WO2023020372A1 - Aza-spirobifluorene compound, preparation comprising same, organic light-emitting element, and display or lighting device - Google Patents
Aza-spirobifluorene compound, preparation comprising same, organic light-emitting element, and display or lighting deviceDownload PDFInfo
- Publication number
- WO2023020372A1 WO2023020372A1PCT/CN2022/111980CN2022111980WWO2023020372A1WO 2023020372 A1WO2023020372 A1WO 2023020372A1CN 2022111980 WCN2022111980 WCN 2022111980WWO 2023020372 A1WO2023020372 A1WO 2023020372A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- substituted
- unsubstituted
- azaspirobifluorene
- layer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
Definitions
- the inventionrelates to an azaspirobifluorene compound, in particular to such azaspirobifluorene compound and a preparation containing it, an organic light-emitting element and a display or lighting device, and belongs to the field of organic semiconductor light-emitting.
- the hole-blocking materialshould have a lower HOMO energy level, a higher electron mobility rate, a higher triplet energy level, a higher oxidation potential and a wider band gap to improve its electron transport ability and hole And the blocking ability of excitons, the excitons are confined in the light-emitting layer, reducing the loss of light energy, thereby greatly improving the efficiency of organic light-emitting devices.
- the object of the present inventionis to provide a nitrogen spirobifluorene compound which has improved electron transport ability and hole and excitation
- the blocking ability of electronsmakes excitons confined in the light-emitting layer, reducing energy loss, thereby further improving the efficiency and life of organic light-emitting devices, and reducing the operating voltage.
- the present inventionprovides a kind of azaspirobifluorene compound, its structural formula is as shown in the following formula (I):
- Ar1 and Ar2are independently selected from substituted or unsubstituted C6-C30 aryl, substituted or unsubstituted C2-C30 heteroaryl; one of X1 to X9 is N, and the others are C-R1; R1 to R3 are independently selected from H, D, F, substituted or unsubstituted C1-C40 alkyl, substituted or unsubstituted C1-C40 alkoxy, substituted or unsubstituted C1-C40 cycloalkyl, substituted Or unsubstituted C1-C40 heteroalkyl, substituted or unsubstituted C6-C40 aryl, substituted or unsubstituted C1-C40 heteroaryl, substituted or unsubstituted C1-C60 silicon, substituted Or one of unsubstituted C6-C60 aromatic fused rings, substituted or unsubstituted C1-C60 heteroaromatic condensed rings,
- the azaspirobifluorene compound of the present inventionis selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
- the above groups that are not deuteratedcan be partially or fully deuterated; the groups that are not fluorinated can be partially or fully fluorinated.
- the above groups that are not deuteratedcan be partially or fully deuterated; the groups that are not fluorinated can be partially or fully fluorinated.
- the azaspirobifluorene compound of the present inventionis selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
- the present inventionalso provides a preparation, wherein the preparation comprises the above-mentioned azaspirobifluorene compound and at least one solvent, and the solvent may be an unsaturated hydrocarbon solvent known to those skilled in the art (such as toluene, xylene, homo Trimethylbenzene, tetrahydronaphthalene, decahydronaphthalene, bicyclohexane, n-butylbenzene, sec-butylbenzene, tert-butylbenzene, etc.), halogenated saturated hydrocarbon solvents (such as carbon tetrachloride, chloroform, dichloromethane, dichloromethane, Chloroethane, chlorobutane, bromobutane, chloropentane, bromopentane, chlorohexane, bromohexane, chlorocyclohexane, bromocyclohexane, etc.), halogenated unsaturated hydrocarbon
- the present inventionalso provides an organic light-emitting element, which includes a cathode layer, an anode layer, and an organic functional layer, and the organic functional layer is at least one layer in a hole injection layer, a hole transport layer, a light-emitting layer, an electron transport layer, and an electron injection layer.
- the organic functional layercomprises the above-mentioned azaspirobifluorene compound.
- the light-emitting layer of the organic light-emitting elementcontains the azaspirobifluorene compound of the present invention and at least one guest material, wherein the volume ratio of the azaspirobifluorene compound to the guest material is 99:1 to 1: 99; the guest material is a fluorescent material or a phosphorescent material.
- the light-emitting layer of the organic light-emitting elementcontains the azaspirobifluorene compound as the first host, another organic compound as the second host, and the azaspirobifluorene compound and the second host
- the volume ratiois 8:2 to 2:8, and the guest material is a fluorescent material or a phosphorescent material.
- the electron transport layer of the organic light-emitting elementcontains the azaspirobifluorene compound of the present invention; the azaspirobifluorene compound can form an electron transport layer alone, or together with a dopant to form an electron transport layer;
- the electron transport layercontains a dopant, the volume ratio of the azaspirobifluorene compound to the dopant is 2:8-8:2; the dopant is an n-type dopant.
- the organic light-emitting element of the present inventioncan be classified as top-emitting, bottom-emitting or double-sided emitting.
- the azaspirobifluorene compound of the present inventioncan also be applied to OLEDs for lighting, flexible OLEDs, and the like.
- the present inventionalso provides a display or lighting device, which includes the above-mentioned organic light-emitting element.
- the azaspirobifluorene compound of the present inventionis applied to organic light-emitting devices, which improves device efficiency, lifespan, film-forming performance, etc., and the spirobifluorene compound of the present invention has a higher triplet state energy level, enabling energy transfer More fully, the transfer of electrons and holes is more balanced, resulting in higher efficiency and lifetime of the device while maintaining a low operating voltage.
- the main feature of the azaspirobifluorene compound of the present inventionis that a substituted triazine group and a substituted pyridine unit are simultaneously introduced, and it is unexpectedly found that the azaspirobifluorene compound of the present invention has the following remarkable effects:
- the azaspirobifluorene compound of the present inventionhas a higher glass transition temperature
- the azaspirobifluorene compound of the present inventionis applied to organic light-emitting devices, which can improve the efficiency of the device. efficiency and longevity;
- the azaspirobifluorene compound of the present inventioncan better balance the transfer of holes and electrons, thereby reducing the operating voltage of organic light-emitting devices.
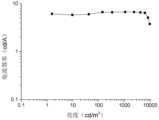
- Fig. 2is a current efficiency-brightness graph of Embodiment 6 of the blue light device of the present invention.
- the OLED device of the present inventioncontains a hole transport layer, and the hole transport material can be preferably selected from known or unknown materials, particularly preferably selected from the following structures, but does not represent the present invention Limited to the following structures:
- the hole transport layer contained in the OLED device of the present inventioncomprises one or more p-type dopants.
- the preferred p-type dopant of the present inventionis the following structure, but it does not mean that the present invention is limited to the following structure:
- the electron transport layercan be selected from one of the reference compounds ET-1 to ET-6 or a spirobifluorene compound similar in structure and the azaspirobifluorene compound of the present invention, but It does not mean that the present invention is limited to the following structures:
- the electron transport layercan be formed of organic materials together with one or more n-type dopants such as LiQ.
- the light-emitting layeris composed of BH-1 as the host and BD-1 as the guest material, and the blue light device is prepared
- the light-emitting layeris composed of GH-1 as the host and GD-1 as the guest material, and the green light device is prepared
- Embodiment 1the synthesis of compound 1
- Embodiment 2the synthesis of compound 2
- Embodiment 3the synthesis of compound 3
- Embodiment 4the synthesis of compound 4
- Embodiment 5the synthesis of compound 5
- Embodiment 6the synthesis of compound 6
- Embodiment 7the synthesis of compound 7
- Embodiment 8the synthesis of compound 8
- Table 1shows the performance test results of the organic light-emitting devices in blue light comparative devices 1-15 and blue light device examples 1-14, wherein the lifetime of LT95 is based on the lifetime of the device in comparative example 1 as 100% for comparison.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
Description
Translated fromChinese本发明涉及一种氮杂螺二芴化合物,具体尤其涉及此类氮杂螺二芴化合物和含有其的制剂、有机发光元件及显示或照明装置,属于有机半导体发光领域。The invention relates to an azaspirobifluorene compound, in particular to such azaspirobifluorene compound and a preparation containing it, an organic light-emitting element and a display or lighting device, and belongs to the field of organic semiconductor light-emitting.
有机发光元件(OLED器件)具有视角广阔、响应速度快、色彩质量高、可实现柔性发光等优点,其具有广阔的应用前景。常见的OLED器件通常包括以下种类的有机材料:空穴注入材料、空穴传输材料、电子传输材料以及各色的发光材料(染料或者掺杂客体材料)和相应的主体材料等。通常,空穴传输材料的电子迁移速率比电子传输材料的电子迁移速率高出两个数量级。因此,为了使电子和空穴在发光层能够很好地复合形成激子并发光,通常在制作有机发光器件中采用空穴阻挡层阻止空穴到达电子传输层。空穴阻挡材料应具有较低的HOMO能级、更高的电子迁移速率、更高的三线态能级及较高的氧化电位和较宽的带隙,以提高其电子的传输能力及空穴和激子的阻挡能力,使激子限制在发光层中,减少光能量的损失,从而大大提高有机发光器件的效率。Organic light-emitting elements (OLED devices) have the advantages of wide viewing angle, fast response speed, high color quality, flexible light emission, etc., and have broad application prospects. Common OLED devices generally include the following types of organic materials: hole injection materials, hole transport materials, electron transport materials, and various colors of light-emitting materials (dye or doped guest materials) and corresponding host materials. Typically, the electron mobility rate of hole transport materials is two orders of magnitude higher than that of electron transport materials. Therefore, in order to make electrons and holes recombine well in the light-emitting layer to form excitons and emit light, a hole-blocking layer is usually used to prevent holes from reaching the electron-transporting layer in the fabrication of organic light-emitting devices. The hole-blocking material should have a lower HOMO energy level, a higher electron mobility rate, a higher triplet energy level, a higher oxidation potential and a wider band gap to improve its electron transport ability and hole And the blocking ability of excitons, the excitons are confined in the light-emitting layer, reducing the loss of light energy, thereby greatly improving the efficiency of organic light-emitting devices.
很多有机材料能够有效地传输空穴,因此,为了提高有机发光器件的发光效率,在很多情况下,往往在阴极一侧额外加一层电子传输/空穴阻挡层,以阻挡空穴传输,从而将载流子复合限制在发光层区域。Many organic materials can effectively transport holes. Therefore, in order to improve the luminous efficiency of organic light-emitting devices, in many cases, an additional electron transport/hole blocking layer is often added on the cathode side to block hole transport, thereby Confine carrier recombination to the light-emitting layer region.
因此,有必要开发高三重态能级空穴阻挡材料(Hole-blocking layer,HBL)或电子传输材料,这样,一方面增加电子与空穴在发光层中的复合几率,减少光能量的损失,大大提高了器件效率;另一方面,高三重态能级有利于使用高效率磷光或TADF材料。Therefore, it is necessary to develop a high triplet energy level hole blocking material (Hole-blocking layer, HBL) or an electron transport material, so that, on the one hand, the recombination probability of electrons and holes in the light-emitting layer is increased, and the loss of light energy is reduced. The device efficiency is greatly improved; on the other hand, the high triplet energy level facilitates the use of high-efficiency phosphorescent or TADF materials.
发明内容Contents of the invention
为了克服现有的有机电子传输材料/空穴阻挡材料中存在的问题,本发明的目的是提供一种氮螺二芴化合物,该氮螺二芴化合物具有提高的电子传输能力及空穴和激子的阻挡能力,使激子限制在发光层中,减少能量的损失,从而进一步提高了有机发光器件的效率和寿命,而且降低了操作电压。In order to overcome the problems existing in the existing organic electron transport materials/hole blocking materials, the object of the present invention is to provide a nitrogen spirobifluorene compound which has improved electron transport ability and hole and excitation The blocking ability of electrons makes excitons confined in the light-emitting layer, reducing energy loss, thereby further improving the efficiency and life of organic light-emitting devices, and reducing the operating voltage.
为了实现本发明的目的,本发明的技术方案如下:In order to realize the purpose of the present invention, technical scheme of the present invention is as follows:
本发明提供了一种氮杂螺二芴化合物,其结构式如下列式(I)所示:The present invention provides a kind of azaspirobifluorene compound, its structural formula is as shown in the following formula (I):
其中,Ar1和Ar2独立选自取代或未取代的C6-C30的芳基、取代或未取代的C2-C30的杂芳基;X1至X9中有一个为N,其他的为C-R1;R1至R3独立选自H、D、F、取代或未取代的C1~C40的烷基、取代或未取代的C1~C40的烷氧基、取代或未取代的C1~C40的环烷基、取代或未取代的C1~C40的杂烷基、取代或未取代的C6~C40的芳基、取代或未取代的C1~C40的杂芳基、取代或未取代的C1~C60的硅基、取代或未取代的C6~C60的芳族稠环、取代或未取代的C1~C60的杂芳族稠环中的一种,n为0至10的整数,m为0或1,p为0至4的整数。Wherein, Ar1 and Ar2 are independently selected from substituted or unsubstituted C6-C30 aryl, substituted or unsubstituted C2-C30 heteroaryl; one of X1 to X9 is N, and the others are C-R1; R1 to R3 are independently selected from H, D, F, substituted or unsubstituted C1-C40 alkyl, substituted or unsubstituted C1-C40 alkoxy, substituted or unsubstituted C1-C40 cycloalkyl, substituted Or unsubstituted C1-C40 heteroalkyl, substituted or unsubstituted C6-C40 aryl, substituted or unsubstituted C1-C40 heteroaryl, substituted or unsubstituted C1-C60 silicon, substituted Or one of unsubstituted C6-C60 aromatic fused rings, substituted or unsubstituted C1-C60 heteroaromatic condensed rings, n is an integer from 0 to 10, m is 0 or 1, and p is 0 to 1 Integer of 4.
优选地,本发明的氮杂螺二芴化合物选自以下结构中的任意一者,但不代表本发明限于此:Preferably, the azaspirobifluorene compound of the present invention is selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
其中,Ar1和Ar2独立选自取代或未取代的C6-C30的芳基、取代或未取代的C2-C30的杂芳基;X1至X9中有一个为N,其他的为C-R1;R1至R3独立选自H、D、F、取代或未取代的C1~C40的烷基、取代或未取代的C1~C40的烷氧基、取代或未取代的C1~C40的环烷基、取代或未取代的C1~C40的杂烷基、取代或未取代的C6~C40的芳基、取代或未取代的C1~C40的杂芳基、取代或未取代的C1~C60的硅基、取代或未取代的C6~C60的芳族稠环、取代或未取代的C1~C60的杂芳族稠环中的一种,n为0至10的整数,m为0或1,p为0至4的整数。Wherein, Ar1 and Ar2 are independently selected from substituted or unsubstituted C6-C30 aryl, substituted or unsubstituted C2-C30 heteroaryl; one of X1 to X9 is N, and the others are C-R1; R1 to R3 are independently selected from H, D, F, substituted or unsubstituted C1-C40 alkyl, substituted or unsubstituted C1-C40 alkoxy, substituted or unsubstituted C1-C40 cycloalkyl, substituted Or unsubstituted C1-C40 heteroalkyl, substituted or unsubstituted C6-C40 aryl, substituted or unsubstituted C1-C40 heteroaryl, substituted or unsubstituted C1-C60 silicon, substituted Or one of unsubstituted C6-C60 aromatic fused rings, substituted or unsubstituted C1-C60 heteroaromatic condensed rings, n is an integer from 0 to 10, m is 0 or 1, and p is 0 to 1 Integer of 4.
更优选地,本发明的氮杂螺二芴化合物的结构式中R选自以下结构中的任意一者,但不代表本发明限于此:More preferably, in the structural formula of the azaspirobifluorene compound of the present invention, R is selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
其中,以上未被氘代的基团,可以被部分或全部氘代;未被氟代的基团可以被部分或全部氟代。Wherein, the above groups that are not deuterated can be partially or fully deuterated; the groups that are not fluorinated can be partially or fully fluorinated.
优选地,本发明的螺二芴化合物的结构式中Ar1和Ar2各自独立地选自以下结构中的任意一者,但不代表本发明限于此:Preferably, in the structural formula of the spirobifluorene compound of the present invention, Ar1 and Ar2 are each independently selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
其中,以上未被氘代的基团,可以被部分或全部氘代;未被氟代的基团可以被部分或全部氟代。Wherein, the above groups that are not deuterated can be partially or fully deuterated; the groups that are not fluorinated can be partially or fully fluorinated.
更优选地,本发明的氮杂螺二芴化合物选自以下结构中的任意一者,但不代表本发明限于此:More preferably, the azaspirobifluorene compound of the present invention is selected from any one of the following structures, but it does not mean that the present invention is limited thereto:
本发明还提供了一种制剂,其中,该制剂包含上述的氮杂螺二芴化合物和至少一种溶剂,该溶剂可以是本领域技术人员公知的不饱和烃溶剂(例如甲苯、二甲苯、均三甲苯、四氢化萘、十氢萘、双环己烷、正丁基苯、仲丁基苯、叔丁基苯等)、卤化饱和烃溶剂(例如四氯化碳、氯仿、二氯甲烷、二氯乙烷、氯丁烷、溴丁烷、氯戊烷、溴戊烷、氯己烷、溴己烷、氯环己烷、溴环己烷等),卤化不饱和烃溶剂(例如氯苯、二氯苯、三氯苯等)或酯类溶剂(例如四氢呋喃、四氢吡喃等醚溶剂,苯甲酸烷基酯等)。The present invention also provides a preparation, wherein the preparation comprises the above-mentioned azaspirobifluorene compound and at least one solvent, and the solvent may be an unsaturated hydrocarbon solvent known to those skilled in the art (such as toluene, xylene, homo Trimethylbenzene, tetrahydronaphthalene, decahydronaphthalene, bicyclohexane, n-butylbenzene, sec-butylbenzene, tert-butylbenzene, etc.), halogenated saturated hydrocarbon solvents (such as carbon tetrachloride, chloroform, dichloromethane, dichloromethane, Chloroethane, chlorobutane, bromobutane, chloropentane, bromopentane, chlorohexane, bromohexane, chlorocyclohexane, bromocyclohexane, etc.), halogenated unsaturated hydrocarbon solvents (such as chlorobenzene, Dichlorobenzene, trichlorobenzene, etc.) or ester solvents (ether solvents such as tetrahydrofuran, tetrahydropyran, etc., alkyl benzoate, etc.).
本发明还提供了有机发光元件,其包括阴极层、阳极层和有机功能层,该有机功能层为空穴注入层、空穴传输层、发光层、电子传输层和电子注入层中至少一层,其中,该有机功能层包含上述的氮杂螺二芴化合物。The present invention also provides an organic light-emitting element, which includes a cathode layer, an anode layer, and an organic functional layer, and the organic functional layer is at least one layer in a hole injection layer, a hole transport layer, a light-emitting layer, an electron transport layer, and an electron injection layer. , wherein the organic functional layer comprises the above-mentioned azaspirobifluorene compound.
优选地,该有机发光元件的发光层中含有本发明的氮杂螺二芴化合物和至少一种客体材料,其中,该氮杂螺二芴化合物与客体材料的体积比为99:1至1:99;该客体材料为荧光材料或磷光材料。Preferably, the light-emitting layer of the organic light-emitting element contains the azaspirobifluorene compound of the present invention and at least one guest material, wherein the volume ratio of the azaspirobifluorene compound to the guest material is 99:1 to 1: 99; the guest material is a fluorescent material or a phosphorescent material.
更优选地,该有机发光元件的发光层中含有所述的氮杂螺二芴化合物作为第一主体,另一种有机化合物作为第二主体,所述氮杂螺二芴化合物与第二主体的体积比为8:2至2:8,客体材料为荧光材料或磷光材料。More preferably, the light-emitting layer of the organic light-emitting element contains the azaspirobifluorene compound as the first host, another organic compound as the second host, and the azaspirobifluorene compound and the second host The volume ratio is 8:2 to 2:8, and the guest material is a fluorescent material or a phosphorescent material.
优选地,该有机发光元件的电子传输层中含有本发明的氮杂螺二芴化合物;所述氮杂螺二芴化合物可以单独形成电子传输层,也可以和掺杂剂共同形成电子传输层;当电子传输层中含有掺杂剂时,所述氮杂螺二芴化合物与掺杂的体积比为2:8~8:2;所述掺杂剂为n型掺杂剂。Preferably, the electron transport layer of the organic light-emitting element contains the azaspirobifluorene compound of the present invention; the azaspirobifluorene compound can form an electron transport layer alone, or together with a dopant to form an electron transport layer; When the electron transport layer contains a dopant, the volume ratio of the azaspirobifluorene compound to the dopant is 2:8-8:2; the dopant is an n-type dopant.
本发明的有机发光元件可以归类为顶发射、底发射或双面发射。本发明的氮杂螺二芴化合物同样可以应用于照明的OLED、柔性OLED等。The organic light-emitting element of the present invention can be classified as top-emitting, bottom-emitting or double-sided emitting. The azaspirobifluorene compound of the present invention can also be applied to OLEDs for lighting, flexible OLEDs, and the like.
本发明还提供了显示或照明装置,其中,其包括上述的有机发光元件。The present invention also provides a display or lighting device, which includes the above-mentioned organic light-emitting element.
本发明的氮杂螺二芴化合物将其应用于有机发光器件,提高了器件效率、寿命、成膜等性能,且本发明的螺二芴化合物由于具有较高的三线态能级,使能量传递更加充分,电子和空穴的传递更加平衡,从而在保持低操作电压的情况下,器件的效率和寿命更高。The azaspirobifluorene compound of the present invention is applied to organic light-emitting devices, which improves device efficiency, lifespan, film-forming performance, etc., and the spirobifluorene compound of the present invention has a higher triplet state energy level, enabling energy transfer More fully, the transfer of electrons and holes is more balanced, resulting in higher efficiency and lifetime of the device while maintaining a low operating voltage.
本发明的氮杂螺二芴化合物的主要特点是同时引入了取代的三嗪基团和取代的吡啶单元,意外地发现,本发明的氮杂螺二芴化合物具有以下显著效果:The main feature of the azaspirobifluorene compound of the present invention is that a substituted triazine group and a substituted pyridine unit are simultaneously introduced, and it is unexpectedly found that the azaspirobifluorene compound of the present invention has the following remarkable effects:
(1)在吡啶环和取代三嗪基团的协同作用下,本发明的氮杂螺二芴化合物具有较高的玻璃化转变温度;(1) Under the synergistic effect of the pyridine ring and the substituted triazine group, the azaspirobifluorene compound of the present invention has a higher glass transition temperature;
(2)与不具有取代吡啶基团的类似化合物相比(如芳基三嗪氮杂螺二芴化合物)相比,本发明的氮杂螺二芴化合物应用于有机发光器件,可以提高器件的效率和寿命;(2) Compared with similar compounds without substituted pyridine groups (such as aryl triazine azaspirobifluorene compounds), the azaspirobifluorene compound of the present invention is applied to organic light-emitting devices, which can improve the efficiency of the device. efficiency and longevity;
(3)本发明的氮杂螺二芴化合物能够较好地平衡空穴和电子的传递,从而降低有机发光器件的操作电压。(3) The azaspirobifluorene compound of the present invention can better balance the transfer of holes and electrons, thereby reducing the operating voltage of organic light-emitting devices.
图1为本发明的有机发光器件的结构示意图;1 is a schematic structural view of an organic light-emitting device of the present invention;
其中,110代表基板,120表示阳极,130表示空穴注入层,140表示空穴传输层,150表示发光层,160表示空穴阻挡层,170表示电子传输层,180表示电子注入层,190表示阴极。Among them, 110 represents the substrate, 120 represents the anode, 130 represents the hole injection layer, 140 represents the hole transport layer, 150 represents the light emitting layer, 160 represents the hole blocking layer, 170 represents the electron transport layer, 180 represents the electron injection layer, 190 represents cathode.
图2为本发明蓝光器件实施例6的电流效率-亮度图。Fig. 2 is a current efficiency-brightness graph of Embodiment 6 of the blue light device of the present invention.
为了使本发明的目的、技术方案及优点更加清楚明白,以下结合具体实施例,对本发明进行进一步详细说明。应当理解,此处所描述的具体实施例仅仅用以解释本发明,并不用于限定本发明。In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with specific embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
在本发明的一种优选实施方式中,本发明的OLED器件中含有空穴传输层,空穴传输材料可以优选自已知或未知的材料,特别优选地选自以下结构,但并不代表本发明限于以下结构:In a preferred embodiment of the present invention, the OLED device of the present invention contains a hole transport layer, and the hole transport material can be preferably selected from known or unknown materials, particularly preferably selected from the following structures, but does not represent the present invention Limited to the following structures:
在本发明的一种优选实施方式中,本发明的OLED器件中含有的空穴传输层,其包含一种或多种p型掺杂剂。本发明优选的p型掺杂剂为以下结构,但并不代表本发明限于以下结构:In a preferred embodiment of the present invention, the hole transport layer contained in the OLED device of the present invention comprises one or more p-type dopants. The preferred p-type dopant of the present invention is the following structure, but it does not mean that the present invention is limited to the following structure:
本发明的一种优选实施方式中,所述的电子传输层可以选自参考化合物ET-1至ET-6的一种或结构类似螺二芴化合物以及本发明的氮杂螺二芴化合物,但并不代表本发明限于以下结构:In a preferred embodiment of the present invention, the electron transport layer can be selected from one of the reference compounds ET-1 to ET-6 or a spirobifluorene compound similar in structure and the azaspirobifluorene compound of the present invention, but It does not mean that the present invention is limited to the following structures:
电子传输层可以有机材料与一种或多种n型掺杂剂(如LiQ)共同形成。The electron transport layer can be formed of organic materials together with one or more n-type dopants such as LiQ.
本发明的一种优选实施方式中,所述的发光层由BH-1作为主体,BD-1作为客体材料组成,制备得到蓝光器件In a preferred embodiment of the present invention, the light-emitting layer is composed of BH-1 as the host and BD-1 as the guest material, and the blue light device is prepared
本发明的一种优选实施方式中,所述的发光层由GH-1作为主体,GD-1作为客体材料组成,制备得到绿光器件In a preferred embodiment of the present invention, the light-emitting layer is composed of GH-1 as the host and GD-1 as the guest material, and the green light device is prepared
合成实施例Synthetic example
上述中间体1到中间体14是通过本领域公知的方法合成得到的。The
实施例1:化合物1的合成Embodiment 1: the synthesis of
在氮气保护下,250毫升的三口烧瓶中装入中间体1(10毫摩尔)、3,5-二苯基-1-氯三嗪(10毫摩尔)、甲苯:105毫升、乙醇:26毫升,接着,加入预先将碳酸钾:5克溶解于H2O:30毫升而成的水溶液,同时通入氮气。加入醋酸钯:0.1克,三苯基膦0.4克在加热回流下搅拌12小时。自然冷却后,分离出有机层,浓缩而得到粗产物。在将该粗产物用二氯甲烷/石油醚混合溶液作为淋洗剂,过硅胶柱得到白色固体,5.2克(产率83%);1H NMR(500MHz):δ7.30-7.51(m,9H),7.61(dd,2H),7.66(d,2H),7.70-7.91(m,5H),8.00(s,1H),8.11(d,1H),8.19-8.24(m,5H),8.68(s,1H),Fast-MS:M/Z实测626.2(M+H)+,理论625.23。Under nitrogen protection, intermediate 1 (10 mmol), 3,5-diphenyl-1-chlorotriazine (10 mmol), toluene: 105 ml, ethanol: 26 ml were charged in a 250 ml three-necked flask , Next, an aqueous solution obtained by dissolving potassium carbonate: 5 g in H2 O: 30 ml in advance was added, and nitrogen gas was blown in at the same time. Palladium acetate: 0.1 g and 0.4 g of triphenylphosphine were added and stirred under reflux for 12 hours. After natural cooling, the organic layer was separated and concentrated to obtain a crude product. The crude product was passed through a silica gel column with a dichloromethane/petroleum ether mixed solution as eluent to obtain a white solid, 5.2 g (yield 83%);1 H NMR (500 MHz): δ7.30-7.51 (m, 9H),7.61(dd,2H),7.66(d,2H),7.70-7.91(m,5H),8.00(s,1H),8.11(d,1H),8.19-8.24(m,5H),8.68 (s,1H), Fast-MS: M/Z found 626.2(M+H)+ , theoretically 625.23.
实施例2:化合物2的合成Embodiment 2: the synthesis of compound 2
化合物2的合成步骤与化合物1类似,选用中间体2作为反应物,产率86%;1H NMR(500MHz):δ7.36-7.58(m,8H),7.66-7.78(m,8H),7.79(d,1H),7.87(d,2H),7.96(s,1H),8.17-8.27(m,4H),8.46(d,2H),9.31(s,1H);Fast-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 2 is similar to that of
实施例3:化合物3的合成Embodiment 3: the synthesis of compound 3
化合物3的合成步骤与化合物1类似,选用中间体3作为反应物,产率81%;1H NMR(500MHz):δ7.33-7.52(m,9H),7.61(dd,2H),7.65(d,2H),7.71-7.88(m,5H),8.01(s,1H),8.27-8.35(m,5H),8.56(d,1H),8.71(s,1H),9.36(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 3 is similar to that of
实施例4:化合物4的合成Embodiment 4: the synthesis of compound 4
化合物4的合成步骤与化合物1类似,选用中间体4作为反应物,产率88%;1H NMR(500 MHz):δ7.42-7.68(m,14H),7.73(d,2H),7.85(s,1H),8.11-8.37(m,6H),8.55(d,1H),8.65(d,2H),9.21(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 4 is similar to that of
实施例5:化合物5的合成Embodiment 5: the synthesis of compound 5
化合物5的合成步骤与化合物1类似,选用中间体5作为反应物,产率72%;1H NMR(500MHz):δ7.45(dd,1H),7.47-7.52(m,6H),7.55(dd,1H),7.65-7.92(m,10H),7.96(d,1H),8.03-8.22(m,5H),8.55-8.61(m,4H),9.34(s,1H);FAST-MS:M/Z实测676.2(M+H)+,理论675.24。The synthesis procedure of compound 5 is similar to that of
实施例6:化合物6的合成Embodiment 6: the synthesis of compound 6
化合物6的合成步骤与化合物1类似,选用中间体6作为反应物,产率78%;1H NMR(500MHz):δ7.40-7.72(m,19H),7.82-8.01(m,9H),8.62(d,2H),9.26(s,1H);FAST-MS:M/Z实测702.3(M+H)+,理论701.26。The synthesis procedure of compound 6 is similar to that of
实施例7:化合物7的合成Embodiment 7: the synthesis of compound 7
化合物7的合成步骤与化合物1类似,选用中间体7作为反应物,产率80%;1H NMR(500MHz):δ7.38(dd,1H),7.40(dd,1H),7.46-7.72(m,13H),7.83(d,1H),7.89-8.12(m,6H),8.33-8.55(m,6H),9.16(s,1H);FAST-MS:M/Z实测676.2(M+H)+,理论675.24。The synthesis procedure of compound 7 is similar to that of
实施例8:化合物8的合成Embodiment 8: the synthesis of compound 8
化合物8的合成步骤与化合物1类似,选用中间体8作为反应物,产率83%;1H NMR(500MHz):δ7.41-7.62(m,10H),7.73-7.81(m,4H),7.93(d,1H),8.11-8.36(m,8H),8.69(d,2H),8.74(d,1H),9.36(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 8 is similar to that of
实施例9:化合物9的合成Embodiment 9: the synthesis of compound 9
化合物9的合成步骤与化合物1类似,选用中间体9作为反应物,产率76%;1H NMR(500MHz):δ7.35(dd,1H),7.45-7.63(m,13H),7.70(d,1H),8.03-8.32(m,8H),8.63(d,1H),8.71(d,1H),8.91(s,1H),9.18(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 9 is similar to that of
实施例10:化合物10的合成Embodiment 10: the synthesis of
化合物10的合成步骤与化合物1类似,选用中间体10作为反应物,产率90%;1H NMR(500MHz):δ7.43-7.74(m,14H),7.75(s,1H),7.92(d,2H),8.11-8.36(m,7H),8.41(d,2H),9.20(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of
实施例11:化合物11的合成Embodiment 11: the synthesis of compound 11
化合物11的合成步骤与化合物1类似,选用中间体11作为反应物,产率82%;1H NMR(500MHz):δ7.45-7.64(m,11H),7.70(d,2H),7.73-7.91(m,3H),7.93(d,1H),8.01(d,1H),8.23-8.34(m,5H),8.56(d,2H),8.74(d,1H),9.08(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 11 is similar to that of
实施例12:化合物12的合成Embodiment 12: the synthesis of compound 12
化合物12的合成步骤与化合物1类似,选用中间体12作为反应物,产率84%;1H NMR(500MHz):δ7.43-7.51(m,8H),7.55-7.71(m,8H),7.85(s,1H),7.93(d,2H),8.29(d,4H),8.76(d,2H),9.03(s,1H),9.26(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 12 is similar to that of
实施例13:化合物13的合成Embodiment 13: the synthesis of compound 13
化合物13的合成步骤与化合物1类似,选用中间体13作为反应物,产率83%;1H NMR(500MHz):δ7.42-7.53(m,9H),7.59(dd,2H),7.68(d,2H),7.75(d,2H),7.86(d,2H),8.23-8.41(m,6H),8.69(d,2H),8.73(d,1H),9.13(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 13 is similar to that of
实施例14:化合物14的合成Embodiment 14: the synthesis of compound 14
化合物14的合成步骤与化合物1类似,选用中间体14作为反应物,产率86%;1H NMR(500MHz):δ7.45(dd,2H),7.48-7.73(m,13H),7.96(d,2H),8.23-8.31(m,5H),8.39(s,1H),8.63(d,2H),8.76(d,1H),8.96(s,1H);FAST-MS:M/Z实测626.2(M+H)+,理论625.23。The synthesis procedure of compound 14 is similar to that of
器件实施例Device embodiment
如图1所示,本发明的有机发光器件包括基板110、阳极120、空穴注入层130、空穴传输层140、发光层150、空穴阻挡层160、电子传输层170、电子注入层180和阴极190。As shown in FIG. 1 , the organic light-emitting device of the present invention includes a
有机发光元件(OLED)的制造:Manufacturing of Organic Light Emitting Devices (OLEDs):
在底发射ITO玻璃基板上将HT-1与P-3(体积比97:3)共蒸形成的空穴注入层(HIL)(10纳米),接着蒸镀HT-1(90纳米)形成空穴传输层(HTL),在空穴传输层上蒸镀HT-10(10纳米)形成电子阻挡层(EBL),随后在电子阻挡层上将主体化合物(BH-1或GH-1)与客体化合物(BD-1或GD-1)共蒸形成的发光层(EML)(25纳米)(其中,BH-1与BD-1的体积比为97:3;GH-1与GD-1的体积比为94:6),最后将ET-1~ET-6或化合物ref-1~ref-9或本发明的化合物1-14与LiQ共蒸以1:1的体积比蒸镀,形成电子传输层(ETL)(35纳米),然后蒸镀阴极Al(40纳米),制造了有机发光元件。A hole injection layer (HIL) (10 nm) formed by co-evaporating HT-1 and P-3 (volume ratio 97:3) on a bottom-emitting ITO glass substrate, followed by evaporation of HT-1 (90 nm) to form a hole injection layer (HIL) Hole transport layer (HTL), evaporate HT-10 (10 nm) on the hole transport layer to form an electron blocking layer (EBL), then host compound (BH-1 or GH-1) and guest Compound (BD-1 or GD-1) co-evaporated light-emitting layer (EML) (25 nanometers) (wherein, the volume ratio of BH-1 and BD-1 is 97:3; the volume ratio of GH-1 and GD-1 ratio is 94:6), and finally ET-1~ET-6 or compound ref-1~ref-9 or compound 1-14 of the present invention and LiQ are co-evaporated with LiQ at a volume ratio of 1:1 to form an electron transport layer (ETL) (35 nanometers), and then evaporated cathode Al (40 nanometers), and manufactured an organic light-emitting device.
蓝光对比例1-15中采用ET-1~ET-6和ref-1~ref-9与LiQ共蒸作为电子传输层,制备了相应的对比OLED器件。In blue light comparative examples 1-15, ET-1-ET-6 and ref-1-ref-9 were co-evaporated with LiQ as the electron transport layer, and corresponding comparative OLED devices were prepared.
表1显示了蓝光对比器件1-15及蓝光器件例1-14中有机发光器件的性能测试结果,其中,LT95寿命是以对比例1中器件的寿命为100%作为对比。Table 1 shows the performance test results of the organic light-emitting devices in blue light comparative devices 1-15 and blue light device examples 1-14, wherein the lifetime of LT95 is based on the lifetime of the device in comparative example 1 as 100% for comparison.
绿光对比器件16则将上述的OLED器件的发光层换成GH-1:GD-1(94:6)共蒸形成的发光层(EML)(25纳米),电子传输层为化合物6与LiQ(1:1)共蒸形成电子传输层。For the green light contrast device 16, the light-emitting layer of the above-mentioned OLED device is replaced by the light-emitting layer (EML) (25 nanometers) formed by co-evaporation of GH-1:GD-1 (94:6), and the electron transport layer is compound 6 and LiQ (1:1) co-evaporated to form the electron transport layer.
绿光器件15则是将GH-1:化合物6:GD-1以以下的体积比60:34:6,共蒸形成光层(EML)(25纳米)。本发明的OLED器件通过本领域公知的标准方法表征,得到器件结果表1。The green light device 15 co-evaporates GH-1:compound 6:GD-1 at the volume ratio of 60:34:6 to form an optical layer (EML) (25 nm). The OLED device of the present invention was characterized by standard methods known in the art, and the device results Table 1 was obtained.
表1Table 1
现有技术常用的电子传输材料为ET-1至ET-6,蓝光对比器件1的电子传输层材料为ET-1,其操作电压为3.8伏,蓝光对比器件2-6、13-15中的电子传输材料应用在蓝光OLED器件中具有较高的效率,同时也具有较长的使用寿命,但是它们的操作电压较高,不满足蓝光发光器件对低能耗的要求,合适的电子传输层材料必须满足蓝光OLED器件的需求;为此需要提供一类化合物,既能提升蓝光器件的效率,提升它的使用寿命,还需要降低其操作电压。The commonly used electron transport materials in the prior art are ET-1 to ET-6. The electron transport layer material of the blue
蓝光对比器件7-12中,在具有三嗪取代的螺二芴体系中增加苯环取代,与对比例2-3相比,器件的电压有所降低,但效率和使用寿命完全没有优势。而在本申请人前期的基础上(CN106831581A),在螺二芴上引入氮原子,形成氮杂螺二芴,可以显著提升蓝光器件的寿命(蓝光对比器件13-15),但电压仍然比较高。本发明将吡啶基团、取代三嗪基团引入到氮杂螺二芴体系中,意外地发现,吡啶基团的引入(化合物1)后,器件例1的操作电压与蓝光对比器件1的持平,电流效率和使用寿命都得到了提升。与蓝光对比例13-15相比,使用本发明的氮杂螺二芴化合物作为电子传输层具有更低的电压,更高的效率以及相对较长的使用寿命(蓝光器件例2-14)。因此,本发明的氮杂螺二芴化合物种同时引入吡啶和取代三嗪;将其应用于有机发光器件,在降低操作电压的同时,从而提升了器件的效率和寿命。In the blue light comparison devices 7-12, the benzene ring substitution was added to the triazine-substituted spirobifluorene system. Compared with the comparative examples 2-3, the voltage of the device was reduced, but there was no advantage in efficiency and service life. On the basis of the applicant's previous work (CN106831581A), nitrogen atoms were introduced into spirobifluorene to form azaspirobifluorene, which can significantly improve the lifespan of blue light devices (blue light comparison devices 13-15), but the voltage is still relatively high . In the present invention, a pyridine group and a substituted triazine group are introduced into the azaspirobifluorene system, and it is unexpectedly found that after the introduction of the pyridine group (compound 1), the operating voltage of device example 1 is equal to that of blue
以上所述,仅为本发明较佳的具体实施方式,但本发明的保护范围并不局限于此,任何熟悉本技术领域的技术人员在本发明揭露的技术范围内,根据本发明的技术方案及其发明构思加以等同替换或改变,都应涵盖在本发明的保护范围之内。The above is only a preferred embodiment of the present invention, but the scope of protection of the present invention is not limited thereto, any person familiar with the technical field within the technical scope disclosed in the present invention, according to the technical solution of the present invention Any equivalent replacement or change of the inventive concepts thereof shall fall within the protection scope of the present invention.
Claims (11)
Translated fromChineseApplications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110933623.4ACN115894446A (en) | 2021-08-14 | 2021-08-14 | Azaspiro-bifluorene compound, preparation containing azaspirobifluorene compound, organic light-emitting element and display or lighting device |
| CN202110933623.4 | 2021-08-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023020372A1true WO2023020372A1 (en) | 2023-02-23 |
Family
ID=85239478
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2022/111980CeasedWO2023020372A1 (en) | 2021-08-14 | 2022-08-12 | Aza-spirobifluorene compound, preparation comprising same, organic light-emitting element, and display or lighting device |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN115894446A (en) |
| WO (1) | WO2023020372A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118221640B (en)* | 2024-05-24 | 2024-09-03 | 浙江华显光电科技有限公司 | An organic compound, an OLED having the same, and an organic light-emitting device |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106831581A (en)* | 2017-02-10 | 2017-06-13 | 上海大学 | A kind of azepine spirobifluorene derivative and preparation method thereof |
| CN107021926A (en)* | 2017-06-12 | 2017-08-08 | 江苏三月光电科技有限公司 | A kind of compound containing azepine spiro fluorene and nitrogenous hexa-member heterocycle and its application on OLED |
| CN108963099A (en)* | 2018-08-20 | 2018-12-07 | 上海大学 | A kind of composition and organic electroluminescent device for organic electroluminescent device |
| CN108997342A (en)* | 2018-08-20 | 2018-12-14 | 上海大学 | A kind of two fluorene compound of more azaspiros and the organic electro-optic device containing the compound |
| CN110229071A (en)* | 2019-06-06 | 2019-09-13 | 北京诚志永华显示科技有限公司 | Fluorene derivative organic electroluminescence device |
| CN110776496A (en)* | 2019-11-18 | 2020-02-11 | 苏州久显新材料有限公司 | 3, 4-diazaspiro fluorene derivative and synthesis method thereof, and electronic device containing 3, 4-diazaspiro fluorene derivative |
| CN111244306A (en)* | 2018-11-29 | 2020-06-05 | 宇瑞(上海)化学有限公司 | A top emission organic light emitting diode unit |
| CN112390780A (en)* | 2019-08-18 | 2021-02-23 | 北京夏禾科技有限公司 | Electron transport material containing azaspirobifluorene |
- 2021
- 2021-08-14CNCN202110933623.4Apatent/CN115894446A/enactivePending
- 2022
- 2022-08-12WOPCT/CN2022/111980patent/WO2023020372A1/ennot_activeCeased
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106831581A (en)* | 2017-02-10 | 2017-06-13 | 上海大学 | A kind of azepine spirobifluorene derivative and preparation method thereof |
| CN107021926A (en)* | 2017-06-12 | 2017-08-08 | 江苏三月光电科技有限公司 | A kind of compound containing azepine spiro fluorene and nitrogenous hexa-member heterocycle and its application on OLED |
| CN108963099A (en)* | 2018-08-20 | 2018-12-07 | 上海大学 | A kind of composition and organic electroluminescent device for organic electroluminescent device |
| CN108997342A (en)* | 2018-08-20 | 2018-12-14 | 上海大学 | A kind of two fluorene compound of more azaspiros and the organic electro-optic device containing the compound |
| CN111244306A (en)* | 2018-11-29 | 2020-06-05 | 宇瑞(上海)化学有限公司 | A top emission organic light emitting diode unit |
| CN110229071A (en)* | 2019-06-06 | 2019-09-13 | 北京诚志永华显示科技有限公司 | Fluorene derivative organic electroluminescence device |
| CN112390780A (en)* | 2019-08-18 | 2021-02-23 | 北京夏禾科技有限公司 | Electron transport material containing azaspirobifluorene |
| CN110776496A (en)* | 2019-11-18 | 2020-02-11 | 苏州久显新材料有限公司 | 3, 4-diazaspiro fluorene derivative and synthesis method thereof, and electronic device containing 3, 4-diazaspiro fluorene derivative |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115894446A (en) | 2023-04-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN113024529B (en) | An organic electroluminescent material and organic electroluminescent device | |
| CN105503766B (en) | A kind of thermal activation delayed fluorescence material and organic electroluminescence device | |
| CN104538559B (en) | A kind of organic electroluminescence display device and method of manufacturing same with rgb pixel area | |
| CN109748898B (en) | Organic electroluminescent compound and its preparation method and organic electroluminescent device | |
| CN104471022B (en) | Organic light-emitting compound containing acridine derivative and organic light-emitting device containing the organic light-emitting compound | |
| CN102437290B (en) | A kind of display of organic electroluminescence blue-light device and preparation method thereof | |
| WO2023024445A1 (en) | Spiro compound, preparation, organic electroluminescent diode, and display device | |
| CN113105442B (en) | Triazine derivative, organic photoelectric element containing triazine derivative and application of organic photoelectric element | |
| CN109796960B (en) | Organic electroluminescent compound, preparation method and application thereof | |
| WO2017215549A1 (en) | Organic electroluminescent compound and application thereof | |
| KR20160019839A (en) | Monoamine material for organic electroluminescent device and organic electroluminescent device using the same | |
| CN112614964B (en) | Composition and organic electroluminescent element comprising same | |
| CN105440004A (en) | Organic electroluminescent material and application thereof | |
| KR20170043099A (en) | Monoamine material for electroluminescence device and electroluminescence device using the same | |
| KR20150066429A (en) | Organic Compound and Organic Light Emitting Diode Devices using the same | |
| KR20200095709A (en) | Organic Light Emitting Material and Organic Light Emitting Diode Having The Same | |
| WO2023020372A1 (en) | Aza-spirobifluorene compound, preparation comprising same, organic light-emitting element, and display or lighting device | |
| CN112670426B (en) | Composition and organic electroluminescent element comprising same | |
| Braveenth et al. | Fluorene core with several modification by using donor type triphenylamine and carbazole derivatives for organic light emitting diodes | |
| CN113402498B (en) | Spirobifluorene compound, preparation, organic light-emitting device, and display or lighting device | |
| CN116425688A (en) | Cyanophenylspirobifluorene compound, formulation containing same, light-emitting element, and display or lighting device | |
| TW201323379A (en) | 1,1'-binaphthyl-4,4'-diamine derivatives for luminescence of organic electroluminescent device and organic electroluminescent device using them | |
| CN113013346B (en) | Ternary composition, organic electroluminescent element containing ternary composition and application of ternary composition | |
| KR20140079078A (en) | Phosphorescent compound and Organic light emitting diode device using the same | |
| Li et al. | High thermal stability fluorene-based hole-injecting material for organic light-emitting devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:22857684 Country of ref document:EP Kind code of ref document:A1 | |
| NENP | Non-entry into the national phase | Ref country code:DE | |
| 122 | Ep: pct application non-entry in european phase | Ref document number:22857684 Country of ref document:EP Kind code of ref document:A1 |