WO2024163039A1 - Cancer treatment using pi3k inhibitors in combination with antibiotics or purified diets - Google Patents
Cancer treatment using pi3k inhibitors in combination with antibiotics or purified dietsDownload PDFInfo
- Publication number
- WO2024163039A1 WO2024163039A1PCT/US2023/081396US2023081396WWO2024163039A1WO 2024163039 A1WO2024163039 A1WO 2024163039A1US 2023081396 WUS2023081396 WUS 2023081396WWO 2024163039 A1WO2024163039 A1WO 2024163039A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cancer
- antibiotics
- days
- pi3k inhibitor
- diet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/429—Thiazoles condensed with heterocyclic ring systems
- A61K31/43—Compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula, e.g. penicillins, penems
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/429—Thiazoles condensed with heterocyclic ring systems
- A61K31/43—Compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula, e.g. penicillins, penems
- A61K31/431—Compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula, e.g. penicillins, penems containing further heterocyclic rings, e.g. ticarcillin, azlocillin, oxacillin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
- A61K31/7036—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin having at least one amino group directly attached to the carbocyclic ring, e.g. streptomycin, gentamycin, amikacin, validamycin, fortimicins
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/14—Peptides containing saccharide radicals; Derivatives thereof, e.g. bleomycin, phleomycin, muramylpeptides or vancomycin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
Definitions
- This inventionrelates generally to methods for cancer treatment using PI3K inhibitors in combination with one or more antibiotics or purified diets.
- PI3Kphosphatidylinositol 3-kinase
- PI3K inhibitorsshow poor and variable responses against solid tumors in the clinic, often due to drug resistance and low drug tolerance. That said, one PI3K inhibitor, BYL-719 (alpelisib), was approved for the treatment of hormone receptorpositive (HER2) tumors, but even in combination with hormone receptor blockade, a majority of patients progressed within 12 months, and many patients experienced side effects including cutaneous reactions and hyperglycemia. A major challenge is finding ways to sensitize tumors to PI3K inhibitors and improve drug tolerability.
- the disclosureprovides a method of treating a cancer.
- the methodcomprises administering to a subject in need thereof (i) a therapeutically effective amount of a phosphoinositide 3-kinase (PI3K) inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (ii) an effective amount of one or more antibiotics or a purified diet.
- PI3Kphosphoinositide 3-kinase
- the one or more antibioticscomprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
- the one or more antibioticscomprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the one or more antibioticscomprise (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
- the one or more antibioticsare administered at one or more doses of from about 5 mg/kg to about 500 mg/kg of body weight of the subject.
- the purified dietcomprises a ketogenic diet, a control purified diet, or a combination thereof.
- the ketogenic dietcomprises 20 wt%, 10 wt%, 5 wt%, or less of proteins.
- the ketogenic dietcomprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
- control purified dietcomprises from about 5 wt% to about 40 wt% of proteins. In some embodiments, the control purified diet comprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates. In some embodiments, the purified diet is administered at one or more doses of from about 10,000 mg/kg to about 500,000 mg/kg of body weight of the subject.
- the PI3K inhibitorcomprises a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor comprises a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
- the PI3K inhibitoris administered at one or more doses of from about 1 mg/kg to about 200 mg/kg of body weight of the subject.
- the PI3K inhibitoris administered to the subject before or after the one or more antibiotics or the purified diet. In some embodiments, the PI3K inhibitor is administered to the subject concurrently with the one or more antibiotics or the purified diet.
- the PI3K inhibitor, the one or more antibiotics, or the purified dietare administered at least every 1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, or 4 weeks.
- the PI3K inhibitor or the one or more antibioticsare administered to the subject intratumorally, intravenously, subcutaneously, intraosseously, orally, transdermally, sublingually, in sustained release, in controlled release, in delayed release, or as a suppository.
- the PI3K inhibitoris contained in the same composition with the one or more antibiotics or the purified diet.
- the canceris selected from the group consisting of chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), non-Hodgkin’s lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), Hodgkin’s lymphoma, B cell acute lymphoblastic leukemia (B-ALL), Burkitt’s lymphoma, Waldenstrom’s macroglobulinemia (WM), Burkitt’s lymphoma, multiple myeloma, and myelofibrosis.
- CLLchronic lymphocytic leukemia
- SLLsmall lymphocytic leukemia
- NHLdiffuse large B cell lymphoma
- FLfollicular lymphoma
- MCLmantle cell lymphoma
- Hodgkin’s lymphomaB cell acute lymphoblastic leukemia
- B-ALLB cell acute
- the canceris a solid tumor selected from the group consisting of bladder cancer, non-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glioma, gastrointestinal stromal tumor, breast cancer, lung cancer, colorectal cancer, thyroid cancer, bone sarcoma, stomach cancer, oral cavity cancer, oropharyngeal cancer, gastric cancer, kidney cancer, liver cancer, prostate cancer, colorectal cancer, esophageal cancer, testicular cancer, gynecological cancer, thyroid cancer, colon cancer, primary central nervous system lymphoma, and brain cancer.
- bladder cancernon-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glio
- the subjectis a mammal. In some embodiments, the subject is a human.
- the methodfurther comprises administering to the subject an additional therapeutic agent or therapy.
- the additional therapeutic agentcomprises a second PI3K inhibitor, a second antibiotic, a second purified diet, or a combination thereof.
- PI3K inhibitora stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof in combination with one or more antibiotics or a purified diet in a method described herein.
- this disclosureprovides a pharmaceutical composition
- a pharmaceutical compositioncomprising: (a) a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof, and (b) one or more antibiotics or a purified diet.
- the one or more antibioticscomprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
- the one or more antibioticscomprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the one or more antibioticscomprise (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
- the purified dietcomprises a ketogenic diet, a control purified diet, or a combination thereof.
- the ketogenic dietcomprises 20 wt%, 10 wt%, 5 wt%, or less of proteins.
- the ketogenic dietcomprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
- the control purified dietcomprises from about 5 wt% to about 40 wt% of proteins.
- the control purified dietcomprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
- the PI3K inhibitorcomprises a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor comprises a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
- this disclosurefurther provides a kit comprising the pharmaceutical composition described herein.
- Fig. 1shows KPC tumors having similar responsiveness to PI3K inhibition on purified diets regardless of macronutrients composition.
- CPDcontrol purified diet
- KDketogenic diet
- LPlow protein
- NPnormal protein
- VehVehicle.
- Figs. 2A, 2B, 2C, 2D, 2E, 2F, 2G, 2H, and 21show that diet source dictates response to PI3K inhibition.
- Fig. 2Ashows tumor volumes of K8484 KPC allografted tumors in mice fed chow, KD, or CPD and treated with the PI3K inhibitor BYL-719 or vehicle control. Statistics represent differences in tumor sizes on day 40 using two-way ANOVA with Sidak’s post hoc.
- Fig. 2Bshows survival curve of the same mice from Fig. 2A.
- FIG. 2Dshows serum glucose levels
- Figs. 2E and 2Fshow serum BYL levels in response to BYL administration on day 17 of the experiment in Fig. 2A. Mice were fasted during the first 4h of the experiment, after which food was put back. Shaded area represents the dark cycle.
- Figs. 2G, 2H, and 21show the results of the principal component analysis of serum metabolome, fecal metabolome, and fecal microbiome at different timepoints after BYL administration for Fig. 2G or at the 8h time point for FIG. 2H and FIG. 21 on day 17.
- n5 for Figs. 2D, 2E, 2F, 2G, 2H, and 21. Values shown are mean ⁇ SEM. **, /? ⁇ 0.01, ***, ? ⁇ 0.001, ****,/? ⁇ 0.0001.
- Fig. 3shows that PI3K inhibition is still enhanced even when a purified diet is supplemented with high fiber.
- Survival curve of K8484 KPC allografted tumors in mice fed CPD supplemented with high or low cellulose as insoluble fiber and inulin as soluble fiber and treated BYL-719 or vehicle control. Diets with low cellulose are plotted together as low fiber, and diets with high cellulose as high fiber, n7-22. Values shown are mean ⁇ SEM.
- Figs. 4A, 4B, 4C, and 4Dshow that gut microbiome ablation sensitizes tumors to PI3K inhibition.
- Statisticsrepresent differences in tumor sizes at endpoint using two-way ANOVA with Sidak’s post hoc. Figs.
- Figs. 5A, 5B, 5C, and 5Dshow that ampicillin sensitizes tumors to PI3K inhibition.
- Fig. 5Bshows body weight of the mice from Fig. 5A.
- Figs. 6A, 6B, 6C, and 6Dshow that purified diet and ampicillin sensitizes tumors to BKM- 120.
- Fig. 6Dshows body weights of mice from Fig. 6C. Values shown are mean ⁇ SEM. ***,£ ⁇ 0.001, ****,£> ⁇ 0.0001.
- This disclosurerelates to an unexpected discovery that antibiotics or purified diets significantly enhance phosphoinositide 3 -kinase (PI3K) inhibition efficacy for cancer. Accordingly, this disclosure provides a novel method for treating a cancer using PI3K inhibitors in combination with antibiotics or purified diets.
- PI3Kphosphoinositide 3 -kinase
- the methodmay include administering to a subject in need thereof (i) a therapeutically effective amount of a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (ii) an effective amount of one or more antibiotics or a purified diet.
- the PI3K inhibitormay be any PI3K inhibitor known in the art.
- the term “PI3K inhibitor”refers to a nucleic acid, peptide, compound, or small organic molecule that binds to and inhibits at least one activity of PI3K.
- the PI3K proteinscan be divided into three classes, class 1 PI3Ks, class 2 PI3Ks, and class 3 PI3Ks.
- Class 1 PI3Ksexist as heterodimers consisting of one of four p 110-catalytic subunits (p 110-alpha, p 110-beta, p 110-delta, and p 110-gamma) and one of two families of regulatory subunits.
- a PI3K inhibitormay target the class 1 PI3K inhibitors.
- a PI3K inhibitordisplays selectivity for one or more isoforms of the class 1 PI3K inhibitors (/. ⁇ ?., selectivity for pl 10-alpha, pl 10-beta, pl 10-delta, and pl lO-gamma or one or more of pl lO-alpha, pl lO-beta, pl lO-delta, and pl lO-gamma).
- a PI3K inhibitor that does not display isoform selectivityis considered a “pan-PI3K inhibitor.”
- a PI3K inhibitormay compete for binding with ATP to the PI3K catalytic domain.
- a selective PI3K inhibitorrefers to an agent that exhibits a 50% inhibitory concentration with respect to PI3K that is at least 10-fold, at least 20-fold, at least 30- fold, at least 50-fold, at least 100-fold, at least 1000- fold, or more, lower than the inhibitor’s IC50 with respect to mTOR and/or other proteins in the pathway.
- PI3K inhibitormay inhibit PI3K with an IC50 (concentration that inhibits 50% of the activity) of about 200 nM or less, about 100 nm or less, about 60 nM or less, about 25 nM, about 10 nM, about 5 nM, about 1 nM, about 100 uM, about 50 pM, about 25 pM, about 10 pM, about 1 pM, or less.
- a PI3K inhibitormay inhibit PI3K with an IC50 from about 2 nM to about 100 nm, from about 2 nM to about 50 nM, or from about 2 nM to about 15 nM.
- a PI3K inhibitormay be a PI3K-alpha inhibitor, a PI3K-gamma inhibitor, a PI3K-delta inhibitor, and a PI3K-gamma/delta inhibitor.
- the PI3K inhibitormay include a PI3K-alpha inhibitor.
- the PI3K inhibitormay include a pan-PI3K inhibitor.
- non-limiting examples of PI3K inhibitors suitable for use in the disclosed methodsmay include Serabelisib, BEZ235, LY294002, GDC-0941, BYL719, GSK2636771, TGX-221, AS25242, CAL-101, IPI-145, MK-2206, GSK690693, GDC-0068, A- 674563, CCT128930, AZD8055, INK128, rapamycin, PF-04691502, everolimus, BI-D1870, H89, PF-4708671, FMK, AT7867, NU7441, PI-103, NU7026, PIK-75, ZSTK474, PP-121, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a combination thereof.
- the PI3K inhibitormay include BYL-719, BKM-120, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a combination thereof.
- PI3K inhibitorsare described, for example, in U.S. Pat. Nos. 8,193,182 and 8,569,323, and U.S. Patent Application Publication Nos. 2012/0184568 Al, 2013/0344061 Al, and 2013/0267521 Al, the disclosures of which are incorporated by reference herein.
- PI3K inhibitorsmay exist in various forms, such as stereoisomers, racemates, derivatives, prodrugs, analogs, or pharmaceutically acceptable salts, that are also suitable for use in the disclosed methods.
- “Isomers”are different compounds that have the same molecular formula. “Stereoisomers” are isomers that differ only in the way the atoms are arranged in space, i.e., having a different stereochemical configuration. “Enantiomers” are a pair of stereoisomers that are non- superimposable mirror images of each other. A 1 : 1 mixture of a pair of enantiomers is a “racemic” mixture. The term “( ⁇ )” is used to designate a racemic mixture where appropriate. “Diastereoisomers” are stereoisomers that have at least two asymmetric atoms, but which are not mirror-images of each other.
- the absolute stereochemistryis specified according to the Cahn- Ingold-Prelog R-S system.
- the stereochemistry at each chiral carboncan be specified by either (R) or (S).
- Resolved compounds whose absolute configuration is unknowncan be designated (+) or (-) depending on the direction (dextro- or levorotatory) in which they rotate plane polarized light at the wavelength of the sodium D line.
- Certain of the compounds described hereincontain one or more asymmetric centers and can thus give rise to enantiomers, diastereomers, and other stereoisomeric forms that can be defined, in terms of absolute stereochemistry, as (R) or (S).
- Stereoisomersare compounds that differ only in their spatial arrangement. Enantiomers are pairs of stereoisomers whose mirror images are not superimposable, most commonly because they contain an asymmetrically substituted carbon atom that acts as a chiral center. “Enantiomer” means one of a pair of molecules that are mirror images of each other and are not superimposable. Diastereomers are stereoisomers that are not related to mirror images, most commonly because they contain two or more asymmetrically substituted carbon atoms. “R” and “S” represent the configuration of substituents around one or more chiral carbon atoms. Thus, “R*” and “S*” denote the relative configurations of substituents around one or more chiral carbon atoms. The symbol in a structural formula represents the presence of a chiral carbon center.
- Racemateor “racemic mixture” means a compound of equimolar quantities of two enantiomers, wherein such mixtures exhibit no optical activity, i.e., they do not rotate the plane of polarized light.
- “Geometric isomer”means isomers that differ in the orientation of substituent atoms in relationship to a carbon-carbon double bond, to a cycloalkyl ring, or to a bridged bicyclic system. Atoms (other than H) on each side of a carbon-carbon double bond may be in an E (substituents are on opposite sides of the carbon-carbon double bond) or Z (substituents are oriented on the same side) configuration.
- “R,” “S,” “St,” “R*,” “E,” “Z,” “cis,” and “trans”indicate configurations relative to the core molecule.
- a “derivative,” as used herein,refers to a chemical substance related structurally to another, i.e., an “original” substance, which can be referred to as a “parent” compound.
- a “derivative”can be made from the structurally related parent compound in one or more steps.
- the phrase “closely related derivative”means a derivative whose molecular weight does not exceed the weight of the parent compound by more than 50%.
- the general physical and chemical properties of a closely related derivativeare also similar to the parent compound.
- “Pharmaceutically active derivative”refers to any compound that, upon administration to the recipient, is capable of providing, directly or indirectly, the activity disclosed herein.
- an “analog”refers to a small organic compound, a nucleotide, a protein, or a polypeptide that possesses similar or identical activity or function(s) as the compound, nucleotide, protein or polypeptide or compound having the desired activity of this disclosure, but need not necessarily include a sequence or structure that is similar or identical to the sequence or structure of the preferred embodiments.
- prodrugrefers to a compound that may be converted under physiological conditions or by solvolysis to a biologically active compound described herein.
- prodrugrefers to a precursor of a biologically active compound that is pharmaceutically acceptable.
- a prodrugmay be inactive when administered to a subject, but is converted in vivo to an active compound, for example, by hydrolysis.
- the prodrug compoundoften offers the advantages of solubility, tissue compatibility, or delayed release in a mammalian organism see, e.g., Bundgaard, H., Design of Prodrugs (1985) (Elsevier, Amsterdam).
- prodrugalso refers to any covalently bonded carriers, which release the active compound in vivo when administered to a subject.
- Prodrugs of an active compound, as described hereinmay be prepared by modifying functional groups present in the active compound in such a way that the modifications are cleaved, either in routine manipulation or in vivo, to yield the active parent compound.
- Prodrugsinclude, for example, compounds wherein a hydroxy, amino or mercapto group is bonded to any group that, when the prodrug of the active compound is administered to a mammalian subject, cleaves to form a free hydroxy, free amino, or free mercapto group, respectively.
- prodrugsinclude, but are not limited to, acetates, formates, and benzoate derivatives of alcohol, various ester derivatives of a carboxylic acid, or acetamide, formamide, and benzamide derivatives of an amine functional group in the active compound.
- Various forms of prodrugsare well known in the art and are described in: (a) The Practice of Medicinal Chemistry, Camille G. Wermuth et al., Ch 31, (Academic Press, 1996); (b) Design of Prodrugs, edited by H. Bundgaard, (Elsevier, 1985); (c) A Textbook of Drug Design and Development, P. Krogsgaard-Larson and H. Bundgaard, eds. Ch 5, pgs 113-191 (Harwood Academic Publishers, 1991); and (d) Hydrolysis in Drug and Prodrug Metabolism, Bernard Testa and Joachim M. Mayer, (Wiley-VCH, 2003).
- the term “pharmaceutically acceptable”refers to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
- pharmaceutically acceptable saltrefers to a salt of the administered compounds prepared from pharmaceutically acceptable non-toxic acids, including inorganic acids, organic acids, solvates, hydrates, or clathrates thereof.
- an “antibiotic” or “antibiotic agent”refers to a substance that has the capacity to inhibit or slow down the growth of or destroy bacteria and/or other microorganisms.
- the antibiotic agentis a bacteriostatic antibiotic agent.
- the antibioticis a bacteriolytic antibiotic agent.
- Non-limiting examples of antibiotic agentsare set forth in the U.S. Patent Publication US 2006/0269485, which is herein incorporated by reference in its entirety.
- the antibiotic agentis selected from the classes including betalactam antibiotics, aminoglycosides, ansa-type antibiotics, anthraquinones, antibiotic azoles, antibiotic glycopeptides, macrolides, antibiotic nucleosides, antibiotic peptides, antibiotic polyenes, antibiotic polyethers, quinolones, antibiotic steroids, sulfonamides, tetracycline, dicarboxylic acids, antibiotic metals, oxidizing agents, substances that release free radicals and/or active oxygen, cationic antimicrobial agents, quaternary ammonium compounds, biguanides, triguanides, bisbiguanides and analogs and polymers thereof and naturally occurring antibiotic compounds.
- betalactam antibioticsaminoglycosides, ansa-type antibiotics, anthraquinones, antibiotic azoles, antibiotic glycopeptides, macrolides, antibiotic nucleosides, antibiotic peptides, antibiotic polyenes, antibiotic polyethers, quinolones, antibiotic steroids, s
- Non-limiting examples of beta-lactam antibioticsmay include 2-(3-alanyl)clavam, 2- hydroxymethylclavam, 8-epi-thienamycin, acetyl-thienamycin, amoxicillin, amoxicillin sodium, amoxicillin trihydrate, amoxicillin-potassium clavulanate combination, ampicillin, ampicillin sodium, ampicillin trihydrate, ampicillin-sulbactam, apalcillin, aspoxicillin, azidocillin, azlocillin, aztreonam, bacampicillin, biapenem, carbenicillin, carbenicillin disodium, carfecillin, carindacillin, carpetimycin, cefacetril, cefaclor, cefadroxil, cefalexin, cefaloridine, cefalotin, cefamandole, cefamandole, cefapirin, cefatrizine, cefatrizine propylene glycol, cefazedone,
- antibioticsmay include ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
- antibioticsmay include ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- antibioticsmay include (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
- the purified dietmay include a ketogenic diet, a control purified diet, or a combination thereof.
- purified dietrefers to a diet that consists substantially of purified and/or processed food components, as opposed to unprocessed food items (such as fruits, vegetables, whole grains, meats, eggs).
- Purified componentsrefer to simple sugars, simple refined carbohydrates from which substantial chemical complexity has been intentionally removed (e.g., white flour, white rice), mixtures of fats (such as oils, butters, or creams), particular fibers (e.g., purified cellulose or insulin), or particular proteins or mixtures of proteins (e.g., casein, whey) purified from more complex starting materials (such as corn, fruit, whole grains, beans, milk, or meats).
- the purified componentsmay be combined together, optionally with water, prior to being fed to a mammal, e.g., in the form of a protein shake or baked good.
- Processed food componentsrefer to foods comprising a substantial amount (e.g., greater than 50%, 75%, or 90%) of purified components, mixed together optionally with non-purified components.
- a processed foodwould be a cake containing sucrose (table sugar, a purified component), butter (a purified component), white flour (a purified component), and eggs (an unprocessed food item).
- the fraction of purified componentsmay be greater than 25%, 50%, 75%, or 90% of total food intake.
- the fraction of purified or processed food componentsmay be greater than 50%, 75%, 90%, or 95% of total food intake. Fraction of purified components can optionally be measured by mass, by dry mass, or by calories.
- ketogenic dietrefers to a high fat and low saccharide diet, which is utilized as a diet for treating patients requiring saccharide-restrictive diets, for example, children with epilepsy.
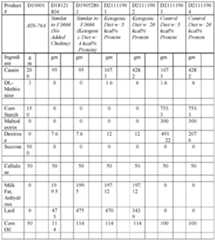
- Non-limiting examples of the ketogenic dietmay include Ketogenic diet (KD, also referred to as KD-LP) - D21111901Mi and Keto normal protein (KD-NP) - D21111902Mi (RESEARCH DIETS) as set forth in Table 1.
- the ketogenic dietmay include 20 wt%, 10 wt%, 5 wt%, or less of proteins. In some embodiments, the ketogenic diet may include about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
- control purified dietrefers to high protein and high carbohydrate diets.
- Nonlimiting examples of the control purified dietmay include Control purified diet (CPD) - D21111904i, High cellulose with 0% Inulin (HC_InO) - D22040601i, High cellulose with 1.9% Inulin (HC Ini.9) - D22040602i, High cellulose with 1.9% Inulin (HC Ini.9) - D22040602i, High cellulose with 8% Inulin (HC_In8) - D22040603i, and Low cellulose with 2.5% Inulin (LC_In2.5) - D22011102i, as set forth in Table 1.
- control purified dietmay include from about 5 wt% to about 40 wt% (e.g, 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%, 10 wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%, 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19 wt%, 20 wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%, 30 wt%, 31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39 wt%, 40 wt%) of proteins.
- wt%e.g,
- control purified dietmay include about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
- Nonlimiting examples of the control purified dietmay include those described in Table 1 in Example 1 of this disclosure.
- cancerrefers to all types of cancer, neoplasm or malignant tumors found in mammals, including leukemias, lymphomas, melanomas, neuroendocrine tumors, carcinomas, and sarcomas.
- Exemplary cancers that may be treated with a compound, pharmaceutical composition, or method provided hereininclude lymphoma, sarcoma, bladder cancer, bone cancer, brain tumor, cervical cancer, colon cancer, esophageal cancer, gastric cancer, head and neck cancer, kidney cancer, myeloma, thyroid cancer, leukemia, prostate cancer, breast cancer (e.g.
- ovarian cancerpancreatic cancer, liver cancer (e.g., hepatocellular carcinoma), lung cancer ( .g., non-small cell lung carcinoma, squamous cell lung carcinoma, adenocarcinoma, large cell lung carcinoma, small cell lung carcinoma, carcinoid, sarcoma), glioblastoma multiforme, glioma, melanoma, prostate cancer, castration-resistant prostate cancer, breast cancer, triple negative breast cancer, glioblastoma, ovarian cancer, lung cancer, squamous cell carcinoma (e.g., head, neck, or esophagus), colorectal cancer, leukemia, acute myeloid leukemia, lymphoma, B cell lymphoma, or multiple myeloma.
- liver cancere.g., hepatocellular carcinoma
- lung cancer.g., non-small cell lung carcinoma, squamous cell lung carcinoma, adenocarcinoma, large cell lung carcinoma,
- Additional examplesinclude, cancer of the thyroid, endocrine system, brain, breast, cervix, colon, head & neck, esophagus, liver, kidney, lung, non-small cell lung, melanoma, mesothelioma, ovary, sarcoma, stomach, uterus or Medulloblastoma, Hodgkin’s Disease, Non-Hodgkin’s Lymphoma, multiple myeloma, neuroblastoma, glioma, glioblastoma multiforme, ovarian cancer, rhabdomyosarcoma, primary thrombocytosis, primary macroglobulinemia, primary brain tumors, cancer, malignant pancreatic insulanoma, malignant carcinoid, urinary bladder cancer, premalignant skin lesions, testicular cancer, lymphomas, thyroid cancer, neuroblastoma, esophageal cancer, genitourinary tract cancer, malignant hypercalcemia, endometrial
- solid tumorrefers to an abnormal mass of tissue that usually does not contain cysts or liquid areas. Solid tumors may be benign or malignant.
- solid tumor cancerrefers to malignant, neoplastic, or cancerous solid tumors. Solid tumor cancers include, but are not limited to, sarcomas, carcinomas, and lymphomas, such as cancers of the lung, breast, triple-negative breast cancer, prostate, colon, rectum, and bladder.
- the canceris selected from cervical cancer, head and neck cancer (including, for example, head and neck squamous cell carcinoma (HNSCC)) glioblastoma, ovarian cancer, sarcoma, pancreatic cancer, bladder cancer, breast cancer, triple-negative breast cancer, and non-small cell lung carcinoma.
- the tissue structure of solid tumorsincludes interdependent tissue compartments, including the parenchyma (cancer cells) and the supporting stromal cells in which the cancer cells are dispersed and which may provide a supporting microenvironment.
- cancermay be chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), non-Hodgkin’ s lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), Hodgkin’s lymphoma, B cell acute lymphoblastic leukemia (B-ALL), Burkitt’s lymphoma, Waldenstrom’s macroglobulinemia (WM), Burkitt’s lymphoma, multiple myeloma, or myelofibrosis.
- CLLchronic lymphocytic leukemia
- SLLsmall lymphocytic leukemia
- NHLdiffuse large B cell lymphoma
- FLfollicular lymphoma
- MCLmantle cell lymphoma
- NHLB cell acute lymphoblastic leukemia
- WMWaldenstrom’s macroglobulinemia
- cancermay be a solid tumor selected from the group consisting of bladder cancer, non-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glioma, gastrointestinal stromal tumor, breast cancer, lung cancer, colorectal cancer, thyroid cancer, bone sarcoma, stomach cancer, oral cavity cancer, oropharyngeal cancer, gastric cancer, kidney cancer, liver cancer, prostate cancer, colorectal cancer, esophageal cancer, testicular cancer, gynecological cancer, thyroid cancer, colon cancer, primary central nervous system lymphoma, and brain cancer.
- bladder cancernon-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glio
- the terms “treating,” “treat,” and “treatment”include preventing a disease, pathologic or medical condition from occurring (e.g., prophylaxis); inhibiting the disease, pathologic or medical condition or arresting its development; relieving or ameliorating the disease, pathologic or medical condition; and/or diminishing symptoms associated with the disease, pathologic or medical condition.
- the terms “treat,” “treatment,” and “treating”can extend to prophylaxis and can include preventing, prevention, lowering, stopping, or reversing the progression or severity of the condition or symptoms being treated.
- the term “treatment”can include medical, therapeutic, and/or prophylactic administration, as appropriate.
- the term “treating” or “treatment”thus can include reversing, reducing, or arresting the symptoms, clinical signs, and underlying pathology of a condition in a manner to improve or stabilize a subject’s condition.
- administeringrefers to the delivery of cells by any route including, without limitation, oral, intranasal, intraocular, intravenous, intraosseous, intraperitoneal, intraspinal, intramuscular, intra-articular, intraventricular, intracranial, intralesional, intratracheal, intrathecal, subcutaneous, intradermal, transdermal, or transmucosal administration.
- the agentis administered to the subject intratumorally, intravenously, subcutaneously, intraosseously, orally, transdermally, sublingually, in sustained release, in controlled release, in delayed release, or as a suppository.
- a compound of the present inventionmay be administered with another therapeutic agent simultaneously or sequentially in separate unit dosage forms or together in a single unit dosage form.
- an “effective amount”refers to an amount effective to treat a disease, disorder, and/or condition, or to bring about a recited effect.
- an effective amountcan be an amount effective to reduce the progression or severity of the condition or symptoms being treated. Determination of a therapeutically effective amount is well within the capacity of persons skilled in the art.
- the term “effective amount”is intended to include an amount of a compound described herein, or an amount of a combination of compounds described herein, e.g., that is effective in treating or preventing a disease or disorder, or treating the symptoms of the disease or disorder, in a host.
- an “effective amount”generally means an amount that provides the desired effect.
- a “therapeutically effective amount” of a compound with respect to the subject method of treatmentrefers to an amount of the compound in a preparation which, when administered as part of a desired dosage regimen (to a mammal, e.g., a human), alleviates a symptom, ameliorates a condition, or slows the onset of disease conditions according to clinically acceptable standards for the disorder or condition to be treated, e.g. , at a reasonable benefit/risk ratio applicable to any medical treatment.
- the actual dosage amount of a composition of this disclosure administered to a subjectcan be determined by physical and physiological factors such as body weight, severity of condition, the type of disease being treated, previous or concurrent therapeutic interventions, idiopathy of the patient, and on the route of administration.
- the practitioner responsible for administrationwill, in any event, determine the concentration of active ingredient(s) in a composition and appropriate dose(s) for the individual subject.
- the PI3K inhibitormay be administered at one or more doses of from about 5 mg/kg to about 200 mg/kg of body weight of the subject, such as about 5 mg/kg of body weight, about 10 mg/kg of body weight, about 20 mg/kg of body weight, about 30 mg/kg of body weight, about 40 mg/kg of body weight, about 50 mg/kg of body weight, about 75 mg/kg of body weight, about 100 mg/kg of body weight, about 125 mg/kg of body weight, about 150 mg/kg of body weight, about 175 mg/kg of body weight, about 200 mg/kg of body weight, or any range derivable therein.
- the antibioticsmay be administered at one or more doses of from about 10 mg/kg to about 500 mg/kg of body weight of the subject, such as about 10 mg/kg of body weight, about 20 mg/kg of body weight, about 30 mg/kg of body weight, about 40 mg/kg of body weight, about 50 mg/kg of body weight, about 75 mg/kg of body weight, about 100 mg/kg of body weight, about 125 mg/kg of body weight, about 150 mg/kg of body weight, about 175 mg/kg of body weight, about 200 mg/kg of body weight, about 250 mg/kg of body weight, about 300 mg/kg of body weight, about 350 mg/kg of body weight, about 400 mg/kg of body weight, about 450 mg/kg of body weight, about 500 mg/kg of body weight, or any range derivable therein.
- the purified dietmay be administered at one or more doses of from about 10,000 mg/kg to about 500,000 mg/kg of body weight of the subject, such as 10,000 mg/kg of body weight, 11,000 mg/kg of body weight, 12,000 mg/kg of body weight, 13,000 mg/kg of body weight, 14,000 mg/kg of body weight, 15,000 mg/kg of body weight, 16,000 mg/kg of body weight, 17,000 mg/kg of body weight, 18,000 mg/kg of body weight, 19,000 mg/kg of body weight, 20,000 mg/kg of body weight, 21,000 mg/kg of body weight, 22,000 mg/kg of body weight, 23,000 mg/kg of body weight, 24,000 mg/kg of body weight, 25,000 mg/kg of body weight, 26,000 mg/kg of body weight, 27,000 mg/kg of body weight, 28,000 mg/kg of body weight, 29,000 mg/kg of body weight, 30,000 mg/kg of body weight, 31 ,000 mg/kg of body weight, 32,000 mg/kg of body weight, 33,000 mg/kg of body weight, 1
- a dosemay be presented in a single dose or as divided doses administered at appropriate intervals, for example, as two, three, four or more sub-doses per day.
- the sub-dose itselfmay be further divided, e.g., into a number of discrete, loosely spaced administrations.
- one or more doses of PI3K inhibitors, antibiotics, or purified dietsmay be administered at least every 1 day, every 2 days, every 3 days, every 4 days, every 5 days, every 6 days, every 7 days, every 8 days, every 9 days, every 10 days, every 11 days, every 12 days, every 13 days, every 14 days, every 15 days, every 16 days, every 17 days, every 18 days, every 19 days, every 20 days, every 21 days, every 22 days, every 23 days, every 24 days, every 25 days, every 26 days, every 27 days, every 28 days, every 29 days, every 30 days, every 31 days, every 32 days, every 33 days, every 34 days, every 35 days, every 36 days, every 37 days, every 38 days, every 39 days, every 40 days, every 41 days, every 42 days, every 43 days, every 44 days, every 45 days, every 46 days, every 47 days, every 48 days, every 49 days, every 50 days, every 51 days, every 52 days, every 53 days, every 54 days, every 55 days, every 56 days,
- the methodmay include administering to the subject an additional therapeutic agent or therapy.
- the additional therapeutic agentmay include a second PI3K inhibitor, a second antibiotic, a second purified diet, or a combination thereof.
- Combination therapyis meant to encompass administration of two or more therapeutic agents in a coordinated fashion and includes, but is not limited to, concurrent dosing.
- combination therapyencompasses both co-administration (e.g., administration of a co-formulation or simultaneous administration of separate therapeutic compositions) and serial or sequential administration, provided that administration of one therapeutic agent is conditioned in some way on the administration of another therapeutic agent.
- one therapeutic agentmay be administered only after a different therapeutic agent has been administered and allowed to act for a prescribed period of time. See, e.g., Kohrt et al. (2011) Blood 117:2423.
- co-administrationrefers to the administration of at least two agent(s) or therapies to a subject. In some embodiments, the coadministration of two or more agents/therapies is concurrent. In some embodiments, a first agent/therapy is administered prior to a second agent/therapy.
- PI3K inhibitorPI3K inhibitor, antibiotic, purified diet
- active ingredientse.g., antibiotic, purified diet
- the combination therapymay be administered as a simultaneous or sequential regimen.
- the combinationmay be administered in two or more administrations.
- the combination therapymay provide synergy and be synergistic, i.e., the effect achieved when the active ingredients used together are greater than the sum of the effects that result from using the compounds separately.
- a synergistic effectmay be attained when the active ingredients are: (1) co-formulated and administered or delivered simultaneously in a combined formulation; (2) delivered by alternation or in parallel as separate formulations; or (3) by some other regimen.
- a synergistic effectmay be attained when the compounds are administered or delivered sequentially, e.g., in separate tablets, pills, capsules, or by different injections in separate syringes.
- an effective dosage of each active ingredientis administered sequentially, i.e., serially, whereas, in combination therapy, effective dosages of two or more active ingredients are administered together.
- a synergistic effectdenotes an effect that is greater than the predicted purely additive effects of the individual compounds of the combination.
- Combination therapyis further described by U.S. Pat. Nos. 11103514, 10702495, 9382215, and 6833373, which include additional active agents that can be combined with the compounds described herein, and additional types of ailments and other conditions that can be treated with a compound or combination of compounds described herein.
- An active agentmay precede or follow treatment of the other agent by intervals ranging from minutes to weeks.
- the other agent and expression constructare applied separately to a cell, one would generally ensure that a significant period of time did not elapse between the time of each delivery, such that the agent and expression construct would still be able to exert an advantageously combined effect on the cell.
- one or more agentsmay be administered within about 1 minute, about 5 minutes, about 10 minutes, about 20 minutes, about 30 minutes, about 45 minutes, about 60 minutes, about 2 hours, about 3 hours, about 4 hours, about 6 hours, about 8 hours, about 9 hours, about 12 hours, about 15 hours, about 18 hours, about 21 hours, about 24 hours, about 28 hours, about 31 hours, about 35 hours, about 38 hours, about 42 hours, about 45 hours, to about 48 hours or more prior to and/or after administering the disclosed active agent.
- an agentmay be administered within from about 1 day, about 2 days, about 3 days, about 4 days, about 5 days, about 6 days, about 8 days, about 9 days, about 12 days, about 15 days, about 16 days, about 18 days, about 20 days, to about 21 days prior to and/or after administering the disclosed active.
- itmay be desirable to extend the time period for treatment significantly; however, where several weeks (e.g., about 1, about 2, about 3, about 4, about 6, or about 8 weeks or more) lapse between the respective administrations.
- compositions to a patientwill follow general protocols for the administration of therapeutics, taking into account the toxicity, if any. It is expected that the treatment cycles will be repeated as necessary. It also is contemplated that various standard therapies or adjunct therapies, as well as surgical intervention, may be applied in combination with the described active agent. These therapies include but are not limited to chemotherapy, radiotherapy, immunotherapy, gene therapy, and surgery.
- compositioncomprising: (a) a PI3K inhibitor, a stereoisomer thereof, an analog thereof, a derivative thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof, and (b) one or more antibiotics or a purified diet.
- compositionor “pharmaceutical composition” refers to a mixture of at least one component useful within the disclosure with other components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, and/or excipients.
- the pharmaceutical compositionfacilitates administration of one or more components of the invention to an organism.
- the PI3K inhibitormay include Serabelisib, BEZ235, LY294002, GDC-0941, BYL719, GSK2636771, TGX-221, AS25242, CAL-101, IP1-145, MK-2206, GSK690693, GDC-0068, A-674563, CCT128930, AZD8O55, INK128, rapamycin, PF-04691502, everolimus, BI-D1870, H89, PF-4708671, FMK, AT7867, NU7441, PI-103, NU7026, PIK-75, ZSTK474, PP-121, or a combination thereof.
- the PI3K inhibitormay include a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor may include a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor may include BYL-719, BKM-120, or a combination thereof.
- antibioticsmay include ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
- one or more antibioticsmay include ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- one or more antibioticsmay include (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
- the purified dietmay include a ketogenic diet, a control purified diet, or a combination thereof.
- the ketogenic dietmay include Ketogenic diet (KD, also referred to as KD-LP) - D21111901Mi and Keto normal protein (KD-NP) - D21111902Mi (RESEARCH DIETS) as set forth in Table 1.
- the ketogenic dietmay include 20 wt%, 10 wt%, 5 wt%, or less of proteins.
- the ketogenic dietmay include about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
- Non-limiting examples of the control purified dietmay include Control purified diet (CPD) - D21111904i, High cellulose with 0% Inulin (HC_InO) - D2204060H, High cellulose with 1.9% Inulin (HC_Inl.9) - D22040602i, High cellulose with 1.9% Inulin (HC_Inl.9) - D22040602i, High cellulose with 8% Inulin (HC_In8) - D22040603i, and Low cellulose with 2.5% Inulin (LC_In2.5) - D22011102i, as set forth in Table 1.
- CPDControl purified diet
- HC_InOHigh cellulose with 0% Inulin
- HC_InOHigh cellulose with 1.9% Inulin
- HC_Inl.9High cellulose with 1.9% Inulin
- HC_In8High cellulose with 8% Inulin
- LC_In2.5Control purified diet
- control purified dietmay include from about 5 wt% to about 40 wt% (e.g., 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%, 10 wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%, 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19 wt%, 20 wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%, 30 wt%, 31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39 wt%, 40 wt%) of proteins.
- the agentscan be formulated in liquid solutions, e.g., in physiologically compatible buffers such as Hank’s solution or Ringer’s solution.
- the agentsmay be formulated in solid form and redissolved or suspended immediately prior to use. Lyophilized forms are also included.
- the pharmaceutical compositionmay be in the form of, for example, a tablet, capsule, liquid capsule, suspension, or liquid.
- the pharmaceutical compositionis, e.g., made in the form of a dosage unit containing a particular amount of the active ingredient.
- the pharmaceutical compositionmay be provided as a tablet or capsule comprising an amount of active ingredient in the range of from about 0.1 to 1000 mg, e.g., from about 0.25 to 250 mg, and, e.g., from about 0.5 to 100 mg.
- a suitable daily dose for a human or other mammalmay vary widely depending on the condition of the patient and other factors, but, can be determined using routine methods.
- any pharmaceutical composition contemplated hereincan, for example, be delivered orally via any acceptable and suitable oral preparations.
- Exemplary oral preparationsinclude, but are not limited to, for example, tablets, troches, lozenges, aqueous and oily suspensions, dispersible powders or granules, emulsions, hard and soft capsules, liquid capsules, syrups, and elixirs.
- Pharmaceutical compositions intended for oral administrationcan be prepared according to any methods known in the art for manufacturing pharmaceutical compositions intended for oral administration.
- a pharmaceutical composition in accordance with the disclosurecan contain at least one agent selected from sweetening agents, flavoring agents, coloring agents, demulcents, antioxidants, and preserving agents.
- a tabletcan, for example, be prepared by admixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one non-toxic pharmaceutically acceptable excipient suitable for the manufacture of tablets.
- excipientsinclude, but are not limited to, for example, inert diluents, such as, for example, calcium carbonate, sodium carbonate, lactose, calcium phosphate, and sodium phosphate; granulating and disintegrating agents, such as, for example, microcrystalline cellulose, sodium croscarmellose, corn starch, and alginic acid; binding agents, such as, for example, starch, gelatin, polyvinylpyrrolidone, and acacia; and lubricating agents, such as, for example, magnesium stearate, stearic acid, and talc.
- a tabletcan either be uncoated or coated by known techniques to either mask the bad taste of an unpleasant-tasting drug or delay disintegration and absorption of the active ingredient in the gastrointestinal tract, thereby sustaining the effects of the active ingredient for a longer period.
- exemplary water-soluble taste-masking materialsinclude, but are not limited to, hydroxypropyl-methylcellulose and hydroxypropyl-cellulose.
- Exemplary time delay materialsinclude, but are not limited to, ethylcellulose and cellulose acetate butyrate.
- Hard gelatin capsulescan, for example, be prepared by mixing at least one compound and/or at least one salt thereof with at least one inert solid diluent, such as, for example, calcium carbonate; calcium phosphate; and kaolin.
- Soft gelatin capsulescan, for example, be prepared by mixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one water-soluble carrier, such as, for example, polyethylene glycol, and at least one oil medium, such as, for example, peanut oil, liquid paraffin, and olive oil.
- at least one water-soluble carriersuch as, for example, polyethylene glycol
- at least one oil mediumsuch as, for example, peanut oil, liquid paraffin, and olive oil.
- An aqueous suspensioncan be prepared, for example, by admixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one excipient suitable for the manufacture of an aqueous suspension.
- excipients suitable for the manufacture of an aqueous suspensionmay include suspending agents, such as, for example, sodium carboxymethylcellulose, methyl cellulose, hydroxypropylmethylcellulose, sodium alginate, alginic acid, polyvinyl -pyrrolidone, gum tragacanth, and gum acacia; dispersing or wetting agents, such as, for example, a naturally-occurring phosphatide, e.g., lecithin; condensation products of alkylene oxide with fatty acids, such as, for example, polyoxyethylene stearate; condensation products of ethylene oxide with long-chain aliphatic alcohols, such as, for example, heptadecaethylene-oxycetanol; condensation products of ethylene oxide with partial esters derived from fatty acids
- An aqueous suspensioncan also contain at least one preservative, such as, for example, ethyl and n-propyl p-hydroxybenzoate; at least one coloring agent; at least one flavoring agent; and/or at least one sweetening agent, including but not limited to, for example, sucrose, saccharin, and aspartame.
- Formulations for parenteral administrationmay be in the form of aqueous or non-aqueous isotonic sterile injection solutions or suspensions. These solutions and suspensions may be prepared from sterile powders or granules using one or more of the carriers or diluents mentioned for use in the formulations for oral administration or by using other suitable dispersing or wetting agents and suspending agents.
- the compoundsmay be dissolved in water, polyethylene glycol, propylene glycol, ethanol, corn oil, cottonseed oil, peanut oil, sesame oil, benzyl alcohol, sodium chloride, tragacanth gum, and/or various buffers. Other adjuvants and modes of administration are well and widely known in the pharmaceutical art.
- the active ingredientmay also be administered by injection as a composition with suitable carriers, including saline, dextrose, or water, or with cyclodextrin i.e., Captisol), cosolvent solubilization (i.e., propylene glycol) or micellar solubilization (i.e., Tween 80).
- suitable carriersincluding saline, dextrose, or water, or with cyclodextrin i.e., Captisol), cosolvent solubilization (i.e., propylene glycol) or micellar solubilization (i.e., Tween 80).
- the sterile injectable preparationmay also be a sterile injectable solution or suspension in a non-toxic, parenterally acceptable diluent or solvent, for example, as a solution in 1,3 -butanediol.
- a non-toxic, parenterally acceptable diluent or solventfor example, as a solution in 1,3 -butanediol.
- acceptable vehicles and solventsthat may be employed are water, Ringer’s solution, and isotonic sodium chloride solution.
- sterile, fixed oilsare conventionally employed as a solvent or suspending medium.
- any bland fixed oilmay be employed, including synthetic mono- or diglycerides.
- fatty acidssuch as oleic acid find use in the preparation of injectables.
- Pharmaceutically acceptable carriers, adjuvants, and vehiclesthat may be used in the pharmaceutical compositions of this disclosure include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, self-emulsifying drug delivery systems (SEDDS) such as alpha-tocopherol polyethylene glycol 1000 succinate, surfactants used in pharmaceutical dosage forms such as Tweens, polyethoxylated castor oil, such as cremophor surfactant (BASF), or other similar polymeric delivery matrices, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances,
- Cyclodextrinssuch as alpha-, beta-, and gammacyclodextrin, or chemically modified derivatives such as hydroxyalkyl cyclodextrins, including 2- and 3-hydroxypropyl-cyclodextrins, or other solubilized derivatives may also be advantageously used to enhance delivery of compounds of the formulae described herein.
- the pharmaceutically active compounds of this disclosurecan be processed in accordance with conventional methods of pharmacy to produce medicinal agents for administration to patients, including humans and other mammals.
- the pharmaceutical compositionsmay be subjected to conventional pharmaceutical operations such as sterilization and/or may contain conventional adjuvants, such as additives, preservatives, stabilizers, wetting agents, emulsifiers, buffers etc. Tablets and pills can additionally be prepared with enteric coatings.
- Such compositionsmay also include adjuvants, such as wetting, sweetening, flavoring, and perfuming agents.
- compositions of this disclosuremay include at least one compound and/or at least one pharmaceutically acceptable salt thereof, and optionally an additional agent selected from any pharmaceutically acceptable carrier, adjuvant, and vehicle.
- Alternate compositions of this disclosuremay include a compound described herein, or a prodrug thereof, and a pharmaceutically acceptable carrier, adjuvant, or vehicle.
- kitscan include (a) a container that contains the composition and optionally (b) informational material.
- the informational materialcan be descriptive, instructional, marketing or other material that relates to the methods described herein and/or the use of the agents for therapeutic benefit.
- kitsmay include instructions for the manufacturing, the therapeutic regimen to be used, and periods of administration.
- the kitmay also include an additional therapeutic agent.
- the kitmay include one or more containers, each with a different reagent.
- the kitmay include a first container that contains the composition and a second container for the additional therapeutic agent.
- the containersmay include a unit dosage of the pharmaceutical composition.
- the kitcan include other ingredients, such as a solvent or buffer, an adjuvant, a stabilizer, or a preservative.
- the kitmay optionally include a device suitable for administration of the composition, e.g.. a syringe or other suitable delivery device.
- the devicemay be provided pre-loaded with one or both of the agents or can be empty, but suitable for loading.
- a “subject” or “subject in need thereof’refers to a human and a non-human animal.
- a non-human animalsinclude all vertebrates, e.g., mammals, such as non- human mammals, non-human primates (particularly higher primates), dog, rodent (e.g., mouse or rat), guinea pig, cat, and rabbit, and non-mammals, such as birds, amphibians, reptiles, etc.
- the subjectis a human.
- the subjectis an experimental animal or animal suitable as a disease model.
- the term “disease” as used hereinis intended to be generally synonymous, and is used interchangeably with, the terms “disorder” and “condition” (as in medical condition), in that all reflect an abnormal condition of the human or animal body or of one of its parts that impairs normal functioning, is typically manifested by distinguishing signs and symptoms, and causes the human or animal to have a reduced duration or quality of life.
- agentis used herein to denote a chemical compound, a mixture of chemical compounds, a biological macromolecule (such as a nucleic acid, an antibody, a protein or portion thereof, e.g. , a peptide), or an extract made from biological materials such as bacteria, plants, fungi, or animal (particularly mammalian) cells or tissues. The activity of such agents may render it suitable as a “therapeutic agent,” which is a biologically, physiologically, or pharmacologically active substance (or substances) that acts locally or systemically in a subject.
- therapeutic agentrefers to a molecule or compound that confers some beneficial effect upon administration to a subject.
- the beneficial effectincludes enablement of diagnostic determinations; amelioration of a disease, symptom, disorder, or pathological condition; reducing or preventing the onset of a disease, symptom, disorder, or condition; and generally counteracting a disease, symptom, disorder, or pathological condition.
- samplecan be a sample of serum, urine plasma, amniotic fluid, cerebrospinal fluid, cells (e.g., antibody-producing cells), or tissue.
- cellse.g., antibody-producing cells
- tissuee.g., tissue
- samplecan be used directly as obtained from a patient or can be pre-treated, such as by filtration, distillation, extraction, concentration, centrifugation, inactivation of interfering components, addition of reagents, and the like, to modify the character of the sample in some manner as discussed herein or otherwise as is known in the art.
- sample and biological sampleas used herein, generally refer to a biological material being tested for and/or suspected of containing an analyte of interest.
- the samplemay be any tissue sample from the subject.
- the samplemay comprise protein from the subject.
- inhibitorand “antagonize,” as used herein, mean to reduce a molecule, a reaction, an interaction, a gene, an mRNA, and/or a protein’s expression, stability, function, or activity by a measurable amount or to prevent entirely.
- Inhibitorsare compounds that, e.g., bind to, partially or totally block stimulation, decrease, prevent, delay activation, inactivate, desensitize, or down- regulate a protein, a gene, and an mRNA stability, expression, function, and activity, e.g., antagonists.
- the term “z/z vitro”refers to events that occur in an artificial environment, e.g., in a test tube or reaction vessel, in cell culture, etc., rather than within a multi-cellular organism.
- in vivorefers to events that occur within a multi-cellular organism, such as a non-human animal.
- the term “approximately” or “about”refers to a range of values that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction (greater than or less than) of the stated reference value unless otherwise stated or otherwise evident from the context (except where such number would exceed 100% of a possible value).
- the term “about”is intended to include values, e.g., weight percents, proximate to the recited range that are equivalent in terms of the functionality of the individual ingredient, the composition, or the embodiment.
- eachwhen used in reference to a collection of items, is intended to identify an individual item in the collection but does not necessarily refer to every item in the collection. Exceptions can occur if explicit disclosure or context clearly dictates otherwise.
- miceMouse husbandry and PDAC tumor models. All mouse work was approved by the Institute Animal Care and Use Committee (IACUC) at Princeton University. Wild type C57BL/6 mice were obtained at 6 to 8 weeks of age from Charles River Laboratories, and tumor inoculations were performed up to a few weeks after arrival. Mice were housed under normal light cycle (7:00- 19:00) and fed chow diet ad lib (PicoLab Rodent 205053*, LabDiet) and had free access to reverse osmosis drinking water until experiments began. For experiments using the tumor chunk model (Fig.
- syngeneic PDAC allograft tumorswere established by harvesting tumors from KPC (LSL- Kras G12L) , p53 R172H , Pdx-Cre) mice, mincing the tissue with surgical scissors into ⁇ 3mm pieces able to pass through a 16g needle, suspending in DMEM medium, mixing with Matrigel (Corning Cat #354234) at a 1 : 1 ratio (v/v), and then injecting 200ul of the mixture subcutaneously into the mouse flank (Yang, L. et al. Med 3, 119-136. e8 (2022)).
- KPC K8484 cell linewas used for the rest of the experiments.
- Cellswere harvested with trypsin, counted in DMEM, suspended in PBS, and mixed at a 1 : 1 ratio with Matrigel. 0.5-1 * 10 6 cells were injected subcutaneously into mouse flanks in a volume of 100-200 pl.
- ketogenic dietall mice were fasted overnight one day before the dietary change.
- Antibioticswere administered in the drinking water as a cocktail of ampicillin (1 g/L), neomycin (1 g/L), metronidazole (1 g/L), and vancomycin (0.5 g/L) or as individual antibiotics. To make the drinking water more palatable, 5% aspartame was added. Drinking water with 5% aspartame was used as control. Table 1. Example purified diets and their ingredients
- PI3K inhibitors BYL-719 (BYL) and BKM-120 (BKM)were dissolved in 0.5% carboxymethylcellulose, sonicated, and given by daily oral gavage (lOul/gr) for 5 days a week (5 days on, 2 days off).
- BYL dosewas 45 mg/kg
- BKM dosewas 25 mg/kg.
- Tumor volumeswere measured twice a week with a caliper, and tumor size was calculated by the formula: 0.5 x (Length x Width x Height).
- micewere fasted ⁇ 2h before drug gavage, and food was put back after the 4h time point when mice were entering the dark cycle.
- PKpharmacokinetics
- Metabolite extraction for LC-MSFor serum samples, 3 pl samples were mixed 117 pl with methanol, vortexed for 30 seconds, and centrifuged at 17,000 g for 10 min at 4°C.
- For fecal samples10-20 mg of fecal pellets were weighed and ground under liquid nitrogen using a cryomill (Restch, Newtown, PA). Next, x40 volume (40 pl extraction solvent per 1 mg tissue) of 40:40:20 methanol: acetonitrile: water with 0.5% formic acid pre-cooled to -20°C was added to the fecal powder, vortexed and incubated on ice for 10 min.
- Water soluble metabolite measurementswere obtained by running samples on Orbitrap Exploris 240 mass spectrometer (Thermo Scientific) coupled with hydrophilic interaction chromatography (HILIC) with electrospray ionization.
- LC separationwas performed on an XBridge BEH Amide column (2.13150 mm, 2.5 pm particle size, 130 A pore size; Waters Corporation) using a gradient of solvent A (95:5 water: acetonitrile with 20 mM of ammonium acetate and 20 mM of ammonium hydroxide, pH 9.45) and solvent B (acetonitrile).
- solvent A95:5 water: acetonitrile with 20 mM of ammonium acetate and 20 mM of ammonium hydroxide, pH 9.45
- solvent Bacetonitrile
- the LC gradientwas: 0 min, 90% B; 2 min, 90% B; 3 min, 75%; 7 min, 75% B; 8 min, 70%; 9 min, 70% B; 10 min, 50% B; 12 min, 50% B; 13 min, 25% B; 14 min, 25% B; 16 min, 0.5% B, 20.5 min, 0.5% B; 21 min, 90% B; 25 min, 90% B.
- Injection volumewas 5-10 pl, and autosampler temperature was 4°C.
- MS scanswere in negative and positive ion switching mode with a resolution of 120,000 at m/z 200 and a scan range of m/z 70-1000 for neg mode and m/z 58-116.5 and m/z 120-1000 for pos mode. Mass spec data was analyzed using El-Maven (v0.12.0, Elucidata).
- 16S sequencingBacterial DNA was extracted from fecal samples using the Power Soil DNA Isolation kit (QIAGEN). A section of the 16S rRNA gene (-250 bp, V4 region) was amplified, and Illumina sequencing libraries were prepared from these amplicons according to previously published protocols and primers. Libraries were further pooled together at equal molar ratios and sequenced on an Illumina HiSeq 2500 Rapid Flowcell or MiSeq as paired-end reads. These reads were 2x150 bp with an average depth of -20,000 reads. Also included were 8 bp index reads, following the manufacturer’s protocol (Illumina, USA).
- Pass-Filter readswere generated from raw sequencing reads using Illumina HiSeq Control Software. Samples were de-multiplexed using the index reads. The DADA2 plugin within QIIME2 version 2018.6 was used to infer Amplicon sequencing variants (ASVs) from the unmerged paired-end sequences. The forward reads were trimmed at 150 bp, and the reverse reads were trimmed at 140 bp, with all other DADA2 as default. Taxonomy was assigned to the resulting ASVs with a naive Bayes classifier trained on the Greengenes database version 13.8, with only the target region of the 16S rRNA gene used to train the classifier. Downstream analyses were performed with MATLAB.
- PI3K inhibition efficacywas tested in a pancreatic KPC allograft model. Notably, BYL showed a similar degree of tumor growth inhibition in all four diets. Unexpectedly, similar efficacy was seen even with the high carb 20% protein diet (Fig. 1), despite the macronutrient composition in this diet being similar to the chow used previously (Hopkins, B. D et al.
- a major difference between grain-based chow and purified dietsis dietary fiber, which is typically high in chow and low in the purified diets (Pellizzon, M. A. & Ricci, M. R. Nutr. Metab. 15, 1-6 (2018)). Indeed, while the chow that was used contained 15.5% (w/w) insoluble fiber and -1.9% soluble fiber, CPD had 2.6% insoluble fiber (as cellulose) and no soluble fiber. To test the importance of fiber on the interplay of PI3K inhibition with purified diets, insoluble fiber was added to a similar level as chow and different amounts of soluble fiber back to CPD, and the effect of BYL treatment on KPC allografts was monitored.
- mice bearing KPC allograftswere fed either chow or CPD and were treated with an antibiotics cocktail which ablates the gut microbiome or control, followed by BYL administration.
- chow-fed mice receiving antibiotics and BYLshowed complete tumor control, similar to mice on CPD with or without antibiotics (Fig. 4A).
- BYL pharmacokinetics (PK) under these conditionswere also monitored, and it was found that similar to CPD, antibiotics treatment led to increased peak, and most notably trough serum BYL levels (Figs. 4C-D).
- antibiotics alone without BYL administrationdid not affect tumor growth, ruling out a direct effect of antibiotics on tumor growth (Fig. 4B).
- each of the antibiotics used in the antibiotics cocktailwas individually tested for its ability to enhance BYL efficacy. It was found that ampicillin, but not the three other antibiotics tested, enhanced BYL efficacy to the same levels as the antibiotics cocktail (Fig. 5A). BYL was better tolerated with ampicillin than with the antibiotics cocktail, as depicted by body weight profile over the course of the experiment (Fig. 5B). BYL PK was enhanced by ampicillin, although to a lesser extent than the antibiotics cocktail (Figs. 5C-D).
- This exampleshows purified diets and antibiotics, such as ampicillin, significantly enhance PI3K inhibition efficacy in pancreatic ductal adenocarcinoma (PDAC) mouse models, as demonstrated for the FDA-approved PI3K-alpha-specific BYL-719 (alpelisib) and for the pan- PI3K inhibitor BKM-120 that is now undergoing phase III clinical trials.
- PDACpancreatic ductal adenocarcinoma
- the resultsindicate that manipulating the gut microbiome composition by purified diets or antibiotics enhances PI3K efficacy by improving drug pharmacokinetics.
- the combination of PI3K inhibitors with purified diets or ampicillinremains efficacious and tolerable over time.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Oncology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Hematology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
CANCER TREATMENT USING PI3K INHIBITORS IN COMBINATION WITH ANTIBIOTICS OR PURIFIED DIETS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority under 35 U.S.C. §119(e) to U.S. Provisional Patent Application No. 63/482,214, filed January 30, 2023. The foregoing application is incorporated by reference herein in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
This invention was made with government support under Grant No. CA163591 awarded by the National Institutes of Health. The government has certain rights in the invention.
FIELD OF THE INVENTION
This invention relates generally to methods for cancer treatment using PI3K inhibitors in combination with one or more antibiotics or purified diets.
BACKGROUND OF THE INVENTION
The phosphatidylinositol 3-kinase (PI3K) signaling pathway plays a crucial role in cellular growth and proliferation and is one of the most frequently activated pathways in cancers. Pancreatic ductal adenocarcinoma (PDAC) is a particularly devastating cancer with low survival rates and has been shown to rely heavily on the PI3K pathway. Significant efforts have been made to develop drugs that target PI3K, with five inhibitors currently approved by the FDA, mainly targeting PI3K-delta for hematological malignancies. Many more PI3K inhibitors have undergone clinical trials for various cancers, including solid tumors. However, despite the promising therapeutic potential of targeting this pathway, PI3K inhibitors show poor and variable responses against solid tumors in the clinic, often due to drug resistance and low drug tolerance. That said, one PI3K inhibitor, BYL-719 (alpelisib), was approved for the treatment of hormone receptorpositive (HER2) tumors, but even in combination with hormone receptor blockade, a majority of patients progressed within 12 months, and many patients experienced side effects including cutaneous reactions and hyperglycemia. A major challenge is finding ways to sensitize tumors to PI3K inhibitors and improve drug tolerability.
Therefore, there is a pressing need for improved therapies for cancer treatment. SUMMARY OF THE INVENTION
This disclosure addresses the need mentioned above in a number of aspects. In one aspect, the disclosure provides a method of treating a cancer. In some embodiments, the method comprises administering to a subject in need thereof (i) a therapeutically effective amount of a phosphoinositide 3-kinase (PI3K) inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (ii) an effective amount of one or more antibiotics or a purified diet.
In some embodiments, the one or more antibiotics comprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
In some embodiments, the one or more antibiotics comprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
In some embodiments, the one or more antibiotics comprise (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
In some embodiments, the one or more antibiotics are administered at one or more doses of from about 5 mg/kg to about 500 mg/kg of body weight of the subject.
In some embodiments, the purified diet comprises a ketogenic diet, a control purified diet, or a combination thereof. In some embodiments, the ketogenic diet comprises 20 wt%, 10 wt%, 5 wt%, or less of proteins. In some embodiments, the ketogenic diet comprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
In some embodiments, the control purified diet comprises from about 5 wt% to about 40 wt% of proteins. In some embodiments, the control purified diet comprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates. In some embodiments, the purified diet is administered at one or more doses of from about 10,000 mg/kg to about 500,000 mg/kg of body weight of the subject.
In some embodiments, the PI3K inhibitor comprises a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor comprises a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
In some embodiments, the PI3K inhibitor is administered at one or more doses of from about 1 mg/kg to about 200 mg/kg of body weight of the subject.
In some embodiments, the PI3K inhibitor is administered to the subject before or after the one or more antibiotics or the purified diet. In some embodiments, the PI3K inhibitor is administered to the subject concurrently with the one or more antibiotics or the purified diet.
In some embodiments, the PI3K inhibitor, the one or more antibiotics, or the purified diet are administered at least every 1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, or 4 weeks.
In some embodiments, the PI3K inhibitor or the one or more antibiotics are administered to the subject intratumorally, intravenously, subcutaneously, intraosseously, orally, transdermally, sublingually, in sustained release, in controlled release, in delayed release, or as a suppository.
In some embodiments, the PI3K inhibitor is contained in the same composition with the one or more antibiotics or the purified diet.
In some embodiments, the cancer is selected from the group consisting of chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), non-Hodgkin’s lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), Hodgkin’s lymphoma, B cell acute lymphoblastic leukemia (B-ALL), Burkitt’s lymphoma, Waldenstrom’s macroglobulinemia (WM), Burkitt’s lymphoma, multiple myeloma, and myelofibrosis.
In some embodiments, the cancer is a solid tumor selected from the group consisting of bladder cancer, non-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glioma, gastrointestinal stromal tumor, breast cancer, lung cancer, colorectal cancer, thyroid cancer, bone sarcoma, stomach cancer, oral cavity cancer, oropharyngeal cancer, gastric cancer, kidney cancer, liver cancer, prostate cancer, colorectal cancer, esophageal cancer, testicular cancer, gynecological cancer, thyroid cancer, colon cancer, primary central nervous system lymphoma, and brain cancer.
In some embodiments, the subject is a mammal. In some embodiments, the subject is a human.
In some embodiments, the method further comprises administering to the subject an additional therapeutic agent or therapy. In some embodiments, the additional therapeutic agent comprises a second PI3K inhibitor, a second antibiotic, a second purified diet, or a combination thereof.
Also within the scope of this disclosure is use of a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof in combination with one or more antibiotics or a purified diet in a method described herein.
In another aspect, this disclosure provides a pharmaceutical composition comprising: (a) a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof, and (b) one or more antibiotics or a purified diet.
In some embodiments, the one or more antibiotics comprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
In some embodiments, the one or more antibiotics comprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
In some embodiments, the one or more antibiotics comprise (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
In some embodiments, the purified diet comprises a ketogenic diet, a control purified diet, or a combination thereof. In some embodiments, the ketogenic diet comprises 20 wt%, 10 wt%, 5 wt%, or less of proteins. In some embodiments, the ketogenic diet comprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates. In some embodiments, the control purified diet comprises from about 5 wt% to about 40 wt% of proteins. In some embodiments, the control purified diet comprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
In some embodiments, the PI3K inhibitor comprises a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor comprises a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
In another aspect, this disclosure further provides a kit comprising the pharmaceutical composition described herein.
The foregoing summary is not intended to define every aspect of the disclosure, and additional aspects are described in other sections, such as the following detailed description. The entire document is intended to be related as a unified disclosure, and it should be understood that all combinations of features described herein are contemplated, even if the combinations of features are not found together in the same sentence, or paragraph, or section of this document. Other features and advantages of the invention will become apparent from the following detailed description. It should be understood, however, that the detailed description and the specific examples, while indicating specific embodiments of the disclosure, are given by way of illustration only, because various changes and modifications within the spirit and scope of the disclosure will become apparent to those skilled in the art from this detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 shows KPC tumors having similar responsiveness to PI3K inhibition on purified diets regardless of macronutrients composition. Mean tumor volumes of KPC tumor chunk allografts. Values shown are mean ± SEM. CPD, control purified diet; KD, ketogenic diet; LP, low protein; NP, normal protein; Veh, Vehicle.
Figs. 2A, 2B, 2C, 2D, 2E, 2F, 2G, 2H, and 21 show that diet source dictates response to PI3K inhibition. Fig. 2A shows tumor volumes of K8484 KPC allografted tumors in mice fed chow, KD, or CPD and treated with the PI3K inhibitor BYL-719 or vehicle control. Statistics represent differences in tumor sizes on day 40 using two-way ANOVA with Sidak’s post hoc. Fig. 2B shows survival curve of the same mice from Fig. 2A. Fig. 2C shows body weights of the same mice from Fig. 2A. n = 7-8 for Figs. 2A, 2B, and 2C. Fig. 2D shows serum glucose levels, and Figs. 2E and 2F show serum BYL levels in response to BYL administration on day 17 of the experiment in Fig. 2A. Mice were fasted during the first 4h of the experiment, after which food was put back. Shaded area represents the dark cycle. Figs. 2G, 2H, and 21 show the results of the principal component analysis of serum metabolome, fecal metabolome, and fecal microbiome at different timepoints after BYL administration for Fig. 2G or at the 8h time point for FIG. 2H and FIG. 21 on day 17. n = 5 for Figs. 2D, 2E, 2F, 2G, 2H, and 21. Values shown are mean ± SEM. **, /?<0.01, ***, ?<0.001, ****,/?<0.0001.
Fig. 3 shows that PI3K inhibition is still enhanced even when a purified diet is supplemented with high fiber. Survival curve of K8484 KPC allografted tumors in mice fed CPD supplemented with high or low cellulose as insoluble fiber and inulin as soluble fiber and treated BYL-719 or vehicle control. Diets with low cellulose are plotted together as low fiber, and diets with high cellulose as high fiber, n = 7-22. Values shown are mean ± SEM.
Figs. 4A, 4B, 4C, and 4D show that gut microbiome ablation sensitizes tumors to PI3K inhibition. Fig. 4A shows tumor volumes of K8484 KPC allografted tumors in mice fed chow or CPD and treated with antibiotics cocktail (Abx) or control and BYL-719 or vehicle control, n = 8- 12. Fig. 4B shows tumor volumes of K8484 KPC allografted tumors in chow-fed mice treated with antibiotics cocktail or control and BYL-719 or vehicle control, n = 7-8. Statistics represent differences in tumor sizes at endpoint using two-way ANOVA with Sidak’s post hoc. Figs. 4C and 4D show serum BYL levels in response to BYL administration on day 16 of the experiment in Fig. 4A. n = 5. Values shown are mean ± SEM. *, ?<0.05, **, <0.01, ****, <0.0001.
Figs. 5A, 5B, 5C, and 5D show that ampicillin sensitizes tumors to PI3K inhibition. Fig. 5 A shows tumor volumes of K8484 KPC allografted tumors in chow fed mice treated with single antibiotics, namely ampicillin (Amp), neomycin (Neo), metronidazole (Metro) or vancomycin (Vanco), cocktail of the four antibiotics (ANVM) or control and also treated with BYL-719 or vehicle control, n = 7-9. Fig. 5B shows body weight of the mice from Fig. 5A. Figs. 5C and 5D show serum BYL levels in response to BYL administration on day 10 of the experiment, H = 5. Values shown are mean ± SEM. **,/?<0.01, ****,/?<0.0001.
Figs. 6A, 6B, 6C, and 6D show that purified diet and ampicillin sensitizes tumors to BKM- 120. Fig. 6A shows tumor volumes of K8484 KPC allografted tumors in mice fed chow, KD or CPD and treated with the PI3K inhibitor BKM-120 (BKM) or vehicle control, n = 8-9. Fig. 6B shows serum BKM levels 22h after BKM administration on day 10 of the experiment in Fig. 6A. n = 5. Fig. 6C shows tumor volumes of K8484 KPC allografted tumors in chow-fed mice treated with ampicillin (Amp), neomycin (Neo), four antibiotics cocktail (ANVM) or control and also treated with BKM-120 or vehicle control, n = 7-9. Fig. 6D shows body weights of mice from Fig. 6C. Values shown are mean ± SEM. ***,£<0.001, ****,£><0.0001.
DETAILED DESCRIPTION OF THE INVENTION
This disclosure relates to an unexpected discovery that antibiotics or purified diets significantly enhance phosphoinositide 3 -kinase (PI3K) inhibition efficacy for cancer. Accordingly, this disclosure provides a novel method for treating a cancer using PI3K inhibitors in combination with antibiotics or purified diets.
Methods for Treating Cancer
In some embodiments, the method may include administering to a subject in need thereof (i) a therapeutically effective amount of a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (ii) an effective amount of one or more antibiotics or a purified diet.
PI3K Inhibitors
The PI3K inhibitor may be any PI3K inhibitor known in the art. As used herein, the term “PI3K inhibitor” refers to a nucleic acid, peptide, compound, or small organic molecule that binds to and inhibits at least one activity of PI3K. The PI3K proteins can be divided into three classes, class 1 PI3Ks, class 2 PI3Ks, and class 3 PI3Ks. Class 1 PI3Ks exist as heterodimers consisting of one of four p 110-catalytic subunits (p 110-alpha, p 110-beta, p 110-delta, and p 110-gamma) and one of two families of regulatory subunits. In some embodiments, a PI3K inhibitor may target the class 1 PI3K inhibitors. In some embodiments, a PI3K inhibitor displays selectivity for one or more isoforms of the class 1 PI3K inhibitors (/.<?., selectivity for pl 10-alpha, pl 10-beta, pl 10-delta, and pl lO-gamma or one or more of pl lO-alpha, pl lO-beta, pl lO-delta, and pl lO-gamma). A PI3K inhibitor that does not display isoform selectivity is considered a “pan-PI3K inhibitor.” In some embodiments, a PI3K inhibitor may compete for binding with ATP to the PI3K catalytic domain. In some embodiments, a selective PI3K inhibitor refers to an agent that exhibits a 50% inhibitory concentration with respect to PI3K that is at least 10-fold, at least 20-fold, at least 30- fold, at least 50-fold, at least 100-fold, at least 1000- fold, or more, lower than the inhibitor’s IC50 with respect to mTOR and/or other proteins in the pathway. In some embodiments, PI3K inhibitor may inhibit PI3K with an IC50 (concentration that inhibits 50% of the activity) of about 200 nM or less, about 100 nm or less, about 60 nM or less, about 25 nM, about 10 nM, about 5 nM, about 1 nM, about 100 uM, about 50 pM, about 25 pM, about 10 pM, about 1 pM, or less. In some embodiments, a PI3K inhibitor may inhibit PI3K with an IC50 from about 2 nM to about 100 nm, from about 2 nM to about 50 nM, or from about 2 nM to about 15 nM.
A PI3K inhibitor may be a PI3K-alpha inhibitor, a PI3K-gamma inhibitor, a PI3K-delta inhibitor, and a PI3K-gamma/delta inhibitor. In some embodiments, the PI3K inhibitor may include a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor may include a pan-PI3K inhibitor. In some embodiments, non-limiting examples of PI3K inhibitors suitable for use in the disclosed methods may include Serabelisib, BEZ235, LY294002, GDC-0941, BYL719, GSK2636771, TGX-221, AS25242, CAL-101, IPI-145, MK-2206, GSK690693, GDC-0068, A- 674563, CCT128930, AZD8055, INK128, rapamycin, PF-04691502, everolimus, BI-D1870, H89, PF-4708671, FMK, AT7867, NU7441, PI-103, NU7026, PIK-75, ZSTK474, PP-121, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a combination thereof.
In some embodiments, the PI3K inhibitor may include BYL-719, BKM-120, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a combination thereof.
Additional PI3K inhibitors are described, for example, in U.S. Pat. Nos. 8,193,182 and 8,569,323, and U.S. Patent Application Publication Nos. 2012/0184568 Al, 2013/0344061 Al, and 2013/0267521 Al, the disclosures of which are incorporated by reference herein.
In some embodiments, PI3K inhibitors may exist in various forms, such as stereoisomers, racemates, derivatives, prodrugs, analogs, or pharmaceutically acceptable salts, that are also suitable for use in the disclosed methods.
“Isomers” are different compounds that have the same molecular formula. “Stereoisomers” are isomers that differ only in the way the atoms are arranged in space, i.e., having a different stereochemical configuration. “Enantiomers” are a pair of stereoisomers that are non- superimposable mirror images of each other. A 1 : 1 mixture of a pair of enantiomers is a “racemic” mixture. The term “(±)” is used to designate a racemic mixture where appropriate. “Diastereoisomers” are stereoisomers that have at least two asymmetric atoms, but which are not mirror-images of each other. The absolute stereochemistry is specified according to the Cahn- Ingold-Prelog R-S system. When a compound is a pure enantiomer, the stereochemistry at each chiral carbon can be specified by either (R) or (S). Resolved compounds whose absolute configuration is unknown can be designated (+) or (-) depending on the direction (dextro- or levorotatory) in which they rotate plane polarized light at the wavelength of the sodium D line. Certain of the compounds described herein contain one or more asymmetric centers and can thus give rise to enantiomers, diastereomers, and other stereoisomeric forms that can be defined, in terms of absolute stereochemistry, as (R) or (S). The present chemical entities, pharmaceutical compositions, and methods are meant to include all such possible isomers, including racemic mixtures, optically pure forms, and intermediate mixtures. Optically active (R)- and (S)-isomers can be prepared using chiral synthons or chiral reagents or resolved using conventional techniques. When the compounds described herein contain olefinic double bonds or other centers of geometric asymmetry, and unless specified otherwise, it is intended that the compounds include both E and Z geometric isomers.
Stereoisomers are compounds that differ only in their spatial arrangement. Enantiomers are pairs of stereoisomers whose mirror images are not superimposable, most commonly because they contain an asymmetrically substituted carbon atom that acts as a chiral center. “Enantiomer” means one of a pair of molecules that are mirror images of each other and are not superimposable. Diastereomers are stereoisomers that are not related to mirror images, most commonly because they contain two or more asymmetrically substituted carbon atoms. “R” and “S” represent the configuration of substituents around one or more chiral carbon atoms. Thus, “R*” and “S*” denote the relative configurations of substituents around one or more chiral carbon atoms. The symbol in a structural formula represents the presence of a chiral carbon center.
“Racemate” or “racemic mixture” means a compound of equimolar quantities of two enantiomers, wherein such mixtures exhibit no optical activity, i.e., they do not rotate the plane of polarized light. “Geometric isomer” means isomers that differ in the orientation of substituent atoms in relationship to a carbon-carbon double bond, to a cycloalkyl ring, or to a bridged bicyclic system. Atoms (other than H) on each side of a carbon-carbon double bond may be in an E (substituents are on opposite sides of the carbon-carbon double bond) or Z (substituents are oriented on the same side) configuration. “R,” “S,” “St,” “R*,” “E,” “Z,” “cis,” and “trans” indicate configurations relative to the core molecule.
A “derivative,” as used herein, refers to a chemical substance related structurally to another, i.e., an “original” substance, which can be referred to as a “parent” compound. A “derivative” can be made from the structurally related parent compound in one or more steps. The phrase “closely related derivative” means a derivative whose molecular weight does not exceed the weight of the parent compound by more than 50%. The general physical and chemical properties of a closely related derivative are also similar to the parent compound. “Pharmaceutically active derivative” refers to any compound that, upon administration to the recipient, is capable of providing, directly or indirectly, the activity disclosed herein.
An “analog” refers to a small organic compound, a nucleotide, a protein, or a polypeptide that possesses similar or identical activity or function(s) as the compound, nucleotide, protein or polypeptide or compound having the desired activity of this disclosure, but need not necessarily include a sequence or structure that is similar or identical to the sequence or structure of the preferred embodiments.
A “prodrug” refers to a compound that may be converted under physiological conditions or by solvolysis to a biologically active compound described herein. Thus, the term “prodrug” refers to a precursor of a biologically active compound that is pharmaceutically acceptable. A prodrug may be inactive when administered to a subject, but is converted in vivo to an active compound, for example, by hydrolysis. The prodrug compound often offers the advantages of solubility, tissue compatibility, or delayed release in a mammalian organism see, e.g., Bundgaard, H., Design of Prodrugs (1985) (Elsevier, Amsterdam). The term “prodrug” also refers to any covalently bonded carriers, which release the active compound in vivo when administered to a subject. Prodrugs of an active compound, as described herein, may be prepared by modifying functional groups present in the active compound in such a way that the modifications are cleaved, either in routine manipulation or in vivo, to yield the active parent compound. Prodrugs include, for example, compounds wherein a hydroxy, amino or mercapto group is bonded to any group that, when the prodrug of the active compound is administered to a mammalian subject, cleaves to form a free hydroxy, free amino, or free mercapto group, respectively. Examples of prodrugs include, but are not limited to, acetates, formates, and benzoate derivatives of alcohol, various ester derivatives of a carboxylic acid, or acetamide, formamide, and benzamide derivatives of an amine functional group in the active compound. Various forms of prodrugs are well known in the art and are described in: (a) The Practice of Medicinal Chemistry, Camille G. Wermuth et al., Ch 31, (Academic Press, 1996); (b) Design of Prodrugs, edited by H. Bundgaard, (Elsevier, 1985); (c) A Textbook of Drug Design and Development, P. Krogsgaard-Larson and H. Bundgaard, eds. Ch 5, pgs 113-191 (Harwood Academic Publishers, 1991); and (d) Hydrolysis in Drug and Prodrug Metabolism, Bernard Testa and Joachim M. Mayer, (Wiley-VCH, 2003).
As used herein, the term “pharmaceutically acceptable” refers to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
As used herein, the term “pharmaceutically acceptable salt” refers to a salt of the administered compounds prepared from pharmaceutically acceptable non-toxic acids, including inorganic acids, organic acids, solvates, hydrates, or clathrates thereof.
Antibiotics
As used herein, an “antibiotic” or “antibiotic agent” refers to a substance that has the capacity to inhibit or slow down the growth of or destroy bacteria and/or other microorganisms. In some embodiments, the antibiotic agent is a bacteriostatic antibiotic agent. In some embodiments, the antibiotic is a bacteriolytic antibiotic agent. Non-limiting examples of antibiotic agents are set forth in the U.S. Patent Publication US 2006/0269485, which is herein incorporated by reference in its entirety.
In some embodiments, the antibiotic agent is selected from the classes including betalactam antibiotics, aminoglycosides, ansa-type antibiotics, anthraquinones, antibiotic azoles, antibiotic glycopeptides, macrolides, antibiotic nucleosides, antibiotic peptides, antibiotic polyenes, antibiotic polyethers, quinolones, antibiotic steroids, sulfonamides, tetracycline, dicarboxylic acids, antibiotic metals, oxidizing agents, substances that release free radicals and/or active oxygen, cationic antimicrobial agents, quaternary ammonium compounds, biguanides, triguanides, bisbiguanides and analogs and polymers thereof and naturally occurring antibiotic compounds.
Non-limiting examples of beta-lactam antibiotics may include 2-(3-alanyl)clavam, 2- hydroxymethylclavam, 8-epi-thienamycin, acetyl-thienamycin, amoxicillin, amoxicillin sodium, amoxicillin trihydrate, amoxicillin-potassium clavulanate combination, ampicillin, ampicillin sodium, ampicillin trihydrate, ampicillin-sulbactam, apalcillin, aspoxicillin, azidocillin, azlocillin, aztreonam, bacampicillin, biapenem, carbenicillin, carbenicillin disodium, carfecillin, carindacillin, carpetimycin, cefacetril, cefaclor, cefadroxil, cefalexin, cefaloridine, cefalotin, cefamandole, cefamandole, cefapirin, cefatrizine, cefatrizine propylene glycol, cefazedone, cefazolin, cefbuperazone, cefcapene, cefcapene pivoxil hydrochloride, cefdinir, cefditoren, cefditoren pivoxil, cefepime, cefetamet, cefetamet pivoxil, cefixime, cefinenoxime, cefinetazole, cefminox, cefminox, cefmolexin, cefodizime, cefonicid, cefoperazone, ceforanide, cefoselis, cefotaxime, cefotetan, cefotiam, cefoxitin, cefozopran, cefpiramide, cefpirome, cefpodoxime, cefpodoxime proxetil, cefprozil, cefquinome, cefradine, cefroxadine, cefsulodin, ceftazidime, cefteram, cefteram pivoxil, ceftezole, ceftibuten, ceftizoxime, ceftriaxone, cefuroxime, cefuroxime axetil, cephalosporin, cephamycin, chitinovorin, ciclacillin, clavulanic acid, clometocillin, cioxacillin, cycloserine, deoxy pluracidomycin, dicloxacillin, dihydro pluracidomycin, epicillin, epithienamycin, ertapenem, faropenem, flomoxef, flucloxacillin, hetacillin, imipenem, lenampicillin, loracarbef, mecillinam, meropenem, metampicillin, meticillin, mezlocillin, moxalactam, nafcillin, northienamycin, oxacillin, panipenem, penamecillin, penicillin, phenethicillin, piperacillin, tazobactam, pivampicillin, pivcefalexin, pivmecillinam, pivmecillinam hydrochloride, pluracidomycin, propicillin, sarmoxicillin, sulbactam, sulbenicillin, talampicillin, temocillin, terconazole, thienamycin, ticarcillin and analogs, salts and derivatives thereof.
In some embodiments, antibiotics may include ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof. In some embodiments, antibiotics may include ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
In some embodiments, antibiotics may include (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
Purified Diets
In some embodiments, the purified diet may include a ketogenic diet, a control purified diet, or a combination thereof.
As used herein, the term “purified diet” refers to a diet that consists substantially of purified and/or processed food components, as opposed to unprocessed food items (such as fruits, vegetables, whole grains, meats, eggs). Purified components refer to simple sugars, simple refined carbohydrates from which substantial chemical complexity has been intentionally removed (e.g., white flour, white rice), mixtures of fats (such as oils, butters, or creams), particular fibers (e.g., purified cellulose or insulin), or particular proteins or mixtures of proteins (e.g., casein, whey) purified from more complex starting materials (such as corn, fruit, whole grains, beans, milk, or meats). In some embodiments, the purified components may be combined together, optionally with water, prior to being fed to a mammal, e.g., in the form of a protein shake or baked good. Processed food components refer to foods comprising a substantial amount (e.g., greater than 50%, 75%, or 90%) of purified components, mixed together optionally with non-purified components. One example of a processed food would be a cake containing sucrose (table sugar, a purified component), butter (a purified component), white flour (a purified component), and eggs (an unprocessed food item). In some embodiments, the fraction of purified components may be greater than 25%, 50%, 75%, or 90% of total food intake. In some embodiments, the fraction of purified or processed food components may be greater than 50%, 75%, 90%, or 95% of total food intake. Fraction of purified components can optionally be measured by mass, by dry mass, or by calories.
A “ketogenic diet” or “KD” refers to a high fat and low saccharide diet, which is utilized as a diet for treating patients requiring saccharide-restrictive diets, for example, children with epilepsy. Non-limiting examples of the ketogenic diet may include Ketogenic diet (KD, also referred to as KD-LP) - D21111901Mi and Keto normal protein (KD-NP) - D21111902Mi (RESEARCH DIETS) as set forth in Table 1.
In some embodiments, the ketogenic diet may include 20 wt%, 10 wt%, 5 wt%, or less of proteins. In some embodiments, the ketogenic diet may include about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
A “control purified diet” or “CPD” refers to high protein and high carbohydrate diets. Nonlimiting examples of the control purified diet may include Control purified diet (CPD) - D21111904i, High cellulose with 0% Inulin (HC_InO) - D22040601i, High cellulose with 1.9% Inulin (HC Ini.9) - D22040602i, High cellulose with 1.9% Inulin (HC Ini.9) - D22040602i, High cellulose with 8% Inulin (HC_In8) - D22040603i, and Low cellulose with 2.5% Inulin (LC_In2.5) - D22011102i, as set forth in Table 1.
In some embodiments, the control purified diet may include from about 5 wt% to about 40 wt% (e.g, 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%, 10 wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%, 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19 wt%, 20 wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%, 30 wt%, 31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39 wt%, 40 wt%) of proteins. In some embodiments, the control purified diet may include about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates. Nonlimiting examples of the control purified diet may include those described in Table 1 in Example 1 of this disclosure.
Administration and dosing regimens
As used herein, the term “cancer” refers to all types of cancer, neoplasm or malignant tumors found in mammals, including leukemias, lymphomas, melanomas, neuroendocrine tumors, carcinomas, and sarcomas. Exemplary cancers that may be treated with a compound, pharmaceutical composition, or method provided herein include lymphoma, sarcoma, bladder cancer, bone cancer, brain tumor, cervical cancer, colon cancer, esophageal cancer, gastric cancer, head and neck cancer, kidney cancer, myeloma, thyroid cancer, leukemia, prostate cancer, breast cancer (e.g. triple negative, ER positive, ER negative, chemotherapy resistant, herceptin resistant, HER2 positive, doxorubicin resistant, tamoxifen resistant, ductal carcinoma, lobular carcinoma, primary, metastatic), ovarian cancer, pancreatic cancer, liver cancer (e.g., hepatocellular carcinoma), lung cancer ( .g., non-small cell lung carcinoma, squamous cell lung carcinoma, adenocarcinoma, large cell lung carcinoma, small cell lung carcinoma, carcinoid, sarcoma), glioblastoma multiforme, glioma, melanoma, prostate cancer, castration-resistant prostate cancer, breast cancer, triple negative breast cancer, glioblastoma, ovarian cancer, lung cancer, squamous cell carcinoma (e.g., head, neck, or esophagus), colorectal cancer, leukemia, acute myeloid leukemia, lymphoma, B cell lymphoma, or multiple myeloma. Additional examples include, cancer of the thyroid, endocrine system, brain, breast, cervix, colon, head & neck, esophagus, liver, kidney, lung, non-small cell lung, melanoma, mesothelioma, ovary, sarcoma, stomach, uterus or Medulloblastoma, Hodgkin’s Disease, Non-Hodgkin’s Lymphoma, multiple myeloma, neuroblastoma, glioma, glioblastoma multiforme, ovarian cancer, rhabdomyosarcoma, primary thrombocytosis, primary macroglobulinemia, primary brain tumors, cancer, malignant pancreatic insulanoma, malignant carcinoid, urinary bladder cancer, premalignant skin lesions, testicular cancer, lymphomas, thyroid cancer, neuroblastoma, esophageal cancer, genitourinary tract cancer, malignant hypercalcemia, endometrial cancer, adrenal cortical cancer, neoplasms of the endocrine or exocrine pancreas, medullary thyroid cancer, medullary thyroid carcinoma, melanoma, colorectal cancer, papillary thyroid cancer, hepatocellular carcinoma, Paget’s Disease of the Nipple, Phyllodes Tumors, Lobular Carcinoma, Ductal Carcinoma, cancer of the pancreatic stellate cells, cancer of the hepatic stellate cells, or prostate cancer.
As used herein, the term “solid tumor” refers to an abnormal mass of tissue that usually does not contain cysts or liquid areas. Solid tumors may be benign or malignant. The term “solid tumor cancer” refers to malignant, neoplastic, or cancerous solid tumors. Solid tumor cancers include, but are not limited to, sarcomas, carcinomas, and lymphomas, such as cancers of the lung, breast, triple-negative breast cancer, prostate, colon, rectum, and bladder. In some embodiments, the cancer is selected from cervical cancer, head and neck cancer (including, for example, head and neck squamous cell carcinoma (HNSCC)) glioblastoma, ovarian cancer, sarcoma, pancreatic cancer, bladder cancer, breast cancer, triple-negative breast cancer, and non-small cell lung carcinoma. The tissue structure of solid tumors includes interdependent tissue compartments, including the parenchyma (cancer cells) and the supporting stromal cells in which the cancer cells are dispersed and which may provide a supporting microenvironment.
In some embodiments, cancer may be chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), non-Hodgkin’ s lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), Hodgkin’s lymphoma, B cell acute lymphoblastic leukemia (B-ALL), Burkitt’s lymphoma, Waldenstrom’s macroglobulinemia (WM), Burkitt’s lymphoma, multiple myeloma, or myelofibrosis.
In some embodiments, cancer may be a solid tumor selected from the group consisting of bladder cancer, non-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glioma, gastrointestinal stromal tumor, breast cancer, lung cancer, colorectal cancer, thyroid cancer, bone sarcoma, stomach cancer, oral cavity cancer, oropharyngeal cancer, gastric cancer, kidney cancer, liver cancer, prostate cancer, colorectal cancer, esophageal cancer, testicular cancer, gynecological cancer, thyroid cancer, colon cancer, primary central nervous system lymphoma, and brain cancer.
As used herein, the terms “treating,” “treat,” and “treatment” include preventing a disease, pathologic or medical condition from occurring (e.g., prophylaxis); inhibiting the disease, pathologic or medical condition or arresting its development; relieving or ameliorating the disease, pathologic or medical condition; and/or diminishing symptoms associated with the disease, pathologic or medical condition. Thus, the terms “treat,” “treatment,” and “treating” can extend to prophylaxis and can include preventing, prevention, lowering, stopping, or reversing the progression or severity of the condition or symptoms being treated. As such, the term “treatment” can include medical, therapeutic, and/or prophylactic administration, as appropriate. The term “treating” or “treatment” thus can include reversing, reducing, or arresting the symptoms, clinical signs, and underlying pathology of a condition in a manner to improve or stabilize a subject’s condition.
As used herein, the term “administering” refers to the delivery of cells by any route including, without limitation, oral, intranasal, intraocular, intravenous, intraosseous, intraperitoneal, intraspinal, intramuscular, intra-articular, intraventricular, intracranial, intralesional, intratracheal, intrathecal, subcutaneous, intradermal, transdermal, or transmucosal administration. In some embodiments, the agent is administered to the subject intratumorally, intravenously, subcutaneously, intraosseously, orally, transdermally, sublingually, in sustained release, in controlled release, in delayed release, or as a suppository. As used herein, the term “combination,” “combined,” and related terms refer to the simultaneous or sequential administration of therapeutic agents in accordance with this disclosure. For example, a compound of the present invention may be administered with another therapeutic agent simultaneously or sequentially in separate unit dosage forms or together in a single unit dosage form.
An “effective amount” refers to an amount effective to treat a disease, disorder, and/or condition, or to bring about a recited effect. For example, an effective amount can be an amount effective to reduce the progression or severity of the condition or symptoms being treated. Determination of a therapeutically effective amount is well within the capacity of persons skilled in the art. The term “effective amount” is intended to include an amount of a compound described herein, or an amount of a combination of compounds described herein, e.g., that is effective in treating or preventing a disease or disorder, or treating the symptoms of the disease or disorder, in a host. Thus, an “effective amount” generally means an amount that provides the desired effect. A “therapeutically effective amount” of a compound with respect to the subject method of treatment refers to an amount of the compound in a preparation which, when administered as part of a desired dosage regimen (to a mammal, e.g., a human), alleviates a symptom, ameliorates a condition, or slows the onset of disease conditions according to clinically acceptable standards for the disorder or condition to be treated, e.g. , at a reasonable benefit/risk ratio applicable to any medical treatment.
The actual dosage amount of a composition of this disclosure administered to a subject can be determined by physical and physiological factors such as body weight, severity of condition, the type of disease being treated, previous or concurrent therapeutic interventions, idiopathy of the patient, and on the route of administration. The practitioner responsible for administration will, in any event, determine the concentration of active ingredient(s) in a composition and appropriate dose(s) for the individual subject.
In some embodiments, the PI3K inhibitor may be administered at one or more doses of from about 5 mg/kg to about 200 mg/kg of body weight of the subject, such as about 5 mg/kg of body weight, about 10 mg/kg of body weight, about 20 mg/kg of body weight, about 30 mg/kg of body weight, about 40 mg/kg of body weight, about 50 mg/kg of body weight, about 75 mg/kg of body weight, about 100 mg/kg of body weight, about 125 mg/kg of body weight, about 150 mg/kg of body weight, about 175 mg/kg of body weight, about 200 mg/kg of body weight, or any range derivable therein.
In some embodiments, the antibiotics may be administered at one or more doses of from about 10 mg/kg to about 500 mg/kg of body weight of the subject, such as about 10 mg/kg of body weight, about 20 mg/kg of body weight, about 30 mg/kg of body weight, about 40 mg/kg of body weight, about 50 mg/kg of body weight, about 75 mg/kg of body weight, about 100 mg/kg of body weight, about 125 mg/kg of body weight, about 150 mg/kg of body weight, about 175 mg/kg of body weight, about 200 mg/kg of body weight, about 250 mg/kg of body weight, about 300 mg/kg of body weight, about 350 mg/kg of body weight, about 400 mg/kg of body weight, about 450 mg/kg of body weight, about 500 mg/kg of body weight, or any range derivable therein.
In some embodiments, the purified diet may be administered at one or more doses of from about 10,000 mg/kg to about 500,000 mg/kg of body weight of the subject, such as 10,000 mg/kg of body weight, 11,000 mg/kg of body weight, 12,000 mg/kg of body weight, 13,000 mg/kg of body weight, 14,000 mg/kg of body weight, 15,000 mg/kg of body weight, 16,000 mg/kg of body weight, 17,000 mg/kg of body weight, 18,000 mg/kg of body weight, 19,000 mg/kg of body weight, 20,000 mg/kg of body weight, 21,000 mg/kg of body weight, 22,000 mg/kg of body weight, 23,000 mg/kg of body weight, 24,000 mg/kg of body weight, 25,000 mg/kg of body weight, 26,000 mg/kg of body weight, 27,000 mg/kg of body weight, 28,000 mg/kg of body weight, 29,000 mg/kg of body weight, 30,000 mg/kg of body weight, 31 ,000 mg/kg of body weight, 32,000 mg/kg of body weight, 33,000 mg/kg of body weight, 34,000 mg/kg of body weight, 35,000 mg/kg of body weight, 36,000 mg/kg of body weight, 37,000 mg/kg of body weight, 38,000 mg/kg of body weight, 39,000 mg/kg of body weight, 40,000 mg/kg of body weight, 41,000 mg/kg of body weight, 42,000 mg/kg of body weight, 43,000 mg/kg of body weight, 44,000 mg/kg of body weight, 45,000 mg/kg of body weight, 46,000 mg/kg of body weight, 47,000 mg/kg of body weight, 48,000 mg/kg of body weight, 49,000 mg/kg of body weight, 50,000 mg/kg of body weight, or any range derivable therein.
In some embodiments, a dose may be presented in a single dose or as divided doses administered at appropriate intervals, for example, as two, three, four or more sub-doses per day. The sub-dose itself may be further divided, e.g., into a number of discrete, loosely spaced administrations. In some embodiments, one or more doses of PI3K inhibitors, antibiotics, or purified diets may be administered at least every 1 day, every 2 days, every 3 days, every 4 days, every 5 days, every 6 days, every 7 days, every 8 days, every 9 days, every 10 days, every 11 days, every 12 days, every 13 days, every 14 days, every 15 days, every 16 days, every 17 days, every 18 days, every 19 days, every 20 days, every 21 days, every 22 days, every 23 days, every 24 days, every 25 days, every 26 days, every 27 days, every 28 days, every 29 days, every 30 days, every 31 days, every 32 days, every 33 days, every 34 days, every 35 days, every 36 days, every 37 days, every 38 days, every 39 days, every 40 days, every 41 days, every 42 days, every 43 days, every 44 days, every 45 days, every 46 days, every 47 days, every 48 days, every 49 days, every 50 days, every 51 days, every 52 days, every 53 days, every 54 days, every 55 days, every 56 days, every 57 days, every 58 days, every 59 days, every 60 days, every 61 days, every 62 days, every 63 days, every 64 days, every 65 days, every 66 days, every 67 days, every 68 days, every 69 days, every 70 days, every 71 days, every 72 days, every 73 days, every 74 days, every 75 days, every 76 days, every 77 days, every 78 days, every 79 days, every 80 days, every 81 days, every 82 days, every 83 days, every 84 days, every 85 days, every 86 days, every 87 days, every 88 days, every 89 days, every 90 days, every 91 days, every 92 days, every 93 days, every 94 days, every 95 days, every 96 days, every 97 days, every 98 days, every 99 days, every 100 days, every 101 days, every 102 days, every 103 days, every 104 days, every 105 days, every 106 days, every 107 days, every 108 days, every 109 days, every 110 days, every 111 days, every 112 days, every 113 days, every 114 days, every 115 days, every 116 days, every 117 days, every 118 days, every 119 days, or every 120 days.
Combination Therapies
In some embodiments, the method may include administering to the subject an additional therapeutic agent or therapy. In some embodiments, the additional therapeutic agent may include a second PI3K inhibitor, a second antibiotic, a second purified diet, or a combination thereof.
“Combination” therapy, as used herein, unless otherwise clear from the context, is meant to encompass administration of two or more therapeutic agents in a coordinated fashion and includes, but is not limited to, concurrent dosing. Specifically, combination therapy encompasses both co-administration (e.g., administration of a co-formulation or simultaneous administration of separate therapeutic compositions) and serial or sequential administration, provided that administration of one therapeutic agent is conditioned in some way on the administration of another therapeutic agent. For example, one therapeutic agent may be administered only after a different therapeutic agent has been administered and allowed to act for a prescribed period of time. See, e.g., Kohrt et al. (2011) Blood 117:2423.
As used herein, the term “co-administration” or “co-administered” refers to the administration of at least two agent(s) or therapies to a subject. In some embodiments, the coadministration of two or more agents/therapies is concurrent. In some embodiments, a first agent/therapy is administered prior to a second agent/therapy. Those of skill in the art understand that the formulations and/or routes of administration of the various agents/therapies used may vary.
It is also possible to combine an agent e.g., PI3K inhibitor, antibiotic, purified diet) with one or more other active ingredients in a unitary dosage form for simultaneous or sequential administration to a patient. The combination therapy may be administered as a simultaneous or sequential regimen. When administered sequentially, the combination may be administered in two or more administrations.
The combination therapy may provide synergy and be synergistic, i.e., the effect achieved when the active ingredients used together are greater than the sum of the effects that result from using the compounds separately. A synergistic effect may be attained when the active ingredients are: (1) co-formulated and administered or delivered simultaneously in a combined formulation; (2) delivered by alternation or in parallel as separate formulations; or (3) by some other regimen. When delivered in alternation therapy, a synergistic effect may be attained when the compounds are administered or delivered sequentially, e.g., in separate tablets, pills, capsules, or by different injections in separate syringes. In general, during alternation therapy, an effective dosage of each active ingredient is administered sequentially, i.e., serially, whereas, in combination therapy, effective dosages of two or more active ingredients are administered together. A synergistic effect denotes an effect that is greater than the predicted purely additive effects of the individual compounds of the combination.
Combination therapy is further described by U.S. Pat. Nos. 11103514, 10702495, 9382215, and 6833373, which include additional active agents that can be combined with the compounds described herein, and additional types of ailments and other conditions that can be treated with a compound or combination of compounds described herein. An active agent may precede or follow treatment of the other agent by intervals ranging from minutes to weeks. In embodiments where the other agent and expression construct are applied separately to a cell, one would generally ensure that a significant period of time did not elapse between the time of each delivery, such that the agent and expression construct would still be able to exert an advantageously combined effect on the cell. For example, in such instances, it is contemplated that one may contact the cell, tissue or organism with two, three, four or more modalities substantially simultaneously (i.e., within less than about a minute) with the disclosed active.
In some embodiments, one or more agents may be administered within about 1 minute, about 5 minutes, about 10 minutes, about 20 minutes, about 30 minutes, about 45 minutes, about 60 minutes, about 2 hours, about 3 hours, about 4 hours, about 6 hours, about 8 hours, about 9 hours, about 12 hours, about 15 hours, about 18 hours, about 21 hours, about 24 hours, about 28 hours, about 31 hours, about 35 hours, about 38 hours, about 42 hours, about 45 hours, to about 48 hours or more prior to and/or after administering the disclosed active agent. In certain other embodiments, an agent may be administered within from about 1 day, about 2 days, about 3 days, about 4 days, about 5 days, about 6 days, about 8 days, about 9 days, about 12 days, about 15 days, about 16 days, about 18 days, about 20 days, to about 21 days prior to and/or after administering the disclosed active. In some embodiments, it may be desirable to extend the time period for treatment significantly; however, where several weeks (e.g., about 1, about 2, about 3, about 4, about 6, or about 8 weeks or more) lapse between the respective administrations.
Administration of the compositions to a patient will follow general protocols for the administration of therapeutics, taking into account the toxicity, if any. It is expected that the treatment cycles will be repeated as necessary. It also is contemplated that various standard therapies or adjunct therapies, as well as surgical intervention, may be applied in combination with the described active agent. These therapies include but are not limited to chemotherapy, radiotherapy, immunotherapy, gene therapy, and surgery.
Pharmaceutical Compositions and Kits
In another aspect, this disclosure provides a pharmaceutical composition comprising: (a) a PI3K inhibitor, a stereoisomer thereof, an analog thereof, a derivative thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof, and (b) one or more antibiotics or a purified diet. As used herein, the term “composition” or “pharmaceutical composition” refers to a mixture of at least one component useful within the disclosure with other components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, and/or excipients. The pharmaceutical composition facilitates administration of one or more components of the invention to an organism.
In some embodiments, the PI3K inhibitor may include Serabelisib, BEZ235, LY294002, GDC-0941, BYL719, GSK2636771, TGX-221, AS25242, CAL-101, IP1-145, MK-2206, GSK690693, GDC-0068, A-674563, CCT128930, AZD8O55, INK128, rapamycin, PF-04691502, everolimus, BI-D1870, H89, PF-4708671, FMK, AT7867, NU7441, PI-103, NU7026, PIK-75, ZSTK474, PP-121, or a combination thereof. In some embodiments, the PI3K inhibitor may include a PI3K-alpha inhibitor. In some embodiments, the PI3K inhibitor may include a pan-PI3K inhibitor. In some embodiments, the PI3K inhibitor may include BYL-719, BKM-120, or a combination thereof.
In some embodiments, antibiotics may include ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
In some embodiments, one or more antibiotics may include ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
In some embodiments, one or more antibiotics may include (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
In some embodiments, the purified diet may include a ketogenic diet, a control purified diet, or a combination thereof. Non-limiting examples of the ketogenic diet may include Ketogenic diet (KD, also referred to as KD-LP) - D21111901Mi and Keto normal protein (KD-NP) - D21111902Mi (RESEARCH DIETS) as set forth in Table 1. In some embodiments, the ketogenic diet may include 20 wt%, 10 wt%, 5 wt%, or less of proteins. In some embodiments, the ketogenic diet may include about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
Non-limiting examples of the control purified diet may include Control purified diet (CPD) - D21111904i, High cellulose with 0% Inulin (HC_InO) - D2204060H, High cellulose with 1.9% Inulin (HC_Inl.9) - D22040602i, High cellulose with 1.9% Inulin (HC_Inl.9) - D22040602i, High cellulose with 8% Inulin (HC_In8) - D22040603i, and Low cellulose with 2.5% Inulin (LC_In2.5) - D22011102i, as set forth in Table 1. In some embodiments, the control purified diet may include from about 5 wt% to about 40 wt% (e.g., 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%, 10 wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%, 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19 wt%, 20 wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%, 30 wt%, 31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39 wt%, 40 wt%) of proteins. In some embodiments, the control purified diet may include about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
Techniques and formulations generally may be found in Remmington’s Pharmaceutical Sciences, Meade Publishing Co., Easton, PA. For systemic administration, injection is preferred, including intramuscular, intravenous, intraperitoneal, and subcutaneous. For injection, the agents can be formulated in liquid solutions, e.g., in physiologically compatible buffers such as Hank’s solution or Ringer’s solution. In addition, the agents may be formulated in solid form and redissolved or suspended immediately prior to use. Lyophilized forms are also included.
For oral administration, the pharmaceutical composition may be in the form of, for example, a tablet, capsule, liquid capsule, suspension, or liquid. The pharmaceutical composition is, e.g., made in the form of a dosage unit containing a particular amount of the active ingredient. For example, the pharmaceutical composition may be provided as a tablet or capsule comprising an amount of active ingredient in the range of from about 0.1 to 1000 mg, e.g., from about 0.25 to 250 mg, and, e.g., from about 0.5 to 100 mg. A suitable daily dose for a human or other mammal may vary widely depending on the condition of the patient and other factors, but, can be determined using routine methods.
Any pharmaceutical composition contemplated herein can, for example, be delivered orally via any acceptable and suitable oral preparations. Exemplary oral preparations include, but are not limited to, for example, tablets, troches, lozenges, aqueous and oily suspensions, dispersible powders or granules, emulsions, hard and soft capsules, liquid capsules, syrups, and elixirs. Pharmaceutical compositions intended for oral administration can be prepared according to any methods known in the art for manufacturing pharmaceutical compositions intended for oral administration. In order to provide pharmaceutically palatable preparations, a pharmaceutical composition in accordance with the disclosure can contain at least one agent selected from sweetening agents, flavoring agents, coloring agents, demulcents, antioxidants, and preserving agents.
A tablet can, for example, be prepared by admixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one non-toxic pharmaceutically acceptable excipient suitable for the manufacture of tablets. Exemplary excipients include, but are not limited to, for example, inert diluents, such as, for example, calcium carbonate, sodium carbonate, lactose, calcium phosphate, and sodium phosphate; granulating and disintegrating agents, such as, for example, microcrystalline cellulose, sodium croscarmellose, corn starch, and alginic acid; binding agents, such as, for example, starch, gelatin, polyvinylpyrrolidone, and acacia; and lubricating agents, such as, for example, magnesium stearate, stearic acid, and talc. Additionally, a tablet can either be uncoated or coated by known techniques to either mask the bad taste of an unpleasant-tasting drug or delay disintegration and absorption of the active ingredient in the gastrointestinal tract, thereby sustaining the effects of the active ingredient for a longer period. Exemplary water-soluble taste-masking materials include, but are not limited to, hydroxypropyl-methylcellulose and hydroxypropyl-cellulose. Exemplary time delay materials include, but are not limited to, ethylcellulose and cellulose acetate butyrate. Hard gelatin capsules can, for example, be prepared by mixing at least one compound and/or at least one salt thereof with at least one inert solid diluent, such as, for example, calcium carbonate; calcium phosphate; and kaolin. Soft gelatin capsules can, for example, be prepared by mixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one water-soluble carrier, such as, for example, polyethylene glycol, and at least one oil medium, such as, for example, peanut oil, liquid paraffin, and olive oil.
An aqueous suspension can be prepared, for example, by admixing at least one compound and/or at least one pharmaceutically acceptable salt thereof with at least one excipient suitable for the manufacture of an aqueous suspension. Non-limiting examples of excipients suitable for the manufacture of an aqueous suspension may include suspending agents, such as, for example, sodium carboxymethylcellulose, methyl cellulose, hydroxypropylmethylcellulose, sodium alginate, alginic acid, polyvinyl -pyrrolidone, gum tragacanth, and gum acacia; dispersing or wetting agents, such as, for example, a naturally-occurring phosphatide, e.g., lecithin; condensation products of alkylene oxide with fatty acids, such as, for example, polyoxyethylene stearate; condensation products of ethylene oxide with long-chain aliphatic alcohols, such as, for example, heptadecaethylene-oxycetanol; condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol, such as, for example, polyoxyethylene sorbitol monooleate; and condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, such as, for example, polyethylene sorbitan monooleate. An aqueous suspension can also contain at least one preservative, such as, for example, ethyl and n-propyl p-hydroxybenzoate; at least one coloring agent; at least one flavoring agent; and/or at least one sweetening agent, including but not limited to, for example, sucrose, saccharin, and aspartame.
Formulations for parenteral administration may be in the form of aqueous or non-aqueous isotonic sterile injection solutions or suspensions. These solutions and suspensions may be prepared from sterile powders or granules using one or more of the carriers or diluents mentioned for use in the formulations for oral administration or by using other suitable dispersing or wetting agents and suspending agents. The compounds may be dissolved in water, polyethylene glycol, propylene glycol, ethanol, corn oil, cottonseed oil, peanut oil, sesame oil, benzyl alcohol, sodium chloride, tragacanth gum, and/or various buffers. Other adjuvants and modes of administration are well and widely known in the pharmaceutical art. The active ingredient may also be administered by injection as a composition with suitable carriers, including saline, dextrose, or water, or with cyclodextrin i.e., Captisol), cosolvent solubilization (i.e., propylene glycol) or micellar solubilization (i.e., Tween 80).
The sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic, parenterally acceptable diluent or solvent, for example, as a solution in 1,3 -butanediol. Among the acceptable vehicles and solvents that may be employed are water, Ringer’s solution, and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose, any bland fixed oil may be employed, including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables. Pharmaceutically acceptable carriers, adjuvants, and vehicles that may be used in the pharmaceutical compositions of this disclosure include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, self-emulsifying drug delivery systems (SEDDS) such as alpha-tocopherol polyethylene glycol 1000 succinate, surfactants used in pharmaceutical dosage forms such as Tweens, polyethoxylated castor oil, such as cremophor surfactant (BASF), or other similar polymeric delivery matrices, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, polyethylene glycol, sodium carboxymethylcellulose, polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers, polyethylene glycol and wool fat. Cyclodextrins such as alpha-, beta-, and gammacyclodextrin, or chemically modified derivatives such as hydroxyalkyl cyclodextrins, including 2- and 3-hydroxypropyl-cyclodextrins, or other solubilized derivatives may also be advantageously used to enhance delivery of compounds of the formulae described herein.
The pharmaceutically active compounds of this disclosure can be processed in accordance with conventional methods of pharmacy to produce medicinal agents for administration to patients, including humans and other mammals. The pharmaceutical compositions may be subjected to conventional pharmaceutical operations such as sterilization and/or may contain conventional adjuvants, such as additives, preservatives, stabilizers, wetting agents, emulsifiers, buffers etc. Tablets and pills can additionally be prepared with enteric coatings. Such compositions may also include adjuvants, such as wetting, sweetening, flavoring, and perfuming agents.
Pharmaceutical compositions of this disclosure may include at least one compound and/or at least one pharmaceutically acceptable salt thereof, and optionally an additional agent selected from any pharmaceutically acceptable carrier, adjuvant, and vehicle. Alternate compositions of this disclosure may include a compound described herein, or a prodrug thereof, and a pharmaceutically acceptable carrier, adjuvant, or vehicle.
The PI3K inhibitors, the antibiotics, the purified diets, the composition or the pharmaceutical composition described herein can be provided in a kit. In some embodiments, the kit includes (a) a container that contains the composition and optionally (b) informational material. The informational material can be descriptive, instructional, marketing or other material that relates to the methods described herein and/or the use of the agents for therapeutic benefit. For example, kits may include instructions for the manufacturing, the therapeutic regimen to be used, and periods of administration. In some embodiments, the kit may also include an additional therapeutic agent. The kit may include one or more containers, each with a different reagent. For example, the kit may include a first container that contains the composition and a second container for the additional therapeutic agent.
The containers may include a unit dosage of the pharmaceutical composition. In addition to the composition, the kit can include other ingredients, such as a solvent or buffer, an adjuvant, a stabilizer, or a preservative. The kit may optionally include a device suitable for administration of the composition, e.g.. a syringe or other suitable delivery device. The device may be provided pre-loaded with one or both of the agents or can be empty, but suitable for loading.
Additional Definitions
To aid in understanding the detailed description of the compositions and methods according to the disclosure, a few express definitions are provided to facilitate an unambiguous disclosure of the various aspects of the disclosure. Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure belongs.
As used herein, a “subject” or “subject in need thereof’ refers to a human and a non-human animal. Examples of a non-human animals include all vertebrates, e.g., mammals, such as non- human mammals, non-human primates (particularly higher primates), dog, rodent (e.g., mouse or rat), guinea pig, cat, and rabbit, and non-mammals, such as birds, amphibians, reptiles, etc. In some embodiments, the subject is a human. In another embodiment, the subject is an experimental animal or animal suitable as a disease model.
The term “disease” as used herein is intended to be generally synonymous, and is used interchangeably with, the terms “disorder” and “condition” (as in medical condition), in that all reflect an abnormal condition of the human or animal body or of one of its parts that impairs normal functioning, is typically manifested by distinguishing signs and symptoms, and causes the human or animal to have a reduced duration or quality of life. The term “agent” is used herein to denote a chemical compound, a mixture of chemical compounds, a biological macromolecule (such as a nucleic acid, an antibody, a protein or portion thereof, e.g. , a peptide), or an extract made from biological materials such as bacteria, plants, fungi, or animal (particularly mammalian) cells or tissues. The activity of such agents may render it suitable as a “therapeutic agent,” which is a biologically, physiologically, or pharmacologically active substance (or substances) that acts locally or systemically in a subject.
The terms “therapeutic agent,” “therapeutic capable agent,” or “treatment agent” are used interchangeably and refer to a molecule or compound that confers some beneficial effect upon administration to a subject. The beneficial effect includes enablement of diagnostic determinations; amelioration of a disease, symptom, disorder, or pathological condition; reducing or preventing the onset of a disease, symptom, disorder, or condition; and generally counteracting a disease, symptom, disorder, or pathological condition.
“Sample,” “test sample,” and “patient sample” may be used interchangeably herein. The sample can be a sample of serum, urine plasma, amniotic fluid, cerebrospinal fluid, cells (e.g., antibody-producing cells), or tissue. Such a sample can be used directly as obtained from a patient or can be pre-treated, such as by filtration, distillation, extraction, concentration, centrifugation, inactivation of interfering components, addition of reagents, and the like, to modify the character of the sample in some manner as discussed herein or otherwise as is known in the art. The terms “sample” and “biological sample,” as used herein, generally refer to a biological material being tested for and/or suspected of containing an analyte of interest. The sample may be any tissue sample from the subject. The sample may comprise protein from the subject.
The terms “inhibit” and “antagonize,” as used herein, mean to reduce a molecule, a reaction, an interaction, a gene, an mRNA, and/or a protein’s expression, stability, function, or activity by a measurable amount or to prevent entirely. Inhibitors are compounds that, e.g., bind to, partially or totally block stimulation, decrease, prevent, delay activation, inactivate, desensitize, or down- regulate a protein, a gene, and an mRNA stability, expression, function, and activity, e.g., antagonists.
Doses are often expressed in relation to body weight. Thus, a dose which is expressed as [g, mg, or other unit]/kg (or g, mg, etc.) usually refers to [g, mg, or other unit] “per kg (or g, mg, etc.) body weight,” even if the term “body weight” is not explicitly mentioned. As used herein, the term “z/z vitro” refers to events that occur in an artificial environment, e.g., in a test tube or reaction vessel, in cell culture, etc., rather than within a multi-cellular organism.
As used herein, the term “in vivo” refers to events that occur within a multi-cellular organism, such as a non-human animal.
It is noted here that, as used in this specification and the appended claims, the singular forms “a,” “an,” and “the” include plural reference unless the context clearly dictates otherwise.
The terms “including,” “comprising,” “containing,” or “having” and variations thereof are meant to encompass the items listed thereafter and equivalents thereof as well as additional subject matter unless otherwise noted.
The phrases “in some embodiments,” “in various embodiments,” and the like are used repeatedly. Such phrases do not necessarily refer to the same embodiment, but they may unless the context dictates otherwise.
The terms “and/or” means any one of the items, any combination of the items, or all of the items with which this term is associated.
The word “substantially” does not exclude “completely,” e.g., a composition which is “substantially free” from Y may be completely free from Y. Where necessary, the word “substantially” may be omitted from the definition of the invention.
As used herein, the term “approximately” or “about,” as applied to one or more values of interest, refers to a value that is similar to a stated reference value. In some embodiments, the term “approximately” or “about” refers to a range of values that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction (greater than or less than) of the stated reference value unless otherwise stated or otherwise evident from the context (except where such number would exceed 100% of a possible value). Unless indicated otherwise herein, the term “about” is intended to include values, e.g., weight percents, proximate to the recited range that are equivalent in terms of the functionality of the individual ingredient, the composition, or the embodiment.
It is to be understood that wherever values and ranges are provided herein, all values and ranges encompassed by these values and ranges, are meant to be encompassed within the scope of the present invention. Moreover, all values that fall within these ranges, as well as the upper or lower limits of a range of values, are also contemplated by the present application.
As used herein, the term “each,” when used in reference to a collection of items, is intended to identify an individual item in the collection but does not necessarily refer to every item in the collection. Exceptions can occur if explicit disclosure or context clearly dictates otherwise.
The use of any and all examples, or exemplary language (e.g., “such as”) provided herein, is intended merely to better illuminate the invention and does not pose a limitation on the scope of the invention unless otherwise claimed. No language in the specification should be construed as indicating any non-claimed element as essential to the practice of the invention. When used in this document, the term “exemplary” is intended to mean “by way of example” and is not intended to indicate that a particular exemplary item is preferred or required.
All methods described herein are performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. In regard to any of the methods provided, the steps of the method may occur simultaneously or sequentially. When the steps of the method occur sequentially, the steps may occur in any order, unless noted otherwise.
In cases in which a method comprises a combination of steps, each and every combination or sub-combination of the steps is encompassed within the scope of the disclosure, unless otherwise noted herein.
Each publication, patent application, patent, and other reference cited herein is incorporated by reference in its entirety to the extent that it is not inconsistent with the present disclosure. Publications disclosed herein are provided solely for their disclosure prior to the filing date of the present invention. Nothing herein is to be construed as an admission that the present invention is not entitled to antedate such publication by virtue of prior invention. Further, the dates of publication provided may be different from the actual publication dates, which may need to be independently confirmed.
It is understood that the examples and embodiments described herein are for illustrative purposes only and that various modifications or changes in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims. Examples
EXAMPLE 1
This example describes the materials and methods used in the subsequent EXAMPLES below.
Mouse husbandry and PDAC tumor models. All mouse work was approved by the Institute Animal Care and Use Committee (IACUC) at Princeton University. Wild type C57BL/6 mice were obtained at 6 to 8 weeks of age from Charles River Laboratories, and tumor inoculations were performed up to a few weeks after arrival. Mice were housed under normal light cycle (7:00- 19:00) and fed chow diet ad lib (PicoLab Rodent 205053*, LabDiet) and had free access to reverse osmosis drinking water until experiments began. For experiments using the tumor chunk model (Fig. 1), syngeneic PDAC allograft tumors were established by harvesting tumors from KPC (LSL- KrasG12L), p53R172H, Pdx-Cre) mice, mincing the tissue with surgical scissors into ~3mm pieces able to pass through a 16g needle, suspending in DMEM medium, mixing with Matrigel (Corning Cat #354234) at a 1 : 1 ratio (v/v), and then injecting 200ul of the mixture subcutaneously into the mouse flank (Yang, L. et al. Med 3, 119-136. e8 (2022)). For the rest of the experiments, KPC K8484 cell line was used. Cells were harvested with trypsin, counted in DMEM, suspended in PBS, and mixed at a 1 : 1 ratio with Matrigel. 0.5-1 * 106 cells were injected subcutaneously into mouse flanks in a volume of 100-200 pl.
Diets and antibiotics treatment. Treatments were started when tumors reached an average volume of- 100 mm3. For experiments with dietary modifications, mice were transferred to a new clean cage with the modified diet and had free access to food throughout the whole experiment, except for some of the pharmacokinetics studies where mice were fasted one time during an experiment for up to seven hours during the light cycle. For experiments containing a ketogenic diet, all mice were fasted overnight one day before the dietary change. Chow was PicoLab Rodent 5053, and all purified diets were purchased from research diets, and provided the following diet codes: Ketogenic diet (KD, also called in fig 1 KD-LP) - D21111901Mi. Keto normal protein (KD- NP) - D21111902Mi. Control purified diet with low protein (CPD-LP) - D21111903i. Control purified diet (CPD) - D21111904i . High cellulose with 0% Inulin (HC InO) - D2204060H. High cellulose with 1.9% Inulin (HC Inl .9) - D22040602i. High cellulose with 1 .9% Inulin (HC_Inl .9) - D22040602i. High cellulose with 8% Inulin (HC_In8) - D22040603i. Low cellulose with 2.5% Inulin (LC_In2.5) - D22011 102i. Antibiotics were administered in the drinking water as a cocktail of ampicillin (1 g/L), neomycin (1 g/L), metronidazole (1 g/L), and vancomycin (0.5 g/L) or as individual antibiotics. To make the drinking water more palatable, 5% aspartame was added. Drinking water with 5% aspartame was used as control. Table 1. Example purified diets and their ingredients
Administration of PI3K inhibitors and tumor volume measurements. PI3K inhibitors BYL-719 (BYL) and BKM-120 (BKM) were dissolved in 0.5% carboxymethylcellulose, sonicated, and given by daily oral gavage (lOul/gr) for 5 days a week (5 days on, 2 days off). When not stated otherwise, BYL dose was 45 mg/kg, and BKM dose was 25 mg/kg. Tumor volumes were measured twice a week with a caliper, and tumor size was calculated by the formula: 0.5 x (Length x Width x Height).
BYL and BKM pharmacokinetics (PK) studies. Where indicated, mice were fasted ~2h before drug gavage, and food was put back after the 4h time point when mice were entering the dark cycle. Around 30 pl of blood was collected from the tail snip before gavage (which also represents ~20-24h after previous drug administration) and at the indicated time points after gavage. Blood was allowed to clot for 30 min at room temperature and then put on ice until further processing. Serum was separated by centrifuging blood at 2000 g for 10 min at 4°C, and was stored at -80°C until further analysis. BYL, BKM, and glucose levels were measured by liquid chromatography coupled to mass spectrometry (LC-MS).
Metabolite extraction for LC-MS. For serum samples, 3 pl samples were mixed 117 pl with methanol, vortexed for 30 seconds, and centrifuged at 17,000 g for 10 min at 4°C. For fecal samples, 10-20 mg of fecal pellets were weighed and ground under liquid nitrogen using a cryomill (Restch, Newtown, PA). Next, x40 volume (40 pl extraction solvent per 1 mg tissue) of 40:40:20 methanol: acetonitrile: water with 0.5% formic acid pre-cooled to -20°C was added to the fecal powder, vortexed and incubated on ice for 10 min. The acid was then neutralized by adding 15% ammonium bicarbonate (NH4HCO3) aqueous solution (8.75% v/v of extraction buffer). Samples were vortexed and centrifuged at 17,000 g for 10 min at 4°C. The clear supernatant was transferred to MS vials and loaded to the mass-spec. To measure the absolute concentration of glucose, 13C-glucose standard was spiked into the extraction buffer. The absolute concentrations of BYL and BKM were obtained by preparing external standard curves with known amounts of drugs for LC-MS. LC-MS. Water soluble metabolite measurements were obtained by running samples on Orbitrap Exploris 240 mass spectrometer (Thermo Scientific) coupled with hydrophilic interaction chromatography (HILIC) with electrospray ionization. LC separation was performed on an XBridge BEH Amide column (2.13150 mm, 2.5 pm particle size, 130 A pore size; Waters Corporation) using a gradient of solvent A (95:5 water: acetonitrile with 20 mM of ammonium acetate and 20 mM of ammonium hydroxide, pH 9.45) and solvent B (acetonitrile). The flow rate was 150 pl/min. The LC gradient was: 0 min, 90% B; 2 min, 90% B; 3 min, 75%; 7 min, 75% B; 8 min, 70%; 9 min, 70% B; 10 min, 50% B; 12 min, 50% B; 13 min, 25% B; 14 min, 25% B; 16 min, 0.5% B, 20.5 min, 0.5% B; 21 min, 90% B; 25 min, 90% B. Injection volume was 5-10 pl, and autosampler temperature was 4°C. MS scans were in negative and positive ion switching mode with a resolution of 120,000 at m/z 200 and a scan range of m/z 70-1000 for neg mode and m/z 58-116.5 and m/z 120-1000 for pos mode. Mass spec data was analyzed using El-Maven (v0.12.0, Elucidata).
16S sequencing. Bacterial DNA was extracted from fecal samples using the Power Soil DNA Isolation kit (QIAGEN). A section of the 16S rRNA gene (-250 bp, V4 region) was amplified, and Illumina sequencing libraries were prepared from these amplicons according to previously published protocols and primers. Libraries were further pooled together at equal molar ratios and sequenced on an Illumina HiSeq 2500 Rapid Flowcell or MiSeq as paired-end reads. These reads were 2x150 bp with an average depth of -20,000 reads. Also included were 8 bp index reads, following the manufacturer’s protocol (Illumina, USA). Pass-Filter reads were generated from raw sequencing reads using Illumina HiSeq Control Software. Samples were de-multiplexed using the index reads. The DADA2 plugin within QIIME2 version 2018.6 was used to infer Amplicon sequencing variants (ASVs) from the unmerged paired-end sequences. The forward reads were trimmed at 150 bp, and the reverse reads were trimmed at 140 bp, with all other DADA2 as default. Taxonomy was assigned to the resulting ASVs with a naive Bayes classifier trained on the Greengenes database version 13.8, with only the target region of the 16S rRNA gene used to train the classifier. Downstream analyses were performed with MATLAB.
Statistical analysis. Data are presented as mean ± SEM for bar and line graphs. GraphPad Prism software version 9.0 was used to create figures and calculate statistics. One-way or two-way analysis of variance (ANOVA) with Dunnett’s or Sidak’s post hoc was used for multi pl e-group comparisons. Heatmaps and principal component analyses were generated using Metaboanalyst 5.0 (https://www. metaboanalyst.ca/). All comparisons were two-tailed, and p < 0.05 was considered to be a statistically significant difference. *p < 0.05, **p < 0.01, ***p < 0.001, ****p
< 0.0001.
EXAMPLE 2
To determine the importance of protein restriction on PI3K inhibition efficacy, four matched purified ingredient diets were designed, including either ketogenic or carbohydrate-rich, containing 5% or 20% protein. Using these diets, the efficacy of the FDA-approved PI3K inhibitor BYL-719 (BYL, alpelisib) was tested in a pancreatic KPC allograft model. Notably, BYL showed a similar degree of tumor growth inhibition in all four diets. Unexpectedly, similar efficacy was seen even with the high carb 20% protein diet (Fig. 1), despite the macronutrient composition in this diet being similar to the chow used previously (Hopkins, B. D et al. Nature 560, 499-503 (2018)) where PI3K inhibitors were unresponsive. These results indicate that purified diets, unrelated to their macronutrient composition, increase PI3K inhibition efficacy, while the drugs may be less effective in rodents receiving typical grain-based chow diets. This is the first observation that purified versus grain-based diets can impact the efficacy of targeted anticancer therapies.
To directly test this hypothesis, the effect of three diets, namely standard grain-based chow, ketogenic diet (5% protein) and carbohydrate-rich purified diet (macronutrient composition matched to chow, hereafter termed control purified diet, CPD) were compared on PI3K inhibition efficacy in pancreatic KPC allograft model. Notably, while diets alone did not affect tumor growth, carb-rich purified diet sensitized tumors to PI3K inhibition to a similar degree as the ketogenic diet (Figs. 2A-B). Importantly, while KD mice treated with BYL kept losing weight resulting in study termination for this group, mice on CPD maintained body weight, indicating that BYL is better tolerated in the context of CPD than KD (Fig. 2C). Serum glucose after drug treatment was comparable between chow and KD. CPD, however, resulted in yet higher serum glucose, indicating the drug may have a stronger systemic effect in this diet or that the control purified diet provides yet greater amounts of readily accessible carbohydrates than chow (Fig. 2D).
Next, blood drug levels under different diets were measured. It was found that purified diets led to significantly higher serum BYL levels, with peak drug levels increasing 1.5 and 2 fold and trough drug levels 30 and 70 fold for KD and CPD compared to chow, respectively (Figs. 2E- F). Principal component analysis of the serum metabolome, fecal metabolome, and fecal microbiome demonstrated that KD and CPD cluster closer to each other than to chow, indicating that overall metabolomic impact is more dependent on the diet’s origin (z.c., grain-based vs. purified) than its macronutrient composition (Figs. 2G-I).
A major difference between grain-based chow and purified diets is dietary fiber, which is typically high in chow and low in the purified diets (Pellizzon, M. A. & Ricci, M. R. Nutr. Metab. 15, 1-6 (2018)). Indeed, while the chow that was used contained 15.5% (w/w) insoluble fiber and -1.9% soluble fiber, CPD had 2.6% insoluble fiber (as cellulose) and no soluble fiber. To test the importance of fiber on the interplay of PI3K inhibition with purified diets, insoluble fiber was added to a similar level as chow and different amounts of soluble fiber back to CPD, and the effect of BYL treatment on KPC allografts was monitored. It was found that PI3K inhibition was still enhanced even when mice consumed purified diets containing high fiber (Fig. 3A). The distinct impact of chow relative to even fiber-rich purified diets was also evident in serum metabolomics, as purified diets containing fiber showed a common metabolite profile which was different from chow (Fig. 3B). These results indicate that there is another hidden factor intrinsic to purified diets besides fiber which may mediate the increased BYL efficacy.
To determine whether gut microbiome, which significantly differed between chow and purified diets (Fig. 21), has a role in the synergy PI3K inhibition with purified diets, mice bearing KPC allografts were fed either chow or CPD and were treated with an antibiotics cocktail which ablates the gut microbiome or control, followed by BYL administration. Unexpectedly, chow-fed mice receiving antibiotics and BYL showed complete tumor control, similar to mice on CPD with or without antibiotics (Fig. 4A). BYL pharmacokinetics (PK) under these conditions were also monitored, and it was found that similar to CPD, antibiotics treatment led to increased peak, and most notably trough serum BYL levels (Figs. 4C-D). Importantly, antibiotics alone without BYL administration did not affect tumor growth, ruling out a direct effect of antibiotics on tumor growth (Fig. 4B). These results indicate that the gut microbiome is critical in determining a tumor’s response to PI3K inhibition.
Next, whether a specific subset of the gut microbial community is responsible for the phenotype was studied. To this end, each of the antibiotics used in the antibiotics cocktail was individually tested for its ability to enhance BYL efficacy. It was found that ampicillin, but not the three other antibiotics tested, enhanced BYL efficacy to the same levels as the antibiotics cocktail (Fig. 5A). BYL was better tolerated with ampicillin than with the antibiotics cocktail, as depicted by body weight profile over the course of the experiment (Fig. 5B). BYL PK was enhanced by ampicillin, although to a lesser extent than the antibiotics cocktail (Figs. 5C-D). Importantly, the ability of purified diet and ampicillin to enhance PI3K inhibition efficacy and PK was not limited to BYL. Similar results were obtained when another pan-PI3K inhibitor, BKM-120, was tested (Figs. 6A-D). In summary, these results strongly indicate that a chow-abundant ampicillinsensitive microbiome suppresses anti -cancer activities of PI3K inhibitors, at least in part via PK.
This example shows purified diets and antibiotics, such as ampicillin, significantly enhance PI3K inhibition efficacy in pancreatic ductal adenocarcinoma (PDAC) mouse models, as demonstrated for the FDA-approved PI3K-alpha-specific BYL-719 (alpelisib) and for the pan- PI3K inhibitor BKM-120 that is now undergoing phase III clinical trials. The results indicate that manipulating the gut microbiome composition by purified diets or antibiotics enhances PI3K efficacy by improving drug pharmacokinetics. Importantly, the combination of PI3K inhibitors with purified diets or ampicillin remains efficacious and tolerable over time.
The foregoing examples and description of the preferred embodiments should be taken as illustrating, rather than as limiting the present disclosure as defined by the claims. As will be readily appreciated, numerous variations and combinations of the features set forth above can be utilized without departing from the present disclosure as set forth in the claims. Such variations are not regarded as a departure from the scope of the disclosure, and all such variations are intended to be included within the scope of the following claims. All references cited herein are incorporated by reference in their entireties.
Claims
1. A method of treating a cancer, comprising administering to a subject in need thereof (i) a therapeutically effective amount of a phosphoinositide 3-kinase (PI3K) inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (ii) an effective amount of one or more antibiotics or a purified diet.
2. The method of claim 1, wherein the one or more antibiotics comprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
3. The method of any one of the preceding claims, wherein the one or more antibiotics comprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
4. The method of any one of the preceding claims, wherein the one or more antibiotics comprises (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
5. The method of any one of the preceding claims, wherein the one or more antibiotics are administered at one or more doses of from about 10 mg/kg to about 500 mg/kg of body weight of the subject.
6. The method of any one of the preceding claims, wherein the purified diet comprises a ketogenic diet, a control purified diet, or a combination thereof.
7. The method of claim 6, wherein the ketogenic diet comprises 20 wt%, 10 wt%, 5 wt%, or less of proteins.
8. The method of any one of claims 6-7, wherein the ketogenic diet comprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
9. The method of any one of claims 6-8, wherein the control purified diet comprises from about 5 wt% to about 40 wt% of proteins.
10. The method of any one of claims 6-9, wherein the control purified diet comprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
11. The method of any one of the preceding claims, wherein the purified diet is administered at one or more doses of from about 10,000 mg/kg to about 500,000 mg/kg of body weight of the subject.
12. The method of any one of the preceding claims, wherein the PI3K inhibitor comprises a PI3K-alpha inhibitor.
13. The method of any one of the preceding claims, wherein the PI3K inhibitor comprises a pan-PI3K inhibitor.
14. The method of any one of the preceding claims, wherein the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
15. The method of any one of the preceding claims, wherein the PI3K inhibitor is administered at one or more doses of from about 5 mg/kg to about 200 mg/kg of body weight of the subject.
16. The method of any one of the preceding claims, wherein the PI3K inhibitor is administered to the subject before or after the one or more antibiotics or the purified diet.
17. The method of any one of the preceding claims, wherein the PI3K inhibitor is administered to the subject concurrently with the one or more antibiotics or the purified diet.
18. The method of any one of the preceding claims, wherein the PI3K inhibitor is contained in the same composition with the one or more antibiotics or the purified diet.
19. The method of any one of the preceding claims, wherein the PI3K inhibitor, the one or more antibiotics, or the purified diet are administered at least every 1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, or 4 weeks.
20. The method of any one of the preceding claims, wherein the PI3K inhibitor or the one or more antibiotics are administered to the subject intratumorally, intravenously, subcutaneously, intraosseously, orally, transdermally, sublingually, in sustained release, in controlled release, in delayed release, or as a suppository.
21. The method of any one of the preceding claims, wherein the cancer is selected from the group consisting of chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), non-Hodgkin’s lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), Hodgkin’s lymphoma, B cell acute lymphoblastic leukemia (B-ALL), Burkitt’s lymphoma, Waldenstrom’s macroglobulinemia (WM), Burkitt’s lymphoma, multiple myeloma, and myelofibrosis.
22. The method of any one of the preceding claims, wherein the cancer is a solid tumor selected from the group consisting of bladder cancer, non-small cell lung cancer, cervical cancer, anal cancer, pancreatic cancer, squamous cell carcinoma including head and neck cancer, renal cell carcinoma, melanoma, ovarian cancer, small cell lung cancer, glioblastoma, glioma, gastrointestinal stromal tumor, breast cancer, lung cancer, colorectal cancer, thyroid cancer, bone sarcoma, stomach cancer, oral cavity cancer, oropharyngeal cancer, gastric cancer, kidney cancer, liver cancer, prostate cancer, colorectal cancer, esophageal cancer, testicular cancer, gynecological cancer, thyroid cancer, colon cancer, primary central nervous system lymphoma, and brain cancer.
23. The method of any one of the preceding claims, wherein the subject is a mammal.
24. The method of any one of the preceding claims, wherein the subject is a human.
25. The method of any one of the preceding claims, further comprising administering to the subject an additional therapeutic agent or therapy.
26. The method of claim 25, wherein the additional therapeutic agent comprises a second PI3K inhibitor, a second antibiotic, a second purified diet, or a combination thereof.
27. Use of a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof in combination with one or more antibiotics or a purified diet in a method according to any one of the preceding claims.
28. A pharmaceutical composition comprising: (a) a PI3K inhibitor, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof, and (b) one or more antibiotics or a purified diet.
29. The pharmaceutical composition of claim 28, wherein the one or more antibiotics comprise ampicillin, neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, or a combination thereof.
30. The pharmaceutical composition of any one of claims 28-29, wherein the one or more antibiotics comprise ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
31. The pharmaceutical composition of any one of claims 28-30, wherein the one or more antibiotics comprise (a) ampicillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, or a pharmaceutically acceptable salt thereof; and (b) any one of neomycin, metronidazole, vancomycin, amoxicillin, piperacillin, ticarcillin, a stereoisomer thereof, a derivative thereof, an analog thereof, a prodrug thereof, a pharmaceutically acceptable salt thereof, and a combination thereof.
32. The pharmaceutical composition of any one of claims 28-31, wherein the purified diet comprises a ketogenic diet, a control purified diet, or a combination thereof.
33. The pharmaceutical composition of claim 32, wherein the ketogenic diet comprises 20 wt%, 10 wt%, 5 wt%, or less of proteins.
34. The pharmaceutical composition of any one of claims 32-33, wherein the ketogenic diet comprises about 5 wt% of proteins, about 93 wt% of fat, and about 2 wt% of carbohydrates.
35. The pharmaceutical composition of any one of claims 32-34, wherein the control purified diet comprises from about 5 wt% to about 40 wt% of proteins.
36. The pharmaceutical composition of any one of claims 32-35, wherein the control purified diet comprises about 20 wt% of proteins, about 12 wt% of fat, and about 68 wt% of carbohydrates.
37. The pharmaceutical composition of any one of claims 28-36, wherein the PI3K inhibitor comprises a PT3K-alpha inhibitor.
38. The pharmaceutical composition of any one of claims 28-37, wherein the PI3K inhibitor comprises a pan-PI3K inhibitor.
39. The pharmaceutical composition of any one of claims 28-38, wherein the PI3K inhibitor comprises BYL-719, BKM-120, or a combination thereof.
40. A kit comprising the pharmaceutical composition of any one of claims 28-39.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363482214P | 2023-01-30 | 2023-01-30 | |
| US63/482,214 | 2023-01-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024163039A1true WO2024163039A1 (en) | 2024-08-08 |
Family
ID=89427269
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2023/081396PendingWO2024163039A1 (en) | 2023-01-30 | 2023-11-28 | Cancer treatment using pi3k inhibitors in combination with antibiotics or purified diets |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024163039A1 (en) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6833373B1 (en) | 1998-12-23 | 2004-12-21 | G.D. Searle & Co. | Method of using an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| US20060269485A1 (en) | 2002-11-29 | 2006-11-30 | Foamix Ltd. | Antibiotic kit and composition and uses thereof |
| US8193182B2 (en) | 2008-01-04 | 2012-06-05 | Intellikine, Inc. | Substituted isoquinolin-1(2H)-ones, and methods of use thereof |
| US20120184568A1 (en) | 2011-01-10 | 2012-07-19 | Pingda Ren | Processes for preparing isoquinolinones and solid forms of isoquinolinones |
| US20130344061A1 (en) | 2012-06-25 | 2013-12-26 | Infinity Pharmaceuticals, Inc. | Treatment of lupus, fibrotic conditions, and inflammatory myopathies and other disorders using pi3 kinase inhibitors |
| US9382215B2 (en) | 2012-03-27 | 2016-07-05 | The Board Of Trustees Of The University Of Illinois | Therapeutic methods and agents for treating myotonic dystrophy |
| US10702495B2 (en) | 2017-02-20 | 2020-07-07 | Nexien Biopharma, Inc. | Method and compositions for treating dystrophies and myotonia |
| WO2020191356A1 (en)* | 2019-03-21 | 2020-09-24 | Goncalves Marcus | Anti-fructose therapy for colorectal and small intestine cancers |
| US11103514B2 (en) | 2010-05-26 | 2021-08-31 | Corcept Therapeutics, Inc. | Treatment of muscular dystrophy |
- 2023
- 2023-11-28WOPCT/US2023/081396patent/WO2024163039A1/enactivePending
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6833373B1 (en) | 1998-12-23 | 2004-12-21 | G.D. Searle & Co. | Method of using an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| US20060269485A1 (en) | 2002-11-29 | 2006-11-30 | Foamix Ltd. | Antibiotic kit and composition and uses thereof |
| US8193182B2 (en) | 2008-01-04 | 2012-06-05 | Intellikine, Inc. | Substituted isoquinolin-1(2H)-ones, and methods of use thereof |
| US8569323B2 (en) | 2009-07-15 | 2013-10-29 | Intellikine, Llc | Substituted isoquinolin-1(2H)-one compounds, compositions, and methods thereof |
| US11103514B2 (en) | 2010-05-26 | 2021-08-31 | Corcept Therapeutics, Inc. | Treatment of muscular dystrophy |
| US20120184568A1 (en) | 2011-01-10 | 2012-07-19 | Pingda Ren | Processes for preparing isoquinolinones and solid forms of isoquinolinones |
| US9382215B2 (en) | 2012-03-27 | 2016-07-05 | The Board Of Trustees Of The University Of Illinois | Therapeutic methods and agents for treating myotonic dystrophy |
| US20130344061A1 (en) | 2012-06-25 | 2013-12-26 | Infinity Pharmaceuticals, Inc. | Treatment of lupus, fibrotic conditions, and inflammatory myopathies and other disorders using pi3 kinase inhibitors |
| US10702495B2 (en) | 2017-02-20 | 2020-07-07 | Nexien Biopharma, Inc. | Method and compositions for treating dystrophies and myotonia |
| WO2020191356A1 (en)* | 2019-03-21 | 2020-09-24 | Goncalves Marcus | Anti-fructose therapy for colorectal and small intestine cancers |
Non-Patent Citations (10)
| Title |
|---|
| "A Textbook of Drug Design and Development", 1991, HARWOOD ACADEMIC PUBLISHERS, pages: 113 - 191 |
| BERNARD TESTAJOACHIM M. MAYER: "Hydrolysis in Drug and Prodrug Metabolism", 2003, WILEY-VCH |
| BUNDGAARD, H.: "Design of Prodrugs", 1985, ELSEVIER |
| CAMILLE G. WERMUTH ET AL.: "The Practice of Medicinal Chemistry", 1996, ACADEMIC PRESS |
| HOPKINS, B. D. ET AL., NATURE, vol. 560, 2018, pages 499 - 503 |
| KOHRT ET AL., BLOOD, vol. 117, 2011, pages 2423 |
| PELLIZZON, M. A.RICCI, M. R., NUTR. METAB., vol. 15, 2018, pages 1 - 6 |
| TAJAN MYLÈNE ET AL: "Dietary Approaches to Cancer Therapy", CANCER CELL, CELL PRESS, US, vol. 37, no. 6, 14 May 2020 (2020-05-14), pages 767 - 785, XP086173162, ISSN: 1535-6108, [retrieved on 20200514], DOI: 10.1016/J.CCELL.2020.04.005* |
| YANG, L. ET AL., MED, vol. 3, 2022, pages 119 - 136,e8 |
| ZHANG JIE ET AL: "A new biological triangle in cancer: intestinal microbiota, immune checkpoint inhibitors and antibiotics", CLINICAL AND TRANSLATIONAL ONCOLOGY, SPRINGER ITALIA SRL, ITALY, SPAIN, vol. 23, no. 12, 14 June 2021 (2021-06-14), pages 2415 - 2430, XP037604282, ISSN: 1699-048X, [retrieved on 20210614], DOI: 10.1007/S12094-021-02659-W* |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12030863B2 (en) | Prodrugs of urolithins and uses thereof | |
| TWI451846B (en) | Quercetin-containing composition | |
| KR100537707B1 (en) | Analgesic Compositions Comprising Anti-epileptic Compounds and Methods of Using Same | |
| EP2953623B1 (en) | Methods of treating topical microbial infections | |
| TWI522357B (en) | Pharmaceutical compositions for the treatment of cancer and other diseases or disorders | |
| US8299033B2 (en) | Iodo-hexose compounds useful to treat cancer | |
| US20090203784A1 (en) | Treatment regimen for n-myc, c-myc, and l-myc amplified and overexpressed tumors | |
| KR20080003429A (en) | Synergistic combination of nonsteroidal anti-inflammatory drugs and alpha-delta-ligand | |
| WO2010025328A1 (en) | Compositions and methods of treatment comprising ceftaroline | |
| EP3011972A1 (en) | Medicinal composition for promoting synthesis of protoporphyrin ix | |
| EP3150208A1 (en) | Theobromine or its derivatives for the treatment or prevention of renal lithiasis | |
| WO2013024589A1 (en) | Prophylactic agent and/or therapeutic agent for sepsis | |
| AU2017347778A1 (en) | Delaying latency to seizure by combinations of ketone supplements | |
| EP3193936B1 (en) | Potentiated antimicrobial agents | |
| WO2024163039A1 (en) | Cancer treatment using pi3k inhibitors in combination with antibiotics or purified diets | |
| WO2022028243A1 (en) | Use of compound amino acids in preparation of drugs enhancing sensitivity of bacteria to antibiotics | |
| WO2002100393A1 (en) | Compositions for ameliorating attention-deficient/hyperactivity disorder | |
| EP3801490B1 (en) | Compounds for use in cancer cachexia | |
| JP5101521B2 (en) | Compositions and methods for sensitizing methicillin-resistant Staphylococcus aureus to oxacillin | |
| WO2004066992A1 (en) | Medicinal composition for treating infection with drug-resistant staphylococcus aureus | |
| JP7738992B2 (en) | Alzheimer's disease-like symptom alleviation | |
| EP3628009A1 (en) | Compositions and methods for improving cognition | |
| CA2973370A1 (en) | Compositions and methods for treating gastrointestinal infections | |
| CA2888791C (en) | Use of a combination of citrulline and glutathione in the treatment of brain damage and cognitive decline | |
| WO2015026708A2 (en) | Amelioration of the effects of friedriech's ataxia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:23832919 Country of ref document:EP Kind code of ref document:A1 | |
| NENP | Non-entry into the national phase | Ref country code:DE |