US8821446B2 - Applicators for microneedles - Google Patents
Applicators for microneedlesDownload PDFInfo
- Publication number
- US8821446B2 US8821446B2US12/009,954US995408AUS8821446B2US 8821446 B2US8821446 B2US 8821446B2US 995408 AUS995408 AUS 995408AUS 8821446 B2US8821446 B2US 8821446B2
- Authority
- US
- United States
- Prior art keywords
- applicator
- skin
- configuration
- outer portion
- microprojection array
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0023—Drug applicators using microneedles
Definitions
- This inventionrelates generally to drug delivery and diagnostics performed with the assistance of microneedles or similar systems of microprojections or microprotrusions.
- Microneedle arrayscan facilitate the passage of drugs through human skin and other biological membranes in circumstances where ordinary transdermal administration is inadequate. Microneedle arrays can also be used to sample fluids found in the vicinity of a biological membrane such as interstitial fluid, which is then tested for the presence of biomarkers.
- microneedle arraysIn recent years it has become more feasible to manufacture microneedle arrays in a way that makes their widespread use financially feasible.
- U.S. Pat. No. 6,451,240discloses some methods of manufacturing microneedle arrays. If the arrays are sufficiently inexpensive, for example, they may be marketed as disposable devices. A disposable device may be preferable to a reusable one in order to avoid the question of the integrity of the device being compromised by previous use and to avoid the potential need of resterilizing the device after each use.
- An intravenous injectiondelivers a precise quantity of an active agent to the circulation.
- a subcutaneous or intramuscular injectiondelivers a precise quantity of an active agent into the tissue, but the quantity of active agent delivered to the circulation and the rate at which active ingredient is delivered are affected by the type of surrounding tissue, circulation, and possibly other factors.
- the resulting blood levelsmay exhibit substantial variation among patients due to metabolism and other factors, but minimal therapeutic levels can be assured for most patients, for example, because the speed of metabolism has an upper limit and because there is long experience with the absorption of many drugs from oral formulations.
- Microneedlesmanipulate the permeability of the skin with respect to the active agent. Variability in the permeability enhancement created by different applications of the microneedles will result in variations in the rate of transfer through the skin, the amount transferred through the skin and the bioavailability. Variability of skin permeability enhancement on the application of a microneedle array can result from application on different patients. Particular concern exists, of course, if the enhancement is small in particular patient populations so that the administration of the drug will not produce a therapeutically effective dosing (e.g., adequate blood levels) in those populations. Concern may arise also if the enhancement is sometimes undesirably small in a patient, even if at other times the enhancement is as expected in that patient, depending on details of how and where the microneedle array is applied.
- a typical microneedle arraycomprises microneedles projecting from a base of a particular thickness, which may be of any shape, for example square, rectangular, triangular, or circular.
- the microneedlesthemselves may have a variety of shapes.
- an arraycould be pressed by hand into skin, it has also been proposed to use a variety of devices to hold the microneedle array as it is being applied or to facilitate in one way or another the process of microneedle array application to the skin or other biological membrane.
- Such devicesmay broadly be referred to as “applicators.” Applicators may for example reduce the variations in force, velocity, and skin tension that occur when a microneedle array is pressed by hand into the skin. Variations in force, velocity and skin tension can result in variations in permeability enhancement.
- microneedle arraysmay be applied to the skin or other biological membrane in order to form microchannels and then more or less immediately withdrawn. In other applications the microneedle array may be held in place for a longer period of time.
- the design of the applicatormay naturally be influenced by how long the microneedles are expected to stay in place.
- microneedle arraysparticularly when the arrays are kept in place for a prolonged period of time, devices to transport the drug substance to the skin may be employed.
- a very simple such devicemay, for example, comprise a reservoir for liquid or solid drug substance which is kept in contact with the base, with the liquid drug substance flowing through small apertures in the base or by diffusion when solid drug substance is used.
- Another device suitable for delivering the drug substance to skinis described in U.S. Published Patent Application No. 2005/0094526.
- Rotary applicatorshave been disclosed in U.S. Published Patent Application No. 2004/0087992. There is some disclosure relating to applicators, for example, in U.S. Pat. Nos. 6,537,242, 6,743,211 and 7,087,035.
- a microneedle applicatorwhich has two roughly concentric portions which may be, for example, a solid disk and an annulus surrounding it.
- a microneedle arrayOn the skin-facing side of the inner portion of the applicator a microneedle array is located. The outer portion of the applicator is placed against the skin, contacting it at a zone. The microneedle array is then pressed towards the skin.
- an applicator for a microprojection arraywhich comprises an inner and an outer portion.
- the microprojection arrayattaches to or is integral with the inner portion.
- the outer portioncomprises a contact zone for contacting skin, and the shape of the applicator may be varied between a first and second stable configurations.
- the inner and outer portionsmay be integral with each other. The variation in the shape of the applicator may be used to press microprojections into the skin.
- an expanding microneedle arraywhich comprises elongated members at an angle to the microneedles.
- the members or the attachments between themare sufficiently flexible to allow the microneedles some limited degree of motion which is perpendicular to the needles.
- the ends of the members which in use will be further from the skinare so arranged that they may be pressed towards the skin with the resulting pressure being transmitted to the microneedles, resulting in the microneedles being moved apart as they are pressed into the skin.
- a laminatebonds to the surface of the skin which is to be penetrated with projections.
- the laminateis stiffer than the skin and thinner than the length of the projections.
- the laminatemay be perforated to permit the projections to pass through the holes in the film.
- the laminatemay be continuous and the projections would penetrate it and the skin.
- the laminatemay be a polymer film or a woven or non-woven fabric with an adhesive to attach it to the skin.
- the laminatemay be a liquid that is applied to the skin and forms a film. The laminate normalizes the extensibility of the skin for all patients reducing the effect of patient-to-patient variability on penetration of the skin with the projections.
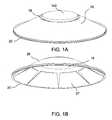
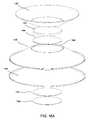
- FIG. 1Aschematically depicts a shape-changing microneedle applicator of the invention as seen from below (i.e., looking at the portion of the application from which the microneedles project).
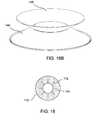
- FIG. 1Bschematically depicts the same applicator in perspective from below with adhesive attached.
- FIG. 1Cdepicts the same applicator in a somewhat different perspective view without adhesive attached.
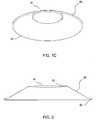
- FIG. 2schematically depicts a similar shape-changing microneedle applicator in cross section.
- FIG. 3Adepicts a different shape-changing microneedle applicator of the invention as seen from above.
- FIG. 3Bdepicts the same applicator in a side view.
- FIG. 3Cdepicts the same applicator as seen looking directly down on it and in cross-sectional view.
- FIG. 4schematically depicts the shapes assumed by the applicators of FIGS. 1A-1C and FIG. 2 when one of them is placed in use.
- FIG. 5schematically depicts an alternate set of shapes which an applicator having the overall shape of FIGS. 1A-1C or FIG. 2 could assume when placed in use.
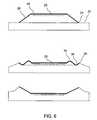
- FIG. 6schematically depicts another set of shapes which an applicator having the overall shape of FIGS. 1A-1C or FIG. 2 could assume when placed in use.
- FIGS. 7A-7Bschematically depict an alternative shape-changing microneedle applicator of the invention.
- FIG. 7Cschematically depicts an application like that of FIGS. 7A-7B with an additional reservoir for fluid to be applied.
- FIG. 7Dis a schematic enlarged view of part of the microneedle array, showing channels through which fluid may pass.
- FIG. 8depicts an approximate force-displacement curve for shape-changing applicators such as those in FIGS. 1A-1C and 2 .
- FIG. 9schematically depicts an expanding microneedle array with elongated members at an angle to the microneedles.
- FIG. 10schematically depicts an alternative embodiment of an expanding microneedle array with elongated members at an angle to the microneedles.
- FIG. 11schematically depicts the operation of a class of devices suitable for tensioning skin as a microneedle array is pressed down on the skin.
- FIG. 12depicts a particular embodiment of the concept set out in FIG. 11 in isometric view.
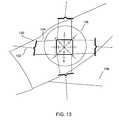
- FIG. 13depicts an arrangement for tensioning skin biaxially in a controlled manner.
- FIG. 14depicts the biaxial strain-stress curves for five human subjects and two thicknesses of silicone rubber, 5 mils and 30 mils.
- FIG. 15(prior art) schematically depicts a general form of the human skin strain-stress curve.
- FIG. 16Aschematically depicts in an exploded view an applicator similar to that of FIGS. 1A-1B with an added component.
- FIG. 16Bschematically depicts the same applicator, not exploded, in perspective.
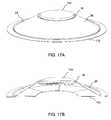
- FIGS. 17A-17Bschematically depict an applicator of the invention, similar to that of FIGS. 1A-1B , with skin adhesive attached.
- FIG. 17Ais a perspective view from above, whereas FIG. 17B is a cross-sectional view.
- FIG. 18schematically depicts a possible form of adhesive for application to skin in connection with certain applicators of the invention.
- FIG. 19A-19Bschematically depict a further embodiment of an applicator of the invention.
- an active ingredientincludes a plurality of active ingredients as well as a single active ingredient
- a temperatureincludes a plurality of temperatures as well as single temperature, and the like.
- microprotrusionsas the type of microprotrusion or microprojection which is being employed. It will be understood by persons of skill in the art that in many cases the same inventive principles apply to the use of other microprotrusions or microprojections to penetrate skin or other biological membranes.
- Other microprotrusions or microprojectionsmay include, for example, microblades as described in U.S. Pat. No. 6,219,574 and Canadian patent application no. 2,226,718, and edged microneedles as described in U.S. Pat. No. 6,652,478.
- the term “downward”is sometimes used to describe the direction in which microprotrusions are pressed into skin, and “upward” to describe the opposite direction.
- the applicatorscan be used where the microprotrusions are pressed into skin at an angle to the direction of the earth's gravity, or even in a direction contrary to that of the earth's gravity.
- microneedles and other protrusions for use with this inventionwill be a function of the manufacturing technology and of the precise application. In general, however, microneedles and other microprotrusions used in practice may be expected to have a length of about 20 to about 1000 microns, more preferably from about 50 to about 750 microns and most preferably from about 100 to about 500 microns. Often it will be desired that the microprotrusions will be long enough to penetrate through the stratum corneum layer of skin at some suitable point of application on the human body, for example the thigh, hip, arm, or torso.
- microneedle arrayfor purposes of this invention is intended to denote a two-dimensional or three-dimensional arrangement of microneedles. The arrangement may be regular according to a repeating geometric pattern or it may be irregular. Similarly, “microprojection array” denotes a two-dimensional or three-dimensional arrangement of microprojections.
- a microneedle applicatorwhich has two roughly concentric portions which may be, for example, a solid disk and an annulus surrounding it.
- a microneedle arrayOn the skin-facing side of the inner portion of the applicator a microneedle array is located. The outer portion of the applicator is placed against the skin, contacting it at a zone. The microneedle array is then pressed towards the skin.
- the outer membermoves upward.
- the zone where the outer member contacts the skinmay have an adhesive or abrasive so that movement of the outer member away from the skin pulls on the skin.
- the microneedle arraymay be pressed sufficiently far down that it lies below the position of the outer member.
- the outer membermay again be provided with an adhesive or abrasive which contacts the skin.
- FIGS. 1A-1Cillustrate an exemplary applicator corresponding to the concentric concept described above.
- FIG. 1Adepicts the applicator as seen from above.
- the outer portioncontacts the skin initially at a contact zone 20 .
- the buttonmay be stiffer than the inner and outer portions of the applicator, for example, by virtue of being thicker.
- FIG. 1Bdepicts the applicator as seen from below, i.e., from the side which is close to skin.
- the microneedle arrayis attached to the underside of the central portion 18 .
- adhesive 27may be attached to part of the underside of the central portion 18 .
- the central and outer portionsare preferably made integrally with each other.
- FIG. 1Cschematically depicts the applicator of FIG. 1A-1B , without adhesive 27 and in a somewhat different perspective view.
- the applicator's outer portion 26has roughly the shape of a truncated cone (frustum of a cone).
- FIG. 2schematically depicts a quite similar applicator in cross-section.
- the applicator's inner member 18 as depicted in FIG. 2has a shape which projects modestly towards the skin.
- the shapehas (in comparison with the applicator of FIGS. 1A-1C ) a more gradually sloping indentation 25 in the center.
- This kind of gradually sloping indentationis useful to allow a slight variability in the pressure exerted on the skin by a microprojection array affixed to the inner member.
- FIG. 3Adepicts a different shape-changing microneedle applicator of the invention as seen from above.
- FIG. 3Bdepicts the same applicator in a side view.
- FIG. 3Cdepicts the same applicator as seen looking directly down on it and in cross-sectional view. It may be seen that the applicator of FIGS. 3A-3C has a shape similar to that of FIGS. 1A-1C except for the presence of eight outwardly pointing members around the edge of the device (for example 114 ) which each have approximately the shape of a quarter cylinder.
- FIG. 4schematically depicts the deformation undergone by the applicators of FIGS. 1A-1C or 2 as they are placed in use.
- the different panels of FIG. 4all show the applicator and the skin 32 to which it is applied in cross section.
- the applicatordeforms elastically.
- a bend 34forms.
- the deformationcontinues until, as the microneedles approach the skin, the relationship between the inner portion of the applicator and the outer member inverts, with the latter extending at an angle away from, rather than towards, the skin.
- the shape change of the applicatormay cause a tensioning of the skin to the extent that the skin moves with it when there is a change of shape.
- the deformed applicatormay be stable in the inverted configuration, so that it remains in that configuration until further force is applied.
- FIG. 5schematically depicts an alternate set of shapes which an applicator having the overall shape of FIGS. 1A-1C or FIG. 2 could assume when placed in use.
- a difference between FIG. 5 and FIG. 4is that the bend 34 has a different directionality. This causes an outer portion of the applicator to remain in contact with the skin in the latter part of the process of deformation.
- FIG. 6schematically depicts another set of shapes which an applicator having the overall shape of FIGS. 1A-1C or FIG. 2 could assume when placed in use.
- a difference between FIG. 6 and FIGS. 4 and 5is that there are two separate bends 34 and 36 in the applicator as depicted in FIG. 6 . With two or more bends, an outer portion of the applicator remains in contact with the skin in the latter part of the process of deformation.
- FIGS. 4-6would generally be expected to require an adhesive applied to some portion of the underside of the outer member.
- a variety of such adhesivesmay be employed.
- a simple two-sided tapesuch as 3M 1513 which contains acrylate with a PET (polyethylene terephthalate) carrier, may be used if cut to an appropriate shape to largely cover a radially outward section of the underside of the outer member, as shown schematically in FIG. 17A .
- an inner member 18 and outer member 26are depicted, as well as a button 142 , much as in FIG. 1A .
- adhesive 170is shown underneath the applicator, touching the skin contacting zone 20 .
- FIG. 17Ba perspective view of the same applicator from below, it is seen that the adhesive 170 is sectioned as in FIG. 18 .
- adhesiveon the outer member of the applicator
- the adhesivefor example may have an annular shape 170 with optional radial cuts such as 172 and 174 , as shown in FIG. 18 .
- the applicatorwould be placed in such a way that the microneedle array approaches the skin through the opening of the annular-shaped adhesive 170 .
- the microneedle arraymay not be attached to the applicator of FIGS. 1A-1C and 2 . Instead, the array may be placed on the skin, and the applicator then placed over the array and caused to invert.
- the outer member of the applicator of FIGS. 1A-1C and 2may have a variety of shapes.
- FIG. 2depicts the outer member as having an approximately straight cross section.
- the cross-sectionmay however alternatively be convex or concave.
- FIG. 16Aschematically depicts in an exploded view an applicator related to that of FIGS. 1A-1C and 2 , which is provided with an additional structure.
- a microneedle array 154(needles are on the lower side which is not visible in the figure). That array is attached via adhesive 152 to the central disk 146 of the shell 148 .
- a skin adhesive 150which is attached to the underside of the shell.
- a further adhesive 144Over the central disc 146 , there is a further adhesive 144 which is used to attach a button or spacer 142 , above which the guide 140 is placed.
- a purpose of the guide 140is to distribute evenly the pressure used to invert the configuration of the shell 148 as it is applied to human skin or another biological membrane, compared to applying pressure directly to the button 142 . This more even distribution of the load is believed to reduce the possibility of the shell 148 buckling asymmetrically.
- the guide 140has a form similar to that of the shell 148 , but is inverted and somewhat smaller.
- FIG. 16Bis a non-exploded schematic view of the applicator of FIG. 16A .
- the guide 140may also permit the applicator to be grasped in different ways which are more convenient for the person applying the microprojection array to skin, for example with thumb and forefinger around its edge.
- a further purpose of the guide 140may be to limit the upward motion of the shell 148 as the applicator inverts configuration.
- the guideis designed so that this is achieved, the applicator's outer portion will contact the guide as the applicator inverts.
- the guide 140under those circumstances serves to define in part the second stable configuration of the applicator.
- FIGS. 7A-7Bschematically depict a different realization of the concentric concept described above.
- FIG. 7Adepicts the applicator prior to use whereas FIG. 7B depicts the applicator in use.
- the upper panelis a perspective view of the applicator whereas the lower panel is a cross section.
- the applicatorcomprises a base which has the shape of a ring 80 .
- the lower side of the ringmay be coated with a suitable adhesive.
- Attached to or integral with that baseis a cylindrical body 82 , which is surmounted by a dome 84 .
- a holder 86 for the microneedlesIn the center of the dome there is a holder 86 for the microneedles, which may also be integral with the remainder of the applicator.
- the applicatoris designed so as to be placed on the skin, to which the adhesive on the ring 80 may adhere. Once the applicator is on the skin, pressure is applied (for example with a finger) to the holder 86 , causing the applicator to invert as shown in FIG. 7B . It may be useful during this application to hold the cylindrical member 82 steady to facilitate the inversion. Upon inversion, the holder 86 contacts the skin, causing the microneedles to penetrate it.
- the applicatormay be so designed that in the inverted position, the holder 86 is slightly below the level of the ring 80 , as shown schematically (not to scale) in FIG. 7B .
- the tendency of the holder 86 to go slightly below the level of the ringmay cause a modest doming of the skin which tensions the skin between the ring and the holder. Such tensioning can facilitate the entry of the microneedles into skin.
- the device described abovemay be used to apply a microneedle array which is not attached to the holder 86 .
- the microneedle arraycan be positioned on the skin or anywhere along the path of the holder as it travels towards the skin. During the change of configuration the holder may exert force on the microneedle array and press it into the skin.
- FIGS. 7A-7BWhile the applicator of FIGS. 7A-7B has been depicted as placing the holder 86 slightly below the level of the ring, it will be understood that the precise position of the holder 86 in use may be adjusted as desired relative to the level of the ring.
- FIG. 7Cwe see the applicator of FIGS. 7A-7B provided with a reservoir 87 , which may be used, for example, for liquids comprising a drug substance.
- the microneedle arraywhen attached to holder 86 , has the needles pointing downward, so that if there is fluid in reservoir 87 , it can flow downward to the channels in the skin created by the microneedle array.
- FIG. 7Dwe see a view of a portion of a microneedle array.
- the arrayhas a base 180 from which microneedles such as 182 project.
- the basehas channels such as 184 and 186 which would permit the flow of liquid from a reservoir such as 87 to the channels in the skin created by the microneedle array.
- a microneedle arraywithout the channels like 184 or 186 in which the drug substance is able to diffuse through the base of the array under conditions of use, or a microneedle array which contains or is coated with drug substance.
- a reservoir such as 87may be supplied with manual or automated equipment (e.g., pumping equipment) for causing transport of drug substance towards the skin.
- Automated equipmentmay be programmed for bolus, sustained, pulsatile, or continuous drug delivery.
- Theremay also be equipment for causing transport of a biological fluid from the skin where the microprojections have penetrated and created microchannels towards the reservoir or towards analytical equipment. Examples of such arrangements are given in U.S. Pat. No. 6,591,124.
- the inversion of configurationwhich is characteristic of certain applicators of the invention may be achieved with manual pressure applied, for example, with the fingers.
- a toolmay be provided which facilitates through leverage or otherwise the application of a pressure suitable for achieving inversion of configuration.
- FIG. 8depicts an approximate load-displacement curve for shape-changing applicators such as those in FIGS. 1A-1C , 2 , and 16 A- 16 B.
- the loadvertical axis
- the displacementhorizontal axis
- FIG. 8was calculated using an approximate formula for the behavior of a Belleville spring which is given in Raymond J. Roark and Warren C. Young, Formulas for Stress and Strain art. 10.8 (5th ed. 1975).
- a threshold force required to make the applicator change configurationmay be between about 0.1 lbf ( ⁇ 0.0449 N) and about 20 lbf ( ⁇ 89 N), more commonly between about 1 lbf and about 10 lbf.
- the required forcemay be dependent on the size of the applicator, and thus more conveniently expressed in units of force per applicator area, for example newtons per square cm, which may range for example from about 0.01 N/cm 2 to 20 N/cm 2 , more commonly 0.1 N/cm 2 to 10 N/cm 2 .
- area applicator areamay conveniently be measured projected on a horizontal plane.
- Applicator design and force appliedmay affect the velocity with which the microprojection array impacts the skin.
- a velocitymay be greater than about 0.01 meters per second (m/s), greater than about 0.05 m/s, about 0.1 m/s, about 0.5 m/s, or about 1 m/s.
- Such a velocitymay be less than about 0.1 m/s, about 0.5 m/s, about 1 m/s, about 2 m/s, about 3 m/s, about 5 m/s, about 10 m/s, or about 20 m/s.
- the applicators of the inventionmay be made of a variety of materials which possess the mechanical properties required, particularly the ability to invert configuration.
- Polymers believed to be suitable for the member or members which invert configurationinclude polyethylene terephthalate (PET), glycol-modified polyethylene terephthalate (PETG), high-impact polystyrene (HIPS), polycarbonate, polyvinylchloride (PVC), polyurethane, polyethylene, polypropylene, silicone rubber, polymethyl methacrylate (PMMA), polyvinyl alcohol (PVA), acrylonitrile-butadiene-styrene (ABS), ethylene vinyl acetate (EVA), phenylene oxide, polysulfones, or natural rubber.
- the member or members which invert configurationmay also be made of laminates, of spring steel or similar alloys, or of composite materials, for example composite materials designed to exhibit a degree of shape memory.
- the applicatorsmay be made by a variety of techniques, such as for example vacuum forming, compression molding, injection molding, casting or stamping.
- biocompatible adhesivesmay be used for attachment of applicators to the skin, for example acrylate adhesives, synthetic rubber adhesives, PIB (polyisobutylene) adhesives, silicone adhesives, and urethanes.
- a variety of abrasivesmay also be used, including for example silicon carbide, aluminum oxide, diamond, cubic zirconium, garnet and cubic boron nitride.
- the applicatorwill protect the tips of the microneedles from damage during handling, shipping, storage and transport.
- the applicators shown above and others of similar designit is believed that as pressure is applied to change them from one stable state to another, the applicators serve as a mechanical fuse. Once a threshold force is reached, the device rapidly inverts permitting the potential energy stored in the force suspended above the skin to be converted into kinetic energy. The portion of the device which holds the microneedle array may tend to impact the microneedles against the skin because the device does not invert until a threshold force is applied and because once the microneedle array holder has deflected past the outer member or members, the energy stored in the device during the application of pressure is released and accelerates the motion of the portions of the device until they reach the inverted configuration.
- the applicatormay be desirable for the applicator to make a sudden noise when it inverts, since that noise provides audible positive feedback to patient or caregiver that the microneedles have been inserted. Inversion of the applicator provides in any event visual positive feedback to patient or caregiver that the microneedles have been inserted. After the applicator has inverted, the applicator may be left in place holding the microneedles in the skin so the drug has ample time to dissolve, for example if it is coated on the microneedles or contained in the microneedles. A color or transparency change may also be used to provide visual feedback in addition to or in lieu of an audible feedback.
- the microneedle arraymay be desired to press the microneedle array one time against the skin. In other uses it may be desired to press the array into the skin a number of times, for example to deliver an active ingredient or other liquid multiple times at same or different rates to the skin through the channels created by the microneedles.
- a microneedle arraywhich comprises elongated members at an angle to the microneedles.
- the members or the attachments between themare sufficiently flexible to allow the microneedles some limited degree of motion which is perpendicular to the needles.
- the ends of the members which in use will be further from the skinare so arranged that they may be pressed towards the skin with the resulting pressure being transmitted to the microneedles, resulting in the microneedles being moved apart as they are pressed into the skin.
- FIG. 9depicts an exemplary embodiment of the microneedle array with elongated members at an angle to the microneedles.
- each microneedleis connected to three other microneedles.
- microneedle 42is connected to microneedles 40 , 44 , and 46 .
- Adjacent microneedlesmove in paths controlled by the links relative to their adjacent microneedles.
- microneedles 40 , 44 , and 46have their bottoms in the plane further from the skin, whereas microneedles 42 and 48 have their bottoms in the plane which is closer to the skin.
- FIG. 9there are elongated members such as 50 which are at an angle to the microneedles.
- the microneedles' basesare connected to the elongated members, as at link 52 .
- a microneedle array of the type shown in FIG. 9would be subjected to pressure on the bases of the microneedles further away from the skin. This pressure would be transmitted to the microneedles whose bases are located in the plane closer to the skin. Those microneedles would press into the skin, penetrating it. The elongated members would also, however, expand and flatten the array as a result of that pressure, spreading the microneedles which are closer to the skin apart from each other, and thus tensioning the skin. The microneedles which are further away from the skin may then penetrate the skin.
- the ends of the links in the plane nearest the skinmay have no microneedles and may be coated with an adhesive. The microneedles are found only in the plane further from the skin.
- the needles on the plane further from the skinmay extend beyond the ends of the links in the nearer plane in order to contact the skin before the nearer plane does.
- FIG. 10schematically depicts a variation on the concept of an elongated member at an angle.
- a microneedle arraywhich is split into two major groups 60 and 62 of microneedles.
- Each major grouphas a base, the two bases being joined by members such as 64 .
- memberssuch as 64 .
- These elongated membersare flexible or flexibly attached to the bases.
- the first microneedles to touch the skinare those at the ends of elongated members such as 66 and 68 .
- the flexibility or flexible coupling of these elongated membersmeans that their ends move both into the skin and also outward away from the bases of the two groups of microneedles 60 and 62 . This outward motion results in a tensioning of the skin in the area in which the bulk of the microneedles will contact, thus facilitating a greater uniformity in the permeation enhancement accomplished by those microneedles.
- FIG. 10is highly schematic.
- microneedlesare represented as simple straight lines, whereas of course they can have complex shapes as shown in the literature.
- the exact dimensions of the elongated members projecting out from the bases of the two major groups 60 and 62 , and their exact angles relative to those bases,are not intended to be depicted by FIG. 10 , which is not to scale.
- FIG. 10Alternatives to FIG. 10 are possible in which, rather than dividing the bulk of the microneedles into two major groups, they are divided into more such groups each with a separate base, but with all or some of the bases connected together. A number of patterns of interconnections of the bases may be employed. The number of microneedles in each of the major groups may be more or less than the number which is schematically depicted in FIG. 10 .
- the elongated members in FIG. 10point in just two directions which are opposite to each other, but an array may be designed to have elongated members pointing in multiple non-collinear directions, for example pointing out at angles of 0, 90, 180, and 270 degrees with respect to some reference.
- the arraysmay have, for example, holes for the passage of active ingredient.
- the arraysmay comprise reservoirs for active agent or may be held by an applicator which contains one or more active ingredient reservoirs.
- the shape of the microneedlesmay be varied according to what is known in the art about microneedle shapes.
- the arrays of FIG. 10may also be fabricated by a variety of techniques as discussed above, for example casting, molding, and embossing.
- a microneedle applicatorwhich has a member which undergoes deformation at a particular applied pressure.
- This deformable memberhelps to ensure more uniform application of the microneedle array on account of the uniformity of the applied pressure achieved by providing visual feedback to the person applying the pressure or by stopping the application of the further pressure through the deformation.
- An exemplary embodimentis depicted schematically in FIGS. 19A-19B .
- the “dome” shaped member 190 in that embodimentis designed to deform at a particular reproducible applied pressure, allowing more uniform application of the microneedle array 192 .
- an applicatormay be provided which, while it does not necessarily have two stable configurations like the applicators of FIGS. 1A-1C , 2 , and 3 A- 3 B, will change shape under pressure in a way which facilitates the application of the microneedle array to skin.
- Such an applicatormay be suitable, for example, where it is desired to apply the microneedle array once and then withdraw it. In such a situation, or in other situations, it may be convenient to maintain the pressure that changes the applicator's shape during the entire time that the microneedle array is in place penetrating the skin.
- a general principleis to have projections from the microneedle array base or member that holds the microneedle array which contact skin in at least two opposite directions in an area of the projections which we may call a contact zone.
- the contact zonemay be designed in such a way as to grasp skin easily, as for example by being coated with a pressure-sensitive adhesive.
- the projectionsexperience an outward force which causes them to push the skin outwards.
- a convenient arrangementis one in which these projections are arranged symmetrically about the microneedle array base. There may for example be four projections acting in four directions, or six projections acting in six directions.
- An exemplary devicedepicted in schematic cross section in FIG. 11 , comprises a base 98 with two or more legs such as 90 radiating from it at an angle (preferably 30 to 60 degrees) to the longitudinal axis.
- the legsare permitted to pivot about the points such as 104 at which they are connected to the base.
- the other end of each legcontacts or is connected to other components that contact the skin 102 .
- the device of FIG. 11is initially in the form depicted in the upper portion of that figure.
- the horizontal areas at the ends of the legs such as 90are in contact with skin 102 .
- the microneedle arrayis attached or integral with the base. Downward pressure is then applied. That causes the microneedle array to move towards the skin, while simultaneously causing the legs such as 90 to move outward. With suitable friction or adhesion between the horizontal ends of the legs and the skin which those horizontal ends contact, the outward movement of the device is expected to cause a tensioning of the skin.
- a device along the lines of FIG. 11may be designed in such a way that the distance between the ends of each leg can decrease once a specific force per unit length (preferably 5 to 40 g/mm, more preferably 5 to 20 g/mm, more preferably 5 to 10 g/mm) is applied along the plane of the skin.
- This forceis measured biaxially; a particular technique for measurement is given in Example 1 below.
- the desirable ranges of forceare believed to be such as to remove creases and redundancy from the skin without actually putting it into a range in which its strain-stress relationship is linear.
- Mechanismssuch as curved sections, pneumatic cylinders, accordioned sections, coil springs, leaf springs or elastomer springs can be used in the legs to make them deflect or deform at a particular load.
- FIG. 12depicts in a schematic isometric view a particular embodiment of the concept which is set out in FIG. 11 .
- FIG. 12we see that there are four curved legs 90 , 92 , 94 , and 96 .
- Each leghas a flat area which may be adhered to skin.
- Pressure on the inner portion 98 of the device towards the skincauses the legs 90 , 92 , 94 , and 96 to extend away from the center. Friction or adhesion to the skin will result in the skin being tensioned as the legs pull away.
- the four corners (e.g., 100 ) in the embodiment of FIG. 12are slit so that they may move apart as pressure is applied to the applicator.
- an adhesivecan be added to the inner portion 98 of the device to hold it in direct contact with the skin for some period of time after downward pressure is removed.
- a skin laminatemay be used.
- the laminatemay be stiffer than the skin and thinner than the length of the needles.
- the laminatemay be perforated to permit the needles to pass through the holes in the laminate.
- the laminatemay be continuous and the needles would penetrate it and the skin.
- the laminatemay be a polymer film or a woven or non-woven fabric with an adhesive to attach it to the skin.
- the laminatemay be a liquid that is applied to the skin and forms a film.
- the laminatemay also help to prevent microbes on the surface of the skin from entering the pathways created in the skin by the projections.
- an antimicrobial activemay be administered together with the active of interest.
- the laminatemay act as reservoir of active drug substance.
- the laminatemay comprise, for example, polyimide-1, vinyl pyrrolidone (VP), polyvinyl pyrrolidone (PVP), a lauryl methacrylate copolymer, albumin, HPMC (hydroxypropyl methylcellulose), copolyvidone (KOLLIDON® VA 64), acrylates, cyanoacrylates, nitrocellulose, pyroxylin, polyphenylmethylsiloxane, polyethylene terephthalate, high density polyethylene, ethylene vinyl acetate, polyurethanes, or blends of polymers in these classes.
- VPvinyl pyrrolidone
- PVPpolyvinyl pyrrolidone
- lauryl methacrylate copolymeralbumin
- HPMChydroxypropyl methylcellulose
- copolyvidoneKLLIDON® VA 64
- acrylatescyanoacrylates
- nitrocellulosepyroxylin
- polyphenylmethylsiloxanepolyethylene terephthalate
- the microneedle arraysmay be attached to the applicators with an adhesive.
- the microneedle arraysmay be fabricated with protuberances suitable for facilitating the attachment of the arrays to the applicator.
- the microneedle arraysmay be welded to the applicator.
- Suitable active agentsinclude the broad classes of compounds such as, by way of illustration and not limitation: analeptic agents; analgesic agents; antiarthritic agents; anticancer agents, including antineoplastic drugs; anticholinergics; anticonvulsants; antidepressants; antidiabetic agents; antidiarrheals; antihelminthics; antihistamines; antihyperlipidemic agents; antihypertensive agents; anti-infective agents such as antibiotics, antifungal agents, antiviral agents and bacteriostatic and bactericidal compounds; antiinflammatory agents; antimigraine preparations; antinauseants; antiparkinsonism drugs; antipruritics; antipsychotics; antipyretics; antispasmodics; antitubercular agents; antiulcer agents; anxiolytics; appetite suppressants; attention deficit disorder and attention deficit hyperactivity disorder drugs; cardiovascular preparations including calcium channel blockers,
- certain drug substancese.g., nitroglycerin
- Other drug substanceswill transport through skin with greater difficulty and, with a practical-sized system for application, only with the assistance of enhancers.
- Other substancesare not suitable for transdermal administration even with available enhancers and thus benefit particularly from the channels which microneedles are able to produce.
- Such substancesinclude, for example, peptidic or other large molecule substances for which oral administration is also not an option.
- peptides and proteinswhich may be used with microneedle arrays are oxytocin, vasopressin, adrenocorticotropic hormone (ACTH), epidermal growth factor (EGF), prolactin, luteinizing hormone, follicle stimulating hormone, luliberin or luteinizing hormone releasing hormone (LHRH), insulin, somatostatin, glucagon, interferon, gastrin, tetragastrin, pentagastrin, urogastrone, secretin, calcitonin, enkephalins, endorphins, kyotorphin, taftsin, thymopoietin, thymosin, thymostimulin, thymic humoral factor, serum thymic factor, tumour necrosis factor, colony stimulating factors, motilin, bombesin, dinorphin, neurotensin, cerulein, bradykinin, urokinase,
- Peptidyl drugsalso include synthetic analogs of LHRH, e.g., buserelin, deslorelin, fertirelin, goserelin, histrelin, leuprolide (leuprorelin), lutrelin, nafarelin, tryptorelin, and pharmacologically active salts thereof.
- LHRHpharmacologically active salts thereof.
- Macromolecular active agents suitable for microneedle array administrationmay also include biomolecules such as antibodies, DNA, RNA, antisense oligonucleotides, ribosomes and enzyme cofactors such as biotin, oligonucleotides, plasmids, and polysaccharides.
- Oligonucleotidesinclude DNA and RNA, other naturally occurring oligonucleotides, unnatural oligonucleotides, and any combinations and/or fragments thereof.
- Therapeutic antibodiesinclude ORTHOCLONE OKT3® (muromonab-CD3), REOPRO® (abciximab), RITUXAN® (rituximab), ZENAPAX® (daclizumab), REMICADE® (infliximab), SIMULECT® (basiliximab), SYNAGIS® (palivizumab), HERCEPTIN® (trastuzumab), MYLOTARG® (gemtuzumab ozogamicin), CROFAB® (Crotalidae Polyvalent Immune Fab Ovine), DIGIFAB® (Digoxin Immune Fab Ovine), CAMPATH® (alemtuzumab), and ZEVALIN® (ibritumomab tiuxetan).
- ORTHOCLONE OKT3®muromonab-CD3
- REOPRO®abciximab
- RITUXAN®rituxim
- Microprojection arraysare advantageously used for the delivery of a variety of vaccines.
- These vaccinesmay include, for example, those approved in the United States for use against anthrax, diphtheria, hepatitis A, hepatitis B, Haemophilus influenzae type b, human papillomavirus, influenza, Japanese encephalitis, Lyme disease, measles, meningococcal and pneumococcal diseases, mumps, pertussis, polio, rabies, rotavirus, rubella, shingles, smallpox, tetanus, tuberculosis, typhoid, varicella, and yellow fever.
- the vaccines being deliveredcan comprise live attenuated or killed bacteria, live attenuated viruses, subunit vaccines, conjugate vaccines, synthetic vaccines, viral vectors, polysaccharide vaccines, and DNA vaccines.
- vaccineswhich may be delivered by means of microprojection arrays may include vaccines (believed to be presently under development) directed against avian (pandemic) influenza virus, Campylobacter sp., Chlamydia sp., Clostridium botulinum, Clostridium difficile , dengue fever virus, E.
- the drug substancemay be coated onto the microneedle arrays. It may be placed in a reservoir from which the drug substance travels to the skin, for example, through the base of the microneedle array, e.g., via small channels formed in the base.
- the drug reservoirmay be part of a button used in pressing the microneedle array against the skin.
- Fluid pressuremay be used, for example, to cause the drug substance to travel from the reservoir.
- Such fluid pressuremay be generated by manual or finger pressure against a flexible wall enclosing the reservoir.
- the fluid pressuremay be generated by a gas-forming chemical reaction in an enclosed area.
- the fluid pressuremay alternatively be generated by a pump such as a bulb pump used, for example, when measuring blood pressure, or an electrically operated pump.
- a pumpsuch as a bulb pump used, for example, when measuring blood pressure, or an electrically operated pump.
- an external source of compressed air or other compressed fluidmay be used to provide a fluid pressure which serves to cause the drug substance to travel from the reservoir to the skin.
- Iontophoresis and electroosmosisbriefly described below, may also be employed to transport the drug substance to the skin where it may further penetrate by means of the channels created by the microneedle arrays.
- the drug substancemay be dissolved or suspended in the microneedles themselves.
- the drug substancemay also be in solid form, for example as a powder, and may during the process of use be mixed with a suitable liquid for transmission to the body.
- the reservoirmay be made of a wide variety of materials. It may, for example, simply be an enclosed area in which the drug substance is stored in a suitable liquid solution.
- the solutionmay, for example, be designed to facilitate absorption under the circumstances of the administration with partial, potentially healing channels existing in the skin.
- the reservoirmay alternatively be a polymeric matrix within which the drug substance is located and from which it may diffuse outwards and towards the skin. Instead of a polymeric matrix, the reservoir could for example comprise a nonwoven material or material of small dimensions onto which a solution comprising the drug substance may adsorb.
- the reservoirmay also encompass, for example, a hydrogel.
- permeation enhancersmay be included in a reservoir together with the drug substance and potentially transport into skin with the active drug substance.
- substancesmay be, for example, permeation enhancers.
- Information about permeation enhancersmay be found, for example, in Tapash K. Ghosh et al., Transdermal and Topical Drug Delivery Systems chapter 11 (Interpharm Press 1997).
- Exemplary permeation enhancersinclude, by way of illustration and not limitation, sulfoxides such as dimethylsulfoxide and decylmethylsulfoxide; ethers such as diethylene glycol monoethyl ether and diethylene glycol monomethyl ether; surfactants such as sodium laurate, sodium lauryl sulfate, cetyltrimethylammonium bromide, benzalkonium chloride, Poloxamer (231, 182, 184), TWEEN® (20, 40, 60, 80) (polysorbates) and lecithin; the 1-substituted azacycloheptan-2-ones, particularly 1-n-dodecylcyclazacycloheptan-2-one; alcohols such as ethanol, propanol, octanol, decanol, benzyl alcohol, and the like; fatty acids such as lauric acid, oleic acid and valeric acid; fatty acid esters such as isopropyl myr
- compositions designed to retard the healing of the channels created in the skin by the microneedlesmay include compositions designed to retard the healing of the channels created in the skin by the microneedles. Certain compositions indicated to have that effect are disclosed in U.S. Published Patent Application No. 2002/0102292.
- the drug substancemay be formulated in a variety of other ways for administration through a microneedle array.
- the pH of the formulationmay be controlled, as for example with glycerol buffers, citrate buffers, borate buffers, phosphate buffers or citric acid-phosphate buffers. It may be found convenient, for example, to include a component in the formulation which facilitates the wetting of the microneedle array by a liquid solution comprising the drug substance.
- the formulationmay also include agents commonly employed to prolong the shelf life of pharmaceutical preparations.
- antimicrobial agentsmay be employed. Suitable antimicrobial agents are typically selected from the group consisting of the methyl and propyl esters of p-hydroxybenzoic acid (i.e., methyl and propyl paraben), sodium benzoate, sorbic acid, imidurea, proteins (i.e., lysozyme), silver salts, and combinations thereof.
- Agents employed to protect the formulation against degradationmay also include, for example, antioxidants such as primary antioxidants which are peroxy free radical scavengers and secondary antioxidants which induce decomposition of hydroperoxides, and thus protect a material from degradation by hydroperoxides.
- antioxidantssuch as primary antioxidants which are peroxy free radical scavengers and secondary antioxidants which induce decomposition of hydroperoxides, and thus protect a material from degradation by hydroperoxides.
- primary antioxidantsare tetrakis [methylene (3,5-di-tert-butyl-4-hydroxyhydrocinnamate)]methane (e.g., IRGANOX®1010, from Ciba-Geigy Corp., Hawthorne, N.Y.) and 1,3,5-trimethyl-2,4,6-tris [3,5-di-t-butyl-4-hydroxy-benzyl]benzene (e.g., ETHANOX®330, from Ethyl Corp.).
- IRGANOX®101010tetrakis [methylene (3,5-di-tert-butyl-4-hydroxyhydrocinnamate)]methane
- IRGANOX®1010101,3,5-trimethyl-2,4,6-tris [3,5-di-t-butyl-4-hydroxy-benzyl]benzene
- ETHANOX®330from Ethyl Corp.
- secondary antioxidantsinclude tris(2,4-di-tert-butylphenyl)phosphite (e.g., IRGAFOS®168, Ciba-Geigy Corp.).
- suitable antioxidantsinclude, for example, ascorbic acid, ascorbic palmitate, tocopherol acetate, propyl gallate, butylhydroxyanisole, butylated hydroxytoluene, IRGANOX®E17 (Ciba-Geigy), IRGANOX®1520 D (Ciba-Geigy), bis(1,2,2,6,6-pentamethyl-4-piperidinyl)-(3,5-di-tert-butyl-4-hydroxybenzyl)butylpropanedioate, (available as TINUVIN®144 from Ciba-Geigy Corp.) or a combination of octadecyl 3,5-di-tert-butyl-4-hydroxyhydrocin
- An applicator of the inventionmay advantageously be combined, for example, with an arrangement to vibrate the microneedle array in order to facilitate insertion.
- the arraymay be vibrated, for example, manually, or with the aid of a manually activated vibrating device, for example one operating on the principle of the tuning fork.
- the microneedle arraymay be vibrated by means of an electric motor, or (usually at a higher frequency such as an ultrasound frequency) by means of a piezoelectric transducer which may be coupled to either the applicator or the array itself.
- the vibrationmay take place during the process of microneedle array insertion, or alternatively the vibration may take place occasionally or episodically after the microneedle array has been inserted, in order to improve the flow characteristics of the channels created by the array, for example when a temporary boost in the rate of transport of drug substance is desired.
- the applicators of the inventionmay also be designed for use in iontophoresis.
- Iontophoresisis a well-known noninvasive technique that may be used to deliver a compound of interest to, or to extract a compound of interest from, a body tissue of a patient.
- two iontophoretic electrodesare placed on a body tissue, typically the skin or mucosa, in order to complete an electrical circuit. At least one of the electrodes is considered to be an active iontophoretic electrode, while the other may be considered as a return, inactive, or indifferent electrode.
- the compound of interestis transported at the active electrode across the tissue as a permeant when a current is applied to the electrodes through the tissue.
- Compound transportmay occur as a result of a direct electrical field effect (e.g., electrophoresis), an indirect electrical field effect (e.g., electroosmosis), electrically induced pore or transport pathway formation (electroporation), or a combination of any of the foregoing.
- a direct electrical field effecte.g., electrophoresis
- an indirect electrical field effecte.g., electroosmosis
- electroly induced pore or transport pathway formationelectroroporation
- the applicators of the inventionare packaged together with a microneedle array and associated reservoir to form a kit comprising at least an applicator and an array.
- the applicatormay be a separate and reusable entity into which a number of microneedle arrays and reservoirs are inserted one after another for application. In such circumstances it may be desired that there be a fixed procedure for sterilizing the applicator between uses, for example autoclaving or washing in a sterile bactericidal liquid.
- a kitmay be designed to maintain the array, or the array and the applicator, sterile.
- a sterilitymay be desired which is comparable to that specified for parenteral dosage forms.
- the packaging for the kit componentscould be constructed from flexible polyvinylchloride or polyolefin thermoplastics of the type used for packaging substantial volumes of intravenous solution.
- the array and/or applicatorcould also be packaged, for example, in sterile stoppered glass, in a laminated foil pouch, or in plastic containers.
- Remingtonthe Science and Practice of Pharmacy (20th ed., Alfonso R. Gennaro ed., Lippincott Williams & Wilkins 2000), chapters 40 and 41, for further information about the formulation of sterile dosage forms and their packages.
- an applicatorchanges configuration and is intended for use with multiple microneedle arrays, it is desirable that the applicator be able to move back and forth from one configuration to another repeatedly without degradation, for example a minimum of about 2 times, about 10 times, about 50 times, or about 100 times.
- a mechanismis constructed for exercising a controlled force in a biaxial way along four perpendicular directions. Such a mechanism is depicted in FIG. 13 .
- Four small metal cylinders such as 130are attached through strings such as 132 to weights (not shown).
- the cylindersare also attached to thin polymeric films such as 134 , which are in turn attached (e.g., via an adhesive) to the skin of a human subject.
- the weightsserve to provide a controllable tension which is biaxial.
- a transparent overlay 138is used to more easily measure strain.
- a base 136is provided to hold the subject's arm and to guide (for example with pulleys) the strings such as 132 which connect the metal cylinders such as 130 to the weights.
- strain-strain curveswere measured for five human subjects, giving the results depicted in FIG. 14 .
- Strainis calculated using the change in the square root of the area of the specimen, i.e., as (sqrt(final area) ⁇ sqrt(initial area))/sqrt(initial area). The figure gives an idea of the variability in the strain-stress curve for human skin.
- Strain-stress curveswere also measured for two thicknesses of silicone rubber. The purpose of this measurement was to correlate the silicone rubber stress-strain curve with that of the human subjects in order to use the silicone rubber as a test bed for different shapes of applicators.
- the applicatoris applied to silicone rubber as if it were human skin.
- the force exerted by the applicatormay be estimated from the strain which it causes on the silicone rubber.
- the stress-strain curves of FIG. 14may be compared with the generalized human skin strain-stress curve of FIG. 15 , which is taken from G. L. Wilkes et al., “The biomechanical properties of skin,” CRC Critical Reviews in Bioengineering, 1:453-495 (1973).
- the curves of FIG. 14tend to correspond to the bottom part of the generalized curve of FIG. 15 , approximately in the portion of that figure which is labeled Phase 2. This may be a desirable area of the strain-stress curve in which to operate for purposes of applying microneedle arrays.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dermatology (AREA)
- Medical Informatics (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Abstract
Description
Claims (28)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/009,954US8821446B2 (en) | 2007-01-22 | 2008-01-22 | Applicators for microneedles |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US88190507P | 2007-01-22 | 2007-01-22 | |
| US12/009,954US8821446B2 (en) | 2007-01-22 | 2008-01-22 | Applicators for microneedles |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20080183144A1 US20080183144A1 (en) | 2008-07-31 |
| US8821446B2true US8821446B2 (en) | 2014-09-02 |
Family
ID=39523503
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/009,954ActiveUS8821446B2 (en) | 2007-01-22 | 2008-01-22 | Applicators for microneedles |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8821446B2 (en) |
| EP (1) | EP2121111B1 (en) |
| JP (2) | JP5553612B2 (en) |
| AU (1) | AU2008209537B2 (en) |
| CA (1) | CA2676221C (en) |
| WO (1) | WO2008091602A2 (en) |
Cited By (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| USD726316S1 (en)* | 2013-10-23 | 2015-04-07 | Claire A. Marquez | Micro-needling tattoo head |
| US9114238B2 (en) | 2007-04-16 | 2015-08-25 | Corium International, Inc. | Solvent-cast microprotrusion arrays containing active ingredient |
| US9381680B2 (en) | 2008-05-21 | 2016-07-05 | Theraject, Inc. | Method of manufacturing solid solution perforator patches and uses thereof |
| US9498524B2 (en) | 2007-04-16 | 2016-11-22 | Corium International, Inc. | Method of vaccine delivery via microneedle arrays |
| US9730624B2 (en) | 2009-03-02 | 2017-08-15 | Seventh Sense Biosystems, Inc. | Delivering and/or receiving fluids |
| US9775551B2 (en) | 2009-03-02 | 2017-10-03 | Seventh Sense Biosystems, Inc. | Devices and techniques associated with diagnostics, therapies, and other applications, including skin-associated applications |
| US9944019B2 (en) | 2012-05-01 | 2018-04-17 | University of Pittsburgh—of the Commonwealth System of Higher Education | Tip-loaded microneedle arrays for transdermal insertion |
| US9962534B2 (en) | 2013-03-15 | 2018-05-08 | Corium International, Inc. | Microarray for delivery of therapeutic agent, methods of use, and methods of making |
| US10188335B2 (en) | 2011-04-29 | 2019-01-29 | Seventh Sense Biosystems, Inc. | Plasma or serum production and removal of fluids under reduced pressure |
| US10195409B2 (en) | 2013-03-15 | 2019-02-05 | Corium International, Inc. | Multiple impact microprojection applicators and methods of use |
| US10226585B2 (en) | 2014-10-01 | 2019-03-12 | Allergan, Inc. | Devices for injection and dosing |
| US10245422B2 (en) | 2013-03-12 | 2019-04-02 | Corium International, Inc. | Microprojection applicators and methods of use |
| US10265477B2 (en) | 2013-05-23 | 2019-04-23 | Allergan, Inc. | Mechanical syringe accessory |
| US10384046B2 (en) | 2013-03-15 | 2019-08-20 | Corium, Inc. | Microarray for delivery of therapeutic agent and methods of use |
| US10384045B2 (en) | 2013-03-15 | 2019-08-20 | Corium, Inc. | Microarray with polymer-free microstructures, methods of making, and methods of use |
| US10433928B2 (en) | 2015-03-10 | 2019-10-08 | Allergan Pharmaceuticals Holdings (Ireland) Unlimited Company | Multiple needle injector |
| US10441768B2 (en) | 2015-03-18 | 2019-10-15 | University of Pittsburgh—of the Commonwealth System of Higher Education | Bioactive components conjugated to substrates of microneedle arrays |
| USD865950S1 (en) | 2017-03-24 | 2019-11-05 | Allergan, Inc. | Syringe device |
| US10543310B2 (en) | 2011-12-19 | 2020-01-28 | Seventh Sense Biosystems, Inc. | Delivering and/or receiving material with respect to a subject surface |
| US10596321B2 (en) | 2016-04-08 | 2020-03-24 | Allergan, Inc. | Aspiration and injection device |
| US10624843B2 (en) | 2014-09-04 | 2020-04-21 | Corium, Inc. | Microstructure array, methods of making, and methods of use |
| US10792427B2 (en) | 2014-05-13 | 2020-10-06 | Allergan, Inc. | High force injection devices |
| US10835163B2 (en) | 2011-04-29 | 2020-11-17 | Seventh Sense Biosystems, Inc. | Systems and methods for collecting fluid from a subject |
| US10857093B2 (en) | 2015-06-29 | 2020-12-08 | Corium, Inc. | Microarray for delivery of therapeutic agent, methods of use, and methods of making |
| US11052231B2 (en) | 2012-12-21 | 2021-07-06 | Corium, Inc. | Microarray for delivery of therapeutic agent and methods of use |
| USD926966S1 (en) | 2018-10-30 | 2021-08-03 | Medrx Co., Ltd. | Package for containing a micro needle patch |
| US11167119B2 (en)* | 2015-12-21 | 2021-11-09 | Medrx Co., Ltd. | Microneedle patch applicator and housing for same |
| US11177029B2 (en) | 2010-08-13 | 2021-11-16 | Yourbio Health, Inc. | Systems and techniques for monitoring subjects |
| US11185673B2 (en) | 2017-05-15 | 2021-11-30 | Fujifilm Corporation | Micro-needle array unit and container |
| US11202895B2 (en) | 2010-07-26 | 2021-12-21 | Yourbio Health, Inc. | Rapid delivery and/or receiving of fluids |
| US20220249818A1 (en)* | 2010-08-13 | 2022-08-11 | Yourbio Health, Inc. | Clinical and/or consumer techniques and devices |
| US11420812B2 (en) | 2019-04-17 | 2022-08-23 | Fujifilm Corporation | Storage container, microneedle unit, storage container group, and method of producing microneedle unit |
| US11419816B2 (en) | 2010-05-04 | 2022-08-23 | Corium, Inc. | Method and device for transdermal delivery of parathyroid hormone using a microprojection array |
| US11654270B2 (en) | 2021-09-28 | 2023-05-23 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11684719B2 (en) | 2013-05-23 | 2023-06-27 | Allergan, Inc. | Methods of treatment using a syringe extrusion accessory |
| US11684763B2 (en) | 2015-10-16 | 2023-06-27 | University of Pittsburgh—of the Commonwealth System of Higher Education | Multi-component bio-active drug delivery and controlled release to the skin by microneedle array devices |
| US11744927B2 (en) | 2009-10-23 | 2023-09-05 | University of Pittsburgh—of the Commonwealth System of Higher Education | Dissolvable microneedle arrays for transdermal delivery to human skin |
| US11744889B2 (en) | 2016-01-05 | 2023-09-05 | University of Pittsburgh—of the Commonwealth System of Higher Education | Skin microenvironment targeted delivery for promoting immune and other responses |
| WO2023168419A3 (en)* | 2022-03-03 | 2023-10-12 | Deka Products Limited Partnership | Systems and apparatuses for medical agent administration |
| US11877848B2 (en) | 2021-11-08 | 2024-01-23 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US11964121B2 (en) | 2021-10-13 | 2024-04-23 | Satio, Inc. | Mono dose dermal patch for pharmaceutical delivery |
| US11992668B2 (en) | 2008-12-02 | 2024-05-28 | Allergan, Inc. | Injection device |
| US12023156B2 (en) | 2021-10-13 | 2024-07-02 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| USD1033641S1 (en) | 2021-12-17 | 2024-07-02 | Biolinq Incorporated | Microneedle array sensor applicator device |
| US12029562B2 (en) | 2021-04-14 | 2024-07-09 | Satio, Inc. | Dermal patch system |
| US12048543B2 (en) | 2021-11-08 | 2024-07-30 | Satio, Inc. | Dermal patch for collecting a physiological sample with removable vial |
| US12053284B2 (en) | 2021-11-08 | 2024-08-06 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US12121353B2 (en) | 2010-11-09 | 2024-10-22 | Yourbio Health, Inc. | Systems and interfaces for blood sampling |
| US12178979B2 (en) | 2021-10-13 | 2024-12-31 | Satio, Inc. | Dermal patch for delivering a pharmaceutical |
| US12186515B2 (en) | 2020-04-28 | 2025-01-07 | Ticona Llc | Microneedle assembly |
| USD1057153S1 (en) | 2022-04-29 | 2025-01-07 | Biolinq Incorporated | Microneedle array sensor applicator device |
| US12214150B2 (en) | 2019-05-16 | 2025-02-04 | University of Pittsburgh—of the Commonwealth System of Higher Education | Microneedle arrays with undercut features for cutaneous and non-cutaneous drug delivery |
| US12214346B2 (en) | 2021-10-13 | 2025-02-04 | Satio, Inc. | Dermal patch with a diagnostic test strip |
| US12440133B2 (en) | 2024-03-29 | 2025-10-14 | Satio, Inc. | Dermal patch for collecting a physiological sample |
Families Citing this family (111)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7828827B2 (en) | 2002-05-24 | 2010-11-09 | Corium International, Inc. | Method of exfoliation of skin using closely-packed microstructures |
| GB0402131D0 (en) | 2004-01-30 | 2004-03-03 | Isis Innovation | Delivery method |
| US7611481B2 (en) | 2004-03-24 | 2009-11-03 | Corium International, Inc. | Transdermal delivery device |
| US8048089B2 (en) | 2005-12-30 | 2011-11-01 | Edge Systems Corporation | Apparatus and methods for treating the skin |
| US10208158B2 (en) | 2006-07-10 | 2019-02-19 | Medipacs, Inc. | Super elastic epoxy hydrogel |
| CA2676221C (en) | 2007-01-22 | 2016-12-20 | Corium International, Inc. | Applicators for microneedles |
| JP2011505520A (en) | 2007-12-03 | 2011-02-24 | メディパックス インコーポレイテッド | Fluid metering device |
| US9220678B2 (en) | 2007-12-24 | 2015-12-29 | The University Of Queensland | Coating method |
| KR20100129269A (en) | 2008-01-04 | 2010-12-08 | 엣지 시스템즈 코포레이션 | Skin treatment device and method |
| US9056193B2 (en) | 2008-01-29 | 2015-06-16 | Edge Systems Llc | Apparatus and method for treating the skin |
| EP2399643A4 (en)* | 2009-02-23 | 2012-08-22 | Medrx Co Ltd | Applicator for microneedle array |
| US20110105952A1 (en)* | 2009-10-30 | 2011-05-05 | Seventh Sense Biosystems, Inc. | Relatively small devices applied to the skin, modular systems, and methods of use thereof |
| WO2011005894A1 (en)* | 2009-07-07 | 2011-01-13 | Naya Touch, Inc. | Dermal roller with therapeutic microstructures |
| WO2011032011A1 (en) | 2009-09-10 | 2011-03-17 | Medipacs, Inc. | Low profile actuator and improved method of caregiver controlled administration of therapeutics |
| US8439896B2 (en)* | 2009-11-13 | 2013-05-14 | The Invention Science Fund I, Llc | Device, system, and method for targeted delivery of anti-inflammatory medicaments to a mammalian subject |
| US8894630B2 (en) | 2009-11-13 | 2014-11-25 | The Invention Science Fund I, Llc | Device, system, and method for targeted delivery of anti-inflammatory medicaments to a mammalian subject |
| US9078863B2 (en)* | 2009-11-13 | 2015-07-14 | The Invention Science Fund I, Llc | Device, system, and method for targeted delivery of anti-inflammatory medicaments to a mammalian subject |
| CN102811754B (en)* | 2010-01-13 | 2017-05-17 | 第七感生物系统有限公司 | Rapid delivery and/or withdrawal of fluids |
| US9750924B2 (en) | 2010-01-22 | 2017-09-05 | Medrx Co., Ltd. | Adhesive patching aid for microneedle adhesive skin patch |
| WO2011094573A1 (en) | 2010-01-28 | 2011-08-04 | Seventh Sense Biosystems, Inc. | Monitoring or feedback systems and methods |
| US9500186B2 (en) | 2010-02-01 | 2016-11-22 | Medipacs, Inc. | High surface area polymer actuator with gas mitigating components |
| JPWO2011105496A1 (en)* | 2010-02-24 | 2013-06-20 | 久光製薬株式会社 | Microneedle device |
| US9687640B2 (en)* | 2010-05-04 | 2017-06-27 | Corium International, Inc. | Applicators for microneedles |
| WO2011148995A1 (en)* | 2010-05-28 | 2011-12-01 | 久光製薬株式会社 | Array with fine protrusions |
| WO2011163347A2 (en) | 2010-06-23 | 2011-12-29 | Seventh Sense Biosystems, Inc. | Sampling devices and methods involving relatively little pain |
| WO2012006677A1 (en) | 2010-07-14 | 2012-01-19 | The University Of Queensland | Patch applying apparatus |
| US20120016308A1 (en) | 2010-07-16 | 2012-01-19 | Seventh Sense Biosystems, Inc. | Low-pressure packaging for fluid devices |
| JP5937092B2 (en) | 2010-10-19 | 2016-06-22 | トラスティーズ オブ タフツ カレッジ | Silk fibroin-based microneedle and method for producing the same |
| WO2012061556A1 (en) | 2010-11-03 | 2012-05-10 | Flugen, Inc. | Wearable drug delivery device having spring drive and sliding actuation mechanism |
| US9089677B2 (en)* | 2011-01-25 | 2015-07-28 | The Regents Of The University Of California | Transcutaneous multimodal delivery system (TMDS) |
| WO2012126784A1 (en) | 2011-03-18 | 2012-09-27 | Universite Libre De Bruxelles | Devices for puncturing a human or animal body's membrane |
| KR101314091B1 (en)* | 2011-07-26 | 2013-10-04 | 연세대학교 산학협력단 | Electro-microneedle assembly for cutaneous gene transfer in-situ and process for preparing the same |
| WO2013038890A1 (en)* | 2011-09-16 | 2013-03-21 | 久光製薬株式会社 | Applicator |
| US9474685B2 (en)* | 2011-09-28 | 2016-10-25 | Sure-Shot Medical Device Inc. | Apparatus for localized dermatological treatment |
| EP2764887B1 (en) | 2011-10-06 | 2020-12-02 | Hisamitsu Pharmaceutical Co., Inc. | Applicator |
| CA2851606C (en)* | 2011-10-12 | 2020-08-04 | 3M Innovative Properties Company | Integrated microneedle array delivery system |
| EP2765927B1 (en)* | 2011-10-12 | 2021-02-24 | Vaxxas Pty Limited | Delivery device |
| US20140236090A1 (en)* | 2011-10-12 | 2014-08-21 | 3M Innovative Properties Company | Integrated microneedle array delivery system |
| GB201120000D0 (en) | 2011-11-20 | 2012-01-04 | Glaxosmithkline Biolog Sa | Vaccine |
| GB201119999D0 (en) | 2011-11-20 | 2012-01-04 | Glaxosmithkline Biolog Sa | Vaccine |
| JP6273543B2 (en)* | 2011-12-26 | 2018-02-07 | 株式会社システック | A hollow liquid chemical container with a needle or a liquid container with a hollow needle containing a liquid medicine, a body fluid collection container with a hollow needle, an integrated carrier equipped with a plurality of these containers, an integrated carrier assembly, a continuous injection apparatus, a continuous body fluid collection apparatus, and , Method for producing integrated carrier |
| US10000605B2 (en) | 2012-03-14 | 2018-06-19 | Medipacs, Inc. | Smart polymer materials with excess reactive molecules |
| EP2662110A1 (en)* | 2012-05-10 | 2013-11-13 | Debiotech S.A. | Device and method for inserting needles |
| JP5903016B2 (en) | 2012-06-27 | 2016-04-13 | コスメディ製薬株式会社 | Protective release sheet for microneedle patch |
| EP2906284B8 (en) | 2012-10-10 | 2021-01-20 | Kindeva Drug Delivery L.P. | Force-controlled applicator for applying a microneedle device to skin |
| WO2014059104A1 (en)* | 2012-10-10 | 2014-04-17 | 3M Innovative Properties Company | Applicator and method for applying a microneedle device to skin |
| WO2014078545A1 (en) | 2012-11-16 | 2014-05-22 | 3M Innovative Properties Company | Force-controlled applicator for applying a microneedle device to skin |
| AU2014222308B2 (en) | 2013-02-28 | 2018-11-08 | Vivasor, Inc. | Transdermal drug delivery device |
| US9861801B2 (en) | 2013-02-28 | 2018-01-09 | Kimberly-Clark Worldwide, Inc. | Drug delivery device |
| EP3437575B1 (en) | 2013-03-15 | 2021-04-21 | Edge Systems LLC | Devices and systems for treating the skin |
| WO2015005143A1 (en)* | 2013-07-11 | 2015-01-15 | 凸版印刷株式会社 | Microneedle unit |
| US20150038897A1 (en)* | 2013-07-30 | 2015-02-05 | Zosano Pharma, Inc. | Low-Profile Microneedle Patch Applicator |
| CN111744101B (en)* | 2013-09-30 | 2023-03-10 | 佐治亚科技研究公司 | Microneedle patches, systems, and methods |
| WO2015111672A1 (en)* | 2014-01-24 | 2015-07-30 | 凸版印刷株式会社 | Microneedle unit |
| JP6414084B2 (en)* | 2014-01-24 | 2018-10-31 | 凸版印刷株式会社 | Microneedle unit and microneedle assembly |
| WO2015115420A1 (en)* | 2014-01-29 | 2015-08-06 | 久光製薬株式会社 | Applicator |
| RU2719937C1 (en) | 2014-04-24 | 2020-04-23 | Джорджия Тек Рисёч Корпорейшн | Microneedles and methods for their production |
| EP4324414A3 (en) | 2014-12-23 | 2024-05-01 | HydraFacial LLC | Devices and methods for treating the skin using a rollerball or a wicking member |
| US11147954B2 (en) | 2015-02-02 | 2021-10-19 | Vaxxas Pty Limited | Microprojection array applicator and method |
| CN106456954A (en)* | 2015-02-13 | 2017-02-22 | 美德阿利克斯株式会社 | Microneedle insertion device and microneedle patch application device |
| US10792857B2 (en) | 2015-03-13 | 2020-10-06 | The University Of North Carolina At Chapel Hill | Polymeric microneedles and rapid additive manufacturing of the same |
| MA41818A (en) | 2015-03-27 | 2018-01-30 | Leo Pharma As | MICRO-NEEDLE STAMP FOR ADMINISTRATION OF AN ACTIVE SUBSTANCE TO THE SKIN |
| JP2018527052A (en)* | 2015-07-08 | 2018-09-20 | エッジ システムズ エルエルシー | Apparatus, system and method for promoting hair growth |
| WO2017045031A1 (en) | 2015-09-18 | 2017-03-23 | Vaxxas Pty Limited | Microprojection arrays with microprojections having large surface area profiles |
| EP3355981A4 (en) | 2015-09-28 | 2019-05-22 | Vaxxas Pty Limited | MICROAILLY NETWORK HAVING IMPROVED SKIN PENETRATION PROPERTIES AND ASSOCIATED METHODS |
| CN109069155B (en) | 2016-03-01 | 2022-06-24 | 基托泰克医疗股份有限公司 | Microstructure-based systems, devices, and methods for wound closure |
| JP6928368B2 (en)* | 2016-05-09 | 2021-09-01 | 学校法人近畿大学 | Method of forming resin microneedles and method of forming three-dimensional pattern |
| JP7116430B2 (en)* | 2016-12-12 | 2022-08-10 | 株式会社 メドレックス | Microneedle patch |
| US11241563B2 (en)* | 2016-12-22 | 2022-02-08 | Johnson & Johnson Consumer Inc. | Microneedle arrays and methods for making and using |
| WO2018176102A1 (en) | 2017-03-31 | 2018-10-04 | Vaxxas Pty Limited | Device and method for coating surfaces |
| DE102017112573A1 (en) | 2017-06-07 | 2018-12-13 | Lts Lohmann Therapie-Systeme Ag | Microneedle system for the application of glucagon-like peptide analogues |
| WO2018227246A1 (en) | 2017-06-13 | 2018-12-20 | Vaxxas Pty Limited | Quality control of substrate coatings |
| CA3071680A1 (en)* | 2017-08-04 | 2019-02-07 | Vaxxas Pty Limited | Compact high mechanical energy storage and low trigger force actuator for the delivery of microprojection array patches (map) |
| DE102017117784A1 (en) | 2017-08-04 | 2019-02-07 | Lts Lohmann Therapie-Systeme Ag | Applicator system comprising a microneedle array having a drug for wound healing |
| DE102017118419A1 (en) | 2017-08-11 | 2019-02-14 | Lts Lohmann Therapie-Systeme Ag | Microneedle array having a color change indicator |
| WO2019046333A1 (en)* | 2017-08-29 | 2019-03-07 | Patchmi, Inc. | Microneedle treatment system |
| GB201716391D0 (en)* | 2017-10-06 | 2017-11-22 | Xobaderm Ltd | Kit for delivery of an active compound into a biological barrier |
| DE102017126501A1 (en)* | 2017-11-10 | 2019-05-16 | Lts Lohmann Therapie-Systeme Ag | Micro needle system for the application of a hepatitis vaccine |
| CA3085633A1 (en)* | 2017-12-14 | 2019-06-20 | Lts Lohmann Therapie-Systeme Ag | Microneedle array comprising an active ingredient in the form of salts |
| KR102238787B1 (en)* | 2018-03-12 | 2021-04-08 | 연세대학교 산학협력단 | Micro-needle Applicator |
| ES3034959T3 (en)* | 2018-03-13 | 2025-08-27 | Medrx Co Ltd | Adhesion member and microneedle patch |
| JP6934109B2 (en) | 2018-05-08 | 2021-09-08 | 富士フイルム株式会社 | Manufacturing method of microneedle array unit |
| JP7476119B2 (en) | 2018-06-29 | 2024-04-30 | ジョンソン アンド ジョンソン コンシューマー インコーポレイテッド | Three-dimensional microfluidic devices for delivery of active substances |
| EP3875140B1 (en) | 2018-10-31 | 2024-07-31 | FUJIFILM Corporation | Microneedle array unit |
| JP7666887B2 (en)* | 2018-12-21 | 2025-04-22 | ロレアル | Kit and beauty method using microneedle sheet |
| SG11202106928PA (en)* | 2018-12-25 | 2021-07-29 | Univ Hirosaki | Medicine administering device and medicine administering system |
| DE102019200558A1 (en)* | 2019-01-17 | 2020-07-23 | Lts Lohmann Therapie-Systeme Ag | Microarray recording |
| DE102019001251A1 (en)* | 2019-02-21 | 2020-08-27 | Lts Lohmann Therapie-Systeme Ag | Microneedle plaster applicator |
| GB201908229D0 (en) | 2019-06-10 | 2019-07-24 | Univ College Cork National Univ Of Ireland | Microneedles and methods for the manufacture thereof |
| EP4076187A1 (en)* | 2019-12-18 | 2022-10-26 | Uprax Microsolutions B.V. | Applicators and methods for applying a microneedle patch to a skin of a subject, and microneedle patches |
| US11291474B2 (en) | 2020-01-06 | 2022-04-05 | Ed F. Nicolas | Skin treatment tool applicator tip |
| KR102368260B1 (en)* | 2020-01-09 | 2022-03-02 | 연세대학교 산학협력단 | Microneedle applicator |
| US11986613B2 (en) | 2020-02-19 | 2024-05-21 | Kitotech Medical, Inc. | Microstructure systems and methods for pain treatment |
| DE102020109157A1 (en) | 2020-04-02 | 2021-10-07 | Lts Lohmann Therapie-Systeme Ag | Carrier element for microneedles as well as microneedle array device |
| WO2021257624A1 (en)* | 2020-06-17 | 2021-12-23 | Biolinq, Inc. | Devices and methods for application of microneedle arrays using radial and axial accelerations |
| JPWO2022176676A1 (en)* | 2021-02-19 | 2022-08-25 | ||
| US12357804B2 (en) | 2021-03-01 | 2025-07-15 | Deka Products Limited Partnership | Medical agent dispensing systems, methods, and apparatuses |
| CA3210623A1 (en)* | 2021-03-01 | 2022-09-09 | Eitan C. Zeira | Delivery device apparatuses, systems, and methods |
| US12161832B2 (en) | 2021-03-01 | 2024-12-10 | Deka Products Limited Partnership | Medical agent dispensing systems, methods, and apparatuses |
| US12377219B2 (en) | 2021-03-01 | 2025-08-05 | Deka Products Limited Partnership | Medical agent dispensing apparatuses, systems, and methods |
| USD1065551S1 (en) | 2021-09-10 | 2025-03-04 | Hydrafacial Llc | Skin treatment device |
| USD1016615S1 (en) | 2021-09-10 | 2024-03-05 | Hydrafacial Llc | Container for a skin treatment device |
| USD1042807S1 (en) | 2021-10-11 | 2024-09-17 | Hydrafacial Llc | Skin treatment tip |
| CN113974615A (en)* | 2021-11-18 | 2022-01-28 | 中山大学 | Tissue fluid detection device and system thereof |
| US11957346B2 (en) | 2022-02-18 | 2024-04-16 | Kitotech Medical, Inc. | Force modulating deep skin staples and instruments |
| MX2024010364A (en)* | 2022-02-23 | 2024-09-02 | Deka Products Lp | Delivery device apparatuses, systems, and methods. |
| WO2023205341A1 (en)* | 2022-04-22 | 2023-10-26 | The Board Of Trustees Of The Leland Stanford Junior University | Compliant polymeric structures having polymeric microneedles and methods for making and using same |
| JP2025530880A (en)* | 2022-09-13 | 2025-09-17 | マイクロン バイオメディカル,インコーポレイテッド | Microneedle patch with force feedback indicator |
| USD1084369S1 (en) | 2023-02-10 | 2025-07-15 | Hydrafacial Llc | Skin treatment tip |
| WO2024263835A1 (en) | 2023-06-23 | 2024-12-26 | Vaxess Technologies, Inc. | Applicator for medicament patch |
| KR102856481B1 (en)* | 2023-11-08 | 2025-09-09 | 경북대학교 산학협력단 | Apparatus for microneedle automatic analysis using magnetic stirring elements and method thereof |
Citations (242)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1554510A (en) | 1924-04-23 | 1925-09-22 | Elsie W Kirby | Massaging device |
| US1770632A (en) | 1928-07-02 | 1930-07-15 | Arthur E Smith | Ampul syringe |
| US2046240A (en) | 1932-07-01 | 1936-06-30 | Thomas W Bayley | Abrasive article |
| US2434407A (en) | 1946-01-05 | 1948-01-13 | Hofe George Douglas | Depilatory device |
| US3675766A (en) | 1970-02-04 | 1972-07-11 | Sol Roy Rosenthal | Multiple puncture injector device |
| US3704194A (en) | 1970-07-02 | 1972-11-28 | Gen Electric | Perforated reinforced plastic member and method for making |
| US3814097A (en) | 1972-02-14 | 1974-06-04 | Ici Ltd | Dressing |
| US3873255A (en) | 1971-01-27 | 1975-03-25 | Johnson & Johnson | Apparatus for producing nonwoven fabric |
| US3918449A (en) | 1973-06-06 | 1975-11-11 | Guerin A Ets | Device for cutaneous therapeutic treatment |
| US3964482A (en) | 1971-05-17 | 1976-06-22 | Alza Corporation | Drug delivery device |
| US4055029A (en) | 1975-03-07 | 1977-10-25 | Heinz Kalbow | Cleaning, scouring and/or polishing pads |
| US4117841A (en) | 1977-02-07 | 1978-10-03 | Anthony Perrotta | Medicated bandage pocket |
| US4151240A (en) | 1976-10-19 | 1979-04-24 | The Procter & Gamble Company | Method for debossing and perforating a running ribbon of thermoplastic film |
| US4180232A (en) | 1977-01-13 | 1979-12-25 | Hardigg James S | Truss panel mold |
| US4342314A (en) | 1979-03-05 | 1982-08-03 | The Procter & Gamble Company | Resilient plastic web exhibiting fiber-like properties |
| US4381963A (en) | 1980-07-30 | 1983-05-03 | The University Of Rochester | Micro fabrication molding process |
| US4395215A (en) | 1981-02-02 | 1983-07-26 | The Procter & Gamble Company | Film forming structure for uniformly debossing and selectively aperturing a resilient plastic web and method for its construction |
| US4402696A (en) | 1979-10-15 | 1983-09-06 | Gulko Bruce N | Folded applicator |
| US4460368A (en) | 1981-10-29 | 1984-07-17 | Almedco, Inc. | Trans-dermal medication system |
| US4460370A (en) | 1981-10-29 | 1984-07-17 | Almedco, Inc. | Trans-dermal medication application cell |
| US4463045A (en) | 1981-03-02 | 1984-07-31 | The Procter & Gamble Company | Macroscopically expanded three-dimensional plastic web exhibiting non-glossy visible surface and cloth-like tactile impression |
| US4509908A (en) | 1981-02-02 | 1985-04-09 | The Procter & Gamble Company | Apparatus for uniformly debossing and aperturing a resilient plastic web |
| US4515168A (en) | 1983-07-22 | 1985-05-07 | Chester Martin H | Clamp-on nerve stimulator and locator |
| US4556441A (en) | 1983-01-24 | 1985-12-03 | Faasse Jr Adrian L | Pharmaceutical packaging method |
| US4585991A (en) | 1982-06-03 | 1986-04-29 | Texas Instruments Incorporated | Solid state multiprobe testing apparatus |
| FR2535602B1 (en) | 1982-11-05 | 1986-06-13 | Stallergenes Lab | SCARIFIER DEVICE |
| US4597961A (en) | 1985-01-23 | 1986-07-01 | Etscorn Frank T | Transcutaneous application of nicotine |
| US4609518A (en) | 1985-05-31 | 1986-09-02 | The Procter & Gamble Company | Multi-phase process for debossing and perforating a polymeric web to coincide with the image of one or more three-dimensional forming structures |
| US4630603A (en) | 1986-02-13 | 1986-12-23 | The Kendall Company | Wound dressing |
| US4743249A (en) | 1986-02-14 | 1988-05-10 | Ciba-Geigy Corp. | Dermal and transdermal patches having a discontinuous pattern adhesive layer |
| US4784737A (en) | 1986-04-18 | 1988-11-15 | The United States Department Of Energy | Electromicroinjection of particles into living cells |
| US4812305A (en) | 1987-11-09 | 1989-03-14 | Vocal Rodolfo S | Well medicine strip |
| EP0312662A1 (en) | 1984-08-24 | 1989-04-26 | David George Vlock | Fluid dispenser |
| EP0156471B1 (en) | 1984-02-16 | 1989-05-03 | The Procter & Gamble Company | Process and apparatus for forming a continuous web, particularly polymeric film, and material produced by the process |
| US4837049A (en) | 1986-06-17 | 1989-06-06 | Alfred E. Mann Foundation For Scientific Research | Method of making an electrode array |
| US4846821A (en) | 1987-08-24 | 1989-07-11 | The Procter & Gamble Company | Substantially fluid-impervious microbubbled polymeric web exhibiting low levels of noise when subjected to movement |
| US4904475A (en) | 1985-05-03 | 1990-02-27 | Alza Corporation | Transdermal delivery of drugs from an aqueous reservoir |
| US4966159A (en) | 1981-12-14 | 1990-10-30 | Maganias Nicholas H | Allergy test strip |
| EP0407063A1 (en) | 1989-07-06 | 1991-01-09 | Connaught Laboratories Limited | Tines structure of clinical applicator |
| SU1641346A1 (en) | 1987-12-08 | 1991-04-15 | Мгту Им.Н.Э.Баумана | Electrostimulation appliance |
| SU1667864A1 (en) | 1989-03-23 | 1991-08-07 | Опытный завод энергетического машиностроения | Electrostimulation device |
| US5051259A (en) | 1987-12-15 | 1991-09-24 | Coloplast A/S | Skin barrier product with discontinuous adhesive layer |
| US5061258A (en) | 1987-08-07 | 1991-10-29 | Martz Joel D | Vapor permeable dressing with releasable medication |
| GB2221394B (en) | 1988-08-05 | 1992-03-04 | Eilert Eilertsen | An injection device |
| US5134079A (en) | 1989-03-27 | 1992-07-28 | International Technidyne Corp. | Fluid sample collection and delivery system and methods particularly adapted for body fluid sampling |
| US5139029A (en) | 1990-04-06 | 1992-08-18 | Henry Fishman | Allergy testing apparatus and method |
| US5156591A (en) | 1990-12-13 | 1992-10-20 | S. I. Scientific Innovations Ltd. | Skin electrode construction and transdermal drug delivery device utilizing same |
| US5158073A (en) | 1990-12-18 | 1992-10-27 | Bukowski Voytek Z | Acupressure foot massage mat |
| US5160315A (en) | 1991-04-05 | 1992-11-03 | Minnesota Mining And Manufacturing Company | Combined adhesive strip and transparent dressing delivery system |
| US5162043A (en) | 1990-03-30 | 1992-11-10 | Alza Corporation | Iontophoretic delivery device |
| US5190558A (en) | 1989-11-08 | 1993-03-02 | Nec Corporation | Method of eliminating stratum corneum from the skin and an instrument to be used therefor |
| US5198192A (en) | 1988-05-18 | 1993-03-30 | Inax Corporation | Apparatus for detecting ingredient in urine, a toilet stool equipped with a urine detecting device and a room for urine detecting facility |
| EP0240593B1 (en) | 1986-03-14 | 1993-05-19 | Drug Delivery Systems Inc. | Improved transdermal drug applicator and electrodes therefor |
| US5215088A (en) | 1989-11-07 | 1993-06-01 | The University Of Utah | Three-dimensional electrode device |
| US5244677A (en) | 1988-12-29 | 1993-09-14 | Minnesota Mining And Manufacturing Company | Application system for drug containing microemulsions |
| US5244711A (en) | 1990-03-12 | 1993-09-14 | Mcneil-Ppc, Inc. | Apertured non-woven fabric |
| US5250067A (en) | 1992-11-30 | 1993-10-05 | Ala Gelfer | Body treatment pad having a multiple number of sharpened skin-penetration protuberances |
| US5250023A (en) | 1989-10-27 | 1993-10-05 | Korean Research Institute on Chemical Technology | Transdermal administration method of protein or peptide drug and its administration device thereof |
| US5252279A (en) | 1991-01-17 | 1993-10-12 | Reinhold Industries | Method for making perforated articles |
| US5256360A (en) | 1992-03-25 | 1993-10-26 | Panasonic Technologies, Inc. | Method of manufacturing a precision micro-filter |
| US5279544A (en) | 1990-12-13 | 1994-01-18 | Sil Medics Ltd. | Transdermal or interdermal drug delivery devices |
| US5308625A (en) | 1992-09-02 | 1994-05-03 | Cygnus Therapeutic Systems | Enhancement of transdermal drug delivery using monoalkyl phosphates and other absorption promoters |
| EP0400249B1 (en) | 1988-01-19 | 1994-06-01 | Perfojet S.A. | Apparatus for perforating a web |
| US5318557A (en) | 1992-07-13 | 1994-06-07 | Elan Medical Technologies Limited | Medication administering device |
| US5320600A (en) | 1993-06-14 | 1994-06-14 | Lambert Wm S | Plural content container for simultaneous ejection |
| US5330452A (en) | 1993-06-01 | 1994-07-19 | Zook Gerald P | Topical medicating device |
| GB2277202A (en) | 1993-03-22 | 1994-10-19 | Peter Leslie Moran | Electrical circuit board manufacture |
| US5362307A (en) | 1989-01-24 | 1994-11-08 | The Regents Of The University Of California | Method for the iontophoretic non-invasive-determination of the in vivo concentration level of an inorganic or organic substance |
| US5383512A (en) | 1993-01-27 | 1995-01-24 | Midwest Research Institute | Method for fabricating a substrate having spaced apart microcapillaries thereon |
| EP0301599B1 (en) | 1987-07-30 | 1995-03-29 | Applied Extrusion Technologies, Inc. | Apertured film and net like fabrics from thermoplastic materials |
| US5457041A (en) | 1994-03-25 | 1995-10-10 | Science Applications International Corporation | Needle array and method of introducing biological substances into living cells using the needle array |
| US5462743A (en) | 1992-10-30 | 1995-10-31 | Medipro Sciences Limited | Substance transfer system for topical application |
| US5476443A (en) | 1993-05-27 | 1995-12-19 | New Dimensions In Medicine, Inc. | Wound dressing product containing a porous layer |
| US5487726A (en) | 1994-06-16 | 1996-01-30 | Ryder International Corporation | Vaccine applicator system |
| US5496304A (en) | 1994-07-20 | 1996-03-05 | University Of Utah Research Foundation | Surgical marking pen |
| US5498235A (en) | 1994-09-30 | 1996-03-12 | Becton Dickinson And Company | Iontophoresis assembly including patch/controller attachment |
| US5503843A (en) | 1994-04-22 | 1996-04-02 | Flora Inc. | Transdermal delivery of alpha adrenoceptor blocking agents |
| US5512219A (en) | 1994-06-03 | 1996-04-30 | Reflexite Corporation | Method of casting a microstructure sheet having an array of prism elements using a reusable polycarbonate mold |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5531675A (en) | 1989-05-10 | 1996-07-02 | Yoo; Tae W. | Micro-acupuncture needle for a finger of a hand |
| US5531855A (en) | 1993-03-22 | 1996-07-02 | Minnesota Mining And Manufacturing Company | Carrier delivered dressing and method of manufacture |
| US5536263A (en) | 1994-03-30 | 1996-07-16 | Lectec Corporation | Non-occulusive adhesive patch for applying medication to the skin |
| US5551953A (en) | 1994-10-31 | 1996-09-03 | Alza Corporation | Electrotransport system with remote telemetry link |
| US5567376A (en) | 1991-08-14 | 1996-10-22 | Chicopee | Method of forming textile-like apertured plastic films |
| US5591139A (en) | 1994-06-06 | 1997-01-07 | The Regents Of The University Of California | IC-processed microneedles |
| US5591123A (en) | 1983-08-18 | 1997-01-07 | Drug Delivery Systems Inc. | Programmable control mounting system for transdermal drug applicator |
| US5611806A (en) | 1994-05-23 | 1997-03-18 | Samsung Electro-Mechanics Co., Ltd. | Skin perforating device for transdermal medication |
| US5645977A (en) | 1995-09-22 | 1997-07-08 | Industrial Technology Research Institute | Method of making molds for manufacturing multiple-lead microstructures |
| US5658515A (en) | 1995-09-25 | 1997-08-19 | Lee; Abraham P. | Polymer micromold and fabrication process |
| US5662127A (en) | 1996-01-17 | 1997-09-02 | Bio-Plas, Inc. | Self-contained blood withdrawal apparatus and method |
| US5676850A (en) | 1991-01-31 | 1997-10-14 | Carnegie Mellon University | Micromechanical barb and method for making the same |
| US5681580A (en) | 1994-05-23 | 1997-10-28 | Samsung Electro-Mechanics Co., Ltd. | Patch-type device for iontophoretic transdermal delivery of insulin |
| US5697901A (en) | 1989-12-14 | 1997-12-16 | Elof Eriksson | Gene delivery by microneedle injection |
| US5704520A (en) | 1993-07-19 | 1998-01-06 | Elan Medical Technologies, Limited | Liquid material dispenser and valve |
| DE19624578A1 (en) | 1996-06-20 | 1998-01-08 | Nancy L Divers | Skin care device |
| US5711761A (en) | 1984-10-29 | 1998-01-27 | Alza Corporation | Iontophoretic drug delivery |
| US5728089A (en) | 1993-06-04 | 1998-03-17 | The Regents Of The University Of California | Microfabricated structure to be used in surgery |
| US5730721A (en) | 1996-01-25 | 1998-03-24 | Vesture Corporation | Medical applicator and method |
| US5735273A (en) | 1995-09-12 | 1998-04-07 | Cygnus, Inc. | Chemical signal-impermeable mask |
| US5756117A (en) | 1992-04-08 | 1998-05-26 | International Medical Asscociates, Inc. | Multidose transdermal drug delivery system |
| US5771890A (en) | 1994-06-24 | 1998-06-30 | Cygnus, Inc. | Device and method for sampling of substances using alternating polarity |
| US5788983A (en) | 1989-04-03 | 1998-08-04 | Rutgers, The State University Of New Jersey | Transdermal controlled delivery of pharmaceuticals at variable dosage rates and processes |
| US5800420A (en) | 1994-11-04 | 1998-09-01 | Elan Medical Technologies Limited | Analyte-controlled liquid delivery device and analyte monitor |
| US5814020A (en) | 1995-09-11 | 1998-09-29 | Elan Medical Technlogies Limited | Medicament delivery device |
| US5843114A (en) | 1994-05-23 | 1998-12-01 | Samsung Electro-Mechanics Co., Ltd. | Skin perforating apparatus for transdermal medication |
| US5848990A (en) | 1993-10-22 | 1998-12-15 | Hoffmann-La Roche Inc. | Device for introducing active substance into a patient |
| US5848991A (en) | 1990-12-13 | 1998-12-15 | Elan Medical Technologies Limited Athlone, Co. | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5848985A (en) | 1996-02-09 | 1998-12-15 | Polytronics, Ltd. | Skin-contact type medical treatment apparatus |
| US5851549A (en) | 1994-05-25 | 1998-12-22 | Becton Dickinson And Company | Patch, with system and apparatus for manufacture |
| US5873849A (en) | 1997-04-24 | 1999-02-23 | Ichor Medical Systems, Inc. | Electrodes and electrode arrays for generating electroporation inducing electrical fields |
| US5879326A (en) | 1995-05-22 | 1999-03-09 | Godshall; Ned Allen | Method and apparatus for disruption of the epidermis |
| EP0796128B1 (en) | 1994-12-09 | 1999-03-10 | Novartis AG | Transdermal system |
| US5938684A (en) | 1997-12-09 | 1999-08-17 | Sierra Self Healing Products, Inc. | Accupressure device for therapeutic relief |
| US5948488A (en) | 1996-04-30 | 1999-09-07 | 3M Innovative Properties Company | Glittering cube-corner article |
| US5962011A (en) | 1993-12-06 | 1999-10-05 | Schering-Plough Healthcare Products, Inc. | Device for delivery of dermatological ingredients |
| US5964729A (en) | 1994-05-23 | 1999-10-12 | Samsung Electro-Mechanics Co., Ltd. | Perforating device for dermal administration |
| US5983136A (en) | 1996-09-17 | 1999-11-09 | Deka Products Limited Partnership | System for delivery of drugs by transport |
| US5987989A (en) | 1996-02-05 | 1999-11-23 | Denso Corporation | Semiconductor physical quantity sensor |
| US5997549A (en) | 1999-02-16 | 1999-12-07 | Sauceda; Charles J. | Wart removing tool |
| US6014584A (en) | 1997-08-01 | 2000-01-11 | Genetronics, Inc. | Electroporation therapy apparatus |
| US6024553A (en) | 1997-12-22 | 2000-02-15 | Mcneil-Ppc, Inc. | Apparatus for supporting a starting web during formation of the apertured web |
| US6036659A (en) | 1998-10-09 | 2000-03-14 | Flexsite Diagnostics, Inc. | Collection device for biological samples and methods of use |
| US6038465A (en) | 1998-10-13 | 2000-03-14 | Agilent Technologies, Inc. | Telemedicine patient platform |
| US6038485A (en) | 1997-06-12 | 2000-03-14 | Axelgaard Manufacturing Co., Ltd. | Current-controlling electrode |
| US6047208A (en) | 1997-08-27 | 2000-04-04 | Becton, Dickinson And Company | Iontophoretic controller |
| US6050988A (en) | 1997-12-11 | 2000-04-18 | Alza Corporation | Device for enhancing transdermal agent flux |
| JP2000146777A (en) | 1998-09-08 | 2000-05-26 | Matsushita Electric Ind Co Ltd | Sample collection method, sample collection device, sample collection container, sample measurement method, sample measurement device, and sample measurement container |
| JP2000147229A (en) | 1998-11-17 | 2000-05-26 | Corning Inc | Duplication of pattern of nanometer scale |
| JP2000164890A (en) | 1998-11-27 | 2000-06-16 | Denso Corp | Semiconductor kinematic value sensor and manufacturing method therefor |
| US6083196A (en) | 1997-12-11 | 2000-07-04 | Alza Corporation | Device for enhancing transdermal agent flux |
| JP2000194142A (en) | 1998-12-25 | 2000-07-14 | Fujitsu Ltd | Pattern forming method and semiconductor device manufacturing method |
| US6091975A (en) | 1998-04-01 | 2000-07-18 | Alza Corporation | Minimally invasive detecting device |
| JP2000232095A (en) | 1999-02-12 | 2000-08-22 | Nippon Telegr & Teleph Corp <Ntt> | Method for forming fine pattern on semiconductor surface |
| US6106751A (en) | 1998-03-18 | 2000-08-22 | The Regents Of The University Of California | Method for fabricating needles via conformal deposition in two-piece molds |
| JP2000232971A (en) | 1999-02-15 | 2000-08-29 | Arkray Inc | Sucked transudate sampling method and device |
| US6120792A (en) | 1998-04-29 | 2000-09-19 | Juni; Jack E. | Medicated skin patch and method for its use |
| US6129696A (en) | 1983-08-18 | 2000-10-10 | Drug Delivery Systems Inc. | Transdermal drug applicator |
| US6132755A (en) | 1995-07-14 | 2000-10-17 | Boehringer Ingelheim Kg | Transcorneal drug-release system |
| US6132449A (en) | 1999-03-08 | 2000-10-17 | Agilent Technologies, Inc. | Extraction and transportation of blood for analysis |
| US6135990A (en) | 1997-12-17 | 2000-10-24 | University Of South Florida | Electroporation device and method |
| JP2000322780A (en) | 1999-05-14 | 2000-11-24 | Yasunori Saotome | Stamper for information recording disk, method of manufacturing the same, information recording disk, and method of manufacturing information recording disk |
| JP2000323461A (en) | 1999-05-11 | 2000-11-24 | Nec Corp | Fine pattern forming device, its manufacture, and method of forming the same |
| US6156336A (en) | 1996-11-27 | 2000-12-05 | Lts Lohmann Therapie-Systeme Gmbh | Method for the low-waste manufacture of diskoid moulded bodies containing active ingredients, and transdermal therapeutic systems containing same |
| JP2001004442A (en) | 1999-06-25 | 2001-01-12 | Matsushita Electric Works Ltd | Infrared sensor and its manufacture |
| US6183770B1 (en) | 1999-04-15 | 2001-02-06 | Acutek International | Carrier patch for the delivery of agents to the skin |
| US6183434B1 (en) | 1996-07-03 | 2001-02-06 | Spectrx, Inc. | Multiple mechanical microporation of skin or mucosa |
| US6187210B1 (en) | 1997-06-30 | 2001-02-13 | The Regents Of The University Of California | Epidermal abrasion device with isotropically etched tips, and method of fabricating such a device |
| EP1086718A1 (en) | 1999-09-22 | 2001-03-28 | Becton Dickinson and Company | Method and apparatus for the transdermal administration of a substance |
| US6216034B1 (en) | 1997-08-01 | 2001-04-10 | Genetronics, Inc. | Method of programming an array of needle electrodes for electroporation therapy of tissue |
| US6219574B1 (en) | 1996-06-18 | 2001-04-17 | Alza Corporation | Device and method for enchancing transdermal sampling |
| JP2001138300A (en) | 1999-08-30 | 2001-05-22 | Canon Inc | Method for producing structure having pores, structure produced by the method, and structure device using the structure |
| US6241701B1 (en) | 1997-08-01 | 2001-06-05 | Genetronics, Inc. | Apparatus for electroporation mediated delivery of drugs and genes |
| JP2001157715A (en) | 1999-09-24 | 2001-06-12 | Becton Dickinson & Co | Method and device suitable for skin abrasion |
| US6256533B1 (en) | 1999-06-09 | 2001-07-03 | The Procter & Gamble Company | Apparatus and method for using an intracutaneous microneedle array |
| US20010023324A1 (en) | 1997-11-03 | 2001-09-20 | Allan Pronovost | Glucose detector and method for diagnosing diabetes |
| US6312612B1 (en) | 1999-06-09 | 2001-11-06 | The Procter & Gamble Company | Apparatus and method for manufacturing an intracutaneous microneedle array |
| US6322808B1 (en)* | 1997-12-11 | 2001-11-27 | Alza Corporation | Device for enhancing transdermal agent flux |
| JP2001341314A (en) | 2000-06-01 | 2001-12-11 | Ricoh Co Ltd | Droplet discharge head and manufacturing method thereof, ink jet recording apparatus and microactuator |
| US6334856B1 (en) | 1998-06-10 | 2002-01-01 | Georgia Tech Research Corporation | Microneedle devices and methods of manufacture and use thereof |
| US20020006355A1 (en) | 2000-07-11 | 2002-01-17 | Bayer Corporation | Hollow microneedle patch |
| US6355054B1 (en) | 1999-11-05 | 2002-03-12 | Ceramoptec Industries, Inc. | Laser system for improved transbarrier therapeutic radiation delivery |
| US20020032415A1 (en) | 1999-12-10 | 2002-03-14 | Trautman Joseph C. | Device and method for enhancing skin piercing by microprotrusions |
| JP2002079499A (en) | 2000-09-08 | 2002-03-19 | Terumo Corp | Method of manufacturing needle-like article, and manufactured needle |
| US20020042589A1 (en) | 2000-10-05 | 2002-04-11 | Thomas Marsoner | Medical injection device |
| US20020045907A1 (en) | 2000-10-16 | 2002-04-18 | The Procter & Gamble Company | Microstructures for treating and conditioning skin |
| US6375978B1 (en) | 1997-12-22 | 2002-04-23 | Alza Corporation | Rate controlling membranes for controlled drug delivery devices |
| US6375627B1 (en) | 2000-03-02 | 2002-04-23 | Agilent Technologies, Inc. | Physiological fluid extraction with rapid analysis |
| US6379324B1 (en) | 1999-06-09 | 2002-04-30 | The Procter & Gamble Company | Intracutaneous microneedle array apparatus |
| JP2002151395A (en) | 2000-11-14 | 2002-05-24 | Kansai Tlo Kk | Method and apparatus for processing material using x- rays |
| US20020082543A1 (en) | 2000-12-14 | 2002-06-27 | Jung-Hwan Park | Microneedle devices and production thereof |
| US20020087182A1 (en) | 2000-10-13 | 2002-07-04 | Trautman Joseph C. | Microblade array impact applicator |
| US20020091357A1 (en) | 2000-10-13 | 2002-07-11 | Trautman Joseph C. | Microprotrusion member retainer for impact applicator |
| US20020096488A1 (en) | 2001-01-24 | 2002-07-25 | Xerox Corporation | Method for fabricating a micro-electro-mechanical fluid ejector |
| JP2002239014A (en) | 2001-02-19 | 2002-08-27 | Sumitomo Precision Prod Co Ltd | Needle-like body and method for manufacturing needle- like body |
| US6440096B1 (en) | 2000-07-14 | 2002-08-27 | Becton, Dickinson And Co. | Microdevice and method of manufacturing a microdevice |
| US20020133129A1 (en) | 2001-03-14 | 2002-09-19 | Francisco Arias | Method of manufacturing microneedle structures using soft lithography and photolithography |
| US20020138049A1 (en) | 1998-06-10 | 2002-09-26 | Allen Mark G. | Microneedle devices and methods of manufacture and use thereof |
| US6476288B1 (en) | 1994-11-28 | 2002-11-05 | The Procter & Gamble Company | Absorbent articles having cuffs and topsheet with skin care composition(s) disposed thereon |
| US20020169411A1 (en) | 2001-05-11 | 2002-11-14 | The Procter & Gamble Co. | Portable interstitial fluid monitoring system |
| US20020177839A1 (en) | 2001-04-20 | 2002-11-28 | Cormier Michel J. N. | Microprojection array having a beneficial agent containing coating |
| US20020177858A1 (en) | 2000-10-16 | 2002-11-28 | Sherman Faiz Feisal | Microstructures and method for treating and conditioning skin which cause less irritation during exfoliation |
| US20020188245A1 (en) | 2001-06-08 | 2002-12-12 | Martin Frank E. | Device for manipulating a needle or abrader array and method of use |
| US6494830B1 (en) | 2000-06-22 | 2002-12-17 | Guidance Interactive Technologies, Inc. | Handheld controller for monitoring/using medical parameters |
| US20020193729A1 (en) | 2001-04-20 | 2002-12-19 | Cormier Michel J.N. | Microprojection array immunization patch and method |
| US6511463B1 (en) | 1999-11-18 | 2003-01-28 | Jds Uniphase Corporation | Methods of fabricating microneedle arrays using sacrificial molds |
| JP2003039399A (en) | 2001-07-31 | 2003-02-13 | Dainippon Printing Co Ltd | Stamp for thin film pattern formation |
| JP2003048160A (en) | 2001-08-09 | 2003-02-18 | Tokyo Denki Univ | Micro-groove processing method and device |
| US6532386B2 (en) | 1998-08-31 | 2003-03-11 | Johnson & Johnson Consumer Companies, Inc. | Electrotransort device comprising blades |
| US6533884B1 (en) | 2000-11-03 | 2003-03-18 | Printpack Illinois, Inc. | Method and system for extrusion embossing |
| US6537242B1 (en)* | 2000-06-06 | 2003-03-25 | Becton, Dickinson And Company | Method and apparatus for enhancing penetration of a member for the intradermal sampling or administration of a substance |
| US6558361B1 (en) | 2000-03-09 | 2003-05-06 | Nanopass Ltd. | Systems and methods for the transport of fluids through a biological barrier and production techniques for such systems |
| US6562014B2 (en) | 1999-12-16 | 2003-05-13 | Alza Corporation | Device and method for enhancing transdermal flux of agents being sampled |
| US20030093089A1 (en) | 1999-10-20 | 2003-05-15 | Greenberg Ronald Allan | Apparatus for variable micro abrasion of human tissue and/or hides using different size and types of abrasive particles |
| US20030093028A1 (en) | 2001-11-09 | 2003-05-15 | Michael Spiegel | Appararus and method for magnetic induction of therapeutic electric fields |
| US6565532B1 (en) | 2000-07-12 | 2003-05-20 | The Procter & Gamble Company | Microneedle apparatus used for marking skin and for dispensing semi-permanent subcutaneous makeup |
| US6585742B2 (en) | 2001-05-08 | 2003-07-01 | Dowling B. Stough | Wart removal method and device |
| US6589202B1 (en) | 2000-06-29 | 2003-07-08 | Becton Dickinson And Company | Method and apparatus for transdermally sampling or administering a substance to a patient |
| US6591133B1 (en) | 2000-11-27 | 2003-07-08 | Microlin Llc | Apparatus and methods for fluid delivery using electroactive needles and implantable electrochemical delivery devices |
| US20030135167A1 (en) | 2001-09-19 | 2003-07-17 | Gonnelli Robert R. | Microneedles, microneedle arrays, and systems and methods relating to same |
| US6611707B1 (en) | 1999-06-04 | 2003-08-26 | Georgia Tech Research Corporation | Microneedle drug delivery device |
| US6611706B2 (en) | 1998-11-09 | 2003-08-26 | Transpharma Ltd. | Monopolar and bipolar current application for transdermal drug delivery and analyte extraction |
| US20030166624A1 (en) | 1996-07-15 | 2003-09-04 | Gale Robert M. | Novel formulations for the administration of fluoxetine |
| US20030187394A1 (en) | 2002-04-02 | 2003-10-02 | Wilkinson Bradley M | Method and device for intradermally delivering a substance |
| US6629949B1 (en) | 2000-05-08 | 2003-10-07 | Sterling Medivations, Inc. | Micro infusion drug delivery device |
| US20030199810A1 (en) | 2001-11-30 | 2003-10-23 | Trautman Joseph Creagan | Methods and apparatuses for forming microprojection arrays |
| US20030208138A1 (en) | 2001-07-09 | 2003-11-06 | Lorin Olson | Micro-needles and methods of manufacture and use thereof |
| US20030220656A1 (en) | 2002-05-24 | 2003-11-27 | Vladimir Gartstein | Method of exfoliation of skin using closely-packed microstructures |
| US20030220610A1 (en)* | 2002-02-04 | 2003-11-27 | Becton Dickinson And Company | Device and method for delivering or withdrawing a substance through the skin |
| US6656147B1 (en) | 2000-07-17 | 2003-12-02 | Becton, Dickinson And Company | Method and delivery device for the transdermal administration of a substance |
| US6685682B1 (en) | 1993-03-22 | 2004-02-03 | 3M Innovative Properties Company | Carrier delivered dressing and method of manufacture |
| US6689103B1 (en) | 1999-05-07 | 2004-02-10 | Scimed Life System, Inc. | Injection array apparatus and method |
| US6691752B2 (en) | 2000-09-15 | 2004-02-17 | Timberjack Inc. | High rotation felling head mechanism |
| JP2004065775A (en) | 2002-08-08 | 2004-03-04 | Sanwa Kagaku Kenkyusho Co Ltd | Device equipped with needle-like structure element |
| US20040087992A1 (en) | 2002-08-09 | 2004-05-06 | Vladimir Gartstein | Microstructures for delivering a composition cutaneously to skin using rotatable structures |
| US20040096455A1 (en) | 2002-08-08 | 2004-05-20 | Yuh-Fun Maa | Transdermal vaccine delivery device having coated microprotrusions |
| US6743211B1 (en)* | 1999-11-23 | 2004-06-01 | Georgia Tech Research Corporation | Devices and methods for enhanced microneedle penetration of biological barriers |
| US20040143211A1 (en) | 2002-08-29 | 2004-07-22 | Haider M. Ishaq | Substance delivery via a rotating microabrading surface |
| US6767341B2 (en) | 2001-06-13 | 2004-07-27 | Abbott Laboratories | Microneedles for minimally invasive drug delivery |
| US6770480B1 (en) | 1998-07-22 | 2004-08-03 | Psimedica Limited | Transferring materials into cells using porous silicon |
| US6778853B1 (en) | 1997-12-17 | 2004-08-17 | University Of South Florida | Electroporation device |
| US6780171B2 (en) | 2002-04-02 | 2004-08-24 | Becton, Dickinson And Company | Intradermal delivery device |
| US20040164454A1 (en) | 2003-02-24 | 2004-08-26 | The Procter & Gamble Company | Method for manufacturing microstructures having multiple microelements with through-holes |
| US20040181203A1 (en) | 1999-12-10 | 2004-09-16 | Cormier Michel J.N. | Skin treatment method and apparatus for sustained transdermal drug delivery |
| US20040204669A1 (en) | 2001-07-05 | 2004-10-14 | Hofmann Gunter A. | Apparatus for electroporation mediated delivery for drugs and genes |
| US20040236271A1 (en) | 1997-12-10 | 2004-11-25 | Felix Theeuwes | Device and method for enhancing transdermal agent flux |
| US6881203B2 (en) | 2001-09-05 | 2005-04-19 | 3M Innovative Properties Company | Microneedle arrays and methods of manufacturing the same |
| US20050089554A1 (en) | 2003-10-24 | 2005-04-28 | Cormier Michel J. | Apparatus and method for enhancing transdermal drug delivery |
| US20050096586A1 (en) | 2003-10-31 | 2005-05-05 | Trautman Joseph C. | Self-actuating applicator for microprojection array |
| US20050163827A1 (en) | 2004-01-23 | 2005-07-28 | Herbert Zech | Transdermal therapeutic delivery system |
| US20050197308A1 (en) | 2000-07-21 | 2005-09-08 | Smithkline Beecham Biologicals S.A. | Vaccines |
| US6945952B2 (en) | 2002-06-25 | 2005-09-20 | Theraject, Inc. | Solid solution perforator for drug delivery and other applications |
| WO2005089857A1 (en) | 2004-03-15 | 2005-09-29 | Unilever Plc | Skin microactivation system |
| US20050228340A1 (en) | 2004-03-24 | 2005-10-13 | Cleary Gary W | Transdermal delivery device |
| US20060076718A1 (en) | 2003-06-02 | 2006-04-13 | The Procter & Gamble Company | Method for manufacturing microstructures having hollow microelements using fluidic jets during a molding operation |
| US7131960B2 (en) | 2000-10-13 | 2006-11-07 | Alza Corporation | Apparatus and method for piercing skin with microprotrusions |
| JP2006341089A (en) | 2005-05-13 | 2006-12-21 | Fujikura Ltd | Medicinal product carrying device and method of manufacturing |
| WO2008011625A2 (en) | 2006-07-21 | 2008-01-24 | Georgia Tech Researh Corporation | Microneedle devices and methods of drug delivery or fluid withdrawal |
| US20080114298A1 (en)* | 2004-11-18 | 2008-05-15 | Cantor Adam S | Low-Profile Microneedle Array Applicator |
| WO2008091602A2 (en) | 2007-01-22 | 2008-07-31 | Corium International, Inc. | Applicators for microneedle arrays |
| US20080195035A1 (en)* | 2005-06-24 | 2008-08-14 | Frederickson Franklyn L | Collapsible Patch and Method of Application |
| US20080269685A1 (en) | 2007-04-16 | 2008-10-30 | Parminder Singh | Solvent-cast microneedle arrays containing active |
| WO2009048607A1 (en) | 2007-10-10 | 2009-04-16 | Corium International, Inc. | Vaccine delivery via microneedle arrays |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE81254T1 (en)* | 1985-02-26 | 1992-10-15 | Univ Johns Hopkins | NEOVASCULARIZATION INHIBITORS AND THEIR PRODUCTION. |
| KR20060037320A (en)* | 2003-07-02 | 2006-05-03 | 알자 코포레이션 | Microprojection Array Immunization Patches and Methods |
| US9119945B2 (en)* | 2006-04-20 | 2015-09-01 | 3M Innovative Properties Company | Device for applying a microneedle array |
- 2008
- 2008-01-22CACA2676221Apatent/CA2676221C/enactiveActive
- 2008-01-22USUS12/009,954patent/US8821446B2/enactiveActive
- 2008-01-22EPEP08724711.0Apatent/EP2121111B1/enactiveActive
- 2008-01-22WOPCT/US2008/000824patent/WO2008091602A2/enactiveApplication Filing
- 2008-01-22AUAU2008209537Apatent/AU2008209537B2/ennot_activeCeased
- 2008-01-22JPJP2009546449Apatent/JP5553612B2/enactiveActive
- 2013
- 2013-07-30JPJP2013157638Apatent/JP2013215621A/ennot_activeWithdrawn
Patent Citations (321)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1554510A (en) | 1924-04-23 | 1925-09-22 | Elsie W Kirby | Massaging device |
| US1770632A (en) | 1928-07-02 | 1930-07-15 | Arthur E Smith | Ampul syringe |
| US2046240A (en) | 1932-07-01 | 1936-06-30 | Thomas W Bayley | Abrasive article |
| US2434407A (en) | 1946-01-05 | 1948-01-13 | Hofe George Douglas | Depilatory device |
| US3675766A (en) | 1970-02-04 | 1972-07-11 | Sol Roy Rosenthal | Multiple puncture injector device |
| US3704194A (en) | 1970-07-02 | 1972-11-28 | Gen Electric | Perforated reinforced plastic member and method for making |
| US3873255A (en) | 1971-01-27 | 1975-03-25 | Johnson & Johnson | Apparatus for producing nonwoven fabric |
| US3964482A (en) | 1971-05-17 | 1976-06-22 | Alza Corporation | Drug delivery device |
| US3814097A (en) | 1972-02-14 | 1974-06-04 | Ici Ltd | Dressing |
| US3918449A (en) | 1973-06-06 | 1975-11-11 | Guerin A Ets | Device for cutaneous therapeutic treatment |
| US4055029A (en) | 1975-03-07 | 1977-10-25 | Heinz Kalbow | Cleaning, scouring and/or polishing pads |
| US4151240A (en) | 1976-10-19 | 1979-04-24 | The Procter & Gamble Company | Method for debossing and perforating a running ribbon of thermoplastic film |
| US4180232A (en) | 1977-01-13 | 1979-12-25 | Hardigg James S | Truss panel mold |
| US4117841A (en) | 1977-02-07 | 1978-10-03 | Anthony Perrotta | Medicated bandage pocket |
| US4342314A (en) | 1979-03-05 | 1982-08-03 | The Procter & Gamble Company | Resilient plastic web exhibiting fiber-like properties |
| US4402696A (en) | 1979-10-15 | 1983-09-06 | Gulko Bruce N | Folded applicator |
| US4381963A (en) | 1980-07-30 | 1983-05-03 | The University Of Rochester | Micro fabrication molding process |
| US4509908A (en) | 1981-02-02 | 1985-04-09 | The Procter & Gamble Company | Apparatus for uniformly debossing and aperturing a resilient plastic web |
| US4395215A (en) | 1981-02-02 | 1983-07-26 | The Procter & Gamble Company | Film forming structure for uniformly debossing and selectively aperturing a resilient plastic web and method for its construction |
| US4463045A (en) | 1981-03-02 | 1984-07-31 | The Procter & Gamble Company | Macroscopically expanded three-dimensional plastic web exhibiting non-glossy visible surface and cloth-like tactile impression |
| US4460368A (en) | 1981-10-29 | 1984-07-17 | Almedco, Inc. | Trans-dermal medication system |
| US4460370A (en) | 1981-10-29 | 1984-07-17 | Almedco, Inc. | Trans-dermal medication application cell |
| US4966159A (en) | 1981-12-14 | 1990-10-30 | Maganias Nicholas H | Allergy test strip |
| US4585991A (en) | 1982-06-03 | 1986-04-29 | Texas Instruments Incorporated | Solid state multiprobe testing apparatus |
| FR2535602B1 (en) | 1982-11-05 | 1986-06-13 | Stallergenes Lab | SCARIFIER DEVICE |
| US4556441A (en) | 1983-01-24 | 1985-12-03 | Faasse Jr Adrian L | Pharmaceutical packaging method |
| US4515168A (en) | 1983-07-22 | 1985-05-07 | Chester Martin H | Clamp-on nerve stimulator and locator |
| US6129696A (en) | 1983-08-18 | 2000-10-10 | Drug Delivery Systems Inc. | Transdermal drug applicator |
| US5591123A (en) | 1983-08-18 | 1997-01-07 | Drug Delivery Systems Inc. | Programmable control mounting system for transdermal drug applicator |
| EP0156471B1 (en) | 1984-02-16 | 1989-05-03 | The Procter & Gamble Company | Process and apparatus for forming a continuous web, particularly polymeric film, and material produced by the process |
| EP0312662A1 (en) | 1984-08-24 | 1989-04-26 | David George Vlock | Fluid dispenser |
| US5711761A (en) | 1984-10-29 | 1998-01-27 | Alza Corporation | Iontophoretic drug delivery |
| US4597961A (en) | 1985-01-23 | 1986-07-01 | Etscorn Frank T | Transcutaneous application of nicotine |
| US4904475A (en) | 1985-05-03 | 1990-02-27 | Alza Corporation | Transdermal delivery of drugs from an aqueous reservoir |
| US4609518A (en) | 1985-05-31 | 1986-09-02 | The Procter & Gamble Company | Multi-phase process for debossing and perforating a polymeric web to coincide with the image of one or more three-dimensional forming structures |
| US4630603A (en) | 1986-02-13 | 1986-12-23 | The Kendall Company | Wound dressing |
| US4743249A (en) | 1986-02-14 | 1988-05-10 | Ciba-Geigy Corp. | Dermal and transdermal patches having a discontinuous pattern adhesive layer |
| EP0240593B1 (en) | 1986-03-14 | 1993-05-19 | Drug Delivery Systems Inc. | Improved transdermal drug applicator and electrodes therefor |
| US4784737A (en) | 1986-04-18 | 1988-11-15 | The United States Department Of Energy | Electromicroinjection of particles into living cells |
| US4837049A (en) | 1986-06-17 | 1989-06-06 | Alfred E. Mann Foundation For Scientific Research | Method of making an electrode array |
| EP0301599B1 (en) | 1987-07-30 | 1995-03-29 | Applied Extrusion Technologies, Inc. | Apertured film and net like fabrics from thermoplastic materials |
| US5061258A (en) | 1987-08-07 | 1991-10-29 | Martz Joel D | Vapor permeable dressing with releasable medication |
| US4846821A (en) | 1987-08-24 | 1989-07-11 | The Procter & Gamble Company | Substantially fluid-impervious microbubbled polymeric web exhibiting low levels of noise when subjected to movement |
| US4812305A (en) | 1987-11-09 | 1989-03-14 | Vocal Rodolfo S | Well medicine strip |
| SU1641346A1 (en) | 1987-12-08 | 1991-04-15 | Мгту Им.Н.Э.Баумана | Electrostimulation appliance |
| US5051259A (en) | 1987-12-15 | 1991-09-24 | Coloplast A/S | Skin barrier product with discontinuous adhesive layer |
| EP0400249B1 (en) | 1988-01-19 | 1994-06-01 | Perfojet S.A. | Apparatus for perforating a web |
| US5730714A (en) | 1988-01-29 | 1998-03-24 | The Regents Of The University Of California | Method for the iontophoretic non-invasive determination of the in vivo concentration level of glucose |
| US5198192A (en) | 1988-05-18 | 1993-03-30 | Inax Corporation | Apparatus for detecting ingredient in urine, a toilet stool equipped with a urine detecting device and a room for urine detecting facility |
| GB2221394B (en) | 1988-08-05 | 1992-03-04 | Eilert Eilertsen | An injection device |
| US5244677A (en) | 1988-12-29 | 1993-09-14 | Minnesota Mining And Manufacturing Company | Application system for drug containing microemulsions |
| US5362307A (en) | 1989-01-24 | 1994-11-08 | The Regents Of The University Of California | Method for the iontophoretic non-invasive-determination of the in vivo concentration level of an inorganic or organic substance |
| SU1667864A1 (en) | 1989-03-23 | 1991-08-07 | Опытный завод энергетического машиностроения | Electrostimulation device |
| US5134079A (en) | 1989-03-27 | 1992-07-28 | International Technidyne Corp. | Fluid sample collection and delivery system and methods particularly adapted for body fluid sampling |
| US5788983A (en) | 1989-04-03 | 1998-08-04 | Rutgers, The State University Of New Jersey | Transdermal controlled delivery of pharmaceuticals at variable dosage rates and processes |
| US5531675A (en) | 1989-05-10 | 1996-07-02 | Yoo; Tae W. | Micro-acupuncture needle for a finger of a hand |
| EP0407063A1 (en) | 1989-07-06 | 1991-01-09 | Connaught Laboratories Limited | Tines structure of clinical applicator |
| US5250023A (en) | 1989-10-27 | 1993-10-05 | Korean Research Institute on Chemical Technology | Transdermal administration method of protein or peptide drug and its administration device thereof |
| US5215088A (en) | 1989-11-07 | 1993-06-01 | The University Of Utah | Three-dimensional electrode device |
| US5190558A (en) | 1989-11-08 | 1993-03-02 | Nec Corporation | Method of eliminating stratum corneum from the skin and an instrument to be used therefor |
| US5697901A (en) | 1989-12-14 | 1997-12-16 | Elof Eriksson | Gene delivery by microneedle injection |
| US5244711A (en) | 1990-03-12 | 1993-09-14 | Mcneil-Ppc, Inc. | Apertured non-woven fabric |
| US5162043A (en) | 1990-03-30 | 1992-11-10 | Alza Corporation | Iontophoretic delivery device |
| US5139029A (en) | 1990-04-06 | 1992-08-18 | Henry Fishman | Allergy testing apparatus and method |
| US5279544A (en) | 1990-12-13 | 1994-01-18 | Sil Medics Ltd. | Transdermal or interdermal drug delivery devices |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5848991A (en) | 1990-12-13 | 1998-12-15 | Elan Medical Technologies Limited Athlone, Co. | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5156591A (en) | 1990-12-13 | 1992-10-20 | S. I. Scientific Innovations Ltd. | Skin electrode construction and transdermal drug delivery device utilizing same |
| US5158073A (en) | 1990-12-18 | 1992-10-27 | Bukowski Voytek Z | Acupressure foot massage mat |
| US5252279A (en) | 1991-01-17 | 1993-10-12 | Reinhold Industries | Method for making perforated articles |
| US5676850A (en) | 1991-01-31 | 1997-10-14 | Carnegie Mellon University | Micromechanical barb and method for making the same |
| US5160315A (en) | 1991-04-05 | 1992-11-03 | Minnesota Mining And Manufacturing Company | Combined adhesive strip and transparent dressing delivery system |
| US5520629A (en) | 1991-04-05 | 1996-05-28 | Minnesota Mining And Manufacturing Company | Combined adhesive strip and transparent dressing delivery system |
| US5997986A (en) | 1991-08-14 | 1999-12-07 | Chicopee | Textile-like apertured plastic films |
| US5567376A (en) | 1991-08-14 | 1996-10-22 | Chicopee | Method of forming textile-like apertured plastic films |
| US5256360A (en) | 1992-03-25 | 1993-10-26 | Panasonic Technologies, Inc. | Method of manufacturing a precision micro-filter |
| US5756117A (en) | 1992-04-08 | 1998-05-26 | International Medical Asscociates, Inc. | Multidose transdermal drug delivery system |
| US5932240A (en) | 1992-04-08 | 1999-08-03 | Americare Technology, Inc. | Multidose transdermal drug delivery system |
| US5318557A (en) | 1992-07-13 | 1994-06-07 | Elan Medical Technologies Limited | Medication administering device |
| US5308625A (en) | 1992-09-02 | 1994-05-03 | Cygnus Therapeutic Systems | Enhancement of transdermal drug delivery using monoalkyl phosphates and other absorption promoters |
| US5462743A (en) | 1992-10-30 | 1995-10-31 | Medipro Sciences Limited | Substance transfer system for topical application |
| US5250067A (en) | 1992-11-30 | 1993-10-05 | Ala Gelfer | Body treatment pad having a multiple number of sharpened skin-penetration protuberances |
| US5383512A (en) | 1993-01-27 | 1995-01-24 | Midwest Research Institute | Method for fabricating a substrate having spaced apart microcapillaries thereon |
| US6685682B1 (en) | 1993-03-22 | 2004-02-03 | 3M Innovative Properties Company | Carrier delivered dressing and method of manufacture |
| US5531855A (en) | 1993-03-22 | 1996-07-02 | Minnesota Mining And Manufacturing Company | Carrier delivered dressing and method of manufacture |
| US6169224B1 (en) | 1993-03-22 | 2001-01-02 | 3M Innovative Properties Company | Carrier delivered dressing and method of manufacture |
| GB2277202A (en) | 1993-03-22 | 1994-10-19 | Peter Leslie Moran | Electrical circuit board manufacture |
| US5738642A (en) | 1993-03-22 | 1998-04-14 | Minnesota Mining And Manufacturing Company | Carrier delivered dressing and method of manufacture |
| US5476443A (en) | 1993-05-27 | 1995-12-19 | New Dimensions In Medicine, Inc. | Wound dressing product containing a porous layer |
| US5330452A (en) | 1993-06-01 | 1994-07-19 | Zook Gerald P | Topical medicating device |
| US5728089A (en) | 1993-06-04 | 1998-03-17 | The Regents Of The University Of California | Microfabricated structure to be used in surgery |
| US5320600A (en) | 1993-06-14 | 1994-06-14 | Lambert Wm S | Plural content container for simultaneous ejection |
| US5704520A (en) | 1993-07-19 | 1998-01-06 | Elan Medical Technologies, Limited | Liquid material dispenser and valve |
| US5848990A (en) | 1993-10-22 | 1998-12-15 | Hoffmann-La Roche Inc. | Device for introducing active substance into a patient |
| US5962011A (en) | 1993-12-06 | 1999-10-05 | Schering-Plough Healthcare Products, Inc. | Device for delivery of dermatological ingredients |
| US5457041A (en) | 1994-03-25 | 1995-10-10 | Science Applications International Corporation | Needle array and method of introducing biological substances into living cells using the needle array |
| US5536263A (en) | 1994-03-30 | 1996-07-16 | Lectec Corporation | Non-occulusive adhesive patch for applying medication to the skin |
| US5503843A (en) | 1994-04-22 | 1996-04-02 | Flora Inc. | Transdermal delivery of alpha adrenoceptor blocking agents |
| US5611806A (en) | 1994-05-23 | 1997-03-18 | Samsung Electro-Mechanics Co., Ltd. | Skin perforating device for transdermal medication |
| US5964729A (en) | 1994-05-23 | 1999-10-12 | Samsung Electro-Mechanics Co., Ltd. | Perforating device for dermal administration |
| US5681580A (en) | 1994-05-23 | 1997-10-28 | Samsung Electro-Mechanics Co., Ltd. | Patch-type device for iontophoretic transdermal delivery of insulin |
| DE19518974C2 (en) | 1994-05-23 | 2000-02-24 | Samsung Electro Mech | Perforation device for administration through the skin |
| US5843114A (en) | 1994-05-23 | 1998-12-01 | Samsung Electro-Mechanics Co., Ltd. | Skin perforating apparatus for transdermal medication |
| US5851549A (en) | 1994-05-25 | 1998-12-22 | Becton Dickinson And Company | Patch, with system and apparatus for manufacture |
| US5512219A (en) | 1994-06-03 | 1996-04-30 | Reflexite Corporation | Method of casting a microstructure sheet having an array of prism elements using a reusable polycarbonate mold |
| US5855801A (en) | 1994-06-06 | 1999-01-05 | Lin; Liwei | IC-processed microneedles |
| US5591139A (en) | 1994-06-06 | 1997-01-07 | The Regents Of The University Of California | IC-processed microneedles |
| US5487726A (en) | 1994-06-16 | 1996-01-30 | Ryder International Corporation | Vaccine applicator system |
| US5771890A (en) | 1994-06-24 | 1998-06-30 | Cygnus, Inc. | Device and method for sampling of substances using alternating polarity |
| US6023629A (en) | 1994-06-24 | 2000-02-08 | Cygnus, Inc. | Method of sampling substances using alternating polarity of iontophoretic current |
| US5496304A (en) | 1994-07-20 | 1996-03-05 | University Of Utah Research Foundation | Surgical marking pen |
| US5498235A (en) | 1994-09-30 | 1996-03-12 | Becton Dickinson And Company | Iontophoresis assembly including patch/controller attachment |
| US5551953A (en) | 1994-10-31 | 1996-09-03 | Alza Corporation | Electrotransport system with remote telemetry link |
| US5807375A (en) | 1994-11-04 | 1998-09-15 | Elan Medical Technologies Limited | Analyte-controlled liquid delivery device and analyte monitor |
| US5800420A (en) | 1994-11-04 | 1998-09-01 | Elan Medical Technologies Limited | Analyte-controlled liquid delivery device and analyte monitor |
| US5820622A (en) | 1994-11-04 | 1998-10-13 | Elan Medical Technologies Limited | Analyte-controlled liquid delivery device and analyte monitor |
| US6476288B1 (en) | 1994-11-28 | 2002-11-05 | The Procter & Gamble Company | Absorbent articles having cuffs and topsheet with skin care composition(s) disposed thereon |
| EP0796128B1 (en) | 1994-12-09 | 1999-03-10 | Novartis AG | Transdermal system |
| US5879326A (en) | 1995-05-22 | 1999-03-09 | Godshall; Ned Allen | Method and apparatus for disruption of the epidermis |
| US6132755A (en) | 1995-07-14 | 2000-10-17 | Boehringer Ingelheim Kg | Transcorneal drug-release system |
| US5814020A (en) | 1995-09-11 | 1998-09-29 | Elan Medical Technlogies Limited | Medicament delivery device |
| US5735273A (en) | 1995-09-12 | 1998-04-07 | Cygnus, Inc. | Chemical signal-impermeable mask |
| US5827183A (en) | 1995-09-12 | 1998-10-27 | Cygnus, Inc. | Method of measuring chemical concentration iontophoretically using impermeable mask |
| US5645977A (en) | 1995-09-22 | 1997-07-08 | Industrial Technology Research Institute | Method of making molds for manufacturing multiple-lead microstructures |
| US5658515A (en) | 1995-09-25 | 1997-08-19 | Lee; Abraham P. | Polymer micromold and fabrication process |
| US5662127A (en) | 1996-01-17 | 1997-09-02 | Bio-Plas, Inc. | Self-contained blood withdrawal apparatus and method |
| US5730721A (en) | 1996-01-25 | 1998-03-24 | Vesture Corporation | Medical applicator and method |
| US5987989A (en) | 1996-02-05 | 1999-11-23 | Denso Corporation | Semiconductor physical quantity sensor |
| US5848985A (en) | 1996-02-09 | 1998-12-15 | Polytronics, Ltd. | Skin-contact type medical treatment apparatus |
| US5948488A (en) | 1996-04-30 | 1999-09-07 | 3M Innovative Properties Company | Glittering cube-corner article |
| US6219574B1 (en) | 1996-06-18 | 2001-04-17 | Alza Corporation | Device and method for enchancing transdermal sampling |
| US7184826B2 (en) | 1996-06-18 | 2007-02-27 | Alza Corporation | Device and method for enhancing transdermal flux of agents being delivered or sampled |
| US6537264B1 (en) | 1996-06-18 | 2003-03-25 | Alza Corp | Device and method for enhancing transdermal flux of agents being sampled |
| US20020016562A1 (en) | 1996-06-18 | 2002-02-07 | Michel J. N. Cormier | Device and method for enhancing transdermal flux of agents being delivered or sampled |
| US6230051B1 (en) | 1996-06-18 | 2001-05-08 | Alza Corporation | Device for enhancing transdermal agent delivery or sampling |
| DE19624578A1 (en) | 1996-06-20 | 1998-01-08 | Nancy L Divers | Skin care device |
| US6183434B1 (en) | 1996-07-03 | 2001-02-06 | Spectrx, Inc. | Multiple mechanical microporation of skin or mucosa |
| US7011844B2 (en) | 1996-07-15 | 2006-03-14 | Alza Corporation | Formulations for the administration of fluoxetine |
| US20030166624A1 (en) | 1996-07-15 | 2003-09-04 | Gale Robert M. | Novel formulations for the administration of fluoxetine |
| US5983136A (en) | 1996-09-17 | 1999-11-09 | Deka Products Limited Partnership | System for delivery of drugs by transport |
| US6156336A (en) | 1996-11-27 | 2000-12-05 | Lts Lohmann Therapie-Systeme Gmbh | Method for the low-waste manufacture of diskoid moulded bodies containing active ingredients, and transdermal therapeutic systems containing same |
| US5873849A (en) | 1997-04-24 | 1999-02-23 | Ichor Medical Systems, Inc. | Electrodes and electrode arrays for generating electroporation inducing electrical fields |
| US6038485A (en) | 1997-06-12 | 2000-03-14 | Axelgaard Manufacturing Co., Ltd. | Current-controlling electrode |
| US6187210B1 (en) | 1997-06-30 | 2001-02-13 | The Regents Of The University Of California | Epidermal abrasion device with isotropically etched tips, and method of fabricating such a device |
| US6241701B1 (en) | 1997-08-01 | 2001-06-05 | Genetronics, Inc. | Apparatus for electroporation mediated delivery of drugs and genes |
| US6055453A (en) | 1997-08-01 | 2000-04-25 | Genetronics, Inc. | Apparatus for addressing needle array electrodes for electroporation therapy |
| US7412284B2 (en) | 1997-08-01 | 2008-08-12 | Genetronics, Inc. | Apparatus for electroporation mediated delivery for drugs and genes |
| US6014584A (en) | 1997-08-01 | 2000-01-11 | Genetronics, Inc. | Electroporation therapy apparatus |
| US6516223B2 (en) | 1997-08-01 | 2003-02-04 | Genetronics, Inc. | Apparatus for electroporation mediated delivery for drugs and genes |
| US6216034B1 (en) | 1997-08-01 | 2001-04-10 | Genetronics, Inc. | Method of programming an array of needle electrodes for electroporation therapy of tissue |
| US20020133137A1 (en) | 1997-08-01 | 2002-09-19 | Hofmann Gunter A. | Apparatus for electroporation mediated delivery for drugs and genes |
| US6181964B1 (en) | 1997-08-01 | 2001-01-30 | Genetronics, Inc. | Minimally invasive apparatus and method to electroporate drugs and genes into tissue |
| US6047208A (en) | 1997-08-27 | 2000-04-04 | Becton, Dickinson And Company | Iontophoretic controller |
| US20010023324A1 (en) | 1997-11-03 | 2001-09-20 | Allan Pronovost | Glucose detector and method for diagnosing diabetes |
| US5938684A (en) | 1997-12-09 | 1999-08-17 | Sierra Self Healing Products, Inc. | Accupressure device for therapeutic relief |
| US20040236271A1 (en) | 1997-12-10 | 2004-11-25 | Felix Theeuwes | Device and method for enhancing transdermal agent flux |
| US6050988A (en) | 1997-12-11 | 2000-04-18 | Alza Corporation | Device for enhancing transdermal agent flux |
| US6083196A (en) | 1997-12-11 | 2000-07-04 | Alza Corporation | Device for enhancing transdermal agent flux |
| US6322808B1 (en)* | 1997-12-11 | 2001-11-27 | Alza Corporation | Device for enhancing transdermal agent flux |
| US6135990A (en) | 1997-12-17 | 2000-10-24 | University Of South Florida | Electroporation device and method |
| US6778853B1 (en) | 1997-12-17 | 2004-08-17 | University Of South Florida | Electroporation device |
| US6024553A (en) | 1997-12-22 | 2000-02-15 | Mcneil-Ppc, Inc. | Apparatus for supporting a starting web during formation of the apertured web |
| US6375978B1 (en) | 1997-12-22 | 2002-04-23 | Alza Corporation | Rate controlling membranes for controlled drug delivery devices |
| US6106751A (en) | 1998-03-18 | 2000-08-22 | The Regents Of The University Of California | Method for fabricating needles via conformal deposition in two-piece molds |
| US6091975A (en) | 1998-04-01 | 2000-07-18 | Alza Corporation | Minimally invasive detecting device |
| US6120792A (en) | 1998-04-29 | 2000-09-19 | Juni; Jack E. | Medicated skin patch and method for its use |
| US6334856B1 (en) | 1998-06-10 | 2002-01-01 | Georgia Tech Research Corporation | Microneedle devices and methods of manufacture and use thereof |
| US20020138049A1 (en) | 1998-06-10 | 2002-09-26 | Allen Mark G. | Microneedle devices and methods of manufacture and use thereof |
| US6503231B1 (en) | 1998-06-10 | 2003-01-07 | Georgia Tech Research Corporation | Microneedle device for transport of molecules across tissue |
| US7332339B2 (en) | 1998-07-22 | 2008-02-19 | Psimedica Limited | Transferring materials into cells using porous silicon |
| US6770480B1 (en) | 1998-07-22 | 2004-08-03 | Psimedica Limited | Transferring materials into cells using porous silicon |
| US20040220535A1 (en) | 1998-07-22 | 2004-11-04 | Psimedica Limited | Transferring materials into cells porous silicon |
| US6532386B2 (en) | 1998-08-31 | 2003-03-11 | Johnson & Johnson Consumer Companies, Inc. | Electrotransort device comprising blades |
| JP2000146777A (en) | 1998-09-08 | 2000-05-26 | Matsushita Electric Ind Co Ltd | Sample collection method, sample collection device, sample collection container, sample measurement method, sample measurement device, and sample measurement container |
| US6036659A (en) | 1998-10-09 | 2000-03-14 | Flexsite Diagnostics, Inc. | Collection device for biological samples and methods of use |
| US6038465A (en) | 1998-10-13 | 2000-03-14 | Agilent Technologies, Inc. | Telemedicine patient platform |
| US7062317B2 (en) | 1998-11-09 | 2006-06-13 | Transpharma Ltd. | Monopolar and bipolar current application for transdermal drug delivery and analyte extraction |
| US20030212397A1 (en) | 1998-11-09 | 2003-11-13 | Zohar Avrahami | Monopolar and bipolar current application for transdermal drug delivery and analyte extraction |
| US6611706B2 (en) | 1998-11-09 | 2003-08-26 | Transpharma Ltd. | Monopolar and bipolar current application for transdermal drug delivery and analyte extraction |
| US6375870B1 (en) | 1998-11-17 | 2002-04-23 | Corning Incorporated | Replicating a nanoscale pattern |
| JP2000147229A (en) | 1998-11-17 | 2000-05-26 | Corning Inc | Duplication of pattern of nanometer scale |
| JP2000164890A (en) | 1998-11-27 | 2000-06-16 | Denso Corp | Semiconductor kinematic value sensor and manufacturing method therefor |
| JP2000194142A (en) | 1998-12-25 | 2000-07-14 | Fujitsu Ltd | Pattern forming method and semiconductor device manufacturing method |
| JP2000232095A (en) | 1999-02-12 | 2000-08-22 | Nippon Telegr & Teleph Corp <Ntt> | Method for forming fine pattern on semiconductor surface |
| JP2000232971A (en) | 1999-02-15 | 2000-08-29 | Arkray Inc | Sucked transudate sampling method and device |
| US5997549A (en) | 1999-02-16 | 1999-12-07 | Sauceda; Charles J. | Wart removing tool |
| US6132449A (en) | 1999-03-08 | 2000-10-17 | Agilent Technologies, Inc. | Extraction and transportation of blood for analysis |
| US6183770B1 (en) | 1999-04-15 | 2001-02-06 | Acutek International | Carrier patch for the delivery of agents to the skin |
| US6689103B1 (en) | 1999-05-07 | 2004-02-10 | Scimed Life System, Inc. | Injection array apparatus and method |
| JP2000323461A (en) | 1999-05-11 | 2000-11-24 | Nec Corp | Fine pattern forming device, its manufacture, and method of forming the same |
| JP2000322780A (en) | 1999-05-14 | 2000-11-24 | Yasunori Saotome | Stamper for information recording disk, method of manufacturing the same, information recording disk, and method of manufacturing information recording disk |
| US7226439B2 (en) | 1999-06-04 | 2007-06-05 | Georgia Tech Research Corporation | Microneedle drug delivery device |
| US20030208167A1 (en) | 1999-06-04 | 2003-11-06 | Prausnitz Mark R. | Microneedle drug delivery device |
| US6611707B1 (en) | 1999-06-04 | 2003-08-26 | Georgia Tech Research Corporation | Microneedle drug delivery device |
| US6931277B1 (en) | 1999-06-09 | 2005-08-16 | The Procter & Gamble Company | Intracutaneous microneedle array apparatus |
| US6312612B1 (en) | 1999-06-09 | 2001-11-06 | The Procter & Gamble Company | Apparatus and method for manufacturing an intracutaneous microneedle array |
| US6256533B1 (en) | 1999-06-09 | 2001-07-03 | The Procter & Gamble Company | Apparatus and method for using an intracutaneous microneedle array |
| US7416541B2 (en) | 1999-06-09 | 2008-08-26 | Corium International, Inc. | Intracutaneous microneedle array apparatus |
| US20020020688A1 (en) | 1999-06-09 | 2002-02-21 | The Procter & Gamble Company | Apparatus and method for manufacturing an intracutaneous microneedle array |
| CA2376285C (en) | 1999-06-09 | 2006-05-09 | Vadim Vladimirovich Yuzhakov | Intracutaneous microneedle array apparatus |
| US6471903B2 (en) | 1999-06-09 | 2002-10-29 | The Procter & Gamble Company | Method for manufacturing an intracutaneous microneedle array |
| US6451240B1 (en) | 1999-06-09 | 2002-09-17 | The Procter & Gamble Company | Method of manufacturing an intracutaneous microneedle array |
| US20050209565A1 (en) | 1999-06-09 | 2005-09-22 | The Procter & Gamble Company | Intracutaneous microneedle array apparatus |
| US6379324B1 (en) | 1999-06-09 | 2002-04-30 | The Procter & Gamble Company | Intracutaneous microneedle array apparatus |
| US6652478B1 (en) | 1999-06-09 | 2003-11-25 | The Procter & Gamble Company | Intracutaneous edged microneedle apparatus |
| JP2001004442A (en) | 1999-06-25 | 2001-01-12 | Matsushita Electric Works Ltd | Infrared sensor and its manufacture |
| US6610463B1 (en) | 1999-08-30 | 2003-08-26 | Canon Kabushiki Kaisha | Method of manufacturing structure having pores |
| JP2001138300A (en) | 1999-08-30 | 2001-05-22 | Canon Inc | Method for producing structure having pores, structure produced by the method, and structure device using the structure |
| JP2001149485A (en) | 1999-09-22 | 2001-06-05 | Becton Dickinson & Co | Method and apparatus for transdermal administration of substances |
| EP1086718A1 (en) | 1999-09-22 | 2001-03-28 | Becton Dickinson and Company | Method and apparatus for the transdermal administration of a substance |
| US20030199812A1 (en) | 1999-09-22 | 2003-10-23 | Becton, Dickinson And Company | Method and apparatus for the transdermal administration of a substance |
| US6960193B2 (en) | 1999-09-22 | 2005-11-01 | Becton, Dickinson And Company | Method and apparatus for the transdermal administration of a substance |
| US6623457B1 (en) | 1999-09-22 | 2003-09-23 | Becton, Dickinson And Company | Method and apparatus for the transdermal administration of a substance |
| EP1086719B1 (en) | 1999-09-24 | 2005-03-23 | Becton Dickinson and Company | Device for abrading skin |
| JP2001157715A (en) | 1999-09-24 | 2001-06-12 | Becton Dickinson & Co | Method and device suitable for skin abrasion |
| CA2316534C (en) | 1999-09-24 | 2009-05-12 | Becton, Dickinson And Company | Method and device for abrading skin |
| US6835184B1 (en) | 1999-09-24 | 2004-12-28 | Becton, Dickinson And Company | Method and device for abrading skin |
| US20030093089A1 (en) | 1999-10-20 | 2003-05-15 | Greenberg Ronald Allan | Apparatus for variable micro abrasion of human tissue and/or hides using different size and types of abrasive particles |
| US6355054B1 (en) | 1999-11-05 | 2002-03-12 | Ceramoptec Industries, Inc. | Laser system for improved transbarrier therapeutic radiation delivery |
| US6511463B1 (en) | 1999-11-18 | 2003-01-28 | Jds Uniphase Corporation | Methods of fabricating microneedle arrays using sacrificial molds |
| US6743211B1 (en)* | 1999-11-23 | 2004-06-01 | Georgia Tech Research Corporation | Devices and methods for enhanced microneedle penetration of biological barriers |
| US20020032415A1 (en) | 1999-12-10 | 2002-03-14 | Trautman Joseph C. | Device and method for enhancing skin piercing by microprotrusions |
| US20040181203A1 (en) | 1999-12-10 | 2004-09-16 | Cormier Michel J.N. | Skin treatment method and apparatus for sustained transdermal drug delivery |
| US7087035B2 (en) | 1999-12-10 | 2006-08-08 | Alza Corporation | Device and method for enhancing skin piercing by microprotrusions |
| US6562014B2 (en) | 1999-12-16 | 2003-05-13 | Alza Corporation | Device and method for enhancing transdermal flux of agents being sampled |
| US6375627B1 (en) | 2000-03-02 | 2002-04-23 | Agilent Technologies, Inc. | Physiological fluid extraction with rapid analysis |
| US6558361B1 (en) | 2000-03-09 | 2003-05-06 | Nanopass Ltd. | Systems and methods for the transport of fluids through a biological barrier and production techniques for such systems |
| US6629949B1 (en) | 2000-05-08 | 2003-10-07 | Sterling Medivations, Inc. | Micro infusion drug delivery device |
| JP2001341314A (en) | 2000-06-01 | 2001-12-11 | Ricoh Co Ltd | Droplet discharge head and manufacturing method thereof, ink jet recording apparatus and microactuator |
| US6537242B1 (en)* | 2000-06-06 | 2003-03-25 | Becton, Dickinson And Company | Method and apparatus for enhancing penetration of a member for the intradermal sampling or administration of a substance |
| JP2003534881A (en) | 2000-06-06 | 2003-11-25 | ベクトン・ディキンソン・アンド・カンパニー | Method and apparatus for enhancing penetration of a piercing member into an intradermal space |
| US6494830B1 (en) | 2000-06-22 | 2002-12-17 | Guidance Interactive Technologies, Inc. | Handheld controller for monitoring/using medical parameters |
| US6589202B1 (en) | 2000-06-29 | 2003-07-08 | Becton Dickinson And Company | Method and apparatus for transdermally sampling or administering a substance to a patient |
| US6603987B2 (en) | 2000-07-11 | 2003-08-05 | Bayer Corporation | Hollow microneedle patch |
| US20020006355A1 (en) | 2000-07-11 | 2002-01-17 | Bayer Corporation | Hollow microneedle patch |
| EP1174078B1 (en) | 2000-07-11 | 2006-11-02 | Bayer Corporation | Hollow microneedle patch |
| US6565532B1 (en) | 2000-07-12 | 2003-05-20 | The Procter & Gamble Company | Microneedle apparatus used for marking skin and for dispensing semi-permanent subcutaneous makeup |
| US6440096B1 (en) | 2000-07-14 | 2002-08-27 | Becton, Dickinson And Co. | Microdevice and method of manufacturing a microdevice |
| US6656147B1 (en) | 2000-07-17 | 2003-12-02 | Becton, Dickinson And Company | Method and delivery device for the transdermal administration of a substance |
| US20050197308A1 (en) | 2000-07-21 | 2005-09-08 | Smithkline Beecham Biologicals S.A. | Vaccines |
| JP2002079499A (en) | 2000-09-08 | 2002-03-19 | Terumo Corp | Method of manufacturing needle-like article, and manufactured needle |
| US6691752B2 (en) | 2000-09-15 | 2004-02-17 | Timberjack Inc. | High rotation felling head mechanism |
| US20020042589A1 (en) | 2000-10-05 | 2002-04-11 | Thomas Marsoner | Medical injection device |
| US20020091357A1 (en) | 2000-10-13 | 2002-07-11 | Trautman Joseph C. | Microprotrusion member retainer for impact applicator |
| US7131960B2 (en) | 2000-10-13 | 2006-11-07 | Alza Corporation | Apparatus and method for piercing skin with microprotrusions |
| US20020087182A1 (en) | 2000-10-13 | 2002-07-04 | Trautman Joseph C. | Microblade array impact applicator |
| US6855131B2 (en) | 2000-10-13 | 2005-02-15 | Alza Corporation | Microprotrusion member retainer for impact applicator |
| US20060095061A1 (en) | 2000-10-13 | 2006-05-04 | Trautman Joseph C | Microblade array impact applicator |
| US20020045859A1 (en) | 2000-10-16 | 2002-04-18 | The Procter & Gamble Company | Microstructures for delivering a composition cutaneously to skin |
| US6821281B2 (en) | 2000-10-16 | 2004-11-23 | The Procter & Gamble Company | Microstructures for treating and conditioning skin |
| US20020045907A1 (en) | 2000-10-16 | 2002-04-18 | The Procter & Gamble Company | Microstructures for treating and conditioning skin |
| US7131987B2 (en) | 2000-10-16 | 2006-11-07 | Corium International, Inc. | Microstructures and method for treating and conditioning skin which cause less irritation during exfoliation |
| CA2422907C (en) | 2000-10-16 | 2009-05-26 | The Procter & Gamble Company | Microstructures for treating and conditioning skin |
| US20020177858A1 (en) | 2000-10-16 | 2002-11-28 | Sherman Faiz Feisal | Microstructures and method for treating and conditioning skin which cause less irritation during exfoliation |
| US20060129174A1 (en) | 2000-10-16 | 2006-06-15 | Corium International, Inc. | Microstructures for delivering a composition cutaneously to skin |
| US7108681B2 (en) | 2000-10-16 | 2006-09-19 | Corium International, Inc. | Microstructures for delivering a composition cutaneously to skin |
| US6533884B1 (en) | 2000-11-03 | 2003-03-18 | Printpack Illinois, Inc. | Method and system for extrusion embossing |
| JP2002151395A (en) | 2000-11-14 | 2002-05-24 | Kansai Tlo Kk | Method and apparatus for processing material using x- rays |
| US6591133B1 (en) | 2000-11-27 | 2003-07-08 | Microlin Llc | Apparatus and methods for fluid delivery using electroactive needles and implantable electrochemical delivery devices |
| US20020082543A1 (en) | 2000-12-14 | 2002-06-27 | Jung-Hwan Park | Microneedle devices and production thereof |
| US20020096488A1 (en) | 2001-01-24 | 2002-07-25 | Xerox Corporation | Method for fabricating a micro-electro-mechanical fluid ejector |
| JP2002301698A (en) | 2001-01-24 | 2002-10-15 | Xerox Corp | Electrostatically actuated device having corrugated multi-layer film structure |
| US6508947B2 (en) | 2001-01-24 | 2003-01-21 | Xerox Corporation | Method for fabricating a micro-electro-mechanical fluid ejector |
| JP2002239014A (en) | 2001-02-19 | 2002-08-27 | Sumitomo Precision Prod Co Ltd | Needle-like body and method for manufacturing needle- like body |
| US7763203B2 (en) | 2001-03-14 | 2010-07-27 | Corium International, Inc. | Method of manufacturing microneedle structures using photolithography |
| US20040146611A1 (en) | 2001-03-14 | 2004-07-29 | The Procter & Gamble Company | Method of manufacturing microneedle structures using soft lithography and photolithography |
| US20020133129A1 (en) | 2001-03-14 | 2002-09-19 | Francisco Arias | Method of manufacturing microneedle structures using soft lithography and photolithography |
| US6663820B2 (en) | 2001-03-14 | 2003-12-16 | The Procter & Gamble Company | Method of manufacturing microneedle structures using soft lithography and photolithography |
| US20020177839A1 (en) | 2001-04-20 | 2002-11-28 | Cormier Michel J. N. | Microprojection array having a beneficial agent containing coating |
| US20020193729A1 (en) | 2001-04-20 | 2002-12-19 | Cormier Michel J.N. | Microprojection array immunization patch and method |
| US6585742B2 (en) | 2001-05-08 | 2003-07-01 | Dowling B. Stough | Wart removal method and device |
| US6591124B2 (en) | 2001-05-11 | 2003-07-08 | The Procter & Gamble Company | Portable interstitial fluid monitoring system |
| US20020169411A1 (en) | 2001-05-11 | 2002-11-14 | The Procter & Gamble Co. | Portable interstitial fluid monitoring system |
| US20020188245A1 (en) | 2001-06-08 | 2002-12-12 | Martin Frank E. | Device for manipulating a needle or abrader array and method of use |
| US7186235B2 (en) | 2001-06-08 | 2007-03-06 | Becton, Dickinson And Company | Device for manipulating a needle or abrader array and method of use |
| US6767341B2 (en) | 2001-06-13 | 2004-07-27 | Abbott Laboratories | Microneedles for minimally invasive drug delivery |
| US20040186419A1 (en) | 2001-06-13 | 2004-09-23 | Cho Steve T. | Microneedles for minimally invasive drug delivery |
| US6980855B2 (en) | 2001-06-13 | 2005-12-27 | Hospira, Inc. | Microneedles for minimally invasive drug delivery |
| US20040204669A1 (en) | 2001-07-05 | 2004-10-14 | Hofmann Gunter A. | Apparatus for electroporation mediated delivery for drugs and genes |
| US20030208138A1 (en) | 2001-07-09 | 2003-11-06 | Lorin Olson | Micro-needles and methods of manufacture and use thereof |
| JP2003039399A (en) | 2001-07-31 | 2003-02-13 | Dainippon Printing Co Ltd | Stamp for thin film pattern formation |
| JP2003048160A (en) | 2001-08-09 | 2003-02-18 | Tokyo Denki Univ | Micro-groove processing method and device |
| US6881203B2 (en) | 2001-09-05 | 2005-04-19 | 3M Innovative Properties Company | Microneedle arrays and methods of manufacturing the same |
| US20030135167A1 (en) | 2001-09-19 | 2003-07-17 | Gonnelli Robert R. | Microneedles, microneedle arrays, and systems and methods relating to same |
| US20030093028A1 (en) | 2001-11-09 | 2003-05-15 | Michael Spiegel | Appararus and method for magnetic induction of therapeutic electric fields |
| US20030199810A1 (en) | 2001-11-30 | 2003-10-23 | Trautman Joseph Creagan | Methods and apparatuses for forming microprojection arrays |
| US6808506B2 (en) | 2002-02-04 | 2004-10-26 | Becton, Dickinson And Company | Device and method for delivering or withdrawing a substance through the skin |
| US20030220610A1 (en)* | 2002-02-04 | 2003-11-27 | Becton Dickinson And Company | Device and method for delivering or withdrawing a substance through the skin |
| US6780171B2 (en) | 2002-04-02 | 2004-08-24 | Becton, Dickinson And Company | Intradermal delivery device |
| US20030187394A1 (en) | 2002-04-02 | 2003-10-02 | Wilkinson Bradley M | Method and device for intradermally delivering a substance |
| US7115108B2 (en) | 2002-04-02 | 2006-10-03 | Becton, Dickinson And Company | Method and device for intradermally delivering a substance |
| US20030220656A1 (en) | 2002-05-24 | 2003-11-27 | Vladimir Gartstein | Method of exfoliation of skin using closely-packed microstructures |
| US6945952B2 (en) | 2002-06-25 | 2005-09-20 | Theraject, Inc. | Solid solution perforator for drug delivery and other applications |
| US20040096455A1 (en) | 2002-08-08 | 2004-05-20 | Yuh-Fun Maa | Transdermal vaccine delivery device having coated microprotrusions |
| JP2004065775A (en) | 2002-08-08 | 2004-03-04 | Sanwa Kagaku Kenkyusho Co Ltd | Device equipped with needle-like structure element |
| US20040087992A1 (en) | 2002-08-09 | 2004-05-06 | Vladimir Gartstein | Microstructures for delivering a composition cutaneously to skin using rotatable structures |
| US7166086B2 (en) | 2002-08-29 | 2007-01-23 | Becton, Dickinson And Company | Substance delivery via a rotating microabrading surface |
| US20040143211A1 (en) | 2002-08-29 | 2004-07-22 | Haider M. Ishaq | Substance delivery via a rotating microabrading surface |
| US20040164454A1 (en) | 2003-02-24 | 2004-08-26 | The Procter & Gamble Company | Method for manufacturing microstructures having multiple microelements with through-holes |
| US7578954B2 (en) | 2003-02-24 | 2009-08-25 | Corium International, Inc. | Method for manufacturing microstructures having multiple microelements with through-holes |
| US20060076718A1 (en) | 2003-06-02 | 2006-04-13 | The Procter & Gamble Company | Method for manufacturing microstructures having hollow microelements using fluidic jets during a molding operation |
| US7572405B2 (en) | 2003-06-02 | 2009-08-11 | Corium International Inc. | Method for manufacturing microstructures having hollow microelements using fluidic jets during a molding operation |
| US20050089554A1 (en) | 2003-10-24 | 2005-04-28 | Cormier Michel J. | Apparatus and method for enhancing transdermal drug delivery |
| US20070027427A1 (en) | 2003-10-31 | 2007-02-01 | Trautman Joseph C | Self-actuating applicator for microprojection array |
| US20050096586A1 (en) | 2003-10-31 | 2005-05-05 | Trautman Joseph C. | Self-actuating applicator for microprojection array |
| US7097631B2 (en) | 2003-10-31 | 2006-08-29 | Alza Corporation | Self-actuating applicator for microprojection array |
| US20050163827A1 (en) | 2004-01-23 | 2005-07-28 | Herbert Zech | Transdermal therapeutic delivery system |
| WO2005089857A1 (en) | 2004-03-15 | 2005-09-29 | Unilever Plc | Skin microactivation system |
| US20100028390A1 (en) | 2004-03-24 | 2010-02-04 | Cleary Gary W | Transdermal Delivery Device |
| US7611481B2 (en) | 2004-03-24 | 2009-11-03 | Corium International, Inc. | Transdermal delivery device |
| US20050228340A1 (en) | 2004-03-24 | 2005-10-13 | Cleary Gary W | Transdermal delivery device |
| US20080114298A1 (en)* | 2004-11-18 | 2008-05-15 | Cantor Adam S | Low-Profile Microneedle Array Applicator |
| JP2006341089A (en) | 2005-05-13 | 2006-12-21 | Fujikura Ltd | Medicinal product carrying device and method of manufacturing |
| US20080195035A1 (en)* | 2005-06-24 | 2008-08-14 | Frederickson Franklyn L | Collapsible Patch and Method of Application |
| WO2008011625A2 (en) | 2006-07-21 | 2008-01-24 | Georgia Tech Researh Corporation | Microneedle devices and methods of drug delivery or fluid withdrawal |
| WO2008091602A2 (en) | 2007-01-22 | 2008-07-31 | Corium International, Inc. | Applicators for microneedle arrays |
| US20080183144A1 (en) | 2007-01-22 | 2008-07-31 | Trautman Joseph C | Applicators for microneedles |
| US20090155330A1 (en) | 2007-04-16 | 2009-06-18 | Corium International, Inc. | Vaccine Delivery via Microneedle Arrays |
| WO2008130587A2 (en) | 2007-04-16 | 2008-10-30 | Corium International, Inc. | Solvent-cast microneedle arrays containing active |
| US20080269685A1 (en) | 2007-04-16 | 2008-10-30 | Parminder Singh | Solvent-cast microneedle arrays containing active |
| WO2009048607A1 (en) | 2007-10-10 | 2009-04-16 | Corium International, Inc. | Vaccine delivery via microneedle arrays |
Non-Patent Citations (39)
| Title |
|---|
| "extend". Macmillan Online Dictionary. .* |
| "extend". Macmillan Online Dictionary. <http://www.macmillandictionary.com/dictionary/american/extend>.* |
| "extend". Merriam-Webster Online Dictionary. .* |
| "extend". Merriam-Webster Online Dictionary. <http://www.merriam-webster.com/dictionary/extend>.* |
| "Heparin Pregnancy and Breast Feeding Warnings", Drugs.com, Accessed Oct. 8, 2009, . |
| "Heparin Pregnancy and Breast Feeding Warnings", Drugs.com, Accessed Oct. 8, 2009, <http://www.drugs.com/pregnancy/heparin.html>. |
| "Heparin Pregnancy and Breast Feeding Warnings". Drugs.com. Accessed Oct. 8, 2009. .* |
| "Heparin Pregnancy and Breast Feeding Warnings". Drugs.com. Accessed Oct. 8, 2009. <http://www.drugs.com/pregnancy/heparin.html>.* |
| Chun, et al., "An array of hollow microcapillaries for the controlled injection of genetic materials into animal/plant cells," IEEE Workshop on Micro Electro Mechanical Systems, pp. 406-411, (1999). |
| Henry, et al., "Microfabricated microneedles: A novel approach to transdermal drug delivery", J. Pharmaceutical Science, vol. 87, No. 8, pp. 922-925, (1998). |
| Henry, et al., "Micromachined microneedles for transdermal delivery of drugs", IEEE Workshop on Micro Electro Mechanical Systems, New York, NY, pp. 494-498, (1998). |
| International Search Report from PCT/US2000/015612 mailed on Sep. 7, 2000. |
| International Search Report from PCT/US2000/015613 mailed on Sep. 6, 2000. |
| International Search Report from PCT/US2000/015614 mailed on Sep. 6, 2000. |
| International Search Report from PCT/US2001/031977 mailed on Apr. 29, 2002. |
| International Search Report from PCT/US2001/031978 mailed on Apr. 29, 2002. |
| International Search Report from PCT/US2002/014624 mailed on Sep. 3, 2002. |
| International Search Report from PCT/US2002/029228 mailed on Apr. 23, 2003. |
| International Search Report from PCT/US2002/029245 mailed on Dec. 27, 2002. |
| International Search Report from PCT/US2004/005382 mailed on Nov. 25, 2004. |
| International Search Report from PCT/US2004/017255 mailed on May 24, 2005. |
| International Search Report from PCT/US2005/009854 mailed on Jul. 3, 2008. |
| International Search Report from PCT/US2008/004943 mailed on Jun. 9, 2009, application published as WO 2008/130587 on Oct. 30, 2008. |
| International Search report from PCT/US2008/011635 mailed on Dec. 19, 2008, application published as WO 2009/048607 on Apr. 16, 2009. |
| Matriano, et al., "Macroflux(R) microprojection array patch technology: A new and efficient approach for intracutaneous immunization", Pharm. Res., vol. 19, No. 1, pp. 63-70, (2002). |
| McAllister, et al., "Micromachined microneedles for transdermal drug delivery", Am. Inst. Chem. Eng., 1998 Annual Meeting, Miami Beach, FL, Nov. 15-20, Drug Delivery II, pp. 1-4. |
| Mikszta, et al., "Improvred genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery", Nat. Med., vol. 8, No. 4, pp. 415-419, (2002). |
| Mikszta, et al., "Protective immunization against inhalation anthrax: A comparison of minimally invasive delivery platforms", J. Inf. Dis., vol. 191, No. 2, pp. 278-288, (2005). |
| Papautsky, et al., "Micromachined Pipette Arrays," MPA, Proceedings-19th international Conference-IEEE/EMBS, Chicago IL, USA, pp. 2281-2284 (1997). |
| Park, et al. "Polymer Microneedles for Controlled-Release Drug Delivery," Pharmaceutical Research, Kluwer Academic Publishers-Plenum Publishers, NE, vol. 23, No. 5, pp. 1008-1019 (2006). |
| PCT Search Report dated Jul. 18, 2008. |
| Prausnitz, "Transdermal delivery of macromolecules: Recent advances by modification of skin's barrier properties", ACS Symposium Series No. 675, Therapeutic Protein and Peptide Formulation and Delivery, American Chemical Society, Washington DC, Chapter 8, pp. 124-153, (1997). |
| Prausnitz, et al., "Transdermal transport efficiency during skin electroporation and iontophoresis", J. Contr. Release, vol. 38, pp. 205-217, (1996). |
| Rydberg et al. "Low-Molecular-Weight Heparin in Preventing and Treating DVT". Am Fam Physician. Mar. 15, 1999; 59(6):1607-12.* |
| Rydberg, et al., "Low-molecular-weight heparin preventing and treating DVT", Am. Fam. Physican, vol. 59, No. 6, pp. 1607-1612, (1999). |
| Sivamani, et al., "Microneedles and transdermal applications", Exp. Opin. Drug Del., vol. 4, No. 1, pp. 19-25, (2007). |
| Wouters, et al., "Microelectrochemical systems for drug delivery", Electrochimica Acta., vol. 42, pp. 3385-3390, (1997). |
| Xia, et al., "Soft Lithography", Angew. Chem. Int. Ed., vol. 37, pp. 551-575, (1998). |
| Xia, et al., "Soft Lithography", Annu. Rev. Mater. Sci., vol. 28, pp. 153-184 (1998). |
Cited By (77)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10238848B2 (en) | 2007-04-16 | 2019-03-26 | Corium International, Inc. | Solvent-cast microprotrusion arrays containing active ingredient |
| US9114238B2 (en) | 2007-04-16 | 2015-08-25 | Corium International, Inc. | Solvent-cast microprotrusion arrays containing active ingredient |
| US9452280B2 (en) | 2007-04-16 | 2016-09-27 | Corium International, Inc. | Solvent-cast microprotrusion arrays containing active ingredient |
| US9498524B2 (en) | 2007-04-16 | 2016-11-22 | Corium International, Inc. | Method of vaccine delivery via microneedle arrays |
| US9381680B2 (en) | 2008-05-21 | 2016-07-05 | Theraject, Inc. | Method of manufacturing solid solution perforator patches and uses thereof |
| US11992668B2 (en) | 2008-12-02 | 2024-05-28 | Allergan, Inc. | Injection device |
| US9775551B2 (en) | 2009-03-02 | 2017-10-03 | Seventh Sense Biosystems, Inc. | Devices and techniques associated with diagnostics, therapies, and other applications, including skin-associated applications |
| US10939860B2 (en) | 2009-03-02 | 2021-03-09 | Seventh Sense Biosystems, Inc. | Techniques and devices associated with blood sampling |
| US9730624B2 (en) | 2009-03-02 | 2017-08-15 | Seventh Sense Biosystems, Inc. | Delivering and/or receiving fluids |
| US10799166B2 (en) | 2009-03-02 | 2020-10-13 | Seventh Sense Biosystems, Inc. | Delivering and/or receiving fluids |
| US12239767B2 (en) | 2009-10-23 | 2025-03-04 | University of Pittsburgh—of the Commonwealth System of Higher Education | Dissolvable microneedle arrays for transdermal delivery to human skin |
| US11744927B2 (en) | 2009-10-23 | 2023-09-05 | University of Pittsburgh—of the Commonwealth System of Higher Education | Dissolvable microneedle arrays for transdermal delivery to human skin |
| US11419816B2 (en) | 2010-05-04 | 2022-08-23 | Corium, Inc. | Method and device for transdermal delivery of parathyroid hormone using a microprojection array |
| US12377044B2 (en) | 2010-05-04 | 2025-08-05 | Panther Life Sciences Corporation | Method and device for transdermal delivery of parathyroid hormone using a microprojection array |
| US11202895B2 (en) | 2010-07-26 | 2021-12-21 | Yourbio Health, Inc. | Rapid delivery and/or receiving of fluids |
| US12076518B2 (en) | 2010-07-26 | 2024-09-03 | Yourbio Health, Inc. | Rapid delivery and/or receiving of fluids |
| US11177029B2 (en) | 2010-08-13 | 2021-11-16 | Yourbio Health, Inc. | Systems and techniques for monitoring subjects |
| US20220249818A1 (en)* | 2010-08-13 | 2022-08-11 | Yourbio Health, Inc. | Clinical and/or consumer techniques and devices |
| US12310728B2 (en) | 2010-11-09 | 2025-05-27 | Yourbio Health, Inc. | Systems and interfaces for blood sampling |
| US12121353B2 (en) | 2010-11-09 | 2024-10-22 | Yourbio Health, Inc. | Systems and interfaces for blood sampling |
| US11253179B2 (en) | 2011-04-29 | 2022-02-22 | Yourbio Health, Inc. | Systems and methods for collection and/or manipulation of blood spots or other bodily fluids |
| US10188335B2 (en) | 2011-04-29 | 2019-01-29 | Seventh Sense Biosystems, Inc. | Plasma or serum production and removal of fluids under reduced pressure |
| US10835163B2 (en) | 2011-04-29 | 2020-11-17 | Seventh Sense Biosystems, Inc. | Systems and methods for collecting fluid from a subject |
| US10543310B2 (en) | 2011-12-19 | 2020-01-28 | Seventh Sense Biosystems, Inc. | Delivering and/or receiving material with respect to a subject surface |
| US9944019B2 (en) | 2012-05-01 | 2018-04-17 | University of Pittsburgh—of the Commonwealth System of Higher Education | Tip-loaded microneedle arrays for transdermal insertion |
| US11052231B2 (en) | 2012-12-21 | 2021-07-06 | Corium, Inc. | Microarray for delivery of therapeutic agent and methods of use |
| US10245422B2 (en) | 2013-03-12 | 2019-04-02 | Corium International, Inc. | Microprojection applicators and methods of use |
| US11110259B2 (en) | 2013-03-12 | 2021-09-07 | Corium, Inc. | Microprojection applicators and methods of use |
| US11565097B2 (en) | 2013-03-15 | 2023-01-31 | Corium Pharma Solutions, Inc. | Microarray for delivery of therapeutic agent and methods of use |
| US9962534B2 (en) | 2013-03-15 | 2018-05-08 | Corium International, Inc. | Microarray for delivery of therapeutic agent, methods of use, and methods of making |
| US10195409B2 (en) | 2013-03-15 | 2019-02-05 | Corium International, Inc. | Multiple impact microprojection applicators and methods of use |
| US10384046B2 (en) | 2013-03-15 | 2019-08-20 | Corium, Inc. | Microarray for delivery of therapeutic agent and methods of use |
| US10384045B2 (en) | 2013-03-15 | 2019-08-20 | Corium, Inc. | Microarray with polymer-free microstructures, methods of making, and methods of use |
| US11684719B2 (en) | 2013-05-23 | 2023-06-27 | Allergan, Inc. | Methods of treatment using a syringe extrusion accessory |
| US10265477B2 (en) | 2013-05-23 | 2019-04-23 | Allergan, Inc. | Mechanical syringe accessory |
| USD726316S1 (en)* | 2013-10-23 | 2015-04-07 | Claire A. Marquez | Micro-needling tattoo head |
| US10792427B2 (en) | 2014-05-13 | 2020-10-06 | Allergan, Inc. | High force injection devices |
| US10624843B2 (en) | 2014-09-04 | 2020-04-21 | Corium, Inc. | Microstructure array, methods of making, and methods of use |
| US11185641B2 (en) | 2014-10-01 | 2021-11-30 | Allergan, Inc. | Devices for injection and dosing |
| US10226585B2 (en) | 2014-10-01 | 2019-03-12 | Allergan, Inc. | Devices for injection and dosing |
| US10433928B2 (en) | 2015-03-10 | 2019-10-08 | Allergan Pharmaceuticals Holdings (Ireland) Unlimited Company | Multiple needle injector |
| US10441768B2 (en) | 2015-03-18 | 2019-10-15 | University of Pittsburgh—of the Commonwealth System of Higher Education | Bioactive components conjugated to substrates of microneedle arrays |
| US10737083B2 (en) | 2015-03-18 | 2020-08-11 | University of Pittsburgh—of the Commonwealth System of Higher Education | Bioactive components conjugated to dissolvable substrates of microneedle arrays |
| US11672964B2 (en) | 2015-03-18 | 2023-06-13 | University of Pittsburgh—of the Commonwealth System of Higher Education | Bioactive components conjugated to substrates of microneedle arrays |
| US10857093B2 (en) | 2015-06-29 | 2020-12-08 | Corium, Inc. | Microarray for delivery of therapeutic agent, methods of use, and methods of making |
| US11684763B2 (en) | 2015-10-16 | 2023-06-27 | University of Pittsburgh—of the Commonwealth System of Higher Education | Multi-component bio-active drug delivery and controlled release to the skin by microneedle array devices |
| US11701507B2 (en) | 2015-12-21 | 2023-07-18 | Medrx Co., Ltd. | Microneedle patch applicator and housing for same |
| US11167119B2 (en)* | 2015-12-21 | 2021-11-09 | Medrx Co., Ltd. | Microneedle patch applicator and housing for same |
| US11744889B2 (en) | 2016-01-05 | 2023-09-05 | University of Pittsburgh—of the Commonwealth System of Higher Education | Skin microenvironment targeted delivery for promoting immune and other responses |
| US11890457B2 (en) | 2016-04-08 | 2024-02-06 | Allergan, Inc. | Aspiration and injection device |
| US10596321B2 (en) | 2016-04-08 | 2020-03-24 | Allergan, Inc. | Aspiration and injection device |
| USD865950S1 (en) | 2017-03-24 | 2019-11-05 | Allergan, Inc. | Syringe device |
| USD867582S1 (en) | 2017-03-24 | 2019-11-19 | Allergan, Inc. | Syringe device |
| USD865949S1 (en) | 2017-03-24 | 2019-11-05 | Allergan, Inc. | Syringe device |
| USD865948S1 (en) | 2017-03-24 | 2019-11-05 | Allergan, Inc. | Syringe device |
| USD866753S1 (en) | 2017-03-24 | 2019-11-12 | Allergan, Inc. | Syringe device |
| US11185673B2 (en) | 2017-05-15 | 2021-11-30 | Fujifilm Corporation | Micro-needle array unit and container |
| USD926966S1 (en) | 2018-10-30 | 2021-08-03 | Medrx Co., Ltd. | Package for containing a micro needle patch |
| US11420812B2 (en) | 2019-04-17 | 2022-08-23 | Fujifilm Corporation | Storage container, microneedle unit, storage container group, and method of producing microneedle unit |
| US12214150B2 (en) | 2019-05-16 | 2025-02-04 | University of Pittsburgh—of the Commonwealth System of Higher Education | Microneedle arrays with undercut features for cutaneous and non-cutaneous drug delivery |
| US12186515B2 (en) | 2020-04-28 | 2025-01-07 | Ticona Llc | Microneedle assembly |
| US12029562B2 (en) | 2021-04-14 | 2024-07-09 | Satio, Inc. | Dermal patch system |
| US11986614B2 (en) | 2021-09-28 | 2024-05-21 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11904127B2 (en) | 2021-09-28 | 2024-02-20 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11672965B2 (en) | 2021-09-28 | 2023-06-13 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11654270B2 (en) | 2021-09-28 | 2023-05-23 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11964121B2 (en) | 2021-10-13 | 2024-04-23 | Satio, Inc. | Mono dose dermal patch for pharmaceutical delivery |
| US12023156B2 (en) | 2021-10-13 | 2024-07-02 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US12214346B2 (en) | 2021-10-13 | 2025-02-04 | Satio, Inc. | Dermal patch with a diagnostic test strip |
| US12178979B2 (en) | 2021-10-13 | 2024-12-31 | Satio, Inc. | Dermal patch for delivering a pharmaceutical |
| US12048543B2 (en) | 2021-11-08 | 2024-07-30 | Satio, Inc. | Dermal patch for collecting a physiological sample with removable vial |
| US12053284B2 (en) | 2021-11-08 | 2024-08-06 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US11877848B2 (en) | 2021-11-08 | 2024-01-23 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| USD1033641S1 (en) | 2021-12-17 | 2024-07-02 | Biolinq Incorporated | Microneedle array sensor applicator device |
| WO2023168419A3 (en)* | 2022-03-03 | 2023-10-12 | Deka Products Limited Partnership | Systems and apparatuses for medical agent administration |
| USD1057153S1 (en) | 2022-04-29 | 2025-01-07 | Biolinq Incorporated | Microneedle array sensor applicator device |
| US12440133B2 (en) | 2024-03-29 | 2025-10-14 | Satio, Inc. | Dermal patch for collecting a physiological sample |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008209537A1 (en) | 2008-07-31 |
| WO2008091602A2 (en) | 2008-07-31 |
| CA2676221C (en) | 2016-12-20 |
| US20080183144A1 (en) | 2008-07-31 |
| JP2010516337A (en) | 2010-05-20 |
| WO2008091602A3 (en) | 2008-09-12 |
| CA2676221A1 (en) | 2008-07-31 |
| AU2008209537B2 (en) | 2013-01-31 |
| JP2013215621A (en) | 2013-10-24 |
| JP5553612B2 (en) | 2014-07-16 |
| EP2121111B1 (en) | 2018-03-14 |
| EP2121111A2 (en) | 2009-11-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8821446B2 (en) | Applicators for microneedles | |
| AU2019200645B2 (en) | Microprojection applicators | |
| US10391290B2 (en) | Microneedle injection apparatus comprising a dual cover | |
| AU2014237357B2 (en) | Multiple impact microprojection applicators and methods of use | |
| JP6251298B2 (en) | Microneedle injection device with reverse actuator | |
| JP2009507576A (en) | Coatable transdermal delivery microprojection assembly |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:TRAUTMAN, JOSEPH C.;WORSHAM, ROBERT WADE;BAYRAMOV, DANIR F.;AND OTHERS;REEL/FRAME:020839/0156;SIGNING DATES FROM 20080317 TO 20080324 Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:TRAUTMAN, JOSEPH C.;WORSHAM, ROBERT WADE;BAYRAMOV, DANIR F.;AND OTHERS;SIGNING DATES FROM 20080317 TO 20080324;REEL/FRAME:020839/0156 | |
| AS | Assignment | Owner name:SILICON VALLEY BANK, CALIFORNIA Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:022634/0119 Effective date:20090430 | |
| AS | Assignment | Owner name:OXFORD FINANCE LLC, AS COLLATERAL AGENT, VIRGINIA Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:027422/0717 Effective date:20111107 | |
| AS | Assignment | Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:OXFORD FINANCE LLC;REEL/FRAME:028884/0179 Effective date:20120816 | |
| AS | Assignment | Owner name:CAPITAL ROYALTY PARTNERS II L.P., TEXAS Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:028895/0961 Effective date:20120713 Owner name:CAPITAL ROYALTY PARTNERS II - PARALLEL FUND "A" L. Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:028895/0961 Effective date:20120713 Owner name:PARALLEL INVESTMENT OPPORTUNITIES PARTNERS II L.P. Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:028895/0961 Effective date:20120713 | |
| AS | Assignment | Owner name:SILICON VALLEY BANK, CALIFORNIA Free format text:SECURITY AGREEMENT;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:028927/0782 Effective date:20120906 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| AS | Assignment | Owner name:CAPITAL ROYALTY PARTNERS II ? PARALLEL FUND ?A? L.P., TEXAS Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 Owner name:CAPITAL ROYALTY PARTNERS II ? PARALLEL FUND ?B? (CAYMAN) L.P., TEXAS Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 Owner name:CAPITAL ROYALTY PARTNERS II (CAYMAN) L.P., TEXAS Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 Owner name:CAPITAL ROYALTY PARTNERS II L.P., TEXAS Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 Owner name:CAPITAL ROYALTY PARTNERS II ? PARALLEL FUND ?A? L. Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 Owner name:CAPITAL ROYALTY PARTNERS II ? PARALLEL FUND ?B? (C Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:034535/0238 Effective date:20141202 | |
| AS | Assignment | Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:SILICON VALLEY BANK;REEL/FRAME:044810/0026 Effective date:20171120 | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 4TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2551) Year of fee payment:4 | |
| AS | Assignment | Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNORS:CAPITAL ROYALTY PARTNERS II L.P.;CAPITAL ROYALTY PARTNERS II - PARALLEL FUND "A" L.P.;CAPITAL ROYALTY PARTNERS II - PARALLEL FUND "B" (CAYMAN) L.P.;AND OTHERS;REEL/FRAME:045513/0686 Effective date:20180305 Owner name:CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNORS:CAPITAL ROYALTY PARTNERS II L.P.;CAPITAL ROYALTY PARTNERS II - PARALLEL FUND "A" L.P.;CAPITAL ROYALTY PARTNERS II - PARALLEL FUND "B" (CAYMAN) L.P.;AND OTHERS;REEL/FRAME:046909/0855 Effective date:20180305 | |
| AS | Assignment | Owner name:CORIUM, INC., CALIFORNIA Free format text:CHANGE OF NAME;ASSIGNOR:CORIUM INTERNATIONAL, INC.;REEL/FRAME:048841/0324 Effective date:20190314 | |
| AS | Assignment | Owner name:HERCULES CAPITAL, INC., AS AGENT, CALIFORNIA Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM, INC.;REEL/FRAME:057597/0907 Effective date:20210923 | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 8TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2552); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment:8 | |
| AS | Assignment | Owner name:CORIUM PHARMA SOLUTIONS, INC., MASSACHUSETTS Free format text:CHANGE OF NAME;ASSIGNOR:CORIUM, INC.;REEL/FRAME:061852/0207 Effective date:20221001 | |
| AS | Assignment | Owner name:CORIUM, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:HERCULES CAPITAL, INC.;REEL/FRAME:061687/0281 Effective date:20221014 Owner name:SILICON VALLEY BANK, AS ADMINISTRATIVE AGENT, CALIFORNIA Free format text:SECURITY INTEREST;ASSIGNOR:CORIUM PHARMA SOLUTIONS, INC.;REEL/FRAME:061689/0581 Effective date:20221014 | |
| AS | Assignment | Owner name:CORIUM, INC., FORMERLY CORIUM INTERNATIONAL, INC., CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST RECORDED AT REEL/FRAME 028927/0782;ASSIGNOR:SILICON VALLEY BANK;REEL/FRAME:062257/0614 Effective date:20221018 | |
| AS | Assignment | Owner name:CORIUM, INC., MASSACHUSETTS Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:HERCULES CAPITAL, INC., AS AGENT;REEL/FRAME:061713/0557 Effective date:20221014 | |
| AS | Assignment | Owner name:CORIUM PHARMA SOLUTIONS, INC., MICHIGAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:CORIUM, INC.;REEL/FRAME:062760/0731 Effective date:20221001 | |
| AS | Assignment | Owner name:CORIUM PHARMA SOLUTIONS, INC., MASSACHUSETTS Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE APPLICATION NUMBER PREVIOUSLY RECORDED AT REEL: 61852 FRAME: 207. ASSIGNOR(S) HEREBY CONFIRMS THE ASSIGNMENT;ASSIGNOR:CORIUM, INC.;REEL/FRAME:071915/0691 Effective date:20221001 |