USRE43714E1 - Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints - Google Patents
Preparation for repairing cartilage defects or cartilage/bone defects in human or animal jointsDownload PDFInfo
- Publication number
- USRE43714E1 USRE43714E1US11/705,575US70557502AUSRE43714EUS RE43714 E1USRE43714 E1US RE43714E1US 70557502 AUS70557502 AUS 70557502AUS RE43714 EUSRE43714 EUS RE43714E
- Authority
- US
- United States
- Prior art keywords
- bone
- cartilage
- bone plate
- subchondral
- tissue
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime, expires
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/3205—Excision instruments
- A61B17/32053—Punch like cutting instruments, e.g. using a cylindrical or oval knife
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30756—Cartilage endoprostheses
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2/4601—Special tools for implanting artificial joints for introducing bone substitute, for implanting bone graft implants or for compacting them in the bone cavity
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1637—Hollow drills or saws producing a curved cut, e.g. cylindrical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00969—Surgical instruments, devices or methods used for transplantation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2835—Bone graft implants for filling a bony defect or an endoprosthesis cavity, e.g. by synthetic material or biological material
- A61F2002/2839—Bone plugs or bone graft dowels
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/30004—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis
- A61F2002/30032—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis differing in absorbability or resorbability, i.e. in absorption or resorption time
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/3006—Properties of materials and coating materials
- A61F2002/30062—(bio)absorbable, biodegradable, bioerodable, (bio)resorbable, resorptive
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30112—Rounded shapes, e.g. with rounded corners
- A61F2002/30113—Rounded shapes, e.g. with rounded corners circular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30143—Convex polygonal shapes hexagonal
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30153—Convex polygonal shapes rectangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30154—Convex polygonal shapes square
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30156—Convex polygonal shapes triangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30159—Concave polygonal shapes
- A61F2002/30172—T-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A61F2002/30233—Stepped cylinders, i.e. having discrete diameter changes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30299—Three-dimensional shapes umbrella-shaped or mushroom-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30317—The prosthesis having different structural features at different locations within the same prosthesis
- A61F2002/30327—The prosthesis having different structural features at different locations within the same prosthesis differing in diameter
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30721—Accessories
- A61F2002/30733—Inserts placed into an endoprosthetic cavity, e.g. for modifying a material property
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30721—Accessories
- A61F2/30749—Fixation appliances for connecting prostheses to the body
- A61F2002/30751—Fixation appliances for connecting prostheses to the body for attaching cartilage scaffolds to underlying bone
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30756—Cartilage endoprostheses
- A61F2002/30759—Mosaicplasty, i.e. using a plurality of individual cartilage plugs for filling a substantial cartilage defect
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30795—Blind bores, e.g. of circular cross-section
- A61F2002/30807—Plurality of blind bores
- A61F2002/30808—Plurality of blind bores parallel
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2002/30929—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth having at least two superposed coatings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2002/30971—Laminates, i.e. layered products
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2002/4631—Special tools for implanting artificial joints the prosthesis being specially adapted for being cemented
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2/4644—Preparation of bone graft, bone plugs or bone dowels, e.g. grinding or milling bone material
- A61F2002/4649—Bone graft or bone dowel harvest sites
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2002/4685—Special tools for implanting artificial joints by means of vacuum
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0004—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof bioabsorbable
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0006—Rounded shapes, e.g. with rounded corners circular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0019—Angular shapes rectangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0021—Angular shapes square
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0023—Angular shapes triangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0052—T-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0069—Three-dimensional shapes cylindrical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0093—Umbrella-shaped, e.g. mushroom-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2240/001—Designing or manufacturing processes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/003—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in adsorbability or resorbability, i.e. in adsorption or resorption time
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0039—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in diameter
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00179—Ceramics or ceramic-like structures
- A61F2310/00293—Ceramics or ceramic-like structures containing a phosphorus-containing compound, e.g. apatite
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00389—The prosthesis being coated or covered with a particular material
- A61F2310/00964—Coating or prosthesis-covering structure made of cartilage
Definitions
- the device according to the inventionallows production and implantation in a manner equally simple as for known devices comprising a bone part and a cartilage layer calescent with the bone part.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
1. Field of the Invention
The present invention lies in the field of medical technology and generally relates to a device for repairing cartilage defects and/or cartilage/bone defects in human or animal joints. More specifically, the device serves to repair defects in the cartilage layer, which in joints covers the bone surface, or of defects that concern this articular cartilage layer and also bone tissue lying thereunder.
2. Description of Related Art
Damage to articular cartilage by way of injuries or involution caused by aging or disease is particularly common in humans. Very often such damage also takes its toll on the bone tissue lying below the articular cartilage. The degree of damage to articular cartilage defects and/or cartilage/bone defects is determined with the help of the Outerbridge scale, with the following categories: superficial fraying (approx. 10% of all cases), cartilage fissure (approx. 28%), fissure down to the bone (approx. 41%), damage involving cartilage and bone (approx. 19%) and other damage such as osteochondritis dissecans and joint fracture (approx. 2% of all detected cases).
Vital cartilage tissue contains living cells by way of whose activity the specific intercellular cartilage matrix is built up during adolescence. However, it contains very little vascularization in the fully grown condition and, therefore, has a very limited regeneration capability. This means that cartilage defects or cartilage bone defects, in particular those defects concerning a relatively large cartilage surface, do not heal by themselves and therefore must be repaired by surgery (Mankin H J: The response of articular cartilage to mechanical injury, Journal of Bone and Joint Surgery (Am) 64A (1982) March: pages 460-466).
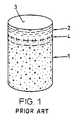
For repairing the named defects it is, for example, suggested to implant devices comprising the tissue to be repaired or a perform of this tissue. Such devices are cylindrical and comprise a cartilage layer on one end face. For implantation a pocket-hole shaped opening or bore is produced in the region of the defect to be repaired and the device is positioned in the bore such that the cartilage layer of the implant faces towards the outside. The bore, independently of the depth of the defect, extends into the healthy bone tissue. The device has a somewhat larger diameter than the bore and the same axial length. Therefore, after implantation there is a radial tension (press fit) between native tissue and the implanted device by way of which the implant is held in the bore. The cartilage surface of the implant is flush with the surrounding native cartilage surface. The devices have, according to the size of the defect, a diameter of 4 to 10 mm (e.g. 5.4 mm for the device and 5.3 mm for the bore) and lengths of approx. 10 to 20 mm.
For larger defects it is suggested to implant a plurality of such cylindrical devices in the defect region in a mosaic manner and to fill out the intermediate spaces between the implants with a suitable material.
The cylindrical devices are for example autologous (auto-transplants). For the repair of an articular cartilage defect concerning a heavily loaded location of a joint, a suitable tissue piece is harvested, for example, from a less loaded location of the same joint and is transplanted into a bore created at the defect location using a hollow drill (Hangody L et al.: Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 1998 Jul., 21(7):751-6).
The cylindrical devices may also originate from a suitable donor (homologous transplants). Also known are suitable heterologue implants or xenotransplants which before implantation are suitably treated, e.g. photo-oxidized (as described in the publication EP-0768332 of Sulzer Innotec), for preventing immune-reaction after implantation or for minimizing such immuno-reaction (immunological deactivation). Such implants are, for example, removed from shoulder joints of slaughtered cattle and have the advantage of being available in much larger numbers than autologous or homologous transplants and of causing no secondary defects on harvesting, which secondary defects must be repaired and lead to new difficulties.
In the publication WO-97/46665 (Sulzer Orthopedics) a suitable device is described of which the bone part consists of bone replacement material and the end-face cartilage layer is grown onto it in vitro from autologous chondrocytes.
In all mentioned devices being made from natural tissue there is a natural connection or coalescence between the end-face cartilage layer and the bone part and there is an outermost bone region (subchondral bone plate) in which the bone tissue is more compact than in other bone regions. The mentioned, partly artificial implants also show the coalescence of cartilage layer and bone part and the artificial bone part is advantageously equipped with a more compact, that is to say less porous, outer layer which serves the cartilage layer as an underlay.
An important function of the subchondral bone plate or an artificial imitation thereof is evidently the prevention of vascularisation of the cartilage layer proceeding from the bone tissue, which would lead to ossification of the cartilage. In addition the subchondral bone plate having a higher density than the inner bone tissue represents a region of higher mechanical strength.
With the devices as mentioned above it is attempted to achieve the following targets:
- The bone part of the device is to allow solid anchoring of the implant by way of a press fit, in a manner such that the implant requires no further fastening means interfering with healthy cartilage regions.
- The coalescence of cartilage layer and bone part in the device is to give the implant stability so that the cartilage layer cannot be detached and removed from the defect location, even if the joint is not immobilized after implantation.
- The cartilage layer of the device Is to have a mechanical strength and elasticity such that the repair location may be fully loaded directly after implantation.
- The cartilage layer is to form a zone in which conditions suitable for the implanted cells or for cells migrating into it after implantation prevail, such that the cell can produce or maintain a fully functional cartilage tissue. This is also to be supported by the subchondral bone plate which separates the cartilage layer from the bone part and which helps to prevent vascularisation proceeding from the bone part.
- The bone part is to represent a zone in which conditions suitable for the implanted cells or for cells migrating into it after implantation prevail, such that they can produce or maintain a fully functional bone tissue.
New trials in which artificially produced defects in joints of sheep have been repaired with auto-transplants, homo-transplants or with hetero-transplants (from cattle tissue) in the previously mentioned manner, show that the healing process after implantation does not proceed as expected.
In particular, it has been shown that the bone part of the implants is not integrated in the native tissue or replaced gradually by new reparative tissue, but that the bone part of the implant undergoes a transformation process with essentially three successive phases. In a first step bone osteoclastic cells (osteoclasts) are stimulated and the implanted bone starts to be resorbed. This first phase is already clearly visible six to eight weeks after implantation. A hollow space (cyst) then arises in the implant and is filled with connective tissue. This second phase reaches a climax after approximately six weeks. In the third and last phase bone-forming cells (osteoblasts) are attracted which convert the connective tissue to bone. This conversion process is concluded after about twelve months. Then the newly created bone structure is so well adapted that the original border between the implant and the surrounding bone tissue can hardly be perceived anymore.
Due to the described, three-phase transformation process comprising a middle phase in which the cartilage layer of the implant is not carried by the bone part capable to do so but by a mechanically inferior cyst, there exists a high risk that the cartilage layer is pressed into this cyst where it can neither fulfill its mechanical nor its biological function and from where it cannot be displaced during the following phases of the healing process. This risk significantly reduces the chances of healing success. Healing with a badly positioned cartilage layer causes negative after-effects.
It is surprising that the trials show the cyst formation at the location of the bone part of an implant in a middle phase of the healing process not only for homologous and heterologous implants, but in particular also for auto-transplants. The initial resorption of the implanted bone tissue does not therefore appear to be an immuno-reaction in which implanted vital material is recognized as foreign and is therefore resorbed. It would appear that it is rather a reaction to implanted, dead material. This means that by cutting off the natural blood supply on harvesting the implant even when harvesting it from viable tissue and even when it is implanted directly after harvesting, the bone tissue loses its viability. In any case, the bone part of the implant is resorbed and is rebuilt only after substantially complete resorption.
It is an object of the invention to provide a device for repairing cartilage defects or cartilage/bone defects in human or animal joints, which device prevents the above described risk of an implanted cartilage layer to sink into the region of the native bone tissue. The device according to the invention allows production and implantation in a manner equally simple as for known devices comprising a bone part and a cartilage layer calescent with the bone part.
The device according to the invention that serves for repairing cartilage defects or cartilage/bone defects in human or animal joints, is based on the finding that the subchondral bone plate is evidently present essentially unchanged when the bone part is completely or to a great extent replaced by connective tissue in the middle critical phase of the above described healing process. This is probably attributed to the fact that the subchondral bone plate, on account of its higher density, is resorbed significantly more slowly than the inner regions of the bone part. Since this subchondral bone plate is mechanically sufficiently stable, sinking of the cartilage layer grown thereon into a cyst underneath is prevented when the subchondral bone plate is supported not only by implanted bone material but in addition by material with different resorption properties such that during the critical healing phase it remains non-displaceable. When the subchondral bone plate of the implant is resorbed after the critical phase, that is to say at a point in time in which the loading capability of the inner implant region is restored again, this will not greatly influence the healing process.
Improved support of the implanted device during the critical healing phase can be realized in essentially two ways.
On the one hand the implant may be formed such that the cartilage layer and the subchondral bone plate of the device have a larger cross section than the bone part. Such a device is implanted into a two stage bore such that the subchondral bone plate of the device is not only supported on the bone part of the implant but also on healthy bone tissue next to the bore set up for repair.
On the other hand the bone part of the device may be equipped with columns having a reduced resorbability. These columns extend axially through the bone part up to the subchondral bone plate. The resorbability of the columns relative to the resorbability of the bone part regions between the columns may be reduced by way of a suitable chemical treatment or by way of producing axial bores in the bone part of the device and filling these with an artificial material more resistant to resorption.
These and further features of the invention will be apparent with reference to the following description and drawings, wherein:
As already mentioned a device as shown inFIG. 1 , is harvested from advantageously vital tissue using a hollow drill and is implanted if possible immediately after harvesting (auto-transplants and homo-transplants), or it is removed from joints of slaughtered animals (e.g. from shoulder joints of slaughtered cattle) and before implantation is subjected to a treatment for immunological deactivation.
As is evident fromFIGS. 2 and 3 , in the critical time period in which at the location of the implanted bone part there is a cyst-like cavity, the subchondral bone plate of the implant is substantially unchanged. This finding is attributed to the higher density of the subchondral bone plate relative to the inner bone tissue and therefore a reduced resorbability. The subchondral bone plate of the implant has evidently different resorption properties than have inner regions of the bone part.
The trials were carried out with auto-transplants and with hetero-transplants. For seven treated animals the repair locations were examined after six months and in ten cases (five animals) cartilage layers were found to be displaced into the cyst cavity, in four cases (two animals) the cartilage layers had remained in place. In none of the cases the cartilage layer was lost into the joint space.
The results of the trials show that evidently adhesion between the cyst and the implanted cartilage layer or the subchondral bone plate respectively is sufficient for preventing removal of the cartilage layer from the repair location, but that the loading capability of the cyst is not sufficient for preventing the cartilage layer from being displaced towards the inside.
Thedevice 10 has in the same manner as the device ofFIG. 1 abone part 1, and on one end face of this, acartilage layer 2 forming acartilage surface 3. In the transition region between thebone part 1 and thecartilage layer 2 there is a subchondral bone plate4. The device has atop part 11 with a larger cross section and abottom part 12 with a smaller cross section. Thetop part 11 comprises essentially thecartilage layer 1 and the subchondral bone plate4, thebottom part 12 essentially corresponds to thebone part 1. Thebottom part 12 has advantageously (but not necessarily) the shape of a circular cylinder or steep angle truncated circular cone and thetop part 11 projects on all sides beyond thebone part 1 and is for example likewise circularly cylindrical.
Auto-transplants and transplants of living donors have advantageously cylindrical top parts since such devices should cause as small as possible harvesting sites. Devices produced from the tissue of slaughtered animals (advantageously cattle or pigs) may without causing problems have head parts with any shape of cartilage surface. This is so due to the easy availability of the material allowing production of wastage. But also in this case it is advantageous to form the bottom part in a manner such that the opening to be made for implantation can be created with a simple tool, for example with a drill.
Adevice 10 with circular cross section at least in the foot region is for example manufactured from a suitable cylindrical device in that the bone part is accordingly machined. This machining may be carried out with a tool In which the cylindrical device is positioned and in which blades are activated to reduce the cross section of thedevice 10 to a predetermined extent at a predetermined or adjustable distance from the cartilage surface.
The opening or bore20 which is to be created in a defect region for implanting thedevice 10 has anouter region 21 adapted to thetop part 11 of thedevice 10 and having a depth down to the region of the native subchondral bone plate4′, and an inner region22 adapted to the bottom part of the device, whose depth is adapted to the shape of the defect to be repaired and to the length of the device to be implanted. The dimensions of thedevice 10 and of thebore 20 are to allow for a press fit in the region of the top part as well as in the region of the bottom part.
A bore20 as shown inFIG. 5 is for example created with a tool comprising a blade with a circular cutting edge and two drills or hollow drills movable relative to the cutting edge in an axially limited manner. The tool is positioned on the defect location and the blade is pressed down to the subchondral bone plate. Then theouter region 21 is drilled out with the first drill, which may be moved beyond the cutting edge of the blade by the thickness of the subchondral bone plate, and whose diameter corresponds essentially to the inner diameter of the blade. Afterwards the inner region22 is drilled out using the second drill, wherein the drilling depth relative to the cutting edge or relative to the end position of the first drill may be adjusted.
For implanting a device in a bore, as shown byFIG. 5 , a tool is used. The tool comprises, for example, a sleeve and a plunger axially movable in the sleeve. The sleeve has an inner cross section that corresponds to the cross section of the top part of the device to be implanted. The plunger advantageously has a cross section roughly equal to the top part; it is longer than the sleeve and has a channel that begins on the end face of the plunger and is connectable to a suction conduit in the region of the other end of the plunger.
For implantation, the end face of the plunger with the channel opening is pushed into the sleeve and using the suction force, a device to be implanted is drawn into the sleeve. Then the sleeve together with the plunger and the device suctioned thereon is positioned over the prepared bore and the device is pressed into the bore with the help of the plunger and where appropriate using a hammer.
It has been shown that resorption of the bone part of an implanted device also affects regions of the native bone tissue bordering the implant. For this reason it is recommended to dimension the protrusion of the top part to about 1 to 2 mm (e.g., bone part with a diameter of approximately 3 mm, top part with a diameter of 5 to 6 mm).
For local reduction of the resorbability of bone material, a treatment with biphosphonate bisphosphonate may be used (“Biophosphonates (“Bisphosphonates in Bone Disease” Herbert Fleisch, the Parthenon Publishing Group, New York and London 1995). As a resorbable material for filling bores for example a hydroxy apatite ceramic material may be used.
As mentioned above, it is possible also to use implants according to the state of the art (FIG. 1 ) for such mosaic-like arrangements for repairing larger defects. The cylindrical devices are implanted as close as possible next to one another, wherein on the one hand there will be gaps in the cartilage layer and on the other hand the regions of native bone tissue between the bone parts of the implants will be very narrow. It has been shown that the chances of healing of such repairs are better in edge regions than in middle regions. One may presume that this is due, on the one hand, to the deficient compactness of the freshly created cartilage layer and, on the other hand, to the deficient repair ability of the greatly reduced native bone tissue between the implants.
The additional advantages described for the repair according toFIG. 8 apply also to the repair according toFIG. 9 .
Claims (34)
1. A device (10) for repairing cartilage defects or cartilage/bone defects in human or animal joints, said device comprising a bone part (11), a cartilage layer (2) grown onto the bone part (1) and forming a cartilage surface (3), and a subchondral bone plate (4) or an imitation of a such a bone plate in a transition region between the bone part (1) and the cartilage layer (2), said device being implantable into an opening or a bore (20) set up in a defective ,region such that the bone part is adapted to be anchored in the healthy bone tissue and the cartilage surface (3) is flush with a native cartilage surface (3′), wherein for supporting the subchondral bone plate (4) and the cartilage layer (2) of the implanted device on at least two materials with differing resorbabilities, a cross-section of the bone part (1) is smaller than a cross section of the subchondral bone plate (4) and the cartilage layer (2) or the bone part has column regions (40,41) that extend to the subchondral bone plate (4) and have a resorbability different from a resorbability of the regions between the column regions.
2. The device according toclaim 1 , further comprising a top part (11) and a bottom part (12), wherein the top part (11) substantially comprises the cartilage layer (2) and the subchondral bone plate (4), and the bottom part (12) substantially corresponds to the bone part and wherein the subchondral bone plate (4) is larger than a cross section parallel to the subchondral bone plate through the bottom part (12) such that the subchondral bone plate (4) of the implanted device (30) is supported on native bone tissue.
3. The device according toclaim 2 , wherein the top part (11) and the bottom part (12) are coaxial circular cylinders.
4. The device according toclaim 2 , wherein the bottom part (12) is a circular cylinder and the top part (11) has a square, rectangular, triangular or hexagonal cartilage surface (3).
5. The device according toclaim 1 , wherein the column regions (40,41) extend perpendicular to the subchondral bone plate (4) through the bone part (1) up to the subchondral bone plate (4), and wherein the column regions (40,41) and regions between the column regions have different resorbabilities.
6. The device according toclaim 5 , wherein the device is essentially cylindrical and the cartilage layer (3) is arranged on one of its end faces.
7. The device according toclaim 5 , wherein a resorption behavior of the column regions (40) is altered with respect to a resorption behavior of the regions between the column regions by way of chemical treatment.
8. The device according toclaim 7 , wherein the column regions (40) consist of bone issue treated with biphosphonate bisphosphonate.
9. The device according toclaim 1 , wherein the column regions (41) consist of a bone replacement material filled into corresponding axial bores.
10. The device according toclaim 1 , wherein said device is made of autologous tissue.
11. The device according toclaim 1 , wherein said device is made of homologous or heterologous, immunologically deactivated tissue.
12. The device according toclaim 11 , wherein said device is made of tissue that is immunologically deactivated by way of photo-oxidation.
13. The device according toclaim 11 , wherein said device consists of tissue removed from slaughtered animals.
14. The device according toclaim 13 , wherein the tissue is removed from cattle or pig's joints.
15. A method for repairing cartilage defects or cartilage/bone defects in human or animal joints using a device (10) according toclaim 2 , comprising the steps of producing in the defect region an opening or bore (20) extending into healthy bone material and implanting the device (10) in the opening or bore, wherein the opening (20) comprises an outer region (21) and an inner region (22), a cross section of the outer region (21) is larger than a cross section of the inner region (22) and wherein the opening (20) is adapted, relative to the device (10), such that the device is implantable with a press fit and the cartilage surface (3) of the implanted device (30) is flush with the native cartilage surface (3′).
16. The method according toclaim 15 , wherein a transition between the outer region (21) and the inner region (22) lies directly below the native subchondral bone plate (4′).
17. The method according toclaim 15 , wherein the opening (20) is produced by drilling.
18. The method according toclaim 15 , wherein, for repairing larger defects, a plurality of devices are implanted in a mosaic-like manner, wherein positions for openings or bores (2) and the shape and size of top parts (11) of the devices (10) are coordinated to one another such that a cartilage surface (3,3′) essentially without interruption is achieved.
19. The method according toclaim 18 , wherein missing bone material between the openings is replaced with a bone replacement material having a resorbability that is different from the resorbability of the bone parts (1) of the devices (10).
20. A device for repairing cartilage defects or cartilage/bone defects in human or animal joints, said device comprising a bone part, a cartilage layer grown onto the bone part and forming a cartilage surface, and a subchondral bone plate or an imitation of a such a bone plate in a transition region between the bone part and the cartilage layer, wherein: (a) resorbability of the bone part is faster than resorbability of the subchondral bone plate or an imitation of a such a bone plate; (b) the bone part of the device comprises columns that have reduced resorbability relative to the rest of the bone part; and (c) the columns extend axially through the bone part up to the subchondral bone plate.
21. The device of claim 20, wherein the subchondral bone plate or an imitation of such a bone plate protrudes on all sides from the bone part.
22. The device of claim 21, wherein the subchondral bone plate or an imitation of such a bone plate has the form of a circular cylinder which is arranged coaxial to the bone part.
23. The device of claim 21, wherein the cartilage layer forms a square, rectangular, triangular, or hexagonal cartilage surface.
24. The device of claim 20, wherein the bone part and the subchondral bone plate or an imitation of a such a bone plate comprise a bone material or a bone substituted material and at least part of the material of the subchondral bone plate or an imitation of a such a bone plate is of a higher density than the material of the bone part.
25. The device of claim 20, wherein the device comprises autologous tissue.
26. The device of claim 20, wherein the device comprises homologous or heterologous, immunologically deactivated tissue.
27. The device of claim 26, wherein the device comprises tissue that is deactivated by photo-oxidation.
28. The device of claim 26, wherein the device comprises tissue removed from at least one slaughtered animal.
29. The device of claim 28, wherein the tissue is removed from a cattle or pig joint.
30. The device of claim 20, wherein the resorbability of the columns has been reduced by chemical treatment.
31. The device of claim 30, wherein the chemical treatment comprises treatment with bisphosphonate.
32. The device of claim 20, wherein the columns comprise axial bores in the bone part that have been filled with a material that is more resistant to resorption than the rest of the bone part.
33. A device for repairing cartilage defects or cartilage/bone defects in human or animal joints, said device comprising a bone part, a cartilage layer grown onto the bone part and forming a cartilage surface, and a subchondral bone plate or an imitation of a such a bone plate in a transition region between the bone part and the cartilage layer, wherein the device is adapted to be implanted into a bore formed in or near a defect region of a human or animal joint, and wherein the bone part comprises at least one column having a resorbability different from the resorbability of the rest of the bone part.
34. The device of claim 33, wherein the at least one column is adapted to bear the subchondral bone plate after the device has been implanted in the bore and the rest of the bone part has been resorbed.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/705,575USRE43714E1 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH229699 | 1999-12-15 | ||
| CH2296/99 | 1999-12-15 | ||
| US11/705,575USRE43714E1 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| US10/149,853US6858042B2 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| PCT/CH2000/000659WO2001043667A1 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE43714E1true USRE43714E1 (en) | 2012-10-02 |
Family
ID=4230469
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/705,575Expired - LifetimeUSRE43714E1 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| US10/149,853Expired - LifetimeUS6858042B2 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/149,853Expired - LifetimeUS6858042B2 (en) | 1999-12-15 | 2000-12-12 | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | USRE43714E1 (en) |
| EP (2) | EP1498089A1 (en) |
| JP (1) | JP2003516803A (en) |
| AT (1) | ATE274875T1 (en) |
| AU (1) | AU783038B2 (en) |
| CA (1) | CA2393776C (en) |
| DE (1) | DE50007640D1 (en) |
| ES (1) | ES2230170T3 (en) |
| WO (1) | WO2001043667A1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080294270A1 (en)* | 2007-05-24 | 2008-11-27 | Zimmer Orthobiologics, Inc. | Differentially processed tissue and processing methods thereof |
| US20090054906A1 (en)* | 2007-08-24 | 2009-02-26 | Zimmer Orthobiologics, Inc. | Medical device and method for delivering an implant to an anatomical site |
| US20110070271A1 (en)* | 2004-10-12 | 2011-03-24 | Truncale Katherine G | Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles |
| US8435305B2 (en) | 2010-08-31 | 2013-05-07 | Zimmer, Inc. | Osteochondral graft delivery device and uses thereof |
| US20130211536A1 (en)* | 2010-02-18 | 2013-08-15 | Biomet Manufacturing Corporation | Method And Apparatus For Augumenting Bone Defects |
| US8801725B2 (en) | 2008-03-10 | 2014-08-12 | Zimmer Orthobiologics, Inc. | Instruments and methods used when repairing a defect on a tissue surface |
| US8906110B2 (en) | 2007-01-24 | 2014-12-09 | Musculoskeletal Transplant Foundation | Two piece cancellous construct for cartilage repair |
| US9113916B2 (en) | 2010-08-31 | 2015-08-25 | Zimmer, Inc. | Drill bit for osteochondral drilling with guiding element and uses thereof |
| WO2019135216A1 (en) | 2018-01-02 | 2019-07-11 | Cartiheal (2009) Ltd. | Implantation tool and protocol for optimized solid substrates promoting cell and tissue growth |
| EP3838195A1 (en) | 2015-11-25 | 2021-06-23 | Subchondral Solutions, Inc. | Methods, systems and devices for repairing anatomical joint conditions |
Families Citing this family (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0896825B1 (en) | 1997-08-14 | 2002-07-17 | Sulzer Innotec Ag | Composition and device for in vivo cartilage repair comprising nanocapsules with osteoinductive and/or chondroinductive factors |
| US8563232B2 (en) | 2000-09-12 | 2013-10-22 | Lifenet Health | Process for devitalizing soft-tissue engineered medical implants, and devitalized soft-tissue medical implants produced |
| EP1099443A1 (en) | 1999-11-11 | 2001-05-16 | Sulzer Orthopedics Ltd. | Transplant/implant device and method for its production |
| USRE43714E1 (en) | 1999-12-15 | 2012-10-02 | Zimmer Orthobiologics, Inc. | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| US6626950B2 (en) | 2001-06-28 | 2003-09-30 | Ethicon, Inc. | Composite scaffold with post anchor for the repair and regeneration of tissue |
| CA2472239A1 (en)* | 2002-01-22 | 2003-07-31 | Pfizer Inc. | 3-(imidazolyl)-2-aminopropanoic acids for use as tafi-a inhibitors for the treatment of thrombotic diseases |
| EP1613364B1 (en)* | 2003-02-26 | 2013-01-16 | Zimmer Orthobiologics, Inc. | Preparation for repairing cartilage tissue, especially articular cartilage defects |
| US7067123B2 (en) | 2003-04-29 | 2006-06-27 | Musculoskeletal Transplant Foundation | Glue for cartilage repair |
| US20050222687A1 (en)* | 2004-04-02 | 2005-10-06 | Gordana Vunjak-Novakovic | Cartilage implant assembly and method for implantation |
| US20050064042A1 (en)* | 2003-04-29 | 2005-03-24 | Musculoskeletal Transplant Foundation | Cartilage implant plug with fibrin glue and method for implantation |
| US7901457B2 (en) | 2003-05-16 | 2011-03-08 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US20090291112A1 (en)* | 2003-05-16 | 2009-11-26 | Truncale Katherine G | Allograft osteochondral plug combined with cartilage particle mixture |
| US7488348B2 (en)* | 2003-05-16 | 2009-02-10 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| GB0325141D0 (en)* | 2003-10-28 | 2003-12-03 | Xiros Plc | Repair of damaged tissue on a bone site |
| JP5086642B2 (en)* | 2003-11-21 | 2012-11-28 | オステオポア・インターナショナル・プライヴェート・リミテッド | Bioabsorbable plug implant and method for bone tissue regeneration |
| WO2005086849A2 (en)* | 2004-03-09 | 2005-09-22 | Osteobiologics, Inc. | Implant scaffold combined with autologous or allogenic tissue |
| US20070185585A1 (en)* | 2004-03-09 | 2007-08-09 | Brat Bracy | Implant Scaffold Combined With Autologous Tissue, Allogenic Tissue, Cultured Tissue, or combinations Thereof |
| US20080220044A1 (en)* | 2007-03-06 | 2008-09-11 | Semler Eric J | Cancellous construct with support ring for repair of osteochondral defects |
| US8071574B2 (en)* | 2005-02-22 | 2011-12-06 | John Dennis Bobyn | Implant improving local bone formation |
| US7815926B2 (en) | 2005-07-11 | 2010-10-19 | Musculoskeletal Transplant Foundation | Implant for articular cartilage repair |
| AU2006292224B2 (en) | 2005-09-19 | 2013-08-01 | Histogenics Corporation | Cell-support matrix and a method for preparation thereof |
| EP1764117A1 (en) | 2005-09-20 | 2007-03-21 | Zimmer GmbH | Implant for the repair of a cartilage defect and method for manufacturing the implant |
| RU2307399C1 (en)* | 2006-03-03 | 2007-09-27 | Федеральное агентство по здравоохранению и социальному развитию Федеральное государственное учреждение "Российский научно-исследовательский институт травматологии и ортопедии им. Р.Р. Вредена Федерального агентства по здравоохранению и социальному развитию" (ФГУ "РНИИТО им. Р.Р. Вредена Росздрава") | Method of simulation of chronic layer-by-layer local defect of knee joint cartilage at experiments on dogs |
| US20080125863A1 (en)* | 2006-11-28 | 2008-05-29 | Mckay William F | Implant designs and methods of improving cartilage repair |
| US7758643B2 (en)* | 2007-02-26 | 2010-07-20 | Biomet Sports Medicine, Llc | Stable cartilage defect repair plug |
| US8435551B2 (en) | 2007-03-06 | 2013-05-07 | Musculoskeletal Transplant Foundation | Cancellous construct with support ring for repair of osteochondral defects |
| US20090024224A1 (en) | 2007-07-16 | 2009-01-22 | Chen Silvia S | Implantation of cartilage |
| EP2224884A2 (en)* | 2007-12-05 | 2010-09-08 | Musculoskeletal Transplant Foundation | Cancellous bone implant for cartilage repair |
| CA2711847C (en)* | 2008-01-09 | 2018-05-08 | Innovative Health Technologies, Llc | Implant pellets and methods for performing bone augmentation and preservation |
| GB0809721D0 (en)* | 2008-05-28 | 2008-07-02 | Univ Bath | Improvements in or relating to joints and/or implants |
| US9289302B2 (en)* | 2008-07-28 | 2016-03-22 | Zimmer, Inc. | Mosaicplasty constructs |
| US8308814B2 (en) | 2009-03-27 | 2012-11-13 | Depuy Mitek, Inc. | Methods and devices for preparing and implanting tissue scaffolds |
| US8241298B2 (en) | 2009-03-27 | 2012-08-14 | Depuy Mitek, Inc. | Methods and devices for delivering and affixing tissue scaffolds |
| US20110257753A1 (en)* | 2009-04-02 | 2011-10-20 | Synvasive Technology, Inc. | Implant having a convex surface surrounding a concave articular surface |
| US20100256758A1 (en)* | 2009-04-02 | 2010-10-07 | Synvasive Technology, Inc. | Monolithic orthopedic implant with an articular finished surface |
| US8556972B2 (en)* | 2009-04-02 | 2013-10-15 | Sevika Holding AG | Monolithic orthopedic implant with an articular finished surface |
| US9744123B2 (en)* | 2009-06-30 | 2017-08-29 | Kensey Nash Corporation | Biphasic implant device providing gradient |
| US10016278B2 (en)* | 2009-06-30 | 2018-07-10 | Dsm Ip Assets B.V. | Biphasic implant device providing joint fluid therapy |
| US20100331998A1 (en)* | 2009-06-30 | 2010-12-30 | Ringeisen Timothy A | Electrokinetic device for tissue repair |
| US8858592B2 (en)* | 2009-11-24 | 2014-10-14 | Covidien Lp | Wound plugs |
| US8994666B2 (en)* | 2009-12-23 | 2015-03-31 | Colin J. Karpfinger | Tactile touch-sensing interface system |
| IT1398443B1 (en)* | 2010-02-26 | 2013-02-22 | Lima Lto S P A Ora Limacorporate Spa | INTEGRATED PROSTHETIC ELEMENT |
| US20110288652A1 (en)* | 2010-05-20 | 2011-11-24 | Indiana University Research & Technology Corporation | Materials and methods for treating critically sized defects in mouse bone |
| EP2389904B1 (en)* | 2010-05-24 | 2013-07-24 | Episurf IP Management AB | Surgical kit for cartilage repair comprising implant and a set of tools |
| EP2389899B1 (en) | 2010-05-24 | 2015-04-29 | Episurf IP Management AB | Method of manufacturing a surgical kit for cartilage repair in a joint |
| EP2389901B8 (en) | 2010-05-24 | 2013-05-15 | Episurf IP Management AB | An implant for cartilage repair |
| EP2389905B1 (en) | 2010-05-24 | 2012-05-23 | Episurf Medical AB | Method of designing a surgical kit for cartilage repair in a joint |
| US8641718B2 (en)* | 2010-10-19 | 2014-02-04 | Biomet Manufacturing, Llc | Method and apparatus for harvesting cartilage for treatment of a cartilage defect |
| US9248020B2 (en)* | 2010-11-17 | 2016-02-02 | Zimmer, Inc. | Ceramic monoblock implants with osseointegration fixation surfaces |
| US8551525B2 (en) | 2010-12-23 | 2013-10-08 | Biostructures, Llc | Bone graft materials and methods |
| US10603049B2 (en) | 2011-09-02 | 2020-03-31 | Episurf Ip-Management Ab | Implant specific drill bit in surgical kit for cartilage repair |
| US11000387B2 (en) | 2011-09-02 | 2021-05-11 | Episurf Ip-Management Ab | Implant for cartilage repair |
| EP2564792A1 (en) | 2011-09-02 | 2013-03-06 | Episurf Medical AB | Modular surgical kit for cartilage repair |
| US9498334B2 (en)* | 2012-03-27 | 2016-11-22 | DePuy Synthes Products, Inc. | Glenoid defect-filling component |
| WO2014130883A2 (en) | 2013-02-22 | 2014-08-28 | Allosource | Cartilage mosaic compositions and methods |
| KR102312720B1 (en) | 2013-03-15 | 2021-10-13 | 알로소스 | Cell repopulated collagen matrix for soft tissue repair and regeneration |
| AU2014236705B2 (en) | 2013-03-15 | 2018-04-12 | Allosource | Perforated osteochondral allograft compositions |
| EP3581153B1 (en)* | 2014-03-11 | 2021-07-14 | The Trustees of Columbia University in the City of New York | Customized bendable osteochondral allografts |
| WO2016004991A1 (en) | 2014-07-09 | 2016-01-14 | Episurf Ip-Management Ab | Customized implant for cartilage repair and corresponding method of design |
| US20170172744A1 (en) | 2014-07-09 | 2017-06-22 | Episurf Ip-Management Ab | Surgical joint implant and a bone-mountable rig |
| US10077420B2 (en) | 2014-12-02 | 2018-09-18 | Histogenics Corporation | Cell and tissue culture container |
| US10702291B2 (en)* | 2015-07-02 | 2020-07-07 | Episurf Ip-Management Ab | System, guide tools and design methods related thereto for performing osteochondral transplantation surgery in a joint |
| US9943414B2 (en)* | 2015-12-30 | 2018-04-17 | Wasas, Llc. | System and method for non-binding allograft subtalar joint implant |
| CN109966029A (en)* | 2017-12-28 | 2019-07-05 | 财团法人工业技术研究院 | Cartilage repair carrier, matched surgical instrument set and cartilage repair system |
| US10786370B2 (en) | 2017-12-28 | 2020-09-29 | Industrial Technology Research Institute | Cartilage repair implant, auxiliary surgical tool kit and cartilage repair system |
| SE543241C2 (en) | 2018-04-27 | 2020-10-27 | Episurf Ip Man Ab | An implant for cartilage and/or bone repair |
| CN116019610B (en)* | 2023-01-09 | 2023-07-25 | 北京大学口腔医学院 | Multi-layer cap type vertical heightening jawbone defect prosthesis and preparation method thereof |
Citations (108)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1703154A (en) | 1927-05-13 | 1929-02-26 | Lanzkron Anna | Vegetable slicer |
| AU717552A (en) | 1952-01-02 | 1952-03-06 | Canadian Patents And Development Limited | Flow distributor valve |
| US3971273A (en) | 1975-02-26 | 1976-07-27 | Leo Peters | Meat slicing board |
| US4589206A (en) | 1984-09-24 | 1986-05-20 | Emery Marcoux | Slicing knife and board |
| GB2175506A (en) | 1985-05-29 | 1986-12-03 | American Hospital Supply Corp | Methods of producing prostheses for replacement of articular cartilage and prostheses so produced |
| US4932973A (en) | 1983-09-30 | 1990-06-12 | El Gendler | Cartilage and bone induction by artificially perforated organic bone matrix |
| US5002583A (en) | 1985-08-13 | 1991-03-26 | Sandu Pitaru | Collagen implants |
| US5112354A (en) | 1989-11-16 | 1992-05-12 | Northwestern University | Bone allograft material and method |
| US5115704A (en) | 1991-06-03 | 1992-05-26 | International Coffee & Tea Inc. | Bread cutter box |
| US5152791A (en) | 1989-12-07 | 1992-10-06 | Olympus Optical Co., Ltd. | Prosthetic artificial bone having ceramic layers of different porosity |
| US5211661A (en) | 1986-05-15 | 1993-05-18 | Sumitomo Cement Co., Ltd. | Artificial living body composite material |
| WO1993015694A1 (en) | 1992-02-14 | 1993-08-19 | Board Of Regents, The University Of Texas System | Multi-phase bioerodible implant/carrier and method of manufacturing and using same |
| US5320626A (en) | 1992-02-19 | 1994-06-14 | Arthrex Inc. | Endoscopic drill guide |
| US5320115A (en) | 1991-01-16 | 1994-06-14 | Applied Biological Concepts | Method and apparatus for arthroscopic knee surgery |
| DE4317448A1 (en)* | 1993-05-19 | 1994-11-24 | Gross Ulrich | Joint replacement |
| US5370692A (en) | 1992-08-14 | 1994-12-06 | Guild Associates, Inc. | Rapid, customized bone prosthesis |
| US5496326A (en) | 1991-06-27 | 1996-03-05 | Johnson; Lanny L. | Fixation screw and method for ligament reconstruction |
| WO1996024310A1 (en) | 1995-02-10 | 1996-08-15 | The Hospital For Joint Diseases, Orthopaedic Institute | Multi-stage collagen-based template or implant for use in the repair of cartilage lesions |
| WO1996024302A1 (en) | 1995-02-07 | 1996-08-15 | Matrix Biotechnologies, Inc. | Surgical implantation of cartilage repair unit |
| WO1996027333A1 (en) | 1995-03-07 | 1996-09-12 | Innovasive Devices, Inc. | Apparatus and methods for articular cartilage defect repair |
| WO1996034955A1 (en) | 1995-05-03 | 1996-11-07 | Warner-Lambert Company | Method of treating cartilaginous diseases with genetically modified chondrocytes |
| US5591234A (en) | 1993-02-01 | 1997-01-07 | Axel Kirsch | Post-surgery orthopedic covering |
| US5603716A (en) | 1995-02-16 | 1997-02-18 | Arthrex Inc. | Method of ligament reconstruction using double socket graft placement and fixation |
| EP0768332A1 (en)* | 1995-10-11 | 1997-04-16 | Sulzer Innotec Ag | Process for photooxidative treatment of collagen-containing tissues |
| US5632747A (en) | 1995-03-15 | 1997-05-27 | Osteotech, Inc. | Bone dowel cutter |
| WO1997046665A1 (en) | 1996-06-04 | 1997-12-11 | Sulzer Orthopedics Ltd. | Method for making cartilage and implants |
| EP0815809A2 (en) | 1996-06-28 | 1998-01-07 | Johnson & Johnson Professional, Inc. | Prosthesis with variable fit and strain distribution |
| US5718707A (en) | 1997-01-22 | 1998-02-17 | Mikhail; W. E. Michael | Method and apparatus for positioning and compacting bone graft |
| US5741685A (en) | 1995-06-07 | 1998-04-21 | Children's Medical Center Corporation | Parenchymal cells packaged in immunoprotective tissue for implantation |
| US5769899A (en) | 1994-08-12 | 1998-06-23 | Matrix Biotechnologies, Inc. | Cartilage repair unit |
| US5785522A (en) | 1992-05-27 | 1998-07-28 | Astra Aktiebolag | Method of treating surgical drill, surgical drill and use of surgical drill |
| WO1998034569A1 (en) | 1997-02-11 | 1998-08-13 | Smith & Nephew, Inc. | Repairing cartilage |
| WO1998056317A1 (en) | 1997-06-11 | 1998-12-17 | Bionx Implants Oy | Joint prosthesis |
| US5853746A (en) | 1991-01-31 | 1998-12-29 | Robert Francis Shaw | Methods and compositions for the treatment and repair of defects or lesions in cartilage or bone using functional barrier |
| AU700349B2 (en) | 1995-06-08 | 1999-01-07 | Axel Kirsch | Cover diaphragm |
| US5876452A (en)* | 1992-02-14 | 1999-03-02 | Board Of Regents, University Of Texas System | Biodegradable implant |
| US5881733A (en) | 1996-09-13 | 1999-03-16 | Depuy Orthopaedic Technology, Inc. | Technique for osteocartilaginous transplantation in a mammalian joint |
| US5885293A (en) | 1997-03-03 | 1999-03-23 | Innovasive Devices, Inc. | Apparatus and method for cutting a surface at a repeatable angle |
| WO1999021497A1 (en) | 1997-10-27 | 1999-05-06 | Sulzer Orthopädie Ag | Method and instruments for repairing endochondral and osteochondral defects |
| US5919196A (en) | 1995-02-16 | 1999-07-06 | Arthrex, Inc. | Method and apparatus for osteochondral autograft transplantation |
| US6013853A (en) | 1992-02-14 | 2000-01-11 | The University Of Texas System | Continuous release polymeric implant carrier |
| US6025538A (en) | 1998-11-20 | 2000-02-15 | Musculoskeletal Transplant Foundation | Compound bone structure fabricated from allograft tissue |
| US6027743A (en) | 1994-06-03 | 2000-02-22 | Stryker Corporation | Manufacture of autogenous replacement body parts |
| US6110178A (en) | 1997-04-25 | 2000-08-29 | Sulzer Orthopadie Ag | Apparatus for the production of endochondral or osteochondral bores |
| US6123731A (en) | 1998-02-06 | 2000-09-26 | Osteotech, Inc. | Osteoimplant and method for its manufacture |
| WO2001005336A1 (en) | 1999-07-16 | 2001-01-25 | Eska Implants Gmbh & Co. | Implant designed to fill up cartilage and bone defects in joints |
| US6179871B1 (en) | 1995-06-07 | 2001-01-30 | Alan A. Halpern | Means for cartilage repair |
| US6193722B1 (en) | 1998-09-29 | 2001-02-27 | Sulzer Orthopaedic Ag | Hollow milling tool |
| WO2001030276A1 (en) | 1999-10-20 | 2001-05-03 | Volkmar Jansson | System for correcting cartilage defects using a cartilage substitute structure |
| US6235035B1 (en) | 1997-04-30 | 2001-05-22 | Societe Implants Diffusion | Bone recovering surgical drill comprising a stop having a colored mark |
| WO2001043667A1 (en) | 1999-12-15 | 2001-06-21 | Sulzer Orthopedics Ltd. | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| US6251143B1 (en)* | 1999-06-04 | 2001-06-26 | Depuy Orthopaedics, Inc. | Cartilage repair unit |
| US20020013627A1 (en) | 1998-10-05 | 2002-01-31 | Ed. Geistlich Soehne Ag Fur Chemische Industrie Switzerland | Method for promoting regeneration of surface cartilage in a damaged joint using multi-layer covering |
| US20020082704A1 (en) | 2000-05-15 | 2002-06-27 | Cryolife, Inc. | Osteochondral transplant techniques |
| US6440141B1 (en) | 2000-07-24 | 2002-08-27 | Oratec Interventions, Inc. | Method and apparatus for treating osteochondral pathologies |
| US6459948B1 (en) | 1996-07-03 | 2002-10-01 | The Trustees Of Columbia University In The City Of New York | Anatomically correct prosthesis and method and apparatus for manufacturing prosthesis |
| US6520964B2 (en) | 2000-05-01 | 2003-02-18 | Std Manufacturing, Inc. | System and method for joint resurface repair |
| US20030036801A1 (en) | 2001-07-16 | 2003-02-20 | Schwartz Herbert E. | Cartilage repair apparatus and method |
| US6530928B1 (en) | 1999-03-23 | 2003-03-11 | Sulzer Orthopedics Ltd. | Instrument, instrument set and a method for the introduction of an osteochondral transplant |
| US6569200B2 (en) | 1998-06-30 | 2003-05-27 | Lifenet | Plasticized soft tissue grafts, and methods of making and using same |
| US6591581B2 (en) | 2000-03-08 | 2003-07-15 | Arthrex, Inc. | Method for preparing and inserting round, size specific osteochondral cores in the knee |
| US6592588B1 (en) | 1995-02-16 | 2003-07-15 | Arthrex, Inc. | Apparatus for osteochondral autograft transplantation |
| US20030135209A1 (en) | 1999-12-03 | 2003-07-17 | Bahaa Seedhom | Fixation technology |
| US6626945B2 (en) | 2000-03-14 | 2003-09-30 | Chondrosite, Llc | Cartilage repair plug |
| US6626950B2 (en) | 2001-06-28 | 2003-09-30 | Ethicon, Inc. | Composite scaffold with post anchor for the repair and regeneration of tissue |
| US6632246B1 (en) | 2000-03-14 | 2003-10-14 | Chondrosite, Llc | Cartilage repair plug |
| US20030216669A1 (en) | 2001-05-25 | 2003-11-20 | Imaging Therapeutics, Inc. | Methods and compositions for articular repair |
| US20030220700A1 (en) | 2002-05-22 | 2003-11-27 | Hammer Joseph J. | Attachment of absorbable tissue scaffolds ot fixation devices |
| US20030225459A1 (en) | 2002-05-31 | 2003-12-04 | Hammer Joseph J. | Attachment of absorbable tissue scaffolds to fixation devices |
| US20030229400A1 (en) | 2002-05-13 | 2003-12-11 | Koichi Masuda | Tissue engineered osteochondral implant |
| US6679917B2 (en) | 2000-05-01 | 2004-01-20 | Arthrosurface, Incorporated | System and method for joint resurface repair |
| US20040034359A1 (en) | 2002-08-09 | 2004-02-19 | Reinhold Schmieding | Retrograde osteochondral autograft transfer system |
| US6699252B2 (en) | 2001-04-17 | 2004-03-02 | Regeneration Technologies, Inc. | Methods and instruments for improved meniscus transplantation |
| US20040059425A1 (en) | 2002-09-20 | 2004-03-25 | Reinhold Schmieding | Method and instrumentation for osteochondral repair using preformed implants |
| US20040073223A1 (en) | 2002-10-15 | 2004-04-15 | Brian Burkinshaw | Method for performing automated microfracture |
| US6727224B1 (en) | 1999-02-01 | 2004-04-27 | Genetics Institute, Llc. | Methods and compositions for healing and repair of articular cartilage |
| US20040147932A1 (en) | 2002-10-15 | 2004-07-29 | Brian Burkinshaw | Device for performing automated microfracture |
| EP1452150A1 (en) | 1997-05-08 | 2004-09-01 | Organogenesis Inc. | Chemical cleaning of biological material |
| US20040176771A1 (en) | 2003-03-07 | 2004-09-09 | Reinhold Schmieding | Retrodrill technique for insertion of autograft, allograft or synthetic osteochondral implants |
| WO2004075940A1 (en) | 2003-02-26 | 2004-09-10 | Zimmer Orthobiologics, Inc. | Preparation for repairing cartilage tissue, especially articular cartilage defects |
| US6793676B2 (en) | 1996-04-05 | 2004-09-21 | Depuy Orthopaedics, Inc. | Method of reconstructing a joint |
| US20040230303A1 (en) | 2003-05-16 | 2004-11-18 | Gomes Katherine A. | Cartilage allograft plug |
| US20050043814A1 (en) | 2003-08-20 | 2005-02-24 | Akihiko Kusanagi | Acellular matrix implanted into an articular cartilage or osteochondral lesion protected with a biodegradable polymer modified to have extended polymerization time and methods for preparation and use thereof |
| US20050043813A1 (en) | 2003-08-20 | 2005-02-24 | Akihiko Kusanagi | Acellular matrix implants for treatment of articular cartilage, bone or osteochondral defects and injuries and method for use thereof |
| US20050064042A1 (en) | 2003-04-29 | 2005-03-24 | Musculoskeletal Transplant Foundation | Cartilage implant plug with fibrin glue and method for implantation |
| WO2005038016A1 (en) | 2003-10-13 | 2005-04-28 | Aesculap Ag & Co. Kg | Cartilage replacement implant and method for producing a cartilage replacement implant |
| US20050209705A1 (en) | 2004-03-09 | 2005-09-22 | Niederauer Gabriele G | Implant scaffold combined with autologous or allogenic tissue |
| US20050222687A1 (en) | 2004-04-02 | 2005-10-06 | Gordana Vunjak-Novakovic | Cartilage implant assembly and method for implantation |
| WO2005092208A1 (en) | 2004-03-03 | 2005-10-06 | Schwartz Biomedical, Llc | Articular cartilage fixation device and method |
| US20050251268A1 (en) | 2003-05-16 | 2005-11-10 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US6988015B1 (en) | 1999-11-02 | 2006-01-17 | Tutogen Medical Gmbh | Bone implant |
| WO2006026981A1 (en) | 2004-09-07 | 2006-03-16 | Technische Universität Dresden | Implant for treating osteochondral defects and method for producing the same |
| US20060060209A1 (en) | 2004-09-23 | 2006-03-23 | Shepard David O | Osteochondral repair using plug fashioned from partial distal allograft femur or condyle |
| US7037339B2 (en) | 2001-09-27 | 2006-05-02 | Zimmer Spine, Inc. | Modular spinal fusion device |
| FR2860423B1 (en) | 2003-10-02 | 2006-06-09 | Tbf Lab | IMPLANT FOR THE TREATMENT OF INSULATED LESIONS OF CARTILAGE BY CHONDROCYTE GRAFT |
| US20060178748A1 (en) | 2004-02-05 | 2006-08-10 | Dinger Fred B Iii | Implants and delivery system for treating defects in articulating surfaces |
| EP1234552B1 (en) | 2001-02-26 | 2006-08-16 | Ethicon, Inc. | Scaffold fixation device for use in articular cartilage repair |
| US20060195188A1 (en) | 2004-11-24 | 2006-08-31 | O'driscoll Shawn W | Biosynthetic composite for osteochondral defect repair |
| US20060247790A1 (en) | 2005-04-30 | 2006-11-02 | Mckay William F | Shaped osteochondral grafts and methods of using same |
| WO2006092718A3 (en) | 2005-03-04 | 2007-01-25 | Fin Ceramica Faenza Spa | Cartilaginiform and osteochondral sustitute comprising a multilayer structure and use thereof |
| US20070093896A1 (en) | 2005-10-26 | 2007-04-26 | Malinin Theodore I | Method and instrumentation for the preparation and transplantation of osteochondral allografts |
| US7291149B1 (en) | 1995-06-07 | 2007-11-06 | Warsaw Orthopedic, Inc. | Method for inserting interbody spinal fusion implants |
| US20070276506A1 (en) | 2006-05-25 | 2007-11-29 | Biomet Manufacturing Corp. | Demineralized osteochondral plug |
| US20070299517A1 (en) | 2006-06-21 | 2007-12-27 | Howmedica Osteonics Corp. | Articular cartilage implant |
| US20080027447A1 (en) | 2006-07-27 | 2008-01-31 | Warsaw Orthopedic, Inc | System and method of harvesting osteochondral plugs |
| US20080051812A1 (en) | 2006-08-01 | 2008-02-28 | Baxano, Inc. | Multi-Wire Tissue Cutter |
| US20120053588A1 (en) | 2010-08-31 | 2012-03-01 | Lozier Antony J | Drill bit for osteochondral drilling with guiding element and uses thereof |
| US20120053642A1 (en) | 2010-08-31 | 2012-03-01 | Lozier Antony J | Osteochondral graft delivery device and uses thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH632923A5 (en)* | 1978-10-06 | 1982-11-15 | Sulzer Ag | Implant for partial replacement of a sliding surface of a human joint |
| JP2951342B2 (en)* | 1989-12-07 | 1999-09-20 | オリンパス光学工業株式会社 | Artificial bone prosthesis |
| ATE120637T1 (en)* | 1990-12-19 | 1995-04-15 | Haerle Anton | BONE REPLACEMENT. |
| HU214568B (en)* | 1994-09-16 | 1998-04-28 | METRIMED Orvosi Műszergyártó Kft. | Instrument-set for implantation of chondrus, like a mosaic |
| US6126919A (en)* | 1997-02-07 | 2000-10-03 | 3M Innovative Properties Company | Biocompatible compounds for pharmaceutical drug delivery systems |
| JP3669107B2 (en)* | 1997-03-17 | 2005-07-06 | 東レ株式会社 | Fiber structure |
- 2000
- 2000-12-12USUS11/705,575patent/USRE43714E1/ennot_activeExpired - Lifetime
- 2000-12-12CACA002393776Apatent/CA2393776C/ennot_activeExpired - Fee Related
- 2000-12-12USUS10/149,853patent/US6858042B2/ennot_activeExpired - Lifetime
- 2000-12-12ESES00979315Tpatent/ES2230170T3/ennot_activeExpired - Lifetime
- 2000-12-12DEDE50007640Tpatent/DE50007640D1/ennot_activeExpired - Lifetime
- 2000-12-12AUAU16857/01Apatent/AU783038B2/ennot_activeCeased
- 2000-12-12EPEP04020622Apatent/EP1498089A1/ennot_activeWithdrawn
- 2000-12-12WOPCT/CH2000/000659patent/WO2001043667A1/enactiveIP Right Grant
- 2000-12-12EPEP00979315Apatent/EP1237511B1/ennot_activeExpired - Lifetime
- 2000-12-12ATAT00979315Tpatent/ATE274875T1/ennot_activeIP Right Cessation
- 2000-12-12JPJP2001544609Apatent/JP2003516803A/enactivePending
Patent Citations (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1703154A (en) | 1927-05-13 | 1929-02-26 | Lanzkron Anna | Vegetable slicer |
| AU717552A (en) | 1952-01-02 | 1952-03-06 | Canadian Patents And Development Limited | Flow distributor valve |
| US3971273A (en) | 1975-02-26 | 1976-07-27 | Leo Peters | Meat slicing board |
| US4932973A (en) | 1983-09-30 | 1990-06-12 | El Gendler | Cartilage and bone induction by artificially perforated organic bone matrix |
| US4589206A (en) | 1984-09-24 | 1986-05-20 | Emery Marcoux | Slicing knife and board |
| US4627853A (en) | 1985-05-29 | 1986-12-09 | American Hospital Supply Corporation | Method of producing prostheses for replacement of articular cartilage and prostheses so produced |
| GB2175506A (en) | 1985-05-29 | 1986-12-03 | American Hospital Supply Corp | Methods of producing prostheses for replacement of articular cartilage and prostheses so produced |
| US5002583A (en) | 1985-08-13 | 1991-03-26 | Sandu Pitaru | Collagen implants |
| US5211661A (en) | 1986-05-15 | 1993-05-18 | Sumitomo Cement Co., Ltd. | Artificial living body composite material |
| US5112354A (en) | 1989-11-16 | 1992-05-12 | Northwestern University | Bone allograft material and method |
| US5152791A (en) | 1989-12-07 | 1992-10-06 | Olympus Optical Co., Ltd. | Prosthetic artificial bone having ceramic layers of different porosity |
| US5320115A (en) | 1991-01-16 | 1994-06-14 | Applied Biological Concepts | Method and apparatus for arthroscopic knee surgery |
| US5853746A (en) | 1991-01-31 | 1998-12-29 | Robert Francis Shaw | Methods and compositions for the treatment and repair of defects or lesions in cartilage or bone using functional barrier |
| US5115704A (en) | 1991-06-03 | 1992-05-26 | International Coffee & Tea Inc. | Bread cutter box |
| US5496326A (en) | 1991-06-27 | 1996-03-05 | Johnson; Lanny L. | Fixation screw and method for ligament reconstruction |
| WO1993015694A1 (en) | 1992-02-14 | 1993-08-19 | Board Of Regents, The University Of Texas System | Multi-phase bioerodible implant/carrier and method of manufacturing and using same |
| US6013853A (en) | 1992-02-14 | 2000-01-11 | The University Of Texas System | Continuous release polymeric implant carrier |
| US5876452A (en)* | 1992-02-14 | 1999-03-02 | Board Of Regents, University Of Texas System | Biodegradable implant |
| US5320626A (en) | 1992-02-19 | 1994-06-14 | Arthrex Inc. | Endoscopic drill guide |
| US5785522A (en) | 1992-05-27 | 1998-07-28 | Astra Aktiebolag | Method of treating surgical drill, surgical drill and use of surgical drill |
| US5370692A (en) | 1992-08-14 | 1994-12-06 | Guild Associates, Inc. | Rapid, customized bone prosthesis |
| US5591234A (en) | 1993-02-01 | 1997-01-07 | Axel Kirsch | Post-surgery orthopedic covering |
| DE4317448A1 (en)* | 1993-05-19 | 1994-11-24 | Gross Ulrich | Joint replacement |
| WO1994026211A1 (en) | 1993-05-19 | 1994-11-24 | Ulrich Gross | Articular surface prosthesis |
| US20050089544A1 (en) | 1994-06-03 | 2005-04-28 | Khouri Roger K. | Manufacture of autogenous replacement body parts |
| US20030064090A1 (en) | 1994-06-03 | 2003-04-03 | Khouri Roger K. | Manufacture of autogenous replacement body parts |
| US6027743A (en) | 1994-06-03 | 2000-02-22 | Stryker Corporation | Manufacture of autogenous replacement body parts |
| US6110482A (en) | 1994-06-03 | 2000-08-29 | Styker Corporation | Manufacture of autogenous replacement body parts |
| US5769899A (en) | 1994-08-12 | 1998-06-23 | Matrix Biotechnologies, Inc. | Cartilage repair unit |
| US5632745A (en) | 1995-02-07 | 1997-05-27 | R&D Biologicals, Inc. | Surgical implantation of cartilage repair unit |
| WO1996024302A1 (en) | 1995-02-07 | 1996-08-15 | Matrix Biotechnologies, Inc. | Surgical implantation of cartilage repair unit |
| WO1996024310A1 (en) | 1995-02-10 | 1996-08-15 | The Hospital For Joint Diseases, Orthopaedic Institute | Multi-stage collagen-based template or implant for use in the repair of cartilage lesions |
| US6592588B1 (en) | 1995-02-16 | 2003-07-15 | Arthrex, Inc. | Apparatus for osteochondral autograft transplantation |
| US5603716A (en) | 1995-02-16 | 1997-02-18 | Arthrex Inc. | Method of ligament reconstruction using double socket graft placement and fixation |
| US5919196A (en) | 1995-02-16 | 1999-07-06 | Arthrex, Inc. | Method and apparatus for osteochondral autograft transplantation |
| US5782835A (en) | 1995-03-07 | 1998-07-21 | Innovasive Devices, Inc. | Apparatus and methods for articular cartilage defect repair |
| WO1996027333A1 (en) | 1995-03-07 | 1996-09-12 | Innovasive Devices, Inc. | Apparatus and methods for articular cartilage defect repair |
| US6017348A (en)* | 1995-03-07 | 2000-01-25 | Innovasive Devices, Inc. | Apparatus and methods for articular cartilage defect repair |
| US5632747A (en) | 1995-03-15 | 1997-05-27 | Osteotech, Inc. | Bone dowel cutter |
| WO1996034955A1 (en) | 1995-05-03 | 1996-11-07 | Warner-Lambert Company | Method of treating cartilaginous diseases with genetically modified chondrocytes |
| US6179871B1 (en) | 1995-06-07 | 2001-01-30 | Alan A. Halpern | Means for cartilage repair |
| US7291149B1 (en) | 1995-06-07 | 2007-11-06 | Warsaw Orthopedic, Inc. | Method for inserting interbody spinal fusion implants |
| US5741685A (en) | 1995-06-07 | 1998-04-21 | Children's Medical Center Corporation | Parenchymal cells packaged in immunoprotective tissue for implantation |
| AU700349B2 (en) | 1995-06-08 | 1999-01-07 | Axel Kirsch | Cover diaphragm |
| EP0768332A1 (en)* | 1995-10-11 | 1997-04-16 | Sulzer Innotec Ag | Process for photooxidative treatment of collagen-containing tissues |
| US5817153A (en) | 1995-10-11 | 1998-10-06 | Sulzer Innotec Ag | Method of photo-oxidative treatment of tissues containing collagen |
| US6793676B2 (en) | 1996-04-05 | 2004-09-21 | Depuy Orthopaedics, Inc. | Method of reconstructing a joint |
| WO1997046665A1 (en) | 1996-06-04 | 1997-12-11 | Sulzer Orthopedics Ltd. | Method for making cartilage and implants |
| US6242247B1 (en)* | 1996-06-04 | 2001-06-05 | Sulzer Orthopedics Ltd. | Method for making cartilage and implants |
| EP0815809A2 (en) | 1996-06-28 | 1998-01-07 | Johnson & Johnson Professional, Inc. | Prosthesis with variable fit and strain distribution |
| US6459948B1 (en) | 1996-07-03 | 2002-10-01 | The Trustees Of Columbia University In The City Of New York | Anatomically correct prosthesis and method and apparatus for manufacturing prosthesis |
| EP0824893B1 (en) | 1996-08-16 | 2005-11-09 | Arthrex Inc | Apparatus for osteochondral autograft transplantation |
| US5881733A (en) | 1996-09-13 | 1999-03-16 | Depuy Orthopaedic Technology, Inc. | Technique for osteocartilaginous transplantation in a mammalian joint |
| US5718707A (en) | 1997-01-22 | 1998-02-17 | Mikhail; W. E. Michael | Method and apparatus for positioning and compacting bone graft |
| US6358253B1 (en) | 1997-02-11 | 2002-03-19 | Smith & Newhew Inc | Repairing cartilage |
| WO1998034569A1 (en) | 1997-02-11 | 1998-08-13 | Smith & Nephew, Inc. | Repairing cartilage |
| US6146385A (en) | 1997-02-11 | 2000-11-14 | Smith & Nephew, Inc. | Repairing cartilage |
| US5885293A (en) | 1997-03-03 | 1999-03-23 | Innovasive Devices, Inc. | Apparatus and method for cutting a surface at a repeatable angle |
| US6110178A (en) | 1997-04-25 | 2000-08-29 | Sulzer Orthopadie Ag | Apparatus for the production of endochondral or osteochondral bores |
| US6235035B1 (en) | 1997-04-30 | 2001-05-22 | Societe Implants Diffusion | Bone recovering surgical drill comprising a stop having a colored mark |
| EP1452150A1 (en) | 1997-05-08 | 2004-09-01 | Organogenesis Inc. | Chemical cleaning of biological material |
| US20060024380A1 (en) | 1997-05-08 | 2006-02-02 | Organogenesis Inc. | Chemical cleaning of biological material |
| WO1998056317A1 (en) | 1997-06-11 | 1998-12-17 | Bionx Implants Oy | Joint prosthesis |
| WO1999021497A1 (en) | 1997-10-27 | 1999-05-06 | Sulzer Orthopädie Ag | Method and instruments for repairing endochondral and osteochondral defects |
| US6123731A (en) | 1998-02-06 | 2000-09-26 | Osteotech, Inc. | Osteoimplant and method for its manufacture |
| US6569200B2 (en) | 1998-06-30 | 2003-05-27 | Lifenet | Plasticized soft tissue grafts, and methods of making and using same |
| US6193722B1 (en) | 1998-09-29 | 2001-02-27 | Sulzer Orthopaedic Ag | Hollow milling tool |
| US20020013627A1 (en) | 1998-10-05 | 2002-01-31 | Ed. Geistlich Soehne Ag Fur Chemische Industrie Switzerland | Method for promoting regeneration of surface cartilage in a damaged joint using multi-layer covering |
| US6025538A (en) | 1998-11-20 | 2000-02-15 | Musculoskeletal Transplant Foundation | Compound bone structure fabricated from allograft tissue |
| US20040192605A1 (en) | 1999-02-01 | 2004-09-30 | Genetics Institute, Llc | Methods and compositions for healing and repair of articular cartilage |
| US6727224B1 (en) | 1999-02-01 | 2004-04-27 | Genetics Institute, Llc. | Methods and compositions for healing and repair of articular cartilage |
| US6530928B1 (en) | 1999-03-23 | 2003-03-11 | Sulzer Orthopedics Ltd. | Instrument, instrument set and a method for the introduction of an osteochondral transplant |
| US6468314B2 (en) | 1999-06-04 | 2002-10-22 | Depuy Orthopaedics, Inc. | Cartilage repair unit |
| US6251143B1 (en)* | 1999-06-04 | 2001-06-26 | Depuy Orthopaedics, Inc. | Cartilage repair unit |
| WO2001005336A1 (en) | 1999-07-16 | 2001-01-25 | Eska Implants Gmbh & Co. | Implant designed to fill up cartilage and bone defects in joints |
| WO2001030276A1 (en) | 1999-10-20 | 2001-05-03 | Volkmar Jansson | System for correcting cartilage defects using a cartilage substitute structure |
| US6988015B1 (en) | 1999-11-02 | 2006-01-17 | Tutogen Medical Gmbh | Bone implant |
| US20030135209A1 (en) | 1999-12-03 | 2003-07-17 | Bahaa Seedhom | Fixation technology |
| US6858042B2 (en) | 1999-12-15 | 2005-02-22 | Zimmer Orthobiologics, Inc. | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| WO2001043667A1 (en) | 1999-12-15 | 2001-06-21 | Sulzer Orthopedics Ltd. | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints |
| US6591581B2 (en) | 2000-03-08 | 2003-07-15 | Arthrex, Inc. | Method for preparing and inserting round, size specific osteochondral cores in the knee |
| US6852125B2 (en) | 2000-03-14 | 2005-02-08 | Chondrosite, Inc. | Cartilage repair plug |
| US6632246B1 (en) | 2000-03-14 | 2003-10-14 | Chondrosite, Llc | Cartilage repair plug |
| US6626945B2 (en) | 2000-03-14 | 2003-09-30 | Chondrosite, Llc | Cartilage repair plug |
| US7029479B2 (en) | 2000-05-01 | 2006-04-18 | Arthrosurface, Inc. | System and method for joint resurface repair |
| US6679917B2 (en) | 2000-05-01 | 2004-01-20 | Arthrosurface, Incorporated | System and method for joint resurface repair |
| US6520964B2 (en) | 2000-05-01 | 2003-02-18 | Std Manufacturing, Inc. | System and method for joint resurface repair |
| US20020082704A1 (en) | 2000-05-15 | 2002-06-27 | Cryolife, Inc. | Osteochondral transplant techniques |
| US6852114B2 (en) | 2000-05-15 | 2005-02-08 | Cryolife, Inc. | Osteochondral transplant techniques |
| US6488033B1 (en) | 2000-05-15 | 2002-12-03 | Cryolife, Inc. | Osteochondral transplant techniques |
| US6440141B1 (en) | 2000-07-24 | 2002-08-27 | Oratec Interventions, Inc. | Method and apparatus for treating osteochondral pathologies |
| EP1234552B1 (en) | 2001-02-26 | 2006-08-16 | Ethicon, Inc. | Scaffold fixation device for use in articular cartilage repair |
| US6699252B2 (en) | 2001-04-17 | 2004-03-02 | Regeneration Technologies, Inc. | Methods and instruments for improved meniscus transplantation |
| US20030216669A1 (en) | 2001-05-25 | 2003-11-20 | Imaging Therapeutics, Inc. | Methods and compositions for articular repair |
| US6626950B2 (en) | 2001-06-28 | 2003-09-30 | Ethicon, Inc. | Composite scaffold with post anchor for the repair and regeneration of tissue |
| US20030036801A1 (en) | 2001-07-16 | 2003-02-20 | Schwartz Herbert E. | Cartilage repair apparatus and method |
| US7037339B2 (en) | 2001-09-27 | 2006-05-02 | Zimmer Spine, Inc. | Modular spinal fusion device |
| US20030229400A1 (en) | 2002-05-13 | 2003-12-11 | Koichi Masuda | Tissue engineered osteochondral implant |
| US20030220700A1 (en) | 2002-05-22 | 2003-11-27 | Hammer Joseph J. | Attachment of absorbable tissue scaffolds ot fixation devices |
| US20030225459A1 (en) | 2002-05-31 | 2003-12-04 | Hammer Joseph J. | Attachment of absorbable tissue scaffolds to fixation devices |
| US20040034359A1 (en) | 2002-08-09 | 2004-02-19 | Reinhold Schmieding | Retrograde osteochondral autograft transfer system |
| US20040059425A1 (en) | 2002-09-20 | 2004-03-25 | Reinhold Schmieding | Method and instrumentation for osteochondral repair using preformed implants |
| US20040147932A1 (en) | 2002-10-15 | 2004-07-29 | Brian Burkinshaw | Device for performing automated microfracture |
| US20040073223A1 (en) | 2002-10-15 | 2004-04-15 | Brian Burkinshaw | Method for performing automated microfracture |
| WO2004075940A1 (en) | 2003-02-26 | 2004-09-10 | Zimmer Orthobiologics, Inc. | Preparation for repairing cartilage tissue, especially articular cartilage defects |
| US20040176771A1 (en) | 2003-03-07 | 2004-09-09 | Reinhold Schmieding | Retrodrill technique for insertion of autograft, allograft or synthetic osteochondral implants |
| US20050064042A1 (en) | 2003-04-29 | 2005-03-24 | Musculoskeletal Transplant Foundation | Cartilage implant plug with fibrin glue and method for implantation |
| WO2004103224A1 (en) | 2003-05-16 | 2004-12-02 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US20050251268A1 (en) | 2003-05-16 | 2005-11-10 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US20040230303A1 (en) | 2003-05-16 | 2004-11-18 | Gomes Katherine A. | Cartilage allograft plug |
| US20050043814A1 (en) | 2003-08-20 | 2005-02-24 | Akihiko Kusanagi | Acellular matrix implanted into an articular cartilage or osteochondral lesion protected with a biodegradable polymer modified to have extended polymerization time and methods for preparation and use thereof |
| US20050043813A1 (en) | 2003-08-20 | 2005-02-24 | Akihiko Kusanagi | Acellular matrix implants for treatment of articular cartilage, bone or osteochondral defects and injuries and method for use thereof |
| US20060083729A1 (en) | 2003-08-20 | 2006-04-20 | Akihiko Kusanagi | Biocompatible tissue sealant for treatment of osteochondral and bone defects using an acellular matrix implant |
| FR2860423B1 (en) | 2003-10-02 | 2006-06-09 | Tbf Lab | IMPLANT FOR THE TREATMENT OF INSULATED LESIONS OF CARTILAGE BY CHONDROCYTE GRAFT |
| WO2005038016A1 (en) | 2003-10-13 | 2005-04-28 | Aesculap Ag & Co. Kg | Cartilage replacement implant and method for producing a cartilage replacement implant |
| US20060178748A1 (en) | 2004-02-05 | 2006-08-10 | Dinger Fred B Iii | Implants and delivery system for treating defects in articulating surfaces |
| WO2005092208A1 (en) | 2004-03-03 | 2005-10-06 | Schwartz Biomedical, Llc | Articular cartilage fixation device and method |
| US20050209705A1 (en) | 2004-03-09 | 2005-09-22 | Niederauer Gabriele G | Implant scaffold combined with autologous or allogenic tissue |
| US20050222687A1 (en) | 2004-04-02 | 2005-10-06 | Gordana Vunjak-Novakovic | Cartilage implant assembly and method for implantation |
| WO2006026981A1 (en) | 2004-09-07 | 2006-03-16 | Technische Universität Dresden | Implant for treating osteochondral defects and method for producing the same |
| US20060060209A1 (en) | 2004-09-23 | 2006-03-23 | Shepard David O | Osteochondral repair using plug fashioned from partial distal allograft femur or condyle |
| US20060195188A1 (en) | 2004-11-24 | 2006-08-31 | O'driscoll Shawn W | Biosynthetic composite for osteochondral defect repair |
| WO2006092718A3 (en) | 2005-03-04 | 2007-01-25 | Fin Ceramica Faenza Spa | Cartilaginiform and osteochondral sustitute comprising a multilayer structure and use thereof |
| US20060247790A1 (en) | 2005-04-30 | 2006-11-02 | Mckay William F | Shaped osteochondral grafts and methods of using same |
| US20070135928A1 (en) | 2005-10-26 | 2007-06-14 | Malinin Theodore I | Osteochondral allografts |
| US20070135917A1 (en) | 2005-10-26 | 2007-06-14 | Malinin Theodore I | Instrumentation for the preparation and transplantation of osteochondral allografts |
| US20070135918A1 (en) | 2005-10-26 | 2007-06-14 | Malinin Theodore I | Instrumentation for the preparation and transplantation of osteochondral allografts |
| US20070093896A1 (en) | 2005-10-26 | 2007-04-26 | Malinin Theodore I | Method and instrumentation for the preparation and transplantation of osteochondral allografts |
| US20070276506A1 (en) | 2006-05-25 | 2007-11-29 | Biomet Manufacturing Corp. | Demineralized osteochondral plug |
| US20070299517A1 (en) | 2006-06-21 | 2007-12-27 | Howmedica Osteonics Corp. | Articular cartilage implant |
| US20080027447A1 (en) | 2006-07-27 | 2008-01-31 | Warsaw Orthopedic, Inc | System and method of harvesting osteochondral plugs |
| US20080051812A1 (en) | 2006-08-01 | 2008-02-28 | Baxano, Inc. | Multi-Wire Tissue Cutter |
| US20120053588A1 (en) | 2010-08-31 | 2012-03-01 | Lozier Antony J | Drill bit for osteochondral drilling with guiding element and uses thereof |
| US20120053642A1 (en) | 2010-08-31 | 2012-03-01 | Lozier Antony J | Osteochondral graft delivery device and uses thereof |
Non-Patent Citations (106)
| Title |
|---|
| "U.S. Appl. No. 12/196,831, Final Office Action Mailed Apr. 12, 2012", 17 pgs. |
| Aichroth, et al, "Biological and Mechanical Problems of Osteoarticular Allografting: The Relation to Clinical Organ Transplantation," The Journal of the Western Pacific Orthopaedic Association, 1971, pp. 25-70, vol. VIII, No. 2, London, England. |
| Akens et al., BMC Musculoskeletal Disorders 2:9 (2001). |
| Akens, et al, "In-vitro and in-vivo studies of osteochondral transplants pretreated with photo-oxidation," Ph.D. Thesis, 2002, pp. 1-100, Ph.D. Program of the Veterinary Faculties of Zurich and Bern. |
| Bakay et al., International Orthopaedics 20:370-372 (1996). |
| Bakay et al., International Orthopaedics 22:277-281 (1998). |
| Bakay, et al., "The alternatives of the application of human lyophilized spongiosa and bone-matrix gelatin," Orvosi Hetilap, 1995, pp. 1891-1896, vol. 136, No. 35, Budapest (Original and Translation enclosed). |
| Baragi et al., Osteoarthritis and Cartilage 5:275-282 (1997). |
| Barber, et al., "COR Osteochondral Repair System," Osteobiologics, Inc., 2006, DePuy Mitek, USA. |
| Beaver, et al., "Fresh Osteochondral Allografts for Post-Traumatic Defects in the Knee," The Journal of Bone and Joint Surgery, 1992, pp. 105-110, vol. 74-B, No. 1, Britain (UK). |
| Bodo et al., Acta Veterinaria Hungarica 48(3):343-354 (2000). |
| Bos, et al., "Immune responses of rats to frozen bone allografts," The Journal of Bone & Joint Surgery, 1983, pp. 239-246, vol. 65, Needham, MA, USA. |
| Brooks, et al., "Immunological Factors in Homogenous Bone Transplantation: IV. The Effect of Various Methods of Preparation and Irradiation on Antingenicity," The Journal of Bone & Joint Surgery, 1963, pp. 1617-1626, vol. 45, Needham, MA, USA. |
| Bugbee, et al., "Osteochondral Allograft Transplantation," Complex Topics in Knee Surgery, 1999, pp. 67-75, vol. 18, No. 1, California, USA. |
| Bugbee, William D., Fresh Osteochondral Allografts, Seminars in Arthroplasty, 2000, pp. 221-226, vol. 11, No. 4, W.B. Saunders Company, San Diego, CA, USA. |
| Burba et al., Journal of Investigative Surgery 5:343-359 (1992). |
| Chalmers, J., "Transplantation immunity in bone homografting," The Journal of Bone & Joint Surgery, 1959, pp. 160-179, vol. 41B, No. 1, London, England. |
| Chu, et al., Articular Cartilage Transplantation-Clinical Results in the Knee, Clinical Orthopaedics and Related Research Journal, 1999, pp. 159-168, No. 360, Lippincott Williams & Watkins, Inc., USA. |
| Convery, et al., "Fresh osteochondral allografting of the femoral condyle," Clinical Orthopaedics and Related Research, 1991, pp. 139-145, No. 273, San Diego, CA, USA. |
| Convery, et al., "Long-term survival of chondrocytes in an osteochondral articular cartilage allograft. A Case Report," The Journal of Bone & Joint Surgery, 1996, pp. 1082-1087, vol. 78, Needham, MA, USA. |
| Convery, et al., "The operative technique of fresh osteochondral allografting of the knee," Operative Techniques in Orthopaedics, 1997, pp. 340-344, vol. 7, No. 4, W.B. Saunders Company, San Diego, CA, USA. |
| Cornell et al., Nature 387:580-583 (Jun. 1997). |
| Csönge et al., Cell and Tissue Banking 3:151-159 (2002). |
| Csönge et al., Cell and Tissue Banking 3:161-168 (2002). |
| Czitrom et al., Clinical Orthopaedics and Related Research, No. 208, pp. 141-145 (Jul. 1986). |
| Delloye et al., Acta Orthopaedica Belgica 57:75-83 (1991). |
| Desjardins, et al., "Incorporation of Fresh and Cryopreserved Bone in Osteochondral Autografts in the Horse," Veterinary Surgery, 1991, pp. 446-452, vol. 20, No. 6, Ontario, Canada. |
| Donald, Paul J., "Cartilage Grafting in Facial Reconstruction with Special Consideration of Irradiated Grafts"*\, Laryngoscope, 1986, pp. 786-806, vol. 96, Sacramento, CA, USA. |
| Ehalt, W, "Bisherige Erfahrungen mit dem plastischen Ersatz von Gelenkknorpel aus der Knochenbank", Verh. Dtsch. Orthop. Ges. 43, (1955), 107-109. |
| Ehalt, W., et al., "Gelenkknorpel-Plastik", Langenbecks Arch. Kiln. Chir. 299, (1962), 768-774. |
| Ehalt, Walther M, "Grafting of joint-cartilage Bone-Blocks from the bank", VI. Congr. Soc. Internat. Chir. Orthop. Traumatol. S., (1954), 419-421. |
| Elves, et al., "A Study of the Humoral Immune Response to Osteoarticular Allografts in the Sheep," Clin. exp. Immunol., 1974, pp. 497-508, vol. 17, Middlesex, UK. |
| Flynn et al., Clinical Orthopaedics and Related Research, No. 303, pp. 38-43 (Jun. 1994). |
| Friedlaender, et al., "Studies on the antigenicity of bone. I. Freeze-dried and deep-frozen bone allografts in rabbits," The Journal of Bone & Joint Surgery, 1976, pp. 854-858, vol. 58, Needham, MA, USA. |
| Friedlaender, Gary E., "Immune Responses to Osteochondral Allografts," Clinical Orthopaedics and Related Research, 1983, pp. 58-68, No. 174, J.B. Lippincott Co., USA. |
| Garrett, Clinical Orthopaedics and Related Research, No. 303, pp. 33-37 (Jun. 1994). |
| Garrett, John C, "Osteochondral Allografts", Instructional Course Lectures, vol. 42, (1993), 355-8. |
| Garrett, John C., "Fresh Osteochondral Allografts for Treatment of Articular Defects in Osteochondritis Dissecans of the Lateral Femoral Condyle in Adults," Clinical Orthopaedics and Related Research, 1994, pp. 33037, No. 303, J. B. Lippincott Company, USA. |
| Garrett, John C., M.D., "Osteochondral Allografts for Reconstruction of Articular Defects of the Knee," AAOS Instructional Course Lectures, 1998, pp. 517-522, vol. 47, USA. |
| Ghazavi, et al., "Fresh Osteochondral Allografts for Post-Traumatic Osteochondral Defects of the Knee," Journal of Bone & Joint Surgery, 1997, pp. 1008-1013, vol. 79-B, No. 6, Toronto, Canada. |
| Gould, Nathaniel, "Trephining Your Way," Orthopedic Clinics of North America, 1973, pp. 157-164, vol. 4, No. 1, Brockton, MA, USA. |
| Gross, "Use of Fresh Osteochondral Allografts to Replace Traumatic Joint Defects," pp. 67-82, Allografts in Orthopaedic Practice (Zitrom and Gross, eds., 1992). |
| Gross, et al., "Reconstruction of Skeletal Deficits at the Knee," Climical Orthopaedics and Related Research, 1983, pp. 96-106, No. 174, J.B. Lippincott Co., Philadelphia, PA, USA. |
| Hangody et al., Autogenous Osteochondral Mosaicplasty 5(3):175-181 (May/Jun. 1997). |
| Hangody, L, et al., "Autogenous osteochondral grafting in the knees of German Shepherd dogs: Radiographic and histological analysis", Hungarian Review of Sports Medicine 35, (1994), 117-123. |
| Hangody, L, et al., "Treatment of localized chondral and osteochondral defects in the knee by a new autogenous osteochondral grafting tenique", Hungarian Review of Sports Medicine 35, (1994), 241-246. |
| Hangody, Laszlo, et al., "Sülyos, körülírt térdízületi porckárosodás sebészi kezelésének új lehetosége (New alternative in the treatment of sever, localized cartilage damages in the knee joint)", Hungarian Journal of Traumatology and Orthopaedics 37, (1994), 237-242. |
| Hetherington et al., BMC Musculoskeletal Disorders 6:36 (2005). |
| Hetherington et al., The Journal of Foot & Ankle Surgery 46(4):223-229 (Jul./Aug. 2007). |
| Hetherington, et al., "Immunologic testing for xeno-derived osteocartilagenous grafts," ICRS, 2002, USA. |
| Hurtig, et al, "Osteochondral Dowel Transplantation for Repair of Focal Defects in the Knee: An Outcome Study Using an Ovine Model," Veterinary Surgery, 1998, pp. 5-16, vol. 27, Alberta, Canada. |
| Hurtig, et al., "Surgical and analytical techniques for articular cartilage transplantation," Veterinary Surgery The Official Journal of The American College of Veterinary Surgeons, Inc., 1992, p. 394, vol. 21, No. 5, J.B. Lippincott Company, Philadelphia, PA, USA. |
| Hurtig, M, et al., "Surgical and analytical techniques for assessment of transplanted articular cartilage: A pilot study", Twenty-ninth Annual Meeting of the Society for Cryobiology Ithaca, New York, USA, (1992), 732. |
| Hurtig, M.B., "Experimental use of small osteochondral grafts for resurfacing the equine third carpal bone," Equine Orthopaedics, 1988, pp. 23-27, Supp. 6, England. |
| Jakob, et al., "Autologous Osteochondral Grafting in the Knee: Indication, Results, and Reflections," Clinical Orthopaedics and Related Research, 2002, pp. 170-184, No. 401, Lippincott Williams & Wilkins, Inc., USA. |
| Jakob, et al., "Isolated articular cartilage lesion: repair or regeneration," Osteoarthritis and Cartilage Journal of the OsteoArthritis Research Society International, 2001, pp. S3-S5, vol. 9, Supplement A, www.idealibrary.com. |
| Jamali, et al., "Donor cell survival in a fresh osteochondral allograft at twenty-nine years," The Journal of Bone & Joint Surgery, 2007, pp. 166-169, vol. 89, Needham, MA, USA. |
| Jimenez, et al., "Experimental Studies onthe Fate of Transplanted Articular Cartilage," Osteochondral Allografts, Biology, Banking and Clinical Applications, 1983, pp. 73-79, Little, Brown & Co., Boston, USA. |
| Kamijou, et al., "Effects of Osteocytes on Osteoinduction i nthe Autogenous Rib Graft in the Rat Mandible," Bone, 1994, pp. 629-637, vol. 15, No. 6, Elsevier Science Ltd., USA. |
| Kawamura et al., Clinical Orthopaedics and Related Research, No. 235, pp. 302-310 (Oct. 1988). |
| Kelley, et al., "Chondrocyte repopulation of allograft cartilage: A preliminary investigation and strategy for developing cartilage matrices for reconstruction," Otolaryngology-Head and Neck Surgery, 2002, pp. 265-270, vol. 127, No. 4, Washington, DC, USA. |
| Kluger, et al, "Removal of the surface layers of human cortical bone allografts restores in vitro osteoclast function reduced by processing and frozen storage," Bone, 2003, pp. 291-296, vol. 32, Elsevier Science, USA. |
| Kwan, et al., "Histological and Biomechanical Assessment of Articular Cartilage from Stored Osteochondral Shell Allografts," Journal of Orthopaedic Research, 1989, pp. 637-644, vol. 7, No. 5, USA. |
| Lexer, E., "Joint transplantation," Clinical Orthopaedics and Related Research, 1985, pp. 4-10, No. 197, USA. |
| Locht et al., The Journal of Bone and Joint Surgery 66-A:328-335 (Mar. 1984). |
| Mahomed et al., Orthopedics 15:1191-1199 (Oct. 1992). |
| Malinin, et al., "Cryopreservation of articular cartilage," Clinical Orthopaedics and Related Research, 1994, pp. 18-32, No. 303, J. B. Lippincott Company, USA. |
| Malinin, et al., Banking of Massive Osteoarticular and Intercalary Bone Allografts-12 Years' Experience, Clinical Orthopaedics and Related Research, 1985, pp. 44-57, No. 197. Miami, FL, USA. |
| Mankin, et al., "Clinical experience with allograft implantation," Clinical Orthopaedics and Related Research, 1983, pp. 69-86, No. 74, J. B. Lippincott Co., USA. |
| Marco et al., International Orthopaedics 17:104-108 (1993). |
| Matsusue, et al., "Arthroscopic Multiple Osteochondral Transplantation to the Chondral Defect in the Knee Associated with Anterior Cruciate Ligament Disruption," Arthroscopy: The Journal of Arthroscopic and Related Surgery, 1993, pp. 318-321, vol. 9, No. 3, Raven Press, Ltd., USA. |
| Maury, et al., "Twenty-five year chondrocyte viability in fresh osteochondral allograft. A Case Report," The Journal of Bone & Joint Surgery, 2007, pp. 159-165, vol. 89, Needham, MA, USA. |
| McDermott et al., Clinical Orthopaedics and Related Research, No. 197, pp. 96-102 (Jul.-Aug. 1985). |
| McPherson, et al., "Creep Behaviour of Osteochondral Grafts in Sheep," 39th Annual Meeting, Orthopaedic Research Society, 1993, p. 227, Alberta, Canada. |
| Meyers et al., The Journal of Bone and Joint Surgery 71-A:704-713 (Jun. 1989). |
| Meyers, Marvin, M.D., "Resurfacing of the Femoral Head with Fresh Osteochondral Allografts," Clinical Orthopaedics and Related Research, 1985, pp. 111-114, No. 197, San Diego, CA, USA. |
| Oakeshott, et al., "A Clinical and Histological Analysis of Failed Fresh Osteochondral Allografts," Clinical Orthopaedics and Related Research, 1988, pp. 283-294, No. 233, Ontario, Canada. |
| Outerbridge, R.E., "Joint surface transplants-a preliminary report," The Journal of the Western Pacific Orthopaedic Association, 1971, pp. 1-15, vol. VIII, No. 1, USA. |
| Pap, et al., "Arthroplasty of the Knee: Experimental and Clinical Experiences," The Journal of Bone & Joint Surgery, 1961, pp. 523-537, vol. 43-A, No. 4, Needham, MA, USA. |
| Pollok et al., Pediatr. Surg. Int. 15:164-167 (1999). |
| Pollok et al., Transplantation Proceedings 29:2131-2133 (1997). |
| Rechenberg, et al., "Changes in subchondral bone in cartilage resurfacing-an experimental study in sheep using different types of osteochondral grafts," OsteoArthritis and Cartilage, 2003, pp. 265-277, vol. 11, Elsevier Science Ltd, Canada. |
| Rodrigo, et al., "Osteocartilaginous Allografts as Compared with Autografts in the Treatment of Knee Joint Osteocartilaginous Defects in Dogs," Clinical Orthopaedics and Related Research, 1978, pp. 342-349, No. 134, J.B. Lippincott Company, USA. |
| Roffman, Moshe, "Autogenous grafting for an osteochondral fracture of the femoral condyle," Acta Orthop Scand, 1995, pp. 571-572, vol. 66, No. 6, Sweden. |
| Schachar, et al., "Fate of massive osteochondral allografts in a feline model," Osteochondral Allografts, Biology, Banking, and Clinical Applications, 1983, pp. 81-101, USA. |
| Schachar, et al., "Metabolic and biochemical status of articular cartilage following cryopreservation and transplantation: a rabbit model," Journal of Orthopaedic Research, 1992, pp. 603-609, vol. 10, Raven Press, New York, USA. |
| Schachar, et al., "The effect of Cryopreservative Agents on the Viability of Frozen Human Articular Cartilage," Canadian Orthopaedic Research Society, 1978, pp. 79-80, Canada. |
| Shahgaldi, et al., "Repair of Cartilage Lesions Using Biological Implants," Journal of Bone & Joint Surgery, 1991, pp. 57-64, vol. 73-B, No. 1, England. |
| Shimizu, et al., "Bone resorption by isolated osteoclasts in living versus devitalized bone," Journal of Bone and Mineral Research, 1990, pp. 411-418, vol. 5, No. 4, Mary Ann Liebert, Inc., Boston, MA, USA. |
| Stevenson et al., The Journal of Bone and Joint Surgery 71-A:1297-1307 (Oct. 1989). |
| Stevenson, et al., "The fate of articular cartilage after transplantation of fresh and cryopreserved tissue antigen-matched and mismatched osteochondral allografts in dogs," The Journal of Bone & Joint Surgery, 1989, pp. 1297-1307, vol. 71-A, No. 9, USA. |
| Toolan, et al., "Development of a novel osteochondral graft for cartilage repair," Novel Ostochondral Graft, 1998, pp. 244-250, John Wiley & Sons, Inc., USA. |
| U.S. Appl. No. 60/024,045, filed Aug. 16, 1996, Reinhold, Schmieding. |
| Volkov, et al., "Use of Allogenous Articular Bone Implants as Substitutes for Autotransplants in Adult Patients," Clinical Orthopaedics and Related Research, 1976, No. 114, pp. 192-202. |
| von Rechenberg et al., OsteoArthritis and Cartilage 11:265-277 (2003). |
| von Rechenberg et al., OsteoArthritis and Cartilage 12:201-216 (2004). |
| von Rechenberg et al., Vet Comp Orthop Traumatol 3:147-156 (2006). |
| Wada, et al., "Architectural remodeling in deep frozen meniscal allografts after total meniscectomy," Arthroscopy: The Journal of Arthroscopic and Related Surgery, 1998, pp. 250-257, vol. 14, No. 3, USA. |
| WO 93/15694, Multi-Phase Bioerodible Implant/Carrier and Method of Manufacturing and Using Same, Publication Date Aug. 19, 1993. |
| WO 96/24302, Surgical Implantation of Cartilage Repair Unit, Publication Date Aug. 15, 1996. |
| WO 96/24310, Multi-Stage Collagen-Based Template or Implant for Use in the Repair of Cartilage Lesions, Publication Date Aug. 15, 1996. |
| WO 96/27333, Apparatus and Methods for Articular Cartilage Defect Repair, Publication Date Sep. 12, 1996. |
| WO 97/46665, Method for Making Cartilage and Implants, Publication Date Dec. 11, 1997. |
| WO 98/56317, Joint Prosthesis, Publication Date Dec. 17, 1998. |
| Wohl, et al., "Assessment of Bone Mechanical Integrity in Osteochondral Grafts," Orthopaedic Research Society, 1994, p. 526, Canada. |
| Yamashita, et al., "The Transplantation of an Autogeneic Osteochondral Fragment for Osteochondritis Dissecans of the Knee," Clinical Orthopaedics and Related Research, 1985, pp. 43-50, No. 201, Japan. |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110070271A1 (en)* | 2004-10-12 | 2011-03-24 | Truncale Katherine G | Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles |
| US8906110B2 (en) | 2007-01-24 | 2014-12-09 | Musculoskeletal Transplant Foundation | Two piece cancellous construct for cartilage repair |
| US20080294270A1 (en)* | 2007-05-24 | 2008-11-27 | Zimmer Orthobiologics, Inc. | Differentially processed tissue and processing methods thereof |
| US20090054906A1 (en)* | 2007-08-24 | 2009-02-26 | Zimmer Orthobiologics, Inc. | Medical device and method for delivering an implant to an anatomical site |
| US8801725B2 (en) | 2008-03-10 | 2014-08-12 | Zimmer Orthobiologics, Inc. | Instruments and methods used when repairing a defect on a tissue surface |
| US20130211536A1 (en)* | 2010-02-18 | 2013-08-15 | Biomet Manufacturing Corporation | Method And Apparatus For Augumenting Bone Defects |
| US9289299B2 (en)* | 2010-02-18 | 2016-03-22 | Biomet Manufacturing, Llc | Method and apparatus for augumenting bone defects |
| US8435305B2 (en) | 2010-08-31 | 2013-05-07 | Zimmer, Inc. | Osteochondral graft delivery device and uses thereof |
| US8753406B2 (en) | 2010-08-31 | 2014-06-17 | Zimmer Inc. | Osteochondral graft delivery device and uses thereof |
| US9113916B2 (en) | 2010-08-31 | 2015-08-25 | Zimmer, Inc. | Drill bit for osteochondral drilling with guiding element and uses thereof |
| EP3838195A1 (en) | 2015-11-25 | 2021-06-23 | Subchondral Solutions, Inc. | Methods, systems and devices for repairing anatomical joint conditions |
| WO2019135216A1 (en) | 2018-01-02 | 2019-07-11 | Cartiheal (2009) Ltd. | Implantation tool and protocol for optimized solid substrates promoting cell and tissue growth |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE274875T1 (en) | 2004-09-15 |
| EP1237511B1 (en) | 2004-09-01 |
| DE50007640D1 (en) | 2004-10-07 |
| ES2230170T3 (en) | 2005-05-01 |
| AU1685701A (en) | 2001-06-25 |
| JP2003516803A (en) | 2003-05-20 |
| US6858042B2 (en) | 2005-02-22 |
| US20030100947A1 (en) | 2003-05-29 |
| EP1498089A1 (en) | 2005-01-19 |
| CA2393776A1 (en) | 2001-06-21 |
| WO2001043667A1 (en) | 2001-06-21 |
| AU783038B2 (en) | 2005-09-15 |
| EP1237511A1 (en) | 2002-09-11 |
| CA2393776C (en) | 2009-02-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| USRE43714E1 (en) | Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints | |
| US6080194A (en) | Multi-stage collagen-based template or implant for use in the repair of cartilage lesions | |
| US8652214B2 (en) | Cartilage replacement implant and method for producing a cartilage replacement implant | |
| EP0808137B1 (en) | Kit for use in repairing cartilage defects | |
| US7662184B2 (en) | Osteoimplant and method for making same | |
| US8795361B2 (en) | Osteochondral plug graft, kit and method | |
| US7427293B2 (en) | Osteochondral plug graft, kit and method | |
| US20070265705A1 (en) | Implant for repairing a cartilage defect | |
| WO2006089359A1 (en) | Replacement bone tissue | |
| AU2005239666B2 (en) | Repairing cartilage defects or cartilage/bone defects in human or animal joints | |
| AU693323C (en) | Multi-stage collagen-based template or implant for use in the repair of cartilage lesions | |
| AU710212C (en) | Fixation method for the attachment of wound repair materials to cartilage defects |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FEPP | Fee payment procedure | Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FPAY | Fee payment | Year of fee payment:12 |