US8016816B2 - Fecal management appliance and method and apparatus for introducing same - Google Patents
Fecal management appliance and method and apparatus for introducing sameDownload PDFInfo
- Publication number
- US8016816B2 US8016816B2US10/929,136US92913604AUS8016816B2US 8016816 B2US8016816 B2US 8016816B2US 92913604 AUS92913604 AUS 92913604AUS 8016816 B2US8016816 B2US 8016816B2
- Authority
- US
- United States
- Prior art keywords
- appliance
- balloon
- distal end
- sleeve
- end portion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime, expires
Links
- 238000000034methodMethods0.000titleabstractdescription14
- 230000002550fecal effectEffects0.000titledescription9
- 239000000463materialSubstances0.000claimsabstractdescription22
- 239000012530fluidSubstances0.000claimsdescription37
- 230000002262irrigationEffects0.000claimsdescription29
- 238000003973irrigationMethods0.000claimsdescription29
- 239000011248coating agentSubstances0.000claimsdescription11
- 238000000576coating methodMethods0.000claimsdescription11
- 210000000664rectumAnatomy0.000claimsdescription11
- 230000008878couplingEffects0.000claimsdescription7
- 238000010168coupling processMethods0.000claimsdescription7
- 238000005859coupling reactionMethods0.000claimsdescription7
- 239000002699waste materialSubstances0.000claimsdescription7
- 229920001296polysiloxanePolymers0.000claimsdescription6
- 230000004888barrier functionEffects0.000claimsdescription5
- 238000003780insertionMethods0.000abstractdescription13
- 230000037431insertionEffects0.000abstractdescription13
- 230000017074necrotic cell deathEffects0.000abstractdescription4
- 210000001519tissueAnatomy0.000description26
- 210000003484anatomyAnatomy0.000description5
- 229920000052poly(p-xylylene)Polymers0.000description5
- 239000008280bloodSubstances0.000description3
- 210000004369bloodAnatomy0.000description3
- 230000006378damageEffects0.000description3
- 230000001627detrimental effectEffects0.000description3
- 230000001105regulatory effectEffects0.000description3
- 230000000451tissue damageEffects0.000description3
- 231100000827tissue damageToxicity0.000description3
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description2
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description2
- 238000011109contaminationMethods0.000description2
- 230000009977dual effectEffects0.000description2
- 238000005259measurementMethods0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 239000004033plasticSubstances0.000description2
- 239000011780sodium chlorideSubstances0.000description2
- 210000005070sphincterAnatomy0.000description2
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description2
- 208000035473Communicable diseaseDiseases0.000description1
- 208000034656ContusionsDiseases0.000description1
- 102100026398Cyclic AMP-responsive element-binding protein 3Human genes0.000description1
- 101710128029Cyclic AMP-responsive element-binding protein 3Proteins0.000description1
- 241000792859EnemaSpecies0.000description1
- 208000002847Surgical WoundDiseases0.000description1
- 206010052428WoundDiseases0.000description1
- 208000027418Wounds and injuryDiseases0.000description1
- 239000000853adhesiveSubstances0.000description1
- 230000001070adhesive effectEffects0.000description1
- 239000003570airSubstances0.000description1
- 210000000436anusAnatomy0.000description1
- 230000015572biosynthetic processEffects0.000description1
- 238000009530blood pressure measurementMethods0.000description1
- 238000004590computer programMethods0.000description1
- 230000001419dependent effectEffects0.000description1
- 238000011161developmentMethods0.000description1
- 230000000694effectsEffects0.000description1
- 239000007920enemaSubstances0.000description1
- 229940095399enemaDrugs0.000description1
- 210000003608feceAnatomy0.000description1
- 206010016766flatulenceDiseases0.000description1
- 238000002347injectionMethods0.000description1
- 239000007924injectionSubstances0.000description1
- 238000001746injection mouldingMethods0.000description1
- 208000014674injuryDiseases0.000description1
- 230000000737periodic effectEffects0.000description1
- 239000002985plastic filmSubstances0.000description1
- 229920006255plastic filmPolymers0.000description1
- 239000004417polycarbonateSubstances0.000description1
- 229920000515polycarbonatePolymers0.000description1
- 230000002265preventionEffects0.000description1
- 238000011084recoveryMethods0.000description1
- 238000011160researchMethods0.000description1
- 230000000717retained effectEffects0.000description1
- 210000004872soft tissueAnatomy0.000description1
- 230000000472traumatic effectEffects0.000description1
- 230000002485urinary effectEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/44—Devices worn by the patient for reception of urine, faeces, catamenial or other discharge; Colostomy devices
- A61F5/445—Colostomy, ileostomy or urethrostomy devices
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/44—Devices worn by the patient for reception of urine, faeces, catamenial or other discharge; Colostomy devices
- A61F5/442—Devices worn by the patient for reception of urine, faeces, catamenial or other discharge; Colostomy devices having irrigation ports or means
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M3/00—Medical syringes, e.g. enemata; Irrigators
- A61M3/02—Enemata; Irrigators
- A61M3/0279—Cannula; Nozzles; Tips; their connection means
- A61M3/0283—Cannula; Nozzles; Tips; their connection means with at least two inner passageways, a first one for irrigating and a second for evacuating
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M3/00—Medical syringes, e.g. enemata; Irrigators
- A61M3/02—Enemata; Irrigators
- A61M3/0279—Cannula; Nozzles; Tips; their connection means
- A61M3/0295—Cannula; Nozzles; Tips; their connection means with inflatable balloon
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/10—Trunk
- A61M2210/1042—Alimentary tract
- A61M2210/1064—Large intestine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0119—Eversible catheters
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
Definitions
- Foley cathetersare large versions of devices commonly used for urinary catheterization. These catheters have balloons located on the exterior of their distal ends and have been used for many years to hold the catheters in place in a patient's rectum. Such catheter systems are frequently used for enema application but are also used for the collection and directing of fecal material from the rectum to a collection system.
- the balloonis inflated to its full size, regardless of the pressure that it exerts on tissue.
- the size of the balloon selectedbecomes critical.
- the choice of balloon sizeis no more than a guess.
- the proximal end of the sleeveis attached to the core. This permits the appliance and the apparatus to function as a unit.
- the present inventionrelates to a fecal management appliance, and to a method and apparatus for introducing the end of the appliance into a body cavity, as set forth in detail in the following specification, and recited in the annexed claims, taken together with the accompanying drawings, wherein like numerals refer to like parts, and in which:
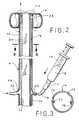
- FIG. 3is a cross-sectional view taken along line 3 - 3 of FIG. 2 ;
- FIG. 5is a view taken along line 5 - 5 of FIG. 4 ;
- Balloon 14is inflated with fluid, such as air, water or saline, through an inflation lumen 16 to a diameter of about 58 mm, with a pressure of less than 52 mm Hg. (1.0 pound per square inch).
- Lumen 16is connected by a Luer type valve connector 17 to an inflation fluid source, such as a syringe 18 .
- the syringeis also used to withdraw the inflation fluid, to deflate the balloon.
- the distal end 10 of element A and balloon 14are both made entirely of soft, compliant material so as not to injure any body tissue. That material may be, for example, silicone.
- a separate introducer apparatusis provided to facilitate introduction and placement of the distal end 10 of element A in the rectum.
- apparatus Dis rigid. It is designed to engage distal end 10 of element A and facilitate its introduction into and positioning within the bowel. Apparatus D is then separated from the medical appliance and removed from the body cavity, leaving only the soft, compliant distal end 10 of element A in the body.
- Irrigation lumen 20extends to a point proximate the edge of distal end 10 of element A and has an open end such that the irrigation fluid can be introduced into the bowel.
- the irrigation fluidis supplied as needed from a source, such as syringe 22 .
- the balloonis inflated and the pressure is regulated remotely from the tubular element.
- the inflation lumen 16extends from the balloon to the inflation fluid source located outside of the body.
- the fluid sourcecan be manually operated or can be regulated by an electronic or mechanical system.
- the same componentscould be supplied without fluid, but with a fluid supply port 25 .
- the caregiverwould supply the fluid and supply the pressure to put in a known range of volume of fluid.
- the syringe plunger backed by the spring 19would act as a pressure gauge.
- the caregiverwould be instructed to stop injecting fluid once the proper pressure is reached. If the fluid injected is not within the prescribed range, the balloon is the wrong size and must be removed.
- the inflation systemcould include a simple pressure gauge 27 attached to lumen 16 to allow the caregiver to only inflate the device to the target pressure.
- This configurationrequires the system to function in the fixed volume state once the pressure is determined on insertion.
- the plunger stemcould contain an integrated or assembled spring that indicates the pressure in the fluid in the syringe barrel. The spring could create a gap between two portions of the plunger stem. As the pressure increases, the spring compresses and the two portions of the plunger move closer together. Scales on the two portions can indicate pressure by their relative position to each other.
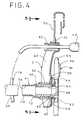
- proximal end 12 of element Ais rotatably connected to adapter plate C by first and second part 30 .
- Part 30is generally tubular in shape and has an outer diameter that is substantially equal to or slightly larger than the inner diameter of element A. Proximate end 12 of element A is received over and fixed on one end of part 30 .
- part 30is rotatably received within part 60 , which has a generally tubular center section.
- part 30is sized such that when snapped into part 60 , there is not too much friction between the part 30 and part 60 to prohibit rotation.
- Plate Cis preferably made of plastic and has a body with a lower, generally circular portion 50 through which opening 34 extends. Part 32 is fixed to one side of portion 50 and acts to cover the end of part 12 . The other side of portion 50 of plate C carries a first inter-engaging part 52 in the form of an annular protrusion or ring welded to its surface. Part 52 surrounds opening 34 in plate C.
- Receptacle Bpreferably takes the form of a standard ostomy pouch 55 .
- inter-engaging parts 52 , 54are shaped so that when the parts are engaged and the pouch is attached to the plate, a fluid tight seal is formed. This seal is strong enough to prevent the weight of the filled pouch from causing accidental attachment of the pouch.
- Plate Cis designed to hang from a stationary object, such as a bed rail 70 .
- a clip 72is provided for that purpose. Clip 72 extends upwardly from portion 60 of plate C and can be received over bed rail 70 , in a conventional manner.
- the present inventionrelates to a medical appliance with an end designed to be introduced into a body cavity that is made entirely of soft, compliant material.
- the balloonis inflated to a predetermined low pressure level to prevent pressure necrosis on the adjacent tissue.
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Epidemiology (AREA)
- Nursing (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Sampling And Sample Adjustment (AREA)
- External Artificial Organs (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
- Medicines Containing Plant Substances (AREA)
- Control And Other Processes For Unpacking Of Materials (AREA)
- Farming Of Fish And Shellfish (AREA)
- Saccharide Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Claims (32)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/929,136US8016816B2 (en) | 2003-09-09 | 2004-08-28 | Fecal management appliance and method and apparatus for introducing same |
| US13/196,375US8827970B2 (en) | 2003-09-09 | 2011-08-02 | Fecal management appliance and method and apparatus for introducing same |
| US14/451,243US10772755B2 (en) | 2003-09-09 | 2014-08-04 | Fecal management appliance and method and apparatus for introducing same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US50121803P | 2003-09-09 | 2003-09-09 | |
| US10/929,136US8016816B2 (en) | 2003-09-09 | 2004-08-28 | Fecal management appliance and method and apparatus for introducing same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/196,375ContinuationUS8827970B2 (en) | 2003-09-09 | 2011-08-02 | Fecal management appliance and method and apparatus for introducing same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20050054996A1 US20050054996A1 (en) | 2005-03-10 |
| US8016816B2true US8016816B2 (en) | 2011-09-13 |
Family
ID=34135377
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/929,136Expired - LifetimeUS8016816B2 (en) | 2003-09-09 | 2004-08-28 | Fecal management appliance and method and apparatus for introducing same |
| US13/196,375Expired - LifetimeUS8827970B2 (en) | 2003-09-09 | 2011-08-02 | Fecal management appliance and method and apparatus for introducing same |
| US14/451,243Active2028-07-30US10772755B2 (en) | 2003-09-09 | 2014-08-04 | Fecal management appliance and method and apparatus for introducing same |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/196,375Expired - LifetimeUS8827970B2 (en) | 2003-09-09 | 2011-08-02 | Fecal management appliance and method and apparatus for introducing same |
| US14/451,243Active2028-07-30US10772755B2 (en) | 2003-09-09 | 2014-08-04 | Fecal management appliance and method and apparatus for introducing same |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US8016816B2 (en) |
| EP (2) | EP2027832B1 (en) |
| JP (2) | JP4768245B2 (en) |
| AT (1) | ATE415897T1 (en) |
| AU (1) | AU2004210541A1 (en) |
| CA (2) | CA2480843C (en) |
| DE (1) | DE602004018080D1 (en) |
| DK (1) | DK1514572T4 (en) |
| ES (1) | ES2321510T5 (en) |
| MX (1) | MX345313B (en) |
| PL (1) | PL1514572T5 (en) |
| PT (1) | PT1514572E (en) |
| ZA (1) | ZA200407195B (en) |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090227971A1 (en)* | 2006-10-17 | 2009-09-10 | C. R. Bard, Inc. | Waste management system |
| WO2013074763A1 (en) | 2011-11-16 | 2013-05-23 | Convatec Technologies, Inc. | Apparatus for preventing over inflation of the retention balloon in medical catheters and airway devices |
| US8777912B2 (en) | 2007-07-22 | 2014-07-15 | C. R. Bard, Inc. | Waste management system |
| US20150099011A1 (en)* | 2001-09-25 | 2015-04-09 | Jenny Colleen McCloskey | Inactivation of papillomavirus |
| US9033944B2 (en) | 2010-08-12 | 2015-05-19 | Colo-Majic Enterprises Ltd. | Ostomy pouch apparatus with closable opening |
| USRE46306E1 (en) | 2008-08-11 | 2017-02-14 | Centurion Medical Products Corporation | Fecal impaction removal tool |
| US9669205B2 (en) | 2013-08-01 | 2017-06-06 | Convatec Technologies Inc. | Self-closing bag connector |
| US20170181837A1 (en)* | 2002-12-23 | 2017-06-29 | Python Medical, Inc. | Implantable digestive tract organ |
| US9808606B2 (en) | 2011-02-17 | 2017-11-07 | Convatec Technologies Inc. | Valve system for inflatable medical device |
| US9839509B2 (en) | 2002-12-23 | 2017-12-12 | Python Medical, Inc. | Stomach peristalsis device and method |
| US9889280B2 (en) | 2013-04-01 | 2018-02-13 | Indiana University Research And Technology Corporation | Rectal catheter configured for pediatric care |
| US10576262B2 (en) | 2015-05-18 | 2020-03-03 | Convatec Technologies Inc. | Spring-loaded bag connector |
| US10667657B2 (en)* | 2018-07-31 | 2020-06-02 | Lien-Kung Wang | Stool drain assembly |
| US10772755B2 (en) | 2003-09-09 | 2020-09-15 | Convatec Technologies Inc. | Fecal management appliance and method and apparatus for introducing same |
| US10842976B2 (en) | 2015-10-29 | 2020-11-24 | Convatec Technologies Inc. | Valve system for inflatable devices |
| US11103260B2 (en) | 2019-07-18 | 2021-08-31 | Medline Industries, Inc. | Fecal impaction removal device |
| US11478625B2 (en) | 2016-01-19 | 2022-10-25 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US11529154B2 (en) | 2018-08-08 | 2022-12-20 | Wilmarc Holdings, Llc | Stool management system |
| US11648380B2 (en) | 2017-12-05 | 2023-05-16 | Jenny Colleen McCloskey | Device for treatment of a body canal and adjacent surfaces |
| US11992646B2 (en) | 2017-03-08 | 2024-05-28 | Wilmarc Holdings, Llc | Catch assembly for releasably connecting fluid conduits |
| US12233232B2 (en) | 2023-04-25 | 2025-02-25 | Wilmarc Holdings, Llc | Genderless aseptic connector |
Families Citing this family (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AUPQ899700A0 (en) | 2000-07-25 | 2000-08-17 | Borody, Thomas Julius | Probiotic recolonisation therapy |
| DE102005028428A1 (en)* | 2005-06-17 | 2006-12-28 | Microcuff Gmbh | Apparatus for gastric feeding and drainage via a transcutaneously applied fistula |
| EP2040653A4 (en) | 2006-05-25 | 2009-07-01 | Hadidi Ahmed Taher El | An inflatable balloon device for fecal incontinence |
| CN101557780B (en)* | 2006-10-26 | 2012-11-14 | 康沃特克科技公司 | Silicone based tube for transporting malodoriforous matter from the human body |
| WO2008103789A2 (en) | 2007-02-22 | 2008-08-28 | Convatec Technologies Inc. | Seal for an ostomy appliance |
| US8936583B2 (en) | 2007-09-28 | 2015-01-20 | Hollister Incorporated | Multi-layer catheter tubes with odor barrier |
| WO2009045874A1 (en)* | 2007-09-28 | 2009-04-09 | Hollister Incorporated | Multi-layer odor barrier tube, and combination odor barrier tube and odor barrier collection bag |
| US8603029B2 (en) | 2007-10-10 | 2013-12-10 | Hospi Corporation | Apparatuses and methods for medication administration |
| EP2280672B1 (en)* | 2008-04-22 | 2016-01-06 | ConvaTec Technologies Inc. | Temporary ostomy appliance |
| WO2009135141A1 (en)* | 2008-05-01 | 2009-11-05 | Bristol-Myers Squibb Company | Rectal drain appliance |
| US20090326490A1 (en)* | 2008-06-30 | 2009-12-31 | Mcmichael Donald Jay | Fecal incontinence collection device and method of use |
| US9775980B2 (en) | 2008-09-23 | 2017-10-03 | Hospi Corporation | Valved enteral administration assembly |
| CA2743167A1 (en)* | 2008-11-17 | 2010-05-20 | C. R. Bard, Inc. | Waste management system |
| US8900116B2 (en)* | 2009-07-14 | 2014-12-02 | Stimatix Gi Ltd. | Inflatable stomal implant |
| US8821464B2 (en) | 2009-07-14 | 2014-09-02 | Stimatix Gi Ltd. | Disposable ostomy assemblies |
| CN102481201B (en) | 2009-07-14 | 2016-03-09 | 斯提马提克斯Gi有限公司 | Ostomy containment device |
| WO2011138727A1 (en) | 2010-05-02 | 2011-11-10 | Stimatix Gi Ltd. | Ostomy port gas release mechanism |
| US8412310B2 (en) | 2009-09-18 | 2013-04-02 | United Medical Innovations, Inc. | Locking syringe with integrated bias member |
| WO2011083451A2 (en) | 2010-01-11 | 2011-07-14 | Motus Gi Medical Technologies Ltd. | Systems and methods for cleaning body cavities |
| US8690848B2 (en) | 2010-03-02 | 2014-04-08 | Ostosolutions, LLC | Closure for ostomy pouch and method thereof |
| US10130506B2 (en) | 2010-03-02 | 2018-11-20 | Ostosolutions, LLC | Closure system for an ostomy pouch and related methods |
| DK2575703T3 (en)* | 2010-05-03 | 2015-08-10 | Convatec Technologies Inc | Drainage device for body fluid |
| WO2011142928A1 (en)* | 2010-05-13 | 2011-11-17 | Convatec Technologies Inc. | Indwelling fecal drainage catheter and fecal collection or ostomy pouch |
| US20130085442A1 (en) | 2010-06-13 | 2013-04-04 | Motus Gi Medical Technologies Ltd. | Systems and methods for cleaning body cavities |
| CA2807242C (en)* | 2010-08-04 | 2017-05-02 | Thomas Julius Borody | Compositions for fecal floral transplantation and methods for making and using them and devices for delivering them |
| KR101197432B1 (en) | 2010-10-15 | 2012-11-06 | 강상윤 | Apparatus for assisting defecation |
| KR200466556Y1 (en) | 2010-11-09 | 2013-04-23 | 퍼시픽 호스피탈 서플라이 컴퍼니 리미티드 | Socket pipe for fecal collection bag and fecal collection bag including the same |
| BR112013022927A2 (en) | 2011-03-09 | 2016-12-06 | Univ Minnesota | composition, methods for replacing or supplementing or modifying a subject's microbiota of the colon, and for treating a subject, and, use of a composition |
| CN108578044A (en) | 2011-03-17 | 2018-09-28 | 康沃特克科技公司 | High barrier elastomer excrement conduit or ostomy bag |
| CN102366643A (en)* | 2011-10-22 | 2012-03-07 | 江苏康诺医疗器械有限公司 | Defecation and excrement-collection mechanism for constipation patients |
| DK2620168T3 (en)* | 2012-01-27 | 2020-06-02 | Hollister Inc | MULTI-LAYER CATTLE HOSE WITH AIR BARRIER |
| WO2014181338A2 (en) | 2013-05-09 | 2014-11-13 | Stimatix Gi Ltd. | Compact ostomy appliance |
| BR112014028052A2 (en) | 2012-05-10 | 2020-10-27 | Stimatix Gi Ltd | ostomy apparatus, ostomy component sealing system, and method of sealing the stoma in an individual's body. |
| WO2013176774A1 (en) | 2012-05-25 | 2013-11-28 | Arizona Board Of Regents | Microbiome markers and therapies for autism spectrum disorders |
| GB2516429A (en)* | 2013-07-19 | 2015-01-28 | Prosys Internat Ltd | Waste management appliance & method |
| US20150051563A1 (en)* | 2013-08-14 | 2015-02-19 | Dale Martin Frimel | Ostomy stoma waste overflow system |
| CA2917047C (en)* | 2013-08-30 | 2019-06-11 | Hollister Incorporated | Device for trans-anal irrigation |
| US20150119836A1 (en)* | 2013-10-29 | 2015-04-30 | Dale Martin Frimel | Ostomy stoma waste overflow process and bag |
| USD783814S1 (en) | 2013-12-09 | 2017-04-11 | B. Braun Medical Sas | Adapter for flatus release |
| USD796029S1 (en) | 2013-12-09 | 2017-08-29 | B. Braun Medical Sas | Colostomy appliance |
| KR101639012B1 (en)* | 2013-12-19 | 2016-07-14 | 경북대학교 산학협력단 | A Device for Protecting the Rectal Anastomosis |
| DK3166661T3 (en) | 2014-07-08 | 2019-04-23 | Hollister Inc | Portable transanal flushing device |
| US10765796B2 (en) | 2014-07-08 | 2020-09-08 | Hollister Incorporated | Trans anal irrigation platform with bed module |
| KR20180026376A (en) | 2015-05-14 | 2018-03-12 | 크레스토보 홀딩스 엘엘씨 | Compositions for fecal flock transplantation, and methods for making and using the same, and devices therefor |
| HUE058209T2 (en) | 2015-05-22 | 2022-07-28 | Univ Arizona State | Methods for treating autism spectrum disorder and associated symptoms |
| US10413441B2 (en)* | 2015-11-18 | 2019-09-17 | Incontinent Control Devices, Inc. | Colostomy balloon plug |
| US20170360848A1 (en) | 2016-06-15 | 2017-12-21 | Arizona Board Of Regents On Behalf Of Arizona State University | Methods for treating autism spectrum disorder and associated symptoms |
| US10849936B2 (en) | 2016-07-01 | 2020-12-01 | Regents Of The University Of Minnesota | Compositions and methods for C. difficile treatment |
| US11577018B2 (en) | 2016-07-08 | 2023-02-14 | Hollister Incorporated | Body cavity irrigation integrated manual controller and pump device, system and method |
| JP6821779B2 (en) | 2016-07-08 | 2021-01-27 | ホリスター・インコーポレイテッドHollister Incorporated | Transanal enema therapy device |
| US20180036352A1 (en) | 2016-08-03 | 2018-02-08 | Crestovo Holdings Llc | Methods for treating ulcerative colitis |
| KR101918685B1 (en) | 2016-09-27 | 2018-11-14 | 주식회사 엔도비전 | Fecal diverting device |
| WO2018071536A1 (en) | 2016-10-11 | 2018-04-19 | Crestovo Holdings Llc | Compositions and methods for treating primary sclerosing cholangitis and related disorders |
| US11026978B2 (en) | 2016-10-11 | 2021-06-08 | Finch Therapeutics Holdings Llc | Compositions and methods for treating multiple sclerosis and related disorders |
| US10092601B2 (en) | 2016-10-11 | 2018-10-09 | Crestovo Holdings Llc | Compositions and methods for treating multiple sclerosis and related disorders |
| EP3534863A1 (en) | 2016-11-03 | 2019-09-11 | Hollister Incorporated | Adjustable bowel treatment arm |
| US11497844B2 (en) | 2016-12-14 | 2022-11-15 | Hollister Incorporated | Transanal irrigation device and system |
| WO2018187467A1 (en) | 2017-04-05 | 2018-10-11 | Crestovo Holdings Llc | Compositions and methods for treating parkinson's disease (pd) and related disorders |
| US11040073B2 (en) | 2017-04-05 | 2021-06-22 | Finch Therapeutics Holdings Llc | Compositions and methods for treating diverticulitis and related disorders |
| EP3630190B1 (en) | 2017-05-26 | 2024-02-21 | Finch Therapeutics Holdings LLC | Lyophilized compositions comprising fecal microbe-based therapeutic agents and methods for making and using same |
| AU2018313766A1 (en) | 2017-08-07 | 2020-02-20 | Finch Therapeutics, Inc. | Compositions and methods for maintaining and restoring a healthy gut barrier |
| EP4368156A3 (en) | 2017-11-09 | 2024-11-06 | ConvaTec Technologies Inc. | Ostomy monitoring system and method |
| GB201721955D0 (en) | 2017-12-27 | 2018-02-07 | Convatec Ltd | Catheter wetting devices |
| GB201721956D0 (en) | 2017-12-27 | 2018-02-07 | Convatec Ltd | Female catheter locator tip |
| CN108478906A (en)* | 2018-05-03 | 2018-09-04 | 苏州高新区建金建智能科技有限公司 | A kind of medical Multi needle optical fiber injection device with activation probiotics function |
| CN108578841A (en)* | 2018-05-03 | 2018-09-28 | 苏州高新区建金建智能科技有限公司 | A kind of medical optical fiber injection needle with medicine liquid heating function |
| CN108578840A (en)* | 2018-05-03 | 2018-09-28 | 苏州高新区建金建智能科技有限公司 | A kind of medical optical fiber injection needle with laser illumination function |
| US11166990B2 (en) | 2018-07-13 | 2021-11-09 | Finch Therapeutics Holdings Llc | Methods and compositions for treating ulcerative colitis |
| WO2020069280A1 (en) | 2018-09-27 | 2020-04-02 | Crestovo Holdings Llc | Compositions and methods for treating epilepsy and related disorders |
| USD893514S1 (en) | 2018-11-08 | 2020-08-18 | 11 Health And Technologies Limited | Display screen or portion thereof with graphical user interface |
| USD1012280S1 (en) | 2018-11-30 | 2024-01-23 | B. Braun Medical Sas | Ostomy device assembly |
| DE102019000474A1 (en)* | 2019-01-24 | 2020-07-30 | Creative Balloons Gmbh | Device for the tampon protection of intestinal anastomoses |
| US11351297B2 (en)* | 2019-02-11 | 2022-06-07 | Ryan Maaskamp | Paddle shaped medicinal dispenser |
| GB2584270B (en)* | 2019-03-26 | 2022-04-20 | Prosys International Ltd | Waste management appliance |
| CA3140906A1 (en) | 2019-06-11 | 2020-12-17 | Convatec Technologies Inc. | Urine collection bags for use with catheter products, kits incorporating the same, and methods therefor |
| CA3147629A1 (en) | 2019-07-19 | 2021-01-28 | Finch Therapeutics Holdings Llc. | Methods and products for treatment of gastrointestinal disorders |
| US20210161701A1 (en)* | 2019-11-09 | 2021-06-03 | Airway Medix S.A. | Bowel waste management systems and methods for use |
| KR102387339B1 (en)* | 2020-03-25 | 2022-04-18 | 주식회사 제이에스알메디컬 | Fecal diverting device |
| US12357494B2 (en) | 2020-10-15 | 2025-07-15 | Convatec Technologies Inc. | Ostomy systems and methods |
| RU205905U1 (en)* | 2021-02-26 | 2021-08-12 | Алексей Владимирович Воронов | DEVICE FOR RECTAL DEPLOYMENT OF FECAL MASSES, FORMATION OF CLESES AND ADMINISTRATION OF MEDICINAL PREPARATIONS INTO THE RECTAL INTESTINE |
| US12023460B1 (en) | 2022-08-25 | 2024-07-02 | Surgin, Inc. | Flow directing medicinal applicator |
Citations (135)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US565386A (en) | 1896-08-04 | Rectal irrigating-dilator | ||
| US2457244A (en) | 1943-06-22 | 1948-12-28 | Otis F Lamson | Medical appliance for control of enemata |
| US2494393A (en) | 1949-02-05 | 1950-01-10 | Otis F Lamson | Removable appliance for use as an artificial dam in cases of rectal incontinence |
| US2813531A (en) | 1955-09-26 | 1957-11-19 | Albert F Lee | Cannula |
| US3459175A (en) | 1966-04-08 | 1969-08-05 | Roscoe E Miller | Medical device for control of enemata |
| US3487837A (en) | 1967-02-06 | 1970-01-06 | Roy A Petersen | Device for holding catheters in position |
| US3509884A (en) | 1967-09-13 | 1970-05-05 | William Bell | Rectal balloon catheter |
| US3543744A (en) | 1968-11-29 | 1970-12-01 | Edwin Lepar | Equipment for administering enemas for radiological purposes |
| US3548828A (en) | 1968-06-14 | 1970-12-22 | John J Vasile | Excrement collecting appliance |
| US3734100A (en) | 1973-05-07 | 1973-05-22 | Medical Products Corp | Catheter tubes |
| US3766920A (en) | 1971-07-21 | 1973-10-23 | Ezem Co | Enemata administering device |
| US3802418A (en) | 1971-02-16 | 1974-04-09 | R Clayton | Colon catheter |
| US3884242A (en) | 1971-03-29 | 1975-05-20 | Mpc Kurgisil | Catheter assembly |
| US3937224A (en) | 1974-04-11 | 1976-02-10 | Uecker Ronald L | Colostomy catheter |
| US3938521A (en) | 1972-06-22 | 1976-02-17 | M.E.D.S. Corporation | Collecting bag |
| DE2447996A1 (en) | 1974-10-09 | 1976-04-22 | Hadar Y Roennqvist | Disposable sanitary faeces collection bag - includes disposable bag fitting into soft plastics tube constricted in centre |
| US3983879A (en) | 1974-07-25 | 1976-10-05 | Western Acadia, Incorporated | Silicone catheter |
| US4013077A (en) | 1974-09-23 | 1977-03-22 | M.E.D.S. Corporation | Collecting device |
| US4019515A (en) | 1975-12-08 | 1977-04-26 | Daniel Kornblum | Enemata administering device |
| FR2326208A1 (en) | 1975-10-01 | 1977-04-29 | Clayton Ralph | Rectal catheter device for anal insertion - has inflatable ring and spacing limiting projection |
| US4030500A (en) | 1974-12-16 | 1977-06-21 | Hadar Yngve Ronnquist | Fecal matter collector |
| US4067335A (en) | 1975-02-12 | 1978-01-10 | Beverley Silvanov | Matter collecting unit |
| US4117847A (en) | 1976-02-05 | 1978-10-03 | Clayton Ralph S | Colon catheter |
| US4121589A (en) | 1975-08-06 | 1978-10-24 | Mcdonnell Roy Edward | Ostomy appliance |
| US4182332A (en) | 1978-02-17 | 1980-01-08 | Delaney Richard P | Rectal catheter |
| US4285341A (en) | 1978-11-22 | 1981-08-25 | Pollack Charles N | Extracorporeal cannula apparatus with retractable intralumenal balloon and method for using same |
| US4344434A (en) | 1981-06-01 | 1982-08-17 | Santa Barbara Medical Foundation Clinic | Ileostomy appliance and method for implanting the same |
| US4368739A (en) | 1979-07-18 | 1983-01-18 | Nelson Jr Richard L | Long intestinal catheter |
| US4403982A (en) | 1978-08-28 | 1983-09-13 | Clayton Ralph S | Colon cleansing system and technique |
| EP0109897A1 (en) | 1982-11-17 | 1984-05-30 | Noel C. Dr. David | Rectal probes for baritic washing |
| US4471782A (en) | 1982-09-30 | 1984-09-18 | Luther Shuffield | Medical implement for use in rectum and method for inserting same |
| US4496356A (en) | 1982-09-29 | 1985-01-29 | Leon Lognion | Anal excretion collecting rectal catheter |
| US4516578A (en) | 1982-09-30 | 1985-05-14 | Luther Shuffield | Rectal device and method of inserting same |
| US4583983A (en) | 1983-10-25 | 1986-04-22 | Einhorn Carol J | Female urinary drainage device |
| US4596554A (en) | 1985-04-19 | 1986-06-24 | Dastgeer Ghulam M | Colo-rectal evacuator |
| US4637814A (en) | 1985-04-05 | 1987-01-20 | Arnold Leiboff | Method and apparatus for intestinal irrigation |
| US4662890A (en) | 1984-10-09 | 1987-05-05 | Waters Instruments, Inc. | Tubular medical prosthesis |
| US4676778A (en) | 1986-10-27 | 1987-06-30 | Nelson Jr Richard L | Long intestinal catheter with sump |
| US4686985A (en) | 1985-05-15 | 1987-08-18 | Lottick Edward A | Anal dilator and occluder |
| EP0246176A2 (en) | 1986-05-12 | 1987-11-19 | Biodan Medical Systems Ltd | Catheter and probe |
| JPS62281955A (en) | 1986-05-30 | 1987-12-07 | 株式会社メデイコン | Urine sampling bag |
| US4721508A (en) | 1984-10-09 | 1988-01-26 | Waters Instruments, Inc. | Ostomy prosthesis |
| US4750902A (en) | 1985-08-28 | 1988-06-14 | Sonomed Technology, Inc. | Endoscopic ultrasonic aspirators |
| US4750488A (en) | 1986-05-19 | 1988-06-14 | Sonomed Technology, Inc. | Vibration apparatus preferably for endoscopic ultrasonic aspirator |
| EP0274415A2 (en) | 1987-01-06 | 1988-07-13 | Howell, Richard Owen | Colonic irrigator |
| US4826481A (en) | 1987-05-27 | 1989-05-02 | Abbott Labs. | Enteral feeding device |
| US4986822A (en) | 1988-09-06 | 1991-01-22 | Anderson Irvin B | Rectal-colon dilator and collector assembly |
| US5041100A (en) | 1989-04-28 | 1991-08-20 | Cordis Corporation | Catheter and hydrophilic, friction-reducing coating thereon |
| JPH0391357U (en) | 1989-12-29 | 1991-09-18 | ||
| US5053023A (en) | 1988-10-25 | 1991-10-01 | Vas-Cath Incorporated | Catheter for prolonged access |
| US5057073A (en) | 1988-04-21 | 1991-10-15 | Vas-Cath Incorporated | Dual lumen catheter |
| GB2243553A (en) | 1990-04-30 | 1991-11-06 | Squibb & Sons Inc | Rectal block |
| US5080650A (en) | 1991-01-28 | 1992-01-14 | Abbott Laboratories | Gastrostomy tube |
| US5171305A (en) | 1991-10-17 | 1992-12-15 | Imagyn Medical, Inc. | Linear eversion catheter with reinforced inner body extension |
| GB2224212B (en) | 1988-09-22 | 1993-01-06 | Aegis Medical Inc | Colonic lavage apparatus |
| US5216898A (en) | 1992-01-14 | 1993-06-08 | Astec Industries, Inc. | Cooling apparatus |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US5261898A (en) | 1990-11-13 | 1993-11-16 | Polin Stanton G | Temporary colostomy apparatus |
| US5279596A (en) | 1990-07-27 | 1994-01-18 | Cordis Corporation | Intravascular catheter with kink resistant tip |
| US5295984A (en) | 1989-12-07 | 1994-03-22 | Ultrafem, Inc. | Vaginal discharge collection device and intravaginal drug delivery system |
| CN2174997Y (en) | 1993-04-26 | 1994-08-24 | 许如良 | Multi-purpose drainage tube for anal and bowels |
| US5342321A (en) | 1993-05-18 | 1994-08-30 | Teleflex, Inc. | Low profile gastrostomy catheter |
| US5404881A (en) | 1990-04-06 | 1995-04-11 | Technomed International | Transrectal probe |
| US5421827A (en) | 1989-05-08 | 1995-06-06 | Temple; John E. | Fecal incontinence device and applicator therefor |
| US5423764A (en) | 1993-06-14 | 1995-06-13 | Fry; William A. | Lavage apparatus |
| US5520669A (en) | 1994-07-19 | 1996-05-28 | Mulholland; Kevin J. | Intra-rectal drain and receptacle for fecal incontinence |
| KR960005818Y1 (en) | 1992-10-30 | 1996-07-16 | 우석형 | Paper guide fixing device |
| US5545149A (en) | 1993-06-25 | 1996-08-13 | Medtronic, Inc. | Method of catheter segment attachment |
| US5569216A (en)* | 1993-12-02 | 1996-10-29 | Kim; Jae H. | Multipurpose colostomy device having balloons on an end thereof |
| US5569218A (en) | 1994-02-14 | 1996-10-29 | Scimed Life Systems, Inc. | Elastic guide catheter transition element |
| US5603698A (en) | 1993-04-13 | 1997-02-18 | Boston Scientific Corporation | Prosthesis delivery system |
| US5632271A (en) | 1996-03-22 | 1997-05-27 | Brain; Archibald I. J. | Laryngeal mask with gastric-drainage feature |
| US5674197A (en) | 1994-07-01 | 1997-10-07 | Cordis Corporation | Controlled flexible catheter |
| US5693036A (en) | 1992-05-12 | 1997-12-02 | E. R. Squibb & Sons Inc. | Method of injection moulding an undercut formation on a circular body and a closure assembly including a coupling element |
| US5697365A (en) | 1996-01-18 | 1997-12-16 | Pell; Donald M. | Endotracheal tube construction and method for intubating a patient |
| US5766209A (en) | 1993-02-19 | 1998-06-16 | Devonec; Marian A. | Prosthesis intended for the treatment of a natural lumen or tract, in particular an endo-urethral prosthesis |
| US5782745A (en) | 1995-11-13 | 1998-07-21 | Benderev; Theodore V. | Devices and methods for assessment and treatment of urinary and fecal incontinence |
| US5785641A (en) | 1996-05-08 | 1998-07-28 | Urocath Corporation | Male indwelling urethral catheter sizing system and insertion method |
| US5791036A (en) | 1996-12-23 | 1998-08-11 | Schneider (Usa) Inc | Catheter transition system |
| US5807314A (en) | 1996-10-11 | 1998-09-15 | Abbott Laboratories | Feeding tube and method for placing same |
| US5860952A (en) | 1996-01-11 | 1999-01-19 | C. R. Bard, Inc. | Corporeal access tube assembly and method |
| US5904701A (en) | 1994-02-14 | 1999-05-18 | Daneshvar; Yousef | Device for aiding procedural and therapeutic interventions of the gastrointestinal tract |
| US5906605A (en) | 1997-01-10 | 1999-05-25 | Cardiac Pathways Corporation | Torquable guiding catheter for basket deployment and method |
| US5911715A (en) | 1994-02-14 | 1999-06-15 | Scimed Life Systems, Inc. | Guide catheter having selected flexural modulus segments |
| CN2325054Y (en) | 1998-04-30 | 1999-06-23 | 关怀敏 | Balloon catheter anal canal |
| US5941860A (en) | 1997-11-03 | 1999-08-24 | Wheeler; Alton D. | Fecal pouch and installation |
| US5964732A (en) | 1997-02-07 | 1999-10-12 | Abbeymoor Medical, Inc. | Urethral apparatus with position indicator and methods of use thereof |
| US5971967A (en) | 1997-08-19 | 1999-10-26 | Abbeymoor Medical, Inc. | Urethral device with anchoring system |
| US5997546A (en) | 1999-01-07 | 1999-12-07 | Ballard Medical Products | Gastric balloon catheter with improved balloon orientation |
| JP3019990B2 (en) | 1990-06-15 | 2000-03-15 | 株式会社ジーシー | Dental unit |
| JP2000167041A (en) | 1998-12-02 | 2000-06-20 | Shigenobu Takane | Defacation device |
| JP3071301U (en) | 2000-02-25 | 2000-08-29 | 株式会社オーセンティックスインターナショナル | Catheter for draining feces |
| JP2000354634A (en) | 1999-06-15 | 2000-12-26 | Shigenobu Takane | Rectal catheter and contrast medium enema device |
| US6171295B1 (en) | 1999-01-20 | 2001-01-09 | Scimed Life Systems, Inc. | Intravascular catheter with composite reinforcement |
| US6217565B1 (en) | 1998-07-16 | 2001-04-17 | Mark Cohen | Reinforced variable stiffness tubing |
| US6240231B1 (en) | 1997-12-22 | 2001-05-29 | Micrus Corporation | Variable stiffness fiber optic shaft |
| US6254570B1 (en) | 1997-04-07 | 2001-07-03 | Vance Products, Inc. | Back-up retention member drainage catheter |
| US6286555B1 (en) | 1997-11-03 | 2001-09-11 | Stm Medizintechnik Starnberg Gmbh | Reinforced roll-back tube for use with endoscopes and the like |
| US6296631B2 (en) | 1998-04-28 | 2001-10-02 | Sean L. Chow | Flow directed catheter |
| US6342052B1 (en) | 1999-11-02 | 2002-01-29 | Hector D. Allende | Anorectal apparatus |
| US20020016607A1 (en)* | 1998-12-01 | 2002-02-07 | Atropos Limited | Medical device comprising an evertable sleeve |
| WO2002026293A1 (en) | 2000-09-28 | 2002-04-04 | Kim Jae Hwang | Improved colostomy device |
| JP2002126094A (en) | 2000-10-24 | 2002-05-08 | Terumo Corp | Connector system |
| CN2489797Y (en) | 2001-04-20 | 2002-05-08 | 胡晓燕 | Balloon-type indwelling catheter for stool |
| JP2002153564A (en) | 2000-11-22 | 2002-05-28 | Shigenobu Takane | Rectum catheter |
| US6406453B1 (en) | 1995-04-26 | 2002-06-18 | Medtronic Xomed, Inc. | Composite ventilation tube |
| US6413228B1 (en) | 1998-12-28 | 2002-07-02 | Pro Duct Health, Inc. | Devices, methods and systems for collecting material from a breast duct |
| US6468245B2 (en) | 1996-11-27 | 2002-10-22 | Colorplast A/S | Irrigation device |
| US20020173771A1 (en) | 2001-05-17 | 2002-11-21 | La Tapachulteca Ii | Lesion restoration catheter for the colon and method for using same |
| US6527755B1 (en) | 2001-04-20 | 2003-03-04 | Fouad A. Salama | Feces control device |
| US20030105485A1 (en)* | 1998-09-09 | 2003-06-05 | Embol-X, Inc. | Introducer/dilator with balloon protection and methods of use |
| US6575934B2 (en) | 2000-12-21 | 2003-06-10 | Advanced Cardiovascular Systems, Inc. | Low profile catheter |
| US6585705B1 (en) | 1999-01-15 | 2003-07-01 | Maginot Catheter Technologies, Inc. | Retractable catheter systems |
| US6635047B2 (en) | 2001-08-06 | 2003-10-21 | Scimed Life Systems, Inc. | Integrated polymer and braid for intravascular catheters |
| US6663614B1 (en) | 2000-11-06 | 2003-12-16 | Advanced Cardiovascular Systems, Inc. | Catheter shaft having variable thickness layers and method of making |
| US20040030380A1 (en)* | 2002-04-24 | 2004-02-12 | Sun Biomedical, Ltd. | Drug-delivery endovascular stent and method for treating restenosis |
| US20040039348A1 (en)* | 2002-08-21 | 2004-02-26 | Kim Jae H. | Bowel management system |
| US6698428B2 (en) | 1996-11-06 | 2004-03-02 | Archibald I. J. Brain | Endotracheal tube construction |
| US6716209B2 (en) | 2002-02-22 | 2004-04-06 | Arnold Robert Leiboff | Loop ostomy device and methods for its use |
| US6719709B2 (en) | 2000-08-31 | 2004-04-13 | Abbeymoor Medical, Inc. | Diagnostic urethral assembly and method |
| US6723084B1 (en) | 1999-01-15 | 2004-04-20 | Maginot Catheter Technologies, Inc. | Catheter systems having multilumen guide catheter and retractable working catheter positioned in at least one lumen thereof |
| US6743219B1 (en) | 2000-08-02 | 2004-06-01 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6743218B2 (en) | 1999-01-15 | 2004-06-01 | Cathlogic, Inc. | Retractable catheter systems and associated methods |
| US6758857B2 (en)* | 2000-11-13 | 2004-07-06 | Acmi Corporation | Treatment catheters with thermally insulated regions |
| US6814718B2 (en) | 2001-01-09 | 2004-11-09 | Rex Medical, L.P | Dialysis catheter |
| US6855137B2 (en) | 2002-03-07 | 2005-02-15 | Visionary Biomedical, Inc. | Catheter shaft with coextruded stiffener |
| US6881209B2 (en) | 2000-05-25 | 2005-04-19 | Cook Incorporated | Medical device including unitary, continuous portion of varying durometer |
| US6960163B2 (en) | 2002-06-13 | 2005-11-01 | Usgi Medical Inc. | Shape lockable apparatus and method for advancing an instrument through unsupported anatomy |
| US7008412B2 (en) | 1998-01-06 | 2006-03-07 | Cathlogic, Inc. | Subcutaneous port catheter system and associated method |
| US7025757B2 (en)* | 2001-02-08 | 2006-04-11 | Medrad, Inc. | Syringe loading devices for use with syringes and medical injectors |
| US7029467B2 (en) | 2002-07-16 | 2006-04-18 | Edwards Lifesciences Corporation | Multiple lumen catheter having a soft tip |
| US20060122709A1 (en) | 1993-02-19 | 2006-06-08 | Devonec Marian A | Prosthesis intended for the treatment of a natural lumen or tract, in particular an endo-urethral prosthesis |
| US7077841B2 (en) | 2001-03-26 | 2006-07-18 | Curon Medical, Inc. | Systems and methods employing a guidewire for positioning and stabilizing external instruments deployed within the body |
| US7122025B1 (en) | 1999-10-06 | 2006-10-17 | Astra Tech Ab | Rectal insertion device |
| US7156100B1 (en) | 1998-10-06 | 2007-01-02 | The Laryngeal Mask Company Ltd. | Laryngeal mask airway device |
Family Cites Families (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1026755A (en) | 1910-12-09 | 1912-05-21 | Birger Lundin | Coin-counting apparatus. |
| GB1026755A (en) | 1963-01-04 | 1966-04-20 | Frederic Eugene Basil Foley | Bag catheters |
| JPS51123399A (en) | 1975-04-18 | 1976-10-28 | Teijin Ltd | Flame proofing of polyester and cellulose blended cloth |
| GB2041760B (en) | 1978-08-28 | 1983-01-26 | Clayton R S | Colon cleansing system and technique |
| US4988822A (en) | 1986-05-29 | 1991-01-29 | Syntex (U.S.A.) Inc. | Intermediates for preparing 5-aroyl-1,2-dihydro-3H-pyrrolo(1,2-A)pyrrole-1,1-dicarboxylates |
| JPH0734819B2 (en) | 1986-12-27 | 1995-04-19 | 富士システムズ株式会社 | Medical Silicone Rubber Tube |
| JPH074427B2 (en) | 1986-12-27 | 1995-01-25 | 富士システムズ株式会社 | Tube with medical cuff |
| US4813422A (en) | 1987-03-06 | 1989-03-21 | Healthcare Technological Resources, Inc. | Bowel control probe and method for controlling bowel incontinence |
| JPH02255155A (en) | 1989-03-30 | 1990-10-15 | Ogita Kazuhide | External urethral opening fixing type catheter to be retained in bladder |
| JPH0371301A (en) | 1989-08-11 | 1991-03-27 | Sanyo Electric Co Ltd | Malfunction preventing method for microcomputer control apparatus |
| JPH0391357A (en) | 1989-09-04 | 1991-04-16 | Canon Inc | facsimile machine |
| SE465017B (en) | 1989-11-24 | 1991-07-15 | Lars Knutson | DEVICE FOR SEGMENTAL PERFUSION / ASPIRATION OF THE ENTREPRENEUR |
| JPH0715542Y2 (en) | 1990-06-04 | 1995-04-12 | アルケア株式会社 | Surgical anastomosis device |
| GB9020218D0 (en) | 1990-09-15 | 1990-10-24 | Smiths Industries Plc | Medico-surgical collection bag assemblies |
| JP2534783Y2 (en) | 1991-02-15 | 1997-05-07 | アルケア株式会社 | Surgical anastomosis device |
| GB9107296D0 (en) | 1991-04-08 | 1991-05-22 | Clinical Product Dev Ltd | Surgical device |
| US5755968A (en) | 1992-07-30 | 1998-05-26 | Stone; Andrew | Dialysis system and method for removing toxic matter from the serum of the large intestine |
| AU669754B2 (en)* | 1992-12-18 | 1996-06-20 | Becton Dickinson & Company | Barrier coating |
| JPH06197977A (en) | 1992-12-28 | 1994-07-19 | Japan Medical Dynamic Marketing Inc | Catheter |
| JPH06210002A (en) | 1993-01-21 | 1994-08-02 | Nippon Zeon Co Ltd | Catheter for alimentary canal |
| US5845125A (en) | 1993-06-21 | 1998-12-01 | Kabushiki Kaisha Toshiba | Debugger using class information and dynamic instance inter-relationships |
| US5419763B1 (en)* | 1994-01-04 | 1997-07-15 | Cor Trak Medical Inc | Prostatic drug-delivery catheter |
| US5429626A (en) | 1994-03-14 | 1995-07-04 | Fenton; Leonard | Ostomy pouch mounting arrangement |
| US5496296A (en) | 1994-06-06 | 1996-03-05 | Dansac A/S | Ostomy appliance with extrudable gasket |
| KR960005818A (en) | 1994-07-06 | 1996-02-23 | 김주용 | Method of Cleaning Semiconductor Devices |
| DE4436796C2 (en) | 1994-07-15 | 2000-11-09 | Via Log Medikalprodukte Gmbh K | Closure means for closing a natural intestinal exit |
| JP2844427B2 (en) | 1994-08-31 | 1999-01-06 | 重信 高根 | Method for producing rectal catheter |
| DK60795A (en) | 1995-05-29 | 1996-11-30 | Coloplast As | Ostomy Collection System |
| JP3019990U (en) | 1995-06-29 | 1996-01-12 | 富士システムズ株式会社 | Medical catheter |
| JP3297268B2 (en) | 1995-09-28 | 2002-07-02 | テルモ株式会社 | catheter |
| JP2001500023A (en) | 1996-02-12 | 2001-01-09 | メンター ユロロジー,インコーポレーテッド | Prostate tissue expander |
| US5722965A (en) | 1996-02-29 | 1998-03-03 | Bristol-Myers Squibb Company | Low profile ostomy system with repositionable pouch |
| JPH09253112A (en) | 1996-03-27 | 1997-09-30 | Shigenobu Takane | Excrement leakage prevention unit and anus protector for inserting appliance |
| JP3252274B2 (en) | 1996-12-21 | 2002-02-04 | 重信 高根 | Rectal catheter |
| US6096057A (en) | 1997-01-30 | 2000-08-01 | Klingenstein; R. James | Fecal incontinence device and method |
| JP3018234B2 (en) | 1997-03-01 | 2000-03-13 | 重信 高根 | Rectal catheter |
| JPH10305057A (en) | 1997-05-06 | 1998-11-17 | Katsuichi Kurashige | Auxiliary orthosis for stoma |
| US6485476B1 (en) | 1998-02-25 | 2002-11-26 | Zassi Medical Evolutions, Inc. | Continent ostomy port |
| JP3560853B2 (en) | 1999-06-18 | 2004-09-02 | 重信 高根 | Rectal insertion working device |
| US6769116B1 (en) | 1999-10-21 | 2004-07-27 | Oracle International Corporation | Diagnostic technique for debugging memory corruption |
| AUPQ641400A0 (en)* | 2000-03-23 | 2000-04-15 | Kleiner, Daniel E. | A device incorporating a hollow member for being positioned along a body cavity of a patient and method of positioning same |
| WO2001083017A1 (en)* | 2000-05-02 | 2001-11-08 | Wilson-Cook Medical, Inc. | Introducer device for catheters o.t.l. with eversible sleeve |
| JP4347705B2 (en) | 2002-04-09 | 2009-10-21 | ファン キム,ジェ | Medical intestinal tract manager for stool bypass of patients with bowel surgery |
| US8016816B2 (en) | 2003-09-09 | 2011-09-13 | Convatec Technologies Inc. | Fecal management appliance and method and apparatus for introducing same |
| US7025557B2 (en) | 2004-01-14 | 2006-04-11 | Concepts Eti, Inc. | Secondary flow control system |
| US7526754B2 (en) | 2005-02-28 | 2009-04-28 | Sap Portals Israel Ltd. | Memory debugging tool |
| US7735069B2 (en) | 2006-02-09 | 2010-06-08 | International Business Machines Corporation | Creating software debug breakpoints activated by specific call patterns |
| JP4852621B2 (en) | 2009-03-03 | 2012-01-11 | インターナショナル・ビジネス・マシーンズ・コーポレーション | Method for tracking allocation location of object in program, computer system and computer program |
- 2004
- 2004-08-28USUS10/929,136patent/US8016816B2/ennot_activeExpired - Lifetime
- 2004-09-08JPJP2004260604Apatent/JP4768245B2/ennot_activeExpired - Lifetime
- 2004-09-08MXMX2009005136Apatent/MX345313B/enunknown
- 2004-09-08ATAT04021323Tpatent/ATE415897T1/enactive
- 2004-09-08ESES04021323Tpatent/ES2321510T5/ennot_activeExpired - Lifetime
- 2004-09-08DKDK04021323.3Tpatent/DK1514572T4/enactive
- 2004-09-08PLPL04021323Tpatent/PL1514572T5/enunknown
- 2004-09-08PTPT04021323Tpatent/PT1514572E/enunknown
- 2004-09-08EPEP08167258.6Apatent/EP2027832B1/ennot_activeExpired - Lifetime
- 2004-09-08EPEP04021323.3Apatent/EP1514572B2/ennot_activeExpired - Lifetime
- 2004-09-08DEDE602004018080Tpatent/DE602004018080D1/ennot_activeExpired - Lifetime
- 2004-09-08ZAZA200407195Apatent/ZA200407195B/enunknown
- 2004-09-09AUAU2004210541Apatent/AU2004210541A1/ennot_activeAbandoned
- 2004-09-09CACA2480843Apatent/CA2480843C/ennot_activeExpired - Fee Related
- 2004-09-09CACA2797832Apatent/CA2797832C/ennot_activeExpired - Fee Related
- 2010
- 2010-11-04JPJP2010246996Apatent/JP5155374B2/ennot_activeExpired - Lifetime
- 2011
- 2011-08-02USUS13/196,375patent/US8827970B2/ennot_activeExpired - Lifetime
- 2014
- 2014-08-04USUS14/451,243patent/US10772755B2/enactiveActive
Patent Citations (145)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US565386A (en) | 1896-08-04 | Rectal irrigating-dilator | ||
| US2457244A (en) | 1943-06-22 | 1948-12-28 | Otis F Lamson | Medical appliance for control of enemata |
| US2494393A (en) | 1949-02-05 | 1950-01-10 | Otis F Lamson | Removable appliance for use as an artificial dam in cases of rectal incontinence |
| US2813531A (en) | 1955-09-26 | 1957-11-19 | Albert F Lee | Cannula |
| US3459175A (en) | 1966-04-08 | 1969-08-05 | Roscoe E Miller | Medical device for control of enemata |
| US3487837A (en) | 1967-02-06 | 1970-01-06 | Roy A Petersen | Device for holding catheters in position |
| US3509884A (en) | 1967-09-13 | 1970-05-05 | William Bell | Rectal balloon catheter |
| US3548828A (en) | 1968-06-14 | 1970-12-22 | John J Vasile | Excrement collecting appliance |
| US3543744A (en) | 1968-11-29 | 1970-12-01 | Edwin Lepar | Equipment for administering enemas for radiological purposes |
| US3802418A (en) | 1971-02-16 | 1974-04-09 | R Clayton | Colon catheter |
| US3884242A (en) | 1971-03-29 | 1975-05-20 | Mpc Kurgisil | Catheter assembly |
| US3766920A (en) | 1971-07-21 | 1973-10-23 | Ezem Co | Enemata administering device |
| US3938521A (en) | 1972-06-22 | 1976-02-17 | M.E.D.S. Corporation | Collecting bag |
| US3734100A (en) | 1973-05-07 | 1973-05-22 | Medical Products Corp | Catheter tubes |
| US3937224A (en) | 1974-04-11 | 1976-02-10 | Uecker Ronald L | Colostomy catheter |
| US3983879A (en) | 1974-07-25 | 1976-10-05 | Western Acadia, Incorporated | Silicone catheter |
| US4013077A (en) | 1974-09-23 | 1977-03-22 | M.E.D.S. Corporation | Collecting device |
| DE2447996A1 (en) | 1974-10-09 | 1976-04-22 | Hadar Y Roennqvist | Disposable sanitary faeces collection bag - includes disposable bag fitting into soft plastics tube constricted in centre |
| US4030500A (en) | 1974-12-16 | 1977-06-21 | Hadar Yngve Ronnquist | Fecal matter collector |
| US4067335A (en) | 1975-02-12 | 1978-01-10 | Beverley Silvanov | Matter collecting unit |
| GB1522391A (en) | 1975-02-12 | 1978-08-23 | Silvanov B | Method and apparatus for collecting human waste matter |
| US4121589A (en) | 1975-08-06 | 1978-10-24 | Mcdonnell Roy Edward | Ostomy appliance |
| FR2326208A1 (en) | 1975-10-01 | 1977-04-29 | Clayton Ralph | Rectal catheter device for anal insertion - has inflatable ring and spacing limiting projection |
| US4019515A (en) | 1975-12-08 | 1977-04-26 | Daniel Kornblum | Enemata administering device |
| US4117847A (en) | 1976-02-05 | 1978-10-03 | Clayton Ralph S | Colon catheter |
| US4182332A (en) | 1978-02-17 | 1980-01-08 | Delaney Richard P | Rectal catheter |
| US4403982A (en) | 1978-08-28 | 1983-09-13 | Clayton Ralph S | Colon cleansing system and technique |
| US4285341A (en) | 1978-11-22 | 1981-08-25 | Pollack Charles N | Extracorporeal cannula apparatus with retractable intralumenal balloon and method for using same |
| US4368739A (en) | 1979-07-18 | 1983-01-18 | Nelson Jr Richard L | Long intestinal catheter |
| US4344434A (en) | 1981-06-01 | 1982-08-17 | Santa Barbara Medical Foundation Clinic | Ileostomy appliance and method for implanting the same |
| US4496356A (en) | 1982-09-29 | 1985-01-29 | Leon Lognion | Anal excretion collecting rectal catheter |
| US4516578A (en) | 1982-09-30 | 1985-05-14 | Luther Shuffield | Rectal device and method of inserting same |
| US4471782A (en) | 1982-09-30 | 1984-09-18 | Luther Shuffield | Medical implement for use in rectum and method for inserting same |
| EP0109897A1 (en) | 1982-11-17 | 1984-05-30 | Noel C. Dr. David | Rectal probes for baritic washing |
| US4583983A (en) | 1983-10-25 | 1986-04-22 | Einhorn Carol J | Female urinary drainage device |
| US4662890A (en) | 1984-10-09 | 1987-05-05 | Waters Instruments, Inc. | Tubular medical prosthesis |
| US4721508A (en) | 1984-10-09 | 1988-01-26 | Waters Instruments, Inc. | Ostomy prosthesis |
| US4637814A (en) | 1985-04-05 | 1987-01-20 | Arnold Leiboff | Method and apparatus for intestinal irrigation |
| US4596554A (en) | 1985-04-19 | 1986-06-24 | Dastgeer Ghulam M | Colo-rectal evacuator |
| US4686985A (en) | 1985-05-15 | 1987-08-18 | Lottick Edward A | Anal dilator and occluder |
| US4750902A (en) | 1985-08-28 | 1988-06-14 | Sonomed Technology, Inc. | Endoscopic ultrasonic aspirators |
| EP0246176A2 (en) | 1986-05-12 | 1987-11-19 | Biodan Medical Systems Ltd | Catheter and probe |
| US4750488A (en) | 1986-05-19 | 1988-06-14 | Sonomed Technology, Inc. | Vibration apparatus preferably for endoscopic ultrasonic aspirator |
| JPS62281955A (en) | 1986-05-30 | 1987-12-07 | 株式会社メデイコン | Urine sampling bag |
| US4676778A (en) | 1986-10-27 | 1987-06-30 | Nelson Jr Richard L | Long intestinal catheter with sump |
| EP0274415A2 (en) | 1987-01-06 | 1988-07-13 | Howell, Richard Owen | Colonic irrigator |
| US4826481A (en) | 1987-05-27 | 1989-05-02 | Abbott Labs. | Enteral feeding device |
| US5057073A (en) | 1988-04-21 | 1991-10-15 | Vas-Cath Incorporated | Dual lumen catheter |
| US4986822A (en) | 1988-09-06 | 1991-01-22 | Anderson Irvin B | Rectal-colon dilator and collector assembly |
| GB2224212B (en) | 1988-09-22 | 1993-01-06 | Aegis Medical Inc | Colonic lavage apparatus |
| US5053023A (en) | 1988-10-25 | 1991-10-01 | Vas-Cath Incorporated | Catheter for prolonged access |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US5041100A (en) | 1989-04-28 | 1991-08-20 | Cordis Corporation | Catheter and hydrophilic, friction-reducing coating thereon |
| US5421827A (en) | 1989-05-08 | 1995-06-06 | Temple; John E. | Fecal incontinence device and applicator therefor |
| US5295984A (en) | 1989-12-07 | 1994-03-22 | Ultrafem, Inc. | Vaginal discharge collection device and intravaginal drug delivery system |
| JPH0391357U (en) | 1989-12-29 | 1991-09-18 | ||
| US5404881A (en) | 1990-04-06 | 1995-04-11 | Technomed International | Transrectal probe |
| GB2243553A (en) | 1990-04-30 | 1991-11-06 | Squibb & Sons Inc | Rectal block |
| JP3019990B2 (en) | 1990-06-15 | 2000-03-15 | 株式会社ジーシー | Dental unit |
| US5279596A (en) | 1990-07-27 | 1994-01-18 | Cordis Corporation | Intravascular catheter with kink resistant tip |
| US5261898A (en) | 1990-11-13 | 1993-11-16 | Polin Stanton G | Temporary colostomy apparatus |
| US5080650A (en) | 1991-01-28 | 1992-01-14 | Abbott Laboratories | Gastrostomy tube |
| US5171305A (en) | 1991-10-17 | 1992-12-15 | Imagyn Medical, Inc. | Linear eversion catheter with reinforced inner body extension |
| US5216898A (en) | 1992-01-14 | 1993-06-08 | Astec Industries, Inc. | Cooling apparatus |
| US5693036A (en) | 1992-05-12 | 1997-12-02 | E. R. Squibb & Sons Inc. | Method of injection moulding an undercut formation on a circular body and a closure assembly including a coupling element |
| KR960005818Y1 (en) | 1992-10-30 | 1996-07-16 | 우석형 | Paper guide fixing device |
| US20060122709A1 (en) | 1993-02-19 | 2006-06-08 | Devonec Marian A | Prosthesis intended for the treatment of a natural lumen or tract, in particular an endo-urethral prosthesis |
| US5766209A (en) | 1993-02-19 | 1998-06-16 | Devonec; Marian A. | Prosthesis intended for the treatment of a natural lumen or tract, in particular an endo-urethral prosthesis |
| US5603698A (en) | 1993-04-13 | 1997-02-18 | Boston Scientific Corporation | Prosthesis delivery system |
| US5984964A (en) | 1993-04-13 | 1999-11-16 | Boston Scientific Corporation | Prothesis delivery system |
| CN2174997Y (en) | 1993-04-26 | 1994-08-24 | 许如良 | Multi-purpose drainage tube for anal and bowels |
| US5342321A (en) | 1993-05-18 | 1994-08-30 | Teleflex, Inc. | Low profile gastrostomy catheter |
| US5423764A (en) | 1993-06-14 | 1995-06-13 | Fry; William A. | Lavage apparatus |
| US5545149A (en) | 1993-06-25 | 1996-08-13 | Medtronic, Inc. | Method of catheter segment attachment |
| US5569216A (en)* | 1993-12-02 | 1996-10-29 | Kim; Jae H. | Multipurpose colostomy device having balloons on an end thereof |
| US5897537A (en) | 1994-02-14 | 1999-04-27 | Scimed Life Systems, Inc. | Guide catheter having a plurality of filled distal grooves |
| US5569218A (en) | 1994-02-14 | 1996-10-29 | Scimed Life Systems, Inc. | Elastic guide catheter transition element |
| US5911715A (en) | 1994-02-14 | 1999-06-15 | Scimed Life Systems, Inc. | Guide catheter having selected flexural modulus segments |
| US5904701A (en) | 1994-02-14 | 1999-05-18 | Daneshvar; Yousef | Device for aiding procedural and therapeutic interventions of the gastrointestinal tract |
| US5674197A (en) | 1994-07-01 | 1997-10-07 | Cordis Corporation | Controlled flexible catheter |
| US5520669A (en) | 1994-07-19 | 1996-05-28 | Mulholland; Kevin J. | Intra-rectal drain and receptacle for fecal incontinence |
| US6406453B1 (en) | 1995-04-26 | 2002-06-18 | Medtronic Xomed, Inc. | Composite ventilation tube |
| US5782745A (en) | 1995-11-13 | 1998-07-21 | Benderev; Theodore V. | Devices and methods for assessment and treatment of urinary and fecal incontinence |
| US5860952A (en) | 1996-01-11 | 1999-01-19 | C. R. Bard, Inc. | Corporeal access tube assembly and method |
| US5697365A (en) | 1996-01-18 | 1997-12-16 | Pell; Donald M. | Endotracheal tube construction and method for intubating a patient |
| US5632271A (en) | 1996-03-22 | 1997-05-27 | Brain; Archibald I. J. | Laryngeal mask with gastric-drainage feature |
| US5785641A (en) | 1996-05-08 | 1998-07-28 | Urocath Corporation | Male indwelling urethral catheter sizing system and insertion method |
| US5807314A (en) | 1996-10-11 | 1998-09-15 | Abbott Laboratories | Feeding tube and method for placing same |
| US6698428B2 (en) | 1996-11-06 | 2004-03-02 | Archibald I. J. Brain | Endotracheal tube construction |
| US6468245B2 (en) | 1996-11-27 | 2002-10-22 | Colorplast A/S | Irrigation device |
| US5791036A (en) | 1996-12-23 | 1998-08-11 | Schneider (Usa) Inc | Catheter transition system |
| US5906605A (en) | 1997-01-10 | 1999-05-25 | Cardiac Pathways Corporation | Torquable guiding catheter for basket deployment and method |
| US5964732A (en) | 1997-02-07 | 1999-10-12 | Abbeymoor Medical, Inc. | Urethral apparatus with position indicator and methods of use thereof |
| US6254570B1 (en) | 1997-04-07 | 2001-07-03 | Vance Products, Inc. | Back-up retention member drainage catheter |
| US5971967A (en) | 1997-08-19 | 1999-10-26 | Abbeymoor Medical, Inc. | Urethral device with anchoring system |
| US5941860A (en) | 1997-11-03 | 1999-08-24 | Wheeler; Alton D. | Fecal pouch and installation |
| US6286555B1 (en) | 1997-11-03 | 2001-09-11 | Stm Medizintechnik Starnberg Gmbh | Reinforced roll-back tube for use with endoscopes and the like |
| EP0913165B1 (en) | 1997-11-03 | 2007-02-28 | invendo medical GmbH | Eversion catheter construction |
| US6240231B1 (en) | 1997-12-22 | 2001-05-29 | Micrus Corporation | Variable stiffness fiber optic shaft |
| US7008412B2 (en) | 1998-01-06 | 2006-03-07 | Cathlogic, Inc. | Subcutaneous port catheter system and associated method |
| US6296631B2 (en) | 1998-04-28 | 2001-10-02 | Sean L. Chow | Flow directed catheter |
| CN2325054Y (en) | 1998-04-30 | 1999-06-23 | 关怀敏 | Balloon catheter anal canal |
| US6217565B1 (en) | 1998-07-16 | 2001-04-17 | Mark Cohen | Reinforced variable stiffness tubing |
| US20030105485A1 (en)* | 1998-09-09 | 2003-06-05 | Embol-X, Inc. | Introducer/dilator with balloon protection and methods of use |
| US7156100B1 (en) | 1998-10-06 | 2007-01-02 | The Laryngeal Mask Company Ltd. | Laryngeal mask airway device |
| US20020016607A1 (en)* | 1998-12-01 | 2002-02-07 | Atropos Limited | Medical device comprising an evertable sleeve |
| JP2000167041A (en) | 1998-12-02 | 2000-06-20 | Shigenobu Takane | Defacation device |
| US6413228B1 (en) | 1998-12-28 | 2002-07-02 | Pro Duct Health, Inc. | Devices, methods and systems for collecting material from a breast duct |
| US5997546A (en) | 1999-01-07 | 1999-12-07 | Ballard Medical Products | Gastric balloon catheter with improved balloon orientation |
| US6743218B2 (en) | 1999-01-15 | 2004-06-01 | Cathlogic, Inc. | Retractable catheter systems and associated methods |
| US6723084B1 (en) | 1999-01-15 | 2004-04-20 | Maginot Catheter Technologies, Inc. | Catheter systems having multilumen guide catheter and retractable working catheter positioned in at least one lumen thereof |
| US6585705B1 (en) | 1999-01-15 | 2003-07-01 | Maginot Catheter Technologies, Inc. | Retractable catheter systems |
| US6171295B1 (en) | 1999-01-20 | 2001-01-09 | Scimed Life Systems, Inc. | Intravascular catheter with composite reinforcement |
| JP2000354634A (en) | 1999-06-15 | 2000-12-26 | Shigenobu Takane | Rectal catheter and contrast medium enema device |
| US7122025B1 (en) | 1999-10-06 | 2006-10-17 | Astra Tech Ab | Rectal insertion device |
| US6342052B1 (en) | 1999-11-02 | 2002-01-29 | Hector D. Allende | Anorectal apparatus |
| JP3071301U (en) | 2000-02-25 | 2000-08-29 | 株式会社オーセンティックスインターナショナル | Catheter for draining feces |
| US6881209B2 (en) | 2000-05-25 | 2005-04-19 | Cook Incorporated | Medical device including unitary, continuous portion of varying durometer |
| US6743219B1 (en) | 2000-08-02 | 2004-06-01 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6719709B2 (en) | 2000-08-31 | 2004-04-13 | Abbeymoor Medical, Inc. | Diagnostic urethral assembly and method |
| WO2002026293A1 (en) | 2000-09-28 | 2002-04-04 | Kim Jae Hwang | Improved colostomy device |
| JP2002126094A (en) | 2000-10-24 | 2002-05-08 | Terumo Corp | Connector system |
| US6663614B1 (en) | 2000-11-06 | 2003-12-16 | Advanced Cardiovascular Systems, Inc. | Catheter shaft having variable thickness layers and method of making |
| US6758857B2 (en)* | 2000-11-13 | 2004-07-06 | Acmi Corporation | Treatment catheters with thermally insulated regions |
| JP2002153564A (en) | 2000-11-22 | 2002-05-28 | Shigenobu Takane | Rectum catheter |
| US6575934B2 (en) | 2000-12-21 | 2003-06-10 | Advanced Cardiovascular Systems, Inc. | Low profile catheter |
| US6814718B2 (en) | 2001-01-09 | 2004-11-09 | Rex Medical, L.P | Dialysis catheter |
| US7390322B2 (en) | 2001-01-09 | 2008-06-24 | Rex Medical, L.P. | Dialysis catheter and methods of insertion |
| US7025757B2 (en)* | 2001-02-08 | 2006-04-11 | Medrad, Inc. | Syringe loading devices for use with syringes and medical injectors |
| US7077841B2 (en) | 2001-03-26 | 2006-07-18 | Curon Medical, Inc. | Systems and methods employing a guidewire for positioning and stabilizing external instruments deployed within the body |
| CN2489797Y (en) | 2001-04-20 | 2002-05-08 | 胡晓燕 | Balloon-type indwelling catheter for stool |
| US6527755B1 (en) | 2001-04-20 | 2003-03-04 | Fouad A. Salama | Feces control device |
| US20020173771A1 (en) | 2001-05-17 | 2002-11-21 | La Tapachulteca Ii | Lesion restoration catheter for the colon and method for using same |
| US6635047B2 (en) | 2001-08-06 | 2003-10-21 | Scimed Life Systems, Inc. | Integrated polymer and braid for intravascular catheters |
| US6716209B2 (en) | 2002-02-22 | 2004-04-06 | Arnold Robert Leiboff | Loop ostomy device and methods for its use |
| US6855137B2 (en) | 2002-03-07 | 2005-02-15 | Visionary Biomedical, Inc. | Catheter shaft with coextruded stiffener |
| US20040030380A1 (en)* | 2002-04-24 | 2004-02-12 | Sun Biomedical, Ltd. | Drug-delivery endovascular stent and method for treating restenosis |
| US6960163B2 (en) | 2002-06-13 | 2005-11-01 | Usgi Medical Inc. | Shape lockable apparatus and method for advancing an instrument through unsupported anatomy |
| US7029467B2 (en) | 2002-07-16 | 2006-04-18 | Edwards Lifesciences Corporation | Multiple lumen catheter having a soft tip |
| US20040039348A1 (en)* | 2002-08-21 | 2004-02-26 | Kim Jae H. | Bowel management system |
| US7147627B2 (en) | 2002-08-21 | 2006-12-12 | Hollister Incorporated | Bowel management system |
| US20090030386A1 (en) | 2002-08-21 | 2009-01-29 | Hollister Incorporated | Bowel management system and waste collection bag therefor |
| US20090030387A1 (en) | 2002-08-21 | 2009-01-29 | Hollister Incorporated | Bowel management system and waste collection bag therefor |
| US20090149824A1 (en) | 2002-08-21 | 2009-06-11 | Hollister Incorporated | Bowel management system |
| US7722583B2 (en) | 2002-08-21 | 2010-05-25 | Hollister Incorporated | Bowel management system and waste collection bag therefor |
Non-Patent Citations (38)
| Title |
|---|
| "A Unique System Designed to Protect Patients and Practitiioners from Fecal Contact and Contamination."; Zassi Medical Evolutions-Bowel Management System; 2003; 10 pages. |
| "Clinical Application of Continent Anal Plug in Bed-Ridden Patents With Intractable Diarrhea," Kim et al. (presented as poster at 2000 Annual Meeting of the ARCRS (Jun. 24-29, 2000), Boston, Massachusetts). |
| A Continent Ileostomy Device, Ann. Surg., Pemberton, J., et al., 197(5):618-26 May 1983. |
| Anal Sphincter Dysfunction in Parkinson's Disease, Mathers et al., Archives of Neurology, vol. 46, No. 10, Oct. 1989. |
| Australian Office Action, dated Apr. 11, 2008 (3 pages). |
| Bowel Management for Fecal Incontinence in Patients With Anorectal Malformations, Peiia, A., J. Pediatric Surg., 33(1):133-7, Jan. 1998. |
| Chronic Constipation and Fecal Incontinence in Children with Neurological and Neuromusular Handicap, Di Lorenzo, C., Journal of Pediatric Gastroenterology & Nutrition: vol. 25, pp. 37-39 (1997). |
| Colostomy Tube: New Device for a Continent Colostomy, Kosorak, P., M.D., Ph.D., Dis. Colon Rectum, vol. 38, No. 7, Jul. 1995. |
| Definition of "few"; Encarta World Dictionary, North American Edition.* |
| European Examination Report, EP 03705823, mailed Apr. 8, 2009 (4 pages). |
| International Search Report for PCT/US03/01594. |
| Jaehwang Kim, M.D., et al. Clinical Application of a Continent Anal Plug in Bedridden Patients with Intractable Diarrhea, Dis Colon Rectum, vol. 44, No. 8, pp. 1162-1167 (Aug. 2001). |
| Japanese Office Action from Japan Patent Application No. 2004530762 (with translation). |
| Japanese Office Action, mailed Jun. 10, 2008, from Japan Patent Application No. 2004-530762 (with translation). |
| Jnl. of the Korean Soc'y of Coloproctology, vol. 14, No. 3 (presented at the 16th conference of the ISUCRS, held in Malmo, Sweden in 1998), "Application of a New Colostomy Device in Incontinent Dog Model" (with translation). Lim, Kim and Shin. |
| Jnl. Of the Korean Soc'y of Coloproctology, vol. 16, No. 3, 2000, "Clinical Application of Continent Anal Plug in Bed-Ridden Patient with Intractable Diarrhea" (with translation). Kim et al. |
| Jnl. of the Korean Soc'y. of Colo proctology, vol. 14, No. 3, "Passive Bowel Movement Effects Using a New Colostomy Device: An Acute Experiment on a Dog" (with translation). Kim et al. |
| Letter from K.H. Shin of Yushin Medical Co., Ltd. to Serjeants, UK, dated Sep. 24, 2009 indicating mailing of samples, which samples Applicant believes to be the same as a Zassi BMS 4CM submitted to the USPTO in this application in a seperate Information Disclosure Statement. |
| Manometric Measurement of Anal Canal Resting Tone-Comparison of a Rectosphinteric Balloon Probe with a Water-Perfused Catheter Assembly, Allen, et al., Digestive Diseases and Sciences, vol. 43, No. 7 (Jul. 1998), pp. 1411-1415. |
| Manometric Measurement of Anal Canal Resting Tone-Comparison ofa Rectosphinteric Balloon Probe with a Water Perfused Catheter Assembly, Allen, et al., Digestive Diseases and Sciences, vol. 43, No. 7 (Jul. 1998), pp. 1411-1415. |
| Neurogenic colorectal dysfunction-use of new antegrade and retrograde colonic wash-out methods, International Medical Soc'y of Paraplegia, Spinal Cord, 2000 Apr. 38(4): 255-261. (Christensen, P., et al.). |
| New Double Balloon Catheter for Enema Examination, Umeda Kazuo et al., Image Information Medical vol. 24 No. 21, Oct. 1992. |
| Office Action, mailed Jun. 22, 2009, from U.S. Appl. No. 11/553,731. |
| Peritoneal Dialysis Access and Exit-Site Care Including Surgical Aspects, Twardowski, Z.J., et al., Chap. 9, Textbook of Peritoneal Dialysis, 2nd Ed., Kluwer Academic Publishers, Dordrecht, The Netherlands, 2000 (pp. 317-362). |
| Photographing Technique to Achieve Good Double Contrast Film of a Complex Sigmoid Colon in an Enema Procedure, Akira Ogawa: Mejiro NT Building Clinic, Therapeutic Research, vol. 13, suppl. 2, 1992. |
| Photographs of a device, which device Applicant believes to be a Zassi BMS 4CM, and which device was made by Zassi Medical, which is owned by Hollister Inc., 2000 Hollister Drive, Libertyville, IL 60048, which device was submitted in an Opposition proceeding against European Patent Application No. 20040021323; photographs taken by Julie Mays on or about Dec. 9, 2010. |
| Poster Abstract, "Clinical Application of a Continent Anal Plug in Bed-Ridden Patients with Intractable Diarrhea", J. Kim, H. Shin, Diseases of the Colon & Rectum, May 2000, vol. 43, No. 5, cover, index, and p. A49. |
| Poster, Clinical Application of Continent Anal Plug in Bed-Ridden Patient With Intractable Diarrhea, Jaehwang Kim, M.D., et al., Jun. 25-29, 2000 presentation at American Society of Colon and Rectal Surgeons in Boston, MA. |
| Problem Solving and Troubleshooting: The Indwelling Catheter, Moore, K., R.N., et al., Jnl. of Wound, Ostomy and Continence Nurses Soc 'y, 1995. |
| Problems in Wet Colostomy Management Following Radical Pelvic Surgery-Use OfA New Giant Balloon Catheter, Amer. Jnl. of Surgery, Sep. 1952, p. 378. |
| Research on Functionalization of Enema Catheter, Ikigaku vol. 69, No. 10 (1999). Eri Nakamura et al. |
| Slide presentation, Clinical Application of a New Colostomy Device, IMSOP, Denmark, 1999. |
| Technique of a Disposable Barium Enema Examination Device. Tsuruoka Masanori, Therapeutic Research vol. 13, suppl. 2, 1992. |
| The Bowel Management Tube: An Effective Means for Controlling Fecal Incontinence, Blair, G.K., et al., Jnl. of Pediatric Surgery, vol. 27, Issue 10, Oct. 1992, pp. 1269-1272. |
| The Enema Continence Catheter in Spina Bifida: Successful Bowel Management. Shandling, B., et al., J. Pediatr. Surg., 22(3):271-3, Mar. 1987. |
| The Procon Incontinence Device: A New Nonsurgical Approach to Preventing Episodes of Fecal Incontinence, The American Journal of Gastroenterology, Giamundo, Paulo, M.D. et al., vol. 97, Issue 9, pp. 2328-2332 (Mar. 26, 2004)(Work presented at Digestive Disease Week, Atlanta, GA, May 20-23, 2001). |
| The Rectal Trumpet: Use of a Nasopharyngeal Airway to Contain Fecal Incontinence in Critically III Patients, Grogan, Tracy A. RN, et al., Jnl. of Wound, Ostomy and Continence Nursing: vol. 29, Issue 4, pp. 193-201, Jul. 2002. |
| Why do patients with faecal impaction have faecal incontinence, Read, N.W., et al., Gut, vol. 27, pp. 283-287 (1986). |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10328101B2 (en)* | 2001-09-25 | 2019-06-25 | Jenny Colleen McCloskey | Inactivation of papillomavirus |
| US20150099011A1 (en)* | 2001-09-25 | 2015-04-09 | Jenny Colleen McCloskey | Inactivation of papillomavirus |
| US9839509B2 (en) | 2002-12-23 | 2017-12-12 | Python Medical, Inc. | Stomach peristalsis device and method |
| US20170181837A1 (en)* | 2002-12-23 | 2017-06-29 | Python Medical, Inc. | Implantable digestive tract organ |
| US10076405B2 (en)* | 2002-12-23 | 2018-09-18 | Python Medical, Inc. | Implantable digestive tract organ |
| US10772755B2 (en) | 2003-09-09 | 2020-09-15 | Convatec Technologies Inc. | Fecal management appliance and method and apparatus for introducing same |
| US20090227971A1 (en)* | 2006-10-17 | 2009-09-10 | C. R. Bard, Inc. | Waste management system |
| US9456920B2 (en) | 2006-10-17 | 2016-10-04 | C. R. Bard, Inc. | Waste management system |
| US9463110B2 (en) | 2006-10-17 | 2016-10-11 | C. R. Bard, Inc. | Waste management system |
| US10660784B2 (en) | 2006-10-17 | 2020-05-26 | C. R. Bard, Inc. | Waste management system |
| US8926577B2 (en) | 2006-10-17 | 2015-01-06 | C. R. Bard, Inc. | Waste management system |
| US8597266B2 (en) | 2006-10-17 | 2013-12-03 | C. R. Bard, Inc. | Waste management system |
| US9855163B2 (en) | 2006-10-17 | 2018-01-02 | C. R. Bard, Inc. | Waste management system |
| US8777912B2 (en) | 2007-07-22 | 2014-07-15 | C. R. Bard, Inc. | Waste management system |
| USRE46306E1 (en) | 2008-08-11 | 2017-02-14 | Centurion Medical Products Corporation | Fecal impaction removal tool |
| US9033944B2 (en) | 2010-08-12 | 2015-05-19 | Colo-Majic Enterprises Ltd. | Ostomy pouch apparatus with closable opening |
| US9808606B2 (en) | 2011-02-17 | 2017-11-07 | Convatec Technologies Inc. | Valve system for inflatable medical device |
| WO2013074763A1 (en) | 2011-11-16 | 2013-05-23 | Convatec Technologies, Inc. | Apparatus for preventing over inflation of the retention balloon in medical catheters and airway devices |
| US9623201B2 (en) | 2011-11-16 | 2017-04-18 | Convatec Technologies Inc. | Apparatus for preventing over inflation of the retention balloon in medical catheters and airway devices |
| US10751493B2 (en) | 2011-11-16 | 2020-08-25 | Convatec Technologies Inc. | Apparatus for preventing over inflation of the retention balloon in medical catheters and airway devices |
| US9889280B2 (en) | 2013-04-01 | 2018-02-13 | Indiana University Research And Technology Corporation | Rectal catheter configured for pediatric care |
| US9669205B2 (en) | 2013-08-01 | 2017-06-06 | Convatec Technologies Inc. | Self-closing bag connector |
| US10507318B2 (en) | 2013-08-01 | 2019-12-17 | Convatec Technologies Inc. | Self-closing bag connector |
| US10576262B2 (en) | 2015-05-18 | 2020-03-03 | Convatec Technologies Inc. | Spring-loaded bag connector |
| US10842976B2 (en) | 2015-10-29 | 2020-11-24 | Convatec Technologies Inc. | Valve system for inflatable devices |
| US11524147B2 (en)* | 2015-10-29 | 2022-12-13 | Convatec Technologies, Inc. | Valve system for inflatable devices |
| US11534594B2 (en) | 2016-01-19 | 2022-12-27 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US11478625B2 (en) | 2016-01-19 | 2022-10-25 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US11478626B2 (en) | 2016-01-19 | 2022-10-25 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US11883624B2 (en) | 2016-01-19 | 2024-01-30 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US12115334B2 (en) | 2016-01-19 | 2024-10-15 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US12144955B2 (en) | 2016-01-19 | 2024-11-19 | Wilmarc Holdings, Llc | Connector system for releasably connecting fluid conduits |
| US11992646B2 (en) | 2017-03-08 | 2024-05-28 | Wilmarc Holdings, Llc | Catch assembly for releasably connecting fluid conduits |
| US11648380B2 (en) | 2017-12-05 | 2023-05-16 | Jenny Colleen McCloskey | Device for treatment of a body canal and adjacent surfaces |
| US10667657B2 (en)* | 2018-07-31 | 2020-06-02 | Lien-Kung Wang | Stool drain assembly |
| US11529154B2 (en) | 2018-08-08 | 2022-12-20 | Wilmarc Holdings, Llc | Stool management system |
| US11103260B2 (en) | 2019-07-18 | 2021-08-31 | Medline Industries, Inc. | Fecal impaction removal device |
| US12233232B2 (en) | 2023-04-25 | 2025-02-25 | Wilmarc Holdings, Llc | Genderless aseptic connector |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2027832A3 (en) | 2009-06-03 |
| ATE415897T1 (en) | 2008-12-15 |
| CA2797832C (en) | 2014-11-18 |
| JP2005118544A (en) | 2005-05-12 |
| PL1514572T3 (en) | 2009-11-30 |
| DK1514572T3 (en) | 2009-04-06 |
| US8827970B2 (en) | 2014-09-09 |
| JP4768245B2 (en) | 2011-09-07 |
| EP1514572B2 (en) | 2021-03-31 |
| JP2011062533A (en) | 2011-03-31 |
| US20050054996A1 (en) | 2005-03-10 |
| EP2027832A2 (en) | 2009-02-25 |
| ES2321510T5 (en) | 2021-11-22 |
| PT1514572E (en) | 2009-03-12 |
| US20110313378A1 (en) | 2011-12-22 |
| AU2004210541A1 (en) | 2005-03-24 |
| EP1514572B1 (en) | 2008-12-03 |
| ZA200407195B (en) | 2006-05-31 |
| US20150005724A1 (en) | 2015-01-01 |
| CA2480843A1 (en) | 2005-03-09 |
| DE602004018080D1 (en) | 2009-01-15 |
| EP2027832B1 (en) | 2020-12-09 |
| JP5155374B2 (en) | 2013-03-06 |
| EP1514572A2 (en) | 2005-03-16 |
| CA2480843C (en) | 2014-04-29 |
| PL1514572T5 (en) | 2021-08-16 |
| MX345313B (en) | 2017-01-25 |
| DK1514572T4 (en) | 2021-07-05 |
| EP1514572A3 (en) | 2006-11-29 |
| US10772755B2 (en) | 2020-09-15 |
| CA2797832A1 (en) | 2005-03-09 |
| ES2321510T3 (en) | 2009-06-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8016816B2 (en) | Fecal management appliance and method and apparatus for introducing same | |
| CN108430562B (en) | Valve system for inflatable device | |
| US4137922A (en) | Dilator for cervical canal | |
| US5167239A (en) | Anchorable guidewire | |
| AU2015287992B2 (en) | Trans anal irrigation platform with bed module | |
| US3937224A (en) | Colostomy catheter | |
| AU2011203584B2 (en) | Fecal management appliance and method and apparatus for introducing same | |
| US11872106B2 (en) | Device and method for controlling fecal incontinence | |
| EP3854431A1 (en) | A nozzle for an enema device and an enema device comprising the nozzle | |
| JPH10502269A (en) | Urinary incontinence prevention device and incontinence prevention law | |
| US20250108158A1 (en) | An enema nozzle comprising retention means, and an enema system comprising said enema nozzle | |
| CA1046376A (en) | Dilator for cervical canal |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:BRISTOL-MYERS SQUIBB COMPANY, NEW YORK Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:GREGORY, CHRISTOPHER C.;REEL/FRAME:015372/0606 Effective date:20041111 | |
| AS | Assignment | Owner name:J.P. MORGAN EUROPE LIMITED, UNITED KINGDOM Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC INC.;REEL/FRAME:021371/0796 Effective date:20080801 Owner name:J.P. MORGAN EUROPE LIMITED,UNITED KINGDOM Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC INC.;REEL/FRAME:021371/0796 Effective date:20080801 | |
| AS | Assignment | Owner name:CONVATEC TECHNOLOGIES INC., NEVADA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BRISTOL-MYERS SQUIBB COMPANY;CONVATEC, INC.;REEL/FRAME:021754/0611 Effective date:20081027 Owner name:CONVATEC TECHNOLOGIES INC.,NEVADA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BRISTOL-MYERS SQUIBB COMPANY;CONVATEC, INC.;REEL/FRAME:021754/0611 Effective date:20081027 | |
| AS | Assignment | Owner name:CONVATEC INC., NEW JERSEY Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:J.P. MORGAN EUROPE LIMITED;REEL/FRAME:021890/0786 Effective date:20081028 Owner name:CONVATEC INC.,NEW JERSEY Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:J.P. MORGAN EUROPE LIMITED;REEL/FRAME:021890/0786 Effective date:20081028 | |
| AS | Assignment | Owner name:J.P. MORGAN EUROPE LIMITED, UNITED KINGDOM Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC TECHNOLOGIES INC.;REEL/FRAME:021901/0419 Effective date:20081028 Owner name:J.P. MORGAN EUROPE LIMITED,UNITED KINGDOM Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC TECHNOLOGIES INC.;REEL/FRAME:021901/0419 Effective date:20081028 | |
| AS | Assignment | Owner name:CONVATEC TECHNOLOGIES, INC., NEVADA Free format text:RELEASE OF SECURITY INTEREST AT 021901/0419;ASSIGNOR:J.P. MORGAN EUROPE LIMITED;REEL/FRAME:025580/0879 Effective date:20101223 | |
| AS | Assignment | Owner name:JPMORGAN CHASE BANK, N.A., AS COLLATERAL AGENT, TEXAS Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC TECHNOLOGIES, INC.;REEL/FRAME:025591/0856 Effective date:20101222 Owner name:JPMORGAN CHASE BANK, N.A., AS COLLATERAL AGENT, TE Free format text:SECURITY AGREEMENT;ASSIGNOR:CONVATEC TECHNOLOGIES, INC.;REEL/FRAME:025591/0856 Effective date:20101222 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| FPAY | Fee payment | Year of fee payment:4 | |
| AS | Assignment | Owner name:UNOMEDICAL A/S, DENMARK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:JPMORGAN CHASE BANK, N.A.;REEL/FRAME:040543/0357 Effective date:20161031 Owner name:CONVATEC TECHNOLOGIES, INC., NEVADA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:JPMORGAN CHASE BANK, N.A.;REEL/FRAME:040543/0357 Effective date:20161031 Owner name:CONVATEC LIMITED, UNITED KINGDOM Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:JPMORGAN CHASE BANK, N.A.;REEL/FRAME:040543/0357 Effective date:20161031 Owner name:UNOMEDICAL LIMITED, UNITED KINGDOM Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:JPMORGAN CHASE BANK, N.A.;REEL/FRAME:040543/0357 Effective date:20161031 | |
| AS | Assignment | Owner name:WILMINGTON TRUST (LONDON) LIMITED, AS COLLATERAL AGENT, UNITED KINGDOM Free format text:SECURITY AGREEMENT;ASSIGNORS:CONVATEC INC.;CONVATEC TECHNOLOGIES INC.;180 MEDICAL, INC.;AND OTHERS;REEL/FRAME:040552/0227 Effective date:20161031 Owner name:WILMINGTON TRUST (LONDON) LIMITED, AS COLLATERAL A Free format text:SECURITY AGREEMENT;ASSIGNORS:CONVATEC INC.;CONVATEC TECHNOLOGIES INC.;180 MEDICAL, INC.;AND OTHERS;REEL/FRAME:040552/0227 Effective date:20161031 | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 8TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1552); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment:8 | |
| AS | Assignment | Owner name:CONVATEC TECHNOLOGIES INC., NEVADA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:WILMINGTON TRUST (LONDON) LIMITED;REEL/FRAME:050831/0001 Effective date:20191024 | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 12TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1553); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment:12 |