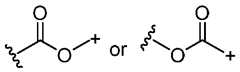WO2024178310A1 - Trop2-directed antibody-drug conjugates and uses thereof - Google Patents
Trop2-directed antibody-drug conjugates and uses thereofDownload PDFInfo
- Publication number
- WO2024178310A1 WO2024178310A1PCT/US2024/017043US2024017043WWO2024178310A1WO 2024178310 A1WO2024178310 A1WO 2024178310A1US 2024017043 WUS2024017043 WUS 2024017043WWO 2024178310 A1WO2024178310 A1WO 2024178310A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amino acid
- adc
- seq
- acid sequence
- phenylalanine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68031—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being an auristatin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6889—Conjugates wherein the antibody being the modifying agent and wherein the linker, binder or spacer confers particular properties to the conjugates, e.g. peptidic enzyme-labile linkers or acid-labile linkers, providing for an acid-labile immuno conjugate wherein the drug may be released from its antibody conjugated part in an acidic, e.g. tumoural or environment
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
Definitions
- This inventionrelates to anti-TROP2 antibodies and antibody-drug conjugates (ADCs) comprising at least one non-naturally-encoded amino acid.
- ADCsantibody-drug conjugates
- Disclosed hereinare anti-TROP2 antibodies with one or more non-naturally encoded amino acids, and further disclosed are antibody drug conjugates wherein the anti-TROP2 antibodies of the invention are conjugated to one or more cytotoxic drug-linker moieties. Further disclosed are methods for using such non-natural amino acid antibody drug conjugates, including therapeutic uses for treating diseases such as cancer.
- Antibody-based therapeuticshave emerged as important components of therapies for an increasing number of human malignancies in such fields as oncology, immunology, inflammatory and infectious diseases. In most cases, the basis of the therapeutic function is the high degree of specificity and affinity the antibody-based drug has for its target antigen. Arming monoclonal antibodies with drugs, toxins, or radionuclides is yet another strategy by which monoclonal antibodies may induce therapeutic effect. By combining the extraordinarily targeting specificity of antibody with the tumor killing power of toxic effector molecules, immunoconjugates permit sensitive discrimination between target and normal tissue thereby resulting in fewer side effects than most conventional chemotherapeutic drugs. The toxins utilized can specifically, stably and irreversibly conjugate to unique sites in the antibody.
- Trophoblast cell surface protein 2also known as: trophoblast antigen 2; calcium signal transducer 2; TR0P2; TROP-2; TACSTD2; GA733-1; or M1S1 is a transmembrane protein that is highly expressed on various epithelial tumors. While the physiological role of TR0P2 remains under investigation, it has been shown to be involved in pathways associated with the proliferation, migration and invasion of cancer cells, including MAPK and PI3K/AKT (Cubas R.
- TROP2 overexpressionhas been associated with enhanced tumor aggressiveness, metastasis, drug resistance, increased tumor cell survival, reduced overall survival (OS) and reduced progression-free survival.
- TROP2is highly expressed in triple negative breast cancer (TNBC), pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC). Overexpression of TROP2 has been correlated with poor prognosis in cancers including breast cancer and NSCLC (Lin H.
- TROP2an important target for the development anti-cancer therapeutics. Challenges towards this end include the known expression of TROP2 in some normal epithelial tissues, including the skin and esophagus (Stepan L.P. et al., J Histochem Cytochem, 59:701-710, 2011). Thus, the potential for on-target toxicity in normal cells that express TROP2 must be factored into the design and development of TROP2-directed therapeutics.
- TROP2-directed ADCsare known in the art.
- PF-06664178also known as RN927C; discontinued
- PF-06664178induced skin rash and mucosal inflammation as dose-limiting toxicities in phase I study in adult patients with advanced solid tumors (King, G.T., Invest New Drugs, 36:836-847, 2018).
- Datopotamab deruxtecan(Dato- DXd, DS- 1062a) contains anti-TROP2 antibody conjugated with topoisomerase I inhibitor DXd via a cleavable linker and has a drug-to-antibody ratio of 4 (Okajima, D. et al., Mol Cancer Ther, 20:2329-2340, 2021).
- TRODELVY(sacituzumab govitecan) is another TROP2-directed ADC and contains antibody hzRS7 (sacituzumab) conjugated with topoisomerase I inhibitor SN-38 via a hydrolyzable linker.
- the present inventionprovides novel TROP2-directed antibody-drug conjugates (ADCs) and uses thereof, including the use of TROP2-directed ADCs for the treatment of diseases such as cancer.
- ADCsnovel TROP2-directed antibody-drug conjugates
- an antibody-drug conjugatecomprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising an anti-TROP2 antibody amino acid sequence, wherein the anti-TROP2 antibody amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
- anti-TROP2anti-trophoblast antigen 2
- the amino acid sequenceis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the one non-natural amino acid at position 114is para-acetyl-L-phenylalanine (pAF).
- an antibody-drug conjugatecomprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein: at least one of the one or more heavy chains comprises one or more non-natural amino acids; and each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
- anti-TROP2anti-trophoblast antigen 2
- the amino acid sequence of at least one of the one or more heavy chainsshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 5; wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 5.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence; wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one or more light chains is SEQ ID NO: 4.
- each cytotoxic moietyis a cytotoxic moiety of Formula (A) or Formula (B): or a pharmaceutically acceptable salt thereof; wherein:
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is independently H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl
- #represents a connection to one of the one or more linkers.
- R 5is not thiazolyl.
- R 5is COOH. In some embodiments, R 6 is H. In some embodiments, R 7 is methyl. In some embodiments, Ar is phenyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is methyl.
- the cytotoxic moietyis less cytotoxic than monomethyl auristatin E (MMAE) in vitro.
- the cytotoxic moietyexhibits a higher in vitro half- maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE.
- IC50in vitro half- maximal inhibitory concentration
- the higher in vitro IC50is at least a two-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 10-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 100-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 1000-fold higher in vitro IC50.
- the microtubule inhibitor-sensitive cell lineis an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay.
- each said cytotoxic moietyis a cytotoxic moiety of Formula (B): wherein:
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- R 6is H. In some embodiments, Ar is phenyl.
- the cytotoxic moietyhas the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
- the ADCis an ADC of Formula (II): wherein:
- Abis an anti-TROP2 antibody comprising an amino acid sequence, wherein the amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L1-CH(L2-)(L3-) is a linker, wherein:
- LIis a first linker unit
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- At least one of LI, L2 and L3is not a bond.
- the amino acid sequenceis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- dis 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
- Ecomprises an oxime. In some embodiments, E has the following structure: /°- +
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (" «') denotes the connection to Ab.
- each LIcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- each of the one or more non-natural amino acidsis para-acetyl-L- phenylalanine (pAF).
- the ADCis an ADC of Formula (I): or a pharmaceutically acceptable salt thereof; wherein:
- the amino acid sequenceis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- dis 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
- Ecomprises an oxime. In some embodiments, E has the following structure:
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (— ) denotes the connection to Ab.
- R qis methyl.
- each Lcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- each of the one or more non-natural amino acidsis para-acetyl-L- phenylalanine (pAF).
- an anti-TROP2 antibodycomprises one or more heavy chains.
- the one or more heavy chainsis two heavy chains.
- the amino acid sequence of each said heavy chainis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- an anti-TROP2 antibodycomprises one or more light chains.
- the one or more light chainsis two light chains.
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 4.
- an anti-TROP2 antibodycomprises two heavy chains and two light chains, wherein: the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
- the one non-natural amino acid at position 114 of each said heavy chainis para-acetyl-L-phenylalanine (pAF).
- an antibody-drug conjugateof Formula (la), (Ib), (Ila) or (lib) : or a pharmaceutically acceptable salt thereof; wherein:
- Abis an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and Formula (Ib) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) and Formula (lib) is a linker, wherein: LI is a first linker unit;
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage covalently joining L or LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab;
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and d is an integer from 1 to 10.
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the amino acid sequence of at least one of the one or more heavy chainsis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- each heavy chain amino acid sequenceis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the one non-natural amino acid at position 114is para-acetyl-L- phenylalanine (pAF).
- the one or more heavy chainsis two heavy chains.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11.
- the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: 4.
- the amino acid sequence of each of the one of the one or more light chainsis the amino acid sequence of SEQ ID NO: 4.
- the one or more heavy chainsis two heavy chains, and the one or more light chains is two light chains.
- dis 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
- the ADCis an ADC of Formula (la): wherein:
- Abis an anti-trophoblast antigen 2 (anti-TR0P2) antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage;
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein at least one of the one or more non-natural amino acids is incorporated into at least one of the one or more heavy chains at position Al 14 based on Kabat numbering.
- the one or more heavy chainsis two heavy chains, and wherein one non-natural amino acid is incorporated into each heavy chain at position Al 14 based on Kabat numbering.
- R 5is not thiazolyl.
- R 5is COOH. In some embodiments, R 6 is H. In some embodiments, R 7 is methyl. In some embodiments, Ar is phenyl
- each Lcomprises at least one unsubstituted alkylene, at least one - (alkylene-O)n-, or both. In some embodiments, each L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, each L is -(CH2CH2) m - (CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF). In some embodiments, each E comprises an oxime. In some embodiments, each E has the following structure:
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (TM») denotes the connection to Ab.
- R qis methyl.
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
- the one or more heavy chainsis two heavy chains, the one or more light chains is two light chains, the one or more non-natural amino acids is two non-natural amino acids.
- dis 2.
- R qis methyl
- R 5is COOH
- R 6is H
- R 7is methyl
- Aris phenyl and L is -(CH2CH2)m-(CH 2 CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
- the ADCis an ADC of Formula (Ic): or a pharmaceutically acceptable salt thereof, wherein:
- Abis an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising one or more nonnatural amino acids; each L is a linker; each E is a linkage covalently joining each linker L with one of the one or more non- natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
- anti-TROP2anti-trophoblast antigen 2
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the amino acid sequence of at least one of the one or more heavy chainsis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- each heavy chain amino acid sequenceis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- the amino acid sequence of at least one of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11.
- the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
- the one or more heavy chainsis two heavy chains. In some embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- dis 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing; wherein at least one moiety is not a bond.
- the peptideis a dipeptide.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, - (alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, - N(R W )-, an amino acid or a dipeptide; wherein each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing; wherein at least one moiety is not a bond.
- Lcomprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, L is -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- each Ecomprises an oxime. In some embodiments, each E has the following structure:
- Rqis unsubstituted Ci-Ce alkyl; + denotes the connection to L; and the wavy line (— ) denotes the connection to Ab.
- R qis methyl.
- R qis methyl and L is -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
- the ADC of Formula (Ic)is an ADC of Formula (Id): or a pharmaceutically acceptable salt thereof, wherein:
- Abis the anti-TROP2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
- dis 2. In some other embodiments, d is 2, 3, or 4.
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the amino acid sequence of at least one of the one or more heavy chainsis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- each heavy chain amino acid sequenceis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- the amino acid sequence of at least one of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11.
- the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2.
- the one non-natural amino acid at position 114 of each said heavy chainis para-acetyl-L-phenylalanine (pAF).
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114; and
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 4.
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- an anti-TROP2 antibodysuch as the anti-TROP2 antibody of an ADC of the present disclosure, is humanized.
- an ADC of the present disclosuredoes not contain a Toll-like receptor (TLR) agonist.
- TLRToll-like receptor
- a pharmaceutical compositioncomprising an ADC of the present disclosure, and at least one pharmaceutically acceptable adjuvant, binder, buffer, carrier, diluent or excipient.
- the pharmaceutical compositionfurther comprises a chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid, or combination thereof.
- a method of treating a disease or condition in a subjectcomprising administering to the subject a therapeutically effective amount of an ADC of the present disclosure, or a pharmaceutical composition comprising subject a therapeutically effective amount of an ADC of the present disclosure.
- the disease or conditionis cancer.
- the canceris a solid tumor.
- the canceris breast cancer, pancreatic cancer, lung cancer, gastric cancer, colorectal cancer or prostate cancer.
- the canceris triplenegative breast cancer (TNBC).
- TNBCtriplenegative breast cancer
- the canceris pancreatic ductal adenocarcinoma (PDAC).
- the canceris non-small cell lung cancer (NSCLC).
- the canceris a TROP2-positive cancer.
- the canceris a Dxd-resistant cancer.
- the canceris a topoisomerase 1 (TOPI) inhibitor-resistant cancer.
- TOPItopoisomerase 1
- FIG. 1shows a representative HPLC chromatogram showing elution of anti-TROP2 light chain, unconjugated heavy chain, and heavy chain conjugated with drug-linker compound 6.
- FIG. 2shows binding kinetic sensorgrams and associated KD values for anti-TROP2 mAb binding to TR0P2 from human (left), cynomolgus (center) and rat (right).
- FIG. 3shows graphical illustrations of cytotoxic activity of anti-TROP2 ADCs against TROP2-expressing BxPC-3 cell line (top left); TROP2-expressing MDA-MB-468 cell line (top right), TROP2-expressing HCC1806 cell line (bottom left) and TROP2-negative Calu-6 cell line (bottom right).
- FIG. 4.shows graphical illustrations of cytotoxic activity of anti-TROP2 ADCs in wildtype and Benchmark-DXd-resistant MDA-MB-468 cells.
- FIG. 5shows a graphical illustration of cytotoxic activity of anti-TROP2 ADCs in a Benchmark-DXd-insensitive JIMT-1 cell line.
- FIG. 6shows a graphical illustration of cytotoxic activity of anti-TROP2 ADCs in human keratinocytes.
- FIG. 7shows a graphical illustration of anti-tumor activity of anti-TROP2 ADCs.
- FIG. 8shows a graphical illustration of binding affinities of TR0P2 mAb and anti-TROP2 ADC in TROP2-expressing MDA-MB-468 cells.
- FIG. 9shows a graphical illustration of antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
- FIG. 10shows another graphical illustration of anti -turn or activity of anti-TROP2 ADCs.
- FIG. 11shows still another graphical illustration of anti-tumor activity of anti-TROP2 ADCs.
- FIG. 12shows a graphical illustration of pharmacokinetic study of anti-TROP2 ADCs.
- acylrepresents -C(O)-alkyl, as defined herein, and is exemplified by acetyl (-C(0)CH3), trifluoroacetyl, propionyl, and butanoyl.
- exemplary unsubstituted acyl groupsinclude from 1 to 6, from 1 to 11, or from 1 to 21 carbons.
- alkylrefers to a branched or straight-chain monovalent saturated aliphatic hydrocarbon radical of 1 to 20 carbon atoms (e.g., 1 to 16 carbon atoms, 1 to 10 carbon atoms, or 1 to 6 carbon atoms).
- An alkyleneis a divalent alkyl group.
- alkyleneby itself or as part of another molecule means a divalent radical derived from an alkane, as exemplified, by (-CH2-) n , wherein n can be 2 to about 100. In some embodiments, n is 1 to 24.
- groupsinclude, but are not limited to, groups having 10 or fewer carbon atoms such as the structures -CH2CH2- and -CH2CH2CH2CH2-.
- alkyleneunless otherwise noted, is also meant to include those groups described herein as “heteroalkylene.” Substituents for alkylene groups are selected from the group of acceptable substituents described herein.
- alkenylrefers to a straight-chain or branched hydrocarbon residue having a carbon-carbon double bond and having 2 to 20 carbon atoms (e.g., 2 to 16 carbon atoms, 2 to 10 carbon atoms, 2 to 6, or 2 carbon atoms).
- alkynylrefers to a straight-chain or branched hydrocarbon residue having a carbon-carbon triple bond and having 2 to 20 carbon atoms (e.g., 2 to 16 carbon atoms, 2 to 10 carbon atoms, 2 to 6, or 2 carbon atoms).
- aminorepresents — N(R N1 )2, wherein each R N1 is, independently, H, OH, NO2, N(R N2 )2, SO2OR N2 , SO2R N2 , SOR N2 , an N-protecting group, alkyl, alkoxy, aryl, arylalkyl, cycloalkyl, acyl (e.g., acetyl, trifluoroacetyl, or others described herein), wherein each of these recited R N1 groups can be optionally substituted; or two R N1 combine to form an alkylene or heteroalkylene, and wherein each R N2 is, independently, H, alkyl, or aryl.
- amino groups of the inventioncan be an unsubstituted amino (i.e., — NH2) or a substituted amino (i.e., — N(R N1 ) 2 ).
- aminois N(R W )-, wherein each R w is independently H or Ci-C 8 alkyl.
- arylrefers to an aromatic mono- or polycarbocyclic radical of 6 to 12 carbon atoms having at least one aromatic ring.
- groupsinclude, but are not limited to, phenyl, naphthyl, 1,2,3,4-tetrahydronaphthyl, 1,2-dihydronaphthyl, indanyl, and IH-indenyl.
- arylenerefers to a divalent aryl radical.
- Non-limiting examples of “arylene”include phenylene, pyridinylene, pyrimidinylene and thiophenylene. Substituents for arylene groups are selected from the group of acceptable substituents described herein.
- arylalkylrepresents an alkyl group substituted with an aryl group.
- exemplary unsubstituted arylalkyl groupsare from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as C1-6 alkyl Ce-io aryl, C1-10 alkyl Ce-io aryl, or C1-20 alkyl Ce-io aryl), such as, benzyl and phenethyl.
- the akyl and the aryleach can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups.
- cyanorepresents a — CN group.
- Carbocyclylrefer to a non-aromatic C3-12 monocyclic, bicyclic, or tricyclic structure in which the rings are formed by carbon atoms.
- Carbocyclyl structuresinclude cycloalkyl groups and unsaturated carbocyclyl radicals.
- cycloalkylrefers to a saturated, non-aromatic, monovalent mono- or polycarbocyclic radical of three to ten, preferably three to six carbon atoms. This term is further exemplified by radicals such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, norbornyl, and adamantyl.
- cycloalkynylrefers to a cyclic radical containing one or more triple bonds. In some embodiments, a cycloalkynyl group is cyclooctynyl group.
- halogenmeans a fluorine (fluoro), chlorine (chloro), bromine (bromo), or iodine (iodo) radical.
- heteroalkylrefers to an alkyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur.
- the heteroalkyl groupcan be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkyl groups.
- Examples of heteroalkyl groupsare an “alkoxy” which, as used herein, refers alkyl-0 — (e.g., methoxy and ethoxy); the alkyl of the alkoxy can be substituted.
- the alkoxy alkyl groupis substituted with amino or alkylamino, or with heterocyclyl, which is optionally substituted.
- a heteroalkyleneis a divalent heteroalkyl group.
- heteroalkenylrefers to an alkenyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur. In some embodiments, the heteroalkenyl group can be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkenyl groups. Examples of heteroalkenyl groups are an “alkenoxy” which, as used herein, refers alkenyl-0 — .
- a heteroalkenyleneis a divalent heteroalkenyl group.
- heteroalkynylrefers to an alkynyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur.
- the heteroalkynyl groupcan be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkynyl groups.
- Examples of heteroalkynyl groupsare an “alkynoxy” which, as used herein, refers alkynyl-0 — .
- a heteroalkynyleneis a divalent heteroalkynyl group.
- heteroarylrefers to an aromatic mono- or polycyclic radical of 5 to 12 atoms having at least one aromatic ring containing one, two, or three ring heteroatoms selected from N, O, and S, with the remaining ring atoms being C. One or two ring carbon atoms of the heteroaryl group may be replaced with a carbonyl group.
- heteroaryl groupsare pyridyl, pyrazoyl, benzooxazolyl, benzoimidazolyl, benzothiazolyl, imidazolyl, oxaxolyl, and thiazolyl.
- heteroarylalkylrepresents an alkyl group substituted with a heteroaryl group.
- exemplary unsubstituted heteroarylalkyl groupsare from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as Ci-6 alkyl C2-9 heteroaryl, C1-10 alkyl C2-9 heteroaryl, or C1-20 alkyl C2-9 heteroaryl).
- the akyl and the heteroaryleach can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups.
- heterocyclyldenotes a mono- or polycyclic radical having 3 to 12 atoms having at least one ring containing one, two, three, or four ring heteroatoms selected from N, O or S, wherein no ring is aromatic.
- heterocyclyl groupsinclude, but are not limited to, morpholinyl, thiomorpholinyl, furyl, piperazinyl, piperidinyl, pyranyl, pyrrolidinyl, tetrahydropyranyl, tetrahydrofuranyl, and 1,3-dioxanyl.
- heterocyclylalkylrepresents an alkyl group substituted with a heterocyclyl group.
- exemplary unsubstituted heterocyclylalkyl groupsare from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as Ci-6 alkyl C2-9 heterocyclyl, C1-10 alkyl C2- 9 heterocyclyl, or C1-20 alkyl C2-9 heterocyclyl).
- the akyl and the heterocyclyleach can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups.
- hydroxylrepresents an — OH group.
- methyleneby itself or as part of another molecule means a divalent radical derived from methane, and can be written as (-CH2-). Substituents for alkylene groups are selected from the group of acceptable substituents described herein.
- metalas used herein refers to a trivalent moiety C(H).
- nitrorepresents an — NO2 group.
- thiolrepresents an — SH group.
- alkyl, alkylene, methylene, methine, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, heteroalky nyl, carbocyclyl (e.g., cycloalkyl), aryl, arylene, heteroaryl, heteroarylene, and heterocyclyl groupsmay be substituted or unsubstituted. When substituted, there will generally be 1 to 4 substituents present, unless otherwise specified.
- Substituentsinclude, for example: aryl (e.g., substituted and unsubstituted phenyl), carbocyclyl (e.g., substituted and unsubstituted cycloalkyl), halogen (e.g., fluoro), hydroxyl, heteroalkyl (e.g., substituted and unsubstituted methoxy, ethoxy, or thioalkoxy), heteroaryl, heterocyclyl, amino (e.g., NH2 or mono- or dialkyl amino), azido, cyano, nitro, or thiol.
- aryle.g., substituted and unsubstituted phenyl
- carbocyclyle.g., substituted and unsubstituted cycloalkyl
- halogene.g., fluoro
- hydroxylhydroxyl
- heteroalkyle.g., substituted and unsubstituted
- Aryl, carbocyclyl (e.g., cycloalkyl), heteroaryl, and heterocyclyl groupsmay also be substituted with alkyl (unsubstituted and substituted such as arylalkyl (e.g., substituted and unsubstituted benzyl)).
- Compounds of the inventioncan have one or more asymmetric carbon atoms and can exist in the form of optically pure enantiomers, mixtures of enantiomers such as, for example, racemates, optically pure diastereoisomers, mixtures of diastereoisomers, diastereoisomeric racemates or mixtures of diastereoisomeric racemates.
- the optically active formscan be obtained for example by resolution of the racemates, by asymmetric synthesis or asymmetric chromatography (chromatography with a chiral adsorbents or eluant). That is, certain of the disclosed compounds may exist in various stereoisomeric forms.
- Stereoisomersare compounds that differ only in their spatial arrangement.
- Enantiomersare pairs of stereoisomers whose mirror images are not superimposable, most commonly because they contain an asymmetrically substituted carbon atom that acts as a chiral center. “Enantiomer” means one of a pair of molecules that are mirror images of each other and are not superimposable. Diastereomers are stereoisomers that are not related as mirror images, most commonly because they contain two or more asymmetrically substituted carbon atoms and represent the configuration of substituents around one or more chiral carbon atoms. Enantiomers of a compound can be prepared, for example, by separating an enantiomer from a racemate using one or more well-known techniques and methods, such as, for example, chiral chromatography and separation methods based thereon.
- Racemateor “racemic mixture” means a compound containing two enantiomers, wherein such mixtures exhibit no optical activity; i.e., they do not rotate the plane of polarized light.
- Geometric isomermeans isomers that differ in the orientation of substituent atoms in relationship to a carbon-carbon double bond, to a cycloalkyl ring, or to a bridged bicyclic system.
- Atoms (other than H) on each side of a carbon-carbon double bondmay be in an E (substituents are on opposite sides of the carbon-carbon double bond) or Z (substituents are oriented on the same side) configuration.
- R,” “S,” “S*,” “R*,” “E,” “Z,” “cis,” and “trans,”indicate configurations relative to the core molecule.
- Certain of the disclosed compoundsmay exist in atropisomeric forms.
- Atropisomersare stereoisomers resulting from hindered rotation about single bonds where the steric strain barrier to rotation is high enough to allow for the isolation of the conformers.
- the compounds of the inventionmay be prepared as individual isomers by either isomer-specific synthesis or resolved from an isomeric mixture.
- Conventional resolution techniquesinclude forming the salt of a free base of each isomer of an isomeric pair using an optically active acid (followed by fractional crystallization and regeneration of the free base), forming the salt of the acid form of each isomer of an isomeric pair using an optically active amine (followed by fractional crystallization and regeneration of the free acid), forming an ester or amide of each of the isomers of an isomeric pair using an optically pure acid, amine or alcohol (followed by chromatographic separation and removal of the chiral auxiliary), or resolving an isomeric mixture of either a starting material or a final product using various well known chromatographic methods.
- the stereochemistry of a disclosed compoundis named or depicted by structure
- the named or depicted stereoisomeris at least 60%, 70%, 80%, 90%, 99% or 99.9%) by weight relative to the other stereoisomers.
- the depicted or named enantiomeris at least 60%, 70%, 80%, 90%, 99% or 99.9% by weight optically pure.
- the depicted or named diastereomeris at least 60%, 70%, 80%, 90%, 99% or 99.9% by weight pure.
- Percent optical purityis the ratio of the weight of the enantiomer or over the weight of the enantiomer plus the weight of its optical isomer. Diastereomeric purity by weight is the ratio of the weight of one diastereomer or over the weight of all the diastereomers.
- the stereochemistry of a disclosed compoundis named or depicted by structure, the named or depicted stereoisomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure relative to the other stereoisomers.
- the depicted or named enantiomeris at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure.
- the depicted or named diastereomeris at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure.
- Percent purity by mole fractionis the ratio of the moles of the enantiomer or over the moles of the enantiomer plus the moles of its optical isomer.
- percent purity by moles fractionis the ratio of the moles of the diastereomer or over the moles of the diastereomer plus the moles of its isomer.
- amino acidrefers to naturally occurring and non-natural or unnatural amino acids, which may be referred to herein as synthetic amino acids, as well as amino acid analogs and amino acid mimetics that function in a manner similar to the naturally occurring amino acids.
- Naturally encoded amino acidsare the 20 common amino acids (alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine) and pyrolysine and selenocysteine.
- Amino acid analogsrefer to compounds that have the same basic chemical structure as a naturally occurring amino acid, by way of example only, an a-carbon that is bound to a hydrogen, a carboxyl group, an amino group, and a functional R group.
- Such analogsmay have modified R groups (by way of example, norleucine) or may have modified peptide backbones while still retaining the same basic chemical structure as a naturally occurring amino acid.
- Non-limiting examples of amino acid analogsinclude homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium.
- Amino acidsmay be referred to herein by either their name, their commonly known three letter symbols or by the one-letter symbols recommended by the IUPAC-IUB Biochemical Nomenclature Commission. Additionally, nucleotides, may be referred to by their commonly accepted single-letter codes.
- amino or carboxy terminus modification grouprefers to any molecule that can be attached to a terminal amine group or terminal carboxy group respectively.
- terminal amine groups or terminal carboxy groupsmay be at the end of polymeric molecules, wherein such polymeric molecules include, but are not limited to, polypeptides, polynucleotides, and polysaccharides.
- Terminus modification groupsinclude but are not limited to, various water- soluble polymers, peptides or proteins.
- terminus modification groupsinclude polyethylene glycol or serum albumin. Terminus modification groups may be used to modify therapeutic characteristics of the polymeric molecule, including but not limited to increasing the serum half-life of peptides, polypeptides or proteins.
- the disclosureprovides novel antibodies and antibody variants.
- antibodyherein refers to a protein consisting of one or more polypeptides substantially encoded by all or part of the antibody genes.
- the immunoglobulin genesinclude, but are not limited to, the kappa, lambda, alpha, gamma (IgGl, IgG2, IgG3, and IgG4), delta, epsilon and mu constant region genes, as well as the myriad immunoglobulin variable region genes.
- Antibody hereinis also meant to include full-length antibodies and antibody fragments, and include antibodies that exist naturally in any organism, antibody variants, engineered antibodies and antibody fragments.
- Antibody hereinis also meant to include intact antibody, monoclonal or polyclonal antibodies.
- Antibody hereinalso encompasses multispecific antibodies and/or bispecific antibodies.
- Antibodies of the present disclosureinclude human antibodies. Human antibodies are usually made of two light chains and two heavy chains each comprising variable regions and constant regions.
- the light chain variable regioncomprises 3 CDRs, identified herein as CDRL1, CDRL2 and CDRL3 flanked by framework regions.
- the heavy chain variable regioncomprises 3 CDRs, identified herein as CDRH1, CDRH2 and CDRH3 flanked by framework regions.
- antibody fragmentrefers to any form of an antibody other than the full- length form.
- Antibody fragments hereininclude antibodies that are smaller components that exist within full-length antibodies, and antibodies that have been engineered, such as antibody variants.
- Antibody fragmentsinclude but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, and variable regions, and alternative scaffold non-antibody molecules, bispecific antibodies, and the like (Maynard & Georgiou, Annu. Rev. Biomed. Eng.
- Another functional substructureis a single chain Fv (scFv), comprised of the variable regions of the immunoglobulin heavy and light chain, covalently connected by a peptide linker (Hu et al., Cancer Research, 56, 3055-3061, 1996).
- scFvsingle chain Fv
- These small (Mr 25,000) proteinsgenerally retain specificity and affinity for antigen in a single polypeptide and can provide a convenient building block for larger, antigen-specific molecules.
- antibodyor “antibodies” specifically includes “antibody fragment” and “antibody fragments.”
- ADCsantibody-drug conjugates
- ADCrefers to an antibody molecule, or fragment thereof, that is covalently bonded to one or more biologically active molecule(s).
- the biologically active moleculemay be conjugated to the antibody through a linker, polymer, or other covalent bond.
- ADCsare a potent class of therapeutic constructs that allow targeted delivery of cytotoxic agents to target cells, such as cancer cells. Because of the targeting function, these compounds show a much higher therapeutic index compared to the same systemically delivered agents.
- ADCshave been developed as intact antibodies or antibody fragments, such as scFvs. The antibody or fragment is linked to one or more copies of drug via a linker that is stable under physiological conditions, but that may be cleaved once inside the target cell.
- antigen-binding fragmentrefers to one or more fragments of an antibody that retain the ability to bind to an antigen. It has been shown that the antigen-binding function of an antibody can be performed by fragments of an intact antibody.
- binding fragments encompassed within the term "antigen-binding fragment" of an antibodyinclude (i) a Fab fragment, a monovalent fragment consisting of the VL, VH, CL and CHI domains; (ii) a F(ab')2 fragment, a bivalent fragment comprising two Fab fragments linked by a disulfide bridge at the hinge region; (iii) a Fd fragment consisting of the VH and CHI domains; (iv) a Fv fragment consisting of the VL and VH domains of a single arm of an antibody, (v) a dAb fragment (Ward et al., Nature 341 :544-546, 1989), which consists of a VH domain; (vi) an isolated complementarity determining region (CDR), e.g., VH CDR3 comprising or not additional sequence (linker, framework region(s) etc.) and (v) a combination of two to six isolated CDRs comprising or not additional sequence (linker, framework
- the two domains of the Fv fragment, VL and VHare coded for by separate genes, they can be joined, using recombinant methods, by a synthetic linker that enables them to be made as a single polypeptide chain in which the VL and VH regions pair to form monovalent molecules (known as single chain Fv (scFv); see e.g., Bird et al., Science 242:423-426, 1988); and (Huston et al., Proc. Natl. Acad. Sci. USA 85:5879-5883, 1988).
- single chain Fvsingle chain Fv
- Such single chain antibodiesare also intended to be encompassed within the term "antigen -binding fragment" of an antibody.
- the antigen-binding fragmentsinclude binding-domain immunoglobulin fusion proteins comprising (i) a binding domain polypeptide (such as a heavy chain variable region, a light chain variable region, or a heavy chain variable region fused to a light chain variable region via a linker peptide) that is fused to an immunoglobulin hinge region polypeptide, (ii) an immunoglobulin heavy chain CH2 constant region fused to the hinge region, and (iii) an immunoglobulin heavy chain CH3 constant region fused to the CH2 constant region.
- the hinge regionmay be modified by replacing one or more cysteine residues with serine residues to prevent dimerization.
- binding-domain immunoglobulin fusion proteinsare further disclosed in US 2003/0118592 and US 2003/0133939. These antibody fragments are obtained using conventional techniques known to those with skill in the art, and the fragments are screened for utility in the same manner as are intact antibodies.
- a typical antigen binding siteis comprised of the variable regions formed by the pairing of a light chain immunoglobulin and a heavy chain immunoglobulin.
- the structure of the antibody variable regionsis very consistent and exhibits very similar structures.
- These variable regionsare typically comprised of relatively homologous framework regions (FR) interspaced with three hypervariable regions termed Complementarity Determining Regions (CDRs).
- CDRsComplementarity Determining Regions
- the overall binding activity of the antigen binding fragmentis often dictated by the sequence of the CDRs.
- the FRsoften play a role in the proper positioning and alignment in three dimensions of the CDRs for optimal antigen binding.
- CDR sequencesare responsible for most antibodyantigen interactions
- it is possible to express recombinant antibodies that shows the properties of specific naturally occurring antibodies by constructing expression vectors that include CDR sequences from the specific naturally occurring antibody grafted onto framework sequences from a different antibody with different propertiessee, e.g., Riechmann, L. et al., Nature 332:323-327, 1998; Jones, P. et al., Nature 321 :522-525, 1986; and Queen, C. et al., Proc. Natl. Acad. USA 86: 10029-10033, 1989).
- Such framework sequencescan be obtained from public DNA databases that include germline antibody gene sequences.
- germline sequenceswill differ from mature antibody gene sequences because they will not include completely assembled variable genes, which are formed by V(D)J joining during B cell maturation. Germline gene sequences will also differ from the sequences of a high affinity secondary repertoire antibody which contains mutations throughout the variable gene but typically clustered in the CDRs. For example, somatic mutations are relatively infrequent in the amino terminal portion of framework region 1 and in the carboxy-terminal portion of framework region 4. Furthermore, many somatic mutations do not significantly alter the binding properties of the antibody. For this reason, it is not necessary to obtain the entire DNA sequence of a particular antibody in order to recreate an intact recombinant antibody having binding properties similar to those of the original antibody. Partial heavy and light chain sequence spanning the CDR regions is typically sufficient for this purpose.
- the partial sequenceis used to determine which germline variable and joining gene segments contributed to the recombined antibody variable genes.
- the germline sequenceis then used to fill in missing portions of the variable regions.
- Heavy and light chain leader sequencesare cleaved during protein maturation and do not contribute to the properties of the final antibody.
- cloned cDNA sequencescan be combined with synthetic oligonucleotides by ligation or PCR amplification.
- the entire variable regioncan be synthesized to create an entirely synthetic variable region clone. This process has certain advantages such as elimination or inclusion of particular restriction sites, or optimization of particular codons.
- the totality or portions of the framework region of the antibody described hereinmay be used in conjunction with the CDRs in order to optimize the affinity, specificity or any other desired properties of the antibody.
- the disclosureconcerns polymers such as a bifunctional polymer.
- Such moietiesmay include, but are not limited to, the side groups on natural or non-natural amino acids or peptides which contain such natural or non-natural amino acids.
- the other moieties that may be linked to the bifunctional linker or bifunctional polymermay be the same or different moieties.
- a bifunctional linkermay have a functional group reactive with a group on a first peptide, and another functional group which is reactive with a group on a second peptide, whereby forming a conjugate that includes the first peptide, the bifunctional linker and the second peptide.
- Many procedures and linker molecules for attachment of various compounds to peptidesare known. See, for example, European Patent Application No. 0188256; U.S. Patent Nos. 4,659,839; 4,414,148; 4,699,784; 4,680,338; and 4,569,789 incorporated herein by reference in their entirety.
- a bi-functional polymer or multi-functional polymermay be any desired length or molecular weight and may be selected to provide a particular desired spacing or conformation between one or more molecules linked to a compound and molecules it binds to, or to the compound.
- bioavailabilityrefers to the rate and extent to which a substance or its active moiety is delivered from a pharmaceutical dosage form and becomes available at the site of action or in the general circulation.
- Increases in bioavailabilityrefers to increasing the rate and extent a substance or its active moiety is delivered from a pharmaceutical dosage form and becomes available at the site of action or in the general circulation.
- an increase in bioavailabilitymay be indicated as an increase in concentration of the substance or its active moiety in the blood when compared to other substances or active moieties.
- biologically active moleculewhen used herein means any substance which can affect any physical or biochemical properties of a biological system, pathway, molecule, or interaction relating to an organism, including but not limited to, viruses, bacteria, bacteriophage, transposon, prion, insects, fungi, plants, animals, and humans.
- biologically active moleculesinclude but are not limited to any substance intended for diagnosis, cure, mitigation, treatment, or prevention of disease in humans or other animals, or to otherwise enhance physical or mental wellbeing of humans or animals.
- biologically active moleculesinclude, but are not limited to, peptides, proteins, enzymes, small molecule drugs, hard drugs, soft drugs, prodrugs, carbohydrates, inorganic atoms or molecules, dyes, lipids, nucleosides, radionuclides, oligonucleotides, toxins, cells, viruses, liposomes, microparticles and micelles.
- Classes of biologically active agentsthat are suitable for use with the methods and compositions described herein include, but are not limited to, drugs, prodrugs, radionuclides, imaging agents, polymers, antibiotics, fungicides, anti-viral agents, anti-inflammatory agents, anti-tumor agents, cardiovascular agents, anti-anxiety agents, hormones, growth factors, steroidal and nonsteroidal agents, microbially derived toxins, and the like.
- modulating biological activityis meant increasing or decreasing the reactivity of a polypeptide, altering the selectivity of the polypeptide, enhancing or decreasing the substrate selectivity of the polypeptide.
- Analysis of modified biological activitycan be performed by comparing the biological activity of the non-natural polypeptide to that of the natural polypeptide.
- the disclosureconcerns amino acids that have been biosynthetically incorporated in the antibody.
- biosyntheticallyrefers to any method utilizing a translation system (cellular or non-cellular), including use of at least one of the following components: a polynucleotide, a codon, a tRNA, and a ribosome.
- non-natural amino acidsmay be “biosynthetically incorporated” into non-natural amino acid polypeptides using the methods and techniques described herein and as is well known in the art. See for example, WO2010/011735 and W02005/074650.
- “conservatively modified variants”applies to both natural and non-natural amino acid and natural and non-natural nucleic acid sequences, and combinations thereof.
- “conservatively modified variants”refers to those natural and non-natural nucleic acids which encode identical or essentially identical natural and non-natural amino acid sequences, or where the natural and non-natural nucleic acid does not encode a natural and non-natural amino acid sequence, to essentially identical sequences.
- the codons GCA, GCC, GCG and GCUall encode the amino acid alanine.
- nucleic acid variationsare “silent variations,” which are one species of conservatively modified variations.
- every natural or non-natural nucleic acid sequence herein which encodes a natural or non-natural polypeptidealso describes every possible silent variation of the natural or non-natural nucleic acid.
- each codon in a natural or non-natural nucleic acidcan be modified to yield a functionally identical molecule. Accordingly, each silent variation of a natural and non-natural nucleic acid which encodes a natural and non-natural polypeptide is implicit in each described sequence.
- amino acid sequencesindividual substitutions, deletions or additions to a nucleic acid, peptide, polypeptide, or protein sequence which alters, adds or deletes a single natural and non-natural amino acid or a small percentage of natural and non-natural amino acids in the encoded sequence is a “conservatively modified variant” where the alteration results in the deletion of an amino acid, addition of an amino acid, or substitution of a natural and non-natural amino acid with a chemically similar amino acid.
- Conservative substitution tables providing functionally similar natural amino acidsare well known in the art.
- Conservative substitution tables providing functionally similar amino acidsare known to those of ordinary skill in the art.
- the following eight groupseach contain amino acids that are conservative substitutions for one another: 1) Alanine (A), Glycine (G); 2) Aspartic acid (D), Glutamic acid (E); 3) Asparagine (N), Glutamine (Q); 4) Arginine (R), Lysine (K); 5) Isoleucine (I), Leucine (L), Methionine (M), Valine (V); 6) Phenylalanine (F), Tyrosine (Y), Tryptophan (W); 7) Serine (S), Threonine (T); and 8) Cysteine (C), Methionine (M) (see, e.g., Creighton, Proteins: Structures and Molecular Properties (W H Freeman & Co.; 2nd edition, 1993).
- drugrefers to any substance used in the prevention, diagnosis, alleviation, treatment, or cure of a disease or condition such as cancer, including but not limited to oral, colorectal, gastric, esophageal, hepatocellular, lung including non-small-cell-lung cancer (NSCLC) and small-cell lung cancer (SCLC), ovarian, breast including triple-negative breast, prostate, pancreatic including pancreatic ductal adenocarcinoma, head and neck, squamous, renal, bladder, cervical, endometrial, thyroid, glioblastoma cancer; a TROP2-positive cancer; a solid tumor; or a blood cancer, including a leukemia, a lymphoma or myeloma.
- a disease or conditionsuch as cancer, including but not limited to oral, colorectal, gastric, esophageal, hepatocellular, lung including non-small-cell-lung cancer (NSCLC) and small-cell lung cancer (SCLC), ova
- DARdrug-to-antibody ratio
- ADCantibody-drug conjugate
- the DAR valuereflects the homogeneity of the ADC population in the composition, and also indicates the amount of “payload” (e.g, drug or drug-linker) that is loaded onto an antibody and can be delivered to a target (e.g., cell or diseased tissue).
- payloade.g, drug or drug-linker
- LC-MSe.g., see Tang, Y.
- an agent, compound or composition being administeredincludes, but is not limited to, a natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, modified non-amino acid polypeptide, or an antibody or variant thereof.
- compositions containing such natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, modified nonnatural amino acid polypeptides, or an antibody or variant thereofcan be administered for prophylactic, enhancing, and/or therapeutic treatments.
- An appropriate “effective” amount in any individual casemay be determined using techniques, such as a dose escalation study.
- “enhance” or “enhancing”means to increase or prolong either in potency or duration a desired effect.
- “enhancing” the effect of therapeutic agentsrefers to the ability to increase or prolong, either in potency or duration, the effect of therapeutic agents on during treatment of a disease, disorder or condition.
- An “enhancing-effective amount,” as used herein,refers to an amount adequate to enhance the effect of a therapeutic agent in the treatment of a disease, disorder or condition. When used in a patient, amounts effective for this use will depend on the severity and course of the disease, disorder or condition, previous therapy, the patient's health status and response to the drugs, and the judgment of the treating physician.
- humanized or chimeric antibodyrefer to a molecule, generally prepared using recombinant techniques, having an antigen binding site derived from an immunoglobulin from a non-human species, (e.g., murine), and the remaining immunoglobulin structure of the molecule based upon the structure and/or sequence of a human immunoglobulin.
- the humanized antibodywill comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the hypervariable loops correspond to those of a non-human immunoglobulin and all or substantially all of the framework residues/regions (FR) are those of a human immunoglobulin sequence.
- the humanized antibodyoptionally also will comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin.
- the humanized forms of rodent antibodieswill essentially comprise the same CDR sequences of the parental rodent antibodies, although certain amino acid substitutions may be included to increase affinity, increase stability of the humanized antibody, or for other reasons.
- CDR loop exchangesdo not uniformly result in an antibody with the same binding properties as the antibody of origin, changes in framework residues (FR), residues involved in CDR loop support, might also be introduced in humanized antibodies to preserve antigen binding affinity.
- the antigen-binding sitemay comprise either complete variable domains fused onto constant domains or only the complementarity determining regions (CDRs) grafted onto appropriate framework regions in the variable domains.
- Antigen binding sitesmay be wild type or modified by one or more amino acid substitutions. This eliminates the constant region as an immunogen in human individuals, but the possibility of an immune response to the foreign variable region remains (LoBuglio, A. F. et al., "Mouse/Human Chimeric Monoclonal Antibody in Man: Kinetics and Immune Response," Proc. Natl. Acad. Sci. (USA) 86:4220-4224, 1989).
- variable regions of both heavy and light chainscontain three complementarity-determining regions (CDRs) which vary in response to the antigens in question and determine binding capability, flanked by four framework regions (FRs) which are relatively conserved in a given species and which putatively provide a scaffolding for the CDRs.
- CDRscomplementarity-determining regions
- FRsframework regions
- the variable regionscan be "humanized” by grafting CDRs derived from nonhuman antibody on the FRs present in the human antibody to be modified.
- Application of this approach to various antibodieshas been reported by Kettleborough, C. A.
- humanized antibodiespreserve all CDR sequences (for example, a humanized mouse antibody which contains all six CDRs from the mouse antibodies).
- humanized antibodieshave one or more CDRs (one, two, three, four, five, six) which are altered with respect to the original antibody, which are also termed one or more CDRs "derived from" one or more CDRs from the original antibody.
- sequences or subsequencesrefers to two or more sequences or subsequences which are the same.
- substantially identicalrefers to two or more sequences which have a percentage of sequential units which are the same when compared and aligned for maximum correspondence over a comparison window, or designated region as measured using comparison algorithms or by manual alignment and visual inspection.
- two or more sequencesmay be “substantially identical” if the sequential units are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region. Such percentages describe the “percent identity” of two or more sequences.
- the identity of a sequencecan exist over a region that is at least about 75-100 sequential units in length, over a region that is about 50 sequential units in length, or, where not specified, across the entire sequence.
- This definitionalso refers to the complement of a test sequence.
- two or more polypeptide sequencesare identical when the amino acid residues are the same, while two or more polypeptide sequences are “substantially identical” if the amino acid residues are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region.
- the identitycan exist over a region that is at least about 75 to about 100 amino acids in length, over a region that is about 50 amino acids in length, or, where not specified, across the entire sequence of a polypeptide sequence.
- two or more polynucleotide sequencesare identical when the nucleic acid residues are the same, while two or more polynucleotide sequences are “substantially identical” if the nucleic acid residues are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region.
- the identitycan exist over a region that is at least about 75 to about 100 nucleic acids in length, over a region that is about 50 nucleic acids in length, or, where not specified, across the entire sequence of a polynucleotide sequence.
- immunogenicityrefers to an antibody response to administration of a therapeutic drug.
- the immunogenicity toward therapeutic non-natural amino acid polypeptidescan be obtained using quantitative and qualitative assays for detection of anti- non-natural amino acid polypeptides antibodies in biological fluids.
- assaysinclude, but are not limited to, Radioimmunoassay (RIA), Enzyme-linked immunosorbent assay (ELISA), luminescent immunoassay (LIA), and fluorescent immunoassay (FIA).
- RIARadioimmunoassay
- ELISAEnzyme-linked immunosorbent assay
- LIAluminescent immunoassay
- FIAfluorescent immunoassay
- isolatedrefers to separating and removing a component of interest from components not of interest. Isolated substances can be in either a dry or semi-dry state, or in solution, including but not limited to an aqueous solution.
- the isolated componentcan be in a homogeneous state or the isolated component can be a part of a pharmaceutical composition that comprises additional pharmaceutically acceptable carriers and/or excipients. Purity and homogeneity may be determined using analytical chemistry techniques including, but not limited to, polyacrylamide gel electrophoresis or high-performance liquid chromatography.
- the componentis described herein as substantially purified.
- nucleic acids or proteinsare “isolated” when such nucleic acids or proteins are free of at least some of the cellular components with which it is associated in the natural state, or that the nucleic acid or protein has been concentrated to a level greater than the concentration of its in vivo or in vitro production.
- a geneis isolated when separated from open reading frames which flank the gene and encode a protein other than the gene of interest.
- linkagerefers to a bond or chemical moiety formed from a chemical reaction between the functional group of one group, such as a linker of the present disclosure, and another molecule.
- bondsmay include, but are not limited to, covalent linkages and non-covalent bonds, while such chemical moieties may include, but are not limited to, esters, carbonates, imines, phosphate esters, hydrazones, acetals, orthoesters, peptide linkages, oximes and oligonucleotide linkages.
- Hydrolytically stable linkagesmean that the linkages are substantially stable in water and do not react with water at useful pH values, including but not limited to, under physiological conditions for an extended period of time, perhaps even indefinitely.
- Hydrolytically unstable or degradable linkagesmean that the linkages are degradable in water or in aqueous solutions, including for example, blood.
- Enzymatically unstable or degradable linkagesmean that the linkage can be degraded by one or more enzymes.
- PEG and related polymersmay include degradable linkages in the polymer backbone or in the linker group between the polymer backbone and one or more of the terminal functional groups of the polymer molecule.
- Such degradable linkagesinclude but are not limited to ester linkages formed by the reaction of PEG carboxylic acids or activated PEG carboxylic acids with alcohol groups on a biologically active agent, wherein such ester groups generally hydrolyze under physiological conditions to release the biologically active agent.
- hydrolytically degradable linkagesinclude but are not limited to carbonate linkages; imine linkages resulted from reaction of an amine and an aldehyde; phosphate ester linkages formed by reacting an alcohol with a phosphate group; hydrazone linkages which are reaction product of a hydrazide and an aldehyde; acetal linkages that are the reaction product of an aldehyde and an alcohol; orthoester linkages that are the reaction product of a formate and an alcohol; peptide linkages formed by an amine group, including but not limited to, at an end of a polymer such as PEG, and a carboxyl group of a peptide; and oligonucleotide linkages formed by a phosphoramidite group, including but not limited to, at the end of a polymer, and a 5' hydroxyl group of an oligonucleotide.
- linkerrefers to any multivalent group that connects, or is capable of connecting, a first group to at least one other group.
- a linkeris a bivalent or a trivalent organic moiety that connects a drug (first group) to a biologically active agent (second group), e.g., via a linkage or adduct moiety, or that connects a drug (first group) to a reactive moiety (second group), wherein the reactive moiety is capable of reacting with a biologically active agent.
- Linkerscan be susceptible to cleavage (cleavable linkers), such as, acid-induced cleavage, photo-induced cleavage, peptidase-induced cleavage, esterase-induced cleavage, and disulfide bond cleavage, and so on, at conditions under which the drug and the at least one other group remains active.
- linkerscan be substantially resistant to cleavage (e.g., stable linker or non-cleavable linker).
- a linkeris a bivalent or trivalent group comprising, or consisting of, at least one moiety, wherein each at least one moiety is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, - (alkylene-O)n-, optionally substituted arylene, -O-, -C(O)-, -C(S)-, -N(R W )-, -S(0)o-2-, an amino acid, a peptide, a disulfide (-S-S-) and a water soluble polymer; and combinations thereof; wherein: each R w is independently H, Ci-Cs alkyl or a bond; and n is an integer of at least 1.
- nis an integer from 1 to 100. In some embodiments, n is an integer from 1 to 10. In some embodiments, n is 1, 2, 3, 4 or 5. In some embodiments, at least one moiety is not a bond. As such, the linker comprises at least one moiety that is not simply a bond.
- the water-soluble polymeris a (polyethylene) glycol (PEG) or modified PEG. In some embodiments, the water-soluble polymer is a polysaccharide. Unless expressly indicated otherwise, no orientation of the linker is implied by the direction in which the formula of the linker group is written.
- the formula -C(O)CH2CH2-represents both - C(O)CH2CH2- and -CEhCEhC O)-.
- the formula - OjCEhCEh-represents both *-C(O)CH2CH2- and - OjCEhCEh-*, wherein * denotes a point of connection, for example, connection to a drug.
- *denotes a point of connection, for example, connection to a drug.
- a linkeris not a bond.
- a linkeris a bivalent moiety that connects a first group and a second group. In some other embodiments, the linker is a trivalent moiety that connects a first group, a second group and a third group. In a non-limiting example, a trivalent moiety is C(H) (i.e., methine) or N. In some other embodiments, a linker is a tetravalent moiety that connects a first group, a second group and a third group.
- a linkerconnects at least a first group and a second group, wherein the first group is a drug, and the second group is a biologically active polypeptide or protein.
- the biologically active polypeptide or proteincontains at least one non-natural amino acid.
- the linkerconnects the drug to a non-natural amino acid of the biologically active polypeptide or protein.
- the biologically active polypeptide or proteinis an antibody.
- the antibody connected to a drug via a linkercan be an antibody-drug conjugate (ADC), such as an ADC of the present disclosure.
- ADCantibody-drug conjugate
- a linkerconnects at least a first group and a second group, wherein the first group is a drug, and the second group is a reactive moiety.
- the second groupis a reactive moiety that is capable of reacting with a biologically active polypeptide or protein.
- the biologically active polypeptide or proteincontains at least one non-natural amino acid.
- the reactive moietyis capable of reacting with a non-natural amino acid of the biologically active polypeptide or protein.
- the biologically active polypeptide or proteinis an antibody.
- a first linker or linker unitis connected to a second linker or linker unit and optionally to a third linker or linker unit, and the combined linkers (a composite linker) connects at least a first group and a second group.
- a composite linker of the present disclosurecan contain 2, 3, 4, 5, 6, 7, 8, 9, 10 or more linker units.
- a first, second and third linker unitare joined together to provide a composite linker that can connect a first group (e.g., a drug) to at least one other group, such as a reactive moiety and/or a biologically active polypeptide or protein (e.g., an antibody.
- the biologically active polypeptide or proteine.g., antibody
- a linkeris linear. In some other embodiments, a linker is branched.
- metaboliterefers to a derivative of a compound, by way of example natural amino acid polypeptide, a non-natural amino acid polypeptide, a modified natural amino acid polypeptide, or a modified non-natural amino acid polypeptide, that is formed when the compound, by way of example natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, or modified non-natural amino acid polypeptide, is metabolized.
- pharmaceutically active metaboliterefers to a biologically active derivative of a compound, by way of example natural amino acid polypeptide, a non-natural amino acid polypeptide, a modified natural amino acid polypeptide, or a modified non-natural amino acid polypeptide, that is formed when such a compound, by way of example a natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, or modified non-natural amino acid polypeptide, is metabolized.
- metabolismrefers to the sum of the processes by which a particular substance is changed by an organism. Such processes include, but are not limited to, hydrolysis reactions and reactions catalyzed by enzymes. Further information on metabolism may be obtained from The Pharmacological Basis of Therapeutics, 9th Edition, McGraw-Hill (1996).
- metabolites of natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non-natural amino acid polypeptidesmay be identified either by administration of the natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non- natural amino acid polypeptides to a host and analysis of tissue samples from the host, or by incubation of natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non-natural amino acid polypeptides with hepatic cells in vitro and analysis of the resulting compounds.
- modifiedrefers to the presence of a change to a natural amino acid, a non-natural amino acid, a natural amino acid polypeptide or a non-natural amino acid polypeptide. Such changes, or modifications, may be obtained by post synthesis modifications of natural amino acids, non-natural amino acids, natural amino acid polypeptides or non-natural amino acid polypeptides, or by co-translational, or by post-translational modification of natural amino acids, non-natural amino acids, natural amino acid polypeptides or non-natural amino acid polypeptides.
- non-natural amino acidrefers to an amino acid that is not one of the 20 common amino acids or pyrolysine or selenocysteine.
- Other terms that may be used synonymously with the term “non-natural amino acid”is “ non-natural ly encoded amino acid,” “unnatural amino acid,” “non-naturally-occurring amino acid,” “synthetic amino acid” and variously hyphenated and non-hyphenated versions thereof.
- non-natural amino acidincludes, but is not limited to, amino acids which occur naturally by modification of a naturally encoded amino acid (including but not limited to, the 20 common amino acids or pyrrolysine and selenocysteine) but are not themselves incorporated into a growing polypeptide chain by the translation complex.
- naturally-occurring amino acids that are not naturally-encodedinclude, but are not limited to, N-acetylglucosaminyl-L-serine, N-acetylglucosaminyl-L-threonine, and O- phosphotyrosine.
- non-natural amino acidincludes, but is not limited to, amino acids which do not occur naturally and may be obtained synthetically or may be obtained by modification of non-natural amino acids.
- nucleic acidrefers to deoxyribonucleotides, deoxyribonucleosides, ribonucleosides or ribonucleotides and polymers thereof in either single- or double-stranded form.
- nucleic acids and nucleic acid polymersinclude, but are not limited to, (i) analogues of natural nucleotides which have similar binding properties as a reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides; (ii) oligonucleotide analogs including, but are not limited to, PNA (peptidonucleic acid), analogs of DNA used in antisense technology (phosphorothioates, phosphoroamidates, and the like); (iii) conservatively modified variants thereof (including but not limited to, degenerate codon substitutions) and complementary sequences and sequence explicitly indicated.
- PNApeptidonucleic acid
- analogs of DNA used in antisense technologyphosphorothioates, phosphoroamidates, and the like
- conservatively modified variants thereofincluding but not limited to, degenerate codon substitutions
- degenerate codon substitutionsmay be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081, 1991; Ohtsuka et al., J. Biol. Chem. 260:2605-2608, 1985; and Rossolini et al., Mol. Cell. Probes 8:91- 98, 1994).
- pharmaceutically acceptablerefers to a material, including but not limited, to a salt, binder, adjuvant, excipient, carrier or diluent, which does not abrogate the biological activity or properties of the compound, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained.
- the disclosureconcerns polymers.
- polymerrefers to a molecule composed of repeated subunits. Such molecules include, but are not limited to, polypeptides, polynucleotides, or polysaccharides or polyalkylene glycols.
- Polymers of the disclosurecan be linear or branched polymeric polyether polyols including, but are not limited to, polyethylene glycol, polypropylene glycol, polybutylene glycol, and derivatives thereof.
- Polymerscan be activated polymers (e.g., activated PEGs) that facilitate conjugation to another group, such as a polypeptide, linker or drug-linker molecule.
- Polymerscan also terminate in a moiety, such as a non-reactive moiety, e.g., alkyl (such as methyl) or alkoxy (such as methoxy).
- polymershave average molecular weights between about 0.1 kDa to about 100 kDa.
- polymersinclude, but are not limited to, between about 100 Da and about 100,000 Da or more.
- the molecular weight of the polymermay be between about 100 Da and about 100,000 Da, including but not limited to, about 100,000 Da, about 95,000 Da, about 90,000 Da, about 85,000 Da, about 80,000 Da, about 75,000 Da, about 70,000 Da, about 65,000 Da, about 60,000 Da, about 55,000 Da, about 50,000 Da, about 45,000 Da, about 40,000 Da, about 35,000 Da, about 30,000 Da, about 25,000 Da, about 20,000 Da, about 15,000 Da, about 10,000 Da, about 9,000 Da, about 8,000 Da, about 7,000 Da, about 6,000 Da, about 5,000 Da, about 4,000 Da, about 3,000 Da, about 2,000 Da, about 1,000 Da, about 900 Da, about 800 Da, about 700 Da, about 600 Da, about 500 Da, 400 Da, about 300 Da, about 200 Da, and about 100 Da.
- molecular weight of the polymeris between about 100 Da and about 50,000 Da. In some embodiments, the molecular weight of the polymer is between about 100 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 1,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 2,000 to about 50,000 Da. In some embodiments, the molecular weight of the polymer is between about 5,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 10,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is within a range of about 100 Da to about 10,000 Da.
- the molecular weight of the polymeris within a range of about 100 Da to about 5,000 Da. In some embodiments, the molecular weight of the polymer is within a range of about 100 Da to about 1,000 Da.
- the polymeris a polyethylene glycol (PEG). In some embodiments, the PEG is a linear PEG. In some embodiments, the PEG is a branched PEG.
- the molecular weight of the linear or branched chain PEGmay be between about 1,000 Da and about 100,000 Da, including but not limited to, about 100,000 Da, about 95,000 Da, about 90,000 Da, about 85,000 Da, about 80,000 Da, about 75,000 Da, about 70,000 Da, about 65,000 Da, about 60,000 Da, about 55,000 Da, about 50,000 Da, about 45,000 Da, about 40,000 Da, about 35,000 Da, about 30,000 Da, about 25,000 Da, about 20,000 Da, about 15,000 Da, about 10,000 Da, about 9,000 Da, about 8,000 Da, about 7,000 Da, about 6,000 Da, about 5,000 Da, about 4,000 Da, about 3,000 Da, about 2,000 Da, and about 1,000 Da.
- the molecular weight of the linear or branched chain PEGis between about 1,000 Da and about 50,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 1,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 5,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 5,000 Da and about 20,000 Da. In other embodiments, the molecular weight of the linear or branched chain PEG is between about 2,000 to about 50,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is within a range of about 100 Da to about 10,000 Da.
- the molecular weight of the linear or branched chain PEGis within a range of about 100 Da to about 5,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is within a range of about 100 Da to about 1,000 Da.
- the PEGis a linear PEG. In some embodiments, the PEG comprises a defined number of repeating (-alkylene-O-) units, such as 2, 4, 6, 8, 10, 12, 14 or more units (e.g., PEG-2, PEG-4, PEG-6, PEG-8, PEG-10, PEG-12, PEG-14). In some embodiments, the PEG is a branched PEG.
- PEGylatingor “PEGylated” is meant to refer to the covalent bonding of a specified moiety to a polyethylene glycol (PEG) molecule.
- the moietycan be present in a drug, a drug-linker, a linker, or a polypeptide or protein.
- the moietyis a hydroxyl group, a carboxylic acid, acyl or an amino group, such as a hydroxyl group, carboxylic acid, acyl or amino group present in a drug, drug-linker, linker or polypeptide.
- the hydroxyl group, carboxylic acid, acyl or amino groupis present in an amino acid.
- the amino acid bearing the hydroxyl group, carboxylic acid, acyl or amino groupis a natural or non-natural amino acid that is present in a polypeptide (e.g., an antibody), linker or drug-linker.
- the methodcan comprise contacting an isolated polypeptide comprising a natural or synthetic amino acid, or contacting a drug-linker comprising a natural or synthetic amino acid, with a water- soluble polymer (e.g., a PEG) comprising a moiety that reacts with the natural or synthetic amino acid.
- the methodcan comprise contacting an isolated a-TROP2 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid.
- the methodcan comprise contacting an isolated a-HER2 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid.
- the methodcan comprise contacting an isolated a-CD70 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid.
- the methodcan comprise reacting a drug-linker comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid.
- polypeptide“peptide” and “protein” are used interchangeably herein to refer to a polymer of amino acid residues. That is, a description directed to a polypeptide applies equally to a description of a peptide and a description of a protein, and vice versa. The terms apply to naturally occurring amino acid polymers as well as amino acid polymers in which one or more amino acid residues is a non-natural amino acid. Additionally, such “polypeptides,” “peptides” and “proteins” include amino acid chains of any length, including full length proteins, including but not limited to antibodies, wherein the amino acid residues are linked by covalent peptide bonds.
- the term “peptide”can be used to refer to short chains of amino acids linked by peptide bonds and containing two or more amino acids linked in a chain. In some embodiments, the peptide contains 2 to 20 amino acids linked in the chain. In some embodiments, the peptide contains 2, 3, 4, 5, 6, 7 or 8 amino acids. As is well understood to a person of ordinary skill in the art, a dipeptide contains two amino acids.
- post-translationally modifiedrefers to any modification of a natural or nonnatural amino acid which occurs after such an amino acid has been translationally incorporated into a polypeptide chain.
- modificationsinclude, but are not limited to, co-translational in vivo modifications, co-translational in vitro modifications (such as in a cell-free translation system), post-translational in vivo modifications, and post-translational in vitro modifications.
- prodrugrefers to an agent that is converted into the parent drug in vivo or in vitro, which does not abrogate the biological activity or properties of the drug, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained.
- Prodrugsare generally drug precursors that, following administration to a subject and subsequent absorption, are converted to an active, or a more active species via some process, such as conversion by a metabolic pathway.
- Some prodrugshave a chemical group present on the prodrug that renders it less active and/or confers solubility or some other property to the drug. Once the chemical group has been cleaved and/or modified from the prodrug the active drug is generated. Prodrugs are converted into active drug within the body through enzymatic or non-enzymatic reactions. Prodrugs may provide improved physiochemical properties such as better solubility, enhanced delivery characteristics, such as specifically targeting a particular cell, tissue, organ or ligand, and improved therapeutic value of the drug.
- prodrugsinclude, but are not limited to, (i) ease of administration compared with the parent drug; (ii) the prodrug may be bioavailable by oral administration whereas the parent is not; and (iii) the prodrug may also have improved solubility in pharmaceutical compositions compared with the parent drug.
- a prodrugincludes a pharmacologically inactive, or reduced activity, derivative of an active drug.
- Prodrugsmay be designed to modulate the amount of a drug or biologically active molecule that reaches a desired site of action through the manipulation of the properties of a drug, such as physiochemical, biopharmaceutical, or pharmacokinetic properties.
- prodruga non-natural amino acid polypeptide which is administered as an ester (the “prodrug”) to facilitate transmittal across a cell membrane where water solubility is detrimental to mobility and that is then metabolically hydrolyzed to the carboxylic acid, the active entity, once inside the cell where water solubility is beneficial.
- Prodrugsmay be designed as reversible drug derivatives, for use as modifiers to enhance drug transport to site-specific tissues.
- prophylactically effective amountrefers to an amount of a composition containing at least one non-natural amino acid polypeptide or at least one modified non-natural amino acid polypeptide prophylactically applied to a patient which will relieve to some extent one or more of the symptoms of a disease, condition or disorder being treated. In such prophylactic applications, such amounts may depend on the patient's state of health, weight, and the like. It is considered well within the skill of the art for one to determine such prophylactically effective amounts by routine experimentation, including, but not limited to, a dose escalation clinical trial.
- recombinant host cellalso referred to as “host cell,” refers to a cell which includes an exogenous polynucleotide, wherein the methods used to insert the exogenous polynucleotide into a cell include, but are not limited to, direct uptake, transduction, f-mating, or other methods known in the art to create recombinant host cells.
- exogenous polynucleotidemay be a nonintegrated vector, including but not limited to a plasmid, or may be integrated into the host genome.
- spacerrefers to an atom or functional group that connects a first group to a second group.
- a spaceris a carbonyl (-C(O)-), -C(O)O-, -C(O)N(R)-, -O-, -S-, -N(R)- wherein each R is H or alkyl.
- the spaceris a bivalent spacer.
- subjectrefers to an animal which is the object of treatment, observation or experiment.
- a subjectmay be, but is not limited to, a mammal including, but not limited to, a human.
- substantially purifiedrefers to a component of interest that may be substantially or essentially free of other components which normally accompany or interact with the component of interest prior to purification.
- a component of interestmay be “substantially purified” when the preparation of the component of interest contains less than about 30%, less than about 25%, less than about 20%, less than about 15%, less than about 10%, less than about 5%, less than about 4%, less than about 3%, less than about 2%, or less than about 1% (by dry weight) of contaminating components.
- a “substantially purified” component of interestmay have a purity level of about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, about 96%, about 97%, about 98%, about 99% or greater.
- a natural amino acid polypeptide or a non-natural amino acid polypeptidemay be purified from a native cell, or host cell in the case of recombinantly produced natural amino acid polypeptides or non-natural amino acid polypeptides.
- a preparation of a natural amino acid polypeptide or a non-natural amino acid polypeptidemay be “substantially purified” when the preparation contains less than about 30%, less than about 25%, less than about 20%, less than about 15%, less than about 10%, less than about 5%, less than about 4%, less than about 3%, less than about 2%, or less than about 1% (by dry weight) of contaminating material.
- the natural amino acid polypeptide or non-natural amino acid polypeptidemay be present at about 30%, about 25%, about 20%, about 15%, about 10%, about 5%, about 4%, about 3%, about 2%, or about 1% or less of the dry weight of the cells.
- the natural amino acid polypeptide or non-natural amino acid polypeptidemay be present in the culture medium at about 5g/L, about 4g/L, about 3g/L, about 2g/L, about Ig/L, about 750mg/L, about 500mg/L, about 250mg/L, about lOOmg/L, about 50mg/L, about lOmg/L, or about Img/L or less of the dry weight of the cells.
- substantially purified natural amino acid polypeptides or non-natural amino acid polypeptidesmay have a purity level of about 30%, about 35%, about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, about 99% or greater as determined by appropriate methods, including, but not limited to, SDS/PAGE analysis, RP-HPLC, SEC, and capillary electrophoresis.
- terapéuticaally effective amountrefers to the amount of a composition containing at least one non-natural amino acid polypeptide and/or at least one modified non-natural amino acid polypeptide administered to a patient already suffering from a disease, condition or disorder, sufficient to cure or at least partially arrest, or relieve to some extent one or more of the symptoms of the disease, disorder or condition being treated.
- the effectiveness of such compositionsdepends on conditions including, but not limited to, the severity and course of the disease, disorder or condition, previous therapy, the patient's health status and response to the drugs, and the judgment of the treating physician.
- therapeutically effective amountsmay be determined by routine experimentation, including but not limited to a dose escalation clinical trial.
- cytotoxicrefers to a cytotoxic compound which can cause harm, disturbances, or death.
- Toxic moietiesinclude, but are not limited to, a drug comprising or consisting of an auristatin, or an analog or derivative thereof.
- a cytotoxic drug or moietyexpressly excludes a topoisomerase I (TOPI) inhibitor.
- a cytotoxic drug or moietyexpressly excludes a TOPI inhibitor, wherein the TOPI inhibitor is exatecan, or a derivative thereof.
- a cytotoxic drug or moietyexpressly excludes exatecan derivative Dxd. In some embodiments, a cytotoxic drug or moiety expressly excludes PF-06380101 (also known as AurOlOl or Auristatin-
- treatinclude alleviating, preventing, abating or ameliorating a disease or condition symptoms, preventing additional symptoms, ameliorating or preventing the underlying metabolic causes of symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition.
- the terms “treat,” “treated,” “treating” or “treatment”,include, but are not limited to, prophylactic and/or therapeutic treatments.
- treatcan refer to the decrease, reduction or amelioration of one or more symptoms or conditions or diseases associated with an antigen related or associated cancer.
- treatcan refer to the administration of an ADC of the present disclosure to a subject in need thereof to decrease, reduce, improve, alter, relieve, affect or ameliorate an antigen related or associated cancer or disease or symptom or condition, or the predisposition toward a condition.
- capable of specific bindingrefers to protein or peptide (e.g., antibody) binding to a predetermined target substance (e.g., an antigen and/or groups of antigens), e.g.
- binding to a target cellor “binding to a cancer cell” is to be understand as referring to protein or peptide (e.g., antibody) binding to a predetermined target substance (e.g. antigen or antigens) that is expressed on such a cell.
- a predetermined target substancee.g. antigen or antigens
- the protein or peptidebinds with an affinity of at least about IxlO 7 Ml, and/or binds to the predetermined target substance (e.g., antigen, antigens or cell) with an affinity that is at least two-fold greater than its affinity for binding to a non-specific control substance (e.g., B SA, casein, non-cancer cells) other than the predetermined target substance or a closely-related target substance.
- a non-specific control substancee.g., B SA, casein, non-cancer cells
- water-soluble polymerrefers to any polymer that is soluble in aqueous solvents.
- water-soluble polymersinclude, but are not limited to, polyethylene glycol, polyethylene glycol propionaldehyde, mono C1-C10 alkoxy or aryloxy derivatives thereof (described in U.S. Patent No.
- water- soluble polymersmay result in changes including, but not limited to, increased water solubility, increased or modulated serum half-life, increased or modulated therapeutic half-life relative to the unmodified form, increased bioavailability, modulated biological activity, extended circulation time, modulated immunogenicity, modulated physical association characteristics including, but not limited to, aggregation and multimer formation, altered receptor binding, altered binding to one or more binding partners, and altered receptor dimerization or multimerization.
- such water- soluble polymersmay or may not have their own biological activity.
- the term “modulated serum half-life”refers to positive or negative changes in the circulating half-life of a modified biologically active molecule relative to its non-modified form.
- the modified biologically active moleculesinclude, but are not limited to, natural amino acid, non-natural amino acid, natural amino acid polypeptide or non-natural amino acid polypeptide.
- serum half-lifeis measured by taking blood samples at various time points after administration of the biologically active molecule or modified biologically active molecule and determining the concentration of that molecule in each sample. Correlation of the serum concentration with time allows calculation of the serum half-life.
- modulated serum half-lifemay be an increased in serum half-life, which may enable an improved dosing regimen or avoid toxic effects.
- increases in serummay be at least about two-fold, at least about three-fold, at least about five-fold, or at least about ten-fold.
- Methods for evaluating serum half-lifeare known in the art and may be used for evaluating the serum half-life of antibodies and antibody drug conjugates of the present disclosure.
- modulated therapeutic half-liferefers to positive or negative change in the half-life of the therapeutically effective amount of a modified biologically active molecule, relative to its non-modified form.
- the modified biologically active moleculesinclude, but are not limited to, natural amino acid, non-natural amino acid, natural amino acid polypeptide or non-natural amino acid polypeptide.
- therapeutic half-lifeis measured by measuring pharmacokinetic and/or pharmacodynamic properties of the molecule at various time points after administration. Increased therapeutic half-life may enable a particular beneficial dosing regimen, a particular beneficial total dose, or avoids an undesired effect.
- the increased therapeutic half-lifemay result from increased potency, increased or decreased binding of the modified molecule to its target, an increase or decrease in another parameter or mechanism of action of the non-modified molecule, or an increased or decreased breakdown of the molecules by enzymes such as, by way of example only, proteases.
- enzymessuch as, by way of example only, proteases.
- the present inventionprovides novel ADCs comprising antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids incorporated at any desired position in the heavy and/or light chain amino acid sequence. Further, the present invention provides ADCs comprising one or more antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids site specifically incorporated in the heavy and/or light chain amino acid sequence conjugated to drug via a linker. In some embodiments, the antibody, antibody fragment or variant thereof is an anti-TROP2 antibody, antibody fragment or variant.
- the inventionprovides anti-TROP2 ADCs comprising antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids incorporated at any desired position in the heavy and/or light chain amino acid sequence.
- the present inventionprovides anti-TROP2 ADCs comprising one or more antibodies, antibody fragments or variants thereof engineered to have one or more non-natural amino acids site specifically incorporated in the heavy and/or light chain amino acid sequence conjugated to drug via a linker.
- Antibody or antibody fragments or variants of the disclosuremay be human, humanized, engineered, non-human, and/or chimeric antibody or antibody fragments.
- An antibody or antibody fragment or variant provided hereinmay comprise two or more amino acid sequences.
- a first amino acid sequencemay comprise a first antibody chain and a second amino acid sequence may comprise a second antibody chain.
- a first antibody chainmay comprise a first amino acid sequence, and a second antibody chain may comprise a second amino acid sequence.
- a chain of an antibodymay refer to an antibody heavy chain, an antibody light chain, or a combination of a region or all of an antibody heavy chain and a region or all of an antibody light chain.
- an antibody provided hereincomprises a heavy chain or fragment or variant thereof, and a light chain or fragment or variant thereof.
- Two amino acid sequences of an antibody, including two antibody chains,may be connected, attached, or linked by one or more disulfide bonds, a chemical linker, a peptide linker, or a combination thereof.
- a chemical linkerincludes a linker via a non-natural amino acid.
- a chemical linkerincludes a linker via one or more nonnatural amino acids.
- a chemical linkercan include a chemical conjugate.
- a peptide linkerincludes any amino acid sequence joining the two amino acid sequences.
- the peptide linkermay comprise 1 or more, 5 or more, 10 or more, 15 or more, 20 or more, 25 or more, 30 or more, 35 or more, 40 or more, 45 or more, 50 or more, 55 or more, 60 or more, 65 or more, 70 or more, 75 or more, 80 or more, 85 or more, 90 or more, 95 or more, 100 or more amino acids.
- the peptide linkermay be a portion of any antibody, including a domain of an antibody, such as a variable domain, CDR1, CDR2, CDR3, and/or a combination of CDRs (complementarity determining regions).
- a heavy and a light chainare connected, attached, or linked, for example, via a peptide linker.
- a heavy chain and a light chainare connected, for example, by one or more disulfide bonds.
- Antibodies, antibody fragments and antibody variants of the disclosuremay interact or engage with an antigen on an effector cell.
- the effector cellcan include, but is not limited to, an immune cell, a genetically modified cell having increase or decrease cytotoxic activity, a cell involved in the host defense mechanism, an anti-inflammatory cell, a leukocyte, a lymphocyte, a macrophage, an erythrocyte, a thrombocyte, a neutrophil, a monocyte, an eosinophil, a basophil, a mast cell, a NK cell, a B-cell, or a T-cell.
- the immune cellmay be a T cell such as a cytotoxic T cell or natural killer T cell.
- the antibody or antibody fragmentmay interact with a receptor on a T-cell such as, but not limited to a T-cell receptor (TCR).
- TCRT-cell receptor
- the TCRmay comprise TCR alpha, TCR beta, TCR gamma, and/or TCR delta or TCR zeta.
- Antibody or antibody fragments of the disclosuremay bind to a receptor on a lymphocyte, dendritic cell, B- cell, macrophage, monocytes, neutrophils and/or NK cells.
- Antibody or antibody fragments of the disclosuremay bind to a cell surface receptor.
- Antibody or antibody fragments of the disclosuremay bind to an antigen receptor, such as for example, a TR0P2 antigen receptor, or a HER2 antigen receptor, or a CD70 antigen receptor.
- Antibody or antibody fragments of the disclosurecan be conjugated to a T-cell surface antigen.
- novel anti-TROP2 antibodiesor the corresponding antibody fragments, and antibody-drug conjugates thereof for use as therapeutic agents.
- novel anti-TROP2 antibodies, antibody fragments or variants thereof with at least one non-natural amino acid or unnaturally encoded amino acidDisclosed herein are novel anti-TROP2 antibodies, antibody fragments or variants thereof with at least one non-natural amino acid or unnaturally encoded amino acid.
- the present inventionprovides anti-TROP2 antibodies having at least one non-natural amino acid that facilitates antibody conjugation to a drug or drug-linker.
- Antibodies, antibody fragments or variants provided in the present disclosuremay be human, humanized, engineered, non-human, and/or chimeric antibody or antibody fragments that bind to the extracellular domain of the target antigen, which can be overexpressed in a number of cancers.
- novel antibodies, compositions and antibody drug conjugates for the treatment and/or diagnosis of antigen-expressing cancersare beneficial, including but not limited to TR0P2- expressing cancers.
- Antibodies or antibody fragments or variants disclosed hereininclude, but are not limited to, analogs, isoforms, mimetics, fragments, or hybrids of anti-TROP2.
- Antibodies or antibody fragments or variants of anti-TROP2 of the present disclosureinclude but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like.
- Antibodies comprising non-natural amino acidsare also disclosed herein.
- the antibody or antibody fragments or variantsinclude but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like.
- the anti-TROP2 antibody or antibody fragments or variantscomprises one or more non-natural amino acids.
- Non-limiting examples of antibodies or antibody fragments or variants of the present disclosurecomprise the sequences listed in Table 1.
- antibody or antibody fragments disclosed hereinare anti-TROP2 antibodies or antibody fragments or variants thereof.
- the anti-TROP2 antibodies or antibody fragments or variants disclosed hereincan be humanized.
- Anti-TR0P2 antibodies or antibody fragments or variants disclosed hereininclude, but are not limited to, anti- TR0P2 analogs, isoforms, mimetics, fragments, or hybrids.
- Anti-TR0P2 antibodies or antibody fragments or variants of the present disclosureinclude but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like.
- the anti-TROP2 antibodies or antibody fragments or variants of the present disclosurecomprise a sequence of SEQ ID NOs: 1 to 17 (Table 1).
- the antibodies, fragments or variants of the present disclosurecan be an anti-TROP2 antibody, fragment or variant.
- the anti-TROP2 antibodyis characterized by the amino acid sequence of its polypeptide chain(s) (e.g., the amino acid sequence of a heavy chain and/or a light chain, each of which can be referred to herein as a heavy chain amino acid sequence and/or a light chain amino acid sequence, respectively).
- polypeptide chain(s)e.g., the amino acid sequence of a heavy chain and/or a light chain, each of which can be referred to herein as a heavy chain amino acid sequence and/or a light chain amino acid sequence, respectively.
- the anti-TROP2 antibody heavy chain amino acid sequence and/or light chain amino acid sequencecan comprise any one of SEQ ID NOs: 1 to 17.
- the anti-TROP2 antibody heavy chain amino acid and/or light chain amino acid sequenceis independently selected from the group consisting of SEQ ID NOs: 1 to 17.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises any one of SEQ ID NOs: 1, 2, 5, and 6; and a light chain amino acid sequence comprises any one of SEQ ID NOs: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17.
- the anti-TROP2 antibody heavy chain amino acid sequenceis selected from the group consisting of SEQ ID NOs: 1, 2, 5, and 6; and a light chain amino acid sequence is selected from the group consisting of SEQ ID NOs: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17.
- a heavy chain amino acid sequenceis SEQ ID NO: 1 or 5.
- a heavy chain amino acid sequenceis SEQ ID NO: 5.
- a heavy chain amino acid sequenceis SEQ ID NO: 2 or 6.
- a heavy chain amino acid sequenceis SEQ ID NO: 6.
- a light chain amino acid sequenceis SEQ ID NO: 3 or 4.
- a light chain amino acid sequenceis SEQ ID NO: 3 or 7. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 5, and a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 5, and a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence is SEQ ID NO: 5, and each light chain amino acid sequence is SEQ ID NO: 4.
- the anti-TROP2 antibodycomprises a heavy chain and a light chain, each characterized by its heavy chain and light chain amino acid sequence, respectively.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 3.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 4.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 7.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 8.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 1, and the light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and a light chain amino acid sequence comprises SEQ ID NO: 13.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and a light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 8.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and a light chain amino acid sequence consists of SEQ ID NO: 12.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, the anti- TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibodycomprises two heavy chains and two light chains, each characterized by its heavy chain and light chain amino acid sequence, respectively.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 3.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1, and each light chain amino acid sequence comprises SEQ ID NO: 4.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 7.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 8.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 14.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 17.
- the anti-TROP2 antibodyconsists of two heavy chains and two light chains, each characterized by its heavy chain and light chain amino acid sequence, respectively.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 3.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 4.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 7.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 8.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 13.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 3.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 4.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 7.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 8.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 9.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and a light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 14.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 3.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence of SEQ ID NO: 4.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 7.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 8.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 9.
- the heavy chain amino acid sequenceconsist of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consist of SEQ ID NO: 14.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibodycomprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 9.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 15.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 17.
- each heavy chain amino acid sequencesconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 3.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 4.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 7.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 8.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 13.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 3.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 4.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 7.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 8.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 9.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 15.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 3.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 4.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 7.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 8.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 9.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 14.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibodycomprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 9.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 15.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 17.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 3.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 4.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 7.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 8.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 13.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequences of SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 3.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 4.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 7.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 8.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 9.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 15.
- the heavy chain amino acid sequencecomprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 17.
- the anti-TROP2 antibody heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 3.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 4.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 7.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 8.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 9.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 14.
- the heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibodycomprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 9.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises of SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 14.
- each heavy chain amino acid sequencecomprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 17. In some embodiments, wherein the anti-TROP2 antibody comprises two heavy chains and two light chains, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 4.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 11.
- each heavy chain amino acid sequenceconsists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 17.
- the anti-TROP2 antibodycomprises a heavy chain, wherein the amino acid sequence of the heavy chain contains amino acid sequence EEM in an Fc constant region.
- said EEM sequencecan be replaced with DEL.
- Anti-TROP2 amino acid sequencesare also disclosed are: all of the sequences in Table 1, wherein X is replaced with any non-natural amino acid; all of the sequences in Table 1, wherein any amino acid is replaced with any non-natural amino acid; all of the sequences in Table 1, wherein X is pAF; all of the heavy chain sequences in Table 1, wherein EEM is replaced with DEL; and all of the heavy chain sequences in Table 1, wherein a non-natural amino acid is site specifically incorporated at position 114, according to Kabat numbering, as well known to the skilled artisan).
- WTWild Type
- HCHeavy Chain
- LCLight Chain
- Xdenotes non-natural amino acid.
- the present disclosureprovides antibodies, antibody fragments or variants comprising at least one non-natural amino acid.
- Introduction of at least one non-natural amino acid into an antibodycan allow for the application of conjugation chemistries that involve specific chemical reactions with one or more non-natural amino acids while not reacting with the commonly occurring 20 amino acids.
- Non-natural amino acid site selectionwas based on surface exposure/site accessibility within the antibody and hydrophobic or neutral amino acid sites were selected to maintain the charge on the antibody.
- Methods for introducing non-natural amino acids inserted into sites in a proteinare described for example in W02010/011735 and in W02005/074650. The present disclosure employs such methodologies and techniques.
- the non-natural amino acids used in the methods and compositions described hereinhave at least one of the following four properties: (1) at least one functional group on the sidechain of the non-natural amino acid has at least one characteristics and/or activity and/or reactivity orthogonal to the chemical reactivity of the 20 common, genetically-encoded amino acids (i.e., alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine), or at least orthogonal to the chemical reactivity of the naturally occurring amino acids present in the polypeptide that includes the non-natural amino acid; (2) the introduced non-natural amino acids are substantially chemically inert toward the 20 common, genetically-encoded amino acids; (3) the non-natural amino acid can be stab
- Non-natural amino acidsmay also include protected or masked oximes or protected or masked groups that can be transformed into an oxime group after deprotection of the protected group or unmasking of the masked group.
- Non-natural amino acidsmay also include protected or masked carbonyl or dicarbonyl groups, which can be transformed into a carbonyl or dicarbonyl group after deprotection of the protected group or unmasking of the masked group and thereby are available to react with hydroxylamines or oximes to form oxime groups.
- Oxime-based non-natural amino acidsmay be synthesized by methods well known in the art, (see for example WO2013/185117 and W02005/074650), including: (a) reaction of a non-natural amino acid containing -ONH2 with a carbonyl- or dicarbonyl-containing reagent; and (b) reaction of a carbonyl- or dicarbonylcontaining non-natural amino acid with a reagent containing -ONH2.
- non-naturally encoded amino acid site selectionis based on surface exposure.
- one possible siteis an amino acid having a solvent accessible surface area ratio of 30% or more, 40% or more, 50% or more, 60% or more, 70% or more, 80% or more, 90% or more, or 95% or more.
- one possible siteis an amino acid having a solvent accessible surface area ratio of about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 95%, or more.
- the solvent accessible surface areacan be calculated based on the DSSP program [Biopolymers, 22, 2577-2637 (1983)], using a crystalline structure analyzing data file of antibodies or antibody fragments registered in Protein data bank (PDB).
- the ratio of the solvent accessible surface area of the amino acid residues of interestcan be calculated by dividing the antibody structural solvent accessible surface area calculated in the above by the solvent accessible surface area of alanine-X-alanine (X represents the amino acid residues of interest).
- Xrepresents the amino acid residues of interest.
- the solvent accessibility of an amino acidcan be determined by a solvent accessibility test in which a functional group on the amino acid (a thiol, amino, or carbonyl group) is functionalized when treated with an electrophilic reagent or a nucleophilic reagent, or the like.
- the functional groupi.e., the thiol, amino, or carbonyl group
- the non-natural amino acid siteis at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, or at least 95% solvent accessible.
- solvent accessibility testinclude, but are not limited to, propargylation of a surface thiol group, or a-bromopyruvate reacting with a surface thiol group, etc.
- Non-natural amino acidsthat may be used in the methods and compositions described herein include, but are not limited to, amino acids comprising amino acids with novel functional groups, amino acids that covalently or noncovalently interact with other molecules, glycosylated amino acids such as a sugar substituted serine, other carbohydrate modified amino acids, ketocontaining amino acids, aldehyde-containing amino acids, amino acids comprising polyethylene glycol or other polyethers, heavy atom substituted amino acids, chemically cleavable and/or photocleavable amino acids, amino acids with an elongated side chains as compared to natural amino acids, including but not limited to, polyethers or long chain hydrocarbons, including but not limited to, greater than about 5 or greater than about 10 carbons, carbon-linked sugar- containing amino acids, redox-active amino acids, amino thioacid containing amino acids, and amino acids comprising one or more toxic moiety.
- antibodiescomprising one or more non-natural amino acids.
- the one or more non-natural amino acidsmay be encoded by a codon that does not code for one of the twenty natural amino acids.
- the one or more non-natural amino acidsmay be encoded by a nonsense codon (stop codon).
- the stop codonmay be an amber codon.
- the amber codonmay comprise a UAG sequence.
- the stop codonmay be an ochre codon.
- the ochre codonmay comprise a UAA sequence.
- the stop codonmay be an opal or umber codon.
- the opal or umber codonmay comprise a UGA sequence.
- the one or more non-natural amino acidsmay be encoded by a four-base codon.
- Non-natural amino acids of the present disclosureinclude, but are not limited to, 1) substituted phenylalanine and tyrosine analogues, such as 4-amino-L-phenylalanine, 4-acetyl-L- phenylalanine, 4-azido-L-phenylalanine, 4-nitro-L-phenylalanine, 3 -methoxy -L-phenylalanine, 4-isopropyl-L-phenylalanine, 3-nitro-L-tyrosine, O-methyl-L-tyrosine and O-phosphotyrosine; 2) amino acids that can be photo-cross-linked, e.g., amino acids with aryl azide or benzophenone groups, such as 4-azidophenylalanine or 4-benzoylphenylalanine; 3) amino acids that have unique chemical reactivity, such as 4-acetyl-L-phenylalanine, 3-acetyl-L-phenylalanine, O-ally
- non-natural amino acidsinclude, but are not limited to, a p-acetylphenylalanine (4-acetyl phenylalanine) (including 4-acetyl-L- phenylalanine, also referred to herein as p-acetyl-L-phenylalanine (pAF)), a 4- boronophenylalanine (pBoF) (e.g., 4-borono-L-phenylalanine, a 4-propargyloxyphenylalanine (pPrF) (e.g., 4-propargyloxy-L-phenylalanine), an O-methyltyrosine (e.g., O-methyl-L-tyrosine), a 3-(2-naphthyl)alanine (NapA) (e.g., 3-(2-naphthyl)-L-alanine), a 3 -methylphenylalanine (e.g., 3-methyl-methylphen
- the one or more non-natural amino acidscan be p- acetylphenylalanine.
- the one or more non-natural amino acidscan be p-acetyl-L-phenylalanine (pAF).
- one or more non-natural amino acidsis selected from the group consisting of 4-acetyl phenylalanine, 3-O-(N-acetyl-beta-D-glucosaminyl)threonine, N4-(0-N- Acetyl-D-glucosaminyl)asparagine, O-allyltyrosine, alpha-N-acetylgalactosamine-O-serine, alpha-N-acetylgalactosamine-O-threonine, 2-aminooctanoic acid, 2-aminophenylalanine, 3- aminophenylalanine, 4-aminophenylalanine, 2-aminotyrosine, 3 -aminotyrosine, 4- azidophenylalanine, 4-benzoylphenylalanine, (2,2-bipyridin-5yl)alanine, 3-boronophenylalanine, 4-boronophenylalanine
- one or more non-natural amino acidsis selected from the group consisting of 4-acetyl-L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N- acetyl-beta-D-glucosaminyl)-L-threonine, N4-(p-N-Acetyl-D-glucosaminyl)-L-asparagine, O- allyl-L-tyrosine, alpha-N-acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L- threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino- L-phenylalanine, 2-amino-L-tyrosine, 3-amino-L-tyrosine,
- an antibody with at least one non-natural amino acidincludes at least one post-translational modification.
- the at least one post- translational modificationcomprises attachment of a molecule including but not limited to, a water-soluble polymer, a derivative of polyethylene glycol, a drug, a second protein or polypeptide or polypeptide analog, an antibody or antibody fragment, a biologically active agent, a small molecule, or any combination of the above or any other desirable compound or substance, comprising a second reactive group to at least one non-natural amino acid comprising a first reactive group utilizing chemistry methodology that is known to one of ordinary skill in the art to be suitable for the particular reactive groups.
- the first reactive groupis an alkynyl moiety (including but not limited to, the non-natural amino acid /?-propargyloxyphenylalanine, where the propargyl group is also sometimes referred to as an acetylene moiety) and the second reactive group is an azido moiety, and [3+2] cycloaddition chemistry methodologies are utilized.
- the first reactive groupis the azido moiety (including but not limited to, the non-natural amino acid /?-azido-L-phenylalanine) and the second reactive group is the alkynyl moiety.
- At least one non-natural amino acidcomprising at least one post-translational modification
- the at least one post-translational modificationcomprises a saccharide moiety.
- the post-translational modificationis made in vivo in a eukaryotic cell or in a non-eukaryotic cell.
- the post-translational modificationis made in vitro.
- the post-translational modificationis made in vitro and in vivo.
- the non-natural amino acidmay be modified to incorporate a chemical group. In some embodiments the non-natural amino acid may be modified to incorporate a ketone group.
- the one or more non-natural amino acidsmay comprise at least one oxime, carbonyl, dicarbonyl, hydroxylamine group or a combination thereof.
- the one or more non-natural amino acidsmay comprise at least one carbonyl, dicarbonyl, alkoxy-amine, hydrazine, acyclic alkene, acyclic alkyne, cyclooctyne, aryl/alkyl azide, norbomene, cyclopropene, transcyclooctene, or tetrazine functional group or a combination thereof.
- the non-natural amino acidis site-specifically incorporated into the antibody, antibody fragment or variant. In some embodiments the non- natural amino acid is site-specifically incorporated into an antibody, antibody fragment or variant.
- Methods for incorporating a non-natural amino acid into a moleculefor example, proteins, polypeptides or peptides, are disclosed in U.S. Patent Nos.: 7,332,571; 7,928,163; 7,696,312; 8,008,456; 8,048,988; 8,809,511; 8,859,802; 8,791,231; 8,476,411; or 9,637,411, (each of which is incorporated herein by reference in its entirety), and in the Examples herein.
- the one or more non-natural amino acidsmay be incorporated by methods known in the art.
- cellbased or cell-free systemsmay be used, and auxotrophic strains may also be used in place of engineered tRNA and synthetase.
- auxotrophic strainsmay also be used in place of engineered tRNA and synthetase.
- orthogonal tRNA synthetaseare used as disclosed in for example, W02002085923A2; W02002086075A2; W02004035743A2; W02007021297A1; W02006068802A2; and W02006069246A2; the contents of each of which are incorporated herein by reference in their entirety.
- Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variantmay comprise modifying one or more amino acid residues in the antibody or antibody fragment or variant. Modifying the one or more amino acid residues in the antibody or antibody fragment or variant may comprise mutating one or more nucleotides in the nucleotide sequence encoding the antibody or antibody fragment or variant. Mutating the one or more nucleotides in the nucleotide sequence encoding the antibody or antibody fragment or variant may comprise altering a codon encoding an amino acid to a nonsense codon.
- Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variantmay comprise modifying one or more amino acid residues in the antibody or antibody fragment or variant to produce one or more amber codons in the antibody or antibody fragment or variant.
- the one or more non-natural amino acidsmay be incorporated into the antibody or antibody fragment or variant in response to an amber codon.
- the one or more non- natural amino acidsmay be site-specifically incorporated into the antibody or antibody fragment or variant.
- Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variantmay comprise one or more genetically encoded non-natural amino acids with orthogonal chemical reactivity relative to the canonical twenty amino acids to site-specifically modify the biologically active molecule or targeting agent.
- Incorporating the one or more non- natural amino acidsmay comprise use of a tRNA/aminoacyl-tRNA synthetase pair to site- specifically incorporate one or more non-natural amino acids at defined sites in the biologically active molecule or targeting agent in response to one or more amber nonsense codon.
- Additional methods for incorporating non-natural amino acidsinclude, but are not limited to, methods disclosed in Chatterjee et al., A Versatile Platform for Single- and Multiple-Unnatural Amino Acid Mutagenesis in Escherichia coli, Biochemistry', 2013; Kazane et ah, J Am Chem Soc, 135(l):340-6, 2013; Kim et al., J Am Chem Soc, 134(24):9918-21, 2012; Johnson et al., Nat Chem Biol, 7(11): 779-86, 2011; and Hutchins et al., J Mol Biol, 406(4):595-603, 2011.
- the one or more non-natural amino acidsmay be produced through selective reaction of one or more natural amino acids.
- the selective reactionmay be mediated by one or more enzymes.
- the selective reaction of one or more cysteines with formylglycine generating enzyme (FGE)may produce one or more formylglycines as described in Rabuka et al., Nature Protocols 7: 1052-1067, 2012.
- FGEformylglycine generating enzyme
- the one or more non-natural amino acidsmay involve a chemical reaction to form a linker.
- the chemical reaction to form the linkermay include a bioorthogonal reaction.
- the chemical reaction to form the linkermay include click chemistry. See for example W02006/050262 incorporated herein by reference in its entirety.
- Any position of the antibody or antibody fragmentis suitable for selection to incorporate a non-natural amino acid, and selection may be based on rational design or by random selection for any or no particular desired purpose. Selection of desired sites may be based on producing a non-natural amino acid polypeptide (which may be further modified or remain unmodified) having any desired property or activity, including but not limited to a receptor binding modulators, receptor activity modulators, modulators of binding to binder partners, binding partner activity modulators, binding partner conformation modulators, dimer or multimer formation, no change to activity or property compared to the native molecule, or manipulating any physical or chemical property of the polypeptide such as solubility, aggregation, or stability.
- the sites identified as critical to biological activitymay also be good candidates for substitution with a non- natural amino acid, again depending on the desired activity sought for the polypeptide.
- Another alternativewould be to simply make serial substitutions in each position on the polypeptide chain with a non-natural amino acid and observe the effect on the activities of the polypeptide. Any means, technique, or method for selecting a position for substitution with a non-natural amino acid into any polypeptide is suitable for use in the methods, techniques and compositions described herein.
- the structure and activity of naturally-occurring mutants of a polypeptide that contain deletionscan also be examined to determine regions of the protein that are likely to be tolerant of substitution with a non-natural amino acid. Once residues that are likely to be intolerant to substitution with non-natural amino acids have been eliminated, the impact of proposed substitutions at each of the remaining positions can be examined using methods including, but not limited to, the three-dimensional structure of the relevant polypeptide, and any associated ligands or binding proteins.
- X-ray crystallographic and NMR structures of many polypeptidesare available in the Protein Data Bank (PDB, see world wide web for rcsb.org), a centralized database containing three-dimensional structural data of large molecules of proteins and nucleic acids, and can be used to identify amino acid positions that can be substituted with non-natural amino acids.
- modelsmay be made investigating the secondary and tertiary structure of polypeptides, if three-dimensional structural data is not available. Thus, the identity of amino acid positions that can be substituted with non-natural amino acids can be determined by the skilled person.
- Exemplary sites of incorporation of a non-natural amino acidinclude, but are not limited to, those that are excluded from potential receptor binding regions, or regions for binding to binding proteins or ligands may be fully or partially solvent exposed, have minimal or no hydrogen-bonding interactions with nearby residues, may be minimally exposed to nearby reactive residues, and/or may be in regions that are highly flexible as predicted by the three- dimensional crystal structure of a particular polypeptide with its associated receptor, ligand or binding proteins
- non-natural amino acidscan be substituted for, or incorporated into, a given position in a polypeptide.
- a particular non-natural amino acidmay be selected for incorporation based on an examination of the three-dimensional crystal structure of a polypeptide with its associated ligand, receptor and/or binding proteins, a preference for conservative substitutions.
- the present disclosurerelates to linkers for intracellular delivery of drug conjugates.
- Many procedures and linker molecules for attachment of various compounds to peptides or proteins, such as antibodies,are known. See, for example, U.S. Patent Nos. 4,671,958, 4,414,148, 4,699,784, 4,680,338, 4,569,789 and 10,550,190; PCT Application Publication Nos. WO2012/166559A1, WO2012/166560A1, WO2013/185117A1, WO2013188740A1,
- a linker of the present disclosurecan comprise one or more linker units.
- a linker comprising one or more linker unitscan bond to one or more drugs.
- Each linker unitcan comprise one or more moieties, each of which may occur one or more times within a given linker unit.
- a linker or linker unit of the present disclosurecan comprise one or more amino acids, wherein each amino acid is the same or different.
- a linker or linker unit of the present disclosurecan comprise one or more unsubstituted alkylene groups, wherein each said unsubstituted alkylene group can be the same or different.
- a linker of formula -alkylene-arylene-alkylene-(O-alkylene) n -, wherein each alkylene is independently selected from the group consisting of -CH2CH2-, -CH2CH2CH2- and - CH2CH2CH2CH2-,includes, but is not limited to, the following species:
- a linkeris a bivalent linker. In some embodiments, a linker is a trivalent linker. In some embodiments, a linker is a tetraval ent linker.
- a linkeror a linker unit, comprises one or more of each of the following moieties: a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, - (alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, - N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each nis independently an integer from 1 to 10.
- the peptideis a dipeptide.
- at least one moietyis not a bond.
- a linkeris not a bond.
- a linker unitis not a bond.
- the dipeptidehas the following structure:
- the N-terminus of a dipeptideis covalently joined to a moiety -
- a linker moietyhas the following structure:
- a linkercomprises one or more of each of the following moieties: a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, - (alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid or a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- a linkeror a linker unit, comprises one or more moieties selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -
- nis independently an integer from 1 to 10
- R wis independently H or Ci- Cs alkyl; or any combination of one or more of each of the foregoing.
- at least one moietyis not a bond.
- a linkeris not a bond.
- a linker unitis not a bond.
- each alkylene moiety of a linker or linker unitis independently - CH 2 -, -(CH 2 ) 2 -, -(CH 2 ) 3 -, -(CH 2 ) 4 -, -(CH 2 ) 5 - or -(CH 2 ) 6 -.
- a linker or linker unitcomprises at least one alkylene group. In some embodiments, a linker or linker unit comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, a linker or linker unit consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
- a linkeris -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10.
- mis 1, 2, 3, 4 or 5, and n is 1, 2, 3, 4 or 5.
- nis 1, 2, 3 or 4.
- mis 1, 2 or 3, and n is 1, 2 or 3.
- mis 1 and n is 3.
- a linkeris a bivalent linker.
- a bivalent linkeris selected from the group consisting of:
- a bivalent linkeris selected from the group consisting of: wherein each m is independently an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, n is 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, n is 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, n is 1, 2 or 3.
- a linkeris a trivalent linker.
- a trivalent linker of the present disclosurecomprises three linker units.
- a linkeris a trivalent linker having the following general structure: wherein LI is a first linker unit, L2 is a second linker unit and L3 is a third linker unit.
- LIis conjugated to an antibody via a linkage, and each of L2 and L3 is covalently bound to a cytotoxic moiety.
- each LI, L2 and L3independently comprises one or more moi eties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene- N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one LI, L2 or L3 is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, each n is independently an integer from 1 to 10.
- each LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O) n -, -(alkylene-N(R w ))n- unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid and a dipeptide; wherein at least one LI, L2 or L3 is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each LI, L2 and L3is independently selected from a bivalent linker or linker unit, as disclosed herein.
- a trivalent linkeris selected from the group consisting of: wherein each m is independently an integer from 1 to 10; and each n is independently an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, each n is independently 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, each n is independently 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, each n is independently 1, 2 or 3.
- a trivalent linkeris selected from the group consisting of:
- each mis independently an integer from 1 to 10; and each n is independently an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, each n is independently 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, each n is independently 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, each n is independently 1, 2 or 3.
- the linkercomprises a water-soluble polymer.
- the linkercomprises a water-soluble polymer and an amino acid, wherein the water-soluble polymer is conjugated to the amino acid.
- the water-soluble polymeris conjugated to a side chain of the amino acid.
- the water-soluble polymeris conjugated to the amino acid via a spacer element.
- a linker of the present disclosurecomprises a water-soluble polymer and an amino acid which is serine, threonine or tyrosine, wherein the water- soluble polymer is conjugated to the side chain -OH group of the serine, threonine or tyrosine.
- the water-soluble polymeris conjugated to the amino acid via a spacer element.
- a linker of the present disclosurecomprises a water-soluble polymer and an amino acid which is cysteine, wherein the water-soluble polymer is conjugated to the side chain -SH group of the cysteine. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element. In some embodiments, a linker of the present disclosure comprises a water-soluble polymer and an amino acid which is aspartic acid or glutamic acid, wherein the water-soluble polymer is conjugated to the side chain carboxylate group of the aspartic acid or glutamic acid. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element.
- a linker of the present disclosurecomprises a water-soluble polymer and an amino acid which is lysine or N E -methyl-lysine, wherein the water-soluble polymer is conjugated to the side chain -N E H(R) group of the lysine or N E -methyl-lysine, wherein R is H or methyl, respectively.
- the water-soluble polymeris conjugated to the amino acid via a spacer element.
- the water-soluble polymeris a polysaccharide.
- the water-soluble polymeris a (polyethylene)glycol (PEG) moiety.
- the PEG moietyhas a molecular weight within a range of about 100 Da to about 100,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 10,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 5,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 1,000 Da. In some other embodiments, the PEG moiety has a molecular weight within a range of about 10,000 Da to about 50,000 Da. In some embodiments, the PEG is linear. In some embodiments, the PEG is branched, multi-armed or dendritic.
- a linker of the present disclosureis connected to a drug and is also connected to a reactive moiety.
- the linkerbridges the drug to the reactive moiety to provide a drug-linker compound.
- the reactive moietycan be one that can react with another moiety of a natural amino acid or a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure.
- the reactive moietyis at least one carbonyl, dicarbonyl, ketone, acyl, -ONH2, alkoxy-amine, hydrazine, acyclic alkene, acyclic alkyne, cyclooctyne, azide, aryl azide, alkyl azide, norbomene, cyclopropene, transcyclooctene, or tetrazine functional group or a combination thereof.
- the reactive moietyis -ONH2.
- the reactive moietyis -ONH2 and the nonnatural amino acid is pAF.
- a linker of the present disclosureis connected to a drug, and is also connected to an antibody, antibody fragment or variant thereof, via a linkage.
- the linkagecan be a product of a reaction between a reactive moiety of a drug-linker compound and another moiety that is present in a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure.
- a linkerbridges a drug and the antibody, antibody fragment or variant thereof, and is covalently bonded to a non-natural amino acid of the antibody, antibody fragment or variant thereof via the linkage.
- the reactive moiety of the drug-linker compoundis -ONH2;
- the non-natural amino acid of the antibody, antibody fragment or variant thereofis para-acetyl-L-phenylalanine (pAF), which contains an acyl group, and the linkage formed between the drug-linker and the non-natural amino acid of the antibody, antibody fragment or variant thereof comprises an oxime.
- pAFpara-acetyl-L-phenylalanine
- the present disclosureprovides a drug or drug-linker.
- a drug-linker of the present disclosurecomprises a drug covalently attached to a linker.
- the drugis a cytotoxic agent or a cytotoxic moiety.
- the cytotoxic agent or moietyis an auristatin, or a derivative or analog thereof.
- a cytotoxic moiety, wherein the cytotoxic moiety is an auristatin, or a derivative or analog thereof,can be a cytotoxic moiety of Formula (A) or Formula (B), as further disclosed herein.
- a cytotoxic moiety of the present disclosureis a cytotoxic moiety of Formula (A): wherein:
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- R 5is not thiazolyl.
- R 5is COOH
- R 6is H
- R 7is Ci-Ce alkyl
- Aris phenyl.
- R 7is methyl.
- the cytotoxic moiety of Formula (A)is selected from the group consisting of: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moietyhas the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl. In some embodiments, R 7 is methyl.
- the cytotoxic moiety of Formula (A)does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl, or wherein R 7 is methyl.
- the cytotoxic moietyis less cytotoxic than monomethyl auristatin E (MMAE) in vitro.
- the cytotoxic moietyexhibits a higher in vitro half-maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE.
- the higher in vitro IC50is at least a two-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 10-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 100-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 1000-fold higher in vitro IC50.
- the microtubule inhibitor-sensitive cell lineis an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay. In some embodiments, the in vitro cytotoxicity assay is an impedance assay, a flow cytometry assay or a luminescent cell viability assay.
- the in vitro cytotoxicity assayis a luminescent cell viability assay, such as a CellTiter Gio assay. Methods of measuring in vitro cytotoxicity are disclosed herein, and are known to a person of ordinary skill in the art.
- a cytotoxic moiety of the present disclosureis a cytotoxic moiety of Formula (B):
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- R 6is H and Ar is phenyl.
- a drug-linker of the present disclosurecan be covalently bound to reactive moiety.
- a drug-linker, wherein the linker is covalently bound to reactive moietycan serve as a precursor compound, for example, for conjugation with a peptide, protein or antibody.
- the reactive moietycan be one that can react with another moiety of a natural amino acid or a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure.
- a drug-linkercomprises a reactive moiety, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O)R c , -C(O)ORa, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, R c is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6.
- a drug-linker of the present disclosureis a drug-linker of Formula (Al):
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl
- Yis a reactive moiety, optionally, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O) R q , -C(O)ORd, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, R q is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6.
- Yis -ONH2.
- R 5is not thiazolyl.
- R 5is COOH
- R 6is H
- R 7is Ci-Ce alkyl
- Aris phenyl.
- R 7is methyl.
- a drug-linker of the present disclosureis a drug-linker of Formula (Bl): wherein:
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl
- Yis a reactive moiety, optionally, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O)R q , -C(O)ORa, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, R q is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6; or Y is H.
- R 6is H and Ar is phenyl.
- the drug or drug-linkeris a drug or drug-linker generated as described in the Examples herein, or in WO2013/185117A1, the contents of which are hereby incorporated by reference in their entirety.
- drug-linker compounds disclosed in Table 2can be employed with anti-TROP2 antibodies, antibody fragments or antibody drug conjugates of the present disclosure.
- the present inventionprovides additional drug-linkers prepared using similar procedures as described herein, including the schemes disclosed in the Examples.
- Additional drug-linker compoundsare engineered by linkage of any possible linker group known in the art or elsewhere.
- the drug-linker compoundsare engineered by linkage of one or more linkers via any chemical or functional reactive positions in the drug or cytotoxic agent. Selection of the chemical or functional reactive positions in the drug for linkage to a linker is assessed as disclosed elsewhere herein, based on structure of the cytotoxic agent, and using the process known in the art or elsewhere to generate a drug-linker.
- a drug linker compound containing a reactive moietyis conjugated to an antibody or antibody fragment by reacting a drug-linker compound with an antibody or antibody fragment (or simply “antibody) containing one or more natural or nonnatural amino acid.
- the conjugation reactionprovides an ADC, wherein drug-linker is conjugated to a natural or non-natural amino acid of the antibody via a covalent linkage, wherein the covalent linkage is a product of the reactive moiety of the drug-linker and an additional moiety present in the natural or non-natural amino acid, wherein the additional moiety can react to form the covalent linkage with the reactive moiety.
- Methods of conjugating drug-linkers to antibodiesare known in the art (see, e.g., Johann, K.
- Non-limiting examples of reactions and linkages formed between drug-linker compounds and natural or nonnatural amino acids incorporated into an antibody of the present disclosureinclude the following.
- the alkynyl groupis a cyclooctynyl group.
- the non-natural amino acidis /?- azido-L-phenylalanine.
- the linkage comprising the 1,2,3-triazolyl moietyhas the following structure: wherein: each s is independently 0 or an integer from 1 to 50; optionally, each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0 or an integer from 1 to 50; optionally, each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to a linker of the drug-linker; and each wavy line ( ⁇ —) denotes connection to the antibody.
- the linkage comprising the 1,4- dihydropyridazinyl moietyhas the following structure: wherein: each R f is independently H or alkyl, optionally unsubstituted Ci-Ce alkyl; each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
- the linkage comprising the oxime moietyhas the following structure: wherein: each R q is independently unsubstituted Ci-Ce alkyl; optionally, each R q is methyl; each + denotes connection to a linker of the drug-linker; and each wavy line (- ⁇ ) denotes connection to the antibody.
- the natural amino acidis cysteine.
- the linkage comprising the a pyrrolidine-2, 5-dione moiety, such as a 3- ( I -sulfaneyl)pyrrolidine-2, 5-dione moietyhas the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
- the reactionis a peptide coupling reaction or other well-known method of forming an amide, each of which can be performed using methods readily understood by a person of ordinary skill in the art.
- the linkage comprising the amide moietyhas the following structure: wherein: each RJ is independently H or alkyl; optionally unsubstituted Ci-Ce alkyl; each + denotes connection to a linker of the drug-linker; and each wavy line ( ⁇ — ) denotes connection to the antibody.
- the natural amino acidis serine, threonine or tyrosine.
- Methods of forming such esters linkagescan be performed using methods readily understood by a person of ordinary skill in the art.
- the linkage comprising the ester moietyhas the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (- ) denotes connection to the antibody.
- a drug-linker comprising a thiol group (-SH)Reaction of a drug-linker comprising a thiol group (-SH) with a natural or nonnatural amino acid comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group, thereby providing a linkage comprising a thioester moiety; or (ii) reaction of a druglinker comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group with a natural or non-natural amino acid comprising a thiol group (-SH), thereby providing a linkage comprising a thioester moiety.
- the natural amino acidis aspartic acid or glutamic acid.
- the natural amino acidis cysteine.
- Methods of forming such thioesters linkagescan be performed using methods readily understood by a person of ordinary skill in the art.
- the linkage comprising the ester moietyhas the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
- the natural amino acidis aspartic acid or glutamic acid.
- the linkagehas the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (- ⁇ ) denotes connection to the antibody.
- a drug-linker comprising a thiol group (-SH)with a natural or non-natural amino acid comprising a thiol group, thereby providing a linkage comprising a disulfide.
- the natural amino acidis cysteine.
- Methods of forming disulfide linkagescan be performed using methods readily understood by a person of ordinary skill in the art.
- the present disclosureprovides drug moieties with linkers that reduce the toxicity of the moiety in vivo while retaining pharmacological activity.
- the toxicity of the linked drugwhen administered to an animal or human, is reduced or eliminated compared to the free toxic group or toxic group derivatives comprising labile linkages, while retaining pharmacological activity.
- increased doses of the linked cytotoxic groupmay be administered to animals or humans with greater safety.
- the nonnatural amino acid polypeptides linked to a drug moietye.g., an auristatin or an auristatin derivative or analog
- the non-natural amino acid polypeptides linked to a drug moietyare efficacious and less toxic compared to the free drug moiety.
- At least one post-translational modification at some position on the polypeptidemay occur.
- the co-translational or post-translational modificationoccurs via the cellular machinery (e.g., glycosylation, acetylation, acylation, lipid- modification, palmitoylation, palmitate addition, phosphorylation, glycolipid-linkage modification, and the like), in many instances, such cellular-machinery-based co-translational or post-translational modifications occur at the naturally occurring amino acid sites on the polypeptide, however, in certain embodiments, the cellular-machinery-based co-translational or post-translational modifications occur on the non-natural amino acid site(s) on the polypeptide.
- the post-translational modificationdoes not utilize the cellular machinery, but the functionality is instead provided by attachment of a molecule (a polymer; a water-soluble polymer; a derivative of polyethylene glycol; a second protein or polypeptide or polypeptide analog; an antibody or antibody fragment; and any combination thereof) comprising a second reactive group to the at least one non-natural amino acid comprising a first reactive group (including but not limited to, non-natural amino acid containing a ketone, aldehyde, acetal, hemiacetal, alkyne, cycloalkyne, azide, oxime, or -ONH2 functional group) utilizing chemistry methodology described herein, or others suitable for the particular reactive groups.
- a moleculea polymer; a water-soluble polymer; a derivative of polyethylene glycol; a second protein or polypeptide or polypeptide analog; an antibody or antibody fragment; and any combination thereof
- a second reactive groupcomprising a second reactive group to the at least one non
- the co-translational or post-translational modificationis made in vivo in a eukaryotic cell or in a non-eukaryotic cell.
- the post-translational modificationis made in vitro not utilizing the cellular machinery. Also included with this aspect are methods for producing, purifying, characterizing and using such a drug-linker containing at least one such co-translationally or post-translationally modified non-natural amino acids.
- reagentscapable of reacting with a drug-linker (e.g., containing a carbonyl or dicarbonyl group, alkyne, cycloalkyne, azide, -ONH2 group, or masked or protected forms thereof, or a moiety Y, as disclosed herein) that is part of a polypeptide so as to produce any of the aforementioned post-translational modifications.
- a drug-linkere.g., containing a carbonyl or dicarbonyl group, alkyne, cycloalkyne, azide, -ONH2 group, or masked or protected forms thereof, or a moiety Y, as disclosed herein
- the resulting post- translationally modified drug-linkercan contain at least one oxime group; the resulting modified oxime-containing drug-linker may undergo subsequent modification reactions.
- methods for producing, purifying, characterizing and using such reagentsthat are capable of any such post-translational modifications of such drug
- the polypeptide or non-natural amino acid linked compositionincludes at least one co-translational or post-translational modification that is made in vivo by one host cell, where the post-translational modification is not normally made by another host cell type.
- the polypeptideincludes at least one co-translational or post-translational modification that is made in vivo by a eukaryotic cell, where the co-translational or post- translational modification is not normally made by a non-eukaryotic cell.
- co- translational or post-translational modificationsexamples include, but are not limited to, glycosylation, acetylation, acylation, lipid-modification, palmitoylation, palmitate addition, phosphorylation, glycolipid-linkage modification, and the like.
- the co-translational or post- translational modificationcomprises attachment of an oligosaccharide to an asparagine by a GlcNAc-asparagine linkage (including but not limited to, where the oligosaccharide comprises (GlcNAc-Man)2-Man-GlcNAc-GlcNAc, and the like).
- the co- translational or post-translational modificationcomprises attachment of an oligosaccharide (including but not limited to, Gal-GalNAc, Gal-GlcNAc, etc.) to a serine or threonine by a GalNAc-serine, a GalNAc-threonine, a GlcNAc-serine, or a GlcNAc-threonine linkage.
- a protein or polypeptidecan comprise a secretion or localization sequence, an epitope tag, a FLAG tag, a polyhistidine tag, a GST fusion, and/or the like.
- glycosylated non-natural amino acid polypeptideis produced in a nonglycosylated form.
- Such a non-glycosylated form of a glycosylated non-natural amino acidmay be produced by methods that include chemical or enzymatic removal of oligosaccharide groups from an isolated or substantially purified or unpurified glycosylated non-natural amino acid polypeptide; production of the non-natural amino acid in a host that does not glycosylate such a non-natural amino acid polypeptide (such a host including, prokaryotes or eukaryotes engineered or mutated to not glycosylate such a polypeptide), the introduction of a glycosylation inhibitor into the cell culture medium in which such a non-natural amino acid polypeptide is being produced by a eukaryote that normally would glycosylate such a polypeptide, or a combination of any such methods.
- non-glycosylated forms of normally-glycosylated non-natural amino acid polypeptidesby normally-glycosylated is meant a polypeptide that would be glycosylated when produced under conditions in which naturally-occurring polypeptides are glycosylated).
- non-glycosylated forms of normally-glycosylated non-natural amino acid polypeptidesmay be in an unpurified form, a substantially purified form, or in an isolated form.
- incorporation of a non-natural amino acid into the antibody or antibody fragmentwill be combined with other additions, substitutions, or deletions within the polypeptide to affect other chemical, physical, pharmacologic and/or biological traits.
- the other additions, substitutions or deletionsmay increase the stability (including but not limited to, resistance to proteolytic degradation) of the polypeptide or increase affinity of the polypeptide for its appropriate receptor, ligand and/or binding proteins.
- the other additions, substitutions or deletionsmay increase the solubility (including but not limited to, when expressed in E. coli or other host cells) of the polypeptide.
- sitesare selected for substitution with a naturally encoded or non-natural amino acid in addition to another site for incorporation of a non-natural amino acid for the purpose of increasing the polypeptide solubility following expression in E. coli, or other recombinant host cells.
- the polypeptidescomprise another addition, substitution, or deletion that modulates affinity for the associated ligand, binding proteins, and/or receptor, modulates (including but not limited to, increases or decreases) receptor dimerization, stabilizes receptor dimers, modulates circulating half-life, modulates release or bio-availability, facilitates purification, or improves or alters a particular route of administration.
- non-natural amino acid polypeptidecan comprise chemical or enzyme cleavage sequences, protease cleavage sequences, reactive groups, antibodybinding domains (including but not limited to, FLAG or poly-His) or other affinity based sequences (including but not limited to, FLAG, poly-His, GST, etc.) or linked molecules (including but not limited to, biotin) that improve detection (including but not limited to, GFP), purification, transport thru tissues or cell membranes, prodrug release or activation, size reduction, or other traits of the polypeptide.
- chemical or enzyme cleavage sequencesincluding but not limited to, FLAG or poly-His
- affinity based sequencesincluding but not limited to, FLAG, poly-His, GST, etc.
- linked moleculesincluding but not limited to, biotin
- ADCsAntibody Drug Conjugates
- Antibody drug conjugates (ADCs) of the present disclosureprovide novel therapeutics or anti-cancer drugs by combining the selectivity of antibodies comprising one or more non- natural amino acids and a cytotoxic agent. Targeted cytotoxic drug delivery into tumor tissue increases the therapeutic window of these agents considerably.
- ADCs of the present disclosurecomprise of an antibody bound to a cytotoxic drug via a linker. Stability of the linker between the antibody and the cytotoxic drug is essential for the ADC integrity in circulation. The successful ADC development for a given target antigen depends on optimization of antibody selection, linker design and stability, drug potency and mode of drug and linker conjugation to the antibody. Linker properties of pH and redox sensitivities and protease susceptibility influence circulatory stability and release of the drug moiety.
- the antibody of the ADCcomprises a full length antibody or fragment thereof that binds to an antigen, and is conjugated to a cytotoxic agent or an immunosuppressive agent, wherein the antibody-drug conjugate exerts: (a) a cytotoxic or cytostatic effect on the antigen-expressing or antigen targeting cell line, or (b) a cytotoxic, cytostatic, or immunosuppressive/immune activating effect on an antigen-expressing immune cell, wherein the conjugation occurs at a non-natural amino acid in the antibody.
- the antigen, antigen-expressing cell, or antigen-targeting cell, or antigen-expressing immune cellis TR0P2.
- the antigen, antigen-expressing cell, or antigen-targeting cell, or antigen-expressing immune cellis a TROP2 antigen, or antigen-targeting cell, or antigenexpressing cell or antigen-expressing immune cell.
- the antibody of the ADCcomprises a full length antibody or fragment thereof that: binds to TROP2 and is conjugated to a cytotoxic agent or an immunosuppressive agent, wherein the antibody-drug conjugate exerts: (a) a cytotoxic or cytostatic effect on a TROP2-expressing cancer cell line, or (b) a cytotoxic, cytostatic, or immunosuppressive/immune activating effect on a TR0P2- expressing immune cell, wherein the conjugation occurs at a non-natural amino acid in the antibody.
- the antibody, variant, or composition of the present disclosuremay be an antibody, variant, or composition that binds to an antigen receptor. In other embodiments the antibody, variant, or composition may be an antibody, variant, or composition that binds to extracellular surface of an antigen receptor. In some embodiments the antibody, variant, or composition of the present disclosure may be an antibody, variant, or composition that has CDRs grafted onto the framework region of the variable region. In other embodiments the antibody, variant, or composition of the present disclosure may be an antibody, variant, or composition that has a non-natural amino acid. In some embodiments the antibody, variant, or composition may be an antibody, variant, or composition that is described by more than one of the embodiments elsewhere herein the present disclosure.
- the antibody, antibody variant or antibody composition(s) disclosed hereinmay be fully humanized. In other embodiments the antibody, antibody variant or antibody composition(s) disclosed herein may be chimeric. In some embodiments the antibody may be an antibody that is full length antibody (Variable + Fc regions), Fab, bispecific, Fab-dimers, Fab-bispecific, Fab-trispecific, bispecific T- cell engagers, dual-affinity re-targeting antibody, IgGl/IgG3 bispecific antibody, diabody, bispecific diabody, scFv-Fc, minibody. In some embodiments, the ADC comprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the antibody. The antibody comprises at least one non-natural amino acid; non-limiting examples of non-natural amino acids are disclosed herein.
- the ADCcomprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the heavy chain of the antibody. In some embodiments, the ADC comprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the light chain of the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the heavy chain of the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the light chain of the antibody.
- the ADCcomprises a full-length antibody conjugated to a drug wherein a first conjugation occurs via a non-natural amino acid in the heavy chain of the antibody, and a second conjugation occurs via a non-natural amino acid in the light chain of the antibody.
- the full-length antibodycomprises two full-length heavy chains and two full-length light chains, wherein a first pair of conjugations occur via a non-natural amino acid in each heavy chain of the antibody, and a second pair of conjugations occur via a non-natural amino acid in each light chain of the antibody.
- the drug of the ADCis a cytotoxic agent or moiety.
- the cytotoxic agent or moietyis an auristatin, or a derivative or analog thereof.
- a cytotoxic moiety, wherein the cytotoxic moiety is an auristatin, or a derivative or analog thereof,can be a cytotoxic moiety of Formula (A) or Formula (B), as disclosed herein.
- the drugis a drug generated as described in the Examples herein.
- the ADCcomprises an antibody, antibody fragment or variant thereof engineered to have one or more non-natural amino acids site specifically incorporated in a heavy chain and/or light chain amino acid sequence, wherein the antibody, antibody fragment or variant thereof is conjugated to a cytotoxic agent or cytotoxic moiety via a linker.
- an antibody-drug conjugatecomprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the amino acid sequence of the anti-TROP2 antibody comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moi eties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
- ADCantibody-drug conjugate
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein at least one of the heavy chain amino acid sequences comprises the one or more nonnatural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
- an ADCcomprising: an anti-TROP2 antibody, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one heavy chain amino acid sequence shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein: at least one of the heavy chain amino acid sequences comprises one or more non-natural amino acids; and each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
- each of the one or more linkersis not a bond.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
- At least one of the amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- the amino acid sequence of at least one of the one or more heavy chainsshares at least 90% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5.
- the one non-natural amino acid of each said heavy chainis para- acetyl-L-phenylalanine (pAF).
- the amino acid sequence of at least one of the one or more heavy chainsshares at least 90% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- the amino acid sequence of each of the one or more light chainsshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7.
- the amino acid sequence of each of the one or more light chainsshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11.
- the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: 4. In some other embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence each of the one or more light chains is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114; and
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- an anti-TROP2 antibody of the present disclosuresuch as the anti- TROP2 antibody of an ADC of the present disclosure, is humanized.
- each cytotoxic moiety of the ADCis a cytotoxic moiety of Formula
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- R 5is not thiazolyl.
- R 5is COOH. In some embodiments, R 6 is H. In some embodiments, R 7 is methyl. In some embodiments, Ar is phenyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- the cytotoxic moiety of Formula (A)does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl, or wherein R 7 is methyl.
- the cytotoxic moietyis less cytotoxic than monomethyl auristatin E (MMAE) in vitro.
- the cytotoxic moietyexhibits a higher in vitro half- maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE.
- IC50in vitro half- maximal inhibitory concentration
- the higher in vitro IC50is at least a two-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 10-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 100-fold higher in vitro IC50.
- the higher in vitro IC50is at least a 1000-fold higher in vitro IC50.
- the microtubule inhibitor-sensitive cell lineis an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay.
- the in vitro cytotoxicity assayis an impedance assay, a flow cytometry assay or a luminescent cell viability assay.
- the in vitro cytotoxicity assayis a luminescent cell viability assay, such as a CellTiter Gio assay. Methods of measuring in vitro cytotoxicity are disclosed herein, and are known to a person of ordinary skill in the art.
- each said cytotoxic moiety of the ADCis a cytotoxic moiety of Formula (B): wherein:
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- R 6is H. In some embodiments, Ar is phenyl.
- the cytotoxic moietyhas the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
- Abis an anti-TR0P2 antibody, wherein the amino acid sequence of the anti-TROP2 antibody comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L is a linker; each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker L to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- each Lis not a bond.
- dis 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, too an amino acid and a peptide; wherein at least one moiety is not a bond; each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(Rw)-, an amino acid and a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each Lcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
- Eis selected from the group consisting of:
- each R>is independently H or unsubstituted Ci-Ce alkyl; each R q is independently unsubstituted Ci-Ce alkyl; each R f is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line denotes connection to Ab.
- Ecomprises an oxime. In some embodiments, E has the following structure:
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (" «) denotes the connection to Ab.
- R qis methyl.
- each Lcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein at least one of the heavy chain amino acid sequences comprises the one or more non- natural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7.
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- the one or more heavy chainsis two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114; and
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- each light chain amino acid sequenceis SEQ ID NO: 11.
- Abis an anti-TROP2 antibody, wherein an anti-TROP2 antibody amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L1-CH(L2-)(L3-) is a linker, wherein:
- LIis a first linker unit
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- At least one of LI, L2 and L3is not a bond.
- dis 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
- each LI, L2 and L3independently comprises one or more moi eties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci- Cs alkyl; or any combination of one or more of each of the foregoing.
- each LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid and a dipeptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each LIcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
- Eis selected from the group consisting of: each R> is independently H or unsubstituted Ci-Ce alkyl; each R q is independently unsubstituted Ci-Ce alkyl; each R f is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line (— ) denotes connection to Ab.
- Ecomprises an oxime. In some embodiments, E has the following structure: /
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (— ) denotes the connection to Ab.
- R qis methyl.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein at least one heavy chain amino acid sequence comprises the one or more non-natural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one heavy chain amino acid sequence shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each light chain amino acid sequence shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences o is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non- natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
- each light chain amino acid sequenceshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains. In some embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and d is 2, and/or the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4, and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- each light chain amino acid sequenceis SEQ ID NO: 11.
- an antibody-drug conjugateof Formula (la), (lb), (Ila) or (lib) : wherein:
- Abis an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and (lb) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) or Formula (lib) is a linker, wherein:
- LIis a first linker unit
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage;
- R 5 of Formula (la) or (lb)is H, COOH, Ci-Ce alkyl or thiazolyl;
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- dis 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
- each L, LI, L2, and L3independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting no of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, - (alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- the peptideis a di
- each L, LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O) n -, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid or a dipeptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each L or each LIcomprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, each L or each LI consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
- each L or each LIis -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
- Eis selected from the group consisting of:
- Ecomprises an oxime. In some embodiments, E has the following structure:
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (" «) denotes the connection to Ab.
- R qis methyl.
- At least one of the one or more non-natural amino acidsis paraacetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, and at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
- each of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4.
- each of the light chain amino acid sequencesis SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2 and/or the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- Abis an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage;
- R 5is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- R 5is not thiazolyl.
- R 5is COOH. In some embodiments, R 6 is H. In some embodiments, R 7 is methyl. In some embodiments, Ar is phenyl
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 100; and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- the peptideis a dipeptide.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(Rw)-, an amino acid and a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each Lcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both. In some embodiments, each n each n is an integer from 1 to 10. In some embodiments, each L is -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
- Eis selected from the group consisting of wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each R q is independently unsubstituted Ci-Ce alkyl; each R f is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line (— ) denotes connection to Ab.
- Ecomprises an oxime. In some embodiments, E has the following structure: /°- +
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (" «) denotes the connection to Ab.
- R qis methyl.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, and wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each heavy chain amino acid sequence is SEQ ID NO: 5. In some embodiments, the one nonnatural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 3 or 7.
- At least one of the light chain amino acid sequenceshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequence shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 4.
- each of the light chain amino acid sequencesis SEQ ID NO: 4. In some other embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- the one or more heavy chainsis two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and d is 1, 2, 3 or 4 and/or the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- R qis methyl
- R 5is COOH
- R 6is H
- R 7is methyl
- Aris phenyl and L is -(CH2CH2)m-(CH 2 CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
- Abis an anti-TR0P2 antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage, wherein each linkage E covalently joins each linker L with one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10; or a pharmaceutically acceptable salt thereof.
- dis 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- the peptideis a dipeptide.
- each Lcomprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid or a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- Lcomprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, L is -(CEECEEjm- CEECEE-Ojn-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
- Eis selected from the group consisting of: wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each R q is independently unsubstituted Ci-Ce alkyl; each R f is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or L 1 ; and each wavy line denotes connection to Ab.
- Ecomprises an oxime.
- Ehas the following structure:
- R qis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (TM») denotes the connection to Ab.
- R qis methyl.
- R qis methyl and L is -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
- At least one of the one or more non-natural amino acidsis para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4.
- each of the light chain amino acid sequencesis SEQ ID NO: 4. In some other embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains. In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2 and/or the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- Abis the anti-TROP2 antibody; wherein the anti-TROP2 antibody comprises one or more non-natural amino acids incorporated therein, such as one or more pAF; wherein each oxime shown in Formula (Id) is a covalent linkage to one of the one or more non-natural amino acids, such as one of the one or more pAF, of the anti-TROP2 antibody Ab, and each methyl group joined to the oxime is a methyl group, such as the methyl group of the pAF; and d is 1, 2, 3, 4, 5, 6, 7 or 8; or a pharmaceutically acceptable salt thereof.
- dis 2. In some other embodiments, d is 4.
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- At least one of the heavy chain amino acid sequencesshares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the heavy chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said nonnatural amino acid is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
- At least one of the light chain amino acid sequencesshares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the at least one of the light chain amino acid sequences is SEQ ID NO: 4.
- each of the light chain amino acid sequencesis SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- the one or more heavy chainsis two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 1, 2, 3 or 4 and/or the ADC has a drug-to-antibody ratio of about 2.
- the one or more heavy chainsis two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4.
- the one non-natural amino acid of each said heavy chain and each said light chainis para-acetyl-L-phenylalanine (pAF).
- the amino acid sequence of each said light chainis the amino acid sequence of SEQ ID NO: 11.
- an ADC of the present disclosuredoes not contain a Toll-like receptor (TLR) agonist.
- TLRToll-like receptor
- an ADCis typically produced as a composition containing a population of ADCs, i.e., a mixture of ADCs that are essentially identical, except for the drug load.
- an ADC compositioncan be characterized by a drug-to-antibody ratio (DAR), which reports on the average number of drugs conjugated to antibody in the ADC composition.
- DARdrug-to-antibody ratio
- the present disclosureprovides an ADC composition comprising a mixture of ADCs, wherein each ADC in the mixture is identical, except that the number of drugs (cytotoxic moieties) or drug-linkers that are conjugated to each ADC can vary.
- an ADC of the present disclosurecomprises a first ADC, a second ADC, a third ADC and a fourth ADC, wherein the first ADC, the second ADC, the third ADC and the fourth ADC are identical, except that the first ADC comprises one drug or druglinker, the second ADC comprises two drugs or drug-linkers, the third ADC comprises three drugs or drug-linkers, and the fourth ADC comprises four drugs or drug-linkers.
- an ADC compositioncomprising:
- an ADC compositioncomprising:
- composition(d) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 4; or a combination of any two or more of the foregoing; wherein the composition is characterized as having a DAR of at least about 1 and at most about 4.
- the ADC compositionis characterized as having a DAR of at least about 2 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 3 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 1 and at most about 2. In some embodiments, the ADC composition is characterized as having a DAR of at least about 1 and at most about 3. In some embodiments, the ADC composition is characterized as having a DAR of at least about 2 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 3 and at most about 4. In some embodiments, the ADC is characterized as having a DAR of about 2. In some other embodiments, the ADC is characterized as having a DAR of about 4.
- an antibody, antibody fragment, variant or drug conjugate with increased serum half-life, water solubility, bioavailability, therapeutic half-life or circulation time, or with modulated immunogenicity, or with modulated biological activityis desired.
- One method of achieving such desired features of the compositions disclosed herein,is by covalent attachment of the polymer polyethylene glycol, (PEG).
- PEGpolymer polyethylene glycol
- the total molecular weight and hydration state of the polymer or polymers attached to the biologically active moleculemust be sufficiently high to impart the advantageous characteristics typically associated with such polymer attachment, such as increased water solubility and circulating half-life, while not adversely impacting the bioactivity of the molecule to which the PEG is attached.
- PEG derivativesare frequently linked to biologically active molecules through reactive chemical functionalities, such as amino acid residues, the N-terminus, and/or carbohydrate moieties.
- PEG derivativesare linked to biologically active molecules through reactive chemical functionalities to improve biophysical properties of the resulting ADC.
- WO99/67291discloses a process for conjugating a protein with PEG, wherein at least one amino acid residue on the protein is substituted with a synthetic amino acid and the protein is contacted with PEG under conditions sufficient to achieve conjugation to the protein.
- antibodyantibody fragments, variant or drug conjugate with increase serum half-life, water solubility, bioavailability, therapeutic half-life, or circulation time, or to modulate immunogenicity, or biological activity is desired.
- One method of achieving such desired features of the composition disclosed herein,is by covalent attachment of the polymer polyethylene glycol, (PEG).
- PEGpolymer polyethylene glycol
- the total molecular weight and hydration state of the polymer or polymers attached to the biologically active moleculemust be sufficiently high to impart the advantageous characteristics typically associated with such polymer attachment, such as increased water solubility and circulating half-life, while not adversely impacting the bioactivity of the molecule to which the PEG is attached.
- PEG derivativesare frequently linked to biologically active molecules through reactive chemical functionalities, such as amino acid residues, the N-terminus, and/or carbohydrate moieties.
- PEG derivativesare linked to biologically active molecules through reactive chemical functionalities to improve biophysical properties of the resulting ADC.
- WO99/67291discloses a process for conjugating a protein with PEG, wherein at least one amino acid residue on the protein is substituted with a synthetic amino acid and the protein is contacted with PEG under conditions sufficient to achieve conjugation to the protein.
- Proteins and other moleculesoften have a limited number of reactive sites available for polymer attachment.
- the sites most suitable for modification via polymer attachmentmay play a significant role in receptor binding, and such sites may be necessary for retention of the biological activity of the molecule therefore making them inappropriate for polymer attachment.
- indiscriminate attachment of polymer chains to such reactive sites on a biologically active moleculeoften leads to a significant reduction or even total loss of biological activity of the polymer-modified molecule
- PEG attachmentcan be directed to a particular position within a protein such that the PEG moiety does not interfere with the function of that protein.
- One method of directing PEG attachmentis to introduce a synthetic amino acid into the protein sequence.
- the protein biosynthetic machinery of the prokaryote Escherichia colican be altered in order to incorporate synthetic amino acids efficiently and with high fidelity into proteins in response to the amber codon, UAG. See, e.g., J. W. Chin et al., J. Amer. Chem. Soc. 124: 9026-9027, 2002; J. W. Chin, & P. G. Schultz, ChemBioChem 3(11): 1135-1137, 2002; J. W. Chin, et al., PNAS USA 99: 11020-11024, 2002; and, L. Wang, & P. G. Schultz, Chem. Comm., 1 : 1-11, 2002.
- a similar methodcan be accomplished with the eukaryote, Saccharomyces cerevisiae (S. cerevisiae) (e.g., J. Chin et al., Science 301 : 964-7, 2003).
- Saccharomyces cerevisiaeSaccharomyces cerevisiae
- a non-natural amino acidcan be incorporated into an antibody, variant or drug conjugate of the present disclosure, providing an attachment site for PEG. See, for example WO2010/011735 and W02005/074650.
- the present disclosureencompasses methodologies and technologies well known in the art. These include conventional methods of mass spectroscopy, NMR, HPLC, protein chemistry, biochemistry, recombinant DNA techniques and pharmacology, within the skill of the art. Compounds of the present disclosure can be synthesized using several processes or schemes employed in the art. See for example, Dubowchik et al., Bioconjugate Chem. 13: 855-869, 2002; Doronina et al., Nature Biotechnology 21(7): 778-784, 2003; WO2012/166560; WO2013/185117, each incorporated herein by reference. Many methodologies and techniques for synthesis of pharmaceutical, diagnostic or therapeutic compounds are well known to one of ordinary skill in the art.
- compositions disclosed hereinmay be used to modulate an immune response.
- Modulation of an immune responsemay comprise stimulating, activating, increasing, enhancing, or up-regulating an immune response.
- Modulation of an immune responsemay comprise suppressing, inhibiting, preventing, reducing, or downregulating an immune response.
- the ADCs of the present inventionmay be used for reducing or inhibiting tumor growth or progression in an antigen-expressing cancer or cancer cell comprising an effective amount of the ADC.
- the inventionprovides a method of treating cancer by administering to a patient a therapeutically-effective amount of an ADC of the invention comprising an antibody or antibody fragment conjugated to a drug-linker disclosed herein.
- the cancer to be treated by an ADC of the present inventionmay be, a breast cancer including triple negative breast cancer (TNBC), a brain cancer, a pancreatic cancer including pancreatic ductal adenocarcinoma, a skin cancer, a lung cancer including non-small cell lung cancer, a liver cancer, a gastric cancer, a gall bladder cancer, a colon cancer (e.g., colorectal cancer (CRC), an ovarian cancer, a prostate cancer, a uterine cancer, a bone cancer, a blood cancer or a cancer or disease or conditions related to any of these cancers.
- the canceris a solid tumor.
- the canceris a TROP2-positive cancer.
- the canceris a Dxd-resistant cancer.
- the canceris a topoisomerase 1 (TOPI) inhibitor resistant cancer.
- the inventionprovides a method of treating cancer by administering to a patient a therapeutically-effective amount of an ADC of the invention.
- the cancermay be an antigen expressing cancer.
- the cancermay be a breast cancer including, but not limited to, triplenegative breast cancer.
- the cancermay be treated by recruiting cytotoxic T cells to the antigen receptor expressing tumor cells.
- the disclosureprovides a method of treating any cancer, disease or condition associated with high expression of antigen receptors by administering to a patient a therapeutically-effective amount of an antibody or ADC of the disclosure.
- the inventionprovides a method of treating a disorder, or condition, or disease, or cancer by administering to a patient a therapeutically-effective amount of an antibody or ADC of the invention.
- the antibody, antibody fragment or variant thereofbinds to a tumor-associated antigen (TAA), wherein the TAA is TR0P2.
- TAAtumor-associated antigen
- the inventionprovides a method of treating a disorder, or condition, or disease, or cancer by administering to a patient a therapeutically-effective amount of an anti-TROP2 antibody or ADC of the invention.
- the disclosureprovides ADCs for use in treating a disease or condition in a cell expressing high TROP2 receptor number.
- the antibodies and ADCs of the disclosureare for use in treating cancer including, but not limited to, ovarian cancer ovarian cancer including, but not limited to, an epithelial, stromal and germ cell tumor.
- the ovarian cancermay comprise a fallopian tube cancer or primary peritoneal carcinoma.
- the cancermay be characterized by high expression of antigen receptors, such as ovarian cancer, for example.
- the cancermay be treated by recruiting cytotoxic T cells to high expressing antigen receptor tumor cells.
- the antibodies of the disclosureare for use in treating inherited diseases, AIDS, or diabetes but is not limited to such.
- the antibodies, compounds or composition or conjugates of the disclosurecan be used in the manufacture of a medicament for treating a disease or condition in a cell expressing high receptor number.
- the antibodies, compounds or composition or conjugates of the disclosurecan be used in the manufacture of a medicament for treating cancer including, but not limited to, breast cancer including triple negative breast cancer, ovarian cancer including, but not limited to, an epithelial, stromal and germ cell tumor.
- the antibodies of the inventioncan be used in the manufacture of a medicament for treating diseases, conditions or cancers related to or associated with expression of an antigen receptor TR0P2.
- the anti-TROP2 antibodies of the inventioncan be used in the manufacture of a medicament for treating diseases, conditions or cancers related to or associated with high TR0P2 receptor numbers.
- the condition to be treatedis a cancer.
- the cancermay be, but is non-limited to, a breast cancer including triple negative breast cancer (TNBC), a brain cancer, a pancreatic cancer including pancreatic ductal adenocarcinoma, a skin cancer, a lung cancer including non-small cell lung cancer, a liver cancer, a gastric cancer, a gall bladder cancer, a colon cancer, an ovarian cancer, a prostate cancer, a uterine cancer, a bone cancer, a solid tumor, or a blood cancer, or a cancer or disease or conditions related to any of these cancers.
- Carcinomasare cancers that begin in the epithelial cells, which are cells that cover the surface of the body, produce hormones, and make up glands.
- carcinomasinclude breast cancer, pancreatic cancer, lung cancer, colon cancer, colorectal cancer, rectal cancer, kidney cancer, bladder cancer, stomach cancer, prostate cancer, liver cancer, ovarian cancer, brain cancer, vaginal cancer, vulvar cancer, uterine cancer, oral cancer, penile cancer, testicular cancer, esophageal cancer, skin cancer, cancer of the fallopian tubes, head and neck cancer, gastrointestinal stromal cancer, adenocarcinoma, cutaneous or intraocular melanoma, cancer of the anal region, cancer of the small intestine, cancer of the endocrine system, cancer of the thyroid gland, cancer of the parathyroid gland, cancer of the adrenal gland, cancer of the urethra, cancer of the renal pelvis, cancer of the ureter, cancer of the endometrium, cancer of the cervix, cancer of the pituitary gland, neoplasms of the central nervous system (CNS), primary CNS lymphoma, brain stem cells, and others.
- the canceris a skin cancer, such as a basal cell carcinoma, squamous, melanoma, nonmelanoma, or actinic (solar) keratosis.
- the canceris any cancer with highly expressed antigen receptor numbers such as, for example, TROP2 antigen receptor numbers.
- a canceris resistant to certain treatments.
- a canceris a Dxd-resistant cancer.
- a canceris a topoisomerase 1 inhibitor-resistant cancer.
- the canceris resistant to treatment with topotecan, irinotecan or belotecan.
- compositions containing an antibody or ADC of the inventionmay be formulated at a strength effective for administration by various means to a human patient experiencing disorders that may be affected by antibody agonists or antagonists, such as but not limited to, anti-proliferatives, anti-inflammatory, or anti-virals are used, either alone or as part of a condition or disease.
- Average quantities of an antibody or ADCmay vary and in particular should be based upon the recommendations and prescription of a qualified physician.
- the exact amount of an antibody or ADCis a matter of preference subject to such factors as the exact type of condition being treated, the condition of the patient being treated, as well as the other ingredients in the composition.
- the disclosurealso provides for administration of a therapeutically effective amount of another active agent such as an anti-cancer chemotherapeutic agent or immunotherapeutic agent but is not limited to such.
- another active agentsuch as an anti-cancer chemotherapeutic agent or immunotherapeutic agent but is not limited to such.
- the amount to be givenmay be readily determined by one skilled in the art based upon therapy with the antibody or ADCs of the invention.
- the antibody, antibody fragments, variants or ADCsfurther comprise a pharmaceutical composition or formulation.
- a pharmaceutical compositioncan employ various pharmaceutically acceptable excipients, stabilizers, buffers, and other components for administration to animals. See, for example, Remington, The Science and Practice of Pharmacy, 19th ed., Gennaro, ed., Mack Publishing Co., Easton, PA, 1995. Identifying suitable composition or formulations for stability, administration to a subject, and activity varies with each compound as a number of components, (for example, purifying, stabilizing components), need to be considered.
- Suitable salts for inclusion into the composition or formulationcan include, but not limited to, sodium chloride, potassium chloride or calcium chloride.
- Buffering and/or stabilizing agentssuch as sodium acetate can be used.
- Suitable bufferscan include phosphate-citrate buffer, phosphate buffer, citrate buffer, L-histidine, L-arginine hydrochloride, bicarbonate buffer, succinate buffer, citrate buffer, and TRIS buffer, either alone or in combination.
- Surfactantscan also be employed, including polysorbates (e.g., polysorbate 80), dodecyl sulfate (SDS), lecithin either alone or in combination.
- the pharmaceutical composition or formulationcan be an aqueous composition or in the form of a reconstituted liquid composition or as a powder.
- the composition or formulationcan have a pH range from about 4.0 to about 7.0 or from about 4.5 to about 6.5 when the formulation is in a liquid form.
- the pHcan be adjusted to provide acceptable stability and administration by the skilled medical practitioner.
- compositioncan be stored in a vial or cartridge, a pen delivery device, a syringe, intravenous administration tubing or an intravenous administration bag but is not limited to such.
- a pharmaceutical composition of the inventioncan be administered as a single dose or followed by one or more subsequent dose(s) minutes, days, or weeks after the first dose. Further administrations may be contemplated as needed to treat, reduce or prevent a cancer, condition, disorder or disease.
- the antibodies, antibody fragments, variants, or ADCs of the present invention disclosuremay be used in conjunction with an additional therapy or treatment including but not limited to surgery, radiation, cryosurgery, thermotherapy, hormone treatment, chemotherapy, vaccines and other immunotherapies.
- additional treatmentcan include a therapeutic agent such as chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid or combination thereof.
- the antibodies, antibody fragments, variants, or ADCs of the inventioncan be administered with one or more immunostimulatory agents to induce or enhance an immune response.
- Immunostimulatory agentsthat can stimulate specific arms of the immune system, such as natural killer (NK) cells that mediate antibody-dependent cell cytotoxicity (ADCC).
- NKnatural killer
- ADCCantibody-dependent cell cytotoxicity
- immunostimulatory agentsinclude, but are not limited to, IL-2, immunostimulatory oligonucleotides (for example, CpG motifs), a-interferon, y-interferon, tumor necrosis factor alpha (TNFa).
- the ADCs of the inventioncan be administered with one or more immunomodulators including, but not limited to, cytokines, chemokines (including, but are not limited to, SLC5 ELC, MIP3a, MIP3 , IP-IO, MIG, and combinations thereof).
- Other therapeutic agentscan be a vaccine that immunizes a subject against an antigen.
- Such vaccinesinclude antigens, with, optionally, one or more adjuvants to induce or enhance an immune response.
- adjuvants of many kindsare well known in the art.
- chemotherapeutic agentsmay include, but are not limited to, erlotinib (TARCEVA®, Genentech/OSI Pharm.), bortezomib (VELCADE®, Millennium Pharm.), fulvestrant (FASLODEX®, AstraZeneca), sutent (SU11248, Pfizer), letrozole (FEMARA®, Novartis), imatinib mesylate (GLEEVEC®, Novartis), PTK787/ZK 222584 (Novartis), oxaliplatin (Eloxatin®, Sanofi), 5-FU (5 -fluorouracil), leucovorin, Rapamycin (Sirolimus, RAPAMUNE®, Wyeth), lapatinib (TYKERB®, GSK572016, Gla
- NMR spectral dataare collected on a 500 MHz Bruker NMR spectrometer. Chemical shifts (5) are reported in ppm and referenced off the deuterium solvent signal. Coupling constants (J) are reported in hertz (Hz). Spin multiplicities are described as: s (singlet), br (broad), d (doublet), dd (doublet of doublets), t (triplet), q (quartet) or m (multiplet).
- AHZacetohydrazide
- DIADDiisopropyl azodi carb oxy late
- DMFDimethylformamide
- HATUl-[Bis(dimethylamino)methylene]-lH- l,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
- MeOHMethanol
- TFATrifluoroacetic acid
- Example 2Protocols for Production of anti-TROP2 Antibodies Containing pAF at Heavy Chain Position 114 (Kabat numbering).
- Gibson Assembly - Anti-TROP2 light chain and heavy chain gBlockswere synthesized at Genewiz (Azanta, South Plainfield, NJ, 07080).
- the primers for amplifying various GOIs containing donor fragmentshad about 25-30 base pair (bp) overlap sequence at their 5 '-termini with the acceptor vector sequences for homologous recombination and were synthesized at Integrated DNA Technologies ((IDT), San Diego, CA)).
- the PCR fragmentswere amplified using high fidelity DNA polymerase mix, Pfu Ultra II Hotstart PCR Master Mix (Agilent Technologies, CA).
- the PCR productswere digested with Dpnl restriction enzyme (NEB) for 2 hours at 37 °C to remove plasmid background followed by column purification using Qiagen PCR column purification kit (Qiagen) and quantitated by Nanodrop (ThermoFisher).
- the acceptor vectorswere linearized by digesting with unique restriction enzymes: Hindlll and EcoRl (NEB, MA) for 4 to 5 hours at supplier’s recommended temperatures, PCR column purified and quantitated.
- the donor inserts and appropriately prepared acceptor vectorswere mixed at a 3 : 1 molar ratio, incubated at 50 °C for 15 min, using Gibson Assembly kit (NEB), and then used for transformation into E. coli NEB 5a strain (NEB).
- the recombinantswere recovered by plating transformed cells on to 2xYT+ 2% Glucose agar plates (Teknova) containing antibiotic carbenicillin, 100 pg/mL. The next day, 4 to 6 well- isolated single colonies were inoculated into 5 mL Super broth + carbenicillin 100 pg/mL (Teknova) media and grown overnight at 37 °C.
- the recombinant plasmidswere isolated using Qiagen’s plasmid DNA mini -prep kit (Qiagen) and verified by DNA sequencing (Eton Biosciences, CA). The complete GOI region plus 200 bp upstream and 200 bp downstream sequences were verified by using gene-specific sequencing primers.
- CHO cells transient transfection grade high-quality large-scale DNA (10 mg or higher)was manufactured at Aldevron (Fargo, North Dakota 58103).
- Transient expression - Platform cell linewas maintained in CD CHO Fusion (SAFC) supplemented with 8 mM L-glutamine (Gibco). Cells were passaged every 3 to 4 days at density of 0.4 million cells per ml. Cells were expanded to large scale to meet transfection volume.
- the culturewas shaking at 190 RPM, 37C, 5% CO2, 80% humidity in incubator (Kuhner) for 5 min.
- PEI Maxliquid, Polysciences
- the incubator temperaturewas shifted from 37 °C to 32 °C, and the shake speed reduced to 160 RPM.
- Additional Cell Boost 4final concentration: 2 g/L
- Cell Boost 7bfinal concentration: 0.1 g/L
- glucosefinal concentration: 2 mM
- the culture media glucose levelwas monitored using glucose meters, and additional glucose was added to the culture when the glucose level was below 2 g/L.
- Viable cell count and viabilitywere measured by Vi-Cell instrument.
- Antibody productionwas measured by Octet using Protein G sensors.
- the culturewas collected on day 7, by spinning at 4000RPM, 20min. in centrifuge. The supernatant was then filtered by 0.2 pm filtration.
- the foregoing transient expression methodcan also be used to generate light chain mutants, such as light chain having amber mutation at VI 10, Al 12 or SI 14, each of which were generated as transients.
- the expression plasmidwas linearized using Pvu I (NEB) digestion for 24 hours. After linearization, the DNA was purified using phenol:chloroform:isoamyl alcohol extraction and dissolved in endotoxin-free water at the concentration of 2.5 pg/pl. Platform cell line was maintained in EX-CELL 302 supplemented with 3 mM L-glutamine and 3 mM GlutaMAX. Cells were passaged every 3 to 4 days seeded at density of 0.3 x 10 6 /ml. One day prior to transfection, cells were seeded at 0.6 x 10 6 /ml.
- transfected cellswere counted, spun down, washed and re-suspended in selection media (50% EX-CELL 302 - 50% CD-CHO with 37.5 pM MSX (Millipore)) for Master- well generation. Limited dilution of cells was performed. Cells were then plated into 96-well flat bottom plate (Corning) at volume of 200uL and at cell density of 2500 cells/well. Five 96-well plates were plated and placed into static 37 °C, 5% CO2, high humidity incubator (Panasonic) for 3 weeks.
- Top 24 cloneswere picked and transferred from the “master” plate into 24 Well plate (Corning) by diluting 180 pL of old culture from each well into 500 pL of same selection media.
- the 24-well platewas placed into static incubator at 37 °C, 5% CO2, 80% humidity (Panasonic) for 4 days.
- Cells from each well24 well static were transferred to 24-Deep Well plate (Thomson Instrument) by dilution of 600 pL of cells into 1400 pL of fresh selection media per well.
- the platewas incubated in shaking incubator (250 RPM, 37 °C, 5% CO2, 80% humidity).
- Example 3Protocols for Production of anti-TROP2 Antibodies Containing pAF at Heavy Chain Position Al 14 (Kabat numbering) and Light Chain Position 121
- Molecular Cloning - CHO cell codon-optimized antibody heavy chain and light chain cDNA sequenceswere obtained from a commercial DNA synthesis service (Integrated DNA Technologies (IDT), San Diego, CA). The synthesized DNA fragments were digested with Hind III and EcoR I (both from New England BioLabs, (NEB), Ipswich, MA) and purified using a PCR purification kit (Qiagen, Valencia, CA). Then the digested antibody gene fragments were ligated into the expression vector via a quick ligation kit (NEB) to yield the constructs for expression of wild type antibody heavy chain and light chain. The resulting plasmids were propagated in E. coli and verified by a DNA sequencing service (Eton Biosciences, San Diego, CA).
- Transient expression - Platform cell linewas maintained in EX-CELL 302 (Sigma) supplemented with 3 mM L-glutamine (Gibco) and 3 mM GlutaMAX (Gibco). Cells were passaged every 3 to 4 days seeded at density of 0.4 million cells per ml. One day prior to transfection, cells were seeded at 0.6 million cells per ml. On day 0, cells were transfected with antibody expression plasmids encoding the light chain and heavy chain using MaxCyte electroporation platform following the instruction manual. After transfection, cells were rested in an empty 125ml shake flask and incubated at 37 °C in a static incubator for 30 min.
- Basal expression media(50% Dynamis - 50% EX-CELL 302 supplemented with 3 mM L-glutamine and 3 mM GlutaMAX) was added to the transfected cells in the shake flask for a final density of 3 x 10 6 /ml.
- the transfected cellswere incubated at 37 °C, 5% CO2 on orbital shaker set to 155 rpm.
- Viable cell count and viabilitywere measured by Vi-Cell instrument.
- Antibody productionwas measured by Octet using Protein G sensors.
- Stable minipool (master well) generationThe expression plasmids (HA114 and LC121) were linearized using Pvu I (NEB) digestion for 4 hours. After linearization, the DNA was purified using phenol:chloroform:isoamyl alcohol extraction and dissolved in endotoxin-free water at the concentration of 1 pg/pl. Platform cell line was maintained in EX-CELL 302 supplemented with
- 3 mM L-glutamine and 3 mM GlutaMAXCells were passaged every 3 to 4 days seeded at density of 0.3 x 10 6 /ml. Two days prior to transfection, cells were seeded at 0.4 x 10 6 /ml. On day 0, 3 x 10 6 cells were transfected with 6 pg of linearized antibody expression plasmids (heavy chain 3 pg + light chain 3 pg) using AMAXA electroporation system following instruction manual. There were 8 reactions of AMAXA transfection executed using total 24 x 10 6 cells and 48pg plasmid DNAs.
- transfected cellswere rested in a T-225 flask in volume of 48ml fresh culture media and incubated in 37 °C static incubator for overnight. On day one, transfected cells were counted, spun down, washed and re-suspended in selection media (50% EX-CELL 302 - 50% CD-CHO with 25 pM MSX), then seeded in 40x 96 well plates at 2500 cell/well/200pl and cultured for three weeks.
- selection media50% EX-CELL 302 - 50% CD-CHO with 25 pM MSX
- Fed-batch expression - Previously generated antibody stable mini-pools(master well) were inoculated into basal expression media (50% Dynamis - 50% EX-CELL 302 supplemented with lx GS, 25 pM MSX, insulin 2 pg/ml, ornithine 0.5 mM, glucose 2 g/L and lx anti-clumping agent) at density of 0.5 x 10 6 /ml in a shake flask on day 0. The cells were incubated at 37 °C, 5% CO2 on orbital shaker set to 150 rpm.
- basal expression media50% Dynamis - 50% EX-CELL 302 supplemented with lx GS, 25 pM MSX, insulin 2 pg/ml, ornithine 0.5 mM, glucose 2 g/L and lx anti-clumping agent
- Clarified Cell culture media containing the target antibody containing non-natural amino acid pAFwas loaded over a protein A ProSep Ultra column (EMD Millipore) equilibrated in 20 mM sodium phosphate, lOOmM sodium chloride, pH 7.5. After loading, the column was washed with buffer A (20 mM sodium phosphate, lOOmM sodium chloride, pH 7.5) followed by wash buffer B (5 mM succinic acid, pH 5.8) to remove host cell contaminants. The target antibody was eluted from the column with elution buffer C (50 mM glycine, 10 mM succinic acid, pH 3.2). The target antibody was pooled, and pH adjusted to pH 5.0 with 2.0 M tris base.
- EMD Milliporeprotein A ProSep Ultra column equilibrated in 20 mM sodium phosphate, lOOmM sodium chloride, pH 7.5. After loading, the column was washed with buffer A (20 mM sodium phosphate, l
- the target antibodywas further purified by loading the conditioned protein A pool over a Capto SP Impres column (GE Healthcare) equilibrated in 30 mM sodium acetate, pH 5.0.
- the target antibodywas eluted from the column with a linear gradient to 100% buffer B (30 mM sodium acetate, 0.5 M sodium chloride, pH 5.0) and fractions containing monomeric antibody were pooled, 0.22 pM filtered, and stored at ⁇ 65 °C until further use.
- TROP2 cell surface levelswere quantified using the Dako QIFIKIT (Agilent, Santa Clara, CA, according to manufacturer’s instruction. Briefly, cells were harvested using StemPro Acccutase cell dissociation reagent, washed with FACS buffer (lx PBS containing 0.2% BSA and 15 mM NaNs) and incubated with mouse anti-TROP2 antibody (clone MR54, eBioScience, San Diego, CA) or isotype control antibody (mouse IgG2a, kappa, eBioScience). After washing, FITC conjugated anti-mouse secondary antibody provided by kit was incubated with cells, set-up beads, and calibration beads. Washed cells and beads were run on FACSCantoII. Lot specific calibration beads were used to generate a standard curve to calculate TROP2 number on the surface of each cell line. TROP2 cell surface numbers were determined as shown in Table 4.
- TR0P2 multi -concentration binding kinetic experimentswere performed on an Octet RED96 (Sartorius) instrument at 30°C.
- Anti-human Fc capture biosensors(Sartorius, cat# 18- 5063) were loaded with purified anti-TROP2 mAb in IX HBS-P+ Buffer (Cytiva, cat# BR-1008- 27). Immobilization levels between 0.8 to 1.0 nm were reached.
- the loaded biosensorswere washed with IX HBS-P+ Buffer to remove any unbound mAb before measuring association and dissociation kinetics.
- TROP2 analyte sampleshuman, cyno, or rat
- IX HBS-P+ Bufferconcentrations ranging from 100 nM to 1.25 nM and transferred to solid-bottom, black 96 well plates (Greiner Bio-One, cat# 655209).
- TROP2 sampleswere allowed to bind to anti-TROP2 mAb loaded biosensors for 90 seconds.
- the dissociation phasewas recorded in wells of a solid black 96-well plate containing IX HBS-P+ Buffer for 300 seconds.
- Anti-TROP2 antibodies containing non-naturally encoded amino acid para-acetyl-L- phenylalanine (pAF) at specified locations in the heavy chain and/or light chainwere buffer exchanged into 50 mM sodium acetate, pH 4.0-4.3, and concentrated to 5-25 mg/mL. 1.5 M acetohydrazide (AHZ), pH 4.0, was added to the concentrated antibody at a final concentration of 100 mM.
- a stock solution of drug-linker compound 6 in water for injection (WFI) or DMSOwas then added to the anti-TROP2 mAb solution at 8-15 molar equivalents drug-linker to mAb. The conjugation reactions were allowed to react for 16-72 hours at 30 °C.
- the antibody conjugateswere purified over a Capto SP Impres column (Cytiva) to remove excess reagents.
- ADCswere then formulated into 50 mM histidine, 2.5% trehalose, pH 6.0 and 0.22 pm filtered.
- Reverse Phase (RP) chromatographywas used to monitor the final drug-to-antibody ratio (DAR) under reducing conditions.
- RP-HPLC analysiswas performed on an Agilent 1200 series HPLC system using an Agilent Stablebond SB-C8, 5 pm, 4.6 x 150 mm column.
- Mobile phase Aconsisted of 0.1% TFA in water and mobile phase B consisted of 0.1% TFA in acetonitrile.
- FIG. 1A representative HPLC chromatogram under reducing conditions (FIG. 1) shows light chain eluting prior to unconjugated heavy chain, followed by conjugated heavy chain with drug-linker compound 6.
- anti-TROP2- HC114pAF-compound-6 ADCalso referred to herein as “TROP2-AS269”.
- Example 8In vitro Cytotoxicity of Anti-TROP2 ADC in TROP2-expressing Cell Lines and a TROP2-negative Cell Line
- Cytotoxicity of anti-TROP2-HCl 14pAF-compound-6 ADCwas tested in multiple TROP2-expressing cell lines and a TROP2 negative cell line with 4 days or 7 days of treatment. Potency of anti-TROP2-HCl 14pAF-compound-6 ADC after 4 days of treatment was similar to that after 7 days of treatment in TROP2-expressing BxPC- 3, MDA-MB-468, or HCC1806 cell lines and Emax (%) was improved after 7 days of treatment (Tables 5 and 6).
- Anti-TROP2-HC114pAF-compound-6 ADCexhibited IC50 values of 0.06-0.25 nM in TR0P2-expressing cell lines and was more potent than Benchmark-DXd ADC at day 7, but no cell killing was observed in TROP2-negative Calu-6 cell line (FIG. 3).
- Example 9In vitro Cytotoxicity of anti-TROP2 ADCs in Benchmark-DXd-resistant Cell Line and Benchmark-DXd-insensitive Cell Line.
- Benchmark-DXd resistant cellswere generation as follows. MDA-MB-468 tumor was collected and isolated from the mice that were treated with anti-TROP2-DXd ADC (also referred to herein as “Benchmark-DXd”). Cells were expanded and cultured in RPML1640 media containing 10% FBS and 1% penicillin/streptomycin, and eventually cultured in RPML1640 media containing 10% FBS, 1% penicillin/streptomycin, and 10 nM Benchmark-DXd for 12 days. Cytotoxicity was evaluated 20 days after Benchmark-DXd removal.
- Tumor cell line cytotoxicitywas assessed as follows. Cells were seeded at 2,500 cells/well into 96-well clear bottom white plate and treated the next day with serially diluted TROP2-AS269, or Benchmark-DXd. The relative viability of the cells was measured using CellTiter-Glo2.0 Reagent 4 days after TROP2-AS269 treatment and 7 days after Benchmark-DXd treatment.
- FIG. 5shows that TROP2-AS269 exhibited cytotoxicity against the Benchmark-DXd insensitive JIMT-1 cell line. While Benchmark-DXd was not potent enough to kill JIMT-1 cells with 7 days of treatment, TROP2-AS269 showed IC50 value of 0.035 nM with >90% of cell killing at a single-digit nM treatment for 4 days.
- Example 10In vitro Cytotoxicity of Anti-TROP2 ADCs in Human Keratinocytes.
- Cytotoxicity in human keratinocyteswas assessed as follows. Cells were seeded at 2,500 cells/well into 96-well clear bottom white plate and treated the next day with serially diluted TROP2-AS269 or an anti-TROP2-auristatin-0101 ADC (also referred to herein as “Benchmark- AurOlOl”). The relative viability of the cells was measured using CellTiter-Glo2.0 Reagent 4 days after TROP2-AS269 or Benchmark- AurOlOl treatment. The results are shown in FIG. 6. TROP2- AS269 exhibited reduced in vitro human keratinocyte cytotoxicity compared to Benchmark- AurOlOl.
- Example 11Anti -tumor Activity of Anti-TROP2 ADCs.
- Human pancreatic BxPC-3 cancer cell linewas cultured in RPMH640 supplemented with 10% FBS and 1% penicillin/streptomycin. 5xl0 6 BxPC-3 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female Nu/Nu mice (Charles River Laboratories). When tumor volume reached approximately 100 mm 3 , mice were randomized and grouped for treatment.
- the single intravenous injectionwas done with vehicle controls, 0.1 mg/kg, 0.3 mg/kg, or 1 mg/kg of anti-TROP2-HCl 14pAF-compound-6 (“TROP2-AS269”) or 1 mg/kg or 3 mg/kg of anti-TROP2-DXd ADC (“Benchmark-DXd”), and tumor size was monitored up to 44 days after treatment. Tumor sizes were measured by calipers and tumor volume was calculated as length x width x width x 0.5. Percent tumor growth inhibition (TGI) was calculated as (1-tumor volume of treated/tumor volume of vehicle control) xlOO. All TROP2-AS269 treated groups reduced BxPC-3 tumor growth with as low as 0.1 mg/kg single administration.
- TGIPercent tumor growth inhibition
- TROP2-AS269 treated groupsreached > 80% of TGI as early as 2 weeks after treatment.
- 0.3 mg/kg of TROP2-AS269 treatmentshowed better TGI than 3 mg/kg of Benchmark-DXd, suggesting that TROP2-AS269 displays about 10-fold increased potency than Benchmark-DXd (FIG. 7).
- there was no sign of toxicitysuch as body weight loss at the single injection of up to 10 mg/kg of TROP2-AS269 (data not shown).
- TROP2 mAb and TROP2-AS269were compared in TROP2-expressing MDA-MB-468 cells.
- Cellswere incubated with serially diluted TROP2 mAb or TROP2-AS269 for 30 min at 4 °C and detected with Alexa Fluor 647 conjugated anti-human F(ab')2 secondary antibody.
- TROP2 mAb and TROP2-AS269showed comparable binding affinities to MDA-MB-468 cells with Kd values of 3.3 and 3.4 nM, respectively.
- ADCCAntibody-dependent cell-mediated cytotoxicity
- TROP2-AS269anti- TROP2-HC114pAF-compound-6
- Benchmark- DXdAnti-TROP2-DXd ADC
- MDA- MD-468 cellswere seeded at 5,000 cells/well in a 96-well white plate. The next day, media was changed to 4% low IgG containing RPMI1640 media and cells were treated with serially diluted TROP2 AS269 or Benchmark-DXd and freshly thawed ADCC Bioassay effector cells.
- Example 14Anti-tumor Activity of Anti-TROP2 ADCs.
- JIMT-1Human breast cancer cell line, JIMT-1, was cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. 5* 10 6 JIMT-1 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female SCID-beige mice (Charles River Laboratories). When tumor volume reached approximately 100 mm 3 , mice were randomized and grouped for treatment.
- HCC827Human lung cancer cell line, HCC827, was cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin/ streptomycin. 5* 10 6 HCC827 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female SCID-beige mice (Charles River Laboratories). When tumor volume reached approximately 100 mm 3 , mice were randomized and grouped for treatment.
- TGIPercent tumor growth inhibition
- TROP2 mAb or TROP2-AS269were administered intravenously to CD-I mice (Charles River Laboratories) at a dose of 3 mg/kg. Whole-blood samples were collected at each time point (0, 1, 2, 6, 24, 48, 72, 96, 168, 336, 504, and 672 hours) and diluted in Casein-PBS blocker. For the total antibody assay, TROP2 mAb or TROP2-AS269 was captured using goat anti-human Fc pAb and detected with a biotinylated anti-human kappa pAb and Streptavidin SULFO-TAG (Meso Scale discovery).
- ADC assayutilized a proprietary anti-AS269 antibody (AMB-20) as a capture antibody, a biotinylated anti-AS269 antibody, and streptavidin SULFO- TAG for detection.
- AMB-20proprietary anti-AS269 antibody
- biotinylated anti-AS269 antibodya biotinylated anti-AS269 antibody
- streptavidin SULFO- TAGstreptavidin SULFO- TAG
- TROP2-AS269The total antibody concentration and the intact ADC concentration from TROP2-AS269 were comparable throughout the time course, similar to the total antibody concentration for unconjugated TROP2 mAb. This indicates that TROP2-AS269 is highly stable in circulation.
- the exposure (AUC )showed values of 4,439 ug*h/mL for TROP2 mAb, 4,867 ug*h/mL for TROP2-AS269 total antibody, and 4,554 ug*h/mL for TROP2-AS269 intact ADC. Furthermore, based on the total antibody assay, the half-lives of TROP2 mAb and TROP2-AS269 were 455 and 479 hours, respectively.
- An antibody-drug conjugatecomprising: an anti-trophoblast antigen 2 (anti-TR0P2) antibody comprising an amino acid sequence, wherein the amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
- ADCanti-trophoblast antigen 2
- ADC of embodiment Alwherein the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 1, 2, 5 or 6.
- ADC of embodiment A2, wherein at least one of the one or more heavy chainshas the amino acid sequence of SEQ ID NO: 5.
- the ADC of embodiment Al, A2 or A3, wherein the anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence; wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17; or wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10 or 11.
- A5The ADC of embodiment A4, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4.
- each said cytotoxic moietyis a cytotoxic moiety of Formula (A) or (B): wherein:
- R 5 of Formula (A)is H, COOH, Ci-Ce alkyl or thiazolyl
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
- each said cytotoxic moietyis a cytotoxic moiety of Formula (A).
- A8The ADC of embodiment A7, wherein the cytotoxic moiety of Formula (A) is selected from the group consisting of: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- ADC of embodiment A8, wherein the cytotoxic moietyhas the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is H or methyl.
- each said cytotoxic moietyis a cytotoxic moiety of Formula (B).
- A12The ADC of embodiment Al l, wherein the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
- A13The ADC of any one of embodiments Al to A12, wherein the ADC is an ADC of Formula
- A14The ADC of any one of embodiments Al to A12, wherein the ADC is an ADC of Formula
- each L1-CH(L2-)(L3-)is one of the one or more linkers, wherein:
- LIis a first linker unit
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- Al 7The ADC of any one of embodiments Al 3 to Al 6, wherein E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4- dihydropyridazine.
- Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4- dihydropyridazine.
- each R>is independently H or unsubstituted Ci-Ce alkyl
- each R qis independently unsubstituted Ci-Ce alkyl
- each R fis independently H or unsubstituted Ci-Ce alkyl
- each sis independently 0, 1, 2, 3, 4, 5 or 6
- each tis independently 0, 1, 2, 3, 4, 5 or 6
- each +denotes connection to L or L 1
- each wavy linedenotes connection to Ab.
- each L, LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more moieties, wherein each L is not a bond, at
- each L, LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O) n -, -(alkylene-N(R w ))n- unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid and a dipeptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each L or LIcomprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10; or each L or LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
- A22The ADC of any one of embodiments A13 to A21, wherein the ADC is an ADC of Formula (I).
- ADC of any one of embodiments Al to A22, wherein each of the one or more nonnatural amino acidsis para-acetyl-L-phenylalanine (pAF).
- each non-natural amino acidis para-acetyl-L- phenylalanine (pAF), and R q is methyl, wherein said methyl is the pAF methyl group.
- pAFpara-acetyl-L- phenylalanine
- A27The ADC of any one of embodiments Al to A26, wherein the anti-TROP2 antibody is humanized.
- A28.The ADC of any one of embodiments A4 to A27, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
- A29The ADC of embodiment A28, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- ADC of any one of embodiments A4 to A27wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 3; and the ADC has a drug-to-antibody ratio of about 2.
- A31The ADC of embodiment A30, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
- pAFpara-acetyl-L-phenylalanine
- A32The ADC of any one of embodiments A4 to A27 , wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 7, wherein SEQ ID NO: 7 is characterized as containing one non-natural amino acid at position 121; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
- each said non-natural amino acidis para-acetyl-L- phenylalanine (pAF).
- A34The ADC of any one of embodiments A4 to A27 , wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
- SEQ ID NO: 8is characterized as containing one non-natural amino acid at position 110;
- SEQ ID NO: 9is characterized as containing one non-natural amino acid at position 112;
- SEQ ID NO: 10is characterized as containing one non-natural amino acid at position 114;
- SEQ ID NO: 11is characterized as containing one non-natural amino acid at position 121; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
- each said non-natural amino acidis para-acetyl-L- phenylalanine (pAF).
- A36The ADC of any one of embodiments Al to A35, wherein the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R 7 is methyl.
- Abis the anti-TROP2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
- ADCantibody-drug conjugate of Formula (la), (lb), (Ila) or (lib):
- Abis an anti-trophoblast antigen 2 (anti-TR0P2) antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and (lb) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) or Formula (lib) is a linker, wherein:
- LIis a first linker unit
- L2is a second linker unit
- L3is a third linker unit; and each E is a linkage;
- R 5 of Formula (la) or (lb)is H, COOH, Ci-Ce alkyl or thiazolyl;
- R 6is H or OH
- R 7is H or Ci-Ce alkyl
- Aris phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
- anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- anti-TROP2 antibodycomprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- B10The ADC of embodiment B7, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5.
- BlThe ADC of embodiment B7, wherein the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5.
- Bl 8The ADC of embodiment Bl 6, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7.
- ADC of embodiment B20, wherein the amino acid sequence of at least one of the one or more light chainsis the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11, or is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17.
- each of the one or more heavy chainscomprises at least one of the one or more non-natural amino acids.
- each Ecomprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4- dihydropyridazine.
- each RJis independently H or unsubstituted Ci-Ce alkyl
- each R qis independently unsubstituted Ci-Ce alkyl
- each R fis independently H or unsubstituted Ci-Ce alkyl
- each sis independently 0, 1, 2, 3, 4, 5 or 6
- each tis independently 0, 1, 2, 3, 4, 5 or 6
- each +denotes connection to L or LI
- each wavy line (- ⁇ )denotes connection to Ab.
- each L, LI, L2, and L3independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each R w is independently H or Ci-Cs alkyl; or any combination of one
- each L, LI, L2 and L3independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(R w ))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(R W )-, an amino acid or a dipeptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each R w is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
- each L or each LIcomprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both.
- each L or each LIconsists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
- each L or each LIis -(CH2CH2) m -(CH2CH2-O) n -; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10.
- each of the one or more nonnatural amino acidsis selected from the group consisting of para-acetyl phenylalanine, 4-acetyl- L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N-acetyl-beta-D-glucosaminyl)-L- threonine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine, O-allyl-L-tyrosine, alpha-N- acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L-threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino-L-
- ADC of any one of embodiments Bl to B49, wherein at least one of the one or more non-natural amino acidsis para-acetyl-L-phenylalanine (pAF).
- each Ecomprises an oxime.
- each Ehas the following structure: wherein R q is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (• ⁇ ') denotes the connection to Ab.
- each non-natural amino acidis para-acetyl-L- phenylalanine (pAF), and R q is methyl, wherein said methyl is the pAF methyl group.
- pAFpara-acetyl-L- phenylalanine
- the amino acid sequence of each of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
- each of the one or more non-natural amino acidsis para-acetyl-L-phenylalanine (pAF).
- Rqis unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (“") denotes the connection to Ab.
- B59.The ADC of any one of embodiments B55 to B58, wherein the ADC is an ADC of Formula (la).
- Abis an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage, wherein each linkage E covalently joins each linker L with one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10; or a pharmaceutically acceptable salt thereof.
- anti-TROP2anti-trophoblast antigen 2
- the anti-TROP2 antibodycomprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
- ADC of embodiment Cl or C2wherein the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
- ADC of any one of embodiments C2 to C5, wherein the amino acid sequence of at least one of the one or more heavy chainsis selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. C7.
- ADC of embodiment C7, wherein the amino acid sequence of at least one of the one or more heavy chainsis the amino acid sequence of SEQ ID NO: 5.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
TROP2-DIRECTED ANTIBODY-DRUG CONJUGATES AND USES THEREOF
CROSS- REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Patent Application No. 63/486,493, filed February 23, 2023, which is entirely incorporated herein by reference.
REFERENCE TO A SEQUENCE LISTING
The instant application contains a Sequence Listing which has been submitted electronically in XML format and is hereby incorporated by reference in its entirety. Said XML copy, created on February 21, 2024, is named AMBX_0246_00_PCT.xml and is 25,192 bytes in size.
FIELD OF THE INVENTION
This invention relates to anti-TROP2 antibodies and antibody-drug conjugates (ADCs) comprising at least one non-naturally-encoded amino acid. Disclosed herein are anti-TROP2 antibodies with one or more non-naturally encoded amino acids, and further disclosed are antibody drug conjugates wherein the anti-TROP2 antibodies of the invention are conjugated to one or more cytotoxic drug-linker moieties. Further disclosed are methods for using such non-natural amino acid antibody drug conjugates, including therapeutic uses for treating diseases such as cancer.
BACKGROUND
Antibody-based therapeutics have emerged as important components of therapies for an increasing number of human malignancies in such fields as oncology, immunology, inflammatory and infectious diseases. In most cases, the basis of the therapeutic function is the high degree of specificity and affinity the antibody-based drug has for its target antigen. Arming monoclonal antibodies with drugs, toxins, or radionuclides is yet another strategy by which monoclonal antibodies may induce therapeutic effect. By combining the exquisite targeting specificity of antibody with the tumor killing power of toxic effector molecules, immunoconjugates permit sensitive discrimination between target and normal tissue thereby resulting in fewer side effects than most conventional chemotherapeutic drugs. The toxins utilized can specifically, stably and irreversibly conjugate to unique sites in the antibody. This unique process of conjugation allows for the precise control of the location of the toxin on the antibody, and also the number of toxins conjugated to each antibody. Both of these features are critical for controlling biophysical characteristics and toxicities associated with ADCs. Trophoblast cell surface protein 2 (also known as: trophoblast antigen 2; calcium signal transducer 2; TR0P2; TROP-2; TACSTD2; GA733-1; or M1S1) is a transmembrane protein that is highly expressed on various epithelial tumors. While the physiological role of TR0P2 remains under investigation, it has been shown to be involved in pathways associated with the proliferation, migration and invasion of cancer cells, including MAPK and PI3K/AKT (Cubas R. et al., Mol Cancer, 9:253, 2010; Guan H. et al., BMC Cancer, 17:486, 2017; Guerra E. et al., Clin Cancer Res, 22:4197-205, 2016). Moreover, TROP2 overexpression has been associated with enhanced tumor aggressiveness, metastasis, drug resistance, increased tumor cell survival, reduced overall survival (OS) and reduced progression-free survival. TROP2 is highly expressed in triple negative breast cancer (TNBC), pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC). Overexpression of TROP2 has been correlated with poor prognosis in cancers including breast cancer and NSCLC (Lin H. et al., Exp Mol Pathol, 94:73-78, 2013; Kobayashi H. et al., Virchows Arch, 457:69-76, 2010; Muhlmann G. et al., J Clin Pathol, 62: 152-158, 2009; Fong D. et al., Mod Pathol, 21 : 186-191, 2008). The foregoing features make TROP2 an important target for the development anti-cancer therapeutics. Challenges towards this end include the known expression of TROP2 in some normal epithelial tissues, including the skin and esophagus (Stepan L.P. et al., J Histochem Cytochem, 59:701-710, 2011). Thus, the potential for on-target toxicity in normal cells that express TROP2 must be factored into the design and development of TROP2-directed therapeutics.
TROP2-directed ADCs are known in the art. PF-06664178 (also known as RN927C; discontinued) contains anti-TROP2 antibody conjugated with tubulin inhibitor AurOlOl via a cleavable linker and has a drug-to-antibody ratio of 2. PF-06664178 induced skin rash and mucosal inflammation as dose-limiting toxicities in phase I study in adult patients with advanced solid tumors (King, G.T., Invest New Drugs, 36:836-847, 2018). Datopotamab deruxtecan (Dato- DXd, DS- 1062a) contains anti-TROP2 antibody conjugated with topoisomerase I inhibitor DXd via a cleavable linker and has a drug-to-antibody ratio of 4 (Okajima, D. et al., Mol Cancer Ther, 20:2329-2340, 2021). TRODELVY (sacituzumab govitecan) is another TROP2-directed ADC and contains antibody hzRS7 (sacituzumab) conjugated with topoisomerase I inhibitor SN-38 via a hydrolyzable linker.
There remains a need for ADCs with improved properties for the treatment of cancer, including TROP2-positive cancers.
SUMMARY OF THE INVENTION The present invention provides novel TROP2-directed antibody-drug conjugates (ADCs) and uses thereof, including the use of TROP2-directed ADCs for the treatment of diseases such as cancer.
In some general aspects, there is provided an antibody-drug conjugate (ADC) comprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising an anti-TROP2 antibody amino acid sequence, wherein the anti-TROP2 antibody amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
In some embodiments, the amino acid sequence is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, the one non-natural amino acid at position 114 is para-acetyl-L-phenylalanine (pAF).
In some other general aspects, there is provided an antibody-drug conjugate (ADC) comprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein: at least one of the one or more heavy chains comprises one or more non-natural amino acids; and each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 5; wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 5.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence; wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one or more light chains is SEQ ID NO: 4.
In some embodiments, each cytotoxic moiety is a cytotoxic moiety of Formula (A) or Formula (B): or a pharmaceutically acceptable salt thereof; wherein:
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is independently H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and
# represents a connection to one of the one or more linkers.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH. In some embodiments, R6 is H. In some embodiments, R7 is methyl. In some embodiments, Ar is phenyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some other embodiments, the cytotoxic moiety of Formula (A) does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is methyl.
In some embodiments, the cytotoxic moiety is less cytotoxic than monomethyl auristatin E (MMAE) in vitro. In some embodiments, the cytotoxic moiety exhibits a higher in vitro half- maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE. In some embodiments, the higher in vitro IC50 is at least a two-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 10-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 100-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 1000-fold higher in vitro IC50. In some embodiments, the microtubule inhibitor-sensitive cell line is an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay.
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
In some embodiments, R6 is H. In some embodiments, Ar is phenyl.
In some embodiments, the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
In some other general aspects, there is provided an ADC of Formula (I) or Formula (II), as disclosed herein.
Ab is an anti-TROP2 antibody comprising an amino acid sequence, wherein the amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L1-CH(L2-)(L3-) is a linker, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, at least one of LI, L2 and L3 is not a bond.
In some embodiments, the amino acid sequence is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
In some embodiments, E comprises an oxime. In some embodiments, E has the following structure: /°-+
/— N
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line ("«') denotes the connection to Ab.
In some embodiments, each LI comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L- phenylalanine (pAF).
In some other aspects, the ADC is an ADC of Formula (I): or a pharmaceutically acceptable salt thereof; wherein:
Ab is the anti-TROP2 antibody; each L is independently one linker of the one or more linkers; each E is independently a linkage covalently joining the one linker L to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; each Drug is a cytotoxic moiety of Formula (A) or Formula (B); and d is an integer from 1 to 100. In some embodiments, each L is not a bond.
In some embodiments, the amino acid sequence is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
In some embodiments, E comprises an oxime. In some embodiments, E has the following structure:
Z - N /0-+
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (— ) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, each L comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L- phenylalanine (pAF).
In some embodiments, an anti-TROP2 antibody comprises one or more heavy chains. In some embodiments, the one or more heavy chains is two heavy chains. In some embodiments, the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
In some embodiments, an anti-TROP2 antibody comprises one or more light chains. In some embodiments, the one or more light chains is two light chains. In some embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4.
In some embodiments, an anti-TROP2 antibody comprises two heavy chains and two light chains, wherein: the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2. In some embodiments, the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some other general aspects, there is provided an antibody-drug conjugate (ADC) of Formula (la), (Ib), (Ila) or (lib) : or a pharmaceutically acceptable salt thereof; wherein:
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and Formula (Ib) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) and Formula (lib) is a linker, wherein: LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage covalently joining L or LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab;
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, the one non-natural amino acid at position 114 is para-acetyl-L- phenylalanine (pAF). In some embodiments, the one or more heavy chains is two heavy chains.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
In some embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
Ab is an anti-trophoblast antigen 2 (anti-TR0P2) antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage;
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein at least one of the one or more non-natural amino acids is incorporated into at least one of the one or more heavy chains at position Al 14 based on Kabat numbering. In some embodiments, the one or more heavy chains is two heavy chains, and wherein one non-natural amino acid is incorporated into each heavy chain at position Al 14 based on Kabat numbering.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH. In some embodiments, R6 is H. In some embodiments, R7 is methyl. In some embodiments, Ar is phenyl
In some embodiments, each L comprises at least one unsubstituted alkylene, at least one - (alkylene-O)n-, or both. In some embodiments, each L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, each L is -(CH2CH2)m- (CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF). In some embodiments, each E comprises an oxime. In some embodiments, each E has the following structure:
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (™») denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the one or more heavy chains is two heavy chains, the one or more light chains is two light chains, the one or more non-natural amino acids is two non-natural amino acids. In some embodiments, d is 2.
In some embodiments, Rq is methyl, R5 is COOH, R6 is H, R7 is methyl, Ar is phenyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
In some general aspects, the ADC is an ADC of Formula (Ic): or a pharmaceutically acceptable salt thereof, wherein:
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising one or more nonnatural amino acids; each L is a linker; each E is a linkage covalently joining each linker L with one of the one or more non- natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
In some embodiments, the one or more heavy chains is two heavy chains. In some embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing; wherein at least one moiety is not a bond. In some embodiments, the peptide is a dipeptide. In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, - (alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, - N(RW)-, an amino acid or a dipeptide; wherein each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing; wherein at least one moiety is not a bond. In some embodiments, L comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, each E comprises an oxime. In some embodiments, each E has the following structure:
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L; and the wavy line (— ) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, Rq is methyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
In some embodiments, the ADC of Formula (Ic) is an ADC of Formula (Id): or a pharmaceutically acceptable salt thereof, wherein:
Ab is the anti-TROP2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
In some embodiments, d is 2. In some other embodiments, d is 2, 3, or 4.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2.
In some embodiments, the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 4. In some embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
In some embodiments, an anti-TROP2 antibody, such as the anti-TROP2 antibody of an ADC of the present disclosure, is humanized.
In some embodiments, an ADC of the present disclosure does not contain a Toll-like receptor (TLR) agonist.
In some general aspects, there is provided a pharmaceutical composition comprising an ADC of the present disclosure, and at least one pharmaceutically acceptable adjuvant, binder, buffer, carrier, diluent or excipient. In some embodiments, the pharmaceutical composition further comprises a chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid, or combination thereof.
In some general aspects, there is provided a method of treating a disease or condition in a subject, the method comprising administering to the subject a therapeutically effective amount of an ADC of the present disclosure, or a pharmaceutical composition comprising subject a therapeutically effective amount of an ADC of the present disclosure.
In some embodiments, the disease or condition is cancer. In some embodiments, the cancer is a solid tumor. In some embodiments, the cancer is breast cancer, pancreatic cancer, lung cancer, gastric cancer, colorectal cancer or prostate cancer. In some embodiments, the cancer is triplenegative breast cancer (TNBC). In some embodiments, the cancer is pancreatic ductal adenocarcinoma (PDAC). In some embodiments, the cancer is non-small cell lung cancer (NSCLC). In some embodiments, the cancer is a TROP2-positive cancer. In some embodiments, the cancer is a Dxd-resistant cancer. In some embodiments, the cancer is a topoisomerase 1 (TOPI) inhibitor-resistant cancer.
INCORPORATION BY REFERENCE
All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication, patent, or patent application was specifically and individually indicated to be incorporated by reference.
BRIEF DESCRIPTION OF THE DRAWINGS
The novel features of the invention are set forth with particularity in the appended claims. A better understanding of the features and advantages of the present invention disclosure will be obtained by reference to the following detailed description that sets forth illustrative embodiments, in which the principles of the invention are utilized, and the accompanying drawings provided.
FIG. 1 shows a representative HPLC chromatogram showing elution of anti-TROP2 light chain, unconjugated heavy chain, and heavy chain conjugated with drug-linker compound 6. FIG. 2 shows binding kinetic sensorgrams and associated KD values for anti-TROP2 mAb binding to TR0P2 from human (left), cynomolgus (center) and rat (right).
FIG. 3 shows graphical illustrations of cytotoxic activity of anti-TROP2 ADCs against TROP2-expressing BxPC-3 cell line (top left); TROP2-expressing MDA-MB-468 cell line (top right), TROP2-expressing HCC1806 cell line (bottom left) and TROP2-negative Calu-6 cell line (bottom right).
FIG. 4. shows graphical illustrations of cytotoxic activity of anti-TROP2 ADCs in wildtype and Benchmark-DXd-resistant MDA-MB-468 cells.
FIG. 5 shows a graphical illustration of cytotoxic activity of anti-TROP2 ADCs in a Benchmark-DXd-insensitive JIMT-1 cell line.
FIG. 6 shows a graphical illustration of cytotoxic activity of anti-TROP2 ADCs in human keratinocytes.
FIG. 7 shows a graphical illustration of anti-tumor activity of anti-TROP2 ADCs.
FIG. 8 shows a graphical illustration of binding affinities of TR0P2 mAb and anti-TROP2 ADC in TROP2-expressing MDA-MB-468 cells.
FIG. 9 shows a graphical illustration of antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
FIG. 10 shows another graphical illustration of anti -turn or activity of anti-TROP2 ADCs.
FIG. 11 shows still another graphical illustration of anti-tumor activity of anti-TROP2 ADCs.
FIG. 12 shows a graphical illustration of pharmacokinetic study of anti-TROP2 ADCs.
DETAILED DESCRIPTION
Before describing the present invention in detail, it is to be understood that this invention is not limited to particular methodologies, or compositions, or biological systems, which can, of course, vary. It is also to be understood that the terminology used herein is for the purpose of describing particular embodiments only and is not intended to be limiting.
While various embodiments have been shown and described herein, it will be obvious to those skilled in the art that such embodiments are provided by way of example only. Numerous variations, changes, and substitutions will now occur to those skilled in the art without departing from the invention. It should be understood that various alternatives to the embodiments of the invention described herein may be employed in practicing the invention. It is intended that the following claims define the scope of the invention and that methods and structures within the scope of these claims and their equivalents be covered thereby.
Definitions Unless otherwise defined herein or below in the remainder of the specification, all technical and scientific terms used herein have the same meaning as commonly understood by those of ordinary skill in the art to which the invention belongs. As used herein and in the appended claims, the singular forms “a,” “an,” and “the” include plural reference unless the context clearly indicates otherwise.
Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood to one of ordinary skill in the art to which the inventions described herein belong. Various methods, materials, and the like, similar or equivalent to those described herein can be used in the practice or testing of the inventions described herein.
All publications and patents mentioned herein are incorporated herein by reference in their entirety for the purpose of describing and disclosing, for example, the chemistry, chemical syntheses, compositions and other methodologies that are described in the publications, which might be used in connection with the presently described inventions. The publications discussed herein are provided solely for their disclosure prior to the filing date of the present application.
Chemical Terms
It is to be understood that the terminology employed herein is for the purpose of describing particular embodiments and is not intended to be limiting.
The term “acyl,” as used herein, represents -C(O)-alkyl, as defined herein, and is exemplified by acetyl (-C(0)CH3), trifluoroacetyl, propionyl, and butanoyl. Exemplary unsubstituted acyl groups include from 1 to 6, from 1 to 11, or from 1 to 21 carbons.
The term “alkyl,” as used herein, refers to a branched or straight-chain monovalent saturated aliphatic hydrocarbon radical of 1 to 20 carbon atoms (e.g., 1 to 16 carbon atoms, 1 to 10 carbon atoms, or 1 to 6 carbon atoms). An alkylene is a divalent alkyl group.
The term “alkylene” by itself or as part of another molecule means a divalent radical derived from an alkane, as exemplified, by (-CH2-)n, wherein n can be 2 to about 100. In some embodiments, n is 1 to 24. By way of example only, such groups include, but are not limited to, groups having 10 or fewer carbon atoms such as the structures -CH2CH2- and -CH2CH2CH2CH2-. The term “alkylene,” unless otherwise noted, is also meant to include those groups described herein as “heteroalkylene.” Substituents for alkylene groups are selected from the group of acceptable substituents described herein.
The term “alkenyl,” as used herein, alone or in combination with other groups, refers to a straight-chain or branched hydrocarbon residue having a carbon-carbon double bond and having 2 to 20 carbon atoms (e.g., 2 to 16 carbon atoms, 2 to 10 carbon atoms, 2 to 6, or 2 carbon atoms). The term “alkynyl,” as used herein, alone or in combination with other groups, refers to a straight-chain or branched hydrocarbon residue having a carbon-carbon triple bond and having 2 to 20 carbon atoms (e.g., 2 to 16 carbon atoms, 2 to 10 carbon atoms, 2 to 6, or 2 carbon atoms).
The term “amino,” as used herein, represents — N(RN1)2, wherein each RN1 is, independently, H, OH, NO2, N(RN2)2, SO2ORN2, SO2RN2, SORN2, an N-protecting group, alkyl, alkoxy, aryl, arylalkyl, cycloalkyl, acyl (e.g., acetyl, trifluoroacetyl, or others described herein), wherein each of these recited RN1 groups can be optionally substituted; or two RN1 combine to form an alkylene or heteroalkylene, and wherein each RN2 is, independently, H, alkyl, or aryl. The amino groups of the invention can be an unsubstituted amino (i.e., — NH2) or a substituted amino (i.e., — N(RN1)2). In some embodiments, amino is N(RW)-, wherein each Rw is independently H or Ci-C8 alkyl.
The term “aryl,” as used herein, refers to an aromatic mono- or polycarbocyclic radical of 6 to 12 carbon atoms having at least one aromatic ring. Examples of such groups include, but are not limited to, phenyl, naphthyl, 1,2,3,4-tetrahydronaphthyl, 1,2-dihydronaphthyl, indanyl, and IH-indenyl.
The term “arylene”, as used herein, refers to a divalent aryl radical. Non-limiting examples of “arylene” include phenylene, pyridinylene, pyrimidinylene and thiophenylene. Substituents for arylene groups are selected from the group of acceptable substituents described herein.
The term “arylalkyl,” as used herein, represents an alkyl group substituted with an aryl group. Exemplary unsubstituted arylalkyl groups are from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as C1-6 alkyl Ce-io aryl, C1-10 alkyl Ce-io aryl, or C1-20 alkyl Ce-io aryl), such as, benzyl and phenethyl. In some embodiments, the akyl and the aryl each can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups.
The term “azido,” as used herein, represents a — N3 group.
The term “cyano,” as used herein, represents a — CN group.
The terms “carbocyclyl,” as used herein, refer to a non-aromatic C3-12 monocyclic, bicyclic, or tricyclic structure in which the rings are formed by carbon atoms. Carbocyclyl structures include cycloalkyl groups and unsaturated carbocyclyl radicals.
The term “carbonyl” as used herein, refers to a divalent C=O group, which can be written as -C(O)-.
The term “cycloalkyl,” as used herein, refers to a saturated, non-aromatic, monovalent mono- or polycarbocyclic radical of three to ten, preferably three to six carbon atoms. This term is further exemplified by radicals such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, norbornyl, and adamantyl. The term “cycloalkynyl” as used herein refers to a cyclic radical containing one or more triple bonds. In some embodiments, a cycloalkynyl group is cyclooctynyl group.
The term “halogen,” as used herein, means a fluorine (fluoro), chlorine (chloro), bromine (bromo), or iodine (iodo) radical.
The term “heteroalkyl,” as used herein, refers to an alkyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur. In some embodiments, the heteroalkyl group can be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkyl groups. Examples of heteroalkyl groups are an “alkoxy” which, as used herein, refers alkyl-0 — (e.g., methoxy and ethoxy); the alkyl of the alkoxy can be substituted. In some embodiments, the alkoxy alkyl group is substituted with amino or alkylamino, or with heterocyclyl, which is optionally substituted. A heteroalkylene is a divalent heteroalkyl group.
The term “heteroalkenyl,” as used herein, refers to an alkenyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur. In some embodiments, the heteroalkenyl group can be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkenyl groups. Examples of heteroalkenyl groups are an “alkenoxy” which, as used herein, refers alkenyl-0 — . A heteroalkenylene is a divalent heteroalkenyl group.
The term “heteroalkynyl,” as used herein, refers to an alkynyl group, as defined herein, in which one or more of the constituent carbon atoms have been replaced by nitrogen, oxygen, or sulfur. In some embodiments, the heteroalkynyl group can be further substituted with 1, 2, 3, or 4 substituent groups as described herein for alkynyl groups. Examples of heteroalkynyl groups are an “alkynoxy” which, as used herein, refers alkynyl-0 — . A heteroalkynylene is a divalent heteroalkynyl group.
The term “heteroaryl,” as used herein, refers to an aromatic mono- or polycyclic radical of 5 to 12 atoms having at least one aromatic ring containing one, two, or three ring heteroatoms selected from N, O, and S, with the remaining ring atoms being C. One or two ring carbon atoms of the heteroaryl group may be replaced with a carbonyl group. Examples of heteroaryl groups are pyridyl, pyrazoyl, benzooxazolyl, benzoimidazolyl, benzothiazolyl, imidazolyl, oxaxolyl, and thiazolyl.
The term “heteroarylalkyl,” as used herein, represents an alkyl group substituted with a heteroaryl group. Exemplary unsubstituted heteroarylalkyl groups are from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as Ci-6 alkyl C2-9 heteroaryl, C1-10 alkyl C2-9 heteroaryl, or C1-20 alkyl C2-9 heteroaryl). In some embodiments, the akyl and the heteroaryl each can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups. The term “heterocyclyl,” as used herein, denotes a mono- or polycyclic radical having 3 to 12 atoms having at least one ring containing one, two, three, or four ring heteroatoms selected from N, O or S, wherein no ring is aromatic. Examples of heterocyclyl groups include, but are not limited to, morpholinyl, thiomorpholinyl, furyl, piperazinyl, piperidinyl, pyranyl, pyrrolidinyl, tetrahydropyranyl, tetrahydrofuranyl, and 1,3-dioxanyl.
The term “heterocyclylalkyl,” as used herein, represents an alkyl group substituted with a heterocyclyl group. Exemplary unsubstituted heterocyclylalkyl groups are from 7 to 30 carbons (e.g., from 7 to 16 or from 7 to 20 carbons, such as Ci-6 alkyl C2-9 heterocyclyl, C1-10 alkyl C2- 9 heterocyclyl, or C1-20 alkyl C2-9 heterocyclyl). In some embodiments, the akyl and the heterocyclyl each can be further substituted with 1, 2, 3, or 4 substituent groups as defined herein for the respective groups.
The term “hydroxyl,” as used herein, represents an — OH group.
The term “methylene” by itself or as part of another molecule means a divalent radical derived from methane, and can be written as (-CH2-). Substituents for alkylene groups are selected from the group of acceptable substituents described herein.
The term “methine” as used herein refers to a trivalent moiety C(H).
The term “nitro,” as used herein, represents an — NO2 group.
The term “thiol,” as used herein, represents an — SH group.
The alkyl, alkylene, methylene, methine, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, heteroalky nyl, carbocyclyl (e.g., cycloalkyl), aryl, arylene, heteroaryl, heteroarylene, and heterocyclyl groups may be substituted or unsubstituted. When substituted, there will generally be 1 to 4 substituents present, unless otherwise specified. Substituents include, for example: aryl (e.g., substituted and unsubstituted phenyl), carbocyclyl (e.g., substituted and unsubstituted cycloalkyl), halogen (e.g., fluoro), hydroxyl, heteroalkyl (e.g., substituted and unsubstituted methoxy, ethoxy, or thioalkoxy), heteroaryl, heterocyclyl, amino (e.g., NH2 or mono- or dialkyl amino), azido, cyano, nitro, or thiol. Aryl, carbocyclyl (e.g., cycloalkyl), heteroaryl, and heterocyclyl groups may also be substituted with alkyl (unsubstituted and substituted such as arylalkyl (e.g., substituted and unsubstituted benzyl)).
Compounds of the invention can have one or more asymmetric carbon atoms and can exist in the form of optically pure enantiomers, mixtures of enantiomers such as, for example, racemates, optically pure diastereoisomers, mixtures of diastereoisomers, diastereoisomeric racemates or mixtures of diastereoisomeric racemates. The optically active forms can be obtained for example by resolution of the racemates, by asymmetric synthesis or asymmetric chromatography (chromatography with a chiral adsorbents or eluant). That is, certain of the disclosed compounds may exist in various stereoisomeric forms. Stereoisomers are compounds that differ only in their spatial arrangement. Enantiomers are pairs of stereoisomers whose mirror images are not superimposable, most commonly because they contain an asymmetrically substituted carbon atom that acts as a chiral center. “Enantiomer” means one of a pair of molecules that are mirror images of each other and are not superimposable. Diastereomers are stereoisomers that are not related as mirror images, most commonly because they contain two or more asymmetrically substituted carbon atoms and represent the configuration of substituents around one or more chiral carbon atoms. Enantiomers of a compound can be prepared, for example, by separating an enantiomer from a racemate using one or more well-known techniques and methods, such as, for example, chiral chromatography and separation methods based thereon. The appropriate technique and/or method for separating an enantiomer of a compound described herein from a racemic mixture can be readily determined by those of skill in the art. “Racemate” or “racemic mixture” means a compound containing two enantiomers, wherein such mixtures exhibit no optical activity; i.e., they do not rotate the plane of polarized light. “Geometric isomer” means isomers that differ in the orientation of substituent atoms in relationship to a carbon-carbon double bond, to a cycloalkyl ring, or to a bridged bicyclic system. Atoms (other than H) on each side of a carbon-carbon double bond may be in an E (substituents are on opposite sides of the carbon-carbon double bond) or Z (substituents are oriented on the same side) configuration. “R,” “S,” “S*,” “R*,” “E,” “Z,” “cis,” and “trans,” indicate configurations relative to the core molecule. Certain of the disclosed compounds may exist in atropisomeric forms. Atropisomers are stereoisomers resulting from hindered rotation about single bonds where the steric strain barrier to rotation is high enough to allow for the isolation of the conformers. The compounds of the invention may be prepared as individual isomers by either isomer-specific synthesis or resolved from an isomeric mixture. Conventional resolution techniques include forming the salt of a free base of each isomer of an isomeric pair using an optically active acid (followed by fractional crystallization and regeneration of the free base), forming the salt of the acid form of each isomer of an isomeric pair using an optically active amine (followed by fractional crystallization and regeneration of the free acid), forming an ester or amide of each of the isomers of an isomeric pair using an optically pure acid, amine or alcohol (followed by chromatographic separation and removal of the chiral auxiliary), or resolving an isomeric mixture of either a starting material or a final product using various well known chromatographic methods. When the stereochemistry of a disclosed compound is named or depicted by structure, the named or depicted stereoisomer is at least 60%, 70%, 80%, 90%, 99% or 99.9%) by weight relative to the other stereoisomers. When a single enantiomer is named or depicted by structure, the depicted or named enantiomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by weight optically pure. When a single diastereomer is named or depicted by structure, the depicted or named diastereomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by weight pure. Percent optical purity is the ratio of the weight of the enantiomer or over the weight of the enantiomer plus the weight of its optical isomer. Diastereomeric purity by weight is the ratio of the weight of one diastereomer or over the weight of all the diastereomers. When the stereochemistry of a disclosed compound is named or depicted by structure, the named or depicted stereoisomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure relative to the other stereoisomers. When a single enantiomer is named or depicted by structure, the depicted or named enantiomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure. When a single diastereomer is named or depicted by structure, the depicted or named diastereomer is at least 60%, 70%, 80%, 90%, 99% or 99.9% by mole fraction pure. Percent purity by mole fraction is the ratio of the moles of the enantiomer or over the moles of the enantiomer plus the moles of its optical isomer. Similarly, percent purity by moles fraction is the ratio of the moles of the diastereomer or over the moles of the diastereomer plus the moles of its isomer. When a disclosed compound is named or depicted by structure without indicating the stereochemistry, and the compound has at least one chiral center, it is to be understood that the name or structure encompasses either enantiomer of the compound free from the corresponding optical isomer, a racemic mixture of the compound or mixtures enriched in one enantiomer relative to its corresponding optical isomer. When a disclosed compound is named or depicted by structure without indicating the stereochemistry and has two or more chiral centers, it is to be understood that the name or structure encompasses a diastereomer free of other diastereomers, a number of diastereomers free from other diastereomeric pairs, mixtures of diastereomers, mixtures of diastereomeric pairs, mixtures of diastereomers in which one diastereomer is enriched relative to the other diastereomer(s) or mixtures of diastereomers in which one or more diastereomer is enriched relative to the other diastereomers. The invention embraces all of these forms.
In embodiments are provided novel amino acid sequences. The term “amino acid” refers to naturally occurring and non-natural or unnatural amino acids, which may be referred to herein as synthetic amino acids, as well as amino acid analogs and amino acid mimetics that function in a manner similar to the naturally occurring amino acids. Naturally encoded amino acids are the 20 common amino acids (alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine) and pyrolysine and selenocysteine. Amino acid analogs refer to compounds that have the same basic chemical structure as a naturally occurring amino acid, by way of example only, an a-carbon that is bound to a hydrogen, a carboxyl group, an amino group, and a functional R group. Such analogs may have modified R groups (by way of example, norleucine) or may have modified peptide backbones while still retaining the same basic chemical structure as a naturally occurring amino acid. Non-limiting examples of amino acid analogs include homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium. Amino acids may be referred to herein by either their name, their commonly known three letter symbols or by the one-letter symbols recommended by the IUPAC-IUB Biochemical Nomenclature Commission. Additionally, nucleotides, may be referred to by their commonly accepted single-letter codes.
An “amino or carboxy terminus modification group” refers to any molecule that can be attached to a terminal amine group or terminal carboxy group respectively. By way of example, such terminal amine groups or terminal carboxy groups may be at the end of polymeric molecules, wherein such polymeric molecules include, but are not limited to, polypeptides, polynucleotides, and polysaccharides. Terminus modification groups include but are not limited to, various water- soluble polymers, peptides or proteins. By way of example only, terminus modification groups include polyethylene glycol or serum albumin. Terminus modification groups may be used to modify therapeutic characteristics of the polymeric molecule, including but not limited to increasing the serum half-life of peptides, polypeptides or proteins.
In some embodiments the disclosure provides novel antibodies and antibody variants. The term “antibody” herein refers to a protein consisting of one or more polypeptides substantially encoded by all or part of the antibody genes. The immunoglobulin genes include, but are not limited to, the kappa, lambda, alpha, gamma (IgGl, IgG2, IgG3, and IgG4), delta, epsilon and mu constant region genes, as well as the myriad immunoglobulin variable region genes. Antibody herein is also meant to include full-length antibodies and antibody fragments, and include antibodies that exist naturally in any organism, antibody variants, engineered antibodies and antibody fragments. Antibody herein is also meant to include intact antibody, monoclonal or polyclonal antibodies. Antibody herein also encompasses multispecific antibodies and/or bispecific antibodies. Antibodies of the present disclosure include human antibodies. Human antibodies are usually made of two light chains and two heavy chains each comprising variable regions and constant regions. The light chain variable region comprises 3 CDRs, identified herein as CDRL1, CDRL2 and CDRL3 flanked by framework regions. The heavy chain variable region comprises 3 CDRs, identified herein as CDRH1, CDRH2 and CDRH3 flanked by framework regions.
The term “antibody fragment” herein refers to any form of an antibody other than the full- length form. Antibody fragments herein include antibodies that are smaller components that exist within full-length antibodies, and antibodies that have been engineered, such as antibody variants. Antibody fragments include but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, and variable regions, and alternative scaffold non-antibody molecules, bispecific antibodies, and the like (Maynard & Georgiou, Annu. Rev. Biomed. Eng. 2:339-76, 2000; Hudson, Curr. Opin. Biotechnol. 9:395-402, 1998). Another functional substructure is a single chain Fv (scFv), comprised of the variable regions of the immunoglobulin heavy and light chain, covalently connected by a peptide linker (Hu et al., Cancer Research, 56, 3055-3061, 1996). These small (Mr 25,000) proteins generally retain specificity and affinity for antigen in a single polypeptide and can provide a convenient building block for larger, antigen-specific molecules. Unless specifically noted otherwise, statements and claims that use the term “antibody” or “antibodies” specifically includes “antibody fragment” and “antibody fragments.”
In embodiments novel antibody drug conjugates (ADCs) are disclosed. The term “antibody-drug conjugate, or “ADC”, as used herein, refers to an antibody molecule, or fragment thereof, that is covalently bonded to one or more biologically active molecule(s). The biologically active molecule may be conjugated to the antibody through a linker, polymer, or other covalent bond. ADCs are a potent class of therapeutic constructs that allow targeted delivery of cytotoxic agents to target cells, such as cancer cells. Because of the targeting function, these compounds show a much higher therapeutic index compared to the same systemically delivered agents. ADCs have been developed as intact antibodies or antibody fragments, such as scFvs. The antibody or fragment is linked to one or more copies of drug via a linker that is stable under physiological conditions, but that may be cleaved once inside the target cell.
The term "antigen-binding fragment", as used herein, refers to one or more fragments of an antibody that retain the ability to bind to an antigen. It has been shown that the antigen-binding function of an antibody can be performed by fragments of an intact antibody. Examples of binding fragments encompassed within the term "antigen-binding fragment" of an antibody include (i) a Fab fragment, a monovalent fragment consisting of the VL, VH, CL and CHI domains; (ii) a F(ab')2 fragment, a bivalent fragment comprising two Fab fragments linked by a disulfide bridge at the hinge region; (iii) a Fd fragment consisting of the VH and CHI domains; (iv) a Fv fragment consisting of the VL and VH domains of a single arm of an antibody, (v) a dAb fragment (Ward et al., Nature 341 :544-546, 1989), which consists of a VH domain; (vi) an isolated complementarity determining region (CDR), e.g., VH CDR3 comprising or not additional sequence (linker, framework region(s) etc.) and (v) a combination of two to six isolated CDRs comprising or not additional sequence (linker, framework region(s) etc.). Furthermore, although the two domains of the Fv fragment, VL and VH, are coded for by separate genes, they can be joined, using recombinant methods, by a synthetic linker that enables them to be made as a single polypeptide chain in which the VL and VH regions pair to form monovalent molecules (known as single chain Fv (scFv); see e.g., Bird et al., Science 242:423-426, 1988); and (Huston et al., Proc. Natl. Acad. Sci. USA 85:5879-5883, 1988). Such single chain antibodies are also intended to be encompassed within the term "antigen -binding fragment" of an antibody. Furthermore, the antigen-binding fragments include binding-domain immunoglobulin fusion proteins comprising (i) a binding domain polypeptide (such as a heavy chain variable region, a light chain variable region, or a heavy chain variable region fused to a light chain variable region via a linker peptide) that is fused to an immunoglobulin hinge region polypeptide, (ii) an immunoglobulin heavy chain CH2 constant region fused to the hinge region, and (iii) an immunoglobulin heavy chain CH3 constant region fused to the CH2 constant region. The hinge region may be modified by replacing one or more cysteine residues with serine residues to prevent dimerization. Such binding-domain immunoglobulin fusion proteins are further disclosed in US 2003/0118592 and US 2003/0133939. These antibody fragments are obtained using conventional techniques known to those with skill in the art, and the fragments are screened for utility in the same manner as are intact antibodies.
A typical antigen binding site is comprised of the variable regions formed by the pairing of a light chain immunoglobulin and a heavy chain immunoglobulin. The structure of the antibody variable regions is very consistent and exhibits very similar structures. These variable regions are typically comprised of relatively homologous framework regions (FR) interspaced with three hypervariable regions termed Complementarity Determining Regions (CDRs). The overall binding activity of the antigen binding fragment is often dictated by the sequence of the CDRs. The FRs often play a role in the proper positioning and alignment in three dimensions of the CDRs for optimal antigen binding. In fact, because CDR sequences are responsible for most antibodyantigen interactions, it is possible to express recombinant antibodies that shows the properties of specific naturally occurring antibodies by constructing expression vectors that include CDR sequences from the specific naturally occurring antibody grafted onto framework sequences from a different antibody with different properties (see, e.g., Riechmann, L. et al., Nature 332:323-327, 1998; Jones, P. et al., Nature 321 :522-525, 1986; and Queen, C. et al., Proc. Natl. Acad. USA 86: 10029-10033, 1989). Such framework sequences can be obtained from public DNA databases that include germline antibody gene sequences. These germline sequences will differ from mature antibody gene sequences because they will not include completely assembled variable genes, which are formed by V(D)J joining during B cell maturation. Germline gene sequences will also differ from the sequences of a high affinity secondary repertoire antibody which contains mutations throughout the variable gene but typically clustered in the CDRs. For example, somatic mutations are relatively infrequent in the amino terminal portion of framework region 1 and in the carboxy-terminal portion of framework region 4. Furthermore, many somatic mutations do not significantly alter the binding properties of the antibody. For this reason, it is not necessary to obtain the entire DNA sequence of a particular antibody in order to recreate an intact recombinant antibody having binding properties similar to those of the original antibody. Partial heavy and light chain sequence spanning the CDR regions is typically sufficient for this purpose. The partial sequence is used to determine which germline variable and joining gene segments contributed to the recombined antibody variable genes. The germline sequence is then used to fill in missing portions of the variable regions. Heavy and light chain leader sequences are cleaved during protein maturation and do not contribute to the properties of the final antibody. To add missing sequences, cloned cDNA sequences can be combined with synthetic oligonucleotides by ligation or PCR amplification. Alternatively, the entire variable region can be synthesized to create an entirely synthetic variable region clone. This process has certain advantages such as elimination or inclusion of particular restriction sites, or optimization of particular codons. Of course, the totality or portions of the framework region of the antibody described herein may be used in conjunction with the CDRs in order to optimize the affinity, specificity or any other desired properties of the antibody.
In some embodiments the disclosure concerns polymers such as a bifunctional polymer. A “bifunctional polymer”, also referred to as a “bifunctional linker”, refers to a polymer comprising two functional groups that are capable of reacting specifically with other moieties to form covalent or non-covalent linkages. Such moieties may include, but are not limited to, the side groups on natural or non-natural amino acids or peptides which contain such natural or non-natural amino acids. The other moieties that may be linked to the bifunctional linker or bifunctional polymer may be the same or different moieties. By way of example only, a bifunctional linker may have a functional group reactive with a group on a first peptide, and another functional group which is reactive with a group on a second peptide, whereby forming a conjugate that includes the first peptide, the bifunctional linker and the second peptide. Many procedures and linker molecules for attachment of various compounds to peptides are known. See, for example, European Patent Application No. 0188256; U.S. Patent Nos. 4,659,839; 4,414,148; 4,699,784; 4,680,338; and 4,569,789 incorporated herein by reference in their entirety. A “multi-functional polymer” also referred to as a “multi-functional linker”, refers to a polymer comprising two or more functional groups that are capable of reacting with other moieties. Such moieties may include, but are not limited to, the side groups on natural or non-natural amino acids or peptides which contain such natural or non-natural amino acids (including but not limited to, amino acid side groups) to form covalent or non-covalent linkages. A bi-functional polymer or multi-functional polymer may be any desired length or molecular weight and may be selected to provide a particular desired spacing or conformation between one or more molecules linked to a compound and molecules it binds to, or to the compound. The term “bioavailability,” as used herein, refers to the rate and extent to which a substance or its active moiety is delivered from a pharmaceutical dosage form and becomes available at the site of action or in the general circulation. Increases in bioavailability refers to increasing the rate and extent a substance or its active moiety is delivered from a pharmaceutical dosage form and becomes available at the site of action or in the general circulation. By way of example, an increase in bioavailability may be indicated as an increase in concentration of the substance or its active moiety in the blood when compared to other substances or active moieties.
The term “biologically active molecule”, “biologically active moiety” or “biologically active agent” when used herein means any substance which can affect any physical or biochemical properties of a biological system, pathway, molecule, or interaction relating to an organism, including but not limited to, viruses, bacteria, bacteriophage, transposon, prion, insects, fungi, plants, animals, and humans. In particular, as used herein, biologically active molecules include but are not limited to any substance intended for diagnosis, cure, mitigation, treatment, or prevention of disease in humans or other animals, or to otherwise enhance physical or mental wellbeing of humans or animals. Examples of biologically active molecules include, but are not limited to, peptides, proteins, enzymes, small molecule drugs, hard drugs, soft drugs, prodrugs, carbohydrates, inorganic atoms or molecules, dyes, lipids, nucleosides, radionuclides, oligonucleotides, toxins, cells, viruses, liposomes, microparticles and micelles. Classes of biologically active agents that are suitable for use with the methods and compositions described herein include, but are not limited to, drugs, prodrugs, radionuclides, imaging agents, polymers, antibiotics, fungicides, anti-viral agents, anti-inflammatory agents, anti-tumor agents, cardiovascular agents, anti-anxiety agents, hormones, growth factors, steroidal and nonsteroidal agents, microbially derived toxins, and the like.
By “modulating biological activity” is meant increasing or decreasing the reactivity of a polypeptide, altering the selectivity of the polypeptide, enhancing or decreasing the substrate selectivity of the polypeptide. Analysis of modified biological activity can be performed by comparing the biological activity of the non-natural polypeptide to that of the natural polypeptide.
In some embodiments the disclosure concerns amino acids that have been biosynthetically incorporated in the antibody. The term “biosynthetically,” as used herein, refers to any method utilizing a translation system (cellular or non-cellular), including use of at least one of the following components: a polynucleotide, a codon, a tRNA, and a ribosome. By way of example, non-natural amino acids may be “biosynthetically incorporated” into non-natural amino acid polypeptides using the methods and techniques described herein and as is well known in the art. See for example, WO2010/011735 and W02005/074650. The term “conservatively modified variants” applies to both natural and non-natural amino acid and natural and non-natural nucleic acid sequences, and combinations thereof. With respect to particular nucleic acid sequences, “conservatively modified variants” refers to those natural and non-natural nucleic acids which encode identical or essentially identical natural and non-natural amino acid sequences, or where the natural and non-natural nucleic acid does not encode a natural and non-natural amino acid sequence, to essentially identical sequences. By way of example, because of the degeneracy of the genetic code, a large number of functionally identical nucleic acids encode any given protein. For instance, the codons GCA, GCC, GCG and GCU all encode the amino acid alanine. Thus, at every position where an alanine is specified by a codon, the codon can be altered to any of the corresponding codons described without altering the encoded polypeptide. Such nucleic acid variations are “silent variations,” which are one species of conservatively modified variations. Thus, by way of example every natural or non-natural nucleic acid sequence herein which encodes a natural or non-natural polypeptide also describes every possible silent variation of the natural or non-natural nucleic acid. One of ordinary skill in the art will recognize that each codon in a natural or non-natural nucleic acid (except AUG, which is ordinarily the only codon for methionine, and TGG, which is ordinarily the only codon for tryptophan) can be modified to yield a functionally identical molecule. Accordingly, each silent variation of a natural and non-natural nucleic acid which encodes a natural and non-natural polypeptide is implicit in each described sequence. As to amino acid sequences, individual substitutions, deletions or additions to a nucleic acid, peptide, polypeptide, or protein sequence which alters, adds or deletes a single natural and non-natural amino acid or a small percentage of natural and non-natural amino acids in the encoded sequence is a “conservatively modified variant” where the alteration results in the deletion of an amino acid, addition of an amino acid, or substitution of a natural and non-natural amino acid with a chemically similar amino acid. Conservative substitution tables providing functionally similar natural amino acids are well known in the art. Conservative substitution tables providing functionally similar amino acids are known to those of ordinary skill in the art. The following eight groups each contain amino acids that are conservative substitutions for one another: 1) Alanine (A), Glycine (G); 2) Aspartic acid (D), Glutamic acid (E); 3) Asparagine (N), Glutamine (Q); 4) Arginine (R), Lysine (K); 5) Isoleucine (I), Leucine (L), Methionine (M), Valine (V); 6) Phenylalanine (F), Tyrosine (Y), Tryptophan (W); 7) Serine (S), Threonine (T); and 8) Cysteine (C), Methionine (M) (see, e.g., Creighton, Proteins: Structures and Molecular Properties (W H Freeman & Co.; 2nd edition, 1993). Such conservatively modified variants are in addition to and do not exclude polymorphic variants, interspecies homologs, and alleles of the compositions described herein. The term “drug,” as used herein, refers to any substance used in the prevention, diagnosis, alleviation, treatment, or cure of a disease or condition such as cancer, including but not limited to oral, colorectal, gastric, esophageal, hepatocellular, lung including non-small-cell-lung cancer (NSCLC) and small-cell lung cancer (SCLC), ovarian, breast including triple-negative breast, prostate, pancreatic including pancreatic ductal adenocarcinoma, head and neck, squamous, renal, bladder, cervical, endometrial, thyroid, glioblastoma cancer; a TROP2-positive cancer; a solid tumor; or a blood cancer, including a leukemia, a lymphoma or myeloma.
The term “drug-to-antibody ratio” (“DAR”) as used herein refers to the average (mean) number of drugs that are conjugated to an antibody in an antibody-drug conjugate (ADC) composition. The DAR value reflects the homogeneity of the ADC population in the composition, and also indicates the amount of “payload” (e.g, drug or drug-linker) that is loaded onto an antibody and can be delivered to a target (e.g., cell or diseased tissue). DAR can be determined by methods known to a person of ordinary skill in the art, for example, LC-MS (e.g., see Tang, Y. et al., Real-Time Analysis on Drug-Antibody Ratio of Antibody-Drug Conjugates for Synthesis, Process Optimization and Quality Control, Sci Rep 7, 7763 (2017). doi: 10.1038/s41598-017- 08151-2; and Chen, Y. Drug-to-antibody ratio (DAR) by UV/Vis spectroscopy, Methods Mol. Biol., 2013;1045:267-73. doi: 10.1007/978-l-62703-541-5_l 6). In a non-limiting example, an ADC can have a population distribution of 20% of drug-loaded antibody, wherein the drug load is two (2) drugs per antibody; 25% of drug-loaded antibody, wherein the drug load is three (3) drugs per antibody; and 55% of drug-loaded antibody, wherein the drug load is four (4) drugs per antibody; thus, in this example, DAR is [(0.2 x 2) + (0.25 x 3) + (0.55 x 4)] = 3.35.
The term “effective amount,” as used herein, refers to a sufficient amount of an agent, compound or composition being administered which will relieve to some extent one or more of the symptoms of the disease or condition being treated. The result can be reduction and/or alleviation of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system. By way of example, an agent, compound or composition being administered includes, but is not limited to, a natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, modified non-amino acid polypeptide, or an antibody or variant thereof. Compositions containing such natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, modified nonnatural amino acid polypeptides, or an antibody or variant thereof can be administered for prophylactic, enhancing, and/or therapeutic treatments. An appropriate “effective” amount in any individual case may be determined using techniques, such as a dose escalation study.
The terms “enhance” or “enhancing” means to increase or prolong either in potency or duration a desired effect. By way of example, “enhancing” the effect of therapeutic agents refers to the ability to increase or prolong, either in potency or duration, the effect of therapeutic agents on during treatment of a disease, disorder or condition. An “enhancing-effective amount,” as used herein, refers to an amount adequate to enhance the effect of a therapeutic agent in the treatment of a disease, disorder or condition. When used in a patient, amounts effective for this use will depend on the severity and course of the disease, disorder or condition, previous therapy, the patient's health status and response to the drugs, and the judgment of the treating physician.
The term "humanized or chimeric antibody" refer to a molecule, generally prepared using recombinant techniques, having an antigen binding site derived from an immunoglobulin from a non-human species, (e.g., murine), and the remaining immunoglobulin structure of the molecule based upon the structure and/or sequence of a human immunoglobulin. In general, the humanized antibody will comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the hypervariable loops correspond to those of a non-human immunoglobulin and all or substantially all of the framework residues/regions (FR) are those of a human immunoglobulin sequence. The humanized antibody optionally also will comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin. The humanized forms of rodent antibodies will essentially comprise the same CDR sequences of the parental rodent antibodies, although certain amino acid substitutions may be included to increase affinity, increase stability of the humanized antibody, or for other reasons. However, as CDR loop exchanges do not uniformly result in an antibody with the same binding properties as the antibody of origin, changes in framework residues (FR), residues involved in CDR loop support, might also be introduced in humanized antibodies to preserve antigen binding affinity. The antigen-binding site may comprise either complete variable domains fused onto constant domains or only the complementarity determining regions (CDRs) grafted onto appropriate framework regions in the variable domains. Antigen binding sites may be wild type or modified by one or more amino acid substitutions. This eliminates the constant region as an immunogen in human individuals, but the possibility of an immune response to the foreign variable region remains (LoBuglio, A. F. et al., "Mouse/Human Chimeric Monoclonal Antibody in Man: Kinetics and Immune Response," Proc. Natl. Acad. Sci. (USA) 86:4220-4224, 1989). Another approach focuses not only on providing human-derived constant regions but modifying the variable regions as well so as to reshape them as closely as possible to human form. It is known that the variable regions of both heavy and light chains contain three complementarity-determining regions (CDRs) which vary in response to the antigens in question and determine binding capability, flanked by four framework regions (FRs) which are relatively conserved in a given species and which putatively provide a scaffolding for the CDRs. When nonhuman antibodies are prepared with respect to a particular antigen, the variable regions can be "humanized" by grafting CDRs derived from nonhuman antibody on the FRs present in the human antibody to be modified. Application of this approach to various antibodies has been reported by Kettleborough, C. A. et al., "Humanization Of A Mouse Monoclonal Antibody By CDR-Grafting: The Importance Of Framework Residues On Loop Conformation," Protein Engineering 4:773-3783,1991; Co, M. S. et al., "Humanized Antibodies For Antiviral Therapy," Proc. Natl. Acad. Sci. (USA) 88:2869- 2873,1991; Carter, P. et al., "Humanization Of An Anti-pl85her2 Antibody For Human Cancer Therapy," Proc. Natl. Acad. Sci. (USA) 89:4285-4289,1992; and Co, M. S. et al., "Chimeric And Humanized Antibodies With Specificity For The CD33 Antigen," J. Immunol. 148: 1149- 1154,1992. In some embodiments, humanized antibodies preserve all CDR sequences (for example, a humanized mouse antibody which contains all six CDRs from the mouse antibodies). In other embodiments, humanized antibodies have one or more CDRs (one, two, three, four, five, six) which are altered with respect to the original antibody, which are also termed one or more CDRs "derived from" one or more CDRs from the original antibody.
The term “identical,” as used herein, refers to two or more sequences or subsequences which are the same. In addition, the term “substantially identical,” as used herein, refers to two or more sequences which have a percentage of sequential units which are the same when compared and aligned for maximum correspondence over a comparison window, or designated region as measured using comparison algorithms or by manual alignment and visual inspection. By way of example only, two or more sequences may be “substantially identical” if the sequential units are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region. Such percentages describe the “percent identity” of two or more sequences. The identity of a sequence can exist over a region that is at least about 75-100 sequential units in length, over a region that is about 50 sequential units in length, or, where not specified, across the entire sequence. This definition also refers to the complement of a test sequence. By way of example only, two or more polypeptide sequences are identical when the amino acid residues are the same, while two or more polypeptide sequences are “substantially identical” if the amino acid residues are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region. The identity can exist over a region that is at least about 75 to about 100 amino acids in length, over a region that is about 50 amino acids in length, or, where not specified, across the entire sequence of a polypeptide sequence. In addition, by way of example only, two or more polynucleotide sequences are identical when the nucleic acid residues are the same, while two or more polynucleotide sequences are “substantially identical” if the nucleic acid residues are about 60% identical, about 65% identical, about 70% identical, about 75% identical, about 80% identical, about 85% identical, about 90% identical, or about 95% identical over a specified region. The identity can exist over a region that is at least about 75 to about 100 nucleic acids in length, over a region that is about 50 nucleic acids in length, or, where not specified, across the entire sequence of a polynucleotide sequence.
The term “immunogenicity,” as used herein, refers to an antibody response to administration of a therapeutic drug. The immunogenicity toward therapeutic non-natural amino acid polypeptides can be obtained using quantitative and qualitative assays for detection of anti- non-natural amino acid polypeptides antibodies in biological fluids. Such assays include, but are not limited to, Radioimmunoassay (RIA), Enzyme-linked immunosorbent assay (ELISA), luminescent immunoassay (LIA), and fluorescent immunoassay (FIA). Analysis of immunogenicity toward therapeutic non-natural amino acid polypeptides involves comparing the antibody response upon administration of therapeutic non-natural amino acid polypeptides to the antibody response upon administration of therapeutic natural amino acid polypeptides.
The term “isolated,” as used herein, refers to separating and removing a component of interest from components not of interest. Isolated substances can be in either a dry or semi-dry state, or in solution, including but not limited to an aqueous solution. The isolated component can be in a homogeneous state or the isolated component can be a part of a pharmaceutical composition that comprises additional pharmaceutically acceptable carriers and/or excipients. Purity and homogeneity may be determined using analytical chemistry techniques including, but not limited to, polyacrylamide gel electrophoresis or high-performance liquid chromatography. In addition, when a component of interest is isolated and is the predominant species present in a preparation, the component is described herein as substantially purified. The term “purified,” as used herein, may refer to a component of interest which is at least 85% pure, at least 90% pure, at least 95% pure, at least 99% or greater pure. By way of example only, nucleic acids or proteins are “isolated” when such nucleic acids or proteins are free of at least some of the cellular components with which it is associated in the natural state, or that the nucleic acid or protein has been concentrated to a level greater than the concentration of its in vivo or in vitro production. Also, by way of example, a gene is isolated when separated from open reading frames which flank the gene and encode a protein other than the gene of interest.
The term “linkage” or “adduct moiety” as used herein refers to a bond or chemical moiety formed from a chemical reaction between the functional group of one group, such as a linker of the present disclosure, and another molecule. Such bonds may include, but are not limited to, covalent linkages and non-covalent bonds, while such chemical moieties may include, but are not limited to, esters, carbonates, imines, phosphate esters, hydrazones, acetals, orthoesters, peptide linkages, oximes and oligonucleotide linkages. Hydrolytically stable linkages mean that the linkages are substantially stable in water and do not react with water at useful pH values, including but not limited to, under physiological conditions for an extended period of time, perhaps even indefinitely. Hydrolytically unstable or degradable linkages mean that the linkages are degradable in water or in aqueous solutions, including for example, blood. Enzymatically unstable or degradable linkages mean that the linkage can be degraded by one or more enzymes. By way of example only, PEG and related polymers may include degradable linkages in the polymer backbone or in the linker group between the polymer backbone and one or more of the terminal functional groups of the polymer molecule. Such degradable linkages include but are not limited to ester linkages formed by the reaction of PEG carboxylic acids or activated PEG carboxylic acids with alcohol groups on a biologically active agent, wherein such ester groups generally hydrolyze under physiological conditions to release the biologically active agent. Other hydrolytically degradable linkages include but are not limited to carbonate linkages; imine linkages resulted from reaction of an amine and an aldehyde; phosphate ester linkages formed by reacting an alcohol with a phosphate group; hydrazone linkages which are reaction product of a hydrazide and an aldehyde; acetal linkages that are the reaction product of an aldehyde and an alcohol; orthoester linkages that are the reaction product of a formate and an alcohol; peptide linkages formed by an amine group, including but not limited to, at an end of a polymer such as PEG, and a carboxyl group of a peptide; and oligonucleotide linkages formed by a phosphoramidite group, including but not limited to, at the end of a polymer, and a 5' hydroxyl group of an oligonucleotide.
The term “linker,” as used herein, refers to any multivalent group that connects, or is capable of connecting, a first group to at least one other group. Typically, a linker is a bivalent or a trivalent organic moiety that connects a drug (first group) to a biologically active agent (second group), e.g., via a linkage or adduct moiety, or that connects a drug (first group) to a reactive moiety (second group), wherein the reactive moiety is capable of reacting with a biologically active agent. Linkers can be susceptible to cleavage (cleavable linkers), such as, acid-induced cleavage, photo-induced cleavage, peptidase-induced cleavage, esterase-induced cleavage, and disulfide bond cleavage, and so on, at conditions under which the drug and the at least one other group remains active. Alternatively, linkers can be substantially resistant to cleavage (e.g., stable linker or non-cleavable linker).
In some embodiments, a linker is a bivalent or trivalent group comprising, or consisting of, at least one moiety, wherein each at least one moiety is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, - (alkylene-O)n-, optionally substituted arylene, -O-, -C(O)-, -C(S)-, -N(RW)-, -S(0)o-2-, an amino acid, a peptide, a disulfide (-S-S-) and a water soluble polymer; and combinations thereof; wherein: each Rw is independently H, Ci-Cs alkyl or a bond; and n is an integer of at least 1. In some embodiments, n is an integer from 1 to 100. In some embodiments, n is an integer from 1 to 10. In some embodiments, n is 1, 2, 3, 4 or 5. In some embodiments, at least one moiety is not a bond. As such, the linker comprises at least one moiety that is not simply a bond. In some embodiments, the water-soluble polymer is a (polyethylene) glycol (PEG) or modified PEG. In some embodiments, the water-soluble polymer is a polysaccharide. Unless expressly indicated otherwise, no orientation of the linker is implied by the direction in which the formula of the linker group is written. By way of example, the formula -C(O)CH2CH2- represents both - C(O)CH2CH2- and -CEhCEhC O)-. In another example, the formula - OjCEhCEh- represents both *-C(O)CH2CH2- and - OjCEhCEh-*, wherein * denotes a point of connection, for example, connection to a drug. In some embodiments, when a selected moiety occurs two or more times in the same linker, the two or more occurrences are not adjacent. In some embodiments, a linker is not a bond.
In some embodiments, a linker is a bivalent moiety that connects a first group and a second group. In some other embodiments, the linker is a trivalent moiety that connects a first group, a second group and a third group. In a non-limiting example, a trivalent moiety is C(H) (i.e., methine) or N. In some other embodiments, a linker is a tetravalent moiety that connects a first group, a second group and a third group.
In some embodiments, a linker connects at least a first group and a second group, wherein the first group is a drug, and the second group is a biologically active polypeptide or protein. In some embodiments, the biologically active polypeptide or protein contains at least one non-natural amino acid. In some embodiments, the linker connects the drug to a non-natural amino acid of the biologically active polypeptide or protein. In some embodiments, the biologically active polypeptide or protein is an antibody. Thus, the antibody connected to a drug via a linker can be an antibody-drug conjugate (ADC), such as an ADC of the present disclosure.
In some other embodiments, a linker connects at least a first group and a second group, wherein the first group is a drug, and the second group is a reactive moiety. In some embodiments, the second group is a reactive moiety that is capable of reacting with a biologically active polypeptide or protein. In some embodiments, the biologically active polypeptide or protein contains at least one non-natural amino acid. Thus, in some embodiments, the reactive moiety is capable of reacting with a non-natural amino acid of the biologically active polypeptide or protein. In some embodiments, the biologically active polypeptide or protein is an antibody.
In some embodiments, a first linker or linker unit is connected to a second linker or linker unit and optionally to a third linker or linker unit, and the combined linkers (a composite linker) connects at least a first group and a second group. A composite linker of the present disclosure can contain 2, 3, 4, 5, 6, 7, 8, 9, 10 or more linker units. In a non-limiting example, a first, second and third linker unit are joined together to provide a composite linker that can connect a first group (e.g., a drug) to at least one other group, such as a reactive moiety and/or a biologically active polypeptide or protein (e.g., an antibody. In some embodiments, the biologically active polypeptide or protein (e.g., antibody) contains a non-natural amino acid.
In some embodiments, a linker is linear. In some other embodiments, a linker is branched.
The term “metabolite,” as used herein, refers to a derivative of a compound, by way of example natural amino acid polypeptide, a non-natural amino acid polypeptide, a modified natural amino acid polypeptide, or a modified non-natural amino acid polypeptide, that is formed when the compound, by way of example natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, or modified non-natural amino acid polypeptide, is metabolized. The term “pharmaceutically active metabolite” or “active metabolite” refers to a biologically active derivative of a compound, by way of example natural amino acid polypeptide, a non-natural amino acid polypeptide, a modified natural amino acid polypeptide, or a modified non-natural amino acid polypeptide, that is formed when such a compound, by way of example a natural amino acid polypeptide, non-natural amino acid polypeptide, modified natural amino acid polypeptide, or modified non-natural amino acid polypeptide, is metabolized.
The term “metabolized,” as used herein, refers to the sum of the processes by which a particular substance is changed by an organism. Such processes include, but are not limited to, hydrolysis reactions and reactions catalyzed by enzymes. Further information on metabolism may be obtained from The Pharmacological Basis of Therapeutics, 9th Edition, McGraw-Hill (1996). By way of example only, metabolites of natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non-natural amino acid polypeptides may be identified either by administration of the natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non- natural amino acid polypeptides to a host and analysis of tissue samples from the host, or by incubation of natural amino acid polypeptides, non-natural amino acid polypeptides, modified natural amino acid polypeptides, or modified non-natural amino acid polypeptides with hepatic cells in vitro and analysis of the resulting compounds.
The term “modified,” as used herein refers to the presence of a change to a natural amino acid, a non-natural amino acid, a natural amino acid polypeptide or a non-natural amino acid polypeptide. Such changes, or modifications, may be obtained by post synthesis modifications of natural amino acids, non-natural amino acids, natural amino acid polypeptides or non-natural amino acid polypeptides, or by co-translational, or by post-translational modification of natural amino acids, non-natural amino acids, natural amino acid polypeptides or non-natural amino acid polypeptides.
The term “non-natural amino acid” refers to an amino acid that is not one of the 20 common amino acids or pyrolysine or selenocysteine. Other terms that may be used synonymously with the term “non-natural amino acid” is “ non-natural ly encoded amino acid,” “unnatural amino acid,” “non-naturally-occurring amino acid,” “synthetic amino acid” and variously hyphenated and non-hyphenated versions thereof. The term “non-natural amino acid” includes, but is not limited to, amino acids which occur naturally by modification of a naturally encoded amino acid (including but not limited to, the 20 common amino acids or pyrrolysine and selenocysteine) but are not themselves incorporated into a growing polypeptide chain by the translation complex. Examples of naturally-occurring amino acids that are not naturally-encoded include, but are not limited to, N-acetylglucosaminyl-L-serine, N-acetylglucosaminyl-L-threonine, and O- phosphotyrosine. Additionally, the term “non-natural amino acid” includes, but is not limited to, amino acids which do not occur naturally and may be obtained synthetically or may be obtained by modification of non-natural amino acids.
The term “nucleic acid,” as used herein, refers to deoxyribonucleotides, deoxyribonucleosides, ribonucleosides or ribonucleotides and polymers thereof in either single- or double-stranded form. By way of example only, such nucleic acids and nucleic acid polymers include, but are not limited to, (i) analogues of natural nucleotides which have similar binding properties as a reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides; (ii) oligonucleotide analogs including, but are not limited to, PNA (peptidonucleic acid), analogs of DNA used in antisense technology (phosphorothioates, phosphoroamidates, and the like); (iii) conservatively modified variants thereof (including but not limited to, degenerate codon substitutions) and complementary sequences and sequence explicitly indicated. By way of example, degenerate codon substitutions may be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081, 1991; Ohtsuka et al., J. Biol. Chem. 260:2605-2608, 1985; and Rossolini et al., Mol. Cell. Probes 8:91- 98, 1994).
The term “pharmaceutically acceptable”, as used herein, refers to a material, including but not limited, to a salt, binder, adjuvant, excipient, carrier or diluent, which does not abrogate the biological activity or properties of the compound, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained. In some embodiments the disclosure concerns polymers. The term “polymer,” as used herein, refers to a molecule composed of repeated subunits. Such molecules include, but are not limited to, polypeptides, polynucleotides, or polysaccharides or polyalkylene glycols. Polymers of the disclosure can be linear or branched polymeric polyether polyols including, but are not limited to, polyethylene glycol, polypropylene glycol, polybutylene glycol, and derivatives thereof. Polymers can be activated polymers (e.g., activated PEGs) that facilitate conjugation to another group, such as a polypeptide, linker or drug-linker molecule. Polymers can also terminate in a moiety, such as a non-reactive moiety, e.g., alkyl (such as methyl) or alkoxy (such as methoxy). Other exemplary embodiments are listed, for example, in commercial supplier catalogs, such as Shearwater Corporation's catalog “Polyethylene Glycol and Derivatives for Biomedical Applications” (2001). By way of example only, such polymers have average molecular weights between about 0.1 kDa to about 100 kDa. Such polymers include, but are not limited to, between about 100 Da and about 100,000 Da or more. The molecular weight of the polymer may be between about 100 Da and about 100,000 Da, including but not limited to, about 100,000 Da, about 95,000 Da, about 90,000 Da, about 85,000 Da, about 80,000 Da, about 75,000 Da, about 70,000 Da, about 65,000 Da, about 60,000 Da, about 55,000 Da, about 50,000 Da, about 45,000 Da, about 40,000 Da, about 35,000 Da, about 30,000 Da, about 25,000 Da, about 20,000 Da, about 15,000 Da, about 10,000 Da, about 9,000 Da, about 8,000 Da, about 7,000 Da, about 6,000 Da, about 5,000 Da, about 4,000 Da, about 3,000 Da, about 2,000 Da, about 1,000 Da, about 900 Da, about 800 Da, about 700 Da, about 600 Da, about 500 Da, 400 Da, about 300 Da, about 200 Da, and about 100 Da. In some embodiments molecular weight of the polymer is between about 100 Da and about 50,000 Da. In some embodiments, the molecular weight of the polymer is between about 100 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 1,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 2,000 to about 50,000 Da. In some embodiments, the molecular weight of the polymer is between about 5,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is between about 10,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the polymer is within a range of about 100 Da to about 10,000 Da. In some embodiments, the molecular weight of the polymer is within a range of about 100 Da to about 5,000 Da. In some embodiments, the molecular weight of the polymer is within a range of about 100 Da to about 1,000 Da. In some embodiments, the polymer is a polyethylene glycol (PEG). In some embodiments, the PEG is a linear PEG. In some embodiments, the PEG is a branched PEG. The molecular weight of the linear or branched chain PEG may be between about 1,000 Da and about 100,000 Da, including but not limited to, about 100,000 Da, about 95,000 Da, about 90,000 Da, about 85,000 Da, about 80,000 Da, about 75,000 Da, about 70,000 Da, about 65,000 Da, about 60,000 Da, about 55,000 Da, about 50,000 Da, about 45,000 Da, about 40,000 Da, about 35,000 Da, about 30,000 Da, about 25,000 Da, about 20,000 Da, about 15,000 Da, about 10,000 Da, about 9,000 Da, about 8,000 Da, about 7,000 Da, about 6,000 Da, about 5,000 Da, about 4,000 Da, about 3,000 Da, about 2,000 Da, and about 1,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 1,000 Da and about 50,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 1,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 5,000 Da and about 40,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is between about 5,000 Da and about 20,000 Da. In other embodiments, the molecular weight of the linear or branched chain PEG is between about 2,000 to about 50,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is within a range of about 100 Da to about 10,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is within a range of about 100 Da to about 5,000 Da. In some embodiments, the molecular weight of the linear or branched chain PEG is within a range of about 100 Da to about 1,000 Da. In some embodiments, the PEG is a linear PEG. In some embodiments, the PEG comprises a defined number of repeating (-alkylene-O-) units, such as 2, 4, 6, 8, 10, 12, 14 or more units (e.g., PEG-2, PEG-4, PEG-6, PEG-8, PEG-10, PEG-12, PEG-14). In some embodiments, the PEG is a branched PEG. The term “PEGylating” or “PEGylated” is meant to refer to the covalent bonding of a specified moiety to a polyethylene glycol (PEG) molecule. In some embodiments, the moiety can be present in a drug, a drug-linker, a linker, or a polypeptide or protein. In some embodiments, the moiety is a hydroxyl group, a carboxylic acid, acyl or an amino group, such as a hydroxyl group, carboxylic acid, acyl or amino group present in a drug, drug-linker, linker or polypeptide. In some embodiments, the hydroxyl group, carboxylic acid, acyl or amino group is present in an amino acid. In some embodiments, the amino acid bearing the hydroxyl group, carboxylic acid, acyl or amino group is a natural or non-natural amino acid that is present in a polypeptide (e.g., an antibody), linker or drug-linker. The method can comprise contacting an isolated polypeptide comprising a natural or synthetic amino acid, or contacting a drug-linker comprising a natural or synthetic amino acid, with a water- soluble polymer (e.g., a PEG) comprising a moiety that reacts with the natural or synthetic amino acid. The method can comprise contacting an isolated a-TROP2 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid. The method can comprise contacting an isolated a-HER2 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid. The method can comprise contacting an isolated a-CD70 ADC polypeptide comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid. The method can comprise reacting a drug-linker comprising a natural or synthetic amino acid with a water-soluble polymer comprising a moiety that reacts with the natural or synthetic amino acid.
The terms “polypeptide,” “peptide” and “protein” are used interchangeably herein to refer to a polymer of amino acid residues. That is, a description directed to a polypeptide applies equally to a description of a peptide and a description of a protein, and vice versa. The terms apply to naturally occurring amino acid polymers as well as amino acid polymers in which one or more amino acid residues is a non-natural amino acid. Additionally, such “polypeptides,” “peptides” and “proteins” include amino acid chains of any length, including full length proteins, including but not limited to antibodies, wherein the amino acid residues are linked by covalent peptide bonds. In some embodiments, the term “peptide” can be used to refer to short chains of amino acids linked by peptide bonds and containing two or more amino acids linked in a chain. In some embodiments, the peptide contains 2 to 20 amino acids linked in the chain. In some embodiments, the peptide contains 2, 3, 4, 5, 6, 7 or 8 amino acids. As is well understood to a person of ordinary skill in the art, a dipeptide contains two amino acids.
The term “post-translationally modified” refers to any modification of a natural or nonnatural amino acid which occurs after such an amino acid has been translationally incorporated into a polypeptide chain. Such modifications include, but are not limited to, co-translational in vivo modifications, co-translational in vitro modifications (such as in a cell-free translation system), post-translational in vivo modifications, and post-translational in vitro modifications.
The terms “prodrug” or “pharmaceutically acceptable prodrug,” as used herein, refers to an agent that is converted into the parent drug in vivo or in vitro, which does not abrogate the biological activity or properties of the drug, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained. Prodrugs are generally drug precursors that, following administration to a subject and subsequent absorption, are converted to an active, or a more active species via some process, such as conversion by a metabolic pathway. Some prodrugs have a chemical group present on the prodrug that renders it less active and/or confers solubility or some other property to the drug. Once the chemical group has been cleaved and/or modified from the prodrug the active drug is generated. Prodrugs are converted into active drug within the body through enzymatic or non-enzymatic reactions. Prodrugs may provide improved physiochemical properties such as better solubility, enhanced delivery characteristics, such as specifically targeting a particular cell, tissue, organ or ligand, and improved therapeutic value of the drug. The benefits of such prodrugs include, but are not limited to, (i) ease of administration compared with the parent drug; (ii) the prodrug may be bioavailable by oral administration whereas the parent is not; and (iii) the prodrug may also have improved solubility in pharmaceutical compositions compared with the parent drug. A prodrug includes a pharmacologically inactive, or reduced activity, derivative of an active drug. Prodrugs may be designed to modulate the amount of a drug or biologically active molecule that reaches a desired site of action through the manipulation of the properties of a drug, such as physiochemical, biopharmaceutical, or pharmacokinetic properties. An example, without limitation, of a prodrug would be a non-natural amino acid polypeptide which is administered as an ester (the “prodrug”) to facilitate transmittal across a cell membrane where water solubility is detrimental to mobility and that is then metabolically hydrolyzed to the carboxylic acid, the active entity, once inside the cell where water solubility is beneficial. Prodrugs may be designed as reversible drug derivatives, for use as modifiers to enhance drug transport to site-specific tissues.
The term “prophylactically effective amount,” as used herein, refers to an amount of a composition containing at least one non-natural amino acid polypeptide or at least one modified non-natural amino acid polypeptide prophylactically applied to a patient which will relieve to some extent one or more of the symptoms of a disease, condition or disorder being treated. In such prophylactic applications, such amounts may depend on the patient's state of health, weight, and the like. It is considered well within the skill of the art for one to determine such prophylactically effective amounts by routine experimentation, including, but not limited to, a dose escalation clinical trial.
The term “recombinant host cell,” also referred to as “host cell,” refers to a cell which includes an exogenous polynucleotide, wherein the methods used to insert the exogenous polynucleotide into a cell include, but are not limited to, direct uptake, transduction, f-mating, or other methods known in the art to create recombinant host cells. By way of example only, such exogenous polynucleotide may be a nonintegrated vector, including but not limited to a plasmid, or may be integrated into the host genome.
The term “spacer” or “spacer element” as used herein refers to an atom or functional group that connects a first group to a second group. In some non-limiting embodiments, a spacer is a carbonyl (-C(O)-), -C(O)O-, -C(O)N(R)-, -O-, -S-, -N(R)- wherein each R is H or alkyl. In some embodiments the spacer is a bivalent spacer.
The term “subject” as used herein, refers to an animal which is the object of treatment, observation or experiment. By way of example only, a subject may be, but is not limited to, a mammal including, but not limited to, a human.
The term “substantially purified,” as used herein, refers to a component of interest that may be substantially or essentially free of other components which normally accompany or interact with the component of interest prior to purification. By way of example only, a component of interest may be “substantially purified” when the preparation of the component of interest contains less than about 30%, less than about 25%, less than about 20%, less than about 15%, less than about 10%, less than about 5%, less than about 4%, less than about 3%, less than about 2%, or less than about 1% (by dry weight) of contaminating components. Thus, a “substantially purified” component of interest may have a purity level of about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, about 96%, about 97%, about 98%, about 99% or greater. By way of example only, a natural amino acid polypeptide or a non-natural amino acid polypeptide may be purified from a native cell, or host cell in the case of recombinantly produced natural amino acid polypeptides or non-natural amino acid polypeptides. By way of example a preparation of a natural amino acid polypeptide or a non-natural amino acid polypeptide may be “substantially purified” when the preparation contains less than about 30%, less than about 25%, less than about 20%, less than about 15%, less than about 10%, less than about 5%, less than about 4%, less than about 3%, less than about 2%, or less than about 1% (by dry weight) of contaminating material. By way of example when a natural amino acid polypeptide or a non-natural amino acid polypeptide is recombinantly produced by host cells, the natural amino acid polypeptide or non-natural amino acid polypeptide may be present at about 30%, about 25%, about 20%, about 15%, about 10%, about 5%, about 4%, about 3%, about 2%, or about 1% or less of the dry weight of the cells. By way of example when a natural amino acid polypeptide or a non-natural amino acid polypeptide is recombinantly produced by host cells, the natural amino acid polypeptide or non-natural amino acid polypeptide may be present in the culture medium at about 5g/L, about 4g/L, about 3g/L, about 2g/L, about Ig/L, about 750mg/L, about 500mg/L, about 250mg/L, about lOOmg/L, about 50mg/L, about lOmg/L, or about Img/L or less of the dry weight of the cells. By way of example, “substantially purified” natural amino acid polypeptides or non-natural amino acid polypeptides may have a purity level of about 30%, about 35%, about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, about 99% or greater as determined by appropriate methods, including, but not limited to, SDS/PAGE analysis, RP-HPLC, SEC, and capillary electrophoresis.
The term “therapeutically effective amount,” as used herein, refers to the amount of a composition containing at least one non-natural amino acid polypeptide and/or at least one modified non-natural amino acid polypeptide administered to a patient already suffering from a disease, condition or disorder, sufficient to cure or at least partially arrest, or relieve to some extent one or more of the symptoms of the disease, disorder or condition being treated. The effectiveness of such compositions depends on conditions including, but not limited to, the severity and course of the disease, disorder or condition, previous therapy, the patient's health status and response to the drugs, and the judgment of the treating physician. By way of example only, therapeutically effective amounts may be determined by routine experimentation, including but not limited to a dose escalation clinical trial.
The term “toxic”, or “toxic moiety” or “toxic group” or “cytotoxic” or “cytotoxic payload” or “payload” or “cytotoxic drug” or “drug” or “cytotoxic moiety” as used herein, refers to a cytotoxic compound which can cause harm, disturbances, or death. Toxic moieties include, but are not limited to, a drug comprising or consisting of an auristatin, or an analog or derivative thereof. In some embodiments, a cytotoxic drug or moiety expressly excludes a topoisomerase I (TOPI) inhibitor. In some embodiments, a cytotoxic drug or moiety expressly excludes a TOPI inhibitor, wherein the TOPI inhibitor is exatecan, or a derivative thereof. In some embodiments, a cytotoxic drug or moiety expressly excludes exatecan derivative Dxd. In some embodiments, a cytotoxic drug or moiety expressly excludes PF-06380101 (also known as AurOlOl or Auristatin-
The terms “treat,” “treated,” “treating” or “treatment”, as used herein, include alleviating, preventing, abating or ameliorating a disease or condition symptoms, preventing additional symptoms, ameliorating or preventing the underlying metabolic causes of symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition. The terms “treat,” “treated,” “treating” or “treatment”, include, but are not limited to, prophylactic and/or therapeutic treatments. The term “treat”, “treated”, “treating” or “treatment” can refer to the decrease, reduction or amelioration of one or more symptoms or conditions or diseases associated with an antigen related or associated cancer. The term “treat”, “treated”, “treating” or “treatment” can refer to the administration of an ADC of the present disclosure to a subject in need thereof to decrease, reduce, improve, alter, relieve, affect or ameliorate an antigen related or associated cancer or disease or symptom or condition, or the predisposition toward a condition. The term "capable of specific binding" refers to protein or peptide (e.g., antibody) binding to a predetermined target substance (e.g., an antigen and/or groups of antigens), e.g. a target substance that is expressed on the surface of a cell; thus the term "binding to a target cell" or "binding to a cancer cell" is to be understand as referring to protein or peptide (e.g., antibody) binding to a predetermined target substance (e.g. antigen or antigens) that is expressed on such a cell. Typically, the protein or peptide (e.g., antibody) binds with an affinity of at least about IxlO7 Ml, and/or binds to the predetermined target substance (e.g., antigen, antigens or cell) with an affinity that is at least two-fold greater than its affinity for binding to a non-specific control substance (e.g., B SA, casein, non-cancer cells) other than the predetermined target substance or a closely-related target substance.
As used herein, the term “water-soluble polymer” refers to any polymer that is soluble in aqueous solvents. Such water-soluble polymers include, but are not limited to, polyethylene glycol, polyethylene glycol propionaldehyde, mono C1-C10 alkoxy or aryloxy derivatives thereof (described in U.S. Patent No. 5,252,714 which is incorporated by reference herein), monomethoxy-polyethylene glycol, polyvinyl pyrrolidone, polyvinyl alcohol, polyamino acids, divinylether maleic anhydride, N-(2-Hydroxypropyl)-methacrylamide, dextran, dextran derivatives including dextran sulfate, polypropylene glycol, polypropylene oxide/ethylene oxide copolymer, polyoxyethylated polyol, heparin, heparin fragments, polysaccharides, oligosaccharides, glycans, cellulose and cellulose derivatives, including but not limited to methylcellulose and carboxymethyl cellulose, serum albumin, starch and starch derivatives, polypeptides, polyalkylene glycol and derivatives thereof, copolymers of polyalkylene glycols and derivatives thereof, polyvinyl ethyl ethers, and alpha-beta-poly[(2-hydroxyethyl)-DL- aspartamide, and the like, or mixtures thereof. By way of example only, coupling of such water- soluble polymers to natural amino acid polypeptides or non-natural polypeptides may result in changes including, but not limited to, increased water solubility, increased or modulated serum half-life, increased or modulated therapeutic half-life relative to the unmodified form, increased bioavailability, modulated biological activity, extended circulation time, modulated immunogenicity, modulated physical association characteristics including, but not limited to, aggregation and multimer formation, altered receptor binding, altered binding to one or more binding partners, and altered receptor dimerization or multimerization. In addition, such water- soluble polymers may or may not have their own biological activity.
As used herein, the term “modulated serum half-life” refers to positive or negative changes in the circulating half-life of a modified biologically active molecule relative to its non-modified form. By way of example, the modified biologically active molecules include, but are not limited to, natural amino acid, non-natural amino acid, natural amino acid polypeptide or non-natural amino acid polypeptide. By way of example, serum half-life is measured by taking blood samples at various time points after administration of the biologically active molecule or modified biologically active molecule and determining the concentration of that molecule in each sample. Correlation of the serum concentration with time allows calculation of the serum half-life. By way of example, modulated serum half-life may be an increased in serum half-life, which may enable an improved dosing regimen or avoid toxic effects. Such increases in serum may be at least about two-fold, at least about three-fold, at least about five-fold, or at least about ten-fold. Methods for evaluating serum half-life are known in the art and may be used for evaluating the serum half-life of antibodies and antibody drug conjugates of the present disclosure.
The term “modulated therapeutic half-life,” as used herein, refers to positive or negative change in the half-life of the therapeutically effective amount of a modified biologically active molecule, relative to its non-modified form. By way of example, the modified biologically active molecules include, but are not limited to, natural amino acid, non-natural amino acid, natural amino acid polypeptide or non-natural amino acid polypeptide. By way of example, therapeutic half-life is measured by measuring pharmacokinetic and/or pharmacodynamic properties of the molecule at various time points after administration. Increased therapeutic half-life may enable a particular beneficial dosing regimen, a particular beneficial total dose, or avoids an undesired effect. By way of example, the increased therapeutic half-life may result from increased potency, increased or decreased binding of the modified molecule to its target, an increase or decrease in another parameter or mechanism of action of the non-modified molecule, or an increased or decreased breakdown of the molecules by enzymes such as, by way of example only, proteases. Methods for evaluating therapeutic half-life are known in the art and may be used for evaluating the therapeutic half-life of antibodies and antibody drug conjugates of the present disclosure.
ADC Antibodies and Antibody Sequences
The present invention provides novel ADCs comprising antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids incorporated at any desired position in the heavy and/or light chain amino acid sequence. Further, the present invention provides ADCs comprising one or more antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids site specifically incorporated in the heavy and/or light chain amino acid sequence conjugated to drug via a linker. In some embodiments, the antibody, antibody fragment or variant thereof is an anti-TROP2 antibody, antibody fragment or variant. In other embodiments the invention provides anti-TROP2 ADCs comprising antibodies, antibody fragments or variants thereof engineered to have one, or more non-natural amino acids incorporated at any desired position in the heavy and/or light chain amino acid sequence. In some embodiments, the present invention provides anti-TROP2 ADCs comprising one or more antibodies, antibody fragments or variants thereof engineered to have one or more non-natural amino acids site specifically incorporated in the heavy and/or light chain amino acid sequence conjugated to drug via a linker.
Antibody or antibody fragments or variants of the disclosure may be human, humanized, engineered, non-human, and/or chimeric antibody or antibody fragments. An antibody or antibody fragment or variant provided herein may comprise two or more amino acid sequences. A first amino acid sequence may comprise a first antibody chain and a second amino acid sequence may comprise a second antibody chain. A first antibody chain may comprise a first amino acid sequence, and a second antibody chain may comprise a second amino acid sequence. A chain of an antibody may refer to an antibody heavy chain, an antibody light chain, or a combination of a region or all of an antibody heavy chain and a region or all of an antibody light chain. As a nonlimiting example, an antibody provided herein comprises a heavy chain or fragment or variant thereof, and a light chain or fragment or variant thereof. Two amino acid sequences of an antibody, including two antibody chains, may be connected, attached, or linked by one or more disulfide bonds, a chemical linker, a peptide linker, or a combination thereof. A chemical linker includes a linker via a non-natural amino acid. A chemical linker includes a linker via one or more nonnatural amino acids. A chemical linker can include a chemical conjugate. A peptide linker includes any amino acid sequence joining the two amino acid sequences. The peptide linker may comprise 1 or more, 5 or more, 10 or more, 15 or more, 20 or more, 25 or more, 30 or more, 35 or more, 40 or more, 45 or more, 50 or more, 55 or more, 60 or more, 65 or more, 70 or more, 75 or more, 80 or more, 85 or more, 90 or more, 95 or more, 100 or more amino acids. The peptide linker may be a portion of any antibody, including a domain of an antibody, such as a variable domain, CDR1, CDR2, CDR3, and/or a combination of CDRs (complementarity determining regions). In some embodiments a heavy and a light chain are connected, attached, or linked, for example, via a peptide linker. In some cases, a heavy chain and a light chain are connected, for example, by one or more disulfide bonds.
Antibodies, antibody fragments and antibody variants of the disclosure may interact or engage with an antigen on an effector cell. The effector cell can include, but is not limited to, an immune cell, a genetically modified cell having increase or decrease cytotoxic activity, a cell involved in the host defense mechanism, an anti-inflammatory cell, a leukocyte, a lymphocyte, a macrophage, an erythrocyte, a thrombocyte, a neutrophil, a monocyte, an eosinophil, a basophil, a mast cell, a NK cell, a B-cell, or a T-cell. In some embodiments the immune cell may be a T cell such as a cytotoxic T cell or natural killer T cell. The antibody or antibody fragment may interact with a receptor on a T-cell such as, but not limited to a T-cell receptor (TCR). The TCR may comprise TCR alpha, TCR beta, TCR gamma, and/or TCR delta or TCR zeta. Antibody or antibody fragments of the disclosure may bind to a receptor on a lymphocyte, dendritic cell, B- cell, macrophage, monocytes, neutrophils and/or NK cells. Antibody or antibody fragments of the disclosure may bind to a cell surface receptor. Antibody or antibody fragments of the disclosure may bind to an antigen receptor, such as for example, a TR0P2 antigen receptor, or a HER2 antigen receptor, or a CD70 antigen receptor. Antibody or antibody fragments of the disclosure can be conjugated to a T-cell surface antigen.
Some cell surface antigens have a high overexpression pattern in a large number of tumors, making them excellent targets in the development of ADCs. Thus, the present disclosure provides novel anti-TROP2 antibodies, or the corresponding antibody fragments, and antibody-drug conjugates thereof for use as therapeutic agents. Disclosed herein are novel anti-TROP2 antibodies, antibody fragments or variants thereof with at least one non-natural amino acid or unnaturally encoded amino acid. The present invention provides anti-TROP2 antibodies having at least one non-natural amino acid that facilitates antibody conjugation to a drug or drug-linker.
Antibodies, antibody fragments or variants provided in the present disclosure may be human, humanized, engineered, non-human, and/or chimeric antibody or antibody fragments that bind to the extracellular domain of the target antigen, which can be overexpressed in a number of cancers. Thus, novel antibodies, compositions and antibody drug conjugates for the treatment and/or diagnosis of antigen-expressing cancers are beneficial, including but not limited to TR0P2- expressing cancers.
Antibodies or antibody fragments or variants disclosed herein include, but are not limited to, analogs, isoforms, mimetics, fragments, or hybrids of anti-TROP2. Antibodies or antibody fragments or variants of anti-TROP2 of the present disclosure include but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like.
Antibodies comprising non-natural amino acids are also disclosed herein. In certain embodiments, the antibody or antibody fragments or variants include but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like. In some embodiments, the anti-TROP2 antibody or antibody fragments or variants comprises one or more non-natural amino acids.
Non-limiting examples of antibodies or antibody fragments or variants of the present disclosure comprise the sequences listed in Table 1. In certain embodiments antibody or antibody fragments disclosed herein are anti-TROP2 antibodies or antibody fragments or variants thereof. In certain embodiments, the anti-TROP2 antibodies or antibody fragments or variants disclosed herein can be humanized. Anti-TR0P2 antibodies or antibody fragments or variants disclosed herein include, but are not limited to, anti- TR0P2 analogs, isoforms, mimetics, fragments, or hybrids. Anti-TR0P2 antibodies or antibody fragments or variants of the present disclosure include but are not limited to Fv, Fc, Fab, and (Fab')2, single chain Fv (scFv), diabodies, triabodies, tetrabodies, bifunctional hybrid antibodies, CDR1, CDR2, CDR3, combinations of CDRs, variable regions, framework regions, constant regions, heavy chains, light chains, alternative scaffold non-antibody molecules, bispecific antibodies and the like. The anti-TROP2 antibodies or antibody fragments or variants of the present disclosure comprise a sequence of SEQ ID NOs: 1 to 17 (Table 1). The antibodies, fragments or variants of the present disclosure can be an anti-TROP2 antibody, fragment or variant.
In certain embodiments, the anti-TROP2 antibody is characterized by the amino acid sequence of its polypeptide chain(s) (e.g., the amino acid sequence of a heavy chain and/or a light chain, each of which can be referred to herein as a heavy chain amino acid sequence and/or a light chain amino acid sequence, respectively).
The anti-TROP2 antibody heavy chain amino acid sequence and/or light chain amino acid sequence can comprise any one of SEQ ID NOs: 1 to 17. In some embodiments, the anti-TROP2 antibody heavy chain amino acid and/or light chain amino acid sequence is independently selected from the group consisting of SEQ ID NOs: 1 to 17. In certain embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises any one of SEQ ID NOs: 1, 2, 5, and 6; and a light chain amino acid sequence comprises any one of SEQ ID NOs: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17. In certain embodiments, the anti-TROP2 antibody heavy chain amino acid sequence is selected from the group consisting of SEQ ID NOs: 1, 2, 5, and 6; and a light chain amino acid sequence is selected from the group consisting of SEQ ID NOs: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 1 or 5. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 5. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 2 or 6. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 6. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 3 or 4. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 3 or 7. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11. In some embodiments, a light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 5, and a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, a heavy chain amino acid sequence is SEQ ID NO: 5, and a light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence is SEQ ID NO: 5, and each light chain amino acid sequence is SEQ ID NO: 4.
In some embodiments, the anti-TROP2 antibody comprises a heavy chain and a light chain, each characterized by its heavy chain and light chain amino acid sequence, respectively. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1, and the light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and a light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and a light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 1 and the light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and a light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, the anti- TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 1 and the light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody comprises two heavy chains and two light chains, each characterized by its heavy chain and light chain amino acid sequence, respectively. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1, and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 1 and each light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody consists of two heavy chains and two light chains, each characterized by its heavy chain and light chain amino acid sequence, respectively. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 1 and each light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and a light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 2 and the light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence of SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence consist of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consist of SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 2 and the light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody comprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 2 and each light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, wherein the anti-TROP2 antibody comprises two heavy chains and two light chains, each heavy chain amino acid sequences consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 2 and each light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 5 and the light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 5 and the light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody comprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 5 and each light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, wherein the anti-TROP2 antibody comprises two heavy chains and two light chains, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 5 and each light chain amino acid sequences of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence comprises SEQ ID NO: 6 and the light chain amino acid sequence comprises SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, the heavy chain amino acid sequence consists of SEQ ID NO: 6 and the light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody comprises two heavy chains and two light chains, wherein each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence comprises of SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence comprises SEQ ID NO: 6 and each light chain amino acid sequence comprises SEQ ID NO: 17. In some embodiments, wherein the anti-TROP2 antibody comprises two heavy chains and two light chains, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 3. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 4. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 7. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 8. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 9. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 10. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 11. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 12. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 13. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 14. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 15. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 16. In some embodiments, each heavy chain amino acid sequence consists of SEQ ID NO: 6 and each light chain amino acid sequence consists of SEQ ID NO: 17.
In some embodiments, the anti-TROP2 antibody comprises a heavy chain, wherein the amino acid sequence of the heavy chain contains amino acid sequence EEM in an Fc constant region. In some embodiments, said EEM sequence can be replaced with DEL.
Table 1. Anti-TROP2 amino acid sequences. Also disclosed are: all of the sequences in Table 1, wherein X is replaced with any non-natural amino acid; all of the sequences in Table 1, wherein any amino acid is replaced with any non-natural amino acid; all of the sequences in Table 1, wherein X is pAF; all of the heavy chain sequences in Table 1, wherein EEM is replaced with DEL; and all of the heavy chain sequences in Table 1, wherein a non-natural amino acid is site specifically incorporated at position 114, according to Kabat numbering, as well known to the skilled artisan). WT: Wild Type; HC: Heavy Chain; LC: Light Chain; X denotes non-natural amino acid.
Non-Natural Amino Acids
The present disclosure provides antibodies, antibody fragments or variants comprising at least one non-natural amino acid. Introduction of at least one non-natural amino acid into an antibody can allow for the application of conjugation chemistries that involve specific chemical reactions with one or more non-natural amino acids while not reacting with the commonly occurring 20 amino acids.
Non-natural amino acid site selection was based on surface exposure/site accessibility within the antibody and hydrophobic or neutral amino acid sites were selected to maintain the charge on the antibody Methods for introducing non-natural amino acids inserted into sites in a protein are described for example in W02010/011735 and in W02005/074650. The present disclosure employs such methodologies and techniques. The non-natural amino acids used in the methods and compositions described herein have at least one of the following four properties: (1) at least one functional group on the sidechain of the non-natural amino acid has at least one characteristics and/or activity and/or reactivity orthogonal to the chemical reactivity of the 20 common, genetically-encoded amino acids (i.e., alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine), or at least orthogonal to the chemical reactivity of the naturally occurring amino acids present in the polypeptide that includes the non-natural amino acid; (2) the introduced non-natural amino acids are substantially chemically inert toward the 20 common, genetically-encoded amino acids; (3) the non-natural amino acid can be stably incorporated into a polypeptide, preferably with the stability commensurate with the naturally-occurring amino acids or under typical physiological conditions, and further preferably such incorporation can occur via an in vivo system; and (4) the non-natural amino acid includes an oxime functional group or a functional group such as -ONH2 that can be transformed into an oxime group by reacting with a reagent, preferably under conditions that do not destroy the biological properties of the polypeptide that includes the non-natural amino acid (unless of course such a destruction of biological properties is the purpose of the modification/transformation), or where the transformation can occur under aqueous conditions at a pH between about 4 and about 8, or where the reactive site on the non-natural amino acid is an electrophilic site. Any number of non-natural amino acids can be introduced into the polypeptide. Non-natural amino acids may also include protected or masked oximes or protected or masked groups that can be transformed into an oxime group after deprotection of the protected group or unmasking of the masked group. Non-natural amino acids may also include protected or masked carbonyl or dicarbonyl groups, which can be transformed into a carbonyl or dicarbonyl group after deprotection of the protected group or unmasking of the masked group and thereby are available to react with hydroxylamines or oximes to form oxime groups. Oxime-based non-natural amino acids may be synthesized by methods well known in the art, (see for example WO2013/185117 and W02005/074650), including: (a) reaction of a non-natural amino acid containing -ONH2 with a carbonyl- or dicarbonyl-containing reagent; and (b) reaction of a carbonyl- or dicarbonylcontaining non-natural amino acid with a reagent containing -ONH2.
In some embodiments, non-naturally encoded amino acid site selection is based on surface exposure. Example, one possible site is an amino acid having a solvent accessible surface area ratio of 30% or more, 40% or more, 50% or more, 60% or more, 70% or more, 80% or more, 90% or more, or 95% or more. In some embodiments, one possible site is an amino acid having a solvent accessible surface area ratio of about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 95%, or more. The solvent accessible surface area can be calculated based on the DSSP program [Biopolymers, 22, 2577-2637 (1983)], using a crystalline structure analyzing data file of antibodies or antibody fragments registered in Protein data bank (PDB).
The ratio of the solvent accessible surface area of the amino acid residues of interest can be calculated by dividing the antibody structural solvent accessible surface area calculated in the above by the solvent accessible surface area of alanine-X-alanine (X represents the amino acid residues of interest). In this connection, there is a case in which two or more PDB files are present on one species of protein, and any one of them can be used in the present invention. Alternatively, the solvent accessibility of an amino acid can be determined by a solvent accessibility test in which a functional group on the amino acid (a thiol, amino, or carbonyl group) is functionalized when treated with an electrophilic reagent or a nucleophilic reagent, or the like. Based on the test results, the functional group (i.e., the thiol, amino, or carbonyl group) can be called, for example, at least 50% solvent accessible when at least 50% of the functional group is functionalized in the test. In some embodiments, the non-natural amino acid site is at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, or at least 95% solvent accessible. Examples of solvent accessibility test include, but are not limited to, propargylation of a surface thiol group, or a-bromopyruvate reacting with a surface thiol group, etc.
Non-natural amino acids that may be used in the methods and compositions described herein include, but are not limited to, amino acids comprising amino acids with novel functional groups, amino acids that covalently or noncovalently interact with other molecules, glycosylated amino acids such as a sugar substituted serine, other carbohydrate modified amino acids, ketocontaining amino acids, aldehyde-containing amino acids, amino acids comprising polyethylene glycol or other polyethers, heavy atom substituted amino acids, chemically cleavable and/or photocleavable amino acids, amino acids with an elongated side chains as compared to natural amino acids, including but not limited to, polyethers or long chain hydrocarbons, including but not limited to, greater than about 5 or greater than about 10 carbons, carbon-linked sugar- containing amino acids, redox-active amino acids, amino thioacid containing amino acids, and amino acids comprising one or more toxic moiety.
In some embodiments disclosed herein are antibodies comprising one or more non-natural amino acids. The one or more non-natural amino acids may be encoded by a codon that does not code for one of the twenty natural amino acids. The one or more non-natural amino acids may be encoded by a nonsense codon (stop codon). The stop codon may be an amber codon. The amber codon may comprise a UAG sequence. The stop codon may be an ochre codon. The ochre codon may comprise a UAA sequence. The stop codon may be an opal or umber codon. The opal or umber codon may comprise a UGA sequence. The one or more non-natural amino acids may be encoded by a four-base codon.
Non-natural amino acids of the present disclosure include, but are not limited to, 1) substituted phenylalanine and tyrosine analogues, such as 4-amino-L-phenylalanine, 4-acetyl-L- phenylalanine, 4-azido-L-phenylalanine, 4-nitro-L-phenylalanine, 3 -methoxy -L-phenylalanine, 4-isopropyl-L-phenylalanine, 3-nitro-L-tyrosine, O-methyl-L-tyrosine and O-phosphotyrosine; 2) amino acids that can be photo-cross-linked, e.g., amino acids with aryl azide or benzophenone groups, such as 4-azidophenylalanine or 4-benzoylphenylalanine; 3) amino acids that have unique chemical reactivity, such as 4-acetyl-L-phenylalanine, 3-acetyl-L-phenylalanine, O-allyl-L- tyrosine, O-2-propyn-l-yl-L-tyrosine, N-(ethylthio)thiocarbonyl-L-phenylalanine and p-(3- oxobutanoyl)-L-phenylalanine; 4) heavy-atom-containing amino acids, e.g., for phasing in X-ray crystallography, such as 4-iodo-L-phenylalanine or 4-bromo-L-phenylalanine; 5) a redox-active amino acid, such as 3,4-dihydroxy-L-phenylalanine; 6) a fluorinated amino acid, such as a 2- fluorophenylalanine (e.g., 2-fluoro-L-phenylalanine), a 3 -fluorophenylalanine (e.g., 3-fluoro-L- phenylalanine) or a 4-fluorophenylalanine (e.g., 4-fluoro-L-phenylalanine; 7) a fluorescent amino acid, such as an amino acid containing a naphthyl, dansyl or 7-aminocoumarin side chain; 8) a photocleavable or photoisomerizable amino acid, such as an amino acid comprising an azobenzyl or nitrobenzyl, e.g., cysteine, serine or tyrosine comprising azobenzyl or nitrobenzyl; 9) a P-amino acid (e.g., a2 or p3 amino acid); 10) a homo-amino acid, such as homoglutamine (e.g., betahomoglutamine) or homophenylalanine (e.g., beta-homophenylalanine); 11) a proline or pyruvic acid derivative; 12) a 3-substituted alanine derivative; 14) a glycine derivative; 15) a linear core amino acid; 16) a diamino acid; 17) a D-amino acid; 18) an N-methyl amino acid; 19) a phosphotyrosine mimetic, such as a carboxymethylphenylalanine (pCmF) (e.g., 4-carboxymethyl- L-phenylalanine); 20) 2-aminooctanoic acid; and 21) an amino acid comprising a saccharide moiety, such as N-acetyl-L-glucosaminyl-L-serine, beta-N-acetylglucosamine-O-serine, N- acetyl-L-galactosaminyl-L-serine, alpha-N-acetylgalactosamine-O-serine, O-(3-O-D-galactosyl- N-acetyl-beta-D-galactosaminyl)-L-serine, N-acetyl-L-glucosaminyl-L-threonine, alpha-N- acetylgalactosamine-O-threonine, 3-O-(N-acetyl-beta-D-glucosaminyl)-L-threonine, N-acetyl-L- glucosaminyl-L-asparagine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine and O-(mannosyl)- L-serine; an amino acid wherein the naturally-occurring N- or O- linkage between the amino acid and the saccharide is replaced by a covalent linkage not commonly found in nature, including but not limited to, an alkene, an oxime, a thioether, an amide and the like; or an amino acid containing saccharides that are not commonly found in naturally-occurring polypeptides, such as 2-deoxy- glucose, 2-deoxy-galactose and the like. Specific examples of non-natural amino acids include, but are not limited to, a p-acetylphenylalanine (4-acetyl phenylalanine) (including 4-acetyl-L- phenylalanine, also referred to herein as p-acetyl-L-phenylalanine (pAF)), a 4- boronophenylalanine (pBoF) (e.g., 4-borono-L-phenylalanine, a 4-propargyloxyphenylalanine (pPrF) (e.g., 4-propargyloxy-L-phenylalanine), an O-methyltyrosine (e.g., O-methyl-L-tyrosine), a 3-(2-naphthyl)alanine (NapA) (e.g., 3-(2-naphthyl)-L-alanine), a 3 -methylphenylalanine (e.g., 3-methyl-L-phenylalanine), an O-allyltyrosine (e.g., O-allyl-L-tyrosine), an O-isopropyltyrosine (e.g., O-isopropyl-L-tyrosine), a dopamine (e.g., L-Dopa), a 4-isopropylphenylalanine (e.g., 4- isopropyl-L-phenylalanine), a 4-azidophenylalanine (pAz) (e.g., 4-azido-L-phenylalanine), a 4- benzoylphenylalanine (pBpF) (e.g., 4-benzoyl-L-phenylalanine), an O-phosphoserine (e.g., O- phospho-L-serine), an O-phosphotyrosine (e.g., O-phospho-L-tyrosine), a 4-iodophenylalanine (pIF) (e.g., 4-iodo-L-phenylalanine, a 4-bromophenylalanine (e.g., 4-bromo-L-phenylalanine), a 4-aminophenylalanine (e.g., 4-amino-L-phenylalanine), a 4-cyanophenylalanine (pCNF) (e.g., 4- cyano-L-phenylalanine, a (8-hydroxyquinolin-3-yl)alanine (HQA) (e.g., (8-hydroxyquinolin-3- yl)-L-alanine), a (2,2-bipyridin-5-yl)alanine (BipyA) (e.g., (2,2-bipyridin-5-yl)-L-alanine), and the like. Additional non-natural amino acids are disclosed in Liu et al. (2010) Annu Rev Biochem, 79:413-44; Wang et al. (2005) Angew Chem Int Ed, 44:34-66; and Published International Application Nos.: WO 2012/166560, WO 2012/166559, WO 2011/028195, WO 2010/037062, WO 2008/083346, WO 2008/077079, WO 2007/094916, WO 2007/079130, WO 2007/070659 and WO 2007/059312, the entire contents of each of which are hereby incorporated by reference herein in their entirety. In some embodiments, the one or more non-natural amino acids can be p- acetylphenylalanine. In some more particular embodiments, the one or more non-natural amino acids can be p-acetyl-L-phenylalanine (pAF).
In some embodiments, one or more non-natural amino acids is selected from the group consisting of 4-acetyl phenylalanine, 3-O-(N-acetyl-beta-D-glucosaminyl)threonine, N4-(0-N- Acetyl-D-glucosaminyl)asparagine, O-allyltyrosine, alpha-N-acetylgalactosamine-O-serine, alpha-N-acetylgalactosamine-O-threonine, 2-aminooctanoic acid, 2-aminophenylalanine, 3- aminophenylalanine, 4-aminophenylalanine, 2-aminotyrosine, 3 -aminotyrosine, 4- azidophenylalanine, 4-benzoylphenylalanine, (2,2-bipyridin-5yl)alanine, 3-boronophenylalanine, 4-boronophenylalanine, 4-bromophenylalanine, p-carboxymethylphenylalanine, 4- carboxyphenylalanine, p-cyanophenylalanine, 3,4-dihydroxyphenylalanine, 4- ethynylphenylalanine, 2-fluorophenylalanine, 3 -fluorophenylalanine, 4-fluorophenylalanine, O- (3-O-D-galactosyl-N-acetyl-beta-D-galactosaminyl)serine, homoglutamine, (8-hydroxyquinolin-
3-yl)alanine, 4-iodophenylalanine, 4-isopropylphenylalanine, O-i-propyltyrosine, 3- isopropyltyrosine, O-mannopyranosyl serine, 2-methoxyphenylalanine, 3 -methoxyphenylalanine,
4-methoxyphenylalanine, 3 -methylphenylalanine, O-methyltyrosine, 3-(2-naphthyl)alanine, 5- nitrohistidine, 4-nitrohistidine, 4-nitroleucine, 2-nitrophenylalanine, 3 -nitrophenylalanine, 4- nitrophenylalanine, 4-nitrotryptophan, 5 -nitrotryptophan, 6-nitrotryptophan, 7 -nitrotry ptophan, 2- nitrotyrosine, 3 -nitrotyrosine, O-phosphoserine, O-phosphotyrosine, 4- propargyloxyphenylalanine, O-2-propyn-l-yltyrosine, 4-sulfophenylalanine and O-sulfotyrosine.
In some further embodiments, one or more non-natural amino acids is selected from the group consisting of 4-acetyl-L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N- acetyl-beta-D-glucosaminyl)-L-threonine, N4-(p-N-Acetyl-D-glucosaminyl)-L-asparagine, O- allyl-L-tyrosine, alpha-N-acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L- threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino- L-phenylalanine, 2-amino-L-tyrosine, 3-amino-L-tyrosine, 4-azido-L-phenylalanine, 4-benzoyl- L-phenylalanine, (2,2-bipyridin-5yl)-L-alanine, 3-borono-L-phenylalanine, 4-borono-L- phenylalanine, 4-bromo-L-phenylalanine, p-carboxymethyl-L-phenylalanine, 4-carboxy-L- phenylalanine, p-cyano-L-phenylalanine, 3, 4-dihydroxy -L-phenylalanine (L-DOPA), 4-ethynyl- L-phenylalanine, 2-fluoro-L-phenylalanine, 3-fluoro-L-phenylalanine, 4-fluoro-L-phenylalanine, O-(3-O-D-galactosyl-N-acetyl-beta-D-galactosaminyl)-L-serine, L-homoglutamine, (8- hydroxyquinolin-3-yl)-L-alanine, 4-iodo-L-phenylalanine, 4-isopropyl-L-phenylalanine, O-i- propyl-L-tyrosine, 3-isopropyl-L-tyrosine, O-mannopyranosyl-L-serine, 2-methoxy-L- phenylalanine, 3 -methoxy -L-phenylalanine, 4-m ethoxy -L-phenylalanine, 3-methyl-L- phenylalanine, O-methyl-L-tyrosine, 3-(2-naphthyl)-L-alanine, 5-nitro-L-histidine, 4-nitro-L- histidine, 4-nitro-L-leucine, 2-nitro-L-phenyl alanine, 3-nitro-L-phenylalanine, 4-nitro-L- phenylalanine, 4-nitro-L-tryptophan, 5-nitro-L-tryptophan, 6-nitro-L-tryptophan, 7-nitro-L- tryptophan, 2-nitro-L-tyrosine, 3-nitro-L-tyrosine, O-phospho-L-serine, O-phospho-L-tyrosine, 4-propargyloxy -L-phenylalanine, O-2-propyn-l-yl-L-tyrosine, 4-sulfo-L-phenylalanine and O- sulfo-L-tyrosine. In some embodiments, the one or more non-natural amino acids can be p-acetyl- L-phenylalanine (pAF). Thus, in some embodiments, each and every one of the one or more nonnatural amino acids is pAF.
In certain embodiments of the disclosure, an antibody with at least one non-natural amino acid includes at least one post-translational modification. In one embodiment, the at least one post- translational modification comprises attachment of a molecule including but not limited to, a water-soluble polymer, a derivative of polyethylene glycol, a drug, a second protein or polypeptide or polypeptide analog, an antibody or antibody fragment, a biologically active agent, a small molecule, or any combination of the above or any other desirable compound or substance, comprising a second reactive group to at least one non-natural amino acid comprising a first reactive group utilizing chemistry methodology that is known to one of ordinary skill in the art to be suitable for the particular reactive groups. For example, the first reactive group is an alkynyl moiety (including but not limited to, the non-natural amino acid /?-propargyloxyphenylalanine, where the propargyl group is also sometimes referred to as an acetylene moiety) and the second reactive group is an azido moiety, and [3+2] cycloaddition chemistry methodologies are utilized. In another example, the first reactive group is the azido moiety (including but not limited to, the non-natural amino acid /?-azido-L-phenylalanine) and the second reactive group is the alkynyl moiety. In certain embodiments of the modified antibody polypeptide of the present disclosure at least one non-natural amino acid, (including but not limited to, non-natural amino acid containing a keto functional group), comprising at least one post-translational modification is used where the at least one post-translational modification comprises a saccharide moiety. In certain embodiments, the post-translational modification is made in vivo in a eukaryotic cell or in a non-eukaryotic cell. In other embodiments the post-translational modification is made in vitro. In another embodiment, the post-translational modification is made in vitro and in vivo.
In some embodiments, the non-natural amino acid may be modified to incorporate a chemical group. In some embodiments the non-natural amino acid may be modified to incorporate a ketone group. The one or more non-natural amino acids may comprise at least one oxime, carbonyl, dicarbonyl, hydroxylamine group or a combination thereof. The one or more non-natural amino acids may comprise at least one carbonyl, dicarbonyl, alkoxy-amine, hydrazine, acyclic alkene, acyclic alkyne, cyclooctyne, aryl/alkyl azide, norbomene, cyclopropene, transcyclooctene, or tetrazine functional group or a combination thereof.
In some embodiments disclosed herein the non-natural amino acid is site-specifically incorporated into the antibody, antibody fragment or variant. In some embodiments the non- natural amino acid is site-specifically incorporated into an antibody, antibody fragment or variant. Methods for incorporating a non-natural amino acid into a molecule, for example, proteins, polypeptides or peptides, are disclosed in U.S. Patent Nos.: 7,332,571; 7,928,163; 7,696,312; 8,008,456; 8,048,988; 8,809,511; 8,859,802; 8,791,231; 8,476,411; or 9,637,411, (each of which is incorporated herein by reference in its entirety), and in the Examples herein. The one or more non-natural amino acids may be incorporated by methods known in the art. For example, cellbased or cell-free systems may be used, and auxotrophic strains may also be used in place of engineered tRNA and synthetase. In certain embodiments, orthogonal tRNA synthetase are used as disclosed in for example, W02002085923A2; W02002086075A2; W02004035743A2; W02007021297A1; W02006068802A2; and W02006069246A2; the contents of each of which are incorporated herein by reference in their entirety. Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variant may comprise modifying one or more amino acid residues in the antibody or antibody fragment or variant. Modifying the one or more amino acid residues in the antibody or antibody fragment or variant may comprise mutating one or more nucleotides in the nucleotide sequence encoding the antibody or antibody fragment or variant. Mutating the one or more nucleotides in the nucleotide sequence encoding the antibody or antibody fragment or variant may comprise altering a codon encoding an amino acid to a nonsense codon. Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variant may comprise modifying one or more amino acid residues in the antibody or antibody fragment or variant to produce one or more amber codons in the antibody or antibody fragment or variant. The one or more non-natural amino acids may be incorporated into the antibody or antibody fragment or variant in response to an amber codon. The one or more non- natural amino acids may be site-specifically incorporated into the antibody or antibody fragment or variant. Incorporating one or more non-natural amino acids into the antibody or antibody fragment or variant may comprise one or more genetically encoded non-natural amino acids with orthogonal chemical reactivity relative to the canonical twenty amino acids to site-specifically modify the biologically active molecule or targeting agent. Incorporating the one or more non- natural amino acids may comprise use of a tRNA/aminoacyl-tRNA synthetase pair to site- specifically incorporate one or more non-natural amino acids at defined sites in the biologically active molecule or targeting agent in response to one or more amber nonsense codon. Additional methods for incorporating non-natural amino acids include, but are not limited to, methods disclosed in Chatterjee et al., A Versatile Platform for Single- and Multiple-Unnatural Amino Acid Mutagenesis in Escherichia coli, Biochemistry', 2013; Kazane et ah, J Am Chem Soc, 135(l):340-6, 2013; Kim et al., J Am Chem Soc, 134(24):9918-21, 2012; Johnson et al., Nat Chem Biol, 7(11): 779-86, 2011; and Hutchins et al., J Mol Biol, 406(4):595-603, 2011. The one or more non-natural amino acids may be produced through selective reaction of one or more natural amino acids. The selective reaction may be mediated by one or more enzymes. In non-limiting examples, the selective reaction of one or more cysteines with formylglycine generating enzyme (FGE) may produce one or more formylglycines as described in Rabuka et al., Nature Protocols 7: 1052-1067, 2012. The one or more non-natural amino acids may involve a chemical reaction to form a linker. The chemical reaction to form the linker may include a bioorthogonal reaction. The chemical reaction to form the linker may include click chemistry. See for example W02006/050262 incorporated herein by reference in its entirety.
Any position of the antibody or antibody fragment is suitable for selection to incorporate a non-natural amino acid, and selection may be based on rational design or by random selection for any or no particular desired purpose. Selection of desired sites may be based on producing a non-natural amino acid polypeptide (which may be further modified or remain unmodified) having any desired property or activity, including but not limited to a receptor binding modulators, receptor activity modulators, modulators of binding to binder partners, binding partner activity modulators, binding partner conformation modulators, dimer or multimer formation, no change to activity or property compared to the native molecule, or manipulating any physical or chemical property of the polypeptide such as solubility, aggregation, or stability. Alternatively, the sites identified as critical to biological activity may also be good candidates for substitution with a non- natural amino acid, again depending on the desired activity sought for the polypeptide. Another alternative would be to simply make serial substitutions in each position on the polypeptide chain with a non-natural amino acid and observe the effect on the activities of the polypeptide. Any means, technique, or method for selecting a position for substitution with a non-natural amino acid into any polypeptide is suitable for use in the methods, techniques and compositions described herein.
The structure and activity of naturally-occurring mutants of a polypeptide that contain deletions can also be examined to determine regions of the protein that are likely to be tolerant of substitution with a non-natural amino acid. Once residues that are likely to be intolerant to substitution with non-natural amino acids have been eliminated, the impact of proposed substitutions at each of the remaining positions can be examined using methods including, but not limited to, the three-dimensional structure of the relevant polypeptide, and any associated ligands or binding proteins. X-ray crystallographic and NMR structures of many polypeptides are available in the Protein Data Bank (PDB, see world wide web for rcsb.org), a centralized database containing three-dimensional structural data of large molecules of proteins and nucleic acids, and can be used to identify amino acid positions that can be substituted with non-natural amino acids. In addition, models may be made investigating the secondary and tertiary structure of polypeptides, if three-dimensional structural data is not available. Thus, the identity of amino acid positions that can be substituted with non-natural amino acids can be determined by the skilled person.
Exemplary sites of incorporation of a non-natural amino acid include, but are not limited to, those that are excluded from potential receptor binding regions, or regions for binding to binding proteins or ligands may be fully or partially solvent exposed, have minimal or no hydrogen-bonding interactions with nearby residues, may be minimally exposed to nearby reactive residues, and/or may be in regions that are highly flexible as predicted by the three- dimensional crystal structure of a particular polypeptide with its associated receptor, ligand or binding proteins
A wide variety of non-natural amino acids can be substituted for, or incorporated into, a given position in a polypeptide. By way of example, a particular non-natural amino acid may be selected for incorporation based on an examination of the three-dimensional crystal structure of a polypeptide with its associated ligand, receptor and/or binding proteins, a preference for conservative substitutions.
Linkers
In some aspects, the present disclosure relates to linkers for intracellular delivery of drug conjugates. Many procedures and linker molecules for attachment of various compounds to peptides or proteins, such as antibodies, are known. See, for example, U.S. Patent Nos. 4,671,958, 4,414,148, 4,699,784, 4,680,338, 4,569,789 and 10,550,190; PCT Application Publication Nos. WO2012/166559A1, WO2012/166560A1, WO2013/185117A1, WO2013188740A1,
WO2013/192360A1, WO2019191728A1, W02020/168017A1, WO2021173889A1, WO2021183832A1 and W02022040596A1; and US Patent Application Publication No. US 2017/0182181A1; the contents of each of which are hereby incorporated by reference in their entirety.
Generally, a linker of the present disclosure can comprise one or more linker units. A linker comprising one or more linker units can bond to one or more drugs.
Each linker unit can comprise one or more moieties, each of which may occur one or more times within a given linker unit. In a non-limiting example, a linker or linker unit of the present disclosure can comprise one or more amino acids, wherein each amino acid is the same or different. In another non-limiting example, a linker or linker unit of the present disclosure can comprise one or more unsubstituted alkylene groups, wherein each said unsubstituted alkylene group can be the same or different.
It is understood that, whenever a linker moiety is independently selected from a group of variables, the independent selection can be made within a given linker. In a non-limiting example, a linker of formula -alkylene-arylene-alkylene-(O-alkylene)n-, wherein each alkylene is independently selected from the group consisting of -CH2CH2-, -CH2CH2CH2- and - CH2CH2CH2CH2-, includes, but is not limited to, the following species:
-CH2CH2CH2CH2-arylene-CH2CH2-(O-CH2CH2CH2)n-,
-CH2CH2CH2-arylene-CH2CH2-(O-CH2CH2)n-,
-CH2CH2-arylene-CH2CH2CH2-(O-(CH2CH2)n- and
- CH2CH2CH2-arylene-CH2CH2CH2CH2-(O-CH2CH2)n-.
In some embodiments, a linker is a bivalent linker. In some embodiments, a linker is a trivalent linker. In some embodiments, a linker is a tetraval ent linker.
In some embodiments, a linker, or a linker unit, comprises one or more of each of the following moieties: a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, - (alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, - N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, each n is independently an integer from 1 to 10. In some embodiments, the peptide is a dipeptide. In some embodiments, at least one moiety is not a bond. Thus, in some embodiments, a linker is not a bond. In some embodiments, a linker unit is not a bond.
In some embodiments, the N-terminus of a dipeptide is covalently joined to a moiety -
C(0)-. In some embodiments, the C-terminus of the dipeptide is covalently joined to a moiety - N(RW)-, wherein Rw is independently H or Ci-Cs alkyl. Thus, in some embodiments, a linker moiety has the following structure:
In some further embodiments, a linker, or linker unit, comprises one or more of each of the following moieties: a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, - (alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid or a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some further embodiments, a linker, or a linker unit, comprises one or more moieties selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -
(alkylene-O)n-, -(alkylene-N(Rw))n-, -C(O)-, -N(RW)-, wherein each n is independently an integer from 1 to 10, and each Rw is independently H or Ci- Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, at least one moiety is not a bond. Thus, in some embodiments, a linker is not a bond. In some embodiments, a linker unit is not a bond.
In some embodiments, each alkylene moiety of a linker or linker unit is independently - CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5- or -(CH2)6-.
In some embodiments, a linker or linker unit comprises at least one alkylene group. In some embodiments, a linker or linker unit comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, a linker or linker unit consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
In some embodiments, a linker is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2, 3, 4 or 5, and n is 1, 2, 3, 4 or 5. In some embodiments, m is 1, 2, 3 or 4, and n is 1, 2, 3 or 4. In some embodiments, m is 1, 2 or 3, and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, a linker is a bivalent linker. In some embodiments, a bivalent linker is selected from the group consisting of:
In some other embodiments, a bivalent linker is selected from the group consisting of: wherein each m is independently an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, n is 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, n is 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, n is 1, 2 or 3.
In some other embodiments, a linker is a trivalent linker. In some embodiments, a trivalent linker of the present disclosure comprises three linker units. In some embodiments, a linker is a trivalent linker having the following general structure: wherein LI is a first linker unit, L2 is a second linker unit and L3 is a third linker unit. In some embodiments, LI is conjugated to an antibody via a linkage, and each of L2 and L3 is covalently bound to a cytotoxic moiety. In some embodiments, each LI, L2 and L3 independently comprises one or more moi eties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene- N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one LI, L2 or L3 is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, each n is independently an integer from 1 to 10. In some embodiments, the peptide is a dipeptide.
In some further embodiments, each LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O)n-, -(alkylene-N(Rw))n- unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide; wherein at least one LI, L2 or L3 is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each LI, L2 and L3 is independently selected from a bivalent linker or linker unit, as disclosed herein.
In some embodiments, a trivalent linker is selected from the group consisting of: wherein each m is independently an integer from 1 to 10; and each n is independently an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, each n is independently 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, each n is independently 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, each n is independently 1, 2 or 3.
In some embodiments, a trivalent linker is selected from the group consisting of:
Z has the following structure: each m is independently an integer from 1 to 10; and each n is independently an integer from 1 to 10. In some embodiments, each m is independently 1, 2, 3, 4 or 5. In some embodiments, each n is independently 1, 2, 3, 4 or 5. In some embodiments, each m is independently 1, 2, 3 or 4. In some embodiments, each n is independently 1, 2, 3 or 4. In some embodiments, each m is independently 1, 2 or 3. In some embodiments, each n is independently 1, 2 or 3.
In some embodiments, the linker comprises a water-soluble polymer.
In some embodiments, the linker comprises a water-soluble polymer and an amino acid, wherein the water-soluble polymer is conjugated to the amino acid. In some embodiments, the water-soluble polymer is conjugated to a side chain of the amino acid. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element.
In some embodiments, a linker of the present disclosure comprises a water-soluble polymer and an amino acid which is serine, threonine or tyrosine, wherein the water- soluble polymer is conjugated to the side chain -OH group of the serine, threonine or tyrosine. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element.
In some embodiments, a linker of the present disclosure comprises a water-soluble polymer and an amino acid which is cysteine, wherein the water-soluble polymer is conjugated to the side chain -SH group of the cysteine. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element. In some embodiments, a linker of the present disclosure comprises a water-soluble polymer and an amino acid which is aspartic acid or glutamic acid, wherein the water-soluble polymer is conjugated to the side chain carboxylate group of the aspartic acid or glutamic acid. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element.
In some embodiments, a linker of the present disclosure comprises a water-soluble polymer and an amino acid which is lysine or NE-methyl-lysine, wherein the water-soluble polymer is conjugated to the side chain -NEH(R) group of the lysine or NE-methyl-lysine, wherein R is H or methyl, respectively. In some embodiments, the water-soluble polymer is conjugated to the amino acid via a spacer element.
In some embodiments, the water-soluble polymer is a polysaccharide.
In some embodiments, the water-soluble polymer is a (polyethylene)glycol (PEG) moiety. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 100,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 10,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 5,000 Da. In some embodiments, the PEG moiety has a molecular weight within a range of about 100 Da to about 1,000 Da. In some other embodiments, the PEG moiety has a molecular weight within a range of about 10,000 Da to about 50,000 Da. In some embodiments, the PEG is linear. In some embodiments, the PEG is branched, multi-armed or dendritic.
In some embodiments, a linker of the present disclosure is connected to a drug and is also connected to a reactive moiety. Thus, the linker bridges the drug to the reactive moiety to provide a drug-linker compound. The reactive moiety can be one that can react with another moiety of a natural amino acid or a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure. In some embodiments, the reactive moiety is at least one carbonyl, dicarbonyl, ketone, acyl, -ONH2, alkoxy-amine, hydrazine, acyclic alkene, acyclic alkyne, cyclooctyne, azide, aryl azide, alkyl azide, norbomene, cyclopropene, transcyclooctene, or tetrazine functional group or a combination thereof. In some embodiments, the reactive moiety is -ONH2. In some embodiments, the reactive moiety is -ONH2 and the nonnatural amino acid is pAF.
In some other embodiments, a linker of the present disclosure is connected to a drug, and is also connected to an antibody, antibody fragment or variant thereof, via a linkage. The linkage can be a product of a reaction between a reactive moiety of a drug-linker compound and another moiety that is present in a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure. Thus, a linker bridges a drug and the antibody, antibody fragment or variant thereof, and is covalently bonded to a non-natural amino acid of the antibody, antibody fragment or variant thereof via the linkage. In some embodiments, the reactive moiety of the drug-linker compound is -ONH2; the non-natural amino acid of the antibody, antibody fragment or variant thereof is para-acetyl-L-phenylalanine (pAF), which contains an acyl group, and the linkage formed between the drug-linker and the non-natural amino acid of the antibody, antibody fragment or variant thereof comprises an oxime.
Drugs and Drug-Linkers
In some aspects, the present disclosure provides a drug or drug-linker. A drug-linker of the present disclosure comprises a drug covalently attached to a linker.
In some aspects of the disclosure, the drug is a cytotoxic agent or a cytotoxic moiety. In some embodiments, the cytotoxic agent or moiety is an auristatin, or a derivative or analog thereof. A cytotoxic moiety, wherein the cytotoxic moiety is an auristatin, or a derivative or analog thereof, can be a cytotoxic moiety of Formula (A) or Formula (B), as further disclosed herein.
Thus, in some embodiments, a cytotoxic moiety of the present disclosure is a cytotoxic moiety of Formula (A): wherein:
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; and wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH, R6 is H, R7 is Ci-Ce alkyl and Ar is phenyl. In some embodiments, R7 is methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is selected from the group consisting of: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl. In some embodiments, R7 is methyl.
In some other embodiments, the cytotoxic moiety of Formula (A) does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl, or wherein R7 is methyl. In some embodiments, the cytotoxic moiety is less cytotoxic than monomethyl auristatin E (MMAE) in vitro. In some embodiments, the cytotoxic moiety exhibits a higher in vitro half-maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE. In some embodiments, the higher in vitro IC50 is at least a two-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 10-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 100-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 1000-fold higher in vitro IC50. In some embodiments, the microtubule inhibitor-sensitive cell line is an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay. In some embodiments, the in vitro cytotoxicity assay is an impedance assay, a flow cytometry assay or a luminescent cell viability assay. In some embodiments, the in vitro cytotoxicity assay is a luminescent cell viability assay, such as a CellTiter Gio assay. Methods of measuring in vitro cytotoxicity are disclosed herein, and are known to a person of ordinary skill in the art.
In some other embodiments, a cytotoxic moiety of the present disclosure is a cytotoxic moiety of Formula (B):
(B); wherein:
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; and wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
In some embodiments, R6 is H and Ar is phenyl.
As disclosed herein, a drug-linker of the present disclosure can be covalently bound to reactive moiety. A drug-linker, wherein the linker is covalently bound to reactive moiety, can serve as a precursor compound, for example, for conjugation with a peptide, protein or antibody. Thus, the reactive moiety can be one that can react with another moiety of a natural amino acid or a non-natural amino acid of a polypeptide, such as an antibody, antibody fragment or variant thereof of the present disclosure.
In some embodiments, a drug-linker comprises a reactive moiety, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O)Rc, -C(O)ORa, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, Rc is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6.
Thus, in some embodiments, a drug-linker of the present disclosure is a drug-linker of Formula (Al):
(Al); wherein:
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; and
Y is a reactive moiety, optionally, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O) Rq, -C(O)ORd, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, Rq is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6.
In some embodiments, Y is -ONH2.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH, R6 is H, R7 is Ci-Ce alkyl and Ar is phenyl. In some embodiments, R7 is methyl.
In some other embodiments, a drug-linker of the present disclosure is a drug-linker of Formula (Bl): wherein:
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and
Y is a reactive moiety, optionally, wherein the reactive moiety comprises -N3, -OH, -SH, -NHRb, -C(O)Rq, -C(O)ORa, -C(O)CH2NH2, an activated ester, -O-NH2, a maleimide, a tetrazine, an alkyne, a cyclooctyne or an (E)-cyclooctene; wherein: Rb is H or unsubstituted Ci-Ce alkyl, Rq is unsubstituted Ci-Ce alkyl, Rd is H, unsubstituted Ci-Ce alkyl or a carboxylic acid protecting group, s is 0, 1, 2, 3, 4, 5 or 6 and t is 0, 1, 2, 3, 4, 5 or 6; or Y is H.
In some embodiments, R6 is H and Ar is phenyl.
In some embodiments the drug or drug-linker is a drug or drug-linker generated as described in the Examples herein, or in WO2013/185117A1, the contents of which are hereby incorporated by reference in their entirety. In some embodiments, drug-linker compounds disclosed in Table 2 can be employed with anti-TROP2 antibodies, antibody fragments or antibody drug conjugates of the present disclosure.
Table 2. Non-limiting Drug-linker Compounds for conjugation with anti-TROP2 antibodies and fragments thereof.
In some embodiments the present invention provides additional drug-linkers prepared using similar procedures as described herein, including the schemes disclosed in the Examples. Additional drug-linker compounds are engineered by linkage of any possible linker group known in the art or elsewhere. The drug-linker compounds are engineered by linkage of one or more linkers via any chemical or functional reactive positions in the drug or cytotoxic agent. Selection of the chemical or functional reactive positions in the drug for linkage to a linker is assessed as disclosed elsewhere herein, based on structure of the cytotoxic agent, and using the process known in the art or elsewhere to generate a drug-linker.
In some embodiments, a drug linker compound containing a reactive moiety is conjugated to an antibody or antibody fragment by reacting a drug-linker compound with an antibody or antibody fragment (or simply “antibody) containing one or more natural or nonnatural amino acid. The conjugation reaction provides an ADC, wherein drug-linker is conjugated to a natural or non-natural amino acid of the antibody via a covalent linkage, wherein the covalent linkage is a product of the reactive moiety of the drug-linker and an additional moiety present in the natural or non-natural amino acid, wherein the additional moiety can react to form the covalent linkage with the reactive moiety. Methods of conjugating drug-linkers to antibodies are known in the art (see, e.g., Johann, K. et al., Polymer Chemistry, 27(11):4396-4407 (2020); Bioconjug Chem., 27(12):2791-2807 (2016); Northrop, B. H. et al., Polymer Chemistry, 18(6):3415-3430 (2015); Axup, J.Y. et al., Proc. Natl. Acad. Sci., 109(40): 16101 - 16016 (2012); Hartmuth, C. et al., Angew. Chem. Int. Ed., 40(11):2004-2021 (2001); Sletten, E.M. and Bertozzi, C. R., Angew. Chem. Int. Ed., 48(38):6974-6998 (2009); W02006/050262A2; and WO2013/185177A1; the contents of each of which are hereby incorporated by reference in their entirety. Non-limiting examples of reactions and linkages formed between drug-linker compounds and natural or nonnatural amino acids incorporated into an antibody of the present disclosure include the following.
A. (i) Reaction of a drug-linker comprising -N3 with a non-natural amino acid comprising an alkynyl group, thereby providing a linkage comprising a 1,2,3-triazolyl moiety; or (ii) reaction of a drug-linker comprising an alkynyl group with a non-natural amino acid comprising -N3, thereby providing a linkage comprising a 1,2,3-triazolyl moiety. In some embodiments, the alkynyl group is a cyclooctynyl group. In some embodiments, the non-natural amino acid is /?- azido-L-phenylalanine. In some embodiments, the linkage comprising the 1,2,3-triazolyl moiety has the following structure: wherein: each s is independently 0 or an integer from 1 to 50; optionally, each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0 or an integer from 1 to 50; optionally, each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to a linker of the drug-linker; and each wavy line (■—) denotes connection to the antibody.
B. (i) Reaction of a drug-linker comprising a tetrazinyl group with a non-natural amino acid comprising an (A)-cyclooctenyl group, thereby providing a linkage comprising a 1,4- dihydropyridazinyl moiety; or (ii) reaction of a drug-linker comprising a tetrazinyl group with a non-natural amino acid comprising an (E)-cyclooctenyl, thereby providing a linkage comprising a 1,4-dihydropyridazinyl moiety. In some embodiments, the linkage comprising the 1,4- dihydropyridazinyl moiety has the following structure: wherein: each Rf is independently H or alkyl, optionally unsubstituted Ci-Ce alkyl; each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
C. (i) Reaction of a drug-linker comprising an -ONH2 group with a non-natural amino acid comprising a carbonyl or ketone group, thereby providing a linkage comprising an oxime moiety; or (ii) reaction of a drug-linker comprising a carbonyl or ketone group with a non-natural amino acid comprising an -ONH2 group, thereby providing a linkage comprising an oxime moiety. In some embodiments, the carbonyl or ketone group is -C(O)Rq, wherein Rq is unsubstituted Ci- Ce alkyl. In some embodiments, Rq is methyl. In some embodiments, the linkage comprising the oxime moiety has the following structure: wherein: each Rq is independently unsubstituted Ci-Ce alkyl; optionally, each Rq is methyl; each + denotes connection to a linker of the drug-linker; and each wavy line (-~^) denotes connection to the antibody.
D. (i) Reaction of a drug-linker comprising a maleimide group with a natural or non- natural amino acid comprising a thiol (-SH), thereby providing a linkage comprising a pyrrolidine- 2, 5-dione moiety, such as a 3-(kl-sulfaneyl)pyrrolidine-2, 5-dione moiety; or (ii) reaction of a drug-linker comprising a thiol (-SH) group with a non-natural amino acid comprising a maleimide group, thereby providing a linkage comprising a pyrrolidine-2, 5-dione moiety, such as a 3-(Xl- sulfaneyl)pyrrolidine-2, 5-dione moiety. In some embodiments, the natural amino acid is cysteine. In some embodiments, the linkage comprising the a pyrrolidine-2, 5-dione moiety, such as a 3- ( I -sulfaneyl)pyrrolidine-2, 5-dione moiety, has the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
E. (i) Reaction of a drug-linker comprising a primary or secondary amine with a natural or non-natural amino acid comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group, thereby providing a linkage comprising an amide moiety; or (ii) reaction of a drug-linker comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group with a natural or non-natural amino acid comprising a primary or secondary amine group, thereby providing a linkage comprising a amide moiety. In some embodiments, the natural amino acid is aspartic acid or glutamic acid. In some other embodiments, the natural amino acid is lysine. In some embodiments, the reaction is a peptide coupling reaction or other well-known method of forming an amide, each of which can be performed using methods readily understood by a person of ordinary skill in the art. In some embodiments, the linkage comprising the amide moiety has the following structure: wherein: each RJ is independently H or alkyl; optionally unsubstituted Ci-Ce alkyl; each + denotes connection to a linker of the drug-linker; and each wavy line (■— ) denotes connection to the antibody.
F. (i) Reaction of a drug-linker comprising a hydroxyl group (-OH) with a natural or non-natural amino acid comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group, thereby providing a linkage comprising an ester moiety; or (ii) reaction of a drug-linker comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group with a natural or non-natural amino acid comprising a hydroxyl group (-OH), thereby providing a linkage comprising an ester moiety. In some embodiments, the natural amino acid is aspartic acid or glutamic acid. In some other embodiments, the natural amino acid is serine, threonine or tyrosine. Methods of forming such esters linkages can be performed using methods readily understood by a person of ordinary skill in the art. In some embodiments, the linkage comprising the ester moiety has the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (- ) denotes connection to the antibody.
G. (i) Reaction of a drug-linker comprising a thiol group (-SH) with a natural or nonnatural amino acid comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group, thereby providing a linkage comprising a thioester moiety; or (ii) reaction of a druglinker comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group with a natural or non-natural amino acid comprising a thiol group (-SH), thereby providing a linkage comprising a thioester moiety. In some embodiments, the natural amino acid is aspartic acid or glutamic acid. In some other embodiments, the natural amino acid is cysteine. Methods of forming such thioesters linkages can be performed using methods readily understood by a person of ordinary skill in the art. In some embodiments, the linkage comprising the ester moiety has the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (— ) denotes connection to the antibody.
H. Reaction of a drug-linker comprising a -C(O)CH2NH2 group with a natural or nonnatural amino acid comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group, thereby providing a linkage comprising a -C(0)CH2NHC(0)- moiety; or (ii) reaction of a drug-linker comprising a carboxylic acid group, a protected carboxylic acid, or an activated ester group with a non-natural amino acid comprising a -C(O)CH2NH2 group, thereby providing a linkage comprising a -C(0)CH2NHC(0)- moiety. In some embodiments, the natural amino acid is aspartic acid or glutamic acid. Methods of forming such linkages can be performed using methods readily understood by a person of ordinary skill in the art. In some embodiments, the linkage has the following structure: wherein: each + denotes connection to a linker of the drug-linker; and each wavy line (-~^) denotes connection to the antibody.
I. Reaction of a drug-linker comprising a thiol group (-SH) with a natural or non-natural amino acid comprising a thiol group, thereby providing a linkage comprising a disulfide. In some embodiments, the natural amino acid is cysteine. Methods of forming disulfide linkages can be performed using methods readily understood by a person of ordinary skill in the art. The present disclosure provides drug moieties with linkers that reduce the toxicity of the moiety in vivo while retaining pharmacological activity. In some embodiments, the toxicity of the linked drug, when administered to an animal or human, is reduced or eliminated compared to the free toxic group or toxic group derivatives comprising labile linkages, while retaining pharmacological activity. In some embodiments, increased doses of the linked cytotoxic group may be administered to animals or humans with greater safety. In certain embodiments, the nonnatural amino acid polypeptides linked to a drug moiety (e.g., an auristatin or an auristatin derivative or analog) provides in vitro and in vivo stability. In some embodiments, the non-natural amino acid polypeptides linked to a drug moiety are efficacious and less toxic compared to the free drug moiety.
In some embodiments, at least one post-translational modification at some position on the polypeptide may occur. In some embodiments the co-translational or post-translational modification occurs via the cellular machinery (e.g., glycosylation, acetylation, acylation, lipid- modification, palmitoylation, palmitate addition, phosphorylation, glycolipid-linkage modification, and the like), in many instances, such cellular-machinery-based co-translational or post-translational modifications occur at the naturally occurring amino acid sites on the polypeptide, however, in certain embodiments, the cellular-machinery-based co-translational or post-translational modifications occur on the non-natural amino acid site(s) on the polypeptide.
In other embodiments, the post-translational modification does not utilize the cellular machinery, but the functionality is instead provided by attachment of a molecule (a polymer; a water-soluble polymer; a derivative of polyethylene glycol; a second protein or polypeptide or polypeptide analog; an antibody or antibody fragment; and any combination thereof) comprising a second reactive group to the at least one non-natural amino acid comprising a first reactive group (including but not limited to, non-natural amino acid containing a ketone, aldehyde, acetal, hemiacetal, alkyne, cycloalkyne, azide, oxime, or -ONH2 functional group) utilizing chemistry methodology described herein, or others suitable for the particular reactive groups. In certain embodiments, the co-translational or post-translational modification is made in vivo in a eukaryotic cell or in a non-eukaryotic cell. In certain embodiments, the post-translational modification is made in vitro not utilizing the cellular machinery. Also included with this aspect are methods for producing, purifying, characterizing and using such a drug-linker containing at least one such co-translationally or post-translationally modified non-natural amino acids.
Also included within the scope of the methods, compositions, strategies and techniques described herein are reagents capable of reacting with a drug-linker (e.g., containing a carbonyl or dicarbonyl group, alkyne, cycloalkyne, azide, -ONH2 group, or masked or protected forms thereof, or a moiety Y, as disclosed herein) that is part of a polypeptide so as to produce any of the aforementioned post-translational modifications. In certain embodiments, the resulting post- translationally modified drug-linker can contain at least one oxime group; the resulting modified oxime-containing drug-linker may undergo subsequent modification reactions. Also included with this aspect are methods for producing, purifying, characterizing and using such reagents that are capable of any such post-translational modifications of such drug-linkers.
In certain embodiments, the polypeptide or non-natural amino acid linked composition includes at least one co-translational or post-translational modification that is made in vivo by one host cell, where the post-translational modification is not normally made by another host cell type. In certain embodiments, the polypeptide includes at least one co-translational or post-translational modification that is made in vivo by a eukaryotic cell, where the co-translational or post- translational modification is not normally made by a non-eukaryotic cell. Examples of such co- translational or post-translational modifications include, but are not limited to, glycosylation, acetylation, acylation, lipid-modification, palmitoylation, palmitate addition, phosphorylation, glycolipid-linkage modification, and the like. In one embodiment, the co-translational or post- translational modification comprises attachment of an oligosaccharide to an asparagine by a GlcNAc-asparagine linkage (including but not limited to, where the oligosaccharide comprises (GlcNAc-Man)2-Man-GlcNAc-GlcNAc, and the like). In another embodiment, the co- translational or post-translational modification comprises attachment of an oligosaccharide (including but not limited to, Gal-GalNAc, Gal-GlcNAc, etc.) to a serine or threonine by a GalNAc-serine, a GalNAc-threonine, a GlcNAc-serine, or a GlcNAc-threonine linkage. In certain embodiments, a protein or polypeptide can comprise a secretion or localization sequence, an epitope tag, a FLAG tag, a polyhistidine tag, a GST fusion, and/or the like. Also included with this aspect are methods for producing, purifying, characterizing and using such polypeptides containing at least one such co-translational or post-translational modification. In other embodiments, the glycosylated non-natural amino acid polypeptide is produced in a nonglycosylated form. Such a non-glycosylated form of a glycosylated non-natural amino acid may be produced by methods that include chemical or enzymatic removal of oligosaccharide groups from an isolated or substantially purified or unpurified glycosylated non-natural amino acid polypeptide; production of the non-natural amino acid in a host that does not glycosylate such a non-natural amino acid polypeptide (such a host including, prokaryotes or eukaryotes engineered or mutated to not glycosylate such a polypeptide), the introduction of a glycosylation inhibitor into the cell culture medium in which such a non-natural amino acid polypeptide is being produced by a eukaryote that normally would glycosylate such a polypeptide, or a combination of any such methods. Also described herein are such non-glycosylated forms of normally-glycosylated non- natural amino acid polypeptides (by normally-glycosylated is meant a polypeptide that would be glycosylated when produced under conditions in which naturally-occurring polypeptides are glycosylated). Of course, such non-glycosylated forms of normally-glycosylated non-natural amino acid polypeptides (or indeed any polypeptide described herein) may be in an unpurified form, a substantially purified form, or in an isolated form.
In some instances, incorporation of a non-natural amino acid into the antibody or antibody fragment will be combined with other additions, substitutions, or deletions within the polypeptide to affect other chemical, physical, pharmacologic and/or biological traits. In some cases, the other additions, substitutions or deletions may increase the stability (including but not limited to, resistance to proteolytic degradation) of the polypeptide or increase affinity of the polypeptide for its appropriate receptor, ligand and/or binding proteins. In some cases, the other additions, substitutions or deletions may increase the solubility (including but not limited to, when expressed in E. coli or other host cells) of the polypeptide. In some embodiments, sites are selected for substitution with a naturally encoded or non-natural amino acid in addition to another site for incorporation of a non-natural amino acid for the purpose of increasing the polypeptide solubility following expression in E. coli, or other recombinant host cells. In some embodiments, the polypeptides comprise another addition, substitution, or deletion that modulates affinity for the associated ligand, binding proteins, and/or receptor, modulates (including but not limited to, increases or decreases) receptor dimerization, stabilizes receptor dimers, modulates circulating half-life, modulates release or bio-availability, facilitates purification, or improves or alters a particular route of administration. Similarly, the non-natural amino acid polypeptide can comprise chemical or enzyme cleavage sequences, protease cleavage sequences, reactive groups, antibodybinding domains (including but not limited to, FLAG or poly-His) or other affinity based sequences (including but not limited to, FLAG, poly-His, GST, etc.) or linked molecules (including but not limited to, biotin) that improve detection (including but not limited to, GFP), purification, transport thru tissues or cell membranes, prodrug release or activation, size reduction, or other traits of the polypeptide.
Antibody Drug Conjugates (ADCs)
Antibody drug conjugates (ADCs) of the present disclosure provide novel therapeutics or anti-cancer drugs by combining the selectivity of antibodies comprising one or more non- natural amino acids and a cytotoxic agent. Targeted cytotoxic drug delivery into tumor tissue increases the therapeutic window of these agents considerably. ADCs of the present disclosure comprise of an antibody bound to a cytotoxic drug via a linker. Stability of the linker between the antibody and the cytotoxic drug is essential for the ADC integrity in circulation. The successful ADC development for a given target antigen depends on optimization of antibody selection, linker design and stability, drug potency and mode of drug and linker conjugation to the antibody. Linker properties of pH and redox sensitivities and protease susceptibility influence circulatory stability and release of the drug moiety.
In some embodiments of the disclosure, the antibody of the ADC comprises a full length antibody or fragment thereof that binds to an antigen, and is conjugated to a cytotoxic agent or an immunosuppressive agent, wherein the antibody-drug conjugate exerts: (a) a cytotoxic or cytostatic effect on the antigen-expressing or antigen targeting cell line, or (b) a cytotoxic, cytostatic, or immunosuppressive/immune activating effect on an antigen-expressing immune cell, wherein the conjugation occurs at a non-natural amino acid in the antibody. In some embodiments, the antigen, antigen-expressing cell, or antigen-targeting cell, or antigen-expressing immune cell is TR0P2. In some embodiments, the antigen, antigen-expressing cell, or antigen-targeting cell, or antigen-expressing immune cell is a TROP2 antigen, or antigen-targeting cell, or antigenexpressing cell or antigen-expressing immune cell. In some embodiments of the disclosure, the antibody of the ADC comprises a full length antibody or fragment thereof that: binds to TROP2 and is conjugated to a cytotoxic agent or an immunosuppressive agent, wherein the antibody-drug conjugate exerts: (a) a cytotoxic or cytostatic effect on a TROP2-expressing cancer cell line, or (b) a cytotoxic, cytostatic, or immunosuppressive/immune activating effect on a TR0P2- expressing immune cell, wherein the conjugation occurs at a non-natural amino acid in the antibody.
In some embodiments, the antibody, variant, or composition of the present disclosure may be an antibody, variant, or composition that binds to an antigen receptor. In other embodiments the antibody, variant, or composition may be an antibody, variant, or composition that binds to extracellular surface of an antigen receptor. In some embodiments the antibody, variant, or composition of the present disclosure may be an antibody, variant, or composition that has CDRs grafted onto the framework region of the variable region. In other embodiments the antibody, variant, or composition of the present disclosure may be an antibody, variant, or composition that has a non-natural amino acid. In some embodiments the antibody, variant, or composition may be an antibody, variant, or composition that is described by more than one of the embodiments elsewhere herein the present disclosure. In some embodiments the antibody, antibody variant or antibody composition(s) disclosed herein may be fully humanized. In other embodiments the antibody, antibody variant or antibody composition(s) disclosed herein may be chimeric. In some embodiments the antibody may be an antibody that is full length antibody (Variable + Fc regions), Fab, bispecific, Fab-dimers, Fab-bispecific, Fab-trispecific, bispecific T- cell engagers, dual-affinity re-targeting antibody, IgGl/IgG3 bispecific antibody, diabody, bispecific diabody, scFv-Fc, minibody. In some embodiments, the ADC comprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the antibody. The antibody comprises at least one non-natural amino acid; non-limiting examples of non-natural amino acids are disclosed herein.
In some embodiment, the ADC comprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the heavy chain of the antibody. In some embodiments, the ADC comprises an antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the light chain of the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the heavy chain of the antibody. In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein the conjugation occurs via a non-natural amino acid in the light chain of the antibody.
In some embodiments, the ADC comprises a full-length antibody conjugated to a drug wherein a first conjugation occurs via a non-natural amino acid in the heavy chain of the antibody, and a second conjugation occurs via a non-natural amino acid in the light chain of the antibody. In some embodiments, the full-length antibody comprises two full-length heavy chains and two full-length light chains, wherein a first pair of conjugations occur via a non-natural amino acid in each heavy chain of the antibody, and a second pair of conjugations occur via a non-natural amino acid in each light chain of the antibody.
In some embodiments, the drug of the ADC is a cytotoxic agent or moiety. In some embodiments, the cytotoxic agent or moiety is an auristatin, or a derivative or analog thereof. A cytotoxic moiety, wherein the cytotoxic moiety is an auristatin, or a derivative or analog thereof, can be a cytotoxic moiety of Formula (A) or Formula (B), as disclosed herein. In some embodiments, the drug is a drug generated as described in the Examples herein. In some embodiments, the ADC comprises an antibody, antibody fragment or variant thereof engineered to have one or more non-natural amino acids site specifically incorporated in a heavy chain and/or light chain amino acid sequence, wherein the antibody, antibody fragment or variant thereof is conjugated to a cytotoxic agent or cytotoxic moiety via a linker.
In some embodiments, there is provided an antibody-drug conjugate (ADC) comprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the amino acid sequence of the anti-TROP2 antibody comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moi eties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the heavy chain amino acid sequences comprises the one or more nonnatural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
Thus, in some embodiments, there is provided an ADC comprising: an anti-TROP2 antibody, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one heavy chain amino acid sequence shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein: at least one of the heavy chain amino acid sequences comprises one or more non-natural amino acids; and each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
In some embodiments, each of the one or more linkers is not a bond.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non- natural amino acids.
In some embodiments, at least one of the amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para- acetyl-L-phenylalanine (pAF).
In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7. In some embodiments, the amino acid sequence of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7.
In some embodiments, the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some embodiments, the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. In some other embodiments, the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, the amino acid sequence each of the one or more light chains is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
In some embodiments, an anti-TROP2 antibody of the present disclosure, such as the anti- TROP2 antibody of an ADC of the present disclosure, is humanized.
In some embodiments, each cytotoxic moiety of the ADC is a cytotoxic moiety of Formula
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH. In some embodiments, R6 is H. In some embodiments, R7 is methyl. In some embodiments, Ar is phenyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some embodiments, the cytotoxic moiety of Formula (A) is: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
In some other embodiments, the cytotoxic moiety of Formula (A) does not have the following structure: wherein # is H or represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl, or wherein R7 is methyl.
In some embodiments, the cytotoxic moiety is less cytotoxic than monomethyl auristatin E (MMAE) in vitro. In some embodiments, the cytotoxic moiety exhibits a higher in vitro half- maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE. In some embodiments, the higher in vitro IC50 is at least a two-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 10-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 100-fold higher in vitro IC50. In some embodiments, the higher in vitro IC50 is at least a 1000-fold higher in vitro IC50. In some embodiments, the microtubule inhibitor-sensitive cell line is an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay. In some embodiments, the in vitro cytotoxicity assay is an impedance assay, a flow cytometry assay or a luminescent cell viability assay. In some embodiments, the in vitro cytotoxicity assay is a luminescent cell viability assay, such as a CellTiter Gio assay. Methods of measuring in vitro cytotoxicity are disclosed herein, and are known to a person of ordinary skill in the art.
In some other embodiments, each said cytotoxic moiety of the ADC is a cytotoxic moiety of Formula (B): wherein:
R6 is H or OH;
R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
In some embodiments, R6 is H. In some embodiments, Ar is phenyl.
In some embodiments, the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
Ab is an anti-TR0P2 antibody, wherein the amino acid sequence of the anti-TROP2 antibody comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L is a linker; each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker L to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, each L is not a bond.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, too an amino acid and a peptide; wherein at least one moiety is not a bond; each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(Rw)-, an amino acid and a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each L comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
In some embodiments, E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line denotes connection to Ab.
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line ("«) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, each L comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the heavy chain amino acid sequences comprises the one or more non- natural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7.
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, each light chain amino acid sequence is SEQ ID NO: 11.
Ab is an anti-TROP2 antibody, wherein an anti-TROP2 antibody amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; each L1-CH(L2-)(L3-) is a linker, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, at least one of LI, L2 and L3 is not a bond.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In some embodiments, d is 1, 2, 3 or 4. In some embodiments, d is 1. In some embodiments, d is 2. In some embodiments, d is 3. In some embodiments, d is 4.
In some embodiments, each LI, L2 and L3 independently comprises one or more moi eties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci- Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each LI comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
In some embodiments, E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
In some embodiments, E is selected from the group consisting of: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line (— ) denotes connection to Ab.
In some embodiments, E comprises an oxime. In some embodiments, E has the following structure: /
/~N0-+
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (— ) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one heavy chain amino acid sequence comprises the one or more non-natural amino acids and shares at least 90% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one heavy chain amino acid sequence shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6 and comprises at least one of the one or more non-natural amino acids.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each light chain amino acid sequence shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences o is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non- natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
In some embodiments, each light chain amino acid sequence shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one light chain amino acid sequence is SEQ ID NO: 4. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains. In some embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and d is 2, and/or the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4, and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, each light chain amino acid sequence is SEQ ID NO: 11.
In some other embodiments, there is provided an antibody-drug conjugate (ADC) of Formula (la), (lb), (Ila) or (lib) : wherein:
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and (lb) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) or Formula (lib) is a linker, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage;
R5 of Formula (la) or (lb) is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
In some embodiments, each L, LI, L2, and L3 independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting no of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, - (alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, the peptide is a dipeptide.
In some embodiments, each L, LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid or a dipeptide; wherein at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each L or each LI comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, each L or each LI consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
In some embodiments, each L or each LI is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line ("«) denotes connection to Ab.
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line ("«) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, at least one of the one or more non-natural amino acids is paraacetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, and at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
In some embodiments, each of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2 and/or the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage;
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
In some embodiments, R5 is not thiazolyl.
In some embodiments, R5 is COOH. In some embodiments, R6 is H. In some embodiments, R7 is methyl. In some embodiments, Ar is phenyl
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 100; and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, the peptide is a dipeptide. In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(Rw)-, an amino acid and a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
In some embodiments, each L comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10. In some embodiments, each L consists of unsubstituted alkylene, -(alkylene-O)n-, or both. In some embodiments, each n each n is an integer from 1 to 10. In some embodiments, each L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine.
In some embodiments, E is selected from the group consisting of wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line (— ) denotes connection to Ab.
In some embodiments, E comprises an oxime. In some embodiments, E has the following structure: /°-+
N
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line ("«) denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each heavy chain has an amino acid sequence, and wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each heavy chain amino acid sequence is SEQ ID NO: 5. In some embodiments, the one nonnatural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 3 or 7.
In some embodiments, at least one of the light chain amino acid sequence shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequence shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one nonnatural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 4; and d is 1, 2, 3 or 4 and/or the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and each heavy chain amino acid sequence is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and each light chain amino acid sequence is SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
In some embodiments, Rq is methyl, R5 is COOH, R6 is H, R7 is methyl, Ar is phenyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
Ab is an anti-TR0P2 antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage, wherein each linkage E covalently joins each linker L with one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10; or a pharmaceutically acceptable salt thereof.
In some embodiments, d is 1, 2, 3, 4, 5, 6, 7 or 8. In some embodiments, d is 1, 2, 3 or 4.
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, the peptide is a dipeptide.
In some embodiments, each L comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid or a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing. In some embodiments, L comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both. In some embodiments, L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both. In some embodiments, L is -(CEECEEjm- CEECEE-Ojn-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10. In some embodiments, m is 1, 2 or 3 and n is 1, 2 or 3. In some embodiments, m is 1 and n is 3.
In some embodiments, E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4-dihydropyridazine. In some embodiments, E is selected from the group consisting of: wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or L 1 ; and each wavy line denotes connection to Ab. In some embodiments, E comprises an oxime. In some embodiments, E has the following structure:
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (™») denotes the connection to Ab. In some embodiments, Rq is methyl.
In some embodiments, Rq is methyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
In some embodiments, at least one of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF). In some embodiments, each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chains amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains. In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2 and/or the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
Ab is the anti-TROP2 antibody; wherein the anti-TROP2 antibody comprises one or more non-natural amino acids incorporated therein, such as one or more pAF; wherein each oxime shown in Formula (Id) is a covalent linkage to one of the one or more non-natural amino acids, such as one of the one or more pAF, of the anti-TROP2 antibody Ab, and each methyl group joined to the oxime is a methyl group, such as the methyl group of the pAF; and d is 1, 2, 3, 4, 5, 6, 7 or 8; or a pharmaceutically acceptable salt thereof.
In some embodiments, d is 2. In some other embodiments, d is 4.
In some embodiments, the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids. In some embodiments, each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
In some embodiments, the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6. In some embodiments, at least one of the heavy chain amino acid sequences is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. In some embodiments, each heavy chain amino acid sequence is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each of the heavy chain amino acid sequences is SEQ ID NO: 5. In some embodiments, the one non-natural amino acid of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the heavy chain amino acid sequences shares at least 90% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences shares at least 95% identity with SEQ ID NO: 6. In some embodiments, at least one of the amino acid sequences shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6. In some embodiments, at least one of the heavy chain amino acid sequences is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering. In some embodiments, each said nonnatural amino acid is para-acetyl-L-phenylalanine (pAF).
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 3 or 7. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 3 or 7.
In some embodiments, at least one of the light chain amino acid sequences shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. In some embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 4, 8, 9, 10 or 11. In some embodiments, the at least one of the light chain amino acid sequences is SEQ ID NO: 4. In some embodiments, each of the light chain amino acid sequences is SEQ ID NO: 4. In some other embodiments, at least one of the light chain amino acid sequences is SEQ ID NO: 12, 13, 14, 15, 16 or 17. In some embodiments, each light chain amino acid sequence is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
In some embodiments, the one or more heavy chains is two heavy chains. In some further embodiments, the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
In some embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF); the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 1, 2, 3 or 4 and/or the ADC has a drug-to-antibody ratio of about 2.
In some other embodiments, the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112; SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4 and/or the drug-to-antibody ratio is about 1, about 2, about 3 or about 4. In some further embodiments, the one non-natural amino acid of each said heavy chain and each said light chain is para-acetyl-L-phenylalanine (pAF). In some more particular embodiments, the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
In some embodiments, an ADC of the present disclosure does not contain a Toll-like receptor (TLR) agonist.
It is understood that an ADC is typically produced as a composition containing a population of ADCs, i.e., a mixture of ADCs that are essentially identical, except for the drug load. As disclosed herein, an ADC composition can be characterized by a drug-to-antibody ratio (DAR), which reports on the average number of drugs conjugated to antibody in the ADC composition.
Thus, in some aspects, the present disclosure provides an ADC composition comprising a mixture of ADCs, wherein each ADC in the mixture is identical, except that the number of drugs (cytotoxic moieties) or drug-linkers that are conjugated to each ADC can vary.
In a non-limiting example, an ADC of the present disclosure comprises a first ADC, a second ADC, a third ADC and a fourth ADC, wherein the first ADC, the second ADC, the third ADC and the fourth ADC are identical, except that the first ADC comprises one drug or druglinker, the second ADC comprises two drugs or drug-linkers, the third ADC comprises three drugs or drug-linkers, and the fourth ADC comprises four drugs or drug-linkers.
In another non-limiting example, there is provided an ADC composition, comprising:
(a) an ADC of Formula (Id), wherein d is 1;
(b) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 2;
(c) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 3;
(d) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 4;
(e) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 5;
(f) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 6;
(g) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 7; and/or
(h) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 8; or a combination of any two or more of the foregoing; wherein the composition is characterized as having a DAR of at least about 1 and at most about 8.
In yet another non-limiting example, there is provided an ADC composition, comprising:
(a) an ADC of Formula (Id), wherein d is 1;
(b) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 2;
(c) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 3; and/or
(d) an ADC of Formula (Id), wherein the ADC is identical to (a), except that d is 4; or a combination of any two or more of the foregoing; wherein the composition is characterized as having a DAR of at least about 1 and at most about 4.
In some embodiments, the ADC composition is characterized as having a DAR of at least about 2 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 3 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 1 and at most about 2. In some embodiments, the ADC composition is characterized as having a DAR of at least about 1 and at most about 3. In some embodiments, the ADC composition is characterized as having a DAR of at least about 2 and at most about 4. In some embodiments, the ADC composition is characterized as having a DAR of at least about 3 and at most about 4. In some embodiments, the ADC is characterized as having a DAR of about 2. In some other embodiments, the ADC is characterized as having a DAR of about 4.
In some aspects of the disclosure, an antibody, antibody fragment, variant or drug conjugate with increased serum half-life, water solubility, bioavailability, therapeutic half-life or circulation time, or with modulated immunogenicity, or with modulated biological activity, is desired. One method of achieving such desired features of the compositions disclosed herein, is by covalent attachment of the polymer polyethylene glycol, (PEG). To maximize the desired properties of PEG, the total molecular weight and hydration state of the polymer or polymers attached to the biologically active molecule must be sufficiently high to impart the advantageous characteristics typically associated with such polymer attachment, such as increased water solubility and circulating half-life, while not adversely impacting the bioactivity of the molecule to which the PEG is attached. PEG derivatives are frequently linked to biologically active molecules through reactive chemical functionalities, such as amino acid residues, the N-terminus, and/or carbohydrate moieties. In some aspects of the present invention, PEG derivatives are linked to biologically active molecules through reactive chemical functionalities to improve biophysical properties of the resulting ADC. WO99/67291 discloses a process for conjugating a protein with PEG, wherein at least one amino acid residue on the protein is substituted with a synthetic amino acid and the protein is contacted with PEG under conditions sufficient to achieve conjugation to the protein.
In some aspects of the disclosure antibody, antibody fragments, variant or drug conjugate with increase serum half-life, water solubility, bioavailability, therapeutic half-life, or circulation time, or to modulate immunogenicity, or biological activity is desired. One method of achieving such desired features of the composition disclosed herein, is by covalent attachment of the polymer polyethylene glycol, (PEG). To maximize the desired properties of PEG, the total molecular weight and hydration state of the polymer or polymers attached to the biologically active molecule must be sufficiently high to impart the advantageous characteristics typically associated with such polymer attachment, such as increased water solubility and circulating half-life, while not adversely impacting the bioactivity of the molecule to which the PEG is attached. PEG derivatives are frequently linked to biologically active molecules through reactive chemical functionalities, such as amino acid residues, the N-terminus, and/or carbohydrate moieties. In some aspects of the present invention, PEG derivatives are linked to biologically active molecules through reactive chemical functionalities to improve biophysical properties of the resulting ADC. WO99/67291 discloses a process for conjugating a protein with PEG, wherein at least one amino acid residue on the protein is substituted with a synthetic amino acid and the protein is contacted with PEG under conditions sufficient to achieve conjugation to the protein.
Proteins and other molecules often have a limited number of reactive sites available for polymer attachment. The sites most suitable for modification via polymer attachment may play a significant role in receptor binding, and such sites may be necessary for retention of the biological activity of the molecule therefore making them inappropriate for polymer attachment. As a result, indiscriminate attachment of polymer chains to such reactive sites on a biologically active molecule often leads to a significant reduction or even total loss of biological activity of the polymer-modified molecule, PEG attachment can be directed to a particular position within a protein such that the PEG moiety does not interfere with the function of that protein. One method of directing PEG attachment is to introduce a synthetic amino acid into the protein sequence. The protein biosynthetic machinery of the prokaryote Escherichia coli (E. coli) can be altered in order to incorporate synthetic amino acids efficiently and with high fidelity into proteins in response to the amber codon, UAG. See, e.g., J. W. Chin et al., J. Amer. Chem. Soc. 124: 9026-9027, 2002; J. W. Chin, & P. G. Schultz, ChemBioChem 3(11): 1135-1137, 2002; J. W. Chin, et al., PNAS USA 99: 11020-11024, 2002; and, L. Wang, & P. G. Schultz, Chem. Comm., 1 : 1-11, 2002. A similar method can be accomplished with the eukaryote, Saccharomyces cerevisiae (S. cerevisiae) (e.g., J. Chin et al., Science 301 : 964-7, 2003). Using this method, a non-natural amino acid can be incorporated into an antibody, variant or drug conjugate of the present disclosure, providing an attachment site for PEG. See, for example WO2010/011735 and W02005/074650.
Methodology and Techniques
The present disclosure encompasses methodologies and technologies well known in the art. These include conventional methods of mass spectroscopy, NMR, HPLC, protein chemistry, biochemistry, recombinant DNA techniques and pharmacology, within the skill of the art. Compounds of the present disclosure can be synthesized using several processes or schemes employed in the art. See for example, Dubowchik et al., Bioconjugate Chem. 13: 855-869, 2002; Doronina et al., Nature Biotechnology 21(7): 778-784, 2003; WO2012/166560; WO2013/185117, each incorporated herein by reference. Many methodologies and techniques for synthesis of pharmaceutical, diagnostic or therapeutic compounds are well known to one of ordinary skill in the art.
The present disclosure, unless otherwise indicated, also encompass conventional techniques of molecular biology (including recombinant techniques), cell biology, biochemistry and immunology, all within the skill of the art. Such techniques are explained fully in the literature, such as, Molecular Cloning: A Laboratory Manual, Third Edition, Cold Spring Harbor Press, Cold Spring Harbor, NY (Sambrook et al. Eds., 2001); Oligonucleotide Synthesis: Methods And Applications (Methods in Molecular Biology), Herdewijn, P., Ed., Humana Press, Totowa, NJ; Oligonucleotide Synthesis (Gait, M. J., Ed., 1984); Methods In Molecular Biology, Humana Press, Totowa, NJ; Cell Biology: A Laboratory Notebook, Academic Press, New York, NY (Cellis, J. E., Ed., 1998); Animal Cell Culture (Freshney, R. I., Ed., 1987); Introduction To Cell And Tissue Culture Plenum Press, New York, NY, (Mather, J. P. and Roberts, P. E., Eds., 1998); Cell And Tissue Culture: Laboratory Procedures John Wiley and Sons, Hoboken, NJ, (Doyle, A. et al., Eds., 1993-8); Methods In Enzymology (Academic Press, Inc.) New York, NY; Weir's Handbook Of Experimental Immunology Wiley-Blackwell Publishers, New York, NY, (Herzenberg, L. A. et al. Eds., 1997); Gene Transfer Vectors For Mammalian Cells Cold Spring Harbor Press, Cold Spring Harbor, NY, (Miller, J. M. et al. Eds., 1987); Current Protocols In Molecular Biology, Greene Pub. Associates, New York, NY, (Ausubel, F. M. et al., Eds., 1987); PCR: The Polymerase Chain Reaction, Birkhauser, Boston, MA, (Mullis, K. et al., Eds., 1994); Current Protocols In Immunology, John Wiley and Sons, Hoboken, NJ, (Coligan, J. E. et al., eds., 1991); Short Protocols In Molecular Biology, Hoboken, NJ, (John Wiley and Sons, 1999); Immunobiology 7 Garland Science, London, UK, (Janeway, C. A. et al., 2007); Antibodies. Stride Publications, Devoran, UK, (P. Finch, 1997); Antibodies: A Practical Approach Oxford University Press, USA, New York, NY, (D. Catty., ed., 1989); Monoclonal Antibodies: A Practical Approach Oxford University Press, USA, New York NY, (Shepherd, P. et al. Eds., 2000); Using Antibodies: A Laboratory Manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, (Harlow, E. et al. Eds., 1998); The Antibodies Harwood Academic Publishers, London, UK, (Zanetti, M. et al. Eds. 1995).
Therapeutic Uses of ADCs
The antibodies or ADCs of the disclosure are useful for treating a wide range of diseases, disorders, conditions, or cancers. Compositions disclosed herein may be used to modulate an immune response. Modulation of an immune response may comprise stimulating, activating, increasing, enhancing, or up-regulating an immune response. Modulation of an immune response may comprise suppressing, inhibiting, preventing, reducing, or downregulating an immune response. In some embodiments, the ADCs of the present invention may be used for reducing or inhibiting tumor growth or progression in an antigen-expressing cancer or cancer cell comprising an effective amount of the ADC.
Disclosed herein are methods of treating a subject for a condition with an ADC or pharmaceutical composition of the disclosure. In some cancers, overexpression of specific cell surface receptors can allow selective targeting of cancerous cells with small molecules or drugs, while minimizing effects on healthy cells. The invention provides a method of treating cancer by administering to a patient a therapeutically-effective amount of an ADC of the invention comprising an antibody or antibody fragment conjugated to a drug-linker disclosed herein. The cancer to be treated by an ADC of the present invention may be, a breast cancer including triple negative breast cancer (TNBC), a brain cancer, a pancreatic cancer including pancreatic ductal adenocarcinoma, a skin cancer, a lung cancer including non-small cell lung cancer, a liver cancer, a gastric cancer, a gall bladder cancer, a colon cancer (e.g., colorectal cancer (CRC), an ovarian cancer, a prostate cancer, a uterine cancer, a bone cancer, a blood cancer or a cancer or disease or conditions related to any of these cancers. In some embodiments, the cancer is a solid tumor. In some embodiments, the cancer is a TROP2-positive cancer. In some embodiments, the cancer is a Dxd-resistant cancer. In some embodiments, the cancer is a topoisomerase 1 (TOPI) inhibitor resistant cancer.
In some embodiments, the invention provides a method of treating cancer by administering to a patient a therapeutically-effective amount of an ADC of the invention. The cancer may be an antigen expressing cancer. The cancer may be a breast cancer including, but not limited to, triplenegative breast cancer. The cancer may be treated by recruiting cytotoxic T cells to the antigen receptor expressing tumor cells. In some embodiments, the disclosure provides a method of treating any cancer, disease or condition associated with high expression of antigen receptors by administering to a patient a therapeutically-effective amount of an antibody or ADC of the disclosure. In some embodiments, the invention provides a method of treating a disorder, or condition, or disease, or cancer by administering to a patient a therapeutically-effective amount of an antibody or ADC of the invention. In some embodiments, the antibody, antibody fragment or variant thereof binds to a tumor-associated antigen (TAA), wherein the TAA is TR0P2. In some embodiments, the invention provides a method of treating a disorder, or condition, or disease, or cancer by administering to a patient a therapeutically-effective amount of an anti-TROP2 antibody or ADC of the invention.
In some aspects, the disclosure provides ADCs for use in treating a disease or condition in a cell expressing high TROP2 receptor number. The antibodies and ADCs of the disclosure are for use in treating cancer including, but not limited to, ovarian cancer ovarian cancer including, but not limited to, an epithelial, stromal and germ cell tumor. The ovarian cancer may comprise a fallopian tube cancer or primary peritoneal carcinoma. The cancer may be characterized by high expression of antigen receptors, such as ovarian cancer, for example. The cancer may be treated by recruiting cytotoxic T cells to high expressing antigen receptor tumor cells. The antibodies of the disclosure are for use in treating inherited diseases, AIDS, or diabetes but is not limited to such. The antibodies, compounds or composition or conjugates of the disclosure can be used in the manufacture of a medicament for treating a disease or condition in a cell expressing high receptor number. The antibodies, compounds or composition or conjugates of the disclosure can be used in the manufacture of a medicament for treating cancer including, but not limited to, breast cancer including triple negative breast cancer, ovarian cancer including, but not limited to, an epithelial, stromal and germ cell tumor. The antibodies of the invention can be used in the manufacture of a medicament for treating diseases, conditions or cancers related to or associated with expression of an antigen receptor TR0P2. The anti-TROP2 antibodies of the invention can be used in the manufacture of a medicament for treating diseases, conditions or cancers related to or associated with high TR0P2 receptor numbers.
In some embodiments the condition to be treated is a cancer. The cancer may be, but is non-limited to, a breast cancer including triple negative breast cancer (TNBC), a brain cancer, a pancreatic cancer including pancreatic ductal adenocarcinoma, a skin cancer, a lung cancer including non-small cell lung cancer, a liver cancer, a gastric cancer, a gall bladder cancer, a colon cancer, an ovarian cancer, a prostate cancer, a uterine cancer, a bone cancer, a solid tumor, or a blood cancer, or a cancer or disease or conditions related to any of these cancers. Carcinomas are cancers that begin in the epithelial cells, which are cells that cover the surface of the body, produce hormones, and make up glands. By way of non-limiting example, carcinomas include breast cancer, pancreatic cancer, lung cancer, colon cancer, colorectal cancer, rectal cancer, kidney cancer, bladder cancer, stomach cancer, prostate cancer, liver cancer, ovarian cancer, brain cancer, vaginal cancer, vulvar cancer, uterine cancer, oral cancer, penile cancer, testicular cancer, esophageal cancer, skin cancer, cancer of the fallopian tubes, head and neck cancer, gastrointestinal stromal cancer, adenocarcinoma, cutaneous or intraocular melanoma, cancer of the anal region, cancer of the small intestine, cancer of the endocrine system, cancer of the thyroid gland, cancer of the parathyroid gland, cancer of the adrenal gland, cancer of the urethra, cancer of the renal pelvis, cancer of the ureter, cancer of the endometrium, cancer of the cervix, cancer of the pituitary gland, neoplasms of the central nervous system (CNS), primary CNS lymphoma, brain stem glioma, and spinal axis tumors. In some instances, the cancer is a skin cancer, such as a basal cell carcinoma, squamous, melanoma, nonmelanoma, or actinic (solar) keratosis. In some embodiments the cancer is any cancer with highly expressed antigen receptor numbers such as, for example, TROP2 antigen receptor numbers. In some embodiments, a cancer is resistant to certain treatments. In some embodiments, a cancer is a Dxd-resistant cancer. In some embodiments, a cancer is a topoisomerase 1 inhibitor-resistant cancer. In some embodiments, the cancer is resistant to treatment with topotecan, irinotecan or belotecan.
The pharmaceutical compositions containing an antibody or ADC of the invention may be formulated at a strength effective for administration by various means to a human patient experiencing disorders that may be affected by antibody agonists or antagonists, such as but not limited to, anti-proliferatives, anti-inflammatory, or anti-virals are used, either alone or as part of a condition or disease. Average quantities of an antibody or ADC may vary and in particular should be based upon the recommendations and prescription of a qualified physician. The exact amount of an antibody or ADC is a matter of preference subject to such factors as the exact type of condition being treated, the condition of the patient being treated, as well as the other ingredients in the composition. The disclosure also provides for administration of a therapeutically effective amount of another active agent such as an anti-cancer chemotherapeutic agent or immunotherapeutic agent but is not limited to such. The amount to be given may be readily determined by one skilled in the art based upon therapy with the antibody or ADCs of the invention.
Pharmaceutical Compositions
In other aspects of the present invention the antibody, antibody fragments, variants or ADCs further comprise a pharmaceutical composition or formulation. Such a pharmaceutical composition can employ various pharmaceutically acceptable excipients, stabilizers, buffers, and other components for administration to animals. See, for example, Remington, The Science and Practice of Pharmacy, 19th ed., Gennaro, ed., Mack Publishing Co., Easton, PA, 1995. Identifying suitable composition or formulations for stability, administration to a subject, and activity varies with each compound as a number of components, (for example, purifying, stabilizing components), need to be considered. Suitable salts for inclusion into the composition or formulation can include, but not limited to, sodium chloride, potassium chloride or calcium chloride. Buffering and/or stabilizing agents such as sodium acetate can be used. Suitable buffers can include phosphate-citrate buffer, phosphate buffer, citrate buffer, L-histidine, L-arginine hydrochloride, bicarbonate buffer, succinate buffer, citrate buffer, and TRIS buffer, either alone or in combination. Surfactants can also be employed, including polysorbates (e.g., polysorbate 80), dodecyl sulfate (SDS), lecithin either alone or in combination.
In some aspects of the present invention, the pharmaceutical composition or formulation can be an aqueous composition or in the form of a reconstituted liquid composition or as a powder. The composition or formulation can have a pH range from about 4.0 to about 7.0 or from about 4.5 to about 6.5 when the formulation is in a liquid form. However, the pH can be adjusted to provide acceptable stability and administration by the skilled medical practitioner.
The composition can be stored in a vial or cartridge, a pen delivery device, a syringe, intravenous administration tubing or an intravenous administration bag but is not limited to such. In other embodiments a pharmaceutical composition of the invention can be administered as a single dose or followed by one or more subsequent dose(s) minutes, days, or weeks after the first dose. Further administrations may be contemplated as needed to treat, reduce or prevent a cancer, condition, disorder or disease.
In some instances, the antibodies, antibody fragments, variants, or ADCs of the present invention disclosure may be used in conjunction with an additional therapy or treatment including but not limited to surgery, radiation, cryosurgery, thermotherapy, hormone treatment, chemotherapy, vaccines and other immunotherapies. In some embodiments such additional treatment can include a therapeutic agent such as chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid or combination thereof.
In other embodiments the antibodies, antibody fragments, variants, or ADCs of the invention can be administered with one or more immunostimulatory agents to induce or enhance an immune response. Immunostimulatory agents that can stimulate specific arms of the immune system, such as natural killer (NK) cells that mediate antibody-dependent cell cytotoxicity (ADCC). Such immunostimulatory agents include, but are not limited to, IL-2, immunostimulatory oligonucleotides (for example, CpG motifs), a-interferon, y-interferon, tumor necrosis factor alpha (TNFa). In other embodiments the ADCs of the invention can be administered with one or more immunomodulators including, but not limited to, cytokines, chemokines (including, but are not limited to, SLC5 ELC, MIP3a, MIP3 , IP-IO, MIG, and combinations thereof). Other therapeutic agents can be a vaccine that immunizes a subject against an antigen. Such vaccines, in some embodiments, include antigens, with, optionally, one or more adjuvants to induce or enhance an immune response. Adjuvants of many kinds are well known in the art.
The chemotherapeutic agent or any agent involved in treating, reducing or preventing a disease, condition or cancer in a subject in need thereof can also be administered in combination with an ADC of the invention disclosure. Chemotherapeutic agents may include, but are not limited to, erlotinib (TARCEVA®, Genentech/OSI Pharm.), bortezomib (VELCADE®, Millennium Pharm.), fulvestrant (FASLODEX®, AstraZeneca), sutent (SU11248, Pfizer), letrozole (FEMARA®, Novartis), imatinib mesylate (GLEEVEC®, Novartis), PTK787/ZK 222584 (Novartis), oxaliplatin (Eloxatin®, Sanofi), 5-FU (5 -fluorouracil), leucovorin, Rapamycin (Sirolimus, RAPAMUNE®, Wyeth), lapatinib (TYKERB®, GSK572016, GlaxoSmithKline), lonafarnib (SCH 66336), sorafenib (BAY43-9006, Bayer Labs.), and gefitinib (IRESSA®, AstraZeneca), AG1478, AG1571 (SU 5271; Sugen), alkylating agents such as thiotepa and CYTOXAN® cyclosphosphamide; alkyl sulfonates such as busulfan, improsulfan and piposulfan; antifolate antineoplastic such as pemetrexed (ALIMTA®, Eli Lilly), aziridines such as benzodopa, carboquone, meturedopa, and uredopa; ethylenimines and methylamelamines including altretamine, triethylenemelamine, triethylenephosphoramide, triethylenethiophosphoramide and trimethylomelamine; acetogenins (especially bullatacin and bullatacinone); a camptothecin (including the synthetic analogue topotecan); bryostatin; cally statin; CC-1065 (including its adozelesin, carzelesin and bizelesin synthetic analogues); cryptophycins (particularly cryptophycin 1 and cryptophycin 8); dolastatin; duocarmycin (including the synthetic analogues, KW-2189 and CB1-TM1); eleutherobin; pancrati statin; a sarcodictyin; spongistatin; nitrogen mustards such as chlorambucil, chlomaphazine, cholophosphamide, estramustine, ifosfamide, mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin, phenesterine, prednimustine, trofosfamide, uracil mustard; nitrosoureas such as carmustine, chlorozotocin, fotemustine, lomustine, nimustine, and ranimnustine; antibiotics such as the enediyne antibiotics, calicheamicin, calicheamicin gamma and calicheamicin omega; dynemicin, including dynemicin A; bisphosphonates, such as clodronate; an esperamicin; as well as neocarzinostatin chromophore and related chromoprotein enediyne antibiotic chromophores, aclacinomysins, actinomycin, anthramycin, azaserine, bleomycins, cactinomycin, carabicin, caminomycin, carzinophilin, chromomycinis, dactinomycin, daunorubicin, detorubicin, 6-diazo-5-oxo-L-norleucine, ADRLAMYCIN® doxorubicin (including morpholino-doxorubicin, cyanomorpholinodoxorubicin, 2-pyrrolino-doxorubicin and deoxydoxorubicin), epirubicin, esorubicin, idarubicin, marcellomycin, mitomycins such as mitomycin C, mycophenolic acid, nogalamycin, olivomycins, peplomycin, potfiromycin, puromycin, quelamycin, rodorubicin, streptonigrin, streptozocin, tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as methotrexate and 5- fluorouracil (5-FU); folic acid analogues such as denopterin, methotrexate, pteropterin, trimetrexate; purine analogs such as fludarabine, 6-mercaptopurine, thiamiprine, thioguanine; pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine, carmofur, cytarabine, dideoxyuridine, doxifluridine, enocitabine, floxuridine; androgens such as calusterone, dromostanolone propionate, epitiostanol, mepitiostane, testolactone; antiandrogens or androgen deprivation therapy; anti-adrenals such as aminoglutethimide, mitotane, trilostane; folic acid replenisher such as frolinic acid; aceglatone; aldophosphamide glycoside; aminolevulinic acid; eniluracil; amsacrine; bestrabucil; bisantrene; edatraxate; defofamine; demecolcine; diaziquone; elformithine; elliptinium acetate; an epothilone; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidainine; maytansinoids such as maytansine and ansamitocins; mitoguazone; mitoxantrone; mopidanmol; nitraerine; pentostatin; phenamet; pirarubicin; losoxantrone; podophyllinic acid; 2- ethylhydrazide; procarbazine; PSK® polysaccharide complex (JHS Natural Products, Eugene, OR); razoxane; rhizoxin; sizofuran; spirogermanium; tenuazonic acid; triaziquone; 2, 2', 2"- tri chlorotri ethylamine; trichothecenes (especially T-2 toxin, verracurin A, roridin A and anguidine); urethan; vindesine; dacarbazine; mannomustine; mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside ("Ara-C"); cyclophosphamide; thiotepa; taxoids, e.g., paclitaxel (TAXOL®, Bristol-Myers Squibb Oncology, Princeton, N.J.), ABRAXANE™ Cremophor-free, albumin, nanoparticle formulation of paclitaxel (American Pharmaceutical Partners, Schaumberg, Ill.), and TAXOTERE® doxetaxel (Rhone-Poulenc Rorer, Antony, France); chloranbucil; GEMZAR® gemcitabine; 6-thioguanine; mercaptopurine; methotrexate; platinum analogs such as cisplatin and carboplatin; vinblastine; platinum; etoposide (VP- 16); ifosfamide; mitoxantrone; vincristine; NAVELBINE® vinorelbine; novantrone; teniposide; edatrexate; daunomycin; aminopterin; xeloda; ibandronate; CPT-11; topoisomerase inhibitor RFS 2000; difluoromethylomithine (DMFO); retinoids such as retinoic acid; capecitabine; and pharmaceutically acceptable salts, acids or derivatives of any of the above.
EXAMPLES
It is understood that the examples and embodiments described herein are for illustrative purposes only and that various modifications or changes in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims.
Example 1: Synthesis of drug-linker compounds
All commercially available anhydrous solvents are used without further purification and stored under a nitrogen atmosphere. Chromatographic purification is performed on CombiFlash Rf from Teledyne ISCO using conditions detailed in the experimental procedure. Preparative HPLC is performed on Shimadzu system using Gemini-NX Cl 8, 5 pm 100 x 30 mm, 150 x 30 mm or 250 x 50 mm column, depending on the scale. Mass spectra (MS) are recorded on a Shimadzu LCMS-2020 system and data are processed using Shimadzu Lab Solutions software. Agilent 1260 Infinity Binary LC coupled with 6230 Accurate-Mass TOFMS system is used for HR-ESI-TOF analysis. NMR spectral data are collected on a 500 MHz Bruker NMR spectrometer. Chemical shifts (5) are reported in ppm and referenced off the deuterium solvent signal. Coupling constants (J) are reported in hertz (Hz). Spin multiplicities are described as: s (singlet), br (broad), d (doublet), dd (doublet of doublets), t (triplet), q (quartet) or m (multiplet).
Abbreviations used in the Examples herein: AHZ: acetohydrazide, DIAD: Diisopropyl azodi carb oxy late, DMF: Dimethylformamide, HATU: l-[Bis(dimethylamino)methylene]-lH- l,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate, MeOH: Methanol, TFA: Trifluoroacetic acid.
Chemical names of compounds were derived from chemical structures using
ChemDraw 20.1.1 (CambridgeSoft).
2-(2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)ethoxy)isoindoline-l, 3-dione
(Compound 1-3): Tetraethylene glycol 1-1 (10g, 51.5mmol), N-hydroxyphthalimide 1-2 (8.4g, 51.15mmol) and triphenylphosphine (17.6g, 67mmol) were dissolved in 300 mL of tetrahydrofuran followed by addition of DIAD (12.8 mL, 61.78 mmol) at 0 °C. The resulting solution was stirred at room temperature overnight, and then concentrated to dryness. The residue was purified by flash column chromatography to give 5.47 g (31%) of compound 1-3.
2-(2-(2-(2-((l,3-dioxoisoindolin-2-yl)oxy)ethoxy)ethoxy)ethoxy)acetaldehyde
(Compound 1-4): To a solution of compound 1-3 (200mg, 0.59mmol) in 15mL dichloromethane was added Dess-Martin periodinane (300mg, 0.71 mmol). The reaction mixture was stirred at ambient temperature overnight. The reaction was quenched with the solution of sodium bisulfite in 15 mL of saturated sodium bicarbonate. The mixture was separated. The organic layer was washed with saturated sodium bicarbonate, brine, dried over sodium sulfate, filtered and concentrated in vacuo. The residue was purified by flash column chromatography to give 150 mg (75%) of compound 1-4.
6
(2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((tert-butoxycarbonyl)amino)-N,3- dimethylbutanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2- methylpropanoic acid (Compound 6-1; Boc-Val-Dil-Dap-OH): Compound 6-1 is available from commercial suppliers including MedChemExpress, located at 1 Deerpark Dr # Q, Monmouth Junction, NJ 08852, as Catalog No.: HY-130961.
Methyl ((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((tert-butoxycarbonyl)amino)-N,3- dimethylbutanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2- methylpropanoyl)-L-phenylalaninate (Compound 6-2): To a solution of compound 6-1 (500 mg, 0.875 mmol) in 3 mL of DMF was added 283 mg of L-phenylalanine methyl ester hydrochloride (commercially available from Sigma-Aldrich; catalog no. P17202), 433 mg of HATU and 581 pL of N-methylmorpholine. The reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was concentrated in vacuo and extracted with ethyl acetate (100 mL x 1, 50 mL x 2). The organic layer was combined and washed with brine, dried over sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography to give 560 mg (76%) of compound 6-2.
Methyl ((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-amino-N,3-dimethylbutanamido)- 3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanoyl)-L- phenylalaninate (Compound 6-3): Compound 6-2 was dissolved in 15 mL 4N HCl/dioxane. The reaction mixture was stirred at room temperature for 2 hours and concentrated in vacuo to give 511 mg of compound 6-3.
Methyl ((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((S)-2-((tert- butoxycarbonyl)(methyl)amino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3- methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanoyl)-L- phenylalaninate (Compound 6-4): To a solution of compound 6-3 (368 mg, 0.55 mmol) in 3 mL of DMF was added 255 mg of Boc-N-methyl-L-valine (commercially available from Sigma- Aldrich; catalog no. 15538), 314mg of HATU and 303 p of N-methylmorpholine. The reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was concentrated in vacuo and extracted with ethyl acetate (100 mL x 1, 50 mL x 2). The organic layer was combined and washed with brine, dried over sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography to give 370 mg (79%) of compound 6-4.
((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((S)-2-((tert- butoxycarbonyl)(methyl)amino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3- methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanoyl)-L- phenylalanine (Compound 6-5): To a solution of compound 6-4 (170mg) in 10 mL MeOH was added 5 eq of IN LiOH. The reaction mixture was stirred at room temperature for 2 hours. The reaction mixture was acidified by IN HC1 and extracted with ethyl acetate washed with brine, dried over sodium sulfate and concentrated in vacuo to give 150 mg (90%) of compound 6-5.
((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-N,3-dimethyl-2-((S)-3-methyl-2- (methylamino)butanamido)butanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3- methoxy-2-methylpropanoyl)-L-phenylalanine (Compound 6-6): Compound 6-5 was dissolved in 4N HCl/dioxane. The reaction mixture was stirred at room temperature for 2 hours and concentrated in vacuo and purified by HPLC to give 150 mg of compound 6-6.
((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((S)-14-((l,3-dioxoisoindolin-2-yl)oxy)-2- isopropyl-3-methyl-6,9,12-trioxa-3-azatetradecanamido)-N,3-dimethylbutanamido)-3- methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanoyl)-L- phenylalanine (Compound 6-7): To a solution of compound 6-6 (50mg, 0.062 mmol) in 1 mL of DMF was added compound 1-4 (63 mg, 0.186 mmol) and 70 pL of acetic acid, followed by addition of 8 mg of sodium cyanoborohydride. The resulting mixture was stirred at ambient temperature for 2 hours. The reaction mixture was diluted with water and purified by HPLC to give 60 mg (80%) of compound 6-7.
((2R,3R)-3-((S)-l-((3R,4S,5S)-4-((S)-2-((S)-14-(aminooxy)-2-isopropyl-3-methyl- 6,9,12-trioxa-3-azatetradecanamido)-N,3-dimethylbutanamido)-3-methoxy-5- methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanoyl)-L-phenylalanine
(Compound 6): Compound 6-7 (60mg, 0.05mmol) was dissolved in 1 mL of DMF. Hydrazine (32 pL) was added. The resulting solution was stirred at ambient temperature for 1 hour. The reaction was quenched with IN hydrochloride solution. The reaction mixture was purified by HPLC to give 33 mg (55%) of compound 6.
Synthesis of Compounds 1-23
Methods for the synthesis of drug-linker compounds 1 to 23 are disclosed in WO2013/185117A1, the contents of which is hereby incorporated herein in its entirety.
Example 2: Protocols for Production of anti-TROP2 Antibodies Containing pAF at Heavy Chain Position 114 (Kabat numbering).
Engineering of Expression Vectors - Anti-TROP2 antibody expression plasmids were engineered by recombination-based cloning method using Gibson Assembly kit (New England Biolabs, MA) in E. coli NEB5a cloning strain (New England Biolabs, MA) as described below. Table 3 shows E. coli cloning host used in this study and its genotypes.
Gibson Assembly - Anti-TROP2 light chain and heavy chain gBlocks (GOIs) were synthesized at Genewiz (Azanta, South Plainfield, NJ, 07080). The primers for amplifying various GOIs containing donor fragments had about 25-30 base pair (bp) overlap sequence at their 5 '-termini with the acceptor vector sequences for homologous recombination and were synthesized at Integrated DNA Technologies ((IDT), San Diego, CA)). The PCR fragments were amplified using high fidelity DNA polymerase mix, Pfu Ultra II Hotstart PCR Master Mix (Agilent Technologies, CA). The PCR products were digested with Dpnl restriction enzyme (NEB) for 2 hours at 37 °C to remove plasmid background followed by column purification using Qiagen PCR column purification kit (Qiagen) and quantitated by Nanodrop (ThermoFisher). The acceptor vectors were linearized by digesting with unique restriction enzymes: Hindlll and EcoRl (NEB, MA) for 4 to 5 hours at supplier’s recommended temperatures, PCR column purified and quantitated. The donor inserts and appropriately prepared acceptor vectors were mixed at a 3 : 1 molar ratio, incubated at 50 °C for 15 min, using Gibson Assembly kit (NEB), and then used for transformation into E. coli NEB 5a strain (NEB).
The recombinants were recovered by plating transformed cells on to 2xYT+ 2% Glucose agar plates (Teknova) containing antibiotic carbenicillin, 100 pg/mL. The next day, 4 to 6 well- isolated single colonies were inoculated into 5 mL Super broth + carbenicillin 100 pg/mL (Teknova) media and grown overnight at 37 °C. The recombinant plasmids were isolated using Qiagen’s plasmid DNA mini -prep kit (Qiagen) and verified by DNA sequencing (Eton Biosciences, CA). The complete GOI region plus 200 bp upstream and 200 bp downstream sequences were verified by using gene-specific sequencing primers.
CHO cells transient transfection grade high-quality large-scale DNA (10 mg or higher) was manufactured at Aldevron (Fargo, North Dakota 58103).
Transient expression - Platform cell line was maintained in CD CHO Fusion (SAFC) supplemented with 8 mM L-glutamine (Gibco). Cells were passaged every 3 to 4 days at density of 0.4 million cells per ml. Cells were expanded to large scale to meet transfection volume.
On the day of transfection, cells were collected for transfection volume at Viable Cell Density of 4 x 106/ml. Cells were then spun at 2000 RPM, for 4 min. at room temperature. The liquid was aspirated by vacuum and the cell pellet was re-suspended with transfection volume of TransFX (Cytiva) supplemented with 8 mM L-glutamine (Gibco), 0.05% Pol oxamer 188 (Sigma), 0.125% Dimethyl Acetamide (Sigma Aldrich). Non-Linearized DNA at the ratio of 2: 1 (HC:LC) at the total amount of 3.2 mgs/L were added to the cells in shake flask. The culture was shaking at 190 RPM, 37C, 5% CO2, 80% humidity in incubator (Kuhner) for 5 min. PEI Max (liquid, Polysciences) was added to culture/DNA at the concentration of 4.8 mgs/L, and immediately, the culture shake flask was placed back into same incubator for 24 hr. The following were added to the culture on day 1 : pAF (final concentration in culture: 1 mM), Cell Boost 4 (GE Healthcare; final concentration in culture: 3.75 g/L), Cell Boost 7b (GE Healthcare; final concentration in culture: 0.2 g/L), Long R3 IGF-1 (Sigma; final concentration in culture: 120 pg/L). The incubator temperature was shifted from 37 °C to 32 °C, and the shake speed reduced to 160 RPM. Additional Cell Boost 4 (final concentration: 2 g/L), Cell Boost 7b (final concentration: 0.1 g/L), and glucose (final concentration: 2 mM) were added on days 3, and 5. The culture media glucose level was monitored using glucose meters, and additional glucose was added to the culture when the glucose level was below 2 g/L. Viable cell count and viability were measured by Vi-Cell instrument. Antibody production was measured by Octet using Protein G sensors. The culture was collected on day 7, by spinning at 4000RPM, 20min. in centrifuge. The supernatant was then filtered by 0.2 pm filtration.
The foregoing transient expression method can also be used to generate light chain mutants, such as light chain having amber mutation at VI 10, Al 12 or SI 14, each of which were generated as transients.
Master-well generation - The expression plasmid was linearized using Pvu I (NEB) digestion for 24 hours. After linearization, the DNA was purified using phenol:chloroform:isoamyl alcohol extraction and dissolved in endotoxin-free water at the concentration of 2.5 pg/pl. Platform cell line was maintained in EX-CELL 302 supplemented with 3 mM L-glutamine and 3 mM GlutaMAX. Cells were passaged every 3 to 4 days seeded at density of 0.3 x 106/ml. One day prior to transfection, cells were seeded at 0.6 x 106/ml. On day 0, 15 x 106 cells were transfected with 25 pg of total linearized antibody expression plasmids using MaxCyte electroporation (OC- 100) platform following instruction manual. After transfection, cells were rested in an empty 125 ml shake flask and incubated in 37 °C static incubator for 20 min. Then 30 ml recovery media (50% EX-CELL 302 (SAFC) - 50% CD-CHO (GIBCO) supplemented with 3mM glutamine and 3mM GlutaMAX) was added into the flask and shaken in incubator. After 48 hours, transfected cells were counted, spun down, washed and re-suspended in selection media (50% EX-CELL 302 - 50% CD-CHO with 37.5 pM MSX (Millipore)) for Master- well generation. Limited dilution of cells was performed. Cells were then plated into 96-well flat bottom plate (Corning) at volume of 200uL and at cell density of 2500 cells/well. Five 96-well plates were plated and placed into static 37 °C, 5% CO2, high humidity incubator (Panasonic) for 3 weeks.
Confluent wells were cherry-picked and transferred to new “Master” 96-well plate by diluting 100 pL of old culture with 100 pL of fresh media (50% EX-CELL 302 + 50% CD CHO + 37.5 pM MSX). The plates were plated and placed into static 37 °C, 5% CO2, high humidity incubator for 4 days. On day 4, 100 pL of cells from each well of “Master” plate were transferred into new “assay” plate which contained 100 pL of 50% EX-CELL 302 + 50% CD CHO + 37.5uM MSX + ImM pAF. The final concentration of pAF is 0.5 mM. After incubating for 4 days, the “assay” plate was spun at 2000 RPM, 4 minutes. The supernatants were subjected to ELISA with capturing antibody Goat ahuman IgG (Southern Biotech) and detected with Goat ahuman Kappa- HRP (Southern Biotech).
Top 24 clones, based upon ELISA data, were picked and transferred from the “master” plate into 24 Well plate (Corning) by diluting 180 pL of old culture from each well into 500 pL of same selection media. The 24-well plate was placed into static incubator at 37 °C, 5% CO2, 80% humidity (Panasonic) for 4 days. Cells from each well (24 well static) were transferred to 24-Deep Well plate (Thomson Instrument) by dilution of 600 pL of cells into 1400 pL of fresh selection media per well. The plate was incubated in shaking incubator (250 RPM, 37 °C, 5% CO2, 80% humidity). After 7 days, cells from each well were diluted with PBS (0.5 mL cells + 0.5 mL PBS) to count by Vicel. Each “master-well” were transferred to 125 mL shake flask (Coming). The dilution was 1.5 mL of cultures into 20 mL of same selection media. The shake flasks were shaking in Kuhner incubator (37 °C, 5% CO2, 80% humidity, 155 RPM) for 5 days. Each of the masterwell clones was passaged at least 1 passage, and 2 freeze vials were frozen. The cultures then being subjected to Fed-batch expression to finalize top 2 master wells.
Fed-batch expression - Generated master-well clones were inoculated into basal expression media (50% Dynamis - 50% EX-CELL 302 supplemented with 25 pM MSX, ornithine 0.5 mM, glucose 2 g/L) at density of 0.5 x 106/ml in a shake flask on day 0. The transfected cells were incubated at 37 °C, 5% CO2 on orbital shaker set to 150 rpm. The following were added to the culture on day 3: pAF (final concentration in culture: 0.5 mM), Cell Boost 4 (GE Healthcare; final concentration in culture: 10 g/L) and Cell Boost 7b (GE Healthcare; final concentration in culture: 0.52 g/L). Long R3 IGF-1 (final concentration in culture: 120 pg/L) was added to the culture on day 5. The culture media glucose level was monitored using glucose meters, and additional glucose was added to the culture up to 6 g/L when the glucose level was below 2 g/L. Viable cell count and viability were measured by Vi-Cell instrument. The supernatant was collected for purification on day 10. Antibody production was measured by Octet using Protein G sensors.
Example 3: Protocols for Production of anti-TROP2 Antibodies Containing pAF at Heavy Chain Position Al 14 (Kabat numbering) and Light Chain Position 121
Molecular Cloning - CHO cell codon-optimized antibody heavy chain and light chain cDNA sequences were obtained from a commercial DNA synthesis service (Integrated DNA Technologies (IDT), San Diego, CA). The synthesized DNA fragments were digested with Hind III and EcoR I (both from New England BioLabs, (NEB), Ipswich, MA) and purified using a PCR purification kit (Qiagen, Valencia, CA). Then the digested antibody gene fragments were ligated into the expression vector via a quick ligation kit (NEB) to yield the constructs for expression of wild type antibody heavy chain and light chain. The resulting plasmids were propagated in E. coli and verified by a DNA sequencing service (Eton Biosciences, San Diego, CA).
Transient expression - Platform cell line was maintained in EX-CELL 302 (Sigma) supplemented with 3 mM L-glutamine (Gibco) and 3 mM GlutaMAX (Gibco). Cells were passaged every 3 to 4 days seeded at density of 0.4 million cells per ml. One day prior to transfection, cells were seeded at 0.6 million cells per ml. On day 0, cells were transfected with antibody expression plasmids encoding the light chain and heavy chain using MaxCyte electroporation platform following the instruction manual. After transfection, cells were rested in an empty 125ml shake flask and incubated at 37 °C in a static incubator for 30 min. Basal expression media (50% Dynamis - 50% EX-CELL 302 supplemented with 3 mM L-glutamine and 3 mM GlutaMAX) was added to the transfected cells in the shake flask for a final density of 3 x 106/ml. The transfected cells were incubated at 37 °C, 5% CO2 on orbital shaker set to 155 rpm. The following were added to the culture on day 1 : pAF (final concentration in culture: 1 mM), Cell Boost 4 (GE Healthcare; final concentration in culture: 3.75 g/L), Cell Boost 7b (GE Healthcare; final concentration in culture: 0.2 g/L), Long R3 IGF-1 (Sigma; final concentration in culture: 120 pg/L) and GlutaMAX (final concentration in culture: 2 mM). The incubator temperature was shifted from 37 °C to 32 °C. Additional Cell Boost 4 (final concentration: 2 g/L), Cell Boost 7b (final concentration: 0.1 g/L), and GlutaMAX (final concentration: 2 mM) was added on days 3 and 5, and supernatant was collected on day 7. The culture media glucose level was monitored using glucose meters, and additional glucose was added to the culture when the glucose level was below
2 g/L. Viable cell count and viability were measured by Vi-Cell instrument. Antibody production was measured by Octet using Protein G sensors.
Stable minipool (master well) generation - The expression plasmids (HA114 and LC121) were linearized using Pvu I (NEB) digestion for 4 hours. After linearization, the DNA was purified using phenol:chloroform:isoamyl alcohol extraction and dissolved in endotoxin-free water at the concentration of 1 pg/pl. Platform cell line was maintained in EX-CELL 302 supplemented with
3 mM L-glutamine and 3 mM GlutaMAX. Cells were passaged every 3 to 4 days seeded at density of 0.3 x 106/ml. Two days prior to transfection, cells were seeded at 0.4 x 106/ml. On day 0, 3 x 106 cells were transfected with 6 pg of linearized antibody expression plasmids (heavy chain 3 pg + light chain 3 pg) using AMAXA electroporation system following instruction manual. There were 8 reactions of AMAXA transfection executed using total 24 x 106 cells and 48pg plasmid DNAs. After transfection, cells were rested in a T-225 flask in volume of 48ml fresh culture media and incubated in 37 °C static incubator for overnight. On day one, transfected cells were counted, spun down, washed and re-suspended in selection media (50% EX-CELL 302 - 50% CD-CHO with 25 pM MSX), then seeded in 40x 96 well plates at 2500 cell/well/200pl and cultured for three weeks. After the clones growing up, cherry-pick of about 1000 clones was done and the clones were consolidated in new 96 well plates, then the screening of the clones went through from static 4-day Batch in 96 well static, 7-day Batch in 24 deep well to 7-day Batch and 12-day Fed Batch in shaking flasks for final top 2 master well generation.
Fed-batch expression - Previously generated antibody stable mini-pools (master well) were inoculated into basal expression media (50% Dynamis - 50% EX-CELL 302 supplemented with lx GS, 25 pM MSX, insulin 2 pg/ml, ornithine 0.5 mM, glucose 2 g/L and lx anti-clumping agent) at density of 0.5 x 106/ml in a shake flask on day 0. The cells were incubated at 37 °C, 5% CO2 on orbital shaker set to 150 rpm. The following were added to the culture on day 3: pAF (final concentration in culture: 0.5 mM), Cell Boost 4 (GE Healthcare; final concentration in culture: 10 g/L) and Cell Boost 7b (GE Healthcare; final concentration in culture: 0.52 g/L). Long R3 IGF-1 (final concentration in culture: 120 pg/L) was added to the culture on day 5. The culture media glucose level was monitored using glucose meters, and additional glucose was added to the culture up to 6 g/L when the glucose level was below 2 g/L. Viable cell count and viability were measured by Vi-Cell instrument. The supernatant was collected for purification on day 10. Antibody production was measured by Octet using Protein G sensors.
Example 4: Purification of Antibodies from EuCODE Expression System
Clarified Cell culture media containing the target antibody containing non-natural amino acid pAF was loaded over a protein A ProSep Ultra column (EMD Millipore) equilibrated in 20 mM sodium phosphate, lOOmM sodium chloride, pH 7.5. After loading, the column was washed with buffer A (20 mM sodium phosphate, lOOmM sodium chloride, pH 7.5) followed by wash buffer B (5 mM succinic acid, pH 5.8) to remove host cell contaminants. The target antibody was eluted from the column with elution buffer C (50 mM glycine, 10 mM succinic acid, pH 3.2). The target antibody was pooled, and pH adjusted to pH 5.0 with 2.0 M tris base. The target antibody was further purified by loading the conditioned protein A pool over a Capto SP Impres column (GE Healthcare) equilibrated in 30 mM sodium acetate, pH 5.0. The target antibody was eluted from the column with a linear gradient to 100% buffer B (30 mM sodium acetate, 0.5 M sodium chloride, pH 5.0) and fractions containing monomeric antibody were pooled, 0.22 pM filtered, and stored at < 65 °C until further use.
Example 5: TROP2 expression levels
TROP2 cell surface levels were quantified using the Dako QIFIKIT (Agilent, Santa Clara, CA, according to manufacturer’s instruction. Briefly, cells were harvested using StemPro Acccutase cell dissociation reagent, washed with FACS buffer (lx PBS containing 0.2% BSA and 15 mM NaNs) and incubated with mouse anti-TROP2 antibody (clone MR54, eBioScience, San Diego, CA) or isotype control antibody (mouse IgG2a, kappa, eBioScience). After washing, FITC conjugated anti-mouse secondary antibody provided by kit was incubated with cells, set-up beads, and calibration beads. Washed cells and beads were run on FACSCantoII. Lot specific calibration beads were used to generate a standard curve to calculate TROP2 number on the surface of each cell line. TROP2 cell surface numbers were determined as shown in Table 4.
Example 6: TR0P2 Binding Cross-reactivity by Bio-Layer Interferometry (BLI)
TR0P2 multi -concentration binding kinetic experiments were performed on an Octet RED96 (Sartorius) instrument at 30°C. Anti-human Fc capture biosensors (Sartorius, cat# 18- 5063) were loaded with purified anti-TROP2 mAb in IX HBS-P+ Buffer (Cytiva, cat# BR-1008- 27). Immobilization levels between 0.8 to 1.0 nm were reached. The loaded biosensors were washed with IX HBS-P+ Buffer to remove any unbound mAb before measuring association and dissociation kinetics. For association phase monitoring, TROP2 analyte samples (human, cyno, or rat) were diluted with IX HBS-P+ Buffer to concentrations ranging from 100 nM to 1.25 nM and transferred to solid-bottom, black 96 well plates (Greiner Bio-One, cat# 655209). TROP2 samples were allowed to bind to anti-TROP2 mAb loaded biosensors for 90 seconds. The dissociation phase was recorded in wells of a solid black 96-well plate containing IX HBS-P+ Buffer for 300 seconds. Data were referenced using a parallel buffer blank subtraction, and the baseline was aligned to the y-axis and smoothed by a Savitzky-Golay filter in the Octet data analysis software version 10.0 (Sartorius). The processed kinetic sensorgrams were globally fitted using the Langmuir model describing a 1 : 1 binding stoichiometry. Binding results are shown in FIG. 2. Example 7: Site Specific Conjugation of Drug-Linkers
Anti-TROP2 antibodies containing non-naturally encoded amino acid para-acetyl-L- phenylalanine (pAF) at specified locations in the heavy chain and/or light chain were buffer exchanged into 50 mM sodium acetate, pH 4.0-4.3, and concentrated to 5-25 mg/mL. 1.5 M acetohydrazide (AHZ), pH 4.0, was added to the concentrated antibody at a final concentration of 100 mM. A stock solution of drug-linker compound 6 in water for injection (WFI) or DMSO was then added to the anti-TROP2 mAb solution at 8-15 molar equivalents drug-linker to mAb. The conjugation reactions were allowed to react for 16-72 hours at 30 °C. The antibody conjugates were purified over a Capto SP Impres column (Cytiva) to remove excess reagents. ADCs were then formulated into 50 mM histidine, 2.5% trehalose, pH 6.0 and 0.22 pm filtered. Reverse Phase (RP) chromatography was used to monitor the final drug-to-antibody ratio (DAR) under reducing conditions. RP-HPLC analysis was performed on an Agilent 1200 series HPLC system using an Agilent Stablebond SB-C8, 5 pm, 4.6 x 150 mm column. Mobile phase A consisted of 0.1% TFA in water and mobile phase B consisted of 0.1% TFA in acetonitrile. The flow rate was 1 mL/minute, the column temperature was 75 °C, and detection was recorded at A214 nm. Elution of the reduced heavy and light chain of the mAb and ADC elution occurred during a 30-60% gradient increase of mobile phase B. A representative HPLC chromatogram under reducing conditions (FIG. 1) shows light chain eluting prior to unconjugated heavy chain, followed by conjugated heavy chain with drug-linker compound 6.
The ADC containing anti-TROP2 mAb, wherein the mAb contains two heavy chains of SEQ ID NO: 5 and two light chains of SEQ ID NO: 4, and wherein compound 6 is conjugated to pAF at Kabat position 114 of each heavy chain (DAR = 2) is referred to herein as anti-TROP2- HC114pAF-compound-6 ADC (also referred to herein as “TROP2-AS269”).
Example 8: In vitro Cytotoxicity of Anti-TROP2 ADC in TROP2-expressing Cell Lines and a TROP2-negative Cell Line
Cells were seeded into 96-well clear bottom white plate at 2,500 cells/well for BxPC-3, MDA-MB-468, and Calu-6 cells or at 2,000 cells/well for HCC 1806 cells and incubated overnight in a 37 °C, 5% CO2 incubator. The next day serially diluted anti-TROP2-HCl 14pAF-compound- 6 ADC (DAR2), Benchmark-DXd ADC, or monomethyl auristatin E (MMAE; MedChemExpress, CAS No.: 474645-27-7) were added to the wells and the plates were incubated for 4 or 7 days. At the end of incubation, luminescence was measured by addition of CellTiter-Glo2.0 Reagent (Promega, Madison, WI) to the room temperature equilibrated plates. The relative cell viability was calculated as a percentage of an untreated control. The half-maximal inhibitory concentration (IC50) was determined by a nonlinear 4-parameter dose-response curve fitting using GraphPad Prism (GraphPad Software, San Diego, CA). The maximal killing (Emax) was determined by subtracting the % viability from 100%.
Cytotoxicity of anti-TROP2-HCl 14pAF-compound-6 ADC (also referred to herein as “TROP2-AS269”) was tested in multiple TROP2-expressing cell lines and a TROP2 negative cell line with 4 days or 7 days of treatment. Potency of anti-TROP2-HCl 14pAF-compound-6 ADC after 4 days of treatment was similar to that after 7 days of treatment in TROP2-expressing BxPC- 3, MDA-MB-468, or HCC1806 cell lines and Emax (%) was improved after 7 days of treatment (Tables 5 and 6). Anti-TROP2-HC114pAF-compound-6 ADC exhibited IC50 values of 0.06-0.25 nM in TR0P2-expressing cell lines and was more potent than Benchmark-DXd ADC at day 7, but no cell killing was observed in TROP2-negative Calu-6 cell line (FIG. 3).
Table 5. IC50 (nM) and Emax (%) of Anti-TROP2-HCl 14pAF-compound-6 in TROP2-expressing cell line at day 4.
Example 9: In vitro Cytotoxicity of anti-TROP2 ADCs in Benchmark-DXd-resistant Cell Line and Benchmark-DXd-insensitive Cell Line.
Benchmark-DXd resistant cells were generation as follows. MDA-MB-468 tumor was collected and isolated from the mice that were treated with anti-TROP2-DXd ADC (also referred to herein as “Benchmark-DXd”). Cells were expanded and cultured in RPML1640 media containing 10% FBS and 1% penicillin/streptomycin, and eventually cultured in RPML1640 media containing 10% FBS, 1% penicillin/streptomycin, and 10 nM Benchmark-DXd for 12 days. Cytotoxicity was evaluated 20 days after Benchmark-DXd removal.
Tumor cell line cytotoxicity was assessed as follows. Cells were seeded at 2,500 cells/well into 96-well clear bottom white plate and treated the next day with serially diluted TROP2-AS269, or Benchmark-DXd. The relative viability of the cells was measured using CellTiter-Glo2.0 Reagent 4 days after TROP2-AS269 treatment and 7 days after Benchmark-DXd treatment.
Potency and Emax were compared between wild-type and Benchmark-DXd resistant MDA-MB-468 cells after 4 or 7 days of anti-TROP2-HCl 14pAF-compound-6 (“TROP2- AS269”) or Benchmark-DXd treatment. The IC50 of Benchmark-Dxd was increased from 0.78 nM in wild-type MDA-MB-468 cells to 47.56 nM in Benchmark-DXd resistant MDA-MB-468 cells; Emax at 100 nM treatment was decreased from 94.9% to 52.5%, respectively. Cytotoxicity of TROP2-AS269 was similar in both wild-type MDA-MB-468 cells and Benchmark-DXd resistant MDA-MB-468 cells (Table 7 and FIG. 4).
FIG. 5 shows that TROP2-AS269 exhibited cytotoxicity against the Benchmark-DXd insensitive JIMT-1 cell line. While Benchmark-DXd was not potent enough to kill JIMT-1 cells with 7 days of treatment, TROP2-AS269 showed IC50 value of 0.035 nM with >90% of cell killing at a single-digit nM treatment for 4 days.
Example 10: In vitro Cytotoxicity of Anti-TROP2 ADCs in Human Keratinocytes.
Cytotoxicity in human keratinocytes was assessed as follows. Cells were seeded at 2,500 cells/well into 96-well clear bottom white plate and treated the next day with serially diluted TROP2-AS269 or an anti-TROP2-auristatin-0101 ADC (also referred to herein as “Benchmark- AurOlOl”). The relative viability of the cells was measured using CellTiter-Glo2.0 Reagent 4 days after TROP2-AS269 or Benchmark- AurOlOl treatment. The results are shown in FIG. 6. TROP2- AS269 exhibited reduced in vitro human keratinocyte cytotoxicity compared to Benchmark- AurOlOl.
Example 11: Anti -tumor Activity of Anti-TROP2 ADCs.
Human pancreatic BxPC-3 cancer cell line was cultured in RPMH640 supplemented with 10% FBS and 1% penicillin/streptomycin. 5xl06 BxPC-3 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female Nu/Nu mice (Charles River Laboratories). When tumor volume reached approximately 100 mm3, mice were randomized and grouped for treatment. The single intravenous injection was done with vehicle controls, 0.1 mg/kg, 0.3 mg/kg, or 1 mg/kg of anti-TROP2-HCl 14pAF-compound-6 (“TROP2-AS269”) or 1 mg/kg or 3 mg/kg of anti-TROP2-DXd ADC (“Benchmark-DXd”), and tumor size was monitored up to 44 days after treatment. Tumor sizes were measured by calipers and tumor volume was calculated as length x width x width x 0.5. Percent tumor growth inhibition (TGI) was calculated as (1-tumor volume of treated/tumor volume of vehicle control) xlOO. All TROP2-AS269 treated groups reduced BxPC-3 tumor growth with as low as 0.1 mg/kg single administration. 0.3 mg/kg or 1 mg/kg of TROP2-AS269 treated groups reached > 80% of TGI as early as 2 weeks after treatment. 0.3 mg/kg of TROP2-AS269 treatment showed better TGI than 3 mg/kg of Benchmark-DXd, suggesting that TROP2-AS269 displays about 10-fold increased potency than Benchmark-DXd (FIG. 7). In addition, there was no sign of toxicity such as body weight loss at the single injection of up to 10 mg/kg of TROP2-AS269 (data not shown).
Example 12: Anti-TROP2 ADC Binding Affinity.
The binding affinities of TROP2 mAb (before conjugation) and anti-TROP2-HCl 14pAF- compound-6 (“TROP2-AS269”) were compared in TROP2-expressing MDA-MB-468 cells. Cells were incubated with serially diluted TROP2 mAb or TROP2-AS269 for 30 min at 4 °C and detected with Alexa Fluor 647 conjugated anti-human F(ab')2 secondary antibody. As seen in FIG. 8, TROP2 mAb and TROP2-AS269 showed comparable binding affinities to MDA-MB-468 cells with Kd values of 3.3 and 3.4 nM, respectively.
Example 13: Antibody-dependent Cell-Mediated Cytotoxicity.
Antibody-dependent cell-mediated cytotoxicity (ADCC) activity was measured with anti- TROP2-HC114pAF-compound-6 (“TROP2-AS269”) and anti-TROP2-DXd ADC (“Benchmark- DXd”) using Promega ADCC reporter bioassay with V-variant system, as directed. Briefly, MDA- MD-468 cells were seeded at 5,000 cells/well in a 96-well white plate. The next day, media was changed to 4% low IgG containing RPMI1640 media and cells were treated with serially diluted TROP2 AS269 or Benchmark-DXd and freshly thawed ADCC Bioassay effector cells. After 6 hours of incubation at 37°C and 5% CO2, luminescence was measured by adding Bio-Gio Luciferase Assay reagent. Fold induction was calculated as an induced luminescence unit divided by a non-treated control luminescence unit after background subtraction. The results are shown in FIG. 9.
Example 14: Anti-tumor Activity of Anti-TROP2 ADCs.
Human breast cancer cell line, JIMT-1, was cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. 5* 106 JIMT-1 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female SCID-beige mice (Charles River Laboratories). When tumor volume reached approximately 100 mm3, mice were randomized and grouped for treatment. Two intravenous Q3W injections were done with vehicle controls, 0.3 mg/kg, 1 mg/kg, or 3 mg/kg of anti-TROP2-HCl 14pAF-compound-6 (“TROP2-AS269”) or anti- TROP2-DXd ADC (“Benchmark-DXd”), and tumor size was monitored up to 47 days after treatment. Tumor sizes were measured by calipers and tumor volume was calculated as length x width x width x 0.5. All TROP2-AS269 treated groups reduced JIMT-1 tumor growth with as low as 0.3 mg/kg administration. JIMT-1 tumor growth inhibition by 1 mg/kg of TROP2-AS269 treatment was greater than 3 mg/kg of Benchmark-DXd, suggesting that TROP2-AS269 displays > 3 -fold increased potency than Benchmark-DXd in JIMT-1 tumor model. The results are shown in FIG. 10. Example 15: Anti-tumor Activity of Anti-TROP2 ADCs.
Human lung cancer cell line, HCC827, was cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin/ streptomycin. 5* 106 HCC827 cells were mixed with Matrigel at a 1 : 1 volumetric ratio and subcutaneously implanted into female SCID-beige mice (Charles River Laboratories). When tumor volume reached approximately 100 mm3, mice were randomized and grouped for treatment. Two intravenous Q3W injections were done with vehicle controls, 0.3 mg/kg, 1 mg/kg, or 3 mg/kg of anti-TROP2-HCl 14pAF-compound-6 (“TROP2-AS269”) or anti- TROP2-DXd ADC (“Benchmark-DXd”), and tumor size was monitored up to 50 days after treatment. Tumor sizes were measured by calipers and tumor volume was calculated as length x width x width x 0.5. Percent tumor growth inhibition (TGI) was calculated as (1-tumor volume of treated/tumor volume of vehicle control) x 100. 0.3 mg/kg of TROP2-AS269 delayed HCC827 tumor growth. 1 mg/kg or 3 mg/kg of TROP2-AS269 treated groups reached > 80% of TGI as early as 2 weeks after the first treatment. 1 mg/kg of TROP2-AS269 treatment showed better TGI than 3 mg/kg of Benchmark-DXd, suggesting that TROP2-AS269 displays > 3-fold increased potency than Benchmark-DXd in HCC827 tumor model. The results are shown in FIG. 11.
Example 16: Pharmacokinetic Study of TROP2-AS269
TROP2 mAb or TROP2-AS269 were administered intravenously to CD-I mice (Charles River Laboratories) at a dose of 3 mg/kg. Whole-blood samples were collected at each time point (0, 1, 2, 6, 24, 48, 72, 96, 168, 336, 504, and 672 hours) and diluted in Casein-PBS blocker. For the total antibody assay, TROP2 mAb or TROP2-AS269 was captured using goat anti-human Fc pAb and detected with a biotinylated anti-human kappa pAb and Streptavidin SULFO-TAG (Meso Scale discovery). For the intact ADC assay utilized a proprietary anti-AS269 antibody (AMB-20) as a capture antibody, a biotinylated anti-AS269 antibody, and streptavidin SULFO- TAG for detection. The electrochemiluminescent signal was detected by the MESO QuickPlex SQ 120MM. Pharmacokinetic parameters were calculated using a noncompartmental model in WinNonlin (Certara). The results are shown in FIG. 12.
The total antibody concentration and the intact ADC concentration from TROP2-AS269 were comparable throughout the time course, similar to the total antibody concentration for unconjugated TROP2 mAb. This indicates that TROP2-AS269 is highly stable in circulation. The exposure (AUC ) showed values of 4,439 ug*h/mL for TROP2 mAb, 4,867 ug*h/mL for TROP2-AS269 total antibody, and 4,554 ug*h/mL for TROP2-AS269 intact ADC. Furthermore, based on the total antibody assay, the half-lives of TROP2 mAb and TROP2-AS269 were 455 and 479 hours, respectively.
Additional non-limiting embodiments of the invention include the following:
Al. An antibody-drug conjugate (ADC) comprising: an anti-trophoblast antigen 2 (anti-TR0P2) antibody comprising an amino acid sequence, wherein the amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
A2. The ADC of embodiment Al, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 1, 2, 5 or 6.
A3. The ADC of embodiment A2, wherein at least one of the one or more heavy chains has the amino acid sequence of SEQ ID NO: 5.
A4. The ADC of embodiment Al, A2 or A3, wherein the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence; wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17; or wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10 or 11.
A5. The ADC of embodiment A4, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4.
A6. The ADC of any one of embodiments Al to A5, wherein each said cytotoxic moiety is a cytotoxic moiety of Formula (A) or (B): wherein:
R5 of Formula (A) is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH; R7 is H or Ci-Ce alkyl; and
Ar is phenyl or pyridinyl; wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; or a pharmaceutically acceptable salt thereof.
A7. The ADC of embodiment A6, wherein each said cytotoxic moiety is a cytotoxic moiety of Formula (A).
A8. The ADC of embodiment A7, wherein the cytotoxic moiety of Formula (A) is selected from the group consisting of: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
A9. The ADC of embodiment A8, wherein the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is H or methyl.
A10. The ADC of any one of embodiments A6 to A9, wherein R7 is methyl.
Al l. The ADC of embodiment A6, wherein each said cytotoxic moiety is a cytotoxic moiety of Formula (B).
A12. The ADC of embodiment Al l, wherein the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers.
A13. The ADC of any one of embodiments Al to A12, wherein the ADC is an ADC of Formula
Ab is the anti-TROP2 antibody; each L is one of the one or more linkers; each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker L to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
A14. The ADC of any one of embodiments Al to A12, wherein the ADC is an ADC of Formula
Ab is the anti-TROP2 antibody; each L1-CH(L2-)(L3-) is one of the one or more linkers, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage; each Drug is a cytotoxic moiety of Formula (A) or (B); and d is an integer from 1 to 100; wherein each E covalently joins one linker unit LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
A15. The ADC of embodiment A13 or A14, wherein d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
A16. The ADC of embodiment A13 or A14, wherein d is 1, 2, 3 or 4.
Al 7. The ADC of any one of embodiments Al 3 to Al 6, wherein E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2,3-triazole or a 1,4- dihydropyridazine. A18. The ADC of embodiment A17, wherein E is selected from the group consisting of: wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or L 1 ; and each wavy line denotes connection to Ab. Al 9. The ADC of any one of embodiments Al 5 to Al 8, wherein each L, LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
A20. The ADC of embodiment A19, wherein each L, LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O)n-, -(alkylene-N(Rw))n- unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
A21. The ADC of any one of embodiments A13 to A20, wherein: each L or LI comprises unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10; or each L or LI consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
A22. The ADC of any one of embodiments A13 to A21, wherein the ADC is an ADC of Formula (I).
A23. The ADC of any one of embodiments Al to A22, wherein each of the one or more nonnatural amino acids is para-acetyl-L-phenylalanine (pAF).
A24. The ADC of any one of embodiments A13 to A22, wherein E comprises an oxime.
A25. The ADC of embodiment A23 or A24, wherein E has the following structure: wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (■~") denotes the connection to Ab.
A26. The ADC of embodiment A25, wherein each non-natural amino acid is para-acetyl-L- phenylalanine (pAF), and Rq is methyl, wherein said methyl is the pAF methyl group.
A27. The ADC of any one of embodiments Al to A26, wherein the anti-TROP2 antibody is humanized. A28. The ADC of any one of embodiments A4 to A27, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
A29. The ADC of embodiment A28, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
A30. The ADC of any one of embodiments A4 to A27 , wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 3; and the ADC has a drug-to-antibody ratio of about 2.
A31. The ADC of embodiment A30, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF).
A32. The ADC of any one of embodiments A4 to A27 , wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 7, wherein SEQ ID NO: 7 is characterized as containing one non-natural amino acid at position 121; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
A33. The ADC of embodiment A32, wherein each said non-natural amino acid is para-acetyl-L- phenylalanine (pAF).
A34. The ADC of any one of embodiments A4 to A27 , wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110; SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and
SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
A35. The ADC of embodiment A34, wherein each said non-natural amino acid is para-acetyl-L- phenylalanine (pAF).
A36. The ADC of any one of embodiments Al to A35, wherein the cytotoxic moiety has the following structure: wherein # represents the connection of the cytotoxic moiety to one of the one or more linkers; and R7 is methyl.
Ab is the anti-TROP2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
A38. The ADC of embodiment A38, wherein d is 2, 3 or 4.
A39. The ADC of embodiment A38, wherein d is 2.
A40. The ADC of any one of embodiments Al to A39, provided that each of the one or more linkers is not a bond.
Bl. An antibody-drug conjugate (ADC) of Formula (la), (lb), (Ila) or (lib):
Ab is an anti-trophoblast antigen 2 (anti-TR0P2) antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and (lb) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) or Formula (lib) is a linker, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage;
R5 of Formula (la) or (lb) is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10; wherein each E covalently joins one linker to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; or a pharmaceutically acceptable salt thereof.
B2. The ADC of embodiment Bl, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
B3. The ADC of embodiment B 1 or B2, wherein the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
B4. The ADC of embodiment B2 or B3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
B5. The ADC of embodiment B2, B3 or B4, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6.
B6. The ADC of any one of embodiments B2 to B5, wherein the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
B7. The ADC of embodiment B2 or B3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 5.
B8. The ADC of embodiment B7, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5.
B9. The ADC of embodiment B7, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5.
B10. The ADC of embodiment B7, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5. Bl 1. The ADC of embodiment B7, wherein the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5.
B12. The ADC of embodiment B2 or B3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 6.
B13. The ADC of embodiment Bl 2, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6.
B14. The ADC of embodiment B12, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6.
Bl 5. The ADC of embodiment Bl 2, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 6.
Bl 6. The ADC of any one of embodiments B3 to Bl 5, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3 or 7.
Bl 7. The ADC of embodiment Bl 6, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7.
Bl 8. The ADC of embodiment Bl 6, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7.
Bl 9. The ADC of embodiment Bl 6, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7.
B20. The ADC of any one of embodiments B3 to Bl 5, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
B21. The ADC of embodiment B20, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
B22. The ADC of embodiment B20, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
B23. The ADC of embodiment B20, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11, or is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17.
B24. The ADC of embodiment B20, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4. B25. The ADC of embodiment B20, wherein the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
B26. The ADC of any one of embodiments B2 to B25, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
B27. The ADC of embodiment B26, wherein each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
B28. The ADC of any one of embodiments B2 to B27, wherein the one or more heavy chains is two heavy chains.
B29. The ADC of any one of embodiments B3 to B27, wherein the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
B30. The ADC of any one of embodiments Bl to B29, wherein d is 1, 2, 3, 4, 5, 6, 7 or 8, or is 1, 2, 3 or 4.
B31. The ADC of any one of embodiments Bl to B30, wherein the ADC is an ADC of Formula
(la).
B32. The ADC of any one of embodiments Bl to B30, wherein the ADC is an ADC of Formula
(lb).
B33. The ADC of any one of embodiments Bl to B30, wherein the ADC is an ADC of Formula (Ila).
B34. The ADC of any one of embodiments Bl to B30, wherein the ADC is an ADC of Formula (lib).
B35. The ADC of any one of embodiments Bl to B34, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein at least one of the one or more non-natural amino acids is incorporated into at least one of the one or more heavy chains at position Al 14 based on Kabat numbering.
B36. The ADC of embodiment B35, wherein the one or more heavy chains is two heavy chains, and wherein one non-natural amino acid is incorporated into each heavy chain at position Al 14 based on Kabat numbering.
B37. The ADC of any one of embodiments Bl to B36, wherein R5 is COOH.
B38. The ADC of any one of embodiments Bl to B37, wherein each E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4- dihydropyridazine.
B39. The ADC of embodiment B38, wherein the 1,2, 3 -triazole or the 1,4-dihydropyridazine is fused to an 8-membered ring.
B40. The ADC of embodiment B38 or B39, wherein E is selected from the group consisting of: wherein: each RJ is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L or LI; and each wavy line (-^ ) denotes connection to Ab.
B41. The ADC of any one of embodiments Bl to B40, wherein each L, LI, L2, and L3 independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
B42. The ADC of embodiment B41, wherein the peptide is a dipeptide.
B43. The ADC of embodiment B41 or B42, wherein each L, LI, L2 and L3 independently comprises one or more moieties, wherein each one or more moieties is independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid or a dipeptide; wherein: each L is not a bond, at least one of LI, L2 and L3 is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
B44. The ADC of embodiment B43, wherein each L or each LI comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both.
B45. The ADC of embodiment B44, wherein each L or each LI consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
B46. The ADC of embodiment B45, wherein each L or each LI is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10.
B47. The ADC of embodiment B46, wherein m is 1, 2 or 3 and n is 1, 2 or 3.
B48. The ADC of embodiment B47, wherein m is 1 and n is 3.
B49. The ADC of any one of embodiments Bl to B48, wherein each of the one or more nonnatural amino acids is selected from the group consisting of para-acetyl phenylalanine, 4-acetyl- L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N-acetyl-beta-D-glucosaminyl)-L- threonine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine, O-allyl-L-tyrosine, alpha-N- acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L-threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino-L-phenylalanine, 2-amino-L- tyrosine, 3-amino-L-tyrosine, 4-azido-L-phenylalanine, 4-benzoyl-L-phenylalanine, (2,2- bipyridin-5yl)-L-alanine, 3-borono-L-phenylalanine, 4-borono-L-phenylalanine, 4-bromo-L- phenylalanine, p-carboxymethyl-L-phenylalanine, 4-carboxy-L-phenylalanine, p-cyano-L- phenylalanine, 3,4-dihydroxy-L-phenylalanine (L-DOPA), 4-ethynyl-L-phenylalanine, 2-fluoro- L-phenylalanine, 3-fluoro-L-phenylalanine, 4-fluoro-L-phenylalanine, O-(3-O-D-galactosyl-N- acetyl-beta-D-galactosaminyl)-L-serine, L-homoglutamine, (8-hydroxyquinolin-3-yl)-L-alanine, 4-iodo-L-phenylalanine, 4-isopropyl-L-phenylalanine, O-i-propyl-L-tyrosine, 3-isopropyl-L- tyrosine, O-mannopyranosyl-L-serine, 2-methoxy-L-phenylalanine, 3-methoxy-L-phenylalanine, 4-methoxy-L-phenylalanine, 3-methyl-L-phenylalanine, O-methyl-L-tyrosine, 3-(2-naphthyl)-L- alanine, 5-nitro-L-histidine, 4-nitro-L-histidine, 4-nitro-L-leucine, 2-nitro-L-phenylalanine, 3- nitro-L-phenylalanine, 4-nitro-L-phenylalanine, 4-nitro-L-tryptophan, 5-nitro-L-tryptophan, 6- nitro-L-tryptophan, 7-nitro-L-tryptophan, 2-nitro-L-tyrosine, 3-nitro-L-tyrosine, O-phospho-L- serine, O-phospho-L-tyrosine, 4-propargyloxy-L-phenylalanine, O-2-propyn-l-yl-L-tyrosine, 4- sulfo-L-phenylalanine and O-sulfo-L-tyrosine.
B50. The ADC of any one of embodiments Bl to B49, wherein at least one of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
B51. The ADC of any one of embodiments Bl to B50, wherein each of the one or more nonnatural amino acids is para-acetyl-L-phenylalanine (pAF).
B52. The ADC of any one of embodiments Bl to B51, wherein each E comprises an oxime.
B53. The ADC of any one of embodiments Bl to B52, wherein each E has the following structure: wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line (•~ ') denotes the connection to Ab.
B54. The ADC of embodiment B53, wherein each non-natural amino acid is para-acetyl-L- phenylalanine (pAF), and Rq is methyl, wherein said methyl is the pAF methyl group.
B55. The ADC of embodiment B3, wherein: the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
B56. The ADC of embodiment B55, wherein the one or more heavy chains is two heavy chains, the one or more light chains is two light chains, the one or more non-natural amino acids is two non-natural amino acids, and d is 2.
B57. The ADC of embodiment B55 or B56, wherein each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L or LI; and the wavy line ("") denotes the connection to Ab. B59. The ADC of any one of embodiments B55 to B58, wherein the ADC is an ADC of Formula (la).
B60. The ADC of embodiment B59, wherein Rq is methyl, R5 is COOH, R6 is H, R7 is methyl, Ar is phenyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody, wherein the anti-TROP2 antibody comprises one or more non-natural amino acids; each L is a linker; each E is a linkage, wherein each linkage E covalently joins each linker L with one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10; or a pharmaceutically acceptable salt thereof.
C2. The ADC of embodiment Cl, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
C3. The ADC of embodiment Cl or C2, wherein the anti-TROP2 antibody comprises one or more light chains, wherein each of the one or more light chains has an amino acid sequence, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
C4. The ADC of embodiment C2 or C3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
C5. The ADC of embodiment C2, C3 or C4, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6.
C6. The ADC of any one of embodiments C2 to C5, wherein the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6. C7. The ADC of embodiment C2 or C3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 5.
C8. The ADC of embodiment C7, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5.
C9. The ADC of embodiment C7, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5.
CIO. The ADC of embodiment C7, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5.
Cl 1. The ADC of embodiment C7, wherein the amino acid sequence of each of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 5.
C12. The ADC of embodiment C2 or C3, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 6.
C13. The ADC of embodiment Cl 2, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6.
C14. The ADC of embodiment C12, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6.
Cl 5. The ADC of embodiment Cl 2, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 6.
Cl 6. The ADC of any one of embodiments C3 to Cl 5, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3 or 7.
Cl 7. The ADC of embodiment Cl 6, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7.
Cl 8. The ADC of embodiment Cl 6, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7.
Cl 9. The ADC of embodiment Cl 6, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7.
C20. The ADC of any one of embodiments C3 to Cl 5, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
C21. The ADC of embodiment C20, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17. C22. The ADC of embodiment C20, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
C23. The ADC of embodiment C20, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4, 8, 9, 10 or 11, or is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17.
C24. The ADC of embodiment C20, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
C25. The ADC of embodiment C20, wherein the amino acid sequence of each of the one of the one or more light chains is the amino acid sequence of SEQ ID NO: 4.
C26. The ADC of any one of embodiments C2 to C25, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
C27. The ADC of embodiment C26, wherein each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids, or wherein each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
C28. The ADC of any one of embodiments C2 to C27, wherein the one or more heavy chains is two heavy chains.
C29. The ADC of any one of embodiments C3 to C27, wherein the one or more heavy chains is two heavy chains, and the one or more light chains is two light chains.
C30. The ADC of any one of embodiments Cl to C29, wherein d is 1, 2, 3, 4, 5, 6, 7 or 8, or is 1, 2, 3 or 4.
C31. The ADC of any one of embodiments Cl to C30, wherein each E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4- dihydropyridazine.
C32. The ADC of embodiment C31, wherein the 1,2, 3 -triazole or the 1,4-dihydropyridazine is fused to an 8-membered ring.
wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L; and each wavy line ("«) denotes connection to Ab.
C34. The ADC of any one of embodiments Cl to C33, wherein each L comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid or a peptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
C35. The ADC of embodiment C34, wherein the peptide is a dipeptide. C36. The ADC of embodiment C34 or C35, wherein each L comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid or a dipeptide; wherein at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl; or any combination of one or more of each of the foregoing.
C37. The ADC of embodiment C36, wherein L comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both.
C38. The ADC of embodiment C37, wherein L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
C39. The ADC of embodiment C38, wherein L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10.
C40. The ADC of embodiment C39, wherein m is 1, 2 or 3 and n is 1, 2 or 3.
C41. The ADC of embodiment C40, wherein m is 1 and n is 3.
C42. The ADC of any one of embodiments Cl to C41, wherein each of the one or more nonnatural amino acids is selected from the group consisting of para-acetyl phenylalanine, 4-acetyl- L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N-acetyl-beta-D-glucosaminyl)-L- threonine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine, O-allyl-L-tyrosine, alpha-N- acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L-threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino-L-phenylalanine, 2-amino-L- tyrosine, 3-amino-L-tyrosine, 4-azido-L-phenylalanine, 4-benzoyl-L-phenylalanine, (2,2- bipyridin-5yl)-L-alanine, 3-borono-L-phenylalanine, 4-borono-L-phenylalanine, 4-bromo-L- phenylalanine, p-carboxymethyl-L-phenylalanine, 4-carboxy-L-phenylalanine, p-cyano-L- phenylalanine, 3,4-dihydroxy-L-phenylalanine (L-DOPA), 4-ethynyl-L-phenylalanine, 2-fluoro- L-phenylalanine, 3-fluoro-L-phenylalanine, 4-fluoro-L-phenylalanine, O-(3-O-D-galactosyl-N- acetyl-beta-D-galactosaminyl)-L-serine, L-homoglutamine, (8-hydroxyquinolin-3-yl)-L-alanine, 4-iodo-L-phenylalanine, 4-isopropyl-L-phenylalanine, O-i-propyl-L-tyrosine, 3-isopropyl-L- tyrosine, O-mannopyranosyl-L-serine, 2-methoxy-L-phenylalanine, 3-methoxy-L-phenylalanine, 4-methoxy-L-phenylalanine, 3-methyl-L-phenylalanine, O-methyl-L-tyrosine, 3-(2-naphthyl)-L- alanine, 5-nitro-L-histidine, 4-nitro-L-histidine, 4-nitro-L-leucine, 2-nitro-L-phenylalanine, 3- nitro-L-phenylalanine, 4-nitro-L-phenylalanine, 4-nitro-L-tryptophan, 5-nitro-L-tryptophan, 6- nitro-L-tryptophan, 7-nitro-L-tryptophan, 2-nitro-L-tyrosine, 3-nitro-L-tyrosine, O-phospho-L- serine, O-phospho-L-tyrosine, 4-propargyloxy-L-phenylalanine, O-2-propyn-l-yl-L-tyrosine, 4- sulfo-L-phenylalanine and O-sulfo-L-tyrosine. C43. The ADC of any one of embodiments Cl to C42, wherein at least one of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
C44. The ADC of any one of embodiments Cl to C43, wherein each of the one or more nonnatural amino acids is para-acetyl-L-phenylalanine (pAF).
C45. The ADC of any one of embodiments Cl to C44, wherein each E comprises an oxime.
C46. The ADC of any one of embodiments Cl to C45, wherein each E has the following structure: wherein Rq is unsubstituted Ci-Ce alkyl; + denotes the connection to L; and the wavy line ( — ) denotes the connection to Ab.
C47. The ADC of embodiment C46, wherein each non-natural amino acid is para-acetyl-L- phenylalanine (pAF), and Rq is methyl, wherein said methyl is the pAF methyl group.
C48. The ADC of embodiment C46 or C47, wherein Rq is methyl and L is -(CEECH^m- CEECEE- O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
Ab is the anti-TROP2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
C50. The ADC of embodiment C49, wherein d is 2, 3 or 4.
C51. The ADC of embodiment C49, wherein d is 2.
C52. The ADC of embodiment C3, C49 or C50, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 4; and d is 2.
C53. The ADC of embodiment C52, wherein the one non-natural amino acid at position 114 of each said heavy chain is para-acetyl-L-phenylalanine (pAF). C54. The ADC of any one of embodiments C3, C49, C50 or C51, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5 or 6, wherein each of SEQ ID NO: 5 and 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, wherein: the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 3; and d is 2; or the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 7, wherein SEQ ID NO: 7 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4.
C55. The ADC of embodiment C3, C49 or C51, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110;
SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and
SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4.
C56. The ADC of embodiment C55, wherein the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 11.
C57. The ADC of embodiment C3, C49 or C51, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110;
SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121; and d is 1, 2, 3 or 4.
C58. The ADC of embodiment C3, C49 or C51, wherein: the one or more heavy chains is two heavy chains, and the amino acid sequence of each said heavy chain is the amino acid sequence of SEQ ID NO: 5 or 6, wherein each of SEQ ID NO: 5 and 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains is two light chains, and the amino acid sequence of each said light chain is the amino acid sequence of SEQ ID NO: 12, 13, 14, 15, 16 or 17, wherein:
SEQ ID NO: 12 is characterized as containing one non-natural amino acid at position 127;
SEQ ID NO: 13 is characterized as containing one non-natural amino acid at position 149;
SEQ ID NO: 14 is characterized as containing one non-natural amino acid at position 156;
SEQ ID NO: 15 is characterized as containing one non-natural amino acid at position 168;
SEQ ID NO: 16 is characterized as containing one non-natural amino acid at position 202;
SEQ ID NO: 17 is characterized as containing one non-natural amino acid at position 205; and d is 1, 2, 3 or 4.
C59. The ADC of any one of embodiments C54 to C58, wherein each said non-natural amino acid is para-acetyl-L-phenylalanine (pAF).
C60. The ADC of any one of embodiments Al to A40, Bl to B60 or Cl to C59, provided that the ADC does not contain a Toll-like receptor (TLR) agonist.
DI. The ADC of any one of embodiments Al to A40 or Bl to B60, provided that the cytotoxic moiety does not have the following structure: wherein R7 is methyl, and # is H or denotes connection to the linker.
D2. The ADC of any one of embodiments Al to A40, Bl to B60 or DI, wherein the cytotoxic moiety has the following structure: wherein R7 is methyl and # is H or denotes connection to the linker.
D3. The ADC of embodiment DI or D2, wherein the cytotoxic moiety is less cytotoxic than monomethyl auristatin E (MMAE) in vitro. D4. The ADC of embodiment DI, D2 or D3, wherein the cytotoxic moiety exhibits a higher in vitro half-maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line compared to MMAE.
D5. The ADC of embodiment D4, wherein the higher in vitro IC50 is at least a two-fold higher in vitro IC50.
D6. The ADC of embodiment D4, wherein the higher in vitro IC50 is at least a 10-fold higher in vitro IC50.
D7. The ADC of embodiment D4, wherein the higher in vitro IC50 is at least a 100-fold higher in vitro IC50.
D8. The ADC of embodiment D4, wherein the higher in vitro IC50 is at least a 1000-fold higher in vitro IC50.
D9. The ADC of any one of embodiments D4 to D8, wherein the microtubule inhibitor-sensitive cell line is an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay.
DIO. The ADC of embodiment D9, wherein the in vitro cytotoxicity assay is an impedance assay, a flow cytometry assay or a luminescent cell viability assay (such as a CellTiter Gio assay).
Dl l. The ADC of embodiment Dl, D2 or D3„ wherein the cytotoxic moiety exhibits reduced in vitro human keratinocyte cytotoxicity when compared to monomethyl auristatin E (MMAE) in vitro.
D12. The ADC of any one of embodiments Dl to Dl l, provided that the ADC does not contain a Toll-like receptor (TLR) agonist.
El. A pharmaceutical composition comprising an ADC of any one of embodiments Al to A40, Bl to B60, Cl to C60 orDl to D12, and at least one pharmaceutically acceptable adjuvant, binder, buffer, carrier, diluent or excipient.
E2. The pharmaceutical composition of embodiment El, further comprising a chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid, or combination thereof.
Fl. A method of treating a disease or condition in a subject, the method comprising administering to the subject a therapeutically effective amount of an ADC of any one of embodiments Al to A40, Bl to B60, Cl to C60 or Dl to D12, or a pharmaceutical composition of any one of embodiment El or E2.
F2. The method of embodiment Fl, wherein the disease or condition is cancer.
F3. The method of embodiment F2, wherein the cancer is a solid tumor. F4. The method of embodiment F2 or F3, wherein the cancer is breast cancer, pancreatic cancer, lung cancer, gastric cancer, colorectal cancer or prostate cancer.
F5. The method of embodiment F2, wherein the cancer is triple-negative breast cancer (TNBC). F6. The method of embodiment F2, wherein the cancer is pancreatic ductal adenocarcinoma (PDAC).
F7. The method of embodiment F2, wherein the cancer is non-small cell lung cancer (NSCLC). F8. The method of any one of embodiments F2 to F7, wherein the cancer is a TROP2-positive cancer.
F9. The method of any one of embodiments F2 to F8, wherein the cancer is a Dxd-resistant cancer. F10. The method of any one of embodiments F2 to F8, wherein the cancer is a topoisomerase 1 (TOPI) inhibitor-resistant cancer.
While certain embodiments of the inventions have been described, these embodiments have been presented by way of example only and are not intended to limit the scope of the disclosure. Indeed, the novel compositions, methods and systems described herein may be embodied in a variety of other forms. Furthermore, various omissions, substitutions and changes in the compositions, systems and methods described herein may be made without departing from the spirit of the disclosure. The accompanying claims and their equivalents are intended to cover such forms or modifications as would fall within the scope and spirit of the disclosure. Accordingly, the scope of the present inventions is defined only by reference to the appended claims.
Features, materials, characteristics, or groups described in conjunction with a particular aspect, embodiment, or example are to be understood to be applicable to any other aspect, embodiment or example described in this section or elsewhere in this specification unless incompatible therewith. All of the features disclosed in this specification (including any accompanying claims, abstract and drawings), and/or all of the compositions, steps of any method or process so disclosed, may be combined in any combination, except combinations where at least some of such features and/or steps are mutually exclusive. The protection is not restricted to the details of any foregoing embodiments. The protection extends to any novel one, or any novel combination, of the features disclosed in this specification (including any accompanying claims, abstract and drawings), or to any novel one, or any novel combination, of the compositions, steps of any method or process so disclosed.
Furthermore, certain features that are described in this disclosure in the context of separate implementations can also be implemented in combination in a single implementation. Conversely, various features that are described in the context of a single implementation can also be implemented in multiple implementations separately or in any suitable subcombination. Although features may be described above as acting in certain combinations, one or more features from a claimed combination can, in some cases, be excised from the combination, and the combination may be claimed as a subcombination or variation of a subcombination.
The features and attributes of the specific embodiments disclosed above may be combined in different ways to form additional embodiments, all of which fall within the scope of the present disclosure. Also, the separation of various system components in the implementations described above should not be understood as requiring such separation in all implementations, and it should be understood that the described components and systems can generally be integrated together in a single product or packaged into multiple products.
For purposes of this disclosure, certain aspects, advantages, and novel features are described herein. Not necessarily all such advantages may be achieved in accordance with any particular embodiment. Thus, for example, those skilled in the art will recognize that the disclosure may be embodied or carried out in a manner that achieves one advantage or a group of advantages as taught herein without necessarily achieving other advantages as may be taught or suggested herein.
As used herein and in the appended claims, the singular forms “a,” “an,” and “the” include plural reference unless the context clearly indicates otherwise.
Conditional language, such as "can," "could," "might," or "may," unless specifically stated otherwise, or otherwise understood within the context as used, is generally intended to convey that certain embodiments include, while other embodiments do not include, certain features, elements, and/or steps. Thus, such conditional language is not generally intended to imply that features, elements, and/or steps are in any way required for one or more embodiments or that one or more embodiments necessarily include logic for deciding, with or without user input or prompting, whether these features, elements, and/or steps are included or are to be performed in any particular embodiment.
Conjunctive language such as the phrase "at least one of X, Y, and Z," unless specifically stated otherwise, is otherwise understood with the context as used in general to convey that an item, term, etc. may be either X, Y, or Z. Thus, such conjunctive language is not generally intended to imply that certain embodiments require the presence of at least one of X, at least one of Y, and at least one of Z.
Language of degree used herein, such as the terms "approximately," "about," "generally," “substantial” and "substantially" as used herein represent a value, amount, quantity or characteristic close to the stated value, amount, quantity or characteristic that still performs a desired function or achieves a desired result. For example, the terms "approximately", "about", "generally," “substantial” and "substantially" may refer to an amount that is within less than 10% of, within less than 5% of, within less than 1% of: within less than 0.1% of, and within less than
0.01% of the stated amount.
As used herein and in the appended claims, the term “comprising” is open ended, and the broadest reasonable interpretation of the term applies. The present disclosure contemplates alternative embodiments wherein the term “consisting of’ can be used in place of each recitation of the term “comprising” herein.
The scope of the present disclosure is not intended to be limited by the specific disclosures of preferred embodiments in this section or elsewhere in this specification, and may be defined by claims as presented in this section or elsewhere in this specification or as presented in the future. The language of the claims is to be interpreted broadly based on the language employed in the claims and not limited to the examples described in the present specification or during the prosecution of the application, which examples are to be construed as non-exclusive.
Claims
1. An antibody-drug conjugate (ADC) comprising: an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising an anti-TROP2 antibody amino acid sequence, wherein the anti-TROP2 antibody amino acid sequence comprises one or more non-natural amino acids and shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6; one or more cytotoxic moieties; and one or more linkers; wherein each of the one or more linkers joins at least one of the one or more cytotoxic moieties to the anti-TROP2 antibody; or a pharmaceutically acceptable salt thereof.
2. The ADC of claim 1, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein the amino acid sequence of at least one of the one or more heavy chains comprises SEQ ID NO: 1, 2, 5 or 6.
3. The ADC of claim 2, wherein the amino acid sequence of at least one of the one or more heavy chains is SEQ ID NO: 5.
4. The ADC of any one of claims 1 to 3, wherein the anti-TROP2 antibody comprises one or more light chains; wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17; wherein the amino acid sequence of at least one of the one or more light chains comprises SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17; or wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3, 4, 7, 8, 9, 10 or 11.
5. The ADC of claim 4, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4.
6. The ADC of any one of claims 1 to 5, wherein each of the one or more cytotoxic moieties is according to Formula (A) or Formula (B): or a pharmaceutically acceptable salt thereof; wherein:
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is independently H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and
# represents a connection to one of the one or more linkers.
7. The ADC of claim 6, wherein each of the cytotoxic moieties is according to Formula (A).
10. The ADC of any one of claims 6 to 9, wherein R7 is methyl.
11. The ADC of claim 6, wherein each of the cytotoxic moieties is according to Formula (B).
J d (I), or a pharmaceutically acceptable salt thereof; wherein:
Ab is the anti-TROP2 antibody; each L is independently one linker of the one or more linkers; each E is independently a linkage covalently joining the one linker L to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab; each Drug is a cytotoxic moiety of Formula (A) or Formula (B); and d is an integer from 1 to 100.
14. The ADC of any one of claims 1 to 12, wherein the ADC is an ADC of Formula (II): or a pharmaceutically acceptable salt thereof; wherein:
Ab is the anti-TROP2 antibody; each L1-CH(L2-)(L3-) is independently one linker of the one or more linkers, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage covalently joining the first linker LI to one of the one or more nonnatural amino acids of the anti-TROP2 antibody Ab; each Drug is a cytotoxic moiety of Formula (A) or Formula (B); and d is an integer from 1 to 100.
15. The ADC of claim 13 or 14, wherein d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
16. The ADC of claim 13 or 14, wherein d is 1, 2, 3 or 4.
17. The ADC of any one of claims 13 to 16, wherein E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4-dihydropyridazine.
18. The ADC of claim 17, wherein E is selected from the group consisting of: wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes a connection to L or LI; and each wavy line (-^) denotes a connection to Ab.
19. The ADC of any one of claims 15 to 18, wherein: each L independently comprises one or more moieties independently selected from the group consisting of methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene- O)n- , -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, - S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide, and a combination thereof; each LI, L2 and L3 independently comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid and a peptide, and a combination thereof, wherein at least one of LI, L2 and L3 is not a bond; each n is independently an integer from 1 to 100; and each Rw is independently H or Ci-Cs alkyl.
20. The ADC of claim 19, wherein: each L independently comprises one or more moieties independently selected from the group consisting of methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene- N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide, and a combination thereof; each LI, L2 and L3 independently comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide, and a combination thereof, wherein at least one of LI, L2 and L3 is not a bond; each n is independently an integer from 1 to 10; and each Rw is independently H or Ci-Cs alkyl.
21. The ADC of any one of claims 13 to 20, wherein: each L or LI independently comprises unsubstituted alkylene, -(alkyl ene-O)n-, or both, wherein each n is an integer from 1 to 10; or each L or LI independently consists of unsubstituted alkylene, -(alkylene-O)n-, or both, wherein each n is an integer from 1 to 10.
22. The ADC of any one of claims 13 to 21, wherein the ADC is an ADC of Formula (I).
23. The ADC of any one of claims 1 to 22, wherein each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
24. The ADC of any one of claims 13 to 22, wherein E comprises an oxime.
26. The ADC of claim 25, wherein each of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF), and wherein Rq is the methyl group of pAF.
27. The ADC of any one of claims 1 to 26, wherein the anti-TROP2 antibody is humanized.
28. The ADC of any one of claims 4 to 27, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 4; and the ADC has a drug-to-antibody ratio of about 2.
29. The ADC of claim 28, wherein the one non-natural amino acid at position 114 of each of the two heavy chains is para-acetyl-L-phenylalanine (pAF).
30. The ADC of any one of claims 4 to 27, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 3; and the ADC has a drug-to-antibody ratio of about 2.
31. The ADC of claim 30, wherein the one non-natural amino acid at position 114 of each of the two heavy chain is para-acetyl-L-phenylalanine (pAF).
32. The ADC of any one of claims 4 to 27, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 7, wherein SEQ ID NO: 7 is characterized as containing one non-natural amino acid at position 121 based on Kabat numbering; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
33. The ADC of claim 32, wherein each of the one non-natural amino acid at position 114 and the one non-natural amino acid at position 121 is para-acetyl-L-phenylalanine (pAF).
34. The ADC of any one of claims 4 to 27, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chain is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110 based on Kabat numbering;
SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112 based on Kabat numbering;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and
SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121 based on Kabat numbering; and the ADC has a drug-to-antibody ratio of about 1, about 2, about 3 or about 4.
35. The ADC of claim 34, wherein each of the non-natural amino acids is para-acetyl-L- phenylalanine (pAF).
(id); or a pharmaceutically acceptable salt thereof; wherein: Ab is the anti-TR0P2 antibody; and d is 1, 2, 3, 4, 5, 6, 7 or 8.
38. The ADC of claim 37, wherein d is 2, 3 or 4.
39. The ADC of claim 38, wherein d is 2.
40. The ADC of any one of claims 1 to 39, provided that each of the one or more linkers is not a bond.
41. An antibody-drug conjugate (ADC) of Formula (la), Formula (lb), Formula (Ila) or Formula (lib): or a pharmaceutically acceptable salt thereof; wherein:
Ab is an anti-trophoblast antigen 2 (anti-TR0P2) antibody, wherein the anti-TR0P2 antibody comprises one or more non-natural amino acids; each L of Formula (la) and Formula (lb) is a linker; each L1-CH(L2-)(L3-) of Formula (Ila) and Formula (lib) is a linker, wherein:
LI is a first linker unit;
L2 is a second linker unit; and
L3 is a third linker unit; and each E is a linkage covalently joining L or LI to one of the one or more non-natural amino acids of the anti-TROP2 antibody Ab;
R5 is H, COOH, Ci-Ce alkyl or thiazolyl;
R6 is H or OH;
R7 is H or Ci-Ce alkyl;
Ar is phenyl or pyridinyl; and d is an integer from 1 to 10.
42. The ADC of claim 41, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
43. The ADC of claim 41 or 42, wherein the anti-TROP2 antibody comprises one or more light chains, wherein the amino acid sequence of at least one of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
44. The ADC of claim 42 or 43, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
45. The ADC of any one of claims 42 to 44, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6.
46. The ADC of any one of claims 42 to 45, wherein the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
47. The ADC of claim 42 or 43, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 5.
48. The ADC of claim 47, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5.
49. The ADC of claim 47, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5.
50. The ADC of claim 47, wherein the amino acid sequence of at least one of the one or more heavy chains is SEQ ID NO: 5.
51. The ADC of claim 47, wherein the amino acid sequence of each of the one or more heavy chains is SEQ ID NO: 5.
52. The ADC of claim 42 or 43, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 6.
53. The ADC of claim 52, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6.
54. The ADC of claim 52, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6.
55. The ADC of claim 52, wherein the amino acid sequence of at least one of the one or more heavy chains is SEQ ID NO: 6.
56. The ADC of any one of claims 43 to 55, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3 or 7.
57. The ADC of claim 56, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7.
58. The ADC of claim 56, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7.
59. The ADC of claim 56, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 3 or 7.
60. The ADC of any one of claims 43 to 55, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
61. The ADC of claim 60, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
62. The ADC of claim 60, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
63. The ADC of claim 60, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4, 8, 9, 10 or 11, or is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
64. The ADC of claim 60, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4.
65. The ADC of claim 60, wherein the amino acid sequence of each of the one of the one or more light chains is SEQ ID NO: 4.
66. The ADC of any one of claims 42 to 65, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
67. The ADC of claim 66, wherein each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
68. The ADC of any one of claims 42 to 67, wherein the one or more heavy chains are two heavy chains.
69. The ADC of any one of claims 43 to 67, wherein the one or more heavy chains are two heavy chains, and wherein the one or more light chains are two light chains.
70. The ADC of any one of claims 41 to 69, wherein d is 1, 2, 3, 4, 5, 6, 7 or 8, or is 1, 2, 3 or 4.
71. The ADC of any one of claims 41 to 70, wherein the ADC is according to Formula (la).
72. The ADC of any one of claims 41 to 70, wherein the ADC is according to Formula (lb).
73. The ADC of any one of claims 41 to 70, wherein the ADC is according to Formula (Ila).
74. The ADC of any one of claims 41 to 70, wherein the ADC is according to Formula (lib).
75. The ADC of any one of claims 41 to 74, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids, and wherein the at least one of the one or more non-natural amino acids is incorporated at position Al 14 based on Kabat numbering.
76. The ADC of claim 75, wherein the one or more heavy chains are two heavy chains, and wherein the at least one of the one or more non-natural amino acids in each of the two heavy chains is incorporated at position Al 14 based on Kabat numbering.
77. The ADC of any one of claims 41 to 76, wherein R5 is COOH.
78. The ADC of any one of claims 41 to 77, wherein each E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4- dihydropyridazine.
79. The ADC of claim 78, wherein the 1,2,3-triazole or the 1,4-dihydropyridazine is fused to an 8-membered ring.
80. The ADC of claim 78 or 79, wherein E is selected from the group consisting of: wherein: each R> is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes a connection to L or LI; and each wavy line ("«) denotes a connection to Ab.
81. The ADC of any one of claims 41 to 80, wherein: each L independently comprises one or more moieties independently selected from the group consisting of methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene- O)n- , -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, - S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid, and a peptide, and a combination thereof; each LI, L2, and L3 independently comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, -(alkyl ene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid, and a peptide, and a combination thereof, wherein at least one of LI, L2 and L3 is not a bond; each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl.
82. The ADC of claim 81, wherein the peptide is a dipeptide.
83. The ADC of claim 81 or 82, wherein: each L independently comprises one or more moieties independently selected from the group consisting of methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene- N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid, and a dipeptide, and a combination thereof; each LI, L2 and L3 independently comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkylene-O)n-, -(alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid, and a dipeptide, and a combination thereof, wherein at least one of LI, L2 and L3 is not a bond; each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl.
84. The ADC of claim 83, wherein each L or each LI comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both.
85. The ADC of claim 84, wherein each L or each LI consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
86. The ADC of claim 85, wherein each L or each LI is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and n is an integer from 1 to 10.
87. The ADC of claim 86, wherein m is 1, 2 or 3 and n is 1, 2 or 3.
88. The ADC of claim 87, wherein m is 1 and n is 3.
89. The ADC of any one of claims 41 to 88, wherein each of the one or more non-natural amino acids is independently selected from the group consisting of para-acetyl phenylalanine, 4-acetyl-
L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N-acetyl-beta-D-glucosaminyl)-L- threonine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine, O-allyl-L-tyrosine, alpha-N- acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L-threonine, 2-aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino-L-phenylalanine, 2-amino-L- tyrosine, 3-amino-L-tyrosine, 4-azido-L-phenylalanine, 4-benzoyl-L-phenylalanine, (2,2- bipyridin-5yl)-L-alanine, 3-borono-L-phenylalanine, 4-borono-L-phenylalanine, 4-bromo-L- phenylalanine, p-carboxymethyl-L-phenylalanine, 4-carboxy-L-phenylalanine, p-cyano-L- phenylalanine, 3,4-dihydroxy-L-phenylalanine (L-DOPA), 4-ethynyl-L-phenylalanine, 2-fluoro- L-phenylalanine, 3-fluoro-L-phenylalanine, 4-fluoro-L-phenylalanine, O-(3-O-D-galactosyl-N- acetyl-beta-D-galactosaminyl)-L-serine, L-homoglutamine, (8-hydroxyquinolin-3-yl)-L-alanine, 4-iodo-L-phenylalanine, 4-isopropyl-L-phenylalanine, O-i-propyl-L-tyrosine, 3-isopropyl-L- tyrosine, O-mannopyranosyl-L-serine, 2-methoxy-L-phenylalanine, 3-methoxy-L-phenylalanine, 4-methoxy-L-phenylalanine, 3-methyl-L-phenylalanine, O-methyl-L-tyrosine, 3-(2-naphthyl)-L- alanine, 5-nitro-L-histidine, 4-nitro-L-histidine, 4-nitro-L-leucine, 2-nitro-L-phenylalanine, 3- nitro-L-phenylalanine, 4-nitro-L-phenylalanine, 4-nitro-L-tryptophan, 5-nitro-L-tryptophan, 6- nitro-L-tryptophan, 7-nitro-L-tryptophan, 2-nitro-L-tyrosine, 3-nitro-L-tyrosine, O-phospho-L- serine, O-phospho-L-tyrosine, 4-propargyloxy-L-phenylalanine, O-2-propyn-l-yl-L-tyrosine, 4- sulfo-L-phenylalanine and O-sulfo-L-tyrosine.
90. The ADC of any one of claims 41 to 89, wherein at least one of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
91. The ADC of any one of claims 41 to 90, wherein each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
92. The ADC of any one of claims 41 to 91, wherein each E comprises an oxime.
94. The ADC of claim 93, wherein each non-natural amino acid is para-acetyl-L-phenylalanine (pAF), and wherein Rq is the methyl group of pAF.
95. The ADC of claim 43, wherein: the amino acid sequence of each of the one or more heavy chains is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and the amino acid sequence of each of the one or more light chains is SEQ ID NO: 4.
96. The ADC of claim 95, wherein: the one or more heavy chains are two heavy chains, the one or more light chains are two light chains, the one or more non-natural amino acids are two non-natural amino acids, and d is 2.
97. The ADC of claim 95 or 96, wherein each of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF).
98. The ADC of any one of claims 95 to 97, wherein E has the following structure: /°“+ z~ bl
Rq ; wherein Rq is unsubstituted Ci-Ce alkyl; + denotes a connection to L or LI; and the wavy line denotes a connection to Ab.
99. The ADC of any one of claims 95 to 98, wherein the ADC is according to Formula (la).
100. The ADC of claim 99, wherein Rq is methyl, R5 is COOH, R6 is H, R7 is methyl, Ar is phenyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and wherein n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
(Ic); or a pharmaceutically acceptable salt thereof, wherein:
Ab is an anti-trophoblast antigen 2 (anti-TROP2) antibody comprising one or more non- natural amino acids; each L is a linker; each E is a linkage covalently joining each linker L with one of the one or more non- natural amino acids of the anti-TROP2 antibody Ab; and d is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
102. The ADC of claim 101, wherein the anti-TROP2 antibody comprises one or more heavy chains, wherein the amino acid sequence of each of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 1, 2, 5 or 6.
103. The ADC of claim 101 or 102, wherein the anti-TROP2 antibody comprises one or more light chains, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
104. The ADC of claim 102 or 103, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% sequence identity with SEQ ID NO: 1, 2, 5 or 6.
105. The ADC of claim 102, 103 or 104, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 1, 2, 5 or 6.
106. The ADC of any one of claims 102 to 105, wherein the amino acid sequence of at least one of the one or more heavy chains is selected from the group consisting of SEQ ID NO: 1, 2, 5 and 6.
107. The ADC of claim 102 or 103, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 5.
108. The ADC of claim 107, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 5.
109. The ADC of claim 107, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 5.
110. The ADC of claim 107, wherein the amino acid sequence of at least one of the one or more heavy chains is SEQ ID NO: 5.
111. The ADC of claim 107, wherein the amino acid sequence of each of the one or more heavy chains is SEQ ID NO: 5.
112. The ADC of claim 102 or 103, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 90% identity with SEQ ID NO: 6.
113. The ADC of claim 112, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 95% identity with SEQ ID NO: 6.
114. The ADC of claim 112, wherein the amino acid sequence of at least one of the one or more heavy chains shares at least 96%, at least 97%, at least 98% or at least 99% identity with SEQ ID NO: 6.
115. The ADC of claim 112, wherein the amino acid sequence of at least one of the one or more heavy chains is the amino acid sequence of SEQ ID NO: 6.
116. The ADC of any one of claims 103 to 115, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 3 or 7.
117. The ADC of claim 116, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 3 or 7.
118. The ADC of claim 116, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 3 or 7.
119. The ADC of claim 116, wherein the amino acid sequence of at least one of the one or more light chains is the amino acid sequence of SEQ ID NO: 3 or 7.
120. The ADC of any one of claims 103 to 115, wherein the amino acid sequence of each of the one or more light chains shares at least 90% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
121. The ADC of claim 120, wherein the amino acid sequence of at least one of the one or more light chains shares at least 95% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
122. The ADC of claim 120, wherein the amino acid sequence of at least one of the one or more light chains shares at least 96% identity, at least 97% identity, at least 98% identity or at least 99% identity with SEQ ID NO: 4, 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17.
123. The ADC of claim 120, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4, 8, 9, 10 or 11, or is SEQ ID NO: 12, 13, 14, 15, 16 or 17.
124. The ADC of claim 120, wherein the amino acid sequence of at least one of the one or more light chains is SEQ ID NO: 4.
125. The ADC of claim 120, wherein the amino acid sequence of each of the one or more light chains is SEQ ID NO: 4.
126. The ADC of any one of claims 102 to 125, wherein at least one of the one or more heavy chains comprises at least one of the one or more non-natural amino acids.
127. The ADC of claim 126, wherein each of the one or more heavy chains comprises at least one of the one or more non-natural amino acids, or wherein each of the one or more heavy chains comprises one of the one or more non-natural amino acids.
128. The ADC of any one of claims 102 to 127, wherein the one or more heavy chains are two heavy chains.
129. The ADC of any one of claims 103 to 127, wherein the one or more heavy chains are two heavy chains, and the one or more light chains are two light chains.
130. The ADC of any one of claims 101 to 129, wherein d is 1, 2, 3, 4, 5, 6, 7 or 8, or is 1, 2, 3 or 4.
131. The ADC of any one of claims 101 to 130, wherein each E comprises an amide, an ester, a thioester, a pyrrolidine-2, 5-dione, an oxime, a disulfide, a 1,2, 3 -triazole or a 1,4- dihydropyridazine.
132. The ADC of claim 131, wherein the 1,2, 3 -triazole or the 1,4-dihydropyridazine is fused to an 8-membered ring.
133. The ADC of claim 131 or 132, wherein E is selected from the group consisting of: wherein: each RJ is independently H or unsubstituted Ci-Ce alkyl; each Rq is independently unsubstituted Ci-Ce alkyl; each Rf is independently H or unsubstituted Ci-Ce alkyl; each s is independently 0, 1, 2, 3, 4, 5 or 6; each t is independently 0, 1, 2, 3, 4, 5 or 6; each + denotes connection to L; and each wavy line (-^ ) denotes connection to Ab.
134. The ADC of any one of claims 101 to 133, wherein: each L comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, substituted alkylene, -(alkylene-O)n-, - (alkylene-N(Rw))n-, unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, -S(0)o-2-, a disulfide (-S-S-), a water-soluble polymer, an amino acid, and a peptide, and a combination thereof; at least one of the one or more moieties is not a bond, each n is independently an integer from 1 to 100, and each Rw is independently H or Ci-Cs alkyl.
135. The ADC of claim 134, wherein the peptide is a dipeptide.
136. The ADC of claim 134 or 135, wherein: each L comprises one or more moieties independently selected from the group consisting of a bond, methine, methylene, unsubstituted alkylene, -(alkyl ene-O)n-, -(alkylene-N(Rw))n- unsubstituted arylene, substituted arylene, -O-, -C(O)-, -N(RW)-, an amino acid and a dipeptide, and a combination thereof; at least one moiety is not a bond, each n is independently an integer from 1 to 10, and each Rw is independently H or Ci-Cs alkyl.
137. The ADC of claim 136, wherein L comprises at least one unsubstituted alkylene, at least one -(alkylene-O)n-, or both.
138. The ADC of claim 137, wherein L consists of one or more unsubstituted alkylene, one or more -(alkylene-O)n-, or both.
139. The ADC of claim 138, wherein L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and wherein n is an integer from 1 to 10.
140. The ADC of claim 139, wherein m is 1, 2 or 3 and n is 1, 2 or 3.
141. The ADC of claim 140, wherein m is 1 and n is 3.
142. The ADC of any one of claims 101 to 141, wherein each of the one or more non-natural amino acids is independently selected from the group consisting of para-acetyl phenylalanine, 4- acetyl-L-phenylalanine (para-acetyl-L-phenylalanine (pAF)), 3-O-(N-acetyl-beta-D- glucosaminyl)-L-threonine, N4-(P-N-Acetyl-D-glucosaminyl)-L-asparagine, O-allyl-L-tyrosine, alpha-N-acetylgalactosamine-O-L-serine, alpha-N-acetylgalactosamine-O-L-threonine, 2- aminooctanoic acid, 2-amino-L-phenylalanine, 3-amino-L-phenylalanine, 4-amino-L- phenylalanine, 2-amino-L-tyrosine, 3-amino-L-tyrosine, 4-azido-L-phenylalanine, 4-benzoyl-L- phenylalanine, (2,2-bipyridin-5yl)-L-alanine, 3-borono-L-phenylalanine, 4-borono-L- phenylalanine, 4-bromo-L-phenylalanine, p-carboxymethyl-L-phenylalanine, 4-carboxy-L- phenylalanine, p-cyano-L-phenylalanine, 3,4-dihydroxy-L-phenylalanine (L-DOPA), 4-ethynyl- L-phenylalanine, 2-fluoro-L-phenylalanine, 3-fluoro-L-phenylalanine, 4-fluoro-L-phenylalanine, O-(3-O-D-galactosyl-N-acetyl-beta-D-galactosaminyl)-L-serine, L-homoglutamine, (8- hydroxyquinolin-3-yl)-L-alanine, 4-iodo-L-phenylalanine, 4-isopropyl-L-phenylalanine, O-i- propyl-L-tyrosine, 3-isopropyl-L-tyrosine, O-mannopyranosyl-L-serine, 2-methoxy-L- phenylalanine, 3 -m ethoxy -L-phenylalanine, 4-m ethoxy -L-phenylalanine, 3-methyl-L- phenylalanine, O-methyl-L-tyrosine, 3-(2-naphthyl)-L-alanine, 5-nitro-L-histidine, 4-nitro-L- histidine, 4-nitro-L-leucine, 2-nitro-L-phenyl alanine, 3-nitro-L-phenylalanine, 4-nitro-L- phenylalanine, 4-nitro-L-tryptophan, 5-nitro-L-tryptophan, 6-nitro-L-tryptophan, 7-nitro-L- tryptophan, 2-nitro-L-tyrosine, 3-nitro-L-tyrosine, O-phospho-L-serine, O-phospho-L-tyrosine, 4-propargyloxy -L-phenylalanine, O-2-propyn-l-yl-L-tyrosine, 4-sulfo-L-phenylalanine and O- sulfo-L-tyrosine.
143. The ADC of any one of claims 101 to 142, wherein at least one of the one or more nonnatural amino acids is para-acetyl-L-phenylalanine (pAF).
144. The ADC of any one of claims 101 to 143, wherein each of the one or more non-natural amino acids is para-acetyl-L-phenylalanine (pAF).
145. The ADC of any one of claims 101 to 144, wherein each E comprises an oxime.
146. The ADC of any one of claims 101 to 145, wherein each E has the following structure: /°-+
>-N Rq wherein:
Rq is unsubstituted Ci-Ce alkyl;
+ denotes the connection to L; and the wavy line (•^) denotes the connection to Ab.
147. The ADC of claim 146, wherein each of the one or more non-natural amino acids is para- acetyl-L-phenylalanine (pAF), and wherein Rq is the methyl group of pAF.
148. The ADC of claim 146 or 147, wherein Rq is methyl and L is -(CH2CH2)m-(CH2CH2-O)n-; wherein m is an integer from 1 to 10; and wherein n is an integer from 1 to 10; optionally, wherein m is 1 and n is 3.
150. The ADC of claim 149, wherein d is 2, 3 or 4.
151. The ADC of claim 149, wherein d is 2.
152. The ADC of claim 103, 149 or 150, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 4; and d is 2.
153. The ADC of claim 152, wherein the one non-natural amino acid at position 114 of each of the two heavy chains is para-acetyl-L-phenylalanine (pAF).
154. The ADC of any one of claims 103, 149, 150 or 151, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the heavy chains is SEQ ID NO: 5 or 6, wherein each of SEQ ID NO: 5 and 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, wherein: the amino acid sequence of each of the two light chains is SEQ ID NO: 3, and d is 2; or the amino acid sequence of each of the two light chain is SEQ ID NO: 7, wherein SEQ ID NO: 7 is characterized as containing one non-natural amino acid at position 121 based on Kabat numbering, and d is 1, 2, 3 or 4.
155. The ADC of claim 103, 149 or 151, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is the SEQ ID NO: 5, wherein SEQ ID NO: 5 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains is SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110 based on Kabat numbering;
SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112 based on Kabat numbering;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121 based on Kabat numbering; and d is 1, 2, 3 or 4.
156. The ADC of claim 155, wherein each of the light chain is SEQ ID NO: 11.
157. The ADC of claim 103, 149 or 151, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chains is SEQ ID NO: 6, wherein SEQ ID NO: 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chains SEQ ID NO: 8, 9, 10 or 11, wherein:
SEQ ID NO: 8 is characterized as containing one non-natural amino acid at position 110 based on Kabat numbering;
SEQ ID NO: 9 is characterized as containing one non-natural amino acid at position 112 based on Kabat numbering;
SEQ ID NO: 10 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; and
SEQ ID NO: 11 is characterized as containing one non-natural amino acid at position 121 based on Kabat numbering; and d is 1, 2, 3 or 4.
158. The ADC of claim 103, 149 or 151, wherein: the one or more heavy chains are two heavy chains, and the amino acid sequence of each of the two heavy chain is SEQ ID NO: 5 or 6, wherein each of SEQ ID NO: 5 and 6 is characterized as containing one non-natural amino acid at position 114 based on Kabat numbering; the one or more light chains are two light chains, and the amino acid sequence of each of the two light chain is SEQ ID NO: 12, 13, 14, 15, 16 or 17, wherein:
SEQ ID NO: 12 is characterized as containing one non-natural amino acid at position 127 based on Kabat numbering;
SEQ ID NO: 13 is characterized as containing one non-natural amino acid at position 149 based on Kabat numbering;
SEQ ID NO: 14 is characterized as containing one non-natural amino acid at position 156 based on Kabat numbering;
SEQ ID NO: 15 is characterized as containing one non-natural amino acid at position 168 based on Kabat numbering; SEQ ID NO: 16 is characterized as containing one non-natural amino acid at position 202 based on Kabat numbering;
SEQ ID NO: 17 is characterized as containing one non-natural amino acid at position 205 based on Kabat numbering; and d is 1, 2, 3 or 4.
159. The ADC of any one of claims 154 to 158, wherein each non-natural amino acid is para- acetyl-L-phenylalanine (pAF).
160. The ADC of any one of claims 1 to 40, 41 to 100 or 101 to 159, provided that the ADC does not contain a Toll-like receptor (TLR) agonist.
163. The ADC of claim 161 or 162, wherein the cytotoxic moiety is less cytotoxic than monomethyl auristatin E (MMAE) in vitro.
164. The ADC of any one of claims 161 to 163, wherein the cytotoxic moiety exhibits a higher in vitro half-maximal inhibitory concentration (IC50) against a microtubule inhibitor-sensitive cancer cell line when compared to MMAE.
165. The ADC of claim 164, wherein the higher in vitro IC50 is at least a two-fold higher.
166. The ADC of claim 164, wherein the higher in vitro IC50 is at least a 10-fold higher.
167. The ADC of claim 164, wherein the higher in vitro IC50 is at least a 100-fold higher.
168. The ADC of claim 164, wherein the higher in vitro IC50 is at least a 1000-fold higher.
169. The ADC of any one of claims 164 to 168, wherein the microtubule inhibitor-sensitive cancer cell line is an SKBR3 cell line or a BxPC3 cell line, and the in vitro IC50 is determined in an in vitro cytotoxicity assay.
170. The ADC of any one of claims 161-169, wherein the cytotoxic moiety displays reduced in vitro human keratinocyte cytotoxicity when compared to monomethyl auristatin E (MMAE) in vitro.
171. The ADC of any one of claims 161 to 170, provided that the ADC does not contain a Toll- like receptor (TLR) agonist.
172. A pharmaceutical composition comprising an ADC of any one of claims 1 to 40, 41 to 100, 101 to 160, or 161 to 171, and at least one pharmaceutically acceptable adjuvant, binder, buffer, carrier, diluent or excipient.
173. The pharmaceutical composition of claim 172, further comprising a chemotherapeutic agent, hormonal agent, antitumor agent, immunostimulatory agent, immunomodulator, corticosteroid, or combination thereof.
174. A method of treating a disease or condition in a subject, the method comprising administering to the subject a therapeutically effective amount of an ADC of any one of claims 1 to 40, 41 to 100, 101 to 160 or 161 to 171, or a pharmaceutical composition of claim 172 or 173.
175. The method of claim 174, wherein the disease or condition is cancer.
176. The method of claim 175, wherein the cancer is a solid tumor.
177. The method of claim 175 or 176, wherein the cancer is breast cancer, pancreatic cancer, lung cancer, gastric cancer, colorectal cancer or prostate cancer.
178. The method of claim 175, wherein the cancer is triple-negative breast cancer (TNBC).
179. The method of claim 175, wherein the cancer is pancreatic ductal adenocarcinoma (PDAC).
180. The method of claim 175, wherein the cancer is non-small cell lung cancer (NSCLC).
181. The method of any one of claims 175 to 180, wherein the cancer is a TROP2-positive cancer.
182. The method of any one of claims 175 to 181, wherein the cancer is a Dxd-resistant cancer.
183. The method of any one of claims 175 to 182, wherein the cancer is a topoisomerase 1 (TOPI) inhibitor-resistant cancer.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363486493P | 2023-02-23 | 2023-02-23 | |
| US63/486,493 | 2023-02-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024178310A1true WO2024178310A1 (en) | 2024-08-29 |
Family
ID=90675229
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/017043PendingWO2024178310A1 (en) | 2023-02-23 | 2024-02-23 | Trop2-directed antibody-drug conjugates and uses thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024178310A1 (en) |
Citations (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4414148A (en) | 1981-04-15 | 1983-11-08 | Sanofi | Anti-cancer drugs for the treatment of melanomas and method for preparing thereof |
| US4569789A (en) | 1984-08-29 | 1986-02-11 | Dana-Farber Cancer Institute, Inc. | Acid-cleavable compound, use in protein conjugates and drug delivery systems |
| EP0188256A2 (en) | 1985-01-14 | 1986-07-23 | NeoRx | Metal radionuclide labeled proteins for diagnosis and therapy |
| US4659839A (en) | 1984-10-10 | 1987-04-21 | Mallinckrodt, Inc. | Coupling agents for radiolabeled antibody fragments |
| US4671958A (en) | 1982-03-09 | 1987-06-09 | Cytogen Corporation | Antibody conjugates for the delivery of compounds to target sites |
| US4680338A (en) | 1985-10-17 | 1987-07-14 | Immunomedics, Inc. | Bifunctional linker |
| US4699784A (en) | 1986-02-25 | 1987-10-13 | Center For Molecular Medicine & Immunology | Tumoricidal methotrexate-antibody conjugate |
| US5252714A (en) | 1990-11-28 | 1993-10-12 | The University Of Alabama In Huntsville | Preparation and use of polyethylene glycol propionaldehyde |
| WO1999067291A2 (en) | 1998-06-22 | 1999-12-29 | Immunex Corporation | Site specific protein modification by mutagenesis |
| WO2002085923A2 (en) | 2001-04-19 | 2002-10-31 | The Scripps Research Institute | In vivo incorporation of unnatural amino acids |
| US20030118592A1 (en) | 2001-01-17 | 2003-06-26 | Genecraft, Inc. | Binding domain-immunoglobulin fusion proteins |
| US20030133939A1 (en) | 2001-01-17 | 2003-07-17 | Genecraft, Inc. | Binding domain-immunoglobulin fusion proteins |
| WO2004035743A2 (en) | 2002-10-16 | 2004-04-29 | The Scripps Research Institute | Site specific incorporation of keto amino acids into proteins |
| WO2005074650A2 (en) | 2004-02-02 | 2005-08-18 | Ambrx, Inc. | Modified human four helical bundle polypeptides and their uses |
| WO2006050262A2 (en) | 2004-11-01 | 2006-05-11 | The Regents Of The University Of California | Compositions and methods for modification of biomolecules |
| WO2006068802A2 (en) | 2004-12-22 | 2006-06-29 | Ambrx, Inc. | COMPOSITIONS OF AMINOACYL-tRNA SYNTHETASE AND USES THEREOF |
| WO2006069246A2 (en) | 2004-12-22 | 2006-06-29 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007021297A1 (en) | 2005-08-18 | 2007-02-22 | Ambrx, Inc. | COMPOSITIONS OF tRNA AND USES THEREOF |
| WO2007059312A2 (en) | 2005-11-16 | 2007-05-24 | Ambrx, Inc. | Methods and compositions comprising non-natural amino acids |
| WO2007070659A2 (en) | 2005-12-14 | 2007-06-21 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007079130A2 (en) | 2005-12-30 | 2007-07-12 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007094916A2 (en) | 2006-01-19 | 2007-08-23 | Ambrx, Inc. | Non-natural amino acid polypeptides having modulated immunogenicity |
| WO2008077079A1 (en) | 2006-12-18 | 2008-06-26 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2008083346A1 (en) | 2006-12-28 | 2008-07-10 | Ambrx, Inc. | Phenazine and quinoxaline substituted amino acids and polypeptides |
| WO2010011735A2 (en) | 2008-07-23 | 2010-01-28 | Ambrx, Inc. | Modified bovine g-csf polypeptides and their uses |
| WO2010037062A1 (en) | 2008-09-26 | 2010-04-01 | Ambrx, Inc. | Non-natural amino acid replication-dependent microorganisms and vaccines |
| WO2012166559A1 (en) | 2011-05-27 | 2012-12-06 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acid linked dolastatin derivatives |
| WO2012166560A1 (en) | 2011-05-27 | 2012-12-06 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acid linked dolastatin derivatives |
| WO2013185117A1 (en) | 2012-06-07 | 2013-12-12 | Ambrx, Inc. | Prostate-specific membrane antigen antibody drug conjugates |
| WO2013185177A1 (en) | 2012-06-12 | 2013-12-19 | Csl Limited | Influenza vaccine |
| WO2013188740A1 (en) | 2012-06-14 | 2013-12-19 | Ambrx, Inc. | Anti-psma antibodies conjugated to nuclear receptor ligand polypeptides |
| WO2013192360A1 (en) | 2012-06-19 | 2013-12-27 | Ambrx, Inc. | Anti-cd70 antibody drug conjugates |
| US9637411B2 (en) | 2010-12-29 | 2017-05-02 | Sumco Corporation | Vitreous silica crucible and method of manufacturing the same |
| US20170182181A1 (en) | 2014-04-04 | 2017-06-29 | Merck Sharp & Dohme Corp. | Phosphate based linkers for intracellular delivery of drug conjugates |
| WO2019191728A1 (en) | 2018-03-29 | 2019-10-03 | Ambrx, Inc. | Humanized anti-prostate-specific membrane antigen (psma) antibody drug conjugates |
| WO2020168017A1 (en) | 2019-02-12 | 2020-08-20 | Ambrx, Inc. | Compositions containing, methods and uses of antibody-tlr agonist conjugates |
| WO2021027851A1 (en)* | 2019-08-12 | 2021-02-18 | 凯惠科技发展(上海)有限公司 | Trop2 antibody, preparation method therefor, and conjugate and use thereof |
| WO2021173889A1 (en) | 2020-02-26 | 2021-09-02 | Ambrx, Inc. | Uses of anti-cd3 antibody folate bioconjugates |
| WO2021183832A1 (en) | 2020-03-11 | 2021-09-16 | Ambrx, Inc. | Interleukin-2 polypeptide conjugates and methods of use thereof |
| WO2022040596A1 (en) | 2020-08-20 | 2022-02-24 | Ambrx, Inc. | Antibody-tlr agonist conjugates, methods and uses thereof |
| WO2023028165A2 (en)* | 2021-08-25 | 2023-03-02 | R.P. Scherer Technologies, Llc | Tumor-associated calcium signal transducer 2 (tacstd2) antibody-maytansine conjugates and methods of use thereof |
| WO2024077277A1 (en)* | 2022-10-07 | 2024-04-11 | Ambrx, Inc. | Drug linkers and antibody conjugates thereof |
- 2024
- 2024-02-23WOPCT/US2024/017043patent/WO2024178310A1/enactivePending
Patent Citations (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4414148A (en) | 1981-04-15 | 1983-11-08 | Sanofi | Anti-cancer drugs for the treatment of melanomas and method for preparing thereof |
| US4671958A (en) | 1982-03-09 | 1987-06-09 | Cytogen Corporation | Antibody conjugates for the delivery of compounds to target sites |
| US4569789A (en) | 1984-08-29 | 1986-02-11 | Dana-Farber Cancer Institute, Inc. | Acid-cleavable compound, use in protein conjugates and drug delivery systems |
| US4659839A (en) | 1984-10-10 | 1987-04-21 | Mallinckrodt, Inc. | Coupling agents for radiolabeled antibody fragments |
| EP0188256A2 (en) | 1985-01-14 | 1986-07-23 | NeoRx | Metal radionuclide labeled proteins for diagnosis and therapy |
| US4680338A (en) | 1985-10-17 | 1987-07-14 | Immunomedics, Inc. | Bifunctional linker |
| US4699784A (en) | 1986-02-25 | 1987-10-13 | Center For Molecular Medicine & Immunology | Tumoricidal methotrexate-antibody conjugate |
| US5252714A (en) | 1990-11-28 | 1993-10-12 | The University Of Alabama In Huntsville | Preparation and use of polyethylene glycol propionaldehyde |
| WO1999067291A2 (en) | 1998-06-22 | 1999-12-29 | Immunex Corporation | Site specific protein modification by mutagenesis |
| US20030118592A1 (en) | 2001-01-17 | 2003-06-26 | Genecraft, Inc. | Binding domain-immunoglobulin fusion proteins |
| US20030133939A1 (en) | 2001-01-17 | 2003-07-17 | Genecraft, Inc. | Binding domain-immunoglobulin fusion proteins |
| WO2002085923A2 (en) | 2001-04-19 | 2002-10-31 | The Scripps Research Institute | In vivo incorporation of unnatural amino acids |
| WO2002086075A2 (en) | 2001-04-19 | 2002-10-31 | The Scripps Research Institute | Methods and composition for the production of orthoganal trna-aminoacyltrna synthetase pairs |
| WO2004035743A2 (en) | 2002-10-16 | 2004-04-29 | The Scripps Research Institute | Site specific incorporation of keto amino acids into proteins |
| WO2005074650A2 (en) | 2004-02-02 | 2005-08-18 | Ambrx, Inc. | Modified human four helical bundle polypeptides and their uses |
| WO2006050262A2 (en) | 2004-11-01 | 2006-05-11 | The Regents Of The University Of California | Compositions and methods for modification of biomolecules |
| WO2006068802A2 (en) | 2004-12-22 | 2006-06-29 | Ambrx, Inc. | COMPOSITIONS OF AMINOACYL-tRNA SYNTHETASE AND USES THEREOF |
| US8476411B2 (en) | 2004-12-22 | 2013-07-02 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US8008456B2 (en) | 2004-12-22 | 2011-08-30 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US7928163B2 (en) | 2004-12-22 | 2011-04-19 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2006069246A2 (en) | 2004-12-22 | 2006-06-29 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US7696312B2 (en) | 2004-12-22 | 2010-04-13 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US8859802B2 (en) | 2004-12-22 | 2014-10-14 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US7332571B2 (en) | 2004-12-22 | 2008-02-19 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US8791231B2 (en) | 2004-12-22 | 2014-07-29 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US8809511B2 (en) | 2004-12-22 | 2014-08-19 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| US8048988B2 (en) | 2004-12-22 | 2011-11-01 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007021297A1 (en) | 2005-08-18 | 2007-02-22 | Ambrx, Inc. | COMPOSITIONS OF tRNA AND USES THEREOF |
| WO2007059312A2 (en) | 2005-11-16 | 2007-05-24 | Ambrx, Inc. | Methods and compositions comprising non-natural amino acids |
| WO2007070659A2 (en) | 2005-12-14 | 2007-06-21 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007079130A2 (en) | 2005-12-30 | 2007-07-12 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2007094916A2 (en) | 2006-01-19 | 2007-08-23 | Ambrx, Inc. | Non-natural amino acid polypeptides having modulated immunogenicity |
| WO2011028195A2 (en) | 2006-12-18 | 2011-03-10 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2008077079A1 (en) | 2006-12-18 | 2008-06-26 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acids and polypeptides |
| WO2008083346A1 (en) | 2006-12-28 | 2008-07-10 | Ambrx, Inc. | Phenazine and quinoxaline substituted amino acids and polypeptides |
| WO2010011735A2 (en) | 2008-07-23 | 2010-01-28 | Ambrx, Inc. | Modified bovine g-csf polypeptides and their uses |
| WO2010037062A1 (en) | 2008-09-26 | 2010-04-01 | Ambrx, Inc. | Non-natural amino acid replication-dependent microorganisms and vaccines |
| US9637411B2 (en) | 2010-12-29 | 2017-05-02 | Sumco Corporation | Vitreous silica crucible and method of manufacturing the same |
| WO2012166560A1 (en) | 2011-05-27 | 2012-12-06 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acid linked dolastatin derivatives |
| WO2012166559A1 (en) | 2011-05-27 | 2012-12-06 | Ambrx, Inc. | Compositions containing, methods involving, and uses of non-natural amino acid linked dolastatin derivatives |
| WO2013185117A1 (en) | 2012-06-07 | 2013-12-12 | Ambrx, Inc. | Prostate-specific membrane antigen antibody drug conjugates |
| WO2013185177A1 (en) | 2012-06-12 | 2013-12-19 | Csl Limited | Influenza vaccine |
| WO2013188740A1 (en) | 2012-06-14 | 2013-12-19 | Ambrx, Inc. | Anti-psma antibodies conjugated to nuclear receptor ligand polypeptides |
| WO2013192360A1 (en) | 2012-06-19 | 2013-12-27 | Ambrx, Inc. | Anti-cd70 antibody drug conjugates |
| US20170182181A1 (en) | 2014-04-04 | 2017-06-29 | Merck Sharp & Dohme Corp. | Phosphate based linkers for intracellular delivery of drug conjugates |
| US10550190B2 (en) | 2014-04-04 | 2020-02-04 | Merck Sharp & Dohme Corp. | Phosphate based linkers for intracellular delivery of drug conjugates |
| WO2019191728A1 (en) | 2018-03-29 | 2019-10-03 | Ambrx, Inc. | Humanized anti-prostate-specific membrane antigen (psma) antibody drug conjugates |
| WO2020168017A1 (en) | 2019-02-12 | 2020-08-20 | Ambrx, Inc. | Compositions containing, methods and uses of antibody-tlr agonist conjugates |
| WO2021027851A1 (en)* | 2019-08-12 | 2021-02-18 | 凯惠科技发展(上海)有限公司 | Trop2 antibody, preparation method therefor, and conjugate and use thereof |
| WO2021173889A1 (en) | 2020-02-26 | 2021-09-02 | Ambrx, Inc. | Uses of anti-cd3 antibody folate bioconjugates |
| WO2021183832A1 (en) | 2020-03-11 | 2021-09-16 | Ambrx, Inc. | Interleukin-2 polypeptide conjugates and methods of use thereof |
| WO2022040596A1 (en) | 2020-08-20 | 2022-02-24 | Ambrx, Inc. | Antibody-tlr agonist conjugates, methods and uses thereof |
| WO2023028165A2 (en)* | 2021-08-25 | 2023-03-02 | R.P. Scherer Technologies, Llc | Tumor-associated calcium signal transducer 2 (tacstd2) antibody-maytansine conjugates and methods of use thereof |
| WO2024077277A1 (en)* | 2022-10-07 | 2024-04-11 | Ambrx, Inc. | Drug linkers and antibody conjugates thereof |
Non-Patent Citations (70)
| Title |
|---|
| "Antibodies: A Practical Approach", 1989, OXFORD UNIVERSITY PRESS |
| "Cell And Tissue Culture: Laboratory Procedures", 1993, JOHN WILEY AND SONS |
| "Current Protocols In Immunology", 1991, JOHN WILEY AND SONS |
| "Gene Transfer Vectors For Mammalian Cells", 1987, COLD SPRING HARBOR PRESS |
| "Introduction To Cell And Tissue Culture", 1998, COLD SPRING HARBOR LABORATORY PRESS |
| "Methods in Molecular Biology", HUMANA PRESS, article "Oligonucleotide Synthesis: Methods And Applications" |
| "Molecular Cloning: A Laboratory Manual", 2001, COLD SPRING HARBOR PRESS |
| "Monoclonal Antibodies: A Practical Approach", 2000, OXFORD UNIVERSITY PRESS |
| "Oligonucleotide Synthesis", 1984 |
| "PCR: The Polymerase Chain Reaction", 1994, BIRKHAUSER |
| "Short Protocols In Molecular Biology", 1999, JOHN WILEY AND SONS |
| "The Pharmacological Basis of Therapeutics", 1996, MCGRAW-HILL |
| AXUP, J.Y. ET AL., PROC. NATL. ACAD. SCI., vol. 109, no. 40, 2012, pages 16101 - 16016 |
| BATZER ET AL., NUCLEIC ACID RES, vol. 19, 1991, pages 5081 |
| BIOCONJUG CHEM., vol. 27, no. 12, 2016, pages 2791 - 2807 |
| BIOPOLYMERS, vol. 22, 1983, pages 2577 - 2637 |
| BIRD ET AL., SCIENCE, vol. 242, 1988, pages 423 - 426 |
| CARTER, P ET AL.: "Humanization Of An Anti-p185her2 Antibody For Human Cancer Therapy", PROC. NATL. ACAD. SCI. (USA, vol. 89, 1992, pages 4285 - 4289, XP000275844, DOI: 10.1073/pnas.89.10.4285 |
| CHATTERJEE ET AL.: "A. Versatile Platform for Single- and Multiple-Unnatural Amino Acid Mutagenesis in Escherichia coli", BIOCHEMISTRY, 2013 |
| CHEN, Y: "Drug-to-antibody ratio (DAR) by UV/Vis spectroscopy", METHODS MOL. BIOL., vol. 1, no. 045, 2013, pages 267 - 73, XP008169798, DOI: 10.1007/978-1-62703-541-5-16 |
| CO, M. S. ET AL.: "Chimeric And Humanized Antibodies With Specificity For The CD33 Antigen", J. IMMUNOL., vol. 148, 1992, pages 1149 - 1154 |
| CO, M. S. ET AL.: "Humanized Antibodies For Antiviral Therapy", PROC. NATL. ACAD. SCI. (USA, vol. 88, 1991, pages 2869 - 2873, XP000200583, DOI: 10.1073/pnas.88.7.2869 |
| CUBAS R ET AL., MOL CANCER, vol. 9, 2010, pages 253 |
| DORONINA ET AL., NATURE BIOTECHNOLOGY, vol. 21, no. 7, 2003, pages 778 - 784 |
| DUBOWCHIK ET AL., BIOCONJUGATE CHEM, vol. 13, 2002, pages 855 - 869 |
| FONG D ET AL., MOD PATHOL, vol. 21, 2008, pages 186 - 191 |
| GEORG FALK ET AL: "Enzyme-Based Labeling Strategies for Antibody-Drug Conjugates and Antibody Mimetics", ANTIBODIES, vol. 7, no. 1, 4 January 2018 (2018-01-04), CH, pages 4, XP055566036, ISSN: 2073-4468, DOI: 10.3390/antib7010004* |
| GUAN H ET AL., BMC CANCER, vol. 17, 2017, pages 486 |
| GUERRA E ET AL., CLIN CANCER RES, vol. 22, 2016, pages 4197 - 205 |
| HARTMUTH, C ET AL., ANGEW. CHEM. INT. ED., vol. 40, no. 11, 2001, pages 2004 - 2021 |
| HU ET AL., CANCER RESEARCH, vol. 56, 1996, pages 3055 - 3061 |
| HUDSON, CURR. OPIN. BIOTECHNOL., vol. 9, 1998, pages 395 - 402 |
| HUSTON ET AL., PROC. NATL. ACAD., vol. 85, 1988, pages 5879 - 5883 |
| HUTCHINS ET AL., J MOL BIOL, vol. 406, no. 4, 2011, pages 595 - 603 |
| J. CHIN ET AL., SCIENCE, vol. 301, 2003, pages 964 - 7 |
| J. W. CHIN ET AL., J. AMER. CHEM. SOC., vol. 124, 2002, pages 9026 - 9027 |
| J. W. CHIN ET AL., PNAS USA, vol. 99, 2002, pages 11020 - 11024 |
| J. W. CHINP. G. SCHULTZ, CHEMBIOCHEM, vol. 3, no. 11, 2002, pages 1135 - 1137 |
| JANEWAY, C. A. ET AL.: "Immunobiology", vol. 7, 2007, GARLAND SCIENCE |
| JOHANN, K ET AL., POLYMER CHEMISTRY, vol. 27, no. 11, 2020, pages 4396 - 4407 |
| JOHNSON ET AL., NAT CHEM BIOL, vol. 7, no. 11, 2011, pages 779 - 86 |
| JONES, P ET AL., NATURE, vol. 321, 1986, pages 522 - 525 |
| KAZANE ET AL., J AM CHEM SOC, vol. 135, no. 1, 2013, pages 340 - 6 |
| KETTLEBOROUGH, C. A. ET AL.: "Humanization Of A Mouse Monoclonal Antibody By CDR-Grafting: The Importance Of Framework Residues On Loop Conformation", PROTEIN ENGINEERING, vol. 4, 1991, pages 773 - 3783 |
| KIM ET AL., J AM CHEM SOC, vol. 134, no. 24, 2012, pages 9918 - 21 |
| KING, G.T., INVEST NEW DRUGS, vol. 36, 2018, pages 836 - 847 |
| KOBAYASHI H ET AL., VIRCHOWS ARCH, vol. 457, 2010, pages 69 - 76 |
| L. WANGP. G. SCHULTZ, CHEM. COMM., vol. 1, 2002, pages 1 - 11 |
| LIN H ET AL., EXP MOL PATHOL, vol. 94, 2013, pages 73 - 78 |
| LIU ET AL., ANNU REV BIOCHEM, vol. 79, 2010, pages 413 - 44 |
| LOBUGLIO, A. F. ET AL.: "Mouse/Human Chimeric Monoclonal Antibody in Man: Kinetics and Immune Response", PROC. NATL. ACAD. SCI. (USA, vol. 86, 1989, pages 4220 - 4224, XP002100301, DOI: 10.1073/pnas.86.11.4220 |
| MAYNARDGEORGIOU, ANNU. REV. BIOMED. ENG., vol. 2, 2000, pages 339 - 76 |
| MUHLMANN G ET AL., J CLIN PATHOL, vol. 62, 2009, pages 152 - 158 |
| no. 474645-27-7 |
| NORTHROP, B. H. ET AL., POLYMER CHEMISTRY, vol. 18, no. 6, 2015, pages 3415 - 3430 |
| OHTSUKA ET AL., J. BIOL. CHEM., vol. 260, 1985, pages 2605 - 2608 |
| OKAJIMA, D ET AL., MOL CANCER THER, vol. 20, 2021, pages 2329 - 2340 |
| P. STROP ET AL: "RN927C, a Site-Specific Trop-2 Antibody-Drug Conjugate (ADC) with Enhanced Stability, Is Highly Efficacious in Preclinical Solid Tumor Models", MOLECULAR CANCER THERAPEUTICS, vol. 15, no. 11, 31 August 2016 (2016-08-31), US, pages 2698 - 2708, XP055658796, ISSN: 1535-7163, DOI: 10.1158/1535-7163.MCT-16-0431* |
| QUEEN, C ET AL., PROC. NATL. ACAD. USA, vol. 86, 1989, pages 10029 - 10033 |
| RABUKA ET AL., NATURE PROTOCOLS, vol. 7, 2012, pages 1052 - 1067 |
| REMINGTON: "The Science and Practice of Pharmacy", 1995, HARWOOD ACADEMIC PUBLISHERS |
| RIECHMANN, L ET AL., NATURE, vol. 332, 1998, pages 323 - 327 |
| ROSSOLINI ET AL., MOL. CELL. PROBES, vol. 8, 1994, pages 91 - 98 |
| SKIDMORE LILLIAN ET AL: "ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers", MOLECULAR CANCER THERAPEUTICS, vol. 19, no. 9, 1 September 2020 (2020-09-01), US, pages 1833 - 1843, XP055933201, ISSN: 1535-7163, Retrieved from the Internet <URL:https://aacrjournals.org/mct/article-pdf/19/9/1833/1866133/1833.pdf> DOI: 10.1158/1535-7163.MCT-19-1004* |
| SLETTEN, E.M.BERTOZZI, C. R., ANGEW. CHEM. INT. ED., vol. 48, no. 38, 2009, pages 6974 - 6998 |
| STEPAN L.P. ET AL., J HISTOCHEM CYTOCHEM, vol. 59, 2011, pages 701 - 710 |
| TANG, Y ET AL.: "Real-Time Analysis on Drug-Antibody Ratio of Antibody-Drug Conjugates for Synthesis, Process Optimization and Quality Control", SCI REP, vol. 7, 2017, pages 7763, XP055693488, DOI: 10.1038/s41598-017-08151-2 |
| WANG ET AL., ANGEW CHEM INT ED, vol. 44, 2005, pages 34 - 66 |
| WARD ET AL., NATURE, vol. 341, 1989, pages 544 - 546 |
| WEIR ET AL.: "Handbook Of Experimental Immunology", 1997, WILEY-BLACKWELL PUBLISHERS |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102864931B1 (en) | Humanized anti-prostate-specific membrane antigen (PSMA) antibody drug conjugate | |
| US20210163620A1 (en) | Trispecific binding molecules against cancers and uses thereof | |
| CA3075087A1 (en) | Anti- folate receptor alpha antibody conjugates and their uses | |
| CN114502200A (en) | Antibody Drug Conjugates | |
| EP4598586A1 (en) | Drug linkers and antibody conjugates thereof | |
| TW202515620A (en) | Targeted treatment of prostate cancers and other tumors by an antibody-drug conjugate | |
| WO2024178310A1 (en) | Trop2-directed antibody-drug conjugates and uses thereof | |
| JP2025506485A (en) | PEGylated antibody hydroxyl-containing drug conjugates | |
| WO2024215515A1 (en) | Drug linkers and antibody conjugates thereof | |
| TW202525345A (en) | Anti-psma adc conjugate compositions and methods of use thereof | |
| WO2024178360A2 (en) | Auristatin analogs and antibody conjugates thereof | |
| KR20250134670A (en) | anti-CD70 antibody-drug conjugate | |
| WO2023103854A1 (en) | Antibody-drug conjugate having improved affinity, and preparation method therefor and application thereof | |
| KR20250117662A (en) | Compositions and methods for protein internalization | |
| HK40044903B (en) | Humanized anti-prostate-specific membrane antigen (psma) antibody drug conjugates | |
| HK40044903A (en) | Humanized anti-prostate-specific membrane antigen (psma) antibody drug conjugates | |
| KR20240125945A (en) | Stapled peptide-antibody conjugates (SPACs) and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:24715696 Country of ref document:EP Kind code of ref document:A1 |