WO2024068518A1 - 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide - Google Patents
3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicideDownload PDFInfo
- Publication number
- WO2024068518A1 WO2024068518A1PCT/EP2023/076346EP2023076346WWO2024068518A1WO 2024068518 A1WO2024068518 A1WO 2024068518A1EP 2023076346 WEP2023076346 WEP 2023076346WWO 2024068518 A1WO2024068518 A1WO 2024068518A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- alkoxy
- methyl
- formula
- hydrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/82—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with three ring hetero atoms
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01P—BIOCIDAL, PEST REPELLANT, PEST ATTRACTANT OR PLANT GROWTH REGULATORY ACTIVITY OF CHEMICAL COMPOUNDS OR PREPARATIONS
- A01P3/00—Fungicides
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
Definitions
- BCS223033FC AF/hz 2023-07-04 -1- 3-HETEROARYL-5-CHLORODIFLUOROMETHYL-1,2,4-OXADIAZOLE AS FUNGICIDE
- the present inventionrelates to 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole compounds as well as the uses thereof for controlling harmful microorganisms, in particular phytopathogenic fungi, in crop protection.
- (Hetero)aryl substituted 5-trifluoromethyl oxadiazole compoundswhich may be useful as HDAC6 and/or HDAC4 inhibitors for treating human diseases are known from WO 2013/080120 and WO 2017/222951.
- (hetero)aryl substituted 5-trifluoromethyl oxadiazole compoundsmay also be useful as crop protection agents to combat or prevent microorganisms’ infestations, such as phytopathogenic fungi (WO2019/122323, WO2018/187553, WO2018/162643, WO2017/178245, WO2017/055473, WO2020/208509, EP 3356335).
- the use of 3-aryl-5-chlorodifluoromethyl-1,2,4-oxadiazoles as fungicidesis disclosed in WO2022/207496, WO2022/207494 and WO2022/129190. Numerous fungicidal agents have been developed until now.
- fungicidal compoundsso as to provide compounds being effective against a broad spectrum of fungi, having lower toxicity, higher selectivity, being used at lower dosage rate to reduce or avoid unfavorable environmental or toxicological effects whilst still allowing effective disease control. It may also be desired to identify further fungicidal compounds to prevent the emergence of fungicides resistances. Furthermore, it may be desired to provide further fungicidal compounds having an improved storage stability and/or a higher weather stability, for example an improved photostability.
- the present inventionprovides new fungicidal compounds which have advantages over known compounds and compositions in at least some of these aspects.
- the present inventionrelates to compounds of formula (I): wherein A, R 1 , R 2 , R 3 and R 4 are as recited herein, as well as their salts, N-oxides and solvates.
- the present inventionalso relates to a composition comprising at least one compound of formula (I) as defined herein and at least one agriculturally suitable auxiliary.
- the present inventionalso relates to the use of a compound of formula (I) as defined herein or a composition as defined herein for controlling harmful microorganisms, in particular phytopathogenic fungi, in crop protection.
- the present inventionalso relates to a method for controlling harmful microorganisms in crop protection, which comprises the step of applying at least one compound of formula (I) as defined herein or a composition as defined herein to the microorganisms and/or their habitat.
- halogenrefers to fluorine, chlorine, bromine or iodine atom.
- C 1 -C 6 -alkylrefers to a saturated, branched or straight hydrocarbon chain having 1, 2, 3, 4, 5 or 6 carbon atoms. Preferably, said hydrocarbon chain has 1, 2, 3 or 4 carbon atoms (“C 1 -C 4 - alkyl”).
- C 1 -C 6 -alkylexamples include methyl, ethyl, propyl (n-propyl), 1-methylethyl (iso-propyl), butyl (n-butyl), 1-methylpropyl (sec-butyl), 2-methylpropyl (iso-butyl), 1,1-dimethylethyl (tert-butyl), pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl, 1,1- dimethylpropyl, 1,2-dimethylpropyl, hexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4- methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3- dimethylbutyl, 3,3-dimethylbutyl, 1-ethy
- C 1 -C 2 -alkylrefers to methyl or ethyl.
- C 2 -C 6 -alkenylrefers to an unsaturated, branched or straight hydrocarbon chain having 2, 3, 4, 5 or 6 carbon atoms and comprising at least one double bond that can be of either the (E)- or (Z)-configuration.
- said hydrocarbon chainhas 3, 4, 5 or 6 carbon atoms (“C 3 -C 6 -alkenyl”).
- C 2 -C 6 -alkenylexamples include but are not limited to C 2 -C 4 -alkenyl groups such as ethenyl (or "vinyl"), prop-2-en-1-yl (or “allyl”), prop-1-en-1-yl, but-3-enyl, but-2-enyl, but-1-enyl, prop-1-en-2-yl (or “isopropenyl”), 2-methylprop-2-enyl, 1-methylprop-2-enyl, 2-methylprop-1-enyl, 1-methylprop-1-enyl and buta-1,3-dienyl.
- C 2 -C 4 -alkenyl groupssuch as ethenyl (or "vinyl"), prop-2-en-1-yl (or “allyl”), prop-1-en-1-yl, but-3-enyl, but-2-enyl, but-1-enyl, prop-1-en-2-yl (or “isopropenyl”),
- C 2 -C 6 -alkynylrefers to a branched or straight hydrocarbon chain having 2, 3, 4, 5 or 6 carbon atoms and comprising at least one triple bond.
- said hydrocarbon chainhas 3, 4, 5 or 6 carbon atoms (“C 3 -C 6 -alkynyl”).
- Examples of C 2 -C 6 -alkynylinclude but are not limited to C 2 - C 4 -alkynyl groups such as ethynyl, prop-1-ynyl, prop-2-ynyl (or “propargyl"), but-1-ynyl, but-2-ynyl, but- 3-ynyl or 1-methylprop-2-ynyl group.
- C 1 -C 6 -haloalkylrefers to a C 1 -C 6 -alkyl group as defined above in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different. Typically, C 1 - C 6 -haloalkyl comprises up to 9 halogen atoms that can be the same or different. Analogously, the term “C 1 -C 4 -haloalkyl” as used herein refer to a corresponding group that contains 1 to 4 carbon atoms.
- C 2 -C 6 -haloalkenyl and “C 3 -C 6 -haloalkenyl” as used hereinrefer to a C 2 -C 6 -alkenyl or, respectively, C 3 -C 6 -alkenyl group as defined above in which one or more hydrogen atoms are replaced with one or more halogen atoms that may be the same or different.
- C 2 -C 6 -haloalkenylcomprises up to 9 halogen atoms that can be the same or different.
- hydroxy-C 1 -C 6 -alkyland “hydroxy-C 1 -C 4 -alkyl”as used herein refer to a C 1 -C 6 -alkyl or, respectively, C 1 -C 4 -alkyl group as defined above in which at least one hydrogen atom is replaced with a hydroxy group.
- cyano-C 1 -C 6 -alkylrefers to a C 1 -C 6 -alkyl group as defined above in which at least one hydrogen atom is replaced with a cyano group.
- amino-C 1 -C 6 -alkylrefers to a C 1 -C 6 -alkyl group as defined above in which at least one hydrogen atom is replaced with an amino group.
- C 1 -C 6 -alkoxyrefers to a group of formula (C 1 -C 6 -alkyl)-O-, in which the term "C 1 -C 6 -alkyl” is as defined herein.
- C 1 -C 4 -alkoxyrefers to a corresponding group containing a "C 1 -C 4 -alkyl” group as defined herein and the term “C 1 -C 2 -alkoxy” as used herein refers to a corresponding group containing a "C 1 -C 2 -alkyl” group as defined herein.
- C 1 -C 6 -alkoxyexamples include methoxy, ethoxy, n-propoxy, 1-methylethoxy, n-butoxy, 1-methylpropoxy, 2- methylpropoxy, 1,1-dimethylethoxy, n-pentoxy, 1-methylbutoxy, 2-methylbutoxy, 3-methylbutoxy, 2,2- dimethylpropoxy, 1-ethylpropoxy, 1,1-dimethylpropoxy, 1,2-dimethylpropoxy, n-hexyloxy, 1- methylpentoxy, 2-methylpentoxy, 3-methylpentoxy, 4-methylpentoxy, 1,1-dimethylbutoxy, 1,2- dimethylbutoxy, 1,3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethylbutoxy, 1- ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy, 1-ethyl-1-methylpropoxy and 1-ethyl-2-methylpropoxy
- C 1 -C 4 -haloalkoxyrefers to a C 1 -C 4 -alkoxy group as defined above in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different.
- C 1 -C 2 -haloalkoxyrefers to a corresponding group that contains 1 or 2 carbon atoms.
- C 1 -C 4 -haloalkoxyexamples are chloromethoxy, bromomethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 1-chloroethoxy, 1-bromoethoxy, 1-fluoroethoxy, 2- fluoroethoxy, 2,2-difluoroethoxy, 2,2,2-trifluoroethoxy, 2-chloro-2-fluoroethoxy, 2-chloro-2,2- BCS223033 FC -4- difluoroethoxy, 2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy, pentafluoroethoxy and 1,1,1- trifluoroprop-2-oxy.
- C 2 -C 6 -alkenyloxy and “C 3 -C 6 -alkenyloxy”refer to groups of the formulae (C 2 -C 6 -alkenyl)- O- and (C 3 -C 6 -alkenyl)-O-, respectively, in which the terms "C 2 -C 6 -alkenyl” and "C 3 -C 6 -alkenyl” are as defined herein.
- C 3 -C 6 -haloalkenyloxyrefers to a C 3 -C 6 -alkenyloxy group as defined herein in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different.
- C 2 -C 6 -alkynyloxyand “C 3 -C 6 -alkynyloxy” refer to groups of the formulae (C 2 -C 6 -alkynyl)- O- and (C 3 -C 6 -alkynyl)-O-, respectively, in which the terms "C 2 -C 6 -alkynyl” and "C 3 -C 6 -alkynyl” are as defined herein.
- C 1 -C 6 -alkylsulfanylrefers to a saturated, linear or branched group of formula (C 1 -C 6 -alkyl)-S-, in which the term “C 1 -C 6 -alkyl” is as defined herein.
- C 1 -C 4 - alkylsulfanylrefers to a corresponding group containing a "C 1 -C 4 -alkyl” group as defined herein.
- C 1 -C 6 -alkylsulfanylexamples include methylsulfanyl, ethylsulfanyl, propylsulfanyl, isopropylsulfanyl, butylsulfanyl, sec-butylsulfanyl, isobutylsulfanyl, tert-butylsulfanyl, pentylsulfanyl, isopentylsulfanyl and hexylsulfanyl group.
- C 1 -C 4 - alkylsulfonylrefers to a corresponding group containing a "C 1 -C 4 -alkyl” group as defined herein.
- C 1 -C 4 -alkylsulfonylexamples include methylsulfonyl, ethylsulfonyl, propylsulfonyl, 1-methyl- ethylsulfonyl, butylsulfonyl, 1-methylpropylsulfonyl, 2-methylpropylsulfonyl and 1,1- dimethylethylsulfonyl.
- C 1 -C 4 - alkylcarbonylrefers to corresponding groups that contains a C 1 -C 4 -alkyl group as defined herein.
- C 1 -C 4 -haloalkylcarbonylrefers to a C 1 -C 4 -alkylcarbonyl as defined above in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different.
- C 1 -C 4 - alkylcarbonyloxyrefers to a corresponding group containing a C 1 -C 4 -alkyl group.
- C 1 -C 4 -haloalkylcarbonyloxyrefers to a C 1 -C 4 -alkylcarbonyloxy as defined above in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different.
- C 1 -C 4 - alkoxycarbonylrefers to a corresponding group containing a C 1 -C 4 -alkoxy group.
- N-(C 1 -C 4 -alkyl)aminorefer to an amino radical having one C 1 -C 4 -alkyl group as defined herein.
- N-(C 1 -C 4 -alkyl)aminoexamples include but are not limited to N-methylamino, N- ethylamino, N-isopropylamino, N-n-propylamino, N-isopropylamino and N-tert-butylamino.
- N,N-di(C 1 -C 4 -alkyl)aminorefers to an amino radical having two independently selected C 1 -C 4 -alkyl groups as defined herein.
- di-(C 1 -C 4 -alkyl)aminoexamples include but are not limited to N,N-dimethylamino, N,N-diethylamino, N,N-diisopropylamino, N-ethyl-N-methylamino, N- methyl-N-n-propylamino, N-isopropyl-N-n-propylamino and N-tert-butyl-N-methylamino.
- N-(C 1 -C 4 -alkylcarbonyl)aminorefers to a group of the formula (C 1 -C 4 - alkylcarbonyl)-NH-, in which the term “C 1 -C 4 -alkylcarbonyl” is as defined herein.
- N-(C 1 -C 2 -alkylcarbonyl)aminorefer to a corresponding group containing a C 1 -C 2 - alkylcarbonyl group.
- C 3 -C 6 -cycloalkylrefers to a saturated, monovalent, monocylic hydrocarbon ring which contains 3, 4, 5 or 6 carbon atoms.
- Examples of C 3 -C 6 -cycloalkylinclude cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
- C 3 -C 6 -cycloalkyloxyrefers to a group of formula (C 3 -C 6 -cycloalkyl)-O-, in which the term "C 3 -C 6 -cycloalkyl" is as defined herein.
- C 3 -C 6 -cycloalkylaminorefers to a group of formula (C 3 -C 6 -cycloalkyl)-NH-, in which the term “C 3 -C 6 -cycloalkyl” is as defined herein.
- arylrefers to an aromatic hydrocarbon ring system in which all of the ring members, which vary from 6 to 14, preferably from 6 to 10, are carbon atoms. The ring system may be monocyclic or fused polycyclic (e.g. bicyclic or tricyclic).
- arylexamples include but are not limited to phenyl, azulenyl, naphthyl and fluorenyl.
- the arylcan be attached to the parent molecular moiety through any carbon atom. It is further understood that when said aryl group is substituted with one or more substituents, said substituent(s) may be at any positions on said aryl ring(s). Particularly, in the case of aryl being a phenyl group, said substituent(s) may occupy one or both ortho positions, one or both meta positions, or the para position, or any combination of these positions.
- the term “4- to 6-membered heterocyclyl” as used hereinrefers to a 4-, 5- or 6-membered monocyclic ring system containing 1, 2 or 3 heteroatoms independently selected from the group consisting of oxygen, nitrogen and sulfur where the ring system is saturated or unsaturated but not aromatic.
- the heterocyclemay comprise one to three nitrogen atoms, or one or two oxygen atoms, or one or two sulfur atoms, or one to three nitrogen atoms and one oxygen atom, or one to three nitrogen atoms and a sulfur atom or one sulfur atom and one oxygen atom.
- saturated 4- to 6-membered heterocyclyl groupsinclude 4-membered rings such as azetidinyl, oxetanyl and thietanyl, 5-membered rings such as tetrahydrofuranyl, 1,3-dioxolanyl, tetrahydrothienyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, triazolidinyl, isoxazolidinyl, oxazolidinyl, oxadiazolidinyl, thiazolidinyl, isothiazolidinyl and thiadiazolidinyl, and 6-membered rings such as piperidinyl, hexahydropyridazinyl, hexahydropyrimidinyl, piperazinyl, triazinanyl, hexahydrotriazinyl, tetrahydropyranyl, dioxanyl, tetra
- unsaturated 4- to 6-membered heterocyclesinclude but are not limited to 5-membered rings such as dihydrofuranyl, 1,3-dioxolyl, dihydrothienyl, pyrrolinyl, dihydroimidazolyl, dihydropyrazolyl, isoxazolinyl, dihydrooxazolyl and dihydrothiazolyl, and 6-membered rings such as pyranyl, thiopyranyl, thiazinyl and thiadiazinyl.
- 5-membered ringssuch as dihydrofuranyl, 1,3-dioxolyl, dihydrothienyl, pyrrolinyl, dihydroimidazolyl, dihydropyrazolyl, isoxazolinyl, dihydrooxazolyl and dihydrothiazolyl
- 6-membered ringssuch as pyranyl, thiopyranyl, thiazinyl and thiadiazinyl.
- the term “5- to 14-membered heteroaryl” as used hereinrefers to an aromatic ring system comprising 1 to 4 heteroatoms independently selected from the group consisting of oxygen, nitrogen and sulfur. If the ring system contains more than one oxygen atom, they are not directly adjacent.
- Aromatic heterocyclesinclude aromatic 5- or 6-membered monocyclic heterocycles and 6- to 14-membered polycyclic (e.g.
- the 5- to 14-membered aromatic heterocyclecan be connected to the parent molecular moiety through any carbon atom or nitrogen atom contained within the heterocycle.
- the term “5- or 6-membered heteroaryl” as used hereinrefers to a 5- or 6-membered monocyclic, aromatic ring system containing 1, 2, 3 or 4 heteroatoms independently selected from the group consisting of oxygen, nitrogen and sulfur.
- Examples of 5-membered heteroarylsinclude but are not limited to furyl (furanyl), thienyl, pyrrolyl, pyrazolyl, imidazolyl, triazolyl, tetrazolyl, isoxazolyl, oxazolyl, oxadiazolyl, oxatriazolyl, isothiazolyl, thiazolyl, thiadiazolyl and thiatriazolyl.
- Examples of 6-membered heteroarylsinclude but are not limited to pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, tetrazinyl.
- 6- to 14-membered polycyclic heteroarylrefers to a 6-, 7-, 8-, 9-, 10-, 11-, 12-, 13- or 14-membered polycyclic (e.g. bicyclic or tricyclic), aromatic ring system containing 1, 2 or 3 heteroatoms independently selected from the group consisting of oxygen, nitrogen and sulfur.
- 6- to 14- membered polycyclic heteroarylsmay consist of a 5- or 6-membered heteroaryl as defined herein fused to an aryl (e.g. phenyl) or to a 5- or 6-membered heteroaryl.
- bicyclic heteroarylsinclude but are not limited to 9-membered rings such as indolyl, indolizinyl, isoindolyl, benzimadozolyl, imidazopyridinyl, indazolyl, benzotriazolyl, purinyl, benzofuranyl, benzothiophenyl, benzothiazolyl, benzoxazolyl and benzisoxazolyl and 10-membered rings such as quinolinyl, isoquinolinyl, cinnolinyl, quinazolinyl, quinoxalinyl, phthalazinyl, naphthyridinyl, pteridinal and benzodioxinyl.
- 9-membered ringssuch as indolyl, indolizinyl, isoindolyl, benzimadozolyl, imidazopyridinyl, indazolyl, benzotriazolyl, puriny
- nitrogen atommay be at the bridgehead (e.g. imidazo[1,2-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, imidazo[1,2- a]pyridinyl, imidazo[2,1-b]oxazolyl, furo[2,3-d]isoxazolyl).
- Examples of tricyclic heteroarylsinclude but are not limited to carbazolyl, acridinyl and phenazinyl. Unless defined otherwise, the definitions for collective terms also apply to these collective terms in composite moieties.
- the groupwhen a group is said to be “substituted”, the group may be substituted with one or more substituents.
- the expression “one or more substituents”refers to a number of substituents that ranges from one to the maximum number of substituents possible based on the number of available bonding sites, provided that the conditions of stability and chemical feasibility are met.
- composite moietyas used herein is to be understood as meaning a moiety that is composed of at least two smaller moieties as defined herein, such as “C 1 -C 4 -alkoxy-C 1 -C 4 -alkoxy-C 1 -C 6 -alkyl”, “C 1 - C 4 -alkylcarbonyloxy-C 1 -C 4 -alkyl”, “C 1 -C 4 -haloalkylcarbonyloxy-C 1 -C 4 -alkyl”, “C 1 -C 4 - alkoxycarbonyloxy-C 1 -C 4 -alkyl”, “C 3 -C 6 -cycloalkyl-C 1 -C 2 -alkyl”, “C 3 -C 6 -cycloalkyl-C 1 -C 2 -alkoxy”, “C 3 -C 6 -cycloalkylcarbonyloxy-C 1 -C 2 -alkyl”, “5-
- leaving groupas used herein is to be understood as meaning a group which is displaced from a compound in a substitution or an elimination reaction, for example a halogen atom, a trifluoromethanesulfonate (“triflate”) group, alkoxy, methanesulfonate, p-toluenesulfonate, etc.
- trimertrifluoromethanesulfonate
- the present inventionrelates to compounds of formula (I) (I) BCS223033 FC -9- wherein A is selected from the group consisting of wherein n is 0, 1 or 2, and where the bond identified by “x” is bonded directly to the oxadiazol ring and the bond identified by “y” is bonded directly to the CR 1 R 2 group, R 1 and R 2 are independently selected from the group consisting of hydrogen, C 1 -C 4 -alkyl, halogen, trifluoromethyl and difluoromethyl, or R 1 and R 2 form, together with the carbon atom to which they are linked, a cyclopropyl ring, which is unsubstituted or substituted with one to three halogen atoms; R 3 is hydrogen, hydroxy, C 1 -C 4 -alkyl, C 1 -C 4 -haloalkyl, C 1 -C 4 -alkoxy, hydroxy-C 1 -C 4 -alkyl, C 1
- the compounds of formula (I)can be used for controlling harmful microorganisms, in particular phytopathogenic fungi, in crop protection.
- the phytopathogenic fungiare selected from the group consisting of the Puccinia species, for example Puccinia recondita, Puccinia graminis or Puccinia striiformis; the Uromyces species, for example Uromyces appendiculatus; and the rust disease pathogens, in particular selected from the group consisting of the Gymnosporangium species, for example Gymnosporangium sabinae; Hemileia species, for example Hemileia vastatrix, and Phakopsora species, for example Phakopsora pachyrhizi or Phakopsora meibomiae.
- the compound of the inventionmay be present in the form of different stereoisomers. These stereoisomers are, for example, enantiomers, diastereomers, atropisomers or geometric isomers. Accordingly, the invention encompasses both pure stereoisomers and any mixture of these isomers. Where a compound can be present in two or more tautomer forms in equilibrium, reference to the compound by means of one tautomeric description is to be considered to include all tautomer forms.
- the compound of the inventionmay be present in the form of the free compound or an agrochemically active salt or N-oxide thereof.
- Agrochemically active saltsinclude acid addition salts of inorganic and organic acids well as salts of customary bases.
- inorganic acidsexamples include hydrohalic acids, such as hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide, sulfuric acid, phosphoric acid and nitric acid, and acidic salts, such as sodium bisulfate and potassium bisulfate.
- hydrohalic acidssuch as hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide
- sulfuric acidphosphoric acid and nitric acid
- acidic saltssuch as sodium bisulfate and potassium bisulfate.
- Useful organic acidsinclude, for example, formic acid, carbonic acid and alkanoic acids such as acetic acid, trifluoroacetic acid, trichloroacetic acid and propionic acid, and also glycolic acid, thiocyanic acid, lactic acid, succinic acid, citric acid, benzoic acid, cinnamic acid, oxalic acid, saturated or mono- or diunsaturated fatty acids having 6 to 20 carbon atoms, alkylsulphuric monoesters, alkylsulphonic acids (sulphonic acids having straight-chain or branched alkyl radicals having 1 to 20 carbon atoms), arylsulphonic acids or aryldisulphonic acids (aromatic radicals, such as phenyl and naphthyl, which bear one or two sulphonic acid groups), alkylphosphonic acids (phosphonic acids having straight-chain or branched alkyl radicals having 1 to 20 carbon atoms), BCS223033 FC -13-
- N-oxidescan be obtained in a simple manner by customary processes, for example by N-oxidation with hydrogen peroxide (H 2 O 2 ), peracids, for example peroxy sulfuric acid or peroxy carboxylic acids, such as meta-chloroperoxybenzoic acid or peroxymonosulfuric acid (Caro ⁇ s acid).
- peracidsfor example peroxy sulfuric acid or peroxy carboxylic acids, such as meta-chloroperoxybenzoic acid or peroxymonosulfuric acid (Caro ⁇ s acid).
- the corresponding N-oxidesmay be prepared starting from the respective compounds using conventional oxidation methods, e.g. by treating the compounds with an organic peracid such as metachloroperbenzoic acid (e.g. WO-A 2003/64572 or J. Med.
- the oxidationmay lead to pure mono-N- oxides or to a mixture of different N-oxides, which can be separated by conventional methods such as chromatography.
- the compound of the inventionmay exist in multiple crystalline and/or amorphous forms. Crystalline forms include unsolvated crystalline forms, solvates and hydrates.
- Solvates of the compounds of the invention or their saltsare stoichiometric compositions of the compounds with solvents.
- Compounds of formula (I)are herein also referred to as “active ingredient(s)”.
- Ais A 1 , A 2 , A 4 , A 6 , A 8 or A 12 , and n is 0 or 1. More preferably, in the above formula (I), A is A 1 , A 2 or A 8 , and n is 0 or 1. In some embodiments, in the above formula (I), A is A 1 or A 8 , and n is 0 or 1. In some other embodiments, in the above formula (I), A is A 1 or A 2 .
- Ais A 8 .
- R 1 and R 2are independently selected from hydrogen, methyl and ethyl, or R 1 and R 2 form, together with the carbon atom to which they are linked, a cyclopropyl ring. More preferably, in the above formula (I), R 1 is hydrogen, methyl or ethyl, and R 2 is hydrogen. In some embodiments, in the above formula (I), R 1 and R 2 are hydrogen.
- R 3is hydrogen, hydroxy, C 1 -C 4 -alkyl, C 1 -C 4 -haloalkyl, C 1 -C 4 - alkoxy, C 1 -C 4 -alkoxycarbonyloxy, C 1 -C 4 -alkylcarbonyl, C 1 -C 4 -alkoxycarbonyl, C 3 -C 6 -cycloalkyl, C 3 -C 6 - cycloalkylamino, C 3 -C 6 -cycloalkyloxy or phenoxy.
- R 3is hydrogen, hydroxy, C 1 -C 4 -alkyl, C 1 -C 4 -alkoxy, C 1 -C 4 - alkoxycarbonyloxy, C 3 -C 4 -cycloalkyl, C 3 -C 4 -cycloalkyloxy or phenoxy.
- R 3is hydrogen, hydroxy, methyl, ethyl, isopropyl, methoxy, ethoxy, iso-propoxy, methoxycarbonyloxy, ethoxycarbonyloxy, isopropylcarbonyloxy, tert- butylcarbonyloxy, cyclopropyl, cyclobutyl, cyclopropyloxy or phenoxy.
- R 5is C 1 -C 6 -alkyl, C 1 -C 6 -haloalkyl, C 1 -C 4 -alkoxy-C 1 -C 4 -alkyl, C 1 -C 4 -haloalkoxy-C 1 -C 4 -alkyl, C 1 - C 4 -alkoxy-C 1 -C 4 -alkoxy-C 1 -C 4 -alkyl, C 1 -C 4 -alkoxycarbonyl-C 1 -C 2 -alkyl, C 3 -C 6 -cycloalkyl, phenyl, benzyl or 5- or 6-membered heteroaryl, wherein said C 3 -C 6 -cycloalkyl, phenyl, benzyl and 5- or 6-membere heteroaryl moieties
- BCS223033 FC -15- R 5is C 1 -C 4 -alkyl, C 1 -C 4 -haloalkyl, C 1 -C 4 -alkoxy-C 1 -C 4 -alkyl, C 1 -C 4 -haloalkoxy-C 1 -C 4 -alkyl or C 1 -C 4 -alkoxy-C 1 -C 4 -alkoxy-C 1 -C 4 -alkyl, C 1 -C 4 -alkoxycarbonyl-C 1 -C 2 -alkyl or C 3 -C 6 - cycloalkyl, R 6 is C 1 -C 6 -alkyl or C 3 -C 4 -cycloalkyl, R 7 is hydrogen or C 1 -C 6 -alkyl, and R 8 is C 1 -C 4 -alkyl or C 1 -C 4 -alkoxy, or R 7 and
- R 5is methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-butyl, tert-butyl, fluoromethyl, 2,2,2-trifluoroethyl, methoxymethyl, ethoxymethyl, 1-methoxyethyl, 2-methoxyethyl, methoxycarbonylmethyl or cyclopropyl, in particular methyl ethyl, methoxymethyl, ethoxymethyl, 1-methoxyethyl or 2-methoxyethyl; R 6 is methyl, ethyl, n-propyl, isopropyl, tert-butyl or cyclopropyl; R 7 is hydrogen, methyl or ethyl, and R 8 is methyl, ethyl, methoxy or ethoxy, or R 7 and R 8 together with the nitrogen to which they are
- the said preferred featurescan also be selected among the more preferred features of each of A, R 1 , R 2 , R 3 and R 4 so as to form more preferred subclasses of compounds according to the invention. This also applies to the preferences with regard to the substituents of the compounds according to the embodiments (Ia), (Ib) and (Ic) mentioned below.
- Ais A 1 , A 2 or A 8 .
- R 1 and R 2are independently selected from hydrogen, methyl and ethyl
- R 3is hydrogen, hydroxy, C 1 -C 4 -alkyl, C 1 -C 4 -alkoxy, C 1 -C 4 -alkoxycarbonyloxy, C 3 -C 4 -cycloalkyl, C 3 -C 4 -cycloalkyloxy or phenoxy
- R 1is hydrogen, methyl or ethyl
- R 2is hydrogen
- R 3is hydrogen, hydroxy, methyl, ethyl, isopropyl, methoxy, ethoxy, iso-propoxy, methoxycarbonyloxy, ethoxycarbonyloxy, isopropylcarbonyloxy, tert-butylcarbonyloxy, cyclopropyl, cyclobutyl, cyclopropyloxy or phenoxy
- the compounds of formula (I)are useful for controlling phytopathogenic fungi in crop protection (use as fungicide).
- the present inventionalso relates to the use of the compounds of formula (I) for controlling phytopathogenic fungi in crop protection.
- the present inventionalso relates to any compounds of formula (I) disclosed in Table 1.
- Intermediates for the preparation of the active ingredientsThe present invention also relates to intermediates for the preparation of compounds of formula (I).
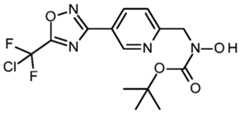
- the present inventionrelates to compounds of formula (V) as well as their acceptable salts, N-oxides or solvates: wherein A, R 1 , R 2 and R 3 have the meanings as defined herein for the compounds of formula (I).
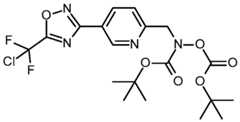
- the present inventionalso relates to compounds of formula (II) as well as their acceptable salts, N-oxides and solvates: (II), BCS223033 FC -22- wherein A, R 1 , R 2 , R 3 and R 4 have the meanings as defined herein for the compounds of formula (I).
- the present inventionalso relates to compounds of formula (III) as well as their acceptable salts, N-oxides and solvates: (III), wherein A, R 1 , R 2 , R 3 and R 4 have the meanings as defined herein for the compounds of formula (I).
- the present inventionalso relates to compounds of formula (XII) as well as their acceptable salts, N- oxides and solvates: (XII), wherein A and R 1 have the meanings as defined herein for the compounds of formula (I).
- the present inventionalso relates to compounds of formula (XV) as well as their acceptable salts, N- oxides and solvates: (XV), wherein A, R 1 and R 3 have the meanings as defined herein for the compounds of formula (I).
- the present inventionalso relates to compounds of formula (IX) as well as their acceptable salts, N-oxides and solvates: BCS223033 FC -23- (IX), wherein A, R 1 and R 2 have the meanings as defined herein for the compounds of formula (I) and W is an halogen, hydroxy, mesylate or triflate group.
- the present inventionalso relates to compounds of formula (X) as well as their acceptable salts, N-oxides and solvates: wherein R 1 , R 2 and A have the meanings as defined herein for the compounds of formula (I).
- Processes for the preparation of compounds of formula (I) and intermediatesThe present invention relates to processes for the preparation of compounds of formula (I) and their intermediates.
- Process P1 BCS223033 FC -24- Amidoximes of formula (II)can be prepared according to known procedures (see for examples WO2013080120), as shown in process P2 by treating nitriles of formula (III) with hydroxylamine (or its hydrochloride salt) in the presence of a base such as triethylamine in a solvent such as ethanol.
- Process P2 Compounds of formula (III)can be commercially available or may be prepared starting from readily available compounds according to known procedures.
- compounds of formula (III)can be prepared, according to process P3, from compounds of formula (IV), wherein LG is a leaving group as for example bromide with a suitable cyanide reagent such as for example zinc cyanide in presence of palladium (0) in a solvent such as N,N-dimethylformamide as described for example in ACS Medicinal Chemistry Letters, 8(9), 919-924, 2017.
- a suitable cyanide reagentsuch as for example zinc cyanide in presence of palladium (0) in a solvent such as N,N-dimethylformamide as described for example in ACS Medicinal Chemistry Letters, 8(9), 919-924, 2017.
- Process P3Compounds of formula (IV) can be commercially available or may be prepared starting from readily available compounds according to known procedures.
- compounds of formula (I)can be prepared, according to process P4, from a compound of formula (V), with a compound of formula (VI) wherein LG1 is a leaving group in presence of a base like for example triethylamine in a solvent such as for example dichloromethane.
- Process P4Compounds of formula (V) may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- BCS223033 FC -25- Compounds of formula (VI)can be commercially available or may be prepared starting from readily available compounds according to known procedures.
- compounds of formula (V)can be prepared, according to process P5, from a compound of formula (VII), wherein PG is a protecting group by using state of the art deprotecting condition like for example trifluoroacetic acid in dichloromethane when PG is a tert-butyloxycarbonyl group.
- Compounds of formula (VII)may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- Compounds of formula (VIII)may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- compounds of formula (VIII)can be prepared, according to process P7, from a compound of formula (IX), wherein W is a leaving group by nucleophilic substitution with a compound of formula (VI) (as described for example in Journal of Organic Chemistry, 78, 5218; 2013 or Tetrahedron, 72, 734; 2016) in presence of a base (like for example potassium carbonate or sodium hydride) in a solvent such as for example acetonitrile or DMF.
- a baselike for example potassium carbonate or sodium hydride
- a solventsuch as for example acetonitrile or DMF.
- compounds of formula (V)can be prepared, according to process P9, by reacting a compound of formula (IX) with a compound of formula (XI) as described for example in Organic Letters 6(14), 2361, 2004 optionally in presence of a base like for example diisopropylethylamine, optionally in presence of a catalyst like for example potassium iodide, in a solvent such as, for example, acetonitrile or dimethylformamide.
- a baselike for example diisopropylethylamine
- a catalystlike for example potassium iodide
- a solventsuch as, for example, acetonitrile or dimethylformamide.
- compounds of formula (IX)can be prepared, according to process P11, from a compound of formula (XIII) by treatment with mesyl chloride or triflic anhydride in presence of a base like for example triethylamine in a solvent such as, for example dichloromethane as described for example in Journal of the American Chemical Society, 135(44), 16288-16291, 2013 or by reaction with an halogenating agent, like for example carbon tetrabromide, optionally in presence of triphenylphosphine in a solvent such as, for example, dichloromethane as described for example in Bioorganic & Medicinal Chemistry Letters, 17(3), 756-760, 2007.
- a baselike for example triethylamine
- a solventsuch as, for example dichloromethane as described for example in Journal of the American Chemical Society, 135(44), 16288-16291, 2013 or by reaction with an halogenating agent, like for example carbon tetrabromide, optionally in presence of trip
- Process P13Compounds of formula (XV) may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- compounds of formula (XV)can be prepared, according to process P14, by reacting a compound of formula (XII) with a compound of formula (XVI) or a salt thereof, optionally in presence of a base such as sodium acetate in a solvent such as ethanol as described for example Organic & Biomolecular Chemistry (2020), 18(34), 6732-6737.
- BCS223033 FC -29- Process P14Compounds of formula (XVI) can be commercially available or may be prepared starting from readily available compounds according to known procedures.
- Compounds of formula (XII)may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- compounds of formula (XII)can be prepared, according to process P15, from a compound of formula (XIII) by treatment with an oxidizing agent such as for example manganese oxide in a solvent such as for example chloroform as described for example in Journal of the American Chemical Society (2019), 141(6), 2274-2278.
- Process P15Compounds of formula (XIII) may be prepared starting from readily available compounds analogously to process P1 and P2 or analogously to process P1, P2 and P3.
- processes P1 to P15can be performed if appropriate in the presence of a solvent and if appropriate in the presence of a base.
- Suitable solvents for carrying out processes P1 to P15 according to the inventionare customary inert organic solvents. Preference is given to using optionally halogenated aliphatic, alicyclic or aromatic hydrocarbons, such as petroleum ether, hexane, heptane, cyclohexane, methylcyclohexane, benzene, toluene, xylene or decalin ; chlorobenzene, dichlorobenzene, dichloromethane, chloroform, carbon tetrachloride, dichlorethane or trichlorethane ; ethers, such as diethyl ether, diisopropyl ether, methyl tert- butyl ether, methyl tert-amyl ether, dioxane, tetrahydrofuran, 1,
- Suitable bases for carrying out processes P1 to P15 according to the inventionare inorganic and organic bases which are customary for such reactions.
- alkaline earth metalalkali metal hydride, alkali metal hydroxides or alkali metal alkoxides, such as sodium hydroxide, sodium hydride, calcium hydroxide, potassium hydroxide, potassium tert-butoxide or other ammonium hydroxide
- alkali metal carbonatessuch as sodium carbonate, potassium carbonate, potassium bicarbonate, sodium bicarbonate, cesium carbonate
- alkali metal or alkaline earth metal acetatessuch as sodium acetate, potassium acetate, calcium acetate and also tertiary amines, such as trimethylamine, triethylamine, diisopropylethylamine, tributylamine, N,N-dimethylaniline, pyridine, N-methylpiperidine, N,N-dimethyl- aminopyridine, 1,4-diazabicyclo
- the reaction temperaturecan independently be varied within a relatively wide range.
- processes according to the inventionare carried out at temperatures between -20°C and 160°C.
- Processes P1 to P15 according to the inventionare generally independently carried out under atmospheric pressure. However, it is also possible to operate under elevated or reduced pressure. Work-up is carried out by customary methods. Generally, the reaction mixture is treated with water and the organic phase is separated off and, after drying, concentrated under reduced pressure. If appropriate, the remaining residue can be freed by customary methods, such as chromatography or recrystallization, from any impurities that can still be present.
- Compounds according to the inventioncan be prepared according to the above described processes.
- compositions/FormulationsThe present invention further relates to compositions, in particular compositions for controlling unwanted microorganisms.
- the compositionmay be applied to the microorganisms and/or in their habitat.
- the compositioncomprises at least one compound of the invention and at least one agriculturally suitable auxiliary, e.g. carrier(s) and/or surfactant(s).
- a carrieris a solid or liquid, natural or synthetic, organic or inorganic substance that is generally inert.
- the carriergenerally improves the application of the compounds, for instance, to plants, plants parts or BCS223033 FC -31- seeds.
- suitable solid carriersinclude, but are not limited to, ammonium salts, in particular ammonium sulfates, ammonium phosphates and ammonium nitrates, natural rock flours, such as kaolins, clays, talc, chalk, quartz, attapulgite, montmorillonite and diatomaceous earth, silica gel and synthetic rock flours, such as finely divided silica, alumina and silicates.
- typically useful solid carriers for preparing granulesinclude, but are not limited to crushed and fractionated natural rocks such as calcite, marble, pumice, sepiolite and dolomite, synthetic granules of inorganic and organic flours and granules of organic material such as paper, sawdust, coconut shells, maize cobs and tobacco stalks.
- suitable liquid carriersinclude, but are not limited to, water, organic solvents and combinations thereof.
- suitable solventsinclude polar and nonpolar organic chemical liquids, for example from the classes of aromatic and nonaromatic hydrocarbons (such as cyclohexane, paraffins, alkylbenzenes, xylene, toluene, tetrahydronaphthalene, alkylnaphthalenes, chlorinated aromatics or chlorinated aliphatic hydrocarbons such as chlorobenzenes, chloroethylenes or methylene chloride), alcohols and polyols (which may optionally also be substituted, etherified and/or esterified, such as ethanol, propanol, butanol, benzylalcohol, cyclohexanol or glycol), ketones (such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone), esters (including fats and oils) and (poly)ethers, unsubstituted and substituted amines, amide
- the carriermay also be a liquefied gaseous extender, i.e. liquid which is gaseous at standard temperature and under standard pressure, for example aerosol propellants such as halohydrocarbons, butane, propane, nitrogen and carbon dioxide.
- aerosol propellantssuch as halohydrocarbons, butane, propane, nitrogen and carbon dioxide.
- Preferred solid carriersare selected from clays, talc and silica.
- Preferred liquid carriersare selected from water, fatty acid amides and esters thereof, aromatic and nonaromatic hydrocarbons, lactams and carbonic acid esters.
- the amount of carriertypically ranges from 1 to 99.99%, preferably from 5 to 99.9%, more preferably from 10 to 99.5%, and most preferably from 20 to 99% by weight of the composition.
- Liquid carriersare typically present in a range of from 20 to 90%, for example 30 to 80% by weight of the composition. Solid carriers are typically present in a range of from 0 to 50%, preferably 5 to 45%, for example 10 to 30% by weight of the composition. If the composition comprises two or more carriers, the outlined ranges refer to the total amount of carriers.
- the surfactantcan be an ionic (cationic or anionic), amphoteric or non-ionic surfactant, such as ionic or non-ionic emulsifier(s), foam former(s), dispersant(s), wetting agent(s), penetration enhancer(s) and any mixtures thereof.
- surfactantsinclude, but are not limited to, salts of polyacrylic acid, BCS223033 FC -32- salts of lignosulfonic acid (such as sodium lignosulfonate), salts of phenolsulfonic acid or naphthalenesulfonic acid, polycondensates of ethylene oxide and/or propylene oxide with fatty alcohols, fatty acids or fatty amines (for example, polyoxyethylene fatty acid esters such as castor oil ethoxylate, polyoxyethylene fatty alcohol ethers, for example alkylaryl polyglycol ethers), substituted phenols (preferably alkylphenols or arylphenols) and ethoxylates thereof (such as tristyrylphenol ethoxylate), salts of sulfosuccinic esters, taurine derivatives (preferably alkyl taurates), phosphoric esters of polyethoxylated alcohols or phenols, fatty esters of polyols (such
- any reference to salts in this paragraphrefers preferably to the respective alkali, alkaline earth and ammonium salts.
- Preferred surfactantsare selected from polyoxyethylene fatty alcohol ethers, polyoxyethylene fatty acid esters, alkylbenzene sulfonates, such as calcium dodecylbenzenesulfonate, castor oil ethoxylate, sodium lignosulfonate and arylphenol ethoxylates, such as tristyrylphenol ethoxylate.
- the amount of surfactantstypically ranges from 5 to 40%, for example 10 to 20%, by weight of the composition.
- auxiliariesinclude water repellents, siccatives, binders (adhesive, tackifier, fixing agent, such as carboxymethylcellulose, natural and synthetic polymers in the form of powders, granules or latices, such as gum arabic, polyvinyl alcohol and polyvinyl acetate, natural phospholipids such as cephalins and lecithins and synthetic phospholipids, polyvinylpyrrolidone and tylose), thickeners and secondary thickeners (such as cellulose ethers, acrylic acid derivatives, xanthan gum, modified clays, e.g. the products available under the name Bentone, and finely divided silica), stabilizers (e.g.
- cold stabilizerspreservatives (e.g. dichlorophene and benzyl alcohol hemiformal), antioxidants, light stabilizers, in particular UV stabilizers, or other agents which improve chemical and/or physical stability), dyes or pigments (such as inorganic pigments, e.g. iron oxide, titanium oxide and Prussian Blue; organic dyes, e.g. alizarin, azo and metal phthalocyanine dyes), antifoams (e.g.
- auxiliariesmineral and vegetable oils, perfumes, waxes, nutrients (including trace nutrients, such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc), protective colloids, thixotropic substances, penetrants, sequestering agents and complex formers.
- the choice of the auxiliariesdepends on the intended mode of application of the compound of the invention and/or on the physical properties of the compound(s).
- the auxiliariesmay be chosen to impart particular properties (technical, physical and/or biological properties) to the compositions or use forms prepared therefrom. The choice of auxiliaries may allow customizing the compositions to specific needs.
- composition of the inventionmay be provided to the end user as ready-for-use formulation, i.e. the compositions may be directly applied to the plants or seeds by a suitable device, such as a spraying or dusting device. Alternatively, the compositions may be provided to the end user in the form of concentrates which have to be diluted, preferably with water, prior to use.
- the composition of the inventioncan be prepared in conventional manners, for example by mixing the compound of the invention with one or more suitable auxiliaries, such as disclosed herein above.
- the compositioncomprises a fungicidally effective amount of the compound(s) of the invention.
- the term "effective amount”denotes an amount, which is sufficient for controlling harmful fungi on cultivated plants or in the protection of materials and which does not result in a substantial damage to the treated plants. Such an amount can vary in a broad range and is dependent on various factors, such as the fungal species to be controlled, the treated cultivated plant or material, the climatic conditions and the specific compound of the invention used.
- the composition according to the inventioncontains from 0.01 to 99% by weight, preferably from 0.05 to 98% by weight, more preferred from 0.1 to 95% by weight, even more preferably from 0.5 to 90% by weight, most preferably from 1 to 80% by weight of the compound of the invention. It is possible that a composition comprises two or more compounds of the invention.
- composition of the inventionmay be in any customary composition type, such as solutions (e.g aqueous solutions), emulsions, water- and oil-based suspensions, powders (e.g. wettable powders, soluble powders), dusts, pastes, granules (e.g. soluble granules, granules for broadcasting), suspoemulsion concentrates, natural or synthetic products impregnated with the compound of the invention, fertilizers and also microencapsulations in polymeric substances.
- the compound of the inventionmay be present in a suspended, emulsified or dissolved form. Examples of particular suitable composition types are solutions, watersoluble concentrates (e.g.
- SLLS
- dispersible concentratesDC
- suspensions and suspension concentratese.g. SC, OD, OF, FS
- emulsifiable concentratese.g. EC
- emulsionse.g. EW, EO, ES, ME, SE
- capsulese.g. CS, ZC
- pastespastilles
- wettable powders or dustse.g. WP, SP, WS, DP, DS
- pressingse.g. BR, TB, DT
- granulese.g. WG, SG, GR, FG, GG, MG
- insecticidal articlese.g.
- compositions typesare defined by the Food and Agriculture Organization of the United Nations (FAO). An overview is given in the "Catalogue of pesticide formulation types and international coding system", Technical Monograph No.2, 6th Ed. May 2008, Croplife International.
- the composition of the inventionis in form of one of the following types: EC, SC, FS, SE, OD and WG, more preferred EC, SC, OD and WG. Further details about examples of composition types and their preparation are given below.
- the outlined amount of compound of the inventionrefers to the total BCS223033 FC -34- amount of compounds of the present invention. This applies mutatis mutandis for any further component of the composition, if two or more representatives of such component, e.g. wetting agent, binder, are present.
- Water-soluble concentratesSL, LS
- surfactante.g. polyoxyethylene fatty alcohol ether
- water-soluble solvente.g. alcohols such as propylene glycol or carbonates such as propylene carbonate
- Dispersible concentrates5-25 % by weight of at least one compound of the invention and 1-10 % by weight surfactant and/or binder (e.g. polyvinylpyrrolidone) are dissolved in such amount of organic solvent (e.g. cyclohexanone) to result in a total amount of 100 % by weight. Dilution with water gives a dispersion.
- Emulsifiable concentratesEC 15-70 % by weight of at least one compound of the invention and 5-10 % by weight surfactant (e.g.
- EmulsionsEW, EO, ES 5-40 % by weight of at least one compound of the invention and 1-10 % by weight surfactant (e.g. a mixture of calcium dodecylbenzenesulfonate and castor oil ethoxylate) are dissolved in 20-40 % by weight water- insoluble organic solvent (e.g. aromatic hydrocarbon).
- water-insoluble organic solvente.g. aromatic hydrocarbon or fatty acid amide
- SC, FSWater-based
- surfactante.g. sodium lignosulfonate and polyoxyethylene fatty alcohol ether
- thickenere.g. xanthan gum
- bindere.g. polyvinylalcohol
- oil-based (OD, OF)In a suitable grinding equipment, e.g. an agitated ball mill, 20-60 % by weight of at least one compound of the invention are comminuted with addition of 2-10 % by weight surfactant (e.g. sodium lignosulfonate and polyoxyethylene fatty alcohol ether), 0.1-2 % by weight thickener (e.g.
- surfactante.g. sodium lignosulfonate and polyoxyethylene fatty alcohol ether
- thickenere.g.
- Water-dispersible granules and water-soluble granules (WG, SG) 50-80 % by weight of at least one compound of the inventionare ground finely with addition of surfactant (e.g. sodium lignosulfonate and polyoxyethylene fatty alcohol ether) and converted to water-dispersible or water-soluble granules by means of technical appliances (e. g. extrusion, spray tower, fluidized bed).
- surfactante.g. sodium lignosulfonate and polyoxyethylene fatty alcohol ether
- the surfactantis used in such amount to result in a total amount of 100 % by weight. Dilution with water gives a stable dispersion or solution of the active substance.
- Water-dispersible powders and water-soluble powders (WP, SP, WS) 50-80 % by weight of at least one compound of the inventionare ground in a rotor-stator mill with addition of 1-8 % by weight surfactant (e.g. sodium lignosulfonate, polyoxyethylene fatty alcohol ether) and such amount of solid carrier, e.g. silica gel, to result in a total amount of 100 % by weight. Dilution with water gives a stable dispersion or solution of the active substance.
- surfactante.g. sodium lignosulfonate, polyoxyethylene fatty alcohol ether
- solid carriere.g. silica gel
- Gel (GW, GF)In an agitated ball mill, 5-25 % by weight of at least one compound of the invention are comminuted with addition of 3-10 % by weight surfactant (e.g. sodium lignosulfonate), 1-5 % by weight binder (e.g. carboxymethylcellulose) and such amount of water to result in a total amount of 100 % by weight. This results in a fine suspension of the active substance. Dilution with water gives a stable suspension of the active substance.
- surfactante.g. sodium lignosulfonate
- bindere.g. carboxymethylcellulose
- fatty acid dimethylamide and cyclohexanone10-25 % by weight surfactant blend (e.g. polyoxyethylene fatty alcohol ether and arylphenol ethoxylate), and such amount of water to result in a total amount of 100 % by weight. This mixture is stirred for 1 h to produce spontaneously a thermodynamically stable microemulsion.
- surfactant blende.g. polyoxyethylene fatty alcohol ether and arylphenol ethoxylate
- This mixtureis stirred for 1 h to produce spontaneously a thermodynamically stable microemulsion.
- Microcapsules (CS)An oil phase comprising 5-50 % by weight of at least one compound of the invention, 0-40 % by weight water-insoluble organic solvent (e.g. aromatic hydrocarbon), 2-15 % by weight acrylic monomers (e.g.
- methylmethacrylate, methacrylic acid and a di- or triacrylateare dispersed into an aqueous solution of a protective colloid (e.g. polyvinyl alcohol). Radical polymerization initiated by a radical initiator results in the formation of poly(meth)acrylate microcapsules.
- a protective colloide.g. polyvinyl alcohol
- Radical polymerization initiated by a radical initiatorresults in the formation of poly(meth)acrylate microcapsules.
- an oil phasecomprising 5-50 % by weight of at least one compound of the invention, 0-40 % by weight water-insoluble organic solvent (e.g. aromatic hydrocarbon), and an isocyanate monomer (e.g. diphenylmethene-4,4'-diisocyanatae) are dispersed into an aqueous solution of a protective colloid (e.g. polyvinyl alcohol).
- a polyaminee.g. hexamethylenediamine
- the monomersamount to 1-10 % by weight of the total CS composition.
- Dustable powders (DP, DS) 1-10 % by weight of at least one compound of the inventionare ground finely and mixed intimately with such amount of solid carrier, e.g. finely divided kaolin, to result in a total amount of 100 % by weight.
- Granules (GR, FG)0.5-30 % by weight of at least one compound of the invention are ground finely and associated with such amount of solid carrier (e.g. silicate) to result in a total amount of 100 % by weight.
- Ultra-low volume liquids1-50 % by weight of at least one compound of the invention are dissolved in such amount of organic solvent, e.g. aromatic hydrocarbon, to result in a total amount of 100 % by weight.
- the compositions types i) to xiii)may optionally comprise further auxiliaries, such as 0.1-1 % by weight preservatives, 0.1-1 % by weight antifoams, 0.1-1 % by weight dyes and/or pigments, and 5-10% by weight antifreezes.
- the compound and the composition of the inventioncan be mixed with other active ingredients like fungicides, bactericides, acaricides, nematicides, insecticides, biological control agents or herbicides. Mixtures with fertilizers, growth regulators, safeners, nitrification inhibitors, semiochemicals and/or other agriculturally beneficial agents are also possible. This may allow to broaden the activity spectrum or to prevent development of resistance.
- the active compounds identified here by their common namesare known and are described, for example, in the pesticide handbook (“The Pesticide Manual” 17th Ed., British Crop Protection Council 2015) or can be found on the Internet (e.g. http://www.alanwood.net/pesticides).
- fungicideswhich could be mixed with the compound and the composition of the invention are: 1) Inhibitors of the ergosterol biosynthesis, for example (1.001) cyproconazole, (1.002) difenoconazole, (1.003) epoxiconazole, (1.004) fenbuconazole, (1.005) fenhexamid, (1.006) fenpropidin, (1.007) fenpropimorph, (1.008) fenpyrazamine, (1.009) Fluoxytioconazole, (1.010) fluquinconazole, (1.011) flutriafol, (1.012) hexaconazole, (1.013) imazalil, (1.014) imazalil sulfate, (1.015) ipconazole, (1.016) ipfentrifluconazole, (1.017) mefentrifluconazole, (1.018) metconazole, (1.019) myclobutanil, (1.020) paclobutra
- Inhibitors of the respiratory chain at complex I or IIfor example (2.001) benzovindiflupyr, (2.002) bixafen, (2.003) boscalid, (2.004) carboxin, (2.005) cyclobutrifluram, (2.006) flubeneteram, (2.007) BCS223033 FC -39- fluindapyr, (2.008) fluopyram, (2.009) flutolanil, (2.010) fluxapyroxad, (2.011) furametpyr, (2.012) inpyrfluxam, (2.013) Isofetamid, (2.014) isoflucypram, (2.015) isopyrazam, (2.016) penflufen, (2.017) penthiopyrad, (2.018) pydiflumetofen, (2.019) pyrapropoyne, (2.020) pyraziflumid, (2.021) sedaxane, (2.022) Thifluzamide (aka trifluzamide), (2.023) 5,8
- Inhibitors of the respiratory chain at complex IIIfor example (3.001) ametoctradin, (3.002) amisulbrom, (3.003) azoxystrobin, (3.004) coumethoxystrobin, (3.005) coumoxystrobin, (3.006) cyazofamid, (3.007) dimoxystrobin, (3.008) enoxastrobin, (3.009) famoxadone, (3.010) fenamidone, (3.011) fenpicoxamid, (3.012) florylpicoxamid, (3.013) flufenoxystrobin, (3.014) fluoxastrobin, (3.015) kresoxim-methyl, (3.016) mandestrobin, (3.017) metarylpicoxamid, (3.018) metominostrobin, (3.019) metyltetraprole, (3.020) orysastrobin, (3.021) picoxystrobin, (3.022) pyraclostrobin, (3.021) pic
- Inhibitors of the mitosis and cell divisionfor example (4.001) carbendazim, (4.002) diethofencarb, (4.003) ethaboxam, (4.004) fluopicolide, (4.005) fluopimomide, (4.006) metrafenone, (4.007) pencycuron, (4.008) pyridachlometyl, (4.009) pyriofenone (chlazafenone), (4.010) thiabendazole, (4.011) thiophanate-methyl, (4.012) zoxamide, (4.013) 3-chloro-5-(4-chlorophenyl)-4-(2,6-difluorophenyl)-6- methylpyridazine, (4.014) 3-chloro-5-(6-chloropyridin-3-yl)-6-methyl-4-(2,4,6- trifluorophenyl)pyridazine, (4.015) 4-(2-bromo-4-fluorophenyl)
- BCS223033 FC -41- 5Compounds capable to have a multisite action, for example (5.001) bordeaux mixture, (5.002) captafol, (5.003) captan, (5.004) chlorothalonil, (5.005) copper hydroxide, (5.006) copper naphthenate, (5.007) copper oxide, (5.008) copper oxychloride, (5.009) copper(2+) sulfate, (5.010) dithianon, (5.011) dodine, (5.012) folpet, (5.013) mancozeb, (5.014) maneb, (5.015) metiram, (5.016) metiram zinc, (5.017) oxine- copper, (5.018) propineb, (5.019) sulfur and sulfur preparations including calcium polysulfide, (5.020) thiram, (5.021) zineb, (5.022) ziram, (5.023) 6-ethyl-5,7-dioxo-6,7-dihydro-5H-
- Compounds capable to induce a host defencefor example (6.001) acibenzolar-S-methyl, (6.002) fosetyl-aluminium, (6.003) fosetyl-calcium, (6.004) fosetyl-sodium, (6.005) isotianil, (6.006) phosphorous acid and its salts, (6.007) probenazole, (6.008) tiadinil.
- Inhibitors of the amino acid and/or protein biosynthesisfor example (7.001) cyprodinil, (7.002) kasugamycin, (7.003) kasugamycin hydrochloride hydrate, (7.004) oxytetracycline, (7.005) pyrimethanil 8) Inhibitors of the ATP production, for example (8.001) silthiofam.
- Inhibitors of the cell wall synthesisfor example (9.001) benthiavalicarb, (9.002) dimethomorph, (9.003) flumorph, (9.004) iprovalicarb, (9.005) mandipropamid, (9.006) pyrimorph, (9.007) valifenalate, (9.008) (2E)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4-yl)-1-(morpholin-4-yl)prop-2-en-1-one, (9.009) (2Z)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4-yl)-1-(morpholin-4-yl)prop-2-en-1-one.
- Inhibitors of the lipid synthesis or transport, or membrane synthesisfor example (10.001) fluoxapiprolin, (10.002) natamycin, (10.003) oxathiapiprolin, (10.004) propamocarb, (10.005) propamocarb hydrochloride, (10.006) propamocarb-fosetylate, (10.007) tolclofos-methyl, (10.008) 1-(4- ⁇ 4-[(5R)-5-(2,6-difluorophenyl)-4,5-dihydro-1,2-oxazol-3-yl]-1,3-thiazol-2-yl ⁇ piperidin-1-yl)-2-[5- methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl]ethanone, (10.009) 1-(4- ⁇ 4-[(5S)-5-(2,6-difluorophenyl)- 4,5-dihydro-1,2-oxazol-3-yl]-1
- Inhibitors of the melanin biosynthesisfor example (11.001) tolprocarb, (11.002) tricyclazole.
- Inhibitors of the nucleic acid synthesisfor example (12.001) benalaxyl, (12.002) benalaxyl-M (kiralaxyl), (12.003) metalaxyl, (12.004) metalaxyl-M (mefenoxam).
- 13) Inhibitors of the signal transductionfor example (13.001) fludioxonil, (13.002) iprodione, (13.003) procymidone, (13.004) proquinazid, (13.005) quinoxyfen, (13.006) vinclozolin.
- BCS223033 FC -45- All named mixing partners of the classes (1) to (15) as described here abovecan be present in the form of the free compound or, if their functional groups enable this, an agrochemically active salt thereof.
- the compound and the composition of the inventionmay also be combined with one or more biological control agents.
- biological controlis defined as control of harmful organisms such as a phytopathogenic fungi and/or insects and/or acarids and/or nematodes by the use or employment of a biological control agent.
- biological control agentis defined as an organism other than the harmful organisms and / or proteins or secondary metabolites produced by such an organism for the purpose of biological control.
- Mutants of the second organismshall be included within the definition of the biological control agent.
- the term “mutant”refers to a variant of the parental strain as well as methods for obtaining a mutant or variant in which the pesticidal activity is greater than that expressed by the parental strain.
- the ”parent strain“is defined herein as the original strain before mutagenesis.
- the parental strainmay be treated with a chemical such as N-methyl-N'-nitro-N-nitrosoguanidine, ethylmethanesulfone, or by irradiation using gamma, x-ray, or UV-irradiation, or by other means well known to those skilled in the art.
- Known mechanisms of biological control agentscomprise enteric bacteria that control root rot by out-competing fungi for space on the surface of the root.
- Bacterial toxinssuch as antibiotics, have been used to control pathogens.
- the toxincan be isolated and applied directly to the plant or the bacterial species may be administered so it produces the toxin in situ.
- a ”variantis a strain having all the identifying characteristics of the NRRL or ATCC Accession Numbers as indicated in this text and can be identified as having a genome that hybridizes under conditions of high stringency to the genome of the NRRL or ATCC Accession Numbers.
- Hybridizationrefers to a reaction in which one or more polynucleotides react to form a complex that is stabilized via hydrogen bonding between the bases of the nucleotide residues.
- the hydrogen bondingmay occur by Watson-Crick base pairing, Hoogstein binding, or in any other sequence-specific manner.
- the complexmay comprise two strands forming a duplex structure, three or more strands forming a multi- stranded complex, a single self-hybridizing strand, or any combination of these.
- Hybridization reactionscan be performed under conditions of different “stringency”. In general, a low stringency hybridization reaction is carried out at about 40 °C in 10 X SSC or a solution of equivalent ionic strength/temperature.
- a moderate stringency hybridizationis typically performed at about 50 °C in 6 X SSC, and a high stringency hybridization reaction is generally performed at about 60 °C in 1 X SSC.
- a variant of the indicated NRRL or ATCC Accession Numbermay also be defined as a strain having a genomic sequence that is greater than 85%, more preferably greater than 90% or more preferably greater than 95% sequence identity to the genome of the indicated NRRL or ATCC Accession Number.
- a BCS223033 FC -46- polynucleotide or polynucleotide region (or a polypeptide or polypeptide region)has a certain percentage (for example, 80%, 85%, 90%, or 95%) of “sequence identity” to another sequence means that, when aligned, that percentage of bases (or amino acids) are the same in comparing the two sequences.
- This alignment and the percent homology or sequence identitycan be determined using software programs known in the art, for example, those described in Current Protocols in Molecular Biology (F. M. Ausubel et al., eds., 1987).
- NRRLis the abbreviation for the Agricultural Research Service Culture Collection, an international depositary authority for the purposes of deposing microorganism strains under the Budapest treaty on the international recognition of the deposit of microorganisms for the purposes of patent procedure, having the address National Center for Agricultural Utilization Research, Agricultural Research service, U.S. Department of Agriculture, 1815 North university Street, Peroira, Illinois 61604 USA.
- ATCCis the abbreviation for the American Type Culture Collection, an international depositary authority for the purposes of deposing microorganism strains under the Budapest treaty on the international recognition of the deposit of microorganisms for the purposes of patent procedure, having the address ATCC Patent Depository, 10801 University Boulevard., Manassas, VA 10110 USA.
- biological control agentswhich may be combined with the compound and the composition of the invention are: (A) Antibacterial agents selected from the group of: (A1) bacteria, such as (A1.01) Bacillus subtilis, in particular strain QST713/AQ713 (available as SERENADE OPTI or SERENADE ASO from Bayer CropScience LP, US, having NRRL Accession No. B21661, U.S. Patent No. 6,060,051); (A1.02) Bacillus sp., in particular strain D747 (available as DOUBLE NICKEL ® from Kumiai Chemical Industry Co., Ltd.), having Accession No. FERM BP-8234, U.S. Patent No.
- Bacillus pumilusin particular strain BU F-33, having NRRL Accession No. 50185 (available as part of the CARTISSA ® product from BASF, EPA Reg. No. 71840- 19); (A1.04) Bacillus subtilis var. amyloliquefaciens strain FZB24 having Accession No. DSM 10271 (available from Novozymes as TAEGRO ® or TAEGRO ® ECO (EPA Registration No.70127-5)); (A1.05) a Paenibacillus sp. strain having Accession No. NRRL B-50972 or Accession No.
- Bacillus subtilis strain BU1814(available as VELONDIS ® PLUS, VELONDIS ® FLEX and VELONDIS ® EXTRA from BASF SE); (A1.07) Bacillus mojavensis strain R3B (Accession No. NCAIM (P) B001389) (WO 2013/034938) from Certis USA LLC, a subsidiary of Mitsui & Co.; (A1.08) Bacillus subtilis CX-9060 from Certis USA LLC, a subsidiary of Mitsui & Co.; (A1.09) Paenibacillus polymyxa, in particular strain AC-1 (e.g.
- NRRL B-21856(available as BLOOMTIME BIOLOGICAL TM FD BIOPESTICIDE from Northwest Agri Products); and (A2) fungi, such as (A2.01) Aureobasidium pullulans, in particular blastospores of strain DSM14940, blastospores of strain DSM 14941 ormixtures of blastospores of strains DSM14940 and DSM14941 (e.g., BOTECTOR ® and BLOSSOM PROTECT ® from bio-ferm, CH); (A2.02) Pseudozyma aphidis (as disclosed in WO2011/151819 by Yissum Research Development Company of the Hebrew University of Jerusalem); (A2.03) Saccharomyces cerevisiae, in particular strains CNCM No.
- Aureobasidium pullulansin particular blastospores of strain DSM14940, blastospores of strain DSM 14941 ormixtures of blastospores of strains DSM14940 and D
- Bacillus subtilis Y1336(available as BIOBAC ® WP from Bion-Tech, Taiwan, registered as a biological fungicide in Taiwan under Registration Nos. 4764, 5454, 5096 and 5277); (B1.07) Bacillus subtilis strain MBI 600 (available as SUBTILEX from BASF SE), having Accession Number NRRL B-50595, U.S. Patent No. 5,061,495; (B1.08) Bacillus subtilis strain GB03 (available as Kodiak® from Bayer AG, DE); (B1.09) Bacillus subtilis var. amyloliquefaciens strain FZB24 having Accession No.
- DSM 10271(available from Novozymes as TAEGRO ® or TAEGRO ® ECO (EPA Registration No. 70127-5)); (B1.10) Bacillus mycoides, isolate J , having Accession No. B-30890 (available as BMJ TGAI ® or WG and LifeGard TM from Certis USA LLC, a subsidiary of Mitsui & Co.); (B1.11) Bacillus licheniformis, in particular strain SB3086 , having Accession No. ATCC 55406, WO 2003/000051 (available as ECOGUARD ® Biofungicide and GREEN RELEAF TM from Novozymes); (B1.12) a Paenibacillus sp. strain having Accession No.
- B-50768; WO 2014/028521Bacillus amyloliquefaciens strain FZB42, Accession No. DSM 23117 (available as RHIZOVITAL ® from ABiTEP, DE); (B1.17) Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 (QUARTZO ® (WG) and PRESENCE ® (WP) from FMC Corporation); (B1.18) BCS223033 FC -48- Bacillus mojavensis strain R3B (Accession No.
- NCAIM (P) B001389)(WO 2013/034938) from Certis USA LLC, a subsidiary of Mitsui & Co.; (B1.19) Paenibacillus polymyxa ssp. plantarum (WO 2016/020371) from BASF SE; (B1.20) Paenibacillus epiphyticus (WO 2016/020371) from BASF SE; (B1.21) Pseudomonas chlororaphis strain AFS009, having Accession No.
- NRRL B-50897, WO 2017/019448e.g., HOWLERTM and ZIO ® from AgBiome Innovations, US
- B1.22Pseudomonas chlororaphis, in particular strain MA342 (e.g. CEDOMON ® , CERALL ® , and CEDRESS ® by Bioagri and Koppert);
- B1.23Streptomyces lydicus strain WYEC108 (also known as Streptomyces lydicus strain WYCD108US) (ACTINO-IRON ® and ACTINOVATE ® from Novozymes);
- B1.24Agrobacterium radiobacter strain K84 (e.g.
- AVOGREEN TMfrom University of Pretoria
- Bacillus methylotrophicus strain BAC-9912from Chinese Academy of Sciences’ Institute of Applied Ecology
- B1.31 Pseudomonas proradixe.g. PRORADIX ® from Sourcon Padena
- B1.32 Streptomyces griseoviridis strain K61also known as Streptomyces galbus strain K61
- DSM 7206Streptomyces griseoviridis strain K61
- MYCOSTOP ®from Verdera
- PREFENCE ®from BioWorks
- Crop Protection 200625, strain A506 (e.g ® .
- B2BLIGHTBAN A506 by NuFarm
- B2 fungifor example: (B2.01) Coniothyrium minitans, in particular strain CON/M/91-8 (Accession No. DSM-9660; e.g. Contans ® from Bayer CropScience Biologics GmbH); (B2.02) Metschnikowia fructicola, in particular strain NRRL Y-30752; (B2.03) Microsphaeropsis ochracea; (B2.04) Trichoderma atroviride, in particular strain SC1 (having Accession No. CBS 122089, WO 2009/116106 and U.S.
- Patent No.8,431,120from Bi-PA
- strain 77BT77 from Andermatt Biocontrol
- strain LU132e.g. Sentinel from Agrimm Technologies Limited
- Trichoderma harzianum strain T-22e.g. Trianum-P from Andermatt Biocontrol or Koppert
- strain Cepa Simb-T5from Simbiose Agro
- Gliocladium roseumalso known as Clonostachys rosea f.
- strain 321Ufrom Adjuvants Plus
- strain ACM941as disclosed in Xue (Efficacy of Clonostachys rosea strain ACM941 and fungicide seed treatments for controlling the root tot complex of field pea, Can Jour Plant Sci 83(3): 519-524), or strain IK726 (Jensen DF, et al. Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain ‘IK726’; Australas Plant Pathol.
- Esquive® WP from Agrauxine, FREsquive® WP from Agrauxine, FR); (B2.11) Trichoderma atroviride, strain no. V08/002387; (B2.12) Trichoderma BCS223033 FC -49- atroviride, strain NMI no. V08/002388; (B2.13) Trichoderma atroviride, strain NMI no. V08/002389; (B2.14) Trichoderma atroviride, strain NMI no. V08/002390; (B2.15) Trichoderma atroviride, strain LC52 (e.g.
- Trichoderma atrovirideTrichoderma atroviride, strain ATCC 20476 (IMI 206040); (B2.17) Trichoderma atroviride, strain T11 (IMI352941/ CECT20498); (B2.18) Trichoderma harmatum; (B2.19) Trichoderma harzianum; (B2.20) Trichoderma harzianum rifai T39 (e.g. Trichodex® from Makhteshim, US); (B2.21) Trichoderma asperellum, in particular, strain kd (e.g. T-Gro from Andermatt Biocontrol); (B2.22) Trichoderma harzianum, strain ITEM 908 (e.g.
- Trianum-PTrianum-P from Koppert
- B2.23Trichoderma harzianum, strain TH35 (e.g. Root-Pro by Mycontrol);
- Trichoderma virensalso known as Gliocladium virens), in particular strain GL-21 (e.g. SoilGard by Certis, US);
- B2.25Trichoderma viride, strain TV1(e.g. Trianum-P by Koppert);
- Ampelomyces quisqualisin particular strain AQ 10 (e.g.
- NM 99/06216e.g., BOTRY- ZEN ® by Botry-Zen Ltd, New Zealand and BOTRYSTOP ® from BioWorks, Inc.
- Verticillium chlamydosporiumB2.45) mixtures of Trichoderma asperellum strain ICC 012 (also known as Trichoderma harzianum ICC012), having Accession No.
- NRRL B-50759(TRICHO PLUS ® from BASF SE); (B2.47) Aspergillus flavus strain NRRL 21882 (products known as AFLA-GUARD ® from Syngenta/ChemChina); BCS223033 FC -50- (B2.48) Chaetomium cupreum (Accession No. CABI 353812) (e.g. BIOKUPRUM TM by AgriLife); (B2.49) Saccharomyces cerevisiae, in particular strain LASO2 (from Agro-Levures et Dérivés), strain LAS117 cell walls (CEREVISANE ® from Lesaffre; ROMEO ® from BASF SE), strains CNCM No. I- 3936, CNCM No.
- Trichoderma virens strain G-41formerly known as Gliocladium virens (Accession No. ATCC 20906) (e.g., ROOTSHIELD ® PLUS WP and TURFSHIELD ® PLUS WP from BioWorks, US); (B2.51) Trichoderma hamatum, having Accession No. ATCC 28012; (B2.52) Ampelomyces quisqualis strain AQ10, having Accession No.
- CNCM I-807e.g., AQ 10 ® by IntrachemBio Italia
- Penicillium steckiiDM 27859; WO 2015/067800) from BASF SE;
- B2.55 Chaetomium globosumavailable as RIVADIOM ® by Rivale
- B2.56Cryptococcus flavescens, strain 3C (NRRL Y-50378);
- B2.58) Dilophosphora alopecuriavailable as TWIST FUNGUS ® );
- B2.60Pseudozyma flocculosa, strain PF-A22 UL (available as SPORODEX ® L by Plant Products Co.
- strain ICC 080IMI CC 392151 CABI
- BIODERMAAGROBIOSOL DE MEXICO, S.A. DE C.V.
- B2.62Trichoderma fertile (e.g. product TrichoPlus from BASF);
- B2.63Muscodor roseus, in particular strain A3-5 (Accession No. NRRL 30548);
- B2.64Simplicillium lanosoniveum;
- C1 bacteriaselected from the group consisting of (C1.01) Bacillus pumilus, in particular strain QST2808 (having Accession No. NRRL No.
- Bacillus subtilisin particular strain QST713/AQ713 (having NRRL Accession No. B-21661 and described in U.S. Patent No. 6,060,051; available as SERENADE ® OPTI or SERENADE ® ASO from Bayer CropScience LP, US); (C1.03) Bacillus subtilis, in particular strain AQ30002 (having Accession Nos. NRRL B-50421 and described in U.S. Patent Application No. 13/330,576); (C1.04) Bacillus subtilis, in particular strain AQ30004 (and NRRL B-50455 and described in U.S. Patent Application No.
- B- 50928)Bacillus amyloliquefaciens SB3281 (ATCC # PTA-7542; WO 2017/205258), (C1.17) BCS223033 FC -51- Bacillus amyloliquefaciens TJ1000 (available as QUIKROOTS ® from Novozymes); (C1.18) Bacillus firmus, in particular strain CNMC I-1582 (e.g. VOTIVO ® from BASF SE); (C1.19) Bacillus pumilus, in particular strain GB34 (e.g.
- YIELD SHIELD ®from Bayer Crop Science, DE
- C1.20Bacillus amyloliquefaciens, in particular strain IN937a
- C1.21Bacillus amyloliquefaciens, in particular strain FZB42 (e.g. RHIZOVITAL ® from ABiTEP, DE)
- C1.22Bacillus amyloliquefaciens BS27 (Accession No.
- NRRL B-5015a mixture of Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 (available as QUARTZO ® (WG), PRESENCE ® (WP) from FMC Corporation); (C1.24) Bacillus cereus, in particular strain BP01 (ATCC 55675; e.g. MEPICHLOR ® from Arysta Lifescience, US); (C1.25) Bacillus subtilis, in particular strain MBI 600 (e.g. SUBTILEX ® from BASF SE); (C1.26) Bradyrhizobium japonicum (e.g.
- OPTIMIZE ® from Novozymes(C1.27) Mesorhizobium cicer (e.g., NODULATOR from BASF SE); (C1.28) Rhizobium leguminosarium biovar viciae (e.g., NODULATOR from BASF SE); (C1.29) Delftia acidovorans, in particular strain RAY209 (e.g. BIOBOOST ® from Brett Young Seeds); (C1.30) Lactobacillus sp. (e.g. LACTOPLANT ® from LactoPAFI); (C1.31) Paenibacillus polymyxa, in particular strain AC-1 (e.g.
- strain Z25(Accession No. CECT 4585); (C1.38) Azorhizobium caulinodans, in particular strain ZB-SK-5; (C1.39) Azotobacter chroococcum, in particular strain H23; (C1.40) Azotobacter vinelandii, in particular strain ATCC 12837; (C1.41) Bacillus siamensis, in particular strain KCTC 13613T; (C1.42) Bacillus tequilensis, in particular strain NII-0943; (C1.43) Serratia marcescens, in particular strain SRM (Accession No. MTCC 8708); (C1.44) Thiobacillus sp. (e.g.
- C2.01Purpureocillium lilacinum (previously known as Paecilomyces lilacinus) strain 251 (AGAL 89/030550; e.g. BioAct from Bayer CropScience Biologics GmbH);
- C2.04Trichoderma atroviride strain CNCM I-1237 (e.g.
- Equive® WP from Agrauxine, FR(C2.05) Trichoderma viride, e.g. strain B35 (Pietr et al., 1993, Zesz. Nauk. A R w Szczecinie 161: 125-137); (C2.06) Trichoderma atroviride strain LC52 (also known as Trichoderma atroviride strain LU132; e.g. Sentinel from Agrimm Technologies Limited); (C2.07) Trichoderma atroviride strain SC1 described in International Application No. PCT/IT2008/000196); (C2.08) Trichoderma asperellum strain kd (e.g.
- T-Grofrom Andermatt Biocontrol
- C2.09Trichoderma asperellum strain Eco-T (Plant Health Products, ZA);
- C2.10)Trichoderma harzianum strain T-22 (e.g. Trianum-P from Andermatt Biocontrol or Koppert);
- C2.11)Myrothecium verrucaria strain AARC-0255 (e.g. DiTeraTM from Valent Biosciences);
- C2.12Penicillium bilaii strain ATCC ATCC20851;
- C2.13Pythium oligandrum strain M1 (ATCC 38472; e.g.
- israelensis strain BMP 141by Becker Microbial Products, IL
- Bacillus thuringiensis israelensis strain BMP 144e.g. AQUABAC ® by Becker Microbial Products IL
- Burkholderia spp.in particular Burkholderia rinojensis strain A396 (also known as Burkholderia rinojensis strain MBI 305) (Accession No. NRRL B- 50319; WO 2011/106491 and WO 2013/032693; e.g.
- MBI-206 TGAI and ZELTO ® from Marrone Bio Innovations(D1.10) Chromobacterium subtsugae, in particular strain PRAA4-1T (MBI-203; e.g. GRANDEVO ® from Marrone Bio Innovations); (D1.11) Paenibacillus popilliae (formerly Bacillus popilliae; e.g. MILKY SPORE POWDER TM and MILKY SPORE GRANULAR TM from St. Gabriel Laboratories); (D1.12) Bacillus thuringiensis subsp. israelensis (serotype H-14) strain AM65-52 (Accession No. ATCC 1276) (e.g.
- israeltaki strain PB 54Bacillus thuringiensis subsp. kurstaki strain SA 11; strain SA 12; (D1.20) Bacillus thuringiensis subsp. kurstaki strain EG 2348; (D1.21) Bacillus thuringiensis var. Colmeri (e.g. TIANBAOBTC by Changzhou Jianghai Chemical Factory); (D1.22) Bacillus thuringiensis subsp. aizawai strain GC-91; (D1.23) Serratia entomophila (e.g. INVADE ® by Wrightson Seeds); (D1.24) Serratia marcescens, in particular strain SRM (Accession No.
- DSM 24665)viruses selected from the group consisting of Adoxophyes orana (summer fruit tortrix) granulosis virus (GV), Cydia pomonella (codling moth) granulosis virus (GV), Helicoverpa armigera (cotton bollworm) nuclear polyhedrosis virus (NPV), Spodoptera exigua (beet armyworm) mNPV, Spodoptera frugiperda (fall armyworm) mNPV, and Spodoptera littoralis (African cotton leafworm) NPV; (F) bacteria and fungi which can be added as 'inoculant' to plants or plant parts or plant organs and which, by virtue of their particular properties, promote plant growth and plant health.
- Examplesare: Agrobacterium spp., Azorhizobium caulinodans, Azospirillum spp., Azotobacter spp., Bradyrhizobium spp., Burkholderia spp., in particular Burkholderia cepacia (formerly known as Pseudomonas cepacia), Gigaspora spp., or Gigaspora monosporum, Glomus spp., Laccaria spp., Lactobacillus buchneri, Paraglomus spp., Pisolithus tinctorus, Pseudomonas spp., Rhizobium spp., in particular Rhizobium trifolii, Rhizopogon spp., Scleroderma spp., Suillus spp., and Streptomyces spp.; and (G) plant extracts and products formed by microorganisms including proteins and secondary metabolites which can be used as
- the compound and the composition of the inventionmay be combined with one or more active ingredients selected from insecticides, acaricides and nematicides.
- BCS223033 FC -54- “Insecticides” as well as the term “insecticidal”refers to the ability of a substance to increase mortality or inhibit growth rate of insects.
- the term “insects”comprises all organisms in the class “Insecta”.
- “Nematicide” and “nematicidal”refers to the ability of a substance to increase mortality or inhibit the growth rate of nematodes.
- nematodecomprises eggs, larvae, juvenile and mature forms of said organism.
- Acaricide and “acaricidal”refers to the ability of a substance to increase mortality or inhibit growth rate of ectoparasites belonging to the class Arachnida, sub-class Acari.
- insecticides, acaricides and nematicides, respectively, which could be mixed with the compound and the composition of the inventionare: (1) Acetylcholinesterase (AChE) inhibitors, preferably carbamates selected from alanycarb, aldicarb, bendiocarb, benfuracarb, butocarboxim, butoxycarboxim, carbaryl, carbofuran, carbosulfan, ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, metolcarb, oxamyl, pirimicarb, propoxur, thiodicarb, thiofanox, triazamate, trimethacarb
- GABA-gated chloride channel blockerspreferably cyclodiene-organochlorines selected from chlordane and endosulfan, or phenylpyrazoles (fiproles) selected from ethiprole and fipronil.
- Sodium channel modulatorspreferably pyrethroids selected from acrinathrin, allethrin, d-cis-trans allethrin, d-trans allethrin, bifenthrin, bioallethrin, bioallethrin s-cyclopentenyl isomer, bioresmethrin, cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda-cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin,
- Nicotinic acetylcholine receptor (nAChR) competitive modulatorspreferably neonicotinoids selected from acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid and thiamethoxam, or nicotine, or sulfoximines selected from sulfoxaflor, or butenolids selected from flupyradifurone, or mesoionics selected from triflumezopyrim, or pyridylidenes selected from Flupyrimin.
- nAChRNicotinic acetylcholine receptor
- Nicotinic acetylcholine receptor (nAChR) allosteric modulatorssite I
- nAChRNicotinic acetylcholine receptor
- site Ipreferably spinosyns selected from spinetoram and spinosad.
- Glutamate-gated chloride channel (GluCl) allosteric modulatorspreferably avermectins/milbemycins selected from abamectin, emamectin benzoate, lepimectin and milbemectin.
- Juvenile hormone mimicspreferably juvenile hormone analogues selected from hydroprene, kinoprene and methoprene, or fenoxycarb or pyriproxyfen.
- Miscellaneous non-specific (multi-site) inhibitorspreferably alkyl halides selected from methyl bromide and other alkyl halides, or chloropicrine or sulphuryl fluoride or borax or tartar emetic or methyl isocyanate generators selected from diazomet and metam.
- Chordotonal organ TRPV channel modulatorspreferably pyridine azomethanes selected from pymetrozine and pyrifluquinazone, or pyropenes selected from afidopyropen.
- Mite growth inhibitors affecting CHS1selected from clofentezine, hexythiazox, diflovidazin and etoxazole.
- Microbial disruptors of the insect gut membranesselected from Bacillus thuringiensis subspecies israelensis, Bacillus sphaericus, Bacillus thuringiensis subspecies aizawai, Bacillus thuringiensis subspecies kurstaki, Bacillus thuringiensis subspecies tenebrionis, and B.t. plant proteins selected from Cry1Ab, Cry1Ac, Cry1Fa, Cry1A.105, Cry2Ab, Vip3A, mCry3A, Cry3Ab, Cry3Bb and Cry34Ab1/35Ab1.
- Inhibitors of mitochondrial ATP synthasepreferably ATP disruptors selected from diafenthiuron, or organotin compounds selected from azocyclotin, cyhexatin and fenbutatin oxide, or propargite or tetradifon.
- Uncouplers of oxidative phosphorylation via disruption of the proton gradientselected from chlorfenapyr, DNOC and sulfluramid.
- Nicotinic acetylcholine receptor channel blockersselected from bensultap, cartap hydrochloride, thiocylam and thiosultap-sodium.
- Moulting disruptorsin particular for Diptera, i.e. dipterans selected from cyromazine.
- Ecdysone receptor agonistspreferably diacylhydrazines selected from chromafenozide, halofenozide, methoxyfenozide and tebufenozide.
- Octopamine receptor agonistsselected from amitraz.
- Mitochondrial complex III electron transport inhibitorsselected from hydramethylnone, acequinocyl, fluacrypyrim and bifenazate.
- Mitochondrial complex I electron transport inhibitorspreferably METI acaricides and insecticides selected from fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad and tolfenpyrad, or rotenone (Derris).
- Voltage-dependent sodium channel blockerspreferably oxadiazines selected from indoxacarb, or semicarbazones selected from metaflumizone.
- Inhibitors of acetyl CoA carboxylasepreferably tetronic and tetramic acid derivatives selected from spirodiclofen, spiromesifen, spiropidion and spirotetramat.
- Mitochondrial complex IV electron transport inhibitorspreferably phosphides selected from aluminium phosphide, calcium phosphide, phosphine and zinc phosphide, or cyanides selected from calcium cyanide, potassium cyanide and sodium cyanide.
- Mitochondrial complex II electron transport inhibitorspreferably beta-ketonitrile derivatives selected from cyenopyrafen and cyflumetofen, or carboxanilides selected from pyflubumide.
- Ryanodine receptor modulatorspreferably diamides selected from chlorantraniliprole, cyantraniliprole, cyclaniliprole, flubendiamide and tetraniliprole.
- Chordotonal organ Modulators(with undefined target site) selected from flonicamid.
- GABA-gated chlorid channel allosteric modulatorspreferably meta-diamides selected from broflanilide, or isoxazoles selected from fluxametamide.
- BCS223033FC -57- (31) Baculoviruses, preferably Granuloviruses (GVs) selected from Cydia pomonella GV and Thaumatotibia leucotreta (GV), or Nucleopolyhedroviruses (NPVs) selected from Anticarsia gemmatalis MNPV, Flucypyriprole and Helicoverpa armigera NPV.
- GVsGranuloviruses
- GVsGranuloviruses
- GVsGranuloviruses
- NPVsNucleopolyhedroviruses
- Nicotinic acetylcholine receptor allosteric modulatorssite II selected from GS-omega/kappa HXTX-Hv1a peptide.
- Calcium-activated potassium channel KCa2 modulatorsselected from acynonapyr.
- Mitochondrial complex III electron transfer inhibitorsnon-Qo site
- flometoquin.UN
- Compounds of unknown or uncertain MoATarget protein responsible for biological activity is unknown, or uncharacterized), selected from azadirachtin, benzoximate, bromopropylate, chinomethionat, dicofol, lime sulfur, mancozeb, pyridalyl, and sulfur.
- UNEBotanical essence including synthetic, extracts and unrefined oils with unknown or uncertain MoA (Target protein responsible for biological activity is unknown, or uncharacterized), selected from Chenopodium ambrosioides near ambrosioides extract and fatty acid monoesters with glycerol or propanediol neem oil.
- UDFFungal agents of unknown or uncertain MoA (Target protein responsible for biological activity is unknown, or uncharacterized), selected from Beauveria bassiana strains, Metarhizium anisopliae strain F52, and Paecilomyces fumosoroseus Apopka strain 97.
- NEMNon-specific mechanical and physical disruptors (Target protein responsible for biological activity is unknown, or uncharacterized), selected from Diatomaceous earth, and mineral oil.
- Further active compoundsselected from Afoxolaner, Benclothiaz, Benzpyrimoxan, Chloroprallethrin, Cryolite, Cyclobutrifluram, Cycloxaprid, Cyetpyrafen, Cyhalodiamide, Cyproflanilide (CAS 2375110- 88-4), Dicloromezotiaz, Dimpropyridaz, epsilon-Metofluthrin, epsilon-Momfluthrin, Fenmezoditiaz, Fluazaindolizine, Fluchlordiniliprole, Fluensulfone, Flufenerim, Flufenoxystrobin, Flufiprole, Fluhexafon, Fluopyram, Fluralaner, Fufenozide, Flupentiofenox, Guadipyr, Heptafluthrin, Imidaclothiz, Indazapyroxamet, Iprodione, Is
- nematicideswhich could be mixed with the compound and the composition of the invention are: (Group N-1) Acetylcholinesterase (AChE) inhibitors, preferably (N-1A) carbamates selected from aldicarb, benfuracarb, carbofuran, carbosulfan and thiodicarb, or (N-1B) organophosphates selected from cadusafos, ethoprofos, fenamiphos, fosthiazate, imicyafos, phorate and terbufos.
- AChEAcetylcholinesterase
- Group N-2Glutamate-gated chloride channel (GluCl) allosteric modulators, preferably avermectins selected from abamectin and emamectin benzoate.
- Group N-3Mitochondrial complex II electron transport inhibitors, especially inhibitors of succinate- coenzyme Q reductase, preferably pyridinylmethyl-benzamides selected from fluopyram.
- Lipid synthesis/growth regulation modulatorsespecially inhibitors of acetyl CoA carboxylase, preferably tetronic and tetramic acid derivatives selected from spirotetramat.
- Group N-UNCompounds of unknown or uncertain mode of action with various chemistries, selected from fluensulfone, fluazaindolizine, furfural, iprodione, tioxazafen and trifluenfuronate.
- BCS223033 FC -60-(Group N-UNX) Compounds of unknown or uncertain mode of action: Presumed multi-site inhibitors, preferably volatile sulphur generators selected from carbon disulphide and dimethyl disulphide (DMDS), or carbon disulphide liberators selected from sodium tetrathiocarbonate, or alkyl halides selected from methyl bromide and methyl iodide (iodomethane), or halogenated hydrocarbons selected from 1,2- dibromo-3-chloropropane (DBCP) and 1,3-dichloropropene, or chloropicrin, or methyl isothiocyanate generators selected from allyl isothiocyanate, diazomet, metam potassium and metam sodium.
- DMDScarbon disulphide and dimethyl disulphide
- iodomethanealkyl halides selected from methyl bromide and methyl iodide (iodomethane)
- DBCP1,2- dibrom
- Bacterial agents(non-Bt) of unknown or uncertain mode of action, preferably bacterium or bacterium-derived, selected from Burkholderia spp., e.g. rinojensis A396, Bacillus spp., e.g. firmus, licheniformis, amyloliquefaciens or subtilis, Pasteuria spp., e.g. penetrans or nishizawae, Pseudomonas spp., e.g. chlororaphis or fluorescens, and Streptomyces spp., e.g. lydicus, dicklowii or albogriseolus.
- Burkholderia spp.e.g. rinojensis A396, Bacillus spp., e.g. firmus, licheniformis, amyloliquefaciens or subtilis, Pasteuria spp
- fungus or fungus-derivedselected from Actinomyces spp., e.g. streptococcus, Arthrobotrys spp., e.g. oligospora, Aspergillus spp., e.g. niger, Muscodor spp., e.g. albus, Myrothecium spp., e.g. verrucaria, Paecilomyces spp., e.g. lilacinus (Purpureocillium lilacinum), carneus or fumosoroseus, Pochonia spp., e.g.
- Botanical or animal derived agentsincluding synthetic extracts and unrefined oils, with unknown or uncertain mode of action, preferably botanical or animal derived agents selected from azadirachtin, camellia seed cake, essential oils, garlic extract, pongamia oil, terpenes, e.g. carvacrol, geraniol and thymol, and Quillaja saponaria extract.
- herbicideswhich could be mixed with the compound and the composition of the invention are: acetochlor, acifluorfen, acifluorfen-methyl, acifluorfen-sodium, aclonifen, alachlor, allidochlor, alloxydim, alloxydim-sodium, ametryn, amicarbazone, amidochlor, amidosulfuron, 4-amino-3-chloro-6- (4-chloro-2-fluoro-3-methylphenyl)-5-fluoropyridine-2-carboxylic acid, aminocyclopyrachlor, aminocyclopyrachlor-potassium, aminocyclopyrachlor-methyl, aminopyralid, aminopyralid- dimethylammonium, aminopyralid-tripromine, amitrole, ammoniumsulfamate, anilofos, asulam, asulam- potassium, asulam sodium, atrazine, azafenidin, azimsulfuron, beflubut
- dicamba-biproaminedicamba-N,N-Bis(3-aminopropyl)methylamine, dicamba-butotyl, dicamba-choline, dicamba-diglycolamine, dicamba-dimethylammonium, dicamba- diethanolamine ammonium, dicamba-diethylammonium, dicamba-isopropylammonium, dicamba- methyl, dicamba-monoethanolamine, dicamba-olamine, dicamba-potassium, dicamba-sodium, dicamba- triethanolamine, dichlobenil, 2-(2,4-dichlorobenzyl)-4,4-dimethyl-1,2-oxazolidin-3-one, 2-(2,5- dichlorobenzyl)-4,4-dimethyl-1,2-oxazolidin-3-one, dichlorprop, dichlorprop-butotyl, dichlorprop- dimethylammonium, dichhlorprop-etex
- Examples of plant growth regulatorswhich could be mixed with the compound and the composition of the invention are: Abscisic acid and related analogues [e.g. (2Z,4E)-5-[6-Ethynyl-1-hydroxy-2,6-dimethyl-4-oxocyclohex- 2-en-1-yl]-3-methylpenta-2,4-dienoic acid, methyl-(2Z,4E)-5-[6-ethynyl-1-hydroxy-2,6-dimethyl-4- oxocyclohex-2-en-1-yl]-3-methylpenta-2,4-dienoate, (2Z,4E)-3-ethyl-5-(1-hydroxy-2,6,6-trimethyl-4- oxocyclohex-2-en-1-yl)penta-2,4-dienoic acid, (2E,4E)-5-(1-hydroxy-2,6,6-trimethyl-4-oxocyclohex-2-en-1-yl)-3-(
- COssometimes referred to as N-acetylchitooligosaccharides, are also composed of GlcNAc residues but have side chain decorations that make them different from chitin molecules [(C 8 H 13 NO 5 ) n , CAS No.1398-61-4] and chitosan molecules [(C 5 H 11 NO 4 ) n , CAS No.9012-76-4]), chitinous compounds, chlormequat chloride, cloprop, cyclanilide, 3-(Cycloprop-1-enyl)propionic acid, 1-[2-(4-cyano-3,5- dicyclopropylphenyl)acetamido]cyclohexanecarboxylic acid, 1-[2-(4-cyano-3- cyclopropylphenyl)acetamido]cyclohexanecarboxylic acid, daminozide, dazomet, dazomet-sodium, n- decanol, dikegulac
- LCOlipo-chitooligosaccharides
- Nodsymbiotic nodulation
- Myc factorsconsist of an oligosaccharide backbone of ⁇ -l,4-linked N-acetyl-D-glucosamine (“GlcNAc”) residues with an N-linked fatty acyl chain condensed at the non-reducing end.
- LCOsdiffer in the number of GlcNAc residues in the backbone, in the length and degree of saturation of the fatty acyl chain and in the substitutions of reducing and non-reducing sugar residues), linoleic acid or derivatives thereof, linolenic acid or derivatives thereof, maleic hydrazide, mepiquat chloride, mepiquat pentaborate, 1- methylcyclopropene, 3-methylcyclopropene, 1-ethylcyclopropene, 1-n-propylcyclopropene, 1- cyclopropenylmethanol, methoxyvinylglycin (MVG), 3’-methyl abscisic acid, 1-(4-methylphenyl)-N-(2- oxo-1-propyl-1,2,3,4-tetrahydroquinolin-6-yl)methanesulfonamide and related substituted tetrahydroquinolin-6-yl)methanes
- Examples of safeners which could be mixed with the compound and the composition of the inventionare: S1) Compounds from the group of heterocyclic carboxylic acid derivatives: S1 a ) Compounds of the dichlorophenylpyrazoline-3-carboxylic acid type (S1 a ), preferably compounds such as 1-(2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline-3-carboxylic acid, ethyl 1- (2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline-3-carboxylate (S1-1) ("mefenpyr- diethyl”), and related compounds as described in WO-A-91/07874; S1 b ) Derivatives of dichlorophenylpyrazolecarboxylic acid (S1 b ), preferably compounds such as ethyl 1-(2,4-dichlorophenyl)-5-methylpyrazo
- S2 aCompounds from the group of the 8-quinolinoxy derivatives (S2): S2 a ) Compounds of the 8-quinolinoxyacetic acid type (S2 a ), preferably 1-methylhexyl (5-chloro-8- quinolinoxy)acetate ("cloquintocet-mexyl") (S2-1), 1,3-dimethylbut-1-yl (5-chloro-8- quinolinoxy)acetate (S2-2), 4-allyloxybutyl (5-chloro-8-quinolinoxy)acetate (S2-3), 1-allyloxyprop-2-yl (5-chloro-8-quinolinoxy)acetate (S2-4), ethyl (5-chloro-8-quinolinoxy)acetate (S2-5), methyl 5-chloro-8- quinolinoxyacetate (S2-6), allyl (5-chloro-8-quinolinoxy)acetate (S2-7), 2-(2-propylideneiminoxy)-1- eth
- S3Active compounds of the dichloroacetamide type (S3), which are frequently used as pre- emergence safeners (soil-acting safeners), for example "dichlormid” (N,N-diallyl-2,2-dichloroacetamide) (S3-1), "R-29148” (3-dichloroacetyl-2,2,5-trimethyl-1,3-oxazolidine) from Stauffer (S3-2), "R-28725" (3-dichloroacetyl-2,2-dimethyl-1,3-oxazolidine) from Stauffer (S3-3), "benoxacor” (4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine) (S3-4), "PPG-1292” (N-allyl-N-[(1,3-dioxolan-2-yl)methyl]dichloroacetamide) from PPG Industries (S3-5), "DKA-24" (N-ally
- S4Compounds from the class of the acylsulfonamides (S4): S4 a ) N-Acylsulfonamides and salts thereof, as described in WO-A-97/45016, S4 b ) Compounds of the 4-(benzoylsulfamoyl)benzamide type and salts thereof, as described in WO- A-99/16744, S4 c ) Compounds from the class of the benzoylsulfamoylphenylureas as described in EP-A-365484, for example 1-[4-(N-2-methoxybenzoylsulfamoyl)phenyl]-3-methylurea, 1-[4-(N-2-methoxybenzoyl- sulfamoyl)phenyl]-3,3-dimethylurea and 1-[4-(N-4,5-dimethylbenzoylsulfamoyl)phenyl]-3-methylurea;
- BCS223033 FC -68- S5)Active compounds from the class of the hydroxyaromatics and the aromatic-aliphatic carboxylic acid derivatives (S5), for example ethyl 3,4,5-triacetoxybenzoate, 3,5-dimethoxy-4-hydroxybenzoic acid, 3,5-dihydroxybenzoic acid, 4-hydroxysalicylic acid, 4-fluorosalicyclic acid, 2-hydroxycinnamic acid, 2,4-dichlorocinnamic acid, as described in WO-A-2004/084631, WO-A-2005/015994, WO-A- 2005/016001.
- S6Active compounds from the class of the 1,2-dihydroquinoxalin-2-ones (S6), for example 1- methyl-3-(2-thienyl)-1,2-dihydroquinoxalin-2-one, 1-methyl-3-(2-thienyl)-1,2-dihydroquinoxaline-2- thione, 1-(2-aminoethyl)-3-(2-thienyl)-1,2-dihydroquinoxalin-2-one hydrochloride, 1-(2- methylsulfonylaminoethyl)-3-(2-thienyl)-1,2-dihydroquinoxalin-2-one, as described in WO-A- 2005/112630.
- S7Compounds from the class of the diphenylmethoxyacetic acid derivatives (S7), e.g. methyl diphenylmethoxyacetate (CAS Reg. No. 41858-19-9) (S7-1), ethyl diphenylmethoxyacetate or diphenylmethoxyacetic acid, as described in WO-A-98/38856. S8) 2-fluoroacrylic acid derivatives as described in WO-A-98/27049.
- S7diphenylmethoxyacetic acid derivatives
- S7-1e.g. methyl diphenylmethoxyacetate
- S7-1ethyl diphenylmethoxyacetate or diphenylmethoxyacetic acid
- S9 active compoundsfrom the class of the 3-(5-tetrazolylcarbonyl)-2-quinolones (S9), for example 1,2-dihydro-4-hydroxy-1-ethyl-3-(5-tetrazolylcarbonyl)-2-quinolone (CAS Reg. No. 219479-18-2), 1,2- dihydro-4-hydroxy-1-methyl-3-(5-tetrazolylcarbonyl)-2-quinolone (CAS Reg. No. 95855-00-8), as described in WO-A-199/000020; S10) N-acylsulfonamides as described in WO-A-2007/023719 and WO-A-2007/023764.
- S93-(5-tetrazolylcarbonyl)-2-quinolones
- S11Active compounds of the oxyimino compound type (S11), which are known as seed-dressing agents, for example “oxabetrinil” ((Z)-1,3-dioxolan-2-ylmethoxyimino(phenyl)acetonitrile) (S11-1), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage, "fluxofenim” (1-(4-chlorophenyl)-2,2,2-trifluoro-1-ethanone O-(1,3-dioxolan-2-ylmethyl)oxime) (S11- 2), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage, and “cyometrinil” or “CGA-43089” ((Z)-cyanomethoxyimino(phenyl)acetonitrile) (S11-3), which is known as a seed-dressing safener for millet
- S12active compounds from the class of the isothiochromanones (S12), for example methyl [(3-oxo- 1H-2-benzothiopyran-4(3H)-ylidene)methoxy]acetate (CAS Reg. No. 205121-04-6) (S12-1) and related compounds from WO-A-1998/13361.
- nitrification inhibitors wichcan be mixed with the compound and the composition of the invention are selected from the group consisting of 2-(3,4-dimethyl-1H-pyrazol-1-yl)succinic acid, 2-(4,5- dimethyl-1H-pyrazol-1-yl)succinic acid, 3,4-dimethyl pyrazolium glycolate, 3,4-dimethyl pyrazolium citrate, 3,4-dimethyl pyrazolium lactate, 3,4-dimethyl pyrazolium mandelate, 1,2,4-triazole, 4-Chloro-3- methylpyrazole, N-((3(5)-methyl-1H-pyrazole-1-yl)methyl)acetamide, N-((3(5)-methyl-1 H-pyrazole-1- yl)methyl)formamide, N-((3(5),4-dimethylpyrazole-1-yl)methyl)formamide, N-((4-chloro-3(5)-methyl
- the compound and the composition of the inventionmay be combined with one or more agriculturally beneficial agents.
- agriculturally beneficial agentsinclude biostimulants, plant growth regulators, plant signal molecules, growth enhancers, microbial stimulating molecules, biomolecules, soil amendments, nutrients, plant nutrient enhancers, etc., such as lipo-chitooligosaccharides (LCO), chitooligosaccharides (CO), chitinous compounds, flavonoids, jasmonic acid or derivatives thereof (e.g., jasmonates), cytokinins, auxins, gibberellins, absiscic acid, ethylene, brassinosteroids, salicylates, macro- and micro-nutrients, linoleic acid or derivatives thereof, linolenic acid or derivatives thereof, karrikins, and beneficial microorganisms (e.g., Rhizobium spp., Bradyrhizobium spp., Sinorhizobium spp., Azorh
- the compound and the composition of the inventionmay be combined with one or more biostimulants.
- Biostimulantsmay enhance metabolic or physiological processes such as BCS223033 FC -71- respiration, photosynthesis, nucleic acid uptake, ion uptake, nutrient delivery, or a combination thereof.
- biostimulantsmay include seaweed extracts (e.g., ascophyllum nodosum; BAYFOLAN ALGAE, Aglukon gmbH, Germany), bacterial extracts (e.g., extracts of one or more diazotrophs, phosphate-solubilizing microorgafjaponisms and/or biopesticides), fungal extracts, humic acids (e.g., potassium humate), fulvic acids, myo-inositol, and/or glycine, protein hydrolysates and amino-acids both from animal BAYFOLAN AMBITION & BAYFOLAN cobre, SICIT, Italy) and plant origin, inorganic compounds (e.g silica) and any combinations thereof.
- seaweed extractse.g., ascophyllum nodosum; BAYFOLAN ALGAE, Aglukon gmbH, Germany
- bacterial extractse.g., extracts of one or more diazotrophs,
- the biostimulantsmay comprise one or more Azospirillum extracts (e.g., an extract of media comprising A. brasilense INTA Az-39), one or more Bradyrhizobium extracts (e.g., an extract of media comprising B. elkanii SEMIA 501, B. elkanii SEMIA 587, B. elkanii SEMIA 5019, B. japonicum NRRL B-50586 (also deposited as NRRL B-59565), B. japonicum NRRL B-50587 (also deposited as NRRL B-59566), B. japonicum NRRL B-50588 (also deposited as NRRL B-59567), B.
- Azospirillum extractse.g., an extract of media comprising A. brasilense INTA Az-39
- one or more Bradyrhizobium extractse.g., an extract of media comprising B. elkanii SEMIA 501
- japonicum NRRL B-50589also deposited as NRRL B-59568
- B. japonicum NRRL B-50590also deposited as NRRL B-59569
- B. japonicum NRRL B-50591also deposited as NRRL B-59570
- B. japonicum NRRL B-50592also deposited as NRRL B-59571
- B. japonicum NRRL B-50593also deposited as NRRL B-59572
- B. japonicum NRRL B-50594also deposited as NRRL B-50493
- B. japonicum NRRL B-50608also deposited as NRRL B-50608, B. japonicum NRRL B-50609, B.
- japonicum NRRL B-50610B. japonicum NRRL B-50611, B. japonicum NRRL B-50612, B. japonicum NRRL B- 50726, B. japonicum NRRL B-50727, B. japonicum NRRL B-50728, B. japonicum NRRL B-50729, B. japonicum NRRL B-50730, B. japonicum SEMIA 566, B. japonicum SEMIA 5079, B. japonicum SEMIA 5080, B. japonicum USDA 6, B. japonicum USDA 110, B. japonicum USDA 122, B. japonicum USDA 123, B. japonicum USDA 127, B.
- japonicum USDA 129 and/or B. japonicum USDA 532Cone or more Rhizobium extracts (e.g., an extract of media comprising R. leguminosarum SO12A-2), one or more Sinorhizobium extracts (e.g., an extract of media comprising S. fredii CCBAU114 and/or S. fredii USDA 205), one or more Penicillium extracts (e.g., an extract of media comprising P. bilaiae ATCC 18309, P. bilaiae ATCC 20851, P. bilaiae ATCC 22348, P. bilaiae NRRL 50162, P.
- Rhizobium extractse.g., an extract of media comprising R. leguminosarum SO12A-2
- Sinorhizobium extractse.g., an extract of media comprising S. fredii CCBAU114 and/or S.
- bilaiae NRRL 50169P. bilaiae NRRL 50776, P. bilaiae NRRL 50777, P. bilaiae NRRL 50778, P. bilaiae NRRL 50777, P. bilaiae NRRL 50778, P. bilaiae NRRL 50779, P. bilaiae NRRL 50780, P. bilaiae NRRL 50781, P. bilaiae NRRL 50782, P. bilaiae NRRL 50783, P. bilaiae NRRL 50784, P. bilaiae NRRL 50785, P.
- radicum FRR 4717P. radicum FRR 4719, P. radicum N93/47267 and/or P. raistrickii ATCC 10490
- Pseudomonas extractse.g., an extract of media comprising P. jessenii PS06
- acaricidal, insecticidal and/or nematicidal extractse.g., an extract of media comprising Bacillus firmus I-1582, Bacillus mycoides AQ726, NRRL B-21664; Beauveria bassiana ATCC-74040, Beauveria bassiana ATCC-74250, BCS223033 FC -72- Burkholderia sp.
- catenulataalso referred to as Gliocladium catenulatum J1446 (PRESTOP®, Verdera, Finland), Coniothyrium minitans CONTANS® (Prophyta, Germany), Cryphonectria parasitica (CNICM, France), Cryptococcus albidus YIELD PLUS® (Anchor Bio-Technologies, South Africa), Fusarium oxysporum BIOFOX® (from S.I.A.P.A., Italy) and FUSACLEAN® (Natural Plant Protection, France), Metschnikowia fructicola SHEMER® (Agrogreen, Israel), Microdochium dimerum ANTIBOT® (Agrauxine, France), Muscodor albus NRRL 30547, Muscodor roseus NRRL 30548, Phlebiopsis gigantea ROTSOP® (Verdera, Finland), Pseudozyma flocculosa SPORODEX® (Plant Products Co.
- the compound and the composition of the inventionmay be combined with one or more lipo-chitooligosaccharides (LCOs), chitooligosaccharides (COs), and/or chitinous compounds.
- LCOslipo-chitooligosaccharides
- COschitooligosaccharides
- LCOssometimes referred to as symbiotic nodulation (Nod) signals (or Nod factors) or as Myc factors, consist of an oligosaccharide backbone of ⁇ -l,4-linked N-acetyl-D-glucosamine (“GlcNAc”) residues with an N-linked fatty acyl chain condensed at the non-reducing end.
- GlcNAcN-acetyl-D-glucosamine
- LCOsmay be included or utilized in various forms of purity and can be used alone or in the form of a culture of LCO-producing bacteria or fungi.
- OPTIMIZE®commercially available from Bayer Company
- Methods to provide substantially pure LCOsinclude removing the microbial cells from a mixture of LCOs and the microbe, or continuing to isolate and purify the LCO molecules through LCO solvent phase separation followed by HPLC chromatography as described, for example, in U.S. Patent No. 5,549,718. Purification can be enhanced by repeated HPLC and the purified LCO molecules can be freeze-dried for long-term storage.
- Compositions and methods of the present disclosuremay comprise analogues, derivatives, hydrates, isomers, salts and/or solvates of LCOs. LCOs may be incorporated into the composition according to the inventionin any suitable amount(s)/concentration(s).
- the composition according to the inventioncomprise about 1 x 10 -20 M to about 1 x 10 -1 M LCO(s).
- the amount/concentration of LCOmay be an amount effective to impart a positive trait or benefit to a plant, such as to enhance the growth and/or yield of the plant to which the composition is applied.
- the LCO amount/concentrationis not effective to enhance the yield of the plant without beneficial contributions from one or more other constituents of the composition, such as CO and/or one or more pesticides.
- the compound and the composition of the inventionmay be combined with any suitable COs, perhaps in combination with one or more LCOs. COs differ from LCOs in that they lack the pendant fatty acid chain that is characteristic of LCOs.
- COssometimes referred to as N-acetylchitooligosaccharides, are also composed of GlcNAc residues but have side chain decorations that make them different from chitin molecules [(C 8 H 13 NO 5 ) n , CAS No.1398-61-4] and chitosan molecules [(C 5 H 11 NO 4 ) n , CAS No. 9012-76-4].
- chitin molecules(C 8 H 13 NO 5 ) n , CAS No.1398-61-4]
- chitosan molecules(C 5 H 11 NO 4 ) n , CAS No. 9012-76-4].
- COsmay be obtained from any suitable source.
- the COmay be derived from an LCO.
- the composition according to the inventioncomprise one or more COs derived from an LCO obtained (i.e., isolated and/or purified) from a strain of Azorhizobium, Bradyrhizobium (e.g., B. japonicum), Mesorhizobium, Rhizobium (e.g., R. leguminosarum), Sinorhizobium (e.g., S. meliloti), or mycorhizzal fungi (e.g., Glomus intraradicus).
- the COmay be synthetic. Methods for the preparation of recombinant COs are known in the art. See, e.g., Cottaz et al., Meth.
- COsmay be included or utilized in various forms of purity and can be used alone or in the form of a culture of CO-producing bacteria or fungi. It is to be understood that the compound and the composition of the invention may be combined with hydrates, isomers, salts and/or solvates of COs. COs may be used in any suitable amount(s)/concentration(s).
- the composition according to the inventionmay comprise about 1 x 10 -20 M to about 1 x 10 -1 M COs.
- the amount/concentration of COmay be an amount effective to impart or confer a positive trait or benefit to a plant, such as to enhance the soil microbial environment, nutrient uptake, or increase the growth and/or yield of the plant to which the composition is applied.
- a CO amount/concentrationmay not be effective to enhance the growth of the plant without beneficial contributions from one or more other ingredients of the composition, such as LCO and/or one or more inoculants, biomolecules, nutrients, or pesticides.
- the compound and the composition of the inventionmay be combined with one or more suitable chitinous compounds, such as, for example, chitin, chitosan, and isomers, salts and solvates thereof.
- Chitins and chitosanswhich are major components of the cell walls of fungi and the exoskeletons of insects and crustaceans, are composed of GlcNAc residues. Chitins and chitosans may be obtained commercially or prepared from insects, crustacean shells, or fungal cell walls. Methods for the preparation of chitin and chitosan are known in the art. See, e.g., U.S. Patent Nos.
- Deacetylated chitins and chitosansmay be obtained that range from less than 35% to greater than 90% deacetylation and cover a broad spectrum of molecular weights, e.g., low molecular weight chitosan oligomers of less than 15kD and chitin oligomers of 0.5 to 2kD; “practical grade” chitosan with a molecular weight of about 15kD; and high molecular weight chitosan of up to 70kD.
- Chitin and chitosan compositions formulated for seed treatmentare commercially available. Commercial products include, for example, ELEXA® (Plant Defense Boosters, Inc.) and BEYONDTM (Agrihouse, Inc.).
- the compound and the composition of the inventionmay be combined with one or more suitable flavonoids, including, but not limited to, anthocyanidins, anthoxanthins, chalcones, coumarins, flavanones, flavanonols, flavans and isoflavonoids, as well as analogues, derivatives, hydrates, isomers, polymers, salts and solvates thereof.
- Flavonoidsare phenolic compounds having the general structure of two aromatic rings connected by a three-carbon bridge. Classes of flavonoids are known in the art. See, e.g., Jain et al., J. Plant Biochem. & Biotechnol.11:1 (2002); and Shaw et al., Environ.
- Flavonoid compoundsmay be isolated from plants or seeds, e.g., as described in U.S. Patents 5,702,752; 5,990,291; and 6,146,668. Flavonoid compounds may also be produced by genetically engineered organisms, such as yeast. See, e.g., Ralston et al., Plant Physiol. 137:1375 (2005).
- the compound and the composition of the inventionmay be combined with one or more flavanones, such as one or more of butin, eriodictyol, hesperetin, hesperidin, homoeriodictyol, isosakuranetin, naringenin, naringin, pinocembrin, poncirin, sakuranetin, sakuranin, and/or sterubin, one or more flavanonols, such as dihydrokaempferol and/or taxifolin, one or more flavans, such as one or more flavan-3-ols (e.g., catechin (C), catechin 3-gallate (Cg), epicatechins (EC), epigallocatechin (EGC) epicatechin 3-gallate (ECg), epigallcatechin 3-gallate (EGCg), epiafzelechin, fisetinidol, gallocatechin (GC), gallcatechin 3-gallate
- Flavonoids and their derivativesmay be included in the present composition in any suitable form, including, but not limited to, polymorphic and crystalline forms. Flavonoids may be included in the composition according to the invention in any suitable amount(s) or concentration(s).
- the amount/concentration of a flavonoid(s)may be an amount effective to impart a benefit to a plant, which may be indirectly through activity on soil microorganisms or other means, such as to enhance plant nutrition and/or yield. According to some embodiments, a flavonoid amount/concentration may not be effective to enhance the nutrition or yield of the plant without the beneficial contributions from one or more other ingredients of the composition, such as LCO, CO, and/or one or more pesticides.
- the compound and the composition of the inventionmay be combined with one or more suitable non- flavonoid nod-gene inducer(s), including, but not limited to, jasmonic acid ([1R-[1 ⁇ ,2 ⁇ (Z)]]-3-oxo-2- (pentenyl)cyclopentaneacetic acid; JA), linoleic acid ((Z,Z)-9,12-Octadecadienoic acid) and/or linolenic acid ((Z,Z,Z)-9,12,15-octadecatrienoic acid), and analogues, derivatives, hydrates, isomers, polymers, salts and solvates thereof.
- suitable non- flavonoid nod-gene inducer(s)including, but not limited to, jasmonic acid ([1R-[1 ⁇ ,2 ⁇ (Z)]]-3-oxo-2- (pentenyl)cyclopentaneacetic acid; JA), linoleic
- Jasmonic acid and its methyl ester, methyl jasmonate (MeJA), collectively known as jasmonates,are octadecanoid-based compounds that occur naturally in some plants (e.g., wheat), fungi (e.g., Botryodiplodia theobromae, Gibbrella fujikuroi), yeast (e.g., Saccharomyces cerevisiae) and bacteria (e.g., Escherichia coli). Linoleic acid and linolenic acid may be produced in the course of the biosynthesis of jasmonic acid.
- fungie.g., Botryodiplodia theobromae, Gibbrella fujikuroi
- yeaste.g., Saccharomyces cerevisiae
- bacteriae.g., Escherichia coli.
- Jasmonates, linoleic acid and linolenic acid (and their derivatives)are reported to be inducers of nod gene expression or LCO production by rhizobacteria. See, e.g., Mabood et al., PLANT PHYSIOL. BIOCHEM. 44(11):759 (2006); Mabood et al., AGR. J.98(2):289 (2006); Mabood et al., FIELD CROPS RES.95(2-3):412 (2006); and Mabood & Smith, Linoleic and linolenic acid induce the expression of nod genes in Bradyrhizobium japonicum USDA 3, PLANT BIOL. (2001).
- estersare compounds in which the carboxyl group of linoleic acid, linolenic acid, or jasmonic acid has been replaced with a --COR group, where R is an --OR 1 group, in which R 1 is: an alkyl group, such as a C 1 -C 8 unbranched or branched alkyl group, e.g., a methyl, ethyl or propyl group; an alkenyl group, such as a C 2 -C 8 unbranched or branched alkenyl group; an alkynyl group, such as a C 2 -C 8 unbranched or branched alkynyl group; an aryl group having, for example, 6 to 10 carbon atoms; or a
- Representative amidesare compounds in which the carboxyl group of linoleic acid, linolenic acid, or jasmonic acid has been replaced with a --COR group, where R is an NR 2 R 3 group, in which R 2 and R 3 are each independently: a hydrogen; an alkyl group, such as a C 1 -C 8 unbranched or branched alkyl group, e.g., a methyl, ethyl or propyl group; an alkenyl group, such as a C 2 -C 8 unbranched or branched alkenyl group; an alkynyl group, such as a C 2 -C 8 unbranched or branched BCS223033 FC -77- alkynyl group; an aryl group having, for example, 6 to 10 carbon atoms; or a heteroaryl group having, for example, 4 to 9 carbon atoms, wherein the heteroatoms in the heteroaryl group can be, for example, N, O
- Estersmay be prepared by known methods, such as acid-catalyzed nucleophilic addition, wherein the carboxylic acid is reacted with an alcohol in the presence of a catalytic amount of a mineral acid.
- Amidesmay also be prepared by known methods, such as by reacting the carboxylic acid with the appropriate amine in the presence of a coupling agent, such as dicyclohexyl carbodiimide (DCC), under neutral conditions.
- Suitable salts of linoleic acid, linolenic acid and jasmonic acidinclude, for example, base addition salts.
- the bases that may be used as reagents to prepare metabolically acceptable base salts of these compoundsinclude those derived from cations such as alkali metal cations (e.g., potassium and sodium) and alkaline earth metal cations (e.g., calcium and magnesium). These salts may be readily prepared by mixing a solution of linoleic acid, linolenic acid, or jasmonic acid with a solution of the base. The salts may be precipitated from solution and collected by filtration, or may be recovered by other means such as by evaporation of the solvent. Non-flavonoid nod-gene inducers may be used in combination with the compound and the composition according to the invention in any suitable amount(s)/concentration(s).
- cationssuch as alkali metal cations (e.g., potassium and sodium) and alkaline earth metal cations (e.g., calcium and magnesium).
- alkali metal cationse.g., potassium and sodium
- the amount/concentration of non-flavonoid nod-gene inducersmay be an amount effective to impart or confer a positive trait or benefit to a plant, such as to enhance the growth and/or yield of the plant to which the composition is applied.
- the amount/concentration of non-flavonoid nod- gene inducersmay not be effective to enhance the growth and/or yield of the plant without beneficial contributions from one or more other ingredients of the composition, such as a LCO, CO and/or one or more pesticides.
- the compound and the composition of the inventionmay be combined with karrakins, including but not limited to 2H-furo[2,3-c]pyran-2-ones, as well as analogues, derivatives, hydrates, isomers, polymers, salts and solvates thereof.
- biologically acceptable salts of karrakinsinclude acid addition salts formed with biologically acceptable acids, examples of which include hydrochloride, hydrobromide, sulphate or bisulphate, phosphate or hydrogen phosphate, acetate, benzoate, succinate, fumarate, maleate, lactate, citrate, tartrate, gluconate; methanesulphonate, benzenesulphonate and p-toluenesulphonic acid.
- Additional biologically acceptable metal saltsmay include alkali metal salts, with bases, examples of which include the sodium and potassium salts.
- Karrakinsmay be incorporated into the composition according to the invention in any suitable amount(s) or concentration(s).
- the amount/concentration of a karrakinmay be an amount or concentration effective to impart or confer a positive trait or benefit to a plant, such as to enhance the growth and/or yield of the plant to which the composition is applied.
- a karrakin amount/concentrationmay not be effective to enhance the growth and/or yield of the plant without beneficial contributions from one or more other ingredients of the composition, such as a LCO, CO and/or one or more pesticides.
- anthocyanidins and/or anthoxanthinssuch as one or more of cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin, flavones (e.g., apigenin, baicalein, chrysin, 7,8-dihydroxyflavone, diosmin, flavoxate, 6- hydroxyflavone, luteolin, scutellarein, tangeritin and/or wogonin) and/or flavonols (e.g., amurensin, astragalin, azaleatin, azalein, fisetin, furanoflavonols galangin, gossypetin, 3-hydroxyflavone, hyperoside, icariin, isoquercetin, kaempferide, kaempferitrin
- the compound and the composition of the inventionmay be combined with gluconolactone and/or an analogue, derivative, hydrate, isomer, polymer, salt and/or solvate thereof.
- Gluconolactonemay be incorporated into the composition according to the inventionin any suitable amount(s)/concentration(s).
- the amount/concentration of a gluconolactone amount/concentrationmay be an amount effective to impart or confer a positive trait or benefit to a plant, such as to enhance the growth and/or yield of the plant to which the composition is applied.
- the gluconolactone amount/concentrationmay not be effective to enhance the growth and/or yield of the plant without beneficial contributions from one or more other ingredients of the composition, such as a LCO, CO and/or one or more pesticides.
- the compound and the composition of the inventionmay be combined with one or more suitable nutrient(s) and/or fertilizer(s), such as organic acids (e.g., acetic acid, citric acid, lactic acid, malic acid, taurine, etc.), macrominerals (e.g., phosphorous, calcium, magnesium, potassium, sodium, iron, etc.), trace minerals (e.g., boron, cobalt, chloride, chromium, copper, fluoride, iodine, iron, manganese, molybdenum, selenium, zinc, etc.), vitamins, (e.g., vitamin A, vitamin B complex (i.e., vitamin B 1 , vitamin B 2 , vitamin B 3 , vitamin B 5 , vitamin B 6 , vitamin B
- the compound and the composition of the inventionmay be combined with macro- and micronutrients of plants or microbes, including phosphorous, boron, chlorine, copper, iron, manganese, molybdenum and/or zinc. According to some embodiments, the compound and the composition of the invention may be combined with one or more beneficial micronutrients.
- Non-limiting examples of micronutrients for use in compositions described hereinmay include vitamins, (e.g., vitamin A, vitamin B complex (i.e., vitamin B1, vitamin B2, vitamin B3, vitamin B5, vitamin B6, vitamin B7, vitamin B8, vitamin B9, vitamin B12, choline) vitamin C, vitamin D, vitamin E, vitamin K, carotenoids ( ⁇ -carotene, ⁇ -carotene, cryptoxanthin, lutein, lycopene, zeaxanthin, etc.), macrominerals (e.g., phosphorous, calcium, magnesium, potassium, sodium, iron, etc.), trace minerals (e.g., boron, cobalt, chloride, chromium, copper, fluoride, iodine, iron, manganese, molybdenum, selenium, zinc, etc.), organic acids (e.g., acetic acid, citric acid, lactic acid, malic acid, taurine, etc.), and combinations thereof (BAYFOLAN secure
- compositionsmay comprise phosphorous, boron, chlorine, copper, iron, manganese, molybdenum, and/or zinc, and combinations thereof.
- phosphorousmay be derived from a rock phosphate source, such as monoammonium phosphate, diammonium phosphate, monocalcium phosphate, super phosphate, triple super phosphate, and/or ammonium polyphosphate, an organic phosphorous source, or a phosphorous source capable of solubilization by one or more microorganisms (e.g., Penicillium bilaiae).
- the compound and the composition of the inventionhave potent microbicidal activity and/or plant defense modulating potential. They can be used for controlling unwanted microorganisms, such as unwanted fungi and bacteria, on plants. They can be particularly useful in crop protection (they control microorganisms that cause plants diseases) or for protecting materials (e.g. industrial materials, timber, storage goods) as described in more details herein below. More specifically, the compound and the composition of the invention can be used to protect seeds, germinating seeds, emerged seedlings, plants, plant parts, fruits, harvest goods and/or the soil in which the plants grow from unwanted microorganisms. Control or controlling as used herein encompasses protective, curative and eradicative treatment of unwanted microorganisms.
- Unwanted microorganismsmay be pathogenic bacteria, pathogenic virus, pathogenic oomycetes or pathogenic fungi, more specifically phytopathogenic bacteria, phytopathogenic virus, phytopathogenic oomycetes or phytopathogenic fungi. As detailed herein below, these phytopathogenic microorganims are the causal agents of a broad spectrum of plants diseases. More specifically, the compound and the composition of the invention can be used as fungicides.
- fungiciderefers to a compound or composition that can be used in crop protection for the control of unwanted fungi, such as Plasmodiophoromycetes, Chytridiomycetes, Zygomycetes, Ascomycetes, Basidiomycetes and Deuteromycetes and/or for the control of Oomycetes.
- the compound and the composition of the inventionmay also be used as antibacterial agent.
- theymay be used in crop protection, for example for the control of unwanted bacteria, such as Pseudomonadaceae, Rhizobiaceae, Xanthomonadaceae, Enterobacteriaceae, Corynebacteriaceae and Streptomycetaceae.
- the compound and the composition of the inventionmay also be used as antiviral agent in crop protection.
- the compound and the composition of the inventionmay have effects on diseases from plant viruses, such as the tobacco mosaic virus (TMV), tobacco rattle virus, tobacco stunt virus (TStuV), tobacco leaf curl virus (VLCV), tobacco nervilia mosaic virus (TVBMV), tobacco necrotic dwarf virus (TNDV), tobacco streak virus (TSV), potato virus X (PVX), potato viruses Y, S, M, and A, potato acuba mosaic virus (PAMV), potato mop-top virus (PMTV), potato leaf-roll virus (PLRV), alfalfa mosaic virus BCS223033 FC -80- (AMV), cucumber mosaic virus (CMV), cucumber green mottlemosaic virus (CGMMV), cucumber yellows virus (CuYV), watermelon mosaic virus (WMV), tomato spotted wilt virus (TSWV), tomato ringspot virus (TomRSV), sugarcane mosaic virus (SCMV), rice drawf virus, rice stripe virus, rice black
- the present inventionalso relates to a method for controlling unwanted microorganisms, such as unwanted fungi, oomycetes and bacteria, on plants comprising the step of applying at least one compound of the invention or at least one composition of the invention to the microorganisms and/or their habitat (to the plants, plant parts, seeds, fruits or to the soil in which the plants grow).
- unwanted microorganismssuch as unwanted fungi, oomycetes and bacteria
- Suitable substrates that may be used for cultivating plantsinclude inorganic based substrates, such as mineral wool, in particular stone wool, perlite, sand or gravel; organic substrates, such as peat, pine bark or sawdust; and petroleum based substrates such as polymeric foams or plastic beads.
- Effective and plant-compatible amountmeans an amount that is sufficient to control or destroy the fungi present or liable to appear on the cropland and that does not entail any appreciable symptom of phytotoxicity for said crops. Such an amount can vary within a wide range depending on the fungus to be controlled, the type of crop, the crop growth stage, the climatic conditions and the respective compound or composition of the invention used. This amount can be determined by systematic field trials that are within the capabilities of a person skilled in the art.
- Plants and plant partsThe compound and the composition of the invention may be applied to any plants or plant parts.
- Plantsmean all plants and plant populations, such as desired and undesired wild plants or crop plants (including naturally occurring crop plants).
- Crop plantsmay be plants which can be obtained by conventional breeding and optimization methods or by biotechnological and genetic engineering methods or combinations of these methods, including the genetically modified plants (GMO or transgenic plants) and the plant cultivars which are protectable and non-protectable by plant breeders’ rights.
- Plant cultivarsare understood to mean plants which have new properties ("traits”) and have been obtained by conventional breeding, by mutagenesis or by recombinant DNA techniques. They can be cultivars, varieties, bio- or genotypes.
- BCS223033 FC -81- Plant partsare understood to mean all parts and organs of plants above and below the ground, such as shoots, leaves, needles, stalks, stems, flowers, fruit bodies, fruits, seeds, roots, tubers and rhizomes.
- the plant partsalso include harvested material and vegetative and generative propagation material, for example cuttings, tubers, rhizomes, slips and seeds.
- Plants which may be treated in accordance with the methods of the inventioninclude the following: cotton, flax, grapevine, fruit, vegetables, such as Rosaceae sp.
- pome fruitssuch as apples and pears, but also stone fruits such as apricots, cherries, almonds and peaches, and soft fruits such as strawberries
- Ribesioidae sp.Juglandaceae sp.
- Betulaceae sp.Anacardiaceae sp., Fagaceae sp., Moraceae sp., Oleaceae sp., Actinidaceae sp., Lauraceae sp., Musaceae sp. (for example banana trees and plantations), Rubiaceae sp.
- Theaceae sp.for example coffee
- Theaceae sp.Sterculiceae sp.
- Rutaceae sp.for example lemons, oranges and grapefruit
- Solanaceae sp.for example tomatoes
- Liliaceae sp.for example lettuce
- Umbelliferae sp.for example lettuce
- Umbelliferae sp.for example lettuce
- Umbelliferae sp.for example lettuce
- Cicurbitaceae sp.for example cucumber
- Alliaceae sp.for example leek, onion
- Papilionaceae sp.for example peas
- major crop plantssuch as Gramineae sp.
- Asteraceae sp.for example sunflower
- Brassicaceae sp.for example white cabbage, red cabbage, broccoli, cauliflower, Brussels sprouts, pak choi, kohlrabi, radishes, and oilseed rape, mustard, horseradish and cress
- Fabacae sp.for example bean, peanuts
- Papilionaceae sp.for example soya bean
- Solanaceae sp.for example potatoes), Chenopodiaceae sp.
- Plants and plant cultivars which may be treated by the above disclosed methodsinclude plants and plant cultivars which are resistant against one or more biotic stresses, i.e. said plants show a better defense against animal and microbial pests, such as against nematodes, insects, mites, phytopathogenic fungi, bacteria, viruses and/or viroids. Plants and plant cultivars which may be treated by the above disclosed methods include those plants which are resistant to one or more abiotic stresses.
- Abiotic stress conditionsmay include, for example, drought, cold temperature exposure, heat exposure, osmotic stress, flooding, increased soil salinity, increased mineral exposure, ozone exposure, high light exposure, limited availability of nitrogen nutrients, limited availability of phosphorus nutrients, shade avoidance.
- Plants and plant cultivars which may be treated by the above disclosed methodsinclude those plants characterized by enhanced yield characteristics. Increased yield in said plants may be the result of, for example, improved plant physiology, growth and development, such as water use efficiency, water retention efficiency, improved nitrogen use, enhanced carbon assimilation, improved photosynthesis, increased germination efficiency and accelerated maturation.
- Yieldmay furthermore be affected by improved plant architecture (under stress and non-stress conditions), including but not limited to, early flowering, flowering control for hybrid seed production, seedling vigor, plant size, internode number and distance, root growth, seed size, fruit BCS223033 FC -82- size, pod size, pod or ear number, seed number per pod or ear, seed mass, enhanced seed filling, reduced seed dispersal, reduced pod dehiscence and lodging resistance.
- Further yield traitsinclude seed composition, such as carbohydrate content and composition for example cotton or starch, protein content, oil content and composition, nutritional value, reduction in anti-nutritional compounds, improved processability and better storage stability.
- Plants and plant cultivars which may be treated by the above disclosed methodsinclude plants and plant cultivars which are hybrid plants that already express the characteristic of heterosis or hybrid vigor which results in generally higher yield, vigor, health and resistance towards biotic and abiotic stresses.
- Transgenic plants, seed treatment and integration eventsThe compound according to the invention can be advantageously used to treat transgenic plants, plant cultivars or plant parts that received genetic material which imparts advantageous and/or useful properties (traits) to these plants, plant cultivars or plant parts. Therefore, it is contemplated that the present invention may be combined with one or more recombinant traits or transgenic event(s) or a combination thereof.
- a transgenic eventis created by the insertion of a specific recombinant DNA molecule into a specific position (locus) within the chromosome of the plant genome.
- the insertioncreates a novel DNA sequence referred to as an “event” and is characterized by the inserted recombinant DNA molecule and some amount of genomic DNA immediately adjacent to/flanking both ends of the inserted DNA.
- Such trait(s) or transgenic event(s)include, but are not limited to, pest resistance, water use efficiency, yield performance, drought tolerance, seed quality, improved nutritional quality, hybrid seed production, and herbicide tolerance, in which the trait is measured with respect to a plant lacking such trait or transgenic event.
- Such advantageous and/or useful propertiesare better plant growth, vigor, stress tolerance, standability, lodging resistance, nutrient uptake, plant nutrition, and/or yield, in particular improved growth, increased tolerance to high or low temperatures, increased tolerance to drought or to levels of water or soil salinity, enhanced flowering performance, easier harvesting, accelerated ripening, higher yields, higher quality and/or a higher nutritional value of the harvested products, better storage life and/or processability of the harvested products, and increased resistance against animal and microbial pests, such as against insects, arachnids, nematodes, mites, slugs and snails.
- Bt Cry or VIP proteinswhich include the CrylA, CryIAb, CryIAc, CryIIA, CryIIIA, CryIIIB2, Cry9c Cry2Ab, Cry3Bb and CryIF proteins or toxic fragments thereof and also hybrids or combinations thereof, BCS223033 FC -83- especially the CrylF protein or hybrids derived from a CrylF protein (e.g. hybrid CrylA-CrylF proteins or toxic fragments thereof), the CrylA-type proteins or toxic fragments thereof, preferably the CrylAc protein or hybrids derived from the CrylAc protein (e.g.
- hybrid CrylAb-CrylAc proteinsor the CrylAb or Bt2 protein or toxic fragments thereof, the Cry2Ae, Cry2Af or Cry2Ag proteins or toxic fragments thereof, the CrylA.105 protein or a toxic fragment thereof, the VIP3Aa19 protein, the VIP3Aa20 protein, the VIP3A proteins produced in the COT202 or COT203 cotton events, the VIP3Aa protein or a toxic fragment thereof as described in Estruch et al.
- any variants or mutants of any one of these proteinsdiffering in some amino acids (1-10, preferably 1-5) from any of the above named sequences, particularly the sequence of their toxic fragment, or which are fused to a transit peptide, such as a plastid transit peptide, or another protein or peptide, is included herein.
- a transit peptidesuch as a plastid transit peptide, or another protein or peptide
- Another and particularly emphasized example of such propertiesis conferred tolerance to one or more herbicides, for example imidazolinones, sulphonylureas, glyphosate or phosphinothricin.
- DNA sequences encoding proteins which confer properties of tolerance to certain herbicides on the transformed plant cells and plantsmention will be particularly be made to the bar or PAT gene or the Streptomyces coelicolor gene described in WO2009/152359 which confers tolerance to glufosinate herbicides, a gene encoding a suitable EPSPS (5-Enolpyruvylshikimat-3-phosphat-synthase) which confers tolerance to herbicides having EPSPS as a target, especially herbicides such as glyphosate and its salts, a gene encoding glyphosate-n-acetyltransferase, or a gene encoding glyphosate oxidoreductase.
- EPSPS5-Enolpyruvylshikimat-3-phosphat-synthase
- herbicide tolerance traitsinclude at least one ALS (acetolactate synthase) inhibitor (e.g. WO2007/024782), a mutated Arabidopsis ALS/AHAS gene (e.g. U.S. Patent 6,855,533), genes encoding 2,4-D- monooxygenases conferring tolerance to 2,4-D (2,4- dichlorophenoxyacetic acid) and genes encoding Dicamba monooxygenases conferring tolerance to dicamba (3,6-dichloro-2- methoxybenzoic acid).
- ALSacetolactate synthase
- a mutated Arabidopsis ALS/AHAS genee.g. U.S. Patent 6,855,533
- genes encoding 2,4-D- monooxygenasesconferring tolerance to 2,4-D (2,4- dichlorophenoxyacetic acid
- genes encoding Dicamba monooxygenasesconferring tolerance to dicamba (3,6-dichloro-2- meth
- DNA sequences encoding proteins which confer properties of resistance to such diseasesmention will particularly be made of the genetic material from glycine tomentella, for example from any one of publically available accession lines PI441001 , PI483224, PI583970, PI446958, PI499939, PI505220, PI499933, PI441008, PI505256 or PI446961 as described in WO2019/103918. Further and particularly emphasized examples of such properties are increased resistance against bacteria and/or viruses owing, for example, to systemic acquired resistance (SAR), systemin, phytoalexins, elicitors and also resistance genes and correspondingly expressed proteins and toxins.
- SARsystemic acquired resistance
- systeminphytoalexins
- elicitorsalso resistance genes and correspondingly expressed proteins and toxins.
- BCS223033 FC -84- Particularly useful transgenic events in transgenic plants or plant cultivarswhich can be treated with preference in accordance with the invention include Event 531/ PV-GHBK04 (cotton, insect control, described in WO2002/040677), Event 1143-14A (cotton, insect control, not deposited, described in WO2006/128569); Event 1143-51B (cotton, insect control, not deposited, described in WO2006/128570); Event 1445 (cotton, herbicide tolerance, not deposited, described in US-A 2002- 120964 or WO2002/034946); Event 17053 (rice, herbicide tolerance, deposited as PTA-9843, described in WO2010/117737); Event 17314 (rice, herbicide tolerance, deposited as PTA-9844, described in WO2010/117735); Event 281-24-236 (cotton, insect control - herbicide tolerance, deposited as PTA-6233, described in WO2005/103266 or US-A 2005-216969); Event 3006-210-
- Event BLRl(oilseed rape, restoration of male sterility, deposited as NCIMB 41193, described in WO2005/074671), Event CE43-67B (cotton, insect control, deposited as DSM ACC2724, described in US-A 2009-217423 or WO2006/128573); Event CE44-69D (cotton, insect control, not deposited, described in US-A 2010- 0024077); Event CE44-69D (cotton, insect control, not deposited, described in WO2006/128571); Event CE46-02A (cotton, insect control, not deposited, described in WO2006/128572); Event COT102 (cotton, insect control, not deposited, described in US-A 2006-130175 or WO2004/039986); Event COT202 (cotton, insect control, not deposited, described in US-A 2007-067868 or WO2005/054479); Event COT203 (cotton, insect control, not deposited, described, described in US-A 2007-067868 or
- transgenic event(s)is provided by the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) and can be found on their website on the world wide web at aphis.usda.gov. For this application, the status of such list as it is/was on the filing date of this application, is relevant.
- the genes/events which impart the desired traits in questionmay also be present in combinations with one another in the transgenic plants.
- transgenic plantswhich may be mentioned are the important crop plants, such as cereals (wheat, rice, triticale, barley, rye, oats), maize, soya beans, potatoes, sugar beet, sugar cane, tomatoes, peas and other types of vegetable, cotton, tobacco, oilseed rape and also fruit plants (with the fruits apples, pears, citrus fruits and grapes), with particular emphasis being given to maize, soya beans, wheat, rice, potatoes, cotton, sugar cane, tobacco and oilseed rape.
- Traits which are particularly emphasizedare the increased resistance of the plants to insects, arachnids, nematodes and slugs and snails, as well as the increased resistance of the plants to one or more herbicides.
- pathogens of fungal diseaseswhich may be treated in accordance with the invention include: diseases caused by powdery mildew pathogens, for example Blumeria species, for example Blumeria graminis; Podosphaera species, for example Podosphaera leucotricha; Sphaerotheca species, for example Sphaerotheca fuliginea; Uncinula species, for example Erysiphe necator; diseases caused by rust disease pathogens, for example Gymnosporangium species, for example Gymnosporangium sabinae; Hemileia species, for example Hemileia vastatrix; Phakopsora species, for example Phakopsora pachyrhizi, Phakopsora meibomiae or Phakopsora euvitis; Puccinia species, for example Puccinia recondita, Puccinia graminis oder Puccinia striiformis; U
- brassicaePhytophthora species, for example Phytophthora infestans; Plasmopara species, for example Plasmopara viticola; Pseudoperonospora species, for example Pseudoperonospora humuli or Pseudoperonospora cubensis; Pythium species, for example Pythium ultimum; leaf blotch diseases and leaf wilt diseases caused, for example, by Alternaria species, for example Alternaria solani; Cercospora species, for example Cercospora beticola; Cladiosporium species, for example Cladiosporium cucumerinum; Cochliobolus species, for example Cochliobolus sativus (conidial form: Drechslera, syn: Helminthosporium) or Cochliobolus miyabeanus; Colletotrichum species, for example Colletotrichum lindemuthanium;
- Pseudomonas speciesfor example Pseudomonas syringae pv. lachrymans
- Erwinia speciesfor example Erwinia amylovora
- Liberibacter speciesfor example Liberibacter asiaticus
- Xyella speciesfor example Xylella fastidiosa
- Ralstonia speciesfor example Ralstonia solanacearum
- Dickeya speciesfor example Dickeya solani
- Clavibacter speciesfor example Clavibacter michiganensis
- Streptomyces speciesfor example Streptomyces scabies.
- Fungal diseases on roots and the stem basecaused, for example, by black root rot (Calonectria crotalariae), charcoal rot (Macrophomina phaseolina), fusarium blight or wilt, root rot, and pod and collar rot (Fusarium BCS223033 FC -91- oxysporum, Fusarium orthoceras, Fusarium semitectum, Fusarium equiseti), mycoleptodiscus root rot (Mycoleptodiscus terrestris), neocosmospora (Neocosmospora vasinfecta), pod and stem blight (Diaporthe phaseolorum), stem canker (Diaporthe phaseolorum var.
- phytophthora rot(Phytophthora megasperma), brown stem rot (Phialophora gregata), pythium rot (Pythium aphanidermatum, Pythium irregulare, Pythium debaryanum, Pythium myriotylum, Pythium ultimum), rhizoctonia root rot, stem decay, and damping-off (Rhizoctonia solani), sclerotinia stem decay (Sclerotinia sclerotiorum), sclerotinia southern blight (Sclerotinia rolfsii), thielaviopsis root rot (Thielaviopsis basicola).
- Mycotoxinsinclude particularly, but not exclusively, the following: deoxynivalenol (DON), nivalenol, 15-Ac-DON, 3-Ac-DON, T2- and HT2- toxin, fumonisins, zearalenon, moniliformin, fusarin, diaceotoxyscirpenol (DAS), beauvericin, enniatin, fusaroproliferin, fusarenol, ochratoxins, patulin, ergot alkaloids and aflatoxins which can be produced, for example, by the following fungi: Fusarium spec., such as F.
- verticillioidesand also by Aspergillus spec., such as A. flavus, A. parasiticus, A. nomius, A. ochraceus, A. clavatus, A. terreus, A. versicolor, Penicillium spec., such as P. verrucosum, P. viridicatum, P. citrinum, P. expansum, P. claviforme, P. roqueforti, Claviceps spec., such as C. purpurea, C. fusiformis, C. paspali, C. africana, Stachybotrys spec. and others.
- Aspergillus spec.such as A. flavus, A. parasiticus, A. nomius, A. ochraceus, A. clavatus, A. terreus, A. versicolor, Penicillium spec., such as P. verrucosum, P. viridicatum, P. citr
- the compound and the composition of the inventionmay also be used in the protection of materials, especially for the protection of industrial materials against attack and destruction by phytopathogenic fungi.
- the compound and the composition of the inventionmay be used as antifouling compositions, alone or in combinations with other active ingredients.
- Industrial materials in the present contextare understood to mean inanimate materials which have been prepared for use in industry.
- industrial materials which are to be protected from microbial alteration or destructionmay be adhesives, glues, paper, wallpaper and board/cardboard, textiles, carpets, leather, wood, fibers and tissues, paints and plastic articles, cooling lubricants and other materials which can be infected with or destroyed by microorganisms.
- Parts of production plants and buildingsfor example cooling-water circuits, cooling and heating systems and ventilation and air-conditioning units, which may be impaired by the proliferation of microorganisms may also be mentioned within the scope of the materials to be BCS223033 FC -92- protected.
- Industrial materials within the scope of the present inventionpreferably include adhesives, sizes, paper and card, leather, wood, paints, cooling lubricants and heat transfer fluids, more preferably wood.
- the compound and the composition of the inventionmay prevent adverse effects, such as rotting, decay, discoloration, decoloration or formation of mould.
- the compound and the composition of the inventionmay also be used against fungal diseases liable to grow on or inside timber.
- Timbermeans all types of species of wood, and all types of working of this wood intended for construction, for example solid wood, high-density wood, laminated wood, and plywood.
- the compound and the composition of the inventionmay be used to protect objects which come into contact with saltwater or brackish water, especially hulls, screens, nets, buildings, moorings and signalling systems, from fouling.
- the compound and the composition of the inventionmay also be employed for protecting storage goods.
- Storage goodsare understood to mean natural substances of vegetable or animal origin or processed products thereof which are of natural origin, and for which long-term protection is desired.
- Storage goods of vegetable originfor example plants or plant parts, such as stems, leaves, tubers, seeds, fruits, grains, may be protected freshly harvested or after processing by (pre)drying, moistening, comminuting, grinding, pressing or roasting.
- Storage goodsalso include timber, both unprocessed, such as construction timber, electricity poles and barriers, or in the form of finished products, such as furniture.
- Storage goods of animal originare, for example, hides, leather, furs and hairs.
- the compound and the composition of the inventionmay prevent adverse effects, such as rotting, decay, discoloration, decoloration or formation of mould.
- Microorganisms capable of degrading or altering industrial materialsinclude, for example, bacteria, fungi, yeasts, algae and slime organisms.
- the compound and the composition of the inventionpreferably act against fungi, especially moulds, wood-discoloring and wood-destroying fungi (Ascomycetes, Basidiomycetes, Deuteromycetes and Zygomycetes), and against slime organisms and algae.
- microorganisms of the following generaAlternaria, such as Alternaria tenuis; Aspergillus, such as Aspergillus niger; Chaetomium, such as Chaetomium globosum; Coniophora, such as Coniophora puetana; Lentinus, such as Lentinus tigrinus; Penicillium, such as Penicillium glaucum; Polyporus, such as Polyporus versicolor; Aureobasidium, such as Aureobasidium pullulans; Sclerophoma, such as Sclerophoma pityophila; Trichoderma, such as Trichoderma viride; Ophiostoma spp., Ceratocystis spp., Humicola spp., Petriella spp., Trichurus spp., Coriolus spp., Gloeophyllum spp., Pleurotus spp., Poria
- the compound and the composition of the inventionmay also be used to protect seeds from unwanted microorganisms, such as phytopathogenic microorganisms, for instance phytopathogenic fungi or phytopathogenic oomycetes.
- seed(s) as used hereininclude dormant seeds, primed seeds, pregerminated seeds and seeds with emerged roots and leaves.
- the present inventionalso relates to a method for protecting seeds from unwanted microorganisms which comprises the step of treating the seeds with the compound or the composition of the invention.
- the treatment of seeds with the compound or the composition of the inventionprotects the seeds from phytopathogenic microorganisms, but also protects the germinating seeds, the emerging seedlings and the plants after emergence from the treated seeds. Therefore, the present invention also relates to a method for protecting seeds, germinating seeds and emerging seedlings.
- the seeds treatmentmay be performed prior to sowing, at the time of sowing or shortly thereafter. When the seeds treatment is performed prior to sowing (e.g.
- the seeds treatmentmay be performed as follows: the seeds may be placed into a mixer with a desired amount of the compound or the composition of the invention, the seeds and the compound or the composition of the invention are mixed until an homogeneous distribution on seeds is achieved. If appropriate, the seeds may then be dried.
- the inventionalso relates to seeds coated with the compound or the composition of the invention.
- the seedsare treated in a state in which it is sufficiently stable for no damage to occur in the course of treatment.
- seedscan be treated at any time between harvest and shortly after sowing. It is customary to use seeds which have been separated from the plant and freed from cobs, shells, stalks, coats, hairs or the flesh of the fruits.
- seeds which have been harvested, cleaned and dried down to a moisture content of less than 15% by weightare possible.
- seeds which, after drying, for example, have been treated with water and then dried again, or seeds just after priming, or seeds stored in primed conditions or pre-germinated seeds, or seeds sown on nursery trays, tapes or paperare typically used.
- the amount of the compound or the composition of the invention applied to the seedsis typically such that the germination of the seed is not impaired, or that the resulting plant is not damaged. This must be ensured particularly in case the compound of the invention would exhibit phytotoxic effects at certain application rates.
- the intrinsic phenotypes of transgenic plantsshould also be taken into consideration when determining the amount of the compound of the invention to be applied to the seed in order to BCS223033 FC -94- achieve optimum seed and germinating plant protection with a minimum amount of compound being employed.
- the compound of the inventioncan be applied as such, directly to the seeds, i.e. without the use of any other components and without having been diluted.
- the composition of the inventioncan be applied to the seeds.
- the compound and the composition of the inventionare suitable for protecting seeds of any plant variety.
- Preferred seedsare that of cereals (such as wheat, barley, rye, millet, triticale, and oats), oilseed rape, maize, cotton, soybean, rice, potatoes, sunflower, beans, coffee, peas, beet (e.g. sugar beet and fodder beet), peanut, vegetables (such as tomato, cucumber, onions and lettuce), lawns and ornamental plants. More preferred are seeds of wheat, soybean, oilseed rape, maize and rice.
- the compound and the composition of the inventionmay be used for treating transgenic seeds, in particular seeds of plants capable of expressing a polypeptide or protein which acts against pests, herbicidal damage or abiotic stress, thereby increasing the protective effect.
- Seeds of plants capable of expressing a polypeptide or protein which acts against pests, herbicidal damage or abiotic stressmay contain at least one heterologous gene which allows the expression of said polypeptide or protein.
- These heterologous genes in transgenic seedsmay originate, for example, from microorganisms of the species Bacillus, Rhizobium, Pseudomonas, Serratia, Trichoderma, Clavibacter, Glomus or Gliocladium.
- These heterologous genespreferably originate from Bacillus sp., in which case the gene product is effective against the European corn borer and/or the Western corn rootworm.
- the heterologous genesoriginate from Bacillus thuringiensis.
- the compound of the inventioncan be applied as such, or for example in the form of as ready-to-use solutions, emulsions, water- or oil-based suspensions, powders, wettable powders, pastes, soluble powders, dusts, soluble granules, granules for broadcasting, suspoemulsion concentrates, natural products impregnated with the compound of the invention, synthetic substances impregnated with the compound of the invention, fertilizers or microencapsulations in polymeric substances.
- Applicationis accomplished in a customary manner, for example by watering, spraying, atomizing, broadcasting, dusting, foaming or spreading-on.
- the compound of the inventionby the ultra-low volume method, via a drip irrigation system or drench application, to apply it in-furrow or to inject it into the soil stem or trunk. It is further possible to apply the compound of the invention by means of a wound seal, paint or other wound dressing.
- the effective and plant-compatible amount of the compound of the invention which is applied to the plants, plant parts, fruits, seeds or soilwill depend on various factors, such as the compound/composition BCS223033 FC -95- employed, the subject of the treatment (plant, plant part, fruit, seed or soil), the type of treatment (dusting, spraying, seed dressing), the purpose of the treatment (curative and protective), the type of microorganisms, the development stage of the microorganisms, the sensitivity of the microorganisms, the crop growth stage and the environmental conditions.
- the application ratescan vary within a relatively wide range, depending on the kind of application.
- the application ratemay range from 0.1 to 10000 g/ha, preferably from 10 to 1000 g/ha, more preferably from 50 to 300 g/ha (in the case of application by watering or dripping, it is even possible to reduce the application rate, especially when inert substrates such as rockwool or perlite are used).
- the application ratemay range from 0.1 to 200 g per 100 kg of seeds, preferably from 1 to 150 g per 100 kg of seeds, more preferably from 2.5 to 25 g per 100 kg of seeds, even more preferably from 2.5 to 12.5 g per 100 kg of seeds.
- the application ratemay range from 0.1 to 10000 g/ha, preferably from 1 to 5000 g/ha. These application rates are merely examples and are not intended to limit the scope of the present invention.
- the compound and the composition of the inventioncan be used in combination with models e.g. embedded in computer programs for site specific crop management, satellite farming, precision farming or precision agriculture. Such models support the site specific management of agricultural sites with data from various sources such as soils, weather, crops (e.g. type, growth stage, plant health), weeds (e.g. type, growth stage), diseases, pests, nutrients, water, moisture, biomass, satellite data or yield with the purpose to optimize profitability, sustainability and protection of the environment.
- the compound of the inventioncan be applied to a crop plant according to appropriate dose regime if a model models the development of a fungal disease and calculates that a threshold has been reached for which it is recommendable to apply the compound of the invention to the crop plant.
- Commercially available systems which include agronomic modelsare e.g. FieldScripts TM from The climate Corporation, Xarvio TM from BASF and AGLogic TM from John Deere.
- the compound of the inventioncan also be used in combination with smart spraying equipment such as e.g.
- Such an equipmentusually includes input sensors (such as e.g. a camera) and a processing unit configured to analyze the input data and configured to provide a decision based on the analysis of the input data to apply the compound of the invention to the crop plants (respectively the weeds) in a specific and precise manner.
- input sensorssuch as e.g. a camera
- processing unitconfigured to analyze the input data and configured to provide a decision based on the analysis of the input data to apply the compound of the invention to the crop plants (respectively the weeds) in a specific and precise manner.
- the use of BCS223033 FC -96- such smart spraying equipmentusually also requires positions systems (e.g.
- GPS receiversto localize recorded data and to guide or to control farm vehicles; geographic information systems (GIS) to represent the information on intelligible maps, and appropriate farm vehicles to perform the required farm action such as the spraying.
- GISgeographic information systems
- fungal diseasescan be detected from imagery acquired by a camera.
- fungal diseasescan be identified and/or classified based on that imagery.
- image processing algorithmscan utilize machine learning algorithms, such as trained neutral networks, decision trees and utilize artificial intelligence algorithms. In this manner, the compounds described herein can be applied only where needed.
- the UV stability of a compound according to the inventioncan be determined as follows: Measurement of the UV stability reported with the half-life time of the photo degradation is performed by irradiating the samples for 24h with the full UV/VIS Spectrum as available on a SUNTEST XLS+ going from 250 nm to 800 nm, followed by an analysis of the analyte and its possible degradation products via reversed phase liquid chromatography with UV-detection coupled to a single quadrupole mass spectrometer using the following method: [ a] The analyte is determined by measurement of LC-UV-MS , with 0.085% (v/v) formic acid in water and 0.1% (v/v) formic acid acetonitrile as eluent (linear gradient from 5% acetonitrile to 95% acetonitrile).
- the analyteis identified and determined via UV and MS-spectrum.
- the half-life timeis determined over the course of 5 time points at 0h, 2h, 4h, 6h and 24h in triplicates each time point. All time points are normalized on detector responses received at 0h.
- the half-life timeis determined fitting the results to a 1 st order degradation function and is returned with the unit [h].
- BCS223033 FC -97-The following table lists the abbreviations used in the Examples section as far as they are not explained within the text body.
- LogP valueis determined by measurement of LC-UV and/or MS and/or ELS, in a neutral range, with 0.001 molar ammonium acetate solution in water and acetonitrile as eluent (linear gradient from 10% acetonitrile to 95% acetonitrile). If more than one LogP value is available within the same method, all the values are given and separated by “+”. Calibration was done with straight-chain alkan2-ones (with 3 to 16 carbon atoms) with known LogP values (measurement of LogP values using retention times with linear interpolation between successive alkanones).
- Lambda-max-valueswere determined using UV-spectra from 200 nm to 400 nm and the peak values of the chromatographic signals.
- the GCMS retention timesare determined on a DB17ms (15m*0.25 ⁇ m*0.25 ⁇ m) column using a 30°C/min gradient from 40°C to 310°C and 1,5mL/min He gaz flow.
- 1 H-NMR data1 H-NMR data of selected examples as provided herein are written in form of 1 H-NMR-peak lists. To each signal peak are listed the ⁇ -value in ppm (parts per million) and the signal intensity in round brackets. Between the ⁇ -value – signal intensity pairs are semicolons as delimiters.
- the peak list of an examplehas therefore the form: ⁇ 1 (intensity 1 ); ⁇ 2 (intensity 2 );........; ⁇ i (intensity i ); hence; ⁇ n (intensity n )
- Intensity of sharp signalscorrelates with the height of the signals in a printed example of a NMR spectrum in cm and shows the real relations of signal intensities. From broad signals several peaks or the middle of BCS223033 FC -99- the signal and their relative intensity in comparison to the most intensive signal in the spectrum can be shown.
- tetramethylsilane and/or the chemical shift of the solventis used, especially in the case of spectra measured in DMSO (dimethyl sulfoxide). Therefore in NMR peak lists, tetramethylsilane peak can occur but not necessarily.
- the 1 H-NMR peak listsare similar to classical 1 H-NMR prints and contains therefore usually all peaks, which are listed at classical NMR-interpretation. Additionally they can show like classical 1 H-NMR prints signals of solvents, stereoisomers of the target compounds, which are also object of the invention, and/or peaks of impurities.
- peaks of solventsfor example peaks of DMSO in DMSO-D 6 and the peak of water are shown in our 1 H-NMR peak lists and have usually on average a high intensity.
- the peaks of stereoisomers of the target compounds and/or peaks of impuritieshave usually on average a lower intensity than the peaks of target compounds (for example with a purity >90%).
- Such stereoisomers and/or impuritiescan be typical for the specific preparation process. Therefore their peaks can help to recognize the reproduction of our preparation process via “side-products-fingerprints”.
- Table 1illustrates in a non-limiting manner examples of compounds of formula (I) according to the invention :
- the compounds of formula (I) which are mentioned in table 1 herein belowwere prepared in accordance with the procedures detailed herein below in connection with specific examples and with the general description of the processes herein disclosed.
- Table 1Compounds according to formula (I) BCS223033 FC -101- Remark: ** linked to oxadiazole
- Table 2illustrates in a non-limiting manner examples of intermediates of formula (V) according to the invention : BCS223033 FC -102- The intermediates of formula (V) which are mentioned in table 2 herein below were prepared in accordance with the procedures detailed herein below in connection with specific examples and with the general description of the processes herein disclosed.
- Table 2Compounds according to formula (V) Remark: ** linked to oxadiazole
- Table 3illustrates in a non-limiting manner examples of intermediates of formula (IX) according to the invention :
- the intermediates of formula (IX) which are mentioned in table 3 herein belowwere prepared in accordance with the procedures detailed herein below in connection with specific examples and with the general description of the processes herein disclosed.
- BCS223033 FC -103- Table 3Compounds according to formula (IX) Remark: ** linked to oxadiazole
- Tables 4illustrates examples of compounds of formula (X) according to the invention : The compounds of formula (X) which are mentioned in table 4 herein below were prepared in accordance with the procedures detailed herein below in connection with specific examples and with the general description of the processes herein disclosed.
- BCS223033 FC -104- Table 4Compounds according to formula (X) Remark: ** linked to oxadiazole
- the NMR data of the compounds of formula (I) disclosed in table 1 and of the intermediates disclosed in tables 2 to 4are listed in table 5 below.
- N-[(5- ⁇ 5-[chloro(difluoro)methyl]-1,2,4-oxadiazol-3-yl ⁇ -2-thienyl)methyl]-O- methylhydroxylamine300 mg, 0.9 mmol
- 1,1'-carbonyldiimidazole732.2 mg, 4.51 mmol, 5.0 equiv
- dichloromethane (12 mL) under argonwas added N,N-diisopropylethylamine (0.94 mL, 5.41 mmol, 6.0 equiv) at room temperature.
- tert-butyl [(tert-butoxycarbonyl)oxy][(5- ⁇ 5-[chloro(difluoro)methyl]-1,2,4-oxadiazol-3- yl ⁇ pyridin-2-yl)methyl]carbamate(1.30 g, 2.72 mmol) in methanol (10 mL) was added a 2.0 M solution of ammonia in methanol (1.64 mL, 3.27 mmol, 1.2 equiv) at room temperature.
- BIOLOGICAL DATAExample: in vivo preventive test on Puccinia recondita (brown rust on wheat) Solvent: 5% by volume of Dimethyl sulfoxide 10% by volume of Acetone Emulsifier: 1 ⁇ l of Tween ® 80 per mg of active ingredient
- the active ingredientswere made soluble and homogenized in a mixture of Dimethyl sulfoxide/Acetone/ /Tween ® 80 and then diluted in water to the desired concentration.
- the young plants of wheatwere treated by spraying the active ingredient prepared as described above. Control plants were treated only with an aqueous solution of Acetone/Dimethyl sulfoxide/ Tween ® 80.
- the plantswere contaminated by spraying the leaves with an aqueous suspension of Puccinia recondita spores.
- the contaminated wheat plantswere incubated for 24 hours at 20°C and at 100% relative humidity and then for 9 days at 20°C and at 70-80% relative humidity.
- BCS223033 FC -115-The test was evaluated 10 days after the inoculation. 0% means an efficacy which corresponds to that of the control plants while an efficacy of 100% means that no disease was observed.
- the young plants of soybeanwere treated by spraying the active ingredient prepared as described above.
- Control plantswere treated only with an aqueous solution of Acetone/Dimethyl sulfoxide/ Tween® 80.
- the plantswere contaminated by spraying the leaves with an aqueous suspension of Phakopsora pachyrhizi spores.
- the contaminated soybean plantswere incubated for 24 hours at 24°C and at 100% relative humidity and then for 10 days at 24°C and at 70-80% relative humidity. The test was evaluated 11 days after the inoculation. 0% means an efficacy which corresponds to that of the control plants while an efficacy of 100% means that no disease was observed.
- the final concentration of DMSO used in the assaywas ⁇ 1%.
- a spore suspension of C. lindemuthianumwas prepared and diluted to the desired spore density.
- Fungicideswere evaluated for their ability to inhibit spores germination and mycelium growth in liquid culture assay.
- the compoundswere added in the desired concentration to the culture medium with spores. After 6 days incubation, fungi-toxicity of compounds was determined by spectrometric measurement of mycelium growth. Inhibition of fungal growth was determined by comparing the absorbance values in wells containing the fungicides with the absorbance in control wells without fungicides.
- 0%means an efficacy which corresponds to that of the untreated control, while an efficacy of 100% means that no disease is observed.
- Emulsifier1 part by weight of polyoxyethylene sorbitan monooleate To produce a suitable preparation of active compound, 1 part by weight of active compound was mixed with the stated amounts of solvent and emulsifier, and the concentrate was diluted with water to the desired concentration.
- Solvent24.5 parts by weight of acetone 24.5 parts by weight of dimethyl sulfoxide
- Emulsifier1 part by weight of polyoxyethylene sorbitan monooleate
- To produce a suitable preparation of active compound1 part by weight of active compound was mixed with the stated amounts of solvent and emulsifier, and the concentrate was diluted with water to the desired concentration.
- To test for long-lasting activityyoung plants were sprayed with the preparation of active compound at the stated rate of application.
- the plantswere placed in an incubation BCS223033 FC -118- cabinet at approximately 24°C and a relative atmospheric humidity of approximately 80 % and a day / night interval of 12h.
- 8 days after the applicationthe plant were inoculated with an aqueous spore suspension of the causal agent of soybean rust (Phakopsora pachyrhizi) and stay for 24h without light in the incubation cabinet at approximately 24°C and a relative atmospheric humidity of 95 %.
- the plantsremained in the incubation cabinet at approximately 24°C and a relative atmospheric humidity of approximately 80 % and a day / night interval of 12h.
- the testwas evaluated 7 days after the inoculation.
- Example: Inhibition of HDAC4/HDAC6 in mammalsThe inhibiting properties of the compounds of the invention towards human HDAC4 and/or HDAC6 were evaluated according to the assay described below: In vitro HDAC inhibition was measured by using the two-step fluorogenic HDAC assay according to Wegener et al. (Wegener D. et al., Analytical Biochemistry 321 (2003): 202-208) and performed in white 384 well plate (Greiner, flat bottom well lumitrac200 for HDAC4 and ProxiPlate-384 Plus ref 6008289 (PERKIN ELMER SAS) for HDAC6).
- the fluorogenic substrates used respectively for human HDAC4 (Active motif, ref.31527) and human HDAC6 (Sigma / SRP0108)are Boc-Lys (Tfa)-AMC (CAS: 97885- 44-4) and Boc-Lys (Ac)-AMC (Enzo Life Sciences /ALX-260-137-M005).
- Enzymeis diluted in HDAC buffer (15 mM Tris pH 8.1; 0.25 mM EDTA; 50 mM NaCl; BSA 1 mg/mL; 0.1% PEG 6000) at the final concentrations of 0.05 ng/ ⁇ L for HDAC4 and 1 ng/ ⁇ L for HDAC6.
- Tested compounds (2 ⁇ l)were diluted in duplicate, at a final concentration range from 100 to 0.02 ⁇ M in final 1% DMSO.
- the enzymatic reactionswere started with the addition of the substrate at a final concentration of 60 ⁇ M and 10 ⁇ M respectively for HDAC4 and HDAC6 substrates.
- a first fluorescence reading(Ex 340 nm/ Em 460 nm for HDAC4 and Ex 390/ Em 460 nm for HDAC6) to obtain blank value is done with Tecan Infinite 1000 microplate reader.
- TSAHDAC inhibitor Sigma /T8552
- trypsinSigma /T0303
- T30 fluorescenceis measured (same conditions as described above). Fluorescence is proportional to the amount of deacetylated substrate, and therefore representative of the human HDAC activity. The blank is subtracted to T30 value well by well for the entire plate, to consider the risk of auto-fluorescence interferences.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental Sciences (AREA)
- Wood Science & Technology (AREA)
- Plant Pathology (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Pest Control & Pesticides (AREA)
- General Health & Medical Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
Claims
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP22198333 | 2022-09-28 | ||
| EP22198333.1 | 2022-09-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024068518A1true WO2024068518A1 (en) | 2024-04-04 |
Family
ID=83506708
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2023/076346CeasedWO2024068518A1 (en) | 2022-09-28 | 2023-09-25 | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024068518A1 (en) |
Citations (203)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2010A (en) | 1841-03-18 | Machine foe | ||
| US2009A (en) | 1841-03-18 | Improvement in machines for boring war-rockets | ||
| US24077A (en) | 1859-05-17 | Window-sash supporter | ||
| US137395A (en) | 1873-04-01 | Improvement in nuts | ||
| DE2906507A1 (en) | 1979-02-20 | 1980-08-28 | Bayer Ag | Plant growth regulating compsn. - contg. alpha amino cyclo:alkanoic acid derivs., e.g. for dwarfing cereals |
| EP0030287A1 (en) | 1979-11-29 | 1981-06-17 | Bayer Ag | 1-Aminocyclopropanecarboxylic acid derivatives, methods for their production, their use as plant-growth regulators and compositions containing such derivatives |
| EP0086750A2 (en) | 1982-02-17 | 1983-08-24 | Ciba-Geigy Ag | Use of quinoline derivatives in the protection of crop plants |
| EP0094349A2 (en) | 1982-05-07 | 1983-11-16 | Ciba-Geigy Ag | Use of quinoline derivatives for the protection of cultivated plants |
| DE3335514A1 (en) | 1983-09-30 | 1985-04-18 | Bayer Ag, 5090 Leverkusen | 1-METHYLAMINO-CYCLOPROPAN-1-CARBONIC ACID DERIVATIVES |
| JPS6087254A (en) | 1983-10-19 | 1985-05-16 | Japan Carlit Co Ltd:The | Novel urea compound and herbicide containing the same |
| US4536207A (en) | 1983-07-26 | 1985-08-20 | Igi Biotechnology, Inc. | Nematocidally active chitin-protein complex |
| EP0174562A2 (en) | 1984-09-11 | 1986-03-19 | Hoechst Aktiengesellschaft | Plant protecting agents based on 1,2,4 - triazole derivatives as well as 1,2,4-triazole derivatives |
| EP0191736A2 (en) | 1985-02-14 | 1986-08-20 | Ciba-Geigy Ag | Use of quinoline derivatives for the protection of crop plants |
| EP0268554A2 (en) | 1986-10-22 | 1988-05-25 | Ciba-Geigy Ag | 1,5-Diphenyl pyrazole-3-carbonic-acid derivatives for the protection of cultured plants |
| EP0269806A1 (en) | 1986-10-04 | 1988-06-08 | Hoechst Aktiengesellschaft | Phenylpyrazole carbonic acid derivatives, their preparation and use as plant growth regulators and antidotes |
| EP0346620A1 (en) | 1988-05-20 | 1989-12-20 | Hoechst Aktiengesellschaft | Plant protection agents containing 1,2,4-triazole derivatives, and the 1,2,4-triazole derivatives |
| WO1990000020A1 (en) | 1988-07-01 | 1990-01-11 | The Bed Board Company Ltd | Appliances for use with beds |
| EP0365484A1 (en) | 1988-10-20 | 1990-04-25 | Ciba-Geigy Ag | Sulfamoyl phenyl ureas |
| WO1991007874A1 (en) | 1989-11-30 | 1991-06-13 | Hoechst Aktiengesellschaft | Pyrazolines for the protection of crops against herbicides |
| WO1991008202A1 (en) | 1989-11-25 | 1991-06-13 | Hoechst Aktiengesellschaft | Isoxazolines, method of preparation thereof, and their use as plant-protection agents |
| US5061495A (en) | 1988-03-07 | 1991-10-29 | Agricultural Genetics Company Limited | Antibiotic derived from b. subtilis |
| US5123951A (en) | 1986-03-31 | 1992-06-23 | Rhone-Poulenc Nederland B.V. | Synergistic plant growth regulator compositions |
| EP0492366A2 (en) | 1990-12-21 | 1992-07-01 | Hoechst Schering AgrEvo GmbH | New 5-chloroquinolin-8-oxyalkanecarbonic acid derivatives, process for their preparation and their use as antidotes for herbicides |
| EP0582198A2 (en) | 1992-08-01 | 1994-02-09 | Hoechst Schering AgrEvo GmbH | Substituted (hetero-)aryle compounds, process for their preparation, those containing compositions and their use as safeners |
| WO1995007897A1 (en) | 1993-09-16 | 1995-03-23 | Hoechst Schering Agrevo Gmbh | Substituted isoxazolines, process for producing them, agents containing them and their use as safeners |
| US5549718A (en) | 1990-04-06 | 1996-08-27 | Centre National De La Recherche Scientifique (C.N.R.S.) | Substance with lipo-oligosaccharide structure capable of acting as plant-specific symbiotic signals, processes for producing them and their applications |
| WO1997017432A1 (en) | 1995-11-06 | 1997-05-15 | Wisconsin Alumni Research Foundation | Insecticidal protein toxins from photorhabdus |
| WO1997045016A1 (en) | 1996-05-29 | 1997-12-04 | Hoechst Schering Agrevo Gmbh | Novel n-acyl sulphonamides, novel mixtures of herbicides and antidotes and their use |
| US5702752A (en) | 1996-03-13 | 1997-12-30 | Archer Daniels Midland Company | Production of isoflavone enriched fractions from soy protein extracts |
| WO1998008932A1 (en) | 1996-08-29 | 1998-03-05 | Dow Agrosciences Llc | Insecticidal protein toxins from $i(photorhabdus) |
| WO1998013361A1 (en) | 1996-09-26 | 1998-04-02 | Novartis Ag | Herbicidal composition |
| WO1998027049A1 (en) | 1996-12-19 | 1998-06-25 | Hoechst Schering Agrevo Gmbh | Novel 2-fluoroacrylic acid derivatives, novel mixtures of herbicides and antidotes and the use thereof |
| WO1998038856A1 (en) | 1997-03-04 | 1998-09-11 | Zeneca Limited | Compositions for safening rice against acetochlor |
| WO1998044140A1 (en) | 1997-04-03 | 1998-10-08 | Dekalb Genetics Corporation | Glyphosate resistant maize lines |
| WO1998050427A1 (en) | 1997-05-05 | 1998-11-12 | Dow Agrosciences Llc | INSECTICIDAL PROTEIN TOXINS FROM $i(XENORHABDUS) |
| WO1999016744A1 (en) | 1997-09-29 | 1999-04-08 | Aventis Cropscience Gmbh | Acylsulfamoyl benzoic acid amides, plant protection agents containing said acylsulfamoyl benzoic acid amides, and method for producing the same |
| JPH11253151A (en) | 1997-11-13 | 1999-09-21 | Kumiai Chem Ind Co Ltd | Rice seedling raising disease control agent |
| US5965545A (en) | 1996-10-15 | 1999-10-12 | State Of Israel, Ministry Of Agriculture, Agricultural Research Organization, The Volcani Center | Compositions and method for controlling fungal disease in plants |
| US5990291A (en) | 1996-06-11 | 1999-11-23 | Protein Technologies International, Inc. | Recovery of isoflavones from soy molasses |
| US6060051A (en) | 1997-05-09 | 2000-05-09 | Agraquest, Inc. | Strain of bacillus for controlling plant diseases and corn rootworm |
| WO2000026356A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N. V. | Glufosinate tolerant rice |
| WO2000026345A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N.V. | Glufosinate tolerant rice |
| US6146668A (en) | 1997-04-28 | 2000-11-14 | Novogen, Inc. | Preparation of isoflavones from legumes |
| WO2001031042A2 (en) | 1999-10-29 | 2001-05-03 | Aventis Cropscience N.V. | Male-sterile brassica plants and methods for producing same |
| US6245551B1 (en) | 1999-03-30 | 2001-06-12 | Agraquest, Inc. | Strain of Bacillus pumilus for controlling plant diseases caused by fungi |
| WO2001041558A1 (en) | 1999-12-08 | 2001-06-14 | Aventis Cropscience N.V. | Hybrid winter oilseed rape and methods for producing same |
| WO2001047952A2 (en) | 1999-12-28 | 2001-07-05 | Bayer Cropscience N.V. | Insecticidal proteins from bacillus thuringiensis |
| WO2001051654A2 (en) | 2000-01-11 | 2001-07-19 | Bayer Cropscience N.V. | Methods and kits for identifying elite event gat-zm1 in biological samples |
| WO2002027004A2 (en) | 2000-09-29 | 2002-04-04 | Monsanto Technology Llc | Glyphosate tolerant wheat plant 33391 and compositions and methods for detection thereof |
| WO2002034048A1 (en) | 2000-10-23 | 2002-05-02 | Syngenta Participations Ag | Agrochemical compositions with quinoline safeners |
| WO2002034946A2 (en) | 2000-10-25 | 2002-05-02 | Monsanto Technology Llc | Cotton event pv-ghgt07(1445) and compositions and methods for detection thereof |
| WO2002036831A2 (en) | 2000-10-30 | 2002-05-10 | Monsanto Technology Llc | Canola event pv-bngt04(rt73) and compositions and methods for detection thereof |
| WO2002040677A2 (en) | 2000-11-20 | 2002-05-23 | Monsanto Technology Llc | Cotton event pv-ghbk04 (531) and compositions and methods for detection thereof |
| WO2002044407A2 (en) | 2000-11-30 | 2002-06-06 | Ses Europe N.V. | Glyphosate resistant transgenic sugar beet characterised by a specific transgene insertion (t227-1), methods and primers for the detection of said insertion |
| US20020102582A1 (en) | 2000-09-13 | 2002-08-01 | Levine Elaine B. | Corn event MON810 and compositions and methods for detection thereof |
| WO2002100163A2 (en) | 2001-06-11 | 2002-12-19 | Monsanto Technology Llc | Cotton event moni5985 and compositions and methods for detection |
| WO2003000051A2 (en) | 2001-06-22 | 2003-01-03 | Drahos David J | Novel biofungicide |
| WO2003013224A2 (en) | 2001-08-06 | 2003-02-20 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for producing and identifying same |
| WO2003052073A2 (en) | 2001-12-17 | 2003-06-26 | Syngenta Participations Ag | Novel corn event |
| US20030126634A1 (en) | 1990-08-09 | 2003-07-03 | Dekalb Genetics Corporation | Methods and compositions for the increase of yield in plants |
| WO2003064572A1 (en) | 2002-01-31 | 2003-08-07 | Exxonmobil Research And Engineering Company | Lubricating oil compositions with improved friction properties |
| WO2004011601A2 (en) | 2002-07-29 | 2004-02-05 | Monsanto Technology, Llc | Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof |
| WO2004039986A1 (en) | 2002-10-29 | 2004-05-13 | Syngenta Participations Ag | Cot102 insecticidal cotton |
| WO2004053062A2 (en) | 2002-12-05 | 2004-06-24 | Monsanto Technology Llc | Bentgrass event asr-368 and compositions and methods for detection thereof |
| WO2004072235A2 (en) | 2003-02-12 | 2004-08-26 | Monsanto Technology Llc | Cotton event mon 88913 and compositions and methods for detection thereof |
| US20040172669A1 (en) | 2003-02-28 | 2004-09-02 | Josef Kraus | Glyphosate tolerant sugar beet |
| WO2004074492A1 (en) | 2003-02-20 | 2004-09-02 | Kws Saat Ag | Glyphosate tolerant sugar beet |
| WO2004084631A1 (en) | 2003-03-26 | 2004-10-07 | Bayer Cropscience Gmbh | Use of aromatic hydroxy compounds as safeners |
| WO2004099447A2 (en) | 2003-05-02 | 2004-11-18 | Dow Agrosciences Llc | Corn event tc1507 and methods for detection thereof |
| US6855533B2 (en) | 1995-04-20 | 2005-02-15 | Basf Corporation | Structure-based designed herbicide resistant products |
| WO2005015994A1 (en) | 2003-08-05 | 2005-02-24 | Bayer Cropscience Gmbh | Use of aromatic compounds as safeners |
| WO2005016001A1 (en) | 2003-08-05 | 2005-02-24 | Bayer Cropscience Gmbh | Safener based on aromatic-aliphatic carboxylic acid derivatives |
| WO2005054480A2 (en) | 2003-12-01 | 2005-06-16 | Syngenta Participations Ag | Insect resistant cotton plants and methods of detecting the same |
| WO2005054479A1 (en) | 2003-12-01 | 2005-06-16 | Syngenta Participations Ag | Insect resistant cotton plants and methods of detecting the same |
| WO2005059103A2 (en) | 2003-12-15 | 2005-06-30 | Monsanto Technology Llc | Corn plant mon88017 and compositions and methods for detection thereof |
| WO2005061720A2 (en) | 2003-12-11 | 2005-07-07 | Monsanto Technology Llc | High lysine maize compositions and methods for detection thereof |
| WO2005074671A1 (en) | 2004-01-30 | 2005-08-18 | Syngenta Participations Ag | Improved fertility restoration for ogura cytoplasmic male sterile brassica and method |
| US20050216969A1 (en) | 2004-03-26 | 2005-09-29 | Dow Agrosciences Llc | Cry1F and Cry1AC transgenic cotton lines and event-specific identification thereof |
| WO2005103301A2 (en) | 2004-03-25 | 2005-11-03 | Syngenta Participations Ag | Corn event mir604 |
| WO2005112630A1 (en) | 2004-05-12 | 2005-12-01 | Bayer Cropscience Gmbh | Quinoxalin-2-one derivatives crop protection agents comprising the same and method for production and use therof |
| WO2006003494A2 (en) | 2004-06-28 | 2006-01-12 | Syngenta Participations Ag | Piperidine derivatives and their use as insecticides, acaricides, molluscicides or nematicides |
| US20060070139A1 (en) | 2004-09-29 | 2006-03-30 | Pioneer Hi-Bred International, Inc. | Corn event DAS-59122-7 and methods for detection thereof |
| WO2006043635A1 (en) | 2004-10-20 | 2006-04-27 | Kumiai Chemical Industry Co., Ltd. | 3-triazolylphenyl sulfide derivative and insecticide/acaricide/nematicide containing the same as active ingredient |
| US7094592B2 (en) | 2001-11-26 | 2006-08-22 | Kumiai Chemical Industry Co., Ltd. | Bacillus sp. D747 strain, plant disease controlling agents and insect pest controlling agents using the same and control method using the agents |
| WO2006098952A2 (en) | 2005-03-16 | 2006-09-21 | Syngenta Participations Ag | Corn event 3272 and methods of detection thereof |
| WO2006108674A2 (en) | 2005-04-08 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a2704-12 and methods and kits for identifying such event in biological samples |
| WO2006108675A2 (en) | 2005-04-11 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a5547-127 and methods and kits for identifying such event in biological samples |
| WO2006128572A1 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce46-02a insecticidal cotton |
| WO2006128573A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce43- 67b, insecticidal transgenic cotton expressing cry1ab |
| WO2006130436A2 (en) | 2005-05-27 | 2006-12-07 | Monsanto Technology Llc | Soybean event mon89788 and methods for detection thereof |
| WO2006128568A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | T342-142, insecticidal transgenic cotton expressing cry1ab |
| WO2006128571A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce44-69d , insecticidal transgenic cotton expressing cry1ab |
| WO2006128569A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | 1143-14a, insecticidal transgenic cotton expressing cry1ab |
| WO2006128570A1 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | 1143-51b insecticidal cotton |
| WO2007017186A1 (en) | 2005-08-08 | 2007-02-15 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for identifying same |
| WO2007023764A1 (en) | 2005-08-26 | 2007-03-01 | Kumiai Chemical Industry Co., Ltd. | Agent for reduction of harmful effect of herbicide and herbicide composition having reduced harmful effect |
| WO2007024782A2 (en) | 2005-08-24 | 2007-03-01 | Pioneer Hi-Bred International, Inc. | Compositions providing tolerance to multiple herbicides and methods of use thereof |
| WO2007023719A1 (en) | 2005-08-22 | 2007-03-01 | Kumiai Chemical Industry Co., Ltd. | Agent for reducing chemical injury and herbicide composition with reduced chemical injury |
| WO2007040280A1 (en) | 2005-10-06 | 2007-04-12 | Nippon Soda Co., Ltd. | Cyclic amine compound and pest control agent |
| WO2007091277A2 (en) | 2006-02-10 | 2007-08-16 | Maharashtra Hybrid Seeds Company Limited (Mahyco) | TRANSGENIC BRINJAL (SOLANUM MELONGENA) EXPRESSING THE CRYlAC GENE |
| WO2007140256A1 (en) | 2006-05-26 | 2007-12-06 | Monsanto Technology, Llc | Corn plant and seed corresponding to transgenic event mon89034 and methods for detection and use thereof |
| WO2007142840A2 (en) | 2006-06-03 | 2007-12-13 | Syngenta Participations Ag | Corn event mir162 |
| US20070292854A1 (en) | 2000-06-22 | 2007-12-20 | Behr Carl F | Corn event PV-ZMGT32(nk603) and compositions and methods for detection thereof |
| WO2008002872A2 (en) | 2006-06-28 | 2008-01-03 | Pioneer Hi-Bred International, Inc. | Soybean event 3560.4.3.5 and compositions and methods for the identification and/or detection thereof |
| US20080064032A1 (en) | 2006-09-13 | 2008-03-13 | Syngenta Participations Ag | Polynucleotides and uses thereof |
| WO2008054747A2 (en) | 2006-10-31 | 2008-05-08 | E. I. Du Pont De Nemours And Company | Soybean event dp-305423-1 and compositions and methods for the identification and/or detection thereof |
| WO2008114282A2 (en) | 2007-03-19 | 2008-09-25 | Maharashtra Hybrid Seeds Company Limited | Transgenic rice (oryza sativa) comprising pe-7 event and method of detection thereof |
| WO2008122406A1 (en) | 2007-04-05 | 2008-10-16 | Bayer Bioscience N.V. | Insect resistant cotton plants and methods for identifying same |
| WO2008131860A2 (en) | 2007-04-30 | 2008-11-06 | Bayer Cropscience Ag | Pyridonecarboxamides crop protection agents containing the same method for production and use thereof |
| WO2008131861A1 (en) | 2007-04-30 | 2008-11-06 | Bayer Cropscience Ag | Use of pyridine-2-oxy-3-carbonamides as safeners |
| US20080289060A1 (en) | 2006-08-24 | 2008-11-20 | Bayer Bioscience N.V. | Herbicide tolerant rice plants and methods for identifying same |
| WO2008151780A1 (en) | 2007-06-11 | 2008-12-18 | Bayer Bioscience N.V. | Insect resistant cotton plants comprising elite event ee-gh6 and methods for identifying same |
| CN101337940A (en) | 2008-08-12 | 2009-01-07 | 国家农药创制工程技术研究中心 | Nitrogen heterocyclic ring dichloro allyl ether compound with insecticidal activity |
| CN101337937A (en) | 2008-08-12 | 2009-01-07 | 国家农药创制工程技术研究中心 | N-benz-3-substituted amino pyrazoles compounds with insecticidal activity |
| US20090130071A1 (en) | 2007-11-15 | 2009-05-21 | Ai-Guo Gao | Soybean Plant And Seed Corresponding To Transgenic Event MON87701 And Methods For Detection Thereof |
| WO2009100188A2 (en) | 2008-02-08 | 2009-08-13 | Dow Agrosciences Llc | Methods for detection of corn event das-59132 |
| WO2009103049A2 (en) | 2008-02-14 | 2009-08-20 | Pioneer Hi-Bred International, Inc. | Plant genomic dna flanking spt event and methods for identifying spt event |
| WO2009102873A1 (en) | 2008-02-15 | 2009-08-20 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87769 and methods for detection thereof |
| US7579183B1 (en) | 2006-12-01 | 2009-08-25 | The United States Of America As Represented By The Secretary Of Agriculture | Saprophytic yeast, Pichia anomala |
| WO2009111263A1 (en) | 2008-02-29 | 2009-09-11 | Monsanto Technology Llc | Corn plant event mon87460 and compositions and methods for detection thereof |
| WO2009116106A1 (en) | 2008-03-21 | 2009-09-24 | Trentino Sviluppo S.P.A. | Trichoderma atroviride sc1 for biocontrol of fungal diseases in plants |
| WO2009152359A2 (en) | 2008-06-11 | 2009-12-17 | Dow Agrosciences Llc | Constructs for expressing herbicide tolerance genes, related plants, and related trait combinations |
| JP2010018586A (en) | 2008-07-14 | 2010-01-28 | Meiji Seika Kaisha Ltd | Substance pf1364, its manufacturing method, producing strain and agricultural/horticultural insecticide having the substance as active ingredient |
| WO2010024976A1 (en) | 2008-08-29 | 2010-03-04 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87754 and methods for detection thereof |
| US20100080887A1 (en) | 2008-09-29 | 2010-04-01 | Monsanto Technology Llc | Soybean Transgenic Event MON87705 and Methods for Detection Thereof |
| WO2010051926A2 (en) | 2008-11-05 | 2010-05-14 | Bayer Cropscience Aktiengesellschaft | New halogen-substituted bonds |
| WO2010052161A2 (en) | 2008-11-06 | 2010-05-14 | Syngenta Participations Ag | Herbicidal compositions |
| CN101715774A (en) | 2008-10-09 | 2010-06-02 | 浙江化工科技集团有限公司 | Preparation and use of compound having insecticidal activity |
| WO2010066780A1 (en) | 2008-12-12 | 2010-06-17 | Syngenta Participations Ag | Spiroheterocyclic n-oxypiperidines as pesticides |
| WO2010076212A1 (en) | 2008-12-19 | 2010-07-08 | Syngenta Participations Ag | Transgenic sugar beet event gm rz13 |
| WO2010077816A1 (en) | 2008-12-16 | 2010-07-08 | Syngenta Participations Ag | Corn event 5307 |
| WO2010080829A1 (en) | 2009-01-07 | 2010-07-15 | Basf Agrochemical Products B.V. | Soybean event 127 and methods related thereto |
| WO2010086790A1 (en) | 2009-01-27 | 2010-08-05 | Lesaffre Et Compagnie | Saccharomyces cerevisiae strains with phytosanitary capabilities |
| CN101838227A (en) | 2010-04-30 | 2010-09-22 | 孙德群 | Safener of benzamide herbicide |
| WO2010117737A1 (en) | 2009-03-30 | 2010-10-14 | Monsanto Technology Llc | Rice transgenic event17053 and methods of use thereof |
| WO2010117735A1 (en) | 2009-03-30 | 2010-10-14 | Monsanto Technology Llc | Transgenic rice event17314 and methods of use thereof |
| EP2248421A1 (en) | 2009-05-07 | 2010-11-10 | GMI - Gregor-Mendel-Institut für Molekulare Pflanzenbiologie GmbH | Accumulation of biomass in plants |
| US20100291039A1 (en) | 2007-12-14 | 2010-11-18 | Kohl Jurgen Anton | Novel micro-organisms controlling plant pathogens |
| WO2011022469A2 (en) | 2009-08-19 | 2011-02-24 | Dow Agrosciences Llc | Aad-1 event das-40278-9, related transgenic corn lines, and event-specific identification thereof |
| WO2011034704A1 (en) | 2009-09-17 | 2011-03-24 | Monsanto Technology Llc | Soybean transgenic event mon 87708 and methods of use thereof |
| WO2011063413A2 (en) | 2009-11-23 | 2011-05-26 | Bayer Bioscience N.V. | Herbicide tolerant soybean plants and methods for identifying same |
| WO2011062904A1 (en) | 2009-11-23 | 2011-05-26 | Monsanto Technology Llc | Transgenic maize event mon 87427 and the relative development scale |
| WO2011066384A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Aad-12 event 416, related transgenic soybean lines, and event-specific identification thereof |
| WO2011066360A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Detection of aad-12 soybean event 416 |
| WO2011075593A1 (en) | 2009-12-17 | 2011-06-23 | Pioneer Hi-Bred International, Inc. | Maize event dp-040416-8 and methods for detection thereof |
| WO2011075595A1 (en) | 2009-12-17 | 2011-06-23 | Pioneer Hi-Bred International, Inc. | Maize event dp-043a47-3 and methods for detection thereof |
| WO2011084632A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-032316-8 and methods for detection thereof |
| WO2011084621A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-004114-3 and methods for detection thereof |
| WO2011085575A1 (en) | 2010-01-15 | 2011-07-21 | 江苏省农药研究所股份有限公司 | Ortho-heterocyclyl formanilide compounds, their synthesis methods and use |
| WO2011106491A2 (en) | 2010-02-25 | 2011-09-01 | Marrone Bio Innovations, Inc. | Isolated bacterial strain of the genus burkholderia and pesticidal metabolites therefrom |
| WO2011151146A1 (en) | 2010-05-31 | 2011-12-08 | Syngenta Participations Ag | Method of crop enhancement |
| WO2011153186A1 (en) | 2010-06-04 | 2011-12-08 | Monsanto Technology Llc | Transgenic brassica event mon 88302 and methods of use thereof |
| WO2011151819A2 (en) | 2010-06-01 | 2011-12-08 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Pseudozyma aphidis as a biocontrol agent against various plant pathogens |
| WO2012033794A2 (en) | 2010-09-08 | 2012-03-15 | Dow Agrosciences Llc | Aad-12 event 1606 and related transgenic soybean lines |
| WO2012034403A1 (en) | 2010-09-14 | 2012-03-22 | 中化蓝天集团有限公司 | Fluoromethoxypyrazole anthranilamide compounds, synthesization methods and uses thereof |
| CN102391261A (en) | 2011-10-14 | 2012-03-28 | 上海交通大学 | N-substituted dioxazine compound as well as preparation method and application thereof |
| WO2012051199A2 (en) | 2010-10-12 | 2012-04-19 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87712 and methods for detection thereof |
| US20120131692A1 (en) | 2010-11-24 | 2012-05-24 | Pioneer Hi-Bred International, Inc. | Brassica gat event dp-073496-4 and compositions and methods for the identification and/or detection thereof |
| WO2012071039A1 (en) | 2010-11-24 | 2012-05-31 | Pioner Hi-Bred International, Inc. | Brassica gat event dp-061061-7 and compositions and methods for the identification and/or detection thereof |
| WO2012075429A1 (en) | 2010-12-03 | 2012-06-07 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8291.45.36.2, related transgenic soybean lines, and detection thereof |
| WO2012075426A1 (en) | 2010-12-03 | 2012-06-07 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8264.44.06.1, related transgenic soybean lines, and detection thereof |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012134808A1 (en) | 2011-03-30 | 2012-10-04 | Monsanto Technology Llc | Cotton transgenic event mon 88701 and methods of use thereof |
| WO2013003558A1 (en) | 2011-06-30 | 2013-01-03 | Monsanto Technology Llc | Alfalfa plant and seed corresponding to transgenic event kk 179-2 and methods for detection thereof |
| WO2013010094A1 (en) | 2011-07-13 | 2013-01-17 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8264.42.32.1, related transgenic soybean lines, and detection thereof |
| WO2013012775A1 (en) | 2011-07-15 | 2013-01-24 | Syngenta Participations Ag | Corn event mzdt09y |
| WO2013032693A2 (en) | 2011-08-27 | 2013-03-07 | Marrone Bio Innovations, Inc. | Isolated bacterial strain of the genus burkholderia and pesticidal metabolites therefrom-formulations and uses |
| WO2013034938A2 (en) | 2011-09-08 | 2013-03-14 | Szegedi Tudományegyetem | A copper resistant, fengycin-producing bacillus mojavensis strain for controlling vegetable pathogens, its use and compositions containing it |
| WO2013050317A1 (en) | 2011-10-03 | 2013-04-11 | Syngenta Limited | Polymorphs of an isoxazoline derivative |
| CN103109816A (en) | 2013-01-25 | 2013-05-22 | 青岛科技大学 | Thiobenzamide compounds and application thereof |
| WO2013080120A1 (en) | 2011-11-28 | 2013-06-06 | Novartis Ag | Novel trifluoromethyl-oxadiazole derivatives and their use in the treatment of disease |
| CN103232431A (en) | 2013-01-25 | 2013-08-07 | 青岛科技大学 | Dihalogenated pyrazole amide compound and its use |
| CN103265527A (en) | 2013-06-07 | 2013-08-28 | 江苏省农用激素工程技术研究中心有限公司 | Anthranilamide compound as well as preparation method and application thereof |
| WO2013144213A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyridinylidene compounds and derivatives for combating animal pests |
| EP2647626A1 (en) | 2012-04-03 | 2013-10-09 | Syngenta Participations AG. | 1-Aza-spiro[4.5]dec-3-ene and 1,8-diaza-spiro[4.5]dec-3-ene derivatives as pesticides |
| WO2013162716A2 (en) | 2012-04-27 | 2013-10-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| CN103524422A (en) | 2013-10-11 | 2014-01-22 | 中国农业科学院植物保护研究所 | Benzimidazole derivative, and preparation method and purpose thereof |
| WO2014028521A1 (en) | 2012-08-14 | 2014-02-20 | Marrone Bio Innovations, Inc. | Bacillus sp. strain with antifungal, antibacterial and growth promotion activity |
| WO2014053450A1 (en) | 2012-10-02 | 2014-04-10 | Bayer Cropscience Ag | Heterocyclic compounds as pesticides |
| US20140213448A1 (en) | 2012-04-27 | 2014-07-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US20140275503A1 (en) | 2013-03-13 | 2014-09-18 | Dow Agrosciences Llc | Process for the preparation of certain triaryl rhamnose carbamates |
| WO2014187846A1 (en) | 2013-05-23 | 2014-11-27 | Syngenta Participations Ag | Tank-mix formulations |
| WO2015067800A1 (en) | 2013-11-11 | 2015-05-14 | Basf Se | Antifungal penicillium strains, fungicidal extrolites thereof, and their use |
| WO2016020371A1 (en) | 2014-08-04 | 2016-02-11 | Basf Se | Antifungal paenibacillus strains, fusaricidin-type compounds, and their use |
| WO2016154297A1 (en) | 2015-03-26 | 2016-09-29 | Bayer Cropscience Lp | A novel paenibacillus strain, antifungal compounds, and methods for their use |
| WO2017019448A1 (en) | 2015-07-24 | 2017-02-02 | AgBiome, Inc. | Modified biological control agents and their uses |
| EP3131131A1 (en) | 2014-04-09 | 2017-02-15 | Sumitomo Chemical Company Limited | Light-emission element, and composition used therein |
| WO2017055473A1 (en) | 2015-10-02 | 2017-04-06 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2017066094A1 (en) | 2015-10-12 | 2017-04-20 | Pioneer Hi-Bred International, Inc. | Biologicals and their use in plants |
| WO2017178245A1 (en) | 2016-04-11 | 2017-10-19 | Basf Se | Substituted oxadiazoles for combating phytopathogenic fungi |
| WO2017205258A1 (en) | 2016-05-26 | 2017-11-30 | Novozymes Bioag A/S | Bacillus and lipochitooligosaccharide for improving plant growth |
| WO2017222951A1 (en) | 2016-06-23 | 2017-12-28 | Merck Sharp & Dohme Corp. | 3-aryl and heteroaryl substituted 5-trifluoromethyl oxadiazoles as histone deacetylase 6 (hdac6) inhibitors |
| EP3356335A1 (en) | 2015-10-02 | 2018-08-08 | Syngenta Participations AG | Microbiocidal oxadiazole derivatives |
| WO2018162643A1 (en) | 2017-03-10 | 2018-09-13 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2018187553A1 (en) | 2017-04-06 | 2018-10-11 | Fmc Corporation | Fungicidal oxadiazoles |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | Prolonged ectoparasite-controlling agent for animal |
| WO2019103918A1 (en) | 2017-11-21 | 2019-05-31 | Syngenta Participations Ag | Novel resistance genes associated with disease resistance in soybeans |
| WO2019122323A1 (en) | 2017-12-22 | 2019-06-27 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019236274A1 (en) | 2018-06-08 | 2019-12-12 | Dow Agrosciences Llc | Molecule having pesticidal utility, and compositions, and processes, related thereto |
| WO2020208509A1 (en) | 2019-04-08 | 2020-10-15 | Pi Industries Limited | Novel oxadiazole compounds for controlling or preventing phytopathogenic fungi |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
- 2023
- 2023-09-25WOPCT/EP2023/076346patent/WO2024068518A1/ennot_activeCeased
Patent Citations (244)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2010A (en) | 1841-03-18 | Machine foe | ||
| US2009A (en) | 1841-03-18 | Improvement in machines for boring war-rockets | ||
| US24077A (en) | 1859-05-17 | Window-sash supporter | ||
| US137395A (en) | 1873-04-01 | Improvement in nuts | ||
| DE2906507A1 (en) | 1979-02-20 | 1980-08-28 | Bayer Ag | Plant growth regulating compsn. - contg. alpha amino cyclo:alkanoic acid derivs., e.g. for dwarfing cereals |
| EP0030287A1 (en) | 1979-11-29 | 1981-06-17 | Bayer Ag | 1-Aminocyclopropanecarboxylic acid derivatives, methods for their production, their use as plant-growth regulators and compositions containing such derivatives |
| EP0086750A2 (en) | 1982-02-17 | 1983-08-24 | Ciba-Geigy Ag | Use of quinoline derivatives in the protection of crop plants |
| EP0094349A2 (en) | 1982-05-07 | 1983-11-16 | Ciba-Geigy Ag | Use of quinoline derivatives for the protection of cultivated plants |
| US4536207A (en) | 1983-07-26 | 1985-08-20 | Igi Biotechnology, Inc. | Nematocidally active chitin-protein complex |
| DE3335514A1 (en) | 1983-09-30 | 1985-04-18 | Bayer Ag, 5090 Leverkusen | 1-METHYLAMINO-CYCLOPROPAN-1-CARBONIC ACID DERIVATIVES |
| JPS6087254A (en) | 1983-10-19 | 1985-05-16 | Japan Carlit Co Ltd:The | Novel urea compound and herbicide containing the same |
| EP0174562A2 (en) | 1984-09-11 | 1986-03-19 | Hoechst Aktiengesellschaft | Plant protecting agents based on 1,2,4 - triazole derivatives as well as 1,2,4-triazole derivatives |
| EP0191736A2 (en) | 1985-02-14 | 1986-08-20 | Ciba-Geigy Ag | Use of quinoline derivatives for the protection of crop plants |
| US5123951A (en) | 1986-03-31 | 1992-06-23 | Rhone-Poulenc Nederland B.V. | Synergistic plant growth regulator compositions |
| EP0269806A1 (en) | 1986-10-04 | 1988-06-08 | Hoechst Aktiengesellschaft | Phenylpyrazole carbonic acid derivatives, their preparation and use as plant growth regulators and antidotes |
| EP0268554A2 (en) | 1986-10-22 | 1988-05-25 | Ciba-Geigy Ag | 1,5-Diphenyl pyrazole-3-carbonic-acid derivatives for the protection of cultured plants |
| US5061495A (en) | 1988-03-07 | 1991-10-29 | Agricultural Genetics Company Limited | Antibiotic derived from b. subtilis |
| EP0346620A1 (en) | 1988-05-20 | 1989-12-20 | Hoechst Aktiengesellschaft | Plant protection agents containing 1,2,4-triazole derivatives, and the 1,2,4-triazole derivatives |
| WO1990000020A1 (en) | 1988-07-01 | 1990-01-11 | The Bed Board Company Ltd | Appliances for use with beds |
| EP0365484A1 (en) | 1988-10-20 | 1990-04-25 | Ciba-Geigy Ag | Sulfamoyl phenyl ureas |
| WO1991008202A1 (en) | 1989-11-25 | 1991-06-13 | Hoechst Aktiengesellschaft | Isoxazolines, method of preparation thereof, and their use as plant-protection agents |
| WO1991007874A1 (en) | 1989-11-30 | 1991-06-13 | Hoechst Aktiengesellschaft | Pyrazolines for the protection of crops against herbicides |
| US5549718A (en) | 1990-04-06 | 1996-08-27 | Centre National De La Recherche Scientifique (C.N.R.S.) | Substance with lipo-oligosaccharide structure capable of acting as plant-specific symbiotic signals, processes for producing them and their applications |
| US20030126634A1 (en) | 1990-08-09 | 2003-07-03 | Dekalb Genetics Corporation | Methods and compositions for the increase of yield in plants |
| EP0492366A2 (en) | 1990-12-21 | 1992-07-01 | Hoechst Schering AgrEvo GmbH | New 5-chloroquinolin-8-oxyalkanecarbonic acid derivatives, process for their preparation and their use as antidotes for herbicides |
| EP0582198A2 (en) | 1992-08-01 | 1994-02-09 | Hoechst Schering AgrEvo GmbH | Substituted (hetero-)aryle compounds, process for their preparation, those containing compositions and their use as safeners |
| WO1995007897A1 (en) | 1993-09-16 | 1995-03-23 | Hoechst Schering Agrevo Gmbh | Substituted isoxazolines, process for producing them, agents containing them and their use as safeners |
| US6855533B2 (en) | 1995-04-20 | 2005-02-15 | Basf Corporation | Structure-based designed herbicide resistant products |
| WO1997017432A1 (en) | 1995-11-06 | 1997-05-15 | Wisconsin Alumni Research Foundation | Insecticidal protein toxins from photorhabdus |
| US5702752A (en) | 1996-03-13 | 1997-12-30 | Archer Daniels Midland Company | Production of isoflavone enriched fractions from soy protein extracts |
| WO1997045016A1 (en) | 1996-05-29 | 1997-12-04 | Hoechst Schering Agrevo Gmbh | Novel n-acyl sulphonamides, novel mixtures of herbicides and antidotes and their use |
| US5990291A (en) | 1996-06-11 | 1999-11-23 | Protein Technologies International, Inc. | Recovery of isoflavones from soy molasses |
| WO1998008932A1 (en) | 1996-08-29 | 1998-03-05 | Dow Agrosciences Llc | Insecticidal protein toxins from $i(photorhabdus) |
| WO1998013361A1 (en) | 1996-09-26 | 1998-04-02 | Novartis Ag | Herbicidal composition |
| US5965545A (en) | 1996-10-15 | 1999-10-12 | State Of Israel, Ministry Of Agriculture, Agricultural Research Organization, The Volcani Center | Compositions and method for controlling fungal disease in plants |
| WO1998027049A1 (en) | 1996-12-19 | 1998-06-25 | Hoechst Schering Agrevo Gmbh | Novel 2-fluoroacrylic acid derivatives, novel mixtures of herbicides and antidotes and the use thereof |
| WO1998038856A1 (en) | 1997-03-04 | 1998-09-11 | Zeneca Limited | Compositions for safening rice against acetochlor |
| US20060059581A1 (en) | 1997-04-03 | 2006-03-16 | Dekalb Genetics Corporation | Method of breeding glyphosate resistant plants |
| WO1998044140A1 (en) | 1997-04-03 | 1998-10-08 | Dekalb Genetics Corporation | Glyphosate resistant maize lines |
| US20050086719A1 (en) | 1997-04-03 | 2005-04-21 | Michael Spencer | Glyphosate resistant maize lines |
| US20050188434A1 (en) | 1997-04-03 | 2005-08-25 | Michael Spencer | Method for plant breeding |
| US6146668A (en) | 1997-04-28 | 2000-11-14 | Novogen, Inc. | Preparation of isoflavones from legumes |
| WO1998050427A1 (en) | 1997-05-05 | 1998-11-12 | Dow Agrosciences Llc | INSECTICIDAL PROTEIN TOXINS FROM $i(XENORHABDUS) |
| US6060051A (en) | 1997-05-09 | 2000-05-09 | Agraquest, Inc. | Strain of bacillus for controlling plant diseases and corn rootworm |
| WO1999016744A1 (en) | 1997-09-29 | 1999-04-08 | Aventis Cropscience Gmbh | Acylsulfamoyl benzoic acid amides, plant protection agents containing said acylsulfamoyl benzoic acid amides, and method for producing the same |
| JPH11253151A (en) | 1997-11-13 | 1999-09-21 | Kumiai Chem Ind Co Ltd | Rice seedling raising disease control agent |
| WO2000026356A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N. V. | Glufosinate tolerant rice |
| WO2000026345A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N.V. | Glufosinate tolerant rice |
| US6468747B1 (en) | 1998-11-03 | 2002-10-22 | Plant Genetic System, N.V. | Glufosinate tolerant rice |
| US6245551B1 (en) | 1999-03-30 | 2001-06-12 | Agraquest, Inc. | Strain of Bacillus pumilus for controlling plant diseases caused by fungi |
| WO2001031042A2 (en) | 1999-10-29 | 2001-05-03 | Aventis Cropscience N.V. | Male-sterile brassica plants and methods for producing same |
| US20030188347A1 (en) | 1999-12-08 | 2003-10-02 | Both Greta De | Hybrid winter oilseed rape and methods for producing same |
| WO2001041558A1 (en) | 1999-12-08 | 2001-06-14 | Aventis Cropscience N.V. | Hybrid winter oilseed rape and methods for producing same |
| WO2001047952A2 (en) | 1999-12-28 | 2001-07-05 | Bayer Cropscience N.V. | Insecticidal proteins from bacillus thuringiensis |
| WO2001051654A2 (en) | 2000-01-11 | 2001-07-19 | Bayer Cropscience N.V. | Methods and kits for identifying elite event gat-zm1 in biological samples |
| US20010029014A1 (en) | 2000-01-11 | 2001-10-11 | Beuckeleer Marc De | Methods and kits for identifying elite event GAT-ZM1 in biological samples |
| US20070292854A1 (en) | 2000-06-22 | 2007-12-20 | Behr Carl F | Corn event PV-ZMGT32(nk603) and compositions and methods for detection thereof |
| US20020102582A1 (en) | 2000-09-13 | 2002-08-01 | Levine Elaine B. | Corn event MON810 and compositions and methods for detection thereof |
| WO2002027004A2 (en) | 2000-09-29 | 2002-04-04 | Monsanto Technology Llc | Glyphosate tolerant wheat plant 33391 and compositions and methods for detection thereof |
| WO2002034048A1 (en) | 2000-10-23 | 2002-05-02 | Syngenta Participations Ag | Agrochemical compositions with quinoline safeners |
| WO2002034946A2 (en) | 2000-10-25 | 2002-05-02 | Monsanto Technology Llc | Cotton event pv-ghgt07(1445) and compositions and methods for detection thereof |
| US20020120964A1 (en) | 2000-10-25 | 2002-08-29 | Rangwala Tasneem S. | Cotton event PV-GHGT07(1445) and compositions and methods for detection thereof |
| US20080070260A1 (en) | 2000-10-30 | 2008-03-20 | Rachel Krieb | Canola event PV-BNGT04(RT73) and compositions and methods for detection thereof |
| WO2002036831A2 (en) | 2000-10-30 | 2002-05-10 | Monsanto Technology Llc | Canola event pv-bngt04(rt73) and compositions and methods for detection thereof |
| WO2002040677A2 (en) | 2000-11-20 | 2002-05-23 | Monsanto Technology Llc | Cotton event pv-ghbk04 (531) and compositions and methods for detection thereof |
| US20090265817A1 (en) | 2000-11-30 | 2009-10-22 | Ses Europe N.V./S.A. | T227-1 flanking sequence |
| WO2002044407A2 (en) | 2000-11-30 | 2002-06-06 | Ses Europe N.V. | Glyphosate resistant transgenic sugar beet characterised by a specific transgene insertion (t227-1), methods and primers for the detection of said insertion |
| WO2002100163A2 (en) | 2001-06-11 | 2002-12-19 | Monsanto Technology Llc | Cotton event moni5985 and compositions and methods for detection |
| US20040250317A1 (en) | 2001-06-11 | 2004-12-09 | Huber Scott A | Cotton event moni5985 and compositions and methods for detection thereof |
| WO2003000051A2 (en) | 2001-06-22 | 2003-01-03 | Drahos David J | Novel biofungicide |
| US20030097687A1 (en) | 2001-08-06 | 2003-05-22 | Linda Trolinder | Herbicide tolerant cotton plants and methods for producing and identifying same |
| WO2003013224A2 (en) | 2001-08-06 | 2003-02-20 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for producing and identifying same |
| US7094592B2 (en) | 2001-11-26 | 2006-08-22 | Kumiai Chemical Industry Co., Ltd. | Bacillus sp. D747 strain, plant disease controlling agents and insect pest controlling agents using the same and control method using the agents |
| WO2003052073A2 (en) | 2001-12-17 | 2003-06-26 | Syngenta Participations Ag | Novel corn event |
| WO2003064572A1 (en) | 2002-01-31 | 2003-08-07 | Exxonmobil Research And Engineering Company | Lubricating oil compositions with improved friction properties |
| US20060095986A1 (en) | 2002-07-29 | 2006-05-04 | Cavato Tracey A | Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof |
| WO2004011601A2 (en) | 2002-07-29 | 2004-02-05 | Monsanto Technology, Llc | Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof |
| WO2004039986A1 (en) | 2002-10-29 | 2004-05-13 | Syngenta Participations Ag | Cot102 insecticidal cotton |
| US20060130175A1 (en) | 2002-10-29 | 2006-06-15 | Ellis Daniel M | Cot102 insecticidal cotton |
| WO2004053062A2 (en) | 2002-12-05 | 2004-06-24 | Monsanto Technology Llc | Bentgrass event asr-368 and compositions and methods for detection thereof |
| US20060162007A1 (en) | 2002-12-05 | 2006-07-20 | Monsanto Technology Llc | Bentgrass event asr-368 and compositions and methods for detection thereof |
| WO2004072235A2 (en) | 2003-02-12 | 2004-08-26 | Monsanto Technology Llc | Cotton event mon 88913 and compositions and methods for detection thereof |
| US20060059590A1 (en) | 2003-02-12 | 2006-03-16 | Monsanto Technology Llc | Cotton event mon 88913 and compositions and methods for detection thereof |
| WO2004074492A1 (en) | 2003-02-20 | 2004-09-02 | Kws Saat Ag | Glyphosate tolerant sugar beet |
| US20040172669A1 (en) | 2003-02-28 | 2004-09-02 | Josef Kraus | Glyphosate tolerant sugar beet |
| WO2004084631A1 (en) | 2003-03-26 | 2004-10-07 | Bayer Cropscience Gmbh | Use of aromatic hydroxy compounds as safeners |
| US20050039226A1 (en) | 2003-05-02 | 2005-02-17 | Dow Agrosciences Llc | Corn event TC1507 and methods for detection thereof |
| WO2004099447A2 (en) | 2003-05-02 | 2004-11-18 | Dow Agrosciences Llc | Corn event tc1507 and methods for detection thereof |
| WO2005016001A1 (en) | 2003-08-05 | 2005-02-24 | Bayer Cropscience Gmbh | Safener based on aromatic-aliphatic carboxylic acid derivatives |
| WO2005015994A1 (en) | 2003-08-05 | 2005-02-24 | Bayer Cropscience Gmbh | Use of aromatic compounds as safeners |
| WO2005054480A2 (en) | 2003-12-01 | 2005-06-16 | Syngenta Participations Ag | Insect resistant cotton plants and methods of detecting the same |
| WO2005054479A1 (en) | 2003-12-01 | 2005-06-16 | Syngenta Participations Ag | Insect resistant cotton plants and methods of detecting the same |
| US20070067868A1 (en) | 2003-12-01 | 2007-03-22 | Negrotto David V | Insect resistant cotton plants and methods of detecting the same |
| WO2005061720A2 (en) | 2003-12-11 | 2005-07-07 | Monsanto Technology Llc | High lysine maize compositions and methods for detection thereof |
| US20070028322A1 (en) | 2003-12-11 | 2007-02-01 | Dizigan Mark A | High lysine maize compositions and methods for detection thereof |
| WO2005059103A2 (en) | 2003-12-15 | 2005-06-30 | Monsanto Technology Llc | Corn plant mon88017 and compositions and methods for detection thereof |
| US20080028482A1 (en) | 2003-12-15 | 2008-01-31 | Beazley Kim A | Corn Plant Mon88017 and Compositions and Methods for Detection Thereof |
| WO2005074671A1 (en) | 2004-01-30 | 2005-08-18 | Syngenta Participations Ag | Improved fertility restoration for ogura cytoplasmic male sterile brassica and method |
| WO2005103301A2 (en) | 2004-03-25 | 2005-11-03 | Syngenta Participations Ag | Corn event mir604 |
| US20080167456A1 (en) | 2004-03-25 | 2008-07-10 | Syngenta Participations Ag | Corn Event MIR604 |
| US20050216969A1 (en) | 2004-03-26 | 2005-09-29 | Dow Agrosciences Llc | Cry1F and Cry1AC transgenic cotton lines and event-specific identification thereof |
| WO2005103266A1 (en) | 2004-03-26 | 2005-11-03 | Dow Agrosciences Llc | Cry1f and cry1ac transgenic cotton lines and event-specific identification thereof |
| US20070143876A1 (en) | 2004-03-26 | 2007-06-21 | Dow Agrosciences Llc | Cry1F and Cry1Ac transgenic cotton lines and event-specific identification thereof |
| WO2005112630A1 (en) | 2004-05-12 | 2005-12-01 | Bayer Cropscience Gmbh | Quinoxalin-2-one derivatives crop protection agents comprising the same and method for production and use therof |
| WO2006003494A2 (en) | 2004-06-28 | 2006-01-12 | Syngenta Participations Ag | Piperidine derivatives and their use as insecticides, acaricides, molluscicides or nematicides |
| US20060070139A1 (en) | 2004-09-29 | 2006-03-30 | Pioneer Hi-Bred International, Inc. | Corn event DAS-59122-7 and methods for detection thereof |
| WO2006043635A1 (en) | 2004-10-20 | 2006-04-27 | Kumiai Chemical Industry Co., Ltd. | 3-triazolylphenyl sulfide derivative and insecticide/acaricide/nematicide containing the same as active ingredient |
| WO2006098952A2 (en) | 2005-03-16 | 2006-09-21 | Syngenta Participations Ag | Corn event 3272 and methods of detection thereof |
| US20060230473A1 (en) | 2005-03-16 | 2006-10-12 | Syngenta Participations Ag | Corn event 3272 and methods for detection thereof |
| US20080320616A1 (en) | 2005-04-08 | 2008-12-25 | Bayer Bioscience N.V. | Elite Event A2407-12 and Methods and Kits for Identifying Such Event in Biological Samples |
| WO2006108674A2 (en) | 2005-04-08 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a2704-12 and methods and kits for identifying such event in biological samples |
| WO2006108675A2 (en) | 2005-04-11 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a5547-127 and methods and kits for identifying such event in biological samples |
| US20080196127A1 (en) | 2005-04-11 | 2008-08-14 | Bayer Bioscience N.V. | Elite Event A5547-127 and Methods and Kits For Identifying Such Event in Biological Samples |
| US20060282915A1 (en) | 2005-05-27 | 2006-12-14 | Monsanto Technology Llc | Soybean event MON89788 and methods for detection thereof |
| WO2006130436A2 (en) | 2005-05-27 | 2006-12-07 | Monsanto Technology Llc | Soybean event mon89788 and methods for detection thereof |
| WO2006128573A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce43- 67b, insecticidal transgenic cotton expressing cry1ab |
| WO2006128568A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | T342-142, insecticidal transgenic cotton expressing cry1ab |
| WO2006128571A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce44-69d , insecticidal transgenic cotton expressing cry1ab |
| US20090217423A1 (en) | 2005-06-02 | 2009-08-27 | Cayley Patricia J | Ce43-67b insecticidal cotton |
| WO2006128569A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | 1143-14a, insecticidal transgenic cotton expressing cry1ab |
| WO2006128570A1 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | 1143-51b insecticidal cotton |
| WO2006128572A1 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce46-02a insecticidal cotton |
| US20100050282A1 (en) | 2005-08-08 | 2010-02-25 | Bayer Bioscience N.V. | Herbicide Tolerant Cotton Plants and Methods for Identifying the Same |
| WO2007017186A1 (en) | 2005-08-08 | 2007-02-15 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for identifying same |
| WO2007023719A1 (en) | 2005-08-22 | 2007-03-01 | Kumiai Chemical Industry Co., Ltd. | Agent for reducing chemical injury and herbicide composition with reduced chemical injury |
| WO2007024782A2 (en) | 2005-08-24 | 2007-03-01 | Pioneer Hi-Bred International, Inc. | Compositions providing tolerance to multiple herbicides and methods of use thereof |
| WO2007023764A1 (en) | 2005-08-26 | 2007-03-01 | Kumiai Chemical Industry Co., Ltd. | Agent for reduction of harmful effect of herbicide and herbicide composition having reduced harmful effect |
| WO2007040282A1 (en) | 2005-10-06 | 2007-04-12 | Nippon Soda Co., Ltd. | Bridged cyclic amine compound and pest control agent |
| WO2007040280A1 (en) | 2005-10-06 | 2007-04-12 | Nippon Soda Co., Ltd. | Cyclic amine compound and pest control agent |
| WO2007091277A2 (en) | 2006-02-10 | 2007-08-16 | Maharashtra Hybrid Seeds Company Limited (Mahyco) | TRANSGENIC BRINJAL (SOLANUM MELONGENA) EXPRESSING THE CRYlAC GENE |
| WO2007140256A1 (en) | 2006-05-26 | 2007-12-06 | Monsanto Technology, Llc | Corn plant and seed corresponding to transgenic event mon89034 and methods for detection and use thereof |
| US20080260932A1 (en) | 2006-05-26 | 2008-10-23 | Anderson Heather M | Corn Plant and Seed Corresponding to Transgenic Event MON89034 and Methods For Detection and Use Thereof |
| WO2007142840A2 (en) | 2006-06-03 | 2007-12-13 | Syngenta Participations Ag | Corn event mir162 |
| US20090300784A1 (en) | 2006-06-03 | 2009-12-03 | Syngenta Participations Ag | Corn event mir162 |
| US20100184079A1 (en) | 2006-06-28 | 2010-07-22 | Pioneer Hi-Bred International, Inc. | Soybean event 3560.4.3.5 and compositions and methods for the identification and detection thereof |
| WO2008002872A2 (en) | 2006-06-28 | 2008-01-03 | Pioneer Hi-Bred International, Inc. | Soybean event 3560.4.3.5 and compositions and methods for the identification and/or detection thereof |
| US20080289060A1 (en) | 2006-08-24 | 2008-11-20 | Bayer Bioscience N.V. | Herbicide tolerant rice plants and methods for identifying same |
| US20080064032A1 (en) | 2006-09-13 | 2008-03-13 | Syngenta Participations Ag | Polynucleotides and uses thereof |
| US20080312082A1 (en) | 2006-10-31 | 2008-12-18 | Kinney Anthony J | Soybean event dp-305423-1 and compositions and methods for the identification and/or detection thereof |
| WO2008054747A2 (en) | 2006-10-31 | 2008-05-08 | E. I. Du Pont De Nemours And Company | Soybean event dp-305423-1 and compositions and methods for the identification and/or detection thereof |
| US7579183B1 (en) | 2006-12-01 | 2009-08-25 | The United States Of America As Represented By The Secretary Of Agriculture | Saprophytic yeast, Pichia anomala |
| WO2008114282A2 (en) | 2007-03-19 | 2008-09-25 | Maharashtra Hybrid Seeds Company Limited | Transgenic rice (oryza sativa) comprising pe-7 event and method of detection thereof |
| WO2008122406A1 (en) | 2007-04-05 | 2008-10-16 | Bayer Bioscience N.V. | Insect resistant cotton plants and methods for identifying same |
| US20100077501A1 (en) | 2007-04-05 | 2010-03-25 | Bayer Bioscience N.V. | Insect resistant cotton plants and methods for identifying same |
| WO2008131861A1 (en) | 2007-04-30 | 2008-11-06 | Bayer Cropscience Ag | Use of pyridine-2-oxy-3-carbonamides as safeners |
| WO2008131860A2 (en) | 2007-04-30 | 2008-11-06 | Bayer Cropscience Ag | Pyridonecarboxamides crop protection agents containing the same method for production and use thereof |
| WO2008151780A1 (en) | 2007-06-11 | 2008-12-18 | Bayer Bioscience N.V. | Insect resistant cotton plants comprising elite event ee-gh6 and methods for identifying same |
| WO2009064652A1 (en) | 2007-11-15 | 2009-05-22 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87701 and methods for detection thereof |
| US20090130071A1 (en) | 2007-11-15 | 2009-05-21 | Ai-Guo Gao | Soybean Plant And Seed Corresponding To Transgenic Event MON87701 And Methods For Detection Thereof |
| US20100291039A1 (en) | 2007-12-14 | 2010-11-18 | Kohl Jurgen Anton | Novel micro-organisms controlling plant pathogens |
| WO2009100188A2 (en) | 2008-02-08 | 2009-08-13 | Dow Agrosciences Llc | Methods for detection of corn event das-59132 |
| WO2009103049A2 (en) | 2008-02-14 | 2009-08-20 | Pioneer Hi-Bred International, Inc. | Plant genomic dna flanking spt event and methods for identifying spt event |
| US20090210970A1 (en) | 2008-02-14 | 2009-08-20 | Pioneer Hi-Bred International, Inc. | Plant Genomic DNA Flanking SPT Event and Methods for Identifying SPT Event |
| WO2009102873A1 (en) | 2008-02-15 | 2009-08-20 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87769 and methods for detection thereof |
| US20110067141A1 (en) | 2008-02-15 | 2011-03-17 | Byron Froman | Soybean plant and seed corresponding to transgenic event mon87769 and methods for detection thereof |
| WO2009111263A1 (en) | 2008-02-29 | 2009-09-11 | Monsanto Technology Llc | Corn plant event mon87460 and compositions and methods for detection thereof |
| US20110138504A1 (en) | 2008-02-29 | 2011-06-09 | Monsanto Technology Llc | Corn plant event mon87460 and compositions and methods for detection thereof |
| WO2009116106A1 (en) | 2008-03-21 | 2009-09-24 | Trentino Sviluppo S.P.A. | Trichoderma atroviride sc1 for biocontrol of fungal diseases in plants |
| US8431120B2 (en) | 2008-03-21 | 2013-04-30 | Trentino Sviluppo S.P.A. | Trichoderma atroviride SC1 for biocontrol of fungal diseases in plants |
| WO2009152359A2 (en) | 2008-06-11 | 2009-12-17 | Dow Agrosciences Llc | Constructs for expressing herbicide tolerance genes, related plants, and related trait combinations |
| JP2010018586A (en) | 2008-07-14 | 2010-01-28 | Meiji Seika Kaisha Ltd | Substance pf1364, its manufacturing method, producing strain and agricultural/horticultural insecticide having the substance as active ingredient |
| CN101337937A (en) | 2008-08-12 | 2009-01-07 | 国家农药创制工程技术研究中心 | N-benz-3-substituted amino pyrazoles compounds with insecticidal activity |
| CN101337940A (en) | 2008-08-12 | 2009-01-07 | 国家农药创制工程技术研究中心 | Nitrogen heterocyclic ring dichloro allyl ether compound with insecticidal activity |
| WO2010024976A1 (en) | 2008-08-29 | 2010-03-04 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87754 and methods for detection thereof |
| US20100080887A1 (en) | 2008-09-29 | 2010-04-01 | Monsanto Technology Llc | Soybean Transgenic Event MON87705 and Methods for Detection Thereof |
| WO2010037016A1 (en) | 2008-09-29 | 2010-04-01 | Monsanto Technology Llc | Soybean transgenic event mon87705 and methods for detection thereof |
| CN101715774A (en) | 2008-10-09 | 2010-06-02 | 浙江化工科技集团有限公司 | Preparation and use of compound having insecticidal activity |
| WO2010051926A2 (en) | 2008-11-05 | 2010-05-14 | Bayer Cropscience Aktiengesellschaft | New halogen-substituted bonds |
| WO2010052161A2 (en) | 2008-11-06 | 2010-05-14 | Syngenta Participations Ag | Herbicidal compositions |
| WO2010066780A1 (en) | 2008-12-12 | 2010-06-17 | Syngenta Participations Ag | Spiroheterocyclic n-oxypiperidines as pesticides |
| WO2010077816A1 (en) | 2008-12-16 | 2010-07-08 | Syngenta Participations Ag | Corn event 5307 |
| WO2010076212A1 (en) | 2008-12-19 | 2010-07-08 | Syngenta Participations Ag | Transgenic sugar beet event gm rz13 |
| WO2010080829A1 (en) | 2009-01-07 | 2010-07-15 | Basf Agrochemical Products B.V. | Soybean event 127 and methods related thereto |
| WO2010086790A1 (en) | 2009-01-27 | 2010-08-05 | Lesaffre Et Compagnie | Saccharomyces cerevisiae strains with phytosanitary capabilities |
| WO2010117737A1 (en) | 2009-03-30 | 2010-10-14 | Monsanto Technology Llc | Rice transgenic event17053 and methods of use thereof |
| WO2010117735A1 (en) | 2009-03-30 | 2010-10-14 | Monsanto Technology Llc | Transgenic rice event17314 and methods of use thereof |
| EP2248421A1 (en) | 2009-05-07 | 2010-11-10 | GMI - Gregor-Mendel-Institut für Molekulare Pflanzenbiologie GmbH | Accumulation of biomass in plants |
| WO2011022469A2 (en) | 2009-08-19 | 2011-02-24 | Dow Agrosciences Llc | Aad-1 event das-40278-9, related transgenic corn lines, and event-specific identification thereof |
| WO2011034704A1 (en) | 2009-09-17 | 2011-03-24 | Monsanto Technology Llc | Soybean transgenic event mon 87708 and methods of use thereof |
| WO2011063413A2 (en) | 2009-11-23 | 2011-05-26 | Bayer Bioscience N.V. | Herbicide tolerant soybean plants and methods for identifying same |
| WO2011062904A1 (en) | 2009-11-23 | 2011-05-26 | Monsanto Technology Llc | Transgenic maize event mon 87427 and the relative development scale |
| WO2011066384A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Aad-12 event 416, related transgenic soybean lines, and event-specific identification thereof |
| WO2011066360A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Detection of aad-12 soybean event 416 |
| WO2011075593A1 (en) | 2009-12-17 | 2011-06-23 | Pioneer Hi-Bred International, Inc. | Maize event dp-040416-8 and methods for detection thereof |
| WO2011075595A1 (en) | 2009-12-17 | 2011-06-23 | Pioneer Hi-Bred International, Inc. | Maize event dp-043a47-3 and methods for detection thereof |
| WO2011084632A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-032316-8 and methods for detection thereof |
| WO2011084621A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-004114-3 and methods for detection thereof |
| WO2011085575A1 (en) | 2010-01-15 | 2011-07-21 | 江苏省农药研究所股份有限公司 | Ortho-heterocyclyl formanilide compounds, their synthesis methods and use |
| WO2011106491A2 (en) | 2010-02-25 | 2011-09-01 | Marrone Bio Innovations, Inc. | Isolated bacterial strain of the genus burkholderia and pesticidal metabolites therefrom |
| CN101838227A (en) | 2010-04-30 | 2010-09-22 | 孙德群 | Safener of benzamide herbicide |
| WO2011151146A1 (en) | 2010-05-31 | 2011-12-08 | Syngenta Participations Ag | Method of crop enhancement |
| WO2011151819A2 (en) | 2010-06-01 | 2011-12-08 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Pseudozyma aphidis as a biocontrol agent against various plant pathogens |
| WO2011153186A1 (en) | 2010-06-04 | 2011-12-08 | Monsanto Technology Llc | Transgenic brassica event mon 88302 and methods of use thereof |
| WO2012033794A2 (en) | 2010-09-08 | 2012-03-15 | Dow Agrosciences Llc | Aad-12 event 1606 and related transgenic soybean lines |
| WO2012034403A1 (en) | 2010-09-14 | 2012-03-22 | 中化蓝天集团有限公司 | Fluoromethoxypyrazole anthranilamide compounds, synthesization methods and uses thereof |
| WO2012051199A2 (en) | 2010-10-12 | 2012-04-19 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87712 and methods for detection thereof |
| US20120131692A1 (en) | 2010-11-24 | 2012-05-24 | Pioneer Hi-Bred International, Inc. | Brassica gat event dp-073496-4 and compositions and methods for the identification and/or detection thereof |
| WO2012071039A1 (en) | 2010-11-24 | 2012-05-31 | Pioner Hi-Bred International, Inc. | Brassica gat event dp-061061-7 and compositions and methods for the identification and/or detection thereof |
| WO2012075429A1 (en) | 2010-12-03 | 2012-06-07 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8291.45.36.2, related transgenic soybean lines, and detection thereof |
| WO2012075426A1 (en) | 2010-12-03 | 2012-06-07 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8264.44.06.1, related transgenic soybean lines, and detection thereof |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012134808A1 (en) | 2011-03-30 | 2012-10-04 | Monsanto Technology Llc | Cotton transgenic event mon 88701 and methods of use thereof |
| WO2013003558A1 (en) | 2011-06-30 | 2013-01-03 | Monsanto Technology Llc | Alfalfa plant and seed corresponding to transgenic event kk 179-2 and methods for detection thereof |
| WO2013010094A1 (en) | 2011-07-13 | 2013-01-17 | Dow Agrosciences Llc | Stacked herbicide tolerance event 8264.42.32.1, related transgenic soybean lines, and detection thereof |
| WO2013012775A1 (en) | 2011-07-15 | 2013-01-24 | Syngenta Participations Ag | Corn event mzdt09y |
| WO2013032693A2 (en) | 2011-08-27 | 2013-03-07 | Marrone Bio Innovations, Inc. | Isolated bacterial strain of the genus burkholderia and pesticidal metabolites therefrom-formulations and uses |
| WO2013034938A2 (en) | 2011-09-08 | 2013-03-14 | Szegedi Tudományegyetem | A copper resistant, fengycin-producing bacillus mojavensis strain for controlling vegetable pathogens, its use and compositions containing it |
| WO2013050317A1 (en) | 2011-10-03 | 2013-04-11 | Syngenta Limited | Polymorphs of an isoxazoline derivative |
| CN102391261A (en) | 2011-10-14 | 2012-03-28 | 上海交通大学 | N-substituted dioxazine compound as well as preparation method and application thereof |
| WO2013080120A1 (en) | 2011-11-28 | 2013-06-06 | Novartis Ag | Novel trifluoromethyl-oxadiazole derivatives and their use in the treatment of disease |
| WO2013144213A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyridinylidene compounds and derivatives for combating animal pests |
| EP2647626A1 (en) | 2012-04-03 | 2013-10-09 | Syngenta Participations AG. | 1-Aza-spiro[4.5]dec-3-ene and 1,8-diaza-spiro[4.5]dec-3-ene derivatives as pesticides |
| WO2013162715A2 (en) | 2012-04-27 | 2013-10-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| WO2013162716A2 (en) | 2012-04-27 | 2013-10-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US20140213448A1 (en) | 2012-04-27 | 2014-07-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| WO2014028521A1 (en) | 2012-08-14 | 2014-02-20 | Marrone Bio Innovations, Inc. | Bacillus sp. strain with antifungal, antibacterial and growth promotion activity |
| WO2014053450A1 (en) | 2012-10-02 | 2014-04-10 | Bayer Cropscience Ag | Heterocyclic compounds as pesticides |
| CN103232431A (en) | 2013-01-25 | 2013-08-07 | 青岛科技大学 | Dihalogenated pyrazole amide compound and its use |
| CN103109816A (en) | 2013-01-25 | 2013-05-22 | 青岛科技大学 | Thiobenzamide compounds and application thereof |
| US20140275503A1 (en) | 2013-03-13 | 2014-09-18 | Dow Agrosciences Llc | Process for the preparation of certain triaryl rhamnose carbamates |
| WO2014187846A1 (en) | 2013-05-23 | 2014-11-27 | Syngenta Participations Ag | Tank-mix formulations |
| CN103265527A (en) | 2013-06-07 | 2013-08-28 | 江苏省农用激素工程技术研究中心有限公司 | Anthranilamide compound as well as preparation method and application thereof |
| CN103524422A (en) | 2013-10-11 | 2014-01-22 | 中国农业科学院植物保护研究所 | Benzimidazole derivative, and preparation method and purpose thereof |
| WO2015067800A1 (en) | 2013-11-11 | 2015-05-14 | Basf Se | Antifungal penicillium strains, fungicidal extrolites thereof, and their use |
| EP3131131A1 (en) | 2014-04-09 | 2017-02-15 | Sumitomo Chemical Company Limited | Light-emission element, and composition used therein |
| WO2016020371A1 (en) | 2014-08-04 | 2016-02-11 | Basf Se | Antifungal paenibacillus strains, fusaricidin-type compounds, and their use |
| WO2016154297A1 (en) | 2015-03-26 | 2016-09-29 | Bayer Cropscience Lp | A novel paenibacillus strain, antifungal compounds, and methods for their use |
| WO2017019448A1 (en) | 2015-07-24 | 2017-02-02 | AgBiome, Inc. | Modified biological control agents and their uses |
| EP3356335A1 (en) | 2015-10-02 | 2018-08-08 | Syngenta Participations AG | Microbiocidal oxadiazole derivatives |
| WO2017055473A1 (en) | 2015-10-02 | 2017-04-06 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2017066094A1 (en) | 2015-10-12 | 2017-04-20 | Pioneer Hi-Bred International, Inc. | Biologicals and their use in plants |
| WO2017178245A1 (en) | 2016-04-11 | 2017-10-19 | Basf Se | Substituted oxadiazoles for combating phytopathogenic fungi |
| WO2017205258A1 (en) | 2016-05-26 | 2017-11-30 | Novozymes Bioag A/S | Bacillus and lipochitooligosaccharide for improving plant growth |
| WO2017222951A1 (en) | 2016-06-23 | 2017-12-28 | Merck Sharp & Dohme Corp. | 3-aryl and heteroaryl substituted 5-trifluoromethyl oxadiazoles as histone deacetylase 6 (hdac6) inhibitors |
| WO2018162643A1 (en) | 2017-03-10 | 2018-09-13 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2018187553A1 (en) | 2017-04-06 | 2018-10-11 | Fmc Corporation | Fungicidal oxadiazoles |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | Prolonged ectoparasite-controlling agent for animal |
| WO2019103918A1 (en) | 2017-11-21 | 2019-05-31 | Syngenta Participations Ag | Novel resistance genes associated with disease resistance in soybeans |
| WO2019122323A1 (en) | 2017-12-22 | 2019-06-27 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019236274A1 (en) | 2018-06-08 | 2019-12-12 | Dow Agrosciences Llc | Molecule having pesticidal utility, and compositions, and processes, related thereto |
| WO2020208509A1 (en) | 2019-04-08 | 2020-10-15 | Pi Industries Limited | Novel oxadiazole compounds for controlling or preventing phytopathogenic fungi |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
Non-Patent Citations (47)
| Title |
|---|
| "Citation of NMR Peaklist Data within Patent Applications", THE RESEARCH DISCLOSURE DATABASE NUMBER 564025 |
| "Technical Monograph No. 2", May 2008, CROPLIFE INTERNATIONAL, article "Catalogue of pesticide formulation types and international coding system" |
| "The Pesticide Manual", 2015, BRITISH CROP PROTECTION COUNCIL |
| ACS MEDICINAL CHEMISTRY LETTERS, vol. 8, no. 9, 2017, pages 919 - 924 |
| BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 17, no. 3, 2007, pages 756 - 760 |
| CAS , no. 1207977-87-4 |
| CAS, no. 1225292-17-0 |
| COTTAZ ET AL., METH. ENG, vol. 7, no. 4, 2005, pages 311 |
| DEMONT-CAULET ET AL., PLANT PHYSIOL., vol. 120, no. 1, 1999, pages 83 |
| DENARIE ET AL., ANN. REV. BIOCHEM, vol. 65, 1996, pages 503 |
| D'HAEZE ET AL., GLYCOBIOL, vol. 12, no. 6, 2002, pages 79R |
| DIAZ ET AL., MOL. PLANT-MICROBE INTERACTIONS, vol. 13, 2000, pages 268 |
| ESTRUCH ET AL., PROC NATL ACAD SCI US A., vol. 93, no. 11, 1996, pages 5389 - 94 |
| HANEL ET AL., PLANTA, vol. 232, 2010, pages 787 |
| HUNGRIA ET AL., SOIL BIOL. BIOCHEM, vol. 29, 1997, pages 819 |
| J. AM. CHEM. SOC., vol. 123, no. 25, 2001, pages 5962 - 5973 |
| J. HETEROCYC. CHEM, vol. 18, no. 7, 1981, pages 1305 - 1308 |
| J. MED. CHEM., vol. 38, no. 11, 1995, pages 1892 - 1903 |
| JAIN ET AL., J. PLANT BIOCHEM. & BIOTECHNOL, vol. 11, no. 1, 2002 |
| JENSEN DF ET AL.: "Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain 'IK726", AUSTRALAS PLANT PATHOL, vol. 36, 2007, pages 95 - 101 |
| JOURNAL OF MEDICINAL CHEMISTRY, vol. 52, no. 21, 2009, pages 6897 |
| JOURNAL OF ORGANIC CHEMISTRY, vol. 54, no. 23, 1989, pages 5574 - 80 |
| JOURNAL OF ORGANIC CHEMISTRY, vol. 78, 2013, pages 5218 |
| JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 135, no. 44, 2013, pages 16288 - 16291 |
| JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 141, no. 6, 2019, pages 2274 - 2278 |
| MABOOD ET AL., AGR. J, vol. 98, no. 2, 2006, pages 289 |
| MABOOD ET AL., FIELD CROPS RES, vol. 95, no. 2-3, 2006, pages 412 |
| MABOOD ET AL., PLANT PHYSIOL. BIOCHEM., vol. 44, no. 11, 2006, pages 759 |
| MABOODSMITH: "Linoleic and linolenic acid induce the expression of nod genes in Bradyrhizobium japonicum USDA 3", PLANT BIOL, 2001 |
| MULLER ET AL., PLANT PHYSIOL, vol. 124, 2000, pages 733 |
| ORGANIC & BIOMOLECULAR CHEMISTRY, vol. 18, no. 34, 2020, pages 6732 - 6737 |
| PIETR ET AL., ZESZ. NAUK. A R W SZCZECINIE, vol. 161, 1993, pages 125 - 137 |
| POCHANAVANICH ET AL., LETT. APPL. MICROBIOL., vol. 35, 2002, pages 17 |
| PROME ET AL., PURE & APPL. CHEM, vol. 70, no. 1, 1998, pages 55 |
| RALSTON ET AL., PLANT PHYSIOL., vol. 137, 2005, pages 1375 |
| ROBINAETAL., TETRAHEDRON, vol. 58, 2002, pages 521 - 530 |
| ROUGE ET AL.: "The Molecular Immunology of Complex Carbohydrates-3", 2011, SPRINGER SCIENCE, article "Docking of Chitin Oligomers and Nod Factors on Lectin Domains of the LysM-RLK Receptors in the Medicago-Rhizobium Symbiosis" |
| SAMAIN ET AL., CARBOHYDRATE RES, vol. 302, 1997, pages 35 |
| SAMAIN ET AL., J. BIOTECHNOL, vol. 72, 1999, pages 33 |
| SHAW ET AL., ENVIRON. MICROBIOL., vol. 11, 2006, pages 1867 - 475 |
| TETRAHEDRON LETTER, vol. 23, no. 24, 1982, pages 2475 |
| TETRAHEDRON, vol. 72, 2016, pages 734 |
| TETRAHEDRON, vol. 74, no. 1, 2018, pages 4236 - 4241 |
| VAN DER HOLST ET AL., CURR. OPIN. STRUC. BIOL, vol. 11, 2001, pages 608 |
| WAN ET AL., PLANT CELL, vol. 21, 2009, pages 1053 |
| WEGENER D. ET AL., ANALYTICAL BIOCHEMISTRY, vol. 321, 2003, pages 202 - 208 |
| XUE: "Efficacy of Clonostachys rosea strain ACM941 and fungicide seed treatments for controlling the root tot complex of field pea", CAN JOUR PLANT SCI, vol. 83, no. 3, pages 519 - 524 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2021255093A1 (en) | Active compound combination | |
| EP3897154A1 (en) | 1,3,4-oxadiazoles and their derivatives as new antifungal agents | |
| US20250280829A1 (en) | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms | |
| WO2021249995A1 (en) | Azabicyclyl-substituted heterocycles as fungicides | |
| WO2022207496A1 (en) | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| CN116157017A (en) | 3- (pyridazin-4-yl) -5, 6-dihydro-4H-1, 2, 4-oxadiazine derivatives as fungicides for crop protection | |
| US20250304566A1 (en) | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine | |
| WO2022207494A1 (en) | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| WO2022129190A1 (en) | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides | |
| EP4295688A1 (en) | Active compound combination | |
| WO2021228734A1 (en) | Triazine and pyrimidine (thio)amides as fungicidal compounds | |
| JP2024529148A (en) | Active compound combinations and antifungal compositions containing them - Patents.com | |
| WO2021255091A1 (en) | 1,3,4-oxadiazoles and their derivatives as fungicides | |
| EP4441049A1 (en) | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds | |
| WO2020182929A1 (en) | Substituted ureas and derivatives as new antifungal agents | |
| WO2024068517A1 (en) | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| WO2021255089A1 (en) | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides | |
| WO2021255170A1 (en) | 1,3,4-oxadiazole pyrimidines as fungicides | |
| WO2020254490A1 (en) | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents | |
| WO2024068518A1 (en) | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| WO2024068519A1 (en) | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| WO2024068520A1 (en) | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide | |
| WO2022129188A1 (en) | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides | |
| WO2021255169A1 (en) | 1,3,4-oxadiazole pyrimidines as fungicides | |
| WO2022058327A1 (en) | Substituted ureas and derivatives as new antifungal agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:23776376 Country of ref document:EP Kind code of ref document:A1 | |
| REG | Reference to national code | Ref country code:BR Ref legal event code:B01A Ref document number:112025005673 Country of ref document:BR | |
| NENP | Non-entry into the national phase | Ref country code:DE | |
| REG | Reference to national code | Ref country code:BR Ref legal event code:B01E Ref document number:112025005673 Country of ref document:BR Free format text:APRESENTE NOVAS FOLHAS DO RELATORIO DESCRITIVO ADAPTADAS AO ART. 26 INCISO II DA PORTARIA/INPI NO 14/2024, UMA VEZ QUE O CONTEUDO ENVIADO NA PETICAO NO 870250034505 DE 29/04/2025 ENCONTRA-SE FORA DA NORMA NO QUE SE REFERE A NUMERACAO DOS PARAGRAFOS. HA ERRO DE NUMERACAO DE PARAGRAFO APOS O NUMERO PARAGRAFO NO 151. A EXIGENCIA DEVE SER RESPONDIDA EM ATE 60 (SESSENTA) DIAS DE SUA PUBLICACAO E DEVE SER REALIZADA POR MEIO DA PETICAO GRU CODIGO DE SERVICO 207. | |
| 122 | Ep: pct application non-entry in european phase | Ref document number:23776376 Country of ref document:EP Kind code of ref document:A1 |