The crude material, which contained the title compound, was carried forward without further purification. LC-MS calc. for C10H14BrN2O [M+H]+: m/z = 257.0, 259.0; Found: 256.9, 258.9. [00509] Step 2.8-Acetyl-3-methoxy-6-methylquinazoline-2,4(1H,3H)-dione [00510] To a solution of 2-amino-3-bromo-N-ethyl-5-methylbenzamide (500 mg, 1.94 mmol) in THF (10.0 mL) was added 1,1’-carbonyldiimidazole (631 mg, 3.89 mmol) and 1,8- diazabicyclo [5.4.0]undec-7-ene (0.871 mL, 5.83 mmol). The mixture was refluxed for 16 h. The reaction mixture was cooled to room temperature and poured into a mixture of ice water (5.0 mL) and sat. NH4Cl (aq.) (5.0 ml). The suspension was stirred for 10 min, and then the solid was collected by filtration to afford the title compound (482 mg, 1.70 mmol, 87.6% yield) as a white solid. LC-MS calc. for C11H12BrN2O2 [M+H]+: m/z = 283.0, 285.0; Found: 283.0, 285.0. [00511] Step 3.2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-6-methyl-3-(methyl- d3)quinazolin-4(3H)-one [00512] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 3-7.1H NMR (400 MHz, DMSO-d6) δ 9.48 (s, 1H), 7.65 (dd, J = 2.1, 1.0 Hz, 1H), 7.39 (d, J = 2.2 Hz, 1H), 7.02 (d, J = 8.7 Hz, 1H), 8.67 (s, br, 1H), 6.67 (d, J = 8.7 Hz, 1H), 5.25 (q, 1H), 4.01 (dq, J = 16.1, 7.0 Hz, 2H), 3.86 (d, J = 10.2 Hz, 1H), 3.73 (d, J = 10.2 Hz, 1H), 3.62 – 3.48 (m, 2H), 2.28 (s, 3H), 1.62 (dt, J = 6.5, 3.4 Hz, 2H), 1.51 (d, , J = 8.7 Hz, 2H), 1.31 (t, J = 6.9 Hz, 3H), 0.59 (td, J = 7.7, 4.6 Hz, 1H), 0.42 (q, J = 4.2 Hz, 1H). LC-MS calc. for C24H27ClN5O3 [M+H]+: m/z = 468.2; Found: 468.1. - 131 -4877-0794-3771.1 105807.005004– PCT Application Examples 45 – 50 [00513] Examples 45 – 50 listed in Tables 5 and 6 were synthesized according to procedures analogous to Example 44. All examples in this table were prepared as the ammonium salt unless otherwise noted. Table 5. Examples 45 – 50 Table 6. Examples 45 – 50 - 132 -4877-0794-3771.1 105807.005004– PCT Application Example 51. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00514] Step 1.8-Bromo-3-methoxy-6-methylquinazoline-2,4(1H,3H)-dione [00515] The title compound was synthesized by procedures analogous to those outlined in Example 44, Steps 1-2, substituting methoxyamine hydrochloride for methylamine in Step 1. LC-MS calc. for C10H10BrN2O3 [M+H]+: m/z = 285.0, 287.0; Found:.284.9, 286.9. [00516] Step 2.8-Acetyl-3-methoxy-6-methylquinazoline-2,4(1H,3H)-dione - 133 -4877-0794-3771.1 105807.005004– PCT Application [00517] The title compound was synthesized by procedures analogous to those outlined in Example 1, Step 5. LC-MS calc. for C12H13N2O4 [M+H]+: m/z = 249.1; Found: 249.0. [00518] Step 3.2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-(1-hydroxyethyl)-3-methoxy-6- methylquinazolin-4(3H)-one [00519] The title compound was synthesized by procedures analogous to those outlined in Example 40, Step 3. for C17H20N3O3 [M+H]+: m/z = 314.1; Found: 314.1. [00520] Step 4.3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00521] The title compound was synthesized by procedures analogous to those outlined in Example 1, Steps 6-7. LC-MS calc. for C23H25ClN5O4 [M+H]+: m/z = 470.2; Found: 470.1. Example 52. 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(tert-butoxy)-6-methyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid [00522] The title compound was synthesized by procedures analogous to those outlined in Example 51, Steps 1-4, substituting O-tert-butylhydroxylamine hydrochloride for 2,2,2- trifluoroethylamine hydrochloride in Step 1. LCMS calc. for C26H31ClN5O4 [M+H]+: 512.2; Found: 512.1. Examples 53 - 70 - 134 -4877-0794-3771.1 105807.005004– PCT Application [00523] Examples 53 - 70 listed in Tables 7 and 8 were synthesized according to procedures analogous to Example 9, [Step 1-3] (Method A), Example 13, [Step 9-10] (Method B), Example 3, Steps 4–5 (method C), or Example 40 (Method D). The appropriate starting materials for Methods A and B can be prepared according to procedures analogous to those reported herein. All examples in this table were prepared as the TFA salt unless otherwise noted. Table 7. Examples 53 – 70 - 135 -4877-0794-3771.1 105807.005004– PCT Application Table 8. Examples 53 – 70 - 136 -4877-0794-3771.1 105807.005004– PCT Application - 137 -4877-0794-3771.1 105807.005004– PCT Application - 138 -4877-0794-3771.1 105807.005004– PCT Application - 139 -4877-0794-3771.1 105807.005004– PCT Application - 140 -4877-0794-3771.1 105807.005004– PCT Application Examples 71 - 79 [00524] Examples 71 - 79 listed in Tables 9 and 10 were synthesized according to procedures analogous to Example 13 (Steps 1-2 and 6-7). The appropriate starting materials can be prepared according to procedures analogous to those reported herein. All examples in this table were prepared as the TFA salt unless otherwise noted. Table 9. Examples 71 – 79 - 141 -4877-0794-3771.1 105807.005004– PCT Application Table 10. Examples 71 – 79 - 142 -4877-0794-3771.1 105807.005004– PCT Application - 143 -4877-0794-3771.1 105807.005004– PCT Application Examples 80 - 83 - 144 -4877-0794-3771.1 105807.005004– PCT Application [00525] Examples 80 – 83 listed in Tables 11 and 12 were synthesized according to procedures analogous to Example 14, Steps 4-5. All examples in this table were prepared as the TFA salt unless otherwise noted. Table 11. Examples 80 – 83 Table 12. Examples 80 – 83 - 145 -4877-0794-3771.1 105807.005004– PCT Application Example 84.6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino] pyridine-2-carboxylic acid - 146 -4877-0794-3771.1 105807.005004– PCT Application [00526] The title compound was synthesized by procedures analogous to those outlined in Example 19, Steps 1–6.1H NMR (300 MHz, DMSO-d6) δ 12.90 (br s, 1H), 8.32 (d, J = 6.0 Hz, 1H), 8.16 (d, J = 2.1 Hz, 1H), 7.68 (d, J = 2.1 Hz, 1H), 7.64 – 7.48 (m, 3H), 7.48 – 7.30 (m, 3H), 7.05 (d, J = 9.0 Hz, 1H), 5.54 - 5.47 (m, 1H), 3.10 (s, 3H), 2.39 (s, 3H), 2.18 (s, 3H), 1.56 (d, J = 6.3 Hz, 3H). LC-MS calc. for C26H25ClN3O3 [M+H]+: m/z= 462.2, 464.2; Found 462.4, 464.4 Example A: PI3K Pathway Activation Assay [00527] The inhibitory activity of compounds was evaluated by measuring phosphorylation of AKT on Ser473 as a readout of the PI3K pathway using HTRF (CisBio catalog number: 64AKSPE). These studies were conducted in the T-47D (heterozygous PIK3CA H1047R) andSKBR3 (PIK3CA WT) cell lines. Cells were maintained in a 37°C incubator at 5% CO2in the following media: T-47D: RPMI 1640, ATCC© Modification (Gibco, A10491-01) supplemented with 10% v/v FBS (Gibco, 26140-079), 1% penicillin streptomycin (Gibco, 15140-122), and 7.4 ug/mL insulin (MilliporeSigma, I9278); SKBR3: McCoy’s 5a (Modified) Medium (Gibco, 16600-082) supplemented with 10% v/v FBS (Gibco, 26140-079). Cells were seeded in 384- well plates at a density of 4,000 cells/well. Compounds dissolved in DMSO were added using a digital dispense (D300E, Tecan) 10-point serial dilution. After two hours of treatment, cells were lysed for thirty minutes and then incubated with detection reagents per HTRF kit material and manufacturer’s instructions. Fluorescence signal was measured with a multimode plate reader (Envision 2105, Perkin Elmer). Fluorescent signal was normalized to background and DMSO controls to obtain percent inhibition/activity for each compound. The results are summarized in Table 13. Table 13. IC50 Values - 147 -4877-0794-3771.1 105807.005004– PCT Application - 148 -4877-0794-3771.1 105807.005004– PCT Application - 149 -4877-0794-3771.1 105807.005004– PCT Application [00528] In Table 9, a “+” denotes an IC50 value of > 10000 nM; a “++” denotes an IC50 value of 500 nM < IC50 ≤ 10000 nM; a “+++” denotes an IC50 value of < 500 nM. [00529] While we have described a number of embodiments of this invention, it is apparent that our basic examples may be altered to provide other embodiments that utilize the compounds and methods of this invention. Therefore, it will be appreciated that the scope of this invention is to be defined by the appended claims rather than by the specific embodiments that have been represented by way of example. - 150 -4877-0794-3771.1
Claims
105807.005004– PCT Application What is claimed: 1. A compound of Formula (I) or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, wherein Ring A is: W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S, or a 5-12 membered heterocyclic group comprising 1-4 heteroatoms selected from N, O, and S, C1-C8 alkyl, haloalkyl, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkenyl, NRcRd, ORb, or SRb; wherein each group is optionally substituted by 1-6 Rf groups; Y is CR2 or N; n is 1, 2, or 3; m is 1, 2 or 3; R6 is -F, -Cl, -Br, -I or -CN; R1 is H, D, ORa, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, -C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, or (C1-C6-alkyl)-Re; wherein said C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, C3-C8 cycloalkyl, -C3-C10 cycloalkenyl, aryl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, or (C1-C6-alkyl)-Re are optionally substituted by 1-6 Rf groups; each R2 and R5 is independently H, D, halogen, C1-C8 alkoxide, C1-C8 alkyl, haloalkyl, - OH, -CN, -NO2, -C2-C6 alkenyl, -C2-C6 alkynyl, aryl, heteroaryl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, -ORa, -SRa, -NRcRd, -NRaRc, -C(O)Rb, -OC(O)Rb, - C(O)ORb, -C(O)NRcRd, -S(O)Rb, -S(O)2NRcRd, -S(O)(=NRb)Rb, -SF5, -P(O)RbRb, - P(O)(ORb)(ORb), -B(ORc)(ORd), -S(O)2Rb, -C(O)NRbORb, -S(O)2ORb, -OS(O)2ORb, or - OPO(ORb)(ORb); wherein said C1-C8 alkyl is optionally substituted by 1-6 R groups selected from H, D, halogen, -OH, -CN, -ORa, -SRa, -NRaRd, or NRcRd; - 151 -4877-0794-3771.1
105807.005004– PCT Application 3. The compound of any one of the preceding claims, wherein the 5-12 membered heterocyclic group is an isoindoline, 3-azabicyclo[3.1.0]hexane, piperidine, pyrrolidine, azetidine, piperazine, 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine or 1,4,5,6-tetrahydropyrrolo[3,4- c]pyrazole group optionally substituted by 1-6 Rf groups. 4. The compound of claim 1, wherein W is a 5-10 membered heteroaryl ring comprising 1-4 heteroatoms selected from N, O, and S optionally substituted by 1-6 Rf groups. 5. The compound of claim 1, wherein W is aryl optionally substituted by 1-6 Rf groups. 6. The compound of any one of claims 1-4, wherein W is NRcRd. 7. The compound of any one of the preceding claims, wherein Y is N. 8. The compound of any one of the preceding claims, wherein Y is CR2. 9. The compound of any one of the preceding claims, wherein n is 1. 10. The compound of any one of claims 1-8, wherein n is 2. 11. The compound of any one of claims 1-8, wherein n is 3. 12. The compound of any one of the preceding claims, wherein m is 1. 13. The compound of any one of claims 1-11, wherein m is 2. 14. The compound of any one of claims 1-11, wherein m is 3. 15. The compound of any one of the preceding claims, wherein R1 is H or C1-C8 alkyl optionally substituted by 1-6 Rf groups. 16. The compound of claim 14, wherein R1 is H. - 153 -4877-0794-3771.1
105807.005004– PCT Application 17. The compound of claim 14, wherein R1 is C1-C8alkyl optionally substituted by 1-6 Rf groups. 18. The compound of claim 16, wherein R1 is methyl. 19. The compound of any one of claims 1-14, wherein R1 is heterocycloalkyl. 20. The compound of claim 19, wherein R1 is tetrahydropyran or tetrahydrofuran. 21. The compound of any one of the preceding claims, wherein at least one R2 is H. 22. The compound of any one of claims 1-21, wherein at least one R2 is C1-C8 alkyl, CD3, CF3, halogen, CN or CHF2. 23. The compound of claim 22, wherein at least one R2 is methyl. 24. The compound of claim 22, wherein at least one R2 is F. 25. The compound of claim 22, wherein at least one R2 is Cl. 26. The compound of claim 22, wherein at least one R2 is CHF2. 27. The compound of claim 22, wherein at least one R2 is CF3. 28. The compound of any one of the preceding claims, wherein R3 is H. 29. The compound of any one of claims 1-27, wherein R3 is C1-C8 alkyl. 30. The compound of claim 29, wherein R3 is methyl. 31. The compound of any one of the preceding claims, wherein R4 is H. 32. The compound of any one of claims 1-30, wherein R4 is C1-C8 alkyl. - 154 -4877-0794-3771.1
105807.005004– PCT Application 33. The compound of claim 32, wherein R4 is methyl. 34. The compound of any one of the preceding claims, wherein at least one R5 is H. 35. The compound of any one of claims 1-34, wherein at least one R5 is -CO2H, -CONH2, - COOCH3, -C(O)H, -CN, or CF3. 36. The compound of any one of the preceding claims, in the form of a pharmaceutically acceptable salt. 37. The compound of claim 1, that is a compound of Formula (II) (II), or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. 38. The compound of claim 1, that is a compound of Formula (III) (III), or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. 39. The compound of claim 1, that is a compound of Formula (IV) - 155 -4877-0794-3771.1
105807.005004– PCT Application (IV), or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. 40. The compound of claim 39, wherein the compound of Formula IV is a compound of Formula IV-1, Formula IV-2, Formula IV-3, or Formula IV-4: (IV-2), (IV-3), (IV-4), or a pharmaceutically acceptable 41. The compound of claim (V) (V), or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof, Wa is a 5-10 membered heteroaryl group comprising 1-4 heteroatoms selected from N, O, and S, or an aryl group, wherein each group is optionally substituted by 1-6 Rf groups. - 156 -4877-0794-3771.1
105807.005004– PCT Application 42. The compounds of V is a compound of Formula V-1, Formula V-2 (V-1), (V-2), or a a 5-10 membered aryl group, 43. (VI), or a pharmaceutically acceptable wherein Wb is a 5-12 membered heterocyclic group from N, O, and S, or a cycloalkyl group, wherein each 1-6 Rf groups. 44. The compounds of claim VI is a compound of Formula VI-1, Formula VI-2, or - 157 -4877-0794-3771.1
105807.005004– PCT Application , , or a membered heterocyclic group, wherein each group is optionally substituted by 1-6 Rf groups. 45. The compound of any one of claims 1-39, 41, or 43, wherein at least one R5 is H, D, F, Cl, Me, or CN. 46. The compound of any each R5 is H. 47. The compound of claim 3-((1-(2-(3-Azabicyclo oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6- 6-Chloro-3-((1-(2-( - -3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-6-fluoro-3-methyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 3-[1-[2-(3-Azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8-yl]ethyl- amino]-6-chloropyridine-2-carboxylic acid; 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-[2-(5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4-oxoquinazolin- 8-yl]ethylamino]pyridine-2-carboxylic acid; - 158 -4877-0794-3771.1
105807.005004– PCT Application 6- dimethyl-4- oxo-3,4- 3-((1- 6- 3-yl)- 3,6-dimethyl- 6- [3.1.0] hexan-3-yl)- 6- dimethyl- 4-oxo-3,4- 6- 8-yl]ethylamino]pyridine-2-carboxylic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-((1R,5S,6s)-6-((pyrrolidine-1-carbonyl)oxy)-3- azabicyclo[3.1.0]hexan-3-yl)-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6- dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-[3,6-dimethyl-2-[1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidin-3-yl]-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-(methoxy(methyl)carbamoyl)-3-azabicyclo[3.1.0]hexan- 3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(6-fluoro-2-((1R,5S,6s)-6-methoxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-(2,7-dimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; 6-Chloro-3-[1-[3,6-dimethyl-2-(1-methylpyrazol-4-yl)-4-oxoquinazolin-8-yl]ethylamino] pyridine-2-carboxylic acid; 3-((1-(2-(4-Benzoylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl) amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4-(cyclobutanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-2-yl)piperazin-1-yl)-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; - 159 -4877-0794-3771.1
105807.005004– PCT Application 6-Chloro-3-((1-(2-hydroxy-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino) picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-4-oxo-2-(4-(pyridin-4-yl)piperazin-1-yl)-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4-(methoxycarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(4-Acetylpiperazin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl) amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4-(cyclopropanecarbonyl)piperazin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3,6-dimethyl-2-(4-(methylsulfonyl)piperazin-1-yl)-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-bromopicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropyrazine-2-carboxylic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinamide; Methyl 3-((1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinate; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinaldehyde; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-chloropicolinonitrile; 2-(3-Azabicyclo[3.1.0]hexan-3-yl)-8-(1-((6-chloro-2-(trifluoromethyl)pyridin-3- yl)amino) ethyl)-3,6-dimethylquinazolin-4(3H)-one; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-cyanopicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-6-fluoropicolinic acid; 6-Chloro-3-((1-(2-((trans-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; - 160 -4877-0794-3771.1
105807.005004– PCT Application 6-Chloro-3-((1-(2-(4,4-dimethylpiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4- dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-((1R,5S,6r)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 5-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8- yl)ethyl)amino)-2-chlorothiazole-4-carboxylic acid; 2-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(difluoromethyl)-6-methyl-4-oxo-3,4- dihydro-quinazolin-8-yl)ethyl)amino)benzoic acid; 6-Chloro-3-((1-(2-(3,3-difluoropyrrolidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-methyl-4-oxo- 3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-methyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-((1-(3-ethyl-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-6- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)-6-chloropicolinic acid; 6-Chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3-ethyl-6-fluoro-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-methoxy-6-methyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 3-((1-(2-(3-Azabicyclo[3.1.0]hexan-3-yl)-3-(tert-butoxy)-6-methyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-[1-[2-[4-(difluoromethyl)piperidin-1-yl]-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethyl- amino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethyl- amino]pyridine-2-carboxylic acid; - 161 -4877-0794-3771.1
105807.005004– PCT Application 3-[1-[2-(5-azaspiro[2.4]heptan-5-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethylamino]-6- chloropyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethyl- amino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-[2-[(2-methylpropan-2-yl)oxycarbonyl]-2,7- diazaspiro[4.4] nonan-7-yl]-4-oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-(2-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3-hydroxy-3-methylazetidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoroazetidin-1-yl)-3,6-dimethyl-4-oxoquinazolin-8-yl]ethyl- amino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-[4-(dimethylcarbamoyl)piperidin-1-yl]-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[3,6-dimethyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3-fluoro-5,7-dihydropyrrolo[3,4-b]pyridin-6-yl)-3,6-dimethyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(5-fluoro-1,3-dihydroisoindol-2-yl)-3,6-dimethyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-((1-(2-(isoindolin-2-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl) amino)picolinic acid; 6-chloro-3-((1-(3,6-dimethyl-4-oxo-2-(6-azaspiro[2.5]octan-6-yl)-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-((3S,4R)-3,4-difluoropyrrolidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydro- quinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-(4,4-difluoropiperidin-1-yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin- 8-yl)ethyl)amino)picolinic acid; 6-chloro-3-[1-[2-(3,3-difluoroazetidin-1-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethyl-amino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-fluoro-3-methyl-2-(2-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; - 162 -4877-0794-3771.1
105807.005004– PCT Application 6-chloro-3-[1-[6-fluoro-3-methyl-2-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-chloro-2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[6-chloro-2-(3,3-difluoropyrrolidin-1-yl)-3-methyl-4-oxoquinazolin-8- yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoropyrrolidin-1-yl)-6-fluoro-3-methyl-4-oxoquinazolin-8- yl]ethyl-amino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(6,6-difluoro-3-azabicyclo[3.1.0]hexan-3-yl)-6-fluoro-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-[1-[2-(3,3-difluoro-4-hydroxypiperidin-1-yl)-6-fluoro-3-methyl-4- oxoquinazolin-8-yl]ethylamino]pyridine-2-carboxylic acid; 6-chloro-3-((1-(6-chloro-2-((1R,5S,6s)-6-hydroxy-3-azabicyclo[3.1.0]hexan-3-yl)-3- methyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 3-((1-(2-((1R,5S,6s)-6-(carbamoyloxy)-3-azabicyclo[3.1.0]hexan-3-yl)-3,6-dimethyl-4- oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)-6-chloropicolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((isopropylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((ethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3-yl)- 3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-chloro-3-((1-(2-((1R,5S,6s)-6-((dimethylcarbamoyl)oxy)-3-azabicyclo[3.1.0]hexan-3- yl)-3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-8-yl)ethyl)amino)picolinic acid; 6-Chloro-3-[1-(2,4,7-trimethyl-1-oxo-3-phenylisoquinolin-5-yl)ethylamino]pyridine-2- carboxylic acid; or a pharmaceutically acceptable salt, N-oxide, or stereoisomer thereof. 48. A pharmaceutical composition comprising a compound according to any one of the preceding claims, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient. 49. A method of treating a disease or disorder associated with modulation of phosphoinositide 3 -kinase (PI3K), comprising administering to a patient in need thereof a - 163 -4877-0794-3771.1
105807.005004– PCT Application therapeutically effective amount of a compound of any one of claims 1-47 or a pharmaceutical composition of claim 48. 50. The method of claim 49, wherein the PI3K is PI3Kα. 51. The method of claim 49 or claim 50, wherein the PI3K associated with the disease or disorder has a H1047R mutation. 52. The method of any one of claims 49-51 wherein the disease or disorder is a cancer. 53. The method of claim 51-52 wherein the cancer is endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, head and neck cancer, breast cancer, brain cancer, cervical cancer, bladder cancer, esophageal cancer, pancreatic cancer, bone cancer, hepatobiliary cancer, medulloblastoma, kidney cancer or prostate cancer. 54. The method of any one of claims 49-51 wherein the disease or disorder is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome), or PIK3CA-related overgrowth syndrome (PROS). 55. A method of inhibiting phosphoinositide 3-kinase (PI3K), comprising administering to a patient in need thereof a therapeutically effective amount of a compound of any one of claims 1- 47 or a pharmaceutical composition of claim 48. 56. A method of treating cancer or a disorder, the method comprising administering to a patient in need thereof a therapeutically effective amount of a compound of any one of claims 1- 47 or a pharmaceutical composition of claim 48. 57. The method of claim 56, wherein the cancer is endometrial cancer, gastric cancer, leukemia, lymphoma, sarcoma, colorectal cancer, lung cancer, ovarian cancer, skin cancer, head and neck cancer, breast cancer, brain cancer, or prostate cancer. 58. The method of claim 57, wherein the cancer is breast cancer. - 164 -4877-0794-3771.1
105807.005004– PCT Application 59. The method of claim 56, wherein the disorder is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome) or PIK3CA-related overgrowth syndrome (PROS). 60. The method of claim 56, wherein the disorder is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome). 61. The method of claim 56, wherein the disorder is PIK3CA-related overgrowth syndrome (PROS). 62. A method of degrading a phosphoinositide 3-kinase (PI3K) protein comprising contacting the PI3K protein with a compound of any one of claims 1-47 or a pharmaceutical composition of claim 48. 63. The method of claim 62, wherein the PI3K is PI3Kα. - 165 -4877-0794-3771.1
59. The method of claim 56, wherein the disorder is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome) or PIK3CA-r elated overgrowth syndrome (PROS).
60. The method of claim 56, wherein the disorder is CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal naevi, scoliosis/skeletal and spinal syndrome).
61. The method of claim 56, wherein the disorder is PIK3CA-related overgrowth syndrome (PROS).
62. A method of degrading a phosphoinositide 3 -kinase (PI3K) protein comprising contacting the PI3K protein with a compound of any one of claims 1-47 or a pharmaceutical composition of claim 48.
63. The method of claim 62, wherein the PI3K is PI3Ka.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363493804P | 2023-04-03 | 2023-04-03 | |
| US63/493,804 | 2023-04-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024211346A1true WO2024211346A1 (en) | 2024-10-10 |
Family
ID=90924456
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/022742PendingWO2024211346A1 (en) | 2023-04-03 | 2024-04-03 | Mutant pi3k-alpha inhibitors and their use as pharmaceuticals |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024211346A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025087295A1 (en)* | 2023-10-25 | 2025-05-01 | 海创药业股份有限公司 | Pi3k inhibitor, and preparation method therefor and use thereof |
Citations (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3657744A (en) | 1970-05-08 | 1972-04-25 | Univ Minnesota | Method for fixing prosthetic implants in a living body |
| US4739762A (en) | 1985-11-07 | 1988-04-26 | Expandable Grafts Partnership | Expandable intraluminal graft, and method and apparatus for implanting an expandable intraluminal graft |
| US4992445A (en) | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5001139A (en) | 1987-06-12 | 1991-03-19 | American Cyanamid Company | Enchancers for the transdermal flux of nivadipine |
| US5023252A (en) | 1985-12-04 | 1991-06-11 | Conrex Pharmaceutical Corporation | Transdermal and trans-membrane delivery of drugs |
| US5040548A (en) | 1989-06-01 | 1991-08-20 | Yock Paul G | Angioplasty mehtod |
| US5061273A (en) | 1989-06-01 | 1991-10-29 | Yock Paul G | Angioplasty apparatus facilitating rapid exchanges |
| US5195984A (en) | 1988-10-04 | 1993-03-23 | Expandable Grafts Partnership | Expandable intraluminal graft |
| US5292331A (en) | 1989-08-24 | 1994-03-08 | Applied Vascular Engineering, Inc. | Endovascular support device |
| US5451233A (en) | 1986-04-15 | 1995-09-19 | Yock; Paul G. | Angioplasty apparatus facilitating rapid exchanges |
| US5496346A (en) | 1987-01-06 | 1996-03-05 | Advanced Cardiovascular Systems, Inc. | Reinforced balloon dilatation catheter with slitted exchange sleeve and method |
| US5674278A (en) | 1989-08-24 | 1997-10-07 | Arterial Vascular Engineering, Inc. | Endovascular support device |
| US6344053B1 (en) | 1993-12-22 | 2002-02-05 | Medtronic Ave, Inc. | Endovascular support device and method |
| WO2021202964A1 (en)* | 2020-04-03 | 2021-10-07 | Petra Pharma Corporation | Allosteric chromenone inhibitors of phosphoinositide 3-kinase (pi3k) for the treatment of diseases associated with p13k modulation |
| WO2022235575A1 (en)* | 2021-05-03 | 2022-11-10 | Petra Pharma Corporation | Allosteric chromenone inhibitors of phosphoinositide 3-kinase (pi3k) for the treatment of disease |
| WO2023060262A1 (en)* | 2021-10-07 | 2023-04-13 | Relay Therapeutics, Inc. | Pi3k-alpha inhibitors and methods of use thereof |
| WO2023078401A1 (en)* | 2021-11-05 | 2023-05-11 | Fochon Biosciences, Ltd. | Compounds as protein kinase inhibitors |
| WO2023159155A1 (en)* | 2022-02-18 | 2023-08-24 | Pivalent Therapeutics, Inc. | Inhibitors of phosphoinositide 3-kinase (pi3k) and uses thereof |
| WO2023207881A1 (en)* | 2022-04-24 | 2023-11-02 | InventisBio Co., Ltd. | Compounds, preparation methods and uses thereof |
| WO2024008122A1 (en)* | 2022-07-07 | 2024-01-11 | 海创药业股份有限公司 | Pi3k inhibitor, preparation method therefor, and use thereof |
| WO2024026424A1 (en)* | 2022-07-27 | 2024-02-01 | Black Diamond Therapeutics, Inc. | Quinazolinone derivatives as and related uses |
| WO2024054469A1 (en)* | 2022-09-08 | 2024-03-14 | Onkure, Inc. | Isoquinolones as pi3k inhibitors |
| WO2024064024A1 (en)* | 2022-09-19 | 2024-03-28 | Onkure, Inc. | ((4-oxo-3,4-dihydroquinazolin-8-yl)methyl)amine derivatives as p13k inhibitors for the treatment of cancer |
- 2024
- 2024-04-03WOPCT/US2024/022742patent/WO2024211346A1/enactivePending
Patent Citations (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3657744A (en) | 1970-05-08 | 1972-04-25 | Univ Minnesota | Method for fixing prosthetic implants in a living body |
| US4739762A (en) | 1985-11-07 | 1988-04-26 | Expandable Grafts Partnership | Expandable intraluminal graft, and method and apparatus for implanting an expandable intraluminal graft |
| US4739762B1 (en) | 1985-11-07 | 1998-10-27 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| US5023252A (en) | 1985-12-04 | 1991-06-11 | Conrex Pharmaceutical Corporation | Transdermal and trans-membrane delivery of drugs |
| US5451233A (en) | 1986-04-15 | 1995-09-19 | Yock; Paul G. | Angioplasty apparatus facilitating rapid exchanges |
| US5496346A (en) | 1987-01-06 | 1996-03-05 | Advanced Cardiovascular Systems, Inc. | Reinforced balloon dilatation catheter with slitted exchange sleeve and method |
| US4992445A (en) | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5001139A (en) | 1987-06-12 | 1991-03-19 | American Cyanamid Company | Enchancers for the transdermal flux of nivadipine |
| US5195984A (en) | 1988-10-04 | 1993-03-23 | Expandable Grafts Partnership | Expandable intraluminal graft |
| US5061273A (en) | 1989-06-01 | 1991-10-29 | Yock Paul G | Angioplasty apparatus facilitating rapid exchanges |
| US5040548A (en) | 1989-06-01 | 1991-08-20 | Yock Paul G | Angioplasty mehtod |
| US5292331A (en) | 1989-08-24 | 1994-03-08 | Applied Vascular Engineering, Inc. | Endovascular support device |
| US5674278A (en) | 1989-08-24 | 1997-10-07 | Arterial Vascular Engineering, Inc. | Endovascular support device |
| US5879382A (en) | 1989-08-24 | 1999-03-09 | Boneau; Michael D. | Endovascular support device and method |
| US6344053B1 (en) | 1993-12-22 | 2002-02-05 | Medtronic Ave, Inc. | Endovascular support device and method |
| WO2021202964A1 (en)* | 2020-04-03 | 2021-10-07 | Petra Pharma Corporation | Allosteric chromenone inhibitors of phosphoinositide 3-kinase (pi3k) for the treatment of diseases associated with p13k modulation |
| WO2022235575A1 (en)* | 2021-05-03 | 2022-11-10 | Petra Pharma Corporation | Allosteric chromenone inhibitors of phosphoinositide 3-kinase (pi3k) for the treatment of disease |
| WO2023060262A1 (en)* | 2021-10-07 | 2023-04-13 | Relay Therapeutics, Inc. | Pi3k-alpha inhibitors and methods of use thereof |
| WO2023078401A1 (en)* | 2021-11-05 | 2023-05-11 | Fochon Biosciences, Ltd. | Compounds as protein kinase inhibitors |
| WO2023159155A1 (en)* | 2022-02-18 | 2023-08-24 | Pivalent Therapeutics, Inc. | Inhibitors of phosphoinositide 3-kinase (pi3k) and uses thereof |
| WO2023207881A1 (en)* | 2022-04-24 | 2023-11-02 | InventisBio Co., Ltd. | Compounds, preparation methods and uses thereof |
| WO2024008122A1 (en)* | 2022-07-07 | 2024-01-11 | 海创药业股份有限公司 | Pi3k inhibitor, preparation method therefor, and use thereof |
| WO2024026424A1 (en)* | 2022-07-27 | 2024-02-01 | Black Diamond Therapeutics, Inc. | Quinazolinone derivatives as and related uses |
| WO2024054469A1 (en)* | 2022-09-08 | 2024-03-14 | Onkure, Inc. | Isoquinolones as pi3k inhibitors |
| WO2024064024A1 (en)* | 2022-09-19 | 2024-03-28 | Onkure, Inc. | ((4-oxo-3,4-dihydroquinazolin-8-yl)methyl)amine derivatives as p13k inhibitors for the treatment of cancer |
Non-Patent Citations (19)
| Title |
|---|
| "Handbook of Clinical Drug Data", 2002, MCGRAW-HILL |
| "Principles of Drug Action", 1990, CHURCHILL LIVINGSTON |
| "Remington's Pharmaceutical Sciences", 1985, MACK PUBLISHING COMPANY, pages: 1418 |
| "Remingtons Pharmaceutical Sciences", 2000, LIPPINCOTT WILLIAMS & WILKINS. |
| "The Pharmacological Basis of Therapeutics", 2001, MCGRAW HILL |
| ANDRE FCIRUELOS ERUBOVSZKY GCAMPONE MLOIBL SRUGO HSIWATA HCONTE PMAYER IAKAUFMAN B: "Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer", N ENGL J MED., vol. 380, no. 20, 16 May 2019 (2019-05-16), pages 1929 - 1940 |
| BADER AGKANG S, VOGT PK. PROC NATL ACAD SCI USA., vol. 103, no. 5, 31 January 2006 (2006-01-31), pages 1475 - 9 |
| BARLAAM BERNARD ET AL: "Discovery of ( R )-8-(1-(3,5-Difluorophenylamino)ethyl)- N , N -dimethyl-2-morpholino-4-oxo-4 H -chromene-6-carboxamide (AZD8186): A Potent and Selective Inhibitor of PI3K[beta] and PI3K[delta] for the Treatment of PTEN-Deficient Cancers", JOURNAL OF MEDICINAL CHEMISTRY, vol. 58, no. 2, 22 January 2015 (2015-01-22), US, pages 943 - 962, XP055813284, ISSN: 0022-2623, Retrieved from the Internet <URL:https://pubs.acs.org/doi/pdf/10.1021/jm501629p> DOI: 10.1021/jm501629p* |
| CHEUNG YMCROMWELL GETOLANEY SMMIN LMCDONNELL ME.: "Factors leading to alpelisib discontinuation in patients with hormone receptor positive, human epidermal growth factor receptor-2 negative breast cancer", BREAST CANCER RES TREAT., vol. 192, no. 2, April 2022 (2022-04-01), pages 303 - 311, XP037721245, DOI: 10.1007/s10549-021-06476-1 |
| FRUMAN, D. A. ET AL.: "The PI3K pathway in human disease", CELL, vol. 170, 2017, pages 605 - 635, XP085153697, DOI: 10.1016/j.cell.2017.07.029 |
| IAVARONE ABERGER AHBIVONA TGLAZAR AJHAMMER GDGIORDANO TKWONG LNMCARTHUR GHUANG CTWARD AD: "Cancer Genome Atlas Research Network", CELL., vol. 173, no. 2, 5 April 2018 (2018-04-05), pages 321 - 337 |
| JANKU FWHELER JJNAING ASTEPANEK VMFALCHOOK GSFU SGARRIDO-LAGUNA ITSIMBERIDOU AMPIHA-PAUL SAMOULDER SL, KURZROCK R. ONCOTARGET., vol. 3, no. 12, December 2012 (2012-12-01), pages 1566 - 75 |
| JOURNAL OF PHARMACEUTICAL SCIENCE, vol. 66, no. 1, 1977, pages 1 - 19 |
| MAAG, H.: "Prodrugs. Biotechnology: Pharmaceutical Aspects", vol. V, 2007, SPRINGER, article "Prodrugs of Carboxylic Acids." |
| MANNING BD: "AKT/PKB Signaling: Navigating the Network", TOKER A. CELL., vol. 169, no. 3, 20 April 2017 (2017-04-20), pages 381 - 405 |
| MARTINEZ-SAEZ OCHIC NPASCUAL TADAMO BVIDAL MGONZALEZ-FARRE BSANFELIU ESCHETTINI FCONTE BBRASO-MARISTANY F, PRAT A. BREAST CANCER RES., vol. 22, no. 1, 13 May 2020 (2020-05-13), pages 45 |
| RUGO HSLACOUTURE MEGONCALVES MDMASHARANI UAAPRO MSO'SHAUGHNESSY JA.: "A multidisciplinary approach to optimizing care of patients treated with alpelisib", BREAST., vol. 61, February 2022 (2022-02-01), pages 156 - 167, XP086949899, DOI: 10.1016/j.breast.2021.12.016 |
| T.W. GREENEP.G.M. WUTS: "Protective Groups in Organic Synthesis", 1999, THE PHARMACEUTICAL PRESS |
| VANHAESEBROECK BPERRY MWDBROWN JRANDRE F, OKKENHAUG K. NAT REV DRUG DISCOV., vol. 20, no. 10, October 2021 (2021-10-01), pages 741 - 769 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025087295A1 (en)* | 2023-10-25 | 2025-05-01 | 海创药业股份有限公司 | Pi3k inhibitor, and preparation method therefor and use thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2023192416A1 (en) | Mutant pi3k-alpha inhibitors and their use as pharmaceuticals | |
| US11673893B2 (en) | CDK inhibitors and their use as pharmaceuticals | |
| EP3170825B1 (en) | Novel pyrrolopyrimidine compounds as inhibitors of protein kinases | |
| KR101829940B1 (en) | Phthalazinone ketone derivative, preparation method thereof, and pharmaceutical use thereof | |
| WO2022147246A1 (en) | Indazole compounds as kinase inhibitors | |
| US12129262B2 (en) | CDK inhibitors and their use as pharmaceuticals | |
| EA024350B1 (en) | Pyrrolotriazinone derivatives as pi3k inhibitors | |
| WO2024086789A2 (en) | Mutant pi3k-alpha inhibitors and their use as pharmaceuticals | |
| WO2016119707A1 (en) | Novel heteroaryl and heterocycle compounds, compositions and methods | |
| US20230265083A1 (en) | Heterocycle CDK Inhibitors And Their Use Thereof | |
| WO2024211346A1 (en) | Mutant pi3k-alpha inhibitors and their use as pharmaceuticals | |
| US20240246993A1 (en) | CDK Inhibitors And Their Use As Pharmaceuticals | |
| US20230144528A1 (en) | CDK Inhibitors And Their Use As Pharmaceuticals | |
| US20230257394A1 (en) | CDK Inhibitors And Their Use As Pharmaceuticals | |
| WO2025038815A1 (en) | Mutant pi3k-alpha inhibitors and their use as pharmaceuticals | |
| WO2024216249A1 (en) | Cdk inhibitors and their use as pharmaceuticals | |
| WO2023247596A1 (en) | Pyrazolothiazole carboxamides and their uses as pdgfr inhibitors | |
| EA049578B1 (en) | CDK (CYCLIN-DEPENDENT KINASE) INHIBITORS AND THEIR USE IN PHARMACEUTICALS | |
| HK1238243A1 (en) | Novel pyrrolopyrimidine compounds as inhibitors of protein kinases | |
| HK1238243B (en) | Novel pyrrolopyrimidine compounds as inhibitors of protein kinases | |
| HK1210471B (en) | Novel pyrrolopyrimidine compounds as inhibitors of protein kinases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24722881 Country of ref document:EP Kind code of ref document:A1 |