US8337492B2 - Ablation catheter - Google Patents
Ablation catheterDownload PDFInfo
- Publication number
- US8337492B2 US8337492B2US12/938,791US93879110AUS8337492B2US 8337492 B2US8337492 B2US 8337492B2US 93879110 AUS93879110 AUS 93879110AUS 8337492 B2US8337492 B2US 8337492B2
- Authority
- US
- United States
- Prior art keywords
- carrier assembly
- carrier
- ablation
- control shaft
- electrodes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related, expires
Links
- 238000002679ablationMethods0.000titleabstractdescription286
- 238000013507mappingMethods0.000claimsabstractdescription57
- 230000000712assemblyEffects0.000claimsdescription14
- 238000000429assemblyMethods0.000claimsdescription14
- 229910001000nickel titaniumInorganic materials0.000claimsdescription11
- HLXZNVUGXRDIFK-UHFFFAOYSA-Nnickel titaniumChemical compound[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni]HLXZNVUGXRDIFK-UHFFFAOYSA-N0.000claimsdescription11
- 210000003492pulmonary veinAnatomy0.000abstractdescription67
- 238000000034methodMethods0.000abstractdescription61
- 210000001519tissueAnatomy0.000description92
- 210000002216heartAnatomy0.000description28
- 230000003902lesionEffects0.000description22
- 239000000463materialSubstances0.000description22
- 206010003658Atrial FibrillationDiseases0.000description21
- 238000010276constructionMethods0.000description20
- 230000000694effectsEffects0.000description19
- 230000006870functionEffects0.000description19
- 238000011282treatmentMethods0.000description16
- 229920002614Polyether block amidePolymers0.000description13
- 206010003119arrhythmiaDiseases0.000description12
- 230000037361pathwayEffects0.000description12
- 238000002604ultrasonographyMethods0.000description12
- 230000008859changeEffects0.000description11
- 210000002837heart atriumAnatomy0.000description11
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description10
- TZCXTZWJZNENPQ-UHFFFAOYSA-Lbarium sulfateChemical compound[Ba+2].[O-]S([O-])(=O)=OTZCXTZWJZNENPQ-UHFFFAOYSA-L0.000description8
- 210000004369bloodAnatomy0.000description8
- 239000008280bloodSubstances0.000description8
- 210000005246left atriumAnatomy0.000description8
- 210000005003heart tissueAnatomy0.000description7
- 229910052751metalInorganic materials0.000description7
- 239000002184metalSubstances0.000description7
- 229910001220stainless steelInorganic materials0.000description7
- 238000001356surgical procedureMethods0.000description7
- 230000007704transitionEffects0.000description7
- 210000005166vasculatureAnatomy0.000description7
- 230000001594aberrant effectEffects0.000description6
- 230000006793arrhythmiaEffects0.000description6
- 238000013153catheter ablationMethods0.000description6
- 239000010935stainless steelSubstances0.000description6
- 239000000853adhesiveSubstances0.000description5
- 230000001070adhesive effectEffects0.000description5
- 230000001746atrial effectEffects0.000description5
- 230000005540biological transmissionEffects0.000description5
- 238000001816coolingMethods0.000description5
- 239000012530fluidSubstances0.000description5
- 230000036961partial effectEffects0.000description5
- 229910052697platinumInorganic materials0.000description5
- 230000033764rhythmic processEffects0.000description5
- 230000000087stabilizing effectEffects0.000description5
- 238000002560therapeutic procedureMethods0.000description5
- 238000004873anchoringMethods0.000description4
- 238000003491arrayMethods0.000description4
- 239000013078crystalSubstances0.000description4
- 230000007246mechanismEffects0.000description4
- 150000002739metalsChemical class0.000description4
- 210000000056organAnatomy0.000description4
- 210000003462veinAnatomy0.000description4
- 229910001006ConstantanInorganic materials0.000description3
- 206010028980NeoplasmDiseases0.000description3
- 239000004809TeflonSubstances0.000description3
- 229920006362Teflon®Polymers0.000description3
- 230000002159abnormal effectEffects0.000description3
- 210000001992atrioventricular nodeAnatomy0.000description3
- 230000008901benefitEffects0.000description3
- 210000004556brainAnatomy0.000description3
- 210000000038chestAnatomy0.000description3
- 238000004891communicationMethods0.000description3
- 230000008602contractionEffects0.000description3
- 230000009977dual effectEffects0.000description3
- 210000003191femoral veinAnatomy0.000description3
- 239000004033plasticSubstances0.000description3
- 229920003023plasticPolymers0.000description3
- 230000002829reductive effectEffects0.000description3
- 210000005245right atriumAnatomy0.000description3
- 239000000126substanceSubstances0.000description3
- 229920001651CyanoacrylatePolymers0.000description2
- MWCLLHOVUTZFKS-UHFFFAOYSA-NMethyl cyanoacrylateChemical compoundCOC(=O)C(=C)C#NMWCLLHOVUTZFKS-UHFFFAOYSA-N0.000description2
- 239000004677NylonSubstances0.000description2
- 238000010317ablation therapyMethods0.000description2
- 229910045601alloyInorganic materials0.000description2
- 239000000956alloySubstances0.000description2
- 230000003126arrythmogenic effectEffects0.000description2
- 210000003157atrial septumAnatomy0.000description2
- 239000000560biocompatible materialSubstances0.000description2
- 230000007423decreaseEffects0.000description2
- 238000003745diagnosisMethods0.000description2
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description2
- 208000035475disorderDiseases0.000description2
- 229940079593drugDrugs0.000description2
- 239000003814drugSubstances0.000description2
- 229920001971elastomerPolymers0.000description2
- 239000000806elastomerSubstances0.000description2
- 238000002001electrophysiologyMethods0.000description2
- 230000007831electrophysiologyEffects0.000description2
- 238000005516engineering processMethods0.000description2
- 238000002594fluoroscopyMethods0.000description2
- 210000000232gallbladderAnatomy0.000description2
- 229910052741iridiumInorganic materials0.000description2
- GKOZUEZYRPOHIO-UHFFFAOYSA-Niridium atomChemical compound[Ir]GKOZUEZYRPOHIO-UHFFFAOYSA-N0.000description2
- 230000001788irregularEffects0.000description2
- 229910052755nonmetalInorganic materials0.000description2
- 150000002843nonmetalsChemical class0.000description2
- 229920001778nylonPolymers0.000description2
- 239000004417polycarbonateSubstances0.000description2
- 229920000515polycarbonatePolymers0.000description2
- 229920000642polymerPolymers0.000description2
- 229920002635polyurethanePolymers0.000description2
- 239000004814polyurethaneSubstances0.000description2
- 230000008569processEffects0.000description2
- 238000005086pumpingMethods0.000description2
- 230000005855radiationEffects0.000description2
- 210000001013sinoatrial nodeAnatomy0.000description2
- 230000003068static effectEffects0.000description2
- 230000000451tissue damageEffects0.000description2
- 231100000827tissue damageToxicity0.000description2
- 238000012546transferMethods0.000description2
- 210000004291uterusAnatomy0.000description2
- 239000010963304 stainless steelSubstances0.000description1
- 208000016216ChoristomaDiseases0.000description1
- 208000032544CicatrixDiseases0.000description1
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000description1
- 208000005189EmbolismDiseases0.000description1
- 206010020772HypertensionDiseases0.000description1
- 206010020850HyperthyroidismDiseases0.000description1
- 241000124008MammaliaSpecies0.000description1
- 241001465754MetazoaSpecies0.000description1
- 206010049171Pulmonary vein stenosisDiseases0.000description1
- 229910000589SAE 304 stainless steelInorganic materials0.000description1
- 206010039509ScabDiseases0.000description1
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description1
- 229910000831SteelInorganic materials0.000description1
- 208000003734Supraventricular TachycardiaDiseases0.000description1
- 208000007536ThrombosisDiseases0.000description1
- RTAQQCXQSZGOHL-UHFFFAOYSA-NTitaniumChemical compound[Ti]RTAQQCXQSZGOHL-UHFFFAOYSA-N0.000description1
- 238000011298ablation treatmentMethods0.000description1
- 230000003213activating effectEffects0.000description1
- 210000001367arteryAnatomy0.000description1
- 206010003668atrial tachycardiaDiseases0.000description1
- 239000011324beadSubstances0.000description1
- 230000023555blood coagulationEffects0.000description1
- 230000036760body temperatureEffects0.000description1
- 201000011510cancerDiseases0.000description1
- 230000000747cardiac effectEffects0.000description1
- 238000013194cardioversionMethods0.000description1
- 230000035602clottingEffects0.000description1
- 150000001875compoundsChemical class0.000description1
- 239000004020conductorSubstances0.000description1
- 229910052802copperInorganic materials0.000description1
- 239000010949copperSubstances0.000description1
- 208000029078coronary artery diseaseDiseases0.000description1
- 210000003748coronary sinusAnatomy0.000description1
- 210000004351coronary vesselAnatomy0.000description1
- 230000006378damageEffects0.000description1
- 230000003247decreasing effectEffects0.000description1
- 238000013461designMethods0.000description1
- 238000011161developmentMethods0.000description1
- 206010012601diabetes mellitusDiseases0.000description1
- 238000002405diagnostic procedureMethods0.000description1
- 235000012489doughnutsNutrition0.000description1
- 238000012377drug deliveryMethods0.000description1
- 239000013013elastic materialSubstances0.000description1
- 238000013195electrical cardioversionMethods0.000description1
- 210000003238esophagusAnatomy0.000description1
- 210000001105femoral arteryAnatomy0.000description1
- 229920002457flexible plasticPolymers0.000description1
- 208000019622heart diseaseDiseases0.000description1
- 230000036540impulse transmissionEffects0.000description1
- 230000002401inhibitory effectEffects0.000description1
- 208000014674injuryDiseases0.000description1
- 238000003780insertionMethods0.000description1
- 230000037431insertionEffects0.000description1
- 239000012212insulatorSubstances0.000description1
- 238000013152interventional procedureMethods0.000description1
- 210000004731jugular veinAnatomy0.000description1
- 210000005248left atrial appendageAnatomy0.000description1
- 230000000670limiting effectEffects0.000description1
- 244000144972livestockSpecies0.000description1
- 230000004807localizationEffects0.000description1
- 230000007257malfunctionEffects0.000description1
- 238000007726management methodMethods0.000description1
- 238000004519manufacturing processMethods0.000description1
- 238000005259measurementMethods0.000description1
- 230000003278mimic effectEffects0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 210000004165myocardiumAnatomy0.000description1
- 238000013021overheatingMethods0.000description1
- RVTZCBVAJQQJTK-UHFFFAOYSA-Noxygen(2-);zirconium(4+)Chemical compound[O-2].[O-2].[Zr+4]RVTZCBVAJQQJTK-UHFFFAOYSA-N0.000description1
- 229920001296polysiloxanePolymers0.000description1
- 238000003825pressingMethods0.000description1
- 230000002265preventionEffects0.000description1
- 230000000644propagated effectEffects0.000description1
- 238000007674radiofrequency ablationMethods0.000description1
- 238000011160researchMethods0.000description1
- 230000002441reversible effectEffects0.000description1
- 208000004124rheumatic heart diseaseDiseases0.000description1
- 231100000241scarToxicity0.000description1
- 230000037387scarsEffects0.000description1
- 238000000926separation methodMethods0.000description1
- 229910001285shape-memory alloyInorganic materials0.000description1
- 239000011780sodium chlorideSubstances0.000description1
- 239000007779soft materialSubstances0.000description1
- 230000006641stabilisationEffects0.000description1
- 238000011105stabilizationMethods0.000description1
- 239000010959steelSubstances0.000description1
- 238000003860storageMethods0.000description1
- 208000024891symptomDiseases0.000description1
- 230000001360synchronised effectEffects0.000description1
- 230000002277temperature effectEffects0.000description1
- 230000001225therapeutic effectEffects0.000description1
- 210000000115thoracic cavityAnatomy0.000description1
- 208000005057thyrotoxicosisDiseases0.000description1
- 239000010936titaniumSubstances0.000description1
- 229910052719titaniumInorganic materials0.000description1
- 230000008733traumaEffects0.000description1
- 210000005243upper chamberAnatomy0.000description1
- 210000001631vena cava inferiorAnatomy0.000description1
- 210000002620vena cava superiorAnatomy0.000description1
- 208000003663ventricular fibrillationDiseases0.000description1
- 206010047302ventricular tachycardiaDiseases0.000description1
- 230000000007visual effectEffects0.000description1
- 238000012800visualizationMethods0.000description1
- 239000011800void materialSubstances0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B18/1492—Probes or electrodes therefor having a flexible, catheter-like structure, e.g. for heart ablation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/1815—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using microwaves
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/02—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00005—Cooling or heating of the probe or tissue immediately surrounding the probe
- A61B2018/00011—Cooling or heating of the probe or tissue immediately surrounding the probe with fluids
- A61B2018/00029—Cooling or heating of the probe or tissue immediately surrounding the probe with fluids open
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/0016—Energy applicators arranged in a two- or three dimensional array
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
- A61B2018/00267—Expandable means emitting energy, e.g. by elements carried thereon having a basket shaped structure
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
- A61B2018/00351—Heart
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
- A61B2018/00351—Heart
- A61B2018/00375—Ostium, e.g. ostium of pulmonary vein or artery
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00571—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for achieving a particular surgical effect
- A61B2018/00577—Ablation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00636—Sensing and controlling the application of energy
- A61B2018/00773—Sensed parameters
- A61B2018/00791—Temperature
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00636—Sensing and controlling the application of energy
- A61B2018/00773—Sensed parameters
- A61B2018/00791—Temperature
- A61B2018/00821—Temperature measured by a thermocouple
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00636—Sensing and controlling the application of energy
- A61B2018/00773—Sensed parameters
- A61B2018/00839—Bioelectrical parameters, e.g. ECG, EEG
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1405—Electrodes having a specific shape
- A61B2018/1435—Spiral
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1467—Probes or electrodes therefor using more than two electrodes on a single probe
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1475—Electrodes retractable in or deployable from a housing
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/1815—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using microwaves
- A61B2018/1861—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using microwaves with an instrument inserted into a body lumen or cavity, e.g. a catheter
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N7/00—Ultrasound therapy
- A61N7/02—Localised ultrasound hyperthermia
- A61N7/022—Localised ultrasound hyperthermia intracavitary
Definitions
- the present inventionrelates generally to catheters and methods for performing targeted tissue ablation in a subject.
- the present inventionprovides devices comprising catheters having distal ends configured to pulmonary vein ostia, and methods for treating conditions (e.g., cardiac arrhythmias) with these and similar devices.
- Tissue ablationis used in numerous medical procedures to treat a patient.
- Ablationcan be performed to remove undesired tissue such as cancer cells.
- Ablation proceduresmay also involve the modification of the tissue without removal, such as to stop electrical propagation through the tissue in patients with an arrhythmia.
- the ablationis performed by passing energy, such as electrical energy, through one or more electrodes causing the tissue in contact with the electrodes to heats up to an ablative temperature.
- Ablation procedurescan be performed on patients with atrial fibrillation by ablating tissue in the heart.
- Mammalian organ functiontypically occurs through the transmission of electrical impulses from one tissue to another. A disturbance of such electrical transmission may lead to organ malfunction.
- One particular area where electrical impulse transmission is critical for proper organ functionis in the heart. Normal sinus rhythm of the heart begins with the sinus node generating an electrical impulse that is propagated uniformly across the right and left atria to the atrioventricular node. Atrial contraction leads to the pumping of blood into the ventricles in a manner synchronous with the pulse.
- Atrial fibrillationrefers to a type of cardiac arrhythmia where there is disorganized electrical conduction in the atria causing rapid uncoordinated contractions that result in ineffective pumping of blood into the ventricle and a lack of synchrony.
- the atrioventricular nodereceives electrical impulses from numerous locations throughout the atria instead of only from the sinus node. This overwhelms the atrioventricular node into producing an irregular and rapid heartbeat.
- bloodpools in the atria that increases a risk for blood clot formation.
- the major risk factors for atrial fibrillationinclude age, coronary artery disease, rheumatic heart disease, hypertension, diabetes, and thyrotoxicosis. Atrial fibrillation affects 7% of the population over age 65.
- Atrial fibrillation treatment optionsare limited. Lifestyle change only assists individuals with lifestyle related atrial fibrillation. Medication therapy assists only in the management of atrial fibrillation symptoms, may present side effects more dangerous than atrial fibrillation, and fail to cure atrial fibrillation. Electrical cardioversion often restores sinus rhythm, but has a high recurrence rate. In addition, if there is a blood clot in the atria, cardioversion may cause the clot to leave the heart and travel to the brain or to some other part of the body, which may lead to stroke. What are needed are new methods for treating atrial fibrillation and other conditions involving disorganized electrical conduction.
- an ablation catheterfor an operator to treat a patient with an arrhythmia.
- the catheterincludes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween.
- the catheterfurther includes a control shaft, coaxially disposed and slidingly received with the lumen of the tubular body member.
- a flexible carrier assemblyis attached to the end of the control shaft and includes at least one ablation and/or mapping elements. Retraction of the control shaft causes the carrier assembly to transition from a compact, near linear configuration, to a helix or partial helix. In a preferred embodiment, the helix is less than 360.degree.
- the carrier assemblycan be withdrawn into a location within the tubular body member.
- the ablation catheterincludes at least two carrier assemblies that can be transitioned between a compact, near linear configuration to a helix or partial helix.
- the cathetercan be placed over a guidewire or includes an integral guidewire tip.
- an ablation catheterfor an operator to treat a patient with an arrhythmia.
- the catheterincludes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween.
- the catheterfurther includes a control shaft, coaxially disposed and slidingly received with the lumen of the tubular body member.
- a flexible carrier assemblyis attached to the end of the control shaft and includes at least one ablation and/or mapping elements in an umbrella tip configuration. Retraction of the control shaft causes the carrier assembly to change shape, such as to conform to tissue surrounding one or more pulmonary veins entering the left atrium of a patient.
- the ablation catheterincludes a second carrier assembly, also in an umbrella tip configuration.
- the catheterincludes an anchoring element, such as a balloon or expandable cage, for stabilizing and/or anchoring the ablation catheter in a pulmonary vein.
- the catheterincludes an ultrasound element for directing ultrasound energy in a circular pattern toward tissue.
- one or more carrier arms of the umbrella tipcan be rotated, stabilized, or otherwise manipulated to better conform to or stabilize with tissue such as pulmonary vein ostial tissue.
- the cathetercan be placed over a guidewire or includes an integral guidewire tip.
- the catheterincludes an advancable spline which can be used to position or stabilize the carrier assembly.
- an ablation catheterfor an operator to treat a patient with an arrhythmia.
- the catheterincludes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween.
- the catheterfurther includes a flexible carrier assembly comprising an inflatable balloon with mounted or embedded ablation and/or mapping elements.
- One such example of a minimally invasive therapyinvolves the treatment of cardiac arrhythmias or irregular heartbeats in which physicians employ specialized cardiac assessment and treatment devices, such as mapping catheters and ablation catheters, to gain access to, diagnose, and treat interior regions of a patient's body.
- cardiac assessment and treatment devicessuch as mapping catheters and ablation catheters
- Such devicesmay include energized electrodes or other ablation assemblies to create lesions or other anatomical effects that disrupt or block electrical pathways through the targeted tissue.
- a specific area of cardiac tissue having aberrant electrically conductive pathwaysis typically initially identified for subsequent treatment.
- This localization or identificationcan include first using a medical device such as a mapping catheter to obtain a baseline electrophysiological map of electrical activity in selected tissue. After mapping and diagnosing aberrant tissue, a physician may decide to treat the patient by ablating the tissue.
- An ablation proceduremay involve creating one or more lesions to electrically isolate tissue believed to be the source of an arrhythmia.
- One type of ablationis the cryotreatment or cryogenic ablation, which entails creating cold temperatures at specific regions of the body or contacting tissue with cold treatment devices to transfer heat from the targeted tissue to the cryogenic element, thus cooling and/or ablating the tissue.
- Such cryotreatmentmay require first repositioning or removing a mapping catheter before placing a second medical device or ablation catheter into contact with the tissue to be treated.
- the physicianmay desire to asses or confirm the efficacy of the treatment by obtaining a second electrophysiological map of the tissue region.
- This subsequent mapping proceduremay involve removal or manipulation of the ablation medical device to allow the desired positioning of the mapping device adjacent to the tissue that was previously treated.
- Each device exchange or manipulationrepresents an added risk to the patient as inserting and removing catheters in the vasculature carries a number of inherent risks, possibly including embolism. Exchanging these various catheters during a procedure can cause inaccuracies or movement in the placement and location of the distal tip a device with respect to the tissue to be mapped or ablated, and may further add to the time required to perform the desired treatment. These potential inaccuracies and extended duration of the particular procedure further increase the risk to the patient undergoing treatment. Accordingly, it would be desirable to provide an integrated apparatus and method of use thereof for both diagnosing aberrant electrical pathways and treating those detected pathways.
- placing and maintaining a medical device in the desired position with correct alignment and positive contact with the selected tissuemay enhance a mapping and ablation treatment and its likelihood of success. It is therefore desirable to provide apparatus and method of use to verify the position of a medical device, positive contact and alignment with the selected tissue, and to evaluate the medical treatment contemporaneously.
- FIG. 1illustrates a side sectional view of an ablation catheter, consistent with present invention, with the distal end inserted into a pulmonary vein of a patient.
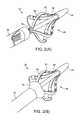
- FIGS. 2 a and 2 billustrates a perspective view of the distal portion of the ablation catheter of FIG. 1 , consistent with the present invention.
- FIG. 3illustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which the device includes a proximal energy delivering carrier assembly and a distal mapping carrier assembly.
- FIG. 4illustrates an ablation catheter handle consistent with the present invention including the dual carrier assemblies of FIG. 3 .
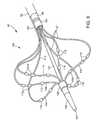
- FIG. 5illustrates a side sectional view of an ablation catheter, consistent with present invention, with the distal end inserted into a pulmonary vein of a patient.
- FIG. 6illustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which the carrier assembly includes one or more carrier arms that can be rotationally positioned.
- FIG. 7illustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which a sleeve can be advanced to manipulate one or more carrier arms of the carrier assembly, and the distal end includes a flexible wire for inserting into a pulmonary vein.
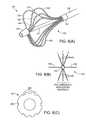
- FIG. 8 aillustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which one or more carrier arms of the carrier assembly are maintained in close proximity with a collar.
- FIG. 8 bis an end view of the ablation catheter of FIG. 8 a.
- FIG. 8 cis an end view of the collar of the ablation catheter of FIG. 8 a.
- FIG. 9illustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which the carrier assembly includes a radially deployable spline that can be deployed in between two carrier arms.
- FIGS. 9 a and 9 billustrate a side view of the distal portion of the ablation catheter of FIG. 9 a , with the spline in partially and fully deployed conditions, respectively, with the carrier arms removed for clarity.
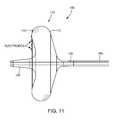
- FIG. 10illustrates a perspective view of the distal portion of an ablation catheter consistent with the present invention, in which the carrier assembly comprises a balloon with fixedly mounted ablation elements.
- FIG. 11illustrates a side view of the distal portion of the ablation catheter of FIG. 10 .
- FIG. 12 aillustrates a perspective view of an ablation catheter consistent with the present invention, wherein the carrier assembly comprises a single carrier arm and the carrier assembly is in the deployed state.
- FIG. 12 billustrates a perspective view of a distal portion of the ablation catheter of FIG. 12 a , in which the carrier assembly is in a fully compacted state.
- FIG. 12 cillustrates a perspective view of a distal portion of the ablation catheter of FIG. 12 b , in which the carrier assembly is in a partially deployed state.
- FIG. 13illustrates a distal portion of an ablation catheter consistent with the present invention, in which the carrier assembly comprises a single carrier arm whose distal end is attached along a different axis than the proximal end.
- FIG. 13 aillustrates a side view of a distal portion of the catheter of FIG. 13 .
- FIG. 13 billustrates a perspective view of a distal portion of the catheter of FIG. 13 .
- FIG. 14illustrates a side sectional view of a distal portion of an ablation catheter consistent with the present invention, in which the carrier assembly comprises a single carrier arm.
- FIG. 15 aillustrates a perspective view of a proximal portion of an ablation catheter consistent with the present invention, including a handle with multiple controls.
- FIG. 15 billustrates a perspective view of a distal portion of the ablation catheter of FIG. 15 a , in which the carrier assembly comprises a single carrier arm and the carrier assembly is in a fully compacted state.
- FIG. 15 cillustrates a perspective view of a distal portion of the ablation catheter of FIG. 15 a , in which the carrier assembly comprises a single carrier arm and the carrier assembly is in the fully deployed state.
- FIG. 15 dillustrates the ablation catheter of FIGS. 15 a through 15 c after having been placed through a transeptal sheath and the carrier assembly deployed and contacting the ostium of the left superior pulmonary vein.
- FIG. 16illustrates a perspective view of an ablation catheter consistent with the present invention, including a first deployable carrier assembly and a second deployable carrier assembly.
- FIG. 16 aillustrates an end view of the ablation catheter of FIG. 16 .
- FIG. 16 billustrates a side sectional view of the distal portion of the ablation catheter of FIG. 16 , wherein the distal carrier assembly is in contact with the lumen of a pulmonary vein and the proximal carrier assembly is in contact with the pulmonary vein ostium.
- FIG. 17illustrates a side view of an ablation catheter consistent with the present invention, including a carrier assembly comprising a single carrier arm that can be fully retracted within a lumen of the shaft of the device.
- FIG. 17 aillustrates a side sectional view of the device of FIG. 17 wherein the carrier assembly has been fully deployed.
- FIG. 17 billustrates a side sectional view of the device of FIG. 17 wherein the carrier assembly has been fully compacted.
- FIG. 17 cillustrates an end view of the device of FIG. 17 a.
- the present inventionprovides catheters for performing targeted tissue ablation in a subject.
- the catheterscomprise a tubular body member having a proximal end and distal end and preferably a lumen extending therebetween.
- the catheteris preferably of the type used for performing intracardiac procedures, typically being percutaneously introduced and advanced from the femoral vein in a patient's leg. Alternative methods involve percutaneous introduction into the jugular vein of the patient's neck, or other anatomical entry point that can be used to access the target location within the patient.
- the catheteris preferably introducable through a sheath and also preferably is advancable over a guidewire.
- the catheterpreferably has a steerable tip that allows precise positioning of the distal portion such as when the distal end of the catheter needs to access a pulmonary vein of the left atrium of the patient's heart.
- the cathetersinclude ablation elements mounted on one or more carrier arms of a flexible carrier assembly. Typical metals chosen for carrier assembly construction include but are not limited to: stainless steel, Nitinol, ElgiloyTM, other alloys and combinations thereof. These ablation elements can be used to ablate and/or map electrical activity of tissue.
- the carrier assemblyis attached to a control shaft that is coaxially disposed and slidingly received within the lumen of the tubular body member. The shape of the carrier assembly is adjusted by advancing or retracting the control shaft, such as to engage one or more ablation elements against cardiac tissue, typically pulmonary vein ostial tissue.
- Arrays of ablation elementsmay be configured in a wide variety of ways and patterns.
- the present inventionprovides devices with multi-dimensional electrode arrays that provide electrical energy, such as radiofrequency (RF) energy, in monopolar (unipolar), bipolar or combined monopolar-bipolar fashion, as well as methods for treating conditions (e.g., atrial fibrillation, supra ventricular tachycardia, atrial tachycardia, ventricular tachycardia, ventricular fibrillation, and the like) with these devices.
- RFradiofrequency
- ablation elementsthat deliver electrical energy to tissue
- other forms and types of energycan be delivered including but not limited to: sound energy such as acoustic energy and ultrasound energy; electromagnetic energy such as electrical, magnetic, microwave and radiofrequency energies; thermal energy such as heat and cryogenic energies; chemical energy such as energy generated by delivery of a drug; light energy such as infrared and visible light energies; mechanical and physical energy; radiation; and combinations thereof.
- the normal functioning of the heartrelies on proper electrical impulse generation and transmission.
- proper electrical generation and transmissionare disrupted or are otherwise abnormal.
- the ablation catheters of the present inventionmay be employed.
- One current method of treating cardiac arrhythmiasis with catheter ablation therapy. Physicians make use of catheters to gain access into interior regions of the body. Catheters with attached electrode arrays or other ablating devices are used to create lesions that disrupt electrical pathways in cardiac tissue. In the treatment of cardiac arrhythmias, a specific area of cardiac tissue having aberrant conductive pathways, such as atrial rotors, emitting or conducting erratic electrical impulses, is initially localized. A user (e.g., a physician) directs a catheter through a main vein or artery into the interior region of the heart that is to be treated. The ablating element or elements are next placed near the targeted cardiac tissue that is to be ablated, such as a pulmonary vein ostium.
- the physiciandirects energy, provided by a source external to the patient, from one or more ablation elements to ablate the neighboring tissue and form a lesion.
- energyprovided by a source external to the patient
- the goal of catheter ablation therapyis to disrupt the electrical pathways in cardiac tissue to stop the emission of and/or prevent the propagation of erratic electric impulses, thereby curing the heart of the disorder.
- atrial fibrillationcurrently available methods and devices have shown only limited success and/or employ devices that are extremely difficult to use or otherwise impractical.
- the ablation catheters of the present inventionallow the generation of lesions of appropriate size and shape to treat conditions involving disorganized electrical conduction (e.g., atrial fibrillation).
- the created lesionsare segmented and localized.
- the lesionsmay be linear or curvilinear, circumferential and partial circumferential, and/or continuous or discontinuous.
- the ablation catheters of the present inventionare also practical in terms of ease-of-use and limiting risk to the patient, as well as significantly reducing procedure times.
- the lesions created by the ablation cathetersare suitable for inhibiting the propagation of inappropriate electrical impulses in the heart for prevention of reentrant arrhythmias.
- the catheters of the present inventioncan perform tissue ablation and/or mapping of electrical signals present in tissue. Patients, such as those with atrial fibrillation, are diagnosed and treated with the herein described mapping and/or ablation procedures.
- the catheters of the present inventionare specifically applicable to mapping and ablation of the pulmonary vein ostia located in the left atrium of the patient's heart. These vein ostia are approximately 1.5 cm in diameter and often are non-circular in geometry, especially when a venous bifurcation is present proximate the ostia.
- the carrier assembly of the present inventionmay include one or more carrier arms that are configured to conform to these circular and non-circular contours of pulmonary vein ostia.
- One or more carrier arms, or groups of carrier armsmay be configured to be independently advancable and retractable, such as to properly engage pulmonary vein ostium tissue.
- the carrier armsare preferably made of Nitinol, and may have round, oval, triangular, rectangular, or trapezoidal cross-sectional geometry.
- the carrier armsmay include compound splines or angles, such as to conform to pulmonary vein ostia and surrounding tissue.
- Each carrier armmay include one or more sensors, such as temperature sensors integral to an ablation element or mounted between two ablation elements, such as to measure tissue temperature and/or blood temperature.
- a temperature sensoris mounted to a carrier arm in a location more distal than the most distal ablation element, when the carrier assembly is in a deployed, ready to deliver ablation energy, configuration.
- Information recorded by the temperature sensorcan be used by an energy delivery unit of the present invention as a threshold to avoid overheating of blood or tissue, as well as regulate power to a target temperature.
- a first carrier armmay have a different property than a second carrier arm, such as a different rigidity, a different number of ablation elements, or a different configuration of sensors such as temperature sensors.
- the catheters of the present inventionmay be configured to be advanced into the heart of a patient over a previously placed guidewire, such as a standard interventional 0.035′′ guidewire.
- the cathetermay include an inner lumen for the majority of its length, through which the guidewire is inserted, or the catheter may include a relatively short sidecar near its distal end, where the guidewire inserted through a lumen of the sidecar.
- the placement over the guidewireallows simplified positioning and re-positioning by an operator.
- the guidewire placementalso provides stability such as to simplify maintaining the position of the catheter during energy delivery, typically 60 seconds.
- the catheters of the present inventionare configured to be inserted through the lumen of a previously placed transeptal sheath, such as a 9.5 French (Fr) steerable transeptal sheath.
- the catheter of the present inventionpreferably include an integral steering mechanism, such as one or more pull wires fixedly attached near a distal portion of the catheter and operably attached to a lever, knob or other control integral to a handle of the catheter.
- the steeringcan be used to deflect the carrier assembly and distal end of the catheter into the left and right pulmonary veins of the left atrium.
- the integral catheter steeringcan be used in conjunction a steerable transeptal sheath. Multiple pull wires can be fixedly mounted 90.degree.
- the tubular body member of the ablation catheteris constructed with sufficient columnar strength and rigidity to allow an operator to apply significant torque to the proximal end that equivalently translates to the catheters distal portions.
- the present inventionincludes one or more systems that include the ablation catheters of the present invention.
- the systemmay further include a guide catheter such as a steerable transeptal sheath that slidingly receives the ablation catheter.
- the systemmay further include an energy delivery unit, such as a unit configured to deliver RF and/or other forms of energy to the ablation elements of the catheter.
- the systemmay further include a mapping unit that receives information recorded from one or more sensors of the ablation catheter, such as an ablation element of the ablation catheter.
- the mapping unitprovides electrical activity information to an operator of the system.
- the mapping unitmay be integral to the energy delivery unit.
- the terms “subject” and “patient”refer to any animal, such as a mammal like livestock, pets, and preferably a human. Specific examples of “subjects” and “patients” include, but are not limited, to individuals requiring medical assistance, and in particular, requiring atrial fibrillation catheter ablation treatment.
- the terms “catheter ablation” or “ablation procedures” or “ablation therapy,” and like terms,refer to what is generally known as tissue destruction procedures.
- Ablationis often used in treating several medical conditions, including abnormal heart rhythms. It can be performed both surgically and non-surgically. Non-surgical ablation is typically performed in a special lab called the electrophysiology (EP) laboratory. During this non-surgical procedure a catheter is inserted into the heart using fluoroscopy for visualization, and then an energy delivery apparatus is used to direct energy to the heart muscle. This energy either “disconnects” or “isolates” the pathway of the abnormal rhythm (depending on the type of ablation). It can also be used to disconnect the conductive pathway between the upper chambers (atria) and the lower chambers (ventricles) of the heart. For individuals requiring heart surgery, ablation can be performed during coronary artery bypass or valve surgery.
- the term “ablation element”refers to an energy delivery element, such as an electrode for delivering electrical energy such as RF energy.
- Ablation elementscan be configured to deliver multiple types of energy, such as ultrasound energy and cryogenic energy, either simultaneously or serially.
- Electrodescan be constructed of a conductive plate, wire coil, or other means of conducting electrical energy through contacting tissue. Electrodes may comprise a laminate construction, such as at least one conductive layer and at least one insulative layer.
- RF electrodespreferably are constructed of platinum or a combination of platinum and iridium. In monopolar energy delivery, the energy is conducted from the electrode, through the tissue to a ground pad, such as a conductive pad attached to the back of the patient. The high concentration of energy at the electrode site causes localized tissue ablation.
- bipolar energy deliverythe energy is conducted from a first electrode to one or more separate electrodes, relatively local to the first electrode, through the tissue between the associated electrodes.
- Bipolar energy deliveryresults in more precise, shallow lesions while monopolar delivery results in deeper lesions. Both monopolar and bipolar delivery provide advantages, and the combination of their use is a preferred embodiment of this application.
- Energycan also be delivered using pulse width modulated drive signals, well known to those of skill in the art. Energy can also be delivered in a closed loop fashion, such as a system with temperature feedback wherein the temperature modifies the type, frequency and or magnitude of the energy delivered.
- Ablation elementsmay have one or more different shapes, such as tubular electrodes mounted around a shaft such as a carrier arm, and other cross-sections such as oval, triangular, rectangular and trapezoidal. Triangular cross sections can be positioned where multiple sides contact tissue for increased energy transfer or multiple sides contact a cooling source such as blood for increased cooling.
- the ablation elementsmay include a heat-sinking element, such as a projecting fin or other increased surface area portion.
- the ablation elementspreferably include an integral temperature sensor, such as a thermocouple comprised of copper and constantan wires that are welded inside a mid portion of an RF electrode.
- an ablation elementcan also be used to record and map electrical activity in tissue.
- one or more ablation elementsmay be configured to only map electrical activity, and not be configured to deliver energy.
- carrier assemblyrefers to a flexible carrier, on which one or more ablation elements are disposed.
- Carrier assembliesinclude one or more carrier arms.
- Carrier assembliesare not limited to any particular size, or shape, and can be configured to be in expanded and unexpanded or compact states.
- carrier armrefers to a wire-like shaft capable of interfacing with electrodes and a control shaft.
- a carrier armis not limited to any size or measurement. Examples include, but are not limited to: stainless steel shafts; Nitinol shafts; titanium shafts; polyurethane shafts; nylon shafts; and steel shafts.
- Carrier armscan be entirely flexible, or may include flexible and rigid segments.
- the term “spiral tip”refers to a carrier assembly configured in its fully expanded state into the shape of a helix or spiral.
- the spiral tipis not limited in the number of spirals it may contain. Examples include, but are not limited to, a wire tip body with one spiral, two spirals, ten spirals, and a half of a spiral.
- the spiralscan lie in a relatively single plane, or in multiple planes.
- a spiral tipmay be configured for energy delivery during an ablation procedure.
- tissuethat has received ablation therapy. Examples include, but are not limited to, scars, scabs, dead tissue, burned tissue and tissue with conductive pathways that have been made highly resistive or disconnected.
- each carrier armmay include one or more ablation elements.
- Each carrier arm of an umbrella tipincludes a proximal arm segment and a distal arm segment, the distal arm segment more distal than the proximal arm segment when the carrier assembly is in a fully expanded condition.
- One or more additional carrier armscan be included which include no ablation elements, such as carrier arms used to provide support or cause a particular deflection.
- An umbrella tip bodyis not limited to any particular size.
- An umbrella tipmay be configured for energy delivery during an ablation procedure.
- carrier arm bend pointrefers to a joint (e.g., junction, flexion point) located on a carrier arm.
- the degree of flexion for a carrier arm bend pointmay range from 0 to 360 degrees.
- the bend portioncan be manufactured such what when the carrier assembly is fully expanded the bend point is positioned in a relatively straight portion, a curved portion, or in a discrete transition from a first direction to a second transition, such as a 45 degree bend transition.
- the bend portioncan include one or more flexing means such as a spring, a reduced diameter segment, or a segment of increased flexibility.
- the term “energy delivery unit”refers to a device configured to operably attach to an ablation catheter and deliver one or more forms of energy to an ablation element.
- the energy delivery unitincludes a user interface which allows an operator to make one or more settings involved in applying the ablative energy.
- the energy unitmay be further configured to receive temperature information from the ablation catheter. The temperature information can provided to an operator and/or be used to provide closed loop energy delivery.
- the energy delivery unitmay include a remote control device that may be maintained in the sterile field of the patient during the ablation procedure.
- the energy delivery unitmay receive a signal from an operator control integral to the ablation catheter that initiates delivery of the ablation energy.
- mapping unitrefers to a device configured to operably attach to an ablation catheter and receive one or more mapping signals from an ablation element or other sensor of an ablation catheter.
- the present inventionprovides structures that embody aspects of the ablation catheter.
- the present inventionalso provides tissue ablation systems and methods for using such ablation systems.
- the illustrated and preferred embodimentsdiscuss these structures and techniques in the context of catheter-based cardiac ablation. These structures, systems, and techniques are well suited for use in the field of cardiac ablation.
- the inventionis applicable for use in other tissue ablation applications such as tumor ablation procedures.
- the various aspects of the inventionhave application in procedures for ablating tissue in the prostrate, brain, gall bladder, uterus, and other regions of the body, preferably regions with an accessible wall or flat tissue surface, using systems that are not necessarily catheter-based.
- FIGS. 1-17show various preferred embodiments of the multifunctional catheters of the present invention.
- the present inventionis not limited to these particular configurations.
- FIG. 1illustrates a preferred embodiment of an ablation catheter of the present invention with an umbrella tip, wherein a carrier assembly includes multiple carrier arms configured to properly engage a pulmonary vein ostium.
- Ablation catheter 50and the other catheter devices of this application, are constructed of biocompatible materials suitable for percutaneous advancement through the vasculature of a patient, and for navigation within the patient's heart.
- Various tubular body members and shaftsare constructed of extruded materials such as Pebax, silicones, polyurethanes, polymers, elastomers, flexible plastics and combinations of these.
- Ablation catheter 50includes a distal tip 94 , which is made of materials to be atraumatic to tissue and which is shown entering the lumen of pulmonary vein 15 such as to provide a stabilizing and/or anchoring function.
- Ablation catheter 50further includes outer shaft 76 that preferably has a diameter between 8 and 9 Fr and is constructed to provide sufficient stability and torque through the procedure.
- Ablation catheter 50includes a carrier assembly of the present invention, carrier assembly 85 , which includes multiple ablation elements 92 mounted to distal carrier arms 88 .
- the ablation elements 92 and other components of carrier assembly 85are configured to flex to conform to pulmonary vein ostia and other applicable tissues.
- Outer shaft 76can be advanced forward to change the shape of carrier assembly 85 and cause one or more ablation elements 92 to contact tissue.
- Outer shaft 76slidingly receives inner shaft 78 , which is fixedly attached to proximal carrier arms 86 .
- Distal carrier arms 88are fixedly attached to cap 15 and the distal end of control shaft 84 .
- Proximal carrier arms 86are pivotally attached to distal carrier arms 88 , such that advancement and retraction of control shaft 84 relative to inner tube 78 causes the diameter of carrier assembly 85 to contract and expand respectively, such as to cause the carrier assembly to expand to a 4-5 mm diameter.
- Inner shaft 78further provides columnar strength to allow an operator to advance inner shaft 78 and cause carrier assembly 85 to properly contact tissue, such as to conform to non-circular pulmonary vein ostia.
- Inner shaft 78preferably is attached to a pull wire (not shown), near its distal end, which is operably connected to a control on the proximal end of device 50 allowing an operator to controllably deflect the distal portion of device 50 .
- the distal end of outer shaft 76includes a shaft tip 82 , configured to radially expand when carrier assembly 85 is retracted.
- the proximal end of outer shaft 76preferably includes a handle, not shown, but including one or more controls, such as knobs or levers, such as to advance and retract inner shaft 78 and control shaft 84 .
- the proximal end of device 50includes one or more connectors for connecting to an energy delivery unit and/or a mapping unit.
- one or more proximal control arms 86are attached to a second control shaft such that the symmetry of the geometry of carrier assembly 85 can be adjusted to conform to asymmetric pulmonary vein ostia.
- device 50is configured to be inserted over a previously placed guidewire.
- FIGS. 2 a and 2 bthe distal portion of device 50 of FIG. 1 is illustrated.
- FIG. 2 adepicts carrier assembly 85 fully expanded, with inner shaft 78 fully advanced.
- FIG. 2 bdepicts inner shaft 78 partially retracted such that proximal arms 85 are being captured and radially compressed by shaft tip 82 , which expands, as shown, to create a smooth transition of carrier assembly 85 into the inner lumen of outer shaft 76 .
- Ablation catheter 40includes an elongate tube, outer shaft 36 , preferably constructed of Pebax material and approximately 6-8 Fr in diameter, which slidingly receives first control shaft 48 .
- First control shaft 48is attached on its distal portion to first carrier assembly 45 , comprising multiple carrier arms and ablation elements configured to deliver energy.
- the proximal end of first control shaft 48is attached to a control on the proximal end of ablation catheter 40 configured to allow an operator to precisely advance and retract first control shaft 48 .
- First control shaft 48includes ring 52 on its distal end that fixedly attaches one end of each distal carrier arm segment 44 to first control shaft 48 .
- Each distal carrier arm segment 44is pivotally attached on its opposite end to one end of a proximal carrier arm segment 42 .
- the opposite end of each proximal arm segment 42is fixedly attached to the distal end of outer shaft 36 via ring 38 .
- Distal carrier arm segments 44 and proximal arm segments 42are constructed of a flexible material, such as Nitinol, and can be resiliently biased in a straight or umbrella tip configuration.
- Advancement and retraction of first control shaft 48changes the diameter of carrier assembly 45 , including a fully compacted (minimal diameter) radial state when first control shaft 48 is fully advanced, and a maximum diameter state when first control shaft 48 is fully retracted.
- Electrodes 46Fixedly mounted to distal arm segments 44 are ablation elements, RF electrodes 46 , configured to deliver energy to tissue to create lesions for disrupting aberrant electrical pathways in the tissue. Electrodes 46 include fins 64 configured to reside in a flow of blood during energy delivery and provide sinking of heat into the circulating blood. Electrodes 46 are configured to deliver monopolar, bipolar or a combination of monopolar and bipolar RF energy as has been described above. Electrodes 46 preferably include integral temperature sensors, such as a thermocouple welded to an internal portion of the electrode 46 .
- Electrode 46 and any integral temperature or other sensorsare attached to wires, not shown, which travel proximally to the proximal portion of ablation catheter 40 for attachment to an energy delivery unit, a mapping unit, and/or another electronic device for sending or receiving signals and/or power.
- First control shaft 48slidingly receives second control shaft 57 .
- Second control shaft 57is attached on its distal portion to second carrier assembly 55 , comprising multiple carrier arms and ablation elements configured to map electrical activity.
- the proximal end of second control shaft 48is attached to a control on the proximal end of ablation catheter 50 configured to allow an operator to precisely advance and retract second control shaft 57 .
- Second control shaft 57includes tip 62 on its distal end that fixedly attaches one end of each distal carrier arm segment 56 to second control shaft 57 .
- Tip 62is preferably constructed of a soft or flexible material such as a soft plastic or elastomer chosen to be atraumatic to tissue, and is preferably radiopaque such as a Pebax material doped with Barium Sulfate.
- Distal tip 62is constructed to help navigation into and stabilization within a pulmonary vein.
- Distal tip 62includes guidewire lumen 63 , which is in fluid communication with an internal lumen of second control shaft 57 , the lumen traveling to and exiting a proximal portion of ablation catheter 40 , such that ablation catheter 40 can be percutaneously inserted into the vasculature of a patient over a guidewire.
- Each distal carrier arm segment 56is pivotally attached on its opposite end to one end of a proximal carrier arm segment 54 .
- the opposite end of each proximal arm segment 54is fixedly attached to the distal end of first control shaft 48 via ring 52 .
- Distal carrier arm segments 56 and proximal arm segments 54are constructed of a flexible material, such as Nitinol, and can be resiliently biased in a straight or umbrella tip configuration.
- Advancement and retraction of second control shaft 57changes the diameter of carrier assembly 55 , including a fully compacted (minimum diameter) radial state when second control shaft 57 is fully advanced, and a maximum diameter state when second control shaft 57 is fully retracted.
- Electrodes 58are constructed of a conductive material such as platinum or a combination of platinum and iridium. Electrodes 58 preferably include integral temperature sensors, such as a thermocouple welded to an internal portion of the electrode 58 . Electrode 58 and any integral temperature or other sensors, are attached to wires, not shown, which travel proximally to the proximal portion of ablation catheter 40 for attachment to a mapping unit, an energy delivery unit, and/or another electronic device for sending or receiving signals and/or power.
- Ablation catheter 40 of FIG. 3includes on its proximal end, a handle, not shown, but preferably of the type described in reference to FIG. 4 and including multiple controls for allowing an operator to: advance and retract first control shaft 48 ; advance and retract second control shaft 57 ; activate energy delivery to one or more of electrodes 46 or 58 ; operate a user interface of an energy delivery unit or mapping unit (both not shown); or perform another function.
- the handleincludes an exit port through which a guidewire, such as a guidewire that has been placed into a pulmonary vein of the patient, can exit.
- Carrier assembly 55is sized such that it can engage the luminal wall of a pulmonary vein
- carrier assembly 45is sized and of sufficient flexibility such that it can engage the ostium of a pulmonary vein, including a non-circular orifice.
- Outer shaft 36is constructed of sufficient material and the handle may be manipulated to apply conforming forces to carrier assembly 55 and/or carrier assembly 45 .
- Both first control shaft 48 and second control shaft 57are configured to transmit sufficient torque to allow an operator to precisely rotationally position carrier assembly 45 and carrier assembly 55 respectively.
- the ablation elements 46 of proximal carrier assembly 45may be configured to additionally or alternatively map electrical activity in tissue.
- the ablation elements 58 of distal carrier assembly 55may be configured to additionally or alternatively delivery ablation energy such as RF energy.
- the carrier arms of carrier assembly 45 and/or carrier assembly 55may include sensors, such as temperature thermocouples, placed within an electrode or mounted to a carrier arm some distance from an electrode, such as midway between two electrodes. Ring 38 and Ring 52 are preferably made of a compressible material, such as a metal which can be crimped in a manufacturing process.
- adhesivesmay be used to fixed one or more carrier arms to a shaft. One or more adhesives may be used to attach distal tip 62 .
- Ablation catheter 40includes a tubular body member, outer shaft 36 , which includes on its distal end, proximal carrier assembly 45 and distal carrier assembly 55 , as have been described in detail in reference to FIG. 3 .
- the proximal end of outer shaft 36is attached to handle 66 , which includes multiple controls: slide 67 , slide 68 and button 69 .
- Slide 67is operably attached to first control shaft 48 .
- Slide 68is operably attached to second control shaft 57 . Movement of slides 67 and 68 change the geometries of first carrier assembly 45 and second carrier assembly 55 as has been described in detail in reference to FIG.
- Button 69is used to initiate energy delivery, such as when first carrier assembly 45 is positioned against a pulmonary vein ostium and ablation catheter 40 is electrically connected to an energy delivery unit, not shown.
- Handle 60includes two pigtails, one which terminates in luer 74 and the other which terminates with electrical connector 72 .
- Luer 74is in fluid communication with guidewire lumen 63 exiting tip 62 such that ablation catheter 40 can be advanced over-the-wire into the vasculature of the patient.
- Electrical connector 72includes multiple connection points for multiple wires that travel within outer shaft 36 and connect to ablation elements and one or more sensors such as temperature sensors included in first carrier assembly 45 and second carrier assembly 55 .
- Electrical connector 72is configured to electrically connect to one or more of: an energy delivery unit; a mapping unit; an electronic device for receiving and/or transmitting signals and/or power such as signals received from temperature or other physiologic sensors of ablation catheter 40 ; and combinations of these.
- Ablation catheter 20includes outer shaft 22 , which slidingly receives control shaft 24 , both of which have similar construction to the tubular body members and other shafts described throughout this application.
- carrier assembly 25mounted to control shaft 24 is carrier assembly 25 , comprising proximal carrier arms 26 and distal carrier arms 28 .
- Proximal carrier arms 26 and distal carrier arms 28are made of a flexible material such as Nitinol wire and may be resiliently biased in the geometry shown or a different geometry such as a radially compact geometry compatible with intravascular insertion.
- Each proximal carrier arm 26is fixedly attached at one end to control shaft 24 .
- Each proximal carrier arms 26is pivotally attached at its opposite end to an end of distal carrier arms 28 .
- the opposite end of each distal carrier arm 28is fixedly attached, at a location more distal, to control shaft 24 , such that a right-angle construction is achieved.
- a first proximal arm 26 and attached distal arm 28 pairis attached 180.degree. from a second proximal arm 26 and attached distal arm 28 pair, as shown in FIG. 5 .
- the two pairsare used to contact pulmonary vein ostium 30 , also as shown.
- Additional carrier arm pairsmay be included, such as a total of four pairs separated by 90.degree.
- centering arms 32Distal to distal carrier arms 28 , are centering arms 32 , configured to center control shaft 24 in the pulmonary vein ostium and/or stabilize the distal portion of ablation catheter 20 such as during delivery of ablation energy of mapping of electrical activity.
- Centering arms 32are of similar construction to carrier arms 26 and 28 , such as a round or flat Nitinol band.
- the distal portion of control shaft 24may include an inflatable balloon configured to center and/or anchor control shaft 24 .
- the centering balloonmay include one or more mapping and/or energy delivery elements.
- the centering and/or stabilizing elements of ablation catheter 20may be integrated into the other ablation devices and catheters described throughout this application. These stabilizing and centering elements are particularly useful when accessing pulmonary vein ostia that are non-circular.
- Control shaft 24includes guidewire lumen 31 , which exits the distal end of control shaft 24 and travels proximally and exits a proximal portion of ablation catheter 20 .
- ablation catheter 20is advanced over a previously placed guidewire that has its distal end placed into a pulmonary vein of the patient.
- ultrasound crystal 21Fixedly mounted to external shaft 24 is ultrasound crystal 21 , a tubular energy delivery element configured to deliver ultrasonic energy along a cone shaped path, such as along the trajectory of proximal carrier arms 26 (dashed lines shown on FIG. 5 ).
- the vector of energy deliverywill cause a relatively circular patterned lesion around the pulmonary vein ostium.
- the ultrasound crystalmay be configured to provide energy in a sector (less that 360.degree.), and the carrier assembly 25 would be rotated and repositioned by an operator between ablations to sequentially create a full circumferential lesion.
- Advancement and retraction of control shaft 24can be used to change the diameter of carrier assembly 25 , such as retraction wherein the proximal portion of carrier assembly 25 is captured within the lumen of outer shaft 22 .
- Centering arms 32are preferably connected to a control shaft, not shown, such that the centering arms can be expanded and contracted.
- the size of the elementis configured to be controlled (e.g. expanded and contracted) from the proximal end of the ablation catheter.
- Ablation catheter 60includes outer shaft 96 , ring 98 , ring 102 , carrier assembly 105 , ring 109 and tip 106 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- Carrier assemblyincludes multiple proximal carrier arms 112 which are each at one end fixedly attached to ring 102 . Proximal carrier arms 112 are each pivotally attached at their opposite end to one end of distal carrier arms 114 .
- each distal carrier arms 114is fixedly attached to control shaft 101 via ring 109 , such that advancement of control shaft 101 relative to outer shaft 96 causes carrier assembly 105 to change shape.
- Full advancement of control shaft 101causes carrier assembly 105 to transition to a compact, minimum diameter configuration, and retraction of control shaft 101 causes carrier assembly 105 to transition to a maximum diameter configuration, such as for contacting pulmonary vein ostia.
- Carrier assembly 105further includes a rotatable arm comprising distal arm segment 108 and proximal arm segment 104 .
- One end of distal arm segment 108is rotatably attached to control shaft 101 via ring 109 .
- the opposite end of distal arm segment 108is pivotally attached to proximal arm segment 104 .
- the opposite end of proximal arm segment 104is fixedly attached to ring 98 , which in turn is fixedly attached to a control shaft, not shown but continuing proximally to a control (such as a lever or knob on a handle, both not shown) configured to allow an operator to precisely rotate carrier arm 104 .
- a controlsuch as a lever or knob on a handle, both not shown
- control shaft 101includes tip 106 , which is preferably made of flexible material to be atraumatic to tissue.
- Tip 106includes a guidewire lumen 107 which continues proximally and exits a proximal portion of ablation catheter 60 such that ablation catheter 60 can be percutaneously advanced over a previously placed guidewire, such as a guidewire placed into a pulmonary vein as has been described hereabove.
- Each distal carrier arms 114includes multiple ablation elements 116 configured to deliver energy to tissue.
- mapping element 118configured to record electrical signals present in tissue.
- Distal carrier arm 108includes multiple ablation elements 115 configured to deliver energy to tissue.
- mapping element 113configured to record electrical signals present in tissue.
- Proximal carrier arm 104can be rotated and remain concentric with the static carrier arms, such that the ablation elements 115 on distal arm segment 108 can be positioned at a specific distance from one or more of the ablation elements 116 on static distal carrier arms 114 .
- the positioning through rotationcan be used to achieve lesions of a specific length or other property, especially when bipolar energy is transmitted between ablation element 115 and one or more ablation elements 116 .
- This configurationprovides simplified use in creating continuous lesions created one sector at a time.
- the rotation of proximal arm segment 104 and distal arm segment 114can also be performed to more properly match the contour of a non-circular pulmonary vein ostium.
- ablation catheter 60includes multiple rotatable carrier arms, such as carrier arms connected to independent or ganged control shafts such that multiple carrier arms and their integral ablation element can be rotated to modify the carrier assembly geometry.
- Ablation catheter 70includes outer shaft 130 , first control shaft 121 , second control shaft 122 , carrier assembly 125 and ring 136 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- Carrier assembly 125includes multiple carrier arms comprising proximal arm segments 124 , 124 a and 124 b , each of which are pivotally attached to distal arm segments 126 , 126 a and 126 b respectively.
- Proximal arm segments 124 and distal arm segments 126 , 126 a and 126 bare fixedly attached on their opposite ends, as has been described hereabove, such that advancement and contraction of control shaft 121 decreases and increases the diameter of carrier assembly 125 , respectively.
- Ablation catheter 70further includes second control shaft 122 which is hollow at least on its distal portion and surround proximal arm segments 124 a and 124 b such that advancement of control shaft 122 causes proximal arm segments 124 a and 124 b , each of which includes ablation elements 128 and mapping element 132 , to move towards each other, changing the geometry of carrier assembly 125 , similar to the geometry change causes by rotating a carrier arm as was described in reference to FIG. 6 .
- Both control shaft 121 and control shaft 122are preferably operably connected to a control such as a knob or lever in a handle on the proximal end of ablation catheter 70 .
- Repositioning of one or more carrier armsmay be performed to increase or decrease the distance between ablation elements, mapping electrodes or other arm-mounted sensors or transducers. Repositioning of the arms may also be performed to better conform to various pulmonary vein anatomies, such as pulmonary veins with non-circular ostia.
- Ablation device 70 of FIG. 7further includes an elongate floppy tip 134 , preferably of a guidewire-like construction, to assist in entering an orifice such as a pulmonary vein lumen, or in maintaining stability during a mapping or ablating procedure.
- ablation device 70includes a guidewire lumen from a proximal portion of the device to a distal portion of the device.
- Ablation catheter 90includes outer shaft 138 , control shaft 141 , carrier assembly 145 and tip 154 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- Carrier assembly 145includes proximal arm segments 144 which are pivotally attached to distal arm segments 146 .
- Each distal arm segment 146includes multiple ablation electrodes 147 and a mapping sensor 148 distal to the ablation electrodes 147 .
- Carrier assembly 145further includes multiple carrier arms 156 , which may be void of electrodes as shown, or may include one or more mapping or ablating electrodes, or other sensor or transducer.
- each proximal carrier arm segmentis circumferentially positioned in a groove 246 of ring 142 .
- Carrier arms 156are similarly positioned in a groove 256 of ring 142 .
- Ring 142is preferably attached to a control shaft, not shown, such that advancement of that control shaft changes the geometry of carrier assembly 145 accordingly. Retraction of control shaft 141 changes the diameter of carrier assembly 145 as has been described in detail hereabove.
- FIG. 8 billustrates an end view of the ablation catheter 90 of FIG. 8 a , showing the rotational orientation of the distal arm segments 146 and carrier arms 156 .
- Carrier arms 156are positioned orthogonal to two sets of three distal carrier arms 146 such as to provide positioning and radial support to distal carrier arms 146 .
- Various configurations of carrier arm geometriescan be provided for handling of various pulmonary vein tissue contours.
- Ring 142may maintain the ablation elements 147 and/or the mapping elements 148 in close proximity.
- ring 142is not advancable (not connected to a control shaft), but included with grooves 256 and grooves 246 to maintain the rotational orientation of the distal carrier arms 146 and the carrier arms 156 such as when force is applied to outer shaft 138 , such as via a handle, the force translated to carrier assembly 145 .
- Ablation catheter 90 bincludes outer shaft 138 , control shaft 141 , carrier assembly 95 and tip 154 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- Carrier assembly 95includes multiple carrier arms 156 in an umbrella tip configuration, one or more including ablation elements, other sensors or transducers, all not shown. As control shaft 141 is advanced and retracted (e.g.
- Control shaft 141slidingly receives an advancable spline 164 , whose distal end resides in recess 162 of control shaft 141 .
- Advancement of spline 164causes its distal end to extend radially out from control shaft 141 as is shown in FIG. 9 a (partially extended) and FIG. 9 b (fully extended).
- spline 164can be advanced to the maximum diameter of carrier arms 156 .
- spline 164can be advanced to a diameter greater than the maximum diameter of carrier arms 156 .
- Spline 164is advance to modify the performance characteristics of carrier assembly 95 , such as to modify the supporting forces applied to tissue.
- spline 164includes one or more ablation elements (e.g. RF electrodes) or other sensors or transducers.
- Ablation catheter 100comprises an inflatable balloon with multiple ablation elements mounted on or embedded in its external surface.
- Ablation catheter 100includes carrier assembly 175 comprising balloon 174 and electrodes 172 , such as RF ablation or electrical signal mapping electrodes.
- Ablation catheter 100further includes an elongate tubular body, outer shaft 166 , which includes an inflation lumen 176 from its proximal portion to the inner cavity of balloon 174 .
- Balloon 174is sealed and fixedly attached to the distal portion of outer shaft 166 .
- Balloon 174may be a compliant or non-compliant balloon, and while shown as a disk or donut shape, may have profiles specific to mimic pulmonary vein ostia and the tissue extending therefrom.
- a guidewire lumenis included from the distal end to a proximal portion of ablation catheter 100 such that ablation catheter may be advanced over a guidewire such as a guidewire that has previously been placed into a pulmonary vein ostium or other applicable orifice. Mapping and/or ablating procedures can be accomplished by an operator applying a force to balloon 174 via shaft 166 , and transmitting ablation energy to electrodes 172 and/or recording electrical activity from electrodes 172 .
- ablation catheter of the present inventionis illustrated in which the carrier assembly includes a single carrier arm that can be positioned into an adjustable, partial circumferential (less than 360.degree.) loop for ablating and/or mapping tissue.
- Ablation device 180includes an elongate tubular body member, outer shaft 182 , with sufficient column and torsion strength to support standard interventional procedures such as those which access the vasculature from a femoral vein or artery and access the patient's heart.
- Outer shaft 182is constructed of biocompatible materials such as Pebax, and preferably includes an inner braid, such as a stainless steel 304 braid.
- outer shaft 182is preferably made of Pebax 7233D and the distal portion of outer shaft 182 is preferably made of Pebax 55/3533D.
- Outer shaft 182is fixedly attached to handle 195 via strain relief 181 .
- control shaft 184 and carrier assembly 185Exiting the distal end of outer shaft 182 are control shaft 184 and carrier assembly 185 , which comprises a single carrier arm 186 . At least one of control shaft 184 and carrier arm 186 can be advanced and/or retracted by manipulating a control on handle 195 , such as rotating knob 193 .
- the proximal end of carrier arm 186is fixedly attached to a distal portion of outer shaft 182 , such as via a crimp and/or adhesives, and outer shaft 184 is slidingly received by outer shaft 182 and operably attached to rotating knob 193 .
- Carrier arm 185preferably made of an elastic material such as Nitinol covered with a sleeve made of Pebax, includes one or more electrodes 188 along its length.
- the electrodes 188may include heat-sinking fins as shown.
- the electrodes 188are preferably made of platinum, and are typically 3 mm long and separated by 1 to 4 mm, with symmetric or asymmetric spacing.
- Four electrodes 188are depicted in FIGS. 12 a through 12 c .
- Four (4) to sixteen (16) electrodes 188are preferred, typically eight (8) to ten (10).
- the presence of multiple, typically uniformly distributed electrodesenables the operator to rapidly identify problematic target areas (undesired electrical activity) and create lesions (ablate) rapidly.
- the multi-electrode geometry of carrier assembly 185precision loop control, and ease of positioning (including over-the-wire positioning and anchoring), enables simplified, customized diagnosis and treatment of heart rhythm disorders such as atrial fibrillation.
- Electrodes 188may be for delivering of ablation energy, for mapping of electrical activity, and/or for performing other functions such as pacing of the heart.
- carrier arm 185may include different types of sensors and/or transducers, such as sensors or transducers that can be applied to the ostium of a vessel to perform a diagnostic and/or therapeutic function.
- the distal end of carrier arm 185is fixedly attached to the distal end of control shaft 184 , the connection point encased by tip 192 .
- Tip 192is of materials and construction to be atraumatic, such as a Pebax tip, which has been doped with Barium Sulfate in order to be radiopaque.
- Tip 192includes guidewire lumen 191 , a through hole which travels proximally, through control shaft 184 , and exits handle 195 at guidewire exit hole 199 , configured such that ablation device 180 can be percutaneously advanced over a guidewire which has had its distal end inserted into a pulmonary vein of the patient.
- Handle 195preferably made of a plastic such as a polycarbonate, includes lever 196 which is operably attached to one or more pull wires, not shown, that travel within a lumen of outer shaft 182 and attach near the distal end of shaft 182 .
- Multiple pull wiresmay be included, such as two pull wires which are attached at a 90.degree. radial separation from each other near the distal end of shaft 182 .
- the two pull wiresmay be attached at the same longitudinal position along the axis of shaft 182 , or may be offset.
- Manipulation of lever 196causes the distal portion of ablation catheter 180 to deflect in one or more planes such that a clinician can manipulate tip 192 into a pulmonary vein or other orifice such as the coronary sinus or other vessel.
- Handle 195also includes plug 198 which is configured to electrically connect to one or more separate devices, such as an energy delivery unit configured to deliver ablation energy to electrodes 188 and/or to receive temperature signals from one or more temperature sensors such as a thermocouple integral to an electrode 188 ; a mapping unit configured to receive electrical signals from one or more electrodes 188 ; a pacing unit configured to deliver electrical energy to electrodes 188 in order to pace the heart of a patient; or another device such as a device which receives and/or transmits signals to one or more functional elements of carrier assembly 185 .
- an energy delivery unitconfigured to deliver ablation energy to electrodes 188 and/or to receive temperature signals from one or more temperature sensors such as a thermocouple integral to an electrode 188
- a mapping unitconfigured to receive electrical signals from one or more electrodes 188
- a pacing unitconfigured to deliver electrical energy to electrodes 188 in order to pace the heart of a patient
- another devicesuch as a device which receives and/or transmits signals to one
- Wiresattach to plug 198 and travel through handle 195 , through outer shaft 182 and attach to electrodes 188 and any other sensors or transducers integral to electrodes 188 or attached to carrier arm 186 .
- the wiresmay be located on the external surface of carrier arm 186 , or travel within a lumen of carrier arm 186 , exiting through a side hole to attach to electrodes 188 .
- carrier assembly 185is shown in a linear configuration such that ablation device 180 can be intraluminally advanced through the vasculature of the patient.
- Carrier assembly 185is placed in this linear configuration by advancing control shaft 184 such as via rotating knob 193 of handle 195 .
- control shaft 184is being retracted, and carrier assembly 185 is transitioning to a partial circumferential (less than 360.degree.) loop. Advancement and retraction of control shaft 184 adjust the geometry of the loop, wherein full advancement causes a near-linear configuration and retraction causes the diameter of carrier assembly 185 to increase.
- Preferred maximum diameters of carrier assembly 185are typically 15-32 mm to accommodate the varied anatomical contours neighboring pulmonary vein ostia (including non-circular ostia).
- the simplified loop-modifying controls of the present inventionallow for rapid positioning by an operator.
- carrier arm 186is resiliently biased (such as with the heat treating of a Nitinol component) in a helical configuration.
- carrier arm 186is resiliently biased in a near-linear configuration. In the configuration where carrier arm 186 includes a wire surrounded by a sleeve, a resilient bias can be provided by the wire or the sleeve.
- the proximal end of carrier arm 186exits outer shaft 182 at a location approximate 90.degree. radially offset from the location that carrier arm 186 is attached to the distal end of control shaft 184 , such offset attachment providing a bias for forming the loop during retraction of control shaft 184 .
- Carrier assembly 185may include a loop of 360.degree. or more.
- outer shaft 182may include a mechanical key to maintain the rotational orientation of control shaft 184 and/or carrier arm 186 .
- Control shaft 184may include an attachment ring near its distal end such as for attachment to the proximal end of carrier arm 186 .
- carrier assembly 185includes at least one temperature sensor more distal than the most distal ablation element delivering ablation energy, such that the maximum distal (e.g. into the pulmonary vein lumen) temperature is always monitored (e.g. to prevent creation of a pulmonary vein stenosis). In the configuration of FIGS.
- both control shaft 184 and carrier arm 186exit the distal end of outer shaft 182 .
- either or both control shaft 184 or carrier arm 186exit a side hole of outer shaft 182 (not shown but near the distal end of outer shaft 182 ).
- control shaft 184may be rotated, such as via a control on handle 195 , to further change the geometry of carrier assembly 185 .
- ablation catheter 180is shown with carrier assembly 185 in linear configuration such as for advancing ablation catheter 180 over guidewire 12 , such as an intraluminal advancement over a guidewire which has been inserted into a femoral vein, and travels to the heart, through the septum separating the right atrium and left atrium (e.g. through a transeptal sheath), and into a pulmonary vein such as the left superior pulmonary vein.
- Carrier assembly 185is placed in this linear, maximally compact configuration by advancing control shaft 184 , such as by manipulating a control on a handle of device 180 as has been described hereabove.
- Carrier arm 186includes electrodes 188 .
- Carrier arm 186has a proximal end fixedly attached to outer shaft 182 via crimp ring 194 .
- Carrier arm 186 distal endis fixedly attached to control shaft 184 at a radial location that is 90.degree. offset from its proximal end attachment (as shown in FIG. 13 ), such that carrier assembly 185 radially expands as shown in FIGS. 13 a and 13 b as control shaft 184 is retracted.
- the distal end of control shaft 184is covered with atraumatic tip 192 , which includes an exit hole in communication with an internal guidewire lumen, not shown but through which guidewire 12 passes.
- Ablation catheter 180includes carrier assembly 186 , which comprises a single carrier arm 186 whose geometry is adjusted by advancing and retracting control shaft 184 , as has been described in detail hereabove.
- Carrier arm 186includes wire shaft 202 , preferably Nitinol or other shaped memory alloy or polymer, surrounded by outer sleeve 203 , preferably Pebax or other biocompatible, soft material.
- Sleeve 203can perform one or more functions, including but not limited to: capturing one or more wires between wire 202 and sleeve 203 ; acting as an insulator; providing an atraumatic boundary (e.g.
- Wire shaft 202preferably is resiliently biased in the loop geometry depicted.
- the proximal end of carrier arm 186is fixedly attached to outer shaft 182 via ring 194 .
- adhesivessuch as cyanoacrylate may be used for fixation.
- ring 194also functions as an electrode, such as a mapping and/or ablation electrode.
- Cap 192is of soft construction to be atraumatic to tissue, and is preferably made of Pebax which has been doped with Barium Sulfate to be radiopaque.
- a guidewire lumen 201exits cap 192 after having passed within control shaft 184 .
- Guidewire lumen 201is surrounded by a braided tube preferably made of Nylon and braided with stainless steel wire. The guidewire lumen 201 travels proximally to an exit port on a handle, not shown but described in detail hereabove.
- Electrodes 188are fixedly mounted to carrier arm 186 , such as with cyanoacrylate (or other adhesive) beads 204 .
- Each electrode 188preferably includes a thermocouple, not shown but preferably a copper-constantan wire junction welded to an internal surface of electrode 188 .
- Each electrode, and any included thermocoupleis attached to one or more wires (attachment not shown), which are grouped with other wires to form wire bundle 210 , which travels proximally and is attached to an electrical port on the proximal end of ablation catheter 180 .
- Carrier arm 184may include other sensors or transducers, some of which may also be attached to wires included in wire bundle 210 , these sensors or transducers placed against tissue such as pulmonary vein ostial tissue to perform a diagnostic or therapeutic procedure in a patient.
- FIG. 14further illustrates a preferred construction of the distal portion of outer shaft 182 .
- distal segment 205preferably made of Pebax 5533 or 6333.
- hinge segment 206proximal to hinge segment 206 is wall 212 .
- Hinge segment 206is preferably made of a software material than distal segment 205 and wall 212 , such as Pebax 3533.
- second anchor ring 211a metal (e.g. stainless steel) ring that is fixedly attached to two (2) pull wires 209 .
- Pull wires 209extend proximally and are attached to a knob, lever or other control integral to a handle, all not shown but operably configured to deflect the distal end of ablation catheter 180 in multiple planes.
- Wall 212surrounds braid 207 and braid 207 surrounds liner 208 .
- Braid 207a standard catheter braid to provide column and torsion support, is preferably made of stainless steel such as 304 stainless steel.
- Liner 208is preferably made of Teflon or another lubricious material that allows one or more shafts, such as control shaft 184 and pull wires 209 , to be slidingly received within a lumen of shaft 182 without significant resistance.
- Ablation catheter 180includes outer shaft 182 , control shaft 184 , ring 194 , carrier assembly 185 and tip 192 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- FIG. 15 aillustrates the proximal portion, including handle, preferably made of a plastic such as polycarbonate.
- Handle 195includes lever 196 and slide 197 , controls used by an operator to adjust the carrier assembly, deflect the distal portion of catheter 180 and/or perform other functions. Handle 195 is fixedly attached to shaft 182 . Extending from handle 195 is pigtail plus 198 , an electrical connector that attaches signal and power wires to one or more components of ablation catheter 180 such as ablation electrodes, mapping electrodes and thermocouples.
- FIG. 15 billustrates carrier assembly 185 in its compacted, linear configuration applicable for over-the-wire intraluminal advancement of ablation catheter 180 , such as to reach the left superior pulmonary vein as depicted in FIG. 15 d .
- control shaft 184has been fully advanced such that carrier arm 186 is pulled tight against control shaft 184 .
- Control shaft 184includes on its distal end tip 192 .
- Within tip 192is guidewire lumen 191 through which a standard interventional guidewire, such as a 0.035′′ guidewire, can be inserted.
- Carrier arm 185is configured, as has been described in detail hereabove, such that upon retraction of control shaft 184 , carrier arm 186 extends laterally into the loop configuration illustrated in FIG. 15 c.
- FIG. 15 dshows a cutaway view of the human heart 10 , showing the major structures of the heart including the left and right atria, and the pulmonary veins 15 .
- the atrial septumseparates the left and right atria.
- the fossa ovalisis a small depression in the atrial septum that may be used as an access pathway to the left atrium from the right atrium, such as with a transeptal puncture device and transeptal sheath.
- the fossa ovaliscan be punctured, and easily reseals and heals after procedure completion.
- arrhythmogenic focialso referred to as drivers or rotors
- the catheters of the present inventionprovide means of creating lesions, including lesions to surround the pulmonary vein ostia, and are easily deployed to identify and ablate the driver and rotor tissue.
- catheter 180is inserted into the right atrium, preferably through the inferior vena cava, as shown in the illustration, or through the superior vena cava.
- Catheter 180is sized for this advancement through the patient's vasculature, such as where the inserted (shaft) diameter is approximately 9 Fr, the shaft length is approximately 115 cm and the overall length is typically 158 cm.
- Catheter 180has been passed through transeptal sheath 11 , which may or may not be a deflectable sheath since catheter 180 preferably includes a deflectable distal portion.
- transeptal sheath 11passes through or penetrates the fossa ovalis, such as over guidewire 12 which may have been placed by a transeptal puncture device.
- Catheter 180is inserted over guidewire 12 and through transeptal sheath 11 such that its distal end enters right superior pulmonary vein 15 's lumen.
- the distal portion of shaft 182has been deflected such that the distal end of shaft 182 is directed toward the lumen of pulmonary vein 15 a .
- Catheter 180carries a structure carrying multiple ablation elements such as RF electrodes, carrier assembly 185 , into the left atrium.
- Carrier assembly 185has been transitioned to expand to a maximal diameter by retracting control shaft 184 , such that multiple ablation elements (ablation and/or mapping elements), electrodes 188 , are in contact with the pulmonary vein ostial tissue.
- Carrier assembly 185is adapted to be deformable such that pressing carrier assembly into pulmonary vein 15 ostium will cause one or more, and preferably all of electrodes 188 to make contact with tissue to be analyzed and/or ablated.
- Each of the electrodes 188is attached via connecting wires and one or more connectors, such as plug 198 , to an energy delivery apparatus, not shown but preferably an RF energy delivery unit which is also attached to a patch electrode, also not shown but preferably a conductive pad attached to the back of the patient.
- the energy delivery unitis configured to delivery RF energy in monopolar, bipolar or combination monopolar-bipolar energy delivery modes, simultaneously or sequentially, with or without “off” or no energy delivered time durations.
- the energy delivery unit 200is configured to also provide electrical mapping of the tissue that is contacted by one or more electrodes integral to carrier assembly 185 .
- a separate mapping unitmay be used, preferably attached to catheter 180 simultaneous with attachment to the energy delivery unit.
- Electrodes 188can also be configured to be mapping electrodes and/or additional electrodes can be integral to carrier assembly 185 to provide a mapping function.
- Carrier assembly 185is configured to be engaged over a pulmonary vein ostium surface to map and/or ablate tissue on the surface.
- Energyis delivered after a proper location of the electrodes 188 is confirmed with a mapping procedure. If conditions are determined to be inadequate, an operator may adjust the shape of carrier assembly 185 (e.g. through advancement or retraction of control shaft 184 ) and/or the operator may reposition carrier assembly 185 against tissue through various manipulations at the proximal end of the ablation catheter 180 . After an ablation step is completed, ablation catheter 180 is repositioned, with or without changing the geometry of carrier assembly 185 , and a similar mapping and ablation step is performed. For each pulmonary vein ostium, this repositioning will typically occur two to three times creating semi-circular lesions that preferably overlap.
- the steerability of the distal portion of shaft 182via a control on handle 195 , is an important function in this repositioning process.
- the clinicianwill perform ablations in the left superior pulmonary vein first, followed by the right superior, left inferior and then the right inferior pulmonary veins.
- the energy delivery unitis configured to delivery both RF energy and ultrasound energy to the identical or different electrodes 188 .
- the energy delivery unitis configured to accept a signal from one or more sensors integral to ablation catheter 180 , not shown, such that the energy delivered can be modified via an algorithm which processes the information received from the one or more sensors.
- a guidewireis inserted through a sidecar present at the distal portion of shaft 182 , avoiding the need for threading the entire the device over the guidewire.
- carrier arm 186is attached to a second control shaft, also slidingly received by outer shaft 182 and connected to a control on handle 195 , such that the carrier assembly 185 geometry can be adjusted by advancing and retracting either the second control shaft or control shaft 184 .
- This dual control shaft designalso allows carrier assembly to be completely retracted within the distal end of control shaft 182 , as is described in reference to FIG. 17 herebelow.
- Ablation catheter 400includes outer shaft 410 , first control shaft 411 , second control shaft 412 , first carrier assembly 430 a , second carrier assembly 430 b and tip 415 , all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.
- Outer shaft 410preferably a braided construction including braid 413 , slidingly receives first control shaft 410 , which in turn slidingly receives second control shaft 411 .
- Outer shaft 410is fixedly attached to handle 420 via strain relief 426 .
- An atraumatic tip 415is fixedly attached to the distal end of second control shaft 412 .
- a guidewire lumen 416exits tip 415 and travels proximally, through second control shaft 412 , first control shaft 411 and outer shaft 410 to handle 420 where it exits via guidewire entry 424 , such that ablation catheter 400 can be percutaneously introduced into a patient over a previously placed guidewire.
- First carrier assembly 430 acomprises a single carrier arm, first carrier arm 433 , which is fixedly attached on its distal end to the distal end of first control shaft 411 and on its proximal end to the distal end of outer shaft 410 .
- First control shaft 411is operably connected on its proximal end to first advancable knob 421 , such that advancement and retraction of knob 421 causes advancement and retraction of first control shaft 411 .
- Advancement and retraction of first control shaft 411causes first carrier assembly 430 a to contract and expand, respectively, as has been described hereabove.
- First carrier assembly 430 aincludes electrodes 431 , and each electrode 431 is preferably at least configured to deliver ablation energy to tissue.
- Second carrier assembly 430 bcomprises a single carrier arm, second carrier arm 434 , which is fixedly attached on its distal end to the distal end of second control shaft 412 and on its proximal end to the distal end of first control shaft 411 .
- Second control shaft 412is operably connected on its proximal end to second advancable knob 422 , such that advancement and retraction of knob 422 causes advancement and retraction of second control shaft 412 .
- Advancement and retraction of first control shaft 411(via advancement and retraction of knob 421 ) causes second carrier assembly 430 b to expand and contract, respectively.
- first carrier assembly 430 a and second carrier assembly 430 bare placed in a minimal diameter configuration.
- Second carrier assembly 430 bincludes electrodes 432 , and each electrode 432 is preferably at least configured to record electrical activity present in tissue.
- Electrodes 431 and electrodes 432preferably include an integral temperature sensor, such as a thermocouple constructed of a copper-constantan bimetallic assembly. Electrodes 431 may be further configured to record electrical signals in tissue and electrodes 432 may be further configured to deliver ablation energy to tissue.
- an integral temperature sensorsuch as a thermocouple constructed of a copper-constantan bimetallic assembly. Electrodes 431 may be further configured to record electrical signals in tissue and electrodes 432 may be further configured to deliver ablation energy to tissue.
- Handle 420also includes lever 423 , which is operably attached to one or more pull wires which extend distally within a lumen, such as a Teflon lined lumen, within outer shaft 410 .
- the one or more pull wiresare fixedly attached to a distal portion of outer shaft 410 causing operator controlled deflection of the distal portion of ablation catheter 400 in one or more planes.
- Handle 420further includes electrical plug 425 which is electrically connected to one or more electrical wires or other conduits, all of which travel distally, along outer shaft 410 to various locations such as electrodes 431 or electrodes 432 , or another sensor or transducer not shown.
- Plug 425is configured to attach to an energy delivery unit such as an RF energy delivery unit, a mapping unit or another device configured to transmit or receive electrical signals or power.
- FIG. 16 aillustrates an end view of the ablation catheter of FIG. 16 .
- First carrier assembly 430 a and second carrier assembly 430 bare both in their maximum diameter configurations.
- FIG. 16 billustrates a side view of the ablation catheter of FIGS. 16 and 16 a inserted into a vessel, such as a pulmonary vein 15 , through its ostium.
- Second carrier assembly 430 bis partially or fully expanded and in contact with the luminal wall of vein 15 .
- First carrier assembly 430 ais partially or fully expanded and engaging the ostium of vein 15 .
- Ablation catheter 180 bis of similar construction to ablation catheter 180 of FIGS. 12 through 14 with common elements having the same reference numbers. For brevity, most of the common construction and common component details will be omitted.
- Ablation catheter 180 bincludes handle 195 with operator controls first slide 197 a , second slide 197 b and lever 196 , each operably attached to a control shaft or other linkage.
- Plug 198is connected to one or more electrical wires or other conduits that travel distally through outer shaft 182 and connect to one or more functional elements of the device, such as electrodes included in carrier assembly 185 .
- ablation catheter 192includes an atraumatic tip 192 , preferably constructed of Pebax which has been doped with Barium Sulfate for radiopacity.
- the ablation catheter 180 b of FIG. 17depicts the carrier assembly 185 in a fully deployed (maximum diameter) configuration, caused by retracting the control shaft via a slide or lever on handle 196 .
- carrier arm carrier assembly 185is in its fully deployed (maximum diameter) condition.
- Carrier arm 186is attached on its distal end to first control shaft 182 .
- carrier arm 186is attached to a second control shaft 213 , which also can be advanced and retracted from a control on handle 195 . Advancement and retraction of both first control shaft 184 and second control shaft 213 can be used, independently or in combination, to change the geometry of carrier assembly 185 .
- ablation catheter 180 ba differentiating feature of ablation catheter 180 b is illustrated where second control shaft 213 has been retracted until carrier arm 186 , including ablation elements 188 , is contained completely within a lumen of outer shaft 182 , such as within a lumen within a Teflon liner.
- carrier arm 186is retracted within outer shaft 182 by retracted first control shaft 184 .
- Carrier arm 186is shown in its less than 360.degree. helix. Also shown are electrodes 188 , typically 2-3 mm in length with symmetric 3 mm spacing between electrodes.
- the systemincludes multiple functional components, such as the ablation catheter and the energy delivery apparatus.
- the ablation catheterconsists of a catheter shaft, a carrier assembly for providing electrodes in a resiliently biased configuration, a control shaft for deploying and withdrawing the carrier assembly, and a coupler for attaching the control shaft to the carrier assembly.
- the carrier assemblyis a support structure which is shiftable from a storage or confined configuration, such as a radially constrained configuration, to a deployed or expanded configuration.
- the carrier assemblycan includes wires, ribbons, cables and struts, made of either metals, non-metals or combinations of both.
- the carrier assemblycan be constructed of one or more materials, including both metals and non-metals. Typical metals chosen for carrier assembly construction include but are not limited to: stainless steel, Nitinol, ElgiloyTM, other alloys and combinations thereof.
- the ablation catheter of the present inventionmay include a steerable outer sheath, or may work in conjunction as a system with a separate steerable outer sheath.
- One or more tubular components of the ablation cathetermay be steerable such as with the inclusion of a controllable pull wire at or near the distal end.
- the ablation catheter of the present inventionmay be inserted over the wire, such as via a lumen within one of the tubular conduits such as within a lumen of the tubular body member or control shaft, or alternatively the catheter may include a rapid exchange sidecar at or near its distal end, consisting of a small projection with a guidewire lumen therethrough.
- a guidewire lumenmay be included solely for the guidewire, or may provide other functions such as a vacuum lumen for an integral suction port integrated at the distal portion of the carrier assembly.
- the ablation catheter of the present inventionfurther includes ablation elements.
- one or more ablation elementsare electrodes configured to deliver RF energy.
- Other forms of energy, alternative or in addition to RF,may be delivered, including but not limited to: acoustic energy and ultrasound energy; electromagnetic energy such as electrical, magnetic, microwave and radiofrequency energies; thermal energy such as heat and cryogenic energies; chemical energy; light energy such as infrared and visible light energies; mechanical energy; radiation; and combinations thereof.
- One or more ablation elementsmay comprise a drug delivery pump or a device to cause mechanical tissue damage such as a forwardly advanceable spike or needle.
- the ablation elementscan deliver energy individually, in combination with or in serial fashion with other ablation elements.
- the ablation elementscan be electrically connected in parallel, in series, individually, or combinations thereof.
- the ablation cathetermay include cooling means to prevent undesired tissue damage and/or blood clotting.
- the ablation elementsmay be constructed of various materials, such as plates of metal and coils of wire for RF energy delivery.
- the electrodescan take on various shapes including shapes used to focus energy such as a horn shape to focus sound energy, and shapes to assist in cooling such as a geometry providing large surface area. Electrodes can vary within a single carrier assembly, such as a spiral array of electrodes or a umbrella tip configuration wherein electrodes farthest from the central axis of the catheter have the largest major axis. Wires and other flexible conduits are attached to the ablation elements, such as electrical energy carrying wires for RF electrodes or ultrasound crystals, and tubes for cryogenic delivery.
- the ablation elements requiring electrical energy to ablaterequire wired connections to an electrical energy power source such as an RF power source.
- an electrical energy power sourcesuch as an RF power source.
- individual pairs of wires for each electrodemay be bulky and compromise the cross-sectional profile of the ablation catheter.
- one or more electrodes, connected in serial fashion such that a reduced number of wires, such as two wires, can be attached to two or more electrodesinclude switching means such that while a first electrode is powered, the remaining electrodes do not transmit ablative energy.
- Switching meansmay be a thermal switch, such that as a first electrodes heats up, a single pole double throw switch change state disconnecting power from that electrode and attaching power to the next electrode in the serial connection.
- This integral temperature switchmay have a first temperature to disconnect the electrode, and a second temperature to reconnect the electrode wherein the second temperature is lower than the first temperature, such as a second temperature below body temperature.
- each electrodeis constructed of materials in their conductive path such that as when the temperature increased and reached a predetermined threshold, the resistance abruptly decreased to near zero, such that power dissipation, or heat, generated by the electrode was also near zero, and more power could be delivered to the next electrode incorporating the above switching means.
- the ablation catheter of the present inventionpreferably includes a handle activating or otherwise controlling one or more functions of the ablation catheter.
- the handlemay include various knobs, such as rotating or sliding knobs which are operably connected to advanceable conduits, or are operably connected to gear trains or cams which are connected to advanceable conduits. These knobs, such as knobs use to deflect a distal portion of a conduit, or to advance or retract the carrier assembly, preferably include a reversible locking mechanism such that a particular tip deflection or deployment amount can be maintained through various manipulations of the system.
- the ablation cathetermay include one or more sensors, such as sensors used to detect chemical activity; light; electrical activity; pH; temperature; pressure; fluid flow or another physiologic parameter. These sensors can be used to map electrical activity, measure temperature, or gather other information that may be used to modify the ablation procedure. In a preferred embodiment, one or more sensors, such as a mapping electrode, can also be used to ablate tissue.
- sensorssuch as sensors used to detect chemical activity; light; electrical activity; pH; temperature; pressure; fluid flow or another physiologic parameter. These sensors can be used to map electrical activity, measure temperature, or gather other information that may be used to modify the ablation procedure.
- one or more sensorssuch as a mapping electrode, can also be used to ablate tissue.
- Numerous components internal to the patientmay include one or more visual markers such as radiopaque markers visible under fluoroscopy, or ultrasound markers.
- Selection of the tissue to be ablatedmay be based on a diagnosis of aberrant conduit or conduits, or based on anatomical location.
- RF energymay be delivered first, followed by another energy type in the same location, such as when a single electrode can deliver more than one type of energy, such as RF and ultrasound energy.
- a first proceduremay be performed utilizing one type of energy, followed by a second procedure utilizing a different form of energy. The second procedure may be performed shortly after the first procedure, such as within four hours, or at a later date such as greater than twenty-four hours after the first procedure.
- Numerous types of tissuecan be ablated utilizing the devices, systems and methods of the present invention.
- the various aspects of the inventionhave application in procedures for ablating tissue in the prostrate, brain, gall bladder, uterus, other organs and regions of the body, and a tumor, preferably regions with an accessible wall or flat tissue surface.
- heart tissueis ablated, such as left atrial tissue.
- an ablation catheter and a heat sensing technologyare included.
- the heat sensing technologyincludes sensor means that may be placed on the chest of the patient, the esophagus or another area in close enough proximity to the tissue being ablated to directly measure temperature effects of the ablation, such as via a temperature sensor, or indirectly such as through the use of an infrared camera.
- a temperature or a surrogate temperaturereaches a threshold, such as an adjustable threshold, the ablation energy is reduced or stopped, to one or more ablation elements.
- the thresholdwill depend on the location of the sensor means, as well as where the ablation energy is being delivered.
- the thresholdmay be adjustable, and may be automatically configured.
- An ablation catheteris provided with multiple carrier assemblies. These carrier assemblies can be removed for the tubular body member of the catheter, or may include multiple tubular body members in the kit.
- the multiple carrier assembliescan have different patterns, different types or amounts of electrodes, and have numerous other configurations including compatibility with different forms of energy.
- the arraymay be used on the heart during open heart surgery, open chest surgery, or minimally invasive thoracic surgery.
- a short catheter or cannula carrying the carrier assembly and its electrodesmay be inserted into the heart, such as through the left atrial appendage or an incision in the atrium wall, to apply the electrodes to the tissue to be ablated.
- the carrier assembly and its electrodesmay be applied to the epicardial surface of the atrium or other areas of the heart to detect and/or ablate arrhythmogenic foci from outside the heart.
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Physics & Mathematics (AREA)
- Otolaryngology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Cardiology (AREA)
- Plasma & Fusion (AREA)
- Electromagnetism (AREA)
- Surgical Instruments (AREA)
- Information Retrieval, Db Structures And Fs Structures Therefor (AREA)
Abstract
Description
This application is a continuation of U.S. patent application Ser. No. 11/471,467, filed Jun. 20, 2006, now U.S. Pat. No. 7,850,685, entitled “Ablation Catheter,” which claims the benefit of U.S. Provisional Patent Application Ser. No. 60/692,416, filed Jun. 20, 2005, which are incorporated by reference herein in its entirety.
N/A
The present invention relates generally to catheters and methods for performing targeted tissue ablation in a subject. In particular, the present invention provides devices comprising catheters having distal ends configured to pulmonary vein ostia, and methods for treating conditions (e.g., cardiac arrhythmias) with these and similar devices.
Tissue ablation is used in numerous medical procedures to treat a patient. Ablation can be performed to remove undesired tissue such as cancer cells. Ablation procedures may also involve the modification of the tissue without removal, such as to stop electrical propagation through the tissue in patients with an arrhythmia. Often the ablation is performed by passing energy, such as electrical energy, through one or more electrodes causing the tissue in contact with the electrodes to heats up to an ablative temperature. Ablation procedures can be performed on patients with atrial fibrillation by ablating tissue in the heart.
Mammalian organ function typically occurs through the transmission of electrical impulses from one tissue to another. A disturbance of such electrical transmission may lead to organ malfunction. One particular area where electrical impulse transmission is critical for proper organ function is in the heart. Normal sinus rhythm of the heart begins with the sinus node generating an electrical impulse that is propagated uniformly across the right and left atria to the atrioventricular node. Atrial contraction leads to the pumping of blood into the ventricles in a manner synchronous with the pulse.
Atrial fibrillation refers to a type of cardiac arrhythmia where there is disorganized electrical conduction in the atria causing rapid uncoordinated contractions that result in ineffective pumping of blood into the ventricle and a lack of synchrony. During atrial fibrillation, the atrioventricular node receives electrical impulses from numerous locations throughout the atria instead of only from the sinus node. This overwhelms the atrioventricular node into producing an irregular and rapid heartbeat. As a result, blood pools in the atria that increases a risk for blood clot formation. The major risk factors for atrial fibrillation include age, coronary artery disease, rheumatic heart disease, hypertension, diabetes, and thyrotoxicosis. Atrial fibrillation affects 7% of the population over age 65.
Atrial fibrillation treatment options are limited. Lifestyle change only assists individuals with lifestyle related atrial fibrillation. Medication therapy assists only in the management of atrial fibrillation symptoms, may present side effects more dangerous than atrial fibrillation, and fail to cure atrial fibrillation. Electrical cardioversion often restores sinus rhythm, but has a high recurrence rate. In addition, if there is a blood clot in the atria, cardioversion may cause the clot to leave the heart and travel to the brain or to some other part of the body, which may lead to stroke. What are needed are new methods for treating atrial fibrillation and other conditions involving disorganized electrical conduction.
Various ablation techniques have been proposed to treat atrial fibrillation, including the Cox-Maze procedure, linear ablation of various regions of the atrium, and circumferential ablation of pulmonary vein ostia. The Cox-Maze procedure and linear ablation procedures are tedious and time-consuming, taking several hours to accomplish. Pulmonary vein ostial ablation is proving to be difficult to do, and has resulted in inadequate results and unacceptable trauma to the pulmonary veins. There is therefore a need for improved atrial ablation products and techniques.
According to a first aspect of the invention, an ablation catheter for an operator to treat a patient with an arrhythmia is disclosed. The catheter includes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween. The catheter further includes a control shaft, coaxially disposed and slidingly received with the lumen of the tubular body member. A flexible carrier assembly is attached to the end of the control shaft and includes at least one ablation and/or mapping elements. Retraction of the control shaft causes the carrier assembly to transition from a compact, near linear configuration, to a helix or partial helix. In a preferred embodiment, the helix is less than 360.degree.
In a preferred embodiment, the carrier assembly can be withdrawn into a location within the tubular body member. In another preferred embodiment, the ablation catheter includes at least two carrier assemblies that can be transitioned between a compact, near linear configuration to a helix or partial helix. In yet another preferred embodiment, the catheter can be placed over a guidewire or includes an integral guidewire tip.
According to another aspect of the invention, an ablation catheter for an operator to treat a patient with an arrhythmia is disclosed. The catheter includes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween. The catheter further includes a control shaft, coaxially disposed and slidingly received with the lumen of the tubular body member. A flexible carrier assembly is attached to the end of the control shaft and includes at least one ablation and/or mapping elements in an umbrella tip configuration. Retraction of the control shaft causes the carrier assembly to change shape, such as to conform to tissue surrounding one or more pulmonary veins entering the left atrium of a patient.
In a preferred embodiment, the ablation catheter includes a second carrier assembly, also in an umbrella tip configuration. In another preferred embodiment, the catheter includes an anchoring element, such as a balloon or expandable cage, for stabilizing and/or anchoring the ablation catheter in a pulmonary vein. In yet another preferred embodiment, the catheter includes an ultrasound element for directing ultrasound energy in a circular pattern toward tissue. In yet another preferred embodiment, one or more carrier arms of the umbrella tip can be rotated, stabilized, or otherwise manipulated to better conform to or stabilize with tissue such as pulmonary vein ostial tissue. In yet another preferred embodiment, the catheter can be placed over a guidewire or includes an integral guidewire tip. In yet another preferred embodiment, the catheter includes an advancable spline which can be used to position or stabilize the carrier assembly.
According to yet another aspect of the invention, an ablation catheter for an operator to treat a patient with an arrhythmia is disclosed. The catheter includes an elongate, flexible tubular body member have a proximal end, a distal end and a lumen therebetween. The catheter further includes a flexible carrier assembly comprising an inflatable balloon with mounted or embedded ablation and/or mapping elements.
One such example of a minimally invasive therapy involves the treatment of cardiac arrhythmias or irregular heartbeats in which physicians employ specialized cardiac assessment and treatment devices, such as mapping catheters and ablation catheters, to gain access to, diagnose, and treat interior regions of a patient's body. Such devices may include energized electrodes or other ablation assemblies to create lesions or other anatomical effects that disrupt or block electrical pathways through the targeted tissue.
In the treatment of cardiac arrhythmias, a specific area of cardiac tissue having aberrant electrically conductive pathways is typically initially identified for subsequent treatment. This localization or identification can include first using a medical device such as a mapping catheter to obtain a baseline electrophysiological map of electrical activity in selected tissue. After mapping and diagnosing aberrant tissue, a physician may decide to treat the patient by ablating the tissue. An ablation procedure may involve creating one or more lesions to electrically isolate tissue believed to be the source of an arrhythmia. One type of ablation is the cryotreatment or cryogenic ablation, which entails creating cold temperatures at specific regions of the body or contacting tissue with cold treatment devices to transfer heat from the targeted tissue to the cryogenic element, thus cooling and/or ablating the tissue.
Such cryotreatment may require first repositioning or removing a mapping catheter before placing a second medical device or ablation catheter into contact with the tissue to be treated. Following the ablation procedure, the physician may desire to asses or confirm the efficacy of the treatment by obtaining a second electrophysiological map of the tissue region. This subsequent mapping procedure may involve removal or manipulation of the ablation medical device to allow the desired positioning of the mapping device adjacent to the tissue that was previously treated.
Each device exchange or manipulation represents an added risk to the patient as inserting and removing catheters in the vasculature carries a number of inherent risks, possibly including embolism. Exchanging these various catheters during a procedure can cause inaccuracies or movement in the placement and location of the distal tip a device with respect to the tissue to be mapped or ablated, and may further add to the time required to perform the desired treatment. These potential inaccuracies and extended duration of the particular procedure further increase the risk to the patient undergoing treatment. Accordingly, it would be desirable to provide an integrated apparatus and method of use thereof for both diagnosing aberrant electrical pathways and treating those detected pathways.
In addition, placing and maintaining a medical device in the desired position with correct alignment and positive contact with the selected tissue may enhance a mapping and ablation treatment and its likelihood of success. It is therefore desirable to provide apparatus and method of use to verify the position of a medical device, positive contact and alignment with the selected tissue, and to evaluate the medical treatment contemporaneously.
The accompanying drawings, which are incorporated in and constitute a part of this specification, illustrate various embodiments of the present invention, and, together with the description, serve to explain the principles of the invention. In the drawings:
Reference will now be made in detail to the present embodiments of the invention, examples of which are illustrated in the accompanying drawings. Wherever possible, the same reference numbers will be used throughout the drawings to refer to the same or like parts.
The present invention provides catheters for performing targeted tissue ablation in a subject. In preferred embodiments, the catheters comprise a tubular body member having a proximal end and distal end and preferably a lumen extending therebetween. The catheter is preferably of the type used for performing intracardiac procedures, typically being percutaneously introduced and advanced from the femoral vein in a patient's leg. Alternative methods involve percutaneous introduction into the jugular vein of the patient's neck, or other anatomical entry point that can be used to access the target location within the patient. The catheter is preferably introducable through a sheath and also preferably is advancable over a guidewire. The catheter preferably has a steerable tip that allows precise positioning of the distal portion such as when the distal end of the catheter needs to access a pulmonary vein of the left atrium of the patient's heart. The catheters include ablation elements mounted on one or more carrier arms of a flexible carrier assembly. Typical metals chosen for carrier assembly construction include but are not limited to: stainless steel, Nitinol, Elgiloy™, other alloys and combinations thereof. These ablation elements can be used to ablate and/or map electrical activity of tissue. The carrier assembly is attached to a control shaft that is coaxially disposed and slidingly received within the lumen of the tubular body member. The shape of the carrier assembly is adjusted by advancing or retracting the control shaft, such as to engage one or more ablation elements against cardiac tissue, typically pulmonary vein ostial tissue.
Arrays of ablation elements, preferably geometrically-adjustable electrode arrays, may be configured in a wide variety of ways and patterns. In particular, the present invention provides devices with multi-dimensional electrode arrays that provide electrical energy, such as radiofrequency (RF) energy, in monopolar (unipolar), bipolar or combined monopolar-bipolar fashion, as well as methods for treating conditions (e.g., atrial fibrillation, supra ventricular tachycardia, atrial tachycardia, ventricular tachycardia, ventricular fibrillation, and the like) with these devices. Alternative to or in combination with ablation elements that deliver electrical energy to tissue, other forms and types of energy can be delivered including but not limited to: sound energy such as acoustic energy and ultrasound energy; electromagnetic energy such as electrical, magnetic, microwave and radiofrequency energies; thermal energy such as heat and cryogenic energies; chemical energy such as energy generated by delivery of a drug; light energy such as infrared and visible light energies; mechanical and physical energy; radiation; and combinations thereof.
As described above, the normal functioning of the heart relies on proper electrical impulse generation and transmission. In certain heart diseases (e.g., atrial fibrillation) proper electrical generation and transmission are disrupted or are otherwise abnormal. In order to diagnose and/or prevent improper impulse generation and transmission from causing an undesired condition, the ablation catheters of the present invention may be employed.
One current method of treating cardiac arrhythmias is with catheter ablation therapy. Physicians make use of catheters to gain access into interior regions of the body. Catheters with attached electrode arrays or other ablating devices are used to create lesions that disrupt electrical pathways in cardiac tissue. In the treatment of cardiac arrhythmias, a specific area of cardiac tissue having aberrant conductive pathways, such as atrial rotors, emitting or conducting erratic electrical impulses, is initially localized. A user (e.g., a physician) directs a catheter through a main vein or artery into the interior region of the heart that is to be treated. The ablating element or elements are next placed near the targeted cardiac tissue that is to be ablated, such as a pulmonary vein ostium. After performing an electrical mapping procedure, the physician directs energy, provided by a source external to the patient, from one or more ablation elements to ablate the neighboring tissue and form a lesion. In general, the goal of catheter ablation therapy is to disrupt the electrical pathways in cardiac tissue to stop the emission of and/or prevent the propagation of erratic electric impulses, thereby curing the heart of the disorder. For treatment of atrial fibrillation, currently available methods and devices have shown only limited success and/or employ devices that are extremely difficult to use or otherwise impractical.
The ablation catheters of the present invention allow the generation of lesions of appropriate size and shape to treat conditions involving disorganized electrical conduction (e.g., atrial fibrillation). The created lesions are segmented and localized. The lesions may be linear or curvilinear, circumferential and partial circumferential, and/or continuous or discontinuous. The ablation catheters of the present invention are also practical in terms of ease-of-use and limiting risk to the patient, as well as significantly reducing procedure times. The lesions created by the ablation catheters are suitable for inhibiting the propagation of inappropriate electrical impulses in the heart for prevention of reentrant arrhythmias.
The catheters of the present invention can perform tissue ablation and/or mapping of electrical signals present in tissue. Patients, such as those with atrial fibrillation, are diagnosed and treated with the herein described mapping and/or ablation procedures. The catheters of the present invention are specifically applicable to mapping and ablation of the pulmonary vein ostia located in the left atrium of the patient's heart. These vein ostia are approximately 1.5 cm in diameter and often are non-circular in geometry, especially when a venous bifurcation is present proximate the ostia. The carrier assembly of the present invention may include one or more carrier arms that are configured to conform to these circular and non-circular contours of pulmonary vein ostia. One or more carrier arms, or groups of carrier arms, may be configured to be independently advancable and retractable, such as to properly engage pulmonary vein ostium tissue. The carrier arms are preferably made of Nitinol, and may have round, oval, triangular, rectangular, or trapezoidal cross-sectional geometry. The carrier arms may include compound splines or angles, such as to conform to pulmonary vein ostia and surrounding tissue. Each carrier arm may include one or more sensors, such as temperature sensors integral to an ablation element or mounted between two ablation elements, such as to measure tissue temperature and/or blood temperature. In a preferred embodiment, a temperature sensor is mounted to a carrier arm in a location more distal than the most distal ablation element, when the carrier assembly is in a deployed, ready to deliver ablation energy, configuration. Information recorded by the temperature sensor can be used by an energy delivery unit of the present invention as a threshold to avoid overheating of blood or tissue, as well as regulate power to a target temperature. A first carrier arm may have a different property than a second carrier arm, such as a different rigidity, a different number of ablation elements, or a different configuration of sensors such as temperature sensors.
The catheters of the present invention may be configured to be advanced into the heart of a patient over a previously placed guidewire, such as a standard interventional 0.035″ guidewire. The catheter may include an inner lumen for the majority of its length, through which the guidewire is inserted, or the catheter may include a relatively short sidecar near its distal end, where the guidewire inserted through a lumen of the sidecar. The placement over the guidewire allows simplified positioning and re-positioning by an operator. The guidewire placement also provides stability such as to simplify maintaining the position of the catheter during energy delivery, typically 60 seconds.
The catheters of the present invention are configured to be inserted through the lumen of a previously placed transeptal sheath, such as a 9.5 French (Fr) steerable transeptal sheath. The catheter of the present invention preferably include an integral steering mechanism, such as one or more pull wires fixedly attached near a distal portion of the catheter and operably attached to a lever, knob or other control integral to a handle of the catheter. The steering can be used to deflect the carrier assembly and distal end of the catheter into the left and right pulmonary veins of the left atrium. The integral catheter steering can be used in conjunction a steerable transeptal sheath. Multiple pull wires can be fixedly mounted 90.degree. apart at separated locations in a distal portion of the catheter to provide multi-axis, precision controlled steering. The tubular body member of the ablation catheter is constructed with sufficient columnar strength and rigidity to allow an operator to apply significant torque to the proximal end that equivalently translates to the catheters distal portions.
The present invention includes one or more systems that include the ablation catheters of the present invention. The system may further include a guide catheter such as a steerable transeptal sheath that slidingly receives the ablation catheter. The system may further include an energy delivery unit, such as a unit configured to deliver RF and/or other forms of energy to the ablation elements of the catheter. The system may further include a mapping unit that receives information recorded from one or more sensors of the ablation catheter, such as an ablation element of the ablation catheter. The mapping unit provides electrical activity information to an operator of the system. The mapping unit may be integral to the energy delivery unit.
Definitions. To Facilitate an Understanding of the Invention, a Number of Terms are Defined Below.
As used herein, the terms “subject” and “patient” refer to any animal, such as a mammal like livestock, pets, and preferably a human. Specific examples of “subjects” and “patients” include, but are not limited, to individuals requiring medical assistance, and in particular, requiring atrial fibrillation catheter ablation treatment.
As used herein, the terms “catheter ablation” or “ablation procedures” or “ablation therapy,” and like terms, refer to what is generally known as tissue destruction procedures. Ablation is often used in treating several medical conditions, including abnormal heart rhythms. It can be performed both surgically and non-surgically. Non-surgical ablation is typically performed in a special lab called the electrophysiology (EP) laboratory. During this non-surgical procedure a catheter is inserted into the heart using fluoroscopy for visualization, and then an energy delivery apparatus is used to direct energy to the heart muscle. This energy either “disconnects” or “isolates” the pathway of the abnormal rhythm (depending on the type of ablation). It can also be used to disconnect the conductive pathway between the upper chambers (atria) and the lower chambers (ventricles) of the heart. For individuals requiring heart surgery, ablation can be performed during coronary artery bypass or valve surgery.
As used herein, the term “ablation element” refers to an energy delivery element, such as an electrode for delivering electrical energy such as RF energy. Ablation elements can be configured to deliver multiple types of energy, such as ultrasound energy and cryogenic energy, either simultaneously or serially. Electrodes can be constructed of a conductive plate, wire coil, or other means of conducting electrical energy through contacting tissue. Electrodes may comprise a laminate construction, such as at least one conductive layer and at least one insulative layer. RF electrodes preferably are constructed of platinum or a combination of platinum and iridium. In monopolar energy delivery, the energy is conducted from the electrode, through the tissue to a ground pad, such as a conductive pad attached to the back of the patient. The high concentration of energy at the electrode site causes localized tissue ablation. In bipolar energy delivery, the energy is conducted from a first electrode to one or more separate electrodes, relatively local to the first electrode, through the tissue between the associated electrodes. Bipolar energy delivery results in more precise, shallow lesions while monopolar delivery results in deeper lesions. Both monopolar and bipolar delivery provide advantages, and the combination of their use is a preferred embodiment of this application. Energy can also be delivered using pulse width modulated drive signals, well known to those of skill in the art. Energy can also be delivered in a closed loop fashion, such as a system with temperature feedback wherein the temperature modifies the type, frequency and or magnitude of the energy delivered. Ablation elements may have one or more different shapes, such as tubular electrodes mounted around a shaft such as a carrier arm, and other cross-sections such as oval, triangular, rectangular and trapezoidal. Triangular cross sections can be positioned where multiple sides contact tissue for increased energy transfer or multiple sides contact a cooling source such as blood for increased cooling. The ablation elements may include a heat-sinking element, such as a projecting fin or other increased surface area portion. The ablation elements preferably include an integral temperature sensor, such as a thermocouple comprised of copper and constantan wires that are welded inside a mid portion of an RF electrode. In a preferred embodiment, an ablation element can also be used to record and map electrical activity in tissue. In an alternative embodiment, one or more ablation elements may be configured to only map electrical activity, and not be configured to deliver energy.
As used herein, the term “carrier assembly” refers to a flexible carrier, on which one or more ablation elements are disposed. Carrier assemblies include one or more carrier arms. Carrier assemblies are not limited to any particular size, or shape, and can be configured to be in expanded and unexpanded or compact states.
As used herein, the term “carrier arm” refers to a wire-like shaft capable of interfacing with electrodes and a control shaft. A carrier arm is not limited to any size or measurement. Examples include, but are not limited to: stainless steel shafts; Nitinol shafts; titanium shafts; polyurethane shafts; nylon shafts; and steel shafts. Carrier arms can be entirely flexible, or may include flexible and rigid segments.
As used herein, the term “spiral tip” refers to a carrier assembly configured in its fully expanded state into the shape of a helix or spiral. The spiral tip is not limited in the number of spirals it may contain. Examples include, but are not limited to, a wire tip body with one spiral, two spirals, ten spirals, and a half of a spiral. The spirals can lie in a relatively single plane, or in multiple planes. A spiral tip may be configured for energy delivery during an ablation procedure.
As used herein, the term “lesion,” or “ablation lesion,” and like terms, refers to tissue that has received ablation therapy. Examples include, but are not limited to, scars, scabs, dead tissue, burned tissue and tissue with conductive pathways that have been made highly resistive or disconnected.
As used herein the term “umbrella tip” refers to a carrier assembly with a geometric center which lies at a point along the axis of the distal portion of the tubular body member, with one or more bendable or hinged carrier arms extending from the geometric center, in an umbrella configuration. Each carrier arm may include one or more ablation elements. Each carrier arm of an umbrella tip includes a proximal arm segment and a distal arm segment, the distal arm segment more distal than the proximal arm segment when the carrier assembly is in a fully expanded condition. One or more additional carrier arms can be included which include no ablation elements, such as carrier arms used to provide support or cause a particular deflection. An umbrella tip body is not limited to any particular size. An umbrella tip may be configured for energy delivery during an ablation procedure.
As used herein, the term “carrier arm bend point” refers to a joint (e.g., junction, flexion point) located on a carrier arm. The degree of flexion for a carrier arm bend point may range from 0 to 360 degrees. The bend portion can be manufactured such what when the carrier assembly is fully expanded the bend point is positioned in a relatively straight portion, a curved portion, or in a discrete transition from a first direction to a second transition, such as a 45 degree bend transition. The bend portion can include one or more flexing means such as a spring, a reduced diameter segment, or a segment of increased flexibility.
As used herein, the term “energy delivery unit” refers to a device configured to operably attach to an ablation catheter and deliver one or more forms of energy to an ablation element. The energy delivery unit includes a user interface which allows an operator to make one or more settings involved in applying the ablative energy. The energy unit may be further configured to receive temperature information from the ablation catheter. The temperature information can provided to an operator and/or be used to provide closed loop energy delivery. The energy delivery unit may include a remote control device that may be maintained in the sterile field of the patient during the ablation procedure. The energy delivery unit may receive a signal from an operator control integral to the ablation catheter that initiates delivery of the ablation energy.
As used herein, the term “mapping unit” refers to a device configured to operably attach to an ablation catheter and receive one or more mapping signals from an ablation element or other sensor of an ablation catheter.
The present invention provides structures that embody aspects of the ablation catheter. The present invention also provides tissue ablation systems and methods for using such ablation systems. The illustrated and preferred embodiments discuss these structures and techniques in the context of catheter-based cardiac ablation. These structures, systems, and techniques are well suited for use in the field of cardiac ablation.
However, it should be appreciated that the invention is applicable for use in other tissue ablation applications such as tumor ablation procedures. For example, the various aspects of the invention have application in procedures for ablating tissue in the prostrate, brain, gall bladder, uterus, and other regions of the body, preferably regions with an accessible wall or flat tissue surface, using systems that are not necessarily catheter-based.
The multifunctional catheters of the present invention have advantages over previous prior art devices.FIGS. 1-17 show various preferred embodiments of the multifunctional catheters of the present invention. The present invention is not limited to these particular configurations.
Theablation elements 92 and other components ofcarrier assembly 85 are configured to flex to conform to pulmonary vein ostia and other applicable tissues.Outer shaft 76 can be advanced forward to change the shape ofcarrier assembly 85 and cause one ormore ablation elements 92 to contact tissue.Outer shaft 76 slidingly receivesinner shaft 78, which is fixedly attached toproximal carrier arms 86.Distal carrier arms 88 are fixedly attached to cap15 and the distal end ofcontrol shaft 84.Proximal carrier arms 86 are pivotally attached todistal carrier arms 88, such that advancement and retraction ofcontrol shaft 84 relative toinner tube 78 causes the diameter ofcarrier assembly 85 to contract and expand respectively, such as to cause the carrier assembly to expand to a 4-5 mm diameter.Inner shaft 78 further provides columnar strength to allow an operator to advanceinner shaft 78 andcause carrier assembly 85 to properly contact tissue, such as to conform to non-circular pulmonary vein ostia.Inner shaft 78 preferably is attached to a pull wire (not shown), near its distal end, which is operably connected to a control on the proximal end ofdevice 50 allowing an operator to controllably deflect the distal portion ofdevice 50.
The distal end ofouter shaft 76 includes ashaft tip 82, configured to radially expand whencarrier assembly 85 is retracted. The proximal end ofouter shaft 76 preferably includes a handle, not shown, but including one or more controls, such as knobs or levers, such as to advance and retractinner shaft 78 andcontrol shaft 84. The proximal end ofdevice 50 includes one or more connectors for connecting to an energy delivery unit and/or a mapping unit. In an alternative embodiment, one or moreproximal control arms 86 are attached to a second control shaft such that the symmetry of the geometry ofcarrier assembly 85 can be adjusted to conform to asymmetric pulmonary vein ostia. In another alternative embodiment,device 50 is configured to be inserted over a previously placed guidewire.
Referring now toFIGS. 2 aand2b, the distal portion ofdevice 50 ofFIG. 1 is illustrated.FIG. 2 adepictscarrier assembly 85 fully expanded, withinner shaft 78 fully advanced.FIG. 2 bdepictsinner shaft 78 partially retracted such thatproximal arms 85 are being captured and radially compressed byshaft tip 82, which expands, as shown, to create a smooth transition ofcarrier assembly 85 into the inner lumen ofouter shaft 76.
Referring nowFIG. 3 , an ablation catheter of the present invention is illustrated comprising two carrier assemblies disposed serially along a single axis, with each carrier assembly in an umbrella tip configuration.Ablation catheter 40 includes an elongate tube,outer shaft 36, preferably constructed of Pebax material and approximately 6-8 Fr in diameter, which slidingly receivesfirst control shaft 48.First control shaft 48 is attached on its distal portion tofirst carrier assembly 45, comprising multiple carrier arms and ablation elements configured to deliver energy. The proximal end offirst control shaft 48, not shown, is attached to a control on the proximal end ofablation catheter 40 configured to allow an operator to precisely advance and retractfirst control shaft 48.First control shaft 48 includesring 52 on its distal end that fixedly attaches one end of each distalcarrier arm segment 44 tofirst control shaft 48. Each distalcarrier arm segment 44 is pivotally attached on its opposite end to one end of a proximalcarrier arm segment 42. The opposite end of eachproximal arm segment 42 is fixedly attached to the distal end ofouter shaft 36 viaring 38. Distalcarrier arm segments 44 andproximal arm segments 42 are constructed of a flexible material, such as Nitinol, and can be resiliently biased in a straight or umbrella tip configuration. Advancement and retraction offirst control shaft 48 changes the diameter ofcarrier assembly 45, including a fully compacted (minimal diameter) radial state whenfirst control shaft 48 is fully advanced, and a maximum diameter state whenfirst control shaft 48 is fully retracted.
Fixedly mounted todistal arm segments 44 are ablation elements,RF electrodes 46, configured to deliver energy to tissue to create lesions for disrupting aberrant electrical pathways in the tissue.Electrodes 46 includefins 64 configured to reside in a flow of blood during energy delivery and provide sinking of heat into the circulating blood.Electrodes 46 are configured to deliver monopolar, bipolar or a combination of monopolar and bipolar RF energy as has been described above.Electrodes 46 preferably include integral temperature sensors, such as a thermocouple welded to an internal portion of theelectrode 46.Electrode 46 and any integral temperature or other sensors, are attached to wires, not shown, which travel proximally to the proximal portion ofablation catheter 40 for attachment to an energy delivery unit, a mapping unit, and/or another electronic device for sending or receiving signals and/or power.
Each distalcarrier arm segment 56 is pivotally attached on its opposite end to one end of a proximalcarrier arm segment 54. The opposite end of eachproximal arm segment 54 is fixedly attached to the distal end offirst control shaft 48 viaring 52. Distalcarrier arm segments 56 andproximal arm segments 54 are constructed of a flexible material, such as Nitinol, and can be resiliently biased in a straight or umbrella tip configuration. Advancement and retraction ofsecond control shaft 57 changes the diameter ofcarrier assembly 55, including a fully compacted (minimum diameter) radial state whensecond control shaft 57 is fully advanced, and a maximum diameter state whensecond control shaft 57 is fully retracted.
Fixedly mounted todistal arm segments 44 are ablation elements,mapping electrodes 58, configured to map electrical activity present in tissue to target areas for creating lesions and/or otherwise assess a patient condition.Electrodes 58 are constructed of a conductive material such as platinum or a combination of platinum and iridium.Electrodes 58 preferably include integral temperature sensors, such as a thermocouple welded to an internal portion of theelectrode 58.Electrode 58 and any integral temperature or other sensors, are attached to wires, not shown, which travel proximally to the proximal portion ofablation catheter 40 for attachment to a mapping unit, an energy delivery unit, and/or another electronic device for sending or receiving signals and/or power.
In an alternative embodiment, theablation elements 46 ofproximal carrier assembly 45 may be configured to additionally or alternatively map electrical activity in tissue. In another alternative embodiment, theablation elements 58 ofdistal carrier assembly 55 may be configured to additionally or alternatively delivery ablation energy such as RF energy. In another alternative embodiment, the carrier arms ofcarrier assembly 45 and/orcarrier assembly 55 may include sensors, such as temperature thermocouples, placed within an electrode or mounted to a carrier arm some distance from an electrode, such as midway between two electrodes.Ring 38 andRing 52 are preferably made of a compressible material, such as a metal which can be crimped in a manufacturing process. In an alternative or additional embodiment, adhesives may be used to fixed one or more carrier arms to a shaft. One or more adhesives may be used to attachdistal tip 62.
Referring Now toFIG. 4 , an ablation catheter of the present invention is illustrated including the dual carrier assemblies of the ablation catheter ofFIG. 3 .Ablation catheter 40 includes a tubular body member,outer shaft 36, which includes on its distal end,proximal carrier assembly 45 anddistal carrier assembly 55, as have been described in detail in reference toFIG. 3 . The proximal end ofouter shaft 36 is attached to handle66, which includes multiple controls: slide67, slide68 andbutton 69.Slide 67 is operably attached tofirst control shaft 48.Slide 68 is operably attached tosecond control shaft 57. Movement ofslides first carrier assembly 45 andsecond carrier assembly 55 as has been described in detail in reference toFIG. 3 . Numerous types of mechanical mechanisms can be incorporated intohandle 66 to operably advance one or more control shafts, such as linear slides, rotating knobs or rotating levers such as knobs connected to cam assemblies, and other mechanisms used to move the shafts forward and back.Button 69 is used to initiate energy delivery, such as whenfirst carrier assembly 45 is positioned against a pulmonary vein ostium andablation catheter 40 is electrically connected to an energy delivery unit, not shown.
Referring now toFIG. 5 , an ablation catheter of the present invention is illustrated wherein an ultrasound crystal forwardly directs ultrasonic energy in a circular pattern.Ablation catheter 20 includesouter shaft 22, which slidingly receivescontrol shaft 24, both of which have similar construction to the tubular body members and other shafts described throughout this application. Mounted to controlshaft 24 iscarrier assembly 25, comprisingproximal carrier arms 26 anddistal carrier arms 28.Proximal carrier arms 26 anddistal carrier arms 28 are made of a flexible material such as Nitinol wire and may be resiliently biased in the geometry shown or a different geometry such as a radially compact geometry compatible with intravascular insertion. Eachproximal carrier arm 26 is fixedly attached at one end to controlshaft 24. Eachproximal carrier arms 26 is pivotally attached at its opposite end to an end ofdistal carrier arms 28. The opposite end of eachdistal carrier arm 28 is fixedly attached, at a location more distal, to controlshaft 24, such that a right-angle construction is achieved. A firstproximal arm 26 and attacheddistal arm 28 pair is attached 180.degree. from a secondproximal arm 26 and attacheddistal arm 28 pair, as shown inFIG. 5 . The two pairs are used to contactpulmonary vein ostium 30, also as shown. Additional carrier arm pairs may be included, such as a total of four pairs separated by 90.degree.
Distal todistal carrier arms 28, are centeringarms 32, configured to centercontrol shaft 24 in the pulmonary vein ostium and/or stabilize the distal portion ofablation catheter 20 such as during delivery of ablation energy of mapping of electrical activity. Centeringarms 32 are of similar construction tocarrier arms arms 32, the distal portion ofcontrol shaft 24 may include an inflatable balloon configured to center and/oranchor control shaft 24. The centering balloon, not shown, may include one or more mapping and/or energy delivery elements. The centering and/or stabilizing elements ofablation catheter 20, such as the centeringarms 32, an inflatable balloon, or other similarly functioning element, may be integrated into the other ablation devices and catheters described throughout this application. These stabilizing and centering elements are particularly useful when accessing pulmonary vein ostia that are non-circular.
Fixedly mounted to centeringarms 32 are mappingelements 34, electrodes configured to record electrical activity found in tissue.Control shaft 24 includesguidewire lumen 31, which exits the distal end ofcontrol shaft 24 and travels proximally and exits a proximal portion ofablation catheter 20. In a preferred method,ablation catheter 20 is advanced over a previously placed guidewire that has its distal end placed into a pulmonary vein of the patient.
Fixedly mounted toexternal shaft 24 isultrasound crystal 21, a tubular energy delivery element configured to deliver ultrasonic energy along a cone shaped path, such as along the trajectory of proximal carrier arms26 (dashed lines shown onFIG. 5 ). The vector of energy delivery will cause a relatively circular patterned lesion around the pulmonary vein ostium. In an alternative embodiment, the ultrasound crystal may be configured to provide energy in a sector (less that 360.degree.), and thecarrier assembly 25 would be rotated and repositioned by an operator between ablations to sequentially create a full circumferential lesion.
Advancement and retraction ofcontrol shaft 24 can be used to change the diameter ofcarrier assembly 25, such as retraction wherein the proximal portion ofcarrier assembly 25 is captured within the lumen ofouter shaft 22. Centeringarms 32 are preferably connected to a control shaft, not shown, such that the centering arms can be expanded and contracted. In alternative embodiments with centering and/or stabilizing balloons, or other similar functional elements, the size of the element is configured to be controlled (e.g. expanded and contracted) from the proximal end of the ablation catheter.
Referring now toFIG. 6 , an ablation catheter of the present invention is illustrated wherein one or more carrier arms can be rotated along the axis of the distal portion of the outer shaft.Ablation catheter 60 includesouter shaft 96,ring 98,ring 102,carrier assembly 105,ring 109 andtip 106, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove. Carrier assembly includes multipleproximal carrier arms 112 which are each at one end fixedly attached toring 102.Proximal carrier arms 112 are each pivotally attached at their opposite end to one end ofdistal carrier arms 114. The opposite end of eachdistal carrier arms 114 is fixedly attached to controlshaft 101 viaring 109, such that advancement ofcontrol shaft 101 relative toouter shaft 96causes carrier assembly 105 to change shape. Full advancement ofcontrol shaft 101 causescarrier assembly 105 to transition to a compact, minimum diameter configuration, and retraction ofcontrol shaft 101 causescarrier assembly 105 to transition to a maximum diameter configuration, such as for contacting pulmonary vein ostia.
The distal end ofcontrol shaft 101 includestip 106, which is preferably made of flexible material to be atraumatic to tissue.Tip 106 includes aguidewire lumen 107 which continues proximally and exits a proximal portion ofablation catheter 60 such thatablation catheter 60 can be percutaneously advanced over a previously placed guidewire, such as a guidewire placed into a pulmonary vein as has been described hereabove.
Eachdistal carrier arms 114 includesmultiple ablation elements 116 configured to deliver energy to tissue. Distal to theablation elements 116 is mappingelement 118 configured to record electrical signals present in tissue.Distal carrier arm 108 includesmultiple ablation elements 115 configured to deliver energy to tissue. Distal to theablation elements 115 is mappingelement 113 configured to record electrical signals present in tissue.Proximal carrier arm 104 can be rotated and remain concentric with the static carrier arms, such that theablation elements 115 ondistal arm segment 108 can be positioned at a specific distance from one or more of theablation elements 116 on staticdistal carrier arms 114. The positioning through rotation can be used to achieve lesions of a specific length or other property, especially when bipolar energy is transmitted betweenablation element 115 and one ormore ablation elements 116. This configuration provides simplified use in creating continuous lesions created one sector at a time. The rotation ofproximal arm segment 104 anddistal arm segment 114 can also be performed to more properly match the contour of a non-circular pulmonary vein ostium.
In an alternative embodiment,ablation catheter 60 includes multiple rotatable carrier arms, such as carrier arms connected to independent or ganged control shafts such that multiple carrier arms and their integral ablation element can be rotated to modify the carrier assembly geometry.
Referring now toFIG. 7 , an ablation catheter is illustrated with a carrier assembly including multiple carrier arms, some of which can be repositioned relative to other carrier arms.Ablation catheter 70 includesouter shaft 130,first control shaft 121,second control shaft 122,carrier assembly 125 andring 136, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.Carrier assembly 125 includes multiple carrier arms comprisingproximal arm segments distal arm segments Proximal arm segments 124 anddistal arm segments control shaft 121 decreases and increases the diameter ofcarrier assembly 125, respectively.
Referring now toFIG. 8 a, an ablation catheter of the present invention is illustrated in which multiple carrier arms are maintained in fixed positions by a ring, such as an advancable ring.Ablation catheter 90 includesouter shaft 138,control shaft 141,carrier assembly 145 andtip 154, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.Carrier assembly 145 includesproximal arm segments 144 which are pivotally attached todistal arm segments 146. Eachdistal arm segment 146 includesmultiple ablation electrodes 147 and amapping sensor 148 distal to theablation electrodes 147.Carrier assembly 145 further includesmultiple carrier arms 156, which may be void of electrodes as shown, or may include one or more mapping or ablating electrodes, or other sensor or transducer.
Referring also toFIG. 8 c, each proximal carrier arm segment is circumferentially positioned in agroove 246 ofring 142.Carrier arms 156 are similarly positioned in agroove 256 ofring 142.Ring 142 is preferably attached to a control shaft, not shown, such that advancement of that control shaft changes the geometry ofcarrier assembly 145 accordingly. Retraction ofcontrol shaft 141 changes the diameter ofcarrier assembly 145 as has been described in detail hereabove.FIG. 8 billustrates an end view of theablation catheter 90 ofFIG. 8 a, showing the rotational orientation of thedistal arm segments 146 andcarrier arms 156.Carrier arms 156 are positioned orthogonal to two sets of threedistal carrier arms 146 such as to provide positioning and radial support todistal carrier arms 146. Various configurations of carrier arm geometries can be provided for handling of various pulmonary vein tissue contours.Ring 142 may maintain theablation elements 147 and/or themapping elements 148 in close proximity.
In an alternative embodiment,ring 142 is not advancable (not connected to a control shaft), but included withgrooves 256 andgrooves 246 to maintain the rotational orientation of thedistal carrier arms 146 and thecarrier arms 156 such as when force is applied toouter shaft 138, such as via a handle, the force translated tocarrier assembly 145.
Referring now toFIG. 9 , an ablation catheter of the present invention is illustrated in which an advancable spline can be radially expanded to improve or otherwise alter the structure and rigidity of a carrier assembly.Ablation catheter 90bincludesouter shaft 138,control shaft 141,carrier assembly 95 andtip 154, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.Carrier assembly 95 includesmultiple carrier arms 156 in an umbrella tip configuration, one or more including ablation elements, other sensors or transducers, all not shown. Ascontrol shaft 141 is advanced and retracted (e.g. via a control on a proximal handle, both not shown),carrier assembly 95 contracts and expands respectively, as has been described hereabove.Control shaft 141 slidingly receives anadvancable spline 164, whose distal end resides inrecess 162 ofcontrol shaft 141. Advancement ofspline 164 causes its distal end to extend radially out fromcontrol shaft 141 as is shown inFIG. 9 a(partially extended) andFIG. 9 b(fully extended). In a preferred embodiment,spline 164 can be advanced to the maximum diameter ofcarrier arms 156. In an alternative embodiment,spline 164 can be advanced to a diameter greater than the maximum diameter ofcarrier arms 156.Spline 164 is advance to modify the performance characteristics ofcarrier assembly 95, such as to modify the supporting forces applied to tissue. In an alternative embodiment,spline 164 includes one or more ablation elements (e.g. RF electrodes) or other sensors or transducers.
Referring now toFIGS. 10 and 11 , an ablation catheter of the present invention is illustrated in which the carrier assembly comprises an inflatable balloon with multiple ablation elements mounted on or embedded in its external surface.Ablation catheter 100 includescarrier assembly 175 comprisingballoon 174 andelectrodes 172, such as RF ablation or electrical signal mapping electrodes.Ablation catheter 100 further includes an elongate tubular body,outer shaft 166, which includes aninflation lumen 176 from its proximal portion to the inner cavity ofballoon 174.Balloon 174 is sealed and fixedly attached to the distal portion ofouter shaft 166. Passage of fluid such as air or saline through the inflation lumen ofouter shaft 166 causesballoon 174 to inflate and remain in an expanded state as long as the fluid pressure is maintained.Balloon 174 may be a compliant or non-compliant balloon, and while shown as a disk or donut shape, may have profiles specific to mimic pulmonary vein ostia and the tissue extending therefrom.
Extending from the distal end of and coaxial toexternal shaft 166 is centeringpost 168 which traverses from the proximal end to the distal end ofballoon 174, and includes a projection configured to engage a pulmonary vein lumen. In a preferred embodiment, a guidewire lumen is included from the distal end to a proximal portion ofablation catheter 100 such that ablation catheter may be advanced over a guidewire such as a guidewire that has previously been placed into a pulmonary vein ostium or other applicable orifice. Mapping and/or ablating procedures can be accomplished by an operator applying a force to balloon174 viashaft 166, and transmitting ablation energy toelectrodes 172 and/or recording electrical activity fromelectrodes 172.
Referring now toFIG. 12 a, ablation catheter of the present invention is illustrated in which the carrier assembly includes a single carrier arm that can be positioned into an adjustable, partial circumferential (less than 360.degree.) loop for ablating and/or mapping tissue.Ablation device 180 includes an elongate tubular body member,outer shaft 182, with sufficient column and torsion strength to support standard interventional procedures such as those which access the vasculature from a femoral vein or artery and access the patient's heart.Outer shaft 182 is constructed of biocompatible materials such as Pebax, and preferably includes an inner braid, such as a stainless steel 304 braid. The proximal portion ofouter shaft 182 is preferably made of Pebax 7233D and the distal portion ofouter shaft 182 is preferably made ofPebax 55/3533D.Outer shaft 182 is fixedly attached to handle195 viastrain relief 181.
Exiting the distal end ofouter shaft 182 arecontrol shaft 184 andcarrier assembly 185, which comprises asingle carrier arm 186. At least one ofcontrol shaft 184 andcarrier arm 186 can be advanced and/or retracted by manipulating a control onhandle 195, such asrotating knob 193. In a preferred embodiment, the proximal end ofcarrier arm 186 is fixedly attached to a distal portion ofouter shaft 182, such as via a crimp and/or adhesives, andouter shaft 184 is slidingly received byouter shaft 182 and operably attached torotating knob 193.Carrier arm 185, preferably made of an elastic material such as Nitinol covered with a sleeve made of Pebax, includes one ormore electrodes 188 along its length. Theelectrodes 188 may include heat-sinking fins as shown. Theelectrodes 188 are preferably made of platinum, and are typically 3 mm long and separated by 1 to 4 mm, with symmetric or asymmetric spacing. Fourelectrodes 188 are depicted inFIGS. 12 athrough12c. Four (4) to sixteen (16)electrodes 188 are preferred, typically eight (8) to ten (10). The presence of multiple, typically uniformly distributed electrodes enables the operator to rapidly identify problematic target areas (undesired electrical activity) and create lesions (ablate) rapidly. The multi-electrode geometry ofcarrier assembly 185, precision loop control, and ease of positioning (including over-the-wire positioning and anchoring), enables simplified, customized diagnosis and treatment of heart rhythm disorders such as atrial fibrillation.
Handle195, preferably made of a plastic such as a polycarbonate, includeslever 196 which is operably attached to one or more pull wires, not shown, that travel within a lumen ofouter shaft 182 and attach near the distal end ofshaft 182. Multiple pull wires may be included, such as two pull wires which are attached at a 90.degree. radial separation from each other near the distal end ofshaft 182. The two pull wires may be attached at the same longitudinal position along the axis ofshaft 182, or may be offset. Manipulation oflever 196 causes the distal portion ofablation catheter 180 to deflect in one or more planes such that a clinician can manipulatetip 192 into a pulmonary vein or other orifice such as the coronary sinus or other vessel.
Handle195 also includes plug198 which is configured to electrically connect to one or more separate devices, such as an energy delivery unit configured to deliver ablation energy toelectrodes 188 and/or to receive temperature signals from one or more temperature sensors such as a thermocouple integral to anelectrode 188; a mapping unit configured to receive electrical signals from one ormore electrodes 188; a pacing unit configured to deliver electrical energy toelectrodes 188 in order to pace the heart of a patient; or another device such as a device which receives and/or transmits signals to one or more functional elements ofcarrier assembly 185. Wires, not shown, attach to plug198 and travel throughhandle 195, throughouter shaft 182 and attach toelectrodes 188 and any other sensors or transducers integral toelectrodes 188 or attached tocarrier arm 186. The wires may be located on the external surface ofcarrier arm 186, or travel within a lumen ofcarrier arm 186, exiting through a side hole to attach toelectrodes 188.
Referring additionally toFIG. 12 b,carrier assembly 185 is shown in a linear configuration such thatablation device 180 can be intraluminally advanced through the vasculature of the patient.Carrier assembly 185 is placed in this linear configuration by advancingcontrol shaft 184 such as viarotating knob 193 ofhandle 195. Referring now toFIG. 12 c,control shaft 184 is being retracted, andcarrier assembly 185 is transitioning to a partial circumferential (less than 360.degree.) loop. Advancement and retraction ofcontrol shaft 184 adjust the geometry of the loop, wherein full advancement causes a near-linear configuration and retraction causes the diameter ofcarrier assembly 185 to increase. Preferred maximum diameters ofcarrier assembly 185 are typically 15-32 mm to accommodate the varied anatomical contours neighboring pulmonary vein ostia (including non-circular ostia). The simplified loop-modifying controls of the present invention allow for rapid positioning by an operator. In a preferred embodiment,carrier arm 186 is resiliently biased (such as with the heat treating of a Nitinol component) in a helical configuration. In an alternative embodiment,carrier arm 186 is resiliently biased in a near-linear configuration. In the configuration wherecarrier arm 186 includes a wire surrounded by a sleeve, a resilient bias can be provided by the wire or the sleeve.
In another alternative embodiment, the proximal end ofcarrier arm 186 exitsouter shaft 182 at a location approximate 90.degree. radially offset from the location thatcarrier arm 186 is attached to the distal end ofcontrol shaft 184, such offset attachment providing a bias for forming the loop during retraction ofcontrol shaft 184.
Referring now toFIGS. 13 ,13aand13b, the ablation catheter ofFIG. 12 ais illustrated. InFIG. 13 ,ablation catheter 180 is shown withcarrier assembly 185 in linear configuration such as for advancingablation catheter 180 overguidewire 12, such as an intraluminal advancement over a guidewire which has been inserted into a femoral vein, and travels to the heart, through the septum separating the right atrium and left atrium (e.g. through a transeptal sheath), and into a pulmonary vein such as the left superior pulmonary vein.Carrier assembly 185 is placed in this linear, maximally compact configuration by advancingcontrol shaft 184, such as by manipulating a control on a handle ofdevice 180 as has been described hereabove.Carrier arm 186 includeselectrodes 188.Carrier arm 186 has a proximal end fixedly attached toouter shaft 182 viacrimp ring 194.Carrier arm 186 distal end is fixedly attached to controlshaft 184 at a radial location that is 90.degree. offset from its proximal end attachment (as shown inFIG. 13 ), such thatcarrier assembly 185 radially expands as shown inFIGS. 13 aand13bascontrol shaft 184 is retracted. The distal end ofcontrol shaft 184 is covered withatraumatic tip 192, which includes an exit hole in communication with an internal guidewire lumen, not shown but through which guidewire12 passes.
Referring now toFIG. 14 , a preferred construction of the ablation catheter ofFIG. 12 ais illustrated.Ablation catheter 180 includescarrier assembly 186, which comprises asingle carrier arm 186 whose geometry is adjusted by advancing and retractingcontrol shaft 184, as has been described in detail hereabove.Carrier arm 186 includeswire shaft 202, preferably Nitinol or other shaped memory alloy or polymer, surrounded byouter sleeve 203, preferably Pebax or other biocompatible, soft material.Sleeve 203 can perform one or more functions, including but not limited to: capturing one or more wires betweenwire 202 andsleeve 203; acting as an insulator; providing an atraumatic boundary (e.g. covering any sharp edges of wire202); and combinations thereof.Wire shaft 202 preferably is resiliently biased in the loop geometry depicted. The proximal end ofcarrier arm 186 is fixedly attached toouter shaft 182 viaring 194. Alternatively or additionally, adhesives such as cyanoacrylate may be used for fixation. In an alternative embodiment,ring 194 also functions as an electrode, such as a mapping and/or ablation electrode.
At its distal end,carrier arm 186 is fixedly attached to cap192 and the distal end ofcontrol shaft 184.Cap 192 is of soft construction to be atraumatic to tissue, and is preferably made of Pebax which has been doped with Barium Sulfate to be radiopaque. Aguidewire lumen 201 exits cap192 after having passed withincontrol shaft 184.Guidewire lumen 201 is surrounded by a braided tube preferably made of Nylon and braided with stainless steel wire. Theguidewire lumen 201 travels proximally to an exit port on a handle, not shown but described in detail hereabove.
Referring now toFIGS. 15 athrough15d, the ablation catheter ofFIG. 12 ais illustrated.Ablation catheter 180 includesouter shaft 182,control shaft 184,ring 194,carrier assembly 185 andtip 192, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.FIG. 15 aillustrates the proximal portion, including handle, preferably made of a plastic such as polycarbonate. Handle195 includeslever 196 and slide197, controls used by an operator to adjust the carrier assembly, deflect the distal portion ofcatheter 180 and/or perform other functions. Handle195 is fixedly attached toshaft 182. Extending fromhandle 195 is pigtail plus198, an electrical connector that attaches signal and power wires to one or more components ofablation catheter 180 such as ablation electrodes, mapping electrodes and thermocouples.
Referring now toFIG. 15 b, the distal end ofcatheter 180 is illustrated including the distal end ofouter shaft 182.FIG. 15 billustratescarrier assembly 185 in its compacted, linear configuration applicable for over-the-wire intraluminal advancement ofablation catheter 180, such as to reach the left superior pulmonary vein as depicted inFIG. 15 d. Referring back toFIG. 15 b,control shaft 184 has been fully advanced such thatcarrier arm 186 is pulled tight againstcontrol shaft 184.Control shaft 184 includes on itsdistal end tip 192. Withintip 192 isguidewire lumen 191 through which a standard interventional guidewire, such as a 0.035″ guidewire, can be inserted.Carrier arm 185 is configured, as has been described in detail hereabove, such that upon retraction ofcontrol shaft 184,carrier arm 186 extends laterally into the loop configuration illustrated inFIG. 15 c.
Referring additionally toFIG. 15 d, the treatment to be accomplished with the devices and method described in this application is illustrated.FIG. 15 dshows a cutaway view of thehuman heart 10, showing the major structures of the heart including the left and right atria, and thepulmonary veins 15. The atrial septum separates the left and right atria. The fossa ovalis is a small depression in the atrial septum that may be used as an access pathway to the left atrium from the right atrium, such as with a transeptal puncture device and transeptal sheath. The fossa ovalis can be punctured, and easily reseals and heals after procedure completion. In a patient suffering from atrial fibrillation, aberrant electrically conducive tissue may be found in the atrial walls, as well as in thepulmonary veins 15. Ablation of these areas, referred to arrhythmogenic foci (also referred to as drivers or rotors), is an effective treatment for atrial fibrillation. The catheters of the present invention provide means of creating lesions, including lesions to surround the pulmonary vein ostia, and are easily deployed to identify and ablate the driver and rotor tissue.
To accomplish this,catheter 180 is inserted into the right atrium, preferably through the inferior vena cava, as shown in the illustration, or through the superior vena cava.Catheter 180 is sized for this advancement through the patient's vasculature, such as where the inserted (shaft) diameter is approximately 9 Fr, the shaft length is approximately 115 cm and the overall length is typically 158 cm.Catheter 180 has been passed throughtranseptal sheath 11, which may or may not be a deflectable sheath sincecatheter 180 preferably includes a deflectable distal portion. When passing into the left atrium,transeptal sheath 11 passes through or penetrates the fossa ovalis, such as overguidewire 12 which may have been placed by a transeptal puncture device.Catheter 180 is inserted overguidewire 12 and throughtranseptal sheath 11 such that its distal end enters right superiorpulmonary vein 15's lumen. The distal portion ofshaft 182 has been deflected such that the distal end ofshaft 182 is directed toward the lumen ofpulmonary vein 15a.Catheter 180 carries a structure carrying multiple ablation elements such as RF electrodes,carrier assembly 185, into the left atrium.Carrier assembly 185 has been transitioned to expand to a maximal diameter by retractingcontrol shaft 184, such that multiple ablation elements (ablation and/or mapping elements),electrodes 188, are in contact with the pulmonary vein ostial tissue.Carrier assembly 185 is adapted to be deformable such that pressing carrier assembly intopulmonary vein 15 ostium will cause one or more, and preferably all ofelectrodes 188 to make contact with tissue to be analyzed and/or ablated. Each of theelectrodes 188 is attached via connecting wires and one or more connectors, such asplug 198, to an energy delivery apparatus, not shown but preferably an RF energy delivery unit which is also attached to a patch electrode, also not shown but preferably a conductive pad attached to the back of the patient.
The energy delivery unit is configured to delivery RF energy in monopolar, bipolar or combination monopolar-bipolar energy delivery modes, simultaneously or sequentially, with or without “off” or no energy delivered time durations. In a preferred embodiment, the energy delivery unit200 is configured to also provide electrical mapping of the tissue that is contacted by one or more electrodes integral tocarrier assembly 185. Alternatively, a separate mapping unit may be used, preferably attached tocatheter 180 simultaneous with attachment to the energy delivery unit.Electrodes 188 can also be configured to be mapping electrodes and/or additional electrodes can be integral tocarrier assembly 185 to provide a mapping function.Carrier assembly 185 is configured to be engaged over a pulmonary vein ostium surface to map and/or ablate tissue on the surface. Energy is delivered after a proper location of theelectrodes 188 is confirmed with a mapping procedure. If conditions are determined to be inadequate, an operator may adjust the shape of carrier assembly185 (e.g. through advancement or retraction of control shaft184) and/or the operator may repositioncarrier assembly 185 against tissue through various manipulations at the proximal end of theablation catheter 180. After an ablation step is completed,ablation catheter 180 is repositioned, with or without changing the geometry ofcarrier assembly 185, and a similar mapping and ablation step is performed. For each pulmonary vein ostium, this repositioning will typically occur two to three times creating semi-circular lesions that preferably overlap. The steerability of the distal portion ofshaft 182, via a control onhandle 195, is an important function in this repositioning process. In a typical procedure, the clinician will perform ablations in the left superior pulmonary vein first, followed by the right superior, left inferior and then the right inferior pulmonary veins.
In a preferred embodiment, the energy delivery unit is configured to delivery both RF energy and ultrasound energy to the identical ordifferent electrodes 188. In another preferred embodiment, the energy delivery unit is configured to accept a signal from one or more sensors integral toablation catheter 180, not shown, such that the energy delivered can be modified via an algorithm which processes the information received from the one or more sensors.
In an alternative embodiment, a guidewire is inserted through a sidecar present at the distal portion ofshaft 182, avoiding the need for threading the entire the device over the guidewire. In another alternative embodiment,carrier arm 186 is attached to a second control shaft, also slidingly received byouter shaft 182 and connected to a control onhandle 195, such that thecarrier assembly 185 geometry can be adjusted by advancing and retracting either the second control shaft orcontrol shaft 184. This dual control shaft design also allows carrier assembly to be completely retracted within the distal end ofcontrol shaft 182, as is described in reference toFIG. 17 herebelow.
Referring now toFIG. 16 , an ablation catheter of the present invention is illustrated comprising a first deployable carrier assembly including multiple ablation electrodes and a more distal second deployable carrier assembly including multiple mapping electrodes.Ablation catheter 400 includesouter shaft 410,first control shaft 411,second control shaft 412,first carrier assembly 430a,second carrier assembly 430bandtip 415, all of which include similar components, materials, construction and function to the same or like parts used in reference to the ablation catheters described hereabove.Outer shaft 410, preferably a braidedconstruction including braid 413, slidingly receivesfirst control shaft 410, which in turn slidingly receivessecond control shaft 411.Outer shaft 410 is fixedly attached to handle420 viastrain relief 426. Anatraumatic tip 415 is fixedly attached to the distal end ofsecond control shaft 412. Aguidewire lumen 416 exitstip 415 and travels proximally, throughsecond control shaft 412,first control shaft 411 andouter shaft 410 to handle420 where it exits viaguidewire entry 424, such thatablation catheter 400 can be percutaneously introduced into a patient over a previously placed guidewire.
Handle420 also includeslever 423, which is operably attached to one or more pull wires which extend distally within a lumen, such as a Teflon lined lumen, withinouter shaft 410. The one or more pull wires are fixedly attached to a distal portion ofouter shaft 410 causing operator controlled deflection of the distal portion ofablation catheter 400 in one or more planes. Handle420 further includeselectrical plug 425 which is electrically connected to one or more electrical wires or other conduits, all of which travel distally, alongouter shaft 410 to various locations such aselectrodes 431 orelectrodes 432, or another sensor or transducer not shown.Plug 425 is configured to attach to an energy delivery unit such as an RF energy delivery unit, a mapping unit or another device configured to transmit or receive electrical signals or power.
Referring now toFIGS. 17 ,17a,17band17c, an ablation catheter of the present invention is illustrated.Ablation catheter 180bis of similar construction toablation catheter 180 ofFIGS. 12 through 14 with common elements having the same reference numbers. For brevity, most of the common construction and common component details will be omitted.Ablation catheter 180bincludes handle195 with operator controls first slide197a,second slide 197bandlever 196, each operably attached to a control shaft or other linkage.Plug 198 is connected to one or more electrical wires or other conduits that travel distally throughouter shaft 182 and connect to one or more functional elements of the device, such as electrodes included incarrier assembly 185. The distal end ofablation catheter 192 includes anatraumatic tip 192, preferably constructed of Pebax which has been doped with Barium Sulfate for radiopacity. Theablation catheter 180bofFIG. 17 depicts thecarrier assembly 185 in a fully deployed (maximum diameter) configuration, caused by retracting the control shaft via a slide or lever onhandle 196.
Referring now toFIG. 17 a, carrierarm carrier assembly 185 is in its fully deployed (maximum diameter) condition.Carrier arm 186 is attached on its distal end tofirst control shaft 182. On its proximal end, instead of being attached to the distal portion ofouter shaft 182 as theablation device 180 ofFIG. 12 a,carrier arm 186 is attached to asecond control shaft 213, which also can be advanced and retracted from a control onhandle 195. Advancement and retraction of bothfirst control shaft 184 andsecond control shaft 213 can be used, independently or in combination, to change the geometry ofcarrier assembly 185.
Referring now toFIG. 17 b, a differentiating feature ofablation catheter 180bis illustrated wheresecond control shaft 213 has been retracted untilcarrier arm 186, includingablation elements 188, is contained completely within a lumen ofouter shaft 182, such as within a lumen within a Teflon liner. In an alternative embodiment,carrier arm 186 is retracted withinouter shaft 182 by retractedfirst control shaft 184.
Referring now toFIG. 17 c, an end view of the device and deployment status ofFIG. 17 ais illustrated.Carrier arm 186 is shown in its less than 360.degree. helix. Also shown areelectrodes 188, typically 2-3 mm in length with symmetric 3 mm spacing between electrodes.
It should be understood that numerous other configurations of the systems, devices and methods described herein can be employed without departing from the spirit or scope of this application. It should be understood that the system includes multiple functional components, such as the ablation catheter and the energy delivery apparatus. The ablation catheter consists of a catheter shaft, a carrier assembly for providing electrodes in a resiliently biased configuration, a control shaft for deploying and withdrawing the carrier assembly, and a coupler for attaching the control shaft to the carrier assembly. The carrier assembly is a support structure which is shiftable from a storage or confined configuration, such as a radially constrained configuration, to a deployed or expanded configuration. The carrier assembly can includes wires, ribbons, cables and struts, made of either metals, non-metals or combinations of both. The carrier assembly can be constructed of one or more materials, including both metals and non-metals. Typical metals chosen for carrier assembly construction include but are not limited to: stainless steel, Nitinol, Elgiloy™, other alloys and combinations thereof.
The ablation catheter of the present invention may include a steerable outer sheath, or may work in conjunction as a system with a separate steerable outer sheath. One or more tubular components of the ablation catheter may be steerable such as with the inclusion of a controllable pull wire at or near the distal end. The ablation catheter of the present invention may be inserted over the wire, such as via a lumen within one of the tubular conduits such as within a lumen of the tubular body member or control shaft, or alternatively the catheter may include a rapid exchange sidecar at or near its distal end, consisting of a small projection with a guidewire lumen therethrough. A guidewire lumen may be included solely for the guidewire, or may provide other functions such as a vacuum lumen for an integral suction port integrated at the distal portion of the carrier assembly.
The ablation catheter of the present invention further includes ablation elements. In preferred embodiments, one or more ablation elements are electrodes configured to deliver RF energy. Other forms of energy, alternative or in addition to RF, may be delivered, including but not limited to: acoustic energy and ultrasound energy; electromagnetic energy such as electrical, magnetic, microwave and radiofrequency energies; thermal energy such as heat and cryogenic energies; chemical energy; light energy such as infrared and visible light energies; mechanical energy; radiation; and combinations thereof. One or more ablation elements may comprise a drug delivery pump or a device to cause mechanical tissue damage such as a forwardly advanceable spike or needle. The ablation elements can deliver energy individually, in combination with or in serial fashion with other ablation elements. The ablation elements can be electrically connected in parallel, in series, individually, or combinations thereof. The ablation catheter may include cooling means to prevent undesired tissue damage and/or blood clotting. The ablation elements may be constructed of various materials, such as plates of metal and coils of wire for RF energy delivery. The electrodes can take on various shapes including shapes used to focus energy such as a horn shape to focus sound energy, and shapes to assist in cooling such as a geometry providing large surface area. Electrodes can vary within a single carrier assembly, such as a spiral array of electrodes or a umbrella tip configuration wherein electrodes farthest from the central axis of the catheter have the largest major axis. Wires and other flexible conduits are attached to the ablation elements, such as electrical energy carrying wires for RF electrodes or ultrasound crystals, and tubes for cryogenic delivery.
The ablation elements requiring electrical energy to ablate require wired connections to an electrical energy power source such as an RF power source. In configurations with large numbers of electrodes, individual pairs of wires for each electrode may be bulky and compromise the cross-sectional profile of the ablation catheter. In an alternative embodiment, one or more electrodes, connected in serial fashion such that a reduced number of wires, such as two wires, can be attached to two or more electrodes, include switching means such that while a first electrode is powered, the remaining electrodes do not transmit ablative energy. Switching means may be a thermal switch, such that as a first electrodes heats up, a single pole double throw switch change state disconnecting power from that electrode and attaching power to the next electrode in the serial connection. This integral temperature switch may have a first temperature to disconnect the electrode, and a second temperature to reconnect the electrode wherein the second temperature is lower than the first temperature, such as a second temperature below body temperature. In an alternative embodiment, each electrode is constructed of materials in their conductive path such that as when the temperature increased and reached a predetermined threshold, the resistance abruptly decreased to near zero, such that power dissipation, or heat, generated by the electrode was also near zero, and more power could be delivered to the next electrode incorporating the above switching means.
The ablation catheter of the present invention preferably includes a handle activating or otherwise controlling one or more functions of the ablation catheter. The handle may include various knobs, such as rotating or sliding knobs which are operably connected to advanceable conduits, or are operably connected to gear trains or cams which are connected to advanceable conduits. These knobs, such as knobs use to deflect a distal portion of a conduit, or to advance or retract the carrier assembly, preferably include a reversible locking mechanism such that a particular tip deflection or deployment amount can be maintained through various manipulations of the system.
The ablation catheter may include one or more sensors, such as sensors used to detect chemical activity; light; electrical activity; pH; temperature; pressure; fluid flow or another physiologic parameter. These sensors can be used to map electrical activity, measure temperature, or gather other information that may be used to modify the ablation procedure. In a preferred embodiment, one or more sensors, such as a mapping electrode, can also be used to ablate tissue.
Numerous components internal to the patient, such as the carrier assembly or electrodes, may include one or more visual markers such as radiopaque markers visible under fluoroscopy, or ultrasound markers.
Selection of the tissue to be ablated may be based on a diagnosis of aberrant conduit or conduits, or based on anatomical location. RF energy may be delivered first, followed by another energy type in the same location, such as when a single electrode can deliver more than one type of energy, such as RF and ultrasound energy. Alternatively or additionally, a first procedure may be performed utilizing one type of energy, followed by a second procedure utilizing a different form of energy. The second procedure may be performed shortly after the first procedure, such as within four hours, or at a later date such as greater than twenty-four hours after the first procedure. Numerous types of tissue can be ablated utilizing the devices, systems and methods of the present invention. For example, the various aspects of the invention have application in procedures for ablating tissue in the prostrate, brain, gall bladder, uterus, other organs and regions of the body, and a tumor, preferably regions with an accessible wall or flat tissue surface. In the preferred embodiment, heart tissue is ablated, such as left atrial tissue.
In another preferred embodiment of the system of the present invention, an ablation catheter and a heat sensing technology are included. The heat sensing technology, includes sensor means that may be placed on the chest of the patient, the esophagus or another area in close enough proximity to the tissue being ablated to directly measure temperature effects of the ablation, such as via a temperature sensor, or indirectly such as through the use of an infrared camera. In the described system, when a temperature or a surrogate temperature reaches a threshold, such as an adjustable threshold, the ablation energy is reduced or stopped, to one or more ablation elements. The threshold will depend on the location of the sensor means, as well as where the ablation energy is being delivered. The threshold may be adjustable, and may be automatically configured.
Numerous kit configurations are also to be considered within the scope of this application. An ablation catheter is provided with multiple carrier assemblies. These carrier assemblies can be removed for the tubular body member of the catheter, or may include multiple tubular body members in the kit. The multiple carrier assemblies can have different patterns, different types or amounts of electrodes, and have numerous other configurations including compatibility with different forms of energy.
Though the ablation device has been described in terms of its preferred endocardial and transcutaneous method of use, the array may be used on the heart during open heart surgery, open chest surgery, or minimally invasive thoracic surgery. Thus, during open chest surgery, a short catheter or cannula carrying the carrier assembly and its electrodes may be inserted into the heart, such as through the left atrial appendage or an incision in the atrium wall, to apply the electrodes to the tissue to be ablated. Also, the carrier assembly and its electrodes may be applied to the epicardial surface of the atrium or other areas of the heart to detect and/or ablate arrhythmogenic foci from outside the heart.
Other embodiments of the invention will be apparent to those skilled in the art from consideration of the specification and practice of the invention disclosed herein. It is intended that the specification and examples be considered as exemplary only, with a true scope and spirit of the invention being indicated by the following claims. In addition, where this application has listed the steps of a method or procedure in a specific order, it may be possible, or even expedient in certain circumstances, to change the order in which some steps are performed, and it is intended that the particular steps of the method or procedure claim set forth herebelow not be construed as being order-specific unless such order specificity is expressly stated in the claim.
Claims (17)
1. A medical device, comprising:
a catheter shaft defining a longitudinal axis;
a first carrier assembly coupled to the catheter shaft and having a first array of electrodes coupled to a plurality of first carrier arms; and
a second carrier assembly coupled to the catheter shaft and having a second array of electrodes coupled to a plurality of second carrier arms;
the first carrier assembly and the second carrier assembly each being independently movable between a configuration substantially parallel to the longitudinal axis, and a radially expanded configuration.
2. The medical device ofclaim 1 , wherein the first carrier assembly is positioned proximal to the second carrier assembly.
3. The medical device ofclaim 2 , further comprising:
a tubular body defining a tubular body lumen;
a first control shaft slidably received within the tubular body lumen, the first control shaft defining a control shaft lumen;
a second control shaft slidably received within the control shaft lumen, wherein a proximal portion of the first carrier assembly is coupled to the tubular body, a distal portion of the first carrier assembly and a proximal portion of the second carrier assembly is coupled to the first control shaft, and a distal portion of the second carrier assembly is coupled to the second control shaft.
4. The medical device ofclaim 3 , wherein:
the first control shaft is independently movable between a first distal position and a first proximal position, such that moving the first control shaft to the first distal position moves the first carrier assembly to the configuration substantially parallel to the longitudinal axis, and moving the first control shaft to the first proximal position moves the first carrier assembly to the radially expanded configuration; and
the second control shaft is independently movable between a second distal position and a second proximal position, such that moving the second control shaft to the second distal position moves the second carrier assembly to the configuration substantially parallel to the longitudinal axis, and moving the second control shaft to the second proximal position moves the second carrier assembly to the radially expanded configuration.
5. The medical device ofclaim 1 , the first array of electrodes having a series of longitudinally spaced electrodes.
6. The medical device ofclaim 1 , wherein the first and second carrier assemblies are each resiliently biased to a primary configuration.
7. The medical device ofclaim 1 , further comprising a mapping element coupled to the first carrier assembly.
8. The medical device ofclaim 7 , wherein the mapping element is distal to the first array of electrodes.
9. The medical device ofclaim 1 , wherein the catheter shaft defines a guidewire lumen.
10. The medical device ofclaim 1 , wherein the catheter shaft has a distal end, the medical device further comprising a tip coupled to the distal end.
11. The medical device ofclaim 1 , wherein the first and second carrier assemblies are made at least partially from nitinol.
12. The medical device ofclaim 1 , further comprising a temperature sensor coupled to an electrode of the first array.
13. The medical device ofclaim 1 , wherein the first and second carrier assemblies longitudinally overlap.
14. The medical device ofclaim 13 , wherein the second carrier assembly is rotatable around the longitudinal axis.
15. The medical device ofclaim 13 , further comprising a control element coupled to the second carrier assembly, wherein manipulation of the control element rotates the second carrier assembly around the longitudinal axis.
16. A medical device, comprising:
a catheter shaft;
a first carrier assembly coupled to the catheter shaft and having a first radially expandable array of electrodes, the electrodes of the first carrier assembly being coupled to a plurality of first carrier arms; and
a second carrier assembly rotatably coupled to the catheter shaft and having a second radially expandable array of electrodes, the electrodes of the first carrier assembly being coupled to a plurality of second carrier arms, the second carrier assembly rotatable about the first carrier assembly.
17. The medical device ofclaim 16 , wherein the catheter shaft has a longitudinal axis, and the first carrier assembly and the second carrier assembly are each movable between a configuration substantially parallel to the longitudinal axis and a radially expanded configuration.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/938,791US8337492B2 (en) | 2005-06-20 | 2010-11-03 | Ablation catheter |
| US13/677,571US8771267B2 (en) | 2005-06-20 | 2012-11-15 | Ablation catheter |
| US14/300,693US8979841B2 (en) | 2005-06-20 | 2014-06-10 | Ablation catheter |
| US14/627,274US9468495B2 (en) | 2005-06-20 | 2015-02-20 | Ablation catheter |
| US15/291,822US20170027640A1 (en) | 2005-06-20 | 2016-10-12 | Ablation catheter |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69241605P | 2005-06-20 | 2005-06-20 | |
| US11/471,467US7850685B2 (en) | 2005-06-20 | 2006-06-20 | Ablation catheter |
| US12/938,791US8337492B2 (en) | 2005-06-20 | 2010-11-03 | Ablation catheter |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/471,467ContinuationUS7850685B2 (en) | 2005-06-20 | 2006-06-20 | Ablation catheter |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/677,571ContinuationUS8771267B2 (en) | 2005-06-20 | 2012-11-15 | Ablation catheter |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20110106074A1 US20110106074A1 (en) | 2011-05-05 |
| US8337492B2true US8337492B2 (en) | 2012-12-25 |
Family
ID=37595714
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/471,467Active2029-05-13US7850685B2 (en) | 2005-06-20 | 2006-06-20 | Ablation catheter |
| US12/938,791Expired - Fee RelatedUS8337492B2 (en) | 2005-06-20 | 2010-11-03 | Ablation catheter |
| US13/677,571ActiveUS8771267B2 (en) | 2005-06-20 | 2012-11-15 | Ablation catheter |
| US14/300,693ActiveUS8979841B2 (en) | 2005-06-20 | 2014-06-10 | Ablation catheter |
| US14/627,274ActiveUS9468495B2 (en) | 2005-06-20 | 2015-02-20 | Ablation catheter |
| US15/291,822AbandonedUS20170027640A1 (en) | 2005-06-20 | 2016-10-12 | Ablation catheter |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/471,467Active2029-05-13US7850685B2 (en) | 2005-06-20 | 2006-06-20 | Ablation catheter |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/677,571ActiveUS8771267B2 (en) | 2005-06-20 | 2012-11-15 | Ablation catheter |
| US14/300,693ActiveUS8979841B2 (en) | 2005-06-20 | 2014-06-10 | Ablation catheter |
| US14/627,274ActiveUS9468495B2 (en) | 2005-06-20 | 2015-02-20 | Ablation catheter |
| US15/291,822AbandonedUS20170027640A1 (en) | 2005-06-20 | 2016-10-12 | Ablation catheter |
Country Status (8)
| Country | Link |
|---|---|
| US (6) | US7850685B2 (en) |
| EP (2) | EP2759276A1 (en) |
| JP (1) | JP2009500052A (en) |
| CN (1) | CN101309651B (en) |
| AU (1) | AU2006262447A1 (en) |
| CA (1) | CA2612679A1 (en) |
| IL (1) | IL188882A0 (en) |
| WO (1) | WO2007001981A2 (en) |
Cited By (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120071870A1 (en)* | 2008-11-11 | 2012-03-22 | Amr Salahieh | Low Profile Electrode Assembly |
| US20130110104A1 (en)* | 2011-10-26 | 2013-05-02 | Medtronic Ablation Frontiers Llc | Semi-circular pulmonary vein ablation catheter |
| US8612022B1 (en) | 2012-09-13 | 2013-12-17 | Invatec S.P.A. | Neuromodulation catheters and associated systems and methods |
| US8774913B2 (en) | 2002-04-08 | 2014-07-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravasculary-induced neuromodulation |
| US8834464B2 (en) | 1999-04-05 | 2014-09-16 | Mark T. Stewart | Ablation catheters and associated systems and methods |
| US8888773B2 (en) | 2012-05-11 | 2014-11-18 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US8934978B2 (en) | 2002-04-08 | 2015-01-13 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for renal neuromodulation |
| US8956352B2 (en) | 2010-10-25 | 2015-02-17 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having multi-electrode arrays for renal neuromodulation and associated systems and methods |
| US8992514B2 (en)* | 2012-02-24 | 2015-03-31 | Isolase, Ltd. | Ablation techniques for the treatment of atrial fibrillation |
| US9011431B2 (en) | 2009-01-12 | 2015-04-21 | Ethicon Endo-Surgery, Inc. | Electrical ablation devices |
| US9078662B2 (en) | 2012-07-03 | 2015-07-14 | Ethicon Endo-Surgery, Inc. | Endoscopic cap electrode and method for using the same |
| US9095321B2 (en) | 2012-11-21 | 2015-08-04 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices having integral multi-helical balloons and methods of making the same |
| WO2015089505A3 (en)* | 2013-12-13 | 2015-09-17 | The Trustees Of The University Of Pennsylvania | Coaxial ablation probe and method and system for real-time monitoring of ablation therapy |
| US9138281B2 (en) | 2002-04-08 | 2015-09-22 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for bilateral renal neuromodulation via catheter apparatuses having expandable baskets |
| US20150313669A1 (en)* | 2014-05-04 | 2015-11-05 | Diros Technology Inc. | Radiofrequency Probes with Retractable Multi-Tined Electrodes |
| US9179974B2 (en) | 2013-03-15 | 2015-11-10 | Medtronic Ardian Luxembourg S.A.R.L. | Helical push wire electrode |
| US9277957B2 (en) | 2012-08-15 | 2016-03-08 | Ethicon Endo-Surgery, Inc. | Electrosurgical devices and methods |
| WO2016040982A1 (en)* | 2014-09-15 | 2016-03-24 | Cathrx Ltd | An irrigated ablation catheter and process thereof |
| US9333031B2 (en) | 2013-04-08 | 2016-05-10 | Apama Medical, Inc. | Visualization inside an expandable medical device |
| US9375268B2 (en) | 2007-02-15 | 2016-06-28 | Ethicon Endo-Surgery, Inc. | Electroporation ablation apparatus, system, and method |
| US9427255B2 (en) | 2012-05-14 | 2016-08-30 | Ethicon Endo-Surgery, Inc. | Apparatus for introducing a steerable camera assembly into a patient |
| US9545290B2 (en) | 2012-07-30 | 2017-01-17 | Ethicon Endo-Surgery, Inc. | Needle probe guide |
| US9572623B2 (en) | 2012-08-02 | 2017-02-21 | Ethicon Endo-Surgery, Inc. | Reusable electrode and disposable sheath |
| US9579149B2 (en) | 2014-03-13 | 2017-02-28 | Medtronic Ardian Luxembourg S.A.R.L. | Low profile catheter assemblies and associated systems and methods |
| US9655677B2 (en) | 2010-05-12 | 2017-05-23 | Shifamed Holdings, Llc | Ablation catheters including a balloon and electrodes |
| US9707035B2 (en) | 2002-04-08 | 2017-07-18 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for catheter-based renal neuromodulation |
| US9795442B2 (en) | 2008-11-11 | 2017-10-24 | Shifamed Holdings, Llc | Ablation catheters |
| US9883910B2 (en) | 2011-03-17 | 2018-02-06 | Eticon Endo-Surgery, Inc. | Hand held surgical device for manipulating an internal magnet assembly within a patient |
| US9895073B2 (en)* | 2015-07-29 | 2018-02-20 | Biosense Webster (Israel) Ltd. | Dual basket catheter |
| EP3300660A1 (en) | 2016-09-29 | 2018-04-04 | Biosense Webster (Israel), Ltd. | Basket catheter conforming to organ using strain-relief elements |
| US9999461B2 (en) | 2011-12-09 | 2018-06-19 | Metavention, Inc. | Therapeutic denervation of nerves surrounding a hepatic vessel |
| US10098694B2 (en) | 2013-04-08 | 2018-10-16 | Apama Medical, Inc. | Tissue ablation and monitoring thereof |
| US10098527B2 (en) | 2013-02-27 | 2018-10-16 | Ethidcon Endo-Surgery, Inc. | System for performing a minimally invasive surgical procedure |
| US10098691B2 (en) | 2009-12-18 | 2018-10-16 | Ethicon Endo-Surgery, Inc. | Surgical instrument comprising an electrode |
| US10105141B2 (en) | 2008-07-14 | 2018-10-23 | Ethicon Endo-Surgery, Inc. | Tissue apposition clip application methods |
| US10258406B2 (en) | 2011-02-28 | 2019-04-16 | Ethicon Llc | Electrical ablation devices and methods |
| US10271899B2 (en) | 2015-03-18 | 2019-04-30 | Medtronic Cryocath Lp | Multi-function device with treatment and sensing capabilities |
| US10278761B2 (en) | 2011-02-28 | 2019-05-07 | Ethicon Llc | Electrical ablation devices and methods |
| US10314603B2 (en) | 2008-11-25 | 2019-06-11 | Ethicon Llc | Rotational coupling device for surgical instrument with flexible actuators |
| US10314649B2 (en) | 2012-08-02 | 2019-06-11 | Ethicon Endo-Surgery, Inc. | Flexible expandable electrode and method of intraluminal delivery of pulsed power |
| US10350004B2 (en) | 2004-12-09 | 2019-07-16 | Twelve, Inc. | Intravascular treatment catheters |
| US10349824B2 (en) | 2013-04-08 | 2019-07-16 | Apama Medical, Inc. | Tissue mapping and visualization systems |
| US10517667B2 (en) | 2014-05-16 | 2019-12-31 | Biosense Webster (Israel) Ltd. | Catheter tip with microelectrodes |
| US10524859B2 (en) | 2016-06-07 | 2020-01-07 | Metavention, Inc. | Therapeutic tissue modulation devices and methods |
| US10631927B2 (en) | 2013-12-23 | 2020-04-28 | Boaz Avitall | Small loop ablation catheter |
| US10736690B2 (en) | 2014-04-24 | 2020-08-11 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation catheters and associated systems and methods |
| US10736693B2 (en) | 2015-11-16 | 2020-08-11 | Apama Medical, Inc. | Energy delivery devices |
| US10779882B2 (en) | 2009-10-28 | 2020-09-22 | Ethicon Endo-Surgery, Inc. | Electrical ablation devices |
| US11213678B2 (en) | 2013-09-09 | 2022-01-04 | Medtronic Ardian Luxembourg S.A.R.L. | Method of manufacturing a medical device for neuromodulation |
| US11395697B2 (en) | 2018-11-14 | 2022-07-26 | Medtronic, Inc. | Devices and methods for preparing a valve for a transcatheter valve replacement procedure |
| US11737851B2 (en) | 2018-06-28 | 2023-08-29 | Cook Medical Technologies Llc | Medical devices for magnetic resonance imaging and related methods |
| US12011212B2 (en) | 2013-06-05 | 2024-06-18 | Medtronic Ireland Manufacturing Unlimited Company | Modulation of targeted nerve fibers |
| US12108983B2 (en) | 2019-05-03 | 2024-10-08 | Biosense Webster (Israel) Ltd. | Device, system and method to ablate cardiac tissue |
| US12408976B2 (en) | 2021-04-26 | 2025-09-09 | Pulse Biosciences, Inc. | Circumferential ablation devices and methods |
| US12408974B2 (en) | 2014-12-03 | 2025-09-09 | Medtronic Ireland Manufacturing Unlimited Company | Systems and methods for modulating nerves or other tissue |
Families Citing this family (779)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7027869B2 (en) | 1998-01-07 | 2006-04-11 | Asthmatx, Inc. | Method for treating an asthma attack |
| US7992572B2 (en) | 1998-06-10 | 2011-08-09 | Asthmatx, Inc. | Methods of evaluating individuals having reversible obstructive pulmonary disease |
| US6488673B1 (en)* | 1997-04-07 | 2002-12-03 | Broncus Technologies, Inc. | Method of increasing gas exchange of a lung |
| US6634363B1 (en) | 1997-04-07 | 2003-10-21 | Broncus Technologies, Inc. | Methods of treating lungs having reversible obstructive pulmonary disease |
| US7921855B2 (en) | 1998-01-07 | 2011-04-12 | Asthmatx, Inc. | Method for treating an asthma attack |
| US7198635B2 (en) | 2000-10-17 | 2007-04-03 | Asthmatx, Inc. | Modification of airways by application of energy |
| US8181656B2 (en)* | 1998-06-10 | 2012-05-22 | Asthmatx, Inc. | Methods for treating airways |
| US20040215235A1 (en) | 1999-11-16 | 2004-10-28 | Barrx, Inc. | Methods and systems for determining physiologic characteristics for treatment of the esophagus |
| US20060095032A1 (en) | 1999-11-16 | 2006-05-04 | Jerome Jackson | Methods and systems for determining physiologic characteristics for treatment of the esophagus |
| US8251070B2 (en) | 2000-03-27 | 2012-08-28 | Asthmatx, Inc. | Methods for treating airways |
| US7104987B2 (en) | 2000-10-17 | 2006-09-12 | Asthmatx, Inc. | Control system and process for application of energy to airway walls and other mediums |
| US8974446B2 (en) | 2001-10-11 | 2015-03-10 | St. Jude Medical, Inc. | Ultrasound ablation apparatus with discrete staggered ablation zones |
| US8347891B2 (en)* | 2002-04-08 | 2013-01-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for performing a non-continuous circumferential treatment of a body lumen |
| US6907884B2 (en) | 2002-09-30 | 2005-06-21 | Depay Acromed, Inc. | Method of straddling an intraosseous nerve |
| US8361067B2 (en) | 2002-09-30 | 2013-01-29 | Relievant Medsystems, Inc. | Methods of therapeutically heating a vertebral body to treat back pain |
| US7258690B2 (en) | 2003-03-28 | 2007-08-21 | Relievant Medsystems, Inc. | Windowed thermal ablation probe |
| US7115127B2 (en)* | 2003-02-04 | 2006-10-03 | Cardiodex, Ltd. | Methods and apparatus for hemostasis following arterial catheterization |
| US7223266B2 (en) | 2003-02-04 | 2007-05-29 | Cardiodex Ltd. | Methods and apparatus for hemostasis following arterial catheterization |
| US10413211B2 (en) | 2003-02-21 | 2019-09-17 | 3Dt Holdings, Llc | Systems, devices, and methods for mapping organ profiles |
| US10172538B2 (en) | 2003-02-21 | 2019-01-08 | 3Dt Holdings, Llc | Body lumen junction localization |
| US8078274B2 (en) | 2003-02-21 | 2011-12-13 | Dtherapeutics, Llc | Device, system and method for measuring cross-sectional areas in luminal organs |
| US7818053B2 (en) | 2003-02-21 | 2010-10-19 | Dtherapeutics, Llc | Devices, systems and methods for plaque type determination |
| US8632469B2 (en)* | 2003-02-21 | 2014-01-21 | 3Dt Holdings, Llc | Devices, systems, and methods for mapping organ profiles |
| US20040226556A1 (en) | 2003-05-13 | 2004-11-18 | Deem Mark E. | Apparatus for treating asthma using neurotoxin |
| DE202004021953U1 (en) | 2003-09-12 | 2013-06-19 | Vessix Vascular, Inc. | Selectable eccentric remodeling and / or ablation of atherosclerotic material |
| US9713730B2 (en) | 2004-09-10 | 2017-07-25 | Boston Scientific Scimed, Inc. | Apparatus and method for treatment of in-stent restenosis |
| US8396548B2 (en) | 2008-11-14 | 2013-03-12 | Vessix Vascular, Inc. | Selective drug delivery in a lumen |
| US8750983B2 (en) | 2004-09-20 | 2014-06-10 | P Tech, Llc | Therapeutic system |
| US20060089637A1 (en) | 2004-10-14 | 2006-04-27 | Werneth Randell L | Ablation catheter |
| US8617152B2 (en) | 2004-11-15 | 2013-12-31 | Medtronic Ablation Frontiers Llc | Ablation system with feedback |
| EP1814478A4 (en) | 2004-11-22 | 2011-05-18 | Cardiodex Ltd | Techniques for heat-treating varicose veins |
| US7429261B2 (en) | 2004-11-24 | 2008-09-30 | Ablation Frontiers, Inc. | Atrial ablation catheter and method of use |
| US7468062B2 (en) | 2004-11-24 | 2008-12-23 | Ablation Frontiers, Inc. | Atrial ablation catheter adapted for treatment of septal wall arrhythmogenic foci and method of use |
| EP2759276A1 (en) | 2005-06-20 | 2014-07-30 | Medtronic Ablation Frontiers LLC | Ablation catheter |
| AU2006268238A1 (en) | 2005-07-11 | 2007-01-18 | Medtronic Ablation Frontiers Llc | Low power tissue ablation system |
| US12433837B2 (en) | 2005-07-22 | 2025-10-07 | The Foundry, Llc | Systems and methods for delivery of a therapeutic agent |
| EP1906923B1 (en) | 2005-07-22 | 2018-01-24 | The Foundry, LLC | Systems and methods for delivery of a therapeutic agent |
| US8657814B2 (en) | 2005-08-22 | 2014-02-25 | Medtronic Ablation Frontiers Llc | User interface for tissue ablation system |
| EP1951129B1 (en) | 2005-10-19 | 2012-11-21 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| US8702694B2 (en) | 2005-11-23 | 2014-04-22 | Covidien Lp | Auto-aligning ablating device and method of use |
| US7997278B2 (en) | 2005-11-23 | 2011-08-16 | Barrx Medical, Inc. | Precision ablating method |
| US7959627B2 (en) | 2005-11-23 | 2011-06-14 | Barrx Medical, Inc. | Precision ablating device |
| US9480552B2 (en)* | 2006-04-26 | 2016-11-01 | The Cleveland Clinic Foundation | Apparatus and method for treating cardiovascular diseases |
| US8019435B2 (en) | 2006-05-02 | 2011-09-13 | Boston Scientific Scimed, Inc. | Control of arterial smooth muscle tone |
| US7729752B2 (en) | 2006-06-13 | 2010-06-01 | Rhythmia Medical, Inc. | Non-contact cardiac mapping, including resolution map |
| US7515954B2 (en) | 2006-06-13 | 2009-04-07 | Rhythmia Medical, Inc. | Non-contact cardiac mapping, including moving catheter and multi-beat integration |
| CN103222894B (en)* | 2006-06-28 | 2015-07-01 | 美敦力Af卢森堡公司 | Methods and systems for thermally-induced renal neuromodulation |
| WO2008014629A2 (en) | 2006-08-03 | 2008-02-07 | Christoph Scharf | Method and device for determining and presenting surface charge and dipole densities on cardiac walls |
| EP2455036B1 (en) | 2006-10-18 | 2015-07-15 | Vessix Vascular, Inc. | Tuned RF energy and electrical tissue characterization for selective treatment of target tissues |
| JP5559539B2 (en) | 2006-10-18 | 2014-07-23 | べシックス・バスキュラー・インコーポレイテッド | System that induces desirable temperature effects on body tissue |
| EP2076198A4 (en) | 2006-10-18 | 2009-12-09 | Minnow Medical Inc | Inducing desirable temperature effects on body tissue |
| EP2120759B1 (en) | 2007-02-15 | 2017-07-19 | Cardionova Ltd. | Intra-atrial apparatus |
| US7867227B2 (en) | 2007-02-22 | 2011-01-11 | A David Slater | Bipolar cardiac ablation system and method |
| US8641711B2 (en) | 2007-05-04 | 2014-02-04 | Covidien Lp | Method and apparatus for gastrointestinal tract ablation for treatment of obesity |
| US8588885B2 (en) | 2007-05-09 | 2013-11-19 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Bendable catheter arms having varied flexibility |
| US8224416B2 (en)* | 2007-05-09 | 2012-07-17 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Basket catheter having multiple electrodes |
| US8641704B2 (en) | 2007-05-11 | 2014-02-04 | Medtronic Ablation Frontiers Llc | Ablation therapy system and method for treating continuous atrial fibrillation |
| US10492729B2 (en) | 2007-05-23 | 2019-12-03 | St. Jude Medical, Cardiology Division, Inc. | Flexible high-density mapping catheter tips and flexible ablation catheter tips with onboard high-density mapping electrodes |
| ITBA20070049A1 (en)* | 2007-06-14 | 2008-12-15 | Massimo Grimaldi | CATHETERS FOR ABLATION TRANSCATETER BY PERCUTANEOUS ROUTE OF HEART ARITHMIA THROUGH BIPOLAR RADIOFREQUENCY |
| US8784338B2 (en) | 2007-06-22 | 2014-07-22 | Covidien Lp | Electrical means to normalize ablational energy transmission to a luminal tissue surface of varying size |
| WO2009009443A1 (en) | 2007-07-06 | 2009-01-15 | Barrx Medical, Inc. | Method and apparatus for gastrointestinal tract ablation to achieve loss of persistent and/or recurrent excess body weight following a weight-loss operation |
| CN102688092B (en) | 2007-07-06 | 2015-04-22 | 柯惠有限合伙公司 | Ablation in the gastrointestinal tract to achieve hemostasis and eradicate lesions with a propensity for bleeding |
| US20100049189A1 (en)* | 2007-07-22 | 2010-02-25 | Duane Dickens | Device and method for treating annular organ structure |
| US8273012B2 (en) | 2007-07-30 | 2012-09-25 | Tyco Healthcare Group, Lp | Cleaning device and methods |
| US8646460B2 (en) | 2007-07-30 | 2014-02-11 | Covidien Lp | Cleaning device and methods |
| JP4925210B2 (en)* | 2007-09-29 | 2012-04-25 | 日本ライフライン株式会社 | Electrode catheter |
| WO2009022537A1 (en)* | 2007-08-11 | 2009-02-19 | Japan Lifeline Co., Ltd. | Electrode catheter |
| JP4925206B2 (en)* | 2007-08-11 | 2012-04-25 | 日本ライフライン株式会社 | Electrode catheter |
| EP2182875A4 (en)* | 2007-08-15 | 2011-08-24 | Cardiodex Ltd | Systems and methods for puncture closure |
| JP5618332B2 (en)* | 2007-09-26 | 2014-11-05 | レトロヴァスキュラー・インコーポレーテッド | Occluded vascular recanalization using high frequency energy |
| US10398393B2 (en) | 2007-10-02 | 2019-09-03 | Stryker European Holdings I, Llc | Dynamic reference method and system for interventional procedures |
| US8315690B2 (en)* | 2007-10-02 | 2012-11-20 | General Electric Company | Dynamic reference method and system for interventional procedures |
| US20090093808A1 (en)* | 2007-10-03 | 2009-04-09 | Gregg A. Vandusseldorp & Associates, Inc. | Partial (non-apical) prostate ablation procedure and device |
| US9474571B2 (en)* | 2007-10-15 | 2016-10-25 | Boston Scientific Scimed, Inc. | Percutaneous tissue ablation probe with occlusive bodies |
| US20090124927A1 (en)* | 2007-11-13 | 2009-05-14 | Chest Innovations, Inc. | Endoscopic system for lung biopsy and biopsy method of insufflating gas to collapse a lung |
| US8906011B2 (en) | 2007-11-16 | 2014-12-09 | Kardium Inc. | Medical device for use in bodily lumens, for example an atrium |
| EP2231060B1 (en)* | 2007-12-10 | 2015-05-27 | Medtronic Ablation Frontiers LLC | Ablation catheter |
| US10660690B2 (en) | 2007-12-28 | 2020-05-26 | St. Jude Medical, Atrial Fibrillation Division, Inc. | System and method for measurement of an impedance using a catheter such as an ablation catheter |
| US8103327B2 (en)* | 2007-12-28 | 2012-01-24 | Rhythmia Medical, Inc. | Cardiac mapping catheter |
| EP2737849A3 (en) | 2008-01-17 | 2014-10-29 | Christoph Scharf | A device and method for the geometric determination of electrical dipole densities on the cardiac wall |
| US8483831B1 (en) | 2008-02-15 | 2013-07-09 | Holaira, Inc. | System and method for bronchial dilation |
| WO2009117523A2 (en) | 2008-03-18 | 2009-09-24 | Circa Medical, Llc | Large surface area temperature sensing device |
| US9833149B2 (en) | 2008-03-18 | 2017-12-05 | Circa Scientific, Llc | Methods, apparatus and systems for facilitating introduction of shaped medical instruments into the body of a subject |
| US8538509B2 (en) | 2008-04-02 | 2013-09-17 | Rhythmia Medical, Inc. | Intracardiac tracking system |
| JP2011519699A (en) | 2008-05-09 | 2011-07-14 | インノブアトイブエ プルモナルイ ソルウトイオンス,インコーポレイティッド | Systems, assemblies and methods for treatment of bronchial trees |
| US8128617B2 (en)* | 2008-05-27 | 2012-03-06 | Boston Scientific Scimed, Inc. | Electrical mapping and cryo ablating with a balloon catheter |
| US8882761B2 (en)* | 2008-07-15 | 2014-11-11 | Catheffects, Inc. | Catheter and method for improved ablation |
| US9402707B2 (en) | 2008-07-22 | 2016-08-02 | Neuravi Limited | Clot capture systems and associated methods |
| WO2010028314A1 (en) | 2008-09-05 | 2010-03-11 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US10028753B2 (en) | 2008-09-26 | 2018-07-24 | Relievant Medsystems, Inc. | Spine treatment kits |
| CA2737374C (en) | 2008-09-26 | 2017-03-28 | Relievant Medsystems, Inc. | Systems and methods for navigating an instrument through bone |
| WO2010039443A1 (en)* | 2008-10-04 | 2010-04-08 | Boston Scientific Scimed, Inc. | Loop structures for supporting diagnostic and/or therapeutic elements in contact with tissue |
| US10695126B2 (en) | 2008-10-06 | 2020-06-30 | Santa Anna Tech Llc | Catheter with a double balloon structure to generate and apply a heated ablative zone to tissue |
| US8137343B2 (en) | 2008-10-27 | 2012-03-20 | Rhythmia Medical, Inc. | Tracking system using field mapping |
| US9044618B2 (en) | 2008-11-05 | 2015-06-02 | Shockwave Medical, Inc. | Shockwave valvuloplasty catheter system |
| US8295902B2 (en)* | 2008-11-11 | 2012-10-23 | Shifamed Holdings, Llc | Low profile electrode assembly |
| EP2355737B1 (en) | 2008-11-17 | 2021-08-11 | Boston Scientific Scimed, Inc. | Selective accumulation of energy without knowledge of tissue topography |
| US20100160906A1 (en)* | 2008-12-23 | 2010-06-24 | Asthmatx, Inc. | Expandable energy delivery devices having flexible conductive elements and associated systems and methods |
| US9339331B2 (en)* | 2008-12-29 | 2016-05-17 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Non-contact electrode basket catheters with irrigation |
| US8600472B2 (en)* | 2008-12-30 | 2013-12-03 | Biosense Webster (Israel), Ltd. | Dual-purpose lasso catheter with irrigation using circumferentially arranged ring bump electrodes |
| US20100274088A1 (en)* | 2009-04-23 | 2010-10-28 | Carl Frederic West | Flexible Medical Instrument |
| CA2703347C (en) | 2009-05-08 | 2016-10-04 | Endosense Sa | Method and apparatus for controlling lesion size in catheter-based ablation treatment |
| US9393068B1 (en) | 2009-05-08 | 2016-07-19 | St. Jude Medical International Holding S.À R.L. | Method for predicting the probability of steam pop in RF ablation therapy |
| DK3117784T3 (en) | 2009-07-08 | 2019-04-08 | Sanuwave Inc | USE OF INTRACORPORAL PRESSURE SHOCK WAVES IN MEDICINE |
| US8280477B2 (en)* | 2009-07-29 | 2012-10-02 | Medtronic Cryocath Lp | Mono-phasic action potential electrogram recording catheter, and method |
| EP2932999A1 (en)* | 2009-07-29 | 2015-10-21 | Medtronic Ablation Frontiers LLC | Mono-phasic action potential electrogram recording catheter |
| US20110028962A1 (en)* | 2009-07-31 | 2011-02-03 | Randell Werneth | Adjustable pulmonary vein ablation catheter |
| WO2011056684A2 (en) | 2009-10-27 | 2011-05-12 | Innovative Pulmonary Solutions, Inc. | Delivery devices with coolable energy emitting assemblies |
| US20110098694A1 (en)* | 2009-10-28 | 2011-04-28 | Ethicon Endo-Surgery, Inc. | Methods and instruments for treating cardiac tissue through a natural orifice |
| WO2011060200A1 (en) | 2009-11-11 | 2011-05-19 | Innovative Pulmonary Solutions, Inc. | Systems, apparatuses, and methods for treating tissue and controlling stenosis |
| US8911439B2 (en) | 2009-11-11 | 2014-12-16 | Holaira, Inc. | Non-invasive and minimally invasive denervation methods and systems for performing the same |
| JP6013186B2 (en) | 2009-11-13 | 2016-10-25 | セント ジュード メディカル インコーポレイテッド | Staggered arrangement of shochu elements |
| US8469953B2 (en) | 2009-11-16 | 2013-06-25 | Covidien Lp | Twin sealing chamber hub |
| US20110144637A1 (en)* | 2009-12-11 | 2011-06-16 | Medtronic Cryocath Lp | Vein Occlusion Devices and Methods for Catheter-Based Ablation |
| US9861438B2 (en) | 2009-12-11 | 2018-01-09 | Biosense Webster (Israel), Ltd. | Pre-formed curved ablation catheter |
| US8906013B2 (en) | 2010-04-09 | 2014-12-09 | Endosense Sa | Control handle for a contact force ablation catheter |
| WO2011126580A2 (en) | 2010-04-09 | 2011-10-13 | Minnow Medical, Inc. | Power generating and control apparatus for the treatment of tissue |
| CA2796347A1 (en) | 2010-04-13 | 2011-10-20 | Sentreheart, Inc. | Methods and devices for pericardial access |
| AU2011241103A1 (en)* | 2010-04-13 | 2012-11-08 | Sentreheart, Inc. | Methods and devices for treating atrial fibrillation |
| US9192790B2 (en) | 2010-04-14 | 2015-11-24 | Boston Scientific Scimed, Inc. | Focused ultrasonic renal denervation |
| US10220134B2 (en) | 2010-04-23 | 2019-03-05 | Mark D. Wieczorek | Transseptal access device and method of use |
| US11419632B2 (en) | 2010-04-23 | 2022-08-23 | Mark D. Wieczorek, P.C. | Transseptal access device and method of use |
| WO2011133977A2 (en) | 2010-04-23 | 2011-10-27 | Assist Medical Llc | Transseptal access device and method of use |
| EP2563255B1 (en)* | 2010-04-26 | 2017-03-22 | Medtronic Ardian Luxembourg S.à.r.l. | Catheter apparatuses and systems for renal neuromodulation |
| US8845631B2 (en)* | 2010-04-28 | 2014-09-30 | Medtronic Ablation Frontiers Llc | Systems and methods of performing medical procedures |
| EP4257065A3 (en)* | 2010-05-05 | 2023-12-27 | ElectroPhysiology Frontiers S.p.A. | Anchored cardiac ablation catheter |
| US9918787B2 (en) | 2010-05-05 | 2018-03-20 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Monitoring, managing and/or protecting system and method for non-targeted tissue |
| US8694074B2 (en) | 2010-05-11 | 2014-04-08 | Rhythmia Medical, Inc. | Electrode displacement determination |
| US9744339B2 (en) | 2010-05-12 | 2017-08-29 | Circa Scientific, Llc | Apparatus for manually manipulating hollow organs |
| US8473067B2 (en) | 2010-06-11 | 2013-06-25 | Boston Scientific Scimed, Inc. | Renal denervation and stimulation employing wireless vascular energy transfer arrangement |
| DE102010026210A1 (en)* | 2010-07-06 | 2012-01-12 | Wolfgang Seidel | Method and device for coagulating body tissue and / or body vessels |
| US9084609B2 (en) | 2010-07-30 | 2015-07-21 | Boston Scientific Scime, Inc. | Spiral balloon catheter for renal nerve ablation |
| US9408661B2 (en)* | 2010-07-30 | 2016-08-09 | Patrick A. Haverkost | RF electrodes on multiple flexible wires for renal nerve ablation |
| US9463062B2 (en) | 2010-07-30 | 2016-10-11 | Boston Scientific Scimed, Inc. | Cooled conductive balloon RF catheter for renal nerve ablation |
| US9358365B2 (en) | 2010-07-30 | 2016-06-07 | Boston Scientific Scimed, Inc. | Precision electrode movement control for renal nerve ablation |
| US9155589B2 (en) | 2010-07-30 | 2015-10-13 | Boston Scientific Scimed, Inc. | Sequential activation RF electrode set for renal nerve ablation |
| US9539046B2 (en)* | 2010-08-03 | 2017-01-10 | Medtronic Cryocath Lp | Cryogenic medical mapping and treatment device |
| WO2012030393A1 (en)* | 2010-08-31 | 2012-03-08 | Synecor Llc | Intravascular electrodes and anchoring devices for transvascular stimulation |
| CN102429721B (en)* | 2010-09-29 | 2015-08-26 | 心诺普医疗技术(北京)有限公司 | A kind of ablation system |
| US9566456B2 (en) | 2010-10-18 | 2017-02-14 | CardioSonic Ltd. | Ultrasound transceiver and cooling thereof |
| EP2629682A1 (en)* | 2010-10-18 | 2013-08-28 | Cardiosonic Ltd. | Separation device for ultrasound element |
| US8585601B2 (en) | 2010-10-18 | 2013-11-19 | CardioSonic Ltd. | Ultrasound transducer |
| WO2012052925A1 (en) | 2010-10-18 | 2012-04-26 | CardioSonic Ltd. | An ultrasound transceiver and control of a thermal damage process |
| US9028417B2 (en) | 2010-10-18 | 2015-05-12 | CardioSonic Ltd. | Ultrasound emission element |
| US9084610B2 (en) | 2010-10-21 | 2015-07-21 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses, systems, and methods for renal neuromodulation |
| ES2683943T3 (en) | 2010-10-22 | 2018-09-28 | Neuravi Limited | Clot capture and removal system |
| US8974451B2 (en) | 2010-10-25 | 2015-03-10 | Boston Scientific Scimed, Inc. | Renal nerve ablation using conductive fluid jet and RF energy |
| WO2012061150A1 (en) | 2010-10-25 | 2012-05-10 | Medtronic Ardian Luxembourg S.a.r.I. | Microwave catheter apparatuses, systems, and methods for renal neuromodulation |
| US9005197B2 (en)* | 2010-10-26 | 2015-04-14 | Cook Medical Technologies Llc | Medical instrument for ablating tissue |
| US9220558B2 (en) | 2010-10-27 | 2015-12-29 | Boston Scientific Scimed, Inc. | RF renal denervation catheter with multiple independent electrodes |
| JP2014501551A (en)* | 2010-10-28 | 2014-01-23 | クック メディカル テクノロジーズ エルエルシー | Ablation device |
| US9028485B2 (en) | 2010-11-15 | 2015-05-12 | Boston Scientific Scimed, Inc. | Self-expanding cooling electrode for renal nerve ablation |
| US9089350B2 (en) | 2010-11-16 | 2015-07-28 | Boston Scientific Scimed, Inc. | Renal denervation catheter with RF electrode and integral contrast dye injection arrangement |
| US9668811B2 (en) | 2010-11-16 | 2017-06-06 | Boston Scientific Scimed, Inc. | Minimally invasive access for renal nerve ablation |
| EP4032486A1 (en) | 2010-11-16 | 2022-07-27 | TVA Medical, Inc. | Devices for forming a fistula |
| US9326751B2 (en) | 2010-11-17 | 2016-05-03 | Boston Scientific Scimed, Inc. | Catheter guidance of external energy for renal denervation |
| US9060761B2 (en) | 2010-11-18 | 2015-06-23 | Boston Scientific Scime, Inc. | Catheter-focused magnetic field induced renal nerve ablation |
| US9023034B2 (en) | 2010-11-22 | 2015-05-05 | Boston Scientific Scimed, Inc. | Renal ablation electrode with force-activatable conduction apparatus |
| US9192435B2 (en) | 2010-11-22 | 2015-11-24 | Boston Scientific Scimed, Inc. | Renal denervation catheter with cooled RF electrode |
| US8560086B2 (en) | 2010-12-02 | 2013-10-15 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Catheter electrode assemblies and methods of construction therefor |
| US20120157993A1 (en) | 2010-12-15 | 2012-06-21 | Jenson Mark L | Bipolar Off-Wall Electrode Device for Renal Nerve Ablation |
| EP3482708B1 (en) | 2010-12-27 | 2021-03-10 | St. Jude Medical International Holding S.à r.l. | Prediction of atrial wall electrical reconnection based on contact force measured duing rf ablation |
| US9044245B2 (en)* | 2011-01-05 | 2015-06-02 | Medtronic Ablation Frontiers Llc | Multipolarity epicardial radiofrequency ablation |
| US9220561B2 (en) | 2011-01-19 | 2015-12-29 | Boston Scientific Scimed, Inc. | Guide-compatible large-electrode catheter for renal nerve ablation with reduced arterial injury |
| US11259867B2 (en) | 2011-01-21 | 2022-03-01 | Kardium Inc. | High-density electrode-based medical device system |
| CA2764494A1 (en) | 2011-01-21 | 2012-07-21 | Kardium Inc. | Enhanced medical device for use in bodily cavities, for example an atrium |
| US9480525B2 (en) | 2011-01-21 | 2016-11-01 | Kardium, Inc. | High-density electrode-based medical device system |
| US9452016B2 (en) | 2011-01-21 | 2016-09-27 | Kardium Inc. | Catheter system |
| EP2667812A1 (en) | 2011-01-28 | 2013-12-04 | Medtronic Ardian Luxembourg S.à.r.l. | Ablation catheter equipped with a shape memory material |
| US11259824B2 (en) | 2011-03-09 | 2022-03-01 | Neuravi Limited | Clot retrieval device for removing occlusive clot from a blood vessel |
| EP4566553A3 (en) | 2011-03-09 | 2025-08-06 | Neuravi Limited | A clot retrieval device for removing occlusive clot from a blood vessel |
| US12076037B2 (en) | 2011-03-09 | 2024-09-03 | Neuravi Limited | Systems and methods to restore perfusion to a vessel |
| US9757044B2 (en) | 2011-03-10 | 2017-09-12 | Acutus Medical, Inc. | Device and method for the geometric determination of electrical dipole densities on the cardiac wall |
| US10278774B2 (en) | 2011-03-18 | 2019-05-07 | Covidien Lp | Selectively expandable operative element support structure and methods of use |
| WO2012134911A1 (en)* | 2011-03-25 | 2012-10-04 | Medtronic Ablation Frontiers Llc | Cooling systems for electrode arrays |
| EP2699153B1 (en) | 2011-04-22 | 2015-12-16 | Topera, Inc. | Flexible electrode assembly for insertion into body lumen or organ |
| WO2012149167A2 (en) | 2011-04-26 | 2012-11-01 | Christopher Gerard Kunis | Method and device for treatment of hypertension and other maladies |
| CN102198015B (en)* | 2011-05-03 | 2013-11-06 | 上海微创电生理医疗科技有限公司 | Retractable spiral laminar ring type electrode catheter |
| US9198706B2 (en)* | 2011-05-12 | 2015-12-01 | Cvdevices, Llc | Systems and methods for cryoblation of a tissue |
| US8909316B2 (en) | 2011-05-18 | 2014-12-09 | St. Jude Medical, Cardiology Division, Inc. | Apparatus and method of assessing transvascular denervation |
| CN103582460B (en) | 2011-06-03 | 2019-03-19 | 帕尔萨维斯库勒公司 | Aneurysm devices and related systems and methods with additional anchoring mechanism |
| US20240050143A1 (en) | 2011-06-14 | 2024-02-15 | Aerin Medical Inc. | Methods and devices to treat nasal airways |
| US11304746B2 (en) | 2011-06-14 | 2022-04-19 | Aerin Medical Inc. | Method of treating airway tissue to reduce mucus secretion |
| US9237924B2 (en) | 2011-06-14 | 2016-01-19 | Aerin Medical, Inc. | Methods and devices to treat nasal airways |
| JP5948412B2 (en)* | 2011-06-21 | 2016-07-06 | クック・メディカル・テクノロジーズ・リミテッド・ライアビリティ・カンパニーCook Medical Technologies Llc | Expandable probe and instrument including it |
| US9220433B2 (en) | 2011-06-30 | 2015-12-29 | Biosense Webster (Israel), Ltd. | Catheter with variable arcuate distal section |
| EP2701790B1 (en)* | 2011-07-11 | 2016-04-20 | Interventional Autonomics Corporation | Intravascular electrodes and anchoring devices for transvascular stimulation |
| US9492113B2 (en) | 2011-07-15 | 2016-11-15 | Boston Scientific Scimed, Inc. | Systems and methods for monitoring organ activity |
| CN103813745B (en) | 2011-07-20 | 2016-06-29 | 波士顿科学西美德公司 | In order to visualize, be directed at and to melt transcutaneous device and the method for nerve |
| EP2734264B1 (en) | 2011-07-22 | 2018-11-21 | Boston Scientific Scimed, Inc. | Nerve modulation system with a nerve modulation element positionable in a helical guide |
| JP6318088B2 (en) | 2011-07-26 | 2018-04-25 | アンフォラ メディカル, インコーポレイテッド | Apparatus and method for modulating pelvic nerve tissue |
| US9662169B2 (en)* | 2011-07-30 | 2017-05-30 | Biosense Webster (Israel) Ltd. | Catheter with flow balancing valve |
| EP2792322B1 (en) | 2011-08-25 | 2017-10-04 | Covidien LP | Systems and devices for treatment of luminal tissue |
| US11413062B2 (en) | 2011-09-13 | 2022-08-16 | Venturemed Group, Inc. | Methods for preparing a zone of attention within a vascular system for subsequent angioplasty with an intravascular catheter device having an expandable incising portion and an integrated embolic protection device |
| US11357533B2 (en) | 2011-09-13 | 2022-06-14 | Venturemed Group, Inc. | Intravascular catheter having an expandable incising portion and abrasive surfaces |
| US10610255B2 (en) | 2011-09-13 | 2020-04-07 | John P. Pigott | Intravascular catheter having an expandable incising portion and medication delivery system |
| EP2755714B1 (en) | 2011-09-13 | 2020-03-11 | Pigott, John, P. | Intravascular catheter having an expandable incising portion |
| US11559325B2 (en) | 2011-09-13 | 2023-01-24 | Venturemed Group, Inc. | Intravascular catheter having an expandable incising portion and grating tool |
| US10463387B2 (en) | 2011-09-13 | 2019-11-05 | John P. Pigott | Intravascular catheter having an expandable incising portion for incising atherosclerotic material located in a blood vessel |
| US12324603B2 (en) | 2011-09-13 | 2025-06-10 | Venturemed Group, Inc. | Intravascular catheter having an expandable incising portion |
| US9351790B2 (en) | 2011-09-17 | 2016-05-31 | M.O.E. Medical Devices Llc | Electrode geometries and method for applying electric field treatment to parts of the body |
| US9427579B2 (en) | 2011-09-29 | 2016-08-30 | Pacesetter, Inc. | System and method for performing renal denervation verification |
| US8498686B2 (en)* | 2011-10-04 | 2013-07-30 | Biosense Webster (Israel), Ltd. | Mapping catheter with spiral electrode assembly |
| US9119625B2 (en) | 2011-10-05 | 2015-09-01 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| WO2013052852A1 (en) | 2011-10-07 | 2013-04-11 | Boston Scientific Scimed, Inc. | Methods and systems for detection and thermal treatment of lower urinary tract conditions |
| WO2013052848A1 (en)* | 2011-10-07 | 2013-04-11 | Boston Scientific Scimed, Inc. | Methods for detection and thermal treatment of lower urinary tract conditions |
| WO2013055826A1 (en) | 2011-10-10 | 2013-04-18 | Boston Scientific Scimed, Inc. | Medical devices including ablation electrodes |
| EP2765940B1 (en) | 2011-10-11 | 2015-08-26 | Boston Scientific Scimed, Inc. | Off-wall electrode device for nerve modulation |
| US9420955B2 (en) | 2011-10-11 | 2016-08-23 | Boston Scientific Scimed, Inc. | Intravascular temperature monitoring system and method |
| US9364284B2 (en) | 2011-10-12 | 2016-06-14 | Boston Scientific Scimed, Inc. | Method of making an off-wall spacer cage |
| US9162046B2 (en) | 2011-10-18 | 2015-10-20 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| EP2768568B1 (en) | 2011-10-18 | 2020-05-06 | Boston Scientific Scimed, Inc. | Integrated crossing balloon catheter |
| CN102462532B (en)* | 2011-10-25 | 2013-03-13 | 张石江 | Conduit system with label testing and ablation functions in blood vessels |
| US8951251B2 (en) | 2011-11-08 | 2015-02-10 | Boston Scientific Scimed, Inc. | Ostial renal nerve ablation |
| WO2013074813A1 (en) | 2011-11-15 | 2013-05-23 | Boston Scientific Scimed, Inc. | Device and methods for renal nerve modulation monitoring |
| US9119632B2 (en) | 2011-11-21 | 2015-09-01 | Boston Scientific Scimed, Inc. | Deflectable renal nerve ablation catheter |
| US9192766B2 (en) | 2011-12-02 | 2015-11-24 | Medtronic Ardian Luxembourg S.A.R.L. | Renal neuromodulation methods and devices for treatment of polycystic kidney disease |
| CN102488552B (en)* | 2011-12-15 | 2015-04-15 | 四川锦江电子科技有限公司 | Manageable spiral electrophysiology catheter |
| US9265969B2 (en) | 2011-12-21 | 2016-02-23 | Cardiac Pacemakers, Inc. | Methods for modulating cell function |
| US9028472B2 (en) | 2011-12-23 | 2015-05-12 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| EP2797534A1 (en) | 2011-12-28 | 2014-11-05 | Boston Scientific Scimed, Inc. | Device and methods for nerve modulation using a novel ablation catheter with polymeric ablative elements |
| US9050106B2 (en) | 2011-12-29 | 2015-06-09 | Boston Scientific Scimed, Inc. | Off-wall electrode device and methods for nerve modulation |
| AU2012362524B2 (en) | 2011-12-30 | 2018-12-13 | Relievant Medsystems, Inc. | Systems and methods for treating back pain |
| JP6317927B2 (en) | 2012-01-09 | 2018-04-25 | ムー・メディカル・デバイスズ・エルエルシーMoe Medical Devices Llc | Plasma assisted skin treatment |
| CA2862862C (en)* | 2012-01-26 | 2022-06-14 | Robert Schwartz | Controlled sympathectomy and micro-ablation systems and methods |
| US9649064B2 (en) | 2012-01-26 | 2017-05-16 | Autonomix Medical, Inc. | Controlled sympathectomy and micro-ablation systems and methods |
| US9168080B2 (en) | 2012-01-27 | 2015-10-27 | Medtronic Cryocath Lp | Balloon catheter |
| US9144449B2 (en) | 2012-03-02 | 2015-09-29 | Csa Medical, Inc. | Cryosurgery system |
| AU2013230781B2 (en) | 2012-03-08 | 2015-12-03 | Medtronic Af Luxembourg S.A.R.L. | Ovarian neuromodulation and associated systems and methods |
| US8934988B2 (en) | 2012-03-16 | 2015-01-13 | St. Jude Medical Ab | Ablation stent with meander structure |
| US10159531B2 (en) | 2012-04-05 | 2018-12-25 | C. R. Bard, Inc. | Apparatus and methods relating to intravascular positioning of distal end of catheter |
| JP2015516846A (en) | 2012-04-05 | 2015-06-18 | バード・アクセス・システムズ,インコーポレーテッド | Device and system for navigating and positioning a central venous catheter within a patient |
| US11759268B2 (en) | 2012-04-05 | 2023-09-19 | C. R. Bard, Inc. | Apparatus and methods relating to intravascular positioning of distal end of catheter |
| US10357304B2 (en) | 2012-04-18 | 2019-07-23 | CardioSonic Ltd. | Tissue treatment |
| US9113929B2 (en) | 2012-04-19 | 2015-08-25 | St. Jude Medical, Cardiology Division, Inc. | Non-electric field renal denervation electrode |
| ITMI20120651A1 (en)* | 2012-04-19 | 2013-10-20 | Stefano Bianchi | SYSTEM FOR THE ABLATION OF CARDIAC FABRIC, IN PARTICULAR OF ATRIAL FABRIC |
| US20140207136A1 (en)* | 2012-05-04 | 2014-07-24 | St. Jude Medical, Inc. | Multiple staggered electrodes connected via flexible joints |
| US9408662B2 (en) | 2012-05-07 | 2016-08-09 | Cook Medical Technologies Llc | Sphincterotome having expandable tines |
| US10660703B2 (en) | 2012-05-08 | 2020-05-26 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices |
| US9439722B2 (en)* | 2012-05-09 | 2016-09-13 | Biosense Webster (Israel) Ltd. | Ablation targeting nerves in or near the inferior vena cava and/or abdominal aorta for treatment of hypertension |
| US11357447B2 (en) | 2012-05-31 | 2022-06-14 | Sonivie Ltd. | Method and/or apparatus for measuring renal denervation effectiveness |
| WO2013184319A1 (en) | 2012-06-04 | 2013-12-12 | Boston Scientific Scimed, Inc. | Systems and methods for treating tissue of a passageway within a body |
| KR101450041B1 (en)* | 2012-06-21 | 2014-10-15 | 주식회사 두배시스템 | Centering tool for a medical catheter |
| US9642673B2 (en)* | 2012-06-27 | 2017-05-09 | Shockwave Medical, Inc. | Shock wave balloon catheter with multiple shock wave sources |
| CN103536352A (en)* | 2012-07-09 | 2014-01-29 | 李莉 | Balloon expanding renal artery sympathetic nerve ablation catheter |
| US9526426B1 (en)* | 2012-07-18 | 2016-12-27 | Bernard Boon Chye Lim | Apparatus and method for assessing tissue composition |
| US10499984B2 (en)* | 2012-07-18 | 2019-12-10 | Bernard Boon Chye Lim | Apparatus and method for assessing tissue treatment |
| US10881459B2 (en)* | 2012-07-18 | 2021-01-05 | Bernard Boon Chye Lim | Apparatus and method for assessing tissue treatment |
| US9592086B2 (en) | 2012-07-24 | 2017-03-14 | Boston Scientific Scimed, Inc. | Electrodes for tissue treatment |
| AU2013300176B2 (en) | 2012-08-06 | 2017-08-17 | Shockwave Medical, Inc. | Low profile electrodes for an angioplasty shock wave catheter |
| US9993295B2 (en) | 2012-08-07 | 2018-06-12 | Covidien Lp | Microwave ablation catheter and method of utilizing the same |
| US9554815B2 (en) | 2012-08-08 | 2017-01-31 | Shockwave Medical, Inc. | Shockwave valvuloplasty with multiple balloons |
| CA2881462C (en) | 2012-08-09 | 2020-07-14 | University Of Iowa Research Foundation | Catheters, catheter systems, and methods for puncturing through a tissue structure |
| US9168004B2 (en)* | 2012-08-20 | 2015-10-27 | Biosense Webster (Israel) Ltd. | Machine learning in determining catheter electrode contact |
| US10321946B2 (en) | 2012-08-24 | 2019-06-18 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices with weeping RF ablation balloons |
| EP3123973A1 (en)* | 2012-08-28 | 2017-02-01 | Boston Scientific Scimed, Inc. | Renal rf ablation system with a movable virtual electrode and related methods of use |
| CN102885649B (en)* | 2012-08-29 | 2015-01-21 | 中国人民解放军第三军医大学第一附属医院 | Radio frequency cable controlled ablation catheter system for removing sympathetic nerve from kidney |
| CN102908189B (en)* | 2012-08-29 | 2015-04-08 | 中国人民解放军第三军医大学第一附属医院 | Multifunctional ablation catheter system for denervation of renal sympathetic nerves |
| CN102885648B (en)* | 2012-08-29 | 2015-03-18 | 中国人民解放军第三军医大学第一附属医院 | Sympathetic nerve denervation ablation catheter system for kidneys |
| CN102908188B (en)* | 2012-08-29 | 2015-04-08 | 中国人民解放军第三军医大学第一附属医院 | Radio frequency ablation (RFA) catheter system for denervation of renal sympathetic nerves |
| EP2890292B1 (en) | 2012-08-31 | 2021-01-13 | Acutus Medical, Inc. | Catheter system for the heart |
| US10588691B2 (en) | 2012-09-12 | 2020-03-17 | Relievant Medsystems, Inc. | Radiofrequency ablation of tissue within a vertebral body |
| US9522012B2 (en) | 2012-09-13 | 2016-12-20 | Shockwave Medical, Inc. | Shockwave catheter system with energy control |
| US9333000B2 (en) | 2012-09-13 | 2016-05-10 | Shockwave Medical, Inc. | Shockwave catheter system with energy control |
| CN104780859B (en) | 2012-09-17 | 2017-07-25 | 波士顿科学西美德公司 | Self-positioning electrode systems and methods for renal neuromodulation |
| US10549127B2 (en) | 2012-09-21 | 2020-02-04 | Boston Scientific Scimed, Inc. | Self-cooling ultrasound ablation catheter |
| US10398464B2 (en) | 2012-09-21 | 2019-09-03 | Boston Scientific Scimed, Inc. | System for nerve modulation and innocuous thermal gradient nerve block |
| CN104869930B (en) | 2012-10-10 | 2020-12-25 | 波士顿科学国际有限公司 | Renal neuromodulation apparatus and methods |
| US9486276B2 (en) | 2012-10-11 | 2016-11-08 | Tva Medical, Inc. | Devices and methods for fistula formation |
| CN102940525A (en)* | 2012-10-17 | 2013-02-27 | 上海安通医疗科技有限公司 | Multi-pole helicoidal radiofrequency ablation conduit |
| US10342608B2 (en)* | 2012-10-18 | 2019-07-09 | The Board Of Trustees Of The Leland Stanford Junior University | Ablation catheter system and method for deploying same |
| US9044575B2 (en) | 2012-10-22 | 2015-06-02 | Medtronic Adrian Luxembourg S.a.r.l. | Catheters with enhanced flexibility and associated devices, systems, and methods |
| EP2908745A4 (en) | 2012-10-22 | 2016-06-15 | Sentreheart Inc | Pericardial access devices and methods |
| ITFR20120013A1 (en)* | 2012-10-23 | 2013-01-22 | Luciana Vitale | CIRCULAR CATHETER FOR MAPPING AND ABLATION GUIDE. |
| US9272132B2 (en) | 2012-11-02 | 2016-03-01 | Boston Scientific Scimed, Inc. | Medical device for treating airways and related methods of use |
| WO2014071161A1 (en) | 2012-11-05 | 2014-05-08 | Relievant Medsystems, Inc. | System and methods for creating curved paths through bone and modulating nerves within the bone |
| WO2014071372A1 (en) | 2012-11-05 | 2014-05-08 | Boston Scientific Scimed, Inc. | Devices for delivering energy to body lumens |
| CN102908191A (en) | 2012-11-13 | 2013-02-06 | 陈绍良 | Multipolar synchronous pulmonary artery radiofrequency ablation catheter |
| US9827036B2 (en)* | 2012-11-13 | 2017-11-28 | Pulnovo Medical (Wuxi) Co., Ltd. | Multi-pole synchronous pulmonary artery radiofrequency ablation catheter |
| US11241267B2 (en) | 2012-11-13 | 2022-02-08 | Pulnovo Medical (Wuxi) Co., Ltd | Multi-pole synchronous pulmonary artery radiofrequency ablation catheter |
| US12082868B2 (en) | 2012-11-13 | 2024-09-10 | Pulnovo Medical (Wuxi) Co., Ltd. | Multi-pole synchronous pulmonary artery radiofrequency ablation catheter |
| CN103830001B (en)* | 2012-11-23 | 2016-12-21 | 四川锦江电子科技有限公司 | Controllable bending spiral ablation catheter |
| CN103830000B (en)* | 2012-11-23 | 2016-03-02 | 四川锦江电子科技有限公司 | Controllable bending open irrigated ablation catheters |
| JP6059737B2 (en)* | 2012-11-30 | 2017-01-11 | 株式会社グッドマン | Ablation catheter |
| US9615878B2 (en) | 2012-12-21 | 2017-04-11 | Volcano Corporation | Device, system, and method for imaging and tissue characterization of ablated tissue |
| US9398933B2 (en) | 2012-12-27 | 2016-07-26 | Holaira, Inc. | Methods for improving drug efficacy including a combination of drug administration and nerve modulation |
| WO2014110579A1 (en)* | 2013-01-14 | 2014-07-17 | Boston Scientific Scimed, Inc. | Renal nerve ablation catheter |
| US20140200639A1 (en) | 2013-01-16 | 2014-07-17 | Advanced Neuromodulation Systems, Inc. | Self-expanding neurostimulation leads having broad multi-electrode arrays |
| US9333111B2 (en) | 2013-02-04 | 2016-05-10 | Hologic, Inc. | Fundus bumper mechanical reference for easier mechanism deployment |
| CN105358070B (en) | 2013-02-08 | 2018-03-23 | 阿库图森医疗有限公司 | Expandable catheter assembly with flexible printed circuit board |
| JP2014184134A (en)* | 2013-02-25 | 2014-10-02 | Inter Noba Kk | Catheter for monitoring biological environment |
| EP3964150A1 (en)* | 2013-03-04 | 2022-03-09 | CSA Medical, Inc. | Cryospray catheters |
| CA2904190C (en) | 2013-03-04 | 2022-08-16 | Csa Medical, Inc. | Cryospray catheters |
| US9179997B2 (en) | 2013-03-06 | 2015-11-10 | St. Jude Medical, Cardiology Division, Inc. | Thermochromic polyvinyl alcohol based hydrogel artery |
| US9474486B2 (en) | 2013-03-08 | 2016-10-25 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Basket for a multi-electrode array catheter |
| WO2014163987A1 (en) | 2013-03-11 | 2014-10-09 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| WO2014143571A1 (en) | 2013-03-11 | 2014-09-18 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| US10716914B2 (en) | 2013-03-12 | 2020-07-21 | St. Jude Medical, Cardiology Division, Inc. | Catheter system |
| US10328238B2 (en) | 2013-03-12 | 2019-06-25 | St. Jude Medical, Cardiology Division, Inc. | Catheter system |
| US9775966B2 (en) | 2013-03-12 | 2017-10-03 | St. Jude Medical, Cardiology Division, Inc. | Catheter system |
| US9381055B2 (en)* | 2013-03-13 | 2016-07-05 | Cryofocus Medtech (Shanghai) Co. Ltd. | Therapeutic cryoablation system |
| US9808311B2 (en) | 2013-03-13 | 2017-11-07 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| US10561509B2 (en) | 2013-03-13 | 2020-02-18 | DePuy Synthes Products, Inc. | Braided stent with expansion ring and method of delivery |
| US9510902B2 (en) | 2013-03-13 | 2016-12-06 | St. Jude Medical, Cardiology Division, Inc. | Ablation catheters and systems including rotational monitoring means |
| US10603157B2 (en) | 2013-03-13 | 2020-03-31 | DePuy Synthes Products, Inc. | Braid implant delivery and retraction device with distal engagement |
| US8876813B2 (en) | 2013-03-14 | 2014-11-04 | St. Jude Medical, Inc. | Methods, systems, and apparatus for neural signal detection |
| ES2708786T3 (en) | 2013-03-14 | 2019-04-11 | Neuravi Ltd | Clot recovery device to remove occlusive clots from a blood vessel |
| US9703317B2 (en) | 2013-03-14 | 2017-07-11 | Biosense Webster (Israel) Ltd. | Dongle with shape memory |
| US9433429B2 (en) | 2013-03-14 | 2016-09-06 | Neuravi Limited | Clot retrieval devices |
| WO2014140092A2 (en) | 2013-03-14 | 2014-09-18 | Neuravi Limited | Devices and methods for removal of acute blockages from blood vessels |
| CN105228683B (en) | 2013-03-14 | 2022-06-10 | Tva医疗公司 | Fistula-forming device and method for forming fistula |
| US9131982B2 (en) | 2013-03-14 | 2015-09-15 | St. Jude Medical, Cardiology Division, Inc. | Mediguide-enabled renal denervation system for ensuring wall contact and mapping lesion locations |
| US9333113B2 (en) | 2013-03-15 | 2016-05-10 | Abbott Cardiovascular Systems Inc. | System and method for denervation |
| US9561070B2 (en) | 2013-03-15 | 2017-02-07 | St. Jude Medical, Cardiology Division, Inc. | Ablation system, methods, and controllers |
| US9974477B2 (en) | 2013-03-15 | 2018-05-22 | St. Jude Medical, Cardiology Division, Inc. | Quantification of renal denervation via alterations in renal blood flow pre/post ablation |
| US10265122B2 (en) | 2013-03-15 | 2019-04-23 | Boston Scientific Scimed, Inc. | Nerve ablation devices and related methods of use |
| US9186212B2 (en) | 2013-03-15 | 2015-11-17 | St. Jude Medical, Cardiology Division, Inc. | Feedback systems and methods utilizing two or more sites along denervation catheter |
| US9179973B2 (en) | 2013-03-15 | 2015-11-10 | St. Jude Medical, Cardiology Division, Inc. | Feedback systems and methods for renal denervation utilizing balloon catheter |
| EP2967734B1 (en) | 2013-03-15 | 2019-05-15 | Boston Scientific Scimed, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| EP3345564A1 (en) | 2013-03-15 | 2018-07-11 | St. Jude Medical, Cardiology Division, Inc. | Multi-electrode ablation system with a controller for determining a thermal gain of each electrode |
| CN105228546B (en) | 2013-03-15 | 2017-11-14 | 波士顿科学国际有限公司 | Medical devices and methods for treating hypertension utilizing impedance compensation |
| US9089414B2 (en) | 2013-03-22 | 2015-07-28 | Edwards Lifesciences Corporation | Device and method for increasing flow through the left atrial appendage |
| WO2014176205A1 (en) | 2013-04-25 | 2014-10-30 | St. Jude Medical, Cardiology Division, Inc. | Electrode assembly for catheter system |
| EP2996754B1 (en) | 2013-05-18 | 2023-04-26 | Medtronic Ardian Luxembourg S.à.r.l. | Neuromodulation catheters with shafts for enhanced flexibility and control and associated devices and systems |
| WO2014188430A2 (en) | 2013-05-23 | 2014-11-27 | CardioSonic Ltd. | Devices and methods for renal denervation and assessment thereof |
| US9351789B2 (en)* | 2013-05-31 | 2016-05-31 | Medtronic Ablation Frontiers Llc | Adjustable catheter for ostial, septal, and roof ablation in atrial fibrillation patients |
| CN105473092B (en) | 2013-06-21 | 2019-05-17 | 波士顿科学国际有限公司 | The medical instrument for renal nerve ablation with rotatable shaft |
| CN105473091B (en) | 2013-06-21 | 2020-01-21 | 波士顿科学国际有限公司 | Renal denervation balloon catheter with co-movable electrode supports |
| US9707036B2 (en) | 2013-06-25 | 2017-07-18 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation using localized indifferent electrodes |
| US9872728B2 (en) | 2013-06-28 | 2018-01-23 | St. Jude Medical, Cardiology Division, Inc. | Apparatuses and methods for affixing electrodes to an intravascular balloon |
| CN105358084B (en) | 2013-07-01 | 2018-11-09 | 波士顿科学国际有限公司 | Medical instrument for renal nerve ablation |
| US20150011991A1 (en) | 2013-07-03 | 2015-01-08 | St. Jude Medical, Cardiology Division, Inc. | Electrode Assembly For Catheter System |
| US10413357B2 (en) | 2013-07-11 | 2019-09-17 | Boston Scientific Scimed, Inc. | Medical device with stretchable electrode assemblies |
| CN105377169B (en) | 2013-07-11 | 2019-04-19 | 波士顿科学国际有限公司 | Devices and methods for neuromodulation |
| US10315014B2 (en) | 2013-07-15 | 2019-06-11 | John P. Pigott | Balloon catheter having a retractable sheath and locking mechanism with balloon recapture element |
| US11202892B2 (en) | 2013-07-15 | 2021-12-21 | John P. Pigott | Balloon catheter having a retractable sheath |
| US10130798B2 (en) | 2013-07-15 | 2018-11-20 | John P. Pigott | Balloon catheter having a retractable sheath and locking mechanism |
| US10828471B2 (en) | 2013-07-15 | 2020-11-10 | John P. Pigott | Balloon catheter having a retractable sheath |
| US9925001B2 (en) | 2013-07-19 | 2018-03-27 | Boston Scientific Scimed, Inc. | Spiral bipolar electrode renal denervation balloon |
| US10342609B2 (en) | 2013-07-22 | 2019-07-09 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation |
| US10695124B2 (en) | 2013-07-22 | 2020-06-30 | Boston Scientific Scimed, Inc. | Renal nerve ablation catheter having twist balloon |
| CN103393464B (en)* | 2013-08-01 | 2016-08-10 | 北京迈迪顶峰医疗科技有限公司 | A kind of RF ablation device |
| US9724151B2 (en) | 2013-08-08 | 2017-08-08 | Relievant Medsystems, Inc. | Modulating nerves within bone using bone fasteners |
| EP3335658B1 (en)* | 2013-08-09 | 2020-04-22 | Boston Scientific Scimed, Inc. | Expandable catheter |
| CN105473093B (en) | 2013-08-22 | 2019-02-05 | 波士顿科学国际有限公司 | Flexible circuit with improved adhesion to renal neuromodulation balloon |
| US9895194B2 (en) | 2013-09-04 | 2018-02-20 | Boston Scientific Scimed, Inc. | Radio frequency (RF) balloon catheter having flushing and cooling capability |
| US9907608B2 (en)* | 2013-09-05 | 2018-03-06 | Mitragen, Inc. | Valve treatment devices, systems, and methods |
| EP3043733A1 (en) | 2013-09-13 | 2016-07-20 | Boston Scientific Scimed, Inc. | Ablation balloon with vapor deposited cover layer |
| CA2922941C (en) | 2013-09-13 | 2021-11-16 | Acutus Medical, Inc. | Devices and methods for determination of electrical dipole densities on a cardiac surface |
| US10687889B2 (en) | 2013-10-11 | 2020-06-23 | Biosense Webster (Israel) Ltd. | Patient-specific pre-shaped cardiac catheter |
| US11246654B2 (en) | 2013-10-14 | 2022-02-15 | Boston Scientific Scimed, Inc. | Flexible renal nerve ablation devices and related methods of use and manufacture |
| EP3057488B1 (en) | 2013-10-14 | 2018-05-16 | Boston Scientific Scimed, Inc. | High resolution cardiac mapping electrode array catheter |
| US9770606B2 (en) | 2013-10-15 | 2017-09-26 | Boston Scientific Scimed, Inc. | Ultrasound ablation catheter with cooling infusion and centering basket |
| US9962223B2 (en) | 2013-10-15 | 2018-05-08 | Boston Scientific Scimed, Inc. | Medical device balloon |
| EP3057521B1 (en) | 2013-10-18 | 2020-03-25 | Boston Scientific Scimed, Inc. | Balloon catheters with flexible conducting wires |
| USD914883S1 (en) | 2013-10-23 | 2021-03-30 | St. Jude Medical, Cardiology Division, Inc. | Ablation generator |
| US10856936B2 (en) | 2013-10-23 | 2020-12-08 | St. Jude Medical, Cardiology Division, Inc. | Electrode assembly for catheter system including thermoplastic-based struts |
| USD774043S1 (en) | 2013-10-23 | 2016-12-13 | St. Jude Medical, Cardiology Division, Inc. | Display screen with graphical user interface for ablation generator |
| USD747491S1 (en) | 2013-10-23 | 2016-01-12 | St. Jude Medical, Cardiology Division, Inc. | Ablation generator |
| US9913961B2 (en) | 2013-10-24 | 2018-03-13 | St. Jude Medical, Cardiology Division, Inc. | Flexible catheter shaft and method of manufacture |
| WO2015061034A1 (en)* | 2013-10-24 | 2015-04-30 | St. Jude Medical, Cardiology Division, Inc. | Flexible catheter shaft and method of manufacture |
| US20150119878A1 (en)* | 2013-10-24 | 2015-04-30 | St. Jude Medical, Cardiology Division, Inc. | Electrode assembly having asymmetric electrode placement |
| US10034705B2 (en) | 2013-10-24 | 2018-07-31 | St. Jude Medical, Cardiology Division, Inc. | High strength electrode assembly for catheter system including novel electrode |
| CN105658163B (en) | 2013-10-25 | 2020-08-18 | 波士顿科学国际有限公司 | Embedded thermocouple in denervation flexible circuit |
| US10420604B2 (en) | 2013-10-28 | 2019-09-24 | St. Jude Medical, Cardiology Division, Inc. | Electrode assembly for catheter system including interlinked struts |
| EP4226881A1 (en) | 2013-10-31 | 2023-08-16 | AtriCure, Inc. | Device for left atrial appendage closure |
| US9861433B2 (en) | 2013-11-05 | 2018-01-09 | St. Jude Medical, Cardiology Division, Inc. | Helical-shaped ablation catheter and methods of use |
| US10682175B2 (en)* | 2013-11-06 | 2020-06-16 | Biosense Webster (Israel) Ltd. | Using catheter position and temperature measurement to detect movement from ablation point |
| EP3091921B1 (en) | 2014-01-06 | 2019-06-19 | Farapulse, Inc. | Apparatus for renal denervation ablation |
| EP3091922B1 (en) | 2014-01-06 | 2018-10-17 | Boston Scientific Scimed, Inc. | Tear resistant flex circuit assembly |
| EP4253024B1 (en) | 2014-01-27 | 2025-02-26 | Medtronic Ireland Manufacturing Unlimited Company | Neuromodulation catheters having jacketed neuromodulation elements and related devices |
| CN106572881B (en) | 2014-02-04 | 2019-07-26 | 波士顿科学国际有限公司 | Alternative placement of thermal sensors on bipolar electrodes |
| US11000679B2 (en) | 2014-02-04 | 2021-05-11 | Boston Scientific Scimed, Inc. | Balloon protection and rewrapping devices and related methods of use |
| WO2015120079A1 (en) | 2014-02-04 | 2015-08-13 | Amphora Medical, Inc. | Devices and methods for treating conditions caused by affarent nerve signals |
| WO2015120325A1 (en) | 2014-02-06 | 2015-08-13 | Acublate, Inc. | Apparatus and method for self-guided ablation |
| US20150230859A1 (en)* | 2014-02-19 | 2015-08-20 | Kevin Mauch | Bi-directional deployment of neuromodulation devices and associated systems and methods |
| CA2939929C (en) | 2014-02-28 | 2023-04-11 | Sentreheart, Inc. | Pericardial access devices and methods |
| US10463424B2 (en) | 2014-03-11 | 2019-11-05 | Medtronic Ardian Luxembourg S.A.R.L. | Catheters with independent radial-expansion members and associated devices, systems, and methods |
| US10285720B2 (en) | 2014-03-11 | 2019-05-14 | Neuravi Limited | Clot retrieval system for removing occlusive clot from a blood vessel |
| US10695534B2 (en) | 2014-03-14 | 2020-06-30 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| JP6739346B2 (en) | 2014-03-25 | 2020-08-12 | アクタス メディカル インクAcutus Medical,Inc. | Method of operating system of cardiac analysis user interface |
| US11154302B2 (en) | 2014-03-31 | 2021-10-26 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| US11076860B2 (en) | 2014-03-31 | 2021-08-03 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| US10398501B2 (en) | 2014-04-24 | 2019-09-03 | St. Jude Medical, Cardiology Division, Inc. | Ablation systems including pulse rate detector and feedback mechanism and methods of use |
| US10610292B2 (en) | 2014-04-25 | 2020-04-07 | Medtronic Ardian Luxembourg S.A.R.L. | Devices, systems, and methods for monitoring and/or controlling deployment of a neuromodulation element within a body lumen and related technology |
| US11826172B2 (en) | 2014-05-06 | 2023-11-28 | St. Jude Medical, Cardiology Division, Inc. | Electrode support structure assembly |
| EP3495018B1 (en) | 2014-05-07 | 2023-09-06 | Farapulse, Inc. | Apparatus for selective tissue ablation |
| WO2015175944A1 (en) | 2014-05-16 | 2015-11-19 | Gary Long | Methods and apparatus for multi-catheter tissue ablation |
| JP2017516620A (en) | 2014-05-23 | 2017-06-22 | アンフォラ メディカル, インコーポレイテッド | Methods and devices for treatment of pelvic conditions |
| CN106413540A (en) | 2014-06-03 | 2017-02-15 | 波士顿科学医学有限公司 | Electrode assembly with atraumatic distal tip |
| US9848795B2 (en) | 2014-06-04 | 2017-12-26 | Boston Scientific Scimed Inc. | Electrode assembly |
| CA2951050A1 (en) | 2014-06-04 | 2015-12-10 | Csa Medical, Inc. | Method and system for consistent, repeatable, and safe cryospray treatment of airway tissue |
| US10118022B2 (en) | 2014-06-05 | 2018-11-06 | St. Jude Medical, Cardiology Division, Inc. | Deflectable catheter shaft section |
| WO2015192018A1 (en) | 2014-06-12 | 2015-12-17 | Iowa Approach Inc. | Method and apparatus for rapid and selective tissue ablation with cooling |
| EP3154463B1 (en) | 2014-06-12 | 2019-03-27 | Farapulse, Inc. | Apparatus for rapid and selective transurethral tissue ablation |
| JP6595513B2 (en) | 2014-06-13 | 2019-10-23 | ニューラヴィ・リミテッド | Device for removal of acute occlusions from blood vessels |
| US10194978B2 (en)* | 2014-06-13 | 2019-02-05 | Medtronic Cryocath Lp | Supporting catheter for use for phrenic nerve pacing |
| US9844645B2 (en) | 2014-06-17 | 2017-12-19 | St. Jude Medical, Cardiology Division, Inc. | Triple coil catheter support |
| US10265086B2 (en) | 2014-06-30 | 2019-04-23 | Neuravi Limited | System for removing a clot from a blood vessel |
| US9918718B2 (en) | 2014-08-08 | 2018-03-20 | DePuy Synthes Products, Inc. | Embolic coil delivery system with retractable mechanical release mechanism |
| US10624697B2 (en) | 2014-08-26 | 2020-04-21 | Covidien Lp | Microwave ablation system |
| WO2016033374A1 (en) | 2014-08-27 | 2016-03-03 | Tva Medical, Inc. | Cryolipopysis devices and methods therefor |
| US10206796B2 (en) | 2014-08-27 | 2019-02-19 | DePuy Synthes Products, Inc. | Multi-strand implant with enhanced radiopacity |
| US9782178B2 (en) | 2014-09-19 | 2017-10-10 | DePuy Synthes Products, Inc. | Vasculature occlusion device detachment system with tapered corewire and heater activated fiber detachment |
| CN104287824B (en)* | 2014-10-11 | 2017-09-15 | 先健科技(深圳)有限公司 | Ablation Catheter Device |
| EP3206613B1 (en) | 2014-10-14 | 2019-07-03 | Farapulse, Inc. | Apparatus for rapid and safe pulmonary vein cardiac ablation |
| US10898096B2 (en) | 2014-10-27 | 2021-01-26 | St. Jude Medical, Cardiology Division, Inc. | Apparatus and method for connecting elements in medical devices |
| US9788893B2 (en) | 2014-11-20 | 2017-10-17 | Biosense Webster (Israel) Ltd. | Catheter with soft distal tip for mapping and ablating tubular region |
| EP3682821B1 (en) | 2014-11-26 | 2022-05-11 | Neuravi Limited | A clot retrieval device for removing an occlusive clot from a blood vessel |
| US10617435B2 (en) | 2014-11-26 | 2020-04-14 | Neuravi Limited | Clot retrieval device for removing clot from a blood vessel |
| US11253278B2 (en) | 2014-11-26 | 2022-02-22 | Neuravi Limited | Clot retrieval system for removing occlusive clot from a blood vessel |
| US9907547B2 (en) | 2014-12-02 | 2018-03-06 | 4Tech Inc. | Off-center tissue anchors |
| CN104605930A (en)* | 2014-12-29 | 2015-05-13 | 上海魅丽纬叶医疗科技有限公司 | Radiofrequency ablation catheter of spiral structure and device with radiofrequency ablation catheter |
| US10603069B2 (en) | 2015-01-13 | 2020-03-31 | John P. Pigott | Intravascular catheter balloon device having a tool for atherectomy or an incising portion for atheromatous plaque scoring |
| EP3244815B1 (en)* | 2015-01-13 | 2020-04-22 | Pigott, John, P. | Intravascular catheter having an expandable portion |
| CN107205774B (en) | 2015-01-28 | 2020-05-29 | 圣犹达医疗用品心脏病学部门有限公司 | Thermal mapping catheter |
| CN104688334A (en)* | 2015-03-23 | 2015-06-10 | 上海魅丽纬叶医疗科技有限公司 | Spiral type radio frequency ablation catheter and equipment with wall-adhered adjusting screw |
| EP3360497B1 (en)* | 2015-02-03 | 2024-05-22 | Shanghai Golden Leaf Med Tec Co., Ltd. | Radio-frequency ablation catheter having spiral structure and device thereof |
| US10376308B2 (en) | 2015-02-05 | 2019-08-13 | Axon Therapies, Inc. | Devices and methods for treatment of heart failure by splanchnic nerve ablation |
| US10603040B1 (en) | 2015-02-09 | 2020-03-31 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| JP6499887B2 (en)* | 2015-03-13 | 2019-04-10 | テルモ株式会社 | Medical device |
| EP3069754B1 (en) | 2015-03-16 | 2017-10-25 | Sorin CRM SAS | In situ implantation accessory for self-contained intracardiac capsule |
| JP6265434B2 (en)* | 2015-03-27 | 2018-01-24 | 日本ライフライン株式会社 | Balloon type ablation catheter and ablation catheter device |
| ES2768107T3 (en) | 2015-04-29 | 2020-06-19 | Innoblative Designs Inc | Cavity tissue removal |
| US11564607B2 (en)* | 2015-04-30 | 2023-01-31 | The Regents Of The University Of Michigan | Method and system for mapping and analyzing cardiac electrical activity |
| US10602983B2 (en) | 2015-05-08 | 2020-03-31 | St. Jude Medical International Holding S.À R.L. | Integrated sensors for medical devices and method of making integrated sensors for medical devices |
| CN115299988A (en) | 2015-05-12 | 2022-11-08 | 阿库图森医疗有限公司 | Ultrasonic sequencing systems and methods |
| US12268433B2 (en) | 2015-05-12 | 2025-04-08 | National University Of Ireland, Galway | Devices for therapeutic nasal neuromodulation and associated methods and systems |
| US10593234B2 (en) | 2015-05-12 | 2020-03-17 | Acutus Medical, Inc. | Cardiac virtualization test tank and testing system and method |
| US10653318B2 (en) | 2015-05-13 | 2020-05-19 | Acutus Medical, Inc. | Localization system and method useful in the acquisition and analysis of cardiac information |
| JP6852898B2 (en)* | 2015-05-13 | 2021-03-31 | 上▲海▼魅▲麗▼▲緯▼叶医▲療▼科技有限公司 | Ripples high frequency ablation catheter with close contact adjustment wire |
| CN105078571B (en)* | 2015-05-13 | 2017-10-17 | 上海魅丽纬叶医疗科技有限公司 | Ripple type radio frequency ablation catheter and its equipment with adherent regulation silk |
| US11259868B2 (en) | 2015-05-13 | 2022-03-01 | Shanghai Golden Leaf Med Tec Co., Ltd. | Corrugated radiofrequency ablation catheter and apparatus thereof |
| DE102015108078A1 (en)* | 2015-05-21 | 2016-11-24 | Aesculap Ag | Electrosurgical coagulation instrument |
| EP3302678A4 (en)* | 2015-05-27 | 2019-02-20 | University of Maryland, Baltimore | APPARATUS AND METHOD FOR PLACING A DEVICE ALONG A WALL OF A BODY LIGHT |
| WO2017024055A1 (en)* | 2015-08-03 | 2017-02-09 | Boston Scientific Scimed, Inc. | Steerable tissue mapping and ablation device |
| US9931487B2 (en) | 2015-08-06 | 2018-04-03 | Boston Scientific Scimed, Inc. | Bidirectional steering control apparatus for a catheter |
| US10492857B2 (en) | 2015-08-06 | 2019-12-03 | Boston Scientific Scimed Inc | Deployment control apparatus for a catheter with a deployable array |
| EP3337387B1 (en) | 2015-08-20 | 2019-09-25 | Boston Scientific Scimed Inc. | Flexible electrode for cardiac sensing and method for making |
| US10524858B2 (en)* | 2015-09-14 | 2020-01-07 | Biosense Webster (Israel) Ltd. | Dual node multiray electrode catheter |
| US10517668B2 (en)* | 2015-09-14 | 2019-12-31 | Boisense Webster (Israel) Ltd. | Dual node multiray electrode catheter |
| US10357173B2 (en)* | 2015-09-14 | 2019-07-23 | Biosense Webster (Israel) Ltd. | Dual multiray electrode catheter |
| EP4205685B1 (en) | 2015-10-21 | 2024-08-28 | St. Jude Medical, Cardiology Division, Inc. | High density electrode mapping catheter |
| CN114668490B (en) | 2015-10-21 | 2025-10-03 | 圣犹达医疗用品心脏病学部门有限公司 | High-density electrode mapping catheter |
| US12207863B2 (en) | 2015-10-29 | 2025-01-28 | Innoblative Designs, Inc. | Screen sphere tissue ablation devices and methods |
| JP6933857B2 (en) | 2015-10-29 | 2021-09-08 | イノブレイティブ デザインズ, インコーポレイテッド | Net spherical tissue ablation device and method |
| JP6785301B2 (en) | 2015-11-04 | 2020-11-18 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | Medical devices and related methods |
| WO2017087195A1 (en) | 2015-11-18 | 2017-05-26 | Shockwave Medical, Inc. | Shock wave electrodes |
| CA3006427A1 (en)* | 2015-12-01 | 2017-06-08 | Symap Medical (Suzhou), Ltd | System and method for mapping functional nerves innervating wall of arteries,3-d mapping and catheters for same |
| SG11201804773PA (en)* | 2015-12-15 | 2018-07-30 | Agency Science Tech & Res | Method and deployable multi-spine apparatus for catheter-based renal denervation |
| US12144541B2 (en) | 2016-01-05 | 2024-11-19 | Boston Scientific Scimed, Inc. | Systems, apparatuses and methods for delivery of ablative energy to tissue |
| US10130423B1 (en) | 2017-07-06 | 2018-11-20 | Farapulse, Inc. | Systems, devices, and methods for focal ablation |
| US10172673B2 (en) | 2016-01-05 | 2019-01-08 | Farapulse, Inc. | Systems devices, and methods for delivery of pulsed electric field ablative energy to endocardial tissue |
| US10660702B2 (en) | 2016-01-05 | 2020-05-26 | Farapulse, Inc. | Systems, devices, and methods for focal ablation |
| US20170189097A1 (en) | 2016-01-05 | 2017-07-06 | Iowa Approach Inc. | Systems, apparatuses and methods for delivery of ablative energy to tissue |
| US10448994B2 (en) | 2016-01-13 | 2019-10-22 | Abbott Cardiovascular Systems Inc. | Method of manufacturing a shaping structure for denervation |
| US10874422B2 (en) | 2016-01-15 | 2020-12-29 | Tva Medical, Inc. | Systems and methods for increasing blood flow |
| CN114042224B (en) | 2016-01-15 | 2024-09-17 | Tva医疗公司 | Device and method for advancing a wire |
| JP7219090B2 (en) | 2016-01-15 | 2023-02-07 | ティーブイエー メディカル, インコーポレイテッド | Systems and methods for gluing vessels |
| WO2017124062A1 (en) | 2016-01-15 | 2017-07-20 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| WO2017136261A1 (en) | 2016-02-02 | 2017-08-10 | Innoblative Designs, Inc. | Cavitary tissue ablation system |
| US10813692B2 (en) | 2016-02-29 | 2020-10-27 | Covidien Lp | 90-degree interlocking geometry for introducer for facilitating deployment of microwave radiating catheter |
| WO2017151431A1 (en) | 2016-03-01 | 2017-09-08 | Innoblative Designs, Inc. | Resecting and coagulating tissue |
| CN109069799B (en)* | 2016-04-08 | 2021-07-16 | 圣犹达医疗用品心脏病学部门有限公司 | Adjustable ring catheter handle for mapping |
| JP6820121B2 (en) | 2016-04-27 | 2021-01-27 | シーエスエー メディカル, インコーポレイテッド | Vision-securing device for medical devices |
| US12364537B2 (en) | 2016-05-02 | 2025-07-22 | Santa Anna Tech Llc | Catheter with a double balloon structure to generate and apply a heated ablative zone to tissue |
| CA3022806A1 (en) | 2016-05-03 | 2017-11-09 | Acutus Medical, Inc. | Cardiac mapping system with efficiency algorithm |
| EP3858277B1 (en) | 2016-05-03 | 2023-02-22 | St. Jude Medical, Cardiology Division, Inc. | Irrigated high density electrode catheter |
| US11871977B2 (en) | 2016-05-19 | 2024-01-16 | Csa Medical, Inc. | Catheter extension control |
| US11331140B2 (en) | 2016-05-19 | 2022-05-17 | Aqua Heart, Inc. | Heated vapor ablation systems and methods for treating cardiac conditions |
| US10285710B2 (en) | 2016-06-01 | 2019-05-14 | DePuy Synthes Products, Inc. | Endovascular detachment system with flexible distal end and heater activated detachment |
| US10905329B2 (en) | 2016-06-09 | 2021-02-02 | Biosense Webster (Israel) Ltd. | Multi-function conducting elements for a catheter |
| EP3471631A4 (en) | 2016-06-16 | 2020-03-04 | Farapulse, Inc. | GUIDE WIRE DISTRIBUTION SYSTEMS, APPARATUSES AND METHODS |
| WO2017223264A1 (en) | 2016-06-23 | 2017-12-28 | St. Jude Medical, Cardiology Division, Inc. | Catheter system and electrode assembly for intraprocedural evaluation of renal denervation |
| WO2018023132A1 (en) | 2016-07-29 | 2018-02-01 | Axon Therepies, Inc. | Devices, systems, and methods for treatment of heart failure by splanchnic nerve ablation |
| EP3500191B1 (en) | 2016-08-17 | 2020-09-23 | Neuravi Limited | A clot retrieval system for removing occlusive clot from a blood vessel |
| US10076428B2 (en) | 2016-08-25 | 2018-09-18 | DePuy Synthes Products, Inc. | Expansion ring for a braided stent |
| US10939963B2 (en) | 2016-09-01 | 2021-03-09 | Covidien Lp | Systems and methods for providing proximity awareness to pleural boundaries, vascular structures, and other critical intra-thoracic structures during electromagnetic navigation bronchoscopy |
| EP3509509B1 (en) | 2016-09-06 | 2021-03-31 | Neuravi Limited | A clot retrieval device for removing occlusive clot from a blood vessel |
| CN109982652B (en) | 2016-09-25 | 2022-08-05 | Tva医疗公司 | Vascular stent device and method |
| US10292851B2 (en) | 2016-09-30 | 2019-05-21 | DePuy Synthes Products, Inc. | Self-expanding device delivery apparatus with dual function bump |
| EP3522807B1 (en) | 2016-10-04 | 2025-07-09 | Avent, Inc. | Cooled rf probes |
| AU2017339980B2 (en) | 2016-10-06 | 2022-08-18 | Shockwave Medical, Inc. | Aortic leaflet repair using shock wave applicators |
| WO2018075389A1 (en) | 2016-10-17 | 2018-04-26 | Innoblative Designs, Inc. | Treatment devices and methods |
| WO2018080985A1 (en) | 2016-10-24 | 2018-05-03 | St. Jude Medical, Cardiology Division, Inc. | Catheter insertion devices |
| US10517708B2 (en) | 2016-10-26 | 2019-12-31 | DePuy Synthes Products, Inc. | Multi-basket clot capturing device |
| US11172858B2 (en) | 2016-10-28 | 2021-11-16 | St. Jude Medical, Cardiology Division, Inc. | Flexible high-density mapping catheter |
| JP6875757B2 (en) | 2016-11-08 | 2021-05-26 | イノブレイティブ デザインズ, インコーポレイテッド | Electrosurgical tissue and vascular seal device |
| US11400205B2 (en) | 2016-11-23 | 2022-08-02 | Biosense Webster (Israel) Ltd. | Balloon-in-balloon irrigation balloon catheter |
| US11806071B2 (en) | 2016-12-22 | 2023-11-07 | Aerin Medical Inc. | Soft palate treatment |
| US11344259B2 (en) | 2017-01-11 | 2022-05-31 | Abbott Cardiovascular Systems Inc. | Expandable member for an electrophysiology catheter |
| US10905853B2 (en) | 2017-01-17 | 2021-02-02 | DePuy Synthes Products, Inc. | System and method for delivering a catheter |
| US12011549B2 (en) | 2017-01-19 | 2024-06-18 | St. Jude Medical, Cardiology Division, Inc. | Sheath visualization |
| US10881497B2 (en) | 2017-01-26 | 2021-01-05 | DePuy Synthes Products, Inc. | Composite vascular flow diverter |
| CN110545739A (en) | 2017-02-23 | 2019-12-06 | 德普伊新特斯产品公司 | aneurysm devices and delivery systems |
| ES2831026T3 (en) | 2017-02-24 | 2021-06-07 | Venturemed Group Inc | Intravascular catheter that has an expandable incision portion and abrasive surfaces |
| WO2018173053A1 (en) | 2017-03-20 | 2018-09-27 | Sonievie Ltd. | Pulmonary hypertension treatment method and/or system |
| JP7125766B2 (en)* | 2017-04-05 | 2022-08-25 | ナショナル・ユニバーシティ・オブ・アイルランド・ガルウェイ | implantable medical device |
| USD827819S1 (en) | 2017-04-07 | 2018-09-04 | St. Jude Medical, Cardiology Division, Inc. | Catheter handle |
| USD827818S1 (en) | 2017-04-07 | 2018-09-04 | St. Jude Medical, Cardiology Division, Inc. | Catheter handle |
| WO2018187856A1 (en)* | 2017-04-12 | 2018-10-18 | Kardium Inc. | Medical device systems and methods including helically configured or twisted, non-helically configured elongate members |
| US9987081B1 (en) | 2017-04-27 | 2018-06-05 | Iowa Approach, Inc. | Systems, devices, and methods for signal generation |
| US10617867B2 (en)* | 2017-04-28 | 2020-04-14 | Farapulse, Inc. | Systems, devices, and methods for delivery of pulsed electric field ablative energy to esophageal tissue |
| US12029545B2 (en) | 2017-05-30 | 2024-07-09 | Biosense Webster (Israel) Ltd. | Catheter splines as location sensors |
| US12220157B2 (en) | 2017-06-05 | 2025-02-11 | St. Jude Medical, Cardiology Division, Inc. | Pulmonary antrum radial-linear ablation devices |
| US10966737B2 (en) | 2017-06-19 | 2021-04-06 | Shockwave Medical, Inc. | Device and method for generating forward directed shock waves |
| US11666379B2 (en) | 2017-07-06 | 2023-06-06 | Biosense Webster (Israel) Ltd. | Temperature controlled short duration ablation with multiple electrodes |
| EP4226859A3 (en)* | 2017-07-07 | 2023-10-04 | St. Jude Medical, Cardiology Division, Inc. | Layered high density electrode mapping catheter |
| US11647935B2 (en) | 2017-07-24 | 2023-05-16 | St. Jude Medical, Cardiology Division, Inc. | Masked ring electrodes |
| EP3658053B1 (en) | 2017-07-26 | 2023-09-13 | Innoblative Designs, Inc. | Minimally invasive articulating assembly having ablation capabilities |
| US11052246B2 (en)* | 2017-07-28 | 2021-07-06 | Medtronic, Inc. | Expandable elements for delivery of electric fields |
| JP7586706B2 (en) | 2017-09-12 | 2024-11-19 | ボストン サイエンティフィック サイムド,インコーポレイテッド | Systems, devices and methods for focal ventricular ablation - Patents.com |
| CN116392238A (en) | 2017-10-13 | 2023-07-07 | 圣犹达医疗用品心脏病学部门有限公司 | Catheters with High Density Mapping Electrodes |
| CN109717942A (en)* | 2017-10-31 | 2019-05-07 | 四川锦江电子科技有限公司 | A kind of cryoablation conduit |
| CN109717943B (en)* | 2017-10-31 | 2021-05-28 | 四川锦江电子科技有限公司 | Cryoablation catheter with mapping function and ablation device |
| US10709462B2 (en) | 2017-11-17 | 2020-07-14 | Shockwave Medical, Inc. | Low profile electrodes for a shock wave catheter |
| CN111278497B (en)* | 2017-11-28 | 2024-01-12 | 圣犹达医疗用品心脏病学部门有限公司 | Lumen control catheter |
| US10561461B2 (en) | 2017-12-17 | 2020-02-18 | Axon Therapies, Inc. | Methods and devices for endovascular ablation of a splanchnic nerve |
| US10806462B2 (en) | 2017-12-21 | 2020-10-20 | DePuy Synthes Products, Inc. | Implantable medical device detachment system with split tube and cylindrical coupling |
| US10751065B2 (en) | 2017-12-22 | 2020-08-25 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| JP7394766B2 (en)* | 2018-01-18 | 2023-12-08 | ファラパルス,インコーポレイテッド | Systems, devices, and methods for focal ablation |
| US12082917B2 (en) | 2018-01-24 | 2024-09-10 | Medtronic Ireland Manufacturing Unlimited Company | Systems, devices, and methods for assessing efficacy of renal neuromodulation therapy |
| US10905430B2 (en) | 2018-01-24 | 2021-02-02 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| CA3089217A1 (en) | 2018-01-26 | 2019-08-01 | Dorin Panescu | Methods and devices for endovascular ablation of a splanchnic nerve |
| WO2019157221A1 (en)* | 2018-02-08 | 2019-08-15 | Texas Medical Center | Gallbladder defunctionalization devices and methods |
| EP3749238B1 (en) | 2018-02-08 | 2023-08-16 | Farapulse, Inc. | Apparatus for controlled delivery of pulsed electric field ablative energy to tissue |
| EP3542853B1 (en) | 2018-03-23 | 2021-05-05 | Heraeus Deutschland GmbH & Co. KG | Manufacturing method for a microlead |
| EP3542854B1 (en) | 2018-03-23 | 2021-06-30 | Heraeus Deutschland GmbH & Co. KG | Manufacturing method for a multi electrode system |
| EP3773267B1 (en)* | 2018-03-27 | 2024-04-24 | Medtronic, Inc. | Devices for aortic valve preparation prior to transcatheter prosthetic valve procedures |
| US10786259B2 (en) | 2018-03-30 | 2020-09-29 | DePuy Synthes Products, Inc. | Split balloon assist device and method for using the same |
| US10918390B2 (en) | 2018-03-30 | 2021-02-16 | DePuy Synthes Products, Inc. | Helical balloon assist device and method for using the same |
| US10806461B2 (en) | 2018-04-27 | 2020-10-20 | DePuy Synthes Products, Inc. | Implantable medical device detachment system with split tube |
| US12194251B2 (en) | 2018-05-01 | 2025-01-14 | Magellan Biomedical Inc. | System and method for device steering, tracking, and navigation of devices for interventional procedures |
| US20190336198A1 (en) | 2018-05-03 | 2019-11-07 | Farapulse, Inc. | Systems, devices, and methods for ablation using surgical clamps |
| WO2019217433A1 (en) | 2018-05-07 | 2019-11-14 | Farapulse, Inc. | Systems, apparatuses and methods for delivery of ablative energy to tissue |
| CN112087978B (en) | 2018-05-07 | 2023-01-17 | 波士顿科学医学有限公司 | epicardial ablation catheter |
| CN119074196A (en) | 2018-05-07 | 2024-12-06 | 波士顿科学医学有限公司 | Systems, devices and methods for filtering high voltage noise induced by pulsed electric field ablation |
| US12156979B2 (en) | 2018-05-21 | 2024-12-03 | St. Jude Medical, Cardiology Division, Inc. | Deflectable catheter shaft with pullwire anchor feature |
| EP3768185B1 (en)* | 2018-05-21 | 2023-06-14 | St. Jude Medical, Cardiology Division, Inc. | Radio-frequency ablation and direct current electroporation catheters |
| US11596412B2 (en) | 2018-05-25 | 2023-03-07 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11058430B2 (en) | 2018-05-25 | 2021-07-13 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11123135B2 (en) | 2018-05-30 | 2021-09-21 | Biosense Webster (Israel) Ltd. | Enhanced large-diameter balloon catheter |
| US10939915B2 (en) | 2018-05-31 | 2021-03-09 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US12114906B2 (en)* | 2018-06-05 | 2024-10-15 | Boston Scientific Scimed, Inc. | Mapping assembly for cryogenic balloon catheter system |
| US10667833B2 (en) | 2018-06-08 | 2020-06-02 | Neuravi Limited | Guidewire with an atraumatic clot-circumventing configured distal end for use in an endovascular medical system |
| US10898216B2 (en) | 2018-06-13 | 2021-01-26 | DePuy Synthes Products, Inc. | Vasculature obstruction capture device |
| US11819229B2 (en) | 2019-06-19 | 2023-11-21 | Boston Scientific Scimed, Inc. | Balloon surface photoacoustic pressure wave generation to disrupt vascular lesions |
| EP3809988B1 (en) | 2018-06-21 | 2023-06-07 | Shockwave Medical, Inc. | System for treating occlusions in body lumens |
| US12102781B2 (en) | 2018-06-29 | 2024-10-01 | Biosense Webster (Israel) Ltd. | Reinforcement for irrigated electrophysiology balloon catheter with flexible-circuit electrodes |
| AU2019204522A1 (en) | 2018-07-30 | 2020-02-13 | DePuy Synthes Products, Inc. | Systems and methods of manufacturing and using an expansion ring |
| AU2019315849A1 (en)* | 2018-08-01 | 2021-03-18 | Adagio Medical, Inc. | Ablation catheter having an expandable treatment portion |
| US10905431B2 (en) | 2018-08-03 | 2021-02-02 | DePuy Synthes Products, Inc. | Spiral delivery system for embolic braid |
| US10456280B1 (en) | 2018-08-06 | 2019-10-29 | DePuy Synthes Products, Inc. | Systems and methods of using a braided implant |
| US10278848B1 (en) | 2018-08-06 | 2019-05-07 | DePuy Synthes Products, Inc. | Stent delivery with expansion assisting delivery wire |
| US11051825B2 (en) | 2018-08-08 | 2021-07-06 | DePuy Synthes Products, Inc. | Delivery system for embolic braid |
| US10813780B2 (en) | 2018-08-08 | 2020-10-27 | DePuy Synthes Products, Inc. | Intraluminal implant delivery system and method |
| WO2020039392A2 (en) | 2018-08-23 | 2020-02-27 | St. Jude Medical, Cardiology Division, Inc. | Curved high density electrode mapping catheter |
| CN113015495A (en) | 2018-09-11 | 2021-06-22 | 阿卡赫特有限公司 | Heating steam ablation system and method for treating heart disease |
| US10842498B2 (en) | 2018-09-13 | 2020-11-24 | Neuravi Limited | Systems and methods of restoring perfusion to a vessel |
| US11071585B2 (en) | 2018-09-14 | 2021-07-27 | Biosense Webster (Israel) Ltd. | Systems and methods of ablating cardiac tissue |
| US10687892B2 (en) | 2018-09-20 | 2020-06-23 | Farapulse, Inc. | Systems, apparatuses, and methods for delivery of pulsed electric field ablative energy to endocardial tissue |
| EP3626212A3 (en) | 2018-09-20 | 2020-07-22 | DePuy Synthes Products, Inc. | Stent with shaped wires |
| US11123077B2 (en) | 2018-09-25 | 2021-09-21 | DePuy Synthes Products, Inc. | Intrasaccular device positioning and deployment system |
| US12082936B2 (en) | 2018-09-27 | 2024-09-10 | St. Jude Medical, Cardiology Division, Inc. | Uniform mapping balloon |
| US11406416B2 (en) | 2018-10-02 | 2022-08-09 | Neuravi Limited | Joint assembly for vasculature obstruction capture device |
| US11918762B2 (en) | 2018-10-03 | 2024-03-05 | St. Jude Medical, Cardiology Division, Inc. | Reduced actuation force electrophysiology catheter handle |
| US11253287B2 (en) | 2018-10-04 | 2022-02-22 | Neuravi Limited | Retrograde blood flow occlusion flushing device |
| US11076861B2 (en) | 2018-10-12 | 2021-08-03 | DePuy Synthes Products, Inc. | Folded aneurysm treatment device and delivery method |
| US12178582B2 (en) | 2018-11-09 | 2024-12-31 | Acutus Medical, Inc. | Systems and methods for calculating patient information |
| US20220008125A1 (en)* | 2018-11-22 | 2022-01-13 | Afreeze Gmbh | Ablation device with adjustable ablation applicator size, ablation system, and method of operating an ablation device |
| US11547473B2 (en)* | 2018-12-11 | 2023-01-10 | Neurent Medical Limited | Systems and methods for therapeutic nasal neuromodulation |
| US11406392B2 (en) | 2018-12-12 | 2022-08-09 | DePuy Synthes Products, Inc. | Aneurysm occluding device for use with coagulating agents |
| US11147562B2 (en) | 2018-12-12 | 2021-10-19 | DePuy Synthes Products, Inc. | Systems and methods for embolic implant detachment |
| US11272939B2 (en) | 2018-12-18 | 2022-03-15 | DePuy Synthes Products, Inc. | Intrasaccular flow diverter for treating cerebral aneurysms |
| JP6808709B2 (en)* | 2018-12-25 | 2021-01-06 | 清明 本間 | Catheter and catheter system |
| US11039944B2 (en) | 2018-12-27 | 2021-06-22 | DePuy Synthes Products, Inc. | Braided stent system with one or more expansion rings |
| US20200205889A1 (en)* | 2018-12-28 | 2020-07-02 | Biosense Webster (Israel) Ltd. | Balloon Catheter with Distal End Having a Recessed Shape |
| US11134953B2 (en) | 2019-02-06 | 2021-10-05 | DePuy Synthes Products, Inc. | Adhesive cover occluding device for aneurysm treatment |
| US11273285B2 (en) | 2019-02-07 | 2022-03-15 | DePuy Synthes Products, Inc. | Ancillary device for detaching implants |
| CN109717945B (en)* | 2019-02-20 | 2024-05-10 | 上海安钛克医疗科技有限公司 | Ablation saccule form control device |
| ES2910600T3 (en) | 2019-03-04 | 2022-05-12 | Neuravi Ltd | Powered Clot Recovery Catheter |
| US11382633B2 (en) | 2019-03-06 | 2022-07-12 | DePuy Synthes Products, Inc. | Strut flow diverter for cerebral aneurysms and methods for preventing strut entanglement |
| CN113660911B (en)* | 2019-03-26 | 2024-07-23 | 泰尔茂株式会社 | medical instruments |
| US11337706B2 (en) | 2019-03-27 | 2022-05-24 | DePuy Synthes Products, Inc. | Aneurysm treatment device |
| US11547471B2 (en)* | 2019-03-27 | 2023-01-10 | Gyrus Acmi, Inc. | Device with loop electrodes for treatment of menorrhagia |
| US11185334B2 (en) | 2019-03-28 | 2021-11-30 | DePuy Synthes Products, Inc. | Single lumen reduced profile occlusion balloon catheter |
| EP3946123B1 (en)* | 2019-04-04 | 2024-05-29 | Boston Scientific Scimed, Inc. | Apparatus for focal ablation |
| US11051928B2 (en) | 2019-04-11 | 2021-07-06 | Neuravi Limited | Floating carotid filter |
| CN111184553B (en)* | 2019-04-29 | 2023-05-09 | 谱创医疗科技(上海)有限公司 | High coverage low profile electrode assembly for angioplasty impact waveguides |
| US11931522B2 (en) | 2019-05-09 | 2024-03-19 | Neuravi Limited | Inflation lumen kink protection and balloon profile |
| US11607531B2 (en) | 2019-05-09 | 2023-03-21 | Neuravi Limited | Balloon catheter with venting of residual air in a proximal direction |
| US11957855B2 (en) | 2019-05-09 | 2024-04-16 | Neuravi Limited | Balloon guide catheter with positive venting of residual air |
| US11571553B2 (en) | 2019-05-09 | 2023-02-07 | Neuravi Limited | Balloon guide catheter with thermally expandable material |
| USD959659S1 (en) | 2019-05-10 | 2022-08-02 | DePuy Synthes Products, Inc. | Implant release handle |
| US11497504B2 (en) | 2019-05-21 | 2022-11-15 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable implanted braid |
| US11672542B2 (en) | 2019-05-21 | 2023-06-13 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable ball segment |
| US11602350B2 (en) | 2019-12-05 | 2023-03-14 | DePuy Synthes Products, Inc. | Intrasaccular inverting braid with highly flexible fill material |
| US11607226B2 (en) | 2019-05-21 | 2023-03-21 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device with corrugations |
| US10653425B1 (en) | 2019-05-21 | 2020-05-19 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US11413046B2 (en) | 2019-05-21 | 2022-08-16 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US11278292B2 (en) | 2019-05-21 | 2022-03-22 | DePuy Synthes Products, Inc. | Inverting braided aneurysm treatment system and method |
| CA3135773A1 (en) | 2019-06-04 | 2020-12-10 | Acutus Medical, Inc. | Systems and methods for performing localization within a body |
| US11406403B2 (en) | 2019-06-14 | 2022-08-09 | Neuravi Limited | Visibility of mechanical thrombectomy device during diagnostic imaging |
| US11109939B2 (en) | 2019-06-14 | 2021-09-07 | DePuy Synthes Products, Inc. | Intravascular devices with radiopaque body markers |
| US11253265B2 (en) | 2019-06-18 | 2022-02-22 | DePuy Synthes Products, Inc. | Pull wire detachment for intravascular devices |
| US12402946B2 (en) | 2019-06-19 | 2025-09-02 | Boston Scientific Scimed, Inc. | Breakdown of laser pulse energy for breakup of vascular calcium |
| US11717139B2 (en) | 2019-06-19 | 2023-08-08 | Bolt Medical, Inc. | Plasma creation via nonaqueous optical breakdown of laser pulse energy for breakup of vascular calcium |
| EP3917426B1 (en) | 2019-06-20 | 2023-09-06 | Axon Therapies, Inc. | Devices for endovascular ablation of a splanchnic nerve |
| US11660427B2 (en) | 2019-06-24 | 2023-05-30 | Boston Scientific Scimed, Inc. | Superheating system for inertial impulse generation to disrupt vascular lesions |
| US12280223B2 (en) | 2019-06-26 | 2025-04-22 | Boston Scientific Scimed, Inc. | Focusing element for plasma system to disrupt vascular lesions |
| WO2020263534A1 (en)* | 2019-06-27 | 2020-12-30 | Verily Life Sciences Llc | Thin film mapping catheter |
| JP2022539155A (en)* | 2019-06-28 | 2022-09-07 | ニューレント メディカル リミテッド | Systems and methods for targeted therapeutic nasal nerve modulation |
| US11426174B2 (en) | 2019-10-03 | 2022-08-30 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant mechanical release |
| US11207494B2 (en) | 2019-07-03 | 2021-12-28 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant distal portion |
| US11266426B2 (en) | 2019-07-10 | 2022-03-08 | DePuy Synthes Products, Inc. | Streamlined treatment of clot removal, angioplasty and prevention of restenosis using a single integrated intravascular device |
| US11266427B2 (en) | 2019-07-10 | 2022-03-08 | Neuravi Limited | Self-expanding intravascular medical device |
| US11395675B2 (en) | 2019-07-11 | 2022-07-26 | DePuy Synthes Products, Inc. | Clot retriever cleaning for reinsertion |
| CN112274238A (en)* | 2019-07-25 | 2021-01-29 | 江苏霆升科技有限公司 | Interventional therapy system and method |
| US12114918B2 (en)* | 2019-08-15 | 2024-10-15 | Biosense Webster (Israel) Ltd. | Dynamic ablation and sensing according to contact of segmented electrodes |
| EP3791815B1 (en) | 2019-09-11 | 2024-06-26 | Neuravi Limited | Expandable mouth catheter |
| AU2020346827A1 (en) | 2019-09-12 | 2022-03-31 | Relievant Medsystems, Inc. | Systems and methods for tissue modulation |
| US12369975B2 (en) | 2019-09-12 | 2025-07-29 | Biosense Webster (Israel) Ltd. | Balloon catheter with force sensor |
| US12376859B2 (en) | 2019-09-17 | 2025-08-05 | DePuy Synthes Products, Inc. | Embolic coil proximal connecting element and stretch resistant fiber |
| US11439403B2 (en) | 2019-09-17 | 2022-09-13 | DePuy Synthes Products, Inc. | Embolic coil proximal connecting element and stretch resistant fiber |
| US10625080B1 (en) | 2019-09-17 | 2020-04-21 | Farapulse, Inc. | Systems, apparatuses, and methods for detecting ectopic electrocardiogram signals during pulsed electric field ablation |
| WO2021061523A1 (en) | 2019-09-24 | 2021-04-01 | Shockwave Medical, Inc. | System for treating thrombus in body lumens |
| WO2021061451A1 (en) | 2019-09-24 | 2021-04-01 | Shockwave Medical, Inc. | Lesion crossing shock wave catheter |
| EP4512350A3 (en) | 2019-09-24 | 2025-05-07 | Shockwave Medical, Inc. | Low profile electrodes for a shock wave catheter |
| WO2021065875A1 (en)* | 2019-09-30 | 2021-04-08 | テルモ株式会社 | Medical device |
| EP4035721B1 (en)* | 2019-09-30 | 2025-08-20 | TERUMO Kabushiki Kaisha | Medical device |
| US11633228B2 (en) | 2019-10-04 | 2023-04-25 | Biosense Webster (Israel) Ltd. | Identifying pulmonary vein occlusion by dimension deformations of balloon catheter |
| US12369974B2 (en) | 2019-10-10 | 2025-07-29 | Biosense Webster (Israel) Ltd. | Touch indication of balloon-catheter ablation electrode via balloon surface temperature measurement |
| US20210121231A1 (en)* | 2019-10-23 | 2021-04-29 | Biosense Webster (Israel) Ltd. | Cardiac mapping catheter with square-spaced electrodes |
| US11712231B2 (en) | 2019-10-29 | 2023-08-01 | Neuravi Limited | Proximal locking assembly design for dual stent mechanical thrombectomy device |
| US20210128183A1 (en) | 2019-10-31 | 2021-05-06 | Neuravi Limited | Thrombectomy and stenting system |
| US11583339B2 (en) | 2019-10-31 | 2023-02-21 | Bolt Medical, Inc. | Asymmetrical balloon for intravascular lithotripsy device and method |
| US12137967B2 (en) | 2019-11-12 | 2024-11-12 | Biosense Webster (Israel) Ltd. | Accurate positioning and shape visualization of balloon catheter ablation tags |
| US12102384B2 (en) | 2019-11-13 | 2024-10-01 | Bolt Medical, Inc. | Dynamic intravascular lithotripsy device with movable energy guide |
| US11376013B2 (en) | 2019-11-18 | 2022-07-05 | DePuy Synthes Products, Inc. | Implant delivery system with braid cup formation |
| US11065047B2 (en) | 2019-11-20 | 2021-07-20 | Farapulse, Inc. | Systems, apparatuses, and methods for protecting electronic components from high power noise induced by high voltage pulses |
| US11497541B2 (en) | 2019-11-20 | 2022-11-15 | Boston Scientific Scimed, Inc. | Systems, apparatuses, and methods for protecting electronic components from high power noise induced by high voltage pulses |
| US11628282B2 (en) | 2019-11-25 | 2023-04-18 | Neuravi Limited | No preparation balloon guide catheter |
| US10842572B1 (en) | 2019-11-25 | 2020-11-24 | Farapulse, Inc. | Methods, systems, and apparatuses for tracking ablation devices and generating lesion lines |
| US11779364B2 (en) | 2019-11-27 | 2023-10-10 | Neuravi Limited | Actuated expandable mouth thrombectomy catheter |
| US11839725B2 (en) | 2019-11-27 | 2023-12-12 | Neuravi Limited | Clot retrieval device with outer sheath and inner catheter |
| US11517340B2 (en) | 2019-12-03 | 2022-12-06 | Neuravi Limited | Stentriever devices for removing an occlusive clot from a vessel and methods thereof |
| CN110974398A (en)* | 2019-12-04 | 2020-04-10 | 宁波胜杰康生物科技有限公司 | Cryoablation system with magnetic navigation |
| US12274497B2 (en) | 2019-12-18 | 2025-04-15 | Bolt Medical, Inc. | Multiplexer for laser-driven intravascular lithotripsy device |
| US11457926B2 (en) | 2019-12-18 | 2022-10-04 | DePuy Synthes Products, Inc. | Implant having an intrasaccular section and intravascular section |
| US11413090B2 (en) | 2020-01-17 | 2022-08-16 | Axon Therapies, Inc. | Methods and devices for endovascular ablation of a splanchnic nerve |
| US11457922B2 (en) | 2020-01-22 | 2022-10-04 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant distal portion |
| US11992241B2 (en) | 2020-01-31 | 2024-05-28 | DePuy Synthes Products, Inc. | System to assist delivery of a mechanical intravascular treatment device |
| US11957354B2 (en) | 2020-02-10 | 2024-04-16 | DePuy Synthes Products, Inc. | Aneurysm implant support device |
| US11432822B2 (en) | 2020-02-14 | 2022-09-06 | DePuy Synthes Products, Inc. | Intravascular implant deployment system |
| IL295739A (en)* | 2020-02-20 | 2022-10-01 | Univ Leland Stanford Junior | A system and method for targeting and treating abnormal biological rhythms |
| JP2021132817A (en)* | 2020-02-26 | 2021-09-13 | テルモ株式会社 | Medical device and medical device set |
| US11633198B2 (en) | 2020-03-05 | 2023-04-25 | Neuravi Limited | Catheter proximal joint |
| US11944327B2 (en) | 2020-03-05 | 2024-04-02 | Neuravi Limited | Expandable mouth aspirating clot retrieval catheter |
| US11672599B2 (en) | 2020-03-09 | 2023-06-13 | Bolt Medical, Inc. | Acoustic performance monitoring system and method within intravascular lithotripsy device |
| US20210290286A1 (en) | 2020-03-18 | 2021-09-23 | Bolt Medical, Inc. | Optical analyzer assembly and method for intravascular lithotripsy device |
| US11883043B2 (en) | 2020-03-31 | 2024-01-30 | DePuy Synthes Products, Inc. | Catheter funnel extension |
| US11707323B2 (en) | 2020-04-03 | 2023-07-25 | Bolt Medical, Inc. | Electrical analyzer assembly for intravascular lithotripsy device |
| US11759217B2 (en) | 2020-04-07 | 2023-09-19 | Neuravi Limited | Catheter tubular support |
| WO2021205229A1 (en) | 2020-04-09 | 2021-10-14 | Neurent Medical Limited | Systems and methods for improving sleep with therapeutic nasal treatment |
| WO2021208847A1 (en)* | 2020-04-13 | 2021-10-21 | 杭州德诺电生理医疗科技有限公司 | Ablation device and preparation method therefor |
| US11717308B2 (en) | 2020-04-17 | 2023-08-08 | Neuravi Limited | Clot retrieval device for removing heterogeneous clots from a blood vessel |
| US11730501B2 (en) | 2020-04-17 | 2023-08-22 | Neuravi Limited | Floating clot retrieval device for removing clots from a blood vessel |
| US11871946B2 (en) | 2020-04-17 | 2024-01-16 | Neuravi Limited | Clot retrieval device for removing clot from a blood vessel |
| US11553961B2 (en)* | 2020-04-30 | 2023-01-17 | Biosense Webster (Israel) Ltd. | Catheter with stretchable irrigation tube |
| US11523831B2 (en) | 2020-04-30 | 2022-12-13 | DePuy Synthes Products, Inc. | Intrasaccular flow diverter |
| US12295654B2 (en) | 2020-06-03 | 2025-05-13 | Boston Scientific Scimed, Inc. | System and method for maintaining balloon integrity within intravascular lithotripsy device with plasma generator |
| US12207870B2 (en) | 2020-06-15 | 2025-01-28 | Boston Scientific Scimed, Inc. | Spectroscopic tissue identification for balloon intravascular lithotripsy guidance |
| US11737771B2 (en) | 2020-06-18 | 2023-08-29 | Neuravi Limited | Dual channel thrombectomy device |
| US11937836B2 (en) | 2020-06-22 | 2024-03-26 | Neuravi Limited | Clot retrieval system with expandable clot engaging framework |
| US11439418B2 (en) | 2020-06-23 | 2022-09-13 | Neuravi Limited | Clot retrieval device for removing clot from a blood vessel |
| US11395669B2 (en) | 2020-06-23 | 2022-07-26 | Neuravi Limited | Clot retrieval device with flexible collapsible frame |
| US20210401490A1 (en)* | 2020-06-29 | 2021-12-30 | Biosense Webster (Israel) Ltd. | Temperature control for ire |
| US11951026B2 (en) | 2020-06-30 | 2024-04-09 | DePuy Synthes Products, Inc. | Implantable medical device detachment system with flexible braid section |
| DE102020208936A1 (en)* | 2020-07-16 | 2022-01-20 | Robert Bosch Gesellschaft mit beschränkter Haftung | Surgical instrument and method of its operation |
| US12310652B2 (en) | 2020-07-24 | 2025-05-27 | Boston Scientific Scimed, Inc. | Hybrid electroporation ablation catheter |
| WO2022020478A1 (en) | 2020-07-24 | 2022-01-27 | Boston Scientific Scimed Inc | Electric field application for single shot cardiac ablation by irreversible electroporation |
| US20220031341A1 (en) | 2020-07-29 | 2022-02-03 | Neuravi Limited | Adhesive-Free Bonded Balloon for a Balloon Guide Catheter With Minimal Outer Profile |
| JP2023538213A (en) | 2020-08-14 | 2023-09-07 | イクテロ メディカル,インコーポレイテッド | Systems, devices, and methods for ablation and defunctionalization of the gallbladder |
| WO2022038546A1 (en) | 2020-08-18 | 2022-02-24 | St. Jude Medical, Cardiology Division, Inc. | High-density electrode catheters with magnetic position tracking |
| US12048479B2 (en)* | 2020-09-10 | 2024-07-30 | Biosense Webster (Israel) Ltd. | Surface mounted electrode catheter |
| US20220071695A1 (en)* | 2020-09-10 | 2022-03-10 | Biosense Webster (Israel) Ltd. | Flex Circuit and Surface Mounted Electrode Catheter |
| CN114246662B (en)* | 2020-09-22 | 2024-09-03 | 苏州华凡创硕医疗科技有限公司 | Cardiac ablation system and method |
| US11864781B2 (en) | 2020-09-23 | 2024-01-09 | Neuravi Limited | Rotating frame thrombectomy device |
| US12082876B1 (en) | 2020-09-28 | 2024-09-10 | Relievant Medsystems, Inc. | Introducer drill |
| US12257437B2 (en) | 2020-09-30 | 2025-03-25 | Covidien Lp | Intravenous phrenic nerve stimulation lead |
| WO2022072385A2 (en) | 2020-09-30 | 2022-04-07 | Boston Scientific Scimed Inc | Pretreatment waveform for irreversible electroporation |
| US12239364B2 (en) | 2020-10-07 | 2025-03-04 | Biosense Webster (Israel) Ltd. | Printed proximal electrodes of an expandable catheter for use as a common electrode |
| US11974803B2 (en) | 2020-10-12 | 2024-05-07 | Biosense Webster (Israel) Ltd. | Basket catheter with balloon |
| US11992232B2 (en) | 2020-10-27 | 2024-05-28 | Shockwave Medical, Inc. | System for treating thrombus in body lumens |
| US12083288B2 (en)* | 2020-12-02 | 2024-09-10 | Biosense Webster (Israel) Ltd. | One-motion handle for steerable catheter |
| IL303202A (en)* | 2020-12-02 | 2023-07-01 | Biosense Webster Israel Ltd | Single-motion handle for an indwelling catheter |
| US11826520B2 (en) | 2020-12-08 | 2023-11-28 | DePuy Synthes Products, Inc. | Catheter designs for enhanced column strength |
| US11786698B2 (en) | 2020-12-08 | 2023-10-17 | DePuy Synthes Products, Inc. | Catheter with textured surface |
| US12016610B2 (en) | 2020-12-11 | 2024-06-25 | Bolt Medical, Inc. | Catheter system for valvuloplasty procedure |
| EP4268150A4 (en) | 2020-12-22 | 2024-12-18 | Relievant Medsystems, Inc. | PREDICTION OF CANDIDATES FOR SPINAL NEUROMODULATION |
| US11937837B2 (en) | 2020-12-29 | 2024-03-26 | Neuravi Limited | Fibrin rich / soft clot mechanical thrombectomy device |
| CN114681038A (en)* | 2020-12-31 | 2022-07-01 | 杭州德诺电生理医疗科技有限公司 | Ablation device and ablation system |
| CN112674865A (en)* | 2021-01-05 | 2021-04-20 | 安杭医疗科技(杭州)有限公司 | Double-ball cage electrode catheter device for tissue ablation in human body cavity |
| EP4277548B1 (en) | 2021-01-12 | 2025-06-04 | Bolt Medical, Inc. | Balloon assembly for valvuloplasty catheter system |
| US11672585B2 (en) | 2021-01-12 | 2023-06-13 | Bolt Medical, Inc. | Balloon assembly for valvuloplasty catheter system |
| US12029442B2 (en) | 2021-01-14 | 2024-07-09 | Neuravi Limited | Systems and methods for a dual elongated member clot retrieval apparatus |
| US11957852B2 (en) | 2021-01-14 | 2024-04-16 | Biosense Webster (Israel) Ltd. | Intravascular balloon with slidable central irrigation tube |
| JP2024504184A (en) | 2021-01-27 | 2024-01-30 | ボストン サイエンティフィック サイムド,インコーポレイテッド | Voltage-controlled pulse sequence for irreversible electroporation ablation |
| CN113143444B (en)* | 2021-01-28 | 2025-01-24 | 上海玄宇医疗器械有限公司 | A cardiac pulse electric field ablation catheter device |
| US11849995B2 (en) | 2021-02-18 | 2023-12-26 | Biosense Webster (Israel) Ltd. | Detection of balloon catheter tissue contact using optical measurement |
| US11872354B2 (en) | 2021-02-24 | 2024-01-16 | Neuravi Limited | Flexible catheter shaft frame with seam |
| US12064130B2 (en) | 2021-03-18 | 2024-08-20 | Neuravi Limited | Vascular obstruction retrieval device having sliding cages pinch mechanism |
| US12357817B2 (en) | 2021-04-06 | 2025-07-15 | Aerin Medical Inc. | Nasal neuromodulation devices and methods |
| EP4312769B1 (en) | 2021-04-16 | 2025-04-09 | Physcade, Inc. | Personalized heart rhythm therapy |
| CN113100919B (en)* | 2021-04-23 | 2025-05-27 | 上海安钛克医疗科技有限公司 | Electrodes, electrophysiology catheters and ablation systems |
| US11648057B2 (en) | 2021-05-10 | 2023-05-16 | Bolt Medical, Inc. | Optical analyzer assembly with safety shutdown system for intravascular lithotripsy device |
| US11974764B2 (en) | 2021-06-04 | 2024-05-07 | Neuravi Limited | Self-orienting rotating stentriever pinching cells |
| US11806075B2 (en) | 2021-06-07 | 2023-11-07 | Bolt Medical, Inc. | Active alignment system and method for laser optical coupling |
| JP7574442B2 (en)* | 2021-06-30 | 2024-10-28 | 日本ライフライン株式会社 | catheter |
| US11998213B2 (en) | 2021-07-14 | 2024-06-04 | DePuy Synthes Products, Inc. | Implant delivery with modified detachment feature and pull wire engagement |
| US11877761B2 (en) | 2021-08-05 | 2024-01-23 | Nextern Innovation, Llc | Systems, devices and methods for monitoring voltage and current and controlling voltage of voltage pulse generators |
| US12089861B2 (en) | 2021-08-05 | 2024-09-17 | Nextern Innovation, Llc | Intravascular lithotripsy system and device |
| US11801066B2 (en) | 2021-08-05 | 2023-10-31 | Nextern Innovation, Llc | Systems, devices and methods for selection of arc location within a lithoplasty balloon spark gap |
| US11896248B2 (en) | 2021-08-05 | 2024-02-13 | Nextern Innovation, Llc | Systems, devices and methods for generating subsonic pressure waves in intravascular lithotripsy |
| US11957369B2 (en) | 2021-08-05 | 2024-04-16 | Nextern Innovation, Llc | Intravascular lithotripsy systems and methods |
| CN117835929A (en) | 2021-08-10 | 2024-04-05 | 菲斯卡德公司 | Treatment system with sensing and ablation catheter for treatment of heart rhythm disorders |
| US12114905B2 (en) | 2021-08-27 | 2024-10-15 | Biosense Webster (Israel) Ltd. | Reinforcement and stress relief for an irrigated electrophysiology balloon catheter with flexible-circuit electrodes |
| CN115721404A (en)* | 2021-08-30 | 2023-03-03 | 四川锦江电子医疗器械科技股份有限公司 | A form-changing catheter and its use method |
| CN114668484B (en)* | 2021-09-13 | 2025-04-25 | 杭州旸瑞医疗器械有限公司 | Ablation device and ablation catheter thereof |
| US12369920B2 (en) | 2021-09-22 | 2025-07-29 | DePuy Synthes Products, Inc. | Introducer sheath having an intentional friction zone to hold in position a delivery system suitable for implantable intravascular devices |
| US11937839B2 (en) | 2021-09-28 | 2024-03-26 | Neuravi Limited | Catheter with electrically actuated expandable mouth |
| US12023098B2 (en) | 2021-10-05 | 2024-07-02 | Shockwave Medical, Inc. | Lesion crossing shock wave catheter |
| US12011186B2 (en) | 2021-10-28 | 2024-06-18 | Neuravi Limited | Bevel tip expandable mouth catheter with reinforcing ring |
| US12433668B1 (en) | 2021-11-08 | 2025-10-07 | Relievant Medsystems, Inc. | Impedance stoppage mitigation during radiofrequency tissue ablation procedures |
| US11751881B2 (en) | 2021-11-26 | 2023-09-12 | DePuy Synthes Products, Inc. | Securement wire withstanding forces during deployment of implantable intravascular treatment device using a delivery and detachment system |
| US11564591B1 (en) | 2021-11-29 | 2023-01-31 | Physcade, Inc. | System and method for diagnosing and treating biological rhythm disorders |
| EP4440462A1 (en)* | 2021-12-01 | 2024-10-09 | Alleima Karlsruhe GmbH | Ablating apparatus |
| US11839391B2 (en) | 2021-12-14 | 2023-12-12 | Bolt Medical, Inc. | Optical emitter housing assembly for intravascular lithotripsy device |
| CN114271928B (en)* | 2021-12-26 | 2023-07-28 | 上海安通医疗科技有限公司 | Combined ablation catheter applicable to radial artery |
| US11844490B2 (en) | 2021-12-30 | 2023-12-19 | DePuy Synthes Products, Inc. | Suture linkage for inhibiting premature embolic implant deployment |
| US20230210592A1 (en)* | 2021-12-30 | 2023-07-06 | Biosense Webster (Israel) Ltd. | Dual balloons for pulmonary vein isolation |
| US20230210589A1 (en)* | 2021-12-30 | 2023-07-06 | Biosense Webster (Israel) Ltd. | Basket Catheter Having Ablation Electrodes and Temperature Sensors |
| US20230210590A1 (en)* | 2021-12-30 | 2023-07-06 | Biosense Webster (Israel) Ltd. | Multi-Form Catheter |
| US11937824B2 (en) | 2021-12-30 | 2024-03-26 | DePuy Synthes Products, Inc. | Implant detachment systems with a modified pull wire |
| US12011171B2 (en) | 2022-01-06 | 2024-06-18 | DePuy Synthes Products, Inc. | Systems and methods for inhibiting premature embolic implant deployment |
| US12220131B2 (en) | 2022-01-06 | 2025-02-11 | DePuy Synthes Products, Inc. | Delivery and detachment system imposing a friction force on a securement wire to minimize movement of an implantable intravascular device |
| US20230225788A1 (en)* | 2022-01-20 | 2023-07-20 | Biosense Webster (Israel) Ltd. | Systems and methods for c-shaped spines forming a spherical basket for improved tissue contact and current delivery |
| US11937825B2 (en) | 2022-03-02 | 2024-03-26 | DePuy Synthes Products, Inc. | Hook wire for preventing premature embolic implant detachment |
| US12137915B2 (en) | 2022-03-03 | 2024-11-12 | DePuy Synthes Products, Inc. | Elongating wires for inhibiting premature implant detachment |
| US11937826B2 (en) | 2022-03-14 | 2024-03-26 | DePuy Synthes Products, Inc. | Proximal link wire for preventing premature implant detachment |
| US12408932B2 (en) | 2022-03-23 | 2025-09-09 | Venturemed Group, Inc. | Intravascular device having feedback elements |
| US20230301712A1 (en)* | 2022-03-25 | 2023-09-28 | Biosense Webster (Israel) Ltd. | Elongated trapezoidal electrodes of a basket catheter and methods of making the same |
| CN114569232B (en)* | 2022-04-11 | 2023-05-05 | 上海安通医疗科技有限公司 | Ultrasonic ablation catheter |
| US20230346464A1 (en)* | 2022-04-28 | 2023-11-02 | Biosense Webster (Israel) Ltd. | Basket catheter with cloverleaf structure to prevent buckling and retention feature for electrodes |
| WO2023212185A1 (en)* | 2022-04-28 | 2023-11-02 | Mayo Foundation For Medical Education And Research | Devices and methods for ablation of tissue |
| US12402886B2 (en) | 2022-06-23 | 2025-09-02 | DePuy Synthes Products, Inc. | Detachment indicator for implant deployment |
| CN115105189A (en)* | 2022-06-30 | 2022-09-27 | 上海睿刀医疗科技有限公司 | Ablation catheter and ablation device |
| US12396730B2 (en) | 2022-09-28 | 2025-08-26 | DePuy Synthes Products, Inc. | Braided implant with detachment mechanism |
| US20240225591A9 (en)* | 2022-10-24 | 2024-07-11 | SoundCath, Inc. | Ultrasonic imaging ablation catheter system and method |
| WO2024136997A1 (en)* | 2022-12-21 | 2024-06-27 | Walzman Daniel Ezra | Catheter with energy delivery member and valve for intravascular lithotripsy |
| US20240216046A1 (en)* | 2022-12-29 | 2024-07-04 | Biosense Webster (Israel) Ltd. | Systems and methods for linear spines forming a spherical basket for improved tissue contact and current delivery |
| CN115778487A (en)* | 2023-02-02 | 2023-03-14 | 上海佳沐垚医疗科技有限公司 | Shock wave balloon catheter and catheter system for targeted therapy |
| US20240260932A1 (en)* | 2023-02-08 | 2024-08-08 | SoundCath, Inc. | INTEGRATED CARDIAC MAPPING AND PIEZOELECTRIC MICROMACHINED ULTRASONIC TRANSDUCER (pMUT) ULTRASONIC IMAGING CATHETER SYSTEM AND METHOD |
| EP4417152A1 (en)* | 2023-02-16 | 2024-08-21 | BIOTRONIK SE & Co. KG | Catheter and method for cardiac surgery generating an interatrial shunt |
| US12376879B2 (en) | 2023-03-03 | 2025-08-05 | Theraheart Inc. | Expandable elements for shunting catheters |
| US12290268B2 (en) | 2023-03-31 | 2025-05-06 | Shockwave Medical, Inc. | Shockwave catheters for treating rhinosinusitis |
| US12290310B2 (en) | 2023-04-06 | 2025-05-06 | Theraheart Inc. | Slicing elements for shunting catheters |
| US12035932B1 (en) | 2023-04-21 | 2024-07-16 | Shockwave Medical, Inc. | Intravascular lithotripsy catheter with slotted emitter bands |
| US20240390053A1 (en)* | 2023-05-26 | 2024-11-28 | Cardiofocus, Inc. | Deployable structures to provide electrodes on the surface of an endoscopically guided laser ablation catheter for use in ablation and electrophysiological mapping |
| US12262943B2 (en) | 2023-06-15 | 2025-04-01 | Theraheart Inc. | Expandable ablation mechanisms for shunting catheters |
| US12220141B2 (en) | 2023-06-29 | 2025-02-11 | Shockwave Medical, Inc. | Catheter system with independently controllable bubble and arc generation |
| US12426904B2 (en) | 2023-11-17 | 2025-09-30 | Shockwave Medical, Inc. | Intravascular lithotripsy catheter with oscillating impactor |
| US12402899B2 (en) | 2023-11-30 | 2025-09-02 | Shockwave Medical, Inc. | Systems, devices, and methods for generating shock waves in a forward direction |
| US12290370B1 (en) | 2024-01-19 | 2025-05-06 | Physcade, Inc. | System and method for mapping activation waves to guide treatment of biological rhythm disorders |
| WO2025171173A1 (en) | 2024-02-08 | 2025-08-14 | IV-X Medical, LLC | Intravascular lithotripsy system |
| US12433620B2 (en) | 2024-02-23 | 2025-10-07 | Shockwave Medical, Inc. | Locus emitter shock wave catheter devices with increased longevity and higher sonic output |
| CN117796901B (en)* | 2024-02-28 | 2024-06-11 | 乐普(北京)医疗器械股份有限公司 | Pulsed electric field ablation catheter |
| US12201354B1 (en)* | 2024-04-01 | 2025-01-21 | Theraheart Inc. | Expandable ablation mechanisms for shunting catheters |
| US12178458B1 (en) | 2024-05-16 | 2024-12-31 | Shockwave Medical, Inc. | Guidewireless shock wave catheters |
Citations (384)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3516412A (en) | 1965-08-16 | 1970-06-23 | Electro Catheter Corp | Bipolar electrode having irregularity at inserting end thereof and method of insertion |
| US3951136A (en) | 1973-10-10 | 1976-04-20 | Vital Signs, Inc. | Multiple purpose esophageal probe |
| US4017903A (en) | 1975-08-27 | 1977-04-12 | Hewlett-Packard Company | Pulse code modulation recording and/or reproducing system |
| US4112952A (en) | 1977-02-11 | 1978-09-12 | The United States Of America As Represented By The Secretary Of Health, Education And Welfare | Electrode for artificial pacemaker |
| US4411266A (en) | 1980-09-24 | 1983-10-25 | Cosman Eric R | Thermocouple radio frequency lesion electrode |
| US4432377A (en) | 1982-01-29 | 1984-02-21 | Medtronic, Inc. | Biomedical lead with ring electrode and method of making same |
| US4660571A (en) | 1985-07-18 | 1987-04-28 | Cordis Corporation | Percutaneous lead having radially adjustable electrode |
| US4785815A (en) | 1985-10-23 | 1988-11-22 | Cordis Corporation | Apparatus for locating and ablating cardiac conduction pathways |
| US4860769A (en) | 1987-11-12 | 1989-08-29 | Thomas J. Fogarty | Implantable defibrillation electrode |
| US4869248A (en) | 1987-04-17 | 1989-09-26 | Narula Onkar S | Method and apparatus for localized thermal ablation |
| SU1512622A1 (en) | 1987-11-12 | 1989-10-07 | Научно-Исследовательский Институт Онкологии И Медицинской Радиологии Мз Бсср | Electrode device for hyperthermia of hollow organs |
| US4882777A (en) | 1987-04-17 | 1989-11-21 | Narula Onkar S | Catheter |
| US4896671A (en) | 1988-08-01 | 1990-01-30 | C. R. Bard, Inc. | Catheter with contoured ablation electrode |
| SU1544396A1 (en) | 1987-12-08 | 1990-02-23 | 1-Й Ленинградский Медицинский Институт Им.Акад.И.П.Павлова | Electrodestruction device |
| US4907589A (en) | 1988-04-29 | 1990-03-13 | Cosman Eric R | Automatic over-temperature control apparatus for a therapeutic heating device |
| US4920980A (en) | 1987-09-14 | 1990-05-01 | Cordis Corporation | Catheter with controllable tip |
| WO1990006079A1 (en) | 1988-11-25 | 1990-06-14 | Sensor Electronics, Inc. | Radiofrequency ablation catheter |
| US4940064A (en) | 1986-11-14 | 1990-07-10 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US4966597A (en) | 1988-11-04 | 1990-10-30 | Cosman Eric R | Thermometric cardiac tissue ablation electrode with ultra-sensitive temperature detection |
| US5010894A (en) | 1988-01-07 | 1991-04-30 | Edhag Knut O | Intravascular electrode lead usable for cardiac defibrillation |
| US5016808A (en) | 1989-09-14 | 1991-05-21 | Cardiac Pacemakers, Inc. | Implantable tapered spiral endocardial lead for use in internal defibrillation |
| SU1690786A1 (en) | 1989-06-30 | 1991-11-15 | Каунасский Медицинский Институт | Electrocardial electrode |
| US5083565A (en) | 1990-08-03 | 1992-01-28 | Everest Medical Corporation | Electrosurgical instrument for ablating endocardial tissue |
| US5100423A (en) | 1990-08-21 | 1992-03-31 | Medical Engineering & Development Institute, Inc. | Ablation catheter |
| US5156151A (en) | 1991-02-15 | 1992-10-20 | Cardiac Pathways Corporation | Endocardial mapping and ablation system and catheter probe |
| US5184621A (en) | 1991-05-29 | 1993-02-09 | C. R. Bard, Inc. | Steerable guidewire having electrodes for measuring vessel cross-section and blood flow |
| WO1993008756A1 (en) | 1991-11-08 | 1993-05-13 | Ep Technologies, Inc. | Radiofrequency ablation with phase sensitive power detection |
| US5215103A (en) | 1986-11-14 | 1993-06-01 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US5228442A (en) | 1991-02-15 | 1993-07-20 | Cardiac Pathways Corporation | Method for mapping, ablation, and stimulation using an endocardial catheter |
| US5230349A (en) | 1988-11-25 | 1993-07-27 | Sensor Electronics, Inc. | Electrical heating catheter |
| US5231995A (en) | 1986-11-14 | 1993-08-03 | Desai Jawahar M | Method for catheter mapping and ablation |
| US5231987A (en) | 1992-04-10 | 1993-08-03 | Random Technologies, Inc. | Time domain reflectometer-integrity testing system and method for implantable electrode |
| US5234004A (en) | 1988-11-21 | 1993-08-10 | Technomed International | Method and apparatus for the surgical treatment of tissues by thermal effect, and in particular the prostate, using a urethral microwave-emitting probe means |
| US5239999A (en) | 1992-03-27 | 1993-08-31 | Cardiac Pathways Corporation | Helical endocardial catheter probe |
| US5255679A (en) | 1992-06-02 | 1993-10-26 | Cardiac Pathways Corporation | Endocardial catheter for mapping and/or ablation with an expandable basket structure having means for providing selective reinforcement and pressure sensing mechanism for use therewith, and method |
| WO1993025273A1 (en) | 1992-06-05 | 1993-12-23 | Cardiac Pathways Corporation | Catheter having needle electrode for radiofrequency ablation |
| US5281213A (en) | 1992-04-16 | 1994-01-25 | Implemed, Inc. | Catheter for ice mapping and ablation |
| US5309910A (en) | 1992-09-25 | 1994-05-10 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US5313943A (en) | 1992-09-25 | 1994-05-24 | Ep Technologies, Inc. | Catheters and methods for performing cardiac diagnosis and treatment |
| US5318525A (en) | 1992-04-10 | 1994-06-07 | Medtronic Cardiorhythm | Steerable electrode catheter |
| WO1994012098A1 (en) | 1992-12-01 | 1994-06-09 | Cardiac Pathways Corporation | Mapping and ablation catheter with individually deployable arms and method |
| US5324284A (en) | 1992-06-05 | 1994-06-28 | Cardiac Pathways, Inc. | Endocardial mapping and ablation system utilizing a separately controlled ablation catheter and method |
| US5330466A (en) | 1992-12-01 | 1994-07-19 | Cardiac Pathways Corporation | Control mechanism and system and method for steering distal extremity of a flexible elongate member |
| US5334193A (en) | 1992-11-13 | 1994-08-02 | American Cardiac Ablation Co., Inc. | Fluid cooled ablation catheter |
| US5342295A (en) | 1993-09-24 | 1994-08-30 | Cardiac Pathways Corporation | Catheter assembly, catheter and multi-port introducer for use therewith |
| US5342357A (en) | 1992-11-13 | 1994-08-30 | American Cardiac Ablation Co., Inc. | Fluid cooled electrosurgical cauterization system |
| US5345936A (en) | 1991-02-15 | 1994-09-13 | Cardiac Pathways Corporation | Apparatus with basket assembly for endocardial mapping |
| US5348554A (en) | 1992-12-01 | 1994-09-20 | Cardiac Pathways Corporation | Catheter for RF ablation with cooled electrode |
| USD351652S (en) | 1993-06-21 | 1994-10-18 | Ep Technologies, Inc. | Steerable medical catheter handle |
| US5364352A (en) | 1993-03-12 | 1994-11-15 | Heart Rhythm Technologies, Inc. | Catheter for electrophysiological procedures |
| US5365926A (en) | 1986-11-14 | 1994-11-22 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US5383917A (en) | 1991-07-05 | 1995-01-24 | Jawahar M. Desai | Device and method for multi-phase radio-frequency ablation |
| US5391147A (en) | 1992-12-01 | 1995-02-21 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and/or radius and method |
| US5397304A (en) | 1992-04-10 | 1995-03-14 | Medtronic Cardiorhythm | Shapable handle for steerable electrode catheter |
| US5400783A (en) | 1993-10-12 | 1995-03-28 | Cardiac Pathways Corporation | Endocardial mapping apparatus with rotatable arm and method |
| US5411025A (en) | 1992-06-30 | 1995-05-02 | Cordis Webster, Inc. | Cardiovascular catheter with laterally stable basket-shaped electrode array |
| US5433198A (en) | 1993-03-11 | 1995-07-18 | Desai; Jawahar M. | Apparatus and method for cardiac ablation |
| US5433739A (en) | 1993-11-02 | 1995-07-18 | Sluijter; Menno E. | Method and apparatus for heating an intervertebral disc for relief of back pain |
| US5445148A (en) | 1992-04-10 | 1995-08-29 | Medtronic Cardiorhythm | Intracardiac electrical potential reference catheter |
| US5462521A (en) | 1993-12-21 | 1995-10-31 | Angeion Corporation | Fluid cooled and perfused tip for a catheter |
| US5462545A (en) | 1994-01-31 | 1995-10-31 | New England Medical Center Hospitals, Inc. | Catheter electrodes |
| US5465717A (en) | 1991-02-15 | 1995-11-14 | Cardiac Pathways Corporation | Apparatus and Method for ventricular mapping and ablation |
| US5471982A (en) | 1992-09-29 | 1995-12-05 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US5487757A (en) | 1993-07-20 | 1996-01-30 | Medtronic Cardiorhythm | Multicurve deflectable catheter |
| US5492119A (en) | 1993-12-22 | 1996-02-20 | Heart Rhythm Technologies, Inc. | Catheter tip stabilizing apparatus |
| US5507802A (en) | 1993-06-02 | 1996-04-16 | Cardiac Pathways Corporation | Method of mapping and/or ablation using a catheter having a tip with fixation means |
| WO1996010961A1 (en) | 1994-10-07 | 1996-04-18 | Ep Technologies, Inc. | Flexible structures for supporting electrode elements |
| US5509411A (en) | 1993-01-29 | 1996-04-23 | Cardima, Inc. | Intravascular sensing device |
| US5533967A (en) | 1992-12-01 | 1996-07-09 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and method |
| US5536267A (en) | 1993-11-08 | 1996-07-16 | Zomed International | Multiple electrode ablation apparatus |
| US5540681A (en) | 1992-04-10 | 1996-07-30 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of tissue |
| US5545193A (en) | 1993-10-15 | 1996-08-13 | Ep Technologies, Inc. | Helically wound radio-frequency emitting electrodes for creating lesions in body tissue |
| US5545200A (en) | 1993-07-20 | 1996-08-13 | Medtronic Cardiorhythm | Steerable electrophysiology catheter |
| US5545161A (en) | 1992-12-01 | 1996-08-13 | Cardiac Pathways Corporation | Catheter for RF ablation having cooled electrode with electrically insulated sleeve |
| WO1996032897A1 (en) | 1995-04-20 | 1996-10-24 | Desai Jawahar M | Apparatus for cardiac mapping and ablation |
| WO1996032885A1 (en) | 1995-04-20 | 1996-10-24 | Desai Jawahar M | Apparatus for cardiac ablation |
| WO1996034558A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Biomedical device having a temperature sensing system |
| WO1996034560A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter with expandable probe |
| WO1996034559A1 (en) | 1995-05-01 | 1996-11-07 | Cordis Webster, Inc. | Unique electrode configurations for cardiovascular electrode catheter with built-in deflection method and central puller wire |
| WO1996034652A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter control system |
| WO1996034650A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Locking mechanism for catheters |
| WO1996034570A1 (en) | 1995-05-01 | 1996-11-07 | Ep Technologies, Inc. | Systems and methods for obtaining desired lesion characteristics while ablating body tissue |
| WO1996034567A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | System for controlling the energy delivered to a patient for ablation |
| WO1996034569A1 (en) | 1995-05-01 | 1996-11-07 | Ep Technologies, Inc. | Systems and methods for ablating body tissue using predicted maximum tissue temperature |
| WO1996034653A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter control system having a pulley |
| US5573533A (en) | 1992-04-10 | 1996-11-12 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of cardiac tissue |
| US5575766A (en) | 1993-11-03 | 1996-11-19 | Daig Corporation | Process for the nonsurgical mapping and treatment of atrial arrhythmia using catheters guided by shaped guiding introducers |
| US5575810A (en) | 1993-10-15 | 1996-11-19 | Ep Technologies, Inc. | Composite structures and methods for ablating tissue to form complex lesion patterns in the treatment of cardiac conditions and the like |
| WO1996036860A2 (en) | 1995-05-01 | 1996-11-21 | Ep Technologies, Inc. | Systems and methods for sensing sub-surface temperatures in body tissue during ablation with actively cooled electrodes |
| US5582609A (en) | 1993-10-14 | 1996-12-10 | Ep Technologies, Inc. | Systems and methods for forming large lesions in body tissue using curvilinear electrode elements |
| US5584830A (en) | 1994-03-30 | 1996-12-17 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of cardiac tissue |
| WO1996039967A1 (en) | 1995-06-07 | 1996-12-19 | Ep Technologies, Inc. | Tissue heating and ablation systems and methods which predict maximum tissue temperature |
| US5588964A (en) | 1992-12-01 | 1996-12-31 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and/or radius and method |
| US5588432A (en) | 1988-03-21 | 1996-12-31 | Boston Scientific Corporation | Catheters for imaging, sensing electrical potentials, and ablating tissue |
| US5595183A (en) | 1995-02-17 | 1997-01-21 | Ep Technologies, Inc. | Systems and methods for examining heart tissue employing multiple electrode structures and roving electrodes |
| US5598848A (en) | 1994-03-31 | 1997-02-04 | Ep Technologies, Inc. | Systems and methods for positioning multiple electrode structures in electrical contact with the myocardium |
| US5601088A (en) | 1995-02-17 | 1997-02-11 | Ep Technologies, Inc. | Systems and methods for filtering artifacts from composite signals |
| US5606974A (en) | 1995-05-02 | 1997-03-04 | Heart Rhythm Technologies, Inc. | Catheter having ultrasonic device |
| US5607462A (en) | 1993-09-24 | 1997-03-04 | Cardiac Pathways Corporation | Catheter assembly, catheter and multi-catheter introducer for use therewith |
| US5620481A (en) | 1991-07-05 | 1997-04-15 | Desai; Jawahar M. | Device for multi-phase radio-frequency ablation |
| WO1997015919A1 (en) | 1995-10-25 | 1997-05-01 | Iomega Corporation | Molded actuator crash stops |
| US5626136A (en) | 1993-04-28 | 1997-05-06 | Cordis Webster, Inc. | Electrophysiology catheter with pre-curved circular tip |
| US5630425A (en) | 1995-02-17 | 1997-05-20 | Ep Technologies, Inc. | Systems and methods for adaptive filtering artifacts from composite signals |
| US5630837A (en) | 1993-07-01 | 1997-05-20 | Boston Scientific Corporation | Acoustic ablation |
| WO1997017893A1 (en) | 1995-11-13 | 1997-05-22 | Heart Rhythm Technologies, Inc. | System and method for analyzing electrogram waveforms |
| WO1997017904A1 (en) | 1995-11-13 | 1997-05-22 | Ep Technologies, Inc. | Flexible tissue ablation elements for making long lesions |
| US5637090A (en) | 1993-10-15 | 1997-06-10 | Ep Technologies, Inc. | Multiple electrode element for mapping and ablating heart tissue |
| US5645082A (en) | 1993-01-29 | 1997-07-08 | Cardima, Inc. | Intravascular method and system for treating arrhythmia |
| US5645064A (en) | 1992-01-29 | 1997-07-08 | Cardima, Inc. | High resolution intravascular signal detection |
| USD381076S (en) | 1995-05-02 | 1997-07-15 | Heart Rhythm Technologies, Inc. | Manipulation handle |
| WO1997025917A1 (en) | 1996-01-19 | 1997-07-24 | Ep Technologies, Inc. | Multi-function electrode structures for electrically analyzing and heating body tissue |
| WO1997025919A1 (en) | 1996-01-19 | 1997-07-24 | Ep Technologies, Inc. | Systems and methods for heating and ablating tissue using multifunctional electrode structures |
| US5657755A (en) | 1993-03-11 | 1997-08-19 | Desai; Jawahar M. | Apparatus and method for cardiac ablation |
| WO1997032525A1 (en) | 1996-03-06 | 1997-09-12 | Cardiac Pathways Corporation | Apparatus for creating linear lesions by ablation |
| US5673695A (en) | 1995-08-02 | 1997-10-07 | Ep Technologies, Inc. | Methods for locating and ablating accessory pathways in the heart |
| WO1997036541A1 (en) | 1996-04-02 | 1997-10-09 | Cordis Webster, Inc. | Electrophysiology catheter with a bullseye electrode |
| US5680860A (en) | 1994-07-07 | 1997-10-28 | Cardiac Pathways Corporation | Mapping and/or ablation catheter with coilable distal extremity and method for using same |
| WO1997040760A1 (en) | 1996-04-30 | 1997-11-06 | Cardiac Crc Nominees Pty. Ltd. | A system for simultaneous unipolar multi-electrode ablation |
| US5687723A (en) | 1993-12-03 | 1997-11-18 | Avitall; Boaz | Mapping and ablation catheter system |
| WO1997042996A1 (en) | 1996-05-13 | 1997-11-20 | Ep Technologies, Inc. | Assemblies for creating compound curves in distal catheter regions |
| US5697928A (en) | 1996-09-23 | 1997-12-16 | Uab Research Foundation | Cardic electrode catheter |
| US5702438A (en) | 1995-06-08 | 1997-12-30 | Avitall; Boaz | Expandable recording and ablation catheter system |
| US5704791A (en) | 1995-03-29 | 1998-01-06 | Gillio; Robert G. | Virtual surgery system instrument |
| US5716389A (en) | 1995-11-13 | 1998-02-10 | Walinsky; Paul | Cardiac ablation catheter arrangement with movable guidewire |
| US5724985A (en) | 1995-08-02 | 1998-03-10 | Pacesetter, Inc. | User interface for an implantable medical device using an integrated digitizer display screen |
| US5733323A (en) | 1995-11-13 | 1998-03-31 | Cordis Corporation | Electrically conductive unipolar vascular sheath |
| US5735280A (en) | 1995-05-02 | 1998-04-07 | Heart Rhythm Technologies, Inc. | Ultrasound energy delivery system and method |
| WO1998018520A2 (en) | 1996-10-28 | 1998-05-07 | Ep Technologies, Inc. | Asymmetric multiple electrode support structures |
| WO1998019611A1 (en) | 1996-11-01 | 1998-05-14 | Cordis Webster, Inc. | Multi-electrode ablation catheter |
| US5766152A (en) | 1996-08-15 | 1998-06-16 | Cardima, Inc. | Intraluminal delivery of tissue lysing medium |
| US5769847A (en) | 1994-06-27 | 1998-06-23 | Ep Technologies, Inc. | Systems and methods for controlling tissue ablation using multiple temperature sensing elements |
| US5769791A (en) | 1992-09-14 | 1998-06-23 | Sextant Medical Corporation | Tissue interrogating device and methods |
| US5769077A (en)* | 1995-12-28 | 1998-06-23 | Pacesetter Ab | Multi-contact implantable electrode cable with a resorbable stiffening element |
| WO1998026724A1 (en) | 1996-12-19 | 1998-06-25 | Ep Technologies, Inc. | Branched structures for supporting multiple electrode elements |
| US5772590A (en) | 1992-06-30 | 1998-06-30 | Cordis Webster, Inc. | Cardiovascular catheter with laterally stable basket-shaped electrode array with puller wire |
| WO1998028039A2 (en) | 1996-12-20 | 1998-07-02 | Ep Technologies, Inc. | Unified switching system for electrophysiological stimulation and signal recording and analysis |
| US5775327A (en) | 1995-06-07 | 1998-07-07 | Cardima, Inc. | Guiding catheter for the coronary sinus |
| US5782828A (en) | 1996-12-11 | 1998-07-21 | Irvine Biomedical, Inc. | Ablation catheter with multiple flexible curves |
| US5782760A (en) | 1995-05-23 | 1998-07-21 | Cardima, Inc. | Over-the-wire EP catheter |
| US5792140A (en) | 1997-05-15 | 1998-08-11 | Irvine Biomedical, Inc. | Catheter having cooled multiple-needle electrode |
| WO1998038913A1 (en) | 1997-03-07 | 1998-09-11 | Ep Technologies, Inc. | Graphical user interface for use with multiple electrode catheters |
| US5820568A (en) | 1996-10-15 | 1998-10-13 | Cardiac Pathways Corporation | Apparatus and method for aiding in the positioning of a catheter |
| US5827272A (en) | 1995-08-07 | 1998-10-27 | Medtronic Cardiorhythm | Simplified torquing electrode catheter |
| US5837001A (en) | 1995-12-08 | 1998-11-17 | C. R. Bard | Radio frequency energy delivery system for multipolar electrode catheters |
| US5849028A (en) | 1997-05-16 | 1998-12-15 | Irvine Biomedical, Inc. | Catheter and method for radiofrequency ablation of cardiac tissue |
| US5857997A (en) | 1994-11-14 | 1999-01-12 | Heart Rhythm Technologies, Inc. | Catheter for electrophysiological procedures |
| US5857464A (en) | 1995-06-07 | 1999-01-12 | Desai; Jawahar M. | Catheter for media injection |
| US5863291A (en) | 1996-04-08 | 1999-01-26 | Cardima, Inc. | Linear ablation assembly |
| US5873865A (en) | 1997-02-07 | 1999-02-23 | Eclipse Surgical Technologies, Inc. | Spiral catheter with multiple guide holes |
| US5876399A (en) | 1997-05-28 | 1999-03-02 | Irvine Biomedical, Inc. | Catheter system and methods thereof |
| US5882333A (en) | 1994-05-13 | 1999-03-16 | Cardima, Inc. | Catheter with deflectable distal section |
| US5885278A (en) | 1994-10-07 | 1999-03-23 | E.P. Technologies, Inc. | Structures for deploying movable electrode elements |
| US5891137A (en) | 1997-05-21 | 1999-04-06 | Irvine Biomedical, Inc. | Catheter system having a tip with fixation means |
| US5891135A (en) | 1996-01-19 | 1999-04-06 | Ep Technologies, Inc. | Stem elements for securing tubing and electrical wires to expandable-collapsible electrode structures |
| US5891027A (en) | 1996-10-21 | 1999-04-06 | Irvine Biomedical, Inc. | Cardiovascular catheter system with an inflatable soft tip |
| US5891138A (en) | 1997-08-11 | 1999-04-06 | Irvine Biomedical, Inc. | Catheter system having parallel electrodes |
| US5893884A (en) | 1997-05-19 | 1999-04-13 | Irvine Biomedical, Inc. | Catheter system having rollable electrode means |
| US5893847A (en) | 1993-03-16 | 1999-04-13 | Ep Technologies, Inc. | Multiple electrode support structures with slotted hub and hoop spline elements |
| US5895417A (en) | 1996-03-06 | 1999-04-20 | Cardiac Pathways Corporation | Deflectable loop design for a linear lesion ablation apparatus |
| US5895355A (en) | 1995-05-23 | 1999-04-20 | Cardima, Inc. | Over-the-wire EP catheter |
| US5897554A (en) | 1997-03-01 | 1999-04-27 | Irvine Biomedical, Inc. | Steerable catheter having a loop electrode |
| US5904680A (en) | 1992-09-25 | 1999-05-18 | Ep Technologies, Inc. | Multiple electrode support structures having optimal bio-mechanical characteristics |
| US5906605A (en) | 1997-01-10 | 1999-05-25 | Cardiac Pathways Corporation | Torquable guiding catheter for basket deployment and method |
| US5910129A (en) | 1996-12-19 | 1999-06-08 | Ep Technologies, Inc. | Catheter distal assembly with pull wires |
| US5911720A (en) | 1996-11-26 | 1999-06-15 | Ep Technologies, Inc. | Ablation catheter with segmented tip |
| US5913854A (en) | 1997-02-04 | 1999-06-22 | Medtronic, Inc. | Fluid cooled ablation catheter and method for making |
| US5916214A (en) | 1995-05-01 | 1999-06-29 | Medtronic Cardiorhythm | Dual curve ablation catheter |
| US5928191A (en) | 1993-07-30 | 1999-07-27 | E.P. Technologies, Inc. | Variable curve electrophysiology catheter |
| US5935063A (en) | 1997-10-29 | 1999-08-10 | Irvine Biomedical, Inc. | Electrode catheter system and methods thereof |
| US5938694A (en) | 1993-11-10 | 1999-08-17 | Medtronic Cardiorhythm | Electrode array catheter |
| US5941845A (en) | 1997-08-05 | 1999-08-24 | Irvine Biomedical, Inc. | Catheter having multiple-needle electrode and methods thereof |
| US5951471A (en) | 1998-03-09 | 1999-09-14 | Irvine Biomedical, Inc. | Catheter-based coronary sinus mapping and ablation |
| US5954719A (en) | 1996-12-11 | 1999-09-21 | Irvine Biomedical, Inc. | System for operating a RF ablation generator |
| US5968040A (en) | 1994-03-04 | 1999-10-19 | Ep Technologies, Inc. | Systems and methods using asymmetric multiple electrode arrays |
| CA2327518A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using controllable duty cycle with alternate phasing |
| CA2328070A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus having high output impedance drivers |
| WO1999056644A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using unipolar and bipolar techniques |
| CA2328064A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Catheter having common lead for electrode and sensor |
| US5992418A (en) | 1997-06-23 | 1999-11-30 | Irvine Biomedical , Inc. | Catheter system having safety means and methods thereof |
| US5997532A (en) | 1997-07-03 | 1999-12-07 | Cardiac Pathways Corporation | Ablation catheter tip with a buffer layer covering the electrode |
| US6001093A (en) | 1993-10-15 | 1999-12-14 | Ep Technologies, Inc. | Systems and methods for creating long, thin lesions in body tissue |
| US6002956A (en) | 1995-05-23 | 1999-12-14 | Cardima, Inc. | Method of treating using an over-the-wire EP catheter |
| US6004269A (en) | 1993-07-01 | 1999-12-21 | Boston Scientific Corporation | Catheters for imaging, sensing electrical potentials, and ablating tissue |
| US6014581A (en) | 1998-03-26 | 2000-01-11 | Ep Technologies, Inc. | Interface for performing a diagnostic or therapeutic procedure on heart tissue with an electrode structure |
| US6023638A (en) | 1995-07-28 | 2000-02-08 | Scimed Life Systems, Inc. | System and method for conducting electrophysiological testing using high-voltage energy pulses to stun tissue |
| US6029091A (en) | 1998-07-09 | 2000-02-22 | Irvine Biomedical, Inc. | Catheter system having lattice electrodes |
| US6033403A (en) | 1998-10-08 | 2000-03-07 | Irvine Biomedical, Inc. | Long electrode catheter system and methods thereof |
| US6032674A (en) | 1992-01-07 | 2000-03-07 | Arthrocare Corporation | Systems and methods for myocardial revascularization |
| US6042580A (en) | 1998-05-05 | 2000-03-28 | Cardiac Pacemakers, Inc. | Electrode having composition-matched, common-lead thermocouple wire for providing multiple temperature-sensitive junctions |
| US6045550A (en) | 1998-05-05 | 2000-04-04 | Cardiac Peacemakers, Inc. | Electrode having non-joined thermocouple for providing multiple temperature-sensitive junctions |
| US6048329A (en) | 1996-12-19 | 2000-04-11 | Ep Technologies, Inc. | Catheter distal assembly with pull wires |
| US6052612A (en) | 1995-06-07 | 2000-04-18 | Desai; Jawahar M. | Catheter for media injection |
| US6053937A (en) | 1995-08-15 | 2000-04-25 | Rita Medical Systems, Inc. | Multiple electrode ablation apparatus and method with cooling element |
| US6056744A (en) | 1994-06-24 | 2000-05-02 | Conway Stuart Medical, Inc. | Sphincter treatment apparatus |
| US6064902A (en) | 1998-04-16 | 2000-05-16 | C.R. Bard, Inc. | Pulmonary vein ablation catheter |
| US6063077A (en) | 1996-04-08 | 2000-05-16 | Cardima, Inc. | Linear ablation device and assembly |
| US6063082A (en) | 1997-11-04 | 2000-05-16 | Scimed Life Systems, Inc. | Percutaneous myocardial revascularization basket delivery system and radiofrequency therapeutic device |
| US6068629A (en) | 1997-02-04 | 2000-05-30 | Medtronic, Inc. | System and methods for tissue mapping and ablation |
| US6070094A (en) | 1994-10-11 | 2000-05-30 | Ep Technologies, Inc. | Systems and methods for guiding movable electrode elements within multiple-electrode structures |
| US6071274A (en) | 1996-12-19 | 2000-06-06 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| US6071281A (en) | 1998-05-05 | 2000-06-06 | Ep Technologies, Inc. | Surgical method and apparatus for positioning a diagnostic or therapeutic element within the body and remote power control unit for use with same |
| US6088610A (en) | 1993-01-29 | 2000-07-11 | Cardima, Inc. | Method and system for using multiple intravascular sensing devices to detect electrical activity |
| US6086581A (en) | 1992-09-29 | 2000-07-11 | Ep Technologies, Inc. | Large surface cardiac ablation catheter that assumes a low profile during introduction into the heart |
| US6096036A (en) | 1998-05-05 | 2000-08-01 | Cardiac Pacemakers, Inc. | Steerable catheter with preformed distal shape and method for use |
| US6099524A (en) | 1994-01-28 | 2000-08-08 | Cardiac Pacemakers, Inc. | Electrophysiological mapping and ablation catheter and method |
| US6106522A (en) | 1993-10-14 | 2000-08-22 | Ep Technologies, Inc. | Systems and methods for forming elongated lesion patterns in body tissue using straight or curvilinear electrode elements |
| US6107699A (en) | 1998-05-22 | 2000-08-22 | Scimed Life Systems, Inc. | Power supply for use in electrophysiological apparatus employing high-voltage pulses to render tissue temporarily unresponsive |
| US6115626A (en) | 1998-03-26 | 2000-09-05 | Scimed Life Systems, Inc. | Systems and methods using annotated images for controlling the use of diagnostic or therapeutic instruments in instruments in interior body regions |
| US6120476A (en) | 1997-12-01 | 2000-09-19 | Cordis Webster, Inc. | Irrigated tip catheter |
| US6129724A (en) | 1993-10-14 | 2000-10-10 | Ep Technologies, Inc. | Systems and methods for forming elongated lesion patterns in body tissue using straight or curvilinear electrode elements |
| US6146381A (en) | 1998-05-05 | 2000-11-14 | Cardiac Pacemakers, Inc. | Catheter having distal region for deflecting axial forces |
| US6146379A (en) | 1993-10-15 | 2000-11-14 | Ep Technologies, Inc. | Systems and methods for creating curvilinear lesions in body tissue |
| US6167291A (en) | 1998-03-12 | 2000-12-26 | Cardima, Inc. | Protected pin connector for an electrophysiology catheter |
| US6165169A (en) | 1994-03-04 | 2000-12-26 | Ep Technologies, Inc. | Systems and methods for identifying the physical, mechanical, and functional attributes of multiple electrode arrays |
| CA2371935A1 (en) | 1999-06-17 | 2000-12-28 | Thomas M. Castellano | Rf ablation apparatus and method having electrode/tissue contact assessment scheme and electrocardiogram filtering |
| US6179833B1 (en) | 1995-06-09 | 2001-01-30 | Engineering & Research Associates, Inc. | Apparatus for thermal ablation |
| US6217573B1 (en) | 1998-12-03 | 2001-04-17 | Cordis Webster | System and method for measuring surface temperature of tissue during ablation |
| US6217576B1 (en) | 1997-05-19 | 2001-04-17 | Irvine Biomedical Inc. | Catheter probe for treating focal atrial fibrillation in pulmonary veins |
| US6226542B1 (en) | 1998-07-24 | 2001-05-01 | Biosense, Inc. | Three-dimensional reconstruction of intrabody organs |
| US6238390B1 (en) | 1998-05-27 | 2001-05-29 | Irvine Biomedical, Inc. | Ablation catheter system having linear lesion capabilities |
| US6241724B1 (en) | 1993-10-19 | 2001-06-05 | Ep Technologies, Inc. | Systems and methods for creating lesions in body tissue using segmented electrode assemblies |
| US6241725B1 (en) | 1993-12-15 | 2001-06-05 | Sherwood Services Ag | High frequency thermal ablation of cancerous tumors and functional targets with image data assistance |
| US6241726B1 (en) | 1997-05-21 | 2001-06-05 | Irvine Biomedical, Inc. | Catheter system having a tip section with fixation means |
| US6241666B1 (en) | 1997-07-03 | 2001-06-05 | Cardiac Pathways Corp. | Ablation catheter tip with a buffer layer covering the electrode |
| US6241728B1 (en) | 1997-12-18 | 2001-06-05 | Medtronic, Inc. | Left atrium ablation catheter and method |
| US6241727B1 (en) | 1998-05-27 | 2001-06-05 | Irvine Biomedical, Inc. | Ablation catheter system having circular lesion capabilities |
| US6245067B1 (en) | 1997-04-16 | 2001-06-12 | Irvine Biomedical, Inc. | Ablation device and methods having perpendicular electrodes |
| US6245089B1 (en) | 1997-03-06 | 2001-06-12 | Scimed Life Systems, Inc. | Distal protection device and method |
| US6251107B1 (en) | 1998-06-25 | 2001-06-26 | Cardima, Inc. | Ep catheter |
| US6256540B1 (en) | 1994-01-28 | 2001-07-03 | Ep Technologies | Systems and methods for examining the electrical characteristic of cardiac tissue |
| US6264664B1 (en) | 2000-03-10 | 2001-07-24 | General Science And Technology Corp. | Surgical basket devices |
| US6267746B1 (en) | 1999-03-22 | 2001-07-31 | Biosense Webster, Inc. | Multi-directional steerable catheters and control handles |
| US6290697B1 (en) | 1998-12-01 | 2001-09-18 | Irvine Biomedical, Inc. | Self-guiding catheter system for tissue ablation |
| US6293943B1 (en) | 1995-06-07 | 2001-09-25 | Ep Technologies, Inc. | Tissue heating and ablation systems and methods which predict maximum tissue temperature |
| US6302880B1 (en) | 1996-04-08 | 2001-10-16 | Cardima, Inc. | Linear ablation assembly |
| US6312425B1 (en) | 1998-05-05 | 2001-11-06 | Cardiac Pacemakers, Inc. | RF ablation catheter tip electrode with multiple thermal sensors |
| US20010039415A1 (en) | 2000-04-27 | 2001-11-08 | Medtronic, Inc. | System and method for assessing transmurality of ablation lesions |
| US6319251B1 (en) | 1998-09-24 | 2001-11-20 | Hosheng Tu | Medical device and methods for treating intravascular restenosis |
| US20010044625A1 (en) | 1998-05-27 | 2001-11-22 | Cary Hata | Catheter for circular tissue ablation and methods thereof |
| US6325797B1 (en) | 1999-04-05 | 2001-12-04 | Medtronic, Inc. | Ablation catheter and method for isolating a pulmonary vein |
| US20010051803A1 (en) | 1991-07-05 | 2001-12-13 | Desai Jawahar M. | Device and method for multi-phase radio-frequency ablation |
| US6332881B1 (en) | 1999-09-01 | 2001-12-25 | Cardima, Inc. | Surgical ablation tool |
| US6332880B1 (en) | 1996-12-19 | 2001-12-25 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| US6360128B2 (en) | 1993-03-16 | 2002-03-19 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US6371955B1 (en) | 1999-08-10 | 2002-04-16 | Biosense Webster, Inc. | Atrial branding iron catheter and a method for treating atrial fibrillation |
| US6375654B1 (en)* | 2000-05-19 | 2002-04-23 | Cardiofocus, Inc. | Catheter system with working portion radially expandable upon rotation |
| US20020065465A1 (en) | 1997-09-26 | 2002-05-30 | Ep Technologies, Inc. | System for recording use of structures deployed in association with heart tissue |
| US6425894B1 (en) | 2000-07-12 | 2002-07-30 | Biosense Webster, Inc. | Ablation catheter with electrode temperature monitoring |
| US6428537B1 (en) | 1998-05-22 | 2002-08-06 | Scimed Life Systems, Inc. | Electrophysiological treatment methods and apparatus employing high voltage pulse to render tissue temporarily unresponsive |
| US6428536B2 (en) | 1996-01-19 | 2002-08-06 | Ep Technologies, Inc. | Expandable-collapsible electrode structures made of electrically conductive material |
| WO2002060523A2 (en) | 2000-12-15 | 2002-08-08 | Brown Tony R | Atrial fibrillation rf treatment device and method |
| US20020126036A1 (en) | 2000-12-21 | 2002-09-12 | Flaherty J. Christopher | Medical apparatus remote control and method |
| US6451015B1 (en) | 1998-11-18 | 2002-09-17 | Sherwood Services Ag | Method and system for menu-driven two-dimensional display lesion generator |
| US6471693B1 (en) | 1999-09-10 | 2002-10-29 | Cryocath Technologies Inc. | Catheter and system for monitoring tissue contact |
| US20020161422A1 (en) | 1996-12-19 | 2002-10-31 | Ep Technologies, Inc. | Structures for supporting porous electrode elements |
| US6475214B1 (en) | 2000-05-01 | 2002-11-05 | Biosense Webster, Inc. | Catheter with enhanced ablation electrode |
| US6477396B1 (en) | 2000-07-07 | 2002-11-05 | Biosense Webster, Inc. | Mapping and ablation catheter |
| US6475213B1 (en) | 1996-01-19 | 2002-11-05 | Ep Technologies, Inc. | Method of ablating body tissue |
| US20020165532A1 (en) | 2001-05-01 | 2002-11-07 | Cardima, Inc. | Helically shaped electrophysiology catheter |
| US6478793B1 (en) | 1999-06-11 | 2002-11-12 | Sherwood Services Ag | Ablation treatment of bone metastases |
| US6487441B1 (en) | 1995-02-17 | 2002-11-26 | Ep Technologies, Inc. | Systems and methods for acquiring and analyzing electrograms in myocardial tissue |
| US6493586B1 (en) | 2000-08-30 | 2002-12-10 | Cardiac Pacemakers, Inc. | Site reversion in cardiac rhythm management |
| US6500167B1 (en) | 1997-09-05 | 2002-12-31 | Biosense Webster, Inc. | Omni-directional steerable catheter |
| US6500172B1 (en) | 1994-08-08 | 2002-12-31 | Ep Technologies, Inc. | Systems and methods for controlling tissue ablation using multiple temperature sensing elements |
| US6517536B2 (en) | 2000-04-27 | 2003-02-11 | Atricure, Inc. | Transmural ablation device and method |
| US6522905B2 (en) | 1993-03-11 | 2003-02-18 | Jawahar M. Desai | Apparatus and method for cardiac ablation |
| US6529756B1 (en) | 1999-11-22 | 2003-03-04 | Scimed Life Systems, Inc. | Apparatus for mapping and coagulating soft tissue in or around body orifices |
| US6542773B2 (en) | 1998-03-26 | 2003-04-01 | Scimed Life Systems, Inc. | Interactive systems and methods for controlling the use of diagnostic or therapeutic instruments in interior body regions |
| US6540744B2 (en) | 1997-06-27 | 2003-04-01 | St. Jude Medical, Daig Division, Inc. | Process and device for the treatment of atrial arrhythmia |
| US6551271B2 (en) | 2001-04-30 | 2003-04-22 | Biosense Webster, Inc. | Asymmetrical bidirectional steerable catheter |
| US6554794B1 (en) | 1997-09-24 | 2003-04-29 | Richard L. Mueller | Non-deforming deflectable multi-lumen catheter |
| US6558378B2 (en) | 1998-05-05 | 2003-05-06 | Cardiac Pacemakers, Inc. | RF ablation system and method having automatic temperature control |
| WO2003041602A2 (en) | 2001-11-13 | 2003-05-22 | Mayo Foundation For Medical Education And Research | Tissue ablation device and methods of using |
| US6569162B2 (en) | 2001-03-29 | 2003-05-27 | Ding Sheng He | Passively self-cooled electrode design for ablation catheters |
| US6569114B2 (en) | 2001-08-31 | 2003-05-27 | Biosense Webster, Inc. | Steerable catheter with struts |
| US6569163B2 (en) | 2001-03-09 | 2003-05-27 | Quantumcor, Inc. | Wireless electrosurgical adapter unit and methods thereof |
| US6574492B1 (en) | 1996-01-08 | 2003-06-03 | Biosense, Inc. | Catheter having multiple arms with electrode and position sensor |
| US6575997B1 (en) | 1999-12-23 | 2003-06-10 | Endovascular Technologies, Inc. | Embolic basket |
| US6583796B2 (en) | 2000-12-14 | 2003-06-24 | Medtronic, Inc. | Method and apparatus for displaying information retrieved from an implanted medical device |
| US20030125730A1 (en) | 2002-01-03 | 2003-07-03 | Afx Inc. | Flexible device for ablation of biological tissue |
| US6605087B2 (en) | 1997-07-21 | 2003-08-12 | St. Jude Medical, Daig Division | Ablation catheter |
| US6607520B2 (en) | 1999-09-15 | 2003-08-19 | The General Hospital Corporation | Coiled ablation catheter system |
| US6613046B1 (en) | 1999-11-22 | 2003-09-02 | Scimed Life Systems, Inc. | Loop structures for supporting diagnostic and therapeutic elements in contact with body tissue |
| US6625482B1 (en) | 1998-03-06 | 2003-09-23 | Ep Technologies, Inc. | Graphical user interface for use with multiple electrode catheters |
| US6628976B1 (en) | 2000-01-27 | 2003-09-30 | Biosense Webster, Inc. | Catheter having mapping assembly |
| US6632223B1 (en) | 2000-03-30 | 2003-10-14 | The General Hospital Corporation | Pulmonary vein ablation stent and method |
| US6635056B2 (en) | 2001-10-09 | 2003-10-21 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using amplitude control |
| US20030199746A1 (en) | 2000-01-27 | 2003-10-23 | Biosense Webster, Inc. | Catheter having mapping assembly |
| US6638223B2 (en) | 2000-12-28 | 2003-10-28 | Ge Medical Systems Global Technology Company, Llc | Operator interface for a medical diagnostic imaging device |
| US6640120B1 (en) | 2000-10-05 | 2003-10-28 | Scimed Life Systems, Inc. | Probe assembly for mapping and ablating pulmonary vein tissue and method of using same |
| US6638275B1 (en) | 2000-10-05 | 2003-10-28 | Medironic, Inc. | Bipolar ablation apparatus and method |
| US20030204186A1 (en) | 2002-04-24 | 2003-10-30 | Biotronik Mess-Und Therapiegerate Gmbh & Co. Ingen | Ablation device for cardiac tissue, in particular for forming linear lesions between two vessel orifices in the heart |
| WO2003089997A2 (en) | 2002-03-15 | 2003-10-30 | C.R. Bard, Inc. | Method and apparatus for control of ablation energy and electrogram acquisition through multiple common electrodes in an electrophysiology catheter |
| US20030204185A1 (en) | 2002-04-26 | 2003-10-30 | Sherman Marshall L. | System and method for monitoring use of disposable catheters |
| DE10218427A1 (en) | 2002-04-24 | 2003-11-06 | Biotronik Mess & Therapieg | Ablation device for cardiac tissue, in particular for creating a circular lesion around a vascular mouth in the heart |
| EP1360938A1 (en) | 2002-05-07 | 2003-11-12 | Irvine Biomedical, Inc. | System for operating an ablation generator with dual energy source |
| US6652517B1 (en)* | 2000-04-25 | 2003-11-25 | Uab Research Foundation | Ablation catheter, system, and method of use thereof |
| US6658279B2 (en) | 1996-10-28 | 2003-12-02 | Ep Technologies, Inc. | Ablation and imaging catheter |
| US6669692B1 (en) | 2000-08-21 | 2003-12-30 | Biosense Webster, Inc. | Ablation catheter with cooled linear electrode |
| US6671533B2 (en) | 2001-10-11 | 2003-12-30 | Irvine Biomedical Inc. | System and method for mapping and ablating body tissue of the interior region of the heart |
| US20040015164A1 (en) | 2002-07-19 | 2004-01-22 | Fuimaono Kristine B. | Atrial ablation catheter and method for treating atrial fibrillation |
| US6690972B2 (en) | 1999-08-20 | 2004-02-10 | Cardiac Pacemakers, Inc. | System and method for detecting and displaying parameter interactions |
| US6702811B2 (en) | 1999-04-05 | 2004-03-09 | Medtronic, Inc. | Ablation catheter assembly with radially decreasing helix and method of use |
| US20040082947A1 (en) | 2002-10-25 | 2004-04-29 | The Regents Of The University Of Michigan | Ablation catheters |
| US6730078B2 (en) | 2002-04-22 | 2004-05-04 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using multi-frequency energy delivery |
| US6738673B2 (en) | 1986-11-14 | 2004-05-18 | Jawahar M. Desai | Method for catheter mapping and ablation |
| US6740080B2 (en) | 2001-08-31 | 2004-05-25 | Cardiac Pacemakers, Inc. | Ablation system with selectable current path means |
| US6743225B2 (en) | 2001-03-27 | 2004-06-01 | Uab Research Foundation | Electrophysiologic measure of endpoints for ablation lesions created in fibrillating substrates |
| US6746446B1 (en) | 2000-08-04 | 2004-06-08 | Cardima, Inc. | Electrophysiological device for the isthmus |
| CA2437140A1 (en) | 2002-12-11 | 2004-06-11 | Cryocor, Inc. | Cold tip rf/ultrasonic ablation catheter |
| US6752804B2 (en) | 2000-12-28 | 2004-06-22 | Cardiac Pacemakers, Inc. | Ablation system and method having multiple-sensor electrodes to assist in assessment of electrode and sensor position and adjustment of energy levels |
| US20040133154A1 (en) | 1996-10-11 | 2004-07-08 | Flaherty J. Christopher | Systems and methods for delivering drugs to selected locations within the body |
| US6761716B2 (en) | 2001-09-18 | 2004-07-13 | Cardiac Pacemakers, Inc. | System and method for assessing electrode-tissue contact and lesion quality during RF ablation by measurement of conduction time |
| US20040143256A1 (en) | 2003-01-21 | 2004-07-22 | Bednarek Michael C. | Ablation catheter and electrode |
| US6771996B2 (en)* | 2001-05-24 | 2004-08-03 | Cardiac Pacemakers, Inc. | Ablation and high-resolution mapping catheter system for pulmonary vein foci elimination |
| US20040158141A1 (en) | 2002-02-28 | 2004-08-12 | Biosense Webster, Inc. | Catheter having circular ablation assembly |
| US20040181249A1 (en) | 2003-03-10 | 2004-09-16 | Pathway Medical Technologies, Inc. | Bearing system to support a rotatable operating head in an intracorporeal device |
| US20040181139A1 (en) | 2001-04-27 | 2004-09-16 | Falwell Gary S. | Method and apparatus for three dimensional mapping of electrical activity in blood vessels and ablation of electrical pathways identified by the three dimension map |
| US20040182384A1 (en) | 1999-09-29 | 2004-09-23 | Alfery David D. | Perilaryngeal oral airway with temperature sensor |
| US6813520B2 (en) | 1996-04-12 | 2004-11-02 | Novacept | Method for ablating and/or coagulating tissue using moisture transport |
| US20040236324A1 (en) | 1995-02-22 | 2004-11-25 | Medtronic, Inc. | Pen-type electrosurgical instrument |
| US20040247164A1 (en) | 2003-06-09 | 2004-12-09 | Simon Furnish | Scanning catheter with position encoder |
| US6830576B2 (en) | 1995-06-07 | 2004-12-14 | Ep Technologies, Inc. | Apparatus for electrically isolating a portion of the atria |
| US20050010095A1 (en)* | 1999-04-05 | 2005-01-13 | Medtronic, Inc. | Multi-purpose catheter apparatus and method of use |
| US20050033137A1 (en) | 2002-10-25 | 2005-02-10 | The Regents Of The University Of Michigan | Ablation catheters and methods for their use |
| US6866662B2 (en) | 2002-07-23 | 2005-03-15 | Biosense Webster, Inc. | Ablation catheter having stabilizing array |
| WO2005027765A1 (en) | 2003-09-01 | 2005-03-31 | Siemens Aktiengesellschaft | Method and device for visually supporting an electrophysiology catheter application in the heart |
| WO2005027766A1 (en) | 2003-09-01 | 2005-03-31 | Siemens Aktiengesellschaft | Method and device for visually assisting the electrophysiological use of a catheter in the heart |
| US20050096644A1 (en) | 2003-10-30 | 2005-05-05 | Hall Jeffrey A. | Energy delivery optimization for RF duty cycle for lesion creation |
| US20050101946A1 (en) | 2003-11-11 | 2005-05-12 | Biosense Webster Inc. | Externally applied RF for pulmonary vein isolation |
| US6893442B2 (en) | 2002-06-14 | 2005-05-17 | Ablatrics, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US6916306B1 (en) | 2000-11-10 | 2005-07-12 | Boston Scientific Scimed, Inc. | Steerable loop structures for supporting diagnostic and therapeutic elements in contact with body tissue |
| CA2492283A1 (en) | 2004-01-14 | 2005-07-14 | Biosense Webster, Inc. | Prediction and assessment of ablation of cardiac tissue |
| US20050177146A1 (en) | 2004-02-10 | 2005-08-11 | Marshall Sherman | System and method for assessing ice ball formation during a cryoablation procedure |
| US20050187545A1 (en) | 2004-02-20 | 2005-08-25 | Hooven Michael D. | Magnetic catheter ablation device and method |
| US6936047B2 (en) | 2000-05-12 | 2005-08-30 | Agility Capital Llc | Multi-channel RF energy delivery with coagulum reduction |
| US6952615B2 (en) | 2001-09-28 | 2005-10-04 | Shutaro Satake | Radiofrequency thermal balloon catheter |
| US6955173B2 (en) | 1997-05-09 | 2005-10-18 | The Regents Of The University Of California | Device and method for forming a circumferential conduction block in a pulmonary vein |
| US20050234444A1 (en) | 2004-04-14 | 2005-10-20 | Hooven Michael D | Electrode and bipolar ablation method using same |
| US6961602B2 (en) | 2001-12-31 | 2005-11-01 | Biosense Webster, Inc. | Catheter having multiple spines each having electrical mapping and location sensing capabilities |
| US6964660B2 (en) | 1997-07-08 | 2005-11-15 | Atrionix, Inc. | Tissue ablation device assembly and method of electrically isolating a pulmonary vein ostium from an atrial wall |
| US6966908B2 (en)* | 1997-07-08 | 2005-11-22 | Atrionix, Inc. | Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall |
| US6973339B2 (en) | 2003-07-29 | 2005-12-06 | Biosense, Inc | Lasso for pulmonary vein mapping and ablation |
| US6987995B2 (en) | 2003-03-12 | 2006-01-17 | Biosense Webster, Inc. | Multifunctional catheter handle |
| US20060030844A1 (en) | 2004-08-04 | 2006-02-09 | Knight Bradley P | Transparent electrode for the radiofrequency ablation of tissue |
| US7001336B2 (en) | 2001-01-30 | 2006-02-21 | Advanced Cardiovascular Systems, Inc. | Method for treating ischemia |
| EP1169976B1 (en) | 2000-07-07 | 2006-04-05 | Biosense Webster, Inc. | Multi-electrode catheter, system and method |
| CA2194061C (en) | 1994-06-27 | 2006-04-11 | David K. Swanson | Systems and methods for sensing temperature within the body |
| US7029470B2 (en) | 2001-04-26 | 2006-04-18 | Medtronic, Inc. | Ablation system and method of use |
| EP1415680B1 (en) | 2002-10-30 | 2006-04-26 | Biosense Webster, Inc. | Multi-tip steerable catheter |
| US20060089637A1 (en) | 2004-10-14 | 2006-04-27 | Werneth Randell L | Ablation catheter |
| US20060095030A1 (en) | 2004-11-04 | 2006-05-04 | Scimed Life Systems, Inc. | Preshaped ablation catheter for ablating pulmonary vein ostia within the heart |
| EP1210021B1 (en) | 1999-03-19 | 2006-05-10 | Atrionix, Inc. | Atrial annulus ablation device |
| WO2006049970A2 (en) | 2004-10-27 | 2006-05-11 | Yuval Carmel | Radio-frequency device for passivation of vascular plaque and method of using same |
| US7047068B2 (en) | 2000-12-11 | 2006-05-16 | C.R. Bard, Inc. | Microelectrode catheter for mapping and ablation |
| WO2006052905A2 (en) | 2004-11-08 | 2006-05-18 | Cardima, Inc. | System and method for performing ablation and other medical procedures using an electrode array with flex circuit |
| US20060106375A1 (en) | 2004-11-15 | 2006-05-18 | Werneth Randell L | Ablation system with feedback |
| US7048734B1 (en) | 1993-10-15 | 2006-05-23 | Ep Technologies, Inc. | Systems and methods for electronically altering the energy emitting characteristics of an electrode array to create different lesion patterns in body tissue |
| US7048756B2 (en) | 2002-01-18 | 2006-05-23 | Apasara Medical Corporation | System, method and apparatus for evaluating tissue temperature |
| EP1658818A1 (en) | 2004-11-23 | 2006-05-24 | Biosense Webster, Inc. | Externally applied rf for pulmonary vein isolation |
| US20060111700A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter and method of use |
| US20060111701A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter adapted for treatment of septal wall arrhythmogenic foci and method of use |
| US20060111708A1 (en) | 2003-01-17 | 2006-05-25 | Vanney Guy P | Ablation catheter assembly having a virtual electrode comprising portholes |
| WO2006055741A1 (en) | 2004-11-17 | 2006-05-26 | Biosense Webster, Inc. | Apparatus for real time evaluation of tissue ablation |
| WO2006055654A1 (en) | 2004-11-15 | 2006-05-26 | Biosense Webster Inc. | Catheter with microfabricated temperature sensing |
| WO2006055658A1 (en) | 2004-11-15 | 2006-05-26 | Biosense Webster Inc. | Catheter with multiple microfabricated temperature sensors |
| EP1125549B1 (en) | 2000-02-18 | 2006-06-07 | Biosense Webster, Inc. | Catheter for generating an electrical map of a chamber of the heart |
| US20060122526A1 (en) | 2004-12-02 | 2006-06-08 | Omer Berenfeld | Method and algorithm for spatially identifying sources of cardiac fibrillation |
| EP1321166B1 (en) | 2001-12-21 | 2006-07-05 | Ethicon Inc. | Expandable intracardiac return electrode and method of use |
| US7077823B2 (en) | 2003-11-19 | 2006-07-18 | Biosense Webster, Inc. | Bidirectional steerable catheter with slidable mated puller wires |
| EP1343427B1 (en) | 2000-12-11 | 2006-07-26 | C.R. Bard, Inc. | Apparatus for mapping |
| EP1690564A1 (en) | 2005-02-14 | 2006-08-16 | Biosense Webster | Steerable catheter with in-plane deflection |
| US7094235B2 (en) | 2001-04-26 | 2006-08-22 | Medtronic, Inc. | Method and apparatus for tissue ablation |
| US20060206109A1 (en) | 2005-03-08 | 2006-09-14 | Boston Scientific Scimed, Inc. | Finger mountable lesion formation devices and methods |
| US7113831B2 (en) | 2000-04-27 | 2006-09-26 | Atricure, Inc. | Transmural ablation device |
| EP1070480B1 (en) | 1999-07-22 | 2006-09-27 | Biosense Webster, Inc. | Vector mapping of three-dimensionally reconstructed intrabody organs |
| US7122031B2 (en) | 1998-02-19 | 2006-10-17 | Curon Medical, Inc. | Graphical user interface for association with an electrode structure deployed in contact with a tissue region |
| US20060241366A1 (en) | 2002-10-31 | 2006-10-26 | Gary Falwell | Electrophysiology loop catheter |
| EP1014874B1 (en) | 1997-05-19 | 2006-12-13 | Boston Scientific Limited | Apparatus for treating tissue with multiple electrodes |
| US7155270B2 (en) | 2003-10-24 | 2006-12-26 | Biosense Webster, Inc. | Catheter with multi-spine mapping assembly |
| US7156843B2 (en) | 2003-09-08 | 2007-01-02 | Medtronic, Inc. | Irrigated focal ablation tip |
| US7163537B2 (en) | 2003-06-02 | 2007-01-16 | Biosense Webster, Inc. | Enhanced ablation and mapping catheter and method for treating atrial fibrillation |
| US20070083195A1 (en) | 2005-07-11 | 2007-04-12 | Werneth Randell L | Low power tissue ablation system |
| US20070083193A1 (en) | 2005-08-22 | 2007-04-12 | Werneth Randell L | User interface for tissue ablation system |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3125450A (en) | 1964-03-17 | Processes for reconstituting meat | ||
| US2376672A (en) | 1943-11-27 | 1945-05-22 | Luella P Dreyling | Poultry litter |
| US4699147A (en) | 1985-09-25 | 1987-10-13 | Cordis Corporation | Intraventricular multielectrode cardial mapping probe and method for using same |
| US5056517A (en) | 1989-07-24 | 1991-10-15 | Consiglio Nazionale Delle Ricerche | Biomagnetically localizable multipurpose catheter and method for magnetocardiographic guided intracardiac mapping, biopsy and ablation of cardiac arrhythmias |
| US5484384A (en)* | 1991-01-29 | 1996-01-16 | Med Institute, Inc. | Minimally invasive medical device for providing a radiation treatment |
| IL103284A0 (en) | 1991-10-31 | 1993-02-21 | Transtech Medical Inc | Transplant protective coating |
| US5866217A (en) | 1991-11-04 | 1999-02-02 | Possis Medical, Inc. | Silicone composite vascular graft |
| US5722401A (en)* | 1994-10-19 | 1998-03-03 | Cardiac Pathways Corporation | Endocardial mapping and/or ablation catheter probe |
| ATE433306T1 (en) | 1997-07-08 | 2009-06-15 | Univ California | DEVICE FOR CIRCUMFERENTIAL ABLATION |
| US6032574A (en)* | 1997-10-06 | 2000-03-07 | Brayton; Darryl D. | Re-configurable modular food processing cells |
| US7198635B2 (en)* | 2000-10-17 | 2007-04-03 | Asthmatx, Inc. | Modification of airways by application of energy |
| US6036689A (en)* | 1998-09-24 | 2000-03-14 | Tu; Lily Chen | Ablation device for treating atherosclerotic tissues |
| JP2001226210A (en) | 2000-02-18 | 2001-08-21 | Hozawa Hiroki | Antiviral agent and method for producing the same |
| US6514250B1 (en)* | 2000-04-27 | 2003-02-04 | Medtronic, Inc. | Suction stabilized epicardial ablation devices |
| US6748255B2 (en)* | 2001-12-14 | 2004-06-08 | Biosense Webster, Inc. | Basket catheter with multiple location sensors |
| CA2552165C (en) | 2003-12-31 | 2013-10-22 | Biosense Webster, Inc. | Circumferential ablation device assembly with dual expandable members |
| WO2005065563A1 (en) | 2003-12-31 | 2005-07-21 | Biosense Webster, Inc | Circumferential ablation device assembly with an expandable member |
| US7504123B2 (en) | 2004-01-09 | 2009-03-17 | Ecolab Inc. | Methods for washing poultry during processing with medium chain peroxycarboxylic acid compositions |
| CN101019411A (en) | 2004-07-29 | 2007-08-15 | 汤姆森特许公司 | System and method for providing visual markers in electronic documents |
| EP2759276A1 (en)* | 2005-06-20 | 2014-07-30 | Medtronic Ablation Frontiers LLC | Ablation catheter |
- 2006
- 2006-06-20EPEP13199213.3Apatent/EP2759276A1/ennot_activeWithdrawn
- 2006-06-20AUAU2006262447Apatent/AU2006262447A1/ennot_activeAbandoned
- 2006-06-20EPEP06773536Apatent/EP1895927A4/ennot_activeWithdrawn
- 2006-06-20CACA002612679Apatent/CA2612679A1/ennot_activeAbandoned
- 2006-06-20USUS11/471,467patent/US7850685B2/enactiveActive
- 2006-06-20WOPCT/US2006/023808patent/WO2007001981A2/enactiveApplication Filing
- 2006-06-20CNCN200680030272XApatent/CN101309651B/ennot_activeExpired - Fee Related
- 2006-06-20JPJP2008517213Apatent/JP2009500052A/enactivePending
- 2008
- 2008-01-17ILIL188882Apatent/IL188882A0/enunknown
- 2010
- 2010-11-03USUS12/938,791patent/US8337492B2/ennot_activeExpired - Fee Related
- 2012
- 2012-11-15USUS13/677,571patent/US8771267B2/enactiveActive
- 2014
- 2014-06-10USUS14/300,693patent/US8979841B2/enactiveActive
- 2015
- 2015-02-20USUS14/627,274patent/US9468495B2/enactiveActive
- 2016
- 2016-10-12USUS15/291,822patent/US20170027640A1/ennot_activeAbandoned
Patent Citations (532)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3516412A (en) | 1965-08-16 | 1970-06-23 | Electro Catheter Corp | Bipolar electrode having irregularity at inserting end thereof and method of insertion |
| US3951136A (en) | 1973-10-10 | 1976-04-20 | Vital Signs, Inc. | Multiple purpose esophageal probe |
| US4017903A (en) | 1975-08-27 | 1977-04-12 | Hewlett-Packard Company | Pulse code modulation recording and/or reproducing system |
| US4112952A (en) | 1977-02-11 | 1978-09-12 | The United States Of America As Represented By The Secretary Of Health, Education And Welfare | Electrode for artificial pacemaker |
| US4411266A (en) | 1980-09-24 | 1983-10-25 | Cosman Eric R | Thermocouple radio frequency lesion electrode |
| US4432377A (en) | 1982-01-29 | 1984-02-21 | Medtronic, Inc. | Biomedical lead with ring electrode and method of making same |
| US4660571A (en) | 1985-07-18 | 1987-04-28 | Cordis Corporation | Percutaneous lead having radially adjustable electrode |
| US4785815A (en) | 1985-10-23 | 1988-11-22 | Cordis Corporation | Apparatus for locating and ablating cardiac conduction pathways |
| US5500011A (en) | 1986-11-14 | 1996-03-19 | Desai; Jawahar M. | Catheter for mapping and ablation and method therefor |
| US5365926A (en) | 1986-11-14 | 1994-11-22 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US6738673B2 (en) | 1986-11-14 | 2004-05-18 | Jawahar M. Desai | Method for catheter mapping and ablation |
| US5397339A (en) | 1986-11-14 | 1995-03-14 | Desai; Jawahar M. | Catheter for mapping and ablation and method therefor |
| US5231995A (en) | 1986-11-14 | 1993-08-03 | Desai Jawahar M | Method for catheter mapping and ablation |
| US5215103A (en) | 1986-11-14 | 1993-06-01 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US4940064A (en) | 1986-11-14 | 1990-07-10 | Desai Jawahar M | Catheter for mapping and ablation and method therefor |
| US4869248A (en) | 1987-04-17 | 1989-09-26 | Narula Onkar S | Method and apparatus for localized thermal ablation |
| US4882777A (en) | 1987-04-17 | 1989-11-21 | Narula Onkar S | Catheter |
| US4920980A (en) | 1987-09-14 | 1990-05-01 | Cordis Corporation | Catheter with controllable tip |
| SU1512622A1 (en) | 1987-11-12 | 1989-10-07 | Научно-Исследовательский Институт Онкологии И Медицинской Радиологии Мз Бсср | Electrode device for hyperthermia of hollow organs |
| US4860769A (en) | 1987-11-12 | 1989-08-29 | Thomas J. Fogarty | Implantable defibrillation electrode |
| SU1544396A1 (en) | 1987-12-08 | 1990-02-23 | 1-Й Ленинградский Медицинский Институт Им.Акад.И.П.Павлова | Electrodestruction device |
| US5010894A (en) | 1988-01-07 | 1991-04-30 | Edhag Knut O | Intravascular electrode lead usable for cardiac defibrillation |
| US5588432A (en) | 1988-03-21 | 1996-12-31 | Boston Scientific Corporation | Catheters for imaging, sensing electrical potentials, and ablating tissue |
| US4907589A (en) | 1988-04-29 | 1990-03-13 | Cosman Eric R | Automatic over-temperature control apparatus for a therapeutic heating device |
| US4896671A (en) | 1988-08-01 | 1990-01-30 | C. R. Bard, Inc. | Catheter with contoured ablation electrode |
| US4966597A (en) | 1988-11-04 | 1990-10-30 | Cosman Eric R | Thermometric cardiac tissue ablation electrode with ultra-sensitive temperature detection |
| US5234004A (en) | 1988-11-21 | 1993-08-10 | Technomed International | Method and apparatus for the surgical treatment of tissues by thermal effect, and in particular the prostate, using a urethral microwave-emitting probe means |
| US5370644A (en) | 1988-11-25 | 1994-12-06 | Sensor Electronics, Inc. | Radiofrequency ablation catheter |
| WO1990006079A1 (en) | 1988-11-25 | 1990-06-14 | Sensor Electronics, Inc. | Radiofrequency ablation catheter |
| US5230349A (en) | 1988-11-25 | 1993-07-27 | Sensor Electronics, Inc. | Electrical heating catheter |
| SU1690786A1 (en) | 1989-06-30 | 1991-11-15 | Каунасский Медицинский Институт | Electrocardial electrode |
| US5016808A (en) | 1989-09-14 | 1991-05-21 | Cardiac Pacemakers, Inc. | Implantable tapered spiral endocardial lead for use in internal defibrillation |
| US5083565A (en) | 1990-08-03 | 1992-01-28 | Everest Medical Corporation | Electrosurgical instrument for ablating endocardial tissue |
| US5100423A (en) | 1990-08-21 | 1992-03-31 | Medical Engineering & Development Institute, Inc. | Ablation catheter |
| US5156151A (en) | 1991-02-15 | 1992-10-20 | Cardiac Pathways Corporation | Endocardial mapping and ablation system and catheter probe |
| US5406946A (en) | 1991-02-15 | 1995-04-18 | Cardiac Pathways Corporation | Endocardial mapping and ablation system and catheter probe and method |
| US5279299A (en) | 1991-02-15 | 1994-01-18 | Cardiac Pathways Corporation | Endocardial mapping and ablation system and catheter probe |
| US5404638A (en) | 1991-02-15 | 1995-04-11 | Cardiac Pathways Corporation | Method of forming a flexible expandable member for use with a catheter probe |
| US5465717A (en) | 1991-02-15 | 1995-11-14 | Cardiac Pathways Corporation | Apparatus and Method for ventricular mapping and ablation |
| US5345936A (en) | 1991-02-15 | 1994-09-13 | Cardiac Pathways Corporation | Apparatus with basket assembly for endocardial mapping |
| US5228442A (en) | 1991-02-15 | 1993-07-20 | Cardiac Pathways Corporation | Method for mapping, ablation, and stimulation using an endocardial catheter |
| US5184621A (en) | 1991-05-29 | 1993-02-09 | C. R. Bard, Inc. | Steerable guidewire having electrodes for measuring vessel cross-section and blood flow |
| US7151964B2 (en) | 1991-07-05 | 2006-12-19 | Desai Jawahar M | Device and method for multi-phase radio-frequency ablation |
| US20010051803A1 (en) | 1991-07-05 | 2001-12-13 | Desai Jawahar M. | Device and method for multi-phase radio-frequency ablation |
| US5693078A (en) | 1991-07-05 | 1997-12-02 | Jawahar M. Desai | Device and method for multi-phase radio-frequency ablation |
| US5620481A (en) | 1991-07-05 | 1997-04-15 | Desai; Jawahar M. | Device for multi-phase radio-frequency ablation |
| US5383917A (en) | 1991-07-05 | 1995-01-24 | Jawahar M. Desai | Device and method for multi-phase radio-frequency ablation |
| US5722975A (en) | 1991-11-08 | 1998-03-03 | E.P. Technologies Inc. | Systems for radiofrequency ablation with phase sensitive power detection and control |
| US5423808A (en) | 1991-11-08 | 1995-06-13 | Ep Technologies, Inc. | Systems and methods for radiofrequency ablation with phase sensitive power detection |
| WO1993008756A1 (en) | 1991-11-08 | 1993-05-13 | Ep Technologies, Inc. | Radiofrequency ablation with phase sensitive power detection |
| US6032674A (en) | 1992-01-07 | 2000-03-07 | Arthrocare Corporation | Systems and methods for myocardial revascularization |
| US5645064A (en) | 1992-01-29 | 1997-07-08 | Cardima, Inc. | High resolution intravascular signal detection |
| US5239999A (en) | 1992-03-27 | 1993-08-31 | Cardiac Pathways Corporation | Helical endocardial catheter probe |
| US5397304A (en) | 1992-04-10 | 1995-03-14 | Medtronic Cardiorhythm | Shapable handle for steerable electrode catheter |
| US5318525A (en) | 1992-04-10 | 1994-06-07 | Medtronic Cardiorhythm | Steerable electrode catheter |
| US5231987A (en) | 1992-04-10 | 1993-08-03 | Random Technologies, Inc. | Time domain reflectometer-integrity testing system and method for implantable electrode |
| US5573533A (en) | 1992-04-10 | 1996-11-12 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of cardiac tissue |
| US5445148A (en) | 1992-04-10 | 1995-08-29 | Medtronic Cardiorhythm | Intracardiac electrical potential reference catheter |
| US5540681A (en) | 1992-04-10 | 1996-07-30 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of tissue |
| US5281213A (en) | 1992-04-16 | 1994-01-25 | Implemed, Inc. | Catheter for ice mapping and ablation |
| US5255679A (en) | 1992-06-02 | 1993-10-26 | Cardiac Pathways Corporation | Endocardial catheter for mapping and/or ablation with an expandable basket structure having means for providing selective reinforcement and pressure sensing mechanism for use therewith, and method |
| US5324284A (en) | 1992-06-05 | 1994-06-28 | Cardiac Pathways, Inc. | Endocardial mapping and ablation system utilizing a separately controlled ablation catheter and method |
| US5281218A (en) | 1992-06-05 | 1994-01-25 | Cardiac Pathways Corporation | Catheter having needle electrode for radiofrequency ablation |
| WO1993025273A1 (en) | 1992-06-05 | 1993-12-23 | Cardiac Pathways Corporation | Catheter having needle electrode for radiofrequency ablation |
| US5782899A (en) | 1992-06-05 | 1998-07-21 | Cardiac Pathways Corporation | Endocardial mapping and ablation system utilizing a separately controlled ablation catheter and method |
| US5578007A (en) | 1992-06-05 | 1996-11-26 | Cardiac Pathways Corporation | Endocardial mapping and ablation system utilizing a separately controlled ablation catheter and method |
| US5411025A (en) | 1992-06-30 | 1995-05-02 | Cordis Webster, Inc. | Cardiovascular catheter with laterally stable basket-shaped electrode array |
| US5772590A (en) | 1992-06-30 | 1998-06-30 | Cordis Webster, Inc. | Cardiovascular catheter with laterally stable basket-shaped electrode array with puller wire |
| US5782239A (en) | 1992-06-30 | 1998-07-21 | Cordis Webster, Inc. | Unique electrode configurations for cardiovascular electrode catheter with built-in deflection method and central puller wire |
| US5769791A (en) | 1992-09-14 | 1998-06-23 | Sextant Medical Corporation | Tissue interrogating device and methods |
| US5313943A (en) | 1992-09-25 | 1994-05-24 | Ep Technologies, Inc. | Catheters and methods for performing cardiac diagnosis and treatment |
| US5309910A (en) | 1992-09-25 | 1994-05-10 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US5904680A (en) | 1992-09-25 | 1999-05-18 | Ep Technologies, Inc. | Multiple electrode support structures having optimal bio-mechanical characteristics |
| US6379352B1 (en) | 1992-09-29 | 2002-04-30 | Ep Technologies, Inc. | Large surface cardiac ablation catherter that assumes a low profile during introduction into the heart |
| US5471982A (en) | 1992-09-29 | 1995-12-05 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US6086581A (en) | 1992-09-29 | 2000-07-11 | Ep Technologies, Inc. | Large surface cardiac ablation catheter that assumes a low profile during introduction into the heart |
| US5334193A (en) | 1992-11-13 | 1994-08-02 | American Cardiac Ablation Co., Inc. | Fluid cooled ablation catheter |
| US5342357A (en) | 1992-11-13 | 1994-08-30 | American Cardiac Ablation Co., Inc. | Fluid cooled electrosurgical cauterization system |
| US5330466A (en) | 1992-12-01 | 1994-07-19 | Cardiac Pathways Corporation | Control mechanism and system and method for steering distal extremity of a flexible elongate member |
| US5697927A (en) | 1992-12-01 | 1997-12-16 | Cardiac Pathways Corporation | Catheter for RF ablation with cooled electrode and apparatus for use therewith |
| US5656029A (en) | 1992-12-01 | 1997-08-12 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and/or radius and method |
| US5527279A (en) | 1992-12-01 | 1996-06-18 | Cardiac Pathways Corporation | Control mechanism and system and method for steering distal extremity of a flexible elongate member |
| US5423811A (en) | 1992-12-01 | 1995-06-13 | Cardiac Pathways Corporation | Method for RF ablation using cooled electrode |
| US5533967A (en) | 1992-12-01 | 1996-07-09 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and method |
| US5545161A (en) | 1992-12-01 | 1996-08-13 | Cardiac Pathways Corporation | Catheter for RF ablation having cooled electrode with electrically insulated sleeve |
| US5658278A (en) | 1992-12-01 | 1997-08-19 | Cardiac Pathways, Inc. | Catheter for RF ablation with cooled electrode and method |
| US5391147A (en) | 1992-12-01 | 1995-02-21 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and/or radius and method |
| US5588964A (en) | 1992-12-01 | 1996-12-31 | Cardiac Pathways Corporation | Steerable catheter with adjustable bend location and/or radius and method |
| US5327889A (en) | 1992-12-01 | 1994-07-12 | Cardiac Pathways Corporation | Mapping and ablation catheter with individually deployable arms and method |
| US5348554A (en) | 1992-12-01 | 1994-09-20 | Cardiac Pathways Corporation | Catheter for RF ablation with cooled electrode |
| WO1994012098A1 (en) | 1992-12-01 | 1994-06-09 | Cardiac Pathways Corporation | Mapping and ablation catheter with individually deployable arms and method |
| US5967978A (en) | 1993-01-29 | 1999-10-19 | Cardima, Inc. | Intravascular sensing device |
| US5960796A (en) | 1993-01-29 | 1999-10-05 | Cardima, Inc. | Intravascular method and device for occluding a body lumen |
| US5685322A (en) | 1993-01-29 | 1997-11-11 | Cardima, Inc. | Intravascular system for treating arrhythmia |
| US5706809A (en) | 1993-01-29 | 1998-01-13 | Cardima, Inc. | Method and system for using multiple intravascular sensing devices to detect electrical activity |
| US5881732A (en) | 1993-01-29 | 1999-03-16 | Cardima, Inc. | Intravascular method and system for treating arrhythmia |
| US5682885A (en) | 1993-01-29 | 1997-11-04 | Cardima, Inc. | Intravascular sensing device |
| US5645082A (en) | 1993-01-29 | 1997-07-08 | Cardima, Inc. | Intravascular method and system for treating arrhythmia |
| US6088610A (en) | 1993-01-29 | 2000-07-11 | Cardima, Inc. | Method and system for using multiple intravascular sensing devices to detect electrical activity |
| US6141576A (en) | 1993-01-29 | 2000-10-31 | Cardima, Inc. | Intravascular sensing device |
| US5509411A (en) | 1993-01-29 | 1996-04-23 | Cardima, Inc. | Intravascular sensing device |
| US5699796A (en) | 1993-01-29 | 1997-12-23 | Cardima, Inc. | High resolution intravascular signal detection |
| US5433198A (en) | 1993-03-11 | 1995-07-18 | Desai; Jawahar M. | Apparatus and method for cardiac ablation |
| US20050148892A1 (en) | 1993-03-11 | 2005-07-07 | Desai Jawahar M. | Apparatus and method for cardiac ablation |
| US5657755A (en) | 1993-03-11 | 1997-08-19 | Desai; Jawahar M. | Apparatus and method for cardiac ablation |
| US6522905B2 (en) | 1993-03-11 | 2003-02-18 | Jawahar M. Desai | Apparatus and method for cardiac ablation |
| US20030181819A1 (en) | 1993-03-11 | 2003-09-25 | Desai Jawahar M. | Apparatus and method for cardiac ablation |
| US5662606A (en) | 1993-03-12 | 1997-09-02 | Heart Rhythm Technologies, Inc. | Catheter for electrophysiological procedures |
| US5364352A (en) | 1993-03-12 | 1994-11-15 | Heart Rhythm Technologies, Inc. | Catheter for electrophysiological procedures |
| US5893847A (en) | 1993-03-16 | 1999-04-13 | Ep Technologies, Inc. | Multiple electrode support structures with slotted hub and hoop spline elements |
| US6360128B2 (en) | 1993-03-16 | 2002-03-19 | Ep Technologies, Inc. | Cardiac mapping and ablation systems |
| US6805131B2 (en) | 1993-03-16 | 2004-10-19 | Ep Technologies, Inc. | Medical device with three dimensional collapsible basket structure |
| US6460545B2 (en) | 1993-03-16 | 2002-10-08 | Ep Technologies, Inc. | Medical device with three dimensional collapsible basket structure |
| US6216044B1 (en) | 1993-03-16 | 2001-04-10 | Ep Technologies, Inc. | Medical device with three dimensional collapsible basket structure |
| US5626136A (en) | 1993-04-28 | 1997-05-06 | Cordis Webster, Inc. | Electrophysiology catheter with pre-curved circular tip |
| US5507802A (en) | 1993-06-02 | 1996-04-16 | Cardiac Pathways Corporation | Method of mapping and/or ablation using a catheter having a tip with fixation means |
| USD351652S (en) | 1993-06-21 | 1994-10-18 | Ep Technologies, Inc. | Steerable medical catheter handle |
| US6004269A (en) | 1993-07-01 | 1999-12-21 | Boston Scientific Corporation | Catheters for imaging, sensing electrical potentials, and ablating tissue |
| US5630837A (en) | 1993-07-01 | 1997-05-20 | Boston Scientific Corporation | Acoustic ablation |
| US5545200A (en) | 1993-07-20 | 1996-08-13 | Medtronic Cardiorhythm | Steerable electrophysiology catheter |
| US5487757A (en) | 1993-07-20 | 1996-01-30 | Medtronic Cardiorhythm | Multicurve deflectable catheter |
| US6074351A (en) | 1993-07-30 | 2000-06-13 | Ep Technologies, Inc. | Variable curve electrophysiology catheter |
| US5928191A (en) | 1993-07-30 | 1999-07-27 | E.P. Technologies, Inc. | Variable curve electrophysiology catheter |
| US5607462A (en) | 1993-09-24 | 1997-03-04 | Cardiac Pathways Corporation | Catheter assembly, catheter and multi-catheter introducer for use therewith |
| US5342295A (en) | 1993-09-24 | 1994-08-30 | Cardiac Pathways Corporation | Catheter assembly, catheter and multi-port introducer for use therewith |
| US5558073A (en) | 1993-10-12 | 1996-09-24 | Cardiac Pathways Corporation | Endocardial mapping apparatus with rotatable arm and method |
| US5400783A (en) | 1993-10-12 | 1995-03-28 | Cardiac Pathways Corporation | Endocardial mapping apparatus with rotatable arm and method |
| US5860920A (en) | 1993-10-14 | 1999-01-19 | Ep Technologies, Inc. | Systems for locating and ablating accessory pathways in the heart |
| US6106522A (en) | 1993-10-14 | 2000-08-22 | Ep Technologies, Inc. | Systems and methods for forming elongated lesion patterns in body tissue using straight or curvilinear electrode elements |
| US6471699B1 (en) | 1993-10-14 | 2002-10-29 | Ep Technologies, Inc. | Systems and methods for forming elongated lesion patterns in body tissue using straight or curvilinear electrode elements |
| US5582609A (en) | 1993-10-14 | 1996-12-10 | Ep Technologies, Inc. | Systems and methods for forming large lesions in body tissue using curvilinear electrode elements |
| US6171306B1 (en) | 1993-10-14 | 2001-01-09 | Ep Technologies, Inc. | Systems and methods for forming large lesions in body tissue using curvilinear electrode elements |
| US6514246B1 (en) | 1993-10-14 | 2003-02-04 | Ep Technologies, Inc. | Systems and methods for forming large lesions in body tissue using curvilinear electrode elements |
| US6129724A (en) | 1993-10-14 | 2000-10-10 | Ep Technologies, Inc. | Systems and methods for forming elongated lesion patterns in body tissue using straight or curvilinear electrode elements |
| US5545193A (en) | 1993-10-15 | 1996-08-13 | Ep Technologies, Inc. | Helically wound radio-frequency emitting electrodes for creating lesions in body tissue |
| US5637090A (en) | 1993-10-15 | 1997-06-10 | Ep Technologies, Inc. | Multiple electrode element for mapping and ablating heart tissue |
| US20010029366A1 (en) | 1993-10-15 | 2001-10-11 | Swanson David K. | Composite structures and methods for ablating tissue to form complex lesion patterns in the treatment of cardiac conditions and the like |
| US5871523A (en) | 1993-10-15 | 1999-02-16 | Ep Technologies, Inc. | Helically wound radio-frequency emitting electrodes for creating lesions in body tissue |
| US6001093A (en) | 1993-10-15 | 1999-12-14 | Ep Technologies, Inc. | Systems and methods for creating long, thin lesions in body tissue |
| US6241754B1 (en) | 1993-10-15 | 2001-06-05 | Ep Technologies, Inc. | Composite structures and methods for ablating tissue to form complex lesion patterns in the treatment of cardiac conditions and the like |
| US7115122B1 (en) | 1993-10-15 | 2006-10-03 | Ep Technologies, Inc. | Composite structures and methods for ablating tissue to form complex lesion patterns in the treatment of cardiac conditions and the like |
| US6146379A (en) | 1993-10-15 | 2000-11-14 | Ep Technologies, Inc. | Systems and methods for creating curvilinear lesions in body tissue |
| US6447506B1 (en) | 1993-10-15 | 2002-09-10 | Ep Technologies, Inc. | Systems and methods for creating long, thin lesions in body tissue |
| US5575810A (en) | 1993-10-15 | 1996-11-19 | Ep Technologies, Inc. | Composite structures and methods for ablating tissue to form complex lesion patterns in the treatment of cardiac conditions and the like |
| US7048734B1 (en) | 1993-10-15 | 2006-05-23 | Ep Technologies, Inc. | Systems and methods for electronically altering the energy emitting characteristics of an electrode array to create different lesion patterns in body tissue |
| US6241724B1 (en) | 1993-10-19 | 2001-06-05 | Ep Technologies, Inc. | Systems and methods for creating lesions in body tissue using segmented electrode assemblies |
| US5433739A (en) | 1993-11-02 | 1995-07-18 | Sluijter; Menno E. | Method and apparatus for heating an intervertebral disc for relief of back pain |
| US5575766A (en) | 1993-11-03 | 1996-11-19 | Daig Corporation | Process for the nonsurgical mapping and treatment of atrial arrhythmia using catheters guided by shaped guiding introducers |
| US5536267A (en) | 1993-11-08 | 1996-07-16 | Zomed International | Multiple electrode ablation apparatus |
| US5938694A (en) | 1993-11-10 | 1999-08-17 | Medtronic Cardiorhythm | Electrode array catheter |
| EP1364677A2 (en) | 1993-11-10 | 2003-11-26 | Medtronic Cardiorhythm | Electrode array catheter |
| US5687723A (en) | 1993-12-03 | 1997-11-18 | Avitall; Boaz | Mapping and ablation catheter system |
| US6241725B1 (en) | 1993-12-15 | 2001-06-05 | Sherwood Services Ag | High frequency thermal ablation of cancerous tumors and functional targets with image data assistance |
| US5462521A (en) | 1993-12-21 | 1995-10-31 | Angeion Corporation | Fluid cooled and perfused tip for a catheter |
| US5492119A (en) | 1993-12-22 | 1996-02-20 | Heart Rhythm Technologies, Inc. | Catheter tip stabilizing apparatus |
| US5711298A (en) | 1994-01-27 | 1998-01-27 | Cardima, Inc. | High resolution intravascular signal detection |
| US5957842A (en) | 1994-01-27 | 1999-09-28 | Cardima, Inc. | High resolution intravascular signal detection |
| US6370435B2 (en) | 1994-01-28 | 2002-04-09 | Ep Technologies, Inc. | Systems and methods for examining the electrical characteristic of cardiac tissue |
| US6256540B1 (en) | 1994-01-28 | 2001-07-03 | Ep Technologies | Systems and methods for examining the electrical characteristic of cardiac tissue |
| US6099524A (en) | 1994-01-28 | 2000-08-08 | Cardiac Pacemakers, Inc. | Electrophysiological mapping and ablation catheter and method |
| US6597955B2 (en) | 1994-01-28 | 2003-07-22 | Ep Technologies, Inc. | Systems and methods for examining the electrical characteristic of cardiac tissue |
| US5462545A (en) | 1994-01-31 | 1995-10-31 | New England Medical Center Hospitals, Inc. | Catheter electrodes |
| US5968040A (en) | 1994-03-04 | 1999-10-19 | Ep Technologies, Inc. | Systems and methods using asymmetric multiple electrode arrays |
| US6216043B1 (en) | 1994-03-04 | 2001-04-10 | Ep Technologies, Inc. | Asymmetric multiple electrode support structures |
| US6165169A (en) | 1994-03-04 | 2000-12-26 | Ep Technologies, Inc. | Systems and methods for identifying the physical, mechanical, and functional attributes of multiple electrode arrays |
| US5584830A (en) | 1994-03-30 | 1996-12-17 | Medtronic Cardiorhythm | Method and system for radiofrequency ablation of cardiac tissue |
| US5598848A (en) | 1994-03-31 | 1997-02-04 | Ep Technologies, Inc. | Systems and methods for positioning multiple electrode structures in electrical contact with the myocardium |
| US5882333A (en) | 1994-05-13 | 1999-03-16 | Cardima, Inc. | Catheter with deflectable distal section |
| US6056744A (en) | 1994-06-24 | 2000-05-02 | Conway Stuart Medical, Inc. | Sphincter treatment apparatus |
| US5769847A (en) | 1994-06-27 | 1998-06-23 | Ep Technologies, Inc. | Systems and methods for controlling tissue ablation using multiple temperature sensing elements |
| CA2194061C (en) | 1994-06-27 | 2006-04-11 | David K. Swanson | Systems and methods for sensing temperature within the body |
| US5680860A (en) | 1994-07-07 | 1997-10-28 | Cardiac Pathways Corporation | Mapping and/or ablation catheter with coilable distal extremity and method for using same |
| US6500172B1 (en) | 1994-08-08 | 2002-12-31 | Ep Technologies, Inc. | Systems and methods for controlling tissue ablation using multiple temperature sensing elements |
| US6214002B1 (en) | 1994-10-07 | 2001-04-10 | Ep Technologies, Inc. | Structures and methods for deploying electrode elements |
| US6544262B2 (en) | 1994-10-07 | 2003-04-08 | Ep Technologies, Inc. | Structures and methods for deploying electrode elements |
| WO1996010961A1 (en) | 1994-10-07 | 1996-04-18 | Ep Technologies, Inc. | Flexible structures for supporting electrode elements |
| US6893439B2 (en) | 1994-10-07 | 2005-05-17 | Ep Technologies, Inc. | Structures and methods for deploying electrode elements |
| US6071282A (en) | 1994-10-07 | 2000-06-06 | Ep Technologies, Inc. | Structures for deploying electrode elements |
| US6939349B2 (en) | 1994-10-07 | 2005-09-06 | Ep Technologies, Inc. | Structures for deploying electrode elements |
| US5885278A (en) | 1994-10-07 | 1999-03-23 | E.P. Technologies, Inc. | Structures for deploying movable electrode elements |
| US6353751B1 (en) | 1994-10-11 | 2002-03-05 | Ep Technologies, Inc. | Systems and methods for guiding movable electrode elements within multiple-electrodes structures |
| US6456864B1 (en) | 1994-10-11 | 2002-09-24 | Ep Technologies, Inc. | Systems and methods for guiding movable electrode elements within multiple-electrode structures |
| US6070094A (en) | 1994-10-11 | 2000-05-30 | Ep Technologies, Inc. | Systems and methods for guiding movable electrode elements within multiple-electrode structures |
| US5857997A (en) | 1994-11-14 | 1999-01-12 | Heart Rhythm Technologies, Inc. | Catheter for electrophysiological procedures |
| US5601088A (en) | 1995-02-17 | 1997-02-11 | Ep Technologies, Inc. | Systems and methods for filtering artifacts from composite signals |
| US6487441B1 (en) | 1995-02-17 | 2002-11-26 | Ep Technologies, Inc. | Systems and methods for acquiring and analyzing electrograms in myocardial tissue |
| US5630425A (en) | 1995-02-17 | 1997-05-20 | Ep Technologies, Inc. | Systems and methods for adaptive filtering artifacts from composite signals |
| US5595183A (en) | 1995-02-17 | 1997-01-21 | Ep Technologies, Inc. | Systems and methods for examining heart tissue employing multiple electrode structures and roving electrodes |
| US20040236324A1 (en) | 1995-02-22 | 2004-11-25 | Medtronic, Inc. | Pen-type electrosurgical instrument |
| US5704791A (en) | 1995-03-29 | 1998-01-06 | Gillio; Robert G. | Virtual surgery system instrument |
| WO1996032897A1 (en) | 1995-04-20 | 1996-10-24 | Desai Jawahar M | Apparatus for cardiac mapping and ablation |
| WO1996032885A1 (en) | 1995-04-20 | 1996-10-24 | Desai Jawahar M | Apparatus for cardiac ablation |
| WO1996034570A1 (en) | 1995-05-01 | 1996-11-07 | Ep Technologies, Inc. | Systems and methods for obtaining desired lesion characteristics while ablating body tissue |
| WO1996034559A1 (en) | 1995-05-01 | 1996-11-07 | Cordis Webster, Inc. | Unique electrode configurations for cardiovascular electrode catheter with built-in deflection method and central puller wire |
| WO1996034569A1 (en) | 1995-05-01 | 1996-11-07 | Ep Technologies, Inc. | Systems and methods for ablating body tissue using predicted maximum tissue temperature |
| US5916214A (en) | 1995-05-01 | 1999-06-29 | Medtronic Cardiorhythm | Dual curve ablation catheter |
| WO1996036860A2 (en) | 1995-05-01 | 1996-11-21 | Ep Technologies, Inc. | Systems and methods for sensing sub-surface temperatures in body tissue during ablation with actively cooled electrodes |
| US5681280A (en) | 1995-05-02 | 1997-10-28 | Heart Rhythm Technologies, Inc. | Catheter control system |
| WO1996034567A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | System for controlling the energy delivered to a patient for ablation |
| US5666970A (en) | 1995-05-02 | 1997-09-16 | Heart Rhythm Technologies, Inc. | Locking mechanism for catheters |
| WO1996034560A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter with expandable probe |
| US5741320A (en) | 1995-05-02 | 1998-04-21 | Heart Rhythm Technologies, Inc. | Catheter control system having a pulley |
| US5596995A (en) | 1995-05-02 | 1997-01-28 | Heart Rhythm Technologies, Inc. | Biomedical device having a temperature sensing system |
| US5606974A (en) | 1995-05-02 | 1997-03-04 | Heart Rhythm Technologies, Inc. | Catheter having ultrasonic device |
| WO1996034558A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Biomedical device having a temperature sensing system |
| CA2222617C (en) | 1995-05-02 | 2002-07-16 | Heart Rhythm Technologies, Inc. | System for controlling the energy delivered to a patient for ablation |
| US5735280A (en) | 1995-05-02 | 1998-04-07 | Heart Rhythm Technologies, Inc. | Ultrasound energy delivery system and method |
| WO1996034653A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter control system having a pulley |
| US5971980A (en) | 1995-05-02 | 1999-10-26 | Heart Rhythm Technologies, Inc. | System for controlling the energy delivered to a patient for ablation |
| WO1996034652A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Catheter control system |
| USD381076S (en) | 1995-05-02 | 1997-07-15 | Heart Rhythm Technologies, Inc. | Manipulation handle |
| WO1996034650A1 (en) | 1995-05-02 | 1996-11-07 | Heart Rhythm Technologies, Inc. | Locking mechanism for catheters |
| US6002956A (en) | 1995-05-23 | 1999-12-14 | Cardima, Inc. | Method of treating using an over-the-wire EP catheter |
| US5782760A (en) | 1995-05-23 | 1998-07-21 | Cardima, Inc. | Over-the-wire EP catheter |
| US5895355A (en) | 1995-05-23 | 1999-04-20 | Cardima, Inc. | Over-the-wire EP catheter |
| US6830576B2 (en) | 1995-06-07 | 2004-12-14 | Ep Technologies, Inc. | Apparatus for electrically isolating a portion of the atria |
| US5775327A (en) | 1995-06-07 | 1998-07-07 | Cardima, Inc. | Guiding catheter for the coronary sinus |
| US6652513B2 (en) | 1995-06-07 | 2003-11-25 | Ep Technologies, Inc. | Tissue heating and ablation systems and methods which predict maximum tissue temperature |
| US20040152980A1 (en) | 1995-06-07 | 2004-08-05 | Desai Jawahar M. | Catheter for media injection |
| US6052612A (en) | 1995-06-07 | 2000-04-18 | Desai; Jawahar M. | Catheter for media injection |
| WO1996039967A1 (en) | 1995-06-07 | 1996-12-19 | Ep Technologies, Inc. | Tissue heating and ablation systems and methods which predict maximum tissue temperature |
| US6021340A (en) | 1995-06-07 | 2000-02-01 | Cardima, Inc. | Guiding catheter for the coronary sinus |
| US5857464A (en) | 1995-06-07 | 1999-01-12 | Desai; Jawahar M. | Catheter for media injection |
| US6293943B1 (en) | 1995-06-07 | 2001-09-25 | Ep Technologies, Inc. | Tissue heating and ablation systems and methods which predict maximum tissue temperature |
| US6701180B1 (en) | 1995-06-07 | 2004-03-02 | Jawahar M. Desai | Catheter for media injection |
| US5702438A (en) | 1995-06-08 | 1997-12-30 | Avitall; Boaz | Expandable recording and ablation catheter system |
| US6179833B1 (en) | 1995-06-09 | 2001-01-30 | Engineering & Research Associates, Inc. | Apparatus for thermal ablation |
| US6212426B1 (en) | 1995-07-28 | 2001-04-03 | Scimed Life Systems, Inc. | Systems and methods for conducting electrophysiological testing using high-voltage energy pulses to stun tissue |
| US6023638A (en) | 1995-07-28 | 2000-02-08 | Scimed Life Systems, Inc. | System and method for conducting electrophysiological testing using high-voltage energy pulses to stun tissue |
| US5724985A (en) | 1995-08-02 | 1998-03-10 | Pacesetter, Inc. | User interface for an implantable medical device using an integrated digitizer display screen |
| US5673695A (en) | 1995-08-02 | 1997-10-07 | Ep Technologies, Inc. | Methods for locating and ablating accessory pathways in the heart |
| US5827272A (en) | 1995-08-07 | 1998-10-27 | Medtronic Cardiorhythm | Simplified torquing electrode catheter |
| US6053937A (en) | 1995-08-15 | 2000-04-25 | Rita Medical Systems, Inc. | Multiple electrode ablation apparatus and method with cooling element |
| WO1997015919A1 (en) | 1995-10-25 | 1997-05-01 | Iomega Corporation | Molded actuator crash stops |
| US5810740A (en) | 1995-11-13 | 1998-09-22 | Heart Rhythm Technologies, Inc. | System and method for analyzing electrogram waveforms |
| US5716389A (en) | 1995-11-13 | 1998-02-10 | Walinsky; Paul | Cardiac ablation catheter arrangement with movable guidewire |
| US5733323A (en) | 1995-11-13 | 1998-03-31 | Cordis Corporation | Electrically conductive unipolar vascular sheath |
| WO1997017893A1 (en) | 1995-11-13 | 1997-05-22 | Heart Rhythm Technologies, Inc. | System and method for analyzing electrogram waveforms |
| WO1997017904A1 (en) | 1995-11-13 | 1997-05-22 | Ep Technologies, Inc. | Flexible tissue ablation elements for making long lesions |
| US5931835A (en) | 1995-12-08 | 1999-08-03 | C. R. Bard | Radio frequency energy delivery system for multipolar electrode catheters |
| US5837001A (en) | 1995-12-08 | 1998-11-17 | C. R. Bard | Radio frequency energy delivery system for multipolar electrode catheters |
| US5769077A (en)* | 1995-12-28 | 1998-06-23 | Pacesetter Ab | Multi-contact implantable electrode cable with a resorbable stiffening element |
| US6574492B1 (en) | 1996-01-08 | 2003-06-03 | Biosense, Inc. | Catheter having multiple arms with electrode and position sensor |
| WO1997025919A1 (en) | 1996-01-19 | 1997-07-24 | Ep Technologies, Inc. | Systems and methods for heating and ablating tissue using multifunctional electrode structures |
| WO1997025917A1 (en) | 1996-01-19 | 1997-07-24 | Ep Technologies, Inc. | Multi-function electrode structures for electrically analyzing and heating body tissue |
| US6428536B2 (en) | 1996-01-19 | 2002-08-06 | Ep Technologies, Inc. | Expandable-collapsible electrode structures made of electrically conductive material |
| US6475213B1 (en) | 1996-01-19 | 2002-11-05 | Ep Technologies, Inc. | Method of ablating body tissue |
| US20030093069A1 (en) | 1996-01-19 | 2003-05-15 | Ep Technologies, Inc. | Expandable-collapsible electrode structures made of electrically conductive material |
| US5891135A (en) | 1996-01-19 | 1999-04-06 | Ep Technologies, Inc. | Stem elements for securing tubing and electrical wires to expandable-collapsible electrode structures |
| US5895417A (en) | 1996-03-06 | 1999-04-20 | Cardiac Pathways Corporation | Deflectable loop design for a linear lesion ablation apparatus |
| US5800482A (en) | 1996-03-06 | 1998-09-01 | Cardiac Pathways Corporation | Apparatus and method for linear lesion ablation |
| WO1997032525A1 (en) | 1996-03-06 | 1997-09-12 | Cardiac Pathways Corporation | Apparatus for creating linear lesions by ablation |
| US6119041A (en) | 1996-03-06 | 2000-09-12 | Cardiac Pathways Corporation | Apparatus and method for linear lesion ablation |
| WO1997036541A1 (en) | 1996-04-02 | 1997-10-09 | Cordis Webster, Inc. | Electrophysiology catheter with a bullseye electrode |
| US6814732B2 (en) | 1996-04-08 | 2004-11-09 | Cardima, Inc. | Linear ablation assembly |
| CA2251041C (en) | 1996-04-08 | 2006-06-06 | Cardima, Inc. | Linear ablation device and assembly |
| US20050065512A1 (en) | 1996-04-08 | 2005-03-24 | Cardima, Inc. | Linear ablation assembly |
| US6063077A (en) | 1996-04-08 | 2000-05-16 | Cardima, Inc. | Linear ablation device and assembly |
| US6302880B1 (en) | 1996-04-08 | 2001-10-16 | Cardima, Inc. | Linear ablation assembly |
| US5863291A (en) | 1996-04-08 | 1999-01-26 | Cardima, Inc. | Linear ablation assembly |
| US6813520B2 (en) | 1996-04-12 | 2004-11-02 | Novacept | Method for ablating and/or coagulating tissue using moisture transport |
| US6346104B2 (en) | 1996-04-30 | 2002-02-12 | Western Sydney Area Health Service | System for simultaneous unipolar multi-electrode ablation |
| US20010020166A1 (en) | 1996-04-30 | 2001-09-06 | Michael Daly | System for simultaneous unipolar multi-electrode ablation |
| WO1997040760A1 (en) | 1996-04-30 | 1997-11-06 | Cardiac Crc Nominees Pty. Ltd. | A system for simultaneous unipolar multi-electrode ablation |
| WO1997042996A1 (en) | 1996-05-13 | 1997-11-20 | Ep Technologies, Inc. | Assemblies for creating compound curves in distal catheter regions |
| US5766152A (en) | 1996-08-15 | 1998-06-16 | Cardima, Inc. | Intraluminal delivery of tissue lysing medium |
| US5697928A (en) | 1996-09-23 | 1997-12-16 | Uab Research Foundation | Cardic electrode catheter |
| US20040133154A1 (en) | 1996-10-11 | 2004-07-08 | Flaherty J. Christopher | Systems and methods for delivering drugs to selected locations within the body |
| US5820568A (en) | 1996-10-15 | 1998-10-13 | Cardiac Pathways Corporation | Apparatus and method for aiding in the positioning of a catheter |
| US5891027A (en) | 1996-10-21 | 1999-04-06 | Irvine Biomedical, Inc. | Cardiovascular catheter system with an inflatable soft tip |
| US6658279B2 (en) | 1996-10-28 | 2003-12-02 | Ep Technologies, Inc. | Ablation and imaging catheter |
| WO1998018520A2 (en) | 1996-10-28 | 1998-05-07 | Ep Technologies, Inc. | Asymmetric multiple electrode support structures |
| WO1998019611A1 (en) | 1996-11-01 | 1998-05-14 | Cordis Webster, Inc. | Multi-electrode ablation catheter |
| US5893885A (en) | 1996-11-01 | 1999-04-13 | Cordis Webster, Inc. | Multi-electrode ablation catheter |
| US5911720A (en) | 1996-11-26 | 1999-06-15 | Ep Technologies, Inc. | Ablation catheter with segmented tip |
| US5782828A (en) | 1996-12-11 | 1998-07-21 | Irvine Biomedical, Inc. | Ablation catheter with multiple flexible curves |
| US5954719A (en) | 1996-12-11 | 1999-09-21 | Irvine Biomedical, Inc. | System for operating a RF ablation generator |
| US7025766B2 (en) | 1996-12-19 | 2006-04-11 | Ep Technologies, Inc. | Structures for supporting multiple electrode elements |
| US6332880B1 (en) | 1996-12-19 | 2001-12-25 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| US7029471B2 (en) | 1996-12-19 | 2006-04-18 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| US20060189975A1 (en) | 1996-12-19 | 2006-08-24 | Whayne James G | Structures For Supporting Multiple Electrode Elements |
| US6048329A (en) | 1996-12-19 | 2000-04-11 | Ep Technologies, Inc. | Catheter distal assembly with pull wires |
| US20020161422A1 (en) | 1996-12-19 | 2002-10-31 | Ep Technologies, Inc. | Structures for supporting porous electrode elements |
| US6071274A (en) | 1996-12-19 | 2000-06-06 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| CA2276755C (en) | 1996-12-19 | 2006-05-16 | Ep Technologies, Inc. | Branched structures for supporting multiple electrode elements |
| US6071279A (en) | 1996-12-19 | 2000-06-06 | Ep Technologies, Inc. | Branched structures for supporting multiple electrode elements |
| US5910129A (en) | 1996-12-19 | 1999-06-08 | Ep Technologies, Inc. | Catheter distal assembly with pull wires |
| US6607505B1 (en) | 1996-12-19 | 2003-08-19 | Ep Technologies, Inc. | Catheter distal assembly with pull wires |
| US6454758B1 (en) | 1996-12-19 | 2002-09-24 | Ep Technologies, Inc. | Loop structures for supporting multiple electrode elements |
| WO1998026724A1 (en) | 1996-12-19 | 1998-06-25 | Ep Technologies, Inc. | Branched structures for supporting multiple electrode elements |
| WO1998028039A2 (en) | 1996-12-20 | 1998-07-02 | Ep Technologies, Inc. | Unified switching system for electrophysiological stimulation and signal recording and analysis |
| US5906605A (en) | 1997-01-10 | 1999-05-25 | Cardiac Pathways Corporation | Torquable guiding catheter for basket deployment and method |
| US5913854A (en) | 1997-02-04 | 1999-06-22 | Medtronic, Inc. | Fluid cooled ablation catheter and method for making |
| US6068629A (en) | 1997-02-04 | 2000-05-30 | Medtronic, Inc. | System and methods for tissue mapping and ablation |
| US5873865A (en) | 1997-02-07 | 1999-02-23 | Eclipse Surgical Technologies, Inc. | Spiral catheter with multiple guide holes |
| US5897554A (en) | 1997-03-01 | 1999-04-27 | Irvine Biomedical, Inc. | Steerable catheter having a loop electrode |
| US6245089B1 (en) | 1997-03-06 | 2001-06-12 | Scimed Life Systems, Inc. | Distal protection device and method |
| EP1011437B1 (en) | 1997-03-07 | 2006-05-31 | Cardima, Inc. | Over-the-wire electrophysiology catheter |
| WO1998038913A1 (en) | 1997-03-07 | 1998-09-11 | Ep Technologies, Inc. | Graphical user interface for use with multiple electrode catheters |
| US6245067B1 (en) | 1997-04-16 | 2001-06-12 | Irvine Biomedical, Inc. | Ablation device and methods having perpendicular electrodes |
| US7044135B2 (en) | 1997-05-09 | 2006-05-16 | The Regents Of The University Of California | Device and method for forming a circumferential conduction block in a pulmonary vein |
| US6955173B2 (en) | 1997-05-09 | 2005-10-18 | The Regents Of The University Of California | Device and method for forming a circumferential conduction block in a pulmonary vein |
| US5792140A (en) | 1997-05-15 | 1998-08-11 | Irvine Biomedical, Inc. | Catheter having cooled multiple-needle electrode |
| US5849028A (en) | 1997-05-16 | 1998-12-15 | Irvine Biomedical, Inc. | Catheter and method for radiofrequency ablation of cardiac tissue |
| US5893884A (en) | 1997-05-19 | 1999-04-13 | Irvine Biomedical, Inc. | Catheter system having rollable electrode means |
| EP1014874B1 (en) | 1997-05-19 | 2006-12-13 | Boston Scientific Limited | Apparatus for treating tissue with multiple electrodes |
| US6217576B1 (en) | 1997-05-19 | 2001-04-17 | Irvine Biomedical Inc. | Catheter probe for treating focal atrial fibrillation in pulmonary veins |
| US5891137A (en) | 1997-05-21 | 1999-04-06 | Irvine Biomedical, Inc. | Catheter system having a tip with fixation means |
| US6241726B1 (en) | 1997-05-21 | 2001-06-05 | Irvine Biomedical, Inc. | Catheter system having a tip section with fixation means |
| US5876399A (en) | 1997-05-28 | 1999-03-02 | Irvine Biomedical, Inc. | Catheter system and methods thereof |
| US6001095A (en) | 1997-06-23 | 1999-12-14 | Irvine Biomedical, Inc. | Catheter system having closely spaced distal bipolar electrodes |
| US5992418A (en) | 1997-06-23 | 1999-11-30 | Irvine Biomedical , Inc. | Catheter system having safety means and methods thereof |
| US6540744B2 (en) | 1997-06-27 | 2003-04-01 | St. Jude Medical, Daig Division, Inc. | Process and device for the treatment of atrial arrhythmia |
| US7118568B2 (en) | 1997-06-27 | 2006-10-10 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Process and device for the treatment of atrial arrhythmia |
| US5997532A (en) | 1997-07-03 | 1999-12-07 | Cardiac Pathways Corporation | Ablation catheter tip with a buffer layer covering the electrode |
| US6241666B1 (en) | 1997-07-03 | 2001-06-05 | Cardiac Pathways Corp. | Ablation catheter tip with a buffer layer covering the electrode |
| US6966908B2 (en)* | 1997-07-08 | 2005-11-22 | Atrionix, Inc. | Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall |
| US6964660B2 (en) | 1997-07-08 | 2005-11-15 | Atrionix, Inc. | Tissue ablation device assembly and method of electrically isolating a pulmonary vein ostium from an atrial wall |
| US20060084966A1 (en) | 1997-07-08 | 2006-04-20 | Maguire Mark A | Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall |
| US6605087B2 (en) | 1997-07-21 | 2003-08-12 | St. Jude Medical, Daig Division | Ablation catheter |
| US5941845A (en) | 1997-08-05 | 1999-08-24 | Irvine Biomedical, Inc. | Catheter having multiple-needle electrode and methods thereof |
| US5891138A (en) | 1997-08-11 | 1999-04-06 | Irvine Biomedical, Inc. | Catheter system having parallel electrodes |
| US6500167B1 (en) | 1997-09-05 | 2002-12-31 | Biosense Webster, Inc. | Omni-directional steerable catheter |
| US6554794B1 (en) | 1997-09-24 | 2003-04-29 | Richard L. Mueller | Non-deforming deflectable multi-lumen catheter |
| US20020065465A1 (en) | 1997-09-26 | 2002-05-30 | Ep Technologies, Inc. | System for recording use of structures deployed in association with heart tissue |
| US6490468B2 (en) | 1997-09-26 | 2002-12-03 | Ep Technologies, Inc. | Systems for recording use of structures deployed in association with heart tissue |
| US6565511B2 (en) | 1997-09-26 | 2003-05-20 | Ep Technologies, Inc. | Systems for recording use of structures deployed in association with heart tissue |
| US5935063A (en) | 1997-10-29 | 1999-08-10 | Irvine Biomedical, Inc. | Electrode catheter system and methods thereof |
| US6063082A (en) | 1997-11-04 | 2000-05-16 | Scimed Life Systems, Inc. | Percutaneous myocardial revascularization basket delivery system and radiofrequency therapeutic device |
| US6120476A (en) | 1997-12-01 | 2000-09-19 | Cordis Webster, Inc. | Irrigated tip catheter |
| US6602242B1 (en) | 1997-12-01 | 2003-08-05 | Biosense Webster, Inc. | Irrigated tip catheter |
| US6241728B1 (en) | 1997-12-18 | 2001-06-05 | Medtronic, Inc. | Left atrium ablation catheter and method |
| US7122031B2 (en) | 1998-02-19 | 2006-10-17 | Curon Medical, Inc. | Graphical user interface for association with an electrode structure deployed in contact with a tissue region |
| US6625482B1 (en) | 1998-03-06 | 2003-09-23 | Ep Technologies, Inc. | Graphical user interface for use with multiple electrode catheters |
| US5951471A (en) | 1998-03-09 | 1999-09-14 | Irvine Biomedical, Inc. | Catheter-based coronary sinus mapping and ablation |
| US6167291A (en) | 1998-03-12 | 2000-12-26 | Cardima, Inc. | Protected pin connector for an electrophysiology catheter |
| US6115626A (en) | 1998-03-26 | 2000-09-05 | Scimed Life Systems, Inc. | Systems and methods using annotated images for controlling the use of diagnostic or therapeutic instruments in instruments in interior body regions |
| US6014581A (en) | 1998-03-26 | 2000-01-11 | Ep Technologies, Inc. | Interface for performing a diagnostic or therapeutic procedure on heart tissue with an electrode structure |
| US6389311B1 (en) | 1998-03-26 | 2002-05-14 | Scimed Life Systems, Inc. | Systems and methods using annotated images for controlling the use of diagnostic or therapeutic instruments in interior body regions |
| US6542773B2 (en) | 1998-03-26 | 2003-04-01 | Scimed Life Systems, Inc. | Interactive systems and methods for controlling the use of diagnostic or therapeutic instruments in interior body regions |
| US6064902A (en) | 1998-04-16 | 2000-05-16 | C.R. Bard, Inc. | Pulmonary vein ablation catheter |
| CA2327518A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using controllable duty cycle with alternate phasing |
| US6050994A (en) | 1998-05-05 | 2000-04-18 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using controllable duty cycle with alternate phasing |
| US6488678B2 (en) | 1998-05-05 | 2002-12-03 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using unipolar and bipolar techniques |
| US6485487B1 (en) | 1998-05-05 | 2002-11-26 | Cardiac Pacemakers, Inc. | RF ablation apparatus having high output impedance drivers |
| US6045550A (en) | 1998-05-05 | 2000-04-04 | Cardiac Peacemakers, Inc. | Electrode having non-joined thermocouple for providing multiple temperature-sensitive junctions |
| CA2328070A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus having high output impedance drivers |
| US6440129B1 (en) | 1998-05-05 | 2002-08-27 | Cardiac Pacemakers, Inc. | Electrode having non-joined thermocouple for providing multiple temperature-sensitive junctions |
| WO1999056644A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using unipolar and bipolar techniques |
| US6049737A (en) | 1998-05-05 | 2000-04-11 | Cardiac Pacemakers, Inc. | Catheter having common lead for electrode and sensor |
| US6059778A (en) | 1998-05-05 | 2000-05-09 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using unipolar and bipolar techniques |
| US20030195501A1 (en) | 1998-05-05 | 2003-10-16 | Sherman Marshall L. | RF ablation system and method having automatic temperature control |
| WO1999056648A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using controllable duty cycle with alternate phasing |
| US6146381A (en) | 1998-05-05 | 2000-11-14 | Cardiac Pacemakers, Inc. | Catheter having distal region for deflecting axial forces |
| US6042580A (en) | 1998-05-05 | 2000-03-28 | Cardiac Pacemakers, Inc. | Electrode having composition-matched, common-lead thermocouple wire for providing multiple temperature-sensitive junctions |
| CA2328064A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Catheter having common lead for electrode and sensor |
| WO1999056647A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus having high output impedance drivers |
| US6558378B2 (en) | 1998-05-05 | 2003-05-06 | Cardiac Pacemakers, Inc. | RF ablation system and method having automatic temperature control |
| US6200314B1 (en) | 1998-05-05 | 2001-03-13 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using unipolar and bipolar techniques |
| US6171305B1 (en) | 1998-05-05 | 2001-01-09 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method having high output impedance drivers |
| CA2327322A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method using unipolar and bipolar techniques |
| US6309385B1 (en) | 1998-05-05 | 2001-10-30 | Cardiac Pacemakers, Inc. | Electrode having composition-matched, common-lead thermocouple wire for providing multiple temperature-sensitive junctions |
| WO1999056649A1 (en) | 1998-05-05 | 1999-11-11 | Cardiac Pacemakers, Inc. | Catheter having common lead for electrode and sensor |
| US6312425B1 (en) | 1998-05-05 | 2001-11-06 | Cardiac Pacemakers, Inc. | RF ablation catheter tip electrode with multiple thermal sensors |
| US6096036A (en) | 1998-05-05 | 2000-08-01 | Cardiac Pacemakers, Inc. | Steerable catheter with preformed distal shape and method for use |
| US6616657B2 (en) | 1998-05-05 | 2003-09-09 | Cardiac Pacemakers, Inc. | RF ablation catheter tip electrode with multiple thermal sensors |
| US6071281A (en) | 1998-05-05 | 2000-06-06 | Ep Technologies, Inc. | Surgical method and apparatus for positioning a diagnostic or therapeutic element within the body and remote power control unit for use with same |
| US6107699A (en) | 1998-05-22 | 2000-08-22 | Scimed Life Systems, Inc. | Power supply for use in electrophysiological apparatus employing high-voltage pulses to render tissue temporarily unresponsive |
| US6428537B1 (en) | 1998-05-22 | 2002-08-06 | Scimed Life Systems, Inc. | Electrophysiological treatment methods and apparatus employing high voltage pulse to render tissue temporarily unresponsive |
| US6238390B1 (en) | 1998-05-27 | 2001-05-29 | Irvine Biomedical, Inc. | Ablation catheter system having linear lesion capabilities |
| US6241727B1 (en) | 1998-05-27 | 2001-06-05 | Irvine Biomedical, Inc. | Ablation catheter system having circular lesion capabilities |
| US20010044625A1 (en) | 1998-05-27 | 2001-11-22 | Cary Hata | Catheter for circular tissue ablation and methods thereof |
| US6251107B1 (en) | 1998-06-25 | 2001-06-26 | Cardima, Inc. | Ep catheter |
| US6029091A (en) | 1998-07-09 | 2000-02-22 | Irvine Biomedical, Inc. | Catheter system having lattice electrodes |
| US6226542B1 (en) | 1998-07-24 | 2001-05-01 | Biosense, Inc. | Three-dimensional reconstruction of intrabody organs |
| US6319251B1 (en) | 1998-09-24 | 2001-11-20 | Hosheng Tu | Medical device and methods for treating intravascular restenosis |
| US6033403A (en) | 1998-10-08 | 2000-03-07 | Irvine Biomedical, Inc. | Long electrode catheter system and methods thereof |
| US6451015B1 (en) | 1998-11-18 | 2002-09-17 | Sherwood Services Ag | Method and system for menu-driven two-dimensional display lesion generator |
| US6290697B1 (en) | 1998-12-01 | 2001-09-18 | Irvine Biomedical, Inc. | Self-guiding catheter system for tissue ablation |
| US6217573B1 (en) | 1998-12-03 | 2001-04-17 | Cordis Webster | System and method for measuring surface temperature of tissue during ablation |
| EP1210021B1 (en) | 1999-03-19 | 2006-05-10 | Atrionix, Inc. | Atrial annulus ablation device |
| US6267746B1 (en) | 1999-03-22 | 2001-07-31 | Biosense Webster, Inc. | Multi-directional steerable catheters and control handles |
| US6572612B2 (en) | 1999-04-05 | 2003-06-03 | Medtronic, Inc. | Ablation catheter and method for isolating a pulmonary vein |
| US20050010095A1 (en)* | 1999-04-05 | 2005-01-13 | Medtronic, Inc. | Multi-purpose catheter apparatus and method of use |
| US6702811B2 (en) | 1999-04-05 | 2004-03-09 | Medtronic, Inc. | Ablation catheter assembly with radially decreasing helix and method of use |
| US6325797B1 (en) | 1999-04-05 | 2001-12-04 | Medtronic, Inc. | Ablation catheter and method for isolating a pulmonary vein |
| EP1182980B1 (en) | 1999-05-11 | 2006-06-28 | Atrionix, Inc. | Tissue ablation device assembly |
| US6478793B1 (en) | 1999-06-11 | 2002-11-12 | Sherwood Services Ag | Ablation treatment of bone metastases |
| CA2371935A1 (en) | 1999-06-17 | 2000-12-28 | Thomas M. Castellano | Rf ablation apparatus and method having electrode/tissue contact assessment scheme and electrocardiogram filtering |
| WO2000078239A2 (en) | 1999-06-17 | 2000-12-28 | Cardiac Pacemakers, Inc. | Rf ablation apparatus and method having electrode/tissue contact assessment scheme and electrocardiogram filtering |
| US6391024B1 (en) | 1999-06-17 | 2002-05-21 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method having electrode/tissue contact assessment scheme and electrocardiogram filtering |
| EP1070480B1 (en) | 1999-07-22 | 2006-09-27 | Biosense Webster, Inc. | Vector mapping of three-dimensionally reconstructed intrabody organs |
| US6371955B1 (en) | 1999-08-10 | 2002-04-16 | Biosense Webster, Inc. | Atrial branding iron catheter and a method for treating atrial fibrillation |
| US6690972B2 (en) | 1999-08-20 | 2004-02-10 | Cardiac Pacemakers, Inc. | System and method for detecting and displaying parameter interactions |
| EP1207798B1 (en) | 1999-09-01 | 2006-06-21 | Cardima, Inc. | Elecrosurgical ablation tool |
| US6332881B1 (en) | 1999-09-01 | 2001-12-25 | Cardima, Inc. | Surgical ablation tool |
| US6471693B1 (en) | 1999-09-10 | 2002-10-29 | Cryocath Technologies Inc. | Catheter and system for monitoring tissue contact |
| US6607520B2 (en) | 1999-09-15 | 2003-08-19 | The General Hospital Corporation | Coiled ablation catheter system |
| US20030208199A1 (en) | 1999-09-15 | 2003-11-06 | David Keane | Coiled ablation catheter system |
| US6960206B2 (en) | 1999-09-15 | 2005-11-01 | The General Hospital Corporation | Coiled ablation catheter system |
| US20040182384A1 (en) | 1999-09-29 | 2004-09-23 | Alfery David D. | Perilaryngeal oral airway with temperature sensor |
| US6613046B1 (en) | 1999-11-22 | 2003-09-02 | Scimed Life Systems, Inc. | Loop structures for supporting diagnostic and therapeutic elements in contact with body tissue |
| US7184811B2 (en)* | 1999-11-22 | 2007-02-27 | Boston Scientific Scimed, Inc. | Apparatus for mapping and coagulating soft tissue in or around body orifices |
| US6529756B1 (en) | 1999-11-22 | 2003-03-04 | Scimed Life Systems, Inc. | Apparatus for mapping and coagulating soft tissue in or around body orifices |
| US6575997B1 (en) | 1999-12-23 | 2003-06-10 | Endovascular Technologies, Inc. | Embolic basket |
| US6628976B1 (en) | 2000-01-27 | 2003-09-30 | Biosense Webster, Inc. | Catheter having mapping assembly |
| US7099711B2 (en) | 2000-01-27 | 2006-08-29 | Biosense Webster, Inc. | Method for mapping electrical activity |
| US20030199746A1 (en) | 2000-01-27 | 2003-10-23 | Biosense Webster, Inc. | Catheter having mapping assembly |
| US6711428B2 (en) | 2000-01-27 | 2004-03-23 | Biosense Webster, Inc. | Catheter having mapping assembly |
| US20030195407A1 (en) | 2000-01-27 | 2003-10-16 | Biosense Webster, Inc. | Method for mapping electrical activity |
| EP1125549B1 (en) | 2000-02-18 | 2006-06-07 | Biosense Webster, Inc. | Catheter for generating an electrical map of a chamber of the heart |
| US6264664B1 (en) | 2000-03-10 | 2001-07-24 | General Science And Technology Corp. | Surgical basket devices |
| US6632223B1 (en) | 2000-03-30 | 2003-10-14 | The General Hospital Corporation | Pulmonary vein ablation stent and method |
| US6652517B1 (en)* | 2000-04-25 | 2003-11-25 | Uab Research Foundation | Ablation catheter, system, and method of use thereof |
| US6893438B2 (en) | 2000-04-25 | 2005-05-17 | Uab Research Foundation | Ablation catheter, system, and method of use thereof |
| US7113831B2 (en) | 2000-04-27 | 2006-09-26 | Atricure, Inc. | Transmural ablation device |
| US6517536B2 (en) | 2000-04-27 | 2003-02-11 | Atricure, Inc. | Transmural ablation device and method |
| US20010039415A1 (en) | 2000-04-27 | 2001-11-08 | Medtronic, Inc. | System and method for assessing transmurality of ablation lesions |
| US6475214B1 (en) | 2000-05-01 | 2002-11-05 | Biosense Webster, Inc. | Catheter with enhanced ablation electrode |
| US6936047B2 (en) | 2000-05-12 | 2005-08-30 | Agility Capital Llc | Multi-channel RF energy delivery with coagulum reduction |
| US6375654B1 (en)* | 2000-05-19 | 2002-04-23 | Cardiofocus, Inc. | Catheter system with working portion radially expandable upon rotation |
| EP1169976B1 (en) | 2000-07-07 | 2006-04-05 | Biosense Webster, Inc. | Multi-electrode catheter, system and method |
| US6477396B1 (en) | 2000-07-07 | 2002-11-05 | Biosense Webster, Inc. | Mapping and ablation catheter |
| US6425894B1 (en) | 2000-07-12 | 2002-07-30 | Biosense Webster, Inc. | Ablation catheter with electrode temperature monitoring |
| US6746446B1 (en) | 2000-08-04 | 2004-06-08 | Cardima, Inc. | Electrophysiological device for the isthmus |
| US6669692B1 (en) | 2000-08-21 | 2003-12-30 | Biosense Webster, Inc. | Ablation catheter with cooled linear electrode |
| US6493586B1 (en) | 2000-08-30 | 2002-12-10 | Cardiac Pacemakers, Inc. | Site reversion in cardiac rhythm management |
| US6638275B1 (en) | 2000-10-05 | 2003-10-28 | Medironic, Inc. | Bipolar ablation apparatus and method |
| US6640120B1 (en) | 2000-10-05 | 2003-10-28 | Scimed Life Systems, Inc. | Probe assembly for mapping and ablating pulmonary vein tissue and method of using same |
| US6916306B1 (en) | 2000-11-10 | 2005-07-12 | Boston Scientific Scimed, Inc. | Steerable loop structures for supporting diagnostic and therapeutic elements in contact with body tissue |
| EP1343427B1 (en) | 2000-12-11 | 2006-07-26 | C.R. Bard, Inc. | Apparatus for mapping |
| US7047068B2 (en) | 2000-12-11 | 2006-05-16 | C.R. Bard, Inc. | Microelectrode catheter for mapping and ablation |
| US6583796B2 (en) | 2000-12-14 | 2003-06-24 | Medtronic, Inc. | Method and apparatus for displaying information retrieved from an implanted medical device |
| US20020120263A1 (en) | 2000-12-15 | 2002-08-29 | Tony R. Brown | Atrial fibrillation RF treatment device and method |
| WO2002060523A2 (en) | 2000-12-15 | 2002-08-08 | Brown Tony R | Atrial fibrillation rf treatment device and method |
| US20020126036A1 (en) | 2000-12-21 | 2002-09-12 | Flaherty J. Christopher | Medical apparatus remote control and method |
| US6638223B2 (en) | 2000-12-28 | 2003-10-28 | Ge Medical Systems Global Technology Company, Llc | Operator interface for a medical diagnostic imaging device |
| US6752804B2 (en) | 2000-12-28 | 2004-06-22 | Cardiac Pacemakers, Inc. | Ablation system and method having multiple-sensor electrodes to assist in assessment of electrode and sensor position and adjustment of energy levels |
| US7001336B2 (en) | 2001-01-30 | 2006-02-21 | Advanced Cardiovascular Systems, Inc. | Method for treating ischemia |
| US6569163B2 (en) | 2001-03-09 | 2003-05-27 | Quantumcor, Inc. | Wireless electrosurgical adapter unit and methods thereof |
| US6743225B2 (en) | 2001-03-27 | 2004-06-01 | Uab Research Foundation | Electrophysiologic measure of endpoints for ablation lesions created in fibrillating substrates |
| US6569162B2 (en) | 2001-03-29 | 2003-05-27 | Ding Sheng He | Passively self-cooled electrode design for ablation catheters |
| US7094235B2 (en) | 2001-04-26 | 2006-08-22 | Medtronic, Inc. | Method and apparatus for tissue ablation |
| US20060195082A1 (en) | 2001-04-26 | 2006-08-31 | Francischelli David E | Method and apparatus for tissue ablation |
| US7029470B2 (en) | 2001-04-26 | 2006-04-18 | Medtronic, Inc. | Ablation system and method of use |
| US20060142753A1 (en) | 2001-04-26 | 2006-06-29 | Medtronic, Inc. | Ablation system and method of use |
| US20040181139A1 (en) | 2001-04-27 | 2004-09-16 | Falwell Gary S. | Method and apparatus for three dimensional mapping of electrical activity in blood vessels and ablation of electrical pathways identified by the three dimension map |
| US6551271B2 (en) | 2001-04-30 | 2003-04-22 | Biosense Webster, Inc. | Asymmetrical bidirectional steerable catheter |
| US6972016B2 (en) | 2001-05-01 | 2005-12-06 | Cardima, Inc. | Helically shaped electrophysiology catheter |
| EP1383437B1 (en) | 2001-05-01 | 2006-12-06 | Cardima, Inc. | Helically shaped electrophysiology catheter |
| US20050015084A1 (en) | 2001-05-01 | 2005-01-20 | Cardima, Inc. | Helically shaped electrophysiology catheter |
| US20020165532A1 (en) | 2001-05-01 | 2002-11-07 | Cardima, Inc. | Helically shaped electrophysiology catheter |
| US6771996B2 (en)* | 2001-05-24 | 2004-08-03 | Cardiac Pacemakers, Inc. | Ablation and high-resolution mapping catheter system for pulmonary vein foci elimination |
| US6569114B2 (en) | 2001-08-31 | 2003-05-27 | Biosense Webster, Inc. | Steerable catheter with struts |
| US6740080B2 (en) | 2001-08-31 | 2004-05-25 | Cardiac Pacemakers, Inc. | Ablation system with selectable current path means |
| US6761716B2 (en) | 2001-09-18 | 2004-07-13 | Cardiac Pacemakers, Inc. | System and method for assessing electrode-tissue contact and lesion quality during RF ablation by measurement of conduction time |
| US6952615B2 (en) | 2001-09-28 | 2005-10-04 | Shutaro Satake | Radiofrequency thermal balloon catheter |
| US6635056B2 (en) | 2001-10-09 | 2003-10-21 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using amplitude control |
| US20040138545A1 (en) | 2001-10-11 | 2004-07-15 | Irvine Biomedical, Inc. | System and method for mapping and ablating body tissue of the interior region of the heart |
| US6671533B2 (en) | 2001-10-11 | 2003-12-30 | Irvine Biomedical Inc. | System and method for mapping and ablating body tissue of the interior region of the heart |
| WO2003041602A2 (en) | 2001-11-13 | 2003-05-22 | Mayo Foundation For Medical Education And Research | Tissue ablation device and methods of using |
| US6669693B2 (en)* | 2001-11-13 | 2003-12-30 | Mayo Foundation For Medical Education And Research | Tissue ablation device and methods of using |
| EP1455667B1 (en) | 2001-12-12 | 2007-01-17 | Medtronic, Inc. | Apparatus for tissue ablation |
| EP1321166B1 (en) | 2001-12-21 | 2006-07-05 | Ethicon Inc. | Expandable intracardiac return electrode and method of use |
| US6961602B2 (en) | 2001-12-31 | 2005-11-01 | Biosense Webster, Inc. | Catheter having multiple spines each having electrical mapping and location sensing capabilities |
| US7099712B2 (en) | 2001-12-31 | 2006-08-29 | Biosense Webster, Inc. | Catheter having multiple spines each having electrical mapping and location sensing capabilities |
| US20030125730A1 (en) | 2002-01-03 | 2003-07-03 | Afx Inc. | Flexible device for ablation of biological tissue |
| US7048756B2 (en) | 2002-01-18 | 2006-05-23 | Apasara Medical Corporation | System, method and apparatus for evaluating tissue temperature |
| US20040158141A1 (en) | 2002-02-28 | 2004-08-12 | Biosense Webster, Inc. | Catheter having circular ablation assembly |
| US20050256521A1 (en) | 2002-03-15 | 2005-11-17 | Kozel Peter D | Method and apparatus for control of ablation energy and electrogram acquisition through multiple common electrodes in an electrophysiology catheter |
| WO2003089997A2 (en) | 2002-03-15 | 2003-10-30 | C.R. Bard, Inc. | Method and apparatus for control of ablation energy and electrogram acquisition through multiple common electrodes in an electrophysiology catheter |
| US6730078B2 (en) | 2002-04-22 | 2004-05-04 | Cardiac Pacemakers, Inc. | RF ablation apparatus and method using multi-frequency energy delivery |
| US20030204186A1 (en) | 2002-04-24 | 2003-10-30 | Biotronik Mess-Und Therapiegerate Gmbh & Co. Ingen | Ablation device for cardiac tissue, in particular for forming linear lesions between two vessel orifices in the heart |
| DE10218427A1 (en) | 2002-04-24 | 2003-11-06 | Biotronik Mess & Therapieg | Ablation device for cardiac tissue, in particular for creating a circular lesion around a vascular mouth in the heart |
| US20030204185A1 (en) | 2002-04-26 | 2003-10-30 | Sherman Marshall L. | System and method for monitoring use of disposable catheters |
| EP1360938A1 (en) | 2002-05-07 | 2003-11-12 | Irvine Biomedical, Inc. | System for operating an ablation generator with dual energy source |
| US6893442B2 (en) | 2002-06-14 | 2005-05-17 | Ablatrics, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US20040015164A1 (en) | 2002-07-19 | 2004-01-22 | Fuimaono Kristine B. | Atrial ablation catheter and method for treating atrial fibrillation |
| US20050119651A1 (en) | 2002-07-19 | 2005-06-02 | Biosense Webster, Inc. | Atrial ablation catheter and method for treating atrial fibrillation |
| EP1384445B1 (en) | 2002-07-23 | 2006-02-15 | Biosense Webster, Inc. | Ablation catheter having stabilizing array |
| US6866662B2 (en) | 2002-07-23 | 2005-03-15 | Biosense Webster, Inc. | Ablation catheter having stabilizing array |
| US20050251132A1 (en) | 2002-10-25 | 2005-11-10 | Regents Of The University Of Michigan | Ablation catheters |
| US20040082947A1 (en) | 2002-10-25 | 2004-04-29 | The Regents Of The University Of Michigan | Ablation catheters |
| US20050033137A1 (en) | 2002-10-25 | 2005-02-10 | The Regents Of The University Of Michigan | Ablation catheters and methods for their use |
| US20050240176A1 (en) | 2002-10-25 | 2005-10-27 | Regents Of The University Of Michigan | Ablation catheters |
| US20070106293A1 (en) | 2002-10-25 | 2007-05-10 | Hakan Oral | Ablation catheters |
| EP1415680B1 (en) | 2002-10-30 | 2006-04-26 | Biosense Webster, Inc. | Multi-tip steerable catheter |
| US20060241366A1 (en) | 2002-10-31 | 2006-10-26 | Gary Falwell | Electrophysiology loop catheter |
| US20040116921A1 (en) | 2002-12-11 | 2004-06-17 | Marshall Sherman | Cold tip rf/ultrasonic ablation catheter |
| JP2004188179A (en) | 2002-12-11 | 2004-07-08 | Cryocor Inc | Low-temperature tip end rf/us ablation catheter |
| CA2437140A1 (en) | 2002-12-11 | 2004-06-11 | Cryocor, Inc. | Cold tip rf/ultrasonic ablation catheter |
| US20060111708A1 (en) | 2003-01-17 | 2006-05-25 | Vanney Guy P | Ablation catheter assembly having a virtual electrode comprising portholes |
| US20040143256A1 (en) | 2003-01-21 | 2004-07-22 | Bednarek Michael C. | Ablation catheter and electrode |
| US20040181249A1 (en) | 2003-03-10 | 2004-09-16 | Pathway Medical Technologies, Inc. | Bearing system to support a rotatable operating head in an intracorporeal device |
| US6987995B2 (en) | 2003-03-12 | 2006-01-17 | Biosense Webster, Inc. | Multifunctional catheter handle |
| US7163537B2 (en) | 2003-06-02 | 2007-01-16 | Biosense Webster, Inc. | Enhanced ablation and mapping catheter and method for treating atrial fibrillation |
| US20040247164A1 (en) | 2003-06-09 | 2004-12-09 | Simon Furnish | Scanning catheter with position encoder |
| US6973339B2 (en) | 2003-07-29 | 2005-12-06 | Biosense, Inc | Lasso for pulmonary vein mapping and ablation |
| WO2005027766A1 (en) | 2003-09-01 | 2005-03-31 | Siemens Aktiengesellschaft | Method and device for visually assisting the electrophysiological use of a catheter in the heart |
| WO2005027765A1 (en) | 2003-09-01 | 2005-03-31 | Siemens Aktiengesellschaft | Method and device for visually supporting an electrophysiology catheter application in the heart |
| US7156843B2 (en) | 2003-09-08 | 2007-01-02 | Medtronic, Inc. | Irrigated focal ablation tip |
| US7155270B2 (en) | 2003-10-24 | 2006-12-26 | Biosense Webster, Inc. | Catheter with multi-spine mapping assembly |
| US20050096644A1 (en) | 2003-10-30 | 2005-05-05 | Hall Jeffrey A. | Energy delivery optimization for RF duty cycle for lesion creation |
| US20050101946A1 (en) | 2003-11-11 | 2005-05-12 | Biosense Webster Inc. | Externally applied RF for pulmonary vein isolation |
| US7077823B2 (en) | 2003-11-19 | 2006-07-18 | Biosense Webster, Inc. | Bidirectional steerable catheter with slidable mated puller wires |
| EP1554986A1 (en) | 2004-01-14 | 2005-07-20 | Biosense Webster, Inc. | Prediction and assessment of ablation of cardiac tissue |
| CA2492283A1 (en) | 2004-01-14 | 2005-07-14 | Biosense Webster, Inc. | Prediction and assessment of ablation of cardiac tissue |
| US20050177146A1 (en) | 2004-02-10 | 2005-08-11 | Marshall Sherman | System and method for assessing ice ball formation during a cryoablation procedure |
| US20050187545A1 (en) | 2004-02-20 | 2005-08-25 | Hooven Michael D. | Magnetic catheter ablation device and method |
| US20050234444A1 (en) | 2004-04-14 | 2005-10-20 | Hooven Michael D | Electrode and bipolar ablation method using same |
| WO2005104972A2 (en) | 2004-04-14 | 2005-11-10 | Atricure, Inc. | Electrode and bipolar ablation method using same |
| WO2006017517A2 (en) | 2004-08-04 | 2006-02-16 | Cardio-Optics, Inc. | Transparent electrode for the radiofrequency ablation of tissue |
| US20060030844A1 (en) | 2004-08-04 | 2006-02-09 | Knight Bradley P | Transparent electrode for the radiofrequency ablation of tissue |
| US20060089637A1 (en) | 2004-10-14 | 2006-04-27 | Werneth Randell L | Ablation catheter |
| WO2006049970A2 (en) | 2004-10-27 | 2006-05-11 | Yuval Carmel | Radio-frequency device for passivation of vascular plaque and method of using same |
| US20060095030A1 (en) | 2004-11-04 | 2006-05-04 | Scimed Life Systems, Inc. | Preshaped ablation catheter for ablating pulmonary vein ostia within the heart |
| WO2006052905A2 (en) | 2004-11-08 | 2006-05-18 | Cardima, Inc. | System and method for performing ablation and other medical procedures using an electrode array with flex circuit |
| WO2006055654A1 (en) | 2004-11-15 | 2006-05-26 | Biosense Webster Inc. | Catheter with microfabricated temperature sensing |
| WO2006055658A1 (en) | 2004-11-15 | 2006-05-26 | Biosense Webster Inc. | Catheter with multiple microfabricated temperature sensors |
| US20060106375A1 (en) | 2004-11-15 | 2006-05-18 | Werneth Randell L | Ablation system with feedback |
| WO2006055741A1 (en) | 2004-11-17 | 2006-05-26 | Biosense Webster, Inc. | Apparatus for real time evaluation of tissue ablation |
| WO2006055733A1 (en) | 2004-11-17 | 2006-05-26 | Biosense Webster, Inc. | Apparatus for real time evaluation of tissue ablation |
| EP1658818A1 (en) | 2004-11-23 | 2006-05-24 | Biosense Webster, Inc. | Externally applied rf for pulmonary vein isolation |
| US20060111701A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter adapted for treatment of septal wall arrhythmogenic foci and method of use |
| US20060111700A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter and method of use |
| US20060111703A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter and method of use |
| US20060111702A1 (en) | 2004-11-24 | 2006-05-25 | Ablation Frontiers, Inc. | Atrial ablation catheter adapted for treatment of septal wall arrhythmogenic foci and method of use |
| US20060122526A1 (en) | 2004-12-02 | 2006-06-08 | Omer Berenfeld | Method and algorithm for spatially identifying sources of cardiac fibrillation |
| EP1690564A1 (en) | 2005-02-14 | 2006-08-16 | Biosense Webster | Steerable catheter with in-plane deflection |
| US20060206109A1 (en) | 2005-03-08 | 2006-09-14 | Boston Scientific Scimed, Inc. | Finger mountable lesion formation devices and methods |
| US20070083195A1 (en) | 2005-07-11 | 2007-04-12 | Werneth Randell L | Low power tissue ablation system |
| US20070083193A1 (en) | 2005-08-22 | 2007-04-12 | Werneth Randell L | User interface for tissue ablation system |
Non-Patent Citations (9)
| Title |
|---|
| European Patent Application 06773536.5 (1895927), Communication pursuant to Rule 114(2) EPC, Third Party Observation, 5 pages. |
| Nademanee et al., "A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate," JACC, vol. 43, No. 11, pp. 2044-2053, 2004. |
| Oral et al., "Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation," Circulation, vol. 108, pp. 2355-2360, 2003. |
| Oral et al., "Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights," Circulation, vol. 106, pp. 1256-1262, 2002. |
| Oral et al.; U.S. Appl. No. 11/932,378 entitled "Ablation catheters and methods for their use," filed Oct. 31, 2007. |
| Oral et al.; U.S. Appl. No. 12/176,115 entitled "Atrial ablation catheter adapted for treatment of septal wall arrhythmogenic foci and method of use," filed Jul. 18, 2008. |
| Sherman et al.; U.S. Appl. No. 12/117,596 entitled RF energy delivery system and method, filed May 8, 2008. |
| Werneth et al.; U.S. Appl. No. 12/116,753 entitled "Ablation therapy system and method for treating continuous atrial fibrillation," filed May 7, 2008. |
| Wittkampf et al., "Radiofrequency ablation with a cooled porous electrode catheter," (abstract) JACC, vol. 11, No. 2, pp. 17a, Feb. 1988. |
Cited By (103)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8834464B2 (en) | 1999-04-05 | 2014-09-16 | Mark T. Stewart | Ablation catheters and associated systems and methods |
| US9554848B2 (en) | 1999-04-05 | 2017-01-31 | Medtronic, Inc. | Ablation catheters and associated systems and methods |
| US9289255B2 (en) | 2002-04-08 | 2016-03-22 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for renal neuromodulation |
| US8774913B2 (en) | 2002-04-08 | 2014-07-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravasculary-induced neuromodulation |
| US8934978B2 (en) | 2002-04-08 | 2015-01-13 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for renal neuromodulation |
| US9675413B2 (en) | 2002-04-08 | 2017-06-13 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for renal neuromodulation |
| US9707035B2 (en) | 2002-04-08 | 2017-07-18 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for catheter-based renal neuromodulation |
| US9138281B2 (en) | 2002-04-08 | 2015-09-22 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for bilateral renal neuromodulation via catheter apparatuses having expandable baskets |
| US10350004B2 (en) | 2004-12-09 | 2019-07-16 | Twelve, Inc. | Intravascular treatment catheters |
| US11272982B2 (en) | 2004-12-09 | 2022-03-15 | Twelve, Inc. | Intravascular treatment catheters |
| US9375268B2 (en) | 2007-02-15 | 2016-06-28 | Ethicon Endo-Surgery, Inc. | Electroporation ablation apparatus, system, and method |
| US10478248B2 (en) | 2007-02-15 | 2019-11-19 | Ethicon Llc | Electroporation ablation apparatus, system, and method |
| US11399834B2 (en) | 2008-07-14 | 2022-08-02 | Cilag Gmbh International | Tissue apposition clip application methods |
| US10105141B2 (en) | 2008-07-14 | 2018-10-23 | Ethicon Endo-Surgery, Inc. | Tissue apposition clip application methods |
| US11744639B2 (en) | 2008-11-11 | 2023-09-05 | Shifamed Holdings Llc | Ablation catheters |
| US10251700B2 (en) | 2008-11-11 | 2019-04-09 | Shifamed Holdings, Llc | Ablation catheters |
| US9717557B2 (en) | 2008-11-11 | 2017-08-01 | Apama Medical, Inc. | Cardiac ablation catheters and methods of use thereof |
| US20120071870A1 (en)* | 2008-11-11 | 2012-03-22 | Amr Salahieh | Low Profile Electrode Assembly |
| US9795442B2 (en) | 2008-11-11 | 2017-10-24 | Shifamed Holdings, Llc | Ablation catheters |
| US9610006B2 (en) | 2008-11-11 | 2017-04-04 | Shifamed Holdings, Llc | Minimally invasive visualization systems |
| US8805466B2 (en)* | 2008-11-11 | 2014-08-12 | Shifamed Holdings, Llc | Low profile electrode assembly |
| US10314603B2 (en) | 2008-11-25 | 2019-06-11 | Ethicon Llc | Rotational coupling device for surgical instrument with flexible actuators |
| US9011431B2 (en) | 2009-01-12 | 2015-04-21 | Ethicon Endo-Surgery, Inc. | Electrical ablation devices |
| US10004558B2 (en) | 2009-01-12 | 2018-06-26 | Ethicon Endo-Surgery, Inc. | Electrical ablation devices |
| US10779882B2 (en) | 2009-10-28 | 2020-09-22 | Ethicon Endo-Surgery, Inc. | Electrical ablation devices |
| US10098691B2 (en) | 2009-12-18 | 2018-10-16 | Ethicon Endo-Surgery, Inc. | Surgical instrument comprising an electrode |
| US9655677B2 (en) | 2010-05-12 | 2017-05-23 | Shifamed Holdings, Llc | Ablation catheters including a balloon and electrodes |
| US11116572B2 (en) | 2010-10-25 | 2021-09-14 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having multi-electrode arrays for renal neuromodulation and associated systems and methods |
| US10076382B2 (en) | 2010-10-25 | 2018-09-18 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having multi-electrode arrays for renal neuromodulation and associated systems and methods |
| US8998894B2 (en) | 2010-10-25 | 2015-04-07 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having multi-electrode arrays for renal neuromodulation and associated systems and methods |
| US8956352B2 (en) | 2010-10-25 | 2015-02-17 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having multi-electrode arrays for renal neuromodulation and associated systems and methods |
| US10258406B2 (en) | 2011-02-28 | 2019-04-16 | Ethicon Llc | Electrical ablation devices and methods |
| US10278761B2 (en) | 2011-02-28 | 2019-05-07 | Ethicon Llc | Electrical ablation devices and methods |
| US9883910B2 (en) | 2011-03-17 | 2018-02-06 | Eticon Endo-Surgery, Inc. | Hand held surgical device for manipulating an internal magnet assembly within a patient |
| US11857250B2 (en) | 2011-10-26 | 2024-01-02 | Medtronic Ablation Frontiers Llc | Semi-circular ablation catheter |
| US10064678B2 (en)* | 2011-10-26 | 2018-09-04 | Medtronic Ablation Frontiers Llc | Semi-circular pulmonary vein ablation catheter |
| US20130110104A1 (en)* | 2011-10-26 | 2013-05-02 | Medtronic Ablation Frontiers Llc | Semi-circular pulmonary vein ablation catheter |
| US10765476B2 (en) | 2011-10-26 | 2020-09-08 | Medtronic Ablation Frontiers Llc | Semi-circular pulmonary vein ablation catheter |
| US10064674B2 (en) | 2011-12-09 | 2018-09-04 | Metavention, Inc. | Methods of modulating nerves of the hepatic plexus |
| US10070911B2 (en) | 2011-12-09 | 2018-09-11 | Metavention, Inc. | Neuromodulation methods to alter glucose levels |
| US10617460B2 (en) | 2011-12-09 | 2020-04-14 | Metavention, Inc. | Neuromodulation for metabolic conditions or syndromes |
| US12029466B2 (en) | 2011-12-09 | 2024-07-09 | Medtronic Ireland Manufacturing Unlimited Company | Neuromodulation for metabolic conditions or syndromes |
| US10856926B2 (en) | 2011-12-09 | 2020-12-08 | Metavention, Inc. | Neuromodulation for metabolic conditions or syndromes |
| US10543034B2 (en) | 2011-12-09 | 2020-01-28 | Metavention, Inc. | Modulation of nerves innervating the liver |
| US9999461B2 (en) | 2011-12-09 | 2018-06-19 | Metavention, Inc. | Therapeutic denervation of nerves surrounding a hepatic vessel |
| US11413089B2 (en) | 2012-02-24 | 2022-08-16 | Cardiofocus, Inc. | Ablation techniques for the treatment of atrial fibrillation |
| US10517669B2 (en) | 2012-02-24 | 2019-12-31 | Isolase Ltd. | Ablation techniques for the treatment of atrial fibrillation |
| US8992514B2 (en)* | 2012-02-24 | 2015-03-31 | Isolase, Ltd. | Ablation techniques for the treatment of atrial fibrillation |
| US9452017B2 (en) | 2012-05-11 | 2016-09-27 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US9855096B2 (en) | 2012-05-11 | 2018-01-02 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US10512504B2 (en) | 2012-05-11 | 2019-12-24 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US8888773B2 (en) | 2012-05-11 | 2014-11-18 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US9138292B2 (en) | 2012-05-11 | 2015-09-22 | Medtronic Ardian Luxembourg S.A.R.L. | Multi-electrode catheter assemblies for renal neuromodulation and associated systems and methods |
| US9427255B2 (en) | 2012-05-14 | 2016-08-30 | Ethicon Endo-Surgery, Inc. | Apparatus for introducing a steerable camera assembly into a patient |
| US11284918B2 (en) | 2012-05-14 | 2022-03-29 | Cilag GmbH Inlernational | Apparatus for introducing a steerable camera assembly into a patient |
| US10206709B2 (en) | 2012-05-14 | 2019-02-19 | Ethicon Llc | Apparatus for introducing an object into a patient |
| US9788888B2 (en) | 2012-07-03 | 2017-10-17 | Ethicon Endo-Surgery, Inc. | Endoscopic cap electrode and method for using the same |
| US9078662B2 (en) | 2012-07-03 | 2015-07-14 | Ethicon Endo-Surgery, Inc. | Endoscopic cap electrode and method for using the same |
| US10492880B2 (en) | 2012-07-30 | 2019-12-03 | Ethicon Llc | Needle probe guide |
| US9545290B2 (en) | 2012-07-30 | 2017-01-17 | Ethicon Endo-Surgery, Inc. | Needle probe guide |
| US9572623B2 (en) | 2012-08-02 | 2017-02-21 | Ethicon Endo-Surgery, Inc. | Reusable electrode and disposable sheath |
| US10314649B2 (en) | 2012-08-02 | 2019-06-11 | Ethicon Endo-Surgery, Inc. | Flexible expandable electrode and method of intraluminal delivery of pulsed power |
| US9277957B2 (en) | 2012-08-15 | 2016-03-08 | Ethicon Endo-Surgery, Inc. | Electrosurgical devices and methods |
| US10342598B2 (en) | 2012-08-15 | 2019-07-09 | Ethicon Llc | Electrosurgical system for delivering a biphasic waveform |
| US9788885B2 (en) | 2012-08-15 | 2017-10-17 | Ethicon Endo-Surgery, Inc. | Electrosurgical system energy source |
| US8612022B1 (en) | 2012-09-13 | 2013-12-17 | Invatec S.P.A. | Neuromodulation catheters and associated systems and methods |
| US9095321B2 (en) | 2012-11-21 | 2015-08-04 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices having integral multi-helical balloons and methods of making the same |
| US10098527B2 (en) | 2013-02-27 | 2018-10-16 | Ethidcon Endo-Surgery, Inc. | System for performing a minimally invasive surgical procedure |
| US11484191B2 (en) | 2013-02-27 | 2022-11-01 | Cilag Gmbh International | System for performing a minimally invasive surgical procedure |
| US9179974B2 (en) | 2013-03-15 | 2015-11-10 | Medtronic Ardian Luxembourg S.A.R.L. | Helical push wire electrode |
| US9888961B2 (en) | 2013-03-15 | 2018-02-13 | Medtronic Ardian Luxembourg S.A.R.L. | Helical push wire electrode |
| US10792098B2 (en) | 2013-03-15 | 2020-10-06 | Medtronic Ardian Luxembourg S.A.R.L. | Helical push wire electrode |
| US11439298B2 (en) | 2013-04-08 | 2022-09-13 | Boston Scientific Scimed, Inc. | Surface mapping and visualizing ablation system |
| US11684415B2 (en) | 2013-04-08 | 2023-06-27 | Boston Scientific Scimed, Inc. | Tissue ablation and monitoring thereof |
| US10098694B2 (en) | 2013-04-08 | 2018-10-16 | Apama Medical, Inc. | Tissue ablation and monitoring thereof |
| US9333031B2 (en) | 2013-04-08 | 2016-05-10 | Apama Medical, Inc. | Visualization inside an expandable medical device |
| US10349824B2 (en) | 2013-04-08 | 2019-07-16 | Apama Medical, Inc. | Tissue mapping and visualization systems |
| US12011212B2 (en) | 2013-06-05 | 2024-06-18 | Medtronic Ireland Manufacturing Unlimited Company | Modulation of targeted nerve fibers |
| US11213678B2 (en) | 2013-09-09 | 2022-01-04 | Medtronic Ardian Luxembourg S.A.R.L. | Method of manufacturing a medical device for neuromodulation |
| US10314648B2 (en)* | 2013-12-13 | 2019-06-11 | The Trustees of the Universoty of Pennsylvania | Coaxial ablation probe and method and system for real-time monitoring of ablation therapy |
| US20160317212A1 (en)* | 2013-12-13 | 2016-11-03 | The Trustees Of The University Of Pennsylvania | Coaxial ablation probe and method and system for real-time monitoring of ablation therapy |
| WO2015089505A3 (en)* | 2013-12-13 | 2015-09-17 | The Trustees Of The University Of Pennsylvania | Coaxial ablation probe and method and system for real-time monitoring of ablation therapy |
| US10631927B2 (en) | 2013-12-23 | 2020-04-28 | Boaz Avitall | Small loop ablation catheter |
| US9579149B2 (en) | 2014-03-13 | 2017-02-28 | Medtronic Ardian Luxembourg S.A.R.L. | Low profile catheter assemblies and associated systems and methods |
| US10433907B2 (en) | 2014-03-13 | 2019-10-08 | Medtronic Ardian Luxembourg S.A.R.L. | Low profile catheter assemblies and associated systems and methods |
| US11464563B2 (en) | 2014-04-24 | 2022-10-11 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation catheters and associated systems and methods |
| US10736690B2 (en) | 2014-04-24 | 2020-08-11 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation catheters and associated systems and methods |
| US20150313669A1 (en)* | 2014-05-04 | 2015-11-05 | Diros Technology Inc. | Radiofrequency Probes with Retractable Multi-Tined Electrodes |
| US10548661B2 (en)* | 2014-05-04 | 2020-02-04 | Diros Technology Inc. | Radiofrequency probes with retractable multi-tined electrodes |
| US12004801B2 (en) | 2014-05-16 | 2024-06-11 | Biosense Webster (Israel) Ltd. | Catheter tip with microelectrodes |
| US10517667B2 (en) | 2014-05-16 | 2019-12-31 | Biosense Webster (Israel) Ltd. | Catheter tip with microelectrodes |
| WO2016040982A1 (en)* | 2014-09-15 | 2016-03-24 | Cathrx Ltd | An irrigated ablation catheter and process thereof |
| US12408974B2 (en) | 2014-12-03 | 2025-09-09 | Medtronic Ireland Manufacturing Unlimited Company | Systems and methods for modulating nerves or other tissue |
| US10271899B2 (en) | 2015-03-18 | 2019-04-30 | Medtronic Cryocath Lp | Multi-function device with treatment and sensing capabilities |
| US9895073B2 (en)* | 2015-07-29 | 2018-02-20 | Biosense Webster (Israel) Ltd. | Dual basket catheter |
| US10736693B2 (en) | 2015-11-16 | 2020-08-11 | Apama Medical, Inc. | Energy delivery devices |
| US10524859B2 (en) | 2016-06-07 | 2020-01-07 | Metavention, Inc. | Therapeutic tissue modulation devices and methods |
| EP3300660A1 (en) | 2016-09-29 | 2018-04-04 | Biosense Webster (Israel), Ltd. | Basket catheter conforming to organ using strain-relief elements |
| US11737851B2 (en) | 2018-06-28 | 2023-08-29 | Cook Medical Technologies Llc | Medical devices for magnetic resonance imaging and related methods |
| US11395697B2 (en) | 2018-11-14 | 2022-07-26 | Medtronic, Inc. | Devices and methods for preparing a valve for a transcatheter valve replacement procedure |
| US11406446B2 (en) | 2018-11-14 | 2022-08-09 | Medtronic, Inc. | Devices and methods for preparing a valve for a transcatheter valve replacement procedure |
| US12108983B2 (en) | 2019-05-03 | 2024-10-08 | Biosense Webster (Israel) Ltd. | Device, system and method to ablate cardiac tissue |
| US12408976B2 (en) | 2021-04-26 | 2025-09-09 | Pulse Biosciences, Inc. | Circumferential ablation devices and methods |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009500052A (en) | 2009-01-08 |
| US9468495B2 (en) | 2016-10-18 |
| IL188882A0 (en) | 2008-04-13 |
| US20150157402A1 (en) | 2015-06-11 |
| US20170027640A1 (en) | 2017-02-02 |
| US8979841B2 (en) | 2015-03-17 |
| EP1895927A4 (en) | 2011-03-09 |
| US20070083194A1 (en) | 2007-04-12 |
| CA2612679A1 (en) | 2007-01-04 |
| CN101309651B (en) | 2011-12-07 |
| US20130116688A1 (en) | 2013-05-09 |
| US8771267B2 (en) | 2014-07-08 |
| CN101309651A (en) | 2008-11-19 |
| EP1895927A2 (en) | 2008-03-12 |
| WO2007001981A3 (en) | 2007-04-05 |
| US20140288552A1 (en) | 2014-09-25 |
| AU2006262447A1 (en) | 2007-01-04 |
| US7850685B2 (en) | 2010-12-14 |
| US20110106074A1 (en) | 2011-05-05 |
| WO2007001981A2 (en) | 2007-01-04 |
| EP2759276A1 (en) | 2014-07-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9468495B2 (en) | Ablation catheter | |
| US9642675B2 (en) | Ablation catheter | |
| US9566113B2 (en) | Low power tissue ablation system | |
| US9757194B2 (en) | RF energy delivery system and method | |
| US8641704B2 (en) | Ablation therapy system and method for treating continuous atrial fibrillation | |
| US20140171942A1 (en) | User interface for tissue ablation system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FEPP | Fee payment procedure | Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| CC | Certificate of correction | ||
| FPAY | Fee payment | Year of fee payment:4 | |
| FEPP | Fee payment procedure | Free format text:MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| LAPS | Lapse for failure to pay maintenance fees | Free format text:PATENT EXPIRED FOR FAILURE TO PAY MAINTENANCE FEES (ORIGINAL EVENT CODE: EXP.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| STCH | Information on status: patent discontinuation | Free format text:PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 | |
| FP | Lapsed due to failure to pay maintenance fee | Effective date:20201225 |