WO2019080537A1 - Therapeutic agent comprising oncolytic virus and car-nk cells, use, kit and method for treating tumor and/or cancer - Google Patents
Therapeutic agent comprising oncolytic virus and car-nk cells, use, kit and method for treating tumor and/or cancerInfo
- Publication number
- WO2019080537A1 WO2019080537A1PCT/CN2018/094003CN2018094003WWO2019080537A1WO 2019080537 A1WO2019080537 A1WO 2019080537A1CN 2018094003 WCN2018094003 WCN 2018094003WWO 2019080537 A1WO2019080537 A1WO 2019080537A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- cancer
- virus
- oncolytic
- tumor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/15—Natural-killer [NK] cells; Natural-killer T [NKT] cells
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4224—Molecules with a "CD" designation not provided for elsewhere
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0646—Natural killers cells [NK], NKT cells
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/59—Reproductive system, e.g. uterus, ovaries, cervix or testes
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
Definitions
- the present inventionis in the field of biotechnology, and in particular, relates to therapeutic agents and applications comprising oncolytic viruses and CAR-NK cells, kits, methods of treating tumors and/or cancer.
- Chimeric Antigen Receptoris an artificially engineered receptor, so any specific receptor can be grafted onto immune effector cells.
- the extracellular specific portion of these receptors used to recognize an antigenis derived from the sequence of the antibody. Because different parts of this receptor have different origins, they are called chimeric receptors.
- CAR-modified immune cellsie, immune cells expressing the chimeric antigen receptor (CAR) are now the most effective and promising tumor cell immunotherapy products.
- NKNatural killer cells
- T cellsperipheral blood mononuclear cells
- NK cellsdo not produce autocrine growth factors such as IL-2, and they do not increase in value when they encounter specific antigens, and have a short life span. They do not need to be equipped with suicide genes. Limit CAR toxicity.
- the killing of tumors by NK cellsdoes not depend on the specific antigen presented by MHC. Therefore, in the immunotherapy application of allogeneic NK cells, immune rejection is not produced, and it is a safer immunity than T cells. Treat candidate cells.
- CARcan avoid the inhibitory signaling pathway in NK cells, while NK cells themselves can express activated receptors and can also utilize antibody-mediated cytotoxicity (ADCC). Therefore, CAR-expressing NK cells are treated in tumors. It is superior to T cells and has a broader application prospect.
- CARincludes an extracellular portion, a transmembrane region, and an intracellular portion.
- immune cells carrying CARthe selection of the extracellular and intracellular portions of CAR and its cooperation with immune cell types are extremely important, which is closely related to the specific killing ability of tumors. At present, in the immunotherapy of tumors, it is still necessary to develop a novel and effective CAR-immune cell drug.
- the way in which the virus treats cancerhas developed quite rapidly in the past two decades.
- One of the biggest advances in viral therapyis the use of tumor cells and normal cells to modify certain viral structures, allowing them to selectively replicate in tumor cells, ultimately achieving the goal of killing tumor cells.
- These modified virusesare collectively referred to as oncolytic viruses according to their functions, and are derived from adenoviruses, herpes viruses, and poxviruses. It has now been found that certain wild-type viruses also have the function of selectively replicating and inducing tumors in tumor cells.
- H101One of the oncolytic virus injections marketed in China is genetically engineered type 5 adenovirus H101, which facilitates replication of the virus in tumor cells.
- H101mainly deletes the 55KD and E3 region gene segments of the E1B region of human type 5 adenovirus, and has the characteristic of specifically replicating in tumor cells to finally cause oncolytic.
- One of the mechanismsis that the 55KD protein encoded by the E1B region of the wild-type adenovirus gene can bind to the p53 protein, thereby inhibiting the clearance of the adenovirus by the p53 gene. Since the virus cannot encode the E1B-55KD protein, the virus cannot replicate in cells normal to p53.
- the virusIn the tumor cells in which the p53 gene is mutated, the virus can be largely replicated because of the inhibition of the p53 gene. In addition, deletion of the E3 region gene fragment allows the virus to be easily recognized and cleared by the immune system (such as NK cells) in vivo, increasing the safety of clinical applications.
- the current viewis that not only the mutation of p53 itself, but also the defect of p53 pathway is beneficial to the selective replication of H101. H101 is administered by intratumoral injection and replicates in a large amount in tumor cells, eventually leading to the dissolution and death of tumor cells.
- the component of the oncolytic virus drug T-Vec approved by the US FDAis genetically engineered type 1 herpes simplex virus HSV-1.
- the ICP34.5 and ICP47 geneswere deleted in T-Vec and the human immune activator granulocyte-macrophage colony-stimulating factor (GM-CSF) gene was inserted, which replicated and expressed GM-CSF in tumor cells.
- GM-CSFhuman immune activator granulocyte-macrophage colony-stimulating factor
- Direct injection into melanoma lesionscan cause tumor cell lysis, thereby rupturing tumor cells, releasing tumor-derived antigens and GM-CSF, and accelerating the anti-tumor immune response.
- the FDA approved T-Vecas a topical treatment for unresectable lesions in patients with melanoma who relapsed after the first surgery.
- Poxvirusis a large-sized virus, and the specificity of the oncolytic virus obtained by genetic modification of wild-type poxvirus in tumor cells is greatly improved.
- some of the oncolytic pox viruseshave deleted the gene that expresses thymidine kinase (TK) in the poxvirus DNA, so that the oncolytic virus cannot replicate and proliferate in normal cells.
- TKthymidine kinase
- tumor cellssynthesize thymidine kinase for replication by oncolytic viruses, so vaccinia virus can replicate in large numbers in tumor cells.
- some of the oncolytic pox viruseshave deleted the gene expressing VGF, which enhances the specific proliferation of the virus in the tumor cells, and finally leads to the dissolution and death of the tumor cells.
- the oncolytic pox virusremoves both the TK gene and the VGF gene.
- the acne oncolytic virushas not yet been marketed.
- vaccinia virushas the advantage of administration which can be administered systemically by intravenous injection and reaching tumor targets.
- the poxvirus genomeis large and facilitates genetic modification that contributes to tumor killing.
- the present inventionprovides therapeutic agents and uses comprising oncolytic viruses and CAR-NK cells, kits, methods of treating tumors and/or cancer.
- the present inventionprovides:
- a therapeutic agentcomprising:
- a first pharmaceutical compositionwherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier;
- the oncolytic virusis capable of selectively replicating in a tumor cell
- the surface of the NK cellis modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that
- the antigen binding domainis derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- amino acid sequence of the intracellular domainis selected from amino acids 62 to 113 of DAP12; preferably, the amino acid sequence of the intracellular domain is SEQ ID NO :5 is shown.
- the second pharmaceutical compositioncomprises the NK cells in a total dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per subject per subject.
- the second pharmaceutical compositioncomprises the NK cells in a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per subject per subject.
- the therapeutic agent according to (1), wherein the oncolytic virusis selected from the group consisting of a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
- the oncolytic virusis selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis Virus, poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
- NK cellsare selected from the group consisting of autologous NK cells and allogeneic NK cells.
- NK cellsare autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
- the therapeutic agent according to (18), wherein the oncolytic effect-containing adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN- 01, and / or ProstAtak TM.
- the active ingredient of the second pharmaceutical compositioncomprises the NK cells in a total dose ranging from 1 x 10 6 to 1 x 10 11 per subject per subject.
- the active ingredient of the second pharmaceutical compositioncomprises the total dose ranging from 6 x 10 7 to 1.2 x 10 10 per NK per patient per subject.
- the tumor and/or cancercomprises lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterus Cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer The treatment is positive for NKG2D ligand and the tumor and/or cancer is treated to become positive for NKG2D ligand.
- a kit for a synergistic combination drug for treating tumors and/or cancercomprising: a first container containing an oncolytic virus and a second container containing NK cells, wherein the first container And the second container are independent; and instructions for indicating the timing and mode of administration; wherein the oncolytic virus is capable of selectively replicating in tumor cells; and wherein the surface of the NK cells is chimeric Antigen receptor modification comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, wherein the antigen binding domain is from NKG2D A ligand binding domain derived from the intracellular signaling region of DAP12.
- the second containercomprises the NK cells in a total dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per person per course of treatment.
- the second containercomprises the NK cells in a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per person per course of treatment.
- the kit according to (26), wherein the oncolytic virusis selected from the group consisting of a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
- the oncolytic virusis selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis Virus, poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
- NK cellsare selected from the group consisting of autologous NK cells and allogeneic NK cells.
- NK cellsare autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
- the tumor and/or cancerincludes lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, Uterine cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer Untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive.
- the oncolytic adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN- 01, and / or ProstAtak TM.
- the NK cellswere dosed to 1 x 10 10 cells/day.
- the second containercomprises the NK cells in a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per person per course of treatment.
- a method of treating a tumor and/or cancercomprising the steps of:
- the surface of the NK cellis modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that
- the antigen binding domainis derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- the oncolytic virusis selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis virus. , poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
- NK cellsare selected from the group consisting of autologous NK cells and allogeneic NK cells.
- NK cellsare autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
- the tumor and/or cancercomprises lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterus Cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer The treatment is positive for NKG2D ligand and the tumor and/or cancer is treated to become positive for NKG2D ligand.
- oncolytic adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS -102, ColoAd1, VCN-01, and / or ProstAtak TM.
- the inventionhas the following advantages and positive effects:
- the chimeric antigen receptor of the present inventionenables the NK cells modified by it (also referred to as "engineered NKG2D ligand-targeted NK cells”) to have strong and specific targeting to tumors positive for expression of various NKG2D ligands.
- the killing activity, the preclinical study of the present inventionhas fully demonstrated that the modified NK cells can significantly reduce or even eliminate the tumor burden in the animal and prolong the survival time of the animal.
- the engineered NKG2D ligand-targeted NK cells of the inventionprovide a new choice for treating NKG2D ligand-positive tumor patients, and have good industrial application prospects.
- the present inventionproposes for the first time the concept of combining an oncolytic virus with the chimeric antigen receptor-modified NK cells of the present invention for treating tumors and/or cancer, and the pharmaceutical composition and method provided based on the concept can be fully utilized.
- the oncolytic virusselectively replicates in tumor cells and kills tumor cells, and further causes subsequent immune responses, while also fully utilizing the function of the NK cells to kill tumor cells, and skillfully utilizing oncolytic
- the characteristic that the virus selectively replicates in tumor cellsmakes the tumor cells containing the oncolytic virus a specific target of NK cells, thereby further enhancing the tumor killing effect of the NK cells.
- oncolytic virusescan stimulate the increase of the expression of various NKG2D ligands on the surface of tumor cells, thereby further cooperating with the chimeric antigen receptor-modified NK cells of the present invention to produce stronger resistance.
- Tumor synergyThe present inventors have found that only the combination of oncolytic virus and the NK cells produces a synergistic effect.
- the oncolytic virus and the NK cellshave the characteristics of recognizing tumor cells, and basically do not cause damage to normal cells, and the combined use of the two has significant advantages in safety and efficacy.
- the present inventionthrough theoretical exploration and experimental research, enables the respective administration dose, application sequence and application interval of the oncolytic virus and the NK cell to achieve the synergistic effect of the maximum efficiency of the combined application of both, while avoiding both Mutual constraints between them to achieve effective treatment of tumors and / or cancer.
- FIG. 1is a schematic diagram showing the construction of a chimeric antigen receptor NKG2D-CD8-DAP12 according to an embodiment of the present invention, wherein “CMV” indicates a CMV promoter sequence, “T7” indicates a T7 promoter sequence, and “5'UTR” indicates a 5'UTR having a Kozak sequence, “SP” indicates a GM-CSF alpha chain signal peptide coding sequence, "NKG2D” indicates a coding sequence of a ligand binding region of NKG2D, and “CD8” indicates a hinge region and a transmembrane region coding sequence of CD8 ⁇ , “DAP12” denotes the intracellular signal region coding sequence of DAP12, “alpha globulin 3'UTR” denotes the 3'UTR of the alpha globulin having a PolyA signal, and “pA 150 " denotes polyA (polyadenylation),
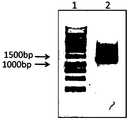
- Figure 2is an electropherogram showing the identification fragment of the expression vector pFastbac1-CD8-DAP12 digested with restriction endonucleases SphI and SalI according to one embodiment of the present invention; wherein lane 1 is a DNA molecular weight marker and lane 2 is an identification fragment .
- Figure 3is an electropherogram showing the identification fragment of the expression vector pFastbac1-NKG2D-CD8-DAP12 digested with restriction endonucleases SphI and NheI according to an embodiment of the present invention; wherein lane 1 is a DNA molecular weight marker, and lane 2 is Identify the fragment.
- Figure 4is a schematic view showing the structure of a recombinant DNA vector pFastbac1-NKG2D-CD8-DAP12 of a chimeric antigen receptor according to an embodiment of the present invention; wherein the clockwise sequence is a forward gene fragment and the counterclockwise is a reverse gene fragment .
- CMVCMV promoter sequence
- T7denotes a T7 promoter sequence
- 5'UTRdenotes a 5'UTR having a Kozak sequence
- GM-CSF ⁇denotes a GM-CSF alpha chain signal peptide coding sequence
- NKG2DThe coding sequence of the ligand binding region of NKG2D
- CD8indicates the hinge region and transmembrane region coding sequence of CD8 ⁇
- DAP12indicates the intracellular signal region coding sequence of DAP12
- alpha globulin 3'UTRindicates The 3'UTR of the alpha globulin of the PolyA signal.
- Figure 5shows the results of analysis of NK cell phenotype by flow cytometry.
- Figure 5Ashows the purity of NK cells when expanded from peripheral blood mononuclear cells for 17 days.
- the abscissaindicates the CD3 expression intensity, and the ordinate indicates the CD56 expression intensity;
- FIG. 5Bshows the expression intensity of NK cells endogenous NKG2D and CD16 when expanded from peripheral blood mononuclear cells for 17 days, the abscissa indicates the intensity of NKG2D expression, and the ordinate indicates CD16. Expression intensity.
- Figure 6shows an electropherogram of a chimeric antigen receptor NKG2D-CD8-DAP12 linearized DNA template synthesized in vitro; Lane 1 is a DNA molecular weight marker and Lane 2 is a linearized DNA template.
- Figure 7shows an electropherogram of mRNA of the chimeric antigen receptor NKG2D-CD8-DAP12 synthesized in vitro; Lane 1 is a molecular weight marker and Lane 2 is an mRNA of NKG2D-CD8-DAP12.
- Figure 8shows the results of flow cytometry detection of chimeric antigen receptor NKG2D-CD8-DAP12 mRNA electroporation NK cells.
- Figure 8Ashows the intensity of endogenous expression of NKG2D by NK cells (where the left peak is the negative control curve and the right peak is the NK cell NKG2D expression intensity curve);
- Figure 8Bshows the intensity of NKG2D expression by NK cells after electroporation (where The left peak is the negative control curve, and the right peak is the NKG2D expression intensity curve after NK cell electrotransformation of NKG2D-CD8-DAP12 mRNA;
- Figure 8Cshows the comparison of the intensity of NKG2D expression after endogenous expression and electrotransfection of NK cells ( That is, 8A and 8B are combined together) (the left peak is a negative control curve, the middle peak is the NK cell NKG2D expression intensity curve, and the right peak is the NKG2D-CD8-DAP12 mRNA expression level after NK
- the abscissaindicates the intensity of NKG2D expression, and the ordinate indicates the relative cell number.
- “NKG2D fluorescence intensity” in the abscissaindicates the fluorescence reading displayed by the flow cytometer when detected with a fluorescent NKG2D antibody.
- Figure 9is a graph showing the results of Elispot analysis of IFN- ⁇ release after mixed culture of chimeric antigen receptor NKG2D-CD8-DAP12-modified NK (NKG2D-CAR-NK) cells and human tumor cells in an embodiment of the present invention.
- mGFP-CAR-NKindicates an mGFP-CAR-modified NK cell group (control group)
- “NKG2D-CAR-NK”indicates a chimeric antigen receptor NKG2D-CD8-DAP12 (i.e., NKG2D-CAR)-modified NK.
- the abscissaindicates the tumor cell group
- the ordinateindicates the relative amount of IFN- ⁇ (expressed as the number of spots per 2.5 ⁇ 10 4 NK cells).
- FIGS. 10A-Gshow NKG2D-CAR-NK versus tumor cells HCT116(A), SKOV3(B), Fadu(C), Detroit(D), HepG2(E), MCF7(F), respectively, according to an embodiment of the present invention
- Figure 11shows the killing effect of adoptively NKG2D-CAR-NK cells on tumor cells; the dotted line shows the results of the chimeric antigen receptor NKG2D-CD8-DAP12 modified NK cell group (shown as "NKG2D-CAR-NK” (6 injections)"); the solid line shows the results of the NK cell reinfusion group (shown as "PBS control group”).
- the abscissaindicates the number of days after inoculation of the tumor
- the ordinateindicates the fluorescence intensity of the tumor cells in the animal recorded by the living imager
- the radiance shown in the ordinaterefers to the number of photons emitted from the surface of the animal per unit time, unit area, and unit radians.
- Figure 12A-Cshows chimeric antigen receptor NKG2D-CD8-DAP12 modified NK cells and chimeric antigen receptor NKG2D-CD8-CD3Z modified NK cells, respectively, for tumor cells SKOV3 (A), Detroit (B), Comparison of the detection results of the killing activity of HCT116(C).
- mGFP-CAR-NKindicates an mGFP-CAR-modified NK cell group
- NKG2D-DAP12-CAR-NKindicates an NKG2D-DAP12-CAR-modified NK cell group
- “NKG2D-CD3Z-CAR-NK”indicates NKG2D-CD3Z-CAR modified NK cell group.
- the abscissaindicates the ratio of effector cells to tumor cells, and the ordinate indicates the proportion of tumor cells that are lysed after killing.
- Figure 13shows the results of flow cytometry detection of the H101-treated tumor cell line Fadu surface NKG2D ligand.
- the black column in the figurerepresents tumor cells without H101 treatment
- the punctate columnrepresents H101-treated tumor cells
- the abscissarepresents different NKG2D ligand staining groups (from left to right, hULBP1, hULBP3, hULBP4, hULBP2/5) /6, MICA/B), the ordinate is the percentage of cells expressing the NKG2D ligand.
- Figure 14shows the results of flow cytometry detection of H101G-treated tumor cell line HepG2 surface NKG2D ligand.
- the black column in the figurerepresents tumor cells without H101 treatment
- the punctate columnrepresents H101-treated tumor cells
- the abscissarepresents different NKG2D ligand staining groups (from left to right, hULBP1, hULBP3, hULBP4, hULBP2/5) /6, MICA/B), the ordinate is the percentage of cells expressing the NKG2D ligand.
- Figure 15is a graph showing the results of detection of the killing activity of the chimeric antigen receptor NKG2D-CD8-DAP12-modified NK cells against tumor cell Fadu.
- the black columnrepresents the tumor cells without H101 treatment
- the dotted pattern columnrepresents the tumor cells after H101 treatment
- "w/o CAR-NK”means that no CAR-NK cells are added, but NK cells are added
- CAR-NKIndicates NK cells supplemented with NKG2D-DAP12-CAR modification.
- the abscissaindicates different experimental groups, and the ordinate indicates the ratio of lysis of tumor cells after killing, and the ratio of effector cells to target cells is 1:1.
- CARincludes an extracellular portion, a transmembrane region, and an intracellular portion.
- the extracellular portionin turn includes an antigen binding domain for recognizing and binding an antigen, and a spacer for spacing the antigen binding domain and the transmembrane region;
- the intracellular portionprimarily includes an intracellular domain for signaling.
- the inventors of the present inventionhave selected a specific combination of an antigen-binding domain and an intracellular domain for NK cells through theoretical research and experimental exploration, and successfully applied the thus developed CAR to NK cells to make the NK cells play.
- a strong targeted tumoricidal activitythereby developing a novel and effective engineered NKG2D ligand-targeted NK cell that is available for selection in tumor immunotherapy.
- the inventionprovides a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane region and an intracellular domain, Characterized in that the antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- NKG2Dis an important receptor regulating NK cell killing activity.
- NKG2D ligandis mainly expressed on the surface of tumor cells and stress cells, and is rarely expressed or even expressed on the surface of normal cells.
- a large number of tumor cellssuch as colorectal cancer cells and ovarian cancer Cells, head and neck cancer cells, lymphatic cancer cells, glioma cells, and the like have a large number of ligands for NKG2D expression.
- the amino acid sequence of the antigen-binding domainis preferably identical to the X-position 216 amino acid sequence of NKG2D, and 73 ⁇ X ⁇ 83, and X is an integer.
- the amino acid sequence of NKG2Dmay be the amino acid sequence of NP_031386.2 in Genbank of NCBI (ie, National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov). That is, the amino acid sequence of the antigen-binding domain is preferably selected from amino acids 73-216 of NKG2D and comprises amino acids 83-216.
- amino acid sequence of the antigen-binding domainis as shown in any one of the following amino acid sequence groups: amino acids 73-216 of NKG2D, amino acids 74-216, amino acids 75-216, 76- 216 amino acids, amino acids 77-216, amino acids 78-216, amino acids 79-216, amino acids 80-216, amino acids 81-216, amino acids 82-216, or 83- 216 amino acids. More preferably, the amino acid sequence of the antigen binding domain is set forth in SEQ ID NO: 3.
- the intracellular domainacts as a signal to activate NK cells.
- the intracellular domain of the CAR originally used for T cellshas only one signaling molecule, usually the receptor-associated Fc ⁇ RI ⁇ of immunoglobulin E (a subunit of a receptor with high affinity for IgE) or T cell antigen receptor signaling.
- the underlying conductive molecule DAP12some intracellular domains comprise a T cell activation domain consisting of one or several T cell activation motifs.
- the present inventorshave found that by combining the antigen-binding domain derived from the ligand binding region of NKG2D described above with the intracellular signal region derived from DAP12, a CAR capable of exerting a strong targeted tumoricidal activity of NK cells can be obtained.
- the amino acid sequence of the intracellular domainis selected from amino acids 62-113 of DAP12, and the amino acid sequence of the intracellular domain is more preferably as set forth in SEQ ID NO: 5.
- the amino acid sequence of DAP12has the Genbank number NP_003323.1.
- the present inventionfurther selects the spacer and transmembrane regions, thereby obtaining a CAR having a specific combination of antigen binding domain-spacer-transmembrane region-intracellular domain.
- the spacer junctionrecognizes and binds to the antigen binding domain and transmembrane region of the antigen.
- the structure of this regionshould be flexible so that the antigen binding domain can be adapted to different orientations to facilitate antigen recognition and binding.
- the simplest form of spacer regionis an immunoglobulin hinge region of IgGl (hinge), it may be a portion of an immunoglobulin C H2 C H3 region.
- the transmembrane regionis typically a hydrophobic alpha helix spanning the cell membrane.
- the spaceris preferably derived from the hinge region of CD8[alpha], which is preferably from the transmembrane region of CD8[alpha].
- CD8is a transmembrane glycosylated membrane protein consisting of two subunits, alpha and beta, which interact with T cell surface receptors to bind T cells to specific antigens. CD8 specifically binds to MHC I and mediates cytotoxic T cells. The killing effect.
- the spacer region and the transmembrane regionconstitute a spacer transmembrane region, and wherein the amino acid sequence of the spacer transmembrane region is identical to the amino acid sequence of the Yth to the 210th position of CD8 ⁇ , and 118 ⁇ Y ⁇ 128, Y is an integer.
- the Genbank number of the amino acid sequence of CD8 ⁇may be NP_001139345.1. That is, the amino acid sequence of the spacer transmembrane region is preferably selected from amino acids 118-210 of CD8 ⁇ and amino acids 128-210.
- amino acid sequence of the spacer transmembrane regionis as shown in any one of the following amino acid sequence groups: amino acids 118-210 of CD8 ⁇ , amino acids 119-210, amino acids 120-210, 121- 210 amino acids, amino acids 122-210, amino acids 123-210, amino acids 124-210, amino acids 125-210, amino acids 126-210, amino acids 127-210, or 128- 210 amino acids.
- amino acid sequence of the spacer transmembrane regionis set forth in SEQ ID NO:4.
- the antigen-binding domain, the spacer, the transmembrane region and the intracellular domainare sequentially connected in series; between the antigen-binding domain and the spacer, between the spacer and the transmembrane region
- the transmembrane region and the intracellular domainare operably linked, for example, may be connected by a joint or directly without a joint.
- a linkeris used between the antigen binding domain and the spacer (the linker is, for example, -Ala-Ser-), and the spacer and transmembrane region, the transmembrane region and the cell
- the internal domainsare directly connected without a joint.
- the amino acid sequence of the chimeric antigen receptoris set forth in SEQ ID NO: 1.
- the chimeric antigen receptorhas an amino acid sequence obtained by replacing, deleting, and/or adding one or more amino acids in the amino acid sequence shown in SEQ ID NO: 1;
- the chimeric antigen receptorhas at least 90%, preferably at least 95%, more preferably at least 99% identity to the amino acid sequence set forth in SEQ ID NO: 1.
- the inventionalso provides an isolated DNA encoding a chimeric antigen receptor of the invention, the DNA comprising an operably linked, sequentially tandem antigen binding domain coding element, a spacer coding element, a transmembrane a region coding element and an intracellular domain coding element, wherein the antigen binding domain coding element is derived from a ligand binding region encoding DNA of NKG2D, and the intracellular domain coding element is derived from the intracellular signal region encoding DNA of DAP12 .
- the nucleotide sequence of the antigen-binding domain coding elementencodes an amino acid sequence of the antigen-binding domain, preferably, the amino acid sequence of the antigen-binding domain is identical to the X-position 216 amino acid sequence of NKG2D, And 73 ⁇ X ⁇ 83, and X is an integer.
- amino acid sequence of the antigen-binding domainis as shown in any one of the following amino acid sequence groups: amino acids 73-216 of NKG2D, amino acids 74-216, amino acids 75-216, 76- 216 amino acids, amino acids 77-216, amino acids 78-216, amino acids 79-216, amino acids 80-216, amino acids 81-216, amino acids 82-216, or 83- 216 amino acids.
- amino acids 73-216 of NKG2Damino acids 74-216, amino acids 75-216, 76- 216 amino acids, amino acids 77-216, amino acids 78-216, amino acids 79-216, amino acids 80-216, amino acids 81-216, amino acids 82-216, or 83- 216 amino acids.
- amino acids 73-216 of NKG2Damino acids 74-216
- amino acids 75-216amino acids 77-216
- amino acids 78-216amino acids 79-216
- amino acids 80-216amino acids 81-216
- the nucleotide sequence of the intracellular domain coding elementencodes the amino acid sequence of the intracellular domain, preferably, the amino acid sequence of the intracellular domain is selected from positions 62-113 of the intracellular signal region of DAP12 Amino acid.
- the nucleotide sequence of the intracellular domain coding elementis set forth in SEQ ID NO: 8.
- the spacer coding elementis derived from the hinge region encoding DNA of CD8 ⁇
- the transmembrane region coding elementis derived from the transmembrane region encoding DNA of CD8 ⁇ .
- the spacer coding element and the transmembrane region coding elementcomprise a spacer transmembrane region coding element, the nucleotide sequence of the spacer transmembrane region coding element encoding an amino acid sequence of the spacer transmembrane region, preferably, The amino acid sequence of the spacer transmembrane region is identical to the amino acid sequence of the Yth position to the 210th position of CD8 ⁇ , and 118 ⁇ Y ⁇ 128, and Y is an integer.
- amino acid sequence of the spacer transmembrane regionis as shown in any one of the following amino acid sequence groups: amino acids 118-210 of CD8 ⁇ , amino acids 119-210, amino acids 120-210, 121- 210 amino acids, amino acids 122-210, amino acids 123-210, amino acids 124-210, amino acids 125-210, amino acids 126-210, amino acids 127-210, or 128- 210 amino acids.
- amino acids 118-210 of CD8 ⁇amino acids 119-210
- amino acids 120-210, 121- 210 amino acids, amino acids 122-210, amino acids 123-210, amino acids 124-210, amino acids 125-210, amino acids 126-210, amino acids 127-210, or 128- 210 amino acidsamino acids 118-210 of CD8 ⁇
- amino acids 119-210amino acids 120-210, 121- 210 amino acids
- amino acids 122-210amino acids 123-210
- amino acids 124-210amino acids 125-210
- the isolated nucleotide sequence encoding the chimeric antigen receptor of the inventionis represented by SEQ ID NO: 2.
- the NKG2D, DAP12 and CD8 ⁇ of the present inventionare preferably derived from humans, and their full-length amino acid sequences and nucleotide sequences are known, and can be inquired from public databases commonly used in the art.
- the present inventionalso provides an isolated mRNA which is transcribed from the DNA encoding the chimeric antigen receptor of the present invention.
- the inventionalso provides a recombinant expression vector comprising a DNA encoding a chimeric antigen receptor according to the invention operably linked to a promoter.
- the recombinant expression vectorcomprises a CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, and a GM-CSF alpha chain signal peptide coding sequence in sequence before the DNA encoding the chimeric antigen receptor according to the present invention. And comprising a 3'UTR of an alpha globulin having a polyA signal following the DNA encoding the chimeric antigen receptor according to the invention.
- the combination of the above-described functional elements of the recombinant expression vector of the present inventioncan promote transcription and translation of DNA and enhance the stability of mRNA.
- the present inventionalso optimizes the structure of each of the above-described action elements to better perform their intended functions.
- CMV promoter sequenceTAGTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAGTTCCGCGTTACATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCCCATTGACGTCAATAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTCCATTGACGTCAATGGGTGGAGTATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATATGCCAAGTACGCCCTATTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGTACATGACCTTATGGGACTTTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTACCATGGTGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAGTCCACCCCATTGACGTCAATGGGAGTTTGTTTGTTTTGGCACCAAAATCAACGGGAC TTTCCAAAATGGAC TTGTTTGTTTTGGCACCAAAATCAA
- the T7 promoter sequenceis TAATACGACTCACTATAG (SEQ ID NO: 17).
- the T7 promoterfunctions to initiate transcription of downstream DNA sequences.
- the sequence of the 5' UTR having the kozak sequenceis AAATAAGAGAGAAAAGAAGAGTAAGAAGAAATATAAGA GCCA CCATG (SEQ ID NO: 18), wherein the sequence underlined is the kozak sequence.
- the function of the 5'UTR with the kozak sequenceis to enhance the translation efficiency of the mRNA.
- the sequence of the GM-CSF alpha chain signal peptide coding sequenceis ATGCTTCTCCTGGTGACAAGCCTTCTGCTCTGTGAGTTACCACACCCAGCATTCCTCCTGATCCCA (SEQ ID NO: 19), and the amino acid sequence thus obtained is MLLLVTSLLLCELPHPAFLLIP (SEQ ID NO: 20).
- the GM-CSF alpha chain signal peptideis a leader sequence that targets the CAR of the present invention to the secretory pathway, and the coding sequence is first translated into a protein together with the CAR in the cell, directing the synthesized protein into the endocrine pathway. The signal peptide has been removed before expression of the CAR on the cell surface.
- the 3'UTR sequence of the alpha globulinis GCTGCCTTCTGCGGGGCTTGCCTTCTGGCCATGCCCTTCTTCTCTCCCTTGCACCTGTACCTCTTGGTCTTTG AATAAA GCCTGAGTAGGAAGT (SEQ ID NO: 21), wherein the sequence underlined is a polyA signal. Its role is to enhance the stability of mRNA.
- the basic backbone of the recombinant expression vectoris a commercially available pFastbac1 vector into which each of the above elements is inserted.
- a DNA double-stranded template in which a positive strand carries a PolyA and an inverted strand carries a corresponding PolyTcan be synthesized from the recombinant expression vector, for example, using a Tail-PCR technique, such that The instability of the DNA template is reduced, and mRNA can be synthesized in vitro.
- the range of the number of A in the PolyA carried in the positive chainis 140-170, preferably 150-170, more preferably It is about 150 (for example, 150).
- the present inventionalso provides a chimeric antigen receptor-modified NK cell whose surface is modified by the chimeric antigen receptor of the present invention.
- modificationmeans that an NK cell expresses a chimeric antigen receptor according to the present invention, that is, a transmembrane region of the chimeric antigen receptor is anchored to a cell membrane of the modified NK cell, The antigen binding domain is located on the cell surface and the intracellular domain is located in the cytoplasm.
- the NK cellsmay be various types of NK cells known and can be obtained by conventional biological methods.
- NK cellsnaturally killer cells
- the phenotypeis CD3 negative CD56.
- Positive single cellsmainly CD16-negative CD56 bright (light) and CD16-positive CD56 dim (dark) two subtypes, respectively, have immunomodulatory and tumor killing in vivo efficacy. Since NK cell action is non-MHC-restricted, there is no need to match the histocompatibility complex of the individual patient in use, that is, NK cells can be used for cell therapy of allogeneic patients, and have wide clinical application value.
- the inventionalso provides a method of preparing a chimeric antigen receptor-modified NK cell according to the invention, comprising the steps of:
- the NK cells described in step 1)can be prepared from peripheral blood mononuclear cells.
- the purity of the NK cells in the method of the inventionmay be > 70%, preferably > 80%.
- NK cell purityrefers to the proportion of NK cells in the total cell population.
- the nucleic acid according to the step 2)is a DNA encoding the chimeric antigen receptor according to the present invention, or an mRNA obtained by transcription of the DNA.
- the transfection described in step 3)can be carried out by cryoelectroporation techniques or lentiviral vectors.
- Transfection using cryoelectroporation techniquescan be carried out in a manner commonly used in the art, such as the literature "Nakazawa Y, Matsuda K, Kurata T, Sueki A, Tanaka M, Sakashita K, Imai C, Wilson MH, Koike K.
- Anti- Proliferative effects of T cells expressing a ligand-based chimeric antigen receptor against CD116 on CD34(+) cells of juvenile myelomonocytic leukemiaJ Hematol Oncol. 2016 Mar.
- Transfection using lentiviral vectorscan be carried out in a manner commonly used in the art, such as the literature "James N. Kochenderfer, Steven A. Feldman, Yangbing Zhao, Hui Xu, Mary A.
- the DNA corresponding to amino acids 83-216 of the human NKG2D protein, the DNA corresponding to amino acids 128-210 of human CD8 ⁇ , and the 62nd of human DAP12are amplified by PCR from the PBMC cDNA library. DNA corresponding to -113 amino acids.
- the three sequences amplifiedwere ligated and ligated to the pFastbac1 vector by molecular cloning techniques to obtain a recombinant expression vector pFastbac1-NKG2D-CD8-DAP12.
- the mRNA of the corresponding sequence of NKG2D-CD8-DAP12was then synthesized.
- the mRNAwas electroporated into the NK cells expanded in vitro by high-efficiency cryoelectroporation to obtain engineered NKG2D ligand-targeted NK cells.
- Each portion of the chimeric antigen receptorcan also be amplified by genomic cDNA of mononuclear cells in venous blood.
- the inventionalso provides the use of a chimeric antigen receptor-modified NK cell according to the invention for the preparation of a medicament for the treatment or prevention of a tumor and/or cancer.
- the tumor and/or canceris positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like.
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the inventionalso provides the use of a chimeric antigen receptor-modified NK cell according to the invention for the preparation of a medicament for detecting a tumor and/or cancer of a host.
- the tumor and/or canceris positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like.
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- a sample of a tumor and/or a cancer cell taken out from a hostmay be contacted with a chimeric antigen receptor-modified NK cell of the present invention at a concentration according to the degree of reaction between the two. It can be judged whether the tumor and/or cancer is NKG2D ligand positive or NKG2D ligand negative.
- the present inventionalso provides a pharmaceutical composition
- a pharmaceutical compositioncomprising the chimeric antigen receptor-modified NK cell according to the present invention as an active ingredient, and a pharmaceutically acceptable adjuvant.
- the pharmaceutical compositionpreferably comprises the chimeric antigen receptor-modified NK cells in a total dose ranging from 1 x 10 6 to 1 x 10 11 per subject per subject; preferably, each treatment lasts for 3 weeks. Days, apply 1-2 times a week.
- the pharmaceutical compositionfurther preferably comprises the chimeric antigen receptor-modified NK cells in a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per subject per treatment; and preferably, each course of treatment is 3 weeks A total of 21 days, 1-2 times a week.
- the patientmay be treated for one or more courses depending on the actual situation and needs.
- the pharmaceutical compositioncan be administered by a suitable route of administration including intravenous administration (for example, intravenous drip administration or intravenous administration) or topical administration (for example, topical administration or local injection). Administration).
- intravenous administrationfor example, intravenous drip administration or intravenous administration
- topical administrationfor example, topical administration or local injection. Administration.
- the inventionalso provides a method of treating a tumor and/or cancer comprising administering to a tumor and/or cancer patient a chimeric antigen receptor modified NK cell according to the invention.
- the tumor and/or canceris positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like.
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the chimeric antigen receptor-modified NK cellsare preferably administered at a dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 cells per patient per course of treatment; preferably, each treatment lasts for 3 weeks for 21 days. Apply 1-2 times a week.
- the dose of the chimeric antigen receptor-modified NK cellsis further preferably a total dose per patient per treatment range of 6 ⁇ 10 7 to 1.2 ⁇ 10 10 cells; and it is also preferred that each treatment has a total of 21 weeks. Days, apply 1-2 times a week.
- the patientmay be treated for one or more courses depending on the actual situation and needs.
- the chimeric antigen receptor-modified NK cellscan be administered by a suitable administration route, such as intravenous administration (for example, intravenous drip administration or intravenous administration) or topical administration (for example, topical drip). Injection or local injection).
- a suitable administration routesuch as intravenous administration (for example, intravenous drip administration or intravenous administration) or topical administration (for example, topical drip). Injection or local injection).
- the inventionalso provides a tool vector comprising, in turn, an operably linked CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, a GM-CSF alpha chain signal peptide coding sequence, and a polyA signal.
- a tool vectorcomprising, in turn, an operably linked CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, a GM-CSF alpha chain signal peptide coding sequence, and a polyA signal.
- the 3'UTR of alpha globulincomprising, in turn, an operably linked CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, a GM-CSF alpha chain signal peptide coding sequence, and a polyA signal.
- tool vectorrefers to an empty vector for insertion of an exogenous DNA fragment in genetic engineering applications.
- the foreign DNA fragmentWhen the foreign DNA fragment is inserted, the foreign DNA fragment is inserted between the GM-CSF ⁇ chain signal peptide coding sequence of the tool vector and the 3'UTR of the ⁇ -globulin having a polyA signal (GM-CSF alpha chain signal peptide coding The sequence may have a multiple cloning site between the 3' UTR of the alpha globulin with a polyA signal).
- the nucleotide sequence of the CMV promoteris set forth in SEQ ID NO: 22, and the nucleotide sequence of the T7 promoter is set forth in SEQ ID NO: 17, the core of the 5'UTR having the kozak sequence.
- the nucleotide sequence of the GM-CSF alpha chain signal peptide coding sequenceis set forth in SEQ ID NO: 19, and the 3'UTR of the alpha globulin having a PolyA signal is shown in SEQ ID NO: 18.
- the nucleotide sequenceis shown in SEQ ID NO:21.
- the tool vector of the present inventionis capable of promoting transcription and translation of the inserted DNA and enhancing the stability of the mRNA.
- the inventors of the present inventionhave also proposed a novel combination therapy based on the above-described chimeric antigen receptor-modified NK cells in combination with systematic thinking.
- the human bodyis a complex system consisting of ten systems, including breathing, circulation, and digestion. These systems work together to make various complex life activities in the human body work normally. Systematic thinking is to conduct a comprehensive investigation of the interaction and interaction of drug effects, diseases, systems and humans from a holistic perspective.
- cytotoxic anti-tumor drugswhen combined with chemotherapy to treat tumors, patients' long-term survival results are not satisfactory, the reason is the lack of systematic thinking.
- the bodywhen a tumor occurs, the body can exert anti-tumor effects through various immune effect mechanisms, and the body's anti-tumor mechanism includes both cellular immunity and humoral immunity. They are closely related and interact with each other and involve a variety of immune effector molecules and effector cells. It is generally believed that cellular immunity plays a leading role in the anti-tumor process, and humoral immunity plays a synergistic role in some cases.
- traditional chemotherapymainly interferes with RNA or DNA synthesis and mitosis. It is mainly for fast-growing cells. It also attacks the normal immune system of the human body while removing tumor cells. As the body's immunity is destroyed, the tumor cells are bound to rise. ".
- the present inventionbelieves that other methods for improving the body immunity can be adopted, and various treatment means can be combined by system to maximize the comprehensive therapeutic effect while minimizing the damage to the immune system.
- the present inventionproposes a novel combination therapy for the combination of an oncolytic virus with a chimeric antigen receptor modified NK cell of the invention for the treatment of tumors and/or cancer.
- the present inventioncan produce a synergistic effect only by combining an oncolytic virus with the NK cells.
- the present inventionprovides a therapeutic agent comprising:
- a first pharmaceutical compositionwherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier;
- the oncolytic virusis capable of selectively replicating in a tumor cell
- the surface of the NK cellis modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that
- the antigen binding domainis derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- the therapeutic agentcan also be understood as a combination of drugs.
- the active ingredient of the first pharmaceutical compositionis the oncolytic virus, and wherein the active ingredient of the second pharmaceutical composition is the NK cell.
- the first pharmaceutical compositioncomprises a therapeutically effective amount of the oncolytic virus, and wherein said second pharmaceutical composition comprises 1 ⁇ 10 5 to 1 ⁇ 10 10 cells / day dose NK cells (for example, the second pharmaceutical composition comprises 1 x 10 7 to 1 x 10 10 cells/day of the NK cells; the second pharmaceutical composition comprises 1 x 10 8 to 5 x 10 9 Cell/day dose of said NK cells; said second pharmaceutical composition comprising 1 x 109 to 4 x 109 cells per day of said NK cells; said second pharmaceutical composition comprising 1 x 10 9 to 3 x 10 9 cells/day of the NK cells).
- the second pharmaceutical compositioncomprises the NK cells in a total dose ranging from 1 x 10 6 to 1 x 10 11 per subject per subject. Still preferably, the second pharmaceutical composition comprises the NK cells in a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per subject per subject.
- the present inventionalso provides a pharmaceutical composition, wherein the active ingredient of the pharmaceutical composition comprises an oncolytic virus and an NK cell, the oncolytic virus being capable of selectively replicating in a tumor cell, the surface of the NK cell being embedded Modification of an antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, wherein the antigen binding domain is derived from The ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- the active ingredient of the pharmaceutical compositionconsists of the oncolytic virus and the NK cells.
- the oncolytic virus and the NK cellsare each independently present in the pharmaceutical composition without mixing with each other.
- the oncolytic viruscontacts the tumor cells by intratumoral or intravenous administration and the infection enters the tumor cells. Since the oncolytic virus is characterized in that it mainly replicates and proliferates in tumor cells, but does not replicate or replicate in normal cells, a large number of oncolytic viruses appear in infected tumor cells, causing tumor cell lysis and death. . The dissolution of tumor cells releases a large number of tumor antigens and proliferating oncolytic viruses. The antigen further activates the immune system in the body, stimulating NK cells and T cells in the body to continue to attack tumor cells that have not yet died, and the new oncolytic virus will Continue to infect tumor cells that have not yet been infected.
- NK cellsare broad-spectrum immune cells that kill tumor cells, and NK cells can distinguish between tumor cells and normal cells. NK contacts and recognizes tumor cells, recognizes it as an abnormal cell, and then kills it through receptor recognition, antibody-targeted recognition (ADCC), granzyme secretion, perforin secretion, and indirect killing of interferon. The effect of dead tumor cells. In vitro experiments have shown that a healthy NK cell can kill 27 tumor cells in a row during its lifetime.
- ADCCantibody-targeted recognition
- NK cellsalso have antiviral functions.
- a normal cellis infected with a virus, as the virus replicates a lot, the cell exhibits an aging lesion, and the composition of the protein cluster reflected on the cell membrane changes.
- the NK cellcan recognize the infected patient sharply and efficiently.
- the cellsby the means described above similar to killing tumor cells, kill the infected cells, thereby achieving the purpose of inhibiting viral replication and proliferation. Subsequently, under the action of factors such as antigen stimulation and interferon, other immune cells will continue to act against the virus.
- the present inventiontakes into consideration the respective characteristics of oncolytic viruses and NK cells, and uses them in combination.
- the antiviral mechanism of NK cellsis equally applicable to tumor cells infected with oncolytic viruses, and is complementary to its anti-tumor mechanism.
- the combinationalso makes tumor cells containing oncolytic virus a specific target of NK cells, thereby enhancing the tumor killing effect of NK cells.
- the oncolytic virusselectively proliferates in cancer cells, plays a role in killing cancer cells in the cell, and can cause changes in protein receptor clusters on the cancer cell membrane, enhancing the recognition of cancer cells by NK cells, and NK cells outside the cancer cells. Attack, the two together to kill cancer cells, have a better therapeutic effect.
- oncolytic virusescan stimulate the increase of the expression of various NKG2D ligands on the surface of tumor cells, thereby further cooperating with the chimeric antigen receptor-modified NK cells of the present invention to produce stronger anti-tumor synergy. effect.
- the wild type virus alonehas a mutually restrictive effect on NK cells.
- the viruscan escape the antiviral killing of NK by stimulating the KIR receptor on the surface of NK, thereby evading the activity of NK;
- NKnot only recognizes and kills the cells infected by the virus, but also inhibits the proliferation of the virus. It can also directly secrete interferon and inhibit viral activity.
- many oncolytic virusesare genetically modified, on the one hand, the specificity of infected tumor cells can be enhanced, and on the other hand, the inhibition of immune cells including NK cells is reduced.
- the oncolytic virus of the present inventionincludes a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
- the gene-mutated virus having oncolytic effectincludes, but is not limited to, an adenovirus, a poxvirus (also known as vaccinia virus), herpes simplex virus (HSV), Measles virus, Semliki Forest virus, vesicular stomatitis virus, poliovirus, and retrovirus; Wild-type viruses that function include (but are not limited to): reovirus, vesicular stomatitis virus, poliovirus, Seneca Valley Virus, Eco type Echo enterovirus, Coxsackie virus, Newcastle disease virus, and maraba virus.
- the exogenous genemay be integrated into the genome of the oncolytic virus, and the exogenous gene includes an exogenous immunoregulatory gene, an exogenously screened gene, an exogenous reporter gene, and the like.
- the foreign genemay also not be integrated into the genome of the oncolytic virus.
- the adenovirusincludes, but is not limited to, a human type 5 adenovirus or a human chimeric adenovirus; specifically including, for example: Onyx-015 (available from Onyx Pharmaceuticals), H101 (available from Shanghai three-dimensional organism) Technology Co., Ltd.), Ad5-yCD/mutTKSR39rep-hIL12 (available from Henry Ford Health System), CG0070 (available from Cold Genesys), DNX-2401 (available from DNAtrix), OBP-301 (available) From Oncolys BioPharma), ONCOS-102 (available from Targovax Oy/Oncos Therapeutics), ColoAd1 (available from PsiOxus Therapeutics), VCN-01 (available from VCN Biosciences), ProstAtak TM (available from Advantagene company) and so on.
- Onyx-015available from Onyx Pharmaceuticals
- H101available from Shanghai three-dimensional organism) Technology Co., Ltd.
- the adenovirusis H101.
- the poxvirusmay be Wyeth strain, WR strain, Listeria strain or Copenhagen strain.
- the poxvirusmay be functionally deficient in the TK gene, functionally deficient in the VGF gene, and functionally deficient in the TK gene and the VGF gene.
- the poxvirusmay also contain other genetic defects, including but not limited to: HA, F14.5L, F4L.
- the poxvirusis functionally deficient in the TK gene and the VGF gene.
- the poxvirusincludes, but is not limited to, Pexa-vac (available from Jennerex Biotherapeutics), JX-963 (available from Jennerex Biotherapeutics), JX-929 (available from Jennerex Biotherapeutics), VSC20 (preparation)
- Pexa-vacavailable from Jennerex Biotherapeutics
- JX-963available from Jennerex Biotherapeutics
- JX-929available from Jennerex Biotherapeutics
- VSC20preparation
- the methodcan be found in the scientific literature: "McCart, JA, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res (2001) 61: 8751-8757.”
- GL- ONC1available from Genelux
- TG6002available from Transgene
- the herpes simplex virusincludes, but is not limited to, HSV-1, HSV-2 herpes simplex virus; specifically including (for example): (available from Amgen), G207 (available from Medigene), HF10 (available from Takara Bio), Seprehvir (available from Virttu Biologics), OrienX010 (available from Beijing Aoyuan and Lili Bio) , NV1020 (available from Catherax) and so on.
- the NK cells of the present inventioninclude autologous NK cells and allogeneic NK cells.
- the NK cellsmay be NK cells obtained by in vitro expansion. Large-scale in vitro expansion culture techniques for NK cells are known and have been largely mature (see, for example, the following scientific literature: "Somanchi SS, Lee DA. Ex Vivo Expansion of Human NK Cells Using K562 Engineered to Express Membrane Bound IL21 .Methods Mol Biol.2016;1441:175-93.” or "Phan MT, Lee SH, Kim SK, Cho D. Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells. Methods Mol Biol. 2016;1441:167-74 .”). Clinical data confirmed that autologous NK cells, haploidentical NK cells (which belong to allogeneic NK cells), and umbilical cord blood were not toxic and side effects after NK cells were returned to humans, and were not long-term dependent, safe and
- the purity of the NK cells which can be used for treatmentmay be: the purity of the autologous NK cells may be 85% or more, and the purity of the allogeneic NK cells may be 90% or more; wherein the proportion of CD3 positive T cells in the allogeneic NK cells is not more than 5 ⁇ 10 5 /kg body weight.
- the present inventionfurther explores optimization of the respective administration dose, administration sequence and administration interval of the oncolytic virus and NK cells, which are critical, which determine the oncolytic virus Anti-tumor efficacy, anti-tumor efficacy of NK cells, and optimal synergistic killing of both tumor cells.
- the pharmaceutical composition or therapeutic agentcomprises a therapeutically effective amount of the oncolytic virus, and the pharmaceutical composition or therapeutic agent comprises a dose of 1 x 10 5 to 1 x 10 10 cells per day.
- Said NK cellsfor example, said pharmaceutical composition or therapeutic agent comprises 1 x 107 to 1 x 10 10 cells/day of said NK cells; said pharmaceutical composition or therapeutic agent comprises 1 x 10 8 to 5 ⁇ 10 9 cells/day of the NK cells; the pharmaceutical composition or therapeutic agent comprising 1 x 10 9 to 4 x 10 9 cells/day of the NK cells; the pharmaceutical composition or treatment
- the agentcomprises 1 x 10 9 to 3 x 10 9 cells/day of the NK cells).
- the pharmaceutical composition or therapeutic agentcomprises a therapeutically effective amount of the oncolytic virus, and the pharmaceutical composition or therapeutic agent comprises a total dose ranging from 1 x 10 6 to 1 x 10 per person per course of treatment. Eleven of said NK cells (for example, said pharmaceutical composition or therapeutic agent comprises a total dose ranging from 6 x 10 7 to 1.2 x 10 10 per NK per subject per patient).
- different preferred clinical dosage rangescan be employed, such as those described in Table 1.
- the oncolytic virusescan be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
- the NK cellscan be administered by administration generally employed in the art, for example, intravenously or topically.
- the oncolytic virus contained in the pharmaceutical composition or therapeutic agent of the present inventionis an oncolytic virus (hereinafter also referred to as "oncolytic adenovirus").
- oncolytic adenovirusan oncolytic virus
- the E1 region and/or E3 region of the oncolytic adenovirusis genetically engineered.
- the oncolytic adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtak TM .
- the active ingredients of the pharmaceutical composition or therapeutic agent of the present inventioninclude 5 ⁇ 10 7 to 5 ⁇ 10 12 VP / day dose of the oncolytic adenovirus (e.g., 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP/day dose of oncolytic adenovirus, 5 ⁇ 10 8 to 1 ⁇ 10 12 VP/day dose of oncolytic adenovirus, 1 ⁇ 10 9 to 5 ⁇ 10 11 VP/day dose of oncolytic adenovirus, 3 ⁇ 10 10 to 3 ⁇ 10 11 VP/day dose of oncolytic adenovirus, etc.) and 1 ⁇ 10 5 to 1 ⁇ 10 10 cells/day of the NK cells (for example, 1 ⁇ 10 7 to 1 ⁇ 10 10 Cell/day dose of said NK cells, 1 x 10 8 to 5 x 10 9 cells/day of said NK cells, 1 x 10 9 to 4 x 10 9 cells/day of said NK cells , 1 ⁇ 10 9 to 3 ⁇ 10 9 cells/day
- ⁇ 10 9 to 5 ⁇ 10 11 VP / day dose of an oncolytic adenovirus3 ⁇ 10 10 to 3 ⁇ 10 11 VP / day dose of the oncolytic adenovirus, etc.
- 1 ⁇ 10 5 1 ⁇ 10 10 cells / day dose of the NK cellse.g., the 1 ⁇ 10 7 to 1 ⁇ 10 10 cells / day dose NK cells, 1 ⁇ 10 8 to 5 ⁇ 10 9 cells / day
- the doseconsists of the NK cells, 1 x 10 9 to 4 x 10 9 cells/day of the NK cells, 1 x 10 9 to 3 x 10 9 cells/day of the NK cells, and the like.
- the active ingredients of the pharmaceutical composition or the therapeutic agent of the present inventioninclude 5 ⁇ 10 7 to 5 ⁇ 10 12 VP / day dose of the oncolytic adenovirus (e.g., 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP/day dose of oncolytic adenovirus, 5 ⁇ 10 8 to 1 ⁇ 10 12 VP/day dose of oncolytic adenovirus, 1 ⁇ 10 9 to 5 ⁇ 10 11 VP/day dose of oncolytic adenovirus, 3 ⁇ 10 10 to 3 ⁇ 10 11 VP/day dose of oncolytic adenovirus, etc.) and the total dose per person per treatment range is 1 ⁇ 10 6 - 1 ⁇ 10 11 of said NK cells (for example, each per person)
- the total dose of the treatmentranges from 6 ⁇ 10 7 to 1.2 ⁇ 10 10 of the NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is from 5 ⁇ 10 7 to 5 ⁇ 10 12 VP/day.
- Dosage of oncolytic adenoviruseg, 5 x 107 to 1.5 x 10 12 VP/day dose of oncolytic adenovirus, 5 x 108 to 1 x 10 12 VP/day dose of oncolytic adenovirus, 1 x 10 9 to 5 ⁇ 10 11 VP / day dose of oncolytic adenovirus, 3 ⁇ 10 10 to 3 ⁇ 10 11 VP / day dose of oncolytic adenovirus, etc.
- the total dose range per person per treatmentis 1 ⁇ 10 6 - 1 ⁇ 10 11 of said NK cells (for example, the total dose per patient per treatment range is 6 ⁇ 10 7 - 1.2 ⁇ 1 0 10 of the NK cells, etc.).
- the active ingredients of the pharmaceutical composition or the therapeutic agent of the present inventioninclude 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day dose of the oncolytic virus H101 (e.g., 5 ⁇ 10 11 to 1.5 ⁇ 10 12 VP / day dose of oncolytic virus H101, etc.) and 1 x 10 5 to 1 x 10 10 cells/day of the NK cells; preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is 5 x 10 7 Up to 1.5 ⁇ 10 12 VP/day dose of oncolytic virus H101 (eg, 5 ⁇ 10 11 to 1.5 ⁇ 10 12 VP/day dose of oncolytic virus H101, etc.) and 1 ⁇ 10 5 to 1 ⁇ 10 10 cells/ A daily dose of the NK cells (for example, 1 x 107 to 1 x 10 10 cells/day of the NK cells, 1 x 10 8 to 5 x 10 9 cells/day of the NK cells, 1 x 10 9 to 4 x 10 9 cells/day dose of the NK cells, 1 x
- the active ingredients of the pharmaceutical composition or therapeutic agent of the present inventioninclude 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day dose of the oncolytic virus H101 (e.g., 5 ⁇ 10 11 to 1.5 ⁇ 10 12 VP/day dose of oncolytic virus H101, etc.) and the total dose range of 1 ⁇ 10 6 -1 ⁇ 10 11 per NK per patient (for example, the total dose range per person per treatment is 6 ⁇ ) 10 7 - 1.2 ⁇ 10 10 of said NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is from 5 x 10 7 to 1.5 x 10 12 VP/day of the oncolytic virus H101 ( For example, 5 x 10 11 to 1.5 x 10 12 VP/day dose of oncolytic virus H101, etc.) and the total dose per person per treatment range is 1 x 10 6 - 1 x 10 11 of said NK cells (for example, Each dose per patient has a total dose ranging from 6 ⁇ 10 7 to 1.2 ⁇ 10 10 of
- the oncolytic virus contained in the pharmaceutical composition or therapeutic agent of the present inventionis a poxvirus having an oncolysis effect (hereinafter also referred to as "anti-tumor poxvirus").
- the oncolytic poxvirusis selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect.
- the oncolytic poxvirusis functionally deficient in the TK gene and/or the VGF gene.
- the oncolytic poxvirusis selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
- the active ingredients of the pharmaceutical composition or therapeutic agent of the present inventioncomprises 1 ⁇ 10 5 to 5 ⁇ 10 9 pfu / day dose oncolytic poxvirus (e.g., 1 ⁇ 10 5 to 3 ⁇ 10 9 a) and 1 ⁇ 10 5 to 1 ⁇ 10 10 cells / day dose pfu / day dose oncolytic poxvirus, 1 ⁇ 10 5 to 1 ⁇ 10 8 pfu / day dose oncolytic poxvirus like NK cells (For example, 1 ⁇ 10 7 to 1 ⁇ 10 10 cells/day of the NK cells, 1 ⁇ 10 8 to 5 ⁇ 10 9 cells/day of the NK cells, 1 ⁇ 10 9 to 4 ⁇ 10 9 cells/day dose of said NK cells, 1 x 10 9 to 3 x 10 9 cells/day of said NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is 1 ⁇ 10 5 to 5 ⁇ 10 9 pfu / day dose of oncolytic pox virus (
- the active ingredients of the pharmaceutical composition or the therapeutic agent of the present inventioncomprises 1 ⁇ 10 5 to 5 ⁇ 10 9 pfu / day dose oncolytic poxvirus (e.g., 1 ⁇ 10 5 to 3 ⁇ 10 9 Pfu/day dose of oncolytic pox virus, 1 ⁇ 10 5 to 1 ⁇ 10 8 pfu/day dose of oncolytic pox virus, etc.) and the total dose range per person per treatment is 1 ⁇ 10 6 -1 ⁇ 10 11
- the NK cellsfor example, the total dose per subject per treatment range is 6 ⁇ 10 7 -1.2 ⁇ 10 10 of the NK cells, etc.
- the active ingredient of the pharmaceutical composition or therapeutic agentis 1 ⁇ 10 5 to 5 ⁇ 10 9 pfu / day dose of oncolytic pox virus (for example, 1 ⁇ 10 5 to 3 ⁇ 10 9 pfu / day dose of oncolytic pox virus, 1 ⁇ 10 5 to 1 ⁇ 10 8 pfu / day dose of oncolytic pox
- the pharmaceutical or therapeutic agents of the present inventionmay also comprise suitable pharmaceutically acceptable excipients.
- the pharmaceutical composition or therapeutic of the present inventionmay further comprise other active ingredients known in the art, such as interleukin-2 (IL-2), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon. - ⁇ (IFN- ⁇ ), tumor necrosis factor- ⁇ (TNF- ⁇ ) and the like.
- IL-2interleukin-2
- GM-CSFgranulocyte-macrophage colony stimulating factor
- IFN- ⁇interferon.
- TNF- ⁇tumor necrosis factor- ⁇
- the pharmaceutical or therapeutic agent of the inventiondoes not comprise bortezomib.
- a pharmaceutical or therapeutic agent of the inventioncomprises one or more pharmaceutically acceptable carriers.
- Pharmaceutical formulationscan be prepared by methods known in the art.
- an active ingredientsuch as a compound can be formulated with a common excipient, a diluent (for example, phosphate buffer or physiological saline), a tissue culture medium, and a carrier (for example, autologous plasma or human serum albumin) as a suspension.
- a diluentfor example, phosphate buffer or physiological saline
- tissue culture mediumfor example, autologous plasma or human serum albumin
- carrierfor example, autologous plasma or human serum albumin
- Other carriersmay include liposomes, micelles, nanocapsules, polymeric nanoparticles, solid lipid particles (see, for example, the literature "E. Koren and V. Torchilin, Life, 63:586-595, 2011").
- Specific methods of formulating the pharmaceutical or therapeutic agents of the present inventioncan be found in the scientific literature and in the patent literature, for
- the inventionprovides a therapeutic comprising: (a) a first pharmaceutical composition, wherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier; and (b) a second pharmaceutical composition, wherein the second pharmaceutical composition comprises NK cells in a second pharmaceutically acceptable carrier; wherein the oncolytic virus is capable of selectively replicating in tumor cells; and wherein the NK cells are
- the surfaceis modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that said antigen binding The domain is derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- the first pharmaceutically acceptable carrier and the second pharmaceutically acceptable carrierare the same. In other embodiments, the first pharmaceutically acceptable carrier and the second pharmaceutically acceptable carrier are different.
- the pharmaceutical composition or therapeutic of the present inventioncan be used for the treatment of various tumors and/or cancers including, but not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal Cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, cervical cancer, lymphoma, stomach cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- the tumor and/or canceris NKG2D ligand positive, including the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive of.
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the pharmaceutical composition or therapeutic agent of the present inventionis administered by first administering the oncolytic virus (for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) to a tumor and/or cancer patient, and then, 18-72 hours after administration of the oncolytic virus (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.) to the tumor and/or cancer
- the oncolytic virusfor example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus
- 18-72 hours after administration of the oncolytic viruseg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.
- the tumor and / Or administration of the NK cells by a cancer patientmeans that the time interval between administration of the first NK cells and the first oncolytic virus administration is 18-72 hours (for example, 20-70 hours, 22-48 hours, 24-48 hours, 30- 48 hours, etc.), or the time interval between administration of the first NK cells and the onset of the oncolytic virus that is most adjacent to it is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48) Hours, 30-48 hours, etc.).
- the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to itis 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30) -48 hours, etc.). Also preferably, the time interval between administration of the first NK cells and administration of the oncolytic virus that is most adjacent to it before is 24-48 hours.
- the oncolytic virus(for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) is administered in a therapeutically effective amount, once a day, continuously administered from 1 to 6
- the NK cellsare administered at a dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per patient per course (for example, the total dose per patient per treatment range is 6 ⁇ 10 7 -1.2 ⁇ 10) 10 ), administered 1-2 times a week for 3 weeks.
- the oncolytic virus(for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) is administered in a therapeutically effective amount, once every 2 days, continuously.
- the NK cellsare administered at a dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per patient per treatment (for example, the total dose range per person per treatment is 6 ⁇ 10 7 - 1.2 ⁇ 10 10 ), 1-2 times a week for 3 weeks.
- the oncolytic viruseg, oncolytic adenovirus, oncolytic pox virus, or oncolytic herpes simplex virus
- the conditions for administering the NK cells to the tumor and/or cancer patientmay be sufficient.
- administration of the oncolytic virus and administration of the NK cellsmay be, for example, first administration of the oncolytic virus once a day for 1-6 days, followed by administration of NK cells at intervals of 18-72 hours, 1-2 weekly administration Times, for 3 consecutive weeks.
- the oncolytic virusis administered first, and the NK cells are administered again 18-72 hours after the oncolytic virus is administered.
- the oncolytic virusis first administered to a tumor and/or cancer patient, the oncolytic virus is administered in a therapeutically effective amount, administered once; and the oncolytic virus is administered
- the NK cellsare administered to the tumor and/or cancer patient from the 18th hour to the 72th hour thereafter, and the NK cells are administered at a dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per person per course of treatment. (for example, the total dose per patient per treatment range is 6 ⁇ 10 7 - 1.2 ⁇ 10 10 ), 1-2 times a week for 3 weeks.
- different preferred clinical dosage rangescan be employed, such as those described in Table 1.
- Oncolytic virusesare capable of selective replication in tumor or cancer cells and peak over time.
- the inventors of the present inventionfound that after a period of replication, the oncolytic virus in the tumor cells promotes killing of tumor cells by NK cells. Therefore, the application interval of the oncolytic virus and NK cells proposed by the present invention achieves a bimodal overlap of the peaks of action of both.
- the inventionalso provides the use of a pharmaceutical or therapeutic agent of the invention in the manufacture of a medicament for the treatment of tumors and/or cancer.
- the tumor and/or cancerincludes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomae.g., head and neck cancer
- liver cancere.g., brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomahead and neck cancer
- liver cancere.g., brain cancer, colorectal cancer
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the present inventionalso provides a kit for synergistic combination therapy for treating tumors and/or cancer, comprising a first container containing the oncolytic virus of the present invention and a ovary containing the NK cells of the present invention a second container, wherein said first container and said second container are independent; and instructions for indicating the timing of administration and mode of administration; wherein said oncolytic virus is capable of selectively replicating in tumor cells;
- the surface of the NK cellis modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane region and an intracellular domain, characterized in that The antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- the kitconsists of separate containers each containing the oncolytic virus of the invention and the NK cells of the invention, respectively, together with instructions for the timing and mode of administration.
- the tumor and/or cancerincludes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomae.g., head and neck cancer
- liver cancere.g., brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomahead and neck cancer
- liver cancere.g., brain cancer, colorectal cancer
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the first container containing the oncolytic viruscomprises a therapeutically effective amount of the oncolytic virus
- the second container containing the NK cellscomprises sufficient to provide 1 x 10 5 to 1 x 10 10 cells per day.
- the dose of the NK cellsfor example, 1 ⁇ 10 7 - 1 ⁇ 10 10 cells / day dose, 1 ⁇ 10 8 to 5 ⁇ 10 9 cells / day dose of the NK cells, 1 ⁇ 10 9 to 4 ⁇ 10 9 cells/day dose of the NK cells, 1 ⁇ 10 9 to 3 ⁇ 10 9 cells/day of the NK cells, etc.).
- the first container containing the oncolytic viruscomprises a therapeutically effective amount of the oncolytic virus
- the second container containing the NK cellscomprises sufficient to provide a total dose range of 1 x 10 per person per course of treatment. 6 - 1 ⁇ 10 11 of said NK cells (e.g., the total dose per subject per treatment range is 6 x 10 7 - 1.2 x 10 10 of said NK cells).
- different preferred clinical dosage rangescan be employed, such as those described in Table 1.
- the oncolytic virusescan be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
- the NK cellscan be administered by administration generally employed in the art, for example, by intravenous administration or topical administration.
- the oncolytic virusis an adenovirus having an oncolytic effect.
- the E1 region and/or E3 region of the oncolytic adenovirusis genetically engineered.
- the oncolytic adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtak TM .
- the first containercomprises a 5 ⁇ 10 7 to 5 ⁇ 10 12 VP / day dose of the oncolytic adenovirus (e.g., 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day Dosage of oncolytic adenovirus, 5 x 108 to 1 x 10 12 VP/day dose of oncolytic adenovirus, 1 x 109 to 5 x 10 11 VP/day dose of oncolytic adenovirus, 3 x 10 10 to 3 ⁇ 10 11 VP/day dose of oncolytic adenovirus, etc.).
- the oncolytic adenoviruse.g., 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day Dosage of oncolytic adenovirus, 5 x 108 to 1 x 10 12 VP/day dose of oncolytic adenovirus, 1 x 109 to 5 x 10 11 VP/day dose of oncolytic adenovirus, 3 x 10 10 to 3 ⁇
- the first containercomprises a 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day dose of the oncolytic virus H101 (e.g., 5 ⁇ 10 11 to 1.5 ⁇ 10 12 VP / day dose of an oncolytic virus H101, etc.).
- the oncolytic virusis an oncolytic virus.
- the oncolytic poxvirusis selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect.
- the oncolytic poxvirusis functionally deficient in the TK gene and/or the VGF gene.
- the oncolytic poxvirusis selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
- the first containercomprises 1 ⁇ 10 5 to 5 ⁇ 10 9 pfu / day dose oncolytic poxvirus (e.g., 1 ⁇ 10 5 to 3 ⁇ 10 9 pfu / day Dosage of oncolytic pox virus, 1 x 10 5 to 1 x 10 8 pfu / day dose of oncolytic pox virus, etc.).
- oncolytic poxviruse.g., 1 ⁇ 10 5 to 3 ⁇ 10 9 pfu / day Dosage of oncolytic pox virus, 1 x 10 5 to 1 x 10 8 pfu / day dose of oncolytic pox virus, etc.
- the inventionalso provides a method of treating a tumor and/or cancer comprising the steps of:
- the tumor and / or a cancer patientadministering the NK cells of the invention, the surface of which is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, Transmembrane and intracellular domains, characterized in that the antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
- 18-72 hours after administration of the oncolytic viruseg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.
- the tumor and / Or administration of the NK cells of the present invention to a cancer patientmeans that the time interval between administration of the first NK cells and the first oncolytic virus administration is 18-72 hours (for example, 20-70 hours, 22-48 hours, 24-48 hours). 30-48 hours, etc., or the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to it before the time is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.).
- the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to itis 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30) -48 hours, etc.). Also preferably, the time interval between administration of the first NK cells and administration of the oncolytic virus that is most adjacent to it before is 24-48 hours.
- the tumor and/or cancerincludes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomae.g., head and neck cancer
- liver cancere.g., brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc.
- lung cancereg, non-small cell lung cancer
- melanomahead and neck cancer
- liver cancere.g., brain cancer, colorectal cancer
- the tumor and/or canceris positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand.
- the treatmentincludes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
- the oncolytic virusis administered in a therapeutically effective amount once a day for 1-6 days; and the NK cells are administered at a total dose per person per course of treatment.
- the rangeis 1 ⁇ 10 6 - 1 ⁇ 10 11 (for example, the total dose per patient per treatment range is 6 ⁇ 10 7 - 1.2 ⁇ 10 10 ), and is administered 1-2 times a week for 3 weeks.
- the oncolytic virusis administered in a therapeutically effective amount once every 2 days for 2-6 days; and the NK cells are administered at a dose of each person.
- the total dose range of treatmentis 1 ⁇ 10 6 -1 ⁇ 10 11 (for example, the total dose range per patient per treatment is 6 ⁇ 10 7 - 1.2 ⁇ 10 10 ), 1-2 times a week for 3 weeks .
- administration of the oncolytic virus and administration of the NK cellsmay be, for example, first administration of the oncolytic virus once a day for 1-6 days, followed by administration of NK cells at intervals of 18-72 hours, 1-2 weekly administration Times, for 3 consecutive weeks).
- the oncolytic virusis administered first, and the NK cells are administered again 18-72 hours after the oncolytic virus is administered.
- the oncolytic virusis first administered to a tumor and/or cancer patient, the oncolytic virus is administered in a therapeutically effective amount, administered once; and the oncolytic virus is administered
- the NK cellsare administered to the tumor and/or cancer patient from the 18th hour to the 72th hour thereafter, and the NK cells are administered at a dose ranging from 1 ⁇ 10 6 to 1 ⁇ 10 11 per person per course of treatment. (for example, the total dose per patient per treatment range is 6 ⁇ 10 7 - 1.2 ⁇ 10 10 ), 1-2 times a week for 3 weeks.
- different preferred clinical dosage rangescan be employed, such as those described in Table 1.
- the method of treating tumors and/or cancer of the present inventionmay be performed one or more times on the patient according to actual conditions and needs.
- the oncolytic virusescan be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
- the NK cellscan be administered by administration generally employed in the art, for example, by intravenous administration or topical administration.
- the oncolytic virusis an adenovirus having an oncolytic effect.
- the E1 region and/or E3 region of the oncolytic adenovirusis genetically engineered.
- the oncolytic adenovirusis selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtak TM .
- the oncolytic virusis an oncolytic adenovirus, and is administered at a dose of 5 ⁇ 10 7 to 5 ⁇ 10 12 VP / day (e.g., 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP/day, 5 ⁇ 10 8 to 1 ⁇ 10 12 VP/day, 1 ⁇ 10 9 to 5 ⁇ 10 11 VP/day, 3 ⁇ 10 10 to 3 ⁇ 10 11 VP/day, etc.).
- the oncolytic virusis an oncolytic virus H101, and which is administered at a dose of 5 ⁇ 10 7 to 1.5 ⁇ 10 12 VP / day (e.g., 5 ⁇ 10 11 to 1.5 ⁇ 10 12 VP / day, etc. ).
- the oncolytic virusis an oncolytic virus.
- the oncolytic poxvirusis selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect.
- the oncolytic poxvirusis functionally deficient in the TK gene and/or the VGF gene.
- the oncolytic poxvirusis selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
- the oncolytic virusis an oncolytic poxvirus and is administered at a dose of from 1 x 10 5 to 5 x 10 9 pfu per day (eg, 1 x 10 5 to 3 x) 10 9 pfu/day, 1 ⁇ 10 5 to 1 ⁇ 10 8 pfu/day, etc.).
- the percent concentration (%) of each reagentrefers to the volume percent concentration (% (v/v)) of the reagent.
- the H101 oncolytic adenovirus used in the following exampleswas purchased from Shanghai 3D organism.
- the PBS used in the following exampleswas purchased from Lonza under the order number BW17-517Q.
- VioBright FITC-conjugated anti-hMICA/B antibody used in the following exampleswas purchased from Miltenyi; PE-conjugated anti-hULBP1 antibody, APC-conjugated anti-hULBP2/5/6 antibody, APC-conjugated anti-hULBP3 antibody, APC-conjugated Anti-hULBP4 antibody was purchased from R&D Systems.
- the Calcein AM used in the following exampleswas purchased from Life Technologies.
- the 24-well cell culture plates used in the following examples(500 ⁇ l per well culture volume) and 96-well cell culture plates (200 ⁇ l per well culture volume) were obtained from Corning.
- PBMCsvenous blood mononuclear cells
- PBMCsMononuclear cells
- RNA extraction kit RNAiso Reagentpurchased from Life Technologies
- the extracted total RNAwas reverse transcribed into cDNA of PBMCs using a reverse transcription kit RevertAicTFirst Strand cDNA Synthesis Kit (purchased from Life Technologies), and stored at -20 ° C until use.
- PCR amplificationwas carried out with primers P1 (SEQ ID NO: 9) and P2 (SEQ ID NO: 10) to obtain an extracellular domain comprising a NKG2D protein of 402 bp in length.
- a fragment of the corresponding DNA coding sequence(nucleotide sequence as shown in SEQ ID NO: 6 in the Sequence Listing) has a Sphl and NheI restriction site and a protective base at both ends, respectively.
- PCR amplification using primers P5 (SEQ ID NO: 13) and P6 (SEQ ID NO: 14)gave an intracellular signal domain containing DAP12 of 156 bp in length (nucleotide sequence such as SEQ ID NO in the sequence listing: A fragment of 8).
- the PCR amplification reaction systemwas the same in each step.
- the PCR reaction conditionswere as described in KAPA HiFi Hot Start Ready Mix (2X) (purchased from Kapa Biosystems), and each reaction system (50 ⁇ L) was as follows:
- Double distilled water21.5 ⁇ L
- the above PCR productwas separated on a 1% (w/v) agarose gel, and DNA fragment recovery was carried out using an agarose gel DNA recovery kit (purchased from Omega Bio Tek).
- the fragment containing the extracellular domain DNA of the NKG2D protein of 402 bp in lengthwas subjected to double digestion with Sphl and NheI, and the digested product was subjected to DNA fragment recovery using an agarose gel DNA recovery kit.
- the commercial vector pFastbac1(Life Technologies) was added to a CMV promoter, a T7 promoter, a 5'UTR with a Kozak sequence, and a coding sequence for a GM-CSF alpha chain signal peptide (SP in Figure 1). ), and a 3'UTR of alpha globulin with a polyA signal to construct the pFastbac1 basic skeleton vector.
- the pFastbac1 basic skeleton vectorwas subjected to double digestion with SphI and SalI, and the digested product was subjected to DNA fragment recovery using an agarose gel DNA recovery kit, and then passed through the CD8 and DAP12 fragments recovered by the previous PCR.
- lane 11000 kb DNA molecular weight marker
- lane 2plasmid pFastbac1-CD8-DAP12 digestion fragment, vector backbone 5276 bp, CD8-DAP12 fragment 405 bp.
- the correct plasmidwas sent to AITbiotech for sequencing of the inserted fusion gene fragment, and the correct recombinant plasmid was named pFastbac1-CD8-DAP12.
- the vector pFastbac1-CD8-DAP12was digested with SphI and NheI, and the digested product was recovered by DNA fragmentation using an agarose gel DNA recovery kit, and then ligated to the extracellular domain fragment of the previously recovered NKG2D protein by T4 DNA.
- Enzyme-linked, linked product-transformed One Chemically Competent TOP10 chemically competent cellswere cultured at 37 ° C for 18 hours, and then picked up, and cultured at 37 ° C, 250 rpm for 6 hours, and then the plasmid was extracted with a plasmid mini-kit.
- the extracted plasmidwas identified by restriction endonuclease SphI and NheI digestion, and the electrophoresis pattern was identified as shown in Fig. 3; wherein, lane 1: 1000 kb DNA molecular weight marker; lane 2: restriction fragment of plasmid pFastbac1-NKG2D-CD8-DAP12, The vector backbone is 5682 bp and the NKG2D fragment is 402 bp.
- the correct plasmidwas sent to AITbiotech for sequencing of the inserted fusion gene fragment, and the recombinant plasmid with the correct sequencing result was named pFastbac1-NKG2D-CD8-DAP12, and the plasmid map is shown in Figure 4.
- PBMCs cells with 20 ⁇ 10 6 cells and 2 mL NK cell activator Ipurchased from Shenzhen Daktronics, DKW35-CYT-NK001
- NK culture mediumwas AIM
- NK cell activator Iwas uniformly mixed in 400 ml of NK medium, inoculated back into a G-Rex 100 cell culture incubator, and culture was continued for 7 days under the same conditions.
- NK cells cultured by this methodwas as high as 90%, as shown in Fig. 5.
- Fig. 5Aindicate that the proportion of CD3-negative and CD56-positive NK cells is 90.2%
- Fig. 5Bindicate that the ratio of NK cell surface receptor NKG2D and CD16 double positive is 96.4%.
- Tail-PCR technologywas used to synthesize large-dose DNA double-stranded templates with PolyA in the positive strand and corresponding PolyT in the reverse strand for in vitro RNA synthesis, which reduced the instability of DNA template.
- the chimeric antigen receptor NKG2D-CD8-DAP12 coding sequencewas subjected to Tail-PCR amplification using the above pFastbac1-NKG2D-CD8-DAP12 vector as a DNA template to synthesize linearization of the chimeric antigen receptor NKG2D-CD8-DAP12 sequence.
- DNA template, Tail-PCR reaction conditionsrefer to the instructions of KAPA HiFiHotStartReadyMix (2X), the reaction system (50 ⁇ L) is as follows:
- pFastbac1-NKG2D-CD8-DAP12 vector DNA template500ng/ ⁇ L: 0.5 ⁇ L
- the above PCR productwas isolated and identified on a 1% (w/v) agarose gel, as shown in FIG.
- the correct productwas identified for in vitro synthesis of the chimeric antigen receptor NKG2D-CD8-DAP12 mRNA.
- In vitro mRNA synthesis kit with capped mRNA synthesis kitas mMESSAGEmMACHINE T7 ULTRA Transcription Kit (available from Invitrogen, USA) or mScript TM RNA system (available from the American Epicentre Corporation). According to the instructions of the kit, and using the reagents provided in the kit for synthesis.
- the in vitro synthesized chimeric antigen receptor NKG2D-CD8-DAP12 mRNA productwas isolated and identified on a 1% (w/v) agarose gel, as shown in FIG. The correct mRNA was identified and stored at -80 ° C for storage.
- NK cells prepared in Preparation Example 2(1 ⁇ 10 7 cells) and 4 ⁇ g of NKG2D-CD8-DAP12 mRNA were mixed in electroporation P3 (product name "P3 Primary Cell” X Kit L”, Lonza, item number V4XP-3012), placed in a 100 ⁇ l Nucleocuvette TM tube (P3Primary Cell) X Kit L, Lonza, item number V4XP-3012), and frozen in an ice bath for 5 minutes. Then using the 4D-Nucleofector TM electroporation instrument (available from Lonza, Inc., Switzerland), choose their own program NK cell electroporation electroporation.
- P3product name "P3 Primary Cell” X Kit L”, Lonza, item number V4XP-3012
- Test Example 1Detection of NKG2D-CAR NK cells in vitro killing ability of human tumor cells
- an ELISPOT assaywas performed to detect the secretion of IFN ⁇ , since the secretion of IFN- ⁇ was positively correlated with the anti-tumor activity of NKG2D-CAR NK cells in adoptive cellular immunotherapy.
- the preparation method of mGFP-CAR NK cellsis the same as that of NKG2D-CAR NK cells (NK cells transfected with NKG2D-CD8-DAP12 mRNA), except that the vector is constructed.
- the outer domain used the mGFP sequence without antigen binding function(its Genbank accession number is YP_002302326.1) as a negative control.
- NK cells transfected with NKG2D-CD8-DAP12 mRNAThe preparation of NK cells transfected with NKG2D-CD8-DAP12 mRNA is shown in Preparation 3.
- NK cells transfected with NKG2D-CD8-DAP12 mRNA or mGFP-CD8-DAP12 mRNAwere associated with human ovarian cancer cells SKOV3, human colorectal cancer cells HCT116 and SW480, human head and neck cancer cells Detroit, human hepatoma cells HepG2 and human neutrophils
- the stromal cell U87was co-cultured on the ELISPOT assay plate, and the ratio of the above NK effector cells to the target cells was 5:1. Each set of experiments was repeated 3 times. After 24 hours of co-cultivation, development and use of the software immunospot for ELISPOT point counting.
- NK cells transfected with NKG2D-CD8-DAP12 mRNAproduced more and stronger IFN- ⁇ than NK cells transfected with mGFP-CD8-DAP12 mRNA (detected by ELISPOT assay kit, purchased from Mabtech The product number is 3420-4HST-1).
- the ELISPOT spots produced by NKG2D-CD8-DAP12 mRNA-transfected NK cellswere significantly higher than the control group, as shown in Figure 9.
- NK cells transfected with NKG2D-CD8-DAP12 mRNA or mGFP-CD8-DAP12 mRNAwere associated with human colorectal cancer cell HCT116, human ovarian cancer cell SKOV3, human head and neck cancer cells Fadu and Detroit, human hepatoma cell HepG2, human breast cancer
- the cell MCF7 and the human myeloma cell KG1were co-cultured in a U-shaped 96-well plate, and the ratio of the above-mentioned NK effector cells to the target cells ranged from 2.5:1 to 10:1. Each set of experiments was repeated 3 times.
- NKG2D-CAR NK cellswere examined using the DELFIA EuTDA cytotoxicity kit (available from PerkinElmer, USA), and the killing effect was calculated by the following formula:
- % specific lysis((experimental group release (reading) - blank group release (reading)) / (maximum release (reading) - blank group release (reading))) x 100
- NKG2D-CAR modified NK cellshave broad and strong tumoricidal activity.
- the killing effect of NKG2D-CAR modified NK cellsis At 10:1, it was significantly higher than the killing effect of the control group, as shown in Figure 10.
- the above resultsfully demonstrate the universality and high efficiency of NKG2D-CAR NK on tumor killing.
- Test Example 2Detection and analysis of NKG2D-CAR NK cells in vivo killing ability of human tumor cells
- NKG2D-CAR NK cellsThe in vivo antitumor effect of NKG2D-CAR NK cells was further tested using a mouse model implanted in human tumors.
- the experimental micewere non-obese diabetic/severe combined immunodeficiency/IL-2R ⁇ cnull (NSG) mice (6-8 weeks, female), and each mouse was implanted with 1 ⁇ 10 7 ovarian cancer cells SKOV3-Luc. .
- NSGnon-obese diabetic/severe combined immunodeficiency/IL-2R ⁇ cnull mice (6-8 weeks, female), and each mouse was implanted with 1 ⁇ 10 7 ovarian cancer cells SKOV3-Luc. .
- Seven days after tumor implantationtumor growth was observed on the IVIS Spectrum imaging platform using in vivo bioimaging (BLI), and tumor growth was photographed using an imager (available from PerkinElmer, USA).
- mice with similar BLI intensitywere randomly divided into 2 groups: phosphate buffered saline (PBS) group, and NKG2D-CAR NK.
- PBSphosphate buffered saline
- NKG2D-CAR NK cell group5 mice per group.
- NKG2D-CAR NK cell groupNKG2D-CAR-modified NK cells prepared by the method shown in Preparation Example 3 were intraperitoneally injected with 1 ⁇ 10 7 cells per mouse, and each mouse in the PBS group was intraperitoneally injected with a dose of 100 ⁇ L. PBS.
- the cell injection protocolwas: two to five injections per week for a total of three weeks (ie, six injections per group).
- micewere closely observed and the development of the tumors was recorded by BLI. All light signals and images were analyzed by Xenogen in vivo imaging software v2.5. As shown in Figure 11, on the 28th day after tumor implantation, tumor growth showed that the tumor growth of mice in the PBS group was rapid, the light signal intensity increased about 10 times that of the 7th day, and all 5 of the NKG2D-CAR NK cell group. Tumor growth in mice was not only inhibited, but the original tumors were eliminated and the light signal intensity decreased to about 10% on day 7. Thus, NKG2D-CAR-modified NK cells can effectively kill tumors in vivo.
- Test Example 3Comparison of different chimeric antigen receptors
- the NK cells modified with the chimeric antigen receptor NKG2D-CD8-DAP12 and the NK cells modified with the chimeric antigen receptor NKG2D-CD8-CD3Zwere tested on tumor cells to compare the killing viability.
- NK cells transfected with NKG2D-CD8-DAP12 mRNA, NKG2D-CD8-CD3z mRNA, or mGFP-CD8-DAP12 mRNAwere co-cultured with human colorectal cancer cell line HCT116, human ovarian cancer cell line SKOV3, and human head and neck cancer cell line Detroit, respectively.
- HCT116human colorectal cancer cell line
- SKOV3human ovarian cancer cell line SKOV3
- human head and neck cancer cell line Detroitrespectively.
- the ratio of the above NK cells to the target cellsranges from 2.5:1 to 10:1.
- Each set of experimentswas repeated 3 times.
- the ability of NKG2D-CAR NK cells to lyse tumor cellswas examined using the DELFIA EuTDA Cytotoxicity Kit (available from PerkinElmer, USA). The killing effect was calculated using the following formula:
- % specific lysis((experimental group release (reading) - blank group release (reading)) / (maximum release (reading) - blank group release (reading))) x 100
- NKG2D-CD8-DAP12 mRNA-modified NK cellshad significantly stronger tumoricidal activity than NKG2D-CD8- in the tumoricidal experiments of human colorectal cancer cell HCT116, human ovarian cancer cell SKOV3, and human head and neck cancer cell Detroit.
- CD3z mRNA modified NK cellsThe chimeric antigen receptor NKG2D-CD8-DAP12 having the specific constituent unit combination created by the present invention has a remarkable therapeutic effect and a good commercialization prospect.
- NKG2D-CD8-CD3z modified NK cellswere prepared in the same manner as NKG2D-CD8-DAP12 modified NK cells except that the intracellular signal domain was constructed using CD3z (the full-length amino acid sequence of Genbank number: NP_932170). .1) The intracellular signal sequence (shown as SEQ ID NO: 23).
- Test Example 4Pretreatment of tumor cells with H101 enhances the in vitro killing ability of NKG2D-CAR NK cells to tumor cells
- NKG2D ligand on the surface of tumor cellsIn order to detect the effect of H101 oncolytic adenovirus treatment on the expression of NKG2D ligand on the surface of tumor cells, the expression of NKG2D ligand on human head and neck cancer cell Fadu and human hepatoma cell HepG2 was detected by flow cytometry.
- the serum-free medium of tumor cells(Fluu medium was purchased from Gibco), HepG2 was cultured and DMEM (purchased from Gibco) was used to dilute H101 virus, Fadu cells were infected at 17 MOI, HepG2 was infected at 34 MOI, and infection was 6 After an hour, discard the virus-containing medium and replace it with standard medium for tumor cells (Fadu medium is RPMI (purchased from Gibco) + 10% FBS (purchased from Sigma), culture of HepG2 and DMEM (purchased from Gibco) + 10% FBS (purchased from Sigma)). Tumor cells without H101 treatment were used as negative controls (the same exchange treatment was performed at the corresponding time points).
- the culturewas continued for 18 hours, and the harvested cells were digested with VioBright FITC-conjugated anti-hMICA/B antibody (Miltenyi), PE-conjugated anti-hULBP1 antibody, APC-conjugated anti-hULBP2/5/6 antibody, and APC-conjugated antibody.
- hULBP3 antibody, APC-conjugated anti-hULBP4 antibodyR&D Systems
- the expression of NKG2D ligandwas detected by flow cytometry (purchased from Novocyte), and each experiment was repeated 3 times. Take the average for statistical analysis.
- Fadu cell assayshows that compared to the negative control (i.e., the legend is shown as "Fadu"), the H101-infected Fadu cell group (i.e., the legend shown as "H101-treated Fadu”) has a tumor cell surface.
- NKG2D ligand MICA/Bwas significantly increased (% of cells expressing MICA/B in the negative control "Fadu” group: 39.1%, percentage of cells expressing MICA/B in the "H101-treated Fadu” group: 50.81%), and ULBP1 was decreased (% of cells expressing ULBP1 in the negative control "Fadu”: 33.4%, percentage of cells expressing ULBP1 in the "H101-treated Fadu” group: 27.11%).
- the HepG2 cell assay resultsare shown in Figure 14, which shows that H101 infected HepG2 cells (i.e., the legend shows "H101-treated HepG2") on the surface of tumor cells compared to the negative control (i.e., the legend shows "HepG2").
- the NKG2D ligand MICA/Bwas significantly increased (% of cells expressing MICA/B in the negative control "HepG2" group: 8.3%, percentage of cells expressing MICA/B in the "H101-treated HepG2" group: 45.37%).
- NK cells transfected with oncolytic adenovirus H101 at the corresponding time points but without NKG2D-CD8-DAP12 mRNAwere transfected as "w/o CAR-NK+H101"group;
- a group of NK cells that were neither treated with oncolytic adenovirus H101 nor transfected with NKG2D-CD8-DAP12 mRNAwere used as the "w/o CAR-NK” group, which was a negative control group.
- Each groupwas subjected to the corresponding liquid exchange operation at the corresponding time; each group of experiments was repeated three times, and the average value was taken for statistical analysis.
- NKG2D-CAR-modified NK cellshad strong tumoricidal activity against Fadu, as shown in Figure 15A (p ⁇ 0.001).
- NKG2D-CAR-modified NK cellsstill had strong tumoricidal activity against Fadu, see Figure 15B (p ⁇ 0.001).
- treatment of Fadu cells with H101 oncolytic adenovirussignificantly increased the tumoricidal activity of NKG2D-CAR-modified NK cells against Fadu, as shown in Fig. 15C (Figs. 15A and 15B are shown in Fig. 15C in the same figure). .
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Immunology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Medicinal Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Translated fromChinese本发明属于生物技术领域,具体而言,涉及包含溶瘤病毒和CAR-NK细胞的治疗剂及应用、药盒、治疗肿瘤和/或癌症的方法。The present invention is in the field of biotechnology, and in particular, relates to therapeutic agents and applications comprising oncolytic viruses and CAR-NK cells, kits, methods of treating tumors and/or cancer.
近几年随着对免疫机制的了解,导致了继手术治疗、化疗、放射性治疗之后又一治疗癌症的新技术的产生,即免疫治疗。癌症的免疫治疗被《Science》杂志评为2013年世界年度科技突破。嵌合抗原受体(Chimeric Antigen Receptor,CAR)是经人工改造的受体,因此可将任意的特异性受体嫁接到免疫效应细胞上。通常,这些受体的用于识别抗原的胞外特异性部分来自抗体的序列。由于这种受体的不同部分有不同的来源,因此被称为嵌合受体。CAR修饰的免疫细胞即表达嵌合性抗原受体(CAR)的免疫细胞,是现在最有效,最有希望的肿瘤细胞免疫治疗产品。In recent years, with the understanding of the immune mechanism, it has led to the emergence of another new technology for treating cancer after surgery, chemotherapy and radiotherapy, namely immunotherapy. The immunotherapy of cancer was named the 2013 World Technology Breakthrough by Science magazine. Chimeric Antigen Receptor (CAR) is an artificially engineered receptor, so any specific receptor can be grafted onto immune effector cells. Typically, the extracellular specific portion of these receptors used to recognize an antigen is derived from the sequence of the antibody. Because different parts of this receptor have different origins, they are called chimeric receptors. CAR-modified immune cells, ie, immune cells expressing the chimeric antigen receptor (CAR), are now the most effective and promising tumor cell immunotherapy products.
自然杀伤细胞(NK)是机体重要的免疫细胞,在外周血单个核细胞组成中占10%左右,是人体抗肿瘤、抗病毒感染的第一道防线。NK细胞与T细胞不同,不会产生自分泌生长因子如IL-2,而且相比T细胞,在遇到特异性抗原时不会增值过快,寿命较短,不需要装备自杀基因,也能限制CAR毒性。除此以外,NK细胞对肿瘤的杀伤不依赖于MHC呈递的特异性抗原,因此,在异体NK细胞的免疫治疗应用上,不会产生免疫排斥作用,是一种比T细胞更为安全的免疫治疗候选细胞。理论上,CAR可以避开NK细胞内抑制性信号通路,同时NK细胞自身可表达激活型受体,还能利用抗体介导的细胞毒作用(ADCC),因此,CAR表达的NK细胞在肿瘤治疗上更优于T细胞,有着更为广阔的应用前景。Natural killer cells (NK) are important immune cells in the body, accounting for about 10% of the composition of peripheral blood mononuclear cells, which is the first line of defense against anti-tumor and anti-viral infections. Unlike T cells, NK cells do not produce autocrine growth factors such as IL-2, and they do not increase in value when they encounter specific antigens, and have a short life span. They do not need to be equipped with suicide genes. Limit CAR toxicity. In addition, the killing of tumors by NK cells does not depend on the specific antigen presented by MHC. Therefore, in the immunotherapy application of allogeneic NK cells, immune rejection is not produced, and it is a safer immunity than T cells. Treat candidate cells. In theory, CAR can avoid the inhibitory signaling pathway in NK cells, while NK cells themselves can express activated receptors and can also utilize antibody-mediated cytotoxicity (ADCC). Therefore, CAR-expressing NK cells are treated in tumors. It is superior to T cells and has a broader application prospect.
CAR包括胞外部分、跨膜区和胞内部分。对于携带CAR的免疫细胞而言,CAR的胞外部分和胞内部分的选择、及其与免疫细胞种类的配合极为重要,这与对肿瘤的特异性杀伤能力密切相关。目前在肿瘤的免疫治疗中,仍然需要开发出可供选择的新型且效果良好的CAR-免疫细胞药物。CAR includes an extracellular portion, a transmembrane region, and an intracellular portion. For immune cells carrying CAR, the selection of the extracellular and intracellular portions of CAR and its cooperation with immune cell types are extremely important, which is closely related to the specific killing ability of tumors. At present, in the immunotherapy of tumors, it is still necessary to develop a novel and effective CAR-immune cell drug.
另一方面,病毒治疗癌症的方法近二十年来发展相当迅速。目前病毒治疗最大的进展之一是利用肿瘤细胞与正常细胞的差异,对某些病毒结构进行改造,使其能选择性地在肿瘤细胞中复制,最终达到杀死肿瘤细胞的目的。这些被改造后的病毒,根据其功能被统称为溶瘤病毒,其来源自腺病毒、疱疹病毒和痘病毒等。目前发现某些野生型病毒也具有在肿瘤细胞中选择性复制而溶瘤的功能。On the other hand, the way in which the virus treats cancer has developed quite rapidly in the past two decades. One of the biggest advances in viral therapy is the use of tumor cells and normal cells to modify certain viral structures, allowing them to selectively replicate in tumor cells, ultimately achieving the goal of killing tumor cells. These modified viruses are collectively referred to as oncolytic viruses according to their functions, and are derived from adenoviruses, herpes viruses, and poxviruses. It has now been found that certain wild-type viruses also have the function of selectively replicating and inducing tumors in tumor cells.
在中国上市的一种溶瘤病毒注射液的成分是经基因改造的5型腺病毒H101,从而有利于病毒在肿瘤细胞中的复制。H101主要是删除了人5型腺病毒的E1B区55KD和E3区基因片段,具有在肿瘤细胞中特异性复制而最终导致溶瘤的特性。其机理之一是野生型腺病毒基因中E1B区编码的55KD蛋白可以与p53蛋白相结合,从而抑制p53基因对腺病毒的清除作用。由于病毒不能编码产生E1B-55KD蛋白,使得病毒在p53正常的细胞中不能复制。而在p53基因突变的肿瘤细胞中,因无p53基因的抑制作用,所以病毒可以大量复制。此外,E3区基因片段的删除使得病毒在体内易于被免疫系统(例如NK细胞)识别并清除,增加了临床应用的安全性。目前的观点是,不仅仅p53本身的突变,只要p53通路的缺陷都有利于H101的选择性复制。H101通过瘤内注射给药,在肿瘤细胞中大量复制,最终导致肿瘤细胞的溶解和死亡。One of the oncolytic virus injections marketed in China is genetically engineered
由美国FDA批准的一种溶瘤病毒药T-Vec的成分是基因工程修饰过了的1型单纯疱疹病毒HSV-1。在T-Vec中删除了ICP34.5和ICP47基因并插入了人免疫激活蛋白粒细胞-巨噬细胞集落刺激因子(GM-CSF)基因,它可以在肿瘤细胞内复制并表达GM-CSF。将其直接注射到黑色素瘤病灶中可造成肿瘤细胞的溶解,从而使肿瘤细胞破裂,并释放出肿瘤源性抗原和GM-CSF,加速抗肿瘤的免疫应答。 然而据安进公司称,该作用的确切机制尚不清楚。2015年10月27日,FDA批准T-Vec作为首次手术后复发的黑色素瘤患者不可切除病灶的局部治疗方案。The component of the oncolytic virus drug T-Vec approved by the US FDA is genetically engineered
痘病毒是一类体积较大的病毒,由野生型痘病毒通过基因修饰得到的溶瘤病毒在肿瘤细胞中复制的特异性大大提高。其中,有的溶瘤痘病毒中删除了痘病毒DNA中表达胸腺嘧啶激酶(Thymidine kinase(TK))的基因,从而使得该溶瘤病毒不能在正常细胞内复制增殖。与正常细胞不同,肿瘤细胞会合成胸腺嘧啶激酶提供给溶瘤病毒复制使用,因此痘溶瘤病毒可以在肿瘤细胞中大量复制。此外有的溶瘤痘病毒中删除了表达VGF的基因,增强了病毒在肿瘤细胞内的特异增殖,最终导致肿瘤细胞的溶解和死亡。还有的溶瘤痘病毒中既删除了TK基因又删除了VGF基因。目前痘溶瘤病毒尚未有上市的产品。与腺病毒和疱疹病毒来源的溶瘤病毒相比,痘溶瘤病毒是具有可通过静脉注射全身给药并且到达肿瘤靶点的给药优势的。此外,痘病毒基因组庞大,便于进行有助于提高肿瘤杀伤性的基因修饰。Poxvirus is a large-sized virus, and the specificity of the oncolytic virus obtained by genetic modification of wild-type poxvirus in tumor cells is greatly improved. Among them, some of the oncolytic pox viruses have deleted the gene that expresses thymidine kinase (TK) in the poxvirus DNA, so that the oncolytic virus cannot replicate and proliferate in normal cells. Unlike normal cells, tumor cells synthesize thymidine kinase for replication by oncolytic viruses, so vaccinia virus can replicate in large numbers in tumor cells. In addition, some of the oncolytic pox viruses have deleted the gene expressing VGF, which enhances the specific proliferation of the virus in the tumor cells, and finally leads to the dissolution and death of the tumor cells. In other cases, the oncolytic pox virus removes both the TK gene and the VGF gene. Currently, the acne oncolytic virus has not yet been marketed. Compared to oncolytic viruses derived from adenovirus and herpes virus, vaccinia virus has the advantage of administration which can be administered systemically by intravenous injection and reaching tumor targets. In addition, the poxvirus genome is large and facilitates genetic modification that contributes to tumor killing.
然而,用溶瘤病毒单独治疗肿瘤的疗效有时并不十分理想,溶瘤病毒与化疗药物联合使用治疗肿瘤时,由于化疗药物对正常细胞同等杀伤,会带来强烈的副作用。However, the efficacy of oncolytic virus alone in the treatment of tumors is sometimes not very satisfactory. When oncolytic viruses are combined with chemotherapeutic drugs to treat tumors, because chemotherapy drugs are equally killing normal cells, they will have strong side effects.
因此,目前在肿瘤和/或癌症的免疫治疗中,仍然需要更加有效的治疗方案和由此开发出的药物。Therefore, there is still a need for more effective treatment regimens and drugs developed thereby in the immunotherapy of tumors and/or cancer.
发明内容Summary of the invention
为解决上述现有技术中所存在的问题,本发明提供了包含溶瘤病毒和CAR-NK细胞的治疗剂及应用、药盒、治疗肿瘤和/或癌症的方法。To address the problems in the prior art described above, the present invention provides therapeutic agents and uses comprising oncolytic viruses and CAR-NK cells, kits, methods of treating tumors and/or cancer.
具体而言,本发明提供了:In particular, the present invention provides:
(1)一种治疗剂,包含:(1) A therapeutic agent comprising:
(a)第一药物组合物,其中该第一药物组合物包含位于第一可药用载体中的溶瘤病毒;和(a) a first pharmaceutical composition, wherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier;
(b)第二药物组合物,其中该第二药物组合物包含位于第二可 药用载体中的NK细胞;(b) a second pharmaceutical composition, wherein the second pharmaceutical composition comprises NK cells in a second pharmaceutically acceptable carrier;
其中所述溶瘤病毒能够选择性地在肿瘤细胞中复制;并且Wherein the oncolytic virus is capable of selectively replicating in a tumor cell;
其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。Wherein the surface of the NK cell is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that In the above, the antigen binding domain is derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
(2)根据(1)所述的治疗剂,其中所述抗原结合结构域的氨基酸序列与NKG2D的第X位-第216位氨基酸序列一致,且73≤X≤83。(2) The therapeutic agent according to (1), wherein the amino acid sequence of the antigen-binding domain is identical to the X-position 216 amino acid sequence of NKG2D, and 73 ≤ X ≤ 83.
(3)根据(1)或(2)所述的治疗剂,其中所述抗原结合结构域的氨基酸序列如SEQ ID NO:3所示。(3) The therapeutic agent according to (1) or (2), wherein the amino acid sequence of the antigen-binding domain is as shown in SEQ ID NO: 3.
(4)根据(1)所述的治疗剂,其中所述胞内结构域的氨基酸序列选自DAP12的第62-113位氨基酸;优选地,所述胞内结构域的氨基酸序列如SEQ ID NO:5所示。(4) The therapeutic agent according to (1), wherein the amino acid sequence of the intracellular domain is selected from amino acids 62 to 113 of DAP12; preferably, the amino acid sequence of the intracellular domain is SEQ ID NO :5 is shown.
(5)根据(1)所述的治疗剂,其中所述间隔区来自CD8α的铰链区,所述跨膜区来自CD8α的跨膜区。(5) The therapeutic agent according to (1), wherein the spacer is derived from a hinge region of CD8α, and the transmembrane region is derived from a transmembrane region of CD8α.
(6)根据(1)或(5)所述的治疗剂,其中所述间隔区和跨膜区构成间隔跨膜区,并且其中所述间隔跨膜区的氨基酸序列与CD8α的第Y位-第210位氨基酸序列一致,且118≤Y≤128。(6) The therapeutic agent according to (1) or (5), wherein the spacer and the transmembrane region constitute a spacer transmembrane region, and wherein the amino acid sequence of the spacer transmembrane region and the Yth position of CD8α- The amino acid sequence of the 210th position is identical, and 118≤Y≤128.
(7)根据(6)所述的治疗剂,其中所述间隔跨膜区的氨基酸序列如SEQ ID NO:4所示。(7) The therapeutic agent according to (6), wherein the amino acid sequence of the spacer transmembrane region is as shown in SEQ ID NO: 4.
(8)根据(1)所述的治疗剂,其中所述嵌合抗原受体的氨基酸序列如SEQ ID NO:1所示。(8) The therapeutic agent according to (1), wherein the amino acid sequence of the chimeric antigen receptor is as shown in SEQ ID NO: 1.
(9)根据(1)所述的治疗剂,其中所述第一药物组合物和所述第二药物组合物各自独立地存在于所述治疗剂中而互不混合。(9) The therapeutic agent according to (1), wherein the first pharmaceutical composition and the second pharmaceutical composition are each independently present in the therapeutic agent without being mixed with each other.
(10)根据(1)所述的治疗剂,其中所述第一药物组合物的活性成分为所述溶瘤病毒,并且其中所述第二药物组合物的活性成分为所述NK细胞。(10) The therapeutic agent according to (1), wherein the active ingredient of the first pharmaceutical composition is the oncolytic virus, and wherein the active ingredient of the second pharmaceutical composition is the NK cell.
(11)根据(1)所述的治疗剂,其中所述第一药物组合物包含治疗有效量的所述溶瘤病毒,并且所述第二药物组合物包含1×105 至1×1010个细胞/天剂量的所述NK细胞。(11) The therapeutic agent according to (1), wherein the first pharmaceutical composition comprises a therapeutically effective amount of the oncolytic virus, and the second pharmaceutical composition comprises 1 × 105 to 1 × 1010 Cell/day dose of the NK cells.
(12)根据(11)所述的治疗剂,其中所述第二药物组合物包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞。优选的是,所述第二药物组合物包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞。(12) The therapeutic agent according to (11), wherein the second pharmaceutical composition comprises the NK cells in a total dose ranging from 1 × 106 to 1 × 1011 per subject per subject. Preferably, the second pharmaceutical composition comprises the NK cells in a total dose ranging from 6 x 107 to 1.2 x 1010 per subject per subject.
(13)根据(1)所述的治疗剂,其中所述溶瘤病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。(13) The therapeutic agent according to (1), wherein the oncolytic virus is selected from the group consisting of a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
(14)根据(1)所述的治疗剂,其中所述溶瘤病毒选自具有溶瘤作用的腺病毒、痘病毒、单纯疱疹病毒、麻疹病毒、塞姆利基森林病毒、水疱性口炎病毒、脊髓灰质炎病毒、逆转录病毒、呼肠孤病毒、塞内卡谷病毒、埃可型肠道病毒、柯萨奇病毒、新城疫病毒和马拉巴病毒。(14) The therapeutic agent according to (1), wherein the oncolytic virus is selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis Virus, poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
(15)根据(1)所述的治疗剂,其中所述NK细胞选自自体NK细胞和异体NK细胞。(15) The therapeutic agent according to (1), wherein the NK cells are selected from the group consisting of autologous NK cells and allogeneic NK cells.
(16)根据(15)所述的治疗剂,其中所述NK细胞为经体外扩增得到的自体NK细胞或经体外扩增得到的异体NK细胞。(16) The therapeutic agent according to (15), wherein the NK cells are autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
(17)根据(1)所述的治疗剂,其中所述溶瘤病毒配制成通过瘤内注射给药或静脉给药;并且其中所述NK细胞配制成通过静脉给药或局部给药。(17) The therapeutic agent according to (1), wherein the oncolytic virus is formulated to be administered by intratumoral injection or intravenously; and wherein the NK cells are formulated to be administered intravenously or topically.
(18)根据(1)-(17)中任一项所述的治疗剂,其中所述溶瘤病毒为具有溶瘤作用的腺病毒。The therapeutic agent according to any one of (1) to (17) wherein the oncolytic virus is an adenovirus having an oncolysis effect.
(19)根据(18)所述的治疗剂,其中所述具有溶瘤作用的腺病毒的E1区和/或E3区是经基因工程改造的。(19) The therapeutic agent according to (18), wherein the E1 region and/or the E3 region of the oncolytic adenovirus is genetically engineered.
(20)根据(18)所述的治疗剂,其中所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。(20) The therapeutic agent according to (18), wherein the oncolytic effect-containing adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN- 01, and / or ProstAtakTM.
(21)根据(1)所述的治疗剂,其中所述第一药物组合物的活性成分包含5×107至5×1012VP/天剂量的溶瘤腺病毒,并且其中所述第二药物组合物的活性成分包含1×105至1×1010个细胞/天剂量 的所述NK细胞。(21) The therapeutic agent according to (1), wherein the active ingredient of the first pharmaceutical composition comprises an oncolytic adenovirus of 5 x 107 to 5 x 1012 VP/day, and wherein the second The active ingredient of the pharmaceutical composition comprises from 1 x 105 to 1 x 1010 cells per day of the NK cells.
(22)根据(21)所述的治疗剂,其中所述第二药物组合物的活性成分包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞。优选的是,所述第二药物组合物的活性成分包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞。(22) The therapeutic agent according to (21), wherein the active ingredient of the second pharmaceutical composition comprises the NK cells in a total dose ranging from 1 x 106 to 1 x 1011 per subject per subject. Preferably, the active ingredient of the second pharmaceutical composition comprises the total dose ranging from 6 x 107 to 1.2 x 1010 per NK per patient per subject.
(23)根据(1)所述的治疗剂,其中所述治疗剂由所述第一药物组合物和所述第二药物组合物组成。(23) The therapeutic agent according to (1), wherein the therapeutic agent consists of the first pharmaceutical composition and the second pharmaceutical composition.
(24)根据(1)-(23)中任一项所述的治疗剂在制备用于治疗肿瘤和/或癌症的药物中的应用。(24) Use of the therapeutic agent according to any one of (1) to (23) for the preparation of a medicament for treating a tumor and/or cancer.
(25)根据(24)所述的应用,其中所述肿瘤和/或癌症包括肺癌、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病;优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。(25) The use according to (24), wherein the tumor and/or cancer comprises lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterus Cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer The treatment is positive for NKG2D ligand and the tumor and/or cancer is treated to become positive for NKG2D ligand.
(26)一种用于治疗肿瘤和/或癌症的具有协同作用的联合药物的药盒,包括:装有溶瘤病毒的第一容器和装有NK细胞的第二容器,其中所述第一容器和所述第二容器是独立的;以及载明给药时机和给药方式的说明书;其中所述溶瘤病毒能够选择性地在肿瘤细胞中复制;并且其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。(26) A kit for a synergistic combination drug for treating tumors and/or cancer, comprising: a first container containing an oncolytic virus and a second container containing NK cells, wherein the first container And the second container are independent; and instructions for indicating the timing and mode of administration; wherein the oncolytic virus is capable of selectively replicating in tumor cells; and wherein the surface of the NK cells is chimeric Antigen receptor modification comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, wherein the antigen binding domain is from NKG2D A ligand binding domain derived from the intracellular signaling region of DAP12.
(27)根据(26)所述的药盒,其中所述第一容器包含治疗有效量的所述溶瘤病毒,并且所述第二容器包含1×105至1×1010个细胞/天剂量的所述NK细胞。(27) The kit of (26), wherein the first container comprises a therapeutically effective amount of the oncolytic virus, and the second container comprises 1 x 105 to 1 x 1010 cells per day A dose of the NK cells.
(28)根据(27)所述的药盒,其中所述第二容器包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞。优选的是,所述第二容器包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞。(28) The kit according to (27), wherein the second container comprises the NK cells in a total dose ranging from 1 × 106 to 1 × 1011 per person per course of treatment. Preferably, the second container comprises the NK cells in a total dose ranging from 6 x 107 to 1.2 x 1010 per person per course of treatment.
(29)根据(26)所述的药盒,其中所述溶瘤病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。(29) The kit according to (26), wherein the oncolytic virus is selected from the group consisting of a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
(30)根据(26)所述的药盒,其中所述溶瘤病毒选自具有溶瘤作用的腺病毒、痘病毒、单纯疱疹病毒、麻疹病毒、塞姆利基森林病毒、水疱性口炎病毒、脊髓灰质炎病毒、逆转录病毒、呼肠孤病毒、塞内卡谷病毒、埃可型肠道病毒、柯萨奇病毒、新城疫病毒和马拉巴病毒。(30) The kit according to (26), wherein the oncolytic virus is selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis Virus, poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
(31)根据(26)所述的药盒,其中所述NK细胞选自自体NK细胞和异体NK细胞。(31) The kit according to (26), wherein the NK cells are selected from the group consisting of autologous NK cells and allogeneic NK cells.
(32)根据(31)所述的药盒,其中所述NK细胞为经体外扩增得到的自体NK细胞或经体外扩增得到的异体NK细胞。(32) The kit according to (31), wherein the NK cells are autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
(33)根据(26)所述的药盒,其中所述肿瘤和/或癌症包括肺癌、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病;优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。(33) The kit according to (26), wherein the tumor and/or cancer includes lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, Uterine cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer Untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive.
(34)根据(26)所述的药盒,其中所述溶瘤病毒配制成通过瘤内注射给药或静脉给药;所述NK细胞配制成通过静脉给药或局部给药。(34) The kit according to (26), wherein the oncolytic virus is formulated to be administered by intratumoral injection or intravenous administration; and the NK cells are formulated to be administered intravenously or topically.
(35)根据(26)-(34)中任一项所述的药盒,其中所述溶瘤病毒为具有溶瘤作用的腺病毒。The kit according to any one of (26) to (34), wherein the oncolytic virus is an adenovirus having an oncolysis effect.
(36)根据(35)所述的药盒,其中所述具有溶瘤作用的腺病毒的E1区和/或E3区是经基因工程改造的。(36) The kit according to (35), wherein the E1 region and/or the E3 region of the oncolytic adenovirus is genetically engineered.
(37)根据(35)所述的药盒,其中所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。(37) The kit according to (35), wherein the oncolytic adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN- 01, and / or ProstAtakTM.
(38)根据(26)所述的药盒,其中所述第一容器包含5×107至5×1012VP/天剂量的溶瘤腺病毒,并且所述第二容器包含1×105 至1×1010个细胞/天剂量的所述NK细胞。(38) The kit according to (26), wherein the first container comprises an oncolytic adenovirus of 5 × 107 to 5 × 1012 VP / day, and the second container comprises 1 × 105 The NK cells were dosed to 1 x 1010 cells/day.
(39)根据(38)所述的药盒,其中所述第二容器包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞。优选的是,所述第二容器包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞。(39) The kit according to (38), wherein the second container comprises the NK cells in a total dose ranging from 1 x 106 to 1 x 1011 per person per course of treatment. Preferably, the second container comprises the NK cells in a total dose ranging from 6 x 107 to 1.2 x 1010 per person per course of treatment.
(40)一种治疗肿瘤和/或癌症的方法,包括以下依次进行的步骤:(40) A method of treating a tumor and/or cancer, comprising the steps of:
1)对肿瘤和/或癌症患者施用溶瘤病毒,该溶瘤病毒能够选择性地在肿瘤细胞中复制;1) administering an oncolytic virus to a tumor and/or cancer patient, the oncolytic virus being capable of selectively replicating in a tumor cell;
2)在施用所述溶瘤病毒之后的第18小时至72小时,对所述肿瘤和/或癌症患者施用NK细胞;2) administering NK cells to said tumor and/or cancer patient from 18 hours to 72 hours after administration of said oncolytic virus;
其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。Wherein the surface of the NK cell is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that In the above, the antigen binding domain is derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
(41)根据(40)所述的方法,其中所述溶瘤病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。(41) The method according to (40), wherein the oncolytic virus is selected from the group consisting of a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect.
(42)根据(40)所述的方法,其中所述溶瘤病毒选自具有溶瘤作用的腺病毒、痘病毒、单纯疱疹病毒、麻疹病毒、塞姆利基森林病毒、水疱性口炎病毒、脊髓灰质炎病毒、逆转录病毒、呼肠孤病毒、塞内卡谷病毒、埃可型肠道病毒、柯萨奇病毒、新城疫病毒和马拉巴病毒。(42) The method according to (40), wherein the oncolytic virus is selected from the group consisting of an oncolytic adenovirus, a poxvirus, a herpes simplex virus, a measles virus, a Semliki forest virus, and a vesicular stomatitis virus. , poliovirus, retrovirus, reovirus, Seneca Valley virus, Echo enterovirus, Coxsackie virus, Newcastle disease virus and Maraba virus.
(43)根据(40)所述的方法,其中所述NK细胞选自自体NK细胞和异体NK细胞。(43) The method according to (40), wherein the NK cells are selected from the group consisting of autologous NK cells and allogeneic NK cells.
(44)根据(43)所述的方法,其中所述NK细胞为经体外扩增得到的自体NK细胞或经体外扩增得到的异体NK细胞。(44) The method according to (43), wherein the NK cells are autologous NK cells obtained by in vitro expansion or allogeneic NK cells obtained by in vitro expansion.
(45)根据(40)所述的方法,其中所述肿瘤和/或癌症包括肺癌、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病;优选地,所述肿瘤和/或癌症是NKG2D配 体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。(45) The method according to (40), wherein the tumor and/or cancer comprises lung cancer, melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterus Cancer, cervical cancer, lymphoma, gastric cancer, esophageal cancer, renal cancer, prostate cancer, pancreatic cancer, leukemia; preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer The treatment is positive for NKG2D ligand and the tumor and/or cancer is treated to become positive for NKG2D ligand.
(46)根据(40)所述的方法,其中所述溶瘤病毒的施用剂量为治疗有效量,每天1次,连续施用1-6天。(46) The method according to (40), wherein the oncolytic virus is administered in a therapeutically effective amount once a day for 1-6 days.
(47)根据(40)所述的方法,其中所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个,每个疗程3周共21天,每周施用1-2次。(47) The method according to (40), wherein the NK cells are administered at a dose ranging from 1×106 to 1×1011 per treatment per subject, and each treatment lasts for 3 weeks for 21 days. Apply 1-2 times a week.
(48)根据(40)所述的方法,其中所述溶瘤病毒的施用剂量为治疗有效量,每2天1次,连续施用2-6天。(48) The method according to (40), wherein the oncolytic virus is administered in a therapeutically effective amount, once every 2 days, for 2-6 consecutive days.
(49)根据(40)所述的方法,其中所述NK细胞的施用剂量为每人每个疗程总剂量范围为6×107-1.2×1010个,每个疗程3周共21天,每周施用1-2次。(49) The method according to (40), wherein the NK cell is administered at a dose ranging from 6×107 to 1.2×1010 per subject per treatment, and each treatment lasts for 3 weeks for 21 days. Apply 1-2 times a week.
(50)根据(40)所述的方法,其中所述溶瘤病毒通过瘤内注射给药或静脉给药;所述NK细胞通过静脉给药或局部给药。(50) The method according to (40), wherein the oncolytic virus is administered by intratumoral injection or intravenous administration; and the NK cells are administered intravenously or topically.
(51)根据(40)-(50)中任一项所述的方法,其中所述溶瘤病毒为具有溶瘤作用的腺病毒。The method of any one of (40) to (50), wherein the oncolytic virus is an adenovirus having an oncolysis effect.
(52)根据(51)所述的方法,其中所述具有溶瘤作用的腺病毒的E1区和/或E3区是经基因工程改造的。(52) The method according to (51), wherein the E1 region and/or the E3 region of the oncolytic adenovirus is genetically engineered.
(53)根据(51)所述的方法,其中所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。(53) The method according to (51), wherein the oncolytic adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS -102, ColoAd1, VCN-01, and / or ProstAtakTM.
(54)根据(40)所述的方法,其中所述溶瘤病毒为具有溶瘤作用的腺病毒,并且其施用剂量为5×107至5×1012VP/天。(54) The method according to (40), wherein the oncolytic virus is an adenovirus having an oncolysis effect, and is administered at a dose of 5 × 107 to 5 × 1012 VP / day.
本发明与现有技术相比具有以下优点和积极效果:Compared with the prior art, the invention has the following advantages and positive effects:
本发明的嵌合抗原受体能够使被其修饰的NK细胞(也称为“工程化NKG2D配体靶向性NK细胞”)对多种NKG2D配体表达阳性的肿瘤具有强烈且特异的靶向杀伤活性,本发明的临床前研究已充分证明该修饰的NK细胞可以明显减小甚至消除动物体内的肿瘤负荷, 延长动物的存活时间。The chimeric antigen receptor of the present invention enables the NK cells modified by it (also referred to as "engineered NKG2D ligand-targeted NK cells") to have strong and specific targeting to tumors positive for expression of various NKG2D ligands. The killing activity, the preclinical study of the present invention has fully demonstrated that the modified NK cells can significantly reduce or even eliminate the tumor burden in the animal and prolong the survival time of the animal.
用本发明的工程化NKG2D配体靶向性NK细胞治疗肿瘤时,可有效避免采用CAR-T治疗时所引起的细胞因子风暴和免疫排斥反应。When treating tumors with the engineered NKG2D ligand-targeted NK cells of the present invention, cytokine storms and immune rejection caused by CAR-T treatment can be effectively avoided.
本发明的工程化NKG2D配体靶向性的NK细胞为治疗NKG2D配体阳性肿瘤患者提供了一种新的选择,具有良好的产业应用前景。The engineered NKG2D ligand-targeted NK cells of the invention provide a new choice for treating NKG2D ligand-positive tumor patients, and have good industrial application prospects.
另外,本发明首次提出将溶瘤病毒与本发明所述嵌合抗原受体修饰的NK细胞联用以治疗肿瘤和/或癌症的构思,基于该构思而提供的药物组合物和方法能够充分发挥溶瘤病毒选择性地在肿瘤细胞中复制并杀死肿瘤细胞、以及进一步引起随后的机体免疫反应的作用,同时还能够充分发挥所述NK细胞杀伤肿瘤细胞的功能,并且巧妙地利用了溶瘤病毒选择性地在肿瘤细胞中复制的特点,使得含有溶瘤病毒的肿瘤细胞成为了NK细胞的特异性靶标,从而进一步增强了所述NK细胞的肿瘤杀伤作用。更重要的是,本发明发现溶瘤病毒可以刺激肿瘤细胞表面多种NKG2D配体表达的增加,从而更进一步地与本发明所述的嵌合抗原受体修饰的NK细胞配合产生更强的抗肿瘤协同作用。本发明发现仅将溶瘤病毒与所述NK细胞联用即可产生协同效果。此外,溶瘤病毒与所述NK细胞均有识别肿瘤细胞的特性,对正常细胞基本不会造成杀伤,两者的联合使用在安全性和疗效性上都有显著的优势。Further, the present invention proposes for the first time the concept of combining an oncolytic virus with the chimeric antigen receptor-modified NK cells of the present invention for treating tumors and/or cancer, and the pharmaceutical composition and method provided based on the concept can be fully utilized. The oncolytic virus selectively replicates in tumor cells and kills tumor cells, and further causes subsequent immune responses, while also fully utilizing the function of the NK cells to kill tumor cells, and skillfully utilizing oncolytic The characteristic that the virus selectively replicates in tumor cells makes the tumor cells containing the oncolytic virus a specific target of NK cells, thereby further enhancing the tumor killing effect of the NK cells. More importantly, the present inventors have found that oncolytic viruses can stimulate the increase of the expression of various NKG2D ligands on the surface of tumor cells, thereby further cooperating with the chimeric antigen receptor-modified NK cells of the present invention to produce stronger resistance. Tumor synergy. The present inventors have found that only the combination of oncolytic virus and the NK cells produces a synergistic effect. In addition, the oncolytic virus and the NK cells have the characteristics of recognizing tumor cells, and basically do not cause damage to normal cells, and the combined use of the two has significant advantages in safety and efficacy.
进一步地,本发明通过理论探索和实验研究,使得溶瘤病毒和所述NK细胞各自的施用剂量、施用顺序和施用间隔能够使两者的联合施用达到最大效率的协同作用,同时避免了两者之间的相互制约,从而达到有效地治疗肿瘤和/或癌症的效果。Further, the present invention, through theoretical exploration and experimental research, enables the respective administration dose, application sequence and application interval of the oncolytic virus and the NK cell to achieve the synergistic effect of the maximum efficiency of the combined application of both, while avoiding both Mutual constraints between them to achieve effective treatment of tumors and / or cancer.
图1为示出本发明一个实施方案的嵌合抗原受体NKG2D-CD8-DAP12的构建示意图,其中“CMV”表示CMV启动子序列,“T7”表示T7启动子序列,“5’UTR”表示具有Kozak序列的5’UTR,“SP”表示GM-CSFα链信号肽编码序列,“NKG2D”表示NKG2D的配体结合区的编码序列,“CD8”表示CD8α的铰链 区和跨膜区编码序列,“DAP12”表示DAP12的胞内信号区编码序列,“α球蛋白3’UTR”表示具有PolyA信号的α球蛋白的3’UTR,“pA150”表示polyA(多聚腺苷酸),其中包含150个A。BRIEF DESCRIPTION OF THE DRAWINGS Fig. 1 is a schematic diagram showing the construction of a chimeric antigen receptor NKG2D-CD8-DAP12 according to an embodiment of the present invention, wherein "CMV" indicates a CMV promoter sequence, "T7" indicates a T7 promoter sequence, and "5'UTR" indicates a 5'UTR having a Kozak sequence, "SP" indicates a GM-CSF alpha chain signal peptide coding sequence, "NKG2D" indicates a coding sequence of a ligand binding region of NKG2D, and "CD8" indicates a hinge region and a transmembrane region coding sequence of CD8α, "DAP12" denotes the intracellular signal region coding sequence of DAP12, "alpha globulin 3'UTR" denotes the 3'UTR of the alpha globulin having a PolyA signal, and "pA150 " denotes polyA (polyadenylation), which comprises 150 A.
图2为示出本发明一个实施方案的表达载体pFastbac1-CD8-DAP12经限制性内切酶SphI和SalI双酶切的鉴定片段的电泳图;其中泳道1为DNA分子量标记,泳道2为鉴定片段。Figure 2 is an electropherogram showing the identification fragment of the expression vector pFastbac1-CD8-DAP12 digested with restriction endonucleases SphI and SalI according to one embodiment of the present invention; wherein
图3为示出本发明一个实施方案的表达载体pFastbac1-NKG2D-CD8-DAP12经限制性内切酶SphI和NheI双酶切的鉴定片段的电泳图;其中泳道1为DNA分子量标记,泳道2为鉴定片段。Figure 3 is an electropherogram showing the identification fragment of the expression vector pFastbac1-NKG2D-CD8-DAP12 digested with restriction endonucleases SphI and NheI according to an embodiment of the present invention; wherein
图4为示出本发明一个实施方案的嵌合型抗原受体的重组DNA载体pFastbac1-NKG2D-CD8-DAP12的结构示意图;其中,顺时针序列为正向基因片段,逆时针为反向基因片段。其中“CMV”表示CMV启动子序列,“T7”表示T7启动子序列,“5’UTR”表示具有Kozak序列的5’UTR,“GM-CSFα”表示GM-CSFα链信号肽编码序列,“NKG2D”表示NKG2D的配体结合区的编码序列,“CD8”表示CD8α的铰链区和跨膜区编码序列,“DAP12”表示DAP12的胞内信号区编码序列,“α球蛋白3’UTR”表示具有PolyA信号的α球蛋白的3’UTR。Figure 4 is a schematic view showing the structure of a recombinant DNA vector pFastbac1-NKG2D-CD8-DAP12 of a chimeric antigen receptor according to an embodiment of the present invention; wherein the clockwise sequence is a forward gene fragment and the counterclockwise is a reverse gene fragment . Wherein "CMV" denotes a CMV promoter sequence, "T7" denotes a T7 promoter sequence, "5'UTR" denotes a 5'UTR having a Kozak sequence, "GM-CSFα" denotes a GM-CSF alpha chain signal peptide coding sequence, "NKG2D "The coding sequence of the ligand binding region of NKG2D, "CD8" indicates the hinge region and transmembrane region coding sequence of CD8α, "DAP12" indicates the intracellular signal region coding sequence of DAP12, and "alpha globulin 3'UTR" indicates The 3'UTR of the alpha globulin of the PolyA signal.
图5示出流式细胞术对NK细胞表型的分析结果。图5A示出从外周血单个核细胞扩增17天时NK细胞的纯度。横坐标表示CD3表达强度,纵坐标表示CD56表达强度;图5B示出从外周血单个核细胞扩增17天时NK细胞内源NKG2D和CD16的表达强度,横坐标表示NKG2D表达强度,纵坐标表示CD16表达强度。Figure 5 shows the results of analysis of NK cell phenotype by flow cytometry. Figure 5A shows the purity of NK cells when expanded from peripheral blood mononuclear cells for 17 days. The abscissa indicates the CD3 expression intensity, and the ordinate indicates the CD56 expression intensity; FIG. 5B shows the expression intensity of NK cells endogenous NKG2D and CD16 when expanded from peripheral blood mononuclear cells for 17 days, the abscissa indicates the intensity of NKG2D expression, and the ordinate indicates CD16. Expression intensity.
图6示出体外合成的嵌合抗原受体NKG2D-CD8-DAP12线性化DNA模板的电泳图;其中泳道1为DNA分子量标记,泳道2为线性化DNA模板。Figure 6 shows an electropherogram of a chimeric antigen receptor NKG2D-CD8-DAP12 linearized DNA template synthesized in vitro;
图7示出体外合成的嵌合抗原受体NKG2D-CD8-DAP12的mRNA的电泳图;其中泳道1为分子量标记,泳道2为NKG2D-CD8-DAP12的mRNA。Figure 7 shows an electropherogram of mRNA of the chimeric antigen receptor NKG2D-CD8-DAP12 synthesized in vitro;
图8示出流式细胞术对嵌合抗原受体NKG2D-CD8-DAP12 mRNA电转NK细胞效率的检测结果。图8A示出NK细胞内源表达NKG2D的强度(其中左侧峰为阴性对照曲线,右侧峰为NK细胞NKG2D表达强度曲线);图8B示出电转染后NK细胞表达NKG2D的强度(其中左侧峰为阴性对照曲线,右侧峰为NK细胞电转NKG2D-CD8-DAP12 mRNA之后NKG2D的表达强度曲线);图8C示出NK细胞内源表达和电转染后表达NKG2D的强度的比较(即,将图8A和8B合并在一起)(其中左侧峰为阴性对照曲线,中间峰为NK细胞NKG2D表达强度曲线,右侧峰为NK细胞电转NKG2D-CD8-DAP12 mRNA之后NKG2D的表达强度曲线)。图中横坐标表示NKG2D表达强度,纵坐标表示相对细胞数。横坐标中“NKG2D荧光强度”表示用带荧光的NKG2D抗体检测时,流式细胞仪所显示的荧光读数。Figure 8 shows the results of flow cytometry detection of chimeric antigen receptor NKG2D-CD8-DAP12 mRNA electroporation NK cells. Figure 8A shows the intensity of endogenous expression of NKG2D by NK cells (where the left peak is the negative control curve and the right peak is the NK cell NKG2D expression intensity curve); Figure 8B shows the intensity of NKG2D expression by NK cells after electroporation (where The left peak is the negative control curve, and the right peak is the NKG2D expression intensity curve after NK cell electrotransformation of NKG2D-CD8-DAP12 mRNA; Figure 8C shows the comparison of the intensity of NKG2D expression after endogenous expression and electrotransfection of NK cells ( That is, 8A and 8B are combined together) (the left peak is a negative control curve, the middle peak is the NK cell NKG2D expression intensity curve, and the right peak is the NKG2D-CD8-DAP12 mRNA expression level after NK cell electroporation. ). In the figure, the abscissa indicates the intensity of NKG2D expression, and the ordinate indicates the relative cell number. "NKG2D fluorescence intensity" in the abscissa indicates the fluorescence reading displayed by the flow cytometer when detected with a fluorescent NKG2D antibody.
图9示出本发明实施例的嵌合抗原受体NKG2D-CD8-DAP12修饰的NK(NKG2D-CAR-NK)细胞与人肿瘤细胞混合培养后,释放IFN-γ的Elispot分析结果。图中“mGFP-CAR-NK”表示mGFP-CAR修饰的NK细胞组(对照组),“NKG2D-CAR-NK”表示嵌合抗原受体NKG2D-CD8-DAP12(即NKG2D-CAR)修饰的NK细胞组。图中横坐标表示肿瘤细胞组别,纵坐标表示相对IFN-γ数量(以每2.5×104个NK细胞中的斑点数表示)。Figure 9 is a graph showing the results of Elispot analysis of IFN-γ release after mixed culture of chimeric antigen receptor NKG2D-CD8-DAP12-modified NK (NKG2D-CAR-NK) cells and human tumor cells in an embodiment of the present invention. In the figure, "mGFP-CAR-NK" indicates an mGFP-CAR-modified NK cell group (control group), and "NKG2D-CAR-NK" indicates a chimeric antigen receptor NKG2D-CD8-DAP12 (i.e., NKG2D-CAR)-modified NK. Cell group. In the figure, the abscissa indicates the tumor cell group, and the ordinate indicates the relative amount of IFN-γ (expressed as the number of spots per 2.5 × 104 NK cells).
图10A-G分别示出本发明实施例的NKG2D-CAR-NK对肿瘤细胞HCT116(A)、SKOV3(B)、Fadu(C)、Detroit(D)、HepG2(E)、MCF7(F)、KG1(G)的杀伤活力检测结果;其中纵坐标表示肿瘤细胞被杀伤后裂解的比例;横坐标表示效应细胞和肿瘤细胞的比例;实线即“NKG2D-CAR-NK”示出嵌合抗原受体NKG2D-CAR修饰的NK细胞组的结果;虚线即“mGFP-CAR-NK”示出嵌合抗原受体mGFP-CAR修饰的NK细胞组(对照组)的结果。10A-G show NKG2D-CAR-NK versus tumor cells HCT116(A), SKOV3(B), Fadu(C), Detroit(D), HepG2(E), MCF7(F), respectively, according to an embodiment of the present invention, The killing activity test result of KG1(G); the ordinate indicates the proportion of tumor cells ruptured after killing; the abscissa indicates the ratio of effector cells to tumor cells; the solid line is “NKG2D-CAR-NK” showing chimeric antigen Results of the NKG2D-CAR-modified NK cell group; the dotted line, "mGFP-CAR-NK", shows the results of the chimeric antigen receptor mGFP-CAR-modified NK cell group (control group).
图11示出过继回输NKG2D-CAR-NK细胞对肿瘤细胞的杀伤作用;虚线示出嵌合抗原受体NKG2D-CD8-DAP12修饰NK细胞组的结果(图中示为“NKG2D-CAR-NK(6次注射)”);实线示出未 进行NK细胞回输组的结果(图中示为“PBS对照组”)。图中横坐标表示接种肿瘤后的天数,纵坐标表示活体成像仪记录的动物体内肿瘤细胞荧光强度,其中纵坐标中所示辐射率(单位为“p/秒/cm2/sr”,即,photons/sec/cm2/steradian)指单位时间、单位面积、单位弧度从动物体表发出的光子数。Figure 11 shows the killing effect of adoptively NKG2D-CAR-NK cells on tumor cells; the dotted line shows the results of the chimeric antigen receptor NKG2D-CD8-DAP12 modified NK cell group (shown as "NKG2D-CAR-NK" (6 injections)"); the solid line shows the results of the NK cell reinfusion group (shown as "PBS control group"). In the figure, the abscissa indicates the number of days after inoculation of the tumor, and the ordinate indicates the fluorescence intensity of the tumor cells in the animal recorded by the living imager, wherein the radiance shown in the ordinate (in units of "p/sec/cm2 /sr", ie, Photons/sec/cm2 /steradian) refers to the number of photons emitted from the surface of the animal per unit time, unit area, and unit radians.
图12A-C分别示出嵌合抗原受体NKG2D-CD8-DAP12修饰的NK细胞和嵌合抗原受体NKG2D-CD8-CD3Z修饰的NK细胞分别对肿瘤细胞SKOV3(A)、Detroit(B)、HCT116(C)的杀伤活力的检测结果比较。图中“mGFP-CAR-NK”表示mGFP-CAR修饰的NK细胞组,“NKG2D-DAP12-CAR-NK”表示NKG2D-DAP12-CAR修饰的NK细胞组,“NKG2D-CD3Z-CAR-NK”表示NKG2D-CD3Z-CAR修饰的NK细胞组。图中横坐标表示效应细胞和肿瘤细胞的比例,纵坐标表示肿瘤细胞被杀伤后裂解的比例。Figure 12A-C shows chimeric antigen receptor NKG2D-CD8-DAP12 modified NK cells and chimeric antigen receptor NKG2D-CD8-CD3Z modified NK cells, respectively, for tumor cells SKOV3 (A), Detroit (B), Comparison of the detection results of the killing activity of HCT116(C). In the figure, "mGFP-CAR-NK" indicates an mGFP-CAR-modified NK cell group, "NKG2D-DAP12-CAR-NK" indicates an NKG2D-DAP12-CAR-modified NK cell group, and "NKG2D-CD3Z-CAR-NK" indicates NKG2D-CD3Z-CAR modified NK cell group. In the figure, the abscissa indicates the ratio of effector cells to tumor cells, and the ordinate indicates the proportion of tumor cells that are lysed after killing.
图13示出流式细胞术对H101处理的肿瘤细胞系Fadu表面NKG2D配体的检测结果。图中黑色柱子代表没有H101处理的肿瘤细胞,点状花纹柱子代表H101处理后的肿瘤细胞;横坐标表示不同的NKG2D配体染色组(从左到右依次为hULBP1、hULBP3、hULBP4、hULBP2/5/6、MICA/B),纵坐标为表达NKG2D配体的细胞数百分比。Figure 13 shows the results of flow cytometry detection of the H101-treated tumor cell line Fadu surface NKG2D ligand. The black column in the figure represents tumor cells without H101 treatment, the punctate column represents H101-treated tumor cells, and the abscissa represents different NKG2D ligand staining groups (from left to right, hULBP1, hULBP3, hULBP4, hULBP2/5) /6, MICA/B), the ordinate is the percentage of cells expressing the NKG2D ligand.
图14示出流式细胞术对H101处理的肿瘤细胞系HepG2表面NKG2D配体的检测结果。图中黑色柱子代表没有H101处理的肿瘤细胞,点状花纹柱子代表H101处理后的肿瘤细胞;横坐标表示不同的NKG2D配体染色组(从左到右依次为hULBP1、hULBP3、hULBP4、hULBP2/5/6、MICA/B),纵坐标为表达NKG2D配体的细胞数百分比。Figure 14 shows the results of flow cytometry detection of H101G-treated tumor cell line HepG2 surface NKG2D ligand. The black column in the figure represents tumor cells without H101 treatment, the punctate column represents H101-treated tumor cells, and the abscissa represents different NKG2D ligand staining groups (from left to right, hULBP1, hULBP3, hULBP4, hULBP2/5) /6, MICA/B), the ordinate is the percentage of cells expressing the NKG2D ligand.
图15示出嵌合抗原受体NKG2D-CD8-DAP12修饰的NK细胞对肿瘤细胞Fadu的杀伤活力的检测结果比较。图中黑色柱子代表没有H101处理的肿瘤细胞,点状花纹柱子代表H101处理后的肿瘤细胞;“w/o CAR-NK”表示没有添加CAR-NK细胞、但添加NK细胞,“CAR-NK”表示添加有NKG2D-DAP12-CAR修饰的NK细胞。图中横坐标表示不同的实验组,纵坐标表示肿瘤细胞被杀伤后裂解的比例,效应细胞和靶细胞的比例为1∶1。Figure 15 is a graph showing the results of detection of the killing activity of the chimeric antigen receptor NKG2D-CD8-DAP12-modified NK cells against tumor cell Fadu. In the figure, the black column represents the tumor cells without H101 treatment, the dotted pattern column represents the tumor cells after H101 treatment; "w/o CAR-NK" means that no CAR-NK cells are added, but NK cells are added, "CAR-NK" Indicates NK cells supplemented with NKG2D-DAP12-CAR modification. In the figure, the abscissa indicates different experimental groups, and the ordinate indicates the ratio of lysis of tumor cells after killing, and the ratio of effector cells to target cells is 1:1.
图中“*”表示p<0.05,“**”表示p<0.01。“***”表示p<0.001。In the figure, "*" indicates p<0.05, and "**" indicates p<0.01. "***" indicates p < 0.001.
以下通过具体实施方式的描述并参照附图对本发明作进一步说明,但这并非是对本发明的限制,本领域技术人员根据本发明的基本思想,可以做出各种修改或改进,但是只要不脱离本发明的基本思想,均在本发明的范围之内。The invention is further described by the following description of the embodiments and with reference to the accompanying drawings, but this is not a limitation of the invention, and those skilled in the art can make various modifications or improvements according to the basic idea of the invention, but The basic idea of the invention is within the scope of the invention.
在本发明中,词语“肿瘤”、“癌症”、“肿瘤细胞”、“癌细胞”涵盖本领域通常认为的含义。In the present invention, the words "tumor", "cancer", "tumor cell", "cancer cell" encompass the meanings generally recognized in the art.
CAR包括胞外部分、跨膜区和胞内部分。胞外部分又包括用于识别和结合抗原的抗原结合结构域、以及用于间隔抗原结合结构域和跨膜区的间隔区;胞内部分主要包括用于信号传递的胞内结构域。对于携带CAR的免疫细胞而言,CAR的功能性部分的选择、及其与免疫细胞种类的配合极为重要,这与对肿瘤的特异性杀伤能力密切相关。本发明的发明人通过理论研究和实验摸索,针对NK细胞选择了特定的抗原结合结构域和胞内结构域的组合,并将由此开发的CAR成功地应用于了NK细胞,使该NK细胞发挥了强烈的靶向杀瘤活性,由此开发出在肿瘤免疫治疗中可供选择的新型且效果良好的工程化NKG2D配体靶向性的NK细胞。CAR includes an extracellular portion, a transmembrane region, and an intracellular portion. The extracellular portion in turn includes an antigen binding domain for recognizing and binding an antigen, and a spacer for spacing the antigen binding domain and the transmembrane region; the intracellular portion primarily includes an intracellular domain for signaling. For immune cells carrying CAR, the selection of the functional part of CAR and its cooperation with immune cell types are extremely important, which is closely related to the specific killing ability of tumors. The inventors of the present invention have selected a specific combination of an antigen-binding domain and an intracellular domain for NK cells through theoretical research and experimental exploration, and successfully applied the thus developed CAR to NK cells to make the NK cells play. A strong targeted tumoricidal activity, thereby developing a novel and effective engineered NKG2D ligand-targeted NK cell that is available for selection in tumor immunotherapy.
本发明所述术语“抗原结合结构域”、“间隔区”、“跨膜区”、“胞内结构域”的定义可参考“《免疫学导论》,于善谦,高等教育出版社,2008”;以及“《Immunobiology》,第七版,Kenneth Murphy,Paul Travers,Mark Walport等”。The definitions of the terms "antigen-binding domain", "spacer", "transmembrane region" and "intracellular domain" of the present invention can be referred to "Introduction to Immunology", Yu Shanqian, Higher Education Press, 2008 "; and "Immunobiology, seventh edition, Kenneth Murphy, Paul Travers, Mark Walport, etc.".
具体而言,本发明提供了一种嵌合抗原受体,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。In particular, the invention provides a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane region and an intracellular domain, Characterized in that the antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
NKG2D是调节NK细胞杀伤活性的重要受体,NKG2D的配体主要表达于肿瘤细胞和应激细胞表面,很少表达甚至不表达于正常细胞表面,大量的肿瘤细胞如结直肠癌细胞、卵巢癌细胞、头颈癌细胞、 淋巴癌细胞、神经胶质瘤细胞等都有大量NKG2D的配体的表达。NKG2D is an important receptor regulating NK cell killing activity. NKG2D ligand is mainly expressed on the surface of tumor cells and stress cells, and is rarely expressed or even expressed on the surface of normal cells. A large number of tumor cells such as colorectal cancer cells and ovarian cancer Cells, head and neck cancer cells, lymphatic cancer cells, glioma cells, and the like have a large number of ligands for NKG2D expression.
本发明进一步发现,所述抗原结合结构域的氨基酸序列优选与NKG2D的第X位-第216位氨基酸序列一致,且73≤X≤83,X为整数。其中,NKG2D的氨基酸序列可为NCBI(即,美国国立生物技术信息中心,网址:https://www.ncbi.nlm.nih.gov)的Genbank中编号为NP_031386.2的氨基酸序列。也就是说,所述抗原结合结构域的氨基酸序列优选选自NKG2D的第73-216位氨基酸、并且包含第83-216位氨基酸。例如,所述抗原结合结构域的氨基酸序列如以下氨基酸序列组中任一氨基酸序列所示:NKG2D的第73-216位氨基酸、第74-216位氨基酸、第75-216位氨基酸、第76-216位氨基酸、第77-216位氨基酸、第78-216位氨基酸、第79-216位氨基酸、第80-216位氨基酸、第81-216位氨基酸、第82-216位氨基酸、或第83-216位氨基酸。更优选地,所述抗原结合结构域的氨基酸序列如SEQ ID NO:3所示。The present invention further found that the amino acid sequence of the antigen-binding domain is preferably identical to the X-position 216 amino acid sequence of NKG2D, and 73 ≤ X ≤ 83, and X is an integer. The amino acid sequence of NKG2D may be the amino acid sequence of NP_031386.2 in Genbank of NCBI (ie, National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov). That is, the amino acid sequence of the antigen-binding domain is preferably selected from amino acids 73-216 of NKG2D and comprises amino acids 83-216. For example, the amino acid sequence of the antigen-binding domain is as shown in any one of the following amino acid sequence groups: amino acids 73-216 of NKG2D, amino acids 74-216, amino acids 75-216, 76- 216 amino acids, amino acids 77-216, amino acids 78-216, amino acids 79-216, amino acids 80-216, amino acids 81-216, amino acids 82-216, or 83- 216 amino acids. More preferably, the amino acid sequence of the antigen binding domain is set forth in SEQ ID NO: 3.
胞内结构域起到信号传递从而活化NK细胞的作用。最初用于T细胞的CAR的胞内结构域只有一个信号分子,通常为免疫球蛋白E的受体相关FcεRIγ(与IgE有高亲和力的受体的一个亚基)或T细胞抗原受体信号的基础传导分子DAP12;有的胞内结构域包含由一种或几种T细胞活化基序组成的T细胞活化结构域。本发明发现,将上述来自NKG2D的配体结合区的抗原结合结构域与来自DAP12的胞内信号区进行组合,可得到能够使NK细胞发挥强烈的靶向杀瘤活性的CAR。优选地,所述胞内结构域的氨基酸序列选自DAP12的第62-113位氨基酸,所述胞内结构域的氨基酸序列更优选如SEQ ID NO:5所示。其中,DAP12的氨基酸序列的Genbank编号为NP_003323.1。The intracellular domain acts as a signal to activate NK cells. The intracellular domain of the CAR originally used for T cells has only one signaling molecule, usually the receptor-associated FcεRIγ of immunoglobulin E (a subunit of a receptor with high affinity for IgE) or T cell antigen receptor signaling. The underlying conductive molecule DAP12; some intracellular domains comprise a T cell activation domain consisting of one or several T cell activation motifs. The present inventors have found that by combining the antigen-binding domain derived from the ligand binding region of NKG2D described above with the intracellular signal region derived from DAP12, a CAR capable of exerting a strong targeted tumoricidal activity of NK cells can be obtained. Preferably, the amino acid sequence of the intracellular domain is selected from amino acids 62-113 of DAP12, and the amino acid sequence of the intracellular domain is more preferably as set forth in SEQ ID NO: 5. Among them, the amino acid sequence of DAP12 has the Genbank number NP_003323.1.
本发明更进一步选择了间隔区和跨膜区,从而获得了具有抗原结合结构域-间隔区-跨膜区-胞内结构域的特定组合的CAR。间隔区连接识别和结合抗原的抗原结合结构域和跨膜区,这一区域的结构应该是易弯曲的,这样可以使抗原结合结构域适应不同的方向,以促进抗原的识别和结合。形式最简单的间隔区是免疫球蛋白IgGl的铰链区(hinge),也可以是免疫球蛋白CH2CH3区的一部分。跨膜区一般是跨越细胞膜的疏水性α螺旋。通过研究和实验摸索,本发明发现,所述 间隔区优选来自CD8α的铰链区,所述跨膜区优选来自CD8α的跨膜区。CD8为跨膜的糖基化膜蛋白,由α和β两个亚基组成,与T细胞表面受体共同作用使T细胞与特定抗原结合,CD8特异性结合MHC I,介导细胞毒性T细胞的杀伤作用。The present invention further selects the spacer and transmembrane regions, thereby obtaining a CAR having a specific combination of antigen binding domain-spacer-transmembrane region-intracellular domain. The spacer junction recognizes and binds to the antigen binding domain and transmembrane region of the antigen. The structure of this region should be flexible so that the antigen binding domain can be adapted to different orientations to facilitate antigen recognition and binding. The simplest form of spacer region is an immunoglobulin hinge region of IgGl (hinge), it may be a portion of an immunoglobulin CH2 CH3 region. The transmembrane region is typically a hydrophobic alpha helix spanning the cell membrane. Through research and experimental exploration, the present inventors have found that the spacer is preferably derived from the hinge region of CD8[alpha], which is preferably from the transmembrane region of CD8[alpha]. CD8 is a transmembrane glycosylated membrane protein consisting of two subunits, alpha and beta, which interact with T cell surface receptors to bind T cells to specific antigens. CD8 specifically binds to MHC I and mediates cytotoxic T cells. The killing effect.
更优选地,所述间隔区和跨膜区构成间隔跨膜区,并且其中所述间隔跨膜区的氨基酸序列与CD8α的第Y位-第210位氨基酸序列一致,且118≤Y≤128,Y为整数。其中,CD8α的氨基酸序列的Genbank编号可为NP_001139345.1。也就是说,所述间隔跨膜区的氨基酸序列优选选自CD8α的第118-210位氨基酸、并且包含第128-210位氨基酸。例如,所述间隔跨膜区的氨基酸序列如以下氨基酸序列组中任一氨基酸序列所示:CD8α的第118-210位氨基酸、第119-210位氨基酸、第120-210位氨基酸、第121-210位氨基酸、第122-210位氨基酸、第123-210位氨基酸、第124-210位氨基酸、第125-210位氨基酸、第126-210位氨基酸、第127-210位氨基酸、或第128-210位氨基酸。More preferably, the spacer region and the transmembrane region constitute a spacer transmembrane region, and wherein the amino acid sequence of the spacer transmembrane region is identical to the amino acid sequence of the Yth to the 210th position of CD8α, and 118≤Y≤128, Y is an integer. Wherein, the Genbank number of the amino acid sequence of CD8α may be NP_001139345.1. That is, the amino acid sequence of the spacer transmembrane region is preferably selected from amino acids 118-210 of CD8α and amino acids 128-210. For example, the amino acid sequence of the spacer transmembrane region is as shown in any one of the following amino acid sequence groups: amino acids 118-210 of CD8α, amino acids 119-210, amino acids 120-210, 121- 210 amino acids, amino acids 122-210, amino acids 123-210, amino acids 124-210, amino acids 125-210, amino acids 126-210, amino acids 127-210, or 128- 210 amino acids.
更优选地,所述间隔跨膜区的氨基酸序列如SEQ ID NO:4所示。More preferably, the amino acid sequence of the spacer transmembrane region is set forth in SEQ ID NO:4.
在本发明的嵌合抗原受体中,抗原结合结构域、间隔区、跨膜区和胞内结构域是依次串联的;抗原结合结构域和间隔区之间、间隔区和跨膜区之间、跨膜区和胞内结构域之间是可操作地连接的,例如可以采用接头连接,也可以不采用接头而直接连接。在本发明一个实施方案中,抗原结合结构域和间隔区之间采用接头连接(所述接头(例如)为-Ala-Ser-),而间隔区和跨膜区之间、跨膜区和胞内结构域之间未采用接头而直接连接。In the chimeric antigen receptor of the present invention, the antigen-binding domain, the spacer, the transmembrane region and the intracellular domain are sequentially connected in series; between the antigen-binding domain and the spacer, between the spacer and the transmembrane region The transmembrane region and the intracellular domain are operably linked, for example, may be connected by a joint or directly without a joint. In one embodiment of the invention, a linker is used between the antigen binding domain and the spacer (the linker is, for example, -Ala-Ser-), and the spacer and transmembrane region, the transmembrane region and the cell The internal domains are directly connected without a joint.
在本发明一个优选的实施方案中,所述嵌合抗原受体的氨基酸序列如SEQ ID NO:1所示。在本发明另一个优选的实施方案中,所述嵌合抗原受体具有在SEQ ID NO:1所示氨基酸序列中替换、删除、和/或添加一个或多个氨基酸而得到的氨基酸序列;例如,所述嵌合抗原受体具有与SEQ ID NO:1所示氨基酸序列至少90%、优选至少95%、更优选至少99%的一致性。In a preferred embodiment of the invention, the amino acid sequence of the chimeric antigen receptor is set forth in SEQ ID NO: 1. In another preferred embodiment of the present invention, the chimeric antigen receptor has an amino acid sequence obtained by replacing, deleting, and/or adding one or more amino acids in the amino acid sequence shown in SEQ ID NO: 1; The chimeric antigen receptor has at least 90%, preferably at least 95%, more preferably at least 99% identity to the amino acid sequence set forth in SEQ ID NO: 1.
本发明还提供了一种分离的、编码本发明所述的嵌合抗原受体 的DNA,该DNA包括可操作地连接的、依次串联的抗原结合结构域编码元件、间隔区编码元件、跨膜区编码元件和胞内结构域编码元件,其特征在于,所述抗原结合结构域编码元件来自NKG2D的配体结合区编码DNA,所述胞内结构域编码元件来自DAP12的胞内信号区编码DNA。The invention also provides an isolated DNA encoding a chimeric antigen receptor of the invention, the DNA comprising an operably linked, sequentially tandem antigen binding domain coding element, a spacer coding element, a transmembrane a region coding element and an intracellular domain coding element, wherein the antigen binding domain coding element is derived from a ligand binding region encoding DNA of NKG2D, and the intracellular domain coding element is derived from the intracellular signal region encoding DNA of DAP12 .
所述抗原结合结构域编码元件的核苷酸序列编码所述抗原结合结构域的氨基酸序列,优选地,所述抗原结合结构域的氨基酸序列与NKG2D的第X位-第216位氨基酸序列一致,且73≤X≤83,X为整数。例如,所述抗原结合结构域的氨基酸序列如以下氨基酸序列组中任一氨基酸序列所示:NKG2D的第73-216位氨基酸、第74-216位氨基酸、第75-216位氨基酸、第76-216位氨基酸、第77-216位氨基酸、第78-216位氨基酸、第79-216位氨基酸、第80-216位氨基酸、第81-216位氨基酸、第82-216位氨基酸、或第83-216位氨基酸。优选地,所述抗原结合结构域编码元件的核苷酸序列如SEQ ID NO:6所示。The nucleotide sequence of the antigen-binding domain coding element encodes an amino acid sequence of the antigen-binding domain, preferably, the amino acid sequence of the antigen-binding domain is identical to the X-position 216 amino acid sequence of NKG2D, And 73 ≤ X ≤ 83, and X is an integer. For example, the amino acid sequence of the antigen-binding domain is as shown in any one of the following amino acid sequence groups: amino acids 73-216 of NKG2D, amino acids 74-216, amino acids 75-216, 76- 216 amino acids, amino acids 77-216, amino acids 78-216, amino acids 79-216, amino acids 80-216, amino acids 81-216, amino acids 82-216, or 83- 216 amino acids. Preferably, the nucleotide sequence of the antigen binding domain coding element is set forth in SEQ ID NO: 6.
所述胞内结构域编码元件的核苷酸序列编码所述胞内结构域的氨基酸序列,优选地,所述胞内结构域的氨基酸序列选自DAP12的胞内信号区的第62-113位氨基酸。优选地,所述胞内结构域编码元件的核苷酸序列如SEQ ID NO:8所示。The nucleotide sequence of the intracellular domain coding element encodes the amino acid sequence of the intracellular domain, preferably, the amino acid sequence of the intracellular domain is selected from positions 62-113 of the intracellular signal region of DAP12 Amino acid. Preferably, the nucleotide sequence of the intracellular domain coding element is set forth in SEQ ID NO: 8.
优选地,所述间隔区编码元件来自CD8α的铰链区编码DNA,所述跨膜区编码元件来自CD8α的跨膜区编码DNA。Preferably, the spacer coding element is derived from the hinge region encoding DNA of CD8α, and the transmembrane region coding element is derived from the transmembrane region encoding DNA of CD8α.
所述间隔区编码元件和所述跨膜区编码元件构成间隔跨膜区编码元件,所述间隔跨膜区编码元件的核苷酸序列编码所述间隔跨膜区的氨基酸序列,优选地,所述间隔跨膜区的氨基酸序列与CD8α的第Y位-第210位氨基酸序列一致,且118≤Y≤128,Y为整数。例如,所述间隔跨膜区的氨基酸序列如以下氨基酸序列组中任一氨基酸序列所示:CD8α的第118-210位氨基酸、第119-210位氨基酸、第120-210位氨基酸、第121-210位氨基酸、第122-210位氨基酸、第123-210位氨基酸、第124-210位氨基酸、第125-210位氨基酸、第126-210位氨基酸、第127-210位氨基酸、或第128-210位氨基酸。优选地, 所述间隔区编码元件的核苷酸序列包含SEQ ID NO:7所示的序列。The spacer coding element and the transmembrane region coding element comprise a spacer transmembrane region coding element, the nucleotide sequence of the spacer transmembrane region coding element encoding an amino acid sequence of the spacer transmembrane region, preferably, The amino acid sequence of the spacer transmembrane region is identical to the amino acid sequence of the Yth position to the 210th position of CD8α, and 118≤Y≤128, and Y is an integer. For example, the amino acid sequence of the spacer transmembrane region is as shown in any one of the following amino acid sequence groups: amino acids 118-210 of CD8α, amino acids 119-210, amino acids 120-210, 121- 210 amino acids, amino acids 122-210, amino acids 123-210, amino acids 124-210, amino acids 125-210, amino acids 126-210, amino acids 127-210, or 128- 210 amino acids. Preferably, the nucleotide sequence of the spacer coding element comprises the sequence set forth in SEQ ID NO:7.
在本发明一个优选的实施方案中,所述分离的、编码本发明所述的嵌合抗原受体的DNA的核苷酸序列如SEQ ID NO:2所示。In a preferred embodiment of the invention, the isolated nucleotide sequence encoding the chimeric antigen receptor of the invention is represented by SEQ ID NO: 2.
本发明所述的NKG2D、DAP12和CD8α均优选来源于人,其全长氨基酸序列和核苷酸序列均为已知的,可以由本领域常用的公开数据库查询到。The NKG2D, DAP12 and CD8α of the present invention are preferably derived from humans, and their full-length amino acid sequences and nucleotide sequences are known, and can be inquired from public databases commonly used in the art.
本发明还提供了一种分离的、由本发明所述的编码嵌合抗原受体的DNA转录得到的mRNA。The present invention also provides an isolated mRNA which is transcribed from the DNA encoding the chimeric antigen receptor of the present invention.
本发明还提供了一种重组表达载体,其含有与启动子有效连接的根据本发明所述的编码嵌合抗原受体的DNA。The invention also provides a recombinant expression vector comprising a DNA encoding a chimeric antigen receptor according to the invention operably linked to a promoter.
优选地,所述重组表达载体在根据本发明所述的编码嵌合抗原受体的DNA之前依次包含CMV启动子、T7启动子、具有kozak序列的5’UTR和GM-CSFα链信号肽编码序列;并且在根据本发明所述的编码嵌合抗原受体的DNA之后包含具有polyA信号的α球蛋白的3’UTR。Preferably, the recombinant expression vector comprises a CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, and a GM-CSF alpha chain signal peptide coding sequence in sequence before the DNA encoding the chimeric antigen receptor according to the present invention. And comprising a 3'UTR of an alpha globulin having a polyA signal following the DNA encoding the chimeric antigen receptor according to the invention.
本发明的重组表达载体的上述作用元件的组合能够促进DNA的转录和翻译,并增强mRNA的稳定性。本发明还对上述各作用元件的结构作了如下优化,从而更好地发挥其应有的功能。The combination of the above-described functional elements of the recombinant expression vector of the present invention can promote transcription and translation of DNA and enhance the stability of mRNA. The present invention also optimizes the structure of each of the above-described action elements to better perform their intended functions.
优选地,在本发明中,CMV启动子序列为TAGTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAGTTCCGCGTTACATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCCCATTGACGTCAATAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTCCATTGACGTCAATGGGTGGAGTATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATATGCCAAGTACGCCCCCTATTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGTACATGACCTTATGGGACTTTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTACCATGGTGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAGTCTCCACCCCATTGACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGGAC TTTCCAAAATGTCGTAACAACTCCGCCCCATTGACGCAAATGGGCGGTAGGCGTGTACGGTGGGAGGTCTATATAAGCAGAGCTGGTTTAGTGAACCGTCAGATC(SEQ ID NO:22)。CMV启动子功能为使下游的DNA序列开始转录。Preferably, in the present invention, CMV promoter sequence TAGTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAGTTCCGCGTTACATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCCCATTGACGTCAATAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTCCATTGACGTCAATGGGTGGAGTATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATATGCCAAGTACGCCCCCTATTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGTACATGACCTTATGGGACTTTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTACCATGGTGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAGTCTCCACCCCATTGACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGGAC TTTCCAAAATGTCGTAACAACTCCGCCCCATTGACGCAAATGGGCGGTAGGCGTGTACGGTGGGAGGTCTATATAAGCAGAGCTGGTTTAGTGAACCGTCAGATC (SEQ ID NO: 22). The CMV promoter functions to initiate transcription of downstream DNA sequences.
优选地,在本发明中,T7启动子序列为TAATACGACTCACTATAG(SEQ ID NO:17)。T7启动子功能为使下游的DNA序列开始转录。Preferably, in the present invention, the T7 promoter sequence is TAATACGACTCACTATAG (SEQ ID NO: 17). The T7 promoter functions to initiate transcription of downstream DNA sequences.
优选地,在本发明中,具有kozak序列的5’UTR的序列为AAATAAGAGAGAAAAGAAGAGTAAGAAGAAATATAAGAGCCACCATG(SEQ ID NO:18),其中下划线所示序列为kozak序列。具有kozak序列的5’UTR的功能为增强mRNA的翻译效率。Preferably, in the present invention, the sequence of the 5' UTR having the kozak sequence is AAATAAGAGAGAAAAGAAGAGTAAGAAGAAATATAAGAGCCACCATG (SEQ ID NO: 18), wherein the sequence underlined is the kozak sequence. The function of the 5'UTR with the kozak sequence is to enhance the translation efficiency of the mRNA.
优选地,在本发明中,GM-CSFα链信号肽编码序列的序列为ATGCTTCTCCTGGTGACAAGCCTTCTGCTCTGTGAGTTACCACACCCAGCATTCCTCCTGATCCCA(SEQ ID NO:19),由此得出的氨基酸序列为MLLLVTSLLLCELPHPAFLLIP(SEQ ID NO:20)。GM-CSFα链信号肽是将本发明的CAR靶向至分泌途径的前导序列,其编码序列首先与CAR一起在细胞内被翻译成蛋白质,引导合成的蛋白进入胞内分泌途径。在细胞表面表达CAR前信号肽已被去除。Preferably, in the present invention, the sequence of the GM-CSF alpha chain signal peptide coding sequence is ATGCTTCTCCTGGTGACAAGCCTTCTGCTCTGTGAGTTACCACACCCAGCATTCCTCCTGATCCCA (SEQ ID NO: 19), and the amino acid sequence thus obtained is MLLLVTSLLLCELPHPAFLLIP (SEQ ID NO: 20). The GM-CSF alpha chain signal peptide is a leader sequence that targets the CAR of the present invention to the secretory pathway, and the coding sequence is first translated into a protein together with the CAR in the cell, directing the synthesized protein into the endocrine pathway. The signal peptide has been removed before expression of the CAR on the cell surface.
优选地,在本发明中,α球蛋白的3’UTR序列为GCTGCCTTCTGCGGGGCTTGCCTTCTGGCCATGCCCTTCTTCTCTCCCTTGCACCTGTACCTCTTGGTCTTTGAATAAAGCCTGAGTAGGAAGT(SEQ ID NO:21),其中下划线所示序列为polyA信号。其作用是增强mRNA的稳定性。Preferably, in the present invention, the 3'UTR sequence of the alpha globulin is GCTGCCTTCTGCGGGGCTTGCCTTCTGGCCATGCCCTTCTTCTCTCCCTTGCACCTGTACCTCTTGGTCTTTGAATAAA GCCTGAGTAGGAAGT (SEQ ID NO: 21), wherein the sequence underlined is a polyA signal. Its role is to enhance the stability of mRNA.
在一个具体实施方案中,所述重组表达载体的基本骨架为市售可得的pFastbac1载体,然后在其中插入上述各元件。In a specific embodiment, the basic backbone of the recombinant expression vector is a commercially available pFastbac1 vector into which each of the above elements is inserted.
由于本发明优化了3’UTR和5’UTR结构,因此可以例如应用Tail-PCR技术由所述重组表达载体合成其中正链带有PolyA、反链带有对应的PolyT的DNA双链模板,这样降低了DNA模板的不稳定性,进而可在体外合成mRNA。所述的正链中所带有的PolyA中的A的个数范围(或者其反链中对应的PolyT中的T的个数范围)为 140-170个,优选为150-170个,更优选为150个左右(例如150个)。Since the present invention optimizes the 3'UTR and 5'UTR structures, a DNA double-stranded template in which a positive strand carries a PolyA and an inverted strand carries a corresponding PolyT can be synthesized from the recombinant expression vector, for example, using a Tail-PCR technique, such that The instability of the DNA template is reduced, and mRNA can be synthesized in vitro. The range of the number of A in the PolyA carried in the positive chain (or the range of the number of T in the corresponding PolyT in the reverse chain) is 140-170, preferably 150-170, more preferably It is about 150 (for example, 150).
本发明还提供了一种嵌合抗原受体修饰的NK细胞,该NK细胞的表面被本发明所述的嵌合抗原受体修饰。The present invention also provides a chimeric antigen receptor-modified NK cell whose surface is modified by the chimeric antigen receptor of the present invention.
本文所用的术语“修饰”是指,NK细胞表达有本发明所述的嵌合抗原受体,即,所述嵌合抗原受体的跨膜区锚定在所修饰的NK细胞的细胞膜上,抗原结合结构域位于细胞表面,胞内结构域位于细胞质内。The term "modification" as used herein means that an NK cell expresses a chimeric antigen receptor according to the present invention, that is, a transmembrane region of the chimeric antigen receptor is anchored to a cell membrane of the modified NK cell, The antigen binding domain is located on the cell surface and the intracellular domain is located in the cytoplasm.
所述NK细胞可以为已知的各种类型的NK细胞,并且可以通过常规的生物学方法得到。NK细胞(自然杀伤细胞)是人体内的一种非特异性的先天免疫细胞,来源于骨髓,在体内几乎所有器官中都有存在,是非特异性免疫系统的重要组成部分,表型为CD3阴性CD56阳性的单一细胞,主要有CD16阴性CD56 bright(亮)和CD16阳性CD56 dim(暗)两种亚型,分别有免疫调节和肿瘤杀伤的体内功效。由于NK细胞作用是非MHC-限制性,使用上没有必要与病人个体的组织相容性复合物相匹配,也就是NK细胞可用于异体病人细胞治疗,具有广泛的临床应用价值。The NK cells may be various types of NK cells known and can be obtained by conventional biological methods. NK cells (natural killer cells) are non-specific innate immune cells in the human body. They are derived from bone marrow and are present in almost all organs of the body. They are an important part of the non-specific immune system. The phenotype is CD3 negative CD56. Positive single cells, mainly CD16-negative CD56 bright (light) and CD16-positive CD56 dim (dark) two subtypes, respectively, have immunomodulatory and tumor killing in vivo efficacy. Since NK cell action is non-MHC-restricted, there is no need to match the histocompatibility complex of the individual patient in use, that is, NK cells can be used for cell therapy of allogeneic patients, and have wide clinical application value.
本发明还提供了一种制备根据本发明所述的嵌合抗原受体修饰的NK细胞的方法,包括以下步骤:The invention also provides a method of preparing a chimeric antigen receptor-modified NK cell according to the invention, comprising the steps of:
1)提供NK细胞;1) providing NK cells;
2)提供编码根据本发明所述的嵌合抗原受体的核酸;2) providing a nucleic acid encoding a chimeric antigen receptor according to the invention;
3)将所述核酸转染入所述NK细胞中。3) Transfecting the nucleic acid into the NK cells.
步骤1)所述的NK细胞可以由外周血单个核细胞制备。本发明方法中NK细胞的纯度可为≥70%,优选为≥80%。NK细胞纯度指NK细胞在总细胞群体中所占比例。步骤2)所述的核酸为根据本发明所述的编码所述嵌合抗原受体的DNA、或由该DNA转录得到的mRNA。步骤3)所述的转染可通过冷冻电穿孔技术或慢病毒载体进行。采用冷冻电穿孔技术进行的转染可采用本领域常用的方式进行,如文献“Nakazawa Y,Matsuda K,Kurata T,Sueki A,Tanaka M,Sakashita K,Imai C,Wilson MH,Koike K.Anti-proliferative effects of T cells expressing a ligand-based chimeric antigen receptor against CD116 on CD34(+)cells of juvenile myelomonocytic leukemia.J Hematol Oncol.2016Mar.”所述。采用慢病毒载体进行的转染可采用本领域常用的方式进行,如文献“James N.Kochenderfer,Steven A.Feldman,Yangbing Zhao,Hui Xu,Mary A.Black,Richard A.Morgan,Wyndham H.Wilson,Ψ and Steven A.Rosenberg.Construction and Pre-clinical Evaluation of an Anti-CD19 Chimeric Antigen Receptor.2009 J Immunother.2009 Sep;32(7):689-702.”和文献“Cooper LJ,Topp MS,Serrano LM,Gonzalez S,Chang WC,Naranjo A,Wright C,Popplewell L,Raubitschek A,Forman SJ,Jensen MC.T-cell clones can be rendered specific for CD19:toward the selective augmentation of the graft-versus-B-lineage leukemia effect.Blood.2003 Feb15;101(4):1637-44.”所述。The NK cells described in step 1) can be prepared from peripheral blood mononuclear cells. The purity of the NK cells in the method of the invention may be > 70%, preferably > 80%. NK cell purity refers to the proportion of NK cells in the total cell population. The nucleic acid according to the step 2) is a DNA encoding the chimeric antigen receptor according to the present invention, or an mRNA obtained by transcription of the DNA. The transfection described in step 3) can be carried out by cryoelectroporation techniques or lentiviral vectors. Transfection using cryoelectroporation techniques can be carried out in a manner commonly used in the art, such as the literature "Nakazawa Y, Matsuda K, Kurata T, Sueki A, Tanaka M, Sakashita K, Imai C, Wilson MH, Koike K. Anti- Proliferative effects of T cells expressing a ligand-based chimeric antigen receptor against CD116 on CD34(+) cells of juvenile myelomonocytic leukemia. J Hematol Oncol. 2016 Mar. Transfection using lentiviral vectors can be carried out in a manner commonly used in the art, such as the literature "James N. Kochenderfer, Steven A. Feldman, Yangbing Zhao, Hui Xu, Mary A. Black, Richard A. Morgan, Wyndham H. Wilson. , Ψ and Steven A. Rosenberg. Construction and Pre-clinical Evaluation of an Anti-CD19 Chimeric Antigen Receptor. 2009 J Immunother. 2009 Sep; 32(7): 689-702." and the literature "Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, Wright C, Popplewell L, Raubitschek A, Forman SJ, Jensen MC. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage Leukemia effect.Blood.2003 Feb15;101(4):1637-44."
在本发明一个具体实施方案中,通过PCR由PBMC cDNA文库扩增得到人NKG2D蛋白的第83-216位氨基酸对应的DNA、人CD8α的第128-210位氨基酸对应的DNA、人DAP12的第62-113位氨基酸对应的DNA。将扩增得到的三种序列连接,然后通过分子克隆技术连接到pFastbac1载体上,得到pFastbac1-NKG2D-CD8-DAP12重组表达载体。然后合成NKG2D-CD8-DAP12对应序列的mRNA。再利用高效冷冻电穿孔技术将mRNA电转进入体外扩增培养的NK细胞,获得工程化NKG2D配体靶向性的NK细胞。In a specific embodiment of the present invention, the DNA corresponding to amino acids 83-216 of the human NKG2D protein, the DNA corresponding to amino acids 128-210 of human CD8α, and the 62nd of human DAP12 are amplified by PCR from the PBMC cDNA library. DNA corresponding to -113 amino acids. The three sequences amplified were ligated and ligated to the pFastbac1 vector by molecular cloning techniques to obtain a recombinant expression vector pFastbac1-NKG2D-CD8-DAP12. The mRNA of the corresponding sequence of NKG2D-CD8-DAP12 was then synthesized. The mRNA was electroporated into the NK cells expanded in vitro by high-efficiency cryoelectroporation to obtain engineered NKG2D ligand-targeted NK cells.
所述嵌合抗原受体的各部分也可由静脉血中单个核细胞的基因组cDNA扩增得到。Each portion of the chimeric antigen receptor can also be amplified by genomic cDNA of mononuclear cells in venous blood.
本发明还提供了根据本发明所述的嵌合抗原受体修饰的NK细胞在制备用于治疗或预防肿瘤和/或癌症的药物中的用途。The invention also provides the use of a chimeric antigen receptor-modified NK cell according to the invention for the preparation of a medicament for the treatment or prevention of a tumor and/or cancer.
所述肿瘤和/或癌症是NKG2D配体阳性的,包括结直肠癌、卵巢癌、头颈癌、骨髓瘤、肝癌、乳腺癌、血液瘤、宫颈癌、神经胶质瘤等。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer is positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
本发明还提供了根据本发明所述的嵌合抗原受体修饰的NK细胞在制备用于检测宿主的肿瘤和/或癌症的药物中的用途。The invention also provides the use of a chimeric antigen receptor-modified NK cell according to the invention for the preparation of a medicament for detecting a tumor and/or cancer of a host.
所述肿瘤和/或癌症是NKG2D配体阳性的,包括结直肠癌、卵巢癌、头颈癌、骨髓瘤、肝癌、乳腺癌、血液瘤、宫颈癌、神经胶质瘤等。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer is positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
在本发明的一个实施方案中,可将从宿主取出的肿瘤和/或癌症细胞的样本与本发明所述的嵌合抗原受体修饰的NK细胞以一定浓度进行接触,根据二者的反应程度可以判断所述肿瘤和/或癌症是NKG2D配体阳性的还是NKG2D配体阴性的。In one embodiment of the present invention, a sample of a tumor and/or a cancer cell taken out from a host may be contacted with a chimeric antigen receptor-modified NK cell of the present invention at a concentration according to the degree of reaction between the two. It can be judged whether the tumor and/or cancer is NKG2D ligand positive or NKG2D ligand negative.
本发明还提供了一种药物组合物,其中该药物组合物包括作为活性成分的根据本发明所述的嵌合抗原受体修饰的NK细胞,及可药用辅料。The present invention also provides a pharmaceutical composition comprising the chimeric antigen receptor-modified NK cell according to the present invention as an active ingredient, and a pharmaceutically acceptable adjuvant.
所述药物组合物优选包含每人每个疗程总剂量范围为1×106-1×1011个的所述嵌合抗原受体修饰的NK细胞;优选的是,每个疗程3周共21天,每周施用1-2次。所述药物组合物进一步优选包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述嵌合抗原受体修饰的NK细胞;还优选的是,每个疗程3周共21天,每周施用1-2次。可以根据实际情况和需要对患者进行一个或多个疗程的治疗。The pharmaceutical composition preferably comprises the chimeric antigen receptor-modified NK cells in a total dose ranging from 1 x 106 to 1 x 1011 per subject per subject; preferably, each treatment lasts for 3 weeks. Days, apply 1-2 times a week. The pharmaceutical composition further preferably comprises the chimeric antigen receptor-modified NK cells in a total dose ranging from 6 x 107 to 1.2 x 1010 per subject per treatment; and preferably, each course of treatment is 3 weeks A total of 21 days, 1-2 times a week. The patient may be treated for one or more courses depending on the actual situation and needs.
所述药物组合物可通过合适的给药途径给药,其给药方式包括静脉给药(例如静脉滴注给药或静脉注射给药)或局部给药(例如局部滴注给药或局部注射给药)。The pharmaceutical composition can be administered by a suitable route of administration including intravenous administration (for example, intravenous drip administration or intravenous administration) or topical administration (for example, topical administration or local injection). Administration).
本发明还提供了一种治疗肿瘤和/或癌症的方法,包括对肿瘤和/或癌症患者施用根据本发明所述的嵌合抗原受体修饰的NK细胞。The invention also provides a method of treating a tumor and/or cancer comprising administering to a tumor and/or cancer patient a chimeric antigen receptor modified NK cell according to the invention.
所述肿瘤和/或癌症是NKG2D配体阳性的,包括结直肠癌、卵巢癌、头颈癌、骨髓瘤、肝癌、乳腺癌、血液瘤、宫颈癌、神经胶质瘤等。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳 性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer is positive for NKG2D ligand, including colorectal cancer, ovarian cancer, head and neck cancer, myeloma, liver cancer, breast cancer, hematoma, cervical cancer, glioma, and the like. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
所述嵌合抗原受体修饰的NK细胞的施用剂量优选为每人每个疗程总剂量范围为1×106-1×1011个细胞;优选的是,每个疗程3周共21天,每周施用1-2次。所述嵌合抗原受体修饰的NK细胞的施用剂量进一步优选为每人每个疗程总剂量范围为6×107-1.2×1010个细胞;还优选的是,每个疗程3周共21天,每周施用1-2次。可以根据实际情况和需要对患者进行一个或多个疗程的治疗。The chimeric antigen receptor-modified NK cells are preferably administered at a dose ranging from 1×106 to 1×1011 cells per patient per course of treatment; preferably, each treatment lasts for 3 weeks for 21 days. Apply 1-2 times a week. The dose of the chimeric antigen receptor-modified NK cells is further preferably a total dose per patient per treatment range of 6×107 to 1.2×1010 cells; and it is also preferred that each treatment has a total of 21 weeks. Days, apply 1-2 times a week. The patient may be treated for one or more courses depending on the actual situation and needs.
所述嵌合抗原受体修饰的NK细胞可通过合适的给药途径给药,其给药方式包括静脉给药(例如静脉滴注给药或静脉注射给药)或局部给药(例如局部滴注给药或局部注射给药)。The chimeric antigen receptor-modified NK cells can be administered by a suitable administration route, such as intravenous administration (for example, intravenous drip administration or intravenous administration) or topical administration (for example, topical drip). Injection or local injection).
本发明还提供了一种工具载体,该工具载体依次包含可操作地连接的CMV启动子、T7启动子、具有kozak序列的5’UTR、GM-CSFα链信号肽编码序列、和具有polyA信号的α球蛋白的3’UTR。The invention also provides a tool vector comprising, in turn, an operably linked CMV promoter, a T7 promoter, a 5'UTR having a kozak sequence, a GM-CSF alpha chain signal peptide coding sequence, and a polyA signal. The 3'UTR of alpha globulin.
术语“工具载体”是指基因工程应用中,用于插入外源DNA片段的空载体。The term "tool vector" refers to an empty vector for insertion of an exogenous DNA fragment in genetic engineering applications.
在插入外源DNA片段时,将外源DNA片段插入到所述工具载体的GM-CSFα链信号肽编码序列与具有polyA信号的α球蛋白的3’UTR之间(GM-CSFα链信号肽编码序列与具有polyA信号的α球蛋白的3’UTR之间可以具有多克隆位点)。When the foreign DNA fragment is inserted, the foreign DNA fragment is inserted between the GM-CSFα chain signal peptide coding sequence of the tool vector and the 3'UTR of the α-globulin having a polyA signal (GM-CSF alpha chain signal peptide coding The sequence may have a multiple cloning site between the 3' UTR of the alpha globulin with a polyA signal).
优选地,所述CMV启动子的核苷酸序列如SEQ ID NO:22所示,T7启动子的核苷酸序列如SEQ ID NO:17所示,所述具有kozak序列的5’UTR的核苷酸序列如SEQ ID NO:18所示,所述GM-CSFα链信号肽编码序列的核苷酸序列如SEQ ID NO:19所示,所述具有PolyA信号的α球蛋白的3’UTR的核苷酸序列如SEQ ID NO:21所示。Preferably, the nucleotide sequence of the CMV promoter is set forth in SEQ ID NO: 22, and the nucleotide sequence of the T7 promoter is set forth in SEQ ID NO: 17, the core of the 5'UTR having the kozak sequence. The nucleotide sequence of the GM-CSF alpha chain signal peptide coding sequence is set forth in SEQ ID NO: 19, and the 3'UTR of the alpha globulin having a PolyA signal is shown in SEQ ID NO: 18. The nucleotide sequence is shown in SEQ ID NO:21.
本发明的工具载体能够促进插入DNA的转录和翻译,并增强mRNA的稳定性。The tool vector of the present invention is capable of promoting transcription and translation of the inserted DNA and enhancing the stability of the mRNA.
在本发明的另一个方面中,本发明的发明人还在上述嵌合抗原受体修饰的NK细胞的基础上,结合系统化思维提出了新的联合疗法。In another aspect of the invention, the inventors of the present invention have also proposed a novel combination therapy based on the above-described chimeric antigen receptor-modified NK cells in combination with systematic thinking.
人体是一个复杂的系统,它是由呼吸、循环、消化等十大系统组成,这些系统协调配合,使人体内各种复杂的生命活动能够正常进行。系统化思考就是从整体观出发,把药物作用、疾病、系统和人体的相互联系、相互作用进行综合考察。The human body is a complex system consisting of ten systems, including breathing, circulation, and digestion. These systems work together to make various complex life activities in the human body work normally. Systematic thinking is to conduct a comprehensive investigation of the interaction and interaction of drug effects, diseases, systems and humans from a holistic perspective.
当前,很多非细胞毒性抗肿瘤药物,在联合化疗治疗肿瘤时,患者的长期生存结果都不甚理想,其原因是缺少了系统思维的考虑。例如,当肿瘤发生后,机体可通过多种免疫效应机制发挥抗肿瘤作用,机体的抗肿瘤机制包括细胞免疫和体液免疫两个方面。它们联系密切,相互影响,涉及多种免疫效应分子和效应细胞。一般认为,细胞免疫在抗肿瘤过程中起到主导作用,体液免疫在某些情况下起协同作用。然而传统化疗主要是干扰RNA或DNA合成以及有丝分裂等,主要针对生长快速的细胞,在清除肿瘤细胞的同时也打击了人体正常的免疫系统,随着机体免疫力被摧跨,肿瘤细胞势必“抬头”。At present, many non-cytotoxic anti-tumor drugs, when combined with chemotherapy to treat tumors, patients' long-term survival results are not satisfactory, the reason is the lack of systematic thinking. For example, when a tumor occurs, the body can exert anti-tumor effects through various immune effect mechanisms, and the body's anti-tumor mechanism includes both cellular immunity and humoral immunity. They are closely related and interact with each other and involve a variety of immune effector molecules and effector cells. It is generally believed that cellular immunity plays a leading role in the anti-tumor process, and humoral immunity plays a synergistic role in some cases. However, traditional chemotherapy mainly interferes with RNA or DNA synthesis and mitosis. It is mainly for fast-growing cells. It also attacks the normal immune system of the human body while removing tumor cells. As the body's immunity is destroyed, the tumor cells are bound to rise. ".
结合上述系统化思维的基础上,本发明认为可以采用其它提高机体免疫的方法,通过系统组合各种治疗手段,以最大可能地提高综合疗效,同时使对免疫系统的伤害最小化。由此,本发明提出了新的联合疗法,将溶瘤病毒与本发明所述的嵌合抗原受体修饰的NK细胞联用以治疗肿瘤和/或癌症。特别是,本发明仅将溶瘤病毒与所述NK细胞联用就能够产生协同效果。Based on the above systematic thinking, the present invention believes that other methods for improving the body immunity can be adopted, and various treatment means can be combined by system to maximize the comprehensive therapeutic effect while minimizing the damage to the immune system. Thus, the present invention proposes a novel combination therapy for the combination of an oncolytic virus with a chimeric antigen receptor modified NK cell of the invention for the treatment of tumors and/or cancer. In particular, the present invention can produce a synergistic effect only by combining an oncolytic virus with the NK cells.
因此,本发明提供了一种治疗剂,包含:Accordingly, the present invention provides a therapeutic agent comprising:
(a)第一药物组合物,其中该第一药物组合物包含位于第一可药用载体中的溶瘤病毒;和(a) a first pharmaceutical composition, wherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier;
(b)第二药物组合物,其中该第二药物组合物包含位于第二可药用载体中的NK细胞;(b) a second pharmaceutical composition, wherein the second pharmaceutical composition comprises NK cells in a second pharmaceutically acceptable carrier;
其中所述溶瘤病毒能够选择性地在肿瘤细胞中复制;并且Wherein the oncolytic virus is capable of selectively replicating in a tumor cell;
其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。Wherein the surface of the NK cell is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that In the above, the antigen binding domain is derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
在一些情况下,所述治疗剂也可以理解为药物的组合。In some cases, the therapeutic agent can also be understood as a combination of drugs.
在一些实施方案中,所述第一药物组合物的活性成分为所述溶瘤病毒,并且其中所述第二药物组合物的活性成分为所述NK细胞。在一些实施方案中,所述第一药物组合物包含治疗有效量的所述溶瘤病毒,并且所述第二药物组合物包含1×105至1×1010个细胞/天剂量的所述NK细胞(例如,所述第二药物组合物包含1×107至1×1010个细胞/天剂量的所述NK细胞;所述第二药物组合物包含1×108至5×109个细胞/天剂量的所述NK细胞;所述第二药物组合物包含1×109至4×109个细胞/天剂量的所述NK细胞;所述第二药物组合物包含1×109至3×109个细胞/天剂量的所述NK细胞)。优选地,所述第二药物组合物包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞。还优选地,所述第二药物组合物包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞。In some embodiments, the active ingredient of the first pharmaceutical composition is the oncolytic virus, and wherein the active ingredient of the second pharmaceutical composition is the NK cell. In some embodiments, the first pharmaceutical composition comprises a therapeutically effective amount of the oncolytic virus, and wherein said second pharmaceutical composition comprises 1 × 105 to 1 × 1010 cells / day dose NK cells (for example, the second pharmaceutical composition comprises 1 x 107 to 1 x 1010 cells/day of the NK cells; the second pharmaceutical composition comprises 1 x 108 to 5 x 109 Cell/day dose of said NK cells; said second pharmaceutical composition comprising 1 x109 to 4 x109 cells per day of said NK cells; said second pharmaceutical composition comprising 1 x 109 to 3 x 109 cells/day of the NK cells). Preferably, the second pharmaceutical composition comprises the NK cells in a total dose ranging from 1 x 106 to 1 x 1011 per subject per subject. Still preferably, the second pharmaceutical composition comprises the NK cells in a total dose ranging from 6 x 107 to 1.2 x 1010 per subject per subject.
本发明还提供了一种药物组合物,其中该药物组合物的活性成分包括溶瘤病毒和NK细胞,所述溶瘤病毒能够选择性地在肿瘤细胞中复制,所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。优选的是,该药物组合物的活性成分由所述溶瘤病毒和所述NK细胞组成。The present invention also provides a pharmaceutical composition, wherein the active ingredient of the pharmaceutical composition comprises an oncolytic virus and an NK cell, the oncolytic virus being capable of selectively replicating in a tumor cell, the surface of the NK cell being embedded Modification of an antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, wherein the antigen binding domain is derived from The ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12. Preferably, the active ingredient of the pharmaceutical composition consists of the oncolytic virus and the NK cells.
优选地,所述溶瘤病毒和所述NK细胞各自独立地存在于所述药物组合物中而互不混合。Preferably, the oncolytic virus and the NK cells are each independently present in the pharmaceutical composition without mixing with each other.
所有溶瘤病毒杀伤肿瘤细胞的机制都大体相似。在不同实施方案中,通过瘤内注射或静脉给药的方式,溶瘤病毒与肿瘤细胞接触,感染进入肿瘤细胞内。由于溶瘤病毒的特性是,其主要在肿瘤细胞内复制增殖,而在正常细胞内低复制或不复制,因此被感染的肿瘤细胞中会出现大量的溶瘤病毒,造成肿瘤细胞的溶解和死亡。肿瘤细胞的溶解会释放出大量的肿瘤抗原和增殖的溶瘤病毒,抗原会进一步激活体内的免疫系统,刺激体内的NK细胞和T细胞继续攻击尚未死亡的肿瘤细胞,同时新的溶瘤病毒会继续感染尚未被感染的肿瘤细胞。The mechanism by which all oncolytic viruses kill tumor cells is generally similar. In various embodiments, the oncolytic virus contacts the tumor cells by intratumoral or intravenous administration and the infection enters the tumor cells. Since the oncolytic virus is characterized in that it mainly replicates and proliferates in tumor cells, but does not replicate or replicate in normal cells, a large number of oncolytic viruses appear in infected tumor cells, causing tumor cell lysis and death. . The dissolution of tumor cells releases a large number of tumor antigens and proliferating oncolytic viruses. The antigen further activates the immune system in the body, stimulating NK cells and T cells in the body to continue to attack tumor cells that have not yet died, and the new oncolytic virus will Continue to infect tumor cells that have not yet been infected.
NK细胞是广谱型杀伤肿瘤细胞的免疫细胞,NK细胞可以辨别 肿瘤细胞与正常细胞的区别。NK通过与肿瘤细胞接触,识别确认其为非正常细胞,然后通过受体识别、抗体靶向识别(ADCC)、颗粒酶分泌、穿孔素分泌和分泌干扰素间接杀伤等多种协同手段,达到杀死肿瘤细胞的效果。体外实验显示,一个健康的NK细胞在生命期内可以连续杀死27个肿瘤细胞。NK cells are broad-spectrum immune cells that kill tumor cells, and NK cells can distinguish between tumor cells and normal cells. NK contacts and recognizes tumor cells, recognizes it as an abnormal cell, and then kills it through receptor recognition, antibody-targeted recognition (ADCC), granzyme secretion, perforin secretion, and indirect killing of interferon. The effect of dead tumor cells. In vitro experiments have shown that a healthy NK cell can kill 27 tumor cells in a row during its lifetime.
NK细胞还具有抗病毒的功能。当正常细胞感染了病毒后,随着病毒的大量复制,细胞体现出衰老病变,体现在细胞膜上的蛋白簇的组成发生变化,在这个过程中,NK细胞就可以敏锐而高效地识别被感染的细胞,通过类似于杀伤肿瘤细胞的上述手段,杀死被感染的细胞,从而达到抑制病毒复制增殖的目的。随后在抗原刺激和干扰素等因子的作用下,其它免疫细胞会持续作用,抵抗病毒。NK cells also have antiviral functions. When a normal cell is infected with a virus, as the virus replicates a lot, the cell exhibits an aging lesion, and the composition of the protein cluster reflected on the cell membrane changes. In this process, the NK cell can recognize the infected patient sharply and efficiently. The cells, by the means described above similar to killing tumor cells, kill the infected cells, thereby achieving the purpose of inhibiting viral replication and proliferation. Subsequently, under the action of factors such as antigen stimulation and interferon, other immune cells will continue to act against the virus.
本发明考虑了溶瘤病毒和NK细胞各自的特点,巧妙地将其联用,在联用时,NK细胞的抗病毒机制对于被溶瘤病毒感染的肿瘤细胞同样适用,并且与其抗肿瘤机制互补。此外,联用还使得含有溶瘤病毒的肿瘤细胞成为了NK细胞的特异性靶标,从而增强了NK细胞的肿瘤杀伤作用。溶瘤病毒选择性地在癌细胞内增殖,在胞内起作用杀伤癌细胞,同时能够导致癌细胞膜上的蛋白受体簇发生变化,增强NK细胞对癌细胞的识别,NK细胞在癌细胞外攻击,两者联合起来协同杀伤癌细胞,具有更好的治疗效果。此外,本发明还发现溶瘤病毒可以刺激肿瘤细胞表面多种NKG2D配体表达的增加,从而更进一步地与本发明所述的嵌合抗原受体修饰的NK细胞配合产生更强的抗肿瘤协同作用。The present invention takes into consideration the respective characteristics of oncolytic viruses and NK cells, and uses them in combination. When used in combination, the antiviral mechanism of NK cells is equally applicable to tumor cells infected with oncolytic viruses, and is complementary to its anti-tumor mechanism. In addition, the combination also makes tumor cells containing oncolytic virus a specific target of NK cells, thereby enhancing the tumor killing effect of NK cells. The oncolytic virus selectively proliferates in cancer cells, plays a role in killing cancer cells in the cell, and can cause changes in protein receptor clusters on the cancer cell membrane, enhancing the recognition of cancer cells by NK cells, and NK cells outside the cancer cells. Attack, the two together to kill cancer cells, have a better therapeutic effect. In addition, the present inventors have also found that oncolytic viruses can stimulate the increase of the expression of various NKG2D ligands on the surface of tumor cells, thereby further cooperating with the chimeric antigen receptor-modified NK cells of the present invention to produce stronger anti-tumor synergy. effect.
单独的野生型病毒与NK细胞之间具有互相制约的作用。一方面,病毒会通过刺激NK表面的KIR类受体,抑制NK的活性,从而逃脱NK的抗病毒杀伤;另一方面,NK除了识别并杀死被病毒感染的细胞,从而抑制病毒的增殖外,还可以直接分泌干扰素,抑制病毒活性。然而,由于很多溶瘤病毒都经过基因修饰,一方面可增强感染肿瘤细胞的特异性,另一方面减少了对包括NK细胞在内的免疫细胞的抑制。The wild type virus alone has a mutually restrictive effect on NK cells. On the one hand, the virus can escape the antiviral killing of NK by stimulating the KIR receptor on the surface of NK, thereby evading the activity of NK; on the other hand, NK not only recognizes and kills the cells infected by the virus, but also inhibits the proliferation of the virus. It can also directly secrete interferon and inhibit viral activity. However, since many oncolytic viruses are genetically modified, on the one hand, the specificity of infected tumor cells can be enhanced, and on the other hand, the inhibition of immune cells including NK cells is reduced.
本发明所述的溶瘤病毒包括具有溶瘤作用的经基因突变的病毒 和具有溶瘤作用的野生型病毒。所述具有溶瘤作用的经基因突变的病毒包括(但不限于):腺病毒(adenovirus)、痘病毒(也称痘苗病毒(vaccinia virus))、单纯疱疹病毒(herpes simplex virus(HSV))、麻疹病毒(measles virus)、塞姆利基森林病毒(Semliki Forest virus)、水疱性口炎病毒(vesicular stomatitis virus)、脊髓灰质炎病毒(poliovirus)和逆转录病毒(retrovirus);所述具有溶瘤作用的野生型病毒包括(但不限于):呼肠孤病毒(reovirus)、水疱性口炎病毒(vesicular stomatitis virus)、脊髓灰质炎病毒、塞内卡谷病毒(Seneca Valley Virus)、埃可型肠道病毒(echo enterovirus)、柯萨奇病毒(Coxsackie virus)、新城疫病毒(Newcastle disease virus)和马拉巴病毒(maraba virus)。The oncolytic virus of the present invention includes a gene-mutated virus having an oncolysis effect and a wild-type virus having an oncolysis effect. The gene-mutated virus having oncolytic effect includes, but is not limited to, an adenovirus, a poxvirus (also known as vaccinia virus), herpes simplex virus (HSV), Measles virus, Semliki Forest virus, vesicular stomatitis virus, poliovirus, and retrovirus; Wild-type viruses that function include (but are not limited to): reovirus, vesicular stomatitis virus, poliovirus, Seneca Valley Virus, Eco type Echo enterovirus, Coxsackie virus, Newcastle disease virus, and maraba virus.
所述溶瘤病毒的基因组中可以整合有外源基因,所述外源基因包括外源免疫调节基因、外源筛选基因、外源报告基因等。所述溶瘤病毒的基因组中也可以不整合任何外源基因。The exogenous gene may be integrated into the genome of the oncolytic virus, and the exogenous gene includes an exogenous immunoregulatory gene, an exogenously screened gene, an exogenous reporter gene, and the like. The foreign gene may also not be integrated into the genome of the oncolytic virus.
所述腺病毒包括(但不限于):人5型腺病毒或人嵌合型腺病毒;具体包括(例如):Onyx-015(可得自Onyx Pharmaceuticals公司)、H101(可得自上海三维生物技术有限公司)、Ad5-yCD/mutTKSR39rep-hIL12(可得自Henry Ford Health System公司)、CG0070(可得自Cold Genesys公司)、DNX-2401(可得自DNAtrix公司)、OBP-301(可得自Oncolys BioPharma公司)、ONCOS-102(可得自Targovax Oy公司/Oncos Therapeutics公司)、ColoAd1(可得自PsiOxus Therapeutics公司)、VCN-01(可得自VCN Biosciences公司)、ProstAtakTM(可得自Advantagene公司)等。The adenovirus includes, but is not limited to, a
优选的是,所述腺病毒为H101。Preferably, the adenovirus is H101.
所述痘病毒可以是惠氏株、WR株、李斯特株或哥本哈根株等。The poxvirus may be Wyeth strain, WR strain, Listeria strain or Copenhagen strain.
所述痘病毒可以是TK基因功能缺陷型的、VGF基因功能缺陷型的、TK基因和VGF基因功能缺陷型的。所述痘病毒还可以包含其它基因功能缺陷,其它基因包括(但不限于):HA、F14.5L、F4L。The poxvirus may be functionally deficient in the TK gene, functionally deficient in the VGF gene, and functionally deficient in the TK gene and the VGF gene. The poxvirus may also contain other genetic defects, including but not limited to: HA, F14.5L, F4L.
优选的是,所述痘病毒是TK基因和VGF基因功能缺陷型的。Preferably, the poxvirus is functionally deficient in the TK gene and the VGF gene.
所述痘病毒包括(但不限于):Pexa-vac(可得自Jennerex Biotherapeutics公司)、JX-963(可得自Jennerex Biotherapeutics公 司)、JX-929(可得自Jennerex Biotherapeutics公司)、VSC20(制备方法可参见科技文献:“McCart,JA,et al.Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes.Cancer Res(2001)61:8751-8757.”)、GL-ONC1(可得自Genelux公司)、TG6002(可得自Transgene公司)等。The poxvirus includes, but is not limited to, Pexa-vac (available from Jennerex Biotherapeutics), JX-963 (available from Jennerex Biotherapeutics), JX-929 (available from Jennerex Biotherapeutics), VSC20 (preparation) The method can be found in the scientific literature: "McCart, JA, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res (2001) 61: 8751-8757."), GL- ONC1 (available from Genelux), TG6002 (available from Transgene), etc.
所述单纯疱疹病毒包括(但不限于):HSV-1、HSV-2型单纯疱疹病毒;具体包括(例如):(可得自Amgen公司)、G207(可得自Medigene公司)、HF10(可得自Takara Bio公司)、Seprehvir(可得自Virttu Biologics公司)、OrienX010(可得自北京奥源和力生物公司)、NV1020(可得自Catherax公司)等。The herpes simplex virus includes, but is not limited to, HSV-1, HSV-2 herpes simplex virus; specifically including (for example): (available from Amgen), G207 (available from Medigene), HF10 (available from Takara Bio), Seprehvir (available from Virttu Biologics), OrienX010 (available from Beijing Aoyuan and Lili Bio) , NV1020 (available from Catherax) and so on.
上述病毒的具体例子如下表1所示。Specific examples of the above viruses are shown in Table 1 below.
表1 本发明所述的溶瘤病毒列表Table 1 List of oncolytic viruses according to the present invention
本发明所述的NK细胞包括自体NK细胞和异体NK细胞。所述NK细胞可以为经体外扩增得到的NK细胞。NK细胞的大规模体外扩增培养技术是已知的,并且已经基本成熟(参见(例如)以下科技文献:“Somanchi SS,Lee DA.Ex Vivo Expansion of Human NK Cells Using K562 Engineered to Express Membrane Bound IL21.Methods Mol Biol.2016;1441:175-93.”或“Phan MT,Lee SH,Kim SK,Cho D.Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells.Methods Mol Biol.2016;1441:167-74.”)。临床数据证实自体NK细胞、半相合异体NK细胞(属于异体NK细胞)、以及脐血制备NK细胞回输人体后均无毒副作用,无长期依赖性,安全有效。The NK cells of the present invention include autologous NK cells and allogeneic NK cells. The NK cells may be NK cells obtained by in vitro expansion. Large-scale in vitro expansion culture techniques for NK cells are known and have been largely mature (see, for example, the following scientific literature: "Somanchi SS, Lee DA. Ex Vivo Expansion of Human NK Cells Using K562 Engineered to Express Membrane Bound IL21 .Methods Mol Biol.2016;1441:175-93." or "Phan MT, Lee SH, Kim SK, Cho D. Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells. Methods Mol Biol. 2016;1441:167-74 ."). Clinical data confirmed that autologous NK cells, haploidentical NK cells (which belong to allogeneic NK cells), and umbilical cord blood were not toxic and side effects after NK cells were returned to humans, and were not long-term dependent, safe and effective.
可用于治疗的NK细胞的纯度范围可以是:自体NK细胞的纯度可为大于等于85%,异体NK细胞的纯度可为大于等于90%;其中的异体NK细胞中CD3阳性T细胞的比例不超过5×105/kg体重。The purity of the NK cells which can be used for treatment may be: the purity of the autologous NK cells may be 85% or more, and the purity of the allogeneic NK cells may be 90% or more; wherein the proportion of CD3 positive T cells in the allogeneic NK cells is not more than 5 × 105 /kg body weight.
在本发明的所述联用治疗方案中,本发明进一步探索优化了溶瘤病毒和NK细胞各自的施用剂量、施用顺序和施用间隔,这几点是 至关重要的,其决定了溶瘤病毒的抗肿瘤疗效、NK细胞的抗肿瘤疗效、以及两者对肿瘤细胞的最佳协同杀伤。In the combination treatment regimen of the present invention, the present invention further explores optimization of the respective administration dose, administration sequence and administration interval of the oncolytic virus and NK cells, which are critical, which determine the oncolytic virus Anti-tumor efficacy, anti-tumor efficacy of NK cells, and optimal synergistic killing of both tumor cells.
因此,优选地,所述药物组合物或治疗剂包含治疗有效量的所述溶瘤病毒,并且所述药物组合物或治疗剂包含1×105至1×1010个细胞/天剂量的所述NK细胞(例如,所述药物组合物或治疗剂包含1×107至1×1010个细胞/天剂量的所述NK细胞;所述药物组合物或治疗剂包含1×108至5×109个细胞/天剂量的所述NK细胞;所述药物组合物或治疗剂包含1×109至4×109个细胞/天剂量的所述NK细胞;所述药物组合物或治疗剂包含1×109至3×109个细胞/天剂量的所述NK细胞)。还优选地,所述药物组合物或治疗剂包含治疗有效量的所述溶瘤病毒,并且所述药物组合物或治疗剂包含每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,所述药物组合物或治疗剂包含每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞)。对于不同的溶瘤病毒,可以采用不同的优选临床剂量范围,例如表1所述的那样。Accordingly, preferably, the pharmaceutical composition or therapeutic agent comprises a therapeutically effective amount of the oncolytic virus, and the pharmaceutical composition or therapeutic agent comprises a dose of 1 x 105 to 1 x 1010 cells per day. Said NK cells (for example, said pharmaceutical composition or therapeutic agent comprises 1 x107 to 1 x 1010 cells/day of said NK cells; said pharmaceutical composition or therapeutic agent comprises 1 x 108 to 5 × 109 cells/day of the NK cells; the pharmaceutical composition or therapeutic agent comprising 1 x 109 to 4 x 109 cells/day of the NK cells; the pharmaceutical composition or treatment The agent comprises 1 x 109 to 3 x 109 cells/day of the NK cells). Still preferably, the pharmaceutical composition or therapeutic agent comprises a therapeutically effective amount of the oncolytic virus, and the pharmaceutical composition or therapeutic agent comprises a total dose ranging from 1 x 106 to 1 x 10 per person per course of treatment.Eleven of said NK cells (for example, said pharmaceutical composition or therapeutic agent comprises a total dose ranging from 6 x 107 to 1.2 x 1010 per NK per subject per patient). For different oncolytic viruses, different preferred clinical dosage ranges can be employed, such as those described in Table 1.
所述溶瘤病毒可采用其各自的本领域通常所采用的给药方式给药,例如通过瘤内注射给药或静脉给药。The oncolytic viruses can be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
NK细胞可采用本领域通常所采用的给药方式给药,例如可通过静脉给药或局部给药。The NK cells can be administered by administration generally employed in the art, for example, intravenously or topically.
在特定的实施方案中,本发明的药物组合物或治疗剂所包含的溶瘤病毒为具有溶瘤作用的腺病毒(下文也称为“溶瘤腺病毒”)。在一些例子中,所述具有溶瘤作用的腺病毒的E1区和/或E3区是经基因工程改造的。在一些例子中,所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。In a specific embodiment, the oncolytic virus contained in the pharmaceutical composition or therapeutic agent of the present invention is an oncolytic virus (hereinafter also referred to as "oncolytic adenovirus"). In some examples, the E1 region and/or E3 region of the oncolytic adenovirus is genetically engineered. In some examples, the oncolytic adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtakTM .
在某些实施方案中,本发明的药物组合物或治疗剂的活性成分包括5×107至5×1012VP/天剂量的溶瘤腺病毒(例如,5×107至1.5×1012VP/天剂量的溶瘤腺病毒、5×108至1×1012VP/天剂量的溶瘤腺病毒、1×109至5×1011VP/天剂量的溶瘤腺病毒、3×1010至3×1011VP/天剂量的溶瘤腺病毒等)和1×105至1×1010个细胞/天剂量 的所述NK细胞(例如,1×107至1×1010个细胞/天剂量的所述NK细胞、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等);优选的是,该药物组合物或治疗剂的活性成分由5×107至5×1012VP/天剂量的溶瘤腺病毒(例如,5×107至1.5×1012VP/天剂量的溶瘤腺病毒、5×108至1×1012VP/天剂量的溶瘤腺病毒、1×109至5×1011VP/天剂量的溶瘤腺病毒、3×1010至3×1011VP/天剂量的溶瘤腺病毒等)和1×105至1×1010个细胞/天剂量的所述NK细胞(例如,1×107至1×1010个细胞/天剂量的所述NK细胞、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等)组成。In certain embodiments, the active ingredients of the pharmaceutical composition or therapeutic agent of the present invention include 5 × 107 to 5 × 1012 VP / day dose of the oncolytic adenovirus (e.g., 5 × 107 to 1.5 × 1012 VP/day dose of oncolytic adenovirus, 5×108 to 1×1012 VP/day dose of oncolytic adenovirus, 1×109 to 5×1011 VP/day dose of oncolytic adenovirus, 3× 1010 to 3 × 1011 VP/day dose of oncolytic adenovirus, etc.) and 1 × 105 to 1 × 1010 cells/day of the NK cells (for example, 1 × 107 to 1 × 1010 Cell/day dose of said NK cells, 1 x 108 to 5 x 109 cells/day of said NK cells, 1 x 109 to 4 x 109 cells/day of said NK cells , 1 × 109 to 3 × 109 cells/day of the NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is from 5 × 107 to 5 × 1012 VP / day Dosage of oncolytic adenovirus (eg, 5 x107 to 1.5 x 1012 VP/day dose of oncolytic adenovirus, 5 x108 to 1 x 1012 VP/day dose of oncolytic adenovirus, 1 x 10. 9 to 5 × 1011 VP / day dose of an oncolytic adenovirus, 3 ×10 10 to 3 × 1011 VP / day dose of the oncolytic adenovirus, etc.) and 1 × 105 1 × 1010 cells / day dose of the NK cells (e.g., the 1 × 107 to 1 × 1010 cells / day dose NK cells, 1 × 108 to 5 × 109 cells / day The dose consists of the NK cells, 1 x 109 to 4 x 109 cells/day of the NK cells, 1 x 109 to 3 x 109 cells/day of the NK cells, and the like.
在另一些实施方案中,本发明的药物组合物或治疗剂的活性成分包括5×107至5×1012VP/天剂量的溶瘤腺病毒(例如,5×107至1.5×1012VP/天剂量的溶瘤腺病毒、5×108至1×1012VP/天剂量的溶瘤腺病毒、1×109至5×1011VP/天剂量的溶瘤腺病毒、3×1010至3×1011VP/天剂量的溶瘤腺病毒等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等);优选的是,该药物组合物或治疗剂的活性成分由5×107至5×1012VP/天剂量的溶瘤腺病毒(例如,5×107至1.5×1012VP/天剂量的溶瘤腺病毒、5×108至1×1012VP/天剂量的溶瘤腺病毒、1×109至5×1011VP/天剂量的溶瘤腺病毒、3×1010至3×1011VP/天剂量的溶瘤腺病毒等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等)组成。In other embodiments, the active ingredients of the pharmaceutical composition or the therapeutic agent of the present invention include 5 × 107 to 5 × 1012 VP / day dose of the oncolytic adenovirus (e.g., 5 × 107 to 1.5 × 1012 VP/day dose of oncolytic adenovirus, 5×108 to 1×1012 VP/day dose of oncolytic adenovirus, 1×109 to 5×1011 VP/day dose of oncolytic adenovirus, 3× 1010 to 3 × 1011 VP/day dose of oncolytic adenovirus, etc.) and the total dose per person per treatment range is 1 × 106 - 1 × 1011 of said NK cells (for example, each per person) The total dose of the treatment ranges from 6×107 to 1.2×1010 of the NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is from 5×107 to 5×1012 VP/day. Dosage of oncolytic adenovirus (eg, 5 x107 to 1.5 x 1012 VP/day dose of oncolytic adenovirus, 5 x108 to 1 x 1012 VP/day dose of oncolytic adenovirus, 1 x 109 to 5 × 1011 VP / day dose of oncolytic adenovirus, 3 × 1010 to 3 × 1011 VP / day dose of oncolytic adenovirus, etc.) and the total dose range per person per treatment is 1 × 106 - 1 × 1011 of said NK cells (for example, the total dose per patient per treatment range is 6 × 107 - 1.2 × 1 010 of the NK cells, etc.).
在一个实施方案中,本发明的药物组合物或治疗剂的活性成分包括5×107至1.5×1012VP/天剂量的溶瘤病毒H101(例如,5×1011至1.5×1012VP/天剂量的溶瘤病毒H101等)和1×105至1×1010个细胞/天剂量的所述NK细胞;优选的是,该药物组合物或治疗剂的活性成分由5×107至1.5×1012VP/天剂量的溶瘤病毒H101(例如,5 ×1011至1.5×1012VP/天剂量的溶瘤病毒H101等)和1×105至1×1010个细胞/天剂量的所述NK细胞(例如,1×107至1×1010个细胞/天剂量的所述NK细胞、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等)组成。In one embodiment, the active ingredients of the pharmaceutical composition or the therapeutic agent of the present invention include 5 × 107 to 1.5 × 1012 VP / day dose of the oncolytic virus H101 (e.g., 5 × 1011 to 1.5 × 1012 VP / day dose of oncolytic virus H101, etc.) and 1 x 105 to 1 x 1010 cells/day of the NK cells; preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is 5 x 107 Up to 1.5×1012 VP/day dose of oncolytic virus H101 (eg, 5 × 1011 to 1.5×1012 VP/day dose of oncolytic virus H101, etc.) and 1×105 to 1×1010 cells/ A daily dose of the NK cells (for example, 1 x107 to 1 x 1010 cells/day of the NK cells, 1 x 108 to 5 x 109 cells/day of the NK cells, 1 x 109 to 4 x 109 cells/day dose of the NK cells, 1 x 109 to 3 x 109 cells/day dose of the NK cells, etc.).
在另一个实施方案中,本发明的药物组合物或治疗剂的活性成分包括5×107至1.5×1012VP/天剂量的溶瘤病毒H101(例如,5×1011至1.5×1012VP/天剂量的溶瘤病毒H101等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等);优选的是,该药物组合物或治疗剂的活性成分由5×107至1.5×1012VP/天剂量的溶瘤病毒H101(例如,5×1011至1.5×1012VP/天剂量的溶瘤病毒H101等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等)组成。In another embodiment, the active ingredients of the pharmaceutical composition or therapeutic agent of the present invention include 5 × 107 to 1.5 × 1012 VP / day dose of the oncolytic virus H101 (e.g., 5 × 1011 to 1.5 × 1012 VP/day dose of oncolytic virus H101, etc.) and the total dose range of 1×106 -1×1011 per NK per patient (for example, the total dose range per person per treatment is 6×) 107 - 1.2 × 1010 of said NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is from 5 x 107 to 1.5 x 1012 VP/day of the oncolytic virus H101 ( For example, 5 x 1011 to 1.5 x 1012 VP/day dose of oncolytic virus H101, etc.) and the total dose per person per treatment range is 1 x 106 - 1
在特定的实施方案中,本发明的药物组合物或治疗剂所包含的溶瘤病毒为具有溶瘤作用的痘病毒(下文也称为“溶瘤痘病毒”)。在一些例子中,所述溶瘤痘病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。在一些例子中,所述溶瘤痘病毒是TK基因和/或VGF基因功能缺陷型的。在一些例子中,所述溶瘤痘病毒选自:Pexa-vac、JX-963、JX-929、VSC20、GL-ONC1、和/或TG6002。In a specific embodiment, the oncolytic virus contained in the pharmaceutical composition or therapeutic agent of the present invention is a poxvirus having an oncolysis effect (hereinafter also referred to as "anti-tumor poxvirus"). In some examples, the oncolytic poxvirus is selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect. In some examples, the oncolytic poxvirus is functionally deficient in the TK gene and/or the VGF gene. In some examples, the oncolytic poxvirus is selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
在某些实施方案中,本发明的药物组合物或治疗剂的活性成分包括1×105至5×109pfu/天剂量的溶瘤痘病毒(例如,1×105至3×109pfu/天剂量的溶瘤痘病毒、1×105至1×108pfu/天剂量的溶瘤痘病毒等)和1×105至1×1010个细胞/天剂量的所述NK细胞(例如,1×107至1×1010个细胞/天剂量的所述NK细胞、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等);优选的是,该药物组合物或治疗剂的活性成分由1×105至5×109pfu/ 天剂量的溶瘤痘病毒(例如,1×105至3×109pfu/天剂量的溶瘤痘病毒、1×105至1×108pfu/天剂量的溶瘤痘病毒等)和1×105至1×1010个细胞/天剂量的所述NK细胞(例如,1×107至1×1010个细胞/天剂量的所述NK细胞、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等)组成。In certain embodiments, the active ingredients of the pharmaceutical composition or therapeutic agent of the present invention comprises 1 × 105 to 5 × 109 pfu / day dose oncolytic poxvirus (e.g., 1 × 105 to 3 × 109 a) and 1 × 105 to 1 × 1010 cells / day dose pfu / day dose oncolytic poxvirus, 1 × 105 to 1 × 108 pfu / day dose oncolytic poxvirus like NK cells (For example, 1 × 107 to 1 × 1010 cells/day of the NK cells, 1 × 108 to 5 × 109 cells/day of the NK cells, 1 × 109 to 4 × 109 cells/day dose of said NK cells, 1 x 109 to 3 x 109 cells/day of said NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is 1 × 105 to 5 × 109 pfu / day dose of oncolytic pox virus (for example, 1 × 105 to 3 × 109 pfu / day dose of oncolytic pox virus, 1 × 105 to 1 × 108 pfu / day dose oncolytic poxvirus, etc.) and 1 × 105 to 1 × 1010 NK cells of said cell / day dose (e.g., the 1 × 107 to 1 × 1010 cells / day dose NK cells, 1×108 to 5×109 cells/day dose of the NK cells, 1×109 to 4×109 cells/day dose NK cells, 1 x 109 to 3 x 109 cells/day dose of the NK cells, etc.).
在另一些实施方案中,本发明的药物组合物或治疗剂的活性成分包括1×105至5×109pfu/天剂量的溶瘤痘病毒(例如,1×105至3×109pfu/天剂量的溶瘤痘病毒、1×105至1×108pfu/天剂量的溶瘤痘病毒等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等);优选的是,该药物组合物或治疗剂的活性成分由1×105至5×109pfu/天剂量的溶瘤痘病毒(例如,1×105至3×109pfu/天剂量的溶瘤痘病毒、1×105至1×108pfu/天剂量的溶瘤痘病毒等)和每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞等)组成。In other embodiments, the active ingredients of the pharmaceutical composition or the therapeutic agent of the present invention comprises 1 × 105 to 5 × 109 pfu / day dose oncolytic poxvirus (e.g., 1 × 105 to 3 × 109 Pfu/day dose of oncolytic pox virus, 1×105 to 1×108 pfu/day dose of oncolytic pox virus, etc.) and the total dose range per person per treatment is 1×106 -1×1011 The NK cells (for example, the total dose per subject per treatment range is 6×107 -1.2×1010 of the NK cells, etc.); preferably, the active ingredient of the pharmaceutical composition or therapeutic agent is 1 × 105 to 5 × 109 pfu / day dose of oncolytic pox virus (for example, 1 × 105 to 3 × 109 pfu / day dose of oncolytic pox virus, 1 × 105 to 1 × 108 pfu / day dose of oncolytic pox virus, etc.) and the total dose per person per treatment range of 1 x 106 - 1 × 1011 of said NK cells (for example, the total dose range per person per treatment is 6 × 107 - 1.2 × 1010 of the NK cells, etc.) composition.
本领域的技术人员可以理解,本发明的药物组合物或治疗剂还可包含合适的可药用的辅料。Those skilled in the art will appreciate that the pharmaceutical or therapeutic agents of the present invention may also comprise suitable pharmaceutically acceptable excipients.
本发明的药物组合物或治疗剂还可以包含本领域已知的其它活性成分,例如白细胞介素-2(IL-2)、粒细胞-巨噬细胞集落刺激因子(GM-CSF)、干扰素-γ(IFN-γ)、肿瘤坏死因子-α(TNF-α)等。The pharmaceutical composition or therapeutic of the present invention may further comprise other active ingredients known in the art, such as interleukin-2 (IL-2), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon. - γ (IFN-γ), tumor necrosis factor-α (TNF-α) and the like.
优选的是,本发明的药物组合物或治疗剂不包含硼替佐米。Preferably, the pharmaceutical or therapeutic agent of the invention does not comprise bortezomib.
在一些实施方案中,本发明的药物组合物或治疗剂包含一种或多种可药用载体。可以通过本领域已知的方法制备药物制剂。例如,可以将化合物等活性成分与常见的赋形剂、稀释剂(例如磷酸盐缓冲液或生理盐水)、组织培养基、和载体(例如自体血浆或人血清白蛋白)进行配制,并作为悬浮剂施用。其它的载体可以包括脂质体、胶团、纳米胶囊、聚合纳米颗粒、固体脂颗粒(例如参见文献“E.Koren and V.Torchilin,Life,63:586-595,2011”)。本发明的药物组合物或 治疗剂的具体配制方法可参见科学文献和专利文献中的描述,例如参见最新版雷明登氏药物科学,Maack出版公司,Easton PA(″Remington′s″)。In some embodiments, a pharmaceutical or therapeutic agent of the invention comprises one or more pharmaceutically acceptable carriers. Pharmaceutical formulations can be prepared by methods known in the art. For example, an active ingredient such as a compound can be formulated with a common excipient, a diluent (for example, phosphate buffer or physiological saline), a tissue culture medium, and a carrier (for example, autologous plasma or human serum albumin) as a suspension. Administration. Other carriers may include liposomes, micelles, nanocapsules, polymeric nanoparticles, solid lipid particles (see, for example, the literature "E. Koren and V. Torchilin, Life, 63:586-595, 2011"). Specific methods of formulating the pharmaceutical or therapeutic agents of the present invention can be found in the scientific literature and in the patent literature, for example, see the latest edition of Remington's Pharmaceutical Sciences, Maack Publishing Company, Easton PA ("Remington's").
在一些实施方案中,本发明提供了一种治疗剂,包含:(a)第一药物组合物,其中该第一药物组合物包含位于第一可药用载体中的溶瘤病毒;和(b)第二药物组合物,其中该第二药物组合物包含位于第二可药用载体中的NK细胞;其中所述溶瘤病毒能够选择性地在肿瘤细胞中复制;并且其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。在一些实施方案中,所述第一可药用载体和第二可药用载体是相同的。在另一些实施方案中,所述第一可药用载体和第二可药用载体是不同的。In some embodiments, the invention provides a therapeutic comprising: (a) a first pharmaceutical composition, wherein the first pharmaceutical composition comprises an oncolytic virus in a first pharmaceutically acceptable carrier; and (b) a second pharmaceutical composition, wherein the second pharmaceutical composition comprises NK cells in a second pharmaceutically acceptable carrier; wherein the oncolytic virus is capable of selectively replicating in tumor cells; and wherein the NK cells are The surface is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane domain and an intracellular domain, characterized in that said antigen binding The domain is derived from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12. In some embodiments, the first pharmaceutically acceptable carrier and the second pharmaceutically acceptable carrier are the same. In other embodiments, the first pharmaceutically acceptable carrier and the second pharmaceutically acceptable carrier are different.
本发明的药物组合物或治疗剂可以用于治疗多种肿瘤和/或癌症,包括但不限于:肺癌(例如非小细胞肺癌)、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病等。优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The pharmaceutical composition or therapeutic of the present invention can be used for the treatment of various tumors and/or cancers including, but not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal Cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, cervical cancer, lymphoma, stomach cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc. Preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive of. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
本发明的药物组合物或治疗剂的施用方法为,对肿瘤和/或癌症患者首先施用所述溶瘤病毒(例如溶瘤腺病毒、溶瘤痘病毒或溶瘤单纯疱疹病毒),然后,在施用所述溶瘤病毒之后的第18-72小时(例如,第20-70小时、第22-48小时、第24-48小时、第30-48小时等),对所述肿瘤和/或癌症患者施用所述NK细胞。“在施用所述溶瘤病 毒之后的第18-72小时(例如,第20-70小时、第22-48小时、第24-48小时、第30-48小时等),对所述肿瘤和/或癌症患者施用所述NK细胞”是指首次NK细胞的施用与首次溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等),或首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等)。优选地,首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等)。还优选地,首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为24-48小时。The pharmaceutical composition or therapeutic agent of the present invention is administered by first administering the oncolytic virus (for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) to a tumor and/or cancer patient, and then, 18-72 hours after administration of the oncolytic virus (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.) to the tumor and/or cancer The patient administers the NK cells. "18-72 hours after administration of the oncolytic virus (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.), the tumor and / Or administration of the NK cells by a cancer patient" means that the time interval between administration of the first NK cells and the first oncolytic virus administration is 18-72 hours (for example, 20-70 hours, 22-48 hours, 24-48 hours, 30- 48 hours, etc.), or the time interval between administration of the first NK cells and the onset of the oncolytic virus that is most adjacent to it is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48) Hours, 30-48 hours, etc.). Preferably, the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to it is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30) -48 hours, etc.). Also preferably, the time interval between administration of the first NK cells and administration of the oncolytic virus that is most adjacent to it before is 24-48 hours.
在本发明的一个优选实施方案中,所述溶瘤病毒(例如溶瘤腺病毒、溶瘤痘病毒或溶瘤单纯疱疹病毒)的施用剂量为治疗有效量,每天1次,连续施用1-6天;并且所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。在本发明的另一个优选实施方案中,所述溶瘤病毒(例如溶瘤腺病毒、溶瘤痘病毒或溶瘤单纯疱疹病毒)的施用剂量为治疗有效量,每2天1次,连续施用2-6天;并且所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。无论本发明采用上述何种实施方案或其它实施方案,只要满足在施用所述溶瘤病毒(例如溶瘤腺病毒、溶瘤痘病毒或溶瘤单纯疱疹病毒)之后的第18小时至72小时,对所述肿瘤和/或癌症患者施用NK细胞的条件即可。其中溶瘤病毒的施用和NK细胞的施用可以为(例如)首先施用溶瘤病毒,每天1次,连续施用1-6天,之后间隔18-72小时再施用NK细胞,每周施用1-2次,连续3周。优选的是,首先施用溶瘤病毒,在溶瘤病毒全部施用之后间隔18-72小时再施用NK细胞。在本发明的一个优选实施方案中,对肿瘤和/或癌症患者首先施用所述溶瘤病毒,所述溶瘤病毒的施用剂量为治疗有效量,施用1次;并且在施用 所述溶瘤病毒之后的第18小时至72小时,对所述肿瘤和/或癌症患者施用所述NK细胞,所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。对于不同的溶瘤病毒,可以采用不同的优选临床剂量范围,例如表1所述的那样。In a preferred embodiment of the invention, the oncolytic virus (for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) is administered in a therapeutically effective amount, once a day, continuously administered from 1 to 6 The NK cells are administered at a dose ranging from 1×106 to 1×1011 per patient per course (for example, the total dose per patient per treatment range is 6×107 -1.2×10)10 ), administered 1-2 times a week for 3 weeks. In another preferred embodiment of the present invention, the oncolytic virus (for example, oncolytic adenovirus, oncolytic pox virus or oncolytic herpes simplex virus) is administered in a therapeutically effective amount, once every 2 days, continuously. 2-6 days; and the NK cells are administered at a dose ranging from 1×106 to 1×1011 per patient per treatment (for example, the total dose range per person per treatment is 6×107 - 1.2 × 1010 ), 1-2 times a week for 3 weeks. Regardless of which embodiment or other embodiments of the invention are employed, as long as the 18th to 72th hours after administration of the oncolytic virus (eg, oncolytic adenovirus, oncolytic pox virus, or oncolytic herpes simplex virus) is satisfied, The conditions for administering the NK cells to the tumor and/or cancer patient may be sufficient. Wherein administration of the oncolytic virus and administration of the NK cells may be, for example, first administration of the oncolytic virus once a day for 1-6 days, followed by administration of NK cells at intervals of 18-72 hours, 1-2 weekly administration Times, for 3 consecutive weeks. Preferably, the oncolytic virus is administered first, and the NK cells are administered again 18-72 hours after the oncolytic virus is administered. In a preferred embodiment of the invention, the oncolytic virus is first administered to a tumor and/or cancer patient, the oncolytic virus is administered in a therapeutically effective amount, administered once; and the oncolytic virus is administered The NK cells are administered to the tumor and/or cancer patient from the 18th hour to the 72th hour thereafter, and the NK cells are administered at a dose ranging from 1×106 to 1×1011 per person per course of treatment. (for example, the total dose per patient per treatment range is 6 × 107 - 1.2 × 1010 ), 1-2 times a week for 3 weeks. For different oncolytic viruses, different preferred clinical dosage ranges can be employed, such as those described in Table 1.
溶瘤病毒能够在肿瘤或癌症细胞中选择性复制,经过一定时间达到峰值。本发明的发明人发现,在经过一段时间的复制之后,肿瘤细胞里的溶瘤病毒会促进NK细胞对肿瘤细胞的杀伤。因此,本发明提出的溶瘤病毒和NK细胞的施用间隔实现了两者作用峰值的双峰重叠。Oncolytic viruses are capable of selective replication in tumor or cancer cells and peak over time. The inventors of the present invention found that after a period of replication, the oncolytic virus in the tumor cells promotes killing of tumor cells by NK cells. Therefore, the application interval of the oncolytic virus and NK cells proposed by the present invention achieves a bimodal overlap of the peaks of action of both.
本发明还提供了本发明所述的药物组合物或治疗剂在制备用于治疗肿瘤和/或癌症的药物中的应用。The invention also provides the use of a pharmaceutical or therapeutic agent of the invention in the manufacture of a medicament for the treatment of tumors and/or cancer.
所述肿瘤和/或癌症包括但不限于:肺癌(例如非小细胞肺癌)、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病等。优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer includes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc. Preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive of. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
本发明还提供了一种用于治疗肿瘤和/或癌症的具有协同作用的联合药物的药盒,包括装有本发明所述溶瘤病毒的第一容器和装有本发明所述NK细胞的第二容器,其中所述第一容器和所述第二容器是独立的;以及载明给药时机和给药方式的说明书;其中所述溶瘤病毒能够选择性地在肿瘤细胞中复制;其中所述NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12 的胞内信号区。优选的是,该药盒由分别独立地装有本发明所述的溶瘤病毒和本发明所述的NK细胞的独立容器组成,以及载明给药时机和给药方式的说明书。The present invention also provides a kit for synergistic combination therapy for treating tumors and/or cancer, comprising a first container containing the oncolytic virus of the present invention and a ovary containing the NK cells of the present invention a second container, wherein said first container and said second container are independent; and instructions for indicating the timing of administration and mode of administration; wherein said oncolytic virus is capable of selectively replicating in tumor cells; The surface of the NK cell is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, a transmembrane region and an intracellular domain, characterized in that The antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12. Preferably, the kit consists of separate containers each containing the oncolytic virus of the invention and the NK cells of the invention, respectively, together with instructions for the timing and mode of administration.
所述肿瘤和/或癌症包括但不限于:肺癌(例如非小细胞肺癌)、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病等。优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer includes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc. Preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive of. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
优选地,所述装有溶瘤病毒的第一容器包含治疗有效量的所述溶瘤病毒,并且装有NK细胞的第二容器包含足够提供1×105至1×1010个细胞/天剂量的所述NK细胞(例如,1×107-1×1010个细胞/天剂量、1×108至5×109个细胞/天剂量的所述NK细胞、1×109至4×109个细胞/天剂量的所述NK细胞、1×109至3×109个细胞/天剂量的所述NK细胞等)。还优选地,所述装有溶瘤病毒的第一容器包含治疗有效量的所述溶瘤病毒,并且装有NK细胞的第二容器包含足够提供每人每个疗程总剂量范围为1×106-1×1011个的所述NK细胞(例如,每人每个疗程总剂量范围为6×107-1.2×1010个的所述NK细胞)。对于不同的溶瘤病毒,可以采用不同的优选临床剂量范围,例如表1所述的那样。Preferably, the first container containing the oncolytic virus comprises a therapeutically effective amount of the oncolytic virus, and the second container containing the NK cells comprises sufficient to provide 1 x 105 to 1 x 1010 cells per day. The dose of the NK cells (for example, 1 × 107 - 1 × 1010 cells / day dose, 1 × 108 to 5 × 109 cells / day dose of the NK cells, 1 × 109 to 4 × 109 cells/day dose of the NK cells, 1 × 109 to 3 × 109 cells/day of the NK cells, etc.). Still preferably, the first container containing the oncolytic virus comprises a therapeutically effective amount of the oncolytic virus, and the second container containing the NK cells comprises sufficient to provide a total dose range of 1 x 10 per person per course of treatment.6 - 1 × 1011 of said NK cells (e.g., the total dose per subject per treatment range is 6 x 107 - 1.2 x 1010 of said NK cells). For different oncolytic viruses, different preferred clinical dosage ranges can be employed, such as those described in Table 1.
所述溶瘤病毒可采用其各自的本领域通常所采用的给药方式给药,例如通过瘤内注射给药或静脉给药。The oncolytic viruses can be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
所述NK细胞可采用本领域通常所采用的给药方式给药,例如可通过静脉给药或局部给药。The NK cells can be administered by administration generally employed in the art, for example, by intravenous administration or topical administration.
在本发明药盒的特定实施方案中,所述溶瘤病毒为具有溶瘤作用的腺病毒。在一些例子中,所述具有溶瘤作用的腺病毒的E1区和 /或E3区是经基因工程改造的。在一些例子中,所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。In a particular embodiment of the kit of the invention, the oncolytic virus is an adenovirus having an oncolytic effect. In some examples, the E1 region and/or E3 region of the oncolytic adenovirus is genetically engineered. In some examples, the oncolytic adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtakTM .
在本发明药盒的特定实施方案中,所述第一容器包含5×107至5×1012VP/天剂量的溶瘤腺病毒(例如,5×107至1.5×1012VP/天剂量的溶瘤腺病毒、5×108至1×1012VP/天剂量的溶瘤腺病毒、1×109至5×1011VP/天剂量的溶瘤腺病毒、3×1010至3×1011VP/天剂量的溶瘤腺病毒等)。In a particular embodiment of the kit of the present invention, the first container comprises a 5 × 107 to 5 × 1012 VP / day dose of the oncolytic adenovirus (e.g., 5 × 107 to 1.5 × 1012 VP / day Dosage of oncolytic adenovirus, 5 x108 to 1 x 1012 VP/day dose of oncolytic adenovirus, 1 x109 to 5 x 1011 VP/day dose of oncolytic adenovirus, 3 x 1010 to 3×1011 VP/day dose of oncolytic adenovirus, etc.).
在一个实施方案中,所述第一容器包含5×107至1.5×1012VP/天剂量的溶瘤病毒H101(例如,5×1011至1.5×1012VP/天剂量的溶瘤病毒H101等)。In one embodiment, the first container comprises a 5 × 107 to 1.5 × 1012 VP / day dose of the oncolytic virus H101 (e.g., 5 × 1011 to 1.5 × 1012 VP / day dose of an oncolytic virus H101, etc.).
在本发明药盒的特定实施方案中,所述溶瘤病毒为溶瘤痘病毒。在一些例子中,所述溶瘤痘病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。在一些例子中,所述溶瘤痘病毒是TK基因和/或VGF基因功能缺陷型的。在一些例子中,所述溶瘤痘病毒选自:Pexa-vac、JX-963、JX-929、VSC20、GL-ONC1、和/或TG6002。In a particular embodiment of the kit of the invention, the oncolytic virus is an oncolytic virus. In some examples, the oncolytic poxvirus is selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect. In some examples, the oncolytic poxvirus is functionally deficient in the TK gene and/or the VGF gene. In some examples, the oncolytic poxvirus is selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
在本发明药盒的特定实施方案中,所述第一容器包含1×105至5×109pfu/天剂量的溶瘤痘病毒(例如,1×105至3×109pfu/天剂量的溶瘤痘病毒、1×105至1×108pfu/天剂量的溶瘤痘病毒等)。In a particular embodiment of the kit of the present invention, the first container comprises 1 × 105 to 5 × 109 pfu / day dose oncolytic poxvirus (e.g., 1 × 105 to 3 × 109 pfu / day Dosage of oncolytic pox virus, 1 x 105 to 1 x 108 pfu / day dose of oncolytic pox virus, etc.).
本发明还提供了一种治疗肿瘤和/或癌症的方法,包括以下依次进行的步骤:The invention also provides a method of treating a tumor and/or cancer comprising the steps of:
1)对肿瘤和/或癌症患者施用本发明所述的溶瘤病毒,该溶瘤病毒能够选择性地在肿瘤细胞中复制;1) administering to a tumor and/or cancer patient an oncolytic virus according to the invention, which is capable of selectively replicating in a tumor cell;
2)在施用所述溶瘤病毒之后的第18-72小时(例如,第20-70小时、第22-48小时、第24-48小时、第30-48小时等),对所述肿瘤和/或癌症患者施用本发明所述的NK细胞,该NK细胞的表面被嵌合抗原受体修饰,该嵌合抗原受体包括可操作地连接的、依次串联的抗原结合结构域、间隔区、跨膜区和胞内结构域,其特征在于,所 述抗原结合结构域来自NKG2D的配体结合区,所述胞内结构域来自DAP12的胞内信号区。2) 18-72 hours after administration of the oncolytic virus (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.), the tumor and / or a cancer patient administering the NK cells of the invention, the surface of which is modified by a chimeric antigen receptor comprising an operably linked, sequentially tandem antigen binding domain, a spacer, Transmembrane and intracellular domains, characterized in that the antigen binding domain is from the ligand binding region of NKG2D, which is derived from the intracellular signaling region of DAP12.
“在施用所述溶瘤病毒之后的第18-72小时(例如,第20-70小时、第22-48小时、第24-48小时、第30-48小时等),对所述肿瘤和/或癌症患者施用本发明所述的NK细胞”是指首次NK细胞的施用与首次溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等),或首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等)。优选地,首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为18-72小时(例如,20-70小时、22-48小时、24-48小时、30-48小时等)。还优选地,首次NK细胞的施用与在其之前最相邻一次的所述溶瘤病毒施用的时间间隔为24-48小时。"18-72 hours after administration of the oncolytic virus (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.), the tumor and / Or administration of the NK cells of the present invention to a cancer patient" means that the time interval between administration of the first NK cells and the first oncolytic virus administration is 18-72 hours (for example, 20-70 hours, 22-48 hours, 24-48 hours). 30-48 hours, etc., or the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to it before the time is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30-48 hours, etc.). Preferably, the time interval between administration of the first NK cell and the onset of the oncolytic virus that is most adjacent to it is 18-72 hours (eg, 20-70 hours, 22-48 hours, 24-48 hours, 30) -48 hours, etc.). Also preferably, the time interval between administration of the first NK cells and administration of the oncolytic virus that is most adjacent to it before is 24-48 hours.
所述肿瘤和/或癌症包括但不限于:肺癌(例如非小细胞肺癌)、黑色素瘤、头颈部癌症、肝癌、脑癌、结直肠癌、膀胱癌、乳腺癌、卵巢癌、子宫癌、宫颈癌、淋巴癌、胃癌、食道癌、肾癌、前列腺癌、胰腺癌、白血病等。优选地,所述肿瘤和/或癌症是NKG2D配体阳性的,包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。在本发明的一个实施方案中,所述肿瘤和/或癌症是NKG2D配体阳性的情况包括所述肿瘤和/或癌症未经处理是NKG2D配体阳性的以及所述肿瘤和/或癌症经处理而成为NKG2D配体阳性的。所述处理包括经药物、放射或生物制剂处理后成为NKG2D配体阳性的。The tumor and/or cancer includes, but is not limited to, lung cancer (eg, non-small cell lung cancer), melanoma, head and neck cancer, liver cancer, brain cancer, colorectal cancer, bladder cancer, breast cancer, ovarian cancer, uterine cancer, Cervical cancer, lymphoma, gastric cancer, esophageal cancer, kidney cancer, prostate cancer, pancreatic cancer, leukemia, etc. Preferably, the tumor and/or cancer is NKG2D ligand positive, including the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated to become NKG2D ligand positive of. In one embodiment of the invention, the tumor and/or cancer is positive for NKG2D ligand comprising the tumor and/or cancer being untreated is NKG2D ligand positive and the tumor and/or cancer is treated It became positive for NKG2D ligand. The treatment includes becoming NKG2D ligand positive after treatment with a drug, radiation or biological agent.
在本发明的一个优选实施方案中,所述溶瘤病毒的施用剂量为治疗有效量,每天1次,连续施用1-6天;并且所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。在本发明的另一个优选实施方案中,所述溶瘤病毒的施用剂量为治疗有效量,每2天1次,连续施用2-6天;并且所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗 程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。无论本发明采用上述何种实施方案或其它实施方案,只要满足在施用所述溶瘤病毒之后的第18小时至72小时,对所述肿瘤和/或癌症患者施用NK细胞的条件即可。其中溶瘤病毒的施用和NK细胞的施用可以是(例如)首先施用溶瘤病毒,每天1次,连续施用1-6天,之后间隔18-72小时再施用NK细胞,每周施用1-2次,连续3周)。优选的是,首先施用溶瘤病毒,在溶瘤病毒全部施用之后间隔18-72小时再施用NK细胞。在本发明的一个优选实施方案中,对肿瘤和/或癌症患者首先施用所述溶瘤病毒,所述溶瘤病毒的施用剂量为治疗有效量,施用1次;并且在施用所述溶瘤病毒之后的第18小时至72小时,对所述肿瘤和/或癌症患者施用所述NK细胞,所述NK细胞的施用剂量为每人每个疗程总剂量范围为1×106-1×1011个(例如,每人每个疗程总剂量范围为6×107-1.2×1010个),每周施用1-2次,连续3周。对于不同的溶瘤病毒,可以采用不同的优选临床剂量范围,例如表1所述的那样。In a preferred embodiment of the present invention, the oncolytic virus is administered in a therapeutically effective amount once a day for 1-6 days; and the NK cells are administered at a total dose per person per course of treatment. The range is 1 × 106 - 1 × 1011 (for example, the total dose per patient per treatment range is 6 × 107 - 1.2 × 1010 ), and is administered 1-2 times a week for 3 weeks. In another preferred embodiment of the present invention, the oncolytic virus is administered in a therapeutically effective amount once every 2 days for 2-6 days; and the NK cells are administered at a dose of each person. The total dose range of treatment is 1 × 106 -1 × 1011 (for example, the total dose range per patient per treatment is 6 × 107 - 1.2 × 1010 ), 1-2 times a week for 3 weeks . Regardless of which embodiment or other embodiments of the invention are employed, as long as the conditions of administration of NK cells to the tumor and/or cancer patient are satisfied from 18 hours to 72 hours after administration of the oncolytic virus. Wherein administration of the oncolytic virus and administration of the NK cells may be, for example, first administration of the oncolytic virus once a day for 1-6 days, followed by administration of NK cells at intervals of 18-72 hours, 1-2 weekly administration Times, for 3 consecutive weeks). Preferably, the oncolytic virus is administered first, and the NK cells are administered again 18-72 hours after the oncolytic virus is administered. In a preferred embodiment of the invention, the oncolytic virus is first administered to a tumor and/or cancer patient, the oncolytic virus is administered in a therapeutically effective amount, administered once; and the oncolytic virus is administered The NK cells are administered to the tumor and/or cancer patient from the 18th hour to the 72th hour thereafter, and the NK cells are administered at a dose ranging from 1×106 to 1×1011 per person per course of treatment. (for example, the total dose per patient per treatment range is 6 × 107 - 1.2 × 1010 ), 1-2 times a week for 3 weeks. For different oncolytic viruses, different preferred clinical dosage ranges can be employed, such as those described in Table 1.
可以根据实际情况和需要对患者进行一次或多次本发明的治疗肿瘤和/或癌症的方法。The method of treating tumors and/or cancer of the present invention may be performed one or more times on the patient according to actual conditions and needs.
所述溶瘤病毒可采用其各自的本领域通常所采用的给药方式给药,例如通过瘤内注射给药或静脉给药。The oncolytic viruses can be administered by their respective modes of administration commonly employed in the art, such as by intratumoral injection or intravenous administration.
所述NK细胞可采用本领域通常所采用的给药方式给药,例如可通过静脉给药或局部给药。The NK cells can be administered by administration generally employed in the art, for example, by intravenous administration or topical administration.
在本发明的方法的特定实施方案中,所述溶瘤病毒为具有溶瘤作用的腺病毒。在一些例子中,所述具有溶瘤作用的腺病毒的E1区和/或E3区是经基因工程改造的。在一些例子中,所述具有溶瘤作用的腺病毒选自:Onyx-015、H101、Ad5-yCD/mutTKSR39rep-hIL12、CG0070、DNX-2401、OBP-301、ONCOS-102、ColoAd1、VCN-01、和/或ProstAtakTM。In a particular embodiment of the method of the invention, the oncolytic virus is an adenovirus having an oncolytic effect. In some examples, the E1 region and/or E3 region of the oncolytic adenovirus is genetically engineered. In some examples, the oncolytic adenovirus is selected from the group consisting of: Onyx-015, H101, Ad5-yCD/mutTKSR39rep-hIL12, CG0070, DNX-2401, OBP-301, ONCOS-102, ColoAd1, VCN-01 , and / or ProstAtakTM .
在本发明的方法的某些实施方案中,所述溶瘤病毒为溶瘤腺病毒,并且其施用剂量为5×107至5×1012VP/天(例如,5×107至1.5×1012VP/天、5×108至1×1012VP/天、1×109至5×1011VP/天、3× 1010至3×1011VP/天等)。In certain embodiments of the method of the present invention, the oncolytic virus is an oncolytic adenovirus, and is administered at a dose of 5 × 107 to 5 × 1012 VP / day (e.g., 5 × 107 to 1.5 × 1012 VP/day, 5×108 to 1×1012 VP/day, 1×109 to 5×1011 VP/day, 3×1010 to 3×1011 VP/day, etc.).
在一个实施方案中,所述溶瘤病毒为溶瘤病毒H101,并且其施用剂量为5×107至1.5×1012VP/天(例如,5×1011至1.5×1012VP/天等)。In one embodiment, the oncolytic virus is an oncolytic virus H101, and which is administered at a dose of 5 × 107 to 1.5 × 1012 VP / day (e.g., 5 × 1011 to 1.5 × 1012 VP / day, etc. ).
在本发明的方法的特定实施方案中,所述溶瘤病毒为溶瘤痘病毒。在一些例子中,所述溶瘤痘病毒选自具有溶瘤作用的经基因突变的病毒和具有溶瘤作用的野生型病毒。在一些例子中,所述溶瘤痘病毒是TK基因和/或VGF基因功能缺陷型的。在一些例子中,所述溶瘤痘病毒选自:Pexa-vac、JX-963、JX-929、VSC20、GL-ONC1、和/或TG6002。In a particular embodiment of the methods of the invention, the oncolytic virus is an oncolytic virus. In some examples, the oncolytic poxvirus is selected from the group consisting of a genetically mutated virus having an oncolysis effect and a wild type virus having an oncolysis effect. In some examples, the oncolytic poxvirus is functionally deficient in the TK gene and/or the VGF gene. In some examples, the oncolytic poxvirus is selected from the group consisting of: Pexa-vac, JX-963, JX-929, VSC20, GL-ONC1, and/or TG6002.
在本发明的方法的某些实施方案中,所述溶瘤病毒为溶瘤痘病毒,并且其施用剂量为1×105至5×109pfu/天(例如,1×105至3×109pfu/天、1×105至1×108pfu/天等)。In certain embodiments of the methods of the invention, the oncolytic virus is an oncolytic poxvirus and is administered at a dose of from 1 x 105 to 5 x 109 pfu per day (eg, 1 x 105 to 3 x) 109 pfu/day, 1×105 to 1×108 pfu/day, etc.).
以下通过例子的方式进一步解释或说明本发明的内容,但这些例子不应被理解为对本发明的保护范围的限制。The contents of the present invention are further explained or illustrated by way of examples, but the examples are not to be construed as limiting the scope of the invention.
例子example
以下除非特别说明,否则以下例子中所用实验方法均使用生物工程领域的常规实验流程、操作、材料和条件进行。Unless otherwise stated, the experimental methods used in the following examples were carried out using conventional experimental procedures, procedures, materials and conditions in the field of bioengineering.
以下除非特别说明,否则各试剂的百分浓度(%)均指该试剂的体积百分浓度(%(v/v))。Unless otherwise indicated, the percent concentration (%) of each reagent refers to the volume percent concentration (% (v/v)) of the reagent.
以下例子中所用人类卵巢癌细胞SKOV3、人类结直肠癌细胞HCT116和SW480、人类头颈癌细胞Fadu和Detroit、人类肝癌细胞HepG2、人类神经胶质瘤细胞U87、人类乳腺癌细胞MCF7、和人类骨髓瘤细胞KG1购自ATCC。Human ovarian cancer cell line SKOV3, human colorectal cancer cells HCT116 and SW480, human head and neck cancer cells Fadu and Detroit, human liver cancer cell HepG2, human glioma cell line U87, human breast cancer cell MCF7, and human myeloma used in the following examples Cell KG1 was purchased from ATCC.
以下例子中所用H101溶瘤腺病毒购自上海三维生物。The H101 oncolytic adenovirus used in the following examples was purchased from Shanghai 3D organism.
以下例子中所用PBS购自Lonza,货号为BW17-517Q。The PBS used in the following examples was purchased from Lonza under the order number BW17-517Q.
以下例子中所用VioBright FITC偶联的抗hMICA/B抗体购自Miltenyi;PE偶联的抗hULBP1抗体、APC偶联的抗hULBP2/5/6抗体、 APC偶联的抗hULBP3抗体、APC偶联的抗hULBP4抗体购自R&D Systems。The VioBright FITC-conjugated anti-hMICA/B antibody used in the following examples was purchased from Miltenyi; PE-conjugated anti-hULBP1 antibody, APC-conjugated anti-hULBP2/5/6 antibody, APC-conjugated anti-hULBP3 antibody, APC-conjugated Anti-hULBP4 antibody was purchased from R&D Systems.
以下例子中所用Calcein AM购自Life Technologies。The Calcein AM used in the following examples was purchased from Life Technologies.
以下例子中所用24孔细胞培养板(每孔培养体积500μl)、96孔细胞培养板(每孔培养体积200μl)均得自:Corning(康宁)公司。The 24-well cell culture plates used in the following examples (500 μl per well culture volume) and 96-well cell culture plates (200 μl per well culture volume) were obtained from Corning.
制备例1:嵌合抗原受体质粒构建Preparation Example 1: Construction of chimeric antigen receptor plasmid
1)静脉血单个核细胞(PBMCs)cDNA的制备1) Preparation of venous blood mononuclear cells (PBMCs) cDNA
取人静脉血于含肝素的真空管中。采用淋巴细胞分离液,密度梯度离心方法分离获得单个核细胞(PBMCs)。Take human venous blood into a vacuum tube containing heparin. Mononuclear cells (PBMCs) were isolated by lymphocyte separation and density gradient centrifugation.
离心沉淀上述PBMCs,用总RNA提取试剂盒RNAiso Reagent(购自Life Technologies)提取细胞的总RNA,-80℃保存备用。提取的总RNA用逆转录试剂盒RevertAicTFirst Strand cDNASynthesis Kit(购自Life Technologies)逆转录得PBMCs的cDNA,-20℃保存备用。The above PBMCs were pelleted by centrifugation, and total RNA of the cells was extracted with a total RNA extraction kit RNAiso Reagent (purchased from Life Technologies), and stored at -80 ° C until use. The extracted total RNA was reverse transcribed into cDNA of PBMCs using a reverse transcription kit RevertAicTFirst Strand cDNA Synthesis Kit (purchased from Life Technologies), and stored at -20 ° C until use.
2)嵌合抗原受体的重组DNA载体pFastbac1-NKG2D-CD8-DAP12的构建2) Construction of recombinant DNA vector pFastbac1-NKG2D-CD8-DAP12 of chimeric antigen receptor
以步骤1)中单个核细胞cDNA为模板,用引物P1(SEQ ID NO:9)、P2(SEQ ID NO:10)进行PCR扩增,获得其中包含长度为402bp的NKG2D蛋白的胞外结构域对应的DNA编码序列(核苷酸序列如序列表中SEQ ID NO:6所示)的片段,其两端分别具有Sphl和NheI酶切位点和保护碱基。利用引物P3(SEQ ID NO:11)、P4(SEQ ID NO:12)进行PCR扩增,获得其中包含长度为249bp的CD8α基因的铰链(hinge)区和跨膜区(核苷酸序列如序列表中SEQ ID NO:7所示)的片段。利用引物P5(SEQ ID NO:13)、P6(SEQ ID NO:14)进行PCR扩增得到其中包含长度为156bp的DAP12的胞内信号结构域(核苷酸序列如序列表中SEQ ID NO:8所示)的片段。各步PCR扩增反应体系相同,PCR反应条件参照KAPA HiFiHotStart ReadyMix(2X)(购自Kapa Biosystems)的说明书,各反应体系(50μL)如下:Using the single nuclear cell cDNA in step 1) as a template, PCR amplification was carried out with primers P1 (SEQ ID NO: 9) and P2 (SEQ ID NO: 10) to obtain an extracellular domain comprising a NKG2D protein of 402 bp in length. A fragment of the corresponding DNA coding sequence (nucleotide sequence as shown in SEQ ID NO: 6 in the Sequence Listing) has a Sphl and NheI restriction site and a protective base at both ends, respectively. PCR amplification using primers P3 (SEQ ID NO: 11) and P4 (SEQ ID NO: 12) to obtain a hinge region and a transmembrane region in which a CD8α gene of 249 bp in length was obtained (nucleotide sequence A fragment of SEQ ID NO: 7 in the list). PCR amplification using primers P5 (SEQ ID NO: 13) and P6 (SEQ ID NO: 14) gave an intracellular signal domain containing DAP12 of 156 bp in length (nucleotide sequence such as SEQ ID NO in the sequence listing: A fragment of 8). The PCR amplification reaction system was the same in each step. The PCR reaction conditions were as described in KAPA HiFi Hot Start Ready Mix (2X) (purchased from Kapa Biosystems), and each reaction system (50 μL) was as follows:
双蒸水:21.5μLDouble distilled water: 21.5μL
2×KAPA HiFiHotStart Uracil+ReadyMix:25μL2×KAPA HiFiHotStart Uracil+ReadyMix: 25μL
上游引物(10μM):1.5μLUpstream primer (10μM): 1.5μL
下游引物(10μM):1.5μLDownstream primer (10μM): 1.5μL
单个核细胞cDNA模板(120ng/ul):0.5μLMononuclear cell cDNA template (120ng/ul): 0.5μL
将上述PCR产物用1%(w/v)的琼脂糖凝胶进行分离,用琼脂糖凝胶DNA回收试剂盒(购自Omega Bio Tek)进行DNA片段回收。将回收片段中包含长度为402bp的NKG2D蛋白的胞外结构域DNA的片段进行Sphl和NheI双酶切反应,酶切产物用琼脂糖凝胶DNA回收试剂盒进行DNA片段回收备用。The above PCR product was separated on a 1% (w/v) agarose gel, and DNA fragment recovery was carried out using an agarose gel DNA recovery kit (purchased from Omega Bio Tek). The fragment containing the extracellular domain DNA of the NKG2D protein of 402 bp in length was subjected to double digestion with Sphl and NheI, and the digested product was subjected to DNA fragment recovery using an agarose gel DNA recovery kit.
按照图1的顺序,将商业化载体pFastbac1(Life Technologies)加入一个CMV启动子,T7启动子,一个拥有Kozak序列的5’UTR,一个GM-CSFα链信号肽的编码序列(图1中的SP),和一个具有polyA信号的阿尔法球蛋白的3’UTR来构建pFastbac1基本骨架载体。将pFastbac1基本骨架载体进行SphI和SalI双酶切反应,酶切产物用琼脂糖凝胶DNA回收试剂盒进行DNA片段回收,然后与之前PCR回收的CD8、DAP12片段通过Seamless Cloning and Assembly Kit(购自Life Technologies)进行连接,连接产物转化OneChemically Competent TOP10化学感受态细胞(购自Life Technologies),37℃培养18小时后挑取单克隆,37℃,250rpm培养6小时后用质粒小提试剂盒(购自Omega Bio Tek)提取质粒。提取的质粒经限制性内切酶SphI和SalI双酶切鉴定,鉴定电泳图见图2;其中,泳道1:1000kb DNA分子量标记;泳道2:质粒pFastbac1-CD8-DAP12的酶切片段,载体骨架5276bp,CD8-DAP12片段405bp。将鉴定正确的质粒送AITbiotech公司对插入的融合基因片段进行测序,将测序结果正确的重组质粒命名为pFastbac1-CD8-DAP12。According to the sequence of Figure 1, the commercial vector pFastbac1 (Life Technologies) was added to a CMV promoter, a T7 promoter, a 5'UTR with a Kozak sequence, and a coding sequence for a GM-CSF alpha chain signal peptide (SP in Figure 1). ), and a 3'UTR of alpha globulin with a polyA signal to construct the pFastbac1 basic skeleton vector. The pFastbac1 basic skeleton vector was subjected to double digestion with SphI and SalI, and the digested product was subjected to DNA fragment recovery using an agarose gel DNA recovery kit, and then passed through the CD8 and DAP12 fragments recovered by the previous PCR. Seamless Cloning and Assembly Kit (purchased from Life Technologies) for connection and connection of product conversion to One Chemically Competent TOP10 chemically competent cells (purchased from Life Technologies), picked up for 18 hours at 37 ° C, picked up the monoclonal, and cultured at 37 ° C, 250 rpm for 6 hours, and then extracted the plasmid with a plasmid miniprep kit (purchased from Omega Bio Tek). The extracted plasmid was identified by restriction endonuclease SphI and SalI digestion, and the electrophoresis pattern was identified as shown in Fig. 2; wherein, lane 1: 1000 kb DNA molecular weight marker; lane 2: plasmid pFastbac1-CD8-DAP12 digestion fragment, vector backbone 5276 bp, CD8-DAP12 fragment 405 bp. The correct plasmid was sent to AITbiotech for sequencing of the inserted fusion gene fragment, and the correct recombinant plasmid was named pFastbac1-CD8-DAP12.
将载体pFastbac1-CD8-DAP12进行SphI和NheI双酶切反应,酶切产物用琼脂糖凝胶DNA回收试剂盒进行DNA片段回收,然后与之前回收的NKG2D蛋白的胞外结构域片段通过T4 DNA连接酶连接,连接产物转化OneChemically Competent TOP10化学感受 态细胞,37℃培养18小时后挑取单克隆,37℃,250rpm培养6小时后用质粒小提试剂盒提取质粒。提取的质粒经限制性内切酶SphI和NheI双酶切鉴定,鉴定电泳图见图3;其中,泳道1:1000kb DNA分子量标记;泳道2:质粒pFastbac1-NKG2D-CD8-DAP12的酶切片段,载体骨架5682bp,NKG2D片段402bp。将鉴定正确的质粒送AITbiotech公司对插入的融合基因片段进行测序,将测序结果正确的重组质粒命名为pFastbac1-NKG2D-CD8-DAP12,其质粒图谱示意图见图4。The vector pFastbac1-CD8-DAP12 was digested with SphI and NheI, and the digested product was recovered by DNA fragmentation using an agarose gel DNA recovery kit, and then ligated to the extracellular domain fragment of the previously recovered NKG2D protein by T4 DNA. Enzyme-linked, linked product-transformed One Chemically Competent TOP10 chemically competent cells were cultured at 37 ° C for 18 hours, and then picked up, and cultured at 37 ° C, 250 rpm for 6 hours, and then the plasmid was extracted with a plasmid mini-kit. The extracted plasmid was identified by restriction endonuclease SphI and NheI digestion, and the electrophoresis pattern was identified as shown in Fig. 3; wherein, lane 1: 1000 kb DNA molecular weight marker; lane 2: restriction fragment of plasmid pFastbac1-NKG2D-CD8-DAP12, The vector backbone is 5682 bp and the NKG2D fragment is 402 bp. The correct plasmid was sent to AITbiotech for sequencing of the inserted fusion gene fragment, and the recombinant plasmid with the correct sequencing result was named pFastbac1-NKG2D-CD8-DAP12, and the plasmid map is shown in Figure 4.
制备例2:NK细胞的制备Preparation Example 2: Preparation of NK cells
将细胞数为20×106个的PBMCs细胞和2mL NK细胞活化剂I(购自深圳达科为,DKW35-CYT-NK001)均匀混合于400ml NK培养液(NK培养液为AIM培养基(购自Life Technologies)+1%人类血清(购自Valley Biomedical,货号HP1022HI)中,接种于一个G-Rex100细胞培养器(购自Wilson Wolf),加入IL2(终浓度:50IU/ml),放置于37℃细胞培养箱中,每隔一天补充全液体积的IL2(终浓度:50IU/ml),培养10天,收集细胞计数,计数完毕取出收集细胞中的20×106个细胞与另外的2mL NK细胞活化剂I均匀混合于400ml NK培养液中,接种回G-Rex100细胞培养器,相同条件继续培养7天。上述第10天时的剩余细胞冻存备用,第17天时的细胞收集计数,取出2×106个细胞用于流式细胞仪(购自BD,型号C6Samplar)进行细胞表型分析。结果显示,用该方法培养出来的NK细胞平均纯度高达90%,见图5。图5A所示结果说明CD3阴性且CD56阳性的NK细胞比例为90.2%,图5B所示结果说明NK细胞表面受体NKG2D和CD16双阳性的比例为96.4%。PBMCs cells with 20×106 cells and 2 mL NK cell activator I (purchased from Shenzhen Daktronics, DKW35-CYT-NK001) were uniformly mixed in 400 ml NK medium (NK culture medium was AIM). Medium (purchased from Life Technologies) + 1% human serum (purchased from Valley Biomedical, item number HP1022HI), inoculated into a G-Rex100 cell culture device (purchased from Wilson Wolf), added with IL2 (final concentration: 50 IU/ml) Place in a 37 ° C cell culture incubator, replenish the whole liquid volume of IL2 every other day (final concentration: 50 IU / ml), culture for 10 days, collect the cell count, and count the 20 × 106 cells in the collected cells. An additional 2 mL of NK cell activator I was uniformly mixed in 400 ml of NK medium, inoculated back into a G-
制备例3:制备嵌合抗原受体修饰的NK细胞Preparation Example 3: Preparation of chimeric antigen receptor-modified NK cells
1)嵌合抗原受体NKG2D-CD8-DAP12 mRNA的体外合成1) In vitro synthesis of chimeric antigen receptor NKG2D-CD8-DAP12 mRNA
优化了3’UTR和5’UTR结构,其序列如上文所述。应用Tail-PCR技术大剂量合成其中正链带有PolyA、反链带有对应的 PolyT的DNA双链模板用来进行试管内RNA合成,减少了DNA模板的不稳定性。嵌合抗原受体NKG2D-CD8-DAP12编码序列以上述pFastbac1-NKG2D-CD8-DAP12载体作为DNA模板进行Tail-PCR扩增,用以合成编码嵌合抗原受体NKG2D-CD8-DAP12序列的线性化DNA模板,Tail-PCR反应条件参照KAPA HiFiHotStartReadyMix(2X)的说明书,反应体系(50μL)如下:The 3' UTR and 5' UTR structures were optimized and the sequences are as described above. Tail-PCR technology was used to synthesize large-dose DNA double-stranded templates with PolyA in the positive strand and corresponding PolyT in the reverse strand for in vitro RNA synthesis, which reduced the instability of DNA template. The chimeric antigen receptor NKG2D-CD8-DAP12 coding sequence was subjected to Tail-PCR amplification using the above pFastbac1-NKG2D-CD8-DAP12 vector as a DNA template to synthesize linearization of the chimeric antigen receptor NKG2D-CD8-DAP12 sequence. DNA template, Tail-PCR reaction conditions refer to the instructions of KAPA HiFiHotStartReadyMix (2X), the reaction system (50 μL) is as follows:
双蒸水(nuclease free):25μLDouble distilled water (nuclease free): 25μL
2X KAPA HiFiHotStart Uracil+ReadyMix:25μL2X KAPA HiFiHotStart Uracil+ReadyMix: 25μL
P7(SEQ ID NO:15)(100μM):0.15μLP7 (SEQ ID NO: 15) (100 μM): 0.15 μL
P8(SEQ ID NO:16)(100μM):0.15μLP8 (SEQ ID NO: 16) (100 μM): 0.15 μL
pFastbac1-NKG2D-CD8-DAP12载体DNA模板(500ng/μL):0.5μLpFastbac1-NKG2D-CD8-DAP12 vector DNA template (500ng/μL): 0.5μL
将上述PCR产物用1%(w/v)的琼脂糖凝胶进行分离鉴定,见图6。鉴定正确的产物用于嵌合抗原受体NKG2D-CD8-DAP12 mRNA的体外合成。用mRNA体外合成试剂盒合成戴帽的mRNA,试剂盒为mMESSAGEmMACHINE T7 ULTRA转录试剂盒(可得自美国Invitrogen公司)或者mScriptTM RNA system(可得自美国Epicentre公司)。按照试剂盒的说明,并用试剂盒提供的试剂进行合成即可。The above PCR product was isolated and identified on a 1% (w/v) agarose gel, as shown in FIG. The correct product was identified for in vitro synthesis of the chimeric antigen receptor NKG2D-CD8-DAP12 mRNA. In vitro mRNA synthesis kit with capped mRNA synthesis kit as mMESSAGEmMACHINE T7 ULTRA Transcription Kit (available from Invitrogen, USA) or mScriptTM RNA system (available from the American Epicentre Corporation). According to the instructions of the kit, and using the reagents provided in the kit for synthesis.
将体外合成的嵌合抗原受体NKG2D-CD8-DAP12 mRNA产物用1%(w/v)的琼脂糖凝胶进行分离鉴定,见图7。鉴定正确的mRNA存放于-80℃保存备用。The in vitro synthesized chimeric antigen receptor NKG2D-CD8-DAP12 mRNA product was isolated and identified on a 1% (w/v) agarose gel, as shown in FIG. The correct mRNA was identified and stored at -80 ° C for storage.
2)对NK细胞进行嵌合抗原受体修饰2) Chimeric antigen receptor modification of NK cells
将制备例2制备的NK细胞(1×107个)与4μgNKG2D-CD8-DAP12 mRNA混合于电转液P3(产品名称“P3 Primary CellX Kit L”,Lonza,货号V4XP-3012),置于100μl NucleocuvetteTM管(P3Primary CellX Kit L,Lonza,货号V4XP-3012),并进行冰浴冷冻5分钟。然后使用4D-NucleofectorTM电转仪(得自瑞士Lonza公司),选择其自带的NK细胞电转程序进行电转。电转后,将细胞取出置于制备例2所述NK培养液中,加入IL2 50 IU/mL和0.5μg/mL的DNase,置于37℃, 5%CO2孵箱中恢复过夜。24小时后,收集细胞,使用流式细胞仪对电转细胞进行鉴定,见图8。图8所示结果说明转染NKG2D-CAR mRNA后,NKG2D的表达强度明显增加。符合要求的细胞(NKG2D-CAR NK细胞)可用于对相关肿瘤的治疗。NK cells prepared in Preparation Example 2 (1 × 107 cells) and 4 μg of NKG2D-CD8-DAP12 mRNA were mixed in electroporation P3 (product name "P3 Primary Cell" X Kit L”, Lonza, item number V4XP-3012), placed in a 100μl NucleocuvetteTM tube (P3Primary Cell) X Kit L, Lonza, item number V4XP-3012), and frozen in an ice bath for 5 minutes. Then using the 4D-NucleofectorTM electroporation instrument (available from Lonza, Inc., Switzerland), choose their own program NK cell electroporation electroporation. After electroporation, the cells were removed and placed in the NK medium described in Preparation Example 2, and
试验例1:NKG2D-CAR NK细胞对人肿瘤细胞体外杀伤能力的检测分析Test Example 1: Detection of NKG2D-CAR NK cells in vitro killing ability of human tumor cells
1)对NKG2D-CAR NK细胞杀伤性细胞因子IFN-γ的释放能力的检测1) Detection of the release ability of NKG2D-CAR NK cell killing cytokine IFN-γ
为了评价肿瘤细胞杀伤效率,进行了ELISPOT实验来检测IFNγ的分泌,因为IFN-γ的分泌与过继细胞免疫治疗时NKG2D-CAR NK细胞的抗肿瘤活性成正相关。In order to evaluate the tumor cell killing efficiency, an ELISPOT assay was performed to detect the secretion of IFNγ, since the secretion of IFN-γ was positively correlated with the anti-tumor activity of NKG2D-CAR NK cells in adoptive cellular immunotherapy.
mGFP-CAR NK细胞(转染mGFP-CD8-DAP12 mRNA的NK细胞)的制备方法与NKG2D-CAR NK细胞(转染NKG2D-CD8-DAP12 mRNA的NK细胞)的制备方法相同,只是载体构建时胞外结构域使用无抗原结合功能的mGFP序列(其Genbank编号为YP_002302326.1),以此作为阴性对照。The preparation method of mGFP-CAR NK cells (NK cells transfected with mGFP-CD8-DAP12 mRNA) is the same as that of NKG2D-CAR NK cells (NK cells transfected with NKG2D-CD8-DAP12 mRNA), except that the vector is constructed. The outer domain used the mGFP sequence without antigen binding function (its Genbank accession number is YP_002302326.1) as a negative control.
转染NKG2D-CD8-DAP12 mRNA的NK细胞的制备如制备例3所示。The preparation of NK cells transfected with NKG2D-CD8-DAP12 mRNA is shown in Preparation 3.
将转染NKG2D-CD8-DAP12 mRNA或者mGFP-CD8-DAP12 mRNA的NK细胞分别与人类卵巢癌细胞SKOV3、人类结直肠癌细胞HCT116和SW480、人类头颈癌细胞Detroit、人类肝癌细胞HepG2和人类神经胶质瘤细胞U87共培养于ELISPOT检测板,上述NK效应细胞与靶标细胞的数量比例为5∶1。每组实验重复3次。经过24小时的共培养后,显影并使用软件immunospot进行ELISPOT点计数。结果显示,NKG2D-CD8-DAP12 mRNA转染的NK细胞与mGFP-CD8-DAP12 mRNA转染的NK细胞相比,产生了更多更强的IFN-γ(通过ELISPOT检测试剂盒检测,购自Mabtech,货号为3420-4HST-1),统计后发现NKG2D-CD8-DAP12 mRNA转染的NK细胞产生的ELISPOT点显著高于对照组,见图9。NK cells transfected with NKG2D-CD8-DAP12 mRNA or mGFP-CD8-DAP12 mRNA were associated with human ovarian cancer cells SKOV3, human colorectal cancer cells HCT116 and SW480, human head and neck cancer cells Detroit, human hepatoma cells HepG2 and human neutrophils The stromal cell U87 was co-cultured on the ELISPOT assay plate, and the ratio of the above NK effector cells to the target cells was 5:1. Each set of experiments was repeated 3 times. After 24 hours of co-cultivation, development and use of the software immunospot for ELISPOT point counting. The results showed that NK cells transfected with NKG2D-CD8-DAP12 mRNA produced more and stronger IFN-γ than NK cells transfected with mGFP-CD8-DAP12 mRNA (detected by ELISPOT assay kit, purchased from Mabtech The product number is 3420-4HST-1). After counting, the ELISPOT spots produced by NKG2D-CD8-DAP12 mRNA-transfected NK cells were significantly higher than the control group, as shown in Figure 9.
2)对NKG2D-CAR NK细胞溶解肿瘤细胞能力的检测2) Detection of the ability of NKG2D-CAR NK cells to lyse tumor cells
将转染NKG2D-CD8-DAP12 mRNA或者mGFP-CD8-DAP12 mRNA的NK细胞分别与人类结直肠癌细胞HCT116、人类卵巢癌细胞SKOV3、人类头颈癌细胞Fadu和Detroit、人类肝癌细胞HepG2、人类乳腺癌细胞MCF7、和人类骨髓瘤细胞KG1共培养于U型96孔板,上述NK效应细胞与靶标细胞的个数比例范围为由2.5∶1到10∶1。每组实验重复3次。经过2小时的共培养后,用DELFIA EuTDA细胞毒性试剂盒(得自美国的PerkinElmer公司)检测NKG2D-CAR NK细胞溶解肿瘤细胞的能力,杀伤效果用如下公式计算:NK cells transfected with NKG2D-CD8-DAP12 mRNA or mGFP-CD8-DAP12 mRNA were associated with human colorectal cancer cell HCT116, human ovarian cancer cell SKOV3, human head and neck cancer cells Fadu and Detroit, human hepatoma cell HepG2, human breast cancer The cell MCF7 and the human myeloma cell KG1 were co-cultured in a U-shaped 96-well plate, and the ratio of the above-mentioned NK effector cells to the target cells ranged from 2.5:1 to 10:1. Each set of experiments was repeated 3 times. After 2 hours of co-cultivation, the ability of NKG2D-CAR NK cells to lyse tumor cells was examined using the DELFIA EuTDA cytotoxicity kit (available from PerkinElmer, USA), and the killing effect was calculated by the following formula:
%特异性裂解=((实验组释放(读数)-空白组释放(读数))/(最大释放(读数)-空白组释放(读数)))×100% specific lysis = ((experimental group release (reading) - blank group release (reading)) / (maximum release (reading) - blank group release (reading))) x 100
结果显示,NKG2D-CAR修饰的NK细胞具有广泛并且强烈的杀瘤活性。如,当结直肠癌细胞HCT116、卵巢癌细胞SKOV3、头颈癌细胞Fadu和Detroit、肝癌细胞HepG2、乳腺癌细胞MCF7、以及骨髓瘤细胞KG1作为靶标时,NKG2D-CAR修饰的NK细胞的杀伤效果在10∶1时均已显著高于对照组的杀伤效果,见图10。以上结果充分说明了NKG2D-CAR NK对肿瘤杀伤的普遍性和高效性。The results show that NKG2D-CAR modified NK cells have broad and strong tumoricidal activity. For example, when colorectal cancer cell HCT116, ovarian cancer cell SKOV3, head and neck cancer cells Fadu and Detroit, liver cancer cell HepG2, breast cancer cell MCF7, and myeloma cell KG1 are targeted, the killing effect of NKG2D-CAR modified NK cells is At 10:1, it was significantly higher than the killing effect of the control group, as shown in Figure 10. The above results fully demonstrate the universality and high efficiency of NKG2D-CAR NK on tumor killing.
试验例2:NKG2D-CAR NK细胞对人肿瘤细胞体内杀伤能力的检测分析Test Example 2: Detection and analysis of NKG2D-CAR NK cells in vivo killing ability of human tumor cells
进一步利用植入人体肿瘤的小鼠模型测试了NKG2D-CAR NK细胞的体内杀瘤效果。实验用小鼠为非肥胖型糖尿病/重症联合免疫缺陷/IL-2Rγcnull(NSG)小鼠(6-8周,雌性),每只小鼠被植入1×107个卵巢癌细胞SKOV3-Luc。肿瘤植入7天后,利用活体生物成像技术(BLI),在IVIS Spectrum成像平台观测到了肿瘤的生长,并用成像仪(得自美国PerkinElmer公司)拍照记录肿瘤的生长情况。拥有相似BLI强度(BLI强度指的是活体成像仪记录的小鼠体内肿瘤细胞荧光强度)的小鼠被随机分为2组,分别为:磷酸盐缓冲液(PBS)组,和NKG2D-CAR NK细胞组,每组5只小鼠。NKG2D-CAR NK细胞组以每只小鼠每次1×107细胞数腹腔注射按制备例3所示方法制 备的NKG2D-CAR修饰的NK细胞,PBS组每只小鼠每次腹腔注射100μL量的PBS。细胞注射方案为:每周二、五注射,总共注射三周(即,每组共6次注射)。密切地观察小鼠的行为和生存情况,并将肿瘤的发展状况由BLI记录。所有的光信号和图片都由Xenogen活体成像软件v2.5记录分析。如图11所示,肿瘤植入后第28天,肿瘤生长状况显示PBS组小鼠肿瘤生长迅速,光信号强度增长约为第7天的10倍,而NKG2D-CAR NK细胞组的全部5只小鼠肿瘤生长不但被抑制,而且原有肿瘤消除明显,光信号强度下降到第7天的约10%。由此可见,NKG2D-CAR修饰的NK细胞能够有效杀死体内肿瘤。The in vivo antitumor effect of NKG2D-CAR NK cells was further tested using a mouse model implanted in human tumors. The experimental mice were non-obese diabetic/severe combined immunodeficiency/IL-2Rγcnull (NSG) mice (6-8 weeks, female), and each mouse was implanted with 1×107 ovarian cancer cells SKOV3-Luc. . Seven days after tumor implantation, tumor growth was observed on the IVIS Spectrum imaging platform using in vivo bioimaging (BLI), and tumor growth was photographed using an imager (available from PerkinElmer, USA). Mice with similar BLI intensity (BLI intensity refers to the fluorescence intensity of tumor cells in mice recorded by a live imager) were randomly divided into 2 groups: phosphate buffered saline (PBS) group, and NKG2D-CAR NK. Cell group, 5 mice per group. In the NKG2D-CAR NK cell group, NKG2D-CAR-modified NK cells prepared by the method shown in Preparation Example 3 were intraperitoneally injected with 1 × 107 cells per mouse, and each mouse in the PBS group was intraperitoneally injected with a dose of 100 μL. PBS. The cell injection protocol was: two to five injections per week for a total of three weeks (ie, six injections per group). The behavior and survival of the mice were closely observed and the development of the tumors was recorded by BLI. All light signals and images were analyzed by Xenogen in vivo imaging software v2.5. As shown in Figure 11, on the 28th day after tumor implantation, tumor growth showed that the tumor growth of mice in the PBS group was rapid, the light signal intensity increased about 10 times that of the 7th day, and all 5 of the NKG2D-CAR NK cell group. Tumor growth in mice was not only inhibited, but the original tumors were eliminated and the light signal intensity decreased to about 10% on day 7. Thus, NKG2D-CAR-modified NK cells can effectively kill tumors in vivo.
试验例3:不同嵌合抗原受体的比较Test Example 3: Comparison of different chimeric antigen receptors
用嵌合抗原受体NKG2D-CD8-DAP12修饰的NK细胞和嵌合抗原受体NKG2D-CD8-CD3Z修饰的NK细胞分别对肿瘤细胞进行实验,比较杀伤活力。The NK cells modified with the chimeric antigen receptor NKG2D-CD8-DAP12 and the NK cells modified with the chimeric antigen receptor NKG2D-CD8-CD3Z were tested on tumor cells to compare the killing viability.
将转染NKG2D-CD8-DAP12 mRNA、NKG2D-CD8-CD3z mRNA、或者mGFP-CD8-DAP12 mRNA的NK细胞分别与人类结直肠癌细胞HCT116、人类卵巢癌细胞SKOV3、和人类头颈癌细胞Detroit共培养于U型96孔板,上述NK细胞与靶标细胞的个数比例范围为由2.5∶1到10∶1。每组实验重复3次。经过2小时的共培养后,用DELFIA EuTDA细胞毒性试剂盒(得自美国的PerkinElmer公司)检测NKG2D-CAR NK细胞溶解肿瘤细胞的能力,杀伤效果的计算应用如下公式:NK cells transfected with NKG2D-CD8-DAP12 mRNA, NKG2D-CD8-CD3z mRNA, or mGFP-CD8-DAP12 mRNA were co-cultured with human colorectal cancer cell line HCT116, human ovarian cancer cell line SKOV3, and human head and neck cancer cell line Detroit, respectively. In the U-shaped 96-well plate, the ratio of the above NK cells to the target cells ranges from 2.5:1 to 10:1. Each set of experiments was repeated 3 times. After 2 hours of co-cultivation, the ability of NKG2D-CAR NK cells to lyse tumor cells was examined using the DELFIA EuTDA Cytotoxicity Kit (available from PerkinElmer, USA). The killing effect was calculated using the following formula:
%特异性裂解=((实验组释放(读数)-空白组释放(读数))/(最大释放(读数)-空白组释放(读数)))×100% specific lysis = ((experimental group release (reading) - blank group release (reading)) / (maximum release (reading) - blank group release (reading))) x 100
结果显示,在人类结直肠癌细胞HCT116、人类卵巢癌细胞SKOV3、和人类头颈癌细胞Detroit的杀瘤实验中,NKG2D-CD8-DAP12 mRNA修饰的NK细胞的杀瘤活性明显强于NKG2D-CD8-CD3z mRNA修饰的NK细胞。说明本发明所创建的具有所述特定构成单元组合的嵌合抗原受体NKG2D-CD8-DAP12具有 显著的疗效和良好的商业化前景。The results showed that NKG2D-CD8-DAP12 mRNA-modified NK cells had significantly stronger tumoricidal activity than NKG2D-CD8- in the tumoricidal experiments of human colorectal cancer cell HCT116, human ovarian cancer cell SKOV3, and human head and neck cancer cell Detroit. CD3z mRNA modified NK cells. The chimeric antigen receptor NKG2D-CD8-DAP12 having the specific constituent unit combination created by the present invention has a remarkable therapeutic effect and a good commercialization prospect.
上述NKG2D-CD8-CD3z修饰的NK细胞的制备方法与NKG2D-CD8-DAP12修饰的NK细胞的制备方法相同,只是载体构建时胞内信号结构域使用CD3z(全长氨基酸序列的Genbank编号为:NP_932170.1)的胞内信号序列(如SEQ ID NO:23所示)。The above NKG2D-CD8-CD3z modified NK cells were prepared in the same manner as NKG2D-CD8-DAP12 modified NK cells except that the intracellular signal domain was constructed using CD3z (the full-length amino acid sequence of Genbank number: NP_932170). .1) The intracellular signal sequence (shown as SEQ ID NO: 23).
试验例4:H101预处理肿瘤细胞可提升NKG2D-CAR NK细胞对肿瘤细胞的体外杀伤能力Test Example 4: Pretreatment of tumor cells with H101 enhances the in vitro killing ability of NKG2D-CAR NK cells to tumor cells
1)H101处理后肿瘤细胞表面NKG2D配体表达的检测1) Detection of NKG2D ligand expression on tumor cell surface after H101 treatment
为了检测H101溶瘤腺病毒处理对肿瘤细胞表面NKG2D配体表达的影响,通过流式细胞术检测了人头颈癌细胞Fadu和人肝癌细胞HepG2表面NKG2D配体的表达。In order to detect the effect of H101 oncolytic adenovirus treatment on the expression of NKG2D ligand on the surface of tumor cells, the expression of NKG2D ligand on human head and neck cancer cell Fadu and human hepatoma cell HepG2 was detected by flow cytometry.
用肿瘤细胞的无血清培养基(Fadu的培养基为RPMI(购自Gibco),HepG2的培养及为DMEM(购自Gibco))稀释H101病毒,以17MOI感染Fadu细胞,以34MOI感染HepG2,感染6小时后,弃掉含有病毒的培养基,换上肿瘤细胞的标准培养基(Fadu的培养基为RPMI(购自Gibco)+10%FBS(购自Sigma),HepG2的培养及为DMEM(购自Gibco)+10%FBS(购自Sigma))。以没有H101处理的肿瘤细胞作为阴性对照(在相应时间点做相同换液处理)。继续培养18小时,消化收获细胞,分别用VioBright FITC偶联的抗hMICA/B抗体(Miltenyi),PE偶联的抗hULBP1抗体、APC偶联的抗hULBP2/5/6抗体、APC偶联的抗hULBP3抗体、APC偶联的抗hULBP4抗体(R&D Systems)于4摄氏度与肿瘤细胞共孵育半个小时,最后通过流式细胞仪(购自Novocyte)检测NKG2D配体的表达,每组实验重复3次,取平均值做统计学分析。The serum-free medium of tumor cells (Fluu medium was purchased from Gibco), HepG2 was cultured and DMEM (purchased from Gibco) was used to dilute H101 virus, Fadu cells were infected at 17 MOI, HepG2 was infected at 34 MOI, and infection was 6 After an hour, discard the virus-containing medium and replace it with standard medium for tumor cells (Fadu medium is RPMI (purchased from Gibco) + 10% FBS (purchased from Sigma), culture of HepG2 and DMEM (purchased from Gibco) + 10% FBS (purchased from Sigma)). Tumor cells without H101 treatment were used as negative controls (the same exchange treatment was performed at the corresponding time points). The culture was continued for 18 hours, and the harvested cells were digested with VioBright FITC-conjugated anti-hMICA/B antibody (Miltenyi), PE-conjugated anti-hULBP1 antibody, APC-conjugated anti-hULBP2/5/6 antibody, and APC-conjugated antibody. hULBP3 antibody, APC-conjugated anti-hULBP4 antibody (R&D Systems) was incubated with tumor cells for 4 hours at 4 ° C. Finally, the expression of NKG2D ligand was detected by flow cytometry (purchased from Novocyte), and each experiment was repeated 3 times. Take the average for statistical analysis.
Fadu细胞试验结果见图13,其中显示,与阴性对照(即,图例显示为“Fadu”)相比,H101感染Fadu细胞组(即,图例显示为“H101处理的Fadu”)的肿瘤细胞表面的NKG2D配体MICA/B显著提高(阴性对照“Fadu”组中表达MICA/B的细胞数百分比:39.1%、“H101处理的Fadu”组中表达MICA/B的细胞数百分比:50.81%),而ULBP1 有所降低(阴性对照“Fadu”中表达ULBP1的细胞数百分比:33.4%、“H101处理的Fadu”组中表达ULBP1的细胞数百分比:27.11%)。The results of the Fadu cell assay are shown in Figure 13, which shows that compared to the negative control (i.e., the legend is shown as "Fadu"), the H101-infected Fadu cell group (i.e., the legend shown as "H101-treated Fadu") has a tumor cell surface. NKG2D ligand MICA/B was significantly increased (% of cells expressing MICA/B in the negative control "Fadu" group: 39.1%, percentage of cells expressing MICA/B in the "H101-treated Fadu" group: 50.81%), and ULBP1 was decreased (% of cells expressing ULBP1 in the negative control "Fadu": 33.4%, percentage of cells expressing ULBP1 in the "H101-treated Fadu" group: 27.11%).
HepG2细胞试验结果见图14,其中显示,与阴性对照(即,图例显示为“HepG2”)相比,H101感染HepG2细胞组(即,图例显示为“H101处理的HepG2”)的肿瘤细胞表面的NKG2D配体MICA/B显著提高(阴性对照“HepG2”组中表达MICA/B的细胞数百分比:8.3%、“H101处理的HepG2”组中表达MICA/B的细胞数百分比:45.37%)。The HepG2 cell assay results are shown in Figure 14, which shows that H101 infected HepG2 cells (i.e., the legend shows "H101-treated HepG2") on the surface of tumor cells compared to the negative control (i.e., the legend shows "HepG2"). The NKG2D ligand MICA/B was significantly increased (% of cells expressing MICA/B in the negative control "HepG2" group: 8.3%, percentage of cells expressing MICA/B in the "H101-treated HepG2" group: 45.37%).
2)NKG2D-CAR NK细胞对H101处理的肿瘤细胞的溶解能力的检测2) Detection of solvency of NKG2D-CAR NK cells on H101-treated tumor cells
第0天,将Fadu接种于细胞培养皿中(培养基为RPMI(购自Gibco))。第1天,以17MOI用H101溶瘤腺病毒感染肿瘤细胞6小时后,弃掉含有病毒的培养基,换上肿瘤细胞的标准培养基(RPMI(购自Gibco)+10%FBS(购自Sigma)),继续培养18小时。收获肿瘤细胞,于37摄氏度用Calcein AM(Life Technologies)染色肿瘤细胞半个小时(终浓度:2μM)(未被裂解的活细胞会被Calcein AM染色而带有绿色荧光),用PBS清洗。按E∶T 1∶1的效靶比将1×105个转染了NKG2D-CD8-DAP12 mRNA的NK细胞与1×105个的经H101处理并染色的Fadu(作为“CAR-NK+H101”组)或没有经H101处理但染色的Fadu(作为“CAR-NK”组)共培养于24孔板,每组实验重复3次。经过6小时的共培养,收获全部细胞,重悬于350μl MACS缓冲液(Miltenyi),通过流式细胞仪(购自Novocyte)检测50μl样本中带绿色荧光的细胞的数目。实验中另设两个实验组:在相应时间点经溶瘤腺病毒H101处理,但不加转染了NKG2D-CD8-DAP12 mRNA的NK细胞,作为“w/o CAR-NK+H101”组;一组既未经溶瘤腺病毒H101处理也未经转染了NKG2D-CD8-DAP12 mRNA的NK细胞处理,作为“w/o CAR-NK”组,该组为阴性对照组。各组均在相应时间做相应换液操作;每组实验重复三次,取平均值做统计学分析。On
%特异性裂解=(1-实验组/阴性对照组)×100% specific lysis = (1 - experimental group / negative control group) × 100
结果显示,NKG2D-CAR修饰的NK细胞对Fadu具有强烈的杀瘤 活性,见图15A(p<0.001)。H101处理肿瘤细胞后,NKG2D-CAR修饰的NK细胞仍然对Fadu具有强烈的杀瘤活性,见图15B(p<0.001)。不仅如此,还发现用H101溶瘤腺病毒处理Fadu细胞后,可显著提升NKG2D-CAR修饰的NK细胞对Fadu的杀瘤活性,见图15C(图15A和15B放在同一图中得到图15C)。The results showed that NKG2D-CAR-modified NK cells had strong tumoricidal activity against Fadu, as shown in Figure 15A (p < 0.001). After treatment of tumor cells with H101, NKG2D-CAR-modified NK cells still had strong tumoricidal activity against Fadu, see Figure 15B (p < 0.001). Moreover, it was found that treatment of Fadu cells with H101 oncolytic adenovirus significantly increased the tumoricidal activity of NKG2D-CAR-modified NK cells against Fadu, as shown in Fig. 15C (Figs. 15A and 15B are shown in Fig. 15C in the same figure). .
以上结果充分说明H101和NKG2D CAR-NK联合使用具有更好的抗肿瘤活性。The above results fully demonstrate that the combined use of H101 and NKG2D CAR-NK has better antitumor activity.
Claims (54)
Translated fromChineseApplications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711027016.1 | 2017-10-27 | ||
| CN201711027016.1ACN107759701B (en) | 2017-10-27 | 2017-10-27 | Chimeric antigen receptor, its modified NK cells, coding DNA, mRNA, expression vector, preparation method and application |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019080537A1true WO2019080537A1 (en) | 2019-05-02 |
Family
ID=61271654
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2018/094003CeasedWO2019080537A1 (en) | 2017-10-27 | 2018-07-02 | Therapeutic agent comprising oncolytic virus and car-nk cells, use, kit and method for treating tumor and/or cancer |
| PCT/CN2018/094005CeasedWO2019080538A1 (en) | 2017-10-27 | 2018-07-02 | Chimeric antigen receptor, nk cell modified by same, coding dna, mrna, expression vector, preparation method and application |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2018/094005CeasedWO2019080538A1 (en) | 2017-10-27 | 2018-07-02 | Chimeric antigen receptor, nk cell modified by same, coding dna, mrna, expression vector, preparation method and application |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN107759701B (en) |
| WO (2) | WO2019080537A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112300288A (en)* | 2019-07-29 | 2021-02-02 | 济南赛尔生物科技股份有限公司 | Chimeric antigen receptor CAR of CIK cell and application thereof |
| EP3842051A4 (en)* | 2018-08-24 | 2022-06-29 | Hangzhou Converd Co., Ltd. | Therapeutic agent comprising nucleic acid and car-modified immune cell and application thereof |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107759701B (en)* | 2017-10-27 | 2021-07-02 | 杭州优善生物科技有限公司 | Chimeric antigen receptor, its modified NK cells, coding DNA, mRNA, expression vector, preparation method and application |
| CN110724199B (en)* | 2018-07-17 | 2023-12-05 | 成都盛世君联生物技术有限公司 | NKG2D CAR-T cell and preparation and application thereof |
| CN110669144B (en)* | 2019-10-12 | 2021-09-17 | 华夏源(上海)细胞基因工程股份有限公司 | Chimeric antigen receptor of targeting B cell mature antigen and application thereof |
| CN111363046A (en)* | 2020-03-11 | 2020-07-03 | 深圳宾德生物技术有限公司 | Chimeric antigen receptor targeting NKG2D, chimeric antigen receptor T cell, and preparation method and application thereof |
| CN114807042B (en)* | 2021-01-22 | 2024-09-03 | 南京助天中科科技发展有限公司 | A chimeric antigen receptor modified NK cell and its preparation method and application |
| CA3209374A1 (en)* | 2021-02-25 | 2022-09-01 | Kambiz MOUSAVI | Engineered expression constructs to increase protein expression from synthetic ribonucleic acid (rna) |
| CN113621073A (en)* | 2021-07-14 | 2021-11-09 | 上海易慕峰生物科技有限公司 | Novel chimeric receptor composition, recombinant vector, cell and application thereof |
| CN117604031B (en)* | 2023-04-20 | 2025-05-09 | 龙岩学院 | An in vitro transcription system for feline coronavirus S protein mRNA and its construction method |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105307671A (en)* | 2013-04-18 | 2016-02-03 | 蒂尔坦生物制药有限公司 | Enhanced Adoptive Cell Therapy |
| WO2016123333A1 (en)* | 2015-01-29 | 2016-08-04 | Regents Of The University Of Minnesota | Chimeric antigen receptors, compositions, and methods |
| WO2016164370A1 (en)* | 2015-04-06 | 2016-10-13 | Ohio State Innovation Foundation | Egfr-directed car therapy for glioblastoma |
| CN107034237A (en)* | 2017-03-31 | 2017-08-11 | 北京呈诺医学科技有限公司 | A kind of CAR NK cell and its preparation method and application |
| CN107759701A (en)* | 2017-10-27 | 2018-03-06 | 杭州优善生物科技有限公司 | Chimeric antigen receptor, the NK cells of its modification, coding DNA, mRNA, expression vector, preparation method and application |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL151873A0 (en)* | 2000-03-24 | 2003-04-10 | Micromet Ag | Multifunctional polypeptides comprising a binding site to an epitope of the nkg2d receptor complex |
- 2017
- 2017-10-27CNCN201711027016.1Apatent/CN107759701B/enactiveActive
- 2018
- 2018-07-02WOPCT/CN2018/094003patent/WO2019080537A1/ennot_activeCeased
- 2018-07-02WOPCT/CN2018/094005patent/WO2019080538A1/ennot_activeCeased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105307671A (en)* | 2013-04-18 | 2016-02-03 | 蒂尔坦生物制药有限公司 | Enhanced Adoptive Cell Therapy |
| WO2016123333A1 (en)* | 2015-01-29 | 2016-08-04 | Regents Of The University Of Minnesota | Chimeric antigen receptors, compositions, and methods |
| WO2016164370A1 (en)* | 2015-04-06 | 2016-10-13 | Ohio State Innovation Foundation | Egfr-directed car therapy for glioblastoma |
| CN107034237A (en)* | 2017-03-31 | 2017-08-11 | 北京呈诺医学科技有限公司 | A kind of CAR NK cell and its preparation method and application |
| CN107759701A (en)* | 2017-10-27 | 2018-03-06 | 杭州优善生物科技有限公司 | Chimeric antigen receptor, the NK cells of its modification, coding DNA, mRNA, expression vector, preparation method and application |
Non-Patent Citations (3)
| Title |
|---|
| "CAR-NK (Current Situation and Development Trend of CAR-NK Anti-Tumor Research", CHINESE JOURNAL OF CANCER BIOTHERAPY, vol. 23, no. 1, 16 August 2903 (2903-08-16), pages 1 - 10* |
| CHEN, X. L.: "A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases", ONCOTARGET, vol. 7, no. 19, 1 April 2016 (2016-04-01), pages 27764 - 27777, XP055493359* |
| VANSEGGELEN, H.: "Chimeric antigen receptor engineered T cells as oncolytic virus carriers", MOLECULAR THERAPY ONCOLYTICS, vol. 2, 9 September 2015 (2015-09-09), pages 15014 - 12, XP055569703* |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3842051A4 (en)* | 2018-08-24 | 2022-06-29 | Hangzhou Converd Co., Ltd. | Therapeutic agent comprising nucleic acid and car-modified immune cell and application thereof |
| US12329826B2 (en) | 2018-08-24 | 2025-06-17 | Hangzhou Converd Co., Ltd. | Therapeutic agents comprising nucleic acids and CAR-modified immune cells, and uses thereof |
| CN112300288A (en)* | 2019-07-29 | 2021-02-02 | 济南赛尔生物科技股份有限公司 | Chimeric antigen receptor CAR of CIK cell and application thereof |
| CN112300288B (en)* | 2019-07-29 | 2022-08-02 | 济南赛尔生物科技股份有限公司 | Chimeric antigen receptor CAR of CIK cell and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107759701B (en) | 2021-07-02 |
| WO2019080538A1 (en) | 2019-05-02 |
| CN107759701A (en) | 2018-03-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI830771B (en) | Therapeutic agents containing nucleic acids and CAR-modified immune cells and their applications | |
| WO2019080537A1 (en) | Therapeutic agent comprising oncolytic virus and car-nk cells, use, kit and method for treating tumor and/or cancer | |
| US10787645B2 (en) | Enhanced adoptive cell therapy | |
| CN109554353B (en) | Isolated recombinant oncolytic poxvirus, pharmaceutical compositions and use thereof in a medicament for the treatment of tumors and/or cancers | |
| US20220339220A1 (en) | Therapeutic agents comprising oncolytic vaccinia viruses and nk cells, and uses thereof for drugs for treatment of tumors and/or cancers | |
| CN118813547A (en) | A double-gene modified CAR-T cell and its application | |
| JP7326663B2 (en) | Pharmaceutical composition containing vaccinia virus and hydroxyurea as active ingredients for the treatment of cancer | |
| US12421502B2 (en) | Enhanced adoptive cell therapy | |
| Hatami et al. | Interleukin-12 encoded by the oncolytic virus VSV-GP enhances therapeutic antitumor efficacy by inducing CD8+ T-cell responses with a long-lived effector cell phenotype | |
| CN113046320B (en) | CAR molecule whose extracellular segment is Vδ1(GTM)Vγ4, CAR-T cell expressing the molecule and use thereof | |
| WO2022048574A1 (en) | Nucleic acid molecule encoding kras gene mutant | |
| WO2024251286A1 (en) | Oncolytic hsv-1 clinical isolate, directed evolution strain, infectious clone and use | |
| WO2025002479A1 (en) | Chimeric antigen receptor based on intracellular region truncation | |
| WO2024062098A1 (en) | Recombinant pseudocowpox virus encoding an interleukin-12 | |
| CN112877293A (en) | CAR molecule with extracellular segment of V delta2(OT3) V gamma9, CAR-T cell expressing same and application thereof | |
| HK1256147B (en) | Pharmaceutical composition and application in medicines for treating tumors and/or cancers thereof | |
| HK1256147A1 (en) | Pharmaceutical composition and application in medicines for treating tumors and/or cancers thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | Ref document number:18871712 Country of ref document:EP Kind code of ref document:A1 | |
| NENP | Non-entry into the national phase | Ref country code:DE | |
| 122 | Ep: pct application non-entry in european phase | Ref document number:18871712 Country of ref document:EP Kind code of ref document:A1 |